Welcome to the new OASIS website! We have academic skills, library skills, math and statistics support, and writing resources all together in one new home.

- Walden University
- Faculty Portal

Common Assignments: Literature Review Matrix
Literature review matrix.
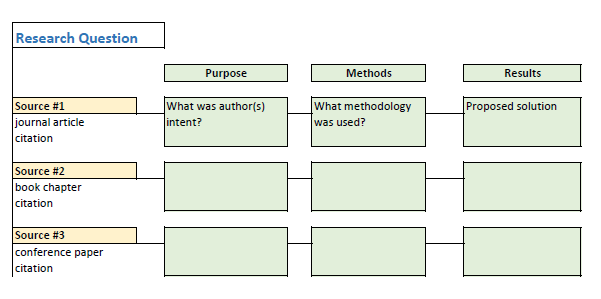
As you read and evaluate your literature there are several different ways to organize your research. Courtesy of Dr. Gary Burkholder in the School of Psychology, these sample matrices are one option to help organize your articles. These documents allow you to compile details about your sources, such as the foundational theories, methodologies, and conclusions; begin to note similarities among the authors; and retrieve citation information for easy insertion within a document.
You can review the sample matrixes to see a completed form or download the blank matrix for your own use.
- Literature Review Matrix 1 This PDF file provides a sample literature review matrix.
- Literature Review Matrix 2 This PDF file provides a sample literature review matrix.
- Literature Review Matrix Template (Word)
- Literature Review Matrix Template (Excel)
Related Resources
Didn't find what you need? Email us at [email protected] .
- Previous Page: Commentary Versus Opinion
- Next Page: Professional Development Plans (PDPs)
- Office of Student Disability Services
Walden Resources
Departments.
- Academic Residencies
- Academic Skills
- Career Planning and Development
- Customer Care Team
- Field Experience
- Military Services
- Student Success Advising
- Writing Skills
Centers and Offices
- Center for Social Change
- Office of Academic Support and Instructional Services
- Office of Degree Acceleration
- Office of Research and Doctoral Services
- Office of Student Affairs
Student Resources
- Doctoral Writing Assessment
- Form & Style Review
- Quick Answers
- ScholarWorks
- SKIL Courses and Workshops
- Walden Bookstore
- Walden Catalog & Student Handbook
- Student Safety/Title IX
- Legal & Consumer Information
- Website Terms and Conditions
- Cookie Policy
- Accessibility
- Accreditation
- State Authorization
- Net Price Calculator
- Cost of Attendance
- Contact Walden
Walden University is a member of Adtalem Global Education, Inc. www.adtalem.com Walden University is certified to operate by SCHEV © 2024 Walden University LLC. All rights reserved.
- Linguistics
- Composition Studies
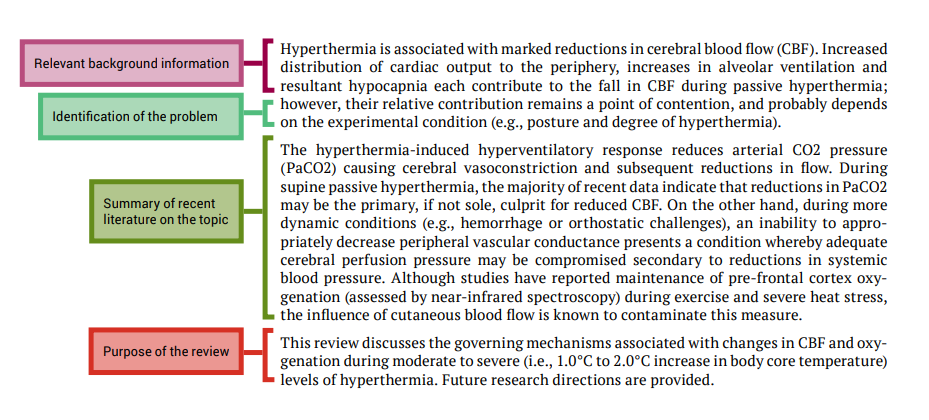
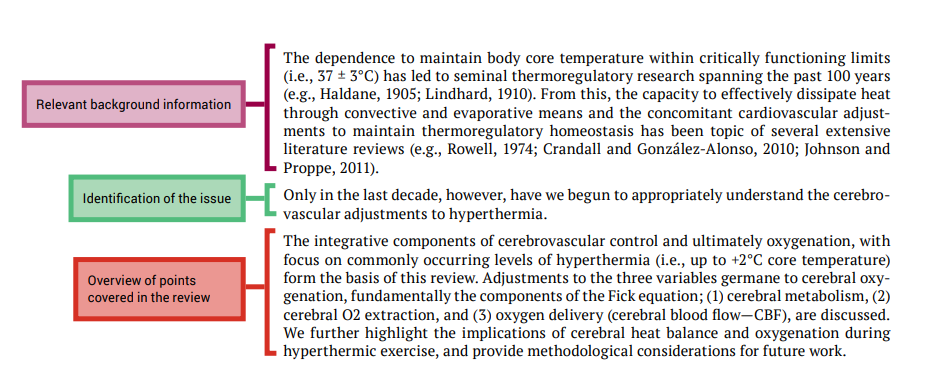
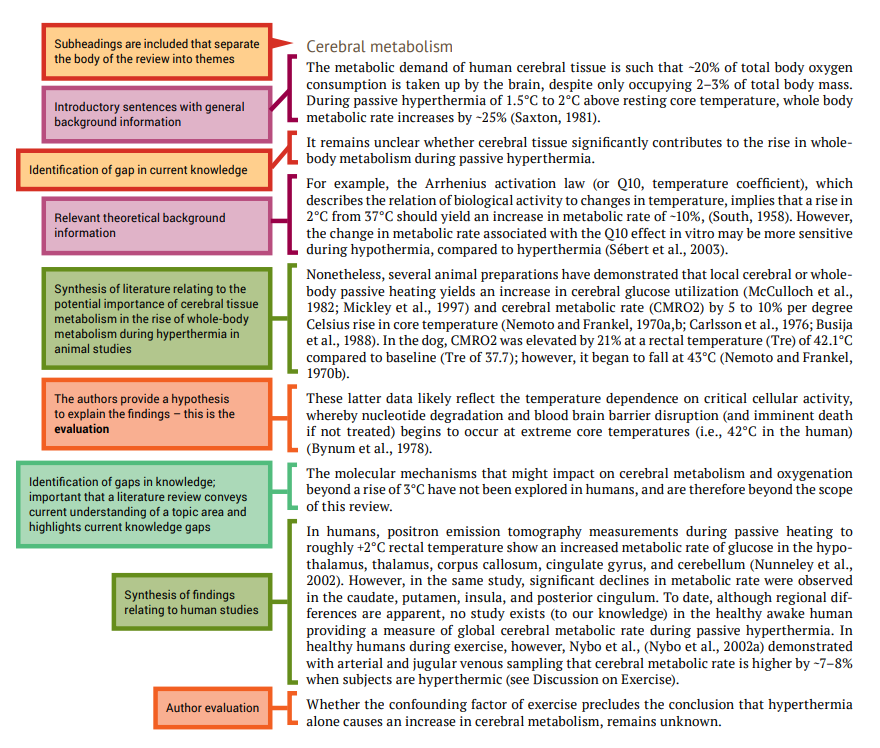
Five tips for developing useful literature summary tables for writing review articles
- Evidence-Based Nursing 24(2)

- Memorial University of Newfoundland

- The University of Sheffield
Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations
- Heart Lung Circ

- Alyne Santos Borges
- Paola Pugian Jardim

- Creativ Nurs

- Richard Booth

- Pearse McCusker

- Hamzah Shahid Rafiq

- Bethany Jackson
- Esther Weir
- Jonathan Mead

- Maryanna Cruz da Costa e Silva Andrade
- Juliana de Melo Vellozo Pereira Tinoco
- Isabelle Andrade Silveira

- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
Methodology
- How to Write a Literature Review | Guide, Examples, & Templates
How to Write a Literature Review | Guide, Examples, & Templates
Published on January 2, 2023 by Shona McCombes . Revised on September 11, 2023.
What is a literature review? A literature review is a survey of scholarly sources on a specific topic. It provides an overview of current knowledge, allowing you to identify relevant theories, methods, and gaps in the existing research that you can later apply to your paper, thesis, or dissertation topic .
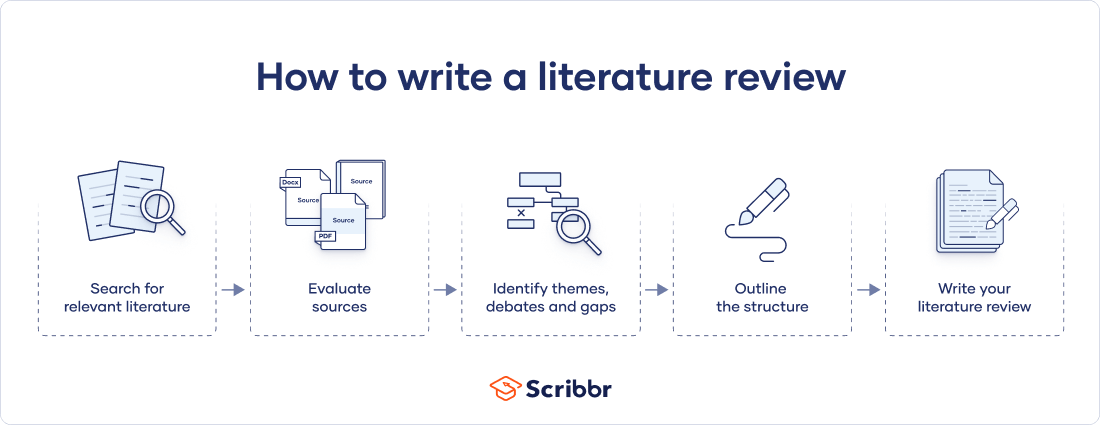
There are five key steps to writing a literature review:
- Search for relevant literature
- Evaluate sources
- Identify themes, debates, and gaps
- Outline the structure
- Write your literature review
A good literature review doesn’t just summarize sources—it analyzes, synthesizes , and critically evaluates to give a clear picture of the state of knowledge on the subject.
Instantly correct all language mistakes in your text
Upload your document to correct all your mistakes in minutes

Table of contents
What is the purpose of a literature review, examples of literature reviews, step 1 – search for relevant literature, step 2 – evaluate and select sources, step 3 – identify themes, debates, and gaps, step 4 – outline your literature review’s structure, step 5 – write your literature review, free lecture slides, other interesting articles, frequently asked questions, introduction.
- Quick Run-through
- Step 1 & 2
When you write a thesis , dissertation , or research paper , you will likely have to conduct a literature review to situate your research within existing knowledge. The literature review gives you a chance to:
- Demonstrate your familiarity with the topic and its scholarly context
- Develop a theoretical framework and methodology for your research
- Position your work in relation to other researchers and theorists
- Show how your research addresses a gap or contributes to a debate
- Evaluate the current state of research and demonstrate your knowledge of the scholarly debates around your topic.
Writing literature reviews is a particularly important skill if you want to apply for graduate school or pursue a career in research. We’ve written a step-by-step guide that you can follow below.
Receive feedback on language, structure, and formatting
Professional editors proofread and edit your paper by focusing on:
- Academic style
- Vague sentences
- Style consistency
See an example

Writing literature reviews can be quite challenging! A good starting point could be to look at some examples, depending on what kind of literature review you’d like to write.
- Example literature review #1: “Why Do People Migrate? A Review of the Theoretical Literature” ( Theoretical literature review about the development of economic migration theory from the 1950s to today.)
- Example literature review #2: “Literature review as a research methodology: An overview and guidelines” ( Methodological literature review about interdisciplinary knowledge acquisition and production.)
- Example literature review #3: “The Use of Technology in English Language Learning: A Literature Review” ( Thematic literature review about the effects of technology on language acquisition.)
- Example literature review #4: “Learners’ Listening Comprehension Difficulties in English Language Learning: A Literature Review” ( Chronological literature review about how the concept of listening skills has changed over time.)
You can also check out our templates with literature review examples and sample outlines at the links below.
Download Word doc Download Google doc
Before you begin searching for literature, you need a clearly defined topic .
If you are writing the literature review section of a dissertation or research paper, you will search for literature related to your research problem and questions .
Make a list of keywords
Start by creating a list of keywords related to your research question. Include each of the key concepts or variables you’re interested in, and list any synonyms and related terms. You can add to this list as you discover new keywords in the process of your literature search.
- Social media, Facebook, Instagram, Twitter, Snapchat, TikTok
- Body image, self-perception, self-esteem, mental health
- Generation Z, teenagers, adolescents, youth
Search for relevant sources
Use your keywords to begin searching for sources. Some useful databases to search for journals and articles include:
- Your university’s library catalogue
- Google Scholar
- Project Muse (humanities and social sciences)
- Medline (life sciences and biomedicine)
- EconLit (economics)
- Inspec (physics, engineering and computer science)
You can also use boolean operators to help narrow down your search.
Make sure to read the abstract to find out whether an article is relevant to your question. When you find a useful book or article, you can check the bibliography to find other relevant sources.
You likely won’t be able to read absolutely everything that has been written on your topic, so it will be necessary to evaluate which sources are most relevant to your research question.
For each publication, ask yourself:
- What question or problem is the author addressing?
- What are the key concepts and how are they defined?
- What are the key theories, models, and methods?
- Does the research use established frameworks or take an innovative approach?
- What are the results and conclusions of the study?
- How does the publication relate to other literature in the field? Does it confirm, add to, or challenge established knowledge?
- What are the strengths and weaknesses of the research?
Make sure the sources you use are credible , and make sure you read any landmark studies and major theories in your field of research.
You can use our template to summarize and evaluate sources you’re thinking about using. Click on either button below to download.
Take notes and cite your sources
As you read, you should also begin the writing process. Take notes that you can later incorporate into the text of your literature review.
It is important to keep track of your sources with citations to avoid plagiarism . It can be helpful to make an annotated bibliography , where you compile full citation information and write a paragraph of summary and analysis for each source. This helps you remember what you read and saves time later in the process.
Don't submit your assignments before you do this
The academic proofreading tool has been trained on 1000s of academic texts. Making it the most accurate and reliable proofreading tool for students. Free citation check included.
Try for free
To begin organizing your literature review’s argument and structure, be sure you understand the connections and relationships between the sources you’ve read. Based on your reading and notes, you can look for:
- Trends and patterns (in theory, method or results): do certain approaches become more or less popular over time?
- Themes: what questions or concepts recur across the literature?
- Debates, conflicts and contradictions: where do sources disagree?
- Pivotal publications: are there any influential theories or studies that changed the direction of the field?
- Gaps: what is missing from the literature? Are there weaknesses that need to be addressed?
This step will help you work out the structure of your literature review and (if applicable) show how your own research will contribute to existing knowledge.
- Most research has focused on young women.
- There is an increasing interest in the visual aspects of social media.
- But there is still a lack of robust research on highly visual platforms like Instagram and Snapchat—this is a gap that you could address in your own research.
There are various approaches to organizing the body of a literature review. Depending on the length of your literature review, you can combine several of these strategies (for example, your overall structure might be thematic, but each theme is discussed chronologically).
Chronological
The simplest approach is to trace the development of the topic over time. However, if you choose this strategy, be careful to avoid simply listing and summarizing sources in order.
Try to analyze patterns, turning points and key debates that have shaped the direction of the field. Give your interpretation of how and why certain developments occurred.
If you have found some recurring central themes, you can organize your literature review into subsections that address different aspects of the topic.
For example, if you are reviewing literature about inequalities in migrant health outcomes, key themes might include healthcare policy, language barriers, cultural attitudes, legal status, and economic access.
Methodological
If you draw your sources from different disciplines or fields that use a variety of research methods , you might want to compare the results and conclusions that emerge from different approaches. For example:
- Look at what results have emerged in qualitative versus quantitative research
- Discuss how the topic has been approached by empirical versus theoretical scholarship
- Divide the literature into sociological, historical, and cultural sources
Theoretical
A literature review is often the foundation for a theoretical framework . You can use it to discuss various theories, models, and definitions of key concepts.
You might argue for the relevance of a specific theoretical approach, or combine various theoretical concepts to create a framework for your research.
Like any other academic text , your literature review should have an introduction , a main body, and a conclusion . What you include in each depends on the objective of your literature review.
The introduction should clearly establish the focus and purpose of the literature review.
Depending on the length of your literature review, you might want to divide the body into subsections. You can use a subheading for each theme, time period, or methodological approach.
As you write, you can follow these tips:
- Summarize and synthesize: give an overview of the main points of each source and combine them into a coherent whole
- Analyze and interpret: don’t just paraphrase other researchers — add your own interpretations where possible, discussing the significance of findings in relation to the literature as a whole
- Critically evaluate: mention the strengths and weaknesses of your sources
- Write in well-structured paragraphs: use transition words and topic sentences to draw connections, comparisons and contrasts
In the conclusion, you should summarize the key findings you have taken from the literature and emphasize their significance.
When you’ve finished writing and revising your literature review, don’t forget to proofread thoroughly before submitting. Not a language expert? Check out Scribbr’s professional proofreading services !
This article has been adapted into lecture slides that you can use to teach your students about writing a literature review.
Scribbr slides are free to use, customize, and distribute for educational purposes.
Open Google Slides Download PowerPoint
If you want to know more about the research process , methodology , research bias , or statistics , make sure to check out some of our other articles with explanations and examples.
- Sampling methods
- Simple random sampling
- Stratified sampling
- Cluster sampling
- Likert scales
- Reproducibility
Statistics
- Null hypothesis
- Statistical power
- Probability distribution
- Effect size
- Poisson distribution
Research bias
- Optimism bias
- Cognitive bias
- Implicit bias
- Hawthorne effect
- Anchoring bias
- Explicit bias
A literature review is a survey of scholarly sources (such as books, journal articles, and theses) related to a specific topic or research question .
It is often written as part of a thesis, dissertation , or research paper , in order to situate your work in relation to existing knowledge.
There are several reasons to conduct a literature review at the beginning of a research project:
- To familiarize yourself with the current state of knowledge on your topic
- To ensure that you’re not just repeating what others have already done
- To identify gaps in knowledge and unresolved problems that your research can address
- To develop your theoretical framework and methodology
- To provide an overview of the key findings and debates on the topic
Writing the literature review shows your reader how your work relates to existing research and what new insights it will contribute.
The literature review usually comes near the beginning of your thesis or dissertation . After the introduction , it grounds your research in a scholarly field and leads directly to your theoretical framework or methodology .
A literature review is a survey of credible sources on a topic, often used in dissertations , theses, and research papers . Literature reviews give an overview of knowledge on a subject, helping you identify relevant theories and methods, as well as gaps in existing research. Literature reviews are set up similarly to other academic texts , with an introduction , a main body, and a conclusion .
An annotated bibliography is a list of source references that has a short description (called an annotation ) for each of the sources. It is often assigned as part of the research process for a paper .
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
McCombes, S. (2023, September 11). How to Write a Literature Review | Guide, Examples, & Templates. Scribbr. Retrieved October 8, 2024, from https://www.scribbr.com/dissertation/literature-review/
Is this article helpful?
Shona McCombes
Other students also liked, what is a theoretical framework | guide to organizing, what is a research methodology | steps & tips, how to write a research proposal | examples & templates, get unlimited documents corrected.
✔ Free APA citation check included ✔ Unlimited document corrections ✔ Specialized in correcting academic texts
- Research Guides
- Vanderbilt University Libraries
- Peabody Library
Literature Reviews
Literature review table.
- Steps to Success: The Literature Review Process
- Literature Reviews Webinar Recording
- Writing Like an Academic
- Publishing in Academic Journals
- Managing Citations This link opens in a new window
- Literature Review Table Template Peabody Librarians have created a sample literature review table to use to organize your research. Feel free to download this file and use or adapt as needed.
- << Previous: Literature Reviews Webinar Recording
- Next: Writing Like an Academic >>
- Last Updated: Oct 1, 2024 12:43 PM
- URL: https://researchguides.library.vanderbilt.edu/peabody/litreviews


Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
7 Writing a Literature Review
Hundreds of original investigation research articles on health science topics are published each year. It is becoming harder and harder to keep on top of all new findings in a topic area and – more importantly – to work out how they all fit together to determine our current understanding of a topic. This is where literature reviews come in.
In this chapter, we explain what a literature review is and outline the stages involved in writing one. We also provide practical tips on how to communicate the results of a review of current literature on a topic in the format of a literature review.
7.1 What is a literature review?

Literature reviews provide a synthesis and evaluation of the existing literature on a particular topic with the aim of gaining a new, deeper understanding of the topic.
Published literature reviews are typically written by scientists who are experts in that particular area of science. Usually, they will be widely published as authors of their own original work, making them highly qualified to author a literature review.
However, literature reviews are still subject to peer review before being published. Literature reviews provide an important bridge between the expert scientific community and many other communities, such as science journalists, teachers, and medical and allied health professionals. When the most up-to-date knowledge reaches such audiences, it is more likely that this information will find its way to the general public. When this happens, – the ultimate good of science can be realised.
A literature review is structured differently from an original research article. It is developed based on themes, rather than stages of the scientific method.
In the article Ten simple rules for writing a literature review , Marco Pautasso explains the importance of literature reviews:
Literature reviews are in great demand in most scientific fields. Their need stems from the ever-increasing output of scientific publications. For example, compared to 1991, in 2008 three, eight, and forty times more papers were indexed in Web of Science on malaria, obesity, and biodiversity, respectively. Given such mountains of papers, scientists cannot be expected to examine in detail every single new paper relevant to their interests. Thus, it is both advantageous and necessary to rely on regular summaries of the recent literature. Although recognition for scientists mainly comes from primary research, timely literature reviews can lead to new synthetic insights and are often widely read. For such summaries to be useful, however, they need to be compiled in a professional way (Pautasso, 2013, para. 1).
An example of a literature review is shown in Figure 7.1.
Video 7.1: What is a literature review? [2 mins, 11 secs]
Watch this video created by Steely Library at Northern Kentucky Library called ‘ What is a literature review? Note: Closed captions are available by clicking on the CC button below.
Examples of published literature reviews
- Strength training alone, exercise therapy alone, and exercise therapy with passive manual mobilisation each reduce pain and disability in people with knee osteoarthritis: a systematic review
- Traveler’s diarrhea: a clinical review
- Cultural concepts of distress and psychiatric disorders: literature review and research recommendations for global mental health epidemiology
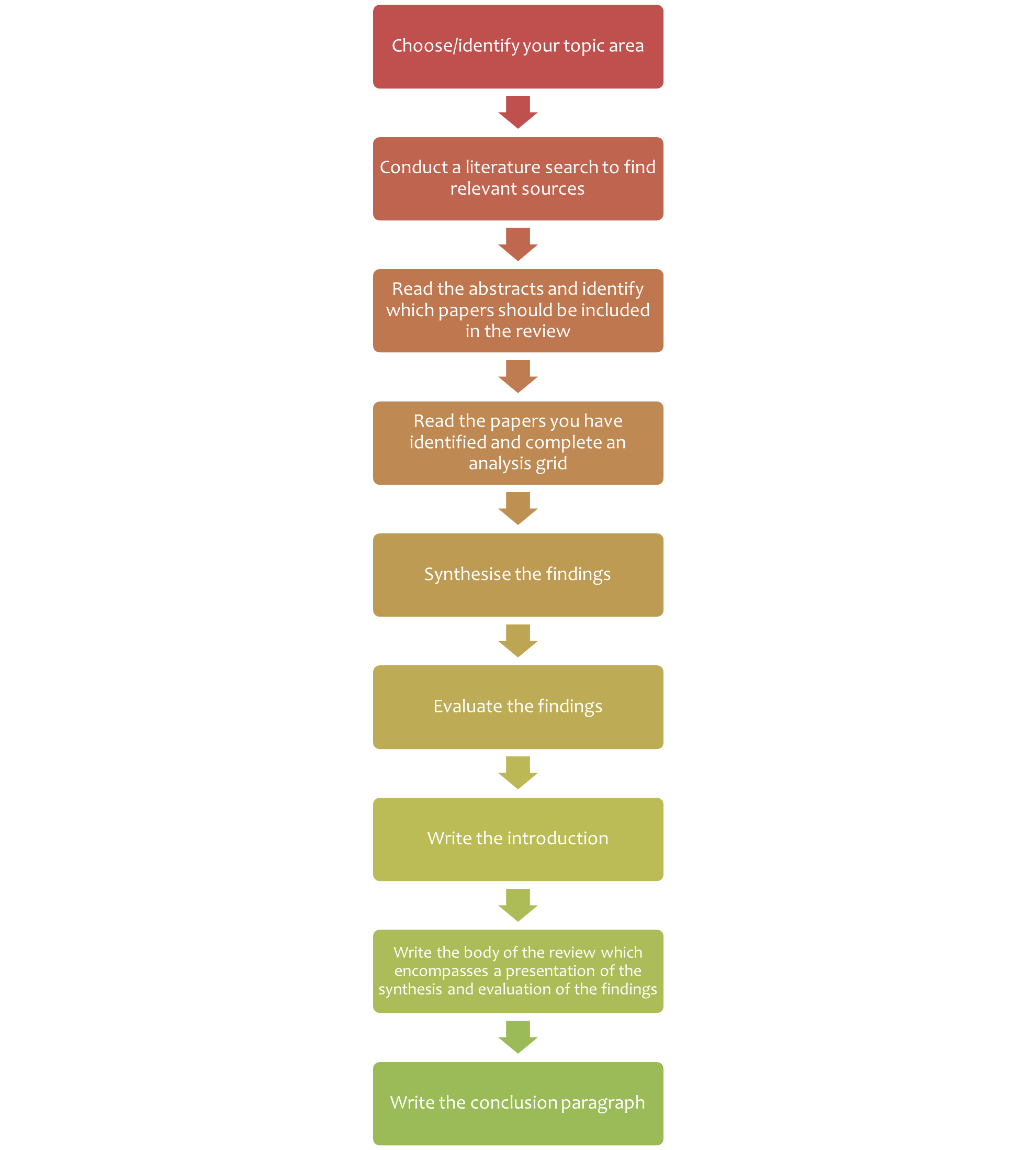
7.2 Steps of writing a literature review
Writing a literature review is a very challenging task. Figure 7.2 summarises the steps of writing a literature review. Depending on why you are writing your literature review, you may be given a topic area, or may choose a topic that particularly interests you or is related to a research project that you wish to undertake.
Chapter 6 provides instructions on finding scientific literature that would form the basis for your literature review.
Once you have your topic and have accessed the literature, the next stages (analysis, synthesis and evaluation) are challenging. Next, we look at these important cognitive skills student scientists will need to develop and employ to successfully write a literature review, and provide some guidance for navigating these stages.
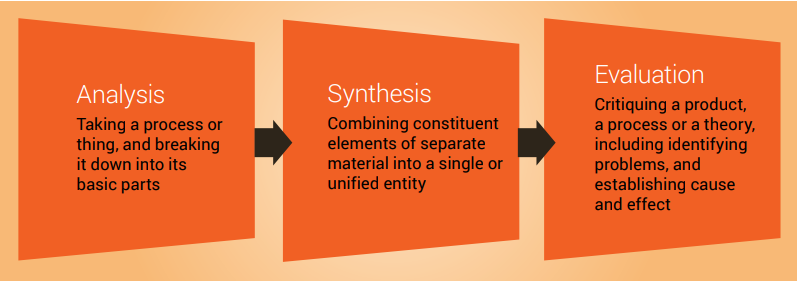
Analysis, synthesis and evaluation
Analysis, synthesis and evaluation are three essential skills required by scientists and you will need to develop these skills if you are to write a good literature review ( Figure 7.3 ). These important cognitive skills are discussed in more detail in Chapter 9.
The first step in writing a literature review is to analyse the original investigation research papers that you have gathered related to your topic.
Analysis requires examining the papers methodically and in detail, so you can understand and interpret aspects of the study described in each research article.
An analysis grid is a simple tool you can use to help with the careful examination and breakdown of each paper. This tool will allow you to create a concise summary of each research paper; see Table 7.1 for an example of an analysis grid. When filling in the grid, the aim is to draw out key aspects of each research paper. Use a different row for each paper, and a different column for each aspect of the paper ( Tables 7.2 and 7.3 show how completed analysis grid may look).
Before completing your own grid, look at these examples and note the types of information that have been included, as well as the level of detail. Completing an analysis grid with a sufficient level of detail will help you to complete the synthesis and evaluation stages effectively. This grid will allow you to more easily observe similarities and differences across the findings of the research papers and to identify possible explanations (e.g., differences in methodologies employed) for observed differences between the findings of different research papers.
Table 7.1: Example of an analysis grid
| [include details about the authors, date of publication and the rationale for the review] | [summarise the aim of the experiment] | [summarise the experiment design, include the subjects used and experimental groups] | [summarise the main findings] | [summarise the conclusion] | [evaluate the paper’s findings, and highlight any terms or physiology concepts that you are unfamiliar with and should be included in your review] |
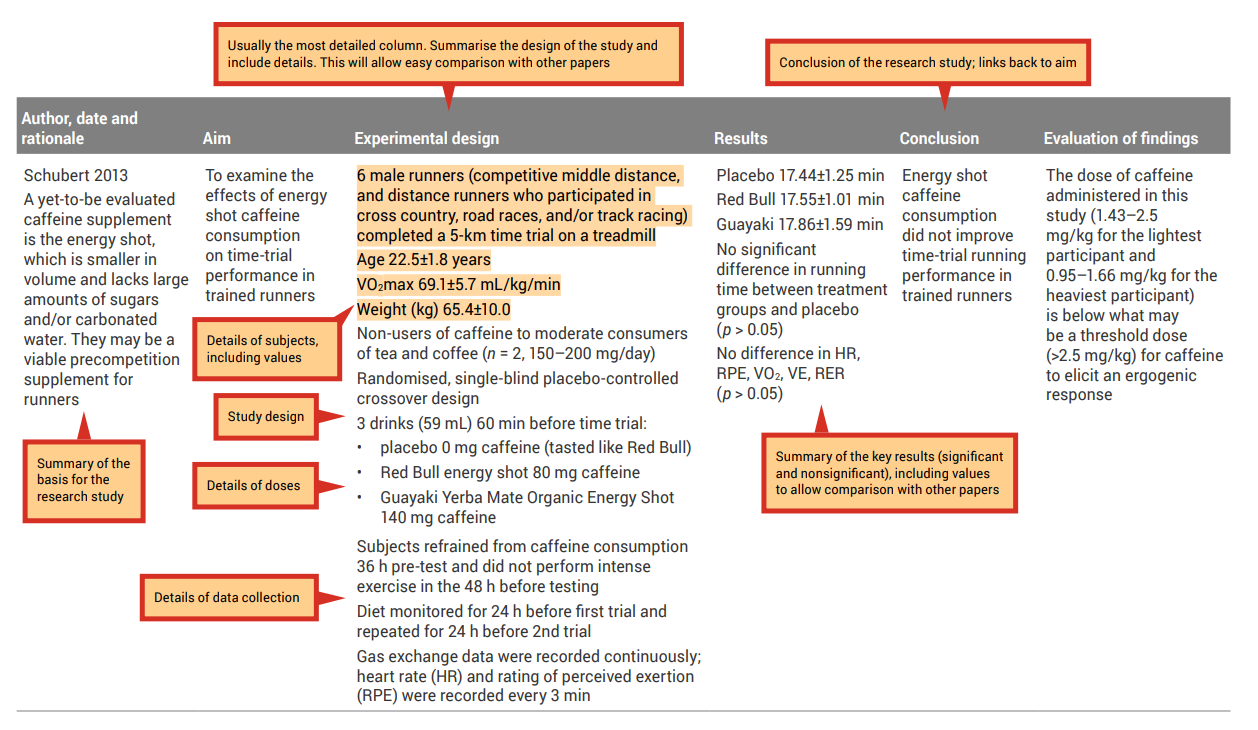
Table 7.3: Sample filled-in analysis grid for research article by Ping and colleagues
| Ping 2010 The effect of chronic caffeine supplementation on endurance performance has been studied extensively in different populations. However, concurrent research on the effects of acute supplementation of caffeine on cardiorespiratory responses during endurance exercise in hot and humid conditions is unavailable | To determine the effect of caffeine supplementation on cardiorespiratory responses during endurance running in hot and humid conditions | 9 heat-adapted recreational male runners Age 25.4±6.9 years Weight (kg) 57.6±8.4 Non-users of caffeine (23.7±12.6 mg/day) Randomised, double-blind placebo-controlled cross-over design (at least 7 days gap between trials to nullify effect of caffeine) Caffeine (5 mg/kg) or placebo ingested as a capsule one hour before a running trial to exhaustion (70% VO2 max on a motorised treadmill in a heat-controlled laboratory (31 °C, 70% humidity) Diet monitored for 3 days before first trial and repeated for 3 days before 2nd trial (to minimise variation in pre-exercise muscle glycogen) Subjects asked to refrain from heavy exercise for 24 h before trials Subjects drank 3 ml of cool water per kg of body weight every 20 min during running trial to stay hydrated Heart rate (HR), core body temperature and rating of perceived exertion (RPE) were recorded at intervals of 10 mins, while oxygen consumption was measured at intervals of 20 min | Mean exhaustion time was 31.6% higher in the caffeine group: • Placebo 83.6±21.4 • Caffeine 110.1±29.3 Running time to exhaustion was significantly higher (p | Ingestion of caffeine improved the endurance running performance, but did not affect heart rate, core body temperature, oxygen uptake or RPE. | The lower RPE during the caffeine trial may be because of the positive effect of caffeine ingestion on nerve impulse transmission, as well as an analgesic effect and psychological effect. Perhaps this is the same reason subjects could sustain the treadmill running for longer in the caffeine trial. |
Source: Ping, WC, Keong, CC & Bandyopadhyay, A 2010, ‘Effects of acute supplementation of caffeine on cardiorespiratory responses during endurance running in a hot and humid climate’, Indian Journal of Medical Research, vol. 132, pp. 36–41. Used under a CC-BY-NC-SA licence.
Step two of writing a literature review is synthesis.
Synthesis describes combining separate components or elements to form a connected whole.
You will use the results of your analysis to find themes to build your literature review around. Each of the themes identified will become a subheading within the body of your literature review.
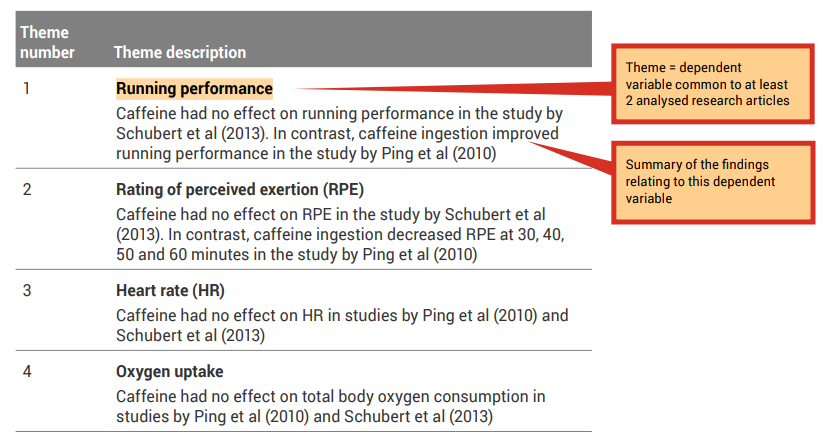
A good place to start when identifying themes is with the dependent variables (results/findings) that were investigated in the research studies.
Because all of the research articles you are incorporating into your literature review are related to your topic, it is likely that they have similar study designs and have measured similar dependent variables. Review the ‘Results’ column of your analysis grid. You may like to collate the common themes in a synthesis grid (see, for example Table 7.4 ).
Step three of writing a literature review is evaluation, which can only be done after carefully analysing your research papers and synthesising the common themes (findings).
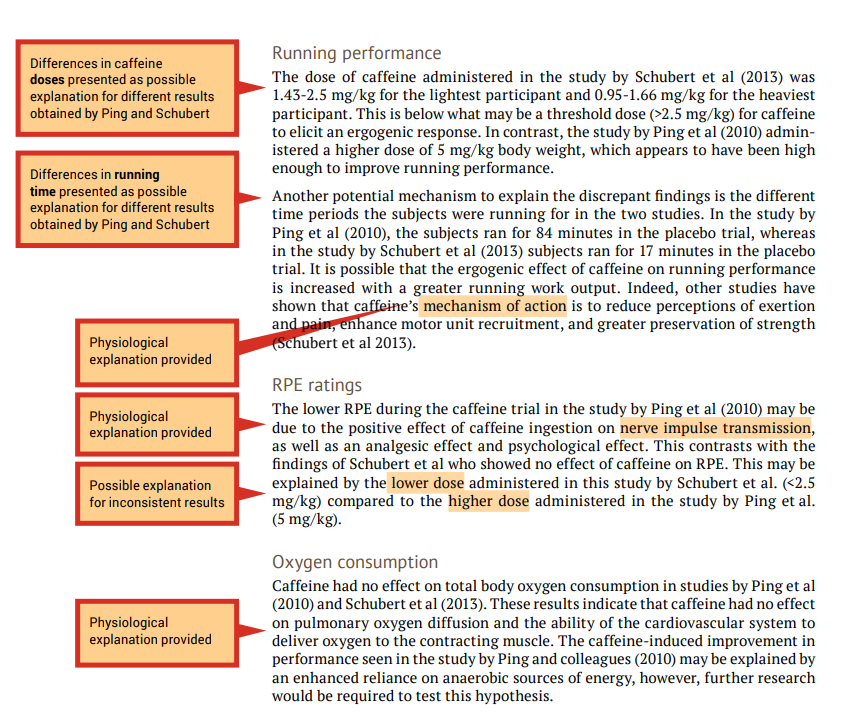
During the evaluation stage, you are making judgements on the themes presented in the research articles that you have read. This includes providing physiological explanations for the findings. It may be useful to refer to the discussion section of published original investigation research papers, or another literature review, where the authors may mention tested or hypothetical physiological mechanisms that may explain their findings.
When the findings of the investigations related to a particular theme are inconsistent (e.g., one study shows that caffeine effects performance and another study shows that caffeine had no effect on performance) you should attempt to provide explanations of why the results differ, including physiological explanations. A good place to start is by comparing the methodologies to determine if there are any differences that may explain the differences in the findings (see the ‘Experimental design’ column of your analysis grid). An example of evaluation is shown in the examples that follow in this section, under ‘Running performance’ and ‘RPE ratings’.
When the findings of the papers related to a particular theme are consistent (e.g., caffeine had no effect on oxygen uptake in both studies) an evaluation should include an explanation of why the results are similar. Once again, include physiological explanations. It is still a good idea to compare methodologies as a background to the evaluation. An example of evaluation is shown in the following under ‘Oxygen consumption’.
7.3 Writing your literature review
Once you have completed the analysis, and synthesis grids and written your evaluation of the research papers , you can combine synthesis and evaluation information to create a paragraph for a literature review ( Figure 7.4 ).
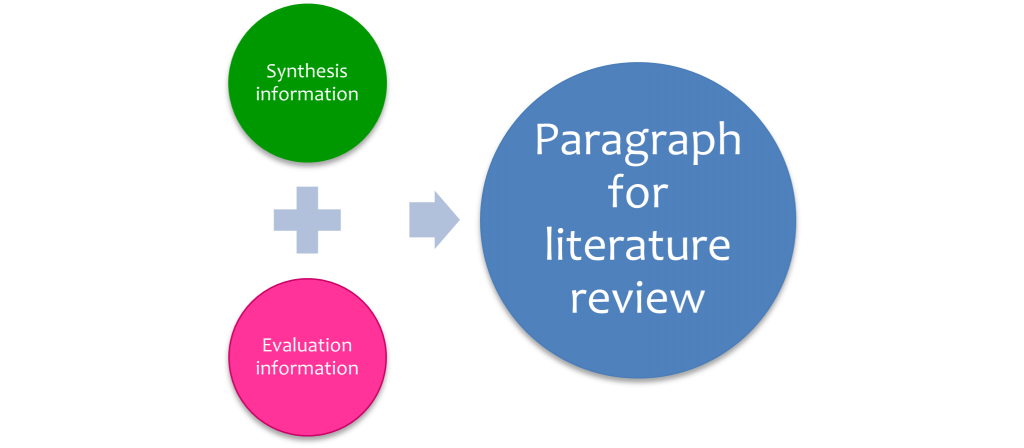
The following paragraphs are an example of combining the outcome of the synthesis and evaluation stages to produce a paragraph for a literature review.
Note that this is an example using only two papers – most literature reviews would be presenting information on many more papers than this ( (e.g., 106 papers in the review article by Bain and colleagues discussed later in this chapter). However, the same principle applies regardless of the number of papers reviewed.

The next part of this chapter looks at the each section of a literature review and explains how to write them by referring to a review article that was published in Frontiers in Physiology and shown in Figure 7.1. Each section from the published article is annotated to highlight important features of the format of the review article, and identifies the synthesis and evaluation information.
In the examination of each review article section we will point out examples of how the authors have presented certain information and where they display application of important cognitive processes; we will use the colour code shown below:

This should be one paragraph that accurately reflects the contents of the review article.
Introduction
The introduction should establish the context and importance of the review
Body of literature review
The reference section provides a list of the references that you cited in the body of your review article. The format will depend on the journal of publication as each journal has their own specific referencing format.
It is important to accurately cite references in research papers to acknowledge your sources and ensure credit is appropriately given to authors of work you have referred to. An accurate and comprehensive reference list also shows your readers that you are well-read in your topic area and are aware of the key papers that provide the context to your research.
It is important to keep track of your resources and to reference them consistently in the format required by the publication in which your work will appear. Most scientists will use reference management software to store details of all of the journal articles (and other sources) they use while writing their review article. This software also automates the process of adding in-text references and creating a reference list. In the review article by Bain et al. (2014) used as an example in this chapter, the reference list contains 106 items, so you can imagine how much help referencing software would be. Chapter 5 shows you how to use EndNote, one example of reference management software.
Click the drop down below to review the terms learned from this chapter.
Copyright note:
- The quotation from Pautasso, M 2013, ‘Ten simple rules for writing a literature review’, PLoS Computational Biology is use under a CC-BY licence.
- Content from the annotated article and tables are based on Schubert, MM, Astorino, TA & Azevedo, JJL 2013, ‘The effects of caffeinated ‘energy shots’ on time trial performance’, Nutrients, vol. 5, no. 6, pp. 2062–2075 (used under a CC-BY 3.0 licence ) and P ing, WC, Keong , CC & Bandyopadhyay, A 2010, ‘Effects of acute supplementation of caffeine on cardiorespiratory responses during endurance running in a hot and humid climate’, Indian Journal of Medical Research, vol. 132, pp. 36–41 (used under a CC-BY-NC-SA 4.0 licence ).
Bain, A.R., Morrison, S.A., & Ainslie, P.N. (2014). Cerebral oxygenation and hyperthermia. Frontiers in Physiology, 5 , 92.
Pautasso, M. (2013). Ten simple rules for writing a literature review. PLoS Computational Biology, 9 (7), e1003149.
How To Do Science Copyright © 2022 by University of Southern Queensland is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License , except where otherwise noted.
Share This Book

Organizing and Creating Information
- Citation and Attribution
What Is a Literature Review?
Review the literature, write the literature review, further reading, learning objectives, attribution.
This guide is designed to:
- Identify the sections and purpose of a literature review in academic writing
- Review practical strategies and organizational methods for preparing a literature review
A literature review is a summary and synthesis of scholarly research on a specific topic. It should answer questions such as:
- What research has been done on the topic?
- Who are the key researchers and experts in the field?
- What are the common theories and methodologies?
- Are there challenges, controversies, and contradictions?
- Are there gaps in the research that your approach addresses?
The process of reviewing existing research allows you to fine-tune your research question and contextualize your own work. Preparing a literature review is a cyclical process. You may find that the research question you begin with evolves as you learn more about the topic.
Once you have defined your research question , focus on learning what other scholars have written on the topic.
In order to do a thorough search of the literature on the topic, define the basic criteria:
- Databases and journals: Look at the subject guide related to your topic for recommended databases. Review the tutorial on finding articles for tips.
- Books: Search BruKnow, the Library's catalog. Steps to searching ebooks are covered in the Finding Ebooks tutorial .
- What time period should it cover? Is currency important?
- Do I know of primary and secondary sources that I can use as a way to find other information?
- What should I be aware of when looking at popular, trade, and scholarly resources ?
One strategy is to review bibliographies for sources that relate to your interest. For more on this technique, look at the tutorial on finding articles when you have a citation .
Tip: Use a Synthesis Matrix
As you read sources, themes will emerge that will help you to organize the review. You can use a simple Synthesis Matrix to track your notes as you read. From this work, a concept map emerges that provides an overview of the literature and ways in which it connects. Working with Zotero to capture the citations, you build the structure for writing your literature review.
| Citation | Concept/Theme | Main Idea | Notes 1 | Notes 2 | Gaps in the Research | Quotation | Page |
How do I know when I am done?
A key indicator for knowing when you are done is running into the same articles and materials. With no new information being uncovered, you are likely exhausting your current search and should modify search terms or search different catalogs or databases. It is also possible that you have reached a point when you can start writing the literature review.
Tip: Manage Your Citations
These citation management tools also create citations, footnotes, and bibliographies with just a few clicks:
Zotero Tutorial
Endnote Tutorial
Your literature review should be focused on the topic defined in your research question. It should be written in a logical, structured way and maintain an objective perspective and use a formal voice.
Review the Summary Table you created for themes and connecting ideas. Use the following guidelines to prepare an outline of the main points you want to make.
- Synthesize previous research on the topic.
- Aim to include both summary and synthesis.
- Include literature that supports your research question as well as that which offers a different perspective.
- Avoid relying on one author or publication too heavily.
- Select an organizational structure, such as chronological, methodological, and thematic.
The three elements of a literature review are introduction, body, and conclusion.
Introduction
- Define the topic of the literature review, including any terminology.
- Introduce the central theme and organization of the literature review.
- Summarize the state of research on the topic.
- Frame the literature review with your research question.
- Focus on ways to have the body of literature tell its own story. Do not add your own interpretations at this point.
- Look for patterns and find ways to tie the pieces together.
- Summarize instead of quote.
- Weave the points together rather than list summaries of each source.
- Include the most important sources, not everything you have read.
- Summarize the review of the literature.
- Identify areas of further research on the topic.
- Connect the review with your research.
- DeCarlo, M. (2018). 4.1 What is a literature review? In Scientific Inquiry in Social Work. Open Social Work Education. https://scientificinquiryinsocialwork.pressbooks.com/chapter/4-1-what-is-a-literature-review/
- Literature Reviews (n.d.) https://writingcenter.unc.edu/tips-and-tools/literature-reviews/ Accessed Nov. 10, 2021
This guide was designed to:
- Identify the sections and purpose of a literature review in academic writing
- Review practical strategies and organizational methods for preparing a literature review
Content on this page adapted from:
Frederiksen, L. and Phelps, S. (2017). Literature Reviews for Education and Nursing Graduate Students. Licensed CC BY 4.0
- << Previous: EndNote
- Last Updated: Jul 17, 2024 3:55 PM
- URL: https://libguides.brown.edu/organize
Brown University Library | Providence, RI 02912 | (401) 863-2165 | Contact | Comments | Library Feedback | Site Map
Library Intranet

Nursing and Allied Health: Building a Summary Table or Synthesis Matrix
- Nursing Library Services
- Library Support for Nursing Accreditation
- Academic Research Libraries (ACRL) Information Literacy Standards for Science
- ACRL Guidelines for Distance Learning
- AACN Resources: Information Literacy
- The Essentials: Core Competencies for Professional Nursing Education
- Accreditation Commision for Education
- ACEN Learning Resources | Definitions & Standards
- Tutoring & Paper Review: College of Nursing & Allied Health Students
- Technology and Collaboration
- Affordable Learning Louisiana
- NIH Virtual Reality Funding
- How to Create Links to Library Resources
- Nursing Resources
- Literature Searching: Overview
- Nursing Resources: Books, Journals and Articles
- Assistive Technology Resources
- Web Accessibility Initiative WC3
- Healthcare Links for Persons With Disabilities
- Accessibility
- Accessibility Tools: Videos
- Braile Institute: Blind and Low Vision Web Accessibility
- Braile Institute: Assistive Technology for the Visually Impaired This link opens in a new window
- Mental Health Resources for Students & Faculty
- Student Activities & Organizations
- Anatomage FAQ's
- APA Reference Worksheet With Examples This link opens in a new window
- APA Style and Grammar Guidelines, 7.0
- What's New in APA Guide, 7.0 This link opens in a new window
- APA Instructional Aids for Professors/Instructors
- APA Handouts & Guides This link opens in a new window
- Sample Papers for Students and Instructors
- Academic Writer Tutorial: Basics This link opens in a new window
- APA Styling Your Paper
- When to cite or not cite a database
- Video: A Step-By-Step Guide for APA Style Student Papers
- Video: Citing Works in Text Using APA 7.0 This link opens in a new window
- Video: Creating References Using APA Guide, 7.0 This link opens in a new window
- Journal Article Reporting Standards
- Digital Information Literacy
- Tips Sheet: Copyright Essentials for Higher Education This link opens in a new window
- Citing National Patient Safety Goals (Joint Commission)
- Citing Agency for Healthcare Research and Quality (AHRQ) in APA format
- Best Nursing Apps
- Writing an Abstract for Your PILT or Special Project
- PILT Poster Presentation Archive
- Academic Success
- Healthcare Career Salaries
- Industry Statistics: Healthcare
- Nursing Organizations
- Radiology Organizations
- Controlled Medical Vocabularies
- COVID-19 Current Vaccines (Medscape)
- COVID-19 Current and Candidate Vaccine Landscape and Tracker (WHO)
- COVID-19: Clinician Care Guidance (CDC.gov) This link opens in a new window
- COVID -19 Contract Tracing & Patient Privacy (Cornell) This link opens in a new window
- COVID-19: Coronavirus Dashboard (Johns Hopkins Epidemiology)
- COVID-19: Coronavirus Guidelines (Up-To-Date) This link opens in a new window
- COVID-19: Critical Care Evidence (Cochrane) This link opens in a new window
- COVID-19: Diagnosis & Treatment (JAMA) This link opens in a new window
- COVID-19: Free Video Access (Jove) This link opens in a new window
- COVID-19: Healthcare Hub (Elsevier) This link opens in a new window
- COVID-19: Keeping Up With A Moving Target (Johns Hopkins Nursing Videos)
- COVID-19: LitCovid Daily Update (NLM)
- COVID 19 - Long Term Health Effects Stemming From COVID-19
- COVID-19: Louisiana Department of Health
- COVID-19: Novel Coronavirus Information Center (Elsevier) This link opens in a new window
- COVID-19 Nursing Resources (Medscape)
- COVID-19: Red Book - Recent Pediatric Updates (AAP)
- COVID-19: Washing Your Hands Thoroughly (NHS)
- COVID-19: Well-Being Initiative (ANF)
- Properly Putting On Your Facemask & Getting a Good Seal (Dr. Scheiner) This link opens in a new window
- Creating Personal Accounts
- Creating a CINAHL MyFolder Account
- Creating a PubMed | MyNCBI Personal Account
- Creating a ProQuest Nursing & Allied Health Premium | MyResearch Folder
- Creating an OVID MyWorkspace Personal Account
- Mobile APPS | CINAHL for Apple and Android Mobile Devices
- My Circulation Login
- Interlibrary Loan Personal Account
- Data Visualization Products
- International Classification of Diseases (ICD)
- Diagnostic and Statistical Manual of Mental Disorders | DSM-5-TR
- [C1] Infections | Infectious Diseases
- [C04] Neoplasms
- [C05] Musculoskeletal Diseases
- [C06] Digestive System Diseases
- [C07] Stomatognathic Diseases
- [C08] Respiratory Tract Diseases
- [C09] Otorhinolaryngologic Diseases
- [C10] Nervous System Diseases
- [C11] Eye Diseases
- [C12] Urogenital Diseases
- [C14] Cardiovascular Diseases
- [C15] Hemic and Lymphatic Diseases
- [C16] Congenital, Hereditary, and Neonatal Diseases and Abnormalities
- [C17] Skin and Connective Tissue Diseases
- [C18] Nutritional and Metabolic Diseases
- [C19] Endocrine System Diseases
- [C20] Immune System Diseases
- [C21] Disorders of Environmental Origin
- [C22] Animal Diseases [Zoonotic diseases]
- [C23] Pathological Conditions, Signs and Symptoms
- [C24] Occupational Diseases
- [C25] Chemically-Induced Disorders
- [C26] Wounds and Injuries
- WHO Drug Information [Relative to Diseases]
- NDDK Patient Education Tool Kit
- Clinical Tools & Patient Education
- NDDK Resources on Medline Plus
- NDDK Open Research
- Dietary Guidelines for Americans
- Physical Activity Guidelines for Americans
- Move Your Way Community Resources
- National Youth Sports Strategy
- President’s Council on Sports, Fitness & Nutrition
- White House Conference on Hunger, Nutrition, and Health
- Equitable Long-Term Recovery and Resilience
- National Health Observances
- Finding Clinical Trials
- NIH News in Health
- Dosage Calculations & Pharmacology
- PICO - EBM Video This link opens in a new window
- PICO Slides
- Fillable CONSAH Pico Form
- Evidence Based Practice for Nursing
- Evidence-Based Nursing: -Step 2
- Evidence Appraisal - Step 3
- Evidence Application - Step 4
- Outcome Evaluation - Step 5
- Evidence Translation - Step 6
- Google Advanced Search for EBM
- Nursing Research Methods
- Faculty Book Request
- Proctor Request Form
- Peer Reviewed Literature: Assessment Goals
- Full Text Finder
- EBSCO e-Books Nursing 2021
- EBSCO eBooks Anesthesia
- EBSCO eBooks Radiology Science & Allied Health
- EBSCO eBooks: Writing About Nursing and Allied Health
- Alzheimers and Dementia
- Statistics on Aging
- CDC Bibliography: Alzheimers & Aging This link opens in a new window
- Health Conditions
- Health Behaviors
- Populations
- Settings and Systems
- Social Determinants of Health
- ILL Interlibrary Loan
- Gestational Diabetes and Fast Foods (MeSH)
- Mobile Resources
- Nursing Theory
- Psychiatric Nursing Journals
- Display, sort and; navigate
- Similar articles
- Cite, save and share
- Citations in PubMed
- All About PubMed Filters
- PubMed Quick Tours and Tutorials
- Evidence Based Practice Tutorial (PubMed) This link opens in a new window
- Developing a Clinical Question This link opens in a new window
- Using PubMed to Find Relevant Articles This link opens in a new window
- Next Steps This link opens in a new window
- Scenario (practice) This link opens in a new window
- Radiology Books and e-Books
- History of Radiology & Radiography
- Radiology: Anatomage This link opens in a new window
- Radiology Anatomy Atlas Viewer
- Advanced Radiographic Research
- Diagnostic Imaging Selected Articles
- Faculty and Administrative Resources
- Radiology Tech and MRI Salaries (Bureau of Labor Statistics)
- Radiology Technician Demand by State
- Review Tools for Graduate Students
- Training & Videos
- Register for an Online Meeting
- Joining an Online Meeting
- Training Videos & Search Examples
- Training Survey
- Sources for Health Statistics
- Ebola and Infectious Diseases
- Nursing Sites
- Writing Research Papers
Building a Summary Table or Synthesis Matrix

Quick Links | Nursing & Allied Health

- NSU Libraries Home
- Contact Your Librarian (Research Assistance)
- Nursing & Allied Health Databases
- Holidays & University Closures
- Review My Paper**
- Student Help Desk : Phone: (318) 357-6696 [email protected]
- Nursing & Alled Health Databases
- Shreveport Library Main Phone: 318.677.3007
- Shreveport Librarian Phone: 318.677.301 3
- Simplifying Synthesis | Download the Article PDF Copy
- Writing a Literature Review and Using a Synthesis Matrix
What a Summary Table or Synthesis Matrix looks like
Use the "Literature Review Matrix Template" as a guideline to help you sort through your thoughts, note important points and think through the similarities and differences:
You are organizing the review by ideas and not by sources . The literature review is not just a summary of the already published works. Your synthesis should show how various articles are linked.

A summary table is also called a synthesis matrix. The table helps you organize and compare information for your systematic review, scholarly report, dissertation or thesis
Synthesis Matrix.
A summary table is also called a synthesis matrix . A summary table helps you record the main points of each source and document how sources relate to each other. After summarizing and evaluating your sources, arrange them in a matrix to help you see how they relate to each other, and apply to each of your themes or variables.
Faculty who typically guide students find it challenging to help students learn how to synthesize material (Blondy, Blakesless, Scheffer, Rubenfeld, Cronin, & Luster-Turner, 2016; Kearney, 2015) . Writers can easily summarize material but seem to struggle to adequately synthesize knowledge about their topic and express that in their writing. So, whether you are writing a student papers, dissertations, or scholarly report it is necessary to learn a few tips and tricks to organize your ideas.
Building a summary table and developing solid synthesis skills is important for nurses, nurse practitioners, and allied health researchers. Quality evidence-based practice initiatives and nursing care and medicine are based on understanding and evaluating the resources and research available, identifying gaps, and building a strong foundation for future work.
Good synthesis is about putting the data gathered, references read, and literature analyzed together in a new way that shows connections and relationships. ( Shellenbarger, 2016 ). The Merriam-Webster dictionary defines synthesis as something that is made by combining different things or the composition or combination of parts or elements so as to form a whole (Synthesis, n.d.).
In other words, building a summary table or synthesis matrix involves taking information from a variety of sources, evaluating that information and forming new ideas or insights in an original way. This can be a new and potentially challenging experience for students and researchers who are used to just repeating what is already in the literature.

Visit Our Libraries

Interlibrary Loan | Shreveport Education Center Library | Eugene P. Watson Memorial Library | NSU Leesville Library
Cammie G. Henry Research Center | Prince Music Media Library
- << Previous: Best Nursing Apps
- Next: Writing an Abstract for Your PILT or Special Project >>
- Last Updated: Sep 5, 2024 2:05 PM
- URL: https://libguides.nsula.edu/NursingandAlliedHealth
Literature Review Basics
- What is a Literature Review?
- Synthesizing Research
- Using Research & Synthesis Tables
- Additional Resources

About the Research and Synthesis Tables
Research Tables and Synthesis Tables are useful tools for organizing and analyzing your research as you assemble your literature review. They represent two different parts of the review process: assembling relevant information and synthesizing it. Use a Research table to compile the main info you need about the items you find in your research -- it's a great thing to have on hand as you take notes on what you read! Then, once you've assembled your research, use the Synthesis table to start charting the similarities/differences and major themes among your collected items.
We've included an Excel file with templates for you to use below; the examples pictured on this page are snapshots from that file.
- Research and Synthesis Table Templates This Excel workbook includes simple templates for creating research tables and synthesis tables. Feel free to download and use!
Using the Research Table

This is an example of a research table, in which you provide a basic description of the most important features of the studies, articles, and other items you discover in your research. The table identifies each item according to its author/date of publication, its purpose or thesis, what type of work it is (systematic review, clinical trial, etc.), the level of evidence it represents (which tells you a lot about its impact on the field of study), and its major findings. Your job, when you assemble this information, is to develop a snapshot of what the research shows about the topic of your research question and assess its value (both for the purpose of your work and for general knowledge in the field).
Think of your work on the research table as the foundational step for your analysis of the literature, in which you assemble the information you'll be analyzing and lay the groundwork for thinking about what it means and how it can be used.
Using the Synthesis Table

This is an example of a synthesis table or synthesis matrix , in which you organize and analyze your research by listing each source and indicating whether a given finding or result occurred in a particular study or article ( each row lists an individual source, and each finding has its own column, in which X = yes, blank = no). You can also add or alter the columns to look for shared study populations, sort by level of evidence or source type, etc. The key here is to use the table to provide a simple representation of what the research has found (or not found, as the case may be). Think of a synthesis table as a tool for making comparisons, identifying trends, and locating gaps in the literature.
How do I know which findings to use, or how many to include? Your research question tells you which findings are of interest in your research, so work from your research question to decide what needs to go in each Finding header, and how many findings are necessary. The number is up to you; again, you can alter this table by adding or deleting columns to match what you're actually looking for in your analysis. You should also, of course, be guided by what's actually present in the material your research turns up!
- << Previous: Synthesizing Research
- Next: Additional Resources >>
- Last Updated: Sep 26, 2023 12:06 PM
- URL: https://usi.libguides.com/literature-review-basics

Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
Chapter 7: Synthesizing Sources
Learning objectives.
At the conclusion of this chapter, you will be able to:
- synthesize key sources connecting them with the research question and topic area.
7.1 Overview of synthesizing
7.1.1 putting the pieces together.
Combining separate elements into a whole is the dictionary definition of synthesis. It is a way to make connections among and between numerous and varied source materials. A literature review is not an annotated bibliography, organized by title, author, or date of publication. Rather, it is grouped by topic to create a whole view of the literature relevant to your research question.

Your synthesis must demonstrate a critical analysis of the papers you collected as well as your ability to integrate the results of your analysis into your own literature review. Each paper collected should be critically evaluated and weighed for “adequacy, appropriateness, and thoroughness” ( Garrard, 2017 ) before inclusion in your own review. Papers that do not meet this criteria likely should not be included in your literature review.
Begin the synthesis process by creating a grid, table, or an outline where you will summarize, using common themes you have identified and the sources you have found. The summary grid or outline will help you compare and contrast the themes so you can see the relationships among them as well as areas where you may need to do more searching. Whichever method you choose, this type of organization will help you to both understand the information you find and structure the writing of your review. Remember, although “the means of summarizing can vary, the key at this point is to make sure you understand what you’ve found and how it relates to your topic and research question” ( Bennard et al., 2014 ).
As you read through the material you gather, look for common themes as they may provide the structure for your literature review. And, remember, research is an iterative process: it is not unusual to go back and search information sources for more material.
At one extreme, if you are claiming, ‘There are no prior publications on this topic,’ it is more likely that you have not found them yet and may need to broaden your search. At another extreme, writing a complete literature review can be difficult with a well-trod topic. Do not cite it all; instead cite what is most relevant. If that still leaves too much to include, be sure to reference influential sources…as well as high-quality work that clearly connects to the points you make. ( Klingner, Scanlon, & Pressley, 2005 ).
7.2 Creating a summary table
Literature reviews can be organized sequentially or by topic, theme, method, results, theory, or argument. It’s important to develop categories that are meaningful and relevant to your research question. Take detailed notes on each article and use a consistent format for capturing all the information each article provides. These notes and the summary table can be done manually, using note cards. However, given the amount of information you will be recording, an electronic file created in a word processing or spreadsheet is more manageable. Examples of fields you may want to capture in your notes include:
- Authors’ names
- Article title
- Publication year
- Main purpose of the article
- Methodology or research design
- Participants
- Measurement
- Conclusions
Other fields that will be useful when you begin to synthesize the sum total of your research:
- Specific details of the article or research that are especially relevant to your study
- Key terms and definitions
- Strengths or weaknesses in research design
- Relationships to other studies
- Possible gaps in the research or literature (for example, many research articles conclude with the statement “more research is needed in this area”)
- Finally, note how closely each article relates to your topic. You may want to rank these as high, medium, or low relevance. For papers that you decide not to include, you may want to note your reasoning for exclusion, such as ‘small sample size’, ‘local case study,’ or ‘lacks evidence to support assertion.’
This short video demonstrates how a nursing researcher might create a summary table.
7.2.1 Creating a Summary Table

Summary tables can be organized by author or by theme, for example:
| Author/Year | Research Design | Participants or Population Studied | Comparison | Outcome |
| Smith/2010 | Mixed methods | Undergraduates | Graduates | Improved access |
| King/2016 | Survey | Females | Males | Increased representation |
| Miller/2011 | Content analysis | Nurses | Doctors | New procedure |
For a summary table template, see http://blogs.monm.edu/writingatmc/files/2013/04/Synthesis-Matrix-Template.pdf
7.3 Creating a summary outline
An alternate way to organize your articles for synthesis it to create an outline. After you have collected the articles you intend to use (and have put aside the ones you won’t be using), it’s time to identify the conclusions that can be drawn from the articles as a group.
Based on your review of the collected articles, group them by categories. You may wish to further organize them by topic and then chronologically or alphabetically by author. For each topic or subtopic you identified during your critical analysis of the paper, determine what those papers have in common. Likewise, determine which ones in the group differ. If there are contradictory findings, you may be able to identify methodological or theoretical differences that could account for the contradiction (for example, differences in population demographics). Determine what general conclusions you can report about the topic or subtopic as the entire group of studies relate to it. For example, you may have several studies that agree on outcome, such as ‘hands on learning is best for science in elementary school’ or that ‘continuing education is the best method for updating nursing certification.’ In that case, you may want to organize by methodology used in the studies rather than by outcome.
Organize your outline in a logical order and prepare to write the first draft of your literature review. That order might be from broad to more specific, or it may be sequential or chronological, going from foundational literature to more current. Remember, “an effective literature review need not denote the entire historical record, but rather establish the raison d’etre for the current study and in doing so cite that literature distinctly pertinent for theoretical, methodological, or empirical reasons.” ( Milardo, 2015, p. 22 ).
As you organize the summarized documents into a logical structure, you are also appraising and synthesizing complex information from multiple sources. Your literature review is the result of your research that synthesizes new and old information and creates new knowledge.
7.4 Additional resources:
Literature Reviews: Using a Matrix to Organize Research / Saint Mary’s University of Minnesota
Literature Review: Synthesizing Multiple Sources / Indiana University
Writing a Literature Review and Using a Synthesis Matrix / Florida International University
Sample Literature Reviews Grid / Complied by Lindsay Roberts
Select three or four articles on a single topic of interest to you. Then enter them into an outline or table in the categories you feel are important to a research question. Try both the grid and the outline if you can to see which suits you better. The attached grid contains the fields suggested in the video .
Literature Review Table
| Author Date | Topic/Focus Purpose | Conceptual Theoretical Framework | Paradigm Methods | Context Setting Sample | Findings | Gaps |
Test Yourself
- Select two articles from your own summary table or outline and write a paragraph explaining how and why the sources relate to each other and your review of the literature.
- In your literature review, under what topic or subtopic will you place the paragraph you just wrote?
Image attribution
Literature Reviews for Education and Nursing Graduate Students Copyright © by Linda Frederiksen is licensed under a Creative Commons Attribution 4.0 International License , except where otherwise noted.
Share This Book
Literature Review Example
A literature review is a summary of the existing knowledge and research on a particular subject. by identifying gaps in the literature, it provides a foundation for future research. as such, it’s a crucial first step in any research project..
What is a literature review?
A literature review serves several purposes:
- identifies knowledge gaps
- evaluates the quality of existing research
- provides a foundation for newly presented research
Looking at existing examples of literature reviews is beneficial to get a clear understanding of what they entail. Find examples of a literature review by using an academic search engine (e.g. Google Scholar). As a starting point, search for your keyword or topic along with the term "literature review".
Example of literature review
Identify the research question or topic, making it as narrow as possible. In this example of a literature review, we review the anxiolytic (anti-anxiety) activity of Piper methysticum , or Kava .
Let's walk through the steps in the process with this literature review example.
Define the research question
First, identify the research question or topic, making it as narrow as possible. In this literature review example, we're examining the effects of urbanization on the migration of birds.
Search for relevant literature
Searching for relevant studies is arguably the most important aspect of the literature review.
Start by identifying keywords and phrases related to the topic and use them to search academic journals and databases ( Google Scholar , BASE , PubMed , etc.). For our example, you might start with "the effects of urbanization on bird migration", but after researching the field, discover that other terms like "avian migration" and "avian populations" are more commonly used.
Search for your keywords in Litmaps to find some initial articles to explore the field from. You can then use Litmaps to find additinal sources and curate a whole library of literature on your topic.

Search for your keywords in Litmaps, and select a starting article. This will return a visualization containing suggestions for relevant articles on your literature review topic. Review these to start curating your library.
Evaluate the sources
Evaluate the relevance and quality of the sources found by reading abstracts of the most relevant articles. Additionally, consider the publication venue, year of publication and other salient measures to identify the reliability and relevance of the source.


Read and analyze the sources
Take notes on the key findings, methodologies, and theoretical frameworks used in the studies.
Use a research-friendly note-taking software, like Obsidian , that provide #tags to keep track of key concepts.
Organize the literature
Organize the literature according to themes, subtopics, or categories, which will help outline the layout of the literature review.

Tag keywords using a tool like Obsidian to help organize papers into subtopics for the review.
Write the literature review
Summarize and synthesize the findings from the sources analyzed. Start with an introduction that defines the research question, followed by the themes, subtopics, or categories identified. After that, provide a discussion or conclusion that addresses any gaps in the literature to motivate future research. Lastly, edit and revise your review to ensure it is well-structured, clear, and concise. The example below is from a review paper, which includes a table comparing the different sources evaluated. Such tables can be useful if you are conducting a comprehensive review.

If you're conducting a comprehensive review, you can include a table of sources reviewed in your process, like the one above from this publication .
Cite and reference the sources
Lastly, cite and reference the sources used in the literature review. Consider any referencing style requirements of the institution or journal you're submitting to. APA is the most common. However, you may need to familiarize yourself with other citation styles such as MLA, Chicago, or MHRA depending on your venue. See the image below for a literature review example APA of references. To cite references you've saved in Litmaps, you can move your saved articles from Litmaps to a reference manager (i.e. Zotero, Mendeley, EndNote, etc.) and then export their bibliography from there. Here's how to export articles from Litmaps.

Use a reference manager tool like Zotero to easily export which makes them easy to manage, like in this APA literature review example.
A successful literature review tells a brief story about the topic at hand and leaves the reader a clear notion of what has been covered. Most importantly, a literature review addresses any gaps in the field and frames newly presented research. Understand the key steps and look at literature review examples in order to create a high quality review.
Header image Forest & Kim Starr, used under Creative Commons BY 3.0

Jump to navigation

Cochrane Training
Chapter 14: completing ‘summary of findings’ tables and grading the certainty of the evidence.
Holger J Schünemann, Julian PT Higgins, Gunn E Vist, Paul Glasziou, Elie A Akl, Nicole Skoetz, Gordon H Guyatt; on behalf of the Cochrane GRADEing Methods Group (formerly Applicability and Recommendations Methods Group) and the Cochrane Statistical Methods Group
Key Points:
- A ‘Summary of findings’ table for a given comparison of interventions provides key information concerning the magnitudes of relative and absolute effects of the interventions examined, the amount of available evidence and the certainty (or quality) of available evidence.
- ‘Summary of findings’ tables include a row for each important outcome (up to a maximum of seven). Accepted formats of ‘Summary of findings’ tables and interactive ‘Summary of findings’ tables can be produced using GRADE’s software GRADEpro GDT.
- Cochrane has adopted the GRADE approach (Grading of Recommendations Assessment, Development and Evaluation) for assessing certainty (or quality) of a body of evidence.
- The GRADE approach specifies four levels of the certainty for a body of evidence for a given outcome: high, moderate, low and very low.
- GRADE assessments of certainty are determined through consideration of five domains: risk of bias, inconsistency, indirectness, imprecision and publication bias. For evidence from non-randomized studies and rarely randomized studies, assessments can then be upgraded through consideration of three further domains.
Cite this chapter as: Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence [last updated August 2023]. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.5. Cochrane, 2024. Available from www.training.cochrane.org/handbook .
14.1 ‘Summary of findings’ tables
14.1.1 introduction to ‘summary of findings’ tables.
‘Summary of findings’ tables present the main findings of a review in a transparent, structured and simple tabular format. In particular, they provide key information concerning the certainty or quality of evidence (i.e. the confidence or certainty in the range of an effect estimate or an association), the magnitude of effect of the interventions examined, and the sum of available data on the main outcomes. Cochrane Reviews should incorporate ‘Summary of findings’ tables during planning and publication, and should have at least one key ‘Summary of findings’ table representing the most important comparisons. Some reviews may include more than one ‘Summary of findings’ table, for example if the review addresses more than one major comparison, or includes substantially different populations that require separate tables (e.g. because the effects differ or it is important to show results separately). In the Cochrane Database of Systematic Reviews (CDSR), all ‘Summary of findings’ tables for a review appear at the beginning, before the Background section.
14.1.2 Selecting outcomes for ‘Summary of findings’ tables
Planning for the ‘Summary of findings’ table starts early in the systematic review, with the selection of the outcomes to be included in: (i) the review; and (ii) the ‘Summary of findings’ table. This is a crucial step, and one that review authors need to address carefully.
To ensure production of optimally useful information, Cochrane Reviews begin by developing a review question and by listing all main outcomes that are important to patients and other decision makers (see Chapter 2 and Chapter 3 ). The GRADE approach to assessing the certainty of the evidence (see Section 14.2 ) defines and operationalizes a rating process that helps separate outcomes into those that are critical, important or not important for decision making. Consultation and feedback on the review protocol, including from consumers and other decision makers, can enhance this process.
Critical outcomes are likely to include clearly important endpoints; typical examples include mortality and major morbidity (such as strokes and myocardial infarction). However, they may also represent frequent minor and rare major side effects, symptoms, quality of life, burdens associated with treatment, and resource issues (costs). Burdens represent the impact of healthcare workload on patient function and well-being, and include the demands of adhering to an intervention that patients or caregivers (e.g. family) may dislike, such as having to undergo more frequent tests, or the restrictions on lifestyle that certain interventions require (Spencer-Bonilla et al 2017).
Frequently, when formulating questions that include all patient-important outcomes for decision making, review authors will confront reports of studies that have not included all these outcomes. This is particularly true for adverse outcomes. For instance, randomized trials might contribute evidence on intended effects, and on frequent, relatively minor side effects, but not report on rare adverse outcomes such as suicide attempts. Chapter 19 discusses strategies for addressing adverse effects. To obtain data for all important outcomes it may be necessary to examine the results of non-randomized studies (see Chapter 24 ). Cochrane, in collaboration with others, has developed guidance for review authors to support their decision about when to look for and include non-randomized studies (Schünemann et al 2013).
If a review includes only randomized trials, these trials may not address all important outcomes and it may therefore not be possible to address these outcomes within the constraints of the review. Review authors should acknowledge these limitations and make them transparent to readers. Review authors are encouraged to include non-randomized studies to examine rare or long-term adverse effects that may not adequately be studied in randomized trials. This raises the possibility that harm outcomes may come from studies in which participants differ from those in studies used in the analysis of benefit. Review authors will then need to consider how much such differences are likely to impact on the findings, and this will influence the certainty of evidence because of concerns about indirectness related to the population (see Section 14.2.2 ).
Non-randomized studies can provide important information not only when randomized trials do not report on an outcome or randomized trials suffer from indirectness, but also when the evidence from randomized trials is rated as very low and non-randomized studies provide evidence of higher certainty. Further discussion of these issues appears also in Chapter 24 .
14.1.3 General template for ‘Summary of findings’ tables
Several alternative standard versions of ‘Summary of findings’ tables have been developed to ensure consistency and ease of use across reviews, inclusion of the most important information needed by decision makers, and optimal presentation (see examples at Figures 14.1.a and 14.1.b ). These formats are supported by research that focused on improved understanding of the information they intend to convey (Carrasco-Labra et al 2016, Langendam et al 2016, Santesso et al 2016). They are available through GRADE’s official software package developed to support the GRADE approach: GRADEpro GDT (www.gradepro.org).
Standard Cochrane ‘Summary of findings’ tables include the following elements using one of the accepted formats. Further guidance on each of these is provided in Section 14.1.6 .
- A brief description of the population and setting addressed by the available evidence (which may be slightly different to or narrower than those defined by the review question).
- A brief description of the comparison addressed in the ‘Summary of findings’ table, including both the experimental and comparison interventions.
- A list of the most critical and/or important health outcomes, both desirable and undesirable, limited to seven or fewer outcomes.
- A measure of the typical burden of each outcomes (e.g. illustrative risk, or illustrative mean, on comparator intervention).
- The absolute and relative magnitude of effect measured for each (if both are appropriate).
- The numbers of participants and studies contributing to the analysis of each outcomes.
- A GRADE assessment of the overall certainty of the body of evidence for each outcome (which may vary by outcome).
- Space for comments.
- Explanations (formerly known as footnotes).
Ideally, ‘Summary of findings’ tables are supported by more detailed tables (known as ‘evidence profiles’) to which the review may be linked, which provide more detailed explanations. Evidence profiles include the same important health outcomes, and provide greater detail than ‘Summary of findings’ tables of both of the individual considerations feeding into the grading of certainty and of the results of the studies (Guyatt et al 2011a). They ensure that a structured approach is used to rating the certainty of evidence. Although they are rarely published in Cochrane Reviews, evidence profiles are often used, for example, by guideline developers in considering the certainty of the evidence to support guideline recommendations. Review authors will find it easier to develop the ‘Summary of findings’ table by completing the rating of the certainty of evidence in the evidence profile first in GRADEpro GDT. They can then automatically convert this to one of the ‘Summary of findings’ formats in GRADEpro GDT, including an interactive ‘Summary of findings’ for publication.
As a measure of the magnitude of effect for dichotomous outcomes, the ‘Summary of findings’ table should provide a relative measure of effect (e.g. risk ratio, odds ratio, hazard) and measures of absolute risk. For other types of data, an absolute measure alone (such as a difference in means for continuous data) might be sufficient. It is important that the magnitude of effect is presented in a meaningful way, which may require some transformation of the result of a meta-analysis (see also Chapter 15, Section 15.4 and Section 15.5 ). Reviews with more than one main comparison should include a separate ‘Summary of findings’ table for each comparison.
Figure 14.1.a provides an example of a ‘Summary of findings’ table. Figure 15.1.b provides an alternative format that may further facilitate users’ understanding and interpretation of the review’s findings. Evidence evaluating different formats suggests that the ‘Summary of findings’ table should include a risk difference as a measure of the absolute effect and authors should preferably use a format that includes a risk difference .
A detailed description of the contents of a ‘Summary of findings’ table appears in Section 14.1.6 .
Figure 14.1.a Example of a ‘Summary of findings’ table
Summary of findings (for interactive version click here )
|
| ||||||
| anyone taking a long flight (lasting more than 6 hours) international air travel compression stockings without stockings | ||||||
| Outcomes | * (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
|
|
| |||||
| (DVT) | See comment | See comment | Not estimable | 2821 (9 studies) | See comment | 0 participants developed symptomatic DVT in these studies |
|
|
| (0.04 to 0.26) | 2637 (9 studies) | ⊕⊕⊕⊕
| ||
|
|
(0 to 3) | |||||
|
| ||||||
|
|
(1 to 8) | |||||
|
|
|
(2 to 15) |
(0.18 to 1.13) | 1804 (8 studies) | ⊕⊕⊕◯
| |
|
Post-flight values measured on a scale from 0, no oedema, to 10, maximum oedema | The mean oedema score ranged across control groups from
| The mean oedema score in the intervention groups was on average
(95% CI –4.9 to –4.5) | 1246 (6 studies) | ⊕⊕◯◯
| ||
|
| See comment | See comment | Not estimable | 2821 (9 studies) | See comment | 0 participants developed pulmonary embolus in these studies |
|
| See comment | See comment | Not estimable | 2821 (9 studies) | See comment | 0 participants died in these studies |
|
| See comment | See comment | Not estimable | 1182 (4 studies) | See comment | The tolerability of the stockings was described as very good with no complaints of side effects in 4 studies |
| *The basis for the is provided in footnotes. The (and its 95% confidence interval) is based on the assumed risk in the intervention group and the of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; GRADE: GRADE Working Group grades of evidence (see explanations). | ||||||
a All the stockings in the nine studies included in this review were below-knee compression stockings. In four studies the compression strength was 20 mmHg to 30 mmHg at the ankle. It was 10 mmHg to 20 mmHg in the other four studies. Stockings come in different sizes. If a stocking is too tight around the knee it can prevent essential venous return causing the blood to pool around the knee. Compression stockings should be fitted properly. A stocking that is too tight could cut into the skin on a long flight and potentially cause ulceration and increased risk of DVT. Some stockings can be slightly thicker than normal leg covering and can be potentially restrictive with tight foot wear. It is a good idea to wear stockings around the house prior to travel to ensure a good, comfortable fit. Participants put their stockings on two to three hours before the flight in most of the studies. The availability and cost of stockings can vary.
b Two studies recruited high risk participants defined as those with previous episodes of DVT, coagulation disorders, severe obesity, limited mobility due to bone or joint problems, neoplastic disease within the previous two years, large varicose veins or, in one of the studies, participants taller than 190 cm and heavier than 90 kg. The incidence for the seven studies that excluded high risk participants was 1.45% and the incidence for the two studies that recruited high-risk participants (with at least one risk factor) was 2.43%. We have used 10 and 30 per 1000 to express different risk strata, respectively.
c The confidence interval crosses no difference and does not rule out a small increase.
d The measurement of oedema was not validated (indirectness of the outcome) or blinded to the intervention (risk of bias).
e If there are very few or no events and the number of participants is large, judgement about the certainty of evidence (particularly judgements about imprecision) may be based on the absolute effect. Here the certainty rating may be considered ‘high’ if the outcome was appropriately assessed and the event, in fact, did not occur in 2821 studied participants.
f None of the other studies reported adverse effects, apart from four cases of superficial vein thrombosis in varicose veins in the knee region that were compressed by the upper edge of the stocking in one study.
Figure 14.1.b Example of alternative ‘Summary of findings’ table
|
| ||||||
|
| ||||||
| children given antibiotics inpatients and outpatient probiotics no probiotics | ||||||
|
|
|
|
|
| ||
|
|
|
| ||||
|
Follow-up: 10 days to 3 months Children < 5 years |
|
| ⊕⊕⊕⊝
Due to risk of bias | Probably decreases the incidence of diarrhoea. | ||
| 1474 (7 studies) |
(0.29 to 0.55) |
|
(6.5 to 12.2) |
(10.1 to 15.8 fewer) | ||
| Children > 5 years |
|
| ⊕⊕⊝⊝
Due to risk of bias and imprecision | May decrease the incidence of diarrhoea. | ||
| 624 (4 studies) |
(0.53 to 1.21) |
|
(5.9 to 13.6) |
(5.3 fewer to 2.4 more) | ||
|
Follow-up: 10 to 44 days 1575 (11 studies) | - |
|
(0.8 to 3.8) |
(1 fewer to 2 more) | ⊕⊕⊝⊝
Due to risk of bias and inconsistency | There may be little or no difference in adverse events. |
|
Follow-up: 10 days to 3 months 897 (5 studies) | - | The mean duration of diarrhoea without probiotics was | - |
(1.18 to 0.02 fewer days) | ⊕⊕⊝⊝
Due to imprecision and inconsistency | May decrease the duration of diarrhoea. |
|
Follow-up: 10 days to 3 months 425 (4 studies) | - | The mean stools per day without probiotics was | - |
(0.6 to 0 fewer) | ⊕⊕⊝⊝
Due to imprecision and inconsistency | There may be little or no difference in stools per day. |
| *The basis for the (e.g. the median control group risk across studies) is provided in footnotes. The (and its 95% confidence interval) is based on the assumed risk in the comparison group and the of the intervention (and its 95% CI). confidence interval; risk ratio. | ||||||
|
Control group risk estimates come from pooled estimates of control groups. Relative effect based on available case analysis High risk of bias due to high loss to follow-up. Imprecision due to few events and confidence intervals include appreciable benefit or harm. Side effects: rash, nausea, flatulence, vomiting, increased phlegm, chest pain, constipation, taste disturbance and low appetite. Risks were calculated from pooled risk differences. High risk of bias. Only 11 of 16 trials reported on adverse events, suggesting a selective reporting bias. Serious inconsistency. Numerous probiotic agents and doses were evaluated amongst a relatively small number of trials, limiting our ability to draw conclusions on the safety of the many probiotics agents and doses administered. Serious unexplained inconsistency (large heterogeneity I = 79%, P value [P = 0.04], point estimates and confidence intervals vary considerably). Serious imprecision. The upper bound of 0.02 fewer days of diarrhoea is not considered patient important. Serious unexplained inconsistency (large heterogeneity I = 78%, P value [P = 0.05], point estimates and confidence intervals vary considerably). Serious imprecision. The 95% confidence interval includes no effect and lower bound of 0.60 stools per day is of questionable patient importance. | ||||||
14.1.4 Producing ‘Summary of findings’ tables
The GRADE Working Group’s software, GRADEpro GDT ( www.gradepro.org ), including GRADE’s interactive handbook, is available to assist review authors in the preparation of ‘Summary of findings’ tables. GRADEpro can use data on the comparator group risk and the effect estimate (entered by the review authors or imported from files generated in RevMan) to produce the relative effects and absolute risks associated with experimental interventions. In addition, it leads the user through the process of a GRADE assessment, and produces a table that can be used as a standalone table in a review (including by direct import into software such as RevMan or integration with RevMan Web), or an interactive ‘Summary of findings’ table (see help resources in GRADEpro).
14.1.5 Statistical considerations in ‘Summary of findings’ tables
14.1.5.1 dichotomous outcomes.
‘Summary of findings’ tables should include both absolute and relative measures of effect for dichotomous outcomes. Risk ratios, odds ratios and risk differences are different ways of comparing two groups with dichotomous outcome data (see Chapter 6, Section 6.4.1 ). Furthermore, there are two distinct risk ratios, depending on which event (e.g. ‘yes’ or ‘no’) is the focus of the analysis (see Chapter 6, Section 6.4.1.5 ). In the presence of a non-zero intervention effect, any variation across studies in the comparator group risks (i.e. variation in the risk of the event occurring without the intervention of interest, for example in different populations) makes it impossible for more than one of these measures to be truly the same in every study.
It has long been assumed in epidemiology that relative measures of effect are more consistent than absolute measures of effect from one scenario to another. There is empirical evidence to support this assumption (Engels et al 2000, Deeks and Altman 2001, Furukawa et al 2002). For this reason, meta-analyses should generally use either a risk ratio or an odds ratio as a measure of effect (see Chapter 10, Section 10.4.3 ). Correspondingly, a single estimate of relative effect is likely to be a more appropriate summary than a single estimate of absolute effect. If a relative effect is indeed consistent across studies, then different comparator group risks will have different implications for absolute benefit. For instance, if the risk ratio is consistently 0.75, then the experimental intervention would reduce a comparator group risk of 80% to 60% in the intervention group (an absolute risk reduction of 20 percentage points), but would also reduce a comparator group risk of 20% to 15% in the intervention group (an absolute risk reduction of 5 percentage points).
‘Summary of findings’ tables are built around the assumption of a consistent relative effect. It is therefore important to consider the implications of this effect for different comparator group risks (these can be derived or estimated from a number of sources, see Section 14.1.6.3 ), which may require an assessment of the certainty of evidence for prognostic evidence (Spencer et al 2012, Iorio et al 2015). For any comparator group risk, it is possible to estimate a corresponding intervention group risk (i.e. the absolute risk with the intervention) from the meta-analytic risk ratio or odds ratio. Note that the numbers provided in the ‘Corresponding risk’ column are specific to the ‘risks’ in the adjacent column.
For the meta-analytic risk ratio (RR) and assumed comparator risk (ACR) the corresponding intervention risk is obtained as:
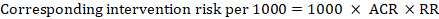
As an example, in Figure 14.1.a , the meta-analytic risk ratio for symptomless deep vein thrombosis (DVT) is RR = 0.10 (95% CI 0.04 to 0.26). Assuming a comparator risk of ACR = 10 per 1000 = 0.01, we obtain:
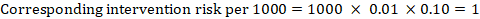
For the meta-analytic odds ratio (OR) and assumed comparator risk, ACR, the corresponding intervention risk is obtained as:
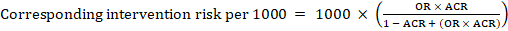
Upper and lower confidence limits for the corresponding intervention risk are obtained by replacing RR or OR by their upper and lower confidence limits, respectively (e.g. replacing 0.10 with 0.04, then with 0.26, in the example). Such confidence intervals do not incorporate uncertainty in the assumed comparator risks.
When dealing with risk ratios, it is critical that the same definition of ‘event’ is used as was used for the meta-analysis. For example, if the meta-analysis focused on ‘death’ (as opposed to survival) as the event, then corresponding risks in the ‘Summary of findings’ table must also refer to ‘death’.
In (rare) circumstances in which there is clear rationale to assume a consistent risk difference in the meta-analysis, in principle it is possible to present this for relevant ‘assumed risks’ and their corresponding risks, and to present the corresponding (different) relative effects for each assumed risk.
The risk difference expresses the difference between the ACR and the corresponding intervention risk (or the difference between the experimental and the comparator intervention).
For the meta-analytic risk ratio (RR) and assumed comparator risk (ACR) the corresponding risk difference is obtained as (note that risks can also be expressed using percentage or percentage points):
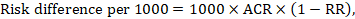
As an example, in Figure 14.1.b the meta-analytic risk ratio is 0.41 (95% CI 0.29 to 0.55) for diarrhoea in children less than 5 years of age. Assuming a comparator group risk of 22.3% we obtain:
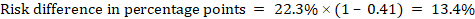
For the meta-analytic odds ratio (OR) and assumed comparator risk (ACR) the absolute risk difference is obtained as (percentage points):
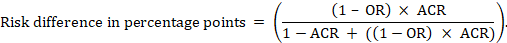
Upper and lower confidence limits for the absolute risk difference are obtained by re-running the calculation above while replacing RR or OR by their upper and lower confidence limits, respectively (e.g. replacing 0.41 with 0.28, then with 0.55, in the example). Such confidence intervals do not incorporate uncertainty in the assumed comparator risks.
14.1.5.2 Time-to-event outcomes
Time-to-event outcomes measure whether and when a particular event (e.g. death) occurs (van Dalen et al 2007). The impact of the experimental intervention relative to the comparison group on time-to-event outcomes is usually measured using a hazard ratio (HR) (see Chapter 6, Section 6.8.1 ).
A hazard ratio expresses a relative effect estimate. It may be used in various ways to obtain absolute risks and other interpretable quantities for a specific population. Here we describe how to re-express hazard ratios in terms of: (i) absolute risk of event-free survival within a particular period of time; (ii) absolute risk of an event within a particular period of time; and (iii) median time to the event. All methods are built on an assumption of consistent relative effects (i.e. that the hazard ratio does not vary over time).
(i) Absolute risk of event-free survival within a particular period of time Event-free survival (e.g. overall survival) is commonly reported by individual studies. To obtain absolute effects for time-to-event outcomes measured as event-free survival, the summary HR can be used in conjunction with an assumed proportion of patients who are event-free in the comparator group (Tierney et al 2007). This proportion of patients will be specific to a period of time of observation. However, it is not strictly necessary to specify this period of time. For instance, a proportion of 50% of event-free patients might apply to patients with a high event rate observed over 1 year, or to patients with a low event rate observed over 2 years.
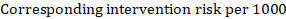
As an example, suppose the meta-analytic hazard ratio is 0.42 (95% CI 0.25 to 0.72). Assuming a comparator group risk of event-free survival (e.g. for overall survival people being alive) at 2 years of ACR = 900 per 1000 = 0.9 we obtain:
so that that 956 per 1000 people will be alive with the experimental intervention at 2 years. The derivation of the risk should be explained in a comment or footnote.
(ii) Absolute risk of an event within a particular period of time To obtain this absolute effect, again the summary HR can be used (Tierney et al 2007):
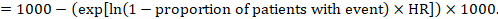
In the example, suppose we assume a comparator group risk of events (e.g. for mortality, people being dead) at 2 years of ACR = 100 per 1000 = 0.1. We obtain:
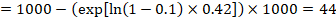
so that that 44 per 1000 people will be dead with the experimental intervention at 2 years.
(iii) Median time to the event Instead of absolute numbers, the time to the event in the intervention and comparison groups can be expressed as median survival time in months or years. To obtain median survival time the pooled HR can be applied to an assumed median survival time in the comparator group (Tierney et al 2007):
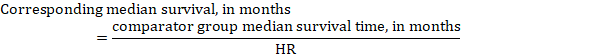
In the example, assuming a comparator group median survival time of 80 months, we obtain:
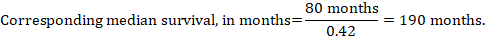
For all three of these options for re-expressing results of time-to-event analyses, upper and lower confidence limits for the corresponding intervention risk are obtained by replacing HR by its upper and lower confidence limits, respectively (e.g. replacing 0.42 with 0.25, then with 0.72, in the example). Again, as for dichotomous outcomes, such confidence intervals do not incorporate uncertainty in the assumed comparator group risks. This is of special concern for long-term survival with a low or moderate mortality rate and a corresponding high number of censored patients (i.e. a low number of patients under risk and a high censoring rate).
14.1.6 Detailed contents of a ‘Summary of findings’ table
14.1.6.1 table title and header.
The title of each ‘Summary of findings’ table should specify the healthcare question, framed in terms of the population and making it clear exactly what comparison of interventions are made. In Figure 14.1.a , the population is people taking long aeroplane flights, the intervention is compression stockings, and the control is no compression stockings.
The first rows of each ‘Summary of findings’ table should provide the following ‘header’ information:
Patients or population This further clarifies the population (and possibly the subpopulations) of interest and ideally the magnitude of risk of the most crucial adverse outcome at which an intervention is directed. For instance, people on a long-haul flight may be at different risks for DVT; those using selective serotonin reuptake inhibitors (SSRIs) might be at different risk for side effects; while those with atrial fibrillation may be at low (< 1%), moderate (1% to 4%) or high (> 4%) yearly risk of stroke.
Setting This should state any specific characteristics of the settings of the healthcare question that might limit the applicability of the summary of findings to other settings (e.g. primary care in Europe and North America).
Intervention The experimental intervention.
Comparison The comparator intervention (including no specific intervention).
14.1.6.2 Outcomes
The rows of a ‘Summary of findings’ table should include all desirable and undesirable health outcomes (listed in order of importance) that are essential for decision making, up to a maximum of seven outcomes. If there are more outcomes in the review, review authors will need to omit the less important outcomes from the table, and the decision selecting which outcomes are critical or important to the review should be made during protocol development (see Chapter 3 ). Review authors should provide time frames for the measurement of the outcomes (e.g. 90 days or 12 months) and the type of instrument scores (e.g. ranging from 0 to 100).
Note that review authors should include the pre-specified critical and important outcomes in the table whether data are available or not. However, they should be alert to the possibility that the importance of an outcome (e.g. a serious adverse effect) may only become known after the protocol was written or the analysis was carried out, and should take appropriate actions to include these in the ‘Summary of findings’ table.
The ‘Summary of findings’ table can include effects in subgroups of the population for different comparator risks and effect sizes separately. For instance, in Figure 14.1.b effects are presented for children younger and older than 5 years separately. Review authors may also opt to produce separate ‘Summary of findings’ tables for different populations.
Review authors should include serious adverse events, but it might be possible to combine minor adverse events as a single outcome, and describe this in an explanatory footnote (note that it is not appropriate to add events together unless they are independent, that is, a participant who has experienced one adverse event has an unaffected chance of experiencing the other adverse event).
Outcomes measured at multiple time points represent a particular problem. In general, to keep the table simple, review authors should present multiple time points only for outcomes critical to decision making, where either the result or the decision made are likely to vary over time. The remainder should be presented at a common time point where possible.
Review authors can present continuous outcome measures in the ‘Summary of findings’ table and should endeavour to make these interpretable to the target audience. This requires that the units are clear and readily interpretable, for example, days of pain, or frequency of headache, and the name and scale of any measurement tools used should be stated (e.g. a Visual Analogue Scale, ranging from 0 to 100). However, many measurement instruments are not readily interpretable by non-specialist clinicians or patients, for example, points on a Beck Depression Inventory or quality of life score. For these, a more interpretable presentation might involve converting a continuous to a dichotomous outcome, such as >50% improvement (see Chapter 15, Section 15.5 ).
14.1.6.3 Best estimate of risk with comparator intervention
Review authors should provide up to three typical risks for participants receiving the comparator intervention. For dichotomous outcomes, we recommend that these be presented in the form of the number of people experiencing the event per 100 or 1000 people (natural frequency) depending on the frequency of the outcome. For continuous outcomes, this would be stated as a mean or median value of the outcome measured.
Estimated or assumed comparator intervention risks could be based on assessments of typical risks in different patient groups derived from the review itself, individual representative studies in the review, or risks derived from a systematic review of prognosis studies or other sources of evidence which may in turn require an assessment of the certainty for the prognostic evidence (Spencer et al 2012, Iorio et al 2015). Ideally, risks would reflect groups that clinicians can easily identify on the basis of their presenting features.
An explanatory footnote should specify the source or rationale for each comparator group risk, including the time period to which it corresponds where appropriate. In Figure 14.1.a , clinicians can easily differentiate individuals with risk factors for deep venous thrombosis from those without. If there is known to be little variation in baseline risk then review authors may use the median comparator group risk across studies. If typical risks are not known, an option is to choose the risk from the included studies, providing the second highest for a high and the second lowest for a low risk population.
14.1.6.4 Risk with intervention
For dichotomous outcomes, review authors should provide a corresponding absolute risk for each comparator group risk, along with a confidence interval. This absolute risk with the (experimental) intervention will usually be derived from the meta-analysis result presented in the relative effect column (see Section 14.1.6.6 ). Formulae are provided in Section 14.1.5 . Review authors should present the absolute effect in the same format as the risks with comparator intervention (see Section 14.1.6.3 ), for example as the number of people experiencing the event per 1000 people.
For continuous outcomes, a difference in means or standardized difference in means should be presented with its confidence interval. These will typically be obtained directly from a meta-analysis. Explanatory text should be used to clarify the meaning, as in Figures 14.1.a and 14.1.b .
14.1.6.5 Risk difference
For dichotomous outcomes, the risk difference can be provided using one of the ‘Summary of findings’ table formats as an additional option (see Figure 14.1.b ). This risk difference expresses the difference between the experimental and comparator intervention and will usually be derived from the meta-analysis result presented in the relative effect column (see Section 14.1.6.6 ). Formulae are provided in Section 14.1.5 . Review authors should present the risk difference in the same format as assumed and corresponding risks with comparator intervention (see Section 14.1.6.3 ); for example, as the number of people experiencing the event per 1000 people or as percentage points if the assumed and corresponding risks are expressed in percentage.
For continuous outcomes, if the ‘Summary of findings’ table includes this option, the mean difference can be presented here and the ‘corresponding risk’ column left blank (see Figure 14.1.b ).
14.1.6.6 Relative effect (95% CI)
The relative effect will typically be a risk ratio or odds ratio (or occasionally a hazard ratio) with its accompanying 95% confidence interval, obtained from a meta-analysis performed on the basis of the same effect measure. Risk ratios and odds ratios are similar when the comparator intervention risks are low and effects are small, but may differ considerably when comparator group risks increase. The meta-analysis may involve an assumption of either fixed or random effects, depending on what the review authors consider appropriate, and implying that the relative effect is either an estimate of the effect of the intervention, or an estimate of the average effect of the intervention across studies, respectively.
14.1.6.7 Number of participants (studies)
This column should include the number of participants assessed in the included studies for each outcome and the corresponding number of studies that contributed these participants.
14.1.6.8 Certainty of the evidence (GRADE)
Review authors should comment on the certainty of the evidence (also known as quality of the body of evidence or confidence in the effect estimates). Review authors should use the specific evidence grading system developed by the GRADE Working Group (Atkins et al 2004, Guyatt et al 2008, Guyatt et al 2011a), which is described in detail in Section 14.2 . The GRADE approach categorizes the certainty in a body of evidence as ‘high’, ‘moderate’, ‘low’ or ‘very low’ by outcome. This is a result of judgement, but the judgement process operates within a transparent structure. As an example, the certainty would be ‘high’ if the summary were of several randomized trials with low risk of bias, but the rating of certainty becomes lower if there are concerns about risk of bias, inconsistency, indirectness, imprecision or publication bias. Judgements other than of ‘high’ certainty should be made transparent using explanatory footnotes or the ‘Comments’ column in the ‘Summary of findings’ table (see Section 14.1.6.10 ).
14.1.6.9 Comments
The aim of the ‘Comments’ field is to help interpret the information or data identified in the row. For example, this may be on the validity of the outcome measure or the presence of variables that are associated with the magnitude of effect. Important caveats about the results should be flagged here. Not all rows will need comments, and it is best to leave a blank if there is nothing warranting a comment.
14.1.6.10 Explanations
Detailed explanations should be included as footnotes to support the judgements in the ‘Summary of findings’ table, such as the overall GRADE assessment. The explanations should describe the rationale for important aspects of the content. Table 14.1.a lists guidance for useful explanations. Explanations should be concise, informative, relevant, easy to understand and accurate. If explanations cannot be sufficiently described in footnotes, review authors should provide further details of the issues in the Results and Discussion sections of the review.
Table 14.1.a Guidance for providing useful explanations in ‘Summary of findings’ (SoF) tables. Adapted from Santesso et al (2016)
|
|
|
|
| , Chi , Tau), or the overlap of confidence intervals, or similarity of point estimates. , describe it as considerable, substantial, moderate or not important. |
|
|
|
|
14.2 Assessing the certainty or quality of a body of evidence
14.2.1 the grade approach.
The Grades of Recommendation, Assessment, Development and Evaluation Working Group (GRADE Working Group) has developed a system for grading the certainty of evidence (Schünemann et al 2003, Atkins et al 2004, Schünemann et al 2006, Guyatt et al 2008, Guyatt et al 2011a). Over 100 organizations including the World Health Organization (WHO), the American College of Physicians, the American Society of Hematology (ASH), the Canadian Agency for Drugs and Technology in Health (CADTH) and the National Institutes of Health and Clinical Excellence (NICE) in the UK have adopted the GRADE system ( www.gradeworkinggroup.org ).
Cochrane has also formally adopted this approach, and all Cochrane Reviews should use GRADE to evaluate the certainty of evidence for important outcomes (see MECIR Box 14.2.a ).
MECIR Box 14.2.a Relevant expectations for conduct of intervention reviews
| Assessing the certainty of the body of evidence ( ) | |
|
| GRADE is the most widely used approach for summarizing confidence in effects of interventions by outcome across studies. It is preferable to use the online GRADEpro tool, and to use it as described in the help system of the software. This should help to ensure that author teams are accessing the same information to inform their judgements. Ideally, two people working independently should assess the certainty of the body of evidence and reach a consensus view on any downgrading decisions. The five GRADE considerations should be addressed irrespective of whether the review includes a ‘Summary of findings’ table. It is helpful to draw on this information in the Discussion, in the Authors’ conclusions and to convey the certainty in the evidence in the Abstract and Plain language summary. |
| Justifying assessments of the certainty of the body of evidence ( ) | |
|
| The adoption of a structured approach ensures transparency in formulating an interpretation of the evidence, and the result is more informative to the user. |
For systematic reviews, the GRADE approach defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the quantity of specific interest. Assessing the certainty of a body of evidence involves consideration of within- and across-study risk of bias (limitations in study design and execution or methodological quality), inconsistency (or heterogeneity), indirectness of evidence, imprecision of the effect estimates and risk of publication bias (see Section 14.2.2 ), as well as domains that may increase our confidence in the effect estimate (as described in Section 14.2.3 ). The GRADE system entails an assessment of the certainty of a body of evidence for each individual outcome. Judgements about the domains that determine the certainty of evidence should be described in the results or discussion section and as part of the ‘Summary of findings’ table.
The GRADE approach specifies four levels of certainty ( Figure 14.2.a ). For interventions, including diagnostic and other tests that are evaluated as interventions (Schünemann et al 2008b, Schünemann et al 2008a, Balshem et al 2011, Schünemann et al 2012), the starting point for rating the certainty of evidence is categorized into two types:
- randomized trials; and
- non-randomized studies of interventions (NRSI), including observational studies (including but not limited to cohort studies, and case-control studies, cross-sectional studies, case series and case reports, although not all of these designs are usually included in Cochrane Reviews).
There are many instances in which review authors rely on information from NRSI, in particular to evaluate potential harms (see Chapter 24 ). In addition, review authors can obtain relevant data from both randomized trials and NRSI, with each type of evidence complementing the other (Schünemann et al 2013).
In GRADE, a body of evidence from randomized trials begins with a high-certainty rating while a body of evidence from NRSI begins with a low-certainty rating. The lower rating with NRSI is the result of the potential bias induced by the lack of randomization (i.e. confounding and selection bias).
However, when using the new Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool (Sterne et al 2016), an assessment tool that covers the risk of bias due to lack of randomization, all studies may start as high certainty of the evidence (Schünemann et al 2018). The approach of starting all study designs (including NRSI) as high certainty does not conflict with the initial GRADE approach of starting the rating of NRSI as low certainty evidence. This is because a body of evidence from NRSI should generally be downgraded by two levels due to the inherent risk of bias associated with the lack of randomization, namely confounding and selection bias. Not downgrading NRSI from high to low certainty needs transparent and detailed justification for what mitigates concerns about confounding and selection bias (Schünemann et al 2018). Very few examples of where not rating down by two levels is appropriate currently exist.
The highest certainty rating is a body of evidence when there are no concerns in any of the GRADE factors listed in Figure 14.2.a . Review authors often downgrade evidence to moderate, low or even very low certainty evidence, depending on the presence of the five factors in Figure 14.2.a . Usually, certainty rating will fall by one level for each factor, up to a maximum of three levels for all factors. If there are very severe problems for any one domain (e.g. when assessing risk of bias, all studies were unconcealed, unblinded and lost over 50% of their patients to follow-up), evidence may fall by two levels due to that factor alone. It is not possible to rate lower than ‘very low certainty’ evidence.
Review authors will generally grade evidence from sound non-randomized studies as low certainty, even if ROBINS-I is used. If, however, such studies yield large effects and there is no obvious bias explaining those effects, review authors may rate the evidence as moderate or – if the effect is large enough – even as high certainty ( Figure 14.2.a ). The very low certainty level is appropriate for, but is not limited to, studies with critical problems and unsystematic clinical observations (e.g. case series or case reports).
Figure 14.2.a Levels of the certainty of a body of evidence in the GRADE approach. *Upgrading criteria are usually applicable to non-randomized studies only (but exceptions exist).
| |
| |
| | ||
|
|
|
|
|
|
| |
|
|
| |||||
|
|
|
|
|
⊕⊕⊕⊕ | ||
|
|
⊕⊕⊕◯ | |||||
|
|
|
⊕⊕◯◯ | ||||
|
|
⊕◯◯◯ | |||||
14.2.2 Domains that can lead to decreasing the certainty level of a body of evidence
We now describe in more detail the five reasons (or domains) for downgrading the certainty of a body of evidence for a specific outcome. In each case, if no reason is found for downgrading the evidence, it should be classified as 'no limitation or not serious' (not important enough to warrant downgrading). If a reason is found for downgrading the evidence, it should be classified as 'serious' (downgrading the certainty rating by one level) or 'very serious' (downgrading the certainty grade by two levels). For non-randomized studies assessed with ROBINS-I, rating down by three levels should be classified as 'extremely' serious.
(1) Risk of bias or limitations in the detailed design and implementation
Our confidence in an estimate of effect decreases if studies suffer from major limitations that are likely to result in a biased assessment of the intervention effect. For randomized trials, these methodological limitations include failure to generate a random sequence, lack of allocation sequence concealment, lack of blinding (particularly with subjective outcomes that are highly susceptible to biased assessment), a large loss to follow-up or selective reporting of outcomes. Chapter 8 provides a discussion of study-level assessments of risk of bias in the context of a Cochrane Review, and proposes an approach to assessing the risk of bias for an outcome across studies as ‘Low’ risk of bias, ‘Some concerns’ and ‘High’ risk of bias for randomized trials. Levels of ‘Low’. ‘Moderate’, ‘Serious’ and ‘Critical’ risk of bias arise for non-randomized studies assessed with ROBINS-I ( Chapter 25 ). These assessments should feed directly into this GRADE domain. In particular, ‘Low’ risk of bias would indicate ‘no limitation’; ‘Some concerns’ would indicate either ‘no limitation’ or ‘serious limitation’; and ‘High’ risk of bias would indicate either ‘serious limitation’ or ‘very serious limitation’. ‘Critical’ risk of bias on ROBINS-I would indicate extremely serious limitations in GRADE. Review authors should use their judgement to decide between alternative categories, depending on the likely magnitude of the potential biases.
Every study addressing a particular outcome will differ, to some degree, in the risk of bias. Review authors should make an overall judgement on whether the certainty of evidence for an outcome warrants downgrading on the basis of study limitations. The assessment of study limitations should apply to the studies contributing to the results in the ‘Summary of findings’ table, rather than to all studies that could potentially be included in the analysis. We have argued in Chapter 7, Section 7.6.2 , that the primary analysis should be restricted to studies at low (or low and unclear) risk of bias where possible.
Table 14.2.a presents the judgements that must be made in going from assessments of the risk of bias to judgements about study limitations for each outcome included in a ‘Summary of findings’ table. A rating of high certainty evidence can be achieved only when most evidence comes from studies that met the criteria for low risk of bias. For example, of the 22 studies addressing the impact of beta-blockers on mortality in patients with heart failure, most probably or certainly used concealed allocation of the sequence, all blinded at least some key groups and follow-up of randomized patients was almost complete (Brophy et al 2001). The certainty of evidence might be downgraded by one level when most of the evidence comes from individual studies either with a crucial limitation for one item, or with some limitations for multiple items. An example of very serious limitations, warranting downgrading by two levels, is provided by evidence on surgery versus conservative treatment in the management of patients with lumbar disc prolapse (Gibson and Waddell 2007). We are uncertain of the benefit of surgery in reducing symptoms after one year or longer, because the one study included in the analysis had inadequate concealment of the allocation sequence and the outcome was assessed using a crude rating by the surgeon without blinding.
(2) Unexplained heterogeneity or inconsistency of results
When studies yield widely differing estimates of effect (heterogeneity or variability in results), investigators should look for robust explanations for that heterogeneity. For instance, drugs may have larger relative effects in sicker populations or when given in larger doses. A detailed discussion of heterogeneity and its investigation is provided in Chapter 10, Section 10.10 and Section 10.11 . If an important modifier exists, with good evidence that important outcomes are different in different subgroups (which would ideally be pre-specified), then a separate ‘Summary of findings’ table may be considered for a separate population. For instance, a separate ‘Summary of findings’ table would be used for carotid endarterectomy in symptomatic patients with high grade stenosis (70% to 99%) in which the intervention is, in the hands of the right surgeons, beneficial, and another (if review authors considered it relevant) for asymptomatic patients with low grade stenosis (less than 30%) in which surgery appears harmful (Orrapin and Rerkasem 2017). When heterogeneity exists and affects the interpretation of results, but review authors are unable to identify a plausible explanation with the data available, the certainty of the evidence decreases.
(3) Indirectness of evidence
Two types of indirectness are relevant. First, a review comparing the effectiveness of alternative interventions (say A and B) may find that randomized trials are available, but they have compared A with placebo and B with placebo. Thus, the evidence is restricted to indirect comparisons between A and B. Where indirect comparisons are undertaken within a network meta-analysis context, GRADE for network meta-analysis should be used (see Chapter 11, Section 11.5 ).
Second, a review may find randomized trials that meet eligibility criteria but address a restricted version of the main review question in terms of population, intervention, comparator or outcomes. For example, suppose that in a review addressing an intervention for secondary prevention of coronary heart disease, most identified studies happened to be in people who also had diabetes. Then the evidence may be regarded as indirect in relation to the broader question of interest because the population is primarily related to people with diabetes. The opposite scenario can equally apply: a review addressing the effect of a preventive strategy for coronary heart disease in people with diabetes may consider studies in people without diabetes to provide relevant, albeit indirect, evidence. This would be particularly likely if investigators had conducted few if any randomized trials in the target population (e.g. people with diabetes). Other sources of indirectness may arise from interventions studied (e.g. if in all included studies a technical intervention was implemented by expert, highly trained specialists in specialist centres, then evidence on the effects of the intervention outside these centres may be indirect), comparators used (e.g. if the comparator groups received an intervention that is less effective than standard treatment in most settings) and outcomes assessed (e.g. indirectness due to surrogate outcomes when data on patient-important outcomes are not available, or when investigators seek data on quality of life but only symptoms are reported). Review authors should make judgements transparent when they believe downgrading is justified, based on differences in anticipated effects in the group of primary interest. Review authors may be aided and increase transparency of their judgements about indirectness if they use Table 14.2.b available in the GRADEpro GDT software (Schünemann et al 2013).
(4) Imprecision of results
When studies include few participants or few events, and thus have wide confidence intervals, review authors can lower their rating of the certainty of the evidence. The confidence intervals included in the ‘Summary of findings’ table will provide readers with information that allows them to make, to some extent, their own rating of precision. Review authors can use a calculation of the optimal information size (OIS) or review information size (RIS), similar to sample size calculations, to make judgements about imprecision (Guyatt et al 2011b, Schünemann 2016). The OIS or RIS is calculated on the basis of the number of participants required for an adequately powered individual study. If the 95% confidence interval excludes a risk ratio (RR) of 1.0, and the total number of events or patients exceeds the OIS criterion, precision is adequate. If the 95% CI includes appreciable benefit or harm (an RR of under 0.75 or over 1.25 is often suggested as a very rough guide) downgrading for imprecision may be appropriate even if OIS criteria are met (Guyatt et al 2011b, Schünemann 2016).
(5) High probability of publication bias
The certainty of evidence level may be downgraded if investigators fail to report studies on the basis of results (typically those that show no effect: publication bias) or outcomes (typically those that may be harmful or for which no effect was observed: selective outcome non-reporting bias). Selective reporting of outcomes from among multiple outcomes measured is assessed at the study level as part of the assessment of risk of bias (see Chapter 8, Section 8.7 ), so for the studies contributing to the outcome in the ‘Summary of findings’ table this is addressed by domain 1 above (limitations in the design and implementation). If a large number of studies included in the review do not contribute to an outcome, or if there is evidence of publication bias, the certainty of the evidence may be downgraded. Chapter 13 provides a detailed discussion of reporting biases, including publication bias, and how it may be tackled in a Cochrane Review. A prototypical situation that may elicit suspicion of publication bias is when published evidence includes a number of small studies, all of which are industry-funded (Bhandari et al 2004). For example, 14 studies of flavanoids in patients with haemorrhoids have shown apparent large benefits, but enrolled a total of only 1432 patients (i.e. each study enrolled relatively few patients) (Alonso-Coello et al 2006). The heavy involvement of sponsors in most of these studies raises questions of whether unpublished studies that suggest no benefit exist (publication bias).
A particular body of evidence can suffer from problems associated with more than one of the five factors listed here, and the greater the problems, the lower the certainty of evidence rating that should result. One could imagine a situation in which randomized trials were available, but all or virtually all of these limitations would be present, and in serious form. A very low certainty of evidence rating would result.
Table 14.2.a Further guidelines for domain 1 (of 5) in a GRADE assessment: going from assessments of risk of bias in studies to judgements about study limitations for main outcomes across studies
|
|
|
|
|
|
| Low risk of bias | Most information is from results at low risk of bias. | Plausible bias unlikely to seriously alter the results. | No apparent limitations. | No serious limitations, do not downgrade. |
| Some concerns | Most information is from results at low risk of bias or with some concerns. | Plausible bias that raises some doubt about the results. | Potential limitations are unlikely to lower confidence in the estimate of effect. | No serious limitations, do not downgrade. |
| Potential limitations are likely to lower confidence in the estimate of effect. | Serious limitations, downgrade one level. | |||
| High risk of bias | The proportion of information from results at high risk of bias is sufficient to affect the interpretation of results. | Plausible bias that seriously weakens confidence in the results. | Crucial limitation for one criterion, or some limitations for multiple criteria, sufficient to lower confidence in the estimate of effect. | Serious limitations, downgrade one level. |
| Crucial limitation for one or more criteria sufficient to substantially lower confidence in the estimate of effect. | Very serious limitations, downgrade two levels. |
Table 14.2.b Judgements about indirectness by outcome (available in GRADEpro GDT)
|
| |||||
|
|
|
| |||
|
| Probably yes | Probably no | No | ||
|
|
|
|
| ||
Intervention:
| Yes | Probably yes | Probably no | No |
|
|
|
|
|
Comparator:
Direct comparison:
Final judgement about indirectness across domains:
|
|
|
|
14.2.3 Domains that may lead to increasing the certainty level of a body of evidence
Although NRSI and downgraded randomized trials will generally yield a low rating for certainty of evidence, there will be unusual circumstances in which review authors could ‘upgrade’ such evidence to moderate or even high certainty ( Table 14.3.a ).
- Large effects On rare occasions when methodologically well-done observational studies yield large, consistent and precise estimates of the magnitude of an intervention effect, one may be particularly confident in the results. A large estimated effect (e.g. RR >2 or RR <0.5) in the absence of plausible confounders, or a very large effect (e.g. RR >5 or RR <0.2) in studies with no major threats to validity, might qualify for this. In these situations, while the NRSI may possibly have provided an over-estimate of the true effect, the weak study design may not explain all of the apparent observed benefit. Thus, despite reservations based on the observational study design, review authors are confident that the effect exists. The magnitude of the effect in these studies may move the assigned certainty of evidence from low to moderate (if the effect is large in the absence of other methodological limitations). For example, a meta-analysis of observational studies showed that bicycle helmets reduce the risk of head injuries in cyclists by a large margin (odds ratio (OR) 0.31, 95% CI 0.26 to 0.37) (Thompson et al 2000). This large effect, in the absence of obvious bias that could create the association, suggests a rating of moderate-certainty evidence. Note : GRADE guidance suggests the possibility of rating up one level for a large effect if the relative effect is greater than 2.0. However, if the point estimate of the relative effect is greater than 2.0, but the confidence interval is appreciably below 2.0, then some hesitation would be appropriate in the decision to rate up for a large effect. Another situation allows inference of a strong association without a formal comparative study. Consider the question of the impact of routine colonoscopy versus no screening for colon cancer on the rate of perforation associated with colonoscopy. Here, a large series of representative patients undergoing colonoscopy may provide high certainty evidence about the risk of perforation associated with colonoscopy. When the risk of the event among patients receiving the relevant comparator is known to be near 0 (i.e. we are certain that the incidence of spontaneous colon perforation in patients not undergoing colonoscopy is extremely low), case series or cohort studies of representative patients can provide high certainty evidence of adverse effects associated with an intervention, thereby allowing us to infer a strong association from even a limited number of events.
- Dose-response The presence of a dose-response gradient may increase our confidence in the findings of observational studies and thereby enhance the assigned certainty of evidence. For example, our confidence in the result of observational studies that show an increased risk of bleeding in patients who have supratherapeutic anticoagulation levels is increased by the observation that there is a dose-response gradient between the length of time needed for blood to clot (as measured by the international normalized ratio (INR)) and an increased risk of bleeding (Levine et al 2004). A systematic review of NRSI investigating the effect of cyclooxygenase-2 inhibitors on cardiovascular events found that the summary estimate (RR) with rofecoxib was 1.33 (95% CI 1.00 to 1.79) with doses less than 25mg/d, and 2.19 (95% CI 1.64 to 2.91) with doses more than 25mg/d. Although residual confounding is likely to exist in the NRSI that address this issue, the existence of a dose-response gradient and the large apparent effect of higher doses of rofecoxib markedly increase our strength of inference that the association cannot be explained by residual confounding, and is therefore likely to be both causal and, at high levels of exposure, substantial. Note : GRADE guidance suggests the possibility of rating up one level for a large effect if the relative effect is greater than 2.0. Here, the fact that the point estimate of the relative effect is greater than 2.0, but the confidence interval is appreciably below 2.0 might make some hesitate in the decision to rate up for a large effect
- Plausible confounding On occasion, all plausible biases from randomized or non-randomized studies may be working to under-estimate an apparent intervention effect. For example, if only sicker patients receive an experimental intervention or exposure, yet they still fare better, it is likely that the actual intervention or exposure effect is larger than the data suggest. For instance, a rigorous systematic review of observational studies including a total of 38 million patients demonstrated higher death rates in private for-profit versus private not-for-profit hospitals (Devereaux et al 2002). One possible bias relates to different disease severity in patients in the two hospital types. It is likely, however, that patients in the not-for-profit hospitals were sicker than those in the for-profit hospitals. Thus, to the extent that residual confounding existed, it would bias results against the not-for-profit hospitals. The second likely bias was the possibility that higher numbers of patients with excellent private insurance coverage could lead to a hospital having more resources and a spill-over effect that would benefit those without such coverage. Since for-profit hospitals are likely to admit a larger proportion of such well-insured patients than not-for-profit hospitals, the bias is once again against the not-for-profit hospitals. Since the plausible biases would all diminish the demonstrated intervention effect, one might consider the evidence from these observational studies as moderate rather than low certainty. A parallel situation exists when observational studies have failed to demonstrate an association, but all plausible biases would have increased an intervention effect. This situation will usually arise in the exploration of apparent harmful effects. For example, because the hypoglycaemic drug phenformin causes lactic acidosis, the related agent metformin was under suspicion for the same toxicity. Nevertheless, very large observational studies have failed to demonstrate an association (Salpeter et al 2007). Given the likelihood that clinicians would be more alert to lactic acidosis in the presence of the agent and over-report its occurrence, one might consider this moderate, or even high certainty, evidence refuting a causal relationship between typical therapeutic doses of metformin and lactic acidosis.
14.3 Describing the assessment of the certainty of a body of evidence using the GRADE framework
Review authors should report the grading of the certainty of evidence in the Results section for each outcome for which this has been performed, providing the rationale for downgrading or upgrading the evidence, and referring to the ‘Summary of findings’ table where applicable.
Table 14.3.a provides a framework and examples for how review authors can justify their judgements about the certainty of evidence in each domain. These justifications should also be included in explanatory notes to the ‘Summary of Findings’ table (see Section 14.1.6.10 ).
Chapter 15, Section 15.6 , describes in more detail how the overall GRADE assessment across all domains can be used to draw conclusions about the effects of the intervention, as well as providing implications for future research.
Table 14.3.a Framework for describing the certainty of evidence and justifying downgrading or upgrading
|
|
|
|
|
| Describe the risk of bias based on the criteria used in the risk-of-bias table. | Downgraded because of 10 randomized trials, five did not blind patients and caretakers. |
|
| Describe the degree of inconsistency by outcome using one or more indicators (e.g. I and P value), confidence interval overlap, difference in point estimate, between-study variance. | Not downgraded because the proportion of the variability in effect estimates that is due to true heterogeneity rather than chance is not important (I = 0%). |
|
| Describe if the majority of studies address the PICO – were they similar to the question posed? | Downgraded because the included studies were restricted to patients with advanced cancer. |
|
| Describe the number of events, and width of the confidence intervals. | The confidence intervals for the effect on mortality are consistent with both an appreciable benefit and appreciable harm and we lowered the certainty. |
|
| Describe the possible degree of publication bias. | 1. The funnel plot of 14 randomized trials indicated that there were several small studies that showed a small positive effect, but small studies that showed no effect or harm may have been unpublished. The certainty of the evidence was lowered. 2. There are only three small positive studies, it appears that studies showing no effect or harm have not been published. There also is for-profit interest in the intervention. The certainty of the evidence was lowered. |
|
| Describe the magnitude of the effect and the widths of the associate confidence intervals. | Upgraded because the RR is large: 0.3 (95% CI 0.2 to 0.4), with a sufficient number of events to be precise. |
|
| The studies show a clear relation with increases in the outcome of an outcome (e.g. lung cancer) with higher exposure levels. | Upgraded because the dose-response relation shows a relative risk increase of 10% in never smokers, 15% in smokers of 10 pack years and 20% in smokers of 15 pack years. |
|
| Describe which opposing plausible biases and confounders may have not been considered. | The estimate of effect is not controlled for the following possible confounders: smoking, degree of education, but the distribution of these factors in the studies is likely to lead to an under-estimate of the true effect. The certainty of the evidence was increased. |
14.4 Chapter information
Authors: Holger J Schünemann, Julian PT Higgins, Gunn E Vist, Paul Glasziou, Elie A Akl, Nicole Skoetz, Gordon H Guyatt; on behalf of the Cochrane GRADEing Methods Group (formerly Applicability and Recommendations Methods Group) and the Cochrane Statistical Methods Group
Acknowledgements: Andrew D Oxman contributed to earlier versions. Professor Penny Hawe contributed to the text on adverse effects in earlier versions. Jon Deeks provided helpful contributions on an earlier version of this chapter. For details of previous authors and editors of the Handbook , please refer to the Preface.
Funding: This work was in part supported by funding from the Michael G DeGroote Cochrane Canada Centre and the Ontario Ministry of Health.
14.5 References
Alonso-Coello P, Zhou Q, Martinez-Zapata MJ, Mills E, Heels-Ansdell D, Johanson JF, Guyatt G. Meta-analysis of flavonoids for the treatment of haemorrhoids. British Journal of Surgery 2006; 93 : 909-920.
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O'Connell D, Oxman AD, Phillips B, Schünemann HJ, Edejer TT, Varonen H, Vist GE, Williams JW, Jr., Zaza S. Grading quality of evidence and strength of recommendations. BMJ 2004; 328 : 1490.
Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt GH. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011; 64 : 401-406.
Bhandari M, Busse JW, Jackowski D, Montori VM, Schünemann H, Sprague S, Mears D, Schemitsch EH, Heels-Ansdell D, Devereaux PJ. Association between industry funding and statistically significant pro-industry findings in medical and surgical randomized trials. Canadian Medical Association Journal 2004; 170 : 477-480.
Brophy JM, Joseph L, Rouleau JL. Beta-blockers in congestive heart failure. A Bayesian meta-analysis. Annals of Internal Medicine 2001; 134 : 550-560.
Carrasco-Labra A, Brignardello-Petersen R, Santesso N, Neumann I, Mustafa RA, Mbuagbaw L, Etxeandia Ikobaltzeta I, De Stio C, McCullagh LJ, Alonso-Coello P, Meerpohl JJ, Vandvik PO, Brozek JL, Akl EA, Bossuyt P, Churchill R, Glenton C, Rosenbaum S, Tugwell P, Welch V, Garner P, Guyatt G, Schünemann HJ. Improving GRADE evidence tables part 1: a randomized trial shows improved understanding of content in summary of findings tables with a new format. Journal of Clinical Epidemiology 2016; 74 : 7-18.
Deeks JJ, Altman DG. Effect measures for meta-analysis of trials with binary outcomes. In: Egger M, Davey Smith G, Altman DG, editors. Systematic Reviews in Health Care: Meta-analysis in Context . 2nd ed. London (UK): BMJ Publication Group; 2001. p. 313-335.
Devereaux PJ, Choi PT, Lacchetti C, Weaver B, Schünemann HJ, Haines T, Lavis JN, Grant BJ, Haslam DR, Bhandari M, Sullivan T, Cook DJ, Walter SD, Meade M, Khan H, Bhatnagar N, Guyatt GH. A systematic review and meta-analysis of studies comparing mortality rates of private for-profit and private not-for-profit hospitals. Canadian Medical Association Journal 2002; 166 : 1399-1406.
Engels EA, Schmid CH, Terrin N, Olkin I, Lau J. Heterogeneity and statistical significance in meta-analysis: an empirical study of 125 meta-analyses. Statistics in Medicine 2000; 19 : 1707-1728.
Furukawa TA, Guyatt GH, Griffith LE. Can we individualize the 'number needed to treat'? An empirical study of summary effect measures in meta-analyses. International Journal of Epidemiology 2002; 31 : 72-76.
Gibson JN, Waddell G. Surgical interventions for lumbar disc prolapse: updated Cochrane Review. Spine 2007; 32 : 1735-1747.
Guyatt G, Oxman A, Vist G, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann H. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336 : 3.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schünemann HJ. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011a; 64 : 383-394.
Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, Devereaux PJ, Montori VM, Freyschuss B, Vist G, Jaeschke R, Williams JW, Jr., Murad MH, Sinclair D, Falck-Ytter Y, Meerpohl J, Whittington C, Thorlund K, Andrews J, Schünemann HJ. GRADE guidelines 6. Rating the quality of evidence--imprecision. Journal of Clinical Epidemiology 2011b; 64 : 1283-1293.
Iorio A, Spencer FA, Falavigna M, Alba C, Lang E, Burnand B, McGinn T, Hayden J, Williams K, Shea B, Wolff R, Kujpers T, Perel P, Vandvik PO, Glasziou P, Schünemann H, Guyatt G. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ 2015; 350 : h870.
Langendam M, Carrasco-Labra A, Santesso N, Mustafa RA, Brignardello-Petersen R, Ventresca M, Heus P, Lasserson T, Moustgaard R, Brozek J, Schünemann HJ. Improving GRADE evidence tables part 2: a systematic survey of explanatory notes shows more guidance is needed. Journal of Clinical Epidemiology 2016; 74 : 19-27.
Levine MN, Raskob G, Landefeld S, Kearon C, Schulman S. Hemorrhagic complications of anticoagulant treatment: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126 : 287S-310S.
Orrapin S, Rerkasem K. Carotid endarterectomy for symptomatic carotid stenosis. Cochrane Database of Systematic Reviews 2017; 6 : CD001081.
Salpeter S, Greyber E, Pasternak G, Salpeter E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2007; 4 : CD002967.
Santesso N, Carrasco-Labra A, Langendam M, Brignardello-Petersen R, Mustafa RA, Heus P, Lasserson T, Opiyo N, Kunnamo I, Sinclair D, Garner P, Treweek S, Tovey D, Akl EA, Tugwell P, Brozek JL, Guyatt G, Schünemann HJ. Improving GRADE evidence tables part 3: detailed guidance for explanatory footnotes supports creating and understanding GRADE certainty in the evidence judgments. Journal of Clinical Epidemiology 2016; 74 : 28-39.
Schünemann HJ, Best D, Vist G, Oxman AD, Group GW. Letters, numbers, symbols and words: how to communicate grades of evidence and recommendations. Canadian Medical Association Journal 2003; 169 : 677-680.
Schünemann HJ, Jaeschke R, Cook DJ, Bria WF, El-Solh AA, Ernst A, Fahy BF, Gould MK, Horan KL, Krishnan JA, Manthous CA, Maurer JR, McNicholas WT, Oxman AD, Rubenfeld G, Turino GM, Guyatt G. An official ATS statement: grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. American Journal of Respiratory and Critical Care Medicine 2006; 174 : 605-614.
Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, Williams JW, Jr., Kunz R, Craig J, Montori VM, Bossuyt P, Guyatt GH. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ 2008a; 336 : 1106-1110.
Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Bossuyt P, Chang S, Muti P, Jaeschke R, Guyatt GH. GRADE: assessing the quality of evidence for diagnostic recommendations. ACP Journal Club 2008b; 149 : 2.
Schünemann HJ, Mustafa R, Brozek J. [Diagnostic accuracy and linked evidence--testing the chain]. Zeitschrift für Evidenz, Fortbildung und Qualität im Gesundheitswesen 2012; 106 : 153-160.
Schünemann HJ, Tugwell P, Reeves BC, Akl EA, Santesso N, Spencer FA, Shea B, Wells G, Helfand M. Non-randomized studies as a source of complementary, sequential or replacement evidence for randomized controlled trials in systematic reviews on the effects of interventions. Research Synthesis Methods 2013; 4 : 49-62.
Schünemann HJ. Interpreting GRADE's levels of certainty or quality of the evidence: GRADE for statisticians, considering review information size or less emphasis on imprecision? Journal of Clinical Epidemiology 2016; 75 : 6-15.
Schünemann HJ, Cuello C, Akl EA, Mustafa RA, Meerpohl JJ, Thayer K, Morgan RL, Gartlehner G, Kunz R, Katikireddi SV, Sterne J, Higgins JPT, Guyatt G, Group GW. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. Journal of Clinical Epidemiology 2018.
Spencer-Bonilla G, Quinones AR, Montori VM, International Minimally Disruptive Medicine W. Assessing the Burden of Treatment. Journal of General Internal Medicine 2017; 32 : 1141-1145.
Spencer FA, Iorio A, You J, Murad MH, Schünemann HJ, Vandvik PO, Crowther MA, Pottie K, Lang ES, Meerpohl JJ, Falck-Ytter Y, Alonso-Coello P, Guyatt GH. Uncertainties in baseline risk estimates and confidence in treatment effects. BMJ 2012; 345 : e7401.
Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JPT. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355 : i4919.
Thompson DC, Rivara FP, Thompson R. Helmets for preventing head and facial injuries in bicyclists. Cochrane Database of Systematic Reviews 2000; 2 : CD001855.
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007; 8 .
van Dalen EC, Tierney JF, Kremer LCM. Tips and tricks for understanding and using SR results. No. 7: time‐to‐event data. Evidence-Based Child Health 2007; 2 : 1089-1090.
For permission to re-use material from the Handbook (either academic or commercial), please see here for full details.

How To Structure Your Literature Review

- Demonstrate your knowledge of the research topic
- Identify the gaps in the literature and show how your research links to these
- Provide the foundation for your conceptual framework (if you have one)
- Inform your own methodology and research design

But wait – is this the right time?
Deciding on the structure of your literature review should come towards the end of the literature review process – after you have collected and digested the literature, but before you start writing the chapter.
In other words, you need to first develop a rich understanding of the literature before you even attempt to map out a structure. There’s no use trying to develop a structure before you’ve fully wrapped your head around the existing research.
Equally importantly, you need to have a structure in place before you start writing , or your literature review will most likely end up a rambling, disjointed mess.
Importantly, don’t feel that once you’ve defined a structure you can’t iterate on it. It’s perfectly natural to adjust as you engage in the writing process. As we’ve discussed before , writing is a way of developing your thinking, so it’s quite common for your thinking to change – and therefore, for your chapter structure to change – as you write.
Need a helping hand?
Like any other chapter in your thesis or dissertation, your literature review needs to have a clear, logical structure. At a minimum, it should have three essential components – an introduction , a body and a conclusion .
Let’s take a closer look at each of these.
1: The Introduction Section
Just like any good introduction, the introduction section of your literature review should introduce the purpose and layout (organisation) of the chapter. In other words, your introduction needs to give the reader a taste of what’s to come, and how you’re going to lay that out. Essentially, you should provide the reader with a high-level roadmap of your chapter to give them a taste of the journey that lies ahead.
Here’s an example of the layout visualised in a literature review introduction:
Your introduction should also outline your topic (including any tricky terminology or jargon) and provide an explanation of the scope of your literature review – in other words, what you will and won’t be covering (the delimitations ). This helps ringfence your review and achieve a clear focus . The clearer and narrower your focus, the deeper you can dive into the topic (which is typically where the magic lies).
Depending on the nature of your project, you could also present your stance or point of view at this stage. In other words, after grappling with the literature you’ll have an opinion about what the trends and concerns are in the field as well as what’s lacking. The introduction section can then present these ideas so that it is clear to examiners that you’re aware of how your research connects with existing knowledge .

2: The Body Section
The body of your literature review is the centre of your work. This is where you’ll present, analyse, evaluate and synthesise the existing research. In other words, this is where you’re going to earn (or lose) the most marks. Therefore, it’s important to carefully think about how you will organise your discussion to present it in a clear way.
The body of your literature review should do just as the description of this chapter suggests. It should “review” the literature – in other words, identify, analyse, and synthesise it. So, when thinking about structuring your literature review, you need to think about which structural approach will provide the best “review” for your specific type of research and objectives (we’ll get to this shortly).
There are (broadly speaking) three options for organising your literature review.

Option 1: Chronological (according to date)
Organising the literature chronologically is one of the simplest ways to structure your literature review. You start with what was published first and work your way through the literature until you reach the work published most recently. Pretty straightforward.
The benefit of this option is that it makes it easy to discuss the developments and debates in the field as they emerged over time. Organising your literature chronologically also allows you to highlight how specific articles or pieces of work might have changed the course of the field – in other words, which research has had the most impact . Therefore, this approach is very useful when your research is aimed at understanding how the topic has unfolded over time and is often used by scholars in the field of history. That said, this approach can be utilised by anyone that wants to explore change over time .

For example , if a student of politics is investigating how the understanding of democracy has evolved over time, they could use the chronological approach to provide a narrative that demonstrates how this understanding has changed through the ages.
Here are some questions you can ask yourself to help you structure your literature review chronologically.
- What is the earliest literature published relating to this topic?
- How has the field changed over time? Why?
- What are the most recent discoveries/theories?
In some ways, chronology plays a part whichever way you decide to structure your literature review, because you will always, to a certain extent, be analysing how the literature has developed. However, with the chronological approach, the emphasis is very firmly on how the discussion has evolved over time , as opposed to how all the literature links together (which we’ll discuss next ).
Option 2: Thematic (grouped by theme)
The thematic approach to structuring a literature review means organising your literature by theme or category – for example, by independent variables (i.e. factors that have an impact on a specific outcome).
As you’ve been collecting and synthesising literature , you’ll likely have started seeing some themes or patterns emerging. You can then use these themes or patterns as a structure for your body discussion. The thematic approach is the most common approach and is useful for structuring literature reviews in most fields.
For example, if you were researching which factors contributed towards people trusting an organisation, you might find themes such as consumers’ perceptions of an organisation’s competence, benevolence and integrity. Structuring your literature review thematically would mean structuring your literature review’s body section to discuss each of these themes, one section at a time.

Here are some questions to ask yourself when structuring your literature review by themes:
- Are there any patterns that have come to light in the literature?
- What are the central themes and categories used by the researchers?
- Do I have enough evidence of these themes?
PS – you can see an example of a thematically structured literature review in our literature review sample walkthrough video here.
Option 3: Methodological
The methodological option is a way of structuring your literature review by the research methodologies used . In other words, organising your discussion based on the angle from which each piece of research was approached – for example, qualitative , quantitative or mixed methodologies.
Structuring your literature review by methodology can be useful if you are drawing research from a variety of disciplines and are critiquing different methodologies. The point of this approach is to question how existing research has been conducted, as opposed to what the conclusions and/or findings the research were.

For example, a sociologist might centre their research around critiquing specific fieldwork practices. Their literature review will then be a summary of the fieldwork methodologies used by different studies.
Here are some questions you can ask yourself when structuring your literature review according to methodology:
- Which methodologies have been utilised in this field?
- Which methodology is the most popular (and why)?
- What are the strengths and weaknesses of the various methodologies?
- How can the existing methodologies inform my own methodology?
3: The Conclusion Section
Once you’ve completed the body section of your literature review using one of the structural approaches we discussed above, you’ll need to “wrap up” your literature review and pull all the pieces together to set the direction for the rest of your dissertation or thesis.
The conclusion is where you’ll present the key findings of your literature review. In this section, you should emphasise the research that is especially important to your research questions and highlight the gaps that exist in the literature. Based on this, you need to make it clear what you will add to the literature – in other words, justify your own research by showing how it will help fill one or more of the gaps you just identified.
Last but not least, if it’s your intention to develop a conceptual framework for your dissertation or thesis, the conclusion section is a good place to present this.

Example: Thematically Structured Review
In the video below, we unpack a literature review chapter so that you can see an example of a thematically structure review in practice.
Let’s Recap
In this article, we’ve discussed how to structure your literature review for maximum impact. Here’s a quick recap of what you need to keep in mind when deciding on your literature review structure:
- Just like other chapters, your literature review needs a clear introduction , body and conclusion .
- The introduction section should provide an overview of what you will discuss in your literature review.
- The body section of your literature review can be organised by chronology , theme or methodology . The right structural approach depends on what you’re trying to achieve with your research.
- The conclusion section should draw together the key findings of your literature review and link them to your research questions.
If you’re ready to get started, be sure to download our free literature review template to fast-track your chapter outline.

Learn More About Lit Review:

Writing A Literature Review: 4 Time-Saving Hacks
🎙️ PODCAST: Ace The Literature Review 4 Time-Saving Tips To Fast-Track Your Literature...

Research Question 101: Everything You Need To Know
Research Question 101 📖 Everything you need to know to write a high-quality research...

Research Question Examples: The Perfect Starting Point
See what quality research questions look like across multiple topic areas, including psychology, business, computer science and more.

Literature Synthesis 101: How To Synthesise The Literature
Literature Syntheis 101 How To Synthesise The Existing Research (With Examples)By: Derek...

The Essential Components of a Literature Review
Literature Review: 3 Essential Ingredients The theoretical framework, empirical research...
📄 FREE TEMPLATES
Research Topic Ideation
Proposal Writing
Literature Review
Methodology & Analysis
Academic Writing
Referencing & Citing
Apps, Tools & Tricks
The Grad Coach Podcast
29 Comments
Great work. This is exactly what I was looking for and helps a lot together with your previous post on literature review. One last thing is missing: a link to a great literature chapter of an journal article (maybe with comments of the different sections in this review chapter). Do you know any great literature review chapters?
I agree with you Marin… A great piece
I agree with Marin. This would be quite helpful if you annotate a nicely structured literature from previously published research articles.
Awesome article for my research.
I thank you immensely for this wonderful guide
It is indeed thought and supportive work for the futurist researcher and students
Very educative and good time to get guide. Thank you
Great work, very insightful. Thank you.
Thanks for this wonderful presentation. My question is that do I put all the variables into a single conceptual framework or each hypothesis will have it own conceptual framework?
Thank you very much, very helpful
This is very educative and precise . Thank you very much for dropping this kind of write up .
Pheeww, so damn helpful, thank you for this informative piece.
I’m doing a research project topic ; stool analysis for parasitic worm (enteric) worm, how do I structure it, thanks.
comprehensive explanation. Help us by pasting the URL of some good “literature review” for better understanding.
great piece. thanks for the awesome explanation. it is really worth sharing. I have a little question, if anyone can help me out, which of the options in the body of literature can be best fit if you are writing an architectural thesis that deals with design?
I am doing a research on nanofluids how can l structure it?
Beautifully clear.nThank you!
Lucid! Thankyou!
Brilliant work, well understood, many thanks
I like how this was so clear with simple language 😊😊 thank you so much 😊 for these information 😊
Insightful. I was struggling to come up with a sensible literature review but this has been really helpful. Thank you!
You have given thought-provoking information about the review of the literature.
Thank you. It has made my own research better and to impart your work to students I teach
I learnt a lot from this teaching. It’s a great piece.
I am doing research on EFL teacher motivation for his/her job. How Can I structure it? Is there any detailed template, additional to this?
You are so cool! I do not think I’ve read through something like this before. So nice to find somebody with some genuine thoughts on this issue. Seriously.. thank you for starting this up. This site is one thing that is required on the internet, someone with a little originality!
I’m asked to do conceptual, theoretical and empirical literature, and i just don’t know how to structure it
Asking questions are actually fastidious thing if you are not understanding anything fully, but this article presents good understanding yet.
thank you SOOO much it is really helpful ..
Submit a Comment Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Submit Comment
- Print Friendly
Purdue Online Writing Lab Purdue OWL® College of Liberal Arts
Writing a Literature Review

Welcome to the Purdue OWL
This page is brought to you by the OWL at Purdue University. When printing this page, you must include the entire legal notice.
Copyright ©1995-2018 by The Writing Lab & The OWL at Purdue and Purdue University. All rights reserved. This material may not be published, reproduced, broadcast, rewritten, or redistributed without permission. Use of this site constitutes acceptance of our terms and conditions of fair use.
A literature review is a document or section of a document that collects key sources on a topic and discusses those sources in conversation with each other (also called synthesis ). The lit review is an important genre in many disciplines, not just literature (i.e., the study of works of literature such as novels and plays). When we say “literature review” or refer to “the literature,” we are talking about the research ( scholarship ) in a given field. You will often see the terms “the research,” “the scholarship,” and “the literature” used mostly interchangeably.
Where, when, and why would I write a lit review?
There are a number of different situations where you might write a literature review, each with slightly different expectations; different disciplines, too, have field-specific expectations for what a literature review is and does. For instance, in the humanities, authors might include more overt argumentation and interpretation of source material in their literature reviews, whereas in the sciences, authors are more likely to report study designs and results in their literature reviews; these differences reflect these disciplines’ purposes and conventions in scholarship. You should always look at examples from your own discipline and talk to professors or mentors in your field to be sure you understand your discipline’s conventions, for literature reviews as well as for any other genre.
A literature review can be a part of a research paper or scholarly article, usually falling after the introduction and before the research methods sections. In these cases, the lit review just needs to cover scholarship that is important to the issue you are writing about; sometimes it will also cover key sources that informed your research methodology.
Lit reviews can also be standalone pieces, either as assignments in a class or as publications. In a class, a lit review may be assigned to help students familiarize themselves with a topic and with scholarship in their field, get an idea of the other researchers working on the topic they’re interested in, find gaps in existing research in order to propose new projects, and/or develop a theoretical framework and methodology for later research. As a publication, a lit review usually is meant to help make other scholars’ lives easier by collecting and summarizing, synthesizing, and analyzing existing research on a topic. This can be especially helpful for students or scholars getting into a new research area, or for directing an entire community of scholars toward questions that have not yet been answered.
What are the parts of a lit review?
Most lit reviews use a basic introduction-body-conclusion structure; if your lit review is part of a larger paper, the introduction and conclusion pieces may be just a few sentences while you focus most of your attention on the body. If your lit review is a standalone piece, the introduction and conclusion take up more space and give you a place to discuss your goals, research methods, and conclusions separately from where you discuss the literature itself.
Introduction:
- An introductory paragraph that explains what your working topic and thesis is
- A forecast of key topics or texts that will appear in the review
- Potentially, a description of how you found sources and how you analyzed them for inclusion and discussion in the review (more often found in published, standalone literature reviews than in lit review sections in an article or research paper)
- Summarize and synthesize: Give an overview of the main points of each source and combine them into a coherent whole
- Analyze and interpret: Don’t just paraphrase other researchers – add your own interpretations where possible, discussing the significance of findings in relation to the literature as a whole
- Critically Evaluate: Mention the strengths and weaknesses of your sources
- Write in well-structured paragraphs: Use transition words and topic sentence to draw connections, comparisons, and contrasts.
Conclusion:
- Summarize the key findings you have taken from the literature and emphasize their significance
- Connect it back to your primary research question
How should I organize my lit review?
Lit reviews can take many different organizational patterns depending on what you are trying to accomplish with the review. Here are some examples:
- Chronological : The simplest approach is to trace the development of the topic over time, which helps familiarize the audience with the topic (for instance if you are introducing something that is not commonly known in your field). If you choose this strategy, be careful to avoid simply listing and summarizing sources in order. Try to analyze the patterns, turning points, and key debates that have shaped the direction of the field. Give your interpretation of how and why certain developments occurred (as mentioned previously, this may not be appropriate in your discipline — check with a teacher or mentor if you’re unsure).
- Thematic : If you have found some recurring central themes that you will continue working with throughout your piece, you can organize your literature review into subsections that address different aspects of the topic. For example, if you are reviewing literature about women and religion, key themes can include the role of women in churches and the religious attitude towards women.
- Qualitative versus quantitative research
- Empirical versus theoretical scholarship
- Divide the research by sociological, historical, or cultural sources
- Theoretical : In many humanities articles, the literature review is the foundation for the theoretical framework. You can use it to discuss various theories, models, and definitions of key concepts. You can argue for the relevance of a specific theoretical approach or combine various theorical concepts to create a framework for your research.
What are some strategies or tips I can use while writing my lit review?
Any lit review is only as good as the research it discusses; make sure your sources are well-chosen and your research is thorough. Don’t be afraid to do more research if you discover a new thread as you’re writing. More info on the research process is available in our "Conducting Research" resources .
As you’re doing your research, create an annotated bibliography ( see our page on the this type of document ). Much of the information used in an annotated bibliography can be used also in a literature review, so you’ll be not only partially drafting your lit review as you research, but also developing your sense of the larger conversation going on among scholars, professionals, and any other stakeholders in your topic.
Usually you will need to synthesize research rather than just summarizing it. This means drawing connections between sources to create a picture of the scholarly conversation on a topic over time. Many student writers struggle to synthesize because they feel they don’t have anything to add to the scholars they are citing; here are some strategies to help you:
- It often helps to remember that the point of these kinds of syntheses is to show your readers how you understand your research, to help them read the rest of your paper.
- Writing teachers often say synthesis is like hosting a dinner party: imagine all your sources are together in a room, discussing your topic. What are they saying to each other?
- Look at the in-text citations in each paragraph. Are you citing just one source for each paragraph? This usually indicates summary only. When you have multiple sources cited in a paragraph, you are more likely to be synthesizing them (not always, but often
- Read more about synthesis here.
The most interesting literature reviews are often written as arguments (again, as mentioned at the beginning of the page, this is discipline-specific and doesn’t work for all situations). Often, the literature review is where you can establish your research as filling a particular gap or as relevant in a particular way. You have some chance to do this in your introduction in an article, but the literature review section gives a more extended opportunity to establish the conversation in the way you would like your readers to see it. You can choose the intellectual lineage you would like to be part of and whose definitions matter most to your thinking (mostly humanities-specific, but this goes for sciences as well). In addressing these points, you argue for your place in the conversation, which tends to make the lit review more compelling than a simple reporting of other sources.
Library FAQs
- Capella FAQs Home
- Capella FAQs
Q. How do I find the Literature Review Summary Table?
- Career Center
- Disability Support
- Doctoral Support
- Learner Records
- Military Support
- Office of Research & Scholarship
- Quantitative Skills Center
- Scholarships & Grants
- Technical Support
- Writing Center
- 8 About the library
- 2 Alumni Library
- 19 Articles
- 8 Bookstore
- 11 Business
- 7 Comprehensive Exams
- 4 Counseling
- 17 Course Readings
- 38 Databases
- 21 Dissertation
- 7 Dissertation Writing
- 5 Education
- 9 Evaluating Sources
- 3 Health Administration
- 7 How do I...
- 2 Human Services
- 2 Information Literacy
- 2 Information Technology
- 7 Interlibrary Loan
- 8 Internet Research
- 13 Journal & Book Locator
- 3 Legal Research
- 14 Library Help
- 3 Literature Reviews
- 2 Methodology
- 1 Non-Library
- 15 Psychology
- 3 Public Health
- 1 Public Safety
- 8 Public Service Leadership
- 14 RefWorks
- 38 Searching
- 1 Social Work
- 17 Technical Issues
Search Library FAQs
Search All FAQs
Answer Last Updated: Jun 28, 2023 Views: 1045
Here are some examples of a literature review summary table: literature review summary table - with examples .
Researchers use literature review summary tables to organize a number of articles in a small space, based on selected criteria.
If you have the need to summarize and organize a lot of literature in a small space, you may want to ask your faculty whether a literature review summary table would be appropriate.
This resource and other tools can also be found in this library guide: Staying Organized & Keeping Track: Research Tools
- Share on Facebook
Was this helpful? Yes 0 No 0
Need Help? Ask a Librarian
SEND US YOUR QUESTION Learner Request Form Faculty & Staff Request Form
MAKE A PHONE APPOINTMENT Schedule a Phone Call with a Librarian
Related Topics
- Dissertation
- Dissertation Writing
The Sheridan Libraries
- Write a Literature Review
- Sheridan Libraries
- Evaluate This link opens in a new window
Get Organized
- Lit Review Prep Use this template to help you evaluate your sources, create article summaries for an annotated bibliography, and a synthesis matrix for your lit review outline.
Synthesize your Information
Synthesize: combine separate elements to form a whole.
Synthesis Matrix
A synthesis matrix helps you record the main points of each source and document how sources relate to each other.
After summarizing and evaluating your sources, arrange them in a matrix or use a citation manager to help you see how they relate to each other and apply to each of your themes or variables.
By arranging your sources by theme or variable, you can see how your sources relate to each other, and can start thinking about how you weave them together to create a narrative.
- Step-by-Step Approach
- Example Matrix from NSCU
- Matrix Template
- << Previous: Summarize
- Next: Integrate >>
- Last Updated: Jul 30, 2024 1:42 PM
- URL: https://guides.library.jhu.edu/lit-review
Be the boss of your literature review
Download this free article summary table template.
When dealing with the literature, summarise the articles you read as you go along. This will ensure that you don't read and forget. Using the Article Summary Table template, you can neatly add a summary of each study to a table. This table is handy because you can easily refer to a specific article without searching through piles of pdfs.
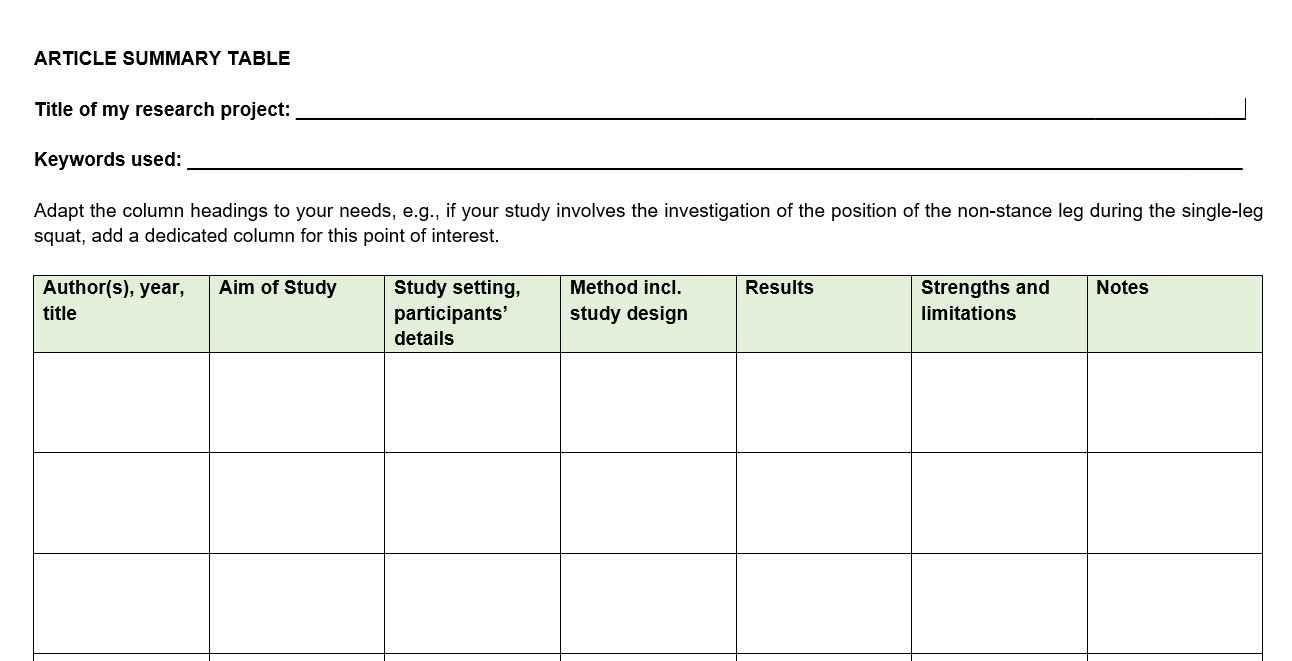
Get the Article Summary Table template in Microsoft Word AND Microsoft Excel - for FREE

IMAGES
VIDEO
COMMENTS
Literature reviews offer a critical synthesis of empirical and theoretical literature to assess the strength of evidence, develop guidelines for practice and policymaking, and identify areas for future research.1 It is often essential and usually the first task in any research endeavour, particularly in masters or doctoral level education. For effective data extraction and rigorous synthesis ...
Literature Review Matrix. As you read and evaluate your literature there are several different ways to organize your research. Courtesy of Dr. Gary Burkholder in the School of Psychology, these sample matrices are one option to help organize your articles. These documents allow you to compile details about your sources, such as the foundational ...
in the summary tables (see figure 1) demonstrates to. the readers that a robust method of data extraction and. synthesis has been followed. Tip 5: create your person alised template for lit ...
Examples of literature reviews. Step 1 - Search for relevant literature. Step 2 - Evaluate and select sources. Step 3 - Identify themes, debates, and gaps. Step 4 - Outline your literature review's structure. Step 5 - Write your literature review.
For example, for a review about the effectiveness of healthcare interven-tions, a literature summary table must include infor-mation about the intervention, its type, content timing, duration, setting, effectiveness, negative consequences, and receivers and implementers' experiences of its usage. Similarly, literature summary tables for articles
Literature Review Table. Literature Review Table Template. Peabody Librarians have created a sample literature review table to use to organize your research. Feel free to download this file and use or adapt as needed. << Previous: Literature Reviews Webinar Recording; Next: Writing Like an Academic >>
This video shows you exactly how to create a summary table for your research articles. It outlines what information should go in the table and provides helpf...
An example of a literature review is shown in Figure 7.1. ... This tool will allow you to create a concise summary of each research paper; see Table 7.1 for an example of an analysis grid. When filling in the grid, the aim is to draw out key aspects of each research paper.
When writing a literature review it is important to start with a brief introduction, followed by the text broken up into subsections and conclude with a summary to bring everything together. A summary table including title, author, publication date and key findings is a useful feature to present in your review (see Table 1 for an example). This ...
Structure. The three elements of a literature review are introduction, body, and conclusion. Introduction. Define the topic of the literature review, including any terminology. Introduce the central theme and organization of the literature review. Summarize the state of research on the topic. Frame the literature review with your research question.
The literature review is not just a summary of the already published works. Your synthesis should show how various articles are linked. Synthesis Matrix or Summary [Table Examples] Some students prefer to place the names of the authors across the top of the chart. Use what works best for you and your instructor or advisor.
Annotated Bibliographies. Annotated bibliographies can help you clearly see and understand the research before diving into organizing and writing your literature review. Although typically part of the "summarize" step of the literature review, annotations should not merely be summaries of each article - instead, they should be critical ...
This is an example of a research table, in which you provide a basic description of the most important features of the studies, articles, and other items you discover in your research.The table identifies each item according to its author/date of publication, its purpose or thesis, what type of work it is (systematic review, clinical trial, etc.), the level of evidence it represents (which ...
A literature review is not an annotated bibliography, organized by title, author, or date of publication. Rather, it is grouped by topic to create a whole view of the literature relevant to your research question. Figure 7.1. Your synthesis must demonstrate a critical analysis of the papers you collected as well as your ability to integrate the ...
Literature Review Example A literature review is a summary of the existing knowledge and research on a particular subject. By identifying gaps in the literature, it provides a foundation for future research. ... The example below is from a review paper, which includes a table comparing the different sources evaluated. Such tables can be useful ...
at each of these in turn.IntroductionThe first part of any literature review is a way of inviting your read. into the topic and orientating them. A good introduction tells the reader what the review is about - its s. pe—and what you are going to cover. It may also specifically tell you.
Some reviews may include more than one 'Summary of findings' table, for example if the review addresses more than one major comparison, or includes substantially different populations that require separate tables (e.g. because the effects differ or it is important to show results separately). ... Figure 14.1.a Example of a 'Summary of ...
See section 2.1 for further sou rces of information on GRADE and SoF tables, and section 2.2 for examples of CCCG reviews that include SoF tables. Creating a SoF: an overview A SoF table presents a summary of the main results of a review, together with an assessment of the quality of the evidence, in a highly standardised format.
Demonstrate your knowledge of the research topic. Identify the gaps in the literature and show how your research links to these. Provide the foundation for your conceptual framework (if you have one) Inform your own methodology and research design. To achieve this, your literature review needs a well-thought-out structure.
Writing a Literature Review. A literature review is a document or section of a document that collects key sources on a topic and discusses those sources in conversation with each other (also called synthesis). The lit review is an important genre in many disciplines, not just literature (i.e., the study of works of literature such as novels and ...
Here are some examples of a Literature Review Summary Table: Literature Review Summary Table - with examples . Researchers use literature review summary tables to organize a number of articles in a small space, based on selected criteria. If you have the need to summarize and organize a lot of literature in a small space, you may want to ask ...
Synthesis Matrix. A synthesis matrix helps you record the main points of each source and document how sources relate to each other. After summarizing and evaluating your sources, arrange them in a matrix or use a citation manager to help you see how they relate to each other and apply to each of your themes or variables. By arranging your ...
Download this FREE Article Summary Table template. When dealing with the literature, summarise the articles you read as you go along. This will ensure that you don't read and forget. Using the Article Summary Table template, you can neatly add a summary of each study to a table. This table is handy because you can easily refer to a specific ...