Ph.D. Program
Graduate studies.
The Genetics Ph.D. program provides opportunities for graduate study in all major areas of modern genetics, including identification and analysis of human disease genes, molecular evolution, gene therapy, statistical genetics, application of model organisms to problems in biology and medicine, and computational and experimental approaches to genome biology.
An underlying theme in our Department is that genetics is not merely a set of tools but a coherent and fruitful way of thinking about biology and medicine. To this end, we emphasize a spectrum of approaches based on molecules, organisms, populations, and genomes.
We provide training through laboratory rotations, dissertation research, seminar series, didactic and interactive coursework, and an annual three-day retreat.
Students receive a competitive stipend ($51,600 for the 2023-24 Academic Year), tuition, health insurance, and a dental care stipend for a full four years. We also encourage students to seek additional fellowships, including but not limited to: NSF GRFP , NIH F31 , NDSEG , Stanford Bio-X fellowship , Stanford DARE , and Stanford CEHG Fellowship .

Lab Rotations
Students rotate through 3 laboratories during their first year in the Genetics Graduate Program. Rotations typically last one quarter each, but can be less and are contingent upon the faculty member agreeing to the rotation request. All Genetics students must rotate with at least 1 Genetics faculty member (primary or secondary appointment). Other rotations may be done with any Bioscience faculty.
While most students start in Fall Quarter, students are encouraged to consider participating in the Advance Summer Institute for a smoother early transition into graduate school. There is a nomination & selection process. The department nominates, so if you are interested please let the department student services officer know. The program is not meant to be a source of summer bridge funding or simply an early rotation opportunity. There are many components to the program that require commitment of time and effort; and the funding, reflects both the expectation of full participation and belief that participants should be compensated for these efforts. The Office of Graduate Education does the selection for ADVANCE. There is no guarantee that if you are nominated that you will be admitted into ADVANCE.
While students may select a thesis laboratory after completing their third rotation, you can do more. Selection of the dissertation research laboratory must be done with the faculty member's approval. Prior to committing to a dissertation laboratory, students are invited to discuss their selection with the Graduate Program Director. Students are welcome to join labs outside of the Genetics Department; if so, they will discuss with the Graduate Program Director whether transferring into that department would be beneficial.
Dissertation Research
Once a student selects a permanent laboratory, they begin their dissertation research that will last for approximately four years. All students are expected to publish at least one first-author paper about their research during this time period, and the work culminates with a thesis defense presentation and written dissertation. See the Genetics Student Handbook for more information.
Coursework, Qualifying Exams, and other Requirements
Students in the Genetics Graduate Program take the Qualifying Examination in the Fall Quarter of their second year of study. There are two parts to the exam, a written research proposal and an oral examination.

Ami Bhatt, Dylan Maghini, and collaborators from the University of the Witwatersrand visit with researchers and staff at the MRC/Wits Public Health and Health Transitions Research Unit in Agincourt, South Africa.

Ami Bhatt, Dylan Maghini, and collaborators tour the MRC/Wits Public Health and Health Transitions Research Unit labs and biobank facility in Agincourt, South Africa.
Service Requirement
Service and outreach are a critical component of a student’s development as a scientist, and offer unique opportunities to learn by interacting with individuals outside the Department. Students are expected to participate in a minimum of 60 hours of service and/or outreach work prior to defending their dissertation.
Supplementary Educational Activities
In addition to your courses, qualifying exams, and dissertation, the Genetics Department has arranged additional educational activities for students. These regularly occurring meetings are:
Current Issues in Genetics (CIG) Two people from the Genetics Department give 20-25 minute presentations about their current work at this weekly Friday meeting. Students in their third year and above are expected to present their work annually. This series gives students the chance to learn about the range of science going on in the department and provides a great opportunity to give formal presentations to peers and colleagues.
Graduate Student Journal Club This weekly journal club is organized completely by graduate students from the Genetics and Developmental Biology Departments. At each meeting, one or two graduate students lead 30 minute discussions on their choice of a recent journal article. For the first three years of the Ph.D. program, each student presents once per academic year.
Refreshments are provided by the graduate students and reimbursed up to the current year limit. Reimbursement requires an original receipt to the Student Services Coordinator.
Frontiers in Biology Every week, the Departments of Genetics, Developmental Biology, and Biochemistry host an external speaker through the “Frontiers in Biology” seminar series. First year students also take a course related to this seminar (GENE 215), where they discuss a relevant paper the day before and meet the speaker after the presentation.
Frontiers is held most Wednesdays at 4pm in Clark Auditorium.
Other Seminar Series There are many other regular seminar series on campus that students choose to attend. Some of the most popular include:
Center of Law and the Biosciences lunchtime talks. See CLB events calendar or subscribe to the listserv
Evolgenome (organized by CEHG). See CEHG website or subscribe to the listserv
Teaching and Mentoring Academy Events. See TMA website or subscribe to the listserv
Discussing Developmental Data (3D). See events schedule
Biomedical Seminars. See events schedule or subscribe to the listserv
Interested in Applying to the Genetics PhD Program?

Attend a Virtual Session
November 4 or 18, 2024 , visit the stanford bioscience booth at sacnas and abrcms, requirements, important forms.
Service Requirement Form
Email Wendy Christiansen
We're sorry but you will need to enable Javascript to access all of the features of this site.
Stanford Online
Genetics and genomics program.
Stanford School of Medicine , Stanford Center for Health Education
Get Started
Thank you for your interest in the Stanford Genetics and Genomics Program!
We are now offering two new programs: Foundations of Genetics and Genomics and Advanced Topics in Genetics and Genomics.
Foundations of Genetics and Genomics
New technologies and breakthroughs in research are impacting the health and medicine industries and allowing for the use of personalized medicine, genetic engineering, and more. But what does this all mean, and how are these innovations occurring? Understanding the core concepts of genes and genomes will help you grasp how researchers and health professionals improve disease diagnosis, prevention, and treatment. From studying the function and structure of chromosomes to examining the genetic codes found in DNA, the Foundations of Genetics and Genomics track will give you the fundamental knowledge needed to understand how we can progress in our work targeting human health and disease and prepare you to explore more advanced topics.
Advanced Topics in Genetics and Genomics
Technologies like CRISPR and stem cell therapies, and research such as those in the fields of epigenetics and biotechnology, are changing how we understand and develop solutions for medicine, biology, and agriculture. The fields of genetics and genomics are constantly evolving from personalized treatment plans based on your genes, lifestyle, and environment to manipulating DNA and editing genetic code. The Advanced Topics in Genetics and Genomics track allows you to dive deeper into the topics you care about and provides you with up-to-date information on cutting-edge research and technologies in the health and medicine industries today.
- Preview Image
- Course/Course #
- Time Commitment
- Availability

Fundamentals of Genetics: The Genetics You Need To Know

Genomics and the Other Omics: The Comprehensive Essentials
Advanced topics in genetics and genomics.

Principles and Practices of Gene Therapy

Understanding Cancer at the Genetic Level

Genetic Engineering and Biotechnology

Stem Cell Therapeutics

Personal Genomics and Your Health

New Frontiers in Cancer Genomics

Epigenetics and Microbiomics in Precision Health

Biology and Applications of the CRISPR/Cas System
Teaching team.

Russ Altman
Kenneth Fong Professor
Bioengineering
Russ Biagio Altman is the Kenneth Fong Professor of Bioengineering, Genetics, Medicine, Biomedical Data Science and (by courtesy) Computer Science) and past chairman of the Bioengineering Department at Stanford University. His primary research interests are in the application of computing and informatics technologies to problems relevant to medicine. He is particularly interested in methods for understanding drug action at molecular, cellular, organism and population levels. His lab studies how human genetic variation impacts drug response (e.g., http://www.pharmgkb.org/). Other work focuses on the analysis of biological molecules to understand the actions, interactions and adverse events of drugs (e.g., http://feature.stanford.edu/). He helps lead an FDA-supported Center of Excellence in Regulatory Science & Innovation.
Dr. Altman holds an AB from Harvard College, and an MD from Stanford Medical School, and a PhD in Medical Information Sciences from Stanford. He received the U.S. Presidential Early Career Award for Scientists and Engineers and a National Science Foundation CAREER Award. He is a fellow of the American College of Physicians (ACP), the American College of Medical Informatics (ACMI), the American Institute of Medical and Biological Engineering (AIMBE), and the American Association for the Advancement of Science (AAAS). He is a member of the National Academy of Medicine (formerly the Institute of Medicine, IOM). He is a past-president, founding board member, and a fellow of the International Society for Computational Biology (ISCB), and a past-president of the American Society for Clinical Pharmacology & Therapeutics (ASCPT). He has chaired the Science Board advising the FDA commissioner, served on the NIH Director’s Advisory Committee, and co-chaired the IOM Drug Forum. He is an organizer of the annual Pacific Symposium on Biocomputing, and a founder of Personalis, Inc. Dr. Altman is board certified in Internal Medicine and in Clinical Informatics. He received the Stanford Medical School graduate teaching award in 2000 and mentorship award in 2014.

Ximena Ares
Licensing Associate
Stanford University
Ximena Ares is a Licensing Associate at the Stanford Office of Technology Licensing (OTL). Dr. Ares received her Ph.D training in Molecular Biology in Buenos Aires, Argentina and completed her postdoctoral training at the University of California, San Francisco in Human Genetics. Later, she was a scientist at Geron Corporation and a Research Fellow at the Molecular Sciences Institute in Berkeley, California. She joined Stanford OTL in 2004, where she manages a portfolio of about 250 life sciences inventions, makes decisions about their intellectual property protection and negotiates license agreements and other contracts.

Euan Ashley
Roger and Joelle Burnell Professor
- School of Medicine
Born and raised in Scotland, Euan Angus Ashley graduated with 1st class Honors in Physiology and Medicine from the University of Glasgow. He completed medical residency and a PhD in molecular cardiology at the University of Oxford before moving to Stanford University where he trained in cardiology and advanced heart failure joining the faculty in 2006. His group is focused on the application of genomics to medicine. In 2010, he led the team that carried out the first clinical interpretation of a human genome. The paper published in the Lancet was the focus of over 300 news stories, became one of the most cited articles in clinical medicine that year, and was featured in the Genome Exhibition at the Smithsonian in DC. The team extended the approach in 2011 to a family of four and now routinely apply genome sequencing to the diagnosis of patients at Stanford hospital where Dr Ashley directs the Clinical Genome Service and the Center for Inherited Cardiovascular Disease. In 2013, Dr Ashley was recognized by the White House Office of Science and Technology Policy for his contributions to Personalized Medicine. In 2014, Dr Ashley became co-chair of the steering committee for the NIH Undiagnosed Diseases Network. Dr Ashley is a recipient of the National Innovation Award from the American Heart Association (AHA) and a National Institutes of Health (NIH) Director’s New Innovator Award. He is a member of the AHA Council on Functional Genomics, and the Institute of Medicine (IOM) of the National Academy of Sciences Roundtable on Translating Genomic-Based Research for Health. He is a peer reviewer for the NIH and the AHA as well as journals including Nature, the New England Journal of Medicine, the Lancet and the Journal of Clinical Investigation. He is co-founder of Personalis Inc, a genome scale genetic diagnostics company. Father to three young Americans, in his ‘spare’ time, he tries to understand American football, plays the saxophone, and conducts research on the health benefits of single malt Scotch whisky.

Laura Attardi
Catharine and Howard Avery Professor
Academic Appointments
- Professor, Radiation Oncology - Radiation and Cancer Biology
- Professor, Genetics
- Member, Bio-X
- Member, Child Health Research Institute
- Member, Stanford Cancer Institute

Israeli Society of Gene and Cell Therapy
Administrative Appointments
Founder of LogicBio Therapeutics, a gene therapy company (2014) Member of the American Society of Gene and Cell therapy (2011)
Honors & Awards
Presidential symposium lecturer at the annual meeting of the American Society for Gene and Cell Therapy (ASGCT) (2014) Recipient of the Child Health Research Institute (CHRI) fellowship (2013) 1st place- Stanford Genetics Department “Big Idea” Contest (2012)
Professional Education
MSc, Tel Aviv University, Tel Aviv, Israel, Genetics (2006) PhD, Tel Aviv University, Tel Aviv, Israel, Genetics (2011) Postdoctoral fellow, Stanford University (2011)

Michael Bassik
Associate Professor, Genetics

Chris Bjornson
Senior Scientific Researcher
Chris Bjornson holds a Ph.D. from the University of Washington and has served as a Research Associate for Calos Lab, Stanford University.

Anne Brunet
Michele And Timothy Barakett Endowed Professor
Anne Brunet is a Professor of Genetics at Stanford University. Dr. Brunet is interested in the molecular mechanisms of aging and longevity, with a particular emphasis on the nervous system. Her lab is interested in identifying pathways involved in delaying aging in response to external stimuli such as availability of nutrients and mates. She also seeks to understand the mechanisms that influence the rejuvenation of old stem cells. Finally, her lab has pioneered the naturally short-lived African killifish as a new model to explore the regulation of aging and age-related diseases.

Senior Scientist, Population Genetics
Katarzyna ("Kasia") Bryc is a Senior Scientist of Population Geneticist at 23andMe. Dr. Bryc has developed statistical models that leverage genetic data to learn about ancient human history and migrations, recent population admixture and other forces shaping the human genome. Her prior research illuminated the genetic population structure of Africans, and the complex admixture of African Americans and Hispanic/Latino populations. Dr. Bryc received a B.A. from Stanford University, and her M.S. and Ph.D. in Biometry at Cornell under Dr. Carlos Bustamante. Prior to joining 23andMe, she was a NIH Ruth L. Kirschstein National Research Fellow at Harvard Medical School with Dr. David Reich, where she developed statistical methods to infer genetic diversity from sequence data.

Michele Calos
Professor, Genetics (Emerita)
Professor, Genetics
Member, Bio-X
Member, Child Health Research Institute
Chair, School of Medicine Appointments and Promotions Committee (2008 - 2010)
- Searle Scholar Award, Searle Family Foundation (1986)
- Graduate Fellowship, National Science Foundation (1979)
- B.A., M.A., Oxford University, Zoology
- Ph.D., Harvard University, Biochemistry & Molecular Biology
- Postdoc., University of Geneva, Biologie Moleculaire
Community and International Work
- Member, Board of Directors, American Society of Gene and Cell Therapy
- Advisory Committee, United States Food and Drug Administration, Bethesda, Maryland

Jan Carette
Associate Professor, Microbiology and Immunology

Mildred Cho
Professor, Pediatrics and Medicine

Emily Crane
Senior Principle Scientific Researcher
Dr. Emily Crane grew up in Palo Alto, California. She left the sunshine state to earn her B.A. in Biology from Carleton College in Northfield, Minnesota. She returned to California in 2005, where she enrolled in graduate school at UC Berkeley and began training as a geneticist with Dr. Barbara Meyer. She studied the connection between gene expression regulation and chromosome structure, earning a Ph.D. in Molecular and Cell Biology in 2011. While pursuing her doctorate she was able to first pair research with teaching as a Graduate Student Instructor for both lab and lecture courses. She is currently a NIH IRACDA postdoctoral fellow at Stanford University, which allows her to do research while also teaching as a visiting professor at San Jose State University. At Stanford she works in Dr. Jin Li’s lab, where she is currently setting up a screening system to look for regulators of RNA editing. Dysregulation of RNA editing has been linked to neurological diseases and cancers, and its complete loss is lethal. Emily is passionate about the rapidly expanding field of personal genomics, which will soon be an indispensable resource for improving patient health.

Christina Curtis
Professor, Genetics and Biomedical Data Science
The Curtis laboratory couples innovative experimental approaches, high-throughput omic technologies, statistical inference and computational modeling to interrogate the evolutionary dynamics of tumor progression and therapeutic resistance. To this end, Dr. Curtis and her team have developed an integrated experimental and computational framework to measure clinically relevant patient-specific parameters and to measure clonal dynamics. Her research also aims to develop a systematic interpretation of genotype/phenotype associations in cancer by leveraging state-of-the-art technologies and robust data integration techniques. For example, using integrative statistical approaches to mine multiple data types she lead a seminal study that redefined the molecular map of breast cancer, revealing novel subgroups with distinct clinical outcomes and subtype-specific drivers.

Barbara Dunn
Final Foods Inc.
Barbara Dunn is a Senior Biocuration Research Scientist in the Department of Genetics at Stanford University, currently working with the Saccharomyces Genome Database in the laboratory of Dr. J. Michael Cherry. She received her A.B. in Botany at Berkeley, and her Ph.D. in Biological Chemistry at Harvard University, where she studied yeast telomeres in the laboratory of Dr. Jack Szostak. Her recent research has focused on using whole-genome DNA and RNA sequencing, ChIP-Seq, array-CGH, and other “omics” methods to broadly explore evolution in yeast, and particularly the genome structures and genome evolution of industrial yeasts (lager, ale, wine, ethanol, bread).

Dianna Fisk
Senior Scientific Curator
Dianna received her B.S. in Biology from Marquette University and her Ph.D. in Molecular Biology, Cell Biology and Genetics from the University of Oregon, where she studied how nuclear and chromosomal gene expression are coordinately regulated, in the laboratory of Dr. Alice Barkan. She then went on to work as a Scientific Curator under Dr. David Botstein and Dr. J. Michael Cherry, at the Saccharomyces Genome Database (SGD). After 13 years of analyzing, assembling and organizing the vast amounts of detailed biochemical and genetic data available on yeast, she switched to interpretation of human genomics data and is now the Senior Biocurator at the Stanford Clinical Genomics Service.

Professor, Medicine and Genetics
Dr. Ford is a medical oncologist and geneticist at Stanford, devoted to studying the genetic basis of breast and GI cancer development, treatment and prevention. Dr. Ford graduated in 1984 Magna Cum Laude (Biology) from Yale University where he later received his M.D. degree from the School of Medicine in 1989. He was a internal medicine resident (1989-91), Clinical Fellow in Medical Oncology (1991-94), Research Fellow of Biological Sciences (1993-97) at Stanford, and joined the faculty in 1998. He is currently Associate Professor of Medicine (Oncology) and Genetics, and Director of the Stanford Cancer Genetics Clinic, at the Stanford University Medical Center. Dr. Ford’s research goals are to understand the role of genetic changes in cancer genes in the risk and development of common cancers. He studies the role of the p53 and BRCA1 tumor suppressor genes in DNA repair, and uses techniques for high-throughput genomic analyses of cancer to identify molecular signatures for targeted therapies. Recently, his team has identified a novel class of drugs that target DNA repair defective breast cancers, and have opened clinical trials at Stanford and nationally using these “PARP inhibitors” for the treatment of women with “triple-negative” breast cancer. Dr. Ford’s clinical interests include the diagnosis and treatment of patients with a hereditary pre-disposition to cancer. He runs the Stanford Cancer Genetics Clinic, that sees patients for genetic counseling and testing of hereditary cancer syndromes, and enters patients on clinical research protocols for prevention and early diagnosis of cancer in high-risk individuals.

Hinco Gierman
VP Precision Oncology

Julie Granka
Principal Scientist, Statistical Genetics
Julie Granka is a biologist and a statistician with expertise in genetics and evolution who currently serves as the Director of Personalized Genomics at Ancestry.com. Dr. Granka has experience developing and applying advanced computational tools to genetic data to understand population history and evolution. During fieldwork in South Africa, she collected and analyzed DNA samples from an African hunter-gatherer population to uncover the genetic basis of human height and skin pigmentation. Dr. Granka has also analyzed numerous other African populations to identify regions of the human genome where positive natural selection has occurred in recent history. In addition, she has studied the genetics of other organisms, including M. tuberculosis, the organism that causes tuberculosis. Dr. Granka received a B.S. in Biometry and Statistics from Cornell University where she worked with Dr. Carlos Bustamante. Afterwards, she received an M.S. in Statistics and a Ph.D. in Biology with Dr. Marcus Feldman at Stanford University.

Hank Greely
Deane F. and Kate Edelman Johnson Professor of Law
- Stanford Law School
Henry T. "Hank" Greely is the Deane F. and Kate Edelman Johnson Professor of Law and Professor, by courtesy, of Genetics at Stanford University. He specializes in ethical, legal, and social issues arising from advances in the biosciences, particularly from genetics, neuroscience, and human stem cell research. He chairs the California Advisory Committee on Human Stem Cell Research and the steering committee of the Stanford University Center for Biomedical Ethics, and directs the Stanford Center for Law and the Biosciences and the Stanford Program in Neuroscience and Society. He serves as a member of the NAS Committee on Science, Technology, and Law; the NIGMS Advisory Council, the Institute of Medicine’s Neuroscience Forum, and the NIH Multi-Center Working Group on the BRAIN Initiative. Professor Greely graduated from Stanford in 1974 and from Yale Law School in 1977. He served as a law clerk for Judge John Minor Wisdom on the United States Court of Appeals for the Fifth Circuit and for Justice Potter Stewart of the United States Supreme Court. He began teaching at Stanford in 1985.

Will Greenleaf
William Greenleaf is an Associate Professor in the Genetics Department at Stanford University School of Medicine, with a courtesy appointment in the Applied Physics Department. He is a member of Bio-X, the Biophysics Program, the Biomedical Informatics Program, and the Cancer Center. He received an A.B. in physics from Harvard University (summa cum laude) in 2002, and received a Gates Fellowship to study computer science for one year in Trinity College, Cambridge, UK (with distinction). After this experience abroad, he returned to Stanford to carry out his Ph.D. in Applied Physics in the laboratory of Steven Block, where he investigated, at the single molecule level, the chemo-mechanics of RNA polymerase and the folding of RNA transcripts. He conducted postdoctoral work in the laboratory of X. Sunney Xie in the Chemistry and Chemical Biology Department at Harvard University, where he was awarded a Damon Runyon Cancer Research Foundation Fellowship, and developed new fluorescence-based high-throughput sequencing methodologies. He moved to Stanford as an Assistant Professor in November 2011. Since beginning his lab, he has been named a Rita Allen Foundation Young Scholar, an Ellison Foundation Young Scholar in Aging (declined), a Baxter Foundation Scholar, and a Chan-Zuckerberg Investigator. His highly interdisciplinary research links molecular biology, computer science, bioengineering, and genomics a to understand how the physical state of the human genome controls gene regulation and biological state. Efforts in his lab are split between building new tools to leverage the power of high-throughput sequencing and cutting-edge microscopies, and bringing these new technologies to bear against basic biological questions of genomic and epigenomic variation. His long-term goal is to unlock an understanding of the physical “regulome” — i.e. the factors that control how the genetic information is read into biological instructions — profoundly impacting our understanding of how cells maintain, or fail to maintain, their state in health and disease.

Arthur Grossman
Professor (by courtesy), Biology
Arthur Grossman has been a Staff Scientist at The Carnegie Institution for Science, Department of Plant Biology since 1982, and holds a courtesy appointment as Professor in the Department of Biology at Stanford University. He has performed research across fields ranging from plant biology, microbiology, marine biology, ecology, genomics, engineering and photosynthesis and initiated large scale algal genomics by leading the Chlamydomonas genome project (sequencing of the genome coupled to transcriptomics). During his tenure at Carnegie, he mentored more than fifteen PhD students and approximately 40 post-doctoral fellows (many of whom have become very successful independent scientists at both major universities and in industry). In 2002 he received the Darbaker Prize (Botanical Society of America) for work on microalgae and in 2009 received the Gilbert Morgan Smith Medal (National Academy of Sciences) for the quality of his publications on marine and freshwater algae. In 2015 he was Vice Chair of the Gordon Research Conference on Photosynthesis and in 2017 was Chair of that same conference (Photosynthetic plasticity: From the environment to synthetic systems). He also gave the Arnon endowed lecture on photosynthesis in Berkeley in March of 2017, has given numerous plenary lectures and received a number of fellowships throughout his career, including the Visiting Scientist Fellowship - Department of Life and Environmental Sciences (DiSVA), Università Politecnica delle Marche (UNIVPM) (Italy, 2014), the Lady Davis Fellowship (Israel, 2011) and most recently the Chaire Edmond de Rothschild (to work IBPC in Paris in 2017-2018). He has been Co-Editor in Chief of Journal of Phycology and has served on the editorial boards of many well-respected biological journals including the Annual Review of Genetics, Plant Physiology, Eukaryotic Cell, Journal of Biological Chemistry, Molecular Plant, and Current Genetics. He has also reviewed innumerable papers and grants, served on many scientific panels that has evaluated various programs for granting agencies [NSF, CNRS, Marden program (New Zealand)] and private companies. He has also served on scientific advisory boards for both nonprofit and for profit companies including Phoenix Bioinformatics, Excelixis, Martex, Solazyme/TerraVia, Checkerspot and Phycoil.

Bethann Hromatka
Senior Director, Medical Affairs
Puma Biotechnology, Inc.

Natalie Jaeger
Senior Scientist
DKFZ German Cancer Research Center
Natalie is a Post-Doctoral Scientist in the laboratory of Professor Michael Snyder at Stanford University. Her duties include applying approaches comprising genome sequencing, transcriptomics, and proteomics to the analysis of human disease, to help understand the molecular basis of disease and aid the development of diagnostics and therapeutics.

Dennis Farrey Family Professor of Genetics
Mark A. Kay, MD, PhD, is the Director of the Program in Human Gene Therapy, and Professor in the Department of Pediatrics and Genetics at Stanford University School of Medicine. Dr. Kay is one of the founders of the American Society of Gene Therapy and served as its President in 2005-2006. Dr. Kay received the E. Mead Johnson Award for Research in Pediatrics in 2000 and was elected to the American Society for Clinical Investigation in 1997. He has organized many national and international conferences, including the first Gordon Conference related to gene therapy.
Kay is respected worldwide for his work in gene therapy for hemophilia and viral hepatitis. He is an Associate Editor of Human Gene Therapy and Molecular Therapy, and a member of the editorial boards of other peer-reviewed publications.
Here at Stanford University, Dr. Kay is involved in many committees, including the Administrative Panel on Biosafety Committee, and Chair of the Berry Foundation Committee. Along with his work in Gene Therapy Dr. Kay is an avid photographer and enjoys spending time outdoors photographing wildlife.

Professor, Developmental Biology (Emeritus)
Dr. Kim's lab's research focuses are in C. elegans aging, human aging, cell lineage analyzer, and ModENCODE.
Students, fellows, and faculty in the Department of Developmental Biology are working at the forefront of basic science research to understand the molecular mechanisms that generate and maintain diverse cell types in many different contexts, including the embryo, various adult organs, and the evolution of different species. Research groups use a wide array of cutting-edge approaches including genetics, genomics, computation, biochemistry, and advanced imaging, in organisms ranging from microbes to humans. This work has connections to many areas of human health and disease, including stem cell biology, aging, cancer, diabetes, arthritis, infectious disease, autoimmune disease, neurological disorders, and novel strategies for stimulating repair or regeneration of body tissues.

Jane Lebkowski
Regenerative Patch Technologies
President of Research and Development, Asterias

Joe Lipsick
Professor, Pathology and Genetics
Since participating in the initial identification of the protein product of the v-Myb oncogene as a postdoctoral fellow, Dr. Lipsick has dedicated his research career to understanding the function of the highly conserved Myb oncogene family. The laboratory has initially focused on the retroviral v-Myb oncogene and its cellular homologue, c-Myb. More recently, they have focused on the fruit fly Drosophila melanogaster as a model organism for understanding the human Myb oncogene family. They created the first null mutants of the sole Drosophila Myb gene, and showed that the absence of Myb resulted in mitotic abnormalities including chromosome condensation defects, aneuploidy, polyploidy, and aberrant spindle formation. In collaboration with the laboratory of Michael Botchan (UC Berkeley), they also showed that Myb was required for the site-specific initiation of DNA replication that occurs during chorion gene amplification in adult ovarian follicle cells. They themselves then showed that the absence of Myb causes a failure in the normal progression of chromosome condensation from heterchromatin to euchromatin. Most recently, they have found that Myb acts in opposition to repressive E2F and RB proteins to epigenetically regulate the expression of key components of the spindle assembly checkpoint and spindle pole regulatory pathways.

Kelly Ormond
Adjunct Professor, Genetics
Kelly Ormond is a genetic counselor (US ABGC certified) and ELSI researcher. She received her MS in Genetic Counseling from Northwestern University (1994) and a post-?graduate certificate in Clinical Medical Ethics from the MacLean Center at the University of Chicago (2001). She joined the Health Ethics and Policy Lab as a Senior Scientist in February 2021, and is an Adjunct Professor in the Department of Genetics at Stanford School of Medicine, Stanford University, California, USA

Matthew Porteus
Sutardja Chuk Professor
Dr. Porteus was raised in California and was a local graduate of Gunn High School before completing A.B. degree in “History and Science” at Harvard University where he graduated Magna Cum Laude and wrote an thesis entitled “Safe or Dangerous Chimeras: The recombinant DNA controversy as a conflict between differing socially constructed interpretations of recombinant DNA technology.” He then returned to the area and completed his combined MD, PhD at Stanford Medical School with his PhD focused on understanding the molecular basis of mammalian forebrain development with his PhD thesis entitled “Isolation and Characterization of TES-1/DLX-2: A Novel Homeobox Gene Expressed During Mammalian Forebrain Development.” After completion of his dual degree program, he was an intern and resident in Pediatrics at Boston Children’s Hospital and then completed his Pediatric Hematology/Oncology fellowship in the combined Boston Chidlren’s Hospital/Dana Farber Cancer Institute program. For his fellowship and post-doctoral research he worked with Dr. David Baltimore at MIT and CalTech where he began his studies in developing homologous recombination as a strategy to correct disease causing mutations in stem cells as definitive and curative therapy for children with genetic diseases of the blood, particularly sickle cell disease. Following his training with Dr. Baltimore, he took an independent faculty position at UT Southwestern in the Departments of Pediatrics and Biochemistry before again returning to Stanford in 2010 as an Associate Professor. During this time his work has been the first to demonstrate that gene correction could be achieved in human cells at frequencies that were high enough to potentially cure patients and is considered one of the pioneers and founders of the field of genome editing—a field that now encompasses thousands of labs and several new companies throughout the world. His research program continues to focus on developing genome editing by homologous recombination as curative therapy for children with genetic diseases but also has interests in the clonal dynamics of heterogeneous populations and the use of genome editing to better understand diseases that affect children including infant leukemias and genetic diseases that affect the muscle. Clinically, Dr. Porteus attends at the Lucille Packard Children’s Hospital where he takes care of pediatric patients undergoing hematopoietic stem cell transplantation.

Vice President of Program Management
Jose loves talking about science, especially to non-scientists. He has been involved in science outreach and education since he first learned of the simplicity and beauty of the structure of DNA. Naturally, Jose went on to graduate school at Stanford where he received a M.A. in Education and a Ph.D. in Developmental Biology. His doctoral work focused on understanding how epigenetic regulators control the biology of adult stem cells. For example, when some of these regulators misbehave, stem cells are lost to the detriment of the tissue they normally maintain. Why? How? Well, Jose still doesn’t know, but he hopes his work helped add one more piece to the never-ending puzzle of scientific research. After finishing his Ph.D., Jose moved to St. Louis, MO and joined Monsanto as part of a rotational leadership program, where he’s been doing a number of fun things both close and far from his science background. His year-long rotations have spanned biotechnology regulation and policy, global technology strategy, and development of molecular detection technologies. All of these rotations have complemented each other and contributed to his passion for sustainably and safely increasing food productivity and agricultural efficiency. Jose’s favorite activity is backpacking and talking about how light his backpack is over an open fire under the Milky Way-splattered sky of the Sierra Nevada. When he’s not outdoors, which is more frequent than he’d like, Jose enjoys good beer (peanut butter chocolate milk stout is real and delicious), good music (Tool), and thoughtful discussions involving science, education and politics.

Jonathan Pritchard
Bing Professor of Population Studies
Jonathan Pritchard is a Professor of Genetics and Biology at Stanford University. He received his BSc in Biology and Mathematics from Penn State University in 1994, and his PhD in Biology at Stanford in 1998. After that he moved to a postdoc in the Department of Statistics at Oxford University and then to his first faculty job at the University of Chicago in 2001. He has been an Investigator of the Howard Hughes Medical Institute since 2008.

Li (Stanley) Qi
Associate Professor

Maria Grazia Roncarolo
George D. Smith Professor
Maria Grazia Roncarolo, MD is the co-director of the Institute for Stem Cell Biology and Regenerative Medicine, the George D. Smith Professor in Stem Cell and Regenerative Medicine, Professor of Pediatrics and of Medicine (blood and marrow transplantation), chief of the Division of Pediatric Stem Cell Transplantation and Regenerative Medicine, and co-director of the Bass Center for Childhood Cancer and Blood Diseases.
Dr. Roncarolo leads efforts to translate scientific discoveries in genetic diseases and regenerative medicine into novel patient therapies, including treatments based on stem cells and gene therapy. A pediatric immunologist by training, she earned her medical degree at the University of Turin, Italy. She spent her early career in Lyon, France, where she focused on severe inherited metabolic and immune diseases, including severe combined immunodeficiency (SCID), better known as the "bubble boy disease." Dr. Roncarolo was a key member of the team that carried out the first stem cell transplants given before birth to treat these genetic diseases.
While studying inherited immune diseases, Dr. Roncarolo discovered a new class of T cells. These cells, called T regulatory type 1 cells, help maintain immune system homeostasis by preventing autoimmune diseases and assisting the immune system in tolerating transplanted cells and organs. Recently, Dr. Roncarolo completed the first clinical trial using T regulatory type 1 cells to prevent severe graft-versus-host disease in leukemia patients receiving blood-forming stem-cell transplants from donors who were not genetic matches.
Dr. Roncarolo worked for several years at DNAX Research Institute for Molecular and Cellular Biology in Palo Alto, where she contributed to the discovery of novel cytokines, cell-signaling molecules that are part of the immune response. She studied the role of cytokines in inducing immunological tolerance and in promoting stem cell growth and differentiation.
Dr. Roncarolo developed new gene-therapy approaches, which she pursued as director of the Telethon Institute for Cell and Gene Therapy at the San Raffaele Scientific Institute in Milan. She was the principal investigator leading the successful gene therapy trial for SCID patients who lack an enzyme critical to DNA synthesis, which is a severe life-threatening disorder. That trial is now considered the gold standard for gene therapy in inherited immune diseases. Under her direction, the San Raffaele Scientific Institute has been seminal in showing the efficacy of gene therapy for otherwise untreatable inherited metabolic diseases and primary immunodeficiencies.
Dr. Roncarolo's goal at Stanford is to build the teams and infrastructure to move stem cell and gene therapy to the clinic quickly and to translate basic science discoveries into patient treatments. In addition, her laboratory continues to work on T regulatory cell-based treatments to induce immunological tolerance after transplantation of donor tissue stem cells. In Nature Medicine, Dr. Roncarolo recently published her discovery of new biomarkers for T regulatory type 1 cells, which will be used to purify the cells and to track them in patients. She also is investigating genetic chronic inflammatory and autoimmune diseases that occur due to impairment in T regulatory cell functions.

Julien Sage
Elaine and John Chambers Professor
Dr. Sage studied biology at the École Normale Supérieure in Paris and did his PhD at the University of Nice and post-doctoral training at MIT. He is currently the Elaine and John Chambers Professor in Pediatric Cancer and a Professor of Genetics at Stanford University where he serves as the co-Director of the Cancer Biology PhD program. For his work on cancer genetics, he has been awarded a Damon Runyon Cancer Research Foundation Scholar Award, a Leukemia and Lymphoma Society Scholar Award, and an R35 Outstanding Investigator Award from the National Cancer Institute. Dr. Sage’s work has focused on the RB tumor suppressor pathway and how inactivation of RB promotes tumorigenesis in children and adult patients. In the past few years, the Sage lab has developed pre-clinical models for small cell lung cancer, an RB-mutant cancer, and has used these models to investigate signaling pathways driving the growth of this cancer type and to identify novel therapeutic targets in this recalcitrant cancer.

Gavin Sherlock
Associate Professor, Genetics Member, Stanford Cancer Institute
Army Breast Cancer Research Fellowship, Department of Defence (1997-1998) Cold Spring Harbor Fellowship, Cold Spring Harbor Laboratory (1996-1997) Prize Studentship, The Wellcome Trust (1991-1994) John Buckley Entrance Scholarship for Science, Manchester University (1988-1991)
B.Sc., Manchester University, Genetics (1991) Ph.D., Manchester University, Molecular Biology (1994)

Michael Snyder
Stanford W. Ascherman Professor of Genetics
Michael Snyder is the Stanford Ascherman Professor and Chair of Genetics and the Director of the Center of Genomics and Personalized Medicine. Dr. Snyder received his Ph.D. training at the California Institute of Technology and carried out postdoctoral training at Stanford University.
He is a leader in the field of functional genomics and proteomics, and one of the major participants of the ENCODE project. His laboratory study was the first to perform a large-scale functional genomics project in any organism, and has launched many technologies in genomics and proteomics. These including the development of proteome chips, high resolution tiling arrays for the entire human genome, methods for global mapping of transcription factor binding sites (ChIP-chip now replaced by ChIP-seq), paired end sequencing for mapping of structural variation in eukaryotes, de novo genome sequencing of genomes using high throughput technologies and RNA-Seq. These technologies have been used for characterizing genomes, proteomes and regulatory networks. Seminal findings from the Snyder laboratory include the discovery that much more of the human genome is transcribed and contains regulatory information than was previously appreciated, and a high diversity of transcription factor binding occurs both between and within species.
He has also combined different state-of–the-art “omics” technologies to perform the first longitudinal detailed integrative personal omics profile (iPOP) of person and used this to assess disease risk and monitor disease states for personalized medicine. He is a cofounder of several biotechnology companies, including Protometrix (now part of Life Tehcnologies), Affomix (now part of Illumina), Excelix, and Personalis, and he presently serves on the board of a number of companies

Barry Starr
Senior Science Writer
Barry received his B.S. from CSU, Chico in Biochemistry. He then went on to graduate school at the University of Oregon where he earned his Ph.D. in biochemistry with Dr. Diane Hawley. During his six years, Barry worked on many aspects of basal RNA polymerase II transcription but Barry’s main contribution to the field was showing that the TATA-binding protein (TBP) recognized its AT-rich sequence entirely through the minor groove. This was deemed impossible at the time. Barry then went on to do a postdoc with Dr. Keith Yamamoto at UCSF where he worked on glucocorticoid receptor mutants. After that Barry entered the world of biotechnology where he was employed at three different companies designing small molecules that could specifically alter gene expression. He then stepped off the standard science track and took a job with Stanford University’s Department of Genetics running an outreach program called Stanford at The Tech. Over the next ten or so years Barry helped design and update a museum exhibition (Genetics: Technology With a Twist), a website (Understanding Genetics), have given over 100 graduate students and postdoctoral fellows the opportunity to improve their communication skills, and have written hundreds of blogs both for the Understanding Genetics website and for KQED QUEST, a local PBS television show.

Lars Steinmetz
Dieter Schwarz Foundation Endowed Professor
Lars Steinmetz studied molecular biophysics and biochemistry at Yale University and conducted his Ph.D. research on genome-wide approaches to study gene function and natural phenotypic diversity at Stanford University. After a brief period of postdoctoral research at the Stanford Genome Technology Center, where he worked on functional genomic technology development, he moved to Europe in 2003. At the European Molecular Biology Laboratory (EMBL) in Heidelberg, Germany, he started his own group, focused on applying functional genomic approaches and high-throughput methods to study complex traits, transcription and the mitochondrial organelle at a systems level. In parallel, he maintained a focused group at the Stanford Genome Technology Center working on technology development. Since 2009, Lars acted as Joint Head of the department of Genome Biology at EMBL.
In October 2013 Lars became Professor of Genetics at Stanford University and Co-Director of the Stanford Genome Technology Center. His lab develops and applies cutting-edge technologies to investigate the function and mechanism of transcription, the genetic basis of complex phenotypes and the genetic and molecular systems underpinning disease. Their ultimate goal is to enable the development of personalized, preventative medicine.
In parallel to his research activities at Stanford, Lars continues to lead his lab at EMBL and acts as Associate Head of Genome Biology and Senior Scientist at EMBL. His Stanford and EMBL labs collaborate very closely.
In addition to his academic endeavours, Lars is a consultant and board member of several companies, advising in the areas of genetics and personalized medicine.

Ruth Tennen
Senior Product Scientist I
Ruth Tennen picked up her first pipette as a summer high-school student in a lab at the University of Connecticut Health Center. She received her bachelor’s degree in molecular biology from Princeton University and her Ph.D. in cancer biology from Stanford University. Her graduate work examined the intersection between epigenetics and disease: how human cells squeeze two meters of DNA into their nuclei while keeping that DNA accessible and dynamic, and how DNA packaging goes awry during cancer and aging. As a graduate student, Ruth shared her love of science by teaching hands-on classes to students at local schools, hospitals, and museums and by blogging on the San Jose Tech Museum’s website.
After completing her Ph.D., Ruth moved to Washington, DC to serve as an AAAS Science & Technology Policy Fellow. Working in the Bureau of African Affairs at the U.S. Department of State, she collaborated with colleagues in DC and at U.S. Embassies abroad to promote scientific capacity building, science education, and entrepreneurship in sub-Saharan Africa. She managed the Apps4Africa program, which challenges young African innovators to develop mobile apps that tackle problems in their communities. She also traveled to South Africa and Ghana, where she delivered lectures and workshops designed to spark the scientific excitement of young learners.
Ruth is currently a Product Scientist at 23andMe. In her free time, Ruth enjoys running, reading, quoting Seinfeld, and cheering for the UConn Huskies.

Sören Turan
Bayer Pharmaceuticals
Postdoc, Genetics
DFG Fellowship (2013)
- Diploma TU-Braunschweig (Germany) 2007
- Dr. rer. nat. Medical School Hannover (Germany)
Research Interest: Gene Therapy, (Stem) Cell Therapy, Genome Engineering, CRISPR/Cas9 gene editing

Monte Winslow
Associate Professor of Genetics and of Pathology
Monte Winslow is an Associate Professor of Genetics and Pathology at Stanford University.

Stacey Wirt Taylor
Commercial Planning Manager
Adaptive Biotechnologies Corp.
Stacey received her B.A. in Biology from Wellesley College and her Ph.D. in Cancer Biology from Stanford University. Her dissertation focused on uncovering new mechanisms for cell cycle control in mouse embryonic stem cells and neural progenitors. She went on to complete a post-doctoral fellowship in genome engineering, where she worked to develop nuclease technology for editing disease-causing mutations in human stem cells. In her spare time, Stacey volunteers at the San Jose Tech Museum, likes to camp and hike throughout Northern California, and is an avid photographer.

Simon H. Stertzer, MD, Professor
Joseph C. Wu, MD, PhD is Director of the Stanford Cardiovascular Institute and Professor in the Department of Medicine (Cardiology) and Department of Radiology (Molecular Imaging Program) at the Stanford University School of Medicine. Dr. Wu received his medical degree from Yale. He completed his medicine internship, residency and cardiology fellowship training at UCLA followed by a PhD (Molecular & Medical Pharmacology) at UCLA. Dr. Wu has received several awards, including the Burroughs Wellcome Foundation Career Award in Medical Sciences, Baxter Foundation Faculty Scholar Award, AHA Innovative Research Award, AHA Established Investigator Award, NIH Director’s New Innovator Award, NIH Roadmap Transformative Award, and Presidential Early Career Award for Scientists and Engineers given out by President Obama. He is on the editorial board of Journal Clinical Investigation, Circulation Research, Circulation Cardiovascular Imaging, JACC Imaging, Human Gene Therapy, Molecular Therapy, Stem Cell Research, and Journal of Nuclear Cardiology. He is a Council Member for the American Society for Clinical Investigation and a Scientific Advisory Board Member for the Keystone Symposia. His clinical activities involve adult congenital heart disease and cardiovascular imaging. His lab research focuses on stem cells, drug discovery, and molecular imaging.
You May Also Like

Medical Statistics Program
Stanford School of Medicine, Stanford Center for Health Education

Artificial Intelligence in Healthcare

Digital Health Product Development
SOM-XCHE0025
- Engineering
- Artificial Intelligence
- Computer Science & Security
- Business & Management
- Energy & Sustainability
- Data Science
- Medicine & Health
- Explore All
- Technical Support
- Master’s Application FAQs
- Master’s Student FAQs
- Master's Tuition & Fees
- Grades & Policies
- HCP History
- Graduate Application FAQs
- Graduate Student FAQs
- Graduate Tuition & Fees
- Community Standards Review Process
- Academic Calendar
- Exams & Homework FAQs
- Enrollment FAQs
- Tuition, Fees, & Payments
- Custom & Executive Programs
- Free Online Courses
- Free Content Library
- School of Engineering
- Graduate School of Education
- Stanford Doerr School of Sustainability
- School of Humanities & Sciences
- Graduate School of Business
- Learning Collaborations
- Stanford Credentials
- What is a digital credential?
- Grades and Units Information
- Our Community
- Get Course Updates
- Giving to Mass General Brigham
- Search the Site Site-wide search 0 items available in list reset search Search Close Search
Gene and Cell Therapy Institute
With the Gene and Cell Therapy Institute, gene and cell therapy research at Mass General Brigham is rapidly advancing, pushing the technological and clinical boundaries of this new frontier.

2024 Research symposium
The Gene and Cell Therapy Institute’s annual research symposium is an exploration of the dynamic research landscape, uniting trailblazers who are driving advancements in gene therapies, cell therapies, and gene editing techniques. The 2024 event (November 12-13) will feature engaging discussions by leading pioneers in gene and cell therapy research and plentiful opportunities for networking and collaboration among attendees.
Uniting cutting-edge research with world-class patient care
Discover the groundbreaking world of gene and cell therapy, where advanced medical technologies are revolutionizing patient care. At Mass General Brigham, we are at the forefront of this exciting frontier, pushing the boundaries of technology and clinical applications.
About the Gene and Cell Therapy Institute
The Gene and Cell Therapy Institute (GCTI) at Mass General Brigham is a hub of innovation and collaboration, uniting more than 400 researchers and clinicians dedicated to advancing gene and cell therapy.
The GCTI is committed to finding targeted and transformative treatments that have the potential to cure diseases or halt their progression. Led by renowned cardiac gene therapy pioneer Roger Hajjar, MD, the GCTI accelerates groundbreaking research, conducts clinical trials, and facilitates the development of FDA-approved treatments.
The Institute’s multidisciplinary approach sets it apart from others in the space. The GCTI leverages expertise from across the entire ecosystem of medical innovation and fosters an environment of shared knowledge and collective impact by bringing together researchers utilizing gene and cell therapy platforms. This collaboration ensures that scientific and clinical goals align seamlessly, ultimately leading to improved patient outcomes.
The close relationships between physicians, researchers, and patients is a critical component of what makes the GCTI successful. This continuous loop, from bench to bedside and back again, enables translation to clinical applications. The GCTI is committed to expediting the pace of discovery and development by bridging the gap between research and practice.
The GCTI operates an annual Spark grant program for Mass General Brigham researchers, providing crucial funding to fuel groundbreaking projects and drive scientific progress. This investment in cutting-edge research ensures that Mass General Brigham remains at the forefront of gene and cell therapy advancements. The GCTI also offers expertise in manufacturing, regulatory, clinical trials, and core laboratory services .
The Gene and Cell Therapy Institute's leadership actively fosters and establishes both internal and external connections to drive forward the advancement of gene and cell therapy treatments and groundbreaking discoveries.
Roger J. Hajjar, MD Head, Gene and Cell Therapy Institute
Marcela V. Maus, MD, PhD Associate Head & Head of Cell Therapies, Gene and Cell Therapy Institute Paula O’Keeffe Endowed Chair, Mass General Cancer Center Director, Cellular Immunotherapy Program, Massachusetts General Hospital Professor of Medicine, Harvard Medical School Attending Physician, Hematopoietic Cell Transplant & Cell Therapy Program, MGH
Nathan L. Yozwiak, PhD Head of Research, Gene and Cell Therapy Institute
Natalie Artzi, PhD Head of Structural Nanomedicines, Gene and Cell Therapy Institute Associate Professor, Harvard Medical School Associate Faculty Member, Wyss Institute Principal Research Scientist, MIT
Angela Shen, MD, MBA Head of Regulatory, Gene and Cell Therapy Institute
Dana M. Hammill, MS, MBA Head of Manufacturing & CMC, Gene and Cell Therapy Institute
Meini S. Shin, MBA Head of Strategic Planning & Operations, Gene and Cell Therapy Institute
Omar Abudayyeh, PhD Director of Gene Editing, Gene and Cell Therapy Institute Assistant Professor of Medicine, Harvard Medical School Investigator, Division of Eng. in Medicine, Department of Medicine, Brigham and Women's Hospital Department of Stem Cell and Regenerative Biology, Harvard University
Robert Alexander Wesselhoeft, PhD Director RNA Therapeutics, Gene and Cell Therapy Institute
Nandhitha Uma Naresh, PhD Project Manager, Gene and Cell Therapy Institute
Isabella M. Zamora, MPH Program Coordinator, Gene and Cell Therapy Institute
Susanti Sugianto, MSF Program Coordinator, Gene and Cell Therapy Institute
Together, we can shape the future of medicine to make a profound impact on patient lives.
What are gene and cell therapies?
Gene and cell therapies are advanced medical treatments aimed at stopping or slowing disease progression. Gene therapy involves replacing or modifying faulty genes with corrected versions, while cell therapy replaces or repairs damaged cells. Gene editing, utilizing tools like CRISPR, enable targeted and programmable repair of damaged gene products. Mass General Brigham offers a range of FDA-approved gene and cell therapy treatments for various medical conditions, including certain types of cancer, hereditary diseases, and blood disorders.
Types of therapies
Gene therapy.
Many diseases can involve a genetic component — a missing gene, multiple copies of a gene, or a gene that is structurally defective. Gene therapy is the process of replacing or modifying genes to correct a deficiency, sometimes by altering the DNA sequence of the target gene or by modifying expression of a gene by turning it off or on. Gene therapy can have an especially profound effect in congenital diseases where the disease is associated with malfunction of a single gene. Researchers are working to identify such genes and develop therapies to replace or modify genes.
Cell therapy
Cell therapy involves replacing or modifying cells to treat disease. Cell therapy can use cells from another person (allogenic) or the person’s own cells (autologous), which may or may not be genetically altered.
Autologous, modified cell therapy is personalized to treat an individual’s condition where scientists take the person’s own cells and genetically change them outside the body, expand the number of modified cells, and then reinfuse these modified cells to produce a therapeutic benefit. Such therapies alter the patient's cells so their immune system can now target and destroy previously stealth diseased cells.
Examples of cells that are modified to treat disease include T-cells (commercialized as chimeric antigen receptor T cells; CAR-T cells), B-cells, Natural Killer (NK) cells, Tumor Infiltrating Lymphocytes (TILs), and Dendritic Cells (DCs).
Gene editing
Gene editing is the modification of a cell’s DNA with great precision using tools, such as CRISPR-based biotechnologies, that enable programmable and targeted repair of damaged gene products.
Mass General Brigham is proud to offer FDA-approved gene and cell therapy treatments for the following conditions:
- CAR T-cell therapy for lymphoma and leukemia
- Multiple myeloma
- Beta-thalassemia
- Retinal dystrophy
- Cartilage defects - knee
- Cerebral adrenoleukodystrophy
- Spinal muscular atrophy
- Neurodegenerative diseases
- Duchenne muscular dystrophy
Gene and Cell Therapy Institute awards
The Gene and Cell Therapy Institute (GCTI) at Mass General Brigham is advancing medical innovation through two awards programs that serve to fund cutting-edge gene and cell therapy projects led by Mass General Brigham investigators.

Clinical trials
Mass General Brigham is actively involved in numerous clinical trials, conducting many studies across the health system. These trials, led by our experts, hold the potential to expand the number of FDA-approved therapies in the coming years. To learn more about clinical trials visit the Mass General Brigham Clinical Trials website or explore ClinicalTrials.gov .

Meet our researchers
Get to know some of the brilliant minds driving research at Mass General Brigham.
Marcela Maus, MD, PhD
Dr. Maus and her research team design and evaluate next generation genetically modified (CAR) T cells as immunotherapy in patients with cancer. Using the immune system as treatment has the potential to induce long-term, durable remissions, and perhaps even cures for some patients. “We’re effectively hacking the immune system so patients’ own bodies can fight their disease,” says Dr. Maus.

Natalie Artzi, PhD
Natalie Artzi, PhD, is an Associate Professor at Harvard Medical School and researcher in the Department of Medicine at Brigham and Women’s Hospital. Dr. Artzi and her team are researching the design of smart biomaterials for disease monitoring. Additionally, they are investigating gene and cell therapy deliveries for a range of diseases and for tissue regeneration applications.

News & highlights
Mass General Brigham and its diverse teams are leading novel approaches in innovation and improved patient care.
Sept 3, 2024
May 7, 2024

March 13, 2024
Feb 12, 2024
Jan 16, 2024

Nov 18, 2023

Nov 13, 2023
Oct 18, 2023
Aug 17, 2023

College of Science
Cell and gene therapies.
Northeastern University's Master of Science in Cell and Gene Therapies is an innovative nonthesis degree program that prepares students for the evolving field of cell and gene therapy. The industry-aligned curriculum provides the knowledge and experience to advance your career.
Within the medical studies, cell and gene therapies is one of the fastest-growing fields. Northeastern's Master of Science in Cell and Gene Therapies is an innovative, nonthesis master's program that brings students the knowledge and skills to understand the key concepts and solve the vital challenges in cell and gene therapy treatments. The curriculum and course contents are designed carefully to cover cell and gene therapy topics in a broad range of introductory topics, regulatory insight, clinical studies, laboratory skills and interpersonal skills critical to today's employers.
Students in the program gain professional experience through Northeastern's signature co-op program. Degree requirements include a mandatory co-op assignment, which allows you to practice skills acquired in coursework. Whether you're a recent graduate, looking to advance your career, or become a change agent, at the end of the program you'll be prepared to innovate, collaborate, and lead as a research, managerial, or technical professional within leading cell and gene therapy organizations.
More Details
Unique features.
Gain interdisciplinary training in a program that combines knowledge of advanced therapies, including cell therapies and gene therapies, with the development of laboratory skills critical to success in today's workplace.
Study in an innovative master's program that lets you earn a nonthesis graduate degree fully integrated with industry projects and clinical studies.
Gain industry-aligned skills and experience in a professional science program that provides both technical training and professional skills that you'll practice in a mandatory co-op.
Enhance your career in the evolving field of cell and gene therapy by graduating with technical skills, knowledge of the regulatory landscape, and laboratory and interpersonal skills.
Career Outlook
- Employment for regenerative medicine-related occupations is growing faster than average across the globe.
- In New England, jobs related to regenerative medicine are expected to grow by 10.8 percent over the next decade—with salaries for professional workers in related occupations above the national average.
- In 2021, the median pay for a genetic counselor was $81,150 annually. (U.S. Bureau of Labor Statistics, 2022)
Testimonials
– dr. sahar tavakoli, program director of ms in cell and gene therapies, looking for something different.
A graduate degree or certificate from Northeastern—a top-ranked university—can accelerate your career through rigorous academic coursework and hands-on professional experience in the area of your interest. Apply now—and take your career to the next level.
Program Costs
Finance Your Education We offer a variety of resources, including scholarships and assistantships.
How to Apply Learn more about the application process and requirements.
Requirements
- Online application
- Application fee
- The Foreign Credential Evaluation (FCE) is a required assessment of all transcripts and documents from non-U.S. accredited post-secondary education institutions. (Review the FCE requirements by country.)
- Personal statement
- Resumé
- 2 letters of recommendation
- GPA 3.0 +
- GRE not required
- Prerequisite: Biology
- Degree earned or in progress at a U.S / Canadian institution
- Degree earned or in progress at an institution where English is the only medium of instruction
- Official exam scores from either the TOEFL iBT (institution code is 3682), IELTS, PTE exam, or Duolingo English Test. Scores are valid for 2 years from the test date.
Are You an International Student? Find out what additional documents are required to apply.
Admissions Details Learn more about the College of Science admissions process, policies, and required materials.
Admissions Dates
| Admissions Deadlines | |
|---|---|
| Domestic Students | August 1 |
| International Students | July 1 |
| Admissions Deadlines | |
|---|---|
| Domestic Students | December 1 |
| International Students | November 1 |
| International currently on a F1 visa in the US | November 1 |
Industry-aligned courses for in-demand careers.
For 100+ years, we’ve designed our programs with one thing in mind—your success. Explore the current program requirements and course descriptions, all designed to meet today’s industry needs and must-have skills.
View curriculum
Northeastern's experience-powered learning model has been at the heart of the university for more than a century. It combines world-class academics with professional practice, allowing you to acquire relevant, real-world skills you can immediately implement in your current workplace. Your Northeastern education can be a dynamic, transformative experience, giving you countless opportunities to grow as a professional and person.
Our Faculty
Northeastern University faculty represents a broad cross-section of professional practices and fields, including finance, education, biomedical science, management, and the U.S. military. They serve as mentors and advisors and collaborate alongside you to solve the most pressing global challenges facing established and emerging markets.

Sahar Tavakoli
By enrolling in Northeastern, you’ll be connected to students at our 13 campuses, as well as 300,000-plus alumni and more than 3,500 employer partners around the world. Our global university system provides you with unique opportunities to think locally and act globally and serves as a platform for scaling ideas, talent, and solutions.
Related Articles

What Is Medicinal Chemistry?

Is a Master’s in Biotechnology Worth It?

Top Biotechnology Salaries in 2024
| --> Master's | Save | |||||
| COMMITMENT | DURATION | TYPE | ||||
| --> Master's | Save | |||||
| COMMITMENT | DURATION | TYPE | ||||
| --> Master's | Save | |||||
| COMMITMENT | DURATION | TYPE | ||||
| --> Master's | Save | |||||
| COMMITMENT | DURATION | TYPE | ||||
- Skip to Content
- Catalog Home
- Institution Home
- Graduate Catalog /
- Perelman School of Medicine /

Cell and Molecular Biology: Gene Therapy and Vaccines, PhD
Related programs.
- Cell and Molecular Biology: Cancer Biology, PhD
- Cell and Molecular Biology: Cell Biology, Physiology, and Metabolism, PhD
- Cell and Molecular Biology: Developmental, Stem Cell, and Regenerative Biology, PhD
- Cell and Molecular Biology: Genetics and Epigenetics, PhD
- Cell and Molecular Biology: Microbiology, Virology, and Parasitology, PhD
Cell and Molecular Biology
The Cell and Molecular Biology Graduate Group (CAMB) is an interdisciplinary graduate program, providing rigorous training in modern cell and molecular biology, preparing students for leadership careers in biomedical research. Within this integrated program are six discipline areas: Cancer Biology ; Cell Biology, Physiology, and Metabolism ; Developmental, Stem Cell and Regenerative Biology ; Gene Therapy and Vaccines ; Genetics and Epigenetics ; and Microbiology, Virology and Parasitology . Program faculty include more than 300 scientists representing 35 departments from the Perelman School of Medicine, the Schools of Arts and Sciences, Dental Medicine, and Veterinary Medicine, Children’s Hospital of Philadelphia, the Wistar Institute and Fox Chase Cancer Center. The research efforts of these scientists are diverse in their focus, experimental system, methodology, and represent the leading edge of basic and translational biomedical science.
Students from colleges and universities around the nation and the world are enrolled in the program, selecting one discipline area based on their scientific interests, yet have access to the full breadth of curricular and research opportunities provided by this large and diverse program. Our students participate in core courses in cell and molecular biology, specialized coursework in one or more discipline areas, and original hypothesis-driven thesis research. Upon completion of the PhD, they pursue successful research careers at top academic institutions, in the biotech and pharmaceutical industries, and in other biomedicine-related career paths.
For more information: http://www.med.upenn.edu/camb/
Gene Therapy and Vaccines
The Gene Therapy and Vaccines Program focuses on the use of animal and human gene transfer for therapeutic purposes and for vaccination. Program faculty conduct research in basic cell biology, molecular biology, developmental biology, molecular physiology, virology and immunology. While the goals of the research are disease-based with the objective of developing prophylactic and therapeutic applications, student training focuses on a basic understanding of disease pathobiology and achieving efficient and effective gene transfer in humans. Students contribute to research of cystic fibrosis, hemophilia, lysosomal storage disease, inherited blindness, cancer, cardiovascular diseases, and immunologic and infectious diseases. Study of vaccines for prophylactic, as well as therapeutic applications are emphasized. Students participate in month research seminar series, as well as present in a bi-weekly research in progress seminar.
For more information: https://www.med.upenn.edu/camb/gtv.shtml
View the University’s Academic Rules for PhD Programs .
Required Courses
| Code | Title | Course Units |
|---|---|---|
| Coursework | ||
| Regulation of the Genome | ||
| Cell Biology | ||
| Foundations in Statistics | ||
| CAMB First Year Seminar | ||
| Molecular Basis of Genetic Therapies | ||
| Immunology for CAMB | ||
| or | Immune Mechanisms | |
| Select one program elective | ||
| Select three electives | ||
| Research | ||
| Lab Rotation | ||
| Pre-Dissertation Lab Rot | ||
| Dissertation | ||
Or other statistics course with approval of the Graduate Group.
The degree and major requirements displayed are intended as a guide for students entering in the Fall of 2024 and later. Students should consult with their academic program regarding final certifications and requirements for graduation.
Sample Plan of Study
| Code | Title | Course Units |
|---|---|---|
| Year 1 | ||
| Fall | ||
| Cell Biology | ||
| CAMB First Year Seminar | ||
| Molecular Basis of Genetic Therapies | ||
| Lab Rotation | ||
| Spring | ||
| Regulation of the Genome | ||
| Immunology for CAMB | ||
| Immune Mechanisms | ||
| Lab Rotation | ||
| Lab Rotation | ||
| Summer | ||
| Pre-Dissertation Lab Rot | ||
| Year 2 | ||
| Fall | ||
| Foundations in Statistics | ||
| Pre-Dissertation Lab Rot | ||
| Spring | ||
| Scientific Writing | ||
| Pre-Dissertation Lab Rot | ||
| Year 3 and Beyond | ||
| Dissertation | ||
Print Options
Print this page.
The PDF will include all information unique to this page.
A PDF of the entire 2024-25 catalog.
A PDF of the 2024-25 Undergraduate catalog.
A PDF of the 2024-25 Graduate catalog.
- MD | PhD Program
- Master's Programs
- PhD Programs
- Postdoctoral Fellows
- Residency & Fellowship
- Non-Degree Programs
- Visiting Students
- Campus Life at U-M
- Health & Wellness
- Building Your Community
- Accessibility & Disability
- Departments
- Centers & Institutes
- Interdisciplinary Programs
- Facts & Figures
- Medical School Leadership
- News & Stories
- Requirements
- Interview Day
- Admissions Chats
- AAMC Michigan's 35 Answers
- AAMC Michigan's 10 Financial Aid Answers
- Admitted Students
- Overview & Highlights
- Patient Interaction
- Chief Concern
- Years 3 & 4
- Learning Informatics
- Training Sites
- Leadership Program
- Global Health & Disparities
- Healthcare Innovation
- Health Policy
- Medical Humanities
- Patient Safety & Quality Improvement
- Scientific Discovery
- Doctoring Course
- Evidence-Based Medicine
- Interprofessional Education
- DEIAJ Curriculum
- Language Opportunities
- Curriculum Diagrams
- Grading & Assessments
- Guideline Budget
- Loans & Eligibility
- Scholarships & Grants
- Documents & Forms
- Tuition Refund Policies
- Consumer Information
- Extra Help for Current Students
- Contact the Office of Financial Aid
- Profiles & Demographics
- Culinary Connections
- Students with Disabilities
- Health & Wellbeing
- Arts & Humanities
- Diversity & Health Equity
- Dual Degrees
- More Possibilities
- Commencement
- Available PhD Programs
- Academic & Social Events
- MSTP Fellows
- Application Process
- Application Requirements
- MD | PhD Curriculum
- Undergrad Summer Program
- Contact the MD | PhD Program
- Bioinformatics
- Biological Chemistry
- Cancer Biology
Cell & Developmental Biology
- Cellular & Molecular Biology
- Genetics and Genomics
- Health Infrastructures & Learning Systems
- Microbiology & Immunology
- Molecular, Cellular & Developmental Biology
- Molecular & Cellular Pathology
- Molecular & Integrative Physiology
- Neuroscience
- Pharmacology
- Recruitment Events
- Interview Weekends
- Certificates & Dual Degrees
- Quantitative & Computational Biology Emphasis
- Training Grants
- Facilities & Resources
- Stipend & Benefits
- Professional Development
- Finding a Position
- Funding Your Postdoc
- Hiring Process
- Postdoc Preview
- International Postdocs
- ACGME Fellowships
- Non-Accredited Fellowships
- Postdoctoral Physician Scientist Training
- Salary & Benefits
- Prerequisites
- Visiting Residents & Fellows
- Application Overview & Requirements
- Tuition & Fees
- Timeline & Curriculum
- Information Sessions
- Program Details
- Undergrad Summer Research
- First Days Survival Guide
- Health Services
- Mental Health
- Health, Spirituality & Religion Program
- For Partners & Families
- Things to Do in Ann Arbor
- Getting Around
- Guiding Tools
- Graduate Medical Education
- Office of Continuing Medical Education
- Office of Faculty Affairs & Faculty Development
- Office of Graduate & Postdoctoral Studies
- Physician Scientist Education & Training
- Office of Medical Student Education
- Points of Blue

Excel in a number of different scientific careers
Program Overview
The Graduate Program in Cell & Developmental Biology (CDB) provides outstanding doctoral training in fundamental aspects of cell and developmental biology, ranging from stem cells to regenerative medicine and organogenesis to cancer biology. Research teams in CDB employ high-resolution microscopy, live single cell imaging, biochemistry-based methods, sophisticated genetics including gene editing, and systems-based strategies to understand the basic building block of life, the cell.
Research activities in CDB place a strong emphasis on quantitative and computational skills, cover gametes as well as highly differentiated and specialized cell types, and involve a broad array of model systems including budding yeast, flies, mice and human samples. CDB graduate students thus receive rigorous and comprehensive training and are integral members of a highly collaborative and collegial environment. Together these elements provide exceptional preparation for graduate students to excel in a number of different scientific careers.
Apply through our PIBS application
CDB faculty work in a very broad range of research areas:
- Aging and degeneration
- Cancer biology
- Cell signaling
- Cellular biophysics
- Computational biology and mathematical modeling
- Developmental genetics
- Gene expression and genomics
- Genetic and molecular models of human disease
- Host-pathogen interactions
- Intracellular transport
- Neurobiology
- Organogenesis and pattern formation in the eye, ear, limb, gut, heart, skeleton, hematopoietic system, urogenital system, reproductive system, and nervous system
- Stem cell biology
- Systems biology
Our faculty and students are extremely interactive and collaborative. Many CDB students have interdisciplinary projects involving multiple laboratories.
CDB is home to several special programs and research facilities:
The Microscopy and Image Analysis Laboratory (MIL) , a facility of more than 3,000 square feet housing state-of-the-art microscopy and imaging equipment, is housed within the Department of Cell and Developmental Biology.
The Center for Organogenesis is an interdisciplinary group of scientists working on basic mechanisms by which organs and tissues are formed and maintained, using this knowledge to create long lasting artificial organs, stem cell therapies or organ transplantation systems that will correct genetic and acquired diseases. The Center has run an NIH-supported training grant for over 15 years.
The Michigan Center for hES Cell Research was established in 2002, with funding from the Medical School and the NIH, for the study of human embryonic stem cells. The Consortium for Stem Cell Therapies is now part of the Taubman Institute and provides training and resources for students and faculty interested in ESC and iPSC stem cell research.
Course requirements are flexible; the goal is to establish a basic working knowledge of current concepts in molecular biology, cell biology, biochemistry, genetics, and neurobiology while allowing students to pursue more advanced coursework related to their research interests, such as developmental biology, bioinformatics, gene expression, signal transduction, etc. In your first year, you will take the PIBS curriculum, which also fulfils CDB requirements. When you choose a research laboratory in CDB, you will enroll in our Seminars in Cell and Developmental Biology course, CDB 801. You will also select additional program courses and electives from the rich offerings at the University of Michigan.
Preliminary Examination
The preliminary exam consists of two checkpoints. The first is the preliminary exam in the form on a paper discussion. For students in good academic standing, this will take place in August at the end of their first year in order to advance to PhD candidacy. The second checkpoint takes place in the second year and is a vital component of the first thesis committee meeting. This checkpoint is comprised of two steps, the writing of a 6-page fellowship (NIH format) and the oral presentation of the proposed research to the thesis committee.
Teaching Requirement
As part of their professional training, CDB students serve as graduate student instructors (GSIs) in a graduate-level course for one semester.
Expected Length of Program
The usual time to degree is approximately 5 to 5 1/2 years.
CDB students run the course CDB 801 where they take turns presenting their research, give and get feedback on their research and presentations, explore career development opportunities, and interact with their fellow students in a supportive and cohesive environment. CDB students also participate in department committees, invite and host seminar speakers, help organize the annual departmental retreat, and plan/attend monthly happy hours.
CDB students have also expanded their skills and knowledge through university certificate programs such as those in teaching and translational science.
In recent years, a number of CDB students have been heavily involved in an outreach program called Developing Future Biologists (DFB). This is a graduate student-led educational organization that organizes and facilitates a week-long summer course aimed at teaching the next generation of biologists the fundamental concepts of developmental biology regardless of race, gender, or socioeconomic status.
In addition to scientific discovery, our mission is to train future leaders in cell and developmental biology, by encouraging high-impact research and providing teaching and mentorship of the highest quality.
Our students have received University and national recognition for their scholarship, research, and teaching, and their work is frequently published in high-impact journals (including recent papers in Nature Cell Biology, Science Signaling, Neuron, PLoS Biology, Current Biology, Developmental Cell, Molecular Cell, PLoS Pathogens, J Neurosci, J Virology, PNAS, J Cell Sci, EMBO J, Cell Metabolism, MCB, JBC, Blood, J Clin Invest, Development, etc.).
CDB graduates have gone on to successful careers in academic science, medical research, scientific consulting, and biotechnology.
Learn more about the Department of Cell & Developmental Biology.
We transform lives through bold discovery, compassionate care and innovative education.
- Diversity, Equity & Inclusion
- Find a Doctor
- Conditions & Treatments
- Patient & Visitor Guide
- Patient Portal
- Clinical Trials
- Research Labs
- Research Centers
- Cores and Resources
- Programs & Admissions
- Our Community
- Departments, Centers & Offices
- About the Medical School
Global Footer Secondary Navigation
- Menu Toggle extended navigation
Genome Editing Research Program
Founding Director: Krzysztof Palczewski, PhD

Our Vision: Cure Genetic Disorders
Introduced just a decade ago, gene editing has swiftly emerged as one of the most promising frontiers in the quest for innovative approaches to restore DNA sequences.
At UC Irvine, our investigators have unlocked the ability to reverse and forestall cell degeneration. Currently, our researchers are tirelessly working to refine a method for directing this therapy to specific cells in need of correction. The pioneering approaches spearheaded by the Genome Editing Research Program, once realized, hold the potential to revolutionize not only the treatment of genetic disorders affecting vision but also a broader spectrum of inherited conditions.
The program has been designated as one of the 12 high-impact research programs to be housed in the Falling Leaves Foundation Medical Innovation Building.
In This New Facility
Current world-renowned scientists and clinicians , alongside newly recruited experts, will collaborate in custom-designed labs to translate discoveries into cures.
Interdisciplinary teams will collaborate closely to model and comprehend inherited disorders, then develop and test therapies for the benefit of patients.
Future leaders will receive training from the forefront of science and medicine.
Indoor and outdoor gathering spaces will facilitate events, speakers and presentations, fostering engagement and dialogue to expedite and share discoveries.
Our Competitive Advantage
Empowered by the distinctive One Health approach of UCI Health Affairs that transcends disciplinary boundaries, the Genome Editing Research Program comprises faculty and staff from the Susan & Henry Samueli College of Health Sciences (encompassing schools of medicine, nursing, pharmacy and pharmaceutical sciences, population and public health) and UCI Health, our regional healthcare delivery system which includes the trailblazing Susan Samueli Integrative Health Institute. By nurturing collaborative efforts among brilliant minds, our teams can surpass the limitations of working in isolation. This collaborative approach enables us to push the boundaries of innovation and make fundamental discoveries in the field of gene therapy.
The strength of the Genome Editing Research Program lies in a critical mass of multifaceted research laboratories, each focusing on complementary aspects of function and disease, ranging from structural biology and genetics to physiology and pharmacology. Dedicated investigators collaborate to model and understand disorders, subsequently developing and testing therapies to benefit patients.
In the realm of retinal research, our program is addressing the urgent need for effective treatments for inherited retinal diseases, which currently have limited therapeutic options. Our innovative use of base and prime editing has led to the correction of mutations responsible for conditions such as Leber Congenital Amaurosis and Retinitis Pigmentosa, not only restoring vision from the retina to the visual cortex but also offering a protective shield for photoreceptors against degeneration. These groundbreaking advancements have been published in prestigious journals such as Cell, Nature Biotechnology, Nature Biomedical Engineering, Nature Communications and PNAS . We are dedicated to refining our genome engineering techniques and developing new delivery methods to ensure our genome editing is safer, more effective and more precise. This effort demonstrates our steadfast commitment to advancing the field of genetic therapy.
Glaucoma is the second leading cause of irreversible blindness worldwide. We are paving the way to innovative treatments through studies that successfully target the mutant MYOC gene using the CRISPR-Cas9 system, significantly reducing mutant myocilin levels in the trabecular meshwork and preventing the onset of glaucoma in models. This research is instrumental in developing targeted delivery systems for various mutations associated with glaucoma. Our holistic approach, combining cutting-edge research with compassionate patient care, ensures our work goes beyond laboratory experiments to make a significant difference in patients’ lives. Through collaborative efforts, we are creating a unique research environment conducive to discovering breakthroughs in the treatment of ocular diseases.
Current Team
We have assembled, and will seek to expand, an exceptional group of scientists with a keen understanding of genome editing to optimize research collaborations. Faculty leadership includes:

Founding Director of the Genome Editing Research Program; Director of the Center for Translational Vision Research

Professor, Ophthalmology, Biomedical Engineering

Associate Professor, Ophthalmology, Biomedical Engineering

Professor, Biomedical Engineering, Molecular Biology and Biochemistry

Professor, Chemistry, Molecular Biology and Biochemistry, Pharmaceutical Sciences

Professor, Ophthalmology, Physiology & Biophysics
Join Us in Making an Impact
We invite philanthropic partners to collaborate with us in establishing a new home for the UCI Genome Editing Research Program within the Falling Leaves Foundation Medical Innovation Building, and in providing essential support for our programmatic funding to drive transformative discoveries.
The architectural design of the Falling Leaves Foundation Medical Innovation Building is tailored to facilitate vibrant collaborations that translate inspiration into tangible results. You have the exclusive opportunity to name the new headquarters for the Genome Editing Research Program and custom-designed laboratories and gathering spaces. As a philanthropic partner, your contribution will ensure that our scientists have the necessary space, resources and facilities to advance their life-changing work.
Research endowments play a pivotal role in sustaining our investigators, who are at the forefront of shaping the future of science and healthcare. External grants cover only a fraction of associated expenses, underscoring the importance of endowed funding in supporting innovative discoveries and pioneering approaches. Endowed grants bear special significance, offering the flexibility to pursue scientific avenues that lead to breakthroughs. Your generous support will enable this vital work to be carried out under the auspices of our philanthropic partners. Additionally, named endowed scholarships will further enrich the training of future generations of leading investigators and healthcare providers in perpetuity.
By partnering with us as a philanthropic sponsor to name an endowed chair, you will establish a lasting connection between your legacy and UCI’s esteemed experts. Ultimately, advancements in biomedical research will drive the development of innovative therapies. Philanthropic contributions towards endowed chairs, research and scholarships ensure sustained support, facilitating the recruitment and retention of top-tier faculty who are instrumental in maintaining UCI’s status as a world-class center of excellence in emerging therapies. Endowed chairs are particularly impactful as they establish a perpetual annual funding stream, empowering the chair-holder to focus their efforts on groundbreaking research initiatives.
- COHS Faculty Assembly
- UCI Research Associates
- Accreditation Information
- UCI Brilliant Campaign
Support Biology
Dei council and dei faculty committee, biology diversity community, mit biology catalyst symposium, honors and awards, employment opportunities, faculty and research, current faculty, in memoriam, areas of research, biochemistry, biophysics, and structural biology, cancer biology, cell biology, computational biology, human disease, microbiology, neurobiology, stem cell and developmental biology, core facilities, video gallery, faculty resources, undergraduate, why biology, undergraduate testimonials, major/minor requirements, general institute requirement, advanced standing exam, transfer credit, current students, subject offerings, research opportunities, biology undergraduate student association, career development, why mit biology, diversity in the graduate program, nih training grant, career outcomes, graduate testimonials, prospective students, application process, interdisciplinary and joint degree programs, living in cambridge, graduate manual: key program info, graduate teaching, career development resources, biology graduate student council, biopals program, postdoctoral, life as a postdoc, postdoc associations, postdoc testimonials, workshops for mit biology postdocs entering the academic job market, responsible conduct of research, postdoc resources, non-mit undergraduates, bernard s. and sophie g. gould mit summer research program in biology (bsg-msrp-bio), bsg-msrp-bio gould fellows, quantitative methods workshop, high school students and teachers, summer workshop for teachers, mit field trips, leah knox scholars program, additional resources, mitx biology, department calendar, ehs and facilities, graduate manual, resources for md/phd students, preliminary exam guidelines, thesis committee meetings, guidelines for graduating, mentoring students and early-career scientists, remembering stephen goldman (1962 – 2022).

genes • gene editing • heredity• evolution • genetic variability • phenotypic variability • horizontal gene transfer • meiosis • recombination • epigenetics • DNA repair and replication • chromosome segregation • cell division • gene regulation • development • aging • pathogenesis • cancer • disease
David Bartel
Iain m. cheeseman, olivia corradin, gerald r. fink, mary gehring, alan d. grossman, leonard p. guarente, michael t. hemann, h. robert horvitz, david housman, siniša hrvatin, tyler jacks, chris a. kaiser, kristin knouse, eric s. lander, michael t. laub, ruth lehmann, troy littleton, david c. page, peter reddien, francisco j. sánchez-rivera, anthony j. sinskey, graham c. walker, yukiko yamashita.

David Bartel studies molecular pathways that regulate eukaryotic gene expression by affecting the stability or translation of mRNAs.

Establishing boundaries of the genetic kind

Growing to greatness: Professor Mary Gehring on plant epigenetics and becoming an HHMI Investigator

In immune cells, X marks the spot(s)

Unusual Labmates: Meet tardigrades, the crafters of nature’s ultimate survival kit

Gene silencing tool has a need for speed

“Vaults” within germ cells offer more than safekeeping

Taking RNAi from interesting science to impactful new treatments

Q&A: Pulin Li on recreating development in the lab
Human Genetics and Genomics, PhD
School of medicine, ph.d. program.
The Johns Hopkins Human Genetics and Genomics Training Program provides training in all aspects of human genetics and genomics relevant to human biology, health and disease.
Advances in human genetics and genomics continue at an astounding rate and increasingly they are being integrated into medical practice. The Human Genetics and Genomics Program aims to educate highly motivated and capable students with the knowledge and experimental tools that will enable them to answer important questions at the interface between genetics and medicine. Ultimately, our trainees will be the leaders in delivering the promise of genetics to human health.
The overall objective of the Human Genetics program is to provide our students with a strong foundation in basic science by exposure to a rigorous graduate education in genetics, genomics, molecular biology, cell biology, biochemistry and biostatistics as well as a core of medically-related courses selected to provide knowledge of human biology in health and disease.
This program is also offered as training for medical students in the combined M.D./Ph.D. program. Students apply to the combined program at the time of application to the M.D. program. (See section entitled Medical Scientist Training Program).
Research Facilities
Research laboratories are well equipped to carry out sophisticated research in all areas of genetics. The proximity to renown clinical facilities of the Johns Hopkins Hospital, including the Department of Genetic Medicine, and Oncology Center provides faculty and students with access to a wealth of material for study. Computer and library facilities are excellent. Laboratories involved in the Human Genetics Program span Johns Hopkins University; consequently supporting facilities are extensive.
Financial Aid
The program is supported by a training grant from the National Institute of General Medical Sciences. These fellowships, which are restricted to United States citizens and permanent United States residents, cover tuition, health care insurance and a stipend during year one. Once a student has joined a thesis lab, all financial responsibilities belong to the mentor. Students are encouraged, however, to apply for fellowships from outside sources (e.g., the National Science Foundation, Fulbright Scholars Program, Howard Hughes Medical Institute) before entering the program.
Applicants for admission should show a strong academic foundation with coursework in biology, chemistry and quantitative analysis. Applicants are encouraged to have exposure to lab research or to data science. A bachelor's degree from a qualified college or university will be required for matriculation. GREs are no longer required.
The Human Genetics and Genomics site has up-to-date information on “ How to Apply .” For questions not addressed on these pages, please access the contact information listed on the program page: Human Genetics and Genomics Training Program | Johns Hopkins Department of Genetic Medicine .
Program Requirements
The program includes the following required core courses: Advanced Topics in Human Genetics, Evolving Concept of the Gene, Molecular Biology and Genomics, Cell Structure and Dynamics, Computational Bootcamp, Pathways and Regulation, Genomic Technologies, Rigor and Reproducibility in Research, and Systems, Genes and Mechanisms of Disease. Numerous elective courses are available and are listed under sponsoring departments.
Our trainees must take a minimum of four electives, one of which must provide computational/statistical training.
The HG program requires the “OPTIONS” Career Curriculum offered by the Professional Development and Career Office. OPTIONS is designed to provide trainees with the skills for career building and the opportunity for career exploration as well as professional development training
Human Genetics trainees also take a two-week course in July at the Jackson Labs in Bar Harbor, Maine entitled "Human and Mammalian Genetics and Genomics: The McKusick Short Course" which covers the waterfront from basic principles to the latest developments in mammalian genetics. The faculty numbers about 50 and consists roughly in thirds of JAX faculty, Hopkins faculty and “guest” faculty comprising outstanding mammalian geneticists from other US universities and around the world.
The courses offered by the faculty of the program are listed below. All courses are open to graduate students from any university program as well as selected undergraduates with permission of the course director.
Trainees must complete three research rotations before deciding on their thesis lab. They must also participate in the Responsible Conduct of Research sessions offered by the Biomedical Program; starting at year 3, students must attend at least two Research Integrity Colloquium lectures per year.
Our trainees participate in weekly journal clubs, department seminars, monthly Science & Pizza presentations as well as workshops given twice a year on diversity, identity and culture.
At the end of the second year, trainees take their Doctoral Board Oral Examination. Annual thesis committee meetings must be held following successful completion of this exam.
Average time for completion is 5.3 years.
| Code | Title | Credits |
|---|---|---|
| Advanced Topics in Human Genetics | 1.5 | |
| Introduction to Rigor and Reproducibility in Reseach | ||
| Evolving Concepts of the Gene | 5 | |
| Introduction to Responsible Conduct of Research | 1 | |
| Human Genetics Boot Camp | 2 | |
| Cell Structure and Dynamics | 1.5 | |
| Molecular Biology and Genomics | 1.5 | |
| Independent Research | 1 - 18 | |
| Systems, genes and mechanisms in disease | 3 | |
| Genomic Technologies: Tools for Illuminating Biology and Dissecting Disease | 1.5 | |
| Understanding Genetic Disease | 0.5 | |
| Pathways and Regulation | 2 |
Graduates from the Human Genetics program pursue careers in academia, medicine, industry, teaching, government, law, as well the private sector. Our trainees are encouraged to explore the full spectrum of professional venues in which their training my provide a strong foundation. Driven by curiosity and a desire for excellence, our trainees stand out as leaders in the chosen arenas of professional life. They are supported in the development of their career plans by a program faculty and administration who are dedicated to their success, and by a myriad of support networks across the Johns Hopkins University, many of which are provided by the Professional Development Career Office of the School of Medicine.
30 gene-editing PhD positions
Filtered by.
- gene-editing
Refine Your Search
- Scholarship 20
- Research Job 10
- United States 9
- Australia 2
- United Kingdom 1
- Umeå University 13
- The University of Iowa 3
- Duke University 2
- Brigham and Women's Hospital/Harvard Medical School-Department of Medicine 1
- Centre for Genomic Regulation 1
- Chalmers University of Technology 1
- Cornell University 1
- Cranfield University 1
- Karolinska Institutet 1
- Monash University 1
- Murdoch University 1
- SciLifeLab 1
- The Ohio State University 1
- University of Göttingen • 1
- University of Iowa Stead Family Department of Pediatrics 1
- Computer Science 7
- Medical Sciences 4
- Chemistry 3
- Materials Science 2
- Earth Sciences 1
- Economics 1
- Humanities 1
- Social Sciences 1
Gene editing to improve salt tolerance in legumes - PhD
at the same time they enhance soil quality. With the support of Azolla Biotech Ltd. and Cranfield University the student will use CRISPR-Cas9 gene editing and Agrobacterium-mediated transformation methods
Researcher—Computational Design Gene Editors (Bioinformatician)
models and gene editing . Our goal is to design novel protein-based gene writers with improved efficacy and safety. The candidate will train large language and diffusion models to provide new protein
Research Assistant/Technician
Parkinson’s Disease. The researcher will endeavor to solve some of the biggest problems in the field, using cutting-edge technologies and concepts, e.g. CRISPR gene editing , stem cell technology, human brain
PhD position in Experimental Physics
-leading research in several fields. Notably, the groundbreaking discovery of the CRISPR-Cas9 gene - editing tool, which was awarded the Nobel Prize in Chemistry, was made here. At Umeå University, everything
POSTDOCTORAL SCHOLAR - ANESTHESIA - OBAL LAB
cell technology. Experience with RNA sequencing, gene editing (CRISPR-Cas9), single particle imaging, and large data set analysis. Proficiency in programming languages like R, python, Matlab Online
Research Assistant Professor
University of Iowa Stead Family Department of Pediatrics seeks a faculty member at the rank of Research Assistant Professor (non-tenure track) level in the research area of gene therapy
to as STING (stimulator of interferon genes ) that is activated by DNA based microbes such as viruses, bacteria, and parasites. He is interested in developing viral oncolytic agents and immunotherapeutic
PhD-student in human geography
Phd student in computing science with a focus on efficiently monitoring and managing of large-scale cloud edge systems, phd position in chemistry, searches related to gene editing.
- gene therapy
- medical sciences
- genetic engineering
- transcription
- genome editing
- human genome
We have 146 crispr PhD Projects, Programmes & Scholarships
All disciplines
All locations
Institution
All Institutions
All PhD Types
All Funding
crispr PhD Projects, Programmes & Scholarships
Hematopoietic specification using single-cell in vivo crispr screen (mok_u25dtp1), phd research project.
PhD Research Projects are advertised opportunities to examine a pre-defined topic or answer a stated research question. Some projects may also provide scope for you to propose your own ideas and approaches.
Competition Funded PhD Project (Students Worldwide)
This project is in competition for funding with other projects. Usually the project which receives the best applicant will be successful. Unsuccessful projects may still go ahead as self-funded opportunities. Applications for the project are welcome from all suitably qualified candidates, but potential funding may be restricted to a limited set of nationalities. You should check the project and department details for more information.
Peptide-mediated delivery of CRISPR-Cas genome editors in the eye
Funded phd project (students worldwide).
This project has funding attached, subject to eligibility criteria. Applications for the project are welcome from all suitably qualified candidates, but its funding may be restricted to a limited set of nationalities. You should check the project and department details for more information.
(MRC DTP) Identifying the functional effects of risk loci in rheumatic disease using CRISPR screens
Self-funded phd- understanding the molecular mechanism of a bacterial genome defence system and its synergy with crispr-cas, self-funded phd students only.
This project does not have funding attached. You will need to have your own means of paying fees and living costs and / or seek separate funding from student finance, charities or trusts.
Investigate cytoskeletal networks controlling cancer cell migration at the systems level using CRISPR-Cas9, microfluidics, and advanced live cell imaging.
Investigate the molecular mechanism controlling feme (fast endophilin mediated endocytosis) in directed cancer cell migration using crispr, biochemistry, and advanced microscopy methods., using crispr in ips cells to modify platelet function, decoding the epigenetic mechanisms of drug resistance in aggressive breast cancers, in vivo reprogramming of extracellular vesicles for targeted drug delivery of genome editors to the central nervous system, using single-cell nanopore long-read sequencing to identify drivers of neurodegenerative disorders, understanding the physiological roles and pathological impacts of er-autophagy, mechanism evaluation of synthetic lethality in cancers of mesenchyme, decoding prokaryotic adaptive immunity and pathways to therapeutic innovation, transcriptional kinases in cancer and developmental disorders, dfg-funded phd position (f/m/x) in protein biochemistry.
FindAPhD. Copyright 2005-2024 All rights reserved.
Unknown ( change )
Have you got time to answer some quick questions about PhD study?
Select your nearest city
You haven’t completed your profile yet. To get the most out of FindAPhD, finish your profile and receive these benefits:
- Monthly chance to win one of ten £10 Amazon vouchers ; winners will be notified every month.*
- The latest PhD projects delivered straight to your inbox
- Access to our £6,000 scholarship competition
- Weekly newsletter with funding opportunities, research proposal tips and much more
- Early access to our physical and virtual postgraduate study fairs
Or begin browsing FindAPhD.com
or begin browsing FindAPhD.com
*Offer only available for the duration of your active subscription, and subject to change. You MUST claim your prize within 72 hours, if not we will redraw.

Create your account
Looking to list your PhD opportunities? Log in here .
Filtering Results
- U.S. Department of Health & Human Services
- National Institutes of Health

En Español | Site Map | Staff Directory | Contact Us
- News & Events
Custom alterations: mending genes for long-lasting effects
Engineered lipid nanoparticles drive durable gene editing in lungs of mice.
Gene-editing therapies —techniques that modify DNA to treat or prevent disease—have the potential to transform the field of drug development. By making precise edits to the genome, problematic genes could be modified or eliminated, representing long-lasting therapies for genetic disorders that currently have no treatment.
Few gene-editing therapies currently exist. Last year, the FDA approved a gene-editing therapy for sickle cell disease, a landmark treatment that uses CRISPR to modify blood stem cells. But like most experimental gene-editing treatments, this therapy requires a stem cell transplant, a costly and time-consuming procedure that involves removing stem cells from a patient, modifying them to correct defects causing the disease, and reintroducing them back to the patient. Finding a way to modify a patient’s stem cells in vivo —without having to remove them from the body—could revolutionize the field of genome editing.
Researchers at UT Southwestern are working to bring in vivo gene editing to the fore. Through rational engineering of lipid nanoparticles, this collaborative team developed a way to effectively target specific organs in the body to precisely deliver therapeutic cargo, including gene-editing molecules. Their research demonstrated that a one-time treatment with their nanoparticles resulted in durable gene editing in mouse lungs for nearly two years. Further, their technique showed promise in correcting a mutation present in a currently untreatable form of cystic fibrosis in several models of the disease. The research was recently published in Science .
“There is a real desire to imagine a one-time injection of a medicine that could correct mutations that are causing and driving diseases,” said senior study author Daniel Siegwart, Ph.D., a professor at UT Southwestern Medical Center. “Our preclinical platform illustrates a potential method to achieve long-term gene editing in the lungs, representing a new treatment approach for a variety of genetic respiratory conditions.”
Getting it SORTed
Lipid nanoparticles, a popular drug delivery method thanks to the success of mRNA COVID-19 vaccines, are typically comprised of four different lipids (fats) that envelop their therapeutic cargo. While these traditional lipid nanoparticles are effective at transporting and protecting their payload, when infused into a patient, they accumulate in one specific organ: the liver.
“Traditional lipid nanoparticles are remarkably similar to low-density lipoprotein (LDL) in terms of their size and chemical composition,” explained Siegwart. “LDL particles naturally travel to the liver to be broken down, so it makes sense that lipid nanoparticles accumulate there as well.”
To enable delivery to tissues other than the liver, Siegwart and colleagues previously developed a new class of particle, termed selective organ targeting (SORT) nanoparticles. In addition to the four conventional lipids found in traditional lipid nanoparticles, SORT nanoparticles contain a fifth lipid that directs the particles to a specific organ. This fifth lipid affects the physiochemical properties of the nanoparticle and attracts distinct plasma proteins to its surface, two factors that influence uptake by different types of tissues in the body.
“We knew that we needed to break the rules of traditional lipid nanoparticle formulations to target tissues other than the liver,” Siegwart said. “Using SORT, we’ve shown that we can direct nanoparticles specifically to the liver, spleen, and lungs of mice. Whether this technique could result in durable, tissue-specific gene editing remained an open question.”
Long-term lung editing
Getting the lipid nanoparticles to the right organ is an important first step. But for effective gene editing, it’s also important to target the right type of cell—specifically, stem cells and progenitor cells, or cells that can become different types of cells.
“It’s estimated that the cells in the lungs of rodents turn over and regenerate every few months or so,” said Siegwart. “If you achieve genome correction in mature cells, the effects will be temporary: as soon as the new cells are born, they will no longer have those corrected events, and malfunctioning genes will be produced again.”
To understand if their SORT nanoparticles could achieve durable genome editing in the lungs, Siegwart and colleagues used a genetically engineered mouse that has the capacity to make a red fluorescent protein—but only if its genome is edited in a specific way. “It’s a beautiful model because it allows us to quantify which specific cell types have been edited by the nanoparticles, and we can easily visualize where these nanoparticles are effective,” he said.
The researchers administered SORT nanoparticles filled with gene-editing molecules to mice and then evaluated lung tissues at ten different time points, ranging from two days to 22 months after the injections. They found that the red fluorescence was uniformly spread throughout the lungs at every time point. Further, cell-specific analyses revealed that genome editing was achieved in multiple different types of cells, and that the editing was sustained over the course of the study, nearly two years after treatment with the nanoparticles.
“When we tracked the animals over time, we found that these genome editing events were completely persistent, and almost two years later, the animals were equally edited as they were on the second day,” Siegwart said. “This indicates that the nanoparticles can successfully edit stem and progenitor cell populations that then differentiate over time into healthy, edited cells.”
Evaluation in cystic fibrosis models
With a method to achieve long-term gene editing in hand, Siegwart and colleagues turned their attention to a genetic lung disease: cystic fibrosis.
Cystic fibrosis is caused by mutations in a chloride pump, a protein that can regulate the concentration of salt inside and outside of the cell, Siegwart explained. “When this protein malfunctions, there’s an improper salt balance, leading to thick and sticky mucus building up in the lungs. This can lead to a whole host of different respiratory issues, infections, and eventually persistent lung damage that may lead to the necessity of having a lung transplant.”
Roughly 90 percent of patients with cystic fibrosis can be treated with a breakthrough medicine that helps this chloride pump function more efficiently. However, some patients have mutations in their genome that lead to a truncated, non-functional form of this protein—or the protein is not made at all. These patients currently have no approved treatment options.
In this backdrop, Siegwart and colleagues focused on one of these “undruggable” cystic fibrosis mutations. They filled their lung SORT nanoparticles with a gene editor that corrects the mutation, turning the truncated chloride pump into the normal version of the protein. Then they evaluated how well their system performed in cystic fibrosis models.
In experiments using patient-derived cystic fibrosis lung cells, the researchers found that lung SORT could correct the faulty gene, effectively restoring the function of the chloride pump by more than 50%. What’s more, in a mouse model carrying this undruggable human cystic fibrosis mutation, the researchers found that lung SORT could achieve gene correction in nearly 50% of lung stem cells.
“Our early results suggest that this technique could someday correct dysfunctional proteins in the lungs, which would absolutely be transformative in the daily lives of cystic fibrosis patients,” Siegwart said.
Jermont Chen, Ph.D., a program director in the Division of Discovery Science and Technology at NIBIB, agreed: “Through clever modifications of standard lipid nanoparticles, this team has laid the groundwork for an in vivo gene editing platform in the lungs, which could potentially be translated to other tissues down the line. While future work in relevant animal models will be required before this technique can be evaluated in humans, the method described here has the potential to lead to long-lasting treatments for patients with genetic conditions.”
Note: Siegwart is a co-founder of the company ReCode Therapeutics, a partner in this study.
This study was supported in part by a grant from NIBIB (R01EB025192).
This science highlight describes a basic research finding. Basic research increases our understanding of human behavior and biology, which is foundational to advancing new and better ways to prevent, diagnose, and treat disease. Science is an unpredictable and incremental process—each research advance builds on past discoveries, often in unexpected ways. Most clinical advances would not be possible without the knowledge of fundamental basic research.
Study reference: Yehui Sun et al., In vivo editing of lung stem cells for durable gene correction in mice. Science 384 ,1196-1202(2024). DOI: 10.1126/science.adk9428
Newsletters

DP Daybreak
Our daily newsletter rounding up all of the top headlines from the DP. Get it Monday-Friday in your inbox. Free.

34th Street Magazine's "Toast" is a semi-weekly newsletter with the latest on Penn's campus culture and arts scene. Delivered Monday-Wednesday-Friday. Free.

Penn, Unbuttoned
Penn, Unbuttoned is Penn's only intentionally satirical newsletter, giving you your weekly dose of comedy from Under the Button every Wednesday. Free.

Quaker Nation
Quaker Nation is a weekly sports newsletter covering all things Penn sports. Delivered Monday mornings to your inbox. Free.

Access Penn. The stories, people, and places that make up one of the country's premier institutions. Get it in your inbox every other Wednesday. Free.
Print Edition of The Daily Pennsylvanian
Get our award-winning print editions of The Daily Pennsylvanian delivered to your doorstep every week.
I've already signed up
The Daily Pennsylvanian is a student-run nonprofit.
Please support us by disabling your ad blocker on our site.
Penn Med, Children’s Hospital of Philadelphia receive $14 million grant for gene-editing research

The National Institutes of Health has awarded a $14 million grant to the Perelman School of Medicine and the Children’s Hospital of Philadelphia for gene-editing research.
This funding, provided through the NIH’s Somatic Cell Genome Editing Program , will support gene-editing research aimed at developing treatments for rare metabolic diseases. Researchers will aim to target developing therapies for urea cycle disorders, which affect approximately one in every 35,000 children, according to the Penn Medicine announcement.
Central to the research is a new, more advanced form of CRISPR technology known as prime editing. This technology allows for more precise changes in the genome, allowing for the correction of any genetic mutation rather than simply replacing DNA bases.
With prime editing, there is potential for the personalized treatment of many rare metabolic diseases, including life-threatening disorders like type I citrullinemia, ASA lyase deficiency, and CPS1 deficiency. Prime editing would allow permanent genetic corrections, mitigating previous challenges of the body’s immune response to treatments.
Derek Griffith named Penn Integrates Knowledge with appointments in Penn Nursing, Penn Med
Penn Health System revenue passes $10 billion for first time, marking nearly a 10% increase
Kiran Musunuru , a professor of cardiovascular medicine and director of Penn Cardiovascular Institute's Genetic and Epigenetic Origins of Disease Program, spoke with Penn Med about prime editing.
“With this technology, we hope to not just manage symptoms, but offer a durable, potentially lifelong cure for these children,” he said.
The SCGE program is specifically designed to tackle diseases resulting from genetic mutations, focusing on developing innovative genome-editing tools and therapies. The four-year grant aims to develop a method that can support the creation of personalized gene-editing therapies for a broad spectrum of rare genetic disorders.
Now entering its second phase, the program is looking to translate gene-editing advancements from the laboratory into clinical practice. During its initial phase, spanning 2018 to 2023, SCGE focused on developing the fundamental tools required for genome editing in non-reproductive cells in the body.
Rebecca Ahrens-Nicklas , an attending physician with the Metabolic Disease Program and the Division of Human Genetics at CHOP, told Penn Med that the program's scope is broader than focusing on one more specific disease.
"We’re focusing on the patient in front of us, whatever variant they have,” Ahrens-Nicklas said. “This approach enables us to treat a wider array of patients who’ve previously had no options.”
Having previously received a grant from the SCGE program, the team is now focused on advancing research toward clinical trials, which it hopes to begin within the next four years.
The Daily Pennsylvanian is an independent, student-run newspaper. Please consider making a donation to support the coverage that shapes the University. Your generosity ensures a future of strong journalism at Penn.
PennConnects
More like this.

HuidaGene Therapeutics Appoints TJ Cradick, PhD as Chief Technology Officer to Lead Delivery Science and Genome Editing Innovations
SHANGHAI and MIDDLETOWN, Del. , Oct. 21, 2024 /PRNewswire/ -- HuidaGene Therapeutics (“HuidaGene”), a global clinical-stage biotechnology company pioneering CRISPR-based programmable genome medicines, today announced the appointment of Dr. TJ Cradick as Chief Technology Officer. In this role, Dr. Cradick will further drive innovation and development of delivery vectors and gene editing tools through computational biology, artificial intelligence (AI), machine learning (ML), and other tools and methodologies.

A recognized leader in genome editing technologies, Dr. Cradick has made significant contributions to the development of nucleases and gene therapy methods, particularly for CRISPR/Cas nucleases, and before that, TAL Effector Nucleases (TALENs) and Zinc Finger Nucleases (ZFNs). His gene editing industry experiences began at Sangamo Therapeutics in 2000, with recent roles at CRISPR Therapeutics and Excision BioTherapeutics. When he was Chief Scientific Officer (CSO) at Excision, Dr. Cradick led the development of the first in-vivo CRISPR-based systemic treatment targeting latent HIV DNA reservoirs, currently under evaluation in clinical trials in the United States . Additionally, as Head of Genome Editing at CRISPR Therapeutics, he contributed to the approval of the groundbreaking therapy Casgevy™, the first-ever approved CRISPR-based treatment.
“We are excited to welcome TJ to the leadership team at this pivotal moment in our growth,” stated Alvin Luk , PhD., MBA, CCRA, co-founder and CEO of HuidaGene. “I have known TJ since before at UCSF, when we were colleagues. I have had the pleasure of working alongside Prof. Yang, who has been instrumental in developing our gene editing tools. TJ will now take the baton to optimize and enhance these tools to further advance our clinical programs. His vast expertise in gene editing will be key in advancing our mission to bring life-changing genomic medicines to patients worldwide.”
Prof. Hui Yang , co-founder and Chief Scientific Advisor at HuidaGene added, “I am delighted to welcome TJ to the team. His remarkable track record applying gene editing technologies to a range of diseases aligns perfectly with HuidaGene’s vision. I am confident that TJ’s leadership will further accelerate our efforts to develop cutting-edge tools and therapies that could transform the lives of patients around the world.”
Dr. Cradick’s achievements include developing gene editing assays, bioinformatics tools, and concepts that are widely used in genome editing research. He holds an undergraduate degree from MIT , an M.A. in Microbiology and Immunology from the University of California, San Francisco , and a PhD in Molecular and Cell Biology from the University of Iowa .
“I am thrilled to join HuidaGene at such an exciting time,” commented Dr. TJ Cradick. “The innovative work being done here has the potential to revolutionize how we approach gene editing. I look forward to collaborating with the talented team on new means to develop genomic medicines.”
About HuidaGene -
SOURCE HuidaGene Therapeutics

- Publications
Seminar on Precision Agriculture and Gene Editing Technology for Sustainable Food Production – Science and Communication
Selangor, Malaysia, & Biopolis, Singapore 18/09/2024 – 19/09/2024 9:00 am – 4:45 pm

Technological change is occurring at an unprecedented rate in the global agri-food value chain. New biotechnologies are a major part of this transformational change, including precision agriculture technology and new plant breeding techniques such as gene editing for targeted modification of plant genomes. In combination with innovative practices for monitoring and managing food and agricultural systems, they are opening up new pathways to delivering sustainable food production systems of the future.
It is, thus, important that key stakeholders along the value chain and regulatory authorities share the latest scientific knowledge on the
safety assessment, updates on status and advancement as well as regulatory approach towards potential adoption of these technologies. There is also increasing recognition of needs to engage and educate the broader community and consumers through innovative communication tools to impart knowledge and mitigate any misperception of these technologies.
This seminar to be held back-to-back in Singapore and Malaysia aims to bring together key stakeholders to share scientific updates and facilitate stakeholders’ engagement, addressing issues and perspectives on new biotechnologies, safety assessment, regulatory development and communication strategies.
Note: Topic and title of presentations are subject to change upon final confirmation with invited speakers.
ABOUT THE SPEAKERS
Dato' Dr Mohamad Zabawi bin Abdul Ghani, Director General, Malaysian Agricultural Research and Development Institute (MARDI), Malaysia
Mr Timothy Harrison, Regional Agricultural Counselor, USDA Foreign Agricultural Service (FAS)
Mr Geoffry Smith, ILSI SEA Region
Prof Wayne Allen Parrott, Professor, University of Georgia, USA
Mr Amin Asyraf Tamizi, Research Officer, Agri-Omics and Bioinformatics Programme, Biotechnology and Nanotechnology Research Centre, MARDI, Malaysia
Dr Kumitaa Theva Das, Senior Lecturer, Institute for Research in Molecular Medicine, Universiti Sains Malaysia, Penang, Malaysia
Dr Tan Yong Quan, Covering Specialist Team Lead, Bioengineering, National Centre for Food Science, Singapore Food Agency, Singapore
Dr Gabriel Romero, Executive Director, Philippines Seed Industry Association (PSIA), Philippines
Dr Mahaletchumy Arujanan, Executive Director, Malaysian Biotechnology Information Center (MABIC), Malaysia
Prof Dr Rofina Yasmin Othman, Honorary Professor, Institute for Advanced Studies, Universiti Malaya, Malaysia
Ms Li Xin Kang, Genetic Modification Advisory Committee (GMAC) Secretariat, Singapore
Prof Paul Teng, Dean and Managing Director, National Institute of Education International (NIEI), Nanyang Technology University, Singapore
Prof. Teng has won numerous awards for his work such as the Eriksson Prize in Plant Pathology (Royal Swedish Academy of Science), an Honorary Doctor of Science (from Murdoch University, Australia) and is a Fellow of the American Phytopathological Society, the International Society of Plant Pathology, and The World Academy of Sciences (TWAS). He is a Past-Chair, Genetic Modification Advisory Committee, Singapore and is also Immediate Past Chairman, International Service for the Acquisition of Agri-biotech Applications (ISAAA) Inc. He has edited/co-edited fifteen books, authored/co-authored four books and published over 250 technical papers. His latest edited book (2024) is “Food Security Issues in Asia”.
Assoc Prof Tan Meng How, Associate Professor, Nanyang Technological University (NTU) & Genetic Modification Advisory Committee (GMAC), Singapore
Dr Andrew D. Powell,CEO, Asia BioBusiness Pte. Ltd., Singapore
Ms Mazlina Banu Jaikubali, Genetic Modification Advisory Committee (GMAC) Secretariat, Singapore

CO-ORGANIZERS
If you have any inquiries, please do not hesitate to contact the following:
For Malaysia: Dr. Rogayah Sekeli and/or Dr. Zulkifli Ahmad Seman Malaysian Agricultural Research and Development Institute (MARDI) Email: [email protected] ; [email protected]
For Singapore: ILSI Southeast Asia Region Singapore Tel: 65 6352 5220 Email: [email protected]
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Designing Allele-Specific Competitive-Extension PCR-Based Assays for High-Throughput Genotyping and Gene Characterization
Ruslan kalendar, alexandr v shustov, ilyas akhmetollayev, ulykbek kairov.
- Author information
- Article notes
- Copyright and License information
Edited by: Pier Paolo Piccaluga , University of Bologna, Italy
Reviewed by: Alsamman M. Alsamman , Mohammed VI Polytechnic University, Morocco
Nosheen Masood , Fatima Jinnah Women University, Pakistan
*Correspondence: Ruslan Kalendar, [email protected]
ORCID: Ruslan Kalendar, orcid.org/0000-0003-3986-2460 ; Alexandr V. Shustov, orcid.org/0000-0001-9880-9382 ; Ilyas Akhmetollayev, orcid.org/0000-0002-6219-4002 ; Ulykbek Kairov, orcid.org/0000-0001-8511-8064
This article was submitted to Molecular Diagnostics and Therapeutics, a section of the journal Frontiers in Molecular Biosciences
Received 2021 Sep 10; Accepted 2022 Feb 2; Collection date 2022.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
Polymerase chain reaction (PCR) is a simple and rapid method that can detect nucleotide polymorphisms and sequence variation in basic research applications, agriculture, and medicine. Variants of PCR, collectively known as allele-specific PCR (AS-PCR), use a competitive reaction in the presence of allele-specific primers to preferentially amplify only certain alleles. This method, originally named by its developers as Kompetitive Allele Specific PCR (KASP), is an AS-PCR variant adapted for fluorescence-based detection of amplification results. We developed a bioinformatic tool for designing probe sequences for PCR-based genotyping assays. Probe sequences are designed in both directions, and both single nucleotide polymorphisms (SNPs) and insertion-deletions (InDels) may be targeted. In addition, the tool allows discrimination of up to four-allelic variants at a single SNP site. To increase both the reaction specificity and the discriminative power of SNP genotyping, each allele-specific primer is designed such that the penultimate base before the primer’s 3′ end base is positioned at the SNP site. The tool allows design of custom FRET cassette reporter systems for fluorescence-based assays. FastPCR is a user-friendly and powerful Java-based software that is freely available ( http://primerdigital.com/tools/ ). Using the FastPCR environment and the tool for designing AS-PCR provides unparalleled flexibility for developing genotyping assays and specific and sensitive diagnostic PCR-based tests, which translates into a greater likelihood of research success.
Keywords: genotyping assay design software, polymerase chain reaction-based markers, diagnostic system, genotyping system, single nucleotide polymorphism, insertion-deletion polymorphism
Introduction
High-throughput technologies for nucleotide sequence analysis and detection of sequence variation have been increasingly used for plant and animal genotyping, forensics, genetic medicine, and other fields of genetic testing. The most frequently encountered sequence variations in any genome are single-nucleotide polymorphisms (SNP) ( Clevenger et al., 2015 ). The primary consideration for selecting a marker type for genotyping is the information content of the polymorphisms and the informative capacity of the test. In this regard, SNPs are very effective markers. A variety of SNP genotyping methods are available, and new methods appear regularly with the aim of reducing cost and increasing throughput. PCR can be adapted for rapid detection of single-base changes in genomic DNA by using a family of closely related methods, such as allele-specific PCR (AS-PCR) ( Ugozzoli and Wallace, 1991 ; Bottema and Sommer, 1993 ; Spierings et al., 2006 ), PCR-amplification of specific alleles (PASA) ( Sommer et al., 1989 ; Sarkar et al., 1990 ), allele-specific amplification (ASA), and amplification refractory mutation system (ARMS) ( Newton et al., 1989 ; Nichols et al., 1989 ; Wu et al., 1989 ). All these methods use specifically designed PCR-primer sets containing allele-specific primers (ASP) in which the allele specificity is determined by the base at or near the 3′ end. In principle, AS-PCR assays can be developed to analyse almost any allelic variation ( Sommer et al., 1989 ; Ye et al., 1992 ).
There currently exists an enormous number and variety of established methods applicable for SNP analysis and genotyping based on distinctly different platforms and approaches ( Kim and Misra, 2007 ). FRET (Fluorescence Resonance Energy Transfer) is one such method and is based on dual fluorescence that is quantified during ligation. Another method is allele-specific PCR with SNP targeting ( Didenko, 2001 ; Kaur et al., 2020 ). Single-plex PCRs for SNP analysis, such as TaqMan (Life Technologies, USA) and SimpleProbe (Roche Applied Science, USA) have been described as valuable additions for marker-assisted selection in plant breeding ( Makhoul et al., 2020 ; Della Coletta et al., 2021 ). Further improvements for AS-PCR include using fluorescence to detect the amplification of a specific allele. Kompetitive Allele Specific PCR (KASP) (LGC Biosearch Technologies, Teddington, UK) and PCR Allele Competitive Extension (PACE) (Integrated DNA Technologies, Inc.) genotyping represent developments to the original AS-PCR approach. These techniques use fluorescence resonance energy transfer (FRET) for signal generation and both allow accurate bi-allelic discrimination of known SNPs and insertion-deletion polymorphisms (InDels). The KASP method has great potential for expanded utilization, as it requires only a slight modification to commonly used procedures for designing ASPs, offers convenient FRET detection, and master-mixes are commercially available ( Wang et al., 2015 ; Ryu et al., 2018 ; Brusa et al., 2021 ). In KASP, PCR amplification is performed using a pair of allele-specific (forward) primers and a single common (reverse) primer. In addition to the common-core primers, the reaction mixture is supplemented with a FRET cassette. This is a duplex of two synthetic complementary oligonucleotides; one is labelled with a fluorescent dye and the other carries a fluorescence quencher. Further, each ASP has a unique 5′ terminal extension (tail), which is complementary to the sequence in the FRET cassette. The oligonucleotides in the FRET cassette are modified such that they do not participate in polymerase-mediated extension steps. The dye-labelled oligonucleotide is capable of annealing to the reverse-complement of the tail sequence in PCR fragments containing one selected allele. During amplification of the allele with participation of the tailed ASP, an amount of DNA increases to which the dye-labelled component anneals. This disrupts the integrity of the FRET cassette and the fluorescent dye is spatially separated from the quencher and thus able to emit fluorescence. Unlike other PCR-based genotyping assays, KASP/PACE requires no labelling of the target-specific primers/probes, which provides additional flexibility in the assay design. Both methods use two reporting cassettes. If a genotype at a given SNP is homozygous, only one of the two possible fluorescent signals will be generated. If the genotype is heterozygous, both fluorescent signals will be generated. KASP/PACE technology is especially suitable for high-volume screening projects, such as in plant breeding. KASP/PACE technology has a key feature, which is utilizing a universal FRET cassette reporter system that eliminates the need for dual-labelled probes. Commercial companies produce PCR additives, called master mix, which contains one or more ‘universal’ FRET cassette(s). In theory, this additive can be used to upgrade existing AS-PCR assays to KASP/PACE, provided that new ASPs are used that have the described cassette-specific tails. For example, a protocol upon which this work is based, uses chemistry consisting of the following two parts. These are the assay mix (template-specific, contains target DNA, two ASPs [for binary SNP] and a single reverse primer) and master mix (not template-specific, combines all reagents required for PCR and in addition contains two different FRET cassettes, one cassette labelled with FAM dye and the other with HEX dye). Each ASP carries a unique tail sequence that corresponds to a FRET cassette. In other FRET AS-PCR methods, such as Amplifluor ( Rickert et al., 2004 ; Fuhrman et al., 2008 ) and STARP ( Rasheed et al., 2016 ; Long et al., 2017 ; Li et al., 2019 ; Wu et al., 2020 ), these two parts are completely separated into non-labelled ASPs such that the last base of the primer’s 3′ end base is positioned on the SNP site, and labelled universal probes (UPs) that carries a fluorophore at the 5′ terminus and a quencher attached in the middle of the universal probes ( Nazarenko et al., 1997 ). Simple and inexpensive ASPs can be designed and ordered for each SNP separately, while the relatively expensive UPs with fluorophores and quenchers are ordered just once for a stock that can be used over a very long time in many different SNP analyses. The principle of ASP-UP is similar to that of Molecular Beacons, with the addition of specialized identical “tags” at the 5′ end of the ASP and the 3′ end of the UP. In this particular case, ASPs are slightly longer ( Myakishev et al., 2001 ). In KASP-related methods, a set of non-labelled ASPs includes two forward primers and a single common reverse primer that act on a competitive basis in conjunction with one of two corresponding UPs with “hair-pin” FRET structures that end with either FAM or HEX/VIC fluorophores. This approach allows for great flexibility in assay design, which translates into a higher overall success rate for SNP genotyping and detection of InDels. This principle of separated ASPs and UPs is used in various methods, including commercially produced Amplifluor (Merck KGaA) and KASP markers (LGC Biosearch Technologies) for fluorescent signal generation that enable bi-allelic discrimination and genotyping of SNPs or InDels.
Developing high-throughput, multiplex genotyping assays require using computerized approaches to design primers and probes and select reaction conditions. In recent years, several software tools have been developed to aid AS-PCR assay development, including GSP ( Wang et al., 2016 ), PrimerSearch-EMBOSS ( http://emboss.open-bio.org/rel/rel6/apps/primersearch.html ), WASP ( Wangkumhang et al., 2007 ), PolyMarker ( Ramirez-Gonzalez et al., 2015 ), KASPspoon ( Alsamman et al., 2019 ), and PUNS ( Boutros and Okey, 2004 ). However, not all of these programs are readily available or broadly applicable; some are no longer actively updated whilst others are extremely narrow in their applicability.
Here we describe a software tool and a modified KASP method that further increases the convenience and power of the genotyping protocol. We have named this allele-specific quantitative PCR (ASQ). The software facilitates the development of assays using KASP and PACE technology and works with SNP and InDel polymorphisms. For all polymorphisms taken into the study, the program produces the thermodynamically optimal combination of ASPs for single-plex or multiplex assays. During ASP design, a user can add a tail sequence (which can be a custom sequence) or a sequence that matches a commercially available aster mix (e.g., manufactured by LGC Biosearch Technologies, or Merck KGaA). If a commercial FRET cassette is not optimal, the program allows use of custom FRET cassettes. The program also computes the optimal reaction conditions to perform KASP. This software will be most useful for multiplex genotyping in a high-performance environment. Furthermore, potential uses are not limited to genotyping. The program can produce tests for selecting mutants, e.g., after genome editing or gene knockout ( Lee et al., 2016 ). Other possible applications include analysis of genetic variations in microorganisms, strain identification, detecting genetic markers of resistance or virulence, and tracing pathogens in epidemiology and medical diagnostics. The ASQ method described here is a tool for assessing the relative amount of different allelic variants in a sample. This method can be useful for a variety of tasks. For example, ASQ can be used to measure a fraction of admixed GMO material in samples of agricultural products or to measure a portion of malignant cells in samples from cancer patients. Distinctive features of the presented program make it unique in its class. These features include computing of multiplex reactions for simultaneous identification of up to four alleles (e.g., 3-state or 4-state polymorphisms); use of input polymorphisms of mixed type (overlapping SNP and InDel sites); and absence of restrictions on the length difference between InDel alleles during genotyping of InDels. The program automatically calculates primers for possible amplification in both directions from the polymorphic site. The program allows the user to include in the protocol commercially available master mixes and FRET cassettes or to create unique FRET cassettes and use custom reagents in designing other assays. The software described herein is integrated into and works inside the FastPCR environment. FastPCR is an integrated software space for developing PCR-based processes. The FastPCR program, including the presented AS-PCR-designing tool, may be used online or downloaded and used in Microsoft Windows ( Kalendar et al., 2011 ; Kalendar et al., 2017 ) and is freely available at http://primerdigital.com/tools/ . This AS-PCR tool simplifies and automates design of genotyping assays, resulting in a greater likelihood of success.
Materials and Methods
Selection of highly informative snp candidates.
SNP data in the SNPforID browser ( http://spsmart.cesga.es/snpforid.php ) were employed in the present study for SNP genotyping of humans in forensic studies. The allelic frequencies were used for screening to select highly informative SNP candidates. As markers with even allelic distributions have high observed heterozygosity and are more informative, 7 of the SNPs were selected with a common 40:60-60:40 allelic distribution in European and Central Asian populations. All of the selected markers are located on autosomal chromosomes ( Supplementary Table S1 ). The marker also known as “C/T(-13910)” located in the MCM6 gene but with influence on the lactase LCT gene (rs4988235) is one of two SNPs that is associated with the primary haplotype associated with hypolactasia, or more commonly known as lactose intolerance in European populations.
Sample Preparation
This work was discussed by the institutional review board and was approved by the ethical committee of the Center for Life Sciences, National Laboratory Astana, Nazarbayev University (protocol #21, 10 October 2017). Institutional written informed consent about nationality declaring, DNA extraction and for further investigation was signed and obtained from the participated individuals.
Informed consent was obtained for each participant. DNA was extracted using QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany). The extracted DNA pellet was diluted in TE buffer (10 mM Tris pH 8.0, 0.1 mM EDTA), and DNA concentration was measured with a NanoDrop spectrophotometer (Thermo Fisher Scientific, USA). Quality was assessed using 1 mg of DNA visualised after running in a 1% agarose gel.
Real-Time PCR Analysis
A QuantStudio-7 Real-Time PCR instrument (Thermo Fisher Scientific, USA) and CFX96 Real-Time PCR Detection System (Bio-Rad, USA) were used. These instruments have detection systems with filters for FAM, VIC/HEX, Cy3, Cy5, and ROX fluorophores. While SNP identity calls were made automatically using software accompanying the instruments, amplification curves were checked for each genotype manually for final allele discrimination. SNP genotyping experiments used at least three to eight biological replicates and were repeated three times.
The PCR conditions employed an altered PCR cocktail composition ( Table 1 ). PCR plates with 96-wells were used with a 15 μl total reaction volume in each well. The PCR mix consisted of the following reagents: 1x OneTaq buffer (total 3 mM MgCl 2 ), 0.2 mM of each dNTP, 0.2 μM of each UP, 0.5 μM quencher oligo, 0.1 μM of each AS primer, 0.3 μM of reverse primer, and 0.5 units of Taq DNA polymerase (NEB). Half of the PCR volume was genomic DNA, adjusted to 5 ng/μl.
PCR cocktail mix composition for the proposed ASQ method of SNP genotyping.
| Component | Concentration | Volume (µl) | Final concentration |
|---|---|---|---|
| 5x OneTaq Buffer (with 9 mM MgCl ) | 5× | 20 | 1× |
| MgCl | 25 mM | 4.8 | 1.2 mM |
| DNTP | 10 mM | 2 | 0.2 mM |
| ASP-F1 | 5 µM | 2 | 0.1 µM |
| ASP-F2 | 5 µM | 2 | 0.1 µM |
| ASP-R | 5 µM | 6 | 0.3 µM |
| UP-FAM | 10 µM | 2 | 0.2 µM |
| UP-HEX | 10 µM | 2 | 0.2 µM |
| Uni-Q | 50 µM | 1 | 0.5 µM |
| Taq DNA Polymerase | 5 units/µl | 0.8 | 0.04 units/µl |
| Milli-Q water | 37.4 | ||
| DNA template | 10 ng/μl | 20 | 2 ng/μl |
| Total | 100 |
The PCR program was optimised and consisted of 95°C, 120 s; 10 cycles of 10 s at 95°C; 20 s at 55°C; 20 s at 68°C; 30 cycles of 10 s at 95°C; 30 s at 68°C; 30 s at 55°C ( Table 2 ). Fluorescence was monitored during the last step at a second annealing. ASQ was performed to simultaneously detect two alleles in a single tube. Each well was examined for the characteristic fluorescent emissions of both fluorescein (FAM channel) and HEX (HEX channel).
PCR protocol for genotyping using advanced ASQ method for SNP genotyping.
| Step | Temperature | Duration (sec) | Notes |
|---|---|---|---|
| 1 | 95°C | 120 | Initial denaturation |
| 2 | 95°C | 10 | First-round denaturation |
| 3 | 55°C | 20 | First-round annealing |
| 4 | 68°C | 20 | First-round extension |
| 5 | 10 cycles repeat for steps 2–4 | ||
| 6 | 95°C | 10 | Second-round denaturation |
| 7 | 68C | 30 | Second-round annealing and extension |
| 8 | 55°C | 30 | FRET cassette annealing and signal read |
| 9 | 30 cycles repeat for steps 6–8 |
Genotyping with SNP calling was determined automatically. Each experiment was repeated twice and technical replicates confirmed a confidence of SNP calls.
KASP Assay Design Method
Standard KASP allows genotyping of only two alternative alleles at any specific site. This is because there are two only FRET cassettes, and thus two fluorochromes, present in commercially available kits. However, advances in real-time PCR equipment allow detection of up to 6 spectrally separated dyes.
In addition, there are approaches to overcome physical spectral dye channels and expand the potential of spectral channels to practically detect an unlimited number of independent targets ( Marras et al., 2019 ). Theoretically, the number of different multiplex targets or alternative alleles that can be identified in a screening assay can be increased significantly by utilizing a unique combination of two colours for the identification of each target allele ( Marras et al., 2019 ). The novel tool described here allow detection of up to 4 spectrally separated dyes ( Figure 1 ). Moreover, the program calculates even more assay possibilities because the ASPs are searched for on both strands and at both sides from a polymorphism site ( Figure 2 ). The user has the possibility to use the single most suitable set of primers from the output or, if desired, alternative sets of primers may be used to target the same site. The tool also allows design combinations of tail sequences to create a multiplex PCR targeting several different polymorphic sites simultaneously. There is a need to incorporate flexibility in a primer-designing algorithm to select AS primers, as placing the 3′-terminal nucleotide on the polymorphic site may not be an optimal solution for some sites. Briefly, to achieve optimal amplification, the tool automatically selects the primer length and the position of its 3′ terminus for each ASP. This kind of optimization is also necessary when working with InDels. For example, for a null-site polymorphism (i.e., one or more nucleotide[s] absent in one allele or there are insertions in other alleles), the ASP sequence to target this allele will be positioned such that the primer’s 3′-terminal sequences are sufficient for annealing. Thus, ASP sequences are properly adjusted for each allelic variant. In case of short InDels (<4 nucleotides), the better solution is to individually design the sequence of the ASP 3′ termini for each variant, which can be achieved with this tool. Thus, the algorithm allows the generation of ASPs with 3′-terminal sequences that are unique for each allelic variant. This tool works within the FastPCR environment, which contains numerous functions that are necessary for the development of PCR primers for most applications. These include complex PCR designs, such as multiplex PCR or Loop-mediated Isothermal Amplification (LAMP). Regardless of the intended application, PCR primer design using FastPCR’s algorithms attempts to generate sets of primers with a high likelihood of success. For this purpose, there is an automatic check for unwanted binding sites (non-specific priming control) in input sequences to prevent the generation of side PCR products. Inputs may be linear or circular sequences. Moreover, the FastPCR environment is expandable, allowing addition of specialized functions for specific tasks.
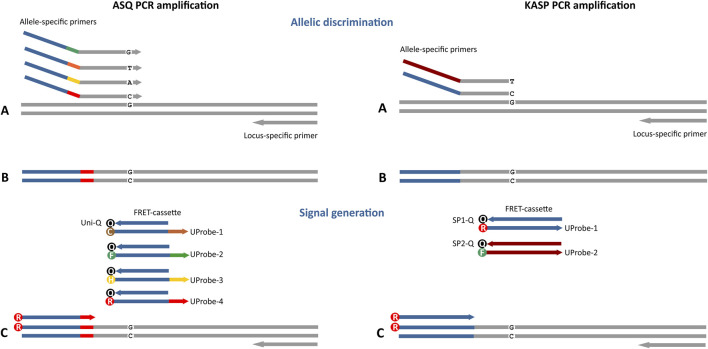
Four-plex fluorescent ASQ assay genotyping system compared to the standard two-plex KASP technology (LGC Biosearch Technologies). The main differences in these approaches are associated with the potential number of simultaneously detectable polymorphic sites (4 in ASQ, 2 in KASP) and the structure of the primers that compose the FRET cassette. For ASQ, the FRET cassette consists of 2 or more of allele-specific primers (ASP) and a fluorescently labelled universal probe (UP) with a single universal quencher probe (Uni-Q). Differences in the tail sequence ASPs and UPs are determined by a unique 6-nt barcode sequence that is not part of the universal tail of the Uni-Q sequence. KASP technology includes 2 variants of ASPs and fluorescently labelled UPs (UP-1/2) with each UP requiring a specific quencher (SP1/2-Q). In addition, the ability to design an allele-specific primer with the SNP site at the penultimate or antepenultimate 3′ base of each allele-specific primer is characteristic of the ASQ method. (A) Both allele-specific primers query the SNP locus. Denaturation of DNA template and annealing ASP to the target, PCR round 1. (B) Formation of a PCR product containing a specific tail sequence that is complementary to allele-specific primers. This PCR product will be used in subsequent PCR cycles as a template for amplification using a specific fluorescently labelled UP (C) . During the first two amplification cycles, a tail sequence is incorporated into the amplicon that is subsequently recognized by a universal, probe-based reporter system.
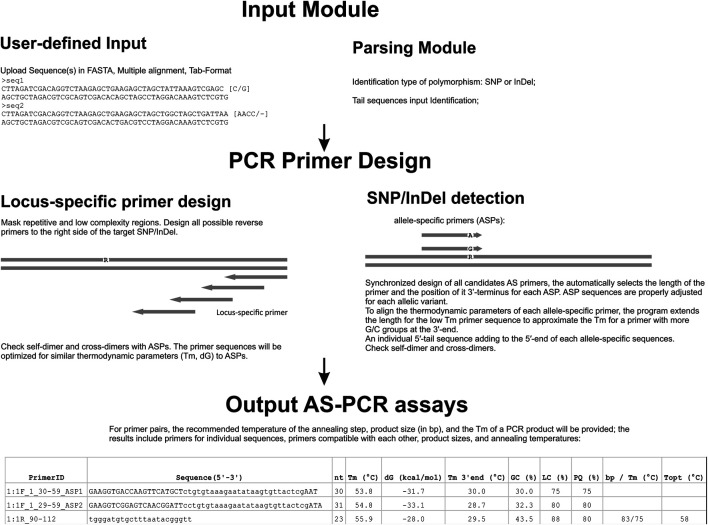
Flowchart of the main steps in the AS-PCR process. Data can be uploaded into FastPCR, which accepts a single sequence or multiple separate DNA sequences in FASTA, tabulated format, multiple alignment, or from clipboard. SNP sequence data are pre-processed by FastPCR prior to execution to collect the SNP position and also to remove non-DNA symbols from the entire sequence. The user sets the various parameters for primer design for the DNA or SNP sequences, such as primer maximum and minimum lengths and T m . Repetitive or low-complexity sequences are excluded from primer design by default (this can be changed by the user). The initial primer design attempts both directions and a reverse primer is designed at the upstream accordingly. An allele-specific primer with the SNP site is designed at the second (penultimate) position of the primer’s 3′ terminus or to any other position in manual or automatic mode. Once the allele-specific primers are designed, an individual 5′-tail sequence is added to the 5′ end of each allele-specific sequence. This combined whole primer sequence is reanalysed for self- and cross-dimerization. Finally, results are returned to the text-editor window.
Components of FastPCR are programmed in Java and thus the program is intrinsically multiplatform. Entering inputs and execution and post-processing of the output can be performed with ease. The selection of the optimal target for KASP primer sets is performed in the same way as for PCR primers. The thermodynamic stability of hairpins is evaluated primarily for the 3′-terminal sequence and, if desired, for the primer’s 5′ terminus as well. The user may select thermodynamic options that will be used to design primer sets (within different templates) or individually set the options for each template in a set of several ones. The program calculates several primer pairs or sets from which the user may choose the best. The user can request a desired product size or instruct the software to search for primer pairs for diagnostic PCR, in which case a whole input sequence will be used to search for the best primer pair. By default, the program selects primers sets with compatible melting temperatures. Alternatively, the user may choose primer sets with similar Gibbs free energy (dG). The tool checks for self-dimer and cross-dimer interactions for all primers. Additionally, users may design compatible primer sets for a predetermined primer (probe) or a list of predetermined primers (probes). This feature can be useful when designing a multiplex assay for different targets. FastPCR allows avoiding non-specific amplification by choosing the best paired primer for a given oligonucleotide to achieve the highest likelihood of success.
To this end, the program chooses primers that avoid repeats or other stable motifs, like potential G-quadruplex sequences. Results with program-suggested primers and primer sets may be exported in common exchange formats (e.g., MS Excel). The following parameters may be placed into spreadsheets: primer name, sequence, location on target, sequence direction, length, melting temperature, GC content, molecular weight, molar extinction coefficient, linguistic complexity (LC), and primer quality (PQ).
For primer pairs, the recommended temperature of the annealing step, product size (in bp), and the T m of a PCR product will be provided. For pairs, the results include primers for individual sequences, primers compatible with each other, product sizes, and annealing temperatures. For all selected primer pairs, the program provides (in tabular form) the compatibility of the two primers in one reaction, including primer-dimers, cross-hybridization, and product size overlaps ( Figure 2 ).
Users will find examples for entering sequences encoding polymorphic sites in the File menu of the FastPCR software. Input sequences can be in FASTA, in tabular formats, or as multiple sequence alignment as given in the examples. Square brackets are used to specify borders of a polymorphic site of interest. The alternative alleles of a SNP are indicated by brackets, e.g., [C/G] (alternative way to code—[S]). Individual allelic variants in a polymorphic site are thus listed as [variant1/variant2] for binary polymorphism or [variant1/variant2/variant3/variant4] for quaternary polymorphism. Alternatively, IUPAC notation can be used for a degenerate base (e.g., FASTA format) ( Supplementary material ). When a polymorphic site contains InDels, the same format with square brackets ([variant1/variant2/variant3/variant4]; two or more different variants per site) is used to describe individual variants. Only differences between the variants must be shown within the brackets ( Supplementary material ). The program allows primers to be designed with the SNP placed at any position with respect to the 3’ end. To do this, the user must specify the desired SNP position using the command with the position number. By default, the program automatically specifies the SNP position at a penultimate base (-aspcr). In addition, for InDel sites, the number of discreditable nucleotides at the 3′ end of each ASP can be automatically determined depending on the polymorphism variant. For InDels, the potential of 3′-end generation for each ASP design option is wider, thus offering the possibility of a more effective discrimination of the various alleles. The program will also determine the minimum length of the 3′ end of each ASP for effective detection of all polymorphisms. The user can define the position of the polymorphic site in any of the positions, such as at a first or penultimate or another base in 3′ end of each ASP to increase reaction specificity and allele discrimination. The 3′-end sequence of each ASP should be designed such that each ASP is unique and specific only to its target. The program uses the sequence from the insertion to generate the 3′-end of ASP sequence for a null-site polymorphism. In the case of a single- or two-base InDel, the following sequence after target site will be used to generate the 3′ end of each ASP. The maximum length that the program can use to develop unique 3′ ends of ASP sequences in the program is 12 nucleotides. This should be sufficient for any InDel polymorphism to generate unique sequences for all 3′ ends of ASPs. The primer sequences will be optimized for similar thermodynamic parameters ( T m , dG) ( Peyret et al., 1999 ; Bommarito et al., 2000 ). To align the thermodynamic parameters of each ASP, the program extends the length for the low- T m primer sequence to approximate the T m for a primer with more G/C groups at the 3′ end.
Design of Universal Probes and Allele-specific Primers and Locus-specific Common Primers
In our experiments, we used four UPs with types of specific tails. Sequences of the four UPs are presented in Table 3 ; an example of one such ASP for the human SNPs is shown. All SNP-specific primers and UPs labelled with FAM, HEX, Cy3, and Cy5 were synthesized locally (National Center for Biotechnology, Nur-Sultan, Kazakhstan). A stock of each probe and a single quencher (Uni-Q) was prepared by dissolving each in TE buffer. A working stock of all four probes and a quencher was used for assays. A Uni-Q was conjugated with BHQ1 at the 3′ end. The sequence of Uni-Q was complementary to all corresponding probes. The quenchers were shorter in nucleotide length (13 nt) than the UPs (19 nt). The T m of this quencher oligonucleotide and the complementary probe was 55°C (for oligonucleotide concentration of 500 nM in 55 mM KCl with 2.2 mM Mg 2+ (3 mM Mg 2+ minus 0.8 mM dNTP) ( Owczarzy et al., 2008 ) as calculated by FastPCR ( Kalendar et al., 2017 ). The UPs were designed to have a higher T m (66–68°C). At an annealing-extension temperature of 60–68°C, the AS primers and UPs can bind the target and induce polymerization without much interference from the lower T m of the quencher oligo. When the temperature is subsequently decreased to 55°C, the Uni-Q binds the tail of the free, single-stranded UPs, but not the double-stranded PCR product. Because the Uni-Q concentration is two- to four-fold higher than that of each UP, most of the free UPs are expected to bind the quencher oligo at 55°C, thus strongly quenching the UP fluorescence. Since the 5′ end of the UP tail is opposite to the 3′ end of the quencher oligo, the interaction is mediated via direct contact-quenching between the 5′ fluorophore and the 3′ quencher present on the tail and quencher oligo, respectively, which for most fluorophores provides stronger quenching than fluorescence resonance energy transfer (FRET) ( Fiandaca et al., 2001 ; Li et al., 2006 ). The 3′ BHQ1 was used as it has a wide range of absorbance wavelengths and is appropriate for quenching multiple fluorophores simultaneously, including the FAM and Cy5 used for multiplex PCR.
Fluorescent probes (UP) and a quencher oligonucleotide (Uni-Q) for Allele-Specific Quantitative PCR (ASQ).
| Primer ID | Sequence (5′-3′) | nt | (°C) | dG (kcal/mole) | GC (%) | LC (%) | Type and fluorescent label |
|---|---|---|---|---|---|---|---|
| Uni-Q | Accgttcagctgg | 13 | 53.3 | −16.7 | 61.5 | 100 | Universal 3′ Quencher (Eclipse Quencher or 3′ Black Hole Quencher 1) |
| UP1 | ccagctgaacggtACGGCA | 19 | 67.4 | −26.4 | 63.2 | 86 | UP1: 5′-FAM |
| UP2 | ccagctgaacggtCGTTGC | 19 | 66.5 | −26.4 | 63.2 | 95 | UP2: 5′-HEX/JOE/VIC |
| UP3 | ccagctgaacggtAGCCGA | 19 | 66.9 | −26.1 | 63.2 | 89 | UP3: 5′-Cy3/TAMRA |
| UP4 | ccagctgaacggtGCGTCA | 19 | 67.9 | −26.8 | 63.2 | 92 | UP4: 5′-Cy5/Liz |
Capital letters used for a unique barcode sequence; underlined letters used for the universal tail in UPs, and Uni-Q.
Melting temperature ( T m ) calculated for oligonucleotide concentration of 200 nM in 55 mM KCl with 2.2 mM Mg 2+ .
Linguistic Complexity (%).
The SNP site in the AS primers of each locus were designed to directly flank the site in both the sense and antisense orientation. The position of the polymorphic site was located in the penultimate base in the 3′ end of each ASP. Amplification primers were designed such that the amplicons ranged in size from 100 to 200 bases and encompassed the SNP site.
The remaining allele-specific region of ASPs was selected to obtain a T m close to 63°C (range 62–64°C, calculated for oligonucleotide concentration of 200 nM, 55 mM K + , in the present of 2.2 mM Mg 2+ using FastPCR software) ( Kalendar et al., 2014 ). The initial primer design was first attempted for the current strain and then a complement strain direction was attempted and a reverse primer was designed at the upstream accordingly. Once the allele-specific primers were designed, an individual 5′-tail sequence was added to the 5′ end of each allele-specific sequence. This combined whole primer sequence was reanalysed for self- and cross-dimerization.
AS-PCR Analysis
The validation of the proposed ASQ method was performed using SNP for genotyping humans. We designed eight ASQ sets for informative SNP candidates that could be used for genotyping humans and tested their genome specificity using PCR assays. Validation tests were performed for ASQ with quantitative PCR-based experimentation. The corresponding primer sequences are listed in Supplementary Table S1 . PCR reactions using primers designed by our tool were performed and the resulting products are presented in Figure 3 . In each case, bands of the correct size were obtained from each PCR. These ASQ sets showed genome specificity and identified SNP alleles in the human genome ( Supplementary Table S1 ). SNP allele discrimination for 16 human genotypes is presented in Figure 3 . These results for SNP for genotyping of humans validated our conclusion that the proposed ASQ method is very accurate and effective for SNP genotyping; we expect similar results in other animal species. In addition, we developed ASQ sets and applied SNP genotyping in the barley genes HvSAP16 and HvSAP8 (controlling stress-associated proteins); these are validated examples and have been accepted for publication ( Baidyussen et al., 2021 ; Kalendar et al., 2022 ). Our tool and the ASQ method are suitable for two-, three-, or four-allelic uniplex reactions but can potentially be used for different SNPs in a multiplex format in a range of applications, including medical or forensic studies or other studies involving SNP genotyping.
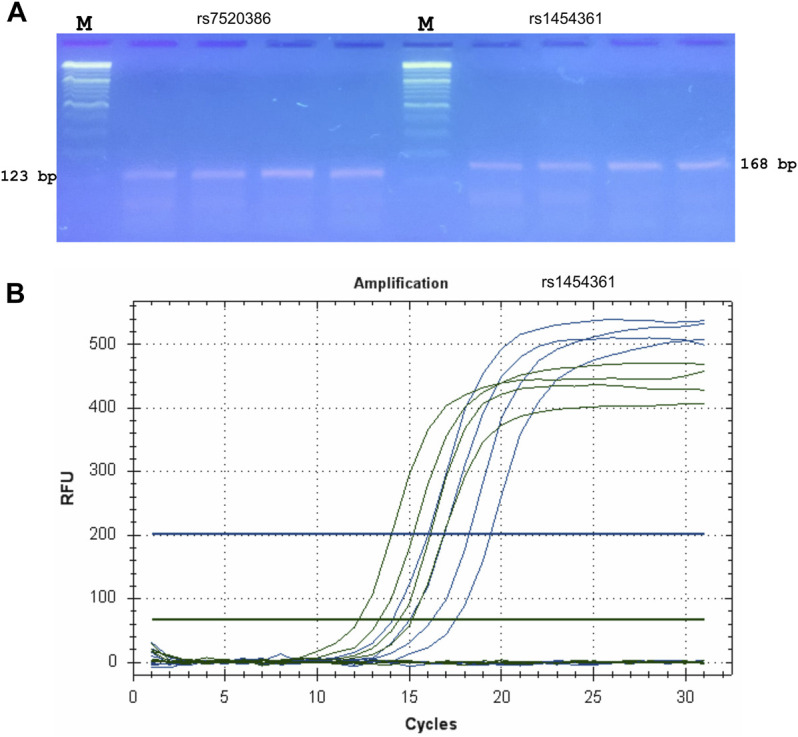
Validation and testing of ASQ method. Probes for ASQ assays were designed using FastPCR to human SNPs (rs7520386 and rs1454361) that were employed for SNP genotyping of humans in forensic studies and PCR products from a series of PCR reactions using these primers were examined by the agarose gel electrophoresis was used without staining (A) . M—Thermo Scientific GeneRuler DNA Ladder Mix (100–10,000 bp) stained with SYBR Green I. Primer sequences are displayed in Supplementary Table S1 . PCR bands of the correct size (123 bp for rs7520386 and 168 bp for rs1454361, respectively) were obtained from each qPCR. (B) qPCR amplification plot for SNP (rs1454361). FAM plot (green) shows amplifications of a A-allele, whereas HEX plot (blue) shows amplifications of a T-allele.
Comparison of KASP Assay Design Tool to Existing Software
We compared the functionality of the tool described here with other web-based software available online. Only software programs capable of AS-PCR designs were compared ( Table 4 ). One example of an AS-PCR-directed program is WASP ( https://bioinfo.biotec.or.th/WASP ) ( Wangkumhang et al., 2007 ). We used example allele sequences published on the WASP website to produce ASPs (primers were computed with WASP and with the KASP/PACE tool) ( Supplementary Data S2 ). It appeared that the primer design in WASP has some limitations, e.g., WASP does not allow addition of user-defined 5′ tail sequences to ASPs. Additionally, WASP searches for ASP binding sites from only one side (upstream) of a polymorphism site. Furthermore, it appeared that when attempting to target an ASP on an SNP site, WASP places an additional deliberate mismatch at the penultimate base of AS primers. This peculiarity in the primer-design algorithm was proposed by WASP developers as they sought to increase specificity of allele discrimination. However, using ASPs with deliberate mismatches in the 3′ terminal region is controversial, at least because such mismatches are known to decrease overall PCR efficiency and may lead to complete PCR failure.
Comparison between the KASP tool (in the FastPCR suite) and other AS-PCR programs (web-based).
| Feature | WASP | PolyMarker | FastPCR |
|---|---|---|---|
| Web site | |||
| Platform | Web server | Web server | Java Web Start (Oracle) |
| Primer-designing algorithm | Primer3 | Primer3 | FastPCR |
| Detection limit of SNP/InDel alleles | 2 | 2 | 2-(4)-any |
| SNP genotyping | yes | yes | yes |
| InDel genotyping | no | no | yes |
| Primer-binding site selection to one side or both sides from the polymorphic site | One side | One side | Both sides |
| Variable base positioning in ASPs (for SNP targeting) related to the primer’s 3′ terminus | At the first base, an additional deliberate mismatch is introduced at the penultimate base | At the first base | At the first, second, or third base, and automatic selection based on thermodynamic calculations |
| Uses multiple sequence alignment of alleles as input | no | yes | yes |
| Allows user-defined 5′ tails in ASPs | no | no | yes |
| Multiplex reaction design | no | no | yes |
| Inclusion of commercial (e.g. LCG or Merck KGaA) or custom FRET cassette | no | no | yes |
| Analysis for primer self-dimers and cross-dimers in all multiplexed primer sets | no | no | yes |
| /dG selection for thermodynamic comparisons | no | no | yes |
| Probe design (TaqMan, MGB) | no | no | yes |
| Identification of low- and high-complexity sequences with automatic adjustment of primer positions | no | no | yes |
| BLAST test | no | yes | no |
| Ability to automatically adjust algorithm to the sequence complexity of an input template | no | no | yes |
| Potential for wide range applications beyond genotyping (such as diagnostics, quantitation of alleles) | no | no | yes |
In contrast, when targeting SNPs our AS-PCR tool automatically places a variable base at the second (penultimate) position of the primer’s 3′ terminus. The selection of an exact position (of the variable base) is driven by thermodynamic considerations. In our opinion, the latter solution provides greater flexibility in equilibrating thermodynamic parameters for all ASPs targeting existing alleles. In this regard, it was published that 3′-terminal and internal oligonucleotide mismatches differ on the effect on duplex stability. Mismatches at the oligonucleotide’s very 3′ terminus actually stabilize its duplex with a template, whereas internal mismatches can be either stabilizing or destabilizing. A destabilizing effect for internal mismatches occurs when there are unfavourable constraints on the geometry of hydrogen bonding in a DNA duplex ( Santalucia and Hicks, 2004 ). In general, the destabilizing effect is more pronounced for mismatches in the penultimate or third position (at the 3′ terminus) and when A or T is a terminal base. These considerations work in our AS-PCR algorithm in a way that the tool generates various candidate ASPs that carry one intended mismatch at different positions and compares their thermodynamic stability. As a result, the program automatically shifts the 3′ terminus of the ASP to produce the best performing ASP. The best performing ASP does not decrease the efficiency of amplification. In our experience, the described approach increases the specificity of allele detection. Importantly, ASPs designed with our AS-PCR tool can be used in PCR driven by either proofreading or non-proofreading DNA polymerases. In our experience, proofreading DNA polymerases (e.g., brands Phire, Phusion from Thermo Scientific) also produce better results when DNA sources for genotyping are not of the highest quality (direct PCR protocol).
A program capable of designing primers for KASP is PolyMarker ( http://www.polymarker.info ) ( Ramirez-Gonzalez et al., 2015 ). We used example template sequences published on the PolyMarker website to produce primers using PolyMarker and our AS-PCR tool. However, for two example sequences (Cadenza1697_chr1A_12142209 1A; BA00343846 5A), PolyMarker did not perform the expected KASP analysis, as it identified two copies of the target region in the hexaploid wheat genome. In contrast, our AS-PCR tool does not impose similar restrictions because our tool is not only able to check for repeats but can also position ASPs in non-repeated regions. Thus, our AS-PCR tool is more suitable for work with polyploid genomes. When our AS-PCR tool analysed a template in the example above, the tool found a non-repeated region at one side of a polymorphic site of interest and generated ASPs. With two different example templates (BA00591935 3B; BA00122841 7D), PolyMarker and our AS-PCR design tool generated ASPs of the same sequences. However, the computed common reverse primers were different. Our AS-PCR tool’s output with the primers’ T m illustrates that this program can design reverse primers with thermodynamic properties very similar to ASPs.
The other differences between these programs (WASP, PolyMarker) favour our AS-PCR design tool, as both programs do not allow for addition of predefined 5′ tails to ASPs and do not check primer thermodynamic compatibility if the ASPs have 5′ tails. PolyMarker searches for primer-binding sites at only one side of a polymorphic site, whereas our tool searches in both directions.
It should be mentioned that the primer-design algorithm in software from other developers (WASP and PolyMarker) is significantly different from ours. This difference becomes evident when a sequence surrounding a polymorphic site has low (linguistic) complexity, as in cases of perfect or imperfect microsatellites (simple sequence repeats, SSRs) or G-rich sequences capable of G/C-quadruplex formation. The algorithm in software from other groups ignores the linguistic complexity and sometimes target primers on sequences with low linguistic complexity. During PCR, such primers will generate unexpected amplifications and may hamper identification of specific alleles ( Kalendar, 2022 ).
An important condition for successful PCR is that all primers in one multiplex reaction must have similar thermodynamic properties ( T m , dG) and be compatible in terms of possible primer-dimers. Our AS-PCR tool is arguably the most sophisticated instrument in these regards, as the tool automatically checks the compatibility of all primers in a multiplex design.
Thus, the main differences between these programs (WASP, PolyMarker) and our AS-PCR tool are that probe sequences are designed in both directions and both SNPs and InDels may be targeted. Our tool automatically places a variable base at the second (penultimate) position of the primer’s 3′ terminus or to any other position in manual or automatic mode. In addition, our tool allows discrimination of up to four-allelic variants at a single SNP site. Finally, our tool allows design of custom FRET cassette reporter systems for fluorescence-based assays.
Allele-specific qPCR and AS-PCR Tool Validation
We propose a modification to the KASP method to an improved and universal ‘Allele-specific qPCR’ (ASQ) for designing target-specific primers for KASP genotyping assays. When compared with KASP, this proposed ASQ method also contains two separate components: an allele-specific part (two or more AS primers targeting the SNP/InDel with identity in the penultimate 3′ nucleotide and specific 5′ tags) and a universal part. There are two or more universal probes (UP-1-4) with corresponding tags and different fluorescent dyes at the 5′ end and a single common UP with a quencher at the 3′ end (Uni-Q) ( Table 3 ). A single common UP Uni-Q at the 3′ terminus carries a universal 3′ quencher (Eclipse with quenching range 390–625 nm; Black Hole Quencher [BHQ1] with quenching range 450–580 nm, Black Hole Quencher [BHQ2] with quenching range 520–650 nm, or Tide Quencher 3 [TQ3] with quenching range 510–620 nm) that effectively quenches a broad range of fluorescent dyes for most fluorescently labelled UPs. The identical sequence of the 13-bp tag-fragment in each UP fully complements those in the Uni-Q. Differences in the tail sequences are determined by short barcode 6-nt sequences that are not part of the universal tag sequence and are located between it and ASP sequence ( Table 3 ). The proposed modified KASP method based on FRET is suitable for single- or multiplex applications and can be used in various approaches for SNP/InDel genotyping ( Figure 1 ). During thermal cycling, the relevant ASP binds to a template and elongates, thus attaching its tail sequence to a newly synthesized strand. The complement of the allele-specific tail sequence is generated during subsequent rounds of PCR, enabling the dye-containing component in a FRET cassette to bind to DNA. Through binding, the dye is no longer quenched and emits fluorescence. Separating of the reactions into template-specific and not template-specific parts allows screening of multiple templates in one run. ASQ protocols use FRET master mixes for generating fluorescent signals with different templates in development of various genotyping assays.
The wide use of sequencing has shown that sequence variations (SNPs and InDels) between individuals of a given species is very common; for example, millions of SNP differences are found between two wheat varieties. It is not always necessary to have access to all this information for comparative studies. For many tasks, a limited number of polymorphic DNA sites is sufficient, and the use of high-density microarrays and NGS is unjustified and excessively costly. Improved AS-PCR techniques (e.g., KASP-related technology) are entirely appropriate in such situations. A limitation of the originally described KASP method is that only two alternative alleles at any specific site can be detected because of the use of individual quencher oligos for each ASP at a FRET cassette. However, planning KASP for the detection of three or four alleles at the same site and for allele identification at different polymorphic sites is possible in one multiplexed reaction. The latter possibility is valuable for those who require multiplexing to decrease sample number and increase throughput. Using a four-plexed fluorescent KASP assay, output during SNP genotyping will be doubled without additional labour or a significant increase in costs.
Here we describe a convenient alternative to originally described fluorescent reporters (FRET cassettes) that employs one universal quencher oligonucleotide. The quencher oligonucleotide is complementary to the UP 5′ tails. The quencher oligonucleotide is also complementary to the 5′ proximal sequence in the fluorescent probes. The quencher oligonucleotide at the 3′ terminus carries a universal quencher that effectively quenches a broad group of fluorescent dyes (such as Eclipse, BHQ1 or BHQ2 or Tide Quencher 2/3). Any of the known non-fluorescent dark quenchers can potentially be used ( Fiandaca et al., 2001 ; Li et al., 2006 ), as this quenching occurs in non-dual-labelled probes through non-FRET (static) quenching mechanisms ( Johansson et al., 2002 ; Marras et al., 2002 ) in situations where the dyes are held close together through hybridization. Static quenching occurs through the formation of a ground-state complex and can be important in dual-labelled “linear” probes ( Parkhurst and Parkhurst, 1995 ; Bernacchi and Mely, 2001 ). Efficient quenching can be obtained via static quenching without the use of molecular beacon stem-loop structures. The oligonucleotide presumably acts as a tether, effectively increasing the relative fluorophore quencher concentration, promoting heterodimer formation.
Differences in the tail UP sequences are determined by a short barcode 6-nt sequence that is not part of the universal tail sequence. The improved KASP makes AS-PCR-based genotyping into a flexible, easy-to-plan, high-throughput, and cost-effective technology. Modern qPCR machines are emerging that allow simultaneous use of four or more optical channels to detect fluorescent signals. Such equipment is poised to be utilized with the improved KASP as it is possible to genotype more SNPs or include more-than-two-variant SNPs.
In this work, we describe a tool working in the FastPCR environment for developing PCR-based genotyping assays. The tool helps design assays for detection of SNPs and InDels. The FastPCR software was originally created with the intention to allow great flexibility when designing PCR assays. Now with KASP capabilities, FastPCR allows for even more sophisticated genotyping assays, which translates into a higher overall success rate and provides the unique ability to genotype mix-type SNP/InDel polymorphisms. Increasing the reaction specificity and discriminative power of an assay, the ASPs are positioned automatically such that the penultimate base at the primer’s 3′ end is placed over the variable base. This feature of the FastPCR algorithm permits the use of proofreading enzymes, which is necessary when working with low-quality DNA templates and with a direct PCR assay. FastPCR expands detection options as it makes possible detection of all four variants at a single SNP site and any form of InDel. Theoretically, it also allows genotyping of several different SNP targets in a multiplexed reaction and genotyping mix-type polymorphisms. This contrasts with the limited ability of standard KASP assays to detect only two variants at a SNP site. FastPCR generates primers to target a polymorphic site from either strand. Custom FRET cassette reporter systems may be devised, and a universal FRET cassette system from LGC Biosearch Technologies may be used in planning the assays.
The AS-PCR design tool may be used to plan high-throughput screening assays to rapidly identify mutant strains of microorganisms, particular genetic lines of higher organisms, or cell lines, such as those produced with genome editing using Transcription Activator-Like Effector Nucleases (TALEN) or Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated protein (CRISPR/Cas) ( Lee et al., 2016 ; Koonin et al., 2017 ; Shmakov et al., 2017 ). An important prospective use of our AS-PCR design tool is rapid testing for specific variations in pathogen genomes. For example, the four-way genotyping assay may provide information on genetically encoded drug resistance or the presence of pathogenicity determinants in a clinical isolate. It may also be possible to rapidly design and deploy genotyping assays for epidemiological studies of circulating pathogens. Another entity presented in this manuscript is a KASP modification for quantitative measurement of the presence of allelic variants. The ASQ method is thus a highly multiplexed alternative to traditional quantitative PCR.
Acknowledgments
The authors wish to thank Derek Ho (The University of Helsinki Language Centre, Finland) for outstanding editing and proofreading of the manuscript.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material , further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Center for Life Sciences, National Laboratory Astana, Nazarbayev University (protocol #21, 10 October 2017). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
RK conceived the idea and provided bioinformatics analyses to design primers, tags, and barcode sequences; RK developed the methods and software; RK and AS performed the experiments and analysed the data. IA performed oligonucleotide synthesis and performed PCR analysis. RK prepared the initial draft of the manuscript. RK, UK, and AS wrote the main manuscript text and RK prepared all figures. UK provide the technical support, IA conceived the experiment. All authors reviewed and approved the final manuscript.
This research was funded by the Science Committee of the Ministry of Education and Science of the Republic of Kazakhstan (Grant No. AP08855353 and AP09058660). This study was also partially funded by Primer Digital Ltd. ( https://primerdigital.com ). Open access funding provided by University of Helsinki (Finland), including Helsinki University Library, via RK.
Conflict of Interest
Author RK is the owner and director of PrimerDigital. Author RK is an employee of PrimerDigital. This affiliation does not alter our adherence to publication policies on sharing data and materials.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmolb.2022.773956/full#supplementary-material
Abbreviations
ASP, allele-specific primer; AS-PCR, allele-specific PCR; ASQ, allele-specific qPCR; dG, Gibbs free energy; FRET, fluorescence resonant energy transfer; InDel, insertion-deletion polymorphism; KASP, kompetitive allele-specific PCR; SNP, single-nucleotide polymorphism; Uni-Q, universal quencher probe; UP, fluorescently-labeled Universal probe.
- Alsamman A. M., Ibrahim S. D., Hamwieh A. (2019). KASPspoon: an In Vitro and In Silico PCR Analysis Tool for High-Throughput SNP Genotyping. Bioinformatics 35, 3187–3190. 10.1093/bioinformatics/btz004 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Baidyussen A., Jatayev S., Khassanova G., Amantayev B., Sereda G., Sereda S., et al. (2021). Expression of Specific Alleles of Zinc-Finger Transcription Factors, HvSAP8 and HvSAP16, and Corresponding SNP Markers, Are Associated with Drought Tolerance in Barley Populations. Ijms 22, 12156. 10.3390/ijms222212156 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bernacchi S., Mely Y. (2001). Exciton Interaction in Molecular Beacons: a Sensitive Sensor for Short Range Modifications of the Nucleic Acid Structure. Nucleic Acids Res. 29, 62e–62. 10.1093/nar/29.13.e62 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bommarito S., Peyret N., Jr J. S., Jr. (2000). Thermodynamic Parameters for DNA Sequences with Dangling Ends. Nucleic Acids Res. 28, 1929–1934. 10.1093/nar/28.9.1929 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bottema C. D. K., Sommer S. S. (1993). PCR Amplification of Specific Alleles: Rapid Detection of Known Mutations and Polymorphisms. Mutat. Research/Fundamental Mol. Mech. Mutagenesis 288, 93–102. 10.1016/0027-5107(93)90211-W [ DOI ] [ PubMed ] [ Google Scholar ]
- Boutros P. C., Okey A. B. (2004). PUNS: Transcriptomic- and Genomic-In Silico PCR for Enhanced Primer Design. Bioinformatics 20, 2399–2400. 10.1093/bioinformatics/bth257 [ DOI ] [ PubMed ] [ Google Scholar ]
- Brusa A., Patterson E. L., Gaines T. A., Dorn K., Westra P., Sparks C. D., et al. (2021). A Needle in a Seedstack: an Improved Method for Detection of Rare Alleles in Bulk Seed Testing through KASP. Pest Manag. Sci. 77, 2477–2484. 10.1002/ps.6278 [ DOI ] [ PubMed ] [ Google Scholar ]
- Clevenger J., Chavarro C., Pearl S. A., Ozias-Akins P., Jackson S. A. (2015). Single Nucleotide Polymorphism Identification in Polyploids: A Review, Example, and Recommendations. Mol. Plant 8, 831–846. 10.1016/j.molp.2015.02.002 [ DOI ] [ PubMed ] [ Google Scholar ]
- Della Coletta R., Qiu Y., Ou S., Hufford M. B., Hirsch C. N. (2021). How the Pan-Genome Is Changing Crop Genomics and Improvement. Genome Biol. 22, 3. 10.1186/s13059-020-02224-8 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Didenko V. V. (2001). DNA Probes Using Fluorescence Resonance Energy Transfer (FRET): Designs and Applications. Biotechniques 31, 1106–1121. 10.2144/01315rv02 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fiandaca M. J., Hyldig-Nielsen J. J., Gildea B. D., Coull J. M. (2001). Self-reporting PNA/DNA Primers for PCR Analysis. Genome Res. 11, 609–613. 10.1101/gr.170401 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fuhrman L. E., Shianna K. V., Aballay A. (2008). High-throughput Isolation and Mapping of C. elegans Mutants Susceptible to Pathogen Infection. PLoS One 3, e2882. 10.1371/journal.pone.0002882 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Johansson M. K., Fidder H., Dick D., Cook R. M. (2002). Intramolecular Dimers: a New Strategy to Fluorescence Quenching in Dual- Labeled Oligonucleotide Probes. J. Am. Chem. Soc. 124, 6950–6956. 10.1021/ja025678o [ DOI ] [ PubMed ] [ Google Scholar ]
- Kalendar R. (2022). A Guide to Using FASTPCR Software for PCR, In Silico PCR, and Oligonucleotide Analysis. Methods Mol. Biol. 2392, 223–243. 10.1007/978-1-0716-1799-1_16 [ DOI ] [ PubMed ] [ Google Scholar ]
- Kalendar R., Baidyussen A., Serikbay D., Zotova L., Khassanova G., Kuzbakova M., et al. (2022). Modified "Allele-specific qPCR" Method for SNP Genotyping Based on FRET. Front. Plant Sci. 12, 747886. 10.3389/fpls.2021.747886 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kalendar R., Khassenov B., Ramankulov Y., Samuilova O., Ivanov K. I. (2017). FastPCR: An In Silico Tool for Fast Primer and Probe Design and Advanced Sequence Analysis. Genomics 109, 312–319. 10.1016/j.ygeno.2017.05.005 [ DOI ] [ PubMed ] [ Google Scholar ]
- Kalendar R., Lee D., Schulman A. H. (2014). FastPCR Software for PCR, In Silico PCR, and Oligonucleotide Assembly and Analysis. Methods Mol. Biol. 1116, 271–302. 10.1007/978-1-62703-764-8_18 [ DOI ] [ PubMed ] [ Google Scholar ]
- Kalendar R., Lee D., Schulman A. H. (2011). Java Web Tools for PCR, In Silico PCR, and Oligonucleotide Assembly and Analysis. Genomics 98, 137–144. 10.1016/j.ygeno.2011.04.009 [ DOI ] [ PubMed ] [ Google Scholar ]
- Kaur A., Kaur P., Ahuja S. (2020). Förster Resonance Energy Transfer (FRET) and Applications Thereof. Anal. Methods 12, 5532–5550. 10.1039/d0ay01961e [ DOI ] [ PubMed ] [ Google Scholar ]
- Kim S., Misra A. (2007). SNP Genotyping: Technologies and Biomedical Applications. Annu. Rev. Biomed. Eng. 9, 289–320. 10.1146/annurev.bioeng.9.060906.152037 [ DOI ] [ PubMed ] [ Google Scholar ]
- Koonin E. V., Makarova K. S., Zhang F. (2017). Diversity, Classification and Evolution of CRISPR-Cas Systems. Curr. Opin. Microbiol. 37, 67–78. 10.1016/j.mib.2017.05.008 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lee H. B., Schwab T. L., Koleilat A., Ata H., Daby C. L., Cervera R. L., et al. (2016). Allele-Specific Quantitative PCR for Accurate, Rapid, and Cost-Effective Genotyping. Hum. Gene Ther. 27, 425–435. 10.1089/hum.2016.011 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Li J., Wang F., Mamon H., Kulke M. H., Harris L., Maher E., et al. (2006). Antiprimer Quenching-Based Real-Time PCR and its Application to the Analysis of Clinical Cancer Samples. Clin. Chem. 52, 624–633. 10.1373/clinchem.2005.063321 [ DOI ] [ PubMed ] [ Google Scholar ]
- Li P., Su T., Wang H., Zhao X., Wang W., Yu Y., et al. (2019). Development of a Core Set of KASP Markers for Assaying Genetic Diversity in Brassica rapa subsp. chinensis Makino. Plant Breed 138, 309–324. 10.1111/pbr.12686 [ DOI ] [ Google Scholar ]
- Long Y. M., Chao W. S., Ma G. J., Xu S. S., Qi L. L. (2017). An Innovative SNP Genotyping Method Adapting to Multiple Platforms and Throughputs. Theor. Appl. Genet. 130, 597–607. 10.1007/s00122-016-2838-4 [ DOI ] [ PubMed ] [ Google Scholar ]
- Makhoul M., Rambla C., Voss-Fels K. P., Hickey L. T., Snowdon R. J., Obermeier C. (2020). Overcoming Polyploidy Pitfalls: a User Guide for Effective SNP Conversion into KASP Markers in Wheat. Theor. Appl. Genet. 133, 2413–2430. 10.1007/s00122-020-03608-x [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Marras S. A. E., Kramer F. R., Tyagi S. (2002). Efficiencies of Fluorescence Resonance Energy Transfer and Contact-Mediated Quenching in Oligonucleotide Probes. Nucleic Acids Res. 30, 122e–122. 10.1093/nar/gnf121 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Marras S. A. E., Tyagi S., Antson D.-O., Kramer F. R. (2019). Color-coded Molecular Beacons for Multiplex PCR Screening Assays. PLoS One 14, e0213906. 10.1371/journal.pone.0213906 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Myakishev M. V., Khripin Y., Hu S., Hamer D. H. (2001). High-throughput SNP Genotyping by Allele-specific PCR with Universal Energy-Transfer-Labeled Primers. Genome Res. 11, 163–169. 10.1101/gr.157901 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nazarenko I. A., Bhatnagar S. K., Hohman R. J. (1997). A Closed Tube Format for Amplification and Detection of DNA Based on Energy Transfer. Nucleic Acids Res. 25, 2516–2521. 10.1093/nar/25.12.2516 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Newton C. R., Graham A., Heptinstall L. E., Powell S. J., Summers C., Kalsheker N., et al. (1989). Analysis of Any point Mutation in DNA. The Amplification Refractory Mutation System (ARMS). Nucl. Acids Res. 17, 2503–2516. 10.1093/nar/17.7.2503 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nichols W. C., Liepnieks J. J., Mckusick V. A., Benson M. D. (1989). Direct Sequencing of the Gene for Maryland/German Familial Amyloidotic Polyneuropathy Type II and Genotyping by Allele-specific Enzymatic Amplification. Genomics 5, 535–540. 10.1016/0888-7543(89)90020-7 [ DOI ] [ PubMed ] [ Google Scholar ]
- Owczarzy R., Moreira B. G., You Y., Behlke M. A., Walder J. A. (2008). Predicting Stability of DNA Duplexes in Solutions Containing Magnesium and Monovalent Cations. Biochemistry 47, 5336–5353. 10.1021/bi702363u [ DOI ] [ PubMed ] [ Google Scholar ]
- Parkhurst K. M., Parkhurst L. J. (1995). Donor-acceptor Distance Distributions in a Double-Labeled Fluorescent Oligonucleotide Both as a Single Strand and in Duplexes. Biochemistry 34, 293–300. 10.1021/bi00001a036 [ DOI ] [ PubMed ] [ Google Scholar ]
- Peyret N., Seneviratne P. A., Allawi H. T., Santalucia J., Jr. (1999). Nearest-Neighbor Thermodynamics and NMR of DNA Sequences with Internal A·A, C·C, G·G, and T·T Mismatches. Biochemistry 38, 3468–3477. 10.1021/bi9825091 [ DOI ] [ PubMed ] [ Google Scholar ]
- Ramirez-Gonzalez R. H., Uauy C., Caccamo M. (2015). PolyMarker: A Fast Polyploid Primer Design Pipeline: Fig. 1. Bioinformatics 31, 2038–2039. 10.1093/bioinformatics/btv069 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rasheed A., Wen W., Gao F., Zhai S., Jin H., Liu J., et al. (2016). Development and Validation of KASP Assays for Genes Underpinning Key Economic Traits in Bread Wheat. Theor. Appl. Genet. 129, 1843–1860. 10.1007/s00122-016-2743-x [ DOI ] [ PubMed ] [ Google Scholar ]
- Rickert A. M., Borodina T. A., Kuhn E. J., Lehrach H., Sperling S. (2004). Refinement of Single-Nucleotide Polymorphism Genotyping Methods on Human Genomic DNA: Amplifluor Allele-specific Polymerase Chain Reaction versus Ligation Detection Reaction-TaqMan. Anal. Biochem. 330, 288–297. 10.1016/j.ab.2004.03.035 [ DOI ] [ PubMed ] [ Google Scholar ]
- Ryu J., Kim W. J., Im J., Kim S. H., Lee K.-S., Jo H.-J., et al. (2018). Genotyping-by-sequencing Based Single Nucleotide Polymorphisms Enabled Kompetitive Allele Specific PCR Marker Development in Mutant Rubus Genotypes. Electron. J. Biotechnol. 35, 57–62. 10.1016/j.ejbt.2018.08.001 [ DOI ] [ Google Scholar ]
- Santalucia J., Jr., Hicks D. (2004). The Thermodynamics of DNA Structural Motifs. Annu. Rev. Biophys. Biomol. Struct. 33, 415–440. 10.1146/annurev.biophys.32.110601.141800 [ DOI ] [ PubMed ] [ Google Scholar ]
- Sarkar G., Cassady J., Bottema C. D. K., Sommer S. S. (1990). Characterization of Polymerase Chain Reaction Amplification of Specific Alleles. Anal. Biochem. 186, 64–68. 10.1016/0003-2697(90)90573-r [ DOI ] [ PubMed ] [ Google Scholar ]
- Shmakov S., Smargon A., Scott D., Cox D., Pyzocha N., Yan W., et al. (2017). Diversity and Evolution of Class 2 CRISPR-Cas Systems. Nat. Rev. Microbiol. 15, 169–182. 10.1038/nrmicro.2016.184 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sommer S. S., Cassady J. D., Sobell J. L., Bottema C. D. K. (1989). A Novel Method for Detecting Point Mutations or Polymorphisms and its Application to Population Screening for Carriers of Phenylketonuria. Mayo Clinic Proc. 64, 1361–1372. 10.1016/s0025-6196(12)65378-6 [ DOI ] [ PubMed ] [ Google Scholar ]
- Spierings E., Drabbels J., Hendriks M., Pool J., Spruyt-Gerritse M., Claas F., et al. (2006). A Uniform Genomic Minor Histocompatibility Antigen Typing Methodology and Database Designed to Facilitate Clinical Applications. PLoS One 1, e42. 10.1371/journal.pone.0000042 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ugozzoli L., Wallace R. (1991). Allele-specific Polymerase Chain Reaction. Methods 2, 42–48. 10.1016/s1046-2023(05)80124-0 [ DOI ] [ PubMed ] [ Google Scholar ]
- Wang B., Tan H.-W., Fang W., Meinhardt L. W., Mischke S., Matsumoto T., et al. (2015). Developing Single Nucleotide Polymorphism (SNP) Markers from Transcriptome Sequences for Identification of Longan (Dimocarpus Longan) Germplasm. Hortic. Res. 2, 14065. 10.1038/hortres.2014.65 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wang Y., Tiwari V. K., Rawat N., Gill B. S., Huo N., You F. M., et al. (2016). GSP: a Web-Based Platform for Designing Genome-specific Primers in Polyploids. Bioinformatics 32, 2382–2383. 10.1093/bioinformatics/btw134 [ DOI ] [ PubMed ] [ Google Scholar ]
- Wangkumhang P., Chaichoompu K., Ngamphiw C., Ruangrit U., Chanprasert J., Assawamakin A., et al. (2007). WASP: a Web-Based Allele-specific PCR Assay Designing Tool for Detecting SNPs and Mutations. BMC Genomics 8, 275. 10.1186/1471-2164-8-275 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wu D. Y., Ugozzoli L., Pal B. K., Wallace R. B. (1989). Allele-specific Enzymatic Amplification of Beta-Globin Genomic DNA for Diagnosis of Sickle Cell Anemia. Proc. Natl. Acad. Sci. 86, 2757–2760. 10.1073/pnas.86.8.2757 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wu Y., Li M., He Z., Dreisigacker S., Wen W., Jin H., et al. (2020). Development and Validation of High-Throughput and Low-Cost STARP Assays for Genes Underpinning Economically Important Traits in Wheat. Theor. Appl. Genet. 133, 2431–2450. 10.1007/s00122-020-03609-w [ DOI ] [ PubMed ] [ Google Scholar ]
- Ye S., Humphries S., Green F. (1992). Allele Specific Amplification by Tetra-Primer PCR. Nucl. Acids Res. 20, 1152. 10.1093/nar/20.5.1152 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
- View on publisher site
- PDF (1.5 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Your privacy is very important to us. When you visit our website, please agree to the use of all cookies. For more information about personal data processing, please go to Privacy Policy .
Partnership
Crispr-cas13, crispr-cas12, scientific publications & presentations, hg-aav platform, animal model platform, manufacturing capabilities, disease focus, press release, get in touch, technology platform.

Back to Latest News
HuidaGene Therapeutics Appoints TJ Cradick, PhD as Chief Technology Officer to Lead Delivery Science and Genome Editing Innovations
SHANGHAI and MIDDLETOWN (DE), October 21, 2024 –HuidaGene Therapeutics (“HuidaGene”), a global clinical-stage biotechnology company pioneering CRISPR-based programmable genome medicines, today announced the appointment of Dr. TJ Cradick as Chief Technology Officer. In this role, Dr. Cradick will further drive innovation and development of delivery vectors and gene editing tools through computational biology, artificial intelligence (AI), machine learning (ML), and other tools and methodologies.


IMAGES
VIDEO
COMMENTS
Graduate Studies. The Genetics Ph.D. program provides opportunities for graduate study in all major areas of modern genetics, including identification and analysis of human disease genes, molecular evolution, gene therapy, statistical genetics, application of model organisms to problems in biology and medicine, and computational and experimental approaches to genome biology.
Recent breakthroughs in genetics and genomics are revolutionizing the way we diagnose and treat diseases. Technologies such as rapid sequencing and gene editing are expanding the field of precision medicine, enabling treatments to be personalized for each patient. The Genetics and Genomics program will equip you with the knowledge and skills you need to keep up-and and get ahead-in this ...
Gene and cell therapies are advanced medical treatments aimed at stopping or slowing disease progression. Gene therapy involves replacing or modifying faulty genes with corrected versions, while cell therapy replaces or repairs damaged cells. Gene editing, utilizing tools like CRISPR, enable targeted and programmable repair of damaged gene ...
Within the medical studies, cell and gene therapies is one of the fastest-growing fields. Northeastern's Master of Science in Cell and Gene Therapies is an innovative, nonthesis master's program that brings students the knowledge and skills to understand the key concepts and solve the vital challenges in cell and gene therapy treatments.
Ranked in 2022, part of Best Science Schools. Genetics and genomics courses examine heredity and DNA and, in bioinformatics, computer programs are used to analyze this genetic information.
The Cell and Molecular Biology Graduate Group (CAMB) is an interdisciplinary graduate program, providing rigorous training in modern cell and molecular biology, preparing students for leadership careers in biomedical research. ... The Gene Therapy and Vaccines Program focuses on the use of animal and human gene transfer for therapeutic purposes ...
The Graduate Program in Cell & Developmental Biology (CDB) provides outstanding doctoral training in fundamental aspects of cell and developmental biology, ranging from stem cells to regenerative medicine and organogenesis to cancer biology. ... sophisticated genetics including gene editing, and systems-based strategies to understand the basic ...
Gene editing and cell engineering have exciting applications to the study and treatment of a range of critical human diseases, including cardiovascular, neurodegenerative, hematologic, immune, metabolic, and hereditary diseases, and cancer. Genetics and Genomic Sciences Faculty. Alessia Baccarini, PhD; Margo Breilyn, MD; Efrat Eliyahu, PhD
Our Competitive Advantage. Empowered by the distinctive One Health approach of UCI Health Affairs that transcends disciplinary boundaries, the Genome Editing Research Program comprises faculty and staff from the Susan & Henry Samueli College of Health Sciences (encompassing schools of medicine, nursing, pharmacy and pharmaceutical sciences, population and public health) and UCI Health, our ...
BMS Student's Work on Revolutionary Gene-Editing Technique. ... (BMS) program is an interdisciplinary graduate research program that equips students with the training and research tools to dissect disease-related biology, from single cells to tissue and organ systems. Students in the BMS program must acquire a level of competence in molecular ...
Diversity in the Graduate Program; NIH Training Grant; ... genes • gene editing • heredity• evolution • genetic variability • phenotypic variability • horizontal gene transfer • meiosis • recombination • epigenetics • DNA repair and replication • chromosome segregation • cell division • gene regulation • development ...
The overall objective of the Human Genetics program is to provide our students with a strong foundation in basic science by exposure to a rigorous graduate education in genetics, genomics, molecular biology, cell biology, biochemistry and biostatistics as well as a core of medically-related courses selected to provide knowledge of human biology in health and disease.
The world stands on the edge of an era when gene editing can address many serious ills plaguing humankind, according to Jennifer Doudna, whose work on the gene editing technique known as CRISPR-Cas9 earned her the 2020 Nobel Prize in chemistry. But first, she said, there is a problem to solve: ensuring that as these technologies become approved to treat and even cure certain human diseases ...
30 gene-editing PhD positions. Filters Search Sort by. relevance listed; Filtered by; PhD gene-editing Remove All ; Refine Your Search. Category. Scholarship 20; Research Job 10; Country. ... Experience with RNA sequencing, gene editing (CRISPR-Cas9), single particle imaging, and large data set analysis. Proficiency in programming languages ...
Search Funded PhD Projects, Programmes & Scholarships in crispr. Search for PhD funding, scholarships & studentships in the UK, Europe and around the world. ... Software Engineering (2) Soil Science ... Delivery of CRISPR editing systems in vivo is a major challenge for clinical translation and therapy development.
NIBIB-funded researchers are working to bring in vivo gene editing to the fore. Through rational engineering of lipid nanoparticles, this collaborative team developed a way to effectively target specific organs in the body to precisely deliver therapeutic cargo, including gene-editing molecules. Their research demonstrated that a one-time treatment with their nanoparticles resulted in durable ...
PhD. Contact. Connect with experts in your field. ... A method of gene editing or gene stacking within a FAD2 loci by cleaving, in a site directed manner, a location in a FAD2 gene in a cell, to ...
The National Institutes of Health has awarded a $14 million grant to the Perelman School of Medicine and the Children's Hospital of Philadelphia for gene-editing research. This funding, provided through the NIH's Somatic Cell Genome Editing Program, will support gene-editing research aimed at developing treatments for rare metabolic diseases.
Gene editing and gene synthesis technologies are inherently dual use, meaning they can be employed for good or ill by sovereign states, nonstate groups, and even individuals. This dual-use dilemma is nothing new. 22 From prehistory to the present, humans have invented tools, from hand axes to drones, that can cause grave damage in the wrong ...
For gene editing in wheat, a Cas9 protein containing one or more signals for nuclear localization is expressed from a codon optimized gene under the control of RNA polymerase II promoters such as CaMV35S or ZmUbi, while the sgRNA is usually expressed from a polymerase III promoter (most commonly, rice or wheat U6 and U3 promoters).
SHANGHAI and MIDDLETOWN, Del., Oct. 21, 2024 /PRNewswire/ -- HuidaGene Therapeutics ("HuidaGene"), a global clinical-stage biotechnology company pioneering CRISPR-based programmable genome medicines, today announced the appointment of Dr. TJ Cradick as Chief Technology Officer. In this role, Dr. Cradick will further drive innovation and development of delivery vectors and gene editing ...
He served a term as a co-director for the NSF Plant Genome Research Program, and currently serves as the past-secretary and annual meeting program chair for the American Society of Plant Biologists. He is actively engaged in training graduate students and postdoctoral fellows, and teaches graduate-level courses in crop genetics.
The program also computes the optimal reaction conditions to perform KASP. This software will be most useful for multiplex genotyping in a high-performance environment. Furthermore, potential uses are not limited to genotyping. The program can produce tests for selecting mutants, e.g., after genome editing or gene knockout (Lee et al., 2016 ...
The Company is advancing clinical programs, including trials of HG004 (granted ODD & RPDD by FDA) 'LIGHT' trial (NCT06088992) and Phase 1/2 international, master-protocol 'STAR' clinical trial (NCT05906953) in RPE65-associated retinal disease, HG202 RNA-editing therapy 'SIGHT-I' first-in-human trial (NCT06031727) and Phase 1 ...