Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts

Ulcerative colitis articles within Nature Reviews Gastroenterology & Hepatology
Review Article | 25 April 2024
Pouchitis: pathophysiology and management
Pouchitis is a common condition that can occur after intestinal surgery. In this Review, Shen discusses our current understanding of the multifactorial pathophysiology, diagnosis and management of pouchitis, primarily in patients with underlying ulcerative colitis.
Year in Review | 07 December 2023
Upgrading therapeutic ambitions and treatment outcomes
Important studies published in 2023 outlined new agents and strategies for the management of inflammatory bowel disease. Therapeutic ambitions for the management of inflammatory bowel disease were raised by the success of combinations of biologic agents in ulcerative colitis and early surgical resection in Crohn’s disease.
- Paulo Gustavo Kotze
- & Severine Vermeire
Review Article | 10 November 2023
Deciphering the different phases of preclinical inflammatory bowel disease
Inflammatory bowel disease (IBD) is an immune-mediated inflammatory disease (IMID). Here, the authors review evidence on the preclinical phase of IBD, outlining and describing the proposed at-risk, initiation and expansion phases. Overlap with other IMIDs is discussed alongside the possible future directions for research into preclinical IBD.
- Jonas J. Rudbaek
- , Manasi Agrawal
- & Tine Jess
In Brief | 08 August 2023
Mirikizumab for inducing and maintaining clinical remission in ulcerative colitis
- Jordan Hindson
Perspective | 20 April 2023
The appendix and ulcerative colitis — an unsolved connection
The appendix is thought to have a role in the pathogenesis of ulcerative colitis but the association remains unclear. In this Perspective, the authors consider the biology of the appendix with respect to its immunological function and the microbiome, and how this relates to its possible involvement in ulcerative colitis.
- Manasi Agrawal
- , Kristine H. Allin
- & Jean-Frederic Colombel
In Brief | 07 March 2023
Two therapeutic antibodies better than one
Clinical Outlook | 27 January 2023
Positioning therapies for the management of inflammatory bowel disease
A careful integration of the effectiveness and safety of the therapies for inflammatory bowel disease, considering patients’ disease risks, treatment complications and preferences, is warranted to inform the positioning of therapies in clinical practice. Precision medicine might help choose the best option for an individual patient.
- Siddharth Singh
Comment | 19 December 2022
Risk minimization of JAK inhibitors in ulcerative colitis following regulatory guidance
The European Medicines Agency safety committee has revisited the label and recommended the use of Janus kinase inhibitors in patients with certain risk factors only if no suitable treatment alternatives are available. Although regulatory decisions are key to place therapeutic options based on safety, broad restrictions might lead to unintended consequences without an individualized benefit–risk evaluation.
- Silvio Danese
- , Virginia Solitano
- & Laurent Peyrin-Biroulet
Clinical Outlook | 16 September 2022
Medical therapy of paediatric inflammatory bowel disease
Antibodies targeting tumour necrosis factor have substantially advanced the treatment of paediatric inflammatory bowel disease. Understanding pharmacokinetics and therapeutic drug monitoring has led to increased efficacy and durability of response. Primary non-response is more common in ulcerative colitis than in Crohn’s disease, highlighting the need for alternative biologic agents and oral small molecules.
- Luca Scarallo
- & Anne M. Griffiths
Review Article | 07 December 2021
Revisiting fibrosis in inflammatory bowel disease: the gut thickens
Intestinal fibrosis is an important feature of inflammatory bowel disease (IBD) that remains poorly understood. Here, D’Alessio and Ungaro et al. review the cellular and molecular mechanisms contributing to intestinal fibrosis and discuss future therapeutic strategies for IBD-related fibrosis.
- Silvia D’Alessio
- , Federica Ungaro
- & Silvio Danese
In Brief | 01 October 2021
Ozanimod is efficacious in ulcerative colitis
- Katrina Ray
Journal Club | 20 September 2021
Inflammatory bowel disease and corticosteroids: the first RCT
- Fernando Gomollón
In Brief | 22 June 2021
Filgotinib for ulcerative colitis
Research Highlight | 04 May 2021
Intercrypt goblet cells — the key to colonic mucus barrier function
News & Views | 05 February 2021
IBD risk prediction using multi-ethnic polygenic risk scores
Inflammatory bowel disease (IBD) has emerged as a global disease, yet identifying those at higher risk of developing IBD remains challenging. A new study highlights the use of a multi-ethnic polygenic risk score to determine risk of inflammatory bowel disease in a large primary care population.
- Ashwin N. Ananthakrishnan
Comment | 20 January 2021
SARS-CoV-2 vaccination in IBD: more pros than cons
Data on the efficacy and safety of SARS-CoV-2 vaccines are now available, but evidence for these vaccines in those who are immunocompromised (including patients with inflammatory bowel diseases) are lacking. As vaccination begins, questions on advantages and disadvantages can be partially addressed using the experience from other vaccines or immune-mediated inflammatory disorders.
- Ferdinando D’Amico
- , Christian Rabaud
News & Views | 01 October 2020
Environmental stimuli and gut inflammation via dysbiosis in mouse and man
A new study sheds further light on the interplay between environmental stimuli, the gut microbiota and intestinal inflammation. Identification of modifiable environmental triggers and the mechanisms by which they act has implications for the prevention and treatment of inflammatory bowel disease.
- Charlie W. Lees
Research Highlight | 03 September 2020
Deciphering the role of CD8 + T cells in IBD: from single-cell analysis to biomarkers
Research Highlight | 21 January 2020
Shining a spotlight on somatic mutations in ulcerative colitis
Research Highlight | 15 October 2019
New trials in ulcerative colitis therapies
- Iain Dickson
Comment | 13 September 2019
Evolving therapeutic goals in ulcerative colitis: towards disease clearance
In ulcerative colitis, treating beyond endoscopic healing has shown a reduction of relapse and hospitalization, pushing for histological remission to be embraced in clinical practice and clinical trials. Here, we propose the concept of disease clearance (symptomatic, endoscopic and histological remission) as the ultimate goal in the treatment of ulcerative colitis.
- , Giulia Roda
In Brief | 29 March 2019
Gut mucosal virome altered in ulcerative colitis
News & Views | 08 March 2019
FMT for ulcerative colitis: closer to the turning point
A new study shows that a sustainable faecal microbiota transplantation (FMT) treatment protocol, including anaerobic sample preparation, induces remission of active ulcerative colitis. The promising results are another piece in the puzzle, but it is not yet possible to draw conclusions and implement the procedure in clinical practice.
- Giovanni Cammarota
- & Gianluca Ianiro
In Brief | 06 April 2018
Autofluorescence inferior for dysplasia surveillance
In Brief | 02 November 2017
The changing epidemiology of IBD
Review Article | 11 October 2017
Environmental triggers in IBD: a review of progress and evidence
A wide variety of environmental triggers have been associated with IBD pathogenesis, including the gut microbiota, diet, pollution and early-life factors. This Review discusses the latest evidence and progress towards better understanding the environmental factors associated with IBD.
- , Charles N. Bernstein
- & Claudio Fiocchi
Review Article | 19 July 2017
Gut microbiota and IBD: causation or correlation?
Changes in the composition and metabolic function of the gut microbiota have been linked to IBD, but a direct causal association has yet to be established in humans. This Review discusses the evidence supporting dysbiosis in the gut microbiota in Crohn's disease and ulcerative colitis, exploring evidence from animal models and the translation to human disease.
- Josephine Ni
- , Gary D. Wu
- & Vesselin T. Tomov
In Brief | 14 June 2017
Phase II trial success for anti-MADCAM1 antibody
Research Highlight | 17 May 2017
Tofacitinib effective in ulcerative colitis
- Conor A. Bradley
Research Highlight | 01 March 2017
FMT induces clinical remission in ulcerative colitis
- Hugh Thomas
News & Views | 07 December 2016
Mucosal healing in ulcerative colitis: what constitutes remission?
Patients with ulcerative colitis in clinical remission are increasingly undergoing colonoscopies to determine endoscopic remission. However, the histological evaluation of biopsy samples provides additional criteria to predict which patients are most likely to undergo relapse, so what is the ideal therapeutic end point for patients with ulcerative colitis?
- Robert H. Riddell
Research Highlight | 05 October 2016
A role for GATA3 in ulcerative colitis
Review Article | 01 September 2016
Acute severe ulcerative colitis: from pathophysiology to clinical management
Acute severe ulcerative colitis (ASUC) is a potentially life-threatening condition that occurs in ∼20% of patients with ulcerative colitis. Here, the authors provide an overview of ASUC from pathophysiology to clinical management (including drug therapy and surgery).
- Pieter Hindryckx
- , Vipul Jairath
- & Geert D'Haens
In Brief | 13 July 2016
Treatment for acute severe ulcerative colitis
- Isobel Leake
News & Views | 05 May 2016
Vitamin D and IBD: moving towards clinical trials
A new study reports that low vitamin D levels are associated with increased morbidity and severity of IBD. A number of issues must now be addressed to enable the optimal design of interventional studies to test whether vitamin D supplementation can improve outcomes in this disease.
- Margherita T. Cantorna
In Brief | 17 February 2016
CT-P13: a safe and effective treatment for IBD
Research Highlight | 24 December 2015
Maintaining the mucosal barrier in intestinal inflammation
In Brief | 18 November 2015
Who benefits the most from etrolizumab in ulcerative colitis?
In Brief | 08 September 2015
Gel-based drug delivery for IBD hits the target
In Brief | 21 April 2015
Faecal transplant from donors no more effective than autologous transplant for treating ulcerative colitis
News & Views | 15 July 2014
Sequential rescue therapy in steroid-refractory ulcerative colitis
Treatment of patients with steroid-refractory ulcerative colitis is still a challenge for physicians. A recent study has evaluated the effectiveness and safety of sequential rescue therapies in this subgroup of patients.
- Paolo Gionchetti
- & Fernando Rizzello
Research Highlight | 24 June 2014
T H 9 cells might have a role in the pathogenesis of ulcerative colitis
In Brief | 17 June 2014
Phase II study reveals potential of etrolizumab as induction therapy for ulcerative colitis
Research Highlight | 22 April 2014
Mouse model reveals how appendicitis protects against ulcerative colitis
- Claire Greenhill
Research Highlight | 18 March 2014
EUS can differentiate Crohn's disease from ulcerative colitis
- Natalie J. Wood
News & Views | 04 March 2014
Which makes patients happier, surgery or anti-TNF therapy?
Quality of life and disability have been compared in patients with ulcerative colitis who were undergoing one of the two current major treatments of choice, proctocolectomy or anti-TNF therapy. The only significant differences between the two groups were increased use of antidiarrhoeal medication and stool frequency in those who underwent surgery.
- Taku Kobayashi
- & Toshifumi Hibi
Review Article | 07 January 2014
Tailoring anti-TNF therapy in IBD: drug levels and disease activity
Despite the proven and often clinically marked efficacy of anti-TNF drugs for IBD, these biologic agents are not immune to treatment failures. Tailoring anti-TNF treatment in IBD mandates considerations of the different clinical scenarios in which therapy failure might occur while bearing in mind an opposite group of patients in whom intensive therapy might be unnecessary.
- Shomron Ben-Horin
- & Yehuda Chowers
In Brief | 17 September 2013
Induction and maintenance therapy for ulcerative colitis—vedolizumab more effective than placebo
News & Views | 13 August 2013
Activity of IBD during pregnancy
Women worry that their IBD will flare during pregnancy. A prospective multicentre study from Europe has now demonstrated that although women with Crohn's disease do not have an increased risk of relapse during pregnancy, women with ulcerative colitis are at increased risk of relapse, both during pregnancy and postpartum.
- Sunanda Kane
News & Views | 30 July 2013
Golimumab in ulcerative colitis: a 'ménage à trois' of drugs
Golimumab, a human anti-TNF antibody, is effective in patients with ulcerative colitis, according to new findings from an international phase III double-blind trial. The addition of this drug makes a ménage à trois of available drugs—comprising infliximab, adalimumab and golimumab—for the treatment of ulcerative colitis.
Browse broader subjects
- Inflammatory diseases
- Inflammatory bowel disease
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Drugs Context
New developments in ulcerative colitis: latest evidence on management, treatment, and maintenance
Kartikeya tripathi.
1 Department of Medicine, St Vincent Hospital, Worcester, MA, USA
Joseph D Feuerstein
2 Department of Medicine and Division of Gastroenterology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA
Ulcerative colitis (UC) is a chronic idiopathic inflammatory disorder that involves any part of the colon starting in the rectum in a continuous fashion presenting typically with symptoms such as bloody diarrhea, abdominal pain, and rectal urgency. UC is diagnosed based on clinical presentation and endoscopic evidence of inflammation in the colon starting in the rectum and extending proximally in the colon. The clinical presentation of the disease usually dictates the choice of pharmacologic therapy, where the goal is to first induce remission and then maintain a corticosteroid-free remission. There are multiple classes of drugs that are available and are used based on the clinical severity of the disease. For mild-to-moderate disease, oral or rectal formulations of 5-aminosalicylic acid are used. In moderate-to-severe UC, corticosteroids are usually used in induction of remission with or without another class of medications such as thiopurines or biologics including anti-tumor necrosis factor, anti-integrins, or Janus kinase inhibitors for maintenance of remission. Up to 15% of the patients may require surgery as they fail to respond to medications and have risk of developing dysplasia secondary to longstanding colitis.
Introduction
Ulcerative colitis (UC) was first described in mid-1800s. 1 It is an idiopathic, chronic inflammatory disorder of the colonic mucosa that commonly involves the rectum and may extend in a proximal and continuous fashion to involve other parts of the colon. 2 The disease typically affects individuals in the second or third decade of life with hallmark clinical symptoms of bloody diarrhea and rectal urgency with tenesmus. 3 , 4 The clinical course is marked by exacerbations and remissions, which may occur spontaneously or in response to treatment changes. 5 , 6 There are multiple drug classes discussed in this review that can be used to treat acute exacerbation of the disease and for maintenance of remission. However, even with medical therapy, up to 15% of patients will require surgery to treat UC or disease complications of dysplasia.
Overall, the incidence of inflammatory bowel disease (IBD) has traditionally been highest in North America and Western Europe with increasing incidence in the mid-20th century. However, incidence of IBD is increasing in emerging populations in continental Asia. 7 , 8 In North America, the incidence of UC is 2.2–14.3 cases per 100,000 persons per year, and its prevalence is 37–246 cases per 100,000 per year. 7 The exact pathogenesis of the disease is not well understood but there are genetic factors that are attributed to the risk of developing the disease accompanied by epithelial barrier defects and environmental factors. Currently, a number of genetic and environmental factors that increase the risk of developing UC are identified. 9 A westernized lifestyle and diet including cessation of tobacco use, fatty diet, stress, and medication use and high socioeconomic status are all associated with the development of IBD. 10 Among many such factors, tobacco smoking and appendectomy are linked to milder disease, fewer hospitalizations, and decreased incidence of UC but the reverse is true for Crohn’s disease. 11 , 12
The diagnosis of UC is based on the clinical presentation and symptoms consistent with the disease and findings on colonoscopy or sigmoidoscopy showing continuous colonic inflammation starting in the rectum. Pathologic findings of chronic colitis confirm the diagnosis.
Disease approach, assessment of clinical severity, and disease management
Initial treatment is based upon disease severity and extent. Patients can present with mild, moderate, or severe disease – stratification based on clinical severity is used to guide medical and pharmacologic management. The goals of treatment are induction of remission followed by maintenance of remission in conjunction with steroid-free treatments in the long-term management. 6 Historically, the Truelove and Witts criteria are utilized to stratify patients with mild, severe, or fulminant colitis ( Table 1 ). Patients categorized as having mild clinical disease have less than four stools per day with or without blood with no signs of systemic toxicity. Mild crampy abdominal pain and tenesmus are common clinical symptoms. In moderate–severe disease, patients have abdominal pain, frequent loose bloody stools (typically more than four per day), and mild anemia not requiring blood transfusions. They also have minimal signs of systemic toxicity such as low grade fever. In contrast, patients with fulminant disease present with over six loose bloody stools with severe abdominal cramps and systemic toxicity such as fever or tachycardia. They also have manifestations such as anemia or an elevated erythrocyte sedimentation rate (ESR)/c-reactive protein (CRP). In addition to assessing patients with the Truelove and Witts criteria, the colon should be evaluated endoscopically either with a sigmoidoscopy or colonoscopy, depending on the clinical presentation and any validated score such as Mayo Endoscopy score or Ulcerative Colitis Endoscopic Index of Severity (UCEIS) should be utilized 13 ( Tables 2 and and3). 3 ). The endoscopic Mayo score classifies disease as mild, moderate, or severe based on the erythema, erosions, ulcers, and/or severe friability. The management of severe and fulminant clinical disease differs from that of mild-to-moderate disease. Historically, step-up therapy was used for treating any flare ups of UC. However, recent evidence suggests a top down approach using effective therapy such as anti-tumor necrosis factor (TNF) often with immunomodulator to control severe disease; thus, patients with severe clinical disease on presentation may be treated with biologics early on as opposed to use of mesalamines that are effective only in mild-to-moderate disease.
Truelove and Witts’ severity index.
Adapted from Sturm et al. 13
Endoscopic Mayo score.
UCEIS (Ulcerative Colitis Endoscopic Index of Severity) descriptors and definitions.
Mild-to-moderate disease
5-aminosalicylate.
There are multiple 5-aminosalicylate (5-ASA) compounds available. One of the first drugs available was sulfasalazine. Sulfasalazine is a prodrug that is partially absorbed in the jejunum and passes to the colon where it is reduced by coliforms to sulfapyridine and its active form, 5-ASA. 14 5-ASA is primarily responsible for efficacy of sulfasalazine. Other 5-ASA products (i.e. mesalamine) are formulated to release in the colon via a number of different mechanisms including both bacterial-mediated release and pH-mediated release. In azo-bond prodrug, the mesalazine is synthesized as a prodrug binding via an azo-bond to a transport molecule. Due to the presence of an azo-bond, it is not absorbed in the upper gastrointestinal tract. The bond is subsequently cleaved by bacterial action in the colon by azoreductase, releasing the active mesalazine component of the drug. In pH-mediated release formulations, the active drug is encapsulated in an enteric coating to control the site of drug release. Other available formulations include time-dependent release, which consists of microspheres of mesalazine encapsulated within a semipermeable membrane that produces time and moisture-dependent release of active drug. Although the different release mechanisms may be of benefit for certain patients, the recent American Gastroenterological Association (AGA) guidelines on mild-to-moderate UC do not suggest changing mesalamine based on release formulation in someone who is not responding adequately to the initial mesalamine release mechanism. 15
The dosing per pill is variable but in general, these medications can be taken once a day or in twice daily regimens. The initial approach for mild-to-moderate disease is to start oral and topical 5-ASA. Oral 5-ASA is started at a full-strength dose of 4.8 g/day for induction of remission. Over time, this can be reduced to a maintenance dose of 2.4 g/day. In patients who have not achieved remission on oral therapy, combining oral and rectal therapy is more effective in inducing remission. 16 – 18
In patients with more limited disease of the rectum and/or sigmoid colon, some patients may opt for only topical rectal treatments and defer oral therapy due to cheaper costs, quicker response time, and typically requiring lesser frequent dosing when compared to oral therapy. However, if the patient fails to respond to topical therapy, then oral therapy should be added to the regimen. Topical therapy with 5-ASA can be given via suppository or enema. 19 – 21
In left-sided colitis and pancolitis, combination therapy has proven to be more effective in achieving remission and its maintenance than isolated oral therapy or isolated topical therapy. 22 In general, 5-ASA drugs start working within 2–4 weeks and they show response in up to 80% of patients (when selected appropriately). 19 Once remission is achieved, patients are continued on the drug for maintenance therapy. Given the safety of the drug and lack of any dose-dependent side-effect profile, some practitioners opt to keep patients on the 4.8 g/day dose while others will lower the dose to 2.4 g/day when dosing for maintenance. There are no data to support doses that are less than 2.4 g/day, and these doses should be avoided. 15
In patients with only mild-to-moderate disease who fail to respond to mesalamine, one can consider adding a steroid-containing foam or enema in combination with 5-ASA therapy. 23 In patients who have an inadequate response to the combination of oral 5-ASA and topical 5-ASA/steroids in 2–4 weeks, the budesonide multimatrix (MMX) formulation can be considered. 24 , 25 Although these steroid formulations are relatively safe given their lack of systemic absorption, they are not as effective as oral prednisone. The initial study importantly compared budesonide MMX to mesalamine and not prednisone. 26 Additionally, none of these steroid formulations are approved for long-term maintenance of remission. 15
Although side effects can happen with both sulfasalazine and 5-ASA, sulfasalazine appears to have a wider range of more serious adverse events. Both anemia and abnormal liver tests are associated with sulfasalazine use and, to that end, patients should have routine complete blood counts (CBC) and liver function tests (LFTs) checked while on sulfasalazine. Additionally, to reduce the risk of anemia, patients should take folic acid 1 mg daily. Other side effects include nausea, headaches, fevers, and rash. Headaches and nausea are often dose dependent but slow titration of the dose can minimize these issues. However, sulfasalazine should be discontinued if the patient experiences idiosyncratic drug reactions such as skin rash, pancreatitis, pneumonitis, and agranulocytosis, and the patient should not be rechallenged. Approximately, 25% of patients stop using sulfasalazine due to its broader side-effect profile compared to mesalamine.
In contrast, mesalamine is a very safe and effective medication. It is extremely rare to develop any side effects on the drug. Up to 3% of patients may experience paradoxical worsening of diarrhea, and stopping the drug may be helpful. 27 Interstitial nephritis is a very rare side effect occurring in less than 0.2% of cases. Routine monitoring of kidney function is recommended to screen for interstitial nephritis. 28
Oral budesonide and rectal budesonide formulations carry little to no risk. Rectal budesonide foam was more efficacious in inducing remission in patients when compared to placebo. 29 Studies show that there is a small change in systemic cortisol levels, but classic steroid-related side effects are not seen with these drugs. There are case reports of steroid-related side effects with oral budesonide when used in high doses for long term. 30
Moderate-to-severe disease
Systemic corticosteroids are typically given first line for induction of remission in cases of moderate-to-severe disease. Oral steroids are used in most cases, but in up to 15% of patients, the disease may present as acute severe ulcerative colitis necessitating hospitalization and intravenous steroids. 31 Steroids are used in the acute inflammatory phase of the disease to assist with induction of remission but should always be bridged with a steroid-sparing agent for a goal of long-term steroid-free maintenance of remission. 6 Intravenous or oral steroids should never be used for long-term therapy as they are associated with a myriad of irreversible side effects such as weight gain, cataracts, osteoporosis, hypothalamic pituitary axis suppression, and immunocompromised state. Several classes of drugs can be used for maintenance of remission including thiopurines, anti-TNF agents, anti-integrins, and Janus kinase inhibitors that are discussed in detail in this review.
Corticosteroids
As stated earlier, corticosteroids are only used for induction of remission. Oral prednisone is usually the first choice of treatment at a dose of 40–60 mg daily. 6 Higher doses have not been shown to be more effective. In most patients, oral steroids are useful for induction of remission; however, if symptoms do not respond adequately, intravenous steroids should be used and the patient should be hospitalized. 32 These patients are at risks of developing complications, and close monitoring in the hospital setting is recommended. Intravenous methylprednisone is usually preferred at a dose of 40–60 mg daily (e.g. 20 mg every 8 hours) over intravenous hydrocortisone that may cause sodium retention. 31 Approximately two-thirds of the patients respond to this treatment. There are no specific recommendations for tapering the steroid dose, but it is advised to transition to an oral prednisone dose of 40–60 mg daily until significant clinical improvement occurs and then taper with a dose of 5–10 mg weekly until a dose of 20 mg is reached, then a tapering of 2.5–5 mg every week is advised. 6 , 33
If there is no meaningful response to the intravenous steroids in acute severe disease within 3–5 days as determined by the Oxford index, steroid-refractory disease should be considered and rescue therapy with other therapeutic entities – either infliximab or cyclosporine – should be initiated. 34
Steroids are very effective in inducing remission but have a number of adverse effects. In addition, they are ineffective in maintaining remission. 35 The frequency and severity of steroid toxicity are substantial and may involve virtually any organ system and many of these complications are irreversible 6 , 36 such as obesity, hypertension, diabetes, cataracts, glaucoma, depression, anxiety, insomnia, irritability, and avascular necrosis. Additionally, the risks of opportunistic infections in patients with inflammatory bowel disease patients using steroids are increased three-fold and are more common over the age of 50 years. 6 , 37 The risks are increased synergistically when steroids are used concomitantly with other immunosuppressive therapies such as infliximab or thiopurines. 37
Thiopurines
Thiopurines (azathioprine [AZA] and 6-mercaptopurine [6-MP]) have a steroid-sparing effect and are used for maintenance of remission when steroids are withdrawn. Thiopurines have no role for induction of remission. AZA and 6-MP are slow-acting medications, and it can take 3 months before therapeutic concentrations are achieved. Hence, a longer course of steroids is often required until the pharmacologic effect of thiopurines is exerted.
AZA and 6-MP have multiple side effects where leukopenia and elevation in transaminases are the most common. These are dose-dependent side effects of the medication and are typically related to the activity of thiopurine methyltransferase (TPMT) enzyme. These adverse effects occur in 10% of the patients and usually in the first month of therapy. 38 It is recommended to monitor CBC and LFTs in patients on treatment with thiopurines frequently when first starting the drug and then periodically thereafter. Given that there is a small risk of mortality secondary to severe leukopenia and infections, the current guidelines recommend testing for TPMT enzymatic activity before starting thiopurines. 39 – 41 Another limiting side effects is intractable nausea. There is 0.3% of the population with homozygous mutations for TPMT, and they have negligible enzyme activity. In these cases, one should avoid using a thiopurine. If the enzymatic activity is intermediate, the starting dose should be reduced by 25–50%. 39 , 42
Thiopurines are also associated with an increased risk of malignancy. There is a risk of non-melanoma skin cancer, and an annual skin exam is advisable to mitigate this risk. Patients should be advised to wear sunscreen and avoid prolonged exposures to the sun. Additionally, there is also an increased risk of lymphoma in patients treated with thiopurines. The incidence is small and is quantified as 1 in 1000-person years. 43 The risk of developing lymphoma is most pronounced in patients with negative Epstein Barr virus at the time drug is initiated, and it is advised to not use thiopurines in these patients. 44 Use of thiopurines over 2 years appears to be a common denominator in cases of hepatosplenic T-cell lymphomas. This is particularly significant in young men under the age of 35. 45
Other nonspecific side effects include abdominal pain, nausea, vomiting, and pancreatitis. Most of these side effects aside from the pancreatitis are self-limited and often dissipate over time. In cases of pancreatitis, however, the drug should be stopped and not reinstituted.
Anti-TNF agents (infliximab, adalimumab, and golimumab)
Different from thiopurines, anti-TNFs can be used for both induction and maintenance of remission. 46 , 47 They are most often used with corticosteroids to induce remission. 48 The American College of Gastroenterology (ACG) and the European Crohn’s and Colitis Organisation (ECCO) recommend the use of infliximab for induction of remission in patients with glucocorticoid-refractory or glucocorticoid-dependent disease. 6 , 49 The recommendations also suggest use in patients with severe disease where standard treatment has failed or is not responding to high-dose steroids in hospital. 6 There are three anti-TNF agents that are approved to be used in moderate-to-severe UC: infliximab, adalimumab, and golimumab. 46 , 50 , 51
Infliximab is a chimeric (combination of human and murine) IgG1 monoclonal antibody that binds with affinity to TNF-ά and neutralizes its biologic activity. 52 Adalimumab and golimumab are 100% human antibodies. There are several clinical trials on infliximab evaluating its efficacy and its use in UC. 50 The number-needed-to-treat is four to induce one case of remission. 53 All anti-TNF agents are effective, with infliximab being slightly more efficacious than adalimumab with regard to inducing a clinical response or mucosal healing, but these results are not well established. 54 Infliximab is an infusion that is typically infused over 2 hours. In contrast, both adalimumab and golimumab are subcutaneous injectables. All of these agents are effective for induction and maintenance of remission in UC. However, only infliximab dosing is weight based and has efficacy in acute severe UC refractory to intravenous steroids. 55
Anti-TNF agents are safe but there are many recognized adverse effects associated with them, which can be minimized by preinitiation testing and careful monitoring after starting the treatment. Injection site reactions are seen in less than 10% of the patients and these reactions are usually mild. Infusion reactions with infliximab can be acute or delayed. Acute reactions occur in the first 24 hours of infusion. True anaphylaxis (IgE mediated) may occur in some patients; however, in most patients it is nonallergic or an anaphylactoid reaction. 56 , 57 If allergic reaction is suspected, the drug should be discontinued, and the patient should not be rechallenged with infliximab.
The most common significant side effect of anti-TNF agents are infections. Most infections are mild, such as common cold, otitis media, and sinusitis. However, these patients are at increased risk of developing serious infections due to their immunocompromised state. All patients must undergo tests to eliminate latent tuberculosis (TB) and chronic hepatitis B infection before starting therapy as both are at increased risk of reactivation if found to be latent in the patient. 58 There are numerous rare side effects associated with anti-TNF therapy. One of the rare but more commonly seen side effects is the nonspecific elevation of liver enzymes. The elevated liver enzymes can be a reaction to the infliximab itself and considered a drug reaction that should reverse with cessation of the drug, but a second condition potentially brought out by infliximab is autoimmune hepatitis that may or may not be directly related to the infliximab. Often in this scenario, the condition persists even with cessation of the infliximab. 59 Periodic testing of liver function tests is recommended. Liver enzymes usually tend to normalize once the drug is discontinued. Although rare, there are case reports of acute liver failure requiring liver transplantation with the use of infliximab. 60
The risk of cancer with anti-TNF therapy is debatable. There appears to be an increased risk of melanoma skin cancer, and a yearly skin exam is advisable. There is conflicting evidence about increased risks of lymphoma; therefore, there are no specific screening recommendations for this while on anti-TNF therapy.
Anti-TNF agents can take up to 6–12 weeks to achieve initial response and mucosal healing. Therapeutic drug monitoring is the new standard of care in treatment of IBD patients. In addition, all anti-TNF agents have risks of developing antibodies altering its efficacy. 61 Hence, checking drug levels and levels of antibodies may allow tailoring of drug dosage or choice of medication to achieve a clinical response or remission. 61 , 62
Calcineurin inhibitors
Cyclosporine has a role in induction of remission in severe-to-fulminant steroid-refractory colitis. Although there are some limited data for the use of tacrolimus, they are not recommended for typical use. Cyclosporine is used as a rescue therapy at select IBD centers, but it does not have a role for long-term therapy. Transition to oral cyclosporine from a continuous infusion is typically performed after patients show response to intravenous cyclosporine. When transitioning to oral cyclosporine, patients are also started on a long-term maintenance plan consisting of thiopurines or anti-integrins. 6 The oral cyclosporine is usually discontinued within 3 months. Even though, over 60% of patients with severe UC respond to intravenous cyclosporine, most will still ultimately require colectomy in 5–7 years. 63
Cyclosporine is administered as a continuous infusion at a dose of 2–4 mg/kg per 24 hours. As studies show similar efficacy and lesser toxicity with the lower dose of 2 mg/kg, many clinicians start with this dose. 64 , 65 It is recommended to maintain intravenous use of glucocorticoids in these patients. Because of the extent of immunosuppression given the steroids, cyclosporine, and a long-term maintenance drug, prophylaxis against pneumocystis pneumonia (PCP) is recommended.
Conversion from the continuous infusion of cyclosporine to oral cyclosporine should be sought early in the course of treatment once a patient shows adequate response to the intravenous dose. Blood levels of cyclosporine should be checked every day to every alternate day with goal levels ranging between 200 and 400 ng/mL in doses 2–4 mg/kg, respectively. Doses can be adjusted based on efficacy and toxicity and rounded off to nearest 25 mg to aid oral conversion, which is calculated by doubling the intravenous dose that led to resolution of symptoms and is administered 12 hours apart. Trough levels are checked before the fourth dose. Levels of 200–300 ng/mL are optimum as levels that are less than 200 ng/mL are associated with loss of response. 66
Patients receiving intravenous cyclosporine should show initial response in 2–3 days of starting treatment, evidenced by clinical resolution of symptoms of abdominal pain, blood in stool, and may have formed stools with normalization of laboratory tests. Before transitioning to oral cyclosporine, patients should be able to tolerate an oral diet. In patients who fail to show resolution of symptoms of severe disease in 72 hours, Clostridioides difficile should be tested and treated if positive. Unfortunately, patients failing to respond within 72 hours likely will need a colectomy.
Patients responding to intravenous cyclosporine and successfully transitioned or oral cyclosporine can be discharged on oral cyclosporine, oral steroids, a long-term steroid sparing drug (e.g. thiopurine or anti-integrin) and PCP prophylaxis with a tapering regimen of steroids over the 4–6 weeks followed by tapering of oral cyclosporine over the ensuing 3 months. Patients who cannot get off steroids should be evaluated for surgery.
Adverse effects are common with use of cyclosporine and sometimes, life threatening. Patients must be monitored for electrolyte abnormalities like hyperkalemia and hypomagnesemia. Nephrotoxicity is a common side effect and is usually reversible after discontinuation of the drug. Neurotoxicity may manifest as mild tremor or sometimes, severe headache, visual abnormality or seizures. 67 Calcineurin-inhibitor pain syndrome is characterized by symmetrical pain in feet and ankles. Symptoms may improve once the drug is stopped or by use of calcium channel blockers. 68
Anti-integrins
Integrins are proteins that regulate migration of leucocytes to the intestines. Vedolizumab is a fully humanized recombinant monoclonal antibody that binds to alpha4–beta7 integrin and prevents migration of leucocytes to the gut. Vedolizumab has shown to be effective and is approved for use to induce and maintain remission in moderate-to-severe active UC. 69 , 70 It is the first anti-integrin approved for use in UC. The initial therapeutic response is usually seen in 6 weeks of treatment, but it can take up to 6 months for the full maximal benefit to be seen.
With regard to safety, vedolizumab is the safest biologic available with minimal side effects such as intestinal infections – attributed to its mechanism of action that is very gut therapeutic. 71 There is a small theoretical risk of developing progressive multifocal leukoencephalopathy (PML), which is a viral infection of the brain resulting in severe disability and death and has been associated with the use of anti-integrins. However, in the initial studies there are no reported cases of PML with vedolizumab. 71 , 72 Upper respiratory tract infections are the most common infections in patients on treatment with vedolizumab. There is no increased incidence of abdominal infections and lower respiratory tract infections with vedolizumab when compared to placebo. 71 Infusion-related reactions are also identified as an adverse event of vedolizumab with an incidence of <5% with most of these reactions being mild to moderate. 71 , 72 These are mostly self-limiting and do not usually require the discontinuation of the drug.
Tofacitinib
Tofacitinib is a Janus kinase inhibitor and was recently licensed in 2018 for treatment of moderate-to-severe active UC. 73 The timing and decision to use is similar to that of anti-TNFs or vedolizumab. It is indicated for treatment of adult patients with moderate-to-severe UC, but it is not recommended for use in combination with other biologics or potent immunosuppressants such as a thiopurine or calcineurin inhibitor. 73 A decision to start treatment with tofacitinib should be based on the patient’s compliance with drug therapy and comfort with the drug’s adverse events profile. In the United States insurance coverage and costs also need to be considered. Initial drug response can be seen in 6 weeks.
Tofacitinib is the first oral formulation of a small molecule that is taken twice a day. It is available in doses of 5 mg and 10 administered twice a day. The lowest effective dose should be used to maintain the response. If adequate therapeutic benefit is not achieved after 16 weeks of 10 mg twice a day dosing, it must be discontinued. Dose adjustment is required in moderate-to-severe renal impairment and it is recommended to cut down to a half-daily dose compared with the dose given to patients with normal renal function. It is not recommended to use tofacitinib in patients with severe hepatic impairment. Half-dosing should also apply to those patients receiving concomitant CYP 3A4 inhibitors such as ketoconazole. 73
Adverse effects of tofacitinib are similar to anti-TNF agents. 74 Serious and sometimes fatal infections due to bacterial, mycobacterial, invasive fungal, viral, or other opportunistic pathogens have been reported in the clinical trials with tofacitinib. 73 Patients with UC on 10 mg twice daily were associated with a greater risk of serious infections compared with those on 5 mg twice daily. Additionally, opportunistic herpes zoster infections including meningoencephalitis, ophthalmologic, and disseminated cutaneous were seen in patients on 10 mg twice daily. 73 To mitigate the risk of zoster activation, it is recommended that these patients should be vaccinated against zoster.
Before starting tofacitinib, patients should be evaluated and tested for latent or active TB. In patients who are tested positive for latent TB, it is recommended to consult an infectious disease specialist to whether or not to initiate anti-TB therapy before starting the treatment with tofacitinib. Other side effects include neutropenia and it is recommended that patients should undergo episodic checking of a CBC with differential. It is also associated with an increase in liver enzymes of up to three times the upper limit of normal. Reduction of dose of tofacitinib in these patients resulted in normalization of liver enzymes. 75
UC is a chronic inflammatory condition where medications are used to induce remission and maintain a steroid-free remission. Up to 15% patients may require colectomy due to inability to control the disease. The choice of medication depends upon the clinical stage of the disease. Contrary to the historical treatment paradigm of a bottom-up versus top-down strategy, now the recommendation is to treat the underlying severity of disease with medications that are most appropriate for that level of disease severity. In cases of mild-to-moderate disease severity, mesalamine is preferred as it is the safest available drug for the management of UC with a 0.2% risk of interstitial nephritis. However, if the disease is not responding adequately to mesalamine or if the disease is categorized as moderate-to-severe, then one should utilize immunosuppressants, and biologics including anti-TNF, anti-integrin, or a small molecule Janus kinase inhibitors. Thiopurines including azathioprine and mercaptopurine have been utilized for decades in the management of UC, but they only have a role in maintenance of remission and can take up to 3 months to achieve efficacy. In contrast, anti-TNF medications including infliximab, adalimumab, and golimumab all have efficacy for induction of remission and maintenance of remission. The drugs are fairly equivalent, but infliximab has greater bioavailability, as it is administered intravenously, and can be dosed based on one’s weight. These drugs have side effects from the immunosuppression but no more than a thiopurine. The safest available biologic is vedolizumab that is a gut-specific anti-integrin. Given its gut specificity it does not carry many side effects. The newest group of drugs is the small molecule Janus kinase inhibitors. Tofacitinib is an oral pill taken twice a day that is likely to be quite desirable to patients given the mode of administration. However, it still retains a side-effect profile that is equal to, or more significant than, anti-TNF medications. All of these drugs should be considered in the appropriate setting based on the severity of the UC. Most importantly, though, no patients should be left on long-term corticosteroids.
Acknowledgements
Contributions: Both authors contributed equally to the preparation of this review. The authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: The authors declare that they have no conflicts of interest. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors are available for download at http://www.drugsincontext.com/wp-content/uploads/2019/03/dic.212572-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2019 Tripathi K, Feuerstein JD. https://doi.org/10.7573/dic.212572 . Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Article URL: https://drugsincontext.com/new-developments-in-ulcerative-colitis:-latest-evidence-on-management,-treatment,-and-maintenance
Provenance: invited; externally peer reviewed.
Peer review comments to author: 8 January 2019
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editor-in-Chief [email protected]
For all permissions, rights and reprints, contact David Hughes [email protected]
- Our Ambition, Purpose, & Values
- Executive Leadership Team
- Board of Directors
- Our Locations
- Gastrointestinal
- Dermatology Products/Aesthetics Devices
- International Pharmaceuticals
- Generics, Neurology and Other
- Vision Care
- U.S. Product List
- Our Pipeline
- U.S. Health Care Compliance Policy
- Declaration of Compliance
- Misconduct Reporting Mechanisms
- Auditing, Monitoring and Risk Assessments
- Training and Awareness
- Policies and Procedures
- Compliance Governance
- U.S. Grants
- Payments to U.S. Health Care Professionals
- Charitable Contributions
- Education and Research Grants
- Patient Assistance Programs
- Responsible Procurement
- Global Quality Management System (GQMS)
- Public Reporting on Product and Service Safety Issues
- Safety Data Sheets
- Responsible Clinical Trials
- INVESTOR RELATIONS HOME
- Stock Chart
- Historical Price Lookup
- Investment Calculator
- Advanced Fundamentals
- Annual Reports Archive
- SEC Filings
- Corporate Governance Documents
- News Releases
- Events and Presentations
- Financial Tear Sheet
- Shareholder Services
- IR and Communications Contacts
- Working at Bausch Health
- Our U.S. Businesses
Salix to Present Late-Breaking Data from Phase 2 Trial of Amiselimod in Active Ulcerative Colitis at Digestive Disease Week 2024
May 17, 2024
LAVAL, Quebec, May 17, 2024 – Bausch Health Companies Inc. (NYSE/TSX: BHC) and its gastroenterology (GI) business, Salix Pharmaceuticals, today announced that they will be presenting data from its Phase 2 trial evaluating Amiselimod as treatment for active ulcerative colitis (UC). The data will be presented at Digestive Disease Week (DDW) 2024 during the IMIBD Late Breakers and Innovations in IBD session on Sunday, May 19, 2024, in Washington, D.C.
“We are pleased to present late-breaking data on Amiselimod, our investigational, oral, sphingosine 1- phosphate (S1P) receptor modulator as a potential treatment for the induction of remission in UC,” said Tage Ramakrishna, M.D., Chief Medical Officer and President of Research & Development, Bausch Health. “The abstract underscores our steadfast commitment to developing new and innovative therapies for patients with UC.”
The research to be featured at DDW 2024 and available via the meeting's online platform is as follows:
- Hanauer, Stephen B. et al. “Amiselimod for the treatment of active ulcerative colitis: a randomized, double-blind, placebo-controlled trial” Abstract #4094796
The Phase 2 clinical trial was a 12-week, double-blind, placebo-controlled, randomized, dose ranging study to evaluate the efficacy and safety of Amiselimod in 320 patients with mildly-to- moderately active UC. Bausch Health announced positive topline results from this study in December 2023.
About Amiselimod Amiselimod is a sphingosine-1-phosphate (S1P) receptor functional antagonist and, by inhibiting the receptor function of the lymphocyte sphingosine-1-phosphate (S1P) receptor, retains lymphocytes sequestered in the lymph nodes and prevents them from contributing to autoimmune reactions.1 Due to this mechanism of action, Amiselimod may potentially be useful for various autoimmune diseases.2 Affinity to S1P1 and S1P5 receptor subtypes, suggests that Amiselimod could potentially have a more pronounced effect on ulcerative colitis related inflammation than compounds with restricted activity on S1P1 receptor subtype exclusively or combined activity on S1P1 and S1P5. 3
About Salix Salix Pharmaceuticals is one of the largest specialty pharmaceutical companies in the world committed to the prevention and treatment of gastrointestinal diseases. For more than 30 years, Salix has licensed, developed, and marketed innovative products to improve patients' lives and provide health care providers with life-changing solutions for many chronic and debilitating conditions. Salix currently markets its product line to U.S. health care providers through an expanded sales force that focuses on gastroenterology, hepatology, pain specialists, and primary care. Salix is headquartered in Bridgewater, New Jersey. For more information about Salix, visit www.Salix.com and connect with us on Twitter and Linkedin
About Bausch Health Bausch Health Companies Inc. (NYSE:BHC)(TSX:BHC) is a global diversified pharmaceutical company enriching lives through our relentless drive to deliver better health outcomes. We develop, manufacture and market a range of products, primarily in gastroenterology, hepatology, neurology, dermatology, medical aesthetic devices, international pharmaceuticals, and eye health, through our controlling interest in Bausch + Lomb. Our ambition is to be a globally integrated healthcare company, trusted and valued by patients, HCPs, employees and investors. For more information, visit www.bauschhealth.com and connect with us on Twitter and LinkedIn .
About DDW Digestive Disease Week® (DDW) is the largest international gathering of physicians, researchers and academics in the fields of gastroenterology, hepatology, endoscopy and gastrointestinal surgery. Jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA), the American Society for Gastrointestinal Endoscopy (ASGE) and the Society for Surgery of the Alimentary Tract (SSAT), DDW is an in-person and online meeting from May 18-21, 2024. The meeting showcases more than 4,400 abstracts and hundreds of lectures on the latest advances in GI research, medicine and technology. More information can be found at www.ddw.org .
- Kunio Sugahara, Yasuhiro Maeda. Amiselimod, a novel sphingosine 1‐phosphate receptor‐1 modulator, has potent therapeutic efficacy for autoimmune diseases, with low bradycardia risk. British Journal of Pharmacology. January 2017.
- Peyrin-Biroulet, Ronald Christopher Modulation of sphingosine-1-phosphate in inflammatory bowel disease. Autoimmunity Reviews. February 2017.
- BiseraStepanovska, AndreaHuwiler . Targeting the S1P receptor signaling pathways as a promising approach for treatment of autoimmune and inflammatory diseases. Pharmacological Research. February 2019.
©2024 Salix Pharmaceuticals or its affiliates. UNB.0018.USA.24


STEPHEN M. ADAMS, MD, ELIZABETH D. CLOSE, MD, AND APARNA P. SHREENATH, MD, PhD
Am Fam Physician. 2022;105(4):406-411
Related Letter to the Editor: New-Onset Ulcerative Colitis in Patients With COVID-19
Author disclosure: No relevant financial relationships.
Ulcerative colitis is a relapsing and remitting inflammatory bowel disease of the large intestine. Risk factors include recent Salmonella or Campylobacter infection and a family history of ulcerative colitis. Diagnosis is suspected based on symptoms of urgency, tenesmus, and hematochezia and is confirmed with endoscopic findings of continuous inflammation from the rectum to more proximal colon, depending on the extent of disease. Fecal calprotectin may be used to assess disease activity and relapse. Medications available to treat the inflammation include 5-aminosalicylic acid, corticosteroids, tumor necrosis factor–alpha antibodies, anti-integrin antibodies, anti-interleukin-12 and -23 antibodies, and Janus kinase inhibitors. Choice of medication and method of delivery depend on the location and severity of mucosal inflammation. Other treatments such as fecal microbiota transplantation are considered experimental, and complementary therapies such as probiotics and curcumin have mixed data. Surgical treatment may be needed for fulminant or refractory disease. Increased risk of colorectal cancer and use of immunosuppressive therapies affect the preventive care needs for these patients.
Ulcerative colitis is a relapsing and remitting inflammatory bowel disease frequently encountered in primary care. This article provides a summary of ulcerative colitis and a review of the available evidence for management.
Epidemiology and Risk Factors
Ulcerative colitis most commonly presents between 15 and 30 years of age and is more common in industrialized nations, with a prevalence of 286 per 100,000 adults in the United States. 1 , 2
Incidence is similar in men and women. 3
Risk factors include urban living; family history of ulcerative colitis; recent Salmonella , Clostridioides difficile , or Campylobacter infection; tobacco cessation; and soda consumption. 4 , 5
Protective factors include history of appendectomy, active tobacco use, tea consumption, and having been breastfed as an infant. 4 , 5

DIFFERENTIAL DIAGNOSIS
Active Salmonella , Shigella , Escherichia coli , Yersinia , Campylobacter , or C. difficile infection should be ruled out using stool studies. 1
Amebic dysentery should be considered if an appropriate travel or exposure history exists. Cytomegalovirus infection should be excluded in immunocompromised patients. 1
Other causes of bloody diarrhea include ischemic colitis, Crohn disease, and colitis caused by medications or radiation. Non-bloody diarrhea can be caused by microscopic colitis, irritable bowel syndrome, celiac disease, or food intolerances. 6
SIGNS AND SYMPTOMS
The most common presenting symptom is bloody diarrhea. Other common symptoms include abdominal pain, tenesmus, and fecal urgency. 1 , 2
Extraintestinal manifestations include arthropathies, erythema nodosum, pyoderma gangrenosum, uveitis, iritis, and primary sclerosing cholangitis. These may be present before the onset of gastrointestinal symptoms. 7 , 8
Overall, extraintestinal manifestations are only 6% more common in patients with inflammatory bowel disease than in the general population and are more common with Crohn disease compared with ulcerative colitis. 8
DIAGNOSTIC TESTING
Lower endoscopy should be performed on all adult patients with suspected ulcerative colitis. 1 , 9
Fecal calprotectin testing has a high negative predictive value and helps to differentiate inflammatory bowel disease from irritable bowel syndrome, but no serum biomarkers alone are sufficient for the diagnosis of ulcerative colitis. 10 A normal fecal calprotectin level (100 mcg per g or less) in children virtually excludes the diagnosis of ulcerative colitis (100% negative predictive value; 95% CI, 98% to 100%). Therefore, in children with a negative fecal calprotectin test, endoscopy can be limited to those whose symptoms persist without another diagnosis. 9 , 11
Bacterial stool culture, including C. difficile toxin assay and stool examination for ova and parasites, should be performed. Other tests, such as complete blood count, erythrocyte sedimentation rate, and measurement of C-reactive protein, may be useful but are nonspecific. 1
Endoscopic evidence of continuous colonic inflammation starting at the rectum with confirmatory biopsies establishes the diagnosis of ulcerative colitis. 1
Elevation in serial measurements of fecal calprotectin predicts relapse, whereas serial values in the normal range predict continued remission over time. 12
INDUCTION AND MAINTENANCE OF REMISSION
The goal of managing patients with ulcerative colitis is to attain mucosal healing with symptom control so that sustained steroid-free remission can be achieved and prevent hospitalizations and surgeries.
Initiation of treatment begins with stratifying disease activity into mild vs. moderate to severe. The American College of Gastroenterology Ulcerative Colitis Activity Index provides a set of criteria to help determine if the disease is in remission, mild, moderate to severe, or fulminant. 1
Therapy and medication delivery modes ( Table 1 ) are based on the location and extent of mucosal inflammation. This is broadly divided into proctitis (i.e., 18 cm from the true anal verge), left-sided colitis (i.e., extending to the splenic flexure), and pancolitis (i.e., extending proximal to the splenic flexure). 1
TREATMENT OF MILD DISEASE
The 2019 guidelines from the American College of Gastroenterology recommend treatment of mild ulcerative proctitis with rectal 5-amino-salicylic acid (5-ASA) therapies. 1
Mild to moderate colitis should be treated with a combination of rectal 5-ASA enemas and oral 5-ASA therapies. Rectal 5-ASA enemas are preferred to rectal steroid formulations. Mesalamine is more potent than sulfasalazine for inducing remission. 1 , 13
Patients who are unresponsive to or intolerant of 5-ASA should use oral budesonide, extended release (Uceris; multimatrix formulation designed to deliver medication to the colonic mucosa). 1
TREATMENT OF MODERATE TO SEVERE DISEASE
First-line therapy for moderate to severe ulcerative colitis is biologics. 14
Biologic agents with or without glucocorticoids and immune modulators should be used to induce and maintain remission. Thiopurines or methotrexate should not be used as monotherapy. 1
Systemic corticosteroids are effective in inducing remission, but dosages and treatment duration should be limited. Other options for inducing remission include tumor necrosis factor–alpha antibodies, anti-integrin antibodies, anti-interleukin antibodies, and Janus kinase inhibitors 1 ( Table 1 ) .
Fecal microbiota transplantation induces remission in some patients with ulcerative colitis, but current use is limited to clinical trials. 15 – 17
SURGICAL TREATMENT
Among patients with ulcerative colitis, 15% will ultimately need colectomy. Indications include failure of medical therapy, toxic megacolon, perforation, uncontrolled hemorrhage, or dysplasia/malignancy. 7
About 50% of patients who undergo colectomy will experience postoperative inflammation of the residual rectal tissue. 18
Predictors for aggressive disease include age younger than 40 years, pancolitis, severe disease activity seen on endoscopy, presence of extraintestinal manifestations, early need for steroids, and elevated inflammatory markers. 1
COMPLEMENTARY MEDICINE
One probiotic (VSL#3) modestly improves symptoms. It also helps to prevent pouchitis, an abnormal immune response in patients susceptible to autoimmune disease that leads to inflammation of the rectal pouch fashioned after a colectomy. 19 – 21
A systematic review of six small studies found that curcumin (2 to 3 g daily) promotes clinical and endoscopic improvement when added to conventional therapy in patients with mild ulcerative colitis. 22
Fish oil does not improve remission rates. 23 , 24
Acupuncture is considered safe in addition to conventional treatment, but high-quality evidence of effectiveness is lacking. 25
LIFESTYLE AND BEHAVIORAL INTERVENTIONS
Exercise and diet interventions help improve symptom burden and quality of life.
A low fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAP) diet reduces symptoms but does not change clinical severity. 26 , 27
Physical activity improves quality of life and the anxiety that commonly affects patients with inflammatory bowel disease. 28
HOSPITAL CARE
Up to 25% of patients with ulcerative colitis will require hospitalization for severe disease.
Early endoscopy should be performed to exclude cytomegalovirus colitis, and C. difficile testing should be ordered.
Avoidance of nonsteroidal anti-inflammatory drugs, opiates, and anticholinergic medications is recommended. Antibiotics should not be used routinely.
Surgical consultation should be obtained for patients not responding to intravenous corticosteroids after three days, or earlier if other surgical indications (e.g., toxic megacolon) arise.
If there is failure of intravenous corticosteroids after three days of treatment, cyclosporine (Sandimmune) or infliximab (Remicade) may be used as rescue therapy. 29
Preventive Care Considerations
Vaccinations should be given according to routine recommendations from the Advisory Committee on Immunization Practices and the Centers for Disease Control and Prevention, paying special attention to additional vaccinations necessary for patients on immunosuppressive therapies. 30
Dual energy x-ray absorptiometry is recommended to check for low bone mineral density in patients with ulcerative colitis, especially those with a history of chronic oral corticosteroid use for three months or more. 31
Skin cancer occurs at higher rates in patients with inflammatory bowel disease. In addition, common therapies for ulcerative colitis increase the risk of melanoma and nonmelanoma skin cancer. 32
The American College of Obstetricians and Gynecologists recommends annual cytology screening for cervical cancer in women on immunosuppressive therapy. 33
Colonoscopy is recommended starting eight years after diagnosis of ulcerative colitis or immediately if primary sclerosing cholangitis is also present because of an increased risk of colorectal cancer. Interval surveillance in those with disease proximal to the sigmoid colon should occur every one to three years based on risk factors and prior endoscopy findings, with annual colonoscopies in patients with concomitant primary sclerosing cholangitis, due to very high risk of developing colorectal cancer. 1 , 34
Most patients with ulcerative colitis experience a mild to moderate course with periods of remission and flare-ups.
Ulcerative colitis does not increase mortality but is associated with high morbidity. 7
ULCERATIVE COLITIS AND COVID-19
Based on a panel of international experts, in the absence of definitive data, the American Gastroenterological Association has concluded that the risk of a patient with ulcerative colitis becoming infected with SARS-CoV-2 is no higher than that of the general population, independent of treatment.
It is unknown whether active inflammation from ulcerative colitis can increase the risk of infection with SARS-CoV-2.
The American Gastroenterological Association recommends ongoing biologic therapy, deeming it safe for patients who are on such medications to continue working in environments with those who have known or suspected SARS-CoV-2 infection.
Patients who test positive for SARS-CoV-2 and whose ulcerative colitis medications are held because of this can restart their medications after 14 days if they do not develop symptoms or if symptoms resolve. 35
A retrospective review of patients vaccinated against SARS-CoV-2 suggests the vaccine effectiveness and adverse event rate in patients with inflammatory bowel disease is similar to the general population. 36
This article updates a previous article on this topic by Adams and Bornemann . 37
Data Sources: This article was based on ACG and AGA Guidelines, the Cochrane database, Essential Evidence Plus, and a PubMed search including meta-analyses, randomized controlled trials, and systematic reviews. Search dates: December 2020 through October 2021.
- Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019;114(3):384-413.
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies [published correction appears in Lancet . 2020; 396(10256): e56]. Lancet. 2017;390(10114):2769-2778.
Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Front Neuroendocrinol. 2014;35(3):347-369.
- Esan OB, Perera R, McCarthy N, et al. Incidence, risk factors, and health service burden of sequelae of Campylobacter and non-typhoidal Salmonella infections in England, 2000–2015: a retrospective cohort study using linked electronic health records. J Infect. 2020;81(2):221-230.
- Piovani D, Danese S, Peyrin-Biroulet L, et al. Environmental risk factors for inflammatory bowel diseases: an umbrella review of meta-analyses. Gastroenterology. 2019;157(3):647-659.e4.
- Arasaradnam RP, Brown S, Forbes A, et al. Guidelines for the investigation of chronic diarrhoea in adults: British Society of Gastroenterology, 3rd ed. Gut. 2018;67(8):1380-1399.
- Fumery M, Singh S, Dulai PS, et al. Natural history of adult ulcerative colitis in population-based cohorts: a systematic review. Clin Gastroenterol Hepatol. 2018;16(3):343-356.e3.
Card TR, Langan SM, Chu TPC. Extra-gastrointestinal manifestations of inflammatory bowel disease may be less common than previously reported. Dig Dis Sci. 2016;61(9):2619-2626.
- Holtman GA, Lisman-van Leeuwen Y, Kollen BJ, et al. Diagnostic accuracy of fecal calprotectin for pediatric inflammatory bowel disease in primary care: a prospective cohort study. Ann Fam Med. 2016;14(5):437-445. Accessed October 14, 2021. https://www.annfammed.org/content/14/5/437.long
- Menees SB, Powell C, Kurlander J, et al. A meta-analysis of the utility of C-reactive protein, erythrocyte sedimentation rate, fecal calprotectin, and fecal lactoferrin to exclude inflammatory bowel disease in adults with IBS. Am J Gastroenterol. 2015;110(3):444-454.
- Walker GJ, Chanchlani N, Thomas A, et al. Primary care faecal calprotectin testing in children with suspected inflammatory bowel disease: a diagnostic accuracy study. Arch Dis Child. 2020;105(10):957-963.
Heida A, Park KT, van Rheenen PF. Clinical utility of fecal calprotectin monitoring in asymptomatic patients with inflammatory bowel disease: a systematic review and practical guide. Inflamm Bowel Dis. 2017;23(6):894-902.
- Ko CW, Singh S, Feuerstein JD, et al.; American Gastroenterological Association Institute Clinical Guidelines Committee. AGA clinical practice guidelines on the management of mild-to-moderate ulcerative colitis. Gastroenterology. 2019;156(3):748-764.
- Feuerstein JD, Isaacs KL, Schneider Y, et al.; AGA Institute Clinical Guidelines Committee. AGA clinical practice guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology. 2020;158(5):1450-1461.
- Paramsothy S, Kamm MA, Kaakoush NO, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017;389(10075):1218-1228.
- Costello SP, Hughes PA, Waters O, et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA. 2019;321(2):156-164.
- Imdad A, Nicholson MR, Tanner-Smith EE, et al. Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database Syst Rev. 2018;(11):CD012774.
- Nguyen N, Zhang B, Holubar SD, et al. Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis. Cochrane Database Syst Rev. 2019;(11):CD001176.
- Tursi A, Brandimarte G, Papa A, et al. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2010;105(10):2218-2227.
Limketkai BN, Wolf A, Parian AM. Nutritional interventions in the patient with inflammatory bowel disease. Gastroenterol Clin North Am. 2018;47(1):155-177.
Lin SC, Cheifetz AS. The use of complementary and alternative medicine in patients with inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2018;14(7):415-425.
- Coelho MR, Romi MD, Ferreira DMTP, et al. The use of curcumin as a complementary therapy in ulcerative colitis: a systematic review of randomized controlled clinical trials. Nutrients. 2020;12(8):2296.
- Scaioli E, Sartini A, Bellanova M, et al. Eicosapentaenoic acid reduces fecal levels of calprotectin and prevents relapse in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2018;16(8):1268-1275.e2.
Turner D, Steinhart AH, Griffiths AM. Omega 3 fatty acids (fish oil) for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2007;(3):CD006443.
- Wang X, Zhao NQ, Sun YX, et al. Acupuncture for ulcerative colitis: a systematic review and meta-analysis of randomized clinical trials. BMC Complement Med Ther. 2020;20(1):309.
- Cox SR, Lindsay JO, Fromentin S, et al. Effects of low FOD-MAP diet on symptoms, fecal microbiome, and markers of inflammation in patients with quiescent inflammatory bowel disease in a randomized trial. Gastroenterology. 2020;158(1):176-188.e7.
- Prince AC, Myers CE, Joyce T, et al. Fermentable carbohydrate restriction (low FODMAP diet) in clinical practice improves functional gastrointestinal symptoms in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016;22(5):1129-1136.
- Eckert KG, Abbasi-Neureither I, Köppel M, et al. Structured physical activity interventions as a complementary therapy for patients with inflammatory bowel disease - a scoping review and practical implications. BMC Gastroenterol. 2019;19(1):115.
Fudman DI, Sattler L, Feuerstein JD. Inpatient management of acute severe ulcerative colitis. J Hosp Med. 2019;14(12):766-773.
Centers for Disease Control and Prevention. Recommended adult immunization schedule by medical condition and other indications, United States, 2021. U.S. Department of Health and Human Services. Last reviewed February 12, 2021. Accessed September 8, 2021. https://www.cdc.gov/vaccines/schedules/hcp/imz/adult-conditions.html#table-conditions
- Cosman F, de Beur SJ, LeBoff MS, et al.; National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis [published correction appears in Osteoporos Int . 2015;26(7):2045–2047]. Osteoporos Int. 2014;25(10):2359-2381.
- Farraye FA, Melmed GY, Lichtenstein GR, et al. ACG clinical guideline: preventive care in inflammatory bowel disease [published correction appears in Am J Gastroenterol . 2017;112(7):1208]. Am J Gastroenterol. 2017;112(2):241-258.
- ACOG Committee on Practice Bulletins—Gynecology. Practice bulletin no. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128(4):e111-e130.
- Lopez A, Pouillon L, Beaugerie L, et al. Colorectal cancer prevention in patients with ulcerative colitis. Best Pract Res Clin Gastroenterol. 2018;32–33:103-109.
- Rubin DT, Feuerstein JD, Wang AY, et al. AGA clinical practice update on management of inflammatory bowel disease during the COVID-19 pandemic: expert commentary. Gastroenterology. 2020;159(1):350-357.
- Hadi YB, Thakkar S, Shah-Khan SM, et al. COVID-19 vaccination is safe and effective in patients with inflammatory bowel disease: analysis of a large multi-institutional research network in the United States. Gastroenterology. 2021;161(4):1336-1339.e3.
Adams SM, Bornemann PH. Ulcerative colitis. Am Fam Physician. 2013;87(10):699-705. Accessed September 8, 2021. https://www.aafp.org/afp/2013/0515/p699.html
Continue Reading
More in AFP
More in pubmed.
Copyright © 2022 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
A comprehensive review and update on ulcerative colitis
Affiliations.
- 1 Department of Internal Medicine, Texas Tech University Health Science Center El Paso, 2000B Transmountain Road, El Paso, TX 79911, USA; Paul L. Foster School of Medicine (PLFSOM), Texas Tech University Health Science Center El Paso, 5001 El Paso Dr, El Paso, TX 79905, USA. Electronic address: [email protected].
- 2 Department of Internal Medicine, Texas Tech University Health Sciences Center, 4800 Alberta Avenue, El Paso, TX 79905, USA.
- 3 Paul L. Foster School of Medicine (PLFSOM), Texas Tech University Health Science Center El Paso, 5001 El Paso Dr, El Paso, TX 79905, USA.
- 4 Department of Family Medicine, Texas Tech University Health Science Center El Paso, 2000B Transmountain Road, El Paso, TX 79911, USA.
- 5 Department of Surgery, Texas Tech University Health Science Center El Paso, 2000B Transmountain Road, El Paso, TX 79911, USA.
- 6 Department of Gastroenterology & Hepatology, University of California San Francisco (UCSF), Fresno, CA, USA.
- 7 Division of Gastroenterology, American University of Beirut, Beirut, Lebanon.
- 8 Division of Gastroenterology, American University of Beirut, Beirut, Lebanon; Division of Gastroenterology, Hepatology and Nutrition, University of Pittsburgh, M2, C Wing, 200 Lothrop Street, Pittsburgh, PA 15213, USA.
- PMID: 30837080
- DOI: 10.1016/j.disamonth.2019.02.004
Ulcerative colitis (UC) is a chronic idiopathic inflammatory bowel disorder of the colon that causes continuous mucosal inflammation extending from the rectum to the more proximal colon, with variable extents. UC is characterized by a relapsing and remitting course. UC was first described by Samuel Wilks in 1859 and it is more common than Crohn's disease worldwide. The overall incidence and prevalence of UC is reported to be 1.2-20.3 and 7.6-245 cases per 100,000 persons/year respectively. UC has a bimodal age distribution with an incidence peak in the 2nd or 3rd decades and followed by second peak between 50 and 80 years of age. The key risk factors for UC include genetics, environmental factors, autoimmunity and gut microbiota. The classic presentation of UC include bloody diarrhea with or without mucus, rectal urgency, tenesmus, and variable degrees of abdominal pain that is often relieved by defecation. UC is diagnosed based on the combination of clinical presentation, endoscopic findings, histology, and the absence of alternative diagnoses. In addition to confirming the diagnosis of UC, it is also important to define the extent and severity of inflammation, which aids in the selection of appropriate treatment and for predicting the patient's prognosis. Ileocolonoscopy with biopsy is the only way to make a definitive diagnosis of UC. A pathognomonic finding of UC is the presence of continuous colonic inflammation characterized by erythema, loss of normal vascular pattern, granularity, erosions, friability, bleeding, and ulcerations, with distinct demarcation between inflamed and non-inflamed bowel. Histopathology is the definitive tool in diagnosing UC, assessing the disease severity and identifying intraepithelial neoplasia (dysplasia) or cancer. The classical histological changes in UC include decreased crypt density, crypt architectural distortion, irregular mucosal surface and heavy diffuse transmucosal inflammation, in the absence of genuine granulomas. Abdominal computed tomographic (CT) scanning is the preferred initial radiographic imaging study in UC patients with acute abdominal symptoms. The hallmark CT finding of UC is mural thickening with a mean wall thickness of 8 mm, as opposed to a 2-3 mm mean wall thickness of the normal colon. The Mayo scoring system is a commonly used index to assess disease severity and monitor patients during therapy. The goals of treatment in UC are three fold-improve quality of life, achieve steroid free remission and minimize the risk of cancer. The choice of treatment depends on disease extent, severity and the course of the disease. For proctitis, topical 5-aminosalicylic acid (5-ASA) drugs are used as the first line agents. UC patients with more extensive or severe disease should be treated with a combination of oral and topical 5-ASA drugs +/- corticosteroids to induce remission. Patients with severe UC need to be hospitalized for treatment. The options in these patients include intravenous steroids and if refractory, calcineurin inhibitors (cyclosporine, tacrolimus) or tumor necrosis factor-α antibodies (infliximab) are utilized. Once remission is induced, patients are then continued on appropriate medications to maintain remission. Indications for emergency surgery include refractory toxic megacolon, colonic perforation, or severe colorectal bleeding.
Copyright © 2019 Elsevier Inc. All rights reserved.
Publication types
- Anti-Inflammatory Agents / therapeutic use
- Colitis, Ulcerative / complications
- Colitis, Ulcerative / diagnosis
- Colitis, Ulcerative / pathology
- Colitis, Ulcerative / therapy*
- Colon / pathology
- Inflammation / diagnosis
- Inflammation / therapy
- Intestinal Mucosa / pathology*
- Quality of Life
- Rectum / pathology
- Severity of Illness Index
- Anti-Inflammatory Agents
- Open access
- Published: 14 May 2024
The development of probiotics and prebiotics therapy to ulcerative colitis: a therapy that has gained considerable momentum
- Jing Guo 1 ,
- Liping Li 1 ,
- Yue Cai 2 &
- Yongbo Kang 1
Cell Communication and Signaling volume 22 , Article number: 268 ( 2024 ) Cite this article
344 Accesses
1 Altmetric
Metrics details
Ulcerative colitis (UC) is increasingly common, and it is gradually become a kind of global epidemic. UC is a type of inflammatory bowel disease (IBD), and it is a lifetime recurrent disease. UC as a common disease has become a financial burden for many people and has the potential to develop into cancer if not prevented or treated. There are multiple factors such as genetic factors, host immune system disorders, and environmental factors to cause UC. A growing body of research have suggested that intestinal microbiota as an environmental factor play an important role in the occurrence and development of UC. Meanwhile, evidence to date suggests that manipulating the gut microbiome may represent effective treatment for the prevention or management of UC. In addition, the main clinical drugs to treat UC are amino salicylate and corticosteroid. These clinical drugs always have some side effects and low success rate when treating patients with UC. Therefore, there is an urgent need for safe and efficient methods to treat UC. Based on this, probiotics and prebiotics may be a valuable treatment for UC. In order to promote the wide clinical application of probiotics and prebiotics in the treatment of UC. This review aims to summarize the recent literature as an aid to better understanding how the probiotics and prebiotics contributes to UC while evaluating and prospecting the therapeutic effect of the probiotics and prebiotics in the treatment of UC based on previous publications.
Introduction
Ulcerative colitis (UC) is a chronic non-specific intestinal inflammatory disease [ 1 ]. UC becomes an important health problem, because it’s high morbidity. Especially in newly industrialized countries [ 2 ]. Research shows that the incidence of UC is 10 to 20 patients per 100,000 people every year [ 3 ]. UC often presents with recurrent attacks. And the inflammatory of UC will become a factor of colon cancer in the long run [ 4 ]. The pathogenic factors of UC are sophisticated, it is related to intestinal microbiota, immune function of the body (For example, UC is closely related with Th2 cells) [ 5 ], genetic factor and environment factor (e.g. life-style, dietary habits) and so on [ 6 ]. wherein, intestinal microbiota is one of the most important factor that arise UC [ 7 ]. Therefore, we can use probiotics to regulate the intestinal flora in the treatment of UC [ 8 , 9 ]. A growing body of research has shown that probiotics and prebiotics can bring about remission the symptoms of UC improving intestinal mucosal homeostasis, ameliorating the intestinal microbiota environment, regulating the body’s immune function. Therefore, probiotics and prebiotics may be a very safe and efficient treatment for UC. At the same time, it can greatly reduce the financial burden of patients. Furthermore, New techniques have made it possible to attempt systematic studies of probiotics prebiotics, which can provide more specific information about their functions and pathological variations. This review summarizes cutting-edge research on probiotics and prebiotics treatment for UC, existing issues in probiotics treatment and prebiotics therapy, the future of probiotics and prebiotics, and microbial therapeutics.
Pathogenesis of UC
Ulcerative colitis is a chronic inflammatory disorder of the gastrointestinal tract. It is characterized by a progressive decline in health. UC is marked by inflammation of the mucosal lining, usually confined to the colon and rectum [ 10 ]. The pathogenesis of UC is closely related to a variety of factors, such as genetics and environment [ 11 ]. Statistically, genetics can only explain 7.5% of the variation in disease and has little predictive power for phenotype. Therefore, it has limited clinical application. Examples of loci associated with increased susceptibility to UC including genes associated with barrier function and human leukocyte antigen, such as HNF4A and CDH1 [ 12 , 13 ]. Environment plays an important role in the development of UC. Such as, living condition, hygiene, diet, etc. While UC is mainly due to immune dysfunction and intestinal barrier dysfunction. Colonic epithelial cells (colonocytes), as the first line of defense of the gut immune system, are closely related to the pathogenesis of the UC. Research findings, the expression of peroxisome proliferator-activated receptor γ (PPAR γ) is reduced in the colonocytes in patients with UC. And the reduced expression of PPAR γ, which is a nuclear receptor that downregulates inflammation, will stimulate an inflammatory cascade responses through a series of immune responses, leading to the production of large quantities of inflammatory factors [ 14 ]. Also, when certain genes in the intestinal epithelium are functionally deficient, it may lead to disruption of the intestinal barrier function [ 10 ]. The deficiency or malfunction of various immune cells and the abnormal expression of cytokines, which play an important signaling function, can also lead to inflammation, which, if prolonged, can lead to the development of UC. The intestinal immune system also involves the intrinsic and adaptive immunity [ 15 ], involving a variety of immune cells and molecules and others. If dendritic cells abundantly express Toll-like receptors (TLR) which can recognize pathogen pattern receptors, this will leads to the activation of several inflammatory signaling pathway, such as NF-κB [ 16 ] and MAPK pathway, triggering an inflammatory response. The production of large amounts of pro-inflammatory factors affects the differentiation of immune cells such as T cell differentiation towards subpopulation. For example, massive activation of Th2 cells leads to high expression of IL-13, which induces apoptosis of epithelial cells and disrupts the integrity of mucosal barrier [ 17 , 18 ]. Other T helper cells also play an important role in UC. And some research suggest that Breg deficiency may also associated with UC [ 19 ]. The damage of the intestinal mucosal barrier is also an important causative factor in UC. Intestinal secretory dysfunction such as decreased secretion of antimicrobial peptides and mucus layer, or structural defects of intestinal barrier including occludin, ZO-1, ZO-2 and so on. It has been found that the disruption of human gut microbiota, the largest collection of microbes within the body [ 20 ], is critical in the progression of UC, but the specific mechanism is not yet clear.
The role of gut microflora in UC
Gut microflora lives on intestinal mucosal and forms bacterial layer. Thus, there is a strong and complex relationship between gut microbiota and gut. Intestinal dysbacteriosis can leads to a decrease in intestinal defense function and immune regulatory function. Furthermore, the decrease of the body immune function and an increase in associated pathogenic factors leading to the intestinal mucosal invasion or exacerbates the gastrointestinal diseases [ 6 ]. Recently, a large number of studies have shown that alterations of intestinal microbiota can play an important role in the occurrence and development of UC. Meanwhile, some studies have shed light on UC subjects exhibiting alterations in the relative abundance of “beneficial” and potentially “harmful” bacteria compared to healthy subjects. The existence of a link between UC and the gut microbiota was indicated based on studies in animals and patients with UC. Changes of gut microbiota together with their-derived products and metabolites account for the important factors to promote UC occurrence. Here, the possible mechanisms of microbiome-gut action in promoting UC occurrence are discussed as well as outlined in Fig. 1 .
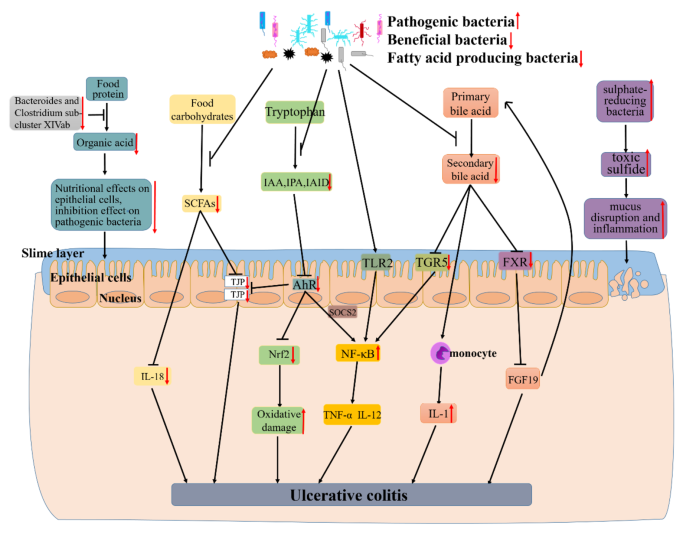
The mechanism of UC caused by dysbiosis of gut microbiota. Research findings, the decline of certain beneficial bacteria inhibits the conversion of food protein into organic acid which can nourish epithelial cells and inhibit pathogenic bacteria. Firmicutes as a major producer of butyrate (a kind of SCFAs), its decline leads to lower intestinal SCFAs. Leading the decreased secretion of epithelial repair cytokine interleukin-18, reduced the integrity of epithelial cells, and inhibited goblet cells secrete mucin and modification of tight junctions. And the decline of some gut microbiota also can lead to a decrease of indoles and their derivatives (e.g., IAA, IPA and IAID) which is produced by tryptophan. Thereby reducing the activation of AhR, a member of the activation of PER-ARNT-SIM (PAS) superfamily of transcription factors. The activation of AhR can inhibited the expression of NF-κB in a manner dependent on suppressor of cytokine signaling 2 (SOCS2). And AhR can also maintains the integrity of intestinal barrier activation by increasing the expressions of intestinal tight junction protein (TJPs) or activating the AhR-Nrf2 pathway. All of these effects were reversed due to the decrease of IAA, IPA or IAID. Thus lead to the increase of inflammatory factors (e.g., TNF-α and IL-17) and oxidative damage. Other researchers found that certain pathogenic bacteria such as Bacteroides (B.) fragilis and capsular lipopolysaccharide A can activate NF-κB signaling pathway and promote the secretion of inflammatory factors. The gut microbiota dysbiosis can also lead to the decreased synthesis of secondary bile acid. And secondary acid act as high-affinity ligands for TGR5 and FXR, its decline can promote NF-κB activation to synthesize inflammatory and the expression of proinflammatory cytokines secreted by monocyte and downregulate the expression of FGF19 and promote the synthesis of bile acids thus increasing its toxicity effect on tissues. As an intestinal pathogen, the increase of sulphate-reducing bacteria leads to cell disintegration and inflammatory via toxic sulfide. All of these can lead to the occurrence and development of UC.
A large number of studies have shown that patients with UC have a decrease in the bacterial diversity of gut microbiota [ 21 ]. Animal study results indicate a close association between gut microbiota and UC. Li et al. found that Firmicutes and Proteobacteria increased, whereas Bacteroidetes decreased in UC rats. And Lactobacillus , Lachnospiraceae_NK4A136_group , Prevotella_9 and Bacteroides were dominant genera in the model group [ 22 ]. Consistent with animal studies, the existence of a link between UC and the gut microbiota was indicated based on studies in patients with UC. Guo et al. also found that the abundance of Bacteroides and Clostridium sub-cluster XIVab as well as the concentration of organic acids significantly decrease by comparing with healthy individuals [ 23 ]. Similarly, Mizoguchi et al. shown that UC patients harbored relatively more abundant Actinobacteria, Proteobacteria and Tenericutes [ 24 ]. A comparison between UC and healthy individuals differed in the composition and diversity of the microbiota, with an upward trend in the Clostridium cluster IX and a decreased Clostridium cluster XIVa in patients with UC [ 25 ]. Consistent with the above results, there is a reduced amounts of bacterial groups from the Clostridium cluster XIVa , and the levels of Bacteroidetes was increased [ 26 ].
In addition, Kotlowski et al. found that the numbers of Escherichia coli were high in the rectal tissue of patients with UC [ 27 ]. By comparing with healthy controls, Xu et al. showed that the inflamed mucosa had more Proteobacteria (e.g. Escherichia–Shigella ) and fewer Firmicutes (e.g., Enterococcus ) [ 28 ]. As demonstrated by Schwiertz et al., Patients with active UC have lower cell counts of Bifidobacterium than healthy controls [ 29 ]. Another study found that the sulfate-reducing bacteria which is the dominant microflora in UC, it may proliferate with the release of toxic sulfide [ 30 ].
Recently, Verma et al. shown that during the active and remission stages of UC cases, the proportions of Bacteroides , Eubacterium , and Lactobacillus spp. are decrease [ 31 ]. Similarly, in another analysis of mucosa-associated flora in UC patients, it was learned that UC patients contained proportionally less Firmicutes , and correspondingly more Bacteroidetes [ 32 ]. Tahara et al. demonstrated that Fusobacterium nucleatum is common which is isolate from human intestinal biopsy from UC, compared to healthy controls [ 33 ].
In keeping with these results, Machiels et al. found that there is a decrease of the Roseburia hominis and Faecalibacterium prausnitzii in patients with UC [ 34 ]. Lepage et al. demonstrated that patients with UC are characterized by more Actinobacteria and Proteobacteria and less bacteria from the Lachnospiraceae and Ruminococcaceae families [ 35 ]. Likewise, a significant reduction was found on the UC mucosa compared with the non-IBD controls, that is levels of Clostridium clostridioforme , the Eubacterium rectale group, Faecalibacterium prausnitzii , Bifidobacteria , Lactobacilli , and Clostridium butyricum [ 36 ]. Consistent with the above results of this study, patients with UC in remission compared to that of controls, there is a loss of Bacteroides , Escherichia , Eubacterium , Lactobacillus , and Ruminococcus spp [ 37 ].
Recently, Hu et al. [ 38 ] found that the decreased of the dominant bacteria that digest food carbohydrates to short chain fatty acid (SCFA) lead to the reduce of intestinal barrier integrity (for example, the decrease of TJPs in colon). Guo et al. [ 23 ] also found that SCFAs can affect the secretion of the epithelial repair cytokine interleukin-18. And they found that the decreased of Bacteroides and Clostridium sub-cluster XIVab leading to the decrease of organic acid, which reduces the trophic effect of organic acid to epithelial cells and the inhibitory effect on pathogenic bacteria [ 39 ]. Agus et al. [ 40 ] found that the reduced of certain intestinal flora inhibited the conversion of tryptophan to indole and its derivatives, and AhR as a receptor of indole and its derivatives, its activation will reduced, thereby inhibiting the intestinal TJP and AhR-Nrf2 pathway, leading to the reduced of intestinal barrier integrity and increased oxidative stress [ 41 ]. Rothhammer et al. [ 42 ] demonstrated that the reduce of AhR can promote the activation of NF-κB pathway in a manner dependent on suppressor of cytokine signaling 2 (SOCS2), then increase the expression of a number of inflammatory factors, including TNF-α and IL-12 et al. It is reported that some bacteria regulate the secretion of TNF-α and IL-12 by activating the NF-κB pathway through TLR2 receptor [ 43 ]. Iracheta et al. [ 44 ] found that primary bile acid are converted to secondary bile acid by gut microorganisms after being secreted into gut through a series of reactions, and that a decline of these gut microorganisms leads to a decrease of secondary bile acid. The decrease of secondary bile acid, which act as high-affinity ligands for TGR5 and FXR, leads to a decreased activation of TGF5 and FXR. The inhibitory effect of TGR5 on NF-κB is reduced, thereby promoting the activation of NF-κB. Reduced activation of FXR down-regulates the expression of FGF19, then its inhibitory effect to hepatic bile acid is declined, leading to a further increase of bile acid and exacerbating the development of inflammation [ 45 ]. And the decrease of secondary bile acids promote the secretion of pro-inflammatory factors by monocytes [ 46 ]. Figliuolo et al. [ 47 ] found that the increase of sulphate-reducing bacteria lead to an increase of toxic sulfide, which cause the disruption of gut epithelial cell and increase intestinal inflammatory.
Taken together, these results provide further insights into a role for gut microbiota in the pathogenesis of UC and might potentially serve as guidance for the interventions of UC by manipulating gut microbiota.
Research advances existing challenges IBD treatment
At present, there are many various treatment methods for IBD. Conventional treatment is the use of pharmacotherapy, including aminosalicylates, corticosteroids (CSs), immunomodulators (e.g., thiopurines (TPs), methotrexate (MTX), and calcineurin inhibitors), and biologics (e.g., pro-inflammatory cytokine inhibitors and integrin antagonists). Surgical resection and other methods including apheresis therapy, antibiotics, probiotics and prebiotics can also be used for treatment [ 48 ]. However, the side effects and high reccurence rate of these substances and methods limit there application. For example, research found, although aminosalicylates have been used in the treatment of IBD for the past 80 years, its efficacy remains controversial. And its mild side effects include diarrhea, nausea, abdominal pain, flatulence and others [ 49 ]. Severe cases can lead to infertility and anemia. CSs inhibits the transcription of certain inflammatory factors [ 50 ] and regulate the expression of certain anti-inflammatory genes [ 51 ] through certain signaling pathways. And it has many side effects, including diabetes mellitus, hypertension, venous thromboembolism (VTE), etc [ 52 ].. Some patients may also have dependence on this medication [ 53 ]. TPs inhibits intestinal inflammatory response by regulating T cell proliferation and activation. But TPs can cause side effects such as liver damage [ 54 ] and gastrointestinal intolerance [ 55 ]. MTS excerts its effects also by downregulating inflammatory factors. But it can cause adverse reactions such as fatigue, diarrhea, pneumonia and rash [ 51 ]. Calcineurin inhibitors also supresses inflammatory responses by interfering with signaling pathways. The incidence of side effects of calcineurin inhibitors is high, including renal function damage, hyperkalemia and infectious diseases and so on [ 56 ]. Anti-TNF therapy will inhibit the secretion of pro-inflammatory factor TNF-α. Anti-IL-12/23 therapy works by inhibiting the production of pro-inflammatory factor IL-12 and IL-23 by antigen-presenting cells. Anti-integrin therapy inhibits the accumulation of white blood cells in intestinal and alleviates intestinal inflammatory. But these biological agents are expensive and many patients may experience unresponsive and intolerant states. Therefore, it is urgent to study effective and safe methods to treat UC.
In the recent years, regulating gut microbiota has become a hot topic in the treatment of UC. Therefore, as a promising method for treating IBD, probiotics act as live microorganisms have therapeutic effects on IBD which is caused by intestinal ecological disorders and other reasons. The treatment of IBD can be achieved through its antioxidant effects [ 57 ], the regulatory effect on gut microbiota [ 58 ], anti-inflammatory effect [ 59 ], the promotion effect to intestinal barrier integrity [ 60 ] and so on. As an indigestible food ingredient, prebiotics can also be used to treat or alleviate UC by regulating the redox system, immune system, etc. It can also selectively regulate colon microbiota, for example, enhancement of beneficial intestinal bacteria and inhibition of the growth of pathogenic microorganisms. All of these suggests that probiotics and prebiotics have a lot of room to develop as new form of treatment.
Effect and mechanism of probiotics and prebiotics in treating UC
Probiotics are nonpathogenic living microorganisms which, when administered in adequate amounts, have been shown to confer health benefits to the host and regulate intestinal microecological balance. Probiotics are widely used in medical application to prevent or treat many diseases, such as obesity [ 61 ], hepatocellular Carcinoma [ 62 ], autoimmune hepatitis [ 63 ], diabetic retinopathy [ 64 ], and alcoholic liver disease [ 65 ] and so on. The therapeutic effects of probiotics on UC have also been confirmed in animals and humans (Tables 1 and 2 ). Thus, therapeutic interventions with probiotics may offer new treatment for UC. Here, the possible effects and mechanisms of probiotics in the treatment of UC are summarized in Fig. 2 .
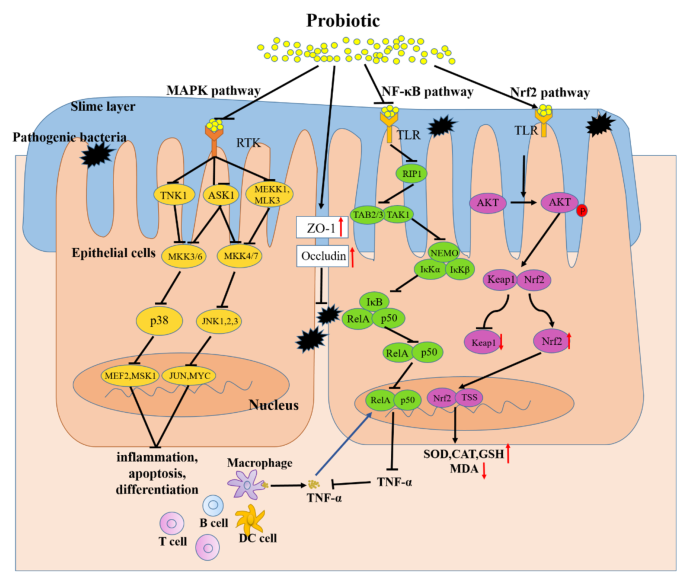
The potential mechanism of probiotics in alleviating Ulcerative Colitis (UC). Probiotics that enter the gut can bind with corresponding receptors (e.g. PTK) which are on the intestinal epithelial cells, then inhibit its stimulation to MAPKKK (e.g. TNK1, ASK1, MEKK1, MLK3), further suppress the activation of MAPKK (e.g. MKK3/6, MKK4/7) which are activated by MAPKKK, thereby inhibiting the activation of MAPK (e.g. p38, JNK1,2,3). Blocking the transcription factor transcribe of relevant genes (e.g. Cyclin D1, Raf). Finally, inhibition the inflammatory, apoptosis, and differentiation activated by this pathway. Meanwhile, probiotics protect the intestinal barrier by increasing the levels of tight junction proteins of ZO-1 and Occludin between intestinal epithelial cells, preventing the invasion of pathogenic microorganisms. In addition, probiotics can bind with its receptors (e.g. TLR) on the intestinal epithelial cells, inhibiting the activation of adaptor protein (e.g. RIP1) and suppressing the recruitment of TAB/TAK complex, thereby inhibiting the ubiquitination degradation of IκB by ubiquitinatingNEMO. Prevents the release of NF-κB proteins (RelA/p50) to nucleus. Ultimately inhibits the transcription of proinflammatory factors (e.g. TNF-β) and reduces the promotion effect of TNFα releasing by macrophages to this pathway. Meanwhile, probiotics act on intestinal epithelial cells-associated receptors (e.g. TLR), then phosphorylate AKT, and inhibit the degradation of Nrf2. Nrf2 enters the nucleus and promotes the expression of a range of cytoprotective genes (e.g. SOD, CAT, GSH).
Probiotics therapy
Experimental studies.
Convincing evidence from animal studies indicate that probiotics treatment can relieve UC (Table 1 ). Wu et al. [ 66 ] found that the use of Bifidobacterium longum CCFM1206 to treat Dextran-Sulfate-Sodium (DSS) induced Colitis mice will promotes the conversion of Glucoraphanin (GRP) to sulforaphane (SFN). SFN help to upregulate the Nrf2 signaling pathway and inhibit the NF-κB activity, which can ameliorate DSS-induced colitis. The result also indicated that the intervention of B.longum CCFM1206 could relieve the dysbiosis of intestinal microbiota. That is, promoted the proportion of Alistipes , Bifidobacterium , Blautia and Lachnospiraceae NK4A136 group and inhibited the proportion of Acinetobacter , and Lachnospiraceae A2 in the gut. Similar study, Han et al. [ 67 ] demonstrated that Bifidobacterium infantis enhances genetic stability by maintaining the balance of gut flora to increase anaphase-promoting complex subunit 7 (APC7) expression in colonic tissues, changing gut flora such as an increase in B.infantis . Then reducing DSS-induced colonic inflammation. Consistent with the above results, Fu et al. [ 68 ] found that Bacteroides xylanisolvens AY11-1 regulate the intestinal microbiota through the efficient degradation of alginate, improving the dysbiosis of intestinal ecology and promoting the growth of beneficial bacteria, for example, the increase of Blautia spp and Prevotellaceae UCG-001. Then ameliorated the symptoms of DSS-induced UC in mice. Wang et al. [ 69 ] revealed that the administration of probiotic Companilactobacillus crustorum MN047 in DSS-induced UC mice resulted in the expression of tight junctions, and down-regulation of pro-inflammatory and chemokine expression. It was also found that an increase of goblet cells, MUCs, TFF3, and TJs in the probiotic group, which demonstrated that the treat with CCMN could enhance the gut barrier function. And confirmed by fecal microbiota transplantation (FMT), the mechanisms of CCMN alleviating UC were partly due to its modulation to gut microbiota. The result showed that an increase in Bacteroidaceae and Burkholderiaceae and a decrease in Akkermansiaceae and Eggerthellaceae . Hu et al. [ 70 ] also found that Selenium-enriched Bifidobacterium longum DD98 administration alleviated the symptoms caused by DSS, inhibited the expression of the pro-inflammatory cytokines, decreased the level of oxidative stress, promoted the expression of tight junction proteins, inhibited the activation of toll-like receptor 4 (TLR4), and regulated the gut flora. They found that after the treatment of Se-B. longum DD98, the phylum of Bacteroidetes decreased and the phylum of Firmicutes increased. All of the above can be effective attenuated DSS-induced colitis in mice. In another study, the results of Han et al.’s [ 71 ] study of Lacticaseibacillus rhamnosus Hao9 in DSS-induced UC mice showed that the use of Hao9 attenuated weight loss which is caused by DSS, lowered DAI scores, attenuated colonic damage and inflammatory infiltrates and promoted the growth of Faecalibaculum and Romboutsia in the gut. The researcher attributed the observed effects of Hao9 on UC to its ability to inhibit lipopolysaccharide-induced intestinal IκB activation of mice. Consistent with the above results, Huang et al. [ 72 ] also showed that Lactobacillus paracasei R3 supplementation improved the general symptoms of murine colitis, attenuated inflammatory cell infiltration and more. And it was showed that the imbalance of Treg/Th17 cell in the intestinal inflammation caused by DSS was restored after treatment with L.p R3. Similarly, Xu et al. [ 73 ] investigated the effect of Saccharomyces boulardii and its postbiotics on DSS-induced UC in mice, showing that both S. boulardii elements and its postbiotics could significantly alleviate weight loss, reduce colonic tissue damage, regulate the balance of pro/anti-inflammatory cytokines in serum and colon, promote the expression of colonic tight junction proteins, and regulate the stability of intestinal microecology in mice. Changing in the bacterial flora were characterized by a significant increase in Turcibacter at the genus level, which collectively attenuate DSS-induced colitis. Komaki et al. [ 74 ] administered Lactococcus lactis subsp.lactis JCM5850 to mices with colitis induced by DSS and found that moderate amounts of L. lactis had a mitigating effect on colitis. In keeping with these results, Hizay et al. [ 75 ] also found that Lactobacillus acidophilus reduces abnormally high levels of serotonin in colon tissue in acetic acid-induced UC and relieves inflammation in intestinal tissue. As with the results above, Gao et al. [ 76 ] made Saccharomyces boulardii into suspension, observing its effect on DSS induced colitis in mice. The results suggested that S. boulardii can alleviate the clinical symotoms of colitis in mice exposed to DSS and the histological lesions. And it was found that the mechanism of S. boulardii to treat UC is inhibite nuclear transcription factor kappa B (NF-κB) and activate nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway. As demonstrated by He [ 77 ] et al., Enterococcus faecium administration prevented DSS-induced intestinal inflammation and intestinal flora dysbiosis and particially repaired the damage to intestinal mucosal barrier and tight junctions. The modulatory effect on intestinal flora was characterized by an increase in Butyricicoccus sp., Lactobacillus sp., and Bifidobacterium sp. and a decrease in Ochrobactrum sp. and Acinetobacter sp. .
By studying the effects of tetrapeptide from maize (TPM) and probiotic (5 Lactobacillus strains: L.animalis- BA12, L.bulgaricus- LB42, L.paracasei- LC86, L.casei- LC89 and L.plantarum- LP90) in mice with DSS-induced UC, Li et al. [ 78 ] found that it could reduce the level of oxidative stress, attenuate the loss of kidney and colon, and regulate the intestinal flora to alleviate the inflammatory effects of UC. Wherein, the modulation effect to gut microbiota in manifested as an increase in Muribaculaceae, Alistipes, Ligilactobacillus and Lactobacillus . Recently, Shang et al. [ 79 ] reported that Bifidobacterium bifidum H3-R2 can effectively alleviate of pathogenesis by inhibiting inflammatory signaling, maintaining intestinal ecological homeostasis, and protecting colonic integrity. B.bifidum H3-R2 administration similarly affected the composition of gut microbiota, showing that B.bifidum H3-R2 caused a significant increase in the abundance of Bifidobacterium and Lactobacillus and a decrease in Enterobacter , Enterococcus and Streptococcus . Chen et al. [ 80 ] also discovered Lactobacillus fermentum ZS40 could inhibit DSS-induced mice colon shortening, colon damage, and intestinal wall thickening. It does so by inhibiting the activation of NF-κB and MAPK signaling pathways, and ultimately relieved inflammation.
To sum up, these results provide important clues for the design and use of more effective probiotic agents to treat UC and may provide new insights into the mechanisms by which host-microbe interactions confer the protective effect. And probiotics as additionally supplemented active micro-organisms, may have better value in clinical applications as drugs in the future [ 81 ].
Clinical studies
There are many contributing factors to UC, but much evidence suggests a strong link between host gut microbes and the treatment of UC pathogenesis, and suggests that mediation of gut microbes is the key to treating UC. Probiotics have been shown to alleviate UC by altering the composition of the gut microbiota and many other ways. A growing number of clinical trials have also demonstrated the therapeutic effects of probiotics in UC (Table 2 ). As early as 2010, Hegazy et al.’s [ 82 ] study showed that administration of probiotics ( Lactobacillus delbruekii and L. fermentum ) not only decreased the NF-κB DNA binding activity, but also reduced the accumulation of leukocytes, and down-regulated levels of pro-inflammatory factors, and thereby ameliorated the severity of the colitis. Similarly, in order to study the long-term effect of probiotics on UC, Palumbo et al. [ 83 ] conducted a clinical study and the results of the study showed that patients in the probiotics ( L. salivarius , L.acidophilus and B.bifidus strain BGN4) treatment group had better outcomes which is reflected through MMDAI. Thus, the use of probiotics may enhance the anti-inflammatory effect. Similar results, Bjarnason et al. [ 84 ] tried to prove the impact of the probiotic Symprove (including Lactobacillus rhamnosus NCIMB 30,174, Lactobacillus plantarum NCIMB 30,173, Lactobacillus acidophilus NCIMB 30,175 and Enterococcus faecium NCIMB 30,176) which contains four naturally occurring bacterial strain for this experiment. Research showed that Symprove are associated with reduced intestinal inflammation in UC patients. In line with these results, Tsuda et al. [ 85 ] gave patients with moderate to severe UC treated with BIO-THREE (containing Streptococcus faecalisa T-110, Clostridium butyricum TO-A and Bacillus mesentericus TO-A). Researchers found that the treated with BIO-THREE were able to improve clinical and endoscopic examinations in about half of UC patients who were intolerant to conventional therapy. And its intake improved intestinal microflora, the main change may be an increase in bifidobacteria. After a six-week study, Agraib et al. [ 86 ] found that patients in the probiotic (containing nine Lactobacillus and five Bifidobacterium species) group had higher levels of anti-inflammatoty and better clinical symptoms compared with the placebo group. Groeger et al. [ 87 ] demonstrated that Bifidobacterium infantis 35,624 achieved palliate effect to UC primarily by reducing intestinal inflammatory biomarkers (e.g. CRP, TNF-α, IL-6). In 2021, the study conducted by Gu et al. [ 88 ] revealed that Akkermansia muciniphila activate aryl hydrocarbon receptor (AhR) signaling, inhibite Kyn pathway (KP) activation, and restore the down-regulation of anti-inflammatory factors through increasing the levels of indoleacetic acid (IAA) and indole acrylic acid (IA) in the tryptophan (Trp) metabolic pathway. Similarly, the mitigation effect of probiotics (containing L.casei Zhang, L.plantarum P-8 and B.animalis subsp. lactis V9) was demonstrated in a trail by Chen et al. [ 89 ] in the treatment of UC. And the researchers found that the probiotic group had more beneficial bacteria, such as Eubacterium ramulus , Pediococcus pentosaceus , Bacteroides fragilis and Weissella cibaria .
All in all, these clinical studies have shown that the effectiveness of treating UC patients with probiotics is increasingly being proven. Above all, probiotics intervention might be a potentially effective approach in the treatment of UC by restoration of gut microbiota. Meanwhile, therapies that may most efficiently bring the disease under control are still being sought.
Prebiotics therapy
Prebiotics are selectively fermentable, non-digestible oligosaccharides, or ingredients. They function to accelerate beneficial bacterial growth and suppress harmful bacterial growth, thus adjusting the balance of gut microbiota. In addition, they can lead to the production of SCFAs, regulate immune response, control gene expression in bacterial cells, and improve absorption of micronutrients. And prebiotics are used to treat a wide variety of disease, such as obesity [ 90 ], chronic enteritis [ 91 ], skin disease [ 92 ] and autism spectrum disorder [ 93 ]. The therapeutic effects of prebiotics on UC have also been confirmed in animal and humans (Tables 3 and 4 ). Thus, Prebiotics can be used as a novel dietary management approach for UC. Here, the possible effects and mechanisms of prebiotics in the treatment of UC are summarized in Fig. 3 .
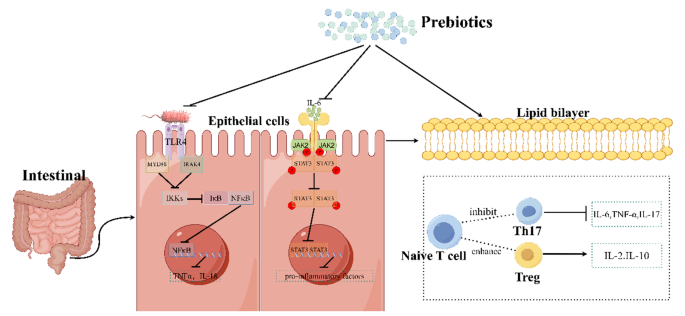
The mechanism of prebiotics in alleviating Ulcerative Colitis in Mice. It was found that the mechanism of prebiotics alleviate UC is probably through inhibiting of the TLR4/NF-κB signaling pathway, the JAK2/STAT3 signaling pathway, and regulating the ratio of T cell subsets. Firstly, prebiotics inhibit the activation effect of lipopolysaccharides from Gram-positive bacteria on TLR4 receptors, thereby inhibiting NF-κB from being released into nucleus and thus reducing the transcription of pro-inflammatory factors. Secondly, prebiotics can inhibit the activation of cytokine receptors by IL-6, thus suppress the entry of STAT3 into the nucleus and likewise inhibit its production of pro-inflammatory factors. Thirdly, prebiotics can inhibit of the conversion of naive T cells into Th17 cells and promote of their conversion into Treg cells, causing an increase of the expression of anti-inflammatory. (This mechanism diagram was drawn by Figdraw ( https://www.figdraw.com ))
Convincing evidence from animal studies indicate that prebiotics treatment can relieve UC. Koleva et al. [ 94 ] showed that fructo-oligosaccharides (FOS) promoted Bifidobacterium spp. and inulin and FOS can all decrease Clostridium cluster XI in rats, while Bifidobacterium spp. and Clostridium cluster XI correlated negatively and positively, respectively, to chronic intestinal inflammation. That is, both this two fructans inhibited intestinal inflammation. Hoentjen et al. [ 95 ] also orally administered a prebiotic combination of chicory-derived long-chain inulin-type fructans and short-chain inulin fraction oligofructose to HLA-B27 transgenic rats and found that this prebiotic can significantly reduce colitis and demonstrated that this effect was not only related to the gut microbiota, but also to immunomodulatory effects. They found that the prebiotic can promote the increase of bifidobacteria and endogenous lactobacilli. In immunomodulation, for example, it is possible to increase TGF-β in cecum. Wang et al. [ 96 ] allowed C57BL/6 mice with UC to receive oral administration of stachyose which is a prebiotic that traditionally extracted from plants for a period of time, and demonstrated the effect of stachyose on the recovery of body weight and found that it can reduced colonic tissue damage, lowered the level of pro-inflammatory cytokines, and restored the dysbiosis of the intestinal microbiota imblance (reduce the abundance of Escherichia_Shigella , Parabacteroides , Romboutsia and Turicibacter and raise the abundance of Alistipes and Roseburia ). In the study of Lunken et al. [ 97 ], they used an adoptive T-cell transfer mice model of colitis to examine the effects of enriching exclusive enteral nutrition (EEN) with inulin-type fructans (IN) (ENN IN) on colitis and found that a less deterioration of the mucus layer, increased butyrate production, and the expansion of anti-inflammatory T-cell subsets, including IL-10 producing Foxp3 + Tregs. And they also found an increased relative abundance of beneficial microbes ( Bifidobacterium spp. and Anaerostipes caccae ) and an reduced relative abundance of potentially pathogenic microbes ( Escherichia Shigella spp.). All of these results continue to prove the benefits of prebiotics in UC. Li et al. [ 98 ] established the DSS-induced mice model of colitis by evaluating the therapeutic effects of prebiotics high-substituted hydroxypropyl cellulose (HHPC) and low-substituted hydroxypropyl cellulose (LHPC) on UC, and the results confirmed that these two prebiotics dose-dependently ameliorated the inflammation in colitis mice, inhibited pro-inflammatory cytokine and regulated the balance of intestinal flora, including increased the relative abundance of Bacteroides and Alloprevotella genus and reduced the relative abundance of Firmicutes. Kanauchi et al. [ 99 ] investigated the effect of Germinated barley foodstuff (GBF), a prebiotic product, on the gut environment and found that it can inhibited the expression of STAT3 and NF-κB, thereby reducing the inflammatory response of the epithelium.
In summary, these animal experiments have showed the good effect of prebiotic therapy alone or in combination to UC. This provides a new direction in the clinical treatment of UC.
Many clinical studies have demonstrated the benefits of prebiotics for people with UC. Oligofructose and Inulin as the oligosaccharide fraction of Raftilose and the oligosaccharide fraction of Raftiline, which was obtained by the extraction of chicory roots, Gibson et al. [ 100 ] have demonstrated the stimulatory effect of these two substances on intestinal bifibacteria, which is a bacterium thought to be beneficial to health through clinical experiment and reduced some pathogenic bacteria that can produce toxins or hydrolyzed proteins, including bacteroides, clostridia, and fusobacteria. Vulevic et al. [ 101 ] found that Galactooligosaccharides (GOSs) promoted the population of beneficial bacteria, especially bifidobacteria and lactobacilli, and reduced numbers of less beneficial bacteria (bacteroides, the C. histolyticum group, E. coli , and Desulfovibrio spp.), and also enhanced the immune response and reduced the production of pro-inflammatory factors. Similarly. Casellas et al. [ 102 ] demonstrated that oligofructose-enriched inulin reduced intestinal inflammation by measuring fecal calprotectin levels in patients. Faghfoori et al. [ 103 ] administrated germinated barley foodstuff (GBF) to patients with UC and showed that GBF were able to reduce serum levels of pro-inflammatory including IL-6, IL-8,TNF-α. As demonstrated by Mitsuyama et al. [ 104 ], by determining the changes of microorganisms in the feces of patients with UC after four weeks of oral administration of GBF, the results proved that prebiotics can increase the concentration of fecal Bifidobacterium and Eubacterium limosum and increase the concentration of colonic butyrate, which is a source of energy for epithelium. And decreased the presence of Bacteroides .
Ryan et al. [ 105 ] conducted in vitro and in vivo experiments and demonstrated the promoting effect of \( {2}^{{\prime }}\) -fucosyllactose ( \( {2}^{{\prime }}\) -FL) which is a prebiotic human milk oligosaccharide on butyric acid producers, including Bifidobacterium , Clostridium cluster XIVa and Roseburia spp. Butyric acid, on the other hand, as a kind of SCFA, can inhibit the inflammatory response. In this study, they also found a significant increase in fecal Faecalibacterium prausnitzii , Anaerotruncus colihominis , and Pseudoflavonifractor species. Consistent with the above results, Suzuki et al. [ 106 ] tested the effectiveness of Bifidogenic growth stimulator (BGS) which is a prebiotic preparation produced by Propionibacterium freudenreichii isolated from Swiss cheese in patients with UC and found that it can selectively stimulated the activation of Bifidobacteria , which not only produced butyrate to nourish colonocytes and inhibited cytokine production and activation of NF-κB pathway, but also improved the balance of the intestinal microflora to maintain intestinal mucosal integrity and prevented intestinal damage. In the clinical study by Li et al. [ 107 ], they demonstrated the potential of Xylo-oligosaccharide (XOS) to alleviate microecological dysbiosis in patients with UC by measuring the effect of XOS on the intestinal flora. They found that XOS promotes the proliferation of Bifidobacteria , which produces a variety of organic acids and inhibits the growth of harmful bacteria by altering their metabolites.
In conclusion, these clinical studies demonstrated the palliative effects of prebiotics on UC, showing that prebiotics hold promise as primary or adjunctive maintenance therapy for UC.
Concluding remarks
UC as a common disease has become a financial burden for many people and has the potential to develop into cancer if not prevented or treated. Therefore, it is important to identify and intervene in a timely manner. The pathogenesis of UC is complex, that’s why it’s important to find a reliable treatment. There is a strong and complex relationship between gut microbiota and gut. Crucially, growing evidence strongly suggests that the gut microbiota plays a pivotal role in intestinal defense function, immune regulatory function, inflammatory responses, as a result, the development and progression of UC. Meanwhile, mechanistic studies have demonstrated these particular species of intestinal commensal bacteria capable of playing either a protective or pathogenic role in UC development. Traditional treatment methods come with a lot of side effects. And probiotics and prebiotics emerge as a new therapeutic modality to modulate the gut microbiota. Based on these, numerous animal and clinical studies have shown that regulating gut microbiota may be an effective strategy to treat UC.
Probiotics being able to confer notable health benefits by modulating the composition of gut microbiota and restoring the physiological bacterial flora. However, while an increasing number of studies have pointed to the therapeutic effects of probiotics on UC, the available data in this field remain limited and the relevant scientific work is still in its early stages. Thus, further research is still necessary. Firstly, due to the complex relationship between gut microbiota and UC, in order to better use probiotics to treat UC, it is necessary to further study the mechanism of intestinal flora affecting the occurrence and development of UC through more animal and clinical experiments. Secondly, we need to know how these probiotics regulate gut microbiota or how they function in the intestinal and what factors contribute to their long-run stability in both health and disease. Changes in certain pathway molecules can be probed to determine the specific mechanism of probiotic treats UC. Meanwhile, in the study of probiotics in the treatment of UC, we should pay more attention to the etiology and pathogenesis. Based on this, the composition and metabolites of probiotics should be of great concern. In particularly, it should be thoroughly studied for their antioxidant effects, anti-inflammatory properties, maintenance of the intestinal homeostasis, regulation of mucosal immune homeostasis, and so on. Some key probiotic components and metabolites may be highly effective postbiotic in the treatment of UC. Thirdly, most medications for the treatment of UC have many adverse effects. Meanwhile, probiotics have great potential as drugs to treat UC. Therefore, it may be more cost-efficient to invest more effort in probiotics than in developing new anti-inflammatory drugs. Fourth, in order to provide more effective probiotics to clinical, we can study the beneficial gut microbiota of healthy humans to dig out more and better probiotics. At the same time, it is necessary to search for the most effective probiotic compositions for the treatment of UC. Fifth, more clinical rationalized trials should be carried out to determine whether probiotics is safe and effective in the treatment of UC. Furthermore, because the composition of the gut microbiota is related to region, ethnicity, and diet, it is necessary to study large samples of people in different regions. Sixth, we must figure out the route of administration of the probiotics as well as the dosage, to ensure the probiotics will maximize the benefits in patient’s body under safe administration. Seventh, in order to make it easier and more convenient for patients to use probiotics, such as how to keep probiotics maintain highly active in some way and make it easier for patients to take, we should further explore the production and preservation of probiotics. Last but not least, to accepted by patients as a reliable treatment, it should be clarified for which patients a particular probiotic is effective, or which is preferable for a single probiotic or a blend of strains. So, there are still many problems it faces. In the future, probiotic therapy may be a potentially useful approach for UC, but research in this area has just started.
Prebiotics offer an exciting new approach to dietary management of gastrointestinal disorders including UC. It has been accepted as a dietary food ingredient that helps to nourish gut microbes, which can improve health and prevent UC. But while many studies to date have demonstrated the beneficial effects of prebiotics in UC, it still faces numerous challenges. Now many studies have the limitation of too small a sample size or lack of a control group, so the evidence for a significant effect of prebiotics is still lacking. The dosage of prebiotics is also a question to be confirmed, if too high a dosage will lead to tolerance, or if a higher dosage of prebiotics will produce better results when well tolerated. With so many types of prebiotics available, it is also deserving of further study as to which prebiotics have better results for which type of UC patients. Although a large number of in vitro and in vivo experiments have confirmed the positive effects of prebiotics, there is still a need for more clinical trials or animal experiments to further evaluate their specific effects.The specific mechanism by which we found that prebiotics alleviate UC remains unclear. It’s worth exploring further. In-depth experiments are needed to further elucidate the role of prebiotics in patients with UC, whether it is their own structure or their metabolites that play a role. And to meet the needs of consumers, new strategies for cost-effective and efficient prebiotics can be developed. Prebiotics, as a food-sourced ingredient for the treatment of UC, offer a new clinical direction, and it is important to study its good effects and side effects as clearly as possible. Therefore, in any case, the prospect of the application of prebiotics in UC is worthy of attention and expectation.
Certainly, in order to gain wider acceptance and recognition for probiotics and prebiotics to treat UC, further research is urgently required.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Hijova E, Soltesova A. Effects of probiotics and prebiotics in ulcerative colitis. Bratislava Med J. 2013;114(09):540–3.
Article CAS Google Scholar
Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. The Worldwide Incidence and Prevalence of Inflammatory Bowel Disease in the 21 St Century: a systematic review of Population-Based studies. Gastroenterology. 2017;152(5):S970–1.
Article Google Scholar
Iheozor-Ejiofor Z, Kaur L, Gordon M, Baines PA, Sinopoulou V, Akobeng AK. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database of Systematic Reviews. 2020;3(3):CD007443.
Mladenova D, Kohonen-Corish MRJ. Mouse models of inflammatory bowel disease - insights into the mechanisms of inflammation-associated Colorectal Cancer. Vivo. 2012;26(4):627–46.
CAS Google Scholar
Li J, Ueno A, Fort Gasia M, Luider J, Wang T, Hirota C, et al. Profiles of Lamina Propria T Helper Cell subsets discriminate between Ulcerative Colitis and Crohnʼs Disease. Inflamm Bowel Dis. 2016;22(8):1779–92.
Article PubMed Google Scholar
Shen Z-H, Zhu C-X, Quan Y-S, Yang Z-Y, Wu S, Luo W-W, et al. Relationship between intestinal microbiota and ulcerative colitis: mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J Gastroenterol. 2018;24(1):5–14.
Article CAS PubMed PubMed Central Google Scholar
Yao SY, Zhao ZX, Wang WJ, Liu XL. Bifidobacterium Longum: Protection against Inflammatory Bowel Disease. Journal of Immunology Research. 2021:8030297.
Zou JF, Shen YM, Chen MJ, Zhang ZM, Xiao SW, Liu C, et al. Lizhong decoction ameliorates ulcerative colitis in mice via modulating gut microbiota and its metabolites. Appl Microbiol Biotechnol. 2020;104(13):5999–6012.
Article CAS PubMed Google Scholar
Rather IA, Majumder R, Alshammari FH, Park JG, Bajpai VK. Ulcerative colitis and probiotics: an overview. Pak J Pharm Sci. 2016;29(5):1877–80.
CAS PubMed Google Scholar
Peyrin-Biroulet L, Sandborn W, Sands BE, Reinisch W, Bemelman W, Bryant RV, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110(9):1324–38.
Kushkevych I, Martinkova K, Vitezova M, Rittmann SKR. Intestinal microbiota and perspectives of the Use of Meta-Analysis for comparison of Ulcerative Colitis studies. J Clin Med. 2021;10(3):462.
Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–24.
Barrett JC, Lee JC, Lees CW, Prescott NJ, Anderson CA, Phillips A, et al. Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the < i > HNF4A region. Nat Genet. 2009;41(12):1330–U99.
Ordas I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380(9853):1606–19.
Sands BE, Kaplan GG. therapeutic review the role of tnfα α in ulcerative colitis.2007;47(8):930-41.
Zhang FX, Kirschning CJ, Mancinelli R, Xu XP, Jin Y, Faure E, et al. Bacterial lipopolysaccharide activates nuclear factor-kappab through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J Biol Chem. 1999;274(12):7611–4.
Selin KA, Hedin CRH, Villablanca EJ. Immunological networks defining the heterogeneity of Inflammatory Bowel diseases. J Crohns Colitis. 2021;15(11):1959–73.
Article PubMed PubMed Central Google Scholar
Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, et al. Interleukin-13 is the Key Effector Th2 Cytokine in Ulcerative Colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129(2):550–64.
Zhu QG, Rui K, Wang SJ, Tian J. Advances of Regulatory B cells in Autoimmune diseases. Front Immunol. 2021;12:12:592914.
Dave M, Higgins PD, Middha S, Rioux KP. The human gut microbioma current knowledge, challenges, and future directions. Translational Res. 2012;160(4):246–57.
He XL, Gao J, Peng L, Hu TT, Wan Y, Zhou MJ, et al. Bacterial O-GlcNAcase genes abundance decreases in ulcerative colitis patients and its administration ameliorates colitis in mice. Gut. 2021;70(10):1872–83.
Li BL, Du PL, Du Y, Zhao DY, Cai YR, Yang Q et al. Luteolin alleviates inflammation and modulates gut microbiota in ulcerative colitis rats. Life Sci. 2021;269:269:119008.
Guo XY, Liu XJ, Hao JY. Gut microbiota in ulcerative colitis: insights on pathogenesis and treatment. J Dig Dis. 2020;21(3):147–59.
Mizoguchi E, Tong M, Li X, Wegener Parfrey L, Roth B, Ippoliti A et al. A Modular Organization of the Human Intestinal Mucosal Microbiota and its Association with Inflammatory Bowel Disease. PLoS ONE. 2013;8(11):e80702.
Andoh A, Ida S, Tsujikawa T, Benno Y, Fujiyama Y. Terminal restriction fragment polymorphism analyses of fecal microbiota in five siblings including two with ulcerative colitis. Clin J Gastroenterol. 2009;2(5):343–5.
Fuentes S, Rossen NG, van der Spek MJ, Hartman JHA, Huuskonen L, Korpela K, et al. Microbial shifts and signatures of long-term remission in ulcerative colitis after faecal microbiota transplantation. ISME J. 2017;11(8):1877–89.
Kotlowski R, Bernstein CN, Sepehri S, Krause DO. High prevalence of Escherichia coli belonging to the B2 + D phylogenetic group in inflammatory bowel disease. Gut. 2007;56(5):669–75.
Xu J, Chen N, Wu Z, Song Y, Zhang Y, Wu N et al. 5-Aminosalicylic acid alters the gut bacterial microbiota in patients with Ulcerative Colitis. Front Microbiol. 2018;9:1274.
Schwiertz A, Jacobi M, Frick J-S, Richter M, Rusch K, Köhler H. Microbiota in Pediatric Inflammatory Bowel Disease. J Pediatr. 2010;157(2):240–e41.
Khalil NA, Walton GE, Gibson GR, Tuohy KM, Andrews SC. In vitrobatch cultures of gut microbiota from healthy and ulcerative colitis (UC) subjects suggest that sulphate-reducing bacteria levels are raised in UC and by a protein-rich diet. Int J Food Sci Nutr. 2013;65(1):79–88.
Verma R, Verma AK, Ahuja V, Paul J. Real-Time Analysis of Mucosal Flora in patients with inflammatory bowel disease in India. J Clin Microbiol. 2010;48(11):4279–82.
Walker AW, Sanderson JD, Churcher C, Parkes GC, Hudspith BN, Rayment N et al. High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol. 2011;11:7.
Tahara T, Shibata T, Kawamura T, Okubo M, Ichikawa Y, Sumi K, et al. Fusobacterium detected in Colonic Biopsy and Clinicopathological features of Ulcerative Colitis in Japan. Dig Dis Sci. 2014;60(1):205–10.
Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, et al. A decrease of the butyrate-producing speciesRoseburia hominisandFaecalibacterium prausnitziidefines dysbiosis in patients with ulcerative colitis. Gut. 2014;63(8):1275–83.
Lepage P, Häsler R, Spehlmann ME, Rehman A, Zvirbliene A, Begun A, et al. Twin study indicates loss of Interaction between Microbiota and Mucosa of patients with Ulcerative Colitis. Gastroenterology. 2011;141(1):227–36.
Fite A, Macfarlane S, Furrie E, Bahrami B, Cummings JH, Steinke DT, et al. Longitudinal analyses of gut mucosal microbiotas in Ulcerative Colitis in Relation to Patient Age and Disease Severity and Duration. J Clin Microbiol. 2013;51(3):849–56.
Ott SJ, Plamondon S, Hart A, Begun A, Rehman A, Kamm MA, et al. Dynamics of the Mucosa-Associated Flora in Ulcerative Colitis patients during remission and clinical relapse. J Clin Microbiol. 2008;46(10):3510–3.
Hu Y, Chen Z, Xu C, Kan S, Chen D. Disturbances of the gut microbiota and microbiota-derived metabolites in Inflammatory Bowel Disease. Nutrients. 2022;14(23):5140.
Andreopoulou M, Tsiouris V, Georgopoulou I. Effects of organic acids on the gut ecosystem and on the performance of broiler chickens. J Hellenic Veterinary Med Soc. 2017;65(4):289.
Agus A, Planchais J, Sokol H. Gut microbiota regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe. 2018;23(6):716–24.
Singh R, Chandrashekharappa S, Bodduluri SR, Baby BV, Hegde B, Kotla NG et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat Commun. 2019;10(1):89.
Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016;22(6):586–97.
Q W, RM M, BA C, M C-D, KJ Z. D G, - A bacterial carbohydrate links innate and adaptive responses through Toll-like. D– 2985109r. (– 0022-1007 (Print)):- 2853-63.
A I-V CDC, K JPAA. K, L A, - FXR and TGR5 Agonists Ameliorate Liver Injury, Steatosis, and Inflammation After. D– 101695860. (– 2471-254X (Electronic)):- 1379-91.
Agus A, Clément K, Sokol H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut: J Br Soc Gastroenterol. 2021;70(6):1174–82.
Wahlstrom A, Kovatcheva-Datchary P, Stahlman M, Backhed F, Marschall H-U. Crosstalk between bile acids and gut microbiota and its impact on farnesoid receptor signalling. Dig Dis. 2017;35(3):246–50.
Figliuolo VR, Coutinho-Silva R, Lara Melo Coutinho CM. Contribution of sulfate-reducing bacteria to homeostasis disruption during intestinal inflammation. Life Sci. 2018;215:145–51.
Cai Z, Wang S, Li J. Treatment of inflammatory bowel disease: a Comprehensive Review. Front Med (Lausanne). 2021;8:765474.
Tremblay L, Pineton de Chambrun G, De Vroey B, Lavogiez C, Delaporte E, Colombel JF, et al. Stevens-Johnson syndrome with sulfasalazine treatment: report of two cases. J Crohns Colitis. 2011;5(5):457–60.
Hayashi R, Wada H, Ito K, Adcock IM. Effects of glucocorticoids on gene transcription. Eur J Pharmacol. 2004;500(1–3):51–62.
Lichtenstein GR, Abreu MT, Cohen R, Tremaine W, American Gastroenterological A. American Gastroenterological Association Institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006;130(3):940–87.
Curkovic I, Egbring M, Kullak-Ublick GA. Risks of inflammatory bowel disease treatment with glucocorticosteroids and aminosalicylates. Dig Dis. 2013;31(3–4):368–73.
Chhaya V, Saxena S, Cecil E, Subramanian V, Curcin V, Majeed A, et al. Steroid dependency and trends in prescribing for inflammatory bowel disease - a 20-year national population-based study. Aliment Pharmacol Ther. 2016;44(5):482–94.
D’Haens G. Systematic review: second-generation vs. conventional corticosteroids for induction of remission in ulcerative colitis. Aliment Pharmacol Ther. 2016;44(10):1018–29.
Jharap B, Seinen ML, de Boer NK, van Ginkel JR, Linskens RK, Kneppelhout JC, et al. Thiopurine therapy in inflammatory bowel disease patients: analyses of two 8-year intercept cohorts. Inflamm Bowel Dis. 2010;16(9):1541–9.
Herrlinger KR, Barthel DN, Schmidt KJ, Buning J, Barthel CS, Wehkamp J, et al. Infliximab as rescue medication for patients with severe ulcerative/indeterminate colitis refractory to tacrolimus. Aliment Pharmacol Ther. 2010;31(9):1036–41.
Moura FA, de Andrade KQ, Dos Santos JCF, Araujo ORP, Goulart MOF. Antioxidant therapy for treatment of inflammatory bowel disease: does it work? Redox Biol. 2015;6:617–39.
Hui L, Wei-Hsien L, Hanying Z, Haotian F, Wen Z, Wei-Lian H et al. Bifidobacterium lactis BL-99 protects mice with osteoporosis caused by colitis via gut inflammation and gut microbiota regulation. Food & Function. 2022;13(3):1482-1494.
Aghamohammad S, Sepehr A, Miri ST, Najafi S, Pourshafie MR, Rohani M. The role of combining Probiotics in Preventing and Controlling inflammation: a focus on the anti-inflammatory and Immunomodulatory effects of Probiotics in an in vitro model of IBD. Can J Gastroenterol Hepatol. 2022:2045572.
White R, Atherly T, Guard B, Rossi G, Wang C, Mosher C, et al. Randomized, controlled trial evaluating the effect of multi-strain probiotic on the mucosal microbiota in canine idiopathic inflammatory bowel disease. Gut Microbes. 2017;8(5):451–66.
Kang Y, Cai Y. The development of probiotics therapy to obesity: a therapy that has gained considerable momentum. Hormones. 2018;17(2):141–51.
Kang Y, Cai Y, Yang Y. The gut Microbiome and Hepatocellular Carcinoma: implications for early diagnostic biomarkers and Novel therapies. Liver Cancer. 2022;11(2):113–25.
Li LP, Kang YB. The gut Microbiome and Autoimmune Hepatitis: implications for early diagnostic biomarkers and Novel therapies. Molecular Nutrition & Food Research; 2023;67(24):e2300043.
Cai Y, Kang YB. Gut microbiota and metabolites in diabetic retinopathy: insights into pathogenesis for novel therapeutic strategies. Biomed Pharmacother. 2023;164:114994.
Liu HX, Kang X, Yang XD, Yang H, Kuang XY, Ren P, et al. Compound Probiotic ameliorates Acute Alcoholic Liver Disease in mice by modulating gut microbiota and maintaining intestinal barrier. Probiotics Antimicrob Proteins. 2023;15(1):185–201.
Wu J, Cui S, Tang X, Zhang Q, Jin Y, Zhao J, et al. Bifidobacterium longum CCFM1206 promotes the Biotransformation of Glucoraphanin to Sulforaphane that contributes to amelioration of Dextran-Sulfate-Sodium-Induced Colitis in mice. J Agric Food Chem. 2023;71(2):1100–12.
Han T, Hu X, Li K, Zhang D, Zhang Y, Li J. Bifidobacterium infantis maintains Genome Stability in Ulcerative Colitis via regulating anaphase-promoting Complex Subunit 7. Front Microbiol. 2021;12:761113.
Fu T, Wang Y, Ma M, Dai W, Pan L, Shang Q et al. Isolation of Alginate-degrading Bacteria from the human gut microbiota and Discovery of Bacteroides xylanisolvens AY11-1 as a Novel Anti-colitis Probiotic Bacterium. Nutrients. 2023;15(6):1352.
Wang T, Shi C, Wang S, Zhang Y, Wang S, Ismael M, et al. Protective effects of Companilactobacillus Crustorum MN047 against Dextran Sulfate Sodium-Induced Ulcerative Colitis: a fecal microbiota transplantation study. J Agric Food Chem. 2022;70(5):1547–61.
Hu Y, Jin X, Gao F, Lin T, Zhu H, Hou X et al. Selenium-enriched Bifidobacterium longum DD98 effectively ameliorates dextran sulfate sodium-induced ulcerative colitis in mice. Front Microbiol. 2022;13:955112.
Han M, Liao W, Si X, Bai C, Gai Z. Protective effects of lacticaseibacillus rhamnosus Hao9 on dextran sulphate sodium-induced ulcerative colitis in mice. J Appl Microbiol. 2022;133(3):2039–49.
Huang J, Yang Z, Li Y, Chai X, Liang Y, Lin B et al. Lactobacillus paracasei R3 protects against dextran sulfate sodium (DSS)-induced colitis in mice via regulating Th17/Treg cell balance. J Translational Med. 2021;19(1):356.
Xu X, Wu J, Jin Y, Huang K, Zhang Y, Liang Z. Both Saccharomyces boulardii and its postbiotics alleviate Dextran Sulfate Sodium-Induced Colitis in mice, Association with modulating inflammation and intestinal microbiota. Nutrients. 2023;15(6):1484.
Komaki S, Haque A, Miyazaki H, Matsumoto T, Nakamura S. Unexpected effect of probiotics by Lactococcus lactis subsp. lactis against colitis induced by dextran sulfate sodium in mice. J Infect Chemother. 2020;26(6):549–53.
Hizay A, Dag K, Oz N, Comak-Gocer EM, Ozbey-Unlu O, Ucak M et al. Lactobacillus acidophilus regulates abnormal serotonin availability in experimental ulcerative colitis. Anaerobe. 2023;80:102710.
Gao H, Li Y, Sun J, Xu H, Wang M, Zuo X, et al. Saccharomyces Boulardii ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in mice by regulating NF-κB and Nrf2 signaling pathways. Oxidative Med Cell Longev. 2021;2021:1–14.
Google Scholar
He W, Ni W, Zhao J, M’Koma A. Enterococcus faecium alleviates Gut Barrier Injury in C57BL/6 mice with Dextran Sulfate Sodium-Induced Ulcerative Colitis. Gastroenterol Res Pract. 2021;2021:1–9.
Li ZG, Zhang S, Xu L, Fang XX, Wan YZ, Yu DH, et al. A tetrapeptide from maize combined with probiotics exerted strong anti-inflammatory effects and modulated gut microbiota in DSS-induced colitis mice. Food Funct. 2022;13(24):12602–18.
Shang JC, Yang S, Tang ZX, Chen YH, Duan BF, Meng XC. Bifidobacterium bifidum H3-R2 and its Molecular Communication within the context of Ulcerative Colitis. J Agric Food Chem. 2022;70(37):11678–88.
Chen ZX, Yi L, Pan YN, Long XY, Mu JF, Yi RK et al. Lactobacillus fermentum ZS40 ameliorates inflammation in mice with Ulcerative Colitis Induced by Dextran Sulfate Sodium. Front Pharmacol. 2021;12:700217.
Ren Z, Hong Y, Huo Y, Peng L, Lv H, Chen J et al. Prospects of probiotic adjuvant drugs in clinical treatment. Nutrients. 2022;14(22):4723.
Hegazy SK. Effect of probiotics on pro-inflammatory cytokines and NF-κB activation in ulcerative colitis. World J Gastroenterol. 2010;16(33):4145-51.
Palumbo VD, Romeo M, Gammazza AM, Carini F, Damiani P, Damiano G, et al. The long-term effects of probiotics in the therapy of ulcerative colitis: a clinical study. Biomedical Papers-Olomouc. 2016;160(3):372–7.
Bjarnason I, Sission G, Hayee B. A randomised, double-blind, placebo-controlled trial of a multi-strain probiotic in patients with asymptomatic ulcerative colitis and Crohn’s disease. Inflammopharmacology. 2019;27(3):465–73.
Tsuda Y, Yoshimatsu Y, Aoki H, Nakamura K, Irie M, Fukuda K, et al. Clinical effectiveness of probiotics therapy (BIO-THREE) in patients with ulcerative colitis refractory to conventional therapy. Scand J Gastroenterol. 2009;42(11):1306–11.
Agraib LM, Yamani MI, Tayyem R, Abu-Sneineh AT, Rayyan YM. Probiotic supplementation induces remission and changes in the immunoglobulins and inflammatory response in active ulcerative colitis patients: a pilot, randomized, double-blind, placebo-controlled study. Clin Nutr ESPEN. 2022;51:83–91.
Groeger D, O’Mahony L, Murphy EF, Bourke JF, Dinan TG, Kiely B, et al. Bifidobacterium infantis35624 modulates host inflammatory processes beyond the gut. Gut Microbes. 2014;4(4):325–39.
Gu ZY, Pei WL, Shen YH, Wang LJ, Zhu J, Zhang Y, et al. Akkermansia muciniphila and its outer protein Amuc_1100 regulates tryptophan metabolism in colitis. Food Funct. 2021;12(20):10184–95.
Chen P, Xu HY, Tang H, Zhao FY, Yang CC, Kwok LY, et al. Modulation of gut mucosal microbiota as a mechanism of probiotics-based adjunctive therapy for ulcerative colitis. Microb Biotechnol. 2020;13(6):2032–43.
Carnahan S, Balzer A, Panchal SK, Brown L. Prebiotics in obesity. Panminerva Med. 2014;56(2):165–75.
Looijer-van Langen MA, Dieleman LA. Prebiotics in chronic intestinal inflammation. Inflamm Bowel Dis. 2009;15(3):454–62.
Baquerizo Nole KL, Yim E, Keri JE. Probiotics and prebiotics in dermatology. J Am Acad Dermatol. 2014;71(4):814–21.
Sanlier N, Kocabas S. The effect of probiotic, prebiotic and gut microbiota on ASD: a review and future perspectives. Crit Rev Food Sci Nutr. 2023;63(15):2319–30.
Koleva PT, Valcheva RS, Sun X, Ganzle MG, Dieleman LA. Inulin and fructo-oligosaccharides have divergent effects on colitis and commensal microbiota in HLA-B27 transgenic rats. Br J Nutr. 2012;108(9):1633–43.
Hoentjen F, Welling GW, Harmsen HJM, Zhang X, Snart J, Tannock GW, et al. Reduction of colitis by prebiotics in HLA-B27 transgenic rats is associated with microflora changes and immunomodulation. Inflamm Bowel Dis. 2005;11(11):977–85.
Wang C, Bai J, Wang B, Yu L, Tian F, Zhao J, et al. Stachyose modulates gut microbiota and alleviates DSS-induced ulcerative colitis in mice. Food Sci Hum Wellness. 2023;12(6):2211–20.
Lunken GR, Tsai K, Schick A, Lisko DJ, Cook L, Vallance BA, et al. Prebiotic enriched exclusive Enteral Nutrition suppresses colitis via gut microbiome modulation and expansion of anti-inflammatory T cells in a mouse model of colitis. Cell Mol Gastroenterol Hepatol. 2021;12(4):1251–66.
Li X, Lv H, Shi F, Song J, Zhang Z. The potential therapeutic effects of hydroxypropyl cellulose on acute murine colitis induced by DSS. Carbohydr Polym. 2022;289:119430.
Kanauchi O, Serizawa I, Araki Y, Suzuki A, Andoh A, Fujiyama Y, et al. Germinated barley foodstuff, a prebiotic product, ameliorates inflammation of colitis through modulation of the enteric environment. J Gastroenterol. 2003;38(2):134.
Gibson GR, Beatty ER, Wang X, Cummings JH, Gibson GR, Beatty ER, Wang X, Cummings JH. Selective stimulation of Bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology. 1995;108(4):975– 82.
Vulevic J, Drakoularakou A, Yaqoob P, Tzortzis G, Gibson GR. Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. Am J Clin Nutr. 2008;88(5):1438–46.
Casellas F, Borruel N, Torrejon A, Varela E, Antolin M, Guarner F, et al. Oral oligofructose-enriched inulin supplementation in acute ulcerative colitis is well tolerated and associated with lowered faecal calprotectin. Aliment Pharmacol Ther. 2007;25(9):1061–7.
Faghfoori Z, Navai L, Shakerhosseini R, Somi MH, Nikniaz Z, Norouzi MF. Effects of an oral supplementation of germinated barley foodstuff on serum tumour necrosis factor-alpha, interleukin-6 and– 8 in patients with ulcerative colitis. Ann Clin Biochem. 2011;48(Pt 3):233–7.
Mitsuyama K, Saiki T, Kanauchi O, Iwanaga T, Sata M. Treatment of ulcerative colitis with germinated barley foodstuff feeding: a pilot study. Aliment Pharmacol Ther. 1998;12(12):1225–30.
Ryan JJ, Monteagudo-Mera A, Contractor N, Gibson GR. Impact of 2’-Fucosyllactose on Gut Microbiota Composition in Adults with Chronic Gastrointestinal Conditions: Batch Culture Fermentation Model and Pilot Clinical Trial Findings. Nutrients. 2021;13(3):938.
Suzuki A, Mitsuyama K, Koga H, Tomiyasu N, Masuda J, Takaki K, et al. Bifidogenic growth stimulator for the treatment of active ulcerative colitis: a pilot study. Nutrition. 2006;22(1):76–81.
Li Z, Li Z, Zhu L, Dai N, Sun G, Peng L, et al. Effects of Xylo-Oligosaccharide on the gut microbiota of patients with Ulcerative Colitis in Clinical Remission. Front Nutr. 2021;8:778542.
Download references
Acknowledgements
Not applicable.
This work was supported by Shanxi Province Natural Science Foundation (Grant No. 202203021221182), Science Research Start-up Fund for Doctor of Shanxi Medical University (Grant No. XD1807), Science Research Start-up Fund for Doctor of Shanxi Province (Grant No.SD1807), Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (Grant No. 2019L0425), and Shanxi Province Science Foundation for Youths (Grant No. 201901D211314).
Author information
Authors and affiliations.
Department of microbiology and immunology, School of Basic Medical Sciences, Shanxi Medical University, Taiyuan, Shanxi, China
Jing Guo, Liping Li & Yongbo Kang
Faculty of Life science and Technology, Kunming University of Science and Technology, Kunming, Yunnan, China
You can also search for this author in PubMed Google Scholar
Contributions
Jing Guo wrote the main manuscript text with the help of Liping Li, Xinwei Huang and Yongbo Kang. All authors reviewed the manuscript.
Corresponding author
Correspondence to Yongbo Kang .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Guo, J., Li, L., Cai, Y. et al. The development of probiotics and prebiotics therapy to ulcerative colitis: a therapy that has gained considerable momentum. Cell Commun Signal 22 , 268 (2024). https://doi.org/10.1186/s12964-024-01611-z
Download citation
Received : 10 October 2023
Accepted : 10 April 2024
Published : 14 May 2024
DOI : https://doi.org/10.1186/s12964-024-01611-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Ulcerative colitis
- Gut microflora
- Inflammation
Cell Communication and Signaling
ISSN: 1478-811X
- General enquiries: [email protected]
Clinical Trials
Ulcerative colitis.
Displaying 69 studies
The purpose of the study is to demonstrate the efficacy and safety, and to assess the pharmacokinetics of adalimumab administered subcutaneously (SC) in pediatric subjects with moderate to severe ulcerative colitis (UC).
The purpose of this study is to evaluate the accuracy of the length-109 probe set panel (a genetic test) in predicting response to golimumab treatment in participants with moderately to severely active ulcerative colitis (UC).
The purpose of this study is to assess the effectiveness and safety of BMS-986165 in ulcerative colitis.
The purpose of this study is to evaluate the effectiveness and safety of vedolizumab intravenous (IV) treatment compared to adalimumab subcutaneous (SC) treatment for patients who have ulcerative colitis.
This is a registry study to evaluate the long-term safety and effectiveness of adalimumab in patients with moderately to severely active UC who are treated as recommended in the product label.
The purpose of this study is to evaluate the effectiveness and safety of continuing ustekinumab as a subcutaneous (injection) maintenance therapy in patients with moderately to severely active ulcerative colitis (UC) who have demonstrated a clinical response to an induction treatment with IV ustekinumab.
This study is an open label, long-term extension study for subjects with moderate to severe ulcerative colitis designed to evaluate long term therapy of CP-690,550.
The purpose of this study is to evaluate the pharmacokinetics (what the body does to the study medication) and safety of subcutaneously (under the skin) administered golimumab in pediatric participants (aged 2 to 17 years) with moderately to severely active Ulcerative Colitis (UC).
The investigators aim to prospectively study the feasibility and clinical impact of hyperbaric oxygen therapy in acute hospitalized moderate to severe ulcerative colitis flares as an adjunct to standard medical treatment. Specifically, we will investigate the impact of hyperbaric oxygen therapy on clinical response/remission and serum and mucosal inflammatory markers. The investigators expect that hyperbaric oxygen therapy will improve patient responsiveness to steroids and avoid progression to second line therapy during hospitalization.
To evaluate safety and efficacy of two dosing regimens in achieving clinical remission at Week 8 in subjects with moderately to severely active Ulcerative Colitis.
The purpose of this study is to investigate the safety and effectiveness of methotrexate for the initial treatment and the maintenance of a steroid free remission, for patients who have active ulcerative colitis.
The purpose of this study is to evaluate the safety and effectiveness of trichuris suis ova (TSO) in ulcerative colitis (UC). We will look at how TSO affects the body's immune response and if there are related changes in participants' UC.
The cause of UC is not well understood. It is believed to be caused from an abnormal immune response to the normal bacteria that live in the gut (intestines and colon). This response acts as an "attack" on the healthy tissue of the bowel by a person's own immune cells which leads to disease.
It is well known that autoimmune diseases ...
The purpose of this study is to assess the long-term safety and effectiveness of repeat treatment with adalimumab in pediatric patients who have ulcerative colitis.
This study is designed to evaluate the efficacy and safety of tofacitinib (CP-690,550) in patients with moderate to severe ulcerative colitis who have failed or be intolerant to one of following treatments for ulcerative colitis: oral steroids, azathiopurine/6-mercaptopurine, or anti-TNF-alpha therapy.
The main objective is to compare prebiotic therapy with placebo for the prevention of pouchitis after closure of diverting ileostomy in patients with an ileal pouch anal anastomosis. This study will also characterize the effects of prebiotics on the fecal microbiota and fecal microbial metabolites and correlate these effects with the primary outcome of development of pouchitis.
The purpose of this study is to assess the efficacy and safety of vedolizumab intravenous (IV) in participants with a proctocolectomy and ileal pouch anal anastomosis for ulcerative colitis (UC) who have developed chronic or recurrent pouchitis, or require continuous antibiotic treatment.
The purpose of this study is to assess the long-term safety of vedolizumab versus other biologic agents in participants with Ulcerative Colitis or Crohn's Disease.
This is a randomized, double blind, placebo controlled, parallel group, multiple dose study to evaluate the efficacy of AMG 181 compared with placebo as measured by the proportion of subjects in remission (total Mayo Score < 2 points with no individual subscore > 1 point) at week 8. After completing all screening assessments and meeting all eligibility criteria, subjects will be randomized to receive placebo or AMG 181 at various doses per protocol. A maximum of approximately 50% of subjects with any prior anti-TNF agent use will be allowed in the study. At the end of the double blind period, ...
This Phase III, double blind, placebo-controlled, multicenter study will investigate the efficacy and safety of etrolizumab in maintenance of remission in patients with moderately to severely active ulcerative colitis (UC) who are naïve to TNF inhibitors and refractory to or intolerant of prior immunosuppressant and/or corticosteroid treatment.
The purpose of this study is to evaluate the efficacy of SHP647 in inducing remission, based on composite score of patient-reported symptoms and centrally read endoscopy, in subjects with moderate to severe ulcerative colitis (UC).
This study comprises three sub-studies. The objective of sub-study 1 was to characterize the dose-response, efficacy, and safety of upadacitinib compared to placebo in inducing clinical remission per Adapted Mayo score (using the Mayo Scoring System for Assessment of Ulcerative Colitis Activity, excluding Physician's Global Assessment [PGA]) in subjects with moderately-to-severely active UC in order to identify the induction dose of upadacitinib for further evaluation in Phase 3 studies including Substudy 2.
The primary objective of Substudy 2 (Phase 3 induction) is to evaluate the efficacy and safety of upadacitinib 45 mg QD compared to placebo in inducing clinical ...
This study seeks to identify predictive fecal, blood, and tissue biomarkers, in which biomarkers can be used to identify responders that will be reflective of disease severity or predict response to Janus Kinase Inhibitor Therapy. The benefits to the sub-study are to develop a population PK/PD model to characterize the relationships between local and systemic drug exposure and clinical, endoscopic, histologic, or biologic response to therapy. Study also seeks to collect safety data from these subjects on Janus Kinase Inhibitor Therapy.
The purpose of this research is to determine if different diets have different effects on the micribiome and inflammation of your colon.
The purpose of this study is to evaluate the effectiveness, safety, pharmacokinetics (PK), and immune response of high and low doses in 3 different weight groups of vedolizumab intravenous (IV) for maintenance of remission in children aged 2 to 17 years, inclusive, who weigh ≥ 10 kg, with moderately to severely active ulcerative colitis (UC).
This study is designed to evaluate the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of TD-1473 in subjects with moderately-to-severely active UC over 28 days. This exploratory study will also serve as a signal seeking endeavor to demonstrate biologic effect associated with TD-1473 through biomarker analysis and clinical, endoscopic, and histologic assessments.
The study proposes to assess whether compared to placebo, CP-690,550 is effective, safe, and tolerable maintenance therapy in subjects with Ulcerative Colitis (UC). The study proposes to assess whether compared to placebo, CP-690,550 maintenance therapy more effectively achieves mucosal healing and improves quality of life in subjects with UC.The study proposes to assess CP-690,550 pharmacokinetic exposure during maintenance therapy in subjects over the age of 18 years with UC.
To assess clinical response to MMX mesalamine/mesalazine between a low and high dose in children and adolescents aged 5-17 years with mild to moderate Ulcerative Colitis (UC) or who are in remission.
The global primary objectives of this study are, in pediatric participants with moderately to severely active ulcerative colitis (UC):
- To evaluate the efficacy of ustekinumab dosing in inducing clinical remission;
- To evaluate the safety profile of ustekinumab;
- To evaluate ustekinumab exposure (pharmacokinetics [PK]).
The United States (US)-specific primary objectives of this study are, in pediatric participants with moderately to severely active UC:
- To evaluate the efficacy of ustekinumab dosing in maintaining clinical remission among participants who were in clinical response in induction;
The purpose of this study is to evaluate the safety, tolerability and microbiome dynamics of SER-287 in subjects with mild-to-moderate ulcerative colitis.
This study will evaluate the effectiveness, safety, and tolerability of GS-5745. It will consist of 2 parts: Induction Study (Cohort 1) and Maintenance Study (Cohort 2). Participants in each part will receive either active GS-5745 or a placebo.
The primary objective of this study is to observe the long-term safety of filgotinib in adults who have completed or met protocol specified efficacy discontinuation criteria in a prior Gilead-sponsored filgotinib treatment study in ulcerative colitis (UC).
The purpose of this study is to obtain long term safety and tolerability data of vedolizumab subcutaneous (vedolizumab SC) in participants with ulcerative colitis (UC) and Crohn's disease (CD).
The purpose of this study is to evaluate safety and efficacy of risankizumab in subjects with ulcerative colitis (UC) in subjects who responded to induction treatment with rizankizumab in a prior AbbVie study of risankizumab in UC. This study consists of three sub-studies: Substudy 1 is a 52-week, randomized, double-blind, placebo-controlled maintenance study; Substudy 2 is 52-week, randomized, exploratory maintenance study; and Substudy 3 is an open-label long-term extension study for subjects who completed Substudy 1 or 2.
This two-part, part 1: open-label extension (OLE) and part 2: safety monitoring (SM) study will examine the efficacy and safety of continued etrolizumab treatment in moderate to severe ulcerative colitis (UC) participants previously enrolled in etrolizumab Phase II/III studies. Participants with moderate to severe UC who were enrolled in the Phase II OLE study (GA27927 [NCT01461317]) or the Phase III studies (GA28948 [NCT02163759], GA28949 [NCT02171429], GA28950 [NCT02100696], GA29102 [NCT02165215], and GA29103 [NCT02136069]) were included. Participants from the Phase II OLE study or the Phase III studies who are not eligible or willing to receive etrolizumab in the OLE-SM study, and ...
The purpose of this study is to assess the integrated effect of normalization of intestinal dysbiosis through a structured semi-vegetarian dietary intervention in active UC patients who will also be under the standard of care medical therapy (vedolizumab).
The purpose of this study is to evaluate the effectiveness and safety of induction and maintenance therapy with TD-1473 in subjects with moderately-to-severely active ulcerative colitis with up to 60 weeks of treatment.
The purpose of the trial is to determine the safety and efficacy of RPC1063 in patients diagnosed with moderate to severe ulcerative colitis.
The primary purpose of this study is to evaluate the effectiveness and safety of treatment with filgotinib on the induction and maintenance of remission in subjects with moderately to severely active ulcerative colitis (UC). Subjects who are biologic-naïve and biologic-experienced will be enrolled in Cohorts A and B, respectively. Treatment assignments will be randomized within each Cohort.
Participants who complete the study, or do not meet protocol response or remission criteria at Week 10, will have the option to enter a separate, long-term extension (LTE) study (Gilead Study GS-US-418-3899).
The primary objectives of this study are to evaluate the safety and efficacy of filgotinib in the induction and maintenance treatment of moderately to severely active ulcerative colitis (UC) in participants who are biologic-naive and biologic-experienced. Participants who complete the study, or do not meet protocol response or remission criteria at Week 10 will have the option to enter a separate, long-term extension (LTE) study (Gilead Study GS-US-418-3899).
The objective of this study is to evaluate the efficacy and safety of Upadacitinib compared to placebo in inducing clinical remission (per Adapted Mayo score) in participants with moderately to severely active ulcerative colitis (UC).
The purpose of this study is to determine the safety profile of long-term vedolizumab IV treatment in pediatric participants with UC or CD.
To evaluate a new oral treatment, ABI-M201 (or placebo) in individuals with mild to moderate Ulcerative Colitis who have sustained an inadequate response to ongoing mesalamine treatment.
Subjects with Ulcerative Colitis who have completed an induction study with PF-00547659 will receive an additional 72 weeks of open-label treatment to evaluate the long-term safety of the drug.
The purpose of this study is to evaluate effectiveness of golimumab in inducing clinical remission as assessed by the Mayo score, in pediatric participants with moderately to severely active ulcerative colitis (UC). In addition, the safety profile of golimumab, in pediatric participants with moderately to severely active UC will be assessed.
This study is designed to evaluate the long-term safety and efficacy of ABT-494 in participants with ulcerative colitis (UC) who have not responded at the end of the induction period in Study M14-234 (Substudies 1 and 2), who have had loss of response during the maintenance period of Study M14-234 (Substudy 3), or who have successfully completed Study M14-234.
The purpose of this research study is to collect clinical information and human samples from patients with IBD. The data collected will be used to create an organized, accessible resource of human samples for biological research in IBD.
The purpose of this study is to evaluate the effectiveness and safety of ustekinumab used as the initial treatment, given by IV, and as maintenance treatment, injected subcutaneously for patients who have moderately to severely active Ulcerative Colitis.
The purpose of this study is to determine whether RPC1063 is effective in the treatment of Ulcerative Colitis (UC).
The purpose of this study is to assess the effectiveness and safety of SER-287 in adults with active mild-to-moderate ulcerative colitis.
This phase III, double blind, placebo-controlled, multicenter study will investigate the efficacy and safety of etrolizumab during induction and maintenance of remission in participants with moderately to severely active ulcerative colitis (UC) who are refractory to or intolerant of TNF inhibitors.
The objectives of Sub-Study 1 are to evaluate the efficacy, safety, and pharmacokinetics of risankizumab as induction treatment in subjects with moderately to severely active ulcerative colitis (UC), and to identify the appropriate induction dose of risankizumab for further evaluation in Sub-Study 2. The objective of Sub-Study 2 is to evaluate the efficacy and safety of risankizumab compared to placebo in inducing clinical remission in subjects with moderately to severely active UC.
The purpose of this study is to evaluate the efficacy of SHP647 as maintenance therapy in participants with moderate to severe ulcerative colitis (UC) who achieved clinical response in induction studies.
Ulcerative Colitis (UC) is a chronic inflammatory disease affecting the mucosal lining of the colon and rectum and the incidence is increasing, but the etiology remains unknown. Patients may require a proctocolectomy due to refractory disease. Prior to an operation, UC is treated with antibiotic therapy, immunomodulatory therapy and immunosuppressive agents. While there is an increasing number of approved biologics for the treatment of UC, there are many patients that still suffer from refractory disease. Thus, alternative mechanisms of therapy are desperately needed.
Treatments that have the potential to reduce mucosal inflammation could alleviate the pathology of luminal UC. This trial ...
The purpose of this study is to demonstrate the impact of an Adult Inflammatory Bowel Disease (IBD) learning health system approach that enables patients and their care teams to coproduce optimal health and high value care.
We would like to collaborate and further develop an ipad-based, interactive quiz game 'Emma'; to identify gaps in knowledge of inflammatory bowel disease in pediatric patients. These gaps can be used to improve patient education.
Understanding of how best to treat inflammatory bowel disease (IBD) has evolved over the last ten years. Evidence now suggests that the most effective therapy early in the course of Crohn's disease (CD) and ulcerative colitis (UC) involves the use of immune suppressing medications such as the anti-Tumor Necrosis Factor (anti-TNF) agents infliximab, adalimumab, and certolizumab. However, many CD and UC patients still ultimately require surgery despite the use of these medications. Side effects of the anti-TNF agents include increased risk of infections due to their effect on the immune system. Little is known about how use of these medications ...
The purpose of this study is to create a group of blood and urine samples and quality of life data from Mayo Clinic patients with a diagnosis of inflammatory bowel disease (IBD). The blood and urine samples and quality of life data will be used for future research of IBD at Mayo Clinic and future research at Mayo Clinic to learn about, prevent, or treat other health problems.
The focus of this study is to retrospectively examine the patterns of use of biological agents and risk factors for discontinuation of these agents in pediatric Crohn’s disease (CD) and ulcerative colitis (UC) within the ICN network. This will be a retrospective longitudinal observational cohort study. The study population for the first portion of the study (Aim 1a below) will be all CD and UC patients in ICN who were diagnosed with IBD prior to age 18. This population will be queried for biologic use prior to age 18. The study population for the remainder of the study will be ...
The purpose of this study is to evaluate vedolizumab pharmacokinetics (PK), safety and tolerability in pediatric participants with moderately to severely active ulcerative colitis or Crohn's disease.
This study will be a multi-center, longitudinal, observational investigation to collect biospecimens (blood, stool and intestinal biopsies) to support the development of specific biomarkers of treatment response to infliximab and vedolizumab in children with IBD.
The focus of the proposed study is to improve understanding of the underlying mechanisms of symptom burden, and the purpose is to describe how sleep health contributes to symptoms and functional performance in adults with Inflammatory Bowel Disease (IBD).
The purpose of this research study is to assess women who are diagnosed with Inflammatory Bowel Disease (IBD) and study the combination of using usual medical treatment with a wellness program designed just for women with IBD and the effect it has on quality of life, stress, and their symptoms
The purpose of this study is to evaluate the safety and tolerability of long-term treatment with SHP647 in participants with moderate to severe Ulcerative Colitis (UC) or Crohn's disease (CD).
Primary Aim
We aim to evaluate: 1) the correlation between patient-reported rectal bleeding and stool frequency and health-related quality of life focused on fatigue, depression and anxiety, and work productivity; and 2) the correlation between the severity of endoscopic inflammation and health-related quality of life focused on fatigue, depression and anxiety, and work productivity.
Secondary Aims
We also aim to evaluate the correlation between the combination of clinical/PRO and the severity of endoscopic inflammation and health-related quality of life focused on fatigue, depression and anxiety, and work productivity.
The purpose of this study is to understand the impact of the COVID pandemic on IBD patients, including infection rates and complications from SARS-COV2, IBD related flares, patient compliance with biologic agents, impact of lockdowns on access to IV infusion centers, need for steroid therapy and admissions for IBD related flares, and modification of medical management.
The purpose of this study is to determine if WATS 3D (The Wide Area Transepithelial Sample with three dimension analysis) can be used to adequately sample the colon and identify dysplasia in patients with Irritable Bowel Disease (IBD) undergoing surveillance.
The purpose of the study is to:
- Map and compare the neoplasia-associated epigenetic field in IBD-CRN, sporadic CRN and controls.
- Measure sensitivity of DNA methylation for the detection of synchronous IBD-CRN as a complement or alternative to histologic diagnosis of dysplasia on random biopsies.
- Measure the density and types of lymphocytes infiltrating IBD-CRN tumors, sporadic tumors and the epigenetic field.
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
Switch language:

J&J’s Tremfya meets endpoints in Phase III ulcerative colitis trial
The company’s monoclonal antibody met primary and secondary endpoints as a maintenance therapy for ulcerative colitis in a Phase III trial.
- Share on Linkedin
- Share on Facebook

Johnson and Johnson (J&J) has announced positive data from the Phase III QUASAR maintenance trial for Tremfya (guselkumab) as a maintenance therapy in patients with moderate to severe active ulcerative colitis.
Tremfya is an interleukin (IL)-23 and CD64-inhibiting monoclonal antibody. It was first approved as a treatment for moderate to severe plaque psoriasis by the US Food and Drug Administration (FDA), in 2017. Tremfya has since been approved to treat psoriatic arthritis.
Go deeper with GlobalData

LOA and PTSR Model - JNJ-4703 in Ulcerative Colitis
Loa and ptsr model - jnj-4804 in ulcerative colitis, premium insights.
The gold standard of business intelligence.
Find out more
Related Company Profiles
Johnson & johnson.
The therapy achieved the primary and nine major secondary endpoints in the placebo-controlled Phase III QUASAR maintenance study (NCT04033445). The participants in the trial were randomised to receive either placebo or one of the two doses of subcutaneous Tremfya – 200mg every four weeks (q4w) or 100mg every eight weeks (q8w).
The trial met its primary endpoint by inducing remission in 50% and 45.2% of the participants in the q4w and q8w treatment groups respectively at 44 weeks, compared to the placebo. Of the participants who were in clinical remission, 67% and 71% of the participants in the q4w and q8w treatment groups respectively also achieved endoscopic remission at 44 weeks.
Clinical response was seen in 74.7% and 77.7% of the participants in the q4w and q8w treatment groups respectively at 44 weeks, compared to 43.2% of participants in the placebo group. Endoscopic improvement was seen in 51.6% and 49.5% of the participants in the q4w and q8w treatment groups respectively at 44 weeks, compared to 18.9% of participants in the placebo group.
The data from the trial was presented at the Digestive Disease Week 2024 taking place in Washington DC from 19 to 21 May.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
J&J’s Tremfya is expected to be the successor to the company’s IL-12/IL-23 inhibitor Stelara (ustekinumab). Stelara is an approved treatment for severe plaque psoriasis, active psoriatic arthritis, and ulcerative colitis. As per J&J’s annual report, the therapy generated $10.8bn in global sales last year, but its future sales are expected to decline with multiple biosimilars entering the market .
J&J is also evaluating Tremfya as a treatment for Crohn’s disease in a Phase II GALAXI trial (NCT03466411). The therapy maintained efficacy for three years, with 54.1% of the participants demonstrating clinical remission.
Sign up for our daily news round-up!
Give your business an edge with our leading industry insights.
More Relevant
MRD endpoint expected to usher new era for MM therapies
Edgewood oncology treats first two subjects in breast cancer trial, vedanta biosciences doses first subject in phase iii c difficile prevention trial, j&j’s tremfya superior to stelara in phase ii/iii crohn’s disease trial, sign up to the newsletter: in brief, your corporate email address, i would also like to subscribe to:.
I consent to Verdict Media Limited collecting my details provided via this form in accordance with Privacy Policy
Thank you for subscribing
View all newsletters from across the GlobalData Media network.
- See us on facebook
- See us on twitter
- See us on youtube
- See us on linkedin
- See us on instagram
Stanford scientists link ulcerative colitis to missing gut microbes
Bacteria normally inhabiting healthy people’s intestines — and the anti-inflammatory metabolites these bacteria produce — are depleted in ulcerative colitis patients, a Stanford study shows.
February 25, 2020 - By Bruce Goldman

Aida Habtezion is the senior author of a study that describes how people with ulcerative colitis have insufficient amounts of a metabolite produced by a family of gut-dwelling bacteria. Steve Castillo
About 1 million people in the United States have ulcerative colitis, a serious disease of the colon that has no cure and whose cause is obscure. Now, a study by Stanford University School of Medicine investigators has tied the condition to a missing microbe.
The microbe makes metabolites that help keep the gut healthy.
“This study helps us to better understand the disease,” said Aida Habtezion , MD, associate professor of gastroenterology and hepatology. “We hope it also leads to our being able to treat it with a naturally produced metabolite that’s already present in high amounts in a healthy gut.”
When the researchers compared two groups of patients — one group with ulcerative colitis, the other group with a rare noninflammatory condition — who had undergone an identical corrective surgical procedure, they discovered that a particular family of bacteria was depleted in patients with ulcerative colitis. These patients also were deficient in a set of anti-inflammatory substances that the bacteria make, the scientists report.
A paper describing the research findings was published online Feb. 25 in Cell Host & Microbe . Habtezion is the senior author. Lead authorship is shared by Sidhartha Sinha , MD, assistant professor of gastroenterology and hepatology, and postdoctoral scholar Yeneneh Haileselassie, PhD.
The discoveries raise the prospect that supplementing ulcerative colitis patients with those missing metabolites — or perhaps someday restoring the gut-dwelling bacteria that produce them — could effectively treat intestinal inflammation in these patients and perhaps those with a related condition called Crohn’s disease, Habtezion said.
A clinical trial to determine whether those metabolites, called secondary bile acids, are effective in treating the disease is now underway at Stanford. Sinha is the trial’s principal investigator, and Habtezion is the co-principal investigator.
Surgery often required
Ulcerative colitis is an inflammatory condition in which the immune system attacks tissue in the rectum or colon. Patients can suffer from heavy bleeding, diarrhea, weight loss and, if the colon becomes sufficiently perforated, life-threatening sepsis.
There is no known cure. While immunosuppressant drugs can keep ulcerative colitis at bay, they put patients at increased risk for cancer and infection. Moreover, not all patients respond, and even when an immunosuppressant drug works initially, its effectiveness can fade with time. About one in five ulcerative colitis patients progress to the point where they require total colectomy, the surgical removal of the colon and rectum, followed by the repositioning of the lower end of the small intestine to form a J-shaped pouch that serves as a rectum.
These “pouch patients” can lead quite normal lives. However, as many as half will develop pouchitis, a return of the inflammation and symptoms they experienced in their initial condition.
The new study began with a clinical observation. “Patients with a rare genetic condition called familial adenomatous polyposis, or FAP, are at extremely high risk for colon cancer,” Habtezion said. “To prevent this, they undergo the exact same surgical procedure patients with refractory ulcerative colitis do.” Yet FAP pouch patients rarely if ever experience the inflammatory attacks on their remaining lower digestive tract that ulcerative-colitis patients with a pouch do, she said.
The Stanford scientists decided to find out why. Their first clue lay in a large difference in levels of a group of substances called secondary bile acids in the intestines of seven FAP patients compared with 17 patients with ulcerative colitis who had undergone the pouch surgery. The investigators measured these metabolite levels by examining the participants’ stool samples.
Primary bile acids are produced in the liver, stored in the gallbladder and released into the digestive tract to help emulsify fats. The vast majority of secreted primary bile acids are taken up in the intestine, where resident bacteria perform a series of enzymatic operations to convert them to secondary bile acids.
Prior research has suggested, without much elaboration or follow-up, that secondary bile acids are depleted in ulcerative colitis patients and in those with a related condition, Crohn’s disease, in which tissue-destroying inflammation can occur in both the colon and the small intestine.
The researchers confirmed that levels of the two most prominent secondary bile acids, deoxycholic acid and lithocholic acid, were much lower in stool specimens taken from the ulcerative colitis pouch patients than from FAP pouch patients. Clearly, the surgical procedure hadn’t caused the depletion.
Diminished microbial diversity
These findings were mirrored by the scientists’ observation that microbial diversity in the specimens from ulcerative colitis pouch patients was diminished. Moreover, the investigators showed that a single bacterial family — Ruminococcaceae — was markedly underrepresented in ulcerative colitis pouch patients compared with FAP pouch patients. A genomic analysis of all the gut bacteria in the participants showed that the genes for making enzymes that convert primary bile acids to secondary bile acids were underrepresented, too. Ruminococcaceae, but few other gut bacteria, carry those genes.
“All healthy people have Ruminococcaceae in their intestines,” Habtezion said. “But in the UC pouch patients, members of this family were significantly depleted.”
Incubating primary bile acids with stool samples from FAP pouch patients, but not from ulcerative colitis pouch patients, resulted in those substances’ effective conversion to secondary bile acids.
In three different mouse models of colitis, supplementation with lithocholic acid and deoxycholic acid reduced infiltration by inflammatory immune cells and levels of several inflammatory signaling proteins and chemicals in the mice’s intestines, the researchers showed. The supplements also mitigated the classic symptoms of colitis in the mice, such as weight loss or signs of colon pathology.
All three mouse models are considered representative of not just ulcerative colitis but inflammatory bowel disease in general, a category that also includes Crohn’s disease. So the findings may apply to Crohn’s disease patients, as well, Habtezion said.
In an ongoing Phase 2 trial at Stanford, Sinha, Habtezion and their colleagues are investigating the anti-inflammatory effects, in 18- to 70-year-old ulcerative colitis pouch patients, of oral supplementation with ursodeoxycholic acid, a naturally occurring secondary bile acid approved by the Food and Drug Administration for treatment of primary biliary sclerosis and for management of gall stones. Information about the trial, which is still recruiting people, is available at https://clinicaltrials.gov/ct2/show/NCT03724175 .
Habtezion is associate dean for academic affairs in the School of Medicine, a faculty fellow of Stanford ChEM-H and a member of Stanford Bio-X , the Stanford Cancer Institute , the Stanford Pancreas Cancer Research Group and the Wu Tsai Neurosciences Institute at Stanford .
Other Stanford co-authors of the study are postdoctoral scholars Min Wang, PhD, Estelle Spear, PhD, Gulshan Singh, PhD, and Hong Namkoong, PhD; former research scientist Linh Nguyen, PhD; former postdoctoral scholar Carolina Tropini, PhD; former gastroenterology medical fellow Davis Sim, MD; research assistant Karolin Jarr; Laren Becker , MD, instructor of gastroenterology and hepatology; Michael Fischbach, PhD, associate professor of bioengineering; and Justin Sonnenburg , PhD, associate professor of microbiology and immunology.
Researchers from the Children’s Hospital of Philadelphia also contributed to the work.
The work was funded by the National Institutes of Health (grants R01DK101119, KL2TR001083 and UL1TR001085), the Ann and Bill Swindells Charitable Trust, the Kenneth Rainin Foundation, and Leslie and Douglas Ballinger.
Stanford’s Department of Medicine also supported the work.

About Stanford Medicine
Stanford Medicine is an integrated academic health system comprising the Stanford School of Medicine and adult and pediatric health care delivery systems. Together, they harness the full potential of biomedicine through collaborative research, education and clinical care for patients. For more information, please visit med.stanford.edu .
Hope amid crisis
Psychiatry’s new frontiers

- Patient Care & Health Information
- Diseases & Conditions
- Ulcerative colitis
- What is ulcerative colitis? A Mayo Clinic expert explains
Listen to gastroenterologist William Faubion, M.D., walk through ulcerative colitis basics.
[Music playing]
William A. Faubion, Jr., M.D., Gastroenterology, Mayo Clinic I'm Dr. Bill Faubion, a gastroenterologist at Mayo Clinic. In this video, we'll cover the basics of ulcerative colitis. What is it? Who gets it? The symptoms, diagnosis, and treatment. Whether you're looking for answers for yourself or someone you love, we're here to give you the best information available.
Ulcerative colitis is an inflammatory bowel disease that causes chronic inflammation and ulcers in the superficial lining of the large intestine, also called the colon. And that includes the rectum. It's estimated that about a million Americans are living with ulcerative colitis, making it the most common form of inflammatory bowel disease. It can be painful and debilitating, occasionally leading to severe complications. It can also be emotionally stressful. And while there is no cure, once you've been diagnosed, treatment can help you get back to a much more normal and comfortable life.
Who gets it?
The exact cause of ulcerative colitis is unknown, but there are things that appear to trigger or aggravate it. It may involve an abnormal immune response against some microorganism in which your tissues are also attacked. Genetics might also play a role. You are at higher risk if a first-degree relative has it. There's also a correlation with age. Although it can show up at any stage of life, most people are diagnosed before the age of 30. And ethnicity is a risk factor. Whites have the highest risk, especially among people of Ashkenazi Jewish descent. While diet and stress don't cause ulcerative colitis, they are known to exacerbate symptoms.
What are the symptoms?
Most people have mild to moderate cases of ulcerative colitis. Although it can be more severe, you may also experience periods of remission when you have no issues at all. A person's symptoms depend on the severity of the case in the area of the colon that's involved. They usually develop over time, and they can include diarrhea, often with blood or pus, fever, fatigue, anemia, loss of appetite and weight loss, abdominal pain and cramping, rectal pain and bleeding, the need for a bowel movement, yet the inability to do so despite the urgency. And in children, delayed growth and development. Over time, ulcerative colitis can lead to other complications, such as severe dehydration, a perforated colon, bone loss, inflammation of your skin, joints and eyes. It can also increase your risk for blood clots and colon cancer. These symptoms don't automatically mean that you have ulcerative colitis. But if you're experiencing anything that concerns you, it's a good idea to make an appointment with your doctor.
How is it diagnosed?
The only way to definitively diagnose ulcerative colitis is with a biopsy after taking a tissue sample through an endoscopic procedure. But first, less invasive things can be done to rule out other causes. First, your doctor will consider your medical history. They may want to run a variety of tests or procedures. And at some point, your general practitioner may refer you to a specialist called a gastroenterologist like myself. A blood test can check for anemia and check for signs of infection. A stool study can test for white blood cells and other specific proteins that point to ulcerative colitis, as well as rule out certain pathogens. A colonoscopy may be needed. This allows your doctor to view the entirety of the large intestine using an endoscope, a small camera mounted on a thin flexible tube. They can take tissue samples for a biopsy at the same time. Or if your colon is extremely inflamed, they may do a flexible sigmoidoscopy, which only goes as far as the rectum and lower or sigmoid colon. If your symptoms are more severe, your doctor may want some imaging done. An abdominal x-ray can rule out serious complications, like a perforated colon. An MRI or CT scan can also be performed for a more detailed view of the bowel, as well as to reveal the extent of the inflammation.
How is it treated?
Although there is no cure for ulcerative colitis there are widely effective treatments, usually involving either drug therapy or surgery. Your doctor can work with you to find things that alleviate your symptoms and in some cases, even bring about long-term remission. Treatments may include anti-inflammatory drugs like corticosteroids and immune system suppressants. Certain targeted therapies directed against the immune system called biologics can help. Antidiarrheals, pain relievers, antispasmodics and iron supplements can help counter other symptoms. And surgery may be required to remove the damaged tissue. In extreme cases, the whole colon may be removed. Which sounds drastic, but this can sometimes be the best option for eliminating the pain and struggle of ulcerative colitis once and for all. Some of these therapies may have side effects themselves. So be sure to review the risks and benefits with your doctor.
Ulcerative colitis can be physically and emotionally challenging, but there are things that can help. Although there's no firm evidence that any foods cause ulcerative colitis, certain things seem to aggravate flare-ups. So a food diary can help you identify personal triggers. Beyond that, limit dairy products, eat small meals, stay hydrated, try to avoid caffeine and alcohol and carbonation. If you're concerned about weight loss or if your diet has become too limited, talk to a registered dietitian. It's important to take care of your mental health, too. Find ways to manage stress, like exercise, breathing and relaxation techniques or biofeedback. Some symptoms like abdominal pain, gas, and diarrhea can cause anxiety and frustration. That can make it difficult to be out in public for any amount of time. It can feel limiting and isolating and lead to depression. So learn as much as you can about ulcerative colitis. Staying informed can help a lot in feeling like you're in control of your condition. Talk to a therapist, especially one familiar with inflammatory bowel disease. Your doctor should be able to give you some recommendations. And you might want to find a support group for people going through the same thing that you are. Ulcerative colitis is a complex disease, but having expert medical care and developing a treatment strategy can make it more manageable and even help patients get back to the freedom of a normal life. Meanwhile, significant advances continue to be made in understanding and treating the disease and getting us closer to curing it or preventing it entirely. If you'd like to learn even more about ulcerative colitis, watch our other related videos or visit mayoclinic.org. We wish you well.
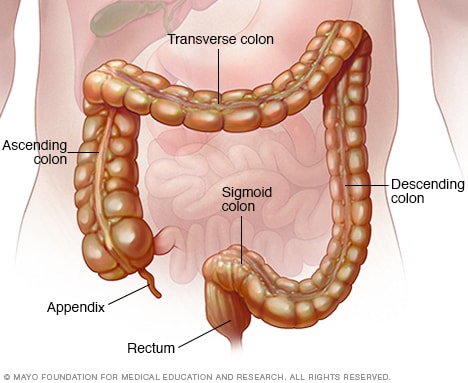
Colon and rectum
The colon is a long tube-like organ in the abdomen. It's the largest part of the large intestine. The colon carries waste to be expelled from the body. The rectum makes up the last several inches of the colon.
Gastroenterology & GI Surgery Blog
Ulcerative colitis (UL-sur-uh-tiv koe-LIE-tis) is an inflammatory bowel disease (IBD) that causes inflammation and ulcers (sores) in your digestive tract. Ulcerative colitis affects the innermost lining of your large intestine, also called the colon, and rectum. In most people, symptoms usually develop over time, rather than suddenly.
Ulcerative colitis can be draining and can sometimes lead to life-threatening complications. While it has no known cure, there are several new treatments that can greatly reduce signs and symptoms of the disease and bring about long-term remission.
Products & Services
- A Book: Mayo Clinic on Crohn’s Disease and Ulcerative Colitis
- A Book: Mayo Clinic on Digestive Health
- Available Digestive Health Products at Mayo Clinic Store
Ulcerative colitis symptoms can vary, depending on the severity of inflammation and where it occurs. Signs and symptoms may include:
- Diarrhea, often with blood or pus
- Rectal bleeding — passing small amount of blood with stool
- Abdominal pain and cramping
- Rectal pain
- Urgency to defecate
- Inability to defecate despite urgency
- Weight loss
- In children, failure to grow
Most people with ulcerative colitis have mild to moderate symptoms. The course of ulcerative colitis may vary, with some people having long periods when it goes away. This is called remission.
Health care providers often classify ulcerative colitis according to its location. Symptoms of each type often overlap. Types of ulcerative colitis include:
- Ulcerative proctitis. Inflammation is confined to the area closest to the anus, also called the rectum. Rectal bleeding may be the only sign of the disease.
- Proctosigmoiditis. Inflammation involves the rectum and sigmoid colon — the lower end of the colon. Symptoms include bloody diarrhea, abdominal cramps and pain, and an inability to move the bowels despite the urge to do so. This is called tenesmus.
- Left-sided colitis. Inflammation extends from the rectum up through the sigmoid and descending portions of the colon. Symptoms include bloody diarrhea, abdominal cramping and pain on the left side, and urgency to defecate.
- Pancolitis. This type often affects the entire colon and causes bouts of bloody diarrhea that may be severe, abdominal cramps and pain, fatigue, and significant weight loss.
When to see a doctor
See your health care provider if you experience a persistent change in your bowel habits or if you have signs and symptoms such as:
- Abdominal pain
- Blood in your stool
- Ongoing diarrhea that doesn't respond to nonprescription medications
- Diarrhea that awakens you from sleep
- An unexplained fever lasting more than a day or two
Although ulcerative colitis usually isn't fatal, it's a serious disease. In some cases, ulcerative colitis may cause life-threatening complications.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
Get the latest health information from Mayo Clinic delivered to your inbox.
Subscribe for free and receive your in-depth guide to digestive health, plus the latest on health innovations and news. You can unsubscribe at any time. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing
Your in-depth digestive health guide will be in your inbox shortly. You will also receive emails from Mayo Clinic on the latest health news, research, and care.
If you don’t receive our email within 5 minutes, check your SPAM folder, then contact us at [email protected] .
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
The exact cause of ulcerative colitis remains unknown. Previously, diet and stress were suspected. However, researchers now know that these factors may aggravate but don't cause ulcerative colitis.
One possible cause is an immune system malfunction. When your immune system tries to fight off an invading virus or bacterium, an irregular immune response causes the immune system to attack the cells in the digestive tract, too.
Heredity also seems to play a role in that ulcerative colitis is more common in people who have family members with the disease. However, most people with ulcerative colitis don't have this family history.
Risk factors
Ulcerative colitis affects about the same number of women and men. Risk factors may include:
- Age. Ulcerative colitis usually begins before the age of 30, but it can occur at any age. Some people may not develop the disease until after age 60.
- Race or ethnicity. Although white people have the highest risk of the disease, it can occur in any race. If you're of Ashkenazi Jewish descent, your risk is even higher.
- Family history. You're at higher risk if you have a close relative, such as a parent, sibling or child, with the disease.
Complications
Possible complications of ulcerative colitis include:
- Severe bleeding
- Severe dehydration
- A rapidly swelling colon, also called a toxic megacolon
- A hole in the colon, also called a perforated colon
- Increased risk of blood clots in veins and arteries
- Inflammation of the skin, joints and eyes
- An increased risk of colon cancer
- Bone loss, also called osteoporosis
Ulcerative colitis care at Mayo Clinic
- Feldman M, et al, eds. Epidemiology, pathogenesis, and diagnosis of inflammatory bowel diseases. In: Sleisenger and Fordtran's Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 11th ed. Elsevier; 2021. https://www.clinicalkey.com. Accessed July 22, 2020.
- Goldman L, et al., eds. Inflammatory bowel disease. In: Goldman-Cecil Medicine. 26th ed. Elsevier; 2020. https://www.clinicalkey.com. Accessed July 22, 2020.
- The facts about inflammatory bowel diseases. Crohn's and Colitis Foundation. https://www.crohnscolitisfoundation.org/. Accessed Sept. 6, 2022.
- Ulcerative colitis. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/digestive-diseases/ulcerative-colitis. Accessed Sept. 6, 2022.
- What is ulcerative colitis? Crohn's and Colitis Foundation. https://www.crohnscolitisfoundation.org/what-is-ulcerative-colitis. Accessed Sept. 6, 2022.
- Kliegman RM, et al. Inflammatory bowel diseases. In: Nelson Textbook of Pediatrics. 21st ed. Elsevier; 2020. https://www.clinicalkey.com. Accessed July 22, 2020.
- AskMayoExpert. Chronic ulcerative colitis. Mayo Clinic; 2019.
- Abraham B, et al. Antibiotics and probiotics in inflammatory bowel disease: When to use them? Frontline Gastroenterology. 2020; doi:10.1136/flgastro-2018-101057.
- What should I eat? Crohn's and Colitis Foundation. https://www.crohnscolitisfoundation.org/diet-and-nutrition/what-should-i-eat. Accessed Sept. 6, 2022.
- Mind-body therapies. Crohn's and Colitis Foundation. https://www.crohnscolitisfoundation.org/complementary-medicine/mind-body-therapies. Accessed Sept. 6, 2022.
- Nguyen H. Allscripts EPSi. Mayo Clinic. April 1, 2022.
- Special IBD diets. Crohn's and Colitis Foundation. https://www.crohnscolitisfoundation.org/diet-and-nutrition/special-ibd-diets. Accessed Sept. 6, 2022.
- Shergill A, et al. Surveillance and management of dysplasia in patients with inflammatory bowel disease. https://www.uptodate.com/contents/search. Accessed Sept. 6, 2022.
- Kashyap PC (expert opinion). Mayo Clinic. Aug. 13, 2020.
- Kane SV (expert opinion). Mayo Clinic. Sept. 12, 2020.
- Xeljanz, Xeljanz XR (tofacitinib): Drug safety communication — Initial safety trial results find increased risk of serious heart-related problems and cancer with arthritis and ulcerative colitis medicine. U.S. Food and Drug Administration. https://www.fda.gov/safety/medical-product-safety-information/xeljanz-xeljanz-xr-tofacitinib-drug-safety-communication-initial-safety-trial-results-find-increased?utm_medium=email&utm_source=govdelivery. Accessed Sept. 6, 2022.
- Khanna S (expert opinion). Mayo Clinic. Aug. 20, 2022.
- Cohen RD, et al. Management of moderate to severe ulcerative colitis in adults. https://www.uptodate.com/contents/search. Accessed Aug. 1, 2022.
- Ulcerative colitis flare-ups: 5 tips to manage them
Associated Procedures
- Acupuncture
- Barium enema
- Colonoscopy
- Flexible sigmoidoscopy
- Ileoanal anastomosis (J-pouch) surgery
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
- Care at Mayo Clinic
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.

IMAGES
VIDEO
COMMENTS
May 27, 2021. LA JOLLA, CA— Ozanimod, the drug invented at Scripps Research that won FDA approval last year for relapsing forms of multiple sclerosis, has been approved in the United States for a second high-need medical condition, ulcerative colitis. The once-daily oral drug, sold by Bristol Myers Squibb under the name Zeposia, can now be ...
Ulcerative colitis is an inflammatory bowel disease that is characterized by chronic inflammation of the colon. ... Latest Research and Reviews ... Important studies published in 2023 outlined new ...
This latest study focuses on ulcerative colitis, a chronic disease characterized by an autoimmune attack against colon cells. For this and other autoimmune diseases, there is no cure. ... of more than 3,100 is responsible for groundbreaking medical advances and is committed to translating science-driven research quickly to new clinical ...
A new aspect of the LUCENT trial program was the inclusion of end points relating to bowel urgency. 21,22 Many patients with ulcerative colitis consider control of bowel movements to be more ...
AltruBio raises $225 million to develop ulcerative colitis drug and other immune checkpoint enhancers. By Jonathan Wosen May 21, 2024. ... At a flashy new research hub, AstraZeneca lays out…
Ulcerative colitis is a chronic disease that is characterized by a dysregulated immune response and chronic inflammation in the colonic mucosa. 1 Conventional therapies such as aminosalicylates ...
Ulcerative colitis is a lifelong inflammatory disease affecting the rectum and colon to a variable extent. In 2023, the prevalence of ulcerative colitis was estimated to be 5 million cases around the world, and the incidence is increasing worldwide. Ulcerative colitis is thought to occur in people with a genetic predisposition following environmental exposures; gut epithelial barrier defects ...
Ulcerative colitis (UC) is a chronic inflammatory bowel disease of unknown aetiology affecting the colon and rectum. Multiple factors, such as genetic background, environmental and luminal factors ...
The therapeutic goal in patients with ulcerative colitis is to induce and maintain long-term remission, because the disease often has a relapsing and remitting course. 16,17 Endoscopic improvement ...
A new study shows that a sustainable faecal microbiota transplantation (FMT) treatment protocol, including anaerobic sample preparation, induces remission of active ulcerative colitis.
AI Shows Potential for Detecting Mucosal Healing in Ulcerative Colitis Ultraprocessed Food Consumption Increases Disease Burden in Ulcerative Colitis 3090D553-9492-4563-8681-AD288FA52ACE
Janus Kinase Inhibitors. Sphingosine 1 Phosphate Receptor Modulators. Ulcerative colitis (UC) is a relapsing and remitting inflammatory disease of the colon with a variable course. Despite advances in treatment, only approximately 40% of patients achieve clinical remission at the end of a year, prompting the exploration of new treatment ...
Scientists are developing new treatments for ulcerative colitis (UC). There is ongoing scientific research into stem cell therapy for UC. Despite such developments, it remains an incurable condition.
AltruBio, after tweaking checkpoint drug, raises $225M for midphase ulcerative colitis program. AltruBio put ALTB-268 through a phase 1 study in healthy volunteers in 2023. (Nuthawut Somsuk/iStock ...
Ulcerative colitis (UC) is a chronic inflammatory disorder defined by mucosal inflammation that involves the colon and rectum in a continuous pattern [ 1, 2, 3 ]. The peak age of onset is 30-40 years old, and men and women are affected equally [ 4 ]. While still not yet fully defined, the pathogenesis of UC is multifactorial and implicates ...
Introduction. The Inflammatory Bowel Diseases (IBDs), namely Ulcerative Colitis (UC) and Crohn's disease (CD) ( Table 1), are chronic immune-mediated conditions with a high prevalence in developed countries (>0.3%) and rapidly increasing incidence in newly industrialised countries (annual percentage change +14.9%) 1, 2.Global prevalence is projected to affect up to 30 million individuals by ...
Introduction. Ulcerative colitis (UC) was first described in mid-1800s.1 It is an idiopathic, chronic inflammatory disorder of the colonic mucosa that commonly involves the rectum and may extend in a proximal and continuous fashion to involve other parts of the colon.2 The disease typically affects individuals in the second or third decade of life with hallmark clinical symptoms of bloody ...
San Francisco-based biotech AltruBio has raised $225 million in an oversubscribed Series B round, the company announced Tuesday. The financing will help the company advance its ulcerative colitis candidate into mid-stage clinical trials. AltruBio's candidate ALTB-268 is a first-in-class immune checkpoint enhancer (ICE) PSGL agonist antibody ...
Acute severe ulcerative colitis (ASUC) is a distinctive ulcerative colitis flare presentation characterised by the presence of systemic inflammation as well as bloody diarrhoea, and occurs at least once in 25% of patients with ulcerative colitis during their disease course. Each episode carries a risk of complications, need for colectomy, and mortality. Little is known about ASUC pathogenesis ...
LAVAL, Quebec, May 17, 2024- Bausch Health Companies Inc. (NYSE/TSX: BHC) and its gastroenterology (GI) business, Salix Pharmaceuticals, today announced that they will be presenting data from its Phase 2 trial evaluating Amiselimod as treatment for active ulcerative colitis (UC).The data will be presented at Digestive Disease Week (DDW) 2024 during the IMIBD Late Breakers and Innovations in ...
Ulcerative colitis most commonly presents between 15 and 30 years of age and is more common in industrialized nations, with a prevalence of 286 per 100,000 adults in the United States. 1, 2 ...
Research update: The latest findings on ulcerative colitis. Ulcerative colitis is a condition involving chronic inflammation of the lining of the large intestine. It leads to the formation of ...
Abstract. Ulcerative colitis (UC) is a chronic idiopathic inflammatory bowel disorder of the colon that causes continuous mucosal inflammation extending from the rectum to the more proximal colon, with variable extents. UC is characterized by a relapsing and remitting course. UC was first described by Samuel Wilks in 1859 and it is more common ...
Ulcerative colitis (UC) is increasingly common, and it is gradually become a kind of global epidemic. UC is a type of inflammatory bowel disease (IBD), and it is a lifetime recurrent disease. UC as a common disease has become a financial burden for many people and has the potential to develop into cancer if not prevented or treated. There are multiple factors such as genetic factors, host ...
Subjects with Ulcerative Colitis who have completed an induction study with PF-00547659 will receive an additional 72 weeks of open-label treatment to evaluate the long-term safety of the drug. ... To evaluate a new oral treatment, ABI-M201 (or placebo) in individuals with mild to moderate Ulcerative Colitis who have sustained an inadequate ...
Image Credit: josefkubes / Shutterstock. Johnson and Johnson (J&J) has announced positive data from the Phase III QUASAR maintenance trial for Tremfya (guselkumab) as a maintenance therapy in patients with moderate to severe active ulcerative colitis. Tremfya is an interleukin (IL)-23 and CD64-inhibiting monoclonal antibody.
The new study began with a clinical observation. "Patients with a rare genetic condition called familial adenomatous polyposis, or FAP, are at extremely high risk for colon cancer," Habtezion said. "To prevent this, they undergo the exact same surgical procedure patients with refractory ulcerative colitis do."
Ulcerative colitis is a complex disease, but having expert medical care and developing a treatment strategy can make it more manageable and even help patients get back to the freedom of a normal life. ... You will also receive emails from Mayo Clinic on the latest health news, research, and care. If you don't receive our email within 5 ...
When inflammatory bowel disease is identified in a new population, ulcerative colitis invariably precedes Crohn's disease and has a higher incidence. ... Lerner Research Institute, Department of ...
A doctor may order a colonoscopy to help diagnose ulcerative colitis (UC), check for complications of UC, or see whether the condition is progressing. UC is a type of inflammatory bowel disease ...