Senior Clinical Research Associate
- Location: Sofia, Milan, Barcelona, Madrid, Roma
- Categories Clinical Monitoring
- __vacancyopjusttionswidget.opt-Business Area__ ICON Full Service & Corporate Support
- __vacancyopjusttionswidget.opt-Remote Working __ Hybrid: Office/Remote

TA Business Partner
- Full Service Division

Send me a message
About the role, this vacancy has now expired. please see similar roles below....
ICON plc is a world-leading healthcare intelligence and clinical research organisation. From molecule to medicine, we advance clinical research providing outsourced services to pharmaceutical, biotechnology, medical device and government and public health organisations.
With our patients at the centre of all that we do, we help to accelerate the development of drugs and devices that save lives and improve quality of life.
Our people are our greatest strength, are at the core of our culture, and the driving force behind our success. ICON people have a mission to succeed and a passion that ensures what we do, we do well.
As a Clinical Research Associate for Medical Devices and Diagnostics Research at ICON, you’ll work within a large-scale, fast-paced environment alongside a close-knit team of highly qualified CRAs and medical devices experts to identify, select, initiate and close-out investigational sites for pre and post market studies, ensuring adherence to applicable regulations and principles of ICH-GCP. We pride ourselves on our amazing company culture, where we work as one team to achieve industry-leading results. The Role: • Working independently and actively to coordinate activities to set up and monitor a study, completing accurate study status reports and maintaining study documentation
• Ensuring safety and rights of patients is maintained throughout the study.
• Working solely on Medical Devices or In Vitro Diagnostic studies, in various phases (First in Man, Registry, Post-market Follow-up…) and in various therapeutics areas (cardiovascular, neurology, oncology, Ophthalmology, Orthopaedics….)
• Ensuring close and regular site contact management by developing strong relationship with site staff and site investigators • Balancing sponsor generated queries efficiently and responsible for study cost-effectiveness • Dependent on level of experience you may assist in training and mentoring less expert CRA’s and/or lead CRA’s working on international projects What you need : • 12 months+ of monitoring experience in pre and post market trials as a CRA • Knowledge of ICG GCP guidelines and expertise to review and evaluate medical data
• Preferably, previous experience with medical devices studies either in another CRO, at a manufacturer or in a hospital. • You will possess excellent written and verbal communication in English • Ability to produce accurate work to tight deadlines within a pressurized environment • You will be asked to travel at least 60% of the time (international and domestic -fly and drive) and should possess a valid driving license
Benefits of Working in ICON:
Our success depends on the knowledge, capabilities and quality of our people. That’s why we are committed to developing our employees in a continuous learning culture – one where we challenge you with engaging work and where every experience adds to your professional development. At ICON, our focus is to provide you with a comprehensive and competitive total reward package that comprises, not only an excellent level of base pay, but also a wide range of variable pay and recognition programs. In addition, our best in class employee benefits, supportive policies and wellbeing initiatives are tailored to support you and your family at all stages of your career - both now, and into the future. ICON, including subsidiaries, is an equal opportunity and inclusive employer and is committed to providing a workplace free of discrimination and harassment. All qualified applicants will receive equal consideration for employment without regard to race, color, religion, sex, sexual orientation, gender identity, national origin, disability or protected veteran status.
If, because of a medical condition or disability, you need a reasonable accommodation for any part of the application process, or in order to perform the essential functions of a position, please let us know through the form below.

ICON and you

ICON history

Career Pathways

Benefits & Rewards

Environmental, Social & Governance

Women in IT

A better career. A better world. A better you.
Day in the life

Teaser label
Content type
Publish date
To excel as a Clinical Research Associate (CRA) in a Clinical Research Organization (CRO), you need a combination of education, skills, and the right mindset. Brazil-based CRA II Debora shares her
Brazil-based CRA II Debora Oh shares her tips on how to become a great CRA and provides insight into life at ICON.

How to progress as a Clinical Research AssociateTo thrive as a Clinical Research Associate (CRA), it is imperative to cultivate a multifaceted skill set and demonstrate unwavering commitment to exce
Senior CRA Yemi Moses recounts her development and shares her career ambitions with ICON plc.

Senior Clinical Research Associate Suzaita Hipolito talks about the satisfaction gained from working in Clinical Research. “What would it look like to wake up every day feeling happy and fulfilled?
Senior Clinical Research Associate, Suzaita Hipolito, talks about how working in Clinical Research gives her happiness and fulfilment.

Suzaita Hipolito
Press play to find out more

Similar jobs at ICON
US, Blue Bell (ICON)
Clinical Monitoring
Real World Solutions
Remote Working
Hybrid: Office/Remote
Business Area
ICON Full Service & Corporate Support
Job Categories
Description
At ICON, it’s our people that set us apart. Our diverse teams enable us to become a better partner to our customers and help us to fulfil our mission to advance and improve patients’ lives. Our ‘Own I
Expiry date

US, Brentwood, TN
Full Service - Development & Commercialisation Solutions
Requirements:Must be located within the United StatesMust have 12+ months of independent monitoring experience as a CRAAt ICON, it’s our people that set us apart. Our diverse teams enable us to become

ICON Strategic Solutions
As Clinical Research Associate (CRA), you will be joining the world's largest & most comprehensive clinical research organization, powered by healthcare intelligence. A CRA is a professional who cont
2023-103904

ICON plc is a world-leading healthcare intelligence and clinical research organisation. From molecule to medicine, we advance clinical research providing outsourced services to pharmaceutical, biotech
2024-110128
Lead Clincial Research Associate (FSP)Location: Home-based, Australia As a Lead CRA, you will be joining the world’s largest & most comprehensive clinical research organisation, powered by healthcare
2024-109668

As a (CRA II, SCRA) you will be joining the world’s largest & most comprehensive clinical research organisation, powered by healthcare intelligence.
2024-110100

Browse popular job categories below or search all jobs above
CRES ACADEMY

Start-Up Specialist

Clinical Research Associate – CRA

Clinical Project Manager – CPM

Patient Report Outcome Specialist

Consenso Informato

Get That Job!
CRES Academy è una scuola di formazione, altamente specializzata nella preparazione alle professioni e nello sviluppo delle competenze più richieste dal mondo della ricerca clinica, la nostra offerta formativa è quanto di più mirato si possa trovare sul mercato. I nostri docenti, selezionati per le loro specifiche competenze nel mondo reale, adottano la metodologia Experience-Based-Learning che caratterizza tutti i corsi CRES, e si riconoscono nei valori dell’etica, della collaborazione e del rispetto delle diversità.
Become CRES Approved Professional
PROFESSIONAL PROGRAMS I nostri programmi professionalizzanti, forniscono tutti gli strumenti e le conoscenze necessarie per cogliere, con padronanza e sicurezza, le molteplici opportunità offerte dal mercato della ricerca clinica.
UP-SKILLING COURSES I corsi di UP-SKILLING sono focalizzati sullo sviluppo di specifiche competenze, e sull’acquisizione di strumenti utili per arricchire il proprio bagaglio professionale.
Con i nostri corsi acquisirai le competenze, l’esperienza e le capacità relazionali per affrontare con sicurezza e successo tutte le sfide reali che potrai incontrare nel coordinamento di uno studio clinico.
- STRATEGIC THINKING
- SOFT SKILLS
- MANAGEMENT TOOLS
Il nostro metodo, basato sul Experience-Based Learning, prevede un approccio immersivo ed esperienziale, basato su esercitazioni real life, che ripercorre tutte le fasi di uno studio clinico. Il percorso formativo si svolge online in modalità sincrona con docenti esperti sia del settore che delle più avanzate tecniche formative. L’apprendimento è assicurato attraverso:
- Esercitazioni e simulazioni di tutti i passaggi necessari al processo di esecuzione di uno studio clinico in tutte le sue fasi
- Corsi a numero limitato, per assicurare lo sviluppo delle competenze individuali per allenare la capacità di lavorare in team
OUR FACULTY
I nostri docenti sono esperti, selezionati per le loro specifiche competenze nell’ambito della ricerca clinica applicata. Alla loro attività di docenza, affiancano un’intensa attività professionale di alto livello nelle strutture più accreditate per la ricerca clinica. La faculty di Cres Academy si distingue per metodo, qualità, esperienza e aggiornamento continuo. I nostri docenti si riconoscono per un mindset caratterizzato da: disciplina, flessibilità e innovazione.
- Per noi la formazione non è solo una mera acquisizione di informazioni e nozioni, ma è anche fornire un metodo per capire come utilizzare gli strumenti acquisiti negli aspetti critici nella gestione di uno studio clinico o progetto di ricerca
- Obiettivo è far crescere una nuova generazione di eccellenza di esperti in ricerca clinica, al fine di poter aumentare la competitività dell’Italia e dei centri italiani, per gli investimenti in ricerca e sviluppo in campo farmaceutico, medicale e biotecnologico
- Aumentare la capacità di lavorare in team, per contribuire alla crescita dell’organizzazione nella quale si lavora
- Dare impulso alla propria carriera per raggiungere i vertici più elevati nella professione
- Dare gli strumenti per imparare a “costruire relazioni” e la mediazione, la leadership
- Fornire le nozioni più complesse nella gestione degli studi clinici attraverso corsi con pratica
PROFESSIONS
Dal 2015 al 2020 le imprese del farmaco hanno investito in Italia +14,5% in ricerca e sviluppo. Il 60% di questi investimenti, è destinato agli studi clinici. Di pari passo aumenta la domanda di personale qualificato per lavorare nella sperimentazione clinica. Cogli l’opportunità di diventare professionista del settore e affrontiamo insieme le sfide del futuro! Parla con il tuo CRES Counsellor

- Comprensione della gestione di uno studio clinico con specifico riferimento alla fase di Start-up
- Conoscere il ruolo del Clinical Start-up Associate in relazione con gli altri membri del Team di ricerca
- Comprensione e preparazione della documentazione essenziale
- Interazione con il Team di ricerca
- Capacità di mediazione e risoluzione dei conflitti
- Negoziazione del contratto
- Pianificazione delle attività
- Analisi di situazioni complesse con Fishbone Diagram
- Sviluppo e implementazione di un CAPA – Corrective Action and Preventative Action
- Centri di ricerca clinica ospedalieri
- Aziende Farmaceutiche e Biomedicali come Start-Up Specialist
- Analisi e gestione di un protocollo di ricerca clinica
- Capacità di Problem Solving
- Pianificazione secondo rispetto delle GCP
- Coordinamento del team
- Coordinamento delle procedure per la conduzione dello studio clinico
- Controllo e gestione di una cartella clinica
- Analisi di un Case Report Form
- Clinical Trial Protocol
- Aziende Farmaceutiche e Biomedicali come Clinical Research Associate
- Composizione di un budget
- Pianificazione
- Impiego di App digitale collaborativa per la programmazione e pianificazione strategica
- Aziende Farmaceutiche e Biomedicali come Professional Clinical Project Manager
- Identification and critical appraisal of relevant PROs measures
- Evaluation of content validity and psychometric properties
- PROs challenges in clinical practice
- Stakeholders’ management
- Strategy for implementation in healthcare service
- Online tools to support the identification of exisiting PROs measures
- Research guidelines for the development, evaluation and implementation of PROs measures
- Planning and management of the 3 Ts: Team, Task, Time
- Research organization
- Healthcare service providers

- un CV snello, modulare e facilmente aggiornabile
- un Personal Profile sintetico e impattante
- un profilo Linkedin dinamico ed efficace
- le tecniche di comunicazione, verbale e non, per gestire il colloquio di lavoro in modo professionale, assertivo e strategico
- PRE-WORKSHOP BRIEF - Un’attività preliminare di testing sulle POTENZIALITA’, di studio del Documento GET THAT JOB – How to write a CV: Do’s & Dont’s
- SESSION 1 Sessione di formazione di gruppo Esercizio: My Personal Communication Matrix, Revisione / perfezionamento del Curriculum Vitae, messa a fuoco del Personal Profile sintetico
- SESSION 2 Revisione / allineamento del profilo Linkedin e THE INTERVIEW SHOW simulazione del colloquio di lavoro (in italiano e in inglese)
- DE-BRIEF conclusivo & feedback
- FRANCESCA SARACINO - Senior Clinical Research Associate e Career Coach
- BARBARA SANTORO – Professional Executive Coach esperto in personal branding and international communication
- DAVIDE INTEGLIA - Senior Clinical Project Manager
CRES Academy utilizza WordPress
Stipendio per Clinical Research Associate in Italia per il 2024
Clinical research associate: qual è lo stipendio medio.
Ricerche correlate

Le persone si chiedono anche

Annunci Attivi con Stipendi

Clinical Research Associate - Florence
Jordan Griffiths has partnered with a growing CRO who are looking for a dedicated Clinical Research Associate (CRA) to join their team in Florence. This is a fantastic opportunity to work on a..
Senior Clinical Research Associate - Italy
Liam Newton has partnered with a huge, internationally recognised CRO who are searching for experienced CRA's to join their rapidly expanding team. This CRO is very dynamic as it h..
Principal Regulatory Medical Writer
CSR's or Clinical Protocols. Ability to work independently and collaborate cross functionally in fast paced environment Strong project management skills and ability to deliver high quality..

R&d - research and development
E WORK SPA Filiale di Roma per un'azienda italiana leader nel monitoraggio di infrastrutture con sensori in fibra ottica e tecnologie tradizionali ricerca un R&D. Research and Development..

Plant Health Scientific Research Officer
We are looking for a Scientific Research Officer in the area of Plant Health. EFSA is committed to supporting the careers of people with visible and invisible disabilities that are encouraged to..
EXPORT MANAGER / BUSINESS DEVELOPMENT - settore PHARMA
Dialogo con le aziende consorziate sul loro sviluppo di nuovi prodotti a medio termine. relazioni quotidiane con uffici esteri. trasferte all'estero e presso le aziende associate..
Employer Branding Trainee - internship
Collaborate with cross functional teams to ensure consistent messaging and branding. Conduct research and analysis to identify trends and best practices in employer branding. Assist in..

Research Scientist needed in Italy
Skills Provision is searching for Research Scientist for a role in Milan, Italy.This position is based in the molecular biology department. Salary & Benefits. 38,000 p a The RoleResearch project..

R&D - RESEARCH AND DEVELOPMENT
E WORK SPA Filiale di Roma per un azienda italiana leader nel monitoraggio di infrastrutture con sensori in fibra ottica e tecnologie tradizionali ricerca un RandD. Research and Development..

Investment Associate
Stai cercando un Investment Associate per la tua azienda? Offerte simili Milano. Addetto a alle Vendite L. 68 99 (Categorie Protette) Milano 32 persone hanno già visualizzato questa offerta..
Associate Tributarista
Descrizione Hunters Group, per importante studio di consulenza societaria e fiscale, è alla ricerca di un a Associate Tributarista Principali responsabilità La risorsa si occuperà, in..
Associate Diritto Amministrativo
Il team è composto da altri tre associate e un praticante, oltre ai due soci di riferimento. Principali.. Luogo di lavoro. Milano Stai cercando un Associate Diritto Amministrativo per la tua azienda? Offerte..

PE Analyst/Associate
Job title. PE Analyst AssociateLocation. Milan, Lombardy, Italy (Hybrid)The Green recruitment company.. They are now looking to expand their team with a PE Analyst Associate in Milan, Italy. Their strategy..

Marketplace Operations Associate
For our headquarter in Milan, We are looking for a process and data driven Marketplace Operations Associate to join our Marketplace Operations Team. Reporting to the Head of Operations Italy..

Receptionist - Community Associate
Regus' BRAND DEL GRUPPO IWG Plc' multinzionale leader nel suo settore' è alla ricerca di un una Receptionist. Community Associate da inserire in stage full time per 6 mesi (con possibilita di..

Sales Associate Michael Kors
Globally minded individuals with a passion for fashion.If you enjoy working in a creative, fast paced environment, then we would love to hear from you!SALES ASSOCIATE"I feel really lucky to be..
Sales Associate -PART-TIME MATERNITY COVER -Vittorio Emanuele
Fast paced environment, then we would love to hear from you!EMEA TALENT AQUISITIONSALES ASSOCIATE"I feel really lucky to be surrounded by such a great team. I'm not only grateful that they've..
EU_Noventa Outlet Sales Associate Part time Fixed term


Stock Associate | Capri | Boutique di lusso
Retail Se non hai ancora trovato l'opportunità professionale giusta, puoi riuscirci con Jobtech , l'agenzia per il lavoro interamente digitale. Candidati come Stock associate per una boutique..

Retail Se non hai ancora trovato l'opportunit professionale giusta, puoi riuscirci con Jobtech, l'agenzia per il lavoro interamente digitale. Candidati come Stock associate per una boutique di..

BANDO BORSA DI STUDIO POST-DOCTORAL POSITION
The IEO operates as a Comprehensive Cancer Center, linking fundamental and applied research to clinical activities, patient care and clinical trials. The department of Experimental Oncology..

Informatore/trice Scientifico/a Emilia Romagna (851651)
Prima multinazionale del lavoro italiana, dedicata a valorizzare le esperienze, le competenze e il potenziale dei candidati in ambito Sales & Marketing, Clinical Research, Regulatory e Operation..

Informatore/trice Scientifico Lombardia (855149)

Informatore/trice Scientifico Lombardia
- Program Overview
The SOCRA Certified Clinical Research Professional (CCRP) program is your gateway to excellence in clinical research. Elevate your career with our internationally recognized certification, tailored for professionals dedicated to upholding the highest standards in the field. Join a community committed to ethical practices, continuous learning, and advancing global health.
The Society of Clinical Research Associates (SOCRA) established the Certification Program for Clinical Research Professionals in order to create an internationally accepted standard of knowledge, education, and experience by which clinical research professionals will be recognized by the clinical research community. Those individuals so recognized may use the "Certified Clinical Research Professional" or "CCRP ® " designation.
Path to Certification
CCRP certification is awarded upon meeting two criteria: a successful written application and a passing CCRP examination score. The benefits of obtaining certification are numerous. It not only validates knowledge, skills, and abilities but also enhances credibility and peer recognition. Career advancement and increased earning potential become tangible outcomes, reflecting a commitment to standards, compliance, and integrity.
Scope and Standards of Practice
The standards upon which this certification program is based have been set forth by SOCRA to promote recognition and continuing excellence in the ethical conduct of clinical trials. It is the goal of SOCRA to encourage members, and assure the competency of certified members, in their knowledge, understanding, and application of the conduct of clinical investigations involving humans in accordance with the ICH Guidelines, the U.S. Code of Federal Regulations, and the ethical principles that guide clinical research. Members are expected to adhere to national, state, local and provincial regulations and to international guidelines published by the International Conference for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) and all applicable federal, state and local laws and policies.
Standards of Practice include an understanding of and application of basic concepts of Good Clinical (Research) Practice, including:
- The Nuremberg Code
- The Belmont Report
- The Declaration of Helsinki
- 21 U.S. Code of Federal Regulations – Parts 11, 50, 56, 312, 812
- 45 U.S. Code of Federal Regulations - Part 46
- ICH Harmonised Guideline for Good Clinical Practice E6(R2), and
- ICH Clinical Safety Data Management: Definitions and Standards for Expedited Reporting (E2A)
- 42 CFR Part 11 (ClinicalTrials.gov)
Certification Exam
The SOCRA Certification Examination is offered in two formats: paper and pencil (at SOCRA sponsored sites), and computer based (at Prometric testing centers or through Home Proctoring).
SOCRA Sponsored Sites: Paper and Pencil
- Hosted exams offered in various location throughout the US and Canada.
- Visit the paper and pencil exam schedule for dates and locations.
- A complete application must be received by the deadline date as stated on the examination schedule.
- Score reports mailed to you in 4-6 weeks after exam.
Computer Based Testing: Testing Centers and Remote Proctoring
- Offered at Prometric testing centers throughout the world or through Home Proctoring
- Click here for a list of test centers.
- Allow 2-4 weeks for application processing.
- Once application is approved, schedule exam at a testing center. Exam sessions are available at least 6 weeks in advance.
- Score reports received immediately upon completion of exam.
Candidate Handbook
For more information, please view the Candidate Handbook.
Certification
- CCRP Certification Quick Facts
- Definition of a Clinical Research Professional
- Certification Program Policies
- Removal of CCRP® Credential
- Verify Certification
- Exam Overview
- Candidate Eligibility
- Application and Fee
- Computer Based Testing Exams
- Paper and Pencil Exams
- Refunds, Rescheduling and Retesting
- SOCRA Sponsored Exam Schedule
- Preparing for the Exam
- Preparation Resources
- Examination Results
- Host an Exam at Your Site
- Apply Online
- Exam Schedule SOCRA Sponsored Sites
- Requirements for Maintaining Certification
- Continuing Education Requirements
- Descriptions of Acceptable CE
- CE Recordkeeping Requirements
- Request for SOCRA CE for Courses / Workshops
- Installment Plan Payment
- Renewal of Certification
- Recertification Audit
- Recertification Learning Module
- Accreditation
Summary of Certification Activities
11,145 CCRPs (as of 12/31/2022)
- 1,391 candidates took CCRP exam
- 73% passed CCRP exam
- 2,649 CCRPs recertified
- 946 candidates took CCRP exam
- 65% passed CCRP exam
- 2,783 CCRPs recertified
- 2,060 candidates took CCRP exam
- 70% passed CCRP exam
- 3,801 CCRPs recertified
- 1,980 candidates took CCRP exam
- 71% passed CCRP exam
- 3,188 CCRPs recertified
- 104 exam sites hosted
- 2,175 candidates took CCRP exam
- 2,491 CCRPs recertified
- 91 exam sites hosted
- 2,141 candidates took CCRP exam
- 2,421CCRPs recertified
CRES Trials
Cres academy.

& Services
Learn how we ignite clinical and professional development in the life sciences industry..
Learn about the future of Clinical Research Associates and what it takes to thrive at a pharmaceutical company as a CRA.

Working With CRES

We strive for excellence in clinical research. We think of training as a tool for personal and team growth and dedicate ourselves to our students.

Flexibility
We design specific training courses for a wide array of specialities. from monitoring & clinical research associates to clinical project managers & data managers..

Becoming more
Whether through face-to-face or virtual classes, we realize professional growth, give direction and enable students to orient themselves in the world of work..
At the heart of CRES lies a mindset to stay open to cooperating with everyone, from patient association representatives to clinicians, life science companies and policy and decision-makers. We aim to foster innovation in healthcare and fill the gap in access to medical treatments and healthcare services.
Davide Integlia , CEO
A track record of successful services & training
CRES was born from the merger between Cres Academy and Cres Trials, CRES supports companies in their approach to clinical research and commercialization, allowing them to modernize and speed up the identification of impactful solutions.
Trial Management
Our versatile and dynamic operational approach, together with a pool of highly motivated in-house Clinical Research Associates, enables us to overcome even most complex challenges and help you meet milestones.
Study Start Up
Study start-up can be unpredictable, and it is vital to keep costs and quality under control while minimizing the time necessary to activate your site.
CRES’ professionals oversee all aspect of your investigation, from site activation to getting your research underway as swiftly as possible.
CRES Executive
The objective of our courses is to build new skills that enhance the organizations in which our students work.
We understand consulting not as an end in itself but as a fundamental intervention to achieve the client’s development objectives .We aim for excellence in clinical research. We think of training as a tool for personal growth and the team in which one works.
CRES Corporate
CRES implements Training consistent with the company’s development objectives.
Through the analysis of the training needs and of the real issues, it allows research team empowerment and teamwork efficiency. CRES aims improve your team and achieve new goals.
Analysis of the Training Needs of the Company Team
Educational Objectives:
- CRES offers companies an analysis of the training needs of the learning team.
- Identifies the individual and group characteristics of the learning team, adapting specific theoretical and practical paths to learners.
Ask Us Anything
Please feel free to contact us. We’re always glad to offer you our support, discuss how to generate new evidences and disseminate results of modern-day advances in medical science.
We will be glad to listen to you, and we will turn back to you with a tailored strategy, a detailed plan of activities, and a mindset to help you advance your goals.
I agree with the Privacy Policy
We're here to help!

Ask us anything.
- About AstraZeneca
Life at AstraZeneca
- Our Locations
- Information Technology
- BioPharmaceuticals
- BioPharmaceuticals R&D
- International
- Enabling functions
- Oncology R&D
- Early Talent
Inclusion & Diversity
- Application Hints & Tips
Keyword Search
City, State, or ZIP
Clinical Research Associate
Are you passionate about making a positive and impactful difference to the lives of others through Clinical Research? Join AstraZeneca as a Senior Clinical Research Associate working on the Biopharmaceutical pipeline.
About AstraZeneca We are a biopharmaceutical company driven by a strong sense of purpose, bringing a patient-centric approach to everything we do. We are bold learners and lateral thinkers, eager to explore out of our comfort zones. Working with cross-functional teams, we are positive collaborators and team players. We are always willing to get involved and support each other.
Equally driven by purpose and performance, we are recognised and respected within the industry and by our peers. We are renowned for doing the right thing, and those we work with – from clinicians to doctors – admire our work and ambitions. Join our team and feel proud to work for a company with an enviable reputation, which stands firm and is well positioned to be a market leader in Australia and New Zealand.
What you’ll do
As a Clinical Research Associate, you will be involved in effective site management across a number of high-profile studies, as well as all aspects of the study from site selection and start-up, initiation, monitoring, and closure. To be successful in this role you will have excellent knowledge of clinical study management including monitoring, study drug handling and data management. You will have experience working in a similar capacity, have excellent knowledge of international guidelines ICH-GCP, relevant local regulations and a sound understanding of the drug development process.
This role is a full time, initial 12-month contract position to be based in Sydney, Melbourne or Brisbane.
Essential for the role
Bachelors degree in related discipline, preferably in life science, or equivalent qualification
Experience working at CRA level
Excellent knowledge of international guidelines ICH‐GCP, basic knowledge of GMP/GDP and good knowledge of relevant local regulations.
Sound medical knowledge and ability to learn relevant AZ Therapeutic Areas.
Good understanding of clinical study management including monitoring, study drug handling and data management.
Good analytical and problem‐solving skills with a solutions focused mindset.
Ability to travel and manage sites across multiple states
Desirable for the role
Ability to work in a fun and collaborative team environment, yet comfortable with autonomy.
Manages change with a positive approach for self, team and the business. Sees change as an opportunity to improve performance and add value to the business.
Ability to look for and champion continuous improvement to deliver high quality clinical trials with reduced budget and in less time.
Demonstrates ability to prioritize and manage multiple tasks with conflicting deadlines.
Experience working within a risk‐based monitoring approach including remote monitoring.
Why AstraZeneca
At AstraZeneca, we share and are motivated by the same purpose: pushing the boundaries of science in order to deliver life-changing medicines to patients around the world. In short, our work has true meaning. As a business, we are committed to people. Our patient first approach informs all our decision making and we invest in team and individual development. Ours is an energetic, fast paced way of working that is solutions focused and collaborative.
AstraZeneca in Australia has been formally recognised as an Employer of Choice for Gender Equality by the Workplace Gender Equality Agency (WGEA) and as a Bronze Tier Employer within the Australian Workplace Equality Index (AWEI). Visit our ANZ Careers Page to learn more about our commitment to fostering a flexible, diverse, and inclusive environment.
So, what’s next?
We want to unlock your potential and inspire you to be your best, challenging you to expand your horizons, extend your knowledge and grow to your fullest. Join us to unlock your best!
If you’re curious to know more, please reach out to Danielle Jones.
Where can I find out more?
Our Social Media, Follow AstraZeneca on LinkedIn https://www.linkedin.com/company/1603/ Our website https://www.astrazeneca.com.au/
AstraZeneca embraces diversity and equality of opportunity. We are committed to building an inclusive and diverse team representing all backgrounds, with as wide a range of perspectives as possible, and harnessing industry-leading skills. We believe that the more inclusive we are, the better our work will be. We welcome and consider applications to join our team from all qualified candidates, regardless of their characteristics. We comply with all applicable laws and regulations on non-discrimination in employment (and recruitment), as well as work authorization and employment eligibility verification requirements.
We’ll keep you up-to-date
Sign up to be the first to receive job updates.
Email Address
Confirm Email
We are AstraZeneca, one of the world’s most forward-thinking and connected BioPharmaceutical companies. Explore our world.
At AstraZeneca, our purpose is to help patients all over the world by delivering life-changing medicines as one collaborative team.
The success of AstraZeneca is founded on innovation, creativity and diversity. Discover what this means for you.

Great culture, great work assignments, supportive management. Rotation opportunity within the company. They value inclusion and diversity.
Drug Discovery and Development
How much does a clinical research associate impact a company’s carbon footprint? More than you might think!
By Michael J. Cohen | May 1, 2024

[Adobe Stock]
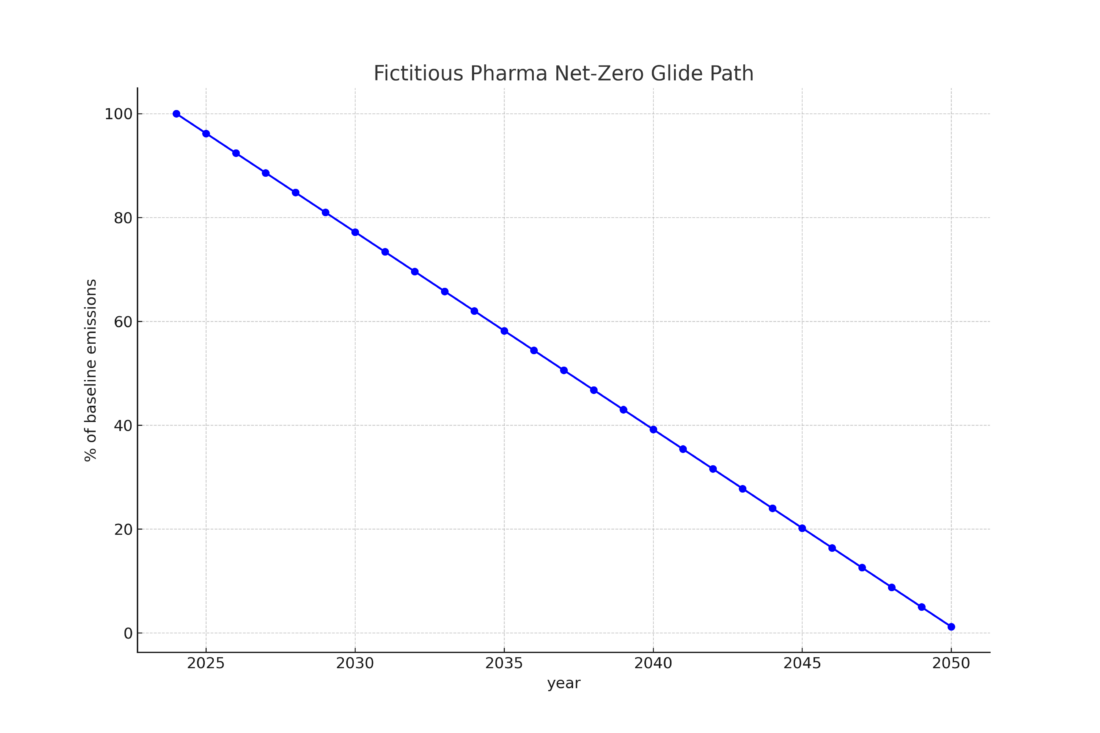
Figure 1: Fictional glide path for Fictitious Pharmaceutical Company (FPC) and its carbon footprint.
As FPC has established goals that extend across its entire organization, clinical trials must also be set on a similar glide path down toward net zero because clinical trials are not exempt from FPC’s corporate sustainability goals. Globally, clinical trials as an industry have a carbon footprint of somewhere between 37 and 100 megatons of greenhouse gas emissions or CO2e. This equates to an annual footprint between the size of Sweden and Belgium .

Figure 2: Johnson & Johnson Innovative Medicine sunburst plot
Returning to FPC and its carbon footprint of clinical studies, how does one walk down this path to net zero? If we consider the literature, there are a few key areas we can focus on, but here we will consider clinical research associate (CRA) monitoring visits, since the flights, trains, rental cars, taxis and hotel stays accumulate greenhouse gas emissions ranging from 4% to 20% of the trial’s total carbon impact. Johnson & Johnson Innovative Medicine has prepared a sunburst plot (see Figure 2) that summarizes some of their literature. In their analysis, they found on-site monitoring accounts for 11% of the entire trial footprint, so they are well within the 4%-20% range of FPC’s clinical trial footprint. For our purposes, we will assume CRA monitoring is 20% of clinical trial carbon footprint.
Returning to FPC, let’s assume it needs to reduce emissions by about 10% by 2026 to keep up with its glide path (Figure 1). Suddenly, CRA monitoring visits seem like a great area on which to focus. If we reduced CRA visits associated emissions by half, we could realize a net reduction of 10% for the entire study.
What does a typical CRA visit look like from a carbon footprint perspective? Let’s assume the average trip includes a flight or a train ride; a car rental, ride share or taxi; and, of course, a hotel stay. We calculated that the average CRA trip emits approximately 550kg CO2e, or about the same annual footprint as someone from Nigeria .
Recognizing there are many different types of CRA visits, for our purposes, we assumed that from a travel perspective, all visit types look the same regarding travel. So how can we reduce the environmental impact of these visits? Sustainable aviation fuel (SAF) is an emerging option to significantly reduce the carbon footprint of air travel, but availability of SAF remains low and certificate schemes are not currently approved by the Greenhouse Gas Protocol. In fact, Virgin Atlantic had the first 100% SAF flight from Heathrow Airport to John F. Kennedy International Airport on November 28, 2023, so clearly this technology is not widely available to cover all CRA trips. FPC could have all clinical trial monitors request “green” ride-share taxis, or rent electric vehicles, but these are not available everywhere, and may only promise “at least 55% fewer carbon emissions” We can provide colleagues with travel footprint data, routing support and other tools to better understand their carbon footprint and help them make more sustainable choices. However, this alone will not make a significant reduction in FPC’s carbon footprint.
So the obvious conclusion is that we have to be more selective on when we visit and who is conducting the visit (namely where they live and how far they are travelling to the site).

Figure 3: Thermo Fisher Scientific’s 360° monitoring model
The PPD clinical research business of Thermo Fisher Scientific has adopted a 360° monitoring model (Figure 3), consisting of continual remote, onsite and centralized monitoring. The model aims to reduce reliance on episodic onsite monitoring and making site monitoring and data review a continual, real-time process. In this model, we strive to assign onsite CRAs within close proximity to a research site (approximately within four-hour roundtrip). This CRA develops a deep site knowledge and relationship with a particular site, but they are only sent when the outcome of the continuous data monitoring or risk indicators triggers the need for an onsite visit. Based on our own internal data, we see 23% fewer CRA visits across almost 40 studies and multiple therapeutic areas. We also see that when a CRA does visit the site, they are locally assigned monitors. This results in an 18.5% reduction in visit emissions when the local monitor does need to conduct in-person visits. If FPC were able to utilize this type of 360 ° monitoring, they could realize a 37% CO2e reduction across multiple therapeutic areas (see Figure 4).

Figure 4: Environmental impact of implementing 360° monitoring across multiple therapeutic areas for 39 studies.
Benefits go beyond reducing environmental impact, as 360 ° monitoring results in faster entry into the electronic data capture system and sites appreciate the reduction in visits and time on site. Overall, 76% of sites rated this monitoring strategy as good as or better than traditional monitoring strategies.
Now, returning to our glide path for FPC and its team deploys 360 ° monitoring-type strategies across their portfolio, they could reduce their global clinical trial emissions by approximately 7% — just in time for the 2026 target!
CRA monitoring is an incredible area of opportunity to deploy strategies that are both better for sites, the CRAs and the environment. Recently, we’ve seen pharma partners reduce in-person source data verification to incredibly low levels (approximately 20%), while other organizations maintain 100% in-person source data verification (SDV). However, challenges remain, such as sites allowing remote access and regulations that may not allow a future of 100% remote SDV. The 360° monitoring model embraces improvements to technology that enable remote review and embraces growing client and regulatory acceptance of risk-based quality management (RBQM) principles enabling our approach to reduce SDV% by reassessing critical data and defining those data points to target and refocus time from SDV/SDR to higher-value on-site activities and deploy continual remote monitoring.

Michael Cohen
There’s a growing notion in clinical research that yesterday’s decentralized clinical trial is today’s clinical trial. This idea that we must decentralize, digitize and modernize trials obviously became paramount during the COVID-19 pandemic, but today we see that these strategies can help improve a study’s resilience, ability to attract and retain diverse patient populations, and improve quality and cost. But these strategies also benefit the environment. Considering CRA monitor visits, the more we can leverage digital and decentralized resources and strategies, the more we can drive environmental sustainability within clinical trials.
Michael J. Cohen, MSc, MBA is senior ∂irector, lead, environmental Sustainability, PPD clinical research business at Thermo Fisher Scientific.
Related Articles Read More >

Top pharma companies ranked by 2023 R&D spend

Real-world evidence use is expanding. What’s behind the change?

From Novartis to Pfizer: A closer look at novel cell and gene therapy pricing and reimbursement strategies

Public vs. private: Who’s leading the charge in H5N1 preparedness?
Search drug discovery & development.
- Home Drug Discovery and Development
- Drug Discovery
- Women in Pharma and Biotech
- Neurological Disease
- Infectious Disease
- Video features
- 2023 Pharma 50
- 2022 Pharma 50
- 2021 Pharma 50

Clinical Research Coordinator Associate
🔍 school of medicine, stanford, california, united states.
Duties include:
- Serve as primary contact with research participants, sponsors, and regulatory agencies. Coordinate studies from startup through close-out.
- Determine eligibility of and gather consent from study participants according to protocol. Assist in developing and implementing a variety of recruitment strategies.
- Coordinate collection of study specimens and processing.
- Collect and manage patient and laboratory data for clinical research projects. Manage research project databases, develop flow sheets and other study related documents, and complete study documents/case report forms.
- Ensure compliance with research protocols, and review and audit case report forms for completion and accuracy with source documents. Prepare regulatory submissions and ensure institutional Review Board renewals are completed.
- Conduct study visits and semi-structured interviews, monitor scheduling of procedures and charges, coordinate documents, and attend monitoring meetings with sponsors, acting as primary contact.
- Assist in coding qualitative interviews, data-cleaning, and participating in the coding process.
- Monitor expenditures and adherence to study budgets and resolve billing issues in collaboration with finance and/or management staff.
- Interact with the principal investigator regularly, ensuring patient safety and adherence to proper study conduct.
- Ensure essential documentation and recording of patient and research data in appropriate files per institutional and regulatory requirements.
- Participate in monitor visits and regulatory audits.
* - Other duties may also be assigned
Stanford University provides pay ranges representing its good faith estimate of what the University reasonably expects to pay for a position. The pay offered to a selected candidate will be determined based on factors such as (but not limited to) the scope and responsibilities of the position, the qualifications of the selected candidate, departmental budget availability, internal equity, geographic location and external market pay for comparable jobs. The hourly range for this position working in the California Bay area is between $31.73 to $36.54 based on commensurate experience and background.
DESIRED QUALIFICATIONS:
- Stanford IRB Knowledge
- Assistant Clinical Research Coordinator experience
· Previous use of RedCap (preferred not required)
EDUCATION & EXPERIENCE (REQUIRED):
Two-year college degree and two years related work experience or a Bachelor’s degree in a related field or an equivalent combination of related education and relevant experience.
KNOWLEDGE, SKILLS AND ABILITIES (REQUIRED):
· Strong interpersonal skills.
· Proficiency with Microsoft Office.
· Knowledge of medical terminology.
CERTIFICATIONS & LICENSES:
Society of Clinical Research Associates or Association of Clinical Research Professionals certification is preferred.
PHYSICAL REQUIREMENTS*:
· Frequently stand, walk, twist, bend, stoop, squat and use fine light/fine grasping.
· Occasionally sit, reach above shoulders, perform desk-based computer tasks, use a telephone and write by hand, lift, carry, push, and pull objects that weigh up to 40 pounds.
· Rarely kneel, crawl, climb ladders, grasp forcefully, sort and file paperwork or parts, rarely lift, carry, push, and pull objects that weigh 40 pounds or more.
* - Consistent with its obligations under the law, the University will provide reasonable accommodation to any employee with a disability who requires accommodation to perform the essential functions of his or her job.
WORKING CONDITIONS:
· Position may at times require the employee to work with or be in areas where hazardous materials and/or exposure to chemicals, blood, body fluid or tissues and risk of exposure to contagious disease and infections.
· May require extended or unusual work hours based on research requirements and business needs.
· Spanish speaking is preferred, as there will be monolingual Spanish participants in our studies.
~ All members of the Department of Pediatrics are engaged in continuous learning and improvement to foster a culture where diversity, equity, inclusion, and justice are central to all aspects of our work. The Department collectively and publicly commits to continuously promoting anti-racism and equity through its policies, programs, and practices at all levels. ~
- Schedule: Full-time
- Job Code: 1013
- Employee Status: Fixed-Term
- Department URL: http://pediatrics.stanford.edu/
- Requisition ID: 103037
- Work Arrangement : Hybrid Eligible
My Submissions
Track your opportunities.
Similar Listings
School of Medicine, Stanford, California, United States
📁 Research
Post Date: Jan 29, 2024
Post Date: Feb 15, 2023
Post Date: Aug 05, 2022
Global Impact We believe in having a global impact
Climate and sustainability.
Stanford's deep commitment to sustainability practices has earned us a Platinum rating and inspired a new school aimed at tackling climate change.
Medical Innovations
Stanford's Innovative Medicines Accelerator is currently focused entirely on helping faculty generate and test new medicines that can slow the spread of COVID-19.
From Google and PayPal to Netflix and Snapchat, Stanford has housed some of the most celebrated innovations in Silicon Valley.
Advancing Education
Through rigorous research, model training programs and partnerships with educators worldwide, Stanford is pursuing equitable, accessible and effective learning for all.
Working Here We believe you matter as much as the work

I love that Stanford is supportive of learning, and as an education institution, that pursuit of knowledge extends to staff members through professional development, wellness, financial planning and staff affinity groups.
School of Engineering

I get to apply my real-world experiences in a setting that welcomes diversity in thinking and offers support in applying new methods. In my short time at Stanford, I've been able to streamline processes that provide better and faster information to our students.
Phillip Cheng
Office of the Vice Provost for Student Affairs

Besides its contributions to science, health, and medicine, Stanford is also the home of pioneers across disciplines. Joining Stanford has been a great way to contribute to our society by supporting emerging leaders.
Denisha Clark
School of Medicine

I like working in a place where ideas matter. Working at Stanford means being part of a vibrant, international culture in addition to getting to do meaningful work.
Office of the President and Provost
Getting Started We believe that you can love your job
Join Stanford in shaping a better tomorrow for your community, humanity and the planet we call home.
- 4.2 Review Ratings
- 81% Recommend to a Friend
View All Jobs

IMAGES
COMMENTS
Visualizza tutti gli annunci di lavoro - Thermo Fisher Scientific - Annunci (Roma) - lavoro - Clinical Research Associate - Roma, Lazio; Ricerca Stipendi: Clinical Research Associate (CRA) Level II / Senior CRA, stipendi - Roma, Lazio; Leggi le domande e risposte più comuni su Thermo Fisher Scientific
22 lavori per Clinical Research Associate disponibili a Roma, Lazio su Indeed.com. Passa a contenuto principale. Cerca lavoro. Recensioni aziendali. Esplora stipendi. Carica il tuo CV. Accedi. Accedi. Indeed aziende/Pubblica un annuncio. Inizio del contenuto principale. Cosa. Dove. Cerca lavoro. Data di pubblicazione. Pubblicato da.
Feb 17, 2024. Current Associate Consultant in Burlington, MA, Massachusetts. Company is generous with 401K match and health insurance options are reasonable. Search Clinical research associate jobs in Italy with company ratings & salaries. 54 open jobs for Clinical research associate in Italy.
Ricerca offerte di lavoro per Clinical research a Roma con valutazioni e stipendi dell'azienda. 44 lavori disponibili per Clinical research a Roma.
An excellent level of English, both written and spoken is a mandatory requirement. A basic level of German language would be preferable. The ability to travel frequently. A high level of attention to detail. The ability to work independently. 40 lavori disponibili come Clinical Research Associate Italy su Indeed.com.
clinical research associate jobs in Roma Roma jobs Novo Nordisk jobs in Roma. Employee reviews at Novo Nordisk Novo Nordisk Overview. ClinChoice. 3.8. Clinical Research Associate II (CRA II) or Senior Clinical Research Associate (SCRA) ... Assist Clinical Research Associates (CRAs) and Regulatory and Start-Up (RSU) team with accurately updating ...
Location: Sofia, Milan, Barcelona, Madrid, Roma Reference: JR106224. Categories Clinical Monitoring __vacancyopjusttionswidget.opt ... As a Clinical Research Associate for Medical Devices and Diagnostics Research at ICON, you'll work within a large-scale, fast-paced environment alongside a close-knit team of highly qualified CRAs and medical ...
The Humanitas Research Center is seeking a highly motivated and dynamic individual to join our neurologic multidisciplinary team as a Clinical Project and Research Laboratory Researcher/Technologist. The candidate will be involved in project management, including informed consent collection and compiling eCRF for data collection.
Il Clinical Research Associate o CRA, è la figura delegata dall'ente proponente di uno studio clinico che organizza, segue e monitora l'andamento di uno studio: dalla fase di selezione del centro alla sua chiusura.Il CRA gestisce la ricerca per lo sviluppo di un farmaco o di un dispositivo medico, coordinando e supervisionando le attività tra il centro sperimentale e l'ente proponente ...
Clinical Research Associate (CRA) Level II / Senior CRA Pharmaceutical Product Development (PPD) Multi location: Remote, Milano, Italy Napoli, Napoli, Italy Bologna, Bologna, Italy Rome, Roma, Italy. Job Description. At PPD, part of Thermo Fisher Scientific, you'll discover meaningful work that makes a positive impact on a global scale. Join ...
Italy. Milan 7. Clinical Research. Clinical Research Associate 14. Clinical Operations 6. Study Start Up 2. Clinical Project Manager 1. Pharmacovigilance 1. Get new jobs for this search by email.
Quanto guadagna un Clinical Research Associate in Italia? Lo stipendio medio per clinical research associate in Italia è € 52 500 all'anno o € 26.92 all'ora. Le posizioni "entry level" percepiscono uno stipendio di € 48 250 all'anno, mentre i lavoratori con più esperienza guadagnano fino a € 82 200 all'anno.
The SOCRA Certified Clinical Research Professional (CCRP) program is your gateway to excellence in clinical research. Elevate your career with our internationally recognized certification, tailored for professionals dedicated to upholding the highest standards in the field. Join a community committed to ethical practices, continuous learning ...
17 lavori per Clinical Research Associate disponibili a 00161 Roma su Indeed.com. Passa a contenuto principale. Cerca lavoro. Recensioni aziendali. Esplora stipendi. Carica il tuo CV. Accedi. Accedi. Indeed aziende/Pubblica un annuncio. Inizio del contenuto principale. Cosa. Dove. Cerca lavoro. Data di pubblicazione.
The ACRP offers the Certified Clinical Research Associate credential. To earn this certification, you must have one of the following: A bachelor's degree and at least 3,000 hours of experience as a CRA. A current CCRC, CPI or ACRP-CP certification and be able to substitute 1,500 hours of work experience.
4,769 Clinical Research Associates jobs available on Indeed.com. Apply to Clinical Research Associate, Clinical Research Coordinator, Senior Clinical Research Coordinator and more!
clinical research associate jobs in Roma Roma jobs Novo Nordisk jobs in Roma. Employee reviews at Novo Nordisk Novo Nordisk Overview. Medpace, Inc. 3.2. Clinical Research Associate. Italy. For those with a medical and/or health/life science background who want to explore the research field and be part of a team bringing pharmaceutical and ...
Working With CRES. Dedication. We strive for excellence in clinical research. We think of training as a tool for personal and team growth and dedicate ourselves to our students. Flexibility. We design specific training courses for a wide array of specialities. From monitoring & Clinical Research Associates to Clinical Project Managers & Data ...
Liked by Roma Parikh. Experienced and passionate Clinical Research Associate with a demonstrated history of…. · Experience: Syneos Health · Education: University of North Carolina at Chapel ...
As a Clinical Research Associate, you will be involved in effective site management across a number of high-profile studies, as well as all aspects of the study from site selection and start-up, initiation, monitoring, and closure. To be successful in this role you will have excellent knowledge of clinical study management including monitoring ...
Clinical Research Associate. Hyderābād. Apply on employer site Apply now. Apply on employer site Apply now) Act as DRL's direct contact with assigned sites, assess and ensure overall integrity of study 2) Implementation and adherence to study protocol at clinical investigational sites, and resolve site issues. Escalate issues that were ...
12 lavori per Cra Clinical Research Associate disponibili a Roma, Lazio su Indeed.com.
Returning to FPC and its carbon footprint of clinical studies, how does one walk down this path to net zero? If we consider the literature, there are a few key areas we can focus on, but here we will consider clinical research associate (CRA) monitoring visits, since the flights, trains, rental cars, taxis and hotel stays accumulate greenhouse gas emissions ranging from 4% to 20% of the trial ...
Posted 5:03:38 AM. Join our client's team as a remote Clinical Research Associate to help drive their clinical trials.…See this and similar jobs on LinkedIn.
The typical day of a clinical research associate includes planning and managing clinical research projects for pharmaceutical companies. They may recruit participants, coordinate schedules, input data, and oversee trials. In their career, clinical researchers may also be in charge of ensuring that researchers follow all local and federal ...
The Clinical Research Coordinator Associate is responsible for the overall management and implementation of an assigned set of multiple research protocols assuring efficiency and regulatory compliance. These studies will be conducted at the Stanford Hospital and Clinics. He/she will work as part of a clinical trials research team and report to ...
Clinical Research Associate / Senior CRA (Italy) Precision for Medicine. Remoto in Milano, Lombardia. You will have previous monitoring experience for Oncology clinical Studies in Italy. This is a remote based position (homebased anywhere in Italy), with travel…. 30+ giorni fa ·.
Collect and manage patient and laboratory data for clinical research projects. Manage research project databases, develop flow sheets and other study related documents, and complete study documents/case report forms. Ensure compliance with research protocols, and review and audit case report forms for completion and accuracy with source documents.
The Division of Pulmonary and Critical Care Medicine at the University of Virginia is currently seeking a Postdoctoral Research Associate to investigate the lung immune and tissue responses to infectious and non-infectious exposures (COPD, ILD, lung transplant, and ARDS/ALI) in the research group of Dr. Y. Michael Shim. This project is a part of two newly funded NIH R01's and will utilize ...