An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Springer Nature - PMC COVID-19 Collection


An Introduction to COVID-19
Simon james fong.
4 Department of Computer and Information Science, University of Macau, Taipa, Macau, China
Nilanjan Dey
5 Department of Information Technology, Techno International New Town, Kolkata, West Bengal India
Jyotismita Chaki
6 School of Information Technology and Engineering, Vellore Institute of Technology, Vellore, Tamil Nadu India
A novel coronavirus (CoV) named ‘2019-nCoV’ or ‘2019 novel coronavirus’ or ‘COVID-19’ by the World Health Organization (WHO) is in charge of the current outbreak of pneumonia that began at the beginning of December 2019 near in Wuhan City, Hubei Province, China [1–4]. COVID-19 is a pathogenic virus. From the phylogenetic analysis carried out with obtainable full genome sequences, bats occur to be the COVID-19 virus reservoir, but the intermediate host(s) has not been detected till now.
A Brief History of the Coronavirus Outbreak
A novel coronavirus (CoV) named ‘2019-nCoV’ or ‘2019 novel coronavirus’ or ‘COVID-19’ by the World Health Organization (WHO) is in charge of the current outbreak of pneumonia that began at the beginning of December 2019 near in Wuhan City, Hubei Province, China [ 1 – 4 ]. COVID-19 is a pathogenic virus. From the phylogenetic analysis carried out with obtainable full genome sequences, bats occur to be the COVID-19 virus reservoir, but the intermediate host(s) has not been detected till now. Though three major areas of work already are ongoing in China to advise our awareness of the pathogenic origin of the outbreak. These include early inquiries of cases with symptoms occurring near in Wuhan during December 2019, ecological sampling from the Huanan Wholesale Seafood Market as well as other area markets, and the collection of detailed reports of the point of origin and type of wildlife species marketed on the Huanan market and the destination of those animals after the market has been closed [ 5 – 8 ].
Coronaviruses mostly cause gastrointestinal and respiratory tract infections and are inherently categorized into four major types: Gammacoronavirus, Deltacoronavirus, Betacoronavirus and Alphacoronavirus [ 9 – 11 ]. The first two types mainly infect birds, while the last two mostly infect mammals. Six types of human CoVs have been formally recognized. These comprise HCoVHKU1, HCoV-OC43, Middle East Respiratory Syndrome coronavirus (MERS-CoV), Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) which is the type of the Betacoronavirus, HCoV229E and HCoV-NL63, which are the member of the Alphacoronavirus. Coronaviruses did not draw global concern until the 2003 SARS pandemic [ 12 – 14 ], preceded by the 2012 MERS [ 15 – 17 ] and most recently by the COVID-19 outbreaks. SARS-CoV and MERS-CoV are known to be extremely pathogenic and spread from bats to palm civets or dromedary camels and eventually to humans.
COVID-19 is spread by dust particles and fomites while close unsafe touch between the infector and the infected individual. Airborne distribution has not been recorded for COVID-19 and is not known to be a significant transmission engine based on empirical evidence; although it can be imagined if such aerosol-generating practices are carried out in medical facilities. Faecal spreading has been seen in certain patients, and the active virus has been reported in a small number of clinical studies [ 18 – 20 ]. Furthermore, the faecal-oral route does not seem to be a COVID-19 transmission engine; its function and relevance for COVID-19 need to be identified.
For about 18,738,58 laboratory-confirmed cases recorded as of 2nd week of April 2020, the maximum number of cases (77.8%) was between 30 and 69 years of age. Among the recorded cases, 21.6% are farmers or employees by profession, 51.1% are male and 77.0% are Hubei.
However, there are already many concerns regarding the latest coronavirus. Although it seems to be transferred to humans by animals, it is important to recognize individual animals and other sources, the path of transmission, the incubation cycle, and the features of the susceptible community and the survival rate. Nonetheless, very little clinical knowledge on COVID-19 disease is currently accessible and details on age span, the animal origin of the virus, incubation time, outbreak curve, viral spectroscopy, dissemination pathogenesis, autopsy observations, and any clinical responses to antivirals are lacking among the serious cases.
How Different and Deadly COVID-19 is Compared to Plagues in History
COVID-19 has reached to more than 150 nations, including China, and has caused WHO to call the disease a worldwide pandemic. By the time of 2nd week of April 2020, this COVID-19 cases exceeded 18,738,58, although more than 1,160,45 deaths were recorded worldwide and United States of America became the global epicentre of coronavirus. More than one-third of the COVID-19 instances are outside of China. Past pandemics that have existed in the past decade or so, like bird flu, swine flu, and SARS, it is hard to find out the comparison between those pandemics and this coronavirus. Following is a guide to compare coronavirus with such diseases and recent pandemics that have reformed the world community.
Coronavirus Versus Seasonal Influenza
Influenza, or seasonal flu, occurs globally every year–usually between December and February. It is impossible to determine the number of reports per year because it is not a reportable infection (so no need to be recorded to municipality), so often patients with minor symptoms do not go to a physician. Recent figures placed the Rate of Case Fatality at 0.1% [ 21 – 23 ].
There are approximately 3–5 million reports of serious influenza a year, and about 250,000–500,000 deaths globally. In most developed nations, the majority of deaths arise in persons over 65 years of age. Moreover, it is unsafe for pregnant mothers, children under 59 months of age and individuals with serious illnesses.
The annual vaccination eliminates infection and severe risks in most developing countries but is nevertheless a recognized yet uncomfortable aspect of the season.
In contrast to the seasonal influenza, coronavirus is not so common, has led to fewer cases till now, has a higher rate of case fatality and has no antidote.
Coronavirus Versus Bird Flu (H5N1 and H7N9)
Several cases of bird flu have existed over the years, with the most severe in 2013 and 2016. This is usually from two separate strains—H5N1 and H7N9 [ 24 – 26 ].
The H7N9 outbreak in 2016 accounted for one-third of all confirmed human cases but remained confined relative to both coronavirus and other pandemics/outbreak cases. After the first outbreak, about 1,233 laboratory-confirmed reports of bird flu have occurred. The disease has a Rate of Case Fatality of 20–40%.
Although the percentage is very high, the blowout from individual to individual is restricted, which, in effect, has minimized the number of related deaths. It is also impossible to monitor as birds do not necessarily expire from sickness.
In contrast to the bird flu, coronavirus becomes more common, travels more quickly through human to human interaction, has an inferior cardiothoracic ratio, resulting in further total fatalities and spread from the initial source.
Coronavirus Versus Ebola Epidemic
The Ebola epidemic of 2013 was primarily centred in 10 nations, including Sierra Leone, Guinea and Liberia have the greatest effects, but the extremely high Case Fatality Rate of 40% has created this as a significant problem for health professionals nationwide [ 27 – 29 ].
Around 2013 and 2016, there were about 28,646 suspicious incidents and about 11,323 fatalities, although these are expected to be overlooked. Those who survived from the original epidemic may still become sick months or even years later, because the infection may stay inactive for prolonged periods. Thankfully, a vaccination was launched in December 2016 and is perceived to be effective.
In contrast to the Ebola, coronavirus is more common globally, has caused in fewer fatalities, has a lesser case fatality rate, has no reported problems during treatment and after recovery, does not have an appropriate vaccination.
Coronavirus Versus Camel Flu (MERS)
Camel flu is a misnomer–though camels have MERS antibodies and may have been included in the transmission of the disease; it was originally transmitted to humans through bats [ 30 – 32 ]. Like Ebola, it infected only a limited number of nations, i.e. about 27, but about 858 fatalities from about 2,494 laboratory-confirmed reports suggested that it was a significant threat if no steps were taken in place to control it.
In contrast to the camel flu, coronavirus is more common globally, has occurred more fatalities, has a lesser case fatality rate, and spreads more easily among humans.
Coronavirus Versus Swine Flu (H1N1)
Swine flu is the same form of influenza that wiped 1.7% of the world population in 1918. This was deemed a pandemic again in June 2009 an approximately-21% of the global population infected by this [ 33 – 35 ].
Thankfully, the case fatality rate is substantially lower than in the last pandemic, with 0.1%–0.5% of events ending in death. About 18,500 of these fatalities have been laboratory-confirmed, but statistics range as high as 151,700–575,400 worldwide. 50–80% of severe occurrences have been reported in individuals with chronic illnesses like asthma, obesity, cardiovascular diseases and diabetes.
In contrast to the swine flu, coronavirus is not so common, has caused fewer fatalities, has more case fatality rate, has a longer growth time and less impact on young people.
Coronavirus Versus Severe Acute Respiratory Syndrome (SARS)
SARS was discovered in 2003 as it spread from bats to humans resulted in about 774 fatalities. By May there were eventually about 8,100 reports across 17 countries, with a 15% case fatality rate. The number is estimated to be closer to 9.6% as confirmed cases are counted, with 0.9% cardiothoracic ratio for people aged 20–29, rising to 28% for people aged 70–79. Similar to coronavirus, SARS had bad results for males than females in all age categories [ 36 – 38 ].
Coronavirus is more common relative to SARS, which ended in more overall fatalities, lower case fatality rate, the even higher case fatality rate in older ages, and poorer results for males.
Coronavirus Versus Hong Kong Flu (H3N2)
The Hong Kong flu pandemic erupted on 13 July 1968, with 1–4 million deaths globally by 1969. It was one of the greatest flu pandemics of the twentieth century, but thankfully the case fatality rate was smaller than the epidemic of 1918, resulting in fewer fatalities overall. That may have been attributed to the fact that citizens had generated immunity owing to a previous epidemic in 1957 and to better medical treatment [ 39 ].
In contrast to the Hong Kong flu, coronavirus is not so common, has caused in fewer fatalities and has a higher case fatality rate.
Coronavirus Versus Spanish Flu (H1N1)
The 1918 Spanish flu pandemic was one of the greatest occurrences of recorded history. During the first year of the pandemic, lifespan in the US dropped by 12 years, with more civilians killed than HIV/AIDS in 24 h [ 40 – 42 ].
Regardless of the name, the epidemic did not necessarily arise in Spain; wartime censors in Germany, the United States, the United Kingdom and France blocked news of the disease, but Spain did not, creating the misleading perception that more cases and fatalities had occurred relative to its neighbours
This strain of H1N1 eventually affected more than 500 million men, or 27% of the world’s population at the moment, and had deaths of between 40 and 50 million. At the end of 1920, 1.7% of the world’s people had expired of this illness, including an exceptionally high death rate for young adults aged between 20 and 40 years.
In contrast to the Spanish flu, coronavirus is not so common, has caused in fewer fatalities, has a higher case fatality rate, is more harmful to older ages and is less risky for individuals aged 20–40 years.
Coronavirus Versus Common Cold (Typically Rhinovirus)
Common cold is the most common illness impacting people—Typically, a person suffers from 2–3 colds each year and the average kid will catch 6–8 during the similar time span. Although there are more than 200 cold-associated virus types, infections are uncommon and fatalities are very rare and typically arise mainly in extremely old, extremely young or immunosuppressed cases [ 43 , 44 ].
In contrast to the common cold, coronavirus is not so prevalent, causes more fatalities, has more case fatality rate, is less infectious and is less likely to impact small children.
Reviews of Online Portals and Social Media for Epidemic Information Dissemination
As COVID-19 started to propagate across the globe, the outbreak contributed to a significant change in the broad technology platforms. Where they once declined to engage in the affairs of their systems, except though the possible danger to public safety became obvious, the advent of a novel coronavirus placed them in a different interventionist way of thought. Big tech firms and social media are taking concrete steps to guide users to relevant, credible details on the virus [ 45 – 48 ]. And some of the measures they’re doing proactively. Below are a few of them.
Facebook started adding a box in the news feed that led users to the Centers for Disease Control website regarding COVID-19. It reflects a significant departure from the company’s normal strategy of placing items in the News Feed. The purpose of the update, after all, is personalization—Facebook tries to give the posts you’re going to care about, whether it is because you’re connected with a person or like a post. In the virus package, Facebook has placed a remarkable algorithmic thumb on the scale, potentially pushing millions of people to accurate, authenticated knowledge from a reputable source.
Similar initiatives have been adopted by Twitter. Searching for COVID-19 will carry you to a page highlighting the latest reports from public health groups and credible national news outlets. The search also allows for common misspellings. Twitter has stated that although Russian-style initiatives to cause discontent by large-scale intelligence operations have not yet been observed, a zero-tolerance approach to network exploitation and all other attempts to exploit their service at this crucial juncture will be expected. The problem has the attention of the organization. It also offers promotional support to public service agencies and other non-profit groups.
Google has made a step in making it better for those who choose to operate or research from home, offering specialized streaming services to all paying G Suite customers. Google also confirmed that free access to ‘advanced’ Hangouts Meet apps will be rolled out to both G Suite and G Suite for Education clients worldwide through 1st July. It ensures that companies can hold meetings of up to 250 people, broadcast live to up to about 100,000 users within a single network, and archive and export meetings to Google Drive. Usually, Google pays an additional $13 per person per month for these services in comparison to G Suite’s ‘enterprise’ membership, which adds up to a total of about $25 per client each month.
Microsoft took a similar move, introducing the software ‘Chat Device’ to help public health and protection in the coronavirus epidemic, which enables collaborative collaboration via video and text messaging. There’s an aspect of self-interest in this. Tech firms are offering out their goods free of charge during periods of emergency for the same purpose as newspapers are reducing their paywalls: it’s nice to draw more paying consumers.
Pinterest, which has introduced much of the anti-misinformation strategies that Facebook and Twitter are already embracing, is now restricting the search results for ‘coronavirus’, ‘COVID-19’ and similar words for ‘internationally recognized health organizations’.
Google-owned YouTube, traditionally the most conspiratorial website, has recently introduced a connection to the World Health Organization virus epidemic page to the top of the search results. In the early days of the epidemic, BuzzFeed found famous coronavirus conspiratorial videos on YouTube—especially in India, where one ‘explain’ with a false interpretation of the sources of the disease racketeered 13 million views before YouTube deleted it. Yet in the United States, conspiratorial posts regarding the illness have failed to gain only 1 million views.
That’s not to suggest that misinformation doesn’t propagate on digital platforms—just as it travels through the broader Internet, even though interaction with friends and relatives. When there’s a site that appears to be under-performing in the global epidemic, it’s Facebook-owned WhatsApp, where the Washington Post reported ‘a torrent of disinformation’ in places like Nigeria, Indonesia, Peru, Pakistan and Ireland. Given the encrypted existence of the app, it is difficult to measure the severity of the problem. Misinformation is also spread in WhatsApp communities, where participation is restricted to about 250 individuals. Knowledge of one category may be readily exchanged with another; however, there is a considerable amount of complexity of rotating several groups to peddle affected healing remedies or propagate false rumours.
Preventative Measures and Policies Enforced by the World Health Organization (WHO) and Different Countries
Coronavirus is already an ongoing epidemic, so it is necessary to take precautions to minimize both the risk of being sick and the transmission of the disease.
WHO Advice [ 49 ]
- Wash hands regularly with alcohol-based hand wash or soap and water.
- Preserve contact space (at least 1 m/3 feet between you and someone who sneezes or coughs).
- Don’t touch your nose, head and ears.
- Cover your nose and mouth as you sneeze or cough, preferably with your bent elbow or tissue.
- Try to find early medical attention if you have fatigue, cough and trouble breathing.
- Take preventive precautions if you are in or have recently go to places where coronavirus spreads.
The first person believed to have become sick because of the latest virus was near in Wuhan on 1 December 2019. A formal warning of the epidemic was released on 31 December. The World Health Organization was informed of the epidemic on the same day. Through 7 January, the Chinese Government addressed the avoidance and regulation of COVID-19. A curfew was declared on 23 January to prohibit flying in and out of Wuhan. Private usage of cars has been banned in the region. Chinese New Year (25 January) festivities have been cancelled in many locations [ 50 ].
On 26 January, the Communist Party and the Government adopted more steps to contain the COVID-19 epidemic, including safety warnings for travellers and improvements to national holidays. The leading party has agreed to prolong the Spring Festival holiday to control the outbreak. Universities and schools across the world have already been locked down. Many steps have been taken by the Hong Kong and Macau governments, in particular concerning schools and colleges. Remote job initiatives have been placed in effect in many regions of China. Several immigration limits have been enforced.
Certain counties and cities outside Hubei also implemented travel limits. Public transit has been changed and museums in China have been partially removed. Some experts challenged the quality of the number of cases announced by the Chinese Government, which constantly modified the way coronavirus cases were recorded.
Italy, a member state of the European Union and a popular tourist attraction, entered the list of coronavirus-affected nations on 30 January, when two positive cases in COVID-19 were identified among Chinese tourists. Italy has the largest number of coronavirus infections both in Europe and outside of China [ 51 ].
Infections, originally limited to northern Italy, gradually spread to all other areas. Many other nations in Asia, Europe and the Americas have tracked their local cases to Italy. Several Italian travellers were even infected with coronavirus-positive in foreign nations.
Late in Italy, the most impacted coronavirus cities and counties are Lombardia, accompanied by Veneto, Emilia-Romagna, Marche and Piedmonte. Milan, the second most populated city in Italy, is situated in Lombardy. Other regions in Italy with coronavirus comprised Campania, Toscana, Liguria, Lazio, Sicilia, Friuli Venezia Giulia, Umbria, Puglia, Trento, Abruzzo, Calabria, Molise, Valle d’Aosta, Sardegna, Bolzano and Basilicata.
Italy ranks 19th of the top 30 nations getting high-risk coronavirus airline passengers in China, as per WorldPop’s provisional study of the spread of COVID-19.
The Italian State has taken steps like the inspection and termination of large cultural activities during the early days of the coronavirus epidemic and has gradually declared the closing of educational establishments and airport hygiene/disinfection initiatives.
The Italian National Institute of Health suggested social distancing and agreed that the broader community of the country’s elderly is a problem. In the meantime, several other nations, including the US, have recommended that travel to Italy should be avoided temporarily, unless necessary.
The Italian government has declared the closing (quarantine) of the impacted areas in the northern region of the nation so as not to spread to the rest of the world. Italy has declared the immediate suspension of all to-and-fro air travel with China following coronavirus discovery by a Chinese tourist to Italy. Italian airlines, like Ryan Air, have begun introducing protective steps and have begun calling for the declaration forms to be submitted by passengers flying to Poland, Slovakia and Lithuania.
The Italian government first declined to permit fans to compete in sporting activities until early April to prevent the potential transmission of coronavirus. The step ensured players of health and stopped event cancellations because of coronavirus fears. Two days of the declaration, the government cancelled all athletic activities owing to the emergence of the outbreak asking for an emergency. Sports activities in Veneto, Lombardy and Emilia-Romagna, which recorded coronavirus-positive infections, were confirmed to be temporarily suspended. Schools and colleges in Italy have also been forced to shut down.
Iran announced the first recorded cases of SARS-CoV-2 infection on 19 February when, as per the Medical Education and Ministry of Health, two persons died later that day. The Ministry of Islamic Culture and Guidance has declared the cancellation of all concerts and other cultural activities for one week. The Medical Education and Ministry of Health has also declared the closing of universities, higher education colleges and schools in many cities and regions. The Department of Sports and Culture has taken action to suspend athletic activities, including football matches [ 52 ].
On 2 March 2020, the government revealed plans to train about 300,000 troops and volunteers to fight the outbreak of the epidemic, and also send robots and water cannons to clean the cities. The State also developed an initiative and a webpage to counter the epidemic. On 9 March 2020, nearly 70,000 inmates were immediately released from jail owing to the epidemic, presumably to prevent the further dissemination of the disease inside jails. The Revolutionary Guards declared a campaign on 13 March 2020 to clear highways, stores and public areas in Iran. President Hassan Rouhani stated on 26 February 2020 that there were no arrangements to quarantine areas impacted by the epidemic and only persons should be quarantined. The temples of Shia in Qom stayed open to pilgrims.
South Korea
On 20 January, South Korea announced its first occurrence. There was a large rise in cases on 20 February, possibly due to the meeting in Daegu of a progressive faith community recognized as the Shincheonji Church of Christ. Any citizens believed that the hospital was propagating the disease. As of 22 February, 1,261 of the 9,336 members of the church registered symptoms. A petition was distributed calling for the abolition of the church. More than 2,000 verified cases were registered on 28 February, increasing to 3,150 on 29 February [ 53 ].
Several educational establishments have been partially closing down, including hundreds of kindergartens in Daegu and many primary schools in Seoul. As of 18 February, several South Korean colleges had confirmed intentions to delay the launch of the spring semester. That included 155 institutions deciding to postpone the start of the semester by two weeks until 16 March, and 22 institutions deciding to delay the start of the semester by one week until 9 March. Also, on 23 February 2020, all primary schools, kindergartens, middle schools and secondary schools were declared to postpone the start of the semester from 2 March to 9 March.
South Korea’s economy is expected to expand by 1.9%, down from 2.1%. The State has given 136.7 billion won funding to local councils. The State has also coordinated the purchase of masks and other sanitary supplies. Entertainment Company SM Entertainment is confirmed to have contributed five hundred million won in attempts to fight the disease.
In the kpop industry, the widespread dissemination of coronavirus within South Korea has contributed to the cancellation or postponement of concerts and other programmes for kpop activities inside and outside South Korea. For instance, circumstances such as the cancellation of the remaining Asian dates and the European leg for the Seventeen’s Ode To You Tour on 9 February 2020 and the cancellation of all Seoul dates for the BTS Soul Tour Map. As of 15 March, a maximum of 136 countries and regions provided entry restrictions and/or expired visas for passengers from South Korea.
The overall reported cases of coronavirus rose significantly in France on 12 March. The areas with reported cases include Paris, Amiens, Bordeaux and Eastern Haute-Savoie. The first coronaviral death happened in France on 15 February, marking it the first death in Europe. The second death of a 60-year-old French national in Paris was announced on 26 February [ 54 ].
On February 28, fashion designer Agnès B. (not to be mistaken with Agnès Buzyn) cancelled fashion shows at the Paris Fashion Week, expected to continue until 3 March. On a subsequent day, the Paris half-marathon, planned for Sunday 1 March with 44,000 entrants, was postponed as one of a series of steps declared by Health Minister Olivier Véran.
On 13 March, the Ligue de Football Professional disbanded Ligue 1 and Ligue 2 (France’s tier two professional divisions) permanently due to safety threats.
Germany has a popular Regional Pandemic Strategy detailing the roles and activities of the health care system participants in the case of a significant outbreak. Epidemic surveillance is carried out by the federal government, like the Robert Koch Center, and by the German governments. The German States have their preparations for an outbreak. The regional strategy for the treatment of the current coronavirus epidemic was expanded by March 2020. Four primary goals are contained in this plan: (1) to minimize mortality and morbidity; (2) to guarantee the safety of sick persons; (3) to protect vital health services and (4) to offer concise and reliable reports to decision-makers, the media and the public [ 55 ].
The programme has three phases that may potentially overlap: (1) isolation (situation of individual cases and clusters), (2) safety (situation of further dissemination of pathogens and suspected causes of infection), (3) prevention (situation of widespread infection). So far, Germany has not set up border controls or common health condition tests at airports. Instead, while at the isolation stage-health officials are concentrating on recognizing contact individuals that are subject to specific quarantine and are tracked and checked. Specific quarantine is regulated by municipal health authorities. By doing so, the officials are seeking to hold the chains of infection small, contributing to decreased clusters. At the safety stage, the policy should shift to prevent susceptible individuals from being harmed by direct action. By the end of the day, the prevention process should aim to prevent cycles of acute treatment to retain emergency facilities.
United States
The very first case of coronavirus in the United States was identified in Washington on 21 January 2020 by an individual who flew to Wuhan and returned to the United States. The second case was recorded in Illinois by another individual who had travelled to Wuhan. Some of the regions with reported novel coronavirus infections in the US are California, Arizona, Connecticut, Illinois, Texas, Wisconsin and Washington [ 56 ].
As the epidemic increased, requests for domestic air travel decreased dramatically. By 4 March, U.S. carriers, like United Airlines and JetBlue Airways, started growing their domestic flight schedules, providing generous unpaid leave to workers and suspending recruits.
A significant number of universities and colleges cancelled classes and reopened dormitories in response to the epidemic, like Cornell University, Harvard University and the University of South Carolina.
On 3 March 2020, the Federal Reserve reduced its goal interest rate from 1.75% to 1.25%, the biggest emergency rate cut following the 2008 global financial crash, in combat the effect of the recession on the American economy. In February 2020, US businesses, including Apple Inc. and Microsoft, started to reduce sales projections due to supply chain delays in China caused by the COVID-19.
The pandemic, together with the subsequent financial market collapse, also contributed to greater criticism of the crisis in the United States. Researchers disagree about when a recession is likely to take effect, with others suggesting that it is not unavoidable, while some claim that the world might already be in recession. On 3 March, Federal Reserve Chairman Jerome Powell reported a 0.5% (50 basis point) interest rate cut from the coronavirus in the context of the evolving threats to economic growth.
When ‘social distance’ penetrated the national lexicon, disaster response officials promoted the cancellation of broad events to slow down the risk of infection. Technical conferences like E3 2020, Apple Inc.’s Worldwide Developers Conference (WWDC), Google I/O, Facebook F8, and Cloud Next and Microsoft’s MVP Conference have been either having replaced or cancelled in-person events with internet streaming events.
On February 29, the American Physical Society postponed its annual March gathering, planned for March 2–6 in Denver, Colorado, even though most of the more than 11,000 physicist attendees already had arrived and engaged in the pre-conference day activities. On March 6, the annual South to Southwest (SXSW) seminar and festival planned to take place from March 13–22 in Austin, Texas, was postponed after the city council announced a local disaster and forced conferences to be shut down for the first time in 34 years.
Four of North America’s major professional sports leagues—the National Hockey League (NHL), National Basketball Association (NBA), Major League Soccer (MLS) and Major League Baseball (MLB) —jointly declared on March 9 that they would all limit the media access to player accommodations (such as locker rooms) to control probable exposure.
Emergency Funding to Fight the COVID-19
COVID-19 pandemic has become a common international concern. Different countries are donating funds to fight against it [ 57 – 60 ]. Some of them are mentioned here.
China has allocated about 110.48 billion yuan ($15.93 billion) in coronavirus-related funding.
Foreign Minister Mohammad Javad Zarif said that Iran has requested the International Monetary Fund (IMF) of about $5 billion in emergency funding to help to tackle the coronavirus epidemic that has struck the Islamic Republic hard.
President Donald Trump approved the Emergency Supplementary Budget Bill to support the US response to a novel coronavirus epidemic. The budget plan would include about $8.3 billion in discretionary funding to local health authorities to promote vaccine research for production. Trump originally requested just about $2 billion to combat the epidemic, but Congress quadrupled the number in its version of the bill. Mr. Trump formally announced a national emergency that he claimed it will give states and territories access to up to about $50 billion in federal funding to tackle the spread of the coronavirus outbreak.
California politicians approved a plan to donate about $1 billion on the state’s emergency medical responses as it readies hospitals to fight an expected attack of patients because of the COVID-19 pandemic. The plans, drawn up rapidly in reaction to the dramatic rise in reported cases of the virus, would include the requisite funds to establish two new hospitals in California, with the assumption that the state may not have the resources to take care of the rise in patients. The bill calls for an immediate response of about $500 million from the State General Fund, with an additional about $500 million possible if requested.
India committed about $10 million to the COVID-19 Emergency Fund and said it was setting up a rapid response team of physicians for the South Asian Association for Regional Cooperation (Saarc) countries.
South Korea unveiled an economic stimulus package of about 11.7 trillion won ($9.8 billion) to soften the effects of the biggest coronavirus epidemic outside China as attempts to curb the disease exacerbate supply shortages and drain demand. Of the 11,7 trillion won expected, about 3.2 trillion won would cover up the budget shortfall, while an additional fiscal infusion of about 8.5 trillion won. An estimated 10.3 trillion won in government bonds will be sold this year to fund the extra expenditure. About 2.3 trillion won will be distributed to medical establishments and would support quarantine operations, with another 3.0 trillion won heading to small and medium-sized companies unable to pay salaries to their employees and child care supports.
The Swedish Parliament announced a set of initiatives costing more than 300 billion Swedish crowns ($30.94 billion) to help the economy in the view of the coronavirus pandemic. The plan contained steps like the central government paying the entire expense of the company’s sick leave during April and May, and also the high cost of compulsory redundancies owing to the crisis.
In consideration of the developing scenario, an updating of this strategy is planned to take place before the end of March and will recognize considerably greater funding demands for the country response, R&D and WHO itself.
Artificial Intelligence, Data Science and Technological Solutions Against COVID-19
These days, Artificial Intelligence (AI) takes a major role in health care. Throughout a worldwide pandemic such as the COVID-19, technology, artificial intelligence and data analytics have been crucial in helping communities cope successfully with the epidemic [ 61 – 65 ]. Through the aid of data mining and analytical modelling, medical practitioners are willing to learn more about several diseases.
Public Health Surveillance
The biggest risk of coronavirus is the level of spreading. That’s why policymakers are introducing steps like quarantines around the world because they can’t adequately monitor local outbreaks. One of the simplest measures to identify ill patients through the study of CCTV images that are still around us and to locate and separate individuals that have serious signs of the disease and who have touched and disinfected the related surfaces. Smartphone applications are often used to keep a watch on people’s activities and to assess whether or not they have come in touch with an infected human.
Remote Biosignal Measurement
Many of the signs such as temperature or heartbeat are very essential to overlook and rely entirely on the visual image that may be misleading. However, of course, we can’t prevent someone from checking their blood pressure, heart or temperature. Also, several advances in computer vision can predict pulse and blood pressure based on facial skin examination. Besides, there are several advances in computer vision that can predict pulse and blood pressure based on facial skin examination.
Access to public records has contributed to the development of dashboards that constantly track the virus. Several companies are designing large data dashboards. Face recognition and infrared temperature monitoring technologies have been mounted in all major cities. Chinese AI companies including Hanwang Technology and SenseTime have reported having established a special facial recognition system that can correctly identify people even though they are covered.
IoT and Wearables
Measurements like pulse are much more natural and easier to obtain from tracking gadgets like activity trackers and smartwatches that nearly everybody has already. Some work suggests that the study of cardiac activity and its variations from the standard will reveal early signs of influenza and, in this case, coronavirus.
Chatbots and Communication
Apart from public screening, people’s knowledge and self-assessment may also be used to track their health. If you can check your temperature and pulse every day and monitor your coughs time-to-time, you can even submit that to your record. If the symptoms are too serious, either an algorithm or a doctor remotely may prescribe a person to stay home, take several other preventive measures, or recommend a visit from the doctor.
Al Jazeera announced that China Mobile had sent text messages to state media departments, telling them about the citizens who had been affected. The communications contained all the specifics of the person’s travel history.
Tencent runs WeChat, and via it, citizens can use free online health consultation services. Chatbots have already become important connectivity platforms for transport and tourism service providers to keep passengers up-to-date with the current transport protocols and disturbances.
Social Media and Open Data
There are several people who post their health diary with total strangers via Facebook or Twitter. Such data becomes helpful for more general research about how far the epidemic has progressed. For consumer knowledge, we may even evaluate the social network group to attempt to predict what specific networks are at risk of being viral.
Canadian company BlueDot analyses far more than just social network data: for instance, global activities of more than four billion passengers on international flights per year; animal, human and insect population data; satellite environment data and relevant knowledge from health professionals and journalists, across 100,000 news posts per day covering 65 languages. This strategy was so successful that the corporation was able to alert clients about coronavirus until the World Health Organization and the Centers for Disease Control and Prevention notified the public.
Automated Diagnostics
COVID-19 has brought up another healthcare issue today: it will not scale when the number of patients increases exponentially (actually stressed doctors are always doing worse) and the rate of false-negative diagnosis remains very high. Machine learning therapies don’t get bored and scale simply by growing computing forces.
Baidu, the Chinese Internet company, has made the Lineatrfold algorithm accessible to the outbreak-fighting teams, according to the MIT Technology Review. Unlike HIV, Ebola and Influenza, COVID-19 has just one strand of RNA and it can mutate easily. The algorithm is also simpler than other algorithms that help to determine the nature of the virus. Baidu has also developed software to efficiently track large populations. It has also developed an Ai-powered infrared device that can detect a difference in the body temperature of a human. This is currently being used in Beijing’s Qinghe Railway Station to classify possibly contaminated travellers where up to 200 individuals may be checked in one minute without affecting traffic movement, reports the MIT Review.
Singapore-based Veredus Laboratories, a supplier of revolutionary molecular diagnostic tools, has currently announced the launch of the VereCoV detector package, a compact Lab-on-Chip device able to detect MERS-CoV, SARS-CoV and COVID-19, i.e. Wuhan Coronavirus, in a single study.
The VereCoV identification package is focused on VereChip technology, a Lab-on-Chip device that incorporates two important molecular biological systems, Polymerase Chain Reaction (PCR) and a microarray, which will be able to classify and distinguish within 2 h MERS-CoV, SARS-CoV and COVID-19 with high precision and responsiveness.
This is not just the medical activities of healthcare facilities that are being charged, but also the corporate and financial departments when they cope with the increase in patients. Ant Financials’ blockchain technology helps speed-up the collection of reports and decreases the number of face-to-face encounters with patients and medical personnel.
Companies like the Israeli company Sonovia are aiming to provide healthcare systems and others with face masks manufactured from their anti-pathogenic, anti-bacterial cloth that depends on metal-oxide nanoparticles.
Drug Development Research
Aside from identifying and stopping the transmission of pathogens, the need to develop vaccinations on a scale is also needed. One of the crucial things to make that possible is to consider the origin and essence of the virus. Google’s DeepMind, with their expertise in protein folding research, has rendered a jump in identifying the protein structure of the virus and making it open-source.
BenevolentAI uses AI technologies to develop medicines that will combat the most dangerous diseases in the world and is also working to promote attempts to cure coronavirus, the first time the organization has based its product on infectious diseases. Within weeks of the epidemic, it used its analytical capability to recommend new medicines that might be beneficial.
Robots are not vulnerable to the infection, and they are used to conduct other activities, like cooking meals in hospitals, doubling up as waiters in hotels, spraying disinfectants and washing, selling rice and hand sanitizers, robots are on the front lines all over to deter coronavirus spread. Robots also conduct diagnostics and thermal imaging in several hospitals. Shenzhen-based firm Multicopter uses robotics to move surgical samples. UVD robots from Blue Ocean Robotics use ultraviolet light to destroy viruses and bacteria separately. In China, Pudu Technology has introduced its robots, which are usually used in the cooking industry, to more than 40 hospitals throughout the region. According to the Reuters article, a tiny robot named Little Peanut is distributing food to passengers who have been on a flight from Singapore to Hangzhou, China, and are presently being quarantined in a hotel.
Colour Coding
Using its advanced and vast public service monitoring network, the Chinese government has collaborated with software companies Alibaba and Tencent to establish a colour-coded health ranking scheme that monitors millions of citizens every day. The mobile device was first introduced in Hangzhou with the cooperation of Alibaba. This applies three colours to people—red, green or yellow—based on their transportation and medical records. Tencent also developed related applications in the manufacturing centre of Shenzhen.
The decision of whether an individual will be quarantined or permitted in public spaces is dependent on the colour code. Citizens will sign into the system using pay wallet systems such as Alibaba’s Alipay and Ant’s wallet. Just those citizens who have been issued a green colour code will be permitted to use the QR code in public spaces at metro stations, workplaces, and other public areas. Checkpoints are in most public areas where the body temperature and the code of individual are tested. This programme is being used by more than 200 Chinese communities and will eventually be expanded nationwide.
In some of the seriously infected regions where people remain at risk of contracting the infection, drones are used to rescue. One of the easiest and quickest ways to bring emergency supplies where they need to go while on an epidemic of disease is by drone transportation. Drones carry all surgical instruments and patient samples. This saves time, improves the pace of distribution and reduces the chance of contamination of medical samples. Drones often operate QR code placards that can be checked to record health records. There are also agricultural drones distributing disinfectants in the farmland. Drones, operated by facial recognition, are often used to warn people not to leave their homes and to chide them for not using face masks. Terra Drone uses its unmanned drones to move patient samples and vaccination content at reduced risk between the Xinchang County Disease Control Center and the People’s Hospital. Drones are often used to monitor public areas, document non-compliance with quarantine laws and thermal imaging.
Autonomous Vehicles
At a period of considerable uncertainty to medical professionals and the danger to people-to-people communication, automated vehicles are proving to be of tremendous benefit in the transport of vital products, such as medications and foodstuffs. Apollo, the Baidu Autonomous Vehicle Project, has joined hands with the Neolix self-driving company to distribute food and supplies to a big hospital in Beijing. Baidu Apollo has also provided its micro-car packages and automated cloud driving systems accessible free of charge to virus-fighting organizations.
Idriverplus, a Chinese self-driving organization that runs electrical street cleaning vehicles, is also part of the project. The company’s signature trucks are used to clean hospitals.
This chapter provides an introduction to the coronavirus outbreak (COVID-19). A brief history of this virus along with the symptoms are reported in this chapter. Then the comparison between COVID-19 and other plagues like seasonal influenza, bird flu (H5N1 and H7N9), Ebola epidemic, camel flu (MERS), swine flu (H1N1), severe acute respiratory syndrome, Hong Kong flu (H3N2), Spanish flu and the common cold are included in this chapter. Reviews of online portal and social media like Facebook, Twitter, Google, Microsoft, Pinterest, YouTube and WhatsApp concerning COVID-19 are reported in this chapter. Also, the preventive measures and policies enforced by WHO and different countries such as China, Italy, Iran, South Korea, France, Germany and the United States for COVID-19 are included in this chapter. Emergency funding provided by different countries to fight the COVID-19 is mentioned in this chapter. Lastly, artificial intelligence, data science and technological solutions like public health surveillance, remote biosignal measurement, IoT and wearables, chatbots and communication, social media and open data, automated diagnostics, drug development research, robotics, colour coding, drones and autonomous vehicles are included in this chapter.
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- BMJ Journals
You are here
- Volume 74, Issue 11
- The COVID-19 pandemic and health inequalities
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0002-1294-6851 Clare Bambra 1 ,
- Ryan Riordan 2 ,
- John Ford 2 ,
- Fiona Matthews 1
- 1 Population Health Sciences Institute, Newcastle University Institute for Health and Society , Newcastle upon Tyne , UK
- 2 School of Clinical Medicine, Cambridge University , Cambridge , UK
- Correspondence to Clare Bambra, Population Health Sciences Institute, Faculty of Medical Sciences, Newcastle University, Newcastle upon Tyne NE1 4LP, UK; clare.bambra{at}newcastle.ac.uk
This essay examines the implications of the COVID-19 pandemic for health inequalities. It outlines historical and contemporary evidence of inequalities in pandemics—drawing on international research into the Spanish influenza pandemic of 1918, the H1N1 outbreak of 2009 and the emerging international estimates of socio-economic, ethnic and geographical inequalities in COVID-19 infection and mortality rates. It then examines how these inequalities in COVID-19 are related to existing inequalities in chronic diseases and the social determinants of health, arguing that we are experiencing a syndemic pandemic . It then explores the potential consequences for health inequalities of the lockdown measures implemented internationally as a response to the COVID-19 pandemic, focusing on the likely unequal impacts of the economic crisis. The essay concludes by reflecting on the longer-term public health policy responses needed to ensure that the COVID-19 pandemic does not increase health inequalities for future generations.
- DEPRIVATION
- Health inequalities
This article is made freely available for use in accordance with BMJ's website terms and conditions for the duration of the COVID-19 pandemic or until otherwise determined by BMJ. You may use, download and print the article for any lawful, non-commercial purpose (including text and data mining) provided that all copyright notices and trade marks are retained.
https://doi.org/10.1136/jech-2020-214401
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Twitter Clare Bambra @ProfBambra.
Funding CB is a senior investigator in the National Institute for Health Research (NIHR) ARC North East and North Cumbria, NIHR Policy Research Unit in Behavioural Science, NIHR School of Public Health Research, the UK Prevention Research Partnership SIPHER: Systems science in Public Health and Health Economics Research consortium, and the Norwegian Research Council Centre for Global Health Inequalities Research. JF is a senior investigator in the NIHR ARC East of England. FM is a senior investigator in the NIHR Policy Research Unit in Ageing and Frailty. The views expressed in this publication are those of the authors and not necessarily those of the funders.
Competing interests We have read and understood the BMJ Group policy on declaration of interests and declare the following interests: none.
Patient consent for publication Not required.
Data sharing statement Data sharing not applicable as no datasets generated and/or analysed for this study.
Provenance and peer review Not commissioned; internally peer reviewed.
Read the full text or download the PDF:

45,000+ students realised their study abroad dream with us. Take the first step today
Meet top uk universities from the comfort of your home, here’s your new year gift, one app for all your, study abroad needs, start your journey, track your progress, grow with the community and so much more.

Verification Code
An OTP has been sent to your registered mobile no. Please verify

Thanks for your comment !
Our team will review it before it's shown to our readers.

- School Education /
Essay On Covid-19: 100, 200 and 300 Words

- Updated on
- Apr 30, 2024
COVID-19, also known as the Coronavirus, is a global pandemic that has affected people all around the world. It first emerged in a lab in Wuhan, China, in late 2019 and quickly spread to countries around the world. This virus was reportedly caused by SARS-CoV-2. Since then, it has spread rapidly to many countries, causing widespread illness and impacting our lives in numerous ways. This blog talks about the details of this virus and also drafts an essay on COVID-19 in 100, 200 and 300 words for students and professionals.
Table of Contents
- 1 Essay On COVID-19 in English 100 Words
- 2 Essay On COVID-19 in 200 Words
- 3 Essay On COVID-19 in 300 Words
- 4 Short Essay on Covid-19
Essay On COVID-19 in English 100 Words
COVID-19, also known as the coronavirus, is a global pandemic. It started in late 2019 and has affected people all around the world. The virus spreads very quickly through someone’s sneeze and respiratory issues.
COVID-19 has had a significant impact on our lives, with lockdowns, travel restrictions, and changes in daily routines. To prevent the spread of COVID-19, we should wear masks, practice social distancing, and wash our hands frequently.
People should follow social distancing and other safety guidelines and also learn the tricks to be safe stay healthy and work the whole challenging time.
Also Read: National Safe Motherhood Day 2023
Essay On COVID-19 in 200 Words
COVID-19 also known as coronavirus, became a global health crisis in early 2020 and impacted mankind around the world. This virus is said to have originated in Wuhan, China in late 2019. It belongs to the coronavirus family and causes flu-like symptoms. It impacted the healthcare systems, economies and the daily lives of people all over the world.
The most crucial aspect of COVID-19 is its highly spreadable nature. It is a communicable disease that spreads through various means such as coughs from infected persons, sneezes and communication. Due to its easy transmission leading to its outbreaks, there were many measures taken by the government from all over the world such as Lockdowns, Social Distancing, and wearing masks.
There are many changes throughout the economic systems, and also in daily routines. Other measures such as schools opting for Online schooling, Remote work options available and restrictions on travel throughout the country and internationally. Subsequently, to cure and top its outbreak, the government started its vaccine campaigns, and other preventive measures.
In conclusion, COVID-19 tested the patience and resilience of the mankind. This pandemic has taught people the importance of patience, effort and humbleness.
Also Read : Essay on My Best Friend
Essay On COVID-19 in 300 Words
COVID-19, also known as the coronavirus, is a serious and contagious disease that has affected people worldwide. It was first discovered in late 2019 in Cina and then got spread in the whole world. It had a major impact on people’s life, their school, work and daily lives.
COVID-19 is primarily transmitted from person to person through respiratory droplets produced and through sneezes, and coughs of an infected person. It can spread to thousands of people because of its highly contagious nature. To cure the widespread of this virus, there are thousands of steps taken by the people and the government.
Wearing masks is one of the essential precautions to prevent the virus from spreading. Social distancing is another vital practice, which involves maintaining a safe distance from others to minimize close contact.
Very frequent handwashing is also very important to stop the spread of this virus. Proper hand hygiene can help remove any potential virus particles from our hands, reducing the risk of infection.
In conclusion, the Coronavirus has changed people’s perspective on living. It has also changed people’s way of interacting and how to live. To deal with this virus, it is very important to follow the important guidelines such as masks, social distancing and techniques to wash your hands. Getting vaccinated is also very important to go back to normal life and cure this virus completely.
Also Read: Essay on Abortion in English in 650 Words
Short Essay on Covid-19
Please find below a sample of a short essay on Covid-19 for school students:
Also Read: Essay on Women’s Day in 200 and 500 words
to write an essay on COVID-19, understand your word limit and make sure to cover all the stages and symptoms of this disease. You need to highlight all the challenges and impacts of COVID-19. Do not forget to conclude your essay with positive precautionary measures.
Writing an essay on COVID-19 in 200 words requires you to cover all the challenges, impacts and precautions of this disease. You don’t need to describe all of these factors in brief, but make sure to add as many options as your word limit allows.
The full form for COVID-19 is Corona Virus Disease of 2019.
Related Reads
Hence, we hope that this blog has assisted you in comprehending with an essay on COVID-19. For more information on such interesting topics, visit our essay writing page and follow Leverage Edu.
Simran Popli
An avid writer and a creative person. With an experience of 1.5 years content writing, Simran has worked with different areas. From medical to working in a marketing agency with different clients to Ed-tech company, the journey has been diverse. Creative, vivacious and patient are the words that describe her personality.
Leave a Reply Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Contact no. *

Connect With Us
45,000+ students realised their study abroad dream with us. take the first step today..

Resend OTP in

Need help with?
Study abroad.
UK, Canada, US & More
IELTS, GRE, GMAT & More
Scholarship, Loans & Forex
Country Preference
New Zealand
Which English test are you planning to take?
Which academic test are you planning to take.
Not Sure yet
When are you planning to take the exam?
Already booked my exam slot
Within 2 Months
Want to learn about the test
Which Degree do you wish to pursue?
When do you want to start studying abroad.
January 2025
September 2025
What is your budget to study abroad?

How would you describe this article ?
Please rate this article
We would like to hear more.
Have something on your mind?

Make your study abroad dream a reality in January 2022 with
India's Biggest Virtual University Fair

Essex Direct Admission Day
Why attend .

Don't Miss Out
- Research article
- Open access
- Published: 04 June 2021
Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews
- Israel Júnior Borges do Nascimento 1 , 2 ,
- Dónal P. O’Mathúna 3 , 4 ,
- Thilo Caspar von Groote 5 ,
- Hebatullah Mohamed Abdulazeem 6 ,
- Ishanka Weerasekara 7 , 8 ,
- Ana Marusic 9 ,
- Livia Puljak ORCID: orcid.org/0000-0002-8467-6061 10 ,
- Vinicius Tassoni Civile 11 ,
- Irena Zakarija-Grkovic 9 ,
- Tina Poklepovic Pericic 9 ,
- Alvaro Nagib Atallah 11 ,
- Santino Filoso 12 ,
- Nicola Luigi Bragazzi 13 &
- Milena Soriano Marcolino 1

On behalf of the International Network of Coronavirus Disease 2019 (InterNetCOVID-19)
BMC Infectious Diseases volume 21 , Article number: 525 ( 2021 ) Cite this article
18k Accesses
37 Citations
14 Altmetric
Metrics details
Navigating the rapidly growing body of scientific literature on the SARS-CoV-2 pandemic is challenging, and ongoing critical appraisal of this output is essential. We aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Nine databases (Medline, EMBASE, Cochrane Library, CINAHL, Web of Sciences, PDQ-Evidence, WHO’s Global Research, LILACS, and Epistemonikos) were searched from December 1, 2019, to March 24, 2020. Systematic reviews analyzing primary studies of COVID-19 were included. Two authors independently undertook screening, selection, extraction (data on clinical symptoms, prevalence, pharmacological and non-pharmacological interventions, diagnostic test assessment, laboratory, and radiological findings), and quality assessment (AMSTAR 2). A meta-analysis was performed of the prevalence of clinical outcomes.
Eighteen systematic reviews were included; one was empty (did not identify any relevant study). Using AMSTAR 2, confidence in the results of all 18 reviews was rated as “critically low”. Identified symptoms of COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%) and gastrointestinal complaints (5–9%). Severe symptoms were more common in men. Elevated C-reactive protein and lactate dehydrogenase, and slightly elevated aspartate and alanine aminotransferase, were commonly described. Thrombocytopenia and elevated levels of procalcitonin and cardiac troponin I were associated with severe disease. A frequent finding on chest imaging was uni- or bilateral multilobar ground-glass opacity. A single review investigated the impact of medication (chloroquine) but found no verifiable clinical data. All-cause mortality ranged from 0.3 to 13.9%.
Conclusions
In this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic were of questionable usefulness. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards.
Peer Review reports
The spread of the “Severe Acute Respiratory Coronavirus 2” (SARS-CoV-2), the causal agent of COVID-19, was characterized as a pandemic by the World Health Organization (WHO) in March 2020 and has triggered an international public health emergency [ 1 ]. The numbers of confirmed cases and deaths due to COVID-19 are rapidly escalating, counting in millions [ 2 ], causing massive economic strain, and escalating healthcare and public health expenses [ 3 , 4 ].
The research community has responded by publishing an impressive number of scientific reports related to COVID-19. The world was alerted to the new disease at the beginning of 2020 [ 1 ], and by mid-March 2020, more than 2000 articles had been published on COVID-19 in scholarly journals, with 25% of them containing original data [ 5 ]. The living map of COVID-19 evidence, curated by the Evidence for Policy and Practice Information and Co-ordinating Centre (EPPI-Centre), contained more than 40,000 records by February 2021 [ 6 ]. More than 100,000 records on PubMed were labeled as “SARS-CoV-2 literature, sequence, and clinical content” by February 2021 [ 7 ].
Due to publication speed, the research community has voiced concerns regarding the quality and reproducibility of evidence produced during the COVID-19 pandemic, warning of the potential damaging approach of “publish first, retract later” [ 8 ]. It appears that these concerns are not unfounded, as it has been reported that COVID-19 articles were overrepresented in the pool of retracted articles in 2020 [ 9 ]. These concerns about inadequate evidence are of major importance because they can lead to poor clinical practice and inappropriate policies [ 10 ].
Systematic reviews are a cornerstone of today’s evidence-informed decision-making. By synthesizing all relevant evidence regarding a particular topic, systematic reviews reflect the current scientific knowledge. Systematic reviews are considered to be at the highest level in the hierarchy of evidence and should be used to make informed decisions. However, with high numbers of systematic reviews of different scope and methodological quality being published, overviews of multiple systematic reviews that assess their methodological quality are essential [ 11 , 12 , 13 ]. An overview of systematic reviews helps identify and organize the literature and highlights areas of priority in decision-making.
In this overview of systematic reviews, we aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Methodology
Research question.
This overview’s primary objective was to summarize and critically appraise systematic reviews that assessed any type of primary clinical data from patients infected with SARS-CoV-2. Our research question was purposefully broad because we wanted to analyze as many systematic reviews as possible that were available early following the COVID-19 outbreak.
Study design
We conducted an overview of systematic reviews. The idea for this overview originated in a protocol for a systematic review submitted to PROSPERO (CRD42020170623), which indicated a plan to conduct an overview.
Overviews of systematic reviews use explicit and systematic methods for searching and identifying multiple systematic reviews addressing related research questions in the same field to extract and analyze evidence across important outcomes. Overviews of systematic reviews are in principle similar to systematic reviews of interventions, but the unit of analysis is a systematic review [ 14 , 15 , 16 ].
We used the overview methodology instead of other evidence synthesis methods to allow us to collate and appraise multiple systematic reviews on this topic, and to extract and analyze their results across relevant topics [ 17 ]. The overview and meta-analysis of systematic reviews allowed us to investigate the methodological quality of included studies, summarize results, and identify specific areas of available or limited evidence, thereby strengthening the current understanding of this novel disease and guiding future research [ 13 ].
A reporting guideline for overviews of reviews is currently under development, i.e., Preferred Reporting Items for Overviews of Reviews (PRIOR) [ 18 ]. As the PRIOR checklist is still not published, this study was reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 statement [ 19 ]. The methodology used in this review was adapted from the Cochrane Handbook for Systematic Reviews of Interventions and also followed established methodological considerations for analyzing existing systematic reviews [ 14 ].
Approval of a research ethics committee was not necessary as the study analyzed only publicly available articles.
Eligibility criteria
Systematic reviews were included if they analyzed primary data from patients infected with SARS-CoV-2 as confirmed by RT-PCR or another pre-specified diagnostic technique. Eligible reviews covered all topics related to COVID-19 including, but not limited to, those that reported clinical symptoms, diagnostic methods, therapeutic interventions, laboratory findings, or radiological results. Both full manuscripts and abbreviated versions, such as letters, were eligible.
No restrictions were imposed on the design of the primary studies included within the systematic reviews, the last search date, whether the review included meta-analyses or language. Reviews related to SARS-CoV-2 and other coronaviruses were eligible, but from those reviews, we analyzed only data related to SARS-CoV-2.
No consensus definition exists for a systematic review [ 20 ], and debates continue about the defining characteristics of a systematic review [ 21 ]. Cochrane’s guidance for overviews of reviews recommends setting pre-established criteria for making decisions around inclusion [ 14 ]. That is supported by a recent scoping review about guidance for overviews of systematic reviews [ 22 ].
Thus, for this study, we defined a systematic review as a research report which searched for primary research studies on a specific topic using an explicit search strategy, had a detailed description of the methods with explicit inclusion criteria provided, and provided a summary of the included studies either in narrative or quantitative format (such as a meta-analysis). Cochrane and non-Cochrane systematic reviews were considered eligible for inclusion, with or without meta-analysis, and regardless of the study design, language restriction and methodology of the included primary studies. To be eligible for inclusion, reviews had to be clearly analyzing data related to SARS-CoV-2 (associated or not with other viruses). We excluded narrative reviews without those characteristics as these are less likely to be replicable and are more prone to bias.
Scoping reviews and rapid reviews were eligible for inclusion in this overview if they met our pre-defined inclusion criteria noted above. We included reviews that addressed SARS-CoV-2 and other coronaviruses if they reported separate data regarding SARS-CoV-2.
Information sources
Nine databases were searched for eligible records published between December 1, 2019, and March 24, 2020: Cochrane Database of Systematic Reviews via Cochrane Library, PubMed, EMBASE, CINAHL (Cumulative Index to Nursing and Allied Health Literature), Web of Sciences, LILACS (Latin American and Caribbean Health Sciences Literature), PDQ-Evidence, WHO’s Global Research on Coronavirus Disease (COVID-19), and Epistemonikos.
The comprehensive search strategy for each database is provided in Additional file 1 and was designed and conducted in collaboration with an information specialist. All retrieved records were primarily processed in EndNote, where duplicates were removed, and records were then imported into the Covidence platform [ 23 ]. In addition to database searches, we screened reference lists of reviews included after screening records retrieved via databases.
Study selection
All searches, screening of titles and abstracts, and record selection, were performed independently by two investigators using the Covidence platform [ 23 ]. Articles deemed potentially eligible were retrieved for full-text screening carried out independently by two investigators. Discrepancies at all stages were resolved by consensus. During the screening, records published in languages other than English were translated by a native/fluent speaker.
Data collection process
We custom designed a data extraction table for this study, which was piloted by two authors independently. Data extraction was performed independently by two authors. Conflicts were resolved by consensus or by consulting a third researcher.
We extracted the following data: article identification data (authors’ name and journal of publication), search period, number of databases searched, population or settings considered, main results and outcomes observed, and number of participants. From Web of Science (Clarivate Analytics, Philadelphia, PA, USA), we extracted journal rank (quartile) and Journal Impact Factor (JIF).
We categorized the following as primary outcomes: all-cause mortality, need for and length of mechanical ventilation, length of hospitalization (in days), admission to intensive care unit (yes/no), and length of stay in the intensive care unit.
The following outcomes were categorized as exploratory: diagnostic methods used for detection of the virus, male to female ratio, clinical symptoms, pharmacological and non-pharmacological interventions, laboratory findings (full blood count, liver enzymes, C-reactive protein, d-dimer, albumin, lipid profile, serum electrolytes, blood vitamin levels, glucose levels, and any other important biomarkers), and radiological findings (using radiography, computed tomography, magnetic resonance imaging or ultrasound).
We also collected data on reporting guidelines and requirements for the publication of systematic reviews and meta-analyses from journal websites where included reviews were published.
Quality assessment in individual reviews
Two researchers independently assessed the reviews’ quality using the “A MeaSurement Tool to Assess Systematic Reviews 2 (AMSTAR 2)”. We acknowledge that the AMSTAR 2 was created as “a critical appraisal tool for systematic reviews that include randomized or non-randomized studies of healthcare interventions, or both” [ 24 ]. However, since AMSTAR 2 was designed for systematic reviews of intervention trials, and we included additional types of systematic reviews, we adjusted some AMSTAR 2 ratings and reported these in Additional file 2 .
Adherence to each item was rated as follows: yes, partial yes, no, or not applicable (such as when a meta-analysis was not conducted). The overall confidence in the results of the review is rated as “critically low”, “low”, “moderate” or “high”, according to the AMSTAR 2 guidance based on seven critical domains, which are items 2, 4, 7, 9, 11, 13, 15 as defined by AMSTAR 2 authors [ 24 ]. We reported our adherence ratings for transparency of our decision with accompanying explanations, for each item, in each included review.
One of the included systematic reviews was conducted by some members of this author team [ 25 ]. This review was initially assessed independently by two authors who were not co-authors of that review to prevent the risk of bias in assessing this study.
Synthesis of results
For data synthesis, we prepared a table summarizing each systematic review. Graphs illustrating the mortality rate and clinical symptoms were created. We then prepared a narrative summary of the methods, findings, study strengths, and limitations.
For analysis of the prevalence of clinical outcomes, we extracted data on the number of events and the total number of patients to perform proportional meta-analysis using RStudio© software, with the “meta” package (version 4.9–6), using the “metaprop” function for reviews that did not perform a meta-analysis, excluding case studies because of the absence of variance. For reviews that did not perform a meta-analysis, we presented pooled results of proportions with their respective confidence intervals (95%) by the inverse variance method with a random-effects model, using the DerSimonian-Laird estimator for τ 2 . We adjusted data using Freeman-Tukey double arcosen transformation. Confidence intervals were calculated using the Clopper-Pearson method for individual studies. We created forest plots using the RStudio© software, with the “metafor” package (version 2.1–0) and “forest” function.
Managing overlapping systematic reviews
Some of the included systematic reviews that address the same or similar research questions may include the same primary studies in overviews. Including such overlapping reviews may introduce bias when outcome data from the same primary study are included in the analyses of an overview multiple times. Thus, in summaries of evidence, multiple-counting of the same outcome data will give data from some primary studies too much influence [ 14 ]. In this overview, we did not exclude overlapping systematic reviews because, according to Cochrane’s guidance, it may be appropriate to include all relevant reviews’ results if the purpose of the overview is to present and describe the current body of evidence on a topic [ 14 ]. To avoid any bias in summary estimates associated with overlapping reviews, we generated forest plots showing data from individual systematic reviews, but the results were not pooled because some primary studies were included in multiple reviews.
Our search retrieved 1063 publications, of which 175 were duplicates. Most publications were excluded after the title and abstract analysis ( n = 860). Among the 28 studies selected for full-text screening, 10 were excluded for the reasons described in Additional file 3 , and 18 were included in the final analysis (Fig. 1 ) [ 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 ]. Reference list screening did not retrieve any additional systematic reviews.
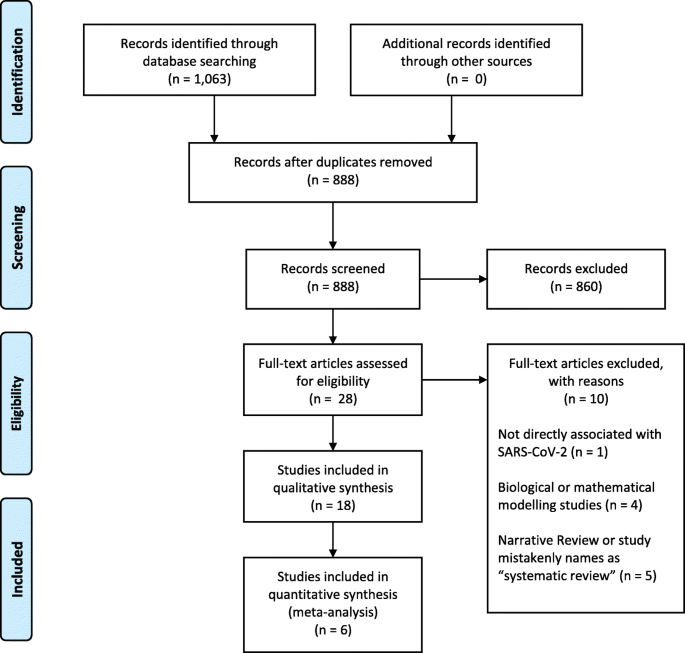
PRISMA flow diagram
Characteristics of included reviews
Summary features of 18 systematic reviews are presented in Table 1 . They were published in 14 different journals. Only four of these journals had specific requirements for systematic reviews (with or without meta-analysis): European Journal of Internal Medicine, Journal of Clinical Medicine, Ultrasound in Obstetrics and Gynecology, and Clinical Research in Cardiology . Two journals reported that they published only invited reviews ( Journal of Medical Virology and Clinica Chimica Acta ). Three systematic reviews in our study were published as letters; one was labeled as a scoping review and another as a rapid review (Table 2 ).
All reviews were published in English, in first quartile (Q1) journals, with JIF ranging from 1.692 to 6.062. One review was empty, meaning that its search did not identify any relevant studies; i.e., no primary studies were included [ 36 ]. The remaining 17 reviews included 269 unique studies; the majority ( N = 211; 78%) were included in only a single review included in our study (range: 1 to 12). Primary studies included in the reviews were published between December 2019 and March 18, 2020, and comprised case reports, case series, cohorts, and other observational studies. We found only one review that included randomized clinical trials [ 38 ]. In the included reviews, systematic literature searches were performed from 2019 (entire year) up to March 9, 2020. Ten systematic reviews included meta-analyses. The list of primary studies found in the included systematic reviews is shown in Additional file 4 , as well as the number of reviews in which each primary study was included.
Population and study designs
Most of the reviews analyzed data from patients with COVID-19 who developed pneumonia, acute respiratory distress syndrome (ARDS), or any other correlated complication. One review aimed to evaluate the effectiveness of using surgical masks on preventing transmission of the virus [ 36 ], one review was focused on pediatric patients [ 34 ], and one review investigated COVID-19 in pregnant women [ 37 ]. Most reviews assessed clinical symptoms, laboratory findings, or radiological results.
Systematic review findings
The summary of findings from individual reviews is shown in Table 2 . Overall, all-cause mortality ranged from 0.3 to 13.9% (Fig. 2 ).
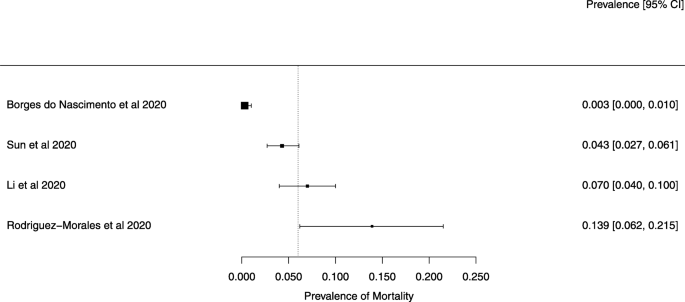
A meta-analysis of the prevalence of mortality
Clinical symptoms
Seven reviews described the main clinical manifestations of COVID-19 [ 26 , 28 , 29 , 34 , 35 , 39 , 41 ]. Three of them provided only a narrative discussion of symptoms [ 26 , 34 , 35 ]. In the reviews that performed a statistical analysis of the incidence of different clinical symptoms, symptoms in patients with COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%), gastrointestinal disorders, such as diarrhea, nausea or vomiting (5.0–9.0%), and others (including, in one study only: dizziness 12.1%) (Figs. 3 , 4 , 5 , 6 , 7 , 8 and 9 ). Three reviews assessed cough with and without sputum together; only one review assessed sputum production itself (28.5%).
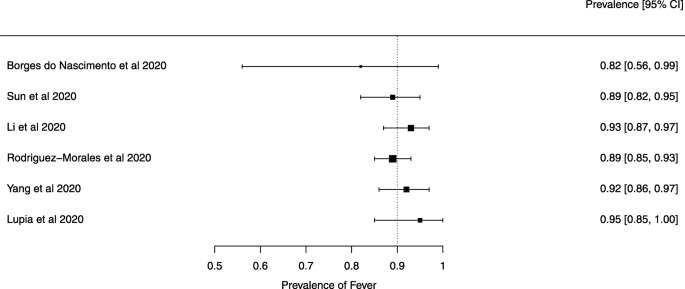
A meta-analysis of the prevalence of fever
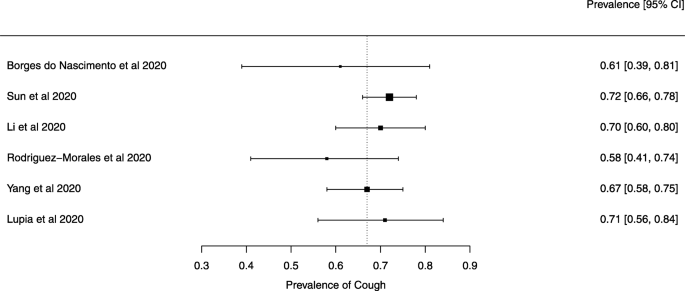
A meta-analysis of the prevalence of cough
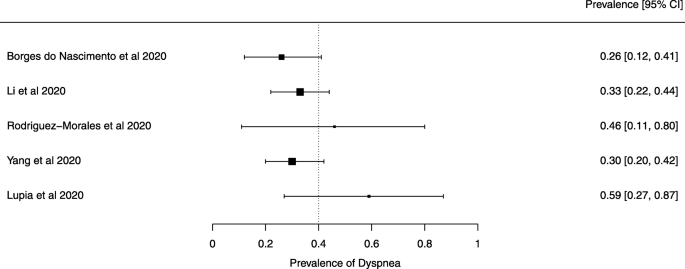
A meta-analysis of the prevalence of dyspnea
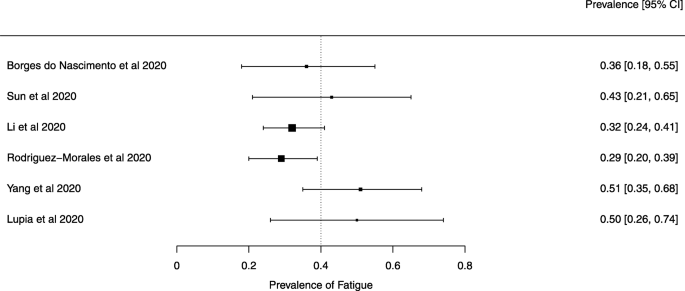
A meta-analysis of the prevalence of fatigue or myalgia
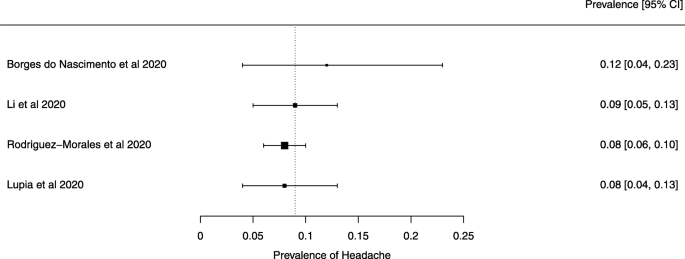
A meta-analysis of the prevalence of headache
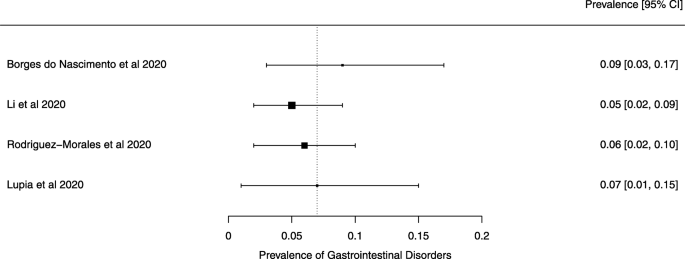
A meta-analysis of the prevalence of gastrointestinal disorders
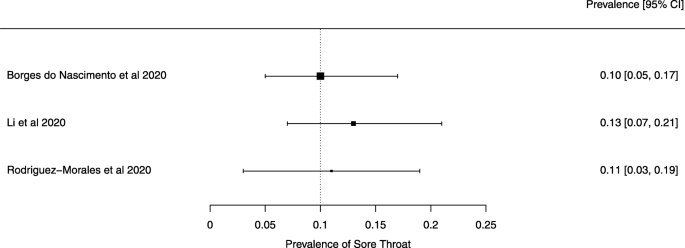
A meta-analysis of the prevalence of sore throat
Diagnostic aspects
Three reviews described methodologies, protocols, and tools used for establishing the diagnosis of COVID-19 [ 26 , 34 , 38 ]. The use of respiratory swabs (nasal or pharyngeal) or blood specimens to assess the presence of SARS-CoV-2 nucleic acid using RT-PCR assays was the most commonly used diagnostic method mentioned in the included studies. These diagnostic tests have been widely used, but their precise sensitivity and specificity remain unknown. One review included a Chinese study with clinical diagnosis with no confirmation of SARS-CoV-2 infection (patients were diagnosed with COVID-19 if they presented with at least two symptoms suggestive of COVID-19, together with laboratory and chest radiography abnormalities) [ 34 ].
Therapeutic possibilities
Pharmacological and non-pharmacological interventions (supportive therapies) used in treating patients with COVID-19 were reported in five reviews [ 25 , 27 , 34 , 35 , 38 ]. Antivirals used empirically for COVID-19 treatment were reported in seven reviews [ 25 , 27 , 34 , 35 , 37 , 38 , 41 ]; most commonly used were protease inhibitors (lopinavir, ritonavir, darunavir), nucleoside reverse transcriptase inhibitor (tenofovir), nucleotide analogs (remdesivir, galidesivir, ganciclovir), and neuraminidase inhibitors (oseltamivir). Umifenovir, a membrane fusion inhibitor, was investigated in two studies [ 25 , 35 ]. Possible supportive interventions analyzed were different types of oxygen supplementation and breathing support (invasive or non-invasive ventilation) [ 25 ]. The use of antibiotics, both empirically and to treat secondary pneumonia, was reported in six studies [ 25 , 26 , 27 , 34 , 35 , 38 ]. One review specifically assessed evidence on the efficacy and safety of the anti-malaria drug chloroquine [ 27 ]. It identified 23 ongoing trials investigating the potential of chloroquine as a therapeutic option for COVID-19, but no verifiable clinical outcomes data. The use of mesenchymal stem cells, antifungals, and glucocorticoids were described in four reviews [ 25 , 34 , 35 , 38 ].
Laboratory and radiological findings
Of the 18 reviews included in this overview, eight analyzed laboratory parameters in patients with COVID-19 [ 25 , 29 , 30 , 32 , 33 , 34 , 35 , 39 ]; elevated C-reactive protein levels, associated with lymphocytopenia, elevated lactate dehydrogenase, as well as slightly elevated aspartate and alanine aminotransferase (AST, ALT) were commonly described in those eight reviews. Lippi et al. assessed cardiac troponin I (cTnI) [ 25 ], procalcitonin [ 32 ], and platelet count [ 33 ] in COVID-19 patients. Elevated levels of procalcitonin [ 32 ] and cTnI [ 30 ] were more likely to be associated with a severe disease course (requiring intensive care unit admission and intubation). Furthermore, thrombocytopenia was frequently observed in patients with complicated COVID-19 infections [ 33 ].
Chest imaging (chest radiography and/or computed tomography) features were assessed in six reviews, all of which described a frequent pattern of local or bilateral multilobar ground-glass opacity [ 25 , 34 , 35 , 39 , 40 , 41 ]. Those six reviews showed that septal thickening, bronchiectasis, pleural and cardiac effusions, halo signs, and pneumothorax were observed in patients suffering from COVID-19.
Quality of evidence in individual systematic reviews
Table 3 shows the detailed results of the quality assessment of 18 systematic reviews, including the assessment of individual items and summary assessment. A detailed explanation for each decision in each review is available in Additional file 5 .
Using AMSTAR 2 criteria, confidence in the results of all 18 reviews was rated as “critically low” (Table 3 ). Common methodological drawbacks were: omission of prospective protocol submission or publication; use of inappropriate search strategy: lack of independent and dual literature screening and data-extraction (or methodology unclear); absence of an explanation for heterogeneity among the studies included; lack of reasons for study exclusion (or rationale unclear).
Risk of bias assessment, based on a reported methodological tool, and quality of evidence appraisal, in line with the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method, were reported only in one review [ 25 ]. Five reviews presented a table summarizing bias, using various risk of bias tools [ 25 , 29 , 39 , 40 , 41 ]. One review analyzed “study quality” [ 37 ]. One review mentioned the risk of bias assessment in the methodology but did not provide any related analysis [ 28 ].
This overview of systematic reviews analyzed the first 18 systematic reviews published after the onset of the COVID-19 pandemic, up to March 24, 2020, with primary studies involving more than 60,000 patients. Using AMSTAR-2, we judged that our confidence in all those reviews was “critically low”. Ten reviews included meta-analyses. The reviews presented data on clinical manifestations, laboratory and radiological findings, and interventions. We found no systematic reviews on the utility of diagnostic tests.
Symptoms were reported in seven reviews; most of the patients had a fever, cough, dyspnea, myalgia or muscle fatigue, and gastrointestinal disorders such as diarrhea, nausea, or vomiting. Olfactory dysfunction (anosmia or dysosmia) has been described in patients infected with COVID-19 [ 43 ]; however, this was not reported in any of the reviews included in this overview. During the SARS outbreak in 2002, there were reports of impairment of the sense of smell associated with the disease [ 44 , 45 ].
The reported mortality rates ranged from 0.3 to 14% in the included reviews. Mortality estimates are influenced by the transmissibility rate (basic reproduction number), availability of diagnostic tools, notification policies, asymptomatic presentations of the disease, resources for disease prevention and control, and treatment facilities; variability in the mortality rate fits the pattern of emerging infectious diseases [ 46 ]. Furthermore, the reported cases did not consider asymptomatic cases, mild cases where individuals have not sought medical treatment, and the fact that many countries had limited access to diagnostic tests or have implemented testing policies later than the others. Considering the lack of reviews assessing diagnostic testing (sensitivity, specificity, and predictive values of RT-PCT or immunoglobulin tests), and the preponderance of studies that assessed only symptomatic individuals, considerable imprecision around the calculated mortality rates existed in the early stage of the COVID-19 pandemic.
Few reviews included treatment data. Those reviews described studies considered to be at a very low level of evidence: usually small, retrospective studies with very heterogeneous populations. Seven reviews analyzed laboratory parameters; those reviews could have been useful for clinicians who attend patients suspected of COVID-19 in emergency services worldwide, such as assessing which patients need to be reassessed more frequently.
All systematic reviews scored poorly on the AMSTAR 2 critical appraisal tool for systematic reviews. Most of the original studies included in the reviews were case series and case reports, impacting the quality of evidence. Such evidence has major implications for clinical practice and the use of these reviews in evidence-based practice and policy. Clinicians, patients, and policymakers can only have the highest confidence in systematic review findings if high-quality systematic review methodologies are employed. The urgent need for information during a pandemic does not justify poor quality reporting.
We acknowledge that there are numerous challenges associated with analyzing COVID-19 data during a pandemic [ 47 ]. High-quality evidence syntheses are needed for decision-making, but each type of evidence syntheses is associated with its inherent challenges.
The creation of classic systematic reviews requires considerable time and effort; with massive research output, they quickly become outdated, and preparing updated versions also requires considerable time. A recent study showed that updates of non-Cochrane systematic reviews are published a median of 5 years after the publication of the previous version [ 48 ].
Authors may register a review and then abandon it [ 49 ], but the existence of a public record that is not updated may lead other authors to believe that the review is still ongoing. A quarter of Cochrane review protocols remains unpublished as completed systematic reviews 8 years after protocol publication [ 50 ].
Rapid reviews can be used to summarize the evidence, but they involve methodological sacrifices and simplifications to produce information promptly, with inconsistent methodological approaches [ 51 ]. However, rapid reviews are justified in times of public health emergencies, and even Cochrane has resorted to publishing rapid reviews in response to the COVID-19 crisis [ 52 ]. Rapid reviews were eligible for inclusion in this overview, but only one of the 18 reviews included in this study was labeled as a rapid review.
Ideally, COVID-19 evidence would be continually summarized in a series of high-quality living systematic reviews, types of evidence synthesis defined as “ a systematic review which is continually updated, incorporating relevant new evidence as it becomes available ” [ 53 ]. However, conducting living systematic reviews requires considerable resources, calling into question the sustainability of such evidence synthesis over long periods [ 54 ].
Research reports about COVID-19 will contribute to research waste if they are poorly designed, poorly reported, or simply not necessary. In principle, systematic reviews should help reduce research waste as they usually provide recommendations for further research that is needed or may advise that sufficient evidence exists on a particular topic [ 55 ]. However, systematic reviews can also contribute to growing research waste when they are not needed, or poorly conducted and reported. Our present study clearly shows that most of the systematic reviews that were published early on in the COVID-19 pandemic could be categorized as research waste, as our confidence in their results is critically low.
Our study has some limitations. One is that for AMSTAR 2 assessment we relied on information available in publications; we did not attempt to contact study authors for clarifications or additional data. In three reviews, the methodological quality appraisal was challenging because they were published as letters, or labeled as rapid communications. As a result, various details about their review process were not included, leading to AMSTAR 2 questions being answered as “not reported”, resulting in low confidence scores. Full manuscripts might have provided additional information that could have led to higher confidence in the results. In other words, low scores could reflect incomplete reporting, not necessarily low-quality review methods. To make their review available more rapidly and more concisely, the authors may have omitted methodological details. A general issue during a crisis is that speed and completeness must be balanced. However, maintaining high standards requires proper resourcing and commitment to ensure that the users of systematic reviews can have high confidence in the results.
Furthermore, we used adjusted AMSTAR 2 scoring, as the tool was designed for critical appraisal of reviews of interventions. Some reviews may have received lower scores than actually warranted in spite of these adjustments.
Another limitation of our study may be the inclusion of multiple overlapping reviews, as some included reviews included the same primary studies. According to the Cochrane Handbook, including overlapping reviews may be appropriate when the review’s aim is “ to present and describe the current body of systematic review evidence on a topic ” [ 12 ], which was our aim. To avoid bias with summarizing evidence from overlapping reviews, we presented the forest plots without summary estimates. The forest plots serve to inform readers about the effect sizes for outcomes that were reported in each review.
Several authors from this study have contributed to one of the reviews identified [ 25 ]. To reduce the risk of any bias, two authors who did not co-author the review in question initially assessed its quality and limitations.
Finally, we note that the systematic reviews included in our overview may have had issues that our analysis did not identify because we did not analyze their primary studies to verify the accuracy of the data and information they presented. We give two examples to substantiate this possibility. Lovato et al. wrote a commentary on the review of Sun et al. [ 41 ], in which they criticized the authors’ conclusion that sore throat is rare in COVID-19 patients [ 56 ]. Lovato et al. highlighted that multiple studies included in Sun et al. did not accurately describe participants’ clinical presentations, warning that only three studies clearly reported data on sore throat [ 56 ].
In another example, Leung [ 57 ] warned about the review of Li, L.Q. et al. [ 29 ]: “ it is possible that this statistic was computed using overlapped samples, therefore some patients were double counted ”. Li et al. responded to Leung that it is uncertain whether the data overlapped, as they used data from published articles and did not have access to the original data; they also reported that they requested original data and that they plan to re-do their analyses once they receive them; they also urged readers to treat the data with caution [ 58 ]. This points to the evolving nature of evidence during a crisis.
Our study’s strength is that this overview adds to the current knowledge by providing a comprehensive summary of all the evidence synthesis about COVID-19 available early after the onset of the pandemic. This overview followed strict methodological criteria, including a comprehensive and sensitive search strategy and a standard tool for methodological appraisal of systematic reviews.
In conclusion, in this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all the reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic could be categorized as research waste. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards to provide patients, clinicians, and decision-makers trustworthy evidence.
Availability of data and materials
All data collected and analyzed within this study are available from the corresponding author on reasonable request.
World Health Organization. Timeline - COVID-19: Available at: https://www.who.int/news/item/29-06-2020-covidtimeline . Accessed 1 June 2021.
COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available at: https://coronavirus.jhu.edu/map.html . Accessed 1 June 2021.
Anzai A, Kobayashi T, Linton NM, Kinoshita R, Hayashi K, Suzuki A, et al. Assessing the Impact of Reduced Travel on Exportation Dynamics of Novel Coronavirus Infection (COVID-19). J Clin Med. 2020;9(2):601.
Chinazzi M, Davis JT, Ajelli M, Gioannini C, Litvinova M, Merler S, et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368(6489):395–400. https://doi.org/10.1126/science.aba9757 .
Article CAS PubMed PubMed Central Google Scholar
Fidahic M, Nujic D, Runjic R, Civljak M, Markotic F, Lovric Makaric Z, et al. Research methodology and characteristics of journal articles with original data, preprint articles and registered clinical trial protocols about COVID-19. BMC Med Res Methodol. 2020;20(1):161. https://doi.org/10.1186/s12874-020-01047-2 .
EPPI Centre . COVID-19: a living systematic map of the evidence. Available at: http://eppi.ioe.ac.uk/cms/Projects/DepartmentofHealthandSocialCare/Publishedreviews/COVID-19Livingsystematicmapoftheevidence/tabid/3765/Default.aspx . Accessed 1 June 2021.
NCBI SARS-CoV-2 Resources. Available at: https://www.ncbi.nlm.nih.gov/sars-cov-2/ . Accessed 1 June 2021.
Gustot T. Quality and reproducibility during the COVID-19 pandemic. JHEP Rep. 2020;2(4):100141. https://doi.org/10.1016/j.jhepr.2020.100141 .
Article PubMed PubMed Central Google Scholar
Kodvanj, I., et al., Publishing of COVID-19 Preprints in Peer-reviewed Journals, Preprinting Trends, Public Discussion and Quality Issues. Preprint article. bioRxiv 2020.11.23.394577; doi: https://doi.org/10.1101/2020.11.23.394577 .
Dobler CC. Poor quality research and clinical practice during COVID-19. Breathe (Sheff). 2020;16(2):200112. https://doi.org/10.1183/20734735.0112-2020 .
Article Google Scholar
Bastian H, Glasziou P, Chalmers I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up? PLoS Med. 2010;7(9):e1000326. https://doi.org/10.1371/journal.pmed.1000326 .
Lunny C, Brennan SE, McDonald S, McKenzie JE. Toward a comprehensive evidence map of overview of systematic review methods: paper 1-purpose, eligibility, search and data extraction. Syst Rev. 2017;6(1):231. https://doi.org/10.1186/s13643-017-0617-1 .
Pollock M, Fernandes RM, Becker LA, Pieper D, Hartling L. Chapter V: Overviews of Reviews. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane. 2020. Available from www.training.cochrane.org/handbook .
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane. 2020; Available from www.training.cochrane.org/handbook .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. The impact of different inclusion decisions on the comprehensiveness and complexity of overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):18. https://doi.org/10.1186/s13643-018-0914-3 .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. A decision tool to help researchers make decisions about including systematic reviews in overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):29. https://doi.org/10.1186/s13643-018-0768-8 .
Hunt H, Pollock A, Campbell P, Estcourt L, Brunton G. An introduction to overviews of reviews: planning a relevant research question and objective for an overview. Syst Rev. 2018;7(1):39. https://doi.org/10.1186/s13643-018-0695-8 .
Pollock M, Fernandes RM, Pieper D, Tricco AC, Gates M, Gates A, et al. Preferred reporting items for overviews of reviews (PRIOR): a protocol for development of a reporting guideline for overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):335. https://doi.org/10.1186/s13643-019-1252-9 .
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med. 2009;3(3):e123–30.
Krnic Martinic M, Pieper D, Glatt A, Puljak L. Definition of a systematic review used in overviews of systematic reviews, meta-epidemiological studies and textbooks. BMC Med Res Methodol. 2019;19(1):203. https://doi.org/10.1186/s12874-019-0855-0 .
Puljak L. If there is only one author or only one database was searched, a study should not be called a systematic review. J Clin Epidemiol. 2017;91:4–5. https://doi.org/10.1016/j.jclinepi.2017.08.002 .
Article PubMed Google Scholar
Gates M, Gates A, Guitard S, Pollock M, Hartling L. Guidance for overviews of reviews continues to accumulate, but important challenges remain: a scoping review. Syst Rev. 2020;9(1):254. https://doi.org/10.1186/s13643-020-01509-0 .
Covidence - systematic review software. Available at: https://www.covidence.org/ . Accessed 1 June 2021.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Borges do Nascimento IJ, et al. Novel Coronavirus Infection (COVID-19) in Humans: A Scoping Review and Meta-Analysis. J Clin Med. 2020;9(4):941.
Article PubMed Central Google Scholar
Adhikari SP, Meng S, Wu YJ, Mao YP, Ye RX, Wang QZ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1):29. https://doi.org/10.1186/s40249-020-00646-x .
Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279–83. https://doi.org/10.1016/j.jcrc.2020.03.005 .
Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–8. https://doi.org/10.1007/s00392-020-01626-9 .
Article CAS PubMed Google Scholar
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(6):577–83. https://doi.org/10.1002/jmv.25757 .
Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63(3):390–1. https://doi.org/10.1016/j.pcad.2020.03.001 .
Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). Eur J Intern Med. 2020;75:107–8. https://doi.org/10.1016/j.ejim.2020.03.014 .
Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505:190–1. https://doi.org/10.1016/j.cca.2020.03.004 .
Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–8. https://doi.org/10.1016/j.cca.2020.03.022 .
Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–95. https://doi.org/10.1111/apa.15270 .
Lupia T, Scabini S, Mornese Pinna S, di Perri G, de Rosa FG, Corcione S. 2019 novel coronavirus (2019-nCoV) outbreak: a new challenge. J Glob Antimicrob Resist. 2020;21:22–7. https://doi.org/10.1016/j.jgar.2020.02.021 .
Marasinghe, K.M., A systematic review investigating the effectiveness of face mask use in limiting the spread of COVID-19 among medically not diagnosed individuals: shedding light on current recommendations provided to individuals not medically diagnosed with COVID-19. Research Square. Preprint article. doi : https://doi.org/10.21203/rs.3.rs-16701/v1 . 2020 .
Mullins E, Evans D, Viner RM, O’Brien P, Morris E. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol. 2020;55(5):586–92. https://doi.org/10.1002/uog.22014 .
Pang J, Wang MX, Ang IYH, Tan SHX, Lewis RF, Chen JIP, et al. Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel coronavirus (2019-nCoV): a systematic review. J Clin Med. 2020;9(3):623.
Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. https://doi.org/10.1016/j.tmaid.2020.101623 .
Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215(1):87–93. https://doi.org/10.2214/AJR.20.23034 .
Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis. J Med Virol. 2020;92(6):612–7. https://doi.org/10.1002/jmv.25735 .
Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–5. https://doi.org/10.1016/j.ijid.2020.03.017 .
Bassetti M, Vena A, Giacobbe DR. The novel Chinese coronavirus (2019-nCoV) infections: challenges for fighting the storm. Eur J Clin Investig. 2020;50(3):e13209. https://doi.org/10.1111/eci.13209 .
Article CAS Google Scholar
Hwang CS. Olfactory neuropathy in severe acute respiratory syndrome: report of a case. Acta Neurol Taiwanica. 2006;15(1):26–8.
Google Scholar
Suzuki M, Saito K, Min WP, Vladau C, Toida K, Itoh H, et al. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117(2):272–7. https://doi.org/10.1097/01.mlg.0000249922.37381.1e .
Rajgor DD, Lee MH, Archuleta S, Bagdasarian N, Quek SC. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20(7):776–7. https://doi.org/10.1016/S1473-3099(20)30244-9 .
Wolkewitz M, Puljak L. Methodological challenges of analysing COVID-19 data during the pandemic. BMC Med Res Methodol. 2020;20(1):81. https://doi.org/10.1186/s12874-020-00972-6 .
Rombey T, Lochner V, Puljak L, Könsgen N, Mathes T, Pieper D. Epidemiology and reporting characteristics of non-Cochrane updates of systematic reviews: a cross-sectional study. Res Synth Methods. 2020;11(3):471–83. https://doi.org/10.1002/jrsm.1409 .
Runjic E, Rombey T, Pieper D, Puljak L. Half of systematic reviews about pain registered in PROSPERO were not published and the majority had inaccurate status. J Clin Epidemiol. 2019;116:114–21. https://doi.org/10.1016/j.jclinepi.2019.08.010 .
Runjic E, Behmen D, Pieper D, Mathes T, Tricco AC, Moher D, et al. Following Cochrane review protocols to completion 10 years later: a retrospective cohort study and author survey. J Clin Epidemiol. 2019;111:41–8. https://doi.org/10.1016/j.jclinepi.2019.03.006 .
Tricco AC, Antony J, Zarin W, Strifler L, Ghassemi M, Ivory J, et al. A scoping review of rapid review methods. BMC Med. 2015;13(1):224. https://doi.org/10.1186/s12916-015-0465-6 .
COVID-19 Rapid Reviews: Cochrane’s response so far. Available at: https://training.cochrane.org/resource/covid-19-rapid-reviews-cochrane-response-so-far . Accessed 1 June 2021.
Cochrane. Living systematic reviews. Available at: https://community.cochrane.org/review-production/production-resources/living-systematic-reviews . Accessed 1 June 2021.
Millard T, Synnot A, Elliott J, Green S, McDonald S, Turner T. Feasibility and acceptability of living systematic reviews: results from a mixed-methods evaluation. Syst Rev. 2019;8(1):325. https://doi.org/10.1186/s13643-019-1248-5 .
Babic A, Poklepovic Pericic T, Pieper D, Puljak L. How to decide whether a systematic review is stable and not in need of updating: analysis of Cochrane reviews. Res Synth Methods. 2020;11(6):884–90. https://doi.org/10.1002/jrsm.1451 .
Lovato A, Rossettini G, de Filippis C. Sore throat in COVID-19: comment on “clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis”. J Med Virol. 2020;92(7):714–5. https://doi.org/10.1002/jmv.25815 .
Leung C. Comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1431–2. https://doi.org/10.1002/jmv.25912 .
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. Response to Char’s comment: comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1433. https://doi.org/10.1002/jmv.25924 .
Download references
Acknowledgments
We thank Catherine Henderson DPhil from Swanscoe Communications for pro bono medical writing and editing support. We acknowledge support from the Covidence Team, specifically Anneliese Arno. We thank the whole International Network of Coronavirus Disease 2019 (InterNetCOVID-19) for their commitment and involvement. Members of the InterNetCOVID-19 are listed in Additional file 6 . We thank Pavel Cerny and Roger Crosthwaite for guiding the team supervisor (IJBN) on human resources management.
This research received no external funding.
Author information
Authors and affiliations.
University Hospital and School of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil
Israel Júnior Borges do Nascimento & Milena Soriano Marcolino
Medical College of Wisconsin, Milwaukee, WI, USA
Israel Júnior Borges do Nascimento
Helene Fuld Health Trust National Institute for Evidence-based Practice in Nursing and Healthcare, College of Nursing, The Ohio State University, Columbus, OH, USA
Dónal P. O’Mathúna
School of Nursing, Psychotherapy and Community Health, Dublin City University, Dublin, Ireland
Department of Anesthesiology, Intensive Care and Pain Medicine, University of Münster, Münster, Germany
Thilo Caspar von Groote
Department of Sport and Health Science, Technische Universität München, Munich, Germany
Hebatullah Mohamed Abdulazeem
School of Health Sciences, Faculty of Health and Medicine, The University of Newcastle, Callaghan, Australia
Ishanka Weerasekara
Department of Physiotherapy, Faculty of Allied Health Sciences, University of Peradeniya, Peradeniya, Sri Lanka
Cochrane Croatia, University of Split, School of Medicine, Split, Croatia
Ana Marusic, Irena Zakarija-Grkovic & Tina Poklepovic Pericic
Center for Evidence-Based Medicine and Health Care, Catholic University of Croatia, Ilica 242, 10000, Zagreb, Croatia
Livia Puljak
Cochrane Brazil, Evidence-Based Health Program, Universidade Federal de São Paulo, São Paulo, Brazil
Vinicius Tassoni Civile & Alvaro Nagib Atallah
Yorkville University, Fredericton, New Brunswick, Canada
Santino Filoso
Laboratory for Industrial and Applied Mathematics (LIAM), Department of Mathematics and Statistics, York University, Toronto, Ontario, Canada
Nicola Luigi Bragazzi
You can also search for this author in PubMed Google Scholar
Contributions
IJBN conceived the research idea and worked as a project coordinator. DPOM, TCVG, HMA, IW, AM, LP, VTC, IZG, TPP, ANA, SF, NLB and MSM were involved in data curation, formal analysis, investigation, methodology, and initial draft writing. All authors revised the manuscript critically for the content. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Livia Puljak .
Ethics declarations
Ethics approval and consent to participate.
Not required as data was based on published studies.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: appendix 1..
Search strategies used in the study.
Additional file 2: Appendix 2.
Adjusted scoring of AMSTAR 2 used in this study for systematic reviews of studies that did not analyze interventions.
Additional file 3: Appendix 3.
List of excluded studies, with reasons.
Additional file 4: Appendix 4.
Table of overlapping studies, containing the list of primary studies included, their visual overlap in individual systematic reviews, and the number in how many reviews each primary study was included.
Additional file 5: Appendix 5.
A detailed explanation of AMSTAR scoring for each item in each review.
Additional file 6: Appendix 6.
List of members and affiliates of International Network of Coronavirus Disease 2019 (InterNetCOVID-19).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Borges do Nascimento, I.J., O’Mathúna, D.P., von Groote, T.C. et al. Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews. BMC Infect Dis 21 , 525 (2021). https://doi.org/10.1186/s12879-021-06214-4
Download citation
Received : 12 April 2020
Accepted : 19 May 2021
Published : 04 June 2021
DOI : https://doi.org/10.1186/s12879-021-06214-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Coronavirus
- Evidence-based medicine
- Infectious diseases
BMC Infectious Diseases
ISSN: 1471-2334
- General enquiries: [email protected]
Our systems are now restored following recent technical disruption, and we’re working hard to catch up on publishing. We apologise for the inconvenience caused. Find out more: https://www.cambridge.org/universitypress/about-us/news-and-blogs/cambridge-university-press-publishing-update-following-technical-disruption
We use cookies to distinguish you from other users and to provide you with a better experience on our websites. Close this message to accept cookies or find out how to manage your cookie settings .
Login Alert
- > Understanding Coronavirus
- > Introduction

Book contents
- Understanding Coronavirus
- Series page
- Copyright page
- Preface to the Revised and Updated Edition
- Preface to the First Edition
- Abbreviations
- 1 Introduction
- 2 How Is the Coronavirus Spreading?
- 3 What Is a Coronavirus?
- 4 How Is the Coronavirus Changing?
- 5 How Did the COVID-19 Outbreak Start and Evolve?
- 6 How Does the COVID-19 Outbreak Compare to the SARS Outbreak in 2003?
- 7 How Does the COVID-19 Outbreak Compare to Seasonal and Pandemic Influenza?
- 8 How Can We Treat the Virus and Prevent Infections?
- Conclusions
- Summary of Common Misunderstandings
- Suggested Further Reading
- Figure and Quotation Credits
1 - Introduction
Published online by Cambridge University Press: 29 September 2021
At the end of December 2019, an outbreak of pneumonia cases of unknown origin was reported in Wuhan, Hubei province, China. The patients presented with high fever and had difficulty breathing. Some, but not all, of these cases were in people who visited the Huanan Seafood Wholesale Market, where, in addition to seafood, a variety of live animals were also sold. Other infections occurred in people staying at a nearby hotel on December 23–27. All tests carried out by the Chinese Center for Disease Control and Prevention for known viruses and bacteria were negative, indicating the presence of a previously unreported agent. A new virus was isolated and its genome sequenced, revealing a similarity with SARS-like coronaviruses found in bats. Although very similar to the virus causing severe acute respiratory syndrome (SARS) in 2003, it was different enough to be considered a new human-infecting coronavirus. Clusters of infected families, together with transmission in medical settings, indicated that the virus had the ability to undergo human-to-human transmission. A month later, by the beginning of February 2020, the virus was found in several countries across the globe, and on March 11, 2020, the World Health Organization (WHO) declared it a global pandemic. The disease caused by the new coronavirus was called coronavirus disease 19, or COVID-19.
Access options
Save book to kindle.
To save this book to your Kindle, first ensure [email protected] is added to your Approved Personal Document E-mail List under your Personal Document Settings on the Manage Your Content and Devices page of your Amazon account. Then enter the ‘name’ part of your Kindle email address below. Find out more about saving to your Kindle .
Note you can select to save to either the @free.kindle.com or @kindle.com variations. ‘@free.kindle.com’ emails are free but can only be saved to your device when it is connected to wi-fi. ‘@kindle.com’ emails can be delivered even when you are not connected to wi-fi, but note that service fees apply.
Find out more about the Kindle Personal Document Service .
- Introduction
- Raul Rabadan , Columbia University, New York
- Book: Understanding Coronavirus
- Online publication: 29 September 2021
- Chapter DOI: https://doi.org/10.1017/9781009090063.004
Save book to Dropbox
To save content items to your account, please confirm that you agree to abide by our usage policies. If this is the first time you use this feature, you will be asked to authorise Cambridge Core to connect with your account. Find out more about saving content to Dropbox .
Save book to Google Drive
To save content items to your account, please confirm that you agree to abide by our usage policies. If this is the first time you use this feature, you will be asked to authorise Cambridge Core to connect with your account. Find out more about saving content to Google Drive .
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Download Free PDF
Essay on corona Virus Covid-19

2020, Malik
My little research on Covid-19
Free related PDFs Related papers
Grisvian Gilchrist Agustin, 2020
SCITECH requirement, 2020
On December 31st, 2020, reports of the first case of Covid-19 had surfaced the city of Wuhan, China. Since then, the outbreak was declared a public health emergency of international concern and reached almost all corners of the world and in a short time it became a pandemic resulting for lots of countries to raise up their walls declaring lockdowns, travel ban, and to practice social distancing. This had lead for the World health Organization (WHO) to work 24/7 to analyze data related to this new type of coronavirus, provide necessary advice, coordinate with different sectors and partners in an international scale, help countries prepare, increase supplies, and manage expert networks. Now, as of April 18th, 2020, there are almost 2 million coronavirus cases, 150 thousand deaths, and 580 thousand recovered across the globe and counting.

The outbreak of Coronavirus Disease (COVID-19) has been declared a Public Health Emergency of International Concern (PHEIC) and the virus has now spread to many countries and territories. While a lot is still unknown about the virus that causes COVID-19, we do know that it is transmitted through direct contact with respiratory droplets of an infected person (generated through coughing and sneezing). Individuals can also be infected from touching surfaces contaminated with the virus and touching their face (e.g., eyes, nose, mouth). While COVID-19 continues to spread it is important that communities take action to prevent further transmission, reduce the impacts of the outbreak and support control measures.

ResearchGate, 2020
This is user friendly article with scientific details and remedy process with my experience. I wrote this to help society and reduce fear among people regarding covid.

International Journal of Research in Pharmaceutical Sciences, 2020

Coronavirus disease (COVID-19) is an infectious disease caused by a newly discovered coronavirus and it has created unexpected world crisis which never happen after second world war. On 30 January 2020, the Director-General of the World Health Organization (WHO) declared the outbreak of COVID-19 to be a Public Health Emergency of International Concern and issued a set of Temporary Recommendations. There is a new public health crisis threatening the world with the emergence and spread of 2019 novel coronavirus (2019-nCoV) or the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The virus originated in bats and was transmitted to humans through yet unknown intermediary animals in Wuhan, Hubei province, China in December 2019. The objective of the paper is to identify the reason to spread COVID-19 and to identify the possible solution to prevent COVID-19. It is a qualitative research. The information has collected studying articles, books and newspapers. People can recover ...

European Journal of Medical and Health Sciences
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or Corona virus is a new type of coronavirus that is transmitted to humans. Corona virus infection called COVID-19 (Corona Virus Disease 2019) was first discovered in the city of Wuhan, China at the end of December 2019. Until March 2, 2020, more than 80 thousand confirmed cases have been reported in China. Of these cases, 49 thousand were identified in Wuhan City. Epidemiologically, the spread or distribution of this disease has a wide social and economic impact on the world. Many literature studies about the COVID-19 outbreak, such as causes, natural history of the disease, even to the preventive and medical treatment. Since the end of 2019 until April 2020, there have been many published literature or literature studies at both national and international levels, so this paper aims to examine literature studies related to COVID-19.

Asian Journal of Advances in Medical Science , 2020
Coronavirus (COVID-19) is an important public health emergency of international concern. Firstly COVID-19 was isolated from Wuhan market in China at 7 Jan. 2020. As of this time, there is no known effective pharmaceutical treatment; although it is much needed for patient contracting severs form of the disease. This virus causes respiratory infection in human including sneezing, coughing, cold and pneumonia while in animal it causes diarrhea and upper respiratory diseases. COVID-19 is transmitted human to human or human to animal via airborne droplets. This disease can be diagnosis on the basis of travel history from infected areas, common symptom or by laboratory confirmations. WHO advised to avoid public place, close contact to Coronavirus infected persons, wearing mask, sanitizing hands and maintain social distancing for prevention. It's also advised infected pensions keep in quarantine center for 14 days or separate from other family member to single separate room, implementation of contact, droplet precaution, and airborne precaution, keep physical distances. Some countries are also using lopinavir/ritonavir, remdesivir, chloroquine and hydroxychloroquine drugs for treatment of Coronavirus but these are not efficient in cure of this disease. The aim of the systematic review of literature is to summarize the evidence regarding COVID-19, symptoms, pathogenesis and prevention/treatment.

COVID 19, 2021
Coronaviruses are a family of viruses that can cause the common cold or more severe diseases such as severe acute respiratory syndrome, Middle East respiratory syndrome, and the coronavirus disease COVID-19.

Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.

IP International Journal of Medical Paediatrics and Oncology

IKSAD PUBLISHING HOUSE, 2020

Frontiers in Public Health, 2022

International Journal of Research in Pharmaceutical Sciences

Journal Transformation Knowledge, 2023

RRJOM, 2020

International Journal of Advanced Academic Studies

Pakistan Journal of Zoology, 2021

Feature Article, 2021

Journal of Medical and Biological Science Research, 2020

Community Service Journal of Indonesia, 2021

International Journal of Research in Medical Sciences, 2020

International Journal of Environmental Research and Public Health, 2020

Related topics
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
- Contact Tracing
- Pandemic Data Initiative
- Webcasts & Videos
- 30-Minute COVID-19 Briefing
- Research Papers
JHU has stopped collecting data as of
After three years of around-the-clock tracking of COVID-19 data from...
Loading Module...
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
PEPFAR Expands COVID-19 Vaccines In Zambia
What to know.
Zambia worked with CDC and partners to expand COVID-19 vaccination. This expansion used a new model in clinics supported by the U.S. President's Emergency Plan for AIDS Relief (PEPFAR) to help get COVID-19 vaccines to people living with HIV. With CDC support, the Ministry of Health (MOH) expanded vaccination to more than 1,000 sites in all 10 of Zambia's provinces as vaccines were available.

Photo essay


IMAGES
VIDEO
COMMENTS
Essay on Covid 19 in English - Governments have had to take severe measures to try and contain the pandemic. The virus has altered our way of life in many ways, including its effects on our health and our economy. ... Download PDF. Essay on Covid -19: In a very short amount of time, coronavirus has spread globally. It has had an enormous impact ...
variation in the e ects of COVID-19 across students. In terms of labor market expectations, on average, students foresee a 13 percentage points decrease in. the probability of. on, a reduction of 2 percent in their reservation wages, a. d a2.3 percent decrease in their expected earn. ID-19 demonstrate that stude.
The COVID-19 global recession is the deepest since the end of World War II (Figure 1). The global economy contracted by 3,5 percent in 2020 according to the April 2021 World Economic Outlook
This essay examines key aspects of social relationships that were disrupted by the COVID-19 pandemic. It focuses explicitly on relational mechanisms of health and brings together theory and emerging evidence on the effects of the COVID-19 pandemic to make recommendations for future public health policy and recovery. We first provide an overview of the pandemic in the UK context, outlining the ...
What is COVID-19 •COVID-19 is the infectious disease caused by the most recently discovered coronavirus1 •This new virus and disease were unknown before the outbreak began in Wuhan, China, in December 2019 •COVID-19 is now a pandemic affecting many countries globally
COVID-19 The COVID-19 pandemic is a catastrophe taking an enormous toll on humanity disrupting lives and livelihoods. The scale and severity of COVID-19 is unprecedented. WHO is working with partners and countries toward a coordinated public health response drive n. by real -time, reliable and actionable data.
A novel coronavirus (CoV) named '2019-nCoV' or '2019 novel coronavirus' or 'COVID-19' by the World Health Organization (WHO) is in charge of the current outbreak of pneumonia that began at the beginning of December 2019 near in Wuhan City, Hubei Province, China [1-4]. COVID-19 is a pathogenic virus. From the phylogenetic analysis ...
increase in global poverty since 1998, when the Asian financial crisis hit. With the new forecasts, global poverty—the share of the world's population living on less than US $1.90 per day—is projected to increase from 8.2 per cent in 2019 to 8.8 per cent in 2020, or from 632 million people to 684 million people.
This essay examines the implications of the COVID-19 pandemic for health inequalities. It outlines historical and contemporary evidence of inequalities in pandemics—drawing on international research into the Spanish influenza pandemic of 1918, the H1N1 outbreak of 2009 and the emerging international estimates of socio-economic, ethnic and geographical inequalities in COVID-19 infection and ...
l host into human populationsThe first human cases of COVID-19, the coronavirus disease caused by SARS-CoV-2, were first reported from Wuhan. Environmental samples taken in a food market in Wuhan were positive for the virus, concentrated in the area where wild and farmed animal trade was present. The market could be the origin of the virus or ...
In conclusion, COVID-19 tested the patience and resilience of the mankind. This pandemic has taught people the importance of patience, effort and humbleness. Also Read: Essay on My Best Friend. Essay On COVID-19 in 300 Words. COVID-19, also known as the coronavirus, is a serious and contagious disease that has affected people worldwide.
Thinking about COVID-19 and the literature review It is less likely in the short-term that the crisis will impact on your literature - which is theoretical or empirical and tends to have a long lead into publication. However, there is great deal of research at the moment around the impact of COVID-19 on family life, healthcare and education.
3. Be a leader in keeping yourself, your school, family and community healthy. Share what you learn about preventing disease with your family and friends, especially with younger children. Model good practices such as sneezing or coughing into your elbow and washing your hands, especially for younger family members. 4.
The spread of the "Severe Acute Respiratory Coronavirus 2" (SARS-CoV-2), the causal agent of COVID-19, was characterized as a pandemic by the World Health Organization (WHO) in March 2020 and has triggered an international public health emergency [].The numbers of confirmed cases and deaths due to COVID-19 are rapidly escalating, counting in millions [], causing massive economic strain ...
The COVID-19 crisis has affected societies and economies around the globe and will permanently reshape our world as it continues to unfold. This collection of essays draws on the diverse insights of the World Economic Forum's Global Risks Report Advisory Board to look ahead and across a broad range of issues. ... Download PDF. Reports ...
A new virus was isolated and its genome sequenced, revealing a similarity with SARS-like coronaviruses found in bats. Although very similar to the virus causing severe acute respiratory syndrome (SARS) in 2003, it was different enough to be considered a new human-infecting coronavirus. Clusters of infected families, together with transmission ...
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or Corona virus is a new type of coronavirus that is transmitted to humans. Corona virus infection called COVID-19 (Corona Virus Disease 2019) was first discovered in the city of Wuhan, China at the end of December 2019. Until March 2, 2020, more than 80 thousand confirmed cases have ...
Download as PDF. The Covid-19 pandemic had completely disrupted lives around the world. With lockdowns and social distancing measures in place, daily life had changed dramatically for people globally. No one was truly prepared for how much of an impact a viral outbreak could have. In this life during pandemic essay, we will discuss how the ...
Coronavirus disease 2019 (COVID-19) Situation Report - 94 HIGHLIGHTS • The Global Outbreak Alert and Response Network (GOARN) has launched a GOARN COVID-19 Knowledge hub. The hub is designed as a central repository of quality public health information, guidance, tools and webinars which can be accessed freely at any point.
The novel coronavirus disease 2019 (COVID-19, caused by SARS-CoV-2) has spread globally since its first report in Wuhan, China on December 31, 2019. On January 30, the Philippines reported its first two imported cases of COVID-19 in a couple from Wuhan. One of them died on February 1st, becoming the first COVID-19 death outside China.
The Johns Hopkins Coronavirus Resource Center has collected, verified, and published local, regional, national and international pandemic data since it launched in March 2020. From the beginning, the information has been freely available to all — researchers, institutions, the media, the public, and policymakers. As a result, the CRC and its data have been cited in many published research ...
Welcome to the module on virology, coronaviruses, and COVID-19. Upon completing this module, you will be able to: Understand the definition of a virus and virology. Explore how viruses make people sick. Discuss different types of viruses, including coronaviruses. Describe characteristics of the COVID-19 virus.
Photo essay. In 2021, CDC, Zambia, and local partners collaborated to expand COVID-19 vaccination to adults living with HIV. Photo: Santos Sanchez/CDC ... The COVID-19 vaccination plan was so successful that the MOH expanded it to more than 1,000 sites in all of Zambia's provinces as vaccines were available. By October 2022, more than 850,000 ...