
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
JavaScript appears to be disabled on this computer. Please click here to see any active alerts .

What is Acid Rain?
Acid rain, or acid deposition, is a broad term that includes any form of precipitation with acidic components, such as sulfuric or nitric acid that fall to the ground from the atmosphere in wet or dry forms. This can include rain, snow, fog, hail or even dust that is acidic.
What Causes Acid Rain?
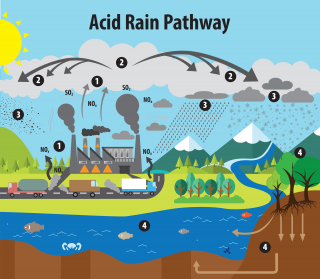
Acid rain results when sulfur dioxide (SO 2 ) and nitrogen oxides (NO X ) are emitted into the atmosphere and transported by wind and air currents. The SO 2 and NO X react with water, oxygen and other chemicals to form sulfuric and nitric acids. These then mix with water and other materials before falling to the ground.
While a small portion of the SO 2 and NO X that cause acid rain is from natural sources such as volcanoes, most of it comes from the burning of fossil fuels. The major sources of SO 2 and NO X in the atmosphere are:
- Burning of fossil fuels to generate electricity. Two thirds of SO 2 and one fourth of NO X in the atmosphere come from electric power generators.
- Vehicles and heavy equipment.
- Manufacturing, oil refineries and other industries.
Winds can blow SO 2 and NO X over long distances and across borders making acid rain a problem for everyone and not just those who live close to these sources.
Forms of Acid Deposition
Wet deposition.
Wet deposition is what we most commonly think of as acid rain . The sulfuric and nitric acids formed in the atmosphere fall to the ground mixed with rain, snow, fog, or hail.
Dry Deposition
Acidic particles and gases can also deposit from the atmosphere in the absence of moisture as dry deposition . The acidic particles and gases may deposit to surfaces (water bodies, vegetation, buildings) quickly or may react during atmospheric transport to form larger particles that can be harmful to human health. When the accumulated acids are washed off a surface by the next rain, this acidic water flows over and through the ground, and can harm plants and wildlife, such as insects and fish.
The amount of acidity in the atmosphere that deposits to earth through dry deposition depends on the amount of rainfall an area receives. For example, in desert areas the ratio of dry to wet deposition is higher than an area that receives several inches of rain each year.
Measuring Acid Rain
Policymakers, research scientists, ecologists, and modelers rely on the National Atmospheric Deposition Program’s (NADP) National Trends Network (NTN) for measurements of wet deposition. The NADP/NTN collects acid rain at more than 250 monitoring sites throughout the US, Canada, Alaska, Hawaii and the US Virgin Islands. Unlike wet deposition, dry deposition is difficult and expensive to measure. Dry deposition estimates for nitrogen and sulfur pollutants are provided by the Clean Air Status and Trends Network (CASTNET). Air concentrations are measured by CASTNET at more than 90 locations.
When acid deposition is washed into lakes and streams, it can cause some to turn acidic. The Long-Term Monitoring (LTM) Network measures and monitors surface water chemistry at over 280 sites to provide valuable information on aquatic ecosystem health and how water bodies respond to changes in acid-causing emissions and acid deposition.
Next, learn about the Effects of Acid Rain .
Or, learn more about:
- Clean Air Markets
- Clean Air Status and Trends Network (CASTNET)
- National Atmospheric Deposition Program (NADP)
- Long-Term Monitoring (LTM) Network

- Why Does Water Expand When It Freezes
- Gold Foil Experiment
- Faraday Cage
- Oil Drop Experiment
- Magnetic Monopole
- Why Do Fireflies Light Up
- Types of Blood Cells With Their Structure, and Functions
- The Main Parts of a Plant With Their Functions
- Parts of a Flower With Their Structure and Functions
- Parts of a Leaf With Their Structure and Functions
- Why Does Ice Float on Water
- Why Does Oil Float on Water
- How Do Clouds Form
- What Causes Lightning
- How are Diamonds Made
- Types of Meteorites
- Types of Volcanoes
- Types of Rocks
What is Acid Rain?
Acid rain, also called acid deposition, is a broad term for any form of precipitation with high concentrations of sulfuric and nitric acids. According to the Environmental Protection Agency (EPA), deposition can be wet such as in rain, fog, snow, mist, or dry, as in dust, gas, and smoke.
The term ‘acid rain’ was used for the first time by Robert Angus Smith in 1852 while examining rainwater reaction from the U.K. and Scotland’s industrial sites.
Where does Acid Rain Occur?
First identified in 1872 in Sweden, scientists in the U.S. started examining the phenomenon in the 1950s. Later in the 1960s and early 1970s, acid rain was recognized as a regional issue that mostly affected Western Europe and North-Eastern America. It is also increasingly found in parts of India and China. Sulfuric acidic rain commonly occurs in Venus.
What Causes Acid Rain and How is it Formed
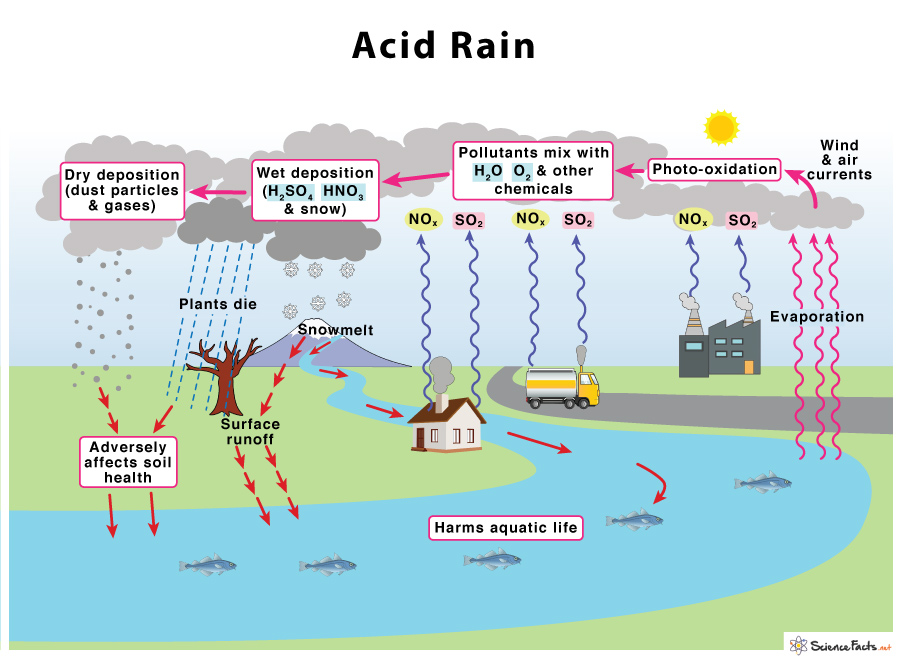
Acid rain forms when two significant pollutants, sulfur dioxide (SO 2 ) and nitrogen oxides (NO X ), are released into the atmosphere due to various natural and human activities. While natural sources such as volcanoes, lightning, or decomposing vegetation emit SO 2 and NO X , acid rain’s primary cause is human-made. The emission of coal, petroleum, and natural gas from the factories, smelting of ore, and automobile emission are all manmade sources.
The process starts when SO 2 and NO X are emitted into the atmosphere, undergo photooxidation in the presence of sunlight and oxygen, and react with water and other components present in the atmosphere. Wind and air currents spread these acidic substances over long distances. They eventually precipitate down in the form of wet deposits such as rain (sulfuric and nitric acids), fog, snow, and mist, or dry deposits as dust, gas, and smoke, adversely affecting soil heath and aquatic life.
How Acidic is Acid Rain?
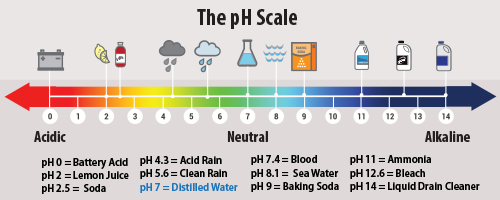
Normal rain is slightly acidic, having a pH of 5.6. It forms when carbon dioxide reacts with water to form weak carbonic acid, which has no harmful effects.
However, acid rain generally has a pH between 4.2 and 4.4. Here, sulfur dioxide and nitrogen dioxide undergo oxidation before reacting with water. The following equations show the formation of acid rain:
Formation of Sulfuric Acid
SO 2 (g) + H 2 O (l) → H 2 SO 3 (aq)
H 2 SO 3 (aq) + O 2 (g) → 2H 2 SO 4 (aq)
Formation of Nitric Acid
N 2 (g) +O 2 (g) → 2NO(g)
2NO (g) + O 2 (g) → 2NO 2 (g)
2NO 2 (g) + H 2 O (l) → HNO 3 (aq) + HNO 2 (aq)
Since nitrous acid is unstable, it eventually oxidizes to nitric acid. Thus, the overall reaction is:
2N 2 (g) + 5O 2 (g) + 2H 2 O (l) → 4HNO 3 (aq)
How does Acid Rain Affect the Environment?
Acid rain nearly affects everything around us. Some significant consequences are listed below:
1) Effects on Soil : It robs the soil of its essential nutrients such as calcium, releasing aluminum that prevents water uptake in plants. It causes a change in soil composition, thus affecting crop production. Acid rain also affects forests, especially those at higher elevations.
2) Effects on Plants : It weakens the trees by washing away the waxy, protective coating on leaves , thus damaging them. Acid rain washes away the essential nutrients and minerals from the soil, causing stunted growth in plants by affecting photosynthesis .
3) Effects on Water Bodies : Acid rain makes lakes, streams, ponds, rivers, and other water bodies more acidic. It adversely affects aquatic life, such as freshwater shrimps, snails, and mussels. Most fish species cannot survive a pH of less than 5. When the pH becomes 4, the lake is dead, which means it becomes devoid of life. Again, the fall of acid rain causes aluminum absorption from the soil, which is carried into the water bodies. The combination of both makes water bodies more toxic for their survival.
Some aquatic species can tolerate acidic pH better than others. Nevertheless, since all ecosystems are interconnected, organisms in one ecosystem depend on the other for their survival. For example, if a fish species disappear, the animals, including the birds that feed on them, will also become extinct.
4) Effects on Building, Monument, and Statue : All structures, especially those made of limestone and sandstone, are mostly affected by the effect of acid rain. The calcium carbonate (limestone) present in the rocks or monuments reacts with sulfuric acid to form calcium sulfate, making them corrode. The soluble substances in the acidic deposition get dissolved in water and then washed away, known as chemical weathering.
Corrosion in Taj Mahal is a real-life example. The chemical reaction causing their corrosion is given below:
CaCO 3 (s) + H 2 SO 4 (aq) → CaSO 4 (s) + H 2 O (l) + CO 2 (g)
Another instance is the Statue of Liberty, which is made of copper. It is also getting damaged by the cumulative action of acid rain and oxidation, thus becoming green.
5) Other Impacts : It causes corrosion of water pipes, which results in the leaching of heavy metals such as lead, iron, and copper into drinking water. This effect can adversely affect humans. Dry precipitation is sometimes associated with heart and lung problems such as asthma and bronchitis.
Solutions to Problems of Acid Rain
The only way to reduce the consequence of acid rain is by checking the emission of nitrogen and sulfur oxides, which can be done by:
- Regulating their emission from coal-based and metal extracting industries by filtering the exhaust before releasing into the environment
- Reducing the dependence on non-renewable energy resources such as coal, petroleum, and natural gas. According to the EPA, this can be done by increasing renewable energy consumption from sunlight, wind, and water.
- Using eco-friendly vehicles instead of petrol or diesel-based ones. The use of catalytic converters filters the exhaust gases from the vehicles before releasing them into the environment.
- Planting more trees or afforestation helps to purify the atmosphere by reducing toxic gases.
- Restoring water-body damage by using powdered limestone that neutralizes the water, a process known as liming.
Advantages of Acid Rain
Here are some examples of acid rain.
- Makes holes in the limestone below ground resulting in caves and groundwater storage and providing habitat for some species
- Sulfuric acid rain limits global warming by offsetting methane’s natural production by microbes in wetland areas, thereby limiting climate change.
Ans . Acid rain is still a massive problem in many countries. However, its effect has reduced many folds in Europe and North America compared to the 1970s and 1980s. In the U.S., the Clean Air Act of 1990 has helped cut sulfur dioxide emissions by 88 percent and nitrogen dioxide down by 50 percent between 1990 and 2017. Countries like India and China are still trying to implement control over the emission of such gases.
- What is Acid Rain? – Epa.gov
- Acid Rain and Water – Usgs.gov
- Acid Rain – Ypte.org.uk
- Acid Rain – Nationalgeographic.com
- Acid Rain – Livescience.com
- Acid Rain – Chem.libretexts.org
- Acid Rain – Safewater.org
Article was last reviewed on Friday, February 3, 2023
Related articles
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Popular Articles
Join our Newsletter
Fill your E-mail Address
Related Worksheets
- Privacy Policy
© 2024 ( Science Facts ). All rights reserved. Reproduction in whole or in part without permission is prohibited.
Acid rain, explained
The fossil fuels that humans burn for energy can come back to haunt us as acid rain.
Acid rain describes any form of precipitation that contains high levels of nitric and sulfuric acids. It can also occur in the form of snow, fog, and tiny bits of dry material that settle to Earth. Normal rain is slightly acidic, with a pH of 5.6, while acid rain generally has a pH between 4.2 and 4.4 .
Causes of acid rain
Rotting vegetation and erupting volcanoes release some chemicals that can cause acid rain, but most acid rain is a product of human activities. The biggest sources are coal-burning power plants , factories, and automobiles.
When humans burn fossil fuels , sulfur dioxide (SO 2 ) and nitrogen oxides (NO x ) are released into the atmosphere. Those air pollutants react with water, oxygen, and other substances to form airborne sulfuric and nitric acid. Winds may spread these acidic compounds through the atmosphere and over hundreds of miles . When acid rain reaches Earth, it flows across the surface in runoff water, enters water systems, and sinks into the soil.

A virtual tree graveyard of Norway spruce in Poland bears the scars of acid rain. Caused when rain droplets absorb air pollution like sulfur and nitrogen oxides, acid rain weakens trees by dissolving nutrients in the soil before plants can use them.
Effects of acid rain
Sulfur dioxide and nitrogen oxides are not primary greenhouse gases that contribute to global warming , one of the main effects of climate change ; in fact, sulfur dioxide has a cooling effect on the atmosphere. But nitrogen oxides contribute to the formation of ground-level ozone , a major pollutant that can be harmful to people. Both gases cause environmental and health concerns because they can spread easily via air pollution and acid rain.
Acid rain has many ecological effects, especially on lakes, streams, wetlands, and other aquatic environments. Acid rain makes such waters more acidic, which results in more aluminum absorption from soil, which is carried into lakes and streams. That combination makes waters toxic to aquatic animals. ( Learn more about the effects of water pollution .)
Some species can tolerate acidic waters better than others. However, in an interconnected ecosystem, what affects some species eventually affects many more throughout the food chain, including non-aquatic species such as birds .
Acid rain and fog also damage forests, especially those at higher elevations. The acid deposits rob the soil of essential nutrients such as calcium and cause aluminum to be released in the soil, which makes it hard for trees to take up water . Acids also harm tree leaves and needles.
The effects of acid rain, combined with other environmental stressors, leave trees and plants less healthy and more vulnerable to cold temperatures, insects, and disease. The pollutants may also inhibit trees' ability to reproduce. Some soils are better able to neutralize acids than others. But in areas where the soil's "buffering capacity" is low, such as parts of the U.S. Northeast, the harmful effects of acid rain are much greater.
Acid deposits damage physical structures such as limestone buildings and cars. And when it takes the form of inhalable fog, acid precipitation can cause health problems in people, including eye irritation and asthma.
What can be done?
The only way to fight acid rain is by curbing the release of the pollutants that cause it. This means burning fewer fossil fuels and setting air-quality standards.
In the U.S., the Clean Air Act of 1990 targeted acid rain, putting in place pollution limits that helped cut sulfur dioxide emissions 88 percent between 1990 and 2017. Air-quality standards have also driven U.S. emissions of nitrogen dioxide down 50 percent in the same time period. These trends have helped red spruce forests in New England and some fish populations , for example, recover from acid rain damage. But recovery takes time, and soils in the northeastern U.S. and eastern Canada have only recently shown signs of stabilizing nutrients .
Acid rain problems will persist as long as fossil fuel use does, and countries such as China that have relied heavily on coal for electricity and steel production are grappling with those effects. One study found that acid rain in China may have even contributed to a deadly 2009 landslide . China is implementing controls for sulfur dioxide emissions, which have fallen 75 percent since 2007 —but India's have increased by half.
YEAR-LONG ADVENTURE for every explorer on your list
Related topics, you may also like.

Inside the Hidden World of Jaguars


This deadly fungus is hitchhiking its way across the world

Is a shoe-free home really better? Scientists may have an answer.

Do Doomsday 'preppers' have a point? Here's what we can learn from them.

What would the world look like without mosquitoes?
- Terms of Use
- Privacy Policy
- Your US State Privacy Rights
- Children's Online Privacy Policy
- Interest-Based Ads
- About Nielsen Measurement
- Do Not Sell or Share My Personal Information
- Nat Geo Home
- Attend a Live Event
- Book a Trip
- Inspire Your Kids
- Shop Nat Geo
- Visit the D.C. Museum
- Learn About Our Impact
- Support Our Mission
- Advertise With Us
- Customer Service
- Renew Subscription
- Manage Your Subscription
- Work at Nat Geo
- Sign Up for Our Newsletters
- Contribute to Protect the Planet
Copyright © 1996-2015 National Geographic Society Copyright © 2015-2024 National Geographic Partners, LLC. All rights reserved
The Harmful Effects of Acid Rain
- University of Strathclyde
- Ithaca College
:max_bytes(150000):strip_icc():format(webp)/ScreenShot2021-04-07at1.57.45PM-bcef177316c94cdf998457c694cce6d5.png)
- University of Tennessee
Ludwig Werle / Getty Images
- Planet Earth
- Climate Crisis
- Recycling & Waste
- Natural Disasters
- Transportation
Acid rain is a serious environmental problem occurring all over the world, particularly in large swaths of the United States and Canada. As the name suggests, it indicates precipitation that is more acidic than normal. It is harmful not only to lakes, streams, and ponds in an area but also to the plants and animals that live within the given ecosystem.
Here is what you need to know about acid rain including why it occurs and what you can do to prevent it.
Acid rain precipitation that forms when acids—typically nitric acid and sulfuric acid—are released from the atmosphere into precipitation. This causes precipitation with pH levels that are lower than normal. Acid rain is predominantly caused by humans' impact on the planet, but there are some natural sources as well.
The term acid rain is also somewhat misleading. Nitric and sulfuric acid can be transported to the Earth from rain but also via snow, sleet, hail, fog, mist, clouds, and dust clouds.
Acid rain is caused by both human and natural sources. Natural causes include volcanoes, lightning, and decaying plant and animal matter. In the United States, fossil-fuel combustion is the primary cause of acid rain.
Burning fossil fuels such as coal, oil, and natural gas for electric power generators releases about two-thirds of all of the sulfuric dioxide and one-quarter of all of the nitrous oxide that is found in our air. Acid rain forms when these chemical pollutants react with the oxygen and water vapor in the air to form nitric acid and sulfuric acid. These acids can combine with precipitation directly over their source. But they can often follow the prevailing winds and blow hundreds of miles away before they return to the surface via acid rain.
When acid rain falls upon an ecosystem, it affects the water supply as well as the plants and animals in that area. In aquatic ecosystems, acid rain can harm fish, insects and other aquatic animals. Lowered pH levels can kill many adult fish, and most fish eggs will not hatch when the pH drops below normal. This drastically alters the biodiversity, food webs and overall well-being of the aquatic environment.
That affects many animals outside of the water, too. When fish die, there is no more food for birds such as the common loon. Acid rain has been linked to thinner eggshells in many bird species such as warblers and other songbirds. Thinner shells mean that fewer chicks will hatch and survive. Acid rain has also been found to damage frogs, salamanders and reptiles in aquatic ecosystems.
Acid rain can be equally damaging to land-based ecosystems. For starters, it drastically changes the chemistry of the soil, lowering the pH and creating an environment where essential nutrients are leached away from the plants that need them. Plants are also directly damaged when acid rain falls on their leaves.
In his book summarizing the research of governmental organizations, such as the Environmental Protection Agency, Don Philpott stated, "Acid rain has been implicated in forest and soil degradation in many areas of the eastern U.S., particularly high elevation forests of the Appalachian Mountains from Maine to Georgia that include areas such as the Shenandoah and Great Smoky Mountain National Parks."
The best way to reduce the incidences of acid rain is to limit the amount of sulfuric dioxide and nitrous oxide that are released into the atmosphere. Since 1990, the Environmental Protection Agency has required companies that emit these two chemicals (namely, companies that burn fossil fuels for the production of electricity,) to make major reductions in their emissions.
The EPA's Acid Rain Program was phased in from 1990 to 2010 with the final sulfuric dioxide cap set at 8.95 million tons for 2010. This is about one-half of the emissions that were emitted from the power sector in 1980.
What Can You Do To Prevent Acid Rain?
Acid rain may feel like a huge problem, but there are actually many things that you can do as an individual to help prevent it. Any step you can take to conserve energy will reduce the amounts of fossil fuels that are burned to produce that energy, thereby reducing the formation of acid rain.
How can you conserve energy? Purchase energy-saving appliances; carpool, use public transportation, walk, or bike whenever possible; keep your thermostat low in the winter and high in the summer; insulate your house; and turn off lights, computers, and appliances when you're not using them.
" Effects of Acid Rain ." Environmental Protection Agency .
" What is Acid Rain? " Environmental Protection Agency.
Hames, Ralph, et al. " Adverse Effects of Acid Rain on the Distribution of the Wood Thrush Hylocichla mustelina in North America ." Proceedings of the National Academy of Science , vol. 99, no. 19, 2002, 11235–11240, doi:10.1073/pnas.172700199
Philpott, Don. Critical Government Documents on the Environment . Bernan Press, 2015.
" Acid Rain Program ." Environmental Protection Agency .
- What Acid Rain Does to the Environment
- How Does Nitrogen Oxide Pollution Affect the Environment?
- Top 5 Ways Water Gets Polluted
- How Sediment Causes Pollution
- How Oil Spills Affect Birds
- What Is Acid Mine Drainage? Definition, Causes, and Examples
- What Is Air Pollution? Definition, Types, and Environmental Impact
- What Is Nutrient Pollution? Causes, Impacts, and Mitigation
- How Much Air Pollution Comes From Cars?
- 6 Common Air Pollutants
- What Is Soil Pollution? Environmental Impacts and Mitigation
- Why Do Oil Spills Happen? Causes, Examples, and Prevention
- What Is Open-Pit Mining? Definition, Examples, and Environmental Impact
- What Is Coal Ash and How Dangerous Is It?
- Mine Tailings and the Environment
- What Does 'Unhealthy Air Quality for Sensitive Groups' Mean?
- Science, Tech, Math ›
- Weather & Climate ›
What You Need to Know About Acid Rain
The Causes, History, and Effects of Acid Rain
Shepard Sherbell/Contributor/Getty Images
- Understanding Your Forecast
- Storms & Other Phenomena
- M.A., Geography, California State University - East Bay
- B.A., English and Geography, California State University - Sacramento
Acid rain is made up of water droplets that are unusually acidic because of atmospheric pollution, most notably the excessive amounts of sulfur and nitrogen released by cars and industrial processes. Acid rain is also called acid deposition because this term includes other forms of acidic precipitation (such as snow).
Acidic deposition occurs in two ways: wet and dry. Wet deposition is any form of precipitation that removes acids from the atmosphere and deposits them on Earth’s surface. Dry deposition polluting particles and gases stick to the ground via dust and smoke in the absence of precipitation. Even though dry, this form of deposition is dangerous as well, because precipitation can eventually wash pollutants into streams, lakes, and rivers.
Acidity itself is determined based on the pH level (the amount of acidity or alkalinity) of the water droplets. The pH scale ranges from 0 to 14, with a lower pH being more acidic, while a high pH is alkaline, and seven is neutral. Normal rainwater is slightly acidic, with a pH range of 5.3-6.0. Acid deposition is anything below that range. It is also important to note that the pH scale is logarithmic, and each whole number on the scale represents a 10-fold change.
Today, acid deposition is present in the northeastern United States, southeastern Canada, and much of Europe, including portions of Sweden, Norway, and Germany. In addition, parts of South Asia (particularly China, Sri Lanka, and southern India) and South Africa are all in danger of being affected by acid deposition in the future.
What Causes Acid Rain?
Acid deposition can be caused by natural sources such as volcanoes , but it is mainly caused by the release of sulfur dioxide and nitrogen oxide during fossil fuel combustion. When these gases are discharged into the atmosphere, they react with the water, oxygen, and other gases already present there to form sulfuric acid, ammonium nitrate, and nitric acid. These acids then disperse over large areas because of wind patterns and fall back to the ground as acid rain or other forms of precipitation.
The gases most responsible for acid deposition are a byproduct of electric power generation and the burning of coal. As such, man-made acid deposition began becoming a significant issue during the Industrial Revolution and was first discovered by a Scottish chemist Robert Angus Smith in 1852. In that year, he discovered the relationship between acid rain and atmospheric pollution in Manchester, England.
Although it was discovered in the 1800s, acid deposition did not gain significant public attention until the 1960s, and the term "acid rain" was coined in 1972. Public attention further increased in the 1970s when the "New York Times" published reports about problems occurring in the Hubbard Brook Experimental Forest in New Hampshire.
Effects of Acid Rain
After studying the Hubbard Brook Forest and other areas, researchers found several important effects of acid deposition on both natural and man-made environments. Aquatic settings are the most clearly affected by acid deposition, however, because acidic precipitation falls directly into them. Both dry and wet deposition also runs off from forests, fields, and roads and flows into lakes, rivers, and streams.
As this acidic liquid flows into larger bodies of water, it is diluted. Howvever, over time, acids can accrue and lower the overall pH of the body of water. Acid deposition also causes clay soils to release aluminum and magnesium , further lowering the pH in some areas. If the pH of a lake drops below 4.8, its plants and animals risk death. It is estimated that around 50,000 lakes in the United States and Canada have a pH below normal (about 5.3 for water). Several hundred of these have a pH too low to support any aquatic life.
Aside from aquatic bodies, acid deposition can significantly affect forests. As acid rain falls on trees, it can make them lose their leaves, damage their bark, and stunt their growth. By damaging these parts of the tree, it makes them vulnerable to disease, extreme weather, and insects. Acid falling on a forest’s soil is also harmful because it disrupts soil nutrients, kills microorganisms in the soil, and can sometimes cause a calcium deficiency. Trees at high altitudes are also susceptible to problems induced by acidic cloud cover as the moisture in the clouds blankets them.
Damage to forests by acid rain is seen all over the world, but the most advanced cases are in Eastern Europe. It’s estimated that in Germany and Poland, half of the forests are damaged, while 30 percent in Switzerland have been affected.
Finally, acid deposition also has an effect on architecture and art because of its ability to corrode certain materials. As acid lands on buildings (especially those constructed with limestone), it reacts with minerals in the stones, sometimes causing them to disintegrate and wash away. Acid deposition can also cause concrete to deteriorate, and it can corrode modern buildings, cars, railroad tracks, airplanes, steel bridges, and pipes above and below ground.
What's Being Done?
Because of these problems and the adverse effects of air pollution has on human health, a number of steps are being taken to reduce sulfur and nitrogen emissions. Most notably, many governments are now requiring energy producers to clean smokestacks with scrubbers that trap pollutants before they are released into the atmosphere and to reduce car emissions with catalytic converters. Additionally, alternative energy sources are gaining more prominence and funding is being put toward the restoration of ecosystems damaged by acid rain worldwide.
"Welcome to the Hubbard Brook Ecosystem Study." Hubbard Brook Ecosystem Study, The Hubbard Brook Research Foundation.
- Monsoons and Their Effect on the Environment
- Is There Any Upside to Global Warming?
- The 1930's Dust Bowl Drought
- What Is La Nina?
- How Forceful Is Torrential Rain?
- An Overview of El Nino and La Nina
- The Types of Flood Events and Their Causes
- Top Weather Songs of the 1990s
- Top 10 Weather Songs of the 1980s
- The Windward Versus Leeward Side of a Mountain
- How Thundersnow Works (and Where to Find It)
- 7 Weather-Related Phobias and What Causes Them
- Thermal Inversion
- What Is a Drought?
- Drought Causes, Stages, and Problems
- The Mount Pinatubo Eruption in the Philippines
Acid rain: Causes, effects and solutions
How acid rain affects nearly everything it touches, and what we can do about it.

Solutions and prevention
Additional resources, bibliography.
Acid rain, or acid deposition, is a broad term that includes any form of precipitation that contains acidic components, such as sulfuric acid or nitric acid. The precipitation is not necessarily wet or liquid; the definition includes dust, gases, rain, snow, fog and hail. The type of acid rain that contains water is called wet deposition. Acid rain formed with dust or gases is called dry deposition.
The precipitation is not necessarily wet or liquid; the definition includes dust, gasses, rain, snow, fog and hail. The type of acid rain that contains water is called wet deposition. Acid rain formed with dust or gasses is called dry deposition.
Causes of acid rain
The term acid rain was coined in 1852 by Scottish chemist Robert Angus Smith, according to the Royal Society of Chemistry, which calls him the "father of acid rain." Smith decided on the term while examining rainwater chemistry near industrial cities in England and Scotland. He wrote about his findings in 1872 in the book " Air and Rain: The Beginnings of a Chemical Climatology ."
In the 1950s, scientists in the United States started studying the phenomenon, and in the 1960s and early 1970s, acid rain became recognized as a regional environmental issue that affected Western Europe and eastern North America.
Though manmade pollutants are currently affecting most acidic precipitation, natural disasters can be a factor as well. For example, volcanoes can cause acid rain by blasting pollutants into the air. These pollutants can be carried around the world in jet streams and turned into acid rain far from the volcano. After an asteroid supposedly wiped out the dinosaurs 65.5 million years ago, sulfur trioxide was blasted into the air. When it hit the air, it turned into sulfuric acid, generating a downpour of acid rain.
Even before that, over 4 billion years ago, it is suspected that the air may have had 10,000 times as much carbon dioxide as today. Geologists from the University of Wisconsin-Madison backed up this theory by studying rocks and publishing the results in a 2008 issue of the journal Earth and Planetary Science Letters. "At [those levels of carbon dioxide], you would have had vicious acid rain and intense greenhouse [effects]. That is a condition that will dissolve rocks," said study team member John Valley.
Sulfur dioxide (SO2) and nitrogen oxides (NOx) released into the air by fossil-fuel power plants, vehicles and oil refineries are the biggest cause of acid rain today, according to the Environmental Protection Agency (EPA). Two thirds of sulfur dioxide and one fourth of nitrogen oxide found in the atmosphere come from electric power generators.
A chemical reaction happens when sulfur dioxide and nitrogen oxides mix with water, oxygen and other chemicals in the air. They then become sulfuric and nitric acids that mix with precipitation and fall to the ground. Precipitation is considered acidic when its pH level is about 5.2 or below. The normal pH of rain is around 5.6.
Environmental affects of acid rain
Acid rain affects nearly everything. Plants, soil, trees, buildings and even statues can be transformed by the precipitation.
Acid rain has been found to be very hard on trees. It weakens them by washing away the protective film on leaves, and it stunts growth. A United States Environmental Protection Agency ( EPA ) study showed that acid rain is particularly hard on trees.
"By providing the only preserved soil in the world collected before the acid rain era, the Russians helped our international team track tree growth for the first time with changes in soil from acid rain," said Greg Lawrence, a U.S. Geological Survey scientist. "We've known that acid rain acidifies surface waters, but this is the first time we've been able to compare and track tree growth in forests that include soil changes due to acid rain."
Acid rain can also change the composition of soil and bodies of water, making them uninhabitable for local animals and plants. For example, healthy lakes have a pH of 6.5 or higher. As acid rain raises the level of acidity, fish tend to die off. Most fish species can't survive a water pH of below 5. When the pH becomes a 4, the lake is considered dead, according to National Atmospheric Deposition Program .
It can additionally deteriorate limestone and marble buildings and monuments, like gravestones.
There are several solutions to stopping human-caused acid rain. Regulating the emissions coming from vehicles and buildings is an important step, according to the EPA. This can be done by restricting the use of fossil fuels and focusing on more renewable energy sources such as solar and wind power.
Related: How do solar panels work?
Also, each person can do their part by reducing their vehicle use. Using public transportation, walking, riding a bike or carpooling is a good start, according to the EPA. People can also reduce their use of electricity, which is widely created with fossil fuels , or switch to a solar plan. Many electricity companies offer solar packages to their customers that require no installation and low costs.

It is also possible to prevent acid rain forming, by adding lime deposits to major water sources. This method has been used to neutralize the Ph levels in the water, which reduced the acidity, for thousands of years, the LA Times reported . These so-called "liming" operations have also been used to restore wildlife. In Wales, a liming operation was conducted in 2003 to restore salmon to the Wye river. The water had become too acidic for the fish to survive, causing them to disappear from the river 18 years earlier, Young People's Trust for the Environment , a U.K. non-profit organization, reported.
Discover key facts about acid rain on Young Peoples Trust for the Environment , watch this National Geographic video about the role of fossil fuels and pollution in creating acid rain, and learn more about what the WWF is doing to reduce emissions.
- Peringe Grennfelt, Anna Engleryd, Martin Forsius, Øystein Hov, Henning Rodhe & Ellis Cowling: Acid rain and air pollution: 50 years of progress in environmental science and policy
- Douglas A.Burns, Julian Aherne, David A.Gay, Christopher M.B.Lehmann: Acid rain and its environmental effects: Recent scientific advances
- Lesley Evans Ogden: Acid Rain: Researchers Addressing Its Lingering Effects
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
Before and after satellite images show lakes appearing across Sahara after deluge of rain soaks desert
Earth from space: Bizarre 'pet cloud' reappears above its favorite spot in New Zealand
How old is planet Earth?
Most Popular
- 2 Euclid telescope reveals 1st section of largest-ever 3D map of the universe — and there's still 99% to go
- 3 1st wheel was invented 6,000 years ago in the Carpathian Mountains, modeling study suggests
- 4 Doctors no longer recommend 'self-checks' for breast cancer — here's what to know
- 5 Key Atlantic current could collapse soon, 'impacting the entire world for centuries to come,' leading climate scientists warn
- Name This field is for validation purposes and should be left unchanged.
- Climate Change
- Policy & Economics
- Biodiversity
- Conservation
Get focused newsletters especially designed to be concise and easy to digest
- ESSENTIAL BRIEFING 3 times weekly
- TOP STORY ROUNDUP Once a week
- MONTHLY OVERVIEW Once a month
- Enter your email *
- Phone This field is for validation purposes and should be left unchanged.
Explainer: What Is Acid Rain?

Acid rain, or acid deposition, is defined or a term that includes any form of precipitation with acidic components, like sulphuric or nitric acid, that falls from the atmosphere in wet or dry forms, including rain, snow, fog, hail and dust.
What Causes Acid Rain?
Acid rain is a result of sulphur dioxide ( SO2) and nitrogen oxides (NOX) being emitted into the atmosphere and transported by wind and air, making it a problem for all, not just those who live near the sources of the pollution. Acid rain consists of sulphuric and nitric acids, formed when aforementioned gases mix and react with water, oxygen and other chemicals, which is then mix with water again and other materials before falling to the ground.
While a small portion of the SO2 and NOX that cause acid rain comes from natural sources such as volcanoes and rotting vegetation, most of it comes from human activities, like the burning of fossil fuels , through electricity generation, oil refineries and vehicles. Over the past few decades, humans have emitted so many different chemicals that the mix of gases in the atmosphere has been changed.
As stated, acid deposition can occur in wet and dry forms. When wet, it is what we most commonly think of as acid rain. This falls as rain, snow, fog or hail. Dry deposition occurs when acidic particles and gases may deposit on surfaces (water bodies, vegetation, buildings) quickly or may react during atmospheric transport to form larger particles that can pose a risk to human health. When these accumulated acids are washed off the surface by the next rain, the acidic water flows over and through the ground and could be harmful to plants and wildlife. The amount of acidity in the atmosphere that occurs through dry deposition depends on the amount of rainfall an area receives.
You might also like: What Are Carbon Sinks?
What Are the Effects of Acid Rain?
While sulphur dioxides actually have a cooling effect on the atmosphere, nitrogen oxides contribute to the formation of ozone, which can be harmful to people and plants. Both of these gases are causes for concern because they can spread easily via air pollution and acid rain.
Acid rain makes waters more acidic, posing a risk to lakes, streams, wetlands and other aquatic environments. Increasing a body of water’s acidity results in more aluminium absorption from soil , which is then transported to lakes and streams. This makes waters toxic to crayfish, clams, fish and other aquatic animals.
While some species can handle acidic waters better than others, in connected ecosystems, there will be a domino effect throughout the food chain, whereby one species dying off because it cannot tolerate acidic rain will leave another without food, killing that species off as well.
Acid rain and fog also damage forests, especially those at higher elevations. The acid deposits rob the soil of nutrients such as calcium and cause aluminium to be released into the soil, which makes it difficult for trees to suck up water. The results are less healthy trees and planets that are more vulnerable to cold temperatures, insects and disease. Additionally, the trees’ ability to reproduce is compromised.
Physical structures such as limestone buildings and cars can also be affected by acid rain. When it takes the form of fog, it can be inhaled and cause health problems, like eye irritation and asthma.
How Do We Measure It?
Acid rain is measured using a pH scale for which 7.0 is neutral. The lower a substance’s pH (less than 7), the more acidic it is; the higher it is (greater than 7), the more alkaline it is.
Normally, rain has a pH of about 5.6, making it slightly acidic because CO2 dissolves into it, forming a weak carbonic acid. However, normal precipitation reacts with alkaline chemicals that can be found in air, soils, bedrock, lakes and streams, the reactions of which usually neutralise natural acids. Acid rain has a pH of between 4.2 and 4.4.
When acid deposition is washed into lakes and streams, it can cause some to turn acidic.
How Do We End It?
Unfortunately, the only way to reverse the occurrence of acid rain is to curb the release of the pollutants that cause it, meaning that we need to burn fewer fossil fuels and set effective air quality standards.
In the US, the Clean Air Act of 1990 implemented pollution limits that helped cut SO2 emissions by 88% between 1990 and 2017. Additionally, air quality standards have also lowered nitrogen dioxide emissions by 50% in the same time period.
Landscapes that have been affected by acid rain can recover from its damage, however it takes time.
More on this topic: How Does Acid Rain Affect the Environment

15 Biggest Environmental Problems of 2024

4 Biggest Environmental Issues in the Philippines in 2024

Water Shortage: Causes and Effects
Hand-picked stories weekly or monthly. We promise, no spam!
- Email This field is for validation purposes and should be left unchanged.
Boost this article By donating us $100, $50 or subscribe to Boosting $10/month – we can get this article and others in front of tens of thousands of specially targeted readers. This targeted Boosting – helps us to reach wider audiences – aiming to convince the unconvinced, to inform the uninformed, to enlighten the dogmatic.

IMAGES
VIDEO
COMMENTS
Acid rain is rain or any other form of precipitation that is unusually acidic, meaning that it has elevated levels of hydrogen ions (low pH). Most water, including drinking water, has a neutral pH that exists between 6.5 and 8.5, but acid rain has a pH level lower than this and ranges from 4–5 on average.
acid rain, precipitation possessing a pH of about 5.2 or below primarily produced from the emission of sulfur dioxide (SO 2) and nitrogen oxides (NO x; the combination of NO and NO 2) from human activities, mostly the combustion of fossil fuels.
Acid rain, or acid deposition, is a broad term that includes any form of precipitation with acidic components, such as sulfuric or nitric acid that fall to the ground from the atmosphere in wet or dry forms. This can include rain, snow, fog, hail or even dust that is acidic.
Acid rain, also called acid deposition, is a broad term for any form of precipitation with high concentrations of sulfuric and nitric acids. According to the Environmental Protection Agency (EPA), deposition can be wet such as in rain, fog, snow, mist, or dry, as in dust, gas, and smoke.
Acid rain describes any form of precipitation that contains high levels of nitric and sulfuric acids. It can also occur in the form of snow, fog, and tiny bits of dry material...
Acid rain is a serious environmental problem occurring all over the world, particularly in large swaths of the United States and Canada. As the name suggests, it indicates precipitation that...
Acid rain can be defined as precipitation that is abnormally acidic due to it containing dissolved pollutants, which make it capable of causing great environmental harm.
Acid rain is made up of water droplets that are unusually acidic because of atmospheric pollution, most notably the excessive amounts of sulfur and nitrogen released by cars and industrial processes. Acid rain is also called acid deposition because this term includes other forms of acidic precipitation (such as snow).
Acid rain, or acid deposition, is a broad term that includes any form of precipitation that contains acidic components, such as sulfuric acid or nitric acid.
Acid rain, or acid deposition, is defined or a term that includes any form of precipitation with acidic components, like sulphuric or nitric acid, that falls from the atmosphere in wet or dry forms, including rain, snow, fog, hail and dust.