Suggestions or feedback?

MIT News | Massachusetts Institute of Technology
- Machine learning
- Social justice
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
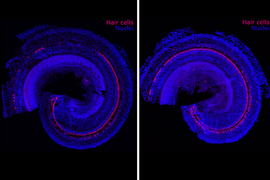
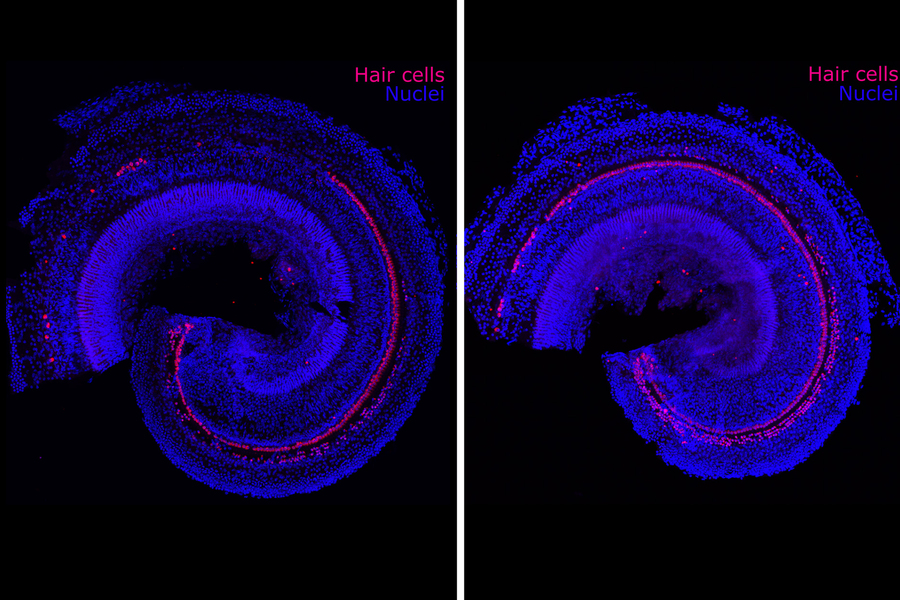
Reversing hearing loss with regenerative therapy
Press contact :, media download.
*Terms of Use:
Images for download on the MIT News office website are made available to non-commercial entities, press and the general public under a Creative Commons Attribution Non-Commercial No Derivatives license . You may not alter the images provided, other than to crop them to size. A credit line must be used when reproducing images; if one is not provided below, credit the images to "MIT."
Previous image Next image
Most of us know someone affected by hearing loss, but we may not fully appreciate how difficult the condition can be. Hearing loss can lead not only to frustration but also social isolation and tinnitus, a debilitating ringing in the ears. It is also closely correlated with dementia.
The biotechnology company Frequency Therapeutics is seeking to reverse hearing loss — not with hearing aids or implants, but with a new kind of regenerative therapy. The company uses small molecules to program progenitor cells, a descendant of stem cells in the inner ear, to create the tiny hair cells that allow us to hear.
Hair cells die off when exposed to loud noises or drugs including certain chemotherapies and antibiotics. Frequency’s drug candidate is designed to be injected into the ear to regenerate these cells within the cochlea. In clinical trials, the company has already improved people’s hearing as measured by tests of speech perception — the ability to understand speech and recognize words.
“Speech perception is the No. 1 goal for improving hearing and the No. 1 need we hear from patients,” says Frequency co-founder and Chief Scientific Officer Chris Loose PhD ’07.
In Frequency’s first clinical study, the company saw statistically significant improvements in speech perception in some participants after a single injection, with some responses lasting nearly two years.
The company has dosed more than 200 patients to date and has seen clinically meaningful improvements in speech perception in three separate clinical studies. Another study failed to show improvements in hearing compared to the placebo group, but the company attributes that result to flaws in the design of the trial.
Now Frequency is recruiting for a 124-person trial from which preliminary results should be available early next year.
The company’s founders, including Loose, MIT Institute Professor Robert Langer, CEO David Lucchino MBA ’06, Senior Vice President Will McLean PhD ’14, and Harvard-MIT Health Sciences and Technology affiliate faculty member Jeff Karp, are already gratified to have been able to help people improve their hearing through the trials. They also believe they’re making important contributions toward solving a problem that impacts more than 40 million people in the U.S. and hundreds of millions more around the world.
“Hearing is such an important sense; it connects people to their community and cultivates a sense of identity,” says Karp, who is also a professor of anesthesia at Brigham and Women’s Hospital. “I think the potential to restore hearing will have enormous impact on society.”
From the lab to patients
In 2005, Lucchino was an MBA student in the MIT Sloan School of Management and Loose was a PhD candidate in chemical engineering at MIT. Langer introduced the two aspiring entrepreneurs, and they started working on what would become Semprus BioSciences, a medical device company that won the MIT $100K Entrepreneurship Competition and later sold at a deal valued at up to $80 million.
“MIT has such a wonderful environment of people interested in new ventures that come from different backgrounds, so we’re able to assemble teams of people with diverse skills quickly,” Loose says.
Eight years after playing matchmaker for Lucchino and Loose, Langer began working with Karp to study the lining of the human gut, which regenerates itself almost every day.
With MIT postdoc Xiaolei Yin, who is now a scientific advisor to Frequency, the researchers discovered that the same molecules that control the gut’s stem cells are also used by a close descendant of stem cells called progenitor cells. Like stem cells, progenitor cells can turn into more specialized cells in the body.
“Every time we make an advance, we take a step back and ask how this could be even bigger,” Karp says. “It’s easy to be incremental, but how do we take what we learned and make a massive difference?”
Progenitor cells reside in the inner ear and generate hair cells when humans are in utero, but they become dormant before birth and never again turn into more specialized cells such as the hair cells of the cochlea. Humans are born with about 15,000 hair cells in each cochlea. Such cells die over time and never regenerate.
In 2012, the research team was able to use small molecules to turn progenitor cells into thousands of hair cells in the lab. Karp says no one had ever produced such a large number of hair cells before. He still remembers looking at the results while visiting his family, including his father, who wears a hearing aid.
“I looked at them and said, ‘I think we have a breakthrough,’” Karp says. “That’s the first and only time I’ve used that phrase.”
The advance was enough for Langer to play matchmaker again and bring Loose and Lucchino into the fold to start Frequency Therapeutics.
The founders believe their approach — injecting small molecules into the inner ear to turn progenitor cells into more specialized cells — offers advantages over gene therapies, which may rely on extracting a patient’s cells, programming them in a lab, and then delivering them to the right area.
“Tissues throughout your body contain progenitor cells, so we see a huge range of applications,” Loose says. “We believe this is the future of regenerative medicine.”
Advancing regenerative medicine
Frequency’s founders have been thrilled to watch their lab work mature into an impactful drug candidate in clinical trials.
“Some of these people [in the trials] couldn’t hear for 30 years, and for the first time they said they could go into a crowded restaurant and hear what their children were saying,” Langer says. “It’s so meaningful to them. Obviously more needs to be done, but just the fact that you can help a small group of people is really impressive to me.”
Karp believes Frequency’s work will advance researchers’ ability to manipulate progenitor cells and lead to new treatments down the line.
“I wouldn't be surprised if in 10 or 15 years, because of the resources being put into this space and the incredible science being done, we can get to the point where [reversing hearing loss] would be similar to Lasik surgery, where you're in and out in an hour or two and you can completely restore your vision,” Karp says. “I think we'll see the same thing for hearing loss.”
The company is also developing a drug for multiple sclerosis (MS), a disease in which the immune system attacks the myelin in the brain and central nervous system. Progenitor cells already turn into the myelin-producing cells in the brain, but not fast enough to keep up with losses sustained by MS patients. Most MS therapies focus on suppressing the immune system rather than generating myelin.
Early versions of that drug candidate have shown dramatic increases in myelin in mouse studies. The company expects to file an investigational new drug application for MS with the FDA next year.
“When we were conceiving of this project, we meant for it to be a platform that could be broadly applicable to multiple tissues. Now we’re moving into the remyelination work, and to me it’s the tip of the iceberg in terms of what can be done by taking small molecules and controlling local biology,” Karp says.
For now, Karp is already thrilled with Frequency’s progress, which hit home the last time he was in Frequency’s office and met a speaker who shared her experience with hearing loss.
“You always hope your work will have an impact, but it can take a long time for that to happen,” Karp says. “It’s been an incredible experience working with the team to bring this forward. There are already people in the trials whose hearing has been dramatically improved and their lives have been changed. That impacts interactions with family and friends. It’s wonderful to be a part of.”
Share this news article on:
Related links.
- Frequency Therapeutics
- Video: Unlocking the Potential of Regenerative Medicine
- Video: Understanding Speech Perception
- Department of Chemical Engineering
- MIT Sloan School of Management
Related Topics
- Innovation and Entrepreneurship (I&E)
- MIT $100K competition
- Biotechnology
- Pharmaceuticals
- Chemical engineering
Related Articles
Creating a permanent bacteria barrier
Previous item Next item
More MIT News

Francis Fan Lee, former professor and interdisciplinary speech processing inventor, dies at 96
Read full story →

Fostering research, careers, and community in materials science
Natural language boosts LLM performance in coding, planning, and robotics

Nuno Loureiro named director of MIT’s Plasma Science and Fusion Center

Studies in empathy and analytics
Science communication competition brings research into the real world
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram
Stay up to date with notifications from The Independent
Notifications can be managed in browser preferences.
UK Edition Change
- UK Politics
- News Videos
- Paris 2024 Olympics
- Rugby Union
- Sport Videos
- John Rentoul
- Mary Dejevsky
- Andrew Grice
- Sean O’Grady
- Photography
- Theatre & Dance
- Culture Videos
- Food & Drink
- Health & Families
- Royal Family
- Electric Vehicles
- Car Insurance deals
- Lifestyle Videos
- UK Hotel Reviews
- News & Advice
- Simon Calder
- Australia & New Zealand
- South America
- C. America & Caribbean
- Middle East
- Politics Explained
- News Analysis
- Today’s Edition
- Home & Garden
- Broadband deals
- Fashion & Beauty
- Travel & Outdoors
- Sports & Fitness
- Sustainable Living
- Climate Videos
- Solar Panels
- Behind The Headlines
- On The Ground
- Decomplicated
- You Ask The Questions
- Binge Watch
- Travel Smart
- Watch on your TV
- Crosswords & Puzzles
- Most Commented
- Newsletters
- Ask Me Anything
- Virtual Events
- Betting Sites
- Online Casinos
- Wine Offers
Thank you for registering
Please refresh the page or navigate to another page on the site to be automatically logged in Please refresh your browser to be logged in
New hope for reversing hearing loss as cell therapy nears first trial
The therapy will build on research that resulted in 40% improved hearing in preclinical tests, researchers said, article bookmarked.
Find your bookmarks in your Independent Premium section, under my profile

Sign up for a full digest of all the best opinions of the week in our Voices Dispatches email
Sign up to our free weekly voices newsletter, thanks for signing up to the voices dispatches email.
A cell therapy to improve hearing could transform the lives of millions of people with hearing loss, new research suggests.
The therapy will see the first clinical trial of a stem cell treatment for hearing loss, building on research that resulted in 40% improved hearing in preclinical tests.
Sheffield-based biotech company Rinri Therapeutics hopes to start clinical trials in the next two years in people with severe-to-profound age-related hearing loss.
According to hearing loss charity RNID, hearing loss affects 12 million people in the UK, and increases as people get older, affecting more than 40% of over-50s and rising to 70% of over-70s.
People with hearing loss can face communication barriers and are less likely to be employed than the general population.
- Parkinson’s blood test could help develop cure, researchers say
- New drug causes ‘complete loss’ of gained weight without ‘any untoward side effects’
- Deaf dog uses sign language to communicate with owner
They are twice as likely to experience mental health problems, including depression, and can be up to five times more likely to develop dementia, the RNID says.
It is thought that some 1.2 million adults in the UK have hearing loss severe enough that they would not be able to hear most conversational speech.
Jennifer Macintosh, 73, from Brighton , who has hearing loss, said: “A treatment for hearing loss would make a great difference because (with) no more hearing aids, I’d be able to take part in larger family gatherings, to take part in more social activities. When I’m in gym classes I would be able to join in the laughter and banter, instead of just standing there wishing I could.
“To start at the beginning of an exercise instead of one movement behind because you copy what is happening, to be able to go to any restaurant without worrying about noise, hear a theatre production without it being hit and miss…
“Also, perhaps more importantly, hear with clarity when you have medical appointments and dental treatment what is going on.”
Rinri’s work was founded on pioneering research into regenerative cell therapy, led by Professor Marcelo Rivolta at the University of Sheffield .
He said: “It is well recognised that hearing loss significantly impacts quality of life, affecting one in five adults in the UK.
A biological solution that can restore hearing significantly would be transformative to people with hearing loss, and we look forward to taking the next step by starting clinical trials in 2025
“A biological solution that can restore hearing significantly would be transformative to people with hearing loss, and we look forward to taking the next step by starting clinical trials in 2025.”
Rinri’s cell therapy, Rincell-1, is for patients with auditory neuropathy, a form of hearing loss which occurs when sounds become disrupted as they travel to the brain.
The company says patients with auditory neuropathy make up 25% of the sensorineural hearing loss community.
Patients will receive Rincell-1, which regenerates auditory neurons and re-establishes the transmission of nerve signals from the inner ear to the auditory centres of the brain to reverse hearing loss.
If clinical trials are successful, the treatment could be available as a treatment to people with hearing loss within the next five to 10 years.
To measure if the therapy is effective, the treatment will be administered with cochlear implants – devices designed to bypass damaged hair cells and directly stimulate auditory neurons.
The implants can act as a recording sensor to pick up signals made by the cochlea as it passes sound information to the auditory nerve, so this will allow researchers to record objective measures of cochlear health, rather than only relying on subjective measures like speech recognition.
Dr Ralph Holme, director of research and insight at RNID, said: “RNID and our supporters are really excited about Rinri’s work, which could result in a life-changing treatment for millions of people with age-related hearing loss caused by damage to the auditory nerve.
“Whilst devices like hearing aids and cochlear implants can be hugely beneficial to people with hearing loss, they are not a complete solution and people still face significant barriers in their daily lives.
“For years, people with hearing loss have dreamed of a future where treatments to restore hearing will be available, and so it’s exciting to see therapies like this which could offer hope to so many approaching clinical trials.”
Subscribe to Independent Premium to bookmark this article
Want to bookmark your favourite articles and stories to read or reference later? Start your Independent Premium subscription today.
New to The Independent?
Or if you would prefer:
Want an ad-free experience?
Hi {{indy.fullName}}
- My Independent Premium
- Account details
- Help centre
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 01 March 2024
A phase I/IIa safety and efficacy trial of intratympanic gamma-secretase inhibitor as a regenerative drug treatment for sensorineural hearing loss
- Anne G. M. Schilder 1 , 2 , 3 ,
- Stephan Wolpert ORCID: orcid.org/0000-0001-5385-8770 4 ,
- Shakeel Saeed 1 , 2 , 3 ,
- Leonie M. Middelink 5 ,
- Albert S. B. Edge ORCID: orcid.org/0000-0001-8641-755X 6 ,
- Helen Blackshaw 1 , 2 ,
- REGAIN Consortium ,
- Kostas Pastiadis 7 &
- Athanasios G. Bibas ORCID: orcid.org/0000-0002-1992-6511 7
Nature Communications volume 15 , Article number: 1896 ( 2024 ) Cite this article
5325 Accesses
112 Altmetric
Metrics details
- Medical research
- Regeneration and repair in the nervous system
- Therapeutics
Inhibition of Notch signalling with a gamma-secretase inhibitor (GSI) induces mammalian hair cell regeneration and partial hearing restoration. In this proof-of-concept Phase I/IIa multiple-ascending dose open-label trial (ISRCTN59733689), adults with mild-moderate sensorineural hearing loss received 3 intratympanic injections of GSI LY3056480, in 1 ear over 2 weeks. Phase I primary outcome was safety and tolerability. Phase lla primary outcome was change from baseline to 12 weeks in average pure-tone air conduction threshold across 2,4,8 kHz. Secondary outcomes included this outcome at 6 weeks and change from baseline to 6 and 12 weeks in pure-tone thresholds at individual frequencies, speech reception thresholds (SRTs), Distortion Product Otoacoustic Emissions (DPOAE) amplitudes, Signal to Noise Ratios (SNRs) and distribution of categories normal, present-abnormal, absent and Hearing Handicap Inventory for Adults/Elderly (HHIA/E). In Phase I ( N = 15, 1 site) there were no severe nor serious adverse events. In Phase IIa ( N = 44, 3 sites) the average pure-tone threshold across 2,4,8 kHz did not change from baseline to 6 and 12 weeks (estimated change −0.87 dB; 95% CI −2.37 to 0.63; P = 0.252 and −0.46 dB; 95% CI −1.94 to 1.03; P = 0.545, respectively), nor did the means of secondary measures. DPOAE amplitudes, SNRs and distribution of categories did not change from baseline to 6 and 12 weeks, nor did SRTs and HHIA/E scores. Intratympanic delivery of LY3056480 is safe and well-tolerated; the trial’s primary endpoint was not met.
Similar content being viewed by others
Deafness: from genetic architecture to gene therapy
Treatments for hearing loss in osteogenesis imperfecta: a systematic review and meta-analysis on their efficacy
Choice of vector and surgical approach enables efficient cochlear gene transfer in nonhuman primate
Introduction.
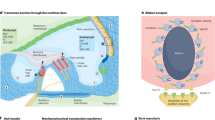
Hearing loss is the most common sensory disorder in humans and an area of significant unmet clinical need 1 , 2 . The most common cause of hearing loss is age-related progressive loss of inner ear sensory hair cells and/or their synapse 3 , 4 . Because in humans these hair cells do not naturally regenerate, hearing loss progresses with age. Current treatment of choice with hearing devices focuses on sound amplification; their benefits in understanding speech in noisy environments are limited, therefore many people choose not to use them 5 , 6 . Importantly, they do not treat the cause of hearing loss. This is where recent discoveries in the molecular pathways leading to hair cell loss and regeneration have opened avenues for novel approaches to the treatment of hearing loss. They have allowed for the identification of therapeutic targets and the development of small molecule drugs that may promote hair cell regeneration 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 .
In young adult mice acutely exposed to noise at a level sufficient to induce loss of hair cells, pharmacological inhibition of Notch signalling with a gamma secretase inhibitor (GSI) upregulated Atoh1, which encodes a bHLH transcription factor required for hair cell differentiation. This approach regenerated hair cells through trans-differentiation of supporting cells and partially restored hearing 14 . Likewise, in an adult guinea pig model of hearing loss due to noise exposure, siRNA silencing of Hes1, an effector of the Notch pathway, caused an induction of new hair cells and partial recovery of hearing 15 .
Following the identification and successful preclinical development of a GSI (LY3056480) with an optimal profile 16 , we designed and delivered a Phase I and IIa clinical trial. Here, we report on the safety and efficacy of this drug, administered intraympanically in adults with mild to moderate sensorineural hearing loss (SNHL).
Characteristics of the patients
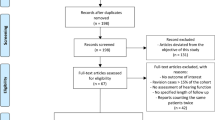
From January 24 to October 17, 2018, 15 patients with mild to moderate hearing loss were enroled in the Phase I trial at the UK site (Fig. 1 ); 3 patients received 3 intratympanic doses of 25 µg LY3056480, 6 received 3 doses of 125 µg, and 3 and 3 patients received 3 doses of 200 µg and 250 µg, respectively. From January 30 to August 5, 2019, 44 patients were enrolled in the Phase IIa trial, 24 at the UK site and 12 and 8 at the German and Greek site, respectively (see patient characteristics in Table 1 ). All received 3 intratympanic doses of 250 µg LY3056480, except 1 patient receiving the diluent-only at the last injection due to a procedural error.
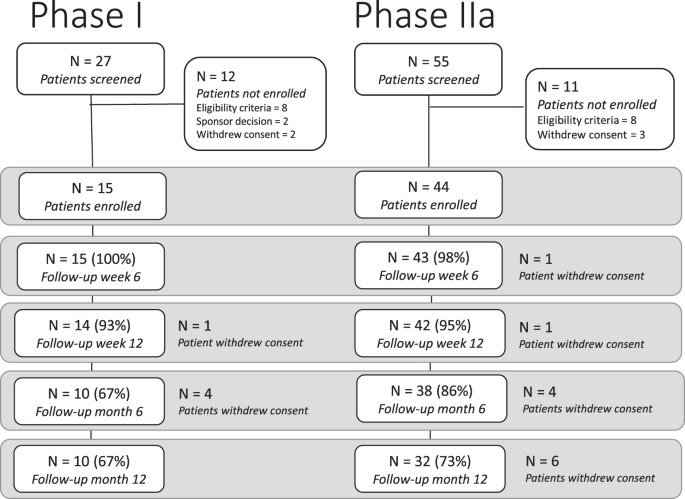
Patient disposition of phase I trial and phase IIa trial.
Safety outcomes
All Phase I patients experienced one or more AEs. In total 174 AEs were reported of which 2 were probably related to the investigational medicinal product (IMP) and 52 probably or definitely related to the procedure. All AEs with a relation to the IMP and/or procedure resolved. No SAEs were reported.
In Phase IIa, all patients experienced one or more AEs. Of the AEs reported (see Table 2 ), 277 (80%) were classified as mild, 70 (20%) as moderate and none as severe. The two reported SAEs were CTCAE Grade 2 (moderate) and considered unrelated to the IMP.
Patients reported fluctuations in tinnitus severity across timepoints (recorded as AE, tinnitus experience, TFI score). Tympanometry readings did not change from baseline to 12 weeks; all intratympanic injection sites closed. See Supplementary Information for all secondary safety outcomes.
Primary efficacy outcome
For the total population of 44 patients with mild to moderate SNHL, the primary endpoint was not met, i.e., the mean pure-tone air-conduction threshold across 2, 4, and 8 kHz did not change from baseline to 12 weeks in the treated ear (Fig. 2 ) (estimated change −0.46 dB; 95% confidence interval [CI] −1.94 to 1.03; P = 0.545).
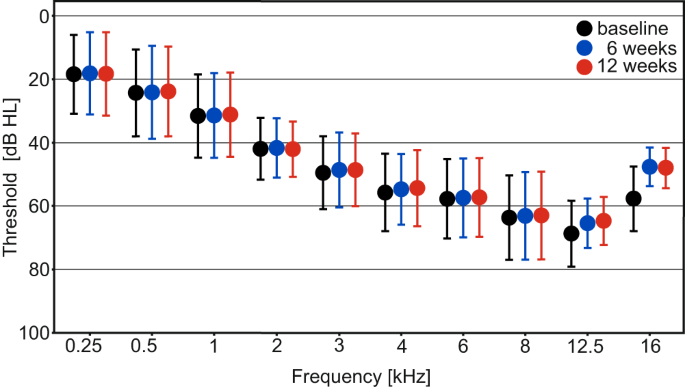
Presented timepoints are baseline (black), 6 (blue) and 12 (red) weeks, error bars indicate standard deviations. Pure-tone thresholds are displayed in decibel hearing level (dB HL), frequency in kilohertz (kHz). Source data are provided as “supplementary data 4 ”.
Secondary efficacy outcomes
The mean pure-tone air-conduction threshold across 2, 4, and 8 kHz did not change from baseline to 6 weeks in the treated ear (estimated change −0.87 dB; 95% CI −2.37 to 0.63; P = 0.252; see Fig. 2 ).
Nineteen patients ( N = 42; 45%) showed an improvement of ≥10 dB (see methods) in one or more individual frequencies (including 12.5 and 16 kHz) from baseline to both 6 and 12 weeks.
Seven patients ( N = 42; 16.7%) showed an improvement of ≥10 dB in two or more adjacent frequencies, or of ≥20 dB in one or more individual frequencies from baseline to both 6 and 12 weeks (see Fig. 3 ). See Supplementary Information for post-hoc analyses of pure-tone audiometry outcomes at 6 and 12 months.
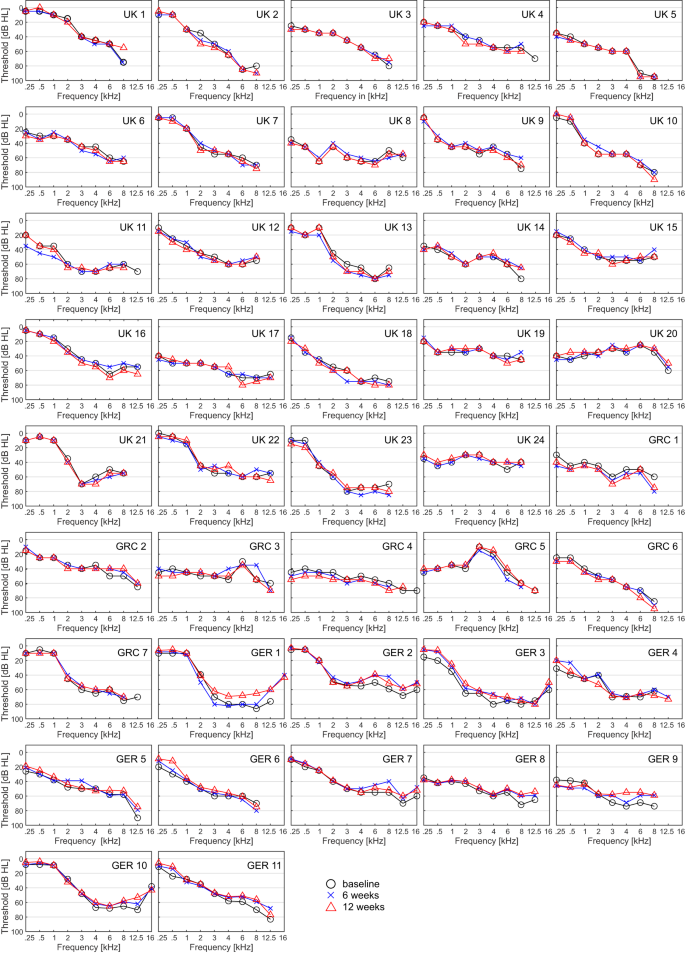
Presented timepoints are baseline (black circle), 6 (blue cross) and 12 (red triangles) weeks. Frequencies not shown indicate that thresholds are beyond the maximum output of the audiometer. Pure-tone thresholds are displayed in decibel hearing level (dB HL), frequency in kilohertz (kHz). Source data are provided as “supplementary data 4 ”.
The mean speech reception threshold in noise, measured with a words-in-babble test and expressed as the SRT50n, defined by the signal-to-noise ratio (SNR) that yields an average response of 50% correctly recognised words, did not change from baseline to 6 weeks (estimated change 0.90 dB; 95% CI −0.09 to +1.88; P = 0.074) and 12 weeks (estimated change 0.08 dB; 95% CI −1.01 to + 1.18; P = 0.881) in the treated ear. See Supplementary Information for post-hoc analyses of speech perception in noise measures at 6 and 12 months.
The mean DPOAE amplitude and signal-to-noise ratio (SNR) did not change from baseline to 6 and 12 weeks. Neither did the distribution of DPOAE categories ‘present-normal, present-abnormal, and absent’ change from baseline to 6 and 12 weeks (Bonferoni correction was used for multiple comparisons). See Supplementary Information for post-hoc analyses of DPOAE outcomes at various time points.
Hearing Handicap inventory of Adults/Elderly (HHIA/E) scores did not change from baseline to 6 weeks ( P = 0.845) and 12 weeks ( P = 0.51).
The mean pure-tone air-conduction threshold across 2, 4, and 8 kHz did not change in the untreated ear from baseline to 6 weeks (estimated change −0.84 dB; 95% CI −2.34 to 0.65; P = 0.265) and to 12 weeks (estimated change −0.86 dB; 95% CI −2.34 to 0.63; P = 0.253). Twenty-one patients ( N = 42; 50%) showed a pure-tone hearing threshold improvement of ≥10 dB in one or more frequencies in the untreated ear at 6 weeks and 22 patients ( N = 42; 52%) at 12 weeks. In 24 patients ( N = 42; 57%) the difference in change from baseline to 6 weeks in pure-tone hearing threshold across 2, 4, and 8 kHz between the treated and untreated ear was ≥10 dB in one or more frequencies, and in 23 patients ( N = 42; 55%) at 12 weeks, respectively.
The mean SRT50n did not change in the untreated ear from baseline to 6 weeks (estimated change 0.03 dB; 95% CI −0.95 to 1.02; P = 0.944) and 12 weeks (estimated change −0.17 dB; 95% CI −1.27 to 0.93; P = 0.759).
This Phase I/IIa trial, developed and delivered by our EU academic-industry consortium, shows that LY3056480 delivered intratympanically in adults with mild-moderate SNHL is safe and well tolerated. Our aim was to test the hypothesis that local administration with the GSI LY3056480 restores outer hair cell function and thereby improves the perception of speech-in-noise, which is the primary unsolved problem for people with hearing loss 17 , 18 . With our trial being the first of a regenerative hearing drug in 2016, we chose a broad set of outcome measures: pure-tone audiometry up to 16 kHz (extended higher frequencies considered key to speech perception) 19 , 20 , speech-in-noise tests as the outcome best reflecting patient experience; and otoacoustic emissions as an objective measure of outer hair cell function 21 .
Although our Phase IIa trial did not meet its primary endpoint of a ≥10 dB change in the average pure-tone air conduction threshold across 2, 4 and 8 kHz at 12 weeks, some patients showed positive changes in predefined secondary pure-tone audiometry and speech perception in noise measures. The Supplementary Information to this article provides additional data and a reflection on the use of DPOAEs in our trial. We feel that further investigation of the use of otoacoustic emissions and the choice of different stimuli such as new frequency primaries, short-pulse DPOAEs 22 and Stimulus-frequency OAEs, may prove valuable for inclusion in future trials for restoration of cochlear function.
Our trial has generated important learnings about the design and delivery of early phase trials of novel hearing approaches. Through a detailed medical history and audiological assessment at the screening visit, checked against our in- and exclusion criteria, we aimed to include patients with SNHL most likely due to outer hair cell loss. We acknowledge that current tests of auditory function do not allow for deep phenotyping and therefore heterogeneity of our patient population in terms of underlying molecular mechanisms of SNHL may have diluted the effects of our highly targeted treatment. Collaborative efforts towards understanding the deep geno-phenotype of hearing and hearing loss are urgently needed 8 , 9 , 23 .
We determined pre-trial stability of patients’ hearing loss based upon their medical history and review of their previous audiograms. Eligibility was confirmed by the screening audiogram within 4 weeks of the first LY3056480 injection. One may argue that a pre-trial lead-in time with multiple audiological assessments would have ascertained stability of hearing loss of our participants.
We chose a traditional 3 + 3 dose escalation study design, starting with the ‘no observed adverse effect level’ dose from our pre-clinical studies and ending with the maximum dose based upon solubility of the formulation and middle ear volume, over an innovative Phase I design 24 . This is because at the time our trial was initiated, there were no data on the safety of novel hearing therapeutics. For future trials one may agree with regulators on more rapid dose escalation or integrated Phase I/II designs. For the same reason, we chose to inject one ear only. Now that more data are available on the safety of hearing regenerative approaches 20 and considering the observed outcomes in the untreated ear, we would recommend future trials adopt a placebo (diluent or saline) -controlled design, using patient-level randomisation.
At the time of development of the trial, we discussed various options for drug administration 25 among our Consortium and with our UK patient panel. At that time, patients shared a strong preference for intratympanic injections in an outpatient setting over a surgical approach that may require a general anaesthetic to deliver the drug directly onto the round window.
Due to the nature of our consortium and its public funding, our trial not only delivered on its drug development milestones, but also generated a wealth of long-term auditory measures for exploration of regeneration mechanisms. Such low-sample-size-high-dimensional datasets however have proven a challenge to existing statistical approaches and clinical interpretation. Going forward, machine learning may offer solutions to unlock these datasets with federated learning across trials.
We conducted the hearing tests as per local guidance, which explains why pure-tone audiometry was measured in 1 dB steps at the German site and 5 dB steps at the UK and Greek site. Whether a smaller step size, improves accuracy and therefore detection of efficacy signals and may explain the differences across trial sites remains open for debate 26 .
Finally, our trial team were contacted by more than 5,000 patients with hearing loss worldwide requesting to take part, illustrating the unmet clinical need this trial addresses.
Trial Oversight
The trial (ISRCTN59733689, registration date 16/5/2017) was designed and coordinated by the REGAIN Consortium ( https://www.regainyourhearing.eu ), supported by an EU Horizon 2020 grant ( https://cordis.europa.eu/project/id/634893 ), sponsored by the Consortium lead Audion Therapeutics BV and overseen by an independent steering committee. The trial protocol was approved by national regulatory authorities, medical ethical committees and local R&D authorities in London (London, Central REC Committee, REC Number: 17/LO/0632), Athens (Hellenic Republic Ministry of Heath National Ethics Committee - Reg. No.: 83052/2018) and Tübingen (Ethik‐Kommission der Medizinischen Fakultät und am Universitätsklinikum Tübingen, Proj. No.: 592/2018AMG1). The trial was conducted in accordance with GCP and principles of the Declaration of Helsinki and enrolment first started after trial registration and ethical approval Patient and study data were monitored by an independent Contract Research Organisation. During the Phase I trial, an independent Data Safety Monitoring Board (DSMB) assessed safety and tolerability (see Supplementary Information page 4).
Eligible for the Phase I/II trial were patients aged from 18 to 80 years with a primary complaint of hearing loss of more than 10 years duration and stable hearing, their history suggesting an age-related, noise-induced or unknown cause, and diagnosed at the trial screening visit with bilateral symmetrical (<15 dB difference between ears) mild to moderate SNHL (mean pure-tone hearing threshold 25 to 60 dB HL across frequencies 0.5, 1, 2, 4 and 8 kHz). Patient sex was self-reported. Excluded were patients presenting with a primary complaint of tinnitus, a ‘true’ air-bone gap >15 dBHL in 3 or more contiguous frequencies between 0.5, 1, 2, 4 kHz, a history of suspected or diagnosed genetic cause of hearing loss, suspected or known diagnosis of inner ear pathology (see Table S1 in the Supplementary Information for details), evidence of acute or chronic middle ear disease and/or surgery), use of ototoxic medication within 12 months of screening, or ongoing or planned systemic or local drug-based therapy for SNHL or tinnitus during the study.
Trial Procedures
This Phase I/ IIa trial was open-label so there was no randomisation or blinding. Patients either self-referred to the local trial teams via the REGAIN website https://www.regainyourhearing.eu/ or were referred by ENT surgeons or audiologists from hospitals serving as Participant Identification Centres (PICs). Trial teams pre-screened potential participants over the phone and gave information about the trial.
Those potentially meeting the inclusion criteria were invited to a screening visit at the trial hospital sites where informed written consent was taken for the trial, and hearing and balance assessments as well as vitals sign measurements were conducted to verify eligibility (see Table 3 ). Patients were not compensated for participation in the study, except for reimbursement of travel expenses.
For the Phase I trial in UK, a Cone Beam CT scan of the temporal bone was made to explore accessibility and permeability of the round window to the study drug. Results were classified into 3 groups: no, up to 50%, and 50-100% opacification of the RWN, where the last group would be excluded from the trial. Because no patients met this exclusion criteria in the Phase I trial, this investigation was taken out of the Phase IIa trial protocol.
At the baseline visit, after confirming eligibility, patients were treated with the study drug in one ear, i.e., the poorer hearing ear according to pure-tone and/or speech audiometry, in case of no difference patients were asked to identify their poorer hearing ear, and if neither applied the ear best accessible for injection was chosen. Under otomicroscopic visual control lidocaine/prilocaine cream (EMLA® cream) was applied onto the tympanic membrane to achieve local anaesthesia; the cream was removed after 30-45 minutes using microsuction. With the patient in supine position 0.5 ml of the study drug was injected into the anterior inferior quadrant of the tympanic membrane with a 25/26-gauge needle and bevel facing in an inferior posterior direction to a depth of 2-3 mm just inferior to the round window niche. Patients remained supine for 30 minutes, their head turned 45 degrees towards the treated ear and advised to not talk, sneeze, and cough. They were monitored for safety for 24 hours after the first injection of the study drug in the Phase I trial and for 4 hours after the second and third injection in the Phase I and all injections in the Phase IIa trial.
In the single-site (UK) Phase I first-in-human trial (from January 24 to October 17, 2018), consecutive cohorts of 3 patients received 3 intratympanic injections of LY3056480 at a specified dose level (ascending doses of 25, 125, 200, 250 µg across cohorts), one week apart in one ear. After dosing each cohort, the Data Safety Monitoring Board assessed the safety and tolerability of the study drug and procedure. In addition, the protocol stated that the trial (Phase I and IIa below) may be discontinued at the discretion of the Coordinating Investigator, Principal Investigator, Sponsor or Independent Ethics Committee based on the occurrence of the following (but not limited to): AEs unknown to date with respect to their nature, severity, and duration; increased frequency and/or severity and/or duration of AEs; medical or ethical reasons affecting the continued performance of the study; cancellation of drug development; notification by regulatory authorities.
In the Phase IIa trial across 3 tertiary care otology services (UK, Greece, Germany; from January 30 to August 5, 2019) all patients received 3 intratympanic injections of 250 µg LY3056480 one week apart in one ear.
Patients were followed up after 6 and 12 weeks, with an optional long term follow up visit at 6 and 12 months. During all trial visits a broad repertoire of hearing assessments were performed: pure-tone audiometry, speech-in-noise, middle ear immittance, DPOAE and TEN test, balance tests (eye movements, head thrust, modified Romberg, Unterberger, bithermal air calorics), quality of life measurements (HHIA/E, TFI, DHI) and general safety assessments (facial nerve and taste function, laboratory, vital signs, ECG). The full trial protocol is provided as “Supplementary Data 1 ” (study protocol version 1.0) and “Supplementary Data 2 ” (study protocol version 5.0).
As per the trial protocol (see Supplementary Information) the primary outcome of Phase I was safety and tolerability in terms of occurrence and severity of treatment and procedure-related local and systemic Adverse Events (AEs). The secondary outcome was the optimal dose of LY3056480 for Phase IIa. Local safety outcomes included changes in hearing in the treated ear, tinnitus, balance and facial nerve function. Systemic safety outcomes included vital signs, haematology, chemistry and electrocardiography. Adverse Events were graded according to Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 27 . The same safety outcomes were assessed in Phase IIa.
The Phase IIa primary efficacy outcome was change in the average pure-tone air-conduction threshold (in dB HL) across 2, 4 and 8 kHz in the treated ear from baseline to 12 weeks. Secondary efficacy outcomes were changes from baseline to 6 and 12 weeks for both the treated and untreated ear in: (1) the average pure-tone air-conduction threshold (in dB HL) across 2, 4 and 8 kHz; (2) pure-tone air-conduction thresholds at individual frequencies (dB HL), including 12.5 and 16 kHz; (3) speech reception threshold in noise, expressed as the SRT50n which is defined by the signal-to-noise ratio (SNR) that yields an average response of 50% correctly recognised words; (4) distortion product otoacoustic emissions (DPOAE) mean amplitude and SNR, for both low-level (65/55 dB SPL) and high-level (70/70 dB SPL) tone primaries; (5) Hearing Handicap Inventory for Adults/Elderly (HHIA/E) score (per participant).
Audiological assessments
Pure-tone audiometry was conducted at 0.25 kHz, 0.5 kHz, 1 kHz, 2 kHz, 3 kHz, 4 kHz, 6 kHz, 8 kHz, 12.5 kHz and 16 kHz, following a ‘down-10/up-5” dB rule (5 dB step-size) in the UK (Otometrics Madsen Astera 2; Sennheiser HDA 300 circum-aural high-frequency headphones; Bone-Conductor Otometrics BC-71) and Greece (Interacoustics Affinity; circumaural Headphones DD45; Sennheiser HDA 300 (circum-aural high-frequency headphones); Bone-conductor Radioear B71) and a down-10/up-1 rule (1 dB step-size) in Germany (AT1000 Auritec; Sennheiser HD300 circum-aural high-frequency headphones; Bone-Conductor Radioear B71W), as per local guidelines. Threshold was defined as the lowest decibel hearing level at which responses occur in at least one half of a series of ascending trials. The minimum number of responses needed to determine the threshold of hearing was two responses out of three presentations at a single level. Blinding of the Audiologists to previous audiometric results was not included in the study design.
Speech perception in noise was measured with a words-in-babble test (WiB) at all sites 28 . Care was taken that the same word lists were not presented at each visit. In UK, the words-in-babble test used lists of 25 monosyllabic meaningful English words as targets, the masking noise was a multi talker babble (Otometrics Madsen Astera 2). The test was presented monaurally in a sound-proof room on a calibrated computer using custom-written Matlab software with Sennheiser HD 125 headphones. The SNR was varied adaptively during the test, with the level of the masker fixed at about 65 dB SPL and the level of the target speech varied. The initial SNR was +20 dB SNR and was decreased after each single correct response (i.e., increasing difficulty) or increased after each incorrect response (i.e., decreasing difficulty) ± 2 dB SNR. The test stopped either at 8 reversals or 25 words. A threshold value was then calculated as the mean of the final six to eight reversals, which is the SNR needed for a performance level of about 50% correct, known also the Speech Reception Threshold (SRT50n).
In Germany, the WiB test used lists of 20 monosyllabic meaningful German words as targets (Freiburger Test), the masking noise was a multi-talker babble. The test was presented monaurally in a sound-proof room on an Auritec AT1000 (Auritec) with Sennheiser HD 125 headphones. The level of the masker was fixed at 65 dB SPL and the level of the target speech varied, starting with the initial SNR of 20 dB. To determine the SRT50n, at least 2 lists of each 20 words were tested at different levels, and SRT was calculated from a regression of the two test lists that were just below and just above the 50% intelligibility.
In Greece, the WiB test used 4 lists of 50 disyllabic phonemically balanced words and multi-talker babble as the masking noise. The test was presented monaurally (Interacoustics Affinity with DD45 headphones) in a sound-proof room. Each of the 4 lists were presented in a random order. The signal intensity level remained constant at 20 dB over the SRT while the babble noise intensity was varied. Each ear was presented with a total of 9 noise levels (−6 dB, −3 dB, −1 dB, 0 dB, +1 dB, +3 dB, +6 dB, +9 dB, + 12 dB), always with this order and starting from the most demanding presentation (−6 dB) to reduce the learning effect. The SRT50n was derived directly from the performance intensity (PI) function.
DPOAE measurements were carried out for both low-level (65/55 dB SPL) and high-level (70/70 dB SPL) tone primaries on the Madsen Capella2 (Natus Medical) in UK and Germany and on TITAN (Interacoustics) in Greece. Settings were: F2/F1 Ratio 1.22; 8 Bands per octave, 3 Blocks, 90 sweeps and 5 retries. At the beginning and at the end of each measurement, the correct fit of the probe in the ear was verified by the device software according to the manufacturer’s recommendation.
Statistical analysis
The number of participants in Phase I was determined by its 3 + 3 design. For Phase IIa we set a recruitment target of 40 patients, based on 87% power to detect a 10 dB change (standard deviation 20 dB) corresponding to an effect size of 0.50 29 .
Data was collected through an eCRF, for Phase I via Open Clinical and for Phase IIa via Castor. Analyses were performed with SAS (v9.4), SPSS (v26), and R (v3.3.1).
As per protocol, all data collected and available was used in the analysis. No imputation of missing data was applied. See the Statistical Analysis Plan (SAP, see “Supplementary Data 3 ”) for details on handling of missing data. The detailed SAP was finalised before database lock.
All Phase IIa analyses were performed according to modified intention-to-treat, including patients with pure-tone audiometry data at baseline and at least once post-LY3056480 administration. The statistical significance level was set at 0.05. Bonferoni correction was used to control for family-wise error rate.
Phase IIa efficacy outcomes were analysed separately for treated and untreated ears (except for Hearing Handicap Inventory for Adults/Elderly) and for the total number of participants across the three trial sites as well as per trial site. A linear mixed-effect model was used to account for repeated measures and the multilevel structure of the pure-tone audiometry, speech-in-noise data and DPOAEs. Patient age, baseline audiometric values, follow-up timepoint, and timepoint-by-treatment interaction were entered as fixed factors. Patient (random intercept) was entered as random factors. An unstructured covariance matrix was used.
See Supplementary Information for the post-hoc exploratory analyses performed for pure-tone audiometry and speech perception in noise measures at the optional follow-up visits 6 and 12 months and for results per trial site.
For pure-tone audiometry we analysed the mean change from baseline to 6 and 12 weeks in the average pure-tone threshold across 2, 4, and 8 kHz, as well as at individual frequencies, including 12.5 and 16 kHz.So far, very few studies of hearing regenerative drugs have been conducted and definitions of clinically important hearing improvement in terms of pure-tone thresholds have not been agreed upon. Some guidance can be deducted from the 1994 American Speech-Language-Hearing Association (ASHA) Guidelines for the Audiologic Management of Individuals Receiving Cochleotoxic Drug Therapy 30 , where a hearing change (in this case decrease) is defined as a 20 dB decrease at any one test frequency; or a 10 dB decrease at any two adjacent test frequencies. Likewise, Campbell et al. 31 define a significant and clinically relevant noise-induced threshold shift as ≥10 dB at one or multiple test frequencies. We therefore also analysed at 6 and 12 weeks the number and percentage of participants showing: (a) a positive change of ≥10 dB in pure-tone air conduction- threshold at any frequency, and (b) a positive change of ≥10 dB in pure-tone air-conduction threshold in two or more adjacent frequencies, or ≥20 dB in a single frequency.
For speech perception in noise we analysed the mean change in SRT50n (see above) from baseline to 6 and 12 weeks. For DPOAEs we analysed the mean amplitude and SNR, for both low-level (65/55 dB SPL) and high-level (70/70 dB SPL) tone primaries as well as the change in overall distribution of DPOAE categories ‘present-normal, present-abnormal, and absent’ from baseline to 6 and 12 weeks, using the MacNemar test.
HHIE and HHIA scores were combined rather than analysed separately, as the scoring mechanisms are the same and have been tested for internal consistency reliability. We analysed the mean change in score from baseline to 6 and 12 weeks, applying the McNemar test.
Differences between the treated and untreated ear in change from baseline to 6 and 12 weeks were expressed as numbers of patients with a difference of >10 dB in one or more frequencies for pure-tone hearing threshold across 2, 4, and 8 kHz. No statistical analysis was performed on these numbers.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The latest version (5.0) of the study protocol, the statistical analysis plan and source data file with demographics, adverse events and pure-tone audiometry data are provided in with this manuscript and its Supplementary Information. Additional Data supporting the current trial findings can be made available from the REGAIN Consortium (Rolf Jan Rutten, [email protected], Audion Therapeutics BV, Amsterdam, the Netherlands). All study data will be shared from the publications onwards until 1 year after publication in a password protected controlled environment, including individual deidentified participant data. Responses will be sent within 2 weeks. Restrictions apply to the availability of these data due to planned regulatory submission. Source data are provided with this paper.
Wilson, B. S., Tucci, D. L., Merson, M. H. & O’Donoghue, G. M. Global hearing health care: new findings and perspectives. Lancet 390 , 2503–2515 (2017).
Article PubMed Google Scholar
www.who.int/publications/i/item/world-report-on-hearing .
Cunningham, L. L. & Tucci, D. L. Hearing Loss in Adults. N. Engl. J. Med. 377 , 2465–2473 (2017).
Article PubMed PubMed Central Google Scholar
Wu, P. Z., O’Malley, J. T., de Gruttola, V. & Liberman, M. C. Age-related hearing loss is dominated by damage to inner ear sensory cells, not the cellular battery that powers them. J. Neurosci. 40 , 6357–6366 (2020).
Article CAS PubMed PubMed Central Google Scholar
Dillon, H., Day, J., Bant, S. & Munro, K. J. Adoption, use and non-use of hearing aids: a robust estimate based on Welsh national survey statistics. Int J. Audio. 59 , 567–573 (2020).
Article Google Scholar
McCormack, A. & Fortnum, H. Why do people fitted with hearing aids not wear them? Int J. Audio. 52 , 360–368 (2013).
Crowson, M. G., Hertzano, R. & Tucci, D. L. Emerging therapies for sensorineural hearing loss. Otol. Neurotol. 38 , 792–803 (2017).
Schilder, A. G. M. et al. Hearing protection, restoration, and regeneration: an overview of emerging therapeutics for inner ear and central hearing disorders. Otol. Neurotol. 40 , 559–570 (2019).
Schilder, A. G. M. et al. Early phase trials of novel hearing therapeutics: avenues and opportunities. Hear Res 380 , 175–186 (2019).
Article CAS PubMed Google Scholar
Isherwood, B., Gonçalves, A. C., Cousins, R. & Holme, R. The global hearing therapeutic pipeline: 2021. Drug Discov. Today 27 , 912–922 (2022).
Cousins, R. Hearing loss drug discovery and medicinal chemistry: current status, challenges, and opportunities. Prog. Med Chem. 61 , 1–91 (2022).
Fujioka, M Y. T. K. S. et al. Topical administration of a gamma-secretase inhibitor LY411575 into the round window niche ameliorates permanent threshold shift in a noise-induced hearing loss model of a non-human primate, the common marmoset. In: 55th Inner ear Biology Workshop (Berlin, 2018) (2018).
Jeon, S. J., Fujioka, M., Kim, S. C. & Edge, A. S. Notch signaling alters sensory or neuronal cell fate specification of inner ear stem cells. J. Neurosci. 31 , 8351–8358 (2011).
Mizutari, K. et al. Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron 77 , 58–69 (2013).
Du, X. et al. Regeneration of cochlear hair cells and hearing recovery through Hes1 modulation with siRNA nanoparticles in adult guinea pigs. Mol. Ther. 26 , 1313–1326 (2018).
Erni, S. T. et al. Hair cell generation in cochlear culture models mediated by novel γ-secretase inhibitors. Front. Cell Dev. Biol. 9 , 710159 (2021).
Dallos, P. & Harris, D. Properties of auditory nerve responses in absence of outer hair cells. J. Neurophysiol. 41 , 365–383 (1978).
Hoben, R., Easow, G., Pevzner, S. & Parker, M. A. Outer hair cell and auditory nerve function in speech recognition in quiet and in background noise. Front. Neurosci. 11 , 157 (2017).
Hunter, L. L. et al. Extended high frequency hearing and speech perception implications in adults and children. Hear Res. 397 , 107922 (2020).
McLean, W. J. et al. Improved speech intelligibility in subjects with stable sensorineural hearing loss following intratympanic dosing of FX-322 in a phase 1b study. Otol. Neurotol. 42 , e849–e857 (2021).
Doosti, A., Lotfi, Y., Moosavi, A., Bakhshi, E. & Talasaz, A. H. Distortion Product Otoacoustic Emission (DPOAE) as an appropriate tool in assessment of otoprotective effects of antioxidants in noise-induced hearing loss (NIHL). Indian J. Otolaryngol. Head. Neck Surg. 66 , 325–329 (2014).
Zelle, D., Lorenz, L., Thiericke, J. P., Gummer, A. W. & Dalhoff, E. Input-output functions of the nonlinear-distortion component of distortion-product otoacoustic emissions in normal and hearing-impaired human ears. J. Acoust. Soc. Am. 141 , 3203 (2017).
Article ADS PubMed PubMed Central Google Scholar
McAlpine, D., Goldman, D. & Schilder, A. G. M. Mind the gap—developing a sustainable pipeline for hearing therapeutics. ENT Audioloy N. 4 , 33–34 (2022).
Google Scholar
Kurzrock, R. et al. Moving beyond 3+3: the future of clinical trial design. Am. Soc. Clin. Oncol. Educ. Book 41 , e133–e144 (2021).
Salt, A. N. & Plontke, S. K. Principles of local drug delivery to the inner ear. Audio. Neurootol. 14 , 350–360 (2009).
Article CAS Google Scholar
Jerlvall, L. & Arlinger, S. A comparison of 2-dB and 5-dB step size in pure-tone audiometry. Scand. Audio. 15 , 51–56 (1986).
National Institutes of Health NCI, Common Terminology Criteria for Adverse Events (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm . (2017).
Spyridakou, C., Rosen, S., Dritsakis, G. & Bamiou, D. E. Adult normative data for the speech in babble (SiB) test. Int J. Audio. 59 , 33–38 (2020).
Suckfuell, M. et al. Efficacy and safety of AM-111 in the treatment of acute sensorineural hearing loss: a double-blind, randomized, placebo-controlled phase II study. Otol. Neurotol. 35 , 1317–1326 (2014).
Association. AS-L-H. Audiologic management of individuals receiving cochleotoxic drug therapy [Guidelines]. Available from www.asha.org/policy . (1994).
Campbell K., Hammill T., Hoffer M., Kil J., Le Prell C. Guidelines for Auditory Threshold Measurement for Significant Threshold Shift. Otol. Neurotol. 37 , e263–e270 (2016).
Download references
Acknowledgements
Horizon 2020, the EU Research and Innovation programme, funded the REGAIN project (ISRCTN number 59733689). Audion Therapeutics BV funding supported long-term follow up of trial participants. We thank Kim Airey, George Shaya, Marta Merida, Glyn Ang, Rishi Mandavia, Nishchay Mehta, Joseph Manjaly, Robert Nash, Andrew Hall, Joanne Palmer, Tanjjnah Ferdous, Natallia Kharytaniuk, Royal National ENT Hospital nursing team, Lamprini Agrapida, Katharina Thum, and Andreas Heyd for their contribution to the REGAIN trial. We are grateful to the participants of this trial whose motivation and support were invaluable to the success of the REGAIN project. We are grateful to the participants of this trial whose motivation and support were invaluable to the success of the REGAIN project.
Author information
Authors and affiliations.
National Institute for Health Research University College London Hospitals Biomedical Research Centre, London, UK
Anne G. M. Schilder, Shakeel Saeed, Helen Blackshaw, Anne Schilder, Elizabeth Arram, Hannah Cooper, Karin Hojgaard & Omursen Yildirim
Ear Institute, University College London, London, UK
Royal National ENT and Eastman Dental Hospitals, University College London Hospitals Trust, London, UK
Anne G. M. Schilder, Shakeel Saeed, Anne Schilder, Elizabeth Arram, Karin Hojgaard, Omursen Yildirim & Sherif Khalil
Department of Otolaryngology, Head and Neck Surgery, University of Tübingen, Tübingen, Germany
Stephan Wolpert, Ernst Dalhoff, Hubert Lowenheim, Marcus Mueller, Thore Schade-Mann, Fritz Schneider & Katerina Vardonikolaki
Middelinc, Utrecht, the Netherlands
Leonie M. Middelink & Leonie Middelink
Department of Otolaryngology, Harvard Medical School, Boston, USA
Albert S. B. Edge & Albert Edge
1st Department of Otolaryngology, Hippocration Hospital Athens, National & Kapodistrian University of Athens, Athens, Greece
Athanasios Bibas, Eleftheria Iliadou, Dimitris Kikidis, Nikos Markatos, Kostas Pastiadis & Athanasios G. Bibas
Nordic Bioscience, Herlev, Denmark
Asger Bilhet
TTopstart BV, Utrecht, the Netherlands
Femke van Diggelen
Audion Therapeutics BV, Amsterdam, the Netherlands
Rolf Jan Rutten & Helmuth van Es
Eli Lilly and Company, Indianapolis, USA
August Wilke
You can also search for this author in PubMed Google Scholar
REGAIN Consortium
- Anne Schilder
- , Stephan Wolpert
- , Shakeel Saeed
- , Leonie Middelink
- , Albert Edge
- , Helen Blackshaw
- , Kostas Pastiadis
- , Athanasios Bibas
- , Elizabeth Arram
- , Asger Bilhet
- , Hannah Cooper
- , Ernst Dalhoff
- , Femke van Diggelen
- , Rolf Jan Rutten
- , Helmuth van Es
- , Karin Hojgaard
- , Eleftheria Iliadou
- , Omursen Yildirim
- , Sherif Khalil
- , Dimitris Kikidis
- , Hubert Lowenheim
- , Nikos Markatos
- , Marcus Mueller
- , Thore Schade-Mann
- , Fritz Schneider
- , Katerina Vardonikolaki
- & August Wilke
Contributions
A.G.M.S. is the coordinating investigator, S.S., S.W. and A.G.B. the principal investigators at the 3 sites. A.G.M.S., S.W., S.S., A.S.B.E., H.B., L.M.M. and A.G.B. designed and planned the trial and its data analysis and interpreted the results. K.P. and A.G.B. performed statistical analyses. The first draft of the manuscript was prepared by A.G.M.S., drafts were reviewed and edited by all authors. All authors made the decision to submit the manuscript for publication and vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol.
Corresponding author
Correspondence to Stephan Wolpert .
Ethics declarations
Competing interests.
A.G.M. Schilder advises hearing industry on clinical trial design and delivery. A.S.B. Edge is a consultant and shareholder of Audion Therapeutics BV. L.M. Middelink is consultant to Audion Therapeutics BV. The remaining authors declare no competing interests
Peer review
Peer review information.
Nature Communications thanks Lechoslaw Turski, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary infomation, peer review file, description of additional supplementary files, supplementary data 1, supplementary data 2, supplementary data 3, reporting summary, source data, source data, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Schilder, A.G.M., Wolpert, S., Saeed, S. et al. A phase I/IIa safety and efficacy trial of intratympanic gamma-secretase inhibitor as a regenerative drug treatment for sensorineural hearing loss. Nat Commun 15 , 1896 (2024). https://doi.org/10.1038/s41467-024-45784-0
Download citation
Received : 10 November 2021
Accepted : 01 February 2024
Published : 01 March 2024
DOI : https://doi.org/10.1038/s41467-024-45784-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Most people are aware of the relationship between healthy eating and vision (remember to eat your carrots so you can see well!). We also are…

Havana Syndrome Sparks Debate Among Experts
Audio-vestibular symptoms are gaining national attention and sparking debate amongst professionals and the public. A CBS 60 Minute report on Sunday, March 31, revisited Havana…

ADHD in Children in the United States
Audiologists sometimes see patients who also present with a diagnosis of attention-deficit/hyperactivity disorder (ADHD). Reuben and Elgaddal (2024) reported the percentage of children in the…
Join the Academy
Academy members receive many benefits for professional development, practice management, and community development.

Latest Issue
Psychiatry’s new frontiers
Hope amid crisis
Recent Issues
- AI explodes Taking the pulse of artificial intelligence in medicine
- Health on a planet in crisis
- Real-world health How social factors make or break us
- Molecules of life Understanding the world within us
- The most mysterious organ Unlocking the secrets of the brain
- All Articles
- The spice sellers’ secret
- ‘And yet, you try’
- Making sense of smell
- Before I go
- My favorite molecule
- View all Editors’ Picks
- Diversity, Equity & Inclusion
- Infectious Diseases
- View All Articles
Hear and now
Better, less costly treatments for hearing loss coming soon
By Julie Greicius
Illustration by Gérard DuBois

As far as Amy Yotopoulos could tell, her father, Edwin Lutz, was changing with age, the way most people do. He had become more introverted and quiet, less engaged. But he was still the same. He was simply finding everything harder to hear.
As his six grandchildren were born and learned to talk, he had difficulty picking up what they were saying. Trying to keep up in any conversation wasn’t just difficult, it was sometimes impossible. “If I didn’t hear something right at the beginning, I just gave up. So I just kind of gave up on listening,” Lutz said.
Lutz, who is now 76, was frustrated with his first pair of hearing aids, which he received about 12 years ago. He would only put them in if his wife, Peggy, suggested it, and then they would squeal or he’d still struggle to hear, only to give up and toss them back in the drawer. Yotopoulos watched as her father grew tired of saying “I can’t hear you” to her and his grandkids. “And it was hard for the kids to take turns, speak clearly and slowly, face Grandpa, et cetera,” she says. “So we all just figured this was the new normal.”
For Yotopoulos, director of the Mind Division of the Stanford Center on Longevity , this new normal was unacceptable. How could a man whose daughter was an expert on healthy aging be unable to address his hearing loss? Yotopoulos was well aware how common it is for aging adults to be afflicted with hearing loss; how rarely they get adequate treatment; and how vast the impact of untreated hearing loss can be on their health, mobility, finances and relationships.
With access to the Stanford Medicine physician-scientists who are making advances in the diagnosis and treatment of hearing loss, Yotopoulos also knew that new solutions were within reach — not just for her dad, but for the millions of people who have hearing loss today or are projected to have it in the future.
According to the National Institutes of Health, “Approximately one in three people between the ages of 65 and 74 has hearing loss, and nearly half of those older than 75 have difficulty hearing.” A review of literature in the October 2017 JAMA Otolaryngology raised that estimate, stating that hearing impairment affects nearly two-thirds of Americans who are 70 and older. Simply put, adults older than 70 are more likely to have hearing loss than to have normal, healthy hearing.
Despite these statistics, fewer than 20 percent of people with hearing loss obtain treatment. The small percentage of people who address the issue, such as by getting hearing aids, don’t usually do so until eight to 10 years after their initial diagnosis — enough time for some of the conditions related to hearing loss to take hold.
Understanding hearing loss
The way we live with hearing loss, however, is in the midst of a revolution, with enormous changes ahead. New approaches to testing and more affordable and effective treatments are clearing the way for healthier hearing in aging adults. Those changes can’t come soon enough. Aging adults are less willing than ever to let hearing loss slow them down and are more open to wearing advanced, in-ear devices.
Yotopoulos says people might be more motivated to take action if they were fully aware of the health consequences of not being treated.
“We can’t say hearing loss causes these issues at this point, but it’s correlated with decreased mental health, such as depression, and increased risk of cognitive decline, dementia and death,” Yotopoulos says. “It’s correlated with your balance, risk of falls and sense of social engagement. And we now know that social isolation has the same mortality and risk factors as smoking a pack of cigarettes a day, or as being obese.”
Because her dad had surmounted other serious health issues, his hearing loss seemed a relatively manageable problem. Still, it was a continual source of frustration. The simple joy of watching British television mysteries with his wife became a complicated activity full of missed moments and him asking questions. Lutz could no longer hear the sounds of the outdoors that he loved. And there was no joy in going to restaurants, where background noise made it impossible to follow conversations.
“I thought he was just kind of calmer or just wanted to watch things,” says Yotopoulos. “And it was a little sad because he wasn’t this ‘Hey let’s play a card game together’ kind of grandpa anymore.”
“Hearing loss is a potent isolator,” says Robert Jackler , MD, the Edward C. and Amy S. Sewall Professor in Otorhinolaryngology, professor of neurosurgery and of surgery at Stanford. “Human communication is nourishing not only to the soul, but also to the mind and, ultimately, the body. People who become isolated and unable to interact with others withdraw into an ever-closing circle that leads to unhappiness and depression for many.”

For older adults, that isolation has far-reaching consequences. “When you lose your hearing, you don’t just lose your ability to hear,” says Matthew Fitzgerald , PhD, assistant professor of otolaryngology-head and neck surgery at Stanford. “There’s evidence to suggest that the brain may reorganize, that the brain will change, when you have hearing loss and are deprived of sound.”
Losing our sense of sound as we age is, for most people, caused by the deterioration of cells deep in the inner ear known as hair cells. “It’s a popular misconception that hearing loss, when you grow older, is ‘nerve deafness.’ In fact, we know that the hearing part of the brain and the hearing nerve remain intact,” explains Jackler, who is chair of the Department of Otolaryngology-Head & Neck Surgery .
Instead, most adults experience a gradual breakdown of “a relatively small population of hair cells that take vibrations in the air and turn them into nerve impulses the brain understands as sound.” Repeated exposure to loud noise — at work sites or concerts, for example — can expedite that breakdown.
“It wasn’t a single event that caused my hearing loss. It was a gradual progression, part of it age, and part of it the environment I was in.”
As a boy growing up on a farm in South Dakota, Lutz was a hunter. “We shot rifles and shotguns,” he says. “And we didn’t wear any hearing protection.” In 1965, when Lutz was 23, he began his service as a helicopter pilot for the Army, serving in the Vietnam War. “Even though we had flight helmets and earplugs, the high frequency of the turbines caused complete high-frequency hearing loss,” says Lutz. In 1987, his military retirement physical showed that Lutz still had hearing in the lower ranges, but over time he began to lose that as well.
“It wasn’t a single event that caused my hearing loss,” says Lutz. “It was a gradual progression, part of it age, and part of it the environment I was in.”
The onset of hearing loss as we age may be gradual, but the accumulated effect on our population is developing into an epidemic of drastic proportions. This is especially true in the United States as the baby boom generation — the 75 million babies born from 1946 to 1964 — has reached or is about to reach senior citizenship. The number of individuals of all ages with mild to complete hearing loss will balloon from just under 44 million today to nearly 55 million in 2030.
With such vast demand for audiological health care services, coupled with recent advances in technology and treatments, the hearing care industry is ripe for transformation. Even as the industry is changing, so are the aging individuals it serves. “It used to be that grandmother sat upstairs and knitted and came down for dinners and was with the grandchildren,” says Jackler. “Now seniors want to live active, full and socially engaged lives. They want to go to seminars, enjoy restaurants and be out with friends until a much later age.”
That’s certainly true for Ed and Peggy Lutz. While most of their friends near their home in the state of Washington travel south for the winter, the Lutzes go north. Ed Lutz is the youngest male member of his ski club at Big White in Kelowna, British Columbia. The oldest member is 90. “I’ve had two strokes and lost a third of my peripheral vision on the left side, so I follow my wife down the slopes now,” he says.
Lutz has also had two heart attacks, remedied by a pacemaker and defibrillator. Yet he and his wife still enjoy hiking and backpacking, sea kayaking, sailing on the lake, playing bridge, golfing, and volunteering at the local community civic center and the annual chamber music festival. “At our age, one can never stop because if you do, you will never get back.”
That desire to stay active and engaged as an aging adult can accentuate the stigma of hearing loss and of using hearing aids. For many adults, losing hearing is a signal of increasing and inevitable physical fragility that can be profoundly difficult to accept. Wearing a hearing aid can feel like having that fragility openly on display. Many would rather live without it.
Couple this with the outrageous prices of hearing aids and the barriers to care feel insurmountable.
High demand for cutting costs of care
Hearing aids cost anywhere from $1,000 to $6,000, which includes the device — or devices, as two are often needed — and the professional services to fit and program them. Few insurance companies provide coverage for hearing aids, though the full coverage the Veterans Health Administration provides is a welcome exception.
Hearing aids are the third most expensive tangible investment most families make, after a house and car. Yet the investment is still a good choice because the use of hearing aids has been shown to mitigate income loss up to $22,000 annually for people with extreme hearing loss. Annual excess medical expenditures for U.S. adults with hearing loss who are 65 and older are estimated at $3.1 billion, according to a study in the Journal of the American Geriatrics Society published in June 2014.
“The profit margin for hearing aids approximates that for popcorn at the movie theater.”
“It’s horrendous how expensive hearing aids are. They are enormously overpriced,” says Jackler. “The profit margin for hearing aids approximates that for popcorn at the movie theater.”
That movie-popcornlike profit margin may soon be a thing of the past, as the new wave of hearing health takes hold. On Aug. 18, 2017, the Over-the-Counter Hearing Aid Act, designed to provide greater public accessibility and affordability for over-the-counter hearing aids, was signed into law. The law will make it easier for people with mild to moderate hearing loss to access hearing aids.
“The challenge,” explains Fitzgerald, “is getting people the right type of care they need for their level of hearing loss. Individuals who have more difficulty communicating will likely be best served by seeing an audiologist, while individuals who have less difficulty communicating should benefit from an over-the-counter device. What’s missing is a way to help triage or help guide patients as to what level of care they should be seeking.”
For family members like Yotopoulos and Peggy Lutz, having a way to help guide Ed Lutz to the right level of care would have simplified things. Recognizing that need — for people with hearing loss and their family members who notice it — Yona Vaisbuch, MD, clinical instructor of otolaryngology at Stanford, is developing an online tool for front-line assessment “to help bring people over the age of 55 to try solutions like hearing aids.”
First conceived in a six-month project with the Stanford Byers Center for Biodesign , the nonprofit website WeHearYou will offer a short video on awareness, a basic hearing screening, an explanation of treatments and an option to input your location to find a provider.
“Today we know that age-related hearing loss doesn’t start when you’re 60 or 70. That’s when it becomes really symptomatic,” says Vaisbuch. “We now know that people in their 30s are already beginning to experience subtle decline.” His hope is that the website will be a way to screen and inform as many people as possible.
“When you have hearing loss, you spend a lot of time just trying to compensate for that,” says Vaisbuch. “We call it the cognitive load. You’re putting all your cognitive effort into hearing, instead of into the other things you’re doing. With time, those brain changes will not be reversible. That’s why we need to treat hearing loss as soon as possible.”
Improving diagnostics
But to adequately treat hearing loss, patients must first have an accurate diagnosis — a key to revolutionized hearing care. Jackler and Fitzgerald are challenging the routine hearing tests of the past and are developing a new approach.
“The way hearing testing has been done for the past 60 years is threshold testing in a quiet room,” says Jackler. “Well, for most people, their problem is not how well they hear really soft whispers in a quiet room.”
“The reality is that when patients walk in the door, the No. 1 complaint they have is the difficulty of understanding speech in the presence of background noise,” says Fitzgerald, who is the chief of audiology at Stanford Health Care and Lucile Packard Children’s Hospital Stanford . “At Stanford, we’re taking the lead in trying to make speech in noise the default test of speech perception in the audiology test battery. This small, but fundamental shift would be one of the most significant changes in how hearing testing is done in this country in decades.”
The change would be particularly beneficial for older people with hearing loss, Fitzgerald says, because “when you get older, the ability to extract speech from background noise gets a little worse than when you’re younger, and the existing test battery doesn’t account for that possibility at all.” Testing a patient’s ability to communicate and understand speech may allow audiologists and physicians to parse the effects of aging from the effects of hearing loss.
While these new measures are part of the standard audiometric testing at Stanford, only some clinics nationally have incorporated them. “They are more often seen as something extra,” says Fitzgerald.
“When you have hearing loss, you spend a lot of time just trying to compensate for that.”
Changing decades of clinical practice doesn’t happen overnight, Fitzgerald says, and some audiologists question what it adds to their practice. His research aims to show how these measures can be readily integrated and to emphasize the additional information that can be gained from their use. The next, ideal step after that research is published, he says, would be for the governing bodies for both audiology and otolaryngology to recommend guidelines for speech in noise testing as part of the baseline audiological evaluation. “I’m optimistic that high-quality published research, in conjunction with maintaining a presence at national meetings, will facilitate this transition that is long overdue,” Fitzgerald says.
The more accurate the measure of a patient’s hearing difficulty, the more accurate and personalized treatment can be. Because hearing difficulty is highly individualized, from the degree and type of hearing loss right down to the shape of the ear, there’s no one-size-fits-all solution. With a broad selection of fittings, features and styles, a variety of hearing aids can drastically improve hearing for people with mild to severe hearing loss right now.
“There’s been more development in hearing aids in the past seven years than in the past 70,” says Gerald Popelka , PhD, adjunct professor of neurosurgery, head of the Stanford Ear Institute Neuromodulation Research Lab and one of the inventors of the first digital hearing aid.
High-tech advancements in devices
Those advances include everything from digital Bluetooth-connected hearing aids that work with devices like televisions and other sound systems to stream audio to designs that fit invisibly in the ear canal or with a low-profile shape around the ear. Each can be programmed to the individual frequency needs of the listener.
Even as today’s technology can readily solve the hearing difficulties of many people, the pace of innovation is still gaining momentum. Solutions to hearing loss are being developed both within the ear and beyond the ear. By the time that Generation Y — the only generation to outnumber baby boomers, and one that’s already accustomed to the regular use of in-ear devices — begins to reach age 60 in 2041, these innovations, along with others we cannot yet imagine, will be available.
“Increasingly,” says Jackler, “wearing something on your ear will be a badge of technological prowess rather than a marker of age and infirmity.” Jackler and his associates in Stanford’s audiology division and the Byers Center for Biodesign envision devices that are capable of doing much more than enhancing hearing for users of any age.
“It turns out that having a telemetry system attached to the human body has very important implications, not the least of which are for hearing,” says Jackler. “If you have something clipped on your ear that communicates out to a computer system, you have the ability to monitor oxygen, glucose, blood pressure and, through little EKG-like sensors on your chest, the electrical activity of your heart.”
All of this health monitoring could be available in the same device — Vaisbuch calls such devices “earables” — that stores and plays your music or audiobooks and connects to your phone or to audio text messaging. Such a device could, for example, alert you if you’re starting to cross the street when a car is coming or discreetly remind you of the name of the host’s spouse when you arrive at a party. As voice-to-text technology improves, it could eliminate the need for a keyboard, reducing carpal tunnel syndrome. It could be programmed to lower background noise and amplify voices in spaces that are often especially challenging for people with hearing loss, such as restaurants or theaters. It could also use noise-cancelling technology to silence the environment when desired.
“The ear becomes an important part of this enriched linkage between the human body and machines,” says Jackler. “You’re looking at a coupling of man and electronic device that really changes our understanding of how diseases function.”
Another intervention that Jackler hopes will become a reality in the near future is a biological cure for hearing loss. Through the Stanford Initiative to Cure Hearing Loss , more than a hundred scientists and technicians are working to cure inner-ear hearing loss — the type that results from hair cell degeneration — which remains incurable today.
“We’re very hopeful that this will come to humans in the next decades and that what was heretofore incurable will become curable.”
Stefan Heller , PhD, the Edward C. and Amy H. Sewall Professor and professor and vice chair of research in otolaryngology, was the first to discover the stem cells within the inner ear of mammals that can be converted to hair cells. “We know that hair cells regenerate in birds, reptiles and amphibians,” says Jackler, “but not particularly in mammals such as humans.”
Under Heller’s leadership, the hearing loss initiative is exploring the manipulation of pluripotent stem cells — foundational stem cells that exist in the patient’s own body, in this case in the ear itself — to restore hair cells and allow patients to hear again. So far, the team has seen success only in mice.
“We’re very hopeful that this will come to humans in the next decades and that what was heretofore incurable will become curable,” says Jackler, “and that we’ll be able to rejuvenate hearing in the elderly, in the child born deaf and in someone who’s lost hearing from a variety of medical conditions.”
Until that cure is available, Jackler is still enthusiastic about the increasing options available to patients. “I have a huge optimism about the future of our ability to help people with hearing loss,” he says.
For two days in March 2017, the Stanford Center on Longevity gathered international experts in a broad range of fields including audiology, psychology, engineering, architecture and health advocacy to focus on improving communication for people with hearing loss. Yotopoulos and her colleagues had organized the conference to increase awareness of the prevalence and risks of untreated hearing loss, and of the need to think about hearing in the context of accessibility for all ages and in all settings. “It’s such an important piece of what Stanford University is doing with education and with creating community,” Yotopoulos says. “If we aren’t able to hear and listen to each other, we don’t have anything.”
Participants at the conference looked at many aspects of hearing, such as how acoustics in public spaces could be enhanced; how hearing tests could be improved; how hearing aid device technology, service, and cost could be transformed for the better; and how close scientists are to a cure. Even as they underscored the coming crisis of hearing loss in a ballooning population of aging adults, they envisioned a world designed to enhance listening and communication.
“Don’t give up if the first pair of hearing aids doesn’t feel right. Let your mind get accustomed to the new sounds.”
Yotopoulos had already seen, personally, the impact of some of these changes. In 2015, her father was fitted with two Bluetooth-enabled hearing aids at a Veterans Affairs clinic in Washington. “The audiologist was great,” says Lutz. “He said, ‘Now you’ve got hearing aids, Ed, but you know you’ve still got to learn how to listen.’ And my wife thought that was really great advice.”
Lutz has his own advice for people with hearing loss: “Don’t give up if the first pair of hearing aids doesn’t feel right. Let your mind get accustomed to the new sounds.”
He’s happy to hear the birds easily again. And he’s able to keep up with the clues when he and his wife watch their favorite mysteries.
“My wife can set the TV volume to what’s comfortable for her, and with the device I can set it for what’s comfortable for me,” says Lutz. “And I’m not afraid to go to a restaurant or a crowded place because I can adjust the ambient noise. I feel more comfortable around people in conversations, and I understand the kids better.”
Being able to communicate easily with his wife has been the most meaningful improvement. “Just being able to hear what she said, rather than having to have her repeat herself three times,” says Lutz. “Now I can hear her more often and I can answer her, so it’s clear, yeah, I’m paying attention to you. We have a pretty good life, but the hearing aids have enhanced it.”
Though they live far apart, Yotopoulos loves talking with her dad on the phone about everything from the activities of her now-teenage kids to the Lutzes’ latest adventures — such as learning to play the guitar and speak Spanish. It’s not only easier for her to communicate with him, but it’s also good to see him as the active, involved person he really is. “Having the hearing aids that actually work for him changed so much,” she says.
Julie Greicius
Julie Greicius is the senior director of external communications in the Office of Communications.
Email the author
Advertisement
A new understanding of tinnitus and deafness could help reverse both
Investigations of the paradoxical link between tinnitus and hearing loss have revealed a hidden form of deafness, paving the way to possible new treatments
By Clare Wilson
17 April 2024

Working as a DJ in Liverpool, UK, a decade ago, James Rand would often leave work hearing strange sounds that he knew weren’t real — a high-pitched whine or a low rumble. These symptoms of tinnitus always disappeared by the time he awoke… until, one day in 2017, they didn’t.
A doctor confirmed that the sounds had probably been caused by Rand’s exposure to loud music for hours at a time. There were no treatments, bar ways to help him get used to it. “I knew I was never going to hear silence again ,” he says. “It was incredibly depressing.”
Today, though, the prospects for treating tinnitus aren’t so bleak. New research has led to neurostimulation devices that reduce the sounds’ volume. Moreover, several treatments are in development that could even silence tinnitus completely. “For the first time, we’re talking about a possible cure,” says Stéphane Maison at Harvard Medical School.
Unravelling the secrets of the vagus nerve will revolutionise medicine
These insights have also shed light on a common cause of hearing loss. In fact, they suggest that some of the same treatments for tinnitus could also restore hearing in people who have become partially deaf with age. “It has completely changed the way we think about hearing loss,” says Maison.
What is tinnitus?
Tinnitus is one of the most common long-term medical conditions, affecting up to a quarter of older adults . While the whining and rumbling experienced by Rand are common forms, others may hear whistling, humming, clicking or even musical hallucinations. The sounds can be intrusive and distracting, sometimes leading to depression, anxiety…
Sign up to our weekly newsletter
Receive a weekly dose of discovery in your inbox! We'll also keep you up to date with New Scientist events and special offers.
To continue reading, subscribe today with our introductory offers
No commitment, cancel anytime*
Offer ends 2nd of July 2024.
*Cancel anytime within 14 days of payment to receive a refund on unserved issues.
Inclusive of applicable taxes (VAT)
Existing subscribers
More from New Scientist
Explore the latest news, articles and features
Odd bump on praying mantis chest is actually world’s weirdest tongue
Subscriber-only
Most brain monitors sold to consumers don't keep your data private
Rat neuron injection lets mice that can’t smell sniff out cookies, brain activity seems to be more complex in baby girls than boys, popular articles.
Trending New Scientist articles
Scientists say new treatment could reverse hearing loss

UNDATED (WKRC) - Researchers may have found a way of reversing hearing loss with a new developing treatment.
Frequency Therapeutics, a spinout of MIT, is researching a new kind of therapy that could repair the hair cells that allow people to hear.
Hair cells detect sound, but they begin dying off after exposure to loud noises or drugs. However, Frequency Therapeutics is testing a way to use small molecules to program progenitor cells, which are descendants of stem cells. They are placed in the inner ear and replace the hair cells that have died.
More than 200 patients have undergone the regenerative therapy in four tests, three of which proved effective.
The company has seen positive results when testing speech perception, which Frequency co-founder and Chief Scientific Officer Chris Loose Ph.D. says , “is the number one goal for improving hearing.”
Frequency Therapeutics hopes to have another trial of 124 people with results available by 2023. If it proves successful, there could be a widespread testing of the new therapy that would be easier to administer to the general public.
Jeff Karp, a Harvard-MIT Health Sciences and Technology affiliate faculty member, says that the treatment might evolve into a procedure that could be conducted in a matter of hours.
Karp said , “I wouldn’t be surprised if in 10 or 15 years, because of the resources being put into this space and the incredible science being done, we can get to the point where reversing hearing loss would be similar to Lasik surgery, where you’re in and out in an hour or two and you can completely restore your vision.”

- Better Hearing Consumer
- Dizziness Depot
- FindHearing
- Hearing News Watch
- Hearing Economics
- Hear The Music
- Hear In Private Practice
- Hearing Technologies
- Hearing and Kids
- This Week in Hearing
- Hearing Technology Innovator Awards
- Submit Your Product

12 Companies Revolutionizing Hearing Restoration and Hearing Loss Prevention

In a not-so-distant past, the concept of advances in medical technology that exist today were deemed virtually impossible. The evolutions of science and technology has ventured beyond the boundaries of our imagination, turning once-unthinkable possibilities into tangible realities. For every obstacle faced, it seems that twice as many new opportunities have emerged.

Enter the dedicated scientists who have poured decades of research into investigating the perplexities of hearing loss reversal.
Investing in Hearing Restoration
Over the last decade, we’ve observed the rise of several companies utilizing diverse methods and approaches, all sharing a common objective: the restoration of hearing.
As progress unfolds, innovative medical solutions are emerging, particularly in the biotechnology sector. These companies are actively developing novel therapies not only to prevent or repair inner-ear damage but also to potentially restore normal hearing. While the aging process naturally impacts various faculties, including hearing, in our contemporary, noise-filled world, hearing loss transcends age boundaries, affecting both the young and the old.
While a multitude of emerging companies is tirelessly devoted to the cause of hearing restoration and related disorders, our focus now turns to some of the prominent organizations that have come to our attention over the past several years:
Drug and Pharmaceutical Approach:
Fennec pharmaceuticals, sound pharmaceuticals, spiral therapeutics.
- Autifony Therapeutics

Acousia Therapeutics
Gene and cell therapy approach:, lineage cell therapeutics, eli lilly (formerly akouos).
- Regeneron (formerly Decibel Therapeutics)
Rinri Therapeutics

PEDMARK® is a groundbreaking therapy specifically indicated to reduce the risk of ototoxicity associated with cisplatin use in pediatric patients with localized, non-metastatic solid tumors.
Cisplatin, a vital chemotherapy drug for pediatric cancers, frequently leads to permanent hearing loss, termed ototoxicity. This condition significantly impacts pediatric cancer survivors, affecting their speech, language, social-emotional development, and academic success.
PEDMARK® represents a unique formulation of sodium thiosulfate in single-dose vials for intravenous use, demonstrating efficacy and safety across two Phase 3 clinical studies—COG ACCL0431 and SIOPEL 6. These studies focused on pediatric cancers typically treated with intensive cisplatin therapy, including hepatoblastoma, germ cell tumors, osteosarcoma, neuroblastoma, medulloblastoma, and other solid tumors.
Annually, more than 10,000 children in the U.S. and Europe may receive platinum-based chemotherapy, exposing them to the risk of ototoxicity . PEDMARK® emerges as a critical preventive agent for this hearing loss, addressing a significant unmet medical need in pediatric oncology. Its approval marks a significant advancement in pediatric cancer care, aiming to mitigate the detrimental effects of cisplatin-induced ototoxicity and improve the quality of life for young cancer patients.

The company’s furthest developed indication is for the treatment of Meniere’s Disease . In the first half of 2023, the company announced that it had completed enrollment in its Phase 3 trial with SPI-1005 involving over 220 patients, examining the drug’s impact on hearing loss and tinnitus. While no longer recruiting, the study will monitor patients for three months and may extend to a 12-month safety study.
One of their key developments is a drug called SPI-1005. It’s a form of ebselen that you take as a pill. This medicine is designed to protect the inner ear from damage caused by loud noises that can lead to permanent hearing loss. SPI-1005 works like an antioxidant, reducing harmful substances in the ear that can affect hearing. It does this by acting as a copy of an enzyme called glutathione peroxidase (GPx), which is important for how the ear functions.
Moreover, Sound Pharmaceuticals has created another drug called SPI-3005. This drug is meant to help with hearing loss caused by certain medicines used in cancer treatment and infections. In tests on animals, SPI-3005 has shown it can protect against hearing loss caused by these drugs without stopping the drugs from doing their job.

Their innovative, award-winning drug delivery platform MICS™ (minimally-invasive cochlear system) is designed to accurately deliver a variety of drugs to the ear, allowing them to remain in the middle ear for extended periods, spanning from weeks to months. This adaptable system can be customized to administer drugs that have anti-inflammatory, otoprotective, and neuroprotective characteristics, aiming to address balance disorders and hearing loss. Its goal is to precisely administer drugs to the cochlea , tackling existing hurdles associated with delivering drugs to the inner ear.
Spiral Therapeutics is advancing its inner ear programs, with plans to submit an Investigational New Drug (IND) application for SPT-2101 in the United States by 2024, demonstrating a commitment to advancing treatments for inner ear disorders.
**Readers interested to learn more about Spiral can check out this March 2023 interview with company CEO Hugo Peris and Chief Medical officer, Dr. Charles Limb

Their lead therapy candidate, AC102, targets acute hearing loss and acute tinnitus, conditions lacking approved drug treatments. AC102 has shown promising results in preclinical studies, indicating its ability to act on sensory cells and neurons critical to the hearing process.
By potentially becoming the first causative therapy for these conditions, AC102 aims to prevent the progression of long-term, chronic hearing loss
AC102, a novel molecule, works by safeguarding and rejuvenating sensory hair cells and their connections to the auditory nerve. This therapeutic compound has exhibited impressive outcomes in models of hearing disorders, significantly improving hearing recovery and preserving auditory nerve connections. Notably, AC102 has demonstrated better efficacy in restoring hearing function compared to corticosteroid treatment, commonly recommended for sudden hearing loss.
The innovative aspect of AC102 lies in its specialized gel formulation, allowing for targeted drug delivery to the inner ear through intratympanic administration (injection through the eardrum). This method enables the drug to reach the inner ear cells directly without the need for frequent applications, reducing systemic exposure and potentially minimizing adverse side effects.
Autifony Therapuetics

They’re studying different types of these Kv3 channels found in the nervous system, muscles, and specific parts of the brain. Autifony’s research involves special molecules that aim to influence these channels, like the compound AUT1, showing potential for treating various hearing-related conditions by enhancing the function of nerve cells in the auditory system.
Studies have demonstrated the positive effects of these compounds on specific nerve cells in the brain responsible for processing sound information.

Acousia’s unique approach involves the development of small molecule drugs designed to both enhance and preserve a patient’s natural hearing by targeting validated cochlear-based elements.
The company’s leading drug candidate, ACOU085, is undergoing clinical trials for the prevention of cisplatin-induced hearing loss—a significant side effect of cisplatin-based chemotherapy affecting many cancer survivors. Their Phase 2 trial , approved by German regulatory authorities, aims to evaluate the effectiveness of ACOU085 in preventing cisplatin-induced ototoxicity in young male testicular cancer patients. This trial assesses various aspects of hearing, including audiometry, speech comprehension, and otoacoustic emissions. Acousia’s previous Phase 1b trial demonstrated the safety, tolerability, and prolonged local action of ACOU085, highlighting its potential for conditions such as cisplatin-induced hearing loss.
Regeneron Pharmaceuticals

The initial findings of DB-OTO show potential in reactivating auditory circuits, which could lead to improved hearing.
Administered via a single intracochlear injection, the therapy displayed positive auditory responses during follow-up assessments lasting up to six weeks, demonstrating enhanced hearing function safely and without adverse effects.
Congenital hearing loss affects 1.7 out of every 1,000 children in the U.S., with otoferlin gene mutations resulting in inactive auditory circuits.
DB-OTO’s preliminary success suggests a path towards restoring clinically significant hearing, aligning with Regeneron’s commitment to advancing gene therapies for auditory medicine. DB-OTO, initially a product of collaboration with Decibel Therapeutics, became part of Regeneron following their acquisition of Decibel Therapeutics .
This therapy, designed as a durable gene therapy utilizing a non-pathogenic virus, received Orphan Drug and Rare Pediatric Disease designations from regulatory agencies, indicating its potential significance in addressing this critical medical need.

The lead therapy candidate, AK-OTOF, focuses on addressing hearing loss caused by mutations in the otoferlin gene (OTOF), with additional programs targeting Usher Type 3A, GJB2-related deafness, and vestibular schwannoma.
In late January 2024, the company reported incredibly promising initial findings from the Phase 1/2 AK-OTOF-101 study, demonstrating restored hearing in the first participant within 30 days of AK-OTOF administration. This breakthrough presented compelling evidence of hearing improvement, with an 11-year-old experiencing restored hearing across tested frequencies, even reaching normal thresholds at some frequencies by Day 30. Notably, the therapy was well tolerated, with no adverse events reported. Prof. John Germiller hailed these results as a significant advancement in gene therapy for hearing loss, underscoring the trial’s collaborative efforts in addressing OTOF-mediated hearing loss.
As the largest pharmaceutical company in the world, Lilly plans to leverage it’s size and expertise to accelerate the development of treatments for people with genetic hearing loss.

Restore Hearing Through Gene Therapy
A major focus for Sensorion is the development of gene therapies to restore hearing in people with certain genetic forms of deafness. The company has two gene therapy programs:
- OTOF-GT aims to restore hearing in those with otoferlin deficiency. This rare condition causing hearing loss from childhood is due to mutations in the OTOF gene. Sensorion’s therapy delivers a normal working copy of the OTOF gene to replace the faulty one.
- GJB2-GT targets GJB2-related hearing loss, one of the most common childhood forms. Mutations in the GJB2 gene lead to loss of the connexin 26 protein vital for hearing. The gene therapy adds a normal GJB2 gene to compensate for the mutant copy.
Both approaches use a modified virus to deliver the gene into the inner ear through an injection. OTOF-GT is furthest along, having started Phase 1/2 human trials in 2022 to test safety. A larger Phase 2 efficacy trial is slated for late 2023. GJB2-GT is at an earlier preclinical stage with first human trials planned in 2024.
Treat Sudden Hearing Loss
Sensorion’s lead drug candidate SENS-401 aims to treat sudden sensorineural hearing loss (SSNHL). This sudden hearing impairment happens in less than 72 hours and can be permanent if untreated. It can also be accompanied by tinnitus and vertigo. SENS-401 protects inner ear cells from damage to try to prevent permanent deafness.
Preventing Hearing Loss in At-Risk Patients
Finally, Sensorion has two preclinical programs for using SENS-401 to prevent hearing loss:
- Cisplatin-induced ototoxicity – Cisplatin chemotherapy frequently causes permanent inner ear damage and hearing loss. SENS-401 may reduce this risk.
- Cochlear implantation – Sensorion has partnered with Cochlear Ltd to test using SENS-401 to prevent hearing loss in patients undergoing cochlear implant surgery . The drug could help preserve remaining natural acoustic hearing in implant recipients.
In summary, Sensorion is leveraging gene therapies, a lead drug candidate, and strategic partnerships to target restoring lost hearing and preventing further loss across a range of inner ear conditions. The company aims to address significant unmet needs through this multifaceted therapeutic approach.
**Readers interested to learn more about Lineage’s work can check out this September 2023 interview with company CEO Nawal Ouzren
Myrtelle Inc.

Myr-201 is designed to send a special gene called TMPRSS3 directly into the inner ear using a method referred to as low-dose recombinant adeno-associated virus (rAAV) gene therapy. Forge Biologics, known for its work in gene therapy, will provide important manufacturing services for Myrtelle’s program at its special gene therapy facility in Columbus, Ohio.
This collaboration is a big move forward for Myrtelle as they work on changing how gene therapy helps people with DFNB8-related hearing loss.
Their treatment involves putting the gene therapy straight into the inner ear, aiming to avoid possible problems that can come with using a lot of gene therapy throughout the body. Tests in animals have shown promising results, suggesting that Myr-201 might help protect important ear cells and improve hearing in mice with DFNB8-related deafness.
Myrtelle’s main goal is to quickly move into Phase 1/2 clinical trials, hoping to offer this new therapy to people dealing with DFNB8-related hearing problems.

Using cutting-edge science originating from Professor Marcelo Rivolta’s groundbreaking work in sensory stem cell biology, Rinri aims to develop effective treatments that target sensory replacement.
Their innovative approach involves deriving auditory sensory cells from renewable human progenitors, focusing on restoring function by developing functional auditory neurons. Preclinical studies have shown promising outcomes, including survival and maturity of auditory neuron progenitors when delivered to the cochlea, resulting in an improvement of hearing threshold by approximately 40% in auditory neuropathy models.
Furthermore, Rinri has made significant progress in refining the manufacturing process of these cells to meet the required standards for initiating clinical trials in humans. The company has also overcome challenges related to accessing the cochlea, a pivotal area for hearing loss treatment, by developing a safe surgical route validated through collaborative efforts with experts in the field.
Rinri is currently preparing for its first human clinical trials involving Rincell-1 , aiming to administer it to adults experiencing severe-to-profound age-related hearing loss or Auditory Neuropathy eligible for cochlear implants under UK NICE guidance (TA566). These trials represent a significant step forward in exploring the potential of regenerative cell therapy as a treatment for various forms of hearing loss.

These cells can be transplanted into a patient’s inner ear to replace damaged or lost auditory nerve cells.
The company’s goal is to address hearing loss caused by issues with nerve fibers that connect the ear’s hair cells to the brain. Instead of focusing solely on amplifying sound signals like hearing aids or cochlear implants, Lineage aims to restore hearing by regenerating these damaged connections using new auditory neurons.
Drawing from their success in replacing retinal cells for macular degeneration with OpRegen, Lineage aims to apply a similar strategy to hearing loss.
They have already achieved initial success in testing these auditory neurons in animal models, partnering with the University of Michigan to understand the optimal placement and survival of these cells. This data will guide future human trials.
The company has moved rapidly from concept to animal testing, leveraging their existing expertise, and anticipates at least two more years of preclinical work before considering human studies. Lineage’s unique approach could potentially revolutionize hearing loss treatments, offering long-lasting repairs that current methods cannot achieve.
**Readers interested to learn more about Lineage’s work can check out this May 2023 interview with company CEO Brian Culley
The ongoing research efforts aimed at restoring hearing highlight the profound impact of hearing loss globally. While some of these innovative solutions have shown promise in trials, there remains much to learn during their implementation.
It’s important to recognize the significance of timely interventions for hearing loss, considering the advancements made by hearing aids and cochlear implants in managing this condition. These developments pave an exciting path for the future of hearing healthcare.
The pace of these developments is rapidly accelerating, underscoring the need to stay informed about the latest trends in this field. Of course, it’s equally vital to seek the guidance of your trusted hearing healthcare practitioner for expert insights on the path forward.
Che-hears to better hearing!
About the Author

Leave a Reply Cancel Reply
Save my name, email, and website in this browser for the next time I comment.

- April 24, 2024 | Shedding Pounds, Dodging Cancer: The Life-Saving Promise of Bariatric Surgery
- April 24, 2024 | Quantum Computing Meets Genomics: The Dawn of Hyper-Fast DNA Analysis
- April 24, 2024 | Scientists Turn to Venus in the Search for Alien Life
- April 24, 2024 | NASA Astronauts Enter Quarantine As Boeing Starliner Test Flight Approaches
- April 23, 2024 | Peeking Inside Protons: Supercomputers Reveal Quark Secrets
New Hope for Hearing Loss Treatment: Researchers Identify 48 Genes Linked to Hearing Loss
By King's College London June 13, 2022
To identify the genes, the researchers used data from 723,266 persons from 17 studies who had clinically diagnosed or self-reported hearing impairment and examined genetic tests previously conducted in centers throughout the globe.
Researchers identified 48 genes that are associated with hearing loss, including 10 new variants.
Hearing loss is a common problem caused by noise, age, disease, and genetics. While using ear protection can help prevent hearing loss due to noise, you may think there is nothing you can do when the cause is age and genetics. However, scientists are working on developing new medicines and gene therapies for these conditions.
Research shows that one in eight people in the United States (13%, or 30 million) aged 12 years or older has hearing loss in both ears, based on standard hearing examinations. Conversations with friends and family can be difficult for those with hearing loss. They may also have difficulty understanding medical advice, reacting to warnings, and hearing doorbells and alarms.
Scientists from King’s College London , the Karolinska Institute , and Erasmus University have recently discovered ten additional genes connected to hearing loss, and located the part of the ear affected.
The results, which were published in the American Journal of Human Genetics on May 16th, 2022, put doubt on the notion that age-related hearing loss is primarily caused by sensory hair cells. The stria vascularis, a region of the cochlea in the ear, is a new target for medicines to aid patients with hearing loss, according to researchers.
The stria vascularis is a region of the cochlea in the ear.
As people grow older, they lose part of their hearing capacity, and by 2050, an estimated 2.4 billion people will have some sort of hearing loss. Hearing loss as people become older is a major contributor to the number of years they spend being disabled, and it’s also a major risk factor for dementia.
The team studied genetic analyses previously carried out in centers around the world using samples from 723,266 people from 17 studies who had clinically diagnosed or self-reported hearing impairment. This meta-analysis is one of the largest conducted in hearing genetics to date. The researchers identified 48 genes linked to hearing loss, including 10 new variants newly linked to hearing.
Further analysis looking at mouse genetics indicated that age-related hearing loss is due to changes in the stria vascularis which is necessary for hearing. The results provide targets for the basis of future research which could improve therapies against hearing loss.
Co-main author Frances Williams, Professor at King’s College London, said: “Our findings identify 10 genes newly linked with hearing loss. This study points to genes we could target for screening purposes, drug development, and even gene therapy in the future. This study provides a solid foundation for ultimately improving therapies against hearing loss.”
Co-main author Christopher R. Cederroth, Associate Professor at the Karolinska Institute, said: “It was hypothesized since the 1970s that the stria vascularis may play a role in hearing loss in humans, but the molecular evidence for this was missing until today.”
Reference: “Genome-wide association meta-analysis identifies 48 risk variants and highlights the role of the stria vascularis in hearing loss” by Natalia Trpchevska, Maxim B. Freidin, Linda Broer, Berthe C. Oosterloo, Shuyang Yao, Yitian Zhou, Barbara Vona, Charles Bishop, Argyro Bizaki-Vallaskangas, Barbara Canlon, Fabio Castellana, Daniel I. Chasman, Stacey Cherny, Kaare Christensen, Maria Pina Concas, Adolfo Correa, Ran Elkon, Estonian Biobank Research Team, Jonas Mengel-From, Yan Gao, Anne B.S. Giersch, Giorgia Girotto, Alexander Gudjonsson, Vilmundur Gudnason, Nancy L. Heard-Costa, Ronna Hertzano, Jacob v.B. Hjelmborg, Jens Hjerling-Leffler, Howard J. Hoffman, Jaakko Kaprio, Johannes Kettunen, Kristi Krebs, Anna K. Kähler, Francois Lallemend, Lenore J. Launer, I-Min Lee, Hampton Leonard, Chuan-Ming Li, Hubert Lowenheim, Patrik K.E. Magnusson, Joyce van Meurs, Lili Milani, Cynthia C. Morton, Antti Mäkitie, Mike A. Nalls, Giuseppe Giovanni Nardone, Marianne Nygaard, Teemu Palviainen, Sheila Pratt, Nicola Quaranta, Joel Rämö, Elmo Saarentaus, Rodolfo Sardone, Claudia L. Satizabal, John M. Schweinfurth, Sudha Seshadri, Eric Shiroma, Eldad Shulman, Eleanor Simonsick, Christopher Spankovich, Anke Tropitzsch, Volker M. Lauschke, Patrick F. Sullivan, Andre Goedegebure, Christopher R. Cederroth, Frances M.K. Williams and Andries Paul Nagtegaal, 16 May 2022, American Journal of Human Genetics. DOI: 10.1016/j.ajhg.2022.04.010
More on SciTechDaily

Ultra-Low-Cost Hearing Aid – $1 Worth of Open Source Parts – Could Address Age-Related Hearing Loss Worldwide

Hearing Loss Treatment Hope After New Genes Identified

New COVID-19 Danger Revealed: SARS-CoV-2 Virus Can Infect the Inner Ear
New Treatment Regenerates Hair Cells in the Inner Ear, Combats Hearing Loss
Newly Developed Drug Treatment Could Potentially Treat Hearing Loss
Hearing Restored in Noise-Deafened Mice

Protein Discovery in the Development of New Hearing Hair Cells Could Lead to Treatments for Hearing Loss
What Is Earwax? Why Do We Have It? And How Do You Remove It?
Be the first to comment on "new hope for hearing loss treatment: researchers identify 48 genes linked to hearing loss", leave a comment cancel reply.
Email address is optional. If provided, your email will not be published or shared.
Save my name, email, and website in this browser for the next time I comment.
- Skip to main content
- Keyboard shortcuts for audio player
- Your Health
- Treatments & Tests
- Health Inc.
- Public Health
How to Thrive as You Age
Got tinnitus a device that tickles the tongue helps this musician find relief.

Allison Aubrey

After using the Lenire device for an hour each day for 12 weeks, Victoria Banks says her tinnitus is "barely noticeable." David Petrelli/Victoria Banks hide caption
After using the Lenire device for an hour each day for 12 weeks, Victoria Banks says her tinnitus is "barely noticeable."
Imagine if every moment is filled with a high-pitched buzz or ring that you can't turn off.
More than 25 million adults in the U.S., have a condition called tinnitus, according to the American Tinnitus Association. It can be stressful, even panic-inducing and difficult to manage. Dozens of factors can contribute to the onset of tinnitus, including hearing loss, exposure to loud noise or a viral illness.
There's no cure, but there are a range of strategies to reduce the symptoms and make it less bothersome, including hearing aids, mindfulness therapy , and one newer option – a device approved by the FDA to treat tinnitus using electrical stimulation of the tongue.
The device has helped Victoria Banks, a singer and songwriter in Nashville, Tenn., who developed tinnitus about three years ago.
"The noise in my head felt like a bunch of cicadas," Banks says. "It was terrifying." The buzz made it difficult for her to sing and listen to music. "It can be absolutely debilitating," she says.

Shots - Health News
Tinnitus bothers millions of americans. here's how to turn down the noise.
Banks tried taking dietary supplements , but those didn't help. She also stepped up exercise, but that didn't bring relief either. Then she read about a device called Lenire, which was approved by the FDA in March 2023. It includes a plastic mouthpiece with stainless steel electrodes that electrically stimulate the tongue. It is the first device of its kind to be approved for tinnitus.
"This had worked for other people, and I thought I'm willing to try anything at this point," Banks recalls.
She sought out audiologist Brian Fligor, who treats severe cases of tinnitus in the Boston area. Fligor was impressed by the results of a clinical trial that found 84% of participants who tried Lenire experienced a significant reduction in symptoms. He became one of the first providers in the U.S. to use the device with his patients. Fligor also served on an advisory panel assembled by the company who developed it.
"A good candidate for this device is somebody who's had tinnitus for at least three months," Fligor says, emphasizing that people should be evaluated first to make sure there's not an underlying medical issue.
Tinnitus often accompanies hearing loss, but Victoria Banks' hearing was fine and she had no other medical issue, so she was a good candidate.
Banks used the device for an hour each day for 12 weeks. During the hour-long sessions, the electrical stimulation "tickles" the tongue, she says. In addition, the device includes a set of headphones that play a series of tones and ocean-wave sounds.
The device works, in part, by shifting the brain's attention away from the buzz. We're wired to focus on important information coming into our brains, Fligor says. Think of it as a spotlight at a show pointed at the most important thing on the stage. "When you have tinnitus and you're frustrated or angry or scared by it, that spotlight gets really strong and focused on the tinnitus," Fligor says.
"It's the combination of what you're feeling through the nerves in your tongue and what you're hearing through your ears happening in synchrony that causes the spotlight in your brain to not be so stuck on the tinnitus," Fligor explains.

A clinical trial found 84% of people who used the device experienced a significant reduction in symptoms. Brian Fligor hide caption
A clinical trial found 84% of people who used the device experienced a significant reduction in symptoms.
"It unsticks your spotlight" and helps desensitize people to the perceived noise that their tinnitus creates, he says.
Banks says the ringing in her ears did not completely disappear, but now it's barely noticeable on most days.
"It's kind of like if I lived near a waterfall and the waterfall was constantly going," she says. Over time, the waterfall sound fades out of consciousness.
"My brain is now focusing on other things," and the buzz is no longer so distracting. She's back to listening to music, writing music, and performing music." I'm doing all of those things," she says.
When the buzz comes back into focus, Banks says a refresher session with the device helps.
A clinical trial found that 84% of people who tried Lenire , saw significant improvements in their condition. To measure changes, the participants took a questionnaire that asked them to rate how much tinnitus was impacting their sleep, sense of control, feelings of well-being and quality of life. After 12 weeks of using the device, participants improved by an average of 14 points.
"Where this device fits into the big picture, is that it's not a cure-all, but it's quickly become my go-to," for people who do not respond to other ways of managing tinnitus, Fligor says.
One down-side is the cost. Banks paid about $4,000 for the Lenire device, and insurance doesn't cover it. She put the expense on her credit card and paid it off gradually.
Fligor hopes that as the evidence of its effectiveness accumulates, insurers will begin to cover it. Despite the cost, more than 80% of participants in the clinical trial said they would recommend the device to a friend with tinnitus.
But, it's unclear how long the benefits last. Clinical trials have only evaluated Lenire over a 1-year period. "How durable are the effects? We don't really know yet," says audiologist Marc Fagelson, the scientific advisory committee chair of the American Tinnitus Association. He says research is promising but there's still more to learn.
Fagelson says the first step he takes with his patients is an evaluation for hearing loss. Research shows that hearing aids can be an effective treatment for tinnitus among people who have both tinnitus and hearing loss, which is much more common among older adults. An estimated one-third of adults 65 years of age and older who have hearing loss, also have tinnitus.
"We do see a lot of patients, even with very mild loss, who benefit from hearing aids," Fagelson says, but in his experience it's about 50-50 in terms of improving tinnitus. Often, he says people with tinnitus need to explore options beyond hearing aids.
Bruce Freeman , a scientist at the University of Pittsburgh Medical Center, says he's benefitted from both hearing aids and Lenire. He was fitted for the device in Ireland where it was developed, before it was available in the U.S.
Freeman agrees that the ringing never truly disappears, but the device has helped him manage the condition. He describes the sounds that play through the device headphones as very calming and "almost hypnotic" and combined with the tongue vibration, it's helped desensitize him to the ring.
Freeman – who is a research scientist – says he's impressed with the results of research, including a study published in Nature, Scientific Reports that points to significant improvements among clinical trial participants with tinnitus.
Freeman experienced a return of his symptoms when he stopped using the device. "Without it the tinnitus got worse," he says. Then, when he resumed use, it improved.
Freeman believes his long-term exposure to noisy instruments in his research laboratory may have played a role in his condition, and also a neck injury from a bicycle accident that fractured his vertebra. "All of those things converged," he says.
Freeman has developed several habits that help keep the high-pitched ring out of his consciousness and maintain good health. "One thing that does wonders is swimming," he says, pointing to the swooshing sound of water in his ears. "That's a form of mindfulness," he explains.
When it comes to the ring of tinnitus, "it comes and goes," Freeman says. For now, it has subsided into the background, he told me with a sense of relief. "The last two years have been great," he says – a combination of the device, hearing aids and the mindfulness that comes from a swim.
This story was edited by Jane Greenhalgh
- ringing in ears
- hearing loss
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 30, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
New clinical practice guideline provides evidence-based recommendations for age-related hearing loss
by American Academy of Otolaryngology
The American Academy of Otolaryngology–Head and Neck Surgery Foundation (AAO-HNSF) has published "Clinical Practice Guideline: Age-Related Hearing Loss" in Otolaryngology–Head and Neck Surgery . This clinical practice guideline (CPG) sheds lights on a global public health problem affecting approximately 466 million people worldwide and identifies quality improvement opportunities and provide clinicians trustworthy, evidence-based recommendations regarding the identification and management of age-related hearing loss (ARHL) in patients 50 years and older.
"Age-related hearing loss is underdiagnosed and undertreated despite being the most common sensory deficit in the aging population . With almost 50% of the population over 75 reporting hearing loss, having a clinical practice guideline allows all clinicians to provide better health care to those with hearing loss based on research and best practices . Not only does this CPG provide screening recommendations and management of the hearing loss, it also educates clinicians and care partners how to communicate with those suffering from hearing loss," said Betty S. Tsai Do, MD, the CPG Development Group Chair.
ARHL is the most common sensory disorder in the older population. Between ages of 65 to 74, one in three adults experiences hearing loss. ARHL is a type of hearing loss that occurs over time as individuals age. It develops gradually and symmetrically, meaning it affects both ears similarly and is associated with various sociodemographic factors and health risks including dementia, depression, cardiovascular disease, and falls. It is caused by both genetic and environmental factors such as exposure to loud noises , medication that can harm the ears, cigarette smoking, and alcohol consumption.
This guideline presents clinicians with an evidenced-based framework, which includes 11 evidenced-based Key Action Statements (KAS), to prioritize identifying patients at risk of ARHL as well as managing it.
"I am excited to see a key action statement focusing on social determinants of health and how it impacts access and patient preferences on the management of hearing health care. The focus of individualized health care is the future of medicine, and as such, it is important that this CPG incorporated that into the recommendations," shared Dr. Tsai Do.
The ARHL CPG recommends that all patients aged 50 and above should be screened for hearing loss because detecting hearing loss early and taking appropriate steps can help minimize the negative effects associated with untreated hearing loss.
"Our guideline development group used the AAO-HNSF methodology to create evidence-based recommendations on the management of age-related hearing loss . We hope that the CPG will be useful in improving awareness and decreasing the morbidity of untreated age-related hearing loss in an effort to improve a patient's overall health and quality of life," said Dr. Tsai Do.
The guideline development group (GDG) consisted of experts in otolaryngology (ear, nose, and throat), otology (ear), audiology (hearing), neurology ( nervous system , including the brain), geriatrics (care of aging population) and primary care. The group also included a consumer representative, a public health expert, and staff members from the AAO-HNSF.
Explore further
Feedback to editors

New study supports psilocybin's potential as an antidepressant
4 hours ago

Global study reveals stark differences between females and males in disease burden causes

Researcher discusses mechanism behind a birth defect affecting brain size
5 hours ago

Study indicates that cancer patients gain important benefits from genome-matched treatments
6 hours ago

Machine learning tool identifies rare, undiagnosed immune disorders through patients' electronic health records

New technique improves T cell-based immunotherapies for solid tumors
7 hours ago

Unraveling the roles of non-coding DNA explains childhood cancer's resistance to chemotherapy

Conscious memories of childhood maltreatment strongly associated with psychopathology
8 hours ago

Study finds private equity expanding to mental health facilities

Organ transplant drug may slow Alzheimer's disease progression
Related stories.
Factors associated with age-related hearing loss differ between males and females, finds study
Mar 6, 2024
Survey highlights lack of awareness with hearing loss
Aug 24, 2022

Video: Hearing aid advice from an audiologist
Oct 30, 2023
Study shows dementia risk for older people with hearing difficulty could be reduced by use of hearing aids
Jan 5, 2024

Hearing screening ups awareness of hearing loss, hearing aid use
Jul 25, 2022
Sudden hearing loss: Update to guideline to improve implementation and awareness
Aug 1, 2019
Recommended for you

First effective treatment found for spitting cobra snakebite
11 hours ago

Identifying risks of human flea infestations in plague-endemic areas of Madagascar
13 hours ago

Neuroscientists find integrity of white brain matter in superagers does not deteriorate, explains sharp memory
Apr 30, 2024

Researchers reveal a new approach for treating degenerative diseases
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
- Health & Wellness
- Hearing Disorders
- Patient Care
- Amplification
- Hearing Aids
- Implants & Bone Conduction
- Tinnitus Devices
- Vestibular Solutions
- Accessories
- Office Services
- Practice Management
- Industry News
- Organizations
- White Papers
- Edition Archive
Select Page
- Hearing Aid Innovations Help Silence Stigma of Hearing Loss and Treatment
Apr 30, 2024 | Hearing Aids | 0 |

By Dan Troast
After years of research, technological advances, and dedicated awareness campaigns, there’s been a notable shift in the social perception of hearing aids related to stigma. It’s becoming increasingly acceptable to wear hearing aids and seek treatment for hearing loss, allowing many of those who experience hearing loss to reclaim their quality of life while simultaneously protecting themselves against future risks.
World Hearing Day, an annual awareness campaign created in 2007 by the World Health Organization, has spread awareness and advocacy for treatment and prevention of hearing loss for the past 17 years. This year, World Hearing Day was focused around continuing to reduce stigma and myths surrounding hearing health. The campaign emphasized that globally, more than 80% of ear and hearing care needs are unmet, causing productivity loss and social exclusion, leading to an annual societal cost of $980 billion per year.
Innovation and Destigmatization Across 17 Years
As a practicing audiologist, I understand the difficulties of delivering quality hearing care and strive to destigmatize hearing loss and treatment among patients, physicians, the media and society in general. Since World Hearing Day began 17 years ago, the state of hearing care has advanced dramatically through consistent improvements in hearing aid technologies and increased knowledge of the progressive nature of hearing loss and its relation to both depression and cognitive impairment.
Utilizing smaller components, new battery technologies, and improved processing, many hearing aids today resemble Bluetooth earbuds in both form and function, which are now so common that they carry no stigma. Smaller devices that offer Bluetooth capabilities are already helping convince more people to treat their hearing loss earlier, delivering numerous health benefits.
The addition of new capabilities has been coupled with more advanced user controls, often accessed through mobile device apps that enable simple, fast adaptation to various sound environments. Convenience has also been upgraded with the advent of rechargeable batteries and charging cases that can provide up to a week of full-time use on a single charge.
Awareness: The First Step to Acceptance
I used to begin discussions with patients by explaining how we can improve their daily experiences and noting that hearing issues, like vision issues, only compound over time when left untreated. Thanks to global advocacy, and perhaps even the widespread use of targeted advertising, my patients now enter my office with much greater knowledge than just a decade ago.
Many of them are aware that hearing loss has been directly linked to depression, anxiety, and mental decline, including being the top modifiable issue linked to dementia, with hearing aid use being linked to an astounding almost 50% reduction in dementia risk among patients considered high-risk based on age or other factors. This is related to reductions in conversations and interactions due to hearing loss that are necessary to maintain language and cognitive skills.
The warning signs are also becoming more widely known thanks to increased awareness campaigns from insurance companies and organizations. Traditionally, it was easy for those with early stages of hearing loss to overlook or write it off as normal until it worsened to have a more significant impact on their communications, relationships, and enjoyment of basic daily activities. Thanks to the heightened awareness and more user-friendly hearing aids, more and more people are seeking early treatment and improving their future risk profile.
Transforming Lives with Top-Tier Tech
Hearing tests and subsequent sampling of hearing aids remains the strongest method for demonstrating the power and effectiveness of modern devices. After determining the severity of an individual’s condition, we can clearly convey how daily experiences will be improved by using hearing aids, from general scenarios like taking phone calls or conversing with loved ones to specific situations like riding a bicycle or adapting to the loudness of a movie theater.
These discussions about quality of life can entice patients to test and adopt hearing aids regardless of their age, past experiences or initial reluctance. Basically, once someone is in the chair, we can more easily convince them of the benefits today than we could 20 years ago because modern devices are simply better in every way. They are less noticeable, last longer, don’t require battery changes, can adapt to solve specific hearing needs, and even provide the noise canceling features popularized by modern headphones and earbuds.
All of these factors make it easier and less intrusive to wear hearing aids on a regular basis, just like the addition of ear hooks made the use of eyeglasses explode nearly 300 years ago. The easier it is to forget that one is wearing an assistive device like a hearing aid, combined with wider applicability to daily needs, the more likely it is that the user will permanently integrate it into their routine. By enhancing comfort and increasing capabilities, the latest devices have undoubtedly helped to reduce stigma around hearing treatment, and will continue to do so as manufacturers pursue ever-more innovative designs and functions.
Hearing care today is more accessible than it’s ever been before. Campaigns like World Hearing Day and innovations by companies like HearUSA to develop and formulate better hearing solutions have led to significant advancements and better quality of life for those with hearing loss. For this to continue, the audiology industry must continue to recognize the potential dangers of hearing loss and educate those affected about the risks, while simultaneously taking strides to make hearing care more accessible to everyone.
Dan Troast, AuD, is an audiologist at HearUSA in Winter Garden, Fla., and has served as chair of the company’s HCP Advisory Board since 2022.
Related Posts
Mccarthy reintroduces hearing aid tax credit with solid backing.
March 23, 2009
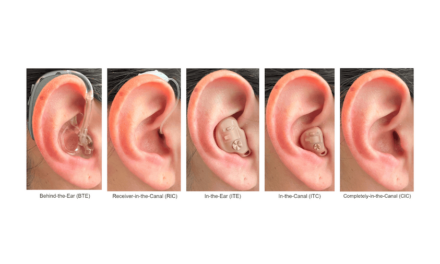
Survey Details OTC Self-Fitting Hearing Aid Users’ Experiences, Perceptions
December 27, 2023
The HR 2003 Dispenser Survey
June 2, 2003
Dan Casey Joins Sonomax as President, Chief Operating Officer
January 17, 2008
Leave a reply Cancel reply
Your email address will not be published. Required fields are marked *
Recent Posts
- ReSound Nexia Hearing Aids Available at VA Audiology Clinics
- Oticon Hearing Aid with User-Intent Sensors Now Available to Veterans
- National Acoustics Laboratories and Ear Science Institute Australia Announce Collaboration
- Availability of Signia’s IX Hearing Aids Expands for Veterans, Military, and American Indians
- New Evidence-Based Recommendations for Age-Related Hearing Loss
New findings pave the way for hearing loss therapies
As we age, many of us will eventually need hearing aids. In some cases, the reason for this may be a signaling pathway that controls auditory sensory cell function and is downregulated with age. Researchers at the University of Basel are uncovering clues.
Hearing loss eventually affects almost everyone: Loud noises or simple aging gradually cause the auditory sensory cells and their synapses in the inner ear to degenerate and die off. The only treatment option is a hearing aid or, in extreme cases, a cochlear implant.
"In order to develop new therapies, we need to better understand what the auditory sensory cells need for proper function," explains Dr. Maurizio Cortada from the Department of Biomedicine at the University of Basel and University Hospital Basel. In collaboration with Professor Michael N. Hall's research group at the Biozentrum, Cortada investigated which signaling pathways influence the so-called sensory "hair cells" in the inner ear. In the process, the researchers discovered a central regulator, as they report in the journal iScience .
This signaling pathway, known by researchers as the mTORC2-signaling pathway, plays an important role, among other things, for cell growth and the cytoskeleton. The role it plays for the hair cells in the inner ear has not previously been studied.
When the researchers removed a central gene of this signaling pathway in the hair cells of the inner ear of mice, the animals gradually lost their hearing. By the age of twelve weeks, they were completely deaf, the authors report in the study.
Fewer synapses
Closer examination indicated that the sensory hair cells in the inner ear lost their sensors without the mTORC2 signaling pathway: hair cells have protuberances similar to tiny hairs that are important for transducing sound into nerve signals. These "tiny hairs" were shortened, as the researchers determined with the use of electron microscopes. The number of synapses that transmit the signals to the auditory nerve was also reduced.
"From other studies, we know that the production of key proteins in this signaling pathway decreases with age," Cortada explains. There may be a connection to the loss of synapses and the reduced function of the auditory sensory cells in the inner ear that leads to hearing loss with increasing age.
"If this is confirmed, it would be a possible starting point for future therapies," says the researcher. The middle and inner ear, for example, would be readily accessible for locally-administered medications or gene therapies. The results could pave the way for the development of such treatment options.
- Hearing Loss
- Diseases and Conditions
- Hearing Impairment
- Hearing impairment
- Sensory system
- Auditory system
- Chemical synapse
- Hallucination
- MMR vaccine
Story Source:
Materials provided by University of Basel . Original written by Angelika Jacobs. Note: Content may be edited for style and length.
Journal Reference :
- Maurizio Cortada, Soledad Levano, Michael N. Hall, Daniel Bodmer. mTORC2 regulates auditory hair cell structure and function . iScience , 2023; 26 (9): 107687 DOI: 10.1016/j.isci.2023.107687
Cite This Page :
Explore More
- Anticoagulant With an On-Off Switch
- Sleep Resets Brain Connections -- At First
- Far-Reaching Effects of Exercise
- Hidden Connections Between Brain and Body
- Novel Genetic Plant Regeneration Approach
- Early Human Occupation of China
- Journey of Inhaled Plastic Particle Pollution
- Earth-Like Environment On Ancient Mars
- A 'Cosmic Glitch' in Gravity
- Time Zones Strongly Influence NBA Results
Trending Topics
Strange & offbeat.
AARP's Brain Health Resource Center offers tips, tools and explainers on brain health.
AARP daily Crossword Puzzle
Hotels with AARP discounts
Life Insurance
AARP Dental Insurance Plans
AARP MEMBERSHIP — $12 FOR YOUR FIRST YEAR WHEN YOU SIGN UP FOR AUTOMATIC RENEWAL
Get instant access to members-only products and hundreds of discounts, a free second membership, and a subscription to AARP the Magazine.
- right_container
Work & Jobs
Social Security
AARP en Español
- Membership & Benefits
AARP Rewards
- AARP Rewards %{points}%
Conditions & Treatments
Drugs & Supplements
Health Care & Coverage
Health Benefits

Staying Fit
Your Personalized Guide to Fitness

AARP Hearing Center
Ways To Improve Your Hearing

Brain Health Resources
Tools and Explainers on Brain Health

A Retreat For Those Struggling
Scams & Fraud
Personal Finance
Money Benefits

View and Report Scams in Your Area

AARP Foundation Tax-Aide
Free Tax Preparation Assistance

AARP Money Map
Get Your Finances Back on Track

How to Protect What You Collect
Small Business
Age Discrimination

Flexible Work
Freelance Jobs You Can Do From Home

AARP Skills Builder
Online Courses to Boost Your Career

31 Great Ways to Boost Your Career

ON-DEMAND WEBINARS
Tips to Enhance Your Job Search

Get More out of Your Benefits

When to Start Taking Social Security

10 Top Social Security FAQs

Social Security Benefits Calculator

Medicare Made Easy
Original vs. Medicare Advantage

Enrollment Guide
Step-by-Step Tool for First-Timers

Prescription Drugs
9 Biggest Changes Under New Rx Law

Medicare FAQs
Quick Answers to Your Top Questions
Care at Home
Financial & Legal
Life Balance

LONG-TERM CARE
Understanding Basics of LTC Insurance

State Guides
Assistance and Services in Your Area

Prepare to Care Guides
How to Develop a Caregiving Plan

End of Life
How to Cope With Grief, Loss
Recently Played
Word & Trivia
Atari® & Retro
Members Only
Staying Sharp
Mobile Apps
More About Games

Right Again! Trivia

Right Again! Trivia – Sports

Atari® Video Games

Throwback Thursday Crossword
Travel Tips
Vacation Ideas
Destinations
Travel Benefits

Beach vacation ideas
Vacations for Sun and Fun

Plan Ahead for Tourist Taxes

AARP City Guide
Discover Seattle

25 Ways to Save on Your Vacation
Entertainment & Style
Family & Relationships
Personal Tech
Home & Living
Celebrities
Beauty & Style

TV for Grownups
Best Reality TV Shows for Grownups

Robert De Niro Reflects on His Life

Looking Back
50 World Changers Turning 50

Sex & Dating
Spice Up Your Love Life

Navigate All Kinds of Connections

Life & Home
Couple Creates Their Forever Home

Store Medical Records on Your Phone?

Maximize the Life of Your Phone Battery

Virtual Community Center
Join Free Tech Help Events

Create a Hygge Haven

Soups to Comfort Your Soul

Your Ultimate Guide to Mulching
Driver Safety
Maintenance & Safety
Trends & Technology

AARP Smart Guide
How to Keep Your Car Running

We Need To Talk
Assess Your Loved One's Driving Skills

AARP Smart Driver Course

Building Resilience in Difficult Times

Tips for Finding Your Calm

Weight Loss After 50 Challenge

Cautionary Tales of Today's Biggest Scams

7 Top Podcasts for Armchair Travelers

Jean Chatzky: ‘Closing the Savings Gap’

Quick Digest of Today's Top News

AARP Top Tips for Navigating Life

Get Moving With Our Workout Series
You are now leaving AARP.org and going to a website that is not operated by AARP. A different privacy policy and terms of service will apply.
Go to Series Main Page
7 Ways to Preserve Hearing When You Already Have Hearing Loss
Wearing hearing aids isn’t enough for protecting your hearing.
Claudia M. Caruana,
If you already are one of the millions who use hearing aids, you know their value: You can hear your family members more clearly or enjoy dinner conversation with friends without saying “What?” all the time.
According to the National Institute of Deafness and Communication Disorders, 30 percent of adults ages 65 to 74 and approximately 50 percent of adults older than 75 have diminished hearing. Hearing loss is on the rise in the United States and is expected to increase by 67 percent by 2060. But you still need to take steps to protect your hearing once you have hearing aids. Unfortunately, aging, plus the noisy world in which we live, can further impair the hearing of those who use hearing aids.

AARP Membership — $12 for your first year when you sign up for Automatic Renewal
Essentially, hearing aids are designed to compensate for a person’s hearing loss and to operate in difficult listening environments, says Ayasakanta Rout, a professor of audiology and director of the Hearing Aid Research Laboratory at James Madison University in Harrisonburg, Virginia. He says hearing aids “do not improve the existing damage already in the inner ear.”
To help prevent additional hearing loss and the problems associated with it, here are seven suggestions that can help.
1. Wear your hearing aids — even when you are at home alone.
“In most cases of age-related hearing loss, there is a slow progression of the degree of hearing difficulty despite the use of hearing aids,” Rout says. “It is critical that hearing aids are programmed exactly to the need of the individual user so that soft sounds are audible, medium sounds are comfortable, and loud sounds are tolerable. If a hearing aid is too loud, it could induce additional hearing loss. That is why the best practices in hearing aid fitting recommend verification of the actual sound levels produced by the hearing aid when worn in the user’s ear.”
Rout adds that hearing aids are designed to make sounds audible and comfortable without being too loud. The aids can selectively reduce background noise.
Individuals with hearing loss should consistently use properly fitted hearing aids, Rout says. That includes when you are at home.
“If you need them and don’t use them, your comprehension of speech will decline,” he says.
“Think of all the things you would not enjoy if you decided not to wear your hearing aids,” says Richard S. Tyler, a professor in the Department of Otolaryngology at the University of Iowa. He adds that hearing aids can improve the user’s quality of life: “Hearing is critical to communication, interacting, appreciating and learning from others. This certainly can impact socialization, friendship and cognition.”

AARP NEWSLETTERS

%{ newsLetterPromoText }%
%{ description }%
Privacy Policy
ARTICLE CONTINUES AFTER ADVERTISEMENT
2. Keep in touch with your hearing care providers.
Clare M. Villanueva, an audiologist affiliated with NYU Langone Medical Center in Brooklyn, New York, recommends that hearing aid wearers see their hearing specialists at least once a year — sooner, if necessary — for a reevaluation of their hearing needs.
AARP® Vision Plans from VSP™
Exclusive vision insurance plans designed for members and their families
“Hearing aids might need to be adjusted, especially if the hearing care provider does another hearing test and finds the results differ from earlier ones,” Villanueva says. The hearing care provider might also determine that the hearing aids need servicing or should be replaced.
Over-the-counter (OTC) hearing aids became available in 2022 for those with mild to moderate hearing loss. They tend to be much less expensive than prescription hearing aids. If you are using over-the-counter hearing aids, follow the manufacturer’s recommendations about the possible need for adjustments. You also may need to contact an audiologist to have your hearing aids adjusted.
3. Use protective earmuffs.
Earmuffs like the ones worn by airport workers on the tarmac can be helpful for protecting your hearing during trying situations — concerts, busy airports, planes or trains or subways, and even when you or a neighbor mows the lawn or uses power tools. (Furry earmuffs will keep your ears warm, but you need the ones designed to cancel out harmful noise.)
Be aware that noise-canceling headphones are not earmuffs and should not be used in their place. Earmuffs, which cover the ear completely, can cost from less than $20 up to hundreds of dollars depending on their noise reduction rating (NRR), which represents the average decibels of sound it can reduce if worn properly. “The higher the NRR the better. Look for an NRR of around 30 dB. You always see airport workers and often construction workers wearing them to preserve their hearing,” Rout says. If you wear hearing aids, “remove them when using the earmuff,” he says.
The reasoning for this is twofold. First, if you wear an earmuff over the hearing aid, there is a greater likelihood of hearing audible feedback (squealing). Second, in loud environments, for example when lawn mowers are used and at construction sites, hearing aids are not effective in amplifying speech over the noise, Rout says.
4. Keep ear protection, such as earplugs, in your purse or pocket.
For those times when noise becomes uncomfortable or unbearable — such as on a subway, at a ballgame or in an amusement park — take out your hearing aids and use foam or silicon earplugs to reduce the noise. Inexpensive ones are available at drugstores. Another option is to use more expensive noise-reducing earplugs, which many musicians use. If you’ve been to a live concert recently and up close to the stage, you might notice that most of the musicians wear ear protection.

Join AARP today for $16 per year. Get instant access to members-only products and hundreds of discounts, a free second membership, and a subscription to AARP The Magazine.
5. Reduce the volume on your headphones.
Do you really need to have music blasting in your ears to enjoy it?
Villanueva recommends “reducing the volume on your iPods or other listening devices and using them for short amounts of time. Find the lowest, most comfortable sound setting on your device. Be aware a specific setting on one device is not necessarily the same on another device.”
6. Check your medications.
Check with your health care provider to see if any of the medications you are prescribed can cause hearing loss (if they are ototoxic). These can include some antibiotics as well as nonsteroidal anti-inflammatory drugs (NSAIDs), diuretics and chemotherapeutic agents. Ask of other drugs might be prescribed instead.
If you have a sudden change in your hearing, see an otolaryngologist, often known as an ear, nose and throat (ENT) specialist, as soon as possible, Villanueva says.
(See: 8 Medications That Can Harm Your Hearing )
7. Make sure you don’t have too much earwax.
One common cause of increased hearing loss is simple earwax, according to Sreekant Cherukuri, M.D., an ear, nose and throat specialist at St. Mary Medical Center in Hobart, Indiana.
“As we age, earwax gets dryer and harder, so it can easily build up without our knowing it and cause some hearing loss,” he says.
The use of hearing aids or earplugs also can push earwax down the ear canal.
Cherukuri recommends cleaning your ears regularly with an over-the-counter ear wash system — not Q-tips — and having your physician or health care provider “look in your ears and check for wax buildup at your next checkup.”
(See: The Ins and Outs of Safe Earwax Removal )
Claudia M. Caruana is a New York-based health and medical writer who uses hearing aids.
Discover AARP Members Only Access
Already a Member? Login

More From AARP

AARP Smart Guide to Seasonal Allergies
Achoo! How to understand and treat your symptoms

Hearing Loss for Dummies
6 Bad Habits for Your Hearing
Protect your ears, avoid these behaviors
Recommended for You
AARP Value & Member Benefits

Learn, earn and redeem points for rewards with our free loyalty program

AARP® Dental Insurance Plan administered by Delta Dental Insurance Company
Dental insurance plans for members and their families

The National Hearing Test
Members can take a free hearing test by phone

AARP® Staying Sharp®
Activities, recipes, challenges and more with full access to AARP Staying Sharp®
SAVE MONEY WITH THESE LIMITED-TIME OFFERS

IMAGES
VIDEO
COMMENTS
Trial participants were aged between 18 and 80, hailed from the UK, Germany, and Greece, and had mild to moderate hearing loss. 15 patients took part in the phase 1 trial, which showed the ...
The findings could benefit efforts to develop new ways to treat and prevent hearing loss, including age-related hearing loss. ... But the new UVA Health research shows these delicate cells have ...
New approach to achieve hearing loss treatment. Previously, Chen's research team studied zebrafish and chickens to uncover which pathways were responsible for inducing the cell division required to regenerate new hair cells. They discovered that two molecular signaling pathways, , were crucial to this process.
MIT spinout Frequency Therapeutics' drug candidate stimulates the growth of hair cells in the inner ear. Frequency Therapeutics, a biotechnology company, is working to repair hearing loss using a novel kind of regenerative treatment rather than hearing aids or implants. The company programs progenitor cells, a descendent of stem cells in the ...
Harvard scientists have developed a new hearing loss treatment that restores hearing via cell reprogramming and regeneration. June 29, 2023 - Researchers at Harvard Medical School are one step closer to restoring sensorineural hearing loss through cell reprogramming and regeneration. If successful, this biotechnology would address a centuries ...
December 20, 2022. A one-time treatment using small molecules to target progenitor cells, pioneered in the lab of a Harvard Stem Cell Institute researcher, may potentially restore hearing lost from some of the most common causes. By Alice McCarthy. Often regarded as a quasi-inevitable consequence of getting older, hearing loss affects a wide ...
It is also closely correlated with dementia. The biotechnology company Frequency Therapeutics is seeking to reverse hearing loss — not with hearing aids or implants, but with a new kind of regenerative therapy. The company uses small molecules to program progenitor cells, a descendant of stem cells in the inner ear, to create the tiny hair ...
Oct. 27, 2021 — Some people are born with hearing loss, while others acquire it with age, infections or long-term noise exposures. In many instances, the tiny hairs in the inner ear's cochlea ...
A cell therapy to improve hearing could transform the lives of millions of people with hearing loss, new research suggests. The therapy will see the first clinical trial of a stem cell treatment ...
Eligible for the Phase I/II trial were patients aged from 18 to 80 years with a primary complaint of hearing loss of more than 10 years duration and stable hearing, their history suggesting an age ...
A team led by Harvard Medical School and Massachusetts Eye and Ear researchers may bring scientists a step closer to developing treatments that regrow the missing cells that cause hearing loss. In a new study published online December 4 in Nature Communications, scientists report a new strategy to induce cell division in the mature inner ear.
This groundbreaking proof-of-concept study unlocks new possibilities for future research, sparking hope for the development of treatments for hearing loss." Reference: "Reversal of an existing hearing loss by gene activation in Spns2 mutant mice" by Elisa Martelletti, Neil J. Ingham and Karen P. Steel, 8 August 2023, Proceedings of the ...
In the News. Harvard scientists have developed a new hearing loss treatment that restores hearing through cell reprogramming and regeneration. Recently, researchers at Harvard University have made another breakthrough in "restoring" sensorineural hearing loss. Through mouse models, the scientists have been working on hair cell regeneration ...
In adults, hearing loss has been shown to affect quality of life, social isolation, depression, and anxiety. The purpose of this review is to advance the understanding of the latest research in the development of new treatments of hearing loss, including gene therapy, stem cell therapy, and pharmacotherapies.
Sensorineural hearing loss is the most common form and is caused by the loss of sensory hair cells in the cochlea. Hair cell loss results from a variety of factors including noise exposure, aging , toxins, infections, and certain antibiotics and anti-cancer drugs. Although hearing aids and cochlear implants can ameliorate the symptoms somewhat ...
The number of individuals of all ages with mild to complete hearing loss will balloon from just under 44 million today to nearly 55 million in 2030. With such vast demand for audiological health care services, coupled with recent advances in technology and treatments, the hearing care industry is ripe for transformation.
Learn about hearing and hearing loss -- causes, prevention, symptoms and treatment options. Read medical research on tinnitus, ear infections, ear tumors and new treatment options for children and ...
Investigations of the paradoxical link between tinnitus and hearing loss have revealed a hidden form of deafness, paving the way to possible new treatments. ... New research has led to ...
Tue, May 3rd 2022 at 6:52 PM. Updated Sun, May 15th 2022 at 9:50 AM. Scientists say new treatment could reverse hearing loss (WKRC file) UNDATED (WKRC) - Researchers may have found a way of ...
Rinri Therapeutics. Rinri Therapeutics, established in 2018, specializes in pioneering regenerative cell therapies for those affected by hearing loss. The company's primary focus is on treating auditory neuron-related hearing loss, such as age-related hearing loss and Auditory Neuropathy.
The researchers identified 48 genes linked to hearing loss, including 10 new variants newly linked to hearing. Further analysis looking at mouse genetics indicated that age-related hearing loss is due to changes in the stria vascularis which is necessary for hearing. The results provide targets for the basis of future research which could ...
Today, cochlear implant hearing performance can be measured in situ, which is a relatively new research method and expected to improve the success rate of cochlear ... Pharmacologic agents applied in the treatment of hearing loss include glutamate inhibitors such as caroverine (Tinnex), derivatives of trimetazidine anti-ischemic agent such as ...
Research shows that hearing aids can be an effective treatment for tinnitus among people who have both tinnitus and hearing loss, which is much more common among older adults. An estimated one ...
With almost 50% of the population over 75 reporting hearing loss, having a clinical practice guideline allows all clinicians to provide better health care to those with hearing loss based on ...
World Hearing Day, an annual awareness campaign created in 2007 by the World Health Organization, has spread awareness and advocacy for treatment and prevention of hearing loss for the past 17 years. This year, World Hearing Day was focused around continuing to reduce stigma and myths surrounding hearing health.
The only treatment option is a hearing aid or, in extreme cases, a cochlear implant. ... (2023, October 5). New findings pave the way for hearing loss therapies. ... 2019 — New research reveals ...
Seventy-seven individuals, a study group of 41 patients diagnosed with ARHL, and a control group of 36 participants without hearing loss were evaluated. Serum samples were collected and used to measure serum BDNF and NT-3 levels with the new Nepenthe enzyme-linked immunosorbent assay method.
(KAS 7) Clinicians should counsel patients with hearing loss on communication strategies and assistive listening devices. (KAS 10) For patients with hearing loss, clinicians should assess if communication goals have been met and if there has been improvement in hearing-related QOL at a subsequent health care encounter or within 1 year.
According to the National Institute of Deafness and Communication Disorders, 30 percent of adults ages 65 to 74 and approximately 50 percent of adults older than 75 have diminished hearing. Hearing loss is on the rise in the United States and is expected to increase by 67 percent by 2060. But you still need to take steps to protect your hearing once you have hearing aids.
Selective listening in competing-talker situations (restaurants, parties, etc.) is an extraordinarily difficult task for many people. For individuals with hearing loss, this difficulty can be so extreme that it seriously impedes communication and participation in daily life. Directional filtering is one of the only proven ways to improve speech understanding in competition, and most hearing ...