An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List


The Importance of Vaccination in the Context of the COVID-19 Pandemic: A Brief Update Regarding the Use of Vaccines
Bruna aparecida souza machado, katharine valéria saraiva hodel, larissa moraes dos santos fonseca, vinícius couto pires, luis alberto brêda mascarenhas, leone peter correia da silva andrade, marcelo albano moret, roberto badaró.
- Author information
- Article notes
- Copyright and License information
Correspondence: [email protected] ; Tel.: +55-(71)-3879-5624
Received 2022 Mar 11; Accepted 2022 Apr 8; Collection date 2022 Apr.
Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( https://creativecommons.org/licenses/by/4.0/ ).
The COVID-19 pandemic has led the world to undertake the largest vaccination campaign in human history. In record time, unprecedented scientific and governmental efforts have resulted in the acquisition of immunizers utilizing different technologies (nucleotide acids, viral vectors, inactivated and protein-based vaccines). Currently, 33 vaccines have already been approved by regulatory agencies in different countries, and more than 10 billion doses have been administered worldwide. Despite the undeniable impact of vaccination on the control of the pandemic, the recurrent emergence of new variants of interest has raised new challenges. The recent viral mutations precede new outbreaks that rapidly spread at global proportions. In addition, reducing protective efficacy rates have been observed among the main authorized vaccines. Besides these issues, several other crucial issues for the appropriate combatting of the pandemic remain uncertain or under investigation. Particularly noteworthy issues include the use of vaccine-boosting strategies to increase protection; concerns related to the long-term safety of vaccines, child immunization reliability and uncommon adverse events; the persistence of the virus in society; and the transition from a pandemic to an endemic state. In this review, we describe the updated scenario regarding SARS-CoV-2 variants and COVID-19 vaccines. In addition, we outline current discussions covering COVID-19 vaccine safety and efficacy, and the future pandemic perspectives.
Keywords: COVID-19, vaccines, SARS-CoV-2, COV, pandemic
1. Introduction
The rapid spread and contagion of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the etiologic agent of coronavirus disease 2019 (COVID-19), raised concern among the public and health authorities worldwide. Shortly after the first case was reported in Wuhan (China), the World Health Organization (WHO) defined COVID-19 as a pandemic [ 1 , 2 ]. Since the pandemic began, one of the main effective and feasible ways to contain the spread of the SARS-CoV-2 has been through vaccination [ 3 ]. Therefore, with unprecedented efforts, multiple vaccine candidates have been identified to prevent SARS-CoV-2 infection in a noticeable period [ 4 ]. The “race” to develop COVID-19 vaccines has been characterized primarily by the use of different technology platforms, such as nucleic acid (mRNA and DNA) [ 5 , 6 , 7 , 8 ], viral vector (replicating and non-replicating) [ 9 , 10 , 11 ], inactivated virus [ 12 ], and protein-based technologies [ 13 ]. Currently, more than 180 vaccines are under clinical development worldwide, while 33 vaccines have already been approved by regulatory agencies in different countries [ 14 ]. As a result of the immense efforts made by governmental and independent organizations, as well as the pharmaceutical and biotech industries, in just over 1 year since the first COVID-19 vaccine was approved for emergency use [ 15 ], more than 10 billion doses have been administered worldwide, representing the largest vaccination program in human history [ 16 ].
Despite this motivating context, the current COVID-19 pandemic scenario is concerning due to the emergence of new SARS-CoV-2 variants. In general, coronaviruses are large RNA viruses, with the largest among them being characterized as non-segmented and single-stranded positive-sense RNA (+ssRNA) [ 17 ]. SARS-CoV-2 has a 29.9 kb genome capable of encoding four structural proteins (spike, envelope, membrane, and nucleocapsid) and sixteen non-structural proteins (nsp1-16) [ 18 ]. Among them, the spike (S) protein of SARS-CoV-2 plays a critical role in the virus, as it is responsible for binding to receptors (angiotensin-converting enzyme 2, or ACE2) on the host cell and determining host tropism [ 19 ]. The S protein is composed of two functional subunits named S1 and S2, which are responsible for binding to the receptor on the host cell and for fusing the viral membrane to that of the host cell, respectively [ 20 , 21 ].
Even though SARS-CoV-2 features molecular tools for nucleotide revision during viral replication, such as the RNA-dependent RNA polymerase complex (RdRP), this process is still error-prone [ 22 ]. Coupled with this, the high prevalence of COVID-19 worldwide has led to the rapid emergence of viral variants that exhibit characteristics differing from the original virus [ 23 ]. Within this context, modifications to the S protein have resulted in increased pathogenicity and higher viral transmission capacity, as well as negatively impacting vaccine efficacy and the performance of available diagnostic kits, which has attracted the attention of health authorities [ 24 , 25 , 26 ]. The WHO and other public health institutions have classified SARS-CoV-2 variants into two main categories: variants of concern (VOCs) and variants of interest (VOIs). In general, VOCs are the SARS-CoV-2 lineages that have been confirmed to be capable of increased transmissibility, virulence, vaccine resistance, decreased immunity acquired from previous infections, and the ability to evade diagnostic detection. VOIs have alterations that may decrease the action of neutralizing antibodies generated from vaccines or previous infections, decrease the effectiveness of available therapies, and have an impact on the transmissibility, severity, or diagnosis of the disease [ 27 ].
In November 2021, a new VOC named Omicron (B.1.1.529) was identified in Botswana (South Africa), and it rapidly escalated to epidemiological proportions [ 28 ]. The Omicron variant has been responsible for the increase in the number of COVID-19 cases in distinct parts of the world in early 2022 [ 29 ], with more than 1 million new cases being reported in a single day in the United States alone, with the Omicron variant dominating in most of them [ 30 ]. The dominance of infections caused by Omicron may be associated with its greater transmissibility, which is up to 3.2 times higher when compared to other VOCs [ 31 , 32 ]. This property has been associated primarily with Omicron’s ability to evade the immune system in individuals previously immunized with the primary vaccination scheme or who have had previous infection through other SARS-CoV-2 lineages [ 33 ]. In addition, Omicron presents important particularities that have resulted in new directions related to COVID-19 dynamics, such as a shorter incubation period (2 to 3 days) when compared to the original strain (5 to 7 days) [ 34 ]; a lower affinity for lung cells, decreasing the severity of respiratory symptoms [ 35 ]; as well as a higher reinfection rate, indicating important immune-system escape mechanisms [ 36 ]. Given these characteristics, it is believed that the COVID-19 situation could take a drastic turn due to transmission of the Omicron variant, as well as other upcoming VOCs with even greater transmission properties, increasing the importance of community immunization [ 37 , 38 ].
Therefore, the unpredictability of how the COVID-19 pandemic will progress in the face of the emergence of new variants has caused health authorities and vaccine developers to search for strategies to mitigate the impacts caused by mutant lineages. The expansion of the eligible population for vaccination, such as the inclusion of children between 5 and 11 years old, and the recommendation for booster doses after primary vaccination are strategies that have fostered discussions related to the efficacy, safety, and the role of vaccines developed at the beginning of the pandemic. Therefore, the aim of this study was to evaluate the expectations related to the COVID-19 pandemic regarding the use of vaccines.
2. Overview of COVID-19 Vaccines and Variants
One of the biggest challenges for vaccination, especially when it comes to airborne viruses—such as flu viruses, for example—is the emergence of new variants caused by mutations in the virus genome. Mutations occur naturally during the replication of any RNA virus due to the instability of the RNA molecules [ 23 ]. In the case of COVID-19, even before it was considered a pandemic, data related to the genomic surveillance of SARS-CoV-2 were available as a useful tool for investigating outbreaks and tracking evolution and possible new waves [ 39 , 40 ]. As a result, more than 25 billion sequences of SARS-CoV-2 have been performed worldwide, and it is estimated that at least two mutations in the viral genome occur per month [ 40 , 41 ]. These additional mutations often result in distinct immune-evasion mechanisms and lead to the appearance of different variants and lineages [ 42 ]. Currently, there are 21 variants of SARS-CoV-2; among them, variants Alpha a (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), and Omicron (B.1.1.529) are considered as VOCs, while Lambda (C.37) and Mu (B.1.621) are considered as VOIs ( Table 1 ). It is emphasized that, in addition to these two categories, there are also variants under monitoring (VUMs), which are those strains that have genetic mutations that may pose a risk in the future, but for which phenotypic and epidemiological changes are currently unclear [ 43 ].
The characteristics of the main SARS-CoV-2 variants of concern or interest, according to WHO [ 27 ].
S—spike; UK—United Kingdom; VOC—variant of concern; VOI—variant of interest.
Because one of their characteristics is lower susceptibility to vaccines and other therapeutic alternatives, VOCs have been intensively monitored [ 44 ]. Modifications found in the structure of the S protein of SARS-CoV-2 have been attributed to the greater ability of the virus to escape the action of neutralizing antibodies [ 43 , 45 ]. Important examples of mutational mechanisms that lead to increased antigenic properties of protein S are the amino acid substitutions that alter the protein epitope, increase receptor-binding avidity, and lead to changes in glycosylation, deletion or insertion of residues, and allosteric structural effects [ 46 ]. These factors strongly contribute to the increased mortality and morbidity of SARS-CoV-2 [ 46 , 47 , 48 ], where the transmissibility can be up to 74% higher when compared to the original strain [ 49 , 50 ].
Since the SARS-CoV-2 S protein is the main target of COVID-19 vaccines, the mutations in this protein are of great concern, especially those which have the corresponding sequences of reference strain Wuhan-Hu-1—that is, no antigens based on different variants are used [ 51 , 52 ]. Thus, the appearance of variants with modifications in the SARS-CoV-2 S protein structure raises questions about the effectiveness of the vaccines available to the population; antibodies derived from the original strain (Wuhan-Hu-1) may have only a partial neutralizing effect against these viruses [ 53 ]. The vaccines BNT162b2 (brand name Comirnaty), mRNA-1273 (brand name Spikevax), CoronaVac, BBIBP-CorV, AZD-1222 (brand name Vaxzevria or Covishield), and Ad26.COV2-S (brand name Janssen COVID-19 Vaccine) are the most widely used around the world for COVID-19 prophylaxis, since all of them use the S protein as the main activator of the immune system [ 54 ]. Therefore, different studies have been published or are being conducted to analyze the efficacy or effectiveness of each of these vaccines against SARS-CoV-2 variants of concern ( Table 2 ).
Efficiency and neutralization activity of major COVID-19 vaccines against variants of concern after primary vaccination.
* Estimated overall effectiveness based on the two mRNA vaccines; ** it has been shown that mutation of D614G does not significantly alter the neutralizing properties of antibodies against SARS-CoV-2, which makes vaccines developed with the wild type efficient. Therefore, different studies have compared the neutralizing activity against protein S with this substitution.
Analysis of several studies shows that the efficiency or effectiveness of vaccines against SARS-CoV-2 variants depends on many factors, including the sample size, demographic factors, host factors, the type of vaccine, the number of doses, a heterologous or homologous booster vaccination scheme, and the time after primary vaccination is completed ( Figure 1 ) [ 44 , 84 , 85 ]. Different authors demonstrated that the application of a booster dose after a certain period is able to increase the humoral immune response against SARS-CoV-2, resulting in increased efficacy or effectiveness of vaccines against the VOC [ 86 , 87 , 88 , 89 , 90 ]. Bruxvoort et al. [ 91 ] found data that reinforced the need for booster dose administration, since primary immunization with mRNA-1273 shows limited protection against the Delta, Alpha, Gamma, and Mu variants. Andrews et al. [ 78 ] reported that administration of the booster dose with BNT162b2 after primary immunization of ChAdOx1, mRNA-1273, or BNT162b2 was able to significantly increase protection against Omicron. Other authors reported that the homologous booster regimen of BNT162b2, mRNA-1273, CoronaVac and BBIBP-CorV vaccines and the heterologous booster with Ad26.COV2.S associated with mRNA-1273 vaccines showed similar performance against Omicron [ 92 , 93 ].
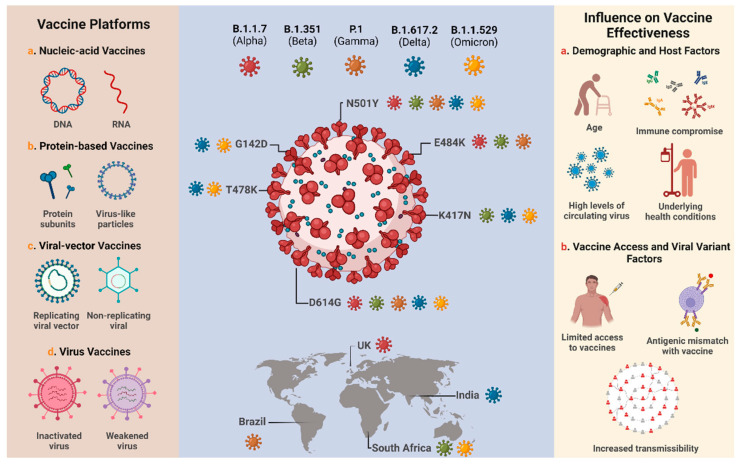
Overview of the major technology platforms used for COVID-19 vaccine development, the SARS-CoV-2 variants of concern and their respective spike protein mutations, and the factors that may influence the effectiveness of available vaccines. Adapted from Tregoning et al. [ 44 ] and Mistry et al. [ 94 ]. Created with BioRender.com (accessed on 17 February 2022).
Data provided by the CDC show that the number of cases and deaths caused by COVID-19 in the United States is higher among unvaccinated individuals when compared to individuals with a full primary vaccination scheme and/or those who have already received a booster dose, regardless of the vaccine administered (BNT162b2, mRNA-1273, or Ad26.COV2-S) [ 95 ]. Among those vaccinated, although the three vaccines showed similar efficacy in reducing COVID-19 infection in the period evaluated (April 2021 to February 2022), it was observed that the number of deaths among individuals vaccinated with Ad26.COV2-S was higher when compared to the mRNA vaccines (BNT162b2 and mRNA-1273) [ 95 ]. In early January 2022, during the wave caused by the Omicron variant, the highest incidence per 100,000 population occurred, where the rates reached 5.44, 2.34, and 1.79 for the Ad26.COV2-S, BNT162b2, and mRNA-1273 vaccines, respectively, after the primary vaccination scheme [ 95 ]. However, it is important to note that the mortality rate among those who received the Ad26.COV2-S vaccine was lower compared to unvaccinated individuals, where the rate was ~9 times higher. Although it has already been reported that the Ad26.COV2-S vaccine can elicit a stable humoral and cellular response over time, data reported by the CDC suggest that the immune response induced by mRNA vaccines may be more effective in reducing mortality rates [ 83 , 96 ]. The greater efficacy observed in mRNA vaccines may be a reflection of their advantages as a technological platform; unlike adenovirus (DNA)-based vaccines, once administered, RNA molecules do not need to cross the nuclear membrane or transcription to start the protein-synthesis process [ 97 ]. Hence, these data encouraged discussions about the immune protection of the Ad26.COV2-S vaccine, particularly with a view to the possible implementation of the intervals required for the application of new booster doses.
Because of this, since the second half of 2021, health authorities around the world have been recommending booster doses for different vaccines to contain the spread of SARS-CoV-2 and consequently control the pandemic in terms of the number of hospitalizations and deaths [ 98 , 99 , 100 , 101 ]. The study by Andrews et al. [ 102 ] conducted in England demonstrated that the administration of a booster dose with mRNA vaccines (BNT162b2 or mRNA-1273) compared to the primary immunization showed an efficiency between 94 and 97% in reducing symptomatic cases of the disease, while against hospitalization or death, the value found ranged between 97% and 99%. Studies conducted in Israel have also shown the same trend, which strengthens the importance of this new vaccination strategy [ 103 , 104 , 105 ]. Furthermore, studies involving computer modeling have shown that the administration of the booster dose will be able to decrease the effective reproduction number (R 0 ), while authors have pointed out that the predicted increase in antibody titers induced by the booster dose provides important protection from infection with SARS-CoV-2 variants [ 106 , 107 , 108 ].
Currently, the application of a new booster dose (usually the fourth dose) has been recommended in different countries due to the emergence of the Omicron variant, which has a greater transmission capacity than the other variants and, consequently, has been associated with an increase in the number of reinfection cases [ 109 , 110 , 111 ]. Initially, the administration of this new dose was directed towards priority groups, such as healthcare workers, immunocompromised individuals, and the elderly; however, new groups are expected to be included soon [ 112 , 113 ]. A preliminary study released by the Israeli Ministry of Health in adults aged >60 years demonstrated that a fourth dose of the BNT162b2 vaccine was able to increase immune protection up to two-fold against SARS-CoV-2 infection and up to three-fold against severe disease when compared to individuals who received only the third dose [ 114 ]. The expectation that new VOCs will emerge over time exposes one of the main challenges associated with developing COVID-19 vaccines, that of using products capable of inducing a robust and/or long-lasting immune response against different variants [ 115 , 116 ].
In early November, a new COVID-19 sublineage named BA.2 was first reported [ 117 ] (2 months after variation BA.1, which rapidly became dominant due to immune-escape mechanisms [ 118 , 119 ]). In a public statement, WHO defined it as an Omicron sublineage, classifying it as a variant of concern. According to the organization, the amino acids differences in structural proteins have possibly conferred a growth advantage when compared to other Omicron sublineages (BA.1, BA. 1.1.), but not greater severity [ 120 ]. In later March, the US Centers for Disease Control and Prevention (CDC) reported that BA.2 was responsible for 55% (50.8–59.1%—95% PI) COVID-19 cases [ 121 ], followed by BA. 1.1 (40.4%, 36.4–44.5%—95% PI). The rapid spread of BA.2 has raised discussions about reinfection and vaccine efficacy. Although BA.2’s ability to evade neutralizing antibodies is unclear, authors have demonstrated evidence that the increasing frequency of BA.2 is probably related to increased transmissibility rather than to enhanced immunologic escape [ 122 ]. On the other hand, initial data from population-level reinfection studies suggest that infection with BA.1 provides strong protection against reinfection with BA.2 [ 123 ]. Recent studies have demonstrated that mRNA vaccines (BNT162b2 and mRNA-1273) provide similar protection against BA.1 (46.6% (95% CI: 33.4–57.2%) and BA.2 51.7% (95% CI: 43.2–58.9%) in the first three months, and this declines to about 10% after 4–6 months. These findings show that protection against BA.2 did not seem to wane any faster than protection against BA.1. Furthermore, in both cases, a second dose was able to recuperate immune protection levels [ 124 ]. Therefore, until now, recent data supported the need for a vaccine targeting the Omicron variant.
Within this context, in January 2022 the companies Pfizer and BioNTech, developers of the BNT162b2 vaccine, started a clinical trial to evaluate the safety, immunogenicity, and tolerability of an Omicron-based vaccine candidate. To this end, the study will be conducted with healthy adults between the ages of 18 and 55 who may be allocated into three distinct cohorts with different dose regimens of the vaccine candidate [ 125 ]. Similarly, the pharmaceutical company Moderna (developer of the mRNA-1273 vaccine) is expected to start a clinical trial for the analysis of a new vaccine candidate against the Omicron variant in the first half of 2022 [ 126 ]. In addition, different institutes have supported the idea of investing in the development of a pan-coronavirus vaccine capable of protecting against several coronaviruses, including the different strains of SARS-CoV-2 [ 126 , 127 ].
Importantly, the development of Omicron-specific vaccines should not completely rule out the use of previously approved vaccines made available to the population, as robust data are still needed to elucidate the induction of the immune response after the first booster dose and its role in controlling infection or disease progression by this variant. Furthermore, one cannot exclude the possible “selective pressure” exerted by vaccines and even by monoclonal antibody therapy in targeting the S protein, since this may have influenced the appearance of new variants with mutations in this region, thus conferring escape mechanisms [ 128 ]. Therefore, new therapeutic targets must be considered for the development of new vaccines.
3. Safety of COVID-19 Vaccines
Due to the pandemic nature of COVID-19 and the various impacts generated in the global health, social and economic sectors, the vaccines against SARS-CoV-2 infection were made available in record time [ 129 ]. This was also because many scientists, manufacturers, and research institutions were already developing innovative technology platforms for new vaccines, which were eventually adapted for COVID-19 prevention. However, since no coronavirus vaccine had been licensed and approved for use in humans previously, the rapid development associated with the limited follow-up time post-vaccination and lack of information about long-term side effects of the vaccines aroused great public concern about the safety profile of the available vaccines [ 130 ]. It is important to note that as mass vaccination progresses, more post-vaccination adverse events are reported [ 131 ]. This demonstrates that vaccine safety information from ongoing clinical trials and surveillance data is important not only for building public confidence, but also for making evidence-based health-policy decisions [ 132 ]. The safety of vaccines is evaluated through adverse event monitoring in randomized controlled trials and safety post-licensure surveillance data after immunization campaigns [ 133 ]. Determining the safety profile of a vaccine is a critical step at the global level and is monitored by the WHO along with manufacturers, health officials, and national regulatory agencies [ 5 , 6 , 134 ], since they are drugs administered in healthy populations. According to the WHO, all available vaccines, including the COVID-19 vaccines, have been rigorously assessed for safety for diverse groups of people, according to age, sex, ethnicity, and medical conditions.
For the mRNA vaccines, the most commonly reported adverse events are local reactions at the injection site, such as pain, redness, and swelling, and systemic reactions, such as headache, myalgia, arthralgia, and chills [ 135 ]. In clinical studies evaluating the mRNA-1273 and BNT162b2 vaccines, the frequency and severity of these adverse events were higher after the administration of the second dose. When it comes to adenoviral vector vaccines, in the case of the AZD 1222 vaccine, pain, fever, chills, muscle ache, headache, and malaise were the most common adverse reactions. Regarding serious adverse events, seven have been associated with the AZD-122 vaccine, including transverse myelitis [ 136 ]. Overall, inactivated virus vaccines such as CoronaVac, BBIBP-CorV, and COVXIN have a good safety profile, with few grade 3 adverse reactions. In the elderly population, studies with vaccines of distinct technologies, such as AZD 1222 (modified adenovirus) and NVX-CoV2373 (protein adjuvant), for example, showed a good antibody response and low reactogenicity events after administration, with a higher incidence and severity of adverse events observed in younger subjects [ 137 ].
Another rare manifestation after vaccination, but which has been reported in different studies, is multisystem inflammatory syndrome (MIS) [ 138 ]. MIS has still poorly understood pathophysiology, however, it is believed to occur due to an exaggerated immune response against SARS-CoV-2 infection due to persistently high levels of IgG and activation of CD8+ T cells [ 139 , 140 ]. Considering the adult population, MIS may result from a delayed and dysregulated immune response and is characterized by the onset of symptoms such as fever, elevated inflammatory markers, as well as multiple-organ involvement (especially of the heart, stomach, and intestines) [ 141 , 142 ]. Different case studies have reported the onset of MIS in adults after immunization with the vaccine based on mRNA [ 143 ], inactivated virus [ 144 ], and adenovirus [ 145 ]. However, a crucial question was demonstrated by Belay et al. [ 146 ], in which the report of MIS after vaccination in adult patients was also associated with prior SARS-CoV-2 infection. These data demonstrate the need for further studies to elucidate the real association between MIS caused purely by vaccination or whether there is a direct relationship with previous viral infection.
It is interesting to highlight that most of the side effects that people experience after COVID-19 vaccination can be attributed to the “nocebo” effect. Nocebo refers to the non-pharmacological adverse effects reported after exposure to a placebo substance, which are usually motivated by the individual’s expectation that, after exposure to a vaccine, drug, or other medical intervention, some disagreeable event will occur [ 147 , 148 ]. Haas et al. [ 149 ] evaluated the frequency of adverse events in the placebo arm in 12 clinical studies of COVID-19 vaccines (mRNA-1273, CoV2 preS dTM, NVX-CoV2373, AZD-1222, BNT162b2, BNT162b1, and SCB-2019) at different phases of clinical development, and the results found demonstrate that while adverse events were mostly reported in the arms receiving the experimental vaccines, subjects receiving the placebo reported a significant frequency of adverse events. These results highlight the importance of critically evaluating the safety of experimental vaccines, especially when some minority groups are known to be resistant to COVID-19 vaccination [ 150 , 151 ].
When it comes to serious adverse events, particular concern has emerged related to the safety of COVID-19 during pregnancy due to reported cases of thrombosis and thrombocytopenia syndrome after vaccination with AZD 1222 in early 2021 [ 152 ]. Despite the devastating consequences of COVID-19 infection in pregnant women and the availability of vaccine safety and efficacy data in different populations, data related to vaccine safety in pregnant women are still limited, since most of the ongoing clinical trials do not include pregnant women [ 153 ]. However, preclinical and toxicological COVID-19 vaccine studies have found no safety concerns with no adverse effect on female reproduction, fertility, fetal or embryonal, or postnatal development, or miscarriage [ 153 , 154 , 155 ]. For mRNA vaccines, surveillance data demonstrated that vaccine-related adverse events in pregnant women were similar to those in non-pregnant women, with pain in the local area of the injection, fatigue, headache, and myalgia being the most frequent local and systemic reactions after vaccination [ 153 ]. Regarding the safety of COVID-19 vaccines for the fetus or breastfeeding infant, various expert panels suggest that mRNA-based and adenovirus vector vaccines do not possess any significant risk [ 156 , 157 ].
COVID-19 Vaccines and Serious Adverse Events: Myocarditis and Pericarditis
Currently, the main safety concerns for COVID-19 vaccines are related to mRNA vaccines, with the emergence of cases of myocarditis and pericarditis. Myocarditis is the inflammation of the heart muscle, while pericarditis is the inflammation of the outer lining of the heart. In both cases, the immune system causes inflammation in response to an infection or some other factor. Both can occur during infections, including SARS-CoV-2 infection. In the case of inflammation caused by mRNA vaccines, one of the first articles involving the evaluation of the incidence of myocarditis after vaccination with Pfizer’s vaccine (BNT162b2 or Comirnaty) was published in October 2021 in the New England Journal of Medicine. The study was conducted with patients in a large Israeli healthcare system who had received at least one dose of the vaccine. The authors reported an estimated incidence of myocarditis of 2.13 cases per 100,000 people; the highest incidence was among male patients between the ages of 16 and 29. Most cases of myocarditis were mild or moderate in severity [ 158 ]. According to the CDC, myocarditis is a rare and serious adverse event that has been associated with mRNA-based COVID-19 vaccines, in this case BNT162b2. The reporting rates of vaccine-associated myocarditis appear to be highest among males aged 12–29 years. As of 31 December 2021, myocarditis among children aged 5–11 years is classified as rare, where 11 Vaccine Adverse Event Reporting System (VAERS)-verified reports were received after the administration of approximately eight million doses of vaccine, and in an active vaccine safety surveillance system, no confirmed reports of myocarditis were observed during the 1–21 days or 1–42 days after 333,000 doses of vaccine were administered to children of the same age. Two deaths following the BNT162b2 vaccine were reported in children with multiple chronic medical conditions, where, in the initial review, no data were found to suggest a causal association between death and vaccination.
It is important to compare the cases among vaccinated and infected ones. The incidence of COVID-19-associated cardiac injury or myocarditis can be 100 times higher than COVID-19 mRNA-vaccine-related myocarditis [ 159 , 160 ]. Another relevant observation is that cases of myocarditis and pericarditis have been reported mainly for mRNA vaccines [ 161 ]; in Brazil, no cases have been reported related to inactivated virus-based vaccines [ 162 ]. The possible mechanisms underlying heart injury side-effects in specific groups were brightly reviewed and hypothesized by Heymans and Cooper [ 159 ], where, according to the authors, mRNA vaccines might trigger immune hyper immunity in a minority population that is genetically susceptible to developing acute myocarditis after viral injury [ 163 ]. In summary, they indicated three potential mechanisms: hormonal differences (the sex-specific distinction can be explained by hormone-related factors); mRNA immune reactivity (genetic variants in HLA genes); and antibodies to SARS-CoV-2 spike glycoproteins cross-reacting with myocardial contractile proteins (genotypes in desmosomal, cytoskeletal or sarcomeric protein).
4. Immunization in Children
Although COVID-19 morbidity and mortality are significantly lower in children than in adults, the risk of severe COVID-19 is not negligible even among healthy individuals. A recent report published by United Nations Children’s Fund (UNICEF), with age- and sex-disaggregated data from 104 countries, shows that children and adolescents under 20 years of age account for 18% of the reported COVID-19 cases and 33% of the population [ 164 ]. In the United States, recent data from the American Academy of Pediatrics show that by 21 February 2022, 12.3 million pediatric COVID-19 cases had been reported since the onset of the pandemic, which represents 15.7% of all confirmed cases in the country [ 121 , 165 ]. In the case of upper-middle-income countries, such as Brazil, the pediatric public has been neglected in terms of pandemic surveillance. Until February 2022, there was no federal public policy promoting early diagnosis at a federal level. This, associated with the absence of a testing strategy and the screening of suspected cases, makes it difficult to obtain data regarding the incidence of COVID-19 in children and adolescents and, consequently, the preventive and prophylactic measures taken by the public [ 166 , 167 ].
In general, most of the cases of the disease in children and adolescents are mild compared to those in adults. However, according to the American Academy of Pediatrics, the incidence of severe disease is 1% and the rate of death is 1/10,000 [ 165 ]. Several cases of multisystemic inflammatory syndrome have also been reported in this population group [ 168 ]. Moreover, even after mild cases of COVID-19, children can also present long-term side effects (known as long COVID-19) [ 121 , 169 ]. It is important to highlight that, according to the CDC, the peak of COVID-19 hospitalization in children and adolescents occurred during the Delta- and Omicron-predominant periods, on 11 September 2021 and 8 January 2022, respectively [ 170 ]. These data highlight how COVID-19 can represent a serious threat to the lives and health of children and adolescents. Therefore, with the development and availability of new vaccines around the world, there is great concern about the vaccination of this population group.
Among adolescents (12–17 years of age), some health and regulatory agencies have granted emergency use authorization for different COVID-19 vaccines: BNT162b2, mRNA 1273, CoronaVac, and Covaxin [ 171 ]. Canada was the first country to approve the vaccine for preventing SARS-CoV-2 infection in May 2021 for this population [ 172 ]. When it comes to children (5–11 years of age), vaccination around the world is not yet widely available. Countries such as the United States, Brazil, Canada, China, Israel, Italy, Chile, Australia, and Japan have already authorized or started the vaccination of children between 5 and 11 years of age ( Table 3 ) [ 173 ]. The vaccines BNT162b2 and CoronaVac are the main vaccines used for the immunization of children. Except for China, Hong Kong, Chile, Ecuador, Indonesia, Cambodia, and Brazil, which have authorized the use of CoronaVac for children, the other countries that have included the vaccination of children in their immunization plans only use BNT162b2 [ 174 ].
Vaccines approved for children immunization worldwide.
AIFA—Agenzia Italiana del Farmaco; Anvisa—Brazilian Health Regulatory Agency; FDA—US Food and Drug Administration; ISP—Instituto de Salud Publica; MHRA—UK Medicines and Healthcare products Regulatory Agency; NMPA—National Medical Products Administration; TGA—Therapeutic Goods Administration.
The decision to immunize children involves a complex risk–benefit analysis. Therefore, it is important to note that the approval of pediatric vaccination for COVID-19 was neither immediate nor widely accepted. Different opinions have generated discussions from the divergent views among regulatory agencies and government officials. For example, in Brazil, after technical analysis, the Anvisa authorized the pediatric version of the BNT162b2 vaccine on 16 December 2021. However, the Ministry of Health of the country did not consider the decision, claiming that the endorsement of the vigilance agency was not enough, initiating an unprecedented medium of public consultation on the subject. This decision was fostered by the resistance of some parents to immunizing their children. According to preliminary data from Oswaldo Cruz Foundation (Fiocruz), this resistance is related to misinformation about vaccination [ 175 ]. After further analysis, vaccination was then approved for children, but not made mandatory, in January 2022 [ 176 ].
Recently, the American Academy of Pediatrics reported that by mid-January 2022, about 8 million US children had received at least one dose of the COVID-19 vaccine, representing 28% of children ages 5–11. Considering both doses, 5.3 million U.S. children are fully vaccinated, representing 19% of children ages 5 to 11 [ 177 ]. In China, more than 84 million children between 3 and 11 have been vaccinated against COVID-19 with CoronaVac, and more than 49 million of them have received a booster dose [ 178 ]. It is important to highlight that the dose level of the BNT162b2 vaccine for children is different from that recommended for adolescents and adults, at 10 and 30 µg, respectively. Another difference is reported in the number of doses per vial, where for adolescents and adults there are six (dosage: 0.3 mL), while for children there are 10 (dosage: 0.2 mL). However, the recommended interval between the first and second dose is the same, regardless of age, and varies according to the regulatory agency of each country (3 weeks, according to the CDC, and 8 weeks according to the Brazilian Health Regulatory Agency—Anvisa) [ 179 ]. For CoronaVac, the recommendations made by Anvisa remain the same as for adults, where the two doses should be applied 28 days (about 4 weeks) apart, and the dosage does not change, remaining at 0.5 mL. The application of CoronaVac is not recommended for immunocompromised children.
The safety related to vaccination among the pediatric public is one of the main concerns of health authorities, as well as of public opinion. However, studies have shown that most of the adverse events related to COVID-19 vaccines have been mild to moderate, and resolve within 24 h [ 192 ]. The incidence of these adverse reactions has been associated with the dose of the vaccine. For the BNT162b2 vaccine, the most common adverse event in the clinical studies with children and adolescents was pain at the injection site. In addition, headaches, fatigue, and fever were also frequently reported. Most adverse events were not serious, and deaths were not reported [ 193 , 194 ]. Notably, a case report following 13 patients with solid tumors also showed that mild to moderate injection-site pain was the most frequent adverse event (found in six patients) [ 195 ]. However, the administration of the BNT162b2 vaccine in the pediatric public has raised different questions regarding the appearance of myocarditis and pericarditis [ 192 ].
When it comes to CoronaVac, the vaccine has already been applied in members of the public from 2 to 17 years old. According to Anvisa, and considering the international scenario, 86% of the adverse events recorded in this age group are of the non-severe type [ 196 ]. In addition, according to the Brazilian Agency, serious adverse events observed after the administration of more than 85 million doses of CoronaVac in Brazil in the public over 18 years old in Brazil are considered rare. Moreover, based on information given by one of the developers of the immunizer (the Butantan Institute), no cases of myocarditis, pericarditis, or thrombosis have been identified in China, where CoronaVac has been administered to the pediatric public for months [ 162 ].
As in adults, there is concern about the emergence of MIS cases after COVID-19 vaccination in children, which is also known as Pediatric Inflammatory Multisystem Syndrome Temporally Associated with SARS-CoV-2 (PIMS-TS) [ 139 ]. MIS among children is also considered to be a dysregulated immune response, where increased levels of neutralizing antibodies have been reported, leading to a condition of hyper inflammation [ 197 ]. Studies conducted in the United States have shown that whether immunization schedules are complete or not, COVID-19 vaccination is safe, whereas cases of MIS in this population after vaccination are rare [ 198 , 199 ]. Specifically, Zambrano et al. [ 199 ] demonstrated that immunization with two doses of an mRNA vaccine was able to prevent MIS in the 12- and 18-year-old population. Studies considering the vaccine-eligible population between the ages of 5 and 11 are still scarce in the literature, exposing, as for other public, the need for more incisive research on MSI.
Regarding efficacy, the BNT162b2 vaccine’s efficiency in preventing symptomatic infection in children is similar to that found for adolescent and adult populations, at >90%, even at a lower dose level. A study is being conducted with about 4650 children, where participants received either the experimental vaccine or a placebo [ 193 , 200 ]. As for CoronaVac, phase III clinical trials are ongoing with 14,000 children aged 6 months to 17 years from five different countries (Chile, Malaysia, Philippines, Turkey, and South Africa); however, preliminary results have shown that the immunizer is well tolerated among the pediatric audience. According to data released by Anvisa, an effectiveness study conducted in Chile demonstrated that CoronaVac has an effectiveness of >90% fourteen days after the second dose in participants between 6 and 16 years old, taking hospitalization into account. However, most of the data related to the mentioned studies are still blinded and should be presented [ 201 ]. It is also noteworthy that the phase I and II trials conducted in China demonstrated the immunogenicity of the immunizer in the population between 3 and 17 years after two doses, whereas in phase I, 71 individuals participated, and 28 days after vaccination, 100% of them showed antibodies. In phase II, the evaluation with 479 children and adolescents showed that in the group that received a dose of 1.5 µg, 96% of the participants showed antibodies, and in the group that received 3 µg, this number was 100% [ 202 ]. These data demonstrate that most COVID-19 vaccines provide good effectiveness and safety; therefore, double-dose vaccinations have been recommended by health authorities.
It is important to highlight that, in addition to the individual long-term health consequences, the decision to vaccinate pediatric groups involves a thorough evaluation of factors such as population-level factors. From this standpoint, it is not possible to mitigate and control pandemics without the immunization of children and adolescents, since this measure also helps to mitigate community transmission, avoid restrictive measures, and support the return of pre-pandemic activities [ 203 , 204 ]. Besides the safety issues mentioned, there are many criteria that must be analyzed when evaluating vaccination in children. These criteria are of high, medium, or low relevance, and should be measured according to their importance as well as the reality of that population, since they are individual and community criteria ( Figure 2 ). However, in the actual scenario, we still need more studies to confirm the long-term safety and efficacy of these vaccines in this population.
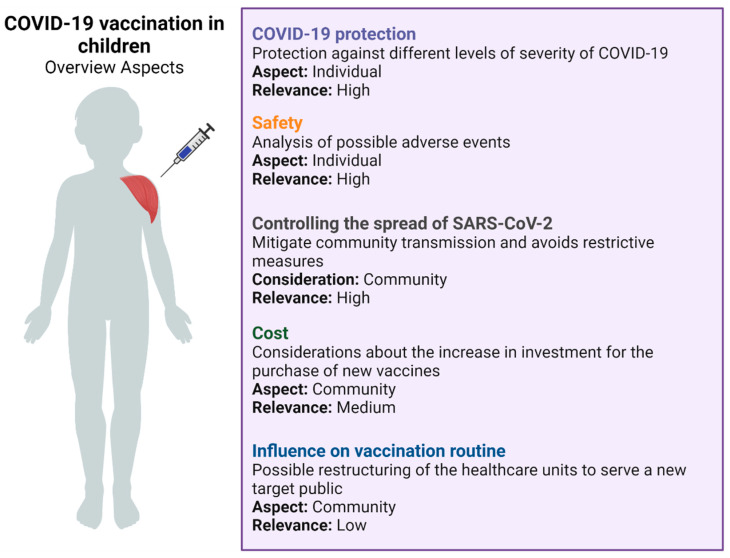
Overview about infantile vaccination for COVID-19. Adapted from Zimmerman et al. [ 203 ]. Created with BioRender.com (accessed on 5 March 2022).
5. COVID-19 Future Perspectives and the Role of Vaccination in this Control
According to epidemiologists, the change in state from pandemic to endemic means that the virus will remain among the population in a reliably predictable way. In this scenario, COVID-19-related transmission, hospitalization, and death will stay stable, following a pattern. This does not necessarily mean that the number of deaths, transmissions, or even severity will get lower; just foreseeable [ 205 ]. Currently, following the advent of vaccines and the emergence of less-lethal variants, mortality rates have decreased globally. According to real-time monitoring data provided by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University, the fatality rates in February 2021 were 3.33% (466,000 daily cases and 15.4 thousand daily deaths). One year later, in February 2022, cases almost quadrupled, but the number of daily deaths declined, resulting in a death rate of 0.38% (3.18 million cases and 12,294,000 deaths) [ 206 , 207 ].Considering this optimistic scenario, some European countries have gradually started to adopt more flexible measures against the disease. These measures assume living with the disease, treating it as an endemic disease. In the context of epidemiological control, managing COVID-19 as an endemic disease entails not following up on all cases. In addition, countries could integrate coronavirus monitoring as with other flu-like diseases, testing representative samples, instead of every symptomatic patient [ 208 ]. However, to classify an infectious disease as endemic, the rate of infections needs to stabilize sufficiently for infection peaks (with seasonal epidemic peaks) to become predictable [ 209 ].
Therefore, although it is recognized that at some point the disease may be redefined as endemic, the WHO is currently against this position [ 210 ]. According to the organization, SARS-CoV-2 still presents many uncertainties due to its high mutation rate, and cannot yet be treated as an endemic disease. Among the key factors that remain uncertain or unknown are the duration of immunity to SARS-CoV-2 from vaccination or prior infection; whether SARS-CoV-2 will become a seasonal infection; or whether new more transmissible, immune-evading, or virulent variants will arise [ 209 ].
Currently, there is no consensus among state governments and health organizations regarding the transition to endemicity. However, most authors argue that the crucial factor for this change is the number of immunized individuals in a population [ 208 , 209 ]. The acquisition of effective vaccines, the intense search for antiviral drugs alternatives, and health-surveillance measures have resulted in a reduction in severe cases and fatalities. In parallel, researchers discuss the impact of the COVID-19 variants on natural immunization [ 211 ]. Telenti et al. [ 209 ] demonstrated that immune protection can affect SARS-CoV-2 transmission. Therefore, it is essential for public health-management strategies (booster doses application, incorporation of new vaccines), as well as providing a transition from pandemic to epidemic.
In this context, wealthy countries with prominent levels of population immunity have shown lower fatality rates, leading them to consider the disease as being manageable and endemic. Remarkably, countries with different vaccinations rates per 100 inhabitants show alarming discrepancies in cumulative death rates. For comparison purposes only, high- to middle-income countries (classification as gross national income (GNI) per capita of World Bank [ 212 ]) such as Trinidad and Tobago (47th GNI position, 1.4 M inhabitants; vaccination per 100 inhabitants: 110.13; death rate: 2.72%), the Bahamas (31st GNI position, 396.91k inhabitants; vaccination per 100 inhabitants: 84.53; death rate: 2.37%), Romania (56th GNI position, 19.13 M inhabitants, vaccination per 100 inhabitants: 87.53; death rate: 2.28%); and Russia (60th GNI position, 145.9 M inhabitants, vaccination per 100 inhabitants: 112.14; death rate: 2.06%) have death rates ~ 2.8 times higher than similar-income countries presenting higher vaccination rates, such as Greece (42nd GNI position, 10.37 M inhabitants; vaccination per 100 inhabitants: 198.34; death rate: 0.9%); Italy (27th GNI position, 60.37 M inhabitants; vaccination per 100 inhabitants: 225.32; death rate: 1.08%); Portugal (37th GNI position, 10.17 M inhabitants; vaccination per 100 inhabitants: 225.45; death rate: 0.6%); Uruguay (46th GNI position, 3.49 M inhabitants; vaccination per 100 inhabitants: 231.5; death rate: 0.81%) [ 213 ].
Unfortunately, the same pattern can be seen comparing low-income countries. Countries with lower access to vaccination have shown markedly higher death rates. For comparison purposes only, Sudan (178th GNI position, 44.91M inhabitants; vaccination per 100 inhabitants: 13.65; death rate: 7.92%); Syria (160th GNI position, 18.28 M inhabitants; vaccination per 100 inhabitants: 18.44; death rate: 5.64%); and Chad (179th GNI position, 16.91 M inhabitants; vaccination per 100 inhabitants: 2.48; death rate: 2.61%) have presented death rates approximately five times higher than Rwanda (174th GNI position, 13.28 M inhabitants; vaccination per 100 inhabitants: 146.28; death rate: 1.13%); Guinea (168th GNI position, 13.5 M inhabitants; vaccination per 100 inhabitants: 44.1; death rate: 1.21%); and Togo (169th GNI position, 8.48M inhabitants; vaccination per 100 inhabitants: 32; death rate: 0.74%) [ 213 ].
However, important aspects of COVID-19 remain unknown or under investigation. These include the varying immunization rates, antibody titers’ waning rates after vaccination or natural infection, high RNA viral instability caused by mutations [ 214 , 215 ], and the impact of heterogeneous pandemic control in countries at distinct stages of economic development. For these reasons, predicting the transition time of COVID-19 to an endemic state remains a considerable challenge.
It is impossible to know exactly what SARS-CoV-2 will become, but among the possible scenarios, the authors consider that most COVID-19 will turn into an influenza-like endemic disease. However, according to the WHO, annually there are 290,000 to 650,000 influenza-related respiratory deaths [ 216 ]. Given that after two years of the pandemic, the number of deaths from COVID-19 is 5.82 million [ 217 ], changing the state from pandemic to endemic will result in a better situation, but not really an ideal one. On the other hand, a more optimistic, but less probable, scenario would be SARS-CoV-2 becoming just another coronavirus species. Commonly, coronaviruses such as SARS-CoV, MERS-CoV, HCoV-NL63, HCoV-229E, HCoV-OC43, and HKU1 present a lower human health impact, with symptoms relating to mild or light respiratory illnesses [ 218 , 219 ].
Finally, the need for the equitable distribution of vaccines in different parts of the world is also highlighted. While high-income countries have a sufficient immunization rate for restrictive measures to be relaxed, low- and middle-income countries face limited and delayed access to COVID-19 vaccines, despite the existence of initiatives to this end, such as COVID-19 Vaccines Global Access (COVAX) [ 220 ]. One of the main consequences of this context is that with high infection rates around the world, coupled with the absence of broad access to vaccines and the establishment of effective immunization campaigns in certain locations, more variants of SARS-CoV-2 will continue to emerge, representing a major concern [ 221 ]. Thus, it is critical that vaccine platforms that have a rapid manufacturing process, as well as distribution without major logistical barriers and easy administration, be made widely available in order to control the advance of COVID-19 [ 222 ].
6. Conclusions
Despite increasing advances in knowledge regarding SARS-CoV-2 and COVID-19, mass vaccination has not yet been enough to stop the pandemic. The emergence of new variants of concern remains an immense challenge, since, despite proven efficacy, vaccines for COVID-19 are not able to prevent viral infection 100% of the time. Thus, health authorities and agencies have adopted the practice of booster doses, thinking about the protection of individuals who are part of at-risk groups. Another concern about COVID-19 vaccination has been the safety of the vaccines due to their rapid development and the lack of knowledge about their long-term effects. Importantly, despite the risks attached to the available vaccines, serious adverse events such as myocarditis and pericarditis seen with RNA vaccines are considered rare, and vaccination is still recommended in the population. It has been shown that vaccination is able to prevent hospitalization of infected individuals and even reduce cases of death, and so it remains the best alternative for the pandemic to finally be considered endemic.
Acknowledgments
The authors thank SENAI CIMATEC and CNPq (Conselho Nacional de Desen- volvimento Científico e Tecnológico) (BAMS is a Technological fellow from CNPq 315351/2018-7), and the National System of the Nanotechnology Laboratories (SisNANO/MCTI/Brazil).

Author Contributions
Conceptualization, B.A.S.M., L.A.B.M., L.P.C.d.S.A. and R.B.; methodology, B.A.S.M., K.V.S.H., L.M.d.S.F., V.C.P., L.A.B.M., L.P.C.d.S.A., M.A.M. and R.B.; validation, B.A.S.M., K.V.S.H., L.M.d.S.F., V.C.P. and R.B.; formal analysis, B.A.S.M., K.V.S.H., L.M.d.S.F., V.C.P., L.A.B.M., L.P.C.d.S.A., M.A.M. and R.B.; investigation, B.A.S.M., K.V.S.H., L.M.d.S.F., V.C.P., L.A.B.M., L.P.C.d.S.A., M.A.M. and R.B.; resources, B.A.S.M., L.A.B.M., L.P.C.d.S.A. and R.B.; writing—original draft preparation, B.A.S.M., K.V.S.H., L.M.d.S.F., V.C.P., L.A.B.M., L.P.C.d.S.A., M.A.M. and R.B.; writing—review and editing, B.A.S.M., K.V.S.H., L.M.d.S.F., V.C.P., L.A.B.M., L.P.C.d.S.A., M.A.M. and R.B.; visualization, B.A.S.M., L.A.B.M., L.P.C.d.S.A. and R.B.; supervision, B.A.S.M., L.A.B.M., L.P.C.d.S.A. and R.B.; project administration, B.A.S.M., L.A.B.M., L.P.C.d.S.A. and R.B. All authors have read and agreed to the published version of the manuscript.
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- 1. Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Pagliusi S., Jarrett S., Hayman B., Kreysa U., Prasad S.D., Reers M., Hong Thai P., Wu K., Zhang Y.T., Baek Y.O., et al. Emerging manufacturers engagements in the COVID-19 vaccine research, development and supply. Vaccine. 2020;38:5418–5423. doi: 10.1016/j.vaccine.2020.06.022. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Gao Q., Bao L., Mao H., Wang L., Xu K., Yang M., Li Y., Zhu L., Wang N., Lv Z., et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369:77–81. doi: 10.1126/science.abc1932. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Callaway E. The race for coronavirus vaccines: A graphical guide. Nature. 2020;580:576–577. doi: 10.1038/d41586-020-01221-y. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Momin T., Kansagra K., Patel H., Sharma S., Sharma B., Patel J., Mittal R., Sanmukhani J., Maithal K., Dey A., et al. Safety and Immunogenicity of a DNA SARS-CoV-2 vaccine (ZyCoV-D): Results of an open-label, non-randomized phase I part of phase I/II clinical study by intradermal route in healthy subjects in India. EClinicalMedicine. 2021;38:101020. doi: 10.1016/j.eclinm.2021.101020. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Machado B.A.S., Hodel K.V.S., Fonseca L.M.d.S., Mascarenhas L.A.B., Andrade L.P.C.d.S., Rocha V.P.C., Soares M.B.P., Berglund P., Duthie M.S., Reed S.G., et al. The Importance of RNA-Based Vaccines in the Fight against COVID-19: An Overview. Vaccines. 2021;9:1345. doi: 10.3390/vaccines9111345. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., Goepfert P.A., Truyers C., Fennema H., Spiessens B., et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., Kovyrshina A.V., Lubenets N.L., Grousova D.M., Erokhova A.S., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Tanriover M.D., Doğanay H.L., Akova M., Güner H.R., Azap A., Akhan S., Köse Ş., Erdinç F.Ş., Akalın E.H., Tabak Ö.F., et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): Interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398:213–222. doi: 10.1016/S0140-6736(21)01429-X. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Yang S., Li Y., Dai L., Wang J., He P., Li C., Fang X., Wang C., Zhao X., Huang E., et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: Two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect. Dis. 2021;21:1107–1119. doi: 10.1016/S1473-3099(21)00127-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. WHO COVID19 Vaccine Tracker. [(accessed on 9 February 2022)]. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines .
- 15. Ledford H. US authorization of first COVID vaccine marks new phase in safety monitoring. Nature. 2020;588:377–378. doi: 10.1038/d41586-020-03542-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Kreier F. Ten billion COVID vaccinations: World hits new milestone. Nature. 2022 doi: 10.1038/d41586-022-00285-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Hu B., Guo H., Zhou P., Shi Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021;19:141–154. doi: 10.1038/s41579-020-00459-7. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., Meng J., Zhu Z., Zhang Z., Wang J., et al. Genome Composition and Divergence of the Novel Coronavirus (2019-nCoV) Originating in China. Cell Host Microbe. 2020;27:325–328. doi: 10.1016/j.chom.2020.02.001. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Wang Q., Zhang Y., Wu L., Niu S., Song C., Zhang Z., Lu G., Qiao C., Hu Y., Yuen K.-Y., et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell. 2020;181:894–904.e9. doi: 10.1016/j.cell.2020.03.045. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 22. Romano M., Ruggiero A., Squeglia F., Maga G., Berisio R. A Structural View of SARS-CoV-2 RNA Replication Machinery: RNA Synthesis, Proofreading and Final Capping. Cells. 2020;9:1267. doi: 10.3390/cells9051267. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 23. Singh D., Yi S.V. On the origin and evolution of SARS-CoV-2. Exp. Mol. Med. 2021;53:537–547. doi: 10.1038/s12276-021-00604-z. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Bian L., Gao Q., Gao F., Wang Q., He Q., Wu X., Mao Q., Xu M., Liang Z. Impact of the Delta variant on vaccine efficacy and response strategies. Expert Rev. Vaccines. 2021;20:1201–1209. doi: 10.1080/14760584.2021.1976153. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Thomas E., Delabat S., Carattini Y.L., Andrews D.M. SARS-CoV-2 and Variant Diagnostic Testing Approaches in the United States. Viruses. 2021;13:2492. doi: 10.3390/v13122492. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Machado B.A.S., Hodel K.V.S., Barbosa-Júnior V.G., Soares M.B.P., Badaró R. The Main Molecular and Serological Methods for Diagnosing COVID-19: An Overview Based on the Literature. Viruses. 2020;13:40. doi: 10.3390/v13010040. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Tracking SARS-CoV-2 Variants. [(accessed on 8 February 2022)]. Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/
- 28. Zhang X., Wu S., Wu B., Yang Q., Chen A., Li Y., Zhang Y., Pan T., Zhang H., He X. SARS-CoV-2 Omicron strain exhibits potent capabilities for immune evasion and viral entrance. Signal Transduct. Target. Ther. 2021;6:430. doi: 10.1038/s41392-021-00852-5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Willyard C. What the Omicron wave is revealing about human immunity. Nature. 2022;602:22–25. doi: 10.1038/d41586-022-00214-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Bhagavathula A.S., Massey P.M., Khubchandani J. COVID-19 testing demand amidst Omicron variant surge: Mass hysteria or population health need? Brain. Behav. Immun. 2022;101:394–396. doi: 10.1016/j.bbi.2022.01.023. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Kumar S., Thambiraja T.S., Karuppanan K., Subramaniam G. Omicron and Delta variant of SARS-CoV-2: A comparative computational study of spike protein. J. Med. Virol. 2022;94:1641–1649. doi: 10.1002/jmv.27526. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Long B., Carius B.M., Chavez S., Liang S.Y., Brady W.J., Koyfman A., Gottlieb M. Clinical update on COVID-19 for the emergency clinician: Presentation and evaluation. Am. J. Emerg. Med. 2022;54:46–57. doi: 10.1016/j.ajem.2022.01.028. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. Kozlov M. How does Omicron spread so fast? A high viral load isn’t the answer. Nature. 2022 doi: 10.1038/d41586-022-00129-z. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Jansen L., Tegomoh B., Lange K., Showalter K., Figliomeni J., Abdalhamid B., Iwen P.C., Fauver J., Buss B., Donahue M. Investigation of a SARS-CoV-2 B.1.1.529 (Omicron) Variant Cluster—Nebraska, November–December 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70:1782–1784. doi: 10.15585/mmwr.mm705152e3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Kozlov M. Omicron’s feeble attack on the lungs could make it less dangerous. Nature. 2022;601:177. doi: 10.1038/d41586-022-00007-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Mallapaty S. COVID reinfections surge during Omicron onslaught. Nature. 2022 doi: 10.1038/d41586-022-00438-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Islam M.R., Hossain M.J. Detection of SARS-CoV-2 Omicron (B.1.1.529) variant has created panic among the people across the world: What should we do right now? J. Med. Virol. 2021;94:1768–1769. doi: 10.1002/jmv.27546. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Islam S., Islam T., Islam M.R. New Coronavirus Variants are Creating More Challenges to Global Healthcare System: A Brief Report on the Current Knowledge. Clin. Pathol. 2022;15:2632010X221075584. doi: 10.1177/2632010X221075584. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Deng X., Gu W., Federman S., du Plessis L., Pybus O.G., Faria N.R., Wang C., Yu G., Bushnell B., Pan C.-Y., et al. Genomic surveillance reveals multiple introductions of SARS-CoV-2 into Northern California. Science. 2020;369:582–587. doi: 10.1126/science.abb9263. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 40. Li J., Lai S., Gao G.F., Shi W. The emergence, genomic diversity and global spread of SARS-CoV-2. Nature. 2021;600:408–418. doi: 10.1038/s41586-021-04188-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Song S., Ma L., Zou D., Tian D., Li C., Zhu J., Chen M., Wang A., Ma Y., Li M., et al. The Global Landscape of SARS-CoV-2 Genomes, Variants, and Haplotypes in 2019nCoVR. Genomics. Proteom. Bioinform. 2020;18:749–759. doi: 10.1016/j.gpb.2020.09.001. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 42. Rathinasamy M., Kandhasamy S. An exploratory study on the propagation of SARS-CoV-2 variants: Omicron is the most predominant variant. J. Med. Virol. 2022:1–8. doi: 10.1002/jmv.27634. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 43. Jiang Y., Wu Q., Song P., You C. The Variation of SARS-CoV-2 and Advanced Research on Current Vaccines. Front. Med. 2022;8:2908. doi: 10.3389/fmed.2021.806641. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Tregoning J.S., Flight K.E., Higham S.L., Wang Z., Pierce B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021;21:626–636. doi: 10.1038/s41577-021-00592-1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 45. Harvey W.T., Carabelli A.M., Jackson B., Gupta R.K., Thomson E.C., Harrison E.M., Ludden C., Reeve R., Rambaut A., Peacock S.J., et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021;19:409–424. doi: 10.1038/s41579-021-00573-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Starr T.N., Greaney A.J., Hilton S.K., Ellis D., Crawford K.H.D., Dingens A.S., Navarro M.J., Bowen J.E., Tortorici M.A., Walls A.C., et al. Deep Mutational Scanning of SARS-CoV-2 Receptor Binding Domain Reveals Constraints on Folding and ACE2 Binding. Cell. 2020;182:1295–1310.e20. doi: 10.1016/j.cell.2020.08.012. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 47. Weisblum Y., Schmidt F., Zhang F., DaSilva J., Poston D., Lorenzi J.C.C., Muecksch F., Rutkowska M., Hoffmann H.-H., Michailidis E., et al. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. Elife. 2020;9:e61312. doi: 10.7554/eLife.61312. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. McCarthy K.R., Rennick L.J., Nambulli S., Robinson-McCarthy L.R., Bain W.G., Haidar G., Duprex W.P. Recurrent deletions in the SARS-CoV-2 spike glycoprotein drive antibody escape. Science. 2021;371:1139–1142. doi: 10.1126/science.abf6950. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 49. Volz E., Mishra S., Chand M., Barrett J.C., Johnson R., Geidelberg L., Hinsley W.R., Laydon D.J., Dabrera G., O’Toole Á., et al. Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England. Nature. 2021;593:266–269. doi: 10.1038/s41586-021-03470-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Davies N.G., Abbott S., Barnard R.C., Jarvis C.I., Kucharski A.J., Munday J.D., Pearson C.A.B., Russell T.W., Tully D.C., Washburne A.D., et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372:eabg3055. doi: 10.1126/science.abg3055. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 51. Dai L., Gao G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021;21:73–82. doi: 10.1038/s41577-020-00480-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 52. Heinz F.X., Stiasny K. Distinguishing features of current COVID-19 vaccines: Knowns and unknowns of antigen presentation and modes of action. npj Vaccines. 2021;6:104. doi: 10.1038/s41541-021-00369-6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 53. Ding C., He J., Zhang X., Jiang C., Sun Y., Zhang Y., Chen Q., He H., Li W., Xie J., et al. Crucial Mutations of Spike Protein on SARS-CoV-2 Evolved to Variant Strains Escaping Neutralization of Convalescent Plasmas and RBD-Specific Monoclonal Antibodies. Front. Immunol. 2021;12:3231. doi: 10.3389/fimmu.2021.693775. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 54. Holder J. COVID World Vaccination Tracker. [(accessed on 6 February 2022)]. Available online: https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html .
- 55. Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., Stowe J., Tessier E., Groves N., Dabrera G., et al. Effectiveness of COVID-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 56. Collie S., Champion J., Moultrie H., Bekker L.-G., Gray G. Effectiveness of BNT162b2 Vaccine against Omicron Variant in South Africa. N. Engl. J. Med. 2022;386:494–496. doi: 10.1056/NEJMc2119270. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 57. Abu-Raddad L.J., Chemaitelly H., Butt A.A. Effectiveness of the BNT162b2 COVID-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N. Engl. J. Med. 2021;385:187–189. doi: 10.1056/NEJMc2104974. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 58. Charmet T., Schaeffer L., Grant R., Galmiche S., Chény O., Von Platen C., Maurizot A., Rogoff A., Omar F., David C., et al. Impact of original, B.1.1.7, and B.1.351/P.1 SARS-CoV-2 lineages on vaccine effectiveness of two doses of COVID-19 mRNA vaccines: Results from a nationwide case-control study in France. Lancet Reg. Health-Eur. 2021;8:100171. doi: 10.1016/j.lanepe.2021.100171. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 59. Wang P., Nair M.S., Liu L., Iketani S., Luo Y., Guo Y., Wang M., Yu J., Zhang B., Kwong P.D., et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593:130–135. doi: 10.1038/s41586-021-03398-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Wang P., Casner R.G., Nair M.S., Wang M., Yu J., Cerutti G., Liu L., Kwong P.D., Huang Y., Shapiro L., et al. Increased resistance of SARS-CoV-2 variant P.1 to antibody neutralization. Cell Host Microbe. 2021;29:747–751.e4. doi: 10.1016/j.chom.2021.04.007. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Wall E.C., Wu M., Harvey R., Kelly G., Warchal S., Sawyer C., Daniels R., Hobson P., Hatipoglu E., Ngai Y., et al. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet. 2021;397:2331–2333. doi: 10.1016/S0140-6736(21)01290-3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 62. Chemaitelly H., Yassine H.M., Benslimane F.M., Al Khatib H.A., Tang P., Hasan M.R., Malek J.A., Coyle P., Ayoub H.H., Al Kanaani Z., et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat. Med. 2021;27:1614–1621. doi: 10.1038/s41591-021-01446-y. [ DOI ] [ PubMed ] [ Google Scholar ]
- 63. Tang P., Hasan M.R., Chemaitelly H., Yassine H.M., Benslimane F.M., Al Khatib H.A., AlMukdad S., Coyle P., Ayoub H.H., Al Kanaani Z., et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat. Med. 2021;27:2136–2143. doi: 10.1038/s41591-021-01583-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 64. Fu Tseng H., Ackerson B.K., Luo Y., Sy L.S., Talarico C.A., Tian Y., Bruxvoort K.J., Tubert J.E., Florea A., Ku J.H., et al. Effectiveness of mRNA-1273 against SARS-CoV-2 omicron and delta variants. medRxiv. 2022 doi: 10.1038/s41591-022-01753-y. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 65. Edara V.-V., Manning K.E., Ellis M., Lai L., Moore K.M., Foster S.L., Floyd K., Davis-Gardner M.E., Mantus G., Nyhoff L.E., et al. mRNA-1273 and BNT162b2 mRNA vaccines have reduced neutralizing activity against the SARS-CoV-2 omicron variant. Cell Rep. Med. 2022;3:100529. doi: 10.1016/j.xcrm.2022.100529. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 66. Choi A., Koch M., Wu K., Dixon G., Oestreicher J., Legault H., Stewart-Jones G.B.E., Colpitts T., Pajon R., Bennett H., et al. Serum Neutralizing Activity of mRNA-1273 against SARS-CoV-2 Variants. J. Virol. 2021;95:e01313-21. doi: 10.1128/JVI.01313-21. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 67. Ranzani O.T., Hitchings M.D.T., Dorion M., D’Agostini T.L., de Paula R.C., de Paula O.F.P., Villela E.F.D.M., Torres M.S.S., de Oliveira S.B., Schulz W., et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of COVID-19 in Brazil: Test negative case-control study. BMJ. 2021;374:n2015. doi: 10.1136/bmj.n2015. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 68. Li X.-N., Huang Y., Wang W., Jing Q.-L., Zhang C.-H., Qin P.-Z., Guan W.-J., Gan L., Li Y.-L., Liu W.-H., et al. Effectiveness of inactivated SARS-CoV-2 vaccines against the Delta variant infection in Guangzhou: A test-negative case–control real-world study. Emerg. Microbes Infect. 2021;10:1751–1759. doi: 10.1080/22221751.2021.1969291. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 69. Cevik M., Grubaugh N.D., Iwasaki A., Openshaw P. COVID-19 vaccines: Keeping pace with SARS-CoV-2 variants. Cell. 2021;184:5077–5081. doi: 10.1016/j.cell.2021.09.010. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 70. Lu L., Mok B.W.-Y., Chen L.-L., Chan J.M.-C., Tsang O.T.-Y., Lam B.H.-S., Chuang V.W.-M., Chu A.W.-H., Chan W.-M., Ip J.D., et al. Neutralization of Severe Acute Respiratory Syndrome Coronavirus 2 Omicron Variant by Sera From BNT162b2 or CoronaVac Vaccine Recipients. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab1041. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 71. Chen Y., Shen H., Huang R., Tong X., Wu C. Serum neutralising activity against SARS-CoV-2 variants elicited by CoronaVac. Lancet Infect. Dis. 2021;21:1071–1072. doi: 10.1016/S1473-3099(21)00287-5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 72. Hu J., Wei X., Xiang J., Peng P., Xu F., Wu K., Luo F., Jin A., Fang L., Liu B., et al. Reduced neutralization of SARS-CoV-2 B.1.617 variant by convalescent and vaccinated sera. Genes Dis. 2021. in press . [ DOI ] [ PMC free article ] [ PubMed ]
- 73. Wang G.-L., Wang Z.-Y., Duan L.-J., Meng Q.-C., Jiang M.-D., Cao J., Yao L., Zhu K.-L., Cao W.-C., Ma M.-J. Susceptibility of Circulating SARS-CoV-2 Variants to Neutralization. N. Engl. J. Med. 2021;384:2354–2356. doi: 10.1056/NEJMc2103022. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 74. Wu D., Zhang Y., Tang L., Wang F., Ye Y., Ma C., Zheng H., Yu W., Cao L., Song Y., et al. Effectiveness of Inactivated COVID-19 Vaccines Against Symptomatic, Pneumonia, and Severe Disease Caused by the Delta Variant: Real World Study and Evidence—China, 2021. China CDC Wkly. 2022;4:57–65. doi: 10.46234/ccdcw2022.009. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 75. Yu X., Wei D., Xu W., Li Y., Li X., Zhang X., Qu J., Yang Z., Chen E. Reduced sensitivity of SARS-CoV-2 Omicron variant to antibody neutralization elicited by booster vaccination. Cell Discov. 2022;8:4. doi: 10.1038/s41421-022-00375-5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 76. Madhi S.A., Baillie V., Cutland C.L., Voysey M., Koen A.L., Fairlie L., Padayachee S.D., Dheda K., Barnabas S.L., Bhorat Q.E., et al. Efficacy of the ChAdOx1 nCoV-19 COVID-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021;384:1885–1898. doi: 10.1056/NEJMoa2102214. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 77. Clemens S.A.C., Folegatti P.M., Emary K.R.W., Weckx L.Y., Ratcliff J., Bibi S., De Almeida Mendes A.V., Milan E.P., Pittella A., Schwarzbold A.V., et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 lineages circulating in Brazil. Nat. Commun. 2021;12:5861. doi: 10.1038/s41467-021-25982-w. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 78. Andrews N., Stowe J., Kirsebom F., Toffa S., Rickeard T., Gallagher E., Gower C., Kall M., Groves N., O’Connell A.-M., et al. Effectiveness of COVID-19 vaccines against the Omicron (B.1.1.529) variant of concern. medRxiv. 2021 doi: 10.1101/2021.12.14.21267615. [ DOI ] [ Google Scholar ]
- 79. Liu C., Ginn H.M., Dejnirattisai W., Supasa P., Wang B., Tuekprakhon A., Nutalai R., Zhou D., Mentzer A.J., Zhao Y., et al. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell. 2021;184:4220–4236.e13. doi: 10.1016/j.cell.2021.06.020. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 80. Mlcochova P., Kemp S.A., Dhar M.S., Papa G., Meng B., Ferreira I.A.T.M., Datir R., Collier D.A., Albecka A., Singh S., et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature. 2021;599:114–119. doi: 10.1038/s41586-021-03944-y. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 81. Dejnirattisai W., Huo J., Zhou D., Zahradník J., Supasa P., Liu C., Duyvesteyn H.M.E., Ginn H.M., Mentzer A.J., Tuekprakhon A., et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell. 2022;185:467–484.e15. doi: 10.1016/j.cell.2021.12.046. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 82. Cameroni E., Bowen J.E., Rosen L.E., Saliba C., Zepeda S.K., Culap K., Pinto D., VanBlargan L.A., De Marco A., di Iulio J., et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature. 2021;602:664–670. doi: 10.1038/s41586-021-04386-2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 83. Barouch D.H., Stephenson K.E., Sadoff J., Yu J., Chang A., Gebre M., McMahan K., Liu J., Chandrashekar A., Patel S., et al. Durable Humoral and Cellular Immune Responses 8 Months after Ad26.COV2.S Vaccination. N. Engl. J. Med. 2021;385:951–953. doi: 10.1056/NEJMc2108829. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 84. Hafiz I., Illian D.N., Meila O., Utomo A.R.H., Susilowati A., Susetya I.E., Desrita D., Siregar G.A., Basyuni M. Effectiveness and Efficacy of Vaccine on Mutated SARS-CoV-2 Virus and Post Vaccination Surveillance: A Narrative Review. Vaccines. 2022;10:82. doi: 10.3390/vaccines10010082. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 85. Huang Z., Su Y., Zhang T., Xia N. A review of the safety and efficacy of current COVID-19 vaccines. Front. Med. 2022;16:39–55. doi: 10.1007/s11684-021-0893-y. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 86. Pajon R., Doria-Rose N.A., Shen X., Schmidt S.D., O’Dell S., McDanal C., Feng W., Tong J., Eaton A., Maglinao M., et al. SARS-CoV-2 Omicron Variant Neutralization after mRNA-1273 Booster Vaccination. N. Engl. J. Med. 2022;386:1088–1091. doi: 10.1056/NEJMc2119912. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 87. Sablerolles R.S.G., Rietdijk W.J.R., Goorhuis A., Postma D.F., Visser L.G., Geers D., Schmitz K.S., Garcia Garrido H.M., Koopmans M.P.G., Dalm V.A.S.H., et al. Immunogenicity and Reactogenicity of Vaccine Boosters after Ad26.COV2.S Priming. N. Engl. J. Med. 2022;386:951–963. doi: 10.1056/NEJMoa2116747. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 88. Zhao X., Li D., Ruan W., Chen Z., Zhang R., Zheng A., Qiao S., Zheng X., Zhao Y., Dai L., et al. Effects of a Prolonged Booster Interval on Neutralization of Omicron Variant. N. Engl. J. Med. 2022;386:894–896. doi: 10.1056/NEJMc2119426. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 89. Sester M., Becker S.L. Boosting immunity after CoronaVac. Lancet. 2022;399:496–497. doi: 10.1016/S0140-6736(22)00095-2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 90. del Rio C., Malani P.N., Omer S.B. Confronting the Delta Variant of SARS-CoV-2, Summer 2021. JAMA. 2021;326:1001. doi: 10.1001/jama.2021.14811. [ DOI ] [ PubMed ] [ Google Scholar ]
- 91. Bruxvoort K.J., Sy L.S., Qian L., Ackerson B.K., Luo Y., Lee G.S., Tian Y., Florea A., Aragones M., Tubert J.E., et al. Effectiveness of mRNA-1273 against delta, mu, and other emerging variants of SARS-CoV-2: Test negative case-control study. BMJ. 2021;375:e068848. doi: 10.1136/bmj-2021-068848. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 92. Garcia-Beltran W.F., St. Denis K.J., Hoelzemer A., Lam E.C., Nitido A.D., Sheehan M.L., Berrios C., Ofoman O., Chang C.C., Hauser B.M., et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022;185:457–466.e4. doi: 10.1016/j.cell.2021.12.033. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 93. Yu X., Qi X., Cao Y., Li P., Lu L., Wang P., Feng Y., Yang J., Wei H., Guo L., et al. Three doses of an inactivation-based COVID-19 vaccine induces cross-neutralizing immunity against the SARS CoV-2 Omicron variant. Emerg. Microbes Infect. 2022;11:749–752. doi: 10.1080/22221751.2022.2044271. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 94. Mistry P., Barmania F., Mellet J., Peta K., Strydom A., Viljoen I.M., James W., Gordon S., Pepper M.S. SARS-CoV-2 Variants, Vaccines, and Host Immunity. Front. Immunol. 2021;12:9244. doi: 10.3389/fimmu.2021.809244. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 95. CDC COVID Data Tracker: Rates of COVID-19 Cases and Deaths by Vaccination Status. [(accessed on 5 April 2022)]; Available online: https://covid.cdc.gov/covid-data-tracker/#rates-by-vaccine-status .
- 96. Johnson A.G., Amin A.B., Ali A.R., Hoots B., Cadwell B.L., Arora S., Avoundjian T., Awofeso A.O., Barnes J., Bayoumi N.S., et al. COVID-19 Incidence and Death Rates Among Unvaccinated and Fully Vaccinated Adults with and Without Booster Doses During Periods of Delta and Omicron Variant Emergence—25 U.S. Jurisdictions, April 4–December 25, 2021. MMWR Morb. Mortal. Wkly. Rep. 2022;71:132–138. doi: 10.15585/mmwr.mm7104e2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 97. Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines-a new era in vaccinology. Nat. Rev. Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 98. Krause P.R., Fleming T.R., Peto R., Longini I.M., Figueroa J.P., Sterne J.A.C., Cravioto A., Rees H., Higgins J.P.T., Boutron I., et al. Considerations in boosting COVID-19 vaccine immune responses. Lancet. 2021;398:1377–1380. doi: 10.1016/S0140-6736(21)02046-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 99. COVID-19 Vaccine Booster Shots. [(accessed on 8 February 2022)]; Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html .
- 100. Comirnaty and Spikevax: EMA Recommendations on Extra Doses and Boosters. [(accessed on 8 February 2022)]. Available online: https://www.ema.europa.eu/en/news/comirnaty-spikevax-ema-recommendations-extra-doses-boosters .
- 101. Ministério da Saúde Ministério da Saúde Anuncia dose de reforço para Vacinação Contra a COVID-19 na Segunda Quinzena de Setembro. [(accessed on 9 February 2022)]; Available online: https://www.gov.br/saude/pt-br/assuntos/noticias/ministerio-da-saude-anuncia-dose-de-reforco-para-vacinacao-contra-a-covid-19-na-segunda-quinzena-de-setembro .
- 102. Andrews N., Stowe J., Kirsebom F., Toffa S., Sachdeva R., Gower C., Ramsay M., Bernal J.L. Effectiveness of COVID-19 booster vaccines against COVID-19 related symptoms, hospitalisation and death in England. Nat. Med. 2022:1. doi: 10.1038/s41591-022-01699-1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 103. Bar-On Y.M., Goldberg Y., Mandel M., Bodenheimer O., Freedman L., Kalkstein N., Mizrahi B., Alroy-Preis S., Ash N., Milo R., et al. Protection of BNT162b2 Vaccine Booster against COVID-19 in Israel. N. Engl. J. Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 104. Arbel R., Hammerman A., Sergienko R., Friger M., Peretz A., Netzer D., Yaron S. BNT162b2 Vaccine Booster and Mortality Due to COVID-19. N. Engl. J. Med. 2021;385:2413–2420. doi: 10.1056/NEJMoa2115624. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 105. Barda N., Dagan N., Cohen C., Hernán M.A., Lipsitch M., Kohane I.S., Reis B.Y., Balicer R.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet. 2021;398:2093–2100. doi: 10.1016/S0140-6736(21)02249-2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 106. Gardner B.J., Kilpatrick A.M. Third doses of COVID-19 vaccines reduce infection and transmission of SARS-CoV-2 and could prevent future surges in some populations: A modeling study. medRxiv. 2021 doi: 10.1101/2021.10.25.21265500. [ DOI ] [ Google Scholar ]
- 107. Keeling M.J., Thomas A., Hill E.M., Thompson R.N., Dyson L., Tildesley M.J., Moore S. Waning, Boosting and a Path to Endemicity for SARS-CoV-2. medRxiv. 2021 doi: 10.1101/2021.11.05.21265977. [ DOI ] [ Google Scholar ]
- 108. Cromer D., Steain M., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Kent S.J., Triccas J.A., Khoury D.S., Davenport M.P. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: A meta-analysis. Lancet Microbe. 2022;3:e52–e61. doi: 10.1016/S2666-5247(21)00267-6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 109. Reardon S. How well can Omicron evade immunity from COVID vaccines? Nature. 2022 doi: 10.1038/d41586-022-00283-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 110. Li M., Lou F., Fan H. SARS-CoV-2 variant Omicron: Currently the most complete “escapee” from neutralization by antibodies and vaccines. Signal Transduct. Target. Ther. 2022;7:28. doi: 10.1038/s41392-022-00880-9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 111. Burki T.K. Fourth dose of COVID-19 vaccines in Israel. Lancet Respir. Med. 2022;10:E19. doi: 10.1016/S2213-2600(22)00010-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 112. Iacobucci G. COVID-19: Fourth vaccine doses—Who needs them and why? BMJ. 2022;376:o30. doi: 10.1136/bmj.o30. [ DOI ] [ PubMed ] [ Google Scholar ]
- 113. Ministry of Health of Canada COVID-19 Vaccine Third Dose Recommendations. [(accessed on 6 April 2022)]; Available online: https://www.health.gov.on.ca/en/pro/programs/publichealth/coronavirus/docs/vaccine/COVID-19_vaccine_third_dose_recommendations.pdf .
- 114. Preliminary Data Analysis: Effectiveness of the Fourth Dose for Older Adults 60 Years of Age and Older. [(accessed on 25 January 2022)]; Available online: https://www.gov.il/en/Departments/news/23012022-01 .
- 115. Watson C. Three, four or more: What’s the magic number for booster shots? Nature. 2022;602:17–18. doi: 10.1038/d41586-022-00200-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 116. Callaway E. Beyond Omicron: What’s next for COVID’s viral evolution. Nature. 2021;600:204–207. doi: 10.1038/d41586-021-03619-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 117. Pango Cov-Lineages. [(accessed on 5 April 2022)]. Available online: httpshttps://cov-lineages.org/lineage_list.htmlsublineage-ba.2 .
- 118. Cele S., Jackson L., Khoury D.S., Khan K., Moyo-Gwete T., Tegally H., San J.E., Cromer D., Scheepers C., Amoako D.G., et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2022;602:654–656. doi: 10.1038/s41586-021-04387-1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 119. Liu L., Iketani S., Guo Y., Chan J.F.W., Wang M., Liu L., Luo Y., Chu H., Huang Y., Nair M.S., et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature. 2022;602:676–681. doi: 10.1038/s41586-021-04388-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 120. WHO Statement on Omicron Sublineage BA.2. [(accessed on 5 April 2022)]. Available online: https://www.who.int/news/item/22-02-2022-statement-on-omicron-sublineage-ba.2 .
- 121. Centers of Disease and Control C. CDC COVID Data Tracker. [(accessed on 5 April 2022)]; Available online: https://covid.cdc.gov/covid-data-tracker/#variant-proportions .
- 122. Yu J., Collier A.-R.Y., Rowe M., Mardas F., Ventura J.D., Wan H., Miller J., Powers O., Chung B., Siamatu M., et al. Neutralization of the SARS-CoV-2 Omicron BA.1 and BA.2 Variants. N. Engl. J. Med. 2022 doi: 10.1056/NEJMc2201849. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 123. Stegger M., Edslev S.M., Sieber R.N., Ingham A.C., Ng K.L., Tang M.-H.E., Alexandersen S., Fonager J., Legarth R., Utko M., et al. Occurrence and significance of Omicron BA.1 infection followed by BA.2 reinfection. medRxiv. 2022 doi: 10.1101/2022.02.19.22271112. [ DOI ] [ Google Scholar ]
- 124. Chemaitelly H., Ayoub H.H., AlMukdad S., Coyle P., Tang P., Yassine H.M., Al-Khatib H.A., Smatti M.K., Hasan M.R., Al-Kanaani Z., et al. Duration of mRNA vaccine protection against SARS-CoV-2 Omicron BA.1 and BA.2 subvariants in Qatar. medRxiv. 2022 doi: 10.1101/2022.03.13.22272308. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 125. Pfizer Pfizer and BioNTech Initiate Study to Evaluate Omicron-Based COVID-19 Vaccine in Adults 18 to 55 Years of Age. [(accessed on 8 February 2022)]. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-initiate-study-evaluate-omicron-based .
- 126. Waltz E. Does the world need an Omicron vaccine? What researchers say. Nature. 2022;602:192–193. doi: 10.1038/d41586-022-00199-z. [ DOI ] [ PubMed ] [ Google Scholar ]
- 127. Morens D.M., Taubenberger J.K., Fauci A.S. Universal Coronavirus Vaccines—An Urgent Need. N. Engl. J. Med. 2022;386:297–299. doi: 10.1056/NEJMp2118468. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 128. Sheridan C. COVID-19 vaccine makers chase variant-ready vaccines. Nat. Biotechnol. 2022;40:141–143. doi: 10.1038/d41587-022-00001-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 129. Kim Y.C., Dema B., Reyes-Sandoval A. COVID-19 vaccines: Breaking record times to first-in-human trials. npj Vaccines. 2020;5:34. doi: 10.1038/s41541-020-0188-3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 130. Dodd R.H., Pickles K., Nickel B., Cvejic E., Ayre J., Batcup C., Bonner C., Copp T., Cornell S., Dakin T., et al. Concerns and motivations about COVID-19 vaccination. Lancet Infect. Dis. 2021;21:161–163. doi: 10.1016/S1473-3099(20)30926-9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 131. Blumenthal K.G., Phadke N.A., Bates D.W. Safety Surveillance of COVID-19 mRNA Vaccines Through the Vaccine Safety Datalink. JAMA. 2021;326:1375. doi: 10.1001/jama.2021.14808. [ DOI ] [ PubMed ] [ Google Scholar ]
- 132. Albalawi O.M., Alomran M.I., Alsagri G.M., Althunian T.A., Alshammari T.M. Analyzing the U.S. Post-marketing safety surveillance of COVID-19 vaccines. Saudi Pharm. J. 2022;30:180–184. doi: 10.1016/j.jsps.2021.12.008. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 133. Kim J.H., Marks F., Clemens J.D. Looking beyond COVID-19 vaccine phase 3 trials. Nat. Med. 2021;27:205–211. doi: 10.1038/s41591-021-01230-y. [ DOI ] [ PubMed ] [ Google Scholar ]
- 134. Coronavirus Disease (COVID-19): Vaccines Safety. [(accessed on 11 February 2022)]. Available online: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-vaccines-safety .
- 135. Pfizer-BioNTech COVID-19 Vaccine Reactions & Adverse Events. [(accessed on 11 February 2022)]; Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html .
- 136. European Medicines Agency Committee for Medicinal Products for Human Use (CHMP) Assessment Report. European Medicines Agency; Amsterdam, The Netherlands: 2021. [ Google Scholar ]
- 137. Soiza R.L., Scicluna C., Thomson E.C. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing. 2021;50:279–283. doi: 10.1093/ageing/afaa274. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 138. Sacco K., Castagnoli R., Vakkilainen S., Liu C., Delmonte O.M., Oguz C., Kaplan I.M., Alehashemi S., Burbelo P.D., Bhuyan F., et al. Immunopathological signatures in multisystem inflammatory syndrome in children and pediatric COVID-19. Nat. Med. 2022:1–13. doi: 10.1038/s41591-022-01724-3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 139. Vogel T.P., Top K.A., Karatzios C., Hilmers D.C., Tapia L.I., Moceri P., Giovannini-Chami L., Wood N., Chandler R.E., Klein N.P., et al. Multisystem inflammatory syndrome in children and adults (MIS-C/A): Case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2021;39:3037–3049. doi: 10.1016/j.vaccine.2021.01.054. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 140. Bartsch Y.C., Wang C., Zohar T., Fischinger S., Atyeo C., Burke J.S., Kang J., Edlow A.G., Fasano A., Baden L.R., et al. Humoral signatures of protective and pathological SARS-CoV-2 infection in children. Nat. Med. 2021;27:454–462. doi: 10.1038/s41591-021-01263-3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 141. Weatherhead J.E., Clark E., Vogel T.P., Atmar R.L., Kulkarni P.A. Inflammatory syndromes associated with SARS-CoV-2 infection: Dysregulation of the immune response across the age spectrum. J. Clin. Investig. 2020;130:6194–6197. doi: 10.1172/JCI145301. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 142. Bastug A., Aslaner H., Aybar Bilir Y., Kemirtlek N., Gursoy F.M., Bastug S., Bodur H. Multiple system inflammatory syndrome associated with SARS-CoV-2 infection in an adult and an adolescent. Rheumatol. Int. 2021;41:993. doi: 10.1007/s00296-021-04843-1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 143. Nune A., Iyengar K.P., Goddard C., Ahmed A.E. Multisystem inflammatory syndrome in an adult following the SARS-CoV-2 vaccine (MIS-V) BMJ Case Rep. 2021;14:e243888. doi: 10.1136/bcr-2021-243888. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 144. Uwaydah A.K., Hassan N.M.M., Abu Ghoush M.S., Shahin K.M.M. Adult multisystem inflammatory syndrome in a patient who recovered from COVID-19 postvaccination. BMJ Case Rep. 2021;14:e242060. doi: 10.1136/bcr-2021-242060. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 145. Park J.W., Yu S.N., Chang S.H., Ahn Y.H., Jeon M.H. Multisystem Inflammatory Syndrome in an Adult after COVID-19 Vaccination: A Case Report and Literature Review. J. Korean Med. Sci. 2021;36:e312. doi: 10.3346/jkms.2021.36.e312. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 146. Belay E.D., Godfred Cato S., Rao A.K., Abrams J., Wyatt Wilson W., Lim S., Newton-Cheh C., Melgar M., DeCuir J., Webb B., et al. Multisystem Inflammatory Syndrome in Adults After Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection and Coronavirus Disease 2019 (COVID-19) Vaccination. Clin. Infect. Dis. 2021:ciab936. doi: 10.1093/cid/ciab936. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 147. Arrow K., Burgoyne L.L., Cyna A.M. Implications of nocebo in anaesthesia care. Anaesthesia. 2022;77:11–20. doi: 10.1111/anae.15601. [ DOI ] [ PubMed ] [ Google Scholar ]
- 148. Graham F. Daily briefing: ‘Nocebo’ effect underlies most COVID vaccine side effects. Nature. 2022 doi: 10.1038/d41586-022-00146-y. [ DOI ] [ PubMed ] [ Google Scholar ]
- 149. Haas J.W., Bender F.L., Ballou S., Kelley J.M., Wilhelm M., Miller F.G., Rief W., Kaptchuk T.J. Frequency of Adverse Events in the Placebo Arms of COVID-19 Vaccine Trials. JAMA Netw. Open. 2022;5:e2143955. doi: 10.1001/jamanetworkopen.2021.43955. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 150. Sever P.P. Nocebo affects after COVID-19 vaccination. Lancet Reg. Health-Eur. 2022;12:100273. doi: 10.1016/j.lanepe.2021.100273. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 151. Razai M.S., Chaudhry U.A.R., Doerholt K., Bauld L., Majeed A. COVID-19 vaccination hesitancy. BMJ. 2021;373:n1138. doi: 10.1136/bmj.n1138. [ DOI ] [ PubMed ] [ Google Scholar ]
- 152. Baker A.T., Boyd R.J., Sarkar D., Teijeira-Crespo A., Chan C.K., Bates E., Waraich K., Vant J., Wilson E., Truong C.D., et al. ChAdOx1 interacts with CAR and PF4 with implications for thrombosis with thrombocytopenia syndrome. Sci. Adv. 2021;7:8213. doi: 10.1126/sciadv.abl8213. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 153. Fu W., Sivajohan B., McClymont E., Albert A., Elwood C., Ogilvie G., Money D. Systematic review of the safety, immunogenicity, and effectiveness of COVID-19 vaccines in pregnant and lactating individuals and their infants. Int. J. Gynecol. Obstet. 2022;156:406–417. doi: 10.1002/ijgo.14008. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 154. Goncu Ayhan S., Oluklu D., Atalay A., Menekse Beser D., Tanacan A., Moraloglu Tekin O., Sahin D. COVID-19 vaccine acceptance in pregnant women. Int. J. Gynecol. Obstet. 2021;154:291–296. doi: 10.1002/ijgo.13713. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 155. Meyyazhagan A., Pushparaj K., Balasubramanian B., Kuchi Bhotla H., Pappusamy M., Arumugam V.A., Easwaran M., Pottail L., Mani P., Tsibizova V., et al. COVID-19 in pregnant women and children: Insights on clinical manifestations, complexities, and pathogenesis. Int. J. Gynecol. Obstet. 2022;156:216–224. doi: 10.1002/ijgo.14007. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 156. Sarwal Y., Sarwal T., Sarwal R. Prioritizing pregnant women for COVID-19 vaccination. Int. J. Gynecol. Obstet. 2021;155:57–63. doi: 10.1002/ijgo.13816. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 157. Sarwal Y., Sarwal T., Sarwal R. Vaccination of pregnant women against COVID-19 in India and Indonesia: Moving beyond the opt-in to the opt-out option. Int. J. Gynecol. Obstet. 2021;155:549–550. doi: 10.1002/ijgo.13930. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 158. Witberg G., Barda N., Hoss S., Richter I., Wiessman M., Aviv Y., Grinberg T., Auster O., Dagan N., Balicer R.D., et al. Myocarditis after COVID-19 Vaccination in a Large Health Care Organization. N. Engl. J. Med. 2021;385:2132–2139. doi: 10.1056/NEJMoa2110737. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 159. Heymans S., Cooper L.T. Myocarditis after COVID-19 mRNA vaccination: Clinical observations and potential mechanisms. Nat. Rev. Cardiol. 2021;19:75–77. doi: 10.1038/s41569-021-00662-w. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 160. Maiese A., Frati P., Del Duca F., Santoro P., Manetti A.C., La Russa R., Di Paolo M., Turillazzi E., Fineschi V. Myocardial Pathology in COVID-19-Associated Cardiac Injury: A Systematic Review. Diagnostics. 2021;11:1647. doi: 10.3390/diagnostics11091647. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 161. Block J.P., Boehmer T.K., Forrest C.B., Carton T.W., Lee G.M., Ajani U.A., Christakis D.A., Cowell L.G., Draper C., Ghildayal N., et al. mm7114e1 Cardiac Complications After SARS-CoV-2 Infection and mRNA COVID-19 Vaccination—PCORnet, United States, January 2021–January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022;71:517–523. doi: 10.15585/mmwr.mm7114e1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 162. Por Unanimidade, CoronaVac é Aprovada Pela Anvisa para uso Emergencial em Crianças de Seis a 17 Anos. [(accessed on 25 January 2022)]; Available online: https://butantan.gov.br/noticias/por-unanimidade-coronavac-e-aprovada-pela-anvisa-para-uso-emergencial-em-criancas-de-seis-a-17-anos-
- 163. Heymans S., Eriksson U., Lehtonen J., Cooper L.T. The Quest for New Approaches in Myocarditis and Inflammatory Cardiomyopathy. J. Am. Coll. Cardiol. 2016;68:2348–2364. doi: 10.1016/j.jacc.2016.09.937. [ DOI ] [ PubMed ] [ Google Scholar ]
- 164. COVID-19 Confirmed Cases and Deaths. [(accessed on 22 February 2022)]. Available online: https://data.unicef.org/resources/covid-19-confirmed-cases-and-deaths-dashboard/
- 165. American Academy of Pediatrics Children and COVID-19: State-Level Data Report. [(accessed on 12 February 2022)]. Available online: https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/
- 166. Barberia L.G., Bastos L.S., Sousa T.C.M. School reopening and COVID-19 in Brazil. Lancet Reg. Health–Am. 2022;5:100149. doi: 10.1016/j.lana.2021.100149. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 167. Ledford H. Should children get COVID vaccines? What the science says. Nature. 2021;595:638–639. doi: 10.1038/d41586-021-01898-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 168. For Parents: Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with COVID-19. [(accessed on 22 February 2022)]; Available online: https://www.cdc.gov/mis/mis-c.html .
- 169. Jiang L., Tang K., Levin M., Irfan O., Morris S.K., Wilson K., Klein J.D., Bhutta Z.A. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect. Dis. 2020;20:e276–e288. doi: 10.1016/S1473-3099(20)30651-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 170. Marks K.J., Whitaker M., Anglin O., Milucky J., Patel K., Pham H., Chai S.J., Kirley P.D., Armistead I., McLafferty S., et al. Hospitalizations of Children and Adolescents with Laboratory-Confirmed COVID-19—COVID-NET, 14 States, July 2021–January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022;71:271–278. doi: 10.15585/mmwr.mm7107e4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 171. World Health Organization . Interim Statement on COVID-19 Vaccination for Children and Adolescents. World Health Organization; Geneva, Switzerland: 2021. [ Google Scholar ]
- 172. Zou X., Cao B. COVID-19 vaccines for children younger than 12 years: Are we ready? Lancet Infect. Dis. 2021;21:1614–1615. doi: 10.1016/S1473-3099(21)00384-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 173. Hale T., Angrist N., Goldszmidt R., Kira B., Petherick A., Phillips T., Webster S., Cameron-Blake E., Hallas L., Majumdar S., et al. A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker) Nat. Hum. Behav. 2021;5:529–538. doi: 10.1038/s41562-021-01079-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 174. Conheça os Países que já Autorizaram a CoronaVac para Crianças a Partir dos 3 Anos—Instituto Butantan. [(accessed on 25 January 2022)]; Available online: https://butantan.gov.br/noticias/conheca-os-paises-que-ja-autorizaram-a-coronavac-para-criancas-a-partir-dos-3-anos .
- 175. Moore D.C.B.C., Nehab M.F., Camacho K.G., Reis A.T., de Junqueira-Marinho M.F., Abramov D.M., Azevedo Z.M.A., de Menezes L.A., dos Santos Salú M., da Silva Figueiredo C.E., et al. Low COVID-19 vaccine hesitancy in Brazil. Vaccine. 2021;39:6262–6268. doi: 10.1016/j.vaccine.2021.09.013. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 176. Ministério da Saúde B. Ministério da Saúde Inclui Crianças de 5 a 11 Anos na Campanha de Vacinação Contra a COVID-19—Português (Brasil) [(accessed on 22 February 2022)]; Available online: https://www.unasus.gov.br/noticia/ministerio-da-saude-inclui-criancas-de-5-a-11-anos-na-campanha-de-vacinacao-contra-a-covid-19 .
- 177. American Academy of Pediatrics Children and COVID-19 Vaccination Trends. [(accessed on 22 February 2022)]. Available online: https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-vaccination-trends/#:~:text=This%20past%20week%20about%2069%2C000,66%25%20receiving%20their%20first%20dose.&text=About%208.1%20million%20children%2012,received%20their%20first%20vaccine%20dose .
- 178. Liu F., Fu H.-D., Mao J.-H. Coronavirus disease 2019 vaccine for children in China: When to start? Mandatory or voluntary? Chin. Med. J. 2021;134:3015–3016. doi: 10.1097/CM9.0000000000001779. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 179. Centers for Disease Control and Prevention Pfizer-BioNTech COVID-19 Vaccine Gray Cap|CDC. [(accessed on 22 February 2022)]; Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/bioNTech-gray-cap-12-and-over.html .
- 180. An Update on Vaccine Roll-Out for 5-11 Year-Olds in the U.S. [(accessed on 22 February 2022)]. Available online: https://www.kff.org/coronavirus-covid-19/issue-brief/an-update-on-vaccine-roll-out-for-5-11-year-olds-in-the-u-s/
- 181. Anvisa Aprova Vacina da Pfizer Contra COVID para Crianças de 5 a 11 Anos. [(accessed on 22 February 2022)]; Available online: https://www.gov.br/anvisa/pt-br/assuntos/noticias-anvisa/2021/anvisa-aprova-vacina-da-pfizer-contra-covid-para-criancas-de-5-a-11-anos .
- 182. Brasil Inicia Vacinação Contra COVID de Crianças Entre 5 e 11 Anos. [(accessed on 22 February 2022)]. Available online: https://www.em.com.br/app/noticia/internacional/2022/01/14/interna_internacional,1337816/brasil-inicia-vacinacao-contra-covid-de-criancas-entre-5-e-11-anos.shtml .
- 183. Coronavirus: First Canadian Kids Receive COVID-19 Vaccines. [(accessed on 22 February 2022)]. Available online: https://www.ctvnews.ca/health/coronavirus/first-canadian-kids-under-12-get-vaccinated-against-covid-19-1.5678502 .
- 184. China Calls on ‘Little Inoculated Warriors’ in Its War on COVID-19. [(accessed on 22 February 2022)]. Available online: https://www.nytimes.com/2021/12/06/business/china-covid-vaccine-children.html .
- 185. China to Offer COVID-19 Vaccine to Children as Young as Three. [(accessed on 22 February 2022)]. Available online: https://www.france24.com/en/live-news/20210608-china-to-offer-covid-19-vaccine-to-children-as-young-as-three .
- 186. Israel OKs COVID-19 Vaccine for Kids Aged 5–11. [(accessed on 22 February 2022)]. Available online: https://globalnews.ca/news/8373256/israel-oks-covid-vaccines-kids/
- 187. Australia Vaccinating Children against COVID-19 from Early Next Year. [(accessed on 22 February 2022)]; Available online: https://www.health.gov.au/news/australia-vaccinating-children-against-covid-19-from-early-next-year .
- 188. AIFA Approva il Vaccino Comirnaty per la Fascia di età 5–11 Anni. [(accessed on 22 February 2022)]; Available online: https://www.aifa.gov.it/documents/20142/1289678/Comunicato_AIFA_674.pdf .
- 189. Japan Approves Pfizer’s COVID-19 Vaccine for Children Aged 5–11. [(accessed on 22 February 2022)]. Available online: https://mainichi.jp/english/articles/20220121/p2g/00m/0na/028000c .
- 190. Wise J. COVID-19: Vaccine will be offered to 5–11 year olds throughout UK. BMJ. 2022;376:o411. doi: 10.1136/bmj.o411. [ DOI ] [ PubMed ] [ Google Scholar ]
- 191. Chile Authorizes Sinovac Vaccine for Kids of 6 and Older. [(accessed on 22 February 2022)]. Available online: https://abcnews.go.com/International/wireStory/chile-authorizes-sinovac-vaccine-kids-older-79863155 .
- 192. Hause A.M., Baggs J., Marquez P., Myers T.R., Gee J., Su J.R., Zhang B., Thompson D., Shimabukuro T.T., Shay D.K. COVID-19 Vaccine Safety in Children Aged 5–11 Years—United States, November 3–December 19, 2021. Morb. Mortal. Wkly. Rep. 2022;70:1755–1760. doi: 10.15585/mmwr.mm705152a1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 193. Walter E.B., Talaat K.R., Sabharwal C., Gurtman A., Lockhart S., Paulsen G.C., Barnett E.D., Muñoz F.M., Maldonado Y., Pahud B.A., et al. Evaluation of the BNT162b2 COVID-19 Vaccine in Children 5 to 11 Years of Age. N. Engl. J. Med. 2022;386:35–46. doi: 10.1056/NEJMoa2116298. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 194. Frenck R.W., Klein N.P., Kitchin N., Gurtman A., Absalon J., Lockhart S., Perez J.L., Walter E.B., Senders S., Bailey R., et al. Safety, Immunogenicity, and Efficacy of the BNT162b2 COVID-19 Vaccine in Adolescents. N. Engl. J. Med. 2021;385:239–250. doi: 10.1056/NEJMoa2107456. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 195. Revon-Riviere G., Ninove L., Min V., Rome A., Coze C., Verschuur A., de Lamballerie X., André N. The BNT162b2 mRNA COVID-19 vaccine in adolescents and young adults with cancer: A monocentric experience. Eur. J. Cancer. 2021;154:30–34. doi: 10.1016/j.ejca.2021.06.002. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 196. Aprovada Ampliação de uso da CoronaVac para Crianças e Adolescentes de 6 a 17 Anos. [(accessed on 25 January 2022)]; Available online: https://www.gov.br/anvisa/pt-br/assuntos/noticias-anvisa/2022/aprovada-ampliacao-de-uso-da-vacina-coronavac-para-criancas-de-6-a-17-anos .
- 197. Weisberg S.P., Connors T.J., Zhu Y., Baldwin M.R., Lin W.-H., Wontakal S., Szabo P.A., Wells S.B., Dogra P., Gray J., et al. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat. Immunol. 2021;22:25–31. doi: 10.1038/s41590-020-00826-9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 198. Yousaf A.R., Cortese M.M., Taylor A.W., Broder K.R., Oster M.E., Wong J.M., Guh A.Y., McCormick D.W., Kamidani S., Schlaudecker E.P., et al. Reported cases of multisystem inflammatory syndrome in children aged 12–20 years in the USA who received a COVID-19 vaccine, December, 2020, through August, 2021: A surveillance investigation. Lancet Child Adolesc. Health. 2022. in press . [ DOI ] [ PMC free article ] [ PubMed ]
- 199. Zambrano L.D., Newhams M.M., Olson S.M., Halasa N.B., Price A.M., Boom J.A., Sahni L.C., Kamidani S., Tarquinio K.M., Maddux A.B., et al. Effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA Vaccination Against Multisystem Inflammatory Syndrome in Children Among Persons Aged 12–18 Years—United States, July–December 2021. MMWR Morb. Mortal. Wkly. Rep. 2022;71:52–58. doi: 10.15585/mmwr.mm7102e1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 200. Kozlov M. What COVID vaccines for young kids could mean for the pandemic. Nature. 2021;599:18–19. doi: 10.1038/d41586-021-02947-z. [ DOI ] [ PubMed ] [ Google Scholar ]
- 201. Santos G. Avaliação da solicitação de ampliação do uso emergencial da vacina Coronavac para a população pediátrica acima de 3 anos de idade. [(accessed on 6 April 2022)];Anvisa. 2022 :1–30. Available online: https://www.gov.br/anvisa/pt-br/assuntos/noticias-anvisa/2022/aprovada-ampliacao-de-uso-da-vacina-coronavac-para-criancas-de-6-a-17-anos/ampliacao-de-uso-pediatrico_coronavac_20012022_final-1-1.pdf . [ Google Scholar ]
- 202. Han B., Song Y., Li C., Yang W., Ma Q., Jiang Z., Li M., Lian X., Jiao W., Wang L., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy children and adolescents: A double-blind, randomised, controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021;21:1645–1653. doi: 10.1016/S1473-3099(21)00319-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 203. Zimmermann P., Pittet L.F., Finn A., Pollard A.J., Curtis N. Should children be vaccinated against COVID-19? Arch. Dis. Child. 2022;107:e1. doi: 10.1136/archdischild-2021-323040. [ DOI ] [ PubMed ] [ Google Scholar ]
- 204. Temsah M.H., Alhuzaimi A.N., Aljamaan F., Bahkali F., Al-Eyadhy A., Alrabiaah A., Alhaboob A., Bashiri F.A., Alshaer A., Temsah O., et al. Parental Attitudes and Hesitancy About COVID-19 vs. Routine Childhood Vaccinations: A National Survey. Front. Public Health. 2021;9:1513. doi: 10.3389/fpubh.2021.752323. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 205. Katzourakis A. COVID-19: Endemic doesn’t mean harmless. Nature. 2022;601:485. doi: 10.1038/d41586-022-00155-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 206. Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 207. Daily Confirmed COVID-19 Cases and Deaths, World. [(accessed on 12 February 2022)]. Available online: https://ourworldindata.org/grapher/daily-covid-cases-deaths?time=2021-02-01..latest&country=~OWID_WRL .
- 208. Emanuel E.J., Osterholm M., Gounder C.R. A National Strategy for the “New Normal” of Life With COVID. JAMA. 2022;327:211. doi: 10.1001/jama.2021.24282. [ DOI ] [ PubMed ] [ Google Scholar ]
- 209. Telenti A., Arvin A., Corey L., Corti D., Diamond M.S., García-Sastre A., Garry R.F., Holmes E.C., Pang P.S., Virgin H.W. After the pandemic: Perspectives on the future trajectory of COVID-19. Nature. 2021;596:495–504. doi: 10.1038/s41586-021-03792-w. [ DOI ] [ PubMed ] [ Google Scholar ]
- 210. Update on Omicron. [(accessed on 12 February 2022)]. Available online: https://www.who.int/news/item/28-11-2021-update-on-omicron .
- 211. Karim S.S.A., Karim Q.A. Omicron SARS-CoV-2 variant: A new chapter in the COVID-19 pandemic. Lancet. 2021;398:2126–2128. doi: 10.1016/S0140-6736(21)02758-6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 212. World Bank GNI per Capita, Atlas Method (Current US$)|Data. [(accessed on 22 February 2022)]. Available online: https://data.worldbank.org/indicator/NY.GNP.PCAP.CD .
- 213. Coronavirus (COVID-19) Vaccinations—Statistics and Research—Our World in Data. [(accessed on 22 February 2022)]. Available online: https://ourworldindata.org/covid-vaccinations .
- 214. Duffy S. Why are RNA virus mutation rates so damn high? PLoS Biol. 2018;16:e3000003. doi: 10.1371/journal.pbio.3000003. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 215. Callaway E. The coronavirus is mutating—Does it matter? Nature. 2020;585:174–177. doi: 10.1038/d41586-020-02544-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 216. World Health Organization . WHO Launches New Global Influenza Strategy. World Health Organization; Geneva, Switzerland: 2019. [ Google Scholar ]
- 217. Mathieu E., Ritchie H., Ortiz-Ospina E., Roser M., Hasell J., Appel C., Giattino C., Rodés-Guirao L. A global database of COVID-19 vaccinations. Nat. Hum. Behav. 2021;5:947–953. doi: 10.1038/s41562-021-01122-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 218. Abdelrahman Z., Li M., Wang X. Comparative Review of SARS-CoV-2, SARS-CoV, MERS-CoV, and Influenza A Respiratory Viruses. Front. Immunol. 2020;11:2309. doi: 10.3389/fimmu.2020.552909. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 219. Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 220. Ramachandran R., Ross J.S., Miller J.E. Access to COVID-19 Vaccines in High-, Middle-, and Low-Income Countries Hosting Clinical Trials. JAMA Netw. Open. 2021;4:e2134233. doi: 10.1001/jamanetworkopen.2021.34233. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 221. COVID is here to stay: Countries must decide how to adapt. Nature. 2022;601:165. doi: 10.1038/d41586-022-00057-y. [ DOI ] [ PubMed ] [ Google Scholar ]
- 222. Technologies to advance COVID-19 vaccine equity. Nat. Biotechnol. 2021;39:1477. doi: 10.1038/s41587-021-01167-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
- View on publisher site
- PDF (1.3 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
- Biochemistry and Molecular Biology
- Biostatistics
- Environmental Health and Engineering
- Epidemiology
- Health Policy and Management
- Health, Behavior and Society
- International Health
- Mental Health
- Molecular Microbiology and Immunology
- Population, Family and Reproductive Health
- Program Finder
- Admissions Services
- Course Directory
- Academic Calendar
- Hybrid Campus
- Lecture Series
- Convocation
- Strategy and Development
- Implementation and Impact
- Integrity and Oversight
- In the School
- In the Field
- In Baltimore
- Resources for Practitioners
- Articles & News Releases
- In The News
- Statements & Announcements
- At a Glance
- Student Life
- Strategic Priorities
- Inclusion, Diversity, Anti-Racism, and Equity (IDARE)
- What is Public Health?
A Top Vaccine Expert Answers Important Questions About a COVID-19 Vaccine
The covid-19 vaccine is on track to become the fastest-developed vaccine in history. that doesn’t mean the process is skipping any critical steps..
Understanding what we know—and still don’t—about a vaccine for COVID-19 can help shed light on its safety and efficacy.
Ruth Karron, MD , is one of the top vaccine experts in the world, serving on vaccine committees for the CDC, the WHO, and the FDA. Karron, who leads the Center for Immunization Research at the Johns Hopkins Bloomberg School of Public Health, recently spoke with Josh Sharfstein and answered a list of important questions about the COVID-19 vaccine.
How close are we to a vaccine?
There are some very encouraging developments. We have a few vaccines now that will go into Phase 3 clinical trials, also known as efficacy trials. That means that those vaccines have passed certain goalposts in terms of initial evaluations of safety and immune response such that they can be evaluated in larger trials.
We know that these vaccines are promising, but we don’t yet know if they are going to work. That’s what the purpose of an efficacy trial is—as well as to provide a broader assessment of safety of the vaccine in a large number of people.
Tell me more about these efficacy trials. What do they actually entail?
They involve large numbers of people: In these particular trials for COVID vaccines, there are going to be about 30,000 people enrolled per trial. Individuals are given a vaccine, and then they are followed both to make sure that the side effects from the vaccine are acceptable and to see whether they develop a SARS-CoV-2 infection along with some symptoms.
These are placebo-controlled trials, meaning that some individuals will get a COVID vaccine and some will get a placebo. Then, the rates of disease will be compared in the people who got placebo and the people who got the vaccine to determine the efficacy of the vaccine.
How successful does a vaccine have to be in one of these studies for it to be considered effective?
Recently, the FDA issued guidance about the development of COVID vaccines. The guidance that they issued to vaccine manufacturers— this is a document that is available to the general public —is that a vaccine would need to be at least 50% effective. This means that an individual who was vaccinated would be 50% less likely to get COVID disease—or whatever the particular endpoint is that’s measured in the trial—than individuals that weren’t vaccinated.
This is a reasonable goal for a number of reasons. Typically, the more severe a disease is, the better chance a vaccine has of preventing that disease. So, a vaccine that’s 50% effective against mild COVID disease—which might be the endpoint that’s measured in a clinical trial, or any evidence of COVID infection with any symptom, which is how a lot of trials are designed—might be more effective against severe disease.
When you have a disease that’s as prevalent as COVID—and if we think about what the U.S. has experienced in the past several months in terms of severe disease and death—even if we were only able to cut those numbers in half, that would be a major achievement.
How long would a vaccine be effective for? If you get 50% effectiveness or more, that’s good news. But if it’s only effective for a few months, that’s not such good news.
Time will tell for that. The short answer is that we don’t yet know. Even for the data we have on the vaccine so far in smaller studies, we haven’t yet had the opportunity to follow individuals for very long. The very first people who got the very first vaccine were immunized in March and it’s only July. So, we don’t know very much about the durability of the immune response in people.
Our hope would be [that protection would last] at least a year or more and then people might need boosters.
It’s also possible that a vaccine might not entirely protect against mild disease. So you might actually experience mild disease and then have a boost in your immune response and not suffer severe disease. From a public health perspective, that would be completely acceptable. If we turned a severe disease not into “ no disease ” but into mild disease, that would be a real victory.
Let’s talk about safety. What are they looking for in a 30,000-person study to figure out whether a vaccine is considered safe enough to use?
Every person who is enrolled in the trial will complete information about the kinds of acute symptoms that you might expect following an infection. People will need to provide information about swelling, redness, tenderness around the injection site, fever, and any other symptoms they might experience in the three to seven days following vaccination.
More long term, people will be looking to make sure that when COVID disease is experienced, there’s not any evidence of more severe disease with vaccination [which is known as disease enhancement].
There was a lot of discussion as these vaccines were being developed of a concern about disease enhancement. This is based on some animal models—not with SARS-CoV-2 but with other coronaviruses. We haven’t seen any evidence of enhanced disease thus far and there are a number of scientific reasons why we don’t think it should occur with these vaccines. But, of course, it’s something we would still watch for very carefully just as with any other safety signal.
How should we think about the possibility of adverse effects that might come up after the period of the vaccine trial?
There are a couple of things to mention about that, and one is that individuals with these trials will be followed for a year or longer. It may be that a vaccine is either approved for emergency use or licensed before all of that long-term follow up is completed. Nevertheless, companies will be obligated to complete that follow up and report those results back to the FDA.
It’s important to enroll older adults in these studies. All of these large efficacy trials will be stratified so there will be some younger adults and some older adults enrolled.
In addition, it’s very likely—and this would not just happen with COVID vaccines, but whenever the FDA licenses vaccines—that there is an obligation for post-licensure assessments. If a COVID vaccine is licensed, the companies will work with the FDA to determine exactly what kind of post-licensure safety assessments will need to be done.
COVID affects certain populations more than others—particularly older adults and people with chronic illnesses. What do these studies need [in order] to address the question of whether a vaccine will be protective for them?
I also think it will be important to enroll older adults across an age span. A 65-year-old is not the same as an 85-year-old. Also, a healthy older adult is not the same as a frail older adult who might be living in a care facility.
We’ll need some information about diverse elderly populations in order to think about how to allocate vaccines. There may also be other alternatives for older adults if they don’t respond well to vaccines. There’s a lot of work going on on development of monoclonal antibodies [ learn more about lab-produced antibodies in a recent podcast episode with Arturo Casadevall ] as an alternative for groups that don’t respond well to vaccines such as elderly, frail adults.
Let’s say there are 30,000 patients in the study and only a few hundred who are over 80 years old. What can you learn about a relatively small population of much older adults that would be informative about that group?
We may not have a large enough number of people in that subgroup to directly look at efficacy of a vaccine. But we might have enough to look at the immune response—the antibody response, for example, of a vaccine.
If, in the course of these trials, we can determine a correlative protection—for example, a laboratory measure like a level of a particular kind of antibody that correlates with protection against COVID disease—we can at least look at the immune responses in that subset of very elderly and decide if they are the same or different than the younger groups’. If they are the same, we may be more comfortable making the leap to say that it’s likely those individuals will also be protected by the vaccine.
So, we will learn more from a vaccine trial than just whether or not a vaccine works. We’re going to find out, perhaps, what predicts whether the vaccine works. That information might help us understand—without having to do a whole new trial—who might be protected by a vaccine.
It’s certainly a hope.
The majority of vaccines that we use today don’t have such a marker of protection and they’re very effective. Just because we can’t detect a marker doesn’t mean that a vaccine is not effective. It means that we’re not smart enough to figure out what that marker should be.
We really hope that there will be such a marker of protection because then we can link that—and, in FDA speak, that’s called “bridging”—to another population where we can just look at that marker of immunity rather than doing a whole efficacy trial.
How should we think about the need for racial and ethnic diversity in these clinical trials?
It’s critically important that we have racial and ethnic diversity.
We know that COVID causes increased rates of severe disease in Latinx and Black populations and in Native American populations. We will certainly want to be able to offer these COVID vaccines to these high-risk populations and encourage their use. But we need to know how well these vaccines work in these populations—if different vaccines work differently—so that we can offer the most effective vaccines.
It would not be an understatement to say that there can be a measure of distrust from some communities that have experienced discrimination from the health care system. How does that play into vaccine research?
It’s really important to engage those communities in a number of ways. One way is to engage local leaders early in the process. Lay leaders and leaders of faith communities can have focus groups to find out what their concerns are and how those can be allayed.
I think a very important issue that has been raised by some people who might potentially volunteer for some of these trials has to do with eventual access. People want to have some sense that if they participate in a trial, not only might they have access to the vaccine at the end of that trial, but their families and their communities would, too. Ensuring access among these high risk and vulnerable communities is really critical.
A clear policy decision to make sure that a vaccine is widely available without charge might actually help with the studies to prove whether or not that vaccine is safe and effective?
That’s absolutely the case. It’s great that you brought up the “without charge” piece, too, because a vaccine that’s made available but costs something to the individual may not be used. Particularly for people who don’t have health insurance or people who are undocumented. It has to be broadly and freely available.
Let’s talk about other specific populations. One of those is pregnant women. We know that they can certainly get COVID-19 and that there are some signs that they can have a more severe course. How do you think about the issue of pregnant women in vaccine studies?
I’ve done some work in this area —particularly with Ruth Faden and Carleigh Krubiner in the Berman Institute of Bioethics —specifically related to ensuring that pregnant women are considered and included in vaccine development and implementation for vaccines against epidemic and pandemic diseases.
When thinking about trials, there needs to be a justification for excluding pregnant women from trials rather than a justification for including them. The justification often is—and certainly is the case with these early COVID vaccines—that we don’t know enough yet about the vaccine or the vaccine platform or the safety of the vaccine to do a study in pregnant people.
With the mRNA vaccine, for example, [the type of vaccine being considered for COVID-19] we don’t currently have a licensed mRNA vaccine. It’s a new platform and we’re just learning about the safety of that platform so it wouldn’t have been appropriate to include pregnant women in the early stage trials.
But these 30,000-person studies are going to be really big studies. They will certainly enroll people of child-bearing potential. And even though there’s what we call an exclusion criterion—women are not supposed to be pregnant at the time they are enrolled, and usually women of child-bearing potential will take a pregnancy test prior to enrollment and immunization—we know from previous experience that it’s quite likely that some women will become pregnant in the months immediately following immunization. It happens quite frequently. So, it’s important for companies and the government to anticipate that this will be the case and to think about how they will systematically collect data from women who do become pregnant during these trials.
It’s not that the data needs to be interpreted cautiously—because pregnant women aren’t being formally randomized and we don’t have that kind of trial design—but there are things that could be learned and it’s important to think now about how to collect those data. It’s also important to think about how pregnant women could be directly included in both trials and deployment later down the road.
What about young children who are less likely to get severe disease? Would your approach to clinical trials be different?
Yes. I think we need to learn a bit more about the epidemiology in children. Fortunately, children don’t seem to suffer from acute COVID disease at the rates that adults do. But we need to learn more about that and we also need to learn from our trials in adults before we make decisions about how and whether children will be included in vaccine trials.
Once we have a vaccine that has made it through these various stages and we’re ready to start immunizing people outside of a pure clinical trial, how close are we to really getting the benefit of the vaccine? How does all the work it takes to develop a vaccine compare to what comes next?
The best vaccine in the world won’t work if it isn’t used.
Use has two parts to it: One is availability and access, and the other part is acceptance.
We need to think about what kind of infrastructure we should be planning now for what we’re going to need to deliver this vaccine. We’ll set priorities; certainly not everyone is going to get a vaccine all at once. But certainly, over time we will expect that all adults will receive the vaccine and perhaps children. So we’ll need to have systems in place that can deliver the vaccine. At the same time, we need to make sure that the vaccine is acceptable. We need to communicate the importance of vaccination to the public and address their concerns so that we can not only be able to deliver vaccines, but have those be accepted by the public.
So, there’s a lot of work to be done. But this isn’t science fiction: We are really on a path to a vaccine for a brand new infectious disease.
Yes. If you think back to the fact that in January, we barely knew what this virus was, and here we are, seven months later, embarking on efficacy trials, it’s really a remarkable accomplishment. We have a lot to do yet, but in the time that we’re assessing the efficacy of these vaccines and making sure that they can be delivered to the public, people really need to stay safe and to do all the things we’ve been encouraging them to do all along.
But we are well on our way to developing vaccines not only for people in the U.S., but for people all over the world.
Public Health On Call
This conversation is excerpted from the July 31 episode of Public Health On Call.
Subscribe to Podcast
Related Content
The superbug fight needs a better business model, astmh's annual meeting: tropmed24 in nola, an update on measles, pertussis, mpox, and other vaccine-preventable diseases, what we know about rwanda’s marburg virus outbreak, johns hopkins bloomberg school of public health and center for urban families begin unique partnership.

IMAGES
VIDEO