CBSE Expert


Case Study Questions of Class 9 Science PDF Download
Download PDF Case Study Questions of Class 9 Science to prepare for the upcoming CBSE Class 9 Exams Exam 2023-24. With the help of our well-trained and experienced faculty, we provide solved examples and detailed explanations for the recently added Class 9 Science case study questions .

Case study questions are based on real or hypothetical scenarios that require students to analyze, evaluate, and apply scientific concepts to solve problems or make informed decisions. They often present a detailed context, providing students with the opportunity to demonstrate their understanding of the subject matter beyond basic recall.
Table of Contents
Class 9 Science: Case Study Questions
The inclusion of case study questions in Class 9 science CBSE is a great way to engage students in critical thinking and problem-solving. By working through real-world scenarios, Class 9 Science students will be better prepared to tackle challenges they may face in their future studies and careers. Class 9 Science Case study questions also promote higher-order thinking skills, such as analysis and synthesis. In addition, case study questions can help to foster creativity and innovation in students. As per the recent pattern of the Class 9 Science examination, a few questions based on case studies/passages will be included in the CBSE Class 9 Science Paper. There will be a paragraph presented, followed by questions based on it.
Chapterwise Case Study Questions of Class 9 Science
- Case Study Questions for Chapter 1 Matter in Our Surroundings
- Case Study Questions for Chapter 2 Is Matter Around Us Pure?
- Case Study Questions for Chapter 3 Atoms and Molecules
- Case Study Questions for Chapter 4 Structure of Atom
- Case Study Questions for Chapter 5 The Fundamental Unit of Life
- Case Study Questions for Chapter 6 Tissues
- Case Study Questions for Chapter 7 Diversity in Living Organisms
- Case Study Questions for Chapter 8 Motion
- Case Study Questions for Chapter 9 Force and Laws of Motion
- Case Study Questions for Chapter 10 Gravitation
- Case Study Questions for Chapter 11 Work and Energy
- Case Study Questions for Chapter 12 Sound
- Case Study Questions for Chapter 13 Why do we Fall ill
- Case Study Questions for Chapter 14 Natural Resources
- Case Study Questions for Chapter 15 Improvement in Food Resources
You can find a wide range of solved case studies on cbseexperts, covering various topics and concepts. Class 9 Science case studies are designed to help you understand the application of various concepts in real-life situations.
Class 9 Science Syllabus
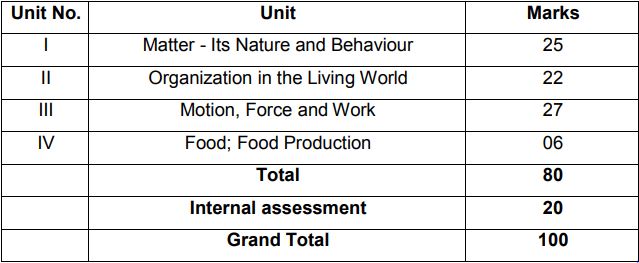
Unit I: Matter-Nature and Behaviour
Definition of matter; solid, liquid, and gas; characteristics – shape, volume, density; change of statementing (absorption of heat), freezing, evaporation (cooling by evaporation), condensation, sublimation.
Nature of matter: Elements, compounds, and mixtures. Heterogeneous and homogenous mixtures, colloids, and suspensions. Physical and chemical changes (excluding separating the components of a mixture).
Particle nature and their basic units: Atoms and molecules, Law of Chemical Combination, Chemical formula of common compounds, Atomic and molecular masses.
Structure of atoms: Electrons, protons and neutrons, Valency, Atomic Number and Mass Number, Isotopes and Isobars.
Unit II: Organization in the Living World
Cell – Basic Unit of life: Cell as a basic unit of life; prokaryotic and eukaryotic cells, multicellular organisms; cell membrane and cell wall, cell organelles and cell inclusions; chloroplast, mitochondria, vacuoles, endoplasmic reticulum, Golgi apparatus; nucleus, chromosomes – basic structure, number.
Tissues, Organs, Organ System, Organism: Structure and functions of animal and plant tissues (only four types of tissues in animals; Meristematic and Permanent tissues in plants).
Unit III: Motio n, Force, and Work
Motion: Distance and displacement, velocity; uniform and non-uniform motion along a straight line; acceleration, distance-time and velocity-time graphs for uniform motion and uniformly accelerated motion, elementary idea of uniform circular motion.
Force and Newton’s laws: Force and Motion, Newton’s Laws of Motion, Action and Reaction forces, Inertia of a body, Inertia and mass, Momentum, Force and Acceleration.
Gravitation: Gravitation; Universal Law of Gravitation, Force of Gravitation of the earth (gravity), Acceleration due to Gravity; Mass and Weight; Free fall. Floatation: Thrust and Pressure. Archimedes’ Principle; Buoyancy.
Work, Energy and Power: Work done by a Force, Energy, power; Kinetic and Potential energy; Law of conservation of energy (excluding commercial unit of Energy).
Sound: Nature of sound and its propagation in various media, speed of sound, range of hearing in humans; ultrasound; reflection of sound; echo.
Unit IV: Food Production
Plant and animal breeding and selection for quality improvement and management; Use of fertilizers and manures; Protection from pests and diseases; Organic farming.
Books for Class 9 Science Exams

Benefits of Case Study Questions
- Enhancing Analytical Skills : Case study questions challenge students to analyze complex scenarios, identify relevant information, and derive meaningful insights. By engaging with these questions, students develop critical analytical skills that are essential for scientific thinking and problem-solving.
- Promoting Critical Thinking : Case study questions encourage students to think critically and evaluate different perspectives. They require students to reason, make logical deductions, and justify their answers with supporting evidence. This process helps in honing their critical thinking abilities, enabling them to approach problems from multiple angles.
- Encouraging Practical Application of Concepts : By presenting real-world or hypothetical situations, case study questions promote the application of scientific concepts in practical scenarios. This application-based approach fosters a deeper understanding of the subject matter and helps students see the relevance of what they learn in the classroom to everyday life.
Case study questions of Class 9 Science provide students with an opportunity to apply their knowledge, enhance analytical skills, and think critically. By understanding the format, benefits, and effective strategies for answering case study questions, students can excel in this form of assessment. While challenges may arise, practicing time management, improving information extraction skills, and enhancing observation abilities will enable students to overcome these obstacles and perform well. Embracing case study questions as a valuable learning tool can contribute to a holistic understanding of scientific concepts and foster problem-solving abilities.
1. What is the purpose of case study questions in Class 9 Science?
Case study questions serve the purpose of evaluating a student’s understanding of scientific concepts, their ability to apply knowledge in real-life situations, and their analytical and critical thinking skills.
2. How can case study questions help improve analytical skills?
Case study questions require students to analyze complex scenarios, identify relevant information, and derive meaningful insights. Regular practice with such questions can significantly enhance analytical skills.
3. Are case study questions difficult to answer?
Case study questions can be challenging due to their comprehensive nature and the need for critical thinking. However, with practice and effective strategies, students can develop the skills necessary to answer them effectively.
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Download India's best Exam Preparation App Now.
Key Features
- Revision Notes
- Important Questions
- Previous Years Questions
- Case-Based Questions
- Assertion and Reason Questions
No thanks, I’m not interested!
myCBSEguide
- Class 9 Science Case...
Class 9 Science Case Study Questions
Table of Contents
myCBSEguide App
Download the app to get CBSE Sample Papers 2023-24, NCERT Solutions (Revised), Most Important Questions, Previous Year Question Bank, Mock Tests, and Detailed Notes.
If you are wondering how to solve class 9 science case study questions, then myCBSEguide is the best platform to choose. With the help of our well-trained and experienced faculty, we provide solved examples and detailed explanations for the recently added Class 9 Science case study questions.
You can find a wide range of solved case studies on myCBSEguide, covering various topics and concepts. Class 9 Science case studies are designed to help you understand the application of various concepts in real-life situations.
The rationale behind Science
Science is crucial for Class 9 students’ cognitive, emotional, and psychomotor development. It encourages curiosity, inventiveness, objectivity, and aesthetic sense.
In the upper primary stage, students should be given a variety of opportunities to engage with scientific processes such as observing, recording observations, drawing, tabulating, plotting graphs, and so on, whereas in the secondary stage, abstraction and quantitative reasoning should take a more prominent role in science teaching and learning. As a result, the concept of atoms and molecules as matter’s building units, as well as Newton’s law of gravitation, emerges.
Science is important because it allows Class 9 Science students to understand the world around us. It helps to find out how things work and to find solutions to problems at the Class 9 Science level. Science is also a source of enjoyment for many people. It can be a hobby, a career, or a source of intellectual stimulation.
Case study questions in Class 9 Science
The inclusion of case study questions in Class 9 science CBSE is a great way to engage students in critical thinking and problem-solving. By working through real-world scenarios, Class 9 Science students will be better prepared to tackle challenges they may face in their future studies and careers. Class 9 Science Case study questions also promote higher-order thinking skills, such as analysis and synthesis. In addition, case study questions can help to foster creativity and innovation in students. As per the recent pattern of the Class 9 Science examination, a few questions based on case studies/passages will be included in the CBSE Class 9 Science Paper. There will be a paragraph presented, followed by questions based on it.
Examples of Class 9 science class case study questions
Class 9 science case study questions have been prepared by myCBSEguide’s qualified teachers. Class 9 case study questions are meant to evaluate students’ knowledge and comprehension of the material. They are not intended to be difficult, but they will require you to think critically about the material. We hope you find Class 9 science case study questions beneficial and that they assist you in your exam preparation.
The following are a few examples of Class 9 science case study questions.
Class 9 science case study question 1
- due to its high compressibility
- large volumes of a gas can be compressed into a small cylinder
- transported easily
- all of these
- shape, volume
- volume, shape
- shape, size
- size, shape
- the presence of dissolved carbon dioxide in water
- the presence of dissolved oxygen in the water
- the presence of dissolved Nitrogen in the water
- liquid particles move freely
- liquid have greater space between each other
- both (a) and (b)
- none of these
- Only gases behave like fluids
- Gases and solids behave like fluids
- Gases and liquids behave like fluids
- Only liquids are fluids
Answer Key:
- (d) all of these
- (a) shape, volume
- (b) the presence of dissolved oxygen in the water
- (c) both (a) and (b)
- (c) Gases and liquids behave like fluids
Class 9 science case study question 2
- 12/32 times
- 18 g of O 2
- 18 g of CO 2
- 18 g of CH 4
- 1 g of CO 2
- 1 g of CH 4 CH 4
- 2 moles of H2O
- 20 moles of water
- 6.022 × 1023 molecules of water
- 1.2044 × 1025 molecules of water
- (I) and (IV)
- (II) and (III)
- (II) and (IV)
- Sulphate molecule
- Ozone molecule
- Phosphorus molecule
- Methane molecule
- (c) 8/3 times
- (d) 18g of CH 4
- (c) 1g of H 2
- (d) (II) and (IV)
- (c) phosphorus molecule
Class 9 science case study question 3
- collenchyma
- chlorenchyma
- It performs photosynthesis
- It helps the aquatic plant to float
- It provides mechanical support
- Sclerenchyma
- Collenchyma
- Epithelial tissue
- Parenchyma tissues have intercellular spaces.
- Collenchymatous tissues are irregularly thickened at corners.
- Apical and intercalary meristems are permanent tissues.
- Meristematic tissues, in its early stage, lack vacuoles, muscles
- (I) and (II)
- (III) and (I)
- Transpiration
- Provides mechanical support
- Provides strength to the plant parts
- None of these
- (a) Collenchyma
- (b) help aquatic plant to float
- (b) Sclerenchyma
- (d) Only (III)
- (c) provide strength to plant parts
Cracking Class 9 Science Case Study Questions
There is no one definitive answer to Class 9 Science case study questions. Every case study is unique and will necessitate a unique strategy. There are, nevertheless, certain general guidelines to follow while answering case study questions.
- To begin, double-check that you understand the Class 9 science case study questions. Make sure you understand what is being asked by reading it carefully. If you’re unclear, seek clarification from your teacher or tutor.
- It’s critical to read the Class 9 Science case study material thoroughly once you’ve grasped the question. This will provide you with a thorough understanding of the problem as well as the various potential solutions.
- Brainstorming potential solutions with classmates or other students might also be beneficial. This might provide you with multiple viewpoints on the situation and assist you in determining the best solution.
- Finally, make sure your answer is presented simply and concisely. Make sure you clarify your rationale and back up your claim with evidence.
A look at the Class 9 Science Syllabus
The CBSE class 9 science syllabus provides a strong foundation for students who want to pursue a career in science. The topics are chosen in such a way that they build on the concepts learned in the previous classes and provide a strong foundation for further studies in science. The table below lists the topics covered in the Class 9 Science syllabus of the Central Board of Secondary Education (CBSE). As can be seen, the Class 9 science syllabus is divided into three sections: Physics, Chemistry and Biology. Each section contains a number of topics that Class 9 science students must study during the course.
CBSE Class 9 Science (Code No. 086)
Theme: Materials Unit I: Matter-Nature and Behaviour Definition of matter; solid, liquid and gas; characteristics – shape, volume, density; change of state-melting (absorption of heat), freezing, evaporation (cooling by evaporation), condensation, sublimation. Nature of matter: Elements, compounds and mixtures. Heterogeneous and homogenous mixtures, colloids and suspensions. Particle nature and their basic units: Atoms and molecules, Law of constant proportions, Atomic and molecular masses. Mole concept: Relationship of mole to mass of the particles and numbers. Structure of atoms: Electrons, protons and neutrons, valency, the chemical formula of common compounds. Isotopes and Isobars.
Theme: The World of the Living Unit II: Organization in the Living World Cell – Basic Unit of life: Cell as a basic unit of life; prokaryotic and eukaryotic cells, multicellular organisms; cell membrane and cell wall, cell organelles and cell inclusions; chloroplast, mitochondria, vacuoles, endoplasmic reticulum, Golgi apparatus; nucleus, chromosomes – basic structure, number. Tissues, Organs, Organ System, Organism: Structure and functions of animal and plant tissues (only four types of tissues in animals; Meristematic and Permanent tissues in plants).
Theme: Moving Things, People and Ideas Unit III: Motion, Force and Work Motion: Distance and displacement, velocity; uniform and non-uniform motion along a straight line; acceleration, distance-time and velocity-time graphs for uniform motion and uniformly accelerated motion, derivation of equations of motion by graphical method; elementary idea of uniform circular motion. Force and Newton’s laws: Force and Motion, Newton’s Laws of Motion, Action and Reaction forces, Inertia of a body, Inertia and mass, Momentum, Force and Acceleration. Elementary idea of conservation of Momentum. Gravitation: Gravitation; Universal Law of Gravitation, Force of Gravitation of the earth (gravity), Acceleration due to Gravity; Mass and Weight; Free fall. Floatation: Thrust and Pressure. Archimedes’ Principle; Buoyancy. Work, energy and power: Work done by a Force, Energy, power; Kinetic and Potential energy; Law of conservation of energy. Sound: Nature of sound and its propagation in various media, speed of sound, range of hearing in humans; ultrasound; reflection of sound; echo.
Theme: Food Unit IV: Food Production Plant and animal breeding and selection for quality improvement and management; Use of fertilizers and manures; Protection from pests and diseases; Organic farming.
PRESCRIBED BOOKS:
- Science-Textbook for class IX-NCERT Publication
- Assessment of Practical Skills in Science-Class IX – CBSE Publication
- Laboratory Manual-Science-Class IX, NCERT Publication
- Exemplar Problems Class IX – NCERT Publication
myCBSEguide: A true helper
There are numerous advantages to using myCBSEguide to achieve the highest results in Class 9 Science.
- myCBSEguide offers high-quality study materials that cover all of the topics in the Class 9 Science curriculum.
- myCBSEguide provides practice questions and mock examinations to assist students in the best possible preparation for their exams.
- On our myCBSEguide app, you’ll find a variety of solved Class 9 Science case study questions covering a variety of topics and concepts. These case studies are intended to help you understand how certain principles are applied in real-world settings
- myCBSEguide is that the study material and practice problems are developed by a team of specialists who are always accessible to assist students with any questions they may have. As a result, students may be confident that they will receive the finest possible assistance and support when studying for their exams.
So, if you’re seeking the most effective strategy to study for your Class 9 Science examinations, myCBSEguide is the place to go!
Test Generator
Create question paper PDF and online tests with your own name & logo in minutes.
Question Bank, Mock Tests, Exam Papers, NCERT Solutions, Sample Papers, Notes
Related Posts
- Competency Based Learning in CBSE Schools
- Class 11 Physical Education Case Study Questions
- Class 11 Sociology Case Study Questions
- Class 12 Applied Mathematics Case Study Questions
- Class 11 Applied Mathematics Case Study Questions
- Class 11 Mathematics Case Study Questions
- Class 11 Biology Case Study Questions
- Class 12 Physical Education Case Study Questions
Leave a Comment
Save my name, email, and website in this browser for the next time I comment.

- Book Solutions
- State Boards
Case Study Questions Class 9 Science Structure of the Atom
Case study questions class 9 science chapter 4 structure of the atom.
CBSE Class 9 Case Study Questions Science Structure of the Atom. Important Case Study Questions for Class 9 Exam. Here we have arranged some Important Case Base Questions for students who are searching for Paragraph Based Questions Structure of the Atom.
At Case Study Questions there will given a Paragraph. In where some Questions will made on that respective Case Based Study. There will various types of marks will given 1 marks, 2 marks, 3 marks or 4 marks.
CBSE Case Study Questions Class 9 Science – Structure of the Atom
Dalton’s atomic theory suggested that the atom was indivisible and indestructible. But the discovery of two fundamental particles (electrons and protons) inside the atom, led to the failure of this aspect of Dalton’s atomic theory. It was then considered necessary to know how electrons and protons are arranged within an atom. For explaining this, many scientists proposed various atomic models. J.J. Thomson was the first one to propose a model for the structure of an atom.
J.J. Thomson (1856- 1940) was a British physicist, He was awarded the Nobel Prize in Physics for his work on the discovery of electrons. Thomson proposed the model of an atom to be similar to that of a Christmas pudding. The electrons, in a sphere of positive charge. We can also think of a watermelon, the positive charge in the atom is spread all over like the red edible part of the watermelon, while the electrons are studded in the positively charged sphere, like the seeds in the watermelon. Thomson proposed that: An atom consists of a positively charged sphere and the electrons are embedded in it. The negative and positive charges are equal in magnitude. So, the atom as a whole is electrically neutral.
(1) Identify the correct statement
Statement 1 – Dalton’s atomic theory suggested that the atom was indivisible and indestructible.
Statement 2 – Electrons and protons are present inside the atom.
Statement 3 – J.J. Thomson was the first one to propose a model for the structure of an atom.
Statement 4 – Protons are positively charged particle.
(b) Both 3 & 4
(c) Both 1 & 2
(d) All of the above
(2) According to Dalton’s Atomic Theory, matter consists of indivisible _______
(a) Molecules
(d) Mixtures
(3) Who was the first to propose atomic theory?
(a) J.J. Thomson
(b) John Dalton
(c) E. Rutherford
(d) Neilsbhore
(4) “Atom is indivisible and indestructible” why this aspect of Dalton’s atomic theory leds to the failure?
(5) Explain the J.J. Thomson’s model for the structure of an atom?
(4) Dalton’s atomic theory suggested that the atom was indivisible and indestructible. But the discovery of two fundamental particles (electrons and protons) inside the atom, led to the failure of this aspect of Dalton’s atomic theory.
(5) Thomson was the first one to propose a model for the structure of an atom:
Postulate 1: An atom consists of a positively charged sphere with electrons embedded in it.
Postulate 2: An atom as a whole is electrically neutral because the negative and positive charges are equal in magnitude
Thomson atomic model is compared to watermelon. Where he considered:
- Watermelon seeds as negatively charged particles
- The red part of the watermelon as positively charged
Rutherford (1871-1937) was known as the ‘Father’ of nuclear physics. He is famous for his work on radioactivity and the discovery of the nucleus of an atom with the gold foil experiment. Ernest Rutherford was interested in knowing how the electrons are arranged within an atom. Rutherford designed an experiment for this. In this experiment, fast moving alpha (α)-particles were made to fall on a thin gold foil. On the basis of his experiment, Rutherford put forward the nuclear model of an atom, which had the following features:
- There is a positively charged centre in an atom called the nucleus. Nearly all the mass of an atom resides in the nucleus.
- The electrons revolve around the nucleus in circular paths.
- The size of the nucleus is very small as compared to the size of the atom.
Drawbacks of Rutherford’s model of the atom: The revolution of the electron in a circular orbit is not expected to be stable. Any particle in a circular orbit would undergo acceleration. During acceleration, charged particles would radiate energy. Thus, the revolving electron would lose energy and finally fall into the nucleus. If this were so, the atom should be highly unstable and hence matter would not exist in the form that we know. We know that atoms are quite stable.
(1) Which of the following scientist was known as the ‘Father of nuclear physics?
(2) Positively charged centre in an atom is termed as
(a) Nucleus
(b) Molecule
(d) Protons
(3) Identify the correct statement
Statement 1 – Positively charged centre in an atom called the nucleus.
Statement 2 – The electrons revolve around the nucleus in circular paths.
Statement 3 – Nearly all the mass of an atom resides in the nucleus.
Statement 4 – The size of the nucleus is very small as compared to the size of the atom.
(4) Write the features of Rutherford’s nuclear model of an atom?
(5) Define Nucleus.
(4) Rutherford put forward the nuclear model of an atom, which had the following features:
(5) There is a positively charged centre in an atom called the nucleus. Nearly all the mass of an atom resides in the nucleus.
Protons are present in the nucleus of an atom. It is the number of protons of an atom, which determines its atomic number. It is denoted by ‘Z’. All atoms of an element have the same atomic number, Z. In fact, elements are defined by the number of protons they possess. For hydrogen, Z = 1, because in hydrogen atom have only one proton is present in the nucleus. Therefore, the atomic number is defined as the total number of protons present in the nucleus of an atom.
The mass of an atom is practically due to protons and neutrons alone. These are present in the nucleus of an atom. Hence protons and neutrons are also called nucleons. Therefore, the mass of an atom resides in its nucleus. For example, mass of carbon is 12 u because it has 6 protons and 6 neutrons, 6 u + 6 u = 12 u. Similarly, the mass of aluminium is 27 u (13 protons+14 neutrons). The mass number is defined as the sum of the total number of protons and neutrons present in the nucleus of an atom. It is denoted by ‘A’.
(1) Atomic number is denoted by
(2) The sum of the total number of protons and neutrons present in the nucleus of an atom.
(a) Atomic number
(b) Mass number
(c) Atomic weight
(d) None of the above
(3) Mass number is denoted by
(4) Identify the correct statement
Statement 1 – Protons are present in the nucleus of an atom.
Statement 2 – Atomic number is the number of protons of an atom.
Statement 3 – Atomic number is denoted by ‘Z’.
Statement 4 – The mass of an atom is due to protons and neutrons alone.
(5) Why mass of carbon is 12 u give the reason?
(5) Mass of carbon is 12 u because it has 6 protons and 6 neutrons, 6 u + 6 u = 12 u.
A number of atoms of some elements have the same atomic number but different mass numbers. For example, hydrogen atom, it has three atomic species, namely Protium, Deuterium and Tritium. The atomic number of each one is 1, but the mass number is 1, 2 and 3, respectively. On the basis of these examples, isotopes are defined as the atoms of the same element, having the same atomic number but different mass numbers. Therefore, we can say that there are three isotopes of hydrogen atom, namely protium, deuterium and tritium.
Many elements consist of a mixture of isotopes. Each isotope of an element is a pure substance. The chemical properties of isotopes are similar but their physical properties are different.
The mass of an atom of any natural element is taken as the average mass of all the naturally occurring atoms of that element. If an element has no isotopes, then the mass of its atom would be the same as the sum of protons and neutrons in it. But if an element occurs in isotopic forms, then we have to know the percentage of each isotopic form and then the average mass is calculated.
Chemical properties of all the isotopes of an element are the same. Some isotopes have special properties which find them useful in various fields. Such as, an isotope of uranium is used as a fuel in nuclear reactors, isotope of cobalt is used in the treatment of cancer, iodine is used in the treatment of goitre.
(1) The atoms of the same element, having the same atomic number but different mass numbers are termed as __________
(a) Isotopes
(b) Protium
(c) Deuterium
(d) Tritium
(2) Which of the following are the isotopes of hydrogen atom.
(a) Protium
(b) Deuterium
(c) Tritium
Statement 1 – Chemical properties of all the isotopes of an element are the same.
Statement 2 – Physical properties are different.
Statement 3 – Chemical properties of all the isotopes of an element are different.
Statement 4 – Physical properties are same.
(b)Both 3 & 4
(4) Give any two uses of isotopes.
(5) Define isotopes.
(4) Isotopes have special properties which find them useful in various fields. Such as,
- An isotope of uranium is used as a fuel in nuclear reactors,
- Isotope of cobalt is used in the treatment of cancer,
- Isotope of iodine is used in the treatment of goitre.
(5) Isotopes are defined as the atoms of the same element, having the same atomic number but different mass numbers.

In order to overcome the objections raised against Rutherford’s model of the atom, Neil’s Bohr put forward the following postulates about the model of an atom:
- Only certain special orbits known as discrete orbits of electrons, are allowed inside the atom.
- While revolving in discrete orbits the electrons do not radiate energy. These orbits or shells are called energy levels. Energy levels in an atom are shown in Fig. A few energy levels in an atom These orbits or shells are represented by the letters K,L,M,N,… or the numbers, n=1,2,3,4,….
(1) The orbits or shells are represented by
(a) Letters
(b) Numbers
(c) Both a & b
(d) Special symbols
(2) These orbits or shells are called
(a) Energy levels
(b) Discrete orbit
(c) Atomic levels
(3) Which of the following book is written by Professor Bohr’s
(a) The Theory of Spectra and Atomic Constitution
(b) Atomic Theory
(c) The Description of Nature
Statement 1 – The orbits or shells are represented by letters only.
Statement 2 – The orbits or shells are represented by numbers only.
Statement 3 – While revolving in discrete orbits the electrons do not radiate energy.
Statement 4 – Certain special orbits known as discrete orbits of electrons.
(a) Both 1 & 2
(5) Write the postulate of Neil’s Bohr model of an atom?
(5) Neil’s Bohr put forward the following postulates about the model of an atom:
- While revolving in discrete orbits the electrons do not radiate energy.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
We have a strong team of experienced Teachers who are here to solve all your exam preparation doubts
Odisha board class 6 geography chapter 3 মানচিত্র অধ্যয়ন, odisha board class 6 geography chapter 2 ভূগোলক-অক্ষাংশ ও দ্রাঘিমা, chhattisgarh state class 9 math chapter 14 solution, the value of work class 6 extra questions.
Sign in to your account
Username or Email Address
Remember Me

Gurukul of Excellence
Classes for Physics, Chemistry and Mathematics by IITians
Join our Telegram Channel for Free PDF Download
Assertion and Reason Questions for Class 9 Science Chapter 3 Atoms and Molecules
- Last modified on: 6 months ago
- Reading Time: 13 Minutes

Here we are providing assertion reason questions for class 9 science. This article covers assertion reason questions based on Class 9 Science Chapter 3 Atoms and Molecules . To check the answer, click on ‘answer’ given below each question. After clicking it will expand.
Direction: In each of the following questions, a statement of Assertion is given followed by a corresponding statement of Reason. Of the statements, mark the correct answer as (a) Both assertion and reason are true and reason is the correct explanation of assertion. (b) Both assertion and reason are true but reason is not the correct explanation of assertion. (c) Assertion is true but reason is false. (d) Assertion is false but reason is true
Question 1. Assertion : The number of particles present in one mole of a substance is fixed. Reason : The mass of one mole of a substance is equal to its relative atomic mass in grams.
Question 2. Assertion : Atoms always combine to form molecule and ions. Reason : Atoms of most element are not able to exist independently.
Question 3. Assertion : Atomicity of ozone is three while that of oxygen is two. Reason : Atomicity is the number of atoms constituting a molecule.
Question 4. Assertion : 1 amu equals to 1.66 x 10 -24 g. Reason : 1.66 x 10 -24 g equal to 1/12 th mass of a C-12 atom.
Question 5. Assertion : On burning magnesium in oxygen, the mass of magnesium oxide formed is equal to the total mass of magnesium and oxygen Reason : In a chemical substance, the elements are always present in a definite proportion.
Question 6. Assertion : 1 mole of and H 2 each O 2 occupy 22.4 L at standard temperature and pressure. Reason : Molar volume for all gases at the standard temperature and pressure has the different values.
Question 7. Assertion : Molecular weight of oxygen is 16. Reason : Atomic weight of oxygen is 16.
Question 8. Assertion : Atomic mass of aluminium is 14. Reason : An atom of aluminium is 27 times heavier than 1/12th of the mass of carbon-12 atom.
Question 9. Assertion : The number of moles of He in 52 g of He is 13. Reason : The number of moles of an atom is the ratio of its given mass to its molar mass.
Question 10. Assertion : The valency of aluminium is 3 and oxygen is 2. Reason : The chemical formula of aluminium oxide is Al 3 O 2 .
Question 11. Assertion : A molecule is the smallest particle of an element or a compound which is capable of free existence. Reason : The number of atoms present in one molecule of the substance is called its atomicity.
Question 12. Assertion : Protons cannot be transferred from one atom to another. Reason : Protons are present deep inside the atom in its nucleus.
Question 13. Assertion : Water molecules always contain hydrogen and oxygen in the ratio 1:8. Reason : Water obeys law of constant proportions irrespective of source and method of preparation.
Question 14. Assertion : Relative atomic mass of the atom of element is the average masses of the atom as compared to the mass of one carbon-12 atom. Reason : Carbon-12 isotope is the standard reference for measuring atomic masses.
Question 15. Assertion : A sodium ion has positive charge. Reason : Sodium ion has more protons than a neutral atom.
Question 16. Assertion : In water, the ratio of mass of hydrogen to the mass of oxygen is always 1:8 whatever the source of water. Reason : According to law of constant proportion, the elements are always present in definite proportion by mass in a chemical substance.
Question 17. Assertion : Ions are always positively charged. Reason : Ions are formed by losing or gaining of electrons.
Question 18. Assertion : One mole of SO 2 contains double the number of molecules present in one mole of O 2 Reason : Molecular weight of SO 2 is double to that of O 2
Question 19. Assertion : When 12 g of CaCO 3 is decomposed, 4.6 g of residue is left and 4.4 g of escapes. Reason : Law of conservation of mass is followed.
Question 20. Assertion : Pure water obtained from different sources such as river, well, spring, sea etc. always contains hydrogen and oxygen combined in the ratio of 1 : 8 by mass. Reason : A chemical compound always contains same elements combined in different fixed proportion by mass.
Question 21. Assertion : Law of conservation of mass holds good for all the reactions. Reason : I states that energy can neither be created nor destroyed in a chemical reaction.
Question 22. Assertion : Atomicity of oxygen is 4. Reason : 1 mole of an element contains 6.023 x 10 23 atoms.
Question 23. Assertion : Atomicity of O 3 is 2. Reason : 1 mole of an element contains 6.023 x 10 23 atoms.
Question 24. Assertion : 52g of He contains 13 x 6.023 x 10 23 atoms. Reason : 1 mole of an element contains 6.023 x 10 23 atoms.
Question 25. Assertion: Atoms can neither be sub-divided, created nor destroyed. Reason: This postulate of Dalton’s theory is the result of law of constant proportion.
Question 26. Assertion: Carbonates are polyatomic ions. Reason: The carbonate ion consists of one carbon atom and three oxygen atoms and carries an overall charge of.
Question 27. Assertion : Number of gram-molecules of SO 2 Cl 2 in 13.5 g of sulphur chloride is 0.1. Reason : Gram molecular mass is equal to two gram molecule.
Question 28. Assertion : The total number of electrons present in 16 g of methane gas is 6.022 x 10 23 Reason : 1 mole of an element contains 6.023 x 10 23 atoms.
Question 29. Assertion : All noble gases are monoatomic. Reason : Noble gases are highly stable and unreactive.
Related Posts
Category lists (all posts).
All categories of this website are listed below with number of posts in each category for better navigation. Visitors can click on a particular category to see all posts related to that category.
- Full Form (1)
- Biography of Scientists (1)
- Assertion Reason Questions in Biology (37)
- Case Study Questions for Class 12 Biology (14)
- DPP Biology for NEET (12)
- Blog Posts (35)
- Career Guidance (1)
- Assertion Reason Questions for Class 10 Maths (14)
- Case Study Questions for Class 10 Maths (15)
- Extra Questions for Class 10 Maths (12)
- Maths Formulas for Class 10 (1)
- MCQ Questions for Class 10 Maths (15)
- NCERT Solutions for Class 10 Maths (4)
- Quick Revision Notes for Class 10 Maths (14)
- Assertion Reason Questions for Class 10 Science (16)
- Case Study Questions for Class 10 Science (14)
- Evergreen Science Book Solutions for Class 10 (17)
- Extra Questions for Class 10 Science (23)
- HOTS for Class 10 Science (17)
- Important Questions for Class 10 Science (10)
- Lakhmir Singh Class 10 Biology Solutions (4)
- Lakhmir Singh Class 10 Chemistry Solutions (5)
- Lakhmir Singh Class 10 Physics Solutions (5)
- MCQ Questions for Class 10 Science (20)
- NCERT Exemplar Solutions for Class 10 Science (16)
- NCERT Solutions for Class 10 Science (15)
- Quick Revision Notes for Class 10 Science (4)
- Study Notes for Class 10 Science (17)
- Assertion Reason Questions for Class 10 Social Science (14)
- Case Study Questions for Class 10 Social Science (24)
- MCQ Questions for Class 10 Social Science (3)
- Topicwise Notes for Class 10 Social Science (4)
- CBSE CLASS 11 (1)
- Assertion Reason Questions for Class 11 Chemistry (14)
- Case Study Questions for Class 11 Chemistry (11)
- Free Assignments for Class 11 Chemistry (1)
- MCQ Questions for Class 11 Chemistry (8)
- Very Short Answer Questions for Class 11 Chemistry (7)
- Assertion Reason Questions for Class 11 Entrepreneurship (8)
- Important Questions for CBSE Class 11 Entrepreneurship (1)
- Assertion Reason Questions for Class 11 Geography (24)
- Case Study Questions for Class 11 Geography (24)
- Assertion Reason Questions for Class 11 History (12)
- Case Study Questions for Class 11 History (12)
- Assertion and Reason Questions for Class 11 Maths (16)
- Case Study Questions for Class 11 Maths (16)
- Formulas for Class 11 Maths (6)
- MCQ Questions for Class 11 Maths (17)
- NCERT Solutions for Class 11 Maths (8)
- Case Study Questions for Class 11 Physical Education (11)
- Assertion Reason Questions for Class 11 Physics (15)
- Case Study Questions for Class 11 Physics (12)
- Class 11 Physics Study Notes (5)
- Concept Based Notes for Class 11 Physics (2)
- Conceptual Questions for Class 11 Physics (10)
- Derivations for Class 11 Physics (3)
- Extra Questions for Class 11 Physics (13)
- MCQ Questions for Class 11 Physics (16)
- NCERT Solutions for Class 11 Physics (16)
- Numerical Problems for Class 11 Physics (4)
- Physics Formulas for Class 11 (7)
- Revision Notes for Class 11 Physics (11)
- Very Short Answer Questions for Class 11 Physics (11)
- Assertion Reason Questions for Class 11 Political Science (20)
- Case Study Questions for Class 11 Political Science (20)
- CBSE CLASS 12 (8)
- Extra Questions for Class 12 Biology (14)
- MCQ Questions for Class 12 Biology (13)
- Case Studies for CBSE Class 12 Business Studies (13)
- MCQ Questions for Class 12 Business Studies (1)
- Revision Notes for Class 12 Business Studies (10)
- Assertion Reason Questions for Class 12 Chemistry (15)
- Case Study Based Questions for Class 12 Chemistry (14)
- Extra Questions for Class 12 Chemistry (5)
- Important Questions for Class 12 Chemistry (15)
- MCQ Questions for Class 12 Chemistry (8)
- NCERT Solutions for Class 12 Chemistry (16)
- Revision Notes for Class 12 Chemistry (7)
- Assertion Reason Questions for Class 12 Economics (9)
- Case Study Questions for Class 12 Economics (9)
- MCQ Questions for Class 12 Economics (1)
- MCQ Questions for Class 12 English (2)
- Assertion Reason Questions for Class 12 Entrepreneurship (7)
- Case Study Questions for Class 12 Entrepreneurship (7)
- Case Study Questions for Class 12 Geography (18)
- Assertion Reason Questions for Class 12 History (8)
- Case Study Questions for Class 12 History (13)
- Assertion Reason Questions for Class 12 Informatics Practices (13)
- Case Study Questions for Class 12 Informatics Practices (11)
- MCQ Questions for Class 12 Informatics Practices (5)
- Assertion and Reason Questions for Class 12 Maths (14)
- Case Study Questions for Class 12 Maths (13)
- Maths Formulas for Class 12 (5)
- MCQ Questions for Class 12 Maths (14)
- Problems Based on Class 12 Maths (1)
- RD Sharma Solutions for Class 12 Maths (1)
- Assertion Reason Questions for Class 12 Physical Education (11)
- Case Study Questions for Class 12 Physical Education (11)
- MCQ Questions for Class 12 Physical Education (10)
- Assertion Reason Questions for Class 12 Physics (16)
- Case Study Based Questions for Class 12 Physics (14)
- Class 12 Physics Conceptual Questions (16)
- Class 12 Physics Discussion Questions (1)
- Class 12 Physics Latest Updates (2)
- Derivations for Class 12 Physics (8)
- Extra Questions for Class 12 Physics (4)
- Important Questions for Class 12 Physics (8)
- MCQ Questions for Class 12 Physics (14)
- NCERT Solutions for Class 12 Physics (18)
- Numerical Problems Based on Class 12 Physics (16)
- Physics Class 12 Viva Questions (1)
- Revision Notes for Class 12 Physics (7)
- Assertion Reason Questions for Class 12 Political Science (16)
- Case Study Questions for Class 12 Political Science (16)
- Notes for Class 12 Political Science (1)
- Assertion Reason Questions for Class 6 Maths (13)
- Case Study Questions for Class 6 Maths (13)
- Extra Questions for Class 6 Maths (1)
- Worksheets for Class 6 Maths (1)
- Assertion Reason Questions for Class 6 Science (16)
- Case Study Questions for Class 6 Science (16)
- Extra Questions for Class 6 Science (1)
- MCQ Questions for Class 6 Science (9)
- Assertion Reason Questions for Class 6 Social Science (1)
- Case Study Questions for Class 6 Social Science (26)
- NCERT Exemplar for Class 7 Maths (13)
- NCERT Exemplar for Class 7 Science (19)
- NCERT Exemplar Solutions for Class 7 Maths (12)
- NCERT Exemplar Solutions for Class 7 Science (18)
- NCERT Notes for Class 7 Science (18)
- Assertion Reason Questions for Class 7 Maths (14)
- Case Study Questions for Class 7 Maths (14)
- Extra Questions for Class 7 Maths (5)
- Assertion Reason Questions for Class 7 Science (18)
- Case Study Questions for Class 7 Science (17)
- Extra Questions for Class 7 Science (19)
- Assertion Reason Questions for Class 7 Social Science (1)
- Case Study Questions for Class 7 Social Science (30)
- Assertion Reason Questions for Class 8 Maths (7)
- Case Study Questions for Class 8 Maths (17)
- Extra Questions for Class 8 Maths (1)
- MCQ Questions for Class 8 Maths (6)
- Assertion Reason Questions for Class 8 Science (16)
- Case Study Questions for Class 8 Science (11)
- Extra Questions for Class 8 Science (2)
- MCQ Questions for Class 8 Science (4)
- Numerical Problems for Class 8 Science (1)
- Revision Notes for Class 8 Science (11)
- Assertion Reason Questions for Class 8 Social Science (27)
- Case Study Questions for Class 8 Social Science (23)
- CBSE Class 9 English Beehive Notes and Summary (2)
- Assertion Reason Questions for Class 9 Maths (14)
- Case Study Questions for Class 9 Maths (14)
- MCQ Questions for Class 9 Maths (11)
- NCERT Notes for Class 9 Maths (6)
- NCERT Solutions for Class 9 Maths (12)
- Revision Notes for Class 9 Maths (3)
- Study Notes for Class 9 Maths (10)
- Assertion Reason Questions for Class 9 Science (16)
- Case Study Questions for Class 9 Science (15)
- Evergreen Science Book Solutions for Class 9 (15)
- Extra Questions for Class 9 Science (22)
- MCQ Questions for Class 9 Science (11)
- NCERT Solutions for Class 9 Science (15)
- Revision Notes for Class 9 Science (1)
- Study Notes for Class 9 Science (15)
- Topic wise MCQ Questions for Class 9 Science (2)
- Topicwise Questions and Answers for Class 9 Science (15)
- Assertion Reason Questions for Class 9 Social Science (15)
- Case Study Questions for Class 9 Social Science (19)
- CHEMISTRY (8)
- Chemistry Articles (2)
- Daily Practice Problems (DPP) (3)
- Books for CBSE Class 9 (1)
- Books for ICSE Class 10 (3)
- Editable Study Materials (8)
- Exam Special for CBSE Class 10 (3)
- H. C. Verma (Concepts of Physics) (13)
- Study Materials for ICSE Class 10 Biology (14)
- Extra Questions for ICSE Class 10 Chemistry (1)
- Study Materials for ICSE Class 10 Chemistry (5)
- Study Materials for ICSE Class 10 Maths (16)
- Important Questions for ICSE Class 10 Physics (13)
- MCQ Questions for ICSE Class 10 Physics (4)
- Study Materials for ICSE Class 10 Physics (8)
- Study Materials for ICSE Class 9 Maths (7)
- Study Materials for ICSE Class 9 Physics (10)
- Topicwise Problems for IIT Foundation Mathematics (4)
- Challenging Physics Problems for JEE Advanced (2)
- Topicwise Problems for JEE Physics (1)
- DPP for JEE Main (1)
- Integer Type Questions for JEE Main (1)
- Integer Type Questions for JEE Chemistry (6)
- Chapterwise Questions for JEE Main Physics (1)
- Integer Type Questions for JEE Main Physics (8)
- Physics Revision Notes for JEE Main (4)
- JEE Mock Test Physics (1)
- JEE Study Material (1)
- JEE/NEET Physics (6)
- CBSE Syllabus (1)
- Maths Articles (2)
- NCERT Books for Class 12 Physics (1)
- NEET Chemistry (13)
- Important Questions for NEET Physics (17)
- Topicwise DPP for NEET Physics (5)
- Topicwise MCQs for NEET Physics (32)
- NTSE MAT Questions (1)
- Physics (1)
- Alternating Current (1)
- Electrostatics (6)
- Fluid Mechanics (2)
- PowerPoint Presentations (13)
- Previous Years Question Paper (3)
- Products for CBSE Class 10 (15)
- Products for CBSE Class 11 (10)
- Products for CBSE Class 12 (6)
- Products for CBSE Class 6 (2)
- Products for CBSE Class 7 (5)
- Products for CBSE Class 8 (1)
- Products for CBSE Class 9 (3)
- Products for Commerce (3)
- Products for Foundation Courses (2)
- Products for JEE Main & Advanced (10)
- Products for NEET (6)
- Products for ICSE Class 6 (1)
- Electrostatic Potential and Capacitance (1)
- Topic Wise Study Notes (Physics) (2)
- Topicwise MCQs for Physics (2)
- Uncategorized (138)
Test series for students preparing for Engineering & Medical Entrance Exams are available. We also provide test series for School Level Exams. Tests for students studying in CBSE, ICSE or any state board are available here. Just click on the link and start test.
Download Books – Exam Special
Sample Papers for CBSE 2025 Exams
- Sample Question Papers for CBSE Class 8 All Subjects (for 2025 Exams)
- Sample Question Papers for CBSE Class 9 All Subjects (for 2025 Exams)
- Sample Question Papers for CBSE Class 10 All Subjects (for 2025 Exams)
- Sample Question Papers for CBSE Class 12 All Subjects (for 2025 Exams)
CBSE Class 10 Most Downloaded Books
- CBSE Important Numerical Problems Class 10 Physics Board Exams
- CBSE Practical Based Questions for Class 10 Science Board Exams
- CBSE Important Diagram Based Questions Class 10 Physics Board Exams
- CBSE Most Repeated Questions for Class 10 Science Board Exams
CBSE Class 12 Most Downloaded Books
- CBSE Important Diagrams & Graphs Asked in Board Exams Class 12 Physics
- CBSE Important Numericals Class 12 Physics Board Exams
- CBSE Important Laws & Principles Class 12 Physics Board Exams
- CBSE Important Definitions Class 12 Physics Board Exams
- Master Organic Conversions CBSE Class 12 Chemistry Board Exams
- CBSE Class 12 Physics Chapterwise Important Questions
CBSE Class 8 Most Downloaded Books
- Worksheets for CBSE Class 8 Maths – Chapterwise
ICSE Class 10
- ICSE Important Numericals Class 10 Physics BOARD Exams (215 Numericals)
- ICSE Important Figure Based Questions Class 10 Physics BOARD Exams (230 Questions)
- ICSE Mole Concept and Stoichiometry Numericals Class 10 Chemistry (65 Numericals)
- ICSE Reasoning Based Questions Class 10 Chemistry BOARD Exams (150 Qs)
- ICSE Important Functions and Locations Based Questions Class 10 Biology
- ICSE Reasoning Based Questions Class 10 Biology BOARD Exams (100 Qs)
- ICSE Reasoning Based Questions Class 10 Geography BOARD Exams
- ICSE Revision Notes for Class 10 Chemistry BOARD Exams
- ICSE Revision Notes for Class 10 Physics BOARD Exams
ICSE Class 9
- ICSE Important Figure Based Questions Class 9 Physics Exams
- ICSE Important Numerical Problems for Class 9 Physics Exams
- ICSE Reasoning Based Questions Class 9 Geography BOARD Exams (150 Qs)
CBSE Chapter-Wise Test Papers
- CBSE Class 9 Science Chapterwise Test Papers
- CBSE Class 10 Science Chapterwise Test Papers
- CBSE Class 10 Maths Chapterwise Test Papers
- CBSE Class 10 Social Science Chapterwise Test Papers
- CBSE Class 12 Physics Chapterwise Test Papers
- CBSE Class 12 Chemistry Chapterwise Test papers
✨ Join our Online NEET Test Series for 499/- Only for 1 Year
2 thoughts on “ Assertion and Reason Questions for Class 9 Science Chapter 3 Atoms and Molecules ”
it helped me a lot thank you all
Assertion: atomic mass of aluminum is 14 is incorrect
Leave a Reply Cancel reply

Editable Study Materials for Your Institute - CBSE, ICSE, State Boards (Maharashtra & Karnataka), JEE, NEET, FOUNDATION, OLYMPIADS, PPTs
Discover more from Gurukul of Excellence
Subscribe now to keep reading and get access to the full archive.
Type your email…
Continue reading

NCERT Solutions for Class 9 Science Chapter 3 Atoms and Molecules

Chapter 3 Atoms and Molecules NCERT Solutions for Class 9 Science
Contact form.
Extra Questions for Class 9 Science Chapter 3 Atoms and Molecules
Extra questions for Class 9 Science Chapter 3 Atoms and Molecules with answers is given below. Our subject expert prepared these solutions as per the latest NCERT textbook. These questions will be helpful to revise the all topics and concepts. CBSE Class 9 extra questions are the most simple and conceptual questions that are prepared by subject experts for the students to study well for the final exams. By solving these extra questions, students can be very efficient in their exam preparations.
Atoms and Molecules Class 9 Science Extra Questions and Answers
Very short answer questions.
1: Define law of conservation of mass. Answer: In a chemical reaction mass can neither be created nor destroyed.
2: Explain law of constant proportion. Answer: In a chemical substance the elements are always present in definite proportions by mass. E.g., In water, the ratio of the mass of hydrogen to the mass of oxygen H : O is always 1:8
3: Who coined the term atom? Answer: John Dalton coined the term atom.
4: Define atom. Answer: The smallest particle of matter, which can take part in a chemical reaction is called atom.
5: Define molecule. Answer: The smallest particle of an element or compound which can exist independently is called molecule.
6: Define atomicity. Answer: The number of atoms constituting a molecule is known as its atomicity.
7: What is atomic mass unit? Answer: The sum of the atomic masses of all the atoms in a molecule of the substance is expressed.in atomic mass unit. E.g., H 2 0 = 1 × 2 + 16 = 18 amu
8: How do atoms exist? Answer: Atoms exist in the form of atom, molecule or ions.
9: Give the atomicity of phosphorous and nitrogen. Answer: The atomicity of phosphorus is P 4 i.e., 4. The atomicity of nitrogen is N 2 i.e., 2.
10: What is an ion? Answer: Charged atom is called as an ion. The ion can be positively charged called cation or negatively charged called anion.
11: Give one example of cation and anion. Answer: Cation = Na + Anion = Cl –
12: Give one difference between cation and anion. Answer: Cations are positively charged ion. Anions are negatively charged ion.
13: Give the chemical formula for ammonium sulphate. Answer: Ammonium sulphate – NH 4 + SO 4 2- Chemical formula – (NH 4 ) 2 S0 4 .
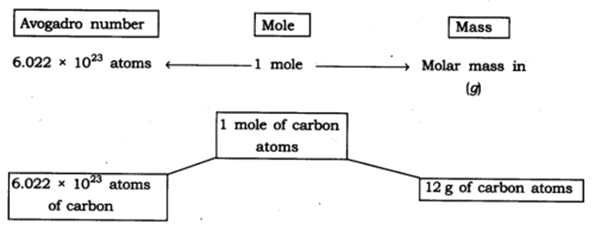
14: What is Avogadro’s constant? Answer: The Avogadro’s constant (6.022 x 10 23 ) is defined as the number of atoms that are present in exactly 12 g of carbon-12.
15: Find the molecular mass of H 2 O. Answer: Molecular mass of H 2 O = (2 × 1) + (16) = 2 + 16 = 18 u
Short Answer Type Questions
1: Give the unit to measure size of atom and give size of hydrogen atom. Answer: The unit to measure size of atom, is nanometer, size of hydrogen atom is 10 -10 m.
2: What is IUPAC, give its one function? Answer: IUPAC is International Union for Pure and Applied Chemistry. It approves the names of elements.
3: Give the Latin name for sodium, potassium, gold and mercury. Answer: Sodium → Natrium, Gold → Aurum Potassium → Kalium, Mercury → Hydrargyrum
4: What is the ratio by mass of combining elements in H 2 O, CO 2 and NH 3 ?
Answer: H 2 O ratio by mass of combining elements 2 : 16 →1 : 8 (H : O) CO 2 ratio by mass of combining elements 12 : 32 → 3 : 08 (C : O) NH 3 ratio by mass of combining elements 14 : 3 → 14 : 3 (N : H)
5: Define valency and give the valency for the following elements: Magnesium, Aluminium, Chlorine and Copper.
Answer: Valency: The combining capacity of an element is called its valency. Valency of the following elements: Magnesium – 2 Aluminium – 3 Chlorine – 1 Copper – 2
6: What is polyatomic ton? Give one example.
Answer: A group of atoms carrying a charge is known as a polyatomic ion. E.g., Ammonium – NH 4 + Nitrate – NO 3 –
7: Write down the formula for: Copper nitrate, calcium sulphate and aluminium hydroxide.
Answer: Chemical formula: Copper nitrate → Cu(NO 3 ) Calcium sulphate → CaSO 4 Aluminium hydroxide Al(OH) 3
8: What is formula unit mass? How is it different from molecular mass?
Answer: The formula unit mass of a substance is a sum of the atomic masses of all atoms in a formula unit of a compound. The constituent particles of formula unit mass are ions and the constituent particles of molecular mass are atoms.
9: Find the number of moles in the following: (i) 50 g of H 2 O (ii) 7 g of Na
Answer: (i) Molar mass of H 2 O = 18 g Given mass of H 2 O = 50 g ∴ No. of moles in 50g of H 2 O = 58/18 = 2.78 moles.
(ii) Molar mass of Na = 23 g Given mass of Na = 7 g ∴ No. of moles in 50g of H 2 O = 7/23 = 0.304 moles.
10: Find the number of atoms in the following: (i) 0.5 mole of C atom (ii) 2 mole of N atom
Answer: (i) 0.5 mole of C atom: Number of atoms in 1 mole of C atom = 6.022 × 10 23 atoms Number of atoms in 0.5 mole of C atom = 6.022 × 10 23 × 0.5 = 3.011 × 10 23 atoms
(ii) 2 mole of N atom: Number of atoms in 1 mole of N atom = 6.022 × 10 23 atoms Number of atoms in 2 mole of N atom = 6.022 × 2 × 10 23 = 1.2044 × 10 24 atoms
11: Find the mass of the following: (i) 6.022 × 10 23 number of O 2 molecules (ii) 1.5 mole of CO 2 molecule
Answer: (i) 6.022 × 10 23 number of 02 molecules: Mass of 1 mole of O 2 molecule = 6.022 × 10 23 molecules = 32 g
(ii) 1.5 mole of CO 2 molecule: Mass of 1 mole of CO 2 molecule = 6.022 × 10 23 molecules = 44 g Mass of 1.5 mole CO 2 molecule = 44 × 1.5 = 66 g
12: Show the relationship between mole, Avogadro number and mass. Answer:
13: What are the rules for writing the symbol of an element?
Answer: IUPAC → International Union of Pure and Applied Chemistry approves name of elements. Symbols are the first one or two letters of the element’s name in English. The first letter of a symbol is always written as a capital letter (upper case) and the second letter as a small letter (lower case). e.g., Hydrogen → H Helium → He Some symbols are taken from the names of elements in Latin, German or Greek. e.g., Symbol of iron is Fe, its Latin name is Ferrum. Symbol of sodium is Na, its Latin name is Natrium. 14: Explain relative atomic mass and relative molecular mass.
Answer: Relative atomic mass: It can be defined as the number of times one atom of given element is heavier than 1/12 th of the mass of an atom of carbon-12. Relative Molecular Mass: It is defined as the number of times one molecule of a substance or given element is heavier than 1/12 th of the mass of one atom of carbon-12. 15: The formula of carbon-dioxide is CO 2 . What information do you get from this formula?
Answer: (i) CO 2 represents carbon-dioxide. (ii) CO 2 is one molecule of carbon-dioxide. (iii) CO 2 is one mole of carbon-dioxide i.e., it contains 6.022 × 10 23 molecules of carbon dioxide. (iv) CO 2 contains 1 atom of carbon and two atoms of oxygen. (v) CO 2 represents 44 g of molar mass.
16: State 3 points of difference between an atom and an ion. Answer:
17: Calculate the formula unit mass of NaCl and CaCl 2 . (Na = 23, Cl = 35.5, Ca = 40)
Answer: Formula unit mass of NaCl = 23 + 35.5 = 58.5 u
Formula unit mass of CaCl 2 = 40 + (2 × 35.5) = 40 + 71 = 111 u
18: The ratio by mass for hydrogen and oxygen in water is given as 1 : 8 respectively. Calculate the ratio by number of atoms for a water molecule. Answer: The ratio by number of atoms for a water molecule are:
Thus, the ratio by number of atoms for water is H : O = 2 : 1.
19: Write down the chemical formula for the following compounds: (a) Aluminium carbonate (b) Calcium sulphide (c) Zinc carbonate (d) Copper phosphate (e) Magnesium bicarbonate (f) Aluminium hydroxide.
Answer: The chemical formula are:
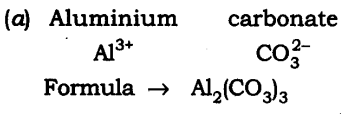
20: Give the atomicity of the following compounds: (a) Ca(OH) 2 (b) Mg(HCO 3 ) 2 (c) Cu 2 O. (d) H 2 SO 4 (e) Al 2 (SO 4 ) 3 (f) MgCl 2
Answer: The atomicity of the molecules are: (a) Ca(OH) 2 → 05 (b) Mg(HCO 3 ) 2 → 11 (c) Cu 2 O → 03 (d) H 2 SO 4 → 07 (e) Al 2 (SO 4 ) 3 → 17 (f) MgCl 2 → 03
21: Explain the difference between 2O, O 2 and O 3 .
Answer: 2O → It represents 2 atoms of oxygen (cannot exist independently). O 2 → It represents one molecule of oxygen (made up of 2 atom) can exist freely. O 3 → It represents one molecule of ozone (made up of 3 atoms) it can exist independently.
Long Answer Type Questions
1: (a) How do atoms exist? (b) What is atomicity? (c) What are polyatomic ions?
Answer: (a) Atoms of some elements are not able to exist independently. For such elements atoms form molecules and ions. In case of metals and inert gases atoms can exist independently.
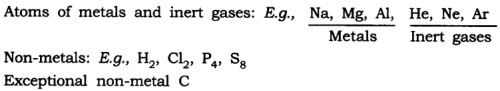
(b) The number of atoms constituting a molecule is known as its atomicity. E.g.,O 3 → atomicity is 3 O 2 → atomicity is 2
(c) Polyatomic ions: When more than two atoms combine together and act like an atom with a charge on it is called polyatomic ion. E.g., OH – , N0 3 – , NH 4 +
2: Calculate (a) the mass of one atom of oxygen (b) the mass of one molecule of oxygen (c) the mass of one mole of oxygen gas (d) the mass of one ion of oxygen (e) the number of atoms in 1 mole of oxygen molecule
Answer: (a) Mass of one atom of oxygen 1 mole of oxygen atom = 16 gm = 6.022 × 10 23 atoms. ∴ Mass of one atom of oxygen = 16/6.022 × 10 23 = 2.65 × 10 23
(b) Mass of one molecule of oxygen 1 molecule of oxygen = O 2 = 2 × 16 = 32 u
(c) Mass of one oxygen gas 1 molecule of oxygen gas is O 2 = 32 u
(d) Mass of one ion of oxygen One mole of oxygen = 6.022 × 10 23 atoms = 16g. 16 Mass of one ion of oxygen = 16/6.022 × 10 23 = 2.65 × 10 23
(e) Number of atoms in one mole of oxygen molecule 1 mole of oxygen molecule i.e. 0 2 = 6.022 × 10 23 molecules. 1 molecule of O 2 = 2 atoms.
∴ Number of atoms in 1 mole of oxygen molecule = 6.022 × 10 23 × 2 atoms = 1.2044 × 10 24 atoms
3: What is meant by atomic mass, gram atomic mass of an element? Why is the mass have different expressions i.e., ‘u’ and ‘g’?
Answer: The atoms are very tiny and their individual mass cannot be calculated as it is negligible. Hence the mass of atoms is expressed in units with respect to a fixed standard. Initially hydrogen atom with mass 1 was taken as standard unit by Dalton. Later, it was replaced by oxygen atom (0=16). But due to the isotopes the masses were found in fractions instead of whole number. Hence, carbon (C=12) isotope was taken as standard unit and was universally accepted. The atomic mass unit is equal to one twelfth (1/12) the mass of an atom of carbon-12, its unit is u.
Gram atomic mass: When the atomic mass of an element is expressed in grams, it is called the gram atomic mass of the element. The mass of atoms, molecules is expressed in ‘u’ and the mass of moles i.e., molar mass is expressed in g.
4: Define a mole. Give the significance of the mole.
Answer: Mole-One mole of any species (atoms, molecules, ions or particles) is that quantity or number having a mass equal to its atomic or molecular mass in grams. 1 mole = 6.022 × 10 23 in number (atoms, molecules, ions or particles)
Significance of the mole 1. A mole gives the number of entities present i.e, 6.022 × 10 23 particles of the substance. 2. Mass of 1 mole is expressed as M grams. 3. Mass of 1 mole = mass of 6.022 × 10 23 atoms of the element.
- Important Questions
- Important Questions Class 9 Chemistry
- Important Questions Class 9 Chemistry Chapter 3 Atoms and Molecules
Class 9 Chemistry Chapter 3 - Atoms and Molecules Important Questions with Answers
Class 9 chemistry important questions with answers are provided here for Chapter 3 Atoms and Molecules. These important questions are based on the CBSE board curriculum and correspond to the most recent Class 9 chemistry syllabus. By practising these Class 9 important questions, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 9 Annual examinations.
Download Class 9 Chemistry Chapter 3 Atoms and Molecules Important Questions with Answers PDF by clicking on the button below. Download PDF

Recommended Video
Atoms and molecules one shot.

Class 9 Chapter 3 Atoms and Molecules Important Questions with Answers
Short answer type questions.
Q1. Which of the following represents a correct chemical formula? Name it.
(b), BiPO 4 represents the correct formulae of bismuth phosphate.
Q2. Write the molecular formulae for the following compounds
(a) Copper (I) bromide
(b) Aluminium (III) nitrate
(c) Calcium (II) phosphate
(d) Iron (III) sulphide
(e) Mercury (I) chloride
(f) Magnesium (I) acetate
(a) The molecular formula of Copper (I) bromide is CuBr.
(b) The molecular formula of Aluminium (III) nitrate is Al(NO 3 ) 3 .
(c) The molecular formula of Calcium (II) phosphate is Ca 3 (PO 4 ) 2 .
(d) The molecular formula of Iron (III) sulphide is Fe 2 S 3 .
(e) The molecular formula of Mercury (I) chloride is HgCl 2 .
(f) The molecular formula of Magnesium (I) acetate is Mg(CH 3 COO) 2 .
Q3. Write the molecular formulae of all the compounds that can be formed by the combination of the following ions.
Cu 2+ , Na + , Fe 3+ , Cl – , SO 4 2- , PO 4 3- .
The molecular formula of the compounds formed by the combination of Cu 2+ , Na + , Fe 3+ , Cl – , SO 4 2- , and PO 4 3- are CuCl 2 , CuSO 4 , NaCl, Na 2 SO 4 , FeCl 3 , and Fe 2 (SO 4 ) 3 .
Q4. Write the cations and anions present (if any) in the following compounds
(a) CH 3 COONa
(d) NH 4 NO 3
(a) The cation and anion present in CH 3 COONa are Na + and CH 3 COO – .
(b) The cation and anion present in NaCI are Na + and Cl – .
(c) There is no cation and anion in H 2 .
(d) The cation and anion present in NH 4 NO 3 are NH 4 + and NH 3 – .
Q5. Give the formulae of the compounds formed from the following sets of elements
(a) Calcium and fluorine
(b) Hydrogen and sulphur
(c) Nitrogen and hydrogen
(d) Carbon and chlorine
(e) Sodium and oxygen
(f) Carbon and oxygen
(a) The formulae of the compound formed by Calcium and fluorine is CaF 2 .
(b) The formulae of the compound formed by Hydrogen and sulphur is H 2 S.
(c) The formulae of the compound formed by Nitrogen and hydrogen is NH 3 .
(d) The formulae of the compound formed by Carbon and chlorine is CCl 4 .
(e) The formulae of the compound formed by Sodium and oxygen is Na 2 O.
(f) The formulae of the compound formed by Carbon and oxygen is CO 2 .
Q6. Which of the following symbols of elements are incorrect? Give their correct symbols
The formula for cobalt, carbon, aluminium, and sodium is incorrect, while the formula for helium is correct.
The correct formulas are enlisted below.
Q7. Give the chemical formulae for the following compounds and compute the ratio by mass of the combining elements in each one of them. (You may use appendix-III).
(a) Ammonia
(b) Carbon monoxide
(c) Hydrogen chloride
(d) Aluminium fluoride
(e) Magnesium sulphide
(a) The chemical formula of ammonia is NH 3, and its mass ratio is Mass of N: Mass of H = 14: 3.
(b) The chemical formula of Carbon monoxide is CO , and its mass ratio is Mass of C: Mass of O = 12: 16 = 3:4.
(c) The chemical formula of Hydrogen chloride is HCl , and its mass ratio is Mass of H: Mass of Cl = 1: 35.5.
(d) The chemical formula of Aluminium fluoride is AlF 3, and its mass ratio is Mass of Al: Mass of F = 27: 19.
(e) The chemical formula of Magnesium sulphide is MgS , and its mass ratio is Mass of Mg: Mass of S = 24: 32 = 3: 4.
Q8. State the number of atoms present in each of the following chemical species
(a) CO 3 2-
(b) PO 3 3-
(c) P 2 O 5
(a) There are four atoms in CO 3 2- .
(b) There are four atoms in PO 3 3- .
(c) There are seven atoms in P 2 O 5 .
(d) There are two atoms in CO.
Q9. What is the fraction of the mass of water due to neutrons?
The mass of one neutron = 1 amu.
The mass of one water molecule = 18 amu.
The oxygen atom has eight neutrons, while the hydrogen atom has 0 neutrons.
So the mass of neutrons in one water molecule is eight amu.
The fraction of mass of water due to neutrons = 8 / 18 = 4 / 9.
Q10. Does the solubility of a substance change with temperature? Explain with the help of an example.
Yes, the solubility of a substance changes with temperature. The solubility generally increases with an increase in temperature.
Example: You can dissolve more sugar in hot water than in cold water.
Q11. Classify each of the following based on their atomicity.
(d) C 2 H 6
(f) H 2 O 2
(g) P 4 O 10
Q12. You are provided with a fine white coloured powder, either sugar or salt. How would you identify it without tasting it?
We can differentiate sugar and salt by
(a) Heating salts separately. Sugar will melt while salt will not.
(b) Dissolving them separately in water. The salt solution will conduct electricity due to Na + ion and Cl – while the sugar solution will not conduct electricity. So, we can immediately tell the difference by testing a drop of the solution with an ohmmeter.
Q13. Calculate the number of moles of magnesium present in a magnesium ribbon weighing 12 g. The molar atomic mass of magnesium is 24g mol -1 .
Mass of magnesium ribbon = 12 g
Molar mass of magnesium = 24 g
Number of moles = Mass / Molar Mass
Number of moles = 12 / 24
Number of moles = 0.5 moles.
Hence, there are half moles of magnesium in a 12 g magnesium ribbon.
Long Answer Type Questions
Q1. Verify by calculating that
(a) 5 moles of CO 2 and 5 moles of H 2 O do not have the same mass.
(b) 240 g of calcium and 240 g of magnesium elements have a mole ratio of 3:5.
(a) The number of moles of CO 2 = 5 moles
Molar mass of CO 2 = 44 g mol -1.
Hence, the mass of 5 moles of CO 2 = the number of moles of CO 2 X molar mass of CO 2.
Hence, the mass of 5 moles of CO 2 = 5 X 44.
Hence, the mass of 5 moles of CO 2 = 220 g.
The number of moles of H 2 O = 5 moles
Molar mass of H 2 O = 18 gmol -1.
Hence, the mass of 5 moles of H 2 O = the number of moles of H 2 O X molar mass of H 2 O .
Hence, the mass of 5 moles of H 2 O = 5 X 18.
Hence, the mass of 5 moles of H 2 O = 90 g.
Thus, we can see the mass of 5 moles of CO 2 and 5 moles of H 2 O is not the same.
(b) Mass of calcium = 240 g
Molar mass of calcium = 40
Number of moles = 240 / 40
Number of moles = 6
Mass of magnesium = 240 g
Number of moles = 240 / 24
Number of moles = 10
Mole ratio of calcium and magnesium = the number of moles of calcium/number of moles of magnesium
Mole ratio of calcium and magnesium = 6 / 10
Mole ratio of calcium and magnesium = 3 / 5
Hence, the mole ratio of 240 g of calcium and 240 g of magnesium is 3:5.
Q2. Find the ratio by mass of the combining elements in the following compounds. (You may use Appendix-III)
(c) H 2 SO 4
(d) C 2 H 5 OH
(f) Ca(OH) 2
(a) The ratio of the mass of CaCO 3
Ca: C: O X 3
40: 12: (16 X 3)
Hence, the ratio of the mass of CaCO 3 is 10: 3: 12.
(b) The ratio of the mass of MgCl 2
24: 35.5 X 2
Hence, the ratio of the mass of MgCl 2 is 24:71.
(c) The ratio of the mass of H 2 SO 4
H X 2: S: O X 4
1 X 2: 32: 64
Hence, the ratio of the mass of H 2 SO 4 is 1: 16: 32.
(d) The ratio of the mass of C 2 H 5 OH
C X 2: H X 6: O
12 X 2: 1 X 6: 16
Hence, the ratio of the mass of C 2 H 5 OH is 12: 3: 8.
(e) The ratio of the mass of NH 3
Hence, the ratio of the mass of NH 3 is 14: 3.
(f) The ratio of the mass of Ca(OH) 2
Ca: O X 2: H X 2
40: 16 X 2: 1 X 2
Hence, the ratio of the mass of Ca(OH) 2 is 20: 16: 1.
Q3. When dissolved in water, calcium chloride dissociates into its ions according to the following equation.
CaCl 2 (aq) → Ca 2+ (aq) + 2 Cl – (aq)
Calculate the number of ions obtained from CaCl 2 when 222 g of it is dissolved in water.
1 mole of CaCl 2 gives 111 g of CaCl 2
∴ 222 g of CaCl 2 is equal to 2 moles of CaCl 2.
Since one formula unit, CaCl 2 gives three ions.
Therefore, 1 mol of CaCl 2 will give 3 moles of ions.
And, 2 moles of CaCl 2 would give 3 X 2 = 6 moles of ions.
The number of ions = the number of moles of ions × Avogadro number.
The number of ions = 6 X 6.022 X 10 23 .
The number of ions = 36.132 X 10 23 .
The number of ions = 3.6132 X 10 24 ions.
Q4. The difference in the mass of 100 moles of sodium atoms and sodium ions is 5.48002 g. Compute the mass of an electron.
Na → Na + + e −
A sodium atom and ion differs by one electron.
For 100 moles each of sodium atoms and ions, there would be a difference of 100 moles of electrons.
Mass of 100 moles of electrons = 5.48002 g
Mass of 1 mole of electron = 5.48002 / 100 g
Mass of one electron = 5.48002 / (100 X 6.022 X 10 23 )
Mass of one electron = 9.1 X 10 −28 g
Mass of one electron = 9.1 X 10 −31 kg
Hence, mass of one electron is equal to 9.1 X 10 −31 kg.
Q5. Cinnabar (HgS) is a prominent ore of mercury. How many grams of mercury are present in 225 g of pure HgS? The molar mass of Hg and S is 200.6 g mol -1 and 32 g mol -1 , respectively.
Molar mass of HgS = molar mass of Hg + molar mass of S
Molar mass of Hg = 200.6 g mol –1
Molar mass of S = 32 g mol –1
Molar mass of HgS = (200.6 + 32) g mol –1
Molar mass of HgS = 232.6 g mol –1
1 molecule of HgS contains one atom of Hg
232.6 g of HgS contains 200.6 g of Hg
225 g of HgS contains (200.6 X 225) / 232.6 of Hg
225 g of HgS contains 45135 / 232.6 of Hg
225 g of HgS contains 194.04g of Hg
Hence, 225 g of HgS contains 194.04g of Hg.
Q6. The mass of one steel screw is 4.11g. Find the mass of one mole of these steel screws. Compare this value with the mass of the Earth (5.98 × 10 24 kg). Which one of the two is heavier, and by how many times?
Mass of one steel screw= 4.11 g.
Thus, the mass of one mole of screw = 4.11 X N A = 4.11 X 6.022 X 10 23.
One mole of screws weighs 2.475 X 10 24 g = 2.475 X 10 21 kg
Ratio of mass of earth to the mass of screw = Mass of the earth / Mass of 1 mole of screws
Ratio of mass of earth to the mass of screw = 5.98 X 10 24 kg / 2.75 X 10 21 kg
Ratio of mass of earth to the mass of screw = 2.4 X 10 3
Hence, the mass of the earth is 2.4 X 10 3 times more than the mass of screw.
Or the earth is 2400 times heavier than a mole of the screw.
Q7. A sample of vitamin C is known to contain 2.58 x10 24 oxygen atoms. How many moles of oxygen atoms are present in the sample?
The number of oxygen atoms in the given sample = 2.58 X 10 24.
We know that 1 mole contains 6.022 X 10 23 oxygen atoms
2.58 X 10 24 oxygen atoms = 2.58 X 10 24 / 6.022 X 10 23
2.58 X 10 24 oxygen atoms = 4.28 moles.
Q8. Raunak took 5 moles of carbon atoms in a container, and Krish took 5 moles of sodium atoms in another container of the same weight. (a) Whose container is heavier? (b) Whose container has more number of atoms?
As both containers are of the same mass, the mass of atoms will decide which will be heavier.
Mass of 5 moles of sodium atoms in Krish’s container = (5 × 23) g = 115 g.
Mass of 5 moles of carbon atom in Raunak’s container =(5 × 12) g = 60 g.
Hence, Krish’s container is heavier.
Q9. Fill in the missing data in Table 3.1
Q10. The visible universe is estimated to contain 1022 stars. How many moles of stars are present in the visible universe?
1 mole of stars = 6.023 X 10 23.
Hence, the number of moles of stars = 10 22 / N A = 10 22 / 6.023 X 10 23 = 0.0166 moles.
Q11. What is the SI prefix for each of the following multiples and submultiples of a unit?
(a) 10 3 : Kilo
(b) 10 -1 : Deci
(c) 10 -2 : Centi
(d) 10 -6 : Micro
(e) 10 -9 : Nano
(f) 10 -12 : Pico
Q12. Express each of the following in kilograms
(a) 5.84 X 10 -3 mg
(b) 58.34 g
(c) 0.584 g
(d) 5.873 X 10 -21 g
(a) 5.84 X 10 –3 mg = 5.84 X 10 –9 kg
(b) 58.34 g = 5.834 X 10 –2 kg
(c) 0.584 g = 5.84 X 10 –4 kg
(d) 5.873 X 10 -21 g = 5.873 X 10 –24 kg
Q13. Compute the difference in masses of 10 3 moles each of magnesium atoms and magnesium ions. (Mass of an electron = 9.1 x 10 -31 kg)
Mg → Mg +2 + 2 e −
A Mg 2+ ion, and Mg atom differs by two electrons.
10 3 moles of Mg 2+ and Mg atoms would differ by 10 3 X 2 moles of electrons.
Mass of 2 X 10 3 moles of electrons = 2 X 10 3 × 6.023 × 10 23 × 9.1 × 10 −31 kg
Mass of 2 X 10 3 moles of electrons = 2 X 6.022 X 9.1 X 10 −5 kg
Mass of 2 X 10 3 moles of electrons = 109.6004 X 10 −5 kg
Mass of 2 X 10 3 moles of electrons = 1.096 X 10 −3 kg
Q14. Which has more number of atoms?
100g of N 2 or 100 g of NH 3.
(i) 100 g N 2 contains = 100 / 28 moles
Number of molecules = (100 X 6.022×10 23 ) / 28
Number of atoms = (100 X 6.022×10 23 X 2) / 28 = 43.01 X 10 23
(ii) 100g NH 3 contains = 100 / 17 moles
100g NH 3 contains = (100 X 6.022 X 10 23 ) / 17 molecules
100g NH 3 contains = (100 X 6.022 X 10 23 X 4) / 17 atoms
100g NH 3 contains = 141.69 X 10 23
Hence, NH 3 would have more atoms than N 2 .
Q15. Compute the number of ions present in 5.85 g of sodium chloride.
5.85 g of NaCl contains 5.85 / 58.5 moles of NaCl particle
5.85 g of NaCl contains 0.1 moles of NaCl particle.
Each NaCl particle contains one Na + and one Cl − ion.
Total moles of ions = 0.1 X 2 = 0.2 moles
No. of ions = 0.2 X 6.022 X 10 23 = 1.2042 X 10 23 ions.
Q16. A gold sample contains 90% of gold and the rest copper. How many atoms of gold are present in one gram of this sample of gold?
As the sample is 90% pure.
So one gram of sample will have 90 / 100 = 0.9 gm of Gold.
The number of moles of Gold = Mass of Gold / Atomic mass of gold.
The number of moles of Gold = 0.9 / 197.
The number of moles of Gold = 0.0046.
According to the mole concept,
Gold’s one mole contains NA atoms = 6.022 X 10 23 atoms.
Hence, 0.0046 moles of Gold will contain = 0.0046 X 6.022 X 10 23.
Hence, 0.0046 moles of Gold will contain = 2.77 X 10 21 atoms.
Q17. What are ionic and molecular compounds? Give examples.
Ionic compounds are those compounds that contain charged species. The charged species are called ions. An ion is a charged particle and can be positively or negatively charged. A positively charged ion is a cation, while a negatively charged ion is an anion. The transfer of electrons forms ionic compounds. Example: sodium chloride and calcium oxide.
Molecular compounds are formed by sharing electrons through covalent bonds. Example: water, ammonia, carbon dioxide.
Q18. Compute the difference in masses of one mole of aluminium atoms and one mole of its ions. (Mass of an electron is 9.1 × 10 -28 g). Which one is heavier?
Mass of 1 mole of aluminium atom = Molar mass of aluminium = 27 g mole −1
An aluminium atom loses three electrons to form an Al 3+ ion.
For one mole of Al 3+ ion, three moles of electrons will be lost.
Hence, the mass of three moles of electrons = 3 X (9.1 X 10 −28 ) X 6.022 X 10 23 g.
Mass of three moles of electrons = 27.3 X 10 −28 X 6.022 X 10 23 g.
Mass of three moles of electrons = 164.400 X 10 −5 g
Mass of three moles of electrons = 0.00164 g
Hence, the molar mass of Al +3 = (27 − 0.00164) g mol − = 26.998 g mol −
Difference in mass = 27 – 26.9984 = 0.0016 g
Hence, aluminium atom is 0.0016 g heavier than aluminium ions.
Q19. A silver ornament of mass ‘m’ gram is polished with gold equivalent to 1% of the mass of silver. Compute the ratio of the number of atoms of gold and silver in the ornament.
Mass of silver = m g
Mass of gold = m / 100 g
Number of atoms of Ag = m / (108 × Na)
Number of atoms of Au = m / (100 × 197 × Na)
Au: Ag ≡ m / (100 × 197 × Na) : m / (108 × Na)
= 108: 100 X 197
= 108: 19700
Q20. The ethane (C 2 H 6 ) gas sample has the same mass as 1.5 x10 20 molecules of methane (CH 4 ). How many C 2 H 6 molecules does the sample of gas contain?
Mass of 1 molecule of CH 4 = 16 / Na g
Mass of 1.5 X 10 20 molecules of methane = (1.5 X 10 20 X 16) / Na
Mass of x molecules of C 2 H 6 is = (1.5 X 10 20 X 16) / Na
We know that mass of 1 molecule of C 2 H 6 = 30 / Na g
Hence, the number of molecules of ethane (x) = (1.5 X 10 20 X 16 X Na) / (30 X Na)
Number of molecules of ethane (x) = 0.8 X 10 20 .
Q21. Fill in the blanks
(a) In a chemical reaction, the sum of the masses of the reactants and products remains unchanged. This is called ______.
(b) A group of atoms carrying a fixed charge on them is called ______
(c) The formula unit mass of Cag(PO4)2 is _____
(d) Formula of sodium carbonate is ______, and that of ammonium sulphate is ______
(a) In a chemical reaction, the sum of the masses of the reactants and products remains unchanged. This is called the Law of conservation of mass.
(b) A group of atoms carrying a fixed charge on them is called a polyatomic ion.
(c) The formula unit mass of Ca 3 (PO 4 ) 2 is 310 g.
(d) Formula of sodium carbonate is Na 2 CO 3 , and that of ammonium sulphate is (NH 4 ) 2 SO 4 .
Q22. Complete the following crossword puzzle (Fig. 3.1) by using the name of the chemical elements. Use the data given in Table 3.2.

Q23. (a) In this crossword puzzle (Fig 3.2), the names of 11 elements are hidden. Symbols of these are given below. Complete the puzzle.

(a) 1. CI: Chlorine
2. H: Hydrogen
3. Ar: Argon
4. O: Oxygen
5. Xe: Xenon
6. N: Nitrogen
7. He: Helium
8. F: Fluorine
9. Kr: Krypton
10. Rn: Radon
11. Ne: Neon
(b) There are five inert gases in this crossword puzzle. Their names and symbols are Argon (Ar), Xenon (Xe), Helium (He), Krypton (Kr) and Radon (Rn).
Q24. Write the formulae for the following and calculate the molecular mass for each of them.
(a) Caustic potash
(b) Baking powder
(c) Limestone
(d) Caustic soda
(e) Ethanol
(f) Common salt
(a) The formulae of Caustic potash is KOH.
The molecular mass of Caustic potash is 39 + 16 + 1 = 56 u.
(b) The formulae of Baking powder is NaHCO 3 .
The molecular mass of Baking powder is 23 + 1 + 12 + 3 X 16 = 84 u.
(c) The formulae of Limestone is CaCO 3 .
The molecular mass of Limestone is 40 + 12 + 3 X 16 = 100 u.
(d) The formulae of Caustic soda is NaOH.
The molecular mass of Caustic soda is 23 + 16 + 1 = 40 u.
(e) The formulae of Ethanol is C 2 H 5 OH.
The molecular mass of 2 × 2 + 5 X 1 + 16 + 1 = 46 u.
(f) The formulae of Common salt is NaCl.
The molecular mass of Common salt is 23 + 35.5 = 58.5 u.
Q25. In photosynthesis, six molecules of carbon dioxide combine with an equal number of water molecules through a complex series of reactions to give a molecule of glucose having a molecular formula C 6 H 12 O 6 . How many grams of water would produce 18 g of glucose? Compute the volume of water consumed, assuming the density of water to be 1g cm -3 .
6 CO 2 + 6 H 2 O → C 6 H 12 O 6 + 6 O 2
1 mole of glucose requires 6 moles of water.
180 g of glucose requires 108 g of water.
1 g of glucose will require 108 / 180 g of water.
18 g of glucose would need (108 X 18) / 180 g of water
18 g of glucose would need = 10.8 g of water
Volume of water used = Mass / Density = 10.8 / 1 = 10.8 cm 3 .
CBSE Class 9 Science Chapter 3 Extra Questions
Q1. What is the compound Al 2 (SO4) 3 , and give the ions present in it?
The given compound is aluminium sulphate.
It contains two ions: Al 3+ and SO 4 2− .
Q2. What is an atomic mass unit’? How is it linked with relative atomic mass?
Atomic mass unit is defined as the 1 / 12 of the mass of carbon – 12 atom C – 12.
The relative atomic mass of an element is the number of times one atom of the element is heavier than the 1 / 12 times the mass of an atom of carbon-12.
Q3. What is Avogadro’s number? Why is it also known as Avogadro’s constant?
Avogadro’s number is the number of particles (atoms, molecules or ions) present in one mole of any substance. It is known as Avogadro’s constant because its value is fixed (6.022 x 10 23 ) irrespective of the nature of the particles.
Q4. Which are the six postulates of Dalton’s atomic theory?
Postulates of Dalton’s atomic theory
1. The matter is composed of indivisible particles called atoms.
2. Atoms of the same element are similar in shape and mass but differ from the atoms of different elements.
3. Atoms can neither be created nor be destroyed.
4. Atoms of different elements combine in fixed, simple, whole-number ratios to form a compound.
5. Atoms of the same element can combine in more than one ratio to form two or more compounds.
6. The atom is the tiniest unit of matter that can take part in a chemical reaction.
Atoms and Molecules – One Shot (Concepts+Questions)

Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Request OTP on Voice Call
Post My Comment
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.
Scholarships
Test Series
Study Materials
- Class 10 Science
- Class 9 Science
- Class 7 Maths
- Class 6 Maths Science
- Class 10 Maths PYPs Science PYPs English PYPs
- Class 12 Maths PYPs Physics PYPs
CBSE Class 9 Notes Science Chapter 3 - Atoms And Molecules
Ancient Indian philosopher Maharishi Kanad proposed that matter is divisible until the tiniest particles, called "Parmanu." Similarly, Greek philosopher Democritus introduced the concept of indivisible particles, "atoms." These ideas were philosophical until the 18th century when scientists began distinguishing elements from compounds. Antoine Lavoisier established the foundation of modern chemistry with his laws of chemical combination.
- 1.0 Laws of Chemical Combination
- Law of conservation of mass : Mass can neither be created nor destroyed in a chemical reaction.
- Law of Definite Proportions : Elements are always present in fixed proportions by mass in a chemical compound.
- 2.0 Dalton’s Atomic Theory Postulates
- All matter is made of tiny particles called atoms and are indivisible.
- Atoms of all elements can neither be created nor destroyed in chemical reactions.
- Atoms of an element are the same in mass and properties.
- Atoms of different elements possess different masses and different properties.
- Atoms combine in small whole-number ratios to form compounds.
- The number and types of atoms in a compound are constant.
Drawbacks of Dalton's Atomic Theory
- Atoms are divisible into subatomic particles (protons, neutrons, electrons).
- Dalton’s theory doesn't explain isotopes, atoms of the same element with different masses.
- 3.0 Elements and Symbols
Dalton introduced a system of notation to represent elements, while Berzelius proposed the symbols used today.
- Berzelius' Symbols of Elements
Element Symbols:
- Single Letters: Elements like Carbon (C), Boron (B), and Oxygen (O) use their first letter.
- Two Letters: Elements like Aluminium (Al) and Chlorine (Cl) use the first letter and another in lowercase.
- Latin Names: Symbols like Iron (Fe) from ferrum and Sodium (Na) from natrium derive from Latin.
Atomic Mass:
- Atomic mass is the mass of an atom of an element.
- The relative atomic mass is how many times an atom of an element is heavier than 1/12th of the mass of a carbon-12 atom.
- A molecule is the smallest particle of an element or compound that can exist independently.
- Examples: H₂O (water), O₂ (oxygen), O₃ (ozone).
- A single hydrogen atom (H) is not a molecule. When atoms bond, molecules such as H 2 (hydrogen gas) or H 2 O (water) form.
The number of atoms constituting a molecule is referred to as atomicity.
A substance formed when two or more elements chemically combine. A compound has a fixed ratio of elements and distinct properties. Examples include Water (H₂O), Glucose (C₆H₁₂O₆), Calcium oxide (CaO), and Sodium chloride (NaCl).
- 4.0 Differences Between a Molecule and a Compound
- A molecule is formed when two or more atoms bond chemically and can consist of the same or different elements. While all compounds are molecules, not all molecules are compounds. Example: Molecular hydrogen (H₂) is a molecule, not a compound. It consists of two hydrogen atoms.
- Compound : A molecule of two or more different elements bonded chemically. Example: Water (H₂O) is a compound of hydrogen and oxygen atoms.
A charged particle is formed when an atom or molecule gains or loses electrons.
- Cation : A positively charged ion. Examples: Na⁺, Ca²⁺.
- Anion : A negatively charged ion. Examples: F⁻, Cl⁻.
- 6.0 Chemical Formula
A chemical formula depicts the composition of a compound by using element symbols along with their corresponding valencies. Here’s a brief overview:
- Symbols of Elements : Represent the elements in a compound.
- Valency : The combining capacity of an element, showing how atoms mix with others. For example, hydrogen (H⁺) has a valency of 1, and oxygen (O²⁻) has a valency of 2.
Rules for Writing Chemical Formulas:
- Balance Valencies : Ensure that the total positive and negative charges are equal.
- Order of Elements : Write the metal before the non-metal. For example, Sodium Chloride is NaCl.
- Polyatomic Ions : If more than one polyatomic ion is present, place them in brackets. For example, calcium nitrate is written as Ca(NO₃)₂.
Writing Formulas for Simple Compounds (Binary Compounds):
- Write the symbols of the elements.
- Note the valencies of each component.
- Crossover the valencies to balance the formula.
The formula for aluminium oxide:
Symbol Al O
↘↙
Valency 3 2
Formula Al 2 O 3
- 7.0 Molecular Mass
- Definition : The total of the atomic masses of all atoms in a molecule is called the molecular mass .
- Calculation : Multiply the atomic mass of each element by the number of its atoms in the molecule, then add these values.
Formula Unit Mass
- The sum of all atoms' atomic masses in an ionic compound's formula unit.
- Calculation : Add the atomic masses of the elements in the formula unit. For example, for Sodium Chloride (NaCl):
(1×23) + (1×35.5) = 58.5 u
- 8.0 Mole Concept
- Mole : A unit representing 6.022 × 10²³ entities (atoms, molecules, ions).
- Avogadro’s Number : 6.022 × 10²³, used to calculate the number of particles in one mole of a substance.
9.0 Solved examples
1. Convert into mole. (a) 12 g of oxygen gas, (b) 20 g of water, (c) 22 g of carbon dioxide.
Solution: To convert grams to moles, use the formula:
Moles = Mass (g) / Molar Mass (g/mol)
(a) 12 g of Oxygen Gas (O₂)
- Molar Mass of O 2 : 2×16 g/mol=32 g/mol
- Moles of O 2 : 12 g / 32 g/mol=0.375 moles
(b) 20 g of Water (H₂O)
- Molar Mass of H 2 O : (2×1)+16=18 g / mol
- Moles of H 2 O : 20 g / 18 g/mol ≈ 1.11 moles
(c) 22 g of Carbon Dioxide (CO₂)
- Molar Mass of CO 2 : 12+(2×16)=44 g / mol
- Moles of CO 2 : 22 g / 44 g/mol=0.5 moles
2. Calculate the molar mass of the following substances. (a) Ethyne, C 2 H 2 (b) Sulphur molecule, S 8 (c) Phosphorus molecule, P 4 (Atomic mass of phosphorus = 31) (d) Hydrochloric acid, HCl (e) Nitric acid, HNO 3
Solution:
To find the molar mass of a substance, the atomic masses of all the atoms present in one mole of the compound are added.
(a) Ethyne (C 2 H 2 )
- Molar Mass :
(2 × mass of C atom ) + (2 × mass of H atom)
=(2×12) + (2×1) = 24 + 2 = 26 g/mol
(b) Sulphur Molecule (S 8 )
- Molar Mass : 8 × Atomic mass of S 8
=8 × 32 = 256 g/mol
(c) Phosphorus Molecule (P 4 )
- Molar Mass : 4 × Atomic mass of P 4
=4 × 31 = 124 g/mol
(d) Hydrochloric Acid (HCl)
- Molar Mass : Atomic mass of H +Atomic mass of Cl
=1 + 35.5 = 36.5 g/mol
(e) Nitric Acid (HNO₃)
- Molar Mass : (Atomic mass of H) + (Atomic mass of N) +(3×Atomic mass of O)
=1+14 +(3×16) =1+14+48=63 g/mol
Table of Contents
- 2.1 Drawbacks of Dalton's Atomic Theory
- 7.1 Formula Unit Mass
- 9.0 Solved examples
Join ALLEN!
(Session 2024 - 25)
CBSE Case Study Questions for Class 9 Science - Pdf PDF Download
Cbse case study questions for class 9 science.
Case based questions for Class 9 Science involve exploring a real-world situation through scientific analysis and inquiry. These questions allow students to make connections between science concepts and the world around them, as well as develop critical thinking skills. For example, a case study may involve challenging a student to determine the cause of an illness in a local population by researching the disease, its symptoms, and the local environment. Through this exercise, students learn how to identify a problem, break it down into parts, and come up with a solution that is supported by evidence. This type of question helps students to understand how science is at the centre of solving real-world problems.
Chapter Wise Case Based Questions for Class 9 Science
Chapter-wise case-based questions for Class 9 Science are a set of questions based on specific chapters or topics covered in the science textbook. These questions are designed to help students apply their understanding of scientific concepts to real-world situations and events.
The CBSE Class 9 Case Based Questions can be accessed from Chapetrwise Links provided below:
Chapter 1: Matter In Our Surroundings
Chapter 2: is matter around us pure.
- Case Based Questions: Is Matter Around Us Pure?
Chapter 3: Atoms And Molecules
- Case Based Questions: Atoms And Molecules
Chapter 4: Structure Of The Atom
- Case Based Questions: Structure Of The Atom
Chapter 5: The Fundamental Unit Of Life
- Case Based Questions: The Fundamental Unit Of Life- 1
- Case Based Questions: The Fundamental Unit Of Life- 2
Chapter 6: Tissues
- Case Based Questions: Tissues- 1
- Case Based Questions: Tissues- 2
Chapter 7: Motion
- Case Based Questions: Motion-1
- Case Based Questions: Motion- 2
Chapter 8: Force And Laws Of Motion
- Case Based Questions: Force And Laws Of Motion
Chapter 9: Gravitation
- Case Based Questions: Gravitation
Chapter 10: Work And Energy
- Case Based Questions: Work And Energy- 1
- Case Based Questions: Work And Energy- 2
Chapter 11: Diversity In Living Organisms
Chapter 12: sound, chapter 13: natural resources, chapter 14: improvement in food resource, chapter 15: why do we fall ill.
- Case Based Questions: Why Do We Fall Ill?
Weightage of Case Based Questions in Class 9 Science
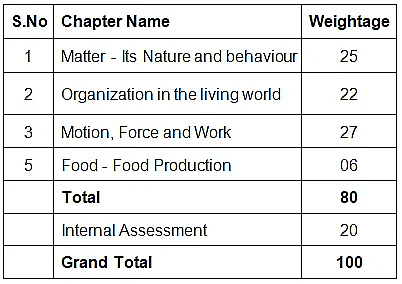
Why are Case Study Questions important in Science Class 9?
- Enhance critical thinking: Case study questions require students to analyze a real-life scenario and think critically to identify the problem and come up with possible solutions. This enhances their critical thinking and problem-solving skills.
- Apply theoretical concepts: Case study questions allow students to apply theoretical concepts that they have learned in the classroom to real-life situations. This helps them to understand the practical application of the concepts and reinforces their learning.
- Develop decision-making skills: Case study questions challenge students to make decisions based on the information provided in the scenario. This helps them to develop their decision-making skills and learn how to make informed decisions.
- Improve communication skills: Case study questions often require students to present their findings and recommendations in written or oral form. This helps them to improve their communication skills and learn how to present their ideas effectively.
- Enhance teamwork skills: Case study questions can also be done in groups, which helps students to develop teamwork skills and learn how to work collaboratively to solve problems.
In summary, case study questions are important in Class 9 because they enhance critical thinking, apply theoretical concepts, develop decision-making skills, improve communication skills, and enhance teamwork skills. They provide a practical and engaging way for students to learn and apply their knowledge and skills to real-life situations.
Class 9 Science Curriculum at Glance
The Class 9 Science curriculum in India covers a wide range of topics and concepts. Here is a brief overview of the Science curriculum at a glance:
- Physics: The Physics section includes topics such as motion, force, work and energy, sound, and light.
- Chemistry: The Chemistry section includes topics such as matter, atoms and molecules, structure of the atom, and chemical reactions.
- Biology: The Biology section includes topics such as cell structure and functions, tissues, diversity in living organisms, natural resources, and environmental management.
- Practical Work: The Science curriculum also includes practical work, where students perform experiments to observe and understand scientific phenomena.
The Class 9 Science curriculum is designed to provide a strong foundation in science and prepare students for higher education in the field. The curriculum is structured to develop critical thinking, problem-solving, and analytical skills, and to promote the application of scientific concepts in real-life situations. The curriculum is also designed to help students prepare for competitive exams and develop a strong scientific base for future academic and professional pursuits.
Students can also access Case Based Questions of all subjects of CBSE Class 9
- Case Based Questions for Class 9 Maths
- Case Based Questions for Class 9 Social Science
- Case Based Questions for Class 9 English
- Case Based Questions for Class 9 Hindi
- Case Based Questions for Class 9 Sanskrit
Frequently Asked Questions (FAQs) on Case Based Questions for Class 9 Science
Are case-based questions on the class 9 science exam.
Yes, case-based questions are often included in science exams at the class 9 level as they test students' ability to apply their scientific knowledge and skills to real-world situations.
How are case-based questions different from traditional science questions?
Traditional science questions typically focus on testing students' knowledge of specific facts, concepts, and theories. Case-based questions, on the other hand, require students to use their knowledge and understanding to analyze and interpret real-world situations and make informed decisions.
How can students prepare for case-based questions in science?
To prepare for case-based questions in science, students should practice analyzing data and interpreting scientific experiments. They should also work on developing their critical thinking and problem-solving skills.
Top Courses for Class 9
Faqs on cbse case study questions for class 9 science - pdf, study material, video lectures, shortcuts and tricks, semester notes, extra questions, past year papers, cbse case study questions for class 9 science - pdf, practice quizzes, previous year questions with solutions, sample paper, viva questions, important questions, objective type questions, mock tests for examination.

CBSE Case Study Questions for Class 9 Science - Pdf Free PDF Download
Importance of cbse case study questions for class 9 science - pdf, cbse case study questions for class 9 science - pdf notes, cbse case study questions for class 9 science - pdf class 9, study cbse case study questions for class 9 science - pdf on the app, welcome back, create your account for free.

Forgot Password
Change country.
- CBSE Class 9 Study Material
CBSE Class 9 Science Important Case Study Questions with Answers for Term 2 Exam 2022 (PDF)
Check important case study questions of cbse class 9 science to prepare for the cbse term 2 exam 2022. all these questions have been put together by subject experts..

CBSE Class 9 Term 2 Exam 2022: Important case based questions for CBSE Class 9 Science are provided here students to prepare for the upcoming Term 2 Exam 2022. All the questions provided below are curated by the subject experts. These questions are really helpful to revise important concepts and prepare the case study questions for the exam. Answers to all questions have been provided for reference. So, students should practice the chapter-wise questions to clearly understand the right way to attempt the case based questions. Download the chapter-wise questions in PDF.
Check some of the important case study questions below:
Q. Read the following and answer the questions :
A student was asked by his teacher to verify the law of conservation of mass in the laboratory. He prepared 5% aqueous solutions of NaCl and Na 2 SO 4 . He mixed 10 mL of both these solutions in a conical flask. He weighed the flask on a balance. He then stirred the flask with a rod and weighed it after sometime. There was no change in mass.
- Was the student able to verify the law of conservation of mass?
- If not, what was the mistake committed by him?
- In your opinion, what he should have done?
- What is the molar mass of Na 2 SO 4 ?
- No, he could not verify the law of conservation of mass in-spite of the fact that there was no change in mass.
- No chemical reaction takes place between NaCl and Na 2 SO 4 . This means that no reaction actually took place in the flask.
- He should have performed the experiment by using aqueous solutions of BaCl 2 and Na 2 SO 4 . A chemical reaction takes place in this case and a white precipitate of BaSO 4 is formed.
- Will the weight of the precipitate be the same as that of the reactants before mixing?
- If not, what she should have done?
- Which law of chemical combination does this support?
- State the law of conservation of mass.
- No, it will not be the same.
- She should have weighed the total contents of the beaker after the reaction and not the precipitate alone.
- It supports the law of conservation of mass.
- Mass can neither be created nor destroyed during a chemical reaction.
Get here latest School , CBSE and Govt Jobs notification and articles in English and Hindi for Sarkari Naukari , Sarkari Result and Exam Preparation . Download the Jagran Josh Sarkari Naukri App .
- Territorial Army Recruitment 2024
- RSMSSB Rajasthan CET Answer Key 2024
- ADRE Grade 4 Admit Card 2024
- MLSU Result 2024
- RSMSSB CET Admit Card 2024
- RSMSSB CET Exam Analysis 2024
- Rajasthan CET Question Paper 2024
- OSSC CGL Admit Card 2024
- RSMSSB Exam Calendar 2024
- Star Sighting Time Today
- Education News
- CBSE Study Material
- CBSE Class 9
Latest Education News
Digital Gujarat Scholarship 2024-25: Direct Link to Apply for Pre-Matric & Post-Matric Scholarship, Check Eligibility
World’s Best School Prizes 2024: Three Indian Schools Top Ranked for Innovation and Community Impact
BSEB Bihar Sent-Up Exam 2024 Date Sheet Released for Class 10, 12, Check Schedule Here
Yogi Adityanath: UP Gov to Ensure 24/7 Power Supply During Festive Season
ITBP Telecommunication Recruitment 2024 Notification: Registration Begins Soon for 526 SI, HC, Constable Posts
SSC CGL Result 2024 Date: Check Expected Date and Other Details
8th Pay Commission: 18 हजार की जगह कितनी होगी मिनिमम बेसिक सैलरी और कितनी बढ़ेगी पेंशन? जानें
ट्रेन छूट जाने पर उसी टिकट पर दूसरी ट्रेन से कैसे करें यात्रा? जानें यहां
Diwali 2024 Holiday: यूपी, बिहार और राजस्थान में दिवाली की कितनी छुट्टी? यहां देखें लिस्ट
[OUT] VMOU Result 2024: यहां देखें MA, BA, BCom, BSW, MBA, MSc, BCA, BEd और अन्य सभी यूजी पीजी डिप्लोमा मार्कशीट
RSMSSB CET Question Paper 2024: Download Rajasthan 12th Level Shift 1 and 2 Unofficial Answer Key PDF
ADRE Grade 4 Admit Card 2024 Out Live Updates: Direct Link to Download Hall Ticket at slrcg4.sebaonline.org, Check Updates
CLAT 2025 Correction Window Closing Today - Check Details Here
Kerala PSC Exam Calendar 2024-25 Released at keralapsc.gov.in: Download Month Wise Schedule Here
AIBE 19 Exam Dates Revised, Check Extended Registration Schedule Here
List of all Chief Justices of India (1950-2024)
Optical Illusion IQ Test: Only 1% Sharpest Eyes Can Find the Mouse Hidden Among Mushrooms In 5 Seconds!
Who is Justice Sanjiv Khanna? The Next CJI to be appointed on November 11th!
CGPSC Recruitment 2024: Apply Online for 341 SI and Subedar Vacancies
RPSC EO RO Exam 2022 Cancel: राजस्थान की ईओ, आरओ की बड़ी भर्ती परीक्षा नकल के कारण रद्द

Class 9 Science Case Study Questions PDF Download
- Post author: studyrate
- Post published:
- Post category: class 9th
- Post comments: 0 Comments
Class 9 Science Case Study Questions play a crucial role in the field of science education as they provide real-life scenarios for students to analyze, apply their knowledge, and develop problem-solving skills. This article aims to present a comprehensive collection of case study questions for Class 9 Science , covering various topics and concepts.
Join our Telegram Channel, there you will get various e-books for CBSE 2024 Boards exams for Class 9th, 10th, 11th, and 12th.

CBSE Class 9 Science Exam will have a set of questions based on case studies in the form of MCQs. The CBSE Class 9 Science Question Bank on Case Studies, provided in this article, can be very helpful to understand the new format of questions. Share this link with your friends.
If you want to want to prepare all the tough, tricky & difficult questions for your upcoming exams, this is where you should hang out. CBSE Case Study Questions for Class 9 will provide you with detailed, latest, comprehensive & confidence-inspiring solutions to the maximum number of Case Study Questions covering all the topics from your NCERT Text Books !
Table of Contents
CBSE Class 9th SCIENCE Chapterwise Case Study Question & Solution
Case study questions provide students with real-life scenarios that require critical thinking and application of scientific concepts. They help students understand the practical application of scientific principles and develop problem-solving skills in various scientific disciplines.
Chapterwise Case Study Questions for Class 9 Science
Inboard exams, students will find the questions based on assertion and reasoning. Also, there will be a few questions based on case studies. In that, a paragraph will be given, and then the MCQ questions based on it will be asked. For Science subjects, there would be 5 case-based sub-part questions, wherein a student has to attempt 4 sub-part questions.
- Case Study Questions for Chapter 1 Matter in Our Surroundings
- Case Study Questions for Chapter 2 Is Matter Around Us Pure?
- Case Study Questions for Chapter 3 Atoms and Molecules
- Case Study Questions for Chapter 4 Structure of Atom
- Case Study Questions for Chapter 5 The Fundamental Unit of Life
- Case Study Questions for Chapter 6 Tissues
- Case Study Questions for Chapter 7 Diversity in Living Organisms
- Case Study Questions for Chapter 8 Motion
- Case Study Questions for Chapter 9 Force and Laws of Motion
- Case Study Questions for Chapter 10 Gravitation
- Case Study Questions for Chapter 11 Work and Energy
- Case Study Questions for Chapter 12 Sound
- Case Study Questions for Chapter 13 Why do we Fall ill
- Case Study Questions for Chapter 14 Natural Resources
- Case Study Questions for Chapter 15 Improvement in Food Resources
The above Case studies for Class 9 Science will help you to boost your scores as Case Study questions have been coming in your examinations. These CBSE Class 9 Science Case Studies have been developed by experienced teachers of schools.studyrate.in for the benefit of Class 10 students.
Class 9 Maths Case Study Questions
Benefits of Case Studies in Science Education
Case studies offer several advantages over traditional teaching methods. Here are some key benefits:
- Real-World Application : Case studies present authentic scenarios, enabling students to understand how scientific concepts are applied in real-life situations.
- Critical Thinking : Analyzing case studies requires students to think critically, make connections, and apply scientific knowledge to solve problems.
- Interdisciplinary Approach : Case studies often involve multiple scientific disciplines, fostering an interdisciplinary understanding of complex issues.
- Engagement and Active Learning : Case studies actively engage students in the learning process, promoting active participation, discussion, and collaboration.
- Skill Development : Case studies develop essential skills such as analytical thinking, problem-solving, and effective communication of scientific concepts.
Importance of Practicing Case Study Questions
Practicing case study questions is crucial for Class 9 Science students to enhance their understanding and application of scientific concepts. Here’s why it is important:
- Application of Knowledge : Case studies allow students to apply their theoretical knowledge to practical situations, bridging the gap between theory and real-world scenarios.
- Developing Analytical Skills : Analyzing case studies improves students’ ability to identify relevant information, make connections, and draw logical conclusions.
- Problem-Solving Skills : Case studies present complex problems that require students to think critically and develop effective problem-solving strategies.
- Enhanced Exam Performance : Practicing case study questions familiarizes students with the format and types of questions they may encounter in exams, leading to improved performance.
Subjects Covered in the Case Study Questions for Class 9 Science
The case study questions for Class 9 Science cover the following subjects:
- Motion and Forces
- Light and Reflection
- Electricity
- Matter and Its Properties
- Atoms and Molecules
- Structure of the Atom
- Chemical Reactions
- Cell: The Fundamental Unit of Life
- Diversity in Living Organisms
- Natural Resources
Tips for Approaching Case Study Questions
To tackle case study questions effectively, consider the following tips:
- Read Carefully : Pay close attention to the details provided in the case study, as they hold crucial information for solving the problem.
- Analyze Methodically : Break down the problem into smaller components and analyze each part systematically.
- Apply Relevant Concepts : Identify the scientific principles relevant to the case study and apply them appropriately.
- Consider Multiple Perspectives : Explore different angles and viewpoints while proposing solutions, taking into account various scientific factors.
- Provide Justifications : Support your answers with scientific explanations and logical reasoning to strengthen your responses.
The Class 9 Science Case Study Questions provided in this article serve as a valuable resource for students seeking to enhance their scientific knowledge and problem-solving skills. By practicing these case studies, students can develop a deeper understanding of scientific concepts and their practical applications. Embrace this opportunity to engage with real-world scenarios and strengthen your scientific acumen.
Q1: Are the Class 9 Science Case Study Questions aligned with the official curriculum?
Yes, the Class 9 Science Case Study Questions presented in this article are aligned with the official curriculum. They cover relevant topics and concepts that students need to study for their exams.
Q2: Can practicing case study questions alone guarantee success in Class 9 Science exams?
Practicing case study questions is an important part of exam preparation, but it should be complemented with a thorough understanding of the subject matter. It is advisable to study the concepts in detail, refer to textbooks, and engage in other learning activities to achieve success in exams.
Q3: Where I Can get Class 9 Science Case Study Questions ?
You can practice Class 9 Science Case Study Questions on schools.studyrate.in for free.
You Might Also Like
Class 9 mcq questions for chapter 9 force and laws of motion with answers, class 9 science case study questions chapter 3 atoms and molecules, class 9 science mcq questions for chapter 14 natural resources with answers, leave a reply cancel reply.
Save my name, email, and website in this browser for the next time I comment.

IMAGES
VIDEO
COMMENTS
Here, we have provided case-based/passage-based questions for Class 9 Science Chapter 3 Atoms and Molecules. Case Study/Passage-Based Questions. Case Study 1: The knowledge of the valencies of various radicals helps us to write the formulae of chemical compounds. The total positive charge on positive ions (cations) is equal to the total ...
The formula of the sulphate of an element X is X2(SO4)3. The formula of nitride of element X will be. The formula of a compound is X3Y. The valencies of elements X and Y will be respectively. Case Study/Passage Based Questions. A mole of an atom is a collection of atoms whose total mass is the number of grams equal to the atomic mass.
Case Study Questions for Class 9 Science Chapter 3 Atoms and Molecules. In CBSE Class 9 Science Paper, Students will have to answer some questions based on Assertion and Reason. There will be a few questions based on case studies and passage based as well. In that, a paragraph will be given, and then questions based on it will be asked.
by experts. Download PDF Case Study Questions of Class 9 Science to prepare for the upcoming CBSE Class 9 Exams Exam 2023-24. With the help of our well-trained and experienced faculty, we provide solved examples and detailed explanations for the recently added Class 9 Science case study questions. Case study questions are based on real or ...
Q2: Calculate the molar mass of Iron (II) Sulphate. [Fe = 56 u, S = 32 u, O = 16 u]Ans: Molar mass of FeSO 4 = 56 + 32 + (16 × 4) = 152 g mol -1. Q3: Write name of (NH4)2SO4Ans: Ammonium sulphate. Q4: Give one example of polyatomic anion.Ans: CO 32- (Carbonate). The document Class 9 Science Chapter 3 Case Based Questions - Atoms and ...
Class 9 science case study question 1. Gases are highly compressible as compared to solids and liquids. The liquefied petroleum gas (LPG) cylinder that we get in our home for cooking or the oxygen supplied to hospitals in cylinders is compressed gas. Compressed natural gas (CNG) is used as fuel these days in vehicles.
Statement 1 - Positively charged centre in an atom called the nucleus. Statement 2 - The electrons revolve around the nucleus in circular paths. Statement 3 - Nearly all the mass of an atom resides in the nucleus. Statement 4 - The size of the nucleus is very small as compared to the size of the atom. (a) Only 2.
Here we are providing assertion reason questions for class 9 science. This article covers assertion reason questions based on Class 9 Science Chapter 3 Atoms and Molecules. To check the answer, click on 'answer' given below each question. After clicking it will expand. Assertion and Reason Questions for Class 9 Science Chapter 3 Atoms and … Continue reading Assertion and Reason Questions ...
Atoms. • Atoms are building blocks of all matter. • According to modern atomic theory, an atom is the smallest particle of an element which takes part in chemical reaction. • Atoms are very small and which can't be seen even through very powerful microscope. • Atomic radius is measured in nanometres.
Chapter-wise NCERT Solutions for Class 9 Science Chapter 3 Atoms and Molecules (Chemistry) solved by Expert Teachers as per NCERT (CBSE) Book guidelines. CBSE Class 9 Chemistry Chapter 3 Atoms and Molecules Exercise Questions with Solutions to help you to revise complete Syllabus and Score More marks.
Class 9 Science Chapter 3 Textbook Questions and Answers. Question 1: In a reaction 5.3 g of sodium carbonate reacted with 6 g of ethanoic acid. The products were 2.2 g of carbon dioxide, 0.9g water and 8.2 g of sodium ethanoate. Show that these observations are in agreement with the law of conservation of mass.
Answer. (a) Mass of one mole of oxygen atoms = 16 g. Then, mass of 0.2 mole of oxygen atoms = 0.2 × 16g = 3.2 g. (b) Mass of one mole of water molecule = 18 g. Then, mass of 0.5 mole of water molecules = 0.5 × 18 g = 9 g. 10. Calculate the number of molecules of sulphur (S8) present in 16 g of solid sulphur.
Significance of the mole. 1. A mole gives the number of entities present i.e, 6.022 × 10 23 particles of the substance. 2. Mass of 1 mole is expressed as M grams. 3. Mass of 1 mole = mass of 6.022 × 10 23 atoms of the element. Extra questions for Class 9 Science Chapter 3 Atoms and Molecules with answers is given below.
By practising these Class 9 important questions, students will be able to quickly review all of the ideas covered in the chapter and prepare for the Class 9 Annual examinations. Download Class 9 Chemistry Chapter 3 Atoms and Molecules Important Questions with Answers PDF by clicking on the button below. Download PDF. Recommended Video
Study Materials. Talk to us. Login. Talk to us CBSE Notes Class 6-10. Class 10. Science. Class 9. Science ... Class 9 Science Chapter 3. CBSE Class 9 Notes Science Chapter 3 - Atoms And Molecules ... Antoine Lavoisier established the foundation of modern chemistry with his laws of chemical combination. 1.0 Laws of Chemical Combination.
Case based questions for Class 9 Science involve exploring a real-world situation through scientific analysis and inquiry. These questions allow students to make connections between science concepts and the world around them, as well as develop critical thinking skills. For example, a case study may involve challenging a student to determine ...
Definition of mole: It is defined as one mole of any species (atoms, molecules, ions or particles) is that quantity in number having a mass equal to its atomic or molecular mass in grams. 1 mole = 6.022 x 10 23 in number. Molar mass = mass of 1 mole → is always expressed in grams and is also known as gram atomic mass.
CBSE Class 9 Science Sample Paper with Solutions for Term 2 Exam 2022. Check some of the important case study questions below: Q. Read the following and answer the questions: A student was asked ...
Thus, masses in the decreasing order are: 0.1 g atom of Ag > 0.1 mole of H 2 SO 4 > 10 23 molecules of CO 2 > 10 23 atoms of Ca > 1 g of carbon. Question 2. Calculate the number of aluminium ions (Al 3+) in 0.056 g of alumina (Al 2 O 3). Answer: Molecular mass of alumina (Al 2 O 3) = 2 × Al 3+ + 3 × O 2-.
Chapterwise Case Study Questions for Class 9 Science. Inboard exams, students will find the questions based on assertion and reasoning. Also, there will be a few questions based on case studies. In that, a paragraph will be given, and then the MCQ questions based on it will be asked. For Science subjects, there would be 5 case-based sub-part ...
Maths Case-Study Qs. VIEW ALL. Discover TopperLearning's online platform for CBSE Class 9 sase studies. Enhance your analytical skills with expert guidance. Start your learning journey today!