An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Rep Pract Oncol Radiother
- v.19(5); 2014 Sep


Case presentation – A five-year survival of the patient with glioblastoma brain tumor
Hubert urbańczyk.
a Centrum Onkologii, Instytut im. Marii Skłodowskiej-Curie, Oddział w Gliwicach, Zakład Radioterapii, Poland
Anita Strączyńska-Niemiec
b Centrum Onkologii, Instytut im. Marii Skłodowskiej-Curie, Oddział w Gliwicach, Zakład Patologii Nowotworów, Poland
Grzegorz Głowacki
Dariusz lange, leszek miszczyk.
This paper presents an atypical case of a patient with brain tumor of the glioblastoma multiforme (GBM) type who achieved a 5-year survival. Some general information is provided including epidemiology, diagnostic and treatment procedures (surgery and radio-chemo-therapy), and prognosis of survival related to GBM. The course of the disease, including its main symptoms, individual reasons for the delay of adjuvant treatment, after the primary surgical treatment, 37-month period of the decease free survival, as well as comprehensive management after the tumor recurrence are also presented. Histopathology confirming the clinical diagnosis is discussed in a separate chapter.
1. Background
Primary brain tumors account for about 2% of all malignant neoplasms in adults. Approximately a half of them represent gliomas, derived from neuroepithelial cells, among which glioblastoma (GBM) is the most common type.
GBM cases represent about 20% of all primary brain tumors in the adult population, and about 75% of all the anaplastic gliomas. 1 The prevalence of GBM is about 2–4 cases per 100,000. It is more common in men than in women, and its incidence increases with age. 2 Only sporadically, GBM can be found in individuals younger than 20 years of age, and its frequency rapidly increases, starting from the 5-th decade of life.
The treatment results of patients diagnosed with GBM are often unsatisfactory, and the outcome is usually poor. Currently, the main standard therapeutic methods include a radical surgical procedure, combined with radio-chemo-therapy. Some innovative methods of radiotherapy based on the application of novel radiosensitizers of corpuscular irradiation or radio-immune-therapy are now being investigated. A median survival time of patients diagnosed with GBM, treated only with the use of neurosurgical procedures are 3–5 months. The application of conventional adjuvant radiotherapy prolongs this average time about 3-fold, with a three-year survival for only about 6% of patients.
The post-treatment survival time depends on many clinical factors, such as general patient condition, age, and histopathological type of the tumor. Simson et al. demonstrated statistically significant longer survival periods among patients in whom the primary tumor location was in the frontal lobe, in comparison to the ones in whom it was located in the parietal or temporal cerebral region (11.4 months vs. 9.6 months vs. 9.1 months, respectively; p = 0.01). 3 Severity of neurological symptoms, limits of the performed surgical procedures, and response to the applied therapy, based on imaging tests, also represent prognostic factors.
Etiology of malignant neoplasms of the central nervous system (CNS) is still unknown. The most common of many probable carcinogens include: nitrosamines, pesticides, herbicides, petrochemical substances, polyvinyl chloride, and electromagnetic irradiation. However, the role of these pro-carcinogenic factors has not been unequivocally proven. 4,5 In contrast, it has been documented that patients exposed to ionizing irradiation have an increased risk of the CNS malignant gliomas. According to the current state of knowledge in the field of molecular biology and genetics of these malignancies, two main hypotheses related to their development have been proposed. The first one includes de novo creation which is related to the loss of heterozygotic properties in chromosomes 9p, 10, 17p, and with the amplification of genes for the EGFR and CDK4 (this type of malignant growth occurs more often in older patients). The second one involves the creation of anaplastic gliomas, through the progression of gliomas with a lower malignancy grade (encountered more often in younger patients). 6,7
Currently, a required standard of therapy for patients with GBM is a combined treatment, including tumor resection, with following concomitant radio-chemo-therapy, and adjuvant chemo-therapy, based on Temozolomide. In patients who undergo non-radical surgery, or who are not treated surgically, the palliative whole brain radiotherapy (WBRT), stereotactic radiation surgery (SRS), or combination of both of these therapeutic methods are used. Also, the application of palliative chemotherapy and symptomatic treatment remain important. In addition, alternatively fractionated radiotherapy, brachytherapy, targeted molecular therapy, radio-immune-therapy, hadrone therapy, or radio-sensitizers can be considered in individual cases.
In 2005, Stupp et al. presented results of a randomized study conducted by EORTC ( European Organization for Research and Treatment of Cancer ) and NCIC ( National Cancer Institute of Canada ), comparing the application of combined radio-chemo-therapy based on Temozolomide and radical radiotherapy alone. The combined management in a statistically significant manner prolonged the total survival time from 12.1 to 14.6 months, and the rate of 2-year survival was 26.5%, compared to 10.4% for radiotherapy alone. 8 The follow-up results, after a longer period of observation, confirmed the previous reports. The 2-, 3-, and 4-year survival rates were 27.3%, 16.7%, and 12.9%, respectively ( p < 0.0001) in the patients’ group treated with a combined therapy, and 11.2%, 4.3%, and 3.8%, in the patients’ group treated with radiotherapy only. 9
Unfortunately, despite the use of Temozolomide, the results are unsatisfactory. The reason for this therapeutic failure is the GBM resistance to most chemotherapeutic agents or rapid development of the GBM as a result of genetic transformations within the tumor cells. The main mechanism of the GBM resistance to alkylating agents, such as temozolomide, procarbazine, or nitrogen mustard derivatives, is the repair of damages caused by these drugs with involvement of protein coded by MGMT ( O 6 -methyl-guanine-DNA methyl-transferase ) gene.
A degree of methylation of the promoter's region of MGMT gene appears to be closely correlated with a therapeutic response of the glioma cells. Hypermethylation of this part of the gene significantly increases treatment efficacy among patients treated with Temozolomide, 10 influencing their survival period, as well. 9
2. Case presentation
The patient is a 38 year old Caucasian male, smoker (about 10–15 cigarettes per day for 20 years), without other relevant family or personal risk factors for neoplasic disease who had suffered from severe headaches and nausea (his first disease symptoms) since August of 2005. He did not seek any medical help until November of 2005, when he presented to his doctor, due to exacerbation of those symptoms. No abnormalities on both physical and neurological examinations were detected at that time.
On December 30th of 2005, the CT scan of his brain was remarkable for the following findings: “An expansive lesion of approximately 5 cm × 3 cm in size, located in the right temporal lobe, with nonhomogenic, post-contrast signal amplification. A large edema surrounding the lesion. A compression of the occipital corner of the right lateral ventricle. A slight enlargement of the supratentorial ventricular system, shifting to the left.”
On January 25th of 2006, the patient underwent surgical therapy, including right temporal craniotomy, with total resection of the tumor. On February 7th of 2006, a histopathology examination (identification number 475,958; Info-Pat, Poznań, Poland), confirmed a diagnosis of the GBM IV stage (according to WHO classification). Microscopic images of the tumor are presented ( Figs. 1–5 ).

Microscopic image of patient's tumor.

After the surgery, the patient was referred to the Institute of Radiation Oncology in Gliwice, Poland, for the post-operative radiotherapy. Although the patient was qualified for this treatment, he did not arrive to the Institute of Radiation Oncology on the day of the scheduled preparatory procedures. The reason for his absence was a simultaneous diagnostic finding of the left lung's tumor, for which the patient underwent a thoracotomy with the wedge tumor resection (for diagnostic purposes). On June 12th of 2006, based on the histopathological examination results, which showed post-tuberculosis lesions, the patient's pulmonary treatment was completed.
In February 2007, approximately 13 months after his brain tumor surgery, the patient again presented to the Institute of Oncology, and according to the follow-up diagnostic work-up, no brain tumor recurrence was found. Due to the absence of tumor, no radiotherapy was considered, and “watchful waiting” was recommended including brain imaging studies (CT or MRI) to be repeated every 3 months. In the face of the atypical disease course, an additional verification of the histopathological diagnosis was also performed, confirming the original findings of the GBM. The patient had remained under close control until February 2009 (37 months from his initial brain tumor surgery), and at that time the brain tumor recurrence was found. His recurrent tumor was located in the primary tumor's bed, and its size was 4 cm × 5.3 cm × 3.5 cm ( Fig. 6 ). However, those findings were not associated with any particular symptoms or abnormalities on subsequent physical or neurological examinations of the patient. On March 16th 2009, the patient underwent another craniotomy with the subtotal tumor resection. (MRI scans after the second craniotomy are shown in Figs. 7 and 8 .) The histopathology examination was again consistent with GBM. During the period from May 11th to June 19th of 2009, the patient received the radiotherapy dose of 60 Gy/30 fractions to the tumor lodge, including the residual tumor, with 2.5 cm of tissue margin. Due to the lack of the patient's consent, no chemotherapy was implemented. During the irradiation period, he had the first seizure episode, and was started on antiepileptic therapy (Depakine 200 mg a day). He continued this therapy for the rest of his life. After the radiotherapy, diagnostic follow-up examinations were conducted every 3 months. At the beginning of March 2010, another recurrence was found, and the tumor was localized in an upper part of the tumor bed, within the previously irradiated area (its size was 3.7 cm × 2.6 cm × 2.3 cm). Surprisingly, the patient had not experienced any symptoms, and his physical and neurological examinations were unremarkable. On March 13th of 2010, the stereotactic radiotherapy, using a single dose of 8 Gy applied to the area of recurrent tumor was performed. Unfortunately, on the control examination, on July 6th of 2010, further progression of the GBM was found. The patient expired on November 15th of 2010, in the local hospital (Zawiercie, Poland), due to the tumor expansion, resulting in cerebral edema, herniation, and multi-organ failure.

CT scan of recurrence tumor.

MRI scan after the second craniotomy.

3. Histopathology examination
On a histopathology specimen, the large areas of thrombotic necrosis, most probably caused by a large tumor size (5 cm × 3 cm) were found. In contrast, no “palisade” necrosis (with the characteristic palisade-like cell arrangements), typical for this type of tumor, was found.
Within vital tumor structures, a high cellular polymorphism was found. Besides some small calls (with hyperchromatic nucleus and scarce amount of cytoplasm), mostly atypical cells (giant, multisided or oval, with numerous nuclei with abnormal shapes, and visible nucleoli) were present. The cells revealed a strongly positive GFAP reaction that can be indicative of their glioma-type origin. Also, some distinctive GBM features, including proliferation of vascular endothelium (focal areas of numerous mitotic figures, in high power field – HPF), were visible. The described microscopic images are presented in Figs. 1–5 (the images of primary and recurrent tumors appear identical).
In this paper, we presented a remarkably long survival period (63 months since the initial onset of symptoms, and 58 months since the primary surgical treatment) of the GBM patient. An important message from our case study that could be useful in the management of many other GBM cases is that the initial complete resection suggests a beneficial role of radical neurosurgery in the early GBM treatment and potential survival period.
Unfortunately, we are unable to indicate the specific reasons for such a long survival of our relatively asymptomatic patient who experienced some disadvantages, including the second malignancy, which caused the delay in the application of his radiotherapy.
Nevertheless, it should be emphasized that personalized, patient-centered approach, using comprehensive diagnostic and therapeutic strategies, as well as vigilant, multi-level follow-up care, should be helpful in explaining different factors, contributing to overall survival. In addition, our single case presentation illustrates several challenges that are common to many GBM patients, and merit further, more individualized research on this devastating disease.
Conflict of interest
None declared.
Financial disclosure

- Weill Cornell Medicine

- Case Studies: Patients With Brain Tumors
- Symptoms of a Brain Tumor
- Diagnosing and Treating a Brain Tumor
- Surgery for Brain Tumors in Adults
- Doctors Who Treat Brain Tumors in Adults
- Cognitive Remediation After Brain Tumor Surgery
What our Patients Say

Our Care Team

- Chair and Neurosurgeon-in-Chief
- Margaret and Robert J. Hariri, MD ’87, PhD ’87 Professor of Neurological Surgery
- Vice Provost of Business Affairs and Integration

- Vice Chair for Clinical Research
- David and Ursel Barnes Professor of Minimally Invasive Brain Surgery
- Professor of Neurosurgery, Neurology, and Otolaryngology
- Director, Center for Epilepsy and Pituitary Surgery
- Co-Director, Surgical Neuro-oncology

- Assistant Professor of Neurological Surgery
- Leon Levy Research Fellow
- Feil Family Brain and Mind Research Institute

- Professor of Radiology in Neurological Surgery

- Associate Professor, Neurological Surgery

- Director, Neurosurgical Radiosurgery
- Professor of Clinical Neurological Surgery

- Chief of Neurological Surgery, NewYork-Presbyterian Queens
- Co-director, Weill Cornell Medicine CSF Leak Program

- Chief of Neurological Surgery, NewYork-Presbyterian Brooklyn Methodist
- Professor, Neurological Surgery
- Director, Brain Metastases Program
- Co-director, William Rhodes and Louise Tilzer-Rhodes Center for Glioblastoma

- Director of Neuro-oncology
- Director, Brain Tumor Center, Sandra and Edward Meyer Cancer Center

- Hematologist/oncologist (Brooklyn)

- Assistant Attending Neurologist, NewYork-Presbyterian Hospital
- Assistant Professor of Neuro-Oncologist
Weill Cornell Medicine Neurological Surgery 525 East 68 Street, Box 99 New York, NY 10065 Phone: 866-426-7787
PERSPECTIVE article
Case report: end-stage recurrent glioblastoma treated with a new noninvasive non-contact oncomagnetic device.

- 1 Kenneth R. Peak Brain and Pituitary Tumor Treatment Center, Department of Neurosurgery, Houston Methodist Neurological Institute, Houston, TX, United States
- 2 Department of Neurosurgery, Houston Methodist Research Institute, Houston, TX, United States
- 3 Department of Neurosurgery, Weill Cornell Medical College, New York, NY, United States
Alternating electric field therapy has been approved for glioblastoma (GBM). We have preclinical evidence for anticancer effects in GBM cell cultures and mouse xenografts with an oscillating magnetic field (OMF) generating device. Here we report OMF treatment of end-stage recurrent glioblastoma in a 53-year-old man who had undergone radical surgical excision and chemoradiotherapy, and experimental gene therapy for a left frontal tumor. He experienced tumor recurrence and progressive enlargement with leptomeningeal involvement. OMF for 5 weeks was well tolerated, with 31% reduction of contrast-enhanced tumor volume and reduction in abnormal T2-weighted Fluid-Attenuated Inversion Recovery volume. Tumor shrinkage appeared to correlate with treatment dose. These findings suggest a powerful new noninvasive therapy for glioblastoma.
Introduction
For glioblastoma (GBM), the most common malignant tumor of the brain in adults, treatment outcome remains dismal. In over 40 years median survival has only shown modest improvement ( 1 ), and standard of care treatment often has negative impact on quality of life ( 2 ). Treatment including radiation and chemotherapy takes a heavy toll. Frequently patients cannot tolerate the completion of the prescribed chemotherapy cycles. Thus, there is a great unmet need for a completely different therapeutic approach with better outcome and less toxicity.
A new FDA-approved treatment involving electric fields alternating at 200 kHz called Optune™ therapy is now available for recurrent GBM as monotherapy and in combination with temozolomide for newly diagnosed GBM ( 3 , 4 ). It is also being tested in clinical trials for other cancers. Its hypothesized mechanism of action involves disruption of tubulin dimers, mitotic spindles, and cell division by electric field-induced dipole alignment and dielectrophoresis ( 5 ). It has a modest effect on survival, increasing median overall survival by 0.6 month in recurrent GBM ( 3 ), and in newly diagnosed GBM by 31% ( 4 ). Even this modest effect is encouraging for patients.
It has been shown that electromagnetic fields (EMF) produce anticancer effects in vitro ( 6 , 7 ). We have conducted preclinical experiments with a new noninvasive wearable device known as an Oncomagnetic device that generates oscillating magnetic fields (OMF) by rotating strong permanent magnets ( 8 , 9 ). The OMF generating components (oncoscillators) of the device can be attached to a helmet and treatment with the device does not require shaving the head. Using the oncoscillators of the device and specially devised patterns of magnet rotations we have produced strong selective anticancer effects in patient derived GBM and xenografted mouse models without causing adverse effects on cultured normal cells and normal mice ( 10 – 12 ). The mechanism of action of OMF differs from Optune™ and involves disruption of the electron transport in the mitochondrial respiratory chain causing elevation of reactive oxygen species and caspase-dependent cancer cell death ( 10 – 12 ).
Here we report evidence of treatment response in the first patient to ever receive this therapy with an untreatable left frontal GBM, treated with a wearable Oncomagnetic device in an FDA-approved Expanded Access Program.
Case Description
The patient is a 53-year-old man who first presented with altered mental status in May 2018. Imaging studies documented a large tumor in the left frontal lobe extending across the midline into the right frontal lobe, with diffuse and extensive infiltration through the corpus callosum. There was mass effect and severe edema. He was taken to the operating room on June 4, 2018, where he underwent left frontal craniotomy and radical excision of the tumor. The tumor was histopathologically confirmed as GBM. At the time of the surgery, the excision extended across the midline into the right frontal lobe. He was enrolled in a herpes simplex virus-thymidine kinase gene therapy program and received viral injection during surgery per protocol. In addition, per protocol, and as standard of care, he received concomitant radiation therapy and chemotherapy with temozolomide.
In August 2019, the patient presented with an area of contrast enhancement on MRI scan along the left ventricle. At first this was thought to be a treatment effect. This area progressively enlarged. Evaluations done before OMF treatment initiation on January 16, March 3, and April 15, 2020, demonstrated a clear recurrence. The tumor abutted the ventricle and there was evidence of leptomeningeal spread. The patient had already had radiation therapy and chemotherapy and the tumor was now progressing. The presence of leptomeningeal disease portends poor outcome, with median survival of 3.5 to 3.9 months ( 13 ).
Because of inadequacy of any standard of care options he was enrolled in an FDA-approved Expanded Access Program (EAP) for compassionate use treatment with the Oncomagnetic device. He signed an informed consent on April 15, 2020. The EAP study was carried out under a protocol approved by the Houston Methodist Research Institute Institutional Review Board.
Oncomagnetic Device
The Oncomagnetic device consists of 3 oncoscillators securely attached to an acrylonitrile butadiene styrene helmet and connected to a microprocessor-based electronic controller operated by a rechargeable battery ( Figure 1 ). Further details regarding the device are given in the Supplementary Appendix . Based on a finite element model-based calculation of the spread of the field and the size and magnetization of the rotated diametrically magnetized neodymium magnets, we estimated that the combined effective field (at least 1 mT in strength) of the 3 oncoscillators covered the entire brain, including the upper part of the brain stem.
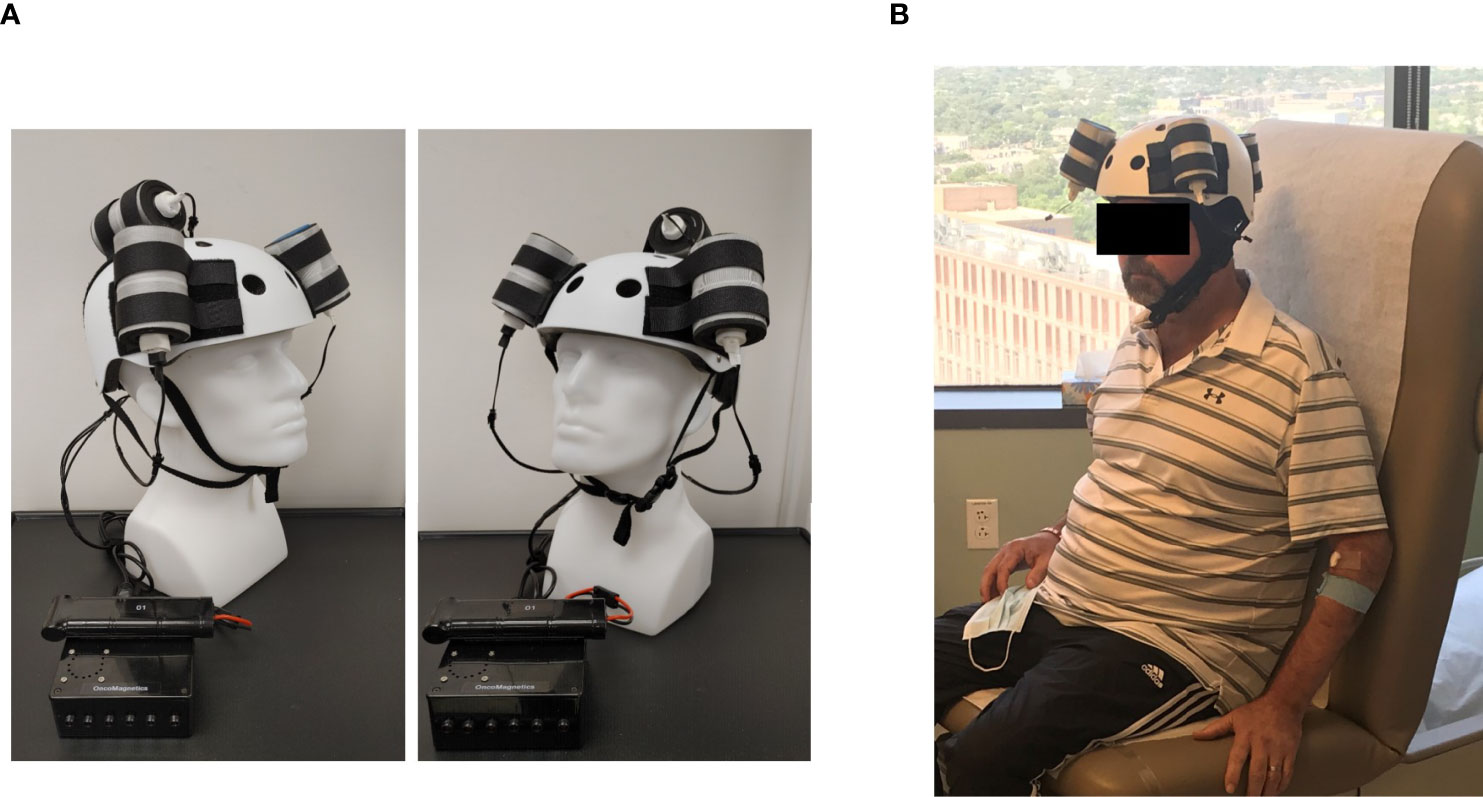
Figure 1 Oncomagnetic Device. (A) Device helmet with 3 oncoscillators securely attached to it. The oncoscillators are connected to a controller box powered by a rechargeable battery. (B) The patient wearing the device helmet with three oncoscillators attached.
Oscillating Magnetic Field Treatment
The treatment consists of intermittent application of an OMF that needs to be generated by rotating permanent magnets in a specific frequency profile and timing pattern to be effective. The patient received this treatment initially in the Peak Center clinic under the supervision of the treating physician and the Principal Investigator (DSB) of this study for the first 3 days. The dose was escalated over this period as follows. On the first day, the treatment was for 2 hours with a 5-min break between the first and the second hour. On the second and third days, it was increased to 2 and 3 2-hour sessions, respectively, with 1-hour breaks between the sessions. The patient’s spouse was trained in the use and care of the device on these days. After this initial supervised phase, the treatment was continued at home unsupervised with the same regimen as on the third day, above. The spouse was instructed to maintain a daily log of the conduct and progress of treatment, and any observed treatment and adverse effects.
Clinical Evaluations and Neuroimaging
The patient was evaluated clinically by the treating physician on each of the 3 days that he received treatment in the clinic and 7, 16, 30 and 44 days after initiation of treatment. Magnetic Resonance Imaging (MRI) scans were done on Days 1, 3, 7, 16, 30 and 44. The Day 1 scan was done before initiation of treatment. All other scans were done after treatment initiation. The treatment was paused on Day 37 because of an unfortunate but unrelated severe closed head injury (CHI). MRI scans were done on a Siemens Magnetom Terra 7T scanner. MRI scans included T1 magnetization prepared rapid gradient echo scans with and without gadolinium contrast, and T2-weighted Fluid-Attenuated Inversion Recovery (FLAIR), T2-weighted Turbo Spin Echo, Diffusion Weighted Imaging, Susceptibility Weighted Imaging, proton Magnetic Resonance spectroscopy and Diffusion Tensor Imaging scans. Treatment effect on contrast-enhanced tumor (CET) was evaluated according to the response assessment in neuro-oncology (RANO) criteria for clinical trials ( 14 ). In addition, an automated software-based method developed in house was used to objectively calculate the CET volume (see below and Supplementary Appendix ).
Data Analysis
Post-contrast T1 anatomical and T2-FLAIR MRI scans at each of the 6 time points were used to determine changes in contrast-enhanced tumor (CET) volume and non-enhanced tumor infiltration, respectively, before and after initiation of treatment. Information on image processing, data normalization and plotting are given in the Supplementary Appendix . Values obtained from pre-treatment clinical scans taken at 2 time points over 3 months before enrollment of the patient were also plotted on the same graph. Because this is a single patient case report, we could not perform any meaningful statistical analysis. However, to obtain a semi-quantitative assessment of the significance of the trend seen with treatment, we analyzed the changes in CET volume using Bayesian logic, given the observed increasing trend at two pre-treatment time points. Accordingly, we assumed that the chance of increase, decrease and no change in the rate of tumor growth was the same at each time point after treatment initiation to calculate the probability of a decrease at each post-treatment initiation time point.
The patient received OMF treatment with the Oncomagnetic device for 36 days. The treatment regimen was changed at various times during this period based on the caregiver reports and clinical findings, as described below.
Clinical Findings
After the initial 3 days of supervised treatment, the patient was seen again by the treating physician in the outpatient clinic on Day 7 from the start of treatment. Because of inattention at baseline, the patient was having difficulty with the length of treatment sessions. They were reduced to 2 hours/day Monday through Friday with Saturday and Sunday off. The Day 16 clinical examination revealed that he was tolerating the treatment sessions well, so they were increased to a total of 3 hours/day (in one-hour increments with 5 min breaks) Monday through Friday and the weekends off. On Day 30 visit, the patient reported headaches related to transient hypertension for which he was taking medication. The treating physician increased blood pressure medication (Valsartan) with improvement. The treatment was paused on Day 36 because of a closed head injury from a fall. Whether the fall was related to the treatment in any way is uncertain. It is worth noting, however, that the patient had experienced several falls before initiation of treatment. At the last follow-up on Day 44 the patient was admitted to the inpatient unit for evaluation of closed head injury and underwent detailed assessment. There were no serious adverse events reported during treatment. The patient’s caregivers reported subjective improvement in speech and cognitive function.
MRI Findings
Evaluation of the T1 post-contrast clinical MRI scans obtained before initiation of treatment showed progression in accordance with the RANO criteria ( Figure 2A ). All scans acquired during treatment showed stable disease, according to these criteria ( Figure 2A ). To obtain an objective quantitative assessment of the CET volume we used an automated MATLAB software-based script. This analysis showed marked changes in CET volume with treatment. Figure 2B shows a plot of the CET volume as a function of time before and after initiation of treatment. It reveals that there was substantial growth of the tumor volume over the 3 months before the treatment. Within the first 3 days of treatment the trend is reversed with the volume steeply decreasing by ~10% on Day 7 and then less steeply by 31% on Day 30. Based on a Bayesian-type assessment of the probability of a decrease in CET volume at each post-treatment initiation time point, the decrease at Day 30 is statistically significant at P = 0.036. The treatment was paused on Day 37. After the pause we see another trend reversal and an increase in CET volume on Day 44.
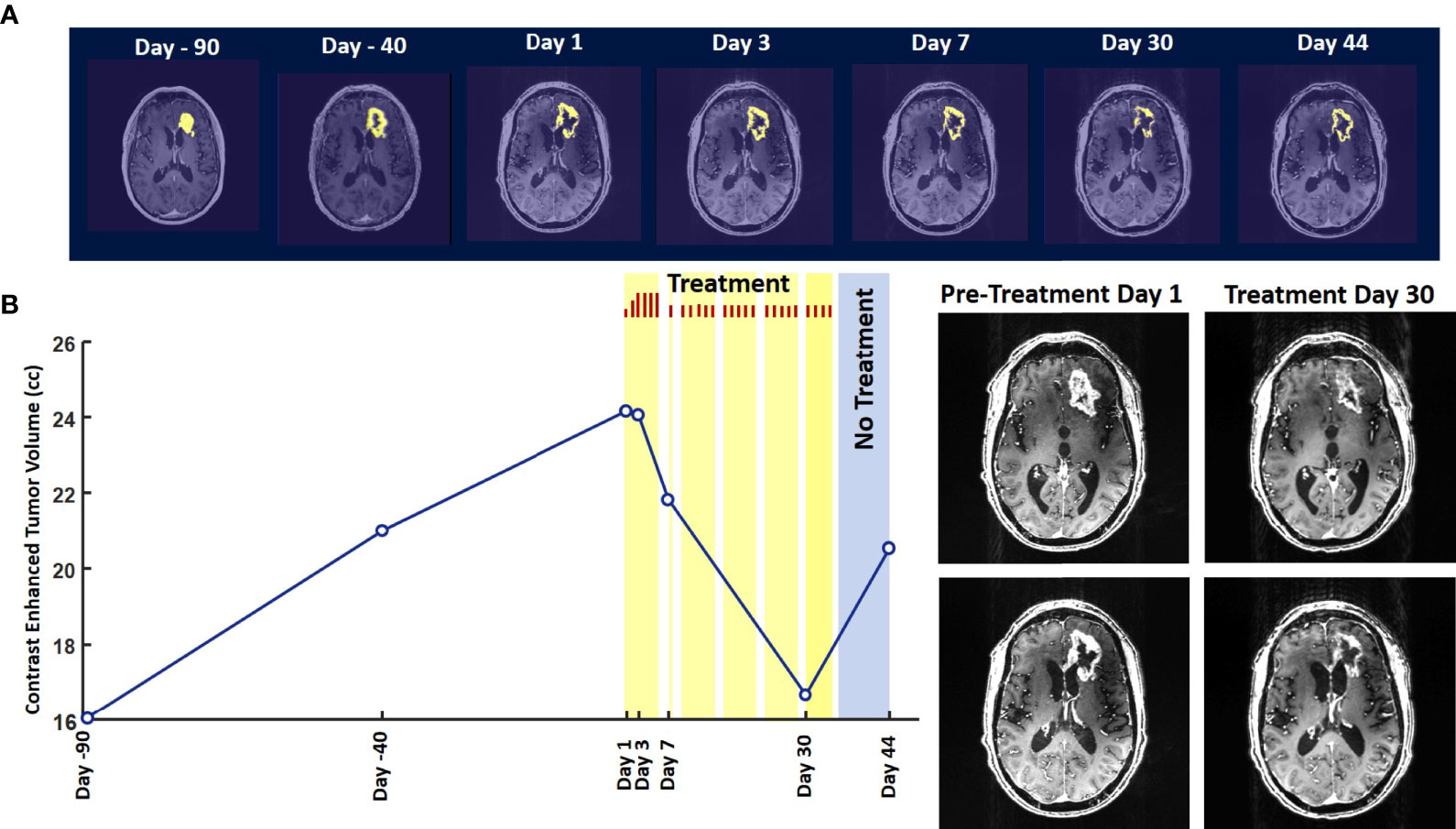
Figure 2 Change in Contrast-Enhanced Tumor Volume. (A) T1-weighted axial post-contrast scans showing the contrast-enhanced tumor (CET) highlighted with an overlayed automated computer program-generated light-yellow mask at different time points (B) Left – A graph showing the change in CET volume over time. The treatment times and durations are shown as red bars and light-yellow highlights. The long pause in treatment is shown as a light-blue highlight. Right – T1-weighted axial post-contrast scans showing CET at two levels along the dorso-ventral axis at Day 1 before treatment and Day 30 of treatment.
The T2-FLAIR data in Figure 3A show changes in enhanced intensity volume of 1 – 11% over time. The decreases in volume are greater after a 3-day pause in treatment on Day 7 and after an 8-day pause on Day 44. These decreases are likely due to reduction in treatment-related cerebral edema and/or reduction in non-contrast enhancing tumor infiltration. The patient died ~3 months after cessation of treatment from the CHI. A brain only autopsy showed a resection cavity in the left frontal lobe (6.0 x 5.0 x 3.5 cm) and recurrent/residual glioblastoma with associated treatment effect (see Figures 3B–E ). Residual/recurrent high-grade glioma was present, including foci of densely cellular tumor, focal microvascular proliferation, and necrosis ( Figure 3C ). In addition, there was prominent treatment effect with pallor and rarefaction of white matter ( Figure 3D ), reactive astrocytosis, infarct-like necrosis ( Figure 3E ) and bizarre nuclear atypia within residual tumor cells. Additional features of treatment effect included dystrophic calcifications ( Figure 3E ).
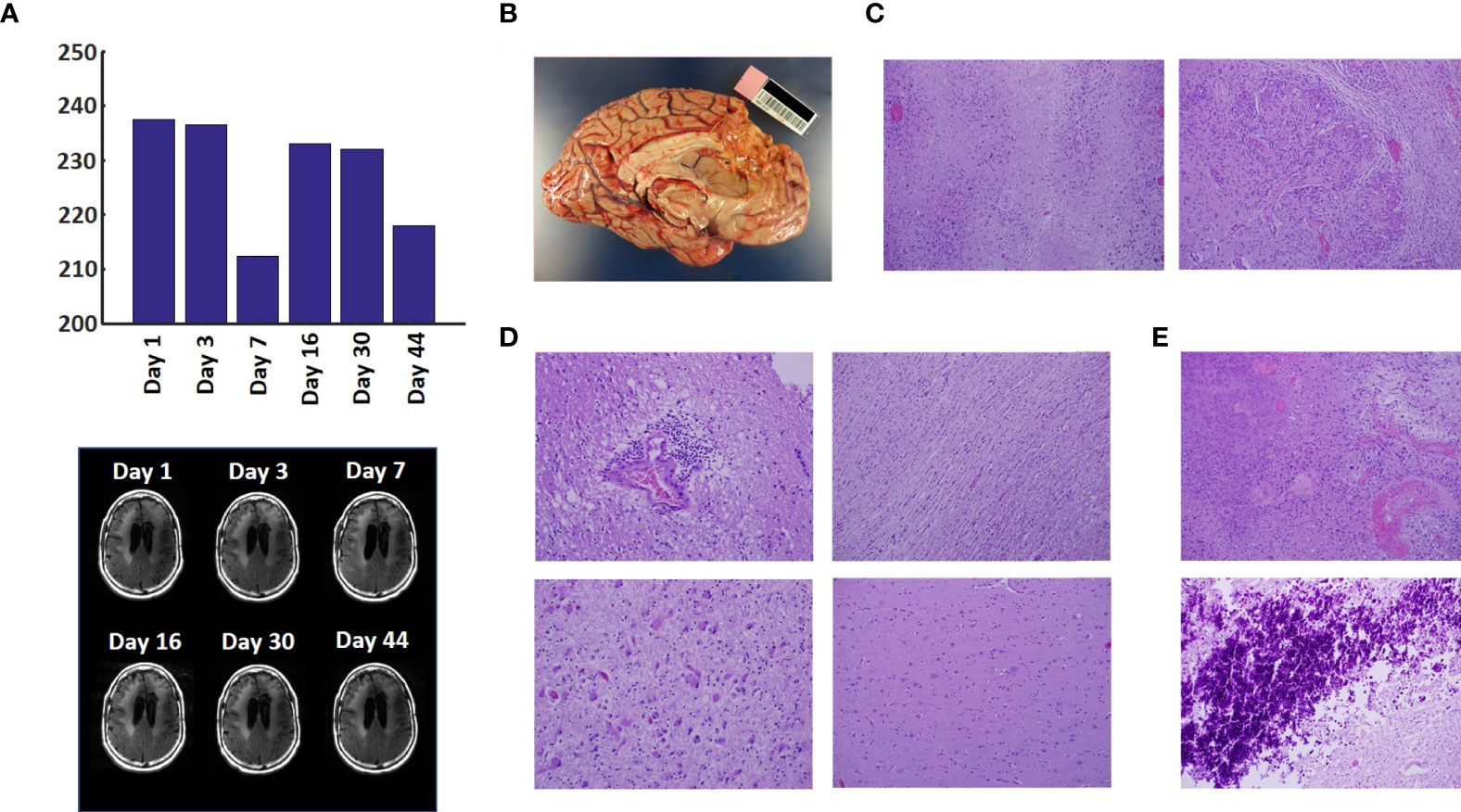
Figure 3 Variation in Enhanced Intensity Volumes in T2-FLAIR MRI Scans and Autopsy Findings. (A) Top – Bar plots of the volumes of T2-FLAIR intensity enhancement in the whole brain at different time points. Overall, there was up to 11% decrease in T2 FLAIR volume over the course of treatment. Bottom – Representative T2-FLAIR images are shown. (B) Left hemisphere of the brain, examined grossly, showing no tumor mass. (C) Photomicrographs of the left cortex showing bland necrosis, residual tumor, and microvascular proliferation with thick-walled vessels. (D) Top left – Microscopic field of the left cingulate cortex showing a focus of rarefied, perivascular inflammation. Bottom left – Cortical field showing rarefied parenchyma and residual tumor cells, enlarged with treatment-type effect that can be seen in GBM. Top right – Micrographic field of the corpus callosum showing thinned, rarefied white matter tract. Bottom right – Field showing relatively uninvolved contralateral (right) cortex. (E) Top – Micrographic field in the left cortex showing infarct-like necrosis (left), tumor (right), and fibrin thrombus (lower right). Bottom – Left cortical field showing necrotic tissue with dystrophic calcification.
The findings of this study indicate that Oncomagnetic device-based OMF therapy is well tolerated by a patient who has end-stage recurrent GBM with leptomeningeal involvement and has no other available effective treatment options. They also demonstrate a clinically significant reduction in CET volume with reductions in non-enhanced tumor volume and/or edema in T2-FLAIR scans. The temporal profile of changes in CET volume also suggests a correlation with the treatment dose and the presence or absence of treatment. When the treatment dose was higher (6 hours/day for 4 days) we see a tumor volume reduction rate of 2.32 cm 3 /day. When it was lower (2 hours/day for 9 days and 3 hours/day for 18 days) the reduction is 1.03 cm 3 /day. Moreover, when the treatment was paused for 8 days the decreasing trend reversed and the CET volume increased, instead. Assuming that the ~1.03 cm 3 /day decreasing trend had continued until the treatment was paused, we can estimate that the CET volume grew at the rate of 1.26 cm 3 /day during the pause. Despite the apparent correlation it is possible that the treatment response is independent of the short-term changes in the treatment dose.
To our knowledge, there is no report in the literature of a noninvasive treatment-related shrinkage of CET volume of GBM at a rate comparable to that seen in this study. One published report on Optune™ therapy has reported that the time course of change in tumor volume in MRI scans shows a ~15% reduction over ~3 months ( 15 ). Besides Optune™, the other type of treatment approved by the FDA and recommended as a standard in National Comprehensive Cancer Network guidelines for recurrent GBM is the anti-vascular endothelial growth factor (VEGF) monoclonal antibody, Bevacizumab ( 16 , 17 ). Bevacizumab treatment response of reduction in tumor volume on MRI scans has been reported to be lower than is observed in the present study ( 18 ). Furthermore, while anti-VEGF drugs in general have mild toxicity profiles and two Phase II trials have shown anti-tumor efficacy ( 19 , 20 ), a subsequent Phase III trial did not show a significant increase in overall survival ( 21 – 23 ).
Noninvasive Oncomagnetic device based OMF therapy appears to be a safe and efficacious new modality of treatment against GBM that potentially has many advantages over existing treatments. The present report has the limitation of the treatment being conducted in only a single patient so far. Extending it to more patients in research studies would provide additional information regarding safety and efficacy.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Houston Methodist Research Institute Institutional Review Board. The patient/participant provided their written informed consent to participate in this study. Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Author Contributions
SH and DB designed the study and drafted the manuscript. SH designed the device used in the study, supervised its construction and testing and quantitively analyzed the imaging data. DB provided medical care to the study subject, supervised the delivery of device treatment, and conducted his clinical assessments. SH, MS, and DB designed the device treatment protocol and interpreted the findings. LN constructed and tested the device and provided device treatment to the study subject. All authors contributed to the article and approved the submitted version.
This work was supported by a grant from the Translational Research Initiative of the Houston Methodist Research Institute to SH and DB, and by Donna and Kenneth Peak, the Kenneth R. Peak Foundation, the John S. Dunn Foundation, the Taub Foundation, the Blanche Green Fund of the Pauline Sterne Wolff Memorial Foundation, the Kelly Kicking Cancer Foundation, the Gary and Marlee Swarz Foundation, the Methodist Hospital Foundation, and the Veralan Foundation. The John S. Dunn Foundation also supports the Distinguished Professorship of MS.
Conflict of Interest
SH, MS, and DB are listed as inventors on a U.S. patent application filed by Houston Methodist Hospital for the device used in this report.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the patient for graciously volunteering to be a research subject in this study and the rest of his family for supporting him. We appreciate the assistance of Dr. Matthew Cykowski, MD, Department of Pathology and Genomic Medicine, who provided pathologic description and images. We thank Blessy S. John and Alvin Saldon for aiding in device construction.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.708017/full#supplementary-material
1. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy Plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N Engl J Med (2005) 352:987–96. doi: 10.1056/NEJMoa043330
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Henriksson R, Asklund T, Poulsen HS. Impact of Therapy on Quality of Life, Neurocognitive Function and Their Correlates in Glioblastoma Multiforme: A Review. J Neurooncol (2011) 104:639–46. doi: 10.1007/s11060-011-0565-x
3. Stupp R, Wong ET, Kanner AA, Steinberg D, Engelhard H, Heidecke V, et al. NovoTTF-100A Versus Physician’s Choice Chemotherapy in Recurrent Glioblastoma: A Randomised Phase III Trial of a Novel Treatment Modality. Eur J Cancer (2012) 48:2192–202. doi: 10.1016/j.ejca.2012.04.011
4. Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B, et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA (2017) 318:2306–16. doi: 10.1001/jama.2017.18718
5. Tuszynski JA, Wenger C, Friesen DE, Preto J. An Overview of Sub-Cellular Mechanisms Involved in the Action of TTFields. Int J Environ Res Public Health 13 (2016) 13:1–23. doi: 10.3390/ijerph13111128
CrossRef Full Text | Google Scholar
6. Saliev T, Begimbetova D, Masoud AR, Matkarimov B. Biological Effects of non-Ionizing Electromagnetic Fields: Two Sides of a Coin. Prog Biophys Mol Biol (2019) 141:25–36. doi: 10.1016/j.pbiomolbio.2018.07.009
7. Jimenez H, Blackman C, Lesser G, Debinski W, Chan M, Sharma S, et al. Use of non-Ionizing Electromagnetic Fields for the Treatment of Cancer. Front Biosci (Landmark Ed) (2018) 23:284–97. doi: 10.2741/4591
8. Helekar SA, Convento S, Nguyen L, John BS, Patel A, Yau JM, et al. The Strength and Spread of the Electric Field Induced by Transcranial Rotating Permanent Magnet Stimulation in Comparison With Conventional Transcranial Magnetic Stimulation. J Neurosci Methods (2018) 309:153–60. doi: 10.1016/j.jneumeth.2018.09.002
9. Helekar SA, Voss HU. Transcranial Brain Stimulation With Rapidly Spinning High-Field Permanent Magnets. IEEE Access (2016) 4:2520–8. doi: 10.1109/ACCESS.2016.2568739
10. Helekar S, Sharpe M, Pichumani K, Ijare O, Nguyen L, Baskin D. CTNI-48. Novel Treatment of End Stage Recurrent Glioblastoma Treated With a Noninvasive Oncomagnetic Device Using Oscillating Magnetic Fields – a New and Powerful Noninvasive Therapy. Neuro-Oncol (2020) 22:ii53–3. doi: 10.1093/neuonc/noaa215.214
11. Helekar S, Hambarde S, Baskin D, Sharpe M. EXTH-13. Potent Anticancer Effects of a New Wearable Noninvasive Oncomagnetic Device: Cellular Mechanisms of Action. Neuro-Oncol (2020) 22:ii89–9. doi: 10.1093/neuonc/noaa215.367
12. Hambarde S, Sharpe M, Baskin D, Helekar S. CBIO-07. Cell Death Induced by an Oscillating Magnetic Field in Patient Derived Glioblastoma Cells is Mediated by Reactive Oxygen Species. Neuro-Oncol (2020) 22:ii17–7. doi: 10.1093/neuonc/noaa215.067
13. Andersen BM, Miranda C, Hatzoglou V, DeAngelis LM, Miller AM. Leptomeningeal Metastases in Glioma: The Memorial Sloan Kettering Cancer Center Experience. Neurology (2019) 92:e2483–91. doi: 10.1212/WNL.0000000000007529
14. Wen PY, Chang SM, Van den Bent MJ, Vogelbaum MA, Macdonald DR, Lee EQ. Response Assessment in Neuro-Oncology Clinical Trials. J Clin Oncol (2017) 35:2439–49. doi: 10.1200/JCO.2017.72.7511
15. Robins HI, Nguyen HN, Field A, Howard S, Salamat S, Deming DA. Molecular Evolution of a Glioblastoma Controlled With Tumor Treating Fields and Concomitant Temozolomide. Front Oncol (2018) 8:451. doi: 10.3389/fonc.2018.00451
16. Kreisl TN, Zhang W, Odia Y, Shih JH, Butman JA, Hammoud D, et al. A Phase II Trial of Single-Agent Bevacizumab in Patients With Recurrent Anaplastic Glioma. Neuro Oncol (2011) 13:1143–50. doi: 10.1093/neuonc/nor091
17. Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab Alone and in Combination With Irinotecan in Recurrent Glioblastoma. J Clin Oncol (2009) 27:4733–40. doi: 10.1200/JCO.2008.19.8721
18. Daniels D, Guez D, Last D, Hoffmann C, Nass D, Talianski A, et al. Early Biomarkers From Conventional and Delayed-Contrast Mri to Predict the Response to Bevacizumab in Recurrent High-Grade Gliomas. AJNR Am J Neuroradiol (2016) 37:2003–9. doi: 10.3174/ajnr.A4866
19. Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Marcello J, Reardon DA, Quinn JA, et al. Bevacizumab Plus Irinotecan in Recurrent Glioblastoma Multiforme. J Clin Oncol (2007) 25:4722–9. doi: 10.1200/JCO.2007.12.2440
20. Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Dowell JM, Reardon DA, Quinn JA, et al. Phase II Trial of Bevacizumab and Irinotecan in Recurrent Malignant Glioma. Clin Cancer Res (2007) 13:1253–9. doi: 10.1158/1078-0432.CCR-06-2309
21. Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, et al. Bevacizumab Plus Radiotherapy-Temozolomide for Newly Diagnosed Glioblastoma. N Engl J Med (2014) 370:709–22. doi: 10.1056/NEJMoa1308345
22. Wick W, Gorlia T, Bendszus M, Taphoorn M, Sahm F, Harting I, et al. Lomustine and Bevacizumab in Progressive Glioblastoma. N Engl J Med (2017) 377:1954–63. doi: 10.1056/NEJMoa1707358
23. Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, et al. A Randomized Trial of Bevacizumab for Newly Diagnosed Glioblastoma. N Engl J Med (2014) 370:699–708. doi: 10.1056/NEJMoa1308573
Keywords: magnetic resonance imaging, contrast enhanced tumor, compassionate use treatment, radiation-type tumor necrosis 2, oscillating magnetic fields
Citation: Baskin DS, Sharpe MA, Nguyen L and Helekar SA (2021) Case Report: End-Stage Recurrent Glioblastoma Treated With a New Noninvasive Non-Contact Oncomagnetic Device. Front. Oncol. 11:708017. doi: 10.3389/fonc.2021.708017
Received: 11 May 2021; Accepted: 21 June 2021; Published: 22 July 2021.
Reviewed by:
Copyright © 2021 Baskin, Sharpe, Nguyen and Helekar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David S. Baskin, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.

Clinical Presentation
Statement of ethics, disclosure statement, a 58-year-old woman with left-sided weakness and a history of a pediatric brain tumor: a case report.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
Shaakir Hasan , Michael J. Gigliotti , Melvin Deutsch , Stacey L. Reed , Rodney E. Wegner; A 58-Year-Old Woman with Left-Sided Weakness and a History of a Pediatric Brain Tumor: A Case Report. Case Rep Oncol 7 May 2018; 11 (1): 131–137. https://doi.org/10.1159/000487430
Download citation file:
- Ris (Zotero)
- Reference Manager
Background: An uncommon but well-established complication of cranial irradiation is secondary neoplasm. This case presentation documents a radiation-induced malignant glioma 55 years after being diagnosed with “cerebral sarcoma,” now defined as atypical meningioma. This not only represents the longest reported latency period for a patient initially receiving over 30 Gy, but also provides a valuable historical perspective of neuro-oncology. Clinical Presentation: A 58-year-old female presenting with progressive left-sided upper and lower extremity weakness with a past medical history significant for “cerebral sarcoma” was diagnosed with glioblastoma multiforme. This patient had previously been treated with resection and adjuvant radiation therapy via a 280-kVP orthovoltage machine and received 3,390 rad to the posterior three-quarters of the skull for “cerebral sarcoma.” Conclusion: A comprehensive investigation of the past medical history helped uncover a mysterious pediatric diagnosis, helped drive the management 5 decades later, and serves as a reminder that seemingly safe interventions may still cause harm.
“Cerebral sarcoma” was defined in the literature as a neoplasm of the meninges prior to 1979, when the World Health Organization classified several subtypes of typical (grade I), atypical (grade II), and anaplastic (grade III) meningioma [ 1 ]. Given long-term toxicity risks, the utilization of radiotherapy in meningiomas remains controversial while postoperative radiotherapy is no longer indicated in pediatric populations [ 2 ].
This is largely because an uncommon but well-established complication of cranial irradiation is a secondary neoplasm [ 3, 4 ]. Notably, 1.3% of glioblastoma cases are associated with a previous exposure to radiation, with a median latency period of 9 years [ 5, 6 ]. Some studies suggest that doses greater than 30 Gy puts the patient at higher risk for malignancy compared to lower doses (less than 18 Gy), although others suggest that there is no threshold dose [ 3, 5 ].
We present the case of a radiation-induced glioblastoma multiforme (GBM) in a 58-year-old female who was treated for a “cerebral sarcoma” 55 years earlier, for which the original records were obtained.
Past Medical History
An otherwise healthy 58-year-old female revealed that in 1962 as a 3-year-old child she had been treated for a “cerebral sarcoma” with surgery and radiation. At the time, she presented with a headache and was diagnosed with an intracranial tumor via a ventriculogram. Excision of the lesion was described as “completely or nearly completely resected,” and per the pathology report was described as a “cerebral sarcoma or meningiosarcoma.” Adjuvant radiotherapy was requested by the neurosurgeon and the administering radiologist reluctantly agreed, noting that “meningiomas are not ordinarily treated with radiotherapy although some may respond, and sarcomas are certainly not radioresponsive. However, careful radiation therapy cannot do any harm and probably may do some good” (Fig. 1 ).

Assessment and plan of the treating radiologist from 1962.
The patient was treated with a 280-kVP orthovoltage machine with 2 lateral fields, prescribed to 14 cm depth on the right and 6 cm depth on the left. She ultimately received 3,390 of a planned 4,000 rad for 39 days in 1962 to the posterior three-fourths of the entire skull. Treatment was discontinued due to an intensely erythematous scalp, although no other toxicities or neurologic deficits were noted.
Examination
The patient presented with a 1-week history of progressively worsening left-sided upper and lower extremity weakness, described as an inability to hold objects in her left hand and frequent falls secondary to a left foot drop. The weakness eventually culminated in an episode where the patient fell out of bed and could not get up. Physical examination demonstrated stable vital signs, diffuse 3/5 strength in the left upper and lower extremities, and a right frontal craniotomy scar with surrounding soft tissue fibrosis (Karnofsky performance status 70).
Pathological Findings
A contrast-enhanced brain MRI demonstrated a 3 × 3 cm right frontoparietal resection cavity surrounded by a 5 × 4 cm area of heterogeneous contrast enhancement extending to the right corona radiata and periventricular white matter with associated cerebral edema (Fig. 2 ). The mass was not technically resectable due to location and biopsy was consistent with GBM, wild-type isocitrate dehydrogenase and unmethylated O 6 -methylguanine DNA methyltransferase (MGMT), with an MIB-1 index of 50% (Fig. 3 ).

T1-weighted brain MRI with contrast at the time of diagnosis of radiation-induced glioblastoma multiforme.

Radiation-induced glioblastoma multiforme demonstrating increased cellularity with marked nuclear atypia, necrosis, and vascular endothelialization.
It was determined that further maximal safe resection would not provide a beneficial therapeutic value, therefore definitive full-dose chemoradiation was recommended. Citing a declining performance status and discontent with the role radiation played in causing her malignancy, the patient ultimately declined treatment. Since identifying information was not used in the context of this case, informed consent for this case presentation was not required.
“Meningiosarcoma” or “cerebral sarcoma” are no longer considered histopathological diagnoses, but our patient likely had a variant of meningioma, which would have an approximately 90% chance of local control in such a scenario [ 7 ]. To this day, the role of radiotherapy in the management of typical and atypical meningiomas remains controversial, although several treatment paradigms have been established since the patient originally presented in 1962 [ 8 ]. For instance, there is virtually no indication for postoperative radiotherapy for meningioma in the pediatric population given the long-term toxicity risks [ 2 ]. As was the case for most radiation therapy in that era, the dose was limited by developing erythema of the scalp, an acute toxicity of little consequence, unlike the late and at that time unknown effect of secondary malignancy.
Cahan et al. [ 9 ] defined parameters of radiation-induced malignant gliomas (RIGMs) as follows: tumors localizing to where radiotherapy was applied, an adequate latency period measured in years, a histology different than that of the original tumor, and the patient should not have an underlying pathology favoring the growth of tumors. In this case, all four parameters were met. Although 80% of patients have a typical latency period within 15 years prior to the development of a secondary malignancy, the longest reported latency period includes a female who was treated for tinea capitis, presumably at a small dose, 61 years preceding the onset of a secondary malignancy [ 5, 10 ]. Prior to this case, the longest latency period between exposure of at least 30 Gy and induction of high-grade glioma was 37 years [ 11 ].
Histologically, radiation-induced GBMs are no different than de novo GBMs; however, there have been conflicting reports of whether RIMGs have greater homogeneity of gene expression [ 3, 12, 13 ]. With a median survival of 11 months, the prognosis for radiation-induced glioblastomas is comparable to that of GBMs with the unmethylated MGMT promoter gene, suggesting that perhaps they are associated with less favorable tumor biology [ 14, 15 ]. It should be noted that the vast majority of reported RIMGs were published before MGMT status testing became commonplace and before adjuvant temozolamide was established as the standard of care [ 16 ]. However, the median survival of RIMGs since 2007 was still 11.5 months [ 5 ].
The ideal management for de novo or secondary GBM in a medically fit patient includes gross total resection followed by adjuvant chemoradiation at a total dose of 6,000 cGy in 30 fractions with concurrent and adjuvant temozolomide [ 16 ]. The risk of neurotoxicity such as brain necrosis theoretically increases in the setting of reirradiation, which may be why only approximately 40% of patients with radiation-induced GBMs received reirradiation as part of their treatment [ 15 ]. Nevertheless, the risk of radionecrosis is minimal with reirradiation to the brain so long as the cumulative dose is less than 100 Gy at 2 Gy per fraction [ 17-19 ]. Furthermore, Paulino et al. [ 15 ] demonstrated that among 85 cases of RIMGs, the 35 patients who underwent reirradiation at a median dose of 50 Gy (range 30–76 Gy) had a median survival of 13 months compared to 8 months of those who were not reirradiated, without additional toxicity. It should be mentioned that potential long-term toxicity of reirradiation to the brain may not have been observed because most patients do not survive long enough to develop it.
This case illustrates how past medical history, going back even 50 years, is instrumental to workup and management. Two uncommon and valuable pieces of information include the patient’s knowledge of her pathology as a 3-year-old and medical records dating back to 1962, both of which helped determine the diagnosis and treatment. The medical records also provide a rare window as to how medicine was practiced 5 decades ago and how it has evolved since then. Importantly, they serve as a humble reminder that there are many aspects of medicine still unknown to clinicians, including the possibility that a seemingly safe therapeutic intervention can still cause harm.
We ensure the accuracy, quality, and integrity of this case report. No identifying patient information was disclosed.
The authors of this paper would like to disclose that they have no financial or other conflicts of interest in relation to this case study and publication.

Email alerts
Citing articles via, related articles.
- Online ISSN 1662-6575
INFORMATION
- Contact & Support
- Information & Downloads
- Rights & Permissions
- Terms & Conditions
- Catalogue & Pricing
- Policies & Information
- People & Organization
- Stay Up-to-Date
- Regional Offices
- Community Voice
SERVICES FOR
- Researchers
- Healthcare Professionals
- Patients & Supporters
- Health Sciences Industry
- Medical Societies
- Agents & Booksellers
Karger International
- S. Karger AG
- P.O Box, CH-4009 Basel (Switzerland)
- Allschwilerstrasse 10, CH-4055 Basel
- Tel: +41 61 306 11 11
- Fax: +41 61 306 12 34
- Contact: Front Office
- Experience Blog
- Privacy Policy
- Terms of Use
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Progress to Advance Care, Treatments, and Outcomes for Brain Tumor Patients
April 25, 2022 , by Brittany Cordeiro, NCI-CONNECT Program Manager
Neuro-Oncologist Dr. Jing Wu reviews brain tumor scans
Read about our advances in care, treatments, and patient outcomes for brain and other central nervous systems tumors in honor of Brain Tumor Awareness Month.
An estimated 700,000 people in the United States are living with a brain tumor. Over 25,000 more will be diagnosed with a cancerous brain or other central nervous system (CNS) tumor this year. The causes are not known. And, while males are slightly more likely to develop brain cancer than females, brain tumors do not discriminate.
In May, the brain tumor community joins together to raise awareness about brain tumors through education. By sharing the rarity and uniqueness of brain and other CNS tumors— 1.3 percent of all new cancers cases and over 130 different types—we can inspire research and clinical studies to improve diagnosis and standardize care.
We also hope to champion progress by sharing the work that NCI-CONNECT and the NCI Center for Cancer Research's Neuro-Oncology Branch (NOB) have completed over the last year to advance care, treatments, and patient outcomes.
Investigating New Therapies and Methods to Improve Outcomes
Nci-connect clinical studies expansion.
In 2021, NCI-CONNECT expanded two clinical studies—an immunotherapy drug trial and a tumor tissue repository study—to its national network of collaborative institutions to reach more people with rare brain and spine tumors and help researchers advance their knowledge of the diseases faster.
The first-of-its-kind immunotherapy drug trial is designed to test whether stimulating the immune system using nivolumab is effective and can shrink (or control the growth or spread of) specific types of recurrent rare brain and spine tumors. The trial first launched in 2017 at the NOB. Now, the trial is being led by NOB Associate Research Physician Marta Penas-Prado, M.D. It is also testing the changes that nivolumab induces in immune cells in peripheral blood during treatment—and whether nivolumab can improve the symptoms of people with these tumors.
The new multi-institutional Tissue Outcomes Study collects tumor tissue and comprehensive clinical data from deceased patients with rare brain and spine tumors, and people who were pregnant at diagnosis (or became pregnant after diagnosis). The study is breaking silos by working across the NCI-CONNECT national network. It is led by Dr. Penas-Prado.
NCI-CONNECT First-in-Human Study
In other firsts, NCI-CONNECT launched a first-in-human phase 1 clinical trial for people with recurrent rare brain and spine tumors. Led by Brett Theeler, M.D., neurologist and neuro-oncologist in the United States Army Medical Corps and NCI-CONNECT clinical collaborator, the trial is studying ONC206, an oral cancer therapy. The trial tests the dosing and safety in adult patients.
NOB Immunotherapy and Glioblastoma Trial
Glioblastoma is the most common type of primary brain cancer. People with this disease or a variant called gliosarcoma have poor long-term outcomes. NOB Chief and NCI-CONNECT Co-Leader Mark Gilbert, M.D., is leading a new clinical trial testing an immunotherapy treatment to slow or stop the spread of cancer cells in people with glioblastoma and gliosarcoma. The trial will also evaluate a test that may help determine who is likely to get an immune response.
We designed the study based on quality science and built-in cutting-edge techniques. The study results should be a very important contribution to the field.
Mark Gilbert, M.D., NOB Chief and NCI-CONNECT Co-Leader
Outcomes Intervention Studies
Under the leadership of NOB Deputy Chief and NCI-CONNECT Co-Leader Terri Armstrong, Ph.D., NCI-CONNECT also launched three studies in 2021 to measure if an intervention helps improve outcomes. Led by Amanda King, Ph.D., an iCURE postdoctoral fellow in the Patient Outcomes Program, a virtual reality study for patients with brain cancer investigates if using a virtual reality headset to deliver relaxation techniques helps to reduce the distress and anxiety that people experience ahead of their magnetic resonance imaging (MRI) scans and clinical appointments.
Led by assistant investigator Dorela Shuboni-Mulligan, Ph.D., CRTA postdoctoral fellow in the Patient Outcomes Program, a sleep observation study measures daytime sleepiness and activity patterns using a smart wearable device. This will provide insight into the impact of sleep disturbance on those with brain cancers.
Led by assistant investigator Alvina Acquaye, psychosocial behavioral specialist for NCI-CONNECT, the CALM therapy intervention study uses individualized therapy to address negative feelings and improve a person’s mood and the way they feel. This study will determine if using the CALM intervention helps to reduce depressive symptoms in people with brain cancer.
Bringing the Community Together to Advance Science and Patient Care
Virtual educational webinars were held to join experts across disciplines with advocates—and provide forums for thoughtful discussion about challenges and solutions.
Targeting CNS Tumor Metabolism
To advance the field of CNS tumor metabolism, NOB Investigator Mioara Larion, Ph.D., and Dr. Gilbert co-led a virtual symposium with the Society for Neuro-Oncology (SNO) on April 6-7, 2021. It was the first conference entirely dedicated to education and research on brain tumor metabolism . Over 500 people registered to attend. The conference brought together clinicians, researchers, trainees, patients, and experts in the field of metabolism.
Survivorship Care in Neuro-Oncology Symposium
NOB Assistant Research Physician Heather Leeper, M.D., and Dr. Armstrong co-led the Survivorship Care in Neuro-Oncology Symposium on June 21-22, 2021. The symposium brought together neuro-oncology providers, researchers, advocates, patients, and care partners virtually to learn, discuss the challenges experienced by people living with brain and spine tumors, and develop survivorship care guidelines. Over 250 people attended the symposium.
Introduction to Career Pathways in Neuro-Oncology
On January 11, 2022, the NOB partnered with SNO to offer trainees of all disciplines the opportunity to learn about neuro-oncology careers in basic science, clinical care, and research. The free virtual event offered insights, opportunities, and strategies shared by world-renowned neuro-oncology professionals to expand a neuro-oncology career. There was also a post-session panel discussion for attendees to share their thoughts with experts.
NIH Tumor Boards and Journal Clubs
To encourage referrals and further collaboration, in 2020 we expanded our virtual journal club and weekly multidisciplinary NIH Neuro-Oncology Tumor Board live sessions to include investigators and cases from participating NCI-CONNECT consortium sites. As of November 2021, 28 providers had presented 41 cases at 24 tumor board meetings with 60 to 70 participants.
Awarding and Recognizing Pioneering Work
National research awards.
In 2021, Lasker Clinical Research Scholar and NOB Investigator Jing Wu, M.D., Ph.D., was accepted into the NExT (NCI Experimental Therapeutics) Program , which provides resources for projects focused on developing therapies for unmet needs. Dr. Wu leads the NOB Translational Research Program, which is dedicated to developing clinical trials for people living with brain and spine tumors. Her work combines both clinical and laboratory research and is making a difference in the field.
Dr. Penas-Prado received an Award for Excellence in Rare CNS Disease at the 2021 SNO Annual Meeting for her abstract on the interim analysis of the immune checkpoint inhibitor nivolumab for people with rare CNS cancers trial. As of March 10, 2021, the interim analysis of the clinical trial determined that the disease control rate exceeded the minimum required to pass the interim analysis in the heavily pretreated cohort and continue the trial.
Ultimately, this multicenter study should advance our understanding of these tumors, set the foundation for future trials, and provide better treatment options.
Marta Penas-Prado, M.D., NOB Associate Research Physician
Dr. Penas-Prado also received the 2022 Center for Cancer Research Staff Clinician Leadership Merit Award for leading our rare CNS tumor clinic and clinical trials, a national tumor board, and an international symposium.
Dr. Leeper received an Award for Excellence in Survivorship at the 2021 SNO Annual Meeting for her abstract with the NOB Patient Outcomes Program on whether economic strain and mood disturbance during the COVID-19 pandemic may have additional impacts on patients’ symptoms and function. Dr. Leeper concluded that worse financial toxicity scores were strongly associated with worse overall symptom burden, worse anxiety and depression scores, and worse overall health-related quality of life scores.
Trainee and Mentor Awards
Postbaccalaureate Research Fellow Yeonju Kim was selected as the Outstanding Post-Graduate Trainee at the 22nd Annual Center for Cancer Research Fellows and Young Investigators Colloquium, where she gave an oral presentation. Kim is mentored by NOB Staff Scientist Orieta Celiku, Ph.D., Dr. Gilbert, and Dr. Armstrong. Her colloquium abstract was titled, "ACROSS: Accrual and Access to Neuro-Oncology trials in the United States. "
Dr. Celiku received the 2022 NCI Staff Scientists and Staff Clinicians Outstanding Mentor Award for her exemplary mentorship in developing the next generation of cancer researchers.
Sharing Progress to Raise Awareness
The NOB and NCI-CONNECT published over 80 abstracts that were presented at national meetings, one journal supplement, and 10 manuscripts. Our work modified two National Comprehensive Cancer Network (NCCN) guidelines for rare CNS tumors in 2020 and 2021. Our progress is made possible by the participation of patients and their care partners in our programs.
To continue our efforts in brain and spine tumor care, treatment, and research—and to improve survival and quality of life for people living with the disease—we encourage you to join us for Brain Tumor Awareness Month in May and share these advances. Educating those living with (or not yet diagnosed with) a brain or spine tumor can make an impact.
A case study of a long-term glioblastoma survivor with unmethylated MGMT and hypermutated genotype
Affiliations.
- 1 Cure Brain Cancer Biomarkers and Translational Research Group, Prince of Wales Clinical School, University of New South Wales, Sydney, New South Wales 2052, Australia.
- 2 Neurospine Clinic, Prince of Wales Hospital, Randwick, New South Wales 2031, Australia.
- 3 University of New South Wales, Sydney, New South Wales 2031, Australia.
- 4 Department of Medical Oncology, Nelune Comprehensive Cancer Centre, Prince of Wales, Hospital, Randwick, New South Wales 2031, Australia.
- PMID: 31160353
- PMCID: PMC6549560
- DOI: 10.1101/mcs.a003251
Effective treatments that extend survival of malignant brain tumor glioblastoma (GBM) have not changed in more than a decade; however, there exists a minority patient group (<5%) whose survival is longer than 3 yr. We herein present a case report of a long-term surviving 51-yr-old female diagnosed with a MGMT unmethylated GBM. The patient was progression-free for 23 mo. Fresh primary and recurrent tumor samples were collected and processed for patient-derived model development. Whole-genome sequencing (WGS) was performed concurrently with additional standard of care diagnostics. WGS revealed a hypermutated genotype in the germline tissue and in both the primary and recurrent tumor samples. Specific to the matched tumors, an average of 30 cancer driver genes were mutated. Noteworthy was the identification of a nonsynonymous mutation in the POLE gene. As a possible instigator of the hypermutational genotype observed in the tumors, we identified nonsynonymous germline mutations within the mismatch repair genes, MLH1 and PMS2 Mutations within these genes are often indicative of the pan-cancer phenotype known as Lynch syndrome; however, their pathogenicity remains unreported. We performed a drug screen of 165 compounds, which identified one compound, YM155, an experimental survivin inhibitor, that showed effectivity to the patient-derived cell lines of both tumors. Treatment selection based on a patient's genome to individualize treatment for GBM patients could potentially be useful in the clinic. This is a promising avenue for further translational research, with larger databases and integrated platforms to increase the efficiency of analyzing and interpreting the individual genomic data of GBM.
Keywords: glioblastoma; glioma.
© 2019 Jue et al.; Published by Cold Spring Harbor Laboratory Press.
Publication types
- Case Reports
- Research Support, Non-U.S. Gov't
- Brain Neoplasms / diagnostic imaging
- Brain Neoplasms / drug therapy
- Brain Neoplasms / genetics*
- DNA Mismatch Repair / genetics
- Drug Screening Assays, Antitumor
- Gene Regulatory Networks
- Germ-Line Mutation
- Glioblastoma / diagnostic imaging
- Glioblastoma / drug therapy
- Glioblastoma / genetics*
- Imidazoles / pharmacology*
- Middle Aged
- Naphthoquinones / pharmacology*
- Neoplasm Recurrence, Local
- Whole Genome Sequencing
- Naphthoquinones
- sepantronium

Brain Tumor and Neuro-Oncology Research
Innovations in brain tumor and neuro-oncology.
- Laser Interstitial Thermotherapy with real-time MRI using the Monteris AutoLitt System
- Brain mapping with functional MRI (fMRI), Diffusion Tensor Imaging (DTI) and/or magnetoencephalography (MEG) to optimize identification of brain tumors and surrounding normal tissues
- Intraoperative MRI to maximize safety and completeness of tumor removal
- New techniques for optical and fluorescence imaging to better identify infiltrating tumor
- Convection Enhanced Delivery (CED): A technology to improve delivery of small anti-tumor molecules and chemotherapy to brain tumors
- Stereotactic Radiosurgery using Gamma Knife or CyberKnife®
Current Clinical Trials
UH provides both standard and advanced nonoperative treatments for patients with brain tumors through our advanced and extensive clinical trials. Many of these are offered in collaboration with the NCI-funded Adult Brain Tumor Consortium (ABTC), a group of 15 elite “Brain Tumor Centers of Excellence” which collaborate to offer the most innovative treatments to patients with brain tumors. Clinical trial offers change frequently, but current offers include:
- Tumor vaccines (immunotherapy) to teach the patient’s own immune system to fight their tumor
- New drugs specifically targeting brain tumor “stem cells” which are resistant to current therapies
- Gene therapy for brain tumors
- New agents targeting “angio-genesis,” the need for tumors to acquire a new blood supply
- Convection Enhanced Deliver (CED). New ways to deliver drugs and immunotoxins specifically to the tumor while avoiding toxicity from the normal brain.
- Innovative combinations of chemotherapeutic agents which are more powerful together than apart
Specific trials include:
- ABTC 0603 – A Phase I/II Trial of Hydroxychloroquine in conjunction with Radiation therapy and concurrent and Adjuvant Temozolomide in Patients with Newly diagnosed Glioblastoma Multiforme (Version 01/08/09)
- ABTC 0703 – Phase I/II study of the poly (ADP-ribose) polymerase-1(PARP-1) inhibitor BSI-201 in patients with newly diagnosed malignant glioma (version 02/06/09)
- ABTC 0904 – A Biomarker and Phase II Study of GDC-0449 in Patients with Recurrent Glioblastoma Multiforme
- ABTC 0901 – An Open Label, Phase 2 Study Evaluating the Safety and Efficacy of IMC-3G3 or IMC-1121B in Patients with Recurrent Glioblastoma Multiforme
How Common Is a Brain Tumor
Have you ever wondered how common brain tumors are? A brain tumor is an abnormal growth of cells within the brain or surrounding areas, and these growths can be either benign (non-cancerous) or malignant (cancerous).
Brain tumors are rare, and less than 1 percent of the population is diagnosed with a malignant brain tumor during their lifetime.
Our aim is to provide comprehensive research on brain tumors, their prevalence, development factors, and the crucial role of early detection and treatment. Let’s take a closer look at brain tumors and their commonality.
Understanding Brain Tumors
A brain tumor is an abnormal growth of cells within the brain or the central spinal canal. It can originate in the brain itself (primary tumors) or spread from other parts of the body (secondary or metastatic tumors). Brain tumors can vary in their growth rates and potential to cause harm.
Types of Brain Tumors
Brain tumors are generally classified into two main categories: benign and malignant.
- Benign tumors : These non-cancerous growths typically grow slowly and do not spread to other body parts. While they can still cause significant health issues by pressing on sensitive brain areas, they are generally less aggressive than malignant tumors.
- Malignant tumors : These are cancerous growths that tend to grow rapidly and invade nearby tissues. Malignant brain tumors can be life-threatening and often require aggressive treatment.
Some common types of brain tumors include gliomas , meningiomas, pituitary adenomas, and schwannomas. Each type has unique characteristics and treatment options.
Symptoms and Diagnosis
The symptoms of brain tumors can vary widely depending on the tumor's size, type, and location within the brain. Common symptoms include headaches, seizures, vision or hearing changes, balance or coordination changes, and cognitive or personality changes.
Diagnosis typically involves a combination of neurological exams, imaging studies (like MRI or CT scans), and biopsy procedures to determine the tumor type and guide treatment planning.

Statistical Overview
With statistics, researchers and healthcare professionals can better understand the scope of the brain tumor challenge and work towards more effective solutions for patients across the globe. Let’s take a look at some statistical trends involving brain tumors:
How Common Are Brain Tumors Globally?
Brain tumors are quite rare when compared to other types of cancer. Globally, the incidence rate of brain and other central nervous system (CNS) tumors is approximately 6.2 per 100,000 people per year. This figure represents both malignant and benign tumors.
Prevalence Rates in Different Age Groups
The prevalence of brain tumors varies across different age groups. In children, brain tumors are the second most common type of cancer after leukemia. For adults, the risk of developing a brain tumor increases with age, with the highest incidence rates observed in individuals over the age of 65.
Gender Differences in Brain Tumor Occurrence
Gender also plays a role in the prevalence of brain tumors. Studies indicate that men are generally more likely to develop brain tumors compared to women. However, certain types of brain tumors, such as meningiomas, are more frequently diagnosed in women.
Brain Tumors in Children vs Adults
Brain tumors are the second most common type of cancer in children, following leukemia. Approximately 20% of all childhood cancers are brain tumors, with around 4,000 new cases diagnosed annually in the United States alone. The most common types of brain tumors in children include medulloblastomas, gliomas, and ependymomas. These tumors can significantly impact a child's development and quality of life, making early diagnosis and treatment essential
Common Types of Brain Tumors in Children
- Medulloblastomas : These are the most common malignant brain tumors in children and typically show up in the cerebellum. They can spread to other parts of the brain and spinal cord.
- Gliomas : These include a variety of tumors such as astrocytomas and glioblastomas, which can range from low-grade (slow-growing) to high-grade (fast-growing and more aggressive).
- Ependymomas : These tumors develop from the ependymal cells lining the ventricles of the brain and the center of the spinal cord. They can happen at any age but are more common in children.
Comparison with Adult Brain Tumor Statistics
Adults with brain tumors account for about 1.4% of all cancers. The incidence of brain tumors increases with age, and the types of tumors commonly seen in adults differ from those in children.
For example, glioblastomas are the most common type of malignant brain tumor in adults, while meningiomas, which are typically benign, are also frequently diagnosed. The prognosis and treatment options for brain tumors can vary significantly between children and adults, highlighting the need for age-specific research and therapeutic approaches.
Support and Resources
Dealing with a brain tumor diagnosis can be overwhelming for both patients and their families. Support groups and resources help provide emotional support, practical advice, and a sense of community.
Resources at Duke University's Tisch Brain Tumor Center
Duke University's Tisch Brain Tumor Center provides a comprehensive range of resources for patients and their families. These include:
- Patient Care and Support Services : The center offers multidisciplinary care teams that include neurologists, oncologists, surgeons, and support staff dedicated to providing personalized treatment plans and ongoing support.
- Educational Materials : Duke University provides extensive educational resources to help patients and families understand their diagnosis, treatment options, and what to expect during the treatment process.
- Clinical Trials and Research : The Tisch Brain Tumor Center is involved in cutting-edge research and clinical trials to develop new and more effective treatments for brain tumors. Patients can participate in these trials, gain access to the latest therapies, and contribute to advancing medical knowledge.
Discover the Best Treatment Options Today
If you’re considering undergoing treatment for a brain tumor, visit our website to request an appointment with one of our leading physicians. Established in 1937, Duke University’s Tisch Brain Tumor Center provides hope and empowerment to anyone facing a brain tumor diagnosis. Passionately leading the pursuit to conquer brain cancer , we hope to be a beacon of hope to all those fighting this ongoing battle.
If you are considering treatment for brain cancer, visit our website to request an appointment with one of our leading physicians.
Interested in joining the team of world-leading Neuro-Oncology doctors and practitioners? Visit our education & training page to learn more. You can also support the clinic by donating funds directly to patient care, research, education, and treatment for brain tumors.
Related readings:
- What Is The Most Common Type of Brain Tumor in Kids?
- How Long Can You Have a Brain Tumor Without Knowing?
- How Active Can You Be with a Brain Cancer Diagnosis?
Frequently Asked Questions About Brain Tumors
What are the chances I have a brain tumor?
The chances of having a brain tumor depend on various factors, including age, genetics, and environmental exposures. Overall, brain and other central nervous system (CNS) tumors are relatively rare, with an incidence rate of approximately 6.2 per 100,000 people per year. The risk increases with age and is higher in individuals with certain hereditary conditions or a family history of brain tumors.
Can you live a full life with a brain tumor?
Many people with benign brain tumors can live full, productive lives, especially if the tumor is slow-growing and does not affect critical brain functions. Even for those with malignant tumors, advances in treatment options, including surgery, radiation, and chemotherapy , have improved survival rates and quality of life.
How common are brain tumors by age?
Brain tumors are more common in certain age groups. They are the second most common type of cancer in children, with approximately 4,000 new cases diagnosed annually in the U.S. For adults, the incidence of brain tumors increases with age, particularly in those over 65.
What are the first signs of a brain tumor?
The first signs of a brain tumor can vary widely depending on the tumor's size, type, and location. Common first symptoms include persistent headaches, seizures, changes in vision or hearing, difficulty with balance or coordination, and cognitive or personality changes
- Português Br
- Journalist Pass
New research platform assesses brain cancer mutations during surgery
Lynda De Widt

Share this:

JACKSONVILLE, Fla. — Brain cancer is difficult to treat when it starts growing, and a prevalent type, known as a glioma , has a poor five-year survival rate. In a new study published in Proceedings of the National Academy of Sciences, Mayo Clinic researchers report on a new surgical platform used during surgery that informs critical decision-making about tumor treatment within minutes. Time is of the utmost importance when dealing with aggressive malignant tumors.
The platform uses mass spectrometry to identify a key gene mutation in brain cancer, known as isocitrate dehydrogenase (IDH) mutations, in real time. Mass spectrometry is a sensitive technique used to analyze substances in tissue samples, including those altered in cancer.
The study involved more than 240 small tissue biopsies from patients undergoing asleep and awake brain surgery for suspected glioma at Mayo Clinic between 2021 and 2023, and an additional 137 biopsies from an international collaborator. Neurosurgeons collected biopsy samples from the core of the tumor to identify the mutations, as well as from areas around it, to assess if the tumor had spread.
Each tissue sample was placed on a glass slide steps away from the patients during ongoing surgery. The samples were analyzed through the mass spectrometer, which allowed researchers to rapidly assess — within two minutes — whether an IDH mutation was present.
The researchers say that, in addition to enabling real-time diagnosis, the platform allows surgeons to determine a patient's prognosis and perform tumor resection to improve patient outcomes. In the future, the new platform will help surgeons take advantage of the window of opportunity in the operating room to tailor treatment to the molecular features of a tumor, a more personalized approach to medicine.
Researchers hope new therapies developed to target IDH mutations can be delivered in the operating room at the time of surgery.

"The ability to identify this mutation during brain surgery means that one day in the future we may be able to treat patients with this specific mutation locally before they leave the operating room," says the study's senior author, Alfredo Quiñones-Hinojosa, M.D. , dean of research and chair of the Department of Neurosurgery at Mayo Clinic in Florida.
"Therefore, we will be able to bring the fight against cancer to the operating room, before chemotherapy and radiation treatments begin, and before the disease has progressed and invaded further." Dr. Quiñones-Hinojosa is also director of the Brain Tumor Stem Cell Research Laboratory .
In the study, researchers were able to diagnose IDH gene mutations with 100% accuracy. They are conducting more research to find other signatures in tumors where the mutation is absent. In addition, they plan to broaden their discoveries to include other types of brain cancers.
This study was supported in part by the National Cancer Institute of the National Institutes of Health under award number R33CA240181. For a full list of authors, funding and disclosures, see the paper .
About Mayo Clinic Mayo Clinic is a nonprofit organization committed to innovation in clinical practice, education and research, and providing compassion, expertise and answers to everyone who needs healing. Visit the Mayo Clinic News Network for additional Mayo Clinic news.
Media contact:
- Kelley Luckstein, Mayo Clinic Communications, [email protected]
- Mayo Clinic Minute: No ‘lesser evil’ when it comes to tobacco use A Mayo Clinic virologist explains FLiRT and why you may need a new COVID-19 vaccination
Related Articles
- Search Menu
- Sign in through your institution
- Advance articles
- Editor's Choice
- Supplements
- Author Guidelines
- Submission Site
- Why Publish With Us?
- Open Access
- Advertising and Corporate Services
- Advertising
- Reprints and ePrints
- Sponsored Supplements
- Branded Books
- Journals Career Network
- About Neuro-Oncology Practice
- About the Society for Neuro-Oncology
- About the European Association of Neuro-Oncology
- Editorial Board
- Dispatch Dates
- Self-Archiving Policy
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Clinical case presentation, initial supportive care, initial diagnostic imaging, epidemiology, standard-of-care treatment, follow-up imaging, prognosis and survivorship.
- < Previous
Case-Based Review : newly diagnosed glioblastoma
- Article contents
- Figures & tables
- Supplementary Data
Derek R. Johnson, Shannon E. Fogh, Caterina Giannini, Timothy J. Kaufmann, Aditya Raghunathan, Philip V. Theodosopoulos, Jennifer L. Clarke, Case-Based Review : newly diagnosed glioblastoma, Neuro-Oncology Practice , Volume 2, Issue 3, September 2015, Pages 106–121, https://doi.org/10.1093/nop/npv020
- Permissions Icon Permissions
Glioblastoma (WHO grade IV astrocytoma) is the most common and most aggressive primary brain tumor in adults. Optimal treatment of a patient with glioblastoma requires collaborative care across numerous specialties. The diagnosis of glioblastoma may be suggested by the symptomatic presentation and imaging, but it must be pathologically confirmed via surgery, which can have dual diagnostic and therapeutic roles. Standard of care postsurgical treatment for newly diagnosed patients involves radiation therapy and oral temozolomide chemotherapy. Despite numerous recent trials of novel therapeutic approaches, this standard of care has not changed in over a decade. Treatment options under active investigation include molecularly targeted therapies, immunotherapeutic approaches, and the use of alternating electrical field to disrupt tumor cell division. These trials may be aided by new insights into glioblastoma heterogeneity, allowing for focused evaluation of new treatments in the patient subpopulations most likely to benefit from them. Because glioblastoma is incurable by current therapies, frequent clinical and radiographic assessment is needed after initial treatment to allow for early intervention upon progressive tumor when it occurs.
A 73-year-old man presented to his local emergency department after experiencing a generalized seizure. He had moderate left-sided weakness in the initial postictal period which quickly resolved. In retrospect, the patient had noted subjective left-hand “clumsiness” for a month prior to the seizure, but had not reported it to his family or physician. A CT scan was obtained in the emergency room and was followed shortly by an MRI (Fig. 1 ). The patient was then referred to our institution for further care.

(A) Unenhanced CT, (B) T2-weighted FLAIR, (C) gradient echo T1-weighted, and (D) post-gadolinium spin echo T1-weighted images depict a relatively circumscribed mass in the left superior temporal lobe with both solid, enhancing components and some cystic or necrotic areas. Moderate edema signal surrounds a portion of the mass.
The presentation of high-grade glioma is variable, depending on the location of the lesion within the brain. Headaches, seizures, and subacutely progressive neurological deficits are all common presenting symptoms.
Antiepileptic Therapy
Patients who present with seizure should be treated with antiepileptic drug (AED) therapy. An optimum AED choice would have rapid efficacy, few side effects, and no drug–drug interactions. In clinical practice, levetiracetam is often chosen as the first-line agent in this setting. 1 Studies have suggested that some AEDs may have direct antitumor effects. For example, valproic acid is a histone deacetylase (HDAC) inhibitor, 2 while levetiracetam is an MGMT inhibitor. 3 However, no impact of AED choice on survival has been proven, so AED choice should be based on efficacy and tolerability.
In patients with high-grade glioma who have not had a seizure, there is no proven role for long-term prophylactic AED therapy, and the American Academy of Neurology recommends against the routine use of prophylactic AEDs outside of the immediate perioperative period. 4 As previous studies of prophylactic AED therapy evaluated older agents in mixed patient populations, some experts question their applicability to current practice. A large trial of lacosamide vs placebo for seizure prophylaxis in patients with high-grade gliomas is ongoing to address this issue. 5
Corticosteroid Therapy
In patients presenting with headaches or focal neurological deficits, symptoms may be due to peritumoral vasogenic edema, which may respond to corticosteroid therapy. Dexamethasone is often started at 16 mg daily in 4 divided doses, and tapered down to the lowest effective dose or discontinued altogether. While this dosing schedule is widely used based on the short pharmacologic half-life of dexamethasone, the biological half-life is in excess of 36 hours, and daily or twice-daily dosing is effective and more convenient for maintenance therapy in most patients. Gastrointestinal prophylaxis and pneumocystis prophylaxis should be considered in patients in whom long-term corticosteroid treatment is anticipated.
Clinical Case Relevance
The patient was started on antiepileptic therapy at the time of his original emergency department visit. He had no further seizures. His exam was pertinent for a Karnofsky Performance Score of 90, and subtle left-sided pronator drift and slowing of rapid hand and foot movements on the left side were his only findings on physical exam. Dexamethasone was not initiated as he did not have symptoms of elevated intracranial pressure, such as headache or papilledema.
Because the symptomatic presentation of brain tumors is nonspecific, the presumptive diagnosis of brain tumor is often made only after imaging. Glioblastoma may be initially imaged with CT, particularly in the emergency department setting, but MRI provides more diagnostic information.
The typical CT appearance of glioblastoma is a mass lesion, often iso- to hyperattenuating (bright) in comparison to normal gray matter, with surrounding hypoattenuation due to infiltrating tumor and vasogenic edema. Contrast-enhanced CT classically reveals a centrally necrotic enhancing mass. Given that vascular proliferation is a hallmark of glioblastoma, intratumoral hemorrhage is common and may be visualized on CT, though it is more frequently identified as microhemorrhages on MR susceptibility-weighted imaging (SWI). Calcification is uncommon in glioblastoma, but can occasionally be seen.
On MRI, nearly all glioblastomas enhance with gadolinium contrast, usually showing a thick, irregular rind of tumor surrounding a necrotic cavity. Heterogeneity of signal intensity and contrast enhancement within glioblastomas and irregularity in shape are expected. Vascular hyperpermeability contributes to surrounding vasogenic edema visible as high signal intensity on T2-weighted images. Hemorrhage may complicate the appearance of glioblastoma, with acute and early subacute hemorrhage appearing hypointense on T2-weighted images and iso- to hyperintense on T1-weighted images. This intrinsic T1 hyperintensity of blood is similar in appearance to gadolinium enhancement, so it is crucial to always compare T1-weighted postcontrast images with T1-weighted precontrast images to ensure accurate judgment of enhancement.
The infiltrative nature of glioblastoma is generally more apparent on MRI than on CT. Mass-like signal abnormality infiltrating along white matter tracts is suggestive of glioma as opposed to other entities. However, distinguishing between nonenhancing infiltrative glial tumor with edema and vasogenic edema from any other etiology can be difficult or impossible. Frequently, glial infiltration and thickening of the cerebral cortex can be appreciated on T2-weighted and T2-weighted FLAIR images, which may help to distinguish gliomas from other neoplasms. Multifocality, distant, or diffuse disease may be seen initially in approximately 13% of glioblastoma cases, with some areas sometimes looking less aggressive than the primary mass. 6 It is also well established that microscopic glial tumor cell infiltration is expected to extend beyond visualized signal abnormality on MRI.
The differential diagnosis of glioblastoma often includes metastasis and CNS lymphoma. Generally speaking, glioblastoma tends to be more irregularly shaped than metastases because of its predilection for spread along white matter tracts, 7 but there is overlap at least in qualitative analysis. Primary CNS lymphoma (PCNSL) in the immunocompetent patient is most often homogeneous in signal intensity and enhancement, though exceptions do occur; while heterogeneity and central necrosis are more common in CNS lymphoma in the immunocompromised.
Advanced MRI techniques including perfusion imaging techniques such as dynamic susceptibility contrast (DSC) imaging, diffusion-weighted imaging (DWI), diffusion tensor imaging (DTI), SWI, and MR spectroscopy may help to distinguish glioblastoma from other tumors. Given the histological hallmark of neovascularity in glioblastoma, increased blood volume (often expressed as rCBV, or relative cerebral blood volume) is expected within at least portions of a glioblastoma. 8–10 On MR spectroscopy, glioblastoma typically has the nonspecific findings of elevated choline and decreased N-acetylaspartate (NAA) and may have elevated lipid and lactate resonances. Generally speaking, the choline:NAA ratio increases with astrocytoma grade. 11 Due to the infiltrative nature of glioblastoma relative to metastases, one may expect greater CBV 12 , 13 and greater choline:creatine 14 , 15 in the peritumoral areas of glioblastoma relative to metastases. rCBV also tends to be greater in enhancing tumor and peritumoral areas of glioblastoma than in CNS lymphoma. 12 Apparent diffusion coefficient tends to be lower in CNS lymphoma than in glioblastoma, given the great hypercellularity of lymphoma. 12 Microhemorrhages on SWI are found in most glioblastomas but rarely in CNS lymphoma. 16 , 17 Differentiation of glioblastoma and lymphoma using multiparametric advanced MRI has also been suggested. 18 Imaging genomic mapping is a burgeoning area of research that has begun to discover associations between MRI features and glioblastoma genotypes and clinical phenotypes. 19
Many published reports using advanced MRI techniques have relied on quantitative analyses, which are currently difficult to standardize across imaging platforms and institutions. For example, with perfusion imaging, there exists great variability in all steps from IV gadolinium bolus injection to scanner platforms used to MRI scan parameters chosen to post-processing software and analysis techniques used. 20 Given the technical variabilities of advanced MR techniques and expected glioblastoma heterogeneity, there are limits to the sensitivity and specificity of these techniques. The standardization of advanced MRI is well recognized as a pressing clinical and research need.
The initial imaging obtained for this patient included a CT and contrast-enhanced MRI, shown in Fig. 1 . Both of these images, and the MRI in particular, were concerning for glioblastoma, and metastasis and non-neuroplastic entities such as infection or demyelination were thought to be significantly less likely.
Surgical resection is the primary treatment for glioblastomas. The goals of surgery are tissue diagnosis, including molecular and genetic tumor analysis, as well as cytoreduction for alleviation of presenting symptoms and improved tumor control. As previously discussed, in the appropriate context imaging can be very suggestive of glioblastoma. However, tissue diagnosis is the standard of care and only in cases of truly inaccessible tumors (such as brainstem lesions) or grave infirmity of the patient, precluding surgical candidacy, should treatment be undertaken without pathological confirmation of disease.
The surgical approach of choice is maximal safe resection. Over the past several years, significant data have accumulated supporting the idea that maximizing the extent of tumor resection positively impacts survival for patients with newly diagnosed glioblastoma. 21 , 22 In a single institution study of 949 patients with high-grade gliomas, more than half of whom were operated on for the first time, the extent of resection was shown to be an independent predictor (gross total resection [GTR] vs near total resection [NTR], NTR vs subtotal resection [STR]) of prolonged survival (median OS 11 months GTR, 9 months NTR, and 5 months STR). 23
The association between extent of resection and survival benefit holds true even for tumors that are difficult to resect. In a study of multicentric high-grade glioma, resection of a dominant lesion was strongly predictive of improved overall survival when compared with biopsy only (12 months vs 4 months). 24 In the setting of insular high-grade gliomas, one of the most technically difficult eloquent cortical areas to access surgically, extent of resection ≥90% of the tumor provided 2-year overall survival of 91% compared with 75% for volumetric resection <90% of the tumor, in addition to improved progression-free survival. 25 The beneficial effect of maximal resection has also been suggested to extend to elderly patients without an increase in mortality or complications. 26
Several technical intraoperative adjuncts have been developed in an effort to maximize the extent of safe resection. 27 , 28 Use of frameless stereotactic guidance, which allows for optimal patient positioning, accurate tailoring of the craniotomy, and safe access trajectory to the tumor, has become standard for resection of glioblastomas. Recent advances have made it possible for intraoperative guidance to integrate imaging tools such as tractography, which allows for identification of motor, speech, and visual pathways, as well as MR spectroscopy to facilitate accurate targeting of presumed higher-grade areas within a heterogeneous tumor. 29 In cases where only a biopsy is planned, such imaging integration allows targeting of regions likely to optimize diagnostic yield and accuracy.
Direct cortical mapping allows identification of motor pathways and, when combined with awake craniotomy used for mapping of language areas, is an important adjunct to surgical resection of lesions in eloquent cortex. 25 , 30 A systematic review of the literature showed that direct cortical mapping decreases late severe neurological deficits from 8.25% to 3.4% and increases the rate of GTR from 58% to 75%. 31
Intraoperative MRI has been used in an attempt to maximize extent of surgical resection and identification of residual resectable tumor. 32 In a study including both high-grade and low-grade tumors, use of intraoperative MRI increased the volumetric extent of resection from 76% to 96%. 33 However, the high installation cost of an intraoperative magnet as well as the complexities involved in its intraoperative use have led to research into alternate ways to identify residual tumor during surgical resection. The utilization of fluorescence guidance has been recently advocated. The use of fluorophores such as 5-aminolevulenic acid (5-ALA) or fluorescein, which accumulate in areas of blood brain disruption, can be a powerful adjunct that allows for the accurate identification of tumor borders and possible residual disease at the time of resection. 34–36 In a systematic review of 10 studies, patients who underwent surgery utilizing 5-ALA for maximizing resection had improved 6-month progression-free survival and overall survival. 37 A multicenter, randomized, phase III trial of 5-ALA-guided surgery found higher rates of gross total resection and 6-month progression-free survival in the 5-ALA group without any increase in adverse events. 38
Following the initial multicenter, randomized trial of implantable carmustine polymer wafers in the treatment of recurrent high-grade glioma, Attenello et al reported their experience with their use during surgery for newly diagnosed high-grade gliomas and found an overall median survival of 13.5 months without any increased incidence of complications. 39 , 40 Although the use of chemotherapy implants appears to be safe in the setting of primary glioblastoma, the relative lack of improved survival and the fact that much of the data regarding the use of chemotherapy wafer implants predates the use of temozolomide (TMZ), has limited enthusiasm for this approach.
Surgery for high-grade gliomas is in general associated with relatively low rates of major complications. In an analysis of the patients in the Glioma Outcomes Project, an overall complication rate of 24% was reported for surgical treatment of newly diagnosed high-grade gliomas. In decreasing frequency, major complications included: depression (11%), worsened neurological status (8.1%), seizures (7.5%), adverse drug reaction (5.2%), DVT (4.2%), intracranial bleeding (1.6%), and pulmonary embolism and wound infection (0.5% each). Perioperative mortality was reported as 1.5%. 41 These results were similar to an earlier study that reported 13% major complications and 1.7% mortality in patient undergoing craniotomy for intraparenchymal tumors. 42 In an analysis of the California Inpatient Database, Marcus et al reported a 30-day readmission rate of 13.2% for patients who underwent surgical treatment for a glioma who were originally discharged home. The most common presentations at readmission were seizures (20.9%) and surgical infection (14.5%). 43
In summary, surgery remains the first and very important treatment modality for a newly diagnosed glioblastoma. Its effectiveness for optimizing overall survival is related to the extent of resection and its safety is dependent on various intraoperative adjuncts that allow for accurate localization of the tumor as well as eloquent cortical areas.
The patient underwent resection of his tumor without use of awake craniotomy or intraoperative MRI. Following surgery, his left-sided weakness was transiently worse but it then improved back to the presurgical baseline. His extensive resection placed him in a more favorable prognostic group than biopsy alone would have. Preoperative and postoperative MRI images are displayed in Fig. 2 .

Post-gadolinium spin echo T1-weighted images (A) before and (B) after surgery.
The histological diagnosis in this case was WHO grade IV astrocytoma (glioblastoma). It was an infiltrative astrocytoma showing areas of high cellularity and brisk mitotic activity (Fig. 3 A), tumor necrosis (Fig. 3 B), and microvascular proliferation (Fig. 3 C). The diagnostic criteria from the WHO (2007) include presence of cytological atypia, mitotic activity, microvascular proliferation, and/or tumor necrosis. 44 Briefly, an infiltrative astrocytoma exhibiting cytological atypia alone, including elongated, irregular and hyperchromatic nuclei, is considered WHO grade II (diffuse astrocytoma). The presence of increased cellularity, nuclear atypia, and mitotic activity warrant a WHO grade III (anaplastic astrocytoma) designation. Tumors that additionally show microvascular proliferation and/or necrosis are WHO grade IV (glioblastoma). Classic microvascular proliferation has the appearance of “glomeruloid tufts,” consisting of multilayered, mitotically active endothelial cells admixed with smooth muscle cells/pericytes (as represented in Fig. 3 C). Although necrosis surrounded by pseudopalisading tumor cells is most characteristic of glioblastoma (Fig. 3 B), both geographic and pseudopalisading tumor necrosis can be present and are associated with similarly dismal prognoses. Astrocytoma grading is based on the highest histological grade. Since infiltrative astrocytomas can have considerable regional heterogeneity, especially toward their infiltrative border into surrounding parenchyma, it is important to assess whether a biopsy sample is representative of the entire tumor by correlating histological, clinical, and radiological findings. A biopsy taken at the periphery of a ring-enhancing mass could well show a low to moderately cellular tumor (as seen in Fig. 3 D) with/without mitoses, prompting an inaccurate diagnosis of diffuse or anaplastic astrocytoma (WHO grade II or III) rather than glioblastoma (WHO grade IV).

The biopsies demonstrated an infiltrating population of atypical astrocytic cells, showing (A) brisk mitotic activity, (B) tumor necrosis, and (C) microvascular proliferation, consistent with a diagnosis of glioblastoma. A biopsy from the periphery of this mass may show (D) a low-to-moderately cellular tumor, with or without mitoses, corresponding to a lower histological grade. In images (A) and (C), photographed at 400× magnification, the scale bars on the bottom right represent 20 µm. In images (B) and (D), photographed at 200× magnification, the scale bars represent 50 µm.

(A) The first MRI following chemoradiotherapy and (B) MRI evidence of tumor progression approximately 2 years later. Both are post-gadolinium spin echo T1-weighted images.
Historically, glioblastomas have been distinguished based on their clinical presentation as primary (de novo) or secondary glioblastomas that develop in progression from a lower grade astrocytoma. Primary glioblastomas, the most common (>90%), develop with a short clinical history without clinical or pathological evidence of a lower grade precursor, and are typically seen in older patients. There are no definite histological features to distinguish primary and secondary glioblastomas. With advancing molecular information, however, it is clear that primary and secondary glioblastoma are two different diseases.
Mutation of isocitrate dehydrogenase (IDH) is frequently seen in low-grade glioma, and is also found in approximately 12% of glioblastomas. 45 The presence of IDH mutation within a glioblastoma is suggestive of secondary glioblastoma, regardless of any previous history of low-grade glioma. 46–50 In glioblastoma, mutations almost exclusively involve residue 132 (R132) of IDH1 resulting in the substitution of arginine. 46 , 51 The presence of IDH1 mutation in glioblastoma has been associated with younger age and relatively longer survival. 52–56 Alterations in receptor tyrosine kinase pathways have also been frequently identified in glioblastomas. 57 Epidermal growth factor receptor (EGFR) activation, either by amplification of wild-type EGFR or by deletion of exons 2–7 that encode the extracellular domain (the variant III mutation) resulting in ligand-independent constitutive activation of EGFR, 47–49 is more commonly seen among primary glioblastomas. Mutations of the TP53 gene are frequent in, but not exclusive of, secondary glioblastomas. 50 , 58 , 59 On the other hand, mutations in the promoter region of the telomerase reverse transcriptase ( TERT ) gene are predominantly found in primary glioblastomas that lack IDH1 mutations, and appear to be associated with worse prognosis. 49 ,60–62
The gene for the DNA-repair enzyme O(6)-methylguanine-DNA methyltransferase ( MGMT ) has a promoter region that is rich in CG dinucleotide (CpG) sites that are normally unmethylated. However, in glioblastomas, the cytosine in these CpG sites may become methylated, resulting in transcriptional silencing of MGMT and subsequent impairment of the DNA-repair process. 63–65 Glioblastomas with MGMT -promoter hypermethylation are unable to repair the DNA damage caused by alkylating agents (such as TMZ), and carry a more favorable prognosis than tumors without methylation of MGMT . 66–69 Tumors with IDH mutation frequently have MGMT promoter methylation. 70
Integrated genomic analysis has revealed subsets of high-grade astrocytomas based on the differential expression of prognostic markers. 71 , 72 Three to four major subgroups are generally well recognized, and their distinction appears to be relatively robust on meta-analysis. 73 At one end of the spectrum is the proneural subgroup, characterized by alterations in markers associated with neurogenesis, strongly associated with IDH1 mutations, and tending to have more favorable outcomes. The mesenchymal subgroup is at the other end of the spectrum, expressing markers usually associated with mesenchymal tissue and increased angiogenesis, associated with loss and/or mutation of the NF1 gene, and tending to have relatively worse outcomes. These expression-based subgroups were also distinguished by their CpG island methylation status. 74 Glioblastomas in the proneural subgroup were more frequently found to show widespread CpG island hypermethylation, termed a glioma CpG island methylator phenotype (G-CIMP), which was not usually seen in tumors of the mesenchymal subgroup.
At present, the diagnosis of glioblastomas per the 2007 WHO guidelines is based on well-established histological features. However, testing for MGMT status is becoming accepted as a standard of care for newly diagnosed glioblastomas, as is screening for IDH1 mutations. As newer therapeutic modalities emerge, there is increasing recognition for the need to communicate the status of prognostically and therapeutically relevant genomic markers to help guide clinical decisions for patient management. In a preliminary attempt to address this, consensus guidelines suggested at an international meeting of neuropathologists recommended that pathologists to provide an integrated diagnosis that incorporates the histological diagnosis and relevant molecular information. 75 For example, the diagnosis of glioblastoma, WHO grade IV, might also include the results of the IDH1 mutation status, MGMT status, and other relevant markers.
Pathology revealed glioblastoma, MGMT methylated. The MGMT methylation status is prognostic of better survival, relative to patients with unmethylated MGMT , and it also may predict response to treatment in some situations, to be discussed later.
Glioblastoma is the most common primary brain tumor in adults. In the United States, the age-adjusted incidence rate is 3.19 (95% confidence interval 3.16–3.21) per 100 000 persons. 76 The lifetime risk of being diagnosed with an invasive cancer of the nervous system, most of which are either glioblastoma or glioblastoma precursors, is approximately 1 in 161, with the risk of dying from an invasive cancer of the nervous system being approximately 1 in 222. 77 Thus, while glioblastoma remains a relatively rare tumor, many patients will be aware of one or more acquaintances or relatives with this condition purely by chance, sometimes raising concern of clustering of tumors by geography or within a family.
Glioblastoma is more common in men than women, as are all infiltrating gliomas. Incidence rises with age, peaking in the 75–84 age range. Thus, though the overall age-adjusted incidence rate of glioblastoma does not appear to be rising, the raw number of tumors diagnosed each year is expected to climb in coming decades due to aging of the population at large. In the United States, glioblastoma is more common amongst non-Hispanic whites than in black, Asian/Pacific Islander, or American Indian/Alaskan native groups.
Ionizing radiation remains the only proven exposure risk factor for glioblastoma. Typically, radiation-induced glioblastoma is seen years after therapeutic radiation for another tumor or medical condition. Diagnostic radiation, for example from CT scanning or even dental X-rays may theoretically confer increased risk of glioma, but this has not yet been confirmed in large-scale epidemiological studies. Nonionizing radiation, specifically related to the use of cellular telephones, has not been convincingly linked to glioma incidence, but this is an area of active investigation. Asthma and atopic disease are associated with lower risk of glioblastoma in multiple studies, with one meta-analysis showing a reduction in glioma risk of 40% in patients with allergies. 78
Many patients with glioblastoma are concerned about possible genetic risk factors, and ask about the advisability of screening for relatives. A heritable component to glioblastoma risk has long been demonstrated by the association between glioblastoma and Mendelian cancer syndromes including the Lynch and Li-Fraumeni syndromes. Recently, genome-wide association studies have revealed single nucleotide polymorphisms (SNPs) associated with glioblastoma risk. These SNPs have been identified within the candidate genes TERT (chromosome 5p15.33), EGFR (7p11.2), CDKN2B (9p21.3), TP53 (17p13.1), and RTEL1 (20q13.33). 79 Other SNP associations such as those within CCDC26 (8q24.21) and PHLDB1 (11q23.3) are mainly associated with IDH-mutant tumors and thus risk of secondary glioblastoma. Most of the currently identified risk SNPs confer only a modest increase in risk of glioma, and thus the absolute risk of glioma remains low even in individuals carrying the risk SNPs.
The patient came from a large family with a history of a number of different tumor types, but no first or second degree relatives with primary brain tumors. He was reassured that his family members were at low risk of primary brain tumor, and no screening studies were recommended.
Glioblastoma is an infiltrative tumor, with residual disease present after surgery, even in cases of radiographic gross total resection. Additional treatment to address this residual tumor is thus necessary as soon as the operative site has healed appropriately. This additional treatment may take the form radiation therapy (RT), chemotherapy, or both, depending on the clinical scenario.
Current Standard-of-Care Therapy
The current standard-of-care treatment for newly diagnosed glioblastoma was established by a landmark trial conducted by the European Organization for Research and Treatment of Cancer (EORTC) and the National Cancer Institute of Canada Trials Group (NCIC). 80 In this trial patients were randomized to RT alone (the previous standard of care) vs RT with concurrent and adjuvant oral TMZ chemotherapy. In this trial, median survival was 12.1 months in the radiation-only arm and 14.6 months in the TMZ arm. More importantly, combined chemoradiotherapy significantly increased the proportion of relatively long-term survivors from 10.9% to 27.2% at two years and from 1.9% to 9.8% at five years. Patients whose tumors had methylation of the promoter for the MGMT gene ( MGMT methylated) had greater benefit from the addition of TMZ (46% 2-year survival vs 27% for the MGMT unmethylated patients), but MGMT unmethylated patients still had incremental benefit from the addition of TMZ (14% 2-year survival vs <2%). 69
Numerous studies have indicated a benefit to using RT in the treatment of gliomas. 81 , 82 A dose of 60 Gy in 2 Gy fractions is the recommended dose based on prior studies indicating that doses up to but not exceeding 60 Gy impact survival. 83 , 84 Despite agreement of a standard dose recommended in both clinical trials and practice, there is tremendous variability in the volume of tissue irradiated. A common approach endorsed by the Radiation Therapy Oncology Group and other cooperative groups is to target the tumor edema with a 2.5-cm margin to a dose of 46 Gy followed by a cone down to the resection cavity and contrast-enhancing tumor with similar margin to a dose of 60 Gy. 85 Other cooperative groups also recommend a staged approach initially including tumor edema with a cone down to the enhancing tumor, but only add a 1-cm margin. Additional strategies include treating the enhancing tumor and resection cavity with a 2–3-cm margin to a total dose of 60 Gy without a staged volume reduction. 80 The volume of brain irradiated in each of these scenarios is substantially different. While studies have demonstrated comparable outcomes comparing conformal radiation to whole brain irradiation, similar comparisons have not been made between these varied radiation approaches. 86
Typically, TMZ is given daily during RT at a dose of 75 mg per square meter of surface area each day, followed by a rest period of approximately a month at the end of radiation. TMZ is then resumed at the dose of 150 mg per square meter on days 1–5 of a single 28-day cycle, and subsequent 28-day cycles are dosed at 200 mg per square meter on days 1–5 if the first adjuvant cycle was well tolerated. In the clinical trial that proved the efficacy of this approach, a total of 6 adjuvant cycles were given. 80 In practice, some physicians recommend more than 6 cycles, though there is currently no definitive data demonstrating that more prolonged regimens are associated with superior survival.
Side Effect Management
The most common symptomatic side effects of treatment are mild fatigue, nausea, and constipation. All patients receiving TMZ should be provided with antiemetic therapy, both to take prior to each TMZ dose to prevent nausea and also for as-needed use. Antiemetics of the serotonin 5-HT3 receptor antagonist class, such as ondansetron and granisetron, are often used for this purpose. TMZ often causes mild thrombocytopenia, but severe thrombocytopenia, neutropenia, and anemia are much less common. Asymptomatic leukopenia is very common in patients on TMZ. Prophylaxis against Pneumocystis jiroveci pneumonia with trimethoprim/sulfamethoxazole or an alternative agent is recommended for the full duration of TMZ therapy.
Common acute radiation side effects include headaches, nausea, exacerbation of presenting symptoms, hair loss, skin reaction at the site of radiation, and fatigue. Less common acute side effects can include dry mouth or altered taste, hearing impairment or seizures. Possible late side effects of radiation include decreased pituitary hormonal production, cataract formation, secondary cancers, and nerve damage. Radiation necrosis can occur and cause symptoms similar to tumor recurrence or stroke. Necrosis is initially managed with steroids but may require more intensive options such as surgery or bevacizumab therapy. 87
Neurocognition can be altered during initial therapy secondary to inflammation, anxiety, and medications, but radiation can also cause cognitive impairment months to years after it is completed, even in the absence of tumor progression. Learning and memory are the most commonly impaired cognitive domains. The extent of impairment may be related to patient-specific factors, but also related to volume of tissue irradiated and location of the radiation field. For example, dose to hippocampal structures has been found to influence extent of neurocognitive dysfunction. 88–90
Elderly Patients and Patients with Poor Performance Status
The pivotal trial that demonstrated the efficacy of TMZ for newly diagnosed glioblastoma did not include patients over the age of 70, leaving unanswered the question of whether this regimen is effective and well tolerated in elderly patients. 80 Moreover, a number of trials have been conducted that suggest, at least in some circumstances, abbreviated or less intensive treatments may be effective in older patients. In a randomized, phase III trial conducted by the Nordic Clinical Brain Tumor Study Group, patients age 60 or older with glioblastoma were randomized to the typical 6 weeks of RT, 2 weeks of hypofractionated RT, or TMZ without RT. 91 The trial demonstrated that both the hypofractionated RT and TMZ monotherapy were superior to 6 weeks of RT with respect to overall survival. Similarly, in the German NOA-08 trial, patients over 65 years of age were randomized to dose-dense TMZ or standard 6-week RT, with similar outcomes in each group. 92 Both the Nordic and NOA-08 trials demonstrated that MGMT methylation was predictive of response to TMZ therapy. Patients whose tumors demonstrated methylation of MGMT had better survival when treated with regimens that contained TMZ, whereas patients with unmethylated MGMT did better when treated with RT. Although both these studies were randomized phase III studies, there are significant limitations in interpreting the data. Notably, 6 weeks of RT with concurrent and adjuvant TMZ was not an arm in either study, so direct comparisons of these alternative approaches to the current standard of care are not possible. In addition, the definition of elderly varied across these trials and across other, retrospective analyses.
Currently, as no therapy has been proven superior or equivalent to standard combined chemoradiotherapy in elderly patients, this therapy is a reasonable option for patients of any age with good performance status who are felt likely to tolerate intensive treatment. In patients with poor performance status or those in whom treatment tolerability is a concern, treatment choice should be informed by MGMT methylation testing. In patients with MGMT methylation, TMZ monotherapy is reasonable, whereas RT monotherapy is an option for patients without MGMT methylation. Given the results of the Nordic trial, as well as a previous trial that demonstrated that abbreviated RT was not inferior to 6-week RT, hypofractionated RT is preferable to the standard six-week schedule in this patient group if not combined with chemotherapy. 93
Optune (NovoTTF-100A)
Recently released information, in the form of a press release and a presentation at a neuro-oncology specialty meeting, suggests that the addition of therapy with alternating electric fields via the Optune device (NovoCure) may prolong both progression-free and overall survival by several months when combined with standard chemoradiotherapy for newly diagnosed glioblastoma. 94 In the trial for which data were presented, Optune therapy was initiated at the time of initiation of adjuvant TMZ therapy and continued until tumor progression. While this result is cause for optimism, the results presented were from an interim data analysis, and publication of the full trial data in a peer-reviewed journal will be necessary before Optune therapy can be critically evaluated for possible inclusion in a new standard of care for glioblastoma.
Recent Clinical Trial Results
After combined chemoradiotherapy became the standard of care, more aggressive dosing of TMZ was tested in hopes that it might provide additional efficacy. RTOG 0525, a large phase III study, compared a dose-dense schedule of TMZ after RT with the standard 5-day schedule. No difference in survival was noted in either MGMT methylated or MGMT unmethylated patients. 85 Cilengitide, a targeted drug that inhibits integrins, was added to standard RT and TMZ in two separate clinical trials, a phase III study in MGMT methylated patients 95 and a phase II study in MGMT unmethylated patients, 96 but did not show improvement in survival in either case. The addition of the antiangiogenic agent bevacizumab, an antibody against VEGF, to standard RT and TMZ was tested in two large phase III trials. While there was some improvement in progression-free survival, no improvement in overall survival was noted in either of these trials. 97 , 98 As such, bevacizumab has not at this time been approved for use in initial treatment of glioblastoma.
Important Ongoing Clinical Trials
There are numerous phase I and II trials around the world testing the addition of other drugs to standard therapy, including targeted agents, cytotoxic agents, and a variety of immune-targeting approaches. In addition, there are trials in progress testing alternative radiation techniques such as proton therapy or imaging-guided radiation dose escalation, and trials testing metabolic approaches against cancer such as variations on the ketogenic diet.
With regard to phase III trials, a randomized, placebo-controlled trial is currently ongoing that tests the addition of an autologous vaccine made from a patient's own tumor and their own dendritic cells (DCVax-L) to initial adjuvant TMZ. 99 Another glioblastoma immunotherapy approach, using the EGFRvIII-targeted experimental cancer vaccine rindopepimut (CDX-110), is being evaluated in the ACT IV trial. 100 Within the elderly population specifically, a randomized, phase III trial comparing hypofractionated RT alone or in combination with TMZ has completed accrual in Canada, Europe, and Japan 101 ; the results are eagerly awaited to further inform whether combination therapy is effective in this patient population.
The patient had excellent performance status and was felt to be a candidate for standard chemoradiotherapy, despite his age of 73. He developed mild nausea and moderate fatigue during chemoradiotherapy, but no life-threatening toxicities. He likewise tolerated adjuvant TMZ well from a symptomatic standpoint, though several cycles had to be briefly delayed due to mild thrombocytopenia. He discontinued TMZ therapy and moved to an observational phase following the sixth adjuvant cycle. Had he not been judged a good candidate for standard therapy, a TMZ-only approach, sparing radiation, would have been an acceptable alternative for an elderly man with an MGMT -methylated tumor.
Initial Postoperative Imaging
Postoperative imaging is strongly recommended within the first 48 hours following surgical resection in order to establish a postoperative baseline. If MRI is obtained, it is important to compare precontrast T1-weighted images with post-contrast T1-weighted images in order to detect true residual enhancement, as blood products in or around the surgical bed are usually present and may cause T1 shortening on both pre-gadolinium and post-gadolinium images. It is also crucial to perform DWI in order to detect any perioperative infarction, which may subsequently gain enhancement in the subacute phase, lose restricted diffusion, and present as a troubling, new enhancing lesion on subsequent MRI follow-ups. 102
Long-term Follow-up
Generally, patients undergo monthly clinical evaluation and blood work during adjuvant TMZ, with MRI every other month. After the completion of TMZ, imaging is recommended every 2 to 3 months until 2 years from the end of treatment. After this time, MRI can be performed less often, provided the patient and physician are comfortable with this approach. Regardless of duration of disease-free survival, clinical evaluation and imaging should take place on at least an annual basis.
It is expected that the imaging appearance of a treated glioblastoma will evolve over time. One hopes for tumor regression after chemoradiation, but a temporary worsening of imaging findings, including increased contrast enhancement and edema signal with mass effect, is very common as a reaction to treated and dying tumor. This phenomenon is known as pseudoprogression, named so because the MRI appearance may be identical to tumor progression when, in fact, subsequent follow-up examinations with no change in therapy show regression of these imaging findings. 103 There is, unfortunately, often no basis on which to discriminate post-treatment related enhancement from enhancement related to viable tumor using standard morphological MRI.
Pseudoprogression may be more common with the combined use of TMZ with RT, it is more common in those with MGMT promoter methylation, and it has been associated with an improved clinical outcome in some cohorts. 104 , 105 The incidence of pseudoprogression is on the order of 30%, 106 depending on how it is judged, and most pseudoprogression occurs within 3 months of the end of RT. However, pseudoprogression occurring after this 3-month period but within the first year is not uncommon, particularly in those with MGMT promoter methylation. 107
The Macdonald criteria has been used as a framework for judging glioblastoma progression or regression, relying mainly upon 2-dimensional maximal diameters of contrast-enhancing lesions. 108 In 2010, the Response Assessment in Neurooncology (RANO) criteria were published, updating the Macdonald criteria in several important ways, while maintaining a reliance upon the product of perpendicular diameters of contrast-enhancing lesions as an indicator of tumor size and status. 109 One very important caveat in the RANO criteria is that, because of the common occurrence of pseudoprogression with modern chemoradiation therapy, a radiographic diagnosis of tumor progression cannot be made within the first 3 months of the end of chemoradiation if an enlarging, enhancing lesion is within the high-dose field of RT. Any apparent tumor progression within the first 3 months after radiation must be closely followed to differentiate early tumor progression from pseudoprogression. Because pseudoprogression occasionally occurs beyond 3 months from the end of radiotherapy, some would advocate early follow-up scans to confirm or deny pseudoprogression if there is apparent radiographic progression occurring even beyond the 3-month window after radiotherapy. RANO also allows for the determination of progression when there is nonenhancing tumoral progression, as nonenhancing tumor progression is not uncommon. 6 New, discrete, masslike abnormalities or new cortical expansion with T2 lengthening suggest nonenhancing tumor progression per RANO criteria. Unfortunately, distinguishing nonenhancing tumor from other treatment-related effects and edema is often difficult or impossible.
Given the complexity of MRI interpretation, other means of glioblastoma treatment response assessment are needed. Estimates of tumor cell proliferation rates and invasion have been made through the analysis of MRI coupled with computational modeling as a means to monitor treated glioblastomas over time. 110 , 111 Physiologic or mechanistic imaging techniques such as dynamic susceptibility contrast (DSC) perfusion imaging, dynamic contrast enhanced perfusion imaging (DCE), DWI, spectroscopy, and PET with various tracers also may aid in distinguishing progressing tumor from treatment-related changes such as pseudoprogression and radiation necrosis, but their implementation has to date been so variable site-to-site that these techniques have not been included in the RANO criteria.
Generally speaking, it is expected that CBV will be elevated with viable high-grade glioma but not radiation necrosis, and possibly not elevated with pseudoprogression, though data for pseudoprogression are as yet less clear. 112–119 However, the variability in perfusion imaging has already been mentioned, there is often overlap between CBV in viable glioblastoma and radiation-induced changes, there is frequently an admixture of viable tumor and radiation-induced changes, and leakage of gadolinium into the interstitium breaks tracer-kinetic modeling assumptions and presents a challenge to accurate determination of CBV. In order to optimize CBV measurement, the use of a leakage-correction option is advised during image postprocessing. Many experts also recommend the use of a gadolinium preload prior to the acquisition of DSC images. Histogram and voxel-wise analyses of perfusion data hold promise for improving the differentiation between viable tumor and treatment-related changes, but they are postprocessing intensive. 120 , 121 Given the frequent admixture of residual glioma and radiation-related changes, determination of a residual/recurrent tumor fraction would be desirable and such a metric using CBV has been shown to correlate with overall survival. 122 Importantly, the relative change in lesion CBV over time may be crucial in judging tumoral stability or progression, 123 though it bears stating explicitly that uniformity in perfusion technique between time points is mandatory for the best chance at fair comparisons.
DCE perfusion imaging, which is generally more technically demanding than DSC perfusion imaging, also holds promise for differentiating recurrent glioblastoma from treatment-related changes, using metrics such as the volume transfer coefficient k trans and initial area under the curve. 118 ,124–126 MR spectroscopy can be technically challenging to perform well and interpret, but after therapy choline and lipid and lactate levels have been shown to correlate with glioma outcomes. 119 , 127 More data on the value of MR spectroscopy in the glioblastoma post-treatment setting is needed. ADC values from DWI imaging, particularly when using advanced analysis techniques such as histogram analysis and functional diffusion maps (fDMs), 128–133 may also help to differentiate recurrent tumor from treatment-related changes. Amino acid PET may also aid in this discrimination better than with FDG PET but these techniques need further evaluation. 134–138 Finally, it is likely that multiparametric approaches with advanced MRI techniques will add to assessment of glioblastoma treatment response. 119
The first MRI following the completion of chemoradiotherapy, shown in Fig. 4 A, demonstrated a rim of contrast enhancement around the resection cavity that had not been visible on the initial postoperative imaging (Fig. 2 B). The patient was asymptomatic, the changes were suspected to be treatment-related, and no change of plan was recommended. He remained clinically and radiographically stable for nearly 2 years until he had tumor progression along the medial margin of his resection cavity, as shown in Fig. 4 B. His KPS at the time of progression was 80, due to increased left-sided weakness, but he was still able to live independently with his wife. After discussing options including continuing to focus on aggressive tumor treatment, potentially at the cost of short-term quality of life, vs prioritizing quality of life and supportive care with hospice, he chose to pursue salvage chemotherapy, the details of which are beyond the scope of this review.
Overall Survival
Survival after diagnosis of glioblastoma has been steadily improving over the course of the last decade, for reasons both known and unknown. The widespread adoption of TMZ for newly diagnosed glioblastoma in and after 2005 was associated with in an increase in population-level survival. 139 Likewise, though a survival benefit has yet to be demonstrated in a randomized prospective trial, population-based data suggest that survival also improved after the FDA approval of bevacizumab for recurrent glioblastoma. 140 Additional improvements in the survival of patients with glioblastoma may be due to incremental improvement in surgery, radiation, and supportive care.
The median survival figure of 14.6 months from the pivotal TMZ trial is often shared with patients with newly diagnosed glioblastoma, but clinical trial median survival numbers have little relevance when predicting the specific prognosis of an individual patient. Many factors can significantly impact survival, including but not limited to age at diagnosis, performance status, extent of resection, MGMT methylation status, and IDH mutation status. Median survival by MGMT methylation status in recent phase III trials is shown in Table 1 . A number of survival prognostication systems have been published, but again these are more relevant to cohorts than patients as individuals. In the long-term follow-up of the pivotal phase III TMZ trial, among patients treated with radiation and TMZ, 27.2% were still alive at 2 years, 16.0% at 3 years, 12.1% at 4 years, and 9.8% at 5 years. 141 Survival to 10 years or longer is very rare.
Survival by MGMT methylation status in recent phase III trials for newly diagnosed glioblastoma
a OS statistics from Stupp Lancet Oncology 2009; PFS statistics from Hegi NEJM 2005. All statistics regard the RT + TMZ trial arm.
Abbreviations: MGMT, O(6)-methylguanine-DNA methyltransferase; OS, overall survival; PFS, progression-free survival; EORTC, European Organisation for Research and Treatment of Cancer; NCIC, National Cancer Institute of Canada; RTOG, Radiation Therapy Oncology Group.
Because glioblastoma is an intrinsically fatal diagnosis, and treatment can cause significant side effects, most tumor treatment plans require the sacrifice of some quality of life in the short term in the hope of gaining duration of life in the longer term. Early in the course of disease, this is a trade-off that most patients readily accept, though some choose to forgo aggressive therapy. With each successive treatment, the expected duration of benefit tends to decrease and toxicity may increase, so the merits of focusing purely on quality of life should be readdressed regularly, for example at the time of clinical or radiographic progression. When the service is available, patients may benefit from speaking with a palliative care physician early in the course of their disease, and maintaining the relationship until the end of their lives. While death may occur precipitously as the result of a pulmonary embolism or intracerebral hemorrhage, most patients die after weeks or rarely months of progressive decline following the decision to discontinue aggressive tumor therapy, and hospice services can be extremely valuable to patients and their families in this situation.
Supportive Care (Long-term)
Most patients with glioblastoma who have experienced a seizure require lifelong AED therapy, particularly if seizures occur during or after initial tumor therapy. There is variability in practice regarding patients who experienced seizure at initial presentation and are seizure-free on anti-epileptic therapy following tumor treatment. Many neuro-oncologists recommend lifelong AED therapy in this situation as well, whereas others will consider tapering patients off of antiepileptic therapy after 1 to 2 years if he patient is interested in doing so and electroencephalogram (EEG) at that time does not demonstrate epileptiform discharges. Of course, freedom from seizures cannot be guaranteed, with or without continuation of antiepileptic therapy. Patients electing to attempt to discontinue AEDs should be counseled about seizure safety and avoiding high-risk activities.
Anticoagulation
Patients with glioblastoma are at significant risk of venous thromboembolism and related complications. There is no proven role for prophylactic anticoagulant therapy to prevent deep venous thrombosis (DVT). 142 Instead, patients should be educated about the symptoms of DVT and pulmonary embolism (PE), and physicians should have a low threshold for obtaining confirmatory testing if these symptoms occur. The diagnosis of glioblastoma is not a contraindication for treatment with systemic anticoagulation if a DVT/PE occurs, even in the setting of antiangiogenic therapy. 143 Treatment with low molecular weight heparin products has been shown to be more effective that oral anticoagulation with warfarin in patients with cancer. 144
Psychological and Emotional Well-being
Symptoms of depression and anxiety are common and undertreated in patients with glioblastoma. Many factors may contribute to depression in patients with glioblastoma, including loss of independence and function, the adjustment to the idea of a significantly shortened life, and possibly a direct biological effect of the tumor on neurotransmitter signaling. 145 Many patients and their families find cancer support groups, or ideally brain-tumor-specific support groups, very beneficial both for practical advice and the knowledge that they are not alone in facing this diagnosis. Antidepressant therapy and/or referral to a mental health professional should be considered for patients with symptoms that extend beyond the range of normal adjustment and negatively impact the quality of their lives.
Despite recent progress, glioblastoma remains an incurable tumor with survival under a year and a half for most patients. Multidisciplinary care is necessary to maximize survival time and preserve quality of life. In appropriately selected patients, aggressive surgery may relieve symptoms and prolong survival. Medical oncologists, radiation oncologists, and neurologists work as a team to design and deliver the initial treatment plan, typically involving the combination of RT and TMZ chemotherapy. Radiologists with expertise in the complexities of glioblastoma imaging help make the initial diagnosis and monitor response to therapy.
For glioblastoma survival to continue to improve, advances in each of these specialties and collaboration between specialties will be required. Advances in neurosurgical technique aided by advanced imaging modalities will allow for more extensive safe tumor resection at time of diagnosis and at time of recurrence. Ongoing clinical trials may help refine the long-recognized role of RT for the treatment of newly diagnosed glioblastoma in general and also define its role relative to that of TMZ in elderly patients. Novel therapeutic strategies, recently with an emphasis on molecularly targeted therapies and immunotherapeutic approaches, also hold the potential to significantly change the care of glioblastoma.
Conflict of interest statement . No conflicts of interest are reported for any author.
Rosati A , Buttolo L , Stefini R et al. . Efficacy and safety of levetiracetam in patients with glioma: a clinical prospective study . Arch Neurol . 2010 ; 67 (3) : 343 – 346 .
Google Scholar
Barker CA , Bishop AJ , Chang M et al. . Valproic acid use during radiation therapy for glioblastoma associated with improved survival . Int J Radiat Oncol Biol Phys . 2013 ; 86 (3) : 504 – 509 .
Bobustuc GC , Baker CH , Limaye A et al. . Levetiracetam enhances p53-mediated MGMT inhibition and sensitizes glioblastoma cells to temozolomide . Neuro Oncol . 2010 ; 12 (9) : 917 – 927 .
Glantz MJ , Cole BF , Forsyth PA et al. . Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology . Neurology . 2000 ; 54 (10) : 1886 – 1893 .
Health USNIo . Lacosamide for Seizure Prophylaxis in High-Grade Gliomas . https://clinicaltrials.gov/ct2/show/NCT01432171 . Accessed February 1, 2015 .
Chamberlain MC . Radiographic patterns of relapse in glioblastoma . J Neurooncol . 2011 ; 101 (2) : 319 – 323 .
Blanchet L , Krooshof PW , Postma GJ et al. . Discrimination between metastasis and glioblastoma multiforme based on morphometric analysis of MR images . AJNR Am J Neuroradiol . 2011 ; 32 (1) : 67 – 73 .
Jackson A , Kassner A , Annesley-Williams D et al. . Abnormalities in the recirculation phase of contrast agent bolus passage in cerebral gliomas: comparison with relative blood volume and tumor grade . AJNR Am J Neuroradiol . 2002 ; 23 (1) : 7 – 14 .
Law M , Yang S , Babb JS et al. . Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade . AJNR Am J Neuroradiol . 2004 ; 25 (5) : 746 – 755 .
Boxerman JL , Schmainda KM , Weisskoff RM . Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not . AJNR Am J Neuroradiol . 2006 ; 27 (4) : 859 – 867 .
Liu ZL , Zhou Q , Zeng QS et al. . Noninvasive evaluation of cerebral glioma grade by using diffusion-weighted imaging-guided single-voxel proton magnetic resonance spectroscopy . J Int Med Res . 2012 ; 40 (1) : 76 – 84 .
Wang S , Kim S , Chawla S et al. . Differentiation between glioblastomas, solitary brain metastases, and primary cerebral lymphomas using diffusion tensor and dynamic susceptibility contrast-enhanced MR imaging . AJNR Am J Neuroradiol . 2011 ; 32 (3) : 507 – 514 .
Cha S , Lupo JM , Chen MH et al. . Differentiation of glioblastoma multiforme and single brain metastasis by peak height and percentage of signal intensity recovery derived from dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging . AJNR Am J Neuroradiol . 2007 ; 28 (6) : 1078 – 1084 .
Chawla S , Zhang Y , Wang S et al. . Proton magnetic resonance spectroscopy in differentiating glioblastomas from primary cerebral lymphomas and brain metastases . J Comput Assist Tomogr . 2010 ; 34 (6) : 836 – 841 .
Server A , Josefsen R , Kulle B et al. . Proton magnetic resonance spectroscopy in the distinction of high-grade cerebral gliomas from single metastatic brain tumors . Acta Radiol . 2010 ; 51 (3) : 316 – 325 .
Radbruch A , Wiestler B , Kramp L et al. . Differentiation of glioblastoma and primary CNS lymphomas using susceptibility weighted imaging . Eur J Radiol . 2013 ; 82 (3) : 552 – 556 .
Deistung A , Schweser F , Wiestler B et al. . Quantitative susceptibility mapping differentiates between blood depositions and calcifications in patients with glioblastoma . PloS One . 2013 ; 8 (3) : e57924 .
Kickingereder P , Wiestler B , Sahm F et al. . Primary central nervous system lymphoma and atypical glioblastoma: multiparametric differentiation by using diffusion-, perfusion-, and susceptibility-weighted MR imaging . Radiology . 2014 ; 272 (3) : 843 – 850 .
Pope WB . Genomics of brain tumor imaging . Neuroimaging Clin N Am . 2015 ; 25 (1) : 105 – 119 .
Willats L , Calamante F . The 39 steps: evading error and deciphering the secrets for accurate dynamic susceptibility contrast MRI . NMR Biomed . 2013 ; 26 (8) : 913 – 931 .
Laws ER , Parney IF , Huang W et al. . Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project . J Neurosurg . 2003 ; 99 (3) : 467 – 473 .
Sanai N , Berger MS . Glioma extent of resection and its impact on patient outcome . Neurosurgery . 2008 ; 62 (4) : 753 – 764 ; discussion 264–756 .
McGirt MJ , Chaichana KL , Gathinji M et al. . Independent association of extent of resection with survival in patients with malignant brain astrocytoma . J Neurosurg . 2009 ; 110 (1) : 156 – 162 .
di Russo P , Perrini P , Pasqualetti F et al. . Management and outcome of high-grade multicentric gliomas: a contemporary single-institution series and review of the literature . Acta Neurochir (Wien) . 2013 ; 155 (12) : 2245 – 2251 .
Sanai N , Polley MY , Berger MS . Insular glioma resection: assessment of patient morbidity, survival, and tumor progression . J Neurosurg . 2010 ; 112 (1) : 1 – 9 .
Holdhoff M , Rosner GL , Alcorn S et al. . ‘Elderly’ patients with newly diagnosed glioblastoma deserve optimal care . J Neurooncol . 2013 ; 113 (2) : 343 – 344 .
Chang SM , Nelson S , Vandenberg S et al. . Integration of preoperative anatomic and metabolic physiologic imaging of newly diagnosed glioma . J Neurooncol . 2009 ; 92 (3) : 401 – 415 .
Farshidfar Z , Faeghi F , Mohseni M et al. . Diffusion tensor tractography in the presurgical assessment of cerebral gliomas . Neuroradiol J . 2014 ; 27 (1) : 75 – 84 .
Gempt J , Soehngen E , Forster S et al. . Multimodal imaging in cerebral gliomas and its neuropathological correlation . Eur J Radiol . 2014 ; 83 (5) : 829 – 834 .
Sanai N , Mirzadeh Z , Berger MS . Functional outcome after language mapping for glioma resection . N Engl J Med . 2008 ; 358 (1) : 18 – 27 .
De Witt Hamer PC , Robles SG , Zwinderman AH et al. . Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis . J Clin Oncol . 2012 ; 30 (20) : 2559 – 2565 .
Maldaun MV , Khawja SN , Levine NB et al. . Awake craniotomy for gliomas in a high-field intraoperative magnetic resonance imaging suite: analysis of 42 cases . J Neurosurg . 2014 ; 121 (4) : 810 – 817 .
Hatiboglu MA , Weinberg JS , Suki D et al. . Impact of intraoperative high-field magnetic resonance imaging guidance on glioma surgery: a prospective volumetric analysis . Neurosurgery . 2009 ; 64 (6) : 1073 – 1081 ; discussion 1081 .
Acerbi F , Broggi M , Eoli M et al. . Fluorescein-guided surgery for grade IV gliomas with a dedicated filter on the surgical microscope: preliminary results in 12 cases . Acta Neurochir (Wien) . 2013 ; 155 (7) : 1277 – 1286 .
Bi WL , Laws ER Jr . Searching for the light: fluorescence guidance in glioma resection . World Neurosurg . 2014 ; 82 (1–2) : 54 – 55 .
Li Y , Rey-Dios R , Roberts DW et al. . Intraoperative fluorescence-guided resection of high-grade gliomas: a comparison of the present techniques and evolution of future strategies . World Neurosurg . 2014 ; 82 (1–2) : 175 – 185 .
Zhao S , Wu J , Wang C et al. . Intraoperative fluorescence-guided resection of high-grade malignant gliomas using 5-aminolevulinic acid-induced porphyrins: a systematic review and meta-analysis of prospective studies . PloS One . 2013 ; 8 (5) : e63682 .
Stummer W , Pichlmeier U , Meinel T et al. . Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial . Lancet Oncol . 2006 ; 7 (5) : 392 – 401 .
Attenello FJ , Mukherjee D , Datoo G et al. . Use of Gliadel (BCNU) wafer in the surgical treatment of malignant glioma: a 10-year institutional experience . Ann Surg Oncol . 2008 ; 15 (10) : 2887 – 2893 .
Brem H , Piantadosi S , Burger PC et al. . Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group . Lancet . 1995 ; 345 (8956) : 1008 – 1012 .
Chang SM , Parney IF , McDermott M et al. . Perioperative complications and neurological outcomes of first and second craniotomies among patients enrolled in the Glioma Outcome Project . J Neurosurg . 2003 ; 98 (6) : 1175 – 1181 .
Sawaya R , Hammoud M , Schoppa D et al. . Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors . Neurosurgery . 1998 ; 42 (5) : 1044 – 1055 ; discussion 1055–1046 .
Marcus LP , McCutcheon BA , Noorbakhsh A et al. . Incidence and predictors of 30-day readmission for patients discharged home after craniotomy for malignant supratentorial tumors in California (1995–2010) . J Neurosurg . 2014 ; 120 (5) : 1201 – 1211 .
Louis DN , Ohgaki H , Wiestler OD et al. . The 2007 WHO classification of tumours of the central nervous system . Vol 114 . 2007/07/10 ed2007 .
Google Preview
Parsons DW , Jones S , Zhang X et al. . An integrated genomic analysis of human glioblastoma multiforme . Science. 2008 ; 321 (5897) : 1807 – 1812 .
Hartmann C , Meyer J , Balss J et al. . Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas . Acta Neuropathol . 2009 ; 118 (4) : 469 – 474 .
Sugawa N , Ekstrand AJ , James CD et al. . Identical splicing of aberrant epidermal growth factor receptor transcripts from amplified rearranged genes in human glioblastomas . Proc Natl Acad Sci USA . 1990 ; 87 (21) : 8602 – 8606 .
Ohgaki H , Dessen P , Jourde B et al. . Genetic pathways to glioblastoma: a population-based study . Cancer Res . 2004 ; 64 (19) : 6892 – 6899 .
Brennan CW , Verhaak RG , McKenna A et al. . The somatic genomic landscape of glioblastoma . Cell. 2013 ; 155 (2) : 462 – 477 .
Louis DN , von Deimling A , Chung RY et al. . Comparative study of p53 gene and protein alterations in human astrocytic tumors . J Neuropathol Exp Neurol . 1993 ; 52 (1) : 31 – 38 .
Yan H , Parsons DW , Jin G et al. . IDH1 and IDH2 mutations in gliomas . N Engl J Med . 2009 ; 360 (8) : 765 – 773 .
SongTao Q , Lei Y , Si G et al. . IDH mutations predict longer survival and response to temozolomide in secondary glioblastoma . Cancer Sci . 2012 ; 103 (2) : 269 – 273 .
Hartmann C , Hentschel B , Wick W et al. . Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas . Acta Neuropathol . 2010 ; 120 (6) : 707 – 718 .
Bujko M , Kober P , Matyja E et al. . Prognostic value of IDH1 mutations identified with PCR-RFLP assay in glioblastoma patients . Mol Diagn Ther . 2010 ; 14 (3) : 163 – 169 .
Yan W , Zhang W , You G et al. . Correlation of IDH1 mutation with clinicopathologic factors and prognosis in primary glioblastoma: a report of 118 patients from China . PloS One . 2012 ; 7 (1) : e30339 .
Metellus P , Coulibaly B , Colin C et al. . Absence of IDH mutation identifies a novel radiologic and molecular subtype of WHO grade II gliomas with dismal prognosis . Acta Neuropathol . 2010 ; 120 (6) : 719 – 729 .
Comprehensive genomic characterization defines human glioblastoma genes and core pathways . Nature . 2008 ; 455 (7216) : 1061 – 1068 .
von Deimling A , Eibl RH , Ohgaki H et al. . p53 mutations are associated with 17p allelic loss in grade II and grade III astrocytoma . Cancer Res . 1992 ; 52 (10) : 2987 – 2990 .
Newcomb EW , Cohen H , Lee SR et al. . Survival of patients with glioblastoma multiforme is not influenced by altered expression of p16, p53, EGFR, MDM2 or Bcl-2 genes . Brain Pathol . 1998 ; 8 (4) : 655 – 667 .
Simon M , Hosen I , Gousias K et al. . TERT promoter mutations: a novel independent prognostic factor in primary glioblastomas . Neuro Oncol . 2015 ; 17 (1) : 45 – 52 .
Nonoguchi N , Ohta T , Oh JE et al. . TERT promoter mutations in primary and secondary glioblastomas . Acta Neuropathol . 2013 ; 126 (6) : 931 – 937 .
Labussiere M , Boisselier B , Mokhtari K et al. . Combined analysis of TERT, EGFR, and IDH status defines distinct prognostic glioblastoma classes . Neurology . 2014 ; 83 (13) : 1200 – 1206 .
Bello MJ , Alonso ME , Aminoso C et al. . Hypermethylation of the DNA repair gene MGMT: association with TP53 G:C to A:T transitions in a series of 469 nervous system tumors . Mutat Res . 2004 ; 554 (1–2) : 23 – 32 .
Nakagawachi T , Soejima H , Urano T et al. . Silencing effect of CpG island hypermethylation and histone modifications on O6-methylguanine-DNA methyltransferase (MGMT) gene expression in human cancer . Oncogene . 2003 ; 22 (55) : 8835 – 8844 .
Esteller M , Garcia-Foncillas J , Andion E et al. . Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents . N Engl J Med . 2000 ; 343 (19) : 1350 – 1354 .
Thon N , Eigenbrod S , Grasbon-Frodl EM et al. . Predominant influence of MGMT methylation in non-resectable glioblastoma after radiotherapy plus temozolomide . J Neurol Neurosurg Psychiatry . 2011 ; 82 (4) : 441 – 446 .
Rivera AL , Pelloski CE , Gilbert MR et al. . MGMT promoter methylation is predictive of response to radiotherapy and prognostic in the absence of adjuvant alkylating chemotherapy for glioblastoma . Neuro Oncol . 2010 ; 12 (2) : 116 – 121 .
Ishii D , Natsume A , Wakabayashi T et al. . Efficacy of temozolomide is correlated with 1p loss and methylation of the deoxyribonucleic acid repair gene MGMT in malignant gliomas . Neurol Med Chir (Tokyo) . 2007 ; 47 (8) : 341 – 349 ; discussion 350 .
Hegi ME , Diserens AC , Gorlia T et al. . MGMT gene silencing and benefit from temozolomide in glioblastoma . N Engl J Med . 2005 ; 352 (10) : 997 – 1003 .
Mulholland S , Pearson DM , Hamoudi RA et al. . MGMT CpG island is invariably methylated in adult astrocytic and oligodendroglial tumors with IDH1 or IDH2 mutations . Int J Cancer . 2012 ; 131 5 : 1104 – 1113 .
Phillips HS , Kharbanda S , Chen R et al. . Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis . Cancer Cell . 2006 ; 9 (3) : 157 – 173 .
Verhaak RG , Hoadley KA , Purdom E et al. . Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1 . Cancer Cell . 2010 ; 17 (1) : 98 – 110 .
Huse JT , Phillips HS , Brennan CW . Molecular subclassification of diffuse gliomas: seeing order in the chaos . Glia . 2011 ; 59 (8) : 1190 – 1199 .
Noushmehr H , Weisenberger DJ , Diefes K et al. . Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma . Cancer Cell . 2010 ; 17 (5) : 510 – 522 .
Louis DN , Perry A , Burger P et al. . International Society Of Neuropathology–Haarlem consensus guidelines for nervous system tumor classification and grading . Brain Pathol . 2014 ; 24 (5) : 429 – 435 .
Ostrom QT , Gittleman H , Farah P et al. . CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010 . Neuro Oncol . 2013 ; 15 (Suppl 2) : ii1 – i56 .
Howlader N , Noone AM , Krapcho M et al. . (eds). SEER Cancer Statistics Review, 1975–2011 . Bethesda, MD : National Cancer Institute . http://seer.cancer.gov/csr/1975_2011/ , based on November 2013 SEER data submission, posted to the SEER web site, April 2014 .
Linos E , Raine T , Alonso A , Michaud D . Atopy and risk of brain tumors: a meta-analysis . J Natl Cancer Inst . 2007 ; 99 (20) : 1544 – 1550 .
Ostrom QT , Bauchet L , Davis FG et al. . The epidemiology of glioma in adults: a “state of the science” review . Neuro Oncol . 2014 ; 16 (7) : 896 – 913 .
Stupp RR , Mason WPWP , van den Bent MJMJ et al. . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma . N Engl J Med . 2005 ; 352 (10) : 987 – 996 .
Shapiro WR , Young DF . Treatment of malignant glioma. A controlled study of chemotherapy and irradiation . Arch Neurol . 1976 ; 33 (7) : 494 – 450 .
Walker MD , Alexander E Jr , Hunt WE et al. . Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial . J Neurosurg . 1978 ; 49 (3) : 333 – 343 .
Salazar OM , Rubin P , Feldstein ML et al. . High dose radiation therapy in the treatment of malignant gliomas: final report . Int J Radiat Oncol Biol Phys . 1979 ; 5 (10) : 1733 – 1740 .
Walker MD , Strike TA , Sheline GE . An analysis of dose-effect relationship in the radiotherapy of malignant gliomas . Int J Radiat Oncol Biol Phys . 1979 ; 5 (10) : 1725 – 1731 .
Gilbert MR , Wang M , Aldape KD et al. . Dose-Dense Temozolomide for Newly Diagnosed Glioblastoma: A Randomized Phase III Clinical Trial . J Clin Oncol . 2013 ; 31 (32) : 4085 – 4091 .
Kita M , Okawa T , Tanaka M et al. . [Radiotherapy of malignant glioma--prospective randomized clinical study of whole brain vs local irradiation] . Gan No Rinsho . 1989 ; 35 (11) : 1289 – 1294 .
Levin VA , Bidaut L , Hou P et al. . Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system . Int J Radiat Oncol Biol Phys . 2011 ; 79 (5) : 1487 – 1495 .
Crossen JR , Garwood D , Glatstein E et al. . Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy . J Clin Oncol . 1994 ; 12 (3) : 627 – 642 .
Gondi V , Hermann BP , Mehta MP et al. . Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors . Int J Radiat Oncol Biol Phys . 2013 ; 85 (2) : 348 – 354 .
Roman DD , Sperduto PW . Neuropsychological effects of cranial radiation: current knowledge and future directions . Int J Radiat Oncol Biol Phys . 1995 ; 31 (4) : 983 – 998 .
Malmström A , Grønberg BH , Marosi C et al. . Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial . Lancet Oncol . 2012 ; 13 (9) : 916 – 926 .
Wick W , Platten M , Meisner C et al. . Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial . Lancet Oncol . 2012 ; 13 (7) : 707 – 715 .
Ferguson M , Rodrigues G , Cao J et al. . Management of high-grade gliomas in the elderly . Semin Radiat Oncol . 2014 ; 24 (4) : 279 – 288 .
Novocure . Novocure Announces the EF-14 Phase III Clinical Trial of Tumor Treating Fields in Patients with Newly Diagnosed Glioblastoma has been Terminated at the Interim Analysis due to Early Success . http://www.novocure.com/~/media/Files/N/Novocure/press-release/2014/201408-EF14-Trial-Results-Press-Release.pdf . Accessed February 1, 2015 .
Stupp R , Hegi ME , Gorlia T et al. . Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071–22072 study): a multicentre, randomised, open-label, phase 3 trial . Lancet Oncol . 2014 ; 15 (10) : 1100 – 1108 .
Nabors LB , Fink KL , Mikkelsen T et al. . Two cilengitide regimens in combination with standard treatment for patients with newly diagnosed glioblastoma and unmethylated MGMT gene promoter: results of the open-label, controlled, randomized phase II CORE study . Neuro Oncol . 2015 ; 17 5 : 708 – 717 .
Chinot OL , Wick W , Mason W et al. . Bevacizumab plus radiotherapy–temozolomide for newly diagnosed glioblastoma . N Engl J Med . 2014 ; 370 (8) : 709 – 722 .
Gilbert MR , Dignam JJ , Armstrong TS et al. . A randomized trial of bevacizumab for newly diagnosed glioblastoma . N Engl J Med . 2014 ; 370 (8) : 699 – 708 .
Health USNIo . Study of a Drug [DCVax®-L] to Treat Newly Diagnosed GBM Brain Cancer . https://clinicaltrials.gov/ct2/show/NCT00045968 . Accessed February 1, 2015 .
Swartz AM , Li QJ , Sampson JH . Rindopepimut: a promising immunotherapeutic for the treatment of glioblastoma multiforme . Immunotherapy . 2014 ; 6 (6) : 679 – 690 .
Health USNIo . Radiation Therapy With or Without Temozolomide in Treating Older Patients With Newly Diagnosed Glioblastoma Multiforme . https://www.clinicaltrials.gov/ct2/show/NCT00045968 . Accessed February 1, 2015 .
Smith JS , Cha S , Mayo MC et al. . Serial diffusion-weighted magnetic resonance imaging in cases of glioma: distinguishing tumor recurrence from postresection injury . J Neurosurg . 2005 ; 103 (3) : 428 – 438 .
Hygino da Cruz LC Jr , Rodriguez I , Domingues RC et al. . Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma . AJNR Am J Neuroradiol . 2011 ; 32 (11) : 1978 – 1985 .
Brandes AA , Franceschi E , Tosoni A et al. . MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients . J Clin Oncol . 2008 ; 26 (13) : 2192 – 2197 .
Gerstner ER , McNamara MB , Norden AD et al. . Effect of adding temozolomide to radiation therapy on the incidence of pseudo-progression . J Neurooncol . 2009 ; 94 (1) : 97 – 101 .
Brandsma D , Stalpers L , Taal W et al. . Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas . Lancet Oncol . 2008 ; 9 (5) : 453 – 461 .
Stuplich M , Hadizadeh DR , Kuchelmeister K et al. . Late and prolonged pseudoprogression in glioblastoma after treatment with lomustine and temozolomide . J Clin Oncol . 2012 ; 30 (21) : e180 – e183 .
Macdonald DR , Cascino TL , Schold SC Jr et al. . Response criteria for phase II studies of supratentorial malignant glioma . J Clin Oncol . 1990 ; 8 (7) : 1277 – 1280 .
Wen PY , Macdonald DR , Reardon DA et al. . Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group . J Clin Oncol . 2010 ; 28 (11) : 1963 – 1972 .
Zaw TM , Pope WB , Cloughesy TF , Lai A et al. . Short-interval estimation of proliferation rate using serial diffusion MRI predicts progression-free survival in newly diagnosed glioblastoma treated with radiochemotherapy . J Neurooncol . 2014 ; 116 (3) : 601 – 608 .
Neal ML , Trister AD , Cloke T et al. . Discriminating survival outcomes in patients with glioblastoma using a simulation-based, patient-specific response metric . PloS One . 2013 ; 8 (1) : e51951 .
Barajas RF Jr , Chang JS , Segal MR et al. . Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging . Radiology . 2009 ; 253 (2) : 486 – 496 .
Kim YH , Oh SW , Lim YJ et al. . Differentiating radiation necrosis from tumor recurrence in high-grade gliomas: assessing the efficacy of 18F-FDG PET, 11C-methionine PET and perfusion MRI . Clin Neurol Neurosurg . 2010 ; 112 (9) : 758 – 765 .
Sugahara T , Korogi Y , Tomiguchi S et al. . Posttherapeutic intraaxial brain tumor: the value of perfusion-sensitive contrast-enhanced MR imaging for differentiating tumor recurrence from nonneoplastic contrast-enhancing tissue . AJNR Am J Neuroradiol . 2000 ; 21 (5) : 901 – 909 .
Hu LS , Baxter LC , Smith KA et al. . Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements . AJNR Am J Neuroradiol . 2009 ; 30 (3) : 552 – 558 .
Mangla R , Singh G , Ziegelitz D et al. . Changes in relative cerebral blood volume 1 month after radiation-temozolomide therapy can help predict overall survival in patients with glioblastoma . Radiology . 2010 ; 256 (2) : 575 – 584 .
Gahramanov S , Raslan AM , Muldoon LL et al. . Potential for differentiation of pseudoprogression from true tumor progression with dynamic susceptibility-weighted contrast-enhanced magnetic resonance imaging using ferumoxytol vs. gadoteridol: a pilot study . Int J Radiat Oncol Biol Phys . 2011 ; 79 (2) : 514 – 523 .
Kim HS , Goh MJ , Kim N et al. . Which combination of MR imaging modalities is best for predicting recurrent glioblastoma? Study of diagnostic accuracy and reproducibility . Radiology . 2014 ; 273 (3) : 831 – 843 .
Seeger A , Braun C , Skardelly M et al. . Comparison of three different MR perfusion techniques and MR spectroscopy for multiparametric assessment in distinguishing recurrent high-grade gliomas from stable disease . Acad Radiol . 2013 ; 20 (12) : 1557 – 1565 .
Kim HS , Kim JH , Kim SH et al. . Posttreatment high-grade glioma: usefulness of peak height position with semiquantitative MR perfusion histogram analysis in an entire contrast-enhanced lesion for predicting volume fraction of recurrence . Radiology . 2010 ; 256 (3) : 906 – 915 .
Tsien C , Galban CJ , Chenevert TL et al. . Parametric response map as an imaging biomarker to distinguish progression from pseudoprogression in high-grade glioma . J Clin Oncol . 2010 ; 28 (13) : 2293 – 2299 .
Hu LS , Eschbacher JM , Heiserman JE et al. . Reevaluating the imaging definition of tumor progression: perfusion MRI quantifies recurrent glioblastoma tumor fraction, pseudoprogression, and radiation necrosis to predict survival . Neuro Oncol . 2012 ; 14 (7) : 919 – 930 .
Boxerman JL , Ellingson BM , Jeyapalan S et al. . Longitudinal DSC-MRI for Distinguishing Tumor Recurrence From Pseudoprogression in Patients With a High-grade Glioma . Am J Clin Oncol . 2014 . [Epub ahead of print] .
Suh CH , Kim HS , Choi YJ et al. . Prediction of pseudoprogression in patients with glioblastomas using the initial and final area under the curves ratio derived from dynamic contrast-enhanced T1-weighted perfusion MR imaging . AJNR Am J Neuroradiol . 2013 ; 34 (12) : 2278 – 2286 .
Yun TJ , Park CK , Kim TM et al. . Glioblastoma Treated with Concurrent Radiation Therapy and Temozolomide Chemotherapy: Differentiation of True Progression from Pseudoprogression with Quantitative Dynamic Contrast-enhanced MR Imaging . Radiology . 2015 ; 274 3 : 830 – 840 .
Shin KE , Ahn KJ , Choi HS et al. . DCE and DSC MR perfusion imaging in the differentiation of recurrent tumour from treatment-related changes in patients with glioma . Clin Radiol . 2014 ; 69 (6) : e264 – e272 .
Li Y , Lupo JM , Parvataneni R et al. . Survival analysis in patients with newly diagnosed glioblastoma using pre- and postradiotherapy MR spectroscopic imaging . Neuro Oncol . 2013 ; 15 (5) : 607 – 617 .
Ellingson BM , Malkin MG , Rand SD et al. . Validation of functional diffusion maps (fDMs) as a biomarker for human glioma cellularity . J Magn Reson Imaging . 2010 ; 31 (3) : 538 – 548 .
Ellingson BM , Malkin MG , Rand SD et al. . Volumetric analysis of functional diffusion maps is a predictive imaging biomarker for cytotoxic and anti-angiogenic treatments in malignant gliomas . J Neurooncol . 2011 ; 102 (1) : 95 – 103 .
Moffat BA , Chenevert TL , Lawrence TS et al. . Functional diffusion map: a noninvasive MRI biomarker for early stratification of clinical brain tumor response . Proc Natl Acad Sci USA 2005 ; 102 (15) : 5524 – 5529 .
Ellingson BM , Cloughesy TF , Lai A et al. . Quantitative probabilistic functional diffusion mapping in newly diagnosed glioblastoma treated with radiochemotherapy . Neuro Oncol . 2013 ; 15 (3) : 382 – 390 .
Pope WB , Qiao XJ , Kim HJ et al. . Apparent diffusion coefficient histogram analysis stratifies progression-free and overall survival in patients with recurrent GBM treated with bevacizumab: a multi-center study . J Neurooncol . 2012 ; 108 (3) : 491 – 498 .
Lutz K , Wiestler B , Graf M et al. . Infiltrative patterns of glioblastoma: Identification of tumor progress using apparent diffusion coefficient histograms . J Magn Reson Imaging . 2014 ; 39 (5) : 1096 – 1103 .
Terakawa Y , Tsuyuguchi N , Iwai Y et al. . Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy . J Nucl Med . 2008 ; 49 (5) : 694 – 699 .
Rachinger W , Goetz C , Popperl G et al. . Positron emission tomography with O-(2-[18F]fluoroethyl)-l-tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas . Neurosurgery . 2005 ; 57 (3) : 505 – 511 ; discussion 505–511 .
Mehrkens JH , Popperl G , Rachinger W et al. . The positive predictive value of O-(2-[18F]fluoroethyl)-L-tyrosine (FET) PET in the diagnosis of a glioma recurrence after multimodal treatment . J Neurooncol . 2008 ; 88 (1) : 27 – 35 .
Chen W , Silverman DH , Delaloye S et al. . 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy . J Nucl Med . 2006 ; 47 (6) : 904 – 911 .
Ledezma CJ , Chen W , Sai V et al. . 18F-FDOPA PET/MRI fusion in patients with primary/recurrent gliomas: initial experience . Eur J Radiol . 2009 ; 71 (2) : 242 – 248 .
Darefsky AS , King JT Jr , Dubrow R . Adult glioblastoma multiforme survival in the temozolomide era: a population-based analysis of Surveillance, Epidemiology, and End Results registries . Cancer . 2012 ; 118 (8) : 2163 – 2172 .
Johnson DR , Leeper HE , Uhm JH . Glioblastoma survival in the United States improved after Food and Drug Administration approval of bevacizumab: a population-based analysis . Cancer . 2013 ; 119 (19) : 3489 – 3495 .
Stupp R , Hegi ME , Mason WP et al. . Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial . Lancet Oncol . 2009 ; 10 (5) : 459 – 466 .
Jo JT , Schiff D , Perry JR . Thrombosis in brain tumors . Semin Thromb Hemost . 2014 ; 40 (3) : 325 – 331 .
Norden AD , Bartolomeo J , Tanaka S et al. . Safety of concurrent bevacizumab therapy and anticoagulation in glioma patients . J Neurooncol . 2012 ; 106 (1) : 121 – 125 .
Lee AY , Levine MN , Baker RI et al. . Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer . N Engl J Med . 2003 ; 349 (2) : 146 – 153 .
Liu R , Page M , Solheim K et al. . Quality of life in adults with brain tumors: current knowledge and future directions . Neuro Oncol . 2009 ; 11 (3) : 330 – 339 .
- chemotherapy regimen
- glioblastoma
- surgical procedures, operative
- diagnostic imaging
- temozolomide
- standard of care
Email alerts
Related articles in pubmed, citing articles via.
- SNO Twitter
- Recommend to your Library
Affiliations
- Online ISSN 2054-2585
- Print ISSN 2054-2577
- Copyright © 2024 Society for Neuro-Oncology
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Brain tumour detection using machine and deep learning: a systematic review
- Published: 23 May 2024
Cite this article

- Novsheena Rasool ORCID: orcid.org/0000-0001-6405-6415 1 &
- Javaid Iqbal Bhat 1
182 Accesses
Explore all metrics
Brain tumors rank as the 1oth leading cause of mortality worldwide, accounting for 85% to 95% of all primary nervous system malignancies. The prevalence of this life-threatening disease is steadily increasing worldwide, highlighting the urgent need for an early and precise diagnosis. Timely identification is critical for initiating effective treatment and improving patient survival chances. Delayed diagnosis significantly elevates the risk of mortality. However, the heterogeneous nature of tumor cells poses challenges for radiologists, making manual diagnosis from magnetic resonance imaging (MRI) images time-consuming and complex. Machine learning (ML) and deep learning (DL) have become useful tools in medical image analysis. These techniques facilitate the automated extraction of intricate patterns and features from MRI images, thereby facilitating a more accurate and efficient tumor diagnosis. Furthermore, these algorithms have demonstrated the capability to handle the intricacy and variability of brain tumor characteristics, thereby improving the diagnostic process. A range of deep learning-based algorithms have been utilized to detect brain tumors, yielding impressive results. The purpose of this paper is to provide an exhaustive examination of the latest techniques used for diagnosing brain tumors from MRI imaging, utilizing machine and deep learning technologies. Moreover, it seeks to outline potential avenues for future exploration within this field. The profound insights gleaned from this comprehensive review are poised to offer invaluable guidance and support to both researchers and medical professionals in the healthcare industry.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Machine learning and deep learning approach for medical image analysis: diagnosis to detection

Brain tumor detection and classification using machine learning: a comprehensive survey

Convolutional neural networks: an overview and application in radiology
Data availability.
There is no such data to be made available.
Bienkowski M, Furtner J, Hainfellner JA (2018) Clinical neuropathology of brain tumors. Handb Clin Neurol 145:477–534
Article Google Scholar
Rasool N, Bhat JI (2023) Glioma brain tumor segmentation using deep learning: A review. In: 2023 10th International Conference on Computing for Sustainable Global Development (INDIACom). IEEE, pp 484-489
Lin Z, Yang R, Li K, Yi G, Li Z, Guo J, Huang G (2020) Establishment of age group classification for risk stratification in glioma patients. BMC Neurol 20:1–11
Article MATH Google Scholar
Ressel A, Fichte S, Brodhun M, Rosahl SK, Gerlach R (2019) WHO grade of intracranial meningiomas differs with respect to patient’s age, location, tumor size and peritumoral edema. J Neuro-Oncol 145:277–286
Grimm SA, Chamberlain MC (2016) Anaplastic astrocytoma. CNS Oncol 5(3):145–157
Tardivo V, Penner F, Garbossa D, Di Perna G, Pacca P, Salvati L, Zenga F (2020) Surgical management of pituitary adenomas: does age matter? Pituitary 23:92–102
Bhojani MS, Van Dort M, Rehemtulla A, Ross BD (2010) Targeted imaging and therapy of brain cancer using theranostic nanoparticles. Mol Pharm 7(6):1921–1929
Kao PY, Ngo T, Zhang A, Chen JW, Manjunath BS (2019) Brain tumor segmentation and tractographic feature extraction from structural MR images for overall survival prediction. In Brainlesion: Glioma, Multiple Sclerosis, Stroke and Traumatic Brain Injuries: 4th International Workshop, BrainLes 2018, Held in Conjunction with MICCAI 2018, Granada, Spain, September 16, 2018, Revised Selected Papers, Part II 4. Springer International Publishing, pp 128-141
Abhisheka B, Biswas SK, Purkayastha B, Das D, Escargueil A (2023) Recent trend in medical imaging modalities and their applications in disease diagnosis: a review. Multimed Tools Appl 1–36
Panduri B, Rao OS (2024) A survey on brain tumour segmentation techniques in deep learning. Int J Intell Syst Appl Eng 12(7s):412–425
MATH Google Scholar
Xiong S, Wu G, Fan X, Feng X, Huang Z, Cao W, Shi Z (2021) MRI-based brain tumor segmentation using FPGA-accelerated neural network. BMC Bioinforma 22:1–15
Wu D, Ma T, Ceritoglu C, Li Y, Chotiyanonta J, Hou Z, Mori S (2016) Resource atlases for multi-atlas brain segmentations with multiple ontology levels based on T1-weighted MRI. Neuroimage 125:120–130
Fathi Kazerooni A, Mohseni M, Rezaei S, Bakhshandehpour G, Saligheh Rad H (2015) Multi-parametric (ADC/PWI/T2-w) image fusion approach for accurate semi-automatic segmentation of tumorous regions in glioblastoma multiforme. Magn Reson Mater Phys Biol Med 28:13–22
Upadhyay N, Waldman A (2011) Conventional MRI evaluation of gliomas. Br J Radiol 84(special_issue_2):S107–S111
Riecker A, Mathiak K, Wildgruber D, Erb M, Hertrich I, Grodd W, Ackermann H (2005) fMRI reveals two distinct cerebral networks subserving speech motor control. Neurology 64(4):700–706
Rajput S, Kapdi RA, Raval MS, Roy M (2023) Interpretable machine learning model to predict survival days of malignant brain tumor patients. Mach Learn: Sci Technol 4(2):025025
Makada M, Matang M (2023) Role of DWI in intracranial pathologies with its comparison to flair and T2w imaging. Int J Acad Med Pharm 5(1):433–437
Google Scholar
Wang L, Wei L, Wang J, Li N, Gao Y, Ma H, Zhang M (2020) Evaluation of perfusion MRI value for tumor progression assessment after glioma radiotherapy: A systematic review and meta-analysis. Medicine 99(52):e23766
Yazdani E, Geramifar P, Karamzade-Ziarati N, Sadeghi M, Amini P, Rahmim A (2024) Radiomics and Artificial Intelligence in Radiotheranostics: A Review of Applications for Radioligands Targeting somatostatin receptors and prostate-specific membrane antigens. Diagnostics 14(2):181
Wani NA, Kumar R, Bedi J (2024) DeepXplainer: An interpretable deep learning-based approach for lung cancer detection using explainable artificial intelligence. Comput Methods Programs Biomed 243:107879
Zhou L, Jiang Y, Li W, Hu J, Zheng S (2024) Shape-Scale Co-Awareness Network for 3D Brain Tumor Segmentation. IEEE Trans Med Imaging
Rasool N, Bhat JI (2023) Unveiling the complexity of medical imaging through deep learning approaches. Chaos Theory Appl 5(4):267–280
Carass A, Roy S, Jog A, Cuzzocreo JL, Magrath E, Gherman A, Pham DL (2017) Longitudinal multiple sclerosis lesion segmentation: resource and challenge. NeuroImage 148:77–102
Porwal P, Pachade S, Kokare M, Deshmukh G, Son J, Bae W, Meriaudeau F (2020) Idrid: Diabetic retinopathy–segmentation and grading challenge. Med Image Anal 59:101561
Andrearczyk V, Oreiller V, Boughdad S, Rest CCL, Elhalawani H, Jreige M, Depeursinge A (2021) Overview of the HECKTOR challenge at MICCAI 2021: automatic head and neck tumor segmentation and outcome prediction in PET/CT images. In 3D head and neck tumor segmentation in PET/CT challenge. Cham: Springer International Publishing, pp 1-37
Weitz P, Valkonen M, Solorzano L, Carr C, Kartasalo K, Boissin C, Rantalainen M (2023) The ACROBAT 2022 challenge: automatic registration of breast cancer tissue. arXiv preprint arXiv:2305.18033
Wang C, Mahbod A, Ellinger I, Galdran A, Gopalakrishnan S, Niezgoda J, Yu Z (2024) FUSeg: The foot ulcer segmentation challenge. Information 15(3):140
Bakas S, Reyes M, Jakab A, Bauer S, Rempfler M, Crimi A, Jambawalikar SR (2018) Identifying the best machine learning algorithms for brain tumor segmentation, progression assessment, and overall survival prediction in the BRATS challenge. arXiv preprint arXiv:1811.02629
Carrete LR, Young JS, Cha S (2022) Advanced imaging techniques for newly diagnosed and recurrent gliomas. Front Neurosci 16:787755
Abd-Ellah MK, Awad AI, Khalaf AA, Hamed HF (2019) A review on brain tumor diagnosis from MRI images: Practical implications, key achievements, and lessons learned. Magn Reson Imaging 61:300–318
Chan S, Siegel EL (2019) Will machine learning end the viability of radiology as a thriving medical specialty? Br J Radiol 92(1094):20180416
Hangel G, Schmitz-Abecassis B, Sollmann N, Pinto J, Arzanforoosh F, Barkhof F, Emblem KE (2023) Advanced MR techniques for preoperative glioma characterization: part 2. J Magn Reson Imaging 57(6):1676–1695
Bauer S, Nolte LP, Reyes M (2011) Fully automatic segmentation of brain tumor images using support vector machine classification in combination with hierarchical conditional random field regularization. In Medical Image Computing and Computer-Assisted Intervention–MICCAI 2011: 14th International Conference, Toronto, Canada, September 18-22, 2011, Proceedings, Part III 14. Springer, Heidelberg, pp 354-361
Mustaqeem A, Javed A, Fatima T (2012) An efficient brain tumor detection algorithm using watershed & thresholding-based segmentation. Int J Image Graph Sig Process 4(10):34
Sharma, M., & Mukharjee, S. (2013). Brain tumor segmentation using genetic algorithm and artificial neural network fuzzy inference system (ANFIS). In Advances in Computing and Information Technology: Proceedings of the Second International Conference on Advances in Computing and Information Technology (ACITY) July 13-15, 2012, Chennai, India-Volume 2. Springer, Heidelberg, pp 329-339
Huang M, Yang W, Wu Y, Jiang J, Chen W, Feng Q (2014) Brain tumor segmentation based on local independent projection-based classification. IEEE Trans Biomed Eng 61(10):2633–2645
Abdel-Maksoud E, Elmogy M, Al-Awadi R (2015) Brain tumor segmentation based on a hybrid clustering technique. Egypt Inform J 16(1):71–81
Dvořák P, Menze B (2016) Local structure prediction with convolutional neural networks for multimodal brain tumor segmentation. In: Medical computer vision: Algorithms for big data: International workshop, MCV 2015, held in conjunction with MICCAI 2015, Munich, Germany, October 9, 2015, revised selected papers 18 . Springer International Publishing, pp 59-71
Havaei M, Davy A, Warde-Farley D, Biard A, Courville A, Bengio Y, Larochelle H (2017) Brain tumor segmentation with deep neural networks. Med Image Anal 35:18–31
Myronenko A (2019) 3D MRI brain tumor segmentation using autoencoder regularization. In Brainlesion: Glioma, Multiple Sclerosis, Stroke and Traumatic Brain Injuries: 4th International Workshop, BrainLes 2018, Held in Conjunction with MICCAI 2018, Granada, Spain, September 16, 2018, Revised Selected Papers, Part II 4. Springer International Publishing, pp 311-320
Sajid S, Hussain S, Sarwar A (2019) Brain tumor detection and segmentation in MR images using deep learning. Arab J Sci Eng 44(11):9249–9261
Akil M, Saouli R, Kachouri R (2020) Fully automatic brain tumor segmentation with deep learning-based selective attention using overlapping patches and multi-class weighted cross-entropy. Med Image Anal 63:101692
Isensee F, Jäger PF, Full PM, Vollmuth P, Maier-Hein KH (2021) nnU-Net for brain tumor segmentation. In Brainlesion: Glioma, Multiple Sclerosis, Stroke and Traumatic Brain Injuries: 6th International Workshop, BrainLes 2020, Held in Conjunction with MICCAI 2020, Lima, Peru, October 4, 2020, Revised Selected Papers, Part II 6. Springer International Publishing, pp 118-132
Jiang Y, Zhang Y, Lin X, Dong J, Cheng T, Liang J (2022) SwinBTS: A method for 3D multimodal brain tumor segmentation using swim transformer. Brain Sci 12(6):797
Zhu Z, He X, Qi G, Li Y, Cong B, Liu Y (2023) Brain tumor segmentation based on the fusion of deep semantics and edge information in multimodal MRI. Inf Fusion 91:376–387
Al Khalil Y, Ayaz A, Lorenz C, Weese J, Pluim J, Breeuwer M (2024) Multi-modal brain tumor segmentation via conditional synthesis with Fourier domain adaptation. Comput Med Imaging Graph 112:102332
Chen M, Shi X, Zhang Y, Wu D, Guizani M (2017) Deep feature learning for medical image analysis with convolutional autoencoder neural network. IEEE Trans Big Data 7(4):750–758
Yue L, Tian D, Chen W, Han X, Yin M (2020) Deep learning for heterogeneous medical data analysis. World Wide Web 23:2715–2737
Xing F, Xie Y, Su H, Liu F, Yang L (2017) Deep learning in microscopy image analysis: A survey. IEEE Trans Neural Netw Learn Syst 29(10):4550–4568
Zhao D, Wang W, Tang T, Zhang YY, Yu C (2023) Current progress in artificial intelligence-assisted medical image analysis for chronic kidney disease: a literature review. Comput Struct Biotechnol J
Bote-Curiel L, Munoz-Romero S, Gerrero-Curieses A, Rojo-Álvarez JL (2019) Deep learning and big data in healthcare: a double review for critical beginners. Appl Sci 9(11):2331
Jafari A, Ganesan A, Thalisetty CSK, Sivasubramanian V, Oates T, Mohsenin T (2018) Sensornet: A scalable and low-power deep convolutional neural network for multimodal data classification. IEEE Trans Circ Syst I: Regular Pap 66(1):274–287
Gezimati M, Singh G (2024) Terahertz Data Extraction and Analysis based on Deep Learning Techniques for Emerging Applications. IEEE Access
Balaban S (2015) Deep learning and face recognition: the state of the art. Biometric and surveillance technology for human and activity identification XII, 9457:68-75
Zhang T, Gao C, Ma L, Lyu M, Kim M (2019) An empirical study of common challenges in developing deep learning applications. In: 2019 IEEE 30th International Symposium on Software Reliability Engineering (ISSRE). IEEE, pp 104-115
Zhang C, Bengio S, Hardt M, Recht B, Vinyals O (2021) Understanding deep learning (still) requires rethinking generalization. Commun ACM 64(3):107–115
Huff DT, Weisman AJ, Jeraj R (2021) Interpretation and visualization techniques for deep learning models in medical imaging. Phys Med Biol 66(4):04TR01
Mohsen H, El-Dahshan ESA, El-Horbaty ESM, Salem ABM (2018) Classification using deep learning neural networks for brain tumors. Future Comput Inf J 3(1):68–71
Sharif MI, Khan MA, Alhussein M, Aurangzeb K, Raza M (2021) A decision support system for multimodal brain tumor classification using deep learning. Complex Intell Syst 1-14
Ahuja S, Panigrahi BK, Gandhi TK (2022) Enhanced performance of Dark-Nets for brain tumor classification and segmentation using colormap-based superpixel techniques. Mach Learn Appl 7:100212
Toğaçar M, Cömert Z, Ergen B (2020) Classification of brain MRI using hyper column technique with convolutional neural network and feature selection method. Expert Syst Appl 149:113274
Díaz-Pernas FJ, Martínez-Zarzuela M, Antón-Rodríguez M, González-Ortega D (2021) A deep learning approach for brain tumor classification and segmentation using a multiscale convolutional neural network. Healthcare 9(2):153 ( MDPI )
Brereton P, Kitchenham BA, Budgen D, Turner M, Khalil M (2007) Lessons from applying the systematic literature review process within the software engineeringdomain. J Syst Softw 80(4):571–583
Kitchenham B, Brereton OP, Budgen D, Turner M, Bailey J, Linkman S (2009) Systematicliteraturereviewsinsoftwareengineering–asystematicliterature review. Inf Softw Technol 51(1):7–15
Kitchenham B, Charters S (2007) Guidelines for performing systematic literature reviews in software engineering. Technical report, ver. 2.3 ebse technical report. ebse
Zhang D, Huang G, Zhang Q, Han J, Han J, Yu Y (2021) Cross-modality deep feature learning for brain tumor segmentation. Pattern Recog 110:107562
Guo Z, Li X, Huang H, Guo N, Li Q (2019) Deep learning-based image segmentation on multimodal medical imaging. IEEE Trans Radiat Plasma Med Sci 3(2):162–169
Aljuaid A, Anwar M (2022) Survey of supervised learning for medical image processing. SN Comput Sci 3(4):292
Raza K, Singh NK (2021) A tour of unsupervised deep learning for medical image analysis. Curr Med Imaging 17(9):1059–1077
Kiranyaz S, Avci O, Abdeljaber O, Ince T, Gabbouj M, Inman DJ (2021) 1D convolutional neural networks and applications: a survey. Mech Syst Signal Process 151:107398
Kaur N, Mittal A, Singh G (2022) Methods for automatic generation of radiological reports of chest radiographs: a comprehensive survey. Multimed Tools Appl 81(10):13409–13439
López-Linares Román K, García Ocaña MI, Lete Urzelai N, González Ballester MÁ, Macía Oliver I (2020) Medical image segmentation using deep learning. Deep Learning in Healthcare: Paradigms and Applications, pp 17-31
Sadad T, Rehman A, Munir A, Saba T, Tariq U, Ayesha N, Abbasi R (2021) Brain tumor detection and multi-classification using advanced deep learning techniques. Microsc Res Tech 84(6):1296–1308
Jin X, Xu C, Feng J, Wei Y, Xiong J, Yan S (2016) Deep learning with s-shaped rectified linear activation units. In: Proceedings of the AAAI Conference on Artificial Intelligence vol 30(1)
Zahoor MM, Qureshi SA, Bibi S, Khan SH, Khan A, Ghafoor U, Bhutta MR (2022) A new deep hybrid boosted and ensemble learning-based brain tumor analysis using MRI. Sensors 22(7):2726
Kulkarni SM, Sundari G (2021) Comparative analysis of performance of deep cnn based framework for brain mri classification using transfer learning. J Eng Sci Technol 16(4):2901–2917
Pereira S, Pinto A, Alves V, Silva CA (2016) Brain tumor segmentation using convolutional neural networks in MRI images. IEEE Trans Med Imaging 35(5):1240–1251
Wang G, Li W, Ourselin S, Vercauteren T (2017) Automatic brain tumor segmentation using cascaded anisotropic convolutional neural networks. In International MICCAI brainlesion workshop. Springer, Cham, pp 178-190
Rasool N, Bhat J (2023) Multimodal Brain Tumor Segmentation using 3D-U-Net. Indian J Nat Prod Resour 8. https://www.researchgate.net/publication/373096976_Multimodal_Brain_Tumor_Segmentation_using_3D-U-Net
Allah AMG, Sarhan AM, Elshennawy NM (2023) Edge U-Net: Brain tumor segmentation using MRI based on deep U-Net model with boundary information. Expert Syst Appl 213:118833
Rai HM, Chatterjee K (2021) 2D MRI image analysis and brain tumor detection using deep learning CNN model LeU-Net. Multimed Tools Appl 80(28):36111–36141
Kumar PS, Sakthivel VP, Raju M, Sathya PD (2023) Brain tumor segmentation of the FLAIR MRI images using novel ResUnet. Biomed Signal Process Control 82:104586
Shaik NS, Cherukuri TK (2022) Multi-level attention network: application to brain tumor classification. Signal Image Video Process 16(3):817–824
Musallam AS, Sherif AS, Hussein MK (2022) A new convolutional neural network architecture for automatic detection of brain tumors in magnetic resonance imaging images. IEEE Access 10:2775–2782. IEEE
Wankhede DS, Rangasamy S (2022) Dynamic architecture based deep learning approach for glioblastoma brain tumor survival prediction. Neurosci Informa 2(4):100062
Abd-Ellah MK, Awad AI, Hamed HF, Khalaf AA (2019) Parallel deep CNN structure for glioma detection and classification via brain MRI Images. In: 2019 31st International Conference on Microelectronics (ICM). IEEE, pp 304-307
Vani N, Sowmya A, Jayamma N (2017) Brain tumor classification using support vector machine. Int Res J Eng Technol (IRJET) 4(7):792–796
Lefkovits L, Lefkovits S, Szilágyi L (2016) Brain tumor segmentation with optimized random forest. In Brainlesion: Glioma, Multiple Sclerosis, Stroke and Traumatic Brain Injuries: Second International Workshop, BrainLes 2016, with the Challenges on BRATS, ISLES and mTOP 2016, Held in Conjunction with MICCAI 2016, Athens, Greece, October 17, 2016, Revised Selected Papers 2. Springer International Publishing, pp 88-99
Gajula S, Rajesh V (2024) An MRI brain tumour detection using logistic regression-based machine learning model. Int J Syst Assur Eng Manag 15(1):124–134
Bashir-Gonbadi F, Khotanlou H (2021) Brain tumor classification using deep convolutional autoencoder-based neural network: multi-task approach. Multimed Tools Appl 80(13):19909–19929
Ding Y, Zhang C, Cao M, Wang Y, Chen D, Zhang N, Qin Z (2021) ToStaGAN: An end-to-end two-stage generative adversarial network for brain tumor segmentation. Neurocomputing 462:141–153
Vu MH, Nyholm T, Löfstedt T (2020) TuNet: End-to-end hierarchical brain tumor segmentation using cascaded networks. In Brainlesion: Glioma, Multiple Sclerosis, Stroke and Traumatic Brain Injuries: 5th International Workshop, BrainLes 2019, Held in Conjunction with MICCAI 2019, Shenzhen, China, October 17, 2019, Revised Selected Papers, Part I 5. Springer International Publishing, pp 174-186
Liu H, Shen X, Shang F, Ge F, Wang F (2019) CU-Net: Cascaded U-Net with loss weighted sampling for brain tumor segmentation. In Multimodal Brain Image Analysis and Mathematical Foundations of Computational Anatomy: 4th International Workshop, MBIA 2019, and 7th International Workshop, MFCA 2019, Held in Conjunction with MICCAI 2019, Shenzhen, China, October 17, 2019, Proceedings 4. Springer International Publishing, pp 102-111
Asif S, Zhao M, Chen X, Zhu Y (2023) BMRI-NET: A deep stacked ensemble model for multi-class brain tumor classification from MRI images. Interdiscip Sci: Comput Life Sci 15(3):499–514
Archana KV, Komarasamy G (2023) A novel deep learning-based brain tumor detection using the Bagging ensemble with K-nearest neighbor. J Intell Syst 32(1):20220206
Dutta S, Bandyopadhyay S (2020) Cross-validated AdaBoost classifier used for brain tumor detection. Preprints
Book MATH Google Scholar
Han L, Kamdar MR (2018) MRI to MGMT: predicting methylation status in glioblastoma patients using convolutional recurrent neural networks. In: Pacific Symposium on Biocomputing 2018: Proceedings of the Pacific Symposium, pp 331-342)
Pitchai R, Supraja P, Victoria AH, Madhavi M (2021) Brain tumor segmentation using deep learning and fuzzy k-means clustering for magnetic resonance images. Neural Process Lett 53(4):2519–2532
Cinar N, Ozcan A, Kaya M (2022) A hybrid DenseNet121-UNet model for brain tumor segmentation from MR Images. Biomed Signal Process Control 76:103647
Ramalakshmi C, Chandran AJ (2014) Automatic brain tumor detection in MR images using neural network-based classification. Int J Comput Sci Netw Secur (IJCSNS) 14(5):38
Kadkhodaei M et al (2016) Automatic segmentation of multimodal brain tumor images based on classification of super-voxels. IEEE 38th Annual International Conference of the Engineering in Medicine and Biology Society (EMBC), pp 5945-5948
Moeskops P, Viergever MA, Mendrik AM, De Vries LS, Benders MJ, Išgum I (2016) Automatic segmentation of MR brain images with a convolutional neural network. IEEE Trans Med imaging 35(5):1252–1261
Anithadevi D, Perumal K (2016) A hybrid approach-based segmentation technique for brain tumor in MRI Images. arXiv preprint arXiv:1603.02447
Chato L, Latifi S (2017) Machine learning and deep learning techniques to predict overall survival of brain tumor patients using MRI images. In: 2017 IEEE 17th international conference on bioinformatics and bioengineering (BIBE). IEEE, pp 9-14
Das S, Aranya ORR, Labiba NN (2019) Brain tumor classification using convolutional neural network. In 2019 1st International Conference on Advances in Science, Engineering and Robotics Technology (ICASERT). IEEE, pp 1-5
Srinivas K, Reddy BRS (2019) Modified kernel based fuzzy clustering for MR brain image segmentation using deep learning. J Eng Adv Technol 8:2249–895
Sultan HH, Salem NM, Al-Atabany W (2019) Multi-classification Deep Learning. International of brain tumor images using deep neural network. IEEE Access 7:69215–69225
Siar M, Teshnehlab M (2019) Brain tumor detection using deep neural network and machine learning algorithm. In 2019 9th international conference on computer and knowledge engineering (ICCKE). IEEE, pp 363-368
Saba T, Mohamed AS, El-Affendi M, Amin J, Sharif M (2020) Brain tumor detection using fusion of hand crafted and deep learning features. Cogn Syst Res 59:221–230
Gupta RK, Bharti S, Kunhare N, Sahu Y, Pathik N (2022) Brain tumor detection and classification using cycle generative adversarial networks. Interdiscip Sci: Comput life Sci 14(2):485–502
Nayak DR, Padhy N, Mallick PK, Bagal DK, Kumar S (2022) Brain tumour classification using noble deep learning approach with parametric optimization through metaheuristics approaches. Computers 11(1):10
Naser MA, Deen MJ (2020) Brain tumor segmentation and grading of lower-grade glioma using deep learning in MRI images. Comput Biol Med 121:103758
Islam KT, Wijewickrema S, O’Leary S (2022) A deep learning framework for segmenting brain tumors using MRI and synthetically generated CT images. Sensors 22(2):523
Arif M, Ajesh F, Shamsudheen S, Geman O, Izdrui D, Vicoveanu D (2022) Brain tumor detection and classification by MRI using biologically inspired orthogonal wavelet transform and deep learning techniques. J Healthc Eng 2022
Győrfi Á, Kovács L, Szilágyi L (2019) Brain tumor detection and segmentation from magnetic resonance image data using ensemble learning methods. In: 2019 IEEE International Conference on Systems, Man and Cybernetics (SMC). IEEE, pp 909-914
Dipu NM, Shohan SA, Salam KA (2021) Brain tumor detection using various deep learning algorithms. In: 2021 International Conference on Science & Contemporary Technologies (ICSCT). IEEE, pp 1-6
Sugimori H, Hamaguchi H, Fujiwara T, Ishizaka K (2021) Classification of type of brain magnetic resonance images with deep learning technique. Magn Reson Imaging 77:180–185
Nalepa J, Lorenzo PR, Marcinkiewicz M, Bobek-Billewicz B, Wawrzyniak P, Walczak M, Hayball MP (2020) Fully-automated deep learning-powered system for DCE-MRI analysis of brain tumors. Artif Intell Med 102:101769
Wang Z, Zhang Z, Liu J, Yi X (2023) Research on Segmentation Method of Brain Tumor Image Based on Deep Learning. In: 2023 3rd International Conference on Electronic Information Engineering and Computer Science (EIECS). IEEE, pp 118-122
Gryska E, Björkman-Burtscher I, Jakola AS, Dunås T, Schneiderman J, Heckemann RA (2022) Deep learning for automatic brain tumour segmentation on MRI: evaluation of recommended reporting criteria via a reproduction and replication study. BMJ Open 12(7):e059000
Walsh J, Othmani A, Jain M, Dev S (2022) Using U-Net network for efficient brain tumor segmentation in MRI images. Healthc Anal 2:100098
Murmu A, Kumar P (2021) Deep learning model-based segmentation of medical diseases from MRI and CT images. In: TENCON 2021-2021 IEEE Region 10 Conference (TENCON). IEEE, pp 608-613
Bloice MD, Stocker C, Holzinger A (2017) Augmentor: an image augmentation library for machine learning. arXiv preprint arXiv:1708.04680
Buslaev A, Iglovikov VI, Khvedchenya E, Parinov A, Druzhinin M, Kalinin AA (2020) Albumentations: fast and flexible image augmentations. Information 11(2):125
Lowekamp BC, Chen DT, Ibáñez L, Blezek D (2013) The design of SimpleITK. Front Neuroinformatics 7:45
Sial AH, Rashdi SYS, Khan AH (2021) Comparative analysis of data visualization libraries Matplotlib and Seaborn in Python. Int J 10(1):45
Waskom ML (2021) Seaborn: statistical data visualization. J Open Source Softw 6(60):3021
Stančin I, Jović A (2019) An overview and comparison of free Python libraries for data mining and big data analysis. In: 2019 42nd International convention on information and communication technology, electronics and microelectronics (MIPRO). IEEE, pp 977-982)
Sartaj (n.d.) Brain Tumor Classification (MRI) Dataset. [Online]. Available: https://www.kaggle.com/sartajbhuvaji/brain-tumor-classification-mri . Accessed 10 Jun 2021
Krishnapriya S, Karuna Y (2023) Pre-trained deep learning models for brain MRI image classification. Front Hum Neorosci 17:1150120
Cheng J, Huang W, Cao S, Yang R, Yang W, Yun Z, Wang Z, Feng Q (2015) Enhanced performance of brain tumor classification via tumor region augmentation and partition. PloS one 10(10):e0140381
Amin J, Sharif M, Gul N, Raza M, Anjum MA, Nisar MW, Bukhari SAC (2020) Brain tumor detection by using stacked autoencoders in deep learning. J Med syst 44:1–12
Zhang D, Huang G, Zhang Q, Han J, Han J, Wang Y, Yu Y (2020) Exploring task structure for brain tumor segmentation from multi-modality MR images. IEEE Trans Image Process 29:9032–9043
Waghmare VK, Kolekar MH (2021) Brain tumor classification using deep learning. Internet Things Healthc Technol 155–175:138
Muhammad K, Khan S, Del Ser J, De Albuquerque VHC (2020) Deep learning for multigrade brain tumor classification in smart healthcare systems: a prospective survey. IEEE Trans Neural Netw Learn Syst 32(2):507–522
Miao K, Basterrechea KF, Hernandez SL, Ahmed OS, Patel MV, Bader KB (2024) Development of Convolutional Neural Network to Segment Ultrasound Images of Histotripsy Ablation. IEEE Trans Biomed Eng
Özyurt F, Sert E, Avci E, Dogantekin E (2019) Brain tumor detection based on Convolutional Neural Network with neutrosophic expert maximum fuzzy sure entropy. Measurement 147:106830
Ranjbarzadeh R, Bagherian Kasgari A, Jafarzadeh Ghoushchi S, Anari S, Naseri M, Bendechache M (2021) Brain tumor segmentation based on deep learning and an attention mechanism using MRI multi-modalities brain images. Sci Rep 11(1):1–17
Alzubaidi L, Bai J, Al-Sabaawi A, Santamaría J, Albahri AS, Al-dabbagh BSN, Gu Y (2023) A survey on deep learning tools dealing with data scarcity: definitions, challenges, solutions, tips, and applications. J Big Data 10(1):46
Download references
Author information
Authors and affiliations.
Department of Computer Science (School of Engineering and Technology), Islamic University of Science & Technology Kashmir, Awantipora, Pulwama, Jammu and Kashmir, India
Novsheena Rasool & Javaid Iqbal Bhat
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Javaid Iqbal Bhat .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Rasool, N., Bhat, J.I. Brain tumour detection using machine and deep learning: a systematic review. Multimed Tools Appl (2024). https://doi.org/10.1007/s11042-024-19333-2
Download citation
Received : 02 November 2022
Revised : 17 April 2024
Accepted : 30 April 2024
Published : 23 May 2024
DOI : https://doi.org/10.1007/s11042-024-19333-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Brain tumor detection
- Deep learning
- Segmentation
- Classification
- Medical image processing
- Machine learning
Advertisement
- Find a journal
- Publish with us
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 03 June 2024
Risk factors for secondary thyroid cancer in patients with breast cancer: a propensity‑matched SEER analysis
- Yizhuo Diao 1 ,
- Ruiqi Wang 1 na1 ,
- Jiaxue Cui 1 na1 ,
- Chenxin Jin 1 ,
- Yongxing Chen 1 &
- Xiaofeng Li 1
Scientific Reports volume 14 , Article number: 12679 ( 2024 ) Cite this article
105 Accesses
Metrics details
- Cancer epidemiology
- Cancer models
With the rapid development of imaging technology and comprehensive treatment in modern medicine, the early diagnosis rate of breast cancer is constantly improving, and the prognosis is also improving; As breast cancer patients survive longer, the risk of developing second primary cancers increases. Since both breast and thyroid are Hormone receptor sensitive organs, which are regulated by hypothalamus pituitary target gland endocrine axis, changes in body endocrine status may lead to the occurrence of these two diseases in succession or simultaneously. This study extracted clinical data and survival outcomes of breast cancer patients registered in the Surveillance, Epidemiology and End Results (SEER) database between 2010 and 2019. After matching the case and controls with propensity scores, the selected patients were randomly split into training and test datasets at a ratio of 7:3. Univariate and multivariate COX proportional regression analysis is used to determine independent risk factors for secondary thyroid cancer and construct a column chart prediction model. Age, ethnicity, whether radiotherapy, tumor primary location, N stage, M stage were identified by Cox regression as independent factors affecting secondary thyroid cancer in patients with breast cancer patients, and a risk factor nomogram was established to predict patients’ 3 and 5 year survival probabilities. The AUC values for 3 and 5 years in the training set were 0.713, 0.707, and the c-index was 0.693 (95% CI 0.67144, 0.71456), and the AUC values for 3 and 5 years in the validation set were 0.681, 0.681, and the c-index was 0.673 (95% CI 0.64164, 0.70436), respectively.
Similar content being viewed by others
Risk of second primary thyroid cancer in cancer survivors

Breast cancer risk among thyroid cancer survivors and the role of I-131 treatment

Impact of thyroid hormone replacement on the risk of second cancer after thyroidectomy: a Korean National Cohort Study
Introduction.
Breast cancer is a malignant tumor that occurs in the Epithelium of the breast gland 1 . It is the “No. 1 public enemy” that threatens the physical and mental health of women around the world today 2 . It ranks first in the incidence and death of cancer among women in most countries around the world 3 . According to the data of the 2023 American Cancer Statistical Report, breast cancer is still one of the three most common cancers among women 4 ,occupying the first place in expected new cases among women; With the rapid development of imaging technology and comprehensive treatment in modern medicine 5 , the early diagnosis rate of breast cancer continues to improve, and the prognosis also improves 6 .The disease-free survival rate and overall survival rate of breast cancer patients have significantly improved; With the prolongation of the survival period of breast cancer patients, the risk of second primary cancer (SPC) may increase 7 ; Thyroid cancer is a malignant tumor originating from thyroid follicular epithelium 8 . It is considered to be an inert tumor 9 and the most common malignant tumor in the Endocrine system and head and neck tumors 10 ; Since both breast and thyroid are Hormone receptor sensitive organs, which are regulated by hypothalamus pituitary target gland endocrine axis, changes in body endocrine status may lead to the occurrence of these two diseases in succession or simultaneously.
Materials and methods
Data sources.
The SEER database containing 13 registry centers prepared by the National Cancer Institute was selected for the data of this study, and a total of 692,555 patients diagnosed with breast cancer from 2010 to 2019 were extracted from the database using the SEER*Stat software program, and a total of 393,722 patients were screened according to the inclusion and exclusion criteria.
Inclusion and exclusion criteria
Inclusion criteria.
(1) Patients diagnosed with breast cancer as the first tumor from 2010 to 2019 (2) Patients with no more than 2 tumors and the second type of tumor is thyroid cancer (3) Female patients (4) Patients with complete clinical data(Includes time of diagnosis, age at diagnosis, marital status at diagnosis, site of origin, mode of diagnostic confirmation, mode of case report, chemotherapy or not, radiotherapy or not, molecular typing, Progesterone Receptor, Estrogen Receptor, T,N, and M typing, type of secondary second tumor, interval between secondary second tumors, survival time, survival status)
Exclusion criteria
(1) Those with benign tumors (2) Proven only at autopsy or death (3) Patients with thyroid cancer occurring within 3 months of breast cancer diagnosis
Independent variables
The study data were converted to categorical variables in order to make the study more intuitive and standardized. Race was categorized as black, white, and other; marital status was categorized as married, unmarried; radiation and chemotherapy status was categorized as yes, no; primary tumor location was categorized as mid-breast, quadrant I, quadrant II, quadrant III, quadrant IV, and other; estrogen receptor (ER), and progesterone receptor (PR) status was categorized as estrogen receptor (ER) and progesterone receptor (PR) status positive and negative; and molecular staging included LuminalA, LuminalB, HER2 overexpression and triple-negative; T staging as T0T1, T2, T3 and T4; N staging as N0, N1, N2 and N3; M staging as M0 and M1; and secondary thyroid cancer status as yes or no.
Statistical methods
R4.2.3 statistical software was used for analysis. Patient standardized incidence rates (SIR) were calculated using the Multiple Principal Standardized Incidence Rates (MP-SIR) module of SEER Stat 8.4.1 software. Due to the large difference in sample sizes between the case and control groups in this study, in order to equalize the distribution of covariates between the groups, equalize confounders, and reduce selection bias, we introduced the propensity score matching (PSM) method 11 , in which the two groups of patients were matched in a ratio of 1:4 12 . Using the R software package MatchIt , the PSM method was used to match the case and control groups according to the year of diagnosis in a 1:4 ratio, and a total of 392,803 patients with unilateral breast cancer were included as controls, and a 1:4 matching of the case and control groups was accomplished based on the year of diagnosis of breast cancer in the 919 case group of thyroid cancer secondary to breast cancer. Patients served as controls. To ensure that the 919 cases were matched to the 3676 controls, caliper distances were chosen to be as small as possible. Propensity scores were calculated using a logistic regression model, and for better matching, the final matching caliper distance was 0.1. The standardized mean difference (SMD) was used to assess the balance of baseline information between the case and control groups after PSM. We considered SMD less than or equal to 0.1 to indicate a good match 11 .
Further univariate and multivariate analyses were performed using the COX proportional risk model to determine the risk factors for secondary thyroid cancer in breast cancer patients. R-studio software was used to randomly divide all the data into training and validation sets in the ratio of 7:3, and χ 2 test and t-test were performed on different variables in the training and validation sets, and then univariate and multivariate Cox regression analyses were carried out on the data in the training set in order to train the model, and the validation set was used to validate the model 13 . A nomogram was created using the R packages rms , foreign and survival for the final filtered variables and the ROC curves were used to create the nomograms under the ROC curves. The area under the ROC curve (AUC value) and C-index were used to evaluate the accuracy of the model, the AUC and C-index range from 0 to 1, the closer to 1 indicates that the model is more accurate, and it is usually considered that the model has a better predictive ability when the AUC reaches more than 0.7; the calibration curve was used to evaluate the degree of calibration of the model, and the closer the calibration curve is to the standard curve, the stronger the predictive ability of the model. Variables with a univariate COX regression of P < 0.1 were included in the multifactor analysis, and the multifactor analysis was included in the final model with the criterion that the difference was considered statistically significant at P < 0.05 14 .
Ethical approval and consent to participate
Not applicable. Data is available in a public database, ethics approval is not applicable.
Standardized incidence rate
The SEER Stat 8.4.1 analysis yielded a SIR result of 14.89 with a 95% CI of 14.02–15.79 for the period 2010–2019, a predicted number of people of 74.16, and a 10 year actual number of people of 1104, with an incidence rate of 159.41/100,000, which leads to the conclusion that patients who already have breast cancer are more likely to develop thyroid cancer than healthy people.
Propensity score matching
919 patients with thyroid cancer secondary to breast cancer after screening as case group and 392,803 patients with solitary breast cancer as control group were included in the propensity score matching, according to the year of diagnosis according to the PSM method to achieve 1:4 matching, and finally a total of 919 cases in the case group and 3676 cases in the control group were obtained, and the results are shown in Table 1 . The R language tableone package automatically uses analysis of variance for continuous variables, which is equivalent to t-test since this data is only divided into two groups. The result before matching was P < 0.001 and the result after matching was P = 1, SMD < 0.001, which can be considered as well balanced between the matched case group and the control group.
Baseline information
The 919 patients with thyroid cancer secondary to breast cancer and the 3676 patients only with breast cancer obtained after propensity score matching, for a total of 4595 patients, were included in subsequent model analyses. The baseline data of the 4595 patients are shown in Table 2 .
Comparison of baseline characteristics
The 4594 patients obtained after propensity score matching were randomly divided into training set and validation set according to the ratio of 7:3, 3219 patients in the training set and 1376 patients in the validation set. t-test was used for continuous variables, and chi-square test was used for categorical variables to characterize the intergroup differences between the training set and the validation set, and the p-values were all greater than 0.05, which proved that there were no intergroup differences in the results of the randomized splitting, and the results are shown in Table 3 .
Results of univariate and multivariate cox regressions
The training set patient data were included in univariate Cox regression analysis for each of the 12 variables. To avoid omission of important variables, 11 variables with P < 0.1 in the univariate Cox regression were included in the multivariate Cox regression. We included the variables with p < 0.1 in the univariate Cox regression analysis to the multivariate Cox model to examine the independent risk factors for second thyroid cancer; when P < 0.05 in multivariate Cox regression analysis, the factor was an independent risk factor affecting patients’ secondary thyroid cancer. The results of this univariate Cox regression showed that age, ethnicity, marital status, primary tumor location, molecular typing, PR status, ER status, whether radiotherapy, whether chemotherapy, T stage, N stage, M stage were the factors affecting the secondary thyroid cancer in breast cancer patients; while the results of multivariate Cox regression showed that age, race, whether radiotherapy, primary tumor location, N-stage, and M-stage were independent risk factors affecting the development of thyroid cancer in patients with breast cancer, and the results are shown in Table 4 .
Creation of nomogram
Variables screened in the multifactorial Cox regression analysis (P < 0.05) were included in the cox proportional risk model, and nomograms were created using R-studio software. Each variable was projected upward for breast cancer patients, and then each score on the scale was summed to obtain a total score, based on which the risk of secondary thyroid cancer in breast cancer patients at 3 and 5 years could be predicted, and the higher the total score the higher the risk, the prediction results are shown in Fig. 1 . Patients with breast cancer who were younger, received radiotherapy, had N1 staging, M1 staging, were white and other race, and had a primary in the inner lower quadrant were at greater risk of secondary thyroid cancer.
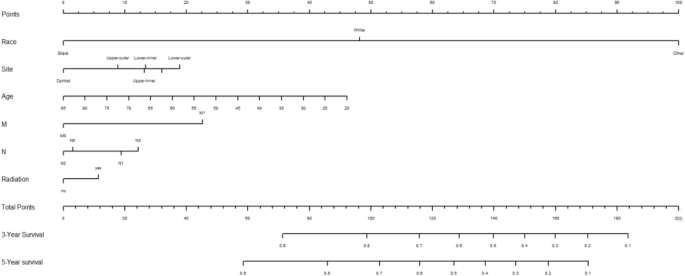
The nomogram of the COX proportional risk model.
Validation of nomogram
Using the ROC curve AUC value and c-index to evaluate the differentiation of the model, we found that, in the Cox proportional risk regression model, the AUC values of three and five years for the patients in the training set (Fig. 2 ) were 0.713, 0.707, and the c -index was 0.693 (95% CI 0.67144, 0.71456), respectively, and the AUC of three and five years for the patients in the validation set (Fig. 3 ) values were 0.681, 0.681,c-index was 0.673 (95% CI 0.64164, 0.70436 respectively), we can consider the model as having moderate predictive power. The calibration plots were plotted using the data from the validation set to evaluate the fit of the established model, and the results showed that in the Cox proportional risk regression model, the calibration curves of 3 and 5 years were close to the dotted line with a 45 ° inclination in the middle, which indicated that the model fit was better.The calibration curves of 3 and 5 years are shown in Fig. 4 .
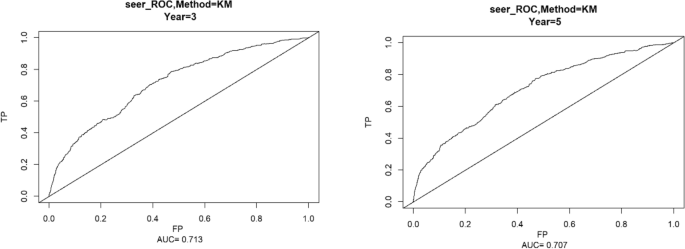
Three and Five year ROC curves for patients in the training center.
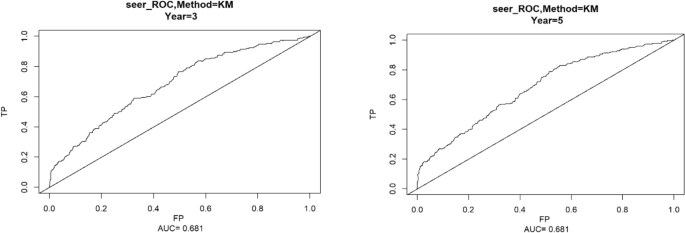
Three and Five year ROC curves for patients in the validation set.
The calibration curves of 3 and 5 years.
Utility and discussion
Breast cancer is known as the world’s number one “red face killer”, according to the latest cancer data released by the World Health Organization, the number of new cases of breast cancer (2.26 million) has exceeded that of lung cancer (2.2 million), and has become the most prevalent cancer in the world 4 . In recent years, more and more general hospitals have set up thyroid breast surgery departments, mainly because it is generally recognized that both the thyroid and the breast are target organs for hormone regulation by the hypothalamic-pituitary endocrine axis, and there are some common pathogenic factors in the two. Therefore, the use of a combination of clinical diagnostics and intelligent means to determine the risk factors for secondary thyroid cancer in breast cancer patients is important for both doctors and patients, and it can guide breast cancer patients for follow-up screening and help clinicians develop treatment plans for the general population and breast cancer survivors.
Although the number of breast cancer patients has been increasing year by year, the low incidence of dual primary cancers, the fact that patients with second primary cancer may be seen in different hospitals, the difficulty of data collection and the long time required for follow-up make the collection of data for the study of dual primary cancers of the breast still difficult. Therefore, this study chooses the U.S. National Cancer Database (SEER), which covers about 30% of the U.S. population, updates patient data in March to April every year, and has a large number of patients with a long follow-up period, making it a more ideal source of data for the study of dual primary cancers.
A total of 692,555 patients diagnosed with breast cancer in the SEER database from 2010 to 2019 were included in this study, and the SEERSTATA software was used to calculate the SIR > 1 for thyroid cancer secondary to breast cancer, and it can be assumed that patients with breast cancer are more likely to develop thyroid cancer compared to cancer-free populations, which is consistent with the findings of previous literature. Due to the large difference in sample size between the case and control groups, in order to minimize bias, we matched 919 patients with thyroid cancer secondary to breast cancer as the case group and 393,722 patients with solitary breast cancer as the control group using propensity score matching. Because the diagnostic criteria, examination instruments, and staging criteria may differ between years, we performed 1:4 propensity score matching according to the year of diagnosis to minimize confounding bias and to include as many control cases as possible to ensure representativeness 12 . The matched 4595 patients were randomly divided into training set and validation set in the ratio of 7:3, and the obtained training set was used to train the cox regression model, and the validation set was used to verify the stability of the model 15 , 16 . The results of univariate cox regression showed that the 11 factors included except ER were independent factors for secondary thyroid cancer in breast cancer patients, and the results of multivariate cox regression showed that age, ethnicity, location of primary tumor, whether or not to have radiotherapy, N-stage, and M-stage were independent factors for secondary thyroid cancer in breast cancer patients.
Contrary to the general impression that age, as a continuous variable with an OR value of less than 1, is considered a protective factor against secondary thyroid cancer from breast cancer, the younger the age, the more likely it is to be secondary thyroid cancer, and previous studies by other scholars have also demonstrated that patients with secondary thyroid cancer after breast cancer were younger compared with those who had breast cancer only 17 , which we believe may be related to the recent years of rejuvenation of the incidence of breast cancer, the early diagnosis and early treatment that makes the breast cancer patient. We believe that this may be related to the recent rejuvenation of breast cancer incidence, prolonged survival due to early diagnosis and early treatment, and the modern lifestyle, where a good prognosis increases the likelihood of a secondary second tumor. However, in some scholars’ studies, age is a risk factor for survival in patients with thyroid cancer secondary to breast cancer relative to patients with solitary breast cancer, and older patients are more likely to have lower survival rates 18 . In conclusion, age is an extremely important factor affecting breast cancer patients with secondary thyroid cancer and warrants further study.
In terms of race, the SEER database has more white profiles due to region, and multivariate cox results show that whiteness and other ethnicities are independent risk factors for secondary thyroid cancers in patients with breast cancer. This may be related to the level of medical care, conditions, and other factors that were co-incorporated into the study, with genetic susceptibility playing a different role in different factors. Mariotto A.B. et al. suggest that the higher incidence of second primary cancers among white women is due to the higher overall survival and screening rates among white women compared to black female populations, and that more comprehensive medical coverage and higher levels of medical care make it easier to diagnose the disease, which would inevitably lead to an increase in the number of diagnoses if these cancers were diagnosed at an earlier stage, which would be consistent with the results of the present study 19 . If these cancers were diagnosed at an early stage, this would inevitably lead to an increase in the number of diagnoses. However the study by Shuting Li et al. suggests that black women with breast cancer should be given attention 13 . However, in a study by Karan Seegobin et al., it was found that there was no significant difference in the incidence of secondary breast and gynecologic cancers between Caucasians and Blacks 20 ,which may be due to the different target second primary cancer disease types in the study, and further research is needed in the future.
Breast cancer patients who receive radiation therapy are more likely to develop secondary thyroid cancers compared to those who do not, a finding that is generally consistent with current clinical opinion that radiation therapy can affect thyroid hormone secretion and thyroid function 21 . The relationship between radiation therapy and the risk of second primary cancers has long been recognized, and it has been demonstrated that radiation therapy is associated with an increased risk of second primary malignancies after exposure 21 . In several observational studies of breast cancer follow-up, the incidence of subsequent secondary acute myeloid leukemia was increased in patients with breast cancer, which may be related to the dose intensity of chemotherapy, the use of adjuvant radiotherapy, and the use of granulocyte colony-stimulating factor (GCSF) 22 . Data from the DBCG (The Danish Breast Cancer Cooperative Group) registry estimate that the proportion of second primary cancers after breast cancer associated with radiation therapy is about 9%. This is consistent with the results from the US SEER database registry 23 . However Grantzau’s analysis found that breast cancer radiotherapy was associated with a small but significant increase in the risk of second cancers for lung, esophageal, and soft tissue cancers, but was not significantly associated with second cancers for thyroid cancer 24 . Another study on the health of the population in Taiwan compared the risk of TC in BC patients who received radiotherapy and those who did not, and the risk of TC in women who received radiotherapy was not significantly higher than that in women who did not receive radiotherapy. This may be related to the selection of data from different ethnic groups in different regions.
In addition, some researchers have suggested that the ER/PR signaling pathway may be a common etiology of breast and thyroid carcinogenesis, and studies of its mechanisms, including the ER pathway and CHEK2 gene mutations in thyroid tissues. In our study, only PR receptor status was an independent factor for secondary thyroid cancer in breast cancer patients. Some researchers have suggested that patients with secondary thyroid cancer have a higher rate of both ER and PR positivity than patients with breast cancer only 25 , 26 .ER has two isoforms, Erα and Erβ, and overexpression of ERα in thyroid cancer tissues and lack of expression of ERβ in peripheral tissues was reported in 2011 27 , and under-expression or deletion of ER can be considered a hallmark of thyroid cancer 28 . Undifferentiated thyroid stem and progenitor cells express lower levels of ERβ compared to differentiated human thyroid cells 29 . Therefore, low levels of ER expression may suggest dedifferentiation of thyroid cancer 27 , 30 . The fact that this paper did not conclude that ER has an effect on secondary thyroid cancer in breast cancer patients may be related to the fact that gene-related variables were not included in this study, and that the SEER database contains only baseline and treatment information and no genetic data, which is a limitation that exists in this study 31 , 32 , 33 .
In AJCC staging, T stage represents the size and extent of the tumor, N stage represents lymph node metastasis, and M stage represents distant spread, with more stages representing more seriousness and worse prognosis. In this study, it was concluded that in N staging, breast cancer patients with N1 stage were more likely to develop secondary thyroid cancer compared to other stages, which may be related to the survival period, and we believe that only if the survival period is long enough, there is a possibility of developing a second primary tumor, while patients with N1 stage had lymph node metastasis, but it was not as serious as N2, N3, and N4 stage, so they were more likely to have a longer survival period; while patients with N0 stage had not No lymph node metastasis has occurred, and from the perspective of disease development, the possibility of secondary cancer is less than that of stage N1.In M stage, M0 stage represents no distant metastasis, and M1 stage represents distant metastasis, and we generally know that tumors with metastases are more likely to cause other cancers.
To summarize, in this study, univariate and multivariate cox analyses were used to screen the influencing factors of secondary thyroid cancer in breast cancer patients, respectively, and a column-line diagram was successfully established, and the calibration curves of the training and validation sets were well fitted, and the AUC and c-index reached significance. It is suggested that the column-line diagram has good predictive ability for the risk of secondary thyroid cancer in breast cancer patients 3 and 5 years after the onset of the disease. However, since the data were obtained from the United States, more studies are needed to verify whether the results obtained by applying this data can be applied to the Chinese population, and the results of this study can provide some references for clinicians 14 .
This study also has some limitations; first, this is a retrospective study, and selection bias due to incomplete data is inevitable. Although we used PSM to avoid selection bias, potential confounders cannot be excluded. Secondly, the Cox model fits well, but the AUC of the validation set is less than 0.7, which represents that the predictive ability of the model is still lacking, which may be related to the fact that breast cancer patients have a good prognosis and a long survival period, while only ten years of data have been observed in the present study, so in the future, we need more follow-up data to improve the model. Because the SEER database itself provides a limited amount of information and the database does not provide any information about genes, we were unable to study the genetic correlation of breast cancer secondary to thyroid cancer at the genetic level. The database has not been updated with data on tumor grading since 2017, so it is not possible to analyze the grading situation, which deserves further study in the future.
Conclusions
In summary, our study suggests that breast cancer patients are more likely to develop thyroid cancer compared to the general population. Among them, age, ethnicity, marital status, primary tumor location, molecular typing, PR status, ER status, whether or not radiotherapy, whether or not chemotherapy, T stage, N stage, and M stage are the factors affecting the secondary thyroid cancer in breast cancer patients; age, ethnicity, whether or not radiotherapy was performed, primary location of the tumor, N stage, and M stage are the independent factors affecting the secondary thyroid cancer in patients with breast cancer patients: the younger the age, the whiter the ethnicity, the whites and the other ethnicities, undergoing radiotherapy, internal lower quadrant and other locations, N1 stage, and M1 stage are more likely to develop thyroid cancer in breast cancer patients.
Data availability
The datasets analyzed during the current study are available in the SEER*Stat software (version 8.4.2, download from https://seer.cancer.gov/data/options.html ). A registration form needs to be completed before using and filtercriteria need to be added. The datasets are also available from the corresponding author on reasonable request.
Huang, J. et al. Effect of breast cancer as the first or second primary cancer on the prognosis of women with thyroid cancer: A SEER database analysis. Transl. Cancer Res. 911 , 6955–6962 (2020).
Article Google Scholar
Bolf, E. L., Sprague, B. L. & Carr, F. E. A linkage between thyroid and breast cancer: A common etiology?. Cancer Epidemiol. Biomark. Prev. 284 , 643–649 (2019).
Bakos, B. et al. Co-occurrence of thyroid and breast cancer is associated with an increased oncogenic SNP burden. BMC Cancer 21 , 1–11 (2021).
Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. A Cancer statistics, 2023.. CA Cancer J. Clin. 73 (1), 17–48 (2023).
Article PubMed Google Scholar
Zhang, W. et al. Metastasis patterns and prognosis in young breast cancer patients: A SEER database analysis[j]. Front. Oncol. 12 , 872862 (2022).
Article CAS PubMed PubMed Central Google Scholar
基于SEER数据库对乳腺癌...原发癌危险因素的回顾性分析_齐欣.pdf>.
Fu, J. et al. The clinical and genetic features in patients coexisting primary breast and thyroid cancers. Front. Endocrinol. 14 , 1136120 (2023).
Dong, L., Lu, J., Zhao, B., Wang, W. & Zhao, Y. Review of the possible association between thyroid and breast carcinoma. World J. Surg. Oncol. 16 , 1–7 (2018).
Ahn, J.-H. et al. The association between vitamin D supplementation and the long-term prognosis of differentiated thyroid cancer patients: A retrospective observational cohort study with propensity score matching. Front. Endocrinol. 14 , 1163671 (2023).
Lim, H., Devesa, S. S., Sosa, J. A., Check, D. & Kitahara, C. M. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA https://doi.org/10.1177/10732748221135447 (2017).
Article PubMed PubMed Central Google Scholar
Cui, J. et al. Association of dietary pattern and Tibetan featured foods with high-altitude polycythemia in Naqu, Tibet: A 1:2 individual-matched case-control study. Front. Nutr. https://doi.org/10.3389/fnut.2022.946259 (2022).
Wang, J., Ning, Y., Du, Y. & Kang, Y. Lymphadenectomy benefits small cell carcinoma of ovary: A population-based analysis. Curr. Oncol. 2910 , 7802–7815 (2022).
Li, S. et al. Clinicopathological features, survival and risk in breast cancer survivors with thyroid cancer: An analysis of the SEER database. BMC Public Health 19 , 1 (2019).
Wang, R. et al. Risk factor analysis and nomogram establishment and verification of brain astrocytoma patients based on SEER database. Sci. Rep. 13 , 1 (2023).
Google Scholar
Qiu, X. et al. Chemotherapy combined with radiotherapy can benefit more unresectable HCC patients with portal and/or hepatic vein invasion: A retrospective analysis of the SEER database. Front. Oncol. https://doi.org/10.3389/fonc.2023.1098686 (2023).
Zhou, Y. et al. The prognostic significance of further axillary dissection for sentinel lymph node micrometastases in female breast cancer: A competing risk analysis using the SEER database. Front. Oncol. https://doi.org/10.3389/fonc.2022.1012646 (2022).
Pu, C.-C., Yin, L. & Yan, J.-M. Risk factors and survival prediction of young breast cancer patients with liver metastases: A population-based study. Front. Endocrinol. https://doi.org/10.3389/fendo.2023.1158759 (2023).
Donin, N. et al. Risk of second primary malignancies among cancer survivors in the United States, 1992 through 2008. Cancer 12219 , 3075–3086 (2016).
Mariotto, A. B., Rowland, J. H., Ries, L. A. G., Scoppa, S. & Feuer, E. J. Multiple cancer prevalence: A growing challenge in long-term survivorship. Cancer Epidemiol. Biomark. Prev. 163 , 566–571 (2007).
Seegobin, K. et al. Pilot study on the occurrence of multiple cancers following cancer-related therapy at the University of Florida, Jacksonville (2011–2016). J Investig. Med. 667 , 1050–1054 (2018).
Praga, C. et al. Risk of acute myeloid leukemia and myelodysplastic syndrome in trials of adjuvant epirubicin for early breast cancer: Correlation with doses of epirubicin and cyclophosphamide. J. Clin. Oncol. 2318 , 4179–91 (2005).
Smith, R. E., Bryant, J., DeCillis, A. & Anderson, S. Acute myeloid leukemia and myelodysplastic syndrome after doxorubicin-cyclophosphamide adjuvant therapy for operable breast cancer: The national surgical adjuvant breast and bowel project experience. J. Clin. Oncol. 217 , 1195–204 (2003).
Sokołowski, M., Mazur, G. & Butrym, A. Breast cancer and synchronous multiple myeloma as a diagnostic challenge: Case report and review of literature. Curr. Probl. Cancer 422 , 231–234 (2018).
Grantzau, T. & Overgaard, J. Risk of second non-breast cancer among patients treated with and without postoperative radiotherapy for primary breast cancer: A systematic review and meta-analysis of population-based studies including 522,739 patients. Radiother. Oncol. 1213 , 402–413 (2016).
Li, S. et al. Clinicopathological features, survival and risk in breast cancer survivors with thyroid cancer: An analysis of the SEER database. BMC Public Health 191 , 1592 (2019).
Ji, J., Zhang, X., Yuan, S., Liu, H. & Yang, L. Survival impact of gastrectomy and chemotherapy for gastric signet ring cell carcinoma with different metastatic lesions: A population-based study. Asian J. Surg. 47 (4), 1769–1775 (2024).
Di Vito, M. et al. Overexpression of estrogen receptor-α in human papillary thyroid carcinomas studied by laser-capture microdissection and molecular biology. Cancer Sci. 10210 , 1921–7 (2011).
Božović, A., Mandušić, V., Todorović, L. & Krajnović, M. Estrogen receptor beta: The promising biomarker and potential target in metastases. Int. J. Mol. Sci. 22 (4), 1656 (2021).
Xu, S., Chen, G., Peng, W., Renko, K. & Derwahl, M. Oestrogen action on thyroid progenitor cells: Relevant for the pathogenesis of thyroid nodules?. J. Endocrinol. 2181 , 125–33 (2013).
Thomas, C. & Gustafsson, J. The different roles of ER subtypes in cancer biology and therapy. Nat. Rev. Cancer 118 , 597–608 (2011).
Lal, G. et al. Risk of subsequent primary thyroid cancer after another malignancy: Latency trends in a population-based study. Ann. Surg. Oncol. 196 , 1887–96 (2012).
Sun, L. M., Lin, C. L., Liang, J. A., Huang, W. S. & Kao, C. H. Radiotherapy did not increase thyroid cancer risk among women with breast cancer: A nationwide population-based cohort study. Int. J. Cancer 13712 , 2896–903 (2015).
An, J. H. et al. A possible association between thyroid cancer and breast cancer. Thyroid 2512 , 1330–8 (2015).
Download references
Acknowledgements
We would like to thank Editage for English language editing.
Author information
These authors contributed equally: Ruiqi Wang and Jiaxue Cui.
Authors and Affiliations
Department of Epidemiology and Health Statistics, Dalian Medical University, 9 Lvshun South Road, Dalian, 116044, Liaoning, China
Yizhuo Diao, Ruiqi Wang, Jiaxue Cui, Chenxin Jin, Yongxing Chen & Xiaofeng Li
You can also search for this author in PubMed Google Scholar
Contributions
Y.D. (first author): design, original draft preparation, software analysis, data analysis and interpretation, manuscript writing, final approval of manuscript. R.W.: data interpretation, final approval of manuscript. J.C: collection data, final approval of manuscript. C.J.: methodology, final approval of manuscript. Y.C.: methodology, final approval of manuscript. X.L.: corresponding author, guide the revision of the article, final approval of manuscript.
Corresponding author
Correspondence to Xiaofeng Li .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Diao, Y., Wang, R., Cui, J. et al. Risk factors for secondary thyroid cancer in patients with breast cancer: a propensity‑matched SEER analysis. Sci Rep 14 , 12679 (2024). https://doi.org/10.1038/s41598-024-59209-x
Download citation
Received : 07 October 2023
Accepted : 08 April 2024
Published : 03 June 2024
DOI : https://doi.org/10.1038/s41598-024-59209-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breast cancer
- Thyroid cancer
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.

IMAGES
VIDEO
COMMENTS
Abstract. This paper presents an atypical case of a patient with brain tumor of the glioblastoma multiforme (GBM) type who achieved a 5-year survival. Some general information is provided including epidemiology, diagnostic and treatment procedures (surgery and radio-chemo-therapy), and prognosis of survival related to GBM.
A Blackout, A Brain Tumor, and Brain Surgery. Fortunately for Stephanie her tumor was low grade, with a much better prognosis than many other types of gliomas. "When treating brain tumors, we often deal with glioblastoma, which is a more serious matter," says Dr. Ramakrishna.
Case Description. The patient is a 53-year-old man who first presented with altered mental status in May 2018. Imaging studies documented a large tumor in the left frontal lobe extending across the midline into the right frontal lobe, with diffuse and extensive infiltration through the corpus callosum. There was mass effect and severe edema.
Cahan et al. defined parameters of radiation-induced malignant gliomas (RIGMs) as follows: tumors localizing to where radiotherapy was applied, an adequate latency period measured in years, a histology different than that of the original tumor, and the patient should not have an underlying pathology favoring the growth of tumors. In this case ...
The temperature was 36.4°C, the heart rate 120 beats per minute, the blood pressure 171/90 mm Hg, the respiratory rate 22 breaths per minute, and the oxygen saturation 95% while the patient was ...
In this study, we retrospectively analyzed a case series of 9 patients who underwent CA via Ommaya reservoir followed by Gamma Knife (GK, Elekta, Stockholm, Sweden) SRS for cystic brain metastases. Our objective is evaluation of volumetric reduction after CA, overall local tumor control, and complications related to this treatment.
NCI-CONNECT First-in-Human Study. In other firsts, NCI-CONNECT launched a first-in-human phase 1 clinical trial for people with recurrent rare brain and spine tumors. Led by Brett Theeler, M.D., neurologist and neuro-oncologist in the United States Army Medical Corps and NCI-CONNECT clinical collaborator, the trial is studying ONC206, an oral ...
Abstract. Effective treatments that extend survival of malignant brain tumor glioblastoma (GBM) have not changed in more than a decade; however, there exists a minority patient group (<5%) whose survival is longer than 3 yr. We herein present a case report of a long-term surviving 51-yr-old female diagnosed with a MGMT unmethylated GBM.
In this case study, we describe a patient with a dementia due to a brain tumor. This unusual cause of dementia illustrates the importance of a thorough evaluation of anyone who experiences relatively sudden changes in cognitive functions. The disorder had ...
A case involving a patient diagnosed with a brain tumor late in her pregnancy highlights strategies for managing such challenging situations. Presentation. A 34-year-old patient, 27 weeks into her first pregnancy, was admitted to the obstetric unit of Hillcrest Hospital, a Cleveland Clinic hospital, for observation. She reported experiencing ...
Brain mapping with functional MRI (fMRI), Diffusion Tensor Imaging (DTI) and/or magnetoencephalography (MEG) to optimize identification of brain tumors and surrounding normal tissues. Intraoperative MRI to maximize safety and completeness of tumor removal. New techniques for optical and fluorescence imaging to better identify infiltrating tumor.
One of these recommendations is early palliative care involvement within 8 weeks of diagnosis in newly diagnosed patients with advanced cancer. 85 In a retrospective study among patients with brain metastases, only about half of the patients received palliative care consultation. 86 ACP in glioblastoma patients was evaluated in another study at ...
Cognitive symptoms are common for patients with brain tumors. Timely identification of problems with neurocognitive function (NCF) is an important aspect of care for these patients, as cognition is critical for complex activities of daily living, 1 work, 2 and functional independence, thus playing a critical role in quality of life. 3 This review details the factors that contribute to NCF ...
For adults, the risk of developing a brain tumor increases with age, with the highest incidence rates observed in individuals over the age of 65. Gender Differences in Brain Tumor Occurrence. Gender also plays a role in the prevalence of brain tumors. Studies indicate that men are generally more likely to develop brain tumors compared to women.
Mass spectrometry is a sensitive technique used to analyze substances in tissue samples, including those altered in cancer. The study involved more than 240 small tissue biopsies from patients undergoing asleep and awake brain surgery for suspected glioma at Mayo Clinic between 2021 and 2023, and an additional 137 biopsies from an international ...
What is a brain tumor? A brain tumor is a growth of abnormal cells in the brain. The anatomy of the brain is very complex, with different parts responsible for different nervous system functions. Brain tumors can develop in any part of the brain or skull, including its protective lining, the underside of the brain (), the brainstem, the sinuses and the nasal cavity, and many other areas.
Clinical Case Relevance. The patient came from a large family with a history of a number of different tumor types, but no first or second degree relatives with primary brain tumors. He was reassured that his family members were at low risk of primary brain tumor, and no screening studies were recommended.
This case study will show how deep learning techniques work in medical image analysis and how they may be used to improve patient outcomes in the detection and treatment of brain tumors. CNN-based models are improving in accuracy and dependability due to their expanding popularity and deep learning developments, which open the door for new ...
A British man who rejected the standard of care to treat his brain cancer has lived with the typically fatal glioblastoma tumor growing very slowly after adopting a ketogenic diet, providing a case study that researchers say reflects the benefits of using the body's own metabolism to fight this particularly aggressive cancer instead of chemo and radiation therapy.
This case demonstrates the need for neurological and psychological departments to work together to provide treatment for brain tumor patients (Reich et al., 2012). Another case study conducted by Marks, Heinrich, and Rosielle (2012) examined the case of 69-year-old man who attempted suicide. The individual had been experiencing depressive ...
Brain tumor patients referred to the Department of Rehabilitation Medicine of a tertiary university hospital for rehabilitation therapy were recruited for this study between Dec 2020 and Jun 2022.
According to the results of a preliminary study of xenografts derived from patients with HER2-positive or HER2-low breast cancer brain metastases and clinical trial results in HER2-positive breast ...
2040 Background: IDH mutant gliomas are the most common primary malignant brain tumors in adults under the age of 50 and accounts for approximately 12% of all glioma diagnoses each year. While lower grade gliomas (LGG) encompassing WHO grades II and III are less aggressive than their higher-grade counterparts, treatment is not curative, and most patients develop tumor recurrence in which there ...
Brain tumors rank as the 1oth leading cause of mortality worldwide, accounting for 85% to 95% of all primary nervous system malignancies. The prevalence of this life-threatening disease is steadily increasing worldwide, highlighting the urgent need for an early and precise diagnosis. Timely identification is critical for initiating effective treatment and improving patient survival chances ...
Data sources. The SEER database containing 13 registry centers prepared by the National Cancer Institute was selected for the data of this study, and a total of 692,555 patients diagnosed with ...
Background and purpose: Diet might be a modifiable factor in preventing cancer by modulating inflammation. This study aims to explore the association between the dietary inflammatory index (DII) score and the risk of bladder cancer (BC). Methods: A total of 112 BC patients and 292 control subjects were enrolled in a case-control trial. Additionally, we tracked a total of 109 BC patients and ...