
Health Services Research Unit, University of Aberdeen, UK
The Health Services Research Unit at the University of Aberdeen is the lead centre for Trial Forge. Randomised controlled trials are the gold standard for evaluating healthcare treatments and thousands are done every year. Tens of billions of pounds of public and private money are invested globally in trials every year.
Some of these resources are being wasted. Despite trials being a cornerstone of evidence-based healthcare, how we make trial process decisions is a largely evidence-free zone. For example, every single trial has to recruit participants but so far methodological research has produced high certainty evidence for only a handful of recruitment interventions. Research questions may not reflect what patients and professionals are most interested in; existing evidence may be ignored; outcomes, especially secondary ones, may simply burden trials with collecting data that are never published. Trials are in desperate need of efficiency improvements.
Trial Forge was set up to help do this and the Aberdeen Centre is where Trial Forge activity is coordinated. Although we are interested in all trial processes, we tend to concentrate on recruitment, retention and data collection. We’re big fans of Studies Within A Trial (SWATs) and formal methods of prioritising unanswered research questions in trial methodology research.

WHAT IS A SWAT?
About aberdeen’s program of work.
Prof Shaun Treweek leads the Aberdeen Trial Forge Centre’s work, along with Dr Heidi Gardner and Adel El Feky . A key additional collaborator here in Aberdeen is Dr Katie Gillies who leads a program of work on trial retention and patient-centred trials that we tap into.
We are running SWATs, mainly in recruitment and retention and work closely with the York Trial Forge Centre and its programme of SWATs. Were also have a portfolio of work around being more aware of the work involved with data collection and, perhaps, whether all those outcomes are really important. Two key projects in this regard are ORINOCO , which is looking at how long data collection takes and DataCat, a wide Trial Forge Collaboration that looks at the categories of data collected in trials, again with the goal of making everyone, us too, more aware of where data collection effort is being put.
We’re into telling the future too. The ESP2 project aims to see if trial managers can predict whether a trial site will meet its recruitment target. It builds on the pilot project ESP, which is described in this Trials paper . ESP2 is much bigger– we now want a 1000 trial manager predictions form around the world. We expect to launch in October 2019: watch this space!
Ongoing Funded Programmes
This is a collaboration with the York Trial Forge Centre. The aim of this programme is to pump prime existing trial teams up to £5,000 to test commonly used strategies for improving trial recruitment and retention by embedding RCTs within already funded trials. The ultimate goal is to make the inclusion of Studies Within A Trial (SWATs) routine when conducting a trial.
More information
Trials are one of the best ways of testing treatments but they can be expensive and time consuming. The amount of data collected has a big influence on both cost and time. We aim to understand how much time trial teams spend collecting the most important trial data (called primary outcomes) compared to the other data they collect (secondary outcomes).
What are the most important unanswered trial process research questions?
There are lots of questions we could ask but what are the most important ones? We’ve worked with the James Lind Alliance, the Health Research Board – Trials Methodology Research Network, Dr Katie Gillies and others to get priority lists for recruitment and retention.
- What are the Top 10 unanswered research questions in trial recruitment research?
- What are the Top 10 unanswered research questions in trial retention research?
Systematic reviews
- What do we know about the effect of recruitment interventions that have been tested in randomised evaluations?
- What do we know about the effect of recruitment interventions that have been tested in non-randomised evaluations?
- What do we know about the factors that affect people’s decisions to take part in a trial or not?
- What do we know about the effect of retention interventions that have been tested in randomised evaluations? Dr Katie Gillies is now leading an update of this review.
- What do we know about the effect of retention interventions that have been tested in non-randomised evaluations?
- What do we know about the factors that affect people’s decisions to continue to stay involved in a trial or not?
Improving site selection

The Estimating Site Performance–2 project (ESP2) project aims to test a tool that just might help. This Trial Forge project is led by trial managers based at the University of Aberdeen, UK and asks trial managers to make predictions about site recruitment after carefully considering eight ‘red flags’ for poor recruitment. These flags (e.g. lack of site staff engagement, or previous poor performance) were developed in the original ESP project, which was published in 2019 in Trials ( https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-019-3287-6 ).
ESP2 needs 1000 trial manager predictions from around the world and launched in October 2019. More details are at https://w3.abdn.ac.uk/hsru/ESP2/ .
USEFUL STUFF
Trial forge guidance 1: what is a study within a trial (swat).
If you want to know what a Study Within A Trial is this document tells you and gives some practical tips on what to do. You can also look at the leaflets in English, French and German at the Trial Forge Resources page.
TRIAL FORGE GUIDANCE 2: HOW TO DECIDE IF A FURTHER STUDY WITHIN A TRIAL (SWAT) IS NEEDED
The orrca project.
PROMETHEUS – This project is funded by the Medical Research Council (MRC). – Grant number MR/R013748/1
ORINOCO – This project is funded by the Chief Scientist Office of Scotland. Grant number HIPS/18/20.
Ethnically diverse communities are prepared to take part in trials but want more inclusive and engaging recruitment, consenting, and retention approaches, as well as more transparency on data collection, storage, and disposal. https://www.jclinepi.com/article/S0895-4356(24)00121-5/fulltext
Stay in the loop

- Skip to content
- About Accessibility on our website

- Staff Directory
Health Services Research Unit
- University Home
Welcome to the

Welcome to the Health Services Research Unit
Established in 1988, the Health Services Research Unit (HSRU) has around 80 staff working in multi-disciplinary teams to deliver high-impact health research.

HSRU has established an internationally recognised portfolio of health services research focusing on two main programmes; Health Care Assessment and Improving Experiences of Care
Public Engagement
HSRU's Public Engagement Group run a number of events and activities for the public throughout the year

Meet the Unit Director, Craig Ramsay
Craig Ramsay is Director of the Health Services Research Unit and Professor of Health Care Evaluation. A statistician by training, he has led the Unit since 2016.

Getting to know Ayodeji Matuluko
Ayodeji tells us about her background in pharmacy and about the new skills she is picking up in her current role as a Research Fellow

Getting to know our Public Involvement Partnership group
Our Public Involvement Partnership group members tell us about their role within HSRU

Shining a light on the Plan-A study
The Plan-A study is aiming to create a decision aid to help women plan their preferred mode of birth (vaginal or caesarean section) during their routine antenatal appointments within the NHS.
Addressing health inequality in randomised trials
Staff news - april 2024, hsru's emma berry awarded the elizabeth russell fellowship, staff news - march 2024, aberdeen-led study aims to reduce inequalities in the diagnosis and treatment of kidney disease, sct 45th annual meeting 19-22 may 2024 boston, usa.

My 2023 Summer Internship at HSRU

What it's like to be involved in implementation research: a personal testimony

Power of Patient Advocacy: My 2023 Summer Internship at HSRU

Reflections on the HSRUK Conference: Perspectives of a Public Contributor
Innovation at the end of life: centre for death and society annual conference, quick links.
- Projects A-Z
- Getting to know
- How to find us
- Opportunities
- Patient and Public Involvement and Engagement
- Publications
- Quality Assurance
- Randomisation Service
- Research Tools
- Sample Size Calculator
- Trials Unit
Scottish Health Economics
Health Economics Research Unit (HERU)

The Health Economics Research Unit (HERU) at the University of Aberdeen was established in 1977. HERU has become one of the leading health economics research centres internationally, with a reputation for delivering both applied and methodological work of the highest quality across a broad range of policy-relevant fields including technology assessment, workforce, person-centred care and public health. HERU receives core funding from the Chief Scientist Office of the Scottish Government Health and Social Care Directorates. Substantial additional funding also comes from competitive research grants, training activities, commissioned research, and the University of Aberdeen.
The aim of HERU is to:
- Research economic approaches to health and health care at standards of international excellence.
- Develop and apply economic techniques to improve health care and population health in Scotland.
- Make available to the health service a body of expertise in health economics.
- Build and sustain capacity in the economics of health.
Our research is organised into four themes:
- Workforce and Organisation of Care – examines how financial and nonfinancial incentives influence the behaviour and performance of the people and the organisations delivering care.
- Health Behaviour – uses economics to understand health behaviour and evaluate health behaviour interventions.
- Assessment of Technologies – conducts economic evaluations to inform NHS decisions on the adoption and withdrawal/modification of health technologies and services. We lead methodological research around the development and application of cost-benefit analysis in health economics, ensuring a person-centred approach to economic evaluations.
- Methods of Benefit Valuation –develops and applies economic methods to help understand what people value, contributing to the aim of person-centred care.
HERU is internationally known for its research developing and applying preference elicitation methods (discrete choice experiments, time preferences and contingent valuation) and economics of the health workforce.
HERU has a wide range of training opportunities available. These are detailed at the SHE Capacity Building page.
[Back to Health Economics in Scotland]
Share this:

- Already have a WordPress.com account? Log in now.
- Subscribe Subscribed
- Copy shortlink
- Report this content
- View post in Reader
- Manage subscriptions
- Collapse this bar
Methodology and reporting characteristics of studies using interrupted time series design in healthcare
Affiliations.
- 1 Health Services Research Unit, University of Aberdeen, Health Sciences Building, Foresterhill, Aberdeen, AB25 2ZD, UK. [email protected].
- 2 Medical Statistics Team, Institute of Applied Health Sciences, University of Aberdeen, Foresterhill, Aberdeen, AB25 2ZD, UK.
- 3 Health Services Research Unit, University of Aberdeen, Health Sciences Building, Foresterhill, Aberdeen, AB25 2ZD, UK.
- PMID: 31272382
- PMCID: PMC6609377
- DOI: 10.1186/s12874-019-0777-x
Background: Randomised controlled trials (RCTs) are considered the gold standard when evaluating the causal effects of healthcare interventions. When RCTs cannot be used (e.g. ethically difficult), the interrupted time series (ITS) design is a possible alternative. ITS is one of the strongest quasi-experimental designs. The aim of this methodological study was to describe how ITS designs were being used, the design characteristics, and reporting in the healthcare setting.
Methods: We searched MEDLINE for reports of ITS designs published in 2015 which had a minimum of two data points collected pre-intervention and one post-intervention. There was no restriction on participants, language of study, or type of outcome. Data were summarised using appropriate summary statistics.
Results: One hundred and sixteen studies were included in the study. Interventions evaluated were mainly programs 41 (35%) and policies 32 (28%). Data were usually collected at monthly intervals, 74 (64%). Of the 115 studies that reported an analysis, the most common method was segmented regression (78%), 55% considered autocorrelation, and only seven reported a sample size calculation. Estimation of intervention effects were reported as change in slope (84%) and change in level (70%) and 21% reported long-term change in levels.
Conclusions: This methodological study identified problems in the reporting of design features and results of ITS studies, and highlights the need for future work in the development of formal reporting guidelines and methodological work.
Keywords: Healthcare interventions; Interrupted time series; Quasi-experimental.
- Interrupted Time Series Analysis / methods
- Interrupted Time Series Analysis / standards*
- Interrupted Time Series Analysis / statistics & numerical data
- MEDLINE / standards
- MEDLINE / statistics & numerical data
- Outcome Assessment, Health Care / methods
- Outcome Assessment, Health Care / standards*
- Outcome Assessment, Health Care / statistics & numerical data
- Regression Analysis
- Research Design / standards*
- Research Design / statistics & numerical data
- Research Report / standards*
Grants and funding
- HSRU2/CSO_/Chief Scientist Office/United Kingdom
- Skip to content
- About Accessibility on our website

- Staff Directory
- Research Tools
- University Home
- Health Services Research Unit
Sample Size calculator for cluster randomised trials
Version 2 of the sample size calculator is in development. Please click here for a beta version. See also this document .
The instruction manual for this program can be downloaded as a pdf file at the link below.
- https://www.abdn.ac.uk/hsru/documents/calculationmanual.pdf
A further description of the calculator can be found in Campbell MK, Thomson S, Ramsay CR, MacLennan GS, Grimshaw JM. Sample size calculator for cluster randomised trials. Comput Biol Med 2004;34:113-125.
If you have any queries about the calculator, please contact Jemma Hudson ( [email protected] ).
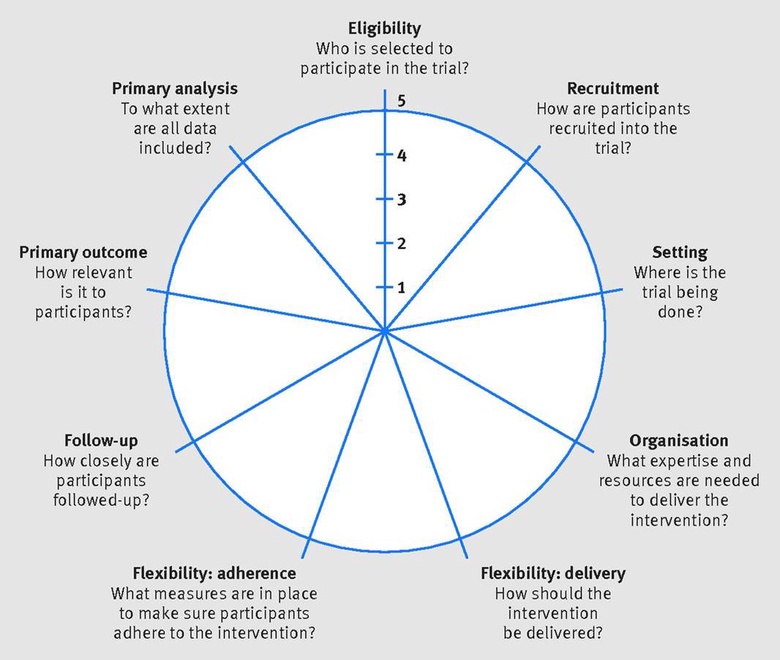
PRECIS-2 Tool
In collaboration with colleagues at other Universities we have developed PRECIS-2, which is a tool to help trialists match their trial design decisions to the needs of the intended users of the trial results. PRECIS-2 has nine domains—eligibility criteria, recruitment, setting, organisation, flexibility (delivery), flexibility (adherence), follow-up, primary outcome, and primary analysis—scored from 1 (very explanatory design approach) to 5 (very pragmatic design approach) to facilitate domain discussion and consensus.
A website to support the use of the tool is available at www.precis-2.org
A description of PRECIS-2 can be found in Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ 2015;350:h2147 ( http://www.bmj.com/content/350/bmj.h2147 )
If you have any queries about the calculator, please contact Shaun Treweek ( [email protected] ).
Database of intra-correlation coefficients (ICCs)
A downloadable excel spreadsheet contains a list of intra-correlation coefficient calculated from a number of different interventions and settings. Also downloadable, is a list of references for the data contained in the spreadsheet.
- Implementation studies: Spreadsheet
- Implementation studies: References
- Surgical studies: Spreadsheet
Contamination in randomised trials
A flowchart was developed by the HTA Contamination in trials project to aid the decision on when a cluster randomised controlled trial might be preferred to a patient randomised controlled trial when contamination of controls are suspected.
- Contamination flowchart
INCLUDE Ethnicity Framework
Trial teams need to do everything possible to make their trial relevant to the people for whom the results are intended to apply (often patients) and those expected to apply them (often healthcare professionals).
The INCLUDE Ethnicity Framework aims to help trial teams think carefully about which ethnic groups should be included in their trial for its results to be widely applicable, and what challenges there may be to making this possible. Having identified potential challenges, the trial team can then consider ways to reduce those challenges. For this to work best, the Framework needs to be used at the trial design stage before funding is in place.
Click here for more information.
INCLUDE Socioeconomic Disadvantage Framework
This framework has been designed to aid researchers, who are designing clinical trials, to consider barriers to including patients from socioeconomically disadvantaged backgrounds in their trial. The framework can also help researchers to develop strategies to attempt to address such barriers in order to improve the design and conduct of clinical research. Although this framework was developed with UK-based clinical trials in mind, aspects may also be relevant to different types of research and research conducted in populations outside of the UK. Whilst this framework focuses on socioeconomic disadvantage, the list of underserved groups in clinical research is extensive and researchers need to be aware of this when identifying barriers to research and developing to strategies to address barriers.
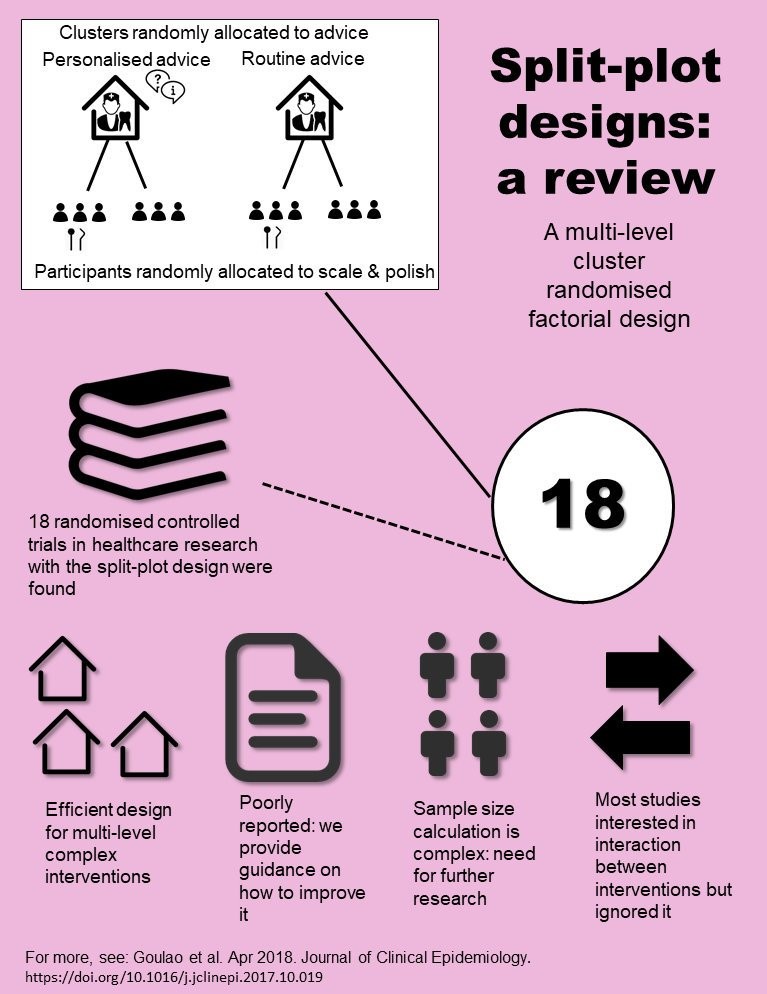
Multilevel factorial cluster randomised controlled trials
Complex randomised controlled trials are required to evaluate complex interventions.
Multilevel factorial cluster randomised controlled trials (C-RCT), also known as split-plot designs, address research questions that are multilevel (interventions aimed at different levels in a structure such as doctors and patients) and have the potential to explore interactions between interventions (whether or not giving two interventions together works any better than giving them separately.
To learn more about this type of design and its statistical implications, please consult Beatriz Goulao’s presentation here .
We have published a set of guidelines to improve the reporting of multilevel factorial cluster randomised controlled trials, which you can see here .
A statistical simulation tutorial to aid the sample size calculation for multilevel factorial cluster randomised controlled trials will be available soon.
Find out about Beatriz’s Goulao's PhD project here.
The DAMOCLES study formulated a list of considerations that would be valuable for a Data Monitoring Committee (DMC) to address at the start of a trial which were developed into a charter.
- Charter for DMCs: template (Word document)
- Worked example of the DAMOCLES charter (Word document)
Beliefs about surgery questionnaire
The REFLUX Study developed and validated a questionnaire for measuring patients Beliefs about surgery.
- Beliefs about surgery questionnaire (PDF)
As part of this programme of research, we have developed tools to support communication of numerical or statistical aspects of trials with patients and the public.
As part of the PoINT project, we developed a guide for numerical aspects developed by Beatriz Goulao and Richard Caie and funded by the Wellcome Trust ISSF fund at the University of Aberdeen: download here , and find out more about the results of the project here .
As part of the INITIAL project, we developed a blog with lay summaries of statistical aspects of trials to support patient and public involvement in statistics in trials.
HSRU provide validated and easy to use online/IVR (telephone) software for randomising patients into clinical trials.
Reasons to choose HSRU for your service:
- Highly experienced staff and reliable online/telephone service.
- Can integrate and export data dynamically to other 3 rd party systems.
- Suitable for a wide variety of trial types, customised to your trial.
- Advice on system design and implementations.
- Ongoing export support via online support tool.
Simple randomisation, block randomisation, stratified block randomisation and minimisation are offered as standard. Novel randomisation can be implemented on request.
Telephone randomisation service
HSRU offer a 24-hour, seven-day automated telephone randomisation service, telephone calls are made to the service using a Freephone number, which is connected to 16 telephone lines. For international calls, there is a standard UK telephone number.
Web-based randomisation service
HSRU offer a secure and robust web-based randomisation system. It is accessible anytime, anywhere and via any device that has a modern web browser and is connected to the Internet.
Standard features
A customisable role-based user access control system, email notifications are built in. The randomisation system generates confirmation emails that can be blinded or unblinded to treatment allocation.
Standard reports summarising randomisation and all data-entry activity, custom reports implemented on request.
All transactions are logged, the trial's audit trail and the list of randomisations can be downloaded and analysed at any time by authorised users
Randomisation simulation data is generated before the study begins, to check balance across stratification factors. The system can be programmed to perform validation checks, such as checking for duplicate Participant Identification Numbers and preventing randomisation from proceeding in such cases.
Drug supply chain management
The randomisation service can also handle drug supply chain. Active and placebo unique treatment pack numbers, batch numbers and expiry dates can be programmed into the system, with the system recording packs delivered to specific site/pharmacies and only allocating medication packs that are available at that site. Notifications can be set up to alert site/pharmacies of low drug supply, and this functionality is configurable as required. For example, notify site/pharmacy when the stock level reaches x amount of packs.
We can offer you an emergency online/telephone code break (unblinding) service which is also available 24 hours a day. Investigators break the blind by simply providing the unique randomisation number, or other identifier used for your trial medication or intervention. Investigators are immediately notified of the unblinded treatment allocation. Trial teams can choose to be notified by email of every new code break.
Invitations are sent by email, links to complete the forms online are included in the emails. Invites and reminders can be sent automatically via our built-in configurable communication manager.
Implementation and cost
The cost of the service depends on how long the service is required and the sample size. The randomisation request form can be found here .
If you require further information relating to planning a new randomisation service or If you would like a demonstration on either the telephone or web-based randomisation service, please contact the Senior IT Development Manager: Mark Forrest

Projects have contributed to guidance documents for reviewing and disseminating results of research.
- Producing information about health and health care interventions: a practical guide (PDF)
- How to identify and assess evidence from diagnostic studies of imaging: an introductory guide (PDF)
- “Sharing trial results directly with trial participants and other stakeholders after the SARS-CoV-2 pandemic hit the UK – experience from the ActWELL trial” https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-021-05340-3
- Help & FAQ
Health Services Research Unit (HSRU)
- Life Sciences & Medicine
- School of Medicine, Medical Sciences & Nutrition
- Website https://www.abdn.ac.uk/hsru/ , http://www.abdn.ac.uk/smd/
- Last Name (descending)
Search results

Lorna Aucott
- School of Medicine, Medical Sciences & Nutrition , Health Services Research Unit (HSRU) - Senior Research Fellow
- School of Medicine, Medical Sciences & Nutrition , Centre for Healthcare Randomised Trials (CHaRT)
- Institute of Applied Health Sciences
Person: Academic Related - Research

Alison Avenell
- School of Medicine, Medical Sciences & Nutrition , Health Services Research Unit (HSRU) - Clinical Chair in Health Services Research
Person: Clinical Academic

Umesh Basavaraju
- School of Medicine, Medical Sciences & Nutrition ,
Person: Honorary

Miriam Brazzelli
- School of Medicine, Medical Sciences & Nutrition , Health Services Research Unit (HSRU) - Personal Chair
Person: Academic, Academic Related - Research

Suzanne Breeman
- School of Medicine, Medical Sciences & Nutrition , Health Services Research Unit (HSRU) - Trial Manager
Person: Academic Related - AST 5-9, Academic Related - Research

Hanne Bruhn
- School of Medicine, Medical Sciences & Nutrition , Health Services Research Unit (HSRU) - Research Fellow

Marion Campbell
- School of Medicine, Medical Sciences & Nutrition , Health Services Research Unit (HSRU) - Chair in HSRU
Person: Academic

Taylor Coffey

Lynda Constable
Person: Academic Related - AST 5-9

David Cooper

Seonaidh Cotton
- School of Medicine, Medical Sciences & Nutrition , Health Services Research Unit (HSRU) - Senior Trial Manager
Person: Academic Related - Management

Moira Cruickshank

Vitri Darlene
- School of Medicine, Medical Sciences & Nutrition , Health Services Research Unit (HSRU) - Research Assistant

Audrey Dawson

Chukwuemeka Emele
- School of Medicine, Medical Sciences & Nutrition , Health Services Research Unit (HSRU) - Senior Programmer
Vikki Entwistle
- School of Medicine, Medical Sciences & Nutrition , Health Services Research Unit (HSRU) - Chair in Health Services Research and Philosophy
- School of Divinity, History & Philosophy , Centre for Knowledge and Society (CEKAS)

Mark Forrest
- School of Medicine, Medical Sciences & Nutrition , Health Services Research Unit (HSRU) - Senior IT Development Manager

Kate Gillies

Beatriz Goulao

Jemma Hudson

Mari Imamura

Kristin Konnyu
- School of Medicine, Medical Sciences & Nutrition , Health Services Research Unit (HSRU) - Lecturer

Louise Locock
- School of Medicine, Medical Sciences & Nutrition , Health Services Research Unit (HSRU) - Professor in Health Services Research

Graeme MacLennan
- School of Medicine, Medical Sciences & Nutrition , Health Services Research Unit (HSRU) - Director (CHaRT)

Ayodeji Matuluko

Dympna McAteer

Sharon McCann
- School of Medicine, Medical Sciences & Nutrition , Health Services Research Unit (HSRU) - Advanced Research Fellow

Kirsty McCormack

Rumana Newlands

Sarah Prowse

Craig Ramsay
- School of Medicine, Medical Sciences & Nutrition , Health Services Research Unit (HSRU) - Director of Health Services Research Unit

George Ramsay
- School of Medicine, Medical Sciences & Nutrition , Health Services Research Unit (HSRU) - Senior Clinical Lecturer
- School of Medicine, Medical Sciences & Nutrition , Aberdeen Cancer Centre

Clare Robertson

Magdalena Rzewuska Diaz
- School of Medicine, Medical Sciences & Nutrition , Health Services Research Unit (HSRU) - Research Fellow, Advanced Research Fellow
- School of Medicine, Medical Sciences & Nutrition , Aberdeen Centre for Health Data Science

Kusum Singal

- School of Medicine, Medical Sciences & Nutrition , Health Services Research Unit (HSRU) - Lecturer (Scholarship), Research Fellow
Person: Academic Related - Scholarship, Academic Related - Research

Ruth Thomas

Shaun Treweek
- School of Medicine, Medical Sciences & Nutrition , Health Services Research Unit (HSRU) - Chair in Health Services Research

Thenmalar Vadiveloo

Samantha Wileman

COMMENTS
Established in 1988, the Health Services Research Unit (HSRU) has around 80 staff working in multi-disciplinary teams to deliver high-impact health research. ... (CHaRT) is located in the Health Services Research Unit (HSRU) as a joint initiative with the University of Aberdeen's Institute of Applied Health Sciences (IAHS). Find out more » ...
The Health Services Research Unit co-hosts the Centre for Healthcare Randomised Trials (CHaRT) a UKCRC registered clinical trials unit. The Unit's work is characterised by being collaborative and multidisciplinary. This involves a range of local, national and international partners. Examples of our recent impact and achievements can be seen here.
Dive into the research topics where Health Services Research Unit (HSRU) is active. These topic labels come from the works of this organisation's members. ... Aberdeen research transforms national policy on the use of robots in prostate surgery. Craig Ramsay (Coordinator) & Clare Robertson (Participant) Impact: Policy, Health and Wellbeing ...
Health Services Research Unit (Organisational unit) Magdalena Rzewuska (Member) 14 Jan 2020 → 14 Jan 2023. Activity: Membership (e.g. panels, networks, committees, etc.) › Member of research network. ... The University of Aberdeen Research Portal data protection policy.
Health Services Research Unit (HSRU) Life Sciences & Medicine; School of Medicine, Medical Sciences & Nutrition ... Aberdeen research transforms national policy on the use of robots in prostate surgery. Craig Ramsay (Coordinator) & Clare Robertson (Participant) Impact: Policy, Health and Wellbeing, Societal. Helping adults with obesity lose ...
The Health Services Research Unit at the University of Aberdeen is the lead centre for Trial Forge. Randomised controlled trials are the gold standard for evaluating healthcare treatments and thousands are done every year. Tens of billions of pounds of public and private money are invested globally in trials every year. Some of these resources are being wasted.
Marion CAMPBELL | Cited by 24,173 | of University of Aberdeen, Aberdeen (ABDN) | Read 314 publications | Contact Marion CAMPBELL. Home; ... ABDN · Health Services Research Unit.
The Unit is located in purpose-built accommodation of the University's Health Sciences Building. This physical co-location of staff in modern well-resourced space provides a vibrant research environment. It also allows early career researchers easy access to senior colleagues, and facilitates the exchange of ideas and collaboration.
Welcome to the Health Services Research Unit. The Unit is supported by the Chief Scientist Office (CSO) of the Scottish Government Health and Social Care Directorates, the University of Aberdeen and competitive grant income. Adrian Grant Memorial Lecture 2023.
The following PhD opportunity is available at the Health Services Research Unit, University of Aberdeen: What are the personal, clinical and social impacts of longer waiting times? A multi-methods study. Supervisers will be Professor Craig Ramsay and Professor Louise Locock. Application deadline: 30 June 2020. Find out more on the University's PhD opportunities page.
Health Services Research Unit; Aberdeen, United Kingdom; Position. Professor (Full) January 2006 - December 2013. University of Dundee. Division of Population Health Sciences ; United Kingdom;
The Health Economics Research Unit (HERU) at the University of Aberdeen was established in 1977. HERU has become one of the leading health economics research centres internationally, with a reputation for delivering both applied and methodological work of the highest quality across a broad range of policy-relevant fields including technology assessment, workforce, person-centred care and ...
What factors influence the uptake of bowel, breast, and cervical cancer screening? An overview of international research Prowse, S., Brazzelli, M. & Treweek, S., 25 Mar 2024, (Accepted/In press) In: European Journal of Public Health. Research output: Contribution to journal › Article › peer-review
University of Aberdeen; Health Services Research Unit; ... Background Policy‐makers and health research funders increasingly require researchers to demonstrate that they have involved patients ...
1 Health Services Research Unit, University of Aberdeen, Health Sciences Building, Foresterhill ... Foresterhill, Aberdeen, AB25 2ZD, UK. 3 Health Services Research Unit, University of Aberdeen, Health Sciences Building, Foresterhill, Aberdeen, AB25 2ZD, UK. PMID: 31272382 PMCID: PMC6609377 DOI: 10.1186/s12874 -019-0777-x ...
Research Tools. Projects undertaken by the Unit have contributed to the development of many different research tools for both researchers and users of research. In collaboration with colleagues at other Universities we have developed tools to help trialists match their trial design decisions to the needs of the intended users of the trial results.
School of Medicine, Medical Sciences & Nutrition, Health Services Research Unit (HSRU) - Senior Research Fellow; School of Medicine, Medical Sciences & Nutrition, Centre for Healthcare Randomised Trials (CHaRT) ... Medical Sciences & Nutrition, Aberdeen Centre for Health Data Science; Person: Academic Related - Research. 2016 2024.
University of Aberdeen; Health Services Research Unit; ... among youth mental health (YMH) services and healthcare commissioners; and to identify barriers and enablers to this practice. Design ...
Graham MOWATT | Cited by 7,266 | of University of Aberdeen, Aberdeen (ABDN) | Read 61 publications | Contact Graham MOWATT
Health Services Research Unit; Aberdeen, United Kingdom; Position. Research Associate; Publications. Publications (25) The BSR-PsA: study protocol for the British Society for Rheumatology ...