73 Bioethics Essay Topic Ideas & Examples
🏆 best bioethics topic ideas & essay examples, 📌 simple & easy bioethics essay titles, 💡 interesting topics to write about bioethics.
- Fifteen Ethical Principles of the Universal Declaration on Bioethics Hence, giving sufficient data and teaching the patient about actual factors, and getting educated consent before exposing a patient to any medical procedure is fundamental.
- Bioethics: Definition, Importance, and Scope In the United States, a National Commission for the Protection of Human Subjects of Biomedical and Behavioural Research, was set up in 1974 under the National Research Act and was charged with the duty of […]
- Concept of Bioethics in Society The classical assumption in the discipline of bioethics is that the field is concerned with the dilemma of determining the most ethically appropriate action in the healthcare setting.
- Genetic Manipulation of Human Embryos: Bioethical Issues Nonetheless, although the modification of human genotype may help in achieving a perfect genetic composition and eliminate a number of genetically transmitted diseases, there is a looming risk. The assembling of genetic makeup to enhance […]
- Bioethics: Which Theory Makes the Most Sense? Out of three well-known theories, Kantian ethics is the most optimal in terms of considering morality and human conduct, unlike natural law theory and utilitarianism which mainly disregard justice or strive to find the correct […]
- Bioethics as an Essential Part of Healthcare Models are interconnected in terms of reliance on internal and external factors of care, yet the narrative medicine model is the most crucial due to its emphasis on transparent communication and attention to patient history.
- The Value of the Human Person in Terms of Bioethical Issues Since the well-being and health of a person is the most valuable thing in life, and abortion is designed to help a woman, the issue of life and health comes before any controversy.
- The Main Principles of Bioethics According to the concept of beneficence, a medical professional has a duty to act in the patient’s best interest rather than their own.
- Bioethics and Its Main Principles The first example of that is that during a study, the researcher must never provide patients’ data to third parties unless it is necessary and agreed upon with the patients.
- Bioethics: Human Organs Markets The sale and use of human organs after the death of a person is a complex ethical and moral issue that people have decided on in various ways at different times. The commercialization of the […]
- Bioethics: Definition and Application The distribution of restricted resources and end-of-life difficulties are two of the most prominent ethical dilemmas and issues in medicine. The importance of debate and exposition in bioethics is a crucial premise.
- Bioethics Principles in Healthcare The principle of autonomy underlies patient-centered care and means the primary postulate of the will of the patient in caring for their health, exceeding the will of the doctor to intervene.
- Bioethics Committees: The Role in Society In engaging the members of science who use medicinal technologies, bioethics committees ensure the youth and the elderly have a voice in the care received.
- International Bioethics and Genetics Genetic discrimination is a problem of bioethical significance in which a patient’s confidential rights are violated to create favorable conditions on the part of the person or company who is the subject of the discriminatory […]
- Human Dignity in Philosophy and Bioethics It is because of the opportunity to master the concepts of good and evil and use them morally a person differs from a thing.
- Bioethical Issues in Health Care – Opioid Overprescribing In order to address the problem of opioid overprescribing in healthcare facilities, administrators should seek to implement and support the application of the so-called analgesic ladder for patients with chronic pain.
- The Human-Subject System of Protection in Bioethics The principles became the foundation and cornerstone for the research regulations and ethical codes that followed the Nuremberg code After the Second World War, the world superpowers focused most of their attention on finding cures […]
- Bioethical Aspect of Animal Research Animal research has made an outstanding contribution to the development of medicine in both scientific and didactical spheres. Even though animal research has been beneficial for medicine development for decades, it is now important to […]
- Bioethics Policy Advocacy Memo The government has the moral obligation to ensure human dignity and at the same time, it is struggling with the implementation of a person’s democratic right to freedom and liberty.
- Bioethical Position on Medical Futility Nursing Advocates for the withdrawal of treatment for severely deformed infants base their arguments on the fact that treatment for severely deformed infants is futile.
- Bioethical Issues in Treating the Terminally Ill On their part, the trans-disciplinary team, led by the head nurse, is guided by the values of offering the greatest good to the patient, information disclosure, and an overriding desire to conform to the set […]
- Bioethical Decision-Making in Nursing Practice The paper will discuss nursing support in the health situation while specifying the role of the nurse as the patients’ advocate.
- Genome: Bioethics and Genetic Engineering Additionally, towards the end of the documentary, the narrator and some of the interviewed individuals explain the problem of anonymity that is also related to genetic manipulations.
- Bioethics. When Abortion Is Morally Permissible Abortion as we all know is the deliberate removal of a foetus from the womb of a female resulting in the death of the foetus.
- Bioethics, Public Policy and Science: Tech Philosophy This research seeks to provide a thorough analysis of the interrelation between stem cells research policies, bioethics and political and public policies and debates.
- Bioethics: Starson vs. Swayne Case There is no denying the importance of the fact that its development was greatly connected with the progress of biology, medicine and anatomy which opened the issues of cloning, genetic transformation.
- “The Triumph of Autonomy in American Bioethics” by Wolpe It is important to note that in the USA the principle of autonomy has overweighed the rest of the principles suggested by Beauchamp and Childress.
- The Essence of Philosophical Bioethics In the second section, the paper discusses the personal opinion of the writer on the effects of the course on personal worldview, self-concept, and other behaviors and activities.
- Jonathan Moreno: Bioethics After the Terror Based on the above facts, Jonathan Moreno in the article Bioethics after the Terror analyses the possible implications of bioethics that could result in tension in the field that has never witnessed major changes in […]
- Human Dignity and Bioethics The current paper is a critical analysis of the controversy surrounding the issue of human dignity and bioethics. On the contrary, it is the ability of the individual and the society in general to set […]
- Bioethics: Artificial Nutrition and Hydration Based on the arguments presented in this discussion, it suffices to mention that withdrawing or withholding of nutrition and hydration remains a controversial issue.
- The Major Components of Bioethics in Healthcare The second component is patient autonomy which refers to the right of a patient to give an informed consent in the course of treatment.
- Bioethics Education and the Development of Nursing Students’ Moral Competence
- Religion and Culture and Their Impact on Bioethics
- Work of a Paediatric Bioethics Centre During the COVID-19 Pandemic First Phase
- Bioethical Conflicts of Gene Therapy
- The Theorisation of ‘Best Interests’ in Bioethical Accounts of Decision-Making
- The Bioethics Surrounding the Concept of Human Cloning
- Difference Between Bioethics & Ethics in Nursing
- Program in Women and Children’s Bioethics
- Exploring Values Among Three Cultures From a Global Bioethics Perspective
- The Uses and Abuses of Moral Theory in Bioethics
- Bioethics Center Breaks Out of Midwest
- Human Rights and Bioethical Considerations of Global Nurse Migration
- Center for Practical Bioethics Steps up During Pandemic
- A Sensitive Period: Bioethics, Human Rights, and Child Development
- Bioethics and Health Informatics in Nursing
- Ethics, Bioethics and Nursing Ethics: Some Working Definitions
- The Role of Philosophy and Philosophers in Bioethics
- Ethical and Philosophical Implications of the Field of Bioethics
- Bioethics: The Law, Medicine, and Ethics of Reproductive Technologies and Genetics
- Modern Assisted Reproductive Technologies and Bioethics in the Islamic Context
- Introduction to Bioethics and Ethical Decision Making
- Genome Editing Among Bioethics and Regulatory Practices
- Bioethics: A Look at Animal Testing in Medicine and Cosmetics in the UK
- American Society for Bioethics and Humanities
- Child Rights and Clinical Bioethics: Historical Reflections on Modern Medicine and Ethics
- Working at the Center for Practical Bioethics
- How Bioethics Can Enrich Medical-Legal Collaborations
- How “Moral” Are the Principles of Biomedical Ethics
- Ethical Aspects of Relationships Between Humans and Research Animals
- Meeting the Publishing Needs of the Bioethics Community
- Bioethicists Can and Should Contribute to Addressing Racism
- Critical Thinkings About Bioethics Mediation
- What Makes an Anti-racist Feminist Bioethics
- Bioethics and Human Rights in the Constitutional Formation of Global Health
- Assisted Reproductive Technologies: A Bioethical Argument for Medicaid Coverage
- The Value of Metaphorical Reasoning in Bioethics
- Art Exhibit Supporting Center for Practical Bioethics Celebrates Wishes of Older Adults
- The Mission of the Center for Practical Bioethics
- The Bioethics of Assisted Reproductive Technology
- Bioethics in Health Policy Development
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2024, March 2). 73 Bioethics Essay Topic Ideas & Examples. https://ivypanda.com/essays/topic/bioethics-essay-topics/
"73 Bioethics Essay Topic Ideas & Examples." IvyPanda , 2 Mar. 2024, ivypanda.com/essays/topic/bioethics-essay-topics/.
IvyPanda . (2024) '73 Bioethics Essay Topic Ideas & Examples'. 2 March.
IvyPanda . 2024. "73 Bioethics Essay Topic Ideas & Examples." March 2, 2024. https://ivypanda.com/essays/topic/bioethics-essay-topics/.
1. IvyPanda . "73 Bioethics Essay Topic Ideas & Examples." March 2, 2024. https://ivypanda.com/essays/topic/bioethics-essay-topics/.
Bibliography
IvyPanda . "73 Bioethics Essay Topic Ideas & Examples." March 2, 2024. https://ivypanda.com/essays/topic/bioethics-essay-topics/.
- Healthcare Policy Essay Titles
- Donation Titles
- Healthcare Reform Essay Ideas
- Genetic Engineering Topics
- Epigenetics Essay Titles
- Euthanasia Titles
- Affordable Care Act Essay Titles
- Intensive Care Research Topics

175 Unique Bioethics Topics To Consider for Academic Paper
Table of Contents
If you are a student who is learning a course in biology or applied science, then for your assignments you can choose bioethics topics. Right now, do you want to write a research paper on bioethics topics? Are you struggling to come up with the best bioethics research topics? Here, in this blog post, we have created a list of the top bioethics topic ideas you can think about for academic writing. Continue reading this blog post and gain interesting bioethics research paper concepts that will help you earn an A+ grade.
What is Bioethics?
Bioethics is a study of the ethical issues that arise from medicine, biology, and technology. It primarily focuses on the philosophical, legal, and social issues that exist in the fields of life sciences, biotechnology, law, public health, theology, healthcare, and medicine. But sometimes it also connects with the ethical questions that are based on the biological environment.
Tips for Selecting a Bioethics Topic for Academic Paper
We hope you are now clear about what bioethics means. Based on the definition of bioethics, you can choose any topic to write about. But remember, not every topic is ideal. Hence, during the bioethics research paper topic selection, make sure to keep the following tips in mind.
- Choose a topic that matches your interest.
- Pick a topic that you have a strong knowledge of because it would be comfortable for you to perform research.
- Give preference to a topic that focuses on any current and latest bioethical issues.
- Your research paper should address a unique topic that is different from the topics that have been already published.
Basically, for writing a research paper, you can think about the following principles of bioethics.
- Principle of non-maleficence
- Principle of respect for autonomy
- Principle of beneficence
- Principle of justice
Medical practices are identified as ethical only if they favor these principles. Therefore, when selecting a topic for a bioethics research paper, consider all these principles.
List of Bioethics Topics and Research Ideas
There are plenty of bioethics topics and research paper topics ideas available. If you are running short of ideas, then feel free to check the list of the best bioethics research paper topic ideas suggested below.
Bioethics Topics for School and College Students
- The history of bioethics.
- Discuss “appeal to disgust”.
- Cloning and reproductive technologies
- Explain utilitarian bioethics.
- What are the major gene therapy vectors?
- Explain veterinary bioethics.
- The principle of double effect.
- Analyze the plant blindness effect in recent years.
- Explain the concept of Dignitas personae.
- Discuss the ethics of uncertain sentience.
- Analyze the playing God ideology in ethics.
- Discuss plant rights in the United States.
- Explain bioprinting.
- Analyze the ethics behind remote-controlled animals.
- Perform a study of Postgenderism.
- Analyze Psychological warfare tactics.
- Cross-Cultural Issues and Diverse Beliefs. Difficult Patient Encounters. Do Not Resuscitate during Anesthesia and Urgent Procedures. Do Not Resuscitate Orders. …
- Futility. HIV and AIDS. Informed Consent. …
- Mistakes. Neonatal ICU Issues. Pandemics. …
- Physician-Patient Relationship. Prenatal Diagnosis. Professionalism.
Also read: A Catalog Of 120 Genetics Research Topics
Common Bioethics Topics
- Discuss the ethical problems with animal testing.
- Explain the legal issues in bioethics.
- Discuss new eugenics.
- Explain biobank ethics.
- Analyze the claims of abortion doping.
- The formation of the International Bioethics Committee.
- What is bio-happiness?
- Explain the important gene therapies.
- The creation of the International Society for Stem Cell Research.
- Focus on the work of Ian Kennedy in bioethics.
- How can physicians handle advanced care planning issues?
- Explain the difference between rationing and futility.
- How does religiosity affect medicine?
- Explain the proportionality between good and bad effect
- Describe the fair distribution of health.
Excellent Bioethics Research Paper Topics
- Analyze the process of biodefense.
- Explain the difference between the means and effects in bioethics.
- How can an agent achieve a good effect even though it’s foreseen?
- Compare experimental intervention and futile intervention.
- Explain the differences between advance care planning and advance directives.
- Is it ethical for physicians to disclose medical mistakes to patients?
- Can a “right to healthcare” be ethically qualified?
- Explain paternalism.
- How can a physician deal with prejudice when addressing HIV/AIDS cases?
- What is moral enhancement?

Top Bioethics Research Topics
- Analyze the concept of morphological freedom.
- Discuss the ethics behind the Oncomouse.
- Explain neuroethics in the United States.
- The ethical problems with cloning.
- Discuss the medical ethics in the military.
- Explain the role of the National Catholic Bioethics Center.
- Analyze the science behind genetically engineered babies.
- Discuss the National Biodefense Strategy of the United Kingdom.
- Discuss the British Operation Cauldron.
- Gene therapy technology.
- Explain the ethics behind Organ transplantation.
- Brain imaging.
- Explore the science behind memory dampening.
- Discuss the Reversal Test.
- The creation of an effective vaccine against COVID-19.
Also read: Great Ethics Topics To Consider For Writing Academic Papers
Interesting Bioethics Topics
- Analyze end-of-life care in the UK.
- The regulation of medical science.
- The Hamster cell.
- Reproductive technology ethics.
- The immortal HeLa human cell line.
- Analyze the process of stem cell doping.
- What are the negative effects of stem cell tourism?
- The retention of the Ebola virus.
- Informed consent in 20 th -century medicine.
- An in-depth look at HEK 293 cells.
- What are neuroenhancement drugs?
- Explain the concept of surrogacy.
- Analyze the ethics behind viral animal testing.
- Bioethics versus epidemiology disciplines.
- The ethics behind brain stimulation technology.
Trending Bioethics Thesis Topics
- Explain the concept of biopolitics.
- Discuss biological warfare.
- What are the new bioweapons?
- Explain bio-risk.
- Is keeping a brain-dead person alive the right choice?
- Discuss biotic ethics.
- What are Stem cell research ethical problems?
- Are GMOs dangerous for humans?
- The effects of plastic surgery.
- Discuss the concept of biopiracy.
- Discuss the ethical issues ‘on the horizon’ in biology and medicine as highlighted by the Nuffield Council on Bioethics
- Compare and contrast the similarities and differences between medical ethics and bioethics
- What should be done with the child of a brain-dead pregnant woman?
- Is it ethical to improve memory functions with brain stimulation?
- ‘End-of-life care’ and ‘Allocation of Medical Resources’ are the two key areas that create bioethical issues: Explain with justifications
- How the National Center for Ethics in Health Care (NCEHC) helps to address bioethical issues and ensure proper healthcare management
- Compare and contrast the bioethical issues that arise in Euthanasia, Eugenics, and Organ Donation
- Discuss the importance of following the principles of bioethics for lab assistants and pharmacists
- Describe the different types of Organ Donation and ethical issues associated with them using real stories in the context of the UK
- Analyze the impact of bioethics on minority communities and society as a whole
Also read: Human Rights Topics to Impress Your Professor
Outstanding Research Topics on Bioethics
- Definitions of death about terminating life.
- Intrinsic value of species.
- Genetic testing issues.
- Artificially sustaining and prolonging life.
- Ethics behind the use of steroids.
- Privacy and Ownership of genetic information.
- Discuss Xenotransplantation.
- Are triage decisions guided by ethical criteria?
- Is Euthanasia ethical?
- Appropriate use of genetic material sampled from indigenous populations.
- Is it ethical to use humans for clinical trials?
- Bioethical issues of Cryonics.
- Explain the use of patient cells for cell lines.
- Discuss artificial exoskeleton.
- Explain the ethics behind the use of growth hormones.
Best Bioethics Research Ideas
- What should a physician do when a patient refuses to undergo an HIV test?
- How do interdisciplinary teams work?
- Explain Bone Conduction.
- Explain the role of a physician’s personal belief in a physician-patient relationship.
- What are the funding policies for old-age care?
- How can doctors address difficulties with advanced care planning without frightening their patients?
- How can doctors counsel patients if they think their relatives would likely disagree with their wishes?
- Should a patient who isn’t likely to stick with the recommended course of treatment be given protease inhibitors?
- When a patient declines to take routine preventive health measures, what should public health doctors do?
- If a doctor admits to making a mistake, does it put them at risk for a malpractice lawsuit?
- When a doctor observes a mistake by a colleague, what should they do?
- Altruism – Medical professionals must put their patients’ needs ahead of their own.
- Accountability – When it comes to matters of public health, doctors should be held responsible by their patients, profession, and society.
- Excellence — Lifelong learning is a must for doctors.
- Duty: Doctors have a responsibility to serve the community and their profession by being attentive, on-call, and available.
Latest Research Topics on Bioethics
- How do medical professionals err?
- Do doctors have an ethical obligation to inform patients about medical errors?
- How does a doctor decide when to inform a patient of a mistake?
- When should doctors notify public health authorities about a communicable disease?
- Can public health care professionals treat patients against their wishes?
- Can admitting mistakes damage a patient’s faith in a doctor?
- What should a doctor do if they see a mistake by a colleague?
- How ought doctors to interact with hospital chaplains?
- What part do a doctor’s personal beliefs play in the doctor-patient relationship?
- How traditional ethics committees can promote shared decision-making between patients (or their surrogates if decisional incapacitated) and their clinicians?
- Discuss about the duties and responsibilities of an attending physician under the Washington Death with Dignity Act
- Is physician aid-in-dying (PAD) the same as euthanasia and ethically permissible? Explain with justifications by focusing on any three developing countries’ acts related to euthanasia
- What if the family of a critical patient asks a registered nurse or physician to withhold the truth from the patient? Is this an ethical practice, if not then explain why?
- Is it justifiable to deceive a patient with a placebo? Explain with appropriate justifications
High-quality Bioethics Essay Topics
- What are the major goals and expected patient outcomes of advanced care planning for old-age patients?
- How should a registered nurse in Australia advise a patient and the patient’s family if she or she does not have anyone to name as a proxy?
- Why euthanasia, eugenics, and organ donation are the major controversial topics in bioethics, and in which countries these are most controversial?
- Compare and contrast the reasons behind the controversies related to end-of-life care and eugenics
- Discuss the difference between an ethics committee and an ethics consultant in the field of bioethics
Popular Bioethics Topics
- Write about healthcare justice.
- Prepare an essay on compulsory vaccination.
- Write an essay about the reproductive cloning of humans.
- Discuss antinatalism in a debate
- Explain the Reversal Test.
- Discuss Mildred Z. Solomon’s contribution to bioethics.
- Write about Bioethics and the feminist side.
- Discuss animal rights in California
- Prepare an essay on herbalism.
- Discuss Islamic bioethics.
Also read: 170 Ethical Essay Topics and Ideas For Students To Focus On
Amazing Bioethics Essay Prompts
- Explain the use of stem cells.
- The rise to power of genetically modified organisms
- Discuss the ethical issues related to abortion.
- Focus on the failure of the President’s Council on Bioethics.
- GMO crops altering wild populations
- Discuss the Three Rs in animal testing
- Discuss doping in a sport of your choice
- Explain state-of-the-art tech for brain imaging
- Analyze the risks of selective reproduction.
- Discuss the risks of having genetic and medical data stolen.
From the list of bioethics topics recommended in this blog post, you can use any topic of your choice for writing a research paper or thesis. In case, you need more bioethics topic ideas or assistance for writing a bioethics research paper, quickly contact us.
Related Post

What is the Claim Letter Format?

85 Fantastic Thesis Topics on Various Subjects

170 Best Business Law Paper Topics To Examine
About author.
Jacob Smith
Jacob Smith guides students with writing research paper topics and theses at greatassignmenthelp.com. Read about the author from this page
https://www.greatassignmenthelp.com/
Comments are closed.
- Featured Posts
Top 100 Java Project Ideas for Beginners and Experts
200 impressive business essay topics, apa vs. mla: know the major differences between the citation styles, 95 trending hackathon project ideas and topics, 95 best data science topics for academic projects, learn how to get good grades in college, learn how to write a leave application for fever, 80 amazing argumentative essay topics and ideas, struggling with assignments.
Expert Help for Your Academic Success
Bioethics: Key Concepts and Research
Two experts in bioethics have curated a reading list of over 20 JSTOR sources on selected issues like: gene-editing, research and treatment, reproduction, disability, genetics, genealogy and race.

Bioethics is a field of inquiry centered around the uses and moral implications of medicine and the bio-sciences. Scholars and researchers come from a very wide variety of professional and disciplinary backgrounds, like medicine, nursing, law, theology, philosophy, history, and other humanities and science disciplines. They employ a range of methodological and theoretical approaches to investigate questions of policy, practice, and meaning in an increasingly technical and medicalized world.

The American biochemist Van Rensselaer Potter is widely credited with introducing the term “bioethics” into the academy in his 1971 book Bioethics: Bridge to the Future . The term “bioethics” was not immediately embraced, though. In fact, neither of the world’s first bioethics research institutes— The Hastings Center (where we work), which was founded in 1969, or The Kennedy Institute of Ethics, founded in 1971—initially used “bioethics” in their names or to describe their work.
The early U.S. national commissions that focused on bioethics issues also shied away from the term. Since the mid to late-1990s, however, the word has become more widely accepted. Bioethics centers can now be found in a growing number of medical schools around the world, many countries have national bioethics commissions, and bioethics courses and degrees are offered in colleges and universities.
This list of essential readings in bioethics is designed to introduce readers to the breadth of writing in the field. Some of the pieces address questions foundational to the field—about reproductive rights, research with human subjects, end -of-life care, and organ donation. Others, such as those about gene editing ancestry testing, consider long standing ethical issues raised by emerging technologies. This list is of course partial and, like the field thus far, has an Anglo-American focus. A relatively young field, bioethics is still expanding its methods and scope.
Theoretical Perspectives
- Albert R. Jonsen et al., “ Special Supplement: The Birth of Bioethics .” The Hastings Center Report (1993).
In 1992, 42 bioethicists who had been active in the field since its inception came together to take stock of what bioethics had accomplished and how it had changed. Warren Reich, a founder of the Kennedy Institute of Ethics, offers a history of the term “bioethics” and the ambivalence that some prominent bioethicists feel about that word.
- James F. Childress and John C. Fletcher, “ Respect for Autonomy .” The Hastings Center Report (1994).
Following revelations of unethical research in the Tuskegee Syphilis Study , a US national commission released a major report, known as The Belmont Report , summarizing the ethical principles for research involving human subjects. These principles—respect for autonomy, beneficence, and justice—were further developed in Tom L. Beauchamp and James F. Childress’ book Principles of Biomedical Ethics , which is still taught in nearly every introductory bioethics course. In this article, Childress and John Fletcher describe the ascension of one principle—respect for autonomy—which they argue deserves a central place in ethical deliberations but must also be tempered by other moral concerns, including care and compassion.
- Ann Bradshaw, “ Yes! There Is an Ethics of Care: An Answer for Peter Allmark .” Journal of Medical Ethics (1996).
Writing as a teacher of nurses, Ann Bradshaw offers historical and modern interpretations of the idea of “caring” that form the basis for an ethic of care. She understands care to not be a value-neutral project, but rather as drawing normatively and descriptively from feminist and religious thought, guided not only by altruism but also by a pursuit of justice.
- Carl E. Schneider, “ Bioethics in the Language of the Law .” The Hastings Center Report (1994).
Schneider argues that moral reasoning within bioethics is often undertaken using legal concepts and language. Law can offer bioethics a rich language and a tool for action, but the social regulatory function of the legal system can also prove inadequate for fully evaluating moral obligations.
- Munyaradzi Felix Murove. “ African Bioethics: An Explanatory Discourse .” Journal for the Study of Religion (2005).
All bioethics is, as this paper notes, culturally conditioned. Western frameworks, which shape much of the scholarship represented in this list, cannot fully describe the contours of ethical reasoning in other cultures. This paper develops an African bioethics that begins with an appreciation of the role of traditional healthcare practices.
Selected Issues in Bioethics
End-of-life care.
- Daniel Callahan, “ Death: “The Distinguished Thing” .” The Hastings Center Report , 2005.
Death, and the myriad ways that dignity may or may not attend it, is one of the enduring themes of bioethics. Daniel Callahan, widely regarded as an originator of the field (and one of the founders of The Hastings Center) asks how we ought to think about the relationship between caring for the dying and the nature of death itself by examining the historical ways that those two concepts have been both conflated and separated.
Defining Death
- Seema K Shah, Robert D Truog, and Franklin G Miller, “ Death and Legal Fictions .” Journal of Medical Ethics (2011).
Advances in life-sustaining treatment and in transplantation medicine have challenged understandings of the definition of death. The introduction in the 1980s of the concept of “brain death” sought to resolve both legal and moral dilemmas by providing additional scientific criteria for determination of death. Shah, Truog, and Miller argue that these changes have created a legal fiction, whereby organs for transplantation are being procured from still-living donors.
Research on Human Subjects
- Charles W. Lidz, and Paul S. Appelbaum. “ The Therapeutic Misconception: Problems and Solutions .” Medical Care (2002).
Clinical research forms the backbone of medical progress, but history is fraught with ethical lapses and oversights that have imperiled human research subjects. One enduring problem is known as the “therapeutic misconception,” in which patients confuse the goals of research and treatment. While medical care is focused on helping a specific patient and is tailored to their needs, research is designed primarily to produce generalizable knowledge, not primarily to help the research subject. This misconception can prevent research subjects from fully appreciating the risks of research or the possibility that they might receive an unproven treatment or even a placebo.
- Nancy E. Kass, Ruth R. Faden, Steven N. Goodman, Peter Pronovost, Sean Tunis, And Tom L. Beauchamp, “ The Research-Treatment Distinction: A Problematic Approach for Determining Which Activities Should Have Ethical Oversight .” The Hastings Center Report (2013).
Since the 1970s, scholars have argued for distinguishing research from treatment, so as to avoid confusion like that described by Lidz and Appelbaum above. The authors of this paper note, however, that distinguishing research from treatment too definitively occludes the fact that for some patients, participation in research is part of their treatment, especially when their illnesses are rare or lack well-established courses of therapy. Thus, adequately protecting patients requires rethinking the research-treatment distinction.
Medical Error
- Nancy Berlinger, “ Avoiding Cheap Grace: Medical Harm, Patient Safety, and the Culture(s) of Forgiveness .” The Hastings Center Report (2003).
Medical errors account for a remarkable number of injuries and deaths. After medical error, patients and families can feel pressure to forgive healthcare providers. Nancy Berlinger argues though that automatic forgiveness amounts to “cheap grace” –it is individual, rather than systemic; a forgiveness achieved without the participation of the injured party; aimed at ending uncomfortable or sad encounters rather than preventing further harm from happening. It asks those who have been harmed to merely ‘do the right thing’ – to forgive, rather than demand change or recompense from those who have erred.
Reproductive Technology
- Eva Feder Kittay, “ Planning a Trip to Italy, Arriving in Holland: The Delusion of Choice in Planning a Family .” International Journal of Feminist Approaches to Bioethics (2010).
New technologies, particularly reproductive ones, purport to offer an ever-expanding range of choices: about if and when to procreate, about who will be genetically related to new offspring, about what kind of health a baby will be born into. Choice is highly valued in many Western cultures, and is strongly defended in much bioethics scholarship. But, Eva Kittay cautions, “choice is not always what it seems and too often it promises what it cannot deliver.”
- John A. Robertson, “ Procreative Liberty and the Control of Conception, Pregnancy, and Childbirth .” Virginia Law Review (1983).
John Robertson argues for an expansion of reproductive freedom beyond the right to access contraception and abortion to include the right to access new reproductive technologies. This additional freedom, which he calls “procreative liberty,” amounts to an additional negative right –the right to be free from government interference in the use of technology to aid reproduction .
- Judith Jarvis Thomson. “ A Defense of Abortion .” Philosophy & Public Affairs (1971).
Debates about abortion infuse many contemporary issues in bioethics. The pro-life argument against abortion is typically premised on the notion that a fetus is a person from the moment of conception. Judith Jarvis Thomson offers a defense of abortion that, contrary to the way the argument usually goes, accepts that premise, using an extended allegory to locate the moral permissibility of abortion instead in the right of the pregnant woman to decide what should happen in and to her body.
Gene Editing
- Brendan P. Foht, “ Gene Editing: New Technology, Old Moral Questions .” The New Atlantis (2016).
Gene editing technologies such as CRISPR-Cas9 are only the latest in the evolution of increasingly precise ways for humans to modify genes. These technologies raise longstanding moral and ethical questions about setting limits, heritable and non-heritable genetic changes, consent, and gratitude. This piece concludes with a pro-life perspective on therapeutic gene editing in humans.
Organ Donation
- Thomas H. Murray, “ Gifts of the Body and the Needs of Strangers .” The Hastings Center Report (1987).
Blood and organ donation raise some of the classic distribution problems in medical ethics: what would a fair matching system look like? Are personal behaviors, or factors such as immigration status, disqualifying? Should donors be compensated for their gifts? In this piece, Thomas Murray considers the context of the “gift” of bodily donations and argues for resisting commercialization.
Disability Rights
- Tom Shakespeare, “ Debating Disability .” Journal of Medical Ethics (2008).
Tom Shakespeare is well known for complicating the distinction between the “medical” and “social” models of disability. The former suggests that disabling traits produce disability, while the latter sees disability as caused by a world unwilling to accommodate people living with different sorts of bodies. Responding to criticism of his book, Disability Rights and Wrongs, Shakespeare details how the field of disability studies can overcome “crude dualism, the better to understand the complex dialectic of disability.”
Enhancing Human Traits
- Erik Parens, “ Authenticity and Ambivalence: Toward Understanding the Enhancement Debate .” The Hastings Center Report (2005).
Many scholars and policymakers have attempted to draw lines between permissible and impermissible uses of biotechnologies by distinguishing between uses that amount to treatments and those that result in enhancement of human traits. Erik Parens reflects on how different notions of authenticity – whether we should primarily be grateful for what we’ve got or creative about improving ourselves – complicate the treatment-enhancement debates.
Genetics, Genealogy, and Race
- Alondra Nelson, “ Bio Science: Genetic Genealogy Testing and the Pursuit of African Ancestry .” Social Studies of Science (2008).
Do genetic ancestry tests ‘geneticize’ racial and ethnic identities? Drawing on ethnographic research conducted with American people of African descent, sociologist Alondra Nelson examines the use of genetics by African Americans who have been cut off from their ancestry due to slavery.
LGBTQ People and Medicine
- Jamie Lindemann Nelson, “ Medicine and Making Sense of Queer Lives .” Hastings Center Report , (2014).
Queer people have had a long and uneasy relationship with the medical establishment, which has by turns offered much-needed care and prejudicial or pathologizing treatment. Noting that medicine extracts a good deal of cultural legitimacy from its “touch of the transcendental,” Jamie Nelson explores the ways that receiving a diagnosis associated with queer identity, such as gender dysphoria, can impact self-understanding.
- Solomon R. Benatar, Abdallah S. Daar, and Peter A. Singer, “ Global Health Ethics: The Rationale for Mutual Caring .” International Affairs (2003).
In our world of staggering and increasing global inequality, bioethics offers insight into how global health needs to be improved by focusing on respect for the dignity of all people and promoting a conception of human flourishing that goes beyond individualistic economic concerns.
- Zahra Meghani and Lisa Eckenwiler, “ Care for the Caregivers? Transnational Justice and Undocumented Non-Citizen Care Workers .” International Journal of Feminist Approaches to Bioethics (2009).
Significant numbers of undocumented workers, often having migrated from the Global South to wealthier nations, are employed as domestic care workers for aging populations. This paper offers insight into some of the injustices these workers confront.

JSTOR is a digital library for scholars, researchers, and students. JSTOR Daily readers can access the original research behind our articles for free on JSTOR.
Get Our Newsletter
Get your fix of JSTOR Daily’s best stories in your inbox each Thursday.
Privacy Policy Contact Us You may unsubscribe at any time by clicking on the provided link on any marketing message.
More Stories

- MeerKAT: The South African Radio Telescope That’s Transformed Our Understanding of the Cosmos

Arakawa and Gins: An Eternal Architecture

The Dangers of Animal Experimentation—for Doctors

Witnessing and Professing Climate Professionals
Recent posts.
- Harvey Houses: Serving the West
- Pakistan’s Ambiguous Islamic Identity
- Building Classroom Discussions around JSTOR Daily Syllabi
- Medieval Whalers, Smart Plants, and Space Mines
Support JSTOR Daily
Sign up for our weekly newsletter.
- Search Menu
- Browse content in Arts and Humanities
- Browse content in Archaeology
- Anglo-Saxon and Medieval Archaeology
- Archaeological Methodology and Techniques
- Archaeology by Region
- Archaeology of Religion
- Archaeology of Trade and Exchange
- Biblical Archaeology
- Contemporary and Public Archaeology
- Environmental Archaeology
- Historical Archaeology
- History and Theory of Archaeology
- Industrial Archaeology
- Landscape Archaeology
- Mortuary Archaeology
- Prehistoric Archaeology
- Underwater Archaeology
- Urban Archaeology
- Zooarchaeology
- Browse content in Architecture
- Architectural Structure and Design
- History of Architecture
- Residential and Domestic Buildings
- Theory of Architecture
- Browse content in Art
- Art Subjects and Themes
- History of Art
- Industrial and Commercial Art
- Theory of Art
- Biographical Studies
- Byzantine Studies
- Browse content in Classical Studies
- Classical History
- Classical Philosophy
- Classical Mythology
- Classical Literature
- Classical Reception
- Classical Art and Architecture
- Classical Oratory and Rhetoric
- Greek and Roman Epigraphy
- Greek and Roman Law
- Greek and Roman Papyrology
- Greek and Roman Archaeology
- Late Antiquity
- Religion in the Ancient World
- Digital Humanities
- Browse content in History
- Colonialism and Imperialism
- Diplomatic History
- Environmental History
- Genealogy, Heraldry, Names, and Honours
- Genocide and Ethnic Cleansing
- Historical Geography
- History by Period
- History of Emotions
- History of Agriculture
- History of Education
- History of Gender and Sexuality
- Industrial History
- Intellectual History
- International History
- Labour History
- Legal and Constitutional History
- Local and Family History
- Maritime History
- Military History
- National Liberation and Post-Colonialism
- Oral History
- Political History
- Public History
- Regional and National History
- Revolutions and Rebellions
- Slavery and Abolition of Slavery
- Social and Cultural History
- Theory, Methods, and Historiography
- Urban History
- World History
- Browse content in Language Teaching and Learning
- Language Learning (Specific Skills)
- Language Teaching Theory and Methods
- Browse content in Linguistics
- Applied Linguistics
- Cognitive Linguistics
- Computational Linguistics
- Forensic Linguistics
- Grammar, Syntax and Morphology
- Historical and Diachronic Linguistics
- History of English
- Language Acquisition
- Language Evolution
- Language Reference
- Language Variation
- Language Families
- Lexicography
- Linguistic Anthropology
- Linguistic Theories
- Linguistic Typology
- Phonetics and Phonology
- Psycholinguistics
- Sociolinguistics
- Translation and Interpretation
- Writing Systems
- Browse content in Literature
- Bibliography
- Children's Literature Studies
- Literary Studies (Asian)
- Literary Studies (European)
- Literary Studies (Eco-criticism)
- Literary Studies (Romanticism)
- Literary Studies (American)
- Literary Studies (Modernism)
- Literary Studies - World
- Literary Studies (1500 to 1800)
- Literary Studies (19th Century)
- Literary Studies (20th Century onwards)
- Literary Studies (African American Literature)
- Literary Studies (British and Irish)
- Literary Studies (Early and Medieval)
- Literary Studies (Fiction, Novelists, and Prose Writers)
- Literary Studies (Gender Studies)
- Literary Studies (Graphic Novels)
- Literary Studies (History of the Book)
- Literary Studies (Plays and Playwrights)
- Literary Studies (Poetry and Poets)
- Literary Studies (Postcolonial Literature)
- Literary Studies (Queer Studies)
- Literary Studies (Science Fiction)
- Literary Studies (Travel Literature)
- Literary Studies (War Literature)
- Literary Studies (Women's Writing)
- Literary Theory and Cultural Studies
- Mythology and Folklore
- Shakespeare Studies and Criticism
- Browse content in Media Studies
- Browse content in Music
- Applied Music
- Dance and Music
- Ethics in Music
- Ethnomusicology
- Gender and Sexuality in Music
- Medicine and Music
- Music Cultures
- Music and Religion
- Music and Media
- Music and Culture
- Music Education and Pedagogy
- Music Theory and Analysis
- Musical Scores, Lyrics, and Libretti
- Musical Structures, Styles, and Techniques
- Musicology and Music History
- Performance Practice and Studies
- Race and Ethnicity in Music
- Sound Studies
- Browse content in Performing Arts
- Browse content in Philosophy
- Aesthetics and Philosophy of Art
- Epistemology
- Feminist Philosophy
- History of Western Philosophy
- Metaphysics
- Moral Philosophy
- Non-Western Philosophy
- Philosophy of Science
- Philosophy of Language
- Philosophy of Mind
- Philosophy of Perception
- Philosophy of Action
- Philosophy of Law
- Philosophy of Religion
- Philosophy of Mathematics and Logic
- Practical Ethics
- Social and Political Philosophy
- Browse content in Religion
- Biblical Studies
- Christianity
- East Asian Religions
- History of Religion
- Judaism and Jewish Studies
- Qumran Studies
- Religion and Education
- Religion and Health
- Religion and Politics
- Religion and Science
- Religion and Law
- Religion and Art, Literature, and Music
- Religious Studies
- Browse content in Society and Culture
- Cookery, Food, and Drink
- Cultural Studies
- Customs and Traditions
- Ethical Issues and Debates
- Hobbies, Games, Arts and Crafts
- Lifestyle, Home, and Garden
- Natural world, Country Life, and Pets
- Popular Beliefs and Controversial Knowledge
- Sports and Outdoor Recreation
- Technology and Society
- Travel and Holiday
- Visual Culture
- Browse content in Law
- Arbitration
- Browse content in Company and Commercial Law
- Commercial Law
- Company Law
- Browse content in Comparative Law
- Systems of Law
- Competition Law
- Browse content in Constitutional and Administrative Law
- Government Powers
- Judicial Review
- Local Government Law
- Military and Defence Law
- Parliamentary and Legislative Practice
- Construction Law
- Contract Law
- Browse content in Criminal Law
- Criminal Procedure
- Criminal Evidence Law
- Sentencing and Punishment
- Employment and Labour Law
- Environment and Energy Law
- Browse content in Financial Law
- Banking Law
- Insolvency Law
- History of Law
- Human Rights and Immigration
- Intellectual Property Law
- Browse content in International Law
- Private International Law and Conflict of Laws
- Public International Law
- IT and Communications Law
- Jurisprudence and Philosophy of Law
- Law and Politics
- Law and Society
- Browse content in Legal System and Practice
- Courts and Procedure
- Legal Skills and Practice
- Primary Sources of Law
- Regulation of Legal Profession
- Medical and Healthcare Law
- Browse content in Policing
- Criminal Investigation and Detection
- Police and Security Services
- Police Procedure and Law
- Police Regional Planning
- Browse content in Property Law
- Personal Property Law
- Study and Revision
- Terrorism and National Security Law
- Browse content in Trusts Law
- Wills and Probate or Succession
- Browse content in Medicine and Health
- Browse content in Allied Health Professions
- Arts Therapies
- Clinical Science
- Dietetics and Nutrition
- Occupational Therapy
- Operating Department Practice
- Physiotherapy
- Radiography
- Speech and Language Therapy
- Browse content in Anaesthetics
- General Anaesthesia
- Neuroanaesthesia
- Browse content in Clinical Medicine
- Acute Medicine
- Cardiovascular Medicine
- Clinical Genetics
- Clinical Pharmacology and Therapeutics
- Dermatology
- Endocrinology and Diabetes
- Gastroenterology
- Genito-urinary Medicine
- Geriatric Medicine
- Infectious Diseases
- Medical Toxicology
- Medical Oncology
- Pain Medicine
- Palliative Medicine
- Rehabilitation Medicine
- Respiratory Medicine and Pulmonology
- Rheumatology
- Sleep Medicine
- Sports and Exercise Medicine
- Clinical Neuroscience
- Community Medical Services
- Critical Care
- Emergency Medicine
- Forensic Medicine
- Haematology
- History of Medicine
- Browse content in Medical Dentistry
- Oral and Maxillofacial Surgery
- Paediatric Dentistry
- Restorative Dentistry and Orthodontics
- Surgical Dentistry
- Browse content in Medical Skills
- Clinical Skills
- Communication Skills
- Nursing Skills
- Surgical Skills
- Medical Ethics
- Medical Statistics and Methodology
- Browse content in Neurology
- Clinical Neurophysiology
- Neuropathology
- Nursing Studies
- Browse content in Obstetrics and Gynaecology
- Gynaecology
- Occupational Medicine
- Ophthalmology
- Otolaryngology (ENT)
- Browse content in Paediatrics
- Neonatology
- Browse content in Pathology
- Chemical Pathology
- Clinical Cytogenetics and Molecular Genetics
- Histopathology
- Medical Microbiology and Virology
- Patient Education and Information
- Browse content in Pharmacology
- Psychopharmacology
- Browse content in Popular Health
- Caring for Others
- Complementary and Alternative Medicine
- Self-help and Personal Development
- Browse content in Preclinical Medicine
- Cell Biology
- Molecular Biology and Genetics
- Reproduction, Growth and Development
- Primary Care
- Professional Development in Medicine
- Browse content in Psychiatry
- Addiction Medicine
- Child and Adolescent Psychiatry
- Forensic Psychiatry
- Learning Disabilities
- Old Age Psychiatry
- Psychotherapy
- Browse content in Public Health and Epidemiology
- Epidemiology
- Public Health
- Browse content in Radiology
- Clinical Radiology
- Interventional Radiology
- Nuclear Medicine
- Radiation Oncology
- Reproductive Medicine
- Browse content in Surgery
- Cardiothoracic Surgery
- Gastro-intestinal and Colorectal Surgery
- General Surgery
- Neurosurgery
- Paediatric Surgery
- Peri-operative Care
- Plastic and Reconstructive Surgery
- Surgical Oncology
- Transplant Surgery
- Trauma and Orthopaedic Surgery
- Vascular Surgery
- Browse content in Science and Mathematics
- Browse content in Biological Sciences
- Aquatic Biology
- Biochemistry
- Bioinformatics and Computational Biology
- Developmental Biology
- Ecology and Conservation
- Evolutionary Biology
- Genetics and Genomics
- Microbiology
- Molecular and Cell Biology
- Natural History
- Plant Sciences and Forestry
- Research Methods in Life Sciences
- Structural Biology
- Systems Biology
- Zoology and Animal Sciences
- Browse content in Chemistry
- Analytical Chemistry
- Computational Chemistry
- Crystallography
- Environmental Chemistry
- Industrial Chemistry
- Inorganic Chemistry
- Materials Chemistry
- Medicinal Chemistry
- Mineralogy and Gems
- Organic Chemistry
- Physical Chemistry
- Polymer Chemistry
- Study and Communication Skills in Chemistry
- Theoretical Chemistry
- Browse content in Computer Science
- Artificial Intelligence
- Computer Architecture and Logic Design
- Game Studies
- Human-Computer Interaction
- Mathematical Theory of Computation
- Programming Languages
- Software Engineering
- Systems Analysis and Design
- Virtual Reality
- Browse content in Computing
- Business Applications
- Computer Security
- Computer Games
- Computer Networking and Communications
- Digital Lifestyle
- Graphical and Digital Media Applications
- Operating Systems
- Browse content in Earth Sciences and Geography
- Atmospheric Sciences
- Environmental Geography
- Geology and the Lithosphere
- Maps and Map-making
- Meteorology and Climatology
- Oceanography and Hydrology
- Palaeontology
- Physical Geography and Topography
- Regional Geography
- Soil Science
- Urban Geography
- Browse content in Engineering and Technology
- Agriculture and Farming
- Biological Engineering
- Civil Engineering, Surveying, and Building
- Electronics and Communications Engineering
- Energy Technology
- Engineering (General)
- Environmental Science, Engineering, and Technology
- History of Engineering and Technology
- Mechanical Engineering and Materials
- Technology of Industrial Chemistry
- Transport Technology and Trades
- Browse content in Environmental Science
- Applied Ecology (Environmental Science)
- Conservation of the Environment (Environmental Science)
- Environmental Sustainability
- Environmentalist Thought and Ideology (Environmental Science)
- Management of Land and Natural Resources (Environmental Science)
- Natural Disasters (Environmental Science)
- Nuclear Issues (Environmental Science)
- Pollution and Threats to the Environment (Environmental Science)
- Social Impact of Environmental Issues (Environmental Science)
- History of Science and Technology
- Browse content in Materials Science
- Ceramics and Glasses
- Composite Materials
- Metals, Alloying, and Corrosion
- Nanotechnology
- Browse content in Mathematics
- Applied Mathematics
- Biomathematics and Statistics
- History of Mathematics
- Mathematical Education
- Mathematical Finance
- Mathematical Analysis
- Numerical and Computational Mathematics
- Probability and Statistics
- Pure Mathematics
- Browse content in Neuroscience
- Cognition and Behavioural Neuroscience
- Development of the Nervous System
- Disorders of the Nervous System
- History of Neuroscience
- Invertebrate Neurobiology
- Molecular and Cellular Systems
- Neuroendocrinology and Autonomic Nervous System
- Neuroscientific Techniques
- Sensory and Motor Systems
- Browse content in Physics
- Astronomy and Astrophysics
- Atomic, Molecular, and Optical Physics
- Biological and Medical Physics
- Classical Mechanics
- Computational Physics
- Condensed Matter Physics
- Electromagnetism, Optics, and Acoustics
- History of Physics
- Mathematical and Statistical Physics
- Measurement Science
- Nuclear Physics
- Particles and Fields
- Plasma Physics
- Quantum Physics
- Relativity and Gravitation
- Semiconductor and Mesoscopic Physics
- Browse content in Psychology
- Affective Sciences
- Clinical Psychology
- Cognitive Psychology
- Cognitive Neuroscience
- Criminal and Forensic Psychology
- Developmental Psychology
- Educational Psychology
- Evolutionary Psychology
- Health Psychology
- History and Systems in Psychology
- Music Psychology
- Neuropsychology
- Organizational Psychology
- Psychological Assessment and Testing
- Psychology of Human-Technology Interaction
- Psychology Professional Development and Training
- Research Methods in Psychology
- Social Psychology
- Browse content in Social Sciences
- Browse content in Anthropology
- Anthropology of Religion
- Human Evolution
- Medical Anthropology
- Physical Anthropology
- Regional Anthropology
- Social and Cultural Anthropology
- Theory and Practice of Anthropology
- Browse content in Business and Management
- Business Strategy
- Business Ethics
- Business History
- Business and Government
- Business and Technology
- Business and the Environment
- Comparative Management
- Corporate Governance
- Corporate Social Responsibility
- Entrepreneurship
- Health Management
- Human Resource Management
- Industrial and Employment Relations
- Industry Studies
- Information and Communication Technologies
- International Business
- Knowledge Management
- Management and Management Techniques
- Operations Management
- Organizational Theory and Behaviour
- Pensions and Pension Management
- Public and Nonprofit Management
- Strategic Management
- Supply Chain Management
- Browse content in Criminology and Criminal Justice
- Criminal Justice
- Criminology
- Forms of Crime
- International and Comparative Criminology
- Youth Violence and Juvenile Justice
- Development Studies
- Browse content in Economics
- Agricultural, Environmental, and Natural Resource Economics
- Asian Economics
- Behavioural Finance
- Behavioural Economics and Neuroeconomics
- Econometrics and Mathematical Economics
- Economic Systems
- Economic History
- Economic Methodology
- Economic Development and Growth
- Financial Markets
- Financial Institutions and Services
- General Economics and Teaching
- Health, Education, and Welfare
- History of Economic Thought
- International Economics
- Labour and Demographic Economics
- Law and Economics
- Macroeconomics and Monetary Economics
- Microeconomics
- Public Economics
- Urban, Rural, and Regional Economics
- Welfare Economics
- Browse content in Education
- Adult Education and Continuous Learning
- Care and Counselling of Students
- Early Childhood and Elementary Education
- Educational Equipment and Technology
- Educational Strategies and Policy
- Higher and Further Education
- Organization and Management of Education
- Philosophy and Theory of Education
- Schools Studies
- Secondary Education
- Teaching of a Specific Subject
- Teaching of Specific Groups and Special Educational Needs
- Teaching Skills and Techniques
- Browse content in Environment
- Applied Ecology (Social Science)
- Climate Change
- Conservation of the Environment (Social Science)
- Environmentalist Thought and Ideology (Social Science)
- Natural Disasters (Environment)
- Social Impact of Environmental Issues (Social Science)
- Browse content in Human Geography
- Cultural Geography
- Economic Geography
- Political Geography
- Browse content in Interdisciplinary Studies
- Communication Studies
- Museums, Libraries, and Information Sciences
- Browse content in Politics
- African Politics
- Asian Politics
- Chinese Politics
- Comparative Politics
- Conflict Politics
- Elections and Electoral Studies
- Environmental Politics
- European Union
- Foreign Policy
- Gender and Politics
- Human Rights and Politics
- Indian Politics
- International Relations
- International Organization (Politics)
- International Political Economy
- Irish Politics
- Latin American Politics
- Middle Eastern Politics
- Political Methodology
- Political Communication
- Political Philosophy
- Political Sociology
- Political Behaviour
- Political Economy
- Political Institutions
- Political Theory
- Politics and Law
- Public Administration
- Public Policy
- Quantitative Political Methodology
- Regional Political Studies
- Russian Politics
- Security Studies
- State and Local Government
- UK Politics
- US Politics
- Browse content in Regional and Area Studies
- African Studies
- Asian Studies
- East Asian Studies
- Japanese Studies
- Latin American Studies
- Middle Eastern Studies
- Native American Studies
- Scottish Studies
- Browse content in Research and Information
- Research Methods
- Browse content in Social Work
- Addictions and Substance Misuse
- Adoption and Fostering
- Care of the Elderly
- Child and Adolescent Social Work
- Couple and Family Social Work
- Developmental and Physical Disabilities Social Work
- Direct Practice and Clinical Social Work
- Emergency Services
- Human Behaviour and the Social Environment
- International and Global Issues in Social Work
- Mental and Behavioural Health
- Social Justice and Human Rights
- Social Policy and Advocacy
- Social Work and Crime and Justice
- Social Work Macro Practice
- Social Work Practice Settings
- Social Work Research and Evidence-based Practice
- Welfare and Benefit Systems
- Browse content in Sociology
- Childhood Studies
- Community Development
- Comparative and Historical Sociology
- Economic Sociology
- Gender and Sexuality
- Gerontology and Ageing
- Health, Illness, and Medicine
- Marriage and the Family
- Migration Studies
- Occupations, Professions, and Work
- Organizations
- Population and Demography
- Race and Ethnicity
- Social Theory
- Social Movements and Social Change
- Social Research and Statistics
- Social Stratification, Inequality, and Mobility
- Sociology of Religion
- Sociology of Education
- Sport and Leisure
- Urban and Rural Studies
- Browse content in Warfare and Defence
- Defence Strategy, Planning, and Research
- Land Forces and Warfare
- Military Administration
- Military Life and Institutions
- Naval Forces and Warfare
- Other Warfare and Defence Issues
- Peace Studies and Conflict Resolution
- Weapons and Equipment

The Oxford Handbook of Bioethics

Bonnie Steinbock is Professor of Philosophy at the University at Albany, where she teaches courses in ethics, applied ethics, philosophy of law, bioethics, public policy, and public health. She is also on the faculty of the Alden March Bioethics Institute at Albany Medical College. She has lectured all over the world on the ethics of reproduction and genetics, and has appeared in various media, including The New York Times, Newsweek, and The Newshour with Jim Lehrer. Her many publications include over 60 articles and a book, Life Before Birth:The Moral and Legal Status of Embryos and Fetuses (Oxford 1992). She has also edited Legal and Ethical Issues in Human Reproduction (Ashgate 2002). She is a Fellow of the Hastings Centre, the Chair of its Fellows Council, and a member of its Board of Directors. She is also a member of the Ethics Committee of the American Society of Reproductive Medicine.
- Cite Icon Cite
- Permissions Icon Permissions
The Oxford Handbook of Bioethics is an authoritative, state-of-the-art guide to current issues in bioethics. Thirty-four contributors reflect the interdisciplinary nature that is characteristic of bioethics, and its increasingly international character. Thirty topics are covered in articles written by some of the world's leading figures in the field, as well as by some newer ‘up-and-comers’. The articles address both perennial issues, such as the methodology of bioethics, autonomy, justice, death, and moral status, and newer issues, such as bio-banking, stem cell research, cloning, pharmacogenomics, and bioterrorism. Other topics concern mental illness and moral agency, the rule of double effect, justice and the elderly, the definition of death, organ transplantation, feminist approaches to commodification of the body, life extension, advance directives, physician-assisted death, abortion, genetic research, population screening, enhancement, research ethics, and the implications of public and global health for bioethics.
Signed in as
Institutional accounts.
- Google Scholar Indexing
- GoogleCrawler [DO NOT DELETE]
Personal account
- Sign in with email/username & password
- Get email alerts
- Save searches
- Purchase content
- Activate your purchase/trial code
- Add your ORCID iD
Institutional access
Sign in with a library card.
- Sign in with username/password
- Recommend to your librarian
- Institutional account management
- Get help with access
Access to content on Oxford Academic is often provided through institutional subscriptions and purchases. If you are a member of an institution with an active account, you may be able to access content in one of the following ways:
IP based access
Typically, access is provided across an institutional network to a range of IP addresses. This authentication occurs automatically, and it is not possible to sign out of an IP authenticated account.
Sign in through your institution
Choose this option to get remote access when outside your institution. Shibboleth/Open Athens technology is used to provide single sign-on between your institution’s website and Oxford Academic.
- Click Sign in through your institution.
- Select your institution from the list provided, which will take you to your institution's website to sign in.
- When on the institution site, please use the credentials provided by your institution. Do not use an Oxford Academic personal account.
- Following successful sign in, you will be returned to Oxford Academic.
If your institution is not listed or you cannot sign in to your institution’s website, please contact your librarian or administrator.
Enter your library card number to sign in. If you cannot sign in, please contact your librarian.
Society Members
Society member access to a journal is achieved in one of the following ways:
Sign in through society site
Many societies offer single sign-on between the society website and Oxford Academic. If you see ‘Sign in through society site’ in the sign in pane within a journal:
- Click Sign in through society site.
- When on the society site, please use the credentials provided by that society. Do not use an Oxford Academic personal account.
If you do not have a society account or have forgotten your username or password, please contact your society.
Sign in using a personal account
Some societies use Oxford Academic personal accounts to provide access to their members. See below.
A personal account can be used to get email alerts, save searches, purchase content, and activate subscriptions.
Some societies use Oxford Academic personal accounts to provide access to their members.
Viewing your signed in accounts
Click the account icon in the top right to:
- View your signed in personal account and access account management features.
- View the institutional accounts that are providing access.
Signed in but can't access content
Oxford Academic is home to a wide variety of products. The institutional subscription may not cover the content that you are trying to access. If you believe you should have access to that content, please contact your librarian.
For librarians and administrators, your personal account also provides access to institutional account management. Here you will find options to view and activate subscriptions, manage institutional settings and access options, access usage statistics, and more.
Our books are available by subscription or purchase to libraries and institutions.
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Rights and permissions
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- BMJ Open Access

A bioethics for all seasons
The last four decades have seen the emergence and flourishing of the field of bioethics and its incorporation into wide-ranging aspects of society, from the clinic or laboratory through to public policy and the media. Yet considerable debate still exists over what bioethics is and how it should be done. In this paper I consider the question of what makes good bioethics. Drawing on historical and contemporary examples, I suggest that bioethics encompasses multiple modes of responding to moral disagreement, and that an awareness of which mode is operational in a given context is essential to doing good bioethics.
What is (good) bioethics?
Imagine, for a moment, being asked to compile a list of what you consider to be the top 10 works in the field of bioethics over the last four decades. Which books and papers spring to mind? If we were all asked to perform the same exercise, no doubt we would each come up with a different selection, though there might well be some overlap. But in trying to make such a list, we would undoubtedly have a sense of what it was we were looking for in our Bioethical Top 10. What, then, is that elusive ‘Oh! factor’ that makes good bioethics?
We might start by considering the influence of a given work in terms of its reach. Some journals produce a list of their most accessed or cited articles, presumably as some indication of the ‘best’ papers on offer. In this age of metrics and statistics, it is certainly tempting to fall back on numerical measures such as citation indices and impact factors—quantifiability as a proxy for quality. Then again, not all papers that are widely read or cited are necessarily good; notoriety does not always equal quality. Indeed, one might facetiously suggest that an effective way to be cited frequently is to be wrong! More seriously, a work that is open both to criticism and further development is of course likely to generate increased citations; the numbers, however, are but a crude reflection of this property and do not supply an explanation or description of what constitutes quality.
Perhaps, then, we ought to search for those works of bioethical scholarship that contain the most rigorous philosophical analysis, or the most elegant argument; or that provide insight into the most pressing issues of the day; or that enable the most effective policy solutions. But how do we measure any of these aspects, or decide which should predominate, in our determination of what we consider to be ‘good’? It is important to recognise that in asking what makes good bioethics we are also inviting an opinion about what we think bioethics is, or should be.
This is a problem that bioethics has confronted repeatedly throughout its relatively short history. In so doing, it has often turned a self-reflective eye to questions of disciplinarity and interdisciplinarity, method and methodology (or lack thereof)—in short, how to define what it is we do. 1 Intertwined with such issues is the question of moral expertise, of what gives us as bioethicists legitimacy to carry out the roles we claim to be able to perform. 2 3
Bioethics has at least some of its roots in philosophy, 4 but we do not, by and large, have an explicitly-developed ‘philosophy of bioethics’. Science produces explanations for observed phenomena and evaluates those explanations according to how well they describe and predict these phenomena. But if bioethics functions analogously, in attempting to elucidate some underlying moral principle, what are the phenomena we are trying to explain?
The role of moral intuitions in bioethics is a contested one, and being led by the ‘moral nose’ is often looked upon as being a poor method of ‘doing bioethics’. Yet bioethical reasoning often relies first on appeals to intuition to establish the basic premises from which axioms can be abstracted and against which more complex problems can be compared and analysed. Can we claim, then, that we are uncovering some sort of ‘moral truth’? This problem both recapitulates perpetual meta-ethical debates over the nature of moral philosophy itself and invokes issues of moral expertise—who is best placed to know or discover the truth, and whose statements should be considered as good moral guidance? How do we decide what makes something a ‘good’ moral explanation, and is producing moral explanations the proper purpose of bioethics?
Considering, almost a decade ago, the question of what bioethicists do and what claim to authority they have in doing it, Baker wrote: “the role of the bioethicist is not that of watchdog, policing and protecting the boundaries of morality, but rather of facilitator, assisting society to reflectively articulate, interpret, and specify our common morality in the context of the rapidly evolving world of biomedicine.” 5 What happens, though, when there is a lack of consensus on a single ‘common morality’? In fact these are the problems in which bioethics is most often called to take a hand—if there were no disagreement over what constituted ‘common morality’, there would be no disagreement over what should be done. The demand for bioethics, then—our justification for existence—can be seen as being generated by the need to respond to a lack of moral consensus within society. The measure of good bioethics on this account is the degree to which the response serves the need. But what form should our responses take and what is their function?
Theory and practice: different bioethical hats?
Presumably we need to have an account of what bioethics is or does, in order to say what constitutes good bioethics, but this does not mean there must be a single account of bioethics or that it must be rigidly or precisely defined. Bioethics can be many things, depending on the particular context and purpose of the endeavour. A primary feature of ‘good’ bioethics, therefore, is ‘using the right tool for the job’. This in turn entails knowing what the job is we are trying to do; knowing (to mix metaphors) which hat we are wearing at any given time. To whom are we talking and for what purpose?
Bioethicists sometimes see themselves as divided up into schools of thought characterised by a particular method or approach. From a more general perspective, however, we might argue that the most significant divide in bioethics is not between virtue ethics and consequentialism, or continental and Anglo-American philosophy, but between theory and practice: bioethics-as-philosophy versus bioethics-as-policy.
Bioethics is frequently described as a branch of practical ethics or applied philosophy. A common bone of contention between differing accounts of bioethics, however, is the extent to which a given piece of work needs to have practical application in order to have value or ‘count’ as bioethics. It is often asserted that bioethics must be ‘action-guiding’, should tell us what to do. Some, indeed, see bioethics as defined by this aim: Sheehan and Dunn, for example, assert that bioethics “must primarily be concerned to address a practical ‘ought’ question ”. 6
In judging what is a worthwhile piece of bioethics, though, we should caution against a too-literal interpretation of practical applicability. An argument does not always have to translate directly into policy or have an immediate and measurable real-world outcome in order to be of some practical value; the ways of moving towards answers to “practical ‘ought’ questions” are not always themselves practical. Consider some of the best known thought experiments generated by bioethics in its early years, for example, involving trolleys and violinists and kittens. Needless to say, such thought experiments do not and are not intended to represent actual situations that are ever going to occur in real life! Moreover, it is worth noting that bioethics does not produce a single ‘solution’ to such problems. The aim of the exercise is not to solve the problem as such, to know what to do should we be confronted with such a situation, but to provide some sort of structure to how we think about the much more complex, messy, squishy problems we encounter in real life—to abstract certain dimensions of these problems and render them susceptible of analysis.
Likewise, some of the terrain into which bioethics has ventured in more recent years has attracted criticism for being too speculative. To give a personal example, when discussing more radical forms of human enhancement, such as living to hundreds of years old or splicing our bodies with machines, I have at least once been asked “Why are you talking about this? This is science fiction! Why aren't we discussing saving the rainforests?” While not discounting the importance of the rainforests, I fervently maintain—and the existence of an ever-growing body of literature on the topic suggests I am not alone in holding this opinion—that an inquiry into the ethics of cyber-enhancement, for example, humans becoming cyborgs, or uploading our brains to computers, can be a worthwhile bioethical enterprise. Its worth, however, is more in what we learn about our present selves and values than in addressing ‘practical ought questions’ about whether we should adopt a technology that does not yet exist.
This is not to say that such pursuits are purely blue-skies bioethics, producing only pure moral knowledge that lacks all practical application (indeed, depending on one's view of how bioethics works to generate moral knowledge, one might deny that such a thing as ‘pure moral knowledge’ can even exist). Using the above example, the philosophical understandings of the ‘human body’ concept and its associated values that we gain through bioethical imaginings of cyber-enhancement can also inform our consideration of more timely ‘ought’ questions regarding current forms of embodiment and the normativities that attach to them, such as disabled/moderately enhanced bodies and their relationships to assistive technologies. Or, to take another example, contemplating how we would or should treat a chimeric mouse or chimpanzee with human-like intelligence—unlikely as it may be that such a being will soon be created—may reveal moral insights that bear upon how we ought to treat non-chimeric mice, chimpanzees and other animals in the present day.
Critics who accuse us of being unrealistic with reference to the thought experiments we invoke, or of dabbling in science fiction with reference to the technological possibilities we imagine, are therefore missing the point. The value of conducting thought experiments that are themselves unrealistic and speculating about technological developments which are as yet only futuristic possibilities lies not least (though also, I would venture to suggest, not only) in what such considerations can tell us about real and present bioethical problems.
So, doing good bioethics can sometimes entail letting our bioethical imaginations run wild. Nevertheless, intersection with the real world remains an important dimension of (and justification for) bioethics, demanding that the work we do be relevant, understandable and applicable, at least when it is offered as a response to a real-world dilemma. This, however, leads us to another potential quagmire of criticism or crisis of bioethical identity: that bioethics is not good philosophy, or that only good philosophy can be called bioethics.
Too philosophical, or not philosophical enough?
Where our aim as bioethicists is to influence policy, it is crucial not only that our arguments are adequate and appropriate to the task but also that they are presented in a manner that will be effective. But what to do when effective presentation involves glossing the underlying philosophy, or when the argument is philosophically sound but contextually or socially inappropriate? The problem of how to walk the line maintaining both philosophical scholarly integrity and the public acceptability necessary to retain credible influence has been a long recognised tension in bioethics.
We may think that good bioethics should aim at “actually convincing people to act differently or to change policy because of the arguments and answers that the bioethicist provides”, 6 but this says nothing about how these arguments and answers should be derived. If this is indeed what we think our job is, then privately reasoning our way to what is morally right and publicly presenting views aimed at changing policy or behaviour to achieve a morally better outcome are two linked but distinct faces of bioethics.
This can be illustrated by reference to two examples from across the lifetime of what we might call contemporary bioethics in this country: the seminal report of the Warnock Committee on embryo research in 1984 that eventually led to the foundation of the Human Fertilisation and Embryology Authority (HFEA), and the policy recommendations produced by the HFEA's own consultation on mitochondrial replacement, 30 years later.
The activities of the Warnock Committee and the resulting Warnock Report have been widely seen as a test case for the role of philosophical bioethics in policymaking. Commentaries at the time criticised the approach and reasoning by which the committee arrived at its recommendations, notably whether the commitment to compromise was an appropriate response to moral pluralism, and whether there was any sound moral basis for selecting the 14-day limit as the ‘bright line’ after which research should be prohibited. But producing watertight philosophical reasoning and dispensing moral expertise was not the committee's self-stated mission. Instead, as Warnock herself argued, the aim was to facilitate a dialogue and process by which the plurality of dissenting moral views could somehow forge a workable policy decision. 7
Thus, as Nelson comments, the choice of the 14-day cut-off “…did not reflect an overwhelming national feeling that individuation is an essential property of a member of the human species. On the contrary, utilitarian considerations concerning potential research benefits played an explicit role in the Committee's judgment about this matter.” 8 The fortuitous intersection of scientific utility (most useful research could be done before this stage), the attributes perceived as important by much of the public, and the use of individuation as a superficially plausible property to support the cut-off point enabled a compromise position on this most intractable issue. While the description of the Warnock Committee report as containing ‘more or less well defended views’ may seem to be damning with faint praise, in fact it was the characterisation of bioethics as a policy process, in the work of the committee itself and in its key recommendations for oversight (eventually taking form as the HFEA), that led to a legacy of what has been for the most part effective public policymaking over the 30 years since, while many other jurisdictions were still struggling to move past moral disagreement and lacking functional policy on the matter.
The work of the Warnock Committee, then, is undeniably one of the most influential pieces of British bioethics, both in its effects on policy and research and in defining what bioethics itself has today become. It may not have been the most philosophically consistent work of all time, but was it ‘good bioethics’? Considering all of the above, I think the answer is yes.
Moving forward to the present day, the work of the HFEA and other policy groups on mitochondrial replacement 9 10 provides another example of favouring the politic over the philosophical to achieve the best policy outcome. Much of the controversy over this technique centred on the significance of genetics, and whether the 0.1% of DNA contained within mitochondria would affect either the child's parentage or identity. The issues of genetic parentage and identity both offer considerable scope for philosophical and conceptual analysis—what makes a parent?; what constitutes identity?—but although such analysis may deepen our understanding of these complex concepts, it is perhaps not immediately useful in a policy-setting context where concerns over ‘three-parent babies’ dominate the media coverage of the subject.
The consultation reports, then, downplayed the significance of mitochondrial DNA in genetic kinship ties and identity, emphasising that it contributed very little to the child's genetic make-up and would not affect any essential characteristics. The latter claim is clearly over-reaching: mitochondrial DNA affects at least one important characteristic, that of health status; otherwise the technique would be pointless. And although a careful consideration of the meaning of ‘parenthood’ might well conclude that mitochondrial donors should not be considered parents, the three-parent issue was sidelined rather than analysed.
At a public meeting to discuss the HFEA consultation in March 2013, comments made by those involved suggest that the de-emphasisation of the importance of genetics to identity was strategic. Genetic parenthood and mtDNA were deliberately dissociated because the three-parent label was seen as too controversial to be publicly acceptable, and the decision to favour donor anonymity and not to regard donors as genetic parents was influenced by fears that the technique might be ‘jeopardised by a three-parent tag’ (D Griffiths 2014, unpublished data).
While this is not the place for an exposition of the moral arguments supporting the use of mitochondrial replacement, such arguments do exist and are in my opinion well-founded. I do not doubt that permission, rather than prohibition, is the morally better policy in this case. The consultation, however, did not take the route of analysing and exploring these arguments philosophically; its recommendations were arrived at by a different but arguably more effective means. If on the basis of this, the technique is allowed to proceed, the better outcome will have been achieved.
I want to suggest, then, that at least in the policy context, it is sometimes less important to be absolutely precise or ‘correct’ (or truthful, if we think there is such a thing as moral truth) in our arguments than to be convincing in order to achieve the desired outcome. If what we care about are consequences, then, in some cases, the means of less than perfect moral reasoning may be justified by the ends of a functional policy that achieves the best outcome. Granted, we must have some underlying idea of what is ‘right’ in order to decide what policy outcome we want to achieve; as bioethicists in whatever capacity, we are not obliged, nor perhaps is it possible, to maintain a fiction of moral neutrality. But the mechanism for achieving the desired outcome does not always have to correspond perfectly to our reasons for thinking it is desirable.
What do we care what other people think? i
In more recent years, another point of contention within bioethics has been the ‘empirical turn’ and (perhaps overstated) disciplinary rivalry between philosophically and sociologically-based approaches to the contested terrain of understanding the effects of biomedical technology on society. This is sometimes conceptualised as a conflict between competing approaches, the normative versus the descriptive. But to see these as mutually exclusive perspectives in the process of doing bioethics is, again, to take too narrow a view of what bioethics itself is and does.
Consider, within the mitochondrial replacement debate discussed above and in reproductive ethics in general, the contested concepts of ‘family’ and ‘parenthood’. An analysis of these may be valuable in addressing many contemporary ethical problems in reproductive medicine, but it must be remembered that these concepts are themselves social constructs; a bioethical analysis of ‘family’ is meaningless without a socially-contextualised account of the concept, and the application and applicability of the results is likewise socially contingent. Sociology is necessary and integral to the bioethical process as a whole, even if isolated elements of our research do not draw directly upon it.
A similar perspective can be applied to the role of public consultations in bioethics. Some scholars scoff: why should we turn to the general public for their ‘inexpert’ opinions regarding matters on which we supposedly have more authority? (Again, the disputed issue of moral expertise arises here). But this mistakes the function of such exercises. The aim of public consultations is (at the risk of drawing a false distinction between ‘bioethicists’ and ‘everyone else’) to tell us, not what ‘we’ should think but, at least to some extent, what ‘they’ think.
One advantage of this might be that we can better target and hone our arguments to serve their intended purpose, but again, to see this as the main or sole aim of public consultations risks caricature or over-simplification. Public engagement in relation to bioethics is not just finding out what ‘the public’ think so we can work out how to convince them of what is right, because we know best; the engagement process is itself important in validating policy decisions. We may perhaps draw an analogy here with the shift from public understanding of science to public engagement—the aim of the exercise is not simply to enlighten the public with our superior scientific or moral knowledge, but to engage the public in dialogue via which science and bioethics, both being social enterprises, can proceed.
This again implies that, in the context of public engagement over ethically contested policy decisions, it is less important to be right or to express a perfectly consistent moral position than it is to arrive, via this process , at a workable solution. This is aptly illustrated by the examples above: policy-oriented bioethics is not identical to philosophical bioethics. It is true that we use apparent moral inconsistencies in policy as a way back into the philosophical debate, to start picking apart moral arguments—a classic example of this is comparing policies on abortion and embryo research and their implications for the moral status of the unborn. But while this analysis goes on, a functional (even if not fully consistent) policy compromise is in place.
Bioethics as negotiation: should we be careful what we say?
A brief diversion: what I have so far said focuses largely on bioethics in the UK context, when in fact the original instruction for this piece was to write about good medical ethics in general. Not wanting to become embroiled in a terminological debate (this is the Journal of Medical Ethics but many of the papers published herein would fall as easily under the heading of ‘bioethics’, were we to attempt to draw a distinction), I simply re-interpreted the theme, but it is perhaps worth taking a step back to comment on one aspect of a potential bio/medical ethics divide. The role of bioethics as public discourse has emerged particularly in the UK, but across the Atlantic, medical ethics has developed as an increasingly professionalised field of expertise, with clinical ethicists appointed to specialist positions within hospitals.
The activities carried out in the capacity of professional clinical ethicist are often very far from the role of the philosophical bioethicist, leading deVries to comment that “[c]linical bioethicists in the US seem to be doing a form of social work or dispute resolution”. 11 Nonetheless, this may also be a valid response to moral conflict, the ethicist's role here being to do with shared or delegated responsibility and accountability for actions between parties and within organisations. This provides us with another potential way of understanding bioethics: clinical ethicists negotiate the personal relationship between private individuals and healthcare organisations; bioethicists negotiate the relationship between the policymakers of the state and the citizens of its publics.
The role of a good negotiator, then, requires attention to diplomacy. Richard Ashcroft describes the role of the bioethicist as that of a ‘public intellectual’; 12 as public intellectuals, we have a responsibility to be aware that our words will be heard by the public and to consider how they may be interpreted. This is another aspect of the ‘multiple-hat problem’ of the bioethicist's differing roles: knowing when and how to exercise discretion over what we say. Again, writing about the Warnock report and whether it represented a bioethical compromise or compromised bioethics, Benjamin suggests that bioethics-as-philosophy should take the ‘no holds barred’ approach “with little or no regard as to immediate acceptability… little or no concern for short-run effects on popular sensibilities” 13 but goes on to acknowledge that bioethicists can play a different role in policy, so long as the distinction is clearly understood and observed.
This may be obvious but bears repeating, given the potential for loss of public trust and consequent impairment to the effective functioning of bioethics should a message drafted while wearing one hat be delivered under another. (Readers of this journal, of course, witnessed a recent unfortunate example of this with the public reception of the idea of ‘after-birth abortion’: 14 15 sound words from philosophers, perhaps rash ones from public intellectuals.) Doing bioethics requires an awareness of the multiple roles that bioethics is called upon to play, and knowing how to balance them is a part of good bioethics.
New horizons: to the next 40 years
Going back, in conclusion, to our hypothetical ‘Bioethical Top 10’, we are perhaps no closer to agreeing on the specific membership of the list, but can say some things about its general content. Good bioethics is likely to have been produced in response to moral disagreement of some sort; lack of moral consensus, rather than presenting an insurmountable obstacle, provides opportunity for discourse and generating new understandings. These understandings may range from theoretical to practical; all are valid spheres for bioethics to operate within, so long as we are aware of what our purpose is in generating them, and conscious of how we present them and to what end. The ‘Oh! factor’ in bioethics comes from something that allows us to approach or understand a problem in a new way; that provides some new insight into the nature of a problem, its intersection with social or regulatory aspects, a concept itself or how that concept is deployed; that moves, however propelled, towards a better world.
In so doing, bioethics achieves one further thing: it opens up space for further reflection and inquiry. It does not have to, nor should we expect it to, provide the definitive solution to all moral problems. If such a Grand Unified Theory of bioethics were possible, the field would be closed rather than open-ended; we would one day know everything there was to know about morality and no more thinking would be possible; and that would be a sorry day. Good bioethics breeds more bioethics. Long may it continue!
Competing interests: None.
Provenance and peer review: Commissioned; internally peer reviewed.
i Without apologies to Richard Feynman.

- Bioethics Articles
- Markkula Center for Applied Ethics
- Focus Areas
- Bioethics Resources
Find information on topics in health care and biotechnology ethics, including end-of-life care, clinical ethics, pandemics, culturally competent care, vulnerable patient populations, organ transplantation, and other topics in bioethics. (For permission to reprint articles, submit requests to [email protected] .)
Articles can also be viewed by the following categories:
For those currently incarcerated, those with a history of incarceration, and their families and communities, incarceration leaves a lasting effect on health and well-being.
China has a long and concerning history of forced organ harvesting which is a clear social justice issue and a severe violation of patient autonomy. Citizens, political leaders, and international committees all play an essential role in ending this practice.
For Muslims living in the United States, determining when a patient has passed on, is not quite as straightforward as ticking a few boxes.
The fitness industry is focused too much on the prioritization of profit, glorification of false realities, and pervasiveness of misinformation, which all have repercussions on individual health and well-being.
The United States significantly outspends other developed countries on prescription medications, and the role of the pharmacy benefit manager is behind much of the extended costs.
Demographic and cultural trends have led to an increased market demand for young and reproductively healthy egg donors, further perpetuating the ethical concerns of current egg donation standards in the United States.
The job of a Child Abuse Pediatricians (CAP) is necessary for recognizing abuse in children, however, their unchecked power has resulted in a vast amount of trauma and pain to families affected by misdiagnosis, violating the physician’s oath to nonmaleficence.
Boarding, the practice of retaining patients in Emergency Department hallway gurneys or chairs instead of rooms, is an unethical practice and a symptom of a broader systemic issue.
Competitive dance is celebrated for fostering a strong work ethic, teamwork, dedication, and self confidence, but not all outcomes are positive. Deep-rooted traditions and a lack of standard codes contribute to ethical concerns that leave young competitive dancers at risk of lasting harmful outcomes.
When considering human embryonic Stem Cells, it is essential to foster robust dialogue, ethical frameworks, and responsible regulation to ensure that the future of hESCs is guided by both scientific progress and ethical reflection. By doing so, we can unlock the full potential of hESCs while upholding the values and principles that define our society.
- More pages:
- What Is Bioethics?
- Johns Hopkins Berman Institute of Bioethics
- Diversity & Inclusion
- Annual Reports
- Meet A Bioethicist
As a world leader in bioethics, the Berman Institute of Bioethics is dedicated to achieving more ethical practices and policies relevant to human health and well-being.
What is bioethics? It’s complicated.
Not even all bioethicists agree on its definition. However, some broadly accepted outlines have emerged as the field has grown in prominence since the 1970s.
New medicines, biomedical procedures, and ways of altering plants and animals are bringing benefits to millions of people. However, these same innovations also have the potential to bring harms or to raise other kinds of ethical questions about their appropriate use.
Bioethics is the multi-disciplinary study of, and response, to these moral and ethical questions.
Bioethical questions often involve overlapping concerns from diverse fields of study including life sciences, biotechnology, public health, medicine, public policy, law, philosophy and theology. They arise in clinical, research, and political arenas, usually in response to advances in biology, health care, and technology, particularly biotechnology.
Although bioethics began as a multi-disciplinary field of study, it is now a full-fledged discipline in its own right. As technology advances ever more quickly, and questions involving its implementation become more complex, bioethics will continue to grow and become increasingly important.
Learn more below about bioethics and the Berman Institute’s leading role in the field.
Study bioethics at the berman institute.
Master of Bioethics (MBE)
Preparing students for bioethics challenges in professional and civic life.
PhD Program
Rigorous training in quantitative and qualitative empirical research.
Postdoctoral fellowships
Intensive immersion in bioethics focused on research and publication.

Meet Our Faculty
Arising almost 30 years ago from an informal bioethics interest group of faculty members dispersed across Johns Hopkins University, the Berman Institute has 47 faculty holding joint appointments at the Hopkins schools of Medicine, Nursing, Public Health, Arts and Sciences, and Advanced International Studies. They are among the most distinguished scholars in the field.
Click here to read more .
- Programs & Events
Search form
- Ethics in Medicine
Bioethics Topics
Please select a topic to view details.
- Department of Health and Human Services
- National Institutes of Health

Chronological Publications
Our interdisciplinary research is published in a range of high impact medical, legal, scientific, philosophical, social science and bioethics journals. The department averages more than 50 publications per year, and has published six books since 2010. Our fellows are expected to produce quality research and often generate multiple first-authored papers during their two year program. Most of our projects are collaborative, and the department encourages faculty and fellows to solicit feedback from colleagues to strengthen our arguments. Notably, we convene bi-weekly Works-In-Progress sessions where developing projects are discussed, critiqued, and refined.
Publications by Author
2023 2022 2021 2020 2019 2018 2017 2016 2015 2014 2013 2012 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 2001 2000 1999 1998 1997 1996 1995 1991 1987.
Bradley E , Wasserman D . The Benefits of Experience Greatly Exceed the Liabilities. Am J Bioeth 2023. 23(1):44-46.
Cheung K, Patch K , Earp BD, Yaden DB. Psychedelics, Meaningfulness, and the "Proper Scope" of Medicine: Continuing the Conversation. Cambridge Quarterly of Healthcare Ethics 2023. Doi: 10.1017/S0963180123000270
Keene AR, Kane NB, Kim SYH , Owen GS. Mental Capacity—Why Look For a Paradigm Shift? Medical Law Review. Doi: 10.1093/medlaw/fwac052.
Kim SYH , Berens N. Risk-Sensitive Decision-Making Capacity: A Comprehensive Defense. Hastings Center Report 2023. 53: TBD, in press.
Kious B, Lewis B, Kim SYH . Epistemic Injustice and the Psychiatrist. Psychological Medicine 2023. 1-5.
Nicholls SG, Taylor HA , James R, Anderson EE, Friesen P, Schonfeld T, Summers EI. A Cross Sectional Survey of Recruitment Practices, Supports, and Perceived Roles for Unaffiliated and Non-scientist Members of IRBs. AJOB Empirical Bioethics 2023. Doi: 10.1080/23294515.2023.2180107
Shen FX, Baum ML, Martinez-Martin N, Miner AS, Abraham M, Brownstein CA, Cortez N, Evans BJ, Germine LT, Glahn DC, Grady C , Holm IA, Hurley EA, Kimble S, Lázaro-Muñoz G, Leary K, Marks M, Monette PJ, Onnela J, O'Rourke P, Rauch SL, Shachar C, Sen S, Vahia I, Vassy JL, Baker JT, Bierer BE, Silverman BC. Returning Individual Research Results from Digital Phenotyping in Psychiatry . Am J Bioeth 2023. Doi: 10.1080/15265161.2023.2180109
Taylor HA . Leveraging the Power of the Centralized IRB Review. Am J Bioeth 2023. 23(6):118-9.
Ulrich C, Grady C . Measuring Moral Distress and its Various Sources. Am J Bioeth 2023. 23(4):63-65.
<< Back to Top >>
Baffoe-Bonnie M . Lived Experience with sickle cell disease: Predictors of altruistic participation in clinical research. Social Science and Medicine 2022. Doi: https://doi.org/10.1016/j.socscimed.2022.115353
Bayefsky MJ, Berkman BE . Access to Expanded Prenatal Genetic Testing: Response to Open Peer Commentaries. American Journal of Bioethics 2022; 22(5):W1-W3
Berens N, Kim SYH . Rapid-Response Treatments for Depression and Requests for Physician-Assisted Death: An Ethical Analysis. Am J Geriatr Psychiatry 2022. doi:10.1016/j.jagp.2022.07.003.
Berens N , Kim SYH . Should Assessments of Decision-Making Capacity Be Risk-Sensitive? A Systematic Review. Frontiers in Psychology 2022. Doi: https://doi.org/10.3389/fpsyg.2022.897144
Berens N , Wasserman D , Wakim P, Bernhard T , Kim SYH . Resource Limitation and 'Forced Irremediability' in Physician-Assisted Death for Nonterminal Mental and Physical Conditions: A Survey of the US Public. Journal of the Academy of Consultation-Liaison Psychiatry 2022. Doi: 10.1016/j.jaclp.2021.12.010
Berens N , Wasserman D . Restricting Access, Stigmatizing Disability? The American Journal of Bioethics 2022. 22(2): 25-27.
Berkman BE , Miner SA , Wendler D , Grady C . The Ethics of Encouraging Employees to Get the COVID-19 Vaccination. Journal of Public Health Policy 2022. 43(2):311-319. Doi: https://doi.org/10.1057/s41271-022-00347-9
Blease C, Cohen IG, Hoffman S . Sharing Clinical Notes: Potential Medical-Legal Benefits and Risks. Journal of the American Medical Association 2022. 327(8):717-718.
Das J, Forlini C, Porcello DM, Rommelfanger KS, Salles A, Atker N, Ankeny R, Araki T, Arie F, Asakawa T, Bennett AJ, Bitsch L, Carter O, Fukuda M, Fukushi T, Hendriks S , Herrera-Ferra K, Ienca M, Illes J, Jeong SJ, Syd L. Neuroscience is ready for neuroethics engagement. Frontiers in Communication 2022. Doi: 10.3389/fcomm.2022.909964
Entwistle JW, Drake DH, Fenton KN , Smith MA, Sade RM. Normothermic Regional Perfusion: Ethical Issues in Thoracic Organ Donation. Cardiothoracic Ethics Forum 2022. 114(1):44-51.
Entwistle JW, Drake DH, Fenton KN , Smith MA, Sade RM. Normothermic regional perfusion: Ethical issues in thoracic organ donation. Journal of Thoracic and Cardiovascular Surgery 2022. Doi: https://doi.org/10.1016/j.jtcvs.2022.01.018
Erzse A, Watson D, Hardy-Johnson P, Kehoe SH, Tugendhaft A, Ward K, Debpuur C, Oduro A, Ofosu W, Danis M , Barker M. Engaging community members in setting priorities for nutrition interventions in rural northern Ghana. PLOS Glob Public Health 2022. 2(9):e0000447.
Euwoso C, Berkman BE , Wonkam A., De Vries J. Should Institutions Fund Feedback of Individual Findings in Genomic Research. Journal of Medical Ethics 2022. Doi: 10.1136/medethics-2021-107992
Federico C, Heagerty PJ, Lantos J, O'Rourke P, Rahimzadeh V, Sugarman J, Weinfurt K, Wendler D , Wilfond BS, Magnus D. Ethical and epistemic issues in the design and conduct of pragmatic stepped-wedge cluster randomized clinical trials. Contemporary Clinical Trials 2022; 115:106703.
Fenton KN . Xenotransplantation: A microcosm of bioethics. Artificial Organs 2022. doi: 10.1111/aor.14260. PMID: 35441722.
Fernandez Lynch H , Taylor HA. How Do Accredited Organizations Evaluate the Quality and Effectiveness of their Human Research Protection Programs: Results from AAHRPP Interviews. AJOB Empirical Bioethics 2022. Doi: https://doi.org/10.1080/23294515.2022.2090641
Grady C . The evolution of research participant as partner: the seminal contributions of Bob Veatch. Theoretical Medicine and Bioethics 2022. DOI 10.1007/s11017-022-09579-y
Green JM, Taylor HA . Editorial: Improving sepsis care: is it research? Promoting clarity in a zone of confusion. Critical Care Medicine 2022; 50(3):516-519.
Hendriks S , Ramos KM, Grady C . Survey of Investigators About Sharing Human Research Data in the Neurosciences. Neurology 2022. Doi: 10.1212/WNL.0000000000200886
Hendriks S , Grady C , Wasserman D , Wendler D , Bianchi DW, Berkman BE . A New Ethical Framework for Assessing the Unique Challenges of Fetal Therapy Trials: Response to Commentaries. American Journal of Bioethics 2022. 22(3):W1-W3. Doi: https://doi.org/10.1080/15265161.2022.2044563
Hendriks S , Grady C , Wasserman D , Wendler D , Bianchi DW, Berkman BE . A New Ethical Framework to Determine Acceptable Risks in Fetal Therapy Trials. Prenatal Diagnosis 2022. Doi: https://doi.org/10.1002/pd.6163
Howard D, Rivlin A, Candilis PJ, Dickert N, Drolen C, Krohmal B, Pavlick M, Wendler D . Surrogate perspectives on patient preference predictors. AJOB Empirical Bioethics 2022. 13:125-135.
Jardas E, Wasserman D , Wendler D . Autonomy-based criticisms of the patient preference predictor. Journal of Medical Ethics 2022. 48:304-310.
Jardas E, Wesley R, Pavlick M, Wendler D , Rid A . Patients' priorities for surrogate decision-making: possible influence of misinformed beliefs. AJOB Empirical Bioethics 2022. 13:137-151.
Kane NB, Ruck Keene A, Owen GS, Kim SYH . Difficult capacity cases – the experience of liaison psychiatrists. An interview study across three jurisdictions. Frontiers in Psychiatry 2022. doi:10.3389/fpsyt.2022.946234.
Kapiriri L, Kiwanuka S, Biemba G, Velez C, Razavi SD, Abelson J, Essue BM, Danis M , Goold S, Noorulhuda M , Nouvet E, Sandman L, Williams I. Priority setting and equity in COVID-19 pandemic plans: a comparative analysis of 18 African countries. Health Policy Plan 2022. 37(3):297-309.
Kim S , Kimmelman J. Practical Steps to Identifying the Research Risk of Pragmatic Trials. Clinical Trials 2022. 00(0): 1-6. Doi: 10.1177/17407745211063476
Matera-Vatnick M, Todman KW, Wakim PG, Sullivan HK, Squires C, Brintnall-Karabelas J, Doernberg S, Danis M . Evaluating the Ability to Consent to Research: A Twenty-Year Track Record. Ethics and Human Research 2022. 44(2):2-17.
McGrew S and Berkman BE . When to Disclose a Borderline Incidental Finding. American Journal of Bioethics 2022. Doi: https://doi.org/10.1080/15265161.2022.2110975
McGrew S , Raskoff S , Berkman BE . When Not to Ask: A Defense of Choice-Masking Nudges in Medical Research. J. Health Care Law and Policy 2022. 25(1): 1-48.
McGrew S , Taylor HA . Adolescents, Parents, and Covid-19 Vaccination―Who Should Decide? New England Journal of Medicine 2022; 386(2):e2.
Miner SA, Berkman BE , Altiery de Jesus V, Jamal L , Grady C . Navigating Pandemic Moral Distress at Home and at Work: Frontline Workers' Experiences . AJOB Empir Bioeth 2022; 13(4):215-225. doi: 10.1080/23294515.2022.2064000
Miner SA, Similuk M, Jamal L , Sapp J, Berkman BE . Genomic tools for health: Secondary findings as findings to be shared. Genetics in Medicine 2022. DOI: https://doi.org/10.1016/j.gim.2022.07.015
Moore CM . Objection or Obstacle: Applying Amartya Sen's Capability Approach to the Conscientious Refusal of Emergency Contraception. International Journal of Feminist Approaches to Bioethics 2022. 15(2):40-50.
Moore CM , Taylor HA . More than Semantics: Abortion Access and Equity. The American Journal of Bioethics 2022. 22(8):68-69. DOI: 10.1080/15265161.2022.2089284
Nicolini ME, Jardas E, Gastmans C, Zarate C, Kim SYH . Irremediability in Psychiatric Euthanasia: Examining the Objective Standard. Psychological Medicine 2022. doi: 10.1017/S0033291722002951
Raskoff S, Thurm A, de Oliveira Miguel H, Kim SYH , Quezado Z. Pain Research and Children with Severe Intellectual Disability: Ethical Challenges and Imperatives. The Lancet Child & Adolescent Health 2022. Doi: 10.1016/S2352-4642(22)00346-7
Razavi SD, Noorulhuda M , Velez C, Kapiriri L, Dreyse BA, Danis M , Essue B, Goold SD, Nouvet E, Williams I. Priority setting for pandemic preparedness and response: A comparative analysis of COVID-19 pandemic plans in 12 countries in the Eastern Mediterranean Region. Health Policy Open 2022. 3:100084.
Robertson C, Hoffman S . Professional Speech at Scale. U.C. Davis Law Review 2022;55(4):2063-2132.
Roesner N , Jamal L , Wasserman D , Berkman BE . Reason-Based Abortion Bans, Disability Rights, and the Future of Prenatal Genetic Testing. American Journal of Law and Medicine 2022. 48(2-3):187-199.
Sade RM, Entwistle JW, Drake DH, Fenton KN , Smith MA. Reply from authors: Tying off brain vessels: Can that be ok? J Thorac Cardiovasc Sur g 2022. 164(2):e93-e94. doi: 10.1016/j.jtcvs.2022.04.041.
Schupmann W, Li X, Wendler D . Acceptable risks in pediatric research: views of the US public. Pediatrics 2022. 149(1):e2021052687.
Spector-Bagdady K, Fernandez Lynch H, Bierer BE, Gelinas L, Hull SC , Magnus D, Meyer MN, Sharp RR, Sugarman J, Wilfond BS, Yearby R, Mohapatra S. Allocation of Opportunities to Participate in Clinical Trials during the Covid-19 Pandemic and Other Public Health Emergencies, Hastings Center Report 2022. 52: 51-58.
Steel R , Wendler D . Distinguishing appropriate from inappropriate conditions on research participation. Bioethics 2022. Doi: 10.1111/bioe.13092
Strassle CL, Schwann B, Berkman BE . Autonomy Concerns with Using Contracts to Enhance Patient Adherence. Bioethics (2022). Doi: 10.1111/bioe.13030
Taylor HA , Dowdy D, Searle A, Stennett A, Dukhanin V, Zwerling AA, Merritt MW. Disadvantage and the Experience of Treatment for Multidrug-Resistant Tuberculosis (MDR-TB). Social Science and Medicine―Qualitative Health Research 2022. 2:100042.
Taylor HA , Hale JF, Centola M, Blodgett A. COVID-19 Test Us: A Case for Embedding Regulatory and Ethics Expertise. IEEE Open Journal of Engineering in Medicine and Biology 2022. 3:167-170.
Taylor HA , Porter KM, Sullivan C , McCormick JB. Current Landscape of Research Ethics Consultation Services: National Survey Results. Journal of Clinical and Translational Research 2022. 6(1):e148.
Ulrich CM, Deatrick JA, Wool J, Huang L, Berlinger N, Grady C . Ethical Challenges Experienced by Clinical Ethicists during COVID-19. AJOB Empirical Bioethics 2022. DOI: 10.1080/23294515.2022.2110965
Ulrich CM, Ratcliffe SJ, Zhou Q, Huang L, Hochheimer C, Gordon T, Naylor KD, Schapira MM, Richmond TS, Grady C , Mao JJ. Association of Perceived Benefit or Burden of Research Participation With Participants' Withdrawal From Cancer Clinical Trials . JAMA Netw Open 2022. 5(11):e2244412. doi:10.1001/jamanetworkopen.2022.44412
Undurraga J, Negussie H, Wendler D . Consent, decisional capacity and guardianship in mental health research. Wellcome Open Research 2022; 7:183. doi: 10.12688/wellcomeopenres.18003.1.
Wasserman D . What Are the Wider Implications of Sparrow's Benefit Argument? The American Journal of Bioethics 2022. 22(9):28-30.
Wendler D . Deceiving research participants: Is it always inconsistent with valid consent? Journal of Medicine and Philosophy 2022. 47:558–571.
Wendler D . Promoting the values for surrogate decision-making. JAMA 2022; 328:243–244.
Wendler D , Kim SYH . Implementing supported decision making in clinical research. International Journal of Geriatric Psychiatry 2022. 38(1): e5860. Doi: 10.1002/gps.5860
Wendler D . Suffering in animal research: the possibility of compensation and the need for limits. Kennedy Institute of Ethics Journal 2022. 32:297–311.
Wendler D , Schupmann W, Li X. Views of IRB members regarding phase 1 pediatric oncology trials. Pediatric Hematology and Oncology 2022. DOI: 10.1080/08880018.2022.2069894.
Wendler D , Sullivan C . Setting Risk Limits and Ensuring Fairness in Learning Health Care. Hastings Center Report 2022. 52(3): 34-36. DOI: 10.1002/hast.1395
Price K, Zionts J . How the God Committee gets Organ Allocation and Xenotransplantation Wrong and Why it Matters. Justice Everywhere 2022. http://justice-everywhere.org/health/how-the-god-committee-gets-organ-allocation-and-xenotransplantation-wrong-and-why-it-matters/
Aas S, Peterson A, Wasserman D . What Justifies the Allocation of Scarce Health Care Resources to Patients with Disorders of Consciousness: Response to Commentaries. AJOB Neuroscience 2021. 12(4): W1-W4.
Aas S and Wasserman D . Bodily Rights in Personal Ventilators? Journal of Applied Philosophy 2021. Doi: 10.1111/japp.12537.
Aguilera B . Trends in clinical trials performed in Chile. Rev Med Chile 2021. 149: 110-118.
Aguilera B , Perez-Gomez J, and DeGrazia D . Should Biomedical Research with Great Apes be Restricted? A Systematic Review of Reasons. BMC Medical Ethics 2021. 22(15): https://doi.org/10.1186/s12910-021-00580-z .
Anjum S, Dean O, Kosa P, Magone MT, King KA, Fitzgibbon E, Kim HJ, Zalewski C, Murphy E, Billioux BJ, Chisholm J, Brewer CC, Krieger C, Elsegeiny W, Scott TL, Wang J, Hunsberger S, Bennett JE, Nath A, Marr KA, Bielekova B , Wendler D , Hammoud DA, Williamson P. Outcomes in previously healthy cryptococcal meningoencephalitis patients treated with pulse - taper corticosteroids for post-infectious inflammatory syndrome. Clinical Infectious Diseases 2021; 73:e2789-e2798.
Bayefsky MJ, Berkman BE . Implementing Expanded Prenatal Genetic Testing: Should Parents Have Access to Any and All Fetal Genetic Information? The American Journal of Bioethics 2021. Doi: 10.1080/15265161.2020.1867933.
Berkman BE , Mastroianni A, Jamal L , Solis C , Taylor H , Hull SC . Repurposing Research Samples in a Pandemic. Ethics and Human Research 2021. 43:1-17.
Berkman BE , Schupmann W . Response to Clayton et al. Genetics in Medicine 2021.
Campbell SM., Stramondo JA, Wasserman, D. How to (Consistently) Reject the Options Argument. Utilitas 2021 . 33(2): 237-245.
Danis M , Fox E, Tarzian AJ, Duke CC. Health care ethics programs in U.S. Hospitals: Results from a national survey. BMC Medical Ethics 2021; 107. Doi: https://doi.org/10.1186/s12910-021-00673-9 .
Danis M . Putting anti-racism into practice as a healthcare ethics consultant. American Journal of Bioethics 2021. 21:36-38. doi: 10.1080/15265161.2020.1861387.
DeGrazia D . Animals and Ethics. Routledge Encyclopedia of Philosophy 2021. DOI: 10.4324/9780415249126-L004-2.
DeGrazia D , Miller FG. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection Challenge Experiments in Nonhuman Primates: An Ethical Perspective. Clinical Infectious Diseases 2021. Doi: 10.1093/cid/ciab278.
Doussau A, Agarwal I, Fojo T, Tannock IF, Grady C. Design of placebo-controlled randomized trials of anticancer agents: Ethical considerations based on a review of published trials. Clin Trials 2021. Online ahead of print. doi: 10.1177/17407745211052474.
Eckstein L, Rid A , Kamuya D, and Shah SK. The essential role of DSMBs in ensuring the ethics of global vaccine trials to address COVID-19. Clinical Infectious Diseases 2021. DOI: 10.1093/cid/ciab239 .
Fabi R, Saloner B, Taylor H . State Policymaking and Stated Reasons: Prenatal Care for Undocumented Immigrants in an Era of Abortion Restriction. The Milbank Quarterly 2021. doi: 10.1111/1468-0009.12519 .
Fenton KN . Ethics of resource allocation to congenital heart surgery in variable-resource contexts. AME Surg J 2021. Doi: https://dx.doi.org/10.21037/asj-21-51.
Fox E, Danis M , Tarzian AJ, Duke CC. Ethics Consultation in U.S. Hospitals: A National Follow-Up Study. American Journal of Bioethics 2021. doi: 10.1080/15265161.2021.1893547 .
Fox E, Danis M , Tarzian AJ, Duke CC. Ethics Consultation in U.S. Hospitals: New Findings about Consultation Practices. AJOB Empirical Bioethics 2021. Doi: https://doi.org/10.1080/23294515.2021.1996117 .
Fox E, Tarzian AJ, Danis M , Duke CC. Ethics consultation in US hospitals: Assessment of training needs. The Journal of Clinical Ethics 2021; 32(3):1-9.
Fox E, Tarzian AJ, Danis M , Duke CC. Ethics Consultation in U.S. Hospitals: Opinions of Ethics Practitioners. American Journal of Bioethics 2021. Doi: 10.1080/15265161.2021.1893550 .
Grady C . Another Cautionary Lesson from COVID Research. The American Journal of Bioethics 2021. 21:12, 36-39, DOI: 10.1080/15265161.2021.1991048.
Grubbs JC, Millum J , Rietmeijer CA, Kilmarx PH. Return of Positive Test Results to Participants in Sexually Transmitted Infection Prevalence Studies. Sexually Transmitted Diseases . 2021.
Hendriks S, Grady C, Wasserman D, Wendler D , Bianchi DW, Berkman B . A New Ethical Framework for Assessing the Unique Challenges of Fetal Therapy Trials. AJOB 2021.
Hendriks S , Pearson SD. Assessing potential cures: are there distinctive elements of value beyond health gain? J Comp Eff Res 2021. doi: 10.2217/cer-2020-0190. Epub ahead of print. PMID: 33663230.
Hsu NS, Hendriks S , Ramos KM, Grady C . Ethical considerations of COVID-19-related adjustments to clinical research. Nat Med 2021. https://doi.org/10.1038/s41591-020-01216-2 .
Hull SC , Nez (Diné) FL, Blome JM. Solidarity as an Aspirational Basis for Partnership with Tribal Communities. The American Journal of Bioethics 2021. 21:10, 14-17. doi: 10.1080/15265161.2021.1965258.
Iyer AA, Millum J, Grady C, Wendler D . Avoiding exploitation in multinational covid-19 vaccine trials. BMJ 2021. 372(n541): doi:10.1136/bmj.n541.
Iyer AA , Saade D, Bharucha-Goebel DX, Foley AR, Averion G, Paredes E, Gray S, Bonnemann CG, Grady C , Hendriks S , Rid A . Ethical challenges Ethical Challenges for a New Wave of Early-Phase Pediatric Gene Therapy Trials. Genetics in Medicine 2021.
Jardas EJ , Wasserman D , Wendler D . "Autonomy-based criticisms of the patient preference predictor." Journal of Medical Ethics (2021).
Jardas E , Wesley R, Pavlick M, Wendler D, Rid A : Patients’ priorities for surrogate decision-making: possible influence of misinformed beliefs. AJOB Empirical Bioethics 2021. Oct 1:1-15.
Kane NB, Keene AR, Owen GS, Kim SY . Applying decision-making capacity criteria in practice: A content analysis of court judgments . Plos one 2021. 16(2):e0246521.
Kapiri L, Kiwanuka S, Biemba G, Velez C, Razavi SD, Abelson J, Essue B, Danis M , Goold S, Noorulhuda M , Nouvet E, Sandman L, Williams I. Priority Setting and Equity in COVID-19 Pandemic Plans: A Comparative Analysis of eighteen African Countries. Health Policy Plan 2021. doi: 10.1093/heapol/czab113
Kim SYH , Kane N, Ruck Keene A, Owen G. Broad Concepts and Messy Realities: Optimizing the Application of Mental Capacity Criteria. Journal of Medical Ethics 2021. DOI: 10.1136/medethics-2021-107571
Kim SYH . Ways of Debating Assisted Suicide and Euthanasia: Implications for Psychiatry. Perspectives in biology and medicine 2021. 64(1):29-43.
Kuylen MN, Kim SY , Keene AR, Owen GS. Should age matter in COVID-19 triage? A deliberative study. Journal of Medical Ethics 2021.
Lyerly AD, Beigi R, Bekker LG, Chi BH, Cohn SE, Diallo DD, Eron J, Faden R, Jaffe E, Kashuba A, Kasule M, Krubiner C, Little M, Mfustso-Bengo J, Mofenson L, Mwapasa V, Mworeko L, Myer L, Penazzato M, Rid A , Shapiro R, Singh JA, Sullivan K, Vicari M, Wambui J, White A, Wickremshire M, Wolf L. Ending the evidence gap for pregnancy, HIV and co-infections: ethics guidance from the PHASES project. Journal of the International AIDS Society 2021. Doi: https://doi.org/10.1002/jia2.25846 .
Meyer M, Gelinas L, Bierer BE, Hull SC , et al. An ethics framework for consolidating and prioritizing COVID-19 clinical trials. Clinical Trials 2021.e-pub ahead of print,pp. 1-8.
Millum J, Bromwich D. Informed Consent: What Must be Disclosed and What Must be Understood? American Journal of Bioethics 2021. 21 (5):46-58.
Miner S . Review of Freezing Fertility Oocyte Cryopreservation and the Gender Politics of Aging. Social Forces 2021. doi: 10.1093/sf/soab061.
Mintz K, Jardas E , Shah S, Grady C, Danis M, Wendler D . Enrolling minors in COVID-19 vaccine trials. Pediatrics 2021; 147(3):e2020040717.
Nicolini M , Gastmans C, Kim S . Psychiatric euthanasia, suicide and the role of gender. The British Journal of Psychiatry 2021. doi:10.1192/bjp.2021.95.
Peterson A, Aas S, Wasserman D . What justifies the allocation of health care resources to patients with disorders of consciousness? AJOB Neuroscience 2021. 12(2-3): 127-139.
Pierson L Millum J. Health Research Priority Setting: Do Grant Review Processes Reflect Ethical Principles? Global Public Health . 2021. Online first.
Pike KJ, Fazio S, Bynum JPW, Travison TG, Wendler D , Mor V. Resources, methods, and data infrastructure to promote research in dementia care, caregiving, and services. Journal of the American Geriatrics Society 2021. doi: 10.1111/jgs.17339.
Rid A , Lipsitch M, Miller FG. The Ethics of Continuing Placebo in SARS-CoV-2 Vaccine Trials. Journal of the American Medical Association 2021. 325(3): 219-220.
Rid A , Shah SK, Miller FG, Danis M , Nicolini M , Ochoa J , Taylor HA , Wendler D , Grady C . So much at stake: ethical trade-offs in accelerating SARS-CoV-2 vaccine development—Response to Gurwitz. Vaccine 2021. 39(7), 1028-1029.
Scholten M, Weller PJ, Kim SY , Vollmann J. Human Rights and Mental Health: Current Developments in Competence Assessment and Supported Decision-Making. Frontiers in Psychiatry 2021. 12:517.
Schupmann W, Miner SA , Sullivan HK , Glover JR, Hall JE, Schurman SH, and Berkman BE . Exploring the Motivations of Research Participants Who Choose Not to Learn Medically Actionable Secondary Genetic Findings about Themselves. Genetics in Medicine 2021.
Schupmann W, Li X, Wendler D . Do the potential medical benefits of phase 1 pediatric oncology trials justify the risks? views of the US public. Journal of Pediatrics 2021. 238:249-258.
Spector-Bagdady K, Fernandez Lynch H, Bierer BE, Gelinas L, Hull SC , Magnus D, Meyer MN, Sharp RR, Sugarman J, Wilfond BS, Yearby R, Mohapatra S. Allocation of Opportunities to Participate in Clinical Trials during the Covid-19 Pandemic and Other Public Health Emergencies. Hastings Center Report 2021. 52:1-8. Doi: 10.1002/hast.1297
Taylor HA, Porter KM , Paquette ET, McCormick J, Tumility E, Arnold JF, Spector-Bagdaddy K, Danis M, Brandt D, Shah J, Wilfond BS, Lee LM. Creating a Research Ethics Consultation Service: Issues to Consider. Ethics and Human Research 2021. 43(5): 18-25.
Taylor HA , Serpico K, Fernandez Lynch H, Baumann J, Anderson EE. A Snapshot of U.S. IRB Review of COVID-19 Research in the Early Pandemic. Journal of Clinical and Translational Science 2021. doi: 10.1017/cts.2021.848.
Taylor HA , Washington D, Wang N, Patel H, Ford D, Kass NE, Ali J. Randomized Comparison of Two Interventions to Enhance Understanding during the Informed Consent Process for Research. Clinical Trials 2021. 18(4): 466-476.
Tarzian AJ, Fox E, Danis M , Duke CC. Ethics Consultation in U.S. Hospitals: Adherence to national practice standards. AJOB Empirical Bioethics 2021. Doi: https://doi.org/10.1080/23294515.2021.1996118 .
Tugendhaft A, Hofman K, Danis M , Kahn K, Erzse A, Twine R, Gold M, and Christofides N. Deliberative engagement methods on health care priority-setting in a rural South African community. Health Policy and Planning 2021. 36:1279-1291.
Ulrich CM, Grady C , Demiris G, Richmond T. The Competing Demands of Patient Privacy and Clinical Research. Ethics & Human Research 2021. 43(1): 25-31. DOI: 10.1002/eahr.500076.
Ulrich C, Knafl K, Foxwell A, Zhou Q, Paidipati C, Tiller D, Ratcliffe S, Wallen G, Richmond T, Naylor M, Gordon T, Grady C , Miller V. Experiences of Patients After Withdrawal From Cancer Clinical Trials. JAMA Netw Open 2021. 4(8):e2120052. doi: 10.1001/jamanetworkopen.2021.20052.
Vickers AJ, Vertosick EA, Carlsson SV, Ehdaie B, Kim SY . Patient accrual and understanding of informed consent in a two-stage consent design. Clinical Trials 2021:1740774520988500.
Weiss EM, Guttmann KF, Olszewski AE, Magnus BE, Li S, Kim SYH et al. Parental Enrollment Decision-Making for a Neonatal Clinical Trial. Journal of Pediatrics 2021. DOI: 10.1016/j.jpeds.2021.08.014.
Wasserman D . How to Compromise on Saving the Most Lives: A Commentary on Hellman and Nicholson,“Rationing and Disability”. Washington and Lee Law Review Online 2021. 78(1): 183-191.
Wendler D . A call for a patient preference predictor. Critical Care Medicine 2021: 49:877-880.
Wendler D . The ethics of mandatory retention of clinical biospecimens for research. Journal of General Internal Medicine 2021; 36:2818-2819.
Wendler D . The Inevitability and Ethics of Inaccurate Screening in Clinical Trials: A Call for Research and Guidance. Ethics and Human Research 2021. 43(4):37-44. doi: 10.1002/eahr.500098.
Wendler D , Anjum S, Williamson P. Innovative treatment as a precursor to clinical research. J Clin Invest 2021. 131(15):e152573. doi: 10.1172/JCI152573.
White A, Grady C , Little M, Sullivan K, Clark K, Ngwu M, Lyerly AD. IRB Decision-Making about Minimal Risk Research with Pregnant Participants. Ethics and Human Research 2021. 43(5):2-17. doi: 10.1002/eahr.500100.
Williams I, Essue B, Nouvet E, Sandman L, Ravazi SD, Noorulhuda M , Goold S, Danis M , Biemba G, Abelson J, Kapiri L. Priority setting during the COVID-19 pandemic: going beyond vaccines. BMJ Global Health 2021. 6(1). doi: 10.1136/bmjgh-2020-004686.
Zionts J , Millum J . How not to count the health benefits of family planning. Journal of Medical Ethics 2021. 1:1-4. Doi: 10.1136/medethics-2021-107668
Aguilera B . Commentary: Ethical allocation of scarce health care resources in the context of the COVID-19 crisis. Medwave 2020;20(5):e7935. doi: 10.5867/medwave.2020.05.7935.
Aguilera B . Nonconscious Pain, Suffering, and Moral Status. Neuroethics 2020. https://doi.org/10.1007/s12152-020-09430-y .
Aguilera B, DeGrazia D, Rid A . Regulating international clinical research: an ethical framework for policy-makers. BMJ Global Health 2020; 5:e002287. doi:10.1136/ bmjgh-2020-002287.
Aguilera B, Wendler D . Should the Belmont report be extended to animal research. Cambridge Quarterly of Healthcare Ethics 2020; 29:58-66.
Asare M, Heckler CE, Peppone LJ, Kamen C, Minasian L, Wendler D , Feige M, Weil C, Long J, Cole S, Onitilo A, Culakova E, Morrow G, Janelsins M. Racial/ethnic differences in comprehension of biospecimen collection. Journal of Cancer Education 2020; 35:292-300.
Belt R, Malavé C, Strassle C . Blog post: Disability and Health in the Age of Triage. Harvard Law Review Blog 2020, https://blog.harvardlawreview.org/disability-and-health-in-the-age-of-triage/ .
Berkman B. Commentary on ‘The right not to know and the obligation not to know’. Journal of Medical Ethics 2020.
Berkman BE , Brody LC, Collins FS and Green ED. Karen Rothenberg’s (Not So) Secret Roles and Contributions at the U.S. National Institutes of Health. Journal of Health Care Law and Policy 2020; 22:167-171.
Cho HL , Grady C , Tarzian A, Povar G, Mangal J, Danis M . Patient and Family Descriptions of Ethical Concerns. Am J Bioeth . 2020; 20(6): 52-64. doi: 10.1080/15265161.2020.1754500.PMID: 32441594.
Cho HL, Huang CJ . Why Mental Health–Related Stigma Matters for Physician Wellbeing, Burnout, and Patient Care. J Gen Intern Med 2020. https://doi.org/10.1007/s11606-019-05173-6 .
Cho HL, Kim SYH , Fitzhugh C, Hsieh M, Tisdale J, Grady C . Motivations and Decision-Making of Adult Sickle Cell Patients in High-Risk Clinical Research. Biol Blood Marrow Transplant . 2020; 26(6): 1225-1232. doi: 10.1016/j.bbmt.2020.03.014. Epub 2020 Mar 19.PMID: 32200120.
Danis M , Povar G, Cho HL, Grady C , Tarzian A, Mangal J. Broadening the Scope of Health Care Ethics Consultation: A Response to Open Peer Commentaries on Patient and Family Description of Ethical Concerns Am J Bioeth . 2020; 20(6): W6-W8.
DeGrazia D. Commentary: On the Possibility of Invertebrate Sentience. Animal Sentience 2020; 29 (15): 1-3.
DeGrazia D . Commentary: Value Theory, Beneficence, and Medical Decision-Making. AJOB 2020; 20(3): 71-73.
Desine S, Hollister BM, Abdallah KE, Persaud A, Hull SC , Bonham VL. The Meaning of Informed Consent: Genome Editing Clinical Trials for Sickle Cell Disease, American Journal of Bioethics: Empirical Bioethics 2020; 11(4):195-207.
Dowdy D, Zwerling AA, Stennett A, Searle A, Dukhanin V, Taylor HA , Merritt MW. Measuring Stigma to Assess the Social Justice Implications of Health-Related Policy Decisions: Application to Novel Treatment Regimens for Multidrug-Resistant Tuberculosis. MDM Policy & Practice 2020; 5(1): 1-12.
Drake D, Morrow CD, Kinlaw K, De Bonis M, Zangrillo A, Sade RM. Cardiothoracic Ethics Forum (incl. Fenton KN ). Cardiothoracic surgeons in pandemics: Ethical Considerations . J Thorac Cardiovasc Surg 2020 Aug;160(2):456-459. Epub 2020 Apr 9.
Drake D, Morrow CD, Kinlaw K, De Bonis M, Zangrillo A, Sade RM. Cardiothoracic Ethics Forum (incl. Fenton KN ). Cardiothoracic surgeons in pandemics: Ethical Considerations. Ann Thorac Surg 2020 Aug;110(2):354-358. Epub 2020 Apr 9.
Entwistle JWC, Fenton KN . Reply: There are limits to autonomy. J Thorac Cardiovasc Surg . 2020 Jul;160(1):e6-e7. Epub 2020 Apr 3.
Entwistle JWC, Fenton KN . Rethinking the ethics of ventricular assist device withdrawal. J Thorac Cardiovasc Surg 2020 Apr;159(4):1328-32. Epub 2019 Dec 4.
Fenton KN , Novick WM, Entwistle JW 3rd, Moffatt-Bruce SD, Sade RM; Cardiothoracic Ethics Forum. Global Health Initiatives in Cardiothoracic Surgery: Ethical Considerations and Guidelines. Ann Thorac Surg 2020 Oct 14: Online ahead of print.
Fenton KN , Novick WM, Entwistle JW 3rd, Moffatt-Bruce SD, Sade RM; Cardiothoracic Ethics Forum. Global Health Initiatives in Cardiothoracic Surgery: Ethical Considerations and Guidelines. J Thorac Cardiovasc Surg 2020 Oct 10: Online ahead of print.
Grady C , Shah S, Miller F, Danis M, Nicolini M, Ochoa J, Taylor H, Wendler D, Rid A . So much at stake: Ethical trade-offs in accelerating SARS-CoV-2 vaccine development. Vaccine . 2020; 38 (6381–6387).
Hiratsuka VY, Hahn MJ, Woodbury RB, Hull SC , Wilson DR, Bonham VL, Dillard DA, and The Alaska Native Genomics Research Workshop Group, et al. Alaska Native genomic research: perspectives from Alaska Native leaders, federal staff, and biomedical researchers. Genet Med 2020; https://doi.org/10.1038/s41436-020-0926-y .
Iyer AA, Barzilay JR, Tabor HK (2020) Patient and Family Social Media Use Surrounding a Novel Treatment for a Rare Genetic Disease: A Qualitative Interview Study. Genetics in Medicine . Epub before print.
Iyer A , Hendriks S , Rid A . Commentary: The Challenge of Selecting Participants Fairly in High-Demand Clinical Trials. AJOB 2020; 20(2): 35-38.
Iyer AA, Hendriks S, Rid A . Advantages of Using Lotteries to Select Participants for High-Demand Covid-19 Treatment Trials. Ethics & Human Research 2020; 42(4): 35-40.
Jamal L, Schupmann W, Berkman BE . An Ethical Framework for Genetic Counseling in the Genomic Era. Journal of Genetic Counseling 2020; 29:718-727 .
Kaewkungwal J, Adams P, Sattabongkot J, Lie RK, Wendler D . Issues and challenges associated with data-sharing in LMICs: perspectives of researchers in Thailand. The American Journal of Tropical Medicine and Hygiene 2020; 103:528-536.
Kim SYH . Comparative Effectiveness Research, Learning Health Systems, and Pragmatic RCTs: The Need for Both Clinical and Research Ethics. Oxford Handbook of Research Ethics 2020. 10.1093/oxfordhb/9780190947750.013.19
Kim SYH, Grady C . Ethics in the time of COVID: What remains the same and what is different. Neurology 2020. https://doi.org/10.1212/WNL.0000000000009520 .
Kim SYH, Mangino D, Nicolini ME . Is this person with dementia (currently) competent to request euthanasia? A complicated and under-explored question. Journal of Medical Ethics 2020. 10.1136/medethics-2020-106091.
Kim SYH , Nicolini ME, Mangino DR , De Vries RG. What we can learn from published reports of euthanasia in persons with dementia: A reply to Marijnisssen et al. American Journal of Geriatric Psychiatry (Epub 2020 July 11) https://doi.org/10.1016/j.jagp.2020.07.005
Kim SYH , Ruck Keene A. A new kind of paternalism in surrogate decision-making? The case of Barnsley Hospitals NHS Foundation Trust v MSP . Journal of Medical Ethics 2020. 10.1136/medethics-2020-106797.
Lengvenyte A, Strumila R, Courtet P, Kim SYH , Olié E. “Nothing hurts less than being dead”. Psychological pain in case descriptions of psychiatric euthanasia and assisted suicide from the Netherlands. Canadian J Psychiatry 2020. (e-pub ahead of print) https://doi.org/10.1177/0706743720931237.
Mangino D, Bernhard T , Wakim P, Kim SYH . Assessing public’s attitudes towards euthanasia and assisted suicide of persons with dementia based on their advance request: an experimental survey of US public. The American Journal of Geriatric Psychiatry 2020. doi: https://doi.org/10.1016/j.jagp.2020.07.013 .
Miner SA , Miller WK, Grady C, Berkman BE . "“It’s Just Another Added Benefit”: Women’s Experiences with Employment-Based Egg Freezing Programs." AJOB Empirical Bioethics 2020; 1-12. doi: 10.1080/23294515.2020.1823908.
Mintz K, Wasserman D. Caring for People with Disabilities: An Ethics of Respect. Hastings Center Report 2020; 50(1): 44-45. https://doi.org/10.1002/hast.1084 .
Morley G, Grady C , McCarthy J, Ulrich CM Covid-19: Ethical Challenges for Nurses. Hastings Cent Rep. 2020; 50(3): 35-39. doi: 10.1002/hast.1110. Epub 2020 May 14.PMID: 32410225 .
Myers CD, Kieffer EC, Fendrick AM, Kim HM, Calhoun K, Szymecko L, LaHahnn L, Ledón C, Danis M , Rowe Z, Goold SD. How Would Low-Income Communities Prioritize Medicaid Spending?. Journal of Health Politics, Policy and Law 2020; 45(3): 373-418.
Nicolini ME, Kim SYH , Churchill ME, Gastmans C. Should euthanasia and assisted suicide for psychiatric disorders be permitted? A systematic review of reasons. Psychological Medicine 2020. https://doi.org/10.1017/S0033291720001543 .
Nicolini ME, Wendler D . Inherent conflict of interest in clinical research: A call for effective guidance. The American Journal of Bioethics 2020; 20(10):94-96. https://doi.org/10.1080/15265161.2020.1806376
Pierson L, Gibert S., Berkman BE, Danis M, Millum J . Allocating Scarce Biospecimens for Use in Research. Journal of Medical Ethics 2020. doi: 10.1136/medethics-2019-105766.
Rid A . Judging the Social Value of Health-Related Research: Current Debate and Open Questions. Perspectives in Biology and Medicine . 2020; 63(2):293-312.
Rid A , Roestenberg M. Judging the social value of controlled human infection studies. Bioethics . 2020; 34:749–763.
Schupmann W, Jamal L, Berkman BE. Re-examining the Ethics of Genetic Counselling in the Genomic Era. Journal of Bioethical Inquiry 2020. https://doi.org/10.1007/s11673-020-09983-w .
Schupmann W , Moreno J. Belmont in Context. Perspectives in Biology and Medicine 2020; 63(2): 220-239.
Schwan B . Responsibility amid the social determinants of health. Bioethics . 2020; 00: 1– 9. https://doi.org/10.1111/bioe.12782 .
Sebo J, DeGrazia D . Can Knowledge Itself Justify Harmful Research? Cambridge Quarterly of Healthcare Ethics 2020; 29: 302-307.
Shah SK, Essack Z, Byron K, Slack C, Reirden D, van Rooyen H, Jones N, Wendler D . Adolescent barriers to HIV prevention research: Are parental consent requirements the biggest obstacle? Journal of Adolescent Health 2020; 67:495-501 .
Shah SK, Miller FG, Darton TC, Duenas D, Emerson C, Fernandez-Lynch H, Jamrozik E, Jecker NS, Kamuya D, Kapulu M, Kimmelman J, MacKay D, Memoli MJ, Murphy SC, Palacios R, Ritchie TL, Roestenberg M, Saxena A, Saylor K, Selgelid MJ, Vaswani V, Rid A . Ethics of controlled human infection to study COVID-19. Science 2020; 368(6493): 832-34.
Shah SK, Miller FG, Darton TC, Duenas D, Emerson C, Lynch HF, Jamrozik E, Jecker NS, Kamuya D, Kapulu M, Kimmelman J… Rid A . Unnecessary hesitancy on human vaccine tests-Response. Science 2020; 369(6500).
Shah SK, and Rid A . Ethics of controlled human infection studies: Past, present and future. Bioethics 2020; 34: 745-748.
Skrzypek JW, Mangino D . Should Animalists Be “Transplanimalists”? Axiomathes 2020. https://doi.org/10.1007/s10516-020-09482-y .
Solis C. How Much Does Slaughter Harm Humanely Raised Animals? Journal of Applied Philosophy 2020. https://doi.org/10.1111/japp.12483 .
Strassle CL and Berkman BE . Prisons and Pandemics. San Diego Law Review 2020; 57(4): 1-43.
Strassle C, Berkman B . Workplace Wellness Programs: Empirical Doubt, Legal Ambiguity, and Conceptual Confusion. William and Mary Law Review 2020 61(6), 1663-1717. https://www.thefreelibrary.com/_/print/PrintArticle.aspx?id=627386479 .
Strassle C, Jardas E, Ochoa J, Berkman B, Danis M, Rid A, Taylor HA . Covid-19 Vaccine Trials and Incarcerated People — The Ethics of Inclusion. New England Journal of Medicine 2020; 383(20): 1897-1899.
Strassle C , Pearson S. A proposed framework for patient engagement throughout the broader research enterprise. Journal of Comparative Effectiveness Research 2020 9(6), 387-393. https://www.futuremedicine.com/doi/10.2217/cer-2019-0175 .
Taylor HA , Mogul D. Commentary. Digital Negotiations. American Journal of Bioethics 2020; 20(10): TBA.
Ulrich C, Rushton C, Grady C . Nurses Confronting coronavirus: challenges met and lessons learned to date. Nursing Outlook . Nov/Dec 2020; 68(6), 838-844. c. https://doi.org/10.1016/j.outlook.2020.08.018
Um S . Book review: Matt Stichter, The Skillfulness of Virtue, Journal of Moral Philosophy (forthcoming).
Um S . Solving the Puzzle of Partiality. Journal of Social Philosophy 2020. DOI:10.1111/josp.12367
Um S . What is a relational virtue? Philosophical Studies 2020. https://doi.org/10.1007/s11098-020-01422-1 .
van der Graaf R, Macklin R, Rid A , et al. Rapid response: Integrating public health programs and research and the CIOMS guidelines. British Medical Journal 2020; 368: m734.
van Dijke I, van Wely M, Berkman BE , Bredenoord AL, Henneman L, Vliegenthart R, Repping S, Hendriks S . Should germline genome editing be allowed? The effect of treatment characteristics on public acceptability. Human Reproduction 2020: 1-14.
Vaswani V, Saxena A, Shah SK, Palacios R, Rid A . Informed consent for controlled human infection studies in low- and middle-income countries: ethical challenges and proposed solutions. Bioethics 2020; 34:809–818.
Warsame R., Riordan L, Jenkins S, Danis M et al. Responsibilities, Strategies, and Practice Factors in Clinical Cost Conversations: a US Physician Survey. J Gen Intern Med 2020. https://doi.org/10.1007/s11606-020-05807-0 .
Wendler D . Minimizing risks is not enough: the relevance of benefits to protecting research participants. Perspectives in Biology and Medicine 2020; 63:346-358.
Wendler D . The Claims of Biospecimen Donors to Credit and Compensation. Trend in Genetics 2020. https://doi.org/10.1016/j.tig.2020.06.005 .
Wendler D . The permissibility of deception in riskier research. Ethics & Human Research 2020; 42:34-40.
Wendler D . When and how to include vulnerable subjects in clinical trials. Clinical Trials 2020; 17(6): 696–702.
Wendler D , Berkman BE. Maximizing the Value of Human Biospecimens: Lessons from the Coronavirus and the Seattle Flu Study. American Journal of Medical Genetics Part A 2020. https://doi.org/10.1002/ajmg.a.61891
Wendler D, Ochoa J, Millum J, Grady C, Taylor HA . COVID-19 Vaccine Trial Ethics Once We Have an Efficacious Vaccine. 3-Dec-2020 DOI: 10.1126/science.abf5084
Wild V, Buyx A, Hurst SA, Munthe C, Rid A , Strech D, Thompson A. COVID-19: Eine Ad-hoc Public-Health-Ethikberatung. Das Gesundheitswesen 2020; 82: 507–513.
Wild V, Buyx A, Hurst SA, Munthe C, Rid A , Strech D, Thompson A. Blog post: Responding to the SARS-CoV-2 pandemic: Experiences of an ad hoc public health ethics consultation. Journal of Medical Ethics blog, April 1, 2020. Available at: https://blogs.bmj.com/medical-ethics/2020/04/01/responding-to-the-sars-cov-2-pandemic-experiences-of-an-ad-hoc-public-health-ethics-consultation/ .
Wild V, Buyx A, Hurst SA, Munthe C, Rid A , Strech D, Thompson A. Covid-19: An ad hoc public health ethics consultation. Gesundheitswesen 2020; 82: 507–513.
Aguilera B, Almonte JC, Irarrázaval R. The sun is shining on the South: advocacy and regulation of conflicts of interest in Chile. BMJ Opinion 2019; online.
Aguilera B, Wendler D . Commentary: Should the Belmont Report Be Extended to Animal Research. Cambridge Quarterly of Healthcare Ethics 2019; 29(1): 58-66.
Berkman B, Brody L, Collins F, Green E. Karen Rothenberg’s (Not So) Secret Roles and Contributions at the U.S. National Institutes of Health. Journal of Health Care Law & Policy 2019; 22(167): 167-171.
Cho HL . Can intersectionality lead to a more accurate diagnosis? AJOB 2019; 19(2): 37-39.
Cho HL , Miller DG, Kim SY . (2019). Understanding people’s ‘unrealistic optimism’ about clinical research participation. J Med Ethics 2019;0:1–6.
Charlton V, Rid A . Innovation as a value in healthcare priority setting: the UK experience. Social Justice Research 2019; 32(2): 208-38.
Chung E, Berkman B . Should Patient Groups Have the Power to Redirect How Their Samples Are Used? American Journal of Bioethics 2019; 19(8): 26-28.
Danis M . Delving into the Details of Evaluating Public Engagement Initiatives. Int J Health Policy Manag 2019; 8(4): 247-249.
Danis M , Goold SD, Schindler M, Hurst SA. The Value of Engaging the Public in CHATing About Healthcare Priorities: A Response to Recent Commentaries. Int J Health Policy Manag 2019; 8(4): 250-252.
Miljeteig I, Defaye F, Wakim P, Desalegn DN, Berhane Y, Norheim O, Danis M . Bedside Rationing Under Resource Constraints-A National Survey of Ethiopian Physicians’ Use of Criteria for Priority Setting. The American Journal of Bioethics 2019; online.
DeGrazia D . Human-Animal Chimeras, ‘Human’ Cognitive Capacities, and Moral Status. Hastings Center Report 2019; 49(5): 33-34.
DeGrazia D , TL Beauchamp. Beyond the 3 Rs to a More Comprehensive Framework of Principles for Animal Research Ethics. ILAR Journal 2019; 0(0): 1-10.
Dickert NW, Wendler D , Devireddy CM, Goldkind SF, Ko YA, Speight CD, Kim SYH . Understanding preferences regarding consent for pragmatic trials in acute care. Clinical Trials 2019; 15:567-578.
Duran D, Asada Y, Millum J , Gezmu M. Harmonizing Health Disparities Measurement. Am J Public Health 2019; 109:S1, S25-S27.
Earl J. Innovative Practice, Clinical Research, and the Ethical Advancement of Medicine. American Journal of Bioethics 2019 19:6
Ezugwu EC, Osamor PE, Wendler D . Ethical issues in denial of church wedding based on couple’s hemoglobin genotype in Enugu, south eastern Nigeria. BMC Medical Ethics 2019; 20:37.
Fabi RE, Taylor HA . Prenatal Care for Undocumented Immigrants: Professional Norms, Ethical Tensions, and Practical Workarounds. Journal of Law, Medicine & Ethics 2019; 47(3): 398-408.
Grady C . Bioethics in the Oversight of Clinical Research: Institutional Review Boards and Data and the Safety Monitoring Boards. Kennedy Institute of Ethics Journal 2019; 29:1
Grady C . The Contribution of Ethics Review to Protection of Human Participants: Comment on Measuring the Quality and Performance of Institutional Review Boards. Journal of Empirical Research on Human Research Ethics 2019; Online first.
Hendriks S , et al. The relative importance of genetic parenthood. Reproductive BioMedicine 2019; 39:1, 103-110.
Hendriks S , Grady C , Ramos KM, Kim SYH , et al. Ethical challenges of risk, informed consent, and posttrial responsibilities in human research with neural devices: a review [published online October 17, 2019]. JAMA Neurol . doi:10.1001/jamaneurol.2019.3523
Huang CJ , Bandettini WP, Danis M . Returning individual research results regarding gadolinium deposition in the brain is the preferable choice. The American Journal of Bioethics 2019; 19:4, 77-78.
Hull Chandros S. Changing the Conversation about The Ethics of Genomics and Health Disparities Research with American Indian and Alaska Native Communities: A Report from the Field. Journal of Health Care for the Poor and Underserved 2019; 30:4, Supplement, 21-26.
Hull Chandros S, Schiffenbauer A. Single IRBs Are Responsible to Ensure Consent Language Effectively Conveys the Local Context. American Journal of Bioethics 2019: 19:4, 85-86.
Jamal L, Schupmann W, Berkman BE . An ethical framework for genetic counseling in the genomic era. J Genet Couns . 2019;00:1–10. https ://doi.org/10.1002/jgc4.1207
Kim SYH . Lives Not Worth Living in Modern Euthanasia Regimes. Journal of Policy and Practice in Intellectual Disabilities 2019;16(2): 134-136. doi:10.1111/jppi.12300
Langlois CM, Bradbury A, Wood EM, Roberts JS, Kim SY , Riviere ME, ... & Langbaum J B. Alzheimer's Prevention Initiative Generation Program: Development of an APOE genetic counseling and disclosure process in the context of clinical trials. Alzheimer's & Dementia: Translational Research & Clinical Interventions. 2019: 5, 705-716.
Levine D, Liederbach E, Johnson L, Kaye E, Spraker-Perlman H, Mandrell B, Pritchard M, Sykes A, Lu Z, Wendler D , Baker J. Are We Meeting the Informational Needs of Cancer Patients and Families? Perception of Physician Communication in Pediatric Oncology. Cancer 2019: published online.
Mangino DR, Nicolini ME , De Vries RG, Kim SYH . Euthanasia and Assisted Suicide of Persons With Dementia in the Netherlands. American Journal of Geriatric Psychiatry 2019.
Miljeteig I, Defaye F, Desalegn D, Danis M . Clinical ethics dilemmas in a low-income setting - a national survey among physicians in Ethiopia. BMC Med Ethics 2019; 20, 63 doi:10.1186/s12910-019-0402-x
Miljeteig I, Defaye FB, Wakim P, Desalegn DN, Berhane Y, Norheim OF, Danis M . Financial risk protection at the bedside: How Ethiopian physicians try to minimize out-of-pocket health expenditures 2019.
Miller W, Berkman B. The Future of Physicians’ First Amendment Freedom: Professional Speech in an Era of Radically Expanded Prenatal Genetic Testing. Washington and Lee Law Review 2019; 76:2/iss3.
Millum J , Beecroft B, Hardcastle TC, Hirshon JM, Hyder AA, Newberry JA, Saenz C. Emergency care research ethics in low- and middle-income countries. BMJ Global Health (2019) 4(S6):e001260.
Millum J , Campbell M, Luna F, Malekzadeh A, Karim Q. Ethical Challenges in Global Health-related Stigma Research. BMC Medicine 2019; 17:84
Millum J, Garnett M. How Payment for Research Participation Can Be Coercive. The American Journal of Bioethics 2019; 19(9): 21-31.
Littlejohns P, et al. Rid A . National Institute of Health and Care Excellence, social values and healthcare priority-setting. Journal of the Royal Society of Medicine 2019; 112(5): 173-179
Nicolini ME, Gastmans C, Kim SYH. Parity Arguments for ‘Physician Aid-in-Dying’ (PAD) for Psychiatric Disorders: Their Structure and Limits. The American Journal of Bioethics 2019; 19(10): 3-7.
Nicolini ME , Peteet JR, Donovan GK , Kim SYH . Euthanasia and assisted suicide of persons with psychiatric disorders: the challenge of personality disorders. Psychological Medicine 2019; 1–8.
Ramos KM1, Grady C , Greely HT, Chiong W, Eberwine J, Farahany N, Johnson LSM, Hyman BT, Hyman S, Rommelfanger KS, Serrano EE, Churchill JD, Gordon JA, Koroshetz WJ. The NIH BRAIN Initiative: Integrating Neuroethics and Neuroscience. Neuron 2019; 101(3):394-398.
Rid A , Littman J, Buyx A. Evaluating the risks of public health programs: Rational antibiotic use and the antimicrobial resistance. Bioethics 2019; 33:734-748.
Ruck Keen A, Kane NB, Kim SYH, Owen GS. Taking Capacity Seriously? Ten Years of Mental Capacity Disputes before England’s Court of Protection. Int J Law Psychiatry 2019; 62:56-76.
Rumbold B, Charlton C, Rid A , Mitchell P, Wilson J, Littlejohns P, Max C, Weale A: Affordability and non-perfectionism in moral action. Ethical Theory and Moral Practice 2019; 22(4):973-991.
Strassle C . Fair subject selection in cystic fibrosis trials. Journal of Cystic Fibrosis 2019; 18:5, e47.
Steel R. Reconceptualising risk-benefit analyses: the case of HIV cure research. J Med Ethics 2019; 0: 1-8.
Sullivan HK, Bayefsky M, Wakim P, Huddleston, K, Biesecker BB , Hull SC , Berkman BE . Views of Pregnant Women on Prenatal Genome Sequencing. Obstetrics and Gynecology 2019; 133: 525-532.
Ulrich CM, Grady C . 2019. Moral Distress and Moral Strength Among Clinicians in Health Care Systems: A Call for Research. NAM Perspectives. Commentary, National Academy of Medicine, Washington, DC. https://doi.org/10.31478/201909c
Ulrich CM, Grady C . Time for Advance Care Planning Discussions during Transitions in Care in Cancer Clinical Trials. JAMA Oncol. 2019; 5:4; 459-460.
Walajahi H . Engaging the “Citizen” in Citizen Science: Who’s Actually Included? The American Journal of Bioethics 2019; 19(8): 31-33.
Walajahi H , Wilson DR, Hull SC . Constructing identities: the implications of DTC ancestry testing for tribal communities. Genetics in Medicine 2019: 1.
Wasserman D . A Case for Greater Risk Tolerance in Internet Use by Adults with Intellectual Disabilities: A Comment on Chalghoumi et al. Ethics & Behavior. 2019; 29(3):223-6.
Wasserman D. Chasing the Elusive Wrongdoing Intuition. Law, Ethics and Philosophy . 2019:63-83.
Wasson K, Adams WH, Berkowitz K, Danis M , et al. What Is the Minimal Competency for a Clinical Ethics Consult Simulation? Setting a Standard for Use of the Assessing Clinical Ethics Skills (ACES) Tool. AJOB Empirical Bioethics 2019; 10:3, 164-172.
Wendlandt B, Ceppe A, Choudhury S, Cox CE, Hanson LC, Danis M , Tulsky JA, Nelson JE, Carson SS. Modifiable Elements of ICU Supportive Care and Communication are Associated with Surrogates’ PTSD Symptoms. Intensive Care Med 2019; doi: 10.1007/s00134-019-05550-z
Wendler D . The value in doing something. Critical Care Medicine 2019; 47:149-151.
Wendler D , Nelson RM, Lantos JD. Ethics rounds: the potential benefits of research may justify certain research risks. Pediatrics 2019; 143:e20181703.
Wilson Y, White A, Akilah Jefferson A, Danis M . Intersectionality in Clinical Medicine: The Need for a Conceptual Framework. The American Journal of Bioethics 2019; 19:2, 8-19.
Zhou Q, Ratcliffe SJ, Grady C , Wang T, Mao J, Ulrich. Cancer Clinical Trial Patient-Participants’ Perceptions about Provider Communication and Dropout Intentions. American Journal of Bioethics 2019; online
Berkman BE, Miller W, Grady C. Is It Ethical to Use Genealogy Data to Solve Crimes? Ann Intern Medicine 2018.
Berkman BE, Lockhart NC, Weil CJ, Carithers LJ, Koester SE, Little AR, Volpi S, Moore HM. Development of a Consensus Approach for Return of Pathology Incidental Findings in the Genotype-Tissue Expression (GTEx) Project. Journal of Medical Ethics 44(9): 643-645 (2018).
Berkman BE and Bayefsky M. Toward the Ethical Allocation of Uterine Transplants. American Journal of Bioethics 18(7): 16-17 (2018).
Berkman BE, Wendler D, Howard D. Reconsidering the Need for Reconsent at 18. Pediatrics 142(2) (2018).
Cho HL, Danis M, Grady C. Correspondence: Post-trial responsibilities beyond post-trial access. Lancet 2018; 391: 1478-1479.
Cho HL, Danis M, Grady C. The ethics of uninsured participants accessing healthcare in biomedical research: A literature review. Clinical Trials 2018; 15(5): 509-521.
Cho HL, Miller DG, Grady C. Beyond Open Communication: A Call for Partnership between Clinical Ethics and Research Ethics Committees. Am J Bioethics 2018 Jan;18(1):52-54.
Danis M, Li L, Nelson JE, Hanson LC, Cox CE, Carson SS, Chai EJ, Keller KL, Tulsky JA. How Surrogate Decision-Makers for Patients with Chronic Critical illness Perceive and Carry Out their Role. Critical Care Medicine. February 16, 2018
Danis M, Millum J, Doernberg S, Memoli M,. Best to Exclude but Pay. AJOB 2018:18:86:-87
Danis M, Hall, W, Williams I, Smith N, Gold M, Coast J, Kapiriri L, Mitton C . Past, present and future challenges in health care priority setting: findings from an international expert survey. Journal of Health Organization and Management. (2018 in press)
Danis M, Hurst M, Schindler M, Goold SD. Swiss-CHAT: Citizens Discuss Priorities for Swiss Health Insurance Coverage. International Journal of Health Policy and Management. (2018 in press)
Danis M, Hurst S, Schindler M, Goold S. Solidarity and Cost Management: Swiss Citizens' Reasons for Priorities Regarding Health Insurance Coverage. Health Expectations (in press 2018)
Danis M, Porter KM, Tayler HA, Cho MK, Wilfond BS. Defining the Scope and Improving the Quality of Clinical Research Ethics Consultation: Response to Open Peer Commentaries About the National Collaborative. AJOB online: 02 Feb 2018
Grady C, Rushton C, Haddad A . An Alternative Account of Clinical Ethics: Leveraging the Strength of the Health Care Team. American Journal of Bioethics. 2018; 18(6): 59-60.
(Grady C) Farahany N et al. The ethics of experimenting with human brain tissue. Nature 2018; 556 (7702): 429-432.
(Grady C) Ulrich C et al. Development and Preliminary Testing of the Perceived Benefit and Burden Scales for Cancer Clinical Trial Participation. Journal of Empirical Research on Human Research Ethics 2018; 13(3): 230-238.
Grady C, Coors M, Bauer L, Edwards K et al. Ethical issues related to clinical research and rare diseases 15th Gordon L. Snider Critical Issues Workshop April 1, 2016. Translational Science of Rare Diseases 2 (2017) 175–194
Grady C, D'Souza P, Allen M, Baumblatt J, Boggiano C.Crotty S, Havenar-Daughton C, Heit A, Hu D, Kunwar N, McElrath J, Lymph node webinar contributors. Conference report Innovative approaches to track lymph node germinal center responses to evaluate development of broadly neutralizing antibodies in human HIV vaccine trials . Vaccine 2018; 36: 5671–5677
Grady C, Ulrich CM, Deshmukh S, Pugh SL, Hanlon A, Watkins Bruner D, Curran W Jr., Attrition in NRG Oncology's Radiation-Based Clinical Trials. Int J Radiat Oncol Biol Phys . 2018 Sep 1;102(1):26-33
Grady C, Osamor PE. Autonomy and couples' joint decision-making in healthcare. BMC Medical Ethics 2018 Jan 11;19(1):3. doi: 10.1186/s12910-017-0241-6
Grady C, Osamor PE. Factors Associated with Women's Health Care Decision-Making Autonomy: Empirical Evidence from Nigeria. J Biosoc Sci . 2018 Jan;50(1):70-85.
Grady C, Ulrich C, Mooney-Doyle K. Communicating with Pediatric Families at End of Life is Not Fantasy. Am J Bioeth . 2018 Jan;18(1):14-16.
Huang CJ, Wasserman D. Deception, Harm, and Expectations of Pain. AJOB Neuroscience 2018; 9(3): 188-189.
Hendriks S, Giesbertz NAA, Bredenoord AL, Repping S. Reasons for being in favour of or against genome modification: a survey of the Dutch general public. Human Reproduction Open 2018; 1-12.
(Hendriks S), Dijke et al. The ethics of clinical applications of germline genome modification: a systematic review of reasons. Human Reproduction . 218; 1-20.
Hendriks, S and Pearson SD. A proposed framework for strengthening regulatory review of innovative reproductive techniques in the United States. Fertility and Sterility, 2018, e-pub September 19.
Hull SC, Splinter K, Holm IA, McDonough TL, Wise AL, Ramoni RB, Members of the Undiagnosed Diseases Network. Implementing the Single Institutional Review Board Model: Lessons from the Undiagnosed Diseases Network. Clin Transl Sci. 2018 Jan:11(1):28-31.
(Kim SYH) Palmer BW et al. Multimedia Aided Consent for Alzheimer's Disease Research. Clinical Gerontologist 2018; 41(1): 20-32.
Kim, S. Y. (2018). Ethical issues in pragmatic trials of "standard‐of‐care" interventions in learning health care systems. Learning Health Systems , 2 (1), e10045.
Kim, S. Y, Tomlinson, T., De Vries, R. G., Kim, H. M., Gordon, L., Ryan, K. A., Krenz, C. D. (2018). Effect of deliberation on the public's attitudes toward consent policies for biobank research. European Journal of Human Genetics , 26 (2), 176.
Kim, S. Y, Dickert, N. W., Wendler, D., Devireddy, C. M., Goldkind, S. F., Ko, Y. A., Speight, C. D. (2018). Consent for Pragmatic Trials in Acute Myocardial Infarction. Journal of the American College of Cardiology , 71 (9), 1051-1053.
Kim, S. Y, De Vries, R. G., Ryan, K. A., Gordon, L., Krenz, C. D., Tomlinson, T., Jewell, S. (2018). Biobanks and the Moral Concerns of Donors: A Democratic Deliberation. Qualitative health research , 1049732318791826.
(Kim, S. Y., Wendler, D), Dickert, N. W., Wendler, D., Devireddy, C. M., Goldkind, S. F., Ko, Y. A., Speight, C. D. (2018). Consent for Pragmatic Trials in Acute Myocardial Infarction. Journal of the American College of Cardiology, 71(9), 1051-1053.
Kim SYH, Conwell Y, Caine ED. Suicide and physician-assisted death for persons with psychiatric disorders: How much overlap? JAMA Psychiatry. 2018. 10.1001/jamapsychiatry.2018.2065
Miller DG, Kim SYH , Dresser R. Advance euthanasia directives: a controversial case and its ethical implications. J Med Ethics . 2018.
Miller WK. Assumption of What: Building Better Market Architecture for Egg Donation. Tennessee Law Review 2018; 86(1).
Millum, J., The Moral Foundations of Parenthood . Oxford University Press (2018)
Millum, J. and D. Wendler 'Randomized Controlled Trials in the Setting of Serious Diseases: Do They Violate the Duty to Rescue?' Journal of Moral Philosophy . 15 (2018): 298-323.
Millum, J. and D. Bromwich 'Understanding, Communication, and Consent' Ergo 5(2) (2018): 45-68.
Millum J, Bromwich, D. 'Lies, Control, and Consent: A Response to Dougherty and Manson' Ethics 128(2) (2018): 446-461.
Millum J, Sharp, D. 'Prioritarianism for Global Health Investments: Identifying the Worst Off' Journal of Applied Philosophy 35(1) (2018): 112-132.
Millum, J. 'The "Reasonable Subject Standard" as an Alternative to the "Best Interest Standard"' American Journal of Bioethics , 18:8 (2018): 66-67
(Pearson SD) Ollendorf DA, Chapman RH . Evaluating and Valuing Drugs for Rare Conditions: No Easy Answers. Value in Health 2018; 21: 547-552.
Pearson SD, Hampson G, Towse A, Dreitlein WB, Henshall C. Gene therapy: evidence, value and affordability in the US health care system. J Comp Eff Res. 2018 Jan;7(1):15-28.
Pearson SD, Carlson JJ, Guzauskas GF, Chapman RH, Synnott PG, Liu S, Russo ET, Brouwer ED, Ollendorf DA. Cost-effectiveness of Drugs to Treat Relapsed/Refractory Multiple Myeloma in the United States. J Manag Care Spec Pharm. 2018 Jan;24(1):29-38.
Pearson SD, Loos AM, Liu S, Segel C, Ollendorf DA, Linder JA. Comparative Effectiveness of Targeted Immunomodulators for the Treatment of Moderate-to-Severe Plaque Psoriasis: A Systematic Review and Network Meta-Analysis. J Am Acad Dermatol. 2018 Jul;79(1):135-144.
Pearson SD, Robinson JC, Howell S. Value-Based Pricing and Patient Access for Specialty Drugs. JAMA . 2018 Jun 5;319(21):2169-2170.
Pearson SD. The ICER Value Framework: Integrating Cost Effectiveness and Affordability in the Assessment of Health Care Value . Value Health . 2018 Mar;21(3):258-265.
Pierson, L. and J. Millum 'Disease Prevalence and the Magnitude of Research Benefits'. American Journal of Bioethics . 18:4 (2018): 73-74
Steel R and Danis M. Blinds and Research Risks. AJOB (2018 in press).
Sullivan HK and Berkman BE. Incidental Findings in Low-Resource Settings. Hastings Center Report 2018; 48(3): 1-9.
Sullivan H, Wendler D, Braverman D . When research regulations and ethics conflict. American Journal of Bioethics 2018; 18: 96-97.
Wasserman D. Fetal Medicine and the Pregnant Woman. Hastings Center Report 2018; 48 (2).
Wasserman D. Review: "Inclusive Ethics: Extending Beneficence and Egalitarian Justice" by Persson, Ingmar, Oxford: Oxford University Press, 2017. Ethics 2018; 128(3): 651-657.
Wasserman D. Review "The Minority Body: A Theory of Disability" by Barnes, Elizabeth, New York: Oxford University Press, 2016. Philosophical Review 2018; 127(2): 251-256.
Wasserman D and Campbell SM . A More "Inclusive" Approach to Enhancement and Disability. In The Ethics of Ability and Enhancement . Palgrave Macmillan 2018: 25-38.
Wasserman, D. "Can a social model of disability encompass mental illness?" In From Disability Theory to Practice: Essays in Honor of Jerome E. Bickenbach . C. Riddle, ed. Rowman & Littlefield, 2018 (pp. 75-99).
Wendler D. Innovative approaches to informed consent for randomized clinical trials: identifying the ethical challenges. Clinical Trials 2018; 15: 17-20.
(Wendler W, Kim SYK) Dickert N et al . Consent for pragmatic trials in acute myocardial infarction. Journal of the American College of Cardiology 2018; 71: 1051-1053.
Wilson YY. White A, Jefferson A, Danis M. Intersectionality in Clinical Medicine . AJOB 2018
Berkman BE. Refuting the Right Not to Know. Journal of Healthcare Law and Policy 2017; 19(1): 1-75.
Berkman B, Bayefsky M. Prenatal Whole Genome Sequencing: An Argument for Professional Self-Regulation. The American Journal of Bioethics 2017; 17(1): 26-28.
Berkman B, Wendler D, Sullivan HK, Grady C. A Proposed Process for Reliably Updating the Common Rule. American Journal of Bioethics 2017; 17(7): 8-14.
(Berkman BE) Odwazny LM. The "Reasonable Person" Standard for Research Informed Consent. American Journal of Bioethics 2017; 17(7): 8-14.
Chen SC, Berkman BE, Hull SC. Recontacting participants for expanded uses of existing samples and data: a case study. Genetics and Medicine 2017; 19(8): 883-889.
Chen SC et al., (Grady C). Phase 1 healthy volunteer willingness to participate and enrollment preferences. Clin Trials 2017; online.
Collier E, Danis M. Participation of Citizen Scientists in Clinical Research and Access to Research Ethics Consultation. AJOB 2017; 17(4): 70-72.
(Danis M) Baltussen R et al. Global Developments in Priority Setting in Health. International Journal of Health Policy and Management 2017; 6(3): 127-128.
(Danis M) Nelson JE et al. The Voice of Surrogate Decision-Makers: Family Responses to Prognostic Information in Chronic Critical Illness. Am J Respiratory and Critical Care Medicine 2017; 196(7): 864-872.
(Danis M) Porter K et al. The Emergence of Clinical Research Ethics Consultation: Insights from a National Collaborative. AJOB 2017.
(Danis M) Porter K et al. Danis - Should priority setting also be concerned about profound socio-economic transformations? A reply to a far too polarized commentary. Int J Health Policy Manag 2017; 6(x): 1-2.
DeGrazia D. On Saving Preterm Infants: A Plea for Sensible Ontology. AJOB 2017; 17(8): 36-37.
DeGrazia D. Procreative Responsibility in View of What Parents Owe Their Children. In The Oxford Handbook of Reproductive Ethics . Oxford University Press 2016; 1-20.
DeGrazia D. Reflections on the Procreative Asymmetry. APA Newsletter on Philosophy and Medicine 2017; 16(2): 1-4.
DeGrazia D. Review: "Inclusive Ethics: Extending Beneficence and Egalitarian Justice" by Ingmar Persson, Oxford University Press 2017. Utilitas 2017.
DeGrazia D. Using Pharmaceuticals to Change Personality: Self-Transformation, Identity, and Authenticity. In Philosophical Issues in Pharmaceutics 2017; 122: 177-188.
(DeGrazia D) Colloca L, Enck P. Reply to Letter to Editor: Using placebos as an opioid-sparing method of pain management. Pain 2017; 158(2): 361-362.
DeGrazia D, Groman M, Lee LM. Defining the Boundaries of a Right to Adequate Protection: A New Lens on Pediatric Research Ethics. Journal of Medicine and Philosophy 2017.
Doernberg S, Hull SC. Harms of Deception in FMR1 Premutation Genotype-Driven Recruitment. AJOB 2017; 17(4): 62-63.
Gibert SH. Closed-Loop Deep Brain Stimulation and Its Compatibility with Autonomous Agency. AJOB Neuroscience 2017; 8(2): 88-90.
Gibert SH, DeGrazia D, Danis M. Ethics of patient activation: exploring its relation to personal responsibility, autonomy and health disparities. Journal of Medical Ethics 2017; 43: 670-675.
Grady C. Response to Carl Elliott. Clin Trials . 2017; online.
Grady C et al. A randomized trial comparing concise and standard consent forms in the START trial. PLoS One 2017; 12(4): e0172607.
Grady C et al. Informed Consent. N Engl J Med . 2017; 376(9): 856-867.
Grady C et al. Motivations, enrollment decisions, and socio-demographic characteristics of healthy volunteers in phase 1 research. Clin Trials 2017; online.
Grady C. Making the Choices Necessary to Make a Difference: The Responsibility of National Bioethics Commissions. Hastings Cent Rep . 2017; 47 Suppl 1: S42-S45.
(Grady C, Wendler D, Kim SYH) Dickert NW et al. Re-framing consent for clinical research: a function-based approach. American Journal of Bioethics 2017; 17:3-11.
(Grady C), Ulrich C. Ethical Issues in Critical Care Nursing, in Critical Care Nursing: A Holistic Approach, 11th edition, P Morton, D Fontaine (eds). Wolters- Kluwer 2017.
(Grady C) Ulrich CM et al. Response to "Nurses at the Table: Nursing, Ethics, and Health Policy". Nurs Outlook 2017; 65(2): 142.
Huang C, Wasserman D. Considering Consent to Research for Patients in Chronic Pain and With Mental Illnesses. American Journal of Bioethics 2017; 17(12): 51-52.
Hull SC, Wilson DR. Beyond Belmont: Ensuring Respect for AI/AN Communities Through Tribal IRBs, Laws, and Policies. AJOB 2017; 17(7): 60-62.
Jefferson AA, Pearson SD. Conflict of Interest in Seminal Hepatitis C Virus and Cholesterol Management Guidelines. JAMA Internal Medicine 2017; 177(3): 352-357.
John TM, Millum J, Wasserman D. How to allocate scarce health resources without discriminating against people with disabilities. Economics & Philosophy 2017; 33(2):161-86. Kalkman S, Kim SYH et al. Ethics of Informed Consent for Pragmatic Trials with New Interventions. Value Health 2017; 20(7): 902-908.
Kim SYH. Ethical issues in pragmatic trials of "standard‐of‐care" interventions in learning health care systems. Learning Health Systems 2017; 2: e10045.
Kim SYH, Flory J, Relton C. Ethics and practice of Trials within Cohorts: An emerging pragmatic trial design. Clinical Trials 2017; online.
(Kim SYH) Eckstein L. Criteria for decision-making capacity: between understanding and evidencing a choice. Journal of Law and Medicine 2017; 24: 678-694.
(Kim SYH) Vickers AJ, Young-Afat DA, Ehdaie B. Just-in-time consent: The ethical case for an alternative to traditional informed consent in randomized trials comparing an experimental intervention with usual care. Clinical Trials 2017; online.
Lie RK et al. (Grady C, Wendler D). Comparative effectiveness research: what to do when experts disagree about risks. BMC Medical Ethics 2017; 18(42).
(Lie RK) Kuhtz-Buschbeck et al. Reassessing Diagrams of Cardiac Mechanics: from Otto Frank and Ernest Starling to Hiroyuki Suga. Perspectives in Biology and Medicine 2017; 59(4): 471-90.
Lie RK, Wendler D. The Guinea Phase III Ebola Vaccine Trial: Lessons for Research Ethics Review in Public Health Emergencies. IRB 2017; 39(2): 1-7.
Miller D, Kim SYH. Euthanasia and physician-assisted suicide not meeting due care criteria in the Netherlands: a qualitative review of review committee judgements. BMJ Open 2017; 7.
Miller D, Kim SYH. Risks of Clinical Research Must Be Reasonable and Necessary. AJOB 2017; 17(10): 79-81.
(Millum J) Barsdorf N. The Social Value of Research and the Worst Off. Bioethics 2017; 31(2): 105-115.
(Millum J) Bromwich D. Informed Consent to HIV Cure Research. Journal of Medical Ethics 2017; 43(2): 108-113.
(Millum J) Mukherjee A et al. Informed consent in dental care and research for the older adult population: A systematic review. Journal of the American Dental Association 2017; 148(4): 211-220.
Nayak R K, Wendler D. Is it important to disclose how treatments are selected in clinical research and clinical care? AJOB Empirical Bioethics 2017; 8(3): 170-177.
Osamor P, Grady C. Factors Associated with Women’s Health Care Decision-Making Autonomy: Empirical Evidence from Nigeria. Journal of Biosocial Science 2017; 10: 1-16.
Pearson SD et al. Indication-specific pricing of pharmaceuticals in the US healthcare system. J Comp Eff Res 2017; online.
(Pearson SD) Sandhu AT. Cost-Effectiveness of Sacubitril-Valsartan in Patients Who Have Heart Failure With Reduced Ejection Fraction. Ann Intern Med 2017; 166(8): 607-608.
(Pearson SD) Sculpher M, Claxton K. Developing a Value Framework: The Need to Reflect the Opportunity Costs of Funding Decisions. Value Health 2017; 20(2): 234-239.
(Pearson SD) Whittington MD et al. Assessing the value of mepolizumab for severe eosinophilic asthma: a cost-effectiveness analysis. Ann Allergy Asthma Immunol 2017; 118(2): 220-225.
Pierson L, Millum J. The limits of research institutions in setting research priorities. Journal of Medical Ethics 2017; online.
Sullivan HK, Miller DG, Huang CJ. Recognizing the Role of the Clinician in Agency-Influencing Interventions. AJOB Neuroscience 2017; 8(2): 71-73.
Wasserman D. Better parenting through biomedical modification: a case for pluralism, deference, and charity. Kennedy Institute of Ethics Journal 2017; 27(2): 217-47.
Wasserman D. Justice, Procreation, and the Costs of Having and Raising Disabled Children. In The Oxford Handbook of Reproductive Ethics . Oxford University Press 2016; 1-18.
Wasserman D. Physical disability, dignity, and physician-assisted death. In Human Dignity and Assisted Death, Muders S (ed.). Oxford University Press; 2017: 85-104.
Wasserman D. Review: "Death or Disability: The Carmentis Machine and Decision-Making for Critically Ill Children" by Dominic Wilkinson, Kennedy Institute of Ethics Journal , 2017;27(1):E-4.
Wasserman D. Review: "One Child: Do We Have a Right to More?" by Sarah Conly, Oxford University Press 2016, 248 pages. Economics & Philosophy 2017; 33(2): 313-319.
Wasserman D, Aas S. Discrimination and disability. In The Routledge Handbook of the Ethics of Discrimination, Lippert-Rasmussen K (ed.); 2017: 231-242.
Wasserman D, Asch A, Blustein J, Putnam D. Cognitive disability and moral status. The Stanford Encyclopedia of Philosophy Fall 2017 Edition, Edward N. Zalta (ed.). https://plato.stanford.edu/archives/fall2017/entries/cognitive-disability/ [ disclaimer ]
Wendler D. A pragmatic analysis of vulnerability in clinical research. Bioethics 2017; 31:515–525.
Wendler D. Innovative approaches to informed consent for randomized clinical trials: Identifying the ethical challenges. Clinical Trials 2017; online.
Wendler D. The Ethics of Research in Lower Income Countries: Double Standards Are Not the Problem. The Journal of Clinical Ethics 2017; 28(3): 239-46.
Wendler D. The theory and practice of surrogate decision making. Hasting Center Report 2017; 47:29-31.
Wendler D et al. (Kim SYH). Targeted consent for research on standard of care interventions in the emergency setting. Critical Care Medicine 2017; 45: e105–e110.
Wendler D, Rid A. In defense of a social value requirement for clinical research. Bioethics 2017; 31(2): 77-86.
Wender D, Wertheimer A. Why is Coerced Consent Worse Than No Consent and Deceived Consent? Journal of Medicine and Philosophy 2017; 42(2): 114-131.
(Wendler D) Levine DR et al. Patients' and parents' needs, attitudes, and perceptions about early palliative care integration in pediatric oncology. JAMA Oncology 2017; 3:1214-1220.
(Wendler D) Rafael Dal-Ré et al. Public preferences on written informed consent for low-risk pragmatic clinical trials in Spain. British Journal of Clinical Pharmacology 2017; 83: 1921-1931.
Voorhoeve A. Balancing small against large burdens. Behavioural Public Policy 2017.
(Voorhoeve A) Rumbold B et al. Universal health coverage, priority setting, and the human right to health. The Lancet 2017.
Voorhoeve A et al. Making Fair Choices on the Path to Universal Health Coverage: Applying Principles to Difficult Cases. Health Systems & Reform 2017.
Bayefsky M, Berkman BE. The Ethics of Allocating Uterine Transplants. Cambridge Quarterly of Health Care Ethics 2016; 25(3): 350-365.
Bayefsky MJ, DeCherney AH, Berkman BE. Compensation for egg donation: a zero-sum game. Fertility and Sterility 2016; 105(5): 1153-1154.
D Braverman, S Doernberg, C Runge, D Howard. OxRec model for assessing risk of recidivism: ethics. Lancet Psychiatry 2016; 3(9): 808-809.
Carson S, Cox, CE, Wallenstein S, Hanson LC, Danis M, Tulsky JA, Chai E, Nelson JE. Effectof Palliative Care-Led Meetings for Families of Patients with Chronic Critical Illness – A Randomized Clinical Trial. JAMA 2016; 316(1): 51-62.
Colloca L, Enck P, DeGrazia D "Relieving Pain Using Dose-Extending Placebos: A Scoping Review," PAIN 157 (2016) 1590–1598.
Dal-Re R, Rid A, Wendler D. The potential exploitation of research participants in high income countries who lack access to health care. British Journal of Clinical Pharmacology 2016; 81: 857-864.
Danis M, Wilson Y, White A. Bioethicists Can and Should Contribute to Addressing Racism. The American Journal of Bioethics 2016; 16(4): 3-12.
Darnell AJ, Austin H, Bluemke DA, Cannon RO, Fischbeck K, Gahl W, Goldman D, Grady C, Greene MH, Holland SM, Hull SC, Porter FD, Resnik, Rubinstein WS, Biesecker LG. A Clinical Service to Support the Return of Secondary Genomic Findings in Human Research. The American Journal of Human Genetics 2016; 98:435-441.
DeGrazia, D. Sentient Nonpersons and the Disvalue of Death. Bioethics 2016; on line February 2016.
DeGrazia, D. Nonhuman Primates, Human Need, and Ethical Constraints. Hastings Center Report 2016; 46(4): 27-28.
DeSante J, DeGrazia D, Danis M. Parents of Adults with Diminished Self-Governance. Cambridge Quarterly of Healthcare Ethics 2016; 25:93-107.
De Vries RG, Tomlinson T, Kim HM, Krenz C, Haggerty, D, Ryan, KA, Kim, SY. Understanding the Public's Reservations about Broad Consent and Study-By Study Consent for Donations to a Biobank: Results of a National Survey. PLoS ONE 2016; 11(7): 1-11.
Dickert NW, Brown J, Cairns CB, Eaves-Leanos A, Goldkind SF, Kim SYH, Nichol G, O'Conor KJ, Scott JD, Sinert R, Wendler D, Wright DW, Silbergleit R. Confronting ethical and regulatory challenges of emergency care research with conscious patients. Annals of Emergency Medicine 2016; 67:538-545.
Doussau A, Geoerger B, Jimenez I, Paoletti X. Innovations for phase I dose-findings in pediatric oncology clinical trials. Contemporary Clinical Trials 2016; 47:217-227.
Doussau A, Grady C. The Ethics of Studying Financial Incentives in Public Health Implementation: Study Design Challenges. American Journal of Bioethics 2016; 16(10):78-80.
Du Toit, J. Is Having Pets Morally Permissible? Journal of Applied Philosophy 2016; 33(3):327-43.
Fins JJ, Kodish E, Cohn F, Danis M, Derse AR, Dubler NN, Goulden B, Kuczewski M, Mercer MB, Pearlman RA, Smsith ML, Tarzian A, Youngner SJ. A Pilor. Evaluation of Portfolios for Quality Attestation of Clinical Ethics Consultants. American Journal of Bioethics 2016; 16(3):17-24.
Gliwa C, Yurkiewicz IR, Lehmann LS, Hull SC, Jones N, Berkman BE. Institutional review board perspectives on obligations to disclose genetic incidental findings to research participants. Genetics in Medicine 2016; 18(7): 705-711.
Goold SD, Myers D, Szymecko L, Collins CC, Martinez S, Ledon C, Campbell TR, Danis M, Cargill SS, Kim HM, Rowe Z. Priorities for Patient-Centered Outcomes Research: The Views of Minority and Underserved Communities. Health Services Research 2016; On line.
Grady C, Nogues I, Wiener L, Wilfond BS, Wendler D. Adolescent research participants' descriptions of medical research. AJOB Empirical Research 2016; 7:1-7.
Grady C, Fauci A. The Role of the Virtuous Investigator in Protecting Human Research Subjects. Perspectives in Biology and Medicine 2016; 59(1): 122-131.
Heazell AEP, Slassakos D, Blencowe H, Burden C, Bhutta ZA, Cacciatore J, Dang N, Das J, Flenady V, Gold KJ, Mensah OK, Millum J, Nuzum D, O-Donoghue K, Redshaw M, Rizvi A, Roberts T, Saraki HET, Storey C, Wojcieszek AM, Downe S for Lancet Preventable Stillbirths Series study group with The Lancet Ending Preventable Stillbirths investigator group. Stillbirths: economic and psychosocial consequences. The Lancet 2016; 387(10018): 6-12.
Johnson R, Rid A, Emanuel E, Wendler D Risks of phase 1 research with healthy participants: A systematic review Clinical Trials 2016; 13:149–160.
Kim S, Miller F. Waivers and Alterations to Consent in Pragmatic Clinical Trials: Respecting the Principle of Respect for Persons. IRB: Ethics & Human Research . 2016;38(1):1-5.
Kim SYH, Wilson R, De Vries R, Ryan KA, Holloway RG, Kieburtz K. Are patients with amyotrophic lateral sclerosis at risk of a therapeutic misconception? Journal of Medical Ethics 2016; on line.
Kim, SYH, Lemmens T. Should assisted dying for psychiatric disorders be legalized in Canada? Canadian Medical Association Journal 2016. On Line.
Kim SYH, De Vries RG, Peteet JR. Euthanasia and Assisted Suicide of Patients With Psychiatric Disorders in the Netherlands 2011-2014. JAMA Psychiatry 2016; 73(4): 362-368.
Kon AA, Davidson JE, Morrison W, Danis M, White DB. Shared Decision Making in ICUs: An American College of Critical Care Medicine and American Thoracic Society Policy Statement. Critical Care Medicine Journal 2016; 44(1): 188-201.
Koretzky M, Bonham VL, Berkman BE, Kruszka P, Adeyemo A, Muenke Max, Hull SC. Towards a more representative morphology: clinical and ethical considerations for including diverse populations in diagnostic genetic atlases. Genetics in Medicine 2016; e-pub 3-10-16.
Osamor PE, Grady C. Women's autonomy in health care decision-making in developing countries: a synthesis of the literature. International Journal of Women's Health 2016; 8: 191-202.
Rulli T, Millum J. Rescuing the duty to rescue. Journal of Medical Ethics 2016; 42:260-264.
Rulli T, Wendler D. The Duty to Take Rescue Precautions. Journal of Applied Philosophy 2016; 33(1).
Sharp D, Wasserman D. Deep Brain Stimulation, Historicism, and Moral Responsibility. Neuroethics 2016. On line.
Sheeler R, Mundell T, Hurst S, Goold SD, Thorsteinsdottir B, Tilburt J, Danis M. Self-Reported Rationing Behavior Among US Physicians: A National Survey. Journal of General Internal Medicine 2016; On Line.
Slutsky J, Tumilty E, Max C, Lu L, Tantivess S, Hauegen RC, Whitty JA, Weale A, Pearson SD, Tugendhaft A, Wang H, Staniszewska S, Weerasuriya K, Ahn J, Cubillos L.Patterns of public participation. Journal of Health Organization and Management 2016; 30(5):751-768.
Wendler D, Wesley B, Pavlick M, Rid A. A new method for making treatment decisions for incapacitated patients: what do patients think about the use of a patient preference predictor? Journal of Medical Ethics 2016; 42: 235-241.
Wendler, D. The Potential for Infrastructure Benefits and the Responsiveness Requirement. American Journal of Bioethics 2016; 16(6): 1-2.
Wendler D, Johnson R When Clinical care is like research: the need for review and consent. Wendler D, Johnson R. When clinical care is like research: the need for review and consent. Theoretical Medicine and Bioethics 2016; 37:193-209.
Wendler D, Shah N, Pulsipher M, Fry T, Grady, C Research involving pediatric stem cell donors: A way forward Clinical Trials 2016; 13:304-310.
Bach PB, Pearson SD. Payer and Policy Maker Steps to Support Value-Based Pricing for Drugs. JAMA , on line 11/30/15.
Barnhill A, Miller FG. The ethics of placebo treatments in clinical practice: a reply to Glackin. Journal of Medical Ethics 2015; 41(8): 673-676. http://www.ncbi.nlm.nih.gov/pubmed/26100361
Bayefsky, M. The Regulatory Gap for Preimplantation Genetic Diagnosis. Hastings Center Report 2015; 45(1): 7-8. http://www.ncbi.nlm.nih.gov/pubmed/25600381
Bayefsky M, Berkman BE. FDA's Proposed Guidance for Laborataory Developed Tests: How Should Regulators Balance the Risks and Promise of Innovation in Clinical Genetics? Food and Drug Law Policy Forum 2015; 5(2):1-16. http://www.ncbi.nlm.nih.gov/pubmed/26448898
Bayefsky, M. Imposing Genetic Diversity: An Imposition on Reproductive Freedom. AJOB 2015;15(6):27-28. http://www.ncbi.nlm.nih.gov/pubmed/26030492
Bayefsky M, Saylor KW, Berkman BE. Parental Consent for the Use of Residual Newborn Screening Bloodspots: Respecting Individual Liberty vs Ensuring Public Health. JAMA 2015; On line 6-8-15. http://www.ncbi.nlm.nih.gov/pubmed/26053685
Berkman BE, Hull SC, Biesecker LG. Scrutinizing the Right Not to Know. AJOB 2015; 15(7): 17-19. http://www.ncbi.nlm.nih.gov/pubmed/26147256
Botkin JR, Belmont JW, Berg JS, Berkman BE, Bombard Y, Holm IA, Levy HP, Ormond KE, Saal HM, Spinner NB, Wilfond BS, McInerney JD. Points to Consider: Eethical, Legal, and Psychosocial Implications of Genetic Testing in Children and Adolescents. The American Journal of Human Genetics 2015; 97: 6-21. http://www.ncbi.nlm.nih.gov/pubmed/26140447
Brody B, Migueles SA, Wendler D. Should All Research Subjects Be Treated the Same? Hastings Center Report 2015; 45(1):17-20. http://www.ncbi.nlm.nih.gov/pubmed/25600384
Bromwich D, Millum J. Disclosure and Consent to Medical Research Participation. Journal of Moral Philosophy 2015; 12:195-219.
Chahal HS, Marseille EA, Tice JA, Pearson SD, Ollendorf DA, Fox RK, Kahn JG. Cost-effectiveness of Early Treatment of Hepatitis C Virus Genotype 1 by State of Liver Fibrosis in a US Treatment-Naïve Population. JAMA Internal Medicine 2015; 176(1): 65-73. http://www.ncbi.nlm.nih.gov/pubmed/26595724
Chen S, McCullumsmith C, Kim SYH. Disclosing the potential impact of placebo controls in antidepressant trials. British Journal of Psychiatry Open . 2015; 1(1): 1-5.
Cho MK, Taylor H, McCormick JB, Anderson N, Barnard D, Boyle MB, Capron AM, Dorfman E, Havard K, Reider C, Sadler J, Schwartz P, Sharp RR, Danis M, Wilfond BS. Building a Central Repository for Research Ethics Consultation Data: A Proposal for a Standard Data Collection Tool. Clin Transl Sci. 2015; 8(4):376-87. http://www.ncbi.nlm.nih.gov/pubmed/25758372
Danis M. Weighing the importance of palliation of symptoms for Ebola patients during the epidemic in West Africa. AJOB 2015;15(4):70-2. http://www.ncbi.nlm.nih.gov/pubmed/25856611
Danis M, Nayak R. Health Policy. Encyclopedia of Global Bioethics Hank ten Have, (Ed.). Springer Verlag Berlin Heidelberg (accessible at: http://www.springerreference.com/docs/html/chapterdbid/398920.html [ disclaimer ] ).
Danis, M., D. Wendler, Kim S. Acceptable Approaches to Enrolling Adults Who Cannot Consent in More Than Minimal Risk Research. AJOB 2015;15: 70-71. http://www.ncbi.nlm.nih.gov/pubmed/26479115
Defaye FB, Desalegn D, Danis M, Hurst S, Berhane Y, Norheim OF, Miljetieg I. A survey of Ethiopian physicians' experiences of bedside rationing: extensive resource scarcity, tough decisions and adverse consequences. BMC Health Services Research 2015;5:467-75. http://www.ncbi.nlm.nih.gov/pubmed/26467298
DeGrazia, D. A reply to critics of Creation Ethics. J Medical Ethics 2015; 41: 423-424. http://www.ncbi.nlm.nih.gov/pubmed/25691666
DeGrazia, D. Ethical Reflections on Genetic Enhancement with the Aim of Enlarging Altruism. Health Care Analysis ; 2015. On line 8-6-15. http://www.ncbi.nlm.nih.gov/pubmed/26246070
DeGrazia D, Sebo J. Necessary Conditions for Morally Responsible Animal Research. Cambridge Quarterly 2015; 24(4): 420-430. http://www.ncbi.nlm.nih.gov/pubmed/26364777
DeGrazia D, Beauchamp TL. Reassessing Animal Research Ethics. Cambridge Quarterly 2015; 24(4): 385-390. http://www.ncbi.nlm.nih.gov/pubmed/26364774
DeGrazia, D. Modal Personhood and Moral Status: A Reply to Kagan's Proposal. Journal of Applied Philosophy , e-pub December 2015.
Denning E, Sharma S, Smolskis M, Touloumi G, Walker S, Babiker A, Clewett M, Emanuel E, Florence E, Papadoupoulos A, Sanchez, Tavel J, Grady C for the International Network for Strategic Initiatives in Global HIV Trials START Study Group. Reported consent processes and demographics: a substudy of the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial. HIV Medicine 2015; 16 (Suppl. 1): 24-29. http://www.ncbi.nlm.nih.gov/pubmed/25711320
Dickert N, Miller FG. Involving patients in enrolment decisions for acute myocardial infarction trials. British Medical Journal 2015; 351:h3791. http://www.ncbi.nlm.nih.gov/pubmed/26223782
Emanuel EJ, Joffe S, Grady C, Wendler D, Persad G. Clinical research: Should patients pay to play? Science Translational Medicine 2015; 7(298): 16. http://www.ncbi.nlm.nih.gov/pubmed/26223299
Emanuel EJ, Bedarida G, Macci K, Gabler NB, Rid A, Wendler D. Quantifying the risks of non-oncology phase I research in healthy volunteers: meta-analysis of phase I studies. British Medical Journal 2015; 350:h3271. http://www.ncbi.nlm.nih.gov/pubmed/26115663
Gelinas, L. Frames, Choice-Reversal, and Consent. Ethical Theory, Moral Practice 2015; 18:1049-1057.
Gliwa C, Yurkiewicz IR, Lehmann LS, Hull SC, Jones N, Berkman BE. Institutional review board perspectives on obligations to disclose genetic incidental findings to research participants. Genetics in Medicine 2015; e-pub November 19, 2015. http://www.ncbi.nlm.nih.gov/pubmed/26583685
Grady C, Nogues I, Wiener L, Wilfond BS, Wendler D. Adolescent research participants' descriptions of medical research. AJOB Empirical Research 2015; on line.
Grady, C. Enduring and Emerging Challenges of Informed Consent. New England Journal of Medicine 2015; 372(9): 855-862. http://www.ncbi.nlm.nih.gov/pubmed/26017840 Grady, C. Surgical Medicine: Imperfect and Extraordinary. Narrative Inquiry in Bioethics 2015; 5(1): 37-43. http://www.ncbi.nlm.nih.gov/pubmed/25981280
Grady C, Eckstein L, Berkman B, Brock D, Cook-Deegan R, Fullerton SM, Greely H, Hansson MG, Hull S, Kim S, Lo B, Pentz R, Rodriguez L, Weil C, Wilfond BS, Wendler D. Broad Consent for Research With biological Samples: Workshop Conclusions. AJOB 2015; 15(9): 34-42. http://www.ncbi.nlm.nih.gov/pubmed/26305750
Grady, C. Institutional Review Boards: Purpose and Challenges. Chest 2015; 148(5): 1-8. http://www.ncbi.nlm.nih.gov/pubmed/26042632
Johnson RA, Wendler D. Challenging the Sanctity of Donorism: Patient Tissue Providers as Payment-Worthy Contributors. Kennedy Institute of Ethics Journal 2015; 25(3): 291-333. http://www.ncbi.nlm.nih.gov/pubmed/26412739
Kantin H, Wendler D. Is There a Role for Assent or Dissent in Animal Research? Cambridge Quarterly 2015; 24(4): 459-472. http://www.ncbi.nlm.nih.gov/pubmed/26364780
Kaptchuk TJ, Miller FG. Placebo Effects in Medicine. New England Journal of Medicine 2015; 373: 8-9. http://www.ncbi.nlm.nih.gov/pubmed/26132938
Kim S YH, Karlawish J, Berkman. Ethics of Genetic and Biomarker Test Disclosures in Neurodegenerative Disease Prevention Trials. Neurology 2015; 84:1-7. http://www.ncbi.nlm.nih.gov/pubmed/25762713
Kim, SYH. ‘Human subjects research' as stigmatized activity: Implications for oversight reform (Commentary on Jonathan Baron's "Some fallacies of human-subjects protection, and some solutions") Cortex 2015Oct;71:417-9 http://www.ncbi.nlm.nih.gov/pubmed/26051212
Kim SYH, Wilson R, DeVries R, Kim HM, Holloway RG, Kieburtz K. "It is not guaranteed that you will benefit": True but misleading? Clinical Trials 2015; 12 (4): 424-431. http://www.ncbi.nlm.nih.gov/pubmed/25963311
Kim SY, Wilson R, De Vries R,Kim HM, Holloway RG, Kieburtz K. Could the High Prevalence of Therapeutic Misconception Partly Be a Measurement Problem? IRB Ethics 2015; 37(4):11-18 http://www.ncbi.nlm.nih.gov/pubmed/26331188
Kim SYH, Miller FG. Ethical complexities in standard of care randomized trials: A case study of morning versus nighttime dosing of blood pressure drugs. Clinical Trials 2015; Dec 12(6): 557-563. http://www.ncbi.nlm.nih.gov/pubmed/26400874
Kim SYH, Miller FG. Varieties of Standard-of-Care Treatment Randomized Trials – Ethical Implications. JAMA 2015; 313(9): 895-896. http://www.ncbi.nlm.nih.gov/pubmed/25591061
Kon AA, Davidson JE, Morrison W, Danis M, White DB. Shared Decision Making in ICUs: An American College of Critical Care Medicine and American Thoracic Society Policy Statement. Critical Care Medicine 2016 Jan;44(1):188-201 http://www.ncbi.nlm.nih.gov/pubmed/26509317 Lantos JD, Wendler D, Septimus E, Wahba S, Madigan R, Bliss G. Considerations in the evaluation and determination of minimal risk in pragmatic clinical trials. Clinical Trials 2015; 12(5): 485-493. http://www.ncbi.nlm.nih.gov/pubmed/26374686
LeWitt PA, Kim S. The pharmacodynamics of placebo. Neurology 2015; 84:766-767. http://www.ncbi.nlm.nih.gov/pubmed/25632090
Lomax GP, Hull SC, Isasi R. The DISCUSS Project: Revised Points to "Consider for the Derivation of Induced Pluripotent Stem Cell Lines From Previously Collected Research Specimens. Stem Cells Translational Medicine 2015; 4:1-7. http://www.ncbi.nlm.nih.gov/pubmed/25561681
Mandava A, Millum J, Berkman BE. When Should Genome Researchers Disclose Misattributed Parentage? Hastings Center Report 2015; 45: 1-9. http://www.ncbi.nlm.nih.gov/pubmed/25677868
Miller FG, Kim Scott YH. Personal Care in Learning Health Care Systems. Kennedy Institute of Ethics Journal 2015; 25(4): 419-435. http://www.ncbi.nlm.nih.gov/pubmed/26775880
Millum J, Sina B, Glass Roger. International Research Ethics Education. JAMA 2015; 313(5): 461-462. http://www.ncbi.nlm.nih.gov/pubmed/25647198
Millum J. Controlling Ebola Trials. American Journal of Bioethics 2015; 15(4): 36-37. http://www.ncbi.nlm.nih.gov/pubmed/25856597
Nachman S, Ahmad A, Amanullah F, Becerra MC, Botgros R, Brigden G, Browning R, Gardiner E, Hafner R, Hesseling A, How C, Jean-Philippe P, Lessem E, Makhene M, Mbelle N, Marais B, McIlleron H, McNeeley DF, Mendel C, Murray S, Navarro E, Anyalechi EG, Procalla AR, Powell C, Powell M, Rigaud M, Rouzier V, Samson P, Schaaf HS, Shah S, Starke J, Swaminathan S, Wobudeya E, Worrell C. Towards early inclusion of children in tuberculosis drugs trials: a consensus statement. Lancet Infectious Diseases 2015; 15(6): 711-720. http://www.ncbi.nlm.nih.gov/pubmed/25957923
Nayak RK, Wendler D, Miller FG, Kim SYH. Pragmatic Randomized Trials Without Standard Informed Consent? Annals of Internal Medicine 2015; 163(5): 356. http://www.ncbi.nlm.nih.gov/pubmed/26215125 Ollendorf DA, Sandhu AT, Pearson SD. Sacubitril-Valsartan for the Treatment of Heart Failure – Effectiveness and Value. JAMA Internal Medicine 2015; Online, December 21, 2015:1-3. http://www.ncbi.nlm.nih.gov/pubmed/26720832
Phillips J, Millum J. Valuing Stillbirths. Bioethics 2015; 29(6) 413-423. http://www.ncbi.nlm.nih.gov/pubmed/25395144
Phillips J, Wendler D. Clarifying substituted judgement: the endorsed life approach. BMJ 2015; 41: 723-730. http://www.ncbi.nlm.nih.gov/pubmed/25360029
Qureshi ZU, Millum J, Blencowe H, Kelley M, Fottrell E, Lawn JE, Costello A, Colbourn T. ‘A Silenced Cry: Should stillbirth be given greater priority on the global health agenda. BMJ 2015. 351: h4620. http://www.ncbi.nlm.nih.gov/pubmed/26400645
Rid A, Wesley R, Pavlick M, Maynard S, Roth K, Wendler D. Patients' priorities for treatment decision making during periods of incapacity: quantitative survey. Palliative and Supportive Care 2015; 13: 1165-1183. http://www.ncbi.nlm.nih.gov/pubmed/25360029
Shah SK, Wendler D, Danis M. Examining the ethics of clinical use of unproven interventions outside of clinical trials during the Ebola epidemic. Am J Bioeth. 2015;15(4):11-6. http://www.ncbi.nlm.nih.gov/pubmed/25856592
Shah NN, Wayne AS, Grady C, Fry T, Wendler D. Children as hematopoietic cell donors in research: when is it approvable? Bone Marrow Transplantation 2015; 50(1): 15-19. http://www.ncbi.nlm.nih.gov/pubmed/25330224
Shah, SK. Experimental Execution. 90 Washington Law Review 147. 2015.
Shah SK, Kasper K, Miller FG. A Narrative Review of the Empirical Evidence on Public Attitudes on Brain Death and Vital Organ Transplantation: The Need for Better Data to Inform Policy. Journal of Medical Ethics 2015; 41(4): 291-296. http://www.ncbi.nlm.nih.gov/pubmed/24769621
Shah SK. Piercing the Veil: The Limits of Brain Death as a Legal Fiction. 48 University of Michigan Journal of Law Reform 301. 2015
Sharp RR, Taylor HA, Brinich MA, Boyle MM, Cho M, Coors M, Danis M, Havard M, Magnus D, Wilfond B. Research Ethics Consultation: Ethical and Professional Practice Challenges and Recommendations. Academic Medicine 2015. http://www.ncbi.nlm.nih.gov/pubmed/25607942
Sharp D, Millum J. The Post-2015 Development Agenda: Keeping Our Focus on the Worst Off. Am. Journal of Tropical Medicine and Hygiene 2015; 92(6): 1087-1089. http://www.ncbi.nlm.nih.gov/pubmed/25846294
Simon RE, Pearson SD, Hur C, Chung RT. Tackling the hepatitis C cost problem: A test case for tomorrow's cures. Hepatology 2015; 62(5): 1334-1336. http://www.ncbi.nlm.nih.gov/pubmed/26359645
Tice JA, Kazi DS, Pearson SD. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Inhibitors for Treatment of High Cholesterol Levels – Effectiveness and Value. JAMA Internal Medicine 2015; 176(1): 107-108. http://www.ncbi.nlm.nih.gov/pubmed/26662572
Tomlinson T, DeVries R, Ryan K, Kim Hyungjin, Lehpamer N, Kim S. Research Letter: Moral Concerns and the Willingness to Donate to a Research Biobank. JAMA 2015; 13 (4): 417-419. http://www.ncbi.nlm.nih.gov/pubmed/25626040
Ulrich CM, Grady C. Cardiopulmonary Resuscitation for Ebola patients: Ethical considerations. Nursing Outlook 2015; 63(1): 16-18. http://www.ncbi.nlm.nih.gov/pubmed/25645475
Weiner L, Viola A, Wilfond BS, Wendler D, Grady C. Contrasting Views of Risk Perception and Influence of Financial Compensation Between Adolescent Research Participants and Their Parents. Journal of Empirical Research on Human Research Ethics 2015; 2015 Feb;10(1):49-58. http://www.ncbi.nlm.nih.gov/pubmed/25742666
Weissman JS, Westrich K, Hargraves JL, Pearson SD, Dubois R, Emond S, Olufajo OA. Translating comparative effectiveness research into Medicaid payment policy: views from medical and pharmacy directors. Journal of Comparative Effectiveness Research 2015; 4(2): 79-88. http://www.ncbi.nlm.nih.gov/pubmed/25825839
Wendler, D. "Targeted" Consent for Pragmatic Clinical Trials. Journal of General Internal Medicine 2015. Online 1-14-15.
Wendler DS, Rid A. Genetic research on biospecimens poses minimal risk. Trends in Genetics; 31(1): 11-15 http://www.ncbi.nlm.nih.gov/pubmed/25530152
Wendler DS, Shah SK. Involving Communities in Deciding What Benefits They Receive in Multinational Research. Journal of Medicine and Philosophy 2015; 40(5): 584-600. http://www.ncbi.nlm.nih.gov/pubmed/26224724
Wertheimer, A. The ethics of promulgating principles of research ethics: the problem of diversion effects. Journal of Law and the Biosciences 2015; 2015 Feb 1;2(1):2-32. http://www.ncbi.nlm.nih.gov/pubmed/25937934
Wherrett DK, Chiang JL, Delamater AM, DiMeglio LA, Gitelman SE, Gottlieb PA, Herold KC, Lovell DJ, Orchard TJ, Ryan CM, Schatz DA, Wendler DS, Greenbaum CJ, Type I Diabetes TrialNet Study Group. Defining Pathways for Development of Disease-Modifying Therapies in Children With Type I Diabetes: A Consensus Report. Diabetes Care 2015; 38: 1975-1985. http://www.ncbi.nlm.nih.gov/pubmed/26404927
White, A. Accelerating the paradigm shift toward inclusion of pregnant women in drug research: Ethical and regulatory considerations. Seminars in Perinatology 2015; 39(7): 537-540. http://www.ncbi.nlm.nih.gov/pubmed/26385413 White A, Danis M, Gillece J. Abuse survivor perspectives on trauma inquiry in obstetrical practice. Arch Women's Ment Health. 2015 E-pub July 21, 2015. http://www.ncbi.nlm.nih.gov/pubmed/26189448
Aramesh K. A Brief History of Biomedical Research Ethics in Iran: Conflict of Paradigms. Developing World Bioethics. 2014, Arp 11 [Epub ahead of print]. http://www.ncbi.nlm.nih.gov/pubmed/24720443
Bekker L, Slack C, Lee S, Shah S, Kapogiannis B. Ethical Issues in Adolescent HIV Research in Resource-Limited Countries. J Acquired Immune Deficiency Syndrome. January 1, 2014 2014;65(Supplement 1):S24-S28. http://www.ncbi.nlm.nih.gov/pubmed/24321980
Berkman B, Hull S. The "Right Not to Know" in the Genomic Era: Time to Break From Tradition? American Journal of Bioethics. 2014;14(3):28-31. http://www.ncbi.nlm.nih.gov/pubmed/24592837
Berkman B, Hull S, Eckstein L. The unintended implications of blurring the line between research and clinical care in a genomic age. Personalized Medicine. 2014;11(3):285-295. http://www.ncbi.nlm.nih.gov/pubmed/25506378
Bharti K, Rao M, Hull S, et al. Developing Cellular Therapies for Retinal Degererative Diseases. Investigative Ophthalmology & Visual Science. 2014;55(2):1191-1202. http://www.ncbi.nlm.nih.gov/pubmed/24573369
Brock D, Park J, Wendler D. Making Treatment Decision for Oneself: Weighing the Value. Hastings Center Report. March-April, 2014 2014;44(2):22-25. http://www.ncbi.nlm.nih.gov/pubmed/24634044
Colloca L. Emotional modulation of placebo analgesia. Pain. 2014;155(4):651. http://www.ncbi.nlm.nih.gov/pubmed/24447512
Colloca L, Grillon C. Understanding Placebo and Nocebo Responses for Pain Management. Curr Pain Headache Rep. 2014;2014(18). http://www.ncbi.nlm.nih.gov/pubmed/24771206
Colloca L, Jonas W, Killen J, Miller F, Shurtleff D. Re-evaluating the Placebo Effect in Medical Practice. Zeitschrift fur Psychologie. 2014;222(3):124-127.
Dal-Re R, Ndebele P, Higgs E, Sewankambo N, Wendler D. Protections for clinical trials in low and middle income countries need strengthening not weakening. BMJ. 2014;349. http://www.ncbi.nlm.nih.gov/pubmed/24996885
Danis M, Abernethy A, Zafar S, et al. A decision exercise to engage cancer patients and families in Deliberation about Medicare Coverage for advanced Cancer Care. BMC Health Services Research. 2014;14:315. http://www.ncbi.nlm.nih.gov/pubmed/25038783
DeGrazia D. Persons, Dolphins, and Human-Nonhuman Chimeras. American Journal of Bioethics. 2014;14(2):17-18. http://www.ncbi.nlm.nih.gov/pubmed/24521328
DeGrazia D. On the Moral Status of Infants and the Cognitively Disabled: A Reply to Jaworska and Tannenbaum. Ethics. April 2014 2014;124(3):543-556.
DeGrazia D. The Case for Moderate Gun Control. Kennedy Institute of Ethics Journal. 2014;24(1):1-25. http://www.ncbi.nlm.nih.gov/pubmed/24783322
DeGrazia D. Review of Jeremy Garnett (ed) The Ethics of Animal Research (Boston, MIT Press, 2012). Journal of Value Inquiry. 2014.
DeGrazia D. Handguns, Moral Rights, and Physical Security. Journal of Moral Philosophy. 2014;On line.
deSante J, Caplan A, Hippen B, Testa G, Lantos J. Was Sarah Murnaghan Treated Justly? Pediatrics. 2014;134(1):155-162. http://www.ncbi.nlm.nih.gov/pubmed/24918227
Eckstein L, Garrett J, Berkman B. A Framework for Analyzing the Ethics of Disclosing Genetic Research Findings. Journal of Law, Medicine and Ethics. 2014;42(2):190-207. http://www.ncbi.nlm.nih.gov/pubmed/25040383
Gliwa C, Pearson S. Evidentiary Rationales for the Choosing Wisely Top 5 Lists. JAMA. 2014;311(14):1443-1444. http://www.ncbi.nlm.nih.gov/pubmed/24715077
Grady C, Wiener L, Abdoler E, et al. Assent in Research: The Voices of Adolescents. Journal of Adolescent Health. 2014. http://www.ncbi.nlm.nih.gov/pubmed/24630932
Hull S, Berkman B. Grappling With Genomic Incidental Findings in the Clinical Realm. Chest. 2014;145(2):226-230. http://www.ncbi.nlm.nih.gov/pubmed/24493507
Hunter T, Siess F, Colloca L. Socially induced placebo analgesia: A comparison of a pre-recorded versus live face-to-face observation. European Journal of Pain. 2014;18:914-922. http://www.ncbi.nlm.nih.gov/pubmed/24347563
Isasi R, Andrews P, Baltz J, et al. Identifiability and Privacy in Pluripotent Stem Cell Research. Cell Stem Cell. April 3, 2014 2014;14:427-430. http://www.ncbi.nlm.nih.gov/pubmed/24702994
Joffe S, Wertheimer A. Determining Minimal Risk for Comparative Effectiveness Research. IRB Ethics. May-June 2014 2014;36(3):16-18. http://www.ncbi.nlm.nih.gov/pubmed/24946508
Johnson R, Danis M, Hafner-Eaton C. US state variation in autism insurance mandates: Balancing access and fairness. Autism. 2014. http://www.ncbi.nlm.nih.gov/pubmed/24789870
Kim S, DeVries R, Parnami S, et al. Are therapeutic motivation and having one's own doctor as researcher sources of therapeutic misconception? Journal of Medical Ethics. 2014. http://www.ncbi.nlm.nih.gov/pubmed/24855070
Kim SY, Miller F. Informed Consent for Pragmatic Trials - The Integrated Consent Model. New England Journal of Medicine. February 20, 2014 2014;370(8):769-772. http://www.ncbi.nlm.nih.gov/pubmed/24552326
King S, Dickert N, Miller F. Learning From FAME: The Need for Sham Controls in Trials of Stable Coronary Disease. JACC: Cardiovascular Interventions. 2014;7(3):342-344. http://www.ncbi.nlm.nih.gov/pubmed/24650413
Klinger R, Colloca L, Bingel U, Flor H. Placebo analgesia: Clinical applications. Pain. 2014;155:1055-1058. http://www.ncbi.nlm.nih.gov/pubmed/24333780
Knebel A, Sharpe V, Danis M, Toomey L, Knickerbocker D. Informing the Gestalt: An Ethical Framework for Allocating Scarce Federal Public Health and Medical Resources to States During Disasters. Disaster Medicine nd PUblic Health Preparedness. 2014;8:79-88. http://www.ncbi.nlm.nih.gov/pubmed/24612854
LeBlanc T, Back A, Danis M, Abernethy A. Electronic Health Records (EHRs) in the Oncology Clinic: How Clinician Interaction With EHRs Can Improve Communication With the Patient. Journal of Oncology Practice. 2014. http://www.ncbi.nlm.nih.gov/pubmed/25027025
Littler K, Millum J, Wassenaar D. The Global Forum for Bioethics in Research: Past, Present, and Future. South African Journal of Bioethics and Law. 2014;7(1):5-8.
Matar A, Garner S, Millum J, Sina B, Silverman H. Curricular Aspects of the Fogarty Bioethics International Training Programs. Journal of Empirical Research on Human Research Ethics. 2014;9(2):12-23. http://www.ncbi.nlm.nih.gov/pubmed/24782069
Miller F. Clinical Research before Informed Consent. Kennedy Institute of Ethics Journal. June 2014 2014;24(2):141-157. http://www.ncbi.nlm.nih.gov/pubmed/25109093
Miller F. Heart Donation Without the Dead Donor Rule. Annals of Thoracic Surgery. 2014;97:1133-1134. http://www.ncbi.nlm.nih.gov/pubmed/24694403
Millum J. Consent Under Pressure: The Puzzle of Third Party Coercion. Ethical Theory and Moral Practice. 2014;17:113-127.
Millum J. The Foundation of the Child's Right to an Open Future. Journal of Social Philosophy. Winter, 2014 2014;45(4):522-538. http://www.ncbi.nlm.nih.gov/pubmed/25558116
Millum J, Sina B. Introduction: International Research Ethics Education. Journal of Empirical Research on Human Research Ethics. 2014;9(2):1-2. http://www.ncbi.nlm.nih.gov/pubmed/24782067
Nayak R, Pearson S. The Ethics of "Fail First": Guidelines and Practical Scenarios for Step Therapy Coverage Policies. Health Affairs. 2014;33(10):1779-1785. http://www.ncbi.nlm.nih.gov/pubmed/25288422
Nayak R, Pearson S, Miller F. Cost-Related Motivations for Conducting Research: Participants Should Be Informed. JAMA. 2014;311(15):1491-1492. http://www.ncbi.nlm.nih.gov/pubmed/24615527
Nayak R, Miller F. Cost-Related Motivations for Research—Reply. JAMA. 2014;312(8):847-848. http://www.ncbi.nlm.nih.gov/pubmed/25157736
Ollendorf D, Pearson S. Through the looking glass: making the design and output of economic models useful for setting medical policy. Journal of comparative Effectiveness Research. 2014 2014;3(1):53-61. http://www.ncbi.nlm.nih.gov/pubmed/24345257
Ollendorf D, Tice J, Pearson S. The Comparative Clinical Effectiveness and Value of Simeprevir and Sofosbuvir for Chronic Hepatitis C Virus Infection. JAMA Internal Medicine. 2014;174(7):1170-1171. http://www.ncbi.nlm.nih.gov/pubmed/24798321
Petersen G, Finnerup N, Colloca L, et al. The magnitude of nocebo effects in pain: A meta-analysis. Pain. 2014;155:1426-1434. http://www.ncbi.nlm.nih.gov/pubmed/24780622
Phillips J, Millum J. Valuing Stillbirths. Bioethics. 2014. http://www.ncbi.nlm.nih.gov/pubmed/25395144
Phillips J, Wendler D. Which Alternatives Should Investigators Disclose to Research Subjects? American Journal of Bioethics. 2014;14(4):54-55. http://www.ncbi.nlm.nih.gov/pubmed/24730499
Pike E, Rothenberg K, Berkman B. Finding Fault? Exploring Legal Duties to Return Incidental Findings in Genomic Research. The Georgetown Law Journal. 2014;102:795-843. http://www.ncbi.nlm.nih.gov/pubmed/25346543
Prasad V, Grady C. The misguided ethics of crossover trials. Contemporary Clinical Trials. 2014;37:167-169. http://www.ncbi.nlm.nih.gov/pubmed/24365533
Rid A, Abdoler E, Roberson-Nay R, Pine D, Wendler D. Evaluating the Risks of Clinical Research: Direct Comparative Analysis. Journal of Child and Adolescent Psychopharmacology. 2014;24(7):390-398. http://www.ncbi.nlm.nih.gov/pubmed/25210944
Rid A, Wendler D. Use of a Patient Preference Predictor to Help Make Medical Decisions for Incapacitated Patients. Journal of Medicine and Philosophy. 2014;39:104-129. http://www.ncbi.nlm.nih.gov/pubmed/24526785
Rid A, Wendler D. Treatment Decision Making for Incapacitated Patients: Is Development and Use of a Patient Preference Predictor Feasible? Journal of Medicine and Philosophy. 2014;39:130-152. http://www.ncbi.nlm.nih.gov/pubmed/24556152
Rulli T, Millum J. Rescuing the duty to rescue. Journal of Medical Ethics. 2014. http://www.ncbi.nlm.nih.gov/pubmed/24790055
Shah SK, Persaud D, Wendler D, Taylor H, Gay H, Kruger M, Grady C.. Research into a functional cure for HIV in neonates: the need for ethical foresight. Lancet Infectious Diseases. 2014;14(9):893-898. http://www.ncbi.nlm.nih.gov/pubmed/24906850
Shah S, Persaud D, Wendler DS, Taylor HA, Gay H, Kruger M, Grady C. Research on very early ART in neonates at risk of HIV infection. The Lancet. 2014;14(9):797. http://www.ncbi.nlm.nih.gov/pubmed/25164194
Sharp D, Palmore T, Grady C. The Ethics of Empowering Patients as Partners in Healthcare-Associated Infection Prevention. Infection Control and Hospital Epidemiology. 2014;35(3):307-309. http://www.ncbi.nlm.nih.gov/pubmed/24521598
Simmons K, Ortiz R, Kossowsky J, Krummenacher P, Grillon C, Pine D, Colloca L. Pain and placebo in pediatrics: A comprehensive review of laboratory and clinical findings. Pain. 2014;155(11):2229-2235. http://www.ncbi.nlm.nih.gov/pubmed/25180010
Truog R, Miller F. Defining death: the importance of scientific candor and transparency. Intensive Care Medicine. 2014;(40)6:885-887. http://www.ncbi.nlm.nih.gov/pubmed/24807081
Ulrich C, Qiuping Z, Hanlon A, Danis M, Grady C. The impact of ethics and work-related factors on nurse practitioners' and physician assistants' views on quality of primary healthcare in the United States. Applied Nursing Research. 2014;27(3):152-156. http://www.ncbi.nlm.nih.gov/pubmed/24613597
Wasserman D, Wertheimer A. In Defense of Bunkering. American Journal of Bioethics. 2014;14(9):42-43. http://www.ncbi.nlm.nih.gov/pubmed/25127277
Wendler D. Should protections for research with humans who cannot consent apply to research with nonhuman primates? Theoretical Medicine and Bioethics. 2014;35(2):157-173. http://www.ncbi.nlm.nih.gov/pubmed/24647873
Wendler D. Justice and Nontherapeutic Pediatric Research. The American Journal of Bioethics. 2014;14(9):13-15. http://www.ncbi.nlm.nih.gov/pubmed/25127265
Wendler D, Miller F. The ethics of peer review in bioethics. Journal of Medical Ethics. 2014 Oct;40(10):697-701. http://www.ncbi.nlm.nih.gov/pubmed/24131903
Wertheimer A. Against Autonomy? Journal of Medical Ethics. 2014;40(5):351-352. http://www.ncbi.nlm.nih.gov/pubmed/24345997
Wertheimer A. Non-completion and informed consent. Journal of Medical Ethics. 2014;40(2):127-130. http://www.ncbi.nlm.nih.gov/pubmed/23371314
Wertheimer A. (Why) should we require consent to participation in research? Journal of Law and the Biosciences 2014.
White A. Responding to Prenatal Disclosure of Past Sexual Abuse. Obstetrics and Gynecology. 2014;123(6):1344 - 1347. http://www.ncbi.nlm.nih.gov/pubmed/24807334
Yeung S, Colagiuri B, Lovibond P, Colloca L. Partial reinforcement, extinction, and placebo analgesia. Pain. 2014;155:1110-1117. http://www.ncbi.nlm.nih.gov/pubmed/24602997
Abdul-Karim R, Berkman BE, Wendler D, et al. Disclosure of incidental findings from next-generation sequencing in pediatric genomic research. Pediatrics. 2013;131(3):564-571. http://www.ncbi.nlm.nih.gov/pubmed/23400601
Blehar MC, Spong C, Grady C, Goldkind SF, Sahin L, Clayton JA. Enrolling Pregnant Women: Issues in Clinical Research. Women's Health Issues. 2013;23(1):e39-e45. http://www.ncbi.nlm.nih.gov/pubmed/23312713
Bravo G, Kim SY, Dubois MF, Cohen CA, Wildeman SM, Graham JE. Surrogate consent for dementia research: factors influencing five stakeholder groups from the SCORES study. IRB. Jul-Aug 2013;35(4):1-11. http://www.ncbi.nlm.nih.gov/pubmed/23926856
Bravo G, Wildeman S, Dubois MF, Kim SY, Cohen C, Graham J, Painter K. Substitute consent practices in the face of uncertainty: a survey of Canadian researchers in aging. Int Psychogeriatr. Aug 8 2013:1-10. http://www.ncbi.nlm.nih.gov/pubmed/23927951
Brim R, Miller F. The potential benefit of the placebo effect in sham-controlled trials: implications for risk-benefit assessments and informed consent. Journal of Medical Ethics. 2013;39:703-707. http://www.ncbi.nlm.nih.gov/pubmed/23239742
Brim R, Pearson SD. The use and reporting of patient-reported outcomes in phase III breast cancer trials. Clinical Trials. 2013;10:243-249. http://www.ncbi.nlm.nih.gov/pubmed/23539108
Brody H, Colloca L. Patient autonomy and provider beneficence are compatible. Hastings Cent Rep. November-December 2013 2013;43(6):6. http://www.ncbi.nlm.nih.gov/pubmed/24249463
Brody H, Miller F. The Research-Clinical Practice Distinction, Learning Health Systems, and Relationships. Hastings Center Report. 2013;43(5):41-47. http://www.ncbi.nlm.nih.gov/pubmed/24092591
Colloca L, Klinger R, Flor H, Bingel U. Placebo analgesia: psychological and neurobiological mechanisms. Pain. Apr 2013;154(4):511-514. http://www.ncbi.nlm.nih.gov/pubmed/23473783
Danis M, Solomon M. Providers, Payers, The Community, And Patients Are All Obliged To Get Patient Activation And Engagement Ethically Right. Health Affairs. 2013;32(2):1-7. http://www.ncbi.nlm.nih.gov/pubmed/23381534
Danis M, Sommers R, Logan J, et al. Exploring Public Attitudes Towards Approaches to Discussing Costs in the Clinical Encounter. J Gen Intern Med. Jul 24 2013. http://www.ncbi.nlm.nih.gov/pubmed/23881272
Domingues D, Jawara M, Martino N, Sinaii N, Grady C. Commonly performed procedures in clinical research: A benchmark for payment. Contemporary Clinical Trials. 2013;33(1):3-4. http://www.ncbi.nlm.nih.gov/pubmed/22580210
Gliwa C, Berkman BE. Do researchers have an obligation to actively look for genetic incidental findings? Am J Bioeth. 2013;13(2):32-42. http://www.ncbi.nlm.nih.gov/pubmed/23391059
Gliwa C, Berkman BE. Response to open peer commentaries on "do researchers have an obligation to actively look for genetic incidental findings?". Am J Bioeth. 2013;13(5):W10-11. http://www.ncbi.nlm.nih.gov/pubmed/23557061
Grady C. Reflections on two decades of bioethics: where we have been and where we are going. Am J Bioeth. 2013;13(1):8-10. http://www.ncbi.nlm.nih.gov/pubmed/23311831
Grady C, Wendler D. Making the transition to a learning health care system. Commentary. Hastings Cent Rep. Jan-Feb 2013;Spec No:S32-33. http://www.ncbi.nlm.nih.gov/pubmed/23315892
Hull S, Colloca L, Avins A, et al. Patients' attitudes about the use of placebo treatments: telephone survey. BMJ. 2013. http://www.ncbi.nlm.nih.gov/pubmed/23819963
Johnson RA, Barrett MS, Sisti DA. The Ethical Boundaries of Patient and Advocate Influence on DSM-5. Harv Rev Psychiatry. Nov-Dec 2013;21(6):334-344. http://www.ncbi.nlm.nih.gov/pubmed/24201823
Johnson RA, Block LF, Danis M. Optimizing the Involvement of Language Interpreters During the Clinical Encounter. J Gen Intern Med. Sep 10 2013. http://www.ncbi.nlm.nih.gov/pubmed/24018627
Kodish E, Fins JJ, Braddock C, 3rd, et al. Quality attestation for clinical ethics consultants: a two-step model from the american society for bioethics and humanities. Hastings Cent Rep. Sep-Oct 2013;43(5):26-36. http://www.ncbi.nlm.nih.gov/pubmed/24092588
Largent E, Grady C, Miller F, Wertheimer A. Misconceptions About Coercion and Undue Influence: Reflections On The Views of IRB Members. Bioethics. 2013;27(9):500-507. http://www.ncbi.nlm.nih.gov/pubmed/22493972
Largent EA, Miller FG, Joffe S. A prescription for ethical learning. Commentary. Hastings Cent Rep. Jan-Feb 2013;Spec No:S28-29. http://www.ncbi.nlm.nih.gov/pubmed/23315890
Lieber SR, Millum J. Preventing sin: the ethics of vaccines against smoking. Hastings Cent Rep. May-Jun 2013;43(3):23-33. http://www.ncbi.nlm.nih.gov/pubmed/23650065
Lindsey JC, Shah SK, Siberry GK, Jean-Philippe P, Levin MJ. Ethical Tradeoffs in Trial Design: Case Study of an HPV Vaccine Trial in HIV-Infected Adolescent Girls in Lower Income Settings. Dev World Bioeth. 2013;13(2):95-104. http://www.ncbi.nlm.nih.gov/pubmed/23725055
Lo B, Grady C. Ethical considerations in HIV cure research: points to consider. Curr Opin HIV AIDS. 2013;8(3):243-249. http://www.ncbi.nlm.nih.gov/pubmed/23422260
Lomax G, Hull S, Lowenthal J, Rao M, Isasi R. The DISCUSS Project: Induced Pluripotent Stem Cell Lines From Previously Collected Research Biospecimens and Informed Consent: Points to Consider. Stem Cells Translational Medicine. 2013;2(10):727-730. http://www.ncbi.nlm.nih.gov/pubmed/23990574
Lowenthal J, Hull S. Framing the "Right to Withdraw" in the Use of Biospecimens for IPSc Research. Ethics in Biology, Engineering & Medicine. 2013;4(1):1-14.
Mandava A, Millum J. Manipulation in the enrollment of research participants. Hastings Cent Rep. 2013;43(2):38-47. http://www.ncbi.nlm.nih.gov/pubmed/23390007
Miller F. Two Philosophical Deaths: Hume and Hitchens. Perspectives in Biology and Medicine. 2013;56(2):251-258. http://www.ncbi.nlm.nih.gov/pubmed/23974505
Miller F, Colloca L, Crouch RA, Kaptchuk TJ. The Placebo. Baltimore: The Johns Hopkins University Press; 2013.
Miller F, Joffe S. Phase 1 oncology trials and informed consent. Journal of Medical Ethics. 2013;39:761-764. http://www.ncbi.nlm.nih.gov/pubmed/23161617
Miller FG, Gelinas L. Nudging, autonomy, and valid consent: context matters. Am J Bioeth. 2013;13(6):12-13. http://www.ncbi.nlm.nih.gov/pubmed/23641836
Millum J, Grady C. The ethics of placebo-controlled trials: Methodological justifications. Contemp Clin Trials. Sep 12 2013;36(2):510-514. http://www.ncbi.nlm.nih.gov/pubmed/24035802
Millum J, Grady C, Keusch G, Sina B. Introduction: The Fogarty International Research Ethics Education and Curriculum Development Progtram in Historical Context. Journal of Empirical Research on Human Research Ethics. 2013;8(5):3-16. http://www.ncbi.nlm.nih.gov/pubmed/24384512
Millum J, Wendler D, Emanuel E. The 50th anniversary of the Declaration of Helsinki: Progress but many remaining challenges. JAMA. 2013;310(20):2143-2144. http://www.ncbi.nlm.nih.gov/pubmed/24141885
Schwartz J, Pearson S. Cost Consideration in the Clinical Guidance Documents of Physician Specialty Societies in the United States. JAMA Internal Medicine. 2013;173(12):1091-7. http://www.ncbi.nlm.nih.gov/pubmed/23649494
Shah S, Grady C. When to start ART in Africa. N Engl J Med. 2013;368(23):2238. http://www.ncbi.nlm.nih.gov/pubmed/23738562
Shah S, Lie RK. Aiming at a moving target: research ethics in the context of evolving standards of care and prevention. J Med Ethics. 2013;39(11):699-702. http://www.ncbi.nlm.nih.gov/pubmed/23322683
Shah S, Wolitz R, Emanuel E. Refocusing the responsiveness requirement. Bioethics. Mar 2013;27(3):151-159. http://www.ncbi.nlm.nih.gov/pubmed/21797911
Shah SK. Does Research with Children Violate the Best Interests Standard? An Empirical and Conceptual Analysis. Northwestern Journal of Law & Social Policy. 2013;8(2):121-173.
Shah SK. Outsourcing ethical obligations: should the revised common rule address the responsibilities of investigators and sponsors? J Law Med Ethics. Jun 2013;41(2):397-410. http://www.ncbi.nlm.nih.gov/pubmed/23802893
Shah SK, Hull SC, Spinner MA, Berkman BE, Sanchez LA, Abdul-Karim R, Hsu AP, Claypool R, Holland SM. What does the duty to warn require? Am J Bioeth. Oct 2013;13(10):62-63. http://www.ncbi.nlm.nih.gov/pubmed/24024817
Sharp D, Wasserman D. Compatibilism and a Political Conception of Autonomy. AJOB Neuroscience. 2013;4(4):55-56.
Sinnott-Armstrong W, Miller FG. What makes killing wrong? J Med Ethics. Jan 2013;39(1):3-7. http://www.ncbi.nlm.nih.gov/pubmed/22267342
Sommers R, Miller F. Forgoing Debriefing in Deceptive Research: Is It Ever Ethical? Ethics and Behavior. 2013;23(2):98-116.
Sprung C, Danis M, Iapichino G, Artigas A, Kesecioglu J, Moreno R, Lippert A, Curtis JR, Meale P, Cohen SL, Levy MM, Truog RD. Triage of intensive care patients: identifying agreement and controversy. Intensive Care Medicine. 2013;39(11):1916-24. http://www.ncbi.nlm.nih.gov/pubmed/23925544
Tilburt J, Wynia M, Sheeler R, Thorsteinsdottir B, James KM, Egginton JS, Liebow M, Hurst S, Danis M, Goold SD. Views of US Physicians About Controlling Health Care Costs. JAMA. 2013;310(4):380-388. http://www.ncbi.nlm.nih.gov/pubmed/23917288
Truog R, Miller F, Halpern S. The Dead-Donor Rule and the Future of Organ Donation. New England Journal of Medicine. 2013;369:1287-1289. http://www.ncbi.nlm.nih.gov/pubmed/24088088
Wendler D. What Should Be Disclosed to Research Participants? American Journal of Bioethics. 2013;13(12):3-8. http://www.ncbi.nlm.nih.gov/pubmed/24256522
Wendler D. Do U.S. Regulations Allow More than Minor Increase over Minimal Risk Pediatric Research? Should They? IRB Ethics. 2013;35(6):1-8. http://www.ncbi.nlm.nih.gov/pubmed/24437000
Wendler D. Broad versus Blanket Consent for Research with Human Biological Samples. Hastings Center Report. September-October 2013 2013;43(5):3-4. http://www.ncbi.nlm.nih.gov/pubmed/24092578
Wendler D. Problems with the Consensus Definition of the Therapeutic Misconception. Journal of Clinical Ethics. 2013;24(4):387-394. http://www.ncbi.nlm.nih.gov/pubmed/24597427
Wertheimer A. Should 'nudge' be salvaged? J Med Ethics. Aug 2013;39(8):498-499. http://www.ncbi.nlm.nih.gov/pubmed/23455011
Wertheimer A. Reply: George Fletcher on Blackmail. In: Christopher R, ed. Fletcher's Essays on Criminal Law. New York: Oxford University Press; 2013:250-253.
Wertheimer A, Miller FG. There are (STILL) no coercive offers. J Med Ethics. May 23 2013. http://www.ncbi.nlm.nih.gov/pubmed/23704780
White A, Danis M. Enhancing patient-centered communication and collaboration by using the electronic health record in the examination room. JAMA. Jun 12 2013;309(22):2327-2328. http://www.ncbi.nlm.nih.gov/pubmed/23757080
White A, Danis M. Patient-physician interactions and electronic health records--reply. JAMA. 2013;310(17):1857-1858. http://www.ncbi.nlm.nih.gov/pubmed/24193089
Abdoler E, Wendler D. Using data to improve surrogate consent for clinical research with incapacitated adults. Journal of empirical research on human research ethics : JERHRE. 2012;7(2):37-50. Epub 2012/05/09. http://www.ncbi.nlm.nih.gov/pubmed/22565582
Berkman BE, Rothenberg KH. Teaching health law. The Journal of law, medicine & ethics : a journal of the American Society of Law, Medicine & Ethics. 2012;40(1):147-53. Epub 2012/03/31. http://www.ncbi.nlm.nih.gov/pubmed/22458470
Brake E, Millum J. Parenthood and Procreation. In: Zalta EN, editor.: The Metaphysics Research Lab, Stanford University; 2012.
Brake E, Millum J. Parenthood and Procreation. Stanford Encyclopedia of Philosophy. Stanford, CA: The Metaphysics Research Lab Center for the Study of Language and Information; 2012.
Brody H, Colloca L, Miller FG. The placebo phenomenon: implications for the ethics of shared decision-making. Journal of general internal medicine. 2012;27(6):739-42. Epub 2012/01/20. http://www.ncbi.nlm.nih.gov/pubmed/22258918
Bush LW, Rothenberg KH. Dialogues, dilemmas, and disclosures: genomic research and incidental findings. Genetics in medicine : official journal of the American College of Medical Genetics. 2012;14(3):293-5. Epub 2012/03/07. http://www.ncbi.nlm.nih.gov/pubmed/22391780
Bush LW, Rothenberg KH. It's Not That Simple! Genomic Research & The Consent Process First in a Series of ELSI Plays and Dramatic Vignettes. Genetics in Medicine. 2012;14:on line supplement www.nature.com/gim .
Bush LW, Rothenberg KH. It's So Complicated! Genomic Research & Disclosure of Incidental Findings Dramatic Vignettes from a Series of ELSI Plays. Genetics in Medicine. 2012;14:on line supplement www.nature.com/gim .
Carson SS, Vu M, Danis M, Camhi SL, Scheunemann LP, Cox CE, et al. Development and validation of a printed information brochure for families of chronically critically ill patients. Critical care medicine. 2012;40(1):73-8. Epub 2011/09/20. http://www.ncbi.nlm.nih.gov/pubmed/21926610
Chan B, Facio FM, Eidem H, Hull SC, Biesecker LG, Berkman BE. Genomic inheritances: disclosing individual research results from whole-exome sequencing to deceased participants' relatives. The American journal of bioethics : AJOB. 2012;12(10):1-8. Epub 2012/09/15. http://www.ncbi.nlm.nih.gov/pubmed/22974017
Colloca L, Finniss D. Nocebo effects, patient-clinician communication, and therapeutic outcomes. JAMA. 2012;307(6):567-8. Epub 2012/02/10. http://www.ncbi.nlm.nih.gov/pubmed/22318275
Danis M, Pesce J. Prospects for acknowledging and addressing the socioeconomic determinants of health in the United States: A response to Goldberg. Social Science and Medicine. 2012:1143-5.
Dominguez D, Jawara M, Martino N, Sinaii N, Grady C. Commonly performed procedures in clinical research: a benchmark for payment. Contemporary clinical trials. 2012;33(5):860-8. Epub 2012/05/15. http://www.ncbi.nlm.nih.gov/pubmed/22580210
Donley G, Hull SC, Berkman BE. Prenatal whole genome sequencing: just because we can, should we? The Hastings Center report. 2012;42(4):28-40. Epub 2012/07/11. http://www.ncbi.nlm.nih.gov/pubmed/22777977
Emanuel EJ, Pearson SD. It Costs More, but Is It Worth More? New York Times. 2012 1/3/12;Sect. Opinionator on line.
Emanuel EJ, Pearson SD. Physician autonomy and health care reform. JAMA. 2012;307(4):367-8. Epub 2012/01/26. http://www.ncbi.nlm.nih.gov/pubmed/22274681
Enama ME, Hu Z, Gordon I, Costner P, Ledgerwood JE, Grady C. Randomization to standard and concise informed consent forms: development of evidence-based consent practices. Contemporary clinical trials. 2012;33(5):895-902. Epub 2012/05/01. http://www.ncbi.nlm.nih.gov/pubmed/22542645
Evans-Lacko SE, Baum N, Danis M, Biddle A, Goold S. Laypersons' choices and deliberations for mental health coverage. Administration and policy in mental health. 2012;39(3):158-69. Epub 2011/04/01. http://www.ncbi.nlm.nih.gov/pubmed/21452017
Friedman A, Robbins E, Wendler D. Which benefits of research participation count as 'direct'? Bioethics. 2012;26(2):60-7. Epub 2010/05/26. http://www.ncbi.nlm.nih.gov/pubmed/20497168
Grady C, Rubinstein YR, Groft SC. Informed consent and patient registry for the rare disease community: Editorial. Contemporary clinical trials. 2012;33(1):3-4. Epub 2011/11/01. http://www.ncbi.nlm.nih.gov/pubmed/22036667
Hughes RC. Individual risk and community benefit in international research. Journal of medical ethics. 2012;38(10):626-9. Epub 2012/05/09. http://www.ncbi.nlm.nih.gov/pubmed/22562945
Hull SC, Chan B, Biesecker LG, Berkman BE. Response to open peer commentaries on "Genomic inheritances: disclosing individual research results from whole-exome sequencing to deceased participants' relatives". The American journal of bioethics : AJOB. 2012;12(12):W9-10. Epub 2012/12/12. http://www.ncbi.nlm.nih.gov/pubmed/23215942
Isasi R, Knoppers BM, Andrews PW, Bredenoord A, Colman A, Hin LE, et al. Disclosure and management of research findings in stem cell research and banking: policy statement. Regenerative medicine. 2012;7(3):439-48. Epub 2012/05/19. http://www.ncbi.nlm.nih.gov/pubmed/22594334
Joffe S, Miller FG. Equipoise: asking the right questions for clinical trial design. Nature reviews Clinical oncology. 2012;9(4):230-5. Epub 2012/01/11. http://www.ncbi.nlm.nih.gov/pubmed/22231753
Kelly B, Rid A, Wendler D. Systematic review: Individuals' goals for surrogate decision-making. Journal of the American Geriatrics Society. 2012;60(5):884-95. Epub 2012/04/04. http://www.ncbi.nlm.nih.gov/pubmed/22469395
Kotwani N, Abdul-Karim R, Danis M. At the Fontlines: Confronting Poverty in Primary Care Medicine. In: Capelli O, editor. Primary Care at a Glance - Hot Topics and New Insights. Rijeka, Croatia: In Tech; 2012. p. 3-25.
Largent E, Grady C, Miller FG, Wertheimer A. Misconceptions About Coercion and Undue Influence: Reflections on the Views of Irb Members. Bioethics. 2012. Epub 2012/04/13. http://www.ncbi.nlm.nih.gov/pubmed/22493972
Largent EA, Grady C, Miller FG, Wertheimer A. Money, coercion, and undue inducement: attitudes about payments to research participants. Irb. 2012;34(1):1-8. Epub 2012/02/18. http://www.ncbi.nlm.nih.gov/pubmed/22338401
Levine D, Cohen K, Wendler D. Shared medical decision-making: considering what options to present based on an ethical analysis of the treatment of brain tumors in very young children. Pediatric blood & cancer. 2012;59(2):216-20. Epub 2012/04/24. http://www.ncbi.nlm.nih.gov/pubmed/22522647
Lowenthal J, Hull SC, Pearson SD. The ethics of early evidence--preparing for a possible breakthrough in Alzheimer's disease. The New England journal of medicine. 2012;367(6):488-90. Epub 2012/08/10. http://www.ncbi.nlm.nih.gov/pubmed/22873528
Lowenthal J, Lipnick S, Rao M, Hull SC. Specimen collection for induced pluripotent stem cell research: harmonizing the approach to informed consent. Stem cells translational medicine. 2012;1(5):409-21. Epub 2012/12/01. http://www.ncbi.nlm.nih.gov/pubmed/23197820
Mackay D. Standard of Care, Professional Obligations, and Distributive Justice. Bioethics. 2012. Epub 2012/09/18. http://www.ncbi.nlm.nih.gov/pubmed/22978692
Mandava A, Pace C, Campbell B, Emanuel E, Grady C. The quality of informed consent: mapping the landscape. A review of empirical data from developing and developed countries. Journal of medical ethics. 2012;38(6):356-65. Epub 2012/02/09. http://www.ncbi.nlm.nih.gov/pubmed/22313664
Miller FG. Clinical equipoise and risk-benefit assessment. Clin Trials. 2012;9(5):621-7. Epub 2012/07/11. http://www.ncbi.nlm.nih.gov/pubmed/22777654
Miller FG. Homage to Henry Beecher (1904-1976). Perspectives in biology and medicine. 2012;55(2):218-29. Epub 2012/05/31. http://www.ncbi.nlm.nih.gov/pubmed/22643759
Miller FG. Is anything lost if we give up clinical equipoise? Clin Trials. 2012;9(5):632-3. Epub 2012/10/13. http://www.ncbi.nlm.nih.gov/pubmed/23060322
Miller FG. Research and complicity: the case of Julius Hallervorden. Journal of medical ethics. 2012;38(1):53-6. Epub 2011/06/23. http://www.ncbi.nlm.nih.gov/pubmed/21693567
Miller FG. The enduring legacy of sham-controlled trials of internal mammary artery ligation. Progress in cardiovascular diseases. 2012;55(3):246-50. Epub 2012/12/12. http://www.ncbi.nlm.nih.gov/pubmed/23217427
Millum J, Skolnik R. Ethical and Human Rights Concerns in Global Health. In: Skolnik R, editor. Global Health 101. 2nd ed: Jones & Bartlet Publishers; 2012. p. 71-86.
Millum J. Canada's new ethical guidelines for research with humans: a critique and comparison with the United States. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2012;184(6):657-61. Epub 2012/01/18. http://www.ncbi.nlm.nih.gov/pubmed/22249987
Millum J. Introduction: case studies in the ethics of mental health research. The Journal of nervous and mental disease. 2012;200(3):230-5. Epub 2012/03/01. http://www.ncbi.nlm.nih.gov/pubmed/22373760
Millum J. Review of N. Richards, The Ethics of Parenthood. Philosophy in Review. 2012;32(2):130-2.
Millum J. Sharing the benefits of research fairly: two approaches. Journal of medical ethics. 2012;38(4):219-23. Epub 2011/09/29. http://www.ncbi.nlm.nih.gov/pubmed/21947808
Pearson SD. Cost, coverage, and comparative effectiveness research: the critical issues for oncology. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(34):4275-81. Epub 2012/10/17. http://www.ncbi.nlm.nih.gov/pubmed/23071229
Rebouche R, Rothenberg KH. Mixed Messages: The Intersection of Prenatal Genetic Testing and Abortion. Howard Law Journal. 2012;55:983-1023.
Rhodes KV, Miller FG. Simulated patient studies: an ethical analysis. The Milbank quarterly. 2012;90(4):706-24. Epub 2012/12/12. http://www.ncbi.nlm.nih.gov/pubmed/23216428
Rothenberg KH, Bush LW. Genes and plays: bringing ELSI issues to life. Genetics in medicine : official journal of the American College of Medical Genetics. 2012;14(2):274-7. Epub 2012/01/14. http://www.ncbi.nlm.nih.gov/pubmed/22241098
Rothenberg KH, Bush LW. Manipulating Fate: Medical Innovations, Ethical Implications, Theatrical Illuminations. Houston Journal of Health Law & Policy. 2012;13.
Rubinstein YR, Groft SC, Chandros SH, Kaneshiro J, Karp B, Lockhart NC, et al. Informed consent process for patient participation in rare disease registries linked to biorepositories. Contemporary clinical trials. 2012;33(1):5-11. Epub 2011/11/01. http://www.ncbi.nlm.nih.gov/pubmed/22036955
Rulli T, Emanuel EJ, Wendler D. The moral duty to buy health insurance. JAMA. 2012;308(2):137-8. Epub 2012/07/12. http://www.ncbi.nlm.nih.gov/pubmed/22782412
Rulli T, Wendler D. Letter of Response to William Smith on "The Moral Duty to Buy Health Insurance". JAMA. 2012;308(16):1628-9.
Schaefer GO, Sinaii N, Grady C. Informing egg donors of the potential for embryonic research: a survey of consent forms from U.S. in vitro fertilization clinics. Fertility and sterility. 2012;97(2):427-33. Epub 2011/12/27. http://www.ncbi.nlm.nih.gov/pubmed/22196714
Solomon BD, Hadley DW, Pineda-Alvarez DE, Kamat A, Teer JK, Cherukuri PF, et al. Incidental medical information in whole-exome sequencing. Pediatrics. 2012;129(6):e1605-11. Epub 2012/05/16. http://www.ncbi.nlm.nih.gov/pubmed/22585771
Solomon BD, Hadley DW, Pineda-Alvarez DE, Program NCS, Kamat A, Teer JK, et al. Incidental Medical Information in Whole-Exome Sequencing. Pediatrics. 2012;129(6):1605-11.
Strech D, Danis M. How can bedside rationing be justified despite coexisting inefficiency? The need for 'benchmarks of efficiency'. Journal of medical ethics. 2012. Epub 2012/12/22. http://www.ncbi.nlm.nih.gov/pubmed/23258082
Ulrich CM, Grady C. Perceptions of Appropriateness of Care in the Intensive Care Unit. JAMA. 2012;307(13):1370-1.
Ulrich CM, Knafl KA, Ratcliffe SJ, Richmond TS, Grady C, Miller-Davis C, et al. Developing a Model of the Benefits and Burdens fo Research Participation in Cancer clinical Trials. American Journal of Bioethics Primary Research. 2012;3(2):10-23.
Ulrich CM, Zhou Q, Ratcliffe SJ, Ye L, Grady C, Watkins-Bruner D. Nurse Practitioners' attitudes about cancer clinical trials and willingness to recommend research participation. Contemporary clinical trials. 2012;33(1):76-84. Epub 2011/10/11. http://www.ncbi.nlm.nih.gov/pubmed/21983623
Wendler D, Abdoler E, Wiener L, Grady C. Views of adolescents and parents on pediatric research without the potential for clinical benefit. Pediatrics. 2012;130(4):692-9. Epub 2012/09/12. http://www.ncbi.nlm.nih.gov/pubmed/22966027
Wendler D. A new justification for pediatric research without the potential for clinical benefit. The American journal of bioethics : AJOB. 2012;12(1):23-31. Epub 2012/01/10. http://www.ncbi.nlm.nih.gov/pubmed/22220955
Wendler D. Consent for research with biological samples: one-time general consent versus a gift model. Annals of internal medicine. 2012;156(8):596-8. Epub 2012/04/18. http://www.ncbi.nlm.nih.gov/pubmed/22508735
Wendler D. The ethics of studying subjects in non-ideal circumstances. Tobacco control. 2012;21(4):385-6. Epub 2012/06/26. http://www.ncbi.nlm.nih.gov/pubmed/22730459
Wendler DS. Time to stop worrying about the therapeutic misconception. The Journal of clinical ethics. 2012;23(3):272-87. Epub 2012/12/22. http://www.ncbi.nlm.nih.gov/pubmed/23256408
Wertheimer A. Voluntary consent: why a value-neutral concept won't work. The Journal of medicine and philosophy. 2012;37(3):226-54. Epub 2012/05/04. http://www.ncbi.nlm.nih.gov/pubmed/22551878
Wiener L, Crum C, Grady C, Merchant M. To friend or not to friend: the use of social media in clinical oncology. Journal of oncology practice / American Society of Clinical Oncology. 2012;8(2):103-6. Epub 2012/10/19. http://www.ncbi.nlm.nih.gov/pubmed/23077437
Abbott L, Grady C. A systematic review of the empirical literature evaluating IRBs: what we know and what we still need to learn. Journal of empirical research on human research ethics : JERHRE. 2011;6(1):3-19. Epub 2011/04/05. http://www.ncbi.nlm.nih.gov/pubmed/21460582
Berkman BE, Kim SC, Wiley LF. Assessing the Impact of Federal Law on Public Health Preparedness. St Louis University Journal of Health Law and Policy. 2011;4(1):155-86.
Colloca L, Miller FG. Harnessing the placebo effect: the need for translational research. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2011;366(1572):1922-30. Epub 2011/05/18. http://www.ncbi.nlm.nih.gov/pubmed/21576150
Colloca L, Miller FG. How placebo responses are formed: a learning perspective. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2011;366(1572):1859-69. Epub 2011/05/18. http://www.ncbi.nlm.nih.gov/pubmed/21576143
Colloca L, Miller FG. Role of expectations in health. Current opinion in psychiatry. 2011;24(2):149-55. Epub 2011/01/21. http://www.ncbi.nlm.nih.gov/pubmed/21248640
Colloca L, Miller FG. The nocebo effect and its relevance for clinical practice. Psychosomatic medicine. 2011;73(7):598-603. Epub 2011/08/25. http://www.ncbi.nlm.nih.gov/pubmed/21862825
Colloca L. Learned placebo analgesia in sequential trials: What are the Pros and Cons? Pain. 2011;152(6):1215-6. Epub 2011/03/08. http://www.ncbi.nlm.nih.gov/pubmed/21377800
Danis M. The Swill Supreme court's thoughtful decision regarding insurance of expensive medications for rare diseases. Bioethica Forum. 2011;4(3):118-9.
Donley G, Danis M. Making the case for talking to patients about the costs of end-of-life care. The Journal of law, medicine & ethics : a journal of the American Society of Law, Medicine & Ethics. 2011;39(2):183-93. Epub 2011/05/13. http://www.ncbi.nlm.nih.gov/pubmed/21561513
Emanuel EJ, Menikoff J. Reforming the regulations governing research with human subjects. The New England journal of medicine. 2011;365(12):1145-50. Epub 2011/07/27. http://www.ncbi.nlm.nih.gov/pubmed/21787202
Fleischman A, Levine C, Eckenwiler L, Grady C, Hammerschmidt DE, Sugarman J. Dealing with the long-term social implications of research. The American journal of bioethics : AJOB. 2011;11(5):5-9. Epub 2011/05/03. http://www.ncbi.nlm.nih.gov/pubmed/21534138
Foulkes MA, Grady C, Spong CY, Bates A, Clayton JA. Clinical research enrolling pregnant women: a workshop summary. J Womens Health (Larchmt). 2011;20(10):1429-32. Epub 2011/08/09. http://www.ncbi.nlm.nih.gov/pubmed/21819233
Goehler A, Ollendorf DA, Jaeger M, Ladapo J, Neumann T, Gazelle GS, et al. A simulation model of clinical and economic outcomes of cardiac CT triage of patients with acute chest pain in the emergency department. AJR American journal of roentgenology. 2011;196(4):853-61. Epub 2011/03/24. http://www.ncbi.nlm.nih.gov/pubmed/21427336
Goldenberg AJ, Hull SC, Wilfond BS, Sharp RR. Patient perspectives on group benefits and harms in genetic research. Public health genomics. 2011;14(3):135-42. Epub 2010/10/13. http://www.ncbi.nlm.nih.gov/pubmed/20938159
Gronowski AM, Moye J, Jr., Wendler DS, Caplan AL, Christman M. The use of human tissues in research: what do we owe the research subjects? Clinical chemistry. 2011;57(4):540-4. Epub 2011/01/06. http://www.ncbi.nlm.nih.gov/pubmed/21205881
Higgins A, Zeddies T, Pearson S. Lessons learned from a multi-payer p8ilot to measure individual physician performance. Health Affairs. 2011;30(4):673-81.
Hrobjartsson A, Kaptchuk TJ, Miller FG. Placebo effect studies are susceptible to response bias and to other types of biases. Journal of clinical epidemiology. 2011;64(11):1223-9. Epub 2011/04/29. http://www.ncbi.nlm.nih.gov/pubmed/21524568
Lantos J, Matlock AM, Wendler D. Clinician integrity and limits to patient autonomy. JAMA. 2011;305(5):495-9. Epub 2011/02/03. http://www.ncbi.nlm.nih.gov/pubmed/21285427
Largent EA, Joffe S, Miller FG. Can research and care be ethically integrated? The Hastings Center report. 2011;41(4):37-46. Epub 2011/08/19. http://www.ncbi.nlm.nih.gov/pubmed/21845922
Lepora C, Millum J. The tortured patient: A medical dilemma. The Hastings Center report. 2011;41(3):38-47. Epub 2011/06/18. http://www.ncbi.nlm.nih.gov/pubmed/21678814
Lev O. Will biomedical enhancements undermine solidarity, responsibility, equality and autonomy? Bioethics. 2011;25(4):177-84. Epub 2009/12/17. http://www.ncbi.nlm.nih.gov/pubmed/20002073
Lie RK, Miller FG. What counts as reliable evidence for public health policy: the case of circumcision for preventing HIV infection. BMC medical research methodology. 2011;11:34. Epub 2011/04/02. http://www.ncbi.nlm.nih.gov/pubmed/21453535
Meissner K, Kohls N, Colloca L. Introduction to placebo effects in medicine: mechanisms and clinical implications. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2011;366(1572):1783-9. Epub 2011/05/18. http://www.ncbi.nlm.nih.gov/pubmed/21576135
Meissner K. The placebo effect and the autonomic nervous system: evidence for an intimate relationship. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2011;366(1572):1808-17. Epub 2011/05/18. http://www.ncbi.nlm.nih.gov/pubmed/21576138
Miller FG, Brody H. Understanding and harnessing placebo effects: clearing away the underbrush. The Journal of medicine and philosophy. 2011;36(1):69-78. Epub 2011/01/12. http://www.ncbi.nlm.nih.gov/pubmed/21220523
Miller FG, Colloca L. The placebo phenomenon and medical ethics: rethinking the relationship between informed consent and risk-benefit assessment. Theoretical medicine and bioethics. 2011;32(4):229-43. Epub 2011/04/12. http://www.ncbi.nlm.nih.gov/pubmed/21479794
Miller FG, Joffe S. Balancing access and evaluation in the approval of new cancer drugs. JAMA. 2011;305(22):2345-6. Epub 2011/06/07. http://www.ncbi.nlm.nih.gov/pubmed/21642688
Miller FG, Joffe S. Equipoise and the dilemma of randomized clinical trials. The New England journal of medicine. 2011;364(5):476-80. Epub 2011/02/04. http://www.ncbi.nlm.nih.gov/pubmed/21288100
Miller FG, Kallmes DF, Buchbinder R. Vertebroplasty and the placebo response. Radiology. 2011;259(3):621-5. Epub 2011/05/24. http://www.ncbi.nlm.nih.gov/pubmed/21602500
Miller FG, Pearson SD. Linking insurance coverage for innovative invasive procedures with participation in clinical research. JAMA. 2011;306(18):2024-5. Epub 2011/11/10. http://www.ncbi.nlm.nih.gov/pubmed/22068996
Miller FG, Wertheimer A. The fair transaction model of informed consent: an alternative to autonomous authorization. Kennedy Institute of Ethics journal. 2011;21(3):201-18. Epub 2011/11/12. http://www.ncbi.nlm.nih.gov/pubmed/22073815
Miller FG. Dispensing with equipoise. The American journal of the medical sciences. 2011;342(4):276-81. Epub 2011/08/02. http://www.ncbi.nlm.nih.gov/pubmed/21804362
Millum J. Post-trial access to antiretrovirals: who owes what to whom? Bioethics. 2011;25(3):145-54. Epub 2009/07/15. http://www.ncbi.nlm.nih.gov/pubmed/19594728
Ollendorf DA, Kuba M, Pearson SD. The diagnostic performance of multi-slice coronary computed tomographic angiography: a systematic review. Journal of general internal medicine. 2011;26(3):307-16. Epub 2010/11/11. http://www.ncbi.nlm.nih.gov/pubmed/21063800
Pesce JE, Kpaduwa CS, Danis M. Deliberation to enhance awareness of and prioritize socioeconomic interventions for health. Soc Sci Med. 2011;72(5):789-97. Epub 2011/02/15. http://www.ncbi.nlm.nih.gov/pubmed/21316832
Polanco FR, Dominguez DC, Grady C, Stoll P, Ramos C, Mican JM, et al. Conducting HIV research in racial and ethnic minority communities: building a successful interdisciplinary research team. The Journal of the Association of Nurses in AIDS Care : JANAC. 2011;22(5):388-96. Epub 2011/02/01. http://www.ncbi.nlm.nih.gov/pubmed/21277228
Rid A, Wendler D. A framework for risk-benefit evaluations in biomedical research. Kennedy Institute of Ethics journal. 2011;21(2):141-79. Epub 2011/06/24. http://www.ncbi.nlm.nih.gov/pubmed/21696094
Rid A, Wendler D. A proposal and prototype for a Research Risk Repository to improve the protection of research participants. Clin Trials. 2011;8(6):705-15. Epub 2011/08/24. http://www.ncbi.nlm.nih.gov/pubmed/21859783
Rivera-Goba MV, Dominguez DC, Stoll P, Grady C, Ramos C, Mican JM. Exploring decision-making of HIV-infected Hispanics and African Americans participating in clinical trials. The Journal of the Association of Nurses in AIDS Care : JANAC. 2011;22(4):295-306. Epub 2011/01/25. http://www.ncbi.nlm.nih.gov/pubmed/21256054
Shah SK, Dawson L, Dixon DO, Lie RK. Should sponsors and DSMBs share interim results across trials? Journal of acquired immune deficiency syndromes. 2011;58(5):433-5. Epub 2011/09/23. http://www.ncbi.nlm.nih.gov/pubmed/21937922
Shah SK, Truog RD, Miller FG. Death and legal fictions. Journal of medical ethics. 2011;37(12):719-22. Epub 2011/08/04. http://www.ncbi.nlm.nih.gov/pubmed/21810923
Shah SK. The Dangers of Using a Relative Standard for Minimal Risk. American Journal of Bioethics. 2011;11(6):22-3.
Smith W, Grady C, Krohmal B, Lazovski J, Wendler D. Empirical evaluation of the need for 'on-going consent' in clinical research. AIDS. 2011;25(1):107-14. Epub 2010/11/16. http://www.ncbi.nlm.nih.gov/pubmed/21076272
Stunkel L, Grady C. More than the money: a review of the literature examining healthy volunteer motivations. Contemporary clinical trials. 2011;32(3):342-52. Epub 2010/12/15. http://www.ncbi.nlm.nih.gov/pubmed/21146635
Wendler D, Rid A. Systematic review: the effect on surrogates of making treatment decisions for others. Annals of internal medicine. 2011;154(5):336-46. Epub 2011/03/02. http://www.ncbi.nlm.nih.gov/pubmed/21357911
Wendler D. How to enroll participants in research ethically. JAMA. 2011;305(15):1587-8. Epub 2011/04/21. http://www.ncbi.nlm.nih.gov/pubmed/21505137
Wendler D. What we worry about when we worry about the ethics of clinical research. Theoretical medicine and bioethics. 2011;32(3):161-80. Epub 2011/03/15. http://www.ncbi.nlm.nih.gov/pubmed/21400219
Wulff KC, Miller FG, Pearson SD. Can coverage be rescinded when negative trial results threaten a popular procedure? The ongoing saga of vertebroplasty. Health Aff (Millwood). 2011;30(12):2269-76. Epub 2011/12/08. http://www.ncbi.nlm.nih.gov/pubmed/22147854
Adikes KA, Hull SC, Danis M. The views of low-income employees regarding mandated comprehensive employee benefits for the sake of health. Soc Work Public Health. 2010;25(1):102-23. Epub 2010/04/15. http://www.ncbi.nlm.nih.gov/pubmed/20391255
Borgerson K, Millum J. A third way: ethics guidance as evidence-informed provisional rules. The American journal of bioethics : AJOB. 2010;10(6):20-2. Epub 2010/06/09. http://www.ncbi.nlm.nih.gov/pubmed/20526963
Chan B, Wendler D. International Guidelines and Ethical Context. AJOB. 2010;1(4):28-30.
Christian MD, Joynt GM, Hick JL, Colvin J, Danis M, Sprung CL. Chapter 7. Critical care triage. Recommendations and standard operating procedures for intensive care unit and hospital preparations for an influenza epidemic or mass disaster. Intensive care medicine. 2010;36 Suppl 1:S55-64. Epub 2010/03/23. http://www.ncbi.nlm.nih.gov/pubmed/20213422
Danis M, Ginsburg M, Goold S. Experience in the United States with public deliberation about health insurance benefits using the small group decision exercise, CHAT. The Journal of ambulatory care management. 2010;33(3):205-14. Epub 2010/06/12. http://www.ncbi.nlm.nih.gov/pubmed/20539147
Danis M, Kotwani N, Garrett J, Rivera I, Davies-Cole J, Carter-Nolan P. Priorities of low-income urban residents for interventions to address the socio-economic determinants of health. Journal of health care for the poor and underserved. 2010;21(4):1318-39. Epub 2010/11/26. http://www.ncbi.nlm.nih.gov/pubmed/21099082
Eichler HG, Bloechl-Daum B, Abadie E, Barnett D, Konig F, Pearson S. Relative efficacy of drugs: an emerging issue between regulatory agencies and third-party payers. Nature reviews Drug discovery. 2010;9(4):277-91. Epub 2010/02/27. http://www.ncbi.nlm.nih.gov/pubmed/20186141
Ellis RD, Sagara I, Durbin A, Dicko A, Shaffer D, Miller L, et al. Comparing the understanding of subjects receiving a candidate malaria vaccine in the United States and Mali. The American journal of tropical medicine and hygiene. 2010;83(4):868-72. Epub 2010/10/05. http://www.ncbi.nlm.nih.gov/pubmed/20889881
Emanuel EJ, Abdoler E, Stunkel L. Research Ethics: How to Treat People who Participate in Research. [Brochure for teaching purposes]. In press 2010.
Figg WD, Smith EK, Price DK, English BC, Thurman PW, Steinberg SM, et al. Disclosing a diagnosis of cancer: where and how does it occur? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(22):3630-5. Epub 2010/07/08. http://www.ncbi.nlm.nih.gov/pubmed/20606078
Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet. 2010;375(9715):686-95. Epub 2010/02/23. http://www.ncbi.nlm.nih.gov/pubmed/20171404
Grady C. Do IRBs protect human research participants? JAMA. 2010;304(10):1122-3. Epub 2010/09/09. http://www.ncbi.nlm.nih.gov/pubmed/20823440
Henderson DK, Dembry L, Fishman NO, Grady C, Lundstrom T, Palmore TN, et al. SHEA guideline for management of healthcare workers who are infected with hepatitis B virus, hepatitis C virus, and/or human immunodeficiency virus. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2010;31(3):203-32. Epub 2010/01/22. http://www.ncbi.nlm.nih.gov/pubmed/20088696
Heyd D, Miller F. Life Plans: do they give meaning to our lives. Monist. 2010;93(1):17-37.
Joynt GM, Loo S, Taylor BL, Margalit G, Christian MD, Sandrock C, et al. Chapter 3. Coordination and collaboration with interface units. Recommendations and standard operating procedures for intensive care unit and hospital preparations for an influenza epidemic or mass disaster. Intensive care medicine. 2010;36 Suppl 1:S21-31. Epub 2010/03/23. http://www.ncbi.nlm.nih.gov/pubmed/20213418
Kotwani N, Danis M. Expanding the Current Health Care Reform Debate: Making the Case for Socio-Econo mic Interventions for Low Income Young Adults. Journal of health care law & policy. 2010;12:17-45.
Largent E. Comments and Opinions: Off Label Letters to the Editor. 2010.
Largent E. Letter to the editor with author response: commentary on "restarting the clock... again". Dimensions of critical care nursing : DCCN. 2010;29(2):103-5; author reply 5. Epub 2010/02/18. http://www.ncbi.nlm.nih.gov/pubmed/20160554
Largent EA, Wendler D, Emanuel E, Miller FG. Is emergency research without initial consent justified?: the consent substitute model. Archives of internal medicine. 2010;170(8):668-74. Epub 2010/04/28. http://www.ncbi.nlm.nih.gov/pubmed/20421549
Lev O, Miller FG, Emanuel EJ. The ethics of research on enhancement interventions. Kennedy Institute of Ethics journal. 2010;20(2):101-13. Epub 2010/07/27. http://www.ncbi.nlm.nih.gov/pubmed/20653248
Lie RK, Millum J. Asian Bioethics: Breaking New Ground. Asian Bioethics Review. 2010;2(3):171-2.
Lie RK. The fair benefits approach revisited. The Hastings Center report. 2010;40(4):3. Epub 2010/07/31. http://www.ncbi.nlm.nih.gov/pubmed/20669771
Litton P, Miller FG. What physician-investigators owe patients who participate in research. JAMA. 2010;304(13):1491-2. Epub 2010/10/07. http://www.ncbi.nlm.nih.gov/pubmed/20924018
Miller FG, Colloca L. Semiotics and the placebo effect. Perspectives in biology and medicine. 2010;53(4):509-16. Epub 2010/11/03. http://www.ncbi.nlm.nih.gov/pubmed/21037405
Miller FG, Truog RD, Brock DW. Moral fictions and medical ethics. Bioethics. 2010;24(9):453-60. Epub 2009/07/15. http://www.ncbi.nlm.nih.gov/pubmed/19594726
Miller FG, Truog RD. Decapitation and the definition of death. Journal of medical ethics. 2010;36(10):632-4. Epub 2010/07/24. http://www.ncbi.nlm.nih.gov/pubmed/20650913
Miller FG, Wertheimer A. The Ethics of Consent. New York: Oxford University Press; 2010. 416 p.
Millum J, Menikoff J. Streamlining ethical review. Annals of internal medicine. 2010;153(10):655-7. Epub 2010/11/17. http://www.ncbi.nlm.nih.gov/pubmed/21079221
Millum J. How should the benefits of bioprospecting be shared? The Hastings Center report. 2010;40(1):24-33. Epub 2010/02/20. http://www.ncbi.nlm.nih.gov/pubmed/20169653
Ollendorf DA, Pearson SD. An integrated evidence rating to frame comparative effectiveness assessments for decision makers. Medical care. 2010;48(6 Suppl):S145-52. Epub 2010/05/18. http://www.ncbi.nlm.nih.gov/pubmed/20473206
Orszag PR, Emanuel EJ. Health care reform and cost control. The New England journal of medicine. 2010;363(7):601-3. Epub 2010/06/18. http://www.ncbi.nlm.nih.gov/pubmed/20554975
Pearson SD, Bach PB. How Medicare could use comparative effectiveness research in deciding on new coverage and reimbursement. Health Aff (Millwood). 2010;29(10):1796-804. Epub 2010/10/06. http://www.ncbi.nlm.nih.gov/pubmed/20921478
Resnik DB, Miller F. The ethics of sham surgery on research subjects with cognitive impairments that affect decision-making capacity. Contemporary clinical trials. 2010;31(5):407-10. Epub 2010/06/24. http://www.ncbi.nlm.nih.gov/pubmed/20570755
Rid A, Emanuel EJ, Wendler D. Evaluating the risks of clinical research. JAMA. 2010;304(13):1472-9. Epub 2010/10/07. http://www.ncbi.nlm.nih.gov/pubmed/20924013
Rid A, Schmidt H. The 2008 Declaration of Helsinki - first among equals in research ethics? The Journal of law, medicine & ethics : a journal of the American Society of Law, Medicine & Ethics. 2010;38(1):143-8. Epub 2010/05/08. http://www.ncbi.nlm.nih.gov/pubmed/20446992
Rid A, Wendler D. Can we improve treatment decision-making for incapacitated patients? The Hastings Center report. 2010;40(5):36-45. Epub 2010/10/23. http://www.ncbi.nlm.nih.gov/pubmed/20964162
Rid A, Wendler D. Risk-benefit assessment in medical research - critical review and open questions. Law, Probability and Risk. 2010;9(3-4):151-77.
Rid A. Faire Allokationsprozesse: zum Verhaltnis von substantieller und prozeduraler Gerechtigkeit in Norman Daniels' Just Health. In: Strech D, Marckmann G, editors. Public Health Ethik. Munster: Verlag; 2010. p. 159-74.
Rid A. Review of "Joachim Boos, Reinhard Merkel, Heiner Raspe, Bettina Schone-Seifert (Hrsg)(2009) Nutzen und Schaden asu klinischer Forschung am Menschen. Abwagung, Equipoise und normative Grundlagen". Ethik in der Medizin. 2010;22(2):167-8.
Rubinstein YR, Groft SC, Bartek R, Brown K, Christensen RA, Collier E, et al. Creating a global rare disease patient registry linked to a rare diseases biorepository database: Rare Disease-HUB (RD-HUB). Contemporary clinical trials. 2010;31(5):394-404. Epub 2010/07/09. http://www.ncbi.nlm.nih.gov/pubmed/20609392
Sachs B. The exceptional ethics of the investigator-subject relationship. The Journal of medicine and philosophy. 2010;35(1):64-80. Epub 2009/12/23. http://www.ncbi.nlm.nih.gov/pubmed/20026526
Saenz C. Virtue ethics and the selection of children with impairments: a reply to Rosalind McDougall. Bioethics. 2010;24(9):499-506. Epub 2009/06/11. http://www.ncbi.nlm.nih.gov/pubmed/19508307
Sapp JC, Hull SC, Duffer S, Zornetzer S, Sutton E, Marteau TM, et al. Ambivalence toward undergoing invasive prenatal testing: an exploration of its origins. Prenatal diagnosis. 2010;30(1):77-82. Epub 2009/11/20. http://www.ncbi.nlm.nih.gov/pubmed/19924734
Schaefer GO, Wertheimer A. The right to withdraw from research. Kennedy Institute of Ethics journal. 2010;20(4):329-52. Epub 2011/02/23. http://www.ncbi.nlm.nih.gov/pubmed/21338028
Shah S, Grady C. Shah and Grady Respond to Onyeabor. Am Journal of Public Health. 2010;100(6):967.
Shah S, Wendler D. Interpretation of the subjects' condition requirement: a legal perspective. The Journal of law, medicine & ethics : a journal of the American Society of Law, Medicine & Ethics. 2010;38(2):365-73. Epub 2010/06/29. http://www.ncbi.nlm.nih.gov/pubmed/20579233
Shah S, Zettler P. From a constitutional right to a policy of exceptions: Abigail Alliance and the future of access to experimental therapy. Yale journal of health policy, law, and ethics. 2010;10(1):135-96. Epub 2010/03/17. http://www.ncbi.nlm.nih.gov/pubmed/20229846
Shah SK, Miller FG. Can we handle the truth? Legal fictions in the determination of death. American journal of law & medicine. 2010;36(4):540-85. Epub 2011/02/10. http://www.ncbi.nlm.nih.gov/pubmed/21302846
Strech D, Hurst S, Danis M. The role of ethics committees and ethics consultation in allocation decisions: a 4-stage process. Medical care. 2010;48(9):821-6. Epub 2010/08/14. http://www.ncbi.nlm.nih.gov/pubmed/20706163
Stunkel L, Benson M, McLellan L, Sinaii N, Bedarida G, Emanuel E, et al. Comprehension and informed consent: assessing the effect of a short consent form. Irb. 2010;32(4):1-9. Epub 2010/09/22. http://www.ncbi.nlm.nih.gov/pubmed/20853797
Tilburt JC, Miller FG, Jenkins S, Kaptchuk TJ, Clarridge B, Bolcic-Jankovic D, et al. Factors that influence practitioners' interpretations of evidence from alternative medicine trials: a factorial vignette experiment embedded in a national survey. Medical care. 2010;48(4):341-8. Epub 2010/04/01. http://www.ncbi.nlm.nih.gov/pubmed/20355265
Tunis SR, Pearson SD. US moves to improve health decisions. BMJ. 2010;341:c3615. Epub 2010/08/25. http://www.ncbi.nlm.nih.gov/pubmed/20732966
Ulrich CM, Hamric AB, Grady C. Moral distress: a growing problem in the health professions? The Hastings Center report. 2010;40(1):20-2. Epub 2010/02/20. http://www.ncbi.nlm.nih.gov/pubmed/20166512
Ulrich CM, Taylor C, Soeken K, O'Donnell P, Farrar A, Danis M, et al. Everyday ethics: ethical issues and stress in nursing practice. Journal of advanced nursing. 2010;66(11):2510-9. Epub 2010/08/26. http://www.ncbi.nlm.nih.gov/pubmed/20735502
Wendler D, Abdoler E. Does it matter whether investigators intend to benefit research subjects? Kennedy Institute of Ethics journal. 2010;20(4):353-70. Epub 2011/02/23. http://www.ncbi.nlm.nih.gov/pubmed/21338029
Wendler D. Are physicians obligated always to act in the patient's best interests? Journal of medical ethics. 2010;36(2):66-70. Epub 2010/02/06. http://www.ncbi.nlm.nih.gov/pubmed/20133397
Wendler D. The Ethics of Pediatric Research. New York: Oxford University Press; 2010. 337 p.
Wertheimer A, Millum J, Schaefer GO. Why adopt a maximin theory of exploitation? The American journal of bioethics : AJOB. 2010;10(6):38-9. Epub 2010/06/09. http://www.ncbi.nlm.nih.gov/pubmed/20526968
Abdoler E. NIH Ethics Education Programs and Initiatives: Training the Next Generation of Clinical and Translational Researchers. Virtual Mentor. 2009;11(4):291-6. Epub 2009/01/01. http://www.ncbi.nlm.nih.gov/pubmed/23195061
Astor A, Lie RK. Physician Migration. In: Mordini E, editor. Ethics and Health in the Global Village: Bioethics, Globalization and Human Rightsq. Roma: CIC Edizioni Internazionali; 2009. p. 259-68.
Berkman BE. Incorporating explicit ethical reasoning into pandemic influenza policies. The Journal of contemporary health law and policy. 2009;26(1):1-19. Epub 2010/02/02. http://www.ncbi.nlm.nih.gov/pubmed/20112616
Beskow LM, Grady C, Iltis AS, Sadler JZ, Wilfond BS. Points to consider: The research ethics consultation service and the IRB. Irb. 2009;31(6):1-9. Epub 2009/12/26. http://www.ncbi.nlm.nih.gov/pubmed/20034184
Caldicott CV, Danis M. Medical ethics contributes to clinical management: teaching medical students to engage patients as moral agents. Medical education. 2009;43(3):283-9. Epub 2009/03/03. http://www.ncbi.nlm.nih.gov/pubmed/19250356
Curlin FA, Rasinski KA, Kaptchuk TJ, Emanuel EJ, Miller FG, Tilburt JC. Religion, clinicians, and the integration of complementary and alternative medicines. J Altern Complement Med. 2009;15(9):987-94. Epub 2009/09/18. http://www.ncbi.nlm.nih.gov/pubmed/19757976
Danis M, Hurst SA. Developing the capacity of ethics consultants to promote just resource allocation. The American journal of bioethics : AJOB. 2009;9(4):37-9. Epub 2009/03/28. http://www.ncbi.nlm.nih.gov/pubmed/19326310
Dickert N, Wendler D. Ancillary care obligations of medical researchers. JAMA. 2009;302(4):424-8. Epub 2009/07/23. http://www.ncbi.nlm.nih.gov/pubmed/19622821
Fitzhugh CD, Hsieh MM, Bolan CD, Saenz C, Tisdale JF. Granulocyte colony-stimulating factor (G-CSF) administration in individuals with sickle cell disease: time for a moratorium? Cytotherapy. 2009;11(4):464-71. Epub 2009/06/11. http://www.ncbi.nlm.nih.gov/pubmed/19513902
Fojo T, Grady C. How much is life worth: cetuximab, non-small cell lung cancer, and the $440 billion question. Journal of the National Cancer Institute. 2009;101(15):1044-8. Epub 2009/07/01. http://www.ncbi.nlm.nih.gov/pubmed/19564563
Goldenberg AJ, Hull SC, Botkin JR, Wilfond BS. Pediatric biobanks: approaching informed consent for continuing research after children grow up. The Journal of pediatrics. 2009;155(4):578-83. Epub 2009/07/15. http://www.ncbi.nlm.nih.gov/pubmed/19595370
Grady C, Edgerly M. Science, technology, and innovation: nursing responsibilities in clinical research. The Nursing clinics of North America. 2009;44(4):471-81. Epub 2009/10/24. http://www.ncbi.nlm.nih.gov/pubmed/19850183
Grady C. Vulnerability in research: individuals with limited financial and/or social resources. The Journal of law, medicine & ethics : a journal of the American Society of Law, Medicine & Ethics. 2009;37(1):19-27. Epub 2009/02/28. http://www.ncbi.nlm.nih.gov/pubmed/19245599
Hanchate A, Kronman AC, Young-Xu Y, Ash AS, Emanuel E. Racial and ethnic differences in end-of-life costs: why do minorities cost more than whites? Archives of internal medicine. 2009;169(5):493-501. Epub 2009/03/11. http://www.ncbi.nlm.nih.gov/pubmed/19273780
Hoffman A, Pearson SD. 'Marginal medicine': targeting comparative effectiveness research to reduce waste. Health Aff (Millwood). 2009;28(4):w710-8. Epub 2009/06/27. http://www.ncbi.nlm.nih.gov/pubmed/19556249
Kotwani N, Danis M. Expanding the Current Health Care Reform Debate: Making the Case for Socia-Economic Interventions for Low Income Young Adults. Journal of health care law & policy. 2009;12(1):17-45.
Koyfman SA, McCabe MS, Emanuel EJ, Grady C. A consent form template for phase I oncology trials. Irb. 2009;31(4):1-8. Epub 2009/08/25. http://www.ncbi.nlm.nih.gov/pubmed/19697538
Largent EA, Miller FG, Pearson SD. Going off-label without venturing off-course: evidence and ethical off-label prescribing. Archives of internal medicine. 2009;169(19):1745-7. Epub 2009/10/28. http://www.ncbi.nlm.nih.gov/pubmed/19858430
Lazovski J, Losso M, Krohmal B, Emanuel EJ, Grady C, Wendler D. Benefits and burdens of participation in a longitudinal clinical trial. Journal of empirical research on human research ethics : JERHRE. 2009;4(3):89-97. Epub 2009/09/17. http://www.ncbi.nlm.nih.gov/pubmed/19754238
Lepora C, Danis M, Wertheimer A. No exceptionalism needed to treat terrorists. The American journal of bioethics : AJOB. 2009;9(10):53-4. Epub 2009/12/10. http://www.ncbi.nlm.nih.gov/pubmed/19998090
Matsui K, Zeid AA, Xinqing Z, Krohmal B, Muthuswamy V, Koo YM, et al. Informed Consent to Future Research on Stored Tissue Samples: the Views of Researchers, Ethics Review Committee Members and Policy Makers in Five Non-Western Countries. Asian Bioethics Review. 2009;1(3):401-16.
Miller FG, Colloca L, Kaptchuk TJ. The placebo effect: illness and interpersonal healing. Perspectives in biology and medicine. 2009;52(4):518-39. Epub 2009/10/27. http://www.ncbi.nlm.nih.gov/pubmed/19855122
Miller FG, Colloca L. The legitimacy of placebo treatments in clinical practice: evidence and ethics. The American journal of bioethics : AJOB. 2009;9(12):39-47. Epub 2009/12/17. http://www.ncbi.nlm.nih.gov/pubmed/20013499
Miller FG, Joffe S. Limits to research risks. Journal of medical ethics. 2009;35(7):445-9. Epub 2009/07/02. http://www.ncbi.nlm.nih.gov/pubmed/19567696
Miller FG, Truog RD. The incoherence of determining death by neurological criteria: a commentary on "Controversies in the determination of death", a White Paper by the President's Council on Bioethics. Kennedy Institute of Ethics journal. 2009;19(2):185-93. Epub 2009/07/25. http://www.ncbi.nlm.nih.gov/pubmed/19623822
Miller FG, Wendler D. The ethics of sham invasive intervention trials. Clin Trials. 2009;6(5):401-2. Epub 2009/10/23. http://www.ncbi.nlm.nih.gov/pubmed/19846893
Miller FG. Death and organ donation: back to the future. Journal of medical ethics. 2009;35(10):616-20. Epub 2009/10/02. http://www.ncbi.nlm.nih.gov/pubmed/19793942
Miller FG. The randomized controlled trial as a demonstration project: an ethical perspective. The American journal of psychiatry. 2009;166(7):743-5. Epub 2009/07/03. http://www.ncbi.nlm.nih.gov/pubmed/19570933
Millum J. Post-Trial Access to Antiretrovirals: Who Owes What To Whom? Bioethics. 2009.
Nalugoda F, Wagman J, Kiddugavu M, Kiwanuka N, Garrett E, Gray RH, et al. Is there coercion or undue inducement to participate in health research in developing countries? An example from Rakai, Uganda. The Journal of clinical ethics. 2009;20(2):141-9. Epub 2009/06/27. http://www.ncbi.nlm.nih.gov/pubmed/19554819
O'Neil CC, Miller FG. When scientists deceive: applying the federal regulations. The Journal of law, medicine & ethics : a journal of the American Society of Law, Medicine & Ethics. 2009;37(2):344-50. Epub 2009/06/06. http://www.ncbi.nlm.nih.gov/pubmed/19493078
Pearson SD, Lieber SR. Financial penalties for the unhealthy? Ethical guidelines for holding employees responsible for their health. Health Aff (Millwood). 2009;28(3):845-52. Epub 2009/05/06. http://www.ncbi.nlm.nih.gov/pubmed/19414897
Pearson SD. From Better Evidence to Better Care: Using Comparative Effectiveness Research to Guide Practice and Policy. The Brookings Institution, 2009 2009. Report No.
Persad G, Wertheimer A, Emanuel EJ. Principles for allocation of scarce medical interventions. Lancet. 2009;373(9661):423-31. Epub 2009/02/03. http://www.ncbi.nlm.nih.gov/pubmed/19186274
Rid A, Bachmann LM, Wettstein V, Biller-Andorno N. Would you sell a kidney in a regulated kidney market? Results of an exploratory study. Journal of medical ethics. 2009;35(9):558-64. Epub 2009/09/01. http://www.ncbi.nlm.nih.gov/pubmed/19717695
Rid A, Biller-Andorno N. Justice in action? Introduction to the mini symposium on Norman Daniels' Just health: meeting health needs fairly. Journal of medical ethics. 2009;35(1):1-2. Epub 2008/12/24. http://www.ncbi.nlm.nih.gov/pubmed/19103933
Rid A, Dinhofer L. Consent. In: Warwick R, Fehily D, Brubaker SA, Eastlund T, editors. Tissue and Cell Donation: An Essential Guide. Malden, MA: Blackwell; 2009. p. 67-97.
Rid A, Schmidt H. [The newly revised Declaration of Helsinki: what do the changes mean from an ethical perspective?]. Dtsch Med Wochenschr. 2009;134(49):2525-8. Epub 2009/11/27. Die erneut uberarbeitete Deklaration von Helsinki. Wie sind die Anderungen aus ethischer Sicht zu beurteilen? http://www.ncbi.nlm.nih.gov/pubmed/19941238
Rid A. Justice and procedure: how does "accountability for reasonableness" result in fair limit-setting decisions? Journal of medical ethics. 2009;35(1):12-6. Epub 2008/12/24. http://www.ncbi.nlm.nih.gov/pubmed/19103936
Sachs B. Extortion and the ethics of "Topping Up". Cambridge quarterly of healthcare ethics : CQ : the international journal of healthcare ethics committees. 2009;18(4):443-5. Epub 2009/09/11. http://www.ncbi.nlm.nih.gov/pubmed/19739335
Saenz C. What is affordable health insurance? The reasonable tradeoff account of affordability. Kennedy Institute of Ethics journal. 2009;19(4):401-14. Epub 2010/03/03. http://www.ncbi.nlm.nih.gov/pubmed/20191951
Schaefer GO, Emanuel EJ, Wertheimer A. The obligation to participate in biomedical research. JAMA. 2009;302(1):67-72. Epub 2009/07/02. http://www.ncbi.nlm.nih.gov/pubmed/19567441
Schulz-Baldes A. How Political is the Future of Health Care? Allocating Scarce Resources in Liberal Democracy. In: Elm S, Willich SN, editors. Quo Vadis Medical Healing? Past Concepts and New Approaches. Berlin: Springer; 2009. p. 3-18.
Shah S, Elmer S, Grady C. Planning for posttrial access to antiretroviral treatment for research participants in developing countries. American journal of public health. 2009;99(9):1556-62. Epub 2009/07/18. http://www.ncbi.nlm.nih.gov/pubmed/19608940
Strech D, Danis M, Lob M, Marckmann G. [Extent and impact of bedside rationing in German hospitals: results of a representative survey among physicians]. Dtsch Med Wochenschr. 2009;134(24):1261-6. Epub 2009/06/06. Ausmass und Auswirkungen von Rationierung in deutschen Krankenhausern. Arztliche Einschatzungen aus einer reprasentativen Umfrage. http://www.ncbi.nlm.nih.gov/pubmed/19499496
Strech D, Persad G, Marckmann G, Danis M. Are physicians willing to ration health care? Conflicting findings in a systematic review of survey research. Health Policy. 2009;90(2-3):113-24. Epub 2008/12/17. http://www.ncbi.nlm.nih.gov/pubmed/19070396
Tilburt JC, Curlin FA, Kaptchuk TJ, Clarridge B, Bolcic-Jankovic D, Emanuel EJ, et al. Alternative medicine research in clinical practice: a US national survey. Archives of internal medicine. 2009;169(7):670-7. Epub 2009/04/15. http://www.ncbi.nlm.nih.gov/pubmed/19364996
Ulrich CM, Grady C. Doing Good with Limited Resources: Is It Good Enough in the Provision of Quality Care? Clinical Scholars Review. 2009;2(1):4-6.
Ulrich CM, Zhou Q, Grady C. Recommending Research Participation to Patients: An Ethical Imperative? Clinical Scholars Review. 2009;2(2):41-4.
Wendler D. Minimal risk in pediatric research as a function of age. Archives of pediatrics & adolescent medicine. 2009;163(2):115-8. Epub 2009/02/04. http://www.ncbi.nlm.nih.gov/pubmed/19188642
Wendler D. Must research participants understand randomization? The American journal of bioethics : AJOB. 2009;9(2):3-8. Epub 2009/01/31. http://www.ncbi.nlm.nih.gov/pubmed/19180378
Wolitz R, Emanuel E, Shah S. Rethinking the responsiveness requirement for international research. Lancet. 2009;374(9692):847-9. Epub 2009/09/08. http://www.ncbi.nlm.nih.gov/pubmed/19733781
Abdoler E, Taylor H, Wendler D. The ethics of phase 0 oncology trials. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(12):3692-7. Epub 2008/06/19. http://www.ncbi.nlm.nih.gov/pubmed/18559585
Applbaum AI, Tilburt JC, Collins MT, Wendler D. A family's request for complementary medicine after patient brain death. JAMA. 2008;299(18):2188-93. Epub 2008/05/15. http://www.ncbi.nlm.nih.gov/pubmed/18477786
Biller-Andorno N, Schaber P, Schulz-Baldes A. Gibt es eine universale Bioethik? Paderborn: Mentis; 2008.
Brendel DH, Miller FG. A plea for pragmatism in clinical research ethics. The American journal of bioethics : AJOB. 2008;8(4):24-31. Epub 2008/06/26. http://www.ncbi.nlm.nih.gov/pubmed/18576248
Brown AP, Wendler DS, Camphausen KA, Miller FG, Citrin D. Performing nondiagnostic research biopsies in irradiated tissue: a review of scientific, clinical, and ethical considerations. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(24):3987-94. Epub 2008/08/20. http://www.ncbi.nlm.nih.gov/pubmed/18711189
Brownlee S, Emanuel EJ. 5 Myths on Our Sick Health Care System. The Washington Post. 2008(B03). Epub Sunday.
Danis M, Farrar A, Grady C, Taylor C, O'Donnell P, Soeken K, et al. Does fear of retaliation deter requests for ethics consultation? Medicine, health care, and philosophy. 2008;11(1):27-34. Epub 2007/10/17. http://www.ncbi.nlm.nih.gov/pubmed/17939060
Daugherty CK, Ratain MJ, Emanuel EJ, Farrell AT, Schilsky RL. Ethical, scientific, and regulatory perspectives regarding the use of placebos in cancer clinical trials. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(8):1371-8. Epub 2008/01/30. http://www.ncbi.nlm.nih.gov/pubmed/18227527
Denny CC, Emanuel EJ. US health aid beyond PEPFAR: the Mother & Child Campaign. JAMA. 2008;300(17):2048-51. Epub 2008/11/06. http://www.ncbi.nlm.nih.gov/pubmed/18984893
Denny CC, Wilfond BS, Peters JA, Giri N, Alter BP. All in the family: disclosure of "unwanted" information to an adolescent to benefit a relative. American journal of medical genetics Part A. 2008;146A(21):2719-24. Epub 2008/10/03. http://www.ncbi.nlm.nih.gov/pubmed/18831063
Emanuel E, Grady C, Menikoff J. Is Longer Always Better. Hastings Center Report. 2008;38(3):10-2.
Emanuel E, Wyden R. A new federal-state partnership in health care: real power for states. JAMA. 2008;300(16):1931-4. Epub 2008/10/23. http://www.ncbi.nlm.nih.gov/pubmed/18940982
Emanuel E. The NIH and bioethics: what should be done? Academic medicine : journal of the Association of American Medical Colleges. 2008;83(6):529-31. Epub 2008/06/04. http://www.ncbi.nlm.nih.gov/pubmed/18520451
Emanuel EJ, Fuchs VR. The perfect storm of overutilization. JAMA. 2008;299(23):2789-91. Epub 2008/06/19. http://www.ncbi.nlm.nih.gov/pubmed/18560006
Emanuel EJ, Fuchs VR. Who really pays for health care? The Chicago Tribune. 2008 March 27, 2008.
Emanuel EJ, Fuchs VR. Who really pays for health care? The myth of "shared responsibility". JAMA. 2008;299(9):1057-9. Epub 2008/03/06. http://www.ncbi.nlm.nih.gov/pubmed/18319416
Emanuel EJ, Wyden R. Why Tie Health Insurance to a Job? The Wall Street Journal. 2008 12/10/08;Sect. A: Op EdA19.
Emanuel EJ. Healthcare, Guaranteed. New York: Public Affairs; 2008. 219 p.
Emanuel EJ. The cost-coverage trade-off: "it's health care costs, stupid". JAMA. 2008;299(8):947-9. Epub 2008/03/04. http://www.ncbi.nlm.nih.gov/pubmed/18314437
Emanuel EJ. The problem with single-payer plans. The Hastings Center report. 2008;38(1):38-41. Epub 2008/03/05. http://www.ncbi.nlm.nih.gov/pubmed/18314808
Emanuel EJ. What are bioethicists doing about health care reform? The Hastings Center report. 2008;38(2):12-3. Epub 2008/05/07. http://www.ncbi.nlm.nih.gov/pubmed/18457221
Emanuel EJ. Will Your Cell Phone Kill You? The New Republic. 2008 April 9, 2008.
Grady C, Danis M, Soeken KL, O'Donnell P, Taylor C, Farrar A, et al. Does ethics education influence the moral action of practicing nurses and social workers? The American journal of bioethics : AJOB. 2008;8(4):4-11. Epub 2008/06/26. http://www.ncbi.nlm.nih.gov/pubmed/18576241
Grady C, Danis M, Soeken KL, O'Donnell P, Taylor C, Farrar A, et al. Response to Peer Commentary on 'Does Ethics Education Influence the Moral Action of Practicing Nurses and Social Workers?". The American Journal of Bioethics. 2008;8(4):W1-W2.
Grady C, Lie RK, Countries PitGUWotA-COoMRWiD. The Ancillary-Care Obligations of Medical Researchers Working in Developing Countries. PLoS medicine. 2008;5(5):e90 (0709-13).
Grady C, Wagman J, Ssekubugu R, Wawer MJ, Serwadda D, Kiddugavu M, et al. Research benefits for hypothetical HIV vaccine trials: The views of Ugandans in the Rakai District. Irb. 2008;30(2):1-7. Epub 2008/06/03. http://www.ncbi.nlm.nih.gov/pubmed/18512653
Hawkins JS, Emanuel EJ. Exploitation and Developing Countries. Princeton, NJ: Princeton University Press; 2008. 320 p.
Hull SC, Sharp RR, Botkin JR, Brown M, Hughes M, Sugarman J, et al. Patients' views on identifiability of samples and informed consent for genetic research. The American journal of bioethics : AJOB. 2008;8(10):62-70. Epub 2008/11/13. http://www.ncbi.nlm.nih.gov/pubmed/19003716
Hull SC, Wilfond BS. What does it mean to be identifiable? The American journal of bioethics : AJOB. 2008;8(10):W7-8. Epub 2008/11/13. http://www.ncbi.nlm.nih.gov/pubmed/19003695
Hurst SA, Reiter-Theil S, Slowther AM, Pegoraro R, Forde R, Danis M. Should ethics consultants help clinicians face scarcity in their practice? Journal of medical ethics. 2008;34(4):241-6. Epub 2008/04/01. http://www.ncbi.nlm.nih.gov/pubmed/18375673
Joffe S, Miller FG. Bench to bedside: mapping the moral terrain of clinical research. The Hastings Center report. 2008;38(2):30-42. Epub 2008/05/07. http://www.ncbi.nlm.nih.gov/pubmed/18457227
Jost TS, Emanuel EJ. Legal reforms necessary to promote delivery system innovation. JAMA. 2008;299(21):2561-3. Epub 2008/06/05. http://www.ncbi.nlm.nih.gov/pubmed/18523225
Keren A. Epistemic Authority, Testimony, and the Transmission of Knowledge. Episteme. 2008.
Lee TH, Emanuel EJ. Tier 4 drugs and the fraying of the social compact. The New England journal of medicine. 2008;359(4):333-5. Epub 2008/07/25. http://www.ncbi.nlm.nih.gov/pubmed/18650510
Lescano AR, Blazes DL, Montano SM, Moran Z, Naquira C, Ramirez E, et al. Research ethics training in Peru: a case study. PloS one. 2008;3(9):e3274. Epub 2008/09/27. http://www.ncbi.nlm.nih.gov/pubmed/18818763
Lev O. Assessing the importance of maintaining soldiers' moral responsibility--possible trade-offs. The American journal of bioethics : AJOB. 2008;8(2):44-5; discussion W4-6. Epub 2008/06/24. http://www.ncbi.nlm.nih.gov/pubmed/18570078
Levy MM, Rapoport J, Lemeshow S, Chalfin DB, Phillips G, Danis M. Association between critical care physician management and patient mortality in the intensive care unit. Annals of internal medicine. 2008;148(11):801-9. Epub 2008/06/04. http://www.ncbi.nlm.nih.gov/pubmed/18519926
Matsui K, Lie RK, Kita Y, Ueshima H. Ethics of future disclosure of individual risk information in a genetic cohort study: a survey of donor preferences. Journal of epidemiology / Japan Epidemiological Association. 2008;18(5):217-24. Epub 2008/09/09. http://www.ncbi.nlm.nih.gov/pubmed/18776708
Miller FG, Emanuel EJ. Quality-improvement research and informed consent. The New England journal of medicine. 2008;358(8):765-7. Epub 2008/02/22. http://www.ncbi.nlm.nih.gov/pubmed/18287598
Miller FG, Gluck JP, Jr., Wendler D. Debriefing and accountability in deceptive research. Kennedy Institute of Ethics journal. 2008;18(3):235-51. Epub 2008/10/22. http://www.ncbi.nlm.nih.gov/pubmed/18935922
Miller FG, Kaptchuk TJ. Deception of subjects in neuroscience: an ethical analysis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(19):4841-3. Epub 2008/05/09. http://www.ncbi.nlm.nih.gov/pubmed/18463235
Miller FG, Kaptchuk TJ. The power of context: reconceptualizing the placebo effect. Journal of the Royal Society of Medicine. 2008;101(5):222-5. Epub 2008/05/09. http://www.ncbi.nlm.nih.gov/pubmed/18463276
Miller FG, Mello MM, Joffe S. Incidental findings in human subjects research: what do investigators owe research participants? The Journal of law, medicine & ethics : a journal of the American Society of Law, Medicine & Ethics. 2008;36(2):271-9, 11. Epub 2008/06/13. http://www.ncbi.nlm.nih.gov/pubmed/18547194
Miller FG, Pearson SD. Coverage with evidence development: ethical issues and policy implications. Medical care. 2008;46(7):746-51. Epub 2008/06/27. http://www.ncbi.nlm.nih.gov/pubmed/18580395
Miller FG, Truog RD. An apology for Socratic bioethics. The American journal of bioethics : AJOB. 2008;8(7):3-7. Epub 2008/09/02. http://www.ncbi.nlm.nih.gov/pubmed/18759171
Miller FG, Truog RD. Rethinking the ethics of vital organ donations. The Hastings Center report. 2008;38(6):38-46. Epub 2009/02/06. http://www.ncbi.nlm.nih.gov/pubmed/19192716
Miller FG, Wendler D. Is it ethical to keep interim findings of randomised controlled trials confidential? Journal of medical ethics. 2008;34(3):198-201. Epub 2008/03/05. http://www.ncbi.nlm.nih.gov/pubmed/18316463
Miller FG. Collaborative research in bioethics. Newsletter on Medicine and Philosophy (American Philosophical Association). 2008;7(2):17-20.
Miller FG. Research on medical records without informed consent. The Journal of law, medicine & ethics : a journal of the American Society of Law, Medicine & Ethics. 2008;36(3):560-6. Epub 2008/10/09. http://www.ncbi.nlm.nih.gov/pubmed/18840249
Millum J. A Biological Alternative to Moral Explanations. The Southern Journal of Philosophy. 2008;46(3):385-407.
Millum J. Are pharmaceutical patents protected by human rights? Journal of medical ethics. 2008;34(11):e25. Epub 2008/11/01. http://www.ncbi.nlm.nih.gov/pubmed/18974405
Millum J. How Do We Acquire Parental Responsibilities? Social Theory and Practice. 2008;34(1):71-93.
Obladen M, Metze B, Henrich W, Aktas A, Czernik C, Schulz-Baldes A. Interdisciplinary surveillance of intraventricular haemorrhage associated conditions in infants <1000 g. Acta Paediatr. 2008;97(6):731-7. Epub 2008/05/08. http://www.ncbi.nlm.nih.gov/pubmed/18460106
O'Donnell P, Farrar A, BrintzenhofeSzoc K, Conrad AP, Danis M, Grady C, et al. Predictors of ethical stress, moral action and job satisfaction in health care social workers. Social work in health care. 2008;46(3):29-51. Epub 2008/06/17. http://www.ncbi.nlm.nih.gov/pubmed/18551828
Persad GC, Elder L, Sedig L, Flores L, Emanuel EJ. The current state of medical school education in bioethics, health law, and health economics. The Journal of law, medicine & ethics : a journal of the American Society of Law, Medicine & Ethics. 2008;36(1):89-94, 4. Epub 2008/03/05. http://www.ncbi.nlm.nih.gov/pubmed/18315764
Persad GC, Little RF, Grady C. Including persons with HIV infection in cancer clinical trials. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(7):1027-32. Epub 2008/03/04. http://www.ncbi.nlm.nih.gov/pubmed/18309938
Rid A. Review of The Cambridge Textbook of Bioethics, Peter A Singer and Adrian M Viens (Eds). Critical care medicine. 2008;36(8):2492-3.
Rid A. Review of The Oxford Handbook of Bioethics, Bonnie Steinbock, ed. Medical Health Care and Philosophy. 2008;11:489.
Rid A. Review of: Norman Daniels 2008 Just Health - Meeting health needs fairly. WHO Bulletin. 2008;86(8):653-4.
Sabik LM, Lie RK. Principles versus procedures in making health care coverage decisions: addressing inevitable conflicts. Theoretical medicine and bioethics. 2008;29(2):73-85. Epub 2008/06/07. http://www.ncbi.nlm.nih.gov/pubmed/18535922
Sabik LM, Lie RK. Priority setting in health care: Lessons from the experiences of eight countries. International journal for equity in health. 2008;7:4. Epub 2008/01/23. http://www.ncbi.nlm.nih.gov/pubmed/18208617
Sachs B. The liberty principle and universal health care. Kennedy Institute of Ethics journal. 2008;18(2):149-72. Epub 2008/07/10. http://www.ncbi.nlm.nih.gov/pubmed/18610783
Schulz-Baldes A, Biller-Andorno N, Schaber P. Einfuhrung. In: Biller-Andorno N, Schaber P, Schulz-Baldes A, editors. Gibt es eine universale Bioethik? Paderborn: Mentis; 2008. p. 9-23.
Schulz-Baldes A. Brauchen wir eine kulturubergreifende Bioethik? Das Beispiel internationaler Forschungskollaborationen. In: Biller-Andorno N, Schaber P, Schulz-Baldes A, editors. Gibt es eine universale Bioethik? Paderborn: Mentis; 2008. p. 27-44.
Schulz-Baldes A. Review of Children in Medical Research by L. Ross Friedman. Medicine Health Care and Philosophy. 2008;11:244-5.
Seidenfeld J, Horstmann E, Emanuel EJ, Grady C. Participants in phase 1 oncology research trials: are they vulnerable? Archives of internal medicine. 2008;168(1):16-20. Epub 2008/01/16. http://www.ncbi.nlm.nih.gov/pubmed/18195190
Shah S. How Lethal Injection Reform Constitutes Impermissible Research on Prisoners. American Criminal Law Review. 2008;45(3):1101-47.
Shalowitz DI, Miller FG. Communicating the results of clinical research to participants: attitudes, practices, and future directions. PLoS medicine. 2008;5(5):e91. Epub 2008/05/16. http://www.ncbi.nlm.nih.gov/pubmed/18479180
Tilburt JC, Emanuel EJ, Kaptchuk TJ, Curlin FA, Miller FG. Prescribing "placebo treatments": results of national survey of US internists and rheumatologists. BMJ. 2008;337:a1938. Epub 2008/10/25. http://www.ncbi.nlm.nih.gov/pubmed/18948346
Tilburt JC, Emanuel EJ, Miller FG. Does the evidence make a difference in consumer behavior? Sales of supplements before and after publication of negative research results. Journal of general internal medicine. 2008;23(9):1495-8. Epub 2008/07/12. http://www.ncbi.nlm.nih.gov/pubmed/18618194
Truog RD, Miller FG. The dead donor rule and organ transplantation. The New England journal of medicine. 2008;359(7):674-5. Epub 2008/08/16. http://www.ncbi.nlm.nih.gov/pubmed/18703469
Varma S, Jenkins T, Wendler D. How do children and parents make decisions about pediatric clinical research? Journal of pediatric hematology/oncology. 2008;30(11):823-8. Epub 2008/11/08. http://www.ncbi.nlm.nih.gov/pubmed/18989159
Varma S, Wendler D. Research involving wards of the state: protecting particularly vulnerable children. The Journal of pediatrics. 2008;152(1):9-14. Epub 2007/12/25. http://www.ncbi.nlm.nih.gov/pubmed/18154889
Wendler D, Grady C. What should research participants understand to understand they are participants in research? Bioethics. 2008;22(4):203-8. Epub 2008/04/15. http://www.ncbi.nlm.nih.gov/pubmed/18405318
Wendler D, Jenkins T. Children's and their parents' views on facing research risks for the benefit of others. Archives of pediatrics & adolescent medicine. 2008;162(1):9-14. Epub 2008/01/09. http://www.ncbi.nlm.nih.gov/pubmed/18180406
Wendler D, Krohmal B, Emanuel EJ, Grady C. Why patients continue to participate in clinical research. Archives of internal medicine. 2008;168(12):1294-9. Epub 2008/06/25. http://www.ncbi.nlm.nih.gov/pubmed/18574086
Wendler D. Is it possible to protect pediatric research subjects without blocking appropriate research? The Journal of pediatrics. 2008;152(4):467-70. Epub 2008/03/19. http://www.ncbi.nlm.nih.gov/pubmed/18346497
Wertheimer A, Miller FG. Payment for research participation: a coercive offer? Journal of medical ethics. 2008;34(5):389-92. Epub 2008/05/02. http://www.ncbi.nlm.nih.gov/pubmed/18448723
Buchanan DR, Miller FG, Wallerstein N. Ethical issues in community-based participatory research: balancing rigorous research with community participation in community intervention studies. Progress in community health partnerships : research, education, and action. 2007;1(2):153-60. Epub 2007/01/01. http://www.ncbi.nlm.nih.gov/pubmed/20208234
Danis M, Goold SD, Parise C, Ginsburg M. Enhancing employee capacity to prioritize health insurance benefits. Health expectations : an international journal of public participation in health care and health policy. 2007;10(3):236-47. Epub 2007/08/07. http://www.ncbi.nlm.nih.gov/pubmed/17678512
Danis M, Lovett F, Sabik L, Adikes K, Cheng G, Aomo T. Low-income employees' choices regarding employment benefits aimed at improving the socioeconomic determinants of health. American journal of public health. 2007;97(9):1650-7. Epub 2007/08/02. http://www.ncbi.nlm.nih.gov/pubmed/17666702
Denny CC, Emanuel EJ, Pearson SD. Why well-insured patients should demand value-based insurance benefits. JAMA. 2007;297(22):2515-8. Epub 2007/06/15. http://www.ncbi.nlm.nih.gov/pubmed/17565086
Denny CC, Grady C. Clinical research with economically disadvantaged populations. Journal of medical ethics. 2007;33(7):382-5. Epub 2007/07/03. http://www.ncbi.nlm.nih.gov/pubmed/17601862
Dickert N, DeRiemer K, Duffy PE, Garcia-Garcia L, Mutabingwa TK, Sina BJ, et al. Ancillary-care responsibilities in observational research: two cases, two issues. Lancet. 2007;369(9564):874-7. Epub 2007/03/14. http://www.ncbi.nlm.nih.gov/pubmed/17350458
Dror DM, Koren R, Ost A, Binnendijk E, Vellakkal S, Danis M. Health insurance benefit packages prioritized by low-income clients in India: three criteria to estimate effectiveness of choice. Soc Sci Med. 2007;64(4):884-96. Epub 2006/12/05. http://www.ncbi.nlm.nih.gov/pubmed/17141931
Emanuel E, Fuchs V. A Comprehensive Cure: Universal Health Care Vouchers. The Hamilton Project. 2007:1-26.
Emanuel E, Fuchs V. Vouchsafe: A new health care plan. The New Republic. 2007(4,806):14-5.
Emanuel EJ, Fuchs VR, Garber AM. Essential elements of a technology and outcomes assessment initiative. JAMA. 2007;298(11):1323-5. Epub 2007/09/20. http://www.ncbi.nlm.nih.gov/pubmed/17878424
Emanuel EJ, Fuchs VR. Beyond Health-Care Band-Aids. Washington Post. 2007 February 8, 2007;Sect. ColumnsA17.
Emanuel EJ, Grady C. Four paradigms of clinical research and research oversight. Cambridge quarterly of healthcare ethics : CQ : the international journal of healthcare ethics committees. 2007;16(1):82-96. Epub 2007/03/10. http://www.ncbi.nlm.nih.gov/pubmed/17345970
Emanuel EJ, Miller FG. Money and distorted ethical judgments about research: ethical assessment of the TeGenero TGN1412 trial. The American journal of bioethics : AJOB. 2007;7(2):76-81. Epub 2007/03/17. http://www.ncbi.nlm.nih.gov/pubmed/17366206
Emanuel EJ. Researching a Bioethical Question. In: Gallin JI, editor. Principles and Practice of Clinical Research. San Diego: Academic Press; 2007. p. 27 - 38.
Emanuel EJ. Unequal Treatment (book review of Medical Apartheid by Harriet A. Washington). New York Times. 2007 January 18, 2007;Sect. Books.
Emanuel EJ. What cannot be said on television about health care. JAMA. 2007;297(19):2131-3. Epub 2007/05/18. http://www.ncbi.nlm.nih.gov/pubmed/17507349
Flory J, Wendler D, Emanuel E. Informed Consent for Research. In: Ashcroft RE, Dawson A, Draper H, McMillan JR, editors. Principles of Health Care Ethics. Second Edition ed. Chichester, UK: John Wiley & Sons, Ltd; 2007. p. 703 - 10.
Friedberg MW, Coltin KL, Pearson SD, Kleinman KP, Zheng J, Singer JA, et al. Does affiliation of physician groups with one another produce higher quality primary care? Journal of general internal medicine. 2007;22(10):1385-92. Epub 2007/06/28. http://www.ncbi.nlm.nih.gov/pubmed/17594130
Grady C. Ethical Principles in Clinical Research. In: Gallin JI, editor. Principles and Practice of Clinical Research. San Diego: Academic Press; 2007. p. 15- 26.
Grady C. Quality improvement and ethical oversight. Annals of internal medicine. 2007;146(9):680-1. Epub 2007/04/18. http://www.ncbi.nlm.nih.gov/pubmed/17438309
Hampson LA, Joffe S, Fowler R, Verter J, Emanuel EJ. Frequency, type, and monetary value of financial conflicts of interest in cancer clinical research. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(24):3609-14. Epub 2007/08/21. http://www.ncbi.nlm.nih.gov/pubmed/17704409
Hardy NM, Grady C, Pentz R, Stetler-Stevenson M, Raffeld M, Fontaine LS, et al. Bioethical considerations of monoclonal B-cell lymphocytosis: donor transfer after haematopoietic stem cell transplantation. British journal of haematology. 2007;139(5):824-31. Epub 2007/11/21. http://www.ncbi.nlm.nih.gov/pubmed/18021093
Henderson GE, Churchill LR, Davis AM, Easter MM, Grady C, Joffe S, et al. Clinical trials and medical care: defining the therapeutic misconception. PLoS medicine. 2007;4(11):e324. Epub 2007/11/30. http://www.ncbi.nlm.nih.gov/pubmed/18044980
Horng SH, Miller FG. Placebo-controlled procedural trials for neurological conditions. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2007;4(3):531-6. Epub 2007/06/30. http://www.ncbi.nlm.nih.gov/pubmed/17599718
Hurst SA, Danis M. A framework for rationing by clinical judgment. Kennedy Institute of Ethics journal. 2007;17(3):247-66. Epub 2008/01/24. http://www.ncbi.nlm.nih.gov/pubmed/18210983
Hurst SA, Forde R, Reiter-Theil S, Slowther AM, Perrier A, Pegoraro R, et al. Physicians' views on resource availability and equity in four European health care systems. BMC health services research. 2007;7:137. Epub 2007/09/04. http://www.ncbi.nlm.nih.gov/pubmed/17764556
Hurst SA, Perrier A, Pegoraro R, Reiter-Theil S, Forde R, Slowther AM, et al. Ethical difficulties in clinical practice: experiences of European doctors. Journal of medical ethics. 2007;33(1):51-7. Epub 2007/01/09. http://www.ncbi.nlm.nih.gov/pubmed/17209113
Hurst SA, Reiter-Theil S, Perrier A, Forde R, Slowther AM, Pegoraro R, et al. Physicians' access to ethics support services in four European countries. Health care analysis : HCA : journal of health philosophy and policy. 2007;15(4):321-35. Epub 2007/10/19. http://www.ncbi.nlm.nih.gov/pubmed/17943449
Kass NE, Medley AM, Natowicz MR, Hull SC, Faden RR, Plantinga L, et al. Access to health insurance: experiences and attitudes of those with genetic versus non-genetic medical conditions. American journal of medical genetics Part A. 2007;143(7):707-17. Epub 2007/02/10. http://www.ncbi.nlm.nih.gov/pubmed/17290434
Kotwani N, Danis M. Tackling the health-poverty nexus: primary care medicine and intersectoral health action. Journal of general internal medicine. 2007;22(11):1632-3. Epub 2007/09/27. http://www.ncbi.nlm.nih.gov/pubmed/17896162
Kotwani N. The media miss key points in scientific reporting. Virtual Mentor. 2007;9(3):188-92. Epub 2007/01/01. http://www.ncbi.nlm.nih.gov/pubmed/23217929
Koyfman SA, Agrawal M, Garrett-Mayer E, Krohmal B, Wolf E, Emanuel EJ, et al. Risks and benefits associated with novel phase 1 oncology trial designs. Cancer. 2007;110(5):1115-24. Epub 2007/07/14. http://www.ncbi.nlm.nih.gov/pubmed/17628485
Krohmal BJ, Emanuel EJ. Access and ability to pay: the ethics of a tiered health care system. Archives of internal medicine. 2007;167(5):433-7. Epub 2007/03/14. http://www.ncbi.nlm.nih.gov/pubmed/17353489
Kurlander JE, Danis M. Organizational Ethics in Health Care. In: Ashcroft RE, Dawson A, Draper H, McMillan JR, editors. Principles of Health Care Ethics. Second Edition ed. Chichester, UK: John Wiley & Sons, Ltd.; 2007. p. 593-600.
Lavery J, Grady C, Wahl E, Emanuel EJ. Ethical Issues in International Biomedical Research. 2007:371.
Levine MA, Wynia MK, Schyve PM, Teagarden JR, Fleming DA, Donohue SK, et al. Improving access to health care: a consensus ethical framework to guide proposals for reform. The Hastings Center report. 2007;37(5):14-9. Epub 2007/10/31. http://www.ncbi.nlm.nih.gov/pubmed/17966833
Lie R. Standard of Care Owed to Participants in Clinical Trials: Different Standards in Different Countries? In: Ashcroft RE, Dawson A, Draper H, McMillan JR, editors. Principles of Health Care Ethics. Second Edition ed. Chichester, UK: John Wiley & Sons, Ltd.; 2007. p. 729-34.
Lie RK. Ethics of placebo controlled trials and the ethics of ancillary care. Rinsho Hyoka (Clinical Evaluation). 2007;35:283-310.
Lie RK. Post-genom forskning. Et prioriteringsproblem. In: Görman U, editor. Att forma vår fremtid. Stockholm: Nordic University Press; 2007. p. 241-51.
Litton P. "Nanoethics"? What's new? The Hastings Center report. 2007;37(1):22-5. Epub 2007/03/14. http://www.ncbi.nlm.nih.gov/pubmed/17348258
Litton P. The Insignificance of Choice and Wallace's Normative Approach to Responsibility. Law and Philosophy. 2007.
Martin AM. Tales publicly allowed: competence, capacity, and religious belief. The Hastings Center report. 2007;37(1):33-40. Epub 2007/03/14. http://www.ncbi.nlm.nih.gov/pubmed/17348262
Matsui K, Lie RK, Kita Y. Two methods of obtaining informed consent in a genetic epidemiological study: effects on understanding. Journal of empirical research on human research ethics : JERHRE. 2007;2(3):39-48. Epub 2007/09/01. http://www.ncbi.nlm.nih.gov/pubmed/19385850
Matsui K, Lie RK. Privacy shakes Japan's statistics on health and welfare. Eubios Journal of Asian and International Bioethics. 2007;17:41-8.
Mehrotra A, Pearson SD, Coltin KL, Kleinman KP, Singer JA, Rabson B, et al. The response of physician groups to P4P incentives. The American journal of managed care. 2007;13(5):249-55. Epub 2007/05/10. http://www.ncbi.nlm.nih.gov/pubmed/17488190
Miller FG, Brody H. Clinical equipoise and the incoherence of research ethics. The Journal of medicine and philosophy. 2007;32(2):151-65. Epub 2007/04/25. http://www.ncbi.nlm.nih.gov/pubmed/17454420
Miller FG, Kaptchuk TJ. Acupuncture trials and informed consent. Journal of medical ethics. 2007;33(1):43-4. Epub 2007/01/09. http://www.ncbi.nlm.nih.gov/pubmed/17209110
Miller FG, Wertheimer A. Facing up to paternalism in research ethics. The Hastings Center report. 2007;37(3):24-34. Epub 2007/07/26. http://www.ncbi.nlm.nih.gov/pubmed/17649900
Millum J, Emanuel EJ. Ethics. The ethics of international research with abandoned children. Science. 2007;318(5858):1874-5. Epub 2007/12/22. http://www.ncbi.nlm.nih.gov/pubmed/18096792
Pearson S, Littlejohns P. Reallocating resources: how should the National Institute for Health and Clinical Excellence guide disinvestment efforts in the National Health Service? Journal of health services research & policy. 2007;12(3):160-5. Epub 2007/08/25. http://www.ncbi.nlm.nih.gov/pubmed/17716419
Rajczi A. A critique of the innovation argument against a national health program. Bioethics. 2007;21(6):316-23. Epub 2007/09/12. http://www.ncbi.nlm.nih.gov/pubmed/17845455
Richardson HS. Gradations of researchers' obligation to provide ancillary care for HIV/AIDS in developing countries. American journal of public health. 2007;97(11):1956-61. Epub 2007/09/29. http://www.ncbi.nlm.nih.gov/pubmed/17901449
Sachs B. Reasons and Requirements. Ethic Theory Moral Practice. 2007;11:73-83.
Schmidt H, Schulz-Baldes A. The 2007 Draft Declaration of Helsinki - Plus ca Change...? Hastings Center Report. 2007.
Shalowitz DI, Garrett-Mayer E, Wendler D. How should treatment decisions be made for incapacitated patients, and why? PLoS medicine. 2007;4(3):e35. Epub 2007/03/29. http://www.ncbi.nlm.nih.gov/pubmed/17388655
Slutsman J, Buchanan D, Grady C. Ethical issues in cancer chemoprevention trials: considerations for IRBs and investigators. Irb. 2007;29(2):1-6. Epub 2007/09/13. http://www.ncbi.nlm.nih.gov/pubmed/17847608
Thiessen C, Ssekubugu R, Wagman J, Kiddugavu M, Wawer MJ, Emanuel E, et al. Personal and community benefits and harms of research: views from Rakai, Uganda. AIDS. 2007;21(18):2493-501. Epub 2007/11/21. http://www.ncbi.nlm.nih.gov/pubmed/18025886
Tilburt JC, Miller FG. Responding to medical pluralism in practice: a principled ethical approach. Journal of the American Board of Family Medicine : JABFM. 2007;20(5):489-94. Epub 2007/09/08. http://www.ncbi.nlm.nih.gov/pubmed/17823467
Ulrich C, O'Donnell P, Taylor C, Farrar A, Danis M, Grady C. Ethical climate, ethics stress, and the job satisfaction of nurses and social workers in the United States. Soc Sci Med. 2007;65(8):1708-19. Epub 2007/07/10. http://www.ncbi.nlm.nih.gov/pubmed/17619068
Varma S, Wendler D. Medical decision making for patients without surrogates. Archives of internal medicine. 2007;167(16):1711-5. Epub 2007/09/12. http://www.ncbi.nlm.nih.gov/pubmed/17846389
Wendler D, Glantz L. A standard for assessing the risks of pediatric research: pro and con. The Journal of pediatrics. 2007;150(6):579-82. Epub 2007/05/23. http://www.ncbi.nlm.nih.gov/pubmed/17517236
Wendler D, Miller FG. Assessing research risks systematically: the net risks test. Journal of medical ethics. 2007;33(8):481-6. Epub 2007/08/01. http://www.ncbi.nlm.nih.gov/pubmed/17664310
Wendler D, Pentz R. How does the collection of genetic test results affect research participants? American journal of medical genetics Part A. 2007;143A(15):1733-8. Epub 2007/07/10. http://www.ncbi.nlm.nih.gov/pubmed/17618487
Wendler D. Logical endings: computers may soon be better than kin at predicting the wishes of the dying. The Economistcom. 2007.
Wertheimer A. Exploitation in Health Care. In: Ashcroft RE, Dawson A, Draper H, McMillan JR, editors. Principles in Health Care Ethics. Second Edition ed. Chichester, UK: John Wiley & Sons, Ltd; 2007. p. 247-54.
Agrawal M, Grady C, Fairclough DL, Meropol NJ, Maynard K, Emanuel EJ. Patients' decision-making process regarding participation in phase I oncology research. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(27):4479-84. Epub 2006/09/20. http://www.ncbi.nlm.nih.gov/pubmed/16983117
Agrawal M, Hampson LA, Emanuel EJ. Ethics of Clinical Oncology Research. In: Chang AE, Ganz PA, Hayes DF, editors. Oncology: An Evidenced Based Approach. New York: Springer; 2006.
Barton J, Goldstein J, Josling T, Steinberg R. The Evolution of the Trade Regime: Politics, Law, and the Economics of the GATT and WTO. Princeton, NJ: Princeton University Press; 2006.
Botkin JR, Clayton EW, Fost NC, Burke W, Murray TH, Baily MA, et al. Newborn screening technology: proceed with caution. Pediatrics. 2006;117(5):1793-9. Epub 2006/05/03. http://www.ncbi.nlm.nih.gov/pubmed/16651338
Buchanan D, Miller FG. Justice in human subjects research. In: Rhodes R, Francis L, Silvers A, editors. The Blackwell Guide to Medical Ethics: Blackwell; 2006.
Buchanan DR, Miller FG. A public health perspective on research ethics. Journal of medical ethics. 2006;32(12):729-33. Epub 2006/12/06. http://www.ncbi.nlm.nih.gov/pubmed/17145915
Buchanan DR, Miller FG. Justice and fairness in the Kennedy Krieger Institute lead paint study: the ethics of public health research on less expensive, less effective interventions. American journal of public health. 2006;96(5):781-7. Epub 2006/03/31. http://www.ncbi.nlm.nih.gov/pubmed/16571697
Burt R, Gottlieb MK. Medical Ethics and the Law. Principles and Practice of Palliative Care and Supportive Oncology. 3rd ed: Lippincott, Williams & Wilkins; 2006.
Danis M, Ginsburg M, Goold SD. The coverage priorities of disabled adult Medi-Cal beneficiaries. Journal of health care for the poor and underserved. 2006;17(3):592-609. Epub 2006/09/09. http://www.ncbi.nlm.nih.gov/pubmed/16960324
Danis M, Linde-Zwirble WT, Astor A, Lidicker JR, Angus DC. How does lack of insurance affect use of intensive care? A population-based study. Critical care medicine. 2006;34(8):2043-8. Epub 2006/06/10. http://www.ncbi.nlm.nih.gov/pubmed/16763518
Danis M. To scan or not to scan? Virtual Mentor. 2006;8(3):135-7. Epub 2006/01/01. http://www.ncbi.nlm.nih.gov/pubmed/23232342
Daugherty CK, Ratain MJ, Emanuel EJ, Farrell AR. Research, Ethical, and Regulatory Perspectives Regarding the Use of Placebos for Terminally Ill Patients With Cancer. ASCO Education Book2006. p. 165-73.
Denny CC, Emanuel EJ. "Physician-assisted suicide among Oregon cancer patients": a fading issue. The Journal of clinical ethics. 2006;17(1):39-42; discussion 3-5. Epub 2006/05/13. http://www.ncbi.nlm.nih.gov/pubmed/16689112
Emanuel E, NIH AoS-. From the Assembly of Scientists Point of View: Accomplishments and Goals of the NIH Assembly of Scientists (AOS). The NIH Catalyst. 2006.
Emanuel E, Wertheimer A. Who Should Get Influenza Vaccine When Not All Can? Science. 2006;312:854-5.
Emanuel EJ, Crouch RA, Grady C, Lie R, Miller FG, Wendler D, editors. The Oxford Textbook of Clinical Research Ethics. New York: Oxford University Press; 2006.
Emanuel EJ, Fuchs VR. Brainstorm: How to Cure US Health Care. Fortune. 2006 November 13, 2006:78.
Emanuel EJ, Lemmens T, Elliot C. Should Society Allow Research Ethics Boards to Be Run As For-Profit Enterprises? PLoS medicine [Internet]. 2006; 3(7):[0001-4 pp.].
Emanuel EJ, Thompson DF. Regulating Congress. In: Globe B, editor. Boston2006.
Emanuel EJ. Cancer in the courts. Drug Addiction. The New Republic. 2006 July 3, 2006:9-12.
Emanuel EJ. Changing premed requirements and the medical curriculum. JAMA. 2006;296(9):1128-31. Epub 2006/09/07. http://www.ncbi.nlm.nih.gov/pubmed/16954492
Emanuel EJ. From the Assembly of Scientists: View Point - Accomplishments and Goals of the NIH Assembly of Scientists (AOS). 2006;2006.
Emanuel EJ. How to Redefine a Medical Education. Chronicle of Higher Education. 2006 October 20, 2006:B12-B3.
Emanuel EJ. Improving How Americans Die. The New Republic - Online. 2006 March 29, 2006.
Gbadegesin S, Wendler D. Protecting communities in health research from exploitation. Bioethics. 2006;20(5):248-53. Epub 2006/11/15. http://www.ncbi.nlm.nih.gov/pubmed/17100008
Ginsburg M, Goold SD, Danis M. (De)constructing "Basic": Consumers Define the Core Elements of Coverage. Health Affairs. 2006;25(6):1648-55.
Gottlieb MK. Executions, Interrogations, and Torture: The Degradation of Role Morality in Medical Law. Yale Journal of Health Policy, Law & Ethics. 2006.
Grady C, Hampson LA, Wallen GR, Rivera-Goba MV, Carrington KL, Mittleman BB. Exploring the ethics of clinical research in an urban community. American journal of public health. 2006;96(11):1996-2001. Epub 2006/10/05. http://www.ncbi.nlm.nih.gov/pubmed/17018826
Grady C, Horstmann E, Sussman JS, Hull SC. The limits of disclosure: what research subjects want to know about investigator financial interests. The Journal of law, medicine & ethics : a journal of the American Society of Law, Medicine & Ethics. 2006;34(3):592-9, 481. Epub 2006/12/06. http://www.ncbi.nlm.nih.gov/pubmed/17144183
Grady C. Ethics of international research: what does responsiveness mean? Virtual Mentor. 2006;8(4):235-40. Epub 2006/01/01. http://www.ncbi.nlm.nih.gov/pubmed/23241623
Gross CP, Krumholz HM, Van Wye G, Emanuel EJ, Wendler D. Does Random Treatment Assignment Cause Harm to Research Participants? PLoS medicine [Internet]. 2006; 3(6):[e188 p.].
Grosse SD, Boyle CA, Kenneson A, Khoury MJ, Wilfond BS. From public health emergency to public health service: the implications of evolving criteria for newborn screening panels. Pediatrics. 2006;117(3):923-9. Epub 2006/03/03. http://www.ncbi.nlm.nih.gov/pubmed/16510675
Hampson LA, Agrawal M, Joffe S, Gross CP, Verter J, Emanuel EJ. Patients' views on financial conflicts of interest in cancer research trials. The New England journal of medicine. 2006;355(22):2330-7. Epub 2006/12/01. http://www.ncbi.nlm.nih.gov/pubmed/17135586
Hauser JM, Chang CH, Alpert H, Baldwin D, Emanuel EJ, Emanuel L. Who's caring for whom? Differing perspectives between seriously ill patients and their family caregivers. The American journal of hospice & palliative care. 2006;23(2):105-12. Epub 2006/04/01. http://www.ncbi.nlm.nih.gov/pubmed/16572748
Henderson GE, Easter MM, Zimmer C, King NM, Davis AM, Rothschild BB, et al. Therapeutic misconception in early phase gene transfer trials. Soc Sci Med. 2006;62(1):239-53. Epub 2005/07/08. http://www.ncbi.nlm.nih.gov/pubmed/16000230
Hurst SA, Danis M. Indecent coverage? Protecting the goals of health insurance from the impact of co-payments. Cambridge quarterly of healthcare ethics : CQ : the international journal of healthcare ethics committees. 2006;15(1):107-13. Epub 2006/03/15. http://www.ncbi.nlm.nih.gov/pubmed/16529313
Hurst SA, Slowther AM, Forde R, Pegoraro R, Reiter-Theil S, Perrier A, et al. Prevalence and determinants of physician bedside rationing: data from Europe. Journal of general internal medicine. 2006;21(11):1138-43. Epub 2006/07/14. http://www.ncbi.nlm.nih.gov/pubmed/16836629
Joffe S, Miller FG. Rethinking risk-benefit assessment for phase I cancer trials. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(19):2987-90. Epub 2006/07/01. http://www.ncbi.nlm.nih.gov/pubmed/16809725
Joffe S, Truog RD, Shurin SB, Emanuel EJ. Ethical Considerations in Pediatric Oncology. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. 5th ed. Phildadelphia: Lippincott, Williams & Wilkins; 2006.
Kantner L, Goold SD, Nowak M, Monroe-Gatrell L, Danis M. Web tool for health insurance design by small groups: usability study. Proceedings of the SIGCH Conference of Human Factors in Computing Systems, 2006. 2006.
Krohmal B, Sobolski GK. Physicians and the Risk of Malevolent Use of Research. Cambridge Quarterly. 2006.
Lie RK, Emanuel EJ, Grady C. Circumcision and HIV prevention research: an ethical analysis. Lancet. 2006;368(9534):522-5. Epub 2006/08/08. http://www.ncbi.nlm.nih.gov/pubmed/16890839
Loud JT, Weissman NE, Peters JA, Giusti RM, Wilfond BS, Burke W, et al. Deliberate deceit of family members: a challenge to providers of clinical genetics services. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(10):1643-6. Epub 2006/04/01. http://www.ncbi.nlm.nih.gov/pubmed/16575016
Malin JL, Schneider EC, Epstein AM, Adams J, Emanuel EJ, Kahn KL. Results of the National Initiative for Cancer Care Quality: how can we improve the quality of cancer care in the United States? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(4):626-34. Epub 2006/01/13. http://www.ncbi.nlm.nih.gov/pubmed/16401682
Martin AM. How to argue for the value of humanity. Pacific Philosophical Quarterly. 2006;87:96-125.
Merritt M, Grady C. Reciprocity and post-trial access for participants in antiretroviral therapy trials. AIDS. 2006;20(14):1791-4. Epub 2006/09/07. http://www.ncbi.nlm.nih.gov/pubmed/16954719
Miller FG, Joffe S. Evaluating the therapeutic misconception. Kennedy Institute of Ethics journal. 2006;16(4):353-66. Epub 2007/09/13. http://www.ncbi.nlm.nih.gov/pubmed/17847601
Miller FG, Rosenstein DL. The nature and power of the placebo effect. Journal of clinical epidemiology. 2006;59(4):331-5. Epub 2006/03/22. http://www.ncbi.nlm.nih.gov/pubmed/16549251
Miller FG, Rosenstein DL. Variance and Dissent: The Nature and Power of the Placebo Affect. J of Clinical Epidemiology. 2006;59:331-5.
Miller FG, Wendler D. The relevance of empirical research in bioethics. Schizophrenia bulletin. 2006;32(1):37-41. Epub 2005/09/30. http://www.ncbi.nlm.nih.gov/pubmed/16192410
Miller FG. Revisiting the Belmont Report: the ethical significance of the distinction between clinical research and medical care. American Philosophical Association Newsletter on Medicine and Philosophy. 2006.
Nelson JE, Angus DC, Weissfeld LA, Puntillo KA, Danis M, Deal D, et al. End-of-life care for the critically ill: A national intensive care unit survey. Critical care medicine. 2006;34(10):2547-53. Epub 2006/08/26. http://www.ncbi.nlm.nih.gov/pubmed/16932230
Novotny TE, Mordini E, Chadwick I, Pedersen JM, Fabgbri F, Lie R, et al. Biomedical Implications of Globalization (BIG): an International Consortium Project of the European Commission. PLoS medicine [Internet]. 2006:[e43 p.]
Pace C, Grady C, Wendler D, Bebchuk JD, Tavel JA, McNay LA, et al. Post-trial access to tested interventions: the views of IRB/REC chair, investigators, and research participants in a multinational HIV/AIDS study. AIDS research and human retroviruses. 2006;22(9):837-41. Epub 2006/09/23. http://www.ncbi.nlm.nih.gov/pubmed/16989607
Pearson SD, Kleinman K, Rusinak D, Levinson W. A trial of disclosing physicians' financial incentives to patients. Archives of internal medicine. 2006;166(6):623-8. Epub 2006/03/29. http://www.ncbi.nlm.nih.gov/pubmed/16567600
Pearson SD, Miller FG, Emanuel EJ. Medicare's requirement for research participation as a condition of coverage: is it ethical? JAMA. 2006;296(8):988-91. Epub 2006/08/24. http://www.ncbi.nlm.nih.gov/pubmed/16926358
Peerzada JM, Schollin J, Hakansson S. Delivery room decision-making for extremely preterm infants in Sweden. Pediatrics. 2006;117(6):1988-95. Epub 2006/06/03. http://www.ncbi.nlm.nih.gov/pubmed/16740840
Peerzada JM, Wendler D. Hematopoietic stem cell transplant research with pediatric donors: when can institutional review boards approve it? Transplantation. 2006;81(12):1616-20. Epub 2006/06/24. http://www.ncbi.nlm.nih.gov/pubmed/16794524
Pentz RD, Billot L, Wendler D. Research on stored biological samples: views of African American and White American cancer patients. American journal of medical genetics Part A. 2006;140(7):733-9. Epub 2006/03/09. http://www.ncbi.nlm.nih.gov/pubmed/16523508
Pentz RD, Joffe S, Emanuel EJ, Schnipper LE, Haskell CM, Tannock IF. ASCO core values. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(36):5780-2. Epub 2006/12/21. http://www.ncbi.nlm.nih.gov/pubmed/17179111
Ravitsky V. Dying with Dignity in a Jewish-Democratic State. In: Hurwitz PJ, Picard J, Steinberg A, editors. Jewish Ethics and the Care of End-of-Life Patients. New Jersey: KTAV Publishing House, Inc; 2006.
Shalowitz D, Garrett-Mayer E, Wendler D. The accuracy of surrogate decision-makers: a systematic review. Archives of internal medicine. 2006;166:493-7.
Shalowitz D, Wendler D. Informed consent for research and authorization under the Health Insurance Portability and Accountability Act Privacy Rule: an integrated approach. Annals of internal medicine. 2006;144(9):685-8. Epub 2006/05/04. http://www.ncbi.nlm.nih.gov/pubmed/16670138
Truog RD, Brock DW, Cook DJ, Danis M, Luce JM, Rubenfeld GD, et al. Rationing in the intensive care unit. Critical care medicine. 2006;34(4):958-63; quiz 71. Epub 2006/02/18. http://www.ncbi.nlm.nih.gov/pubmed/16484912
Tunis SR, Pearson SD. Coverage options for promising technologies: Medicare's 'coverage with evidence development'. Health Aff (Millwood). 2006;25(5):1218-30. Epub 2006/09/13. http://www.ncbi.nlm.nih.gov/pubmed/16966717
Ulrich CM, Danis M, Ratcliffe SJ, Garrett-Mayer E, Koziol D, Soeken KL, et al. Ethical conflict in nurse practitioners and physician assistants in managed care. Nursing research. 2006;55(6):391-401. Epub 2006/11/30. http://www.ncbi.nlm.nih.gov/pubmed/17133146
Wendler D, Cornelio M. Overcoming language barriers in medical care. Pediatric blood & cancer. 2006;47(6):747. Epub 2006/04/22. http://www.ncbi.nlm.nih.gov/pubmed/16628678
Wendler D, Kington R, Madans J, Van Wye G, Christ-Schmidt H, Pratt L, et al. Are Racial and Ethnic Mnorities Less Willing to Participate in Health Research? PLoS medicine [Internet]. 2006; 3(2):[e19 p.].
Wendler D, Varma S. Minimal risk in pediatric research. The Journal of pediatrics. 2006;149(6):855-61. Epub 2006/12/02. http://www.ncbi.nlm.nih.gov/pubmed/17137907
Wendler D. Clinical research, clinical tragedies, and the assumpton of responsibility. Journal of Organizational Ethics. 2006:46-9.
Wendler D. One-time general consent for research on biological samples. BMJ. 2006;332(7540):544-7. Epub 2006/03/04. http://www.ncbi.nlm.nih.gov/pubmed/16513715
Wendler D. One-time general consent for research on biological samples: is it compatible with the health insurance portability and accountability act? Archives of internal medicine. 2006;166(14):1449-52. Epub 2006/07/26. http://www.ncbi.nlm.nih.gov/pubmed/16864754
Wendler D. Three steps to protecting pediatric research participants from excessive risks. PLoS clinical trials. 2006;1(5):e25. Epub 2006/10/04. http://www.ncbi.nlm.nih.gov/pubmed/17016542
Wendler DS, Shah S. How can medical training and informed consent be reconciled with volume-outcome data? The Journal of clinical ethics. 2006;17(2):149-57. Epub 2006/08/18. http://www.ncbi.nlm.nih.gov/pubmed/16913150
Wendler DS. Assent in paediatric research: theoretical and practical considerations. Journal of medical ethics. 2006;32(4):229-34. Epub 2006/04/01. http://www.ncbi.nlm.nih.gov/pubmed/16574878
Astor A, Akhtar T, Matallana MA, Muthuswamy V, Olowu FA, Tallo V, et al. Physician migration: views from professionals in Colombia, Nigeria, India, Pakistan and the Philippines. Soc Sci Med. 2005;61(12):2492-500. Epub 2005/06/15. http://www.ncbi.nlm.nih.gov/pubmed/15953667
Barton JH, Emanuel EJ. The patents-based pharmaceutical development process: rationale, problems, and potential reforms. JAMA. 2005;294(16):2075-82. Epub 2005/10/27. http://www.ncbi.nlm.nih.gov/pubmed/16249422
Brody H, Miller FG, Bogdan-Lovis E. Evidence-based medicine: watching out for its friends. Perspectives in biology and medicine. 2005;48(4):570-84. Epub 2005/10/18. http://www.ncbi.nlm.nih.gov/pubmed/16227668
Buchanan D, Miller FG. Principles of early stopping of randomized trials for efficacy: a critique of equipoise and an alternative nonexploitation ethical framework. Kennedy Institute of Ethics journal. 2005;15(2):161-78. Epub 2005/09/10. http://www.ncbi.nlm.nih.gov/pubmed/16149206
Chen DT, Rosenstein DL, Muthappan P, Hilsenbeck SG, Miller FG, Emanuel EJ, et al. Research with stored biological samples: what do research participants want? Archives of internal medicine. 2005;165(6):652-5. Epub 2005/03/30. http://www.ncbi.nlm.nih.gov/pubmed/15795341
Danis M, Nowak M, Benavides E, Goold SD. Development and evaluation of a computer decision exercise for consumer participation in insurance benefit planning. The Forum. 2005;10(2).
Diallo DA, Doumbo OK, Plowe CV, Wellems TE, Emanuel EJ, Hurst SA. Community permission for medical research in developing countries. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2005;41(2):255-9. Epub 2005/06/29. http://www.ncbi.nlm.nih.gov/pubmed/15983925
DuVal G, Gensler G, Danis M. Ethical dilemmas encountered by clinical researchers. The Journal of clinical ethics. 2005;16(3):267-76. Epub 2005/11/24. http://www.ncbi.nlm.nih.gov/pubmed/16302553
Emanuel EJ, Currie XE, Herman A. Undue inducement in clinical research in developing countries: is it a worry? Lancet. 2005;366(9482):336-40. Epub 2005/07/26. http://www.ncbi.nlm.nih.gov/pubmed/16039339
Emanuel EJ, Emanuel LL. End-of-Life Care. In: Braunwald E, Fauci AS, Kasper DL, Hauser SL, Jameson JL, editors. Harrison's Principles of Internal Medicine 16th Edition. New York: McGraw-Hill; 2005.
Emanuel EJ, Fuchs VR. Getting Covered. Boston Review. 2005.
Emanuel EJ, Fuchs VR. Health care vouchers--a proposal for universal coverage. The New England journal of medicine. 2005;352(12):1255-60. Epub 2005/03/25. http://www.ncbi.nlm.nih.gov/pubmed/15788504
Emanuel EJ, Fuchs VR. Solved! It covers everyone. It cuts costs. It can get through Congress. Why Universal Health Care Vouchers is the next big idea. Washington Monthly. 2005:20-14.
Emanuel EJ. Depression, euthanasia, and improving end-of-life care. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(27):6456-8. Epub 2005/08/24. http://www.ncbi.nlm.nih.gov/pubmed/16116150
Emanuel EJ. Response to Commentators on "Undue Inducement: Nonsense on Stilts? American Journal of Bioethics. 2005;5(5):W8-W11.
Emanuel EJ. The virtues of dirt. In: Tribune.com C, editor. Chicago: Chicago Tribune; 2005.
Flory J, Emanuel EJ. Recent History of End-of-Life Care and Implications for the Future. In: Galston AW, Peppard CZ, editors. Expanding Horizons in Bioethics. Netherlands: Springer Verlag; 2005.
Fuchs VR, Emanuel EJ. Health care reform: why? What? When? Health Aff (Millwood). 2005;24(6):1399-414. Epub 2005/11/15. http://www.ncbi.nlm.nih.gov/pubmed/16284011
Goold SD, Biddle AK, Klipp G, Hall CN, Danis M. Choosing Healthplans All Together: a deliberative exercise for allocating limited health care resources. Journal of health politics, policy and law. 2005;30(4):563-601. Epub 2005/12/02. http://www.ncbi.nlm.nih.gov/pubmed/16318163
Gottleib MK. Singleton v. Norris: precursor to Abu Ghraib? The importance of role integrity in medicine. Newsletter on philosophy and medicine / American Philosophical Association. 2005;5(1):11-25. Epub 2006/11/23. http://www.ncbi.nlm.nih.gov/pubmed/17111539
Grady C, Dickert N, Jawetz T, Gensler G, Emanuel E. An analysis of U.S. practices of paying research participants. Contemporary clinical trials. 2005;26(3):365-75. Epub 2005/05/25. http://www.ncbi.nlm.nih.gov/pubmed/15911470
Grady C. Ethical Issues in Critical Care. In: Morton P, Fontaine D, Hudak CBB, editors. Critical Care Nursing: A Holistic Approachy. 8th ed. Philadelphia: Lippincott, Williams and Wilkins; 2005.
Grady C. Payment of clinical research subjects. The Journal of clinical investigation. 2005;115(7):1681-7. Epub 2005/07/12. http://www.ncbi.nlm.nih.gov/pubmed/16007244
Grady C. The challenge of assuring continued post-trial access to beneficial treatment. Yale journal of health policy, law, and ethics. 2005;5(1):425-35. Epub 2005/03/04. http://www.ncbi.nlm.nih.gov/pubmed/15742586
Hampson LA, Emanuel EJ. The prognosis for changes in end-of-life care after the Schiavo case. Health Aff (Millwood). 2005;24(4):972-5. Epub 2005/07/14. http://www.ncbi.nlm.nih.gov/pubmed/16012137
Hawkins JS, Emanuel EJ. Clarifying confusions about coercion. The Hastings Center report. 2005;35(5):16-9. Epub 2005/11/22. http://www.ncbi.nlm.nih.gov/pubmed/16295260
Horstmann E, McCabe MS, Grochow L, Yamamoto S, Rubinstein L, Budd T, et al. Risks and benefits of phase 1 oncology trials, 1991 through 2002. The New England journal of medicine. 2005;352(9):895-904. Epub 2005/03/05. http://www.ncbi.nlm.nih.gov/pubmed/15745980
Hurst SA, Hull SC, DuVal G, Danis M. How physicians face ethical difficulties: a qualitative analysis. Journal of medical ethics. 2005;31(1):7-14. Epub 2005/01/07. http://www.ncbi.nlm.nih.gov/pubmed/15634746
Hurst SA, Hull SC, DuVal G, Danis M. Physicians' responses to resource constraints. Archives of internal medicine. 2005;165(6):639-44. Epub 2005/03/30. http://www.ncbi.nlm.nih.gov/pubmed/15795339
Kaptchuk TJ, Miller FG. Viewpoint: what is the best and most ethical model for the relationship between mainstream and alternative medicine: opposition, integration, or pluralism? Academic medicine : journal of the Association of American Medical Colleges. 2005;80(3):286-90. Epub 2005/03/01. http://www.ncbi.nlm.nih.gov/pubmed/15734812
King NM, Henderson GE, Churchill LR, Davis AM, Hull SC, Nelson DK, et al. Consent forms and the therapeutic misconception: the example of gene transfer research. Irb. 2005;27(1):1-8. Epub 2005/04/20. http://www.ncbi.nlm.nih.gov/pubmed/15835063
Lands LC, Allen J, Cloutier M, Leigh M, McColley S, Murphy T, et al. Pediatric Assembly of American Thoracic Society Subcommittee ATS Consensus Statement: research opportunities and challenges in pediatric pulmonology. American Journal of Respiratory Critical Care Medicine. 2005;172(6):776-80.
Lie R. Research ethics and evidence based medicine. In: Meulen RT, editor. Evidence-based Practice in Medicine and Health Care: A Discussion of the Ethical Issues. New York: Springer-Verlag Berlin Heidelberg; 2005.
Lieb JR, Gollust S, Hull SC, Wilfond BS. Carrier Screening Panels for Ashkenazi Jews: Is more better? Genetics in Medicine. 2005;7:185-90.
Litton P, Miller FG. A normative justification for distinguishing the ethics of clinical research from the ethics of medical care. The Journal of law, medicine & ethics : a journal of the American Society of Law, Medicine & Ethics. 2005;33(3):566-74. Epub 2005/10/26. http://www.ncbi.nlm.nih.gov/pubmed/16240736
Litton P. ADHD, values, and the self. The American journal of bioethics : AJOB. 2005;5(3):65-7; discussion W10-2. Epub 2005/07/12. http://www.ncbi.nlm.nih.gov/pubmed/16006360
Litton P. The "Abuse Excuse" in Capital Sentencing Trials: Is it relevant to responsibility, punishment, or neither? American Criminal Law Review. 2005;42(3):1027-72.
Martin AM, Emanuel EJ. Risky Business, review of Lesser Harms: The Morality of Risk in Medical Research. Health Affairs. 2005;24(3):877-8.
Martin AM, Peerzada J. The expressive meaning of enhancement. The American journal of bioethics : AJOB. 2005;5(3):25-7; discussion W4-9. Epub 2005/07/12. http://www.ncbi.nlm.nih.gov/pubmed/16006366
Merritt M. Moral conflict in clinical trials. Ethics. 2005;115(2):306-30. Epub 2005/09/01. http://www.ncbi.nlm.nih.gov/pubmed/16127863
Meulen RT, Biller-Andorno N, Lenk C, Lie R. Evidence-based Practice in Medicine and Health Care: A Discussion of the Ethical Issues. New York: Springer-Verlag Berlin Heidelberg; 2005.
Miller FG, Brody H. Enhancement technologies and professional integrity. The American journal of bioethics : AJOB. 2005;5(3):15-7; discussion W4-9. Epub 2005/07/12. http://www.ncbi.nlm.nih.gov/pubmed/16006375
Miller FG, Brody H. Viewpoint: professional integrity in industry-sponsored clinical trials. Academic medicine : journal of the Association of American Medical Colleges. 2005;80(10):899-904. Epub 2005/09/28. http://www.ncbi.nlm.nih.gov/pubmed/16186605
Miller FG, Fins JJ. Protecting human subjects in brain research: a pragmatic perspective. In: Illes J, editor. Neuroethics. New York: Oxford University Press; 2005.
Miller FG, Moreno JD. Informed consent and the ethics of clinical research: reply to commentaries. Journal of Clinical Ethics. 2005;16:376-9.
Miller FG, Moreno JD. The state of research ethics: a tribute to John C. Fletcher. The Journal of clinical ethics. 2005;16(4):355-64. Epub 2006/02/02. http://www.ncbi.nlm.nih.gov/pubmed/16447523
Miller FG, Wendler D, Swartzman LC. Deception in research on the placebo effect. PLoS medicine. 2005;2(9):e262. Epub 2005/09/22. http://www.ncbi.nlm.nih.gov/pubmed/16173830
Miller FG, Wendler D. Direct-to-consumer advertising and physician prescribing. JAMA. 2005;294(6):678; author reply -9. Epub 2005/08/11. http://www.ncbi.nlm.nih.gov/pubmed/16091566
Miller FG. Does research ethics rest on a mistake? The American journal of bioethics : AJOB. 2005;5(1):34-6; author reply W15-8. Epub 2005/07/23. http://www.ncbi.nlm.nih.gov/pubmed/16036654
Miller FG. Ethical issues in surgical research. Thoracic surgery clinics. 2005;15(4):543-54. Epub 2005/11/10. http://www.ncbi.nlm.nih.gov/pubmed/16276819
Miller FG. The case for a code of ethics for bioethicists: some reasons for skepticism. The American journal of bioethics : AJOB. 2005;5(5):50-2. Epub 2005/09/24. http://www.ncbi.nlm.nih.gov/pubmed/16179312
Miller FG. William James, faith, and the placebo effect. Perspectives in biology and medicine. 2005;48(2):273-81. Epub 2005/04/19. http://www.ncbi.nlm.nih.gov/pubmed/15834199
Muthappan P, Forster H, Wendler D. Research advance directives: protection or obstacle? The American journal of psychiatry. 2005;162(12):2389-91. Epub 2005/12/07. http://www.ncbi.nlm.nih.gov/pubmed/16330609
Pace C, Emanuel EJ, Chuenyam T, Duncombe C, Bebchuk JD, Wendler D, et al. The quality of informed consent in a clinical research study in Thailand. Irb. 2005;27(1):9-17. Epub 2005/04/20. http://www.ncbi.nlm.nih.gov/pubmed/15835065
Pace C, Talisuna A, Wendler D, Maiso F, Wabwire-Mangen F, Bakyaita N, et al. Quality of parental consent in a Ugandan malaria study. American journal of public health. 2005;95(7):1184-9. Epub 2005/06/04. http://www.ncbi.nlm.nih.gov/pubmed/15933235
Pace CA, Emanuel EJ. The ethics of research in developing countries: assessing voluntariness. Lancet. 2005;365(9453):11-2. Epub 2005/01/11. http://www.ncbi.nlm.nih.gov/pubmed/15639664
Permanand G, Lie R. Globalization, liberalization of trade and health. In: Mordini E, editor. Bioethical Implications of Globalization. Rome: CIC Edizioni Internationali; 2005.
Ravitsky V, Wendler D. Dissolving the dilemma over forced treatment. Lancet. 2005;365(9470):1525-6. Epub 2005/05/04. http://www.ncbi.nlm.nih.gov/pubmed/15866294
Ravitsky V. Timers on ventilators. BMJ. 2005;330(7488):415-7. Epub 2005/02/19. http://www.ncbi.nlm.nih.gov/pubmed/15718544
Rosenstein DL, Miller FG. Ethical Issues in Psychosomatic Medicine. The American Psychiatric Press; 2005.
Sabik L, Pace CA, Forster-Gertner HP, Wendler D, Bebchuk JD, Tavel JA, et al. Informed consent: practices and views of investigators in a multinational clinical trial. Irb. 2005;27(5):13-8. Epub 2006/01/24. http://www.ncbi.nlm.nih.gov/pubmed/16425476
Schechter AN, Miller FG. To publish or not to publish? The Journal of laboratory and clinical medicine. 2005;145(1):9-11. Epub 2005/01/26. http://www.ncbi.nlm.nih.gov/pubmed/15668655
Shalowitz DI, Miller FG. Disclosing individual results of clinical research: implications of respect for participants. JAMA. 2005;294(6):737-40. Epub 2005/08/11. http://www.ncbi.nlm.nih.gov/pubmed/16091577
Sobolski GK, Barton JH, Emanuel EJ. Technology licensing: lessons from the US experience. JAMA. 2005;294(24):3137-40. Epub 2005/12/29. http://www.ncbi.nlm.nih.gov/pubmed/16380596
Sobolski GK. Biotechnology products and university-based science. JAMA. 2005;293(23):2862; author reply 3. Epub 2005/06/16. http://www.ncbi.nlm.nih.gov/pubmed/15956629
Solli HM, da Silva AB, Lie RK, Bruusgaard D. [Biomedical model of disease and criteria of distributive justice in disability pension cases]. Tidsskrift for den Norske laegeforening : tidsskrift for praktisk medicin, ny raekke. 2005;125(23):3293-6. Epub 2005/12/06. Biomedisinsk sykdomsmodell og rettferdig fordeling av uforepensjon. http://www.ncbi.nlm.nih.gov/pubmed/16327858
Sugarman J, Getz K, Speckman JL, Byrne MM, Gerson J, Emanuel EJ. The cost of institutional review boards in academic medical centers. The New England journal of medicine. 2005;352(17):1825-7. Epub 2005/04/29. http://www.ncbi.nlm.nih.gov/pubmed/15858200
ter Meulen R, Biller-Andorno N, Lenk C, Lie RK, editors. Evidenced-based Practice in Medicine and Health Care. Berlin, Heidelberg, New York: Springer-Verlag; 2005.
Ulrich CM, Danis M, Koziol D, Garrett-Mayer E, Hubbard R, Grady C. Does it pay to pay? A randomized trial of prepaid financial incentives and lottery incentives in surveys of nonphysician healthcare professionals. Nursing research. 2005;54(3):178-83. Epub 2005/05/18. http://www.ncbi.nlm.nih.gov/pubmed/15897793
Ulrich CM, Wallen GR, Feister A, Grady C. Respondent burden in clinical research: when are we asking too much of subjects? Irb. 2005;27(4):17-20. Epub 2005/10/14. http://www.ncbi.nlm.nih.gov/pubmed/16220630
Weiss R. Medical Studies and the Average American Kid. Washington Post. 2005 August 22, 2005;Sect. A05.
Wendler D, Belsky L, Thompson KM, Emanuel EJ. Quantifying the federal minimal risk standard: implications for pediatric research without a prospect of direct benefit. JAMA. 2005;294(7):826-32. Epub 2005/08/18. http://www.ncbi.nlm.nih.gov/pubmed/16106008
Wendler D, Emanuel EJ. What is a "minor" increase over minimal risk? The Journal of pediatrics. 2005;147(5):575-8. Epub 2005/11/18. http://www.ncbi.nlm.nih.gov/pubmed/16291344
Wendler D, Pace C, Talisuna AO, Maiso F, Grady C, Emanuel E. Research on stored biological samples: the views of Ugandans. Irb. 2005;27(2):1-5. Epub 2005/06/14. http://www.ncbi.nlm.nih.gov/pubmed/15948324
Wendler D. Protecting subjects who cannot give consent: toward a better standard for "minimal" risks. The Hastings Center report. 2005;35(5):37-43. Epub 2005/11/22. http://www.ncbi.nlm.nih.gov/pubmed/16295263
Wilfond BS, Candotti F. When eligibility criteria clash with personal treatment choice: a dilemma of clinical research. In: Kodish E, editor. Ethics and Research in Children. New York: Oxford University Press; 2005.
Wilfond BS, Gollust SE. Policy issues for expanding newborn screening programs: the cystic fibrosis newborn screening experience in the United States. The Journal of pediatrics. 2005;146(5):668-74. Epub 2005/05/05. http://www.ncbi.nlm.nih.gov/pubmed/15870672
Wilfond BS, Parad RB, Fost N. Balancing benefits and risks for cystic fibrosis newborn screening: implications for policy decisions. The Journal of pediatrics. 2005;147(3 Suppl):S109-13. Epub 2005/10/06. http://www.ncbi.nlm.nih.gov/pubmed/16202773
Wilfond BS, Ravitsky V. On the proliferation of bioethics sub-disciplines: do we really need "genethics" and "neuroethics"? The American journal of bioethics : AJOB. 2005;5(2):20-1; discussion W3-4. Epub 2005/07/23. http://www.ncbi.nlm.nih.gov/pubmed/16036690
Barton J. Discussion note: Patents in Pharmaceutical R&D by Carlos Correa. In: Organziation BotWH, editor. 2004.
Belsky L, Emanuel EJ. Conflicts of Interest and Preserving the Objectivity of Scientific Research, a review of Science in the Private Interest by Sheldon Krimsky. Health Affairs. 2004;23(1):268-70.
Belsky L, Lie R, Mattoo A, Emanuel EJ, Sreenivasan G. The general agreement on trade in services: implications for health policymakers. Health Aff (Millwood). 2004;23(3):137-45. Epub 2004/05/27. http://www.ncbi.nlm.nih.gov/pubmed/15160811
Belsky L, Richardson HS. Medical researchers' ancillary clinical care responsibilities. BMJ. 2004;328(7454):1494-6. Epub 2004/06/19. http://www.ncbi.nlm.nih.gov/pubmed/15205296
Brock D. The Democracy Problem in Mental Health Care Priority Setting. In: Nelson J, editor. Rationing Sanity: Ethical Issues in Managed Mental Health Care. Washington, DC: Georgetown University Press; 2004.
Clarke EB, Luce JM, Curtis JR, Danis M, Levy M, Nelson J, et al. A content analysis of forms, guidelines, and other materials documenting end-of-life care in intensive care units. Journal of critical care. 2004;19(2):108-17. Epub 2004/07/06. http://www.ncbi.nlm.nih.gov/pubmed/15236144
Coffey MJ, Wilfond B, Ross LF. Ethical assessment of clinical asthma trials including children subjects. Pediatrics. 2004;113(1 Pt 1):87-94. Epub 2004/01/02. http://www.ncbi.nlm.nih.gov/pubmed/14702454
Danis M, Biddle AK, Goold SD. Enrollees choose priorities for Medicare. The Gerontologist. 2004;44(1):58-67. Epub 2004/02/24. http://www.ncbi.nlm.nih.gov/pubmed/14978321
Danis M, Hanson L. Cross Cutting Issues: Ethics. In: Burton SD, Reitt B, Eisner J, editors. New Frontiers in Geriatric Research: An Agenda for Surgical and Related Medical Specialties2004.
Danis M. Evidence-Based Medicine and Managed Care. In: Meulen R, Biller-Andorno N, Lenk C, Lie R, editors. Ethical Issues of Evidence Based Medicine. Dordrecht: Kluwer Academic Publishers; 2004.
Danis M. How will we respond to chronic critical illness? Critical care medicine. 2004;32(7):1617-8. Epub 2004/07/09. http://www.ncbi.nlm.nih.gov/pubmed/15241118
Danis M. The survival benefit of intensive care. Critical care medicine. 2004;32(8):1791-2. Epub 2004/08/03. http://www.ncbi.nlm.nih.gov/pubmed/15286563
DuVal G, Clarridge B, Gensler G, Danis M. A national survey of U.S. internists' experiences with ethical dilemmas and ethics consultation. Journal of general internal medicine. 2004;19(3):251-8. Epub 2004/03/11. http://www.ncbi.nlm.nih.gov/pubmed/15009780
Emanuel E, Grady C, Lie R, Wendler D. Moral Standards for Research in Developing Countries: From "Reasonable Availability" to "Fair Benefits". Hastings Center Report. 2004;34(3):17-27.
Emanuel EJ, Emanuel LL. Palliative and End-of-Life Care. In: Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, editors. Harrison's Principles of Internal Medicine. New York: McGraw-Hill; 2004.
Emanuel EJ, Fairclough DL, Wolfe P, Emanuel LL. Talking with terminally ill patients and their caregivers about death, dying, and bereavement: is it stressful? Is it helpful? Archives of internal medicine. 2004;164(18):1999-2004. Epub 2004/10/13. http://www.ncbi.nlm.nih.gov/pubmed/15477434
Emanuel EJ, Wood A, Fleischman A, Bowen A, Getz KA, Grady C, et al. Oversight of human participants research: identifying problems to evaluate reform proposals. Annals of internal medicine. 2004;141(4):282-91. Epub 2004/08/18. http://www.ncbi.nlm.nih.gov/pubmed/15313744
Emanuel EJ. Bioethics in the Practice of Medicine. In: Goldman L, Ausiello D, editors. Cecil Textbook of Medicine. 22nd ed. Philadelphia: Saunders; 2004.
Emanuel EJ. Ending concerns about undue inducement. The Journal of law, medicine & ethics : a journal of the American Society of Law, Medicine & Ethics. 2004;32(1):100-5. Epub 2004/05/22. http://www.ncbi.nlm.nih.gov/pubmed/15152431
Emanuel EJ. Living wills: are durable powers of attorney better? The Hastings Center report. 2004;34(6):5-6; author reply -7. Epub 2005/01/26. http://www.ncbi.nlm.nih.gov/pubmed/15666885
Emanuel EJ. Review of The Rights of Patients: The Authoritative ACLU Guide to the Rights of Patients (An American Civil Liberties Union Handbook) by George J. Annas. New England Journal of Medicine. 2004;351(7):724-6.
Flory J, Emanuel E. Interventions to improve research participants' understanding in informed consent for research: a systematic review. JAMA. 2004;292(13):1593-601. Epub 2004/10/07. http://www.ncbi.nlm.nih.gov/pubmed/15467062
Flory J, Young-Xu Y, Gurol I, Levinsky NG, Ash AS, Emanuel EJ. Trends: Place of Death: US Trends Since 1980. Health Affairs. 2004;23(3):194-200.
Flory JH, Emanuel EJ. History of End of Life Care. In: Galston AW, Peppard CZ, editors. New Dimensions in Bioethics. Baltimore: Johns Hopkins University Press; 2004.
Flory JH, Kitcher P. Global health and the scientific research agenda. Philosophy & public affairs. 2004;32(1):36-65. Epub 2005/01/22. http://www.ncbi.nlm.nih.gov/pubmed/15658019
Freund CL, Clayton EW, Wilfond BS. Natural settings trials--improving the introduction of clinical genetic tests. The Journal of law, medicine & ethics : a journal of the American Society of Law, Medicine & Ethics. 2004;32(1):106-10. Epub 2004/05/22. http://www.ncbi.nlm.nih.gov/pubmed/15152432
Goold SD, Green SA, Biddle AK, Benavides E, Danis M. Will insured citizens give up benefit coverage to include the uninsured? Journal of general internal medicine. 2004;19(8):868-74. Epub 2004/07/10. http://www.ncbi.nlm.nih.gov/pubmed/15242473
Grady C. Ethics of vaccine research. Nature immunology. 2004;5(5):465-8. Epub 2004/04/30. http://www.ncbi.nlm.nih.gov/pubmed/15116109
Henderson GE, Davis AM, King NM, Easter MM, Zimmer CR, Rothschild BB, et al. Uncertain benefit: investigators' views and communications in early phase gene transfer trials. Molecular therapy : the journal of the American Society of Gene Therapy. 2004;10(2):225-31. Epub 2004/08/06. http://www.ncbi.nlm.nih.gov/pubmed/15294169
Hull SC, Glanz K, Steffen A, Wilfond BS. Recruitment approaches for family studies: attitudes of index patients and their relatives. Irb. 2004;26(4):12-7. Epub 2004/09/29. http://www.ncbi.nlm.nih.gov/pubmed/15449410
Hull SC, Gooding H, Klein AP, Warshauer-Baker E, Metosky S, Wilfond BS. Genetic research involving human biological materials: a need to tailor current consent forms. Irb. 2004;26(3):1-7. Epub 2004/07/30. http://www.ncbi.nlm.nih.gov/pubmed/15281193
Hurst SA, Teagarden JR, Garrett E, Emanuel EJ. Conserving scarce resources: willingness of health insurance enrollees to choose cheaper options. The Journal of law, medicine & ethics : a journal of the American Society of Law, Medicine & Ethics. 2004;32(3):496-9. Epub 2004/10/20. http://www.ncbi.nlm.nih.gov/pubmed/15490596
Hurst SA. When patients refuse assessment of decision-making capacity: how should clinicians respond? Archives of internal medicine. 2004;164(16):1757-60. Epub 2004/09/15. http://www.ncbi.nlm.nih.gov/pubmed/15364668
Joffe S, Harrington DP, George SL, Emanuel EJ, Budzinski LA, Weeks JC. Satisfaction of the uncertainty principle in cancer clinical trials: retrospective cohort analysis. BMJ. 2004;328(7454):1463. Epub 2004/05/28. http://www.ncbi.nlm.nih.gov/pubmed/15163611
Kass NE, Hull SC, Natowicz MR, Faden RR, Plantinga L, Gostin LO, et al. Medical privacy and the disclosure of personal medical information: the beliefs and experiences of those with genetic and other clinical conditions. American journal of medical genetics Part A. 2004;128A(3):261-70. Epub 2004/06/25. http://www.ncbi.nlm.nih.gov/pubmed/15216547
Levine C, Faden R, Grady C, Hammerschmidt D, Eckenwiler L, Sugarman J. "Special scrutiny": a targeted form of research protocol review. Annals of internal medicine. 2004;140(3):220-3. Epub 2004/02/06. http://www.ncbi.nlm.nih.gov/pubmed/14757620
Levine C, Faden R, Grady C, Hammerschmidt D, Eckenwiler L, Sugarman J. The limitations of "vulnerability" as a protection for human research participants. The American journal of bioethics : AJOB. 2004;4(3):44-9. Epub 2005/09/30. http://www.ncbi.nlm.nih.gov/pubmed/16192138
Lie R, editor. Ethical issues in bioterrorism research. International Conference on Ethical Implications of Research into the Prevention of Bioterrorism; 2004; Brussels: European Commission.
Lie RK, Emanuel E, Grady C, Wendler D. The standard of care debate: the Declaration of Helsinki versus the international consensus opinion. Journal of medical ethics. 2004;30(2):190-3. Epub 2004/04/15. http://www.ncbi.nlm.nih.gov/pubmed/15082816
Lie RK. Health, human rights and mobilization of resources for health. BMC international health and human rights. 2004;4(1):4. Epub 2004/10/12. http://www.ncbi.nlm.nih.gov/pubmed/15473899
Lie RK. Research ethics and evidence based medicine. Journal of medical ethics. 2004;30(2):122-5. Epub 2004/04/15. http://www.ncbi.nlm.nih.gov/pubmed/15082802
Miller FG, Emanuel EJ, Rosenstein DL, Straus SE. Ethical issues concerning research in complementary and alternative medicine. JAMA. 2004;291(5):599-604. Epub 2004/02/06. http://www.ncbi.nlm.nih.gov/pubmed/14762039
Miller FG, Kaptchuk TJ. Sham procedures and the ethics of clinical trials. Journal of the Royal Society of Medicine. 2004;97(12):576-8. Epub 2004/12/03. http://www.ncbi.nlm.nih.gov/pubmed/15574854
Miller FG, Silverman HJ. The ethical relevance of the standard of care in the design of clinical trials. American journal of respiratory and critical care medicine. 2004;169(5):562-4. Epub 2004/01/01. http://www.ncbi.nlm.nih.gov/pubmed/14701713
Miller FG, Wendler D. Assessing the ethics of ethics research: a case study. Irb. 2004;26(2):9-12. Epub 2004/04/09. http://www.ncbi.nlm.nih.gov/pubmed/15069971
Miller FG. Research ethics and misguided moral intuition. The Journal of law, medicine & ethics : a journal of the American Society of Law, Medicine & Ethics. 2004;32(1):111-6. Epub 2004/05/22. http://www.ncbi.nlm.nih.gov/pubmed/15152433
Peerzada JM, Richardson DK, Burns JP. Delivery room decision-making at the threshold of viability. The Journal of pediatrics. 2004;145(4):492-8. Epub 2004/10/14. http://www.ncbi.nlm.nih.gov/pubmed/15480373
Rajczi A. Making risk-benefit assessments of medical research protocols. The Journal of law, medicine & ethics : a journal of the American Society of Law, Medicine & Ethics. 2004;32(2):338-48, 192. Epub 2004/08/11. http://www.ncbi.nlm.nih.gov/pubmed/15301198
Rajczi A. Why are there no expert teachers of virtue? Educational Theory. 2004;53(4):389-400.
Ravitsky V. Posthumous reproduction guidelines in Israel. The Hastings Center report. 2004;34(2):6-7. Epub 2004/05/26. http://www.ncbi.nlm.nih.gov/pubmed/15156829
Richardson HS, Belsky L. The ancillary-care responsibilities of medical researchers. An ethical framework for thinking about the clinical care that researchers owe their subjects. The Hastings Center report. 2004;34(1):25-33. Epub 2004/04/22. http://www.ncbi.nlm.nih.gov/pubmed/15098404
Rosenstein DL, Miller FG. Ethical Issues. In: Levenson JL, editor. Textbook of Psychosomatic Medicine. Washington, DC: American Psychiatric Publishing, Inc; 2004.
Schneider EC, Malin JL, Kahn KL, Emanuel EJ, Epstein AM. Developing a system to assess the quality of cancer care: ASCO's national initiative on cancer care quality. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(15):2985-91. Epub 2004/07/31. http://www.ncbi.nlm.nih.gov/pubmed/15284249
Shah S, Whittle A, Wilfond B, Gensler G, Wendler D. How do institutional review boards apply the federal risk and benefit standards for pediatric research? JAMA. 2004;291(4):476-82. Epub 2004/01/30. http://www.ncbi.nlm.nih.gov/pubmed/14747505
Shalowitz D, Emanuel E. Euthanasia and physician-assisted suicide: implications for physicians. The Journal of clinical ethics. 2004;15(3):232-6. Epub 2005/01/06. http://www.ncbi.nlm.nih.gov/pubmed/15630865
Shalowitz DI, Wolf MS. Shared decision-making and the lower literate patient. The Journal of law, medicine & ethics : a journal of the American Society of Law, Medicine & Ethics. 2004;32(4):759-64. Epub 2005/04/06. http://www.ncbi.nlm.nih.gov/pubmed/15807364
Silverman HJ, Miller FG. Control group selection in critical care randomized controlled trials evaluating interventional strategies: An ethical assessment. Critical care medicine. 2004;32(3):852-7. Epub 2004/04/20. http://www.ncbi.nlm.nih.gov/pubmed/15090973
Taylor HA, Wilfond BS. Ethical issues in newborn screening research: lessons from the Wisconsin cystic fibrosis trial. The Journal of pediatrics. 2004;145(3):292-6. Epub 2004/09/03. http://www.ncbi.nlm.nih.gov/pubmed/15343177
Tucker HA, Wilfond BS. Ethical Issues in Newborn Screening Research: Lessons from the Wisconsin Cystic Fibrosis Trial. Journal of Pediatrics. 2004;145:292-6.
Ulrich CM, Grady C, Wendler D. Palliative care: a supportive adjunct to pediatric phase I clinical trials for anticancer agents? Pediatrics. 2004;114(3):852-5. Epub 2004/09/03. http://www.ncbi.nlm.nih.gov/pubmed/15342863
Ulrich CM, Grady C. Financial incentives and response rates in nursing research. Nursing research. 2004;53(2):73-4. Epub 2004/04/16. http://www.ncbi.nlm.nih.gov/pubmed/15084990
Wendler D, Emanuel E. Assessing the ethical and practical wisdom of surrogate consent for living organ donation. JAMA. 2004;291(6):732-5. Epub 2004/02/12. http://www.ncbi.nlm.nih.gov/pubmed/14871918
Wendler D, Emanuel EJ, Lie RK. The standard of care debate: can research in developing countries be both ethical and responsive to those countries' health needs? American journal of public health. 2004;94(6):923-8. Epub 2004/07/14. http://www.ncbi.nlm.nih.gov/pubmed/15249290
Wendler D, Emanuel EJ. Ethics of Surrogate Consent for Living Organ Donation. JAMA. 2004;292(14):1684-5.
Wendler D, Forster H. Why we need legal standards for pediatric research. The Journal of pediatrics. 2004;144(2):150-3. Epub 2004/02/05. http://www.ncbi.nlm.nih.gov/pubmed/14760251
Wendler D, Miller FG. Deception in the pursuit of science. Archives of internal medicine. 2004;164(6):597-600. Epub 2004/03/24. http://www.ncbi.nlm.nih.gov/pubmed/15037487
Wendler D. Can we ensure that all research subjects give valid consent? Archives of internal medicine. 2004;164(20):2201-4. Epub 2004/11/10. http://www.ncbi.nlm.nih.gov/pubmed/15534155
Wendler D. Risk standards for pediatric research: rethinking the Grimes ruling. Kennedy Institute of Ethics journal. 2004;14(2):187-98. Epub 2004/07/30. http://www.ncbi.nlm.nih.gov/pubmed/15281189
Whittle A, Shah S, Wilfond B, Gensler G, Wendler D. Institutional review board practices regarding assent in pediatric research. Pediatrics. 2004;113(6):1747-52. Epub 2004/06/03. http://www.ncbi.nlm.nih.gov/pubmed/15173501
Wood A, Grady C, Emanuel EJ. Regional ethics organizations for protection of human research participants. Nature medicine. 2004;10(12):1283-8. Epub 2004/12/08. http://www.ncbi.nlm.nih.gov/pubmed/15580245
Agrawal M, Emanuel EJ. Ethics of phase 1 oncology studies: reexamining the arguments and data. JAMA. 2003;290(8):1075-82. Epub 2003/08/28. http://www.ncbi.nlm.nih.gov/pubmed/12941681
Agrawal M. Voluntariness in clinical research at the end of life. Journal of pain and symptom management. 2003;25(4):S25-32. Epub 2003/04/15. http://www.ncbi.nlm.nih.gov/pubmed/12691694
Astor A, Sreenivasan G. Providing free care to the uninsured: how much should physicians give? Annals of internal medicine. 2003;139(9):W78. Epub 2003/11/05. http://www.ncbi.nlm.nih.gov/pubmed/14597477
Aulisio M, Brock D, Winslade W. Whose Virtue? Which Character? Doing Ethics Consultation: No Time for Ivory Towers, Ethics Consultation in Health Care. Baltimore: Johns Hopkins University Press; 2003.
Brock D. Empirical Ethics, Moral Philosophy, and the Democracy Problem. In: Murray C, Salomon J, Mathers C, Lopez A, Lozano J, editors. Summary Measures of Population Health. Geneva: World Health Organization; 2003.
Brock D. Ethical Issues in the Use of Cost Effectiveness Analysis for the Prioritization of Health Care Resources. In: Khusfh G, Englehardt T, editors. Bioethics: A Philosophical Overview. Dordrecht, Germany: Kluwer Publishers; 2003.
Brock D. Ethics and Age-Dependent Rationing in Medicine: A Consequentialist View (German translation). In: Lautherbach K, editor. Ethics and Age Dependent Rationing in Medicine. Stuttgart, Germany: Schattauer; 2003.
Brock D. Fairness and Health; Separability of Health and Well-Being. In: Murray C, Salomon J, Mathers C, Lopez A, Lozano J, editors. Summary Measures of Population Health. Geneva: World Health Organization; 2003.
Brock D. Genetic Engineering. In: Frey RG, Wellman CH, editors. Companion to Applied Ethics. London: Blackwell Publishers; 2003.
Brock D. Preventing Genetically Transmitted Disabilities While Respecting Persons With Disabilities. In: Wasserman D, Wachloroit R, Bickenbach J, editors. Quality-of-Life and Human Difference. New York: Cambridge University Press; 2003.
Brock D. Separate Spheres and Indirect Benefits. In: Organization WH, editor. Making Choices in Health: WHO Guide to Cost-Effectiveness Analysis. Geneva: World Health Organization; 2003.
Brock D. Surrogate Decision Making. Encyclopedia of Bioethics, 2nd Ed. New York: Macmillan; 2003.
Brock D. The Democracy Problem in Mental Health Care Priority Setting. In: Boyle P, editor. Managed Care in Mental Health. Washington, DC: Georgetown University Press; 2003.
Brock D. The Misplaced Role of Urgency in Allocation of Persistently Scarce Life-Saving Organs. In: Daar A, Gutmann T, Lard W, editors. Ethics in Organ Transplantation. Lengerich, Germany: Pabst Science Publishers; 2003.
Brock DW. Precommitment in bioethics: some theoretical issues. Texas law review. 2003;81(7):1805-21. Epub 2004/10/14. http://www.ncbi.nlm.nih.gov/pubmed/15478264
Brock DW. Separate spheres and indirect benefits. Cost effectiveness and resource allocation : C/E. 2003;1(1):4. Epub 2003/05/30. http://www.ncbi.nlm.nih.gov/pubmed/12773217
Brody H, Miller FG. The clinician-investigator: unavoidable but manageable tension. Kennedy Institute of Ethics journal. 2003;13(4):329-46. Epub 2004/03/31. http://www.ncbi.nlm.nih.gov/pubmed/15049297
Chen DT, Miller FG, Rosenstein DL. Clinical research and the physician-patient relationship. Annals of internal medicine. 2003;138(8):669-72. Epub 2003/04/16. http://www.ncbi.nlm.nih.gov/pubmed/12693890
Chen DT, Miller FG, Rosenstein DL. Ethical aspects of research into the etiology of autism. Mental retardation and developmental disabilities research reviews. 2003;9(1):48-53. Epub 2003/02/15. http://www.ncbi.nlm.nih.gov/pubmed/12587138
Churchill LR, Nelson DK, Henderson GE, King NM, Davis AM, Leahey E, et al. Assessing benefits in clinical research: why diversity in benefit assessment can be risky. Irb. 2003;25(3):1-8. Epub 2003/10/23. http://www.ncbi.nlm.nih.gov/pubmed/14569987
Clarke EB, Curtis JR, Luce JM, Levy M, Danis M, Nelson J, et al. Quality indicators for end-of-life care in the intensive care unit. Critical care medicine. 2003;31(9):2255-62. Epub 2003/09/23. http://www.ncbi.nlm.nih.gov/pubmed/14501954
Danis M, Lavizzo-Mourey R. Respecting Diversity in Geriatric Palliative Care. In: Meier D, S. Morrison E, editors. Geriatric Palliative Care. New York: Oxford University Press; 2003.
Earle CC, Emanuel EJ. Patterns of care studies: creating "an environment of watchful concern". Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21(24):4479-80. Epub 2003/11/05. http://www.ncbi.nlm.nih.gov/pubmed/14597747
Emanuel EJ, Crouch RA, Arras J, Moreno JD, Grady C, editors. Ethical and Regulatory Aspects of Clinical Research: Readings and Commentary. Baltimore: Johns Hopkins University Press; 2003.
Emanuel EJ, Fuchs VR. The Universal Cure. In: Times TNY, editor. New York2003.
Emanuel EJ, Joffe S. Ethical Aspects of Caring for Patients with Cancer. In: Kufe DW, editor. Cancer Medicine. London: BC Decker, Inc.; 2003.
Emanuel EJ, Schnipper LE, Kamin DY, Levinson J, Lichter AS. The costs of conducting clinical research. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21(22):4145-50. Epub 2003/10/16. http://www.ncbi.nlm.nih.gov/pubmed/14559889
Emanuel EJ, Young-Xu Y, Levinsky NG, Gazelle G, Saynina O, Ash AS. Chemotherapy use among Medicare beneficiaries at the end of life. Annals of internal medicine. 2003;138(8):639-43. Epub 2003/04/16. http://www.ncbi.nlm.nih.gov/pubmed/12693886
Emanuel EJ. Review of The Case Against Assisted Suicide: For the Right to End-of-Life Care, Kathleen Foley and Herbert Hendin, eds. JAMA. 2003;289(2):233-4.
Emanuel EJ. SARS 1 year later: what we have learned. In: Tribune C, editor. Chicago2003.
Emanuel EJ. The lessons of SARS. Annals of internal medicine. 2003;139(7):589-91. Epub 2003/10/08. http://www.ncbi.nlm.nih.gov/pubmed/14530230
Gollust S, Wilfond BS. Population Carrier Screening: Psychological Impact. In: Cooper DN, editor. Nature Encyclopedia of the Human Genome. 1st ed. London, New York, Tokyo: Macmillan Publishers Ltd, Nature Publishing Group; 2003. p. 618-21.
Gollust SE, Wilfond BS, Hull SC. Direct-to-consumer sales of genetic services on the Internet. Genetics in medicine : official journal of the American College of Medical Genetics. 2003;5(4):332-7. Epub 2003/07/17. http://www.ncbi.nlm.nih.gov/pubmed/12865763
Gross ML, Ravitsky V. Israel: bioethics in a Jewish-democratic state. Cambridge quarterly of healthcare ethics : CQ : the international journal of healthcare ethics committees. 2003;12(3):247-55. Epub 2003/08/02. http://www.ncbi.nlm.nih.gov/pubmed/12889328
Horng S, Grady C. Misunderstanding in clinical research: distinguishing therapeutic misconception, therapeutic misestimation, and therapeutic optimism. Irb. 2003;25(1):11-6. Epub 2003/07/02. http://www.ncbi.nlm.nih.gov/pubmed/12833900
Horng S, Miller FG. Ethical framework for the use of sham procedures in clinical trials. Critical care medicine. 2003;31(3 Suppl):S126-30. Epub 2003/03/11. http://www.ncbi.nlm.nih.gov/pubmed/12626957
Hurst SA, Mauron A. Assisted suicide and euthanasia in Switzerland: allowing a role for non-physicians. BMJ. 2003;326(7383):271-3. Epub 2003/02/01. http://www.ncbi.nlm.nih.gov/pubmed/12560284
Kass NE, Natowicz MR, Hull SC, Faden RR, Plantinga L, Gostin LO, et al. The use of medical records in research: what do patients want? The Journal of law, medicine & ethics : a journal of the American Society of Law, Medicine & Ethics. 2003;31(3):429-33. Epub 2003/11/25. http://www.ncbi.nlm.nih.gov/pubmed/14626550
Koogler TK, Wilfond BS, Ross LF. Lethal language, lethal decisions. The Hastings Center report. 2003;33(2):37-41. Epub 2003/05/23. http://www.ncbi.nlm.nih.gov/pubmed/12760119
Lavery JV, Upshur RE, Sharp RR, Hofman KJ. Ethical issues in international environmental health research. International journal of hygiene and environmental health. 2003;206(4-5):453-63. Epub 2003/09/16. http://www.ncbi.nlm.nih.gov/pubmed/12971701
Lie R. The absolute ethical requirement of individual, informed consent: a commentary on Barrett and Parker. Monash bioethics review. 2003;22(3):18-22. Epub 2003/12/20. http://www.ncbi.nlm.nih.gov/pubmed/14682317
Masur H, Emanuel E, Lane HC. Severe acute respiratory syndrome: providing care in the face of uncertainty. JAMA. 2003;289(21):2861-3. Epub 2003/05/08. http://www.ncbi.nlm.nih.gov/pubmed/12734146
Miller FG, Brody H. A critique of clinical equipoise. Therapeutic misconception in the ethics of clinical trials. The Hastings Center report. 2003;33(3):19-28. Epub 2003/07/12. http://www.ncbi.nlm.nih.gov/pubmed/12854452
Miller FG, Fletcher JC, Humber JM, editors. The Nature and Prospect of Bioethics. Totowa, NJ: Humana Press; 2003.
Miller FG, Rosenstein DL. The therapeutic orientation to clinical trials. The New England journal of medicine. 2003;348(14):1383-6. Epub 2003/04/04. http://www.ncbi.nlm.nih.gov/pubmed/12672867
Miller FG, Wendler D, Wilfond B. When do the federal regulations allow placebo-controlled trials in children? The Journal of pediatrics. 2003;142(2):102-7. Epub 2003/02/14. http://www.ncbi.nlm.nih.gov/pubmed/12584527
Miller FG. Clinical research with healthy volunteers: an ethical framework. Journal of investigative medicine : the official publication of the American Federation for Clinical Research. 2003;51 Suppl 1:S2-5. Epub 2003/04/01. http://www.ncbi.nlm.nih.gov/pubmed/12664947
Miller FG. Ethical issues in research with healthy volunteers: risk-benefit assessment. Clinical pharmacology and therapeutics. 2003;74(6):513-5. Epub 2003/12/10. http://www.ncbi.nlm.nih.gov/pubmed/14663453
Miller FG. Sham surgery: an ethical analysis. The American journal of bioethics : AJOB. 2003;3(4):41-8. Epub 2004/01/28. http://www.ncbi.nlm.nih.gov/pubmed/14744332
Pace C, Emanuel EJ. What we don't know about informed consent. SciDevNet [Internet]. 2003 August 28, 2003.
Pace C, Miller FG, Danis M. Enrolling the uninsured in clinical trials: an ethical perspective. Critical care medicine. 2003;31(3 Suppl):S121-5. Epub 2003/03/11. http://www.ncbi.nlm.nih.gov/pubmed/12626956
Pearson SD, Sabin JE, Emanuel EJ. "No Margin, No Mission" Health Care Organizations and the Quest for Ethical Excellence in Competitive Markets. New York: Oxford University Press; 2003. 176 p.
Plantinga L, Natowicz MR, Kass NE, Hull SC, Gostin LO, Faden RR. Disclosure, confidentiality, and families: experiences and attitudes of those with genetic versus nongenetic medical conditions. American journal of medical genetics Part C, Seminars in medical genetics. 2003;119C(1):51-9. Epub 2003/04/22. http://www.ncbi.nlm.nih.gov/pubmed/12704638
Ravitsky V. Embryonic stem cell research and therapeutic cloning: An Israeli point of view. In: Honnefelder, Lanzerath, editors. Biomedical Research and Reproduction: Scientific Aspects - Ethical, Legal, and Social Implications. Bonn: Bonn University Press; 2003.
Sreenivasan G. Does informed consent to research require comprehension? Lancet. 2003;362(9400):2016-8. Epub 2003/12/20. http://www.ncbi.nlm.nih.gov/pubmed/14683665
Sugarman J, Eckenwiler LA, Emanuel EJ. Research oversight through new lenses: the consortium to examine clinical research ethics. Irb. 2003;25(1):9-10. Epub 2003/07/02. http://www.ncbi.nlm.nih.gov/pubmed/12833899
Ulrich CM, Grady C. Research mentors: an understated value? Nursing research. 2003;52(3):139. Epub 2003/06/07. http://www.ncbi.nlm.nih.gov/pubmed/12792253
Ulrich CM, Soeken KL, Miller N. Ethical conflict associated with managed care: views of nurse practitioners. Nursing research. 2003;52(3):168-75. Epub 2003/06/07. http://www.ncbi.nlm.nih.gov/pubmed/12792257
Wendler D, Shah S, Whittle A, Wilfond BS. Nonbeneficial research with individuals who cannot consent: is it ethically better to enroll healthy or affected individuals? Irb. 2003;25(4):1-4. Epub 2003/12/03. http://www.ncbi.nlm.nih.gov/pubmed/14649246
Wendler D, Shah S. Should children decide whether they are enrolled in nonbeneficial research? The American journal of bioethics : AJOB. 2003;3(4):1-7. Epub 2004/01/28. http://www.ncbi.nlm.nih.gov/pubmed/14744301
Wertheimer A. Consent to Sexual Relations. Cambridge, UK: Cambridge University Press; 2003. 293 p.
Agrawal M, Danis M. End-of-life care for terminally ill participants in clinical research. Journal of palliative medicine. 2002;5(5):729-37. Epub 2003/02/08. http://www.ncbi.nlm.nih.gov/pubmed/12572972
Agrawal M, Emanuel E. Death and dignity: dogma disputed. Lancet. 2002;360(9350):1997-8. Epub 2002/12/31. http://www.ncbi.nlm.nih.gov/pubmed/12504390
Agrawal M, Emanuel EJ. Attending to psychologic symptoms and palliative care. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20(3):624-6. Epub 2002/02/01. http://www.ncbi.nlm.nih.gov/pubmed/11821440
Agrawal M, Emanuel EJ. Review: The Payne/Coyne/Smith Article. Oncology. 2002;16(6):808-11.
Biller N LRKtMR. Evidence based medicine as an instrument for rational health policy. Health Care Analysis. 2002;10:261-75.
Brock D. Health Resource Allocation for Vulnerable Populations. In: Danis M, Clancy C, Churchill LR, editors. Ethical Dimensions of Health Policy. New York: Oxford University Press; 2002.
Brock D. Obligaciones Eticas Para Prevenir Danos Transmitidos Geneticamente. In: Hansberg O, Platts M, editors. Responsabilidad y Libertad. Coyoacan: Universidad Nacional Autonoma de Mexico; 2002.
Brock D. Priority to the Worst Off in Health Care Resource Prioritization. In: Battin M, Rhodes R, Silvers A, editors. Medicine and Social Justice. New York: Oxford University Press; 2002.
Brock D. The Separability of Health and Well-Being. In: Murray CJ, Salomon J, Mathers C, Lopez A, editors. Summary Measures of Population Health. Geneva: World Health Organization; 2002.
Brock D. The Trade-off Between Equity and Choice: Ensuring Fair Procedures. In: Neuberger J, New B, editors. Hidden Assets: Values and Decision Making in the NHS. London: The King's Fund; 2002.
Brock DW. Human cloning and our sense of self. Science. 2002;296(5566):314-6. Epub 2002/04/16. http://www.ncbi.nlm.nih.gov/pubmed/11951036
Buchanan A, Brock D, Daniels N, Wikler D. Genetica y Justicia. New York: Cambridge University Press; 2002.
Charney DS, Nemeroff CB, Lewis L, Laden SK, Gorman JM, Laska EM, et al. National Depressive and Manic-Depressive Association consensus statement on the use of placebo in clinical trials of mood disorders. Archives of general psychiatry. 2002;59(3):262-70. Epub 2002/03/07. http://www.ncbi.nlm.nih.gov/pubmed/11879164
Chen DT, Miller FG, Rosenstein DL. Enrolling decisionally impaired adults in clinical research. Medical care. 2002;40(9 Suppl):V20-9. Epub 2002/09/13. http://www.ncbi.nlm.nih.gov/pubmed/12226582
Chen DT. Review: In Two Minds: A Casebook of Psychiatric Ethics, D. Dickenson and KWM Fulford, editors. Psychiatric Services. 2002;53(8):1-2.
Danis M, Biddle AK, Dorr Goold S. Insurance benefit preferences of the low-income uninsured. Journal of general internal medicine. 2002;17(2):125-33. Epub 2002/02/14. http://www.ncbi.nlm.nih.gov/pubmed/11841528
Danis M, Clancy C, Churchill LR, editors. Ethical Dimensions of Health Policy. New York: Oxford University Press; 2002.
Danis M, Sepinwall A. Regulation of the global marketplace for the sake of health. The Journal of law, medicine & ethics : a journal of the American Society of Law, Medicine & Ethics. 2002;30(4):667-76. Epub 2003/02/04. http://www.ncbi.nlm.nih.gov/pubmed/12561272
Davis AM, Hull SC, Grady C, Wilfond BS, Henderson GE. The invisible hand in clinical research: the study coordinator's critical role in human subjects protection. The Journal of law, medicine & ethics : a journal of the American Society of Law, Medicine & Ethics. 2002;30(3):411-9. Epub 2002/12/25. http://www.ncbi.nlm.nih.gov/pubmed/12497701
Dickert N, Emanuel E, Grady C. Paying research subjects: an analysis of current policies. Annals of internal medicine. 2002;136(5):368-73. Epub 2002/03/05. http://www.ncbi.nlm.nih.gov/pubmed/11874309
Emanuel E, Grady C, Lie R, Wendler D, Countries PitCoEAoRiD. Fair Benefits for Research in Developing Countries. Science. 2002;298:2133-4.
Emanuel E. Health care reform: still possible. The Hastings Center report. 2002;32(2):32-4. Epub 2002/05/10. http://www.ncbi.nlm.nih.gov/pubmed/11998767
Emanuel E. Introduction to occupational medical ethics. Occup Med. 2002;17(4):549-58. Epub 2002/09/13. http://www.ncbi.nlm.nih.gov/pubmed/12225926
Emanuel EJ, Ash A, Yu W, Gazelle G, Levinsky NG, Saynina O, et al. Managed care, hospice use, site of death, and medical expenditures in the last year of life. Archives of internal medicine. 2002;162(15):1722-8. Epub 2002/08/03. http://www.ncbi.nlm.nih.gov/pubmed/12153375
Emanuel EJ, Titlow K. Evaluating community-based health initiatives: identifying the characteristics of successful initiatives and evaluations. Journal of health politics, policy and law. 2002;27(1):105-8. Epub 2002/04/11. http://www.ncbi.nlm.nih.gov/pubmed/11942416
Emanuel EJ. Euthanasia and physician-assisted suicide: a review of the empirical data from the United States. Archives of internal medicine. 2002;162(2):142-52. Epub 2002/02/13. http://www.ncbi.nlm.nih.gov/pubmed/11802747
Emanuel EJ. Institutional review board reform. The New England journal of medicine. 2002;347(16):1285-6. Epub 2002/10/24. http://www.ncbi.nlm.nih.gov/pubmed/12393832
Emanuel EJ. Patient v Population: Resolving the Ethical Dilemmas Posed by Treating Patients as Members of Populations. In: Danis M, Clancy C, Churchill LR, editors. Ethical Dimensions of Health Policy. New York: Oxford University Press; 2002.
Emanuel EJ. Research a Bioethical Question. In: Gallin J, editor. Principles and Practice of Clinical Research. New York: Academic Press; 2002.
Emanuel EJ. Review: Setting Limits Fairly: Can We Learn to Share Medical Resources?, by N. Daniels and JE Sabin. New England Journal of Medicine. 2002;347(12):953-4.
Forster HP, Schwartz J, DeRenzo E. Reducing legal risk by practicing patient-centered medicine. Archives of internal medicine. 2002;162(11):1217-9. Epub 2002/06/01. http://www.ncbi.nlm.nih.gov/pubmed/12038938
Freund CL, Wilfond BS. Emerging ethical issues in pharmacogenomics: from research to clinical practice. American journal of pharmacogenomics : genomics-related research in drug development and clinical practice. 2002;2(4):273-81. Epub 2002/11/08. http://www.ncbi.nlm.nih.gov/pubmed/12421098
Gollust SE, Hull SC, Wilfond BS. Limitations of direct-to-consumer advertising for clinical genetic testing. JAMA. 2002;288(14):1762-7. Epub 2002/10/09. http://www.ncbi.nlm.nih.gov/pubmed/12365961
Gooding HC, Wilfond B, Boehm K, Biesecker BB. Unintended messages: the ethics of teaching genetic dilemmas. The Hastings Center report. 2002;32(2):37-9. Epub 2002/05/10. http://www.ncbi.nlm.nih.gov/pubmed/11998769
Grady C. Ethical Principles in Clinical Research. In: Gallin J, editor. Principles and Practice of Clinical Research. New York: Academic Press; 2002.
Grady C. Recruitment of research subjects. PM&D/ SOCRA Source. 2002:33-5.
Grady C. Thinking further about value: commentary on "A taxonomy of value in clinical research". Irb. 2002;24(6):7-8. Epub 2003/04/10. http://www.ncbi.nlm.nih.gov/pubmed/12683395
Horng S, Emanuel EJ, Wilfond B, Rackoff J, Martz K, Grady C. Descriptions of benefits and risks in consent forms for phase 1 oncology trials. The New England journal of medicine. 2002;347(26):2134-40. Epub 2002/12/27. http://www.ncbi.nlm.nih.gov/pubmed/12501226
Horng S, Miller FG. Is placebo surgery unethical? The New England journal of medicine. 2002;347(2):137-9. Epub 2002/07/12. http://www.ncbi.nlm.nih.gov/pubmed/12110744
Jayasinghe S, Mendis N, Lie R. Use of disability adjusted life years in health planning: a plea for caution. The Ceylon medical journal. 2002;47(2):61-3. Epub 2002/07/27. http://www.ncbi.nlm.nih.gov/pubmed/12140881
Killen J, Grady C, Folkers GK, Fauci AS. Ethics of clinical research in the developing world. Nature reviews Immunology. 2002;2(3):210-5. Epub 2002/03/27. http://www.ncbi.nlm.nih.gov/pubmed/11913072
Lavery JV. A Culture of ethical conduct in research: the proper goal of capacity building in international reserach ethics. In: Wh HOCoM, Health WGGPGfH, editors. WHO Commission on Macroeconomics and Health; Geneva2002.
Lie RK, Schotsmans PT, Hansen B, Meulenbergs T, editors. Healthy thoughts: European Perspectives on Health Care Ethics. Leuven: Peeters Verlag; 2002.
Lie RK. Randomised controlled clinical trials. In: Lie Rk SPe, editor. Healthy Thoughts, Perspectives on Health Care Ethics. Leuven: Peeters Verlag; 2002. p. 7-26.
Lie RK. The ethics of the patient physician relationship. In: Lie RKSP, editor. Healthy Thoughts Perspectives on Health Care Ethics. Leuven: Peeters Verlag; 2002. p. 7-26.
Lie RK. The HIV perinatal transmission studies and the debate about the revision of the Helsinki Declaration. In: Lie Rk SPe, editor. Healthy Thoughts Perspectives on Health Care Ethics. Leuven: Peeters Verlag; 2002. p. 189-206.
Merritt M. Review:From Detached Concern to Empathy: Humanizing Medical Practice, by J. Halpern. Hastings Center Report. 2002;32(5):45-6.
Miller FG, Brody H. What makes placebo-controlled trials unethical? The American journal of bioethics : AJOB. 2002;2(2):3-9. Epub 2002/08/22. http://www.ncbi.nlm.nih.gov/pubmed/12189059
Miller FG, Rosenstein DL. Reporting of ethical issues in publications of medical research. Lancet. 2002;360(9342):1326-8. Epub 2002/11/05. http://www.ncbi.nlm.nih.gov/pubmed/12414226
Miller FG, Shorr AF. Ethical assessment of industry-sponsored clinical trials: a case analysis. Chest. 2002;121(4):1337-42. Epub 2002/04/12. http://www.ncbi.nlm.nih.gov/pubmed/11948071
Miller FG, Shorr AF. Unnecessary use of placebo controls: the case of asthma clinical trials. Archives of internal medicine. 2002;162(15):1673-7. Epub 2002/08/03. http://www.ncbi.nlm.nih.gov/pubmed/12153369
Miller FG. Ethical significance of ethics-related empirical research. Journal of the National Cancer Institute. 2002;94(24):1821-2. Epub 2002/12/19. http://www.ncbi.nlm.nih.gov/pubmed/12488467
Parascandola M, Hawkins J, Danis M. Patient autonomy and the challenge of clinical uncertainty. Kennedy Institute of Ethics journal. 2002;12(3):245-64. Epub 2002/12/11. http://www.ncbi.nlm.nih.gov/pubmed/12472078
Rajczi A. The moral theory behind moral dilemmas. American Philosophical Quarterly. 2002;39(4):373-83.
Robertson DW, Bedell R, Lavery JV, Upshur R. What kind of evidence do we need to justify humanitarian medical aid? Lancet. 2002;360(9329):330-3. Epub 2002/07/31. http://www.ncbi.nlm.nih.gov/pubmed/12147390
Slutsman J, Emanuel LL, Fairclough D, Bottorff D, Emanuel EJ. Managing end-of-life care: comparing the experiences of terminally Ill patients in managed care and fee for service. Journal of the American Geriatrics Society. 2002;50(12):2077-83. Epub 2002/12/11. http://www.ncbi.nlm.nih.gov/pubmed/12473022
Sreenivasan G. Errors about Errors: Virtue Theory and Trait Attribution. Mind. 2002;111:47-68.
Sreenivasan G. International justice and health: a proposal. Ethics & international affairs. 2002;16(2):81-90. Epub 2005/02/16. http://www.ncbi.nlm.nih.gov/pubmed/15709281
Ulrich C, Wallen GR, Grady C. Nurse staffing levels and quality of care in hospitals. New England Journal of Medicine. 2002;347(14):1118-9.
Ulrich CM, Wallen GR, Grady C. Research vulnerability and patient advocacy: balance-seeking perspectives for the clinical nurse scientist? Nursing research. 2002;51(2):71. Epub 2002/05/02. http://www.ncbi.nlm.nih.gov/pubmed/11984375
Weatherall D, Brock D, Chee HL, Research MotWHOACoH. Genomics and World Health. Geneva: World Health Organization; 2002.
Weiss SC, Kimball AB, Liewehr DJ, Blauvelt A, Turner ML, Emanuel EJ. Quantifying the harmful effect of psoriasis on health-related quality of life. Journal of the American Academy of Dermatology. 2002;47(4):512-8. Epub 2002/09/25. http://www.ncbi.nlm.nih.gov/pubmed/12271293
Wendler D, Emanuel E. The debate over research on stored biological samples: what do sources think? Archives of internal medicine. 2002;162(13):1457-62. Epub 2002/07/02. http://www.ncbi.nlm.nih.gov/pubmed/12090881
Wendler D, Martinez RA, Fairclough D, Sunderland T, Emanuel E. Views of potential subjects toward proposed regulations for clinical research with adults unable to consent. The American journal of psychiatry. 2002;159(4):585-91. Epub 2002/04/02. http://www.ncbi.nlm.nih.gov/pubmed/11925296
Wendler D, Prasad K, Wilfond B. Does the current consent process minimize the risks of genetics research? American journal of medical genetics. 2002;113(3):258-62. Epub 2002/11/20. http://www.ncbi.nlm.nih.gov/pubmed/12439893
Wendler D, Rackoff J. Consent for continuing research participation: what is it and when should it be obtained? Irb. 2002;24(3):1-6. Epub 2003/07/10. http://www.ncbi.nlm.nih.gov/pubmed/12848185
Wendler D, Rackoff JE, Emanuel EJ, Grady C. The ethics of paying for children's participation in research. The Journal of pediatrics. 2002;141(2):166-71. Epub 2002/08/17. http://www.ncbi.nlm.nih.gov/pubmed/12183709
Wendler D. What research with stored samples teaches us about research with human subjects. Bioethics. 2002;16(1):33-54. Epub 2002/06/14. http://www.ncbi.nlm.nih.gov/pubmed/12061383
Wilfond B, Rothenberg LS. Ethical issues in cystic fibrosis newborn screening: from data to public health policy. Current opinion in pulmonary medicine. 2002;8(6):529-34. Epub 2002/10/24. http://www.ncbi.nlm.nih.gov/pubmed/12394162
Wilfond BS, Geller G, Lerman C, Audrain-McGovern J, Shields AE. Ethical issues in conducting behavioral genetics research: the case of smoking prevention trials among adolescents. Journal of health care law & policy. 2002;6(1):73-88. Epub 2004/03/17. http://www.ncbi.nlm.nih.gov/pubmed/15017952
Agrawal M, Steinberg D. Should a medical practice accept a gift with strings attached? Med Ethics (Burlingt Mass). 2001:3, 7. Epub 2004/12/09. http://www.ncbi.nlm.nih.gov/pubmed/15584189
Brock D. Cuestiones eticas relacionadas con el uso del analisis costo-efectividad para asignar prioridades a los recursos del area de la salud. Perspectivas Bioethicas. 2001;6(12):25-49.
Burton SL, Randel L, Titlow K, Emanuel EJ. The ethics of Pharmaceutical benefit management. Health Aff (Millwood). 2001;20(5):150-63. Epub 2001/09/18. http://www.ncbi.nlm.nih.gov/pubmed/11558699
Danis M, Ethics Committee ACoCCMS. Recommendations for non-heart beating organ donation. Critical care medicine. 2001;29(9):1826-31.
Danis M, Hanson L, Garrett JM. Experimental methods. In: Sugarman J, Sulmasy DP, editors. Methods in Medical Ethics. Washington, DC: Georgetown Univesity Press; 2001.
Danis M. Role of ethnicity, race, religion, and socio-economic status in end-of-life care in the ICU. In: Curtis JR, Rubenfeld GD, editors. Managing Death in the Intensiv Care Unit: The Transition from Cure to Comfort. Oxford, UK: Oxford University Press; 2001.
DeVita MA, Ethics Committee ACoCCMSoCCM. Recommendations for nonheartbeating organ donation. Critical care medicine. 2001;29(9):1826-31.
DuVal G, Sartorius L, Clarridge B, Gensler G, Danis M. What triggers requests for ethics consultations? Journal of medical ethics. 2001;27 Suppl 1:i24-9. Epub 2001/04/21. http://www.ncbi.nlm.nih.gov/pubmed/11314608
Emanuel EJ, Irwin M, Gunning KF, Quill TE, Saunders P. Assisted suicide and cancer. The lancet oncology. 2001;2(3):179-84. Epub 2002/03/21. http://www.ncbi.nlm.nih.gov/pubmed/11902571
Emanuel EJ, Miller FG. The ethics of placebo-controlled trials--a middle ground. The New England journal of medicine. 2001;345(12):915-9. Epub 2001/09/22. http://www.ncbi.nlm.nih.gov/pubmed/11565527
Emanuel LL, Alpert HR, Emanuel EE. Concise screening questions for clinical assessments of terminal care: the needs near the end-of-life care screening tool. Journal of palliative medicine. 2001;4(4):465-74. Epub 2002/01/19. http://www.ncbi.nlm.nih.gov/pubmed/11798478
Fetters MD, Churchill L, Danis M. Conflict resolution at the end of life. Critical care medicine. 2001;29(5):921-5. Epub 2001/05/30. http://www.ncbi.nlm.nih.gov/pubmed/11378597
Forster H, Emanuel EJ, Grady C. International Health Law. The International Lawyer. 2001;35(2):713-4.
Forster H. Legal Trends in Bioethics. Journal of Clincal Ethics. 2001;12(2):176 - 85.
Forster HP, Emanuel E, Grady C. The 2000 revision of the Declaration of Helsinki: a step forward or more confusion? Lancet. 2001;358(9291):1449-53. Epub 2001/11/14. http://www.ncbi.nlm.nih.gov/pubmed/11705513
Forster HP, Shah S. Legal Trends in Bioethics. Journal of Clincal Ethics. 2001;12(3):319-30.
Forzley M, Forster HP, Shah S, Howe EG. Public International Law. The International Lawyer. 2001:2 - 11.
Grady C. Clinical research: the power of the nurse. The American journal of nursing. 2001;101(9):11. Epub 2001/09/26. http://www.ncbi.nlm.nih.gov/pubmed/11570376
Grady C. Money for research participation: does in jeopardize informed consent? The American journal of bioethics : AJOB. 2001;1(2):40-4. Epub 2002/04/16. http://www.ncbi.nlm.nih.gov/pubmed/11951886
Grady C. Payment of research subjects. SOCRA Source. 2001:36-7.
Green SA, Bloch S. Working in a flawed mental health care system: an ethical challenge. The American journal of psychiatry. 2001;158(9):1378-83. Epub 2001/09/05. http://www.ncbi.nlm.nih.gov/pubmed/11532719
Hedenfalk I, Duggan D, Chen Y, Radmacher M, Bittner M, Simon R, et al. Gene-expression profiles in hereditary breast cancer. The New England journal of medicine. 2001;344(8):539-48. Epub 2001/02/24. http://www.ncbi.nlm.nih.gov/pubmed/11207349
Hilden JM, Emanuel EJ, Fairclough DL, Link MP, Foley KM, Clarridge BC, et al. Attitudes and practices among pediatric oncologists regarding end-of-life care: results of the 1998 American Society of Clinical Oncology survey. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19(1):205-12. Epub 2001/01/03. http://www.ncbi.nlm.nih.gov/pubmed/11134214
Hull SC, Prasad K. Reading between the lines: direct-to-consumer advertising of genetic testing in the USA. Reproductive health matters. 2001;9(18):44-8. Epub 2002/01/05. http://www.ncbi.nlm.nih.gov/pubmed/11765398
Hull SC, Taylor HA, Kass NE. Qualitative Methods. In: Sugarman J, Sulmasy DP, editors. Methods in Medical Ethics. Washington, DC: Georgetown University Press; 2001.
Levinsky NG, Yu W, Ash A, Moskowitz M, Gazelle G, Saynina O, et al. Influence of age on Medicare expenditures and medical care in the last year of life. JAMA. 2001;286(11):1349-55. Epub 2001/09/19. http://www.ncbi.nlm.nih.gov/pubmed/11560540
Miller FG, Brody H. The internal morality of medicine: an evolutionary perspective. The Journal of medicine and philosophy. 2001;26(6):581-99. Epub 2001/12/06. http://www.ncbi.nlm.nih.gov/pubmed/11735051
Miller FG, Grady C. The ethical challenge of infection-inducing challenge experiments. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2001;33(7):1028-33. Epub 2001/08/31. http://www.ncbi.nlm.nih.gov/pubmed/11528576
Nelson JE, Danis M. End-of-life care in the intensive care unit: where are we now? Critical care medicine. 2001;29(2 Suppl):N2-9. Epub 2001/03/03. http://www.ncbi.nlm.nih.gov/pubmed/11228566
Nelson RM, Botkin JR, Kodish ED, Levetown M, Truman JT, Wilfond BS, et al. Ethical issues with genetic testing in pediatrics. Pediatrics. 2001;107:1451-5.
Parascandola M. Cigarettes and the US Public Health Service in the 1950s. American journal of public health. 2001;91(2):196-205. Epub 2001/02/24. http://www.ncbi.nlm.nih.gov/pubmed/11211627
Randel L, Pearson SD, Sabin JE, Hyams T, Emanuel EJ. How managed care can be ethical. Health Aff (Millwood). 2001;20(4):43-56. Epub 2001/07/21. http://www.ncbi.nlm.nih.gov/pubmed/11463089
Shah S. Other recent developments in international health law. The International Lawyer. 2001;35(2):713-4.
Shah S. Review: Transplantation Ethics by Robert Veatch. Progress in Transplantation. 2001;11(4):298-9.
Silverman H, Hull SC, Sugarman J. Variability among institutional review boards' decisions within the context of a multicenter trial. Critical care medicine. 2001;29(2):235-41. Epub 2001/03/14. http://www.ncbi.nlm.nih.gov/pubmed/11246299
Smith M, Forster H. Review: Margin of Error: The Ethics of Mistakes in the Practice of Meidcine SB Rubin, L Zoloth, eds. JAMA. 2001;285(16):1234-5.
Sreenivasan G. A proliferation of liberties. Philosophy and Phenomenological Research. 2001;63(1):229-37.
Sreenivasan G. Judicial review and individual self-rule. Revista Argentina de Teoria Juridica. 2001;2(2):1-8.
Sreenivasan G. Opportunity is not the key. American Journal of Bioethics. 2001;1(2).
Sreenivasan G. Understanding alien morals. Philosophy and Phenomenological Research. 2001;62(1):1-31.
Truog RD, Cist AF, Brackett SE, Burns JP, Curley MA, Danis M, et al. Recommendations for end-of-life care in the intensive care unit: The Ethics Committee of the Society of Critical Care Medicine. Critical care medicine. 2001;29(12):2332-48. Epub 2002/01/22. http://www.ncbi.nlm.nih.gov/pubmed/11801837
Weiss SC, Emanuel LL, Fairclough DL, Emanuel EJ. Understanding the experience of pain in terminally ill patients. Lancet. 2001;357(9265):1311-5. Epub 2001/05/10. http://www.ncbi.nlm.nih.gov/pubmed/11343734
Wendler D, Dickert N. The consent process for cadaveric organ procurement: how does it work? How can it be improved? JAMA. 2001;285(3):329-33. Epub 2001/02/15. http://www.ncbi.nlm.nih.gov/pubmed/11176844
Wendler D, Prasad K. Core safeguards for clinical research with adults who are unable to consent. Annals of internal medicine. 2001;135(7):514-23. Epub 2001/10/02. http://www.ncbi.nlm.nih.gov/pubmed/11578155
Wendler D, Rackoff JE. Informed consent and respecting autonomy--what's a signature got to do with it? Irb. 2001;23(3):1-4. Epub 2002/01/16. http://www.ncbi.nlm.nih.gov/pubmed/11789523
Agich GJ, Forster H. Conflicts of interest and management in managed care. Cambridge quarterly of healthcare ethics : CQ : the international journal of healthcare ethics committees. 2000;9(2):189-204. Epub 2000/04/01. http://www.ncbi.nlm.nih.gov/pubmed/10742862
Burger IM, Wilfond BS. Limitations of informed consent for in utero gene transfer research: implications for investigators and institutional review boards. Human gene therapy. 2000;11(7):1057-63. Epub 2000/05/16. http://www.ncbi.nlm.nih.gov/pubmed/10811234
Burton SD. An Ethicist's Evaluation of Drug Coverage. Drug Benefit Trends. 2000;12(2):58-9.
Clancy C, Danis M. Setting Priorities "American Style". In: Coulter A, Ham C, editors. The Global Challenge of Health Care Rationing. Philadelphia: Open University Press; 2000.
Danis M. Deciding whether to withdraw life-support in critically ill children: insightful data on hard choices. Critical care medicine. 2000;28(5):1685-6. Epub 2000/06/02. http://www.ncbi.nlm.nih.gov/pubmed/10834753
Danis M. Role of ethnicity, race, religion, and socio-economic status in end of life care in the ICU. In: Curtis JR, Rubenfeld GD, editors. The Transition from Cure to Comfort: Managing Death in the Intensive Care Unit. Oxford: Oxford University Press; 2000.
Davis DS. Legal trends in bioethics. The Journal of clinical ethics. 2000;11(1):94-5. Epub 2001/10/20. http://www.ncbi.nlm.nih.gov/pubmed/11645768
Emanuel EJ, Fairclough D, Clarridge BC, Blum D, Bruera E, Penley WC, et al. Attitudes and practices of U.S. oncologists regarding euthanasia and physician-assisted suicide. Annals of internal medicine. 2000;133(7):527-32. Epub 2000/10/03. http://www.ncbi.nlm.nih.gov/pubmed/11015165
Emanuel EJ, Fairclough DL, Emanuel LL. Attitudes and desires related to euthanasia and physician-assisted suicide among terminally ill patients and their caregivers. JAMA. 2000;284(19):2460-8. Epub 2000/11/14. http://www.ncbi.nlm.nih.gov/pubmed/11074775
Emanuel EJ, Fairclough DL, Slutsman J, Emanuel LL. Understanding economic and other burdens of terminal illness: the experience of patients and their caregivers. Annals of internal medicine. 2000;132(6):451-9. Epub 2000/03/25. http://www.ncbi.nlm.nih.gov/pubmed/10733444
Emanuel EJ, Wendler D, Grady C. What makes clinical research ethical? JAMA. 2000;283(20):2701-11. Epub 2000/05/20. http://www.ncbi.nlm.nih.gov/pubmed/10819955
Emanuel EJ. Justice and managed care. Four principles for the just allocation of health care resources. The Hastings Center report. 2000;30(3):8-16. Epub 2000/06/22. http://www.ncbi.nlm.nih.gov/pubmed/10862365
Fetters M, Danis M. Death with dignity: cardiopulmonary resuscitatin in the United States and Japan. In: Englehardt T, editor. Hoshino Festchrift. Boston: Kluwar Academic Press; 2000.
Fetters M, Danis M. We live too short and die too long: on Japanese and US Physicians' caregiving practicing and approaches to whithholding life-sustaining treatments. In: Long S, editor. Caregiving for the Elderly in Japan and the US. London: Routledge; 2000.
Fins JJ, Miller FG. Enrolling decisionally incapacitated subjects in neuropsychiatric research. CNS spectrums. 2000;5(10):32-40. Epub 2007/07/17. http://www.ncbi.nlm.nih.gov/pubmed/17632450
Forster H, Agich GJ. Conflicts of interest and management in managed care. Cambridge Quarterly. 2000;9(2):189-204.
Forster H, Ramsey E. The law meets reproductive technology: the prospect of human cloning. In: Lauritzen P, editor. Cloning and the Future of Human Embryo Research. Oxford: Oxford University PRess; 2000.
Hull SC, Kass NE. Adults with cystic fibrosis and (in)fertility: how has the health care system responded? Journal of andrology. 2000;21(6):809-13. Epub 2000/12/06. http://www.ncbi.nlm.nih.gov/pubmed/11105906
Merritt M. Virtue ethics and situationist personality psychology. Ethical Theory and Moral Practice. 2000;3:365-83.
Miller FG, editor. Frontiers in Bioethics: Essays Dedicated to John C. Fletcher. Hagerstown, MD: university Publishing Group; 2000.
Miller FG. Placebo-controlled trials in psychiatric research: an ethical perspective. Biological psychiatry. 2000;47(8):707-16. Epub 2000/04/22. http://www.ncbi.nlm.nih.gov/pubmed/10773177
Salem-Schatz S, Emanuel EJ, Weeks JC. Symptoms and suffering at the end of life in children with cancer. New England Journal of Medicine. 2000;342(5):326-33.
Smith FO, Thomson BG. Umbilical cord blood collection, banking, and transplantation: current status and issues relevant to perinatal caregivers. Birth. 2000;27(2):127-35. Epub 2001/03/17. http://www.ncbi.nlm.nih.gov/pubmed/11251491
Sreenivasan G. What is the general will? The Philosophical Review. 2000;109(4):545-81.
Titlow K, Randel L, Clancy CM, Emanuel EJ. Drug coverage decisions: the role of dollars and values. Health Aff (Millwood). 2000;19(2):240-7. Epub 2000/03/16. http://www.ncbi.nlm.nih.gov/pubmed/10718038
Weijer C, Emanuel EJ. Ethics. Protecting communities in biomedical research. Science. 2000;289(5482):1142-4. Epub 2000/09/02. http://www.ncbi.nlm.nih.gov/pubmed/10970227
Wendler D. Informed consent, exploitation and whether it is possible to conduct human subjects research without either one. Bioethics. 2000;14(4):310-39. Epub 2002/01/05. http://www.ncbi.nlm.nih.gov/pubmed/11758586
Wilfond BS, Thomson E. Models of Public Health Genetics Policy Development. In: Khoury MJ, Burke W, Thompson EJ, editors. Genetics and Public Health in the 21st Centure: Using Genetic Information to Improve Health and Disease. New York: Oxford University Press; 2000.
Wilfond BS. Genetic testing. In: Sugarman J, editor. Twenty Common Problems: Ethics in Primary Care. New York: McGraw Hill; 2000.
Willems DL, Daniels ER, van der Wal G, van der Maas PJ, Emanuel EJ. Attitudes and practices concerning the end of life: A comparison between physicians from the United States and from the Netherlands. Archives of internal medicine. 2000;160(1):63-8.
Wolfe J, Grier HE, Klar N, Levin SB, Ellenbogen JM, Salem-Schatz S, et al. Symptoms and suffering at the end of life in children with cancer. The New England journal of medicine. 2000;342(5):326-33. Epub 2000/02/03. http://www.ncbi.nlm.nih.gov/pubmed/10655532
Wolfe J, Klar N, Grier HE, Duncan J, Salem-Schatz S, Emanuel EJ, et al. Understanding of prognosis among parents of children who died of cancer: impact on treatment goals and integration of palliative care. JAMA. 2000;284(19):2469-75. Epub 2000/11/14. http://www.ncbi.nlm.nih.gov/pubmed/11074776
Burton SL. Why liberals should embrace managed care. Journal of health politics, policy and law. 1999;24(5):911-9. Epub 2000/01/01. http://www.ncbi.nlm.nih.gov/pubmed/10615600
Danis M, Federman D, Fins JJ, Fox E, Kastenbaum B, Lanken PN, et al. Incorporating palliative care into critical care education: principles, challenges, and opportunities. Critical care medicine. 1999;27(9):2005-13. Epub 1999/10/03. http://www.ncbi.nlm.nih.gov/pubmed/10507632
Dickert N, Grady C. What's the price of a research subject? Approaches to payment for research participation. The New England journal of medicine. 1999;341(3):198-203. Epub 1999/07/15. http://www.ncbi.nlm.nih.gov/pubmed/10403861
Emanuel E, Rackoff J. What will it take to restore patient trust? Business and health. 1999:61-4.
Emanuel EJ, Fairclough DL, Slutsman J, Alpert H, Baldwin D, Emanuel LL. Assistance from family members, friends, paid care givers, and volunteers in the care of terminally ill patients. The New England journal of medicine. 1999;341(13):956-63. Epub 1999/09/25. http://www.ncbi.nlm.nih.gov/pubmed/10498492
Emanuel EJ. Choice and representation in health care. Medical care research and review : MCRR. 1999;56 Suppl 1:113-40. Epub 1999/06/04. http://www.ncbi.nlm.nih.gov/pubmed/10354680
Emanuel EJ. Death's Door: The End of Euthanasia? The New Republic. 1999 May 17:15-6.
Emanuel EJ. Eight is too many: the case against octuplets. The New Republic. 1999 January 25:10-1.
Emanuel EJ. The End of Euthanasia? Death's Door. The New Republic. 1999:15-6.
Emanuel EJ. What is the great benefit of legalizing euthanasia or physician-assisted suicide? Ethics. 1999;109(3):629-42. Epub 2001/10/20. http://www.ncbi.nlm.nih.gov/pubmed/11657615
Grady C. Ethics and genetic testing. Advances in internal medicine. 1999;44:389-411. Epub 1999/02/04. http://www.ncbi.nlm.nih.gov/pubmed/9929717
Grady C. Grappling with global concerns in the search for an HIV vaccine. The Journal of the Association of Nurses in AIDS Care : JANAC. 1999;10(1):17-20. Epub 1999/02/06. http://www.ncbi.nlm.nih.gov/pubmed/9934666
Jaworska A. Respecting the margins of agency: Alzheimer's patients and the capacity to value. Philosophy & public affairs. 1999;28(2):105-38. Epub 2001/10/20. http://www.ncbi.nlm.nih.gov/pubmed/11657616
Miller FG, Rosenstein DL. Independent capacity assessment: a critique. BioLaw. 1999;Special Section:432-9.
Parascandola M. Just say Whoa: before new medicines are approved, they have to pass some very tough tests. In: Post TW, editor. Washington, DC1999.
Titlow K, Emanuel E. Employer decisions and the seeds of backlash. Journal of health politics, policy and law. 1999;24(5):941-7. Epub 2000/01/01. http://www.ncbi.nlm.nih.gov/pubmed/10615603
Titlow KI, Rackoff JE, Emanuel EJ. What will it take to restore patient trust? Business and health. 1999:61-4.
Weijer C, Goldsand G, Emanuel EJ. Protecting communities in research: current guidelines and limits of extrapolation. Nature genetics. 1999;23(3):275-80. Epub 1999/11/05. http://www.ncbi.nlm.nih.gov/pubmed/10545946
Wendler D. The importance of autonomy not being all-important. BioLaw. 1999;S:445-51.
Wendler D. Understanding the 'conservative' view on abortion. Bioethics. 1999;13(1):32-56. Epub 2001/10/20. http://www.ncbi.nlm.nih.gov/pubmed/11657058
Wilfond BS, Taussig LM. Cystic Fibrosis: Clinical Overview. In: Taussig LM, Landau LI, editors. Textbook of Pediatric Pulmonary Medicine. St. Louis: Mosby-Year Book; 1999. p. 982-90.
Wilfond BS. Genetic testing of umbilical cord samples: the role of parental permission and non-disclosure of gtenetic information. Cancer Research, Therapy and Control. 1999;8:347-9.
Wolfe J, Fairclough DL, Clarridge BR, Daniels ER, Emanuel EJ. Stability of attitudes regarding physician-assisted suicide and euthanasia among oncology patients, physicians, and the general public. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999;17(4):1274. Epub 1999/11/24. http://www.ncbi.nlm.nih.gov/pubmed/10561189
Biddle AK, DeVellis RF, Henderson G, Fasick SB, Danis M. The health insurance puzzle: a new approach to assessing patient coverage preferences. Journal of community health. 1998;23(3):181-94. Epub 1998/06/06. http://www.ncbi.nlm.nih.gov/pubmed/9615294
Burke W, Thomson E, Khoury MJ, McDonnell SM, Press N, Adams PC, et al. Hereditary hemochromatosis: gene discovery and its implications for population-based screening. JAMA. 1998;280(2):172-8. Epub 1998/07/21. http://www.ncbi.nlm.nih.gov/pubmed/9669792
Danis M, Grady C. Institutional Review Board review and consent for research: what's behind the statistics? Critical care medicine. 1998;26(9):1488-9. Epub 1998/09/29. http://www.ncbi.nlm.nih.gov/pubmed/9751583
Danis M. Improving end-of-life care in the intensive care unit: what's to be learned from outcomes research? New Horiz. 1998;6(1):110-8. Epub 1998/03/21. http://www.ncbi.nlm.nih.gov/pubmed/9508265
Emanuel EJ, Battin MP. What are the potential cost savings from legalizing physician-assisted suicide? The New England journal of medicine. 1998;339(3):167-72. Epub 1998/07/17. http://www.ncbi.nlm.nih.gov/pubmed/9664094
Emanuel EJ, Daniels ER, Fairclough DL, Clarridge BR. The practice of euthanasia and physician-assisted suicide in the United States: adherence to proposed safeguards and effects on physicians. JAMA. 1998;280(6):507-13. Epub 1998/08/26. http://www.ncbi.nlm.nih.gov/pubmed/9707132
Emanuel EJ, Goldman L. Protecting patient welfare in managed care: six safeguards. Journal of health politics, policy and law. 1998;23(4):635-59. Epub 1998/08/27. http://www.ncbi.nlm.nih.gov/pubmed/9718517
Emanuel EJ, Patterson WB. Ethics of randomized clinical trials. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1998;16(1):365-6; discussion 6-71. Epub 1998/01/24. http://www.ncbi.nlm.nih.gov/pubmed/9440766
Emanuel EJ. A world of research subjects. The Hastings Center report. 1998;28(6):25. Epub 1998/12/30. http://www.ncbi.nlm.nih.gov/pubmed/9868607
Emanuel EJ. The blossoming of bioethics at NIH. Kennedy Institute of Ethics journal. 1998;8(4):455-66. Epub 2001/10/20. http://www.ncbi.nlm.nih.gov/pubmed/11657322
Emanuel EJ. The future of euthanasia and physician-assisted suicide: beyond rights talk to informed public policy. Minnesota law review. 1998;82(4):983-1014. Epub 2002/02/28. http://www.ncbi.nlm.nih.gov/pubmed/11865919
Grady C, Anderson R, Chase GA. Fatigue in HIV-infected men receiving investigational interleukin-2. Nursing research. 1998;47(4):227-34. Epub 1998/07/31. http://www.ncbi.nlm.nih.gov/pubmed/9683118
Grady C. Ethics, Genetics, and Nursing Practice. In: Jenkins LD, Francomano C, editors. Genetics in Clinical Practice. Boston: James Bartlett Publishers; 1998.
Grady C. Science in the service of healing. The Hastings Center report. 1998;28(6):34-8. Epub 1998/12/30. http://www.ncbi.nlm.nih.gov/pubmed/9868609
Miller FG, Rosenstein DL, DeRenzo EG. Professional integrity in clinical research. JAMA. 1998;280(16):1449-54. Epub 1998/11/04. http://www.ncbi.nlm.nih.gov/pubmed/9801009
Mischler EH, Wilfond BS, Fost N, Laxova A, Reiser C, Sauer CM, et al. Cystic fibrosis newborn screening: impact on reproductive behavior and implications for genetic counseling. Pediatrics. 1998;102(1 Pt 1):44-52. Epub 1998/07/04. http://www.ncbi.nlm.nih.gov/pubmed/9651412
Pearson SD, Sabin JE, Emanuel EJ. Ethical guidelines for physician compensation based on capitation. The New England journal of medicine. 1998;339(10):689-93. Epub 1998/09/03. http://www.ncbi.nlm.nih.gov/pubmed/9725929
Wendler D. Innateness as an explanatory concept. Biology and Philosophy. 1998;11(1):89-116.
Wendler D. When should "riskier" subjects be excluded from research participation? Kennedy Institute of Ethics journal. 1998;8(3):307-27. Epub 2001/10/20. http://www.ncbi.nlm.nih.gov/pubmed/11656935
Werner L, Septimus A, Grady C. Psychological support and ethical issues for the child and family. In: Weijert C, editor. Pediatric AIDS. Baltimore: Lippincott, Williams, and Wilkins; 1998.
Grady C. HIV Disease: Ethical Considerations for Clinicians. In: DeVita V, Hellman S, Rosenberg S, Lunan J, Essex M, Fauci AS, editors. Genetics in AIDS: Etiology, Diagnosis, Prevention, and Treatment. Philadelphia: JB Lippincott; 1997.
Wendler D. Deception in medical and behavioral research: is it ever acceptable? The Milbank quarterly. 1996;74(1):87-114. Epub 1996/01/01. http://www.ncbi.nlm.nih.gov/pubmed/8596525
Wendler D. Locke's acceptance of innate concepts. Australasian Journal of Philosophy. 1996;74(3):467-83.
Wertheimer A. Exploitation. Princeton, New Jersey: Princeton University Press; 1996. 316 p.
Grady C. The Search for an AIDS Vaccine. Bloomington, IN: Indiana University Press; 1995. 193 p.
Emanuel EJ. The Ends of Human Life: Medical Ethics in a Liberal Polity. Cambridge, MA: Harvard University Press; 1991. 307 p.
Wertheimer A. Coercion. Princeton, NJ: Princeton University Press; 1987. 318 p.
You are now leaving the NIH Clinical Center website.
This external link is provided for your convenience to offer additional information. The NIH Clinical Center is not responsible for the availability, content or accuracy of this external site.
The NIH Clinical Center does not endorse, authorize or guarantee the sponsors, information, products or services described or offered at this external site. You will be subject to the destination site’s privacy policy if you follow this link.
More information about the NIH Clinical Center Privacy and Disclaimer policy is available at http://www.cc.nih.gov/disclaimers.html
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

Bioethics news, resources and funding for global health researchers
Fogarty aims to strengthen research bioethics expertise in developing countries by providing resources and information to bioethicists, research ethics committee members, researchers, students, and everyone else involved in international health research. In keeping with its wider commitment to building the capacity to conduct research in low- and middle-income countries (LMICs), Fogarty believes it is important to build in-country bioethics capacity. This enables local ethics review of research, improves research into the distinctive ethical problems in LMICs, and increases the representation of different voices in on-going global discussions of the ethics of health research.
Bioethics Resources
- For Teachers and Students - teaching tools, career path advice, and online education information
- For Investigators and Ethics Committees - regulations, guidelines, practical aids, committee information
- For Bioethics Researchers - journals and granting agencies
- Bioethics Materials in Other Languages - networks, trainings, journals, and guidelines and regulations in a variety of languages
Video: How to develop a bioethics research proposal - Drs. Annette Rid and Robert Steel from the Department of Bioethics at the NIH Clinical Center off guidance on how to develop a research proposal for one of the Department’s programs aimed at training the next generation of bioethics researchers.

COVID-19 Bioethics Resources
- Coronavirus bioethics resources for researchers
Recent Bioethics News
- Public health and research ethics education: the experience of developing a new cadre of bioethicists at a Ugandan institution , co-authored by Fogarty grant recipients Gertrude N. Kiwanuka and Sana Loue BMC Medical Education , January 3, 2024
- Ethical considerations for biobanking and use of genomics data in Africa: a narrative review , co-authored by Fogarty trainees Mary Amoakoh-Coleman and James Abugri BMC Medical Ethics , December 5, 2023
Related Fogarty News and Information

- Fortifying research ethics at Kinshasa University Global Health Matters , March/April 2024
Diversifying voices in bioethics
- A seat at the table
- Bioethics researchers meet at NIH to reflect on the past, look to the future
- Expanding bioethics from community engagement to genetics in Uganda
- Bioethics program perseveres in wartime Ukraine
NIH News and Resources
- NIH on Research Involving Human Subjects from the NIH Office of Extramural Research
- NIH Office of Clinical Research and Bioethics Policy from the NIH Office of Science Policy
- NIH Clinical Center Department of Bioethics
- National Institute of Environmental Health Sciences (NIEHS) Bioethics Resources
- National Human Genome Research Institute (NHGRI) Bioethics Resources
- National Library of Medicine (NLM) Bioethics Resources
Other US Government Resources
- Department of Health and Human Services (HHS) resources:
- International resources from the HHS Office for Human Research Protections (OHRP)
- Secretary's Advisory Committee on Human Research Protections (SACHRP)
- Archive: Presidential Commission for the Study of Bioethical Issues 2009-2017
Other Online Resources
- Global Forum on Bioethics in Research (GFBR)
- Johns Hopkins University: PREVENT (Pregnancy Research Ethics for Vaccines, Epidemics, and New Technologies) Project
- TRUST Equitable Research Partnerships :
- Global code of conduct for research in resource-poor settings , available in English, as well as in other languages , including Arabic, Chinese, French, German, Hindi, Japanese, Portuguese, Russian and Spanish
- Public Health Emergency Preparedness and Response Ethics Network (PHEPREN) : Led by WHO and supported by key partners including Fogarty/NIH. Currently focusing on the COVID-19 pandemic, offers resources, policy briefs, webinars and an ethics advisory platform.
- Free online course: Strengthening Stakeholder Engagement Through Ethics Review - HIV Prevention Trials - Register through their portal to gain free access to online, on-demand course from AVAC: Global Advocacy for HIV Prevention.
Updated April 30, 2024
Center for Bioethics Papers
The Department serves as the hub for interdisciplinary research and collaboration on topics across four research areas in biomedical ethics: neuro- and mental healthcare ethics, health policy, behavioral economics, research ethics, global bioethics, and the ethics of healthcare allocation. Our health policy research follows three tracks: reducing low-value services; economic and health impacts of policies, such as smoking cessation and workplace wellness; and implementation sciences, with specific effort towards replicating effective programs in the healthcare delivery system.
Publication Type
Results per page, search results.
- Publication Research Issues in Genetic Testing of Adolescents for Obesity ( 2004-08-01 ) Segal, Mary E ; Sankar, Pamela ; Reed, Danielle R Show more Obesity is often established in adolescence, and advances are being made in identifying its genetic underpinnings. We examine issues related to the eventual likelihood of genetic tests for obesity targeted to adolescents: family involvement; comprehension of the test’s meaning; how knowledge of genetic status may affect psychological adaptation; minors’ ability to control events; parental/child autonomy; ability to make informed medical decisions; self-esteem; unclear distinctions between early/late onset for this condition; and social stigmatization. The public health arena will be important in educating families about possible future genetic tests for obesity. Show more
- Publication Successes and Failures of Hospital Ethics Committees: A National Survey of Ethics Committee Chairs ( 2002-01-01 ) McGee, Glenn E ; Caplan, Arthur L. ; Spanogle, Joshua P ; Asch, David A. ; Penny, Dina Show more In 1992, the Joint Commission on the Accreditation of Healthcare Organizations (JCAHO) passed a mandate that all its approved hospitals put in place a means for addressing ethical concerns. Although the particular process the hospital uses to address such concerns—ethics consultant, ethics forum, ethics committee—may vary, the hospital or healthcare ethics committee (HEC) is used most often. In a companion study to that reported here, we found that in 1998 over 90% of U.S. hospitals had ethics committees, compared to just 1% in 1983, and that many have some and a few have sweeping clinical powers in hospitals. Show more
- Publication Desktop Medicine ( 2010-11-10 ) Karlawish, Jason Show more
- Publication A Pilot Survey on the Licensing of DNA Inventions ( 2003-09-01 ) Henry, Michelle R ; Cho, Mildred K ; Weaver, Meredith A ; Merz, Jon F Show more Despite ethical concerns about gene patents, virtually no empirical evidence exists to support claims about either positive or negative effects, and extremely little is known about the intellectual property protection strategies of firms and universities. This article discusses the results of a pilot study to examine patenting and licensing philosophies, policies, and practices of different types of institutions and to describe the contractual conditions for licensing DNA sequence inventions. Show more
- Publication Open Label Extension Studies and the Ethical Design of Clinical Trials ( 2001-08-01 ) Cassarett, David ; Karlawish, Jason ; Sankar, Pamela ; Hirschman, Karen ; Asch, David A. Show more
- Publication The Triumph of Autonomy in Bioethics and Commercialism in American Healthcare ( 2007-01-01 ) Moreno, Jonathan Show more Justifying his proposal for "health savings accounts," which would allow individuals to set aside tax-free dollars against future healthcare needs, President Bush has said that "Health savings accounts all aim at empowering people to make decisions for themselves." Who could disagree with such a sentiment? Although bioethicists may be among those who express skepticism that personal health savings accounts will be part of the needed "fix" of our healthcare financing system, self determination has long been part of their mantra. Indeed, the field of bioethics played an important role in advancing this idea in the medical world when physician paternalism was regnant. Has its popularity caused it to become so vapid as to be ripe for misuse? Show more
- Publication Pilot Study: Does the White Coat Influence Research Participation? ( 2002-07-01 ) Merz, Jon F ; Rebbeck, Timothy R ; Sankar, Pamela ; Meagher, Emma A Show more In health care, the white coat symbolizes professionalism, trustworthiness, and competence; it also represents power. This suggests that the wearing of a white coat could influence the decisions of potential subjects who are asked to participate in clinic-based research. Show more
- Publication Bioethics Consultation in the Private Sector ( 2002-06-01 ) Brody, Baruch ; Dubbler, Nancy ; Caplan, Arthur L. ; Blustein, Jeff ; Kahn, Jeffrey P ; Kass, Nancy ; Moreno, Jonathan ; Lo, Bernard ; Sugarman, Jeremy ; Zoloth, Laurie Show more The members of a task force on bioethics consultation report their conclusions. The task force was convened by the American Society for Bioethics and Humanities and the American Society of Law, Medicine, and Ethics, although these groups do not endorse the group's conclusions. Two commentaries follow, and an essay by science reporter Nell Boyce sets the scene. Show more
- Publication Ethical Considerations in Treating the Horse with Laminitis ( 2004-03-01 ) Fiester, Autumn ; Mann, Lori Show more The nature of laminitis - its unpredictable course, the severe pain and disability it causes, the lengthy convalescence it requires even when cured - poses challenging ethical quandaries for the clinicians who treat it and the owners whose horses suffer from it. Unique among equine ailments, this disease places owners and clinicians in the untenable position of trying to balance considerations that are very difficult to weigh against each other: the animal's pain, the unknown disease trajectory, the questionable possibility of full recovery, the limited usefulness of the animal post-laminitis, the financial drain of treatment, the financial loss of a formerly productive horse, the expense of maintaining a "pasture potato," the animal's frustration or distress during convalescence, etc. The pressing question in every case of laminitis is: where should we draw the line? The answer to this question will not only be different in every individual case of laminitis, but different owners and clinicians will often have divergent views even regarding the same case. In an ethical terrain that is so clearly "gray," absolutes are unlikely to be found. Instead, our essay hopes to clarify the ethical considerations involved in treating a horse with laminitis to facilitate the decision-making process regarding the specific cases encountered by clinicians in the field. Show more
- Publication Older Adults’ Attitudes Toward Enrollment of Noncompetent Subjects Participating in Alzheimer’s Research ( 2009-02-01 ) Karlawish, Jason ; Rubright, Jonathan ; Casarett, David ; Cary, Mark ; Ten Have, Thomas ; Sankar, Pamela Show more OBJECTIVE: Research that seeks to enroll noncompetent patients with Alzheimer’s disease without presenting any potential benefit to participants is the source of substantial ethical controversy. The authors used hypothetical Alzheimer’s disease studies that included either a blood draw or a blood draw and lumbar puncture to explore older persons’ attitudes on this question. METHOD: Face-to-face interviews were conducted with 538 persons age 65 and older. Questions explored participants’ understanding of research concepts, their views on enrolling persons with Alzheimer’s disease in research, and their preferences regarding having a proxy decision maker, granting advance consent, and granting their proxy leeway to override the participant’s decision. Additional questions assessed altruism, trust, value for research, and perceptions of Alzheimer’s disease. RESULTS: The majority (83%) were willing to grant advance consent to a blood draw study, and nearly half (48%) to a blood draw plus lumbar puncture study. Most (96%) were willing to identify a proxy for research decision making, and most were willing to grant their proxy leeway over their advance consent: 81% for the blood draw study and 70% for the blood draw plus lumbar puncture study. Combining the preferences for advance consent and leeway, the proportion who would permit being enrolled in the blood draw and lumbar puncture studies, respectively, were 92% and 75%. Multivariate models showed that willingness to be enrolled in research was most strongly associated with a favorable attitude toward biomedical research. CONCLUSIONS: Older adults generally support enrolling noncompetent persons with Alzheimer’s disease into research that does not present a benefit to subjects. Willingness to grant their proxy leeway over advance consent and a favorable attitude about biomedical research substantially explain this willingness. Show more
- 1 (current)
Usage statistics
- Frequently downloaded items
- Most read researchers
- Popular subjects
- International readership
DSpace software copyright © 2002-2024 LYRASIS
- Cookie settings
- Privacy policy
- End User Agreement
- Send Feedback
Thesis Helpers
Find the best tips and advice to improve your writing. Or, have a top expert write your paper.
Bioethics Topics For Your Research Paper Or Thesis

College and university students that are pursuing biology or applied science programs are required to write essays and papers on bioethics topics. But, what is bioethics? Well, bioethics is an interdisciplinary approach to addressing moral dilemmas and quandaries that arise from the study of medical science and applied biology. Sometimes it is a bit difficult to write it by yourself. That is why an excellent solution might be to use our biology thesis writing service .
Most bioethical topics involve the application of religious values, philosophical principles, and societal mores. They also involve human judgment when it comes to deciding on the life and death of humans, medical treatment, and health, as well as, how humans relate with living organisms, and environmental issues.
What Makes Great Bioethics Topics?
Based on the above bioethics definition, students can choose many topics to write about. However, not every topic is ideal for every student. As such, learners should know what makes great topics for their papers or essays. Here are some of the things that make great bioethics paper topics prepered by our best thesis writers.
Interesting to the students because they will work with them and spend time reading about them. Related to the latest and current topics to make the final work publishable. Different from what has already been published because students should say something unique and new to have their papers published.
It’s also important to ensure that the chosen topics address the current bioethical issues. Perhaps, what makes selecting a topic for research difficult is determining what issues in bioethics are worth researching and writing about. So, what are bioethical issues?
4 Principles of Bioethics that are Worth Writing About
Students can write papers on any of the four principles of bioethics. These are:
- Principle of respect for autonomy
- Principle of non-maleficence
- Principle of justice
- Principle of beneficence
Medical practices are considered ethical if they respect these principles. Therefore, bioethics research paper topics should be selected and analyzed with these principles in mind. Due to the broad nature of bioethics as a study field, topics in this research area can be classified into the following categories.
Professionalism Bioethical Topics
Physicians are trained and experienced professionals that understand the meaning of professionalism. Here are examples of bioethical issues that touch on this profession.
- Altruism – Physicians are obliged to attend to their patients’ best interests instead of self-interest.
- Accountability – Physicians should be accountable to patients, profession, and society on public health issues.
- Excellence – Physicians are obliged to commit to life-long learning.
- Duty – Physicians should be responsive and available on call and commit to serving within the community and profession.
- Integrity and Honor – A physician should commit to being truthful, straightforward, and fair in their professional and interactions with their patients.
The list of bioethics topics that touch on the professionalism of physicians can be longer than this. But learners should choose topics that enable them to explore what they find interesting and the latest.
Principle of Non-Maleficence Topics
According to the principle of non-maleficence, physicians should not intentionally inflict injury or harm to patients, either via acts of omission or commissions. Here are some of the most controversial bioethical topics in this category:
- Discuss the nature of an act concerning the double effect principle
- Explain the distinction between the means and effects in bioethics
- Explain the proportionality between good and bad effect
- The agent’s intention- How can an agent intend to achieve good effect even though it’s foreseen?
- Withhold or withdraw life-sustaining treatment- Which one is lesser or greater harm for self?
Readers can apply different criteria to write papers on bioethics research topics. Nevertheless, each of them requires extensive research and consideration of the basic principles of bioethics.
Advance Care Planning Bioethical Topics
Advance care planning entails helping patients with their decision-making capacity. Some of the most interesting bioethics topics in this category include:
- How can physicians raise advanced care planning issues without scaring their patients?
- How can physicians advise patients if they believe that family members are likely to disagree with their wishes?
- Where and when should physicians start advance care planning?
- Who should physicians approach when it comes to advance care planning?
- What are the differences between advance care planning and advance directives?
This category has some of the most common bioethical issues today especially among patients with chronic conditions.
HIV And AIDS Biomedical Ethics Topics
Physicians face several ethical issues when dealing with individuals that live with HIV and AIDS. Such issues, therefore, can’t miss in a bioethics topics list. They include:
- What should a physician do when a patient refuses to undergo an HIV test?
- When should the HIV status of a patient be reported to the public health department?
- Should protease inhibitors be prescribed to a patient that is unlikely to follow the treatment regimen to the end?
- How can a physician facilitate communication between the members of a family?
- How can a physician deal with prejudice when addressing HIV/AIDS cases?
These bioethics debate topics address sensitive issues that practitioners face almost every day. As such, they require careful research and adherence to the principles of bioethics.
Medical Futility Topics
Medical futility is the intervention that may not yield significant benefits for a patient. Some of the biomedical ethics topics that touch on medical futility include:
- What ethical obligations do physicians have when an intervention is considered futile by a healthcare provider?
- Who decides that treatment should be considered futile?
- What happens when a family or patient requests for an intervention that is considered futile by a healthcare team?
- What’s the difference between rationing and futility?
- What’s the difference between experimental intervention and futile intervention?
Public Health Ethics Topics
Students can write about bioethical issues in healthcare. Some of the topics in this category include:
- When should physicians report a communicable disease to public health authorities?
- Can public health physicians provide medical treatment against the will of a patient?
- What should public health physicians do when a patient refuses to undergo regular preventive health measures?
- What is the fair distribution of health?
- Explain what paternalism is and whose responsibility health is.
These are also great bioethical issues in nursing. They can be used to research and write papers and essays in colleges and universities.
List Of Bioethical Issues
Several bioethical issues topics can be used to understand and address mistakes in this field. They include:
- How do physicians make mistakes?
- Is it an ethical duty for physicians to disclose medical mistakes to patients?
- How can a physician decide to tell their patient about a mistake?
- Can disclosing mistakes undermine the trust a patient has in a physician?
- Does a physician risk facing a malpractice suit if they disclose a mistake?
- What should a physician do if they see their colleague make a mistake?
Interdisciplinary Team Issues Topics
Some of the interdisciplinary team issues that amount to bioethics issues worth studying include:
- How do interdisciplinary teams work?
- Who should be in charge of a team in an operating room?
- What ethical obligations do interdisciplinary team members have when it comes to patient care?
- What is meant by “respectful” views exchange?
- How should members handle disagreements in a multidisciplinary team?
Resource Allocation Bioethics Research Topics
Some resource allocation issues form great bioethics essay topics for any type of assignment, from a short essay to a biology dissertation . These include:
- What should guide rationing decisions?
- Are triage decisions guided by ethical criteria?
- Discuss the major macro-allocation concerns
- Should allocation decisions be based on the quality of life judgments?
- Can a “right to healthcare” be ethically qualified?
Spirituality And Medicine Topics
In most cases, a list of bioethical topics will include issues of spirituality and medicine. Examples of topics in this category include:
- How does religiosity affect medicine?
- What should medicine attend to spirituality?
- How should physicians take a spiritual history?
- How should physicians work with the hospital chaplains?
- What’s the role of a physician’s personal belief in a physician-patient relationship?
This is not an exhaustive bioethical issues list. We advise you to take a look at epidemiology topics as well. Students should read, watch, and listen to bioethics news to stay updated on the latest issues in their field that are worth researching and writing about.
Get Help Writing Bioethics Essay
Now that you have a great list of bioethical topics, you might be thinking ‘How can I get someone to write my dissertation for me ?” Consider referring to high quality thesis writing services. Our service offers the help of the best experts, who are all educated and perfect native English speakers and writers. They will provide with an advanced, well researched essay or thesis that your professor will admire.

Make PhD experience your own
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
All Research Papers
The Anscombe Bioethics Centre is supported by the Catholic Church in England and Wales, Scotland, and Ireland, but has also always relied on donations from generous individuals, friends and benefactors.
FIND OUT MORE MAKE A DONATION
Cookie notice
We use cookies on our website. They help us to know a little bit about you and how you use our website. They are stored locally on your computer or mobile device. If you continue without changing your settings, we’ll assume that you are happy to receive all cookies. However, you can change your cookie settings at any time. Go to the cookie policy for more information and to update your preferences.
Your cookie preferences
We use cookies and similar technologies on our website. Cookies are text files containing small amounts of information, which your computer or mobile device downloads when you visit a website. When you return to websites, or visit websites that use the same cookies, they recognise these cookies and therefore your browsing device.
We use different types of cookies for different things, such as:
- Analysing how you use our website
- Giving you a better, more personalised experience
- Recognising when you’ve sign in
We split our cookies into three distinct categories. You can turn Functional and Performance cookies on and off right here. Strictly necessary cookies can’t be turned off.
Strictly necessary cookies
These cookies are essential in order to enable you to move around the website and use its features. Without these cookies services you have asked for cannot be provided.
Some examples of how we use these cookies are:
- Signing into our website
- Remembering previous actions such as text entered into a form when navigating back to a page in the same session
- Remembering security settings
Project-based learning in bioethics education
- Published: 15 May 2024
Cite this article

- Joseph Tham ORCID: orcid.org/0000-0002-6514-4107 1
Higher education has become more student-centered as the Bologna process assigns students more time to study and research. Online teaching has been needed during the pandemic, which can be challenging regarding didactic and assessment. This paper analyzes project-based learning (PBL) as a form of teaching and assessing students in a bioethics course on reproductive ethics. The team project was the final assessment of the Faculty of Bioethics core curriculum course, "Bioethics, Technology and Procreation,” offered to two student groups in the 2019–2020 school year. The analysis of the results of PBL is descriptive qualitative with semi-quantitative data from student feedback. Forty students were presented with a detailed methodology of team projects and were encouraged to form teams of 3 to 5. They need to develop a team project with creativity and pastoral sensibility that will communicate the content assimilated in class to a selected target audience, with adequate means to measure the impact of their project on their target. Thirty-eight students formed ten teams and presented ten projects. Each team had 2–4 encounters with the professor and 6–10 encounters among team members. Six of the ten projects were categorized as didactic, three mediatic, one didactic-mediatic and 0 artistic. The grades of this final assignment ranged from 7 to 10, with an average of 8.7 out of 10. The survey feedback demonstrated high satisfaction, as students discovered new values in teamwork and pastoral applications. However, this innovation can be more time-consuming for the professor and the students, requiring more significant time management, teamwork and communication competency.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
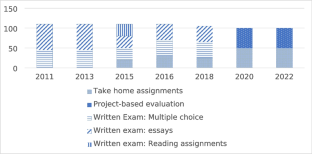
Título de Experto en Docencia Universitaria, Universidad Francisco de Vitoria, Escuela de Posgrado y Formación Permanente, www.postgrado.ufv.es
AAC&U. (n.d.). VALUE Rubrics—Teamwork . AAC&U. Retrieved February 27, 2024, from https://www.aacu.org/initiatives/value-initiative/value-rubrics/value-rubrics-teamwork
Al Moush, S. and A. Farhat. 2023. The power of authentic assessment in the age of AI. Faculty Focus | Higher Ed Teaching & Learning . https://www.facultyfocus.com/articles/educational-assessment/the-power-of-authentic-assessment-in-the-age-of-ai/ . Accessed 28 Feb 2024
Bender, W.N. 2012. Project-based learning: Differentiating instruction for the 21st century . Thousand Oaks: Corwin / SAGE Publications.
Google Scholar
Buck Institute for Education. n.d. What is PBL? PBLWorks. Retrieved February 28, 2024, from https://www.pblworks.org/what-is-pbl
Congregation for the Doctrine of the Faith. 1987. Donum Vitae, instruction on respect for human life . http://www.vatican.va/roman_curia/congregations/cfaith/documents/rc_con_cfaith_doc_19870222_respect-for-human-life_en.html . Accessed 28 Feb 2024
Congregation for the Doctrine of the Faith. 2008. Instruction Dignitas Personae on certain bioethical questions . http://www.vatican.va/roman_curia/congregations/cfaith/documents/rc_con_cfaith_doc_20081208_dignitas-personae_en.html . Accessed 28 Feb 2024
Czarnecki, D. 2015. Moral women, immoral technologies: How devout women negotiate gender, religion, and assisted reproductive technologies. Gender and Society 29 (5): 716–742.
Article Google Scholar
Edutopia. (n.d.). Project-based learning (PBL) . Edutopia. Retrieved February 28, 2024, from https://www.edutopia.org/project-based-learning
Graduate Management Admission Council. 2020. The bologna accord: A European revolution with global implications . https://web.archive.org/web/20200324105130/https://www.gmac.com/why-gmac/gmac-news/gmnews/2005/january-february/the-bologna-accord-a-european-revolution-with-global-implications . Accessed 28 Feb 2024
Kovácsné Pusztai, K. 2021. Evaluation of project-based learning. Acta Didactica Napocensia 14 (1):64–75. https://doi.org/10.24193/adn.14.1.5
Learning Leap Consultants. n.d. The role of Bloom’s taxonomy in project based learning – Learning leap consultants . Retrieved February 28, 2024, from https://learningleapconsultants.com/role-blooms-taxonomy-projectbased-learning/
PBLWorks (Director). 2010. Project based learning: Explained. https://www.youtube.com/watch?v=LMCZvGesRz8 . Accessed 28 Feb 2024
Ramos-Ramos, P. and A. M. B. Nicolás. 2022. Teaching dilemmas and student motivation in project-based learning in secondary education. Interdisciplinary Journal of Problem-Based Learning 16(1):Article 1. https://doi.org/10.14434/ijpbl.v16i1.33056
Sgreccia, E. 2012. Personalist bioethics: Foundations and applications . J. A. D. Camillo & M. J. Miller, Trans. 1 edition. National Catholic Bioethics Center.
The Bologna Process and the European Higher Education Area | European Education Area . (n.d.). Retrieved February 20, 2024, from https://education.ec.europa.eu/education-levels/higher-education/inclusive-and-connected-higher-education/bologna-process . Accessed 28 Feb 2024
Vial Correa, J. D. D. and E. Sgreccia. 2005. The dignity of human procreation and reproductive technologies: Anthropological and ethical aspects . Libreria Editrice Vaticana. http://www.academiavita.org/_pdf/assemblies/10/the_dignity_of_human_procreation_and_reproductive_technologies.pdf . Accessed 28 Feb 2024
Vogler, J.S., P. Thompson, D.W. Davis, B.E. Mayfield, P.M. Finley, and D. Yasseri. 2018. The hard work of soft skills: Augmenting the project-based learning experience with interdisciplinary teamwork. Instructional Science 46 (3): 457–488. https://doi.org/10.1007/s11251-017-9438-9 .
YourEduAction. 2014. Cos’è L’Apprendimento Basato sul Progetto (Project Based Learning). Your Edu Action . https://www.youreduaction.it/project-based-learning/ . Accessed 28 Feb 2024
Download references
Author information
Authors and affiliations.
Faculty of Bioethics, Regina Apostolorum Pontifical Ateneo, Rome, Italy
Joseph Tham
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Joseph Tham .
Ethics declarations
Competing interests, additional information, publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1: Course syllabus (abridged for this publication)
Description: This course seeks to help the students understand the issues and debates surrounding infertility and ART. The student should assimilate the scientific data, the ethical positions, and the challenges of a secular mindset on these issues. Learning is attained through lessons, team and individual assignments, forums, debates and presentations to demonstrate the capacity to translate the acquired knowledge into pastoral projects.
Objective: This course seeks to help the students understand the issues and debates surrounding infertility and ART. The student should be able to assimilate the personalist bioethics position that coincides with the Catholic magisterium and should be able to defend this position in the current secular mindset, translating this knowledge into pastoral projects that could help propagate this understanding.
Competence: Students graduating from this course should have acquired knowledge about infertility problems, treatment options, and ethical positions. They should have assimilated the personalist bioethics vision and be able to explain and defend this position and apply it with pastoral creativity and sensibility.
Course content:
The human meaning of procreation : a) Today's vision of procreation; b) Personalist view of sexuality and procreation; c) Procreate and produce: two different human acts.
Scientific data : a) Infertility: causes, diagnosis, and treatment; b) natural methods; c) NaProTechnology; Assisted Reproductive Technologies (ART).
Ethics of ART : a) Judgment according to DP; b) Judgment according to the Magisterium; c) Surrogate motherhood; d) Laws and policy on procreation; e) Destiny for frozen embryos; f) Pastoral aspects.
Methodology
The course will consist of lectures with activities, forums and student presentations. Outside the classroom, the students will need to prepare weekly assignments that are individual or in teams. The final assessment can be a team or individual project demonstrating the content's assimilation and the ability to apply it to a particular audience pastorally.
Credits and distribution of work time
Twelve hours of classes and 18 h of four assignments, including essays, comparison charts, case studies, film forums, and personal readings. 20 h of a final team project (PBL).
Learning outcome
Summarize scientific data on infertility's causes, investigation and treatments. (NFP, NaProTechnology, ART)
Analyze, evaluate and contrast the modern secular mentality on fertility problems (sexuality, procreation, family) with those of the Catholic and personalist vision.
Offer a personal critique of the arguments for and against using ART in infertility treatments.
Analyze and offer ethical judgment on cases related to fertility treatments.
Designate pastoral projects on infertility that may influence society in favor of the culture of life.
Appendix 2: Instructions for PBL final team project
Please follow these steps.
Forming a team.
The number of the team should be at most six students. A smaller team of three to four might work better.
If you decide to work alone or have a team with more than six students, you need special approval from the professor.
The size of the team has a bearing on the final grade—since the larger the team, the more time and energy the project will require to carry out. The project should be proportionate to the time expected on the average of 20 h per student in the team.
Inform the professor of the formation of the team by ____________________.
Proposal of the target and the deliverable or project.
The team should ideally have a leader, a secretary and a spokesperson. The secretary should help with the minutes and final report describing the team project process. The spokesperson could be the one to present the project at the end.
Discuss which target audience is best suited for this project among the team members. Remember, the idea is to devise a project covering the course content's three areas (mentality, scientific data, and ethics) for a specific target. This stage is crucial. You can choose from different possibilities. It might be necessary to do some research in order to decide on the best target.
Discuss also among the team members the project that would be most effective in helping to convince or enlighten the target you have chosen based on the content material of the course. Preliminary research might also be necessary.
Consider the method of measuring the impact. That is, how do you know if the deliverable has been or will be effective in communicating the objective content?
A 1–2 page proposal could be drafted and sent to the professor at this stage. He can give you feedback, either written or by Zoom meeting, to discuss the merits and defects of the proposal. The proposal could take several encounters and exchanges. Once the professor gives you the okay, the team can start to work on the project.
The deliverable
The team should now divide the work among its members reasonably and fairly.
Refer to the rubrics that will give an idea about what is requested in formulating this deliverable. Some suggested research is offered below.
What is the target audience like? What are their background characteristics? How do you reach them? How do you learn more about them and their attitudes toward the subject matter (surveys, literature searches, interviews, etc.)?
How is the deliverable going to reach out to the target audience? How will they learn about it? What kind of recruitment or promotion would be needed? Would the deliverable address their real needs, pastorally speaking (how to verify that)? Are similar resources or activities available as alternatives, and what can I learn from these alternatives?
Does the content of the deliverable correspond to the three areas of content seen in the course? Does the deliverable express an understanding of the mentality of the target audience and address their concerns in a sensitive and pastoral manner? Does the deliverable cover the basic scientific knowledge proportionate to the target's level of education? Are the ethical principles and messages given in a way suitable to the target audience's needs and concerns?
How is the impact factor measured? Is the means of measurement specific to the target audience's background and needs? Is the means of measurement objective and quantifiable?
While finalizing the deliverable, the team should send a progress report to the professor about the advances and difficulties. There should be at least one Zoom encounter with the professor so that he can give his feedback and observations.
Ideally, the project should be delivered to the target audience, and the report should record or describe the process. The impact should also be recorded and reported. This can be part of the final presentation.
The deliverable and the report should be handed to the professor before the final presentation.
A report should describe the entire process of the team project. This report allows the professor to evaluate the competence of teamwork among the members.
It should describe the responsibilities and tasks of the different members.
It should describe the different encounters and decisions of the team.
It should describe how the team arrives at the target, deliverable, and impact measurement decisions.
It should describe the different investigations, the debates, the obstacles, and the team's agreements.
The presentation
Each team has 20 min to present on the final evaluation day.
The presentation is focused on the deliverable, the target and the impact.
The presenter should explain the nature of this deliverable, its goal and objective, and why the team believes it will effectively communicate the course content to the target audience.
If the project has been delivered, what are the results and impact? If not, how will the impact be measured?
It is followed by 10 min of questions and answers by the other teams and the professor.
Appendix 3: Assessment rubrics
Appendix 4: questionnaire.
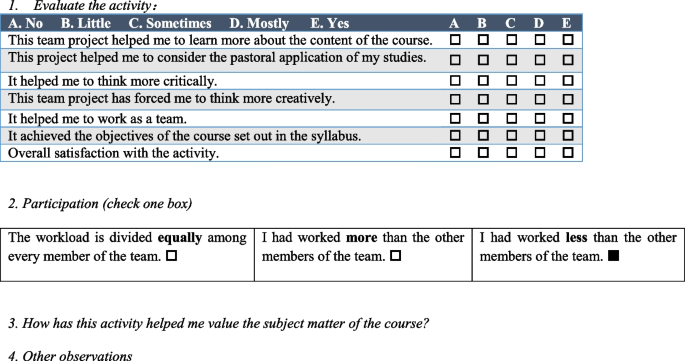
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Tham, J. Project-based learning in bioethics education. International Journal of Ethics Education (2024). https://doi.org/10.1007/s40889-024-00191-3
Download citation
Accepted : 17 April 2024
Published : 15 May 2024
DOI : https://doi.org/10.1007/s40889-024-00191-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Project-based learning
- Reproductive ethics
- Medical ethics
- Catholic theology
- Find a journal
- Publish with us
- Track your research
- Skip to navigation
- Skip to content
Elm Stories
Charm city colloquium explores boundaries and bioethics.

The 2024 colloquium explored the legal and ethical issues that arise when science and medicine cross geopolitical boundaries.
Photo: Diane Hoffmann, director, Law and Health Care Program, and Jacob A. France Professor of Health Care Law, and Jeffrey Khan, director of the Johns Hopkins Berman Institute of Bioethics.
The Charm City Colloquium on Law & Bioethics (CCCLB), a unique partnership between the University of Maryland Francis King Carey School of Law’s Law and Health Care Program and the Johns Hopkins Berman Institute of Bioethics, convened its third gathering May 2-3, 2024.
This year’s event, hosted by Maryland Carey Law, explored the legal and ethical issues that arise when science and medicine cross geopolitical boundaries, especially in contested areas like reproductive rights, new medical practices, and public health.
Faculty from both institutions work together to plan the colloquium — alternately hosting the gathering on their respective campuses. Attendees include faculty, students, and a combination of external experts in law, bioethics, and translational research.
Unlike more formal conferences, the Charm City Colloquium, which launched in 2019, aims to provide scholars with an opportunity for informal dialogue on current topics at the intersection of health policy, law, and ethics. To attend, participants submit a short paper addressing the colloquium’s theme. This year for the first time, the event included faculty who responded to a call for papers.
Diane Hoffmann, JD , Jacob A. France Professor of Health Law at Maryland Carey Law and director of the Law and Health Care Program, welcomed attendees May2, stating, “We are thrilled to be back in person once again after the pandemic, and joined in our efforts by the Johns Hopkins Institute for Clinical and Translational Research,” which she also thanked for generously co-sponsoring the 2024 event through a grant from the National Center for Advancing Translational Research.
Hoffmann also introduced the Berman Institute’s director, Jeffrey Kahn, PhD, MPH, and thanked him for the institute’s support and partnership.
She continued, “Together we created the concept of a colloquium to foster good conversations, strong articles, and a vibrant community of interdisciplinary scholars.”
The opening reception May 2 was followed by a panel titled, “Abortion: Right Here, Wrong There,” moderated by Leslie Meltzer Henry, JD, PhD , who holds dual appointments at Maryland Carey Law and the Berman Institute. The panelists discussed topics such as the far-reaching implications of the Dobbs decision on pregnant patients, abortion bans in Catholic hospitals, and incarcerated individuals’ access to reproductive health care before and after Dobbs .
On May 3, the colloquium continued with panels on health care, regulating new medical practices, public health, and research ethics. Sessions were moderated by faculty from both institutions, including the law school’s Kathleen Hoke, JD , and Professor Emeritus Karen Rothenberg, JD, MP A, who both hold joint appointments at the Berman Institute. Liza Vertinsky, JD, PhD , a professor at Maryland Carey Law, also presented on a panel, sharing her work related to the misalignment between private incentives and public health needs in the regulation of emerging technologies, particularly artificial intelligence.
This year’s planning committee members included Maryland Carey Law’s Rebecca Hall, JD , managing director of the Law and Health Care Program, as well as professors Henry, Hoffmann, Rothenberg, and Natalie Ram, JD . The Johns Hopkins planning committee contributors included Berman Institute faculty Joseph Ali, JD; Debra Mathews, PhD, MA; Alan Regenberg, MBE; and Travis Rieder, PhD, as well as Megan Kasimatis Singleton, JD, MBE, CIP, of the Johns Hopkins University School of Medicine.
In addition to the colloquium, Maryland Carey Law and the Berman Institute offer a dual JD/Master of Bioethics (MBE) program designed to prepare students for a diverse range of careers in the law and bioethics. Current dual-degree students Marc LeVan, Class of 2026, and Sarah Royka, Class of 2026, attended the colloquium with faculty members from both of their academic institutions.
Hoffmann noted that “the collaboration is important because it brings together diverse perspectives and expertise to address complex issues that have far-reaching implications.”
The 2024 Charm City Colloquium met and exceeded its goal — scholars made valuable connections and received thoughtful feedback on their cutting-edge work. One attendee noted she “underestimated how thought-provoking, engaging, and fun” the colloquium would be.
The Maryland Carey Law Journal of Health Care Law & Policy will publish a symposium issue devoted to articles that develop out of the event.
It is expected that the next Charm City Colloquium will take place at the Berman Institute in the 2025-2026 academic year.
You Might Like

Cultural Relativity and Acceptance of Embryonic Stem Cell Research
Article sidebar.
Main Article Content
There is a debate about the ethical implications of using human embryos in stem cell research, which can be influenced by cultural, moral, and social values. This paper argues for an adaptable framework to accommodate diverse cultural and religious perspectives. By using an adaptive ethics model, research protections can reflect various populations and foster growth in stem cell research possibilities.
INTRODUCTION
Stem cell research combines biology, medicine, and technology, promising to alter health care and the understanding of human development. Yet, ethical contention exists because of individuals’ perceptions of using human embryos based on their various cultural, moral, and social values. While these disagreements concerning policy, use, and general acceptance have prompted the development of an international ethics policy, such a uniform approach can overlook the nuanced ethical landscapes between cultures. With diverse viewpoints in public health, a single global policy, especially one reflecting Western ethics or the ethics prevalent in high-income countries, is impractical. This paper argues for a culturally sensitive, adaptable framework for the use of embryonic stem cells. Stem cell policy should accommodate varying ethical viewpoints and promote an effective global dialogue. With an extension of an ethics model that can adapt to various cultures, we recommend localized guidelines that reflect the moral views of the people those guidelines serve.
Stem cells, characterized by their unique ability to differentiate into various cell types, enable the repair or replacement of damaged tissues. Two primary types of stem cells are somatic stem cells (adult stem cells) and embryonic stem cells. Adult stem cells exist in developed tissues and maintain the body’s repair processes. [1] Embryonic stem cells (ESC) are remarkably pluripotent or versatile, making them valuable in research. [2] However, the use of ESCs has sparked ethics debates. Considering the potential of embryonic stem cells, research guidelines are essential. The International Society for Stem Cell Research (ISSCR) provides international stem cell research guidelines. They call for “public conversations touching on the scientific significance as well as the societal and ethical issues raised by ESC research.” [3] The ISSCR also publishes updates about culturing human embryos 14 days post fertilization, suggesting local policies and regulations should continue to evolve as ESC research develops. [4] Like the ISSCR, which calls for local law and policy to adapt to developing stem cell research given cultural acceptance, this paper highlights the importance of local social factors such as religion and culture.
I. Global Cultural Perspective of Embryonic Stem Cells
Views on ESCs vary throughout the world. Some countries readily embrace stem cell research and therapies, while others have stricter regulations due to ethical concerns surrounding embryonic stem cells and when an embryo becomes entitled to moral consideration. The philosophical issue of when the “someone” begins to be a human after fertilization, in the morally relevant sense, [5] impacts when an embryo becomes not just worthy of protection but morally entitled to it. The process of creating embryonic stem cell lines involves the destruction of the embryos for research. [6] Consequently, global engagement in ESC research depends on social-cultural acceptability.
a. US and Rights-Based Cultures
In the United States, attitudes toward stem cell therapies are diverse. The ethics and social approaches, which value individualism, [7] trigger debates regarding the destruction of human embryos, creating a complex regulatory environment. For example, the 1996 Dickey-Wicker Amendment prohibited federal funding for the creation of embryos for research and the destruction of embryos for “more than allowed for research on fetuses in utero.” [8] Following suit, in 2001, the Bush Administration heavily restricted stem cell lines for research. However, the Stem Cell Research Enhancement Act of 2005 was proposed to help develop ESC research but was ultimately vetoed. [9] Under the Obama administration, in 2009, an executive order lifted restrictions allowing for more development in this field. [10] The flux of research capacity and funding parallels the different cultural perceptions of human dignity of the embryo and how it is socially presented within the country’s research culture. [11]
b. Ubuntu and Collective Cultures
African bioethics differs from Western individualism because of the different traditions and values. African traditions, as described by individuals from South Africa and supported by some studies in other African countries, including Ghana and Kenya, follow the African moral philosophies of Ubuntu or Botho and Ukama , which “advocates for a form of wholeness that comes through one’s relationship and connectedness with other people in the society,” [12] making autonomy a socially collective concept. In this context, for the community to act autonomously, individuals would come together to decide what is best for the collective. Thus, stem cell research would require examining the value of the research to society as a whole and the use of the embryos as a collective societal resource. If society views the source as part of the collective whole, and opposes using stem cells, compromising the cultural values to pursue research may cause social detachment and stunt research growth. [13] Based on local culture and moral philosophy, the permissibility of stem cell research depends on how embryo, stem cell, and cell line therapies relate to the community as a whole. Ubuntu is the expression of humanness, with the person’s identity drawn from the “’I am because we are’” value. [14] The decision in a collectivistic culture becomes one born of cultural context, and individual decisions give deference to others in the society.
Consent differs in cultures where thought and moral philosophy are based on a collective paradigm. So, applying Western bioethical concepts is unrealistic. For one, Africa is a diverse continent with many countries with different belief systems, access to health care, and reliance on traditional or Western medicines. Where traditional medicine is the primary treatment, the “’restrictive focus on biomedically-related bioethics’” [is] problematic in African contexts because it neglects bioethical issues raised by traditional systems.” [15] No single approach applies in all areas or contexts. Rather than evaluating the permissibility of ESC research according to Western concepts such as the four principles approach, different ethics approaches should prevail.
Another consideration is the socio-economic standing of countries. In parts of South Africa, researchers have not focused heavily on contributing to the stem cell discourse, either because it is not considered health care or a health science priority or because resources are unavailable. [16] Each country’s priorities differ given different social, political, and economic factors. In South Africa, for instance, areas such as maternal mortality, non-communicable diseases, telemedicine, and the strength of health systems need improvement and require more focus [17] Stem cell research could benefit the population, but it also could divert resources from basic medical care. Researchers in South Africa adhere to the National Health Act and Medicines Control Act in South Africa and international guidelines; however, the Act is not strictly enforced, and there is no clear legislation for research conduct or ethical guidelines. [18]
Some parts of Africa condemn stem cell research. For example, 98.2 percent of the Tunisian population is Muslim. [19] Tunisia does not permit stem cell research because of moral conflict with a Fatwa. Religion heavily saturates the regulation and direction of research. [20] Stem cell use became permissible for reproductive purposes only recently, with tight restrictions preventing cells from being used in any research other than procedures concerning ART/IVF. Their use is conditioned on consent, and available only to married couples. [21] The community's receptiveness to stem cell research depends on including communitarian African ethics.
c. Asia
Some Asian countries also have a collective model of ethics and decision making. [22] In China, the ethics model promotes a sincere respect for life or human dignity, [23] based on protective medicine. This model, influenced by Traditional Chinese Medicine (TCM), [24] recognizes Qi as the vital energy delivered via the meridians of the body; it connects illness to body systems, the body’s entire constitution, and the universe for a holistic bond of nature, health, and quality of life. [25] Following a protective ethics model, and traditional customs of wholeness, investment in stem cell research is heavily desired for its applications in regenerative therapies, disease modeling, and protective medicines. In a survey of medical students and healthcare practitioners, 30.8 percent considered stem cell research morally unacceptable while 63.5 percent accepted medical research using human embryonic stem cells. Of these individuals, 89.9 percent supported increased funding for stem cell research. [26] The scientific community might not reflect the overall population. From 1997 to 2019, China spent a total of $576 million (USD) on stem cell research at 8,050 stem cell programs, increased published presence from 0.6 percent to 14.01 percent of total global stem cell publications as of 2014, and made significant strides in cell-based therapies for various medical conditions. [27] However, while China has made substantial investments in stem cell research and achieved notable progress in clinical applications, concerns linger regarding ethical oversight and transparency. [28] For example, the China Biosecurity Law, promoted by the National Health Commission and China Hospital Association, attempted to mitigate risks by introducing an institutional review board (IRB) in the regulatory bodies. 5800 IRBs registered with the Chinese Clinical Trial Registry since 2021. [29] However, issues still need to be addressed in implementing effective IRB review and approval procedures.
The substantial government funding and focus on scientific advancement have sometimes overshadowed considerations of regional cultures, ethnic minorities, and individual perspectives, particularly evident during the one-child policy era. As government policy adapts to promote public stability, such as the change from the one-child to the two-child policy, [30] research ethics should also adapt to ensure respect for the values of its represented peoples.
Japan is also relatively supportive of stem cell research and therapies. Japan has a more transparent regulatory framework, allowing for faster approval of regenerative medicine products, which has led to several advanced clinical trials and therapies. [31] South Korea is also actively engaged in stem cell research and has a history of breakthroughs in cloning and embryonic stem cells. [32] However, the field is controversial, and there are issues of scientific integrity. For example, the Korean FDA fast-tracked products for approval, [33] and in another instance, the oocyte source was unclear and possibly violated ethical standards. [34] Trust is important in research, as it builds collaborative foundations between colleagues, trial participant comfort, open-mindedness for complicated and sensitive discussions, and supports regulatory procedures for stakeholders. There is a need to respect the culture’s interest, engagement, and for research and clinical trials to be transparent and have ethical oversight to promote global research discourse and trust.
d. Middle East
Countries in the Middle East have varying degrees of acceptance of or restrictions to policies related to using embryonic stem cells due to cultural and religious influences. Saudi Arabia has made significant contributions to stem cell research, and conducts research based on international guidelines for ethical conduct and under strict adherence to guidelines in accordance with Islamic principles. Specifically, the Saudi government and people require ESC research to adhere to Sharia law. In addition to umbilical and placental stem cells, [35] Saudi Arabia permits the use of embryonic stem cells as long as they come from miscarriages, therapeutic abortions permissible by Sharia law, or are left over from in vitro fertilization and donated to research. [36] Laws and ethical guidelines for stem cell research allow the development of research institutions such as the King Abdullah International Medical Research Center, which has a cord blood bank and a stem cell registry with nearly 10,000 donors. [37] Such volume and acceptance are due to the ethical ‘permissibility’ of the donor sources, which do not conflict with religious pillars. However, some researchers err on the side of caution, choosing not to use embryos or fetal tissue as they feel it is unethical to do so. [38]
Jordan has a positive research ethics culture. [39] However, there is a significant issue of lack of trust in researchers, with 45.23 percent (38.66 percent agreeing and 6.57 percent strongly agreeing) of Jordanians holding a low level of trust in researchers, compared to 81.34 percent of Jordanians agreeing that they feel safe to participate in a research trial. [40] Safety testifies to the feeling of confidence that adequate measures are in place to protect participants from harm, whereas trust in researchers could represent the confidence in researchers to act in the participants’ best interests, adhere to ethical guidelines, provide accurate information, and respect participants’ rights and dignity. One method to improve trust would be to address communication issues relevant to ESC. Legislation surrounding stem cell research has adopted specific language, especially concerning clarification “between ‘stem cells’ and ‘embryonic stem cells’” in translation. [41] Furthermore, legislation “mandates the creation of a national committee… laying out specific regulations for stem-cell banking in accordance with international standards.” [42] This broad regulation opens the door for future global engagement and maintains transparency. However, these regulations may also constrain the influence of research direction, pace, and accessibility of research outcomes.
e. Europe
In the European Union (EU), ethics is also principle-based, but the principles of autonomy, dignity, integrity, and vulnerability are interconnected. [43] As such, the opportunity for cohesion and concessions between individuals’ thoughts and ideals allows for a more adaptable ethics model due to the flexible principles that relate to the human experience The EU has put forth a framework in its Convention for the Protection of Human Rights and Dignity of the Human Being allowing member states to take different approaches. Each European state applies these principles to its specific conventions, leading to or reflecting different acceptance levels of stem cell research. [44]
For example, in Germany, Lebenzusammenhang , or the coherence of life, references integrity in the unity of human culture. Namely, the personal sphere “should not be subject to external intervention.” [45] Stem cell interventions could affect this concept of bodily completeness, leading to heavy restrictions. Under the Grundgesetz, human dignity and the right to life with physical integrity are paramount. [46] The Embryo Protection Act of 1991 made producing cell lines illegal. Cell lines can be imported if approved by the Central Ethics Commission for Stem Cell Research only if they were derived before May 2007. [47] Stem cell research respects the integrity of life for the embryo with heavy specifications and intense oversight. This is vastly different in Finland, where the regulatory bodies find research more permissible in IVF excess, but only up to 14 days after fertilization. [48] Spain’s approach differs still, with a comprehensive regulatory framework. [49] Thus, research regulation can be culture-specific due to variations in applied principles. Diverse cultures call for various approaches to ethical permissibility. [50] Only an adaptive-deliberative model can address the cultural constructions of self and achieve positive, culturally sensitive stem cell research practices. [51]
II. Religious Perspectives on ESC
Embryonic stem cell sources are the main consideration within religious contexts. While individuals may not regard their own religious texts as authoritative or factual, religion can shape their foundations or perspectives.
The Qur'an states:
“And indeed We created man from a quintessence of clay. Then We placed within him a small quantity of nutfa (sperm to fertilize) in a safe place. Then We have fashioned the nutfa into an ‘alaqa (clinging clot or cell cluster), then We developed the ‘alaqa into mudgha (a lump of flesh), and We made mudgha into bones, and clothed the bones with flesh, then We brought it into being as a new creation. So Blessed is Allah, the Best of Creators.” [52]
Many scholars of Islam estimate the time of soul installment, marked by the angel breathing in the soul to bring the individual into creation, as 120 days from conception. [53] Personhood begins at this point, and the value of life would prohibit research or experimentation that could harm the individual. If the fetus is more than 120 days old, the time ensoulment is interpreted to occur according to Islamic law, abortion is no longer permissible. [54] There are a few opposing opinions about early embryos in Islamic traditions. According to some Islamic theologians, there is no ensoulment of the early embryo, which is the source of stem cells for ESC research. [55]
In Buddhism, the stance on stem cell research is not settled. The main tenets, the prohibition against harming or destroying others (ahimsa) and the pursuit of knowledge (prajña) and compassion (karuna), leave Buddhist scholars and communities divided. [56] Some scholars argue stem cell research is in accordance with the Buddhist tenet of seeking knowledge and ending human suffering. Others feel it violates the principle of not harming others. Finding the balance between these two points relies on the karmic burden of Buddhist morality. In trying to prevent ahimsa towards the embryo, Buddhist scholars suggest that to comply with Buddhist tenets, research cannot be done as the embryo has personhood at the moment of conception and would reincarnate immediately, harming the individual's ability to build their karmic burden. [57] On the other hand, the Bodhisattvas, those considered to be on the path to enlightenment or Nirvana, have given organs and flesh to others to help alleviate grieving and to benefit all. [58] Acceptance varies on applied beliefs and interpretations.
Catholicism does not support embryonic stem cell research, as it entails creation or destruction of human embryos. This destruction conflicts with the belief in the sanctity of life. For example, in the Old Testament, Genesis describes humanity as being created in God’s image and multiplying on the Earth, referencing the sacred rights to human conception and the purpose of development and life. In the Ten Commandments, the tenet that one should not kill has numerous interpretations where killing could mean murder or shedding of the sanctity of life, demonstrating the high value of human personhood. In other books, the theological conception of when life begins is interpreted as in utero, [59] highlighting the inviolability of life and its formation in vivo to make a religious point for accepting such research as relatively limited, if at all. [60] The Vatican has released ethical directives to help apply a theological basis to modern-day conflicts. The Magisterium of the Church states that “unless there is a moral certainty of not causing harm,” experimentation on fetuses, fertilized cells, stem cells, or embryos constitutes a crime. [61] Such procedures would not respect the human person who exists at these stages, according to Catholicism. Damages to the embryo are considered gravely immoral and illicit. [62] Although the Catholic Church officially opposes abortion, surveys demonstrate that many Catholic people hold pro-choice views, whether due to the context of conception, stage of pregnancy, threat to the mother’s life, or for other reasons, demonstrating that practicing members can also accept some but not all tenets. [63]
Some major Jewish denominations, such as the Reform, Conservative, and Reconstructionist movements, are open to supporting ESC use or research as long as it is for saving a life. [64] Within Judaism, the Talmud, or study, gives personhood to the child at birth and emphasizes that life does not begin at conception: [65]
“If she is found pregnant, until the fortieth day it is mere fluid,” [66]
Whereas most religions prioritize the status of human embryos, the Halakah (Jewish religious law) states that to save one life, most other religious laws can be ignored because it is in pursuit of preservation. [67] Stem cell research is accepted due to application of these religious laws.
We recognize that all religions contain subsets and sects. The variety of environmental and cultural differences within religious groups requires further analysis to respect the flexibility of religious thoughts and practices. We make no presumptions that all cultures require notions of autonomy or morality as under the common morality theory , which asserts a set of universal moral norms that all individuals share provides moral reasoning and guides ethical decisions. [68] We only wish to show that the interaction with morality varies between cultures and countries.
III. A Flexible Ethical Approach
The plurality of different moral approaches described above demonstrates that there can be no universally acceptable uniform law for ESC on a global scale. Instead of developing one standard, flexible ethical applications must be continued. We recommend local guidelines that incorporate important cultural and ethical priorities.
While the Declaration of Helsinki is more relevant to people in clinical trials receiving ESC products, in keeping with the tradition of protections for research subjects, consent of the donor is an ethical requirement for ESC donation in many jurisdictions including the US, Canada, and Europe. [69] The Declaration of Helsinki provides a reference point for regulatory standards and could potentially be used as a universal baseline for obtaining consent prior to gamete or embryo donation.
For instance, in Columbia University’s egg donor program for stem cell research, donors followed standard screening protocols and “underwent counseling sessions that included information as to the purpose of oocyte donation for research, what the oocytes would be used for, the risks and benefits of donation, and process of oocyte stimulation” to ensure transparency for consent. [70] The program helped advance stem cell research and provided clear and safe research methods with paid participants. Though paid participation or covering costs of incidental expenses may not be socially acceptable in every culture or context, [71] and creating embryos for ESC research is illegal in many jurisdictions, Columbia’s program was effective because of the clear and honest communications with donors, IRBs, and related stakeholders. This example demonstrates that cultural acceptance of scientific research and of the idea that an egg or embryo does not have personhood is likely behind societal acceptance of donating eggs for ESC research. As noted, many countries do not permit the creation of embryos for research.
Proper communication and education regarding the process and purpose of stem cell research may bolster comprehension and garner more acceptance. “Given the sensitive subject material, a complete consent process can support voluntary participation through trust, understanding, and ethical norms from the cultures and morals participants value. This can be hard for researchers entering countries of different socioeconomic stability, with different languages and different societal values. [72]
An adequate moral foundation in medical ethics is derived from the cultural and religious basis that informs knowledge and actions. [73] Understanding local cultural and religious values and their impact on research could help researchers develop humility and promote inclusion.
IV. Concerns
Some may argue that if researchers all adhere to one ethics standard, protection will be satisfied across all borders, and the global public will trust researchers. However, defining what needs to be protected and how to define such research standards is very specific to the people to which standards are applied. We suggest that applying one uniform guide cannot accurately protect each individual because we all possess our own perceptions and interpretations of social values. [74] Therefore, the issue of not adjusting to the moral pluralism between peoples in applying one standard of ethics can be resolved by building out ethics models that can be adapted to different cultures and religions.
Other concerns include medical tourism, which may promote health inequities. [75] Some countries may develop and approve products derived from ESC research before others, compromising research ethics or drug approval processes. There are also concerns about the sale of unauthorized stem cell treatments, for example, those without FDA approval in the United States. Countries with robust research infrastructures may be tempted to attract medical tourists, and some customers will have false hopes based on aggressive publicity of unproven treatments. [76]
For example, in China, stem cell clinics can market to foreign clients who are not protected under the regulatory regimes. Companies employ a marketing strategy of “ethically friendly” therapies. Specifically, in the case of Beike, China’s leading stem cell tourism company and sprouting network, ethical oversight of administrators or health bureaus at one site has “the unintended consequence of shifting questionable activities to another node in Beike's diffuse network.” [77] In contrast, Jordan is aware of stem cell research’s potential abuse and its own status as a “health-care hub.” Jordan’s expanded regulations include preserving the interests of individuals in clinical trials and banning private companies from ESC research to preserve transparency and the integrity of research practices. [78]
The social priorities of the community are also a concern. The ISSCR explicitly states that guidelines “should be periodically revised to accommodate scientific advances, new challenges, and evolving social priorities.” [79] The adaptable ethics model extends this consideration further by addressing whether research is warranted given the varying degrees of socioeconomic conditions, political stability, and healthcare accessibilities and limitations. An ethical approach would require discussion about resource allocation and appropriate distribution of funds. [80]
While some religions emphasize the sanctity of life from conception, which may lead to public opposition to ESC research, others encourage ESC research due to its potential for healing and alleviating human pain. Many countries have special regulations that balance local views on embryonic personhood, the benefits of research as individual or societal goods, and the protection of human research subjects. To foster understanding and constructive dialogue, global policy frameworks should prioritize the protection of universal human rights, transparency, and informed consent. In addition to these foundational global policies, we recommend tailoring local guidelines to reflect the diverse cultural and religious perspectives of the populations they govern. Ethics models should be adapted to local populations to effectively establish research protections, growth, and possibilities of stem cell research.
For example, in countries with strong beliefs in the moral sanctity of embryos or heavy religious restrictions, an adaptive model can allow for discussion instead of immediate rejection. In countries with limited individual rights and voice in science policy, an adaptive model ensures cultural, moral, and religious views are taken into consideration, thereby building social inclusion. While this ethical consideration by the government may not give a complete voice to every individual, it will help balance policies and maintain the diverse perspectives of those it affects. Embracing an adaptive ethics model of ESC research promotes open-minded dialogue and respect for the importance of human belief and tradition. By actively engaging with cultural and religious values, researchers can better handle disagreements and promote ethical research practices that benefit each society.
This brief exploration of the religious and cultural differences that impact ESC research reveals the nuances of relative ethics and highlights a need for local policymakers to apply a more intense adaptive model.
[1] Poliwoda, S., Noor, N., Downs, E., Schaaf, A., Cantwell, A., Ganti, L., Kaye, A. D., Mosel, L. I., Carroll, C. B., Viswanath, O., & Urits, I. (2022). Stem cells: a comprehensive review of origins and emerging clinical roles in medical practice. Orthopedic reviews , 14 (3), 37498. https://doi.org/10.52965/001c.37498
[2] Poliwoda, S., Noor, N., Downs, E., Schaaf, A., Cantwell, A., Ganti, L., Kaye, A. D., Mosel, L. I., Carroll, C. B., Viswanath, O., & Urits, I. (2022). Stem cells: a comprehensive review of origins and emerging clinical roles in medical practice. Orthopedic reviews , 14 (3), 37498. https://doi.org/10.52965/001c.37498
[3] International Society for Stem Cell Research. (2023). Laboratory-based human embryonic stem cell research, embryo research, and related research activities . International Society for Stem Cell Research. https://www.isscr.org/guidelines/blog-post-title-one-ed2td-6fcdk ; Kimmelman, J., Hyun, I., Benvenisty, N. et al. Policy: Global standards for stem-cell research. Nature 533 , 311–313 (2016). https://doi.org/10.1038/533311a
[4] International Society for Stem Cell Research. (2023). Laboratory-based human embryonic stem cell research, embryo research, and related research activities . International Society for Stem Cell Research. https://www.isscr.org/guidelines/blog-post-title-one-ed2td-6fcdk
[5] Concerning the moral philosophies of stem cell research, our paper does not posit a personal moral stance nor delve into the “when” of human life begins. To read further about the philosophical debate, consider the following sources:
Sandel M. J. (2004). Embryo ethics--the moral logic of stem-cell research. The New England journal of medicine , 351 (3), 207–209. https://doi.org/10.1056/NEJMp048145 ; George, R. P., & Lee, P. (2020, September 26). Acorns and Embryos . The New Atlantis. https://www.thenewatlantis.com/publications/acorns-and-embryos ; Sagan, A., & Singer, P. (2007). The moral status of stem cells. Metaphilosophy , 38 (2/3), 264–284. http://www.jstor.org/stable/24439776 ; McHugh P. R. (2004). Zygote and "clonote"--the ethical use of embryonic stem cells. The New England journal of medicine , 351 (3), 209–211. https://doi.org/10.1056/NEJMp048147 ; Kurjak, A., & Tripalo, A. (2004). The facts and doubts about beginning of the human life and personality. Bosnian journal of basic medical sciences , 4 (1), 5–14. https://doi.org/10.17305/bjbms.2004.3453
[6] Vazin, T., & Freed, W. J. (2010). Human embryonic stem cells: derivation, culture, and differentiation: a review. Restorative neurology and neuroscience , 28 (4), 589–603. https://doi.org/10.3233/RNN-2010-0543
[7] Socially, at its core, the Western approach to ethics is widely principle-based, autonomy being one of the key factors to ensure a fundamental respect for persons within research. For information regarding autonomy in research, see: Department of Health, Education, and Welfare, & National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research (1978). The Belmont Report. Ethical principles and guidelines for the protection of human subjects of research.; For a more in-depth review of autonomy within the US, see: Beauchamp, T. L., & Childress, J. F. (1994). Principles of Biomedical Ethics . Oxford University Press.
[8] Sherley v. Sebelius , 644 F.3d 388 (D.C. Cir. 2011), citing 45 C.F.R. 46.204(b) and [42 U.S.C. § 289g(b)]. https://www.cadc.uscourts.gov/internet/opinions.nsf/6c690438a9b43dd685257a64004ebf99/$file/11-5241-1391178.pdf
[9] Stem Cell Research Enhancement Act of 2005, H. R. 810, 109 th Cong. (2001). https://www.govtrack.us/congress/bills/109/hr810/text ; Bush, G. W. (2006, July 19). Message to the House of Representatives . National Archives and Records Administration. https://georgewbush-whitehouse.archives.gov/news/releases/2006/07/20060719-5.html
[10] National Archives and Records Administration. (2009, March 9). Executive order 13505 -- removing barriers to responsible scientific research involving human stem cells . National Archives and Records Administration. https://obamawhitehouse.archives.gov/the-press-office/removing-barriers-responsible-scientific-research-involving-human-stem-cells
[11] Hurlbut, W. B. (2006). Science, Religion, and the Politics of Stem Cells. Social Research , 73 (3), 819–834. http://www.jstor.org/stable/40971854
[12] Akpa-Inyang, Francis & Chima, Sylvester. (2021). South African traditional values and beliefs regarding informed consent and limitations of the principle of respect for autonomy in African communities: a cross-cultural qualitative study. BMC Medical Ethics . 22. 10.1186/s12910-021-00678-4.
[13] Source for further reading: Tangwa G. B. (2007). Moral status of embryonic stem cells: perspective of an African villager. Bioethics , 21(8), 449–457. https://doi.org/10.1111/j.1467-8519.2007.00582.x , see also Mnisi, F. M. (2020). An African analysis based on ethics of Ubuntu - are human embryonic stem cell patents morally justifiable? African Insight , 49 (4).
[14] Jecker, N. S., & Atuire, C. (2021). Bioethics in Africa: A contextually enlightened analysis of three cases. Developing World Bioethics , 22 (2), 112–122. https://doi.org/10.1111/dewb.12324
[15] Jecker, N. S., & Atuire, C. (2021). Bioethics in Africa: A contextually enlightened analysis of three cases. Developing World Bioethics, 22(2), 112–122. https://doi.org/10.1111/dewb.12324
[16] Jackson, C.S., Pepper, M.S. Opportunities and barriers to establishing a cell therapy programme in South Africa. Stem Cell Res Ther 4 , 54 (2013). https://doi.org/10.1186/scrt204 ; Pew Research Center. (2014, May 1). Public health a major priority in African nations . Pew Research Center’s Global Attitudes Project. https://www.pewresearch.org/global/2014/05/01/public-health-a-major-priority-in-african-nations/
[17] Department of Health Republic of South Africa. (2021). Health Research Priorities (revised) for South Africa 2021-2024 . National Health Research Strategy. https://www.health.gov.za/wp-content/uploads/2022/05/National-Health-Research-Priorities-2021-2024.pdf
[18] Oosthuizen, H. (2013). Legal and Ethical Issues in Stem Cell Research in South Africa. In: Beran, R. (eds) Legal and Forensic Medicine. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-32338-6_80 , see also: Gaobotse G (2018) Stem Cell Research in Africa: Legislation and Challenges. J Regen Med 7:1. doi: 10.4172/2325-9620.1000142
[19] United States Bureau of Citizenship and Immigration Services. (1998). Tunisia: Information on the status of Christian conversions in Tunisia . UNHCR Web Archive. https://webarchive.archive.unhcr.org/20230522142618/https://www.refworld.org/docid/3df0be9a2.html
[20] Gaobotse, G. (2018) Stem Cell Research in Africa: Legislation and Challenges. J Regen Med 7:1. doi: 10.4172/2325-9620.1000142
[21] Kooli, C. Review of assisted reproduction techniques, laws, and regulations in Muslim countries. Middle East Fertil Soc J 24 , 8 (2020). https://doi.org/10.1186/s43043-019-0011-0 ; Gaobotse, G. (2018) Stem Cell Research in Africa: Legislation and Challenges. J Regen Med 7:1. doi: 10.4172/2325-9620.1000142
[22] Pang M. C. (1999). Protective truthfulness: the Chinese way of safeguarding patients in informed treatment decisions. Journal of medical ethics , 25(3), 247–253. https://doi.org/10.1136/jme.25.3.247
[23] Wang, L., Wang, F., & Zhang, W. (2021). Bioethics in China’s biosecurity law: Forms, effects, and unsettled issues. Journal of law and the biosciences , 8(1). https://doi.org/10.1093/jlb/lsab019 https://academic.oup.com/jlb/article/8/1/lsab019/6299199
[24] Wang, Y., Xue, Y., & Guo, H. D. (2022). Intervention effects of traditional Chinese medicine on stem cell therapy of myocardial infarction. Frontiers in pharmacology , 13 , 1013740. https://doi.org/10.3389/fphar.2022.1013740
[25] Li, X.-T., & Zhao, J. (2012). Chapter 4: An Approach to the Nature of Qi in TCM- Qi and Bioenergy. In Recent Advances in Theories and Practice of Chinese Medicine (p. 79). InTech.
[26] Luo, D., Xu, Z., Wang, Z., & Ran, W. (2021). China's Stem Cell Research and Knowledge Levels of Medical Practitioners and Students. Stem cells international , 2021 , 6667743. https://doi.org/10.1155/2021/6667743
[27] Luo, D., Xu, Z., Wang, Z., & Ran, W. (2021). China's Stem Cell Research and Knowledge Levels of Medical Practitioners and Students. Stem cells international , 2021 , 6667743. https://doi.org/10.1155/2021/6667743
[28] Zhang, J. Y. (2017). Lost in translation? accountability and governance of Clinical Stem Cell Research in China. Regenerative Medicine , 12 (6), 647–656. https://doi.org/10.2217/rme-2017-0035
[29] Wang, L., Wang, F., & Zhang, W. (2021). Bioethics in China’s biosecurity law: Forms, effects, and unsettled issues. Journal of law and the biosciences , 8(1). https://doi.org/10.1093/jlb/lsab019 https://academic.oup.com/jlb/article/8/1/lsab019/6299199
[30] Chen, H., Wei, T., Wang, H. et al. Association of China’s two-child policy with changes in number of births and birth defects rate, 2008–2017. BMC Public Health 22 , 434 (2022). https://doi.org/10.1186/s12889-022-12839-0
[31] Azuma, K. Regulatory Landscape of Regenerative Medicine in Japan. Curr Stem Cell Rep 1 , 118–128 (2015). https://doi.org/10.1007/s40778-015-0012-6
[32] Harris, R. (2005, May 19). Researchers Report Advance in Stem Cell Production . NPR. https://www.npr.org/2005/05/19/4658967/researchers-report-advance-in-stem-cell-production
[33] Park, S. (2012). South Korea steps up stem-cell work. Nature . https://doi.org/10.1038/nature.2012.10565
[34] Resnik, D. B., Shamoo, A. E., & Krimsky, S. (2006). Fraudulent human embryonic stem cell research in South Korea: lessons learned. Accountability in research , 13 (1), 101–109. https://doi.org/10.1080/08989620600634193 .
[35] Alahmad, G., Aljohani, S., & Najjar, M. F. (2020). Ethical challenges regarding the use of stem cells: interviews with researchers from Saudi Arabia. BMC medical ethics, 21(1), 35. https://doi.org/10.1186/s12910-020-00482-6
[36] Association for the Advancement of Blood and Biotherapies. https://www.aabb.org/regulatory-and-advocacy/regulatory-affairs/regulatory-for-cellular-therapies/international-competent-authorities/saudi-arabia
[37] Alahmad, G., Aljohani, S., & Najjar, M. F. (2020). Ethical challenges regarding the use of stem cells: Interviews with researchers from Saudi Arabia. BMC medical ethics , 21 (1), 35. https://doi.org/10.1186/s12910-020-00482-6
[38] Alahmad, G., Aljohani, S., & Najjar, M. F. (2020). Ethical challenges regarding the use of stem cells: Interviews with researchers from Saudi Arabia. BMC medical ethics , 21(1), 35. https://doi.org/10.1186/s12910-020-00482-6
Culturally, autonomy practices follow a relational autonomy approach based on a paternalistic deontological health care model. The adherence to strict international research policies and religious pillars within the regulatory environment is a great foundation for research ethics. However, there is a need to develop locally targeted ethics approaches for research (as called for in Alahmad, G., Aljohani, S., & Najjar, M. F. (2020). Ethical challenges regarding the use of stem cells: interviews with researchers from Saudi Arabia. BMC medical ethics, 21(1), 35. https://doi.org/10.1186/s12910-020-00482-6), this decision-making approach may help advise a research decision model. For more on the clinical cultural autonomy approaches, see: Alabdullah, Y. Y., Alzaid, E., Alsaad, S., Alamri, T., Alolayan, S. W., Bah, S., & Aljoudi, A. S. (2022). Autonomy and paternalism in Shared decision‐making in a Saudi Arabian tertiary hospital: A cross‐sectional study. Developing World Bioethics , 23 (3), 260–268. https://doi.org/10.1111/dewb.12355 ; Bukhari, A. A. (2017). Universal Principles of Bioethics and Patient Rights in Saudi Arabia (Doctoral dissertation, Duquesne University). https://dsc.duq.edu/etd/124; Ladha, S., Nakshawani, S. A., Alzaidy, A., & Tarab, B. (2023, October 26). Islam and Bioethics: What We All Need to Know . Columbia University School of Professional Studies. https://sps.columbia.edu/events/islam-and-bioethics-what-we-all-need-know
[39] Ababneh, M. A., Al-Azzam, S. I., Alzoubi, K., Rababa’h, A., & Al Demour, S. (2021). Understanding and attitudes of the Jordanian public about clinical research ethics. Research Ethics , 17 (2), 228-241. https://doi.org/10.1177/1747016120966779
[40] Ababneh, M. A., Al-Azzam, S. I., Alzoubi, K., Rababa’h, A., & Al Demour, S. (2021). Understanding and attitudes of the Jordanian public about clinical research ethics. Research Ethics , 17 (2), 228-241. https://doi.org/10.1177/1747016120966779
[41] Dajani, R. (2014). Jordan’s stem-cell law can guide the Middle East. Nature 510, 189. https://doi.org/10.1038/510189a
[42] Dajani, R. (2014). Jordan’s stem-cell law can guide the Middle East. Nature 510, 189. https://doi.org/10.1038/510189a
[43] The EU’s definition of autonomy relates to the capacity for creating ideas, moral insight, decisions, and actions without constraint, personal responsibility, and informed consent. However, the EU views autonomy as not completely able to protect individuals and depends on other principles, such as dignity, which “expresses the intrinsic worth and fundamental equality of all human beings.” Rendtorff, J.D., Kemp, P. (2019). Four Ethical Principles in European Bioethics and Biolaw: Autonomy, Dignity, Integrity and Vulnerability. In: Valdés, E., Lecaros, J. (eds) Biolaw and Policy in the Twenty-First Century. International Library of Ethics, Law, and the New Medicine, vol 78. Springer, Cham. https://doi.org/10.1007/978-3-030-05903-3_3
[44] Council of Europe. Convention for the protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine: Convention on Human Rights and Biomedicine (ETS No. 164) https://www.coe.int/en/web/conventions/full-list?module=treaty-detail&treatynum=164 (forbidding the creation of embryos for research purposes only, and suggests embryos in vitro have protections.); Also see Drabiak-Syed B. K. (2013). New President, New Human Embryonic Stem Cell Research Policy: Comparative International Perspectives and Embryonic Stem Cell Research Laws in France. Biotechnology Law Report , 32 (6), 349–356. https://doi.org/10.1089/blr.2013.9865
[45] Rendtorff, J.D., Kemp, P. (2019). Four Ethical Principles in European Bioethics and Biolaw: Autonomy, Dignity, Integrity and Vulnerability. In: Valdés, E., Lecaros, J. (eds) Biolaw and Policy in the Twenty-First Century. International Library of Ethics, Law, and the New Medicine, vol 78. Springer, Cham. https://doi.org/10.1007/978-3-030-05903-3_3
[46] Tomuschat, C., Currie, D. P., Kommers, D. P., & Kerr, R. (Trans.). (1949, May 23). Basic law for the Federal Republic of Germany. https://www.btg-bestellservice.de/pdf/80201000.pdf
[47] Regulation of Stem Cell Research in Germany . Eurostemcell. (2017, April 26). https://www.eurostemcell.org/regulation-stem-cell-research-germany
[48] Regulation of Stem Cell Research in Finland . Eurostemcell. (2017, April 26). https://www.eurostemcell.org/regulation-stem-cell-research-finland
[49] Regulation of Stem Cell Research in Spain . Eurostemcell. (2017, April 26). https://www.eurostemcell.org/regulation-stem-cell-research-spain
[50] Some sources to consider regarding ethics models or regulatory oversights of other cultures not covered:
Kara MA. Applicability of the principle of respect for autonomy: the perspective of Turkey. J Med Ethics. 2007 Nov;33(11):627-30. doi: 10.1136/jme.2006.017400. PMID: 17971462; PMCID: PMC2598110.
Ugarte, O. N., & Acioly, M. A. (2014). The principle of autonomy in Brazil: one needs to discuss it ... Revista do Colegio Brasileiro de Cirurgioes , 41 (5), 374–377. https://doi.org/10.1590/0100-69912014005013
Bharadwaj, A., & Glasner, P. E. (2012). Local cells, global science: The rise of embryonic stem cell research in India . Routledge.
For further research on specific European countries regarding ethical and regulatory framework, we recommend this database: Regulation of Stem Cell Research in Europe . Eurostemcell. (2017, April 26). https://www.eurostemcell.org/regulation-stem-cell-research-europe
[51] Klitzman, R. (2006). Complications of culture in obtaining informed consent. The American Journal of Bioethics, 6(1), 20–21. https://doi.org/10.1080/15265160500394671 see also: Ekmekci, P. E., & Arda, B. (2017). Interculturalism and Informed Consent: Respecting Cultural Differences without Breaching Human Rights. Cultura (Iasi, Romania) , 14 (2), 159–172.; For why trust is important in research, see also: Gray, B., Hilder, J., Macdonald, L., Tester, R., Dowell, A., & Stubbe, M. (2017). Are research ethics guidelines culturally competent? Research Ethics , 13 (1), 23-41. https://doi.org/10.1177/1747016116650235
[52] The Qur'an (M. Khattab, Trans.). (1965). Al-Mu’minun, 23: 12-14. https://quran.com/23
[53] Lenfest, Y. (2017, December 8). Islam and the beginning of human life . Bill of Health. https://blog.petrieflom.law.harvard.edu/2017/12/08/islam-and-the-beginning-of-human-life/
[54] Aksoy, S. (2005). Making regulations and drawing up legislation in Islamic countries under conditions of uncertainty, with special reference to embryonic stem cell research. Journal of Medical Ethics , 31: 399-403.; see also: Mahmoud, Azza. "Islamic Bioethics: National Regulations and Guidelines of Human Stem Cell Research in the Muslim World." Master's thesis, Chapman University, 2022. https://doi.org/10.36837/ chapman.000386
[55] Rashid, R. (2022). When does Ensoulment occur in the Human Foetus. Journal of the British Islamic Medical Association , 12 (4). ISSN 2634 8071. https://www.jbima.com/wp-content/uploads/2023/01/2-Ethics-3_-Ensoulment_Rafaqat.pdf.
[56] Sivaraman, M. & Noor, S. (2017). Ethics of embryonic stem cell research according to Buddhist, Hindu, Catholic, and Islamic religions: perspective from Malaysia. Asian Biomedicine,8(1) 43-52. https://doi.org/10.5372/1905-7415.0801.260
[57] Jafari, M., Elahi, F., Ozyurt, S. & Wrigley, T. (2007). 4. Religious Perspectives on Embryonic Stem Cell Research. In K. Monroe, R. Miller & J. Tobis (Ed.), Fundamentals of the Stem Cell Debate: The Scientific, Religious, Ethical, and Political Issues (pp. 79-94). Berkeley: University of California Press. https://escholarship.org/content/qt9rj0k7s3/qt9rj0k7s3_noSplash_f9aca2e02c3777c7fb76ea768ba458f0.pdf https://doi.org/10.1525/9780520940994-005
[58] Lecso, P. A. (1991). The Bodhisattva Ideal and Organ Transplantation. Journal of Religion and Health , 30 (1), 35–41. http://www.jstor.org/stable/27510629 ; Bodhisattva, S. (n.d.). The Key of Becoming a Bodhisattva . A Guide to the Bodhisattva Way of Life. http://www.buddhism.org/Sutras/2/BodhisattvaWay.htm
[59] There is no explicit religious reference to when life begins or how to conduct research that interacts with the concept of life. However, these are relevant verses pertaining to how the fetus is viewed. (( King James Bible . (1999). Oxford University Press. (original work published 1769))
Jerimiah 1: 5 “Before I formed thee in the belly I knew thee; and before thou camest forth out of the womb I sanctified thee…”
In prophet Jerimiah’s insight, God set him apart as a person known before childbirth, a theme carried within the Psalm of David.
Psalm 139: 13-14 “…Thou hast covered me in my mother's womb. I will praise thee; for I am fearfully and wonderfully made…”
These verses demonstrate David’s respect for God as an entity that would know of all man’s thoughts and doings even before birth.
[60] It should be noted that abortion is not supported as well.
[61] The Vatican. (1987, February 22). Instruction on Respect for Human Life in Its Origin and on the Dignity of Procreation Replies to Certain Questions of the Day . Congregation For the Doctrine of the Faith. https://www.vatican.va/roman_curia/congregations/cfaith/documents/rc_con_cfaith_doc_19870222_respect-for-human-life_en.html
[62] The Vatican. (2000, August 25). Declaration On the Production and the Scientific and Therapeutic Use of Human Embryonic Stem Cells . Pontifical Academy for Life. https://www.vatican.va/roman_curia/pontifical_academies/acdlife/documents/rc_pa_acdlife_doc_20000824_cellule-staminali_en.html ; Ohara, N. (2003). Ethical Consideration of Experimentation Using Living Human Embryos: The Catholic Church’s Position on Human Embryonic Stem Cell Research and Human Cloning. Department of Obstetrics and Gynecology . Retrieved from https://article.imrpress.com/journal/CEOG/30/2-3/pii/2003018/77-81.pdf.
[63] Smith, G. A. (2022, May 23). Like Americans overall, Catholics vary in their abortion views, with regular mass attenders most opposed . Pew Research Center. https://www.pewresearch.org/short-reads/2022/05/23/like-americans-overall-catholics-vary-in-their-abortion-views-with-regular-mass-attenders-most-opposed/
[64] Rosner, F., & Reichman, E. (2002). Embryonic stem cell research in Jewish law. Journal of halacha and contemporary society , (43), 49–68.; Jafari, M., Elahi, F., Ozyurt, S. & Wrigley, T. (2007). 4. Religious Perspectives on Embryonic Stem Cell Research. In K. Monroe, R. Miller & J. Tobis (Ed.), Fundamentals of the Stem Cell Debate: The Scientific, Religious, Ethical, and Political Issues (pp. 79-94). Berkeley: University of California Press. https://escholarship.org/content/qt9rj0k7s3/qt9rj0k7s3_noSplash_f9aca2e02c3777c7fb76ea768ba458f0.pdf https://doi.org/10.1525/9780520940994-005
[65] Schenker J. G. (2008). The beginning of human life: status of embryo. Perspectives in Halakha (Jewish Religious Law). Journal of assisted reproduction and genetics , 25 (6), 271–276. https://doi.org/10.1007/s10815-008-9221-6
[66] Ruttenberg, D. (2020, May 5). The Torah of Abortion Justice (annotated source sheet) . Sefaria. https://www.sefaria.org/sheets/234926.7?lang=bi&with=all&lang2=en
[67] Jafari, M., Elahi, F., Ozyurt, S. & Wrigley, T. (2007). 4. Religious Perspectives on Embryonic Stem Cell Research. In K. Monroe, R. Miller & J. Tobis (Ed.), Fundamentals of the Stem Cell Debate: The Scientific, Religious, Ethical, and Political Issues (pp. 79-94). Berkeley: University of California Press. https://escholarship.org/content/qt9rj0k7s3/qt9rj0k7s3_noSplash_f9aca2e02c3777c7fb76ea768ba458f0.pdf https://doi.org/10.1525/9780520940994-005
[68] Gert, B. (2007). Common morality: Deciding what to do . Oxford Univ. Press.
[69] World Medical Association (2013). World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA , 310(20), 2191–2194. https://doi.org/10.1001/jama.2013.281053 Declaration of Helsinki – WMA – The World Medical Association .; see also: National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. (1979). The Belmont report: Ethical principles and guidelines for the protection of human subjects of research . U.S. Department of Health and Human Services. https://www.hhs.gov/ohrp/regulations-and-policy/belmont-report/read-the-belmont-report/index.html
[70] Zakarin Safier, L., Gumer, A., Kline, M., Egli, D., & Sauer, M. V. (2018). Compensating human subjects providing oocytes for stem cell research: 9-year experience and outcomes. Journal of assisted reproduction and genetics , 35 (7), 1219–1225. https://doi.org/10.1007/s10815-018-1171-z https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6063839/ see also: Riordan, N. H., & Paz Rodríguez, J. (2021). Addressing concerns regarding associated costs, transparency, and integrity of research in recent stem cell trial. Stem Cells Translational Medicine , 10 (12), 1715–1716. https://doi.org/10.1002/sctm.21-0234
[71] Klitzman, R., & Sauer, M. V. (2009). Payment of egg donors in stem cell research in the USA. Reproductive biomedicine online , 18 (5), 603–608. https://doi.org/10.1016/s1472-6483(10)60002-8
[72] Krosin, M. T., Klitzman, R., Levin, B., Cheng, J., & Ranney, M. L. (2006). Problems in comprehension of informed consent in rural and peri-urban Mali, West Africa. Clinical trials (London, England) , 3 (3), 306–313. https://doi.org/10.1191/1740774506cn150oa
[73] Veatch, Robert M. Hippocratic, Religious, and Secular Medical Ethics: The Points of Conflict . Georgetown University Press, 2012.
[74] Msoroka, M. S., & Amundsen, D. (2018). One size fits not quite all: Universal research ethics with diversity. Research Ethics , 14 (3), 1-17. https://doi.org/10.1177/1747016117739939
[75] Pirzada, N. (2022). The Expansion of Turkey’s Medical Tourism Industry. Voices in Bioethics , 8 . https://doi.org/10.52214/vib.v8i.9894
[76] Stem Cell Tourism: False Hope for Real Money . Harvard Stem Cell Institute (HSCI). (2023). https://hsci.harvard.edu/stem-cell-tourism , See also: Bissassar, M. (2017). Transnational Stem Cell Tourism: An ethical analysis. Voices in Bioethics , 3 . https://doi.org/10.7916/vib.v3i.6027
[77] Song, P. (2011) The proliferation of stem cell therapies in post-Mao China: problematizing ethical regulation, New Genetics and Society , 30:2, 141-153, DOI: 10.1080/14636778.2011.574375
[78] Dajani, R. (2014). Jordan’s stem-cell law can guide the Middle East. Nature 510, 189. https://doi.org/10.1038/510189a
[79] International Society for Stem Cell Research. (2024). Standards in stem cell research . International Society for Stem Cell Research. https://www.isscr.org/guidelines/5-standards-in-stem-cell-research
[80] Benjamin, R. (2013). People’s science bodies and rights on the Stem Cell Frontier . Stanford University Press.
Mifrah Hayath
SM Candidate Harvard Medical School, MS Biotechnology Johns Hopkins University
Olivia Bowers
MS Bioethics Columbia University (Disclosure: affiliated with Voices in Bioethics)
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License .
Cities as Engines of Opportunities: Evidence from Brazil
Are developing-world cities engines of opportunities for low-wage earners? In this study, we track a cohort of young low-income workers in Brazil for thirteen years to explore the contribution of factors such as industrial structure and skill segregation on upward income mobility. We find that cities in the south of Brazil are more effective engines of upward mobility than cities in the north and that these differences appear to be primarily related to the exposure of unskilled workers to skilled co-workers, which in turn reflects industry composition and complexity. Our results suggest that the positive effects of urbanization depend on the skilled and unskilled working together, a form of integration that is more prevalent in the cities of southern Brazil than in northern cities. This segregation, which can decline with specialization and the division of labor, may hinder the ability of Brazil's northern cities to offer more opportunities for escaping poverty.
We acknowledge the support of Cristian Jara-Figueroa in the initial conceptualization of the empirical strategy. Barza and Viarengo gratefully acknowledges the financial support received from the Swiss National Science Foundation (Principal Investigator: Prof. Dr. Martina Viarengo; Research Grant n. 100018-176454). Hidalgo acknowledges the support of the Agence Nationale de la Recherche grant number ANR-19-P3IA-0004, the 101086712-LearnData-HORIZON-WIDERA-2022-TALENTS-01 financed by European Research Executive Agency (REA) (https://cordis.europa.eu/project/id/101086712), IAST funding from the French National Research Agency (ANR) under grant ANR-17-EURE-0010 (Investissements d'Avenir program), and the European Lighthouse of AI for Sustainability [grant number 101120237-HOR-IZON-CL4-2022-HUMAN-02]. The usual caveats apply. The views expressed herein are those of the authors and do not necessarily reflect the views of the National Bureau of Economic Research.
I have received speaking fees from organizations that organize members that invest in real estate markets, including the National Association of Real Estate Investment Managers, the Pension Real Estate Association and the Association for International Real Estate Investors.
MARC RIS BibTeΧ
Download Citation Data
Working Groups
Mentioned in the news, more from nber.
In addition to working papers , the NBER disseminates affiliates’ latest findings through a range of free periodicals — the NBER Reporter , the NBER Digest , the Bulletin on Retirement and Disability , the Bulletin on Health , and the Bulletin on Entrepreneurship — as well as online conference reports , video lectures , and interviews .

Suggestions or feedback?
MIT News | Massachusetts Institute of Technology
- Machine learning
- Social justice
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
New treatment could reverse hair loss caused by an autoimmune skin disease
Press contact :, media download.
*Terms of Use:
Images for download on the MIT News office website are made available to non-commercial entities, press and the general public under a Creative Commons Attribution Non-Commercial No Derivatives license . You may not alter the images provided, other than to crop them to size. A credit line must be used when reproducing images; if one is not provided below, credit the images to "MIT."
Previous image Next image
Researchers at MIT, Brigham and Women’s Hospital, and Harvard Medical School have developed a potential new treatment for alopecia areata, an autoimmune disorder that causes hair loss and affects people of all ages, including children.
For most patients with this type of hair loss, there is no effective treatment. The team developed a microneedle patch that can be painlessly applied to the scalp and releases drugs that help to rebalance the immune response at the site, halting the autoimmune attack.
In a study of mice, the researchers found that this treatment allowed hair to regrow and dramatically reduced inflammation at the treatment site, while avoiding systemic immune effects elsewhere in the body. This strategy could also be adapted to treat other autoimmune skin diseases such as vitiligo, atopic dermatitis, and psoriasis, the researchers say.
“This innovative approach marks a paradigm shift. Rather than suppressing the immune system, we’re now focusing on regulating it precisely at the site of antigen encounter to generate immune tolerance,” says Natalie Artzi, a principal research scientist in MIT’s Institute for Medical Engineering and Science, an associate professor of medicine at Harvard Medical School and Brigham and Women’s Hospital, and an associate faculty member at the Wyss Institute of Harvard University.
Artzi and Jamil R. Azzi, an associate professor of medicine at Harvard Medical School and Brigham and Women’s Hospital, are the senior authors of the new study , which appears in the journal Advanced Materials . Nour Younis, a Brigham and Women’s postdoc, and Nuria Puigmal, a Brigham and Women’s postdoc and former MIT research affiliate, are the lead authors of the paper.
The researchers are now working on launching a company to further develop the technology, led by Puigmal, who was recently awarded a Harvard Business School Blavatnik Fellowship.
Direct delivery
Alopecia areata, which affects more than 6 million Americans, occurs when the body’s own T cells attack hair follicles, leading the hair to fall out. The only treatment available to most patients — injections of immunosuppressant steroids into the scalp — is painful and patients often can’t tolerate it.
Some patients with alopecia areata and other autoimmune skin diseases can also be treated with immunosuppressant drugs that are given orally, but these drugs lead to widespread suppression of the immune system, which can have adverse side effects.
“This approach silences the entire immune system, offering relief from inflammation symptoms but leading to frequent recurrences. Moreover, it increases susceptibility to infections, cardiovascular diseases, and cancer,” Artzi says.
A few years ago, at a working group meeting in Washington, Artzi happened to be seated next to Azzi (the seating was alphabetical), an immunologist and transplant physican who was seeking new ways to deliver drugs directly to the skin to treat skin-related diseases.
Their conversation led to a new collaboration, and the two labs joined forces to work on a microneedle patch to deliver drugs to the skin. In 2021, they reported that such a patch can be used to prevent rejection following skin transplant. In the new study, they began applying this approach to autoimmune skin disorders.
“The skin is the only organ in our body that we can see and touch, and yet when it comes to drug delivery to the skin, we revert to systemic administration. We saw great potential in utilizing the microneedle patch to reprogram the immune system locally,” Azzi says.
The microneedle patches used in this study are made from hyaluronic acid crosslinked with polyethylene glycol (PEG), both of which are biocompatible and commonly used in medical applications. With this delivery method, drugs can pass through the tough outer layer of the epidermis, which can’t be penetrated by creams applied to the skin.
“This polymer formulation allows us to create highly durable needles capable of effectively penetrating the skin. Additionally, it gives us the flexibility to incorporate any desired drug,” Artzi says. For this study, the researchers loaded the patches with a combination of the cytokines IL-2 and CCL-22. Together, these immune molecules help to recruit regulatory T cells, which proliferate and help to tamp down inflammation. These cells also help the immune system learn to recognize that hair follicles are not foreign antigens, so that it will stop attacking them.
Hair regrowth
The researchers found that mice treated with this patch every other day for three weeks had many more regulatory T cells present at the site, along with a reduction in inflammation. Hair was able to regrow at those sites, and this growth was maintained for several weeks after the treatment ended. In these mice, there were no changes in the levels of regulatory T cells in the spleen or lymph nodes, suggesting that the treatment affected only the site where the patch was applied.
In another set of experiments, the researchers grafted human skin onto mice with a humanized immune system. In these mice, the microneedle treatment also induced proliferation of regulatory T cells and a reduction in inflammation.
The researchers designed the microneedle patches so that after releasing their drug payload, they can also collect samples that could be used to monitor the progress of the treatment. Hyaluronic acid causes the needles to swell about tenfold after entering the skin, which allows them to absorb interstitial fluid containing biomolecules and immune cells from the skin.
Following patch removal, researchers can analyze samples to measure levels of regulatory T cells and inflammation markers. This could prove valuable for monitoring future patients who may undergo this treatment.
The researchers now plan to further develop this approach for treating alopecia, and to expand into other autoimmune skin diseases.
The research was funded by the Ignite Fund and Shark Tank Fund awards from the Department of Medicine at Brigham and Women’s Hospital.
Share this news article on:
Press mentions, healthday news.
MIT researchers have developed microneedle patches that are capable of restoring hair growth in alopecia areata patients, reports Ernie Mundell for HealthDay . The team’s approach includes a, “patch containing myriad microneedles that is applied to the scalp,” writes Mundell. “It releases drugs to reset the immune system so it stops attacking follicles.”
Previous item Next item
Related Links
- Natalie Artzi
- Institute for Medical Engineering and Science
Related Topics
- Drug delivery
- Health sciences and technology
- Institute for Medical Engineering and Science (IMES)
Related Articles

A sprayable gel could make minimally invasive surgeries simpler and safer
Patch that delivers drug, gene, and light-based therapy to tumor sites shows promising results

MIT researchers design tailored tissue adhesives
More mit news.
Researchers develop a detector for continuously monitoring toxic gases
Read full story →

The beauty of biology

Navigating longevity with industry leaders at MIT AgeLab PLAN Forum

Jeong Min Park earns 2024 Schmidt Science Fellowship
Scientists use generative AI to answer complex questions in physics

New tool empowers users to fight online misinformation
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram

IMAGES
VIDEO
COMMENTS
Fifteen Ethical Principles of the Universal Declaration on Bioethics. Hence, giving sufficient data and teaching the patient about actual factors, and getting educated consent before exposing a patient to any medical procedure is fundamental. We will write. a custom essay specifically for you by our professional experts.
Basically, for writing a research paper, you can think about the following principles of bioethics. Principle of non-maleficence. Principle of respect for autonomy. Principle of beneficence. Principle of justice. Medical practices are identified as ethical only if they favor these principles. Therefore, when selecting a topic for a bioethics ...
9 minutes. The icon indicates free access to the linked research on JSTOR. Bioethics is a field of inquiry centered around the uses and moral implications of medicine and the bio-sciences. Scholars and researchers come from a very wide variety of professional and disciplinary backgrounds, like medicine, nursing, law, theology, philosophy ...
Research Ethics Use of humans for clinical trials (testing new treatments, devices, or drugs) Human testing in vulnerable populations or in less developed countries Use of animals in medical research, dissection, or in testing of personal care products Appropriate use of genetic material sampled from indigenous populations Other Health care justice
The Oxford Handbook of Bioethics is an authoritative, state-of-the-art guide to current issues in bioethics. Thirty-four contributors reflect the interdisciplinary nature that is characteristic of bioethics, and its increasingly international character. Thirty topics are covered in articles written by some of the world's leading figures in the ...
Voices in Bioethics is currently seeking submissions on philosophical and practical topics, both current and timeless. Papers addressing access to healthcare, the bioethical implications of recent Supreme Court rulings, environmental ethics, data privacy, cybersecurity, law and bioethics, economics and bioethics, reproductive ethics, research ethics, and pediatric bioethics are sought.
USA.gov. On February 26, 2020, the Board on Health Sciences Policy of the National Academies of Sciences, Engineering, and Medicine hosted a 1-day public workshop in Washington, DC, to examine current and emerging bioethical issues that might arise in the context of biomedical research and to consider research topics in bioethics that could ...
Bioethics is a journal that publishes content tackling the ethics of the most pressing issues in the biomedical and life-sciences, ranging from the use of AI in health care to organ transplants, ageing, and stem cell research. Bioethics offers researchers cutting edge analyses of real-life ethical, legal and policy problems, as well as of the fundamental concepts, principles and theories that ...
Introduction. The overall aim of systematic reviews is to provide readers with an unbiased overview on specific topics discussed in the literature. 1,2 In the interdisciplinary field of bioethics, systematic reviews can synthesize normative or empirical literature as well as a mix of both. On one hand, systematic reviews of normative literature provide an overview of ethical issues, arguments ...
Publishes research on bioethics and the body with a multi discipline perspective from STEAM, law, philosophy, social and political sciences and theology. ... Inquiries about the suitability of papers or to discuss potential topics for the Essay section or special issues can be made to [email protected].
Voices in Bioethics is currently seeking submissions on philosophical and practical topics, both current and timeless. Papers addressing access to healthcare, the bioethical implications of recent Supreme Court rulings, environmental ethics, data privacy, cybersecurity, law and bioethics, economics and bioethics, reproductive ethics, research ethics, and pediatric bioethics are sought.
The last four decades have seen the emergence and flourishing of the field of bioethics and its incorporation into wide-ranging aspects of society, from the clinic or laboratory through to public policy and the media. Yet considerable debate still exists over what bioethics is and how it should be done. In this paper I consider the question of ...
Bioethics Articles. Find information on topics in health care and biotechnology ethics, including end-of-life care, clinical ethics, pandemics, culturally competent care, vulnerable patient populations, organ transplantation, and other topics in bioethics. (For permission to reprint articles, submit requests to [email protected] .)
Bioethics is the multi-disciplinary study of, and response, to these moral and ethical questions. Bioethical questions often involve overlapping concerns from diverse fields of study including life sciences, biotechnology, public health, medicine, public policy, law, philosophy and theology. They arise in clinical, research, and political ...
Bioethics Topics. Advance Care Planning & Advance Directives. Breaking Bad News. Clinical Ethics and Law. Complementary Medicine. Confidentiality. Cross-Cultural Issues and Diverse Beliefs. Difficult Patient Encounters. Do Not Resuscitate during Anesthesia and Urgent Procedures.
Voices in Bioethics is currently seeking submissions on philosophical and practical topics, both current and timeless. Papers addressing access to healthcare, the bioethical implications of recent Supreme Court rulings, environmental ethics, data privacy, cybersecurity, law and bioethics, economics and bioethics, reproductive ethics, research ethics, and pediatric bioethics are sought.
Research in bioethics has a long history at Harvard Medical School, going back at least to the 1960s and the work of Dr. Henry Beecher, an anesthesiologist at Massachusetts General Hospital and an early scholar of ethical issues in medicine. ... Beecher's 1966 paper on "Ethics and Clinical Research" disabused Americans of this pretense ...
Chronological Publications. Our interdisciplinary research is published in a range of high impact medical, legal, scientific, philosophical, social science and bioethics journals. The department averages more than 50 publications per year, and has published six books since 2010. Our fellows are expected to produce quality research and often ...
This enables local ethics review of research, improves research into the distinctive ethical problems in LMICs, and increases the representation of different voices in on-going global discussions of the ethics of health research. Bioethics Resources. For Teachers and Students - teaching tools, career path advice, and online education information
Center for Bioethics Papers. The Department serves as the hub for interdisciplinary research and collaboration on topics across four research areas in biomedical ethics: neuro- and mental healthcare ethics, health policy, behavioral economics, research ethics, global bioethics, and the ethics of healthcare allocation.
4 Principles of Bioethics that are Worth Writing About. Students can write papers on any of the four principles of bioethics. These are: Principle of respect for autonomy. Principle of non-maleficence. Principle of justice. Principle of beneficence. Medical practices are considered ethical if they respect these principles.
The Consultation took place via a series of set questions, with multiple choice answers and space to give explanations of the answer. The below is the submission on behalf of the Anscombe Centre composed by our Education and Research Officer, Dr Chris Wojtulewicz. 16 January 2023. Euthanasia & Assisted Suicide (EAS)
Higher education has become more student-centered as the Bologna process assigns students more time to study and research. Online teaching has been needed during the pandemic, which can be challenging regarding didactic and assessment. This paper analyzes project-based learning (PBL) as a form of teaching and assessing students in a bioethics course on reproductive ethics. The team project was ...
Attendees include faculty, students, and a combination of external experts in law, bioethics, and translational research. Unlike more formal conferences, the Charm City Colloquium, which launched in 2019, aims to provide scholars with an opportunity for informal dialogue on current topics at the intersection of health policy, law, and ethics.
Working Paper 32444. DOI 10.3386/w32444. Issue Date May 2024. E-cigarette licensure laws (ELLs) require retailers to obtain a state license to sell e-cigarettes over the counter. This study is the first to comprehensively explore the effect of ELL adoption on youth and adult tobacco product use. Using data from the State Youth Risk Behavior ...
DOI 10.3386/w32445. Issue Date May 2024. WHO estimates that as many as 1 in 6 individuals of reproductive age worldwide are affected by infertility. This paper uses rich administrative population-wide data from Sweden to construct and characterize the universe of infertility treatments, and to then quantify the private costs of infertility, the ...
Voices in Bioethics is currently seeking submissions on philosophical and practical topics, both current and timeless. Papers addressing access to healthcare, the bioethical implications of recent Supreme Court rulings, environmental ethics, data privacy, cybersecurity, law and bioethics, economics and bioethics, reproductive ethics, research ethics, and pediatric bioethics are sought.
Working Paper 32426. DOI 10.3386/w32426. Issue Date May 2024. Are developing-world cities engines of opportunities for low-wage earners? In this study, we track a cohort of young low-income workers in Brazil for thirteen years to explore the contribution of factors such as industrial structure and skill segregation on upward income mobility. We ...
Nour Younis, a Brigham and Women's postdoc, and Nuria Puigmal, a Brigham and Women's postdoc and former MIT research affiliate, are the lead authors of the paper. The researchers are now working on launching a company to further develop the technology, led by Puigmal, who was recently awarded a Harvard Business School Blavatnik Fellowship.
The coexistence of several definitions of green jobs and measurement instruments gives room for mismatches between those concepts and their application to research questions. This paper first presents an organizing framework for the existing definitions, measurement instruments, and policy frameworks.