Case study: treatment-resistant schizophrenia
WELLCOME CENTRE HUMAN NEUROIMAGING/SCIENCE PHOTO LIBRARY
Learning objectives
After reading this article, individuals should be able to:
- Describe the management of schizophrenia;
- Understand pharmaceutical issues that occur during treatment with antipsychotics, especially clozapine ;
- Explain how the Mental Health Act 1983 impacts on care;
- Understand the importance of multidisciplinary and patient-centred care in managing psychosis.
Around 0.5–0.7% of the UK population is living with schizophrenia. Of these individuals, up to one-third are classified as treatment-resistant. This is defined as schizophrenia that has not responded to two different antipsychotics [1,2] .
Clozapine is the most effective treatment for such patients [3] . It is recommended by the National Institute for Health and Care Excellence (NICE)[4], and is the only licensed medicine for this patient group [4,5] . For treatment-responsive patients, there should be a collaborative approach when choosing a treatment [4] . More information on the recognition and management of schizophrenia can be found in a previous article here , and in accompanying case studies here .
This case study aims to explore a patient’s journey in mental health services during a relapse of schizophrenia. It also aims to highlight good practice for communicating with patients with severe mental illness in all settings, and in explaining the role of clozapine.

Case presentation
Mr AT is a male, aged 26 years, who has been diagnosed with paranoid schizophrenia. He moved to the UK with his family from overseas five years ago. He lives with his parents in a small flat in London. His mother calls the police after he goes missing, finding his past two months’ medication untouched.
He is found at an airport, attempting to go through security without a ticket. He is confused and paranoid about the police asking him to come with them.
He is taken to A&E and is medically cleared (see Box 1) [6] .
Box 1: Common differentials for psychotic symptoms
Medical conditions can present as psychosis. These include:
- Intoxication/effects of drugs (cannabis, stimulants, opioids, corticosteroids);
- Cerebrovascular disease;
- Temporal lobe epilepsy.
Mr AT’s history is taken by a psychiatrist, and his crisis plan sought (as per NICE recommendations) but he does not have one [7] .
He has been under the care of mental health services for two years and disputes his diagnosis of paranoid schizophrenia. He was admitted to a psychiatric hospital 18 months ago where he was prescribed the antipsychotic amisulpride at 600mg daily.
He is teetotal, smokes ten cigarettes a day and smokes cannabis every day. His BMI is 26 and he has hypercholesterolaemia (total cholesterol = 6.1mmol/L, reference range <5mmol/L) but all other tests are normal.
He has no allergies. His only medication is amisulpride 600mg each morning, which he does not take.
Medicines reconciliation
Mr AT is transferred to a psychiatric ward and placed under Section 2 of the Mental Health Act , allowing detention for up to 28 days for assessment and treatment (see Box 2).
Box 2: The Mental Health Act 1983
This legislation allows for the detention and treatment of patients with serious mental illness, where urgent care is required. This is often referred to as “sectioning”.
It includes regulations about treatment against a patient’s consent to safeguard patients’ liberty, which become more stringent with longer detentions.
Patients may only be given medication to treat their mental illness without their consent and may refuse physical health treatment.
He denies any mental illness and tells the team they are conspiring with MI6. He is visibly experiencing auditory hallucinations: seen by him talking to himself and looking to empty corners of the room. Amisulpride is re-prescribed at 300mg, which he declines to take.
A pharmacy technician completes a medicines reconciliation and contacts the care coordinator. The technician provides information about Mr AT’s treatment and feels he is still unwell as he has continued to express paranoid beliefs about his neighbours and MI6.
The ward pharmacist speaks to the patient. As per NICE guidance on medicines adherence , they adopt a non-judgemental attitude [8] . Mr AT is provided with information on the benefits and side effects of the medication and is asked open questions regarding his reluctance to take it. For more information on non-adherence to medicines and mental illness, see Box 3 [9] .
Box 3: Medicines adherence and mental health
Adherence to medication is similar for both physical and mental health medicines: only about 50% of patients are adherent.
Side effects and lack of involvement in decision making often lead to poor adherence.
In mental illness, other factors are:
- Denial of illness (poor ‘insight’);
- Lack of contact by services;
- Cultural factors, such as family, religious or personal beliefs around mental illness or medication.
Mr AT reports gynaecomastia and impotence, and says that he will not take any antipsychotics as they are “poison designed by MI6”, although is unable to concentrate on the discussion owing to hearing voices.
He is prescribed clonazepam 1mg twice daily owing to his distress, which is to be reduced as treatment controls his psychosis. He is offered nicotine replacement therapy but decides to use an e-cigarette on the ward.
He is unable to weigh up information to make decisions owing to his chaotic thinking and is felt to not have capacity to make decisions on his treatment. The team debates what treatment to offer.
Patient preference
Mr AT refuses all options presented to him. A decision is made to administer against his will and aripiprazole is chosen as it is less likely to cause hyperprolactinaemia and sexual dysfunction. He then agrees to take tablets “if it will get me out of hospital”.
![treatment resistant schizophrenia case study Table 1: Common side effects of antipsychotics[9]](https://pharmaceutical-journal.com/wp-content/uploads/2022/01/clozapine-schizophrenia-Table-1-Common-side-effects-WM.jpg)
After eight weeks of treatment with orodispersible aripiprazole 15mg, Mr AT is able have a more coherent conversation, but is hallucinating and distressed. He is clearly under treated. The pharmacist attempts to complete a side-effect rating scale ( Glasgow Antipsychotic Side-effect Scale [GASS] ) but he declines. He is pacing around the ward in circles: it is felt he may be experiencing akathisia (restlessness) — a common side effect of antipsychotics (see Table 1 ).
Treatment review
The team feels clozapine is the best option owing to the treatment failure of two antipsychotics.
The team suggests this to Mr AT. He refuses, stating the ward is experimenting on him with new medication and he refuses to take another antipsychotics.
The pharmacist meets the patient with an occupational therapist to discuss what his goals are. Mr AT states he wants to go to college to become a carpenter. They discuss routes to achieve this, which all involve the first step of leaving hospital and the conclusion that clozapine is the best way to achieve this. The pharmacist clarifies the patient’s aripiprazole will not continue once clozapine is established. They leave information about clozapine with the patient and offer to return to discuss it further.
Mr AT agrees to take clozapine a week later (see Box 4) [10–14] . Aripiprazole is tapered and stopped.
Box 4: Clozapine characteristics
Clozapine significantly prolongs life and improves quality of life [10] . Delaying clozapine is associated with poorer outcomes for patients [11] .
Clozapine is under-prescribed owing to healthcare professionals’ anxiety and unfamiliarity around its use [12–14] .
It causes neutropenia in up to 3% of patients so regular monitoring is required . Twice-weekly monitoring is needed if neutrophils are <2 x10 9 /L. Most patients should stop clozapine if neutrophils are <1.5×10 9 /L. These ranges can differ from some laboratory definitions of neutropenia.
Other side effects include sedation, hypersalivation and weight gain. See Table 2 for red flags for serious side effects.
Clozapine is titrated up slowly to avoid cardiovascular complications. A treatment break of >48 hours warrants specialist advice for a retitration plan.
The pharmacist meets with Mr AT to discuss clozapine. He is told that this is likely to be a long-term treatment. The pharmacist acknowledges that the patient disagrees with his diagnosis, but this treatment is likely to prevent him from returning to hospital.
He is started on clozapine at 12.5mg at night, which is slowly increased. Pre- and post-dose monitoring of his vital signs is completed.
On day nine of the titration, his pulse is 115bpm. He otherwise feels well and blood tests show no signs of myocarditis (see Table 2), so the titration is continued but slowed.
After 3 weeks he is taking 150mg twice daily of clozapine and his symptoms have significantly improved: he is regularly bathing, not visibly hallucinating and engaging with staff.
The pharmacy technician completes a GASS form. Mr AT reports constipation, hypersalivation and sedation.
A pharmacist meets the patient to reiterate important counselling points, and discuss questions he may have about his treatment and how to manage side effects. Medication changes are made with the patients’ input:
- His constipation is monitored with a stool chart and he is started on senna 15mg at night;
- He is started on hyoscine hydrobromide 300 micrograms at night for salivation;
- He is switched to clozapine 300mg once daily at night to simplify his regime and reduce daytime sedation. His clonazepam is reduced and stopped.
Smoking is discussed owing to tobacco’s role as an enzyme inducer (more information on tobacco smoking and its potential drug interactions can be found in a previous article here ). Mr AT states he will continue to use an e-cigarette for now. He is informed that if he starts smoking again, his clozapine may become less effective and he should immediately inform his team.
He is discharged a few weeks later via a home treatment team and attends a clinic once weekly. On each attendance, he has a full blood count taken and analysed on site. He is assessed by a pharmacy technician and nurse for side effects and adherence to treatment, and his smoking status is clarified.
The technician asks what he thinks the clozapine has done for him. Mr AT states he is still unsure about having a mental illness, but recognises that clozapine has helped him out of hospital and intends to continue taking it.
![treatment resistant schizophrenia case study Table 2: Red flags with clozapine[9]](https://pharmaceutical-journal.com/wp-content/uploads/2022/01/clozapine-schizophrenia-Table-2-Red-flags-with-clozapine-WM.jpg)
Good practice in the pharmaceutical care of psychosis involves:
- Active patient involvement in discussions on treatment decisions;
- Regular review of treatment: discussing efficacy, side effects and the patient’s view and understanding of treatment;
- Multidisciplinary approaches to helping patients choose treatment;
- For patients who dispute their diagnosis and the need for treatment, open dialogue is important. Such discussions should involve the patient’s goals, which are likely to be shared by the team (rapid discharge, preventing admissions, reducing distress);
- Information about treatment should be provided regularly in both written and verbal form;
- Where appropriate, involve carers/next of kin in decision making and information sharing.
Important points
- Schizophrenia affects 1 in 200 people, meaning such patients will present regularly in all settings;
- Patients with acute psychosis, who are in recovery, may be managed by specialist teams, who are the best source of information for a patient’s care;
- Collaborating with the patient on a viable long-term treatment plan improves adherence;
- Clozapine is recommended where two antipsychotics have failed;
- Clozapine is a high-risk medicine, but the risks are manageable;
- Hydrocarbons produced by smoking (but not nicotine replacement therapy, e-cigarettes or chewing tobacco) induce the enzyme CYP1A2, which reduces clozapine levels markedly (up to 20–60%). Starting or stopping smoking could precipitate relapse or induce toxicity, respectively.
- 1 Conley RR, Kelly DL. Management of treatment resistance in schizophrenia. Biological Psychiatry. 2001; 50 :898–911. doi: 10.1016/s0006-3223(01)01271-9
- 2 Gillespie AL, Samanaite R, Mill J, et al. Is treatment-resistant schizophrenia categorically distinct from treatment-responsive schizophrenia? a systematic review. BMC Psychiatry. 2017; 17 . doi: 10.1186/s12888-016-1177-y
- 3 Taylor DM. Clozapine for Treatment-Resistant Schizophrenia: Still the Gold Standard? CNS Drugs. 2017; 31 :177–80. doi: 10.1007/s40263-017-0411-6
- 4 Psychosis and schizophrenia in adults: prevention and management. NICE. 2014. https://www.nice.org.uk/guidance/cg178/ (accessed Jan 2022).
- 5 Clozaril 25 mg tablets. Electronic medicines compendium. 2020. https://www.medicines.org.uk/emc/product/4411/smpc (accessed Jan 2022).
- 6 Psychosis and schizophrenia: what else might it be? NICE. 2020. https://cks.nice.org.uk/topics/psychosis-schizophrenia/diagnosis/differential-diagnosis/ (accessed Jan 2022).
- 7 Service user experience in adult mental health: improving the experience of care for people using adult NHS mental health services. NICE. 2011. https://www.nice.org.uk/guidance/cg136/ (accessed Jan 2022).
- 8 Medicines adherence: involving patients in decisions about prescribed medicines and supporting adherence . NICE. 2009. https://www.nice.org.uk/guidance/cg76/ (accessed Jan 2022).
- 9 Taylor D, Barnes T, Young A. The Maudsley Prescribing Guidelines in Psychiatry . 13th ed. Hoboken, New Jersey: : Wiley 2018.
- 10 Meltzer HY, Burnett S, Bastani B, et al. Effects of Six Months of Clozapine Treatment on the Quality of Life of Chronic Schizophrenic Patients. PS. 1990; 41 :892–7. doi: 10.1176/ps.41.8.892
- 11 Üçok A, Çikrikçili U, Karabulut S, et al. Delayed initiation of clozapine may be related to poor response in treatment-resistant schizophrenia. International Clinical Psychopharmacology. 2015; 30 :290–5. doi: 10.1097/yic.0000000000000086
- 12 Whiskey E, Barnard A, Oloyede E, et al. An Evaluation of the Variation and Underuse of Clozapine in the United Kingdom. SSRN Journal. 2020. doi: 10.2139/ssrn.3716864
- 13 Nielsen J, Dahm M, Lublin H, et al. Psychiatrists’ attitude towards and knowledge of clozapine treatment. J Psychopharmacol. 2009; 24 :965–71. doi: 10.1177/0269881108100320
- 14 Verdoux H, Quiles C, Bachmann CJ, et al. Prescriber and institutional barriers and facilitators of clozapine use: A systematic review. Schizophrenia Research. 2018; 201 :10–9. doi: 10.1016/j.schres.2018.05.046
- This article was corrected on 31 January 2022 to clarify that tobacco is an enzyme inducer, not an enzyme inhibitor
Useful structured introduction to the subject for clinical purposes
Thank you Amrit for your feedback, we are pleased that you found this article useful.
Michael Dowdall, Executive Editor, Research & Learning
Please note that smoking causes enzyme INDUCTION not INHIBITION as stated. (Via aromatic polyhydrocarbons, not nicotine)
Hi James. Thank you for bringing this to our attention. This has now been corrected. Hannah Krol, Deputy Chief Subeditor
Only with Herbal formula I was able to cure my schizophrenia Illness with the product I purchase from Dr Sims Gomez Herbs A Clinic in South Africa
Cancel reply
You must be logged in to post a comment.
You might also be interested in…

More than 100 extra pharmacists employed in mental health community teams since 2019/2020

More than 40% of people with ADHD waiting at least two years to access mental health service, study finds

Pharmacy leaders ‘disappointed’ at cuts to free NHS mental health service
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Global health
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 14, Issue 4
- Treatment-resistant schizophrenia characterised by dopamine supersensitivity psychosis and efficacy of asenapine
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Nagara Takao ,
- Toshiya Murai and
- Hironobu Fujiwara
- Psychiatry , Kyoto University Hospital , Kyoto , Japan
- Correspondence to Dr Nagara Takao; takao.nagara{at}gmail.com
Dopamine supersensitivity psychosis (DSP) frequently arises with long-term antipsychotic treatment and accounts for a significant proportion of treatment-resistant schizophrenia. The mechanism underlying DSP is thought to be a compensatory increase in dopamine receptor density in the striatum caused by long-term antipsychotic treatment. Previous animal studies have reported that antipsychotics increase serotonin 5-HT2A receptor density in the striatum and that 5-HT2A receptor blockers suppress dopamine-sensitive psychomotor activity, which may be linked to the pathophysiology of DSP. In this paper, we describe a patient who was hospitalised with treatment-resistant schizophrenia. Following treatment with high-dose antipsychotic polypharmacy for 10 weeks, the patient experienced worsening of psychotic and extrapyramidal symptoms. The patient was then started on second-generation antipsychotic asenapine while other antipsychotics were tapered off, resulting in improvement of these symptoms. Retrospectively, we presumed that the high-dose antipsychotic polypharmacy caused DSP, which was effectively treated by the potent 5-HT2A receptor antagonism of asenapine.
- schizophrenia
- psychiatry (drugs and medicines)
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/bcr-2021-242495
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Antipsychotics play a crucial role in the treatment of psychotic disorders, such as schizophrenia. However, their administration requires close monitoring because of their potential to induce a variety of adverse events, which include exacerbation of psychotic symptoms and side effects, such as drug-induced parkinsonism. Of note, dopamine supersensitivity psychosis (DSP) is thought to explain the phenomenon in which patients often relapse following slight dose reductions, discontinuation or switching of antipsychotics. Chouinard et al estimated that 50% of treatment-resistant schizophrenia cases are related to DSP. 1 A possible mechanism is that long-term antipsychotic treatment causes a compensatory increase in dopamine receptor density in the striatum, which leads to a dopamine supersensitive state. 2–5 Moreover, recent studies have shown that the prevalence rate of DSP in patients with treatment-resistant schizophrenia in Japan is as high as 70%. 6 The differences in the proportions of patients with DSP may be explained by the common use of high-dose antipsychotic polypharmacy in Japan. 5 7 From the perspective of treatment strategies for DSP, one possible molecular mechanism involves the interaction between dopaminergic and serotonergic neurotransmission. Previous animal studies have reported that antipsychotics increase receptor density of serotonin 5-HT2A, a serotonergic receptor subtype, in the striatum and that 5-HT2A receptor antagonists suppress dopamine-sensitive psychomotor activity, which may be linked to the pathophysiology of DSP. 8 We report a case of a male patient with schizophrenia, who developed DSP following high-dose antipsychotic polypharmacy. Symptoms improved following treatment with asenapine, an atypical antipsychotic that has high 5-HT2A antagonist binding potential.
Case presentation
Mr A, a single man aged 44 years, suddenly ran outdoors 13 years earlier with the belief that he was contacting his old friend telepathically. He was referred to our hospital that year and we observed symptoms of delusions, hallucinations and castrophrenia. His previous medical history included purulent meningitis in infancy, hyperlipidaemia, hyperuricaemia, hypertension and obesity.
He was diagnosed with schizophrenia (ICD-10 code F20.0) and started receiving antipsychotic therapy at our hospital. He was prescribed maximum doses of risperidone, olanzapine, quetiapine and aripiprazole at each previous hospitalisation because of a worsening of psychotic symptoms. These maximum doses were taken over to an outpatient setting and gradually reduced to 2 mg/day of risperidone in the last year. He two times experienced rebound psychosis during switching of antipsychotics, 13 and 6 years earlier. The first episode occurred after switching 8 mg/day of risperidone to 20 mg/day of olanzapine and the second after switching 600 mg/day of quetiapine to 20 mg/day of olanzapine, during which oromandibular dyskinetic movement was observed. These symptoms, alongside a history of high-dose antipsychotic medication, suggest DSP at an earlier period. His latest hospital admission was voluntary and was because of a slight irritation (day 0). Because his symptoms were not causing significant distress, he was followed up without any changes in prescription. However, on day 7, he developed prominent thought disorder, delusions of being injured by broken glass and a variety of visual and auditory hallucinations. Therefore, risperidone was increased to 8 mg/day for the following 2 weeks, and quetiapine was initiated at 200 mg/day (day 17) and slowly increased to 600 mg/day over the next 2 weeks, with an expectation of anxiolytic and sedative drug effects. However, the patients showed inadequate response to these medications. From day 28, risperidone was gradually replaced with olanzapine, and at day 50, olanzapine was increased to 30 mg/day. Despite temporary symptomatic improvement, we observed further aggravation of psychotic symptoms when chlorpromazine equivalence reached 1850 mg (day 59). During the course of the abovementioned antipsychotic medication reinforcement and replacement ( figure 1 ), he developed extrapyramidal symptoms (EPS) of akathisia and stuttering; the latter was suspected to be a tardive dyskinetic movement of the tongue. Given the risk of metabolic side effects and the inadequate sedative effect, quetiapine was tapered and discontinued, while brexpiprazole was gradually increased to 4 mg; however, the patient experienced no symptomatic improvement. We considered using clozapine because the patient met the definition of treatment-resistant schizophrenia, and evidence has suggested that clozapine is the treatment of choice for treatment-resistant schizophrenia. 9–11 However, the patient’s mother did not agree to the administration of clozapine because of fear of side effects, such as agranulocytosis. Finally, he was treated with asenapine, which was initiated at 10 mg/day (day 84) and increased to 20 mg/day (day 93). Olanzapine and brexpiprazole were tapered and discontinued.
- Download figure
- Open in new tab
- Download powerpoint
Reinforcement and replacement of antipsychotic medication worsened psychotic symptoms. Starting asenapine and tapering off other antipsychotics resulted in improvement of psychotic and extrapyramidal symptoms. ASP,asenapine; BRX, brexpiprazole; OLZ, olanzapine; QTP, quetiapine; RIS,risperidone.
Outcome and follow-up
Four weeks of treatment of 20 mg/day of asenapine resulted in gradual improvement of psychotic symptoms and EPS. He subsequently willingly participated in an occupational therapy programme and was discharged on day 229.
In our patient, increasing antipsychotics worsened psychotic symptoms, which was against our expectations. He fulfilled the clinical characteristics of DSP, as reported by Chouinard et al , which are (1) improvement in acute psychotic symptoms, at least initially, by increasing antipsychotic dose, (2) at least 3 months of cumulative exposure to antipsychotics, (3) tolerance to antipsychotic effects, (4) rapid relapse following drug discontinuation, dose reduction or switching of antipsychotics, (5) worsening of psychotic symptoms with life stressors, such as minor life events or daily hassles, (6) appearance of new or more severe psychotic symptoms, (7) treatment resistance and (8) movement disorders, such as tardive dyskinesia. 5 12 These symptoms were all observed during the most recent hospitalisation. However, his previous experiences of rebound psychosis and oromandibular dyskinetic movement during switching of antipsychotics suggest that he had developed DSP during an earlier period.
We question whether a period of 4 weeks for improvement of the established DSP state with asenapine was too short. A therapeutic course of several months is usually suggested for clinical cases of treatment-resistant schizophrenia with DSP. 13 However, an animal study demonstrated rapid (within minutes to an hour) suppression of DSP-like activity using a 5-HT2A antagonist, 8 which corresponds to several hours in humans. 14 This may explain the rapid therapeutic effect induced by the agent in our patient. In light of this, it is possible that improvement of an established DSP state can be achieved within several days or weeks.
Several human studies have demonstrated that the underlying mechanism of DSP is a compensatory increase in dopamine D2 receptor density in the striatum caused by long-term administration of antipsychotics. 4 5 Both lower and higher antipsychotic doses have been shown to induce dopamine supersensitive states in animal studies, but only the higher dose increased the number of striatal D2 receptors. 3 15 Moreover, the relationship between DSP and 5-HT2A receptor function has also been demonstrated in an animal study that showed that chronic exposure to antipsychotic medications increases amphetamine-induced locomotion relative to antipsychotic-naïve rats, which is indicative of a dopamine supersensitive state. In contrast, ritanserin and MDL100,907, both 5-HT2A receptor antagonists, suppress locomotion in antipsychotic-treated rats. Additionally, haloperidol was shown to decrease 5-HT2A receptor density in the prelimbic cortex and nucleus accumbens core and increase 5-HT2A receptor density in the caudate–putamen. 8 It should also be noted that asenapine exhibits the highest affinity for 5-HT2A receptors within second-generation antipsychotics. 16 Given these findings, it is possible that, in our patient, DSP was induced by high-dose antipsychotic polypharmacy, and dopamine sensitivity was reduced by 5-HT2A receptor blockade by asenapine. To date, there has only been one report that suggested a positive effect of asenapine on DSP, which was in a patient with delusional disorder whose DSP was thought to have been induced by ziprasidone. 17
Other than the action on the 5-HT2A system, there are other antipsychotics profiles of note that are closely related to DSP: half-life period, affinity to dopamine D2 receptors and dopamine partial agonists. First, the instability of drug blood levels accounts for the instability of psychiatric symptoms. 5 Long half-life period, sustained release or long-acting injectable antipsychotics are considered to be effective for preventing DSP because of the stability of blood drug levels. 18 Half-life periods of representative antipsychotics are 20 hours for risperidone, 19 7 hours for quetiapine, 20 33 hours for olanzapine, 21 91 hours for brexpiprazole 22 and 24 hours for asenapine. 23 Asenapine has the advantage of a longer half-life period than that of risperidone or quetiapine. Second, antipsychotics that have higher affinity to dopamine D2 receptors are presumed to be effective in the treatment of DSP. 24 Asenapine has higher affinity to dopamine D2 receptors than that of risperidone, quetiapine or olanzapine. 11 25–27 Finally, according to animal studies, a dopamine partial agonist is likely to be effective for DSP. 28 29 However, they may exacerbate psychotic symptoms in cases where DSP has already developed. 30 This would explain why brexpiprazole, which is a dopamine partial agonist, was ineffective in our case.
In conclusion, this case presents an important clinical implication for use of antipsychotic agents. Both long-term use as well as high-dose polypharmacy of antipsychotics should be avoided as much as possible because of their potential to cause DSP due to increases in D2 receptor density and changes in 5-HT2A receptor density across various brain regions. Antipsychotics with strong 5-HT2A antagonism may be effective for the treatment of DSP.
Learning points
This case presents an important clinical implication for the use of antipsychotic agents.
Both long-term use and high-dose polypharmacy of antipsychotics should be avoided as much as possible because they may cause dopamine supersensitivity psychosis (DSP).
Antipsychotics with potent 5-HT2A antagonism may be effective for DSP.
- Chouinard G ,
- Chouinard V-A
- Samaha A-N ,
- Reckless GE ,
- Seeman P , et al
- Stewart J , et al
- Silvestri S ,
- Seeman MV ,
- Negrete JC , et al
- Kanahara N ,
- Yamanaka H , et al
- Xiang Y-T ,
- Ungvari GS ,
- Correll CU , et al
- Charron A ,
- Servonnet A , et al
- Al-Haj Haasan N ,
- Li C , et al
- Remington G ,
- Agid O , et al
- Chouinard V-A , et al
- Komatsu N , et al
- Ramos-Miguel A , et al
- Minassian A ,
- Rajkumar RP
- Tadokoro S ,
- Kanahara N , et al
- Janssen Pharmaceuticals, Inc
- Callaghan JT ,
- Bergstrom RF ,
- Ptak LR , et al
- Brexpiprazole RJA
- Scheidemantel T ,
- Korobkova I ,
- Rej S , et al
- Tallerico T
- Leysen JE ,
- Janssen PM ,
- Megens AA , et al
- Jensen NH ,
- Rodriguiz RM ,
- Caron MG , et al
- Bymaster FP ,
- Rasmussen K ,
- Calligaro DO , et al
- Okamura N ,
- Sekine Y , et al
- Akazawa H ,
- Ohgi Y , et al
- Sasaki T , et al
Contributors NT: manuscript preparation, preparation of images. TM: review & editing. HF: primary consultant and revision of manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Read the full text or download the PDF:
Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic
RELATED TOPICS
INTRODUCTION
Patients who do not respond adequately to antipsychotics should be reevaluated to rule out or address causes other than nonresponsiveness to medication (ie, pseudoresistance). Current medication and psychosocial interventions should be optimized. Treatment strategies for patients who remain incompletely responsive to antipsychotic medications include changes to antipsychotic doses and drugs, use of clozapine (in eligible patients), and drug augmentation.
This topic addresses the evaluation and management of treatment-resistant schizophrenia. The epidemiology, pathogenesis, clinical manifestations, course, assessment, diagnosis and treatment of schizophrenia are reviewed separately, as are the presentation and treatment of anxiety and depression co-occurring with schizophrenia and guidelines for prescribing clozapine. (See "Schizophrenia in adults: Epidemiology and pathogenesis" and "Schizophrenia in adults: Clinical features, assessment, and diagnosis" and "Schizophrenia in adults: Maintenance therapy and side effect management" and "Schizophrenia in adults: Pharmacotherapy with long-acting injectable antipsychotic medication" and "Co-occurring schizophrenia and substance use disorder: Epidemiology, clinical features, assessment, and diagnosis" and "Anxiety in schizophrenia" and "Depression in schizophrenia" and "Schizophrenia in adults: Guidelines for prescribing clozapine" .)
● Minimal TRRIP criteria for treatment-resistant schizophrenia – Based on cross-sectional clinical assessment supplemented by collateral sources of information (eg, medical record documentation, caregiver’s report).
Switch language:

Newron’s add-on schizophrenia therapy finds success in Phase II/III trial
The therapy met endpoints in the Phase II/III trial, with Newron considering a pivotal Phase III study in patients with treatment-resistant schizophrenia.
- Share on Linkedin
- Share on Facebook

Italian biopharma Newron Pharmaceuticals ’s add-on schizophrenia treatment, evenamide, has met primary and secondary endpoints in a Phase II/III trial.
The placebo-controlled Phase II/III study (EudraCT Number: 2020-006062-36) evaluated evenamide as an add-on therapy with a second-generation antipsychotic including clozapine in 291 patients with chronic schizophrenia.
Related Company Profiles
Newron pharmaceuticals spa, karuna therapeutics inc, acadia pharmaceuticals inc.
The trial met its primary endpoint by showing an improvement in the symptom severity, measured using the Positive and Negative Syndrome Scale (PANSS) Total Score. The therapy also demonstrated improvement in the Clinical Global Impression of Severity (CGI-S), a secondary endpoint.
Evenamide had a favourable safety and tolerability profile, with 25% of the participants experiencing at least one adverse event. However, three participants discontinued the trial due to adverse events, two who were in the treatment group and one in the placebo group who died during the trial. Commonly observed evenamide-related adverse events were headache, vomiting and nasopharyngitis.
Following the release of the topline results, Newron’s stock price was up by 17.8% at market open on 30 April, compared to market close on the previous day. The company said it is also planning a “potentially pivotal” placebo-controlled Phase III trial evaluating evenamide as an add-on treatment in patients with treatment-resistant schizophrenia.
Various companies have invested in developing therapies for schizophrenia. One of the most anticipated therapies in the field is Karuna Therapeutics ’ KarXT (xanomeline-trospium) , which is a dual M1/M4 muscarinic agonist that could reduce both positive and negative symptoms of the neurological disorder. The Prescription Drug User Fee Act (PDUFA) date for KarXT is 26 September 2024. If approved, the drug would represent a new class of medication on offer to those with schizophrenia.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
Some companies have also faced setbacks in their development for the treatment of schizophrenia. In March 2024, Acadia Pharmaceuticals ’ schizophrenia therapy, pimavanserin, failed to meet the primary endpoint in its Phase III ADVANCE-2 clinical trial. The company shut down the programme, stating that it does not intend to conduct any further clinical trials with pimavanserin.
Last month, the US Food and Drug Administration placed a clinical hold on the Phase I trial of Neumora Therapeutics’ schizophrenia therapy, NMRA-266. The decision was based on the preclinical data for NMRA-266, showing that the drug triggered convulsions in rabbits.
Sign up for our daily news round-up!
Give your business an edge with our leading industry insights.
More Relevant
Arsenal doses first subject in Phase I/II renal cell carcinoma trial
Denovo biopharma’s glioma gene therapy trial receives cirm grant, trials to watch: four als drugs to keep an eye on, central nervous system remains most researched therapy area for dcts, sign up to the newsletter: in brief, your corporate email address, i would also like to subscribe to:.
I consent to Verdict Media Limited collecting my details provided via this form in accordance with Privacy Policy
Thank you for subscribing
View all newsletters from across the GlobalData Media network.
- Introduction
- Conclusions
- Article Information
a Three participants who were randomized to placebo withdrew before receiving trial drug (1 met a protocol-specified withdrawal criterion and 2 withdrew consent).
b The safety population included all participants who received at least 1 dose of trial drug.
c The modified intent-to-treat (mITT) population, used for all efficacy analyses, included all participants randomized who received at least 1 dose of trial drug, had a baseline Positive and Negative Syndrome Scale (PANSS) assessment, and at least 1 postbaseline PANSS assessment.
All efficacy analyses were performed using the mITT analysis set, defined as all randomized participants who received at least 1 dose of trial medication at baseline and at least 1 postbaseline PANSS assessment (xanomeline-trospium chloride n = 114, placebo n = 120). LS indicates least squares.
a P < .05.
b P < .01.
c P < .001.
eAppendix. Inclusion and Exclusion Criteria
eTable 1. Efficacy Measures at Week 5 (mITT Population)
eTable 2. Primary and Supportive Analyses for Primary End Point: Change From Baseline at Week 5 in PANSS Total Score
eTable 3. Treatment-Emergent Adverse Events Leading to Trial Discontinuation
eFigure. Mean Systolic and Diastolic Blood Pressure Measures Recorded at 2 Hours Post Dose (Cmax)
Trial Protocol
Data Sharing Statement
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
Kaul I , Sawchak S , Walling DP, et al. Efficacy and Safety of Xanomeline-Trospium Chloride in Schizophrenia : A Randomized Clinical Trial . JAMA Psychiatry. Published online May 01, 2024. doi:10.1001/jamapsychiatry.2024.0785
Manage citations:
© 2024
- Permissions
Efficacy and Safety of Xanomeline-Trospium Chloride in Schizophrenia : A Randomized Clinical Trial
- 1 Karuna Therapeutics, Boston, Massachusetts
- 2 CenExel, Garden Grove, California
- 3 Department of Psychiatry, University of Texas Southwestern Medical School, Dallas
- 4 Department of Psychiatry, Indiana University School of Medicine, Indianapolis
Question Is xanomeline-trospium chloride efficacious and well tolerated in people with schizophrenia experiencing acute psychosis?
Findings In this phase 3, double-blind, randomized, placebo-controlled trial in 256 people with schizophrenia, xanomeline-trospium was associated with a statistically significant and clinically meaningful reduction in Positive and Negative Syndrome Scale total score compared with placebo. Xanomeline-trospium was generally well tolerated; the most common adverse events were primarily gastrointestinal effects, which were mild or moderate in intensity and generally transient in nature.
Meaning EMERGENT-3 confirms previously reported clinical trials (EMERGENT-1 and EMERGENT-2) demonstrating that xanomeline-trospium is efficacious and well tolerated in people with schizophrenia experiencing acute psychosis.
Importance A significant need exists for new antipsychotic medications with different mechanisms of action, greater efficacy, and better tolerability than existing agents. Xanomeline is a dual M 1 /M 4 preferring muscarinic receptor agonist with no direct D 2 dopamine receptor blocking activity. KarXT combines xanomeline with the peripheral muscarinic receptor antagonist trospium chloride with the goal of reducing adverse events due to xanomeline-related peripheral muscarinic receptor activation. In prior trials, xanomeline-trospium chloride was effective in reducing symptoms of psychosis and generally well tolerated in people with schizophrenia.
Objective To evaluate the efficacy and safety of xanomeline-trospium vs placebo in adults with schizophrenia.
Design, Setting, and Participants EMERGENT-3 ( NCT04738123 ) was a phase 3, multicenter, randomized, double-blind, placebo-controlled, 5-week trial of xanomeline-trospium in people with schizophrenia experiencing acute psychosis, conducted between April 1, 2021, and December 7, 2022, at 30 inpatient sites in the US and Ukraine. Data were analyzed from February to June 2023.
Interventions Participants were randomized 1:1 to receive xanomeline-trospium chloride (maximum dose xanomeline 125 mg/trospium 30 mg) or placebo for 5 weeks.
Main Outcomes and Measures The prespecified primary end point was change from baseline to week 5 in Positive and Negative Syndrome Scale (PANSS) total score. Secondary outcome measures were change from baseline to week 5 in PANSS positive subscale score, PANSS negative subscale score, PANSS Marder negative factor score, Clinical Global Impression–Severity score, and proportion of participants with at least a 30% reduction in PANSS total score. Safety and tolerability were also evaluated.
Results A total of 256 participants (mean [SD] age, 43.1 [11.8] years; 191 men [74.6%]; 156 of 256 participants [60.9%] were Black or African American, 98 [38.3%] were White, and 1 [0.4%] was Asian) were randomized (125 in xanomeline-trospium group and 131 in placebo group). At week 5, xanomeline-trospium significantly reduced PANSS total score compared with placebo (xanomeline-trospium , −20.6; placebo, −12.2; least squares mean difference, −8.4; 95% CI, −12.4 to −4.3; P < .001; Cohen d effect size, 0.60). Discontinuation rates due to treatment-emergent adverse events (TEAEs) were similar between the xanomeline-trospium (8 participants [6.4%]) and placebo (7 participants [5.5%]) groups. The most common TEAEs in the xanomeline-trospium vs placebo group were nausea (24 participants [19.2%] vs 2 participants [1.6%]), dyspepsia (20 participants [16.0%] vs 2 participants [1.6%]), vomiting (20 participants [16.0%] vs 1 participant [0.8%]), and constipation (16 participants [12.8%] vs 5 participants [3.9%]). Measures of extrapyramidal symptoms, weight gain, and somnolence were similar between treatment groups.
Conclusions and Relevance Xanomeline-trospium was efficacious and well tolerated in people with schizophrenia experiencing acute psychosis. These findings, together with the previously reported and consistent results from the EMERGENT-1 and EMERGENT-2 trials, support the potential of xanomeline-trospium to be the first in a putative new class of antipsychotic medications without D 2 dopamine receptor blocking activity.
Trial Registration ClinicalTrials.gov Identifier: NCT04738123
Currently approved antipsychotic medications act primarily by blocking D 2 dopamine receptors either as antagonists or partial agonists. 1 Although these medications have demonstrated measurable efficacy for treating the positive symptoms of schizophrenia, they have limited efficacy for negative and cognitive symptoms. Moreover, positive symptoms are refractory or resistant to treatment in 20% to 30% of patients 2 - 4 and adverse effects such as extrapyramidal motor symptoms, risk for developing tardive dyskinesia (TD), weight gain, and somnolence contribute to poor tolerability and adherence. 5 , 6 There is a significant need for antipsychotics with different mechanisms of action, improved efficacy, and better safety and tolerability than current treatments.
Imbalances between muscarinic acetylcholine and dopamine neurotransmitter systems have been implicated in the pathophysiology of schizophrenia. 7 Targeting muscarinic receptors may present a novel treatment option for people with schizophrenia. 7 Xanomeline-trospium chloride (KarXT) combines the dual M 1 /M 4 preferring muscarinic receptor agonist xanomeline with the peripherally restricted muscarinic receptor antagonist trospium chloride. In the phase 2 EMERGENT-1 trial ( NCT03697252 ) 8 and phase 3 EMERGENT-2 trial ( NCT04659161 ) 9 in people with schizophrenia experiencing acute psychosis, xanomeline-trospium significantly improved positive and negative symptoms compared with placebo and was well tolerated without many of the problematic adverse effects associated with currently approved antipsychotic medications (eg, extrapyramidal motor symptoms, weight gain, somnolence, and hyperprolactinemia). Here, we report results from the phase 3 EMERGENT-3 trial, which was designed to assess the efficacy, safety, and tolerability of xanomeline-trospium in people with schizophrenia experiencing acute psychosis.
EMERGENT-3 ( NCT04738123 ) was a phase 3, multicenter, randomized, double-blind, placebo-controlled trial conducted at 18 inpatient sites in the US and 12 inpatient sites in Ukraine between April 1, 2021, and December 7, 2022. The design of EMERGENT-3 was identical to EMERGENT-2 9 and very similar to EMERGENT-1. 8 Ukraine was closed to further enrollment after the start of the Russia-Ukraine conflict in February 2022. The trial was conducted in accordance with the principles of the Declaration of Helsinki, the International Council of Harmonization guidelines for Good Clinical Practice, and the relevant regulations in the countries in which the research was conducted. The protocol and written informed consent were approved by a centralized institutional review board (WCG, Princeton, New Jersey). This trial followed Consolidated Standards of Reporting Trials ( CONSORT ) guidelines.
EMERGENT-3 enrolled adults aged 18 to 65 years with a diagnosis of schizophrenia established by comprehensive psychiatric evaluation based on criteria in the DSM-V , 10 and confirmed by Mini International Neuropsychiatric Interview for Schizophrenia and Psychotic Disorders version 7.0.2. 11 A baseline Positive and Negative Syndrome Scale (PANSS) 12 total score of 80 to 120 was required, with a score of 4 or higher (ie, reflective of symptoms that are moderate or worse in severity) on at least 2 of the following positive scale items: P1, delusions; P2, conceptual disorganization; P3, hallucinatory behavior; and P6, suspiciousness/persecution. A Clinical Global Improvement–Severity (CGI-S) 13 score of at least 4 at screening and baseline was also required. People with a primary disorder other than schizophrenia within the 12 months preceding screening or a history of treatment resistance to antipsychotic medication were excluded. Participants with at least 20% improvement in PANSS total score between screening and baseline were also excluded. Per the US Food and Drug Administration directive on diversity in clinical trials, it is important to have representation proportional to the disease burden found in the general public; race was self-reported and entered by study staff in electronic data capture (Medidata RAVE, Medidata). There were no a priori indications that there would/will be racial differences in response or tolerability.Inclusion and exclusion criteria are provided in the eAppendix in Supplement 1 .
Eligible participants were randomized (1:1 ratio; stratified by site) to receive twice-daily oral xanomeline-trospium or placebo using a computer-generated participant identification number and randomization schedule created by Veristat version 9.4 (SAS). Treatment group assignments were concealed from participants, trial and laboratory personnel, investigators, statisticians, and the sponsor.
Xanomeline-trospium and placebo were supplied as identical, matching capsules. Trial medication was started on day 1 of the 5-week treatment period. Xanomeline-trospium was dosed beginning with twice-daily 50-mg xanomeline and 20-mg trospium for the first 2 days and then twice-daily 100-mg xanomeline and 20-mg trospium for days 3 through 7. Beginning on day 8, there was flexible dosing with an optional dose increase to a maximum of twice-daily 125-mg xanomeline and 30-mg trospium based on tolerability as assessed by the investigator, with the option to return to 100-mg xanomeline and 20-mg trospium based on tolerability. No dose changes were allowed during the last 2 weeks of the trial. The dosing regimen was identical to that used in the EMERGENT-1 8 and EMERGENT-2 9 trials.
CGI-S and PANSS scores were assessed at screening and baseline, and then weekly throughout the 5-week treatment period, beginning at week 1 for CGI-S and week 2 for PANSS. Spontaneous adverse events (AEs) were recorded at each visit. Orthostatic vital signs were measured supine and standing (after 2 minutes) at screening and on days 1, 3, 7, 8, 14, 21, 28, 32, and 35. Vital signs were recorded 2 hours after the morning dose at each postbaseline visit to correspond to maximum concentration. The Simpson-Angus Scale, Barnes Akathisia Rating Scale, and Abnormal Involuntary Movement Scale (AIMS) were assessed at baseline and weekly during the trial. A safety follow-up visit was performed at week 6 for participants not enrolling in the long-term, open-label, follow-up trial (EMERGENT-4).
The prespecified primary end point was change from baseline to week 5 in PANSS total score. Prespecified secondary end points were change from baseline to week 5 in PANSS positive subscale score, PANSS negative subscale score, 12 PANSS Marder negative factor score, 14 and CGI-S score, 13 as well as the proportion of participants with at least a 30% reduction from baseline to week 5 in PANSS total score. The trial included pharmacokinetic and exploratory end points not reported here.
Safety was assessed by monitoring for spontaneous AEs after administration of the first dose of trial medication on day 1 until the time of discharge on day 35. The Simpson-Angus Scale was used to measure drug-related extrapyramidal motor symptoms (range, 0 to 40; higher scores indicate greater drug-induced parkinsonian symptoms) 15 ; the Barnes Akathisia Rating Scale was used to assess akathisia (range, 0 to 14; higher scores indicate greater symptoms of akathisia) 16 ; and the AIMS was used to assess risk of TD (range, 0 to 28; rating of 2 or higher on the global severity item indicates evidence of TD). 17 Body weight, vital signs, and clinical laboratory values were also assessed.
Assuming a between-treatment group mean (SD) difference in change from baseline to week 5 in PANSS total score of 8 (16) points based on results from EMERGENT-1 and other similar antipsychotic registration trials, it was estimated that a sample size of approximately 172 participants (86 per group) would achieve 90.3% power for a 2-sided test at α of .05. With an anticipated attrition rate of 30%, enrollment of 246 participants was planned.
Baseline demographics were reported descriptively using the intent-to-treat (ITT) population. All efficacy analyses were performed using the modified ITT (mITT) population, defined as all randomized participants who received at least 1 dose of the trial drug and had 1 baseline PANSS assessment and at least 1 postbaseline PANSS assessment. The difference in change from baseline to week 5 in PANSS total score was estimated using a mixed model for repeated measures (MMRM; SAS version 9.4 [SAS Institute]) with likelihood-based modeling to handle incomplete data. The model included terms for treatment group (xanomeline-trospium or placebo), visit (weeks 2, 3, 4 and 5), treatment by visit interaction, site, age, sex, and baseline PANSS score. Least squares (LS) mean change from baseline, SE, and LS mean difference between the xanomeline-trospium and placebo groups at week 5 along with the 95% CI and a 2-sided P value were calculated for the primary end point. A 2-sided P value of ≤.05 was considered statistically significant. Continuous secondary end points were analyzed in the same manner as the primary efficacy analysis. Cohen d effect size was calculated using the absolute value of the difference in LS mean change in score from baseline at week 5 between the xanomeline-trospium and placebo groups divided by the pooled SD estimated from the MMRM.
A supportive analysis was conducted with the MMRM approach used for the primary analysis, using the completer population, defined as all mITT participants who had a valid PANSS total score at week 5. Missing data assumptions used in the primary analysis were also evaluated with sensitivity analyses using multiple imputation approaches. Finally, a post hoc sensitivity analysis was performed in the intent-to-treat (ITT) population, defined as all participants who were randomized to treatment.
For the PANSS responder analysis, PANSS items were rescaled from a range of 1 through 7 to 0 through 6 or, equivalently, PANSS total scores were floor-adjusted by subtracting 30 points from baseline and postbaseline scores. This floor adjustment was prespecified in the statistical analysis plan. The percentage of PANSS responders at each week was calculated and summarized by treatment group. The percentage of PANSS responders at week 5 was compared between treatment groups (xanomeline-trospium and placebo) using the Cochran-Mantel-Haenszel test stratified by site.
To account for multiplicity, a fixed sequence testing procedure was used for all efficacy variables, which was also used in the EMERGENT-1 and EMERGENT-2 trials. The primary end point was tested first, followed by secondary efficacy outcomes measures in the following order: change from baseline to week 5 for xanomeline-trospium vs placebo in PANSS positive subscale score, PANSS negative subscale score, and PANSS Marder negative factor score, followed by CGI-S score and then the proportion of participants with at least a 30% reduction in PANSS total score (responders).
Safety analyses were performed in all randomized participants receiving at least 1 dose of xanomeline-trospium or placebo (safety population). Safety and tolerability data were summarized descriptively by treatment group and time point as appropriate. The number and percentage of participants with any treatment-emergent AEs (TEAEs), any serious TEAE, any severe TEAE, and any TEAE leading to trial drug discontinuation were summarized by treatment group. Mean change (SD) from baseline was calculated for measures of body weight and vital signs, as well as the Simpson-Angus Rating Scale, Barnes Akathisia Rating Scale, and AIMS scores, and summarized by treatment group. Data were analyzed from February to June 2023.
A total of 431 individuals were screened, and 256 participants were randomized to receive xanomeline-trospium (125 participants) or placebo (131 participants) ( Figure 1 ). The safety population included 253 participants (xanomeline-trospium, 125 participants; placebo, 128 participants), and the mITT population included 234 participants (xanomeline-trospium, 114 participants; placebo, 120 participants). A total of 46 participants (36.8%) in the xanomeline-trospium group and 38 participants (29.0%) in the placebo group discontinued the trial early, with the most common reasons being withdrawal of consent followed by AEs.
Baseline demographics and characteristics were similar between treatment groups ( Table 1 ). The majority of participants were men (191 of 256 participants [74.6%]); 156 of 256 participants (60.9%) were Black or African American, 98 (38.3%) were White, and 1 (0.4%) was Asian; the mean (SD) age was 43.1 (11.8) years. A total of 21 of 125 participants (16.8%) in the xanomeline-trospium group compared with 27 of 131 (20.6%) in the placebo group were from Ukraine. Mean (SD) baseline PANSS total scores were 97.3 (8.9) in the xanomeline-trospium group and 96.7 (8.9) in the placebo group.
For the primary end point, xanomeline-trospium was associated with a statistically significant 8.4-point greater reduction from baseline to week 5 in PANSS total score compared with placebo; the LS mean (SE) change from baseline to week 5 in PANSS total score was −20.6 (1.6) in the xanomeline-trospium group compared with −12.2 (1.6) in the placebo group (LS mean difference, −8.4; 95% CI, −12.4 to −4.3; P < .001; Cohen d effect size, 0.60) ( Figure 2 A; eTable 1 in Supplement 1 ). Among participants receiving xanomeline-trospium, a statistically significant improvement in PANSS total score vs placebo was evident starting at week 2 (first postbaseline rating) and was enhanced through the end of the trial (week 5). A supportive analysis of change from baseline to week 5 in PANSS total score for completers, a sensitivity analysis using placebo-based multiple imputation, and an exploratory post hoc analysis in the ITT population supported the primary analysis (eTable 2 in Supplement 1 ).
The LS mean (SE) change from baseline to week 5 in the PANSS positive subscale score was −7.1 (0.5) in the xanomeline-trospium group compared with −3.6 (0.5) in the placebo group (LS mean difference, −3.5; 95% CI, −4.7 to −2.2; P < .001) ( Figure 2 B; eTable 1 in Supplement 1 ). The prespecified PANSS negative subscale score did not meet statistical significance at week 5, although the LS mean change from baseline in PANSS negative subscale score and PANSS Marder negative factor score achieved statistical significance at week 4 ( Figure 2 C and D; eTable 1 in Supplement 1 ). Although the prespecified secondary end point of CGI-S was not formally tested given the prespecified hierarchical testing procedure, the LS mean (SE) change from baseline to week 5 in CGI-S score was −1.1 (0.1) in the xanomeline-trospium group compared with −0.6 (0.1) in the placebo group (LS mean difference, −0.5; 95% CI, −0.8 to −0.3; nominal P < .001) ( Figure 2 E; eTable 1 in Supplement 1 ). Finally, the percentage of PANSS responders (at least 30% improvement from baseline in floor-adjusted PANSS total scores) at week 5 was 50.6% in the xanomeline-trospium group, compared with 25.3% in the placebo group (rate difference, 35.4%; 95% CI, 10.8% to 38.5%; nominal P < .01) ( Figure 3 ; eTable 1 in Supplement 1 ).
Treatment-emergent AEs (TEAEs) were reported in 88 participants (70.4%) in the xanomeline-trospium group compared with 64 (50.0%) in the placebo group ( Table 2 ). The most common TEAEs occurring in at least 5% of participants receiving xanomeline-trospium and at a rate at least twice that observed in the placebo group were nausea (24 participants [19.2%] vs 2 participants [1.6%]), dyspepsia (20 participants [16.0%] vs 2 participants [1.6%]), vomiting (20 participants [16.0%] vs 1 participant [0.8%]), constipation (16 participants [12.8%] vs 5 participants [3.9%]), hypertension (8 participants [6.4%] vs 2 participants [1.6%]), and diarrhea (7 participants [5.6%] vs 1 participants [0.8%]) ( Table 2 ). Commonly reported cholinergic TEAEs were all mild to moderate in intensity, generally began within the first 2 weeks of treatment, and were transient in nature. The percentages of participants who discontinued treatment due to a TEAE were similar between the xanomeline-trospium group (8 participants [6.4%]) and placebo group (7 participants [5.5%]) (eTable 3 in Supplement 1 ). One serious TEAE (gastroesophageal reflux disease) was reported in the xanomeline-trospium group compared with none in the placebo group.
No clinically meaningful changes from baseline to week 5 on any of the extrapyramidal motor symptom scales were detected between the xanomeline-trospium and placebo groups; across scales, participants had low mean scores at baseline and minimal change from baseline to the end of the trial. The mean (SD) change from baseline to week 5 in scores for the Barnes Rating Scale was −0.1 (0.75) points for xanomeline-trospium vs −0.1 (0.88) points for placebo, and the mean (SD) change in Simpson-Angus Scale score for extrapyramidal motor symptoms was −0.1 (0.56) points vs −0.1 (0.36) points, respectively ( Table 2 ). No cases of TD were reported in either treatment group; mean (SD) change from baseline to week 5 in AIMS score was 0.0 (0.45) in the xanomeline-trospium group and 0.0 (0.15) in the placebo group ( Table 2 ). Symptoms of akathisia were reported by 4 participants (3.2%) in the xanomeline-trospium group and 2 participants (1.6%) in the placebo group; all cases of akathisia were reported at the same trial center and none were deemed related to the trial drug in the opinion of the investigator. Three TEAEs of akathisia (2 in the placebo group, 1 in the xanomeline-trospium group) were ongoing at the end of treatment.
Xanomeline-trospium was not associated with weight gain or somnolence compared with placebo. The mean (SD) change in body weight from baseline to week 5 was 1.4 (3.4) kg in the xanomeline-trospium group and 2.0 (3.1) kg in the placebo group ( Table 2 ). Five (4.0%) participants receiving xanomeline-trospium and 3 (2.3%) receiving placebo had an increase in body weight reported as a TEAE. The number of participants who experienced at least 7% increase in body weight from baseline to week 5 was 5 (6.4%) in the xanomeline-trospium group and 12 (13.0%) in the placebo group. The incidence of somnolence was 1.6% and 0% in participants receiving xanomeline-trospium and placebo, respectively.
There were small increases in mean systolic and diastolic blood pressure as measured at peak plasma concentration (Cmax; 2 hours post dose) in the xanomeline-trospium group compared with placebo, which peaked after 1 week (2 to 3 mm Hg) and partially attenuated by the end of the trial (eFigure 1 in Supplement 1 ). Hypertension was reported as a TEAE more frequently in the xanomeline-trospium group than the placebo group (8 participants [6.4%] vs 2 participants [1.6%], respectively). TEAEs of hypertension were largely transient elevations of blood pressure (higher than 140 mm Hg systolic or higher than 90 mm Hg diastolic) and most resolved during the trial. In participants with a TEAE of hypertension, mean blood pressure at the end of the trial was similar to baseline and did not lead to trial discontinuation. No increases in QTc interval were observed with xanomeline-trospium . Xanomeline-trospium treatment was associated with an increase from baseline in supine heart rate compared with placebo that peaked at day 8 (mean [SD], 13.0 [15.30] bpm vs 4.4 [14.02] bpm) and decreased through the end of the trial (mean [SD], 11.2 [14.99] bpm vs 5.9 [13.70] bpm).
In the phase 3 EMERGENT-3 trial in people with schizophrenia experiencing acute psychosis, xanomeline-trospium was associated with a statistically significant and clinically meaningful 8.4-point greater reduction in PANSS total score compared with placebo at week 5. Statistically significant improvements in PANSS total score were observed with xanomeline-trospium starting at week 2 (first postbaseline measure) and enhanced through the end of the trial (week 5). The treatment effect sizes observed for the primary end point in this trial (0.60), the EMERGENT-1 trial (0.75), 8 and the EMERGENT-2 trial (0.61) 9 were robust; the median treatment effect size reported across 105 trials of antipsychotics in the treatment of acute psychosis in people with schizophrenia was 0.42. 6 Treatment with xanomeline-trospium also resulted in a significantly greater reduction from baseline to week 5 in positive symptoms than placebo as measured by the PANSS positive subscale.
Xanomeline-trospium was well tolerated with an adverse effect profile substantially consistent with the previously reported EMERGENT-1 8 and EMERGENT-2 9 trials. The overall percentage of participants discontinuing treatment was similar between the xanomeline-trospium (36.8%) and placebo groups (29.0%), as was the percentage of people discontinuing due to TEAEs (6.4% vs 5.5%, respectively). The most common TEAEs in the xanomeline-trospium group were primarily gastrointestinal in nature; these TEAEs were all mild or moderate in intensity and improved in the first 2 to 3 weeks of the trial.
TEAS of hypertension were more common with xanomeline-trospium (8 participants [6.4%]) than placebo (2 participants [1.6%]). However, mean changes in systolic and diastolic blood pressure assessed at 2 hours postdose (ie, the approximate Cmax) were similar between the xanomeline-trospium and placebo treatment groups and remained relatively flat over the course of the 5-week trial; hypertension TEAEs were mostly mild, transient elevations of blood pressure in a small number of participants. The effect of xanomeline-trospium treatment on blood pressure is being evaluated in an ongoing ambulatory blood pressure monitoring trial in people with schizophrenia. As in prior trials, there was an increase in heart rate in the xanomeline-trospium group compared with the placebo group that decreased in magnitude by the end of the trial.
The EMERGENT-3 trial had limitations typical of registration trials in acute psychosis. First, the duration of the trial was 5 weeks; schizophrenia is a lifelong illness and longer trials are needed to assess the durability of the effect and long-term safety and tolerability of xanomeline-trospium. Xanomeline-trospium was associated with rapid improvement in psychotic symptoms starting at week 2 and it continued to separate from placebo at week 5, suggesting additional improvement may occur at later time points. Two 52-week, open-label trials (EMERGENT-4 and EMERGENT-5) are currently under way to assess the longer-term effects of xanomeline-trospium in an outpatient setting. Second, the EMERGENT-3 trial did not include an active comparator group and, thus, only cross-trial comparisons can be made as opposed to direct comparisons of xanomeline-trospium with other currently available antipsychotic medications. Additionally, recruitment of participants from the US and Ukraine was planned. The Russia-Ukraine conflict commenced after recruitment started, and Ukraine was closed to further enrollment.
In EMERGENT-3, in adults with schizophrenia experiencing acute psychosis, xanomeline-trospium was associated with statistically significant and clinically meaningful improvement in schizophrenia symptoms and was generally well tolerated. The efficacy, safety, and tolerability of xanomeline-trospium in EMERGENT-3 were substantially consistent with the results reported for the EMERGENT-1 and EMERGENT-2 trials. Together, these results demonstrate that xanomeline-trospium has the potential to be the first in a new class of antipsychotic medications targeting muscarinic receptors and an alternative to D 2 dopamine receptor antagonists not associated with the adverse effects of EPS, weight gain, or somnolence.
Accepted for Publication: February 22, 2024.
Published Online: May 1, 2024. doi:10.1001/jamapsychiatry.2024.0785
Open Access: This is an open access article distributed under the terms of the CC-BY-NC-ND License . © 2024 Kaul I et al. JAMA Psychiatry .
Corresponding Author: Steven M. Paul, MD, Karuna Therapeutics, 99 High St, 26th Floor, Boston, MA 02110 ( [email protected] ).
Author Contributions: Drs Kaul and Brannan had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Kaul, Walling, Tamminga, Breier, Paul, Brannan.
Acquisition, analysis, or interpretation of data: Kaul, Sawchak, Walling, Breier, Zhu, Miller, Paul, Brannan.
Drafting of the manuscript: Kaul, Paul, Brannan.
Critical review of the manuscript for important intellectual content: All authors.
Statistical analysis: Kaul, Breier, Zhu.
Obtained funding: Miller, Paul.
Administrative, technical, or material support: Kaul, Sawchak, Miller, Paul.
Supervision: Kaul, Walling, Miller, Paul, Brannan.
Conflict of Interest Disclosures: Dr Walling reported receiving grants from AbbVie, Acadia, Alkermes, Allergan, Avanir, Biogen, Biohaven, Boehringer Ingelheim, Cerevel, Indivior, Intra-Cellular, Janssen, Johnson and Johnson, Karuna Therapeutics, Lundbeck, Lupin, Lyndra, Merck, Minerva, Navitor, Neurocrine, Novartis, Noven, Otskuka, Pfizer, Recognify, Roche, Sunovion, and Takeda outside the submitted work; and serving as a consultant to Boehringer Ingelheim, Biogen, Janssen, Lyndra, and Otsuka. Dr Tamminga reported receiving personal fees from Karuna, Kynexis, and Merck outside the submitted work. Dr Breier reported receiving personal fees from Karuna, Bioxcel, and Neumarker outside the submitted work. Dr Miller reported a patent for US10,238,643 issued, a patent for US10,265,311 issued, a patent for US10,369,143 issued, a patent for US10,369,144 issued, a patent for US10,695,339 issued, a patent for US Appl. No. 18/461,741 pending, a patent for US Appl. No. 16/950,203 pending, and a patent for US Appl. No. 18/454,177 pending 83555. No other disclosures were reported.
Funding/Support: This trial was sponsored by Karuna Therapeutics.
Role of the Funder/Sponsor: Karuna Therapeutics, Syneos Health, and Veristat designed the EMERGENT-3 trial, developed the protocol, and created the Statistical Analysis Plan together. Data were collected through an internet-based electronic case-report form managed by Medidata (RAVE), as well as study tablets provided by Signant Health. The sponsor had read-only access to RAVE, and did not have access to unblinded data or the randomization codes, which were held by Signant Health and Veristat, respectively, until finalization of the database. Data management and site monitoring were performed by Syneos Health. The finalized database was electronically transferred to Veristat where unblinding and statistical analyses were performed. Veristat transferred the information to Karuna.
Data Sharing Statement: See Supplement 3 .
Additional Contributions: The authors thank Allyson Lehrman, DPM, Matthew Jacobson, CMPP, and Paula Stuckart of Apollo Medical Communications, part of Helios Global Group, for medical writing and editorial assistance, which was funded by Karuna Therapeutics.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
REVIEW article
A review on the pharmacology of cariprazine and its role in the treatment of negative symptoms of schizophrenia.

- 1 Department of Psychiatry, Sneka Mind Care Hospital, Tirunelveli, Tamil Nadu, India
- 2 Medical Affairs and Clinical Research, Sun Pharmaceutical Industries Limited, Mumbai, India
- 3 Medical Affairs and Clinical Research, Sun Pharma Laboratories Limited, Mumbai, India
- 4 Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences (NIMHANS), Bangalore, India
Management of negative symptoms is one of the most challenging and important unmet needs of schizophrenia treatment. Negative symptoms together with positive symptoms result in significant psychosocial impairment and poor quality of life. Existing studies on atypical antipsychotics reported limited treatment adherence due to higher prevalence of treatment-emergent adverse events, such as diabetes, weight gain, hyperlipidemia, hyperprolactinemia and hypertension. A compound with greater affinity for dopamine D2/D3 receptors may improve negative symptoms, mood, and cognitive impairment associated with schizophrenia. In 2015, the US FDA has approved cariprazine, a partial D2/D3 agonist for treatment of schizophrenia, mania or mixed episodes. Midlands and Lancashire Commissioning Support Unit, UK (2019) has particularly suggested cariprazine for the treatment of predominant negative symptoms of schizophrenia. India’s Central Drugs Standard Control Organization (CDSCO) has approved cariprazine in 2021 for the treatment of schizophrenia, manic or mixed episodes associated with bipolar I disorder. A ten-fold greater affinity for D3 receptors and partial agonism to serotonin receptors, along with longer half-life make cariprazine distinct when compared with other atypical antipsychotics. Cariprazine is also reported to have fewer incidents of metabolic and hormonal adverse events, and has been shown to provide better relapse prevention. Recent evidence indicates promising effect of cariprazine in ameliorating negative symptoms as well as psychotic symptoms in patients with schizophrenia. In addition, improved adherence to treatment (adjunctive/monotherapy) with cariprazine in patients having inadequate response to an ongoing antipsychotic treatment has also been clinically established. This review presents the evidence-based safety and efficacy of cariprazine for treatment of predominant negative symptoms of schizophrenia.
1 Introduction
Schizophrenia is a complex neuropsychiatric disorder, presenting with positive (hallucinations and delusions), negative (blunted affect, alogia, anhedonia, asociality and avolition), and cognitive (impaired retrieval of information like thinking, learning and memorizing) symptoms ( 1 ). In addition to these symptoms, patients with schizophrenia often experience affective symptoms (depression and anxiety) that is associated with increased risk of suicide and poor quality of life ( 2 ). Positive symptoms are primarily monitored to diagnose an active state of schizophrenia, but negative symptoms are significant contributors of poor psychosocial functioning and performance, impacting the patient’s quality of life ( 3 ). Identification of negative symptoms can be sometimes challenging due to their insidious onset, paucity of psychotic signs and similarity with other clinical features of schizophrenia, resulting in delayed treatment outcomes ( 1 ). The fundamental pathophysiological mechanism of negative symptoms is different from positive symptoms ( 3 ). Hyperdopaminergic state of dopamine D2 receptor in the mesolimbic area is related to positive symptom prognosis, while hypodopaminergic dysregulation of the prefrontal cortex leads to negative symptoms ( 1 ). Only a decade ago, the focus from positive symptoms has shifted to the negative symptoms. Since then, very few pharmaceutical agents have been studied that successfully met the therapeutic target of negative symptoms ( 4 ). Typical (first generation) and atypical (second and third generations) antipsychotics are primarily used to modulate the dopaminergic function ( 5 ). The mechanism of action of first generation antipsychotics (FGAs) lack preference-based blocking of dopamine pathway, resulting in extrapyramidal symptoms (dyskinesia, akathisia and tremors), hyperprolactinemia-associated sexual dysfunction and aggravation of negative symptoms ( 6 ). The second generation antipsychotics (SGAs) are combined D2 and serotonin 5-HT 2A receptor antagonists with lower risk for developing extrapyramidal symptoms (EPS) ( 7 ). Second generation antipsychotics are effective for negative symptoms, but result in several treatment-emergent side effects including diabetes, ketoacidosis, weight gain, hyperlipidemia, hypercholesterolemia, hypertriglyceridemia, hypertension and metabolic syndrome ( 6 , 8 , 9 ).
Negative symptoms of schizophrenia have been consistently related to poor treatment outcome. A well-tolerated and long-term effective treatment option for negative symptoms is one of the most important unmet needs that is to be addressed. Globally approved third generation antipsychotic (TGA), cariprazine is distinct from other antipsychotics and has partial agonist activity at dopamine D3/D2 and serotonin 5-HT 1A receptors ( 10 ). Because of its dopamine-dependent partial agonism to D2 and D3 receptors, cariprazine is less likely to cause weight gain, metabolic disorder and hyperprolactinemia ( 6 ). This review attempts to outline the safety and efficacy of cariprazine for the treatment of predominant negative symptoms of schizophrenia.
2 Epidemiology of schizophrenia
According to World Health Organization (2022), schizophrenia affects about 24 million people worldwide, i.e., 1 in 300 ( 11 ). It is one of the top 15 causes of global disability ( 12 ). In India, the prevalence of schizophrenia is 1.5-2.5/1000 people, with an annual rate of 0.35-0.38/1000 and 0.44/1000 people from urban and rural area, respectively ( 13 ). Many factors including migration, drug abuse, urbanicity (stress, noise and pollution), childhood traumas, psychosocial factors, infections, cannabis use, birth during winter (high chances of respiratory infections and inadequate vitamin D synthesis), and obstetric complications during fetal, childhood, adolescence and early adult life can increase the risk of developing schizophrenia ( 14 ). The onset of schizophrenia mostly occurs during late adolescence and early adulthood, and happens earlier in males (average age 18 years) than females (average age 25 years) ( 1 ). Late onset of schizophrenia (after the age of 44 years) accounts for 15-20% of all cases ( 15 ).
3 Overview of negative symptoms of schizophrenia
Prevalence of negative symptoms is 75% in patients having schizophrenia and 68% in patients with schizoaffective disorder ( 16 ). Sometimes negative symptoms appear before the onset of positive symptoms (73% prevalence) or in the same month of developing positive symptoms (20% prevalence) ( 17 ). Ninety percent patients with first psychotic episode can develop at least one negative symptom, whereas 35-70% can still suffer from negative symptoms post-treatment ( 17 ).
The five constructs of negative symptoms are blunted affect, alogia, anhedonia, asociality and avolition ( 3 ) that cluster into two domains: the expressive domain (blunted affect and alogia) and the experiential domain (anhedonia, asociality and avolition); latter has a larger effect on the real-world functioning ( 18 ). Moreover, negative symptoms can be primary or secondary depending on their etiology ( Figure 1 ). Primary negative symptoms for more than one year manifest deficit syndrome of schizophrenia and patients without these symptoms are considered to suffer from non-deficit schizophrenia ( 19 ). The severity of negative symptoms is also described by persistent (persisting over time, in spite of antipsychotic treatment), predominant (greater severity than co-occurring positive symptoms) and prominent (at least three moderate symptoms or two severe symptoms) negative symptoms ( 17 , 20 ).
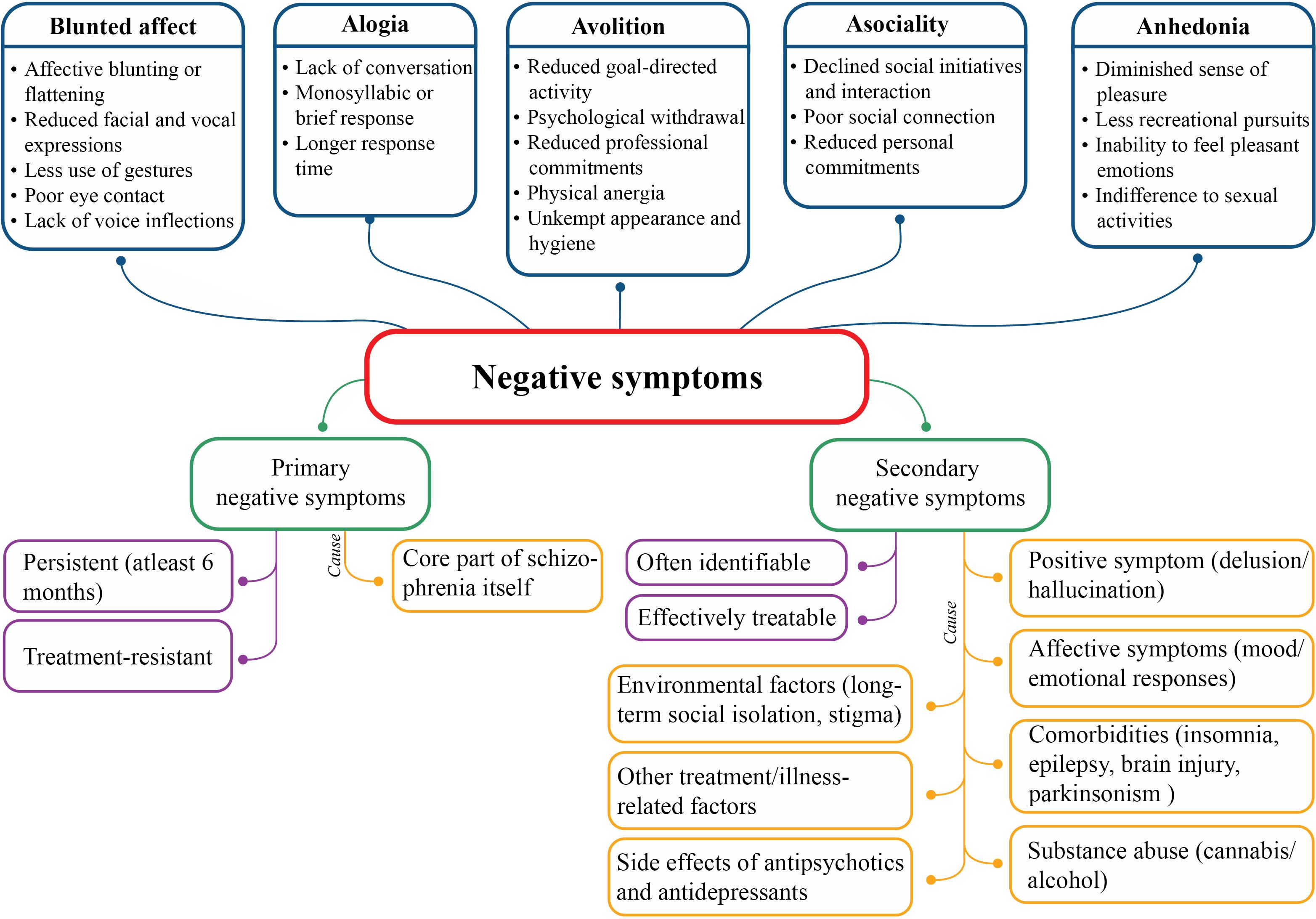
Figure 1 Nature, classification and etiology of negative symptoms.
Pathology of negative symptoms includes decreased dopamine transmission in mesocortical pathways, along with decreased serotonergic, glutamatergic and noradrenergic transmission. Of note, hypodopaminergic functioning in the prefrontal lobe and additional mesolimbic structures are responsible for diminished motivation and reward-related processes, leading to negative symptoms ( 17 , 21 ). Inhibition of glutamate neurotransmission after antagonizing N-methyl-D-aspartate (NMDA) receptors may results in negative symptoms of schizophrenia ( 17 ). Brain regions associated with expressive domain are rostral anterior cingulate cortex, amygdala, and ventrolateral prefrontal cortex; and with experiential domain are dorsal and ventral striatum, dorsolateral prefrontal cortex, anterior cingulate cortex and orbitofrontal cortex ( 21 , 22 ).
Genetic factors, prenatal complications and poor premorbid adjustments prior to development of psychotic illnesses are contributing factors for onset of negative symptoms ( 17 ). Males are more prone to develop negative symptoms, especially anhedonia and avolition ( 23 ). Negative symptoms greatly affect disease prognosis, physical and psychological health, and personal and social relationships ( 24 – 28 ), as reduced functioning of mental health, health utility and expert-rated quality of life were reported ( 29 ). During early phase of the syndrome, negative symptoms increase the risk of self-harm that can persist up to 7 years since first psychiatric visit, and impact different domains of real-life functioning ( 18 ). Level of negative symptoms in elderly population is equivalent to that of younger schizophrenia population ( 30 ). Anxiety and depression are the central symptoms of population with predominant negative symptoms, hence clinicians must pay attention to these symptoms too, and not only to negative symptoms ( 31 ).

4 Assessment of negative symptoms of schizophrenia
Quantitative (frequency, duration and intensity) and qualitative (difference between anticipatory and consummatory aspects of anhedonia; or difference between behavioral and experiential aspects) aspects of negative symptoms is assessed using validated instruments ( 18 ). Different scales for standardized assessments of negative symptoms which are used either by professionals or by the patient ( 17 , 20 ) is presented in Table 1 .
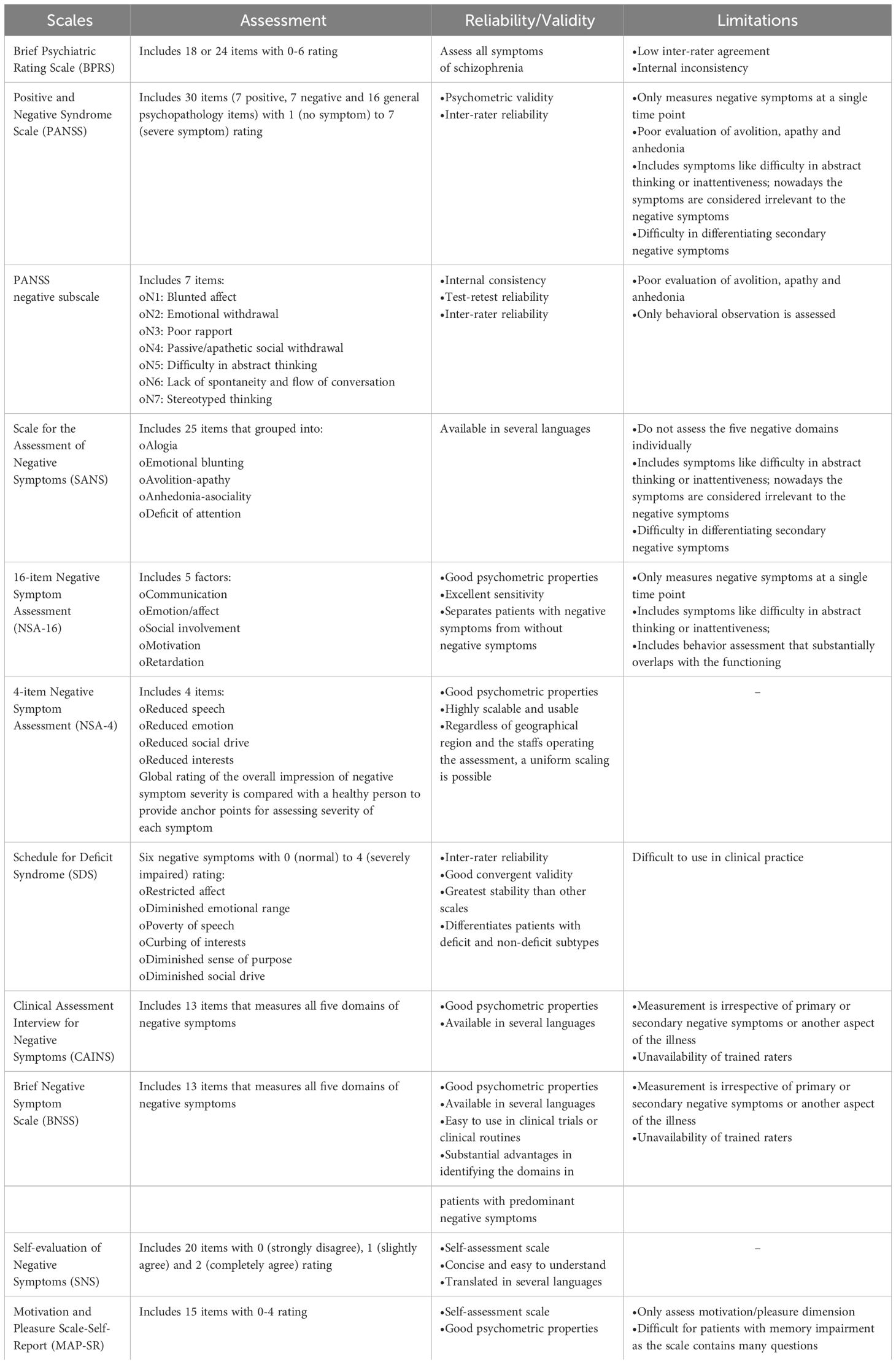
Table 1 Various assessment scales for negative symptoms of schizophrenia.
Brief Psychiatric Rating Scale (BPRS), Positive and Negative Syndrome Scale (PANSS) and Scale for the Assessment of Negative Symptoms (SANS) are used for diagnosis of deficit schizophrenia ( 20 ). In first episode of schizophrenia, patient’s phenomenological variety of negative symptoms can be evaluated with PANSS based experiential factor (‘poor rapport’, ‘passive/apathetic social withdrawal’, ‘active social avoidance’ and ‘lack of spontaneity’) and expressive factor (‘blunted affect’, ‘emotional withdrawal’ and ‘motor retardation’) ( 32 ). Brief Negative Symptom Rating Scale (BNSS) and Clinical Assessment Interview for Negative Symptoms (CAINS) have been developed by National Institute of Mental Health (NIMH) as the ‘next generation’ scale. In the USA, 16-item Negative Symptom Assessment (NSA-16) and SANS are recommended but not PANSS negative symptoms subscale, because of its inadequate coverage; whereas, the European drug authority has endorsed the use of SANS and PANSS negative symptoms subscale in clinical studies ( 9 ). Many European countries have approved the use of Self-evaluation of Negative Symptoms (SNS) scale ( 18 ).
5 Current treatment options for negative symptoms of schizophrenia and their limitations
Treatment of negative symptoms is challenging as no particular guidelines are available related to treatment algorithms and maintenance of the treatment; patients with treatment-resistant schizophrenia usually develop prominent negative symptoms ( 33 , 34 ). The European regulatory guidelines and commentary issued by the US regulators have different perspectives with respect to treatment of schizophrenia, as the former recommend splitting negative symptoms from other domain of the disease, while the latter suggested lumping all the aspects of the disease together ( 9 ). The World Federation of Societies of Biological Psychiatry guidelines recommended use of FGA for secondary but not for primary negative symptoms of schizophrenia ( 35 ). Antipsychotic treatment is recommended by the American Psychiatric Association (APA) and the British Association for Psychopharmacology (BAP) for improvement and remission of both positive and negative symptoms; National Institute for Health and Care Excellence (NICE) and Canadian Psychiatric Association (CPA) suggested this treatment approach for improving functioning and quality of life. Reduced hospitalization and mortality with antipsychotic therapy are demonstrated by APA and CPA ( 34 ). United Nations High Commissioner for Refugees (UNHCR) recommended switching from FGA to SGA in case of ineffective treatment of negative symptoms ( 34 ). Likewise, the European Psychiatric Association guidelines recommended switching to SGA in patients not responding to FGA, along with social skill training and psychosocial rehabilitation ( 20 ).
First generation antipsychotics exhibits narrow efficacy spectrum in managing negative symptoms of schizophrenia ( 7 ). Second generation antipsychotics were introduced in the late 80s ( 24 ) with a promise to yield higher treatment efficacy, better receptor binding properties and lower side effects compared to FGAs ( 36 ). Significant difference in the pharmacological properties and side effect profiles exist between FGAs [fluphenazine, haloperidol, perphenazine and pimozide (D 2 antagonists) and chlorpromazine, loxapine, thioridazine and trifluoperazine (D 2 and 5-HT 2 antagonists)], SGAs [iloperidone, lurasidone, olanzapine and ziprasidone (D 2 and 5-HT 2 antagonists), asenapine, clozapine, paliperidone and risperidone (5-HT 2 , D 2 and norepinephrine α 2 antagonist) and quetiapine (D 2 and 5-HT 2 antagonist and norepinephrine transporter reuptake inhibitor)], and TGAs [aripiprazole and brexpiprazole (D 2 and 5-HT 1A partial agonist and 5-HT 2A antagonists)] ( 37 , 38 ). Clozapine is considered as the best evidence-based therapeutic option for treatment-resistant schizophrenia ( 39 ). Higher efficacy of clozapine than other SGAs is reported for management of schizophrenia and schizophrenia-like psychoses ( 40 ). Despite its efficacy, 40% patients with treatment-resistant schizophrenia were reported to be non-respondent to clozapine treatment ( 41 ). Nielsen et al. reported improvement in negative symptoms after treatment with aripiprazole due to its partial D2 receptor agonist effect; however, no improvement in cognitive functions was found ( 42 ). Another study on patients with schizophrenia-spectrum disorders reported lower efficacy of aripiprazole in terms of improvement in PANSS negative score and CGI-S score ( 43 ). Although brexpiprazole has shown greater efficacy in improving negative symptoms ( 44 ), but common adverse effects associated with brexpiprazole are akathisia, headache, somnolence, weight gain and altered triglyceride level. Long-term risk and benefits of brexpiprazole are also not well-established ( 45 ).
Treatment-emergent adverse events are frequent with SGAs that commonly include akathisia, EPS, weight gain, sedation, insomnia, hyperprolactinemia and metabolic changes ( 46 ). Other adverse events include periorbital edema, parotitis, (inflammation of parotid gland/s) and pseudopheochromocytoma, i.e., severe paroxysmal hypertension ( 39 ). Lobos et al. reported higher incidence of akathisia with olanzapine, elevated glucose, triglycerides and prolactin levels with olanzapine and clozapine, hypercholesterolemia and hypersalivation with clozapine and low sexual drive with clozapine and risperidone treatment ( 40 ). Clozapine is also associated with other side effects viz. EPS, agranulocytosis, drooling, sedation, headache, dizziness, tremor, tachycardia, lengthening of corrected QT (QT C ), weight gain, hypotension, visual abnormality, sweating, dry mouth, constipation, dyslipidemia and flexural intertrigo ( 39 ). Increase in prolactin level and EPS with amisulpride treatment and weight gain and elevated serum lipid and prolactin levels with amisulpride, aripiprazole, and olanzapine treatment were reported ( 43 , 47 ). Additionally, evidence-based international guidelines revealed that SGAs have only moderate effect on negative symptoms; antidepressants and glutamatergic compounds are necessary to use additionally to overcome the disease burden ( 9 ). Schizophrenia patient data from 20 placebo-controlled trials reported prominent negative symptoms (8-33.1%), predominant negative symptoms (14.9%) and European Medicines Association (EMA) criteria-based negative symptoms (12.2-45.5%) even after 6 weeks of active treatment with SGA ( 48 ).
Poor outcomes with FGAs, and major side effects and inadequate response to SGAs leave a gap regarding the most appropriate treatment of negative symptoms, which is a long-standing challenge for schizophrenia management. Recently, a review on mental health care in central and eastern Europe suggested that many countries across the Europe have incorporated cariprazine as the first-line treatment for negative symptoms ( 49 ). Both the EMA and the US Food and Drug Administration (FDA) have approved cariprazine for schizophrenia management ( 50 ). The position statement of Polish Psychiatric Association on the use of D2/D3 receptor partial agonists highlighted the benefits of cariprazine in the management of predominant and persistent negative symptoms ( 51 ).
6 Cariprazine: a novel third generation antipsychotic
Cariprazine was approved in 2015 by the US FDA and later in 2018 in the UK ( 5 , 7 ). The antipsychotic is approved in the US for treatment of schizophrenia, mania or mixed episodes, and depressive episodes related to bipolar I disorder and as adjunctive therapy to anti-depressants for the treatment of major depressive disorders, whereas in Europe it is approved for treatment of schizophrenia ( 26 ). Midlands and Lancashire Commissioning Support Unit 2019 has also recommended cariprazine for the treatment of predominant negative symptoms of schizophrenia ( 52 ). In 2021, India’s national regulatory body for cosmetics, pharmaceuticals and medical devices, Central Drugs Standard Control Organization (CDSCO) approved cariprazine for the treatment of schizophrenia, manic or mixed episodes associated with bipolar I disorder ( 53 ).
Cariprazine is available in capsule form with doses of 1.5, 3, 4.5, or 6 mg for schizophrenia treatment ( 6 ). At clinically relevant doses, cariprazine appeared to have higher occupancies of D2 and D3 receptors ( 54 ). Cariprazine dose of 1.5 mg/day results in 69% occupancy of both D2 and D3 receptors, and 3 mg/day for 14 days leads to 90% occupancy, suggesting adequate efficacy ( 6 ). Efficacy, tolerability and safety of cariprazine in patients having acute exacerbation of schizophrenia is established at a daily dose of 3 or 6 mg ( 55 ). More rapid onset of action (by 1 to 2 weeks) is achieved at a daily cariprazine dose of ≥3 mg than 1.5 mg; however, efficacy of cariprazine at 6 th week remains same with both higher and lower doses ( 56 ). Improvement in PANSS total score and CGI-S with cariprazine was reported at a dose of 1.5, 3 and 4.5 mg/day in one study ( 57 ), and at 3-6 or 6-9 mg/day in another study ( 58 ). Cariprazine dose of 4.5–6 mg/day improves negative symptoms ( 26 ); 3-6 or 6-9 mg/day improves PANSS and CAINS negative symptom scores ( 54 ) and 3, 6 or 9 mg/day lowers the chances of relapse ( 59 ). Cariprazine is also effective for patients with schizophrenia and concomitant substance use disorder, as it appeared to reduce cravings of illicit drugs/alcohol in such patients ( 60 ).
6.1 Unique aspect of cariprazine’s pharmacology
Cariprazine is a potent D2/D3 partial agonist with preferential binding to D3 receptors ( 61 ). This differs from two other TGAs like aripiprazole and brexpiprazole, by its distinct receptor-binding characteristics not only at dopamine D2/D3 receptors, but also at serotonin 5HT1A, 5HT2B, 5HT2A, 5HT2C, and histamine H1 receptors ( 62 ). Cariprazine acts as an antagonist when dopamine activity is normal and as partial agonist when the activity is low, depending on the available dopamine ( 63 ). This feature of cariprazine is proven effective for treatment of predominant primary negative symptoms of schizophrenia ( 24 , 64 ). It is especially recommended for elder patients, as cariprazine results in procognitive and antidepressant effects due to its partial agonism towards D2/D3 receptors ( 7 ). Cariprazine also acts as an antagonist to 5-HT 2B and a partial agonist to 5-HT 1A . Its strong affinity towards 5-HT 1B receptor is the reason for reduced EPS and akathisia; however, the clinical relevance of antagonism to serotonin 5-HT 2B receptors is unknown. Partial agonism of cariprazine to 5-HT 1A receptors lowers depressant effects of schizophrenia, and weak antagonism to 5-HT 2C and H1 receptors reduces risk of weight gain, metabolic abnormalities and sedation than olanzapine and quetiapine ( 63 ). Additionally, cariprazine has a lower or negligible affinity for noradrenergic, histaminergic, and cholinergic receptors ( 65 ). Because of its lower inhibition of dopaminergic neurotransmission in the striatum, cariprazine has lower risk of developing EPS than other atypical antipsychotics ( 63 ). The receptor binding affinities of different anti-psychotics in comparison with cariprazine is shown in Table 2 ( 5 , 36 , 66 – 69 ).
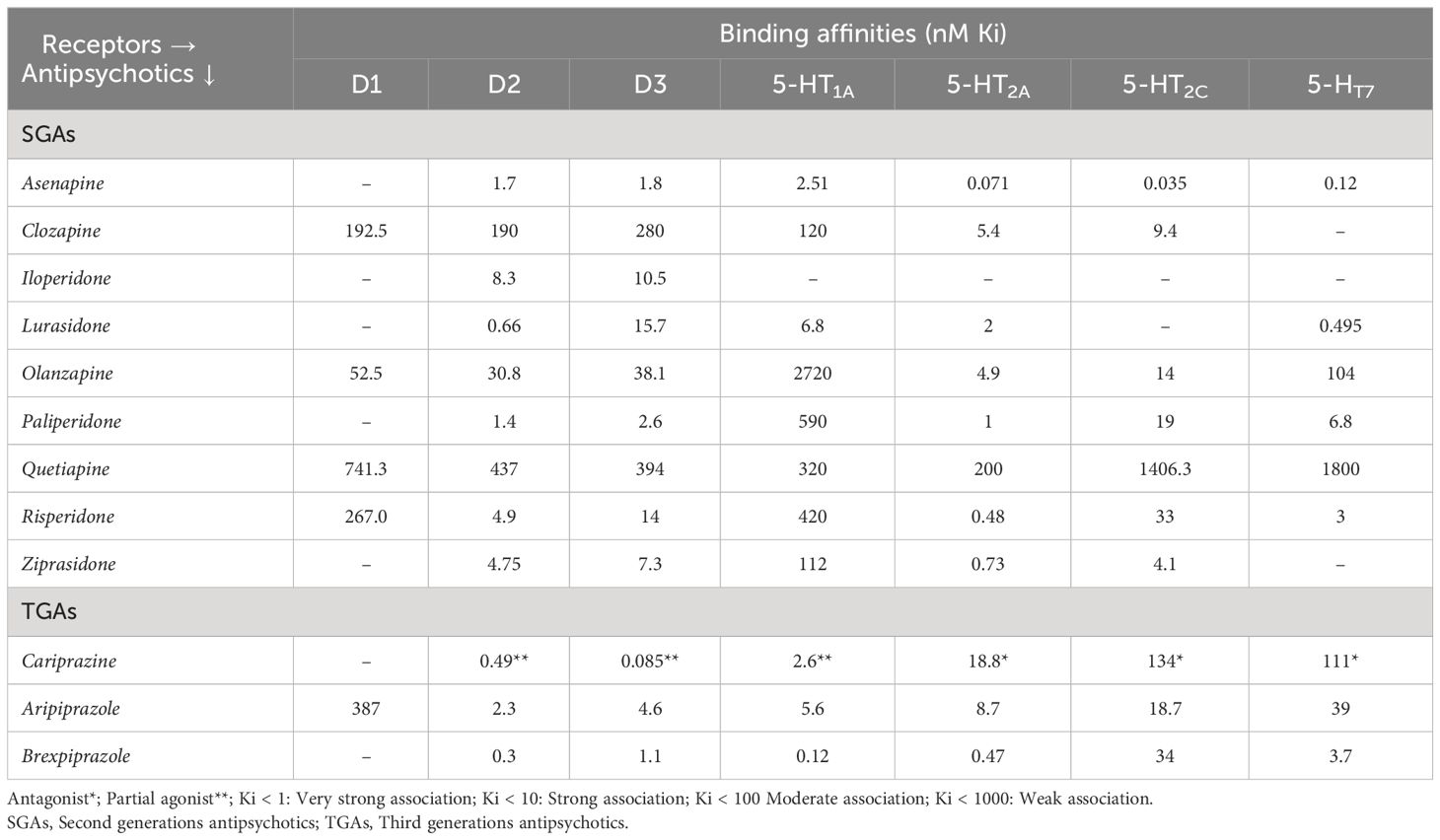
Table 2 Receptor binding affinities of cariprazine in comparison to other antipsychotics.
Greater affinity for D3 receptor together with actions of serotonin receptors makes cariprazine a potential antipsychotic for alleviating the negative symptoms. Moreover, these symptoms are responsible for poor social functioning, impacting patient’s daily functioning and quality of life. Efficacy of cariprazine is well-established in treating negative as well as cognitive and affective symptoms of schizophrenia, thus improving social behavior of the patient. For this reason cariprazine is regarded as a ‘socializing drug’ ( 70 ). In addition to ten-fold higher affinity for D3 receptors, cariprazine adds exceptional values to schizophrenia management because of its long half-life and broad-spectrum efficacy and safety ( 3 , 5 , 71 ). A remarkably longer half-life of the active metabolites of cariprazine, desmethyl cariprazine (DCAR) and didesmethyl cariprazine (DDCAR), of 2-4 days and 1-3 weeks ( 67 ) respectively, prevents patients from experiencing incidence of relapse even after accidentally missing dose. Early and late efficacy are offered by DCAR and DDCAR, respectively; with both depicting mean concentrations of 400% and 30% respectively even after 12 weeks of cariprazine administration ( 56 ). Cariprazine provides a significantly longer time to relapse (defined by occurrence of psychiatric hospitalization, worsening of symptom scores, aggression or violence or suicidal tendency) and lower chance of relapse ( 59 ).
It may be noted that, because cariprazine and its active metabolites have long half-lives, the active moiety would take several weeks to reach steady state; this is unlikely to be a problem as efficacy has anyway been demonstrated in clinical trials. However, because of the long half-lives, the active moiety would take long to wash out. This could be positive if patients do not take the drug for one or more days or temporarily discontinue the treatment, as the drug is still in the body. The long half-lives also obviate the risk of a drug discontinuation syndrome. However, the long half-lives could be negative if rapid reduction of blood levels is desired, as when patients experience adverse effects or become pregnant ( 72 ).
6.2 Clinical evidences on cariprazine in management of negative symptoms of schizophrenia
6.2.1 efficacy of cariprazine treatment.
The broad-spectrum efficacy of cariprazine in treatment of schizophrenia and predominant negative symptoms in terms of reduction in blunted affect, emotional withdrawal, passive/apathetic social withdrawal, poor rapport and difficulty in abstract thinking according to PANSS score is established ( 3 ). Although antipsychotic monotherapy is recommended for schizophrenia treatment, with the evidence of efficacy of polypharmacy in the real world, monotherapy is often challenged ( 6 ). On the other hand, adverse effects of using multiple antipsychotics disapproved the idea of polypharmacy ( 20 ). Available findings on cariprazine monotherapy or adjunctive therapy for negative symptom treatment are summarized in Table 3 . Németh et al. conducted a phase III randomized trial in eleven European countries, and found a significant improvement in predominant negative symptoms with cariprazine than risperidone, starting from ~3 months of treatment, as well as a greater treatment adherence. Moreover, the improvement was independent of EPS, positive and depressive symptoms ( 71 ). A recently published study found that a single trajectory best described improvement of negative symptoms with cariprazine: there was steady improvement all through the trial with most improvement occurring during the first 4 weeks ( 76 ). Another study demonstrated effectiveness of cariprazine monotherapy in reducing PANSS negative subscale items and PANSS-derived factors by week 26; in comparison to risperidone, the efficacy of cariprazine in negative symptom improvement was an exclusive effect of the antipsychotic only ( 3 ). Cariprazine showed to have higher improvement in moderate/severe negative symptoms in patients with acute schizophrenia compared to aripiprazole ( 24 ). A lower number needed to treat (NNT) indicates therapeutic effects of a drug compared to the comparator, based on the visible improvements ( 77 ). The NNT of cariprazine is lower than risperidone (n=3 vs. 6) and aripiprazole (n=3 vs. 19) in achieving PANSS factor score for negative symptoms, suggesting that cariprazine dose of 1.5–3 mg/day is sufficient to accomplish positive outcomes than risperidone and aripiprazole ( 26 ). A small uncontrolled, open label study in patients with early psychosis found that the mean negative PANSS score decreased from 26 (at baseline) to 11 (at 6 months) in patients who tolerated cariprazine (1.5-3.0 mg/day) and responded to it ( 78 ). Treatment-resistant or drug-naïve schizophrenia has shown improvement with cariprazine treatment ( 1 , 75 ). Steady state of paranoid delusions and aggressiveness was achieved with 2 weeks of cariprazine treatment ( 79 ). Cariprazine as adjunctive or monotherapy also resulted in remission of negative symptoms ( 5 , 25 , 64 , 74 ).
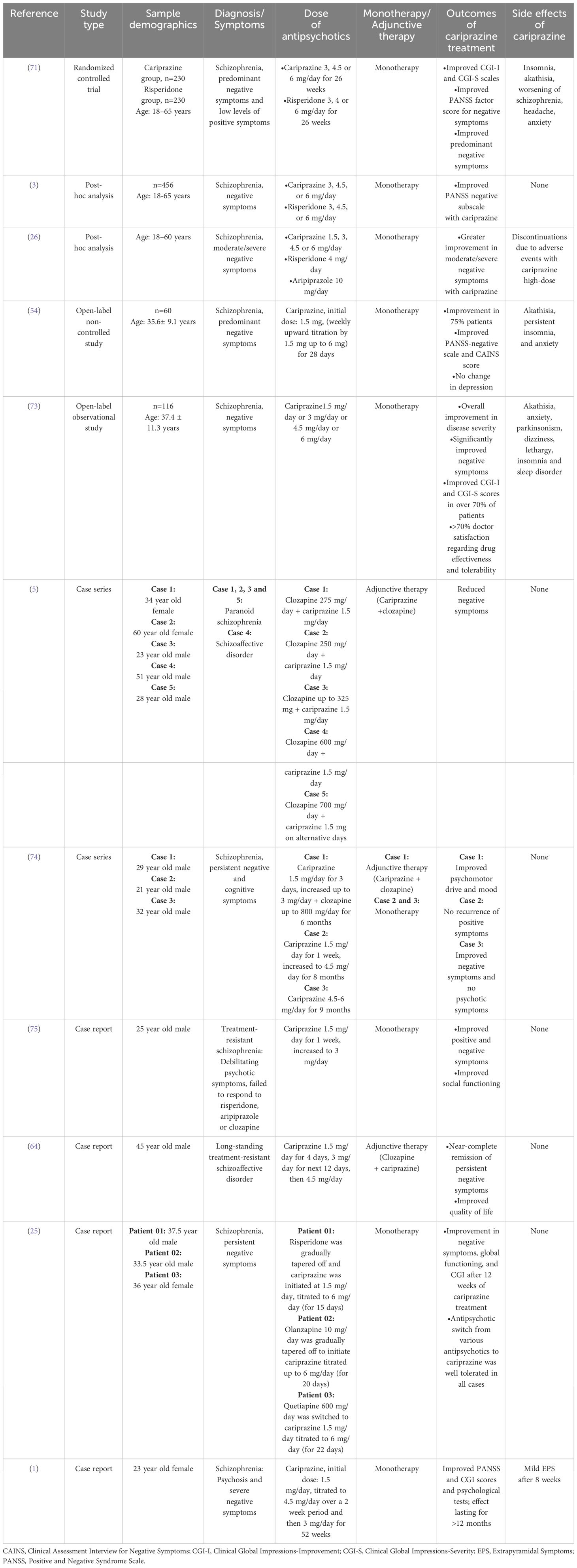
Table 3 Summary of clinical evidence of cariprazine for management of negative symptoms of schizophrenia.
6.2.2 Safety of cariprazine treatment
The most common adverse reactions with cariprazine treatment (incidence rate of ≥ 5%) are EPS and akathisia in patients with schizophrenia; EPS, akathisia, dyspepsia, vomiting, somnolence, and restlessness in bipolar mania; nausea, akathisia, restlessness and EPS in bipolar depression; and akathisia, restlessness, fatigue, constipation, nausea, insomnia, increased appetite, dizziness, and EPS in adjunctive treatment of major depressive disorder ( 80 ). Previous studies on safety and tolerability of cariprazine monotherapy demonstrated that treatment with cariprazine is generally well-tolerated and lowers total cholesterol, low-density lipoprotein, high-density lipoprotein and triglyceride levels in patients with schizophrenia ( 81 , 82 ). Long term safety of cariprazine monotherapy in adults with schizophrenia is established by Cutler et al.; safety and tolerability remained consistent up to one year ( 83 ). Normal electrocardiogram (ECG) and occurrence of mild/moderate treatment-emergent adverse events (akathisia, insomnia, headache and weight increased; anxiety and tremor) over the course of 53 weeks of cariprazine treatment was found in patients with acute exacerbation of schizophrenia. Safety and tolerability of cariprazine in terms of vital signs, body weight, clinical laboratory tests and ECG has been recorded by a post hoc analysis including four short (6 weeks) and four long (≥6 months) term studies. The study reported that cariprazine has a good safety profile and is well-tolerated with lower rates of treatment-emergent adverse events, independent of the treatment durations ( 84 ). Another post hoc analysis of pooled data from three short term (6 weeks) trials recorded safety of cariprazine in both early (<5 years) and late (>15 years) stage schizophrenia patients. Although insomnia, akathisia, EPS and headache occurred in both groups but discontinuation from the study was not related to the adverse events ( 85 ). Insomnia, akathisia, constipation, anxiety, nausea and vomiting are reported to occur with cariprazine treatment in patients with negative symptoms of schizophrenia ( Table 3 ). However, the side effects have lower occurrence rate than other available SGAs ( 86 ). Discontinuation of cariprazine due to treatment-emergent adverse events is as low as 9% ( 62 ). Despite the recorded side effects, ~70% clinicians rated cariprazine’s effectiveness and tolerability as ‘satisfactory’ or ‘very satisfactory’ ( 73 ). If long-term efficacy and tolerability is the chief concern with negative symptom treatment then cariprazine may be used as the first-line treatment for both prominent negative symptoms and severe positive symptoms ( 25 ). Observing the clinical changes in negative symptoms with cariprazine, it can be suggested as a good treatment option for predominant negative symptoms of schizophrenia.
This review article provides new insights on the possible use of cariprazine for negative symptom management ( Figure 2 ). In a nutshell, negative symptoms of schizophrenia hinder patient’s quality of life and treatment options are limited. Antipsychotic management of negative symptoms is recommended by various international guidelines. However, FGAs are ineffective for treatment of negative symptoms when they are secondary to positive symptoms, and SGAs have partial benefits on negative symptoms due to frequent incidence of treatment-related side effects. Cariprazine, a recently approved antipsychotic, has high affinity and occupancy for D2/D3 receptors, partial agonism to 5-HT 1A and antagonism to 5-HT 2B receptors, and longer half-life which is efficacious in management of patients with negative symptoms of schizophrenia. The drug appears to be superior to available SGAs with lower incidence of metabolic disorders and relapse. Therefore, cariprazine can be used as a viable alternative to other antipsychotics for predominant negative symptom treatment. More clinical trials need to be conducted to confirm the beneficial effect of cariprazine for treatment of negative symptoms over other antipsychotics.
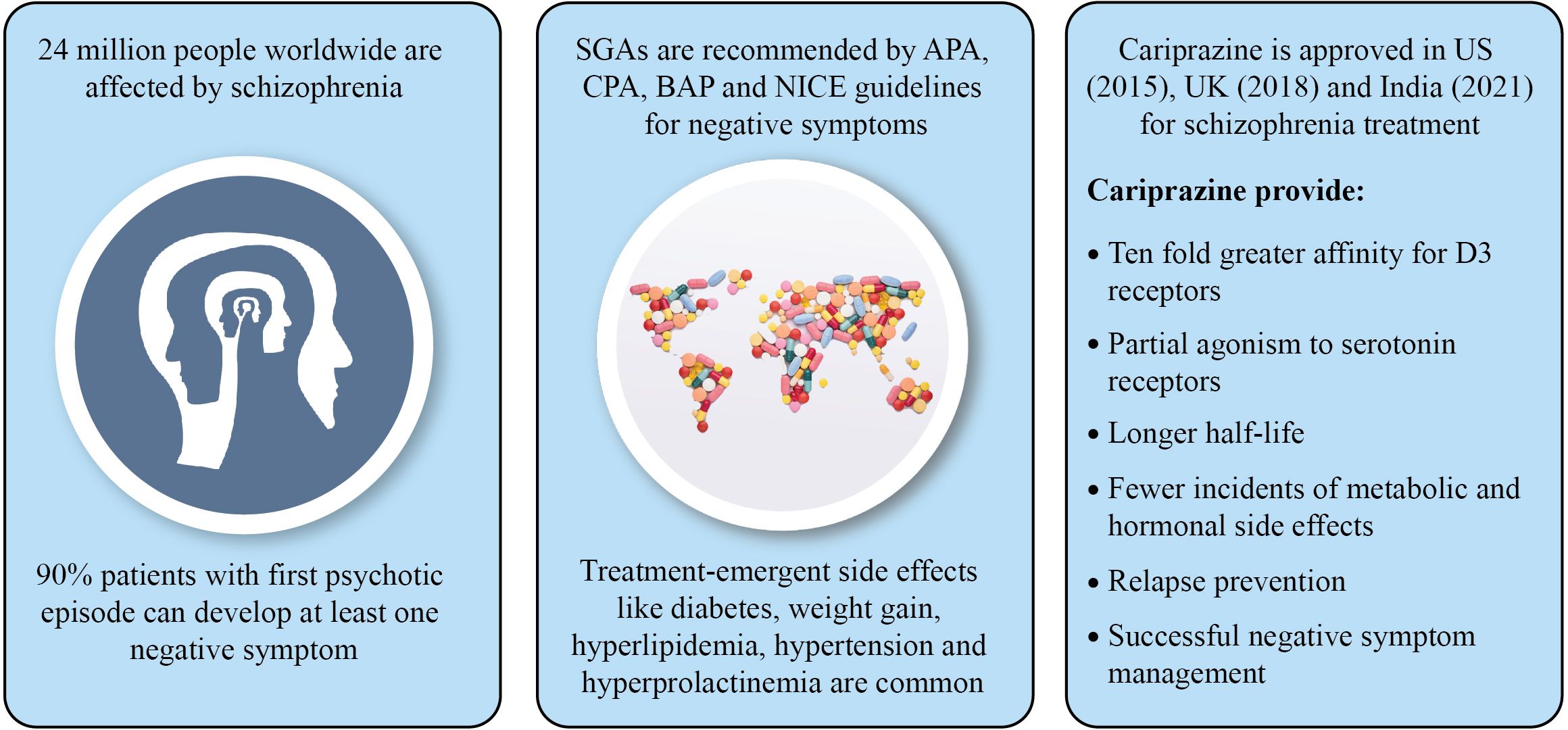
Figure 2 Summary of the study.
Author contributions
PS: Validation, Writing – review & editing. PD: Data curation, Investigation, Writing – original draft. AS: Data curation, Investigation, Writing – original draft. SD: Data curation, Investigation, Writing – original draft. CK: Data curation, Investigation, Writing – original draft. AM: Validation, Writing – review & editing. SM: Validation, Writing – review & editing. CA: Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This review article was supported by Sun Pharma Laboratories Limited, Mumbai, India.
Acknowledgments
The authors are grateful to WorkSure ® India for providing medical writing assistance for this manuscript.
Conflict of interest
Author PD was employed by the company Sun Pharmaceutical Industries Limited. Authors AS, SD, CK, AM, and SM were employed by the company Sun Pharma Laboratories Limited.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Molnar MJ, Jimoh IJ, Zeke H, Palásti Á, Fedor M. Early-onset schizophrenia with predominantly negative symptoms: A case study of a drug-naive female patient treated with cariprazine. Front Pharmacol . (2020) 11:477. doi: 10.3389/fphar.2020.00477
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Morrissette DA, Stahl SM. Affective symptoms in schizophrenia. Drug Discovery Today Ther Strateg . (2011) 8:3–9. doi: 10.1016/j.ddstr.2011.10.005
CrossRef Full Text | Google Scholar
3. Fleischhacker W, Galderisi S, Laszlovszky I, Szatmári B, Barabássy Á, Acsai K, et al. The efficacy of cariprazine in negative symptoms of schizophrenia: Post hoc analyses of PANSS individual items and PANSS-derived factors. Eur Psychiatry . (2019) 58:1–9. doi: 10.1016/j.eurpsy.2019.01.015
4. Fadgyas Stanculete M, Capatina O. The many faces of negative symptoms in schizophrenia. Psychos - Phenomenol Psychopathol Pathophysiol . (2022). doi: 10.5772/intechopen.98412
5. Oloyede E, Clark I, Mace S, Whiskey E, Taylor D. Clozapine augmentation with cariprazine for negative symptoms: a case series and literature review. Ther Adv Vaccines . (2022) 12:1–9. doi: 10.1177/https
6. Edinoff A, Ruoff MT, Ghaffar YT, Rezayev A, Jani D, Kaye AM, et al. Cariprazine to treat schizophrenia and bipolar disorder in adults. Psychopharmacol Bull . (2020) 50:83–117.
PubMed Abstract | Google Scholar
7. Reddy Mukku S, Nadella R, Kornapalli S. Cariprazine for late-life psychiatric illness: A review on therapeutic potential and challenges. J Geriatr Ment Heal . (2021) 8:77. doi: 10.4103/jgmh.jgmh_43_21
8. Fabrazzo M, Cipolla S, Camerlengo A, Perris F, Catapano F. Second-generation antipsychotics’ Effectiveness and tolerability: A review of real-world studies in patients with schizophrenia and related disorders. J Clin Med . (2022) 11(15):4530. doi: 10.3390/jcm11154530
9. Möller HJ, Czobor P. Pharmacological treatment of negative symptoms in schizophrenia. Eur Arch Psychiatry Clin Neurosci . (2015) 265:567–78. doi: 10.1007/s00406-015-0596-y
10. McCormack PL. Cariprazine: first global approval. Drugs . (2015) 75:2035–43. doi: 10.1007/s40265-015-0494-7
11. World Health Organization. Schizophrenia (2022). Available at: https://www.who.int/news-room/fact-sheets/detail/schizophrenia .
Google Scholar
12. Sabe M, Zhao N, Crippa A, Kaiser S. Antipsychotics for negative and positive symptoms of schizophrenia: dose-response meta-analysis of randomized controlled acute phase trials. NPJ Schizophr . (2021) 7(11):43. doi: 10.1038/s41537-021-00171-2
13. Mathew VK, Sam KG, Samuel B, Das AK. Epidemiology of schizophrenia in an Indian hospital. Res J Pharm Technol . (2020) 13(1):219–23. doi: 10.5958/0974-360x.2020.00044.x
14. Janoutová J, Janáčková P, Šerý O, Zeman T, Ambroz P, Kovalová M, et al. Epidemiology and risk factors of Schizophrenia. Neuroendocrinol Lett . (2016) 37:1–8.
15. Folsom DP, Lebowitz BD, Lindamer LA, Palmer BW, Patterson TL, Jeste DV. Schizophrenia in late life: Emerging issues. Dialogues Clin Neurosci . (2006) 8:45–52. doi: 10.31887/dcns.2006.8.1/dfolsom
16. Mosolov SN, Yaltonskaya PA. Primary and secondary negative symptoms in schizophrenia. Front Psychiatry . (2022) 12:766692. doi: 10.3389/fpsyt.2021.766692
17. Correll CU, Schooler NR. Negative symptoms in schizophrenia: A review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr Dis Treat . (2020) 16:519–34. doi: 10.2147/NDT.S225643
18. Giordano GM, Caporusso E, Pezzella P, Galderisi S. Updated perspectives on the clinical significance of negative symptoms in patients with schizophrenia. Expert Rev Neurother . (2022) 22:541–55. doi: 10.1080/14737175.2022.2092402
19. Alabaf S, Kirkpatrick B, Chen S, Cardinal RN, Fernandez-Egea E. Early versus late risk factors for deficit and nondeficit schizophrenia. Rev Psiquiatr Salud Ment . (2022) 15:38–46. doi: 10.1016/j.rpsm.2021.03.002
20. Galderisi S, Mucci A, Dollfus S, Nordentoft M, Falkai P, Kaiser S, et al. EPA guidance on assessment of negative symptoms in schizophrenia. Eur Psychiatry . (2021) 64(1):e23. doi: 10.1192/j.eurpsy.2021.11
21. Wu Q, Wang X, Wang Y, Long YJ, Zhao JP, Wu RR. Developments in biological mechanisms and treatments for negative symptoms and cognitive dysfunction of schizophrenia. Neurosci Bull . (2021) 37:1609–24. doi: 10.1007/s12264-021-00740-6
22. Brisch R, Saniotis A, Wolf R, Bielau H, Bernstein HG, Steiner J, et al. The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: Old fashioned, but still in vogue. Front Psychiatry . (2014) 5:47. doi: 10.3389/fpsyt.2014.00047
23. Barendse MEA, Lara GA, Guyer AE, Swartz JR, Taylor SL, Shirtcliff EA, et al. Sex and pubertal influences on the neurodevelopmental underpinnings of schizophrenia: A case for longitudinal research on adolescents. Schizophr Res . (2023) 252:231–41. doi: 10.1016/j.schres.2022.12.011
24. Căpăţînă O, Micluţia I, Fadgyas−stănculete M. Current perspectives in treating negative symptoms of schizophrenia: A narrative review (Review). Exp Ther Med . (2021) 21(3):276. doi: 10.3892/etm.2021.9707
25. Vasiliu O. Case report: cariprazine efficacy in young patients diagnosed with schizophrenia with predominantly negative symptoms. Front Psychiatry . (2021) 12:786171. doi: 10.3389/fpsyt.2021.786171
26. Earley W, Guo H, Daniel D, Nasrallah H, Durgam S, Zhong Y, et al. Efficacy of cariprazine on negative symptoms in patients with acute schizophrenia: A post hoc analysis of pooled data. Schizophr Res . (2019) 204:282–8. doi: 10.1016/j.schres.2018.08.020
27. Bokhari SQ, Bokhari QM, Mariam A, Majeed R. Correlation between quality of life and positive and negative symptoms of schizophrenia. Pakistan J Med Heal Sci . (2015) 9:367–70.
28. Desalegn D, Girma S, Tessema W, Yeshigeta E, Kebeta T. Quality of Life and Associated Factors among Patients with Schizophrenia Attending Follow-Up Treatment at Jimma Medical Center, Southwest Ethiopia: A Cross-Sectional Study. Psychiatry J . (2020) 2020:1–7. doi: 10.1155/2020/4065082
29. Karow A, Wittmann L, Schöttle D, Schäfer I, Lambert M. The assessment of quality of life in clinical practice in patients with schizophrenia. Dialogues Clin Neurosci . (2014) 16:185–95. doi: 10.31887/dcns.2014.16.2/akarow
30. Cohen CI, Natarajan N, Araujo M, Solanki D. Prevalence of negative symptoms and associated factors in older adults with schizophrenia spectrum disorder. Am J Geriatr Psychiatry . (2013) 21:100–7. doi: 10.1016/j.jagp.2012.10.009
31. Demyttenaere K, Anthonis E, Acsai K, Correll CU. Depressive symptoms and PANSS symptom dimensions in patients with predominant negative symptom schizophrenia: A network analysis. Front Psychiatry . (2022) 13:795866. doi: 10.3389/fpsyt.2022.795866
32. Pelizza L, Leuci E, Maestri D, Quattrone E, Paulillo G, Pellegrini P, et al. Negative symptoms in first episode schizophrenia: Results from the “parma early psychosis” program. Eur Psychiatry . (2021) 64(S1):S168–8. doi: 10.1192/j.eurpsy.2021.446
33. Tsapakis EM, Dimopoulou T, Tarazi FI. Clinical management of negative symptoms of schizophrenia: An update. Pharmacol Ther . (2015) 153:135–47. doi: 10.1016/j.pharmthera.2015.06.008
34. Correll CU, Martin A, Patel C, Benson C, Goulding R, Kern-Sliwa J, et al. Systematic literature review of schizophrenia clinical practice guidelines on acute and maintenance management with antipsychotics. Schizophrenia . (2022) 8:1–10. doi: 10.1038/s41537-021-00192-x
35. Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthøj B, Gattaz WF, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia–a short version for primary care. Int J Psychiatry Clin Pract . (2017) 21:82–90. doi: 10.1080/13651501.2017.1291839
36. Pilla Reddy V, Kozielska M, Suleiman AA, Johnson M, Vermeulen A, Liu J, et al. Pharmacokinetic-pharmacodynamic modelling of antipsychotic drugs in patients with schizophrenia: Part II: The use of subscales of the PANSS score. Schizophr Res . (2013) 146:153–61. doi: 10.1016/j.schres.2013.02.010
37. Ricci V, De Berardis D, Maina G. Third-generation antipsychotics and lurasidone in the treatment of substance-induced psychoses: A narrative review. Healthc . (2024) 12:1–15. doi: 10.3390/healthcare12030339
38. Keepers GA, Fochtmann LJ, Anzia JM, Benjamin S, Lyness JM, Mojtabai R, et al. The American psychiatric association practice guideline for the treatment of patients with schizophrenia. The American Journal of Psychiatry . (Washington, DC: American Psychiatric Association Publishing) (2020). pp. 868–872 p. doi: 10.1176/appi.ajp.2020.177901.
39. De Fazio P, Gaetano R, Caroleo M, Cerminara G, Maida F, Bruno A, et al. Rare and very rare adverse effects of clozapine. Neuropsychiatr Dis Treat . (2015) 11:1995–2003. doi: 10.2147/NDT.S83989
40. Lobos C, Komossa K, Rummel-Kluge C, Hunger H, Schmid F, Schwarz S, et al. Clozapine versus other atypical anti psychotics for schizophrenia. Cochrane Database Syst Rev . (2010) 11(11):CD006633. doi: 10.1002/14651858.cd006633.pub2
41. Siskind D, Siskind V, Kisely S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry . (2017) 62:772–7. doi: 10.1177/0706743717718167
42. Nielsen MØ, Kristensen TD, Borup Bojesen K, Glenthøj BY, Lemvigh CK, Ebdrup BH. Differential effects of aripiprazole and amisulpride on negative and cognitive symptoms in patients with first-episode psychoses. Front Psychiatry . (2022) 13:834333. doi: 10.3389/fpsyt.2022.834333
43. Johnsen E, Kroken RA, Løberg EM, Rettenbacher M, Joa I, Larsen TK, et al. Amisulpride, aripiprazole, and olanzapine in patients with schizophrenia-spectrum disorders (BeSt InTro): a pragmatic, rater-blind, semi-randomised trial. Lancet Psychiatry . (2020) 7:945–54. doi: 10.1016/S2215-0366(20)30341-2
44. Ricci V, Paggi A, Cristofori E, Passarello E, Maina G. Efficacy of brexpiprazole for treatment persistent negative symptoms in three schizophrenic patients: A case series. Psychiatry Res Case Rep . (2022) 1:100040. doi: 10.1016/j.psycr.2022.100040
45. Diefenderfer LA, Iuppa C. Brexpiprazole: A review of a new treatment option for schizophrenia and major depressive disorder. Ment Heal Clin . (2017) 7:207–12. doi: 10.9740/mhc.2017.09.207
46. Stroup TS, Gray N. Management of common adverse effects of antipsychotic medications. World Psychiatry . (2018) 17:341–56. doi: 10.1002/wps.20567
47. Liang Y, Yu X. Effectiveness of amisulpride in Chinese patients with predominantly negative symptoms of schizophrenia: A subanalysis of the ESCAPE study. Neuropsychiatr Dis Treat . (2017) 13:1703–12. doi: 10.2147/NDT.S140905
48. Rabinowitz J, Werbeloff N, Caers I, Mandel FS, Stauffer V, Menard F, et al. Negative symptoms in schizophrenia - the remarkable impact of inclusion definitions in clinical trials and their consequences. Schizophr Res . (2013) 150:334–8. doi: 10.1016/j.schres.2013.06.023
49. Bitter I, Mohr P, Raspopova N, Szulc A, Samochowiec J, Micluia IV, et al. Assessment and treatment of negative symptoms in schizophrenia—A regional perspective. Front Psychiatry . (2022) 12:820801. doi: 10.3389/fpsyt.2021.820801
50. Cerveri G, Gesi C, Mencacci C. Pharmacological treatment of negative symptoms in schizophrenia: Update and proposal of a clinical algorithm. Neuropsychiatr Dis Treat . (2019) 15:1525–35. doi: 10.2147/NDT.S201726
51. Wichniak A, Siwek M, Rymaszewska J, Janas-Kozik M, Wolańczyk T, Bieńkowski P, et al. The position statement of the Working Group of the Polish Psychiatric Association on the use of D2/D3 dopamine receptor partial agonists in special populations. Psychiatr Pol . (2021) 55:967–87. doi: 10.12740/PP/140287
52. Midlands and Lancashire Commissioning Support Unit. New Medicine Assessment Cariprazine (Reagila ® ▼) For the treatment of schizophrenia in adults. In: Lancash South Cumbria Med Manag Gr (2019). Available at: https://www.midlandsandlancashirecsu.nhs.uk/ .
53. Central Drugs Standard Control Organization. List of new drugs approved in the year 2021 till date (2021). Available at: https://cdsco.gov.in/opencms/opencms/en/Approval_new/Approved-New-Drugs/ .
54. Ivanov SV, Smulevich AB, Voronova EI, Yakhin KK, Beybalaeva TZ, Katok AA. Early clinical effects of novel partial D3/D2 agonist cariprazine in schizophrenia patients with predominantly negative symptoms (Open-label, non-controlled study). Front Psychiatry . (2022) 12:770592. doi: 10.3389/fpsyt.2021.770592
55. Durgam S, Cutler A, Lu K, Migliore R, Ruth A, Laszlovszky I, et al. Cariprazine in acute exacerbation of schizophrenia: a fixed-dose, phase 3, randomized, double-blind, placebo-and active-controlled trial. J Clin Psychiatry . (2015) 76:2310. doi: 10.1111/bdi.12238
56. Campbell RH, Diduch M, Gardner KN, Thomas C. Review of cariprazine in management of psychiatric illness. Ment Heal Clin . (2017) 7:221–9. doi: 10.9740/mhc.2017.09.221
57. Durgam S, Starace A, Li D, Migliore R, Ruth A, Németh G, et al. An evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: A phase II, randomized clinical trial. Schizophr Res . (2014) 152:450–7. doi: 10.1016/j.schres.2013.11.041
58. Kane JM, Zukin S, Wang Y, Lu K, Ruth A, Nagy K, et al. Efficacy and safety of cariprazine in acute exacerbation of schizophrenia: results from an international, phase III clinical trial. J Clin Psychopharmacol . (2015) 35:367–73. doi: 10.1097/JCP.0000000000000346
59. Durgam S, Earley W, Li R, Li D, Lu K, Laszlovszky I, et al. Long-term cariprazine treatment for the prevention of relapse in patients with schizophrenia: A randomized, double-blind, placebo-controlled trial. Schizophr Res . (2016) 176:264–71. doi: 10.1016/j.schres.2016.06.030
60. Fagiolini A, Alcalá JÁ, Aubel T, Bienkiewicz W, Bogren MMK, Gago J, et al. Treating schizophrenia with cariprazine: From clinical research to clinical practice. Real world experiences and recommendations from an International Panel. Ann Gen Psychiatry . (2020) 19:1–11. doi: 10.1186/s12991-020-00305-3
61. Stahl SM. Drugs for psychosis and mood: Unique actions at D3, D2, and D1 dopamine receptor subtypes. CNS Spectr . (2017) 22:375–84. doi: 10.1017/S1092852917000608
62. Citrome L. Cariprazine for acute and maintenance treatment of adults with schizophrenia: an evidence-based review and place in therapy. Neuropsychiatr Dis Treat . (2018) 14:2563–77. doi: 10.2147/NDT.S169369
63. Do A, Keramatian K, Schaffer A, Yatham L. Cariprazine in the treatment of bipolar disorder: within and beyond clinical trials. Front Psychiatry . (2021) 12:769897. doi: 10.3389/fpsyt.2021.769897
64. Bogren M, Soltesz M, Hjorth S. Remission of persistent negative symptoms and psychosocial consequences by combined clozapine and cariprazine treatment in a patient with long-standing treatment-resistant schizoaffective disorder. Front Psychiatry . (2022) 13:887547. doi: 10.3389/fpsyt.2022.887547
65. Aubel T. Cariprazine: Patients with treatment-resistant schizophrenia. Neuropsychiatr Dis Treat . (2021) 17:2327–32. doi: 10.2147/NDT.S315653
66. Seneca N, Finnema S, Laszlovszky I, Kiss B, Horváth A, Pásztor G, et al. Occupancy of dopamine D 2 and D 3 and serotonin 5-HT 1A receptors by the novel antipsychotic drug candidate, cariprazine (RGH-188), in monkey brain measured using positron emission tomography. Psychopharmacol (Berl) . (2011) 218:579–87. doi: 10.1007/s00213-011-2343-z
67. Stahl SM, Laredo S, Morrissette DA. Cariprazine as a treatment across the bipolar I spectrum from depression to mania: mechanism of action and review of clinical data. Ther Adv Psychopharmacol . (2020) 10:1–11. doi: 10.1177/https
68. Kaar SJ, Natesan S, McCutcheon R, Howes OD. Antipsychotics: Mechanisms underlying clinical response and side-effects and novel treatment approaches based on pathophysiology. Neuropharmacology . (2020) 172:107704. doi: 10.1016/j.neuropharm.2019.107704
69. Watanabe Y, Yamada S, Otsubo T, Kikuchi T. Brexpiprazole for the treatment of schizophrenia in adults: An overview of its clinical efficacy and safety and a psychiatrist’s perspective. Drug Des Devel Ther . (2020) 14:5559–74. doi: 10.2147/DDDT.S240859
70. Morozov P, Bekker R, Bykov Y. Cariprazine ‘ s potential in improving social dysfunction in patients with schizophrenia: A perspective. Front Psychiatry . (2022) 13:868751. doi: 10.3389/fpsyt.2022.868751
71. Németh G, Laszlovszky I, Czobor P, Szalai E, Szatmári B, Harsányi J, et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double-blind, controlled trial. Lancet . (2017) 389:1103–13. doi: 10.1016/S0140-6736(17)30060-0
72. Andrade C. Psychotropic drugs with long half-lives: implications for drug discontinuation, occasional missed doses, dosing interval, and pregnancy planning. J Clin Psychiatry . (2022) 83(4):22F14593. doi: 10.4088/JCP.22f14593
73. Rancans E, Dombi ZB, Mátrai P, Barabássy Á, Sebe B, Skrivele I, et al. The effectiveness and safety of cariprazine in schizophrenia patients with negative symptoms and insufficient effectiveness of previous antipsychotic therapy: an observational study. Int Clin Psychopharmacol . (2021) 36:154–61. doi: 10.1097/YIC.0000000000000351
74. Viegas F, Ferreira T, Campos C. Using cariprazine to ameliorate negative symptoms and metabolic side effects of clozapine and paliperidone – clinical cases. Neuropsychiatr Dis Treat . (2022) 18:1145–9. doi: 10.2147/NDT.S343747
75. Montgomery A, Rogowska M, Dratcu L. Cariprazine — an alternative treatment for clozapine-resistant schizophrenia? Clin Psychopharmacol Neurosci . (2023) 21:202–6. doi: 10.9758/cpn.2023.21.1.202
76. Leucht S, Dombi ZB, Szabó P, Barabássy Á, Levine SZ. Single trajectory treatment response for predominant negative symptoms: Post-hoc analysis of a clinical trial with cariprazine and risperidone. Schizophr Res . (2023) 261:24–30. doi: 10.1016/j.schres.2023.09.004
77. Mohr P, Masopust J, Kopeˇcek M. Dopamine receptor partial agonists: do they differ in their clinical efficacy? Front Psychiatry . (2022) 12:781946. doi: 10.3389/fpsyt.2021.781946
78. Pappa S, Kalniunas A, Maret J. Cariprazine for negative symptoms in early psychosis: a pilot study with a 6-month follow-up. Front Psychiatry . (2023) 14:1183912. doi: 10.3389/fpsyt.2023.1183912
79. Machetanz L, Lau S, Kirchebner J. Cariprazine in offender patient with acute psychosis and aggressive behavior: Case report. Psychiatry Res Case Rep . (2023) 2:100094. doi: 10.1016/j.psycr.2022.100094
80. VRAYLAR™ (cariprazine) capsules, for oral use. Ref ID 5095981 (2022). Available at: https://shorturl.at/EFHZ4 .
81. Earley W, Durgam S, Lu K, Laszlovszky I, Debelle M, Kane JM. Safety and tolerability of cariprazine in patients with acute exacerbation of schizophrenia: A pooled analysis of four phase II/III randomized, double-blind, placebo-controlled studies. Int Clin Psychopharmacol . (2017) 32:319–28. doi: 10.1097/YIC.0000000000000187
82. Nasrallah HA, Earley W, Cutler AJ, Wang Y, Lu K, Laszlovszky I, et al. The safety and tolerability of cariprazine in long-term treatment of schizophrenia: A post hoc pooled analysis. BMC Psychiatry . (2017) 17:1–13. doi: 10.1186/s12888-017-1459-z
83. Cutler AJ, Durgam S, Wang Y, Migliore R, Lu K, Laszlovszky I, et al. Evaluation of the long-term safety and tolerability of cariprazine in patients with schizophrenia: results from a 1-year open-label study. CNS Spectr . (2018) 23:39–50. doi: 10.1017/S1092852917000220
84. Barabássy Á, Sebe B, Acsai K, Laszlovszky I, Szatmári B, Earley WR, et al. Safety and tolerability of cariprazine in patients with schizophrenia: A pooled analysis of eight phase II/III studies. Neuropsychiatr Dis Treat . (2021) 17:957–70. doi: 10.2147/NDT.S301225
85. Falkai P, Dombi ZB, Acsai K, Barabássy Á, Schmitt A, Németh G. The efficacy and safety of cariprazine in the early and late stage of schizophrenia: a post hoc analysis of three randomized, placebo-controlled trials. CNS Spectr . (2023) 28:104–11. doi: 10.1017/S1092852921000997
86. Altınbaş K, Guloksuz S, Oral ET. Clinical potential of cariprazine in the treatment of acute mania. Psychiatr Danub . (2013) 25:207–13.
Keywords: atypical antipsychotics, cariprazine, negative symptoms, schizophrenia, pharmacology, socializing drug, third-generation antipsychotics, D3 receptor
Citation: Selvan P, Devkare P, Shetty A, Dharmadhikari S, Khandhedia C, Mane A, Mehta S and Andrade C (2024) A review on the pharmacology of cariprazine and its role in the treatment of negative symptoms of schizophrenia. Front. Psychiatry 15:1385925. doi: 10.3389/fpsyt.2024.1385925
Received: 14 February 2024; Accepted: 29 March 2024; Published: 22 April 2024.
Reviewed by:
Copyright © 2024 Selvan, Devkare, Shetty, Dharmadhikari, Khandhedia, Mane, Mehta and Andrade. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Prashant Devkare, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 27 April 2024
The burden of schizophrenia in the Middle East and North Africa region, 1990–2019
- Saeid Safiri 1 , 2 ,
- Maryam Noori 3 ,
- Seyed Aria Nejadghaderi 4 , 5 ,
- Ali Shamekh 6 ,
- Mark J. M. Sullman 7 , 8 ,
- Gary S. Collins 9 , 10 &
- Ali-Asghar Kolahi 11
Scientific Reports volume 14 , Article number: 9720 ( 2024 ) Cite this article
167 Accesses
11 Altmetric
Metrics details
- Epidemiology
- Schizophrenia
Schizophrenia ranks as the third-most common cause of disability among mental disorders globally. This study presents findings on the prevalence, incidence and years lived with disability (YLDs) as a result of schizophrenia in the Middle East and North Africa (MENA), stratified by age, sex and sociodemographic index (SDI). We collected publicly accessible data from the Global Burden of Disease (GBD) study 2019. This study reports the burden of schizophrenia, from 1990 to 2019, for the 21 countries that comprise MENA. In 2019, MENA exhibited an age-standardised point prevalence of 248.2, an incidence rate of 14.7 and an YLD rate of 158.7 per 100,000, which have not changed substantially between 1990 and 2019. In 2019, the age-standardised YLD rate was highest in Qatar and lowest in Afghanistan. No MENA countries demonstrated noteworthy changes in the burden of schizophrenia from 1990 to 2019. Furthermore, in 2019, the highest number of prevalent cases and the point prevalence were observed among those aged 35–39, with a higher prevalence among males in almost all age categories. Additionally, in 2019, the age-standardised YLD rates in MENA were below the worldwide average. Finally, there was a positive correlation between the burden of schizophrenia and the SDI from 1990 to 2019. The disease burden of schizophrenia has remained relatively stable over the past thirty years. Nevertheless, as the regional life-expectancy continues to increase, the burden of schizophrenia is also expected to rise. Therefore, early planning for the increase in the burden of the disease is urgently needed in the region.
Similar content being viewed by others
Incidence, prevalence, and global burden of schizophrenia - data, with critical appraisal, from the Global Burden of Disease (GBD) 2019
Incidence, prevalence, and global burden of ADHD from 1990 to 2019 across 204 countries: data, with critical re-analysis, from the Global Burden of Disease study
The burden of mental disorders in Asian countries, 1990–2019: an analysis for the global burden of disease study 2019
Introduction.
Schizophrenia is defined as a cognitive and behavioral disorder that affects early brain development and manifests itself through several psychotic symptoms, including hallucinations, delusions, and disorganised behavior and speech 1 . The prognosis for patients with schizophrenia can vary from making a full recovery to a lifelong need for care, and patients typically have a life expectancy which is roughly twenty years less than that of the general population 1 , 2 . Psychiatric symptoms typically first appear during late adolescence or early adulthood, and suicidal behaviors are the most frequent cause of death early in the course of the disease 3 . Schizophrenia has also been linked to several comorbid conditions, which is partially as a result of the high prevalence of drug abuse and cigarette smoking, unhealthy lifestyles, and the potential impact of anti-psychotic medications on promoting obesity. These conditions predispose the patients to a higher rate of metabolic syndrome, diabetes, cardiovascular disorders, and respiratory diseases 4 , 5 .
In 2019, schizophrenia was the 42nd leading cause of disability among people of all ages and the 22nd among individuals aged 25–49 years old 6 , 7 . The lifetime prevalence of schizophrenia has been estimated to be just below 1% 8 . In 2019, the global age-standardised prevalence of schizophrenia was 287.4 per 100,000, and this rate was approximately the same as in 1990 6 . Also in 2019, schizophrenia accounted for 12.1% of all disability-adjusted-life-years (DALYs) attributable to mental disorders, and was surpassed only by depressive (37.4%) and anxiety (22.9%) disorders 6 . The highest incidence of schizophrenia was found in those aged 20–24, with no significant sex-based differences in the incidence rate 9 .
Several reports have been published in recent years discussing mental disorders, and more specifically the burden of schizophrenia at the regional level and across the world 6 , 9 , 10 , 11 , 12 , 13 . However, none of these articles have exclusively focused on the attributable burden of schizophrenia in the Middle East and North Africa (MENA) region. The countries located in MENA vary considerably in terms of socioeconomic profile, health system coverage and capacities, and healthcare infrastructures and provisions 14 , 15 . During the past three decades, the MENA region has witnessed several enhancements in health outcomes, resulting in rising life expectancies and decreased neonatal mortality 16 . Consequently, in parallel with increasing longevity, it is expected that the prevalence of chronic conditions, such as mental disorders, will continue to grow in MENA. Furthermore, as a stigmatized disease, schizophrenia is often overlooked among affected patients, especially in developing countries. Moreover, as the socioeconomic status of a country decreases the stigma of mental disorders increases, potentially leading to an underestimation of the burden of schizophrenia in lower socio-economic countries. Therefore, investigating the epidemiology of schizophrenia in the MENA region is of paramount interest 17 . Consequently, this study utilized data from the Global Burden of Disease (GBD) study 2019 to present the burden of schizophrenia in MENA from 1990 to 2019, stratified by sex, age and socio-demographic index (SDI).
The Global Burden of Disease (GBD) study, which was established by the Institute of Health Metrics and Evaluation (IHME), measures the burden of diseases and injuries in over 200 countries and territories. Although schizophrenia is a relatively common mental problem, its burden has not been quantified across all global regions. Therefore, this study presents an assessment of the burden of schizophrenia from 1990 to 2019 for all countries in MENA. There are 21 countries in MENA, which are: Afghanistan, Algeria, Bahrain, Egypt, Iran, Iraq, Jordan, Kuwait, Lebanon, Libya, Morocco, Oman, Palestine, Qatar, Saudi Arabia, Sudan, the Syrian Arab Republic, Tunisia, Turkey, the United Arab Emirates and Yemen. A full description of the methodology utilised by IHME to model the burden of disease has been previously described 7 , 16 , 18 . The GBD 2019 estimates, which cover the period 1990–2019, are available at the following links: http://ghdx.healthdata.org/gbd-results-tool and https://vizhub.healthdata.org/gbd-compare/ .
Case definition and data sources
Schizophrenia is a serious mental disorder which is characterised by a large number of symptoms, including: delusions, hallucinations, diminished interest, flat affect, thought disorders, and emotional withdrawal. The GBD disease modelling process only included data from studies that diagnosed schizophrenia using either the Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria (DSM-IV-TR: 295.10-295.30, 295.60, 295.90) or the International Classification of Diseases (ICD) criteria (ICD 10: F20). The diagnostic criteria encompass the following key elements: (1) Presence of at least two of the following symptoms, each enduring for a substantial part of a one-month period (a shorter duration if effectively treated): (i) Delusions, (ii) Hallucinations, (iii) Disorganised speech (e.g., frequent incoherence or derailment), (iv) Markedly disorganised or catatonic behavior, (v) Negative symptoms (i.e., affective flattening, alogia, or avolition); (2) Dysfunction at work and socially; (3) Persistence of the disorder’s signs and symptoms for a duration of six months or more; (4) Exclusions included substance abuse, schizoaffective and mood disorders, and/or general medical conditions, as well as any connection to pervasive developmental disorders 7 .
IHME conducted a systematic review for schizophrenia, which encompassed searching the scientific literature (i.e., PsycInfo, Embase, and PubMed), examining the grey literature, and consultation with an expert. As part of the GBD project, the electronic databases are searched biennially for mental disorders, including schizophrenia. The last systematic review for schizophrenia was carried out in GBD 2017, with the next review being due in GBD 2020. However, consulting the expert and searching the grey literature produced new data sources in GBD 2019 7 .
The inclusion criteria applied were as follows: (1) published after 1980; (2) cases were defined using DSM or ICD criteria; (3) inclusion of sufficient methodological details and sample characteristics for assessing study quality; and (4) samples that represented the general population. Specifically excluded were samples from inpatients or pharmacological treatments, case studies, veterans, or refugee cases. There were no constraints placed on the publication language. The data sources utilised to model the schizophrenia burden are accessible at this website: https://ghdx.healthdata.org/gbd-2019/data-input-sources 7 .
Data processing and disease model
When necessary, the data extraction process involved three different age and sex splitting procedures: (1) The available estimates were divided into specific five-year age groups by sex. For example, in studies which reported the prevalence in broad age ranges separately for males and females (e.g., 15–65 year old men and women individually), and in cases where studies had smaller age groups without sex separation (e.g., prevalence among 15 to 29 year olds, then in 30 to 70 year olds, for both sexes combined), the sex ratios reported and uncertainty ranges were used to divide the age specific estimates by sex. (2) Meta-Regression with Bayesian priors, Regularisation, and Trimming (MR-BRT) was used to split the remaining data. This method involved matching sex-specific estimates for each parameter, according to location, age, and year. MR-BRT regression was then employed to model the pooled sex ratios, along with their associated uncertainty bounds. These pooled sex ratios were then utilised to split the estimates in the dataset. The prevalence ratio between males and females was 1.17 (95% uncertainty interval (UI) 0.60–1.75). 3. For prevalence estimates covering age categories spanning 25 years or more, the age pattern estimated by DisMod-MR 2.1 was used to split the data into five-year age groups. It’s important to note that the DisMod-MR model used for estimating the age pattern did not contain any previously age split data 7 .
IHME utilised DisMod MR 2.1, using the standard GBD 2019 decomposition structure, to estimate the data related to schizophrenia. At each stage of the decomposition process, IHME compared the new model with the best model from GBD 2017 and the best model from the previous stage. If substantial differences were observed between models, these variances were thoroughly explored and elucidated. In cases where it was deemed necessary, adjustments were implemented to the dataset or the model priors. When outliers were identified, they were included or excluded based upon a re-examination of their quality and methodology.
Initially, all epidemiological parameters were integrated into the modelling process. It was believed, based on the literature on schizophrenia and discussion with the expert that no cases of schizophrenia occurred before the age of 10 or after the age of 80. Furthermore, the remission rate was restricted to a maximum of 0.04, in line with the data in the dataset. In areas lacking available data, prevalence estimates were informed by location-level covariates. Only one location-level covariate, lag distributed income (LDI), was utilised to model the prevalence of schizophrenia.
Compilation of results
The two sequelae (acute and residual) of schizophrenia, along with their corresponding disability weights (DWs), can be found in Table S1 . To calculate the years lived with disability (YLDs), the prevalence estimates for each sequela were multiplied by their respective DWs. The YLDs and DALYs were the same, since there was no mortality due to schizophrenia. All estimates were standardised using the GBD standard population. 95% uncertainty intervals (UIs) were included with all estimates and were generated by producing 1000 iterations at each stage of the estimation process. The final estimates represented the mean values over the 1000 iterations, and the 95% UIs were indicated as the 25th and 975th values among the numerically ordered iterations.
Smoothing Spline models 19 was employed to investigate the relationship the socio-demographic index (SDI) has with the burden of schizophrenia. The SDI is a composite model that contains per capita income, mean number of years attending school (aged 15 and above), and the fertility rate in women aged 25 or less. The SDI ranges from 0 to 1, representing the spectrum from the lowest to the highest development level 7 . The estimates for the point prevalence and annual incidence were obtained from the GBD website ( http://ghdx.healthdata.org/gbd-results-tool ) and all visual representations were created with R software (Version 3.5.2).
Ethics approval and consent to participate
The present study was approved by Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (IR.SBMU.RETECH.REC.1401.387).
The Middle East and North Africa region
In 2019, there were 1.6 million (95% UI: 1.3 to 1.9) prevalent cases of schizophrenia. In addition, the age-standardised point prevalence was 248.2 (203.9 to 294.9) per 100,000, which has hardly changed since 1990 [0.5% (-1.2 to 2.0)] (Tables 1 and S2 ). There were 97.7 thousand (79.8 to 119.7) incident cases of schizophrenia in 2019, with an age-standardised rate of 14.7 (12.1 to 17.9) per 100,000, which did not differ from 1990 [− 1% (− 2.7 to 0.7)] (Tables 1 and S3 ). A total of 1.0 million (0.7 to 1.3) YLDs were attributable to schizophrenia in 2019, having an age-standardised rate of 158.7 (113.2 to 207.8) YLDs per 100,000 population. This rate also has not changed since 1990 [0.4% (− 2.2 to 3.1)] (Tables 1 and S4 ).
Country level
The age-standardised point prevalence of schizophrenia varied from 217.8 to 285.0 cases per 100,000 in the region. Qatar [285.0 (225.4 to 351.1)], the United Arab Emirates [275.3 (218.5 to 337.2)] and Kuwait [273.8 (216.4 to 334.0)] were the three highest in 2019. Conversely, Afghanistan [217.8 (176.2 to 266.6)], Yemen [225.7 (180.7 to 273.9)] and Sudan [232.7 (186.1 to 284.0)] were the three lowest (Table S2 ). Figure 1 A presents the age-standardised point prevalence estimates of schizophrenia by country, separately for men and women, in 2019.
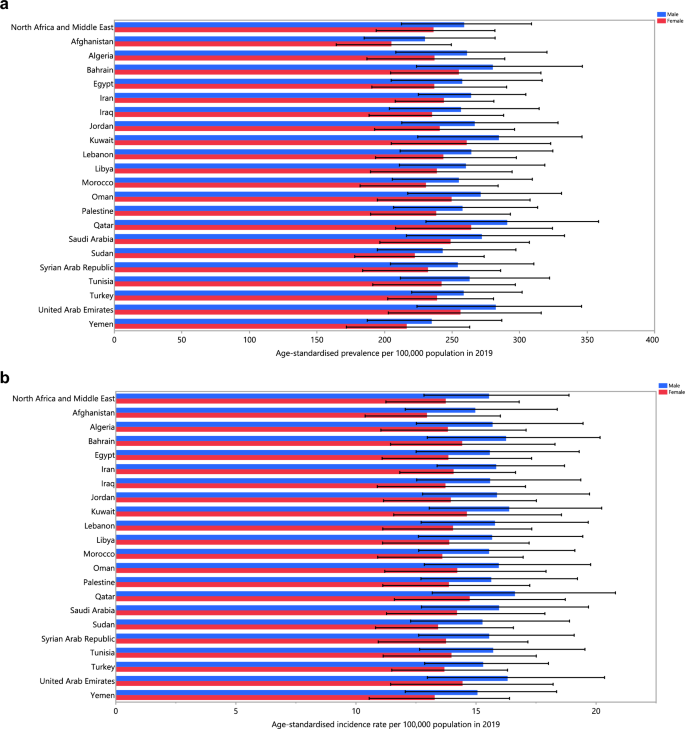
Age-standardised point prevalence ( A ), incidence rate ( B ), and YLD rate ( C ) of schizophrenia per 100,000 population in the Middle East and North Africa region in 2019, by sex and country. YLD years lived with disability. (Generated from data available from http://ghdx.healthdata.org/gbd-results-tool ).
The age-standardised incidence rate of schizophrenia in 2019 varied from 14.0 to 16.2 cases per 100,000 in the region. Qatar [16.2 (12.9 to 20.3)], the United Arab Emirates [15.7 (12.5 to 19.5)] and Kuwait [15.5 (12.4 to 19.3)] had the highest rates, with the lowest being in Afghanistan [14.0 (11.3 to 17.1)], Yemen [14.2 (11.4 to 17.4)] and Sudan [14.3 (11.7 to 17.7)] (Table S3 ). Figure 1 B presents the age-standardised incidence rates of schizophrenia by country, separately for males and females, in 2019.
The age-standardised YLD rate of schizophrenia in 2019 ranged from 135.6 to 182.5 cases (per 100,000) in the region. Qatar [182.5 (125.7 to 245.0)], the United Arab Emirates [176.5 (123.7 to 235.0)] and Kuwait [175.6 (121.0 to 234.3)] had the highest rates, while Afghanistan [135.6 (96.4 to 180.8)], Yemen [143.3 (100.6 to 191.3)] and Sudan [149.1 (104.2 to 199.5)] were lowest (Table S4 ). Figure 1 C presents the age-standardised YLD rates of schizophrenia by country, separately for males and females, in 2019.
The age-standardised prevalence, incidence and YLD rates of schizophrenia did not change significantly in any MENA countries from 1990 to 2019 (Tables S2 – S4 ). The changes in the age-standardised incidence, prevalence, and YLD rates for each country are depicted in Fig. 2 A–C, broken down by sex, for the period 1990–2019.
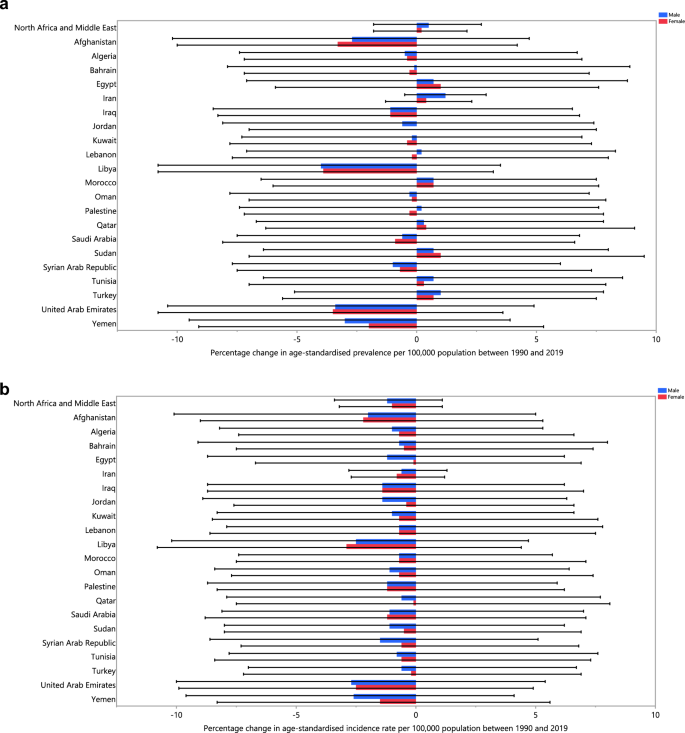
The percentage change in the age-standardised point prevalence ( A ), incidence rate ( B ), and YLD rate ( C ) of schizophrenia in the Middle East and North Africa region from 1990 to 2019, by sex and country. (Generated from data available from http://ghdx.healthdata.org/gbd-results-tool ).
Age and sex patterns
The total number of prevalent cases and the prevalence estimates in 2019 increased sharply for both sexes, starting from the 10–14 age range, reaching their highest level in those aged 35–39, before decreasing with age (Fig. 3 A). Similarly, the number of incidence cases and the incidence rates began to rise from the 10–14 age range, for both sexes, were highest in the 20–24 age range and then declined with age (Fig. 3 B). Furthermore, the YLD numbers rose with increasing age in both sex groups and peaked in those aged 30–34 years old, and then reduced with age. The pattern was similar for the YLD rate, but in both sexes the highest rate was seen in those aged 35–39 years old (Fig. 3 C). Males had a higher prevalence, incidence and YLD cases in all age categories. Likewise, males had higher prevalence, incidence and YLD rates of schizophrenia up to 80–84 years old, while the prevalence and YLD rates were higher for females in all remaining age groups.
Numbers of prevalent cases and point prevalence per 100,000 population ( A ), number of incidence cases and incidence rate per 100,000 population ( B ) and the number of YLDs and YLD rate per 100,000 population ( C ) for schizophrenia in the Middle East and North Africa region, by age and sex in 2019; Dotted and dashed lines indicate 95% upper and lower uncertainty intervals, respectively. YLD years lived with disability. (Generated from data available from http://ghdx.healthdata.org/gbd-results-tool ).
The schizophrenia associated YLD rates in 2019 were below the global rates for both sexes over 20 years of age (ratio of MENA/global YLD rate < 1). For both sexes, people aged 10–19 years of age exhibited YLD rates that were close to the global rate (ratio of MENA/global YLD rate = 1). The YLD rate in females aged 80 and older was 0.7 times the global rate in 2019. Furthermore, in 2019 males had similar YLD ratios (ratio of MENA/global YKD rate = 1), to those in 1990, in most age groups except for 15–19, 40–44 and 95 + years old, which had higher ratios than in 1990. Similarly, in 2019 the YLD ratios (ratio of MENA/global YLD rate = 1) for females increased in the 15–19, 75–79 and older than 90 age-groups, compared to 1990, while all other age-groups had similar rates (Fig. 4 ).
Ratio of the Middle East and North Africa region to the global schizophrenia YLD rate by age and sex, 1990 and 2019. YLD years lived with disability. (Generated from data available from http://ghdx.healthdata.org/gbd-results-tool ).
Relationship with socio-demographic index (SDI)
An almost linear positive association was evident between SDI and the YLD rate of schizophrenia between 1990 and 2019. In general, countries located within the region exhibited a steady rise in YLD rates, from 1990 to 2019, with increases in their SDIs. Qatar was the only country that had actual rates that were higher than those expected from 1990 to 2019, while all other countries had rates below the expected level (Fig. 5 ).
Age-standardised YLD rates of schizophrenia for 21 countries and territories, by SDI during 1990–2019; Expected values based on the Socio-demographic Index and disease rates in all locations are shown as the black line. Each point shows the observed age-standardised YLD rate for each country during 1990–2019. YLD years lived with disability, SDI Socio-demographic Index (Generated from data available from http://ghdx.healthdata.org/gbd-results-tool ).
This article presents an analysis of the burden of schizophrenia in MENA, encompassing the prevalence, incidence, and YLDs, using the most recent GBD 2019 data. This study is the first to present current information on the regional and national burden of schizophrenia in the MENA region. Previous studies were either been restricted to an individual country or investigated multiple causes with limited epidemiological data.
According to the latest research on the global burden of mental diseases, schizophrenia affects far fewer patients than several other mental conditions, but the YLDs attributable to this disorder are amongst the highest of these conditions 6 . Schizophrenia presents with a wide range of clinical symptoms and signs, and also varies greatly in the severity level. Schizophrenia requires lifelong treatment, which is demanding for both the patients and their families. Furthermore, some patients may develop resistance to conventional therapies, as their condition exacerbates with more frequent relapses 20 . These patients are also at a higher risk of suicide attempts and assault, further impacting the patient, their family, and their caregivers 21 , 22 . Due to economic crises, rapid population growth, a shortage of healthcare staff, weak coverage, political issues, and the stigmatizing attitudes of the general population against mental illnesses, many of the healthcare systems in the MENA region are yet to reach their full potential and provide acceptable standards of care. As a result, mismanagement, misdiagnosis, or missed cases might commonly occur 23 . Thus, the true burden of schizophrenia and the disability it imposes is expected to be far higher than the estimates reported here. Drug abuse, alcoholism, and smoking are common in schizophrenic patients, which can lead to comorbidities such as malnutrition, diabetes, vascular events, blood-borne infections, and chronic obstructive pulmonary disease (COPD), causing additional disability and mortality 24 . Although these comorbidities have a global importance, the impact is even larger in economically troubled healthcare systems, which is the situation in many MENA countries. Taken together, to alleviate the burden of schizophrenia, there is an urgent need for a plan to solve the widening socioeconomic disparities and implement measures to reduce the stigma associated with schizophrenia as soon as possible.
In line with the global trend for schizophrenia, the age-standardised prevalence, incidence, and YLDs in the region did not vary significantly between 1990 and 2019 6 . In general, countries which had higher age-standardised prevalence also had higher age-standardized incidence, and YLDs (i.e., Qatar, United Arab Emirates, and Kuwait). This same pattern was also the case for the countries which showed the lowest rates (i.e. Afghanistan, Yemen, and Sudan). Moreover, schizophrenia is linked to decreased fertility in both sexes, with males experiencing a more pronounced impact 25 . This can be attributed to the behavioral and social characteristics associated with schizophrenia. It is anticipated that decreased fertility will increase due to the ongoing delayed marriage patterns, even though the age of onset for schizophrenia will remain unchanged 26 . Natural selection is expected to reduce the population frequencies of genes associated with reduced fertility. Nonetheless, the prevalence of schizophrenia continues to be high, not only in the MENA region but also globally, with the frequency of the disease showing no significant change in recent decades 27 . This is commonly known as a "Darwinian paradox" 26 . Multiple hypotheses have been proposed to explain how schizophrenia evades the influence of natural selection, but the exact mechanism remains an enigma 28 , 29 , 30 . A plausible explanation for the unchanged prevalence of schizophrenia, despite its association with decreased fertility, is that the genetic factors contributing to schizophrenia may also confer advantages related to the development of essential human characteristics, including language, complex cognitive skills, and other favorable brain functions 31 . This hypothesis is substantiated by the presence of enhanced recent evolutionary markers near the loci linked to schizophrenia 31 , 32 . However, the evolutionary puzzle of schizophrenia remains complex and requires further research to be fully understood.
As illustrated in Fig. 2 A–C, the highest incidence of schizophrenia was observed in the 15 to 39 age group, and the disease’s prevalence peaked among those aged 20 to 54 years old, after which it gradually decreased with increasing age. The peak incidence starts earlier in life (20 to 24 age group) and the prevalence peaks in the 35 to 39 age group, and then reduces with age. This pattern was also seen for the YLD rates. The presented data emphasises the need for screening and intervention before the peak ages in the incidence, and also underlines the increased need for social, mental, and healthcare support during the peaks in the prevalence and YLDs. As the disease gets more chronic, and particularly when accompanied by more frequent relapses (either due to the nature of the disease or by mismanagement), more YLDs are observed and thus more access to medical care and social support is required to prevent treatment resistant conditions and worse outcomes, such as suicide, overdose, or domestic violence 33 . In almost all age groups, men showed higher prevalence, incidence and YLD values and rates, but these differences were not statistically significant. The changes in incidence, prevalence, and YLDs observed in both sexes generally show a decrease from 1990 to 2019 in most countries. Interestingly, the percentage changes in the incidence were negative in all MENA countries. Nevertheless, none of the changes were statistically significant, and thus should be carefully interpreted with regards to future planning and policy making.
The MENA YLD rates were below those found globally for all age groups, with the exception of those aged 10 to 19 year olds. This can be explained through the vast medical and non-medical problems faced by most countries in MENA. The burden of communicable diseases are substantially higher in MENA, than globally, and thus chronic conditions such as mental disorders might not receive the appropriate priority level for their management and treatment 34 . Furthermore, the burden of schizophrenia remained unchanged from 1990 to 2019 in most age groups, except for the elderly ages, which have increased.
As displayed in Fig. 4 , SDI has a positive linear relationship with the age-standardised YLD rate in MENA. These results should be carefully interpreted as there are major gaps between the countries showing the lowest values and those with the highest. Countries such as Afghanistan, Yemen, and Sudan were embroiled in prolonged conflicts during much of the measurement period, and their healthcare systems have been severely affected by their unbalanced economies and political problems 35 , 36 . Consequently, the low burden of schizophrenia in these countries is likely to be highly biased and artificially underestimated. In contrast, economically stable and high-income countries in this region have shown a higher burden of schizophrenia, which can be attributed to their more efficient healthcare systems and screening strategies. An alternative explanation for this finding might be that the high level of urbanisation and high density housing in the high income countries is related to the higher incidence of schizophrenia, due to elevated levels of stress and pollution in these areas 37 , 38 . While GBD continues to improve on the data and methodologies for estimating the burden of mental disorders, including schizophrenia, several challenges need acknowledging. Firstly, there were a large number of locations without high-quality raw data. Secondly, quantifying and eliminating all variation caused by measurement error in our prevalence estimates is a challenging task. Although IHME has refined the methodology to address known sources of bias (e.g., case definitions or survey methods), there are still very few data points available to inform such adjustments. Additionally, there is a paucity of research on the risk factors of mental disorders which can be used as predictive covariates in our epidemiological models 39 .
The present article highlights the importance of cautiously interpreting the currently available epidemiological information on the burden of schizophrenia in MENA, since the gathered data are prone to several biases. Thus, presumably the low burden of this condition might increase substantially in the future, as the healthcare systems start to screen and identify more patients. The most important aspect in preventing any future rise in the burden of schizophrenia lies in the efficient screening and prompt identification of patients, and then effectively treating these patients using a holistic approach. By reducing the prevalence of this mental condition, the burden of its related comorbidities and problems will also be addressed, significantly contributing to the overall health of the communities and the countries. Finally, it is important not to underestimate the significance of stigma directed towards people with psychiatric disorders. Initiatives aimed at increasing awareness about schizophrenia among patients, their families and their social networks can contribute significantly to reducing the disability associated with the disease.
Data availability
The data used for these analyses are all publicly available at http://ghdx.healthdata.org/gbd-results-tool .
Kahn, R. S. et al. Schizophrenia. Nat. Rev. Dis. Primers. 1 (1), 15067 (2015).
Article PubMed Google Scholar
Laursen, T. M., Nordentoft, M. & Mortensen, P. B. Excess early mortality in schizophrenia. Annu. Rev. Clin. Psychol. 10 (1), 425–448 (2014).
McGrath, J., Saha, S., Chant, D. & Welham, J. Schizophrenia: A concise overview of incidence, prevalence, and mortality. Epidemiol. Rev. 30 (1), 67–76 (2008).
Hoang, U., Stewart, R. & Goldacre, M. J. Mortality after hospital discharge for people with schizophrenia or bipolar disorder: Retrospective study of linked English hospital episode statistics, 1999–2006. BMJ. 343 , 5422 (2011).
Article Google Scholar
Lambert, T. J., Velakoulis, D. & Pantelis, C. Medical comorbidity in schizophrenia. Med. J. Austral. 178 (9), S67 (2003).
PubMed Google Scholar
Collaborators, G. M. D. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 9 (2), 137–150 (2022).
Vos, T. et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet. 396 (10258), 1204–1222 (2020).
Perälä, J. et al. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch. Gen. Psychiatry. 64 (1), 19–28 (2007).
He, H. et al. Trends in the incidence and DALYs of schizophrenia at the global, regional and national levels: Results from the Global Burden of Disease Study 2017. Epidemiol. Psychiatr. Sci. 29 , 891 (2020).
Charlson, F. J. et al. Global epidemiology and burden of schizophrenia: findings from the global burden of disease study 2016. Schizophr. Bull. 44 (6), 1195–1203 (2018).
Article PubMed PubMed Central Google Scholar
Whiteford, H. A., Ferrari, A. J., Degenhardt, L., Feigin, V. & Vos, T. The global burden of mental, neurological and substance use disorders: An analysis from the Global Burden of Disease Study 2010. PLoS ONE. 10 (2), e0116820 (2015).
Charara, R. et al. The burden of mental disorders in the eastern Mediterranean region, 1990–2013. PLoS ONE. 12 (1), e0169575 (2017).
Mokdad, A. H. et al. The burden of mental disorders in the Eastern Mediterranean region, 1990–2015: Findings from the global burden of disease 2015 study. Int. J. Public Health. 63 , 25–37 (2018).
Mandil, A., Chaaya, M. & Saab, D. Health status, epidemiological profile and prospects: Eastern Mediterranean region. Int. J. Epidemiol. 42 (2), 616–626 (2013).
Lozano, R. et al. Measuring universal health coverage based on an index of effective coverage of health services in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet. 396 (10258), 1250–1284 (2020).
Wang, H. et al. Global age-sex-specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950–2019: A comprehensive demographic analysis for the Global Burden of Disease Study 2019. Lancet. 396 (10258), 1160–1203 (2020).
Alonso, J. et al. Association of perceived stigma and mood and anxiety disorders: Results from the World Mental Health Surveys. Acta Psychiatr. Scand. 118 (4), 305–314 (2008).
Article CAS PubMed PubMed Central Google Scholar
Murray, C. J. et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet. 396 (10258), 1223–1249 (2020).
Wang, Y. Smoothing Splines: Methods and Applications (Chapman and Hall/CRC, 2019).
Google Scholar
Potkin, S. G. et al. The neurobiology of treatment-resistant schizophrenia: Paths to antipsychotic resistance and a roadmap for future research. NPJ Schizophr. 6 (1), 1 (2020).
Kowalec, K. et al. Increased schizophrenia family history burden and reduced premorbid IQ in treatment-resistant schizophrenia: A Swedish National Register and Genomic Study. Mol. Psychiatry. 26 (8), 4487–4495 (2021).
Shiraishi, N. & Reilly, J. Positive and negative impacts of schizophrenia on family caregivers: A systematic review and qualitative meta-summary. Soc. Psychiatry Psychiatr. Epidemiol. 54 (3), 277–290 (2019).
Balkhi, B., Alshayban, D. & Alotaibi, N. M. Impact of healthcare expenditures on healthcare outcomes in the Middle East and North Africa (MENA) region: A cross-country comparison, 1995–2015. Front. Public Health. 8 , 962 (2021).
Brink, M. et al. Excess medical comorbidity and mortality across the lifespan in schizophrenia: A nationwide Danish register study. Schizophr. Res. 206 , 347–354 (2019).
Bassett, A. S., Bury, A., Hodgkinson, K. A. & Honer, W. G. Reproductive fitness in familial schizophrenia. Schizophr. Res. 21 (3), 151–160 (1996).
Pearlson, G. D. & Folley, B. S. Schizophrenia, psychiatric genetics, and Darwinian psychiatry: An evolutionary framework. Schizophr. Bull. 34 (4), 722–733 (2008).
Solmi, M. et al. Incidence, prevalence, and global burden of schizophrenia-data, with critical appraisal, from the Global Burden of Disease (GBD). Mol. Psychiatry. 2023 , 1–9 (2019).
González-Peñas, J. et al. Recent natural selection conferred protection against schizophrenia by non-antagonistic pleiotropy. Sci. Rep. 13 (1), 15500 (2023).
Article ADS PubMed PubMed Central Google Scholar
Liu, C., Everall, I., Pantelis, C. & Bousman, C. Interrogating the evolutionary paradox of schizophrenia: A novel framework and evidence supporting recent negative selection of schizophrenia risk alleles. Front. Genet. 10 , 389 (2019).
Nichols, C. Is there an evolutionary advantage of schizophrenia?. Pers. Individ. Differ. 46 (8), 832–838 (2009).
Srinivasan, S. et al. Genetic markers of human evolution are enriched in schizophrenia. Biol. Psychiatry. 80 (4), 284–292 (2016).
Article CAS PubMed Google Scholar
Xu, K., Schadt, E. E., Pollard, K. S., Roussos, P. & Dudley, J. T. Genomic and network patterns of schizophrenia genetic variation in human evolutionary accelerated regions. Mol. Biol. Evol. 32 (5), 1148–1160 (2015).
Siskind, D. et al. Rates of treatment-resistant schizophrenia from first-episode cohorts: Systematic review and meta-analysis. Br. J. Psychiatry. 220 (3), 115–120 (2021).
Bizri, A. R. et al. The burden of invasive vaccine-preventable diseases in adults in the Middle East and North Africa (MENA) region. Infect. Dis. Ther. 10 (2), 663–685 (2021).
Raad, I. I., Chaftari, A.-M., Dib, R. W., Graviss, E. A. & Hachem, R. Emerging outbreaks associated with conflict and failing healthcare systems in the Middle East. Infect. Control Hosp. Epidemiol. 39 (10), 1230–1236 (2018).
Naal, H., El Koussa, M., El Hamouch, M., Hneiny, L. & Saleh, S. A systematic review of global health capacity building initiatives in low-to middle-income countries in the Middle East and North Africa region. Glob. Health. 16 (1), 56 (2020).
Ventriglio, A., Torales, J., Castaldelli-Maia, J. M., De Berardis, D. & Bhugra, D. Urbanization and emerging mental health issues. CNS Spectr. 26 (1), 43–50 (2020).
Colodro-Conde, L. et al. Association between population density and genetic risk for schizophrenia. JAMA Psychiatry. 75 (9), 901–910 (2018).
Lu, Y. et al. Genetic risk scores and family history as predictors of schizophrenia in Nordic registers. Psychol. Med. 48 (7), 1201–1208 (2017).
Download references
Acknowledgements
We would like to thank the Institute for Health Metrics and Evaluation staff and its collaborators who prepared these publicly available data. We would also like to thank the Clinical Research Development Unit of Tabriz Valiasr Hospital, Tabriz University of Medical Sciences, Tabriz, Iran for their assistance in this research.
The Bill and Melinda Gates Foundation, who were not involved in any way in the preparation of this manuscript, funded the GBD study. The Shahid Beheshti University of Medical Sciences, Tabriz, Iran (Grant No. 43002510) also supported the present report.
Author information
Authors and affiliations.
Neurosciences Research Center, Aging Research Institute, Tabriz University of Medical Sciences, Tabriz, Iran
Saeid Safiri
Clinical Research Development Unit of Tabriz Valiasr Hospital, Tabriz University of Medical Sciences, Tabriz, Iran
Student Research Committee, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
Maryam Noori
HIV/STI Surveillance Research Center, WHO Collaborating Center for HIV Surveillance, Institute for Futures Studies in Health, Kerman University of Medical Sciences, Kerman, Iran
Seyed Aria Nejadghaderi
Systematic Review and Meta-analysis Expert Group (SRMEG), Universal Scientific Education and Research Network (USERN), Tehran, Iran
Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran
Ali Shamekh
Department of Life and Health Sciences, University of Nicosia, Nicosia, Cyprus
Mark J. M. Sullman
Department of Social Sciences, University of Nicosia, Nicosia, Cyprus
Centre for Statistics in Medicine, NDORMS, Botnar Research Centre, University of Oxford, Oxford, UK
Gary S. Collins
NIHR Oxford Biomedical Research Centre, Oxford University Hospitals NHS Foundation Trust, Oxford, UK
Social Determinants of Health Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Ali-Asghar Kolahi
You can also search for this author in PubMed Google Scholar
Contributions
SS and AAK designed the study. SS analysed the data and performed the statistical analyses. SS, MN, SAN, AS, MJMS, GSC, and AAK drafted the initial manuscript. All authors reviewed the drafted manuscript for critical content. All authors approved the final version of the manuscript.
Corresponding authors
Correspondence to Saeid Safiri or Ali-Asghar Kolahi .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary tables., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Safiri, S., Noori, M., Nejadghaderi, S. et al. The burden of schizophrenia in the Middle East and North Africa region, 1990–2019. Sci Rep 14 , 9720 (2024). https://doi.org/10.1038/s41598-024-59905-8
Download citation
Received : 12 July 2023
Accepted : 16 April 2024
Published : 27 April 2024
DOI : https://doi.org/10.1038/s41598-024-59905-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Middle East and North Africa
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Associations between structural brain changes and blood neurofilament light chain protein in treatment-resistant schizophrenia
Affiliations.
- 1 Neuropsychiatry, The Royal Melbourne Hospital, Parkville, VIC, Australia.
- 2 Melbourne Medical School, The University of Melbourne, Parkville, VIC, Australia.
- 3 Melbourne Neuropsychiatry Centre, Department of Psychiatry, The University of Melbourne, Parkville, VIC, Australia.
- 4 Centre for Youth Mental Health, The University of Melbourne, Parkville, VIC, Australia.
- 5 Orygen, Parkville, VIC, Australia.
- 6 Department of Medicine, Royal Melbourne Hospital, The University of Melbourne, Parkville, VIC, Australia.
- 7 Melbourne School of Psychological Sciences, The University of Melbourne, Parkville, VIC, Australia.
- 8 Clinical Memory Research Unit, Department of Clinical Sciences, Faculty of Medicine, Lund University, Malmö, Sweden.
- 9 Memory Clinic, Skåne University Hospital, Malmö, Sweden.
- 10 Visiting Professor, King's College London.
- 11 Department of Medical Genetics, University of Calgary.
- 12 Department of Psychiatry, University of Melbourne, Parkville, VIC, Australia.
- 13 Mental Health and Wellbeing Services, Western Health, St Albans VIC, Australia.
- 14 Monash Institute of Pharmaceutical Sciences (MIPS), Faculty of Pharmacy and Pharmaceutical Sciences Monash University, Parkville, VIC, Australia.
- PMID: 38645076
- PMCID: PMC11030485
- DOI: 10.1101/2024.04.07.24305362
Background and hypothesis: Around 30% of people with schizophrenia are refractory to antipsychotic treatment (treatment-resistant schizophrenia; TRS). While abnormal structural neuroimaging findings, in particular volume and thickness reductions, are often observed in schizophrenia, it is anticipated that biomarkers of neuronal injury like neurofilament light chain protein (NfL) can improve our understanding of the pathological basis underlying schizophrenia. The current study aimed to determine whether people with TRS demonstrate different associations between plasma NfL levels and regional cortical thickness reductions compared with controls.
Study design: Measurements of plasma NfL and cortical thickness were obtained from 39 individuals with TRS, and 43 healthy controls. T1-weighted magnetic resonance imaging sequences were obtained and processed via FreeSurfer. General linear mixed models adjusting for age and weight were estimated to determine whether the interaction between diagnostic group and plasma NfL level predicted lower cortical thickness across frontotemporal structures and the insula.
Study results: Significant (false discovery rate corrected) cortical thinning of the left ( p = 0.001, η 2 p = 0.104) and right ( p < 0.001, η 2 p = 0.167) insula was associated with higher levels of plasma NfL in TRS, but not in healthy controls.
Conclusions: The association between regional thickness reduction of the insula bilaterally and plasma NfL may reflect a neurodegenerative process during the course of TRS. The findings of the present study suggest that some level of cortical degeneration localised to the bilateral insula may exist in people with TRS, which is not observed in the normal population.
Keywords: Schizophrenia; biomarker; neurofilament; neuroimaging; treatment-resistant.
Publication types
Grants and funding.
- R01 AG083740/AG/NIA NIH HHS/United States

Who Relapses From Schizophrenia?
Factors that indicate risk for rehospitalizations and emergency room visits..
Posted April 29, 2024 | Reviewed by Michelle Quirk
- What Is Psychosis?
- Find counselling to treat psychosis
- A study identified a large population of patients with schizophrenia and examined factors related to relapse.
- Factors of relapse had to do with race, insurance access, and medication nonadherence, among others.
- Other studies have confirmed these observations in smaller-scale populations.

A large review article recently published in Nature has revealed some perhaps long-suspected information about people living with schizophrenia and schizoaffective disorder who tend to relapse .
The authors’ methods in this study tried something a little different. Instead of relying on insurance claims data or only using small populations, they searched a system of large electronic health records (EHRs) to analyze the relationships between relapse episodes and characteristics such as race, disease, insurance status, and more. The data, which included 8119 patients, came from October 15, 2016, to December 31, 2021, and focused on patients who received care for at least 12 months.
Who Had Relapses?
What they defined as relapse was an emergency room visit or an inpatient hospitalization for their condition. Of the total patients in the study, 30.52 percent experienced a relapse, while 69.48 percent experienced no relapse. The patients were primarily male and accounted for 54.72 percent of the demographic, while 54.23 percent were white non-Hispanic or Latino.
Even though the patient population consisted mostly of white males, the study showed that those who were more likely to relapse were more likely to be Black (34.68 percent of Blacks), other Pacific Islander (58.33 percent of other Pacific Islanders), and Latino (35.36 percent of Latinos) compared to whites.
Those who relapsed also were more likely to be under Medicaid or Medicare, with a 33 percent and 22 percent increased prevalence, respectively. They were more likely, at a 33 percent increased prevalence, to have a diagnosis of substance abuse alongside their diagnosis of schizophrenia and schizoaffective disorder. They also were more likely to have more encounters with health care, indicating that those who frequent hospitals are more likely to visit them again.
These patients are more likely to be prescribed more medications than those who did not relapse, and the most common prescription written tends to be for atypical antipsychotics when typical antipsychotics haven’t worked.
Oddly, patients who relapsed were less likely to have diagnoses of obesity, hypertension, and diabetes. There were no sex differences in the likelihood of relapse episodes.
Other Findings
In a literature review that included 145 other manuscripts, similar factors were observed for determining who was more at risk for relapse.
The most common factors were nonadherence to treatments like medications, stress and depression , and substance use. Those who were more likely to relapse were also less likely to be privately insured, which may imply that those who cannot work or gain private insurance through other means like family members or spouses are more likely to relapse.
What this study confirms is an instinct many who work in the industry perhaps have already observed. Those who are more at risk for relapse episodes need more targeted treatment solutions so that people living with schizophrenia and schizoaffective disorder can reduce their interaction with substance abuse and increase their adherence to prescribed treatments.
Rivelli, A., Fitzpatrick, V., Nelson, M., Laubmeier, K., Zeni, C., & Mylavarapu, S. (2024). Real-world predictors of relapse in patients with schizophrenia and schizoaffective disorder in a large health system. Schizophrenia , 10 (1), 28.

Sarah An Myers is a writer with a Master of Arts in psychology and behavioral neuroscience from the University of Missouri-St. Louis. She researches novel computational and therapeutic methods for treating and diagnosing mental disorders.
- Find a Therapist
- Find a Treatment Center
- Find a Psychiatrist
- Find a Support Group
- Find Online Therapy
- International
- New Zealand
- South Africa
- Switzerland
- Asperger's
- Bipolar Disorder
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Therapy Center NEW
- Diagnosis Dictionary
- Types of Therapy

Understanding what emotional intelligence looks like and the steps needed to improve it could light a path to a more emotionally adept world.
- Emotional Intelligence
- Gaslighting
- Affective Forecasting
- Neuroscience

IMAGES
VIDEO
COMMENTS
For treatment-responsive patients, there should be a collaborative approach when choosing a treatment [4] . More information on the recognition and management of schizophrenia can be found in a previous article here, and in accompanying case studies here. This case study aims to explore a patient's journey in mental health services during a ...
Treatment resistant schizophrenia (TRS) refers to the significant proportion of schizophrenia patients who continue to have symptoms and poor outcomes despite treatment. ... The second study, the Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS) (Lewis et al., ... Thus far, only two case reports have been presented ...
Well over 3 decades have passed since Kane and colleagues ( 1) conducted the seminal study of clozapine for patients with treatment-resistant schizophrenia (TRS). The study was seminal because of the pronounced treatment response (30% of patients) over 6 weeks of clozapine therapy compared, under double-blind conditions, with chlorpromazine (4% ...
The neurobiology of treatment-resistant schizophrenia: paths to antipsychotic resistance and a roadmap for future research ... A recent study in first-episode patients found evidence that this was the case ... McCutcheon R, Howes OD. Brain-imaging studies of treatment-resistant schizophrenia: a systematic review. Lancet Psychiatry. 2016; 3:451 ...
Well over 3 decades have passed since Kane and colleagues conducted the seminal study of clozapine for patients with treatment-resistant schizophrenia (TRS).The study was seminal because of the pronounced treatment response (30% of patients) over 6 weeks of clozapine therapy compared, under double-blind conditions, with chlorpromazine (4% response).
Overall, said McKenna, his study suggests DBS holds promise for treatment-resistant schizophrenia. The case study by Cascella and colleagues is "clearly encouraging, particularly in view of the full remission of hallucinations and other positive symptoms with immediate return of hallucinations when the stimulation was turned off unknown to ...
The lifetime prevalence of schizophrenia is estimated to be approximately 0.7% (McGrath et al. 2008; Moreno-Küstner et al. 2018; van der Werf et al. 2014), although findings vary depending on the study location, demographic characteristics of the sample, the approach used for case-finding, the method used for diagnostic confirmation, and the ...
Chouinard et al estimated that 50% of treatment-resistant schizophrenia cases are related to DSP.1 A possible mechanism is that long-term antipsychotic treatment causes a compensatory increase in dopamine receptor density in the striatum, which leads to a dopamine supersensitive state.2-5 Moreover, recent studies have shown that the ...
Our study identifies several candidate predictors that could potentially be included in future prediction models for treatment-resistant schizophrenia. Notably, established risk factors for schizophrenia did not predict treatment resistance, suggesting that treatment-resistant disease might be a distinct subtype of schizophrenia and not merely a more severe form.
The case meets the criteria for treatment-resistant schizophrenia (TRS) as per the consensus guidelines of the Treatment Response and Resistance in Psychosis Working Group, given the reasons outlined above . The patient had active and persistent symptoms for over 12 weeks with impaired functioning and at least 6 weeks of treatment with an ...
T reatment-resistant schizophrenia (TRS) represents a major clinical challenge. The broad definition of TRS requires nonresponse to at least 2 sequential antipsychotic trials of sufficient dose, duration, and adherence. Several demographic, clinical, and neurologic predictors are associated with TRS. Primary (or early) TRS is present from the ...
resistant" or "clozapine -resistant schizophrenia" if they also fail to respond to a clozapine treatment trial. ECT has been considered as a possible augmentation of clozapine treatment for ultra-resistant patients. In a small comparison study, Masoudzadeh et al. found that combination therapy of clozapine and ECT is more effective than ...
Objectives: Although clozapine is considered the only effective pharmacological option for patients with treatment-resistant schizophrenia (TRS), around 30-40% of patients show clozapine resistance. Modified electroconvulsive therapy augmentation is reportedly clinically effective for clozapine-resistant schizophrenia, but few case reports have described the efficacy of combining clozapine ...
Epidemiologic studies of treatment-resistant schizophrenia and practice guidelines on its treatment are based on varying definitions of treatment-resistant schizophrenia, limiting the utility of the results . In response, in 2017 an international panel of experts, the Treatment Response and Resistance in Psychosis (TRRIP) Working Group ...
Evaluation of a Collaborative Care Program for Patients With Treatment-Resistant Schizophrenia: Protocol for a Multiple Case Study ... These case studies will focus on an Early Psychosis Intervention Team and 2 Flexible Assertive Community treatment teams in the Netherlands. Data will be collected from patient records as well as through ...
Treatment-resistant symptoms occur in about a third of patients with schizophrenia and are associated with a substantial reduction in their quality of life. The development of new treatment options for clozapine-resistant schizophrenia constitutes a crucial, unmet need in psychiatry. Additionally, an overview of past and possible future research avenues to optimise the early detection ...
Treatment-resistant schizophrenia (TRS), the persistence of positive symptoms despite ≥2 trials of adequate dose and duration of antipsychotic medication with documented adherence, is a serious ...
Treatment-resistant schizophrenia, affecting approximately 20-30% of patients with schizophrenia, has a high burden both for patients and healthcare services. ... As this was a case-control study including all patients with TRS and a ratio of two non-TRS patients for every TRS patient, the prevalence of TRS could not be calculated. The two ...
Case Studies: Stahl's Essential Psychopharmacology - November 2023. ... Case 17: The "standard treatment" is earning a "D": treatment-resistant schizophrenia; Jeffrey R. Strawn, University of Cincinnati, Ohio, Stephen M. Stahl, University of California, San Diego;
Clozapine remains the only licensed medication for treatment-resistant schizophrenia and has substantial mortality, quality of life, and health economic benefits when prescribed. It remains considerably underused in clinical practice worldwide,1 with feedback from clinicians and patients that the use of blood tests for monitoring the effects of the drug is the major barrier to increased uptake ...
Material and methods: A case-control study included treatment-resistant patients and good responders. Patients were stratified in two groups based on the established criteria for treatment-resistant schizophrenia using BPRS and PANSS. The study was approved by the ethical committees (references: CEHDF1017; HPC-017-2017) and all patients/legal ...
In this case report, we explore the case of a 28-year-old male patient diagnosed with schizophrenia who relapsed following COVID-19 infection and failed to respond to clozapine, which had induced remission in the past. Case Presentation The patient is a 28-year-old, single, South Asian male, with a history of treatment-resistant schizophrenia
Italian biopharma Newron Pharmaceuticals's add-on schizophrenia treatment, evenamide, has met primary and secondary endpoints in a Phase II/III trial.. The placebo-controlled Phase II/III study (EudraCT Number: 2020-006062-36) evaluated evenamide as an add-on therapy with a second-generation antipsychotic including clozapine in 291 patients with chronic schizophrenia.
The company also hopes to use the data to make the case for a "potentially pivotal" phase 3 trial testing evenamide over one year in patients with treatment-resistant schizophrenia.
The treatment effect sizes observed for the primary end point in this trial (0.60), the EMERGENT-1 trial (0.75), 8 and the EMERGENT-2 trial (0.61) 9 were robust; the median treatment effect size reported across 105 trials of antipsychotics in the treatment of acute psychosis in people with schizophrenia was 0.42. 6 Treatment with xanomeline ...
Treatment-resistant or drug-naïve schizophrenia has shown improvement with cariprazine treatment (1, 75). Steady state of paranoid delusions and aggressiveness was achieved with 2 weeks of cariprazine treatment . Cariprazine as adjunctive or monotherapy also resulted in remission of negative symptoms (5, 25, 64, 74).
Introduction. Treatment-resistant schizophrenia (TRS) affects ~30% of people with a diagnosis of schizophrenia. 1 TRS is defined as nonresponse to at least two trials of antipsychotic medication of adequate dose and duration, 2 at which point, the antipsychotic clozapine is indicated. Interestingly, clozapine does not work better than other antipsychotics in first-episode cases. 3 Recent work ...
Schizophrenia ranks as the third-most common cause of disability among mental disorders globally. This study presents findings on the prevalence, incidence and years lived with disability (YLDs ...
Background and hypothesis: Around 30% of people with schizophrenia are refractory to antipsychotic treatment (treatment-resistant schizophrenia; TRS). While abnormal structural neuroimaging findings, in particular volume and thickness reductions, are often observed in schizophrenia, it is anticipated that biomarkers of neuronal injury like neurofilament light chain protein (NfL) can improve ...
Key points. A study identified a large population of patients with schizophrenia and examined factors related to relapse. Factors of relapse had to do with race, insurance access, and medication ...