- Open access
- Published: 10 November 2020

Case study research for better evaluations of complex interventions: rationale and challenges
- Sara Paparini ORCID: orcid.org/0000-0002-1909-2481 1 ,
- Judith Green 2 ,
- Chrysanthi Papoutsi 1 ,
- Jamie Murdoch 3 ,
- Mark Petticrew 4 ,
- Trish Greenhalgh 1 ,
- Benjamin Hanckel 5 &
- Sara Shaw 1
BMC Medicine volume 18 , Article number: 301 ( 2020 ) Cite this article
17k Accesses
41 Citations
35 Altmetric
Metrics details
The need for better methods for evaluation in health research has been widely recognised. The ‘complexity turn’ has drawn attention to the limitations of relying on causal inference from randomised controlled trials alone for understanding whether, and under which conditions, interventions in complex systems improve health services or the public health, and what mechanisms might link interventions and outcomes. We argue that case study research—currently denigrated as poor evidence—is an under-utilised resource for not only providing evidence about context and transferability, but also for helping strengthen causal inferences when pathways between intervention and effects are likely to be non-linear.
Case study research, as an overall approach, is based on in-depth explorations of complex phenomena in their natural, or real-life, settings. Empirical case studies typically enable dynamic understanding of complex challenges and provide evidence about causal mechanisms and the necessary and sufficient conditions (contexts) for intervention implementation and effects. This is essential evidence not just for researchers concerned about internal and external validity, but also research users in policy and practice who need to know what the likely effects of complex programmes or interventions will be in their settings. The health sciences have much to learn from scholarship on case study methodology in the social sciences. However, there are multiple challenges in fully exploiting the potential learning from case study research. First are misconceptions that case study research can only provide exploratory or descriptive evidence. Second, there is little consensus about what a case study is, and considerable diversity in how empirical case studies are conducted and reported. Finally, as case study researchers typically (and appropriately) focus on thick description (that captures contextual detail), it can be challenging to identify the key messages related to intervention evaluation from case study reports.
Whilst the diversity of published case studies in health services and public health research is rich and productive, we recommend further clarity and specific methodological guidance for those reporting case study research for evaluation audiences.
Peer Review reports
The need for methodological development to address the most urgent challenges in health research has been well-documented. Many of the most pressing questions for public health research, where the focus is on system-level determinants [ 1 , 2 ], and for health services research, where provisions typically vary across sites and are provided through interlocking networks of services [ 3 ], require methodological approaches that can attend to complexity. The need for methodological advance has arisen, in part, as a result of the diminishing returns from randomised controlled trials (RCTs) where they have been used to answer questions about the effects of interventions in complex systems [ 4 , 5 , 6 ]. In conditions of complexity, there is limited value in maintaining the current orientation to experimental trial designs in the health sciences as providing ‘gold standard’ evidence of effect.
There are increasing calls for methodological pluralism [ 7 , 8 ], with the recognition that complex intervention and context are not easily or usefully separated (as is often the situation when using trial design), and that system interruptions may have effects that are not reducible to linear causal pathways between intervention and outcome. These calls are reflected in a shifting and contested discourse of trial design, seen with the emergence of realist [ 9 ], adaptive and hybrid (types 1, 2 and 3) [ 10 , 11 ] trials that blend studies of effectiveness with a close consideration of the contexts of implementation. Similarly, process evaluation has now become a core component of complex healthcare intervention trials, reflected in MRC guidance on how to explore implementation, causal mechanisms and context [ 12 ].
Evidence about the context of an intervention is crucial for questions of external validity. As Woolcock [ 4 ] notes, even if RCT designs are accepted as robust for maximising internal validity, questions of transferability (how well the intervention works in different contexts) and generalisability (how well the intervention can be scaled up) remain unanswered [ 5 , 13 ]. For research evidence to have impact on policy and systems organisation, and thus to improve population and patient health, there is an urgent need for better methods for strengthening external validity, including a better understanding of the relationship between intervention and context [ 14 ].
Policymakers, healthcare commissioners and other research users require credible evidence of relevance to their settings and populations [ 15 ], to perform what Rosengarten and Savransky [ 16 ] call ‘careful abstraction’ to the locales that matter for them. They also require robust evidence for understanding complex causal pathways. Case study research, currently under-utilised in public health and health services evaluation, can offer considerable potential for strengthening faith in both external and internal validity. For example, in an empirical case study of how the policy of free bus travel had specific health effects in London, UK, a quasi-experimental evaluation (led by JG) identified how important aspects of context (a good public transport system) and intervention (that it was universal) were necessary conditions for the observed effects, thus providing useful, actionable evidence for decision-makers in other contexts [ 17 ].
The overall approach of case study research is based on the in-depth exploration of complex phenomena in their natural, or ‘real-life’, settings. Empirical case studies typically enable dynamic understanding of complex challenges rather than restricting the focus on narrow problem delineations and simple fixes. Case study research is a diverse and somewhat contested field, with multiple definitions and perspectives grounded in different ways of viewing the world, and involving different combinations of methods. In this paper, we raise awareness of such plurality and highlight the contribution that case study research can make to the evaluation of complex system-level interventions. We review some of the challenges in exploiting the current evidence base from empirical case studies and conclude by recommending that further guidance and minimum reporting criteria for evaluation using case studies, appropriate for audiences in the health sciences, can enhance the take-up of evidence from case study research.
Case study research offers evidence about context, causal inference in complex systems and implementation
Well-conducted and described empirical case studies provide evidence on context, complexity and mechanisms for understanding how, where and why interventions have their observed effects. Recognition of the importance of context for understanding the relationships between interventions and outcomes is hardly new. In 1943, Canguilhem berated an over-reliance on experimental designs for determining universal physiological laws: ‘As if one could determine a phenomenon’s essence apart from its conditions! As if conditions were a mask or frame which changed neither the face nor the picture!’ ([ 18 ] p126). More recently, a concern with context has been expressed in health systems and public health research as part of what has been called the ‘complexity turn’ [ 1 ]: a recognition that many of the most enduring challenges for developing an evidence base require a consideration of system-level effects [ 1 ] and the conceptualisation of interventions as interruptions in systems [ 19 ].
The case study approach is widely recognised as offering an invaluable resource for understanding the dynamic and evolving influence of context on complex, system-level interventions [ 20 , 21 , 22 , 23 ]. Empirically, case studies can directly inform assessments of where, when, how and for whom interventions might be successfully implemented, by helping to specify the necessary and sufficient conditions under which interventions might have effects and to consolidate learning on how interdependencies, emergence and unpredictability can be managed to achieve and sustain desired effects. Case study research has the potential to address four objectives for improving research and reporting of context recently set out by guidance on taking account of context in population health research [ 24 ], that is to (1) improve the appropriateness of intervention development for specific contexts, (2) improve understanding of ‘how’ interventions work, (3) better understand how and why impacts vary across contexts and (4) ensure reports of intervention studies are most useful for decision-makers and researchers.
However, evaluations of complex healthcare interventions have arguably not exploited the full potential of case study research and can learn much from other disciplines. For evaluative research, exploratory case studies have had a traditional role of providing data on ‘process’, or initial ‘hypothesis-generating’ scoping, but might also have an increasing salience for explanatory aims. Across the social and political sciences, different kinds of case studies are undertaken to meet diverse aims (description, exploration or explanation) and across different scales (from small N qualitative studies that aim to elucidate processes, or provide thick description, to more systematic techniques designed for medium-to-large N cases).
Case studies with explanatory aims vary in terms of their positioning within mixed-methods projects, with designs including (but not restricted to) (1) single N of 1 studies of interventions in specific contexts, where the overall design is a case study that may incorporate one or more (randomised or not) comparisons over time and between variables within the case; (2) a series of cases conducted or synthesised to provide explanation from variations between cases; and (3) case studies of particular settings within RCT or quasi-experimental designs to explore variation in effects or implementation.
Detailed qualitative research (typically done as ‘case studies’ within process evaluations) provides evidence for the plausibility of mechanisms [ 25 ], offering theoretical generalisations for how interventions may function under different conditions. Although RCT designs reduce many threats to internal validity, the mechanisms of effect remain opaque, particularly when the causal pathways between ‘intervention’ and ‘effect’ are long and potentially non-linear: case study research has a more fundamental role here, in providing detailed observational evidence for causal claims [ 26 ] as well as producing a rich, nuanced picture of tensions and multiple perspectives [ 8 ].
Longitudinal or cross-case analysis may be best suited for evidence generation in system-level evaluative research. Turner [ 27 ], for instance, reflecting on the complex processes in major system change, has argued for the need for methods that integrate learning across cases, to develop theoretical knowledge that would enable inferences beyond the single case, and to develop generalisable theory about organisational and structural change in health systems. Qualitative Comparative Analysis (QCA) [ 28 ] is one such formal method for deriving causal claims, using set theory mathematics to integrate data from empirical case studies to answer questions about the configurations of causal pathways linking conditions to outcomes [ 29 , 30 ].
Nonetheless, the single N case study, too, provides opportunities for theoretical development [ 31 ], and theoretical generalisation or analytical refinement [ 32 ]. How ‘the case’ and ‘context’ are conceptualised is crucial here. Findings from the single case may seem to be confined to its intrinsic particularities in a specific and distinct context [ 33 ]. However, if such context is viewed as exemplifying wider social and political forces, the single case can be ‘telling’, rather than ‘typical’, and offer insight into a wider issue [ 34 ]. Internal comparisons within the case can offer rich possibilities for logical inferences about causation [ 17 ]. Further, case studies of any size can be used for theory testing through refutation [ 22 ]. The potential lies, then, in utilising the strengths and plurality of case study to support theory-driven research within different methodological paradigms.
Evaluation research in health has much to learn from a range of social sciences where case study methodology has been used to develop various kinds of causal inference. For instance, Gerring [ 35 ] expands on the within-case variations utilised to make causal claims. For Gerring [ 35 ], case studies come into their own with regard to invariant or strong causal claims (such as X is a necessary and/or sufficient condition for Y) rather than for probabilistic causal claims. For the latter (where experimental methods might have an advantage in estimating effect sizes), case studies offer evidence on mechanisms: from observations of X affecting Y, from process tracing or from pattern matching. Case studies also support the study of emergent causation, that is, the multiple interacting properties that account for particular and unexpected outcomes in complex systems, such as in healthcare [ 8 ].
Finally, efficacy (or beliefs about efficacy) is not the only contributor to intervention uptake, with a range of organisational and policy contingencies affecting whether an intervention is likely to be rolled out in practice. Case study research is, therefore, invaluable for learning about contextual contingencies and identifying the conditions necessary for interventions to become normalised (i.e. implemented routinely) in practice [ 36 ].
The challenges in exploiting evidence from case study research
At present, there are significant challenges in exploiting the benefits of case study research in evaluative health research, which relate to status, definition and reporting. Case study research has been marginalised at the bottom of an evidence hierarchy, seen to offer little by way of explanatory power, if nonetheless useful for adding descriptive data on process or providing useful illustrations for policymakers [ 37 ]. This is an opportune moment to revisit this low status. As health researchers are increasingly charged with evaluating ‘natural experiments’—the use of face masks in the response to the COVID-19 pandemic being a recent example [ 38 ]—rather than interventions that take place in settings that can be controlled, research approaches using methods to strengthen causal inference that does not require randomisation become more relevant.
A second challenge for improving the use of case study evidence in evaluative health research is that, as we have seen, what is meant by ‘case study’ varies widely, not only across but also within disciplines. There is indeed little consensus amongst methodologists as to how to define ‘a case study’. Definitions focus, variously, on small sample size or lack of control over the intervention (e.g. [ 39 ] p194), on in-depth study and context [ 40 , 41 ], on the logic of inference used [ 35 ] or on distinct research strategies which incorporate a number of methods to address questions of ‘how’ and ‘why’ [ 42 ]. Moreover, definitions developed for specific disciplines do not capture the range of ways in which case study research is carried out across disciplines. Multiple definitions of case study reflect the richness and diversity of the approach. However, evidence suggests that a lack of consensus across methodologists results in some of the limitations of published reports of empirical case studies [ 43 , 44 ]. Hyett and colleagues [ 43 ], for instance, reviewing reports in qualitative journals, found little match between methodological definitions of case study research and how authors used the term.
This raises the third challenge we identify that case study reports are typically not written in ways that are accessible or useful for the evaluation research community and policymakers. Case studies may not appear in journals widely read by those in the health sciences, either because space constraints preclude the reporting of rich, thick descriptions, or because of the reported lack of willingness of some biomedical journals to publish research that uses qualitative methods [ 45 ], signalling the persistence of the aforementioned evidence hierarchy. Where they do, however, the term ‘case study’ is used to indicate, interchangeably, a qualitative study, an N of 1 sample, or a multi-method, in-depth analysis of one example from a population of phenomena. Definitions of what constitutes the ‘case’ are frequently lacking and appear to be used as a synonym for the settings in which the research is conducted. Despite offering insights for evaluation, the primary aims may not have been evaluative, so the implications may not be explicitly drawn out. Indeed, some case study reports might properly be aiming for thick description without necessarily seeking to inform about context or causality.
Acknowledging plurality and developing guidance
We recognise that definitional and methodological plurality is not only inevitable, but also a necessary and creative reflection of the very different epistemological and disciplinary origins of health researchers, and the aims they have in doing and reporting case study research. Indeed, to provide some clarity, Thomas [ 46 ] has suggested a typology of subject/purpose/approach/process for classifying aims (e.g. evaluative or exploratory), sample rationale and selection and methods for data generation of case studies. We also recognise that the diversity of methods used in case study research, and the necessary focus on narrative reporting, does not lend itself to straightforward development of formal quality or reporting criteria.
Existing checklists for reporting case study research from the social sciences—for example Lincoln and Guba’s [ 47 ] and Stake’s [ 33 ]—are primarily orientated to the quality of narrative produced, and the extent to which they encapsulate thick description, rather than the more pragmatic issues of implications for intervention effects. Those designed for clinical settings, such as the CARE (CAse REports) guidelines, provide specific reporting guidelines for medical case reports about single, or small groups of patients [ 48 ], not for case study research.
The Design of Case Study Research in Health Care (DESCARTE) model [ 44 ] suggests a series of questions to be asked of a case study researcher (including clarity about the philosophy underpinning their research), study design (with a focus on case definition) and analysis (to improve process). The model resembles toolkits for enhancing the quality and robustness of qualitative and mixed-methods research reporting, and it is usefully open-ended and non-prescriptive. However, even if it does include some reflections on context, the model does not fully address aspects of context, logic and causal inference that are perhaps most relevant for evaluative research in health.
Hence, for evaluative research where the aim is to report empirical findings in ways that are intended to be pragmatically useful for health policy and practice, this may be an opportune time to consider how to best navigate plurality around what is (minimally) important to report when publishing empirical case studies, especially with regards to the complex relationships between context and interventions, information that case study research is well placed to provide.
The conventional scientific quest for certainty, predictability and linear causality (maximised in RCT designs) has to be augmented by the study of uncertainty, unpredictability and emergent causality [ 8 ] in complex systems. This will require methodological pluralism, and openness to broadening the evidence base to better understand both causality in and the transferability of system change intervention [ 14 , 20 , 23 , 25 ]. Case study research evidence is essential, yet is currently under exploited in the health sciences. If evaluative health research is to move beyond the current impasse on methods for understanding interventions as interruptions in complex systems, we need to consider in more detail how researchers can conduct and report empirical case studies which do aim to elucidate the contextual factors which interact with interventions to produce particular effects. To this end, supported by the UK’s Medical Research Council, we are embracing the challenge to develop guidance for case study researchers studying complex interventions. Following a meta-narrative review of the literature, we are planning a Delphi study to inform guidance that will, at minimum, cover the value of case study research for evaluating the interrelationship between context and complex system-level interventions; for situating and defining ‘the case’, and generalising from case studies; as well as provide specific guidance on conducting, analysing and reporting case study research. Our hope is that such guidance can support researchers evaluating interventions in complex systems to better exploit the diversity and richness of case study research.
Availability of data and materials
Not applicable (article based on existing available academic publications)
Abbreviations
Qualitative comparative analysis
Quasi-experimental design
Randomised controlled trial
Diez Roux AV. Complex systems thinking and current impasses in health disparities research. Am J Public Health. 2011;101(9):1627–34.
Article Google Scholar
Ogilvie D, Mitchell R, Mutrie N, M P, Platt S. Evaluating health effects of transport interventions: methodologic case study. Am J Prev Med 2006;31:118–126.
Walshe C. The evaluation of complex interventions in palliative care: an exploration of the potential of case study research strategies. Palliat Med. 2011;25(8):774–81.
Woolcock M. Using case studies to explore the external validity of ‘complex’ development interventions. Evaluation. 2013;19:229–48.
Cartwright N. Are RCTs the gold standard? BioSocieties. 2007;2(1):11–20.
Deaton A, Cartwright N. Understanding and misunderstanding randomized controlled trials. Soc Sci Med. 2018;210:2–21.
Salway S, Green J. Towards a critical complex systems approach to public health. Crit Public Health. 2017;27(5):523–4.
Greenhalgh T, Papoutsi C. Studying complexity in health services research: desperately seeking an overdue paradigm shift. BMC Med. 2018;16(1):95.
Bonell C, Warren E, Fletcher A. Realist trials and the testing of context-mechanism-outcome configurations: a response to Van Belle et al. Trials. 2016;17:478.
Pallmann P, Bedding AW, Choodari-Oskooei B. Adaptive designs in clinical trials: why use them, and how to run and report them. BMC Med. 2018;16:29.
Curran G, Bauer M, Mittman B, Pyne J, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012;50(3):217–26. https://doi.org/10.1097/MLR.0b013e3182408812 .
Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ. 2015 [cited 2020 Jun 27];350. Available from: https://www.bmj.com/content/350/bmj.h1258 .
Evans RE, Craig P, Hoddinott P, Littlecott H, Moore L, Murphy S, et al. When and how do ‘effective’ interventions need to be adapted and/or re-evaluated in new contexts? The need for guidance. J Epidemiol Community Health. 2019;73(6):481–2.
Shoveller J. A critical examination of representations of context within research on population health interventions. Crit Public Health. 2016;26(5):487–500.
Treweek S, Zwarenstein M. Making trials matter: pragmatic and explanatory trials and the problem of applicability. Trials. 2009;10(1):37.
Rosengarten M, Savransky M. A careful biomedicine? Generalization and abstraction in RCTs. Crit Public Health. 2019;29(2):181–91.
Green J, Roberts H, Petticrew M, Steinbach R, Goodman A, Jones A, et al. Integrating quasi-experimental and inductive designs in evaluation: a case study of the impact of free bus travel on public health. Evaluation. 2015;21(4):391–406.
Canguilhem G. The normal and the pathological. New York: Zone Books; 1991. (1949).
Google Scholar
Hawe P, Shiell A, Riley T. Theorising interventions as events in systems. Am J Community Psychol. 2009;43:267–76.
King G, Keohane RO, Verba S. Designing social inquiry: scientific inference in qualitative research: Princeton University Press; 1994.
Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Q. 2004;82(4):581–629.
Yin R. Enhancing the quality of case studies in health services research. Health Serv Res. 1999;34(5 Pt 2):1209.
CAS PubMed PubMed Central Google Scholar
Raine R, Fitzpatrick R, Barratt H, Bevan G, Black N, Boaden R, et al. Challenges, solutions and future directions in the evaluation of service innovations in health care and public health. Health Serv Deliv Res. 2016 [cited 2020 Jun 30];4(16). Available from: https://www.journalslibrary.nihr.ac.uk/hsdr/hsdr04160#/abstract .
Craig P, Di Ruggiero E, Frohlich KL, E M, White M, Group CCGA. Taking account of context in population health intervention research: guidance for producers, users and funders of research. NIHR Evaluation, Trials and Studies Coordinating Centre; 2018.
Grant RL, Hood R. Complex systems, explanation and policy: implications of the crisis of replication for public health research. Crit Public Health. 2017;27(5):525–32.
Mahoney J. Strategies of causal inference in small-N analysis. Sociol Methods Res. 2000;4:387–424.
Turner S. Major system change: a management and organisational research perspective. In: Rosalind Raine, Ray Fitzpatrick, Helen Barratt, Gywn Bevan, Nick Black, Ruth Boaden, et al. Challenges, solutions and future directions in the evaluation of service innovations in health care and public health. Health Serv Deliv Res. 2016;4(16) 2016. https://doi.org/10.3310/hsdr04160.
Ragin CC. Using qualitative comparative analysis to study causal complexity. Health Serv Res. 1999;34(5 Pt 2):1225.
Hanckel B, Petticrew M, Thomas J, Green J. Protocol for a systematic review of the use of qualitative comparative analysis for evaluative questions in public health research. Syst Rev. 2019;8(1):252.
Schneider CQ, Wagemann C. Set-theoretic methods for the social sciences: a guide to qualitative comparative analysis: Cambridge University Press; 2012. 369 p.
Flyvbjerg B. Five misunderstandings about case-study research. Qual Inq. 2006;12:219–45.
Tsoukas H. Craving for generality and small-N studies: a Wittgensteinian approach towards the epistemology of the particular in organization and management studies. Sage Handb Organ Res Methods. 2009:285–301.
Stake RE. The art of case study research. London: Sage Publications Ltd; 1995.
Mitchell JC. Typicality and the case study. Ethnographic research: A guide to general conduct. Vol. 238241. 1984.
Gerring J. What is a case study and what is it good for? Am Polit Sci Rev. 2004;98(2):341–54.
May C, Mort M, Williams T, F M, Gask L. Health technology assessment in its local contexts: studies of telehealthcare. Soc Sci Med 2003;57:697–710.
McGill E. Trading quality for relevance: non-health decision-makers’ use of evidence on the social determinants of health. BMJ Open. 2015;5(4):007053.
Greenhalgh T. We can’t be 100% sure face masks work – but that shouldn’t stop us wearing them | Trish Greenhalgh. The Guardian. 2020 [cited 2020 Jun 27]; Available from: https://www.theguardian.com/commentisfree/2020/jun/05/face-masks-coronavirus .
Hammersley M. So, what are case studies? In: What’s wrong with ethnography? New York: Routledge; 1992.
Crowe S, Cresswell K, Robertson A, Huby G, Avery A, Sheikh A. The case study approach. BMC Med Res Methodol. 2011;11(1):100.
Luck L, Jackson D, Usher K. Case study: a bridge across the paradigms. Nurs Inq. 2006;13(2):103–9.
Yin RK. Case study research and applications: design and methods: Sage; 2017.
Hyett N, A K, Dickson-Swift V. Methodology or method? A critical review of qualitative case study reports. Int J Qual Stud Health Well-Being. 2014;9:23606.
Carolan CM, Forbat L, Smith A. Developing the DESCARTE model: the design of case study research in health care. Qual Health Res. 2016;26(5):626–39.
Greenhalgh T, Annandale E, Ashcroft R, Barlow J, Black N, Bleakley A, et al. An open letter to the BMJ editors on qualitative research. Bmj. 2016;352.
Thomas G. A typology for the case study in social science following a review of definition, discourse, and structure. Qual Inq. 2011;17(6):511–21.
Lincoln YS, Guba EG. Judging the quality of case study reports. Int J Qual Stud Educ. 1990;3(1):53–9.
Riley DS, Barber MS, Kienle GS, Aronson JK, Schoen-Angerer T, Tugwell P, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol. 2017;89:218–35.
Download references
Acknowledgements
Not applicable
This work was funded by the Medical Research Council - MRC Award MR/S014632/1 HCS: Case study, Context and Complex interventions (TRIPLE C). SP was additionally funded by the University of Oxford's Higher Education Innovation Fund (HEIF).
Author information
Authors and affiliations.
Nuffield Department of Primary Care Health Sciences, University of Oxford, Oxford, UK
Sara Paparini, Chrysanthi Papoutsi, Trish Greenhalgh & Sara Shaw
Wellcome Centre for Cultures & Environments of Health, University of Exeter, Exeter, UK
Judith Green
School of Health Sciences, University of East Anglia, Norwich, UK
Jamie Murdoch
Public Health, Environments and Society, London School of Hygiene & Tropical Medicin, London, UK
Mark Petticrew
Institute for Culture and Society, Western Sydney University, Penrith, Australia
Benjamin Hanckel
You can also search for this author in PubMed Google Scholar
Contributions
JG, MP, SP, JM, TG, CP and SS drafted the initial paper; all authors contributed to the drafting of the final version, and read and approved the final manuscript.
Corresponding author
Correspondence to Sara Paparini .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Paparini, S., Green, J., Papoutsi, C. et al. Case study research for better evaluations of complex interventions: rationale and challenges. BMC Med 18 , 301 (2020). https://doi.org/10.1186/s12916-020-01777-6
Download citation
Received : 03 July 2020
Accepted : 07 September 2020
Published : 10 November 2020
DOI : https://doi.org/10.1186/s12916-020-01777-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Qualitative
- Case studies
- Mixed-method
- Public health
- Health services research
- Interventions
BMC Medicine
ISSN: 1741-7015
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- Write for Us
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 21, Issue 1
- What is a case study?
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Roberta Heale 1 ,
- Alison Twycross 2
- 1 School of Nursing , Laurentian University , Sudbury , Ontario , Canada
- 2 School of Health and Social Care , London South Bank University , London , UK
- Correspondence to Dr Roberta Heale, School of Nursing, Laurentian University, Sudbury, ON P3E2C6, Canada; rheale{at}laurentian.ca
https://doi.org/10.1136/eb-2017-102845
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
What is it?
Case study is a research methodology, typically seen in social and life sciences. There is no one definition of case study research. 1 However, very simply… ‘a case study can be defined as an intensive study about a person, a group of people or a unit, which is aimed to generalize over several units’. 1 A case study has also been described as an intensive, systematic investigation of a single individual, group, community or some other unit in which the researcher examines in-depth data relating to several variables. 2
Often there are several similar cases to consider such as educational or social service programmes that are delivered from a number of locations. Although similar, they are complex and have unique features. In these circumstances, the evaluation of several, similar cases will provide a better answer to a research question than if only one case is examined, hence the multiple-case study. Stake asserts that the cases are grouped and viewed as one entity, called the quintain . 6 ‘We study what is similar and different about the cases to understand the quintain better’. 6
The steps when using case study methodology are the same as for other types of research. 6 The first step is defining the single case or identifying a group of similar cases that can then be incorporated into a multiple-case study. A search to determine what is known about the case(s) is typically conducted. This may include a review of the literature, grey literature, media, reports and more, which serves to establish a basic understanding of the cases and informs the development of research questions. Data in case studies are often, but not exclusively, qualitative in nature. In multiple-case studies, analysis within cases and across cases is conducted. Themes arise from the analyses and assertions about the cases as a whole, or the quintain, emerge. 6
Benefits and limitations of case studies
If a researcher wants to study a specific phenomenon arising from a particular entity, then a single-case study is warranted and will allow for a in-depth understanding of the single phenomenon and, as discussed above, would involve collecting several different types of data. This is illustrated in example 1 below.
Using a multiple-case research study allows for a more in-depth understanding of the cases as a unit, through comparison of similarities and differences of the individual cases embedded within the quintain. Evidence arising from multiple-case studies is often stronger and more reliable than from single-case research. Multiple-case studies allow for more comprehensive exploration of research questions and theory development. 6
Despite the advantages of case studies, there are limitations. The sheer volume of data is difficult to organise and data analysis and integration strategies need to be carefully thought through. There is also sometimes a temptation to veer away from the research focus. 2 Reporting of findings from multiple-case research studies is also challenging at times, 1 particularly in relation to the word limits for some journal papers.
Examples of case studies
Example 1: nurses’ paediatric pain management practices.
One of the authors of this paper (AT) has used a case study approach to explore nurses’ paediatric pain management practices. This involved collecting several datasets:
Observational data to gain a picture about actual pain management practices.
Questionnaire data about nurses’ knowledge about paediatric pain management practices and how well they felt they managed pain in children.
Questionnaire data about how critical nurses perceived pain management tasks to be.
These datasets were analysed separately and then compared 7–9 and demonstrated that nurses’ level of theoretical did not impact on the quality of their pain management practices. 7 Nor did individual nurse’s perceptions of how critical a task was effect the likelihood of them carrying out this task in practice. 8 There was also a difference in self-reported and observed practices 9 ; actual (observed) practices did not confirm to best practice guidelines, whereas self-reported practices tended to.
Example 2: quality of care for complex patients at Nurse Practitioner-Led Clinics (NPLCs)
The other author of this paper (RH) has conducted a multiple-case study to determine the quality of care for patients with complex clinical presentations in NPLCs in Ontario, Canada. 10 Five NPLCs served as individual cases that, together, represented the quatrain. Three types of data were collected including:
Review of documentation related to the NPLC model (media, annual reports, research articles, grey literature and regulatory legislation).
Interviews with nurse practitioners (NPs) practising at the five NPLCs to determine their perceptions of the impact of the NPLC model on the quality of care provided to patients with multimorbidity.
Chart audits conducted at the five NPLCs to determine the extent to which evidence-based guidelines were followed for patients with diabetes and at least one other chronic condition.
The three sources of data collected from the five NPLCs were analysed and themes arose related to the quality of care for complex patients at NPLCs. The multiple-case study confirmed that nurse practitioners are the primary care providers at the NPLCs, and this positively impacts the quality of care for patients with multimorbidity. Healthcare policy, such as lack of an increase in salary for NPs for 10 years, has resulted in issues in recruitment and retention of NPs at NPLCs. This, along with insufficient resources in the communities where NPLCs are located and high patient vulnerability at NPLCs, have a negative impact on the quality of care. 10
These examples illustrate how collecting data about a single case or multiple cases helps us to better understand the phenomenon in question. Case study methodology serves to provide a framework for evaluation and analysis of complex issues. It shines a light on the holistic nature of nursing practice and offers a perspective that informs improved patient care.
- Gustafsson J
- Calanzaro M
- Sandelowski M
Competing interests None declared.
Provenance and peer review Commissioned; internally peer reviewed.
Read the full text or download the PDF:
- Open access
- Published: 01 December 2022
Case study research and causal inference
- Judith Green ORCID: orcid.org/0000-0002-2315-5326 1 ,
- Benjamin Hanckel 2 ,
- Mark Petticrew 3 ,
- Sara Paparini 4 &
- Sara Shaw 5
BMC Medical Research Methodology volume 22 , Article number: 307 ( 2022 ) Cite this article
6774 Accesses
4 Citations
17 Altmetric
Metrics details
Case study methodology is widely used in health research, but has had a marginal role in evaluative studies, given it is often assumed that case studies offer little for making causal inferences. We undertook a narrative review of examples of case study research from public health and health services evaluations, with a focus on interventions addressing health inequalities. We identified five types of contribution these case studies made to evidence for causal relationships. These contributions relate to: (1) evidence about system actors’ own theories of causality; (2) demonstrative examples of causal relationships; (3) evidence about causal mechanisms; (4) evidence about the conditions under which causal mechanisms operate; and (5) inference about causality in complex systems. Case studies can and do contribute to understanding causal relationships. More transparency in the reporting of case studies would enhance their discoverability, and aid the development of a robust and pluralistic evidence base for public health and health services interventions. To strengthen the contribution that case studies make to that evidence base, researchers could: draw on wider methods from the political and social sciences, in particular on methods for robust analysis; carefully consider what population their case is a case ‘of’; and explicate the rationale used for making causal inferences.
Peer Review reports
Case study research is widely used in studies of context in public health and health services research to make sense of implementation and service delivery as enacted across complex systems. A recent meta-narrative review identified four broad, overlapping traditions in this body of work: developing and testing complex interventions; analysing change in organisations; undertaking realist evaluations; and studying complex change naturalistically [ 1 ]. Case studies can provide essential thick description of interventions, context and systems; qualitative understanding of the mechanisms of interventions; and evidence of how interventions are adapted in the ‘real’ world [ 2 , 3 ].
However, in evaluative health research, case study designs remain relegated to a minor, supporting role [ 4 , 5 ], typically at the bottom of evidence hierarchies. This relegation is largely due to assumptions that they offer little for making the kinds of causal claims that are essential to evaluating the effects of interventions. The strengths of deep, thick studies of specific cases are conventionally set against the benefits of ‘variable-based’ designs, with the former positioned as descriptive, exploratory or illustrative, and the latter as providing the strongest evidence for making causal claims about the links between interventions and outcomes. In conventional hierarchies of evidence, the primary evidence for making causal claims comes from randomised controlled trials (RCTs), in which the linear relationship between a change in one phenomenon and a later change in another can be delineated from other causal factors. The classic account of causality drawn on in epidemiology requires identifying that the relationship between two phenomena is characterised by co-variation; time order; a plausible relationship; and a lack of competing explanations [ 6 ]. The theoretical and pragmatic limitations of RCT designs for robust and generalizable evaluation of interventions in complex systems are now well-rehearsed [ 2 , 7 , 8 , 9 , 10 ]. In theory, though, random selection from a population to intervention exposure maximises ability to make causal claims: randomisation minimises risks of confounding, and enables both an unbiased estimate of the effect size of the intervention and extrapolation to the larger population [ 6 ]. Guidance for evaluations in which the intervention cannot be manipulated, such as in natural experiments, therefore typically focuses on methods for addressing threats to validity from non-random allocation in order to strengthen the credibility of probabilistic causal effect estimates [ 4 , 11 ].
This is, however, not the only kind of causal logic. Case study research typically draws on other logics for understanding causation and making causal inferences. We illustrate some of the contributions made by case studies, drawing on a narrative review of research relating to one particularly enduring and complex problem: inequalities in health. The causal chains linking interventions to equity outcomes are long and complex, with recognised limitations in the evidence base for ‘what works’ [ 12 ]. Case study research, we argue, has a critical role to play in making claims about whether, how and why interventions reduce, mitigate, or exacerbate inequalities. Our examples are drawn from a broader review of case study research [ 1 ] and supporting literature reviews [ 5 ], from which we focused on cases which had an explanatory aim, and which shed light on how interventions in public health or health services might reduce, create or sustain inequality. In this paper, we: i) outline some different kinds of evidence relevant to causal relationships that can be derived from case study research; ii) outline what is needed for case study research to contribute to explanatory, as well as exploratory claims; and iii) advocate for greater clarity in reporting case study research to foster discoverability.
Cases and causes
There are considerable challenges in defining case study designs or approaches in ways that adequately delineate them from other research designs. Yin [ 13 ], for instance, one of the most highly cited source texts on case studies in health research [ 1 ], resists providing a definition, instead suggesting case study research is more a strategy for doing empirical research. Gerring [ 14 ] defines case study research as: “ an intensive study of a single unit for the purpose of understanding a larger class of (similar) units ” (p342, emphasis in original). This definition is useful in suggesting the basis for the inferences drawn from cases, and the need to consider the relationships between the ‘case’ (and phenomena observed within it) and the population from which it is drawn. Gerring notes that studies of single cases may have a greater “affinity” for descriptive aims, but that they can furnish “evidence for causal propositions” ( [ 14 ], p347). Case studies are, he suggests, more likely to be useful in elucidating deterministic causes: those conditions that are necessary and/or sufficient for an outcome, whereas variable based designs have advantages for demonstrating probabilistic causation, where the aim is to estimate the likelihood of two phenomena being causally related. Case studies provide evidence for the mechanisms of causal relationships (e.g. through process tracing, through observing two variables interacting in the real world) and corroboration of causal relationships (for instance, through pattern matching).
Gerring’s argument, drawing on political science examples, is that there is nothing epistemologically distinct about research using the case study: rather, it has particular affinities with certain styles of causal modelling. We take this as a point of departure to consider not whether case studies can furnish evidence to help with causal inference in health research, but rather how they have done this. From our examples on case study research on inequalities in health, we identify the kinds of claims that relate to causality that were made. We note that some relate to (1) Actors’ accounts of causality : that is, the theories of those studied about if, how and why interventions work. Other types of claim use various kinds of comparative analytic logic to elucidate evidence of causal relationships between phenomena. These claims include: (2) Demonstrations of causal relationships – in which evidence from one case is sufficient for identifying a plausible causal relationship; (3) Mechanisms – evidence of the mechanisms through which causal relationships work; (4) Conditions —evidence of the conditions under which such mechanisms operate; and (5) Complex causality —evidence for outcomes that arise from complex causality within a system. This list is neither mutually exclusive, nor exhaustive: many case studies aim to do several of these (and some more). It is also a pragmatic rather than theoretical list, focusing on the kinds of evidence claimed by researchers rather than the formal methodological underpinnings of causal claims (for a discussion of the latter, see Rohlfing [ 15 ]).
What kinds of causal evidence do case studies provide?
Actors’ accounts of causality.
This is perhaps the most common kind of evidence provided by case study research. Case studies, through in-depth research on the actors within systems, can generate evidence about how those actors themselves account for causal relationships between interventions and outcomes. This is an overt aim of many realist evaluation studies, which focus on real forces or processes that exist in the world that can provide insight into causal mechanisms for change.
Ford and colleagues [ 16 ], for example, used a series of five case studies of local health systems to explore socio-economic inequalities in unplanned hospital admission. Cases were selected on the basis of either narrowing or widening inequalities in admission, with a realist evaluation focused on delineating the context-mechanisms-outcome (CMO) configurations in each setting, to develop a broader theory of change for addressing inequalities. The case study approach used a mix of methods, including drawing on documentary data to assess the credibility of mechanisms proposed by health providers. The authors identified 17 distinct CMO configurations; and five factors that were related to trends for inequalities in emergency admissions, including health service factors (primary care workforce challenges, case finding and proactive case management) and those external to the health service (e.g., financial constraints on public services, residential gentrification). Ford and colleagues noted that none of the CMO configurations were clearly associated with improved or worsening trends in inequalities in admission.
Clearly, actors’ accounts of causality are not in themselves evidence of causality. Ford and colleagues noted that they interrogated accounts for plausibility (e.g. that interventions mentioned were prior to effects claimed) and triangulated these accounts with other sources of data, but that inability to empirically corroborate the hypothesized CMO links limited their ability to make claims about causal inference. This is crucial: actors in a system may be aware of the forces and processes shaping change but unaware of counterfactuals, and they are unlikely to have any privileged insight into whether factors are causal or simply co-occurring (see, for instance, Milton et. al. [ 17 ] on how commonly cited ‘barriers’ in accounts of not doing evaluations are also evident in actors’ accounts of doing successful evaluations). Over-interpretation of qualitative accounts of insiders’ claims about causal relationships as if they provide conclusive evidence of causal relationships is poor methodology.
This does not mean that actors’ accounts are not of value. First, in realist evaluation, as in Ford and colleagues’ study [ 16 ], these accounts provide the initial theories of change for thinking about the potential causal pathways in logic models of interventions. Second, insiders’ accounts of causality are part of the system that is being explained. An example comes from Mead and colleagues [ 18 ], who used a case study drawing largely on qualitative interviews to explore “how local actors from public health, and the wider workforce, make sense of and work on social inequalities in health” ( [ 18 ] p168). This used a case study of a partnership in northwest England to address an enduring challenge in inequalities policy: the tendency for policies that address upstream health determinants to transform, in practice, to focus more on behavioural and individual level factors . Local public health actors in the partnership recognised the structural causes of unequal health outcomes, yet discourses of policy action tended to focus only on the downstream, more individualising levels of health, and on personal choice and agency as targets for intervention. Professionals conceptualised action on inequality as relating only to the health of the poorest, rather than as a problem of a gradient in health outcomes across society. There was a geographical localism in their approach, which framed particular places as constellations of health and social problems. Drawing on theory from figurational sociology, Mead and colleagues note that actors’ own accounts are the starting point of an analysis, which then puts those accounts into play with theory about how such discourses are reproduced. The researchers suggest that partnership working itself exacerbated the individualising frameworks used to orient action, as it became a hegemonic framing, reducing the possibilities for partnerships to transform health inequalities. Here, then, a case study approach is used to shed light on the causes of a common failure in policies addressing inequalities. The authors take seriously the divergence of actors’ own accounts of causality and those of other sources, and analyse these as part of the system.
Finally, insider accounts should be taken seriously as contributing to evidence about causal inference through shedding light on the complex looping effects of theoretical models of causality and public accounts. For instance, Smith and Anderson [ 19 ], drawing on a meta-ethnographic literature review of ‘lay theorising’ about health inequalities, note that, counter to common assumptions, public understanding of the structural causes of health inequalities is sophisticated: but that it may be disavowed to avoid stigma and shame and to reassert some agency. This is an important finding for informing knowledge exchange, suggesting that further ‘awareness raising’ may be unnecessary for policy change, and counter-productive in needlessly increasing stigma and shame.
Demonstrations of causal relationships
When strategically sampled, and rooted in a sound theoretical framework, studies of single cases can provide evidence for generalizable causal inferences. The strongest examples are perhaps those that operate as ‘black swans’ for deterministic claims, in that one case may be all that is needed to show that a commonly held assumption is not generalizable. That is, a case study can demonstrate unequivocally that one phenomenon is not inevitably related to another. These can come from cases sampled because they are extreme or unusual. Prior’s [ 20 ] study of a single man in a psychiatric institution in Northern Ireland, for instance, showed that, counter to Goffman’s [ 21 ] original theory of how ‘total institutions’ lead to stigmatisation and depersonalisation, the effects of institutionalisation depended on context—in this case, how the institution related to the local community and the availability of alternative sources of self-worth available to residents.
Strategically sampled typical cases can also provide demonstrative evidence of causal relationships. To take the enduring health services challenge of inequalities in self-referral to emergency care, Hudgins and Rising’s [ 22 ] case study of a single patient is used to debunk a common assumption that high use of emergency care is related to inappropriate care-seeking by low-income patients. They look in detail at the case of “a 51-year-old low-income, recently insured, African American man in Philadelphia (USA) who had two recent ED [emergency department] visits for evaluation of frequent headaches and described fear of being at risk for a stroke.” ( [ 22 ] p50). Drawing on theories of structural violence and patient subjectivity, they use this single case to shed light on why emergency department use may appear inappropriate to providers. They analyse the interplay of gender roles, employment, and insurance status in generating competing drivers of health seeking, and point to the ways in which current policies deterring self-referral do not align well with micro- and macro-level determinants of service use. The study authors also note that because their methods generate data on ‘why’ as well ‘what’ people do, they can “lay the groundwork” ( [ 22 ], p54] for developing future interventions. Here, again, a single case is sufficient. In understanding the causal pathways that led to this patient’s use of emergency care, it is clear why policies addressing inequalities through deterring low-income users would be unlikely to work.
Mechanisms: how causal relationships operate
A strength of case study approaches compared with variable-based designs is furnishing evidence of how causal relationships operate, deriving from both direct observations of causal processes and from analysis of comparisons within and between cases. All cases contain multiple observations; variations can be observed over time and space, across or within cases [ 14 ]. Observing regularities, co-variation and deviant or surprising findings, and then using processes of analytic induction [ 23 ] or abductive logic [ 24 ] to derive, develop and test causal theories using observations from the case, can build a picture of causal pathways.
Process tracing is one formal qualitative methodology for doing this. Widely used in political and policy studies, but less in health evaluations [ 25 ], process tracing links outcomes with their causes, focusing on the mechanisms that link events on causal pathways, and on the strength of evidence for making connections on that causal chain. This requires sound theoretical knowledge (such that credible hypotheses can be developed), well described cases (ideally at different time points), observed causal processes (the activities that transfer causes to effects), and careful assessment of evidence against tests of varying strength for the necessity and sufficiency for accepting or rejecting a candidate hypothesis [ 26 , 27 ]. In health policy, process tracing methods have been combined to good effect with quantitative measures to examine casual processes leading to outcomes of interest. Campbell et. al. [ 28 ], for instance, used process tracing to look at four case studies of countries that had made progress towards universal health coverage (measured through routine data on maternal and neonatal health indicators), to identify key causal factors related to health care workforce.
An example of the use of process tracing in evaluation comes from Lohmann and colleagues’ [ 25 ] case study of a single country, Burkina Faso, to examine why performance based financing (PBF) fails to improve equity. PBF, coupled with interventions to improve health care take up among the poor, aims to improve health equity in low and middle-income countries, yet impact evaluations suggest that these benefits are typically not realised. This case study drew on data from the quantitative impact assessment; programme documentation; the intervention process evaluation; and primary qualitative research for the process tracing, in the light of the theory of change of the intervention. Lohmann and colleagues [ 25 ] identified that a number of conditions that would have been necessary for the intervention to work had not been met (such as eligible patients not receiving the card needed to access health care or providers not receiving timely reimbursement). A key finding was that although implementation challenges were a partial cause of policy failure, other causal conditions were external to the intervention, such as lack of attention to the non-health care costs incurred by the poorest to access care. Again, a single case, if there are good grounds for extrapolating to similar contexts (i.e., those in which transport is required to access health care), is enough to demonstrate a necessary part of the causal pathway between PBF and intended equity outcomes.
Conditions under which causal mechanisms operate
The example of ‘transport access’ as a necessary condition for PBF interventions to ‘work’ also illustrates a fourth type of causal evidence: that relating to the transferability of interventions. Transferable causal claims are essential for useful evidence: “(f)or policy and practice we do not need to know ‘it works somewhere’. We need evidence for ‘it-will-work-for-us’ claims: the treatment will produce the desired outcome in our situation as implemented there” ( [ 8 ] p1401). Some causal mechanisms operate widely (using a parachute will reduce injury from jumping from a plane; taking aspirin will relieve pain); others less so. In the context of health services and public health research, few interventions are likely to be widely generalizable, as the mechanisms will operate differently across contexts [ 7 ]. This context dependency is at the heart of realist evaluations, with the assumption that underlying causal mechanisms require particular contexts in order to operate, hence the focus on ‘how, where, and for whom’ interventions work [ 29 ]. Making useful claims therefore requires other kinds of evidence, relating to what Cartwright and Munro [ 30 ] call the ‘capacities’ of the intervention: what power it has to work reliably, what stops it working, what other conditions are needed for it to work. This evidence is critical for assessing whether an intervention is likely to work in a given context and to assess the intended and unintended consequences of intervention adoption and implementation. Cartwright and Munro’s recommendation is therefore to study causal powers rather than causes. That is, as well as interrogating whether the intervention ‘causes’ a particular outcome, it is also necessary to address the potential for and stability of that causal effect. To do that entails addressing a broader range of questions about the causal relationship, such as how the intervention operates in order to bring about changes in outcomes; what other conditions need to be present; what might constrain this effect; what other factors within the system also promote or constrain those effects; and what happens when different capacities interact? [ 30 ]. Case study research can be vital in providing this kind of evidence on the capacities of interventions [ 31 ].
One example is from Gibson and colleagues [ 32 ], who use within-case comparisons to shed light on why a ‘social prescribing’ intervention may have different effects across socioeconomic classes. These interventions, typically entailing link workers who connect people with complex health care needs to local services and resources, are often framed as a way to address enduring health inequalities. Drawing on sociological theory on how social class is reproduced through socially structured and unequal distribution of resources (‘capitals’), and through how these shape people’s practices and dispositions, Gibson and colleagues [ 32 ] explicate how capitals and dispositions shaped encounters with the intervention. Their analysis of similarities and differences within their case (of different clients) in the context of theory enables them to abstract inferences from the case. Drawing out the ways in which more advantaged clients mobilised capital in their pursuit of health, with dispositions more closely aligned to the intervention, they unravel classed differences in ability to benefit from the intervention, with less advantaged clients inevitably having ‘shorter horizons’ focused on day to day challenges: “This challenges the claim that social prescribing can reduce inequalities, instead suggesting it has the potential to exacerbate existing inequalities” ( [ 32 ], p6).
Case studies can shed light on the capacities of interventions to improve or exacerbate inequalities, including identifying unforeseen consequences. Hanckel and colleagues [ 33 , 34 ], for example, used a case study approach to explore implementation of a physical health intervention involving whole classes of children running for 15 min each day in the playground in schools in south London, UK. This documented considerable adaption of the intervention at the level of school, class and pupil, and identified different pathways through which the intervention might impact on inequalities. In terms of access, the intervention appeared to be equitable, in that there was no evidence of disproportionate roll out to schools with more affluent pupils or to those with fewer minority ethnic pupils [ 33 ]. However, identifying the ‘capacities’ of the intervention also identified other pathways through which it could have negative equity effects. The authors found that in practice, the intervention emphasised body weight rather than physical activity, and intervention roll-out reinforced class and ethnicity-based stigmatising discourses about lower income neighbourhoods [ 34 ].
Complex causality
There is increasing recognition that the systems that reproduce unequal health outcomes are complex: that is, that they consist of multiple interacting components that cannot be studied in isolation, and that change is likely to be non-linear, characterised by, for instance, phase shifts or feedback loops [ 35 ]. This has two rather different implications. First, case study designs can be particularly beneficial for taking a system perspective on interventions. Case studies enable a focus on aspects that are not well explicated through other designs, such as how context interacts with interventions within systems [ 7 ], or on how multiple conditional pathways might link interventions and outcomes [ 36 ]. Second, when causation is not linear, but ‘emergent’, in that it is not reducible to the accumulated changes at lower levels, evaluation designs focused on only one outcome at one level (such as weight loss in individuals) may fail to identify important effects. Case studies have an invaluable role here in unpacking and surfacing these effects at different levels within the systems within which interventions and services are delivered. One example is transport systems, which have been the focus of considerable public health interest to encourage more ‘active’ modes, in which more of the population walk or cycle, and fewer drive. However, more simplistic evaluations looking at one part of a causal chain (such as that between traffic calming interventions and local mode shift) may fail to appreciate how systems are dynamic, and that causation might be emergent. This is evident in a case study of transport policy impacts from Sheller [ 37 ], who takes the case of Philadelphia, USA, to reveal how this post-car trend has racialized effects that can exacerbate inequality. Weaving in data from participant observations, historical documentary sources and statistical evidence of declining car use, Sheller documents the racialized impacts of transport policies which may have reduced car use and encouraged active modes overall, but which have largely prioritised ‘young white’ mobility in the context of local gentrification and neglect of public transit.
One approach to synthesising evidence from multiple case studies to make claims about complex causation is Qualitative Comparative Analysis (QCA), which combines quantitative methods (based on Boolean algebra) with detailed qualitative understanding of a small to medium N sample of cases. This has strengths for identifying multiple pathways to outcomes, asymmetrical sets of conditions which lead to success or failure, or ‘conjunctural causation’, whereby some conditions are only causally linked to outcomes in relation to others [ 38 ]. There is growing interest in using these approaches in evaluative health studies [ 39 ]. One example relating to the effectiveness of interventions addressing inequalities in health comes from Blackman and colleagues [ 36 ], who explored configurations of conditions which did or did not lead to narrowing inequalities in teenage conception rates across a series of local areas as cases. This identified some surprising findings, including that ‘basic’ rather than good or exemplary standards of commissioning were associated with narrowing the equity gap, and that the proportion of minority ethnic people in the population was a key condition.
Not all case study research aims to contribute to causal inference, and neither should it [ 1 , 5 , 40 ]. However, it can. We have identified five ways in which case study evidence has contributed to causal explanations in relation to a particularly intractable challenge: inequalities in health. It is therefore time to stop claiming that case study designs have only a supporting role to play in evaluative health research. To develop a theoretical evidence base on ‘what works’, and how, in health services and public health, particularly around complex issues such as addressing unequal health outcomes, we need to draw on a greater range of evidential resources for informing decisions than is currently used. Best explanations are unlikely to be made from single studies based on one kind of causality, but instead will demand some kind of evidential pluralism [ 41 ]. That is, one single study, of any design, is unlikely to generate evidence for all links in complex causal chains between an intervention and health outcomes. We need a bricolage of evidence from a diverse range of designs [ 42 ] to make robust and credible cases for what will improve health and health equity. This will include evidence from case studies, both from single and small N studies, and from syntheses of findings from multiple cases.
Our focus on case studies that shed light on interventions for health inequalities identified the critical role that case studies can play in theorising, illuminating and making sense of: system actors’ own causal reasoning; whether there are causal links between intervention and outcome; what mechanism(s) might link them; when, where and for whom these causal relationships operate; and how unequal outcomes can be generated from the operation of complex systems. These examples draw on a range of different theoretical and methodological approaches, often from the wider political and social sciences. The approaches illustrated are rooted in very different, even incompatible, philosophical traditions: what researchers understand by ‘causality’ is diverse [ 43 ]. However, there are two commonalities across this diversity that suggest some conditions for producing good case studies that can generate evidence to support causal inferences. The first is the need for theoretically informed and comparative analysis. As Gerring [ 14 ] notes, causal inferences rely on comparisons – across units or time within a case, or between cases. It is comparison that drives the ability to make claims about the potential of interventions to produce change in outcomes of interest, and under what conditions. There are a range of approaches to qualitative data analysis, and choice of method has to be appropriate for the kinds of causal logics being explicated, and the availability of data on particular phenomena within the case. Typically, though, this will require analysis that goes beyond descriptive thematic analysis [ 31 ]. Approaches such as process tracing or analytic induction require both fine-grained and rigorous comparative analysis, and a sound theoretical underpinning that provides a framework for making credible inferences about the relationships between phenomena within the case and to the wider population from which the case is selected.
This leads to the second commonality: the need to clarify what the case is a case ‘of’, and how it relates to other candidate cases. What constitutes a ‘case’ is inevitably study specific. The examples we have drawn on include: PBF in a country [ 25 ], transport systems in a city [ 37 ], and a social prescribing intervention in primary care [ 32 ]. Clearly, in other contexts, each of these ‘cases’ could be sampling units within variable based studies (of financing systems, or countries; of infrastructures systems, or cities in a state; of particular kinds of service intervention, or primary care systems). Conversely, these cases could be populations within which lower level phenomena (districts, neighbourhoods, patients) are studied. What leads to appropriate generalisations about causal claims is a sound theorisation of the similarities and particularities of the case compared with other candidate cases: how Burkina Faso has commonalities with, or differences from, other settings in which PBF has failed to improve equity; or the contexts of gentrification and residential churn that make Philadelphia similar to other cities in the US; or the ways in which class-based dispositions and practices intersect with similar types of service provisions.
A critical question remains: How can well-conducted case study evidence be better integrated into the evidence base? Calls for greater recognition for case study designs within health research are hardly new: Flyvberg’s advocacy for a greater role for case studies in the social sciences [ 44 ] has now been cited around 20,000 times, and calls for methodological pluralism in health research go back decades [ 42 , 45 , 46 ]. Yet, case studies remain somewhat neglected, with ongoing misconceptions about their limited role, despite calls for evidence based medicine to incorporate evidence for mechanisms as complementary to evidence of correlation, rather than as inferior [ 47 ]. Even where the value of case studies for contributing to causal inference is recognised, searching for good evidence is not straightforward. Case studies are neither consistently defined nor necessarily well reported. Some of the examples in this paper do not use the term ‘case study’ in the title or abstract, although they meet our definition. Conversely, many small scale qualitative studies describe themselves as ‘case studies’, but focus on thick description rather than generalisability, and are not aiming to contribute to evaluative evidence. It is therefore challenging, currently, to undertake a more systematic review of empirical material. Forthcoming guidance on reporting case studies of context in complex systems aims to aid discoverability and transparency of reporting (Shaw S, et al: TRIPLE C Reporting Principles for Case study evaluations of the role of Context in Complex interventions, under review). This recommends including ‘case study’ in the title, clarifying how terms are used, and explicating the philosophical base of the study. To further advance the usefulness of case study evidence, we suggest that where an aim is to contribute to causal explanations, researchers should, in addition, specify their rationales for making causal inferences, and identify what broader class of phenomena their case is a case ‘of’.
Conclusions
Case study research can and does contribute to evidence for causal inferences. On challenging issues such as addressing health inequalities, we have shown how case studies provide more than detailed description of context or process. Contributions include: describing actors’ accounts of causal relationships; demonstrating theoretically plausible causal relationships; identifying mechanisms which link cause and effect; identifying the conditions under which causal relationships hold; and researching complex causation.
Availability of data and materials
Not applicable; no new data generated in this study.
For the purpose of open access, the author has applied a ‘Creative Commons Attribution (CC BY) licence to any Author Accepted Manuscript version arising.
Abbreviations
Context-mechanism-outcome
Performance based financing
Qualitative Comparative Analysis
Randomised controlled trial
Paparini S, Papoutsi C, Murdoch J, Green J, Petticrew M, Greenhalgh T, Shaw SE. Evaluating complex interventions in context: systematic, meta-narrative review of case study approaches. BMC medical research methodology. 2021;21(1):1–22. https://doi.org/10.1186/s12874-021-01418-32 .
Byrne D. Evaluating complex social interventions in a complex world. Evaluation. 2013;19(3):217–28.
Article Google Scholar
Craig PD, Di Ruggiero E, Frohlich KL, Mykhalovskiy E, White M. On behalf of the Canadian Institutes of Health Research (CIHR)–National Institute for Health Research (NIHR) context guidance authors group. Taking account of context in population health intervention research: guidance for producers, users and funders of research. Southampton: NIHR Evaluation, Trials and Studies Coordinating Centre. 2018.
Craig P, Katikireddi SV, Leyland A, Popham F. Natural experiments: an overview of methods, approaches, and contributions to public health intervention research. Annu Rev Public Health. 2017;20(38):39. https://doi.org/10.1146/annurev-publhealth-031816-044327 .
Paparini S, Green J, Papoutsi C, Murdoch J, Petticrew M, Greenhalgh T, Hanckel B, Shaw S. Case study research for better evaluations of complex interventions: rationale and challenges. BMC Med. 2020;18(1):1–6. https://doi.org/10.1186/s12916-020-01777-6 .
Shadish WR, Cook TD, Campbell DT. Experimental and quasi-experimental designs for generalized causal inference. Mifflin and Company: Houghton; 2002.
Google Scholar
Wells M, Williams B, Treweek S, Coyle J, Taylor J. Intervention description is not enough: evidence from an in-depth multiple case study on the untold role and impact of context in randomised controlled trials of seven complex interventions. Trials. 2012;13(1):1–7.
Cartwright N. A philosopher’s view of the long road from RCTs to effectiveness. The Lancet. 2011;377(9775):1400–1.
Cartwright N. What evidence should guidelines take note of? J Eval Clin Pract. 2018;24(5):1139–44.
Article PubMed Google Scholar
Deaton A, Cartwright N. Understanding and misunderstanding randomized controlled trials. Soc Sci Med. 2018;1(210):2–1.
Wing C, Simon K, Bello-Gomez RA. Designing difference in difference studies: best practices for public health policy research. Annu Rev Public Health. 2018;39(1):453–69.
Petticrew M, Tugwell P, Welch V, Ueffing E, Kristjansson E, Armstrong R, Doyle J, Waters E. Better evidence about wicked issues in tackling health inequities. J Public Health. 2009;31(3):453–6.
Yin R. Case Study Research and Applications: Design and Methods. 6th Edition. Thousand Oaks: Sage; 2018.
Gerring J. What is a case study and what is it good for? Am pol Sci Rev. 2004;98(2):341–54.
Rohlfing I. Case studies and causal inference: An integrative framework. Springer. 2012.
Book Google Scholar
Ford J, Knight J, Brittain J, Bentley C, Sowden S, Castro A, Doran T, Cookson R. Reducing inequality in avoidable emergency admissions: Case studies of local health care systems in England using a realist approach. J Health Serv Res Policy. 2022;27(1):31–40.
Milton S, Petticrew M, Green J. Why do local authorities undertake controlled evaluations of health impact? A qualitative case study of interventions in housing. Public Health. 2014;128(12):1112–7.
Article CAS PubMed Google Scholar
Mead R, Thurston M, Bloyce D. From public issues to personal troubles: individualising social inequalities in health within local public health partnerships. Crit Public Health. 2022;32(2):168–80.
Smith KE, Anderson R. Understanding lay perspectives on socioeconomic health inequalities in Britain: a meta-ethnography. Sociol Health Illn. 2018;40(1):146–70.
Prior PM. Surviving psychiatric institutionalisation: a case study. Sociol Health Illn. 1995;17(5):651–67.
Goffman E. Essays on the social situation of mental patients and other inmates. New York: Doubelday; 1961.
Hudgins A, Rising KL. Fear, vulnerability and sacrifice: Drivers of emergency department use and implications for policy. Soc Sci Med. 2016;1(169):50–7.
Robinson WS. The logical structure of analytic induction. Case study method: key issues, key texts. Am Sociol Rev. 1951;16(6):812–8.
Peirce, C. S ‘Abduction and induction’ in Buchler J (ed) Philosophical Writings of Peirce. New York, Dover Publications, (1955 [1901]).
Lohmann J, Koulidiati JL, Robyn PJ, Somé PA, De Allegri M. Why did performance-based financing in Burkina Faso fail to achieve the intended equity effects? A process tracing study. Soc Sci Med. 2022;25:115065.
Mahoney J. The logic of process tracing tests in the social sciences. Sociol Methods Res. 2012;41(4):570–97.
Beach D, Pedersen RB. Process-tracing methods: Foundations and guidelines. University of Michigan Press. 2019.
Campbell J, Buchan J, Cometto G, David B, Dussault G, Fogstad H, Fronteira I, Lozano R, Nyonator F, Pablos-Méndez A, Quain EE. Human resources for health and universal health coverage: fostering equity and effective coverage. Bull World Health Organ. 2013;91:853–63.
Article PubMed PubMed Central Google Scholar
Pawson R, Tilley N. Scientific Realist Evaluation. Evaluation for the 21st century: A handbook. 1997 Jan 28:405.
Cartwright N, Munro E. The limitations of randomized controlled trials in predicting effectiveness. J Eval Clin Pract. 2010;16(2):260–6.
Green J, Roberts H, Petticrew M, Steinbach R, Goodman A, Jones A, Edwards P. Integrating quasi-experimental and inductive designs in evaluation: a case study of the impact of free bus travel on public health. Evaluation. 2015;21(4):391–406.
Gibson K, Pollard TM, Moffatt S. Social prescribing and classed inequality: A journey of upward health mobility? Soc Sci Med. 2021;1(280):114037.
Hanckel B, Ruta D, Scott G, Peacock JL, Green J. The Daily Mile as a public health intervention: a rapid ethnographic assessment of uptake and implementation in South London. UK BMC Public Health. 2019;19(1):1–4.
Hanckel B, Milton S, Green J. Unruly bodies: resistance,(in) action and hysteresis in a public health intervention. Social Theory Health. 2021;19(3):263–81.
Greenhalgh T, Papoutsi C. Studying complexity in health services research: desperately seeking an overdue paradigm shift. BMC Med. 2018;16(1):1–6.
Blackman T, Wistow J, Byrne D. Using qualitative comparative analysis to understand complex policy problems. Evaluation. 2013;19(2):126–40.
Sheller M. Racialized mobility transitions in Philadelphia: connecting urban sustainability and transport justice. City Society. 2015;27(1):70–91.
Ragin CC. Using qualitative comparative analysis to study causal complexity. Health Serv Res. 1999;34(5 Pt 2):1225.
CAS PubMed PubMed Central Google Scholar
Hanckel B, Petticrew M, Thomas J, Green J. The use of Qualitative Comparative Analysis (QCA) to address causality in complex systems: a systematic review of research on public health interventions. BMC Public Health. 2021;21(1):1–22.
Thomas G. A typology for the case study in social science following a review of definition, discourse, and structure. Qual Inq. 2011;17(6):511–21.
Johnson RB, Russo F, Schoonenboom J. Causation in mixed methods research: The meeting of philosophy, science, and practice. J Mixed Methods Res. 2019;13(2):143–62.
Ogilvie D, Bauman A, Foley L, Guell C, Humphreys D, Panter J. Making sense of the evidence in population health intervention research: building a dry stone wall. BMJ Glob Health. 2020;5(12):e004017. https://doi.org/10.1136/bmjgh-2020-004017 .
Rohlfing I, Zuber CI. Check your truth conditions! Clarifying the relationship between theories of causation and social science methods for causal inference. Sociol Methods Res. 2021;50(4):1623–59.
Flyvbjerg B. Five misunderstandings about case-study research. Qual Inq. 2006;12(2):219–45.
Baum F. Researching public health: behind the qualitative-quantitative methodological debate. Soc Sci Med. 1995;40(4):459–68.
Petticrew M, Roberts H. Evidence, hierarchies, and typologies: horses for courses. J Epidemiol Community Health. 2003;57(7):527–9.
Article CAS PubMed PubMed Central Google Scholar
Clarke B, Gillies D, Illari P, Russo F, Williamson J. Mechanisms and the evidence hierarchy. Topoi. 2014;33(2):339–60.
Download references
Acknowledgements
The research underpinning this paper was conducted as part of the Triple C study. We gratefully acknowledge the input of the wider study team, and that of the participants at a workshop held to discuss forthcoming guidance on reporting case study research.
The research was funded by the Medical Research Council (MR/S014632/1). JG is supported with funding from the Wellcome Trust (WT203109/Z/16/Z). Additional funding for SP and SS salaries over the course of the study was provided by the UK National Institute for Health Research Oxford Biomedical Research Centre (BRC-1215–20008), Wellcome Trust (WT104830MA; 221457/Z/20/Z) and the University of Oxford's Higher Education Innovation Fund.
The views and opinions expressed herein are those of the authors. Funding bodies had no input to the design of the study and collection, analysis, and interpretation of data or preparation of this paper.
Author information
Authors and affiliations.
Wellcome Centre for Cultures & Environments of Health, University of Exeter, Exeter, UK
Judith Green
Institute for Culture and Society, Western Sydney University, Sydney, Australia
Benjamin Hanckel
Department of Public Health, Environments & Society, London School of Hygiene & Tropical Medicine, London, UK
Mark Petticrew
Wolfson Institute of Population Health, Queen Mary University of London, London, UK
Sara Paparini
Nuffield Department of Primary Care Health Sciences, University of Oxford, Oxford, UK
You can also search for this author in PubMed Google Scholar
Contributions
BH, JG and MP drafted the first version of the paper, which was revised with theoretical input from SS and SP. All authors contributed to the paper and have reviewed and approved the final manuscript.
Corresponding author
Correspondence to Judith Green .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Green, J., Hanckel, B., Petticrew, M. et al. Case study research and causal inference. BMC Med Res Methodol 22 , 307 (2022). https://doi.org/10.1186/s12874-022-01790-8
Download citation
Received : 12 August 2022
Accepted : 10 November 2022
Published : 01 December 2022
DOI : https://doi.org/10.1186/s12874-022-01790-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Causal inference
- Public health
- Health services research
BMC Medical Research Methodology
ISSN: 1471-2288
- General enquiries: [email protected]
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
Methodology
- What Is a Case Study? | Definition, Examples & Methods
What Is a Case Study? | Definition, Examples & Methods
Published on May 8, 2019 by Shona McCombes . Revised on November 20, 2023.
A case study is a detailed study of a specific subject, such as a person, group, place, event, organization, or phenomenon. Case studies are commonly used in social, educational, clinical, and business research.
A case study research design usually involves qualitative methods , but quantitative methods are sometimes also used. Case studies are good for describing , comparing, evaluating and understanding different aspects of a research problem .
Table of contents
When to do a case study, step 1: select a case, step 2: build a theoretical framework, step 3: collect your data, step 4: describe and analyze the case, other interesting articles.
A case study is an appropriate research design when you want to gain concrete, contextual, in-depth knowledge about a specific real-world subject. It allows you to explore the key characteristics, meanings, and implications of the case.
Case studies are often a good choice in a thesis or dissertation . They keep your project focused and manageable when you don’t have the time or resources to do large-scale research.
You might use just one complex case study where you explore a single subject in depth, or conduct multiple case studies to compare and illuminate different aspects of your research problem.
Prevent plagiarism. Run a free check.
Once you have developed your problem statement and research questions , you should be ready to choose the specific case that you want to focus on. A good case study should have the potential to:
- Provide new or unexpected insights into the subject
- Challenge or complicate existing assumptions and theories
- Propose practical courses of action to resolve a problem
- Open up new directions for future research
TipIf your research is more practical in nature and aims to simultaneously investigate an issue as you solve it, consider conducting action research instead.
Unlike quantitative or experimental research , a strong case study does not require a random or representative sample. In fact, case studies often deliberately focus on unusual, neglected, or outlying cases which may shed new light on the research problem.
Example of an outlying case studyIn the 1960s the town of Roseto, Pennsylvania was discovered to have extremely low rates of heart disease compared to the US average. It became an important case study for understanding previously neglected causes of heart disease.
However, you can also choose a more common or representative case to exemplify a particular category, experience or phenomenon.
Example of a representative case studyIn the 1920s, two sociologists used Muncie, Indiana as a case study of a typical American city that supposedly exemplified the changing culture of the US at the time.
While case studies focus more on concrete details than general theories, they should usually have some connection with theory in the field. This way the case study is not just an isolated description, but is integrated into existing knowledge about the topic. It might aim to:
- Exemplify a theory by showing how it explains the case under investigation
- Expand on a theory by uncovering new concepts and ideas that need to be incorporated
- Challenge a theory by exploring an outlier case that doesn’t fit with established assumptions
To ensure that your analysis of the case has a solid academic grounding, you should conduct a literature review of sources related to the topic and develop a theoretical framework . This means identifying key concepts and theories to guide your analysis and interpretation.
There are many different research methods you can use to collect data on your subject. Case studies tend to focus on qualitative data using methods such as interviews , observations , and analysis of primary and secondary sources (e.g., newspaper articles, photographs, official records). Sometimes a case study will also collect quantitative data.
Example of a mixed methods case studyFor a case study of a wind farm development in a rural area, you could collect quantitative data on employment rates and business revenue, collect qualitative data on local people’s perceptions and experiences, and analyze local and national media coverage of the development.
The aim is to gain as thorough an understanding as possible of the case and its context.
Receive feedback on language, structure, and formatting
Professional editors proofread and edit your paper by focusing on:
- Academic style
- Vague sentences
- Style consistency
See an example

In writing up the case study, you need to bring together all the relevant aspects to give as complete a picture as possible of the subject.
How you report your findings depends on the type of research you are doing. Some case studies are structured like a standard scientific paper or thesis , with separate sections or chapters for the methods , results and discussion .
Others are written in a more narrative style, aiming to explore the case from various angles and analyze its meanings and implications (for example, by using textual analysis or discourse analysis ).
In all cases, though, make sure to give contextual details about the case, connect it back to the literature and theory, and discuss how it fits into wider patterns or debates.
If you want to know more about statistics , methodology , or research bias , make sure to check out some of our other articles with explanations and examples.
- Normal distribution
- Degrees of freedom
- Null hypothesis
- Discourse analysis
- Control groups
- Mixed methods research
- Non-probability sampling
- Quantitative research
- Ecological validity
Research bias
- Rosenthal effect
- Implicit bias
- Cognitive bias
- Selection bias
- Negativity bias
- Status quo bias
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
McCombes, S. (2023, November 20). What Is a Case Study? | Definition, Examples & Methods. Scribbr. Retrieved April 9, 2024, from https://www.scribbr.com/methodology/case-study/
Is this article helpful?
Shona McCombes
Other students also liked, primary vs. secondary sources | difference & examples, what is a theoretical framework | guide to organizing, what is action research | definition & examples, what is your plagiarism score.
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Call for Papers
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 7, Issue 2
- Fundamentals of case study research in family medicine and community health
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0003-1141-7613 Sergi Fàbregues 1 and
- Michael D Fetters 2
- 1 Department of Psychology and Education , Universitat Oberta de Catalunya , Barcelona , Spain
- 2 Department of Family Medicine , University of Michigan , Ann Arbor , Michigan , USA
- Correspondence to Dr Sergi Fàbregues, Department of Psychology and Education, Universitat Oberta de Catalunya, Rambla del Poblenou, 156, 08018, Barcelona, Spain; sfabreguesf{at}uoc.edu
The aim of this article is to introduce family medicine researchers to case study research, a rigorous research methodology commonly used in the social and health sciences and only distantly related to clinical case reports. The article begins with an overview of case study in the social and health sciences, including its definition, potential applications, historical background and core features. This is followed by a 10-step description of the process of conducting a case study project illustrated using a case study conducted about a teaching programme executed to teach international family medicine resident learners sensitive examination skills. Steps for conducting a case study include (1) conducting a literature review; (2) formulating the research questions; (3) ensuring that a case study is appropriate; (4) determining the type of case study design; (5) defining boundaries of the case(s) and selecting the case(s); (6) preparing for data collection; (7) collecting and organising the data; (8) analysing the data; (9) writing the case study report; and (10) appraising the quality. Case study research is a highly flexible and powerful research tool available to family medicine researchers for a variety of applications.
- Case study research
- research design
- mixed methods
- family practice
- primary care
- general practice
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0
https://doi.org/10.1136/fmch-2018-000074
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Significance statement
Given their potential for answering ‘how’ and ‘why’ questions about complex issues in their natural setting, case study designs are being increasingly used in the health sciences. Conducting a case study can, however, be a complex task because of the possibility of combining multiple methods and the need to choose between different types of case study designs. In order to introduce family medicine and community health researchers to the fundamentals of case study research, this article reviews its definition, potential applications, historical background and main characteristics. It follows on with a practical, step-by-step description of the case study process that will be useful to researchers interested in implementing this research design in their own practice.
Introduction
This article provides family medicine and community health researchers a concise resource to conduct case study research. The article opens with an overview of case study in the social and health sciences, including its definition, potential applications, historical background and core features. This is followed by a 10-step description of the process of conducting a case study project, as described in the literature. These steps are illustrated using a case study about a teaching programme executed to teach international medical learners sensitive examination skills. The article ends with recommendations of useful articles and textbooks on case study research.
Origins of case study research
Case study is a research design that involves an intensive and holistic examination of a contemporary phenomenon in a real-life setting. 1–3 It uses a variety of methods and multiple data sources to explore, describe or explain a single case bounded in time and place (ie, an event, individual, group, organisation or programme). A distinctive feature of case study is its focus on the particular characteristics of the case being studied and the contextual aspects, relationships and processes influencing it. 4 Here we do not include clinical case reports as these are beyond the scope of this article. While distantly related to clinical case reports commonly used to report unusual clinical case presentations or findings, case study is a research approach that is frequently used in the social sciences and health sciences. In contrast to other research designs, such as surveys or experiments, a key strength of case study is that it allows the researcher to adopt a holistic approach—rather than an isolated approach—to the study of social phenomena. As argued by Yin, 3 case studies are particularly suitable for answering ‘how’ research questions (ie, how a treatment was received) as well as ‘why’ research questions (ie, why the treatment produced the observed outcomes).
Given its potential for understanding complex processes as they occur in their natural setting, case study increasingly is used in a wide range of health-related disciplines and fields, including medicine, 5 nursing, 6 health services research 1 and health communication. 7 With regard to clinical practice and research, a number of authors 1 5 8 have highlighted how insights gained from case study designs can be used to describe patients’ experiences regarding care, explore health professionals’ perceptions regarding a policy change, and understand why medical treatments and complex interventions succeed or fail.
In anthropology and sociology, case study as a research design was introduced as a response to the prevailing view of quantitative research as the primary way of undertaking research. 9 From its beginnings, social scientists saw case study as a method to obtain comprehensive accounts of social phenomena from participants. In addition, it could complement the findings of survey research. Between the 1920s and 1960s, case study became the predominant research approach among the members of the Department of Sociology of the University of Chicago, widely known as ‘The Chicago School’. 10 11 During this period, prominent sociologists, such as Florian Znaniecki, William Thomas, Everett C Hughes and Howard S Becker, undertook a series of innovative case studies (including classical works such as The Polish peasant in Europe and America or Boys in White ), which laid the foundations of case study designs as implemented today.
In the 1970s, case study increasingly was adopted in the USA and UK in applied disciplines and fields, such as education, programme evaluation and public policy research. 12 As a response to the limitations of quasi-experimental designs for undertaking comprehensive programme evaluations, researchers in these disciplines saw in case studies—either alone or in combination with experimental designs—an opportunity to gain additional insights into the outcomes of programme implementation. In the mid-1980s and early 1990s, the case study approach became recognised as having its own ‘logic of design’ (p46). 13 This period coincides with the publication of a considerable number of influential articles 14–16 and textbooks 4 17 18 on case study research.
These publications were instrumental in shaping contemporary case study practice, yet they reflected divergent views about the nature of case study, including how it should be defined, designed and implemented (see Yazan 19 for a comparison of the perspectives of Yin, Merriam and Stake, three leading case study methodologists). What these publications have in common is that case study revolves around four key features.
First, case study examines a specific phenomenon in detail by performing an indepth and intensive analysis of the selected case. The rationale for case study designs, rather than more expansive designs such as surveys, is that the researcher is interested in investigating the particularity of a case, that is, the unique attributes that define an event, individual, group, organisation or programme. 2 Second, case study is conducted in natural settings where people meet, interact and change their perceptions over time. The use of the case study design is a choice in favour of ‘maintaining the naturalness of the research situation and the natural course of events’ (p177). 20
Third, case study assumes that a case under investigation is entangled with the context in which it is embedded. This context entails a number of interconnected processes that cannot be disassociated from the case, but rather are part of the study. The case study researcher is interested in understanding how and why such processes take place and, consequently, uncovering the interactions between a case and its context. Research questions concerning how and why phenomena occur are particularly appropriate in case study research. 3
Fourth, case study encourages the researcher to use a variety of methods and data types in a single study. 20 21 These can be solely qualitative, solely quantitative or a mixture of both. The latter option allows the researcher to gain a more comprehensive understanding of the case and improve the accuracy of the findings. The four above-mentioned key features of case study are shown in table 1 , using the example of a mixed methods case study evaluation. 22
- View inline
Key features of case study as presented by Shultz et al 22
There are many potential applications for case study research. While often misconstrued as having only an exploratory role, case study research can be used for descriptive and explanatory research (p7–9). 3 Family medicine and community health researchers can use case study research for evaluating a variety of educational programmes, clinical programmes or community programmes.
Case study illustration from family medicine
In the featured study, Japanese family medicine residents received standardised patient instructor-based training in female breast, pelvic, male genital and prostate examinations as part of an international training collaboration to launch a new family medicine residency programme. 22 From family medicine residents, trainers and staff, the authors collected and analysed data from post-training feedback, semistructured interviews and a web-based questionnaire. While the programme was perceived favourably, they noted barriers to reinforcement in their home training programme, and taboos regarding gender-specific healthcare appear as barriers to implementing a similar programme in the home institution.
A step-by-step description of the process of carrying out a case study
As shown in table 2 and illustrated using the article by Shultz et al , 22 case study research generally includes 10 steps. While commonly conducted in this order, the steps do not always occur linearly as data collection and analysis may occur over several iterations or implemented with a slightly different order.
Ten steps for conducting a case study
Examples of published studies using the four types of case study designs suggested by Yin 3
Step 1. Conduct a literature review
During the literature review, researchers systematically search for publications, select those most relevant to the study’s purpose, critically appraise them and summarise the major themes. The literature review helps researchers ascertain what is and is not known about the phenomenon under study, delineate the scope and research questions of the study, and develop an academic or practical justification for the study. 23
Step 2. Formulate the research questions
Research questions critically define in operational terms what will be researched and how. They focus the study and play a key role in guiding design decisions. Key decisions include the case selection and choice of a case study design most suitable for the study. According to Fraenkel et al , 24 the key attributes of good research questions are (1) feasibility, (2) clarity, (3) significance, (4) connection to previous research identified in the literature and (5) compliance with ethical research standards.
Step 3. Ensure that a case study is appropriate
Before commencing the study, researchers should ensure that case study design embodies the most appropriate strategy for answering the study questions. The above-noted four key features—in depth examination of phenomena, naturalness, a focus on context and the use of a combination of methods—should be reflected in the research questions as well as subsequent design decisions.
Step 4. Determine the type of case study design
Researchers need to choose a specific case study design. Sometimes, researchers may define the case first (step 5), for example, in a programme evaluation, and the case may need to be defined before determining the type. Yin’s 3 typology is based on two dimensions, whether the study will examine a single case or multiple cases, and whether the study will focus on a single or multiple units of analysis. Figure 1 illustrates these four types of design using a hypothetical example of a programme evaluation. Table 3 shows an example of each type from the literature.
- Download figure
- Open in new tab
- Download powerpoint
Types of case study designs. 3 21
In type 1 holistic single case design , researchers examine a single programme as the sole unit of analysis. In type 2 embedded single case design , the interest is not exclusively in the programme, but also in its different subunits, including sites, staff and participants. These subunits constitute the range of units of analysis. In type 3 holistic multiple case design , researchers conduct a within and cross-case comparison of two or more programmes, each of which constitutes a single unit of analysis. A major strength of multiple case designs is that they enable researchers to develop an in depth description of each case and to identify patterns of variation and similarity between the cases. Multiple case designs are likely to have stronger internal validity and generate more insightful findings than single case designs. They do this by allowing ‘examination of processes and outcomes across many cases, identification of how individual cases might be affected by different environments, and the specific conditions under which a finding may occur’ (p583). 25 In type 4 embedded multiple case design , a variant of the holistic multiple case design, researchers perform a detailed examination of the subunits of each programme, rather than just examining each case as a whole.
Step 5. Define the boundaries of the case(s) and select the case(s)
Miles et al 26 define a case as ‘a phenomenon of some sort occurring in a bounded context’ (p28). What is and is not the case and how the case fits within its broader context should be explicitly defined. As noted in step 4, this step may occur before choice of the case study type, and the process may actually occur in a back-and-forth fashion. A case can entail an individual, a group, an organisation, an institution or a programme. In this step, researchers delineate the spatial and temporal boundaries of the case, that is, ‘when and where it occurred, and when and what was of interest’ (p390). 9 Aside from ensuring the coherence and consistency of the study, bounding the case ensures that the planned research project is feasible in terms of time and resources. Having access to the case and ensuring ethical research practice are two central considerations in case selection. 1
Step 6. Prepare to collect data
Before beginning the data collection, researchers need a study protocol that describes in detail the methods of data collection. The protocol should emphasise the coherence between the data collection methods and the research questions. According to Yin, 3 a case study protocol should include (1) an overview of the case study, (2) data collection procedures, (3) data collection questions and (4) a guide for the case study report. The protocol should be sufficiently flexible to allow researchers to make changes depending on the context and specific circumstances surrounding each data collection method.
Step 7. Collect and organise the data
While case study is often portrayed as a qualitative approach to research (eg, interviews, focus groups or observations), case study designs frequently rely on multiple data sources, including quantitative data (eg, surveys or statistical databases). A growing number of authors highlight the ways in which the use of mixed methods within case study designs might contribute to developing ‘a more complete understanding of the case’ (p902), 21 shedding light on ‘the complexity of a case’ (p118) 27 or increasing ‘the internal validity of a study’ (p6). 1 Guetterman and Fetters 21 explain how a qualitative case study can also be nested within a mixed methods design (ie, be considered the qualitative component of the design). An interesting strategy for organising multiple data sources is suggested by Yin. 3 He recommends using a case study database in which different data sources (eg, audio files, notes, documents or photographs) are stored for later retrieval or inspection. See guidance from Creswell and Hirose 28 for conducting a survey and qualitative data collection in mixed methods and DeJonckheere 29 on semistructured interviewing.
Step 8. Analyse the data
Bernard and Ryan 30 define data analysis as ‘the search for patterns in data and for ideas that help explain why these patterns are there in the first place’ (p109). Depending on the case study design, analysis of the qualitative and quantitative data can be done concurrently or sequentially. For the qualitative data, the first step of the analysis involves segmenting the data into coding units, ascribing codes to data segments and organising the codes in a coding scheme. 31 Depending on the role of theory in the study, an inductive, data-driven approach can be used where meaning is found in the data, or a deductive, concept-driven approach can be adopted where predefined concepts derived from the literature, or previous research, are used to code the data. 32 The second step involves searching for patterns across codes and subsets of respondents, so major themes are identified to describe, explain or predict the phenomenon under study. Babchuk 33 provides a step-by-step guidance for qualitative analysis in this issue. When conducting a single case study, the within-case analysis yields an in depth, thick description of the case. When the study involves multiple cases, the cross-comparison analysis elicits a description of similarities and divergence between cases and may generate explanations and theoretical predictions regarding other cases. 26
For the quantitative part of the case study, data are entered in statistical software packages for conducting descriptive or inferential analysis. Guetterman 34 provides a step-by-step guidance on basic statistics. In case study designs where both data strands are analysed simultaneously, analytical techniques include pattern matching, explanation building, time-series analysis and creating logic models (p142–167). 3
Step 9. Write the case study report
The case study report should have the following three characteristics. First, the description of the case and its context should be sufficiently comprehensive to allow the reader to understand the complexity of the phenomena under study. 35 Second, the data should be presented in a concise and transparent manner to enable the reader to question, or to re-examine, the findings. 36 Third, the report should be adapted to the interests and needs of its primary audience or audiences (eg, academics, practitioners, policy-makers or funders of research). Yin 3 suggests six formats for organising case study reports, namely linear-analytic, comparative, chronological, theory building, suspense and unsequenced structures. To facilitate case transferability and applicability to other similar contexts, the case study report must include a detailed description of the case.
Step 10. Appraise quality
Although presented as the final step of the case study process, quality appraisal should be considered throughout the study. Multiple criteria and frameworks for appraising the quality of case study research have been suggested in the literature. Yin 3 suggests the following four criteria: construct validity (ie, the extent to which a study accurately measures the concepts that it claims to investigate), internal validity (ie, the strength of the relationship between variables and findings), external validity (ie, the extent to which the findings can be generalised) and reliability (ie, the extent to which the findings can be replicated by other researchers conducting the same study). Yin 37 also suggests using two separate sets of guidelines for conducting case study research and for appraising the quality of case study proposals. Stake 4 presents a 20-item checklist for critiquing case study reports, and Creswell and Poth 38 and Denscombe 39 outline a number of questions to consider. Since these quality frameworks have evolved from different disciplinary and philosophical backgrounds, the researcher’s approach should be coherent with the epistemology of the study. Figure 2 provides a quality appraisal checklist adapted from Creswell and Poth 38 and Denscombe. 39
Checklist for evaluating the quality of a case study. 38 39
The challenges to conducting case study research include rationalising the literature based on literature review, writing the research questions, determining how to bound the case, and choosing among various case study purposes and designs. Factors held in common with other methods include analysing and presenting the findings, particularly with multiple data sources.
Other resources
Resources with more in depth guidance on case study research include Merriam, 17 Stake 4 and Yin. 3 While each reflects a different perspective on case study research, they all provide useful guidance for designing and conducting case studies. Other resources include Creswell and Poth, 38 Swanborn 2 and Tight. 40 For mixed methods case study designs, Creswell and Clark, 27 Guetterman and Fetters, 21 Luck et al , 6 and Plano Clark et al 41 provide guidance. Byrne and Ragin’s 42 The SAGE Handbook of Case-Based Methods and Mills et al ’s 43 Encyclopedia of case study research provide guidance for experienced case study researchers.
Conclusions
Family medicine and community health researchers engage in a wide variety of clinical, educational, research and administrative programmes. Case study research provides a highly flexible and powerful research tool to evaluate rigorously many of these endeavours and disseminate this information.
Acknowledgments
The authors would like to acknowledge the help of Dick Edelstein and Marie-Hélène Paré in editing the final manuscript.
- Cresswell K ,
- Robertson A , et al
- Walshe CE ,
- Caress AL ,
- Chew-Graham C , et al
- Jackson D ,
- Adelman C ,
- Eurepos G ,
- Eurepos G , et al
- Lincoln YS ,
- Eisenhardt KM
- Hijmans E ,
- Guetterman TC ,
- Shultz CG ,
- Yajima A , et al
- Fraenkel J ,
- Huberman A ,
- Creswell J ,
- DeJonckheere M
- Bernard H ,
- Guetterman T
- Denscombe M
- Plano Clark VL ,
- Walton JB , et al
- Little SH ,
- Motohara S ,
- Miyazaki K , et al
- Peterson JC ,
- Rogers EM ,
- Cunningham-Sabo L , et al
- Halladay JR ,
- Reed D , et al
Contributors SF and MDF conceived and drafted the manuscript, and approved the final version of the manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; internally peer reviewed.
Read the full text or download the PDF:
- Privacy Policy
Buy Me a Coffee

Home » Case Study – Methods, Examples and Guide
Case Study – Methods, Examples and Guide
Table of Contents

A case study is a research method that involves an in-depth examination and analysis of a particular phenomenon or case, such as an individual, organization, community, event, or situation.
It is a qualitative research approach that aims to provide a detailed and comprehensive understanding of the case being studied. Case studies typically involve multiple sources of data, including interviews, observations, documents, and artifacts, which are analyzed using various techniques, such as content analysis, thematic analysis, and grounded theory. The findings of a case study are often used to develop theories, inform policy or practice, or generate new research questions.
Types of Case Study
Types and Methods of Case Study are as follows:
Single-Case Study
A single-case study is an in-depth analysis of a single case. This type of case study is useful when the researcher wants to understand a specific phenomenon in detail.
For Example , A researcher might conduct a single-case study on a particular individual to understand their experiences with a particular health condition or a specific organization to explore their management practices. The researcher collects data from multiple sources, such as interviews, observations, and documents, and uses various techniques to analyze the data, such as content analysis or thematic analysis. The findings of a single-case study are often used to generate new research questions, develop theories, or inform policy or practice.
Multiple-Case Study
A multiple-case study involves the analysis of several cases that are similar in nature. This type of case study is useful when the researcher wants to identify similarities and differences between the cases.
For Example, a researcher might conduct a multiple-case study on several companies to explore the factors that contribute to their success or failure. The researcher collects data from each case, compares and contrasts the findings, and uses various techniques to analyze the data, such as comparative analysis or pattern-matching. The findings of a multiple-case study can be used to develop theories, inform policy or practice, or generate new research questions.
Exploratory Case Study
An exploratory case study is used to explore a new or understudied phenomenon. This type of case study is useful when the researcher wants to generate hypotheses or theories about the phenomenon.
For Example, a researcher might conduct an exploratory case study on a new technology to understand its potential impact on society. The researcher collects data from multiple sources, such as interviews, observations, and documents, and uses various techniques to analyze the data, such as grounded theory or content analysis. The findings of an exploratory case study can be used to generate new research questions, develop theories, or inform policy or practice.
Descriptive Case Study
A descriptive case study is used to describe a particular phenomenon in detail. This type of case study is useful when the researcher wants to provide a comprehensive account of the phenomenon.
For Example, a researcher might conduct a descriptive case study on a particular community to understand its social and economic characteristics. The researcher collects data from multiple sources, such as interviews, observations, and documents, and uses various techniques to analyze the data, such as content analysis or thematic analysis. The findings of a descriptive case study can be used to inform policy or practice or generate new research questions.
Instrumental Case Study
An instrumental case study is used to understand a particular phenomenon that is instrumental in achieving a particular goal. This type of case study is useful when the researcher wants to understand the role of the phenomenon in achieving the goal.
For Example, a researcher might conduct an instrumental case study on a particular policy to understand its impact on achieving a particular goal, such as reducing poverty. The researcher collects data from multiple sources, such as interviews, observations, and documents, and uses various techniques to analyze the data, such as content analysis or thematic analysis. The findings of an instrumental case study can be used to inform policy or practice or generate new research questions.
Case Study Data Collection Methods
Here are some common data collection methods for case studies:
Interviews involve asking questions to individuals who have knowledge or experience relevant to the case study. Interviews can be structured (where the same questions are asked to all participants) or unstructured (where the interviewer follows up on the responses with further questions). Interviews can be conducted in person, over the phone, or through video conferencing.
Observations
Observations involve watching and recording the behavior and activities of individuals or groups relevant to the case study. Observations can be participant (where the researcher actively participates in the activities) or non-participant (where the researcher observes from a distance). Observations can be recorded using notes, audio or video recordings, or photographs.
Documents can be used as a source of information for case studies. Documents can include reports, memos, emails, letters, and other written materials related to the case study. Documents can be collected from the case study participants or from public sources.
Surveys involve asking a set of questions to a sample of individuals relevant to the case study. Surveys can be administered in person, over the phone, through mail or email, or online. Surveys can be used to gather information on attitudes, opinions, or behaviors related to the case study.
Artifacts are physical objects relevant to the case study. Artifacts can include tools, equipment, products, or other objects that provide insights into the case study phenomenon.
How to conduct Case Study Research
Conducting a case study research involves several steps that need to be followed to ensure the quality and rigor of the study. Here are the steps to conduct case study research:
- Define the research questions: The first step in conducting a case study research is to define the research questions. The research questions should be specific, measurable, and relevant to the case study phenomenon under investigation.
- Select the case: The next step is to select the case or cases to be studied. The case should be relevant to the research questions and should provide rich and diverse data that can be used to answer the research questions.
- Collect data: Data can be collected using various methods, such as interviews, observations, documents, surveys, and artifacts. The data collection method should be selected based on the research questions and the nature of the case study phenomenon.
- Analyze the data: The data collected from the case study should be analyzed using various techniques, such as content analysis, thematic analysis, or grounded theory. The analysis should be guided by the research questions and should aim to provide insights and conclusions relevant to the research questions.
- Draw conclusions: The conclusions drawn from the case study should be based on the data analysis and should be relevant to the research questions. The conclusions should be supported by evidence and should be clearly stated.
- Validate the findings: The findings of the case study should be validated by reviewing the data and the analysis with participants or other experts in the field. This helps to ensure the validity and reliability of the findings.
- Write the report: The final step is to write the report of the case study research. The report should provide a clear description of the case study phenomenon, the research questions, the data collection methods, the data analysis, the findings, and the conclusions. The report should be written in a clear and concise manner and should follow the guidelines for academic writing.
Examples of Case Study
Here are some examples of case study research:
- The Hawthorne Studies : Conducted between 1924 and 1932, the Hawthorne Studies were a series of case studies conducted by Elton Mayo and his colleagues to examine the impact of work environment on employee productivity. The studies were conducted at the Hawthorne Works plant of the Western Electric Company in Chicago and included interviews, observations, and experiments.
- The Stanford Prison Experiment: Conducted in 1971, the Stanford Prison Experiment was a case study conducted by Philip Zimbardo to examine the psychological effects of power and authority. The study involved simulating a prison environment and assigning participants to the role of guards or prisoners. The study was controversial due to the ethical issues it raised.
- The Challenger Disaster: The Challenger Disaster was a case study conducted to examine the causes of the Space Shuttle Challenger explosion in 1986. The study included interviews, observations, and analysis of data to identify the technical, organizational, and cultural factors that contributed to the disaster.
- The Enron Scandal: The Enron Scandal was a case study conducted to examine the causes of the Enron Corporation’s bankruptcy in 2001. The study included interviews, analysis of financial data, and review of documents to identify the accounting practices, corporate culture, and ethical issues that led to the company’s downfall.
- The Fukushima Nuclear Disaster : The Fukushima Nuclear Disaster was a case study conducted to examine the causes of the nuclear accident that occurred at the Fukushima Daiichi Nuclear Power Plant in Japan in 2011. The study included interviews, analysis of data, and review of documents to identify the technical, organizational, and cultural factors that contributed to the disaster.
Application of Case Study
Case studies have a wide range of applications across various fields and industries. Here are some examples:
Business and Management
Case studies are widely used in business and management to examine real-life situations and develop problem-solving skills. Case studies can help students and professionals to develop a deep understanding of business concepts, theories, and best practices.
Case studies are used in healthcare to examine patient care, treatment options, and outcomes. Case studies can help healthcare professionals to develop critical thinking skills, diagnose complex medical conditions, and develop effective treatment plans.
Case studies are used in education to examine teaching and learning practices. Case studies can help educators to develop effective teaching strategies, evaluate student progress, and identify areas for improvement.
Social Sciences
Case studies are widely used in social sciences to examine human behavior, social phenomena, and cultural practices. Case studies can help researchers to develop theories, test hypotheses, and gain insights into complex social issues.
Law and Ethics
Case studies are used in law and ethics to examine legal and ethical dilemmas. Case studies can help lawyers, policymakers, and ethical professionals to develop critical thinking skills, analyze complex cases, and make informed decisions.
Purpose of Case Study
The purpose of a case study is to provide a detailed analysis of a specific phenomenon, issue, or problem in its real-life context. A case study is a qualitative research method that involves the in-depth exploration and analysis of a particular case, which can be an individual, group, organization, event, or community.
The primary purpose of a case study is to generate a comprehensive and nuanced understanding of the case, including its history, context, and dynamics. Case studies can help researchers to identify and examine the underlying factors, processes, and mechanisms that contribute to the case and its outcomes. This can help to develop a more accurate and detailed understanding of the case, which can inform future research, practice, or policy.
Case studies can also serve other purposes, including:
- Illustrating a theory or concept: Case studies can be used to illustrate and explain theoretical concepts and frameworks, providing concrete examples of how they can be applied in real-life situations.
- Developing hypotheses: Case studies can help to generate hypotheses about the causal relationships between different factors and outcomes, which can be tested through further research.
- Providing insight into complex issues: Case studies can provide insights into complex and multifaceted issues, which may be difficult to understand through other research methods.
- Informing practice or policy: Case studies can be used to inform practice or policy by identifying best practices, lessons learned, or areas for improvement.
Advantages of Case Study Research
There are several advantages of case study research, including:
- In-depth exploration: Case study research allows for a detailed exploration and analysis of a specific phenomenon, issue, or problem in its real-life context. This can provide a comprehensive understanding of the case and its dynamics, which may not be possible through other research methods.
- Rich data: Case study research can generate rich and detailed data, including qualitative data such as interviews, observations, and documents. This can provide a nuanced understanding of the case and its complexity.
- Holistic perspective: Case study research allows for a holistic perspective of the case, taking into account the various factors, processes, and mechanisms that contribute to the case and its outcomes. This can help to develop a more accurate and comprehensive understanding of the case.
- Theory development: Case study research can help to develop and refine theories and concepts by providing empirical evidence and concrete examples of how they can be applied in real-life situations.
- Practical application: Case study research can inform practice or policy by identifying best practices, lessons learned, or areas for improvement.
- Contextualization: Case study research takes into account the specific context in which the case is situated, which can help to understand how the case is influenced by the social, cultural, and historical factors of its environment.
Limitations of Case Study Research
There are several limitations of case study research, including:
- Limited generalizability : Case studies are typically focused on a single case or a small number of cases, which limits the generalizability of the findings. The unique characteristics of the case may not be applicable to other contexts or populations, which may limit the external validity of the research.
- Biased sampling: Case studies may rely on purposive or convenience sampling, which can introduce bias into the sample selection process. This may limit the representativeness of the sample and the generalizability of the findings.
- Subjectivity: Case studies rely on the interpretation of the researcher, which can introduce subjectivity into the analysis. The researcher’s own biases, assumptions, and perspectives may influence the findings, which may limit the objectivity of the research.
- Limited control: Case studies are typically conducted in naturalistic settings, which limits the control that the researcher has over the environment and the variables being studied. This may limit the ability to establish causal relationships between variables.
- Time-consuming: Case studies can be time-consuming to conduct, as they typically involve a detailed exploration and analysis of a specific case. This may limit the feasibility of conducting multiple case studies or conducting case studies in a timely manner.
- Resource-intensive: Case studies may require significant resources, including time, funding, and expertise. This may limit the ability of researchers to conduct case studies in resource-constrained settings.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

Questionnaire – Definition, Types, and Examples

Observational Research – Methods and Guide

Quantitative Research – Methods, Types and...

Qualitative Research Methods

Explanatory Research – Types, Methods, Guide

Survey Research – Types, Methods, Examples
The curious case of case study research
Affiliations.
- 1 Lee Kong Chian School of Medicine, Nanyang Technological University Ringgold Standard Institution, Singapore City, Singapore.
- 2 Division of Medical Education, Clinical Research Centre, Dalhousie University, Halifax, NS, Canada.
- 3 Community Health Sciences, Foothills Medical Centre, University of Calgary, Calgary, AB, Canada.
- PMID: 33905143
- DOI: 10.1111/medu.14544
The conceptualisation of 'good' medical education research as hypothesis testing to identify universal truths that are generalisable across contexts has been challenged. Joining this conversation, the field of health professions education research is complex and contextual and there are ways of examining and reporting locally based activities and innovations, which can be of general value. This position leads to a focus on case study research (CSR), inquiry bound in time and place that generates thick descriptions and close interpretations to reach explanations. CSR has grown in sophistication in recent years and can inform practice and advance the science of medical and health professions education. The authors evaluated the current state of the science of CSR in the medical education literature by identifying and reviewing 160 papers. Most articles presented as 'case studies' were not in fact CSR. Moreover, most articles failed to go beyond a 'we did this' account. The authors explore definitions of CSR, and they examine dominant CSR methodologists, Yin, Stake and Merriam, and their respective approaches to CSR. They then set out some of the basic tenets of CSR (case definition, methods of data collection and analysis) and consider the logics of CSR (its structures, purposes, assumptions and symbols). CSR challenges are considered next (such as emic and etic perspectives; ethical complexities; generalisability; quality; and reporting and reflexivity). The authors conclude that context is a mechanism, which needs to be understood, and rigorous CSR provides the structures and criticality to do so, opening up new areas of understanding and inquiry.
© 2021 John Wiley & Sons Ltd and The Association for the Study of Medical Education.
- Biomedical Research*
- Education, Medical*
- Research Design

- Policy & Compliance
- Clinical Trials
NIH Definition of Clinical Trial Case Studies
The case studies provided below are designed to help you identify whether your study would be considered by NIH to be a clinical trial. Expect the case studies and related guidance to evolve over the upcoming year. For continuity and ease of reference, case studies will retain their original numbering and will not be renumbered if cases are revised or removed.
The simplified case studies apply the following four questions to determine whether NIH would consider the research study to be a clinical trial:
- Does the study involve human participants?
- Are the participants prospectively assigned to an intervention?
- Is the study designed to evaluate the effect of the intervention on the participants?
- Is the effect being evaluated a health-related biomedical or behavioral outcome?
If the answer to all four questions is “yes,” then the clinical study would be considered a clinical trial according to the NIH definition.
See this page for more information about the NIH definition of a clinical trial.
General Case Studies
Institute or center specific case studies.
The study involves the recruitment of research participants who are randomized to receive one of two approved drugs. It is designed to compare the effects of the drugs on the blood level of a protein.
- Does the study involve human participants? Yes, the study involves human participants.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, one of two drugs.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of the drugs on the level of the protein in the participants’ blood.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, the level of a protein, is a health-related biomedical outcome.
The study involves the recruitment of research participants with condition Y to receive a drug that has been approved for another indication. It is designed to measure the drug’s effects on the level of a biomarker associated with the severity of condition Y.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, the approved drug.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the drug’s effect on the level of the biomarker.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, the level of a biomarker, is a health-related biomedical outcome.
The study involves the recruitment of research participants with condition X to receive investigational compound A. It is designed to assess the pharmacokinetic properties of compound A.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, compound A.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate how the body interacts with compound A
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, pharmacokinetic properties, is a health-related biomedical outcome.
The study involves the recruitment of research participants with disease X to receive an investigational drug. It is designed to assess safety and determine the maximum tolerated dose of the drug.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, the investigational drug.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to assess safety and determine the maximum tolerated dose of the investigational drug.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, safety and maximum tolerated dose, is a health-related biomedical outcome.
The study involves the recruitment of research participants with disease X to receive a chronic disease management program. It is designed to assess usability and to determine the maximum tolerated dose of the chronic disease program (e.g., how many in-person and telemedicine visits with adequate adherence).
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, the chronic disease management program.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to determine the maximum tolerated dose of the program to obtain adequate adherence.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, tolerable intensity and adequate adherence of the intervention, is a health-related outcome.
The study involves the recruitment of research participants with disease X to receive either an investigational drug or a placebo. It is designed to evaluate the efficacy of the investigational drug to relieve disease symptoms.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, the investigational drug or placebo.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of the investigational drug on the participants’ symptoms.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, relief of symptoms, is a health-related outcome.
The study involves the recruitment of research participants with disease X to receive an investigational drug. It is designed to assess whether there is a change in disease progression compared to baseline. There is no concurrent control used in this study.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of the investigational drug on the subject’s disease progression.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, disease progression, is a health-related outcome.
The study involves the recruitment of research participants with disease X to test an investigational in vitro diagnostic device (IVD). It is designed to evaluate the ability of the device to measure the level of an antibody in blood.
- Are the participants prospectively assigned to an intervention? No, in this context the IVD would not be considered an intervention. The IVD is being used to test its ability to measure antibody levels, but not to test its effects on any health-related biomedical or behavioral outcomes.
The study involves the recruitment of research participants with disease X to be evaluated with an investigational in vitro diagnostic device (IVD). The study is designed to evaluate how knowledge of certain antibody levels impacts clinical management of disease.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to an intervention, measurement of an antibody level, with the idea that knowledge of that antibody level might affect clinical management.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate how knowledge of the level of an antibody might inform treatment.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being measured, how blood antibody levels inform treatment, is a health-related outcome.
The study involves the recruitment of healthy volunteers who will be randomized to different durations of sleep deprivation (including no sleep deprivation as a control) and who will have stress hormone levels measured. It is designed to determine whether the levels of stress hormones in blood rise in response to different durations of sleep deprivation.
- Does the study involve human participants? Yes, the healthy volunteers are human participants.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to an intervention, different durations of sleep deprivation followed by a blood draw.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to measure the effect of different durations of sleep deprivation on stress hormone levels.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, stress hormone levels, is a health-related biomedical outcome.
The study involves the analysis of de-identified, stored blood samples and de-identified medical records of patients with disease X who were treated with an approved drug. The study is designed to evaluate the level of a protein in the blood of patients that is associated with therapeutic effects of the drug.
- Does the study involve human participants? No, the study does not involve human participants because only de-identified samples and information are used.
The study involves the analysis of identifiable, stored blood samples and identified medical records of patients with disease X who were treated with an approved drug. The study is designed to evaluate the level of a protein in the blood of patients that is associated with therapeutic effects of the drug.
- Does the study involve human participants? Yes, patients are human participants because the blood and information are identifiable.
- Are the participants prospectively assigned to an intervention? No, secondary research with biospecimens or health information is not a clinical trial.
The study involves the recruitment of a healthy volunteers whose blood is drawn for genomic analysis. It is designed to identify the prevalence of a genetic mutation in the cohort and evaluate potential association between the presence of the mutation and the risk of developing a genetic disorder.
- Are the participants prospectively assigned to an intervention? No, sample collection (blood draw) is not an intervention in this context.
Physicians report that some patients being treated with drug A for disease X are also experiencing some improvement in a second condition, condition Y. The study involves the recruitment of research participants who have disease X and condition Y and are being treated with drug A. The participants are surveyed to ascertain whether they are experiencing an improvement in condition Y.
- Are the participants prospectively assigned to an intervention? No, participants are not prospectively assigned to receive an intervention as they are receiving drugs as part of their clinical care. The surveys are being used for measurement, not to modify a biomedical or behavioral outcome.
The study involves the recruitment of patients with disease X who are receiving one of three standard therapies as part of their clinical care. It is designed to assess the relative effectiveness of the three therapies by monitoring survival rates using medical records over a few years.
- Are the participants prospectively assigned to an intervention? No, there is no intervention. The therapies are prescribed as part of clinical care; they are not prospectively assigned for the purpose of the study. The study is observational.
The study involves the recruitment of research participants with disease X vs. healthy controls and comparing these participants on a range of health processes and outcomes including genomics, biospecimens, self-report measures, etc. to explore differences that may be relevant to the development of disease X.
- Are the participants prospectively assigned to an intervention? No, the measures needed to assess the outcomes are not interventions in this context, as the study is not intended to determine whether the measures modify a health-related biomedical or behavioral outcome.
The study involves the recruitment of healthy volunteers for a respiratory challenge study; participants are randomized to receive different combinations of allergens. The study evaluates the severity and mechanism of the immune response to different combinations of allergens introduced via inhalation.
- Does the study involve human participants? Yes, healthy volunteers are human participants.
- Are the participants prospectively assigned to an intervention? Yes, healthy volunteers are prospectively assigned to randomly selected combinations of allergens.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is evaluating the effects of different combinations of allergens on the immune response in healthy individuals.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the study evaluates the severity and mechanism of the immune reaction to allergens, which are health-related biomedical outcomes.
The study involves the recruitment of research participants with Alzheimer’s disease (AD) to evaluate the effects of an investigational drug on memory, and retention and recall of information.
- Are the participants prospectively assigned to an intervention? Yes, participants are prospectively assigned to receive the investigational drug.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is evaluating the effects of the drug on participants’ memory.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the study evaluates memory, and retention and recall of information in the context of AD.
The study involves the recruitment of individuals to receive a new behavioral intervention for sedentary behavior. It is designed to measure the effect of the intervention on hypothesized differential mediators of behavior change.
- Are the participants prospectively assigned to an intervention? Yes, participants are prospectively assigned to receive a behavioral intervention.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is evaluating the effects of the intervetion on mediators of behavior change.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, mediators of behavior change, are behavioral outcomes relevant to health.
The study involves the recruitment of patients with disease X to be evaluated with a new visual acuity task. It is designed to evaluate the ability of the new task to measure visual acuity as compared with the gold standard Snellen Test
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to an intervention, the new visual acuity test.
- Is the study designed to evaluate the effect of the intervention on the participants? No, the study is designed to evaluate the ability of the new visual acuity test to measure visual acuity as compared to the gold standard Snellen Test, but not to modify visual acuity.
The study involves the recruitment of research participants with CHF who were hospitalized before or after implementation of the Medicare incentives to reduce re-hospitalizations. Morbidity, mortality, and quality of life of these participants are evaluated to compare the effects of these Medicare incentives on these outcomes.
- Are the participants prospectively assigned to an intervention? No, the intervention (incentives to reduce re-hospitalization) were assigned by Medicare, not by the research study.
The study involves the recruitment of healthcare providers to assess the extent to which being provided with genomic sequence information about their patients informs their treatment of those patients towards improved outcomes.
- Does the study involve human participants? Yes, both the physicians and the patients are human participants.
- Are the participants prospectively assigned to an intervention? Yes, physicians are prospectively assigned to receive genomic sequence information, which is the intervention.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of intervening with physicians, on the treatment they provide to their patients.
- Is the effect being evaluated a health-related, biomedical, or behavioral outcome? Yes, the effect being evaluated, the extent to which providing specific information to physicians informs the treatment of patients, is a health-related outcome.
The study involves the recruitment of research participants with a behavioral condition to receive either an investigational behavioral intervention or a behavioral intervention in clinical use. It is designed to evaluate the effectiveness of the investigational intervention compared to the intervention in clinical use in reducing the severity of the obsessive compulsive disorder.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to an intervention, either the investigational intervention or an intervention in clinical use.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate whether the investigational intervention is as effective as the standard intervention, at changing behavior.
- Is the effect being evaluated a health-related, biomedical, or behavioral outcome? Yes, the effect being evaluated, the interventions’ effectiveness in reducing the severity of the condition, is a health-related behavioral outcome.
The study involves the recruitment of physicians who will be randomly assigned to use a new app or an existing app, which cues directed interviewing techniques. The study is designed to determine whether the new app is better than the existing app at assisting physicians in identifying families in need of social service support. The number of community service referrals will be measured.
- Does the study involve human participants? Yes, both the physicians and the families are human participants.
- Are the participants prospectively assigned to an intervention? Yes, physicians are prospectively assigned to use one of two apps, which are the interventions.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of intervening with physicians, on social service support referral for families.
- Is the effect being evaluated a health-related, biomedical, or behavioral outcome? Yes, the effect being evaluated, the number of referrals, is a health-related outcome.
The study involves the recruitment of parents to participate in focus groups to discuss topics related to parental self-efficacy and positive parenting behaviors. It is designed to gather information needed to develop an intervention to promote parental self-efficacy and positive parenting behaviors.
- Does the study involve human participants? Yes, the parents are human participants.
- Are the participants prospectively assigned to an intervention? No, a focus group is not an intervention.
The study involves the recruitment of healthy volunteers to test a new behavioral intervention. It is designed to evaluate the effect of a meditation intervention on adherence to exercise regimens and quality of life to inform the design of a subsequent, fully-powered trial.
- Does the study involve human participants? Yes, study participants are human participants.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to a behavioral intervention.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of the intervention on adherence, and quality of life.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, adherence and quality of life are health-related outcomes.
A study will test the feasibility a mobile phone app designed to increase physical activity. A group of sedentary individuals will use the app for a week while their interactions with the app are monitored. The number of interactions with the app will be measured, as well as any software issues. Participants will also complete a survey indicating their satisfaction with and willingness to use the app, as well as any feedback for improvement. The app’s effect on physical activity, weight, or cardiovascular fitness will not be evaluated.
- Does the study involve human participants? Yes, sedentary individuals will be enrolled.
- Are the participants prospectively assigned to an intervention? The participants will interact with the app for a week.
- Is the study designed to evaluate the effect of the intervention on the participants? No. While the participants’ interactions are monitored (steps or heart rate may be recorded in this process), the study is NOT measuring the effect of using the app ON the participant. The study is only measuring the usability and acceptability of the app, and testing for bugs in the software. The effect on physical activity is NOT being measured.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? N/A
The study involves the recruitment of healthy family members of patients hospitalized for disease X to test two CPR training strategies. Participants will receive one of two training strategies. The outcome is improved CPR skills retention.
- Does the study involve human participants? Yes, family members of patients are human participants.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to one of two CPR educational strategies.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of educational strategies on CPR skills.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, retention of CPR skills is a health-related behavioral outcome.
The study involves the recruitment of research participants in three different communities (clusters) to test three CPR training strategies. The rate of out-of- hospital cardiac arrest survival will be compared.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive one of three types of CPR training, which is the intervention.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of different CPR training strategies on patient survival rates post cardiac arrest.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, out-of-hospital cardiac arrest survival is a health-related outcome.
A study involves the recruitment of school children to evaluate two different tools for monitoring food intake. Food consumption behavior will be measured by asking children to activate a pocket camera during meals and to use a diary to record consumed food. The accuracy of the two food monitoring methods in measuring energy intake will be assessed.
- Does the study involve human participants? Yes, children are human participants.
- Are the participants prospectively assigned to an intervention? No, in this context the monitoring methods would not be considered an intervention. The study is designed to test the accuracy of two monitoring methods, but not to test the effect on any health-related biomedical or behavioral outcomes.
A study involves the recruitment of school children to evaluate two different tools for monitoring food intake. Food consumption behavior will be measured by asking children to activate a pocket camera during meals and to use a diary to record consumed food. Changes to eating behavior will be assessed.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to two food monitoring methods.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to determine whether using the monitoring methods changes eating behavior.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, eating behavior is a health-related outcome.
A study involves the recruitment of children at two schools to monitor eating behavior. Children’s food choices will be monitored using a remote food photography method. Food consumption and the accuracy of food monitoring methods will be assessed.
- Does the study involve human participants? Yes, the children participating in this study are human participants.
- Are the participants prospectively assigned to an intervention? No, not in this context. The study involves observing and measuring eating behavior, but not modifying it. This is an observational study.
A study involves the recruitment of children at two schools to evaluate their preferences for graphics and colors used in healthy food advertisements. Children will be presented with multiple health advertisements and their preferences for graphics and colors will be assessed.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to see different advertisements.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the advertisements.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? No, preferences are not health-related biomedical or behavioral outcomes.
The study involves ambulatory patients who have new-onset stable angina and who are recruited from community practices. They are randomized to undergo CT angiography or an exercise stress test of the doctor’s choice. To keep the trial pragmatic, the investigators do not prescribe a protocol for how physicians should respond to test results. The study is designed to determine whether the initial test (CT angiography or stress test) affects long-term rates of premature death, stroke, or myocardial infarctions.
- Are the participants prospectively assigned to an intervention? Yes, the participants are randomized to undergo CT angiography or an exercise stress test.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to determine whether the initial test done affects long-term rates of certain clinical events.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, premature death, stroke, and myocardial infarction are health-related biomedical outcomes.
The study involves patients who present with stable angina to community practices. As part of their routine care some of their physicians refer them for CT angiography, while others refer them for exercise stress tests. The study is designed to see whether or not there's an association between the type of test that is chosen and long-term risk of death, stroke, or myocardial infarction.
- Are the participants prospectively assigned to an intervention? No, the intervention is not prospectively assigned by the investigators. Rather, the intervention, in this case diagnostic study, occurs as part of routine clinical care.
The investigators conduct a longitudinal study of patients with schizophrenia. Their physicians, as part of their standard clinical care, prescribe antipsychotic medication. The investigators conduct an imaging session before starting treatment; they repeat imaging 4-6 weeks later.
- Does the study involve human participants? Yes.
- Are the participants prospectively assigned to an intervention? No, not in this context. Antipsychotic medications are given as part of clinical care, not as part of a prospective, approved research protocol.
The investigators conduct a longitudinal study of patients with schizophrenia. Their physicians, as part of their standard clinical care, prescribe antipsychotic medication. As part of the research protocol, all participants will be prescribed the same dose of the antipsychotic medication. The investigators conduct an imaging session before starting treatment; they repeat imaging 4-6 weeks later.
- Are the participants prospectively assigned to an intervention? Yes, although participants are all receiving antipsychotic medication as part of their standard medical care, the dose of the antipsychotic medication is determined by the research protocol, rather than individual clinical need.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of a dose of antipsychotic medication on brain function.
- Is the effect being evaluated a health-related biomedical or behavioral outcome ? Yes, brain function measured by imaging is a health-related outcome.
The study involves recruitment of healthy volunteers who will wear a thermal compression device around their legs. This pilot study is designed to examine preliminary performance and safety of a thermal compression device worn during surgery. Investigators will measure core temperature, comfort, and presence of skin injury in 15-minute intervals.
- Are the participants prospectively assigned to an intervention? Yes, participants are assigned to wear a thermal compression device.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of the thermal compression device on participant core temperature, comfort, and presence of skin injury.
- Is the effect being evaluated a health-related biomedical or behavioral outcome ? Yes, participant core temperature, comfort, and presence of skin injury are health-related biomedical outcomes.
The study involves collection of data on hospitalizations for various acute illnesses among people who live close to a border between two states that have recently implemented different laws related to public health (e.g. smoking regulations, soda taxes). The investigators want to take advantage of this “natural experiment” to assess the health impact of the laws.
- Does the study involve human participants? Yes, the study involves human participants.
- Are the participants prospectively assigned to an intervention? No, the interventions were assigned by state laws and state of residence, not by the research study.
The study involves recruitment of healthy volunteers to engage in working memory tasks while undergoing transcranial magnetic stimulation (TMS) to induce competing local neuronal activity. The study is measuring task performance to investigate the neural underpinnings of working memory storage and processing.
- Are the participants prospectively assigned to an intervention? Yes, healthy volunteers are prospectively assigned to receive TMS stimulation protocols during a working memory task.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is evaluating the effects of local TMS stimulation on working memory performance and oscillatory brain activity in healthy individuals.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the study evaluates working memory processes, which are health-related biomedical outcomes.
The study involves recruitment of healthy volunteers to engage in a social valuation task while dopamine tone in the brain is manipulated using tolcapone, an FDA-approved medication. The study aims to understand the role of dopamine in social decision-making and to search for neural correlates of this valuation using fMRI.
- Are the participants prospectively assigned to an intervention? Yes, healthy volunteers are prospectively assigned to receive tolcapone during a social valuation task.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is evaluating the effects of modulating dopamine tone on social decision-making. Although this study uses an FDA-approved drug to modulate dopamine tone, the goal of this intervention is to understand the role of dopamine in a fundamental phenomenon (social valuation), and not to study the mechanism of action of the drug or its clinical effects.
The career development candidate proposes to independently lead a study to test a new drug A on patients with disease X. Patients will be randomized to a test and control group, with the test group receiving one dose of drug A per week for 12 months and controls receiving placebo. To assess presence, number, and type of any polyps, a colonoscopy will be performed. To assess biomarkers of precancerous lesions, colon mucosal biopsies will be collected. Complete blood count will be measured, and plasma will be stored for potential biomarker evaluation.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, drug A or placebo.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of drug A and placebo on the presence and type of polyps.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, the presence and type of polyps, is a health-related biomedical outcome.
Ancillary Study to Case Study #42b: Some types of drug A being evaluated in Case Study #42a have been reported to impact renal function. An internal medicine fellow performs an ancillary study where stored plasma from Case Study #42a will be evaluated for multiple biomarkers of renal function.
- Does the study involve human participants? Yes, patients are human participants because the plasma and information are identifiable.
- Are the participants prospectively assigned to an intervention? No, because the assignment of participants to an intervention occurs as part of an existing, separately funded clinical trial. This proposal would be considered an ancillary study that is not an independent clinical trial.
Ancillary Study to Case Study #42a: An internal medicine fellow designs an independent ancillary trial where a subset of patients from the parent trial in Case Study #42a will also receive drug B, based on the assumption that a two-drug combination will work significantly better than a single drug at both improving renal function and reducing polyps. The test subjects will be evaluated for renal function via plasma clearance rates at 6 and 12 months after initiation of drugs A and B.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to receive an intervention, drugs A and B.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of drugs A and B on renal function.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the effect being evaluated, renal function, is a health-related biomedical outcome.
A group of healthy young adults will perform a Go/No-Go task while undergoing fMRI scans. The purpose of the study is to characterize the pattern of neural activation in the frontal cortex during response inhibition, and the ability of the participant to correctly withhold a response on no-go
- Does the study involve human participants? Yes, healthy young adults will be enrolled in this study.
- Are the participants prospectively assigned to an intervention? Yes, the participants will be prospectively assigned to perform a Go/No-Go task, which involves different levels of inhibitory control.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of the Go/No-Go task on neural activation in the frontal cortex. The study will measure inhibitory control and the neural systems being engaged. In this study, the Go/No-Go task is the independent variable, and behavioral performance and the associated fMRI activations are the dependent variables.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the neural correlates of inhibitory control and behavioral performance are health-related biomedical outcomes.
A group of adolescents will participate in a longitudinal study examining changes in executive function over the course of a normal school year. Color naming performance on the standard version of the Stroop test will be obtained. All measures will be compared at multiple time points during the school year to examine changes in executive function. The purpose is to observe changes in executive function and to observe if differences exist in the Stroop effect over the course of the school year for these adolescents.
- Does the study involve human participants? Yes, adolescents will be enrolled in this study.
- Are the participants prospectively assigned to an intervention? No, there is no intervention in this study and no independent variable manipulated. The adolescents are not prospectively assigned to an intervention, but instead the investigator will examine variables of interest (including the Stroop test) over time. The Stroop effect is used as a measurement of point-in-time data.
- Is the study designed to evaluate the effect of the intervention on the participants? No, there is no intervention. Performance on the Stroop test is a well-established measure of executive function and the test is not providing an independent variable of interest here. It is not being used to manipulate the participants or their environment. The purpose is simply to obtain a measure of executive function in adolescents over the course of the school year.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? N/A. No effect of an intervention is being evaluated.
A group of participants with social anxiety will perform an experimentally manipulated Stroop test. In this variant of the Stroop test, the stimuli presented are varied to include emotional and neutral facial expressions presented in different colors. Participants are instructed to name the colors of the faces presented, with the expectation that they will be slower to name the color of the emotional face than the neutral face. The purpose of the study is to examine the degree to which participants with social anxiety will be slower to process emotional faces than neutral faces.
- Does the study involve human participants? Yes, participants with social anxiety will be enrolled in this study.
- Are the participants prospectively assigned to an intervention? Yes, the participants will be prospectively assigned to perform a modified Stroop test using different colored emotional/neutral faces to explore emotional processing in people with social anxiety. Note that the independent variable is the presentation of emotional vs neutral faces.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to measure the effect of emotional valence (i.e. emotional faces) on participant response time to name the color. The purpose is to determine whether the response time to emotional faces is exaggerated for people with social anxiety as compared to neutral faces. Note that the response time to name the colors is the dependent variable in this study.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the processing of emotional information is a health-related biomedical outcome.
The study involves healthy volunteers and compares temporal SNR obtained with a new fMRI pulse sequence with that from another sequence.
- Are the participants prospectively assigned to an intervention? No, in this context the different pulse sequences would not be considered an intervention. The pulse sequences are not being used to modify any biomedical or behavioral outcome; rather the investigator is comparing performance characteristics of the two pulse sequences.
The study is designed to demonstrate that a new imaging technology (e.g. MRI, PET, ultrasound technologies, or image processing algorithm) is equivalent to, or has better sensitivity/specificity than a standard of care imaging technology. Aim one will use the new imaging technology and the gold standard in ten healthy volunteers. Aim Two will use the new imaging technology and the gold standard before and after a standard care procedure in ten patients. In both aims the performance of the new technology will be compared to the gold standard. No clinical care decisions will be made based on the use of the device in this study.
- Does the study involve human participants? YES. Aim one will study ten healthy volunteers, and aim two will study ten patient volunteers.
- Are the participants prospectively assigned to an intervention? Yes, participants will be prospectively assigned to be evaluated with a new imaging technology and the gold standard technology.
- Is the study designed to evaluate the effect of the intervention on the participants? No, the study is not measuring the effect of the technologies ON the human subjects. The study is determining if the new technology is equivalent or better than the gold standard technology. No effect on the participant is being measured.
An investigator proposes to add secondary outcomes to an already funded clinical trial of a nutritional intervention. The trial is supported by other funding, but the investigator is interested in obtaining NIH funding for studying oral health outcomes. Participants in the existing trial would be assessed for oral health outcomes at baseline and at additional time points during a multi-week dietary intervention. The oral health outcomes would include measures of gingivitis and responses to oral health related quality of life questionnaires. Oral fluids would be collected for analysis of inflammatory markers and microbiome components.
- Are the participants prospectively assigned to an intervention? No, because the assignment of participants to an intervention (and the administration of the intervention) occur as part of an existing, separately funded clinical trial. This proposal would be considered an ancillary study that leverages an already existing clinical trial.
The goal of the project is to use functional neuroimaging to distinguish patients with temporomandibular disorders (TMD) who experience TMD pain through centralized pain processes from those with TMD related to peripheral pain. Pain processing in a study cohort of TMD patients and healthy controls will be measured through functional magnetic resonance neuroimaging (fMRI) following transient stimulation of pain pathways through multimodal automated quantitative sensory testing (MAST QST). TMD patients will receive study questionnaires to better correlate the extent to which TMD pain centralization influences TMD prognosis and response to standard of care peripherally targeted treatment (prescribed by physicians, independently of the study).
- Are the participants prospectively assigned to an intervention? No, not in this context. The transient stimulation of pain pathways and the fMRI are being performed to measure and describe brain activity, but not to modify it.
An investigator proposes to perform a study of induced gingivitis in healthy humans, to study microbial colonization and inflammation under conditions of health and disease. During a 3-week gingivitis induction period, each study participant will use a stent to cover the teeth in one quadrant during teeth brushing. A contralateral uncovered quadrant will be exposed to the individual's usual oral hygiene procedures, to serve as a control. Standard clinical assessments for gingivitis will be made and biospecimens will be collected at the point of maximal induced gingivitis, and again after normal oral hygiene is resumed. Biospecimens will be assessed for microbial composition and levels of inflammation-associated chemokines.
- Are the participants prospectively assigned to an intervention? Yes, the participants are prospectively assigned to an intervention, abstaining from normal oral hygiene for a portion of the mouth, to induce gingivitis.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to evaluate the effect of the induced gingivitis on microbial composition and levels of inflammatory chemokines in oral samples.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, the microbial composition and chemokine levels in oral samples are health-related biomedical outcomes.
The study will enroll older adults with hearing loss, comparing the effectiveness of enhanced hearing health care (HHC) to usual HHC. In addition to routine hearing-aid consultation and fitting, participants randomized to enhanced HCC will be provided patient-centered information and education about a full range of hearing assistive technologies and services. Study outcomes include the utilization of technology or services, quality of life, communication abilities, and cognitive function.
- Does the study involve human participants? Yes, the study enrolls older adults with hearing loss.
- Are the participants prospectively assigned to an intervention? Yes, participants are randomized to receive enhanced HCC or usual HCC interventions.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study will evaluate enhanced HCC’s effectiveness in modifying participant behavior and biomedical outcomes.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, rate of technology/service utilization is a behavioral outcome and quality of life, communications, and cognition are biomedical outcomes that may be impacted by the interventions.
The study involves the recruitment of obese individuals who will undergo a muscle biopsy before and after either exercise training or diet-induced weight loss. Sarcolemmal 1,2-disaturated DAG and C18:0 ceramide species and mitochondrial function will be measured. Levels will be correlated with insulin sensitivity.
- Are the participants prospectively assigned to an intervention? Yes, the participants are assigned to either exercise training or a diet.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to compare the effects of the interventions on muscle metabolism.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, muscle metabolism/signaling is a health-related outcome.
The study involves the recruitment of participants with type 2 diabetes who will undergo a muscle biopsy before and after a fast to measure acetylation on lysine 23 of the mitochondrial solute carrier adenine nucleotide translocase 1 (ANT1). Levels will be related to rates of fat oxidation.
- Are the participants prospectively assigned to an intervention? Yes, the participants are assigned to undergo a fast.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to compare the effects of the fast on molecular parameters of metabolism.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, metabolism is a health-related outcome.
Insulin-resistant and insulin-sensitive nondiabetic adults who have a parent with type 2 diabetes will be followed over time to understand the role of mitochondrial dysfunction in the development of diabetes. Oral glucose tolerance tests will be performed annually to measure insulin sensitivity and glycemic status. Participants will also undergo a brief bout of exercise, and mitochondrial ATP synthesis rates will be measured by assessing the rate of recovery of phosphocreatine in the leg muscle, using 31P magnetic resonance spectroscopy.
- Are the participants prospectively assigned to an intervention? No, the participants are not assigned to an intervention; the OGTT and 31P MRS are measures.
Participants with chronic kidney disease will be recruited to receive one of two drug agents. After 6 weeks of therapy, subjects will undergo vascular function testing and have measures of oxidative stress evaluated in their plasma and urine. Results of the function testing and the oxidative stress biomarkers will be related to drug treatment.
- Are the participants prospectively assigned to an intervention? Yes, the participants are assigned to receive two different drugs.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to compare the effects of the drugs on vascular function.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, vascular function is a health-related outcome.
Participants with Autosomal Dominant Polycystic Kidney Disease will be recruited to receive an oral curcumin therapy or placebo and the participants will undergo vascular function testing, renal imaging to assess kidney size, and assessment of oxidative stress biomarkers in urine and plasma after an ascorbic acid challenge. Changes in these outcomes will be related to oral therapy.
- Are the participants prospectively assigned to an intervention? Yes, the participants are assigned to receive medication or placebo.
- Is the study designed to evaluate the effect of the intervention on the participants? Yes, the study is designed to compare the effects of the drugs on vascular function and kidney size.
- Is the effect being evaluated a health-related biomedical or behavioral outcome? Yes, vascular function and kidney size are health-related outcomes.
Kidney transplant recipients will be recruited to undergo an experimental imaging procedure at several timepoints up to 4 months post-transplantation. Output from the images will be related to pathological assessments of the transplant as well as clinical measures of renal function.
- Are the participants prospectively assigned to an intervention? No, the participants are not assigned to receive an intervention. They undergo transplantation as part of their routine clinical care. The imaging procedure is a measure and not an intervention.
The study proposes the development of a novel probe to assess clearance of a nutritional metabolite in a given disease state. The probe is a GMP grade, deuterated, intravenously administered tracer and clearance is assessed by mass spectrometry analysis of serial blood draws. Participants will either receive a micronutrient supplement or will receive no supplementation. The clearance rate of the probe will be compared in the two groups, to understand the performance of the probe.
- Are the participants prospectively assigned to an intervention? Yes, the participants are assigned to receive either a micronutrient supplement or nothing.
- Is the study designed to evaluate the effect of the intervention on the participants? No, the intervention is being used to assess the performance of the probe and is not looking at an effect on the participant.
- Are the participants prospectively assigned to an intervention? Yes, the participants are assigned to receive a controlled diet for three days.
- Is the study designed to evaluate the effect of the intervention on the participants? No, the intervention (controlled diet) is being used to minimize exogenous dietary sources of oxalate in the participants prior to the labeled tracer infusion. The study will not be evaluating the effect of the diet on the participants.
This page last updated on: April 28, 2021
- Bookmark & Share
- E-mail Updates
- Help Downloading Files
- Privacy Notice
- Accessibility
- National Institutes of Health (NIH), 9000 Rockville Pike, Bethesda, Maryland 20892
- NIH... Turning Discovery Into Health
Journal of Medical Case Reports
In the era of evidence-based practice, we need practice-based evidence. The basis of this evidence is the detailed information from the case reports of individual people which informs both our clinical research and our daily clinical care. Each case report published in this journal adds valuable new information to our medical knowledge. Prof Michael Kidd AO, Editor-in- Chief

Join the Editorial Board
We are recruiting Associate Editors to join our Editorial Board. Learn more about the role and how to apply here !

- Meet the Editors
Get to know the Editors behind Journal of Medical Case Reports !

megaflopp / Getty Images / iStock
Requirements for case reports submitted to JMCR
• Patient ethnicity must be included in the Abstract under the Case Presentation section.
• Consent for publication is a mandatory journal requirement for all case reports . Written informed consent for publication must be obtained from the patient (or their parent or legal guardian in the case of children under 18, or from the next of kin if the patient has died). For more information, please see our editorial policies .
Report of the Month
Superior mesenteric vein thrombosis due to covid-19 vaccination.
Vaccines have made a significant contribute to sowing the spread of the COVID-19 infection. However, side effects of the vaccination are beginning to appear, and one of which, thrombosis, is a particular problem as it can cuase serious complications. While cases of splanchnic venous thrombosis (SVT) after ChAdOx1 nCoV-19 vaccinations have been reported, cases of SVT mRNA-1273 vaccines are rare.
In this case report, clinicians describe a patient presenting with superior mesentric vein thrombosis following a COVID-19 vaccination, and examine the relationship between the mRNA-1273 vaccines and intestinal ischemia.
- Most accessed
Dilated cardiomyopathy due to hypocalcaemia: a case report
Authors: Nilushka Rupasinghe, Priyanga Ranasinghe and Leonard Wanninayake
Paratesticular cellular angiofibroma: a case report
Authors: Takaya Murashima, Kazutaka Kida, Toshihiro Gi, Takuya Hida, Masato Fujii, Takahiro Nagai, Hiroki Takamori, Shoichiro Mukai, Yuichiro Sato and Toshiyuki Kamoto
Anaplastic lymphoma kinase-positive pulmonary inflammatory myofibroblastic tumour: a case report
Authors: Daniel Tong, Julia Chisholm, Brendan Madden and Merina Ahmed
Documentation of a novel FBP1 gene mutation in the Arabian ethnicity: a case report
Authors: Maher Almousa, Mohammad Aljomaa, Shekhey Hamey and Diana Alasmar
Acute gastric dilatation with segmented abdominal paresis as a rare manifestation of herpes zoster: a case report and review of the literature
Authors: Toshihiko Yagyu, Yoshikazu Yakami and Tomoki Bando
Most recent articles RSS
View all articles
An itchy erythematous papular skin rash as a possible early sign of COVID-19: a case report
Authors: Alice Serafini, Peter Konstantin Kurotschka, Mariabeatrice Bertolani and Silvia Riccomi
Red ear syndrome precipitated by a dietary trigger: a case report
Authors: Chung Chi Chan and Susmita Ghosh
How to choose the best journal for your case report
Authors: Richard A. Rison, Jennifer Kelly Shepphird and Michael R. Kidd
The Erratum to this article has been published in Journal of Medical Case Reports 2017 11 :287
COVID-19 with repeated positive test results for SARS-CoV-2 by PCR and then negative test results twice during intensive care: a case report
Authors: Masafumi Kanamoto, Masaru Tobe, Tomonori Takazawa and Shigeru Saito
Recurrent knee arthritis diagnosed as juvenile idiopathic arthritis with a 10-year asymptomatic period after arthroscopic synovectomy: a case report
Authors: Atsushi Teramoto, Kota Watanabe, Yuichiro Kii, Miki Kudo, Hidenori Otsubo, Takuro Wada and Toshihiko Yamashita
Most accessed articles RSS
A Guide to Writing and Using Case Reports
This thematic series, published in 2016, provides a valuable resource for clinicians who are considered producing a case report. It comprises of a special editorial series of guides on writing, reviewing and using case reports.

Aims and scope
Journal of Medical Case Reports will consider any original case report that expands the field of general medical knowledge, and original research relating to case reports.
Case reports should show one of the following:
- Unreported or unusual side effects or adverse interactions involving medications
- Unexpected or unusual presentations of a disease
- New associations or variations in disease processes
- Presentations, diagnoses and/or management of new and emerging diseases
- An unexpected association between diseases or symptoms
- An unexpected event in the course of observing or treating a patient
- Findings that shed new light on the possible pathogenesis of a disease or an adverse effect
Suitable research articles include but are not limited to: N of 1 trials, meta-analyses of published case reports, research addressing the use of case reports and the prevalence or importance of case reporting in the medical literature and retrospective studies that include case-specific information (age, sex and ethnicity) for all patients.
Article accesses
Throughout 2022, articles were accessed from the journal website more than 4.17 million times; an average of over 11 ,400 accesses per day.

Latest Tweets
Your browser needs to have JavaScript enabled to view this timeline
Peer Review Mentoring Scheme
The Editors at Journal of Medical Case Reports endorse peer review mentoring of early career researchers.
If you are a senior researcher or professor and supervise an early career researcher with the appropriate expertise, we invite you to co-write and mentor them through the peer review process. Find out how to express your interest in the scheme here .
Call for Papers
The Journal of Medical Case Reports is calling for submissions to our Collection on COVID-19 – a look at the past, present and future of the pandemic . Guest Edited by Dr. Jean Karl Soler, The Family Practice Malta, Malta

About the Editor-in-Chief
Professor Michael Kidd AO FAHMS is foundation Director of the Centre for Future Health Systems at the University of New South Wales in Sydney, Australia, and Professor of Global Primary Care and Future Health Systems with the Nuffield Department of Primary Care Health Sciences at the University of Oxford. During the COVID-19 pandemic, Prof Kidd was the Deputy Chief Medical Officer and Principal Medical Advisor with the Australian Government Department of Health and Aged Care, and Professor of Primary Care Reform at the Australian National University. He holds honorary appointments with the University of Toronto, the University of Melbourne, Flinders University, and the Murdoch Children's Research Institute, and is the Emeritus Director of the World Health Organization Collaborating Centre on Family Medicine and Primary Care. He is an elected Fellow of the Australian Academy of Health and Medical Sciences (FAHMS). In the 2023 King's Birthday Honours List he was made an Officer of the Order of Australia. Prof Kidd served as president of the World Organization of Family Doctors (WONCA) from 2013-2016, and as president of the Royal Australian College of General Practitioners from 2002-2006. He is the founder and Editor-in-Chief of the Journal of Medical Case Reports, the world's first PubMed-listed journal devoted to publishing case reports from all medical disciplines.
- Editorial Board
- Manuscript editing services
- Instructions for Editors
- Sign up for article alerts and news from this journal
Annual Journal Metrics
2022 Citation Impact 1.0 - 2-year Impact Factor 0.628 - SNIP (Source Normalized Impact per Paper) 0.284 - SJR (SCImago Journal Rank)
2023 Speed 33 days submission to first editorial decision for all manuscripts (Median) 148 days submission to accept (Median)
2023 Usage 4,048,208 downloads 2,745 Altmetric mentions
- More about our metrics

- Follow us on Twitter
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Case Study Research Method in Psychology
Saul Mcleod, PhD
Editor-in-Chief for Simply Psychology
BSc (Hons) Psychology, MRes, PhD, University of Manchester
Saul Mcleod, PhD., is a qualified psychology teacher with over 18 years of experience in further and higher education. He has been published in peer-reviewed journals, including the Journal of Clinical Psychology.
Learn about our Editorial Process
Olivia Guy-Evans, MSc
Associate Editor for Simply Psychology
BSc (Hons) Psychology, MSc Psychology of Education
Olivia Guy-Evans is a writer and associate editor for Simply Psychology. She has previously worked in healthcare and educational sectors.
On This Page:
Case studies are in-depth investigations of a person, group, event, or community. Typically, data is gathered from various sources using several methods (e.g., observations & interviews).
The case study research method originated in clinical medicine (the case history, i.e., the patient’s personal history). In psychology, case studies are often confined to the study of a particular individual.
The information is mainly biographical and relates to events in the individual’s past (i.e., retrospective), as well as to significant events that are currently occurring in his or her everyday life.
The case study is not a research method, but researchers select methods of data collection and analysis that will generate material suitable for case studies.
Freud (1909a, 1909b) conducted very detailed investigations into the private lives of his patients in an attempt to both understand and help them overcome their illnesses.
This makes it clear that the case study is a method that should only be used by a psychologist, therapist, or psychiatrist, i.e., someone with a professional qualification.
There is an ethical issue of competence. Only someone qualified to diagnose and treat a person can conduct a formal case study relating to atypical (i.e., abnormal) behavior or atypical development.
Famous Case Studies
- Anna O – One of the most famous case studies, documenting psychoanalyst Josef Breuer’s treatment of “Anna O” (real name Bertha Pappenheim) for hysteria in the late 1800s using early psychoanalytic theory.
- Little Hans – A child psychoanalysis case study published by Sigmund Freud in 1909 analyzing his five-year-old patient Herbert Graf’s house phobia as related to the Oedipus complex.
- Bruce/Brenda – Gender identity case of the boy (Bruce) whose botched circumcision led psychologist John Money to advise gender reassignment and raise him as a girl (Brenda) in the 1960s.
- Genie Wiley – Linguistics/psychological development case of the victim of extreme isolation abuse who was studied in 1970s California for effects of early language deprivation on acquiring speech later in life.
- Phineas Gage – One of the most famous neuropsychology case studies analyzes personality changes in railroad worker Phineas Gage after an 1848 brain injury involving a tamping iron piercing his skull.
Clinical Case Studies
- Studying the effectiveness of psychotherapy approaches with an individual patient
- Assessing and treating mental illnesses like depression, anxiety disorders, PTSD
- Neuropsychological cases investigating brain injuries or disorders
Child Psychology Case Studies
- Studying psychological development from birth through adolescence
- Cases of learning disabilities, autism spectrum disorders, ADHD
- Effects of trauma, abuse, deprivation on development
Types of Case Studies
- Explanatory case studies : Used to explore causation in order to find underlying principles. Helpful for doing qualitative analysis to explain presumed causal links.
- Exploratory case studies : Used to explore situations where an intervention being evaluated has no clear set of outcomes. It helps define questions and hypotheses for future research.
- Descriptive case studies : Describe an intervention or phenomenon and the real-life context in which it occurred. It is helpful for illustrating certain topics within an evaluation.
- Multiple-case studies : Used to explore differences between cases and replicate findings across cases. Helpful for comparing and contrasting specific cases.
- Intrinsic : Used to gain a better understanding of a particular case. Helpful for capturing the complexity of a single case.
- Collective : Used to explore a general phenomenon using multiple case studies. Helpful for jointly studying a group of cases in order to inquire into the phenomenon.
Where Do You Find Data for a Case Study?
There are several places to find data for a case study. The key is to gather data from multiple sources to get a complete picture of the case and corroborate facts or findings through triangulation of evidence. Most of this information is likely qualitative (i.e., verbal description rather than measurement), but the psychologist might also collect numerical data.
1. Primary sources
- Interviews – Interviewing key people related to the case to get their perspectives and insights. The interview is an extremely effective procedure for obtaining information about an individual, and it may be used to collect comments from the person’s friends, parents, employer, workmates, and others who have a good knowledge of the person, as well as to obtain facts from the person him or herself.
- Observations – Observing behaviors, interactions, processes, etc., related to the case as they unfold in real-time.
- Documents & Records – Reviewing private documents, diaries, public records, correspondence, meeting minutes, etc., relevant to the case.
2. Secondary sources
- News/Media – News coverage of events related to the case study.
- Academic articles – Journal articles, dissertations etc. that discuss the case.
- Government reports – Official data and records related to the case context.
- Books/films – Books, documentaries or films discussing the case.
3. Archival records
Searching historical archives, museum collections and databases to find relevant documents, visual/audio records related to the case history and context.
Public archives like newspapers, organizational records, photographic collections could all include potentially relevant pieces of information to shed light on attitudes, cultural perspectives, common practices and historical contexts related to psychology.
4. Organizational records
Organizational records offer the advantage of often having large datasets collected over time that can reveal or confirm psychological insights.
Of course, privacy and ethical concerns regarding confidential data must be navigated carefully.
However, with proper protocols, organizational records can provide invaluable context and empirical depth to qualitative case studies exploring the intersection of psychology and organizations.
- Organizational/industrial psychology research : Organizational records like employee surveys, turnover/retention data, policies, incident reports etc. may provide insight into topics like job satisfaction, workplace culture and dynamics, leadership issues, employee behaviors etc.
- Clinical psychology : Therapists/hospitals may grant access to anonymized medical records to study aspects like assessments, diagnoses, treatment plans etc. This could shed light on clinical practices.
- School psychology : Studies could utilize anonymized student records like test scores, grades, disciplinary issues, and counseling referrals to study child development, learning barriers, effectiveness of support programs, and more.
How do I Write a Case Study in Psychology?
Follow specified case study guidelines provided by a journal or your psychology tutor. General components of clinical case studies include: background, symptoms, assessments, diagnosis, treatment, and outcomes. Interpreting the information means the researcher decides what to include or leave out. A good case study should always clarify which information is the factual description and which is an inference or the researcher’s opinion.
1. Introduction
- Provide background on the case context and why it is of interest, presenting background information like demographics, relevant history, and presenting problem.
- Compare briefly to similar published cases if applicable. Clearly state the focus/importance of the case.
2. Case Presentation
- Describe the presenting problem in detail, including symptoms, duration,and impact on daily life.
- Include client demographics like age and gender, information about social relationships, and mental health history.
- Describe all physical, emotional, and/or sensory symptoms reported by the client.
- Use patient quotes to describe the initial complaint verbatim. Follow with full-sentence summaries of relevant history details gathered, including key components that led to a working diagnosis.
- Summarize clinical exam results, namely orthopedic/neurological tests, imaging, lab tests, etc. Note actual results rather than subjective conclusions. Provide images if clearly reproducible/anonymized.
- Clearly state the working diagnosis or clinical impression before transitioning to management.
3. Management and Outcome
- Indicate the total duration of care and number of treatments given over what timeframe. Use specific names/descriptions for any therapies/interventions applied.
- Present the results of the intervention,including any quantitative or qualitative data collected.
- For outcomes, utilize visual analog scales for pain, medication usage logs, etc., if possible. Include patient self-reports of improvement/worsening of symptoms. Note the reason for discharge/end of care.
4. Discussion
- Analyze the case, exploring contributing factors, limitations of the study, and connections to existing research.
- Analyze the effectiveness of the intervention,considering factors like participant adherence, limitations of the study, and potential alternative explanations for the results.
- Identify any questions raised in the case analysis and relate insights to established theories and current research if applicable. Avoid definitive claims about physiological explanations.
- Offer clinical implications, and suggest future research directions.
5. Additional Items
- Thank specific assistants for writing support only. No patient acknowledgments.
- References should directly support any key claims or quotes included.
- Use tables/figures/images only if substantially informative. Include permissions and legends/explanatory notes.
- Provides detailed (rich qualitative) information.
- Provides insight for further research.
- Permitting investigation of otherwise impractical (or unethical) situations.
Case studies allow a researcher to investigate a topic in far more detail than might be possible if they were trying to deal with a large number of research participants (nomothetic approach) with the aim of ‘averaging’.
Because of their in-depth, multi-sided approach, case studies often shed light on aspects of human thinking and behavior that would be unethical or impractical to study in other ways.
Research that only looks into the measurable aspects of human behavior is not likely to give us insights into the subjective dimension of experience, which is important to psychoanalytic and humanistic psychologists.
Case studies are often used in exploratory research. They can help us generate new ideas (that might be tested by other methods). They are an important way of illustrating theories and can help show how different aspects of a person’s life are related to each other.
The method is, therefore, important for psychologists who adopt a holistic point of view (i.e., humanistic psychologists ).
Limitations
- Lacking scientific rigor and providing little basis for generalization of results to the wider population.
- Researchers’ own subjective feelings may influence the case study (researcher bias).
- Difficult to replicate.
- Time-consuming and expensive.
- The volume of data, together with the time restrictions in place, impacted the depth of analysis that was possible within the available resources.
Because a case study deals with only one person/event/group, we can never be sure if the case study investigated is representative of the wider body of “similar” instances. This means the conclusions drawn from a particular case may not be transferable to other settings.
Because case studies are based on the analysis of qualitative (i.e., descriptive) data , a lot depends on the psychologist’s interpretation of the information she has acquired.
This means that there is a lot of scope for Anna O , and it could be that the subjective opinions of the psychologist intrude in the assessment of what the data means.
For example, Freud has been criticized for producing case studies in which the information was sometimes distorted to fit particular behavioral theories (e.g., Little Hans ).
This is also true of Money’s interpretation of the Bruce/Brenda case study (Diamond, 1997) when he ignored evidence that went against his theory.
Breuer, J., & Freud, S. (1895). Studies on hysteria . Standard Edition 2: London.
Curtiss, S. (1981). Genie: The case of a modern wild child .
Diamond, M., & Sigmundson, K. (1997). Sex Reassignment at Birth: Long-term Review and Clinical Implications. Archives of Pediatrics & Adolescent Medicine , 151(3), 298-304
Freud, S. (1909a). Analysis of a phobia of a five year old boy. In The Pelican Freud Library (1977), Vol 8, Case Histories 1, pages 169-306
Freud, S. (1909b). Bemerkungen über einen Fall von Zwangsneurose (Der “Rattenmann”). Jb. psychoanal. psychopathol. Forsch ., I, p. 357-421; GW, VII, p. 379-463; Notes upon a case of obsessional neurosis, SE , 10: 151-318.
Harlow J. M. (1848). Passage of an iron rod through the head. Boston Medical and Surgical Journal, 39 , 389–393.
Harlow, J. M. (1868). Recovery from the Passage of an Iron Bar through the Head . Publications of the Massachusetts Medical Society. 2 (3), 327-347.
Money, J., & Ehrhardt, A. A. (1972). Man & Woman, Boy & Girl : The Differentiation and Dimorphism of Gender Identity from Conception to Maturity. Baltimore, Maryland: Johns Hopkins University Press.
Money, J., & Tucker, P. (1975). Sexual signatures: On being a man or a woman.
Further Information
- Case Study Approach
- Case Study Method
- Enhancing the Quality of Case Studies in Health Services Research
- “We do things together” A case study of “couplehood” in dementia
- Using mixed methods for evaluating an integrative approach to cancer care: a case study
- Introduction
- Conclusions
- Article Information
The shaded area indicates the upper and lower limits of the 95% CIs.
DOAC indicates direct oral anticoagulant; IRR, incidence rate ratio; and VKA, vitamin K antagonist.
eMethods 1. Interaction
eMethods 2. Time-Conditional Propensity Score-Matched Analysis
eFigure. Flowchart of Patient Selection in Study Cohort and Case-Control Selection
eTable 1. ICD-10 Codes Used to Define Major Bleeding
eTable 2. Crude and Adjusted IRRs of Major Bleeding Associated With the Continuous Duration of Concomitant Use of SSRIs With OACs, Compared With OAC Use Alone
eTable 3. Crude and Adjusted IRRs of Major Bleeding Associated With Concomitant Use of SSRIs With OACs, Stratified by Age, Sex, History of Bleeding, and History of Chronic Kidney Disease
eTable 4. Crude and Adjusted IRRs of Major Bleeding Associated With Concomitant Use of Strong and Moderate SSRIs With OACs
eTable 5. Crude and Adjusted IRRs of Any Bleeding Associated With Concomitant Use of SSRIs With OACs, Compared With OAC Use Alone
eTable 6. Assessment of Additive and Multiplicative Interaction Between SSRIs and OACs, With Respect to Major Bleeding
eTable 7. Crude and Adjusted IRRs of Major Bleeding Associated With Concomitant Use of SSRIs With OACs, Varying the Exposure Assessment Window
eTable 8. Crude and Adjusted IRRs of Major Bleeding Associated With Concomitant Use of SSRIs With OACs, With Covariates Measured Prior to Cohort Entry
eTable 9. Crude and Adjusted IRRs of Major Bleeding Associated With Concomitant Use of SSRIs With OACs, After Multiple Imputation of Missing BMI and Smoking Values
eTable 10. Crude and Adjusted IRRs of Major Bleeding Associated With Concomitant Use of SSRIs With OACs, Compared With OAC Use Alone, by Type of OAC and Excluding Patients With Valvular AF
eTable 11. Adjusted HRs of Major Bleeding Associated With Concomitant Use of SSRIs With OACs Compared With OAC Use Alone, in a Time-Conditional Propensity Score-Matched Analysis
eTable 12. Crude and Adjusted IRRs of Major Bleeding Associated With Concomitant Use of SSRIs With OACs, With Adjustment for Additional Comedications Interacting With OACs
eReferences.
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Rahman AA , Platt RW , Beradid S , Boivin J , Rej S , Renoux C. Concomitant Use of Selective Serotonin Reuptake Inhibitors With Oral Anticoagulants and Risk of Major Bleeding. JAMA Netw Open. 2024;7(3):e243208. doi:10.1001/jamanetworkopen.2024.3208
Manage citations:
© 2024
- Permissions
Concomitant Use of Selective Serotonin Reuptake Inhibitors With Oral Anticoagulants and Risk of Major Bleeding
- 1 Department of Epidemiology, Biostatistics and Occupational Health, McGill University, Montreal, Quebec, Canada
- 2 Centre for Clinical Epidemiology, Lady Davis Institute for Medical Research, Jewish General Hospital, Montreal, Quebec, Canada
- 3 Department of Pediatrics, McGill University, Montreal, Quebec, Canada
- 4 Department of Psychiatry, McGill University, Montreal, Quebec, Canada
- 5 Department of Neurology and Neurosurgery, McGill University, Montreal, Quebec, Canada
- 6 Department of Medicine, McGill University, Montreal, Quebec, Canada
Question Is there an association between concomitant use of selective serotonin reuptake inhibitors (SSRIs) and oral anticoagulants (OACs) and the risk of major bleeding among patients with atrial fibrillation compared with OAC use alone?
Findings In this nested case-control study comprising 42 190 cases with major bleeding matched to 1 156 641 controls, concomitant SSRI and OAC use was associated with a 33% increased risk of major bleeding compared with OAC use alone; this risk was highest in the first few months of concomitant use and was substantially lower after 6 months.
Meaning This study suggests that concomitant use of SSRIs and OACs may be a risk factor for bleeding and should be closely monitored, particularly within the initial months of treatment.
Importance Selective serotonin reuptake inhibitors (SSRIs) are commonly prescribed antidepressants associated with a small increased risk of major bleeding. However, the risk of bleeding associated with the concomitant use of SSRIs and oral anticoagulants (OACs) has not been well characterized.
Objectives To assess whether concomitant use of SSRIs with OACs is associated with an increased risk of major bleeding compared with OAC use alone, describe how the risk varies with duration of use, and identify key clinical characteristics modifying this risk.
Design, Setting, and Participants A population-based, nested case-control study was conducted among patients with atrial fibrillation initiating OACs between January 2, 1998, and March 29, 2021. Patients were from approximately 2000 general practices in the UK contributing to the Clinical Practice Research Datalink. With the use of risk-set sampling, for each case of major bleeding during follow-up, up to 30 controls were selected from risk sets defined by the case and matched on age, sex, cohort entry date, and follow-up duration.
Exposures Concomitant use of SSRIs and OACs (direct OACs and vitamin K antagonists [VKAs]) compared with OAC use alone.
Main Outcomes and Measures The main outcome was incidence rate ratios (IRRs) of hospitalization for bleeding or death due to bleeding.
Results There were 42 190 patients with major bleeding (mean [SD] age, 74.2 [9.3] years; 59.8% men) matched to 1 156 641 controls (mean [SD] age, 74.2 [9.3] years; 59.8% men). Concomitant use of SSRIs and OACs was associated with an increased risk of major bleeding compared with OACs alone (IRR, 1.33; 95% CI, 1.24-1.42). The risk peaked during the initial months of treatment (first 30 days of use: IRR, 1.74; 95% CI, 1.37-2.22) and persisted for up to 6 months. The risk did not vary with age, sex, history of bleeding, chronic kidney disease, and potency of SSRIs. An association was present both with concomitant use of SSRIs and direct OACs compared with direct OAC use alone (IRR, 1.25; 95% CI, 1.12-1.40) and concomitant use of SSRIs and VKAs compared with VKA use alone (IRR, 1.36; 95% CI, 1.25-1.47).
Conclusions and Relevance This study suggests that among patients with atrial fibrillation, concomitant use of SSRIs and OACs was associated with an increased risk of major bleeding compared with OAC use alone, requiring close monitoring and management of risk factors for bleeding, particularly in the first few months of use.
Antidepressant medications are among the most frequently prescribed class of drugs worldwide, with up to 19% of individuals aged 60 years or older in the US reporting use of an antidepressant over the past 30 days. 1 Selective serotonin reuptake inhibitors (SSRIs) are the most widely used antidepressant medications and are often recommended over other classes of antidepressants for the treatment of major depressive disorder due to their comparable efficacy and favorable safety profile. 2 , 3 However, SSRIs have been shown to increase the risk of major bleeding, 4 - 14 possibly owing to their inhibition of platelet activation during hemostasis. 2 Although the absolute risk remains low for most individuals who use SSRIs, 11 , 12 , 15 coprescription with drugs such as oral anticoagulants (OACs) may be consequential. Concomitant use of SSRIs and OACs is common given the prevalence of mental health disorders. 16
Some observational studies have assessed the association between concomitant use of SSRIs and OACs and the risk of major bleeding. However, some had notable limitations, including exposure misclassification, 17 possible informative censoring, 18 , 19 residual confounding, 19 - 21 and limited statistical power. 20 , 22 - 24 Gaps in evidence that may inform the coprescription of SSRIs and OACs include whether the risk varies with demographic or clinical characteristics or between direct OACs (DOACs) and vitamin K antagonists (VKAs). In addition, data on the risk of specific types of major bleeding are limited. 25
To address these knowledge gaps, we conducted a population-based, nested case-control study to assess whether the concomitant use of SSRIs and OACs was associated with the risk of major bleeding compared with OAC use alone among patients with atrial fibrillation (AF). We also assessed whether the risk varied by duration of use, relevant demographic and other risk factors, potency of SSRIs, and OAC type.
In this population-based, nested case-control study, we used the UK Clinical Practice Research Datalink (CPRD GOLD and Aurum databases), a large primary care database of electronic medical records that contains demographic and lifestyle information, medical diagnoses, prescriptions, and referrals for more than 60 million patients from more than 2000 general practices. 26 , 27 These data are representative of the UK population in terms of age, sex, and race and ethnicity. 26 , 27 Drug prescriptions issued by the general practitioner are automatically recorded at the time of prescription. 26 , 27 Quality control audits of the CPRD are regularly conducted to ensure the accuracy and completeness of data. 26 , 27 The CPRD was linked with the Hospital Episodes Statistics repository, which contains details of inpatient and day case admissions, 28 and the Office for National Statistics database, which contains electronic death certificates. 29 The study protocol was approved by the CPRD Research Data Governance (No. 22_001906) and the Research Ethics Board of the Jewish General Hospital in Montreal, Canada, which also waived the need for patient informed consent as the data were deidentified. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline. 30
We conducted a population-based study with a nested case-control approach to analysis because of its computational efficiency compared with a full-cohort analysis given the time-varying nature of both medications of interest, the size of the cohort, and the long duration of follow-up. 31 We first identified all patients aged 18 years or older with an incident diagnosis of AF between January 2, 1998, and March 29, 2021, and at least 1 year of registration with the practice before AF diagnosis. From this base cohort, we selected those with a prescription for an OAC (apixaban, dabigatran, edoxaban, rivaroxaban, or warfarin) after AF diagnosis, with the date of first prescription defined as study cohort entry. We excluded patients who received OACs any time before cohort entry or SSRIs 6 months prior to cohort entry. We also excluded patients with hyperthyroidism in the year prior to cohort entry because AF in association with hyperthyroidism rarely requires long-term oral anticoagulation. Patients meeting these criteria were followed up until a first major bleeding event, death, end of registration with the practice, or end of the study period (March 29, 2021), whichever occurred first.
We identified cases as patients with a first recorded diagnosis of major bleeding during follow-up, defined as hospitalization with a primary diagnosis of major bleeding or death with bleeding as the primary cause, using relevant International Statistical Classification of Diseases and Related Health Problems, Tenth Revision ( ICD-10 ) codes (eTable 1 in Supplement 1 ); elective hospitalizations were not considered. ICD-10 codes for bleeding have shown good positive predictive values between 81% and 95%. 32 - 34 The index date was the date of hospital admission. For each case, we randomly selected up to 30 controls among the cohort members from the risk sets defined by the case. Each risk set, at each case’s index date, included all individuals who did not experience major bleeding and thus were still at risk up to that point in follow-up time, matched on age, sex, calendar date of cohort entry (±6 months), and duration of follow-up. Thus, as per the risk-set sampling approach, cases were eligible for selection as controls prior to becoming a case, and patients may have been selected as controls for multiple cases. 35 , 36 The index date for controls was the date resulting in the same duration of follow-up for cases and controls.
We identified prescriptions of SSRIs (citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, or sertraline) and OACs for all cases and their matched controls between cohort entry and the index date. Exposure was defined in 4 mutually exclusive categories: concomitant use of SSRIs and OACs, OAC use alone, nonuse, and other use. We considered patients as concomitant users of SSRIs and OACs if the duration of their last prescription for both medications covered or ended 30 days before the index date. Similarly, we considered patients as users of OACs alone if their last prescription for an OAC covered or ended 30 days before the index date, without a prescription for an SSRI in this period—this was the reference category. Users of SSRIs alone, non-SSRI antidepressants alone, or non-SSRI antidepressants concomitantly with SSRIs and/or OACs were classified separately (other use). Finally, nonusers were those not exposed to any medications of interest on or 30 days before the index date.
We adjusted all models for the following comorbidities based on substantive knowledge, measured at or earlier than 365 days (1 year) before the index date: smoking, alcohol abuse, body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) (<25, 25-29.9, or ≥30.0), depression, hypertension, diabetes, stroke or transient ischemic attack, coronary artery disease, congestive heart failure, peripheral arterial disease, disorders of hemostasis, cancer (other than nonmelanoma skin cancer), liver disease, chronic kidney disease, chronic obstructive pulmonary disease, anemia, and venous thromboembolism. We also included history of bleeding at any time before cohort entry and the time between incident AF diagnosis and first OAC prescription. Diabetes and hypertension were defined using diagnostic codes or relevant medications. All models were also adjusted for use of the following drugs measured between 365 days (1 year) and 730 days (2 years) prior to the index date: angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, β-blockers, calcium channel blockers, thiazide diuretics, other diuretics, antiplatelets, lipid-lowering drugs (including statins), antipsychotics, non-SSRI antidepressants, nonsteroidal anti-inflammatory drugs, proton pump inhibitors, and H 2 receptor blockers. We considered the number of hospitalizations in the year before cohort entry as a surrogate marker for overall health. Finally, we adjusted for socioeconomic status using the Index of Multiple Deprivation, categorized in deciles. 37
We used conditional logistic regression to compute odds ratios of major bleeding associated with concomitant use of SSRIs and OACs compared with OAC use alone, adjusting for the covariates listed. In a nested case-control approach, odds ratios are unbiased estimators of incidence rate ratios (IRRs) with very limited loss in precision. 36 In secondary analyses, we assessed whether the risk of major bleeding varied according to age, sex, chronic kidney disease, history of bleeding, type of OAC (DOACs or VKAs), and potency of SSRIs (strong or moderate serotonin reuptake inhibitors based on the dissociation constant). 38 Next, among patients continuously exposed to OACs and concomitantly exposed to SSRIs and OACs at the index date, we investigated whether the risk of major bleeding varied with the duration of continuous concomitant use of SSRIs in 3 prespecified categories (≤30 days, 31-180 days, or >180 days) compared with OAC use alone. These categories were selected because SSRIs were reported to exert antiplatelet action as early as 2 to 3 weeks after initiation. 39 , 40 We defined continuous exposure to SSRIs and OACs separately, allowing a 30-day grace period between consecutive prescriptions where patients were still considered exposed. Patients were then considered concomitant users on any given day if exposed to both drugs on that day. In addition, we used a restricted cubic spline with 5 interior knots to produce a smooth curve of the IRR as a function of continuous duration of use. We also estimated the risk in specific anatomical locations, including gastrointestinal bleeding, intracranial hemorrhage, and other major bleeding. We assessed the risk of any bleeding associated with concomitant use of SSRIs and OACs. For this analysis, we repeated the selection of cases and controls already described, with cases defined using relevant diagnostic codes in primary electronic medical records. Finally, we assessed whether an interaction was present between SSRIs and OACs with respect to the risk of major bleeding on both the additive and multiplicative scales (eMethods 1 in Supplement 1 ). In other words, we assessed whether the joint association of the 2 exposures departed from the sum or product of their individual associations with the risk of bleeding, although an additive interaction has been described as most indicative of biological or mechanistic interaction. 41 , 42
We performed 4 sensitivity analyses to assess the robustness of the results. First, to explore potential exposure misclassification, we considered only prescriptions that covered the index date and, next, those that covered or ended within 15 days before the index date. Second, to account for the potential adjustment for covariates affected by exposure, all covariates were measured at or prior to cohort entry. Third, we implemented multiple imputation by chained equations for missing values of BMI and smoking, combining results from 5 imputed datasets. 43 Fourth, we repeated the analysis by type of OAC, excluding patients with a history of valvular surgery or rheumatic valvular disease before cohort entry because DOACs are not indicated for patients with valvular AF. 44 - 46
We conducted a supplementary time-conditional propensity score–matched analysis to further explore the potential for residual confounding. 47 , 48 In brief, among the base cohort of patients with incident AF initiating OACs, we matched each patient initiating SSRIs to a patient using OACs alone up to that point in time with the same age (±1 year), sex, calendar date of OAC initiation (±1 year), and time-conditional propensity score (eMethods 2 in Supplement 1 ). Finally, we conducted a post hoc analysis, repeating the primary analysis with additional adjustment for the following comedications reported to interact with OACs, measured between 1 and 2 years before the index date: clarithromycin, erythromycin, penicillin, azole antifungals, quinidine, amiodarone, dronedarone, propafenone, allopurinol, oral corticosteroids, tamoxifen, valproic acid, cyclosporin, tacrolimus, disulfiram, methylphenidate, and sulfamethoxazole. All analyses were performed with a 2-sided hypothesis test, and P < .05 was considered statistically significant, without adjustment for multiple comparisons, using SAS, version 9.4 (SAS Institute Inc).
After applying all eligibility criteria, the cohort included 331 305 patients (mean [SD] age, 73.7 [10.8] years; 57.1% men) with incident AF initiating OACs (eFigure in Supplement 1 ). During a mean (SD) follow-up of 4.6 (4.0) years, 42 391 patients were hospitalized with major bleeding, yielding an incidence rate of 27.9 per 1000 person-years (95% CI, 27.7-28.2 per 1000 person-years). Among those, 42 190 cases (mean [SD] age, 74.2 [9.3] years; 59.8% men) were matched to 1 156 641 controls (mean [SD] age, 74.2 [9.3] years; 59.8% men). As anticipated, risk factors for major bleeding were more prevalent among cases than controls ( Table 1 ).
Concomitant use of SSRIs and OACs was associated with an increased risk of major bleeding compared with OAC use alone (IRR, 1.33; 95% CI, 1.24-1.42) ( Table 2 ). The risk was the highest during the first 30 days of continuous use (IRR, 1.74; 95% CI, 1.37-2.22), and decreased thereafter (eTable 2 in Supplement 1 ). This trend was also observed when modeling the IRR flexibly as a spline of the duration of continuous use ( Figure 1 ). The risk did not vary according to age, sex, history of major bleeding, chronic kidney disease ( Figure 2 ; eTable 3 in Supplement 1 ), or potency of SSRIs (eTable 4 in Supplement 1 ). The risk of major bleeding was associated with concomitant use of SSRIs and DOACs compared with DOACs alone (IRR, 1.25; 95% CI, 1.12-1.40) and with concomitant use of SSRIs and VKAs compared with VKAs alone (IRR, 1.36; 95% CI, 1.25-1.47) ( Table 3 ). With respect to types of major bleeding, the association was present for intracranial hemorrhage, gastrointestinal bleeding, and other major bleeding ( Table 2 ). Last, concomitant use of SSRIs and OACs was also associated with the risk of any bleeding (IRR, 1.22; 95% CI, 1.16-1.28) compared with OAC use alone (eTable 5 in Supplement 1 ).
In the assessment of interaction, a small superadditive interaction may have been present, although the estimate was not statistically significant (relative excess risk due to interaction [RERI], 0.10; 95% CI, −0.07 to 0.27) (eTable 6 in Supplement 1 ). Based on the estimated RERI, the interaction may be associated with approximately 5% of all major bleeding. In addition, there was limited evidence of a multiplicative interaction. Results from sensitivity analyses were consistent with those of the primary analysis (eTables 7-10 in Supplement 1 ). Finally, the association remained in the time-conditional propensity score–matched analysis, although slightly attenuated (adjusted hazard ratio, 1.23; 95% CI, 1.08-1.40) (eTable 11 in Supplement 1 ), and was consistent in the post hoc analysis (eTable 12 in Supplement 1 ).
In this population-based, nested case-control study, the concomitant use of SSRIs and OACs was associated with a 33% increased risk of major bleeding. The association was the strongest for the first few months of concomitant use. The overall risk remained consistent regardless of age, sex, potency of SSRIs, history of major bleeding, or chronic kidney disease as well as type of OAC. Concomitant use was individually associated with gastrointestinal bleeding, intracranial hemorrhage, and other major bleeding. Interaction between SSRIs and OACs, if any, was limited.
In light of these findings, the risk of major bleeding may be a pertinent safety consideration for patients using SSRIs and OACs concomitantly. This finding has been echoed in the summary of product characteristics for different OACs, which describes SSRIs as interacting drugs given that they independently increase the risk of bleeding. Although clinical guidelines for the management of major depressive disorder have acknowledged the risk of bleeding associated with SSRIs, the potential for interaction with OACs was either not discussed or based on very limited evidence. 49 , 50 Likewise, guidelines from Canadian, US, and European cardiology associations for the management of AF suggest consideration of drug-drug interactions when prescribing OACs, 44 - 46 with nonsteroidal anti-inflammatory drugs being the only class of drugs cited. 45 Although the European Heart Rhythm Association lists SSRIs as drugs with pharmacodynamic interactions with DOACs, no evidence was cited. 51
The risk of major bleeding associated with the concomitant use of SSRIs and OACs has been assessed in previous observational studies, although results were inconsistent. 7 , 17 - 20 , 22 - 24 , 52 Limitations of previous studies included residual confounding, 19 , 20 , 22 , 24 varying exposure definitions, limited statistical power, 20 , 22 - 24 and the assessment of the concomitant use of SSRIs and OACs being a secondary objective. 7 , 18 In line with our results, a systematic review and meta-analysis of 8 observational studies suggested an increased risk of major bleeding associated with the concomitant use of SSRIs and OACs (hazard ratio, 1.35; 95% CI, 1.14-1.58) compared with OAC use alone. 25 However, several knowledge gaps were identified, including the risk of major bleeding in important patient subgroups. The present study confirmed that compared with OACs alone, concomitant use of SSRIs and OACs increased the risk of major bleeding among patients 60 years of age or older and among both sexes. One study previously assessed this association in these patient subgroups; however, statistical power was limited. 22 Furthermore, we showed that the association remained similar irrespective of patients’ history of major bleeding or chronic kidney disease, both important factors in the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile International Normalized Ratio, Elderly [>65 Years], Drugs/Alcohol Concomitantly) score for major bleeding risk. 53 Our findings also suggested that the concomitant use of SSRIs with both DOACs and VKAs was associated with an increased risk of major bleeding, with a possible lower risk with DOACs, although 95% CIs overlapped. A cohort study of patients from the ROCKET-AF (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Embolism and Stroke Trial in Atrial Fibrillation) trial suggested that the concomitant use of SSRIs and warfarin may increase the risk of major bleeding compared with rivaroxaban; however, the results were limited by high uncertainty and potential for selection bias and confounding. 20 In another nested case-control study of nursing home residents, similar increases in risk were associated with the concomitant use of SSRIs and DOACs and of SSRIs and VKAs; however, statistical power was low. 23
The increased risk of major bleeding with the concomitant use of SSRIs and OACs may occur through multiple mechanisms of action. During hemostasis, serotonin is released by platelets to enhance platelet activation and aggregation and prime them to interact with coagulation factors. 54 Selective serotonin reuptake inhibitors block the serotonin reuptake transporter on platelet membranes and reduce serotonin content within platelets by up to 80% to 90%, 39 , 40 decreasing the potency of hemostasis over time. In addition, some SSRIs, such as fluoxetine and fluvoxamine, inhibit the 1A2 and 2C9 isozymes of the hepatic cytochrome P450 enzyme, which play a key role in the metabolism of warfarin. 55 Nonetheless, the interaction analysis suggests that the joint association of SSRIs and OACs is mainly owing to their individual risks of major bleeding; hence, any additional risk posed by pharmacokinetic interaction is likely minimal.
Although the increased risk of major bleeding does not suggest withholding treatment with either SSRIs or OACs, measures can be taken to mitigate this risk. Studies suggest that DOACs have lower potential for pharmacokinetic interactions with SSRIs than VKAs, and guidelines also recommend them over VKAs for the management of nonvalvular AF. 44 - 46 , 55 , 56 Taken together with the findings in this study, DOACs may also be preferred for patients concomitantly using SSRIs. On the other hand, the risk of major bleeding was similar between SSRIs with more potent inhibition and SSRIs with less potent serotonin inhibition; thus; changing the SSRI may not be associated with bleeding risk. Finally, coprescription of proton pump inhibitors has also been suggested to prevent gastrointestinal bleeding. 51 , 57 Overall, risk factors for bleeding should be monitored and managed to improve the safety of the concomitant use of SSRIs and OACs. 51 Close monitoring is particularly essential within the first few months of concomitant use.
This study has notable strengths. First, the selection of a large study population from routine care settings enhanced generalizability and provided sufficient statistical power to generate precise estimates in primary and secondary analyses. Second, selection bias was unlikely because we analyzed a well-defined cohort and used a nested case-control approach. Third, the assessment of additive and multiplicative interactions provided evidence suggesting that any biological interaction between use of SSRIs and OACs and the risk of major bleeding may only be marginally synergistic. 42
This study also has some limitations. Residual confounding may affect the results given the observational nature of the study. The baseline risk for major bleeding may differ between patients concomitantly using SSRIs and those who were not. To mitigate potential bias, we adjusted for several potential confounders, including some lifestyle risk factors (such as BMI, smoking, and alcohol abuse). Furthermore, the results remained consistent in the time-conditional propensity score–matched analysis and in a post hoc analysis adjusted for additional comedications. Another consideration is that prescriptions recorded in the CPRD are those issued by general practitioners; hence, misclassification of exposure is possible if patients do not follow the treatment regimen. Prescriptions also do not include those issued by specialists, although AF as well as mild and moderate depression are managed mainly by general practitioners in the UK. 58 , 59 To explore the potential for misclassification, we varied the exposure assessment window in sensitivity analyses, which produced results consistent with the main results. Finally, outcome misclassification through inaccurate recording of bleeding in the Hospital Episodes Statistics repository may occur. In addition, the physician’s judgment may be influenced by knowledge of patient treatment. To mitigate bias, we considered only primary diagnoses and did not include elective hospitalizations.
In this large population-based, nested case-control study of patients with AF, the concomitant use of SSRIs and OACs was associated with an increased risk of major bleeding compared with OACs alone. To minimize the risk of bleeding, individual modifiable risk factors should be controlled, and patients should be closely monitored, particularly during the first few months of concomitant use.
Accepted for Publication: January 26, 2024.
Published: March 22, 2024. doi:10.1001/jamanetworkopen.2024.3208
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2024 Rahman AA et al. JAMA Network Open .
Corresponding Author: Christel Renoux, MD, PhD, Centre for Clinical Epidemiology, Lady Davis Institute for Medical Research, Jewish General Hospital, 3755 Côte Sainte Catherine H 416.1, Montreal, QC H3T 1E2, Canada ( [email protected] ).
Author Contributions: Mr Rahman and Dr Renoux had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Rahman, Platt, Boivin, Rej, Renoux.
Acquisition, analysis, or interpretation of data: Rahman, Platt, Beradid, Rej, Renoux.
Drafting of the manuscript: Rahman, Boivin, Renoux.
Critical review of the manuscript for important intellectual content: Rahman, Platt, Beradid, Rej, Renoux.
Statistical analysis: Rahman, Beradid, Renoux.
Administrative, technical, or material support: Renoux.
Supervision: Platt, Boivin, Rej, Renoux.
Conflict of Interest Disclosures: Dr Platt reported receiving personal fees from Biogen, Boehringer Ingelheim, Merck, Nant Pharma, and Pfizer outside the submitted work. Dr Rej reported receiving a Clinician-Scientist Salary Award from Fonds de Recherche Quebec Sante; receiving grants from Mitacs; serving on a steering committee for AbbVie; and holding shares in Aifred outside the submitted work. No other disclosures were reported.
Funding/Support: Mr Rahman was supported by a Tomlinson Doctoral Fellowship from McGill University, a Canada Graduate Scholarship–Doctoral from the Canadian Institutes of Health Research, and a stipend from the Drug Safety and Effectiveness Cross-Training Program.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement 2 .
Additional Contributions: The authors would like to thank Sophie Dell’Aniello, MSc, Centre for Clinical Epidemiology, Lady Davis Institute for Medical Research, Jewish General Hospital, for providing technical support in the analysis of data. She was not compensated for her contribution.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Frontiers in Medicine
- Healthcare Professions Education
- Research Topics
Impact of Technology on Human Behaviors in Medical Professions Education
Total Downloads
Total Views and Downloads
About this Research Topic
Human behaviors are essential in understanding how individuals engage in medical science academic activities. Healthcare systems across the globe have witnessed a significant shift in recent years by integrating technology in innovating new methods and practices to improve educational practices. Therefore, ...
Keywords : healthcare education, medical education, teachers’ behavior, students’ behavior, human behavior, technology in medical sciences, program development, curriculum development, teacher and student performance
Important Note : All contributions to this Research Topic must be within the scope of the section and journal to which they are submitted, as defined in their mission statements. Frontiers reserves the right to guide an out-of-scope manuscript to a more suitable section or journal at any stage of peer review.
Topic Editors
Topic coordinators, recent articles, submission deadlines, participating journals.
Manuscripts can be submitted to this Research Topic via the following journals:
total views
- Demographics
No records found
total views article views downloads topic views
Top countries
Top referring sites, about frontiers research topics.
With their unique mixes of varied contributions from Original Research to Review Articles, Research Topics unify the most influential researchers, the latest key findings and historical advances in a hot research area! Find out more on how to host your own Frontiers Research Topic or contribute to one as an author.
- Open access
- Published: 10 April 2024
“So at least now I know how to deal with things myself, what I can do if it gets really bad again”—experiences with a long-term cross-sectoral advocacy care and case management for severe multiple sclerosis: a qualitative study
- Anne Müller ORCID: orcid.org/0000-0002-2456-2492 1 ,
- Fabian Hebben ORCID: orcid.org/0009-0003-6401-3433 1 ,
- Kim Dillen 1 ,
- Veronika Dunkl 1 ,
- Yasemin Goereci 2 ,
- Raymond Voltz 1 , 3 , 4 ,
- Peter Löcherbach 5 ,
- Clemens Warnke ORCID: orcid.org/0000-0002-3510-9255 2 &
- Heidrun Golla ORCID: orcid.org/0000-0002-4403-630X 1
on behalf of the COCOS-MS trial group represented by Martin Hellmich
BMC Health Services Research volume 24 , Article number: 453 ( 2024 ) Cite this article
Metrics details
Persons with severe Multiple Sclerosis (PwsMS) face complex needs and daily limitations that make it challenging to receive optimal care. The implementation and coordination of health care, social services, and support in financial affairs can be particularly time consuming and burdensome for both PwsMS and caregivers. Care and case management (CCM) helps ensure optimal individual care as well as care at a higher-level. The goal of the current qualitative study was to determine the experiences of PwsMS, caregivers and health care specialists (HCSs) with the CCM.
In the current qualitative sub study, as part of a larger trial, in-depth semi-structured interviews with PwsMS, caregivers and HCSs who had been in contact with the CCM were conducted between 02/2022 and 01/2023. Data was transcribed, pseudonymized, tested for saturation and analyzed using structuring content analysis according to Kuckartz. Sociodemographic and interview characteristics were analyzed descriptively.
Thirteen PwsMS, 12 caregivers and 10 HCSs completed interviews. Main categories of CCM functions were derived deductively: (1) gatekeeper function, (2) broker function, (3) advocacy function, (4) outlook on CCM in standard care. Subcategories were then derived inductively from the interview material. 852 segments were coded. Participants appreciated the CCM as a continuous and objective contact person, a person of trust (92 codes), a competent source of information and advice (on MS) (68 codes) and comprehensive cross-insurance support (128 codes), relieving and supporting PwsMS, their caregivers and HCSs (67 codes).
Conclusions
Through the cross-sectoral continuous support in health-related, social, financial and everyday bureaucratic matters, the CCM provides comprehensive and overriding support and relief for PwsMS, caregivers and HCSs. This intervention bears the potential to be fine-tuned and applied to similar complex patient groups.
Trial registration
The study was approved by the Ethics Committee of the University of Cologne (#20–1436), registered at the German Register for Clinical Studies (DRKS00022771) and in accordance with the Declaration of Helsinki.
Peer Review reports
Introduction
Multiple sclerosis (MS) is the most frequent and incurable chronic inflammatory and degenerative disease of the central nervous system (CNS). Illness awareness and the number of specialized MS clinics have increased since the 1990s, paralleled by the increased availability of disease-modifying therapies [ 1 ]. There are attempts in the literature for the definition of severe MS [ 2 , 3 ]. These include a high EDSS (Expanded disability Status Scale [ 4 ]) of ≥ 6, which we took into account in our study. There are also other factors to consider, such as a highly active disease course with complex therapies that are associated with side effects. These persons are (still) less disabled, but may feel overwhelmed with regard to therapy, side effects and risk monitoring of therapies [ 5 , 6 ].
Persons with severe MS (PwsMS) develop individual disease trajectories marked by a spectrum of heterogeneous symptoms, functional limitations, and uncertainties [ 7 , 8 ] manifesting individually and unpredictably [ 9 ]. This variability can lead to irreversible physical and mental impairment culminating in complex needs and daily challenges, particularly for those with progressive and severe MS [ 5 , 10 , 11 ]. Such challenges span the spectrum from reorganizing biographical continuity and organizing care and everyday live, to monitoring disease-specific therapies and integrating palliative and hospice care [ 5 , 10 ]. Moreover, severe MS exerts a profound of social and economic impact [ 9 , 12 , 13 , 14 ]. PwsMS and their caregivers (defined in this manuscript as relatives or closely related individuals directly involved in patients’ care) often find themselves grappling with overwhelming challenges. The process of organizing and coordinating optimal care becomes demanding, as they contend with the perceived unmanageability of searching for, implementing and coordinating health care and social services [ 5 , 15 , 16 , 17 ].
Case management (CM) proved to have a positive effect on patients with neurological disorders and/or patients with palliative care needs [ 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 ]. However, a focus on severe MS has been missed so far Case managers primarily function as: (1) gatekeeper involving the allocation of necessary and available resources to a case, ensuring the equitable distribution of resources; as (2) broker assisting clients in pursuing their interests, requiring negotiation to provide individualized assistance that aligns as closely as possible with individual needs and (3) advocate working to enhance clients’ individual autonomy, to advocate for essential care offers, and to identify gaps in care [ 25 , 26 , 27 , 28 , 29 ].
Difficulties in understanding, acting, and making decisions regarding health care-related aspects (health literacy) poses a significant challenge for 54% of the German population [ 30 ]. Additionally acting on a superordinate level as an overarching link, a care and case management (CCM) tries to reduce disintegration in the social and health care system [ 31 , 32 ]. Our hypothesis is that a CCM allows PwsMS and their caregivers to regain time and resources outside of disease management and to facilitate the recovery and establishment of biographical continuity that might be disrupted due to severe MS [ 33 , 34 ].
Health care specialists (HCSs) often perceive their work with numerous time and economic constraints, especially when treating complex and severely ill individuals like PwsMS and often have concerns about being blamed by patients when expectations could not be met [ 35 , 36 ]. Our hypothesis is that the CCM will help to reduce time constraints and free up resources for specialized tasks.
To the best of our knowledge there is no long-term cross-sectoral and outreaching authority or service dedicated to assisting in the organization and coordination of the complex care concerns of PwsMS within the framework of standard care addressing needs in health, social, financial, every day and bureaucratic aspects. While some studies have attempted to design and test care programs for persons with MS (PwMS), severely affected individuals were often not included [ 37 , 38 , 39 ]. They often remain overlooked by existing health and social care structures [ 5 , 9 , 15 ].
The COCOS-MS trial developed and applied a long-term cross-sectoral CCM intervention consisting of weekly telephone contacts and monthly re-assessments with PwsMS and caregivers, aiming to provide optimal care. Their problems, resources and (unmet) needs were assessed holistically including physical health, mental health, self-sufficiency and social situation and participation. Based on assessed (unmet) needs, individual care plans with individual actions and goals were developed and constantly adapted during the CCM intervention. Contacts with HCSs were established to ensure optimal care. The CCM intervention was structured through and documented in a CCM manual designed for the trial [ 40 , 41 ].
Our aim was to find out how PwsMS, caregivers and HCSs experienced the cross-sectoral long-term, outreaching patient advocacy CCM.
This study is part of a larger phase II, randomized, controlled clinical trial “Communication, Coordination and Security for people with severe Multiple Sclerosis (COCOS-MS)” [ 41 ]. This explorative clinical trial, employing a mixed-method design, incorporates a qualitative study component with PwsMS, caregivers and HCSs to enrich the findings of the quantitative data. This manuscript focuses on the qualitative data collected between February 2022 and January 2023, following the Consolidated Criteria for Reporting Qualitative Research (COREQ) guidelines [ 42 ].
Research team
Three trained authors AM, KD and FH (AM, female, research associate, M.A. degree in Rehabilitation Sciences; KD, female, researcher, Dr. rer. medic.; FH, male, research assistant, B.Sc. degree in Health Care Management), who had no prior relationship with patients, caregivers or HCSs conducted qualitative interviews. A research team, consisting of clinical experts and health services researchers, discussed the development of the interview guides and the finalized category system.
Theoretical framework
Interview data was analyzed with the structuring content analysis according to Kuckartz. This method enables a deductive structuring of interview material, as well as the integration of new aspects found in the interview material through the inductive addition of categories in an iterative analysis process [ 43 ].
Sociodemographic and interview characteristics were analyzed descriptively (mean, median, range, SD). PwsMS, caregivers and HCSs were contacted by the authors AM, KD or FH via telephone or e-mail after providing full written informed consent. Participants had the option to choose between online interviews conducted via the GoToMeeting 10.19.0® Software or face-to-face. Peasgood et al. (2023) found no significant differences in understanding questions, engagement or concentration between face-to-face and online interviews [ 44 , 45 ]. Digital assessments were familiar to participants due to pandemic-related adjustments within the trial.
Out of 14 PwsMS and 14 caregivers who were approached to participate in interviews, three declined to complete interviews, resulting in 13 PwsMS (5 male, 8 female) and 12 caregiver (7 male, 5 female) interviews, respectively (see Fig. 1 ). Thirty-one HCSs were contacted of whom ten (2 male, 8 female) agreed to be interviewed (see Fig. 2 ).
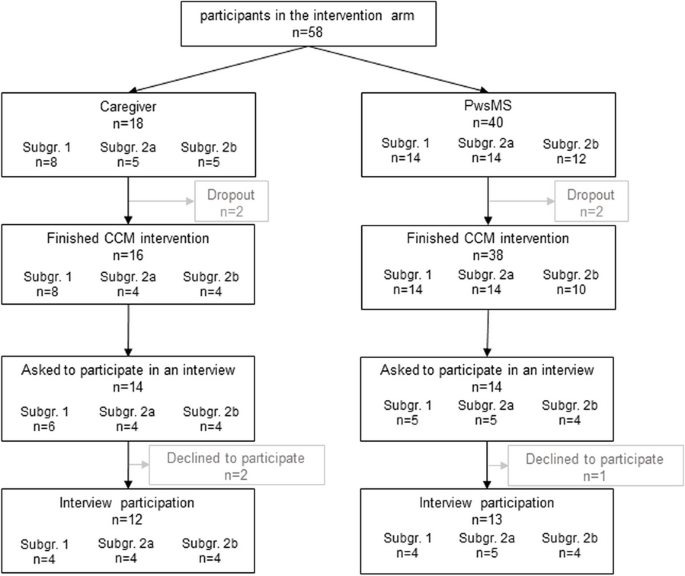
Flowchart of PwsMS and caregiver participation in the intervention group of the COCOS-MS trial. Patients could participate with and without a respective caregiver taking part in the trial. Therefore, number of caregivers does not correspond to patients. For detailed inclusion criteria see also Table 1 in Golla et al. [ 41 ]
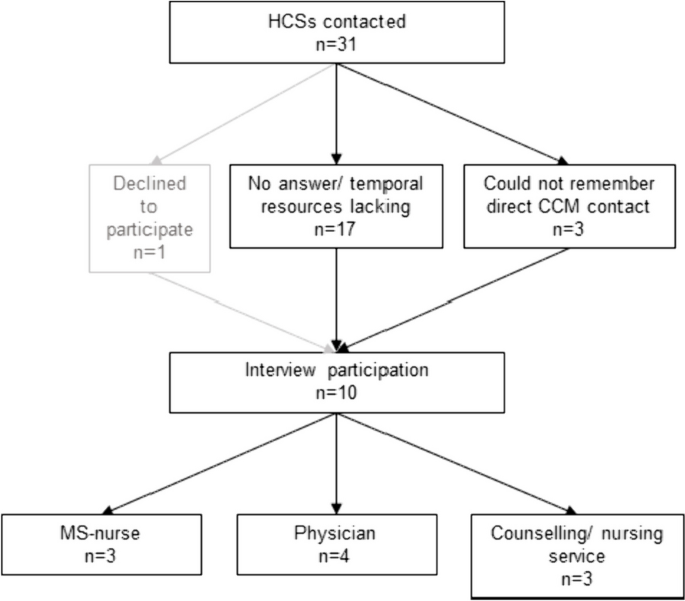
Flowchart of HCSs interview participation
Setting and data collection
Interviews were carried out where participants preferred, e.g. at home, workplace, online, and no third person being present. In total, we conducted 35 interviews whereof 7 interviews face-to-face (3 PwsMS, 3 caregivers, 1 HCS).
The research team developed a topic guide which was meticulously discussed with research and clinical staff to enhance credibility. It included relevant aspects for the evaluation of the CCM (see Tables 1 and 2 , for detailed topic guides see Supplementary Material ). Patient and caregiver characteristics (covering age, sex, marital status, living situation, EDSS (patients only), subgroup) were collected during the first assessment of the COCOS-MS trial and HCSs characteristics (age, sex, profession) as well as interview information (length and setting) were collected during the interviews. The interview guides developed for this study addressed consistent aspects both for PwsMS and caregivers (see Supplementary Material ):
For HCSs it contained the following guides:
Probing questions were asked to get more specific and in-depth information. Interviews were carried out once and recorded using a recording device or the recording function of the GoToMeeting 10.19.0® Software. Data were pseudonymized (including sensitive information, such as personal names, dates of birth, or addresses), audio files were safely stored in a data protection folder. The interview duration ranged from 11 to 56 min (mean: 23.9 min, SD: 11.1 min). Interviews were continued until we found that data saturation was reached. Audio recordings were transcribed verbatim by an external source and not returned to participants.
Data analysis
Two coders (AM, FH) coded the interviews. Initially, the first author (AM) thoroughly reviewed the transcripts to gain a sense of the interview material. Using the topic guide and literature, she deductively developed a category system based on the primary functions of CM [ 25 , 26 , 27 , 28 , 29 ]. Three interviews were coded repeatedly for piloting, and inductive subcategories were added when new themes emerged in the interview material. This category system proved suitable for the interview material. The second coder (FH) familiarized himself with the interview material and category system. Both coders (AM, FH) independently coded all interviews, engaging in discussions and adjusting codes iteratively. The finalized category system was discussed and consolidated in a research workshop and within the COCOS-MS trial group and finally we reached an intercoder agreement of 90% between the two coders AM and FH, computed by the MAXQDA Standard 2022® software.
We analyzed sociodemographic and interview characteristics using IBM SPSS Statistics 27® and Excel 2016®. Transcripts were managed and analyzed using MAXQDA Standard 2022®.
Participants were provided with oral and written information about the trial and gave written informed consent. Ethical approvals were obtained from the Ethics Committee of the University of Cologne (#20–1436). The trial is registered in the German Register for Clinical Studies (DRKS) (DRKS00022771) and is conducted under the Declaration of Helsinki.
Characteristics of participants and interviews
PwsMS participating in an interview were mainly German (84.6%), had a mean EDSS of 6.8 (range: 6–8) and MS for 13.5 years (median: 14; SD: 8.1). For detailed characteristics see Table 3 .
Most of the interviewed caregivers (9 caregivers) were the partners of the PwsMS with whom they lived in the same household. For further details see Table 3 .
HCSs involved in the study comprised various professions, including MS-nurse (3), neurologist (2), general physician with further training in palliative care (1), physician with further training in palliative care and pain therapist (1), housing counselling service (1), outpatient nursing service manager (1), participation counselling service (1).
Structuring qualitative content analysis
The experiences of PwsMS, caregivers and HCSs were a priori deductively assigned to four main categories: (1) gatekeeper function, (2) broker function, (3) advocacy function [ 25 , 26 , 27 , 28 , 29 ] and (4) Outlook on CCM in standard care, whereas the subcategories were developed inductively (see Fig. 3 ).
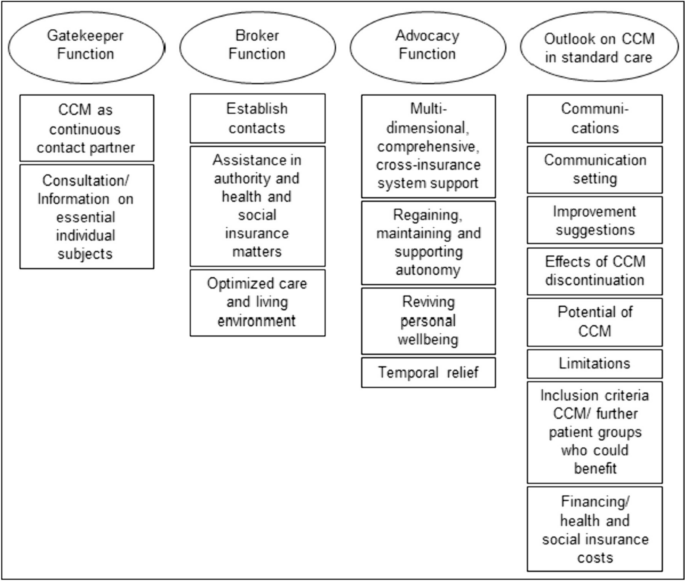
Category system including main and subcategories of the qualitative thematic content analysis
The most extensive category, housing the highest number of codes and subcodes, was the “ Outlook on CCM in standard care ” (281 codes). Following this, the category “ Advocacy Function ” contained 261 codes. The “ Broker Function ” (150 codes) and the “ Gatekeeper Function ” (160 codes) constituted two smaller categories. The majority of codes was identified in the caregivers’ interviews, followed by those of PwsMS (see Table 4 ). Illustrative quotes for each category and subcategory can be found in Table 5 .
Persons with severe multiple sclerosis
In the gatekeeper function (59 codes), PwsMS particularly valued the CCM as a continuous contact person . They appreciated the CCM as a person of trust who was reliably accessible throughout the intervention period. This aspect, with 41 codes, held significant importance for PwsMS.
Within the broker function (44 codes), establishing contact was most important for PwsMS (22 codes). This involved the CCM as successfully connecting PwsMS and caregivers with physicians and therapists, as well as coordinating and arranging medical appointments, which were highly valued. Assistance in authority and health and social insurance matters (10 codes) was another subcategory, where the CCM encompassed support in communication with health insurance companies, such as improving the level of care, assisting with retirement pension applications, and facilitating rehabilitation program applications. Optimized care (12 codes) resulted in improved living conditions and the provision of assistive devices through the CCM intervention.
The advocacy function (103 codes) emerged as the most critical aspect for PwsMS, representing the core of the category system. PwsMS experienced multidimensional, comprehensive, cross-insurance system support from the CCM. This category, with 43 statements, was the largest within all subcategories. PwsMS described the CCM as addressing their concerns, providing help, and assisting with the challenges posed by the illness in everyday life. The second-largest subcategory, regaining, maintaining and supporting autonomy (25 codes), highlighted the CCM’s role in supporting self-sufficiency and independence. Reviving personal wellbeing (17 codes) involved PwsMSs’ needs of regaining positive feelings, improved quality of life, and a sense of support and acceptance, which could be improved by the CCM. Temporal relief (18 codes) was reported, with the CCM intervention taking over or reducing tasks.
Within the outlook on CCM in standard care (84 codes), eight subcategories were identified. Communications was described as friendly and open (9 codes), with the setting of communication (29 codes) including the frequency of contacts deemed appropriate by the interviewed PwsMS, who preferred face-to-face contact over virtual or telephone interactions. Improvement suggestions for CCM (10 codes) predominantly revolved around the desire for the continuation of the CCM beyond the trial, expressing intense satisfaction with the CCM contact person and program. PwsMS rarely wished for better cooperation with the CCM. With respect to limitations (7 codes), PwsMS distinguished between individual limitations (e.g. when not feeling ready for using a wheelchair) and overriding structural limitations (e.g. unsuccessful search for an accessible apartment despite CCM support). Some PwsMS mentioned needing the CCM earlier in the course of the disease and believed it would beneficial for anyone with a chronic illness (6 codes).
In the gatekeeper function (75 codes), caregivers highly valued the CCM as a continuous contact partner (33 codes). More frequently than among the PwsMS interviewed, caregivers valued the CCM as a source of consultation/ information on essential individual subjects (42 codes). The need for basic information about the illness, its potential course, treatment and therapy options, possible supportive equipment, and basic medical advice/ information could be met by the CCM.
Within the broker function (63 codes), caregivers primarily experienced the subcategory establish contacts (24 codes). They found the CCM as helpful in establishing and managing contact with physicians, therapists and especially with health insurance companies. In the subcategory assistance in authority and health and social insurance matters (22 codes), caregivers highlighted similar aspects as the PwsMS interviewed. However, there was a particular emphasis on assistance with patients' retirement matters. Caregivers also valued the optimization of patients’ care and living environment (17 codes) in various life areas during the CCM intervention, including improved access to assistive devices, home modification, and involvement of a household support and/ or nursing services.
The advocacy function, with 115 codes, was by far the broadest category . The subcategory multidimensional, comprehensive, cross-insurance system support represented the largest subcategory of caregivers, with 70 statements. In summary, caregivers felt supported by the CCM in all domains of life. Regaining, maintaining and supporting autonomy (11 codes) and reviving personal wellbeing (8 codes) in the form of an improved quality of life played a role not only for patients but also for caregivers, albeit to a lower extend. Caregivers experienced temporal relief (26 codes) as the CCM undertook a wide range of organizational tasks, freeing up more needed resources for their own interests.
For the Outlook on CCM in standard care , caregivers provided various suggestions (81 codes). Similar to PwsMS, caregivers felt that setting (home based face-to-face, telephone, virtual) and frequency of contact were appropriate (10 codes, communication setting ) and communications (7 codes) were recognized as open and friendly. However, to avoid conflicts between caregiver and PwsMS, caregivers preferred meeting the CCM separately from the PwsMS in the future. Some caregivers wished the CCM to specify all services it might offer at the beginning, while others emphasized not wanting this. Like PwsMS, caregivers criticized the CCM intervention being (trial-related) limited to one year, regardless of whether further support was needed or processes being incomplete (13 codes, improvement suggestions ). After the CCM intervention time had expired, the continuous contact person and assistance were missed and new problems had arisen and had to be managed with their own resources again (9 codes, effects of CCM discontinuation ), which was perceived as an exhausting or unsolvable endeavor. Caregivers identified analogous limitations (8 codes), both individual and structural. However, the largest subcategory, was the experienced potential of CCM (27 codes), reflected in extremely high satisfaction with the CCM intervention. Like PwsMS, caregivers regarded severe chronically ill persons in general as target groups for a CCM (7 codes) and would implement it even earlier, starting from the time of diagnosis. They considered a CCM to be particularly helpful for patients without caregivers or for caregivers with limited (time) resources, as it was true for most caregivers.
Health care specialists
In the gatekeeper function (26 codes) HCSs particularly valued the CCM as a continuous contact partner (18 codes). They primarily described their valuable collaboration with the CCM, emphasizing professional exchange between the CCM and HCSs.
Within the broker function (43 codes), the CCM was seen as a connecting link between patients and HCSs, frequently establishing contacts (18 codes). This not only improved optimal care on an individual patient level (case management) but also at a higher, superordinate care level (care management). HCSs appreciated the optimized care and living environment (18 codes) for PwsMS, including improved medical and therapeutic access and the introduction of new assistive devices. The CCM was also recognized as providing assistance in authority and health and social matters (7 codes) for PwsMS and their caregivers.
In the advocacy function (43 codes), HCSs primarily reported temporal relief through CCM intervention (23 codes). They experienced this relief, especially as the CCM provided multidimensional, comprehensive, and cross-insurance system support (15 codes) for PwsMS and their caregivers. Through this support, HCSs felt relieved from time intensive responsibilities that may not fall within their area of expertise, freeing up more time resources for their actual professional tasks.
The largest category within the HCSs interviews was the outlook on CCM in standard care (116 codes). In the largest subcategory, HCSs made suggestions for further patient groups who could benefit (38 codes) from a CCM. Chronic neurological diseases like neurodegenerative diseases (e.g. amyotrophic lateral sclerosis), typical and atypical Parkinson syndromes were mentioned. HCSs considered the enrollment of the CCM directly after the diagnosis of these complex chronic diseases. Additionally, chronic progressive diseases in general or oncological diseases, which may also run chronically, were regarded worthwhile for this approach. HCSs also provided suggestions regarding improvement (21 codes). They wished e.g. for information or contact when patients were enrolled to the CCM, regular updates, exchange and collaborative effort. On the other hand, HCSs reported, that their suggestions for improvement would hardly be feasible due to their limited time resources. Similar to patients and caregivers, HCSs experienced structural limits (13 codes), which a CCM could not exceed due to overriding structural limitations (e.g. insufficient supply of (household) aids, lack of outreach services like psychotherapists, and long processing times on health and pension insurers' side). HCSs were also asked about their opinions on financial resources (14 codes) of a CCM in standard care. All interviewed HCSs agreed that CCM would initially cause more costs for health and social insurers, but they were convinced of cost savings in the long run. HCSs particularly perceived the potential of the CCM (20 codes) through the feedback of PwsMS, highlighting the trustful relationship enabling individualized help for PwsMS and their caregivers.
Persons with severe multiple sclerosis and their caregivers
The long-term cross-sectoral CCM intervention implemented in the COCOS-MS trial addressed significant unmet needs of PwsMS and their caregivers which previous research revealed as burdensome and hardly or even not possible to improve without assistance [ 5 , 6 , 9 , 10 , 33 , 35 , 46 ]. Notably, the CCM service met the need for a reliable, continuous contact partner, guiding patients through the complexities of regulations, authorities and the insurance system. Both, PwsMS and their caregivers highly valued the professional, objective perspective provided by the CCM, recognizing it as a source of relief, support and improved care in line with previous studies [ 37 , 47 ]. Caregivers emphasized the CCM’s competence in offering concrete assistance and information on caregiving and the fundamentals of MS, including bureaucratic, authority and insurances matters. On the other hand, PwsMS particularly appreciated the CCMs external reflective and advisory function, along with empathic social support tailored to their individual concerns. Above all, the continuous partnership of trust, available irrespective of the care sector, was a key aspect that both PwsMS and their caregivers highlighted. This consistent support was identified as one of the main components in the care of PwsMS in previous studies [ 5 , 33 , 35 ].
As the health literacy is inadequate or problematic for 54% of the German population and disintegration in the health and social care system is high [ 30 , 31 , 32 ], the CCM approach serves to enhance health literacy and reduce disintegration of PwsMS and their caregivers by providing cross-insurance navigational guidance in the German health and social insurance sector on a superordinate level. Simultaneously PwsMS and caregivers experienced relief and gained more (time) resources for all areas of life outside of the disease and its management, including own interests and establishing biographical continuity. This empowerment enables patients to find a sense of purpose beyond their illness, regain autonomy, and enhance social participation, reducing the feeling of being a burden to those closest to them. Such feelings are often experienced as burdensome and shameful by PwsMS [ 6 , 48 , 49 , 50 ]. Finding a sense of purpose beyond the illness also contributes to caregivers perceiving their loved ones not primarily as patient but as individuals outside of the disease, reinforcing valuable relationships such as partners, siblings, or children, strengthening emotional bonds. These factors are also highly relevant and well-documented in a suicide-preventive context, as the suicide rate is higher in persons diagnosed with neurological disorders [ 19 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 ] and the feeling of being a burden to others, loss of autonomy, and perceived loss of dignity are significant factors in patients with severe chronic neurological diseases for suicide [ 50 , 57 ].
The temporal relief experienced by the CCM was particularly significant for HCSs and did not only improve the satisfaction of HCSs but also removed unfulfilled expectations and concerns about being blamed by patients when expectations could not be met, which previous studied elaborated [ 35 , 36 ]. Moreover, the CCM alleviated the burden on HCSs by addressing patients’ concerns, allowing them to focus on their own medical responsibilities. This aspect probably reduced the dissatisfaction that arises when HCSs are expected to address issues beyond their medical expertise, such as assistive devices, health and social insurance, and the organization and coordination of supplementary therapies, appointments, and contacts [ 35 , 36 , 61 ]. Consequently, the CCM reduced difficulties of HCSs treating persons with neurological or chronical illnesses, which previous research identified as problematic.
HCSs perceive their work as increasingly condensed with numerous time and economic constraints, especially when treating complex and severely ill individuals like PwsMS [ 36 ]. This constraint was mentioned by HCSs in the interviews and was one of the main reasons why they were hesitant to participate in interviews and may also be an explanation for a shorter interview duration than initially planned in the interview guides. The CCM’s overarching navigational competence in the health and social insurance system was particularly valued by HCSs. The complex and often small-scale specialties in the health and social care system are not easily manageable or well-known even for HCSs, and dealing with them can exceed their skills and time capacities [ 61 ]. The CCM played a crucial role in keeping (temporal) resources available for what HCSs are professionally trained and qualified to work on. However, there remains a challenge in finding solutions to the dilemma faced by HCSs regarding their wish to be informed about CCM procedures and linked with each other, while also managing the strain of additional requests and contact with the CCM due to limited (time) resources [ 62 ]. Hudon et al. (2023) suggest that optimizing time resources and improving exchange could involve meetings, information sharing via fax, e-mail, secure online platforms, or, prospectively, within the electronic patient record (EPR). The implementation of an EPR has shown promise in improving the quality of health care and time resources, when properly implemented [ 63 , 64 ]. The challenge lies ineffective information exchange between HCSs and CCM for optimal patient care. The prospect of time saving in the long run and at best for a financial incentive, e.g., when anchoring in the Social Security Code, will help best to win over the HCSs.If this crucial factor can be resolved, there is a chance that HCSs will thoroughly accept the CCM as an important pillar, benefiting not only PwsMS but also other complex patient groups, especially those with long-term neurological or complex oncological conditions that might run chronically.
Care and case management and implications for the health care system
The results of our study suggest that the cross-sectoral long-term advocacy CCM in the COCOS-MS trial, with continuous personal contacts at short intervals and constant reevaluation of needs, problems, resources and goals, is highly valued by PwsMS, caregivers, and HCSs. The trial addresses several key aspects that may have been overlooked in previous studies which have shown great potential for the integration of case management [ 17 , 47 , 62 , 65 , 66 ]. However, they often excluded the overriding care management, missed those patient groups with special severity and complexity who might struggle to reach social and health care structures independently or the interventions were not intended for long-term [ 22 , 37 ]. Our results indicate that the CCM intervention had a positive impact on PwsMS and caregivers as HCSs experienced them with benefits such as increased invigoration, reduced demands, and enhanced self-confidence. However, there was a notable loss experienced by PwsMS and caregivers after the completion of the CCM intervention, even if they had stabilized during the intervention period. The experiences of optimized social and health care for the addressed population, both at an individual and superordinate care level, support the integration of this service into standard care. Beyond the quantitatively measurable outcomes and economic considerations reported elsewhere [ 16 , 20 , 21 ], our results emphasize the importance of regaining control, self-efficacy, self-worth, dignity, autonomy, and social participation. These aspects are highlighted as preventive measures in suicidal contexts, which is particularly relevant for individuals with severe and complex illnesses [ 19 , 50 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 ]. Our findings further emphasize the societal responsibilities to offer individuals with severe and complex illnesses the opportunity to regain control and meaningful aspects of life, irrespective of purely economic considerations. This underscores the need for a comprehensive evaluation that not only takes into account quantitative measures but also the qualitative aspects of well-being and quality of life when making recommendations of a CCM in standard care.
The study by J. Y. Joo and Huber (2019) highlighted that CM interventions aligned with the standards of the Case Management Society of America varied in duration, ranging from 1 month to 15.9 years, and implemented in community- or hospital-based settings. However, they noted a limitation in understanding how CM processes unfold [ 67 ]. In contrast, our trial addressed this criticism by providing transparent explanations of the CCM process, which also extends to a superordinate care management [ 40 , 41 ]. Our CCM manual [ 40 ] outlines a standardized and structured procedure for measuring and reevaluating individual resources, problems, and unmet needs on predefined dimensions. It also identifies goals and actions at reducing unmet needs and improving the individual resources of PwsMS and caregivers. Importantly, the CCM manual demonstrates that the CCM process can be structured and standardized, while accounting for the unique aspects of each individual’s serious illness, disease courses, complex needs, available resources, and environmental conditions. Furthermore, the adaptability of the CCM manual to other complex chronically ill patient groups suggests the potential for a standardized approach in various health care settings. This standardized procedure allows for consistency in assessing and addressing the individual needs of patients, ensuring that the CCM process remains flexible while maintaining a structured and goal-oriented framework.
The discussion about the disintegration in the social and health care system and the increasing specialization dates back to 2009 [ 31 , 32 ]. Three strategies were identified to address this issue: (a) “driver-minimizing” [Treiberminimierende], (b) “effect-modifying” [Effektmodifizierende] and (c) “disintegration-impact-minimizing” [Desintegrationsfolgenminimierende] strategies. “Driver-minimizing strategies” involve comprehensive and radical changes within the existing health and social care system, requiring political and social pursuit. “Disintegration-impact-minimizing strategies” are strategies like quality management or tele-monitoring, which are limited in scope and effectiveness. “Effect-modifying strategies”, to which CCM belongs, acknowledges the segmentation within the system but aims to overcome it through cooperative, communicative, and integrative measures. CCM, being an “effect-modifying strategy”, operates the “integrated segmentation model” [Integrierte Segmentierung] rather than the “general contractor model” [Generalunternehmer-Modell] or “total service provider model” [Gesamtdienstleister-Modell] [ 31 , 32 ]. In this model, the advantage lies in providing an overarching and coordinating service to link different HCSs and services cross-sectorally. The superordinate care management aspect of the CCM plays a crucial role in identifying gaps in care, which is essential for future development strategies within the health and social care system. It aims to find or develop (regional) alternatives to ensure optimal care [ 17 , 23 , 24 , 68 , 69 ], using regional services of existing health and social care structures. Therefore, superordinate care management within the CCM process is decisive for reducing disintegration in the system.
Strengths and limitations
The qualitative study results of the explorative COCOS-MS clinical trial, which employed an integrated mixed-method design, provide valuable insights into the individual experiences of three leading stakeholders: PwsMS, caregivers and HCSs with a long-term cross-sectoral CCM. In addition to in-depth interviews, patient and caregiver reported outcome measurements were utilized and will be reported elsewhere. The qualitative study’s strengths include the inclusion of patients who, due to the severity of their condition (e.g. EDSS mean: 6.8, range: 6–8, highly active MS), age (mean: 53.9 years, range: 36–73 years) family constellations, are often underrepresented in research studies and often get lost in existing social and health care structures. The study population is specific to the wider district region of Cologne, but the broad inclusion criteria make it representative of severe MS in Germany. The methodological approach of a deductive and inductive structuring content analysis made it possible to include new findings into an existing theoretical framework.
However, the study acknowledges some limitations. While efforts were made to include more HCSs, time constraints on their side limited the number of interviews conducted and might have biased the results. Some professions are underrepresented in the interviews. Complex symptoms (e.g. fatigue, ability to concentrate), medical or therapeutic appointments and organization of the everyday live may have been reasons for the patients’ and caregivers’ interviews lasting shorter than initially planned.
The provision of functions of a CCM, might have pre-structured the answers of the participants.
At current, there is no support system for PwsMS, their caregivers and HCSs that addresses their complex and unmet needs comprehensively and continuously. There are rare qualitative insights of the three important stakeholders: PwsMS, caregivers and HCSs in one analysis about a supporting service like a CCM. In response to this gap, we developed and implemented a long-term cross-sectoral advocacy CCM and analyzed it qualitatively. PwsMS, their caregivers and HCSs expressed positive experiences, perceiving the CCM as a source of relief and support that improved care across various aspects of life. For patients, the CCM intervention resulted in enhanced autonomy, reviving of personal wellbeing and new established contacts with HCSs. Caregivers reported a reduced organizational burden and felt better informed, and HCSs experienced primarily temporal relief, allowing them to concentrate on their core professional responsibilities. At a higher level of care, the study suggests that the CCM contributed to a reduction in disintegration within the social and health care system.
The feedback from participants is seen as valuable for adapting the CCM intervention and the CCM manual for follow-up studies, involving further complex patient groups such as neurological long-term diseases apart from MS and tailoring the duration of the intervention depending on the complexity of evolving demands.
Availability of data and materials
Generated and/or analyzed datasets of participants are available from the corresponding author on reasonable request to protect participants. Preliminary partial results have been presented as a poster during the EAPC World Congress in June 2023 and the abstract has been published in the corresponding abstract booklet [ 70 ].
Abbreviations
Amyotrophic lateral sclerosis
- Care and case management
Case management
Central nervous system
Communication, Coordination and security for people with multiple sclerosis
Consolidated criteria for reporting qualitative research
German register for clinical studies
Extended disability status scale
Electronic patient record
Quality of life
Multiple sclerosis
Koch-Henriksen N, Magyari M. Apparent changes in the epidemiology and severity of multiple sclerosis. Nat Rev Neurol. 2021;17:676–88. https://doi.org/10.1038/s41582-021-00556-y .
Article PubMed Google Scholar
Ellenberger D, Flachenecker P, Fneish F, Frahm N, Hellwig K, Paul F, et al. Aggressive multiple sclerosis: a matter of measurement and timing. Brain. 2020;143:e97. https://doi.org/10.1093/brain/awaa306 .
Article PubMed PubMed Central Google Scholar
Edmonds P, Vivat B, Burman R, Silber E, Higginson IJ. Loss and change: experiences of people severely affected by multiple sclerosis. Palliat Med. 2007;21:101–7. https://doi.org/10.1177/0269216307076333 .
Kurtzke JF. Rating neurologic impairment in multiple rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology. 1983;33(11):1444–52.
Article CAS PubMed Google Scholar
Galushko M, Golla H, Strupp J, Karbach U, Kaiser C, Ernstmann N, et al. Unmet needs of patients feeling severely affected by multiple sclerosis in Germany: a qualitative study. J Palliat Med. 2014;17:274–81. https://doi.org/10.1089/jpm.2013.0497 .
Borreani C, Bianchi E, Pietrolongo E, Rossi I, Cilia S, Giuntoli M, et al. Unmet needs of people with severe multiple sclerosis and their carers: qualitative findings for a home-based intervention. PLoS One. 2014:e109679. https://doi.org/10.1371/journal.pone.0109679 .
Yamout BI, Alroughani R. Multiple Sclerosis. Semin Neurol. 2018;38:212–25. https://doi.org/10.1055/s-0038-1649502 .
Nissen N, Lemche J, Reestorff CM, Schmidt M, Skjerbæk AG, Skovgaard L, et al. The lived experience of uncertainty in everyday life with MS. Disabil Rehabil. 2022;44:5957–63. https://doi.org/10.1080/09638288.2021.1955302 .
Strupp J, Hartwig A, Golla H, Galushko M, Pfaff H, Voltz R. Feeling severely affected by multiple sclerosis: what does this mean? Palliat Med. 2012;26:1001–10. https://doi.org/10.1177/0269216311425420 .
Strupp J, Voltz R, Golla H. Opening locked doors: Integrating a palliative care approach into the management of patients with severe multiple sclerosis. Mult Scler J. 2016;22:13–8.
Article CAS Google Scholar
Kraft AK, Berger K. Kernaspekte einer bedarfsgerechten Versorgung von Patienten mit Multipler Sklerose : Inanspruchnahme ambulanter Leistungen und „shared decision making“ [Core aspects of a needs-conform care of patients with multiple sclerosis : Utilization of outpatient services and shared decision making]. Nervenarzt. 2020;91:503–10. https://doi.org/10.1007/s00115-020-00906-z .
Doshi A, Chataway J. Multiple sclerosis, a treatable disease. Clin Med (Lond). 2017;17:530–6. https://doi.org/10.7861/clinmedicine.17-6-530 .
Kobelt G, Thompson A, Berg J, Gannedahl M, Eriksson J. New insights into the burden and costs of multiple sclerosis in Europe. Mult Scler. 2017;23:1123–36. https://doi.org/10.1177/1352458517694432 .
Conradsson D, Ytterberg C, Engelkes C, Johansson S, Gottberg K. Activity limitations and participation restrictions in people with multiple sclerosis: a detailed 10-year perspective. Disabil Rehabil. 2021;43:406–13. https://doi.org/10.1080/09638288.2019.1626919 .
Sorensen PS, Giovannoni G, Montalban X, Thalheim C, Zaratin P, Comi G. The Multiple Sclerosis Care Unit. Mult Scler J. 2019;5:627–36.
Article Google Scholar
Tan H, Yu J, Tabby D, Devries A, Singer J. Clinical and economic impact of a specialty care management program among patients with multiple sclerosis: a cohort study. Mult Scler. 2010;16:956–63. https://doi.org/10.1177/1352458510373487 .
Article CAS PubMed PubMed Central Google Scholar
Oeseburg B, Wynia K, Middel B, Reijneveld SA. Effects of case management for frail older people or those with chronic illness: a systematic review. Nurs Res. 2009;58:201–10.
Aiken LS, Butner J, Lockhart CA, Volk-Craft BE, Hamilton G, Williams FG. Outcome evaluation of a randomized trial of the PhoenixCare intervention: program of case management and coordinated care for the seriously chronically ill. J Palliat Med. 2006;9:111–26. https://doi.org/10.1089/jpm.2006.9.111 .
Kuhn U, Düsterdiek A, Galushko M, Dose C, Montag T, Ostgathe C, Voltz R. Identifying patients suitable for palliative care—a descriptive analysis of enquiries using a Case Management Process Model approach. BMC Res Notes. 2012;5:611. https://doi.org/10.1186/1756-0500-5-611 .
Leary A, Quinn D, Bowen A. Impact of proactive case management by multiple sclerosis specialist nurses on use of unscheduled care and emergency presentation in multiple sclerosis: a case study. Int J MS Care. 2015;17:159–63. https://doi.org/10.7224/1537-2073.2014-011 .
Strupp J, Dose C, Kuhn U, Galushko M, Duesterdiek A, Ernstmann N, et al. Analysing the impact of a case management model on the specialised palliative care multi-professional team. Support Care Cancer. 2018;26:673–9. https://doi.org/10.1007/s00520-017-3893-3 .
Wynia K, Annema C, Nissen H, de Keyser J, Middel B. Design of a Randomised Controlled Trial (RCT) on the effectiveness of a Dutch patient advocacy case management intervention among severely disabled Multiple Sclerosis patients. BMC Health Serv Res. 2010;10:142. https://doi.org/10.1186/1472-6963-10-142 .
Ewers M, Schaeffer D, editors. Case Management in Theorie und Praxis. Bern: Huber; 2005.
Google Scholar
Neuffer M. Case Management: Soziale Arbeit mit Einzelnen und Familien. 5th ed. Weinheim, Basel: Beltz Juventa; 2013.
Case Management Society of America. The standards of practice for case management. 2022.
Deutsche Gesellschaft für Care und Case Management e.V., editor. Case Management Leitlinien: Rahmenempfehlung, Standards und ethische Grundlagen. 2nd ed. Heidelberg: Medhochzwei; 2020.
Monzer M. Case Management Grundlagen. 2nd ed. Heidelberg: Medhochzwei; 2018.
Wissert M. Grundfunktionen und fachliche Standards des Unterstützungsmanagements. Z Gerontol Geriat. 1998;31(5):331–7.
Wissert M. Tools und Werkzeuge beim Case Management: Die Hilfeplanung. Case Manag. 2007;1:35–7.
Schaeffer D, Berens E-M, Vogt D. Health literacy in the German population. Dtsch Arztebl Int. 2017;114:53–60. https://doi.org/10.3238/arztebl.2017.0053 .
Pfaff H, Schulte H. Der onkologische Patient der Zukunft. Onkologe. 2012;18:127–33. https://doi.org/10.1007/s00761-011-2201-y .
Pfaff H, Kowalski C, Ommen O. Modelle zur Analyse von Integration und Koordination im Versorgungssystem. In: Ameldung, Sydow, Windeler, editor. Vernetzung im Gesundheitswesen: Wettbewerb und Kooperation. Stuttgart: Kohlhammer Verlag; 2009. p. 75–90.
Golla H, Mammeas S, Galushko M, Pfaff H, Voltz R. Unmet needs of caregivers of severely affected multiple sclerosis patients: A qualitative study. Palliat Support Care. 2015;13(6):1685–93.
Golla H, Galushko M, Pfaff H, Voltz R. Multiple sclerosis and palliative care - perceptions of severely affected multiple sclerosis patients and their health professionals: a qualitative study. BMC Palliat Care. 2014;13:11. https://doi.org/10.1186/1472-684x-13-11 .
Golla H, Galushko M, Pfaff H, Voltz R. Unmet needs of severely affected multiple sclerosis patients: the health professionals’ view. Palliat Med. 2012;26:139–51. https://doi.org/10.1177/0269216311401465 .
Methley AM, Chew-Graham CA, Cheraghi-Sohi S, Campbell SM. A qualitative study of patient and professional perspectives of healthcare services for multiple sclerosis: implications for service development and policy. Health Soc Care Community. 2017;25:848–57. https://doi.org/10.1111/hsc.12369 .
Kalb R, Costello K, Guiod L. Case management services to meet the complex needs of patients with multiple sclerosis in the community—the successes and challenges of a unique program from the national multiple sclerosis society. US Neurology. 2019;15:27–31.
Krüger K, Fricke LM, Dilger E-M, Thiele A, Schaubert K, Hoekstra D, et al. How is and how should healthcare for people with multiple sclerosis in Germany be designed?-The rationale and protocol for the mixed-methods study Multiple Sclerosis-Patient-Oriented Care in Lower Saxony (MS-PoV). PLoS One. 2021;16:e0259855. https://doi.org/10.1371/journal.pone.0259855 .
Ivancevic S, Weegen L, Korff L, Jahn R, Walendzik A, Mostardt S, et al. Effektivität und Kosteneffektivät von Versorgungsmanagement-Programmen bei Multipler Sklerose in Deutschland – Eine Übersichtsarbeit. Akt Neurol. 2015;42:503–8. https://doi.org/10.1055/s-0035-1564111 .
Müller A, Dillen K, Dojan T, Ungeheuer S, Goereci Y, Dunkl V, et al. Development of a long-term cross-sectoral case and care management manual for patients with severe multiple sclerosis and their caregivers. Prof Case Manag. 2023;28:183–93. https://doi.org/10.1097/NCM.0000000000000608 .
Golla H, Dillen K, Hellmich M, Dojan T, Ungeheuer S, Schmalz P, et al. Communication, Coordination, and Security for People with Multiple Sclerosis (COCOS-MS): a randomised phase II clinical trial protocol. BMJ Open. 2022;12:e049300. https://doi.org/10.1136/bmjopen-2021-049300 .
Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19:349–57. https://doi.org/10.1093/intqhc/mzm042 .
Kuckartz U. Qualitative Inhaltsanalyse: Methoden, Praxis, Computerunterstützung. 4th ed. Weinheim: Beltz Juventa; 2018.
Akyirem S, Ekpor E, Aidoo-Frimpong GA, Salifu Y, Nelson LE. Online interviews for qualitative health research in Africa: a scoping review. Int Health. 2023. https://doi.org/10.1093/inthealth/ihad010 .
Peasgood T, Bourke M, Devlin N, Rowen D, Yang Y, Dalziel K. Randomised comparison of online interviews versus face-to-face interviews to value health states. Soc Sci Med. 2023;323:115818. https://doi.org/10.1016/j.socscimed.2023.115818 .
Giordano A, Cimino V, Campanella A, Morone G, Fusco A, Farinotti M, et al. Low quality of life and psychological wellbeing contrast with moderate perceived burden in carers of people with severe multiple sclerosis. J Neurol Sci. 2016;366:139–45. https://doi.org/10.1016/j.jns.2016.05.016 .
Joo JY, Liu MF. Experiences of case management with chronic illnesses: a qualitative systematic review. Int Nurs Rev. 2018;65(1):102–1113.
Freeman J, Gorst T, Gunn H, Robens S. “A non-person to the rest of the world”: experiences of social isolation amongst severely impaired people with multiple sclerosis. Disabil Rehabil. 2020;42:2295–303. https://doi.org/10.1080/09638288.2018.1557267 .
National Institute for Health and Care Excellence. Multiple sclerosis: Management of multiple sclerosis in primary and secondary care. 2014.
Erdmann A, Spoden C, Hirschberg I, Neitzke G. The wish to die and hastening death in amyotrophic lateral sclerosis: A scoping review. BMJ Support Palliat Care. 2021;11:271–87. https://doi.org/10.1136/bmjspcare-2020-002640 .
Erlangsen A, Stenager E, Conwell Y, Andersen PK, Hawton K, Benros ME, et al. Association between neurological disorders and death by suicide in Denmark. JAMA. 2020;323:444–54. https://doi.org/10.1001/jama.2019.21834 .
Kalb R, Feinstein A, Rohrig A, Sankary L, Willis A. Depression and suicidality in multiple sclerosis: red flags, management strategies, and ethical considerations. Curr Neurol Neurosci Rep. 2019;19:77. https://doi.org/10.1007/s11910-019-0992-1 .
Feinstein A, Pavisian B. Multiple sclerosis and suicide. Mult Scler. 2017;23:923–7. https://doi.org/10.1177/1352458517702553 .
Marrie RA, Salter A, Tyry T, Cutter GR, Cofield S, Fox RJ. High hypothetical interest in physician-assisted death in multiple sclerosis. Neurology. 2017;88:1528–34. https://doi.org/10.1212/WNL.0000000000003831 .
Gauthier S, Mausbach J, Reisch T, Bartsch C. Suicide tourism: a pilot study on the Swiss phenomenon. J Med Ethics. 2015;41:611–7. https://doi.org/10.1136/medethics-2014-102091 .
Fischer S, Huber CA, Imhof L, MahrerImhof R, Furter M, Ziegler SJ, Bosshard G. Suicide assisted by two Swiss right-to-die organisations. J Med Ethics. 2008;34:810–4. https://doi.org/10.1136/jme.2007.023887 .
Strupp J, Ehmann C, Galushko M, Bücken R, Perrar KM, Hamacher S, et al. Risk factors for suicidal ideation in patients feeling severely affected by multiple sclerosis. J Palliat Med. 2016;19:523–8. https://doi.org/10.1089/jpm.2015.0418 .
Spence RA, Blanke CD, Keating TJ, Taylor LP. Responding to patient requests for hastened death: physician aid in dying and the clinical oncologist. J Oncol Pract. 2017;13:693–9. https://doi.org/10.1200/JOP.2016.019299 .
Monforte-Royo C, Villavicencio-Chávez C, Tomás-Sábado J, Balaguer A. The wish to hasten death: a review of clinical studies. Psychooncology. 2011;20:795–804. https://doi.org/10.1002/pon.1839 .
Blanke C, LeBlanc M, Hershman D, Ellis L, Meyskens F. Characterizing 18 years of the death with dignity act in Oregon. JAMA Oncol. 2017;3:1403–6. https://doi.org/10.1001/jamaoncol.2017.0243 .
Methley A, Campbell S, Cheraghi-Sohi S, Chew-Graham C. Meeting the mental health needs of people with multiple sclerosis: a qualitative study of patients and professionals. Disabil Rehab. 2017;39(11):1097-105. https://doi.org/10.1080/09638288.2016.1180547 .
Hudon C, Bisson M, Chouinard M-C, Delahunty-Pike A, Lambert M, Howse D, et al. Implementation analysis of a case management intervention for people with complex care needs in primary care: a multiple case study across Canada. BMC Health Serv Res. 2023;23:377. https://doi.org/10.1186/s12913-023-09379-7 .
Beckmann M, Dittmer K, Jaschke J, Karbach U, Köberlein-Neu J, Nocon M, et al. Electronic patient record and its effects on social aspects of interprofessional collaboration and clinical workflows in hospitals (eCoCo): a mixed methods study protocol. BMC Health Serv Res. 2021;21:377. https://doi.org/10.1186/s12913-021-06377-5 .
Campanella P, Lovato E, Marone C, Fallacara L, Mancuso A, Ricciardi W, Specchia ML. The impact of electronic health records on healthcare quality: a systematic review and meta-analysis. Eur J Public Health. 2016;26:60–4. https://doi.org/10.1093/eurpub/ckv122 .
García-Hernández M, González de León B, Barreto-Cruz S, Vázquez-Díaz JR. Multicomponent, high-intensity, and patient-centered care intervention for complex patients in transitional care: SPICA program. Front Med (Lausanne). 2022;9:1033689. https://doi.org/10.3389/fmed.2022.1033689 .
Meisinger C, Stollenwerk B, Kirchberger I, Seidl H, Wende R, Kuch B, Holle R. Effects of a nurse-based case management compared to usual care among aged patients with myocardial infarction: results from the randomized controlled KORINNA study. BMC Geriatr. 2013. https://doi.org/10.1186/1471-2318-13-115 .
Joo JY, Huber DL. Case management effectiveness on health care utilization outcomes: a systematic review of reviews. West J Nurs Res. 2019;41:111–33. https://doi.org/10.1177/0193945918762135 .
Stergiopoulos V, Gozdzik A, Misir V, Skosireva A, Connelly J, Sarang A, et al. Effectiveness of housing first with intensive case management in an ethnically diverse sample of homeless adults with mental illness: a randomized controlled trial. PLoS One. 2015;10:e0130281. https://doi.org/10.1371/journal.pone.0130281 .
Löcherbach P, Wendt R, editors. Care und Case Management: Transprofessionelle Versorgungsstrukturen und Netzwerke. 1st ed. Stuttgart: Kohlhammer; 2020.
EAPC2023 Abstract Book. Palliat Med. 2023;37:1–302. https://doi.org/10.1177/02692163231172891 .
Download references
Acknowledgements
We would like to thank all the patients, caregivers and health care specialists who volunteered their time to participate in an interview and the trial, Carola Janßen for transcribing the interviews, Fiona Brown for translating the illustrative quotes and Beatrix Münzberg, Kerstin Weiß and Monika Höveler for data collection in the quantitative study part.
COCOS-MS Trial Group
Anne Müller 1 , Fabian Hebben 1 , Kim Dillen 1 , Veronika Dunkl 1 , Yasemin Goereci 2 , Raymond Voltz 1,3,4 , Peter Löcherbach 5 , Clemens Warnke 2 , Heidrun Golla 1 , Dirk Müller 6 , Dorthe Hobus 1 , Eckhard Bonmann 7 , Franziska Schwartzkopff 8 , Gereon Nelles 9 , Gundula Palmbach 8 , Herbert Temmes 10 , Isabel Franke 1 , Judith Haas 10 , Julia Strupp 1 , Kathrin Gerbershagen 7 , Laura Becker-Peters 8 , Lothar Burghaus 11 , Martin Hellmich 12 , Martin Paus 8 , Solveig Ungeheuer 1 , Sophia Kochs 1 , Stephanie Stock 6 , Thomas Joist 13 , Volker Limmroth 14
1 Department of Palliative Medicine, Faculty of Medicine and University Hospital, University of Cologne, Cologne, Germany
2 Department of Neurology, Faculty of Medicine and University Hospital, University of Cologne, Cologne, Germany
3 Center for Integrated Oncology Aachen Bonn Cologne Düsseldorf (CIO ABCD), University of Cologne, Cologne, Germany
4 Center for Health Services Research (ZVFK), University of Cologne, Cologne, Germany
5 German Society of Care and Case Management e.V. (DGCC), Münster, Germany
6 Institute for Health Economics and Clinical Epidemiology (IGKE), Faculty of Medicine and University Hospital, University of Cologne, Cologne, Germany
7 Department of Neurology, Klinikum Köln, Cologne, Germany
8 Clinical Trials Centre Cologne (CTCC), Faculty of Medicine and University Hospital, University of Cologne, Cologne, Germany
9 NeuroMed Campus, MedCampus Hohenlind, Cologne, Germany
10 German Multiple Sclerosis Society Federal Association (DMSG), Hannover, Germany
11 Department of Neurology, Heilig Geist-Krankenhaus Köln, Cologne, Germany
12 Institute of Medical Statistics and Computational Biology (IMSB), Faculty of Medicine and University Hospital, University of Cologne, Cologne, Germany
13 Academic Teaching Practice, University of Cologne, Cologne, Germany
14 Department of Neurology, Klinikum Köln-Merheim, Cologne, Germany
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the Innovation Funds of the Federal Joint Committee (G-BA), grant number: 01VSF19029.
Author information
Authors and affiliations.
Department of Palliative Medicine, Faculty of Medicine and University Hospital, University of Cologne, Cologne, Germany
Anne Müller, Fabian Hebben, Kim Dillen, Veronika Dunkl, Raymond Voltz & Heidrun Golla
Department of Neurology, Faculty of Medicine and University Hospital, University of Cologne, Cologne, Germany
Yasemin Goereci & Clemens Warnke
Center for Integrated Oncology Aachen Bonn Cologne Düsseldorf (CIO ABCD), University of Cologne, Cologne, Germany
Raymond Voltz
Center for Health Services Research, University of Cologne, Cologne, Germany
German Society of Care and Case Management E.V. (DGCC), Münster, Germany
Peter Löcherbach
You can also search for this author in PubMed Google Scholar
- Anne Müller
- , Fabian Hebben
- , Kim Dillen
- , Veronika Dunkl
- , Yasemin Goereci
- , Raymond Voltz
- , Peter Löcherbach
- , Clemens Warnke
- , Heidrun Golla
- , Dirk Müller
- , Dorthe Hobus
- , Eckhard Bonmann
- , Franziska Schwartzkopff
- , Gereon Nelles
- , Gundula Palmbach
- , Herbert Temmes
- , Isabel Franke
- , Judith Haas
- , Julia Strupp
- , Kathrin Gerbershagen
- , Laura Becker-Peters
- , Lothar Burghaus
- , Martin Hellmich
- , Martin Paus
- , Solveig Ungeheuer
- , Sophia Kochs
- , Stephanie Stock
- , Thomas Joist
- & Volker Limmroth
Contributions
HG, KD, CW designed the trial. HG, KD obtained ethical approvals. HG, KD developed the interview guidelines with help of the CCM (SU). AM was responsible for collecting qualitative data, developing the code system, coding, analysis of the data and writing the first draft of the manuscript, thoroughly revised and partly rewritten by HG. FH supported in collecting qualitative data, coding and analysis of the interviews. KD supported in collecting qualitative data. AM, FH, KD, VD, YG, RV, PL, CW, HG discussed and con-solidated the finalized category system. AM, FH, KD, VD, YG, RV, PL, CW, HG read and commented on the manuscript and agreed to the final version.
Authors’ information
Not applicable.
Corresponding author
Correspondence to Anne Müller .
Ethics declarations
Ethics approval and consent to participate.
Participants were provided with oral and written information about the trial and provided written informed consent. Ethical approval was obtained from the Ethics Committee of the University of Cologne (#20–1436). The trial is registered in the German Register for Clinical Studies (DRKS) (DRKS00022771) and is conducted under the Declaration of Helsinki.
Consent for publication
Competing interests.
Clemens Warnke has received institutional support from Novartis, Alexion, Sanofi Genzyme, Janssen, Biogen, Merck and Roche. The other authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Müller, A., Hebben, F., Dillen, K. et al. “So at least now I know how to deal with things myself, what I can do if it gets really bad again”—experiences with a long-term cross-sectoral advocacy care and case management for severe multiple sclerosis: a qualitative study. BMC Health Serv Res 24 , 453 (2024). https://doi.org/10.1186/s12913-024-10851-1
Download citation
Received : 23 November 2023
Accepted : 11 March 2024
Published : 10 April 2024
DOI : https://doi.org/10.1186/s12913-024-10851-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cross-sectoral
- Qualitative research
- Health care specialist
- Severe multiple sclerosis
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Heart Views
- v.18(3); Jul-Sep 2017
Guidelines To Writing A Clinical Case Report
What is a clinical case report.
A case report is a detailed report of the symptoms, signs, diagnosis, treatment, and follow-up of an individual patient. Case reports usually describe an unusual or novel occurrence and as such, remain one of the cornerstones of medical progress and provide many new ideas in medicine. Some reports contain an extensive review of the relevant literature on the topic. The case report is a rapid short communication between busy clinicians who may not have time or resources to conduct large scale research.
WHAT ARE THE REASONS FOR PUBLISHING A CASE REPORT?
The most common reasons for publishing a case are the following: 1) an unexpected association between diseases or symptoms; 2) an unexpected event in the course observing or treating a patient; 3) findings that shed new light on the possible pathogenesis of a disease or an adverse effect; 4) unique or rare features of a disease; 5) unique therapeutic approaches; variation of anatomical structures.
Most journals publish case reports that deal with one or more of the following:
- Unusual observations
- Adverse response to therapies
- Unusual combination of conditions leading to confusion
- Illustration of a new theory
- Question regarding a current theory
- Personal impact.
STRUCTURE OF A CASE REPORT[ 1 , 2 ]
Different journals have slightly different formats for case reports. It is always a good idea to read some of the target jiurnals case reports to get a general idea of the sequence and format.
In general, all case reports include the following components: an abstract, an introduction, a case, and a discussion. Some journals might require literature review.
The abstract should summarize the case, the problem it addresses, and the message it conveys. Abstracts of case studies are usually very short, preferably not more than 150 words.
Introduction
The introduction gives a brief overview of the problem that the case addresses, citing relevant literature where necessary. The introduction generally ends with a single sentence describing the patient and the basic condition that he or she is suffering from.
This section provides the details of the case in the following order:
- Patient description
- Case history
- Physical examination results
- Results of pathological tests and other investigations
- Treatment plan
- Expected outcome of the treatment plan
- Actual outcome.
The author should ensure that all the relevant details are included and unnecessary ones excluded.
This is the most important part of the case report; the part that will convince the journal that the case is publication worthy. This section should start by expanding on what has been said in the introduction, focusing on why the case is noteworthy and the problem that it addresses.
This is followed by a summary of the existing literature on the topic. (If the journal specifies a separate section on literature review, it should be added before the Discussion). This part describes the existing theories and research findings on the key issue in the patient's condition. The review should narrow down to the source of confusion or the main challenge in the case.
Finally, the case report should be connected to the existing literature, mentioning the message that the case conveys. The author should explain whether this corroborates with or detracts from current beliefs about the problem and how this evidence can add value to future clinical practice.
A case report ends with a conclusion or with summary points, depending on the journal's specified format. This section should briefly give readers the key points covered in the case report. Here, the author can give suggestions and recommendations to clinicians, teachers, or researchers. Some journals do not want a separate section for the conclusion: it can then be the concluding paragraph of the Discussion section.
Notes on patient consent
Informed consent in an ethical requirement for most studies involving humans, so before you start writing your case report, take a written consent from the patient as all journals require that you provide it at the time of manuscript submission. In case the patient is a minor, parental consent is required. For adults who are unable to consent to investigation or treatment, consent of closest family members is required.
Patient anonymity is also an important requirement. Remember not to disclose any information that might reveal the identity of the patient. You need to be particularly careful with pictures, and ensure that pictures of the affected area do not reveal the identity of the patient.
- Open access
- Published: 11 April 2024
Guillain-barré syndrome (GBS) with antecedent chikungunya infection: a case report and literature review
- Sreelakshmi V. 1 na1 ,
- Amrita Pattanaik ORCID: orcid.org/0000-0002-1562-0347 1 na1 ,
- Srilatha Marate 1 ,
- Reeta S Mani 2 ,
- Aparna R. Pai 3 &
- Chiranjay Mukhopadhyay 1
Neurological Research and Practice volume 6 , Article number: 21 ( 2024 ) Cite this article
Metrics details
Guillain-Barré Syndrome (GBS) is an autoimmune neuropathy. Antecedent infections have been seen to be significant triggering factors for developing GBS. Among them, arboviral infections are rapidly gaining importance as significant triggers, especially in the areas where they are endemic. Chikungunya, an arboviral infection that usually causes a self-limiting acute febrile illness can lead to GBS as one its severe complications. Herein, we describe a case of a 21-year-old female who presented with weakness in all four limbs and paresthesia. Nerve conduction study and cerebrospinal fluid (CSF) analysis showed axonal, demyelinating motor and sensory neuropathy with albuminocytological dissociation indicating Acute Motor and Sensory Axonal Neuropathy (AMSAN) variant of GBS. Serum IgM antibodies against ganglioside GM1 were detected. Anti-Chikungunya IgM antibodies were found in both serum and CSF samples. The patient was initiated with Intravenous Immunoglobulin (IVIG) therapy. In view of hypoxia, she was intubated and was on mechanical ventilation. After 2 weeks of being comatose, the patient gradually improved and was discharged with no sequelae.
A literature review on antecedent infections in GBS is presented alongside the case report to better understand the association of GBS with antecedent infections, especially the endemic arboviral infections like Chikungunya, Dengue and Zika. This will help in reinforcing the significance of having robust surveillance and public health control measures for infectious diseases.
Guillain-Barré Syndrome (GBS) is a rare but serious autoimmune disorder, affecting the peripheral nervous system (PNS). Highlighting the magnitude of the problem, globally, it has an annual incidence of 1–2 cases per 100,000 people [ 1 ]. However, regional differences in the incidence rate has been observed. As per the prevalence surveys done in Europe, Asia, America, and Australia, 0.4 to 4 GBS cases per 100,000 people have been reported annually [ 2 ]. In Asia, an annual incidence of 1.71, 1.82, 0.42 GBS cases per 100,000 people have been observed in China, South Korea, and Japan respectively [ 3 , 4 ]. These cases have been more frequently reported during the monsoon season in some regions [ 2 , 5 ]. However, contradicting this, in other geographical locations, studies have reported the peak of GBS cases in summer and winter, thus pointing towards a regional variation in the seasonality of the cases. Some studies also suggest that there is no discernible seasonal fluctuation [ 6 , 7 ]. Increased incidence in GBS is also observed during outbreaks and pandemics. A recent instance is the significant rise in GBS cases following the COVID-19 pandemic [ 8 , 9 , 10 ]. Although the exact cause of GBS is still unknown, it is speculated to be a post-infectious condition since 2/3rd of the patients suffer from some form of infectious disease before the neurological condition sets in [ 11 , 12 ]. Many bacterial and viral infections have been implicated in triggering the immune system against nerves, damaging it. As a consequence, weakness and tingling sensation in the extremities progressing to acute flaccid paralysis is seen [ 12 , 13 ]. Less than one-third of the patients with GBS require mechanical ventilation due to respiratory muscle weakness. A mortality rate of 1–18% has been reported in such patients [ 14 , 15 ]. Early diagnosis and treatment with intravenous immunoglobulin or plasmapheresis are effective in reducing the severity of the illness and aid in the recovery of the patients [ 12 ]. GBS is mostly a monophasic condition, but recurrence is observed in about 3–10% of the patients. It may occur at any age, however, higher incidences have been observed in adults compared to children with a predominance seen in males [ 16 , 17 ].
GBS is a heterogeneous disorder having regional variation with respect to the clinical presentation, electrophysiological subtype, and outcome [ 18 ]. The lack of definitive cause and effective treatment is a major challenge that clinicians are facing even after 100 years since the reporting of the first case of GBS. Herein, we present a clinical case of fulminant GBS with antecedent Chikungunya infection. Chikungunya infection is an acute febrile illness usually associated with rashes and arthralgia, transmitted through Aedes aegypti and Aedes albopictus mosquitoes [ 19 , 20 ]. Although meningitis, encephalitis, and GBS have been documented as consequences of a severe acute Chikungunya infection, neurological complications are uncommon [ 21 ]. Neurological complications of Chikungunya infection, including one case of GBS were first observed during the outbreak of 1964 in Madras, India [ 22 ].
The objectives of this review include: (i) reviewing the antecedent infections in GBS, (ii) illustrating the pathogenesis of GBS, (iii) describing the clinical features, and (iv) summarizing the treatment and management.
Case presentation
A 21-year-old female presented with rapid progressive weakness involving all four limbs associated with sensory symptoms in the form of paraesthesia followed by numbness. A month prior, she suffered from high-grade fever, chills, rigors with no other symptoms. There was no laboratory confirmed etiological diagnosis. No other premorbid conditions were found as well.
On initial examination, patient had respiratory distress with hypoxia, in view of which she was intubated and was put on control mode of ventilation. Neurological examination revealed tetraparesis, with paraesthesia of the extremities. There was bilateral but asymmetric facial palsy. Deep tendon reflexes were abolished. Pupils were sluggishly reactive. Routine blood examination revealed hyponatremia (126 mEq/L), anaemia with Vitamin B12 and folate deficiency, mild hypokalaemia (3.4 mmol/L), and elevated inflammatory markers. Nerve conduction study and CSF analysis identified axonal, demyelinating motor and sensory neuropathy with albuminocytological dissociation which indicated the Acute Motor Sensory Axonal Neuropathy (AMSAN) variant of GBS. Patient’s serum was positive for IgM antibodies against GM1 ganglioside; IgM antibodies against Chikungunya virus were found to be present in both serum and CSF. IgM antibodies against other prevalent arboviruses like Dengue, Zika, Japanese encephalitis virus, West Nile virus were not detected in both serum and CSF samples.
IVIG therapy (0.4 g/kg/day for 5 days) along with other supportive measures were immediately initiated in the patient. Nevertheless, she continued to deteriorate and 2 days later, ocular movements were lost with power in limbs worsening to zero. The EEG demonstrated severe encephalopathy with theta waves, suggestive of deep coma attributed to hyponatremia and sedation during intubation. She remained comatose with Glasgow Coma Scale score of 3 even after the completion of one course of IVIG therapy. Therefore, another cycle of IVIG therapy was administered. Despite a normal MRI report, a diagnosis of Bickerstaff brainstem encephalitis was considered because of the clinical picture that was presented.
After 10 days, the patient could move her lip, jaw, shoulders, proximal limbs and showed distal flickering. She was eventually removed from ventilatory support. Even with protective measures in place, due to facial weakness, exposure keratitis occurred and was managed by lid taping by the ophthalmology consultant. While intubated, the patient developed paralytic ileus for a few hours which was managed conservatively with bowel rest and intravenous fluid therapy. Physiotherapy and swallowing exercises were started along with iron, vitamin B12, and folate supplementation and continued after discharge. At discharge, she was hemodynamically stable with power of 4/5 in all four limbs, she was able to swallow semi- solids, and maintained saturation in room air.
Literature review: antecedent infections in GBS
Methodology.
We conducted the literature search through multiple search engines such as PubMed, Scopus, Medline, Google Scholar, and ScienceDirect using the search terms: “Guillain-Barré Syndrome”, “GBS”, “autoimmunity”, “antecedent infection”, “bacteria”, “viruses”, “molecular mimicry”, “arboviruses in GBS”, “Chikungunya infection in GBS”, “intravenous immunoglobulins”, “plasmapheresis”. Thereafter, the search was limited to observational and interventional studies on Guillain-Barré Syndrome with antecedent infections. Only articles published in English language were considered. Additionally, relevant studies were identified through reference analysis. The articles available from 1st January 1990 until 31st December 2023, under the above-mentioned criteria were scanned and relevant studies were included in this narrative review. The results are presented under four headings: Antecedent infections causing GBS, Pathogenesis of GBS, Clinical manifestations and Diagnosis, and Treatment and Prognosis.
In addition, all the published cases of GBS with antecedent Chikungunya were included in this literature review. Up to 31st December, 2023, nine papers were published and a total of 33 cases have been described (Table 1 ). There was no gender prevalence, and the average age of patients studied was 51.
Antecedent infections causing GBS
Though the definitive cause of GBS is still unknown, it has been noted that antecedent infections within two to four weeks were present in 2-3rd of the GBS patients before the onset of neurological symptoms [ 11 , 12 ]. A wide spectrum of infectious agents including bacteria and viruses have been reported to cause GBS. Among them, the commonest agent is the Campylobacter jejuni , a Gram-negative bacteria causing gastroenteritis which accounts for 33% of GBS cases in the western countries, and 45–60% in China and Japan [ 11 , 23 , 24 , 25 , 26 , 27 , 28 ]. Other bacteria like Mycoplasma pneumoniae and Haemophilus influenzae have also been reported to trigger GBS [ 11 , 29 ].
Several viral infections have been associated with triggering GBS and have been reported worldwide. A case-control study conducted in Netherlands in 154 GBS patients reported antecedent infections with CMV, EBV, Parainfluenza 1 virus, Influenza A, Influenza B, Adenovirus, Herpes simplex virus (HSV), Varicella zoster virus (VZV) [ 11 ]. Another study conducted in China with 150 GBS patients reported antecedent infections with Influenza A virus, Influenza B virus, Hepatitis E virus (HEV), Hepatitis A virus (HAV), Dengue virus, Cytomegalovirus (CMV), Epstein- Barr virus (EBV), Herpes simplex virus (HSV), Varicella- zoster virus (VZV), and Rubella virus [ 29 ]. Cytomegalovirus has been found to be the most common viral infection which triggers the immune system leading to GBS [ 11 , 29 , 30 , 31 ]. Following CMV, Epstein Barr virus is found to be the next common antecedent viral infection causing approximately 10% of total GBS cases [ 11 , 29 , 32 ]. GBS cases in children are mostly due to antecedent respiratory viral infections [ 33 ]. Antecedent infection with HEV, HAV, and Hepatitis B virus were also reported to cause GBS [ 34 , 35 , 36 ]. Rarely, infections with Adenovirus, Rubella were also observed as antecedent event in GBS patients [ 37 ].
Among the arboviruses, antecedent Zika virus infection has been widely studied in GBS. The 2013 Zika virus outbreak in French Polynesia was followed by an increased number of GBS cases [ 38 ]. The Zika virus epidemic that started in Brazil in 2016 and disseminated to 15 countries and territories increased the GBS global incidence rate 2.6 times [ 39 , 40 ]. Other arboviral infections including Dengue, Chikungunya, and Japanese Encephalitis have also been implicated in the pathogenesis of GBS. In a multinational case-control study conducted in Brazil, Argentina, and Malaysia during 2017–2019 to understand the association of GBS with antecedent arboviral infections, it was found that 55% (27/49) of patients had recent infections. Arboviral infections caused due to Dengue and Chikungunya virus were found in 4% of these cases [ 41 ]. Another study conducted in Mexico to predict the incidence of GBS owing to arboviral infections reported antecedent Zika and Dengue infections in GBS but intriguingly, GBS was not associated with antecedent Chikungunya infection [ 42 ]. Among studies conducted in India, one done in the northern region reported 11.5% (3/26) antecedent Dengue infection in GBS [ 43 ]. In another study conducted in southern India, it was found that 79.3% of GBS patients studied had antecedent infections, of which viral infections with Chikungunya virus, Japanese Encephalitis virus, and Dengue virus were prominent [ 44 ].
While the occurrence of GBS following Chikungunya infection is uncommon, it is recognised as a potential trigger for GBS, particularly during outbreaks and in regions where the virus is endemic. During the Chikungunya outbreak at Reunion Island, France in 2005–2006, Lebrun et al. reported 2 cases of GBS with antecedent Chikungunya infection. In both the patients, Chikungunya infection was confirmed by the presence anti-Chikungunya IgM and IgG antibodies in serum and CSF [ 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 ]. Antecedent Chikungunya infections in GBS reported globally in the last two decades are depicted in ( Table 1 ) .
Pathogenesis of GBS
Th pathophysiology of GBS has been the subject of numerous studies, and research is still ongoing. The most accepted mechanism of pathogenesis is “molecular mimicry” at the B-cell level, particularly in the axonal variant ( Fig. 1 ) . Previous studies found a structural similarity between lipopolysaccharide on the cell membrane of C. jejuni and the glycan molecule in the ganglioside, and the antibody produced against the bacteria led to nerve cell damage through cross-reactive immunological responses [ 54 , 55 , 56 ]. Supporting this, the animal studies carried out in “acute motor axonal neuropathy rabbit model” observed molecular mimicry in acute motor axonal neuropathy (AMAN) type of GBS [ 57 ]. Anti-ganglioside antibodies have been recorded in 36% of GBS patients [ 58 ]. These antibodies have been shown to have different peripheral nerve targets. Anti-GD1a antibodies bind to paranodal myelin, nodes of Ranvier, and neuromuscular junction. Anti-GM1 and anti-GQ1B antibodies bind to a peripheral nerve or neuromuscular junction [ 59 ]. This anti-ganglioside antibody mediated disease progression through complement cascade activation and formation of membrane attack complex plays a major role in the degeneration of nerve components [ 60 ].
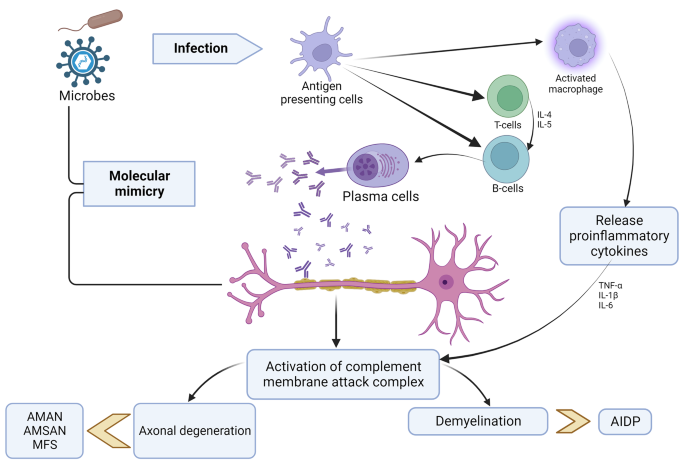
AIDP- “Acute Inflammatory Demyelinating Polyneuropathy,” AMAN- “Acute Motor Axonal Neuropathy,” AMSAN- “Acute Motor and Sensory Axonal Neuropathy,” MFS- “Miller Fisher Syndrome,” TNF- “Tumor Necrosis Factor,” IL- “Interleukin”.
There are a few studies done in arboviral infections that have demonstrated anti-ganglioside antibodies. Dutta et al. observed an increase in anti-GD1a, GD1b, GT1b, and GQ1b antibodies in GBS patients with antecedent Japanese Encephalitis infection [ 61 ]. In a study conducted in immunocompetent mice, an increase in anti-GD1a and GD1b antibodies was observed in Zika virus associated GBS [ 62 ]. In another study involving GBS patients with antecedent Zika virus infection, antibody against GD3 ganglioside was found to be in high [ 63 ]. In GBS patients who had a prior COVID-19 infection, antibodies against GM1, GM2, GD1a, and GQ1b have been identified [ 64 , 65 , 66 ]. In the AIDP variant, autoantibodies against cell adhesion proteins like neurofascin, NRCAM, and contactin-2 localized at Ranvier’s nodes have been suggested as possible targets [ 67 ]. Similar evidence is not well documented for Chikungunya infection. Anti-ganglioside screening has been sparsely done in GBS cases associated with antecedent Chikungunya infection. Although molecular mimicry is speculated to be the most likely mechanism for such cases, there is a lack of evidence that demonstrates viral structure mimicking neural structures. There are two pathways of pathogenesis of Neuro-Chikungunya proposed. One is the direct viral CNS infection which may explain the quick onset of neurological symptoms in GBS patients with antecedent Chikungunya infection (as short as 2 or 3 days) and the second is the PNS affection by autoimmune mechanisms. However, there is a need to generate more evidence on it as well as on the possibility of viral mutations leading to neurological complications [ 68 , 69 ].
Apart from anti-ganglioside antibodies, the epineurium and endoneurium of the nerves have demonstrated infiltration of T lymphocytes in GBS. CD4 + and CD8 + T lymphocytes, as well as macrophages, are observed in greater numbers. It is suggested that the activated T lymphocytes in infections release proinflammatory cytokines, that further activate the complement system leading to demyelination and axonal degeneration. TNF-α produced by the infiltrating T cells has a direct myelinotoxic effect on myelinated fibers, causing demyelination [ 70 ]. The exact mechanisms of pathogenesis, however, still remains unclear.
Clinical manifestations and diagnosis
GBS is caused by the autoimmune attack of peripheral nerves. After the onset of symptoms, it may take 24 hours to four weeks to reach the nadir. The clinical symptoms involved in GBS include weakness and tingling sensation of upper and/or lower limbs which may progress to paralysis of leg, arm, or facial muscles if left untreated [ 12 ].. Some patients may suffer from facial nerve palsy, oculomotor weakness, or oropharyngeal weakness. 10 to 30% GBS patients suffer from severe respiratory muscle weakness within 2 to 4 weeks and require mechanical ventilation [ 71 ]. Keesen et al. observed that in well-characterised GBS patients, respiratory involvement was more distinctively associated with antecedent Chikungunya infection as compared to other arboviral etiology [ 72 ]. Autonomic manifestations like ileus, urinary retention, fever, tachycardia or bradycardia, hypertension or hypotension are also reported in the GBS patients [ 73 , 74 , 75 ].. Fulminant GBS cases reported are characterized by the “absence of brainstem reflexes, complete tetraplegia, and respiratory arrest” [ 76 ]. Rarely, papilledema, facial myokymia, hearing loss, meningeal signs, change in mental status, vocal cord paralysis are also seen in GBS patients [ 77 ]. (Fig. 2 ) shows the clinical features seen in the different variants of GBS.
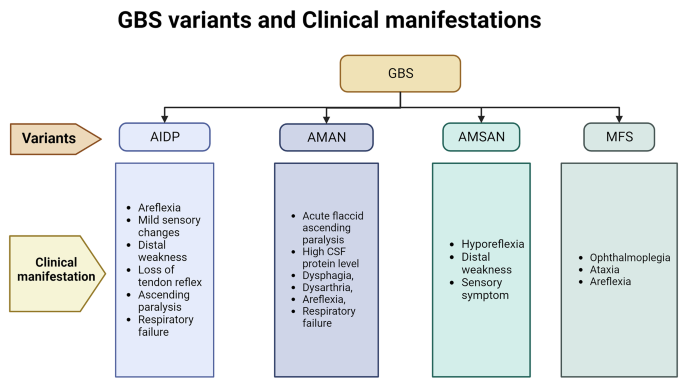
GBS variant and Clinical manifestation
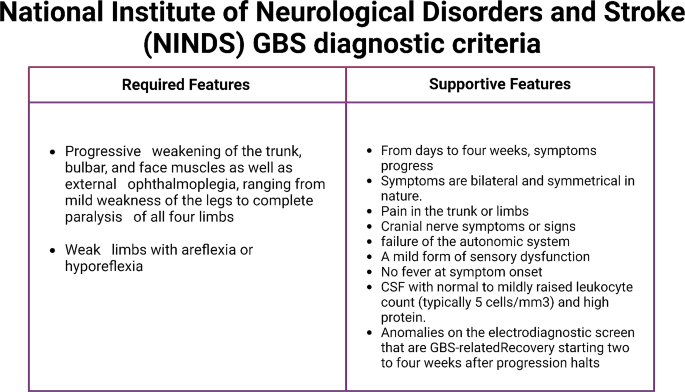
National Institute of Neurological Disorders and Stroke (NINDS) GBS diagnostic criteria
AIDP- “Acute Inflammatory Demyelinating Polyneuropathy,” AMAN- “Acute Motor Axonal Neuropathy,” AMSAN- “Acute Motor and Sensory Axonal Neuropathy,” MFS- “Miller Fisher Syndrome”.
A diagnosis of GBS is mostly based on the clinical history and physical examination. “National Institute of Neurological Disorders and Stroke (NINDS)” has published the GBS diagnostic criteria that is being widely followed (Fig. 3 ) [ 78 ]. The CSF examination and electrophysiological studies provide additional details about the condition. Routine laboratory investigations such as complete blood count, tests for blood glucose, electrolyte estimation, kidney and liver function tests are done in order to exclude other underlying pathologies. Anti-ganglioside antibodies found in the serum aid in identifying the GBS variant. In GBS, CSF protein level is elevated with a normal cell count. CSF examination is also done to find albumin-cytological dissociation [ 78 ]. The severity of the symptoms can be assessed by nerve conduction studies (NCS) and electromyography, which can aid in distinguishing between the axonal and demyelinating forms. Radiological studies can aid in cases where CSF and nerve conduction studies are inconclusive. Spine Magnetic Resonance Imaging (MRI) can exclude other conditions like compressive polyradiculopathy and transverse myelitis [ 79 ].
Treatment and prognosis
Primarily IVIg (0.4 g/kg for 5 days) and plasma exchange (200–250 ml plasma/kg body weight in five sessions) are the recommended treatments for GBS which are quite effective. Due to better availability and ease of administration, IVIg is preferred over plasma exchange. Despite therapy, there is a significant mortality of 3–10% [ 78 , 80 ].
ICU admission is warranted in about 22% of the patients with GBS due to increasing respiratory insufficiency, as was the case here; severe dysfunction in swallowing, cardiac abnormalities and in rapid progression of weakness. Respiratory distress requiring mechanical ventilation has been reported in cases with antecedent Chikungunya infection. In such cases, both acute and long-term mortality are low [ 78 , 81 ].
Antiviral treatment can be considered in patients with GBS who have an ongoing treatable viral infection; however, preceding infections have usually resolved before the onset of weakness [ 78 ]. Arboviral infections including Chikungunya infection is managed by supportive measures. A few novel antiviral compounds and monoclonal antibodies are being evaluated against Chikungunya virus [ 82 , 83 , 84 ]..
Severe disability is seen in 20% of cases which require neurorehabilitation. Some of the delayed complications of GBS include neuropathic pain, postural hypotension, and fatigue. These may persist for months together [ 80 ].
In conclusion, although GBS is considered as a rare disease, there is a perceptible increase in the incidence, especially during outbreaks and pandemics of infectious disease. Many studies mentioned in the literature review have concurred the same. Here we have reported a case of GBS associated with antecedent Chikungunya infection. Chikungunya infection most often causes a self-limiting disease; however, severe complications have been reported occasionally. The case described in this paper adds to the few cases of Chikungunya infection preceding GBS reported globally. This will help physiciansunderstand the clinical course and managesuch cases better. As per the evidence in literature, it is worth noting that acute Arboviral infections including Chikungunya infection lead to GBS in a short period of time that usually ranges from a few days to a few weeks. Most of these cases require ICU support due to frequently associated respiratory insufficiency. Documentation of such cases can help public health policy makers to undertake more surveillance programs and implement appropriate control measures to reduce the burden of Arboviral infections. Along with it, an understanding of the pathogenesis may lead to the development of more directed preventive and therapeutic interventions.
Data availability
Not applicable.
Abbreviations
Guillain Barré Syndrome
Acute Inflammatory Demyelinating Polyneuropathy
Acute Motor Axonal Neuropathy
Acute Motor and Sensory Axonal Neuropathy
Miller Fisher Syndrome
Pharyngeal Cervical Brachial
Bickerstaff’s brainstem encephalitis
Intravenous Immunoglobulin
Lower Motor Neuron
Magnetic Resonance Imaging
Endotracheal tube
Electroencephalogram
Polymerase Chain Reaction
Antigen-presenting cells
Cytomegalovirus
Epstein Barr virus
Cluster of differentiation
Real-time reverse transcriptase polymerase chain reaction
Bragazzi, N. L., Kolahi, A. A., Nejadghaderi, S. A., Lochner, P., Brigo, F., Naldi, A., et al. (2021). Global, regional, and national burden of Guillain–Barré syndrome and its underlying causes from 1990 to 2019. J Neuroinflammation , 18 (1), 264. https://doi.org/10.1186/s12974-021-02319-4 .
Article PubMed PubMed Central Google Scholar
Kuwabara, S. (2004). Guillain-Barré syndrome: Epidemiology, pathophysiology and management. Drugs , 64 (6), 597–610. https://doi.org/10.2165/00003495-200464060-00003 .
Article PubMed Google Scholar
Zheng, P., Tian, D. C., Xiu, Y., Wang, Y., & Shi, F. D. (2021). Incidence of Guillain-Barré syndrome (GBS) in China: A national population-based study. The Lancet Regional Health Western Pacific , 18 , 100302. https://doi.org/10.1016/j.lanwpc.2021.100302 .
Matsui, N., Nodera, H., Kuzume, D., Iwasa, N., Unai, Y., et al. (2018). Guillain-Barré syndrome in a local area in Japan, 2006–2015: An epidemiological and clinical study of 108 patients. European Journal of Neurology , 25 (5), 718–724. https://doi.org/10.1111/ene.13569 .
Article CAS PubMed Google Scholar
Mathew, T., Srinivas, M., Nadig, R., Arumugam, R., & Sarma, G. R. (2014). Seasonal and monthly trends in the occurrence of Guillain-Barre syndrome over a 5-year period: A tertiary care hospital-based study from South India. Annals of Indian Academy of Neurology , 17 (2), 239–241. https://doi.org/10.4103/0972-2327.132662 .
Boucquey, D., Sindic, C. J., Lamy, M., Delmée, M., Tomasi, J. P., & Laterre, E. C. (1991). Clinical and serological studies in a series of 45 patients with Guillain-Barré syndrome. Journal of the Neurological Sciences , 104 (1), 56–63. https://doi.org/10.1016/0022-510x(91)90216-t .
Meshram, R. M., Merchant, S., Bokade, C. M., Bhongade, S., Patil, S., et al. (2016). Seasonal Variation in Childhood Guillain- Barre Syndrome in Central India. J Pediatr Neonatal Care , 5 (6), 00201. https://doi.org/10.15406/jpnc.2016.05.00201 .
Article Google Scholar
Pimentel, V., Luchsinger, V. W., Carvalho, G. L., Alcará, A. M., Esper, N. B., Marinowic, D., Zanirati, G., & da Costa, J. C. (2023). Guillain-Barré syndrome associated with COVID-19: A systematic review. Brain, behavior, & immunity - health , 28 , 100578. https://doi.org/10.1016/j.bbih.2022.100578 .
Bueso, T., Montalvan, V., Lee, J., Gomez, J., Ball, S., Shoustari, A., Julayanont, P., & Jumper, C. (2021). Guillain-Barre Syndrome and COVID-19: A case report. Clinical Neurology and Neurosurgery , 200 , 106413. https://doi.org/10.1016/j.clineuro.2020.106413 .
Chakraborty, U., Hati, A., & Chandra, A. (2021). Covid-19 associated Guillain-Barré syndrome: A series of a relatively uncommon neurological complication. Diabetes & Metabolic Syndrome , 15 (6), 102326. https://doi.org/10.1016/j.dsx.2021.102326 .
Article CAS Google Scholar
Jacobs, B. C., Rothbarth, P. H., van der Meché, F. G., Herbrink, P., Schmitz, P. I., de Klerk, M. A., & van Doorn, P. A. (1998). The spectrum of antecedent infections in Guillain-Barré syndrome: A case-control study. Neurology , 51 (4), 1110–1115. https://doi.org/10.1212/wnl.51.4.1110 .
Guillain–Barré syndrome (2023). https://www.who.int/news-room/fact-sheets/detail/guillain-barré-syndrome . Accessed 08 Aug 2023.
Jasem, J., Marof, K., Nawar, A., Khalaf, Y., Aswad, S., Hamdani, F., Islam, M., & Kalil, A. (2013). Guillain-Barré syndrome as a cause of acute flaccid paralysis in Iraqi children: A result of 15 years of nation-wide study. BMC Neurology , 13 , 195. https://doi.org/10.1186/1471-2377-13-195 .
Melone, M. A., Heming, N., Meng, P., Mompoint, D., Aboab, J., Clair, B., Salomon, J., Sharshar, T., Orlikowski, D., Chevret, S., & Annane, D. (2020). Early mechanical ventilation in patients with Guillain-Barré syndrome at high risk of respiratory failure: A randomized trial. Annals of Intensive care , 10 (1), 128. https://doi.org/10.1186/s13613-020-00742-z .
Article CAS PubMed PubMed Central Google Scholar
Netto, A. B., Taly, A. B., Kulkarni, G. B., Rao, U. G., & Rao, S. (2011). Mortality in mechanically ventilated patients of Guillain Barré Syndrome. Annals of Indian Academy of Neurology , 14 (4), 262–266. https://doi.org/10.4103/0972-2327.91942 .
Arami, M. A., Yazdchi, M., & Khandaghi, R. (2006). Epidemiology and characteristics of Guillain-Barré syndrome in the northwest of Iran. Annals of Saudi Medicine , 26 (1), 22–27. https://doi.org/10.5144/0256-4947.2006.22 .
Nagappa, M., Rahul, W., Sinha, S., Bindu, P. S., Mathuranath, P. S., Rao, S., et al. (2017). Guillain Barre Syndrome in the elderly: Experience from a tertiary-care hospital in India. Journal of Clinical Neuroscience: Official Journal of the Neurosurgical Society of Australasia , 46 , 45–49. https://doi.org/10.1016/j.jocn.2017.08.048 .
Doets, A. Y., Verboon, C., van den Berg, B., Harbo, T., Cornblath, & IGOS Consortium. (2018). Regional variation of Guillain-Barré syndrome. Brain: A Journal of Neurology , 141 (10), 2866–2877. https://doi.org/10.1093/brain/awy232 .
Chikungunya virus| CDC (2023). https://www.cdc.gov/chikungunya/index.html . Accessed 14 Jun 2023.
Ojeda Rodriguez, J. A., Haftel, A., & Walker, I. I. I. (2023). Chikungunya Fever. In: StatPearls. Treasure Island (FL): StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK534224/ . Accessed 14 Jun 2023.
Gonçalves Júnior, J., de Oliveira Bringel, M., Rodrigues de Morais, L. (2022). Chikungunya Neurological Manifestations: A Systematic Literature Review IntechOpen . doi: https://doi.org/10.5772/intechopen.95525 .
Thiruvengadam, K. V., Kalyanasundaram, V., & Rajgopal, J. (1965). Clinical and pathological studies on chikungunya fever in Madras city. The Indian Journal of Medical Research , 53 (8), 729–744.
CAS PubMed Google Scholar
Guillain-Barré Syndrome| Campylobacter| CDC (2022). https://www.cdc.gov/campylobacter/guillain-barre.html . Accessed 09 Aug 2023.
Hao, Q., Saida, T., Kuroki, S., Nishimura, M., Nukina, M., Obayashi, H., & Saida, K. (1998). Antibodies to gangliosides and galactocerebroside in patients with Guillain-Barré syndrome with preceding Campylobacter jejuni and other identified infections. Journal of Neuroimmunology , 81 (1–2), 116–126. https://doi.org/10.1016/s0165-5728(97)00166-5 .
Constant, O. C., Bentley, C. C., Denman, A. M., Lehane, J. R., & Larson, H. E. (1983). The Guillain-Barré syndrome following Campylobacter enteritis with recovery after plasmapheresis. The Journal of Infection , 6 (1), 89–91. https://doi.org/10.1016/s0163-4453(83)95881-4 .
Molnar, G. K., Mertsola, J., & Erkko, M. (1982). Guillain-Barré syndrome associated with campylobacter infection. British Medical Journal (Clinical Research ed) , 285 (6342), 652. https://doi.org/10.1136/bmj.285.6342.652 .
Pryor, W. M., Freiman, J. S., Gillies, M. A., & Tuck, R. R. (1984). Guillain-Barré syndrome associated with Campylobacter infection. Australian and New Zealand Journal of Medicine , 14 (5), 687–688. https://doi.org/10.1111/j.1445-5994.1984.tb05033.x .
Speed, B., Kaldor, J., & Cavanagh, P. (1984). Guillain-Barré syndrome associated with Campylobacter jejuni enteritis. The Journal of Infection , 8 (1), 85–86. https://doi.org/10.1016/s0163-4453(84)93516-3 .
Hao, Y., Wang, W., Jacobs, B. C., Qiao, B., Chen, M., Liu, D., Feng, X., & Wang, Y. (2019). Antecedent infections in Guillain-Barré syndrome: A single-center, prospective study. Annals of Clinical and Translational Neurology , 6 (12), 2510–2517. https://doi.org/10.1002/acn3.50946 .
Mamishi, S., Ashrafi, M. R., Mohammadi, M., Zamani, G., Pourakbari, B., Mahmoudi, S., & Aziz-Ahari, S. (2021). Cytomegalovirus infection and Guillain-Barré syndrome: The First Case-Control Study in Iran. Iranian Journal of Child Neurology , 15 (4), 35–41. https://doi.org/10.22037/ijcn.v15i4.31285 .
Leung, J., Sejvar, J. J., Soares, J., & Lanzieri, T. M. (2020). Guillain-Barré syndrome and antecedent cytomegalovirus infection, USA 2009–2015. Neurological Sciences: Official Journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology , 41 (4), 885–891. https://doi.org/10.1007/s10072-019-04156-z .
Abidoye, O., Raybon-Rojas, E., & Ogbuagu, H. (2022). A rare case of Epstein-Barr Virus: Infectious mononucleosis complicated by Guillain-Barré Syndrome. Cureus , 14 (1), e21085. https://doi.org/10.7759/cureus.21085 .
Gupta, M., Monjazeb, S., Rosser, T., Santoro, J. D., & Ahsan, N. (2023). A case of Pediatric Guillain-Barré Syndrome after respiratory syncytial virus infection. Pediatric Neurology , 146 , 129–131. https://doi.org/10.1016/j.pediatrneurol.2023.06.013 .
Zheng, X., Yu, L., Xu, Q., Gu, S., & Tang, L. (2018). Guillain-Barre syndrome caused by hepatitis E infection: Case report and literature review. BMC Infectious Diseases , 18 (1), 50. https://doi.org/10.1186/s12879-018-2959-2 .
Blecker, E., & Ehtsham, M. (2021). Guillain-Barré Syndrome Likely due to Relapsing Hepatitis A. Case reports in hepatology , 2021 , 5570027. https://doi.org/10.1155/2021/5570027 .
Wei, J., & Duan, S. (2021). Severe Guillain-Barré syndrome associated with chronic hepatitis B: A case report and literature review. Medicine , 100 (48), e27989. https://doi.org/10.1097/MD.0000000000027989 .
Figueiredo, C. A., Klautau, G. B., Afonso, A. M., Castrignano, S. B., Oliveira, M. I., et al. (2008). Isolation and genotype analysis of Rubella virus from a case of Guillain-Barré syndrome. Journal of Clinical Virology: The Official Publication of the Pan American Society for Clinical Virology , 43 (3), 343–345. https://doi.org/10.1016/j.jcv.2008.07.015 .
Cao-Lormeau, V. M., Blake, A., Mons, S., Lastère, S., Roche, C., Vanhomwegen, J., Dub, T., et al. (2016). Guillain-Barré syndrome outbreak associated with Zika virus infection in French polynesia: A case-control study. Lancet (London England) , 387 (10027), 1531–1539. https://doi.org/10.1016/S0140-6736(16)00562-6 .
Koike, H., Chiba, A., & Katsuno, M. (2021). Emerging infection, vaccination, and Guillain-Barré syndrome: A review. Neurology and Therapy , 10 (2), 523–537. https://doi.org/10.1007/s40120-021-00261-4 .
Assesssment and management of Guillain-Barré syndrome in the context of Zika virus infection: interim guidance update (2016). https://www.who.int/publications-detail-redirect/WHO-ZIKV-MOC-16.4-Rev.1 . Accessed 8 Jun 2023.
Leonhard, S. E., Tan, C. Y., van der Eijk, A. A., Reisin, R. R., Franken, S. C., Huizinga, R., et al. (2021). Antecedent infections in Guillain-Barré syndrome in endemic areas of arbovirus transmission: A multinational case-control study. Journal of the Peripheral Nervous System: JPNS , 26 (4), 449–460. https://doi.org/10.1111/jns.12469 .
Arriaga-Nieto, L., Hernández-Bautista, P. F., Vallejos-Parás, A., Grajales-Muñiz, C., et al. (2022). Predict the incidence of Guillain Barré Syndrome and Arbovirus infection in Mexico, 2014–2019. PLOS Global Public Health , 2 (3), e0000137. https://doi.org/10.1371/journal.pgph.0000137 .
Verma, R., Sharma, P., Garg, R. K., Atam, V., Singh, M. K., & Mehrotra, H. S. (2011). Neurological complications of dengue fever: Experience from a tertiary center of north India. Annals of Indian Academy of Neurology , 14 (4), 272–278. https://doi.org/10.4103/0972-2327.91946 .
Dutta, D., Debnath, M., Nagappa, M., Das, S. K., Wahatule, R., Sinha, S., Taly, A. B., & Ravi, V. (2021). Antecedent infections in Guillain-Barré syndrome patients from south India. Journal of the Peripheral Nervous System: JPNS , 26 (3), 298–306. https://doi.org/10.1111/jns.12459 .
Lebrun, G., Chadda, K., Reboux, A. H., Martinet, O., & Gaüzère, B. A. (2009). Guillain-Barré syndrome after chikungunya infection. Emerging Infectious Diseases , 15 (3), 495–496. https://doi.org/10.3201/eid1503.071482 .
Lemant, J., Boisson, V., Winer, A., Thibault, L., André, H., Tixier, F., Lemercier, M., Antok, E., Cresta, M. P., Grivard, P., et al. (2008). Serious acute Chikungunya virus infection requiring intensive care during the Reunion Island outbreak in 2005–2006. Critical care Medicine , 36 (9), 2536–2541. https://doi.org/10.1097/CCM.0b013e318183f2d2 .
Balavoine, S., Pircher, M., Hoen, B., Herrmann-Storck, C., Najioullah, F., Madeux, B., Signate, A., Valentino, R., Lannuzel, A., Saint Louis, M., Cassadou, S., Cabié, A., & Schepers, K. (2017). Guillain-Barré Syndrome and Chikungunya: Description of all cases diagnosed during the 2014 outbreak in the French West Indies. The American Journal of Tropical Medicine and Hygiene , 97 (2), 356–360. https://doi.org/10.4269/ajtmh.15-0753 .
Villamil-Gómez, W., Silvera, L. A., Páez-Castellanos, J., & Rodriguez-Morales, A. J. (2016). Guillain-Barré syndrome after Chikungunya infection: A case in Colombia. Enfermedades Infecciosas y Microbiologia Clinica , 34 (2), 140–141. https://doi.org/10.1016/j.eimc.2015.05.012 .
Matos, A. M. B., Carvalho, M., Malta, F. M., Rodrigues, D. L., Félix, C. L., Pannuti, A. C., Lima, C. S., Espósito, A. D. D. R., Santos, D. L. A. D., von Glehn, L. M. B., Colares, F., da Fonseca, J. K. B., de Oliveira, B. A. L., A. C. P., & Romano, C. M. (2020). High proportion of Guillain-Barré syndrome associated with chikungunya in Northeast Brazil. Neurology(R) Neuroimmunology & Neuroinflammation , 7 (5), e833. https://doi.org/10.1212/NXI.0000000000000833 .
do Rosário, M. S., de Jesus, P. A. P., Farias, D. S., Novaes, M. A. C., Francisco, M. V. L. O., Santos, C. S., Moura, D., Lima, F. W. M., Alcantara, L. C. J., & de Siqueira, I. C. (2022). Guillain-Barré Syndrome and Miller Fisher Syndrome in Association with an arboviral outbreak: A Brazilian Case Series. Frontiers in Medicine , 9 , 911175. https://doi.org/10.3389/fmed.2022.911175 .
Agarwal, A., Vibha, D., Srivastava, A. K., Shukla, G., & Prasad, K. (2017). Guillain-Barre syndrome complicating Chikungunya virus infection. Journal of Neurovirology , 23 (3), 504–507. https://doi.org/10.1007/s13365-017-0516-1 .
Mahto, S. K., Gupta, P. K., Singh, A., & Meena, R. C. (2018). Atypical neurological manifestations of Chikungunya Fever: Two case reports. Indian Journal of Critical care Medicine: peer-reviewed Official Publication of Indian Society of Critical Care Medicine , 22 (4), 306–308. https://doi.org/10.4103/ijccm.IJCCM_459_17 .
Hameed, S., & Khan, S. (2019). Rare variant of Guillain-Barré syndrome after chikungunya viral fever. BMJ case Reports , 12 (4), e228845. https://doi.org/10.1136/bcr-2018-228845 .
van den Berg, B., Walgaard, C., Drenthen, J., Fokke, C., Jacobs, B. C., & van Doorn, P. A. (2014). Guillain-Barré syndrome: Pathogenesis, diagnosis, treatment and prognosis. Nature Reviews Neurology , 10 (8), 469–482. https://doi.org/10.1038/nrneurol.2014.121 .
Nyati, K. K., & Nyati, R. (2013). Role of Campylobacter jejuni infection in the pathogenesis of Guillain-Barré syndrome: An update. BioMed Research International , 2013 , 852195. https://doi.org/10.1155/2013/852195 .
Finsterer, J. (2022). Triggers of Guillain-Barré Syndrome: Campylobacter jejuni predominates. International Journal of Molecular Sciences , 23 (22), 14222. https://doi.org/10.3390/ijms232214222 .
Shahrizaila, N., & Yuki, N. (2011). Guillain-barré syndrome animal model: the first proof of molecular mimicry in human autoimmune disorder. Journal of biomedicine & biotechnology , 2011 , 829129. https://doi.org/10.1155/2011/829129 .
Saeed, M. L., Baloch, K., Mahmud, B., Khan, S. N., Qureshi, M. T., Shad, M. S. S., Hussain, Z. S., Munawar, S. W., Qadeer, K., A., & Abdullah, A. (2019). Role of anti-ganglioside antibodies in the diagnosis of Guillain-Barré Syndrome as an alternate investigation. Cureus , 11 (5), e4625. https://doi.org/10.7759/cureus.4625 .
Goodfellow, J. A., Bowes, T., Sheikh, K., Odaka, M., Halstead, S. K., Humphreys, P. D., Wagner, E. R., Yuki, N., Furukawa, K., Furukawa, K., Plomp, J. J., & Willison, H. J. (2005). Overexpression of GD1a ganglioside sensitizes motor nerve terminals to anti-GD1a antibody-mediated injury in a model of acute motor axonal neuropathy. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience , 25 (7), 1620–1628. https://doi.org/10.1523/JNEUROSCI.4279-04.2005 .
Susuki, K., Rasband, M. N., Tohyama, K., Koibuchi, K., Okamoto, S., Funakoshi, K., Hirata, K., Baba, H., & Yuki, N. (2007). Anti-GM1 antibodies cause complement-mediated disruption of sodium channel clusters in peripheral motor nerve fibers. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience , 27 (15), 3956–3967. https://doi.org/10.1523/JNEUROSCI.4401-06.2007 .
Dutta, D., Debnath, M., Seshagiri, D. V., Nair, B. V. S., Das, S. K., Wahatule, R., Sinha, S., Ravi, V., Taly, A. B., & Nagappa, M. (2022). Impact of antecedent infections on the antibodies against gangliosides and Ganglioside complexes in Guillain-Barré syndrome: A correlative study. Annals of Indian Academy of Neurology , 25 (3), 401–406. https://doi.org/10.4103/aian.aian_121_22 .
Beaver, J. T., Mills, L. K., Swieboda, D., Lelutiu, N., Esser, E. S., Antao, O. Q., Scountzou, E., Williams, D. T., Papaioannou, N., Littauer, E. Q., & Skountzou, I. (2020). Zika virus-induced neuro-ocular pathology in immunocompetent mice correlates with anti-ganglioside autoantibodies. Human Vaccines & Immunotherapeutics , 16 (9), 2092–2108. https://doi.org/10.1080/21645515.2020.1775459 .
Nico, D., Conde, L., Rivera-Correa, J. L., Vasconcelos-Dos-Santos, A., et al. (2018). Prevalence of IgG autoantibodies against GD3 Ganglioside in Acute Zika Virus infection. Frontiers in Medicine , 5 , 25. https://doi.org/10.3389/fmed.2018.00025 .
Simões, J. L. B., & Bagatini, M. D. (2021). Purinergic Signaling of ATP in COVID-19 Associated Guillain-Barré Syndrome. Journal of Neuroimmune Pharmacology: The Official Journal of the Society on NeuroImmune Pharmacology , 16 (1), 48–58. https://doi.org/10.1007/s11481-020-09980-1 .
Dufour, C., Co, T. K., & Liu, A. (2021). GM1 ganglioside antibody and COVID-19 related Guillain Barre Syndrome - a case report, systemic review and implication for vaccine development. Brain Behavior & immunity - health , 12 , 100203. https://doi.org/10.1016/j.bbih.2021.100203 .
Chan, M., Han, S. C., Kelly, S., Tamimi, M., Giglio, B., & Lewis, A. (2021). A Case Series of Guillain-Barré Syndrome after COVID-19 infection in New York. Neurology Clinical Practice , 11 (4), e576–e578. https://doi.org/10.1212/CPJ.0000000000000880 .
Ziganshin, R. H., Ivanova, O. M., Lomakin, Y. A., Belogurov, A. A. Jr, Kovalchuk, S. I., Azarkin, I. V., Arapidi, G. P., Anikanov, N. A., Shender, V. O., Piradov, M. A., Suponeva, N. A., Vorobyeva, A. A., Gabibov, A. G., Ivanov, V. T., & Govorun, V. M. (2016). The pathogenesis of the demyelinating form of Guillain-Barre Syndrome (GBS): Proteo-peptidomic and immunological profiling of physiological fluids. Molecular & Cellular Proteomics: MCP , 15 (7), 2366–2378. https://doi.org/10.1074/mcp.M115.056036 .
Article CAS PubMed Central Google Scholar
Ferreira da Silva, I. R., Frontera, J. A., Moreira, & Nascimento, O. J. (2016). News from the battlefront: Zika virus-associated Guillain-Barré syndrome in Brazil. Neurology , 87 (15), e180–e181. https://doi.org/10.1212/WNL.0000000000003024 .
Cerny, T., Schwarz, M., Schwarz, U., Lemant, J., Gérardin, P., & Keller, E. (2017). The range of neurological complications in Chikungunya Fever. Neurocritical care , 27 (3), 447–457. https://doi.org/10.1007/s12028-017-0413-8 .
Nyati, K. K., & Prasad, K. N. (2014). Role of cytokines and toll-like receptors in the immunopathogenesis of Guillain-Barré syndrome. Mediators of Inflammation , 2014 , 758639. https://doi.org/10.1155/2014/758639 .
Fokke, C., van den Berg, B., Drenthen, J., Walgaard, C., van Doorn, P. A., & Jacobs, B. C. (2014). Diagnosis of Guillain-Barré syndrome and validation of Brighton criteria. Brain: A Journal of Neurology , 137 (Pt 1), 33–43. https://doi.org/10.1093/brain/awt285 .
Keesen, T. S. L., de Almeida, R. P., Gois, B. M., Peixoto, R. F., Pachá, A. S. C., Vieira, F. C. F., Paixão, M., Cazzaniga, R., Boyton, R. J., & Altmann, D. M. (2017). Guillain-Barré syndrome and arboviral infection in Brazil. The Lancet Infectious Diseases , 17 (7), 693–694. https://doi.org/10.1016/S1473-3099(17)30333-X .
Ho, T. W., Hsieh, S. T., Nachamkin, I., Willison, H. J., Sheikh, K., Kiehlbauch, J., Flanigan, K., McArthur, J. C., Cornblath, D. R., McKhann, G. M., & Griffin, J. W. (1997). Motor nerve terminal degeneration provides a potential mechanism for rapid recovery in acute motor axonal neuropathy after Campylobacter infection. Neurology , 48 (3), 717–724. https://doi.org/10.1212/wnl.48.3.717 .
Lehmann, H. C., Jangouk, P., Kierysch, E. K., Meyer zu Hörste, G., Hartung, H. P., & Kieseier, B. C. (2010). Autoantibody-mediated dysfunction of sympathetic neurons in guillain-barre syndrome. Archives of Neurology , 67 (2), 203–210. https://doi.org/10.1001/archneurol.2009.331 .
van Doorn, P. A., Ruts, L., & Jacobs, B. C. (2008). Clinical features, pathogenesis, and treatment of Guillain-Barré syndrome. The Lancet Neurology , 7 (10), 939–950. https://doi.org/10.1016/S1474-4422(08)70215-1 .
Nakamura, Y., Motoki, M., Hirose, T., Hosokawa, T., Ishida, S., & Arawaka, S. (2019). Fulminant Guillain-Barré syndrome showing severe pharyngeal-cervical-brachial weakness in the recovery phase: A case report. BMC Neurology , 19 (1), 145. https://doi.org/10.1186/s12883-019-1376-5 .
Mustafa, A., Adio, B., & Yarahmadi, A. (2021). Descending paralysis as an atypical presentation of Guillain-Barré Syndrome. SAGE open Medical case Reports , 9 , 2050313X20986662. https://doi.org/10.1177/2050313X20986662 .
Leonhard, S. E., Mandarakas, M. R., Gondim, F. A. A., Bateman, K., Ferreira, M. L. B., Cornblath, D. R., van Doorn, P. A., Dourado, M. E., Hughes, R. A. C., Islam, B., Kusunoki, S., Pardo, C. A., Reisin, R., Sejvar, J. J., Shahrizaila, N., Soares, C., Umapathi, T., Wang, Y., Yiu, E. M., Willison, H. J., & Jacobs, B. C. (2019). Diagnosis and management of Guillain-Barré syndrome in ten steps. Nature Reviews Neurology , 15 (11), 671–683. https://doi.org/10.1038/s41582-019-0250-9 .
Singh, G., Bell, D., Rasuli, B., et al. (2021). Guillain-Barré syndrome. Reference Article Radiopaedia org . https://doi.org/10.53347/rID-7533 . https://radiopaedia.org/articles/guillain-barre-syndrome-2 Accessed on 05 Oct 2023.
Head, V. A., & Wakerley, B. R. (2016). Guillain-Barré syndrome in general practice: Clinical features suggestive of early diagnosis. The British Journal of General Practice: The Journal of the Royal College of General Practitioners , 66 (645), 218–219. https://doi.org/10.3399/bjgp16X684733 .
Witsch, J., Galldiks, N., Bender, A., Kollmar, R., Bösel, J., Hobohm, C., Günther, A., Schirotzek, I., Fuchs, K., & Jüttler, E. (2013). Long-term outcome in patients with Guillain-Barré syndrome requiring mechanical ventilation. Journal of Neurology , 260 (5), 1367–1374. https://doi.org/10.1007/s00415-012-6806-x .
Abdelnabi, R., Kovacikova, K., Moesslacher, J., Donckers, K., Battisti, V., Leyssen, P., Langer, T., Puerstinger, G., Quérat, G., Li, C., Decroly, E., Tas, A., Marchand, A., Chaltin, P., Coutard, B., van Hemert, M., Neyts, J., & Delang, L. (2020). Novel class of Chikungunya Virus small molecule inhibitors that targets the viral Capping Machinery. Antimicrobial Agents and Chemotherapy , 64 (7), e00649–e00620. https://doi.org/10.1128/AAC.00649-20 .
Feibelman, K. M., Fuller, B. P., Li, L., LaBarbera, D. V., & Geiss, B. J. (2018). Identification of small molecule inhibitors of the Chikungunya virus nsP1 RNA capping enzyme. Antiviral Research , 154 , 124–131. https://doi.org/10.1016/j.antiviral.2018.03.013 .
Kovacikova, K., & van Hemert, M. J. (2020). Small-molecule inhibitors of Chikungunya Virus: Mechanisms of Action and Antiviral Drug Resistance. Antimicrobial Agents and Chemotherapy , 64 (12), e01788–e01720. https://doi.org/10.1128/AAC.01788-20 .
Download references
Acknowledgements
Author information.
V Sreelakshmi and Amrita Pattanaik contributed equally to this work and share first authorship.
Authors and Affiliations
Manipal Institute of Virology, Manipal Academy of Higher Education, Manipal, 576104, Karnataka, India
Sreelakshmi V., Amrita Pattanaik, Srilatha Marate & Chiranjay Mukhopadhyay
Department of Neurovirology, National Institute of Mental Health and Neurosciences (NIMHANS), Karnataka, Bengaluru, India
Reeta S Mani
Department of Neurology, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, 576104, Karnataka, India
Aparna R. Pai
You can also search for this author in PubMed Google Scholar
Contributions
SV prepared the first draft of the manuscript and performed the virological laboratory investigations. AP was involved in conceptualisation, literature research, and manuscript editing and review. SM was responsible for literature search and manuscript editing. ARP was responsible for patient recruitment, interpretation of clinical data, manuscript editing and review. RSM and CM were responsible for critically revising the paper for important intellectual content and overall supervision. All authors have read and approved the final manuscript.
Corresponding authors
Correspondence to Amrita Pattanaik or Aparna R. Pai .
Ethics declarations
Ethics approval and consent to participate.
The additional virological investigations done were a part of the study titled, ‘Screening for antecedent viral infections in Guillain Barre Syndrome’ that was approved by the Institutional Ethical Committee (IEC), Kasturba Medical College, and Kasturba Hospital (Approval ID: 167/2022).
Consent for publication
Written informed consent was obtained from the patient for the publication of this report.
Competing interests
The authors declare that they have no competing interests.
Authors’ information
Manipal Institute of Virology, Manipal Academy of Higher Education, Manipal, Karnataka, India.
Sreelakshmi V, Amrita Pattanaik, Srilatha Marate, Chiranjay Mukhopadhyay.
Department of Neurovirology, National Institute of Mental Health and Neurosciences (NIMHANS), Bengaluru, Karnataka, India.
Reeta S Mani.
Department of Neurology, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, Karnataka, India.
Aparna R Pai.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
V., S., Pattanaik, A., Marate, S. et al. Guillain-barré syndrome (GBS) with antecedent chikungunya infection: a case report and literature review. Neurol. Res. Pract. 6 , 21 (2024). https://doi.org/10.1186/s42466-024-00315-6
Download citation
Received : 02 December 2023
Accepted : 04 March 2024
Published : 11 April 2024
DOI : https://doi.org/10.1186/s42466-024-00315-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Guillain-Barré Syndrome
- Antecedent infections
- Arboviral infections
- Chikungunya
- Molecular mimicry
- Intravenous immunoglobulin
Neurological Research and Practice
ISSN: 2524-3489
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Open access
- Published: 10 April 2024
Phenotypes and outcome of diffuse pulmonary non-amyloid light chain deposition disease
- François Lestelle 1 ,
- Catherine Beigelman 2 ,
- David Rotzinger 2 ,
- Salim Si-Mohamed 3 ,
- Mouhamad Nasser 1 ,
- Lidwine Wemeau 4 ,
- Sandrine Hirschi 5 ,
- Grégoire Prevot 6 ,
- Antoine Roux 7 ,
- Vincent Bunel 8 ,
- Emmanuel Gomez 9 ,
- Laurent Sohier 10 ,
- Helene Morisse Pradier 11 ,
- Martine Reynaud Gaubert 12 ,
- Anne Gondouin 13 ,
- Romain Lazor 14 ,
- Jean-Charles Glerant 15 ,
- Françoise Thivolet Bejui 16 ,
- Magali Colombat 17 ,
- Vincent Cottin 1 , 18 &
the OrphaLung network
Respiratory Research volume 25 , Article number: 159 ( 2024 ) Cite this article
Metrics details
Light chain deposition disease (LCDD) is a very rare entity. Clinical manifestations of LCDD vary according to the organs involved. Data on pulmonary LCDD are scarce and limited to small series or case reports. This study aimed to describe the characteristics and outcome of diffuse pulmonary non-amyloid LCDD localized to the lungs.
Study design and methods
A multicenter retrospective cohort study was conducted. Clinical characteristics were collected, and chest CTs were centrally reviewed. The diagnosis of pulmonary non-amyloid LCDD was confirmed by immunohistochemistry.
Thirty-one cases were identified (68% female), with a median age at diagnosis of 50 years (IQR 20). Baseline FEV1/FVC was < 0.70 in 45% of patients. Mean ( ± SD) FEV1 and DLCO were 86% ± 26.2 and 52% ± 23.9, respectively. CT revealed peculiar patterns of thin-walled cysts (58%) and thin-walled cystic bronchiectases (27%). Increased serum kappa light chain was found in 87% of patients. Histological analysis showed kappa light chain deposits in all patients, except one with lambda chain deposits. Median annual FEV1 decline was 127 ml (IQR 178) and median DLCO decline was 4.3% (IQR 4.3). Sixteen patients received immunomodulatory treatment or chemotherapy; serum light chain levels decreased in 9 cases (75%), without significant improvement in FEV1 ( p = 0.173). Overall, 48% of patients underwent bilateral lung transplantation. Transplant-free survival at 5 and 10 years were 70% and 30%, respectively. An annual FEV1 decline greater than 127 ml/year was associated with increased risk of death or transplantation ( p = 0.005).
Conclusions
Diffuse pulmonary LCDD is characterised by female predominance, a peculiar imaging pattern with bronchiectasis and/or cysts, progressive airway obstruction and severe DLCO impairment, and poor outcome. Lung transplantation is a treatment of choice.
Take-home message
Diffuse pulmonary light chain deposition disease is characterised by female predominance, a peculiar imaging pattern with bronchiectasis and/or cysts, progressive airway obstruction and diffusion capacity impairment, and poor outcome.
Plain language summary
Diffuse pulmonary light chain deposition disease is an exceedingly rare disease of the lungs, whereby parts of abnormal antibodies (proteins involved in host defense especially against microorganisms) deposit in the lungs. This disease is characterised by a peculiar pattern on chest CT, with dilation of bronchi and formation of air-filled cysts (holes), and progresses in several years to chronic respiratory insufficiency.
Immunoglobulins can cause specific forms of lung involvement. Their physiochemical properties and size are important pathogenetic determinants. Two forms of immunoglobulin light chains (LC) can be deposited in tissues: amyloid [ 1 ] and non-amyloid. Light chain deposition disease (LCDD) is a term restricted to the non-amyloid forms of LC deposition.
LCDD is a rare multisystemic entity described by Randall in 1976 [ 2 ] as the deposition of a nonfibrillary, amorphous material that does not have a β-pleated sheet configuration and consequently does not bind Congo red nor have apple-green birefringence with Congo red stain. Contrary to LC amyloidosis [ 3 ], LCDD is mostly composed of kappa LC. Moreover, electronic microscopy does not show a fibrillary pattern but electron-dense granular deposits along basement membranes [ 4 ]. The diagnosis of LCDD is established by immunohistological analysis of affected organs. It requires a formalin-fixed paraffin-embedded sample for microscopic examination and a frozen sample for immunofluorescence analysis with anti-kappa and anti-lambda antibodies. When a frozen tissue sample is not available, mass spectrometry on formalin-fixed paraffin-embedded tissue can be used [ 5 ].
Clinical manifestations of LCDD vary according to the organs involved. Lung involvement appears to be very uncommon but may be underrecognised especially when the deposition of LCs is limited to the lung. Since its first report in 1988 [ 6 ], pulmonary LCDD has been described as either nodular or diffuse [ 7 , 8 ]. The nodular form is generally seen in patients who have no evidence of plasma cell dyscrasia. Diffuse LCDD is characterised by parenchymal cysts, or airway involvement [ 9 , 10 ] including bronchiectasis [ 11 ]. Pathologically, diffuse LCDD is characterised by LC deposits along the basement membranes of the alveolar, bronchial, and vascular walls. The putative pathophysiology of cyst formation involves the degradation of elastic fibers by matrix metalloproteinases [ 12 ].
The clinical symptoms reported in previous studies are chest discomfort, haemoptysis, and progressive dyspnea leading to chronic respiratory failure [ 13 , 14 , 15 ]. Although no treatment is validated, chemotherapy is often prescribed to control monoclonal LC secretion in the serum [ 16 ]. Lung transplantation may be performed [ 17 ].
Data on pulmonary LCDD are scarce and limited to small series or case reports. The main objectives of this study were to: 1) describe the clinical, functional and radiological characteristics at presentation, 2) determine lung function during follow-up and estimate time to transplantation or death.
Patient selection and data collection
This retrospective multicentre study was conducted in the French OrphaLung network, a cooperative group of lung specialists. Patient data regarding clinical, laboratory, functional, radiological characteristics, and outcome were collected using a case report form. Patients were considered eligible if the diagnosis of pulmonary LCDD was confirmed by immunohistochemistry. The exclusion criteria were uncertain diagnosis, solitary pulmonary nodules, and predominance of amyloid deposits.
Ethical consideration
This study was conducted with respect to the Declaration of Helsinki. It was approved by the ethics committee of the Hospices Civils de Lyon and was registered with the national data protection agency (Commission Nationale de l’Informatique et des Libertés, number 20–075). According to the legislation in place at the time of the study, informed consent signature was waived, but each patient was informed by a written letter and could object to the use of their personal data. Several patients were reported in previous publications [ 10 , 11 , 12 , 14 , 17 , 18 ].
Pulmonary function tests
Lung volumes were measured by plethysmography, and forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) by flow–volume curve, using GLI reference equations. Carbon monoxide transfer factor (DLco) was assessed with the single-breath method. Hypoxemia was defined as a partial pressure of oxygen in arterial blood (PaO2) < 80 mmHg.
Chest computed tomography (CT)
Two expert chest radiologists (SSM, DR) blinded to the clinical data reviewed the baseline and latest available computed tomography (CT) images during follow-up and before lung transplantation. If consensus was not obtained, a third expert radiologist (CB) settled the description. A CT grading system was used to document cyst number, size of the largest cyst, shape (round, oval, irregular), internal septation, and distribution. Bronchiectases were classified by their appearance (cylindric, varicose, cystic), their proximal or distal distribution, and the presence of bronchial wall thickening. Then, based on imaging, the predominant imaging pattern was classified as bronchiectatic, cystic, or mixed. To evaluate temporal changes in imaging findings, the last available follow-up CT was compared to the baseline CT.
Pathological assessment
Histology reports of lung biopsies were collected and centrally reviewed by an expert pathologist in the field (MC) to confirm the diagnosis of LCDD, using previously described criteria [ 5 ], i.e. biopsies had to stain negative with Congo red and demonstrate LC deposits on frozen tissue under immunofluorescence microscopy. When frozen tissue was unavailable, mass spectrometry was used for diagnostic confirmation [ 19 ].
Statistical analysis
Although normal distribution could not be assumed due to the small sample size and the expected heterogeneity in disease behavior, Shapiro–Wilk test was used to check for normal and skewed distributions. Non-parametric tests were used in the absence of normality. Continuous variables are presented as means (percentages) ± standard deviation (SD) or as median (range).
The Wilcoxon signed-rank test was applied to compare pre- and post-treatment lung function decline, and the Mann–Whitney U test was used to compare measures between groups. Chi-squared test, Fisher’s exact test and Student’s t test were used where appropriate. For each patient, the estimated annual decline rate in DLCO and FEV1 expressed in %/year and in mL/year, respectively, were calculated by linear regression. The average ∆FEV1 and ΔDLCO were calculated in patients who had completed a minimum of 24 months of follow-up and a minimum of four measures. Event-free survival, defined as the time from the first consultation to transplantation or death from any cause, was estimated using the Kaplan–Meier survival method, and univariate Cox regression was performed to assess hazard ratios (HRs) with 95% confidence intervals.
Statistical significance was set at p < 0.05 (two-tailed). IBM SPSS Statistics for Windows, Version 25.0. (IBM Corp, Armonk, NY) and RStudio (v1.3.959) were used for statistical analyses.
Baseline characteristics
Out of 61 patients identified between 1998 and 2020 in 12 French centres and one Swiss centre, 31 met the eligibility criteria and were included in the analysis (Figure S 1 ). Overall, 68% of patients were women and the mean ± SD age at diagnosis was 50 ± 10.7 years. The median (range) interval between the onset of symptoms and diagnosis was 4 (1–30) years. Twenty (67%) patients were current or former smokers with a median of 23.8 (2.5–120) pack-years. All patients except two had dyspnea (Table 1 ).
At baseline, FEV1/FVC was < 0.70 in 45% of patients. FEV1 was lower than 80% of the predicted value in 43% of patients. DLCO was 52 ± 24% (Table 1 ). Hypoxemia was present in 68% of patients.
Haematological characteristics and biology
Bone marrow biopsy or aspiration performed in 21 cases showed > 10% of plasma cells in three patients. A bone marrow B-cell clone search was performed in seven cases and was found in four. Immunofixation identified a circulating monoclonal component as IgM in 52% of cases. Kappa/lambda ratio of serum LC was increased in 21 patients. Free monoclonal kappa LC was increased in the serum of 21/23 patients with a mean level of 236.2 ( ± 344.4) mg/L (Table 1 ). An auto immune workup, including antinuclear and antineutrophilic cytoplasmic antibodies, performed in 22 patients, was negative in all but one patient with anti-SSA and anti-SSB antibodies.
Histology and immunohistochemistry
The most frequently used method to obtain tissue was videothoracoscopic lung biopsy (36%) (Figure S 2 A). The pathological examination of lung biopsies demonstrated cystic destruction and bronchiolar dilatation in all cases (Figure S 2 B). In all patients, biopsy specimens showed Congo red negative eosinophilic deposits infiltrating alveolar walls, small airways, and/or vessels. Immunofluorescence assay of frozen tissues was performed in 25 cases, showing LC deposition stained using anti-kappa antibodies in 19 patients, as illustrated in Figure S 2 C. In contrast, anti-lambda antibody staining confirmed the diagnosis in only one patient. Electron microscopy, performed in five patients, revealed granular dense electron deposits in all cases. Mass spectrometry-based analysis of biopsy, performed in 19 cases, showed that the main constituent of the deposits in all cases was the presence of peptides belonging to the constant region of the immunoglobulin kappa chain. A lymphoplasmocytic infiltrate was found in 13 cases. A search for B-cell clone in the lung was performed by PCR in 9 cases and revealed a clone in all patients.
Fiberoptic bronchoscopy
Fiberoptic bronchoscopy was performed in 24 patients. The macroscopic appearance of the bronchial mucosa was inflammatory in 29% of patients, normal in 42%, showed bronchial distorsions in 12.5%, and bronchomalacia in 8%. Bronchial biopsies were performed in 11 cases, including 8 with immunohistochemical analysis (positive in 5), and 3 with proteomic analysis (positive in all 3 cases).
Baseline CT characteristics
The most frequent abnormalities were plurifocal and sometimes extensive lung cysts (100%) and cystic bronchiectasis (77%). Cysts were bilateral and of regular shape and were characterised by internal septation (73%), bronchovascular topography (77%), and thin walls (Supplement Figure S 3 ). There was a wide variation in cyst size; the largest cysts were found abutting the pleura (Table 2 ). The patient population was then split according to whether patients presented with a cystic, bronchiectasis, or mixed CT pattern. FEV1 was significantly lower, and airflow obstruction was significantly more severe in the cystic pattern compared to the bronchiectasis pattern (Table 3 ).
Extrapulmonary manifestations
In four cases, LC deposits were found in salivary glands, one case being associated with primary Sjögren syndrome. Kidney tissue was available in three cases and no deposit was found. Interventricular septum thickening on cardiac ultrasonography was found in none of the cases. No patient had congestive heart failure. Left ventricular ejection fraction was preserved in all cases, and diastolic dysfunction was absent. Low voltage was not present on electrocardiograms. Right heart catheterisation was performed in 11 patients (Supplement Table S 1 ).
Lung function and CT follow-up
Follow-up data were available for FEV1 in 26 patients and for DLCO in 17 patients. The median duration of lung function follow-up was 75 (2–180) months. The median annual overall FEV1 decline was 127 ml/year (2.2 – 877), and the median DLCO annual decline was 4.3%/year (1.9 – 8). Imaging follow-up was available for 16 patients with a median interval between the first and last chest CT of 62 (7–139) months. Cysts increased in size in 13 patients and in number in ten patients (Fig. 1 ). The number of bronchiectases increased in nine patients.
Long-term change in chest CT features in 3 patients showing 3 patterns. A and B CT scan at baseline and after 8 years in a patient with diffuse cystic bronchiectasis. C and D CT scan at baseline and after 1 year in a patient with regular round cysts of various sizes. E and F CT scan at baseline in a patient with micronodules and interlobular reticulation, and 7 years later with multiple cysts and ground glass attenuation
Nonpecific treatment left to the discretion of each investigator included inhaled glucocorticoids ( n = 16), long-acting beta-agonists ( n = 15), long-acting muscarinic antagonist ( n = 6), long-term macrolide treatment ( n = 9). In 16 cases, a treatment was prescribed to attempt treating the disease by targeting the underlying production of LC. In all cases except 4, the first line of chemotherapy was different for each patient, varying according to centres (Supplementary figure S 4 ).
Among the 16 patients treated, ten had evaluable measurements of serum-free LC before and after systemic treatment. A normalisation or reduction > 90% of serum-free LC kappa was obtained only in three patients. Only eight patients had evaluable annual FEV1 before and after systemic treatment. The annual decline in FEV1 did not differ significantly between the pre-and post-treatment periods ( p = 0.73; Supplementary Table S 2 ).
Long-term supplemental nasal oxygen was used in 19 patients (66%). Fifteen patients underwent double lung transplantation, with a median interval between diagnosis and lung transplantation of 5 years (1–11). In 4/15 cases, the diagnosis of LCDD was made before the transplantation. No patient requiring a lung transplant was excluded from receiving it due to contraindications and no patient died on the lung transplant waiting list.
Survival and prognostic factors
When comparing patients deceased or transplanted with those alive or not transplanted, there were significant differences in age at onset of symptoms, baseline FEV1% predicted, FEV1/FVC, and ∆FEV1 (Table 4 ).
Three patients died at the age of 45, 55, and 68 years, all of them due to progressive respiratory failure. The median transplant-free survival was 9 years (Fig. 2 ). There was no significant difference in transplant-free survival between men and women, between smokers and non-smokers, between treated and non-treated, or between cystic and bronchiectatic patterns. A rapid decline in FEV1, defined as ∆FEV1 > 127 ml/year (median value), was associated with an increased risk of death or transplantation ( p = 0.005) (Fig. 2 ).
Kaplan–Meier estimates of transplant-free survival. A transplant-free survival in all patients. B Yellow and blue curves correspond to Kaplan Meier estimates of the transplant-free survival for patients with FEV1 decline greater or less than 127 ml/year ( P value for log rank test). C Yellow and blue curves correspond to Kaplan Meier estimates of the transplant-free survival for patients with “bronchiectasis pattern” and “cystic pattern”, respectively ( P value for log rank test)
The present cohort of diffuse pulmonary LCDD, which is the largest to date, shows a female predominance, an increase in serum free monoclonal kappa LC at diagnosis, and a predominance of thin-walled lung cysts and bronchectases on CT. Moreover, pulmonary LCDD appears as a progressive airflow obstruction associated with a poor prognosis and limited response to chemotherapy. Double lung transplantation should be considered as a curative option in this population, and patients should be referred early for transplantation.
Diffuse pulmonary LCDD localized to the lungs is less well described than other cystic lung diseases such as lymphangioleiomyomatosis or pulmonary Langerhans cell histiocytosis. In the patients’ files, the disease was often reported as “unclassifiable diffuse cystic lung disease”, “idiopathic diffuse bronchiectasis”, or “atypical emphysema” before LCDD was considered. The diagnosis is probably often missed since IF is rarely applied to lung biopsies by pulmonary pathologists and frozen material is rarely available, although it is considered the diagnostic method of choice. In these situations, mass spectrometry may confirm the diagnosis, but is not widely available. Paraffin IF was not performed in the present cohort but a prior study suggested that this technique is feasible and could rescue some difficult diagnoses [ 20 ]. The pathological diagnosis was based on the findings of videothoracoscopic lung biopsy, bronchial biopsy, and/or lung explant. Interestingly, bronchial biopsy tissue analysis was sufficient to confirm the diagnosis in a large proportion of the patients in whom the diagnosis was suspected, and in the cases where IF could be performed on frozen bronchial biopsies. Hence, physicians should be alerted to use adequate tissue fixation methods (i.e. frozen tissue) when a diagnosis of LCDD is considered.
Previous studies clearly demonstrated that diffuse pulmonary LCDD differs clinically from the systemic multivisceral form and from the pulmonary nodular form of the disease [ 21 ]. Unlike patients in two recent reports [ 22 , 23 ], the patients herein rarely had underlying autoimmune conditions, in particular primary Sjögren syndrome. Nodular pulmonary LCDD may be associated with Sjögren syndrome more often than the cystic presentation [ 24 ].
The present results indicate that young females more frequently present with respiratory manifestations of LCDD. The most common lung function abnormality at baseline was decreased DLCO, probably related to the damage induced by LC deposits along the alveolar-capillary membrane, as observed in lung amyloidosis [ 25 ]. Airflow obstruction defined by FEV1/FVC < 70% was also common.
The CT findings of the present study were relatively heterogeneous. In contrast to the findings of Sheard et al. [ 26 ], pulmonary involvement primarily consisted of varicose bronchiectasis without nodular infiltrates, except in one case. The coexistence (and possible communication [ 12 ]) of thin-walled bronchiectasis and cystic changes is somewhat characteristic of diffuse pulmonary LCDD when compared to other cystic lung diseases, in particular lymphangioleiomyomatosis and Birt-Hogg-Dubé syndrome. Cysts in pulmonary LCDD are regular in shape with a predominance of lower distribution [ 27 ]. During follow-up, the size and the number of bronchiectases and pulmonary cysts increased remarkably. However, in some cases, distinguishing these two patterns was not possible, with extensive emphysema-like changes in severe forms, and it is conceivable that both bronchiectases and cyst result from a common mechanisme of elastolysis. Noteworthy, the majority of the patients herein were current or ex-smokers, and it cannot be excluded that tobacco smoking may have played a role in pulmonary lesion formation.
Monoclonal gammopathy is the presence in the serum of a monoclonal immunoglobulin produced by a B-cell clone. This biological anomaly fits with the concept of monoclonal gammopathy of clinical significance, in which the clone induces severe organ damage [ 28 ]. Herein, the free LC ratio was abnormal in the vast majority of patients, making it a plausible screening tool. Nevertheless, the serum level of kappa LC at baseline was not associated with a greater probability of lung transplant or death. As shown by the plasmacytosis greater than 10% found in certain patients who underwent bone marrow biopsy or aspiration, although the association of LCDD with haematological malignancy is possible [ 13 , 29 ], the absence of bone marrow abnormality upon immunohistology should not exclude pulmonary LCDD. In such cases, LC may be produced within the lungs [ 17 ]. No evidence of extrapulmonary LC deposition was observed in the present cohort, except in accessory salivary glands. As a result, pulmonary LCDD may be considered in the appropriate context, even in the absence of kidney dysfunction or proteinuria.
During follow-up, inter-patient variability in FEV1 decline was observed, however, the decline in lung function was generally faster than that seen in chronic obstructive pulmonary disease [ 30 ] or in other multiple cystic lung diseases [ 31 , 32 , 33 ], and diffusion capacity was severely altered. Patients who required a lung transplantation had a higher rate of FEV1 decline than non-transplanted patients. Consequently, lung function surveillance at 6–12 months intervals is of paramount importance to assess disease progression and identify possible transplantation candidates, especially when ΔFEV1 > 127 ml/year.
There is no validated therapeutic strategy for LCDD localized to the lung. The main treatment approach is to target the synthesis of monoclonal proteins, using the same treatments as those employed in multiple myeloma. In the present cohort, various medications were used (rituximab, alkylating agents, proteasome inhibitor, dexamethasone, antimetabolite) targeting the underlying production of light chains, as well as autologous blood stem cell transplantation. Using an exploratory analysis, no change in the annual rate of FEV1 decline before and after treatment was observed, whereas improved renal function was reported in patients treated with melphalan and prednisone [ 34 ] or autologous stem cell transplantation for renal LCDD [ 35 ]. The fact that tissue damage induced by LC deposits is irreversible may explain the absence of clinical improvement despite serum LC titers that tended to decrease upon treatment. It remains to be determined whether treatments allowing LC serum levels to return to normal may slow disease progression. Lung volume reduction surgery might be considered as an alternative to chemotherapy in selected cases [ 18 ].
Lung transplantation is currently the main treatment option in patients with respiratory failure resulting from LCDD, with no reported recurrence in the transplanted lungs [ 17 ]. This is in line with the relatively long median lung transplant-free survival found herein. However, the present analysis showed that there was a significant difference in lung-transplant free survival according to FEV1 decline, underlying the possible need for some patients to undergo transplantation as early as possible. In contrast, kidney transplantation is not considered an option in end-stage renal disease, as further kidney damage cannot be prevented in the transplanted organ [ 36 ]. In case of cardiac LCDD, LC deposits were identified as early as 3 months after heart transplantation [ 37 ].
This study has several limitations, including the relatively small sample size and the retrospective design. Second, its multicentric nature represents a source of variability in pulmonary function test measurements. Third, the lack of standardised therapy may have contributed to the lack of significant treatment benefit observed on lung function decline.
In conclusion, in this cohort of patients with diffuse pulmonary LCDD, women were more commonly affected than men. Low DLCO was the most commonly observed lung function abnormality. Rapid annual FEV1 decline was associated with a greater risk of death or transplantation. Systemic haematologic treatment did not reduce the annual lung function decline. Prospective larger studies are eagerly awaited.
Availability of data and materials
Data are available upon request to the corresponding author.
Abbreviations
Computed tomography
Diffusing capacity for carbon monoxide
Transfer coefficient for the lung for carbon monoxide
Forced vital capacity
Forced expiratory volume in one second
Immunofluorescence
Light chain
- Light chain deposition disease
Arterial oxygen partial pressure
Total lung capacity
Buxbaum JN, Chuba JV, Hellman GC, Solomon A, Gallo GR. Monoclonal immunoglobulin deposition disease: light chain and light and heavy chain deposition diseases and their relation to light chain amyloidosis. Clinical features, immunopathology and molecular analysis. Ann Intern Med. 1990;112:455–64.
Article CAS PubMed Google Scholar
Randall RE, Williamson WC, Mullinax F, Tung MY, Still WJ. Manifestations of systemic light chain deposition. Am J Med. 1976;60:293–9.
Buxbaum J, Gallo G. Nonamyloidotic monoclonal immunoglobulin deposition disease. Light-chain, heavy-chain, and light-and heavy-chain deposition diseases. Hematol Oncol Clin North Am. 1999;13:1235–48.
Lin J, Markowitz GS, Valeri AM, Kambham N, Sherman WH, Appel GB, D’Agati VD. Renal monoclonal immunoglobulin deposition disease: the disease spectrum. J Am Soc Nephrol. 2001;12:1482–92.
Article PubMed Google Scholar
Colombat M, Holifanjaniaina S, Guillonneau F, Mal H, Hirschi S, Reynaud-Gaubert M, Stern M. Mass spectrometry-based proteomic analysis: a good diagnostic tool for cystic lung light chain deposition disease. Am J Respir Crit Care Med. 2013;188:404–5.
Kijner CH, Yousem SA. Systemic light chain deposition disease presenting as multiple pulmonary nodules. A case report and review of the literature. Am J Surg Pathol. 1988;12:405–13.
Khoor A, Myers JL, Tazelaar HD, Kurtin PJ. Amyloid-like pulmonary nodules, including localized light-chain deposition: clinicopathologic analysis of three cases. Am J Clin Pathol. 2004;121:200–4.
Mezzanotte JN, Gibbons-Fideler IS, Shilo K, Lustberg M, Devarakonda S. Nodular pulmonary deposition disease in a patient with the acquired immunodeficiency syndrome: a case report. J Med Case Rep. 2020;14:64.
Article PubMed PubMed Central Google Scholar
Miró O, Fernández-Solá J, Gómez-Angelats E, Andreu MV, Solé M. [Tracheobronchomegaly associated with light chain deposition disease]. Arch Bronconeumol. 1994;30:508–10.
Colombat M, Gounant V, Mal H, Callard P, Milleron B. Light chain deposition disease involving the airways: diagnosis by fibreoptic bonchoscopy. Eur Respir J. 2007;29:1057–60.
Girard N, Vasiljevic A, Cottin V, Falchero L, Meyronet D, Thivolet-Bejui F, Cordier JF. Respiratory failure with diffuse bronchiectases and cryoglobulinaemia. Eur Respir J. 2008;31:1374–8.
Colombat M, Caudroy S, Lagonotte E, Mal H, Danel C, Stern M, Fournier M, Birembaut P. Pathomechanisms of cyst formation in pulmonary light chain deposition disease. Eur Respir J. 2008;32:1399–403.
Kato T, Muto H, Hishima T, Kawashima M, Nagai H, Matsui H, Shimada M, Hebisawa A, Doki N, Miyawaki S, Ohashi K. A 56-Year-old woman with multiple pulmonary cysts and severe chest Pain. Chest. 2018;153:e105–12.
Colombat M, Stern M, Groussard O, Droz D, Brauner M, Valeyre D, Mal H, Taille C, Monnet I, Fournier M, Herson S, Danel C. Pulmonary cystic disorder related to light chain deposition disease. Am J Respir Crit Care Med. 2006;173:777–80.
Gorospe Sarasúa L, Pacios-Blanco RE, Arrieta P, Chinea-Rodríguez A. Intracystic hemorrhage in a patient with pulmonary cystic disorder related to light-chain deposition disease. Arch Bronconeumol. 2017;53:285–7.
Borgne AL, Prévot G, Rouquette I, Huynh A, Têtu L, Projetti F, Carreiro M, Borie R, Jaccard A, Recher C, Didier A. Blood stem cell transplantation to treat cystic lung light chain deposition disease. Eur Respir J. 2015;46:1199–202.
Hirschi S, Colombat M, Kessler R, Reynaud-Gaubert M, Stern M, Chenard MP, Métivier AC, Jeung MY, Mal H. Lung transplantation for advanced cystic lung disease due to nonamyloid kappa light chain deposits. Ann Am Thorac Soc. 2014;11:1025–31.
Delaey P, Plawny L, Nchimi A, Hirschi S, Weingertner N, Santelmo N, Wirtz G. [Effect of surgery of pulmonary cysts related to immunoglobulin light chain deposits]. Rev Mal Respir. 2020;37:180–6.
Camus M, Hirschi S, Prevot G, Chenard MP, Mal H, Stern M, Reynaud-Gaubert M, Gilhodes J, Burlet-Schiltz O, Brousset P, Colombat M. Proteomic evidence of specific IGKV1-8 association with cystic lung light chain deposition disease. Blood. 2019;133:2741–4.
Gibier JB, Perbet R, Lopez B, Colombat M, Dubois R, Humez S, Terriou L, Copin MC, Gnemmi V. Paraffin immunofluorescence increases light-chain detection in Extra-renal Light Chain Amyloidosis and other Light-Chain-Associated diseases. Arch Pathol Lab Med. 2021;145:352–8.
Colombat M, Mal H, Copie-Bergman C, Diebold J, Damotte D, Callard P, Fournier M, Farcet JP, Stern M, fau-Larue MH. Primary cystic lung light chain deposition disease: a clinicopathologic entity derived from unmutated B cells with a stereotyped IGHV4-34/IGKV1 receptor. Blood. 2008;112:2004–12.
Baqir M, Moua T, White D, Yi ES, Ryu JH. Pulmonary nodular and cystic light chain deposition disease: a retrospective review of 10 cases. Respir Med. 2020;164:105896.
Wei P, Tao R, Liu Y, Xie H, Jiang S, Yu D, Lu H, Cao W. Pulmonary light chain deposition disease: a case series and literature review. Annals Transl Med. 2020;8:588.
Article CAS Google Scholar
Arrossi AV, Merzianu M, Farver C, Yuan C, Wang SH, Nakashima MO, Cotta CV. Nodular pulmonary light chain deposition disease: an entity associated with Sjögren syndrome or marginal zone lymphoma. J Clin Pathol. 2016;69:490–6.
Milani P, Basset M, Russo F, Foli A, Palladini G, Merlini G. The lung in amyloidosis. Eur Respir Rev. 2017;26:170046.
Sheard S, Nicholson AG, Edmunds L, Wotherspoon AC, Hansell DM. Pulmonary light-chain deposition disease: CT and pathology findings in nine patients. Clin Radiol. 2015;70:515–22.
Park HJ, Chae EJ, Do KH, Lee SM, Song JW. Differentiation between Lymphangioleiomyomatosis and Birt-Hogg-Dubé Syndrome: analysis of pulmonary cysts on CT images. AJR Am J Roentgenol. 2019;212:766–72.
Fermand JP, Bridoux F, Dispenzieri A, Jaccard A, Kyle RA, Leung N, Merlini G. Monoclonal gammopathy of clinical significance: a novel concept with therapeutic implications. Blood. 2018;132:1478–85.
Clayden RC, Macdonald D, Oikonomou A, Cheung MC. Cystic lung disease with kappa light chain deposition in newly diagnosed multiple myeloma. Br J Haematol. 2020;188:201.
Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, Calverley PM, Celli B, Coxson HO, Crim C, Lomas DA, MacNee W, Miller BE, Silverman EK, Tal-Singer R, Wouters E, Rennard SI. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365:1184–92.
Daccord C, Cottin V, Prévot G, Uzunhan Y, Mornex JF, Bonniaud P, Borie R, Briault A, Collonge-Rame MA, Crestani B, Devouassoux G, Freynet O, Gondouin A, Hauss PA, Khouatra C, Leroy S, Marchand-Adam S, Marquette C, Montani D, Naccache JM, Nadeau G, Poulalhon N, Reynaud-Gaubert M, Salaun M, Wallaert B, Cordier JF, Faouzi M, Lazor R. Lung function in Birt-Hogg-Dubé syndrome: a retrospective analysis of 96 patients. Orphanet J Rare Dis. 2020;15:120.
Article CAS PubMed PubMed Central Google Scholar
Taveira-DaSilva AM, Stylianou MP, Hedin CJ, Hathaway O, Moss J. Decline in lung function in patients with lymphangioleiomyomatosis treated with or without progesterone. Chest. 2004;126:1867–74.
Tazi A, de Margerie C, Naccache JM, Fry S, Dominique S, Jouneau S, Lorillon G, Bugnet E, Chiron R, Wallaert B, Valeyre D, Chevret S. The natural history of adult pulmonary Langerhans cell histiocytosis: a prospective multicentre study. Orphanet J Rare Dis. 2015;10:30.
Heilman RL, Velosa JA, Holley KE, Offord KP, Kyle RA. Long-term follow-up and response to chemotherapy in patients with light-chain deposition disease. Am J Kidney Dis. 1992;20:34–41.
Royer B, Arnulf B, Martinez F, Roy L, Flageul B, Etienne I, Ronco P, Brouet JC, Fermand JP. High dose chemotherapy in light chain or light and heavy chain deposition disease. Kidney Int. 2004;65:642–8.
Leung N, Lager DJ, Gertz MA, Wilson K, Kanakiriya S, Fervenza FC. Long-term outcome of renal transplantation in light-chain deposition disease. Am J Kidney Dis. 2004;43:147–53.
Aimo A, Vergaro G, Pucci A, Bernazzali S, Maccherini M, Buda G, Passino C, Merlini G, Emdin M. Cardiac light-chain deposition disease relapsing in the transplanted heart. Amyloid. 2017;24:135–7. OrphaLung network (collaborators) 19 Yurdagül UZUNHAN, Avicenne hospital, APHP, Paris Nord university, Paris, France 20 Stéphane JOUNEAU, Pontchaillou Hospital, Rennes, France.
Download references
Acknowledgements
We thank the patients for agreeing to participate in this study. The authors would like to thank R. LAVRUT (Lyon, France), M. RABANT (Paris, France) and R. DUBOIS (Lille, France) for histopathological contribution and pathology slides; V. LANDEL for manuscript preparation;
OrphaLung network (collaborators)
19 Yurdagül UZUNHAN, Avicenne hospital, APHP, Paris Nord university, Paris, France.
20 Stéphane JOUNEAU, Pontchaillou Hospital, Rennes, France.
Author information
Authors and affiliations.
Hospices Civils de Lyon, Centre de Référence Coordinateur Des Maladies Pulmonaires Rares (OrphaLung), Hôpital Louis Pradel, Service de Pneumologie, 69677, Lyon, France
François Lestelle, Mouhamad Nasser & Vincent Cottin
Service de Radiologie Et de Radiologie Interventionnelle, Hôpital Universitaire de Lausanne, Université de Lausanne, Lausanne, Suisse
Catherine Beigelman & David Rotzinger
Hospices Civils de Lyon, Hôpital Louis Pradel, Service de Radiologie, Lyon 69677U1206, Université de Lyon, INSA-Lyon, Université Claude Bernard Lyon 1, UJM-Saint Etienne, CNRS, Inserm, CREATIS, UMR 5220, F‐69621, 7 Avenue Jean Capelle O, 69100, Villeurbanne, France
Salim Si-Mohamed
Centre de Référence Constitutif Des Maladies Pulmonaires Rares (OrphaLung), CHU Lille, Service de Pneumologie, Lille, France
Lidwine Wemeau
Centre de Compétence Des Maladies Pulmonaires Rares (OrphaLung), CHU Strasbourg, Service de Pneumologie, Strasbourg, France
Sandrine Hirschi
Centre de Compétence Des Maladies Pulmonaires Rares (OrphaLung), CHU Toulouse, Hôpital LarreyUniversité Paul Sabatier, Toulouse, France
Grégoire Prevot
Service de Pneumologie Et de Transplantation Pulmonaire, Hopital Foch, Suresnes, France
Antoine Roux
Service de Pneumologie B Et de Transplantation Pulmonaire, AP-HP, Hôpital Bichat Claude-Bernard, Inserm U1152, Paris, France
Vincent Bunel
Centre de Compétence Des Maladies Pulmonaires Rares (OrphaLung), CHU Nancy, Service de Pneumologie, Nancy, France
Emmanuel Gomez
Centre Hospitalier Bretagne Sud, Service de Pneumologie, Lorient, France
Laurent Sohier
Centre de Compétence Des Maladies Pulmonaires Rares (OrphaLung), CHU Rouen, Service de Pneumologie, Rouen, France
Helene Morisse Pradier
Service de Pneumologie Et Transplantation Pulmonaire, CHU Marseille Nord, Aix-Marseille Université Marseille, Assistance Publique-Hôpitaux de Marseille, Marseille, France
Martine Reynaud Gaubert
Centre de Compétence Des Maladies Pulmonaires Rares (OrphaLung), CHU Besançon, Service de Pneumologie, Besançon, France
Anne Gondouin
Service de Pneumologie, Centre Hospitalier Universitaire Vaudois, Lausanne, CH, Suisse
Romain Lazor
Hospices Civils de Lyon, Hôpital Louis Pradel, Service d’explorations Fonctionnelles Respiratoires, 69677, Lyon, France
Jean-Charles Glerant
Hospices Civils de Lyon, Hôpital Louis Pradel, Service d’Anatomopathologie, 69677, Lyon, France
Françoise Thivolet Bejui
CHU Toulouse, Institut Universitaire du Cancer de Toulouse, Service d’anatomie Et Cytologie Pathologiques, Toulouse, France
Magali Colombat
UMR754, INRAE; Member of RespiFil and ERN-LUNG, Université, Claude Bernard Lyon 1, Lyon, France
Vincent Cottin
You can also search for this author in PubMed Google Scholar
- Yurdagül Uzunhan
- & Stéphane Jouneau
Contributions
François Lestelle: conception of the work; acquisition, analysis, and interpretation of data for the work; AND Drafting the work; AND Final approval of the version to be published; AND Agreement to be accountable for all aspects of the work. Catherine Beigelman: data acquisition and interpretation, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. David Rotzinger: data acquisition and interpretation, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Salim Si-Mohamed: data acquisition and interpretation, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Mouhamad Nasser: data acquisition, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Lidwine Wemeau: data acquisition, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Sandrine Hirschi: data acquisition, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Grégoire Prevot: data acquisition, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Antoine Roux: data acquisition, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Vincent Bunel: data acquisition, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Emmanuel Gomez: data acquisition, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Laurent Sohier: data acquisition, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Helene Morisse Pradier: data acquisition, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Martine Reynaud Gaubert: data acquisition, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Anne Gondouin: data acquisition and interpretation, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Romain Lazor: data acquisition, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Jean-Charles Glerant: data interpretation, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Françoise Thivolet Bejui: data acquisition, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Magali Colombat: data acquisition, AND revising of manuscript, AND approval of final version, AND agreement to be accountable of the work. Vincent Cottin (guarantor): conception and design of the work; analysis, and interpretation of data for the work; AND Drafting and revising the work; AND Final approval of the version to be published; AND Agreement to be accountable for all aspects of the work.
Corresponding author
Correspondence to Vincent Cottin .
Ethics declarations
Ethics approval and consent to participate.
This study was conducted with respect to the Declaration of Helsinki. It was approved by the ethics committee of the Hospices Civils de Lyon and was registered with the national data protection agency (Commission Nationale de l’Informatique et des Libertés, number 20–075). According to the legislation in place at the time of the study, informed consent signature was waived, but each patient was informed by a written letter and could object to the use of their personal data.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Lestelle, F., Beigelman, C., Rotzinger, D. et al. Phenotypes and outcome of diffuse pulmonary non-amyloid light chain deposition disease. Respir Res 25 , 159 (2024). https://doi.org/10.1186/s12931-024-02798-y
Download citation
Received : 18 October 2023
Accepted : 01 April 2024
Published : 10 April 2024
DOI : https://doi.org/10.1186/s12931-024-02798-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Bronchiectasis
- Lung transplantation
Respiratory Research
ISSN: 1465-993X
- General enquiries: [email protected]
Jump to navigation
- Bahasa Malaysia

Are midwife continuity of care models versus other models of care for childbearing women better for women and their babies?
Key messages
Women or their babies who received midwife continuity of care models were less likely to experience a caesarean section or instrumental birth with forceps or a ventouse suction cup, and may be less likely to experience an episiotomy (a cut made by a healthcare professional into the perineum and vaginal wall). They were more likely to experience spontaneous vaginal birth.
Women who experienced midwife continuity of care models reported more positive experiences during pregnancy, labour, and postpartum. Additionally, there were cost savings in the antenatal (care during pregnancy) and intrapartum (care during labour and birth) period.
Further evidence may change our results, and future research should focus on the impact on women with social risk factors, and those with medical complications, and understanding the implementation and scaling up of midwife continuity of care models, with emphasis on low- and middle-income countries.
What are midwife continuity of care models?
Midwife continuity of care models provide care from the same midwife or team of midwives during pregnancy, birth, and the early parenting period in collaboration with obstetric and specialist teams when required.
What did we want to find out?
We wanted to find out how outcomes differed for women or their babies who received a midwife continuity of care model compared to other models of care.
Our main outcomes were: spontaneous vaginal birth, caesarean section, regional anaesthesia (spinal or epidural block to numb the lower part of the body), intact perineum (the area between the anus and the vulva), fetal loss after 24 weeks gestation, preterm birth, and neonatal death.
We also looked at a range of other outcomes, including women’s experience and cost.
What did we do?
We searched for studies that compared midwife continuity of care models with other models of care for pregnant women. We compared and summarised the results of the studies and rated our confidence in the evidence based on factors such as study methods and size.
What did we find?
We found 17 studies involving 18,533 women in Australia, Canada, China, Ireland, and the United Kingdom.
Many of these studies largely focused on women with a lower risk of complications at the start of pregnancy, or those drawn from a specific geographical location. Midwives continued to provide midwifery care in collaboration with specialist and obstetric teams if women developed complications in pregnancy, birth, and postpartum.
Our main results
Women or their babies who received midwife continuity of care models compared to those receiving other models of care were less likely to experience a caesarean section or instrumental vaginal delivery, and may be less likely to experience an episiotomy. They were more likely to experience a spontaneous vaginal birth.
Midwife continuity care models probably make little or no difference to the likelihood of having an intact perineum, and may have little or no impact on the likelihood of preterm birth.
We are uncertain about the effect of midwife continuity of care models on regional anaesthesia, fetal loss after 24 weeks' gestation, and neonatal death.
Women who experienced care from midwife continuity of care models reported more positive experiences during pregnancy, labour, and postpartum. Additionally, there were cost savings in the antenatal and intrapartum period.
What are the limitations of the evidence?
Our confidence in these findings varies and further evidence may change our results. For instance, it is not always clear if the people assessing the outcomes knew which type of care the women received.The evidence for fetal loss after 24 weeks' gestation and neonatal death is based on a very small number of cases and there are not enough studies to be certain about some results. We lack data on important aspects like maternal health status after birth, neonatal readmissions, or infant health status.
Few studies included a specific focus on women at high risk of complications, and none focused on women from disadvantaged backgrounds, indicating a need for future research in these areas. This highlights the need for more comprehensive and diverse studies to strengthen our understanding and confidence in these findings, particularly in varied populations and across different healthcare settings.
Future research should focus on the impact on women with social risk factors, and those with medical complications, and understanding the implementation and scaling up of midwife continuity of care models, with emphasis on low- and middle-income countries.
How up-to-date is this evidence?
This is an update of our previous review. We included evidence up to 17 August 2022.
Women receiving midwife continuity of care models were less likely to experience a caesarean section and instrumental birth, and may be less likely to experience episiotomy. They were more likely to experience spontaneous vaginal birth and report a positive experience. The certainty of some findings varies due to possible risks of bias, inconsistencies, and imprecision of some estimates.
Future research should focus on the impact on women with social risk factors, and those at higher risk of complications, and implementation and scaling up of midwife continuity of care models, with emphasis on low- and middle-income countries.
Midwives are primary providers of care for childbearing women globally and there is a need to establish whether there are differences in effectiveness between midwife continuity of care models and other models of care. This is an update of a review published in 2016.
To compare the effects of midwife continuity of care models with other models of care for childbearing women and their infants.
We searched the Cochrane Pregnancy and Childbirth Trials Register, ClinicalTrials.gov, and the WHO International Clinical Trials Registry Platform (ICTRP) (17 August 2022), as well as the reference lists of retrieved studies.
All published and unpublished trials in which pregnant women are randomly allocated to midwife continuity of care models or other models of care during pregnancy and birth.
Two authors independently assessed studies for inclusion criteria, scientific integrity, and risk of bias, and carried out data extraction and entry. Primary outcomes were spontaneous vaginal birth, caesarean section, regional anaesthesia, intact perineum, fetal loss after 24 weeks gestation, preterm birth, and neonatal death. We used GRADE to rate the certainty of evidence.
We included 17 studies involving 18,533 randomised women. We assessed all studies as being at low risk of scientific integrity/trustworthiness concerns. Studies were conducted in Australia, Canada, China, Ireland, and the United Kingdom. The majority of the included studies did not include women at high risk of complications. There are three ongoing studies targeting disadvantaged women.
Primary outcomes
Based on control group risks observed in the studies, midwife continuity of care models, as compared to other models of care, likely increase spontaneous vaginal birth from 66% to 70% (risk ratio (RR) 1.05, 95% confidence interval (CI) 1.03 to 1.07; 15 studies, 17,864 participants; moderate-certainty evidence), likely reduce caesarean sections from 16% to 15% (RR 0.91, 95% CI 0.84 to 0.99; 16 studies, 18,037 participants; moderate-certainty evidence), and likely result in little to no difference in intact perineum (29% in other care models and 31% in midwife continuity of care models, average RR 1.05, 95% CI 0.98 to 1.12; 12 studies, 14,268 participants; moderate-certainty evidence). There may be little or no difference in preterm birth (< 37 weeks) (6% under both care models, average RR 0.95, 95% CI 0.78 to 1.16; 10 studies, 13,850 participants; low-certainty evidence).
We are very uncertain about the effect of midwife continuity of care models on regional analgesia (average RR 0.85, 95% CI 0.79 to 0.92; 15 studies, 17,754 participants, very low-certainty evidence), fetal loss at or after 24 weeks gestation (average RR 1.24, 95% CI 0.73 to 2.13; 12 studies, 16,122 participants; very low-certainty evidence), and neonatal death (average RR 0.85, 95% CI 0.43 to 1.71; 10 studies, 14,718 participants; very low-certainty evidence).
Secondary outcomes
When compared to other models of care, midwife continuity of care models likely reduce instrumental vaginal birth (forceps/vacuum) from 14% to 13% (average RR 0.89, 95% CI 0.83 to 0.96; 14 studies, 17,769 participants; moderate-certainty evidence), and may reduce episiotomy 23% to 19% (average RR 0.83, 95% CI 0.77 to 0.91; 15 studies, 17,839 participants; low-certainty evidence).
When compared to other models of care, midwife continuity of care models likely result in little to no difference in postpartum haemorrhage (average RR 0.92, 95% CI 0.82 to 1.03; 11 studies, 14,407 participants; moderate-certainty evidence) and admission to special care nursery/neonatal intensive care unit (average RR 0.89, 95% CI 0.77 to 1.03; 13 studies, 16,260 participants; moderate-certainty evidence). There may be little or no difference in induction of labour (average RR 0.92, 95% CI 0.85 to 1.00; 14 studies, 17,666 participants; low-certainty evidence), breastfeeding initiation (average RR 1.06, 95% CI 1.00 to 1.12; 8 studies, 8575 participants; low-certainty evidence), and birth weight less than 2500 g (average RR 0.92, 95% CI 0.79 to 1.08; 9 studies, 12,420 participants; low-certainty evidence).
We are very uncertain about the effect of midwife continuity of care models compared to other models of care on third or fourth-degree tear (average RR 1.10, 95% CI 0.81 to 1.49; 7 studies, 9437 participants; very low-certainty evidence), maternal readmission within 28 days (average RR 1.52, 95% CI 0.78 to 2.96; 1 study, 1195 participants; very low-certainty evidence), attendance at birth by a known midwife (average RR 9.13, 95% CI 5.87 to 14.21; 11 studies, 9273 participants; very low-certainty evidence), Apgar score less than or equal to seven at five minutes (average RR 0.95, 95% CI 0.72 to 1.24; 13 studies, 12,806 participants; very low-certainty evidence) and fetal loss before 24 weeks gestation (average RR 0.82, 95% CI 0.67 to 1.01; 12 studies, 15,913 participants; very low-certainty evidence). No maternal deaths were reported across three studies.
Although the observed risk of adverse events was similar between midwifery continuity of care models and other models, our confidence in the findings was limited. Our confidence in the findings was lowered by possible risks of bias, inconsistency, and imprecision of some estimates.
There were no available data for the outcomes: maternal health status, neonatal readmission within 28 days, infant health status, and birth weight of 4000 g or more.
Maternal experiences and cost implications are described narratively. Women receiving care from midwife continuity of care models, as opposed to other care models, generally reported more positive experiences during pregnancy, labour, and postpartum. Cost savings were noted in the antenatal and intrapartum periods in midwife continuity of care models.

IMAGES
VIDEO
COMMENTS
A case study is a research approach that is used to generate an in-depth, multi-faceted understanding of a complex issue in its real-life context. It is an established research design that is used extensively in a wide variety of disciplines, particularly in the social sciences. A case study can be defined in a variety of ways (Table 5 ), the ...
Origins of case study research. Case study is a research design that involves an intensive and holistic examination of a contemporary phenomenon in a real-life setting. 1-3 It uses a variety of methods and multiple data sources to explore, describe or explain a single case bounded in time and place (ie, an event, individual, group, organisation or programme).
Editorial. Introduction. Case reports and case series or case study research are descriptive studies to present patients in their natural clinical setting. Case reports, which generally consist of three or fewer patients, are prepared to illustrate features in the practice of medicine and potentially create new research questions that may contribute to the acquisition of additional knowledge ...
Case study research, as an overall approach, is based on in-depth explorations of complex phenomena in their natural, or real-life, settings. ... To this end, supported by the UK's Medical Research Council, we are embracing the challenge to develop guidance for case study researchers studying complex interventions. Following a meta-narrative ...
The popularity of case study research methodology in Health Services Research (HSR) has grown over the past 40 years. 1 This may be attributed to a shift towards the use of implementation research and a newfound appreciation of contextual factors affecting the uptake of evidence-based interventions within diverse settings. 2 Incorporating context-specific information on the delivery and ...
The case study approach allows in-depth, multi-faceted explorations of complex issues in their real-life settings. The value of the case study approach is well recognised in the fields of business, law and policy, but somewhat less so in health services research. Based on our experiences of conducting several health-related case studies, we ...
Case study is a research methodology, typically seen in social and life sciences. There is no one definition of case study research.1 However, very simply… 'a case study can be defined as an intensive study about a person, a group of people or a unit, which is aimed to generalize over several units'.1 A case study has also been described as an intensive, systematic investigation of a ...
Case study methodology is widely used in health research, but has had a marginal role in evaluative studies, given it is often assumed that case studies offer little for making causal inferences. We undertook a narrative review of examples of case study research from public health and health services evaluations, with a focus on interventions addressing health inequalities. We identified five ...
Revised on November 20, 2023. A case study is a detailed study of a specific subject, such as a person, group, place, event, organization, or phenomenon. Case studies are commonly used in social, educational, clinical, and business research. A case study research design usually involves qualitative methods, but quantitative methods are ...
The aim of this article is to introduce family medicine researchers to case study research, a rigorous research methodology commonly used in the social and health sciences and only distantly related to clinical case reports. The article begins with an overview of case study in the social and health sciences, including its definition, potential applications, historical background and core ...
Defnition: A case study is a research method that involves an in-depth examination and analysis of a particular phenomenon or case, such as an individual, organization, community, event, or situation. It is a qualitative research approach that aims to provide a detailed and comprehensive understanding of the case being studied.
A case study is one of the most commonly used methodologies of social research. This article attempts to look into the various dimensions of a case study research strategy, the different epistemological strands which determine the particular case study type and approach adopted in the field, discusses the factors which can enhance the effectiveness of a case study research, and the debate ...
A case-control study is a type of medical research investigation often used to help determine the cause of a disease, particularly when investigating a disease outbreak or rare condition.
This position leads to a focus on case study research (CSR), inquiry bound in time and place that generates thick descriptions and close interpretations to reach explanations. CSR has grown in sophistication in recent years and can inform practice and advance the science of medical and health professions education.
A case study is a research approach that is used to generate an in-depth, multi-faceted understanding of a complex issue in its real-life context. It is an established research design that is used extensively in a wide variety of disciplines, particularly in the social sciences. A case study can be defined in a variety of ways (Table.
It is best to simply tell the story and let the outcome speak for itself. With these points in mind, let's begin the process of writing the case study: Title page: Title: The title page will contain the full title of the article. Remember that many people may find our article by searching on the internet.
The study involves the recruitment of patients with disease X who are receiving one of three standard therapies as part of their clinical care. It is designed to assess the relative effectiveness of the three therapies by monitoring survival rates using medical records over a few years. Case #13b.
However, research showing how case management affects health care is mixed. This study systematically synthesizes and critically evaluates evidence in systematic reviews of health care utilization outcomes from case management interventions for the care of chronic illnesses.
Suitable research articles include but are not limited to: N of 1 trials, meta-analyses of published case reports, research addressing the use of case reports and the prevalence or importance of case reporting in the medical literature and retrospective studies that include case-specific information (age, sex and ethnicity) for all patients.
CCRE, Center for Clinical and Research Ethics, Responsible Conduct of Research, PI Program, Case Studies, Ethics Educational Programming
Case studies are in-depth investigations of a person, group, event, or community. Typically, data is gathered from various sources using several methods (e.g., observations & interviews). The case study research method originated in clinical medicine (the case history, i.e., the patient's personal history). In psychology, case studies are ...
Clinical Case Studies 12/6/2018 2 . warnings can help to ease the toll of heat -related illness and prevention may ease the burden of such events on the health care system. Integrating weather modeling and public health intervention to address vulnerable populations may ease the burden of heat stress on individuals and the health care system.
In this population-based, nested case-control study, we used the UK Clinical Practice Research Datalink (CPRD GOLD and Aurum databases), a large primary care database of electronic medical records that contains demographic and lifestyle information, medical diagnoses, prescriptions, and referrals for more than 60 million patients from more than ...
We invite researchers, practitioners, teachers, and students in all medical science disciplines to submit their research papers, encompassing Quantitative studies, Qualitative studies, Empirical Case studies, Mixed-Method studies, Experimental Research, and Review studies. This Research Topic welcomes articles about but not only limited to the ...
This study is part of a larger phase II, randomized, controlled clinical trial "Communication, Coordination and Security for people with severe Multiple Sclerosis (COCOS-MS)" [].This explorative clinical trial, employing a mixed-method design, incorporates a qualitative study component with PwsMS, caregivers and HCSs to enrich the findings of the quantitative data.
The Viet Nam Preterm Birth Biomarker study was a case-cohort study of PTB and term deliveries. ... serum was aliquoted within 2 h of collection for −80 °C storage at the Oxford University Clinical Research Unit (Ho Chi Minh City, Viet Nam). At the end of the study, samples were shipped to Sera Prognostics in two batches in liquid nitrogen ...
A case report is a detailed report of the symptoms, signs, diagnosis, treatment, and follow-up of an individual patient. Case reports usually describe an unusual or novel occurrence and as such, remain one of the cornerstones of medical progress and provide many new ideas in medicine. Some reports contain an extensive review of the relevant ...
Guillain-Barré Syndrome (GBS) is an autoimmune neuropathy. Antecedent infections have been seen to be significant triggering factors for developing GBS. Among them, arboviral infections are rapidly gaining importance as significant triggers, especially in the areas where they are endemic. Chikungunya, an arboviral infection that usually causes a self-limiting acute febrile illness can lead to ...
Background Light chain deposition disease (LCDD) is a very rare entity. Clinical manifestations of LCDD vary according to the organs involved. Data on pulmonary LCDD are scarce and limited to small series or case reports. This study aimed to describe the characteristics and outcome of diffuse pulmonary non-amyloid LCDD localized to the lungs. Study design and methods A multicenter ...
We are very uncertain about the effect of midwife continuity of care models compared to other models of care on third or fourth-degree tear (average RR 1.10, 95% CI 0.81 to 1.49; 7 studies, 9437 participants; very low-certainty evidence), maternal readmission within 28 days (average RR 1.52, 95% CI 0.78 to 2.96; 1 study, 1195 participants; very ...