TheScholarsRepository@LLU: Digital Archive of Research, Scholarship & Creative Works
- < Previous
Home > ETD > 1444

Loma Linda University Electronic Theses, Dissertations & Projects
The public health impact of maternal smoking cessation during pregnancy : the san bernardino county experience.
Michael Batech
Most studies of maternal tobacco cessation during pregnancy have estimated measures of effect that can be useful in identifying the exposure as an etiologic risk factor in maternal and child health. However, in the case of maternal tobacco use there is overwhelming evidence for a causal role in adverse infant outcomes, and public health needs to focus on measures of potential impact in order to better prioritize and allocate resources toward interventions. The aims of this dissertation were: 1) to investigate the public health impact of smoking cessation during pregnancy among mothers giving birth in San Bernardino County, California; and 2) to examine the relationship between indices of prenatal care utilization and smoking cessation during pregnancy.
The first study showed that, among 65,228 total live births in 2007 and 2008, a single low birth weight or preterm outcome in the county could have been prevented either by at least 35 mothers quitting smoking during pregnancy or by 25 mothers being never smokers during pre-pregnancy.
The second study among 4,059 women reporting tobacco use in San Bernardino County found a dose-response relationship for prenatal care initiation in which women were more likely to quit smoking for every two months earlier they initiated their prenatal care visits. Examining the adequacy of received prenatal care services indicated a threefold increase in odds of smoking cessation for women receiving more than half the number of prenatal care visits recommended by the American College of Obstetricians and Gynecologists.
Based on the findings from these studies of San Bernardino County we can conclude that there is a burden of adverse infant outcomes due to maternal smoking that can be reduced by relatively small numbers of women quitting smoking during pregnancy. We gained the additional insight from analysis of maternal smokers that indicated that those with adequate prenatal care utilization were most likely to quit smoking during their pregnancy.
LLU Discipline
Epidemiology
School of Public Health
First Advisor
Pramil N. Singh
Second Advisor
Jayakaran Job
Third Advisor
Serena Tonstad
Degree Name
Doctor of Public Health (DrPH)
Degree Level
Year degree awarded, date (title page), library of congress/mesh subject headings.
Pregnant women -- Tobacco use; Smoking cessation -- San Bernardino County; Health promotion -- San Bernardino County; Smoking -- in pregnancy; Smoking Cessation; Maternal Exposure -- adverse effects; Maternal Welfare; Prenatal Care -- utilization -- San Bernardino County; Health Promotion -- utilization -- San Bernardino County; Pregnancy Outcome -- San Bernardino County -- statistics -- 2007-2008; Infant, Newborn -- San Bernardino County -- statistics -- 2007-2008; Cohort Studies.
Dissertation
Digital Format
Digital publisher.
Loma Linda University Libraries
Usage Rights
This title appears here courtesy of the author, who has granted Loma Linda University a limited, non-exclusive right to make this publication available to the public. The author retains all other copyrights.
Recommended Citation
Batech, Michael, "The Public Health Impact of Maternal Smoking Cessation during Pregnancy : The San Bernardino County Experience" (2013). Loma Linda University Electronic Theses, Dissertations & Projects . 1444. https://scholarsrepository.llu.edu/etd/1444
Loma Linda University Electronic Theses and Dissertations
Collection Website
http://scholarsrepository.llu.edu/etd/
Loma Linda University. Del E. Webb Memorial Library. University Archives
Since March 15, 2022
Included in
Epidemiology Commons , Maternal and Child Health Commons , Public Health Education and Promotion Commons
- Collections
- Disciplines
Advanced Search
- Notify me via email or RSS
Author Corner
- Faculty Research (Pure)
Home | About | FAQ | My Account | Accessibility Statement
Privacy Copyright
- Open access
- Published: 15 October 2015
Short and long term health effects of parental tobacco smoking during pregnancy and lactation: a descriptive review
- G. Banderali 1 , 2 ,
- A. Martelli 3 ,
- M. Landi 4 ,
- F. Moretti 1 , 2 , 5 ,
- F. Betti 1 , 2 ,
- G. Radaelli 1 , 2 ,
- C. Lassandro 1 , 2 , 5 &
- E. Verduci 1 , 2
Journal of Translational Medicine volume 13 , Article number: 327 ( 2015 ) Cite this article
50k Accesses
209 Citations
25 Altmetric
Metrics details
A great deal of attention has been focused on adverse effects of tobacco smoking on conception, pregnancy, fetal, and child health. The aim of this paper is to discuss the current evidence regarding short and long-term health effects on child health of parental smoking during pregnancy and lactation and the potential underlying mechanisms. Studies were searched on MEDLINE ® and Cochrane database inserting, individually and using the Boolean ANDs and ORs, ‘pregnancy’, ‘human lactation’, ‘fetal growth’, ‘metabolic outcomes’, ‘obesity’, ‘cardiovascular outcomes’, ‘blood pressure’, ‘brain development’, ‘respiratory outcomes’, ‘maternal or paternal or parental tobacco smoking’, ‘nicotine’. Publications coming from the reference list of studies were also considered from MEDLINE. All sources were retrieved between 2015-01-03 and 2015-31-05. There is overall consistency in literature about negative effects of fetal and postnatal exposure to parental tobacco smoking on several outcomes: preterm birth, fetal growth restriction, low birth weight, sudden infant death syndrome, neurodevelopmental and behavioral problems, obesity, hypertension, type 2 diabetes, impaired lung function, asthma and wheezing. While maternal smoking during pregnancy plays a major role on adverse postnatal outcomes, it may also cumulate negatively with smoking during lactation and with second-hand smoking exposure. Although this review was not strictly designed as a systematic review and the PRISMA Statement was not fully applied it may benefit the reader with a promptly and friendly readable update of the matter. This review strengthens the need to plan population health policies aimed to implement educational programs to hopefully minimize tobacco smoke exposure during pregnancy and lactation.
Great attention has been focused on adverse effects of tobacco smoking on health, also early in life [ 1 ]. Although a decrease in smoking prevalence among pregnant women has been observed in almost all Western countries [ 1 ] the prevalence is far from the 2 % goal fixed by “Healthy People—2010” in USA [ 2 ]. Indeed, the European perinatal health report showed that more than 10 % of women smoke during pregnancy with values ranging from 5 % in Lithuania to 22 % in France [ 3 ].
Tobacco smoke is a complex, dynamic and reactive mixture containing an estimated 5000 chemicals [ 4 ]. Tobacco smoke is an aerosol of liquid droplets (the particulate phase) suspended in a mixture of gases and semi-volatile compounds [ 5 ]. The particulate phase is characterized by several compounds, such as the polycyclic aromatic hydrocarbons, tobacco-specific nitrosamines, phytosterols and metals [ 5 ]. Some compounds, termed semi-volatiles, e.g. phenol and the cresols (phenolics), are distributed between the particulate and gaseous phases [ 5 ]. The gaseous phase contains mainly nitrogen and oxygen but also several combustion products, such as carbon monoxide (CO), carbon dioxide and nitric oxide [ 5 ]. Nicotine, an alkaloid produced in the roots of the tobacco plant, is the crucial addictive compound of tobacco smoke [ 6 ]. It is the most important pharmacologically active compound in tobacco smoke [ 5 ].
Aim of the review
Currently there is lack of reviews amalgamating short and long-term effects on child health of maternal and paternal smoking early in life. The aim of this paper is to discuss the current evidence regarding the short and long-term health effects of parental smoking during pregnancy and lactation and the potential underlying mechanisms.
Methodology
Eligibility criteria.
The populations of interest were children from birth to 18 years, their mothers (during pregnancy and lactation) and fathers. Inclusion criteria were: type of article (meta-analysis, multicenter study, review, systematic review, observational study, case–control study, longitudinal/prospective study, retrospective study, randomized controlled trial), publication date (2005–2015), species (both human and animal), English language. Texts available only on abstract form were excluded.
Information sources, search strategy and study selection
Publications were searched on MEDLINE ® and Cochrane database inserting terms individually and using the Boolean ANDs and ORs. Other publications coming from the reference list of studies extracted from MEDLINE, Cochrane database and from the personal reference databases of the authors were also evaluated. In the search strategy the following terms were included: ‘pregnancy’, ‘human lactation’, ‘fetal growth’, ‘metabolic outcomes’, ‘obesity’, ‘cardiovascular outcomes’, ‘blood pressure’, ‘brain development’, ‘respiratory outcomes’, ‘maternal or paternal or parental tobacco smoking’, ‘nicotine’. All sources were retrieved between 2015-01-03 and 2015-31-05. The data screening and extraction were conducted independently by two authors and any variances resolved between them.
Parental tobacco smoking during pregnancy
It is well known that maternal smoking during pregnancy is associated with several fetal and developmental complications with increased risk of significant long-term consequences [ 7 ]. Indeed, nicotine is able to cross the placenta and therefore may affect fetal development [ 6 ]. Several other substances contained in tobacco smoke may cross the placenta and enter the fetal circulation, as CO which can interfere with the unborn child’s oxygen supply, or polycyclic aromatic hydrocarbons and tobacco-specific nitrosamines [ 8 ].
Fetal growth
Smoking during pregnancy has important effects on fetal growth. Several studies have shown that maternal smoking during pregnancy could decrease birth weight and significantly increases the risk of low birth weight births (<2500 g, LBW) and preterm birth [ 9 – 11 ] showing also dose-dependent and time specific associations [ 9 , 11 ]. Recently resulted that for each additional pack smoked during pregnancy, there was a 2.8-g decrease in neonatal body mass (0.7-g decrease in fatty mass and 2.1 g decrease in free-fatty mass), showing a dose-dependent association between prenatal smoking and neonatal body mass composition [ 11 ]. Moreover the paternal smoking and the environmental tobacco smoke may have a role. The effect of paternal smoking on small for gestational age (SGA) and LBW has been evaluated in several studies but there are inconsistent results [ 9 ], although a significant association with environmental tobacco exposure was observed [ 12 ]. Different mechanisms have been suggested to explain how maternal smoking may affect intrauterine growth and birth weight. CO, contained in tobacco, has a great affinity to hemoglobin and accordingly increases carboxyhemoglobin levels in umbilical arteries, inhibiting oxygen delivery to the cells and causing fetal hypoxia [ 9 ]. Moreover it has been showed that the resistance indices of uterine arteries and umbilical artery rised in accordance with increasing levels of tobacco smoking exposure in a dose-dependent manner [ 13 ]. Low weight birth could be the effect of the increase in resistance indices with consequent reduced amount of blood and oxygen transported to the fetus [ 13 ]. However, CO has also great affinity to the other biological molecules that bind oxygen such as myoglobin, cytochrome P450 and cytochrome c oxidase (COX), or mitochondrial respiratory chain complex IV [ 14 ]. The observed increase in apoptosis seems to be caused probably by mitochondrial dysfunction due to CO-mediated COX inhibition [ 14 ].
Moreover, maternal smoking could be related to docosahexaenoic acid (DHA) supply to fetus. Indeed maternal smoking during pregnancy may progressively impair DHA synthesis and/or maternal transfer that has been associated with restricted fetal growth [ 15 ].
Lastly, fetal growth restriction due to tobacco smoking in pregnancy could also be the result of epigenetic mechanisms. Indeed exposure to tobacco smoke in utero has been related with changes in DNA methylation of genes associated with growth restriction (e.g. CYP1A1 promoter) [ 16 – 18 ].
Brain development
Maternal smoking can modulate fetal brain development and function. A reduced brain size and alterations in brain functions were observed in infants exposed to prenatal smoking compared to unexposed infants [ 19 ]. Moreover, in the same review, a meta-analysis of nine papers with information on prenatal smoking exposure and head circumference at birth reported that, on average, the head circumference of the infants exposed to maternal smoking during pregnancy was 0.5 cm smaller than the unexposed [ 19 ]. The underlying mechanisms of these effects might include the nicotine modulating axonal path finding, synapse formation in neurons and CO leading to fetal hypoxia and interfering with fetal brain development, and epigenetic changes (such as the regulation, by DNA methylation, of the brain-derived neurotrophic factor BDNF-gene, important for normal brain development) [ 19 ].
Later obesity and related comorbidities
There is growing concern that perinatal exposure to chemical insults may play a significant role in the increased incidence of obesity and metabolic disorders [ 20 ]. A recent meta-analysis of 17 studies found that children of mothers who smoked during pregnancy had an increased risk of obesity at a mean age of 9 years compared with children of non-smoker mothers [ 20 ]. Similarly another meta-analysis [ 21 ], reported that children whose mothers smoked during pregnancy had a 50 % increased risk of later overweight compared with children whose mothers did not smoke during pregnancy. An Australian Prospective Birth Cohort Study [ 22 ] found that mean BMI and the prevalence of overweight and obesity among adolescents whose mothers smoked before and/or after pregnancy but not during pregnancy were similar to outcomes in adolescents whose mothers had never smoked. These findings may suggest some evidence for a direct effect of maternal smoking in utero on the later development of obesity in offspring [ 22 ], as suggested also by another recent study [ 23 ]. Moreover a dose–response association between maternal pregnancy smoking and obesity could exist [ 23 ]. However it is important to point out that in these studies BMI is used as primary outcome but this approach could not provide correct information about body composition. Leary et al. [ 24 ] found that maternal smoking at any time during pregnancy has been associated with higher total fat mass (assessed by Dual-energy X-ray absorptiometry) in the offspring at mean age 9.9 years [ 24 ].
Recently a study reported that parental smoking during maternal pregnancy may be associated with an increased risk of childhood overweight [ 25 ]. Also other studies found an association between paternal smoking and BMI or adiposity [ 24 , 26 ]. However further studies are needed to find a possible link between paternal smoking and risk of obesity in children.
Different mechanisms and pathways have been proposed to explain the association between smoking during pregnancy and risk of overweight and obesity such as thrifty phenotype theory, postnatal catch-up growth and neurotransmitter or endocrine imbalances [ 27 ]. Maternal smoking during pregnancy may result in lower fetal growth and more rapid postnatal weight gain, which are both associated with risk of adiposity later in life [ 21 ]. Additionally Ino [ 20 ] suggested two different mechanisms to explain the development of obesity in offspring of mothers who smoked. Firstly, obesity in the offspring of mothers affected by nicotine-inducted starvation during early gestation could be due to altered hypothalamic regulatory mechanisms of energy intake and expenditure [ 20 ]. Secondly, fetal exposure to nicotine seems to cause abnormal cell proliferation, differentiation and synaptic activity in the brain and the peripheral autonomic pathways [ 20 ]. However, as tobacco smoke contains a great number of chemicals, it is difficult to fully understand the mechanisms which could determine obesity later in life.
Later cardiovascular outcomes
Maternal smoking during pregnancy may have a persistent influence also on offspring cardiovascular health. Indeed prenatal tobacco exposure has been associated with lower Fetal Heart Rate Variability (HRV) in utero [ 28 ].
Hypertension is considered another of the health consequences associated with in utero exposure to tobacco smoking [ 7 ]. Different studies have found an association of maternal smoking during pregnancy with higher systolic or diastolic blood pressure (BP) in childhood [ 29 – 31 ]. Moreover, it has been suggested that prenatal maternal smoking could influence child BP with a persistent effect even if mother quits months before pregnancy [ 29 ]. A recent study [ 32 ] showed that maternal smoking during pregnancy may have a small long-term effect on late adolescent/young adult offsprings BP but whether this association persists later in life is uncertain.
The involved mechanisms to explain elevated blood pressure in offspring, associated with maternal tobacco smoking, could be endothelial dysfunction, changes in renal structure and function, and alterations in perivascular adipose tissue, important modulator of vascular function [ 7 ]. Maternal smoking was also associated with long-lasting reprogramming of infant blood pressure control mechanisms [ 33 ]. Indeed infants exposed to nicotine during fetal life have a “hyperreactive” autonomic system in the first few weeks of postnatal life and a different parasympathetic and sympathetic control at 1 year of age, which consequently may cause abnormal BP control on tilt testing [ 33 ]. Moreover a positive relationship between maternal smoking in pregnancy and shortened telomere length of the fetus at birth was shown [ 34 ]. However it should be pointed out that a study found a similar effect of maternal and paternal smoking on offspring BP [ 35 ], suggesting that differences in child BP associated with maternal smoking may be not a result of biological effects on the intrauterine environment but rather a marker for parental factors [ 35 ].
Overall these findings suggest that one of strategies for prevention of adulthood cardiovascular disease (in particularly, high BP) should be focused on avoiding tobacco smoking during the conception and pregnancy period.
Respiratory outcomes
Maternal smoking during pregnancy has been associated with increased risk of wheezing, asthma, airway hyper responsiveness, impaired lung function, bronchitis [ 36 ].
A recent systematic review and meta-analysis [ 37 ] reported that in utero tobacco exposure is associated with increased risk of asthma and wheeze in children and adolescents up to the age of 18 years, with the strongest effect for incidence of asthma in children aged ≤2 years.
Childhood asthma is a chronic inflammatory disease of the airways, characterized by a dysregulated T-helper (TH) type-2 response to inhaled antigens with the consequent production of IgE. From the observation that maternal smoke during pregnancy was associated with increased neonatal TH2 cytokine responses to allergens [ 38 ] and that nicotine could increase the production of cellular mediators, which in turn enhance TH2 activity and increase the production of immunoglobulin, it has been suggested that in utero tobacco exposure may enhance allergic inflammatory responses [ 36 ].
Different studies reported that in utero tobacco exposure has been associated with a decrease in lung function, with a reduction of tidal and forced expiratory flow rates, suggestive of the affection of small airway development [ 39 ]. A recent study found that this lower lung function seems to persist into adolescence with an independent increased risk of asthma [ 40 ]. In utero tobacco exposure could increase oxidative stress in the lungs with a consequent reduced alveolarization and impaired lung development [ 39 ]. However further research is needed to better understand these mechanisms.
Tobacco smoking during lactation
One of the most significant and susceptible periods after birth in which parental tobacco smoke may have critical effects is during lactation and breastfeeding. Breastfeeding by a mother who smokes is a key source of infant exposure to tobacco compounds as nicotine is readily available in breast milk [ 41 ].
It has been suggested that the deleterious effects of nicotine transferred into breast milk depend on the number of cigarettes consumed by the mother per day and also on the time interval between the last inhaled cigarette and the beginning of breastfeeding [ 42 , 43 ]. The amount of nicotine found in breast milk is more than double that nicotine circulating in maternal serum (according to some studies 2.9 greater) and this is a relevant matter because is not exactly known when infants develop the capability to completely metabolize nicotine after absorption [ 42 , 43 ]. Both the quantitative and qualitative profile of breast milk components are negatively modulated with early and late effects on the organism. For example maternal smoking habit, either during pregnancy and lactation, has been associated with a reduced content of n-3 long-chain polyunsaturated fatty acid (LC-PUFA) in breast milk [ 44 , 45 ]. A recent study has shown that smoking mothers have different dietary characteristics but the maternal dietary pattern seems not to justify the differences in milk LC-PUFA content, suggesting an important role of the mammary gland in synthesizing and secreting LC-PUFA into the milk [ 45 ]. Indeed, in vitro, a dose-dependent relationship between maternal smoking and the inhibition of the enzyme Δ5-desaturase, involved in the synthesis of n-3-LC-PUFA from the precursor in mammary gland cells, has been showed [ 44 ].
Smoking mothers represent a risk group for short duration of breastfeeding because they are less likely to breastfeed [ 42 ]. Several explanations for premature stopping of breastfeeding practice have been suggested: smoking dose-dependent adverse effects on lactational process, the smoking mothers feelings about their milk supply as inadequate, infants behaviors exacerbated by tobacco smoking (e.g. colic and crying) that may promote other types of feeding [ 42 ].
Other than a shorter duration of breastfeeding, evidences that maternal smoking during lactation has adverse effects on the infant refer particularly to neurobehavioral disorders, sudden infant death syndrome (SIDS), metabolic and respiratory outcomes [ 7 , 42 , 43 , 46 ].
Results of an experimental study indicate that infants sleep and wake patterns seem to be affected immediately after their mother smoked [ 42 ]. These sleep disruptions could be related to nicotine: its the stimulant effects and the direct and indirect inhibitory activity on neuronal sleep promoting functions. Tobacco smoking exposure may also induce irritability, excessive crying, lassitude, colic and pallor early in life, active sleep deprivation in neonates and may also be associated with later memory and learning deficits [ 42 , 43 ].
The relationship between SIDS and neonatal tobacco exposure may be caused by an impaired ability of adrenomedullary chromaffin cells to respond to hypoxic stress due to nicotine [ 7 , 42 ].
Moreover different studies have investigated the negative effects of the nicotine in breast milk involved in organ dysfunction and related diseases in adulthood [ 7 , 43 , 46 ]. Recent animal model studies showed that maternal smoking may induce histopathological changes in the lung and liver of lactating offspring through the inhibition of those mechanisms that prevent from intracellular oxidative stress [ 7 , 43 ]. Additionally, some authors reported also a relationship between nicotine exposure during lactation and pancreatic β-cells depletion with subsequent impaired glucose homeostasis [ 7 , 43 ]. Further human investigations are needed to support these findings.
Although the risk of overweight in childhood after prenatal exposure to tobacco smoking is established [ 20 , 21 ], this association about the exposure in early postnatal life and especially during lactation needs to be better studied. Recent evidence has demonstrated a negative cumulative effect concerning the postnatally exposure to smoking that would worsen the risk of overweight compared with smoking exposure only during pregnancy [ 41 , 47 ]. Given the protective, even if modest [ 48 ], role of breastfeeding against obesity, it seems that in a developmental critical period, as lactation, tobacco compounds via breast milk of smoking mothers in a dose-dependent relationship may be able to contrast the beneficial properties of human breast milk on the risk of overweight [ 41 , 47 ].
Nevertheless, in 2001, the Committee on Drugs of the American Academy of Pediatrics has not placed nicotine (and thus smoking) as a drug contraindicated during breastfeeding [ 49 ]. Awaiting more data on this topic, they suggest that breastfeeding and parental smoking may be less detrimental to child health than bottle-feeding and parental smoking [ 49 ]. Therefore benefits of breastfeeding, for both mothers and children, outweigh the risks of smoking exposure and breast milk remains the normal feeding practice even if the mother does not stop smoking [ 42 ].
An infant can be exposed to tobacco smoke compounds not only via breast milk but also through passive smoking or by the contact with tobacco smoke residues, on parental and infant clothing, bedding, household items etc. [ 50 ]. Therefore, further studies are needed to assess the additional effects of simultaneous second- and third-hand smoking exposure on infant health.
Results and conclusions
Findings from this article indicate that prenatal and early postnatal periods have a critical role in the individual outcome, as Barker affirms: “Much of human development is completed during the first 1000 days after conception” [ 51 ]. There is overall consistency in literature about negative effects of fetal and postnatal exposure to parental tobacco smoking on several outcomes: preterm birth, fetal growth restriction, low birth weight, sudden infant death syndrome, neurodevelopmental and behavioral problems, obesity, hypertension, type 2 diabetes, impaired lung function, asthma and wheezing (Table 1 ). While maternal smoking during pregnancy plays a major role on adverse postnatal outcomes, it may also cumulate negatively with smoking during lactation and with second-hand smoking exposure.
This review aimed to discuss the current evidence regarding the short and long-term effects of parental tobacco smoking during a critical period of life (pregnancy and lactation). It should be pointed out that it was not strictly designed a priori as a systematic review, and that the PRISMA Statement was not fully applied (including PICOS). Although this is a weakness, a descriptive approach might benefit the reader with a promptly and friendly readable update of the matter.
The results of this review strengthens the need to plan population health policies aimed to implement educational programs to hopefully minimize tobacco smoke exposure during pregnancy and lactation. Mothers should be strongly encouraged to stop smoking especially during pregnancy and lactation. Parents should know that exposure to prenatal and postnatal tobacco smoking is associated with different adverse outcomes and that tobacco smoking is one of the most preventable causes of infant morbidity and mortality [ 52 ]. However, breastfeeding is the best way of infant feeding even if a mother doesn’t quit smoking during lactation [ 49 ]. Amalgamating results from different studies, this paper could be a useful tool to educate and increase awareness about adverse health effects of parental tobacco smoking for children. Additionally, it could be a starting point for the conception of new health promotion and public health campaigns that emphasize the importance of parental smoking cessation.
Abbreviations
body mass index
blood pressure
carbon monoxide
cytochrome c oxidase
docosahexaenoic acid
heart rate variability
low birth weight
long chain polyunsaturated fatty acids
small for gestational age
sudden infant death syndrome
Lauria L, Lamberti A, Grandolfo M. Smoking behaviour before, during, and after pregnancy: the effect of breastfeeding. Sci World J. 2012;. doi: 10.1100/2012/154910 .
Google Scholar
Office of Disease Prevention and Health Promotion and U.S. Department of Health and Human Services, “Healthy People 2010 Objectives,” http://www.healthypeople.gov . Accessed 24 March 2015.
EURO-PERISTAT. European Perinatal Health Report by the EURO-PERISTAT project in collaboration with SCPE, EUROCAT & EURONEOSTAT, 2008. http://www.europeristat.com . Accessed 24 Mar 2015.
Talhout R, Schulz T, Florek E, van Benthem J, Wester P, Opperhuizen A. Hazardous compounds in tobacco smoke. Int J Environ Res Public Health. 2011;8:613–28.
Article PubMed Central PubMed Google Scholar
Thielen A, Klus H, Müller L. Tobacco smoke: unraveling a controversial subject. Exp Toxicol Pathol. 2008;60:141–56.
Article CAS PubMed Google Scholar
Lisboa PC, de Oliveira E, de Moura EG. Obesity and endocrine dysfunction programmed by maternal smoking in pregnancy and lactation. Front Physiol. 2012;3:437.
Bruin JE, Gerstein HC, Holloway AC. Long-term consequences of fetal and neonatal nicotine exposure: a critical review. Toxicol Sci. 2010;116:364–74.
Article PubMed Central CAS PubMed Google Scholar
Schneider S, Huy C, Schütz J, Diehl K. Smoking cessation during pregnancy: a systematic literature review. Drug Alcohol Rev. 2010;29:81–90.
Article PubMed Google Scholar
Ko TJ, Tsai LY, Chu LC, Yeh SJ, Leung C, Chen CY, et al. Parental smoking during pregnancy and its association with low birth weight, small for gestational age, and preterm birth offspring: a birth cohort study. Pediatr Neonatol. 2014;55:20–7.
Blatt K, Moore E, Chen A, Van Hook J, DeFranco E. Association of reported trimester-specific smoking cessation with fetal growth restriction. Obstet Gynecol. 2015;125:1452–9.
Harrod CS, Reynolds RM, Chasan-Taber L, Fingerlin TE, Glueck DH, Brinton JT, Dabelea D. Quantity and timing of maternal prenatal smoking on neonatal body composition: the healthy start study. J Pediatr. 2014;165:707–12.
Leonardi-Bee J, Smyth A, Britton J, Coleman T. Environmental tobacco smoke and fetal health: systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2008;93:F351–61.
Machado J de B, Filho Plínio VM, Petersen GO, Chatkin JM. Quantitative effects of tobacco smoking exposure on the maternal-fetal circulation. BMC Pregnancy Childbirth. 2011;11:24–9. doi: 10.1186/1471-2393-11-24
Article Google Scholar
Garrabou G, Hernàndez AS, Catalán García M, Morén C, Tobías E, Córdoba S. Molecular basis of reduced birth weight in smoking pregnant women: mitochondrial dysfunction and apoptosis. Addict Biol. 2014;. doi: 10.1111/adb.12183 .
PubMed Google Scholar
Agostoni C, Galli C, Riva E, Colombo C, Giovannini M, Marangoni F. Reduced docosahexaenoic acid synthesis may contribute to growth restriction in infants born to mothers who smoke. J Pediatr. 2005;147:854–6.
Knopik VS, Maccani MA, Francazio S, McGeary JE. The epigenetics of maternal cigarette smoking during pregnancy and effects in child development. Dev Psychopathol. 2012;24:1377–90.
Lee KW, Richmond R, Hu P, French L, Shin J, Bourdon C, et al. Prenatal exposure to maternal cigarette smoking and DNA Methylation: epigenome-wide association in a discovery sample of adolescents and replication in an independent cohort at birth through 17 years of age. Environ Health Perspect. 2015;123:193–9.
PubMed Central PubMed Google Scholar
Pirini F, Guida E, Lawson F, Mancinelli A, Guerrero-Preston R. Nuclear and mitochondrial DNA alterations in newborns with prenatal exposure to cigarette smoke. Int J Environ Res Public Health. 2015;12:1135–55.
Ekblad M, Korkeila J, Lehtonen L. Smoking during pregnancy affects foetal brain development. Acta Paediatr. 2015;104:12–8.
Ino T. Maternal smoking during pregnancy and offspring obesity: meta-analysis. Pediatr Int. 2010;52:94–9.
Oken E, Levitan EB, Gillman MW. Maternal smoking during pregnancy and child overweight: systematic review and meta analysis. Int J Obes. 2008;32:201–10.
Article CAS Google Scholar
Al Mamun A, Lawlor DA, Alati R, O’Callaghan MJ, Williams GM, Najman JM. Does maternal smoking during pregnancy have a direct effect on future offspring obesity? Evidence from a prospective birth cohort study. Am J Epidemiol. 2006;164:317–25.
Koshy G, Delpisheh A, Brabin BJ. Dose response association of pregnancy cigarette smoke exposure, childhood stature, overweight and obesity. Eur J Public Health. 2011;21:286–91.
Leary SD, Smith GD, Rogers IS, Reilly JJ, Wells JC, Ness AR. Smoking during pregnancy and offspring fat and lean mass in childhood. Obesity. 2006;14:2284–93.
Durmuş B, Heppe DH, Taal HR, Manniesing R, Raat H, Hofman A, et al. Parental smoking during pregnancy and total and abdominal fat distribution in school-age children: the Generation R Study. Int J Obes. 2014;38:966–72.
Kwok MK, Schooling CM, Lam TH, Leung GM. Paternal smoking and childhood overweight: evidence from the Hong Kong “Children of 1997”. Pediatrics. 2010;126:e46–56.
Raum E, Küpper-Nybelen J, Lamerz A, Hebebrand J, Herpertz-Dahlmann B, Brenner H. Tobacco smoke exposure before, during, and after pregnancy and risk of overweight at age 6. Obesity. 2011;19:2411–7.
Zeskind PS, Gingras JL. Maternal cigarette-smoking during pregnancy disrupts rhythms in fetal heart rate. J Pediatr Psychol. 2006;31:5–14.
Oken E, Huh SY, Taveras EM, Rich-Edwards JW, Gillman MW. Associations of maternal prenatal smoking with child adiposity and blood pressure. Obes Res. 2005;13:2021–8.
Geerts CC, Grobbee DE, van der Ent CK, de Jong BM, van der Zalm MM, van Putte-Katier N, Kimpen JL, Uiterwaal CS. Tobacco smoke exposure of pregnant mothers and blood pressure in their newborns: results from the wheezing illnesses study Leidsche Rijn birth cohort. Hypertension. 2007;50:572–8.
Brion MJ, Leary SD, Lawlor DA, Smith GD, Ness AR. Modifiable maternal exposures and offspring blood pressure: a review of epidemiological studies of maternal age, diet, and smoking. Pediatr Res. 2008;63:593–8.
Högberg L, Cnattingius S, Lundholm C, D’Onofrio BM, Långström N, Iliadou AN. Effects of maternal smoking during pregnancy on offspring blood pressure in late adolescence. J Hypertens. 2012;30:693–9.
Cohen G, Jeffery H, Lagercrantz H, Katz-Salamon M. Long-term reprogramming of cardiovascular function in infants of active smokers. Hypertension. 2010;55:722–8.
Salihu HM, Pradhan A, King L, et al. Impact of intrauterine tobacco exposure on fetal telomere length. Am J Obstet Gynecol. 2015;212.e1:205.e8.
Brion MJ, Leary SD, Smith GD, Ness AR. Similar associations of parental prenatal smoking suggest child blood pressure is not influenced by intrauterine effects. Hypertension. 2007;49:1422–8.
Cheraghi M, Salvi S. Environmental tobacco smoke (ETS) and respiratory health in children. Eur J Pediatr. 2009;168:897–905.
Burke H, Leonardi-Bee J, Hashim A, Pine-Abata H, Chen Y, Cook DG, et al. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics. 2012;129:735–44.
Doherty SP, Grabowski J, Hoffman C, Ng SP, Zelikoff JT. Early life insult from cigarette smoke may be predictive of chronic diseases later in life. Biomarkers. 2009;14:97–101.
Maritz GS, Harding R. Life-long programming implications of exposure to tobacco smoking and nicotine before and soon after birth: evidence for altered lung development. Int J Environ Res Public Health. 2011;8:875–98.
Hollams EM, de Klerk NH, Holt PG, Sly PD. Persistent effects of maternal smoking during pregnancy on lung function and asthma in adolescents. Am J Respir Crit Care Med. 2014;189:401–7.
Wen X, Shenassa ED, Paradis AD. Maternal smoking, breastfeeding, and risk of childhood overweight: findings from a national cohort. Matern Child Health J. 2013;17:746–55.
Mennella JA, Yourshaw LM, Morgan LK. Breastfeeding and smoking: short-term effects on infant feeding and sleep. Pediatrics. 2007;120:497–502.
Primo CC, Ruela PB, Brotto LD, Garcia TR, Lima Ede F. Effects of maternal nicotine on breastfeeding infants. Rev Paul Pediatr. 2013;31:392–7.
Verduci E, Banderali G, Barberi S, Radaelli G, Lops A, Betti F, et al. Epigenetic effects of human breast milk. Nutrients. 2014;6:1711–24.
Szlagatys-Sidorkiewicz A, Martysiak-Zurowska D, Krykowski G, Zagierski M, Kaminska B. Maternal smoking modulates fatty acid profile of breast milk. Acta Paediatr. 2013;102:e353–9.
Guedes HT, Souza LS. Exposure to maternal smoking in the first year of life interferes in breast-feeding protective effect against the onset of respiratory allergy from birth to 5 yr. Pediatr Allergy Immunol. 2009;20:30–4.
Moller SE, Ajslev TA, Andersen CS, Dalgard C. Sorensen Thorkild I.A. Risk of childhood overweight after exposure to tobacco smoking in prenatal and early postnatal life. PLoS One. 2014;9:e109184.
Lefebvre CM, John RM. The effect of breastfeeding on childhood overweight and obesity: a systematic review of the literature. J Am Assoc Nurse Pract. 2014;26:386–401.
AAP-American Academy of Pediatrics Committee on drugs. Transfer of drugs and other chemicals into human milk. Pediatrics. 2001;108:776–89.
Merritt TA, Mazela J, Adamczak A, Merritt T. The impact of second-hand tobacco smoke exposure on pregnancy outcomes, infant health, and the threat of third-hand smoke exposure to our environment and to our children. Przegl Lek. 2012;69:717–20.
Barker DJ. Sir Richard Doll lecture: developmental origins of chronic disease. Public Health. 2012;26:185–9.
Magee SR, Bublitz MH, Orazine C, Brush B, Salisbury A, Niaura R, Stroud LR. The relationship between maternal-fetal attachment and cigarette smoking over pregnancy. Matern Child Health J. 2014;18:1017–22.
Download references
Authors’ contributions
GB had primary responsibility for manuscript management, and contributed to the writing of the manuscript. AM, ML, CL, FM, FB, GR, performed critically the literature research about this issue and contributed to the writing of the manuscript. EV supervised the editorial project and contributed to the writing of the manuscript. All authors read and approved the final manuscript.
Competing interests
All the authors declare that they have no conflict of interest or financial support for this study.
Author information
Authors and affiliations.
Department of Pediatrics, San Paolo Hospital, Via A Di Rudinì 8, 20142, Milan, Italy
G. Banderali, F. Moretti, F. Betti, G. Radaelli, C. Lassandro & E. Verduci
Department of Health Sciences, University of Milan, Via A Di Rudinì 8, 20142, Milan, Italy
U.O.C. Pediatria Presidio Ospedaliero Garbagnate Milanese Azienda Ospedaliera G. Salvini, Milan, Italy
A. Martelli
Pediatrician Primary Care, Institute of Biomedicine and Molecular Immunology, National Research Council, CNR, Palermo, Italy
Nutritional Sciences, University of Milan, Milan, Italy
F. Moretti & C. Lassandro
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to E. Verduci .
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Banderali, G., Martelli, A., Landi, M. et al. Short and long term health effects of parental tobacco smoking during pregnancy and lactation: a descriptive review. J Transl Med 13 , 327 (2015). https://doi.org/10.1186/s12967-015-0690-y
Download citation
Received : 22 June 2015
Accepted : 07 October 2015
Published : 15 October 2015
DOI : https://doi.org/10.1186/s12967-015-0690-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Tobacco smoking
- Fetal programming
- Environment
Journal of Translational Medicine
ISSN: 1479-5876
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Open access
- Published: 19 December 2018
Health outcomes of maternal smoking during pregnancy and postpartum period for the mother and infant: protocol for an umbrella review
- Tuba Saygın Avşar ORCID: orcid.org/0000-0002-4143-3852 1 ,
- Hugh McLeod 1 &
- Louise Jackson 1
Systematic Reviews volume 7 , Article number: 235 ( 2018 ) Cite this article
3022 Accesses
3 Citations
3 Altmetric
Metrics details
Internationally, tobacco smoking is a leading cause of mortality, morbidity and health inequality. In England, despite increasing awareness about importance of public health interventions to reduce smoking, about 10% of pregnant women are known to be smokers at the time of delivery. There are many systematic reviews investigating the impact of maternal smoking during pregnancy on particular health conditions. Hence, this overview of systematic reviews, which aims to include all health conditions for mother and infant caused by smoking during pregnancy, is timely.
CINAHL, EMBASE, MEDLINE, PsycINFO, Web of Science, CRD Database (includes DARE, NHSEED and HTA) and HMIC databases will be searched for systematic reviews investigating the effects of smoking during pregnancy. Only reviews written in English and published by 31/12/17 will be included. Studies focussed on low-income countries will be excluded. Study selection and quality assessment will be completed by two reviewers independently. To assess the quality of included studies, the Centre for Reviews and Dissemination checklist for systematic reviews will be utilised.
Existing systematic reviews focus on the impact of smoking during pregnancy on a specific health condition. This review aims to analyse current evidence on the overall health outcomes associated with smoking whilst pregnant by providing an overview of evidence from systematic reviews.
Systematic review registration
PROSPERO CRD42018086350 .
Peer Review reports
Introduction
Smoking is the highest preventable cause of numerous health problems worldwide [ 1 ]. Seven million people die every year because of smoking in the world and more than 18% of adults smoke daily in OECD countries [ 2 ]. In England, 16% of all deaths were attributed to smoking in 2015 [ 3 ]. Smoking during pregnancy is responsible for many avoidable health conditions and deaths across the countries [ 4 ]. Smoking status at delivery was 10.5% in the UK in 2016/2017, and the estimated annual cost to the NHS was up to £87 million in 2010 [ 5 , 6 ].
Smoking is the leading cause of inequalities in health across and within countries [ 7 ], and there is a negative correlation between education and income levels and smoking [ 8 ]. In line with this, the smoking status of pregnant women at the time of delivery is higher in deprived areas of England, being 27% in Blackpool compared to 2% in Central London [ 5 ].
There are many systematic reviews investigating the impact of maternal smoking during pregnancy on individual health conditions, but there have been few studies seeking to review evidence across the range of health conditions caused by maternal smoking during pregnancy. In 2010, Godfrey et al. [ 6 ] reported a scoping review of the health outcomes associated with smoking during pregnancy. However, their strategy focused on search terms for a limited number of smoking-related health conditions which meant that the review may not have captured some relevant health conditions. In addition, quality assessment of the included studies and reviews was not conducted. Several narrative reviews have surveyed short- and long-term effects of maternal smoking during pregnancy and lactation and presented evidence around the topic [ 9 , 10 ]. Nevertheless, these reviews did not systematically assess all available evidence, instead mostly focussed on the negative health effects of nicotine exposure reported in some studies. Considering the large number of published systematic reviews of observational studies regarding the impact of maternal smoking during pregnancy on different health outcomes, an overall evaluation of the current evidence is timely.
Objective of the review
This review seeks to investigate the impact of smoking during pregnancy and the postpartum period on health outcomes for the mother and infant in developed country settings to inform future research and health policy.
Methods/design
This umbrella review is designed in line with the objectives and guideline provided by Cochrane Handbook for Systematic Reviews of Interventions [ 11 ].
Inclusion criteria
Studies will be included based on the following eligibility criteria.
Smoking behaviour, tobacco regulations, access to care for pregnant women, and other related factors are different in high- and middle-income countries compared to low-income countries, and consequently, health outcomes of smoking during pregnancy may not be the same [ 12 , 13 , 14 , 15 , 16 , 17 ]. For this reason, systematic reviews of studies focussing on low-income countries will be excluded [ 18 ]. There will not be any exclusions based on age or social groups.
Intervention/effect
This review will focus on the health impacts of maternal smoking during pregnancy and the postpartum period. Therefore, studies that investigated the effect of maternal smoking during pregnancy and postpartum period will be included.
The comparator is defined as pregnant or postpartum women who have never smoked or who have quit smoking.
Outcome measure
The primary outcome measures for this review are the health outcomes of smoking during pregnancy and the postpartum period for the mother and infant. Outcomes include pregnancy-related clinical problems and long-term adverse health outcomes for the infant. Measures may include odds ratios and relative risks for smoking women and their children compared to non-smoking women and their children.
Study design
Only systematic reviews published in a peer-reviewed journal will be included in the review.
For pragmatic reasons, this review will only include systematic reviews written in English.
Publication date
This study will include systematic reviews published up to 31 December 2017.
Search strategy
A scoping search was conducted using MEDLINE with the words “pregnant women”, “pregnant smokers”, “maternal smoking”, “health outcomes”, “cost outcomes”, and “QALYs”. Then, the InterTASC Information Specialists Sub-Groups (ISSG) filter was used to identify keywords. Additionally, the keywords of five systematic reviews in relevant topics were reviewed. Identified keywords were discussed with two experts to crosscheck. The chosen search terms are shown in Additional file 1 : Appendix 1.
The literature search strategy of this review is defined as follows:
The selected keywords within each concept will be combined with “OR”, and concepts will be combined with “AND”.
The inclusion criteria will be piloted by the reviewers independently in order to maximise the consistency of the study selection process.
CINAHL, EMBASE, MEDLINE, PsycINFO, Web of Science, CRD Database (includes DARE, NHSEED and HTA) and HMIC databases will be searched with those identified keywords. In addition, references of selected studies will be searched for relevant articles.
The phase of screening for eligibility will be conducted by two reviewers independently. Any discrepancies will be resolved by discussion with a third reviewer.
Data extraction
A data extraction sheet was created which covers the lead author’s name and publication year, study design and the databases searched, number of studies included, main outcomes, and some other methodological information (Additional file 1 : Appendix 2). The extraction tool will be piloted. One reviewer (TS) will extract the data and another reviewer (HM) will check the extracted data to minimise any bias.
Data management
Data management will be done by using ENDNOTE and Microsoft Excel software.
Quality assessment
The Centre for Reviews and Dissemination’s practical checklist for conducting a critical appraisal of systematic reviews was modified according to the needs of the current study [ 19 ]. For instance, questions on protocol, publication bias and heterogeneity were added (Additional file 1 : Appendix 3). Quality assessment will be done by two reviewers independently. Any discrepancies will be solved through discussion or involvement of a third reviewer.
Analysis and presentation of the results
The study selection process will be summarised by using a PRISMA diagram [ 20 ]. A narrative analysis of the data gathered via the systematic review will be undertaken. As the study will include reviews focussing on varied health conditions, no sub-group analysis has been planned. Results will be presented in accordance with the Cochrane Handbook for Systematic Reviews of Interventions guidelines [ 11 ].
Existing systematic reviews focus on the impact of smoking during pregnancy on particular health conditions. This review aims to draw a broader picture of the current evidence by including systematic reviews that investigated any health outcome associated with smoking whilst pregnant.
Abbreviations
Centre for Reviews and Dissemination
Database of Abstracts of Reviews of Effects
The Healthcare Management Information Consortium
Health Technology Assessment
NHS Economic Evaluation Database
Preferred Reporting Items for Systematic Review and Meta-Analysis
WHO. Report on the global tobacco epidemic: monitoring tobacco use and prevention policies. Geneva; 2017.
OECD. Health at a glance: OECD indicators. Paris: OECD; 2017.
Google Scholar
NHS Digital. Statistics on smoking, England; NHS; 2017. https://www.gov.uk/government/statistics/statistics-on-smoking-england-2017 . Accessed 11 Dec 2018.
Samet, Jonathan M, Yoon, Soon-Young & WHO Tobacco Free Initiative. Women and the tobacco epidemic : challenges for the 21st century / edited by Jonathan M. Samet, Soon-Young Yoon. Geneva: World Health Organization; 2001. http://www.who.int/iris/handle/10665/66799 . Accessed 11 Dec 2018.
Health and Social Care Information Centre. Statistics on women’s smoking status at time of delivery: England; NHS; 2016. https://digital.nhs.uk/data-and-information/publications/statistical/statistics-on-smoking/statistics-on-smoking-england-2017-pas . Accessed 11 Dec 2018.
Godfrey C, Pickey KE, Parrott S, Mdege N, Eapen D. Estimating the costs to the NHS of smoking in pregnancy: Public Health Reseach Consortium; York; 2010.
Marmot M. Social determinants of health inequalities. Lancet. 2005;365(9464):1099–104.
Article Google Scholar
Schaap MM, van Agt HME, Kunst AE. Identification of socioeconomic groups at increased risk for smoking in European countries: looking beyond educational level. Nicotine Tob Res. 2008;10(2):359–69.
Banderali G, Martelli A, Landi M, Moretti F, Betti F, Radaelli G, Lassandro C, Verduci E. Short and long term health effects of parental tobacco smoking during pregnancy and lactation: a descriptive review. J Transl Med. 2015;13:327.
Article CAS Google Scholar
Bruin JE, Gerstein HC, Holloway AC. Long-term consequences of fetal and neonatal nicotine exposure: a critical review. Toxicol Sci. 2010;116(2):364–74.
Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. England: Wiley-Blackwell; 2011.
Caleyachetty R, Tait CA, Kengne AP, Corvalan C, Uauy R, Echouffo-Tcheugui JB. Tobacco use in pregnant women: analysis of data from demographic and health surveys from 54 low-income and middle-income countries. Lancet Glob Health. 2014:2(9):e513–20.
Barros F, Victora C, Barros A, Santos I, Albernaz E, Matijasevich A, Domingues MR, Sclowitz I, Hallal P, Silveira M, et al. The challenge of reducing neonatal mortality in middle-income countries: findings from three Brazilian birth cohorts in 1982, 1993, and 2004. Lancet. 2005;365(9462):847–54.
Finlayson K, Downe S. Why do women not use antenatal services in low- and middle-income countries? A meta-synthesis of qualitative studies. PLoS Med. 2013;10(1). https://doi.org/10.1371/journal.pmed.1001373 .
World Health Organisation (WHO) and National Cancer Institute (NCI). The economics of tobacco and tobacco control. In: NCI Tobacco Control Monograph Series. Geneva; 2017.
Pereira PP, Da Mata FA, Figueiredo AC, de Andrade KR, Pereira MG. Maternal active smoking during pregnancy and low birth weight in the Americas: a systematic review and meta-analysis. Nicotine Tob Res. 2017;19(5):497–505.
Zhang D, Cui H, Zhang L, Huang Y, Zhu J, Li X. Is maternal smoking during pregnancy associated with an increased risk of congenital heart defects among offspring? A systematic review and meta-analysis of observational studies. J Matern Fetal Neonatal Med. 2017;30(6):645–57.
OECD Members and partners, 2018, [ http://www.oecd.org/about/membersandpartners/ ]. Accessed 11 Dec 2018.
CRD. CRD’s guidance for undertaking reviews in health care: Centre for reviews and dissemination: University of York: York; 2009.
Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta analyses: the PRISMA statement. PLoS Med. 2009;6(7). https://doi.org/10.1371/journal.pmed.1000097 .
Download references
Acknowledgements
This review is planned as a part of Tuba Saygın Avşar’s PhD study at the University of Birmingham.
Availability of data and materials
Not applicable.
Author information
Authors and affiliations.
Health Economics Unit, Institute of Applied Health Research, College of Medical and Dental Sciences, University of Birmingham, Edgbaston, Birmingham, B15 2TT, UK
Tuba Saygın Avşar, Hugh McLeod & Louise Jackson
You can also search for this author in PubMed Google Scholar
Contributions
The search strategy for the database search was developed by all authors through discussion. TSA prepared the first draft for the protocol; HM and LJ provided feedback to improve the protocol. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Tuba Saygın Avşar .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:.
Appendix 1: Sample search strategy from MEDLINE. Appendix 2: Data extraction tool. Appendix 3: Critical appraisal checklist for systematic reviews. (DOCX 23 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Saygın Avşar, T., McLeod, H. & Jackson, L. Health outcomes of maternal smoking during pregnancy and postpartum period for the mother and infant: protocol for an umbrella review. Syst Rev 7 , 235 (2018). https://doi.org/10.1186/s13643-018-0900-9
Download citation
Received : 12 April 2018
Accepted : 28 November 2018
Published : 19 December 2018
DOI : https://doi.org/10.1186/s13643-018-0900-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Smoking during pregnancy
- Health outcomes
- Overview of reviews
- Umbrella review
- Systematic review
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Open access
- Published: 08 August 2022
Maternal smoking during pregnancy and health outcomes in offspring
- Hongjiang Wu ORCID: orcid.org/0000-0003-2193-1114 1 &
- Andrea O. Y. Luk ORCID: orcid.org/0000-0002-5244-6069 1
BMC Medicine volume 20 , Article number: 296 ( 2022 ) Cite this article
1691 Accesses
2 Altmetric
Metrics details
The Original Article was published on 12 August 2022
Maternal smoking during pregnancy (MSDP) is an important public health issue that adversely impacts health outcomes of both children and mothers, contributing to low birthweight, preterm birth, miscarriage, and ectopic pregnancy [ 1 ]. Despite increasing public awareness on the detrimental effects of MSDP, the proportion of women who smoke during pregnancy is still high in some countries. The global prevalence of MSDP was estimated to be 1.7% in 2015, and it varied substantially across regions, being the most prevalent in Europe (8.1%) and Americas (5.9%) and the least prevalent in Africa (0.8%) and Eastern Mediterranean (0.9%) [ 2 ].
Maternal smoking and risk of type 1 diabetes in the offspring
Type 1 diabetes represents less than 5% of the total cases of diabetes globally, but it is one of the most common chronic diseases of childhood [ 3 ]. In contrast to many other health outcomes, several large cohort studies suggested that exposure to maternal smoking may decrease the risk of type 1 diabetes in the offspring [ 4 , 5 , 6 ], although this was not confirmed by all studies [ 7 ]. However, observational studies are limited by issues of potential confounding. The observed inverse association between MSDP and offspring risk of type 1 diabetes in cohort studies is prone to bias due to unmeasured confounders, such as shared genetic and early life environmental factors within families. These factors are frequently correlated with MSDP to influence offspring health outcomes.
To control for potential confounders from unmeasured familial background factors, apart from a traditional cohort study design, Wei et al. [ 8 ] used a family-based, nested case-control study by matching children with type 1 diabetes to their siblings and cousins who were free of diabetes. Sibling and cousin comparisons are quasi-experimental designs, which use design features to help minimise confounding effects from genetic and environmental factors that are shared by family members [ 9 ]. The study by Wei et al. included about three million children born in Sweden between 1983 and 2014 and followed them until 2020. The overall proportion of children exposed to MSDP in this study population was high, with a prevalence of 15.7%. The study found that children exposed to MSDP had a 22% (95% CI: 18%, 25%) reduced risk of developing type 1 diabetes during childhood compared to their unexposed siblings. Similar association was observed in cousin comparison analysis (odds ratio: 0.72, 95% CI: 0.66–0.79) as well as in cohort analysis (hazard ratio: 0.78, 95% CI: 0.75–0.82). For comparison purpose, the authors also reported the association between MSDP and offspring type 2 diabetes using the same study designs. The findings showed that there was an increase in risk of type 2 diabetes in children exposed to MSDP in cohort analysis, but this association was attenuated to null in sibling analysis, indicating confounding effects of unmeasured familial factors. Mechanisms through which MSDP influences type 1 diabetes in the offspring are not clear and needs further investigation. One possible explanation is the immunosuppressive effects of nicotine, which may prevent the development of autoimmune diseases, such as type 1 diabetes, by promoting anti-inflammatory processes.
Maternal smoking and adverse health outcomes
Despite evidence demonstrating a benefit effect of MSDP on offspring type 1 diabetes risk, MSDP should never be considered as intervention to prevent type 1 diabetes in the offspring because of its many other adverse health effects on both children and mothers [ 1 , 10 ]. An umbrella review showed that MSDP is associated with increased risks of 20 infant-related and seven mother-related health conditions [ 1 ]. Most of these association are very likely to be causal and some can have long-term impacts across different phases of life from perinatal to adulthood. MSDP is associated with 3-fold increased risk of sudden infant death syndrome, 2-fold increased risk of asthma, and 1.5-fold increased risk of low birth weight, stillbirth, and obesity in the offspring. Women who are exposed to smoking during pregnancy are estimated to have 2.5-fold increased risk of spontaneous miscarriage in assisted reproduction and ectopic pregnancy. Since MSDP is a modified risk factor, its short-term and long-term detrimental effects are avoidable. This underscores the importance of continuous prevention and intervention efforts to reduce prevalence of MSDP and increase smoking cessation earlier before pregnancy, which would significantly benefit not only mother’s own health but also offspring outcomes.
Conclusions
The study by Wei et al. [ 8 ] provided strong evidence of a lower risk of type 1 diabetes for children exposed to MSDP as compared to unexposed children, by using family-based designs of sibling and cousin comparison analyses. However, this should not preclude the development and implementation of tobacco control policies in pregnant women. Family-based, quasi-experimental designs improve causal inference by reducing unmeasured confounding bias from genetic and early environmental risk factors and is therefore a useful approach in studying the associations between maternal exposures and offspring outcomes. Further research is warranted to understand the mechanisms linking MSDP and type 1 diabetes in the offspring.
Availability of data and materials
Not applicable.
Avşar TS, McLeod H, Jackson L. Health outcomes of smoking during pregnancy and the postpartum period: an umbrella review. BMC Pregnancy Childbirth. 2021;21(1):254. https://doi.org/10.1186/s12884-021-03729-1 .
Article PubMed PubMed Central Google Scholar
Lange S, Probst C, Rehm J, Popova S. National, regional, and global prevalence of smoking during pregnancy in the general population: a systematic review and meta-analysis. Lancet Glob Health. 2018;6(7):e769–76. https://doi.org/10.1016/s2214-109x(18)30223-7 .
Article PubMed Google Scholar
Green A, Hede SM, Patterson CC, Wild SH, Imperatore G, Roglic G, et al. Type 1 diabetes in 2017: global estimates of incident and prevalent cases in children and adults. Diabetologia. 2021. https://doi.org/10.1007/s00125-021-05571-8 .
Begum M, Pilkington RM, Chittleborough CR, Lynch JW, Penno M, Smithers LG. Effect of maternal smoking during pregnancy on childhood type 1 diabetes: a whole-of-population study. Diabetologia. 2020;63(6):1162–73. https://doi.org/10.1007/s00125-020-05111-w .
Haynes A, Cooper MN, Bower C, Jones TW, Davis EA. Maternal smoking during pregnancy and the risk of childhood type 1 diabetes in Western Australia. Diabetologia. 2014;57(3):469–72. https://doi.org/10.1007/s00125-013-3122-7 .
Article CAS PubMed Google Scholar
Edstorp J, Lampousi AM, Carlsson S. Parental smoking, type 1 diabetes, and islet autoantibody positivity in the offspring: a systematic review and meta-analysis. Diabet Med. 2022;39(6):e14830. https://doi.org/10.1111/dme.14830 .
Article CAS PubMed PubMed Central Google Scholar
Mattsson K, Jönsson I, Malmqvist E, Larsson HE, Rylander L. Maternal smoking during pregnancy and offspring type 1 diabetes mellitus risk: accounting for HLA haplotype. Eur J Epidemiol. 2015;30(3):231–8. https://doi.org/10.1007/s10654-014-9985-1 .
Wei YW, Andersson T, Edstorp J, Löfvenborg JE, Talbäck M, Feychting M, et al. Maternal smoking during pregnancy and type 1 diabetes in the offspring: a nationwide register-based study with family-based designs. BMC Medicine. 2022. https://doi.org/10.1186/s12916-022-02447-5 .
D'Onofrio BM, Lahey BB, Turkheimer E, Lichtenstein P. Critical need for family-based, quasi-experimental designs in integrating genetic and social science research. Am J Public Health. 2013;103 Suppl 1(Suppl 1):S46–55. https://doi.org/10.2105/ajph.2013.301252 .
Article Google Scholar
Howell MP, Jones CW, Herman CA, Mayne CV, Fernandez C, Theall KP, et al. Impact of prenatal tobacco smoking on infant telomere length trajectory and ADHD symptoms at 18 months: a longitudinal cohort study. BMC Medicine. 2022;20(1):153. https://doi.org/10.1186/s12916-022-02340-1 .
Download references
Acknowledgements
Author information, authors and affiliations.
Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Hong Kong, Special Administrative Regional, People’s Republic of China
Hongjiang Wu & Andrea O. Y. Luk
You can also search for this author in PubMed Google Scholar
Contributions
Both authors contributed to the content and writing of the commentary. Both authors read and approved the final manuscript.
Corresponding author
Correspondence to Hongjiang Wu .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
H Wu is an editorial board member of BMC Medicine. A Luk declares no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Wu, H., Luk, A.O.Y. Maternal smoking during pregnancy and health outcomes in offspring. BMC Med 20 , 296 (2022). https://doi.org/10.1186/s12916-022-02507-w
Download citation
Received : 01 August 2022
Accepted : 01 August 2022
Published : 08 August 2022
DOI : https://doi.org/10.1186/s12916-022-02507-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Maternal smoking
- Type 1 diabetes
- Perinatal epidemiology
BMC Medicine
ISSN: 1741-7015
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Login Admin
- eTheses Home
- Depositing eTheses
- About Nottingham eTheses
- Nottingham ePrints
Background Smoking during pregnancy is the leading modifiable risk factor for poor maternal and infant health outcomes. Pregnancy-related health problems associated with smoking during pregnancy include complications during labour, increased risk of miscarriage, premature birth, stillbirth and low birthweight. Despite this, around 12% of pregnant women in the United Kingdom (UK), 13% in the United States and 20% in France continue to smoke during pregnancy. A Cochrane review of 136 studies found that nicotine replacement Therapy (NRT) is proven to be effective amongst non-pregnant smokers, however a Cochrane review of eight studies found its efficacy in pregnancy to be uncertain. It is unclear whether we can ascertain a conclusion from this review as it may be subject to error due to repetitive testing, furthermore there may be insufficient power in the meta-analyses. Trial Sequential Analysis (TSA) is a method which could overcome these issues. This thesis provides an overview of TSA and applies the method to a systematic review of NRT use in pregnancy. This thesis also presents an alternative use for TSA, where it can be used for trial sample size estimation. In most studies investigating NRT use for smoking cessation in pregnancy, women are instructed to discontinue use of nicotine patches if they have even brief smoking lapses. This is due to concerns that concomitant smoking and NRT use could increase exposure to nicotine and potentially more tobacco smoke toxins if they smoke heavily when using NRT. In 2014, the ‘Study of Nicotine Patch in Pregnancy’ (SNIPP) trial, a large randomised controlled trial (RCT) investigating NRT used in pregnancy for smoking cessation reported that it did not increase either smoking cessation rates or birth weights. This study was unique as participants were told that they could continue using nicotine patches during smoking lapses. Using data from this trial, this thesis aims to explore whether concurrent smoking and NRT use resulted in changes in nicotine intake as well as smoking behaviour. This thesis also uses this trial to explore whether NRT use and changes in expired air carbon monoxide throughout pregnancy have an impact on birthweight. Methods Systematic review and meta-analysis To determine the efficacy and safety of NRT for smoking cessation in later pregnancy, systematic review methods were used following standard Cochrane methods. The primary outcome was smoking cessation at the latest time point in pregnancy at which this was measured, and secondary outcomes were safety related. Meta-analyses were conducted where appropriate. Trial Sequential Analysis Trial sequential analysis was used to investigate whether there is sufficient evidence available to come to a firm conclusion on the efficacy of nicotine replacement therapy in pregnancy. Trial Sequential Analysis is a methodology that can be used in systematic reviews and meta-analyses to control random errors, and to assess whether further trials need to be conducted. We employ this method to the data from the systematic review, to assess whether there is sufficient evidence to conclude a clinically important treatment effect, no evidence of an effect, or lack of evidence. This thesis goes on to explain an alternative use for Trial Sequential Analysis, where it can be used to estimate trial sample sizes for one or more trials investigating a behavioural smoking cessation intervention. We show how data from a new, planned trial can be combined with data from the earlier trials using Trial Sequential Analysis to assess the intervention’s effects. Using feasibility and pilot trials of a behavioural smoking cessation intervention, data are combined to estimate the sample size that one or more future RCTs would need to recruit, to provide a more decisive answer regarding intervention benefit. Analysis of the SNIPP trial The final study in this thesis used data from 402 women recruited to the SNIPP trial. Paired t-tests, linear regression, interaction tests, and within-individual variability analysis techniques were employed to answer the following questions: (1) does concurrent smoking and NRT use result in changes in nicotine, and other indicators of smoking intensity?; (2) do these changes differ between NRT or placebo patch use?. Results Systematic review and meta-analysis Compared to placebo and non‐placebo controls, there was low‐certainty evidence that NRT increased the likelihood of smoking abstinence in later pregnancy (RR 1.37, 95% CI 1.08 to 1.74; I² = 34%, 9 studies, 2336 women). There was unclear evidence of an effect in placebo‐controlled RCTs (RR 1.21, 95% CI 0.95 to 1.55; I² = 0%, 6 studies, 2063 women), whereas non‐placebo‐controlled trials showed clearer evidence of a benefit (RR 8.55, 95% CI 2.05 to 35.71; I² = 0%, 3 studies, 273 women). Trial Sequential Analysis The meta-analysis was not adequately powered to provide a strong conclusion, and TSA estimates that further placebo-controlled trials with approximately 10,741 participants in total are needed to arrive at a firm conclusion. Analysis of the SNIPP trial (1) In the nicotine patch group, there was no change in saliva cotinine concentrations between baseline and 2-weeks post quit date (ratio of geometric means = 0.94, 95% CI=0.83 to 1.07; p=0.37, Bayes factor=0.15). However, there was a reduction in reported number of daily cigarettes smoked (mean difference -6, 95% CI’s -7 to -5, p<0.001) and in CO concentrations (mean difference -3.0ppm, 95% CI’s -4.2 to -1.9, p<0.001). (2) These changes were not significantly different from changes in the placebo group except for cigarette consumption which reduced more in the placebo group (p=0.046). Conclusions • NRT used for smoking cessation in pregnancy may increase smoking cessation rates in late pregnancy. However, this evidence is of low certainty, as the effect was not evident when potentially biased, non‐placebo‐controlled RCTs were excluded from the analysis. • According to TSA, there is uncertainty regarding the efficacy of NRT use for smoking cessation during pregnancy compared to control, and further placebo-controlled trials are needed to arrive at a firm conclusion. • Although TSA suggests more research is required for a firm conclusion, the general trend appears that NRT as it has previously been trialled, may not be effective for smoking cessation in pregnant women. Further trials should focus on what can be done differently in future. For example, using higher dose NRT or encouraging better adherence to treatment may produce more positive outcomes. • Our findings suggest that when pregnant women use nicotine patches as part of a quit attempt, but they also smoke, they smoke less than they did before the quit attempt started. This means that their exposure to the toxic products of burnt tobacco is reduced. • Despite having similar cotinine exposure to that from cigarette smoking, pregnant women who use nicotine patches and smoke, smoke less and exhale less CO, so their exposure to other tobacco smoke toxins is likely to be lower too.
Actions (Archive Staff Only)
- Study protocol
- Open access
- Published: 12 May 2021
Stress- and smoke free pregnancy study protocol: a randomized controlled trial of a personalized eHealth intervention including heart rate variability-biofeedback to support pregnant women quit smoking via stress reduction
- Willeke van Dijk 1 ,
- Mirjam Oosterman 2 ,
- Imke Jansen 1 ,
- Wieke de Vente 3 &
- Anja Huizink 1
BMC Public Health volume 21 , Article number: 905 ( 2021 ) Cite this article
3175 Accesses
7 Citations
1 Altmetric
Metrics details
Maternal smoking and stress during pregnancy are associated with adverse health effects for women themselves and are risk factors for adverse developmental outcomes of the unborn child. Smoking and stress seem to be intertwined in various ways. First, the majority of smoking pregnant women is of lower socio-economic status, which is associated with higher levels of perceived stress. Second, smoking women often report to smoke because they feel stressed. Third, quitting smoking often increases perceived stress levels initially. Therefore, effective interventions are needed to support women with smoking cessation by reducing stress. The aim of this study is to test the effectiveness of an eHealth intervention on stress reduction and smoking cessation.
Methods/design
The Stress- and Smoke Free Start of Life (SSFSL) study is a randomized controlled trial (RCT) comparing a personalized eHealth intervention with a control condition. Inclusion criteria for the women are: (1) > 18 years of age, (2) < 28 weeks pregnant at recruitment, (3) currently smoking. Consenting participants will be randomly assigned to the intervention or control group. Participants allocated to the intervention group will receive an 8-week intervention delivered on their smartphone. The application includes psycho-education on pregnancy, stress, and smoking (cessation); stress-management training consisting of Heart Rate Variability-biofeedback; and a personalized stop-smoking-plan. Participants in the control condition will be invited to visit a webpage with information on pregnancy, stress, and smoking (cessation). Study outcomes will be collected via online questionnaires, at four timepoints: pre-intervention (baseline; t0), post-intervention (8 weeks + 1 day after t0; t1), follow up at two weeks after birth (t2), and follow up at three months after birth (t3). The primary outcome measure is self-reported smoking cessation. Secondary outcomes include daily self-reported number of cigarettes smoked, perceived stress, pregnancy experience, birth outcomes, and negative affectivity scores of the baby. Moreover, the mediating effect of stress reduction on smoking cessation will be examined, and possible moderators will be tested.
If the eHealth intervention is effective in smoking cessation among pregnant smoking women, it can be implemented as a tool into the health care in the Netherlands.
Trial registration
Netherlands Trial Register, ID: NL8156 . Registered on 11 November 2019.
Peer Review reports
Introduction
Worldwide, substantial numbers of women who smoke, continue to smoke during pregnancy. Recently estimated prevalence of smoking pregnant women in the European Region was 8.1%, which was the highest percentage when compared to the world average of 1.7% [ 1 ]. Even though prevalence rates of pregnant smoking women have been decreasing over the years [ 2 ], these numbers are yet of concern for several reasons. Maternal smoking has serious implications for the developing fetus and the mother. Maternal smoking increases the risk of miscarriage [ 3 ], premature delivery, lower birth weight [ 4 ], and congenital abnormalities [ 5 ]. Among the single newborns born in the Netherlands with small-for-gestational age, 17% of the cases was found to be related to maternal smoking during pregnancy [ 6 ]. Negative effects of prenatal maternal smoking are not only observed in the early years of the child, but have also been associated with negative health outcomes later in life, such as asthma [ 7 ] and obesity [ 8 ]. Moreover, maternal smoking during pregnancy has been linked to reduced academic achievement and decreased cognitive abilities of children [ 9 , 10 ] and to neurobehavioral problems in childhood [ 11 , 12 , 13 ].
Despite the growing body of evidence on the negative consequences of prenatal smoking for child development and despite extensive efforts to reduce the prevalence of prenatal smoking, a relatively large group of expecting mothers continues to smoke during pregnancy [ 1 ]. Factors associated with continued smoking during pregnancy are socioeconomic status (SES), nicotine dependence, and stress experienced during pregnancy [ 14 , 15 ]. In general, women of low SES experience socioeconomic inequalities when it comes to financial resources, stability in living situation, general health, including more stress-related health problems and unhealthy lifestyle habits such as smoking [ 16 , 17 ]. Stress plays an important role in initiating smoking and maintenance of smoking [ 18 , 19 ]. According to the stress-coping model, smoking causes a temporarily reduction of negative affect and an increase in positive affect, which can be an explanation for the use of smoking as a coping strategy in situations of high stress [ 20 ]. Neurobiological models of addiction suggest that both stress and nicotine exposure trigger stress and reward circuits in the brain explaining why stress can increase the urge to smoke [ 18 ]. Moreover, high levels of stress hinder changing unhealthy lifestyle behaviors, such as smoking [ 21 ]. Increased activation of brain stress systems are thought to reduce an individual’s ability to cope with extra stressors and may strengthen the effects of acute nicotine exposure during abstinence, thereby contributing to the increased vulnerability to relapse caused by stress during periods of abstinence [ 19 ]. Elevated stress levels may thus explain why expecting women may fail to quit smoking, because pregnancy is a critical life event. Indeed, during pregnancy, many women experience some additional levels of psychosocial and pregnancy-related stress [ 22 ], due to physical changes related to pregnancy, concerns about childbirth and parenting, or relationship difficulties. These extra pregnancy related stressors can thus make smoking cessation even more challenging.
While stress thus seems to be a maintaining factor of smoking, stress experienced during pregnancy itself has also been associated with adverse offspring outcomes. Research has shown that prenatal stress is associated with delayed motor and mental development, and temperamental variation in infants and older children [ 23 , 24 , 25 , 26 ]. In addition, pregnancy-specific anxiety predicted more difficult child temperament, as indicated by negative emotional reactivity, fearfulness and falling reactivity [ 27 ].
Thus, as prenatal stress and smoking are both related to negative child outcomes, and stress seems to play a role in the maintenance of smoking, interventions targeting both stress and smoking may be highly effective. Unfortunately, existing programs aimed at smoking cessation have been mostly ineffective with high relapse rates, specifically among vulnerable women with low SES and high levels of perceived stress [ 14 , 28 , 29 ]. An important element that is missing in most smoking cessation programs thus far, is stress management. This element seems essential for smoking cessation programs, especially those targeting low SES women, since effective stress-coping techniques may help women quit smoking during pregnancy and reduce long-term relapse risk. Investing in relapse prevention is important, because relapse within the first six months after birth is common, with a pooled mean proportion of women that have re-started smoking at 6 months of 43% [ 30 ].
A method that has shown to reduce stress is Heart Rate Variability-biofeedback (HRV-BF) [ 31 ]. HRV is determined by the variation in time between consecutive heartbeats as assessed by the interval between successive R-wave peaks in an electrocardiogram (ECG). Higher HRV has been associated with more favorable physical and mental health outcomes [ 32 , 33 , 34 ]. The aim of HRV-BF is to increase HRV and stimulate cardiac regulation [ 35 ]. HRV is influenced by physiological factors such as respiration, and respiratory sinus arrhythmia (RSA) is the component of HRV that reflects the variation in heart rate due to respiratory activity [ 35 ]. A mechanism that regulates cardiac activity is the baroreflex [ 36 ], which activity is triggered by changes in blood pressure in the body’s large blood vessels in order to achieve homeostasis [ 35 ]. Physiological activity, such as breathing, affect baroreflex functioning [ 34 ]. Through HRV-BF, people learn a method of paced breathing with visual feedback about their HRV in order to detect the frequency at which HRV is maximized. Maximized HRV causes increases in baroreflex amplitude, which can improve reflex efficiency and, in turn, can positively affect autonomic activity modulation [ 36 ].
Recent research of our team showed that HRV-BF training was effective in reducing feelings of stress in pregnant and non-pregnant women [ 37 ]. These results are in line with a meta-analysis, including community and clinical adult samples, reporting that HRV-BF was associated with a large reduction of self-reported stress and anxiety [ 38 ]. HRV-BF may be regarded as an effective stress-reduction intervention, and may be beneficial for pregnant women to support them quit smoking while suffering from stress.
During the last decades, more and more health care is being offered via the Internet, for instance by means of eHealth platforms [ 39 , 40 ]. Benefits of eHealth are the high accessibility for a large group of people and the usually low cost it entails. Moreover, eHealth is unrestricted by time and place and might reach populations that otherwise do not ask for support, for instance as a result of possible stigmatization. As stigmatization is a common problem among pregnant smoking women [ 41 ], and considering the many benefits of eHealth for this group [ 42 ], an app-intervention to support pregnant women quit smoking is promising. A recent systematic review supports this, as results show that eHealth approaches were moderately effective on smoking abstinence in a general population of adult smokers [ 43 ]. They also emphasize the use of an interactive and tailored approach of smoking cessation interventions.
Considering the benefits of eHealth, we have developed an application including HRV-BF, targeting pregnant women who want to quit smoking. HRV-BF is easy to learn and maintain in daily life, and at the same time requires little efforts. Thus, HRV-BF could be highly appropriate for pregnant smoking women with low SES, who would like to quit smoking. In order to gain knowledge about the underlying mechanisms and conditional factors of treatment effectiveness, various factors that are associated with stress and smoking need to be studied. Factors that may affect the treatment effect obtained in stress reduction and smoking cessation are: pregnancy experience [ 21 ], pregnancy-related anxiety [ 44 ], self-efficacy regarding smoking cessation [ 45 ], a person’s motivation to quit smoking [ 46 ], and COVID-19 related stress [ 47 ]. Moreover, focusing on indirect treatment effects is also relevant in this transitional and developmental context. For example, factors associated with parenting, such as parenting self-efficacy [ 48 , 49 , 50 ] and the bond that the mother feels with her child [ 51 , 52 ] may also increase when women experience less stress and quit smoking. Parenting factors such as parenting self-efficacy and a warm mother-child bond have been associated with positive effects on child development (e.g. [ 48 , 53 ]).
The current study
The current study will examine the effectiveness of a personalized 8-week lasting eHealth intervention “Together with Eva”, consisting of adapted parts of existing quit-smoking and stress-management interventions and an HRV-BF prototype application by means of a randomized controlled trial. “Together with Eva” includes psycho-education on stress, smoking (cessation), and pregnancy; a personalized stop-smoking plan; stress reducing exercises, comprising of HRV-biofeedback, and problem-solving techniques. Participants in the control group will receive access to a webpage developed for this study where they can read information comparable to the psycho-education of the intervention group. This information is however summarized and not interactive. Overall, the main research question of this study is the following:
Is the app intervention “Together with Eva” an effective intervention to support pregnant women to quit smoking during pregnancy and stay abstinent after giving birth? The primary outcome measure is self-reported smoking cessation. Secondary objectives of this study are to investigate whether;
the intervention is effective on secondary outcomes (i.e. smoking behavior, perceived stress, birth outcomes, negative affectivity, parenting self-efficacy, pregnancy-related anxiety, feelings of mother-child bonding, and COVID-19 related stress),
the intervention effect on smoking cessation is mediated by the reduction of perceived stress, and whether;
variables such as program usage and experience, self-efficacy regarding smoking cessation, intrinsic motivation for life style behavior change, and pregnancy experience moderate the treatment effect.
Methods and analysis
Trial design.
This study is a two-arm, parallel, single blinded randomized controlled trial (RCT) including four measurement waves: pre-intervention or baseline (t0), post-intervention (t1), two weeks following birth (t2) and three months after birth (t3). Pregnant, smoking women will be randomized to one of two groups: 1) personalized eHealth intervention (“Together with Eva”) on the smartphone followed for 8 weeks; or 2) active control condition which includes psycho-education on stress, smoking, and pregnancy through a website. The protocol is developed in accordance with the Standard Protocol Items: Recommendations for Interventional Trials guidelines (SPIRIT; Fig. 1 ). The trial is registered with the Netherlands Trial Register on November 112,019. The registration ID is NL8156. Ethical approval was obtained from the Medical Ethical Committee of the VU Medical Center (METc VUmc 2020/065/NL71342.029.20).
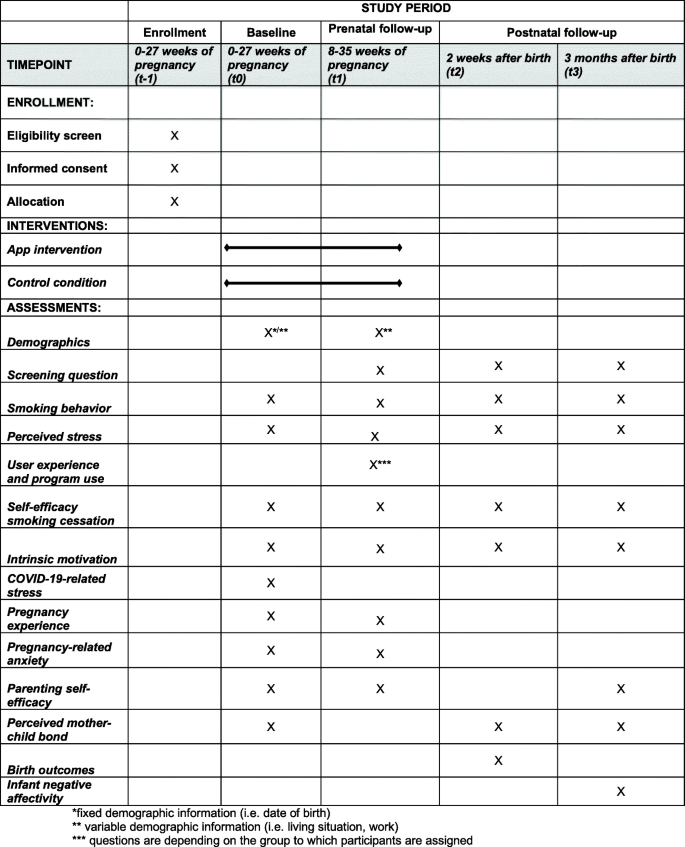
SPIRIT Flow Diagram of the Stress And Smoke Free Pregnancy Study
Participants and recruitment procedure
Participants will be recruited through midwifery practices, specialized departments of hospitals in the Netherlands and through advertisements on reliable websites focusing on pregnancy over a 1.5-year period (July 2020–February 2022). Health care professionals will be offered flyers to hand out to potential participants. Additionally, they will receive an information sheet with the inclusion criteria, short sentences for study explanation, and a list of frequently asked questions so that they can easily explain the study to potential participants. Women are eligible for participation if they are older than 18 years, are less then 28 weeks pregnant (to enable intervention completion in view of the risk for premature birth in pregnant smokers), if they smoke on a regular basis (at least smoking 1 day/week), have an intention to quit smoking during pregnancy, and if they have sufficient command of the Dutch language. Women with severe psychiatric disorders, women who abuse other drugs (e.g. alcohol, cannabis, cocaine, and GHB), and who are participating in other intensive stop-smoking interventions will be excluded from participation. The focus of our recruitment will be on women with low SES. However, women with high SES are also allowed to participate.
Sample size calculation
To ensure acceptable power for the main analysis of the intervention effect on smoking cessation at post-intervention, an a priori sample size calculation was performed by the program G*Power [ 54 ]. Using a power of 80% an alpha of 0.05 and an F-test, we need to include 160 participants (80 participants in each arm) in order to be able to detect a small difference (effect size of 0.20) between the intervention group and the control group on the primary outcome measure, smoking cessation at t1.
The participants will be informed about the study via obstetric practices, gynecologists, or general practitioners, but can also sign up for participation by own registration via a form on the project’s website. Pregnant women, who are visiting their health professional, will be given a brief explanation via a flyer. Women, who are willing to participate, will be asked to scan the QR-code on the flyer and will then be directed to an online registration form where they will fill in the contact details. This information will then be sent to a secured mailbox which can only be accessed by the main researcher. Subsequently, registered participants will be called by the researcher or research assistant who will explain the study in more detail. If participants are willing to participate they will be sent an information letter and consent form, which they will be asked to read, sign and send back. Then, women will be called to screen for exclusion criteria. Eligible women will receive an invitation email with a link to the first online questionnaire (t0). Participants will be assigned an identification number, which will be used throughout the study. To ensure confidentiality, the file including both the participants’ names and identification numbers will be saved with a password that can only be accessed by the principal researcher. After completion of the t0 questionnaire, participants will be randomized. This will be done by sending an email to an independent researcher who has access to the randomization list. The randomization list is created with blocks of 2, 4, and 6 participants, each containing equal numbers of participants allocated to the intervention and active control group, using an online randomization program. The independent researcher will send an email with information about the assigned group. Participants assigned to the intervention group receive an email with a link to download the application on their smartphones. The control group will be sent a link to the website with the psycho-education module, which they can access as frequently as they desire. After 8 weeks, participants will receive an invitation to complete the second questionnaire (t1). Two follow-up questionnaires will be sent, the first at two weeks after birth (t2) and the other at three months after birth (t3). Participants will receive 7.50 euros on their bank account for each completed questionnaire.
Intervention
The eHealth intervention “Together with Eva” of the current study consists of an 8 weeks lasting intervention offered on the participant’s smartphone. The intervention consists of an integration of an existing smoking cessation online intervention ( www.mijnkoersroken.nl ; developed by Trimbos institute) and an HRV-BF prototype application, Time2Breathe [ 55 ]. The smoking cessation intervention was adapted and personalized, and the HRV-BF prototype application was further developed by Aan Zee into an application including the actual HRV measurement and feedback Software Development Kit (SDK), developed by Happitech. Participants use the application at their own pace. The application consists of different components (see below), which will be supported by short instruction videos to explain the intended use.
Personalized stop-smoking plan
After registration in the app, participants will design a personalized stop-smoking plan in which the participant chooses a specific date on which she wants to quit smoking. Moreover, intrinsic motivation for the intended quitting attempt is enhanced by listing personal advantages of quitting and disadvantages of smoking. These lists will be referred to in the exercises in the app. Participants will also designate a person in their social network, who can be approached for help and support in times of craving. The phone number of this person will be saved in the app. When participants click on the button ‘I would like to call someone’, they will immediately be directed to call this number.
Smoking diary
During the 8 weeks of the intervention, participants will receive daily notifications to fill out their diary. In this diary, participants will report if and how many cigarettes they have smoked. This self-report on their smoking behavior will be used to enable positive feedback. For instance, if a woman has been able to refrain from smoking for a day, a week, and so on, she will receive a positive notification such as “Yes, keep it up!”. Participants can navigate through the diary, which gives a clear overview of the quitting process.
Personalized stress reduction training
Another element of the intervention is a personalized stress-reduction training. This training consists of two components: (a) HRV-BF, and (b) psycho-education including personalized and evidence-based problem-solving techniques.
The HRV-BF module in the “Together with Eva”-app teaches the participant the technique of slow-paced diaphragmatic breathing through guided breathing exercises. Breathing in synchrony with the baroreflex, also called resonance frequency, results in a substantial increase in HRV [ 36 ]. In the first week of the intervention, participants are instructed to exercise slow diaphragmatic breathing, also called ‘belly-breathing’, without using their phones. From the second week of the intervention onwards, breathing exercises are guided by a breath pacer and visual feedback based on continuous HRV measurement developed by Happitech (Heart Rhythm SDK version 1 Happitech). HRV is measured through photo plethysmography (PPG) with use of the camera and flash on the mobile phone. Throughout the 8-week intervention period, the participant’s resonance frequency is assessed four times using a series of breathing exercises at the following fixed paces of 6.5, 6.0, 5.5, 5.0, 4.5 breaths/min, which is in accordance with resonance frequency protocols (e.g. [ 35 ]). The pace resulting in the highest frequency power peak in the low frequency (LF) power band (i.e., 0.04–0.15 Hz) relative to the total power, defined as the 0.02–0.5 Hz power band of HR data, is considered resonance frequency and is the advised breathing pace for the participant. During the regular breathing exercises, the participant is guided towards optimal breathing through the color of the breath pacer, which turns dark purple as the LF power peak increases (see Fig. 2 ). Panel 1 shows the instruction to breathe in; panel 2 instructs to hold the breath for 1 s; panel 3 instructs to breathe out; and panel 4 shows the adjustment of the color, becoming darker purple, when breathing more in synchrony with one’s baroreflex, which is the desired result of the breathing exercise. Breathing exercises are practiced twice a day at a time convenient for the participant. In the first week, breathing exercises last 5 min, whereas from the fourth week onwards, they last 15 min. After performing a breathing exercise, participants are asked about their experience, as indicated by a happy, neutral, or sad smiley, and whether they felt dizzy or nauseous during the exercise. The daily duration of the paced breathing with HRV-BF and the information about one’s experience will become visible in the diary.

Screenshots of Instructions for Breathing in the HRV-BF Module of the Application
Participants assigned to the intervention group will receive a phone call from the researcher or research assistant directly after randomization. The researcher will then instruct the participants to fill out the stop plan and will discuss when the participant is planning to use the app and do the breathing exercises. Participants are instructed to start using to app on that same day or the next day. Based on this schedule, researchers will have weekly phone calls with the participants to monitor the planning and, if necessary, adapt it for the following week.
Psycho-education on pregnancy, relaxation, and smoking cessation including personalized and evidence-based problem-solving techniques
A psycho-education module, called ‘Lessons’, offers participants written information about pregnancy, stress and relaxation, and smoking and smoking cessation. The lessons about pregnancy provide written information that matches with the gestational age of the unborn (estimated date of birth filled in at registration). In the lessons about stress and relaxation, participants can for instance read about what stress does to the body and mind, tips on stress management and how feelings of tension can be reduced, which is supported by evidence-based problem-solving exercises. With these problem-solving techniques, women can address their own problems or stressful situations, with guidance from the eHealth application. An important first step in this stress reduction is making a stress diary using notes written with pencil and paper, to identify stressful situations and problems. Next, women will be asked to make a clear difference between problems that can be solved and those that cannot be solved, but need to be accepted. Acceptance and tolerance for the unsolvable problems and associated emotions are boosted by building awareness that emotions related to these problems will pass. Then, active problem-solving techniques are built up in subsequent steps to deal with the potentially solvable problems: (1) the identification of a stressful situation or problem that causes stress that may be solvable, (2) the definition of one’s goal and how the problem hampers that goal, (3) finding solutions, also through seeking social support, (4) planning solutions, (5) implementing solutions, (6) taking stock: reviewing one’s situation again and see if there is a continuous need to deal with the problem more actively. In the lessons about smoking cessation, information about the effects of stopping smoking cigarettes on your body and mind is provided, and participants receive tips to refrain from smoking. Also, frequently asked questions about smoking and quitting will be discussed. New lessons will appear every week. If participants do not use the application as intended, they will be reminded by a text message, or a phone call if needed. Contact information of the researcher and of a person independent of the study is mentioned under ‘Contact’ so that the participants will always be able to ask questions if things are unclear or if they need help with exercises. Questions will be answered within 24 h on weekdays.
The craving-button and the smoked-button
A craving button ‘Help, I feel the urge to smoke’ is included on the home-screen of the app, which can be pressed when the participants expriences craving. When participants click this button, they will be asked several questions to find out what kind of information or exercise is desired to provide them with distraction and/or support to resist the sense of craving. Participants will either be recommended to talk to someone (i.e. social support that they have designated in their stop plan), to do something relaxing or distracting (e.g. a slow breathing exercise, going outside), or to read something (psycho-education modules). Furthermore, the button ‘Oops, I have smoked’ will help participants to deal with negative thoughts after they have smoked.
Control condition
The control condition consists of a website designed specifically for this study consisting of psycho-education on pregnancy, smoking during pregnancy, and stress. The information on the website is a summary of the psycho-education modules on stress, smoking and smoking cessation, and pregnancy included in the app-intervention. Since the website does not include exercises, it is less personalized, less intensive and interactive when compared to the intervention. This webpage can be visited as often as the woman desires and can be accessed on smartphone or computer.
Outcome measures
At four different time points, women will receive online questionnaires. Each questionnaire will take approximately 15–20 min to complete. The first questionnaire is a baseline questionnaire (t0, before randomization) including the main outcomes and demographic information (i.e. date of birth, weeks of pregnancy, ethnicity, educational level, employment status, relationship status, smoking behavior partner, living conditions). Eight weeks and one day after group assignment, participants will receive the second questionnaire (t1), through which post-intervention measurements will take place. Follow-up questionnaires will be sent two weeks after birth (t2) and three months after birth (t3). In Fig. 1 , an overview of the outcome measurements for each time point is depicted. Prior to t1, t2, and t3, a screening question will appear asking about the health of the baby. In case of pregnancy loss or if the baby died after birth, participants will be advised to quit the study participation. They will still receive the agreed compensation for the completed questionnaires.
Primary outcome
- Smoking cessation
Smoking cessation and abstinence will be assessed using a self-report question ‘Do you smoke?’ (yes/no). If answered with ‘yes’, an additional question asking whether she smokes on a daily basis will indicate the frequency of the smoking behaviour.
Secondary outcomes
Smoking behavior.
If participants indicate that they (still) smoke, they will be asked the number of cigarettes smoked per day. Thereby, it can be assessed whether the participant’s smoking behavior changed between the different time points.
Birth outcomes
Birth outcomes such as gender, birth date, gestational age and weight at birth will be assessed with self-constructed questions. Moreover, 1- and 5-min Apgar scores will be asked. These scores can be found in a booklet that women usually receive after delivery in the Netherlands. In case these scores are unknown to the women, consent will be asked to contact the midwifery practice in order to obtain these scores.
Infant temperament
Infant temperament will be measured at 3 months of age using the short version of the Infant Behavior Questionnaire (IBQ-R; [ 56 ]). From the total of 14 scales, the scales ‘Distress to Limitations’ and ‘Fear’, each consisting of 16 items, were used in this study. The mother will be asked to rate the frequency that her baby engaged in specific day-to-day behaviors during the previous week or two weeks on a scale from 1 (never) to 7 (always). Examples for items are ‘After sleeping, how often did the baby cry if someone doesn’t come within a few minutes?’ and ‘When introduced to an unfamiliar adult, how often did the baby refuse to go to the unfamiliar person?’. In a sample of Dutch mothers who reported on the behavior of their 4-month old babies, internal consistency of both the ‘Distress to Limitations’ and ‘Fear’ scale has been found to be good ( α = .75 and .77, respectively; [ 57 ]).
Pregnancy-related anxiety
Pregnancy-related anxiety will be assessed using the PRAQ-R2 [ 58 ], a short version of the Pregnancy-Related Anxiety Questionnaire (PRAQ; [ 59 ]), suited for both women pregnant for the first time, as for women who already have children. The PRAQ-R2 consists of ten items divided into the three subscales (1) Fear of birth (3 items), (2) Worries about Bearing a Physically or Mentally Handicapped Child and complications during and after childbirth (4 items), and (3) Concern about own Appearance (3 items). Examples of items are ‘I am worried about the pain of contractions and the pain during delivery’ and ‘I am worried about the fact that I shall not regain my figure after delivery’, which can be answered on a 5-item Likert scale ranging from 1 (‘Does not apply to me at all’) to 5 (‘Does apply to me a lot’). Higher scores indicate more pregnancy anxiety. Internal consistency of the questionnaire for both nulliparous and parous women is acceptable (Cronbach’s α = 0.82–0.85; [ 58 ]).
Parenting self-efficacy
Parenting self-efficacy will be assessed by the Dutch version of the Maternal Self-efficacy in the Nurturing Role Questionnaire (SENR; [ 60 ]) during and after the pregnancy. The SENR has both a prenatal and postnatal version and both versions contain 16 situations regarding (expected, for the prenatal version) feelings of competences in taking care of a child, such as ‘I feel competent in my role as a parent’ and ‘I feel like I am not prepared for parenthood’, answered on a 7-point Likert scale ranging from 1 (Completely disagree) to 7 (Completely agree). A total score will be generated by summing up scores from each item, with higher scores indicating higher parental self-efficacy. Internal reliability has been found to be high ( α = .86–.89; [ 61 ]).
Experienced stress
Experienced stress will be measured using the Dutch, short version of the Perceived Stress Scale (PSS; [ 62 , 63 ]). This scale consists of 10 items about subjective experiences of unpredictability, lack of control, and burden during the last month, e.g. ‘In the last month, how often have you been angered because of things that were outside of your control?’ Participants indicate on a 4-point scale from ‘never’ to ‘always’ to what extend the items apply to them. Higher scores indicate higher perceived stress. Items and answer categories have been adapted to make it more suitable for our target group. Previous studies reported acceptable to good internal consistency of the PSS (Cronbach’s α 0.78–0.91; [ 64 ]).
User experience
User experience of both the application ‘Together with Eva’ and the webpage ( https://www.fgb.vu.nl/nl/onderzoek/stress-en-rookvrij-zwanger ) of the control condition will be assessed with several self-constructed questions, such as ‘How many times have you used the app/webpage’, answered by a 5-point Likert scale ranging from 0 (Not much/Never) to 4 (Every day). If they indicate that they have not used the application, they will be asked for the reason. Moreover, attractiveness and ease of use of the application will be assessed with 6 items from the Dutch translation of the User Experience Questionnaire (UEQ; [ 65 ]). Participants will answer the question ‘Please indicate what you think about the app’ and ‘Please indicate what you think about the breathing exercises’, answered on a scale from 1 (negative) to 7 (positive), with varying specifications such as ‘not interesting/ interesting’ or ‘unattractive/attractive’. Participants from the intervention group will also be asked if they were motivated to do a breathing exercise when they were stressed and whether they would recommend the application to a pregnant friend who would like to quit smoking.

Self-efficacy regarding smoking cessation
Self-efficacy regarding smoking cessation will be assessed by six items from a questionnaire developed by [ 66 ]. Participants are asked to indicate whether they are able to refrain from smoking if they find themselves in specific situations, for instance when they feel stressed or tense and when someone offers them a cigarette. Questions can be answered by 0 (never) to 3 (always). Higher scores indicate higher self-efficacy regarding smoking cessation.
Perceived mother-child bond
Mother-child bond will be measured using six items derived from a questionnaire developed for Generations 2 study, which is based on the Treatment Self-Regulation Questionnaire (TSRQ; [ 67 ]). This questionnaire has a prenatal version asking about the future mother-child bond, and a postnatal version asking about the current bond of the mother and child. In the first question, the participant will be asked the extent to which she assumes a good relationship with her child is important, which can be answered by five categories ranging from 0 (does not apply to me at all) to 5 (does apply to me a lot). If the participant indicates that the relationship with her child is important to her (answer options 3–5), five items will appear asking about the reason for striving a good relationship. If the participant indicates that relationship with her child is not important (answering options 1–2), she will be asked whether she ever thinks about the relationship with her child. Higher scores indicate a stronger perceived mother-child bond. Internal consistency of the TSRQ was found to be acceptable [ 67 ].
Pregnancy experience
Pregnancy experience will be measured by the Dutch translation of the Pregnancy Experience Scale (PES; [ 68 ]), ‘Beleving van de Zwangerschapsschaal- verkorte versie’ [ 55 ]. Of the total scale, consisting of the two subscales Uplifts (positive pregnancy experiences) and Hassles (negative pregnancy experiences), only the 10-item Uplift scale will be used in this study. Examples of items are ‘How much the baby is moving’ and ‘Making or thinking about nursery arrangements’ and participants will be asked the extent to which a particular experience makes them happy, answered on a 4-item Likert scale ranging from 0 (not at all) to 4 (very much). Higher scores indicate a more positive pregnancy experience. The BZS-K Uplift scale has good internal consistency ( α = .83) and good intraclass correlation coefficients ( ICC = .72–.84; [ 55 ]).
Intrinsic motivation
Intrinsic motivation for behavior change will be asked by five questions based on questions from the Treatment Self-Regulation Questionnaire (TSRQ) [ 69 ]. Examples of questions are ‘I think that quitting smoking is best for my health’ and ‘I think that quitting smoking is best for the health of my baby’ answered on a five-point Likert scale ranging from (1 = totally disagree, 5 = totally agree). Higher scores indicate higher intrinsic motivation to quit smoking. Internal consistency of the TSRQ was found to be acceptable [ 69 ].
Level of addiction
The ‘Heaviness of Smoking Index’ (HSI; [ 70 ]) will be used to assess the level of smoking addiction of the women. The question on the number of cigarettes smoked on a day in combination with a question asking about the time to first smoking in the morning generates the ‘Heaviness of smoking index’. The question on time to first smoking can be answered with ‘within 5 minutes’ (3 points), ‘after 6-30 minutes’ (2 points), ‘31 to 60 min’ (1 point), or ‘after 60 minutes’ (0 points). To create a total HSI score, the answer to the question about the number of cigarettes smoked on a day are categorized in four groups and each group will be given a different amount of points: 0 (10 or fewer), 1 (11 to 20), 2 (21–30), 3 (31 or more). Scores of the two questions will be summed in order to obtain three levels of addiction; low addiction (score between 0 and 2), moderate addiction (3, 4), high addiction (5, 6).
COVID 19-related stress
COVID-19-related concerns will be assessed using self-constructed questions. Participants will be asked to answer questions such as “Have you been infected by the COVID-19 virus?” (yes/no), “How much do you worry about the COVID-19 virus?”, and “How much do you worry about your own health and the health of your unborn child?”, which can be answered on a scale from 0 (Not worried at all) to 10 (Extremely worried). From the scale questions a sum score will be calculated with higher scores indicating more COVID-19-related concerns.
History of psychological complaints
History of psychological complaints will be asked using three self-constructed questions, i.e. “Did you receive treatment for psychological complaints in the past year?” (yes/no), and if so, “For which complaints have you been treated?” and “Do you still experience complaints?” (yes/no). Answers to these questions will be categorized in 0 (never received treatment), 1 (history of treatment), 2 (currently experiencing complaints).
Depression symptoms
Depression symptoms will be assessed using the 7-item ‘Depression’ subscale of the Depression, Anxiety, Stress Scale-21 (DASS short form; [ 71 , 72 ]). Participants are asked to indicate the extent to which they had experienced specific symptoms over the previous week on a 4-point Likert scale ranging from 0 (Never) to 3 (Most of the time). Higher scores indicate higher levels of depressive symptoms. Internal consistency of the depression subscale has found to be good (Cronbach’s α = .91; [ 71 ]).
Reasons of smoking
Reasons of smoking will be asked with six self-constructed questions, e.g. ‘I smoke because it makes me calm’ and ‘I smoke because I am bored’ (1 = totally disagree, 5 = totally agree).
Birth characteristics
Complications and hospitalization during and after childbirth of the mother and child will be asked by questions such as “Did you have any complications during delivery?” and “Was your baby admitted to the hospital immediately after birth or in the first week after birth?”. When answered “yes”, questions will appear asking about the type of complications and reason for hospitalization.
In order to improve comprehension of specific parts of the questionnaires were adapted to our target group, consisting of women of lower SES. Some items under ‘Experienced stress’, ‘Depression’, ‘Intrinsic motivation’ are adapted. Also, answer categories of ‘Experienced stress’, ‘Self-efficacy regarding smoking cessation’ (0 = never to 3 = always) and ‘Perceived mother-child bond’ were reduced or adapted to increase consistency among questionnaires and to improve clarification.
Statistical analyses
The study will be performed according to the Consolidated Standards of Reporting Trials (CONSORT) Statement (see Fig. 3 ). All analysis will be done in SPSS version 26.0 and RStudio version 1.1.456 and a significance level of 0.05 will be used. Analyses will be performed conforming to intention-to-treat analysis, so that all randomized participants will be analyzed. We will explore baseline data to examine differences between the two different conditions and between the study completers and drop-outs. We will control for relevant covariates (i.e. SES, treatment history, maternal age, gestational age at birth, level of addiction, birth complications, way of delivery) by adding them to the models. Prior to the main analyses, outliers will be checked. Demographic outcomes will be compared using chi-square tests and independent sample t-tests.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram
To examine the effectiveness of the eHealth intervention on the primary outcome, the number of women who quit smoking during pregnancy measured at post-intervention and follow-up, General Estimating Equations (GEE) using a logistic model will be performed. Potential covariates that will be included in the model are maternal age, depressive symptoms, gestational age, employment status, education level, level of addiction, and partner’s smoking status. To investigate the effectiveness of the intervention on secondary outcomes (i.e., smoking behavior, perceived stress, birth outcomes, negative affectivity, self-efficacy, mother-child bonding, intrinsic motivation, parenting self-efficacy, pregnancy-related anxiety) linear mixed models will be performed for each outcome variable separately. Furthermore, we will test whether the effect of the intervention (independent variable) on smoking cessation (dependent variable) is mediated by a change in perceived stress using a GEE model. Moreover, it will be tested whether program usage and experience, self-efficacy regarding smoking cessation, feelings of mother-child bonding, intrinsic motivation for life style behavior change, and pregnancy experience moderate the effect on smoking cessation (primary outcome), smoking behaviour, and perceived stress by adding the variables to the GEE model.
Patient and public involvement
The design of the content of both the eHealth intervention and the control condition was achieved by co-creating processes with focus groups of women with low socioeconomic backgrounds, who either successfully quit smoking or struggled with quitting smoking during pregnancy. Data collection is carried out in collaboration with midwives and nurses from various midwifery practices and hospitals throughout the Netherlands.
The objective of this study is to examine the effectiveness of an eHealth intervention to help pregnant smoking women quit smoking. In contrast to other smoking-cessation interventions, this app-intervention particularly focuses on teaching women new stress-coping techniques. Such a component has been missing in existing stop-smoking programs. Stress is a common factor among smokers that hampers quitting and may result in relapse, in particular for women of low socioeconomic backgrounds . Smoking is a common behavior to alleviate feelings of tension. Despite this co-occurrence of smoking with high levels of stress among low SES pregnant women, we still lack an intervention that also supports pregnant women in learning new stress-coping skills. Learning new stress-coping skills might not only help these women quit smoking during their pregnancy, but can also help women stay abstinent after the birth of their baby.
‘Together with Eva’ is developed for women with low SES by means of easy and understandable language, attractive design, many repetitions of both text and exercises, positive feedback, and instruction videos of the different components of the application (i.e. stop-smoking plan, diary, breathing exercises). This was done in co-creation with women from the target group. Thereby, we expect to achieve the most beneficial results for this group of smoking pregnant women. As, once developed, the intervention can be made available to users for a low price, is easily accessible, and easy to implement into the health care system, we expect that this app-intervention will be a useful tool both for health care professionals to offer to their patients and for the pregnant women to receive support with quitting smoking during pregnancy. All in all, a personalized intervention for this target group is needed to further reduce inequalities in perinatal and maternal outcomes in in high income countries.
Status of the trial
After receiving permission by the Medical Ethics Committee to start with the inclusion of participants, the first participants were included in July 2020. Currently, data collection is ongoing. The main results are expected to be published in 2022.
Availability of data and materials
The datasets generated and/or analyzed during the current study will be available from the corresponding author on reasonable request.
Abbreviations
Acceptance and Commitment Therapy
Beleving van de Zwangerschap Schaal
Cognitive Behavior Therapy
Consolidated Standards of Reporting Trials
Depression, Anxiety, Stress Scale
Heart Rate Variability
Heart Rate Variability Bio-feedback
Heaviness of Smoking Index
Infant Behavior Questionnaire
Medical Ethics Committee
Netherlands Trial Register
Pregnancy Experience Scale
Pregnancy-related Anxiety Questionnaire
Perceived Stress Scale
Randomized Controlled Trial
Software Developer Kit
Self-efficacy in the Nurturing Role questionnaire
Socio-economic status
Stress- and Smoke Free Start of Life
Treatment Self-regulation Questionnaire
baseline assessment
second assessment
third assessment (first follow-up)
fourth assessment (second follow-up)
User Experience Questionnaire
VU University Medical Center
Lange S, Probst C, Rehm J, Popova S. National, regional, and global prevalence of smoking during pregnancy in the general population: a systematic review and meta-analysis. Lancet Glob Heal. 2018;6(7):e769–76. https://doi.org/10.1016/S2214-109X(18)30223-7 .
Article Google Scholar
Reitsma MB, Fullman N, Ng M, Salama JS, Abajobir A, Abate KH, et al. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990-2015: a systematic analysis from the global burden of disease study 2015. Lancet. 2017;389(10082):1885–906. https://doi.org/10.1016/S0140-6736(17)30819-X .
Pineles BL, Park E, Samet JM. Systematic review and meta-analysis of miscarriage and maternal exposure to tobacco smoke during pregnancy. Am J Epidemiol. 2014;179(7):807–23. https://doi.org/10.1093/aje/kwt334 .
Article PubMed PubMed Central Google Scholar
Jaddoe VWV, Troe EJWM, Hofman A, Mackenbach JP, Moll HA, Steegers EAP, et al. Active and passive maternal smoking during pregnancy and the risks of low birthweight and preterm birth: the generation R study. Paediatr Perinat Epidemiol. 2008;22(2):162–71. https://doi.org/10.1111/j.1365-3016.2007.00916.x .
Article PubMed Google Scholar
Leite M, Albieri V, Kjaer SK, Jensen A. Maternal smoking in pregnancy and risk for congenital malformations: results of a Danish register-based cohort study. Acta Obstet Gynecol Scand. 2014;93(8):825–34. https://doi.org/10.1111/aogs.12433 .
Lanting CI, Buitendijk SE, Crone MR, Segaar D, Gravenhorst JB, van Wouwe JP. Clustering of socioeconomic, behavioural, and neonatal risk factors for infant health in pregnant smokers. PLoS One. 2009;4(12):e8363.
Neuman Å, Hohmann C, Orsini N, Pershagen G, Eller E, Kjaer HF, et al. Maternal smoking in pregnancy and asthma in preschool children: a pooled analysis of eight birth cohorts. Am J Respir Crit Care Med. 2012;186(10):1037–43. https://doi.org/10.1164/rccm.201203-0501OC .
Rayfield S, Plugge E. Systematic review and meta-analysis of the association between maternal smoking in pregnancy and childhood overweight and obesity. J Epidemiol Community Health. 2017;71(2):162–73. https://doi.org/10.1136/jech-2016-207376 .
Clifford A, Lang L, Chen R. Effects of maternal cigarette smoking during pregnancy on cognitive parameters of children and young adults: a literature review. Neurotoxicol Teratol [Internet]. 2012;34(6):560–570. Available from: https://doi.org/10.1016/j.ntt.2012.09.004
Kristjansson AL, Thomas S, Lilly CL, Thorisdottir IE, Allegrante JP, Sigfusdottir ID. Maternal smoking during pregnancy and academic achievement of offspring over time: a registry data-based cohort study. Prev Med (Baltim) [internet]. 2018;113(may):74–79. Available from: https://doi.org/10.1016/j.ypmed.2018.05.017 .
Huizink AC, Mulder EJ. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neurosci Biobehav Rev. 2006;30(1):24–41. https://doi.org/10.1016/j.neubiorev.2005.04.005 .
Article CAS PubMed Google Scholar
Cornelius MD, De Genna NM, Leech SL, Willford JA, Goldschmidt L, Day NL. Effects of prenatal cigarette smoke exposure on neurobehavioral outcomes in 10-year-old children of adolescent mothers. Neurotoxicol Teratol [Internet] 2011;33(1):137–144. Available from: https://doi.org/10.1016/j.ntt.2010.08.006
Gaysina D, Fergusson DM, Leve LD, Horwood J, Reiss D, Shaw DS, et al. Maternal smoking during pregnancy and offspring conduct problems: evidence from 3 independent genetically sensitive research designs. JAMA Psychiatry. 2013;70(9):956–63. https://doi.org/10.1001/jamapsychiatry.2013.127 .
Boucher J, Konkle ATM. Understanding inequalities of maternal smoking—bridging the gap with adapted intervention strategies. Int J Environ Res Public Health. 2016;13(3):282.
Article PubMed Central Google Scholar
Riaz M, Lewis S, Naughton F, Ussher M. Predictors of smoking cessation during pregnancy: a systematic review and meta-analysis. Addiction. 2018;113(4):610–22.
Mackenbach JP, Stirbu I, Roskam AJR, Schaap MM, Menvielle G, Leinsalu M, et al. Socioeconomic inequalities in health in 22 European countries. N Engl J Med. 2008;358(23):2468–81. https://doi.org/10.1056/NEJMsa0707519 .
Lam JR, Tyler J, Scurrah KJ, Reavley NJ, Dite GS. The association between socioeconomic status and psychological distress: a within and between twin study. Twin Res Hum Genet. 2019;22(5):312–20. https://doi.org/10.1017/thg.2019.91 .
Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158(4):343–59. https://doi.org/10.1007/s002130100917 .
Richards JM, Stipelman BA, Bornovalova MA, Daughters SB, Sinha R, Lejuez CW. Biological mechanisms underlying the relationship between stress and smoking: state of the science and directions for future work. Biol Psychol [Internet] 2011;88(1):1–12. Available from: https://doi.org/10.1016/j.biopsycho.2011.06.009
Shiffman S. Relapse following smoking cessation: a situational analysis. J Consult Clin Psychol. 1982;50(1):71–86. https://doi.org/10.1037/0022-006X.50.1.71 .
Schneider S, Huy C, Schütz J, Diehl K. Smoking cessation during pregnancy: a systematic literature review. Drug Alcohol Rev. 2010;29(1):81–90. https://doi.org/10.1111/j.1465-3362.2009.00098.x .
Woods SM, Melville JL, Guo Y, Fan MY, Gavin A. Psychosocial stress during pregnancy. Am J Obstet Gynecol [Internet]. 2010;202(1):61.e1–61.e7. Available from: https://doi.org/10.1016/j.ajog.2009.07.041
Huizink AC, De Medina PGR, Mulder EJ, Visser GH, Buitelaar JK. Psychological measures of prenatal stress as predictors of infant temperament. J Am Acad Child Adolesc Psychiatry. 2002;41(9):1078–85. https://doi.org/10.1097/00004583-200209000-00008 .
Pesonen AK, Räikkönen K, Heinonen K, Komsi N, Järvenpää AL, Strandberg T. A transactional model of temperamental development: evidence of a relationship between child temperament and maternal stress over five years. Soc Dev. 2008;17(2):326–40. https://doi.org/10.1111/j.1467-9507.2007.00427.x .
Huizink AC, Robles De Medina PG, EJH M, Visser GHA, Buitelaar JK. Stress during pregnancy is associated with developmental outcome in infancy. J Child Psychol Psychiatry Allied Discip. 2003;44(6):810–8. https://doi.org/10.1111/1469-7610.00166 .
Moe V, von Soest T, Fredriksen E, Olafsen KS, Smith L. The multiple determinants of maternal parenting stress 12 months after birth: The contribution of antenatal attachment style, adverse childhood experiences, and infant temperament. Front Psychol. 2018;9:1987.
Nolvi S, Karlsson L, Bridgett DJ, Korja R, Huizink AC, Kataja EL, et al. Maternal prenatal stress and infant emotional reactivity six months postpartum. J Affect Disord. 2016;199:163–70. https://doi.org/10.1016/j.jad.2016.04.020 .
Beenackers MA, Nusselder WJ, Oude Groeniger J, van Lenthe FJ. Het terugdringen van gezondheidsachterstanden: een systematisch overzicht van kansrijke en effectieve interventies. Rapport in opdracht van FondsNutsOhra. Rotterdam: Erasmus MC; 2015.
Springvloet L, Hopman P, Kleinjan M, de Josselin de Jong S, van Laar M. Effectiviteit van stoppen-met-roken interventies voor zwangere vrouwen. Nationaal Expertisecentrum Tabaksontmoediging. Utrecht: Trimbos Instituut; 2016.
Jones M, Lewis S, Parrott S, Wormall S, Coleman T. Re-starting smoking in the postpartum period after receiving a smoking cessation intervention: a systematic review. Addiction. 2016;111(6):981–90. https://doi.org/10.1111/add.13309 .
Lehrer PM, Gevirtz R. Heart rate variability biofeedback: How and why does it work? Front Psychol. 2014;5:756.
Friedman BH, Thayer JF. Anxiety and autonomic flexibility: a cardiovascular approach. Biol Psychol. 1998;47(3):243–63. https://doi.org/10.1016/S0301-0511(97)00027-6 .
Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Ann Behav Med. 2009;37(2):141–53. https://doi.org/10.1007/s12160-009-9101-z .
Lanfranchi PA, Somers VK. Arterial baroreflex function and cardiovascular variability: interactions and implications. Am J Physiol Integr Comp Physiol. 2002;283(4):815–26.
Lehrer PM, Vaschillo E, Vaschillo B. Resonant frequency biofeedback training to increase cardiac variability: rationale and manual for training. Appl Psychophysiol Biofeedback. 2000;25(3):177–91.
Lehrer PM, Vaschillo E, Vaschillo B, Lu SE, Eckberg DL, Edelberg R, et al. Heart rate variability biofeedback increases baroreflex gain and peak expiratory flow. Psychosom Med. 2003;65(5):796–805. https://doi.org/10.1097/01.PSY.0000089200.81962.19 .
van der Zwan JE, Huizink AC, Lehrer PM, Koot HM, de Vente W. The effect of heart rate variability biofeedback training on mental health of pregnant and non-pregnant women: a randomized controlled trial. Int J Environ Res Public Health. 2019;2:16(6).
Google Scholar
Goessl VC, Curtiss JE, Hofmann SG. The effect of heart rate variability biofeedback training on stress and anxiety: a meta-analysis. Psychol Med. 2017;47(15):2578–86. https://doi.org/10.1017/S0033291717001003 .
World Health Organisation. World Health Organization. mHealth: New Horizons for Health Through Mobile Technologies, vol. 3. Geneva: Global Observatory for eHealth Series; 2011.
Schnoor K, Wouters MJM, Ossendorp BC, Hoogerhuis PM, Suijkerbuijk AWM. Verkenning e-healthmonitor: de digitale transitie in de zorg in beeld. 2020.
Greaves L, Poole N, Okoli CTC, Hemsing N, Qu A, Bialystok L, et al. Expecting to quit: a best- practices review of smoking cessation interventions for pregnant and post-partum women. 2nd ed. Vancouver: British Columbia Centre of Excellence for Women’s Health; 2011.
Van Den Heuvel JFM, Groenhof TK, Veerbeek JHW, Van Solinge WW, Lely AT, Franx A, et al. eHealth as the next-generation perinatal care: An overview of the literature. J Med Internet Res. 2018;20(6):e202.
Do HP, Tran BX, Le Pham Q, Nguyen LH, Tran TT, Latkin CA, et al. Which eHealth interventions are most effective for smoking cessation? A systematic review. Patient Prefer Adherence. 2018;12:2065–84. https://doi.org/10.2147/PPA.S169397 .
Goedhart G, van der Wal MF, Cuijpers P, Bonsel GJ. Psychosocial problems and continued smoking during pregnancy. Addict Behav [Internet] 2009;34(4):403–406. Available from: https://doi.org/10.1016/j.addbeh.2008.11.006
Clyde M, Pipe A, Els C, Reid R, Tulloch H. Factor structure of the smoking cessation self-efficacy questionnaire among smokers with and without a psychiatric diagnosis. Psychol Addict Behav. 2017;31(2):162–70. https://doi.org/10.1037/adb0000250 .
Curry SJ, Grothaus L, McBride C, Lando H, Pirie P. Motivation for smoking cessation among pregnant women. Psychol Addict Behav. 2001;15(2):126–32. https://doi.org/10.1037/0893-164X.15.2.126 .
Lebel C, MacKinnon A, Bagshawe M, Tomfohr-Madsen L, Giesbrecht G. Elevated depression and anxiety symptoms among pregnant individuals during the COVID-19 pandemic. J affect Disord [internet]. 2020;277:5–13. Available from: https://doi.org/10.1016/j.jad.2020.07.126 .
Jones TL, Prinz RJ. Potential roles of parental self-efficacy in parent and child adjustment: a review. Clin Psychol Rev. 2005;25(3):341–63. https://doi.org/10.1016/j.cpr.2004.12.004 .
Verena D, Klein A, Tanja J, Soren K, Susan S. Improved parental self-efficacy reduces stress in women receiving home visitation in a longitudinal study [internet]. 2017. Available from: www.rroij.com
Kunseler FC, Willemen AM, Oosterman M, Schuengel C. Changes in parenting self-efficacy and mood symptoms in the transition to parenthood: A bidirectional association. Parenting. 2014;14(3-4):215–34. https://doi.org/10.1080/15295192.2014.972758 .
Lindgren K. Relationships among maternal-fetal attachment, prenatal depression, and health practices in pregnancy. Res Nurs Health. 2001;24(3):203–17. https://doi.org/10.1002/nur.1023 .
Alhusen JL, Gross D, Hayat MJ, Woods AB, Sharps PW. The influence of maternal–fetal attachment and health practices on neonatal outcomes in low-income, urban women. Res Nurs Health [Internet]. 2012;35(2):112–20. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3624763/pdf/nihms412728.pdf . https://doi.org/10.1002/nur.21464 .
de Cock ESA, Henrichs J, Vreeswijk CMJM, Maas AJBM, Rijk CHAM, van Bakel HJA. Continuous feelings of love? The parental bond from pregnancy to toddlerhood. J Fam Psychol. 2016;30(1):125–34. https://doi.org/10.1037/fam0000138 .
Faul F, Erdfelder E, Lang AG, Buchner A. G* power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–91. https://doi.org/10.3758/BF03193146 .
van der Zwan JE, de Vente W, Koot HM, Huizink AC. Validation of the Dutch version of the pregnancy experience scale. Midwifery. 2017;50:16–20. https://doi.org/10.1016/j.midw.2017.03.018 .
Gartstein MA, Rothbart MK. Studying infant temperament via the Revised Infant Behavior Questionnaire. Vol. 26, Infant Behavior & Development. 2003.
Desmarais E, Majdandžić M, Gartstein MA, Bridgett DJ, French BF. Cross-cultural differences in temperament: comparing paternal ratings of US and Dutch infants. Eur J Dev Psychol [internet]. 2019;16(2):137–151. Available from: https://doi.org/10.1080/17405629.2017.1356713 .
Huizink AC, Delforterie MJ, Scheinin NM, Tolvanen M, Karlsson L, Karlsson H. Adaption of pregnancy anxiety questionnaire–revised for all pregnant women regardless of parity: PRAQ-R2. Arch Womens Ment Health. 2016;19(1):125–32. https://doi.org/10.1007/s00737-015-0531-2 .
Van den Bergh BRH. The influence of maternal emotions during pregnancy on fetal and neonatal behavior. J Prenat Perinat Psychol Health. 1990;5(2):119.
Pedersen FA, Bryan Y, Huffman L, Del Carmen R. Constructions of self and offspring in the pregnancy and early infancy periods. Kansas: Paper presented at the Biennial Meeting of the Society for Research in Child Development; 1989.
Verhage ML, Oosterman M, Schuengel C. Parenting self-efficacy predicts perceptions of infant negative temperament characteristics, not vice versa. J Fam Psychol. 2013;27(5):844–9. https://doi.org/10.1037/a0034263 .
Cohen S, Williamson G. Perceived stress in a probability sample of the U.S. In: Spacapam S, Oskamp S, editors. The social psychology of health: Claremont Symposium on Applied Social Psychology. Newbury Park: Sage; 1988.
Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–96. https://doi.org/10.2307/2136404 .
Mughal MK, Giallo R, Arnold P, Benzies K, Kehler H, Bright K, et al. Trajectories of maternal stress and anxiety from pregnancy to three years and child development at 3 years of age: findings from the all our families (AOF) pregnancy cohort. J Affect Disord [internet]. 2018;234:318–26. Available from: https://doi.org/10.1016/j.jad.2018.02.095 .
Schrepp M, Hinderks A, Thomaschewski J. Design and evaluation of a short version of the user experience questionnaire (UEQ-S). Int J Interact Multimed Artif Intell. 2017;4(6):103.
Mudde AN, Willemsen MC, Kremers S, de Vries H. Meetinstrumenten voor onderzoek naar roken en stoppen met roken. 2006.
Williams GC, Grow VM, Freedman ZR, Ryan RM, Deci EL. Motivational predictors of weight loss and weight-loss maintenance. J Pers Soc Psychol. 1996;70(1):115–26. https://doi.org/10.1037/0022-3514.70.1.115 .
DiPietro JA, Ghera MM, Costigan K, Hawkins M. Measuring the ups and downs of pregnancy stress. J Psychosom Obstet Gynecol. 2004;25(3–4):189–201. https://doi.org/10.1080/01674820400017830 .
Article CAS Google Scholar
Levesque CS, Williams GC, Elliot D, Pickering MA, Bodenhamer B, Finley PJ. Validating the theoretical structure of the treatment self-regulation questionnaire (TSRQ) across three different health behaviors. Health Educ Res. 2007;22(5):691–702. https://doi.org/10.1093/her/cyl148 .
Chabrol H, Niezborala M, Chastan E, De Leon J. Comparison of the heavy smoking index and of the Fagerstrom test for nicotine dependence in a sample of 749 cigarette smokers. Addict Behav. 2005;30(7):1474–7. https://doi.org/10.1016/j.addbeh.2005.02.001 .
De Beurs E, van Dyck R, Lange A, De DASS BR. Een vragenlijst voor het meten van depressie, angst en stress. Gedragstherapie. 2001;34:35–53.
Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the depression anxiety stress scales (DASS) with the Beck depression and anxiety inventories. Behav Res Ther. 1995;33(3):335–43. https://doi.org/10.1016/0005-7967(94)00075-U .
Download references
Acknowledgements
The Stress- and Smoke Free Pregnancy study is made possible by financial support from the Netherlands Organization for Health Research and Development (grant No. 54-30031-04). The design of the e-health intervention would not have been possible without AanZee (app developer) and Happitech (Heart Rhythm SDK developer).
This research is funded by the Netherlands Organization for Health Research and Development ZonMw, grand No. 54–30031-04.
Author information
Authors and affiliations.
Department of Clinical, Neuro and Developmental Psychology, Amsterdam Public Health research institute, Vrije Universiteit Amsterdam, Van der Boechorststraat 7, 1081, BT, Amsterdam, the Netherlands
Willeke van Dijk, Imke Jansen & Anja Huizink
Department of Clinical Child and Family Studies, Faculty of Behavioural and Movement Sciences, Vrije Universiteit Amsterdam, Amsterdam, the Netherlands
Mirjam Oosterman
Research Institute of Child Development and Education, University of Amsterdam, Amsterdam, the Netherlands
Wieke de Vente
You can also search for this author in PubMed Google Scholar
Contributions
AH and MO were involved in the conceptualization and design of the study. WD drafted the manuscript. IJ and WDV worked on the app development, particularly on the HRV-BF module. All authors edited the manuscript and read and approved the final manuscript.
Corresponding author
Correspondence to Willeke van Dijk .
Ethics declarations
Ethics approval and consent to participate.
Ethical approval has been obtained from the Medical Ethical Committee of the Medical Centre of the Vrije Universiteit in Amsterdam (METc VUmc) on June 25 2020 (NL71342.029.20/ 2020.065). An amendment, describing extra COVID-19 measures to be included in the study, has been approved by the METc VUmc on September 11 2020. All participants need to give a written informed consent to participate in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
van Dijk, W., Oosterman, M., Jansen, I. et al. Stress- and smoke free pregnancy study protocol: a randomized controlled trial of a personalized eHealth intervention including heart rate variability-biofeedback to support pregnant women quit smoking via stress reduction. BMC Public Health 21 , 905 (2021). https://doi.org/10.1186/s12889-021-10910-w
Download citation
Received : 14 April 2021
Accepted : 26 April 2021
Published : 12 May 2021
DOI : https://doi.org/10.1186/s12889-021-10910-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Stress reduction
- HRV-biofeedback
BMC Public Health
ISSN: 1471-2458
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 28 February 2018
Maternal smoking during pregnancy and offspring overweight: is there a dose–response relationship? An individual patient data meta-analysis
- Lucia Albers ORCID: orcid.org/0000-0002-2430-3167 1 ,
- Christina Sobotzki 1 ,
- Oliver Kuß 2 ,
- Teresa Ajslev 3 ,
- Rosangela FL Batista 4 ,
- Heloisa Bettiol 5 ,
- Bernard Brabin 6 , 7 , 8 ,
- Stephen L Buka 9 ,
- Viviane C Cardoso 5 ,
- Vicki L Clifton 10 ,
- Graham Devereux 11 ,
- Stephen E Gilman ORCID: orcid.org/0000-0002-8331-6419 12 , 13 , 14 , 15 ,
- Luke E Grzeskowiak 10 ,
- Joachim Heinrich 16 ,
- Sandra Hummel 17 , 18 ,
- Geir W Jacobsen 19 ,
- Graeme Jones 20 ,
- Gibby Koshy 6 ,
- Camilla Schmidt Morgen 3 ,
- Emily Oken 21 ,
- Tomas Paus 22 ,
- Zdenka Pausova 23 ,
- Sheryl L Rifas-Shiman 21 ,
- Andrea J Sharma 24 ,
- Antônio AM da Silva 4 ,
- Thorkild IA Sørensen 3 , 25 ,
- Elisabeth Thiering 26 ,
- Stephen Turner 11 ,
- Torstein Vik 27 &
- Rüdiger von Kries 1
International Journal of Obesity volume 42 , pages 1249–1264 ( 2018 ) Cite this article
1174 Accesses
40 Citations
4 Altmetric
Metrics details
- Epidemiology
- Risk factors
Background/objectives
A number of meta-analyses suggest an association between any maternal smoking in pregnancy and offspring overweight obesity. Whether there is a dose–response relationship across number of cigarettes and whether this differs by sex remains unclear.
Subject/methods
Studies reporting number of cigarettes smoked during pregnancy and offspring BMI published up to May 2015 were searched. An individual patient data meta-analysis of association between the number of cigarettes smoked during pregnancy and offspring overweight (defined according to the International Obesity Task Force reference) was computed using a generalized additive mixed model with non-linear effects and adjustment for confounders (maternal weight status, breastfeeding, and maternal education) and stratification for sex.
Of 26 identified studies, 16 authors provided data on a total of 238,340 mother–child-pairs. A linear positive association was observed between the number of cigarettes smoked and offspring overweight for up to 15 cigarettes per day with an OR increase per cigarette of 1.03, 95% CI = [1.02–1.03]. The OR flattened with higher cigarette use. Associations were similar in males and females. Sensitivity analyses supported these results.
Conclusions
A linear dose–response relationship of maternal smoking was observed in the range of 1–15 cigarettes per day equally in boys and girls with no further risk increase for doses above 15 cigarettes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
251,40 € per year
only 20,95 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Precision stratification of prognostic risk factors associated with outcomes in gestational diabetes mellitus: a systematic review
Zhila Semnani-Azad, Romy Gaillard, … Wei Perng

Association of maternal caffeine intake during pregnancy with low birth weight, childhood overweight, and obesity: a meta-analysis of cohort studies
Feng Jin & Chong Qiao

Decreased head circumference at birth associated with maternal tobacco smoke exposure during pregnancy on the Japanese prospective birth cohort study
Tadashi Shiohama, Aya Hisada, … the Japan Environment Children’s Study (JECS) Group
Rayfield S, Plugge E. Systematic review and meta-analysis of the association between maternal smoking in pregnancy and childhood overweight and obesity. J Epidemiol Community Health. 2016;71:162–73. https://doi.org/10.1136/jech-2016-207376
Article PubMed Google Scholar
Riedel C, Schönberger K, Yang S, Koshy G, Chen Y-C, Gopinath B, et al. Parental smoking and childhood obesity: higher effect estimates for maternal smoking in pregnancy compared to paternal smoking - a meta-analysis. Int J Epidemiol. 2014;43:1593–606.
PubMed Google Scholar
Ino T. Maternal smoking during pregnancy and offspring obesity: meta-analysis. Pediatr Int. 2010;52:94–99.
Oken E, Levitan EB, Gillman MW. Maternal smoking during pregnancy and child overweight: systematic review and meta-analysis. Int J Obes. 2008;32:201–10.
CAS Google Scholar
Zinkhan EK, Lang BY, Yu B, Wang Y, Jiang C, Fitzhugh M, et al. Maternal tobacco smoke increased visceral adiposity and serum corticosterone levels in adult male rat offspring. Pediatr Res. 2014;76:17–23.
CAS PubMed Google Scholar
Gao Y-J, Holloway AC, Zeng Z, Lim GE, Petrik JJ, Foster WG, et al. Prenatal exposure to nicotine causes postnatal obesity and altered perivascular adipose tissue function. Obes Res. 2005;13:687–92.
Somm E, Schwitzgebel VM, Vauthay DM, Camm EJ, Chen CY, Giacobino J-P, et al. Prenatal nicotine exposure alters early pancreatic islet and adipose tissue development with consequences on the control of body weight and glucose metabolism later in life. Endocrinology. 2008;149:6289–99.
Oliveira E, Moura EG, Santos-Silva AP, Fagundes ATS, Rios AS, Abreu-Villaça Y, et al. Short- and long-term effects of maternal nicotine exposure during lactation on body adiposity, lipid profile, and thyroid function of rat offspring. J Endocrinol. 2009;202:397–405.
Holloway AC, Lim GE, Petrik JJ, Foster WG, Morrison KM, Gerstein HC. Fetal and neonatal exposure to nicotine in Wistar rats results in increased beta cell apoptosis at birth and postnatal endocrine and metabolic changes associated with type 2 diabetes. Diabetologia. 2005;48:2661–6.
Yousefi M, Karmaus W, Zhang H, Ewart S, Arshad H, Holloway JW. The methylation of the LEPR/LEPROT genotype at the promoter and body regions influence concentrations of leptin in girls and BMI at age 18 years if their mother smoked during pregnancy. Int J Mol Epidemiol Genet. 2013;4:86–100.
CAS PubMed PubMed Central Google Scholar
Rhee KE, Phelan S, McCaffery J. Early determinants of obesity: genetic, epigenetic, and in utero influences. Int J Pediatr. 2012;2012:1–9.
Google Scholar
Joubert BR, Felix JF, Yousefi P, Bakulski KM, Just AC, Breton C, et al. DNA methylation in newborns and maternal smoking in pregnancy: genome-wide consortium meta-analysis. Am J Hum Genet. 2016;98:680–96.
Lee KWK, Richmond R, Hu P, French L, Shin J, Bourdon C, et al. Prenatal exposure to maternal cigarette smoking and DNA methylation: epigenome-wide association in a discovery sample of adolescents and replication in an independent cohort at birth through 17 years of age. Environ Health Perspect. 2015;123:193–9.
Leary SD, Smith GD, Rogers IS, Reilly JJ, Wells JC, Ness AR. Smoking during pregnancy and offspring fat and lean mass in childhood. Obes Silver Spring. 2006;14:2284–93.
Kleiser C, Schaffrath Rosario A, Mensink GB, Prinz-Langenohl R, Kurth BM. Potential determinants of obesity among children and adolescents in Germany: results from the cross-sectional KiGGS Study. BMC Public Health. 2009;9:46.
PubMed PubMed Central Google Scholar
Plachta-Danielzik S, Kehden B, Landsberg B, Schaffrath Rosario A, Kurth BM, Arnold C, et al. Attributable risks for childhood overweight: evidence for limited effectiveness of prevention. Pediatrics. 2012;130:e865–e871.
Harris HR, Willett WC, Michels KB. Parental smoking during pregnancy and risk of overweight and obesity in the daughter. Int J Obes. 2005 ;2013:1356–63.
Iliadou AN, Koupil I, Villamor E, Altman D, Hultman C, Långström N, et al. Familial factors confound the association between maternal smoking during pregnancy and young adult offspring overweight. Int J Epidemiol. 2010;39:1193–202.
Gilman SE, Gardener H, Buka SL. Maternal smoking during pregnancy and children’s cognitive and physical development: a causal risk factor? Am J Epidemiol. 2008;168:522–31.
Eliopoulos C, Klein J, Phan MK, Knie B, Greenwald M, Chitayat D, et al. Hair concentrations of nicotine and cotinine in women and their newborn infants. JAMA. 1994;271:621–3.
Eliopoulos C, Klein J, Chitayat D, Greenwald M, Koren G. Nicotine and cotinine in maternal and neonatal hair as markers of gestational smoking. Clin Investig Med Médecine Clin Exp. 1996;19:231–42.
Møller SE, Ajslev TA, Andersen CS, Dalgård C, Sørensen TIA. Risk of childhood overweight after exposure to tobacco smoking in prenatal and early postnatal life. PLoS ONE. 2014;9:e109184.
Grzeskowiak LE, Hodyl NA, Stark MJ, Morrison JL, Clifton VL. Association of early and late maternal smoking during pregnancy with offspring body mass index at 4 to 5 years of age. J Dev Orig Health Dis. 2015;6:485–92.
Howe LD, Matijasevich A, Tilling K, Brion MJ, Leary SD, Smith GD, et al. Maternal smoking during pregnancy and offspring trajectories of height and adiposity: comparing maternal and paternal associations. Int J Epidemiol. 2012;41:722–32.
Koshy G, Delpisheh A, Brabin BJ. Dose response association of pregnancy cigarette smoke exposure, childhood stature, overweight and obesity. Eur J Public Health. 2011;21:286–91.
Sharma AJ, Cogswell ME, Li R. Dose-response associations between maternal smoking during pregnancy and subsequent childhood obesity: effect modification by maternal race/ethnicity in a low-income US cohort. Am J Epidemiol. 2008;168:995–1007.
Widerøe M, Vik T, Jacobsen G, Bakketeig LS. Does maternal smoking during pregnancy cause childhood overweight? Paediatr Perinat Epidemiol. 2003;17:171–9.
von Kries R, Bolte G, Baghi L, Toschke AM, Group GMES. Parental smoking and childhood obesity--is maternal smoking in pregnancy the critical exposure? Int J Epidemiol. 2008;37:210–6.
Hill SY, Shen S, Locke Wellman J, Rickin E, Lowers L. Offspring from families at high risk for alcohol dependence: increased body mass index in association with prenatal exposure to cigarettes but not alcohol. Psychiatry Res. 2005;135:203–16.
Chen A, Pennell ML, Klebanoff MA, Rogan WJ, Longnecker MP. Maternal smoking during pregnancy in relation to child overweight: follow-up to age 8 years. Int J Epidemiol. 2006;35:121–30.
Reilly JJ, Armstrong J, Dorosty AR, Emmett PM, Ness A, Rogers I, et al. Early life risk factors for obesity in childhood: cohort study. BMJ. 2005;330:1357.
Power C, Jefferis BJ. Fetal environment and subsequent obesity: a study of maternal smoking. Int J Epidemiol. 2002;31:413–9.
Dior UP, Lawrence GM, Sitlani C, Enquobahrie D, Manor O, Siscovick DS, et al. Parental smoking during pregnancy and offspring cardio-metabolic risk factors at ages 17 and 32. Atherosclerosis. 2014;235:430–7.
Durmuş B, Ay L, Hokken-Koelega ACS, Raat H, Hofman A, Steegers EAP, et al. Maternal smoking during pregnancy and subcutaneous fat mass in early childhood. The Generation R Study. Eur J Epidemiol. 2011;26:295–304.
Gorog K, Pattenden S, Antova T, Niciu E, Rudnai P, Scholtens S, et al. Maternal smoking during pregnancy and childhood obesity: results from the CESAR Study. Matern Child Health J. 2011;15:985–92.
Huang DYC, Lanza HI, Anglin MD. Trajectory of adolescent obesity: exploring the impact of prenatal to childhood experiences. J Child Fam Stud. 2014;23:1090–101.
Dahlhoff M, Pfister S, Blutke A, Rozman J, Klingenspor M, Deutsch MJ, et al. Peri-conceptional obesogenic exposure induces sex-specific programming of disease susceptibilities in adult mouse offspring. Biochim Biophys Acta. 2014;1842:304–17.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12.
Cole TJ. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240
Sachdev HS, Fall CHD, Osmond C, Lakshmy R, Dey Biswas SK, Leary SD, et al. Anthropometric indicators of body composition in young adults: relation to size at birth and serial measurements of body mass index in childhood in the New Delhi birth cohort. Am J Clin Nutr. 2005;82:456–66.
Ylihärsilä H, Kajantie E, Osmond C, Forsén T, Barker DJ, Eriksson JG. Body mass index during childhood and adult body composition in men and women aged 56-70 y. Am J Clin Nutr. 2008;87:1769–75.
Bayer O, Krüger H, von Kries R, Toschke AM. Factors associated with tracking of BMI: a meta-regression analysis on BMI tracking. Obes Silver Spring Md. 2011;19:1069–76.
VanderWeele TJ, Mumford SL, Schisterman EF. Conditioning on intermediates in perinatal epidemiology. Epidemiol Camb Mass. 2012;23:1–9.
Rubin DB. Multiple imputation for nonresponse in surveys. Hoboken, NJ: Wiley-Interscience; 2004.
Lin X, Zhang D. Inference in generalized additive mixed modelsby using smoothing splines. J R Stat Soc Ser B Stat Methodol. 1999;61:381–400.
Jones G, Riley M, Dwyer T. Maternal smoking during pregnancy, growth, and bone mass in prepubertal children. J Bone Miner Res J Am Soc Bone Miner Res. 1999;14:146–51.
Suzuki K, Kondo N, Sato M, Tanaka T, Ando D, Yamagata Z. Gender differences in the association between maternal smoking during pregnancy and childhood growth trajectories: multilevel analysis. Int J Obes. 2011;35:53–59.
Suzuki K, Kondo N, Sato M, Tanaka T, Ando D, Yamagata Z. Maternal smoking during pregnancy and childhood growth trajectory: a random effects regression analysis. J Epidemiol. 2012;22:175–8.
Riedel C, Fenske N, Muller MJ, Plachta-Danielzik S, Keil T, Grabenhenrich L, et al. Differences in BMI z-scores between offspring of smoking and nonsmoking mothers: a longitudinal study of German children from birth through 14 years of age. Env Health Perspect. 2014;122:761–7.
Silva AA, Barbieri MA, Cardoso VC, Batista RF, Simões VM, Vianna EO, et al. Prevalence of non-communicable diseases in Brazilian children: follow-up at school age of two Brazilian birth cohorts of the 1990’s. BMC Public Health. 2011;11:486.
Boerschmann H, Pflüger M, Henneberger L, Ziegler A-G, Hummel S. Prevalence and predictors of overweight and insulin resistance in offspring of mothers with gestational diabetes mellitus. Diabetes Care. 2010;33:1845–9.
Oken E, Huh SY, Taveras EM, Rich-Edwards JW, Gillman MW. Associations of maternal prenatal smoking with child adiposity and blood pressure. Obes Res. 2005;13:2021–8.
Syme C, Abrahamowicz M, Mahboubi A, Leonard GT, Perron M, Richer L, et al. Prenatal exposure to maternal cigarette smoking and accumulation of intra-abdominal fat during adolescence. Obes Silver Spring Md. 2010;18:1021–5.
Pausova Z, Paus T, Abrahamowicz M, Bernard M, Gaudet D, Leonard G, et al. Cohort profile: the Saguenay Youth Study (SYS). Int J Epidemiol. 2016;46:e19 https://doi.org/10.1093/ije/dyw023
Article PubMed Central Google Scholar
Thiering E, Brüske I, Kratzsch J, Thiery J, Sausenthaler S, Meisinger C, et al. Prenatal and postnatal tobacco smoke exposure and development of insulin resistance in 10 year old children. Int J Hyg Environ Health. 2011;214:361–8.
Prabhu N, Smith N, Campbell D, Craig LC, Seaton A, Helms PJ, et al. First trimester maternal tobacco smoking habits and fetal growth. Thorax. 2010;65:235–40.
Williams RL. Intrauterine growth curves: intra- and international comparisons with different ethnic groups in California. Prev Med. 1975;4:163–72.
Fried PA, Watkinson B, Gray R. Growth from birth to early adolescence in offspring prenatally exposed to cigarettes and marijuana. Neurotoxicol Teratol. 1999;21:513–25.
Kuhle S, Allen AC, Veugelers PJ. Prevention potential of risk factors for childhood overweight. Can J Public Health Rev Can St Publique. 2010;101:365–8.
Cavlek T, Cavlek M, Bozikov J, Sturz B, Grsic K. Influende of parental smoking on children’s growth and weight at birth and age 6. Paediatr Croat. 2010;54:117–23.
Taylor AE, Davey Smith G, Bares CB, Edwards AC, Munafo MR. Partner smoking and maternal cotinine during pregnancy: implications for negative control methods. Drug Alcohol Depend. 2014;139:159–63.
Mansi G, Raimondi F, Pichini S, Capasso L, Sarno M, Zuccaro P, et al. Neonatal urinary cotinine correlates with behavioral alterations in newborns prenatally exposed to tobacco smoke. Pediatr Res. 2007;61:257–61.
Laubenthal J, Zlobinskaya O, Poterlowicz K, Baumgartner A, Gdula MR, Fthenou E, et al. Cigarette smoke-induced transgenerational alterations in genome stability in cord blood of human F1 offspring. FASEB J. 2012;26:3946–56.
Kenny PJ. Common cellular and molecular mechanisms in obesity and drug addiction. Nat Rev Neurosci. 2011;12:638–51.
Haghighi A, Schwartz DH, Abrahamowicz M, Leonard GT, Perron M, Richer L, et al. Prenatal exposure to maternal cigarette smoking, amygdala volume, and fat intake in adolescence. JAMA Psychiatry. 2013;70:98–105.
Rolls ET. Taste, olfactory and food texture reward processing in the brain and obesity. Int J Obes. 2005 ;2011:550–61.
Bray GA, Popkin BM. Dietary fat intake does affect obesity! Am J Clin Nutr. 1998;68:1157–73.
Tappy L. Thermic effect of food and sympathetic nervous system activity in humans. Reprod Nutr Dev. 1996;36:391–7.
Wrase J, Makris N, Braus DF, Mann K, Smolka MN, Kennedy DN, et al. Amygdala volume associated with alcohol abuse relapse and craving. Am J Psychiatry. 2008;165:1179–84.
Primeaux SD, York DA, Bray GA. Neuropeptide Y administration into the amygdala alters high fat food intake. Peptides. 2006;27:1644–51.
Boghossian S, Park M, York DA. Melanocortin activity in the amygdala controls appetite for dietary fat. Am J Physiol Regul Integr Comp Physiol. 2010;298:R385–R393.
Grabenhorst F, Rolls ET, Parris BA, d’Souza AA. How the brain represents the reward value of fat in the mouth. Cereb Cortex. 2010;20:1082–91. (N Y N 1991).
Trygg K, Lund-Larsen K, Sandstad B, Hoffman HJ, Jacobsen G, Bakketeig LS. Do pregnant smokers eat differently from pregnant non-smokers? Paediatr Perinat Epidemiol. 1995;9:307–19.
Llaquet H, Pichini S, Joya X, Papaseit E, Vall O, Klein J, et al. Biological matrices for the evaluation of exposure to environmental tobacco smoke during prenatal life and childhood. Anal Bioanal Chem. 2010;396:379–99.
Jacqz-Aigrain E, Zhang D, Maillard G, Luton D, André J, Oury JF. Maternal smoking during pregnancy and nicotine and cotinine concentrations in maternal and neonatal hair. BJOG Int J Obstet Gynaecol. 2002;109:909–11.
Toschke AM, Montgomery SM, Pfeiffer U, von Kries R. Early intrauterine exposure to tobacco-inhaled products and obesity. Am J Epidemiol. 2003;158:1068–74.
Venditti CC, Smith GN. Self-reported cigarette smoking status imprecisely quantifies exposure in pregnancy. Open J Obstet Gynecol. 2012;02:56–61.
Pickett KE, Rathouz PJ, Kasza K, Wakschlag LS, Wright R. Self-reported smoking, cotinine levels, and patterns of smoking in pregnancy. Paediatr Perinat Epidemiol. 2005;19:368–76.
Talge NM, Mudd LM, Sikorskii A, Basso O. United States birth weight reference corrected for implausible gestational age estimates. Pediatrics. 2014;133:844–53.
Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med. 28. 1998;17:407–29.
Voigt M, Schneider K, Jährig K. Analyse des Geburtengutes des Jahrgangs 1992 der Bundesrepublik Deutschland. Geburtshilfe Frauenheilkd. 1996;56:550–8.
Dobbins TA, Sullivan EA, Roberts CL, Simpson JM. Australian national birthweight percentiles by sex and gestational age, 1998-2007. Med J Aust. 3. 2012;197:291–4.
Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6.
Download references
Acknowledgements
We want to thank the funders of the individual studies: the UK Medical Research Council and the Wellcome Trust (Grant ref: 102215/2/13/2) and the University of Bristol, the Danish National Research Foundation, Pharmacy Foundation, the March of Dimes Birth Defects Foundation, the Augustinus Foundation, and the Health Foundation, the US NICHD (contracts no. 1-HD-4-2803 and no. 1-HD-1-3127, R01 HD HD034568), the NHMRC, the CNPq (Portuguese acronym for the National Research Council—grant 523474/96-2) and FAPESP (Portuguese acronym for the São Paulo State Research Council—grant 00/0908-7). We would like to thank the participating families of all studies for the use of data. For the ASPAC study, we want to thank the midwives for their help in recruiting families, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses. This work was supported by the Deutschen Forschungsgesellschaft (German Research Foundation, DFG) [KR 1926/9-1, KU1443/4-1]. Dr. Gilman’s contribution was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Author information
Authors and affiliations.
Division of Epidemiology, Institute of Social Paediatrics and Adolescents Medicine, Ludwig-Maximilians-University Munich, Munich, Germany
Lucia Albers, Christina Sobotzki & Rüdiger von Kries
German Diabetes Center, Institute of Biometrics and Epidemiology, Düsseldorf, 40225, Germany
Department of Clinical Epidemiology (formerly Institute of Preventive Medicine), Bispebjerg and Frederiksberg Hospitals, The Capital Region, Denmark
Teresa Ajslev, Camilla Schmidt Morgen & Thorkild IA Sørensen
Departamento de Saúde Pública, Universidade Federal do Maranhão, São Luís, MA, Brazil
Rosangela FL Batista & Antônio AM da Silva
Departamento de Puericultura e Pediatria, Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo, São Paulo, Brazil
Heloisa Bettiol & Viviane C Cardoso
Child and Reproductive Health Group, Liverpool School of Tropical Medicine, Liverpool, UK
Bernard Brabin & Gibby Koshy
Department of Community Child Health,Royal Liverpool Children’s Hospital, NHS Trust Alder Hey, Liverpool, UK
Bernard Brabin
Emma Kinderziekenhuis, Academic Medical Centre, University of Amsterdam, Amsterdam, The Netherlands
Department of Epidemiology, Brown University School of Public Health, Providence, Rhode Island, USA
Stephen L Buka
Adelaide Medical School, The Robinson Research Institute, The University of Adelaide, Adelaide, South Australia, Australia
Vicki L Clifton & Luke E Grzeskowiak
Child Health, University of Aberdeen, Aberdeen, UK
Graham Devereux & Stephen Turner
Health Behavior Branch, Division of Intramural Population Health Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD, USA
Stephen E Gilman
Department of Social and Behavioral Sciences, Harvard TH Chan School of Public Health, Boston, MA, USA
Department of Epidemiology, Harvard TH Chan School of Public Health, Boston, MA, USA
Department of Mental Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA
Institute of Occupational, Social, and Environmental Medicine, University Hospital, Helmholtz Zentrum München, German Research Center for Environmental Health, Institute of Occupational, Social, and Environmental Medicine, University Hospital, Neuherberg, Germany
Joachim Heinrich
Forschergruppe Diabetes der Technischen Universität München, Munich, Germany
Sandra Hummel
Institut für Diabetesforschung der Forschergruppe Diabetes e.V. am Helmholtz Zentrum München, Munich, Germany
Department of Public Health and General Practice, NTNU, Norwegian University of Science and Technology, Trondheim, Norway
Geir W Jacobsen
Menzies Institute for Medical Research, University of Tasmania, Hobart, Tasmania, Australia
Graeme Jones
Obesity Prevention Program, Department of Population Medicine, Harvard Medical School, Harvard Pilgrim Health Care Institute, Boston, MA, USA
Emily Oken & Sheryl L Rifas-Shiman
Rotman Research Institute and Departments of Psychology and Psychiatry, University of Toronto, Toronto, Canada
Hospital for Sick Children and Departments of Physiology and Nutritional Sciences, University of Toronto, Toronto, Canada
Zdenka Pausova
Centers for Disease Control and Prevention, Atlanta, USA
Andrea J Sharma
Novo Nordisk Foundation Centre for Basic Metabolic Research, and Department of Public Health, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark
Thorkild IA Sørensen
Helmholtz Zentrum München, German Research Center for Environmental Health, Institute of Epidemiology I, Neuherberg, Germany
Elisabeth Thiering
Department of Laboratory Medicine, Children and Women’s Health, Norwegian University of Science and Technology, Trondheim, Norway
Torstein Vik
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Lucia Albers .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflict of interest.
Electronic supplementary material
Table s1(docx 49 kb), figure legends for supplemantal figures(docx 12 kb), supplemental figure 1(tif 928 kb), supplemental figure 2(tif 938 kb), supplemental figure 3(tif 881 kb), supplemental figure 4(tif 797 kb), supplemental figure 5(tif 850 kb), supplemental figure 6(tif 900 kb), supplemental figure 7(tif 825 kb), supplemental figure 8(tif 804 kb), rights and permissions.
Reprints and permissions
About this article
Cite this article.
Albers, L., Sobotzki, C., Kuß, O. et al. Maternal smoking during pregnancy and offspring overweight: is there a dose–response relationship? An individual patient data meta-analysis. Int J Obes 42 , 1249–1264 (2018). https://doi.org/10.1038/s41366-018-0050-0
Download citation
Received : 18 July 2017
Revised : 13 November 2017
Accepted : 27 December 2017
Published : 28 February 2018
Issue Date : July 2018
DOI : https://doi.org/10.1038/s41366-018-0050-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Maternal smoking, nutritional factors at different life stage, and the risk of incident type 2 diabetes: a prospective study of the uk biobank.
- Wenbo Jiang
BMC Medicine (2024)
Comparing body composition between the sweet-liking phenotypes: experimental data, systematic review and individual participant data meta-analysis
- Rhiannon Mae Armitage
- Vasiliki Iatridi
- Martin Richard Yeomans
International Journal of Obesity (2024)
Smoking during pregnancy is associated with child overweight independent of maternal pre-pregnancy BMI and genetic predisposition to adiposity
- Theresia M. Schnurr
- Lars Ängquist
- Camilla S. Morgen
Scientific Reports (2022)
Early life exposures are associated with appetitive traits in infancy: findings from the BiTwin cohort
- Alexandra Costa
- Sarah Warkentin
- Andreia Oliveira
European Journal of Nutrition (2022)
Racial Disparities in Obesity Treatment Among Children and Adolescents
- Veronica R. Johnson
- Nonyerem O. Acholonu
- Fatima Cody Stanford
Current Obesity Reports (2021)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Bibliography
- More Referencing guides Blog Automated transliteration Relevant bibliographies by topics
- Automated transliteration
- Relevant bibliographies by topics
- Referencing guides
Dissertations / Theses on the topic 'Smoking during pregnancy'
Create a spot-on reference in apa, mla, chicago, harvard, and other styles.
Consult the top 44 dissertations / theses for your research on the topic 'Smoking during pregnancy.'
Next to every source in the list of references, there is an 'Add to bibliography' button. Press on it, and we will generate automatically the bibliographic reference to the chosen work in the citation style you need: APA, MLA, Harvard, Chicago, Vancouver, etc.
You can also download the full text of the academic publication as pdf and read online its abstract whenever available in the metadata.
Browse dissertations / theses on a wide variety of disciplines and organise your bibliography correctly.
Lindqvist, Rune. "Smoking cessation during pregnancy /." Stockholm : [Karolinska Univ. Press], 2001. http://diss.kib.ki.se/2001/91-7349-034-2/.
Mercelina-Roumans, Patricia E. A. M. "Smoking during pregnancy the haematological status of smoking and non-smoking pregnant women and their offspring /." Maastricht : Maastricht : UPM, Universitaire Pers Maastricht ; University Library, Maastricht University [Host], 1996. http://arno.unimaas.nl/show.cgi?fid=7388.
Morales, Andres Waldo. "Smoking during pregnancy : an attachment theory perspective." Thesis, King's College London (University of London), 2005. https://kclpure.kcl.ac.uk/portal/en/theses/smoking-during-pregnancy--an-attachment-theory-perspective(7fef30b0-75d9-4bf9-8e94-7621bbc952e3).html.
Makin, Judy Carleton University Dissertation Psychology. "Passive smoking during pregnancy: long term effects?" Ottawa, 1989.
Fletcher, Tifani R., Lana McGrady, Andrea D. Clements, and Beth A. Bailey. "Perceptions of Smoking Cessation Barriers During Pregnancy." Digital Commons @ East Tennessee State University, 2013. https://dc.etsu.edu/etsu-works/7263.
Fletcher, Tifani R., Andrea D. Clements, Beth A. Bailey, and Lana McGrady. "Stress, Self-Esteem, and Smoking During Pregnancy." Digital Commons @ East Tennessee State University, 2012. https://dc.etsu.edu/etsu-works/7266.
Wang, Liang, Hadii M. Mamadu, J. L. Anderson, and Arsham Alamian. "Maternal Smoking During Pregnancy and Risk of Adolescent Obesity." Digital Commons @ East Tennessee State University, 2012. https://dc.etsu.edu/etsu-works/1405.
Jones, Matthew John. "The development of the 'Economic impacts of Smoking In Pregnancy' (ESIP) model for measuring the impacts of smoking and smoking cessation during pregnancy." Thesis, University of Nottingham, 2015. http://eprints.nottingham.ac.uk/30604/.
De, Roo Lisa Anne. "Smoking during first pregnancy and the risk of breast cancer /." Thesis, Connect to this title online; UW restricted, 2004. http://hdl.handle.net/1773/10884.
Letourneau, Alyssa Rose. "Timing and Predictors of Postpartum Return to Smoking in Women Who Quit Smoking During Pregnancy." Yale University, 2007. http://ymtdl.med.yale.edu/theses/available/etd-06282006-112209/.
Cooke, Margaret Community Medicine UNSW. "Barriers to the systematic provision of smoking cessation education during pregnancy." Awarded by:University of New South Wales. Community Medicine, 1998. http://handle.unsw.edu.au/1959.4/19041.
Appleton, Peter Leonard. "Smoking cessation and social support during pregnancy : a longitudinal study of the impact of close relationship support on smoking cessation in pregnancy." Thesis, University of Liverpool, 1994. http://ethos.bl.uk/OrderDetails.do?uin=uk.bl.ethos.240393.
Jones, Michael Edwin. "Pre-natal and early life risk factors for diabetes, cryptorchism and inguinal hernia in children." Thesis, London School of Hygiene and Tropical Medicine (University of London), 1996. http://researchonline.lshtm.ac.uk/682237/.
Abrahamsson, Agneta, Jane Springett, Leif Karlsson, and Torgny Ottosson. "Making sense of the challenge of smoking cessation during pregnancy : a phenomenographic approach." Högskolan Kristianstad, Institutionen för hälsovetenskaper, 2005. http://urn.kb.se/resolve?urn=urn:nbn:se:hkr:diva-975.
Jaakkola, Niina. "Passive smoking during pregnancy and early childhood : occurrence, determinants, health effects and prevention." Helsinki : University of Helsinki, 2002. http://ethesis.helsinki.fi/julkaisut/laa/kansa/vk/jaakkola/.
John, Kevin H., Tifani R. Fletcher, Andrea D. Clements, Beth A. Bailey, and Lana McGrady. "Perceptions of Smoking Health Risks During Pregnancy: Comparison of Smokers and Non-Smokers." Digital Commons @ East Tennessee State University, 2012. https://dc.etsu.edu/etsu-works/7270.
Vanderbloemen, Laura. "Smoking during pregnancy and child mental health and wellbeing : evidence, policy and practice." Thesis, University of York, 2013. http://etheses.whiterose.ac.uk/5656/.
Thakur, Geeta Angeli. "Maternal smoking during pregnancy: An environmental factor indexing a more homogenous subgroup of ADHD." Thesis, McGill University, 2013. http://digitool.Library.McGill.CA:80/R/?func=dbin-jump-full&object_id=114156.
Petersen, Zainonisa. "Smoking cessation during pregnancy : a person-centred approach among disadvantaged women in South Africa." Doctoral thesis, Umeå universitet, Epidemiologi och global hälsa, 2011. http://urn.kb.se/resolve?urn=urn:nbn:se:umu:diva-39595.
Lucas, Kevin. "The relationship between beliefs, attitudes, negative affect and changes in smoking behaviour during pregnancy." Thesis, University of Sussex, 1993. http://ethos.bl.uk/OrderDetails.do?uin=uk.bl.ethos.358177.
Klöfvermark, Josefin. "Smoking during pregnancy by duration of residence among immigrants in Sweden 1991-2012 : A study on health inequalities." Thesis, Stockholms universitet, Centrum för forskning om ojämlikhet i hälsa (CHESS), 2016. http://urn.kb.se/resolve?urn=urn:nbn:se:su:diva-132647.
Bailey, Beth, Judy G. McCook, Andrea Clements, and Lana McGrady. "Quitting Smoking During Pregnancy and Birth Outcomes: Evidence of Gains Following Cessation by Third Trimester." Digital Commons @ East Tennessee State University, 2011. https://dc.etsu.edu/etsu-works/7189.
Bailey, Beth A., Judy G. McCook, A. L. Hodge, Andrea D. Clements, and Lana McGrady. "Quitting Smoking During Pregnancy and Birth Outcomes: Evidence of Gains Following Cessation by Third Trimester." Digital Commons @ East Tennessee State University, 2011. https://dc.etsu.edu/etsu-works/7278.
Mutemwa, Muyunda. "Maternal nicotine expose during gestation and lactation induce premature aging of the lungs of the offspring." Thesis, University of the Western Cape, 2009. http://etd.uwc.ac.za/index.php?module=etd&action=viewtitle&id=gen8Srv25Nme4_7850_1297919907.
Tobacco smoking remains one of the leading causes of death worldwide. Despite all the efforts made by governments, researchers and communities to educate women about the dangerous effects of tobacco smoke and nicotine, smoking during pregnancy continues to be a common habit and accounts for a significant percentage of fetal morbidity and mortality. The offspring is, as a result, exposed to nicotine through the blood and the milk of the mother. Nicotine is therefore expected to interact with the developing fetus and the offspring of mothers who smoke or use Nicotine Replacement therapy for smoking cessation, resulting in the interference with normal fetal lung development. Maternal cigarette smoke or nicotine exposure produces adverse effects in the lungs of offspring, these include intrauterine growth retardation, low birth weight, premature birth, reduced pulmonary function at birth, and a high occurrence of respiratory illnesses after birth. The main objectives of this study were to determine: 1) the effects of maternal nicotine exposure during gestation and lactation on lung development in the offspring, 2) if there is evidence of premature aging of the lungs of the lungs of the nicotine exposed offspring, and 3) whether tomato juice can have protective effects on the fetal lung development and function in the offspring. From the study, it was established that maternal nicotine exposure had no significant effect on the growth parameters of the offspring. However, it results in the late onset of gradual parenchymal damage which resembles premature aging. The study also found that the consumption of tomato juice may have protective effects on the premature aging of the lungs of the offspring.
Ingall, Georgina Ruth. "Understanding the complexities of smoking and quitting during pregnancy : An examination of the psychological determinants and attitudes." Thesis, University of Surrey, 2011. http://ethos.bl.uk/OrderDetails.do?uin=uk.bl.ethos.535702.
Jennings-Hobbs, Ruth. "Couple dynamics and maternal smoking cessation during pregnancy : a qualitative examination of nulliparous women and their partners." Thesis, University of Essex, 2018. http://repository.essex.ac.uk/21877/.
Dhalwani, Nafeesa N. "Using primary care data to assess population-level estimates of maternal smoking and nicotine replacement therapy during pregnancy." Thesis, University of Nottingham, 2014. http://eprints.nottingham.ac.uk/27761/.
Steele, Mary. "Development of a theory and evidence informed intervention to promote smoking cessation during pregnancy using narrative, text-messages and images as modes of delivery." Thesis, University of Stirling, 2015. http://hdl.handle.net/1893/22064.
Pertersen, Zainonisa. "Women's knowledge, practices and beliefs of smoking during pregnancy : a study of low income pregnant coloured women attending public sector antenatal clinics in South Africa." Master's thesis, University of Cape Town, 2005. http://hdl.handle.net/11427/3451.
Allston, Julie. "Factors characterizing stages of change for smoking during pregnancy: General risk knowledge, personal risk perceptions, motives, reasons and decisional balance." Thesis, University of Ottawa (Canada), 1995. http://hdl.handle.net/10393/10284.
Bailey, Beth A., Judy G. McCook, Alexis Hodge, and Lana McGrady. "Infant Birth Outcomes Among Substance Using Women: Why Quitting Smoking during Pregnancy Is Just as Important as Quitting Illicit Drug Use." Digital Commons @ East Tennessee State University, 2011. https://dc.etsu.edu/etsu-works/7173.
Bailey, Beth A., David Wood, Nathaniel Justice, and Darshan Shah. "Smoking During Pregnancy as a Risk Factor for Development and Severity of Neonatal Abstinence Syndrome Severity Among Newborns Prenatally Exposed to Opioids." Digital Commons @ East Tennessee State University, 2018. https://dc.etsu.edu/etsu-works/7666.
Negrão, Mary Elly Alves. "Associação entre consumo de tabaco e álcool na gestação e desenvolvimento infantil na coorte do pré natal de Ribeirão Preto/SP, 2010/13." Universidade de São Paulo, 2016. http://www.teses.usp.br/teses/disponiveis/17/17144/tde-29082016-115516/.
Tikanmäki, M. (Marjaana). "Preterm birth and parental and pregnancy related factors in association with physical activity and fitness in adolescence and young adulthood." Doctoral thesis, Oulun yliopisto, 2018. http://urn.fi/urn:isbn:9789526219233.
Wang, Chia Yin, and 王佳音. "Smoking cessation experience of women during pregnancy." Thesis, 2009. http://ndltd.ncl.edu.tw/handle/70650400552330078413.
"Smoking and Pregnant: Criminological Factors Associated with Maternal Cigarette Smoking and Marijuana Use during Pregnancy." Master's thesis, 2014. http://hdl.handle.net/2286/R.I.25066.
Cooke, Margaret. "Barriers to the systematic provision of smoking cessation education during pregnancy /." 1999. http://www.library.unsw.edu.au/~thesis/adt-NUN/public/adt-NUN2000.0014/index.html.
Gilligan, Conor. "Aboriginal and Torres Strait Islander women: an examination of smoking during pregnancy." 2008. http://hdl.handle.net/1959.13/29578.
Uphoff, E. P., Neil A. Small, and K. E. Pickett. "Using birth cohort data to assess the impact of the UK 2008-2010 economic recession on smoking during pregnancy." 2018. http://hdl.handle.net/10454/16240.
Chiu, Hsien-Tsai, and 邱顯財. "Effects of Maternal Cigarette Smoking and Environmental Tobacco Smoke during Pregnancy on Birth Outcomes." Thesis, 2005. http://ndltd.ncl.edu.tw/handle/22932803991866412769.
Haskins, Amy E. "Smoking during pregnancy: Patterns of use and maternal and birth outcomes among Hispanic women." 2008. https://scholarworks.umass.edu/dissertations/AAI3336960.
Šídová, Markéta. "Kouření tabáku a motivace ke změně v souvislosti s těhotenstvím." Master's thesis, 2013. http://www.nusl.cz/ntk/nusl-328253.
Hebeler, Charlotte J. "Smoking and hospital costs during pregnancy and the first year of life a dissertation submitted in partial fulfillment ... for the degree of Doctor of Public Health (Health Management and Policy) ... /." 2004. http://catalog.hathitrust.org/api/volumes/oclc/69001788.html.
(8797178), Emily Rolan. "CONTEXTUAL INFLUENCES OF PRENATAL AND POSTNATAL ENVIRONMENTS ON EXECUTIVE FUNCTION RISK FOR ADOLESCENT SUBSTANCE USE." Thesis, 2020.
Due to the great transitions and turmoil uniquely attributed to the period of adolescence, youth experience a greater risk for substance use and the multitude of concerns that coincide with the early onset of substance use. Many biological and environmental factors have been investigated as predictors of adolescent substance use. Executive function and disruptive behaviors are two important individual characteristics linked to adolescent substance use. Both smoking during pregnancy and sibling relationships are separate contexts that can mitigate or exacerbate the associations of executive function and adolescent substance use. The present study focuses on development of substance use through executive function deficits and disruptive behavior, while considering smoking during pregnancy and sibling relationships as unique moderators of these pathways. This work addresses a novel, interrelated set of questions with a series of three studies. The central hypothesis driving this program of research is that smoking during pregnancy and sibling relationships are under-studied contexts that can mitigate or exacerbate the associations of executive function, disruptive behavior, and adolescent substance use. This dissertation examines whether: (1) executive function mediates the smoking during pregnancy-disruptive behavior association and smoking during pregnancy exacerbates the executive function-disruptive behavior association, (2) smoking during pregnancy exacerbates the association between executive function and disruptive behavior during adolescence using a sibling comparison design, and (3) sibling relationship quality moderates developmental trajectories of executive function on the transition from disruptive problems to adolescent substance use using a high-risk, longitudinal sample. Findings challenge the link between exposure to smoking during pregnancy and both executive function and disruptive behavior. Further, these findings reinforce the need to utilize genetically-informed designs when examining potential effects of smoking during pregnancy. Additionally, this dissertation found support for the link between executive function and disruptive behavior, but not executive function and substance use.
Advertisement
Smokeless tobacco use in pregnancy: an integrative review of the literature
- Published: 04 May 2014
- Volume 59 , pages 599–608, ( 2014 )
Cite this article
- Angela Ratsch 1 &
- Fiona Bogossian 2
903 Accesses
20 Citations
2 Altmetric
Explore all metrics
To systematically critique and summarise the available evidence on the outcomes of smokeless tobacco use in pregnancy to inform the public health response.
In March 2013, a search was conducted of observational studies where the exposure to smokeless tobacco during pregnancy and maternal, placental and/or neonatal outcomes was assessed. Two reviewers extracted data and completed quality assessment of the literature utilizing the Agency for Healthcare Research and Quality criteria (West et al. 2002 ).
The search resulted in 211 articles, 21 (10 %) of which met the final criteria for integrative review. Ten (10) of the studies are from India, seven (7) from Sweden, two (2) from Alaska and one (1) each from South Africa and Pakistan.
Conclusions
Many studies lacked sufficient power to estimate precise risks. Most reports were hindered by imprecise measures of exposure and lack of confounding variable control. However, there were indications that maternal smokeless tobacco use increases rates of stillbirth, low birth weight and alters the male:female live birth ratio. Maternal smokeless tobacco use may not be safe for mother or foetus.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
The human cost of tobacco chewing among pregnant women in india: a systematic review and meta-analysis.
Rizwan A. Suliankatchi & Dhirendra N. Sinha

Health outcomes of maternal smoking during pregnancy and postpartum period for the mother and infant: protocol for an umbrella review
Tuba Saygın Avşar, Hugh McLeod & Louise Jackson
Short and long term health effects of parental tobacco smoking during pregnancy and lactation: a descriptive review
G. Banderali, A. Martelli, … E. Verduci
Aagaard-Tillery KM, Porter TF, Lane RH, Varner MW, Lacoursiere DY (2008) In utero tobacco exposure is associated with modified effects of maternal factors on fetal growth. Am J Obstet Gynecol 198 (1):66 e61–66. doi: 10.1016/j.ajog.2007.06.078
Agrawal P, Chansoriya M, Kaul KK (1983) Effect of tobacco chewing by mothers on placental morphology. Indian Pediatr 20(8):561–565
CAS PubMed Google Scholar
Ashfaq M, Channa MA, Malik MA, Khan D (2008) Morphological changes in human placenta of wet snuff users. J Ayub Med Coll Abbottabad: JAMC 20(2):110–113
PubMed Google Scholar
Baba S, Wikstrom AK, Stephansson O, Cnattingius S (2012) Influence of smoking and snuff cessation on risk of preterm birth. Eur J Epidemiol 27(4):297–304. doi: 10.1007/s10654-012-9676-8
Article PubMed Google Scholar
Benowitz NL, Porchet H, Sheiner L, Jacob P (1988) Nicotine absorption and cardiovascular effects with smokeless tobacco use: comparison with cigarettes and nicotine gum. Clin Pharmacol Ther 44(1):23–28. doi: 10.1038/clpt.1988.107
Article CAS PubMed Google Scholar
Bruin JE, Gerstein HC, Holloway AC (2010) Long-term consequences of fetal and neonatal nicotine exposure: a critical review. Toxicol Sci 116(2):364–374. doi: 10.1093/toxsci/kfq103
Article CAS PubMed Central PubMed Google Scholar
Burritt C, Gustafsson K (2013) Sweden’s Snus tobacco invades, but Americans Prefer Snuff. http://www.businessweek.com/articles/2013-07-03/swedens-snus-tobacco-invades-but-americans-prefer-snuff . Accessed 1 Aug 2013
Caldwell B, Sumner W, Crane J (2012) A systematic review of nicotine by inhalation: is there a role for the inhaled route? Nicotine Tob Res 14(10):1127–1139. doi: 10.1093/ntr/nts009
Cope GF, Nayyar P, Holder R (2001) Measurement of nicotine intake in pregnant women—associations to changes in blood cell count. Nicotine Tob Res 3(2):119–122. doi: 10.1080/14622200110042645
Dahlstrom A, Ebersjo C, Lundell B (2004) Nicotine exposure in breastfed infants. Acta Paediatr 93(6):810–816. doi: 10.1111/j.1651-2227.2004.tb03023.x
Deshmukh JS, Motghare DD, Zodpey SP, Wadhva SK (1998) Low birth weight and associated maternal factors in an urban area. Indian Pediatr 35(1):33–36
England LJ, Levine RJ, Mills JL, Klebanoff MA, Yu KF, Cnattingius S (2003) Adverse pregnancy outcomes in snuff users. Am J Obstet Gynecol 189(4):939–943. doi: 10.1067/S0002-9378(03)00661-6
England LJ, Grauman A, Qian C, Wilkins DG, Schisterman EF, Yu KF, Levine RJ (2007) Misclassification of maternal smoking status and its effects on an epidemiologic study of pregnancy outcomes. Nicotine Tob Res 9(10):1005–1013. doi: 10.1080/14622200701491255
England LJ, Kim SY, Tomar SL, Ray CS, Gupta PC, Eissenberg T, Cnattingius S, Bernert JT, Tita AT, Winn DM, Djordjevic MV, Lambe M, Stamilio D, Chipato T, Tolosa JE (2010) Non-cigarette tobacco use among women and adverse pregnancy outcomes. Acta Obstet Gynecol Scand 89(4):454–464. doi: 10.3109/00016341003605719
England LJ, Kim SY, Shapiro-Mendoza CK, Wilson HG, Kendrick JS, Satten GA, Lewis CA, Whittern P, Tucker MJ, Callaghan WM (2012) Maternal smokeless tobacco use in Alaska Native women and singleton infant birth size. Acta Obstet Gynecol Scand 91(1):93–103. doi: 10.1111/j.1600-0412.2011.01273.x
Ginzel KH, Maritz GS, Marks DF, Neuberger M, Pauly JR, Polito JR, Schulte-Hermann R, Slotkin TA (2007) Critical review: nicotine for the fetus, the infant and the adolescent? J Health Psychol 12(2):215–224. doi: 10.1177/1359105307074240
Giovino GA, Mirza SA, Samet JM, Gupta PC, Jarvis MJ, Bhala N, Peto R, Zatonski W, Hsia J, Morton J, Palipudi KM, Asma S (2012) Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross-sectional household surveys. Lancet 380(9842):668–679. doi: 10.1016/s0140-6736(12)61085-x
Gunnerbeck A, Wikstrom AK, Bonamy AK, Wickstrom R, Cnattingius S (2011) Relationship of maternal snuff use and cigarette smoking with neonatal apnea. Pediatrics 128(3):503–509. doi: 10.1542/peds.2010-3811
Gupta PC, Subramoney S (2004) Smokeless tobacco use, birth weight, and gestational age: population based, prospective cohort study of 1217 women in Mumbai, India. BMJ 328(7455):1538. doi: 10.1136/bmj.38113.687882.EB
Article PubMed Central PubMed Google Scholar
Gupta PC, Subramoney S (2006) Smokeless tobacco use and risk of stillbirth. Epidemiology 17(1):47–51. doi: 10.1097/01.ede.0000190545.19168.c4
Hurt RD, Renner CC, Patten CA, Ebbert JO, Offord KP, Schroeder DR, Enoch CC, Gill L, Angstman SE, Moyer TP (2005) Iqmik—a form of smokeless tobacco used by pregnant Alaska natives: nicotine exposure in their neonates. J Matern Fetal Neonatal Med 17(4):281–289. doi: 10.1080/14767050500123731
Benowitz NL, Hukkanen J, Jacob P, III (2009) Nicotine chemistry, metabolism, kinetics and biomarkers. In: Henningfield JE, London ED, Pogun S (eds) Nicotine psychopharmacology, vol 192. Handbook of experimental pharmacology. Springer, Berlin, pp 29–60. doi: 10.1007/978-3-540-69248-5_2
Jauniaux E, Burton GJ (2007) Morphological and biological effects of maternal exposure to tobacco smoke on the feto-placental unit. Early Human Dev 83(11):699–706. doi: 10.1016/j.earlhumdev.2007.07.016
Article CAS Google Scholar
Khalki H, Khalki L, Aboufatima R, Ouachrif A, Mountassir M, Benharref A, Chait A (2012) Prenatal exposure to tobacco extract containing nicotinic alkaloids produces morphological and behavioral changes in newborn rats. Pharmacol Biochem Behav 101(3):342–347. doi: 10.1016/j.pbb.2012.01.020
Krishna K (1978) Tobacco chewing in pregnancy. Br J Obstet Gynaecol 85(10):726–728. doi: 10.1111/j.1471-0528.1978.tb15591.x
Krishnamurthy S, Joshi S (1993) Gender differences and low birth weight with maternal smokeless tobacco use in pregnancy. J Trop Pediatr 39(4):253–254. doi: 10.1093/tropej/39.4.253
Lambers DS, Clark KE (1996) The maternal and fetal physiologic effects of nicotine. Semin Perinatol 20(2):115–126. doi: 10.1016/S0146-0005(96)80079-6
Law KL, Stroud LR, LaGasse LL, Niaura R, Liu J, Lester BM (2003) Smoking during pregnancy and newborn neurobehavior. Pediatrics 111(6 Pt 1):1318–1323. doi: 10.1542/peds.111.6.1318
Mehta A, Shukla S (1990) Tobacco and pregnancy. J Obstet Gynaecol India 40(2):156–160
Google Scholar
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62(10):1006–1012
Pratinidhi A, Gandham S, Shrotri A, Patil A, Pardeshi S (2010) Use of ‘Mishri’ a smokeless form of tobacco during pregnancy and its perinatal outcome. Indian J Commun Med 35(1):14–18. doi: 10.4103/0970-0218.62547
Article Google Scholar
Ratsch A, Steadman KJ, Bogossian F (2010) The pituri story: a review of the historical literature surrounding traditional Australian Aboriginal use of nicotine in Central Australia. J Ethnobiol Ethnomed 6:26. doi: 10.1186/1746-4269-6-26
Reeves S, Bernstein I (2008) Effects of maternal tobacco-smoke exposure on fetal growth and neonatal size. Expert Rev Obstetr Gynecol 3(6):719–730. doi: 10.1586/17474108.3.6.719
Richter P, Spierto FW (2003) Surveillance of smokeless tobacco nicotine, pH, moisture, and unprotonated nicotine content. Nicotine Tob Res 5(6):885–889. doi: 10.1080/14622200310001614647
Rogers JM (2009) Tobacco and pregnancy. Reprod Toxicol 28(2):152–160. doi: 10.1016/j.reprotox.2009.03.012
Sanderson S, Tatt ID, Higgins JP (2007) Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol 36(3):666–676. doi: 10.1093/ije/dym018
Sarkar S, Bhide M, Bhide A, Raghavan S, Maru G, Bhide S (1992) Transplacental exposure of human foetuses to nicotine/cotinine in masheri addict mothers and consequent loss of weight in babies at birth. Paper presented at the Nitroso Compounds: Biological Mechanisms, Exposures and Cancer Etiology Conference, Kailua-Kona, Hawaii, USA, November 1–2, 1991
Shafey O, Eriksen M, Ross H, Mackey J (2009) The tobacco atlas. American Cancer Society. http://www.afro.who.int/en/clusters-a-programmes/hpr/health-risk-factors/tobacco/tobacco-country-profiles.html . Accessed 20 June 2013
Slotkin T (1997) Expert opinions: Nicotine: interview with professor theodore slotkin. ABC Online—Quantum. Australia Broadcasting Corporation, Australia
Slotkin TA, Lappi SE, McCook EC, Lorber BA, Seidler FJ (1995) Loss of neonatal hypoxia tolerance after prenatal nicotine exposure: implications for sudden infant death syndrome. Brain Res Bull 38(1):69–75. doi: 10.1016/0361-9230(95)00073-N
Slotkin TA, McCook EC, Seidler FJ (1997) Cryptic brain cell injury caused by fetal nicotine exposure is associated with persistent elevations of c-fos protooncogene expression. Brain Res 750(1–2):180–188. doi: 10.1016/S0006-8993(96)01345-5
Steyn K, de Wet T, Saloojee Y, Nel H, Yach D (2006) The influence of maternal cigarette smoking, snuff use and passive smoking on pregnancy outcomes: the Birth to Ten Study. Paediatr Perinat Epidemiol 20(2):90–99. doi: 10.1111/j.1365-3016.2006.00707.x
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 283(15):2008–2012. doi: 10.1001/jama.283.15.2008
Subramoney S, Gupta PC (2008) Anemia in pregnant women who use smokeless tobacco. Nicotine Tob Res 10(5):917–920. doi: 10.1080/14622200802027206
Verma RC, Chansoriya M, Kaul KK (1983) Effect of tobacco chewing by mothers on fetal outcome. Indian Pediatr 20(2):105–111
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology 18(6):800–804. doi: 10.1097/EDE.0b013e3181577654
West S, King V, Carey T, Lohr K, McKoy N, Sutton S, Lux L (2002) Systems to rate the strength of scientific evidence. Evidence Report/Technology Assessment No. 472002. (trans: U.S. Department of Health and Human Services), Rockville
Wikstrom AK, Cnattingius S, Galanti MR, Kieler H, Stephansson O (2010a) Effect of Swedish snuff (snus) on preterm birth. BJOG 117(8):1005–1010. doi: 10.1111/j.1471-0528.2010.02575.x
Wikstrom AK, Cnattingius S, Stephansson O (2010b) Maternal use of Swedish snuff (snus) and risk of stillbirth. Epidemiology 21(6):772–778. doi: 10.1097/EDE.0b013e3181f20d7e
Wikstrom AK, Stephansson O, Cnattingius S (2010c) Tobacco use during pregnancy and preeclampsia risk: effects of cigarette smoking and snuff. Hypertension 55(5):1254–1259. doi: 10.1161/HYPERTENSIONAHA.109.147082
World Health Organization (2011a) WHO Report on the global tobacco epidemic, 2011: warning about the dangers of tobacco. World Health Organisation, Geneva
World Health Organization (2011b) Crude smokeless tobacco prevalence in WHO member states. World Health Organisation, Geneva
Download references
Acknowledgments
AR is supported by a Queensland Health—Office of Health and Medical Research Fellowship, The University of Queensland—School of Nursing and Midwifery-Elizabeth A. Davies Scholarship, and the Queensland Centaur Memorial Fund for Nurses Scholarship. The University of Queensland has provided a FirstLink Grant to the larger program of research of which this review forms a part.
Author information
Authors and affiliations.
Queensland Health and School of Nursing and Midwifery, The University of Queensland, Brisbane, Australia
Angela Ratsch
School of Nursing and Midwifery, The University of Queensland, Brisbane, Australia
Fiona Bogossian
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Angela Ratsch .
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (PDF 85 kb)
Supplementary material 2 (pdf 15 kb), supplementary material 3 (pdf 207 kb), supplementary material 4 (pdf 361 kb), rights and permissions.
Reprints and permissions
About this article
Ratsch, A., Bogossian, F. Smokeless tobacco use in pregnancy: an integrative review of the literature. Int J Public Health 59 , 599–608 (2014). https://doi.org/10.1007/s00038-014-0558-6
Download citation
Received : 14 October 2013
Revised : 14 April 2014
Accepted : 16 April 2014
Published : 04 May 2014
Issue Date : August 2014
DOI : https://doi.org/10.1007/s00038-014-0558-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Smokeless tobacco
- Maternal outcomes
- Perinatal outcomes
- Find a journal
- Publish with us
- Track your research
- Introduction
- Conclusions
- Article Information
Estimates of cumulative risk of (ie, proportion diagnosed with) any SMI by age among those with and without any SDP exposure in the full cohort (A) and in siblings discordant for SDP exposure only (B). Shaded areas are pointwise 95% CIs.
Hazard ratios from Cox proportional hazards regression models for any SMI (A), bipolar disorder (B), schizophrenia spectrum disorders (C), and SMI with substance use disorder (D). Population models (model 1) were adjusted only for offspring sex and parity. Adjusted models (model 2) additionally included maternal and paternal covariates. Cousin fixed-effects models (model 3) compared discordant cousins and included all covariates. Sibling fixed-effects models (model 4) compared discordant siblings and included offspring and paternal covariates and maternal age at childbirth; maternal covariates that could not differ among siblings were excluded. Error bars indicate 95% CIs. Moderate smoking during pregnancy: 1-9 cigarettes per day; high smoking during pregnancy: ≥10 cigarettes per day. Y-axes are natural log-scaled.
eTable 1. International Classification of Diseases (ICD) Codes
eTable 2. Discordant Cousin and Sibling Samples
eTable 3. Sensitivity Analyses: First-Born Cousins, Additional Outcomes, and Missing Data
eTable 4. Sensitivity Analyses: Population Associations
eFigure. Kaplan-Meier Plots for Bipolar Disorder, Schizophrenia Spectrum Disorders, Severe Mental Illness and Substance Use Disorder, and Any Substance Use Disorder
- Causal Inference in Psychiatric Epidemiology JAMA Psychiatry Editorial June 1, 2017 Kenneth S. Kendler, MD
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
Quinn PD , Rickert ME , Weibull CE, et al. Association Between Maternal Smoking During Pregnancy and Severe Mental Illness in Offspring . JAMA Psychiatry. 2017;74(6):589–596. doi:10.1001/jamapsychiatry.2017.0456
Manage citations:
© 2024
- Permissions
Association Between Maternal Smoking During Pregnancy and Severe Mental Illness in Offspring
- 1 Department of Psychological and Brain Sciences, Indiana University, Bloomington
- 2 Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden
- 3 Astrid Lindgren Children's Hospital, Karolinska University Hospital, Stockholm, Sweden
- 4 School of Medical Sciences, Örebro University, Örebro, Sweden
- Editorial Causal Inference in Psychiatric Epidemiology Kenneth S. Kendler, MD JAMA Psychiatry
Question Does exposure to maternal smoking during pregnancy increase the risk of severe mental illness in offspring?
Findings In a population-based cohort of 1.7 million Swedish offspring, maternal smoking during pregnancy was associated with an increased risk of severe mental illness in offspring. However, sibling comparisons, which ruled out all genetic and environmental confounders that make siblings similar, revealed much weaker and statistically nonsignificant associations.
Meaning This study suggests that much of the association between smoking during pregnancy and severe mental illness in offspring is likely explained by familial confounding rather than by causal teratogenic effects.
Importance Several recent population-based studies have linked exposure to maternal smoking during pregnancy to increased risk of severe mental illness in offspring (eg, bipolar disorder, schizophrenia). It is not yet clear, however, whether this association results from causal teratogenic effects or from confounding influences shared by smoking and severe mental illness.
Objective To examine the association between smoking during pregnancy and severe mental illness in offspring, adjusting for measured covariates and unmeasured confounding using family-based designs.
Design, Setting, and Participants This study analyzed population register data through December 31, 2013, for a cohort of 1 680 219 individuals born in Sweden from January 1, 1983, to December 31, 2001. Associations between smoking during pregnancy and severe mental illness in offspring were estimated with adjustment for measured covariates. Cousins and siblings who were discordant on smoking during pregnancy and severe mental illness were then compared, which helped to account for unmeasured genetic and environmental confounding by design.
Exposures Maternal self-reported smoking during pregnancy, obtained from antenatal visits.
Main Outcomes and Measures Severe mental illness, with clinical diagnosis obtained from inpatient and outpatient visits and defined using International Classification of Diseases codes for bipolar disorder and schizophrenia spectrum disorders.
Results Of the 1 680 219 offspring included in the analysis, 816 775 (48.61%) were female. At the population level, offspring exposed to moderate and high levels of smoking during pregnancy had greater severe mental illness rates than did unexposed offspring (moderate smoking during pregnancy: hazard ratio [HR], 1.25; 95% CI, 1.19-1.30; high smoking during pregnancy: HR, 1.51; 95% CI, 1.44-1.59). These associations decreased in strength with increasing statistical and methodologic controls for familial confounding. In sibling comparisons with within-family covariates, associations were substantially weaker and nonsignificant (moderate smoking during pregnancy: HR, 1.09; 95% CI, 0.94-1.26; high smoking during pregnancy: HR, 1.14; 95% CI, 0.96-1.35). The pattern of associations was consistent across subsets of severe mental illness disorders and was supported by further sensitivity analyses.
Conclusions and Relevance This population- and family-based study failed to find support for a causal effect of smoking during pregnancy on risk of severe mental illness in offspring. Rather, these results suggest that much of the observed population-level association can be explained by measured and unmeasured factors shared by siblings.
Maternal smoking during pregnancy (SDP) is associated with a breadth of adverse offspring outcomes, including pregnancy-related, neurodevelopmental, and behavioral problems. 1 - 3 In particular, recent studies have provided novel evidence of associations between SDP and offspring bipolar disorder, 4 schizophrenia, 5 and related outcomes. 6 These associations raise the possibility that SDP exposure has causal teratogenic effects on the risk of severe mental illness (SMI). 7 Because nicotine and carbon monoxide cross the placenta and may directly and indirectly (eg, via hypoxia) affect fetal neurodevelopment, 8 this hypothesis is biologically plausible. Moreover, 8% of pregnant women in the United States smoke, 9 meaning that SDP exposure could represent a potentially modifiable and important source of risk for SMI. 10 Recent editorials have made explicit causal claims regarding teratogenic effects of SDP on offspring neurodevelopment and psychopathology. 11 , 12
Understanding the processes underlying associations between SDP and offspring SMI is essential to evaluating a teratogenic hypothesis. Some animal research is consistent with a possible SDP effect on schizophrenia risk, 7 whereas prior human observational studies have found inconsistent evidence, 13 - 18 possibly because of differences in methodologies and statistical power. 5 One study of bipolar disorder, for example, found that the SDP association was greatly attenuated when adjusted for parental characteristics, 19 suggesting that family background, rather than a teratogenic effect, may explain the association.
Tests of teratogenic SDP hypotheses that use observational data are, more broadly, subject to the serious threat of confounding from unmeasured familial factors, including other prenatal exposures, postnatal environments, and genetically transmitted risk of SMI and other psychopathology (ie, passive gene-environment correlation). 2 , 20 , 21 Indeed, there is reason to suspect that familial factors may contribute to associations between maternal SDP and offspring SMI, as the association between an individual’s own smoking and risk of psychosis is partially explained by shared familial risk. 22 Numerous sibling comparison studies have demonstrated substantial familial confounding of associations between SDP and offspring outcomes, including intellectual performance, academic achievement, externalizing psychopathology, and criminality, 1 , 23 - 28 with few exceptions. 29 - 32 This confounding appears to result from shared genetic influences in mothers and offspring. 33 , 34 Thus, there is a clear need for well-powered studies that can more fully account for familial confounding in the association between SDP and offspring SMI.
The present study used population-level data and family-based comparisons of cousins and siblings to examine the association between SDP and multiple indices of offspring SMI. 35 - 37 Sibling comparisons, in particular, rule out all genetic and environmental influences that make siblings similar to one another, producing a strong test of a teratogenic hypothesis. To our knowledge, this study is the first such family-based examination of SDP and offspring SMI.
We analyzed data through December 31, 2013, from single-birth offspring born in Sweden from January 1, 1983, to December 31, 2001. As described previously, 24 , 25 these data were drawn from the following merged Swedish population registers: (1) Medical Birth Register (live births and antenatal care visits in Sweden since 1973), 38 (2) Multi-Generation Register (familial relationships for individuals born since 1932 and living in Sweden since 1961), 39 (3) National Patient Register (nationwide inpatient psychiatric hospital admissions since 1987 and outpatient specialist visits since 2001), 40 (4) National Crime Register (criminal convictions for those aged 15 years or older since 1973), 41 (5) Education Register (highest level of formal education since 1990), 42 (6) Cause of Death Register (dates and causes of death since 1961), 43 and (7) Migration Register (dates of emigration out of Sweden since 1915). 44
Of the 1 878 371 single births during the included period, 1 809 183 (96.3%) offspring were still living in Sweden at age 12 years and were therefore included in this study. We sequentially excluded offspring missing data on paternity (n = 9532), SDP (n = 110 101), paternal (n = 7002) or maternal (n = 2280) educational level, or paternal (n = 35) or maternal (n = 14) nationality, resulting in a complete case cohort of 1 680 219 offspring (89.5% of the target population of single births). This study was approved by the Indiana University Institutional Review Board and the Regional Ethical Review Board in Stockholm.
From 1983 onward, mothers reported their daily cigarette smoking quantity at the first antenatal visit, typically in the first trimester. We contrasted moderate SDP (1-9 cigarettes per day) and high SDP (≥10 cigarettes per day) with no reported smoking ( Table 1 ). The antenatal SDP variable has been used extensively in research and has demonstrated expected associations with multiple maternal 34 and offspring 24 , 25 behavioral outcomes. In addition, in a validity study of antenatal visits, only 6% of women who denied smoking had cotinine levels consistent with active smoking. 45
We evaluated associations between SDP and first inpatient or outpatient SMI diagnosis in offspring, requiring offspring to be at least age 12 years to receive a diagnosis. We defined SMI diagnoses with International Classification of Diseases, Ninth Revision , and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision , codes for bipolar disorder and schizophrenia spectrum disorders, which we adapted from prior Swedish register–based research (eTable 1 in the Supplement ). 46 , 47 The probability of any SMI diagnosis by age 25 years was 1.3% ( Table 1 ). Because comorbidity with substance use disorder (SUD) appears to explain much of the risk of violence among patients with SMI, 21 we also identified patients with SMI and SUD, with onset defined as the first diagnosis of either disorder.
For each offspring, we included covariates for sex, parity, and maternal and (biological) paternal ages at childbirth. We additionally included covariates for maternal and paternal nationality and the highest level of completed education, as well as any registrations for criminal conviction, hospitalization for SMI, hospitalization for suicidal behavior, and hospitalization for SUD ( Table 1 ).
Follow-up person-time at risk for SMI varied across offspring and was right-censored by the earliest occurrence of death, first emigration out of Sweden, or the end of the study. To account for this censoring, we used survival analysis to evaluate associations between SDP and first SMI diagnosis in offspring, with offspring age as the timescale. 48
First, we plotted cumulative risk (ie, 1 − Kaplan-Meier estimates) for SMI conditional on SDP exposure in (1) all offspring and (2) the subset of siblings discordant for SDP. Second, we estimated hazard ratios (HRs) using 4 Cox proportional hazards regressions. Model 1 (population) examined population associations, with offspring sex and parity covariates and robust SEs to account for clustering of offspring born to the same mother. Model 2 (adjusted) additionally adjusted for maternal and paternal ages at childbirth and the other measured parental covariates.
Model 3 (cousin) and model 4 (sibling) compared discordant cousins or siblings in the same family, independent of all influences that make family members similar to each other. The sibling comparisons, in particular, enabled strong tests of a causal teratogenic SDP hypothesis. 35 - 37 If an observed association persisted in model 4, the result would be consistent with the hypothesis that SDP increases the risk of offspring SMI. In contrast, to the extent that the association was attenuated, the result would fail to support this causal hypothesis and would instead suggest that the population association resulted from familial confounding.
We estimated sibling and cousin associations using fixed-effects (stratified Cox) regression. 49 The sibling models were stratified on mothers. These models allowed the baseline hazard function to differ across groups of offspring born to distinct mothers. We also included measured covariates that could vary within families. The complete case cohort included a total of 1 245 299 siblings born to 536 772 distinct mothers. Of these families, 7201 (1.3%) were discordant on SMI. Among these, 1501 families (20.8%) with 3615 offspring varied in SDP exposure (no, moderate, or high smoking).
The cousin models included all possible nonsibling pairs of offspring within a maternal-grandmother–descended extended family (ie, all pairs of offspring of sisters). These models were stratified on cousin pairs, with robust SEs to adjust for nonindependence introduced by including individual cousins in multiple cousin pairs. The cousin comparisons included 612 191 offspring in 746 063 pairs of cousins who descended from 152 887 distinct maternal grandmothers. Of these cousin pairs, 7697 (1.0%) were discordant on SMI, among whom 3140 (40.8%; descended from 1799 maternal grandmothers) were discordant on SDP (eTable 2 in the Supplement provides exposure and outcome discordant samples for the other outcomes).
Because of their reliance on within-family discordance, sibling comparisons can be subject to bias due to carryover effects from 1 sibling to another (eg, if an offspring’s SDP exposure influences siblings’ SMI risk), measurement error, or unmeasured within-family confounding. 36 , 50 Sibling comparisons may also have reduced power or generalizability. We used 6 alternative approaches to examine the validity of the sibling comparison associations. First, we included cousin comparisons. Although they rule out less familial confounding by design (because of differences among sisters in the maternal generation), cousin comparisons are less likely to be biased by carryover effects. Cousins are also more likely to be differentially exposed, which increases power and generalizability. Second, to further examine generalizability, we estimated population associations in families with multiple vs single included offspring (the latter are excluded from sibling comparisons because they cannot provide information). Third, to evaluate any possible effect of decreases over time in the prevalence of SDP, 51 we stratified population analyses by time. Fourth, to further examine associations independent of carryover and birth-order effects, we compared first-born cousins. Fifth, to examine the possibility that low power or SDP measurement error resulted in underestimated within-family associations, we conducted logistic regressions predicting birth outcomes generally understood to be causally affected by SDP (ie, small for gestational age [birthweight >2 SDs below the gestational age–specific mean] and preterm birth [gestational age <37 weeks]). 1 , 2 , 23 , 51 These models additionally controlled for year of birth. Finally, to examine the potential effect of missing SDP data, we conducted analyses assuming that all missing values were either high SDP or no SDP. We analyzed data using SAS, version 9.4 (SAS Institute Inc), and Stata, version 13.1 (StataCorp).
Offspring with any SDP exposure had a greater risk of any SMI than did offspring without SDP exposure ( Figure 1 ). In contrast, the difference in risks was greatly attenuated when we limited the Kaplan-Meier estimates to discordantly exposed siblings, which was inconsistent with a teratogenic hypothesis.
Cox proportional hazards regressions confirmed this pattern ( Table 2 and Figure 2 ). At the population level, offspring with moderate SDP had a 25% greater rate of any SMI than did offspring with no SDP (HR, 1.25; 95% CI, 1.19-1.30), and offspring with high SDP had a 51% greater rate (HR, 1.51; 95% CI, 1.44-1.59). Adjusting for confounding via measured parental covariates began to attenuate these associations (moderate SDP: HR, 1.12; 95% CI, 1.07-1.17; high SDP: HR, 1.27, 95% CI; 1.20-1.33), whereas the cousin comparison did not substantially attenuate the associations further (moderate SDP: HR, 1.13; 95% CI, 1.04-1.24; high SDP: HR, 1.23; 95% CI, 1.11-1.36). Sibling comparison associations were weaker still and were not statistically significant (moderate SDP: HR, 1.09; 95% CI, 0.94-1.26; high SDP: HR, 1.14; 95% CI, 0.96-1.35).
Disorder-specific results are reported in Table 2 and Figure 2 (eFigure in the Supplement presents disorder-specific Kaplan-Meier estimates). For bipolar disorder, the pattern of associations was similar to that of any SMI. At the population level, moderate SDP was associated with a 29% greater rate of offspring bipolar disorder compared with no SDP, and high SDP was associated with a 54% greater rate. However, in the sibling comparison, the moderate (6% greater rate) and high (15% greater rate) SDP associations were greatly attenuated and not statistically significant. For offspring schizophrenia spectrum disorders, most of the association could be attributed to confounding from measured parental covariates in the adjusted model, and the sibling comparison associations were again not statistically significant. For comorbid SMI and SUD in offspring, the population association was stronger (eg, a greater rate by a factor of 233% for high SDP), as was the attenuation across models, such that high SDP was associated with only a 5% greater—and not statistically significant—rate in the sibling comparison. We found a similar pattern of results for any offspring SUD diagnosis (eTable 3 in the Supplement ).
Most sensitivity analyses explored the association between SDP and any offspring SMI (eTable 3 and eTable 4 in the Supplement ). First, as described above, cousin comparisons were consistent in suggesting that much of the association was attributable to familial confounding. Second, population associations were modestly but not substantively larger in multiple-offspring compared with single-included-offspring families, indicating that the pattern of results in Table 2 could not be explained by the exclusion of single-offspring families from the sibling comparisons. Third, the population associations were larger in later birth years. Although this difference was not consistent with a teratogenic hypothesis, it suggested that the processes underlying the associations may be changing. Fourth, first-born cousin fixed-effects comparisons showed attenuated and nonsignificant associations independent of carryover or birth-order effects. Fifth, inconsistent with the possibility that low power or SDP measurement error explained the attenuated SMI associations, we found the expected offspring-birth-outcome sibling-comparison associations between SDP and small for gestational age (moderate SDP: odds ratio [OR], 1.63; 95% CI, 1.50-1.77; high SDP: OR, 1.83; 95% CI, 1.66-2.02) and preterm birth (moderate SDP: OR, 1.13; 95% CI, 1.06-1.20; high SDP: OR, 1.22; 95% CI, 1.13-1.32). Finally, results did not meaningfully differ regardless of the coding of missing SDP values.
The need to identify environmental risk factors for SMI that are strongly associated, modifiable, and, most important, causal has generated interest in findings of associations between maternal SDP and offspring bipolar disorder and schizophrenia. 52 , 53 Using data from nearly 1.7 million offspring, the present study replicated these associations at the population level. 4 , 5 Consistent with prior population-based studies, children of mothers who smoked heavily during pregnancy had approximately 1.5 times the rate of developing bipolar disorder or schizophrenia spectrum disorders as did children of mothers who did not smoke during pregnancy.
Critically, however, adjustment for measured parental covariates and unmeasured familial confounding largely attenuated these associations. Indeed, for each SMI outcome, sibling comparisons with within-family covariates yielded small, nonsignificant differences between offspring discordant for exposure to SDP. Some 4 , 5 —but not all 19 —recent studies have found associations between SDP and an increased risk of offspring SMI even after controlling for measured parental psychiatric factors and other potential confounders, as did most of our adjusted models. However, we found that associations attenuated even further in sibling comparisons. This pattern highlights the value of family-based approaches that use relatedness to rule out passive gene-environment correlation and other familial factors rather than relying on measured covariates that may not be assessed or modeled comprehensively. 36
Moreover, additional strengths of the present study increase confidence in the results. The sample was population based, including 89.5% of single births in Sweden from 1983 to 2001 and 14 215 offspring with SMI diagnoses. Data on SDP and SMI were collected prospectively and from differing sources (maternal self-report and clinical diagnoses, respectively). Sibling and cousin comparisons and other sensitivity analyses supported the overall findings independent of the assumptions and limitations specific to each approach (eg, decreased power and generalizability in sibling comparisons). Taken in sum, these data provide strong evidence that much of the population association between SDP and offspring SMI results from shared risk for both outcomes rather than from a true teratogenic effect. If a causal effect of SDP exposure on offspring SMI risk exists, our results suggest that it is likely to be substantially weaker than would be expected based on the population associations alone.
One question unanswered by the present results is the exact nature of the familial confounding. Prior quantitative genetic studies, 33 , 34 as well as in vitro fertilization–based studies of genetically unrelated mother-offspring pairs, 54 , 55 point to genetic confounding, with an adoption study providing evidence against postnatal environmental confounding. 56 At the same time, there is emerging evidence that associations between smoking and SMI also reflect multiple environmentally mediated pathways. 22 , 52 Indeed, we found that population associations between SDP and offspring SMI strengthened over time. One possible explanation for this change is that, as smoking has become less prevalent, women who smoked—and smoked during pregnancy—more recently were those with the greatest liability for substance abuse and other psychopathology. 57 As a result, the extent of confounding from familial psychopathology liability may have expanded. Research is needed to characterize the sources of confounding observed here.
Sibling comparisons have several limitations that we could not fully address. First, they are susceptible to confounding from unmeasured factors that make siblings different from one another. The within-family associations may, therefore, overestimate or underestimate the true SDP effect. However, to explain the weak and nonsignificant sibling differences found here, unmeasured within-family confounders would have to be positively associated with SDP and negatively associated with offspring SMI (or vice versa). Nevertheless, evidence from other advanced observational approaches with complementary strengths (eg, instrumental variable analyses, in vitro fertilization–based designs, negative controls) 15 , 20 , 58 is needed to strengthen conclusions from this study. 59 Second, the maternal report assessment of SDP occurred at a single visit, relied on self-report, and did not permit us to explore details of timing, fine-grained smoking frequency, or quitting smoking. Measurement error and, especially, limited phenotyping, have been raised as potential challenges for register-based approaches. 60 However, previous studies have supported the validity of the SDP measure, and we found expected within-family associations with pregnancy-related outcomes, suggesting that measurement error alone cannot account for the main findings. Third, we analyzed a sample of Swedish offspring that included SMI onsets through the first 3 decades of life. We do not know the extent to which our results will generalize to other populations or to later-onset SMI. Finally, our analyses cannot determine whether SDP is more strongly associated with offspring SMI in specific subgroups.
There is extensive and clear evidence that exposure to SDP—in addition to smoking itself—has severe adverse consequences. 52 This study did not, however, support a causal teratogenic hypothesis specific to offspring SMI, which matches prior family-based studies of SDP and a range of other offspring cognitive, behavioral, and psychiatric outcomes. 1 , 2 Within-family and other advanced designs appear to hold promise for the continued evaluation of environmental risk factors for SMI.
Accepted for Publication: February 18, 2017.
Corresponding Author: Patrick D. Quinn, PhD, Department of Psychological and Brain Sciences, Indiana University, 1101 E 10th St, Bloomington, IN 47405 ( [email protected] ).
Published Online: May 3, 2017. doi:10.1001/jamapsychiatry.2017.0456
Author Contributions: Dr Rickert had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Quinn, Rickert, Weibull, Johansson, Lichtenstein, Larsson, D'Onofrio.
Acquisition, analysis, or interpretation of data: Quinn, Rickert, Lichtenstein, Almqvist, Larsson, Iliadou, D'Onofrio.
Drafting of the manuscript: Quinn.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Rickert.
Obtained funding: Quinn, Lichtenstein, Almqvist, Larsson, D'Onofrio.
Administrative, technical, or material support: Lichtenstein, Iliadou.
Supervision: Larsson, D'Onofrio.
Conflict of Interest Disclosures: Dr Lichtenstein has served as a speaker for Medice and Dr Larsson has served as a speaker for Eli Lilly and Shire and has received a research grant from Shire, all outside the submitted work. No other disclosures were reported.
Funding/Support: This project was supported by National Institute of Mental Health grant R01MH102221; National Institute on Drug Abuse grant K99DA040727; the Indiana Clinical and Translational Sciences Institute: Pediatric Project Development Team; the Swedish Research Council (2013-2280, 2011-2492, and, through the Swedish Initiative for Research on Microdata in the Social and Medical Sciences framework, 340-2013-5867); and the Swedish Research Council for Health, Working Life, and Welfare (2012-1678).
Role of the Funder/Sponsor: The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: Kyle Gerst, MS (Indiana University, Bloomington), assisted with statistical analyses. There was no financial compensation.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Cigarette and E-Cigarette Perceptions About Harm During Pregnancy
Affiliation.
- 1 Health, Human Performance and Recreation, University of Arkansas, Fayetteville, AR.
- PMID: 38598822
- DOI: 10.1097/NNR.0000000000000742
Background: Research suggests that pregnancy status (prior, current, and future intention) is associated with differences in perceived harm of e-cigarette use during pregnancy. However, perceptions of health outcomes attributed to cigarette and e-cigarette use during pregnancy have not been explored among a sample of pregnant women who smoke.
Objectives: The purpose of this study was to explore differences in perceived harms of cigarette and e-cigarette use and perceived birth and health outcomes associated with cigarette and e-cigarette use during pregnancy among a sample of pregnant women who currently smoked.
Methods: Using a cross-sectional online survey, we examined perceptions about cigarette and e-cigarette use during pregnancy among a sample of U.S. pregnant women (n = 267) who smoked in the past 30 days. Participants were grouped into categories based on e-cigarette use status (current, past, and never e-cigarette users). Differences between e-cigarette use status and perceived harm (absolute of cigarettes, e-cigarettes, and relative of e-cigarettes) and perceived health outcomes attributed to smoking/e-cigarette use were examined.
Results: Among our sample, 45.7%, 39.7%, and 14.6% were current, ever, and never e-cigarette users, respectively. Associations existed between e-cigarette use status and absolute perceived harm of cigarettes, relative perceived harm of e-cigarettes, and perceived health outcomes. Current e-cigarette users believed pregnant women who smoked cigarettes were more likely to lose a child due to miscarriage or sudden infant death syndrome or give birth to a child with low birth weight, reduced lung function, cleft lip, reduced brain function, or attention-deficit/hyperactivity disorder than never e-cigarette users. No associations were found between perceived birth and health outcomes of e-cigarette use by e-cigarette user status.
Discussion: Pregnant women who smoked and used e-cigarettes had lower risk perceptions about e-cigarette use during pregnancy than those who only smoked. Health messages and research about the harms of nicotine exposure during pregnancy should address the risks of dual-use versus only e-cigarette use. Additionally, messages about the relative harm of e-cigarettes compared to cigarettes are needed for pregnant women who smoke and have trouble quitting.
Copyright © 2024 Wolters Kluwer Health, Inc. All rights reserved.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.12(7); 2022

Effect of maternal cigarette smoking and alcohol consumption during pregnancy on birth weight and cardiometabolic risk factors in infants, children and adolescents: a systematic review protocol
Tammy charlene hartel.
1 Medical Bioscience, University of the Western Cape, Bellville, Western Cape, South Africa
Eunice Bolanle Turawa
2 Burden of Disease Research Unit, South African Medical Research Council, Tygerberg, South Africa
André Oelofse
Juléy janice abigail de smidt, associated data.
bmjopen-2022-061811supp001.pdf
bmjopen-2022-061811supp002.pdf
Introduction
Tobacco smoking and alcohol consumption during pregnancy are particularly prevalent in low socioeconomic status populations, with an adverse association with birth outcomes and cardiometabolic risk factors. However, the direct and indirect effects of prenatal cigarette smoking and alcohol consumption during pregnancy on cardiometabolic risk in offspring have been rather inconsistent. This may be attributed to multiple factors, such as the amount and timing of exposure to tobacco smoking and alcohol during pregnancy; the influence of maternal, environmental and socioeconomic factors; or how risk factors were defined by individual researchers and studies. Therefore, this review aims to provide a summary of the most recent evidence on birth outcomes and cardiometabolic risk in children associated with alcohol and/or tobacco exposure in utero.
Methods and analysis
PubMed, Scopus and Web of Science will be searched to identify published articles from 1 January 2001. Clinical studies that investigate the association between maternal cigarette smoking or alcohol consumption and birth weight and cardiometabolic risk factors in infants, children and adolescents will be included. Prospective cohort, case-control studies and birth cohort studies will be eligible for inclusion. Grey literature will be searched including conference proceedings, Google Scholar and the ProQuest Dissertation and Theses database. Only studies published in English will be included, with no restrictions regarding country, race or gender. Two independent reviewers will conduct the literature search and article screening. Eligibility criteria will be based on the population (infants, children, adolescents), exposure (maternal cigarette smoking, alcohol consumption or both), comparator (control group with no exposure during pregnancy) and outcomes (birth weight and cardiometabolic risk factors). Quality assessment and risk of bias will be assessed using a risk of bias tool for observational studies, and data will be extracted for analysis using a researcher-generated data extraction form. A meta-analysis will be performed to estimate pooled effect sizes if there are sufficient good-quality studies available. Sources of heterogeneity will be explored using subgroup analysis.
Ethics and dissemination
Ethical clearance will not be required as this review will extract publicly available secondary data. Findings from this review will be disseminated via publication in a peer-review journal.
PROSPERO registration number
CRD42021286630.
Strengths and limitations of this study
- This review will synthesise literature from primary human studies that investigate the association, correlation or causation between alcohol and/or tobacco exposure during pregnancy and birth weight or cardiometabolic risk factors in offspring.
- A comprehensive synthesis of all accessible data on outcomes of maternal cigarette smoking and/or alcohol consumption during pregnancy on birth weight and cardiometabolic risk factors in children will make use of a standardised risk of bias tool.
- The Grading of Recommendations, Assessment, Development and Evaluations will be used to assess the quality and to strengthen the evidence from the review.
- Potential publication bias might limit the review; databases will therefore be searched to find unpublished studies such as thesis dissertations and conference proceedings to minimise the risk of publication bias.
- The review will be limited to evidence from an approximately two-decade period from 2001 to the final search.
In 2020, the global prevalence of tobacco smoking among women was 6.5%. 1 This was attributed to a substantial decline in tobacco smoking of >40% in high socioeconomic countries over the past 50 years, however, there has been little to no decline in low-income and middle-income countries. 1 Globally, 32.4% of women 16 years and older were reported to be current alcohol consumers. 2 Despite the general decline in alcohol consumption and tobacco smoking among women globally, tobacco smoking remains a significant health burden, particularly among women of low socioeconomic status. 1 Women in low socioeconomic status communities were more likely to consume alcohol or use narcotics, concurrently with tobacco smoking during pregnancy, compared with women of higher socioeconomic status. 3–5 In addition, the global prevalence of illicit narcotic use during pregnancy was 1.6% in 2020, with studies reporting a 7.4 times higher prevalence after toxicological analysis. 3
In South Africa, 61.2% of women reported alcohol consumption, 56.3% reported smoking tobacco only and 37.4% reported concomitant use of tobacco and alcohol during pregnancy. 4 Women of low education level, of low economic status and those that had mental health disorders were more likely to smoke during pregnancy, relapse after pregnancy and were less likely to quit smoking, compared with women with a higher education level and income status. 6 This, invariably, exacerbates the adverse effects on their offspring during and after pregnancy. 6 Similarly, biochemical analysis revealed that more than one in five pregnant women did not report their smoking status, 7 resulting in under-reported smoking and drinking patterns among pregnant women.
In the past decade, the research field of the Developmental Origins of Health and Disease (DOHaD) has significantly increased, with evidence growing on the detrimental effects of alcohol and tobacco exposure, in utero, on the development of cardiometabolic risk factors in adulthood. 8 9 Cardiometabolic risk factors include increased central adiposity, elevated triglycerides, decreased high-density lipoprotein cholesterol, elevated blood pressure and hyperglycaemia, predisposes individuals to developing type 2 diabetes mellitus and cardiovascular disease (CVD). 10 Magge et al 10 described metabolic syndrome (MetS) as having at least three of these risk factors. Despite the challenges of defining MetS in children and adolescents, Magge et al 10 emphasise that clinical screening should rather shift the focus to cardiometabolic risk factors to address the major risks associated with MetS. Moreover, cardiometabolic risk factors were reported in offspring that were exposed to tobacco smoke or alcohol in utero, which needs further investigation. 11–18 Furthermore, infants born to mothers who smoke, or those that consume alcohol during pregnancy, were reported to have an increased risk of adverse birth outcomes, such as low birth weight. 7 19 This is augmented in infants born to mothers who smoked tobacco and consumed alcohol during pregnancy compared with those born to mothers that smoked only, consumed alcohol only or abstained completely. 20 Therefore, birth weight is an important risk factor for CVD and was shown to be significantly associated with body mass index (BMI), skinfold thickness and adolescent obesity, 21 22 dyslipidaemia, altered glucose metabolism, hypertension as well as low-grade inflammation. 23
Whether infants are born small or large for their gestational age, both have been reported to increase their risk of overweight or obesity later in life, due to catch-up growth. 23–25 Moreover, Koklu et al 26 reported a significantly higher aortic intima-media thickness in infants that were born small for gestational age, compared with infants born appropriate for their gestational age, that was also associated with hypertriglyceridaemia. 26 Low birth weight, as well as small for gestational age was associated with higher carotid intima-media thickness values in children and linked to metabolic abnormalities, such as dyslipidaemia, abdominal obesity, hypertension and development of insulin resistance later in life. 24 27 28 Thus, overweight children or children with obesity who were born with low birth weight are more likely to present with cardiometabolic risk factors, such as elevated systolic and diastolic blood pressures, triglycerides and low-density lipoprotein cholesterol blood concentrations. 29 It is evident that tobacco smoking and alcohol use, during pregnancy, results in adverse birth outcomes which increases cardiometabolic health risks in both childhood and adulthood. 30 Therefore, the DOHaD may play a significant role in the development of cardiometabolic diseases in low-income and middle-income countries as poverty, malnutrition, licit and illicit narcotic use during pregnancy, as well as low birth weight are often prevalent in low socioeconomic regions. 12
In summary, to help prevent CVD later in life, it is important to assess adverse birth outcomes in infants and cardiometabolic risk factors in early childhood, including low birth weight, dyslipidaemia, abdominal obesity, hypertension, high blood glucose and insulin resistance. 31 In South Africa, this is a concern as research has shown that tobacco smoking and alcohol consumption is highly prevalent in low socioeconomic populations while pregnant. 4 20 32–34 However, the evidence investigating the relationship between exposure to alcohol and tobacco during pregnancy and cardiometabolic outcomes in offspring is not consistent, warranting the present review. Therefore, the direct and indirect effects of maternal cigarette smoking and/or alcohol consumption during pregnancy on cardiometabolic risk in offspring merits further investigation. This review aims to provide a summary of up-to-date evidence on the relationship between alcohol and/or tobacco exposure in utero, birth outcomes and cardiometabolic outcomes in children.
Justification for this review
Previous systematic reviews have addressed cardiorenal outcomes, body composition and metabolic outcomes in offspring exposed to alcohol during pregnancy, 14 35 as well as maternal smoking during pregnancy and child diabetes mellitus type 2, whereas only a few systematic reviews addressed maternal smoking during pregnancy and childhood overweight and obesity. 31 36–38 Prenatal smoking and cardiometabolic risk factors were recently studied, but only among adults over 18 years of age. 8 Therefore, none of the latter has included all cardiometabolic risk factors in offspring and both maternal cigarette smoking and alcohol consumption during pregnancy. In addition, a review conducted in 2013 investigated maternal smoking during pregnancy and MetS in children and requires updating. 39 Therefore, a systematic review will be conducted to summarise the most recent evidence-based birth outcomes and cardiometabolic outcomes in children and adolescents in association with alcohol use and/or maternal cigarette smoking during pregnancy.
Aim, research question and objectives
To provide up-to-date evidence-based birth outcomes and cardiometabolic outcomes in children associated with maternal cigarette smoking only, maternal alcohol consumption only or both maternal cigarette smoking and alcohol use during pregnancy.
Research question
What are the associated effects of cigarette smoking and/or alcohol consumption during pregnancy on birth weight, obesity, hypertension, diabetes, dyslipidaemia and vascular dysfunction?
Primary objective: to assess birth weight and cardiometabolic risk factors associated with maternal cigarette smoking and/or alcohol consumption during pregnancy.
Secondary objectives:
- Identify potential mediators in the development of cardiometabolic risk factors and vascular dysfunction in offspring exposed to alcohol consumption and/or cigarette smoking during pregnancy.
- Identify gaps in the literature and provide recommendations for future clinical studies.
Protocol reporting and registration
The methods for this review were developed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) and PRISMA 2020 statement. 40 41 The systematic review protocol was registered with the international prospective register of systematic reviews (PROSPERO): CRD42021286630.
Criteria for consideration of studies
Types of studies.
Table 1 shows the list of inclusion and exclusion criteria. Types of studies will include human clinical studies that study the association, correlation or causation between maternal cigarette smoking and alcohol consumption and cardiometabolic risk factors in their children. Observational studies: prospective studies (cohort studies, birth cohort studies and prospective case-control studies) will be included. Data from primary population-based studies will be included only. Grey literature such as conference proceedings, theses and dessertations will be searched on the ProQuest Dissertation and Theses database, South African National Electronic Thesis and Dissertations Portal as well as Google Scholar to reduce the risk of publication bias.
Inclusion and exclusion criteria
Types of participants
Paediatric patients are defined as:
- Infants, children and adolescents between the ages of 0 and 19 years.
- Infants, children or adolescents exposed to maternal cigarette smoking only, maternal alcohol consumption only or both maternal cigarette smoking and alcohol during pregnancy.
Main outcome measures
- Birth outcomes: low birth weight, birth weight.
- Anthropometry: BMI, overweight, obesity, waist circumference.
- Blood pressure: systolic blood pressure, diastolic blood pressure, hypertension, elevated blood pressure.
- Diabetes mellitus, hyperglycaemia.
- Dyslipidaemia, hypertriglyceridaemia, high low-density lipoprotein cholesterol, low high-density lipoprotein cholesterol.
- Vascular dysfunction, increased intima-media thickness.
Search strategy
Using the comprehensive search terms, all relevant articles published, in English, from 2001, and indexed in PubMed, Google Scholar, Scopus and Web of Science will be identified for inclusion. Relevant articles will be retrieved from 2001 to gather evidence over a two-decade period. The chosen period signalled the period when significant evidence based on the current topic were published, we reckon that articles published before this period may not be relevant or of high quality to generate the robust evidence we are looking for in the current systematic review. The search strategies will incorporate both medical subject headings (MH) and free-text terms, and will be adapted to suit each database using applicable controlled vocabulary ( online supplemental file 1 ). An experienced information specialist will review the search terms to provide inputs and to ensure that search terms are relevant and optimally sensitive to identify eligible studies. An example of the search strategy in PubMed is displayed in table 2 , and in Scopus and Web of Science as shown in online supplemental tables 1 and 2 , respectively. Each of the terms for exposure to maternal cigarette smoking or maternal alcohol consumption will be combined with each of the outcome terms described.
Search strategy developed in PubMed
Supplementary data
Electronic searches.
Relevant studies will be searched for and retrieved from three databases: PubMed, Scopus and Web of Science. In table 2 , each of the terms for exposure to maternal cigarette smoking or alcohol consumption will be combined with each of the outcome terms described.
Selection of studies
The initial electronic database search will be performed to obtain all eligible records. Search results for each database will be exported to Endnote, where duplicates will be removed, and the number recorded. At least two reviewers will, independently, screen the titles and abstracts for potential eligible studies and exclude irrelevant studies using the Rayyan intelligent systematic reviews tool. Disagreements will be resolved by discussion and consensus between the two reviewers or through the third reviewer’s contribution. Eligibility criteria will be based on the PECO approach, an acronym representing the population, exposure, comparator and outcome. 42 P (population) of interest are infants, children and adolescents, the E (exposure) is maternal cigarette smoking, maternal alcohol consumption or both, the C (comparator) is the control group with no exposure to smoking and alcohol during pregnancy and the O (outcomes) of interest are birth weight and cardiometabolic risk factors. Thereafter, the full text will be retrieved and exported to Endnote and reviewed independently by the two reviewers for a final selection of studies for inclusion into the review. Authors will be contacted if full texts cannot be retrieved. Experts in the field will be consulted to identify unpublished studies.
Studies will be selected if (ⅰ) relevant to the topic, (ⅱ) study design is epidemiological observational studies such as prospective cohort studies, birth cohort studies and case-control studies, (ⅲ) having exposure to maternal cigarette smoking or maternal alcohol consumption, (ⅳ) having pregnant women as initially enrolled participants, (ⅴ) specified at least one adverse birth or child health outcome as an outcome of the investigation, (ⅵ) investigated the association, correlation or causation, (ⅶ) having the largest sample size if multiple studies used the same cohort. Only studies published in English will be included, however, there will be no restrictions regarding country, race and gender. Studies focussing on mental health, fetal alcohol syndrome, behavioural changes or cancer as the main outcome will be excluded. Any disagreement about the inclusion of studies will be resolved through the consultation of a third reviewer.
In addition to the database search, reference lists of selected articles will be examined to identify relevant studies that have not been captured in the database search. The PRISMA flow chart template will be used to summarise the search and selection of eligible studies. 43 Reasons for any exclusion of studies will be documented.
Quality and risk of bias assessment
Quality assessment and risk of bias will be measured using the Newcastle Ottawa scale ( http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp ) for cohort and case-control studies. Cohort and case-control studies will be awarded a maximum score of nine stars. High-quality studies will be defined as a quality score of 7 or above and a quality score below 7 will be deemed low quality.
Data extraction and management
All data extracted from the selected studies will be captured into a Microsoft Excel document. The following data will be extracted from each selected study: authors, date of publication, country of data collection, population age at outcome assessment, sample size, study design, study setting, data collection method, type of exposure, source of data on maternal cigarette smoking/alcohol consumption, the measure of the dependent variables, birth or child health outcomes, definition of the outcome, statistical methods used to measure the outcome, adjusted covariates and effect estimates ( online supplemental appendix A ). Effect estimates will include relative risks (95% CI) or adjusted ORs (95% CI). Findings will include all birth outcomes or cardiometabolic outcomes tested for an association with exposure to alcohol and cigarette smoking during pregnancy. Thereafter, the data will be extracted and stored on Review Manager (Revman Cochrane) for analyses and reporting. PRISMA guidelines will be used to summarise and report the review findings. 43
Data synthesis and analysis
Data analysis.
Findings from each study will be reported as a risk ratio or an OR with the 95% CI for dichotomous variables or mean difference with the SD for continuous variables. Studies with similar types of outcomes will be grouped to obtain feasible results on an overall estimate of effect. Due to the anticipated heterogeneity in included studies, a random-effects meta-analysis will be considered.
Assessment of heterogeneity
Heterogeneity will be evaluated through visual inspection of forest plots to judge the extent of CI overlap. In addition, heterogeneity will be tested by the I 2 test. The I 2 statistic will classify heterogeneity. Heterogeneity among studies is expected due to risk factors defined differently according to age in children and adolescents. Therefore, the methods used to measure each health outcome are suspected to be different in children compared with adolescents. Additionally, heterogeneity may be due to characteristics such as gender, maternal health, maternal BMI and socioeconomic status. If the data allow, subgroup analysis will be performed. Similar risk factors will be grouped for comparison across studies. Risk factors according to age groups such as children or adolescents will also be grouped.
Meta-analysis
Where there is sufficient reporting and a low degree of heterogeneity, a meta-analysis will be performed. Quantitative data will be pooled from individual studies to determine the pooled effect estimate with a fixed model preferred. A meta-analysis will be performed using Review Manager V.5.3 (Cochrane). After the meta-analysis, the Grading of Recommendations, Assessment, Development and Evaluations (Cochrane) will be used to score and assess the overall certainty in the evidence for each outcome included in the analysis.
Subgroup analysis
If the data allow, subgroup analysis will be performed. Similar risk factors will be grouped for comparison across studies. Risk factors according to age groups such as children or adolescents will also be grouped. Subgroup analysis will be performed on additional risk factors which may play a role in the adverse health outcomes (such as socioeconomic disadvantage, poor nutrition, second-hand smoke exposure, ethnicity, maternal stress, maternal mental health disorders, gestational diabetes, maternal BMI, poor dietary intake, physical inactivity, adolescent substance use and tobacco smoking). The pooled effect estimate will not be determined if the source of heterogeneity cannot be explained through subgroup analysis. In addition, if a meta-analysis cannot be performed due to few articles, insufficient reporting of data or a high degree of heterogeneity, a narrative synthesis will be reported.
Publication bias
Begg’s funnel chart will be used to perform a visual inspection and evaluation of publication bias on the selected data. This is to exclude any publication bias.
Patient and public involvement
It was not appropriate or possible to involve patients or the public in the design, conduct, reporting or dissemination plans of our research.
The review will extract publicly available secondary data and therefore does not require ethical review. The results will be submitted for publication in a peer-review journal.
Supplementary Material
Correction notice: This article has been corrected since it was published. The affiliation for Eunice Bolanle Turawa has been updated.
Contributors: TCH, JJADeS and AO conceptualised and developed the systematic review protocol. TCH wrote the first draft of the protocol. EBT and TCH developed the search strategy and EBT edited the manuscript. Both TCH and EBT will independently screen and select the title and abstracts of articles. Thereafter, the full text will be retrieved for a final review and selection. Any discrepancies regarding the eligibility of articles will be resolved through consensus and, in the event of a disagreement regarding the inclusion of articles, a consultation with a third reviewer will be held. Reasons for the exclusion of studies will be reported. TCH will extract, analyse and synthesise the data under the critical guidance of EBT. All authors will critically revise the successive drafts of the manuscript and approve the final version.
Funding: This research was made possible through funding by the Council for Scientific and Industrial Research (CSIR).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication.
Not applicable.
Smoking During Pregnancy
Health effects of smoking and secondhand smoke on pregnancies.

- Women who smoke have more difficulty becoming pregnant and have a higher risk of never becoming pregnant. 2,4
- Smoking during pregnancy can cause tissue damage in the unborn baby, particularly in the lung and brain, and some studies suggests a link between maternal smoking and cleft lip. 1,2
- Studies also suggest a relationship between tobacco and miscarriage. Carbon monoxide in tobacco smoke can keep the developing baby from getting enough oxygen. Tobacco smoke also contains other chemicals that can harm unborn babies. 1,2
Health Effects of Smoking and Secondhand Smoke on Babies
- Mothers who smoke are more likely to deliver their babies early. Preterm delivery is a leading cause of death, disability, and disease among newborns. 1,2
- One in every five babies born to mothers who smoke during pregnancy has low birth weight. Mothers who are exposed to secondhand smoke while pregnant are more likely to have lower birth weight babies. Babies born too small or too early are not as healthy. 1,2,3
- Both babies whose mothers smoke while pregnant and babies who are exposed to secondhand smoke after birth are more likely to die from sudden infant death syndrome (SIDS) than babies who are not exposed to cigarette smoke. 1,2,3 Babies whose mothers smoke are about three times more likely to die from SIDS. 1
- Babies whose mothers smoke while pregnant or who are exposed to secondhand smoke after birth have weaker lungs than other babies, which increases the risk for many health problems. 1,2,3
- Smoking reduces a woman’s chances of getting pregnant. 1,2
- Smoking during pregnancy increases the risk for pregnancy complications. 1,2
- Tobacco smoke harms babies before and after they are born. 1,3
Additional Resources
Highlights from the surgeon general’s report.
- Smoking Among Adults: Reproductive Health
- Impact on Unborn Babies, Infants, Children, and Adolescents
- Tobacco Use and Reproductive Outcomes
Related Links
- Tobacco Use and Pregnancy
- Smoking, Pregnancy, and Babies
- CDC’s National Center on Birth Defects and Developmental Disabilities
- U.S. Department of Health and Human Services. A Report of the Surgeon General: How Tobacco Smoke Causes Disease: What It Means to You . Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2010 [accessed 2012 May 10].
- U.S. Department of Health and Human Services. A Report of the Surgeon General: Highlights: Overview of Finding Regarding Reproductive Health . [ PDF –542 KB] . Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2010 [accessed 2012 May 10].
- U.S. Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General: Secondhand Smoke: What It Means To You . Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2006 [accessed 2012 May 10].
- U.S. Department of Health and Human Services. The Health Consequences of Smoking: What It Means to You . Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2004 [accessed 2012 June 15].
To receive email updates about Smoking & Tobacco Use, enter your email address:
- Tips From Former Smokers ®
- Division of Cancer Prevention and Control
- Lung Cancer
- National Comprehensive Cancer Control Program
- Division of Reproductive Health

Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.

IMAGES
VIDEO
COMMENTS
Background It has been shown that active exposure to tobacco is associated with adverse pregnancy outcomes including, but not limited to, intrauterine fetal death, reduced fetal weight, and higher risk of preterm birth. We want to investigate these effects in a high-income country. Methods This cross-sectional study examined 20,843 pregnant women who delivered over 10 years at the Maternity ...
Results. Nineteen thousand five hundred fifty-four pregnant women met the inclusion criteria and 2,714 (13.9%) of them were smokers. Even after adjusting for confounding factors, smoking during pregnancy was associated with preterm birth, birthweight < 2500 g, intrauterine growth restriction, neonatal respiratory and gastrointestinal diseases, transfer to the neonatal intensive care unit, and ...
The aims of this dissertation were: 1) to investigate the public health impact of smoking cessation during pregnancy among mothers giving birth in San Bernardino County, California; and 2) to examine the relationship between indices of prenatal care utilization and smoking cessation during pregnancy. The first study showed that, among 65,228 ...
Introduction Tobacco smoking and alcohol consumption during pregnancy are particularly prevalent in low socioeconomic status populations, with an adverse association with birth outcomes and cardiometabolic risk factors. However, the direct and indirect effects of prenatal cigarette smoking and alcohol consumption during pregnancy on cardiometabolic risk in offspring have been rather inconsistent.
Introduction. Smoking cigarettes throughout pregnancy is one of the most important preventable causes of adverse pregnancy outcomes (Hofhuis et al., 2003; Rauschert et al., 2019).Despite the well documented adverse health effects of maternal smoking and many efforts to reduce its prevalence, 20-30% of women who smoke, continue smoking during pregnancy and lactation (Dietz et al., 2010 ...
1. Tobacco Smoking during Pregnancy: Women's Perception of the Usefulness of Smoking Cessation Interventions. According to the World Health Organization (WHO) [], tobacco smoking is a public health problem with multiple risks and consequences for the general population.Tobacco consumption directly causes the death of more than 7 million people in the world, and about 1.2 million died due to ...
A great deal of attention has been focused on adverse effects of tobacco smoking on conception, pregnancy, fetal, and child health. The aim of this paper is to discuss the current evidence regarding short and long-term health effects on child health of parental smoking during pregnancy and lactation and the potential underlying mechanisms. Studies were searched on MEDLINE® and Cochrane ...
Objective: The objective of this review was twofold: i) to comprehensively identify the best available evidence about the experiences of women who smoked tobacco during pregnancy or postnatally (or both) concerning health care providers' interactions with them about their smoking, when such interactions occurred during contact for prenatal or postnatal health care in any health care setting ...
Background. Smoking is the highest preventable cause of numerous health problems worldwide [].Seven million people die every year because of smoking in the world and more than 18% of adults smoke daily in OECD countries [].In England, 16% of all deaths were attributed to smoking in 2015 [].Smoking during pregnancy is responsible for many avoidable health conditions and deaths across the ...
Purpose To investigate whether maternal cigarette smoking during pregnancy is a risk factor for developing GDM. Methods MEDLINE, Scopus, CENTRAL and Google Scholar databases were searched from inception to December 2022 to identify eligible original articles. A systematic review and meta-analysis (weighted data, random-effects model) were performed. The primary outcome was the development of ...
Maternal smoking during pregnancy (MSDP) is an important public health issue that adversely impacts health outcomes of both children and mothers, contributing to low birthweight, preterm birth, miscarriage, and ectopic pregnancy [].Despite increasing public awareness on the detrimental effects of MSDP, the proportion of women who smoke during pregnancy is still high in some countries.
The prevalence of tobacco smoking during pregnancy throughout the world is estimated at 1.7 % (95 % CI 0.0-4.5 %) (LE3). In France, the prevalence of active smoking is approximately 30 % pre-conception, 20-24 % in the first trimester and 14-20 % in the third trimester of pregnancy (LE3). There is great disparity according to different ...
Background Smoking during pregnancy is the leading modifiable risk factor for poor maternal and infant health outcomes. Pregnancy-related health problems associated with smoking during pregnancy include complications during labour, increased risk of miscarriage, premature birth, stillbirth and low birthweight. Despite this, around 12% of pregnant women in the United Kingdom (UK), 13% in the ...
Worldwide, substantial numbers of women who smoke, continue to smoke during pregnancy. Recently estimated prevalence of smoking pregnant women in the European Region was 8.1%, which was the highest percentage when compared to the world average of 1.7% [].Even though prevalence rates of pregnant smoking women have been decreasing over the years [], these numbers are yet of concern for several ...
smoking during pregnancy and physician education in implementing all 5A's in daily practice. KEYWORDS: Smoking, Pregnancy, Urban, Rural, Smoking Deception ... dissertation process, especially in the area of childcare. For most of our married life we have been students, and now that this chapter is coming to a close I look ...
Several recent meta-analyses showed a strong associations between maternal smoking during pregnancy and offspring overweight and obesity with pooled odds ratios (ORs) ranging from 1.33 to 1.60 [1 ...
Exposure to tobacco smoke affects all stages of human reproduction. Tobacco smoking affects both male and female fecundity. Maternal cigarette smoking is associated with increased risks for ectopic pregnancy, premature rupture of membranes, abruptio pla-centae, placenta previa, miscarriage, stillbirth, preterm birth, low birth weight, small for
The third study of this dissertation evaluated the association between smoking during pregnancy and risk of preterm birth and small-for-gestational-age (SGA). The strengths of this study included the assessment of smoking at two time points in pregnancy and the evaluation of preterm birth subtypes.
Tobacco use among women and during pregnancy. Tobacco consumption is the main cause of preventable death globally with tobacco considered to be the only consumer product legally on sale that kills such a high percentage of its users [].Epidemiological studies over the last 30 years have concluded that the total mortality of smokers is increased within the range of 50% to 116%, depending on the ...
Objectives To systematically critique and summarise the available evidence on the outcomes of smokeless tobacco use in pregnancy to inform the public health response. Methods In March 2013, a search was conducted of observational studies where the exposure to smokeless tobacco during pregnancy and maternal, placental and/or neonatal outcomes was assessed. Two reviewers extracted data and ...
Key Points. Question Does exposure to maternal smoking during pregnancy increase the risk of severe mental illness in offspring?. Findings In a population-based cohort of 1.7 million Swedish offspring, maternal smoking during pregnancy was associated with an increased risk of severe mental illness in offspring. However, sibling comparisons, which ruled out all genetic and environmental ...
Pregnant women who smoked and used e-cigarettes had lower risk perceptions about e-cigarette use during pregnancy than those who only smoked. Health messages and research about the harms of nicotine exposure during pregnancy should address the risks of dual-use versus only e-cigarette use. ... and perceived health outcomes attributed to smoking ...
In South Africa, 61.2% of women reported alcohol consumption, 56.3% reported smoking tobacco only and 37.4% reported concomitant use of tobacco and alcohol during pregnancy. 4 Women of low education level, of low economic status and those that had mental health disorders were more likely to smoke during pregnancy, relapse after pregnancy and ...
caused by smoking during pregnancy. Fetal growth: Mothers who smoke during pregnancy are more likely to deliver babies with low birth weight, even if the babies are full term. Mothers who smoke during pregnancy are also more likely to deliver their babies early. Low birth weight and preterm delivery are leading causes of infant disability and ...
Smoking during pregnancy can cause tissue damage in the unborn baby, particularly in the lung and brain, and some studies suggests a link between maternal smoking and cleft lip. 1,2. Studies also suggest a relationship between tobacco and miscarriage. Carbon monoxide in tobacco smoke can keep the developing baby from getting enough oxygen.