- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution


Search form
- Advanced search
- Search responses
- Search blogs
- Impact of vaping on...
Impact of vaping on respiratory health
Linked editorial.
Protecting children from harms of vaping
- Related content
- Peer review
- Andrea Jonas , clinical assistant professor
- Division of Pulmonary, Allergy, and Critical Care, Department of Medicine, Stanford University, Stanford, CA, USA
- Correspondence to A Jonas andreajonas{at}stanford.edu
Widespread uptake of vaping has signaled a sea change in the future of nicotine consumption. Vaping has grown in popularity over the past decade, in part propelled by innovations in vape pen design and nicotine flavoring. Teens and young adults have seen the biggest uptake in use of vape pens, which have superseded conventional cigarettes as the preferred modality of nicotine consumption. Relatively little is known, however, about the potential effects of chronic vaping on the respiratory system. Further, the role of vaping as a tool of smoking cessation and tobacco harm reduction remains controversial. The 2019 E-cigarette or Vaping Use-Associated Lung Injury (EVALI) outbreak highlighted the potential harms of vaping, and the consequences of long term use remain unknown. Here, we review the growing body of literature investigating the impacts of vaping on respiratory health. We review the clinical manifestations of vaping related lung injury, including the EVALI outbreak, as well as the effects of chronic vaping on respiratory health and covid-19 outcomes. We conclude that vaping is not without risk, and that further investigation is required to establish clear public policy guidance and regulation.
Abbreviations
BAL bronchoalveolar lavage
CBD cannabidiol
CDC Centers for Disease Control and Prevention
DLCO diffusing capacity of the lung for carbon monoxide
EMR electronic medical record
END electronic nicotine delivery systems
EVALI E-cigarette or Vaping product Use-Associated Lung Injury
LLM lipid laden macrophages
THC tetrahydrocannabinol
V/Q ventilation perfusion
Introduction
The introduction of vape pens to international markets in the mid 2000s signaled a sea change in the future of nicotine consumption. Long the mainstay of nicotine use, conventional cigarette smoking was on the decline for decades in the US, 1 2 largely owing to generational shifts in attitudes toward smoking. 3 With the advent of vape pens, trends in nicotine use have reversed, and the past two decades have seen a steady uptake of vaping among young, never smokers. 4 5 6 Vaping is now the preferred modality of nicotine consumption among young people, 7 and 2020 surveys indicate that one in five US high school students currently vape. 8 These trends are reflected internationally, where the prevalence of vape products has grown in both China and the UK. 9 Relatively little is known, however, regarding the health consequences of chronic vape pen use. 10 11 Although vaping was initially heralded as a safer alternative to cigarette smoking, 12 13 the toxic substances found in vape aerosols have raised new questions about the long term safety of vaping. 14 15 16 17 The 2019 E-cigarette or Vaping product Use-Associated Lung Injury (EVALI) outbreak, ultimately linked to vitamin E acetate in THC vapes, raised further concerns about the health effects of vaping, 18 19 20 and has led to increased scientific interest in the health consequences of chronic vaping. This review summarizes the history and epidemiology of vaping, and the clinical manifestations and proposed pathophysiology of lung injury caused by vaping. The public health consequences of widespread vaping remain to be seen and are compounded by young users of vape pens later transitioning to combustible cigarettes. 4 21 22 Deepened scientific understanding and public awareness of the potential harms of vaping are imperative to confront the challenges posed by a new generation of nicotine users.
Sources and selection criteria
We searched PubMed and Ovid Medline databases for the terms “vape”, “vaping”, “e-cigarette”, “electronic cigarette”, “electronic nicotine delivery”, “electronic nicotine device”, “END”, “EVALI”, “lung injury, diagnosis, management, and treatment” to find articles published between January 2000 and December 2021. We also identified references from the Centers for Disease Control and Prevention (CDC) website, as well as relevant review articles and public policy resources. Prioritization was given to peer reviewed articles written in English in moderate-to-high impact journals, consensus statements, guidelines, and included randomized controlled trials, systematic reviews, meta-analyses, and case series. We excluded publications that had a qualitative research design, or for which a conflict of interest in funding could be identified, as defined by any funding source or consulting fee from nicotine manufacturers or distributors. Search terms were chosen to generate a broad selection of literature that reflected historic and current understanding of the effects of vaping on respiratory health.
The origins of vaping
Vaping achieved widespread popularity over the past decade, but its origins date back almost a century and are summarized in figure 1 . The first known patent for an “electric vaporizer” was granted in 1930, intended for aerosolizing medicinal compounds. 23 Subsequent patents and prototypes never made it to market, 24 and it wasn’t until 1979 that the first vape pen was commercialized. Dubbed the “Favor” cigarette, the device was heralded as a smokeless alternative to cigarettes and led to the term “vaping” being coined to differentiate the “new age” method of nicotine consumption from conventional, combustible cigarettes. 25 “Favor” cigarettes did not achieve widespread appeal, in part because of the bitter taste of the aerosolized freebase nicotine; however, the term vaping persisted and would go on to be used by the myriad products that have since been developed.

Timeline of vape pen invention to widespread use (1970s-2020)
- Download figure
- Open in new tab
- Download powerpoint
The forerunner of the modern vape pen was developed in Beijing in 2003 and later introduced to US markets around 2006. 26 27 Around this time, the future Juul Laboratories founders developed the precursor of the current Juul vape pen while they were students at the Stanford Byers-Center for Biodesign. 28 Their model included disposable cartridges of flavored nicotine solution (pods) that could be inserted into the vape pen, which itself resembled a USB flash drive. Key to their work was the chemical alteration of freebase nicotine to a benzoate nicotine salt. 29 The lower pH of the nicotine salt resulted in an aerosolized nicotine product that lacked a bitter taste, 30 and enabled manufacturers to expand the range of flavored vape products. 31 Juul Laboratories was founded a decade later and quickly rose to dominate the US market, 32 accounting for an estimated 13-59% of the vape products used among teens by 2020. 6 8 Part of the Juul vape pen’s appeal stems from its discreet design, as well as its ability to deliver nicotine with an efficiency matching that of conventional cigarettes. 33 34 Subsequent generations of vape pens have included innovations such as the tank system, which allowed users to select from the wide range of different vape solutions on the market, rather than the relatively limited selection available in traditional pod based systems. Further customizations include the ability to select different vape pen components such as atomizers, heating coils, and fluid wicks, allowing users to calibrate the way in which the vape aerosol is produced. Tobacco companies have taken note of the shifting demographics of nicotine users, as evidenced in 2018 by Altria’s $12.8bn investment in Juul Laboratories. 35
Vaping terminology
At present, vaping serves as an umbrella term that describes multiple modalities of aerosolized nicotine consumption. Vape pens are alternatively called e-cigarettes, electronic nicotine delivery systems (END), e-cigars, and e-hookahs. Additional vernacular terms have emerged to describe both the various vape pen devices (eg, tank, mod, dab pen), vape solution (eg, e-liquid, vape juice), as well as the act of vaping (eg, ripping, juuling, puffing, hitting). 36 A conventional vape pen is a battery operated handheld device that contains a storage chamber for the vape solution and an internal element for generating the characteristic vape aerosol. Multiple generations of vape pens have entered the market, including single use, disposable varieties, as well as reusable models that have either a refillable fluid reservoir or a disposable cartridge for the vape solution. Aerosol generation entails a heating coil that atomizes the vape solution, and it is increasingly popular for devices to include advanced settings that allow users to adjust features of the aerosolized nicotine delivery. 37 38 Various devices allow for coil temperatures ranging from 110 °C to over 1000 °C, creating a wide range of conditions for thermal degradation of the vape solution itself. 39 40
The sheer number of vape solutions on the market poses a challenge in understanding the impact of vaping on respiratory health. The spectrum of vape solutions available encompasses thousands of varieties of flavors, additives, and nicotine concentrations. 41 Most vape solutions contain an active ingredient, commonly nicotine 42 ; however, alternative agents include tetrahydrocannabinol (THC) or cannabidiol (CBD). Vape solutions are typically composed of a combination of a flavorant, nicotine, and a carrier, commonly propylene glycol or vegetable glycerin, that generates the characteristic smoke appearance of vape aerosols. Some 450 brands of vape now offer more than 8000 flavors, 41 a figure that nearly doubled over a three year period. 43 Such tremendous variety does not account for third party sellers who offer users the option to customize a vape solution blend. Addition of marijuana based products such as THC or CBD requires the use of an oil based vape solution carrier to allow for extraction of the psychoactive elements. Despite THC vaping use in nearly 9% of high schoolers, 44 THC vape solutions are subject to minimal market regulation. Finally, a related modality of THC consumption is termed dabbing, and describes the process of inhaling aerosolized THC wax concentrate.
Epidemiology of vaping
Since the early 2000s, vaping has grown in popularity in the US and elsewhere. 8 45 Most of the 68 million vape pen users are concentrated in China, the US, and Europe. 46 Uptake among young people has been particularly pronounced, and in the US vaping has overtaken cigarettes as the most common modality of nicotine consumption among adolescents and young adults. 47 Studies estimate that 20% of US high school students are regular vape pen users, 6 48 in contrast to the 5% of adults who use vape products. 2 Teen uptake of vaping has been driven in part by a perception of vaping as a safer alternative to cigarettes, 49 50 as well as marketing strategies that target adolescents. 33 Teen use of vape pens is further driven by the low financial cost of initiation, with “starter kits” costing less than $25, 51 as well as easy access through peer sales and inconsistent age verification at in-person and online retailers. 52 After sustained growth in use over the 2010s, recent survey data from 2020 suggest that the number of vape pen users has leveled off among teens, perhaps in part owing to increased perceived risk of vaping after the EVALI outbreak. 8 53 The public health implications of teen vaping are compounded by the prevalence of vaping among never smokers (defined as having smoked fewer than 100 lifetime cigarettes), 54 and subsequent uptake of cigarette smoking among vaping teens. 4 55 Similarly, half of adults who currently vape have never used cigarettes, 2 and concern remains that vaping serves as a gateway to conventional cigarette use, 56 57 although these results have been disputed. 58 59 Despite regulation limiting the sale of flavored vape products, 60 a 2020 survey found that high school students were still predominantly using fruit, mint, menthol, and dessert flavored vape solutions. 48 While most data available surround the use of nicotine-containing vape products, a recent meta-analysis showed growing prevalence of adolescents using cannabis-containing products as well. 61
Vaping as harm reduction
Despite facing ongoing questions about safety, vaping has emerged as a potential tool for harm reduction among cigarette smokers. 12 27 An NHS report determined that vaping nicotine is “around 95% less harmful than cigarettes,” 62 leading to the development of programs that promote vaping as a tool of risk reduction among current smokers. A 2020 Cochrane review found that vaping nicotine assisted with smoking cessation over placebo 63 and recent work found increased rates of cigarette abstinence (18% v 9.9%) among those switching to vaping compared with conventional nicotine replacement (eg, gum, patch, lozenge). 64 US CDC guidance suggests that vaping nicotine may benefit current adult smokers who are able to achieve complete cigarette cessation by switching to vaping. 65 66
The public health benefit of vaping for smoking cessation is counterbalanced by vaping uptake among never smokers, 2 54 and questions surrounding the safety of chronic vaping. 10 11 Controversy surrounding the NHS claim of vaping as 95% safer than cigarettes has emerged, 67 68 and multiple leading health organizations have concluded that vaping is harmful. 42 69 Studies have demonstrated airborne particulate matter in the proximity of active vapers, 70 and concern remains that secondhand exposure to vaped aerosols may cause adverse effects, complicating the notion of vaping as a net gain for public health. 71 72 Uncertainty about the potential chronic consequences of vaping combined with vaping uptake among never smokers has complicated attempts to generate clear policy guidance. 73 74 Further, many smokers may exhibit “dual use” of conventional cigarettes and vape pens simultaneously, further complicating efforts to understand the impact of vape exposure on respiratory health, and the role vape use may play in smoking cessation. 12 We are unable to know with certainty the extent of nicotine uptake among young people that would have been seen in the absence of vaping availability, and it remains possible that some young vape pen users may have started on conventional cigarettes regardless. That said, declining nicotine use over the past several decades would argue that many young vape pen users would have never had nicotine uptake had vape pens not been introduced. 1 2 It remains an open question whether public health measures encouraging vaping for nicotine cessation will benefit current smokers enough to offset the impact of vaping uptake among young, never smokers. 75
Vaping lung injury—clinical presentations
Vaping related lung injury: 2012-19.
The potential health effects of vape pen use are varied and centered on injury to the airways and lung parenchyma. Before the 2019 EVALI outbreak, the medical literature detailed case reports of sporadic vaping related acute lung injury. The first known case was reported in 2012, when a patient presented with cough, diffuse ground glass opacities, and lipid laden macrophages (LLM) on bronchoalveolar lavage (BAL) return in the context of vape pen use. 76 Over the following seven years, an additional 15 cases of vaping related acute lung injury were reported in the literature. These cases included a wide range of diffuse parenchymal lung disease without any clear unifying features, and included cases of eosinophilic pneumonia, 77 78 79 hypersensitivity pneumonitis, 80 organizing pneumonia, 81 82 diffuse alveolar hemorrhage, 83 84 and giant cell foreign body reaction. 85 Although parenchymal lung injury predominated the cases reported, additional cases detailed episodes of status asthmaticus 86 and pneumothoraces 87 attributed to vaping. Non-respiratory vape pen injury has also been described, including cases of nicotine toxicity from vape solution ingestion, 88 89 and injuries sustained owing to vape pen device explosions. 90
The 2019 EVALI outbreak
In the summer of 2019 the EVALI outbreak led to 2807 cases of idiopathic acute lung injury in predominantly young, healthy individuals, which resulted in 68 deaths. 19 91 Epidemiological work to uncover the cause of the outbreak identified an association with vaping, particularly the use of THC-containing products, among affected individuals. CDC criteria for EVALI ( box 1 ) included individuals presenting with respiratory symptoms who had pulmonary infiltrates on imaging in the context of having vaped or dabbed within 90 days of symptom onset, without an alternative identifiable cause. 92 93 After peaking in September 2019, EVALI case numbers steadily declined, 91 likely owing to identification of a link with vaping, and subsequent removal of offending agents from circulation. Regardless, sporadic cases continue to be reported, and a high index of suspicion is required to differentiate EVALI from covid-19 pneumonia. 94 95 A strong association emerged between EVALI cases and the presence of vitamin E acetate in the BAL return of affected individuals 96 ; however, no definitive causal link has been established. Interestingly, the EVALI outbreak was nearly entirely contained within the US with the exception of several dozen cases, at least one of which was caused by an imported US product. 97 98 99 The pattern of cases and lung injury is most suggestive of a vape solution contaminant that was introduced into the distribution pipeline in US markets, leading to a geographically contained pattern of lung injury among users. CDC case criteria for EVALI may have obscured a potential link between viral pneumonia and EVALI, and cases may have been under-recognized following the onset of the covid-19 pandemic.
CDC criteria for establishing EVALI diagnosis
Cdc lung injury surveillance, primary case definitions, confirmed case.
Vape use* in 90 days prior to symptom onset; and
Pulmonary infiltrate on chest radiograph or ground glass opacities on chest computed tomography (CT) scan; and
Absence of pulmonary infection on initial investigation†; and
Absence of alternative plausible diagnosis (eg, cardiac, rheumatological, or neoplastic process).
Probable case
Pulmonary infiltrate on chest radiograph or ground glass opacities on chest CT; and
Infection has been identified; however is not thought to represent the sole cause of lung injury OR minimum criteria** to exclude infection have not been performed but infection is not thought to be the sole cause of lung injury
*Use of e-cigarette, vape pen, or dabbing.
†Minimum criteria for absence of pulmonary infection: negative respiratory viral panel, negative influenza testing (if supported by local epidemiological data), and all other clinically indicated infectious respiratory disease testing is negative.
EVALI—clinical, radiographic, and pathologic features
In the right clinical context, diagnosis of EVALI includes identification of characteristic radiographic and pathologic features. EVALI patients largely fit a pattern of diffuse, acute lung injury in the context of vape pen exposure. A systematic review of 200 reported cases of EVALI showed that those affected were predominantly men in their teens to early 30s, and most (80%) had been using THC-containing products. 100 Presentations included predominantly respiratory (95%), constitutional (87%), and gastrointestinal symptoms (73%). Radiological studies mostly featured diffuse ground glass opacities bilaterally. Of 92 cases that underwent BAL, alveolar fluid samples were most commonly neutrophil predominant, and 81% were additionally positive for LLM on Oil Red O staining. Lung biopsy was not required to achieve the diagnosis; however, of 33 cases that underwent tissue biopsy, common features included organizing pneumonia, inflammation, foamy macrophages, and fibrinous exudates.
EVALI—outcomes
Most patients with EVALI recovered, and prognosis was generally favorable. A systematic review of identified cases found that most patients with confirmed disease required admission to hospital (94%), and a quarter were intubated. 100 Mortality among EVALI patients was low, with estimates around 2-3% across multiple studies. 101 102 103 Mortality was associated with age over 35 and underlying asthma, cardiac disease, or mental health conditions. 103 Notably, the cohorts studied only included patients who presented for medical care, and the samples are likely biased toward a more symptomatic population. It is likely that many individuals experiencing mild symptoms of EVALI did not present for medical care, and would have self-discontinued vaping following extensive media coverage of the outbreak at that time. Although most EVALI survivors recovered well, case series of some individuals show persistent radiographic abnormalities 101 and sustained reductions in DLCO. 104 105 Pulmonary function evaluation of EVALI survivors showed normalization in FEV 1 /FVC on spirometry in some, 106 while others had more variable outcomes. 105 107 108
Vaping induced lung injury—pathophysiology
The causes underlying vaping related acute lung injury remain interesting to clinicians, scientists, and public health officials; multiple mechanisms of injury have been proposed and are summarized in figure 2 . 31 109 110 Despite increased scientific interest in vaping related lung injury following the EVALI outbreak, the pool of data from which to draw meaningful conclusions is limited because of small scale human studies and ongoing conflicts due to tobacco industry funding. 111 Further, insufficient time has elapsed since widespread vaping uptake, and available studies reflect the effects of vaping on lung health over a maximum 10-15 year timespan. The longitudinal effects of vaping may take decades to fully manifest and ongoing prospective work is required to better understand the impacts of vaping on respiratory health.

Schematic illustrating pathophysiology of vaping lung injury
Pro-inflammatory vape aerosol effects
While multiple pathophysiological pathways have been proposed for vaping related lung injury, they all center on the vape aerosol itself as the conduit of lung inflammation. Vape aerosols have been found to harbor a number of toxic substances, including thermal degradation products of the various vape solution components. 112 Mass spectrometry analysis of vape aerosols has identified a variety of oxidative and pro-inflammatory substances including benzene, acrolein, volatile organic compounds, and propylene oxide. 16 17 Vaping additionally leads to airway deposition of ultrafine particles, 14 113 as well as the heavy metals manganese and zinc which are emitted from the vaping coils. 15 114 Fourth generation vape pens allow for high wattage aerosol generation, which can cause airway epithelial injury and tissue hypoxia, 115 116 as well as formaldehyde exposure similar to that of cigarette smoke. 117 Common carrier solutions such as propylene glycol have been associated with increased airway hyper-reactivity among vape pen users, 31 118 119 and have been associated with chronic respiratory conditions among theater workers exposed to aerosolized propylene glycol used in the generation of artificial fog. 120 Nicotine salts used in pod based vape pen solutions, including Juul, have been found to penetrate the cell membrane and have cytotoxic effects. 121
The myriad available vape pen flavors correlate with an expansive list of chemical compounds with potential adverse respiratory effects. Flavorants have come under increased scrutiny in recent years and have been found to contribute to the majority of aldehyde production during vape aerosol production. 122 Compounds such as cinnamaldehyde, 123 124 2,5-dimethylpyrazine (chocolate flavoring), 125 and 2,3-pentanedione 126 are common flavor additives and have been found to contribute to airway inflammation and altered immunological responses. The flavorant diacetyl garnered particular attention after it was identified on mass spectrometry in most vape solutions tested. 127 Diacetyl is most widely associated with an outbreak of diacetyl associated bronchiolitis obliterans (“popcorn lung”) among workers at a microwave popcorn plant in 2002. 128 Identification of diacetyl in vape solutions raises the possibility of development of a similar pattern of bronchiolitis obliterans among individuals who have chronic vape aerosol exposure to diacetyl-containing vape solutions. 129
Studies of vape aerosols have suggested multiple pro-inflammatory effects on the respiratory system. This includes increased airway resistance, 130 impaired response to infection, 131 and impaired mucociliary clearance. 132 Vape aerosols have further been found to induce oxidative stress in lung epithelial cells, 133 and to both induce DNA damage and impair DNA repair, consistent with a potential carcinogenic effect. 134 Mice chronically exposed to vape aerosols developed increased airway hyper-reactivity and parenchymal changes consistent with chronic obstructive pulmonary disease. 135 Human studies have been more limited, but reveal increased airway edema and friability among vape pen users, as well as altered gene transcription and decreased innate immunity. 136 137 138 Upregulation of neutrophil elastase and matrix metalloproteases among vape users suggests increased proteolysis, potentially putting those patients at risk of chronic respiratory conditions. 139
THC-containing products
Of particular interest during the 2019 EVALI outbreak was the high prevalence of THC use among EVALI cases, 19 raising questions about a novel mechanism of lung injury specific to THC-containing vape solutions. These solutions differ from conventional nicotine based products because of the need for a carrier capable of emulsifying the lipid based THC component. In this context, additional vape solution ingredients rose to attention as potential culprits—namely, THC itself, which has been found to degrade to methacrolein and benzene, 140 as well as vitamin E acetate which was found to be a common oil based diluent. 141
Vitamin E acetate has garnered increasing attention as a potential culprit in the pathophysiology of the EVALI outbreak. Vitamin E acetate was found in 94% of BAL samples collected from EVALI patients, compared with none identified in unaffected vape pen users. 96 Thermal degradation of vitamin E acetate under conditions similar to those in THC vape pens has shown production of ketene, alkene, and benzene, which may mediate epithelial lung injury when inhaled. 39 Previous work had found that vitamin E acetate impairs pulmonary surfactant function, 142 and subsequent studies have shown a dose dependent adverse effect on lung parenchyma by vitamin E acetate, including toxicity to type II pneumocytes, and increased inflammatory cytokines. 143 Mice exposed to aerosols containing vitamin E acetate developed LLM and increased alveolar protein content, suggesting epithelial injury. 140 143
The pathophysiological insult underlying vaping related lung injury may be multitudinous, including potentially compound effects from multiple ingredients comprising a vape aerosol. The heterogeneity of available vape solutions on the market further complicates efforts to pinpoint particular elements of the vape aerosol that may be pathogenic, as no two users are likely to be exposed to the same combination of vape solution products. Further, vape users may be exposed to vape solutions containing terpenes, medium chain triglycerides, or coconut oil, the effects of which on respiratory epithelium remain under investigation. 144
Lipid laden macrophages
Lipid laden alveolar macrophages have risen to prominence as potential markers of vaping related lung injury. Alveolar macrophages describe a scavenger white blood cell responsible for clearing alveolar spaces of particulate matter and modulating the inflammatory response in the lung parenchyma. 145 LLM describe alveolar macrophages that have phagocytosed fat containing deposits, as seen on Oil Red O staining, and have been described in a wide variety of pulmonary conditions, including aspiration, lipoid pneumonia, organizing pneumonia, and medication induced pneumonitis. 146 147 During the EVALI outbreak, LLM were identified in the alveolar spaces of affected patients, both in the BAL fluid and on both transbronchial and surgical lung biopsies. 148 149 Of 52 EVALI cases reported in the literature who underwent BAL, LLM were identified in over 80%. 19 100 101 148 149 150 151 152 153 Accordingly, attention turned to LLM as not only a potential marker of lung injury in EVALI, but as a possible contributor to lung inflammation itself. This concern was compounded by the frequent reported use of oil based THC vape products among EVALI patients, raising the possibility of lipid deposits in the alveolus resulting from inhalation of THC-containing vape aerosols. 154 The combination of LLM, acute lung injury, and inhalational exposure to an oil based substance raised the concern for exogenous lipoid pneumonia. 152 153 However, further evaluation of the radiographic and histopathologic findings failed to identify cardinal features that would support a diagnosis of exogenous lipoid pneumonia—namely, low attenuation areas on CT imaging and foreign body giant cells on histopathology. 155 156 However, differences in the particle size and distribution between vape aerosol exposure and traditional causes of lipoid pneumonia (ie, aspiration of a large volume of an oil-containing substance), could reasonably lead to differences in radiographic appearance, although this would not account for the lack of characteristic histopathologic features on biopsy that would support a diagnosis of lipoid pneumonia.
Recent work suggests that LLM reflect a non-specific marker of vaping, rather than a marker of lung injury. One study found that LLM were not unique to EVALI and could be identified in healthy vape pen users, as well as conventional cigarette smokers, but not in never smokers. 157 Interestingly, this work showed increased cytokines IL-4 and IL-10 among healthy vape users, suggesting that cigarette and vape pen use are associated with a pro-inflammatory state in the lung. 157 An alternative theory supports LLM presence reflecting macrophage clearance of intra-alveolar cell debris rather than exogenous lipid exposure. 149 150 Such a pattern would be in keeping with the role of alveolar macrophages as modulating the inflammatory response in the lung parenchyma. 158 Taken together, available data would support LLM serving as a non-specific marker of vape product use, rather than playing a direct role in vaping related lung injury pathogenesis. 102
Clinical aspects
A high index of suspicion is required in establishing a diagnosis of vaping related lung injury, and a general approach is summarized in figure 3 . Clinicians may consider the diagnosis when faced with a patient with new respiratory symptoms in the context of vape pen use, without an alternative cause to account for their symptoms. Suspicion should be especially high if respiratory complaints are coupled with constitutional and gastrointestinal symptoms. Patients may present with non-specific markers indicative of an ongoing inflammatory process: fevers, leukocytosis, elevated C reactive protein, or elevated erythrocyte sedimentation rate. 19

Flowchart outlining the procedure for diagnosing a vaping related lung injury
Vaping related lung injury is a diagnosis of exclusion. Chest imaging via radiograph or CT may identify a variety of patterns, although diffuse ground glass opacities remain the most common radiographic finding. Generally, patients with an abnormal chest radiograph should undergo a chest CT for further evaluation of possible vaping related lung injury.
Exclusion of infectious causes is recommended. Testing should include evaluation for bacterial and viral causes of pneumonia, as deemed appropriate by clinical judgment and epidemiological data. Exclusion of common viral causes of pneumonia is imperative, particularly influenza and SARS-CoV-2. Bronchoscopy with BAL should be considered on a case-by-case basis for those with more severe disease and may be helpful to identify patients with vaping mediated eosinophilic lung injury. Further, lung biopsy may be beneficial to exclude alternative causes of lung injury in severe cases. 92
No definitive therapy has been identified for the treatment of vaping related lung injury, and data are limited to case reports and public health guidance on the topic. Management includes supportive care and strong consideration for systemic corticosteroids for severe cases of vaping related lung injury. CDC guidance encourages consideration of systemic corticosteroids for patients requiring admission to hospital, or those with higher risk factors for adverse outcomes, including age over 50, immunosuppressed status, or underlying cardiopulmonary disease. 100 Further, given case reports of vaping mediated acute eosinophilic pneumonia, steroids should be implemented in those patients who have undergone a confirmatory BAL. 77 79
Additional therapeutic options include empiric antibiotics and/or antivirals, depending on the clinical scenario. For patients requiring admission to hospital, prompt subspecialty consultation with a pulmonologist can help guide management. Outpatient follow-up with chest imaging and spirometry is recommended, as well as referral to a pulmonologist. Counseling regarding vaping cessation is also a core component in the post-discharge care for this patient population. Interventions specific to vaping cessation remain under investigation; however, literature supports the use of behavioral counseling and/or pharmacotherapy to support nicotine cessation efforts. 66
Health outcomes among vape pen users
Health outcomes among chronic vape pen users remains an open question. To date, no large scale prospective cohort studies exist that can establish a causal link between vape use and adverse respiratory outcomes. One small scale prospective cohort study did not identify any spirometric or radiographic changes among vape pen users over a 3.5 year period. 159 Given that vaping remains a relatively novel phenomenon, many users will have a less than 10 “pack year” history of vape pen use, arguably too brief an exposure period to reflect the potential harmful nature of chronic vaping. Studies encompassing a longer period of observation of vape pen users have not yet taken place, although advances in electronic medical record (EMR) data collection on vaping habits make such work within reach.
Current understanding of the health effects of vaping is largely limited to case reports of acute lung injury, and health surveys drawing associations between vaping exposure and patient reported outcomes. Within these limitations, however, early work suggests a correlation between vape pen use and poorer cardiopulmonary outcomes. Survey studies of teens who regularly vape found increased frequencies of respiratory symptoms, including productive cough, that were independent of smoking status. 160 161 These findings were corroborated in a survey series identifying more severe asthma symptoms and more days of school missed owing to asthma among vape pen users, regardless of cigarette smoking status. 162 163 164 Studies among adults have shown a similar pattern, with increased prevalence of chronic respiratory conditions (ie, asthma or chronic obstructive pulmonary disease) among vape pen users, 165 166 and higher risk of myocardial infarction and stroke, but lower risk of diabetes. 167
The effects of vaping on lung function as determined by spirometric studies are more varied. Reported studies have assessed lung function after a brief exposure to vape aerosols, varying from 5-60 minutes in duration, and no longer term observational cohort studies exist. While some studies have shown increased airway resistance after vaping exposure, 130 168 169 others have shown no change in lung function. 137 170 171 The cumulative exposure of habitual vape pen users to vape aerosols is much longer than the period evaluated in these studies, and the impact of vaping on longer term respiratory heath remains to be seen. Recent work evaluating ventilation-perfusion matching among chronic vapers compared with healthy controls found increased ventilation-perfusion mismatch, despite normal spirometry in both groups. 172 Such work reinforces the notion that changes in spirometry are a feature of more advanced airways disease, and early studies, although inconsistent, may foreshadow future respiratory impairment in chronic vapers.
Covid-19 and vaping
The covid-19 pandemic brought renewed attention to the potential health impacts of vaping. Studies investigating the role of vaping in covid-19 prevalence and outcomes have been limited by the small size of the populations studied and results have been inconsistent. Early work noted a geographic association in the US between vaping prevalence and covid-19 cases, 173 and a subsequent survey study found that a covid-19 diagnosis was five times more likely among teens who had ever vaped. 174 In contrast, a UK survey study found no association between vaping status and covid-19 infection rates, although captured a much smaller population of vape pen users. 175 Reports of nicotine use upregulating the angiotensin converting enzyme 2 (ACE-2) receptor, 176 which serves as the binding site for SARS-CoV-2 entry, raised the possibility of increased susceptibility to covid-19 among chronic nicotine vape pen users. 177 178 Further, vape use associated with sharing devices and frequent touching of the mouth and face were posited as potential confounders contributing to increased prevalence of covid-19 in this population. 179
Covid-19 outcomes among chronic vape pen users remain an open question. While smoking has been associated with progression to more severe infections, 180 181 no investigation has been performed to date among vaping cohorts. The young average age of chronic vape pen users may prove a protective factor, as risk of severe covid-19 infection has been shown to increase with age. 182 Regardless, a prudent recommendation remains to abstain from vaping to mitigate risk of progression to severe covid-19 infection. 183
Increased awareness of respiratory health brought about by covid-19 and EVALI is galvanizing the changing patterns in vape pen use. 184 Survey studies have consistently shown trends toward decreasing use among adolescents and young adults. 174 185 186 In one study, up to two thirds of participants endorsed decreasing or quitting vaping owing to a combination of factors including difficulty purchasing vape products during the pandemic, concerns about vaping effects on lung health, and difficulty concealing vape use while living with family. 174 Such results are reflected in nationwide trends that show halting growth in vaping use among high school students. 8 These trends are encouraging in that public health interventions countering nicotine use among teens may be meeting some measure of success.
Clinical impact—collecting and recording a vaping history
Vaping history in electronic medical records.
Efforts to prevent, diagnose, and treat vaping related lung injury begin with the ability of our healthcare system to identify vape users. Since vaping related lung injury remains a diagnosis of exclusion, clinicians must have a high index of suspicion when confronted with idiopathic lung injury in a patient with vaping exposure. Unlike cigarette use, vape pen use is not built into most EMR systems, and is not included in meaningful use criteria for EMRs. 187 Retrospective analysis of outpatient visits showed that a vaping history was collected in less than 0.1% of patients in 2015, 188 although this number has been increasing. 189 190 In part augmented by EMR frameworks that prompt collection of data on vaping history, more recent estimates indicate that a vaping history is being collected in up to 6% of patients. 191 Compared with the widespread use of vaping, particularly among adolescent and young adult populations, this number remains low. Considering generational trends in nicotine use, vaping will likely eventually overcome cigarettes as the most common mode of nicotine use, raising the importance of collecting a vaping related history. Further, EMR integration of vaping history is imperative to allow for retrospective, large scale analyses of vape exposure on longitudinal health outcomes at a population level.
Practical considerations—gathering a vaping history
As vaping becomes more common, the clinician’s ability to accurately collect a vaping history and identify patients who may benefit from nicotine cessation programs becomes more important. Reassuringly, gathering a vaping history is not dissimilar to asking about smoking and use of other tobacco products, and is summarized in box 2 . Collecting a vaping history is of particular importance for providers caring for adolescents and young adults who are among the highest risk demographics for vape pen use. Adolescents and young adults may be reluctant to share their vaping history, particularly if they are using THC-containing or CBD-containing vape solutions. Familiarity with vernacular terms to describe vaping, assuming a non-judgmental approach, and asking parents or guardians to step away during history taking will help to break down these barriers. 192
Practical guide to collecting a vaping history
Ask with empathy.
Young adults may be reluctant to share history of vaping use. Familiarity with vaping terminology, asking in a non-judgmental manner, and asking in a confidential space may help.
Ask what they are vaping
Vape products— vape pens commonly contain nicotine or an alternative active ingredient, such as THC or CBD. Providers may also inquire about flavorants, or other vape solution additives, that their patient is consuming, particularly if vaping related lung injury is suspected.
Source— ask where they source their product from. Sources may include commercially available products, third party distributors, or friends or local contacts.
Ask how they are vaping
Device— What style of device are they using?
Frequency— How many times a day do they use their vape pen (with frequent use considered >5 times a day)? Alternatively, providers may inquire how long it takes to deplete a vape solution cartridge (with use of one or more pods a day considered heavy use).
Nicotine concentration— For individuals consuming nicotine-containing products, clinicians may inquire about concentration and frequency of use, as this may allow for development of a nicotine replacement therapy plan.
Ask about other inhaled products
Clinicians should ask patients who vape about use of other inhaled products, particularly cigarettes. Further, clinicians may ask about use of water pipes, heat-not-burn devices, THC-containing products, or dabbing.
The following provides a practical guide on considerations when collecting a vaping history. Of note, collecting a partial history is preferable to no history at all, and simply recording whether a patient is vaping or not adds valuable information to the medical record.
Vape use— age at time of vaping onset and frequency of vape pen use. Vape pen use >5 times a day would be considered frequent. Alternatively, clinicians may inquire how long it takes to deplete a vape solution pod (use of one or more pods a day would be considered heavy use), or how frequently users are refilling their vape pens for refillable models.
Vape products— given significant variation in vape solutions available on the market, and variable risk profiles of the multitude of additives, inquiring as to which products a patient is using may add useful information. Further, clinicians may inquire about use of nicotine versus THC-containing vape solutions, and whether said products are commercially available or are customized by third party sellers.
Concurrent smoking— simultaneous use of multiple inhaled products is common among vape users, including concurrent use of conventional cigarettes, water pipes, heat-not-burn devices, and THC-containing or CBD-containing products. Among those using marijuana products, gathering a history regarding the type of product use, the device, and the modality of aerosol generation may be warranted. Gathering such detailed information may be challenging in the face of rapidly evolving product availability and changing popular terminology. Lastly, clinicians may wish to inquire about “dabbing”—the practice of inhaling heated butane hash oil, a concentrated THC wax—which may also be associated with lung injury. 193
Future directions
Our understanding of the effects of vaping on respiratory health is in its early stages and multiple trials are under way. Future work requires enhanced understanding of the effects of vape aerosols on lung biology, such as ongoing investigations into biomarkers of oxidative stress and inflammation among vape users (clinicaltrials.gov NCT03823885 ). Additional studies seek to elucidate the relation between vape aerosol exposure and cardiopulmonary outcomes among vape pen users ( NCT03863509 , NCT05199480 ), while an ongoing prospective cohort study will allow for longitudinal assessment of airway reactivity and spirometric changes among chronic vape pen users ( NCT04395274 ).
Public health and policy interventions are vital in supporting both our understanding of vaping on respiratory health and curbing the vaping epidemic among teens. Ongoing, large scale randomized controlled studies seek to assess the impact of the FDA’s “The Real Cost” advertisement campaign for vaping prevention ( NCT04836455 ) and another trial is assessing the impact of a vaping prevention curriculum among adolescents ( NCT04843501 ). Current trials are seeking to understand the potential for various therapies as tools for vaping cessation, including nicotine patches ( NCT04974580 ), varenicline ( NCT04602494 ), and text message intervention ( NCT04919590 ).
Finally, evaluation of vaping as a potential tool for harm reduction among current cigarette smokers is undergoing further evaluation ( NCT03235505 ), which will add to the body of work and eventually lead to clear policy guidance.
Several guidelines on the management of vaping related lung injury have been published and are summarized in table 1 . 194 195 196 Given the relatively small number of cases, the fact that vaping related lung injury remains a newer clinical entity, and the lack of clinical trials on the topic, guideline recommendations reflect best practices and expert opinion. Further, published guidelines focus on the diagnosis and management of EVALI, and no guidelines exist to date for the management of vaping related lung injury more generally.
Summary of clinical guidelines
- View inline
Conclusions
Vaping has grown in popularity internationally over the past decade, in part propelled by innovations in vape pen design and nicotine flavoring. Teens and young adults have seen the biggest uptake in use of vape pens, which have superseded conventional cigarettes as the preferred modality of nicotine consumption. Despite their widespread popularity, relatively little is known about the potential effects of chronic vaping on the respiratory system, and a growing body of literature supports the notion that vaping is not without risk. The 2019 EVALI outbreak highlighted the potential harms of vaping, and the consequences of long term use remain unknown.
Discussions regarding the potential harms of vaping are reminiscent of scientific debates about the health effects of cigarette use in the 1940s. Interesting parallels persist, including the fact that only a minority of conventional cigarette users develop acute lung injury, yet the health impact of sustained, longitudinal cigarette use is unquestioned. The true impact of vaping on respiratory health will manifest over the coming decades, but in the interval a prudent and time tested recommendation remains to abstain from consumption of inhaled nicotine and other products.
Questions for future research
How does chronic vape aerosol exposure affect respiratory health?
Does use of vape pens affect respiratory physiology (airway resistance, V/Q matching, etc) in those with underlying lung disease?
What is the role for vape pen use in promoting smoking cessation?
What is the significance of pulmonary alveolar macrophages in the pathophysiology of vaping related lung injury?
Are particular populations more susceptible to vaping related lung injury (ie, by sex, demographic, underlying comorbidity, or age)?
Series explanation: State of the Art Reviews are commissioned on the basis of their relevance to academics and specialists in the US and internationally. For this reason they are written predominantly by US authors
Contributors: AJ conceived of, researched, and wrote the piece. She is the guarantor.
Competing interests: I have read and understood the BMJ policy on declaration of interests and declare the following interests: AJ receives consulting fees from DawnLight, Inc for work unrelated to this piece.
Patient involvement: No patients were directly involved in the creation of this article.
Provenance and peer review: Commissioned; externally peer reviewed.
- ↵ US Department of Health and Human Services. The health consequences of smoking—50 years of progress: a report of the Surgeon General. 2014. doi: 10.1037/e510072014-001 .
- Cornelius ME ,
- Loretan CG ,
- Marshall TR
- Fetterman JL ,
- Benjamin EJ ,
- Barrington-Trimis JL ,
- Leventhal AM ,
- Cullen KA ,
- Gentzke AS ,
- Sawdey MD ,
- ↵ Centers for Disease Control and Prevention. Surgeon General’s Advisory on E-cigarette Use Among Youth. 2018. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/surgeon-general-advisory/index.html
- Leventhal A ,
- Johnston L ,
- O’Malley PM ,
- Patrick ME ,
- Barrington-Trimis J
- ↵ Foundation for a Smoke-Free World https://www.smokefreeworld.org/published_reports/
- Kaisar MA ,
- Calfee CS ,
- Matthay MA ,
- ↵ Royal College of Physicians (London) & Tobacco Advisory Group. Nicotine without smoke: tobacco harm reduction: a report. 2016. https://www.rcplondon.ac.uk/projects/outputs/nicotine-without-smoke-tobacco-harm-reduction
- ↵ National Academies of Sciences, Engineering, and Medicine. Public health consequences of e-cigarettes. 2018. doi: 10.17226/24952 OpenUrl CrossRef
- Manigrasso M ,
- Buonanno G ,
- Stabile L ,
- Agnihotri R
- LeBouf RF ,
- Koutrakis P ,
- Christiani DC
- Perrine CG ,
- Pickens CM ,
- Boehmer TK ,
- Lung Injury Response Epidemiology/Surveillance Group
- Layden JE ,
- Esposito S ,
- Spindle TR ,
- Eissenberg T ,
- Kendler KS ,
- McCabe SE ,
- ↵ Joseph R. Electric vaporizer. 1930.
- ↵ Gilbert HA. Smokeless non-tobacco cigarette. 1965.
- ↵ An Interview With A 1970’s Vaping Pioneer. Ashtray Blog. 2015 . https://www.ecigarettedirect.co.uk/ashtray-blog/2014/06/favor-cigarette-interview-dr-norman-jacobson.html (2014).
- Benowitz N ,
- ↵ National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Population Health and Public Health Practice, & Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems. Public Health Consequences of E-Cigarettes . (National Academies Press (US), 2018).
- ↵ Tolentino J. The Promise of Vaping and the Rise of Juul. The New Yorker . 2018 https://www.newyorker.com/magazine/2018/05/14/the-promise-of-vaping-and-the-rise-of-juul
- ↵ Bowen A, Xing C. Nicotine salt formulations for aerosol devices and methods thereof. 2014.
- Madden DR ,
- McConnell R ,
- Gammon DG ,
- Marynak KL ,
- ↵ Jackler RK, Chau C, Getachew BD, et al. JUUL Advertising Over its First Three Years on the Market. Stanford Research into the Impact of Tobacco Advertising, Stanford University School of Medicine. 2019. https://tobacco-img.stanford.edu/wp-content/uploads/2021/07/21231836/JUUL_Marketing_Stanford.pdf
- Prochaska JJ ,
- ↵ Richtel M, Kaplan S. Juul May Get Billions in Deal With One of World’s Largest Tobacco Companies. The New York Times . 2018.
- ↵ Truth Initiative. Vaping Lingo Dictionary: A guide to popular terms and devices. 2020. https://truthinitiative.org/research-resources/emerging-tobacco-products/vaping-lingo-dictionary
- Williams M ,
- Pepper JK ,
- MacMonegle AJ ,
- Nonnemaker JM
- ↵ The Physics of Vaporization. Jupiter Research. 2020. https://www.jupiterresearch.com/physics-of-vaporization/
- Bonnevie E ,
- ↵ National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. E-Cigarette Use Among Youth and Young Adults: A Report of the Surgeon General. 2016. https://www.ncbi.nlm.nih.gov/books/NBK538680/
- Trivers KF ,
- Phillips E ,
- Stefanac S ,
- Sandner I ,
- Grabovac I ,
- ↵ Knowledge-Action-Change. Burning Issues: The Global State of Tobacco Harm Reduction 2020. 2020. https://gsthr.org/resources/item/burning-issues-global-state-tobacco-harm-reduction-2020
- Creamer MR ,
- Park-Lee E ,
- Gorukanti A ,
- Delucchi K ,
- Fisher-Travis R ,
- Halpern-Felsher B
- Amrock SM ,
- ↵ Buy JUUL Products | Shop All JUULpods, JUUL Devices, and Accessories | JUUL. https://www.juul.com/shop
- Schiff SJ ,
- Kechter A ,
- Simpson KA ,
- Ceasar RC ,
- Braymiller JL ,
- Barrington-Trimis JL
- Moustafa AF ,
- Rodriguez D ,
- Audrain-McGovern J
- Berhane K ,
- Stjepanović D ,
- Hitchman SC ,
- Bakolis I ,
- Plurphanswat N
- Warner KE ,
- Cummings KM ,
- ↵ FDA finalizes enforcement policy on unauthorized flavored cartridge-based e-cigarettes that appeal to children, including fruit and mint. FDA . 2020 . https://www.fda.gov/news-events/press-announcements/fda-finalizes-enforcement-policy-unauthorized-flavored-cartridge-based-e-cigarettes-appeal-children
- ↵ Henderson, E. E-cigarettes: an evidence update. 113.
- Hartmann-Boyce J ,
- McRobbie H ,
- Lindson N ,
- Phillips-Waller A ,
- ↵ Centers for Disease Control and Prevention. Electronic Cigarettes. 2020. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/index.htm l
- Patnode CD ,
- Henderson JT ,
- Thompson JH ,
- Senger CA ,
- Fortmann SP ,
- Whitlock EP
- Kmietowicz Z
- ↵ World Health Organization. E-cigarettes are harmful to health. 2020. https://www.who.int/news/item/05-02-2020-e-cigarettes-are-harmful-to-health
- Lachireddy K ,
- Sleiman M ,
- Montesinos VN ,
- Kalkhoran S ,
- Filion KB ,
- Kimmelman J ,
- Eisenberg MJ
- McCauley L ,
- Aoshiba K ,
- Nakamura H ,
- Wiggins A ,
- Hudspath C ,
- Kisling A ,
- Hostler DC ,
- Sommerfeld CG ,
- Weiner DJ ,
- Mantilla RD ,
- Darnell RT ,
- Khateeb F ,
- Agustin M ,
- Yamamoto M ,
- Cabrera F ,
- ↵ Long. Diffuse Alveolar Hemorrhage Due to Electronic Cigarette Use | A54. CRITICAL CARE CASE REPORTS: ACUTE HYPOXEMIC RESPIRATORY FAILURE/ARDS. https://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2016.193.1_MeetingAbstracts.A1862
- Ring Madsen L ,
- Vinther Krarup NH ,
- Bergmann TK ,
- Bradford LE ,
- Rebuli ME ,
- Jaspers I ,
- Clement KC ,
- Loughlin CE
- Bonilla A ,
- Alamro SM ,
- Bassett RA ,
- Osterhoudt K ,
- Slaughter J ,
- ↵ Health CO. on S. and. Smoking and Tobacco Use; Electronic Cigarettes. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html (2019).
- ↵ For State, Local, Territorial, and Tribal Health Departments | Electronic Cigarettes | Smoking & Tobacco Use | CDC. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease/health-departments/index.html (2019).
- ↵ 2019 Lung Injury Surveillance Primary Case Definitions. 2.
- Callahan SJ ,
- Collingridge DS ,
- Armatas C ,
- Heinzerling A ,
- Blount BC ,
- Karwowski MP ,
- Shields PG ,
- Lung Injury Response Laboratory Working Group
- ↵ Government of Canada. Vaping-associated lung illness. 2020. https://www.canada.ca/en/public-health/services/diseases/vaping-pulmonary-illness.html (2020).
- Marlière C ,
- De Greef J ,
- Casanova GS ,
- Blagev DP ,
- Guidry DW ,
- Grissom CK ,
- Werner AK ,
- Koumans EH ,
- Chatham-Stephens K ,
- Lung Injury Response Mortality Working Group
- Tsirilakis K ,
- Yenduri NJS ,
- Guillerman RP ,
- Anderson B ,
- Serajeddini H ,
- Knipping D ,
- Brasky TM ,
- Pisinger C ,
- Godtfredsen N ,
- Jensen RP ,
- Pankow JF ,
- Strongin RM ,
- Lechasseur A ,
- Altmejd S ,
- Turgeon N ,
- Goessler W ,
- Chaumont M ,
- van de Borne P ,
- Bernard A ,
- Kosmider L ,
- Sobczak A ,
- Aldridge K ,
- Afshar-Mohajer N ,
- Koehler K ,
- Varughese S ,
- Teschke K ,
- van Netten C ,
- Beyazcicek O ,
- Onyenwoke RU ,
- Khlystov A ,
- Samburova V
- Lavrich KS ,
- van Heusden CA ,
- Lazarowski ER ,
- Carson JL ,
- Pawlak EA ,
- Lackey JT ,
- Sherwood CL ,
- O’Sullivan M ,
- Vallarino J ,
- Flanigan SS ,
- LeBlanc M ,
- Kullman G ,
- Simoes EJ ,
- Wambui DW ,
- Vardavas CI ,
- Anagnostopoulos N ,
- Kougias M ,
- Evangelopoulou V ,
- Connolly GN ,
- Behrakis PK
- Sussan TE ,
- Gajghate S ,
- Thimmulappa RK ,
- Hossain E ,
- Perveen Z ,
- Lerner CA ,
- Sundar IK ,
- Garcia-Arcos I ,
- Geraghty P ,
- Baumlin N ,
- Coakley RC ,
- Mascenik T ,
- Staudt MR ,
- Hollmann C ,
- Radicioni G ,
- Coakley RD ,
- Kalathil SG ,
- Bogner PN ,
- Goniewicz ML ,
- Thanavala YM
- Massey JB ,
- Matsumoto S ,
- Traber MG ,
- Ranpara A ,
- Stefaniak AB ,
- Williams K ,
- Fernandez E ,
- Basset-Léobon C ,
- Lacoste-Collin L ,
- Courtade-Saïdi M
- Boland JM ,
- Maddock SD ,
- Cirulis MM ,
- Tazelaar HD ,
- Mukhopadhyay S ,
- Dammert P ,
- Kalininskiy A ,
- Davidson K ,
- Brancato A ,
- Heetderks P ,
- Dicpinigaitis PV ,
- Trachuk P ,
- Suhrland MJ
- Khilnani GC
- Simmons A ,
- Freudenheim JL ,
- Hussell T ,
- Cibella F ,
- Caponnetto P ,
- Schweitzer RJ ,
- Williams RJ ,
- Vindhyal MR ,
- Munguti C ,
- Vindhyal S ,
- Lappas AS ,
- Tzortzi AS ,
- Konstantinidi EM ,
- Antoniewicz L ,
- Brynedal A ,
- Lundbäck M ,
- Ferrari M ,
- Boulay MÈ ,
- Boulet LP ,
- Morissette MC
- Kizhakke Puliyakote AS ,
- Elliott AR ,
- Anderson KM ,
- Crotty Alexander LE ,
- Jackson SE ,
- McAlinden KD ,
- McKelvey K ,
- Patanavanich R ,
- NVSS - Provisional Death Counts for COVID-19 - Executive Summary
- Sokolovsky AW ,
- Hertel AW ,
- Micalizzi L ,
- Klemperer EM ,
- Peasley-Miklus C ,
- Villanti AC
- Henricks WH
- Young-Wolff KC ,
- Klebaner D ,
- Mowery DL ,
- Don’t Forget to Ask
- Stephens D ,
- Siegel DA ,
- Jatlaoui TC ,
- Lung Injury Response Clinical Working Group ,
- Ramalingam SS ,
- Schuurmans MM
Advertisement
How bad is vaping for your health? We’re finally getting answers
As more of us take up vaping and concerns rise about the long-term effects, we now have enough data to get a grip on the health impact – and how it compares to smoking
By Graham Lawton
6 December 2023

Klaus Kremmerz
AS THE old joke goes, when I read about the dangers of smoking, I gave up reading. If you are a vaper, you might feel like you want to stop reading now. Don’t: you need to know this.
I am a vaper. Like many others, I used to smoke and switched to vaping for health reasons. I plan to quit completely, but I haven’t managed it yet. I am sure vaping is better for me than smoking, but I am also sure it is worse than not vaping. I cough in the morning and feel massively addicted to the nicotine. I don’t even really know what I am inhaling. I worry that it will be hard to quit, that I am causing long-term damage to my body and that by vaping, I am susceptible to slipping back down the slope to cigarettes. I also have the same worries for the teenagers I see coming out of school and immediately enveloping themselves in sweet-smelling clouds.
Is CBD a wonder drug or waste of money? Here's what the evidence says
As vaping has increased throughout the Western world, these fears have been repeated often. Part of last month’s King’s Speech in the UK focused on new legislation aiming to create a smoke-free generation in part by cracking down on youth vaping. Worldwide, there have been calls for tougher regulation and more investigation into vaping’s health effects as increasing numbers of children admit to taking up the habit.
But there hasn’t been a huge amount to say on whether fears over health effects are well-founded – until…
Sign up to our weekly newsletter
Receive a weekly dose of discovery in your inbox! We'll also keep you up to date with New Scientist events and special offers.
To continue reading, subscribe today with our introductory offers
No commitment, cancel anytime*
Offer ends 15 December 2024.
*Cancel anytime within 14 days of payment to receive a refund on unserved issues.
Inclusive of applicable taxes (VAT)
Existing subscribers
More from New Scientist
Explore the latest news, articles and features

Never mind the health benefits, there are green reasons to stop vaping
Subscriber-only

Ozempic and Wegovy could help people quit smoking

Cannabis vaping liquids contain lead and other toxic metals

Why does the UK want to ban disposable vapes and when will it happen?
Popular articles.
Trending New Scientist articles
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest, how to fight depression faster..

Plastics are everywhere, even in our bodies

Threat of mosquito-borne diseases rises in U.S. with global temperature

Studies show that about 9 percent of the population and nearly 28 percent of high school students are e-cigarette users.
Diego Cervo/iStock by Getty Images
Restricted airways, scarred lung tissue found among vapers
MGH News and Public Affairs
Small study looks at chronic e-cigarette users, seeing partial improvement once they stop
Chronic use of e-cigarettes, commonly known as vaping, can result in small airway obstruction and asthma-like symptoms, according to researchers at Harvard-affiliated Massachusetts General Hospital.
More like this

Teen vaping rising fast, research says

Survey of oncologists finds knowledge gap on medical marijuana

Chemical flavorings found in e-cigarettes linked to respiratory disease
In the first study to microscopically evaluate the pulmonary tissue of e-cigarette users for chronic disease, the team found in a small sample of patients fibrosis and damage in the small airways, similar to the chemical inhalation damage to the lungs typically seen in soldiers returning from overseas conflicts who had inhaled mustard or similar types of noxious gases. The study was published in New England Journal of Medicine Evidence .
“All four individuals we studied had injury localized to the same anatomic location within the lung, manifesting as small airway-centered fibrosis with constrictive bronchiolitis, which was attributed to vaping after thorough clinical evaluations excluded other possible causes,” says lead author Lida Hariri, an associate professor of pathology at Harvard Medical School and a pathologist and physician investigator at MGH. “We also observed that when patients ceased vaping, they had a partial reversal of the condition over one to four years, though not complete due to residual scarring in the lung tissue.”
A huge increase in vaping, particularly among young adults and adolescents, has occurred in the United States, with studies showing about 9 percent of the population and nearly 28 percent of high school students are e-cigarette users. Unlike cigarette smoking, however, the long-term health risks of chronic vaping are largely unknown.
In order to determine the underlying pathophysiology of vaping-related symptoms, the MGH team examined a cohort of four patients, each with a three- to eight-year history of e-cigarette use and chronic lung disease. All patients underwent detailed clinical evaluation, including pulmonary function tests, high resolution chest imaging, and surgical lung biopsy. Constrictive bronchiolitis, or narrowing of the small airways due to fibrosis within the bronchiolar wall, was observed in each patient. So was significant overexpression of MUC5AC, a gel-forming protein in the mucus layer of the airway that has been seen in airway cell and sputum samples of individuals who vape. In addition, three of the four patients had evidence of mild emphysema consistent with their former combustible cigarette smoking history, though researchers concluded this was distinct from the findings of constrictive bronchiolitis seen in the patient cohort.
Because the same type of lung damage was observed in all patients, as well as partial improvement in symptoms after e-cigarette usage was stopped, researchers concluded that vaping was the most likely cause after thorough evaluation and exclusion of other possible causes. “Our investigation shows that chronic pathological abnormalities can occur in vaping exposure,” says senior author David Christiani, a professor of medicine at HMS and a physician investigator at Mass General Research Institute. “Physicians need to be informed by scientific evidence when advising patients about the potential harm of long-term vaping, and this work adds to a growing body of toxicological evidence that nicotine vaping exposures can harm the lung.”
A hopeful sign from the study was that three of the four patients showed improvements in their pulmonary function tests and high-resolution computed tomography (HRCT) chest imaging after they ceased vaping. “While there is growing evidence to show that vaping is a risky behavior with potential long-term health consequences for users,” says Hariri, “our research also suggests that quitting can be beneficial and help to reverse some of the disease.”
The study was funded by the National Institutes of Health.
Share this article
You might like.
Hope flags when medications fail, isolating and endangering patients. Backed by a major grant, 2 Harvard scientists are focused on reducing the distance between diagnosis and recovery.
We ingest equivalent of credit card per week — how worried should we be? In ‘Harvard Thinking,’ experts discuss how to minimize exposure, possible solutions.

Experts fear more cases of West Nile virus, EEE (and possibly Zika, Dengue fever) as warm seasons get longer, wetter
What happened when a meteorite the size of four Mount Everests hit Earth?
Giant impact had silver lining for life, according to new study
Do phones belong in schools?
Banning cellphones may help protect classroom focus, but school districts need to stay mindful of students’ sense of connection, experts say.
How to apply cool-headed reason to red-hot topics
Michael J. Sandel brings back wildly popular ‘Justice’ course amid time of strained discourse on college campuses
- Open access
- Published: 18 May 2021
An updated overview of e-cigarette impact on human health
- Patrice Marques ORCID: orcid.org/0000-0003-0465-1727 1 , 2 ,
- Laura Piqueras ORCID: orcid.org/0000-0001-8010-5168 1 , 2 , 3 &
- Maria-Jesus Sanz ORCID: orcid.org/0000-0002-8885-294X 1 , 2 , 3
Respiratory Research volume 22 , Article number: 151 ( 2021 ) Cite this article
425k Accesses
178 Citations
389 Altmetric
Metrics details
The electronic cigarette ( e-cigarette ), for many considered as a safe alternative to conventional cigarettes, has revolutionised the tobacco industry in the last decades. In e-cigarettes , tobacco combustion is replaced by e-liquid heating, leading some manufacturers to propose that e-cigarettes have less harmful respiratory effects than tobacco consumption. Other innovative features such as the adjustment of nicotine content and the choice of pleasant flavours have won over many users. Nevertheless, the safety of e-cigarette consumption and its potential as a smoking cessation method remain controversial due to limited evidence. Moreover, it has been reported that the heating process itself can lead to the formation of new decomposition compounds of questionable toxicity. Numerous in vivo and in vitro studies have been performed to better understand the impact of these new inhalable compounds on human health. Results of toxicological analyses suggest that e-cigarettes can be safer than conventional cigarettes, although harmful effects from short-term e-cigarette use have been described. Worryingly, the potential long-term effects of e-cigarette consumption have been scarcely investigated. In this review, we take stock of the main findings in this field and their consequences for human health including coronavirus disease 2019 (COVID-19).
Electronic nicotine dispensing systems (ENDS), commonly known as electronic cigarettes or e-cigarettes , have been popularly considered a less harmful alternative to conventional cigarette smoking since they first appeared on the market more than a decade ago. E-cigarettes are electronic devices, essentially consisting of a cartridge, filled with an e-liquid, a heating element/atomiser necessary to heat the e-liquid to create a vapour that can be inhaled through a mouthpiece, and a rechargeable battery (Fig. 1 ) [ 1 , 2 ]. Both the electronic devices and the different e-liquids are easily available in shops or online stores.
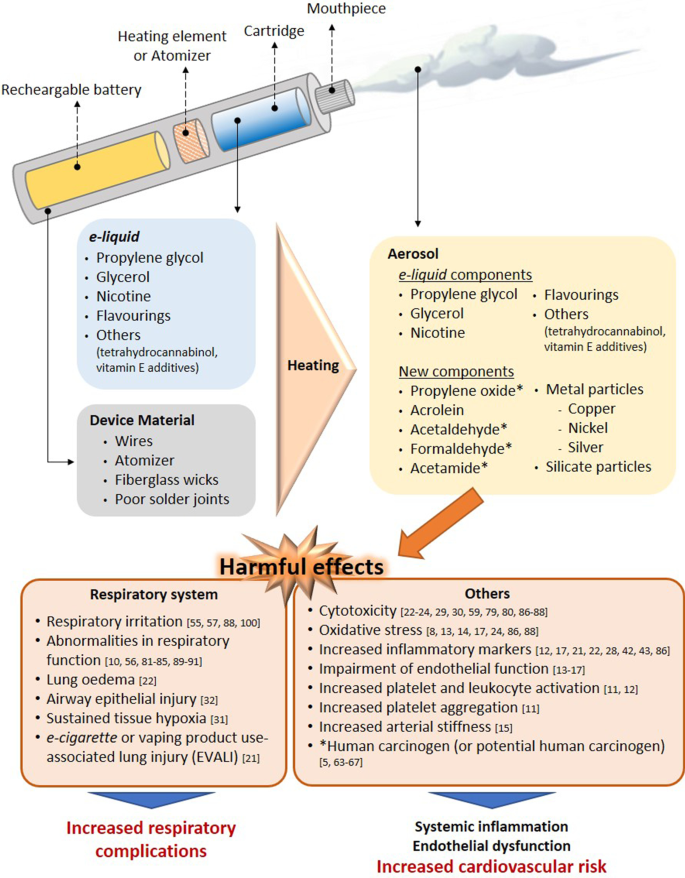
Effect of the heating process on aerosol composition. Main harmful effects documented. Several compounds detected in e-cigarette aerosols are not present in e-liquid s and the device material also seems to contribute to the presence of metal and silicate particles in the aerosols. The heating conditions especially on humectants, flavourings and the low-quality material used have been identified as the generator of the new compounds in aerosols. Some compounds generated from humectants (propylene glycol and glycerol) and flavourings, have been associated with clear airways impact, inflammation, impairment of cardiovascular function and toxicity. In addition, some of them are carcinogens or potential carcinogens
The e-liquid typically contains humectants and flavourings, with or without nicotine; once vapourised by the atomiser, the aerosol (vapour) provides a sensation similar to tobacco smoking, but purportedly without harmful effects [ 3 ]. However, it has been reported that the heating process can lead to the generation of new decomposition compounds that may be hazardous [ 4 , 5 ]. The levels of nicotine, which is the key addictive component of tobacco, can also vary between the commercially available e-liquids, and even nicotine-free options are available. For this particular reason, e-cigarettes are often viewed as a smoking cessation tool, given that those with nicotine can prevent smoking craving, yet this idea has not been fully demonstrated [ 2 , 6 , 7 ].
Because e-cigarettes are combustion-free, and because most of the damaging and well-known effects of tobacco are derived from this reaction, there is a common and widely spread assumption that e-cigarette consumption or “vaping” is safer than conventional cigarette smoking. However, are they risk-free? Is there sufficient toxicological data on all the components employed in e-liquids ? Do we really know the composition of the inhaled vapour during the heating process and its impact on health? Can e-cigarettes be used to curb tobacco use? Do their consumption impact on coronavirus disease 2019 (COVID-19)? In the present review, we have attempted to clarify these questions based on the existing scientific literature, and we have compiled new insights related with the toxicity derived from the use of these devices.
Effect of e-cigarette vapour versus conventional cigarette exposure: in vivo and in vitro effects
Numerous studies have been performed to evaluate the safety/toxicity of e-cigarette use both in vivo and in in vitro cell culture.
One of the first studies in humans involved the analysis of 9 volunteers that consumed e-cigarettes , with or without nicotine, in a ventilated room for 2 h [ 8 ]. Pollutants in indoor air, exhaled nitric oxide (NO) and urinary metabolite profiles were analysed. The results of this acute experiment revealed that e-cigarettes are not emission-free, and ultrafine particles formed from propylene glycol (PG) could be detected in the lungs. The study also suggested that the presence of nicotine in e-cigarettes increased the levels of NO exhaled from consumers and provoked marked airway inflammation; however, no differences were found in the levels of exhaled carbon monoxide (CO), an oxidative stress marker, before and after e-cigarette consumption [ 8 ]. A more recent human study detected significantly higher levels of metabolites of hazardous compounds including benzene, ethylene oxide, acrylonitrile, acrolein and acrylamide in the urine of adolescent dual users ( e-cigarettes and conventional tobacco consumers) than in adolescent e-cigarette -only users (Table 1 ) [ 9 ]. Moreover, the urine levels of metabolites of acrylonitrile, acrolein, propylene oxide, acrylamide and crotonaldehyde, all of which are detrimental for human health, were significantly higher in e-cigarette -only users than in non-smoker controls, reaching up to twice the registered values of those from non-smoker subjects (Table 1 ) [ 9 ]. In line with these observations, dysregulation of lung homeostasis has been documented in non-smokers subjected to acute inhalation of e-cigarette aerosols [ 10 ].
Little is known about the effect of vaping on the immune system. Interestingly, both traditional and e-cigarette consumption by non-smokers was found to provoke short-term effects on platelet function, increasing platelet activation (levels of soluble CD40 ligand and the adhesion molecule P-selectin) and platelet aggregation, although to a lesser extent with e-cigarettes [ 11 ]. As found with platelets, the exposure of neutrophils to e-cigarette aerosol resulted in increased CD11b and CD66b expression being both markers of neutrophil activation [ 12 ]. Additionally, increased oxidative stress, vascular endothelial damage, impaired endothelial function, and changes in vascular tone have all been reported in different human studies on vaping [ 13 , 14 , 15 , 16 , 17 ]. In this context, it is widely accepted that platelet and leukocyte activation as well as endothelial dysfunction are associated with atherogenesis and cardiovascular morbidity [ 18 , 19 ]. In line with these observations the potential association of daily e-cigarettes consumption and the increased risk of myocardial infarction remains controversial but benefits may occur when switching from tobacco to chronic e-cigarette use in blood pressure regulation, endothelial function and vascular stiffness (reviewed in [ 20 ]). Nevertheless, whether or not e-cigarette vaping has cardiovascular consequences requires further investigation.
More recently, in August 2019, the US Centers for Disease Control and Prevention (CDC) declared an outbreak of the e-cigarette or vaping product use-associated lung injury (EVALI) which caused several deaths in young population (reviewed in [ 20 ]). Indeed, computed tomography (CT scan) revealed local inflammation that impaired gas exchange caused by aerosolised oils from e-cigarettes [ 21 ]. However, most of the reported cases of lung injury were associated with use of e-cigarettes for tetrahydrocannabinol (THC) consumption as well as vitamin E additives [ 20 ] and not necessarily attributable to other e-cigarette components.
On the other hand, in a comparative study of mice subjected to either lab air, e-cigarette aerosol or cigarette smoke (CS) for 3 days (6 h-exposure per day), those exposed to e-cigarette aerosols showed significant increases in interleukin (IL)-6 but normal lung parenchyma with no evidence of apoptotic activity or elevations in IL-1β or tumour necrosis factor-α (TNFα) [ 22 ]. By contrast, animals exposed to CS showed lung inflammatory cell infiltration and elevations in inflammatory marker expression such as IL-6, IL-1β and TNFα [ 22 ]. Beyond airway disease, exposure to aerosols from e-liquids with or without nicotine has also been also associated with neurotoxicity in an early-life murine model [ 23 ].
Results from in vitro studies are in general agreement with the limited number of in vivo studies. For example, in an analysis using primary human umbilical vein endothelial cells (HUVEC) exposed to 11 commercially-available vapours, 5 were found to be acutely cytotoxic, and only 3 of those contained nicotine [ 24 ]. In addition, 5 of the 11 vapours tested (including 4 that were cytotoxic) reduced HUVEC proliferation and one of them increased the production of intracellular reactive oxygen species (ROS) [ 24 ]. Three of the most cytotoxic vapours—with effects similar to those of conventional high-nicotine CS extracts—also caused comparable morphological changes [ 24 ]. Endothelial cell migration is an important mechanism of vascular repair than can be disrupted in smokers due to endothelial dysfunction [ 25 , 26 ]. In a comparative study of CS and e-cigarette aerosols, Taylor et al . found that exposure of HUVEC to e-cigarette aqueous extracts for 20 h did not affect migration in a scratch wound assay [ 27 ], whereas equivalent cells exposed to CS extract showed a significant inhibition in migration that was concentration dependent [ 27 ].
In cultured human airway epithelial cells, both e-cigarette aerosol and CS extract induced IL-8/CXCL8 (neutrophil chemoattractant) release [ 28 ]. In contrast, while CS extract reduced epithelial barrier integrity (determined by the translocation of dextran from the apical to the basolateral side of the cell layer), e-cigarette aerosol did not, suggesting that only CS extract negatively affected host defence [ 28 ]. Moreover, Higham et al . also found that e-cigarette aerosol caused IL-8/CXCL8 and matrix metallopeptidase 9 (MMP-9) release together with enhanced activity of elastase from neutrophils [ 12 ] which might facilitate neutrophil migration to the site of inflammation [ 12 ].
In a comparative study, repeated exposure of human gingival fibroblasts to CS condensate or to nicotine-rich or nicotine-free e-vapour condensates led to alterations in morphology, suppression of proliferation and induction of apoptosis, with changes in all three parameters greater in cells exposed to CS condensate [ 29 ]. Likewise, both e-cigarette aerosol and CS extract increased cell death in adenocarcinomic human alveolar basal epithelial cells (A549 cells), and again the effect was more damaging with CS extract than with e-cigarette aerosol (detrimental effects found at 2 mg/mL of CS extract vs. 64 mg/mL of e-cigarette extract) [ 22 ], which is in agreement with another study examining battery output voltage and cytotoxicity [ 30 ].
All this evidence would suggest that e-cigarettes are potentially less harmful than conventional cigarettes (Fig. 2 ) [ 11 , 14 , 22 , 24 , 27 , 28 , 29 ]. Importantly, however, most of these studies have investigated only short-term effects [ 10 , 14 , 15 , 22 , 27 , 28 , 29 , 31 , 32 ], and the long-term effects of e-cigarette consumption on human health are still unclear and require further study.
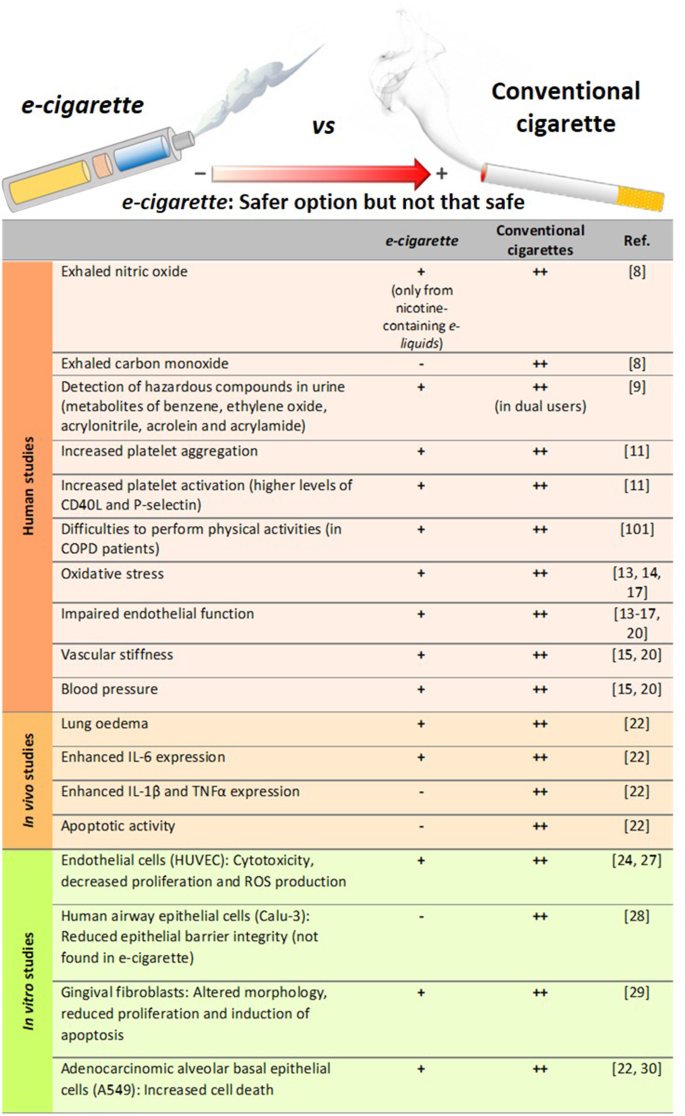
Comparison of the degree of harmful effects documented from e-cigarette and conventional cigarette consumption. Human studies, in vivo mice exposure and in vitro studies. All of these effects from e-cigarettes were documented to be lower than those exerted by conventional cigarettes, which may suggest that e-cigarette consumption could be a safer option than conventional tobacco smoking but not a clear safe choice
Consequences of nicotine content
Beyond flavour, one of the major issues in the e-liquid market is the range of nicotine content available. Depending on the manufacturer, the concentration of this alkaloid can be presented as low , medium or high , or expressed as mg/mL or as a percentage (% v/v). The concentrations range from 0 (0%, nicotine-free option) to 20 mg/mL (2.0%)—the maximum nicotine threshold according to directive 2014/40/EU of the European Parliament and the European Union Council [ 33 , 34 ]. Despite this normative, however, some commercial e-liquids have nicotine concentrations close to 54 mg/mL [ 35 ], much higher than the limits established by the European Union.
The mislabelling of nicotine content in e-liquids has been previously addressed [ 8 , 34 ]. For instance, gas chromatography with a flame ionisation detector (GC-FID) revealed inconsistencies in the nicotine content with respect to the manufacturer´s declaration (average of 22 ± 0.8 mg/mL vs. 18 mg/mL) [ 8 ], which equates to a content ~ 22% higher than that indicated in the product label. Of note, several studies have detected nicotine in those e-liquids labelled as nicotine-free [ 5 , 35 , 36 ]. One study detected the presence of nicotine (0.11–6.90 mg/mL) in 5 of 23 nicotine-free labelled e-liquids by nuclear magnetic resonance spectroscopy [ 35 ], and another study found nicotine (average 8.9 mg/mL) in 13.6% (17/125) of the nicotine-free e-liquids as analysed by high performance liquid chromatography (HPLC) [ 36 ]. Among the 17 samples tested in this latter study 14 were identified to be counterfeit or suspected counterfeit. A third study detected nicotine in 7 of 10 nicotine-free refills, although the concentrations were lower than those identified in the previous analyses (0.1–15 µg/mL) [ 5 ]. Not only is there evidence of mislabelling of nicotine content among refills labelled as nicotine-free, but there also seems to be a history of poor labelling accuracy in nicotine-containing e-liquids [ 37 , 38 ].
A comparison of the serum levels of nicotine from e-cigarette or conventional cigarette consumption has been recently reported [ 39 ]. Participants took one vape from an e-cigarette , with at least 12 mg/mL of nicotine, or inhaled a conventional cigarette, every 20 s for 10 min. Blood samples were collected 1, 2, 4, 6, 8, 10, 12 and 15 min after the first puff, and nicotine serum levels were measured by liquid chromatography-mass spectrometry (LC–MS). The results revealed higher serum levels of nicotine in the conventional CS group than in the e-cigarette group (25.9 ± 16.7 ng/mL vs. 11.5 ± 9.8 ng/mL). However, e-cigarettes containing 20 mg/mL of nicotine are more equivalent to normal cigarettes, based on the delivery of approximately 1 mg of nicotine every 5 min [ 40 ].
In this line, a study compared the acute impact of CS vs. e-cigarette vaping with equivalent nicotine content in healthy smokers and non-smokers. Both increased markers of oxidative stress and decreased NO bioavailability, flow-mediated dilation, and vitamin E levels showing no significant differences between tobacco and e-cigarette exposure (reviewed in [ 20 ]). Inasmuch, short-term e-cigarette use in healthy smokers resulted in marked impairment of endothelial function and an increase in arterial stiffness (reviewed in [ 20 ]). Similar effects on endothelial dysfunction and arterial stiffness were found in animals when they were exposed to e-cigarette vapor either for several days or chronically (reviewed in [ 20 ]). In contrast, other studies found acute microvascular endothelial dysfunction, increased oxidative stress and arterial stiffness in smokers after exposure to e-cigarettes with nicotine, but not after e-cigarettes without nicotine (reviewed in [ 20 ]). In women smokers, a study found a significant difference in stiffness after smoking just one tobacco cigarette, but not after use of e-cigarettes (reviewed in [ 20 ]).
It is well known that nicotine is extremely addictive and has a multitude of harmful effects. Nicotine has significant biologic activity and adversely affects several physiological systems including the cardiovascular, respiratory, immunological and reproductive systems, and can also compromise lung and kidney function [ 41 ]. Recently, a sub-chronic whole-body exposure of e-liquid (2 h/day, 5 days/week, 30 days) containing PG alone or PG with nicotine (25 mg/mL) to wild type (WT) animals or knockout (KO) mice in α7 nicotinic acetylcholine receptor (nAChRα7-KO) revealed a partly nAChRα7-dependent lung inflammation [ 42 ]. While sub-chronic exposure to PG/nicotine promote nAChRα7-dependent increased levels of different cytokines and chemokines in the bronchoalveolar lavage fluid (BALF) such as IL-1α, IL-2, IL-9, interferon γ (IFNγ), granulocyte-macrophage colony-stimulating factor (GM-CSF), monocyte chemoattractant protein-1 (MCP-1/CCL2) and regulated on activation, normal T cell expressed and secreted (RANTES/CCL5), the enhanced levels of IL-1β, IL-5 and TNFα were nAChRα7 independent. In general, most of the cytokines detected in BALF were significantly increased in WT mice exposed to PG with nicotine compared to PG alone or air control [ 42 ]. Some of these effects were found to be through nicotine activation of NF-κB signalling albeit in females but not in males. In addition, PG with nicotine caused increased macrophage and CD4 + /CD8 + T-lymphocytes cell counts in BALF compared to air control, but these effects were ameliorated when animals were sub-chronically exposed to PG alone [ 42 ].
Of note, another study indicated that although RANTES/CCL5 and CCR1 mRNA were upregulated in flavour/nicotine-containing e-cigarette users, vaping flavour and nicotine-less e-cigarettes did not significantly dysregulate cytokine and inflammasome activation [ 43 ].
In addition to its toxicological effects on foetus development, nicotine can disrupt brain development in adolescents and young adults [ 44 , 45 , 46 ]. Several studies have also suggested that nicotine is potentially carcinogenic (reviewed in [ 41 ]), but more work is needed to prove its carcinogenicity independently of the combustion products of tobacco [ 47 ]. In this latter regard, no differences were encountered in the frequency of tumour appearance in rats subjected to long-term (2 years) inhalation of nicotine when compared with control rats [ 48 ]. Despite the lack of carcinogenicity evidence, it has been reported that nicotine promotes tumour cell survival by decreasing apoptosis and increasing proliferation [ 49 ], indicating that it may work as a “tumour enhancer”. In a very recent study, chronic administration of nicotine to mice (1 mg/kg every 3 days for a 60-day period) enhanced brain metastasis by skewing the polarity of M2 microglia, which increases metastatic tumour growth [ 50 ]. Assuming that a conventional cigarette contains 0.172–1.702 mg of nicotine [ 51 ], the daily nicotine dose administered to these animals corresponds to 40–400 cigarettes for a 70 kg-adult, which is a dose of an extremely heavy smoker. We would argue that further studies with chronic administration of low doses of nicotine are required to clearly evaluate its impact on carcinogenicity.
In the aforementioned study exposing human gingival fibroblasts to CS condensate or to nicotine-rich or nicotine-free e-vapour condensates [ 29 ], the detrimental effects were greater in cells exposed to nicotine-rich condensate than to nicotine-free condensate, suggesting that the possible injurious effects of nicotine should be considered when purchasing e-refills . It is also noteworthy that among the 3 most cytotoxic vapours for HUVEC evaluated in the Putzhammer et al . study, 2 were nicotine-free, which suggests that nicotine is not the only hazardous component in e-cigarettes [ 24 ] .
The lethal dose of nicotine for an adult is estimated at 30–60 mg [ 52 ]. Given that nicotine easily diffuses from the dermis to the bloodstream, acute nicotine exposure by e-liquid spilling (5 mL of a 20 mg/mL nicotine-containing refill is equivalent to 100 mg of nicotine) can easily be toxic or even deadly [ 8 ]. Thus, devices with rechargeable refills are another issue of concern with e-cigarettes , especially when e-liquids are not sold in child-safe containers, increasing the risk of spilling, swallowing or breathing.
These data overall indicate that the harmful effects of nicotine should not be underestimated. Despite the established regulations, some inaccuracies in nicotine content labelling remain in different brands of e-liquids . Consequently, stricter regulation and a higher quality control in the e-liquid industry are required.
Effect of humectants and their heating-related products
In this particular aspect, again the composition of the e-liquid varies significantly among different commercial brands [ 4 , 35 ]. The most common and major components of e-liquids are PG or 1,2-propanediol, and glycerol or glycerine (propane-1,2,3-triol). Both types of compounds are used as humectants to prevent the e-liquid from drying out [ 2 , 53 ] and are classified by the Food and Drug Administration (FDA) as “Generally Recognised as Safe” [ 54 ]. In fact, they are widely used as alimentary and pharmaceutical products [ 2 ]. In an analysis of 54 commercially available e-liquids , PG and glycerol were detected in almost all samples at concentrations ranging from 0.4% to 98% (average 57%) and from 0.3% to 95% (average 37%), respectively [ 35 ].
With regards to toxicity, little is known about the effects of humectants when they are heated and chronically inhaled. Studies have indicated that PG can induce respiratory irritation and increase the probability of asthma development [ 55 , 56 ], and both PG and glycerol from e-cigarettes might reach concentrations sufficiently high to potentially cause irritation of the airways [ 57 ]. Indeed, the latter study established that one e-cigarette puff results in a PG exposure of 430–603 mg/m 3 , which is higher than the levels reported to cause airway irritation (average 309 mg/m 3 ) based on a human study [ 55 ]. The same study established that one e-cigarette puff results in a glycerol exposure of 348–495 mg/m 3 [ 57 ], which is close to the levels reported to cause airway irritation in rats (662 mg/m 3 ) [ 58 ].
Airway epithelial injury induced by acute vaping of PG and glycerol aerosols (50:50 vol/vol), with or without nicotine, has been reported in two randomised clinical trials in young tobacco smokers [ 32 ]. In vitro, aerosols from glycerol only-containing refills showed cytotoxicity in A549 and human embryonic stem cells, even at a low battery output voltage [ 59 ]. PG was also found to affect early neurodevelopment in a zebrafish model [ 60 ]. Another important issue is that, under heating conditions PG can produce acetaldehyde or formaldehyde (119.2 or 143.7 ng/puff at 20 W, respectively, on average), while glycerol can also generate acrolein (53.0, 1000.0 or 5.9 ng/puff at 20 W, respectively, on average), all carbonyls with a well-documented toxicity [ 61 ]. Although, assuming 15 puffs per e-cigarette unit, carbonyls produced by PG or glycerol heating would be below the maximum levels found in a conventional cigarette combustion (Table 2 ) [ 51 , 62 ]. Nevertheless, further studies are required to properly test the deleterious effects of all these compounds at physiological doses resembling those to which individuals are chronically exposed.
Although PG and glycerol are the major components of e-liquids other components have been detected. When the aerosols of 4 commercially available e-liquids chosen from a top 10 list of “ Best E-Cigarettes of 2014” , were analysed by gas chromatography-mass spectrometry (GC–MS) after heating, numerous compounds were detected, with nearly half of them not previously identified [ 4 ], thus suggesting that the heating process per se generates new compounds of unknown consequence. Of note, the analysis identified formaldehyde, acetaldehyde and acrolein [ 4 ], 3 carbonyl compounds with known high toxicity [ 63 , 64 , 65 , 66 , 67 ]. While no information was given regarding formaldehyde and acetaldehyde concentrations, the authors calculated that one puff could result in an acrolein exposure of 0.003–0.015 μg/mL [ 4 ]. Assuming 40 mL per puff and 15 puffs per e-cigarette unit (according to several manufacturers) [ 4 ], each e-cigarette unit would generate approximately 1.8–9 μg of acrolein, which is less than the levels of acrolein emitted by a conventional tobacco cigarette (18.3–98.2 μg) [ 51 ]. However, given that e-cigarette units of vaping are not well established, users may puff intermittently throughout the whole day. Thus, assuming 400 to 500 puffs per cartridge, users could be exposed to up to 300 μg of acrolein.
In a similar study, acrolein was found in 11 of 12 aerosols tested, with a similar content range (approximately 0.07–4.19 μg per e-cigarette unit) [ 68 ]. In the same study, both formaldehyde and acetaldehyde were detected in all of the aerosols tested, with contents of 0.2–5.61 μg and 0.11–1.36 μg, respectively, per e-cigarette unit [ 68 ]. It is important to point out that the levels of these toxic products in e-cigarette aerosols are significantly lower than those found in CS: 9 times lower for formaldehyde, 450 times lower for acetaldehyde and 15 times lower for acrolein (Table 2 ) [ 62 , 68 ].
Other compounds that have been detected in aerosols include acetamide, a potential human carcinogen [ 5 ], and some aldehydes [ 69 ], although their levels were minimal. Interestingly, the existence of harmful concentrations of diethylene glycol, a known cytotoxic agent, in e-liquid aerosols is contentious with some studies detecting its presence [ 4 , 68 , 70 , 71 , 72 ], and others finding low subtoxic concentrations [ 73 , 74 ]. Similar observations were reported for the content ethylene glycol. In this regard, either it was detected at concentrations that did not exceed the authorised limit [ 73 ], or it was absent from the aerosols produced [ 4 , 71 , 72 ]. Only one study revealed its presence at high concentration in a very low number of samples [ 5 ]. Nevertheless, its presence above 1 mg/g is not allowed by the FDA [ 73 ]. Figure 1 lists the main compounds detected in aerosols derived from humectant heating and their potential damaging effects. It would seem that future studies should analyse the possible toxic effects of humectants and related products at concentrations similar to those that e-cigarette vapers are exposed to reach conclusive results.
Impact of flavouring compounds
The range of e-liquid flavours available to consumers is extensive and is used to attract both current smokers and new e-cigarette users, which is a growing public health concern [ 6 ]. In fact, over 5 million middle- and high-school students were current users of e-cigarettes in 2019 [ 75 ], and appealing flavours have been identified as the primary reason for e-cigarette consumption in 81% of young users [ 76 ]. Since 2016, the FDA regulates the flavours used in the e-cigarette market and has recently published an enforcement policy on unauthorised flavours, including fruit and mint flavours, which are more appealing to young users [ 77 ]. However, the long-term effects of all flavour chemicals used by this industry (which are more than 15,000) remain unknown and they are not usually included in the product label [ 78 ]. Furthermore, there is no safety guarantee since they may harbour potential toxic or irritating properties [ 5 ].
With regards to the multitude of available flavours, some have demonstrated cytotoxicity [ 59 , 79 ]. Bahl et al. evaluated the toxicity of 36 different e-liquids and 29 different flavours on human embryonic stem cells, mouse neural stem cells and human pulmonary fibroblasts using a metabolic activity assay. In general, those e-liquids that were bubblegum-, butterscotch- and caramel-flavoured did not show any overt cytotoxicity even at the highest dose tested. By contrast, those e-liquids with Freedom Smoke Menthol Arctic and Global Smoke Caramel flavours had marked cytotoxic effects on pulmonary fibroblasts and those with Cinnamon Ceylon flavour were the most cytotoxic in all cell lines [ 79 ]. A further study from the same group [ 80 ] revealed that high cytotoxicity is a recurrent feature of cinnamon-flavoured e-liquids. In this line, results from GC–MS and HPLC analyses indicated that cinnamaldehyde (CAD) and 2-methoxycinnamaldehyde, but not dipropylene glycol or vanillin, were mainly responsible for the high cytotoxicity of cinnamon-flavoured e-liquids [ 80 ]. Other flavouring-related compounds that are associated with respiratory complications [ 81 , 82 , 83 ], such as diacetyl, 2,3-pentanedione or acetoin, were found in 47 out of 51 aerosols of flavoured e-liquids tested [ 84 ] . Allen et al . calculated an average of 239 μg of diacetyl per cartridge [ 84 ]. Assuming again 400 puffs per cartridge and 40 mL per puff, is it is possible to estimate an average of 0.015 ppm of diacetyl per puff, which could compromise normal lung function in the long-term [ 85 ].
The cytotoxic and pro-inflammatory effects of different e-cigarette flavouring chemicals were also tested on two human monocytic cell lines—mono mac 6 (MM6) and U937 [ 86 ]. Among the flavouring chemicals tested, CAD was found to be the most toxic and O-vanillin and pentanedione also showed significant cytotoxicity; by contrast, acetoin, diacetyl, maltol, and coumarin did not show any toxicity at the concentrations assayed (10–1000 µM). Of interest, a higher toxicity was evident when combinations of different flavours or mixed equal proportions of e-liquids from 10 differently flavoured e-liquids were tested, suggesting that vaping a single flavour is less toxic than inhaling mixed flavours [ 86 ]. Also, all the tested flavours produced significant levels of ROS in a cell-free ROS production assay. Finally, diacetyl, pentanedione, O-vanillin, maltol, coumarin, and CAD induced significant IL-8 secretion from MM6 and U937 monocytes [ 86 ]. It should be borne in mind, however, that the concentrations assayed were in the supra-physiological range and it is likely that, once inhaled, these concentrations are not reached in the airway space. Indeed, one of the limitations of the study was that human cells are not exposed to e-liquids per se, but rather to the aerosols where the concentrations are lower [ 86 ]. In this line, the maximum concentration tested (1000 µM) would correspond to approximately 80 to 150 ppm, which is far higher than the levels found in aerosols of some of these compounds [ 84 ]. Moreover, on a day-to-day basis, lungs of e-cigarette users are not constantly exposed to these chemicals for 24 h at these concentrations. Similar limitations were found when five of seven flavourings were found to cause cytotoxicity in human bronchial epithelial cells [ 87 ].
Recently, a commonly commercialized crème brûlée -flavoured aerosol was found to contain high concentrations of benzoic acid (86.9 μg/puff), a well-established respiratory irritant [ 88 ]. When human lung epithelial cells (BEAS-2B and H292) were exposed to this aerosol for 1 h, a marked cytotoxicity was observed in BEAS-2B but not in H292 cells, 24 h later. However, increased ROS production was registered in H292 cells [ 88 ].
Therefore, to fully understand the effects of these compounds, it is relevant the cell cultures selected for performing these assays, as well as the use of in vivo models that mimic the real-life situation of chronic e-cigarette vapers to clarify their impact on human health.
The e-cigarette device
While the bulk of studies related to the impact of e-cigarette use on human health has focused on the e-liquid components and the resulting aerosols produced after heating, a few studies have addressed the material of the electronic device and its potential consequences—specifically, the potential presence of metals such as copper, nickel or silver particles in e-liquids and aerosols originating from the filaments and wires and the atomiser [ 89 , 90 , 91 ].
Other important components in the aerosols include silicate particles from the fiberglass wicks or silicone [ 89 , 90 , 91 ]. Many of these products are known to cause abnormalities in respiratory function and respiratory diseases [ 89 , 90 , 91 ], but more in-depth studies are required. Interestingly, the battery output voltage also seems to have an impact on the cytotoxicity of the aerosol vapours, with e-liquids from a higher battery output voltage showing more toxicity to A549 cells [ 30 ].
A recent study compared the acute effects of e-cigarette vapor (with PG/vegetable glycerine plus tobacco flavouring but without nicotine) generated from stainless‐steel atomizer (SS) heating element or from a nickel‐chromium alloy (NC) [ 92 ]. Some rats received a single e-cigarette exposure for 2 h from a NC heating element (60 or 70 W); other rats received a similar exposure of e-cigarette vapor using a SS heating element for the same period of time (60 or 70 W) and, a final group of animals were exposed for 2 h to air. Neither the air‐exposed rats nor those exposed to e-cigarette vapor using SS heating elements developed respiratory distress. In contrast, 80% of the rats exposed to e-cigarette vapor using NC heating units developed clinical acute respiratory distress when a 70‐W power setting was employed. Thus, suggesting that operating units at higher than recommended settings can cause adverse effects. Nevertheless, there is no doubt that the deleterious effects of battery output voltage are not comparable to those exerted by CS extracts [ 30 ] (Figs. 1 and 2 ).
E-cigarettes as a smoking cessation tool
CS contains a large number of substances—about 7000 different constituents in total, with sizes ranging from atoms to particulate matter, and with many hundreds likely responsible for the harmful effects of this habit [ 93 ]. Given that tobacco is being substituted in great part by e-cigarettes with different chemical compositions, manufacturers claim that e -cigarette will not cause lung diseases such as lung cancer, chronic obstructive pulmonary disease, or cardiovascular disorders often associated with conventional cigarette consumption [ 3 , 94 ]. However, the World Health Organisation suggests that e-cigarettes cannot be considered as a viable method to quit smoking, due to a lack of evidence [ 7 , 95 ]. Indeed, the results of studies addressing the use of e-cigarettes as a smoking cessation tool remain controversial [ 96 , 97 , 98 , 99 , 100 ]. Moreover, both FDA and CDC are actively investigating the incidence of severe respiratory symptoms associated with the use of vaping products [ 77 ]. Because many e-liquids contain nicotine, which is well known for its powerful addictive properties [ 41 ], e-cigarette users can easily switch to conventional cigarette smoking, avoiding smoking cessation. Nevertheless, the possibility of vaping nicotine-free e-cigarettes has led to the branding of these devices as smoking cessation tools [ 2 , 6 , 7 ].
In a recently published randomised trial of 886 subjects who were willing to quit smoking [ 100 ], the abstinence rate was found to be twice as high in the e-cigarette group than in the nicotine-replacement group (18.0% vs. 9.9%) after 1 year. Of note, the abstinence rate found in the nicotine-replacement group was lower than what is usually expected with this therapy. Nevertheless, the incidence of throat and mouth irritation was higher in the e-cigarette group than in the nicotine-replacement group (65.3% vs. 51.2%, respectively). Also, the participant adherence to the treatment after 1-year abstinence was significantly higher in the e-cigarette group (80%) than in nicotine-replacement products group (9%) [ 100 ].
On the other hand, it is estimated that COPD could become the third leading cause of death in 2030 [ 101 ]. Given that COPD is generally associated with smoking habits (approximately 15 to 20% of smokers develop COPD) [ 101 ], smoking cessation is imperative among COPD smokers. Published data revealed a clear reduction of conventional cigarette consumption in COPD smokers that switched to e-cigarettes [ 101 ]. Indeed, a significant reduction in exacerbations was observed and, consequently, the ability to perform physical activities was improved when data was compared with those non-vapers COPD smokers. Nevertheless, a longer follow-up of these COPD patients is required to find out whether they have quitted conventional smoking or even vaping, since the final goal under these circumstances is to quit both habits.
Based on the current literature, it seems that several factors have led to the success of e-cigarette use as a smoking cessation tool. First, some e-cigarette flavours positively affect smoking cessation outcomes among smokers [ 102 ]. Second, e-cigarettes have been described to improve smoking cessation rate only among highly-dependent smokers and not among conventional smokers, suggesting that the individual degree of nicotine dependence plays an important role in this process [ 97 ]. Third, the general belief of their relative harmfulness to consumers' health compared with conventional combustible tobacco [ 103 ]. And finally, the exposure to point-of-sale marketing of e-cigarette has also been identified to affect the smoking cessation success [ 96 ].
Implication of e-cigarette consumption in COVID-19 time
Different reports have pointed out that smokers and vapers are more vulnerable to SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) infections or more prone to adverse outcomes if they suffer COVID-19 [ 104 ]. However, while a systematic review indicated that cigarette smoking is probably associated with enhanced damage from COVID-19, a meta-analysis did not, yet the latter had several limitations due to the small sample sizes [ 105 ].
Interestingly, most of these reports linking COVID-19 harmful effects with smoking or vaping, are based on their capability of increasing the expression of angiotensin-converting enzyme 2 (ACE2) in the lung. It is well known that ACE2 is the gate for SARS-CoV-2 entrance to the airways [ 106 ] and it is mainly expressed in type 2 alveolar epithelial cells and alveolar macrophages [ 107 ]. To date, most of the studies in this field indicate that current smokers have higher expression of ACE2 in the airways (reviewed by [ 108 ]) than healthy non-smokers [ 109 , 110 ]. However, while a recent report indicated that e-cigarette vaping also caused nicotine-dependent ACE2 up-regulation [ 42 ], others have revealed that neither acute inhalation of e-cigarette vapour nor e-cigarette users had increased lung ACE2 expression regardless nicotine presence in the e-liquid [ 43 , 110 ].
In regard to these contentions, current knowledge suggests that increased ACE2 expression is not necessarily linked to enhanced susceptibility to SARS-CoV-2 infection and adverse outcome. Indeed, elderly population express lower levels of ACE2 than young people and SARS-CoV-2/ACE2 interaction further decreases ACE2 expression. In fact, most of the deaths provoked by COVID-19 took place in people over 60 years old of age [ 111 ]. Therefore, it is plausible that the increased susceptibility to disease progression and the subsequent fatal outcome in this population is related to poor angiotensin 1-7 (Ang-1-7) generation, the main peptide generated by ACE2, and probably to their inaccessibility to its anti-inflammatory effects. Furthermore, it seems that all the efforts towards increasing ACE2 expression may result in a better resolution of the pneumonic process associated to this pandemic disease.
Nevertheless, additional complications associated to COVID-19 are increased thrombotic events and cytokine storm. In the lungs, e-cigarette consumption has been correlated to toxicity, oxidative stress, and inflammatory response [ 32 , 112 ]. More recently, a study revealed that while the use of nicotine/flavour-containing e-cigarettes led to significant cytokine dysregulation and potential inflammasome activation, none of these effects were detected in non-flavoured and non-nicotine-containing e-cigarettes [ 43 ]. Therefore, taken together these observations, e-cigarette use may still be a potent risk factor for severe COVID-19 development depending on the flavour and nicotine content.
In summary, it seems that either smoking or nicotine vaping may adversely impact on COVID-19 outcome. However, additional follow up studies are required in COVID-19 pandemic to clarify the effect of e-cigarette use on lung and cardiovascular complications derived from SARS-CoV-2 infection.
Conclusions
The harmful effects of CS and their deleterious consequences are both well recognised and widely investigated. However, and based on the studies carried out so far, it seems that e-cigarette consumption is less toxic than tobacco smoking. This does not necessarily mean, however, that e-cigarettes are free from hazardous effects. Indeed, studies investigating their long-term effects on human health are urgently required. In this regard, the main additional studies needed in this field are summarized in Table 3 .
The composition of e-liquids requires stricter regulation, as they can be easily bought online and many incidences of mislabelling have been detected, which can seriously affect consumers’ health. Beyond their unknown long-term effects on human health, the extended list of appealing flavours available seems to attract new “never-smokers”, which is especially worrying among young users. Additionally, there is still a lack of evidence of e-cigarette consumption as a smoking cessation method. Indeed, e-cigarettes containing nicotine may relieve the craving for smoking, but not the conventional cigarette smoking habit.
Interestingly, there is a strong difference of opinion on e-cigarettes between countries. Whereas countries such as Brazil, Uruguay and India have banned the sale of e-cigarettes , others such as the United Kingdom support this device to quit smoking. The increasing number of adolescent users and reported deaths in the United States prompted the government to ban the sale of flavoured e-cigarettes in 2020. The difference in opinion worldwide may be due to different restrictions imposed. For example, while no more than 20 ng/mL of nicotine is allowed in the EU, e-liquids with 59 mg/dL are currently available in the United States. Nevertheless, despite the national restrictions, users can easily access foreign or even counterfeit products online.
In regard to COVID-19 pandemic, the actual literature suggests that nicotine vaping may display adverse outcomes. Therefore, follow up studies are necessary to clarify the impact of e-cigarette consumption on human health in SARS-CoV-2 infection.
In conclusion, e-cigarettes could be a good alternative to conventional tobacco cigarettes, with less side effects; however, a stricter sale control, a proper regulation of the industry including flavour restriction, as well as further toxicological studies, including their chronic effects, are warranted.
Availability of data and materials
Not applicable.
Abbreviations
Angiotensin-converting enzyme 2
Angiotensin 1-7
Bronchoalveolar lavage fluid
Cinnamaldehyde
US Centers for Disease Control and Prevention
Carbon monoxide
Chronic obstructive pulmonary disease
Coronavirus disease 2019
Cigarette smoke
Electronic nicotine dispensing systems
e-cigarette or vaping product use-associated lung injury
Food and Drug Administration
Gas chromatography with a flame ionisation detector
Gas chromatography-mass spectrometry
Granulocyte–macrophage colony-stimulating factor
High performance liquid chromatography
Human umbilical vein endothelial cells
Interleukin
Interferon γ
Liquid chromatography-mass spectrometry
Monocyte chemoattractant protein-1
Matrix metallopeptidase 9
α7 Nicotinic acetylcholine receptor
Nickel‐chromium alloy
Nitric oxide
Propylene glycol
Regulated on activation, normal T cell expressed and secreted
Reactive oxygen species
Severe acute respiratory syndrome coronavirus 2
Stainless‐steel atomizer
Tetrahydrocannabinol
Tumour necrosis factor-α
Hiemstra PS, Bals R. Basic science of electronic cigarettes: assessment in cell culture and in vivo models. Respir Res. 2016;17(1):127.
Article PubMed PubMed Central CAS Google Scholar
Bertholon JF, Becquemin MH, Annesi-Maesano I, Dautzenberg B. Electronic cigarettes: a short review. Respiration. 2013;86(5):433–8.
Article CAS PubMed Google Scholar
Rowell TR, Tarran R. Will chronic e-cigarette use cause lung disease? Am J Physiol Lung Cell Mol Physiol. 2015;309(12):L1398–409.
Article CAS PubMed PubMed Central Google Scholar
Herrington JS, Myers C. Electronic cigarette solutions and resultant aerosol profiles. J Chromatogr A. 2015;1418:192–9.
Hutzler C, Paschke M, Kruschinski S, Henkler F, Hahn J, Luch A. Chemical hazards present in liquids and vapors of electronic cigarettes. Arch Toxicol. 2014;88(7):1295–308.
Pokhrel P, Herzog TA, Muranaka N, Fagan P. Young adult e-cigarette users’ reasons for liking and not liking e-cigarettes: a qualitative study. Psychol Health. 2015;30(12):1450–69.
Article PubMed PubMed Central Google Scholar
Harrell PT, Simmons VN, Correa JB, Padhya TA, Brandon TH. Electronic nicotine delivery systems (“e-cigarettes”): review of safety and smoking cessation efficacy. Otolaryngol Head Neck Surg. 2014;151(3):381–93.
Schober W, Szendrei K, Matzen W, Osiander-Fuchs H, Heitmann D, Schettgen T, et al. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health. 2014;217(6):628–37.
Rubinstein ML, Delucchi K, Benowitz NL, Ramo DE. Adolescent exposure to toxic volatile organic chemicals from E-cigarettes. Pediatrics. 2018;141(4):e20173557.
Article PubMed Google Scholar
Staudt MR, Salit J, Kaner RJ, Hollmann C, Crystal RG. Altered lung biology of healthy never smokers following acute inhalation of E-cigarettes. Respir Res. 2018;19(1):78.
Nocella C, Biondi-Zoccai G, Sciarretta S, Peruzzi M, Pagano F, Loffredo L, et al. Impact of tobacco versus electronic cigarette smoking on platelet function. Am J Cardiol. 2018;122(9):1477–81.
Higham A, Rattray NJW, Dewhurst JA, Trivedi DK, Fowler SJ, Goodacre R, et al. Electronic cigarette exposure triggers neutrophil inflammatory responses. Respir Res. 2016;17(1):56.
Antoniewicz L, Bosson JA, Kuhl J, Abdel-Halim SM, Kiessling A, Mobarrez F, et al. Electronic cigarettes increase endothelial progenitor cells in the blood of healthy volunteers. Atherosclerosis. 2016;255:179–85.
Carnevale R, Sciarretta S, Violi F, Nocella C, Loffredo L, Perri L, et al. Acute impact of tobacco vs electronic cigarette smoking on oxidative stress and vascular function. Chest. 2016;150(3):606–12.
Vlachopoulos C, Ioakeimidis N, Abdelrasoul M, Terentes-Printzios D, Georgakopoulos C, Pietri P, et al. Electronic cigarette smoking increases aortic stiffness and blood pressure in young smokers. J Am Coll Cardiol. 2016;67(23):2802–3.
Franzen KF, Willig J, Cayo Talavera S, Meusel M, Sayk F, Reppel M, et al. E-cigarettes and cigarettes worsen peripheral and central hemodynamics as well as arterial stiffness: a randomized, double-blinded pilot study. Vasc Med. 2018;23(5):419–25.
Caporale A, Langham MC, Guo W, Johncola A, Chatterjee S, Wehrli FW. Acute effects of electronic cigarette aerosol inhalation on vascular function detected at quantitative MRI. Radiology. 2019;293(1):97–106.
von Hundelshausen P, Schmitt MM. Platelets and their chemokines in atherosclerosis-clinical applications. Front Physiol. 2014;5:294.
Google Scholar
Landmesser U, Hornig B, Drexler H. Endothelial function: a critical determinant in atherosclerosis? Circulation. 2004;109(21 Suppl 1):Ii27-33.
PubMed Google Scholar
Münzel T, Hahad O, Kuntic M, Keaney JF, Deanfield JE, Daiber A. Effects of tobacco cigarettes, e-cigarettes, and waterpipe smoking on endothelial function and clinical outcomes. Eur Heart J. 2020;41:4057–70.
Javelle E. Electronic cigarette and vaping should be discouraged during the new coronavirus SARS-CoV-2 pandemic. Arch Toxicol. 2020;94(6):2261–2.
Husari A, Shihadeh A, Talih S, Hashem Y, El Sabban M, Zaatari G. Acute exposure to electronic and combustible cigarette aerosols: effects in an animal model and in human alveolar cells. Nicotine Tob Res. 2016;18(5):613–9.
Zelikoff JT, Parmalee NL, Corbett K, Gordon T, Klein CB, Aschner M. Microglia activation and gene expression alteration of neurotrophins in the hippocampus following early-life exposure to E-cigarette aerosols in a murine model. Toxicol Sci. 2018;162(1):276–86.
Putzhammer R, Doppler C, Jakschitz T, Heinz K, Forste J, Danzl K, et al. Vapours of US and EU market leader electronic cigarette brands and liquids are cytotoxic for human vascular endothelial cells. PLoS One. 2016;11(6):e0157337.
Bernhard D, Pfister G, Huck CW, Kind M, Salvenmoser W, Bonn GK, et al. Disruption of vascular endothelial homeostasis by tobacco smoke: impact on atherosclerosis. Faseb J. 2003;17(15):2302–4.
Newby DE, Wright RA, Labinjoh C, Ludlam CA, Fox KA, Boon NA, et al. Endothelial dysfunction, impaired endogenous fibrinolysis, and cigarette smoking: a mechanism for arterial thrombosis and myocardial infarction. Circulation. 1999;99(11):1411–5.
Taylor M, Jaunky T, Hewitt K, Breheny D, Lowe F, Fearon IM, et al. A comparative assessment of e-cigarette aerosols and cigarette smoke on in vitro endothelial cell migration. Toxicol Lett. 2017;277:123–8.
Herr C, Tsitouras K, Niederstraßer J, Backes C, Beisswenger C, Dong L, et al. Cigarette smoke and electronic cigarettes differentially activate bronchial epithelial cells. Respir Res. 2020;21(1):67.
Alanazi H, Park HJ, Chakir J, Semlali A, Rouabhia M. Comparative study of the effects of cigarette smoke and electronic cigarettes on human gingival fibroblast proliferation, migration and apoptosis. Food Chem Toxicol. 2018;118:390–8.
Otreba M, Kosmider L. E-cigarettes: voltage- and concentration-dependent loss in human lung adenocarcinoma viability. J Appl Toxicol. 2018;38(8):1135–43.
Chaumont M, Bernard A, Pochet S, Melot C, El Khattabi C, Reye F, et al. High-wattage E-cigarettes induce tissue hypoxia and lower airway injury: a randomized clinical trial. Am J Respir Crit Care Med. 2018;198(1):123–6.
Chaumont M, van de Borne P, Bernard A, Van Muylem A, Deprez G, Ullmo J, et al. Fourth generation e-cigarette vaping induces transient lung inflammation and gas exchange disturbances: results from two randomized clinical trials. Am J Physiol Lung Cell Mol Physiol. 2019;316(5):L705–19.
European Parliament and the council of the European Union. Directive 2014/40/EU. 2014 (updated April 29, 2014). https://ec.europa.eu/health//sites/health/files/tobacco/docs/dir_201440_en.pdf . Accessed 17 April 2020.
Cameron JM, Howell DN, White JR, Andrenyak DM, Layton ME, Roll JM. Variable and potentially fatal amounts of nicotine in e-cigarette nicotine solutions. Tob Control. 2014;23(1):77–8.
Hahn J, Monakhova YB, Hengen J, Kohl-Himmelseher M, Schussler J, Hahn H, et al. Electronic cigarettes: overview of chemical composition and exposure estimation. Tob Induc Dis. 2014;12(1):23.
Omaiye EE, Cordova I, Davis B, Talbot P. Counterfeit electronic cigarette products with mislabeled nicotine concentrations. Tob Regul Sci. 2017;3(3):347–57.
Buettner-Schmidt K, Miller DR, Balasubramanian N. Electronic cigarette refill liquids: child-resistant packaging, nicotine content, and sales to minors. J Pediatr Nurs. 2016;31(4):373–9.
Jackson R, Huskey M, Brown S. Labelling accuracy in low nicotine e-cigarette liquids from a sampling of US manufacturers. Int J Pharm Pract. 2019;28(3):290–4.
Yingst JM, Foulds J, Veldheer S, Hrabovsky S, Trushin N, Eissenberg TT, et al. Nicotine absorption during electronic cigarette use among regular users. PLoS One. 2019;14(7):e0220300.
Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: implications for research protocol standards definition and for public health authorities’ regulation. Int J Environ Res Public Health. 2013;10(6):2500–14.
Mishra A, Chaturvedi P, Datta S, Sinukumar S, Joshi P, Garg A. Harmful effects of nicotine. Indian J Med Paediatr Oncol. 2015;36(1):24–31.
Wang Q, Sundar IK, Li D, Lucas JH, Muthumalage T, McDonough SR, et al. E-cigarette-induced pulmonary inflammation and dysregulated repair are mediated by nAChR α7 receptor: role of nAChR α7 in SARS-CoV-2 Covid-19 ACE2 receptor regulation. Respir Res. 2020;21(1):154.
Lee AC, Chakladar J, Li WT, Chen C, Chang EY, Wang-Rodriguez J, et al. Tobacco, but not nicotine and flavor-less electronic cigarettes, induces ACE2 and immune dysregulation. Int J Mol Sci. 2020;21(15):5513.
Article CAS PubMed Central Google Scholar
England LJ, Bunnell RE, Pechacek TF, Tong VT, McAfee TA. Nicotine and the developing human: a neglected element in the electronic cigarette debate. Am J Prev Med. 2015;49(2):286–93.
Yuan M, Cross SJ, Loughlin SE, Leslie FM. Nicotine and the adolescent brain. J Physiol. 2015;593(16):3397–412.
Holbrook BD. The effects of nicotine on human fetal development. Birth Defects Res C Embryo Today. 2016;108(2):181–92.
Sanner T, Grimsrud TK. Nicotine: carcinogenicity and effects on response to cancer treatment—a review. Front Oncol. 2015;5:196.
Waldum HL, Nilsen OG, Nilsen T, Rørvik H, Syversen V, Sanvik AK, et al. Long-term effects of inhaled nicotine. Life Sci. 1996;58(16):1339–46.
Cucina A, Dinicola S, Coluccia P, Proietti S, D’Anselmi F, Pasqualato A, et al. Nicotine stimulates proliferation and inhibits apoptosis in colon cancer cell lines through activation of survival pathways. J Surg Res. 2012;178(1):233–41.
Wu SY, Xing F, Sharma S, Wu K, Tyagi A, Liu Y, et al. Nicotine promotes brain metastasis by polarizing microglia and suppressing innate immune function. J Exp Med. 2020;217(8):e20191131.
Roemer E, Stabbert R, Rustemeier K, Veltel DJ, Meisgen TJ, Reininghaus W, et al. Chemical composition, cytotoxicity and mutagenicity of smoke from US commercial and reference cigarettes smoked under two sets of machine smoking conditions. Toxicology. 2004;195(1):31–52.
Mayer B. How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century. Arch Toxicol. 2014;88(1):5–7.
Brown CJ, Cheng JM. Electronic cigarettes: product characterisation and design considerations. Tob Control. 2014;23(Suppl 2):ii4-10.
Food and Drug Administration. SCOGS (Select Committee on GRAS Substances). 2019 (updated April 29, 2019). https://www.accessdata.fda.gov/scripts/fdcc/index.cfm?set=SCOGS&sort=Sortsubstance&order=ASC&startrow=251&type=basic&search= . Accessed 14 April 2020.
Wieslander G, Norback D, Lindgren T. Experimental exposure to propylene glycol mist in aviation emergency training: acute ocular and respiratory effects. Occup Environ Med. 2001;58(10):649–55.
Choi H, Schmidbauer N, Sundell J, Hasselgren M, Spengler J, Bornehag CG. Common household chemicals and the allergy risks in pre-school age children. PLoS One. 2010;5(10):e13423.
Kienhuis AS, Soeteman-Hernandez LG, Bos PMJ, Cremers HWJM, Klerx WN, Talhout R. Potential harmful health effects of inhaling nicotine-free shisha-pen vapor: a chemical risk assessment of the main components propylene glycol and glycerol. Tob Induc Dis. 2015;13(1):15.
Renne RA, Wehner AP, Greenspan BJ, Deford HS, Ragan HA, Westerberg RB, et al. 2-Week and 13-week inhalation studies of aerosolized glycerol in rats. Inhal Toxicol. 1992;4(2):95–111.
Article CAS Google Scholar
Behar R, Wang Y, Talbot P. Comparing the cytotoxicity of electronic cigarette fluids, aerosols and solvents. Tob Control. 2018;27(3):325.
Massarsky A, Abdel A, Glazer L, Levin ED, Di Giulio RT. Neurobehavioral effects of 1,2-propanediol in zebrafish (Danio rerio). Neurotoxicology. 2018;65:111–24.
Geiss O, Bianchi I, Barrero-Moreno J. Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours. Int J Hyg Environ Health. 2016;219(3):268–77.
Counts ME, Morton MJ, Laffoon SW, Cox RH, Lipowicz PJ. Smoke composition and predicting relationships for international commercial cigarettes smoked with three machine-smoking conditions. Regul Toxicol Pharmacol. 2005;41(3):185–227.
Agency for Toxic Substances & Disease Registry. Toxicological Profile for Formaldehyde. 2019 (updated September 26, 2019). https://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=220&tid=39 . Accessed 9 April 2020.
Agency for Toxic Substances & Disease Registry. Toxicological Profile for Acrolein. 2019 (updated September 26, 2019). https://www.atsdr.cdc.gov/toxprofiles/TP.asp?id=557&tid=102 . Accessed 9 April 2020
Moghe A, Ghare S, Lamoreau B, Mohammad M, Barve S, McClain C, et al. Molecular mechanisms of acrolein toxicity: relevance to human disease. Toxicol Sci. 2015;143(2):242–55.
Seitz HK, Stickel F. Acetaldehyde as an underestimated risk factor for cancer development: role of genetics in ethanol metabolism. Genes Nutr. 2010;5(2):121–8.
Faroon O, Roney N, Taylor J, Ashizawa A, Lumpkin MH, Plewak DJ. Acrolein health effects. Toxicol Ind Health. 2008;24(7):447–90.
Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23(2):133–9.
Farsalinos KE, Voudris V. Do flavouring compounds contribute to aldehyde emissions in e-cigarettes? Food Chem Toxicol. 2018;115:212–7.
Kavvalakis MP, Stivaktakis PD, Tzatzarakis MN, Kouretas D, Liesivuori J, Alegakis AK, et al. Multicomponent analysis of replacement liquids of electronic cigarettes using chromatographic techniques. J Anal Toxicol. 2015;39(4):262–9.
Etter JF, Zather E, Svensson S. Analysis of refill liquids for electronic cigarettes. Addiction. 2013;108(9):1671–9.
Etter JF, Bugey A. E-cigarette liquids: constancy of content across batches and accuracy of labeling. Addict Behav. 2017;73:137–43.
Varlet V, Farsalinos K, Augsburger M, Thomas A, Etter JF. Toxicity assessment of refill liquids for electronic cigarettes. Int J Environ Res Public Health. 2015;12(5):4796–815.
McAuley TR, Hopke PK, Zhao J, Babaian S. Comparison of the effects of e-cigarette vapor and cigarette smoke on indoor air quality. Inhal Toxicol. 2012;24(12):850–7.
Cullen KA, Gentzke AS, Sawdey MD, Chang JT, Anic GM, Wang TW, et al. e-Cigarette use among youth in the United States, 2019. JAMA. 2019;322(21):2095–103.
Villanti AC, Johnson AL, Ambrose BK, Cummings KM, Stanton CA, Rose SW, et al. Flavored tobacco product use in youth and adults: findings from the first wave of the PATH Study (2013–2014). Am J Prev Med. 2017;53(2):139–51.
Food and Drug Administration. Vaporizers, E-Cigarettes, and other Electronic Nicotine Delivery Systems (ENDS) 2020 (updated April 13, 2020). https://www.fda.gov/tobacco-products/products-ingredients-components/vaporizers-e-cigarettes-and-other-electronic-nicotine-delivery-systems-ends . Accessed 15 April 2020
Omaiye EE, McWhirter KJ, Luo W, Tierney PA, Pankow JF, Talbot P. High concentrations of flavor chemicals are present in electronic cigarette refill fluids. Sci Rep. 2019;9(1):2468.
Bahl V, Lin S, Xu N, Davis B, Wang YH, Talbot P. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod Toxicol. 2012;34(4):529–37.
Behar R, Davis B, Wang Y, Bahl V, Lin S, Talbot P. Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicol In Vitro. 2014;28(2):198–208.
Morgan DL, Flake GP, Kirby PJ, Palmer SM. Respiratory toxicity of diacetyl in C57BL/6 mice. Toxicol Sci. 2008;103(1):169–80.
Hubbs AF, Cumpston AM, Goldsmith WT, Battelli LA, Kashon ML, Jackson MC, et al. Respiratory and olfactory cytotoxicity of inhaled 2,3-pentanedione in Sprague-Dawley rats. Am J Pathol. 2012;181(3):829–44.
Vas CA, Porter A, Mcadam K. Acetoin is a precursor to diacetyl in e-cigarette liquids. Food Chem Toxicol. 2019;133:110727.
Allen JG, Flanigan SS, LeBlanc M, Vallarino J, MacNaughton P, Stewart JH, et al. Flavoring chemicals in E-cigarettes: diacetyl, 2,3-pentanedione, and acetoin in a sample of 51 products, including fruit-, candy-, and cocktail-flavored E-cigarettes. Environ Health Perspect. 2016;124(6):733–9.
Park RM, Gilbert SJ. Pulmonary impairment and risk assessment in a diacetyl-exposed population: microwave popcorn workers. J Occup Environ Med. 2018;60(6):496–506.
Muthumalage T, Prinz M, Ansah KO, Gerloff J, Sundar IK, Rahman I. Inflammatory and oxidative responses induced by exposure to commonly used e-cigarette flavoring chemicals and flavored e-liquids without nicotine. Front Physiol. 2017;8:1130.
Sherwood CL, Boitano S. Airway epithelial cell exposure to distinct e-cigarette liquid flavorings reveals toxicity thresholds and activation of CFTR by the chocolate flavoring 2,5-dimethypyrazine. Respir Res. 2016;17(1):57.
Pinkston R, Zaman H, Hossain E, Penn AL, Noël A. Cell-specific toxicity of short-term JUUL aerosol exposure to human bronchial epithelial cells and murine macrophages exposed at the air–liquid interface. Respir Res. 2020;21(1):269.
Williams M, Villarreal A, Bozhilov K, Lin S, Talbot P. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS One. 2013;8(3):e57987.
Mikheev VB, Brinkman MC, Granville CA, Gordon SM, Clark PI. Real-time measurement of electronic cigarette aerosol size distribution and metals content analysis. Nicotine Tob Res. 2016;18(9):1895–902.
Williams M, Bozhilov K, Ghai S, Talbot P. Elements including metals in the atomizer and aerosol of disposable electronic cigarettes and electronic hookahs. PLoS One. 2017;12(4):e0175430.
Kleinman MT, Arechavala RJ, Herman D, Shi J, Hasen I, Ting A, et al. E-cigarette or vaping product use-associated lung injury produced in an animal model from electronic cigarette vapor exposure without tetrahydrocannabinol or vitamin E oil. J Am Heart Assoc. 2020;9(18):e017368.
Patnode CD, Henderson JT, Thompson JH, Senger CA, Fortmann SP, Whitlock EP. Behavioral counseling and pharmacotherapy interventions for tobacco cessation in adults, including pregnant women: a review of reviews for the U.S. preventive services task force. Ann Intern Med. 2015;163(8):608–21.
Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol. 2014;34(3):509–15.
Bansal V, Kim K-H. Review on quantitation methods for hazardous pollutants released by e-cigarette (EC) smoking. Trends Analyt Chem. 2016;78:120–33.
Mantey DS, Pasch KE, Loukas A, Perry CL. Exposure to point-of-sale marketing of cigarettes and E-cigarettes as predictors of smoking cessation behaviors. Nicotine Tob Res. 2019;21(2):212–9.
Selya AS, Dierker L, Rose JS, Hedeker D, Mermelstein RJ. The role of nicotine dependence in E-cigarettes’ potential for smoking reduction. Nicotine Tob Res. 2018;20(10):1272–7.
Kalkhoran S, Glantz SA. E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis. Lancet Respir Med. 2016;4(2):116–28.
Levy DT, Yuan Z, Luo Y, Abrams DB. The relationship of e-cigarette use to cigarette quit attempts and cessation: insights from a large, nationally representative U.S. survey. Nicotine Tob Res. 2017;20(8):931–9.
Article PubMed Central Google Scholar
Hajek P, Phillips-Waller A, Przulj D, Pesola F, Myers Smith K, Bisal N, et al. A randomized trial of E-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019;380(7):629–37.
Polosa R, Morjaria JB, Caponnetto P, Prosperini U, Russo C, Pennisi A, et al. Evidence for harm reduction in COPD smokers who switch to electronic cigarettes. Respir Res. 2016;17(1):166.
Litt MD, Duffy V, Oncken C. Cigarette smoking and electronic cigarette vaping patterns as a function of e-cigarette flavourings. Tob Control. 2016;25(Suppl 2):ii67–72.
Palmer AM, Brandon TH. How do electronic cigarettes affect cravings to smoke or vape? Parsing the influences of nicotine and expectancies using the balanced-placebo design. J Consult Clin Psychol. 2018;86(5):486–91.
Majmundar A, Allem JP, Cruz TB, Unger JB. Public health concerns and unsubstantiated claims at the intersection of vaping and COVID-19. Nicotine Tob Res. 2020;22(9):1667–8.
Berlin I, Thomas D, Le Faou A-L, Cornuz J. COVID-19 and smoking. Nicotine Tob Res. 2020;22(9):1650–2.
Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh C-L, Abiona O, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–3.
Wang K, Gheblawi M, Oudit GY. Angiotensin Converting Enzyme 2: A Double-Edged Sword. Circulation. 2020;142(5):426–8.
Sharma P, Zeki AA. Does vaping increase susceptibility to COVID-19? Am J Respir Crit Care Med. 2020;202(7):1055–6.
Brake SJ, Barnsley K, Lu W, McAlinden KD, Eapen MS, Sohal SS. Smoking upregulates angiotensin-converting enzyme-2 receptor: a potential adhesion site for novel coronavirus SARS-CoV-2 (Covid-19). J Clin Med. 2020;9(3):841.
Zhang H, Rostamim MR, Leopold PL, Mezey JG, O’Beirne SL, Strulovici-Barel Y, et al. Reply to sharma and zeki: does vaping increase susceptibility to COVID-19? Am J Respir Crit Care Med. 2020;202(7):1056–7.
Cheng H, Wang Y, Wang GQ. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med Virol. 2020;92(7):726–30.
Lerner CA, Sundar IK, Yao H, Gerloff J, Ossip DJ, McIntosh S, et al. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS ONE. 2015;10(2):e0116732.
Download references
Acknowledgements
The authors gratefully acknowledge Dr. Cruz González, Pulmonologist at University Clinic Hospital of Valencia (Valencia, Spain) for her thoughtful suggestions and support.
This work was supported by the Spanish Ministry of Science and Innovation [Grant Number SAF2017-89714-R]; Carlos III Health Institute [Grant Numbers PIE15/00013, PI18/00209]; Generalitat Valenciana [Grant Number PROMETEO/2019/032, Gent T CDEI-04/20-A and AICO/2019/250], and the European Regional Development Fund.
Author information
Authors and affiliations.
Department of Pharmacology, Faculty of Medicine, University of Valencia, Avda. Blasco Ibañez 15, 46010, Valencia, Spain
Patrice Marques, Laura Piqueras & Maria-Jesus Sanz
Institute of Health Research INCLIVA, University Clinic Hospital of Valencia, Valencia, Spain
CIBERDEM-Spanish Biomedical Research Centre in Diabetes and Associated Metabolic Disorders, ISCIII, Av. Monforte de Lemos 3-5, 28029, Madrid, Spain
Laura Piqueras & Maria-Jesus Sanz
You can also search for this author in PubMed Google Scholar
Contributions
All authors discussed and agreed to the scope of the manuscript and contributed to the development of the manuscript at all stages. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Maria-Jesus Sanz .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors of the manuscript declare no conflicts of interest and take sole responsibility for the writing and content of the manuscript. None of the authors have been involved in legal or regulatory matters related to the contents of this paper.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Marques, P., Piqueras, L. & Sanz, MJ. An updated overview of e-cigarette impact on human health. Respir Res 22 , 151 (2021). https://doi.org/10.1186/s12931-021-01737-5
Download citation
Received : 22 October 2020
Accepted : 03 May 2021
Published : 18 May 2021
DOI : https://doi.org/10.1186/s12931-021-01737-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Electronic cigarette
- E-cigarette
- Flavourings
- Smoking cessation tool
Respiratory Research
ISSN: 1465-993X
- General enquiries: [email protected]

Create Free Account or
- Acute Coronary Syndromes
- Anticoagulation Management
- Arrhythmias and Clinical EP
- Cardiac Surgery
- Cardio-Oncology
- Cardiovascular Care Team
- Congenital Heart Disease and Pediatric Cardiology
- COVID-19 Hub
- Diabetes and Cardiometabolic Disease
- Dyslipidemia
- Geriatric Cardiology
- Heart Failure and Cardiomyopathies
- Invasive Cardiovascular Angiography and Intervention
- Noninvasive Imaging
- Pericardial Disease
- Pulmonary Hypertension and Venous Thromboembolism
- Sports and Exercise Cardiology
- Stable Ischemic Heart Disease
- Valvular Heart Disease
- Vascular Medicine
- Clinical Updates & Discoveries
- Advocacy & Policy
- Perspectives & Analysis
- Meeting Coverage
- ACC Member Publications
- ACC Podcasts
- View All Cardiology Updates
- Earn Credit
- View the Education Catalog
- ACC Anywhere: The Cardiology Video Library
- CardioSource Plus for Institutions and Practices
- ECG Drill and Practice
- Heart Songs
- Nuclear Cardiology
- Online Courses
- Collaborative Maintenance Pathway (CMP)
- Understanding MOC
- Image and Slide Gallery
- Annual Scientific Session and Related Events
- Chapter Meetings
- Live Meetings
- Live Meetings - International
- Webinars - Live
- Webinars - OnDemand
- Certificates and Certifications
- ACC Accreditation Services
- ACC Quality Improvement for Institutions Program
- CardioSmart
- National Cardiovascular Data Registry (NCDR)
- Advocacy at the ACC
- Cardiology as a Career Path
- Cardiology Careers
- Cardiovascular Buyers Guide
- Clinical Solutions
- Clinician Well-Being Portal
- Diversity and Inclusion
- Infographics
- Innovation Program
- Mobile and Web Apps
Study Links E-Cigarette Use with Higher Risk of Heart Failure
Large study adds to growing body of evidence that vaping may harm the heart.
Apr 02, 2024
Contact: Nicole Napoli , [email protected], 202-669-1465
WASHINGTON (Apr 02, 2024) -
People who use e-cigarettes are significantly more likely to develop heart failure compared with those who have never used them, according to one of the largest prospective studies to date investigating possible links between vaping and heart failure. The findings are being presented at the American College of Cardiology’s Annual Scientific Session.
Heart failure is a condition affecting more than 6 million U.S. adults in which the heart becomes too stiff or too weak to pump blood as effectively as it should. It can often lead to debilitating symptoms and frequent hospitalizations as people age. Electronic nicotine products, which include e-cigarettes, vape pens, hookah pens, personal vaporizers and mods, e-cigars, e-pipes and e-hookahs, deliver nicotine in aerosol form without combustion. Since they were first introduced in the U.S. in the late 2000s, electronic nicotine products have often been portrayed as a safer alternative to smoking, but a growing body of research has led to increased concern about potential negative health effects.
“More and more studies are linking e-cigarettes to harmful effects and finding that it might not be as safe as previously thought,” said Yakubu Bene-Alhasan, MD, a resident physician at MedStar Health in Baltimore and the study’s lead author. “The difference we saw was substantial. It’s worth considering the consequences to your health, especially with regard to heart health.”
For the study, researchers used data from surveys and electronic health records in All of Us, a large national study of U.S. adults run by the National Institutes of Health, to analyze associations between e-cigarette use and new diagnoses of heart failure in 175,667 study participants (an average age of 52 years and 60.5% female). Of this sample, 3,242 participants developed heart failure within a median follow-up time of 45 months.
The results showed that people who used e-cigarettes at any point were 19% more likely to develop heart failure compared with people who had never used e-cigarettes. In calculating this difference, researchers accounted for a variety of demographic and socioeconomic factors, other heart disease risk factors and participants’ past and current use of other substances, including alcohol and tobacco products. The researchers also found no evidence that participants’ age, sex or smoking status modified the relationship between e-cigarettes and heart failure.
Breaking the data down by type of heart failure, the increased risk associated with e-cigarette use was statistically significant for heart failure with preserved ejection fraction (HFpEF)—in which the heart muscle becomes stiff and does not properly fill with blood between contractions. However, this association was not significant for heart failure with reduced ejection fraction (HFrEF)—in which the heart muscle becomes weak and the left ventricle does not squeeze as hard as it should during contractions. Rates of HFpEF have risen in recent decades, which has led to an increased focus on determining risk factors and improving treatment options for this type of heart failure.
The findings align with previous studies conducted in animals, which signaled e-cigarette use can affect the heart in ways that are relevant to the heart changes involved in heart failure. Other studies in humans have also shown links between e-cigarette use and some risk factors associated with developing heart failure. However, previous studies attempting to assess the direct connection between e-cigarette use and heart failure have been inconclusive, which Bene-Alhasan said is due to the inherent limitations of the cross-sectional study designs, smaller sample sizes and the smaller number of heart failure events seen in previous research.
Researchers said the new study findings point to a need for additional investigations of the potential impacts of vaping on heart health, especially considering the prevalence of e-cigarette use among younger people. Surveys indicate that about 5% to 10% of U.S. teens and adults use e-cigarettes. In 2018, the U.S. Surgeon General called youth e-cigarette use an epidemic and warned about the health risks associated with nicotine addiction.
“I think this research is long overdue, especially considering how much e-cigarettes have gained traction,” Bene-Alhasan said. “We don’t want to wait too long to find out eventually that it might be harmful, and by that time a lot of harm might already have been done. With more research, we will get to uncover a lot more about the potential health consequences and improve the information out to the public.”
Bene-Alhasan also said e-cigarettes are not recommended as a tool to quit smoking, since many people may continue vaping long after they quit smoking. The U.S. Centers for Disease Control and Prevention recommends a combination of counseling and medications as the best strategy for quitting smoking.
Researchers said that the study’s prospective observational design allows them to infer, but not conclusively determine, a causal relationship between e-cigarette use and heart failure. However, with its large sample size and detailed data on substance use and health information, Bene-Alhasan said the study is one of the most comprehensive studies to assess this relationship to date.
For more information about the health effects of e-cigarettes, visit CardioSmart.org/StopSmoking .
Bene-Alhasan will present the study, “Electronic Nicotine Product Use Is Associated with Incident Heart Failure - The All of Us Research Program,” on Sunday, April 7, 2024, at 3:15 p.m. ET / 19:15 UTC in Hall B4-5.
ACC.24 will take place April 6-8, 2024, in Atlanta, bringing together cardiologists and cardiovascular specialists from around the world to share the newest discoveries in treatment and prevention. Follow @ACCinTouch , @ACCMediaCenter and #ACC24 for the latest news from the meeting.
The American College of Cardiology (ACC) is the global leader in transforming cardiovascular care and improving heart health for all. As the preeminent source of professional medical education for the entire cardiovascular care team since 1949, ACC credentials cardiovascular professionals in over 140 countries who meet stringent qualifications and leads in the formation of health policy, standards and guidelines. Through its world-renowned family of JACC Journals, NCDR registries, ACC Accreditation Services, global network of Member Sections, CardioSmart patient resources and more, the College is committed to ensuring a world where science, knowledge and innovation optimize patient care and outcomes. Learn more at ACC.org .
< Back to Listings
You must be logged in to save to your library.
Jacc journals on acc.org.
- JACC: Advances
- JACC: Basic to Translational Science
- JACC: CardioOncology
- JACC: Cardiovascular Imaging
- JACC: Cardiovascular Interventions
- JACC: Case Reports
- JACC: Clinical Electrophysiology
- JACC: Heart Failure
- Current Members
- Campaign for the Future
- Become a Member
- Renew Your Membership
- Member Benefits and Resources
- Member Sections
- ACC Member Directory
- ACC Innovation Program
- Our Strategic Direction
- Our History
- Our Bylaws and Code of Ethics
- Leadership and Governance
- Annual Report
- Industry Relations
- Support the ACC
- Jobs at the ACC
- Press Releases
- Social Media
- Book Our Conference Center
Clinical Topics
- Chronic Angina
- Congenital Heart Disease and Pediatric Cardiology
- Diabetes and Cardiometabolic Disease
- Hypertriglyceridemia
- Invasive Cardiovascular Angiography and Intervention
- Pulmonary Hypertension and Venous Thromboembolism
Latest in Cardiology
Education and meetings.
- Online Learning Catalog
- Products and Resources
- Annual Scientific Session
Tools and Practice Support
- Quality Improvement for Institutions
- Accreditation Services
- Practice Solutions
Heart House
- 2400 N St. NW
- Washington , DC 20037
- Contact Member Care
- Phone: 1-202-375-6000
- Toll Free: 1-800-253-4636
- Fax: 1-202-375-6842
- Media Center
- Advertising & Sponsorship Policy
- Clinical Content Disclaimer
- Editorial Board
- Privacy Policy
- Registered User Agreement
- Terms of Service
- Cookie Policy
© 2024 American College of Cardiology Foundation. All rights reserved.
Latest Cochrane Review finds high certainty evidence that nicotine e-cigarettes are more effective than traditional nicotine-replacement therapy (NRT) in helping people quit smoking
17 November 2022
Research led by the University of Oxford, and funded by Cancer Research UK, has found the strongest evidence yet that e-cigarettes, also known as ‘vapes’, help people to quit smoking better than traditional nicotine replacement therapies, such as patches and chewing gums.
New evidence published today in the Cochrane Library finds high certainty evidence that people are more likely to stop smoking for at least six months using nicotine e-cigarettes, or ‘vapes’, than using nicotine replacement therapies, such as patches and gums. Evidence also suggested that nicotine e-cigarettes led to higher quit rates than e-cigarettes without nicotine, or no stop smoking intervention, but less data contributed to these analyses. The updated Cochrane review includes 78 studies in over 22,000 participants – an addition of 22 studies since the last update in 2021.
Smoking is a significant global health problem. According to the World Health Organisation (WHO), in 2020, 22.3% of the global population used tobacco, despite it killing up to half of its users. Stopping smoking reduces the risk of lung cancer, heart attacks and many other diseases. Though most people who smoke want to quit, many find it difficult to do so permanently. Nicotine patches and gum are safe, effective and widely used methods to help individuals quit.
E-cigarettes heat liquids with nicotine and flavourings, allowing users to ‘vape’ nicotine instead of smoking. Data from the review showed that if six in 100 people quit by using nicotine replacement therapy, eight to twelve would quit by using electronic cigarettes containing nicotine. This means an additional two to six people in 100 could potentially quit smoking with nicotine containing electronic cigarettes.
Dr Jamie Hartmann-Boyce, Associate Professor at the University of Oxford, Editor of the Cochrane Tobacco Addiction Group, and an author of the new publication, said: “Electronic cigarettes have generated a lot of misunderstanding in both the public health community and the popular press since their introduction over a decade ago. These misunderstandings discourage some people from using e-cigarettes as a stop smoking tool. Fortunately, more and more evidence is emerging and provides further clarity. With support from Cancer Research UK, we search for new evidence every month as part of a living systematic review. We identify and combine the strongest evidence from the most reliable scientific studies currently available.
For the first time, this has given us high-certainty evidence that e-cigarettes are even more effective at helping people to quit smoking than traditional nicotine replacement therapies, like patches or gums.”
In studies comparing nicotine e-cigarettes to nicotine replacement treatment, significant side effects were rare. In the short-to-medium term (up to two years), nicotine e-cigarettes most typically caused throat or mouth irritation, headache, cough, and feeling nauseous. However, these effects appeared to diminish over time.
Dr Nicola Lindson, University Research Lecturer at the University of Oxford, Cochrane Tobacco Addiction Group’s Managing Editor, and author of the publication said: “E-cigarettes do not burn tobacco; and as such they do not expose users to the same complex mix of chemicals that cause diseases in people smoking conventional cigarettes. E-cigarettes are not risk free, and shouldn’t be used by people who don’t smoke or aren’t at risk of smoking. However, evidence shows that nicotine e-cigarettes carry only a small fraction of the risk of smoking. In our review, we did not find evidence of substantial harms caused by nicotine containing electronic cigarettes when used to quit smoking. However, due to the small number of studies and lack of data on long-term nicotine-containing electronic cigarette usage – usage over more than two years – questions remain about long-term effects.”
The researchers conclude that more evidence, particularly about the effects of newer e-cigarettes with better nicotine delivery than earlier ones, is needed to assist more people quit smoking. Longer-term data is also needed.
Michelle Mitchell, chief executive at Cancer Research UK, said: “We welcome this report which adds to a growing body of evidence showing that e-cigarettes are an effective smoking cessation tool. We strongly discourage those who have never smoked from using e-cigarettes, especially young people. This is because they are a relatively new product and we don’t yet know the long term health effects.“
“While the long term effects of vaping are still unknown, the harmful effects of smoking are indisputable – smoking causes around 55,000 cancer deaths in the UK every year. Cancer Research UK supports balanced evidence-based regulation on e-cigarettes from UK governments which maximises their potential to help people stop smoking, whilst minimising the risk of uptake among others.”
This work was supported by Cancer Research UK [A ref. A29845]
Notes to Editors:
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD010216.pub7/full
doi: 10.1002/14651858.CD010216.pub7 Lead authors: Associate Professor at the Nuffield Department of Primary Care Health Sciences, University of Oxford and Editor of the Cochrane Tobacco Addiction Group, Dr Jamie Hartmann-Boyce and Senior Researcher at the Nuffield Department of Primary Care Health Sciences, University of Oxford and Managing Editor of the Cochrane Tobacco Addiction Group, Dr Nicola Lindson.
To speak to a team member about this project please contact Dr. Hartmann-Boyce, [email protected] or Dr. Lindson, [email protected] .
About Cochrane Cochrane is a global independent network of researchers, professionals, patients, carers, and people interested in health. Cochrane produces reviews which study all the best available evidence generated through research and make it easier to inform decisions about health. These are called systematic reviews. Cochrane is a not-for profit organization with collaborators from more than 130 countries working together to produce credible, accessible health information that is free from commercial sponsorship and other conflicts of interest. Our work is recognized as representing an international gold standard for high-quality, trusted information. Find out more at cochrane.org
The University of Oxford Oxford University has been placed number 1 in the Times Higher Education World University Rankings for the seventh year running, and 2 in the QS World Rankings 2022. At the heart of this success is our ground-breaking research and innovation. Oxford is world-famous for research excellence and home to some of the most talented people from across the globe. Our work helps the lives of millions, solving real-world problems through a huge network of partnerships and collaborations. The breadth and interdisciplinary nature of our research sparks imaginative and inventive insights and solutions. Through its research commercialisation arm, Oxford University Innovation, Oxford is the highest university patent filer in the UK and is ranked first in the UK for university spinouts, having created more than 200 new companies since 1988. Over a third of these companies have been created in the past three years. The university is a catalyst for prosperity in Oxfordshire and the United Kingdom, contributing £15.7 billion to the UK economy in 2018/19, and supports more than 28,000 full time jobs.
Oxford University’s Medical Sciences Division : The Medical Sciences Division is an internationally recognised centre of excellence for biomedical and clinical research and teaching and is the largest of the four academic divisions within the University. Over 5000 academics, researchers, NHS clinicians and GPs, and administrative staff, 1500 graduate and 1600 undergraduate students, together contribute to our extensive and exemplary research, teaching, and clinical portfolios. We aim to be the best university biomedical institution in Europe and amongst the best five biomedical institutions in the world and have been ranked number one for the last twelve years in the Times Higher Education World University Rankings for clinical, pre-clinical and health sciences - the only non-North American institution to be top-ranked by THE in any subject discipline.
Within the division, the Nuffield Department of Primary Care Health Sciences has been one of the world’s most important primary care centres for over 20 years; leading world-class research and training to rethink the way healthcare is delivered in general practice and other primary care settings. The department’s main research focus is on the prevention, early diagnosis, and management of common illness, bringing together academics from many diverse backgrounds to work together to produce benefits for the NHS, for populations and for patients. www.phc.ox.ac.uk .
DISCOVER MORE
- Support Oxford's research
- Partner with Oxford on research
- Study at Oxford
- Research jobs at Oxford
You can view all news or browse by category
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 22 October 2022
A systematic review of the effects of e-cigarette use on lung function
- Lucy Honeycutt 1 ,
- Katherine Huerne 1 , 2 ,
- Alanna Miller 1 ,
- Erica Wennberg 1 ,
- Kristian B. Filion 1 , 3 ,
- Roland Grad 1 , 4 ,
- Andrea S. Gershon 5 ,
- Carolyn Ells ORCID: orcid.org/0000-0002-4593-454X 1 , 2 , 4 ,
- Genevieve Gore 6 ,
- Andrea Benedetti 3 , 7 ,
- Brett Thombs 1 , 3 , 8 &
- Mark J. Eisenberg ORCID: orcid.org/0000-0002-1296-0661 1 , 3 , 9
npj Primary Care Respiratory Medicine volume 32 , Article number: 45 ( 2022 ) Cite this article
21k Accesses
11 Citations
40 Altmetric
Metrics details
- Epidemiology
Given the increasing use of e-cigarettes and uncertainty surrounding their safety, we conducted a systematic review to determine the effects of e-cigarettes on measures of lung function. We systematically searched EMBASE, MEDLINE, and PsycINFO databases via Ovid, the Cochrane CENTRAL database, and the Web of Science Core from 2004 until July 2021, identifying 8856 potentially eligible studies. A total of eight studies (seven studying immediate effects and one long-term effects, 273 total participants) were included. The risk of bias was assessed using the Risk of Bias in Non-randomized Studies—of Interventions (ROBINS-I) and Cochrane risk of bias tools. These studies suggest that vaping increases airway resistance but does not appear to impact forced expiratory volume in one second (FEV 1) , forced vital capacity (FVC), or FEV 1 /FVC ratio. However, given the limited size and follow-up duration of these studies, larger, long-term studies are required to further determine the effects of e-cigarettes on lung function.
Similar content being viewed by others
Efficacy and safety of inhaled heparin in asthmatic and chronic obstructive pulmonary disease patients: a systematic review and a meta-analysis
Are COPD self-management mobile applications effective? A systematic review and meta-analysis
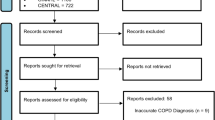
Wearable technology interventions in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis
Introduction.
The first electronic cigarette (e-cigarette) was patented and marketed in 2004 1 . Since then, e-cigarette use (or “vaping”) has grown exponentially across the globe 2 . As the use of vaping devices evolves with policy, the consequences of vaping on health are becoming an increasingly important public health issue. E-cigarettes are being studied for harm reduction in individuals who use cigarettes and as a smoking cessation aid, as they are believed to be less harmful to health than smoking 3 . However, there is increasing evidence demonstrating adverse respiratory effects of vaping compared to vaping abstinence. In particular, an outbreak of E-Cigarette and Vaping-Associated Lung Illness (EVALI) brought the short-term respiratory consequences of vaping into question, especially if cannabis or THC-containing products are used 4 . Other short-term respiratory changes that have been linked to vaping include increased airway resistance 5 , breathing difficulty 6 , and transient lung inflammation 7 . Vaping has also been associated with chronic respiratory conditions such as asthma 8 and chronic bronchitis 9 . Despite these reports, the short- and long-term respiratory safety of vaping is still largely unknown. Several small studies have examined the effects of e-cigarettes on lung function, including measures such as forced expiratory volume in one second (FEV 1 ), forced vital capacity (FVC), and airway resistance. However, no evidence syntheses have been completed on this topic. Therefore, we conducted a systematic review to determine the effects of vaping on measures of lung function.
Our systematic review was conducted following a protocol developed prior to initiating the review, which was registered on the PROSPERO register of systematic reviews ( CRD42021227121 ) 10 . This systematic review is reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines 11 .
Search strategy and study selection
Using a search strategy (Supplementary Tables 1 – 5 ) developed by an experienced health sciences librarian (G.G.), we systematically searched EMBASE, MEDLINE, and PsycINFO databases via Ovid, the Cochrane CENTRAL database, and the Web of Science Core from 2004 (the year of the first e-cigarette patent) until July 12, 2021. We additionally conducted a gray literature search by searching the websites of key governmental and public health organizations (the World Health Organization, Health Canada, the US Centers for Disease Control and Prevention, the US Food and Drug Administration, the Canadian Center on Substance Use and Addiction, the European Centre for Disease Prevention and Control, and the European Public Health Association). Additional articles were identified by manually searching the reference lists of included publications as well as SCOPUS and Google Scholar (first ten pages). Articles were included if they reported quantitative primary data on changes in lung function associated with vaping, defined as the use of any device that functions by transforming an e-liquid to an aerosol using metal coils, among human participants of any age. Studies of cells and those conducted in animals were excluded. Studies using heat-not-burn devices were also excluded, as these do not meet the above definition of vaping. Eligible studies included randomized controlled trials (RCTs), non-randomized studies of interventions (NRSIs), and cohort studies; cross-sectional studies and case reports were excluded. We included studies that used non-users of both vaping devices and conventional cigarettes as a comparison group and those that used a pre- and post-design in which individuals acted as their own controls. Inclusion was not restricted by language or country of publication. Abstracts and conference proceedings were included if sufficient data could be extracted from these publications.
Search results were downloaded from databases into reference management software (EndNote X9) or manually added (e.g., for gray literature results). Duplicates were removed in EndNote and entries were uploaded to Covidence (Veritas Health Innovation, Melbourne, Australia), a systematic review software. Two reviewers (L.H. and K.H.) independently screened the titles and abstracts of all identified publications for eligibility. Citations considered potentially eligible by either reviewer, based on the pre-specified review inclusion/exclusion criteria (Supplementary Table 6 ), were retrieved for full-text screening and assessed for inclusion. The reasons for exclusion after full-text review were annotated in Covidence and any disagreements were resolved by consensus or a third reviewer (A.H-L.).

Data extraction
Two independent reviewers (L.H and K.H.) extracted methodological, demographic, and outcome data from included studies in duplicate; disagreements were detected in Covidence and were resolved by consensus or, if necessary, by a third reviewer (A.H-L.). Extracted data included study characteristics (first author, journal, year of publication, years(s) of data collection, funding, data source, study design, recruitment strategy, duration of follow-up, country of origin, sample size); population characteristics (sex, gender, age, race, ethnicity, socioeconomic status, dose/frequency of e-cigarette use, conventional cigarette smoking status, smoked cannabis use); and vaping behavior, including the type of vaping device used (e.g., disposable e-cigarette vs. pod device such as JUUL), vaping products used (e.g., nicotine cartridges exclusively vs. THC cartridges exclusively vs. dual use of nicotine and THC products), and source of the vaping product (informal [i.e., friends, family members, or dealers] vs. commercial [i.e., vape shops, stores, dispensaries]).
Initially, extracted outcomes of primary interest were respiratory signs and symptoms, as these are important to patients and are the early signs of respiratory disease. Secondary outcomes included: findings on lung function; Computed tomography (CT) findings of emphysema, airway remodeling, and small airway loss; respiratory-related quality of life and exercise limitations; incidence and/or prevalence of respiratory disease as well as exacerbations of previous respiratory disease; and health care resource use including respiratory disease-related ambulatory care, emergency department visits, and hospitalization. Given the limited number of studies available and the heterogeneity of the data extracted from these studies, no meta-analysis was conducted.
Risk of bias
The risk of bias in included publications was assessed independently by two reviewers (L.H. and K.H.), and discrepancies were resolved by consensus or, if necessary, by a third reviewer (A.H-L.). The risk of bias of included non-randomized studies (pre-post studies, NRSI with non-vaping reference group, cohort study) was assessed using the Risk of Bias in Non-randomized Studies—of Interventions (ROBINS-I) tool 12 . The ROBINS-I tool evaluates intervention-specific outcomes for a study through seven domains which assess the risk of bias pre-intervention, at-intervention, and post-intervention. For each outcome of interest extracted from an included study, the risk of bias within each domain was reported as “low”, “moderate”, “serious”, or “critical”. Included RCTs were assessed using the Cochrane Collaboration’s Tool for Assessing Risk of Bias (ROB V1) 13 . Similar to ROBINS-I, this tool evaluates the risk of bias through the assessment of five domains; for each outcome of interest extracted from an included study, the risk of bias for each domain was reported as “low risk of bias”, “high risk of bias”, or “unclear risk of bias.” All eligible publications were included in the qualitative synthesis regardless of their assessed risk of bias.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
As our search did not identify studies which focused on the broad outcomes detailed above, we chose to limit our focus to studies on lung function. Our database searches identified 14,307 potentially eligible studies (Fig. 1 ). After duplicates were removed, 8856 titles and abstracts were assessed. After this initial screening, 44 full texts were retrieved and reviewed in further detail, yielding eight studies eligible for inclusion.
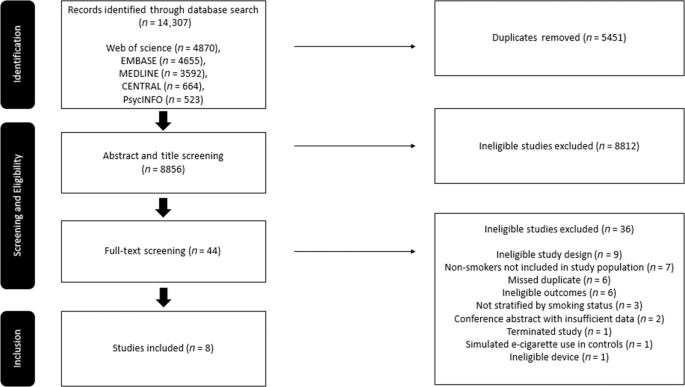
PRISMA flow diagram of included studies assessing the effect of e-cigarettes on lung function.
Study and participant characteristics
Of the eight included studies (273 total participants), seven 14 , 15 , 16 , 17 , 18 , 19 , 20 involved short-term exposure to e-cigarettes with immediate outcome assessment, and the remaining study followed vapers and non-vapers over 3.5 years 21 (Table 1 ). This prospective cohort study examined 21 participants (12 nonsmokers and nine vapers) at means of 12 (standard deviation: 1), 24 (2), and 42 (2) months after baseline 21 . Of the seven short-term studies, four were NRSIs (three pre-post studies 14 , 15 , 16 and one NRSI with a non-vaping reference group 20 ) and three were RCTs 17 , 18 , 19 . Among these seven studies, two included 70–80 participants 14 , 15 and five included 10–30 participants 16 , 17 , 18 , 19 , 20 . Exposures varied in terms of e-cigarettes, e-liquids, and vaping session timings. Most studies did not expand on their definition of “non-smoker/non-vaper” 15 , 16 , 18 , 19 , 20 , 21 , but two studies clarified that these participants were never-smokers 14 , 17 . One of these two studies further elaborated that participants had no exposure to tobacco products or e-cigarettes 17 . Few studies gave detailed information on the type of e-cigarette used. Three studies reported a specific brand or product (Blu 17 , eGo 16 , Joytech elips-C series 18 , Puff bar 20 ). Polosa et al. listed some of the various e-cigarettes used by participants throughout the longitudinal study, including standard refillable (eGo style products) and more advanced refillable (Provari, Innokin, Joytech, eVIC, Avatar Puff) 21 . The remaining studies did not report a specific brand, though one study described the e-cigarette used as a “1 st generation e-cigarette popular in Greece” 15 . All studies clarified whether the e-cigarettes used during the study contained nicotine.
The included RCTs ( n = 3) 17 , 18 , 19 had an unclear risk of bias, with each study demonstrating an unclear risk of bias in 3+ domains (Table 2 ). This was primarily due to missing information in the manuscripts required to make an adequate judgment, such as a lack of detail surrounding randomization. The risk associated with the blinding of participants and personnel was judged to be low for all 3 included RCTs. These studies were not blinded, and one was placebo-controlled. However, it was judged that this lack of blinding would not influence measures of lung function. Of the included non-randomized studies ( n = 5) 14 , 15 , 16 , 20 , 21 , four 14 , 15 , 16 , 20 were judged to be at moderate risk of bias and one 21 was found to have a serious risk of bias (Table 3 ). The most consistent source of bias in these studies was bias due to confounding, with only one 16 study judged to have a low risk of bias due to confounding. Of the remaining four studies, three 14 , 15 , 20 were found to have a moderate risk of bias due to confounding and one 21 was found to be at serious risk of bias due to confounding, with important confounding variables not accounted for in the design or analysis.
Effects of E-cigarette use on lung function
Seven studies 14 , 15 , 16 , 17 , 18 , 19 , 20 reported immediate measures of lung function after short-term exposure to e-cigarettes (Table 4 ), including FEV 1 , FVC, and FEV 1 /FVC. Two studies, Boulay et al. and Staudt. et al. suggested no changes in FEV 1 or FEV 1 /FVC after vaping among nonsmokers 17 , 19 . Kizhakke Puliyakote et al. observed lower baseline FEV 1 and FEV 1 /FVC values among nonsmokers compared to vapers 20 . Coppeta et al. found a decrease in FEV 1 and FEV 1 /FVC among nonsmokers after 1 min of vaping; however, these values returned to baseline after 15 min 16 .
Airway resistance and specific airway conductance after 10 min of vaping were measured in two 14 , 15 of the seven short-term studies (Table 4 ). Both Palamidas et al. 2013 and 2017 suggested that vaping increased airway resistance and decreased specific airway conductance among nonsmokers and smokers with and without respiratory disease. Oxygen saturation was assessed in four studies 15 , 17 , 19 , 20 . Three studies suggested no changes after vaping, with only Palamidas et. al. 2017 suggesting decreased oxygen saturation following vaping among smokers with and without asthma 15 .
Long-term changes (3.5 years) in lung function measurements were assessed in only one small ( n = 21) study (Polosa 2017) 21 . This study suggested that FEV 1 , FVC, FEV 1 /FVC, and forced mid-expiratory flow (FEF 25-75 ) did not change over time among vapers and non-vapers (Table 5 ).
This systematic review was designed to determine the effect of vaping on measures of lung function. We found that there were only eight studies in the literature assessing this issue, all of which were small, and only one examined longer-term outcomes (3.5 years follow-up). In general, these studies suggest that there are no acute changes associated with vaping. However, airway resistance and conductance may be influenced by e-cigarettes, with two studies reporting changes in these values in multiple population subgroups. It is important to note that there were few studies available for this systematic review and that most of these studies focused on the acute effects of vaping; therefore, these results are suggestive but not definitive, and future research must be conducted in this area. Furthermore, three of the included studies had an unclear risk of bias, four had a moderate risk of bias, and one had a serious risk of bias, which further limits the interpretation of this review’s findings.
In addition to the limitations above, this review lacks subgroup analyses or a meta-analysis. This is due to the heterogeneity of the included studies, both in terms of study design and outcomes. Few studies were eligible for this review due to the variation in study designs and definitions of e-cigarettes and smoking status. For example, some studies included both conventional cigarette smokers and nonsmokers in their definition of “non-vapers” and did not analyze data separately based on conventional smoking status. Other studies used a “sham” vaping session for controls where either an e-cigarette with an empty cartridge (i.e., without e-liquid) or second-hand smoke were used. More commonly, studies were conducted on smokers only, without nonsmokers as a comparison group. Future studies could analyze subgroups based on both smoking and vaping status to allow for a more detailed quantitative analysis.
E-cigarettes are becoming more popular for recreational use and are being studied for harm reduction among smokers as a smoking cessation aid, as they are believed to be less harmful to health than smoking. However, there are limited data available and virtually no long-term studies assessing how prolonged e-cigarette use could impact lung function. As the use of vaping devices evolves and becomes more widespread, the health consequences of vaping are becoming an increasingly important public health issue. This is a knowledge gap that must be addressed. Knowledge of the safety of e-cigarettes, particularly their long-term safety, will inform public health policy and e-cigarette regulations, as well as the guidance clinicians, offer to their patients on smoking harm reduction. For these policies, regulations, and guidelines to be developed, we must understand how e-cigarettes can influence one’s health. This includes establishing the effects of e-cigarettes on clinical outcomes such as respiratory symptoms (cough, dyspnea), measures of lung function, and risk of developing respiratory disease. Further research is required to elucidate the short- and long-term consequences of vaping to determine whether e-cigarettes are truly a “safer” alternative to traditional cigarettes for smoking cessation or for recreational use. Future studies should be long-term, have large sample sizes, and may include different types of e-cigarettes as well as conventional cigarettes for comparison. In addition, it is important for future research to include clinical outcomes as described above. This will allow for better translation of the research findings to help inform clinical decision-making.
Data availability
No additional data were available, as this study is a knowledge synthesis that relied on aggregate, published results available in the public domain. Any inquiries should be directed to the corresponding author.
Foulds, J., Veldheer, S. & Berg, A. Electronic cigarettes (e‐cigs): views of aficionados and clinical/public health perspectives. Int. J. Clin. Pract. 65 , 1037–1042 (2011).
Article CAS Google Scholar
Bozier, J. et al. The evolving landscape of e-cigarettes: a systematic review of recent evidence. Chest 157 , 1362–1390 (2020).
CDC. About electronic cigarettes (E-cigarettes). https://www.cdc.gov/tobacco/basic_information/ecigarettes/about-e-cigarettes.html (2020)
Blount, B. C. et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N. Engl. J. Med. 382 , 697–705 (2020).
Palamidas, A. et al. Acute effect of an e-cigarette with and without nicotine on lung function. Tob. Induc. Dis. 12 , A34–A34 (2014).
PubMed Central Google Scholar
Wang, J. B. et al. Cigarette and e-cigarette dual use and risk of cardiopulmonary symptoms in the Health eHeart Study. PLoS ONE 13 , e0198681 (2018).
Article Google Scholar
Chaumont, M. et al. Fourth generation e-cigarette vaping induces transient lung inflammation and gas exchange disturbances: results from two randomized clinical trials. Am. J. Physiol. Lung Cell Mol. Physiol. 316 , L705–l19 (2019).
Schweitzer, R. J., Wills, T. A., Tam, E., Pagano, I. & Choi, K. E-cigarette use and asthma in a multiethnic sample of adolescents. Prev. Med. 105 , 226–231 (2017).
McConnell, R. et al. Electronic cigarette use and respiratory symptoms in adolescents. Am. J. Respir. Crit. Care Med. 195 , 1043–1049 (2017).
Booth, A. et al. The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Syst. Rev. 1 , 2 (2012).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339 , b2535 (2009).
Sterne, J. A. et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355 , i4919 (2016).
Higgins, J. P. T. et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343 , d5928 (2011).
Palamidas, A. et al. Acute effect of an e-cigarette with and without nicotine on lung function. Eur. Respir. J. 42 , P1054 (2013).
Google Scholar
Palamidas, A. et al. Acute effects of short term use of ecigarettes on airways physiology and respiratory symptoms in smokers with and without airway obstructive diseases and in healthy non smokers. Tob. Prev. Cessat. 3 , 5 (2017).
Coppeta, L., Magrini, A., Pietroiusti, A., Perrone, S. & Grana, M. Effects of smoking electronic cigarettes on pulmonary function and environmental parameters. Open Public Health J. 11 , 360–368 (2018).
Staudt, M. R., Salit, J., Kaner, R. J., Hollmann, C. & Crystal, R. G. Altered lung biology of healthy never smokers following acute inhalation of E-cigarettes. Respir. Res. 19 , 78 (2018).
Ferrari, M. et al. Short-term effects of a nicotine-free e-cigarette compared to a traditional cigarette in smokers and non-smokers. BMC Pulm. Med. 15 , 120 (2015).
Boulay, M.-È., Henry, C., Bossé, Y., Boulet, L.-P. & Morissette, M. C. Acute effects of nicotine-free and flavour-free electronic cigarette use on lung functions in healthy and asthmatic individuals. Respir. Res. 18 , 33 (2017).
Puliyakote, A. S. K. et al. Vaping disrupts ventilation-perfusion matching in asymptomatic users. J. Appl. Physiol. 130 , 308–317 (2021).
Polosa, R. et al. Health impact of E-cigarettes: a prospective 3.5-year study of regular daily users who have never smoked. Sci. Rep. 7 , 13825 (2017).
Download references
Acknowledgements
The authors would like to thank Jenna Glidden and Andrea Hebert-Losier for their assistance with study screening, data abstraction, and risk of bias assessment. The authors would also like to thank Francesca Frati, who peer-reviewed the search strategy. This work was funded by the Canadian Institutes for Health Research (#HEV-172891). The funder of the study had no role in study design, data collection, data analysis, data interpretation, writing of the report, or decision to submit for publication. Dr. Filion is supported by a Senior Research Scholar award from the Fonds de recherche du Québec – Santé and a William Dawson Scholar award from McGill University. Dr. Thombs was supported by a Tier 1 Canada Research Chair.
Author information
Authors and affiliations.
Lady Davis Institute for Medical Research, Jewish General Hospital/McGill University, Montreal, QC, Canada
Lucy Honeycutt, Katherine Huerne, Alanna Miller, Erica Wennberg, Kristian B. Filion, Roland Grad, Carolyn Ells, Brett Thombs & Mark J. Eisenberg
Biomedical Ethics Unit, Departments of Medicine and Social Studies of Medicine, and Division of Experimental Medicine, McGill University, Montreal, QC, Canada
Katherine Huerne & Carolyn Ells
Departments of Medicine and of Epidemiology, Biostatistics and Occupational Health, McGill University, Montreal, QC, Canada
Kristian B. Filion, Andrea Benedetti, Brett Thombs & Mark J. Eisenberg
Department of Family Medicine, McGill University, Montreal, QC, Canada
Roland Grad & Carolyn Ells
Division of Respirology, Department of Medicine, Sunnybrook Health Sciences Centre and the University of Toronto, Toronto, ON, Canada
Andrea S. Gershon
Schulich Library of Physical Sciences, Life Sciences, and Engineering, McGill University, Montreal, QC, Canada
Genevieve Gore
Respiratory Epidemiology and Clinical Research Unit, Centre for Outcomes Research and Evaluation, Research Institute of the McGill University Health Centre, Montreal, QC, Canada
Andrea Benedetti
Departments of Psychiatry, Psychology, and Biomedical Ethics Unit, McGill University, Montreal, QC, Canada
Brett Thombs
Division of Cardiology, Jewish General Hospital/McGill University, Montreal, QC, Canada
Mark J. Eisenberg
You can also search for this author in PubMed Google Scholar
Contributions
G.G. performed the search. L.H. and K.H. screened studies, extracted data, and performed a risk of bias assessment of included studies. L.H. drafted the manuscript. All authors contributed to the study design and interpretation of results, revised the manuscript for important intellectual content, and approved the final version of the manuscript. M.J.E. supervised the study and is the guarantor.
Corresponding author
Correspondence to Mark J. Eisenberg .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Honeycutt, L., Huerne, K., Miller, A. et al. A systematic review of the effects of e-cigarette use on lung function. npj Prim. Care Respir. Med. 32 , 45 (2022). https://doi.org/10.1038/s41533-022-00311-w
Download citation
Received : 09 May 2022
Accepted : 06 October 2022
Published : 22 October 2022
DOI : https://doi.org/10.1038/s41533-022-00311-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Gender differences in e-cigarette knowledge, attitudes, and practice among adults in bahrain: a cross-sectional study.
- Adel Salman AlSayyad
- Bayan Abduljalil Alajaimi
- Azhar Faisal Hasan
Discover Public Health (2024)
Our contribution to systematic review and meta-analysis in primary care respiratory medicine
- Tiago Maricoto
- Ioanna Tsiligianni
npj Primary Care Respiratory Medicine (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Introduction
- Article Information
eFigure. Participants Selection Flow Chart
eTable. Calculation of Smoking History Variables
eReferences.
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Digital Health
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Sexual Health
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Wang Q , Jiang C , Hsu ML, et al. E-Cigarette Use and Lung Cancer Screening Uptake. JAMA Netw Open. 2024;7(7):e2419648. doi:10.1001/jamanetworkopen.2024.19648
Manage citations:
© 2024
- Permissions
E-Cigarette Use and Lung Cancer Screening Uptake
- 1 University Hospitals Seidman Cancer Center, Cleveland, Ohio
- 2 Division of Solid Tumor Oncology, Case Western Reserve University, Cleveland, Ohio
- 3 Division of Hematology and Oncology, Department of Internal Medicine, University of Texas Southwestern Medical Center, Dallas
- 4 Division of General Internal Medicine, Icahn School of Medicine at Mount Sinai, New York, New York
- 5 Division of Pulmonary, Critical Care and Sleep Medicine, Icahn School of Medicine at Mount Sinai, New York, New York
- 6 Department of Family, Population and Preventive Medicine, Stony Brook University, Stony Brook, New York
- 7 Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy
Electronic cigarettes (e-cigarettes) have gained popularity since 2007 and have become the second most commonly used tobacco product among US adults in 2021. 1 E-cigarettes have been increasingly used as tobacco cessation aids. 2 , 3 However, there are concerns about the potential long-term cancer risks associated with e-cigarette use. 4 Since the recommendations from the US Preventive Services Task Force (USPSTF) endorsing lung cancer screening (LCS) in 2013 (revised in 2021), uptake has increased but remains low. 5 , 6 The association between e-cigarette use and LCS uptake remains unknown.
This cross-sectional study followed the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline and was exempt for human participants review from the institutional review board at University Hospitals. Informed consent was not required because data were deidentified. Individuals who ever smoked cigarettes and were eligible for LCS according to the USPSTF 2021 recommendations (ie, age 50 to 80 years, 20 pack-years or more smoking history, currently smoking or quit 15 years ago or less) were selected from the 2022 Behavioral Risk Factor Surveillance System (BRFSS) (eMethods and eTable in Supplement 1 ). 5 The χ 2 (for categorical variables) and Kruskal-Wallis (for continuous variables) tests were used to compare baseline demographic characteristics between individuals who underwent LCS vs did not undergo LCS. Logistic regression was used to calculate the association between LCS uptake and e-cigarette use, after adjusting for potential confounders. We also stratified LCS uptake by time since last LCS—ever having an LCS vs having an up-to-date LCS (uptake within the past 1 year). Analyses were weighted per the BRFSS guidelines using SAS version 9.4 (SAS Institute) and R version 2024.04.1 (R Project for Statistical Computing). The significance level was set at 2-sided α < 0.05.
Of 22 713 eligible individuals, the median (IQR) age was 62 (56-67) years and the sample (weighted percentage) included 12 198 males (56.4%), 19 136 non-Hispanic White individuals (77.7%), 1239 non-Hispanic Black individuals (8.6%), 6122 individuals (26.7%) who underwent LCS, and 3472 individuals (14.6%) who were up-to-date with LCS testing ( Table 1 ). Individuals who underwent LCS were older, more likely to have lower income, comorbidities, had a routine check-up last year, reported poor general health, resided in the Northeast region of the US, and were less likely to be uninsured. They also had a higher pack-year of smoking, had recently quit smoking (individuals who previously used cigarettes), had attempted to quit in the past year (individuals who currently use cigarettes), and were more likely to report never using e-cigarettes compared with those who did not undergo LCS ( Table 1 ). Overall, individuals currently using e-cigarettes had 21% lower odds of having undergone LCS (odds ratio [OR], 0.79; 95% CI, 0.62-1.00) compared with individuals who never used e-cigarettes after adjusting for confounders, with similar trends found in individuals who previously used combustible cigarettes (OR, 0.73; 95% CI, 0.52-1.04) ( Table 2 ). After stratifying by time since last LCS, individuals currently using e-cigarettes had 33% lower odds of being up-to-date with LCS (OR, 0.67; 95% CI, 0.51-0.88) than individuals who never use e-cigarettes ( Table 2 ). Similar findings were seen among individuals who previously used combustible cigarettes and currently use e-cigarettes; they had 46% lower odds of being up-to-date with LCS (OR, 0.54; 95% CI, 0.37-0.80) ( Table 2 ).
In this cross-sectional study, e-cigarette use was independently associated with lower use of LCS, particularly among individuals who had quit smoking combustible cigarettes. Emerging research suggests that e-cigarettes contain definite and probable carcinogens and cause similar cancer-associated gene deregulations as combustible tobacco. 4 However, it has been shown that two-thirds of individuals currently using e-cigarettes consider e-cigarettes to be less harmful than combustible cigarettes. 3 Thus, individuals who use e-cigarettes may have lower awareness of lung cancer risks. Limitations of this study include cross-sectional study design, self-reported smoking and LCS information, and inability to assess the impact of switching between cigarettes and e-cigarettes on LCS uptake. Our results highlighted the importance of raising awareness and rectifying misconceptions of e-cigarette use. Former smokers who use e-cigarettes remain at increased risk of lung cancer and should be targeted by interventions to improve adherence to LCS.
Accepted for Publication: April 30, 2024.
Published: July 2, 2024. doi:10.1001/jamanetworkopen.2024.19648
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2024 Wang Q et al. JAMA Network Open .
Corresponding Author: Qian Wang, MD, MPH, Case Western Reserve University, 11100 Euclid Ave, Cleveland, OH 44106 ( [email protected] ).
Author Contributions: Dr Wang had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Wang, Boffetta, Kong.
Acquisition, analysis, or interpretation of data: Wang, Jiang, Hsu, Wisnivesky, Dowlati, Kong.
Drafting of the manuscript: Wang, Dowlati, Kong.
Critical review of the manuscript for important intellectual content: All authors.
Statistical analysis: Wang, Wisnivesky, Boffetta.
Administrative, technical, or material support: Dowlati.
Supervision: Dowlati, Boffetta, Kong.
Conflict of Interest Disclosures: Dr Hsu reported receiving personal fees from Regeneron, Mirati, Astra Zeneca, and Amgen outside the submitted work. Dr Wisnivesky reported receiving personal fees from Sanofi, Pharmaceutical Product Development, and Banook and grants from Regeneron, Sanofi, and Axella outside the submitted work. Dr Dowlati reported receiving personal fees from Astra Zeneca and Amgen outside the submitted work. No other disclosures were reported.
Data Sharing Statement: See Supplement 2 .
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
News releases.
News Release
Wednesday, October 26, 2022
NIH-funded studies show damaging effects of vaping, smoking on blood vessels
Combining e-cigarettes with regular cigarettes may increase health risks.

Long-term use of electronic cigarettes, or vaping products, can significantly impair the function of the body’s blood vessels, increasing the risk for cardiovascular disease. Additionally, the use of both e-cigarettes and regular cigarettes may cause an even greater risk than the use of either of these products alone. These findings come from two new studies supported by the National Heart, Lung, and Blood Institute (NHLBI), part of the National Institutes of Health (NIH).
The findings, which appear today in the journal Arteriosclerosis, Thrombosis, and Vascular Biology , add to growing evidence that long-term use of e-cigarettes can harm a person’s health. Researchers have known for years that tobacco smoking can cause damage to blood vessels. However, the effects of e-cigarettes on cardiovascular health have been poorly understood. The two new studies – one on humans, the other on rats – aimed to change that.
“In our human study, we found that chronic e-cigarettes users had impaired blood vessel function, which may put them at increased risk for heart disease,” said Matthew L. Springer, Ph.D., a professor of medicine in the Division of Cardiology at the University of California in San Francisco, and leader of both studies. “It indicates that chronic users of e-cigarettes may experience a risk of vascular disease similar to that of chronic smokers.”
In this first study, Springer and his colleagues collected blood samples from a group of 120 volunteers that included those with long-term e-cigarette use, long-term cigarette smoking, and those who didn't use. The researchers defined long-term e-cigarette use as more than five times/week for more than three months and defined long-term cigarette use as smoking more than five cigarettes per day.
They then exposed each of the blood samples to cultured human blood vessel (endothelial) cells in the laboratory and measured the release of nitric oxide, a chemical marker used to evaluate proper functioning of endothelial cells. They also tested cell permeability, the ability of molecules to pass through a layer of cells to the other side. Too much permeability makes vessels leaky, which impairs function and increases the risk for cardiovascular disease.
The researchers found that blood from participants who used e-cigarettes and those who smoked caused a significantly greater decrease in nitric oxide production by the blood vessel cells than the blood of nonusers. Notably, the researchers found that blood from those who used e-cigarettes also caused more permeability in the blood vessel cells than the blood from both those who smoked cigarettes and nonusers. Blood from those that used e-cigarettes also caused a greater release of hydrogen peroxide by the blood vessel cells than the blood of the nonusers. Each of these three factors can contribute to impairment of blood vessel function in people who use e-cigarettes, the researchers said.
In addition, Springer and his team discovered that e-cigarettes had harmful cardiovascular effects in ways that were different from those caused by tobacco smoke. Specifically, they found that blood from people who smoked cigarettes had higher levels of certain circulating biomarkers of cardiovascular risks, and the blood people who used e-cigarettes had elevated levels of other circulating biomarkers of cardiovascular risks.
“These findings suggest that using the two products together, as many people do, could increase their health risks compared to using them individually,” Springer said. “We had not expected to see that.”
In the second study, the researchers tried to find out if there were specific components of cigarette smoke or e-cigarette vapor that were responsible for blood vessel damage. In studies using rats, they exposed the animals to various substances found in tobacco smoke or e-cigarettes. These included nicotine, menthol (a cigarette additive), the gases acrolein and acetaldehyde (two chemicals found in both tobacco smoke and e-cigarette vapors), and inert carbon nanoparticles to represent the particle-like nature of smoke and e-cigarette vapor.
Using special arterial flow measurements, the researchers demonstrated that blood vessel damage does not appear to be caused by a specific component of cigarette smoke or e-cigarette vapor. Instead, they said, it appears to be caused by airway irritation that triggers biological signals in the vagus nerve that somehow leads to blood vessel damage, possibly through an inflammatory process. The vagus is a long nerve extending from the brain that connects the airway to the rest of the nervous system and plays a key role in heart rate, breathing, and other functions. The researchers showed that detaching the nerve in rats prevented blood vessel damage caused by tobacco smoke, demonstrating its key role in this process.
“We were surprised to find that there was not a single component that you could remove to stop the damaging effect of smoke or vapors on the blood vessels,” Springer said. “As long as there’s an irritant in the airway, blood vessel function may be impaired.”
The finding has implications for efforts to regulate tobacco products and e-cigarettes, as it underscores how difficult it is to pinpoint any one ingredient in them that is responsible for blood vessel damage. “What I like to tell people is this: Just breathe clean air and avoid using these products,” Springer said.
Lisa Postow, Ph.D., an NHLBI program officer in NHLBI’s Division of Lung Diseases, agreed that the study results “provide further evidence that exposure to e-cigarettes could lead to harmful cardiovascular health effects.” She added that more data is needed to fully understand the health effects of e-cigarettes. The NIH and others are continuing to explore this area.
Research reported in the e-cigarette study was funded by NHLBI grants U54HL147127, P50HL120163, and R01HL120062 and the U.S. Food and Drug Administration Center for Tobacco Products (FDA CTP); and grant P50CA180890 from the National Cancer Institute at the NIH and FDA CTP. Research reported in the cigarette smoke/-vagal nerve study was supported by NHLBI grants R01HL120062 and U54HL147127 and FDA CTP and grant P50CA180890 from the National Cancer Institute at the NIH and FDA CTP. For additional funding details, please see the full journal articles.
About the National Heart, Lung, and Blood Institute (NHLBI): NHLBI is the global leader in conducting and supporting research in heart, lung, and blood diseases and sleep disorders that advances scientific knowledge, improves public health, and saves lives. For more information, visit www.nhlbi.nih.gov .
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov .
NIH…Turning Discovery Into Health ®
Chronic e-cigarette use impairs endothelial function on the physiological and cellular levels. Arteriosclerosis, Thrombosis, and Vascular Biology. DOI: 10.1161/ATVBAHA.121.317749
Impairment of Endothelial Function by Cigarette Smoke is not Caused by a Specific Smoke Constituent, but by Vagal Input from the Airway. Arteriosclerosis, Thrombosis, and Vascular Biology. DOI: 10.1161/ATVBAHA.122.318051
Connect with Us
- More Social Media from NIH
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

The Vaping and Patterns of e-Cigarette Use Research Study: Protocol for a Web-Based Cohort Study
Jeffrey j hardesty , mph, elizabeth crespi , mph, qinghua nian , phd, joshua k sinamo , bsc, alison b breland , phd, thomas eissenberg , phd, kevin welding , phd, ryan david kennedy , phd, joanna e cohen , phd.
- Author information
- Article notes
- Copyright and License information
Corresponding Author: Jeffrey J Hardesty [email protected]
Corresponding author.
Received 2022 May 6; Revision requested 2022 Oct 4; Revised 2022 Dec 13; Accepted 2023 Jan 18; Collection date 2023.
This is an open-access article distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in JMIR Research Protocols, is properly cited. The complete bibliographic information, a link to the original publication on https://www.researchprotocols.org , as well as this copyright and license information must be included.
In total, 3.2% of American adults report using e-cigarettes every day or some days. The Vaping and Patterns of E-cigarette Use Research (VAPER) Study is a web-based longitudinal survey designed to observe patterns in device and liquid use that suggest the benefits and unintended consequences of potential e-cigarette regulations. The heterogeneity of the e-cigarette devices and liquids on the market, the customizability of the devices and liquids, and the lack of standardized reporting requirements result in unique measurement challenges. Furthermore, bots and survey takers who submit falsified responses are threats to data integrity that require mitigation strategies.
This paper aims to describe the protocols for 3 waves of the VAPER Study and discuss recruitment and data processing experiences and lessons learned, including the benefits and limitations of bot- and fraudulent survey taker–related strategies.
American adults (aged ≥21 years) who use e-cigarettes ≥5 days per week are recruited from up to 404 Craigslist catchment areas covering all 50 states. The questionnaire measures and skip logic are designed to accommodate marketplace heterogeneity and user customization (eg, different skip logic pathways for different device types and customizations). To reduce reliance on self-report data, we also require participants to submit a photo of their device. All data are collected using REDCap (Research Electronic Data Capture; Vanderbilt University). Incentives are US $10 Amazon gift codes delivered by mail to new participants and electronically to returning participants. Those lost to follow-up are replaced. Several strategies are applied to maximize the odds that participants who receive incentives are not bots and are likely to possess an e-cigarette (eg, required identity check and photo of a device).
In total, 3 waves of data were collected between 2020 and 2021 (wave 1: n=1209; wave 2: n=1218; wave 3: n=1254). Retention from waves 1 to 2 was 51.94% (628/1209), and 37.55% (454/1209) of the wave 1 sample completed all 3 waves. These data were mostly generalizable to daily e-cigarette users in the United States, and poststratification weights were generated for future analyses. Our data offer a detailed examination of users’ device features and specifications, liquid characteristics, and key behaviors, which can provide more insights into the benefits and unintended consequences of potential regulations.
Conclusions
Relative to existing e-cigarette cohort studies, this study methodology has some advantages, including efficient recruitment of a lower-prevalence population and collection of detailed data relevant to tobacco regulatory science (eg, device wattage). The web-based nature of the study requires several bot- and fraudulent survey taker–related risk-mitigation strategies, which can be time-intensive. When these risks are addressed, web-based cohort studies can be successful. We will continue to explore methods for maximizing recruitment efficiency, data quality, and participant retention in subsequent waves.
International Registered Report Identifier (IRRID)
DERR1-10.2196/38732
Keywords: internet, web-based, cohort, survey, e-cigarettes, electronic nicotine delivery systems, ENDS, tobacco, recruitment, data collection, strategies, lessons learned, mobile phone
Introduction
In the United States, 3.2% of adults, 7.6% of young adults [ 1 ], and 11.3% of high school students [ 2 ] use e-cigarettes (every day or some days for adults and use in the past 30 days for high school students), and the US Food and Drug Administration Center for Tobacco Products is interested in data-driven regulations that maximize public health benefits while minimizing unintended consequences [ 3 ].
Our team is conducting a web-based longitudinal survey to observe patterns that suggest the benefits and unintended consequences of three potential e-cigarette regulations: (1) limits on nicotine, (2) constraints on nicotine flux (ie, nicotine emitted over time) [ 4 ], and (3) reduction in flavor availability. Electronic nicotine delivery systems (ENDS) are e-cigarettes that heat a nicotine liquid into an aerosol that can be inhaled by a user, whereas electronic nonnicotine delivery systems (ENNDS) heat a nonnicotine-containing liquid (ENNDS are included in the study as ENDS users may become ENNDS users and vice versa). Both include a battery that powers a heating element, such as a metal coil, which is in contact with the liquid. Device size, shape, materials, features (eg, coil modifiability), and specifications (eg, battery voltage, coil resistance, and device wattage) vary considerably. The liquids are typically made of a propylene glycol and vegetable glycerin solution that contains flavorings and nicotine, a psychoactive and addictive drug [ 5 ].
Unlike cigarettes, which are all relatively similar in design, the heterogeneity of ENDS and ENNDS devices, settings, and liquid characteristics results in a highly customizable user experience. This heterogeneity and a lack of standardized reporting requirements for device specifications and liquid characteristics can lead to measurement challenges. For example, some manufacturers report liquid nicotine concentration as a percentage of the liquid solution, whereas others report it in milligrams per milliliter. These inconsistencies may partially explain why reporting liquid nicotine concentration accurately in surveys is challenging for some users [ 6 , 7 ]. In addition, liquid manufacturers sometimes inaccurately label nicotine concentration [ 8 - 10 ], and device manufacturers often do not publicize specifications such as device wattage, voltage, and resistance, thus creating additional challenges for researchers even after collecting the brand and model of a user’s device. These data are critical for evaluating nicotine and toxicant emissions and delivery. For example, device wattage is a predictor of nicotine emissions and delivery to the blood [ 11 , 12 ].
Web-based survey methods also present challenges that must be addressed in both a preventive and ad hoc manner; such challenges include recruitment of lower-prevalence populations and navigating bots [ 13 ] and fraudulent survey takers [ 14 ], who primarily aim to deceive researchers for the purpose of receiving incentives. Nevertheless, web-based survey methods are an increasingly used avenue to recruit participants, collect data, and provide incentives across public health research domains, including ENDS and ENNDS research, and they may have several benefits over traditional methods, such as convenience for participants and researchers, scalability, reduced costs, and safety during extraordinary times such as the COVID-19 pandemic.
Studies that address these measurement and data integrity (eg, bots) challenges can offer a more detailed examination of frequent ENDS and ENNDS users’ device features and specifications, liquid characteristics, and user behaviors while simultaneously benefiting from improved convenience, scalability, reduced costs, and safety. Moreover, through an improved understanding of the relationships among device type, features, specifications, liquid characteristics, and key user behaviors such as nicotine dependence, regulators may gain a more precise understanding of how regulating devices and liquids may positively or negatively affect users before implementing a regulation.
Our aim is to describe the protocols for waves 1 to 3 of the Vaping and Patterns of E-cigarette Use Research (VAPER) Study, a web-based longitudinal cohort study of ENDS and ENNDS users (aged ≥21 years) who use devices ≥5 days per week and have a residential address in the United States. We also discuss our recruitment and data processing experiences and lessons learned, along with the benefits and limitations of implementing our strategies for mitigating measurement-, bot-, and fraudulent survey taker–related challenges.
The protocols for waves 1 to 3 are similar. Key differences will be addressed; where none are discussed, similar protocols were followed in all waves and will also be followed in future waves. Additional technical details can be found in Multimedia Appendix 1 . All data are collected using REDCap (Research Electronic Data Capture; Vanderbilt University), a free, secure, and robust data collection platform.
The VAPER Study is a cohort study conducted on the web, including participant recruitment, data collection, and incentive delivery to participants. A self-selection sampling method is used. Recruitment-related information for the baseline survey is posted on Craigslist Jobs and Gigs boards and directs potential participants to click a hyperlink to a study-specific landing page with a welcome message hosted on the Virginia Commonwealth University website (the web page is not accessible through other avenues unless the hyperlink is shared by participants). After providing informed consent, participants complete a registration form requesting the following information: name, email address, mobile phone number, residential address, and date of birth. Participants then review the information they provided and complete a phone number authentication that contains a unique link to the REDCap screener and survey. Before starting the survey, all participants are reminded of actions that can result in their disqualification (also present in the consent form). Upon survey completion, identity verification, and review of submitted data, participants are mailed a US $10 Amazon gift code ( Figure 1 ).
Flow diagram of baseline survey participants from wave 3.
Participants who submit valid baseline surveys and indicate an interest in participating in additional surveys are invited to complete a follow-up survey in subsequent waves. Invitations are sent to their mobile phones and email addresses, and the links to the survey are tied to their previously established record ID number. Returning participants are greeted with a welcome back message and, before completing the screener, are provided with the opportunity to review the consent form again, are notified that they will receive their gift codes electronically, are asked to review and update their contact information (if necessary), and are again reminded of actions that can result in their disqualification ( Figure 2 ).

Flow diagram of follow-up survey participants from wave 3.
To replace those lost to follow-up, new participants are recruited to complete the baseline survey, resulting in 2 concurrent REDCap surveys per wave: one for baseline participants and another for follow-up participants. Therefore, a new baseline cohort is created in each wave, with all previously established cohorts taking a concurrent and identical follow-up survey ( Table 1 ). The measures in the questionnaire evolve as changes in the marketplace are recognized, as new information is learned about the quality of the questions and response options, and as the VAPER Study team fields requests from the funders and colleagues working on related laboratory-based projects. This evolution creates a layer of complexity when analyzing data longitudinally but will not affect cross-sectional analyses. For longitudinal data analyses, steps are taken to ensure that the underlying questions and response options are comparable. More specifically, a data workbook is used to track variable names for each wave and survey (ie, baseline and follow-up surveys); whether edits have been made to the question or response option text; and if “yes,” what those edits were. Manuscript authors can then determine whether their measures can be used across waves in consultation with our statistician and wider team.
Surveys taken in each wave, by cohort.
a N/A: not applicable.
Participants are ENDS and ENNDS users who typically use e-cigarettes at least 5 days per week, are aged ≥21 years, and have a residential address in the United States. Adults are recruited for several reasons: (1) more adults (10.9 million) [ 15 ] than youth (2.2 million) [ 16 ] use ENDS and ENNDS; (2) school-based recruitment methodologies are slower, more expensive, and less scalable; and (3) most of our hypothesized relationships among devices, liquids, and user behaviors are expected to be present irrespective of age (eg, a negative correlation between device power and liquid nicotine concentration). The age of 21 years was chosen over 18 years because of institutional review board (IRB) guidance and state and federal legislation that raised the minimum age of purchase to 21 years. Users who use “At least five days per week” are recruited as we are primarily interested in within-person longitudinal data. Our rationale is that frequent ENDS and ENNDS users are more likely to continue using and remain in the sample compared with users using <5 days per week, thereby increasing the quality of the longitudinal data.
The intended sample size (N=900) was determined by assuming an effect size of 10% (for t tests to detect differences between 2 dependent means using a 2-tailed test), Cronbach α<.05, and power of 0.85. After adjusting for an anticipated loss to follow-up rate of 25%, we determined that a baseline sample of 1200 participants was required to ensure adequate power for the study duration.
Recruitment
Craigslist is used to recruit baseline survey participants. It has a high volume of website traffic, a user base interested in earning income, affordable rates for posting messages targeted to potential participants in the United States, and a track record of success in helping tobacco control [ 17 ] and other public health researchers [ 18 ] recruit participants. Craigslist postings include a photo and text indicating that we are recruiting ENDS and ENNDS users and will compensate participants with a US $10 Amazon gift code ( Figure 3 ). Note that social media (ie, Facebook and Instagram) and vape shop customer recruitment were attempted early in wave 1 but, because of cost and efficiency reasons, were replaced by a Craigslist-focused strategy. Craigslist was used to recruit most of our wave 1 sample and to replace all participants lost to follow-up in waves 2 and 3.
An example Craigslist posting from wave 3.
The Craigslist postings are posted on the Jobs and Gigs boards in as many as 404 geographic locations per wave, including all 50 US states ( Multimedia Appendix 1 ). Both boards were used in most geographic locations, but sometimes only 1 board was used. Geographic locations were selected based on population estimates of major US cities and states, with preference given to the most populous catchment areas (note that Craigslist regions can cover a single city, regions, or large geographic areas spanning entire states). To optimize recruitment efficiency, Craigslist postings are reposted at varying time intervals primarily based on the number of competing advertisers displacing our postings to a lower position on the page (eg, New York City boards need to be reposted 2 times per week, whereas others are reposted as infrequently as once per month). Other metrics are tracked and considered as well, including the fluctuating volume of clicks from different locations over the course of a survey wave (measured in real time using Google Analytics) and the number of participants completing the survey from each location (higher-yielding locations tend to be reposted more frequently).
Communication Strategy for Maximal Retention
To invite participants who previously submitted valid surveys to complete additional survey waves, our team sends 2 pairs of emails and SMS text messages per week for 2 weeks at the start of each wave. Afterward, 1 pair of emails and SMS text messages is sent per week for 6 weeks to all participants who have not yet completed the survey. The subject lines and message content of the reminders vary from week to week to attempt to appeal to different audiences. Note that, at the start of wave 2, tests were conducted to select the most effective communication content (eg, varied email subject lines) and frequency of communications (ie, 1 vs 2 pairs of emails and SMS text messages sent per week for 2 weeks, with each pair sent simultaneously). The tests were conducted using a small subset of the wave 1 sample (n=148), and we found no statistically significant differences in valid survey completion rates or the rates of opting out of SMS text messages, and the absolute number of SMS text message opt-outs was minimal (n=5).
Engagement Strategy
Loss to follow-up is common in longitudinal studies. To increase engagement and minimize loss to follow-up, our participants are sent an annual postcard indicating that they are eligible for a raffle. The annual raffle has 4 winners, with each receiving a US $100 Amazon gift code. Completing 2 surveys will earn participants 10 chances to win the raffle, and completing 3 surveys will earn them 50 chances.
Measures in the questionnaire are from or derived from those found in the PhenX Toolkit (RTI International; a web-based catalog of high-priority measures), validated measures, or measures used in large national surveys whenever possible and appropriate. We developed measures in all other instances. Randomization of questions and response options is not used; however, adaptive questioning to reduce the number and complexity of the questions is used (eg, questions are tailored based on self-reported device features). The number of questions per page is limited to 1 whenever possible; however, it is necessary to have more than 1 question per page when participants are asked to further specify information or in instances where the questions are easier to respond to in sequence (eg, grid-style questions). The number of pages varies widely based on the devices and liquids used and related behaviors. To ensure completeness, all participants must provide a response to continue to the next page (an error message is received when participants attempt to continue without completing all the questions). To mitigate data integrity issues, a back button is not used.
The usability and technical functionality of the questionnaire are rigorously tested by the study team before fielding the questionnaire in each wave. All permutations of skip logic and response option constraints are tested, and before the first wave, mock participants not familiar with the study completed the questionnaire and provided feedback on wording of the prompts, questions, response options, functionality of specific features (eg, photo upload), and overall user experience. Although these actions greatly mitigate technical issues and improve user experience, participants who complete the study during each wave often provide comments and suggestions that are incorporated as well.
To discern the impacts of potential regulations, outcome measures include current ENDS and ENNDS use (≥5 days per week), current combustible cigarette use (past 30 days), product switching (eg, devices, device settings, and liquid nicotine concentration and flavor), do-it-yourself flavor mixing (ie, mixing their own flavored solution typically by mixing a combination of propylene glycol, vegetable glycerin, nicotine, and flavorings), ENDS dependence, and respiratory symptoms. Psychosocial mediators include quitting intentions, perceived risk and severity, and outcome expectancies related to use experience. Moderators include sociodemographic factors, tobacco cigarette history, ENDS and ENNDS history, and reasons for use. Full details for select measures are available in the following sections; the full questionnaire is available on our study website [ 19 ].
ENDS and ENNDS use was assessed using the following question: “How many days in a typical week do you use an e-cigarette or vaping device to vape e-liquids with or without nicotine?” Response options included “I do not use an e-cigarette or vaping device to vape e-liquids with or without nicotine in a typical week,” “1 day,” “2 days,” “3 days,” “4 days,” “5 days,” “6 days,” and “7 days.”
Dependence is measured using the E-cigarette Dependence Scale. Participants receive the following prompt: “The following questions are about your E-CIGARETTE use only. Please respond to each question or statement by marking the most appropriate response.” The statements include “I find myself reaching for my e-cigarette without thinking about it,” “I drop everything to go out and buy e-cigarettes or e-juice,” “I vape more before going into a situation where vaping is not allowed,” and “When I haven’t been able to vape for a few hours, the craving gets intolerable.” Response options include “Almost Always,” “Often,” “Sometimes,” “Rarely,” and “Never.”
Quitting intentions are assessed using the following questions: “Are you planning to quit vaping” and “Are you planning to quit smoking cigarettes.” Response options include “Within the next month,” “Between 1-6 months from now,” “Sometime in the future, beyond 6 months,” and “Not planning to quit.” For cigarettes, only those who indicate that they have smoked ≥100 cigarettes in their lifetime and a cigarette in the past 30 days are asked the question.
The questions and skip logic are designed to accommodate marketplace heterogeneity and user customization. For example, there are different skip logic pathways for participants with disposable devices; disposable pod– or cartridge-based devices; and refillable pod–, cartridge-, or tank-based devices. These pathways allow our team to tailor the questionnaire to each participant’s situation and experiences with the expectation of creating a better user experience for the participants, higher retention, and higher-quality data. Another byproduct of this approach is that it allows for inquiry about the devices, liquids, and related behaviors that do not apply to all participants, such as the addition of extra nicotine to one’s liquid (often called “nicotine boosters” or “nicotine shots”). Such behavior would only be applicable to participants who refill their device from a bottle of liquid. Please note that these device and liquid questions are not validated.
Device and Liquid Data Collection
Previous studies have suggested that self-report data alone may not be a viable strategy for capturing accurate device and liquid data [ 6 , 7 ]. To minimize reliance on these data, we require participants to submit valid photos of their most commonly used device, the current visual display screen (powered on) if available, and the most commonly used liquid for the device if available. Following a standard operating procedure, submitted photos are reviewed to identify the brand and model of the device. When the brand or model are not immediately apparent or found with the aid of a Google search, we use unique features, colors, and text on the device to conduct a Google image search. Upon identifying the brand and model, key variables are collected from manufacturer, academic, retailer, and review websites. YouTube product reviews are also helpful in understanding whether certain features are present when information is not readily available on the aforementioned websites (eg, adjustable airflow). To mitigate issues related to inconsistent reporting of device features and specifications and liquid characteristics across websites, data are collected preferentially from (1) manufacturer sites, (2) academic manuscripts, (3) retailer sites, (4) review sites, and (5) YouTube. Key variables collected for devices include wattage, voltage, resistance, coil modifiability, power modifiability, airflow adjustability, disposable versus reusable, and pod- or cartridge-based device versus tank-based device. Liquid variables include brand, flavor (primary and secondary), container size (mL), nicotine concentration (mg/mL), nicotine formulation (free base vs protonated), propylene glycol percentage, and vegetable glycerin percentage.
Missing Data
Missing data is a multifaceted issue. Participants may self-report not knowing the details of the products used and their related settings and specifications. Photos are also vulnerable to user error, with some participants not following instructions (eg, blurry images), resulting in unidentifiable device brands and models, visual display settings, and liquid characteristics. In addition, some web-based sources do not report all device features, specifications, and liquid characteristics.
We use several preventive strategies to mitigate these issues. First, by collecting device and liquid data via self-report and from photos and web-based sources and creating a combined variable that prioritizes photo and web-based data, we minimize missing data and reliance on participant expertise on the product details. Furthermore, participants are required to answer each question to advance and complete the questionnaire, resulting in minimal missing data for all questions without “Don’t know” and “Prefer not to answer” response options (responses with identifying information were removed). Finally, for the collection of data from photo and web-based sources, participants are provided with instructions for submitting photos, and a comprehensive standard operating procedure is adhered to by the team to ensure that web-based data are reliably collected.
Despite these preventive efforts, missing data can still occur; therefore, we implement 3 additional post hoc strategies specific to device wattage, voltage, and coil resistance. The first strategy is to purchase the most prevalent devices in the sample with missing data for 2 or more of these device specifications, disassemble the devices, and directly measure the voltage and resistance using a multimeter when one or both values are missing. The second strategy is to use a power calculator (ie, a mathematical formula for calculating wattage, voltage, or resistance) when a single specification is missing. The third strategy is used when three conditions are met: (1) the device’s range (eg, 0-80 W) is known, (2) the participant’s current setting within the range is unknown (eg, the picture is blurry or there is no visual display), and (3) the setting is not self-reported by the participant. Under these conditions, we estimate the participant’s setting by calculating the average midpoint for the device type (eg, the average refillable tank user used 35% of the allowable wattage range in wave 3). The average midpoint (eg, 35%) is then applied to the specific device’s known range.
Data Integrity and Security
Rigorous measures are taken to prevent bots and fraudulent survey takers from subverting data integrity. All participants who register and complete the baseline survey provide identifying data and agree to participate in identity verification procedures. LexisNexis (RELX corporation), a third-party identity search engine, is used to verify identities before providing incentives. In instances where the LexisNexis database is not sufficient to identify participants, they are asked to provide a photo ID or utility bill that contains information confirming their identity.
Bot protections include a Completely Automated Public Turing test to tell Computers and Humans Apart (CAPTCHA), authentication of a mobile phone number, attention-checking questions, disabling the back button, and manual review of open-ended responses. The CAPTCHA is a well-known test that requests users to perform simple tasks that humans can accomplish easily but unsophisticated bots cannot. Authentication is used to confirm that phone numbers are real and to generate a new randomly generated survey link. By having 2 REDCap forms linked in this way, bots cannot easily generate new identities and immediately proceed to take the survey. Attention-checking questions not only help us identify participants quickly moving through the survey but also help identify unsophisticated bots who may be providing random answers to the questions. The back button is disabled to prevent participants who build bots from more easily learning the questionnaire and skip logic, thus making it more time-intensive to build a sophisticated bot capable of providing answers that closely mimic real users. The manual review of open-ended responses is particularly important as bots often use similar responses across multiple survey submissions. By screening the new survey submissions on a weekly basis for repeated phrases (particularly uncommon phrases), spelling, and formatting errors, we can more easily identify problematic cases and adjust the strategy as needed. However, the most sophisticated bots may be capable of circumventing these procedures and may be indistinguishable from real users. These strategies are meant to mitigate the chances of having bot-related issues.
Fraudulent survey takers are participants who are savvy enough to enroll in and complete surveys for which they are not qualified. Similar to bots, they may also attempt to take a survey multiple times. By using identity checks, the risk that participants will take the survey multiple times is lower (note that they can use another person’s identity); however, they may not be truthful about their e-cigarette use behavior. To further mitigate the risk of enrolling fraudulent survey takers, we use clear warnings against misuse, require participants to submit a photo of their most used device from the past week, review each photo to verify that it is valid (ie, instructions were followed), and mail incentives. Our warning statement is placed in the consent form and at the start of the surveys: “As a reminder, any perceived attempt to speed through the survey, take the survey more than once, or provide false or misleading information will result in your disqualification from the survey and forfeiture of any promised incentives.” This provides us with maximum flexibility for determining who should not receive an incentive and be included in our final sample. Requiring that participants submit a photo of their most used device from the past week is essential to ensuring that they are in possession of a device and, thus, more likely to be ENDS or ENNDS users. Photo submissions of their most commonly used devices from the past week are rigorously reviewed for the following evidence: the objects featured in the photo are not ENDS or ENNDS devices, the photos are downloaded from the internet, the photos are staged in a store, the photos are identical or nearly identical to submissions by other unique IDs, and the photo-coded brand and model do not match the self-reported brand and model for 3 devices that were provided as examples in the self-report brand and model question; that is, “What is the brand AND model of the device (e.g., JUUL, Vaporesso Luxe, Voopoo Drag 2, etc.)?”
Other rigorous data quality checks are also performed before participants receive their incentives. The checks include identifying the use of non-English or non-Spanish alphabet characters in open-ended responses; verifying that a proper mailing address has been provided; confirming that participants do not submit more than 1 survey within or across survey waves; and verifying that more than the minimal number of questions have been answered (skip logic is such that this is highly improbable), completion time is >5 minutes, and 2 attention-checking questions are answered appropriately (both require correct responses).
Data security is ensured through the use of REDCap and Twilio. REDCap is a secure web application that is used to build web-based surveys and databases. It collects any type of data and is geared toward supporting data capture on a server for category-1 data (ie, confidential data). Access to files with identifying information is restricted to approved research team members, all of whom are trained in standards of research privacy and confidentiality and who have secure passwords required to sign onto the data server. When off-campus, virtual private network services are used to access the server. Twilio is a web service used to send a private survey link to respondents. Participants’ phone numbers do not remain in Twilio’s logs but are removed shortly after being completed, which is done for security and privacy concerns.
The incentive for completing the 15-minute survey is a US $10 Amazon gift code. Once submissions are determined to be preliminarily valid after the initial data quality review and participants’ identities are confirmed, incentives are mailed to the physical address provided by baseline survey participants and emailed to follow-up survey participants. The data quality check and identity verification are typically completed within 3 days of submission, and incentives are mailed on a weekly basis. Baseline survey participants are mailed their incentives as a form of delayed gratification to deter them from attempting to take the survey multiple times. Post office box addresses and other nonresidential mailboxes are not accepted to prevent multiple submissions. Occasionally, incentives are returned to the sender, indicating that a false address was provided, the participant moved, or a typo was present in the provided address. These record IDs are reviewed more closely for other data quality issues (eg, responses indicating that they were likely not ENDS or ENNDS users). If other survey data are found to be of low quality, the corresponding participants are dropped from the data set and not invited to future waves but are still eligible to receive their incentive for the wave in question. To facilitate the emailing of gift codes to follow-up participants, Rybbon (BHN Rewards), a digital gift code delivery service, is used.
Final Data Quality Checks
Once data cleaning procedures are completed after each survey wave, additional data quality checks are completed when preliminarily valid records are flagged. These include instances of photos with multiple devices or liquids present, poor photo quality, photos or survey responses with non–propylene glycol and vegetable glycerin solutions (eg, tetrahydrocannabinol or cannabidiol), age of first use occurring before ENDS and ENNDS were commercially available in the United States (baseline only), select examples of REDCap skip logic not working as intended (investigated by REDCap; the software bug remains unknown), and incentives being returned to the sender (as previously described). When at least one flag is identified, all the survey responses are reviewed for additional evidence of poor data quality. For example, additional evidence may include the self-reported device brand and model not matching the photo brand and model or self-reported liquid flavors not matching their respective photos. A scoring system is used to determine whether the issues found warrant exclusion from the current and future waves of data collection. All excluded participants still receive their incentive for the survey wave in question.
Ethics Approval
The IRB at the Virginia Commonwealth University (HM20015004) approved the study protocol. The Johns Hopkins Bloomberg School of Public Health IRB (9277) approved reliance on the Virginia Commonwealth University IRB.
Recruitment and Retention
Funding for the VAPER Study began on September 1, 2018, and will conclude on August 31, 2023. Data collection for waves 1 to 3 was completed over 3 periods. Wave 1 was completed between May 18, 2020, and October 16, 2020 (n=1209); wave 2 was completed between December 10, 2020, and April 21, 2021 (n=1218); and wave 3 was completed between September 2, 2021, and November 18, 2021 (n=1254). Partially completed questionnaires are not analyzed.
Upon conclusion of wave 2, the retention rate of cohort 1 (baseline survey participants from wave 1; n=1209) was 51.94% (628/1209), and 5.29% (64/1209) of participants opted out of SMS text message reminders. For wave 3, a total of 37.55% (454/1209) of cohort 1 completed the survey, with 33.25% (402/1209) completing the wave 2 and 3 surveys (52/1209, 4.3% completed the survey for wave 3 but not for wave 2). A total of 7.03% (85/1209) of participants opted out of SMS text message reminders. Cohort 2 (baseline survey participants from wave 2; n=590) had a wave 3 retention rate of 44.2% (261/590), and 6.4% (38/590) of participants opted out of the SMS text message reminders.
Generalizability
For each wave, our wave 1 to 3 frequent users were largely generalizable to daily users of e-cigarettes in the United States (1185/1254, 94.5% of our wave 3 sample used ENDS or ENNDS 7 days per week; Table 2 ). There was no statistically significant difference between our wave 3 sample and the weighted 2019 Tobacco Use Supplement to the Current Population Survey data in terms of age/gender/race ( P =.18) and region ( P =.42). Compared with the Tobacco Use Supplement to the Current Population Survey, our wave 3 sample had a higher percentage of frequent ENDS or ENNDS users with an income of US <$60,000 (928/1225, 75.76% vs 804,024/1,537,547, 52.3%; P <.001). Applying poststratification weighting can help improve the representativeness of the data.
A comparison of wave 3 and Tobacco Use Supplement to the Current Population Survey (TUS-CPS) 2019 frequencies for 3 weighting strategies.
a VAPER: Vaping and Patterns of E-cigarette Use Research.
b Missing data: n=21.
c White includes single race White; non-White includes all other single races, including American Indian or Alaska Native, Asian or Asian American, Black or African American, Native Hawaiian or Pacific Islander, other, and multirace.
d Denotes a statistically significant difference between VAPER and TUS-CPS data at P <.001.
e Missing data: n=29.
Poststratification weighting normally requires the sample size of a subgroup to be >20. Thus, for the VAPER Study, creating 1 weight that covers all sociodemographic variables, including gender, age, race, income, and region, is not acceptable as the cell sizes would be under the minimum threshold. Therefore, 3 separate weights are available: a gender, age, and race weight; an income weight; and a geographic region weight. The variables for the gender, age, and race weight are dichotomized as men and women, <35 years and ≥35 years, and White and non-White populations. For the annual income weight, data are dichotomized as US <$60,000 and US ≥$60,000. Finally, for the geographic region weight, data are categorized into “Northeast,” “Midwest,” “South,” and “West.” The specific poststratification weight used in the dissemination of the survey findings will be hypothesis-driven and based on whether the characteristics incorporated into the weight are expected to be correlated highly with the primary outcome of interest.
Planned Analyses
Analyses will be designed to observe patterns that suggest the benefits and unintended consequences of potential regulations. Our data offer a detailed examination of frequent ENDS and ENNDS users’ device features and specifications, liquid characteristics, and behaviors. A better understanding of how devices and liquids relate to one another and may be associated with key behaviors such as nicotine dependence can provide regulators with a more precise understanding of how regulating features, specifications, and characteristics may positively or negatively affect users before implementing a regulation. We intend to examine these relationships for three potential regulations: (1) limits on nicotine, (2) constraints on nicotine flux (ie, nicotine emitted over time), and (3) reduction in flavor availability ( Textbox 1 ).
Hypotheses regarding the 3 potential regulations for electronic nicotine delivery systems (ENDS).
Limits on nicotine
As of May 2016, the European Union limits nicotine concentration in liquids; the US Food and Drug Administration (FDA) may consider a similar action. Hypotheses will be informed by the latest available data at the time of analysis, but a priori, we hypothesize that device wattage will be correlated inversely with nicotine concentration and directly with the amount of liquid consumed and that, across concentration, more dependent ENDS users are using higher-power devices. Over time, users who switch to lower-concentration liquids will begin using higher-power devices. This pattern would highlight how limiting liquid nicotine concentration to control nicotine delivery may fail when higher-wattage devices are available.
Constraints on nicotine flux
Nicotine flux is a function of device specifications and liquid characteristics (ie, power and nicotine concentration) and helps determine user nicotine exposure; it can be predicted mathematically, suggesting that a flux-based product regulatory standard is possible. We will investigate flux standards by studying device wattage, nicotine concentration, and user dependence. Again, hypotheses will be informed by the latest available data at the time, but a priori, we hypothesize that higher flux conditions (eg, greater wattage and nicotine concentration) will be associated with greater dependence, lower flux conditions will be associated with less dependence, and transitions from lower to high flux conditions will be accompanied by higher flux. This pattern highlights nicotine flux as a potential regulatory target.
Reduction in flavor availability
The FDA began prioritizing the enforcement of premarket of ENDS products in 2021 [ 20 , 21 ]. This action has likely decreased the variety of liquid flavors sold. A priori, we hypothesize that this decrease may lead users to change their preferred flavor for their most commonly used device, change their device specifications, or change their behavior to maintain their nicotine delivery. Transition patterns will highlight how reducing flavor availability may affect behavior.
Our primary aims are to evaluate the 3 potential regulations; however, the relative novelty of our methods for collecting detailed device features and specifications and liquid characteristics along with a new practice of analyzing device and liquid pairings rather than analyzing them separately warrants additional supportive and foundational analyses, respectively. As such, our team has identified additional priorities, including but not limited to examining the percentage of agreement between self-reported responses and photo data collection of devices and liquids, mitigating the impact of bots and fraudulent survey takers, and identifying common combinations of device specifications and liquid characteristics (and transitions).
There is no one primary analytic approach; statistical tests will vary based on the research question, measures used, and cross-sectional versus longitudinal nature of the analysis. In general, for longitudinal analyses, generalized estimating equations will be used to account for the variable times between survey waves.
The VAPER Study uses a web-based longitudinal cohort design to observe patterns that suggest the benefits and unintended consequences of 3 potential Food and Drug Administration regulations. A priori, we hypothesize that we will identify relationships among device features and specifications, liquid characteristics, and user behavior. A better understanding of these relationships, particularly longitudinally, may allow regulators to better understand how regulations positively or negatively affect user health.
Most population surveys about ENDS and ENNDS are unable to describe device features and specifications, liquid characteristics, and user behavior in a detailed manner because of measurement-related challenges such as a highly customizable user experience [ 22 ] and a lack of standardized reporting requirements for device features and specifications and liquid characteristics [ 9 ]. Consequently, surveys often oversimplify use. For instance, surveys sometimes presume that a single device is used and request that participants indicate the device type, usually with predefined definitions that may not keep pace with market innovations (particularly salient for longitudinal surveys) [ 23 , 24 ] or terminology used by all participants [ 25 ]. Surveys that use such an approach also ignore the possibility that some users use multiple ENDS and ENNDS in varying amounts and that different device and liquid combinations (within and across device types) may affect nicotine and toxicant emissions and delivery [ 26 ]. Our study demonstrates potential solutions to these measurement challenges through survey questions that allow participants to describe their most commonly used device and most commonly used liquid for that device through the use of adaptive questions. In addition, we require valid photos of their most commonly used device and request photos of their most commonly used liquids for that device, thereby allowing our team to determine the device type, features, and specifications and liquid characteristics based on the photo and related website coded data independent of self-reported responses.
Web-based surveys have become increasingly common; however, reporting of contemporary methodologies to maximize data integrity and mitigate the impact of bots and fraudulent survey takers and best practices for de novo recruitment is lacking. Moreover, technology to evade basic survey protections has evolved since the Checklist for Reporting Results of Internet E-Surveys was developed in 2004, and the use of these technologies has become more common. For example, verifying that a sample does not contain duplicative IP addresses and use of “cookies” can be overcome through the use of virtual private network service providers that allow users easy access to hundreds of servers worldwide and the clearing of cookies (or use of another device), respectively. Our survey was able to address these challenges using a variety of strategies, such as identity verification.
Recruitment modalities beyond de novo recruitment were explored and given consideration for this study, including the use of existing panels. Although appropriate for other study designs and aims, existing panels were not considered a viable option for the VAPER Study. Existing panels at well-established research firms did not contain a sample large enough to recruit our population of interest (ie, ENDS and ENNDS users vaping ≥5 days per week). Mechanical Turk, a commonly used web-based panel, was considered as well but was also not large enough for our lower-prevalence population. Panels that aggregate participants from multiple panels are an option that might have yielded a large enough sample for wave 1; however, the recruitment methodologies are highly heterogeneous. In addition, they may not be large enough to replace those lost to follow-up in multiple waves. We were also advised by a company offering this aggregation service that high loss to follow-up rates should be expected; thus, panels that aggregate participants are not ideal for longitudinal studies.
Social media (eg, Facebook and Instagram) recruitment was attempted in consultation with a market research firm at the start of wave 1, but several challenges were encountered. Despite being an academic survey on ENDS and ENNDS use, our advertisements were repeatedly deleted by Facebook and Instagram for including images and text related to ENDS and ENNDS. Appealing these decisions became a regular and time-consuming phenomenon that was never resolved. Furthermore, the advertisements appeared to generate clicks, presumably by social media users interested in the survey; however, the number of advertisement clicks did not match the landing page traffic, and few valid surveys were submitted. Our approach to tracking landing page traffic was investigated for setup errors, and alternative back-end solutions were attempted, but no strategy improved our traffic. The high costs (US $280 per valid participant) combined with slow recruitment (approximately 5 valid participants per week) led us to switch to a Craigslist-focused recruitment strategy. Subsequently, wave 3 costs have decreased to US $10 per valid participant, and recruitment for the baseline survey has increased to approximately 53 valid participants per week.
Processing data and managing missing data are not without challenges. Coding photos and reviewing and abstracting manufacturer, retailer, and review site data are time-intensive and have practical limitations, including participants who do not follow photo submission instructions and inconsistent and incomplete device features and specifications available on the web. The resulting missing data present challenges, particularly given a lack of validated device and liquid questions and that some participants indicate that they do not know the details of their device features and specifications, settings, and liquid characteristics. Our solution is to implement comprehensive preventive and post hoc strategies that maximize the use of multiple data sources (ie, photos, self-report, and disassembled devices), tools (ie, multimeter), and formulas (ie, power calculator and an average midpoint calculation for each device type). The decisions were considered carefully based on the best available information; however, we cannot rule out the possibility that our underlying data sources, tools, or formulas are biased or inaccurate (eg, product packaging and labeling and manufacturer, academic, retailer, and review websites). Ultimately, we believe that the benefits of our approach outweigh the unknowns and are an opportunity to understand more deeply the interplay between devices, liquids, and user behavior as it pertains to regulations. Other strategies could be valuable, and our approach may change as better data sources emerge and more is learned about this topic.
The loss to follow-up rate is higher than initially presumed based on the expected loss to follow-up rates in more traditional cohort surveys. This presumption based on traditional cohort surveys was made after we were unable to find any web-based cohort surveys that reported their loss to follow-up rates during study design planning in 2019. The higher-than-expected rate is not readily explainable and is a matter of speculation. Our assumption is that it is owing to one or more of the following: (1) web-based cohort survey participants require larger incentives, (2) participants (and email providers) may believe that follow-up survey email and SMS text message invitations and reminders are spam, (3) participants may self-select out of the cohort survey if they no longer use e-cigarettes, and (4) participant commitment to web-based cohort surveys is lower given the lack of an in-person connection with the study team. We will attempt to lower the rate through several strategies. These include increasing the incentive to US $30 per participant, sending postcards to participants to remind them of their involvement with the study and our annual raffle, creating a password-protected study website for participants that contains announcements and descriptive data for participants to review, and allowing follow-up participants who no longer use the devices ≥5 days per week to complete the survey or a shorter survey if they have not used the devices in the past 30 days.
Furthermore, our team is committed to improving the survey with each successive data collection wave. We will continue to monitor and adjust the details of our Craigslist strategy, such as the number of cities and boards used, as we learn more about the recruitment and cost-efficiency of “jobs” versus “gigs” postings and specific locations. We are also considering alterations to the raffles based on the frequency of waves completed and participant follow-up rates and are exploring additional engagement strategies to increase retention rates. Other options include birthday and holiday messages, a website containing updates on findings and survey-related announcements, and alternative incentive structures for follow-up survey participants. Moreover, participants are encouraged to provide us with feedback at the end of the survey: “We would like to continually improve this survey. If you have any comments or suggestions about this survey, please provide them here.” Feedback is reviewed during each wave for isolated issues (eg, survey response corrections) and at the end of the survey wave for consistent feedback that may warrant improvements to the survey. For example, participants noted that our survey responses did not include options for wrapping one’s own device coils and adding extra nicotine drops to liquids. We subsequently edited the response options and added a question to account for these behaviors.
Another key lesson learned was the importance of bot and fraudulent survey taker mitigation strategies. These comprehensive steps were taken in consultation with experts after an initial attempt in 2019 to recruit participants using social media advertisements failed. At the time, the VAPER Study allowed for anonymous survey participants, had minimal review of data before incentive delivery by email, and used fraud detection software intended to prevent multiple completions by each participant. Initially, recruitment began slowly (as expected) but accelerated quickly, raising substantial concerns that led us to halt data collection. Survey submissions (n=1624) were investigated for evidence of bots and fraudulent survey takers, and only 22.35% (363/1624) of the survey completions were assessed to be likely valid. We subsequently restarted recruitment in May 2020 (wave 1) and implemented the aforementioned risk-mitigation strategy. As a result of these steps and the transition to a Craigslist posting strategy, the recruitment pace stabilized, and data quality has appeared adequate. The final wave 1 sample does not include those from our failed attempt at recruitment and data collection in 2019.
The VAPER Study has demonstrated that web-based recruitment and data collection for cohort studies is a promising approach that may offer benefits to researchers and participants, including convenience, scalability, reduced costs, and safety during extraordinary times such as the COVID-19 pandemic. More specifically, this study design has allowed us to recruit a generalizable and nationwide sample relatively quickly and cheaply, recruit a lower-prevalence population successfully, and disseminate high-level findings to regulators quickly. To the best of our knowledge, no other tobacco policy research team is collecting such detailed data on the scale at which we are operating, which differentiates the value of these data from those of other studies. These data better position us to address questions about the relationships among devices, liquids, and user behavior that relate to the benefits and unintended consequences of possible regulations. However, strong risk-mitigation strategies are essential to ensure data quality [ 13 , 14 ], and steps such as identity verification and manual review of photo data quality before sending the incentive are time-intensive and have required a team of individuals at 2 universities. Other limitations include a limited sample size for specific questions (because of skip logic and rare behaviors), missing data resulting from inconsistent and incomplete device and liquid data available on the web, website-reported data on devices and liquids that may or may not correlate with laboratory-measured device specifications (eg, coil resistance) or liquid characteristics (eg, nicotine concentration), and participants unable to recall or who may be misinformed about their device features and specifications or liquid characteristics. Future plans include further optimizing our recruitment and data processing procedures and conducting 2 waves of data collection over the next 12-month period.
Acknowledgments
The research reported in this publication was supported by the National Institute on Drug Abuse and the Food and Drug Administration Center for Tobacco Products under award U54DA036105. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
Abbreviations
Completely Automated Public Turing test to tell Computers and Humans Apart
electronic nicotine delivery systems
electronic nonnicotine delivery systems
institutional review board
Research Electronic Data Capture
Vaping and Patterns of E-cigarette Use Research
Craigslist posting locations and frequencies for waves 1 to 3.
Data Availability
We intend to make deidentified data, codebooks, documentation, and research protocols available on a password-protected website by 2026 in accordance with the National Institutes of Health policy (notice: NOT-OD-03-032) released on February 26, 2003 [ 27 ], and IRB-approved protocols. Before or after the data are available on the aforementioned website, any investigator interested in collaboration or in using the data for their own work is invited to contact the corresponding author. They will be asked to submit a 1-page abstract of their proposed research, including purpose, analytical plan, and dissemination plans. An executive leadership committee will review these proposals and decide on each based on the individual merits (if a proposal is received after the end of the funding period, the principal investigator will review it). Review criteria and prioritization of projects include the potential of the proposed work to advance public health; qualifications of the applicant; potential for publication; potential for future funding; and enhancement of the scientific, geographic, and demographic diversity of the Vaping and Patterns of E-cigarette Use Research Study research portfolio.
Conflicts of Interest: TE is a paid consultant in litigation against the tobacco industry and also the electronic cigarette industry and is named on a patent for a device that measures the puffing behavior of electronic cigarette users, on another patent application for a smartphone app that determines electronic cigarette device and liquid characteristics, and on a third patent application for a smoking cessation intervention.
- 1. Creamer MR, Wang TW, Babb S, Cullen KA, Day H, Willis G, Jamal A, Neff L. Tobacco product use and cessation indicators among adults - United States, 2018. MMWR Morb Mortal Wkly Rep. 2019 Nov 15;68(45):1013–9. doi: 10.15585/mmwr.mm6845a2. doi: 10.15585/mmwr.mm6845a2. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Park-Lee E, Ren C, Sawdey MD, Gentzke AS, Cornelius M, Jamal A, Cullen KA. Notes from the field: e-cigarette use among middle and high school students - National Youth Tobacco Survey, United States, 2021. MMWR Morb Mortal Wkly Rep. 2021 Oct 01;70(39):1387–9. doi: 10.15585/mmwr.mm7039a4. doi: 10.15585/mmwr.mm7039a4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Research Priorities. U.S. Food and Drug Administration. 2019. [2022-12-13]. https://www.fda.gov/tobacco-products/research/research-priorities .
- 4. Eissenberg T, Shihadeh A. Nicotine flux: a potentially important tool for regulating electronic cigarettes. Nicotine Tob Res. 2015 Feb;17(2):165–7. doi: 10.1093/ntr/ntu208. https://europepmc.org/abstract/MED/25332456 .ntu208 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Domino EF. Nicotine: a unique psychoactive drug--arousal with skeletal muscle relaxation. Psychopharmacol Bull. 1986;22(3):870–4. [ PubMed ] [ Google Scholar ]
- 6. Rudy AK, Leventhal AM, Goldenson NI, Eissenberg T. Assessing electronic cigarette effects and regulatory impact: challenges with user self-reported device power. Drug Alcohol Depend. 2017 Oct 01;179:337–40. doi: 10.1016/j.drugalcdep.2017.07.031. https://europepmc.org/abstract/MED/28843084 .S0376-8716(17)30417-9 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Crespi E, Hardesty JJ, Nian Q, Sinamo J, Welding K, Kennedy RD, Cohen JE. Agreement between self-reports and photos to assess e-cigarette device and liquid characteristics in wave 1 of the vaping and patterns of e-cigarette use research study: web-based longitudinal cohort study. J Med Internet Res. 2022 Apr 27;24(4):e33656. doi: 10.2196/33656. https://www.jmir.org/2022/4/e33656/ v24i4e33656 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Taylor A, Dunn K, Turfus S. A review of nicotine-containing electronic cigarettes-trends in use, effects, contents, labelling accuracy and detection methods. Drug Test Anal. 2021 Feb;13(2):242–60. doi: 10.1002/dta.2998. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Raymond BH, Collette-Merrill K, Harrison RG, Jarvis S, Rasmussen RJ. The nicotine content of a sample of e-cigarette liquid manufactured in the United States. J Addict Med. 2018;12(2):127–31. doi: 10.1097/ADM.0000000000000376. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Jackson R, Huskey M, Brown S. Labelling accuracy in low nicotine e-cigarette liquids from a sampling of US manufacturers. Int J Pharm Pract. 2020 Jun;28(3):290–4. doi: 10.1111/ijpp.12596. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Hiler M, Karaoghlanian N, Talih S, Maloney S, Breland A, Shihadeh A, Eissenberg T. Effects of electronic cigarette heating coil resistance and liquid nicotine concentration on user nicotine delivery, heart rate, subjective effects, puff topography, and liquid consumption. Exp Clin Psychopharmacol. 2020 Oct;28(5):527–39. doi: 10.1037/pha0000337. https://europepmc.org/abstract/MED/31855003 .2019-78483-001 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Talih S, Balhas Z, Eissenberg T, Salman R, Karaoghlanian N, El Hellani A, Baalbaki R, Saliba N, Shihadeh A. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: measurements and model predictions. Nicotine Tob Res. 2015 Feb;17(2):150–7. doi: 10.1093/ntr/ntu174. https://europepmc.org/abstract/MED/25187061 .ntu174 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Pozzar R, Hammer MJ, Underhill-Blazey M, Wright AA, Tulsky JA, Hong F, Gundersen DA, Berry DL. Threats of bots and other bad actors to data quality following research participant recruitment through social media: cross-sectional questionnaire. J Med Internet Res. 2020 Oct 07;22(10):e23021. doi: 10.2196/23021. https://www.jmir.org/2020/10/e23021/ v22i10e23021 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 14. Guest JL, Adam E, Lucas IL, Chandler CJ, Filipowicz R, Luisi N, Gravens L, Leung K, Chavanduka T, Bonar EE, Bauermeister JA, Stephenson R, Sullivan PS. Methods for authenticating participants in fully web-based mobile app trials from the iReach project: cross-sectional study. JMIR Mhealth Uhealth. 2021 Aug 31;9(8):e28232. doi: 10.2196/28232. https://mhealth.jmir.org/2021/8/e28232/ v9i8e28232 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. E-cigarettes: Facts, stats and regulations. Truth Initiative. 2018. [2022-12-13]. https://truthinitiative.org/research-resources/emerging-tobacco-products/e-cigarettes-facts-stats-and-regulations .
- 16. Drillinger M. E-cig on the rise among middle and high school students. Healthline. 2022. Oct 13, [2022-12-13]. https://www.healthline.com/health-news/e-cig-on-the-rise-among-middle-and-high-school-students .
- 17. Fagan P, Pohkrel P, Herzog T, Pagano I, Vallone D, Trinidad DR, Sakuma KL, Sterling K, Fryer CS, Moolchan E. Comparisons of three nicotine dependence scales in a multiethnic sample of young adult menthol and non-menthol smokers. Drug Alcohol Depend. 2015 Apr 01;149:203–11. doi: 10.1016/j.drugalcdep.2015.02.005. https://europepmc.org/abstract/MED/25744873 .S0376-8716(15)00086-1 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Freisthler B, Gruenewald PJ, Tebben E, Shockley McCarthy K, Price Wolf J. Understanding at-the-moment stress for parents during COVID-19 stay-at-home restrictions. Soc Sci Med. 2021 Jun;279:114025. doi: 10.1016/j.socscimed.2021.114025. https://europepmc.org/abstract/MED/34004571 .S0277-9536(21)00357-9 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Article Resource Page. Institute for Global Tobacco Control. 2022. [2022-12-13]. https://www.globaltobaccocontrol.org/en/article-resource-page .
- 20. Deeming Tobacco Products To Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act; Restrictions on the Sale and Distribution of Tobacco Products and Required Warning Statements for Tobacco Products. The Daily Journal of the United States Government. 2016. Oct 5, [2022-12-13]. https://www.federalregister.gov/documents/2016/05/10/2016-10685/deeming-tobacco-products-to-be-subject-to-the-federal-food-drug-and-cosmetic-act-as-amended-by-the . [ PubMed ]
- 21. Hahn SM. Coronavirus (COVID-19) update: court grants FDA’s request for extension of premarket review submission deadline for certain tobacco products because of impacts from COVID-19. U.S. Food and Drug Administration. 2020. [2022-12-13]. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-court-grants-fdas-request-extension-premarket-review-submission-deadline .
- 22. Soule E, Bansal-Travers M, Grana R, McIntosh S, Price S, Unger JB, Walton K. Electronic cigarette use intensity measurement challenges and regulatory implications. Tob Control. 2023 Jan;32(1):124–9. doi: 10.1136/tobaccocontrol-2021-056483. http://tobaccocontrol.bmj.com/lookup/pmidlookup?view=long&pmid=34059553 .tobaccocontrol-2021-056483 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 23. Weaver SR, Kim H, Glasser AM, Sutfin EL, Barrington-Trimis J, Payne TJ, Saddleson M, Loukas A. Establishing consensus on survey measures for electronic nicotine and non-nicotine delivery system use: current challenges and considerations for researchers. Addict Behav. 2018 Apr;79:203–12. doi: 10.1016/j.addbeh.2017.11.016. https://europepmc.org/abstract/MED/29173942 .S0306-4603(17)30426-4 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. O'Connor R, Durkin SJ, Cohen JE, Barnoya J, Henriksen L, Hill SE, Malone RE. Thoughts on neologisms and pleonasm in scientific discourse and tobacco control. Tob Control. 2021 Jun 18;30(4):359–60. doi: 10.1136/tobaccocontrol-2021-056795. [ DOI ] [ Google Scholar ]
- 25. Alexander JP, Coleman BN, Johnson SE, Tessman GK, Tworek C, Dickinson DM. Smoke and vapor: exploring the terminology landscape among electronic cigarette users. Tob Regul Sci. 2016 Jul 01;2(3):204–13. doi: 10.18001/TRS.2.3.1. https://europepmc.org/abstract/MED/27430008 . [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. El-Hellani A, Salman R, El-Hage R, Talih S, Malek N, Baalbaki R, Karaoghlanian N, Nakkash R, Shihadeh A, Saliba NA. Nicotine and carbonyl emissions from popular electronic cigarette products: correlation to liquid composition and design characteristics. Nicotine Tob Res. 2018 Jan 05;20(2):215–23. doi: 10.1093/ntr/ntw280. https://europepmc.org/abstract/MED/27798087 .ntw280 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Final NIH statement on sharing research data. National Institutes of Health (NIH) 2003. Feb 26, [2023-02-20]. https://grants.nih.gov/grants/guide/notice-files/NOT-OD-03-032.html .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data availability statement.
- View on publisher site
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Jump to navigation
- Bahasa Indonesia
- Bahasa Malaysia

Latest Cochrane Review finds high certainty evidence that nicotine e-cigarettes are more effective than traditional nicotine-replacement therapy (NRT) in helping people quit smoking

A Cochrane review has found the strongest evidence yet that e-cigarettes, also known as ‘vapes’, help people to quit smoking better than traditional nicotine replacement therapies, such as patches and chewing gums.
New evidence published today in the Cochrane Library finds high certainty evidence that people are more likely to stop smoking for at least six months using nicotine e-cigarettes, or ‘vapes’, than using nicotine replacement therapies, such as patches and gums. Evidence also suggested that nicotine e-cigarettes led to higher quit rates than e-cigarettes without nicotine, or no stop smoking intervention, but less data contributed to these analyses. The updated Cochrane review includes 78 studies in over 22,000 participants – an addition of 22 studies since the last update in 2021.
Smoking is a significant global health problem. According to the World Health Organisation (WHO), in 2020, 22.3% of the global population used tobacco, despite it killing up to half of its users. Stopping smoking reduces the risk of lung cancer, heart attacks and many other diseases. Though most people who smoke want to quit, many find it difficult to do so permanently. Nicotine patches and gum are safe, effective and widely used methods to help individuals quit.
E-cigarettes heat liquids with nicotine and flavourings, allowing users to ‘vape’ nicotine instead of smoking. Data from the review showed that i f six in 100 people quit by using nicotine replacement therapy, eight to twelve would quit by using electronic cigarettes containing nicotine. This means an additional two to six people in 100 could potentially quit smoking with nicotine containing electronic cigarettes.
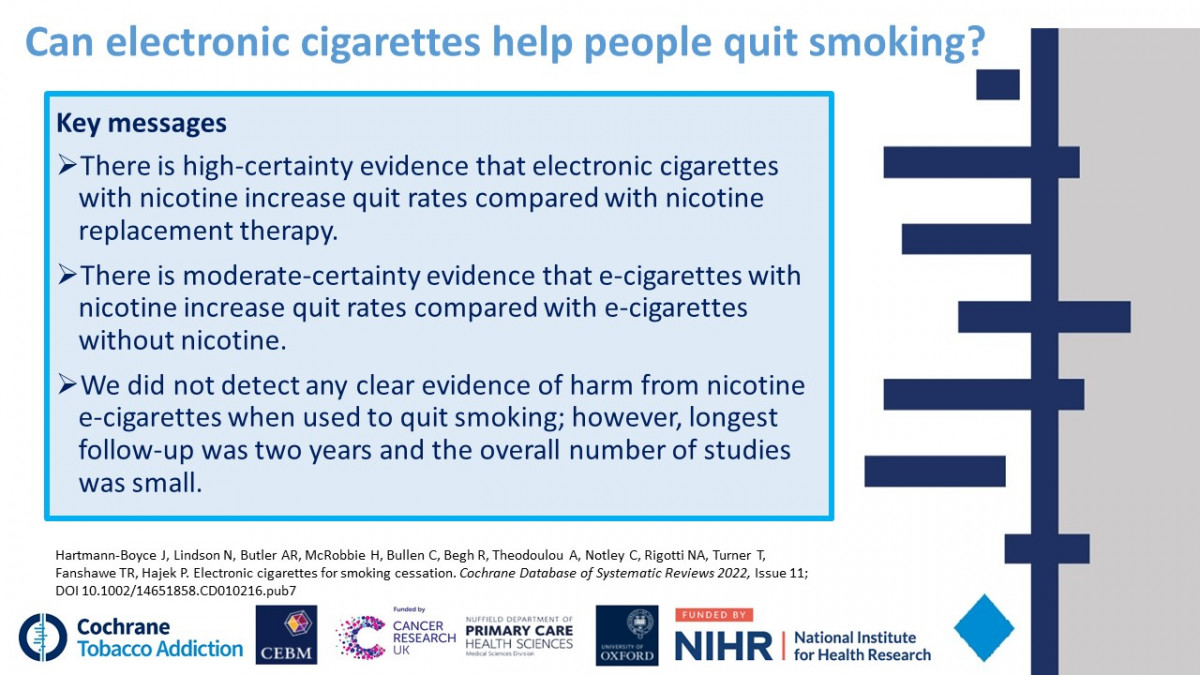
Dr Jamie Hartmann-Boyce, Associate Professor at the University of Oxford, Editor of the Cochrane Tobacco Addiction Group, and an author of the new publication, said:
“Electronic cigarettes have generated a lot of misunderstanding in both the public health community and the popular press since their introduction over a decade ago. These misunderstandings discourage some people from using e-cigarettes as a stop smoking tool. Fortunately, more and more evidence is emerging and provides further clarity. With support from Cancer Research UK, we search for new evidence every month as part of a living systematic review. We identify and combine the strongest evidence from the most reliable scientific studies currently available. For the first time, this has given us high-certainty evidence that e-cigarettes are even more effective at helping people to quit smoking than traditional nicotine replacement therapies, like patches or gums.”
In studies comparing nicotine e-cigarettes to nicotine replacement treatment, significant side effects were rare. In the short-to-medium term (up to two years), nicotine e-cigarettes most typically caused throat or mouth irritation, headache, cough, and feeling nauseous. However, these effects appeared to diminish over time.
Dr Nicola Lindson, University Research Lecturer at the University of Oxford, Cochrane Tobacco Addiction Group’s Managing Editor, and author of the publication said:
“ E-cigarettes do not burn tobacco; and as such they do not expose users to the same complex mix of chemicals that cause diseases in people smoking conventional cigarettes. E-cigarettes are not risk free, and shouldn’t be used by people who don’t smoke or aren’t at risk of smoking. However, evidence shows that nicotine e-cigarettes carry only a small fraction of the risk of smoking. In our review, we did not find evidence of substantial harms caused by nicotine containing electronic cigarettes when used to quit smoking. However, due to the small number of studies and lack of data on long-term nicotine-containing electronic cigarette usage – usage over more than two years – questions remain about long-term effects.”
The researchers conclude that more evidence, particularly about the effects of newer e-cigarettes with better nicotine delivery than earlier ones, is needed to assist more people quit smoking. Longer-term data is also needed.
Michelle Mitchell, chief executive at Cancer Research UK, said:
“We welcome this report which adds to a growing body of evidence showing that e-cigarettes are an effective smoking cessation tool. We strongly discourage those who have never smoked from using e-cigarettes, especially young people. This is because they are a relatively new product and we don’t yet know the long term health effects. While the long term effects of vaping are still unknown, the harmful effects of smoking are indisputable – smoking causes around 55,000 cancer deaths in the UK every year. Cancer Research UK supports balanced evidence-based regulation on e-cigarettes from UK governments which maximises their potential to help people stop smoking, whilst minimising the risk of uptake among others.”
- Read the full Cochrane review and plain language summary
- Learn more about Cochrane Tobacco Addition Group
- Science Media Centre: Expert reaction to cochrane review on electronic cigarettes for smoking cessation

Hartmann-Boyce J, Lindson N, Butler AR, McRobbie H, Bullen C, Begh R, Theodoulou A, Notley C, Rigotti NA, Turner T, Fanshawe TR, Hajek P. Electronic cigarettes for smoking cessation. Cochrane Database of Systematic Reviews 2022, Issue 11. Art. No.: CD010216. DOI: 10.1002/14651858.CD010216.pub7
This work was supported by Cancer Research UK [A ref. A29845]
To speak to a team member about this project please contact Dr. Hartmann-Boyce, [email protected] or Dr. Lindson, [email protected] .
- Help & Support
Where There’s Smoke: New Research Publication Lights Fire about Dangers of Vaping
by Stacey Sturner | January 12, 2023
- E-Cigarettes
The American Journal of Preventive Medicine recently published a new paper, titled “ Cigarette-E-cigarette Transitions and Respiratory Symptom Development ,” which assessed the respiratory health effects of 16 tobacco product transitions, including from non-use to e-cigarette use.
Funded in part by the American Lung Association, the study suggests e-cigarette initiation among nonusers and subsequent cigarette smoking may cause significant lung health impacts. These results reinforce the urgency for robust e-cigarette regulations, as well as demonstrate that additional research is needed to better determine the specific harms of e-cigarettes.
“The topline finding that e-cigarette initiation among nonusers is associated with increased respiratory morbidity is an important point to emphasize given continued high rates of e-cigarette usage among youth and young adult never smokers in the U.S.,” stated Andrew Stokes, PhD , assistant professor of global health at the Boston University School of Public Health and senior author of the paper. “It adds to our body of scientific evidence urgently calling for the public health intervention in support of more stringent regulatory e-cigarette standards.”
Dr. Stokes was a 2020-2022 recipient of the Lung Association’s Public Policy Research Award, which aims to empower scientists who are impacting lung health. In February 2022, he likewise served as senior author of a paper published in the American Journal of Respiratory and Critical Care Medicine , revealing young adults who use e-cigarettes are more likely to develop respiratory issues within one year of vaping.
The latest study, like the one published in February, used data from the Food and Drug Administration’s (FDA) Population Assessment of Tobacco and Health (PATH) cohort, a longitudinal study tracking changes in tobacco use over time among participants. Among 33,231 observations from 13,528 unique participants, the study authors found that nonusers who started e-cigarette use had 62% greater rate of wheezing.
Albert Rizzo, MD, Chief Medical Officer of the Lung Association, added, “Anything that can be done to help curb the e-cigarette epidemic is an important step forward. This research further amplifies our organization’s warning against e-cigarette use due to the resulting health ramifications. It’s a mission-critical public policy initiative, now and always.”
“What is exceedingly clear is that e-cigarette initiation among nonusers is associated with increased respiratory morbidity,” continued Dr. Stokes. “We’re just starting to scratch the surface in our systematic identification of behavioral patterns most closely tied to respiratory events.”
The Lung Association has called on the FDA to end the sale of all flavored tobacco products, including flavored e-cigarettes. Flavors attract youth and the high levels of nicotine found in many e-cigarettes quickly hook kids. States including California and Massachusetts, as well as Washington, D.C. and multiple other cities have passed legislation to end the sale of flavored tobacco products in their states.
In addition, the Lung Association has called on federal officials to do more to ensure youth who are addicted to vaping and other tobacco products have resources to help them end their addiction.
To learn more about e-cigarette risks or a list of proven-effective cessation programs available to help youth and adults quit all tobacco products for good, please visit: Lung.org/quit-smoking .
Related Blogs
Vaping essential oils: hidden health danger you need to know about.
September 6, 2024
ZYN 101: What to Know About Big Tobacco’s Latest Addiction
July 31, 2024
“I Almost Died”: a Vaping Horror Story
May 8, 2024
Blog last updated: August 25, 2023
Double the Lifesaving Impact
Flu and RSV can be deadly and this season, your 2X matched donation provides critical lung health resources to people most at risk. Donate now and help save a life.
Your Gift 2X Matched
Make a Donation
Your tax-deductible donation funds lung disease and lung cancer research, new treatments, lung health education, and more.
Become a Lung Health Insider
Join over 700,000 people who receive the latest news about lung health, including research, lung disease, air quality, quitting tobacco, inspiring stories and more!
Thank you! You will now receive email updates from the American Lung Association.
Select Your Location
Select your location to view local American Lung Association events and news near you.
Change Language
Lung helpline.
Talk to our lung health experts at the American Lung Association. Our service is free and we are here to help you.
1-800-LUNG-USA
(1-800-586-4872)

RESOURCES INFORMATION LIBRARY
Research, data & articles.
This resource is collection of the best and latest research on vaping and other reduced-harm products, medical journal articles, as well as journalistic pieces organized by topic that contain valuable data, information, and perspectives on tobacco harm reduction, vaping, and safer nicotine products.
Our intention is update this resource with additional sources as they become available, so please check back often.
Jump to Topic…
Science // Smoking Cessation Science // Harm Reduction Science // Health Effects Science // Flavors Science // Youth Usage Science // Smokeless Tobacco Science // Regulatory Effects News // Smoking Cessation News // Harm Reduction News // COVID-19 News // EVALI News // Youth Usage News // Social Justice News // Taxes News // Tobacco Control
Highlighted Resources
These resources are some of the highest rated, latest research on vaping.
Balancing Consideration of the Risks and Benefits of E-Cigarettes
Nicotine without smoke: tobacco harm reduction, electronic cigarettes for smoking cessation, a randomized trial of e-cigarettes versus nicotine-replacement therapy, educational videos, recommended videos, what vaping did.

Vaping: what people are getting wrong | The Economist

Vaping: a more balanced message | Michigan Public Health
Why bans of low-risk nicotine alternatives to smoking in lmic's will do more harm than good, vaping demystified.

The E-Cigarette Summit 2019, London | Ethan Nadelman Presentation

Why Health Groups Lie About Vaping

Vaporized: U.K. Government Promotes Vaping As Smoking Cessation Tool | CNBC Prime

Marc Slis, Vape Shop Owner - Michigan Testimony
Recommended reading.

Stop Smoking Start Vaping
Dr. Colin Mendelsohn

Velvet Glove, Iron Fist
Christopher Snowden

The Rediscovery of Tobacco
Jacob Grier

The Cigarette Century
Allan M. Brandt

The Cigarette
Sarah Milov

Cigarette Wars
Cassandra Tate

Sander L. Gilman & Zhou Xun

Cigarettes Are Sublime
Richard Klein

For Smokers Only

Ashes to Ashes
Richard Kluger

Say Why To Drugs
Dr. Suzi Gage
Scientific Research / Articles
Smoking cessation, harm reduction, health effects, youth usage, smokeless tobacco, regulatory effects, news media / blog articles, social justice, tobacco control.
This site uses functional cookies and external scripts to improve your experience.
Privacy settings
Privacy Settings
This site uses functional cookies and external scripts to improve your experience. Which cookies and scripts are used and how they impact your visit is specified on the left. You may change your settings at any time. Your choices will not impact your visit.
NOTE: These settings will only apply to the browser and device you are currently using.
Google Analytics
By continuing to browse or by clicking ‘Accept’, you agree to the storing of cookies on your device to enhance your site experience and for analytical purposes.
Together we are beating cancer
- Cancer types
- Breast cancer
- Bowel cancer
- Lung cancer
- Prostate cancer
- Cancers in general
- Clinical trials
- Causes of cancer
- Coping with cancer
- Managing symptoms and side effects
- Mental health and cancer
- Money and travel
- Death and dying
- Cancer Chat forum
- Health Professionals
- Cancer Statistics
- Cancer Screening
- Learning and Support
- NICE suspected cancer referral guidelines
- Make a donation
- By cancer type
- Leave a legacy gift
- Donate in Memory
- Find an event
- Race for Life
- Charity runs
- Charity walks
- Search events
- Relay for Life
- Volunteer in our shops
- Help at an event
- Help us raise money
- Campaign for us
- Do your own fundraising
- Fundraising ideas
- Get a fundraising pack
- Return fundraising money
- Fundraise by cancer type
- Set up a Cancer Research UK Giving Page
- Find a shop or superstore
- Become a partner
- Cancer Research UK for Children & Young People
- Our Play Your Part campaign
- Brain tumours
- Skin cancer
- All cancer types
- By cancer topic
- New treatments
- Cancer biology
- Cancer drugs
- All cancer subjects
- All locations
- By Researcher
- Professor Duncan Baird
- Professor Fran Balkwill
- Professor Andrew Biankin
- See all researchers
- Our achievements timeline
- Our research strategy
- Involving animals in research
- Research opportunities
- For discovery researchers
- For clinical researchers
- For population researchers
- In drug discovery & development
- In early detection & diagnosis
- For students & postdocs
- Our funding schemes
- Career Development Fellowship
- Discovery Programme Awards
- Clinical Trial Award
- Biology to Prevention Award
- View all schemes and deadlines
- Applying for funding
- Start your application online
- How to make a successful applicant
- Funding committees
- Successful applicant case studies
- How we deliver research
- Our research infrastructure
- Events and conferences
- Our research partnerships
- Facts & figures about our funding
- Develop your research career
- Recently funded awards
- Manage your research grant
- Notify us of new publications
- Find a shop
- Volunteer in a shop
- Donate goods to a shop
- Our superstores
- Shop online
- Wedding favours
- Cancer Care
- Flower Shop
- Our eBay store
- Shoes and boots
- Bags and purses
- We beat cancer
- We fundraise
- We develop policy
- Our global role
- Our organisation
- Our strategy
- Our Trustees
- CEO and Executive Board
- How we spend your money
- Early careers
- Your development
Cancer News
- For Researchers
- For Supporters
- Press office
- Publications
- Update your contact preferences
- About cancer
- Get involved
- Our research
- Funding for researchers
The latest news, analysis and opinion from Cancer Research UK
- Science & Technology
- Health & Medicine
- Personal Stories
- Policy & Insight
- Charity News
- Health & Medicine
Can vaping cause changes in our cells?
20 March 2024
You may have seen recent media coverage of a study that looked at changes in different types of cells from people who smoked and people who vaped. In this article, we take a closer look at what the researchers did, what they found and what the results of the study could mean.
Does this study show that vaping causes cancer?
No. The type of change that this study looked at is different from changes to a cell’s DNA sequence (mutations) . This study shows that some changes were there but not what they might be doing. So, we don’t have enough information yet to understand what these findings mean in terms of any potential health effects.
E-cigarettes haven’t been around for long enough for us to know what their longer-term health effects could be . So, vaping isn’t risk-free and children and people who have never smoked shouldn’t vape. But research overall still finds that legal vaping is far less harmful than smoking and can help people who smoke to stop.
What did the researchers do?
The researchers looked at chemical ‘marks’ that add information to the genetic code in our DNA. This is called epigenetics. It’s a bit like highlighting or adding notes to a page in a book – the words themselves don’t change, but we read them differently. The epigenetic marks affect how our cells ‘read’ the instructions in their genes.
Epigenetic changes ‘turn on’ or ‘turn off’ genes. They’re a way for cells to respond to what’s happening around them by following the instructions from the right gene(s). Epigenetic changes can be temporary and reverse when they’re no longer needed, whereas genetic mutations in our DNA tend to be permanent.
In this study, the researchers looked into a type of epigenetic change called methylation, i n which a small molecule (made up of one carbon atom and three hydrogen atoms, a ‘methyl group’) gets attached to some of the building blocks of DNA. In particular, they focused on methylation in different types of cells in people who smoked but also looked at it in a small number of people who vaped and another small group of people who used smokeless tobacco.
What did the study find?
The study found methylation changes in cells taken from people who smoked, including cheek (which are directly exposed to smoke), cervical (which aren’t) and blood cells. The kind of changes seen varied depending on the type of cell. As mentioned above, epigenetic changes can be temporary, enabling cells to respond to their environment and then stop when the response is no longer needed. The researchers found variation in methylation patterns depending on how long someone had smoked for, and some changes were only seen in samples from people who currently smoked and not in people who had stopped smoking.
The researchers also compared methylation changes in cell DNA samples from people who vaped with those from people who smoked. They found some similarities between the kinds of changes seen in people who vaped and people who smoked.
What does it mean?
This research is at an early stage, so we don’t have enough information yet to understand what the findings mean. For example, the study looked at some changes that were seen in cell DNA samples, not how the cells with those changes were behaving compared to cells without the changes.
When two things occur together, it could mean that:
- one is causing the other;
- they could also be there by chance; or
- they could both be caused by a third factor that we don’t know yet.
So, the epigenetic changes could be part of our cells’ response to cigarette smoke or e-cigarette vapour, they could be unrelated or they could be caused by something else that was affecting both the study participants who smoked and the participants who vaped.
There were also some limitations to what the study was able to do, for example, participants who vaped may have smoked in the past, so we can’t be completely sure that the changes seen weren’t caused by previous smoking.
Should I stop vaping?
If you used to smoke and are vaping to help you to stay off tobacco, the most important thing is not to go back to smoking. So, if you’re thinking of stopping vaping, make sure you only do so if you think you won’t start smoking again. If you think there’s a risk that you’ll smoke if you stop vaping, talk to your free local stop smoking service, GP or pharmacist for advice on stopping vaping or switching to an alternative stop smoking tool.
Because vaping isn’t risk free, it’s important that children and people who have never smoked don’t start to vape. By contrast, however, decades of research have proven the harmful effects of smoking, which kills one person every five minutes in the UK. Research so far has found that e-cigarettes are far less harmful than smoking and can help people to stop. So, if you smoke and want to stop , e-cigarettes are an option.
What could epigenetics research mean in the future?
Epigenetics is an interesting area of research and we look forward to seeing what future studies can tell us about cancer and how it develops.
What are the UK governments doing about smoking and vaping?
In October 2023, the UK Government announced plans to create the first ever smokefree generation by raising the age of sale of tobacco as well as action to tackle youth vaping . Today, those plans are being introduced in Parliament as the Tobacco and Vapes Bill.
Under the Bill, anyone born on or after 1 January 2009 will never legally be able to be sold tobacco.
As an individual who once believed in the promise of vaping as a safer alternative to smoking, I embarked on a journey that ultimately led me to a profound realization: prioritizing my health over convenience was paramount.
It’s undeniable that traditional tobacco products wreak havoc on our bodies, causing irreversible damage to our lungs and increasing the risk of life-threatening diseases like cancer. In my quest for a healthier alternative, I turned to vaping, hoping to break free from the chains of tobacco addiction.
However, my experience with vaping proved to be far from the panacea I had envisioned. Despite its touted benefits, vaping failed to satisfy my cravings and instead left me feeling depleted and unwell. Dehydration, coupled with a lack of essential nutrients from improper eating habits, took its toll on my body, manifesting in alarming symptoms such as liver pain and debilitating fatigue.
Concerned for my well-being, I sought medical advice and underwent a liver scan, revealing unsettling fatty deposits in my liver—a stark reminder of the detrimental effects of vaping on my health. Faced with this sobering reality, I made a conscious decision to reclaim control of my life and pursue a healthier lifestyle.
Quitting vaping marked the beginning of a remarkable transformation. Freed from the shackles of addiction, I experienced a newfound sense of vitality and vigor. The burden of liver pain lifted, replaced by an abundance of energy and vitality that I had long forgotten.
Today, I stand as living testimony to the power of resilience and determination in overcoming adversity. By embracing the simple pleasures of life and relinquishing the harmful habits that once held me captive, I have unlocked a world of boundless possibilities.
To those who may find themselves ensnared by the allure of vaping or any other harmful habit, I implore you to heed this cautionary tale. Embrace the gift of life with gratitude and reverence, for it is a precious treasure to be cherished and nurtured.
In closing, let us remember that true fulfillment lies not in succumbing to the temptations of vice, but in embracing the purity of existence and breathing the air as nature intended. As I bask in the radiance of newfound health and vitality, I invite you to join me on this transformative journey towards a brighter, healthier future.
Yours in health and healing,
I think until there is solid proof that vaping is as bad as cigarettes then people should stop putting fake news on the internet. Smoking contains over 7000 toxins, including tar that they use on the roads , vaping has about 5 substances in and is actually around 95% safer than smoking . My Dr adviced me to switch to vaping to quit the cancer sticks , which I did , my breathing has improved loads and my horrid,rattly smokers cough I had for years had gone , I no longer get out of breath just walking up the stairs. I agree if people have never smoked then they shouldn’t just start vaping and kids shouldn’t do it either . But my Dr said the nail thing in vapes is nicotine, which is not harmful at all , it’s just addictive. We breath in toxins every time we leave the house , with all the car fumes and pollution that’s in the air . The reason the government don’t want people quitting smoking and start vaping is because there’s millions weeks millions of pounds to be made in smoking and killing millions of people a year . There is no money to be made in the vaping . What’s more important, people’s health or money … The government are the most untrustworthy ,lying snakes on the planet. And until there is concrete proof people should vape to quit the cancer sticks.
Every way of smoking, including vaping, is, in my layman understanding, a huge cancer risk. After all, you inhale smoke with plenty of dangerous ingredients that go right up your brain, down your throat and also down your lungs and no one could ever convince me that this is healthy or free of any cancer danger. There are other articles on this (great) website, which clearly explain that the greatest dangers of developing cancer are drinking alcohol and smoking. Luckily, I have never smoked anything in my entire life and I have no interest in starting this, in fact I am very disgusted if someone on the streets is smoking and I have to pass by or even walk behind that person, which makes me holding my breath until I got away, yet, from my observation while walking the streets, when somebody is vaping the clouds are way thicker and the smell seems to go way farther than the smell of burning tobacco. To me, those are clearly warning signs that vaping may even be far more dangerous, also when it comes to cancer development, than smoking cigarettes, although I highly advise to stay away from any kind of smoking anyway. And I would not be surprised if later studies will finally figure out that vaping is indeed coming with a far greater risk than smoking cigarettes, although, again, my layman advise is to just stay away from smoking anything, no matter what it is.
WOAH! This shared a lot of info about how bad vaping is!
Cancer research has a responsibility to protect the public, not the Corporations pushing Vapes on the population under the guise of “safer than cigarettes”. This is akin to saying there is no evidence that walking across the motorway with a blindfold on is unsafe because there is no evidence to suggest you would get run over (as no one has carried out a real study to confirm or deny this)
This nanny state will not stop me vaping it saved me from smoking and your never stop children smoking just like cannabis
Nobody has ever asked my husband about his vaping – e-cigarette use despite having lung cancer🙀 what is going on?
Methyl is not a molecule (the closest molecule to it is methane). It is usually called a group (or, maybe, a moiety).
Highlighted content
Selenium: could a common food supplement help prevent the spread of cancer, when lung cancer killed my dad, the first thing i did was light up a cigarette, 30 years of brca: how the discovery of two breast cancer genes continues to drive progress, how smoking makes breast cancer radiotherapy more complicated, cancer research uk makes unprecedented £173m commitment to world-class research in cambridge, cervical cancer treatment breakthrough cuts risk of death by 40%, cancer metabolism: finding how fast-growing cancers get their energy, “it must be more than just bad luck – it must be genetic”: three women with brca gene mutations share their experiences, more like this, cancer waiting times: latest updates and analysis, £600,000 given to create the world’s first ovarian cancer prevention vaccine, related topics.
- Research and trials
- Cancer controversies
- Epigenetics
- Cancer in the news

IMAGES
VIDEO
COMMENTS
The new scientific statement, "Cardiopulmonary Impact of Electronic Cigarettes and Vaping Products," details the latest usage data and trends, identifies current health impacts, highlights existing basic and clinical scientific evidence surrounding e-cigarettes and recommends research priorities to further understand the short- and long ...
Widespread uptake of vaping has signaled a sea change in the future of nicotine consumption. Vaping has grown in popularity over the past decade, in part propelled by innovations in vape pen design and nicotine flavoring. Teens and young adults have seen the biggest uptake in use of vape pens, which have superseded conventional cigarettes as the preferred modality of nicotine consumption ...
Health. How bad is vaping for your health? We're finally getting answers. As more of us take up vaping and concerns rise about the long-term effects, we now have enough data to get a grip on the ...
29 September 2022. Vaping substantially less harmful than smoking, largest review of its kind finds. New research from the Institute of Psychiatry, Psychology & Neuroscience (IoPPN) at King's College London has found that the use of vaping products rather than smoking leads to a substantial reduction in exposure to toxicants that promote cancer, lung disease and cardiovascular disease.
Chronic use of e-cigarettes, commonly known as vaping, can result in small airway obstruction and asthma-like symptoms, according to researchers at Harvard-affiliated Massachusetts General Hospital. In the first study to microscopically evaluate the pulmonary tissue of e-cigarette users for chronic disease, the team found in a small sample of ...
Little is known about the effect of vaping on the immune system. Interestingly, both traditional and e-cigarette consumption by non-smokers was found to provoke short-term effects on platelet function, increasing platelet activation (levels of soluble CD40 ligand and the adhesion molecule P-selectin) and platelet aggregation, although to a lesser extent with e-cigarettes [].
NIH-funded studies show damaging effects of vaping, smoking on blood vessels. October 26, 2022, 2:00 PM EDT. Combining e-cigarettes with regular cigarettes may increase health risks. Long-term use of electronic cigarettes, or vaping products, can significantly impair the function of the body's blood vessels, increasing the risk for ...
A. Contact: Nicole Napoli , [email protected], 202-669-1465. WASHINGTON (Apr 02, 2024) -. People who use e-cigarettes are significantly more likely to develop heart failure compared with those who have never used them, according to one of the largest prospective studies to date investigating possible links between vaping and heart failure.
"While the long term effects of vaping are still unknown, the harmful effects of smoking are indisputable - smoking causes around 55,000 cancer deaths in the UK every year. Cancer Research UK supports balanced evidence-based regulation on e-cigarettes from UK governments which maximises their potential to help people stop smoking, whilst ...
The scientific statement from the American Heart Association, published Monday in the journal Circulation, highlights the latest usage data and scientific evidence showing health effects of e-cigarette use, also called vaping. It also recommends research priorities to better understand how these products may affect people's health over time.
Shields is one of few researchers who has already probed human lungs for e-cigarettes' effects. Last month, his group published a paper in Cancer Prevention Research that compared 15 healthy volunteers who used e-cigarettes without nicotine for 4 weeks with 15 people who never smoked or vaped. (He did the study before concerns about acute lung ...
Vaping by adolescents is a concern because of the risks of nicotine addiction and because of reports of an association between vaping and acute lung injury. This nationally representative survey ...
Further research is required to elucidate the short- and long-term consequences of vaping to determine whether e-cigarettes are truly a "safer" alternative to traditional cigarettes for ...
Youth vaping rates in the US have dropped sharply over the past year, with teenagers reporting the lowest levels seen in a decade, according to the 2024 National Youth Tobacco Survey (NYTS). A half-million fewer teens reported using e-cigarettes in the past 30 days during 2024 than 2023—a nearly 25% 1-year decline, according to the US Centers for Disease Control and Prevention (CDC).
Electronic cigarettes (e-cigarettes) have gained popularity since 2007 and have become the second most commonly used tobacco product among US adults in 2021. 1 E-cigarettes have been increasingly used as tobacco cessation aids. 2,3 However, there are concerns about the potential long-term cancer risks associated with e-cigarette use. 4 Since the recommendations from the US Preventive Services ...
Gloved hands of lab technician conducts research on electronic cigarettes, or e-cigs, and vaping pens, inside a laboratory environment. CDC/ Von Roebuck Long-term use of electronic cigarettes, or vaping products, can significantly impair the function of the body's blood vessels, increasing the risk for cardiovascular disease.
Abstract Background. In total, 3.2% of American adults report using e-cigarettes every day or some days. The Vaping and Patterns of E-cigarette Use Research (VAPER) Study is a web-based longitudinal survey designed to observe patterns in device and liquid use that suggest the benefits and unintended consequences of potential e-cigarette regulations.
While the long term effects of vaping are still unknown, the harmful effects of smoking are indisputable - smoking causes around 55,000 cancer deaths in the UK every year. Cancer Research UK supports balanced evidence-based regulation on e-cigarettes from UK governments which maximises their potential to help people stop smoking, whilst ...
Vaping and electronic cigarette (e-cigarette) use have grown exponentially in the past decade, particularly among youth and young adults. Cigarette smoking is a risk factor for both cardiovascular and pulmonary disease. Because of their more limited ingredients and the absence of combustion, e-cigarettes and vaping products are often touted as safer alternative and potential tobacco-cessation ...
The American Journal of Preventive Medicine recently published a new paper, titled "Cigarette-E-cigarette Transitions and Respiratory Symptom Development," which assessed the respiratory health effects of 16 tobacco product transitions, including from non-use to e-cigarette use.. Funded in part by the American Lung Association, the study suggests e-cigarette initiation among nonusers and ...
This resource is collection of the best and latest research on vaping and other reduced-harm products, medical journal articles, as well as journalistic pieces organized by topic that contain valuable data, information, and perspectives on tobacco harm reduction, vaping, and safer nicotine products. Our intention is update this resource with ...
So, vaping isn't risk-free and children and people who have never smoked shouldn't vape. But research overall still finds that legal vaping is far less harmful than smoking and can help people who smoke to stop. What did the researchers do? The researchers looked at chemical 'marks' that add information to the genetic code in our DNA.