This website may not work correctly because your browser is out of date. Please update your browser .


Health research saves lives
Here at the Health Research Council of New Zealand, we support high-quality, high-impact research that improves the lives of all New Zealanders.
More than $126m invested a year
Thousands of lives saved in NZ and abroad
Supporting research careers nationwide

Want to know what the HRC is investing in?

Funding for community-led research available now

See our latest news and announcements

Our success stories
Celebrating 30 years of health achievements.
Find out how the HRC is helping to advance Māori health
Latest news.

Celebrating 30 years of health services research

Record number of health delivery grants awarded

NZ researchers win medals for game-changing studies into cervical cancer and rheumatic fever
Back to top
Follow the HRC on X
Print this page

NZACRes was established in 2004 as a professional association for clinical researchers in New Zealand. Membership is open to all those who have an interest in clinical research and as such, we represent a wide range of industry members from the private and public sectors. Our goal is to promote and support clinical research within New Zealand through the provision of networking and educational opportunities, industry resources, and collaborations to address industry issues and create solutions. NZACRes also provides the clinical research community with a unified voice and a means for industry consultation.
Regulations, hdec template updates, hdec piscf templates updated.
Main PISCF, Pregnancy and Reproductive Risks templates have been updated.
Aspiring CRAs
Aspiring cra’s article.
If you are interested in being a CRA and are not sure where to start, read the Aspiring CRA’s article.
HDEC Application Tips
The document provides advice to optimise your chance of gaining Health and Disability Ethics Committee (HDEC) approval.
Our Partners

© 2024 NZACRes. Privacy Policy | Website Design by Jade Creative
- New Zealand Advantage
- Regulatory Approach
- Ethics Approval
- Materials Shipping
- Contract Templates
- MedTech Contract Templates
- Costing Tool
- Standardised Indemnity
- Decentralised Clinical Trial (DCT) SOP’s
- Video Library
- Guidance Information & Documents
- Useful Links
- Online Resources
- LinkedIn Tips
- Registration

Aotearoa Clinical Trials is a network of hospital based clinical trial sites with over 20 years experience in clinical trials in Aotearoa - New Zealand
We achieve fast, high quality outcomes
Investigators
We partner with you in the delivery of outstanding quality research.
Participants
Clinical trials may help you - it will certainly help future generations.

Recruiting trials
A list of all current trials being conducted at Aotearoa Clinical Trials
Trial Sites
ACTT operates at multiple sites based in New Zealand Hospitals
Areas of Interest
A list of therapeutic areas and specialist investigators involved
Reach out to one of our team members
By working closely with partners right across the New Zealand health sector, we create greater insight into improved disease management as well as more equitable access to innovative healthcare.
International Certification
Aoteoroa Clinical Trials is the FIRST clinical trial site network in New Zealand to achieve GCSA Certification - the Global Quality Standard for Clinical Research Sites and IAOCR Workforce Quality Accreditation (WQA). These awards acknowledge ACTT as a centre of excellence for clinical trials.

- Why Engage With Us
- Our Investigators
- Sponsors and Partners
- Diabetes and Obesity
- Commercial Trials
- Why Participate
- Middlemore Hospital
- Whangārei Hospital
- Latest News
- Document Centre
Recruiting Trials
- +64 9 270 9758
- [email protected]
You are using a very outdated browser, which means that some of the features may not work as intented. Please upgrade your browser to improve your experience.
Loading ...
The application is loading, please wait.
Volunteer for clinical research
We have a number of open studies for both healthy volunteers and those with existing conditions. Our friendly, expert team ensure that every participant receives outstanding attention and care.
For Volunteers
Help create a healthier community
Join our community of volunteers and help us advance medicine. Our friendly, expert team ensure that every participant receives outstanding attention and care, taking you through the process efficiently with ongoing support and guidance.
For Researchers: Run a trial
Taking medical advancements further
To achieve ground-breaking results and study success, you need a partner with renowned expertise and a proven track record. Find out how Optimal is centred on facilitating the delivery of high-quality data, with rapid enrolment, to accelerate your next clinical development program.
For Researchers: Why NZ?
Get it right the first time
New Zealand boasts one of the fastest regulatory and streamlined online ethics review system, enabling Optimal to provide confidence around start up timelines. This isn’t the only reason why other companies are choosing to do business in New Zealand. Click below to receive a copy of our free guide to find out how you can accelerate your clinical program.
Every day is valuable
Leading today’s research, for a healthier tomorrow.
Optimal was founded with a core focus – to drive the advancement of medicines for improved quality of life for people across the globe. Our combined expertise across a wide range of therapeutic areas, our dedicated research centre and access to a diverse population database, enables us to deliver high-quality data, with rapid enrolment, achieving ground-breaking results and study success.
Rapid enrolment with high-quality data for ground-breaking results
Trials moving health forward
Safe and Secure
Fast and efficient process
It’s about the people.

Rapid, Quality, Trusted
Reach out to new zealand to run your clinical trials.
Optimal is strategically positioned in the world’s most rapidly growing clinical trial region, AsiaPac. On the global stage, New Zealand boasts one of the fastest regulatory and streamlined online ethics review system, enabling us to provide the expertise and service to pharmaceutical companies and CROs wanting to facilitate high-quality and ethically sound studies.
Don’t have time to read the website? Click below to receive a free copy of our guide: "Successful Clinical Trials: How to accelerate your clinical program; get your first patient in quickly and meet your clinical trial milestones”.
Who our clients are
We reach out to both international and national partners, regularly collaborating with global biopharma and start-up biotechs, supporting global programs under FDA and EMA regulations. We foster strong relationships with international and local CROs and vendors.

Logistics providers

Accredited lab providers

“I enjoyed being part of this study, the doctors and nurses were very friendly and gave clear instructions and descriptions of everything that I needed to do. The atmosphere was very friendly, and I was made to feel very welcome. Given the opportunity I would definitely take part in another study.”
“The staff were professional, helpful and I knew I could trust them. They were efficient without being rushed and remained compassionate toward my condition. Optimal Clinical Trials made participating easy -They all treated me with respect, explained things fully and they made sure I knew what was happening before starting and throughout the study. There was ample opportunity to ask questions and help available at all times. I felt totally confident in Dr M. and the nurses. I thoroughly enjoyed being involved in the trial and hope to do others and definitely recommend him and his team to others.”
“Optimal Clinical Trials were a pleasure to be involved with and maintained the utmost professionalism at all times. While undergoing the trial I found I was able to focus entirely on the study at hand because everything else was taken care of and it helped that a member of the team was always on hand to guide me through any difficulties or questions I had. I would not hesitate to undergo another trial with Dr M or his team, or to recommend that others do the same.”
Have a question?
Whether you are a medical researcher or a potential volunteer who wants more information on our current studies, we're here to help.
A Trusted Hand in Research
Seek a partner with a global reputation for stability, quality and diversity.
Consistently achieving first in world patient enrolment, we partner with global biopharma and start-up biotechs, support international programs with the FDA and EMA, and foster strong relationships with CROs, vendors and participants, to ensure the best possible journey for your medicine to market.
People Helping People
Play your part in greater discoveries.
Without volunteers, giving their time, energy and commitment to our medical journey, nothing can be achieved. Our team of experts ensure that participants receive the care, guidance and monitoring they deserve when participating in a trial from beginning to end.
- MyAucklandUni
- Student Services Online
- Class search
- Student email
- Change my password
- MyCDES+ (job board)
- Course outlines
- Learning essentials
- Libraries and Learning Services
- Forms, policies and guidelines
- New students
- Enrol in courses
- Campus card
- Postgraduate students
- Summer school
- AskAuckland
- Student Hubs
- Student IT Hub
- Student Health and Counselling
- Harassment, bullying, sexual assault and other violence
- Complaints and incidents
- Career Development and Employability Services (CDES)
- Ratonga Hauātanga Tauira | Student Disability Services (SDS)
- Rainbow support
- Covid-19 information for our community
- Emergency information
- Report concerns, incidents and hazards
- Health and safety topics
- Staff email
- Staff intranet
- ResearchHub
- PeopleSoft HR
- Forms register
- Careers at the University
- Education Office
- Early childhood centres
- University Calendar
- Opportunities
- Update your details
- Make a donation
- Publications
- Photo galleries
- Video and audio
- Career services
- Virtual Book Club
- Library services
- Alumni benefits
- Office contact details
- Alumni and friends on social media
- No events scheduled for today You have no more events scheduled for today
- Next event:
- Show {0} earlier events Show {0} earlier event
- Event_Time Event_Name Event_Description
- My Library Account
- Change Password
- Edit Profile
- My GPA Grade Point Average About your GPA GPA not available Why can't I see my GPA?
- My Progress
- Points Required Completed points My Progress Progress not available All done!
- Student hubs
- Health and counselling
- All support
- Health, safety and well-being
Breadcrumbs List.
- Liggins Institute
- About the institute
- Facilities and expertise
- You are currently on: Clinical research
Clinical research
Our researchers are investigating how early life events affect long-term health, growth and development, as well as the impact of nutrition and exercise on health in adults. This has resulted in the identification of four main research themes: Healthy Mothers, Healthy Babies; Nutrition for Life-long Health; Determinants of a Healthy Life, and Research to Reality.
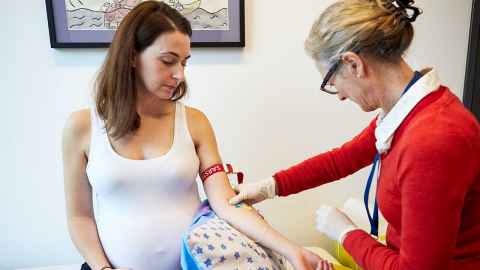
Many Liggins researchers are practising clinicians. This enables them to follow groups of children and young people as they grow up, to assess potential health risks that could be associated with their pre-birth environment or treatments they received around the time of birth. Other research focuses on the role of nutrition in healthy aging, the causes of food intolerances, and the impact of exercise on muscle strength and mass.
Randomised controlled trials and observational studies are carried out in the Liggins’ purpose-built Clinical Research Unit or at Auckland City Hospital, a teaching hospital across the road from the Liggins Institute. All clinical studies are approved by the Northern Regional or multi-site ethics committees.
Clinical Research Unit
The clinic was built, equipped and operates thanks to a large gift from the late Maurice Paykel and Mrs Agnes Paykel. It was officially opened in 2005 by Dame Silvia Cartwright, the (then) Governor General of New Zealand.
Our bright and child friendly Clinical Research Unit is fully-equipped for a wide range of clinical studies, including detailed metabolic, obesity and nutritional assessments, and neurocognitive testing in children and adults. The unit provides for a range of public good and pharma funded studies, with onsite parking available for participants. The clinic is a welcoming environment with the following facilities:
- six beds (in three rooms) for assessment and treatment
- a room with six armchairs for short duration studies
- small, quiet rooms for questionnaires and psychological studies
- a kitchen and eating area
- a sunny waiting area with lots of toys and books
Clinic facilities include:
- DEXA (Dual Energy X-ray Absorptiometry) scanner that allows measurement of body composition in children and adults to determine the relative amounts of fat and lean tissue. It is also the most accurate way to measure bone mineral density.
- Peripheral QCT (Quantitative Computed Tomography) scanner which precisely measures cortical bone density, the most accurate index of actual bone density and strength. This tool can be used in infants, children and adults.
- PEA POD (Infant Body Composition) machine which uses whole body densitometry to determine body composition (fat and fat-free mass) in infants weighing between 1 and 8 kg.
- Equipment to perform sophisticated cardiovascular, exercise and endocrine assessments.
- Portable ultrasound which can be used for a range of functions including carotid intimal thickness assessment and flow mediated vasodilation (an accurate measure of peripheral vascular function).
- Leonardo Jump Plate. This measures jump power as well as balance and is a useful tool in children and adults with impaired gait or motor function.
- Developmental testing suite with video recording.
- Phlebotomy and sample collection rooms.
- Laboratory and sample processing areas.

Clinical research team
The clinic is staffed by a friendly team of paediatric endocrinologists, paediatricians, paediatric and research nurses and administrative staff. Paediatric fellows, PhD and masters students also work in the unit. All staff involved in the set up, conduct and closure of clinical trials maintain current training in ICH-GCP and understand the requirements of The Guideline on the Regulation of Therapeutic Products in New Zealand Part 11: regulatory approval and good clinical practice requirements.
Using the facilities
If you’re interested in using the Clinical Research Unit for research, please contact paykelcru@auckland.ac.nz
Related links
- Why take part in a clinical study?
- Clinical studies in pregnancy
How do clinical trials work in New Zealand?
Information on ethics process, principles of research conduct, regulatory considerations, and research governance.
Researchers initiating clinical trials in New Zealand must take into consideration trial protocol and/or design, resource issues, ethics review, regulatory oversight, institutional policies, research governance and many other issues.
Standardised Indemnity and Compensation Agreements (sICA) have been developed by the New Zealand Association of Clinical Research (NZACRes) and are widely used within the New Zealand clinical trial industry. You can access sICA templates on the NZACRes website .
New Zealand has a world-class record of accomplishment with early phase and proof of concept trials validated by independent, clean and accurate clinical data. Overview of New Zealand Regulatory Enivronment
Medsafe is the Medicines and Medical Devices Regulatory Authority for New Zealand. Medsafe administers the regulatory application for clinical trials under Section 30 of the Medicines Act 1981, involving the use of new medicines, unregistered medicines, scientific assessment of gene technology and medical devices. Approvals are issued by Medsafe under a delegation from the Director-General of Health. Medsafe receives and processes applications, liaises with the relevant Health Research Council committee (Standing Committee on Therapeutic Trials (SCOTT) or Gene Technology Advisory Committee (GTAC)) and the applicant, and issues approval letters as part of the approval scheme for clinical trials. Further information about the scheme can be found in the Guideline on the Regulation of Therapeutic Products in New Zealand – Part 11: Clinical Trials – Regulatory Approval and Good Clinical Practice Requirements. You can find the current regulatory guidance on the Medsafe website. One component of the clinical trials scheme is the self-certification of sites that have study participants in residence while the clinical trial medicines are administered. Further information about the self-certification scheme can be found in Section 4 of the Guideline. A list of sites for which self-certification has been lodged with Medsafe can be reviewed on the Medsafe website.
Section 30 of the Medicines Act 1981 authorises the Director-General of Health to approve a clinical trial involving the use of new and unregistered medicines on the recommendation of the Health Research Council of New Zealand (HRC). The HRC maintains two standing committees to consider clinical trial applications and make recommendations to the Director-General. The Standing Committee on Therapeutic Trials (SCOTT) considers applications for new pharmaceutical-type medicines, and the Gene Technology Advisory Committee (GTAC) considers applications for trials involving new and unregistered gene and other biotechnology therapies. The Terms of Reference for these committees are published on the HRC website . The sponsor of a clinical trial should read these documents before submitting a regulatory approval application, as they provide guidance on the committee processes and the data requirements for applications to be considered by each committee.
Ethics committee approval is a separate process from regulatory approval under Section 30 of the Medicines Act 1981 and is not administered by Medsafe. The New Zealand Health and Disability Ethics Committee (HDEC) administers the ethics approval system, which applies to interventional clinical trials regardless of whether they are trials that require approval under Section 30 of the Medicines Act. Clinical trials that require sample collection and storage, or the use of disclosure of health information are in most cases also subject to ethics approval. In New Zealand only one ethics committee review is required per trial, and this covers all sites. Requirements relating to New Zealand HDEC approval of clinical trials are provided on the New Zealand HDEC website. Locality Authorisation for Ethics The term “Locality Authorisation” refers specifically to the process used by the NZ Health and Disability Ethics Committees (HDECs) for ascertaining that all local governance issues have been addressed at sites participating in a clinical trial. Locality Authorisation approval is required by all sites. To gain full approval for a clinical trial, an application requires approval from HDEC plus a Locality Authorisation. Guides, templates, and forms can be found on the HDEC website.
A national costing tool has been developed by the New Zealand Association of Clinical Research (NZACRes) and is widely used by sites to facilitate accurate and transparent trial costing and to support transparent price discussions. You can access the costing tool on the NZACRes website .
The Medicines Act 1981*, Misuse of Drugs Act 1975, and other legislation control the supply of medicines and medical devices in New Zealand. Ministry of Health approval is required for a trial before medicines can be imported into New Zealand. Detailed information about New Zealand’s medicine control requirements and relevant legislation can be found on the Ministry of Health’s website . Regulatory guidance for importing medical devices into New Zealand can be found on Medsafe’s website. *Note: The Therapeutic Products Bill passed Parliament in July 2023 and became the Therapeutic Products Act (2023). You can follow updates on regulatory changes here.
In accordance with Section 30 of the Medicines Act 1981, an application for a clinical trial of a new or unregistered medicine for approval for distribution in New Zealand must be lodged by, or in the name of, a person or company residing in New Zealand. Further guidance is also available in Section 3.3 of the Medsafe Guideline on the Regulation of Therapeutic Products in New Zealand .
All imports into New Zealand are subject to the Ministry for Primary Industries (MPI) and New Zealand Customs Import regulations. Detailed information about New Zealand’s importing requirements can be found on the MPI website. You can read more about materials shipping on the NZ Association of Clinical Research (NZACRes) website.
The aim is to provide accurate and balanced information for patients. The focus of discussions should be to raise awareness of clinical trials and discuss the risks and benefits of participation. This is to ensure that patients can make a fully informed decision on participation in a clinical trial study. A Patient Information Form and Consent Form must be provided to the potential participant and completed. You can access templates on the HEDC website.
From 1 January 2011 all clinical trials conducted in New Zealand are expected to be conducted in accordance with the Note for Guidance on Good Clinical Practice (CPMP/ICH/135/95). This applies whether or not approval under the Medicines Act 1981 is required for the trial. In some cases, requirements set out in CPMP/ICH/135/95 do not cover or conflict with provisions in the Medicines Act or in other relevant New Zealand legislation (e.g. legislation relating to reporting requirements or the retention of records). For this reason, some of the requirements specified in CPMP/ICH/135/95 must be modified to achieve compliance with New Zealand law. Medsafe Guideline on the Regulation of Therapeutic Products in New Zealand.
Medical device developers looking for a rapid, cost effective means of gaining early proof of concept for their products should consider conducting their initial clinical trials in New Zealand. The simple, one ethics committee approval process in New Zealand does not currently require U.S FDA Investigational Device Exemption (IDE) filing prior to the start of the study. More information on Medical Devices – Regulatory Guidance on the Medsafe website.
Standardised Clinical Trial Research Agreements (sCTRA) have been developed by the New Zealand Association of Clinical Research (NZACRes) and are widely used within the New Zealand clinical trial industry. You can access sCTRA templates on the NZACRes website .
In July 2022, New Zealand’s, twenty District Health Boards (DHBs) were disestablished. To begin reforming the health system , the DHBs’ functions were merged into Te Whatu Ora – Health New Zealand, which now leads the day-to-day running of the system for the whole country. Te Whatu Ora also assumed the operational functions of the Ministry of Health, such as managing national contracts. An interim Māori Health Authority was also established in September 2021, ahead of the creation of Te Aka Whai Ora – Māori Health Authority as an autonomous legal entity in July 2022. Te Aka Whai Ora’s role is to provide a more consistent, national leadership of health service delivery with a Te Ao Māori perspective. New Zealanders receive healthcare through a mixture of private and publicly funded services. The public healthcare system is primarily funded through general taxation and is therefore only available to NZ residents.New Zealand’s districts range in population size from approximately 30,000 at the smallest to over 500,000 at the largest. There are currently over 150 hospitals listed as certified providers (private and public) in New Zealand. You can view certified provider maps on the Ministry of Health website . Te Whatu Ora regions and Public Academic Tertiary Institutions have a research office or similar. The research offices facilitate close working relationships between researchers, clinical staff, ethics committees, funding bodies and commercial sponsors. These offices act as the central entry point for the approval of research and ensures that governing policies and procedure are adhered to. Contact details for certified clinical trial sites can be found on the Medsafe website.
All research involving Te Whatu Ora – Health New Zealand sites must receive approval from the relevant Research Review Committee before commencing. The relevant Te Whatu Ora site’s Research Office registers the project and organises the review process. The approval process will involve: • Research Office Application Form • Protocol • Ethics Application • Research Budget • Participant Information Sheets and Consent Forms • Contract or Clinical Trial Agreement • Indemnity and Compensation Agreement • Investigator Brochure • Funding Letter • Evidence of consultation with Māori • Locality Authorisation for Ethics • Scientific peer review • Ethics Approval Letter You can review an example approval process on the Te Whatu Ora – Te Toka Tumai Auckland (previously Auckland DHB) site.
As part of conducting research within Te Whatu Ora – Health New Zealand, an applicant must demonstrate responsiveness to Māori. The Research Application has a section titled Responsiveness to Māori which must be completed for all projects. In most cases, the review is performed either by the Māori Advisor for Research or a similar entity. The Te Whatu Ora Research Office facilitates the review. On completion of the Māori research assessment, a letter of support will be sent to you showing that a process of formal research review has taken place.
NZ Glossary
Use the below to search through some NZ-specific clinical terms. The clinicaltrials.gov site provides a comprehensive glossary of common site terms.
The Accident Compensation Corporation (ACC) provides comprehensive, no-fault personal injury cover for all New Zealand residents and visitors to New Zealand.
Online registry of clinical trials being undertaken in Australia, New Zealand and elsewhere.
ANZCTR website
Any change to the terms of a study, including to the protocol or other supporting documentation, made after a Health and Disability Ethics Committee has approved the study.
A group of independent scientists who monitor the safety and scientific integrity of a clinical trial. The group can recommend to the study sponsor that the study be stopped if it is not effective, if it is causing harm to participants, or if it is not likely to serve its scientific purpose. Committee members are chosen based on the scientific skills and knowledge needed to monitor the particular study. Also referred to as a Data Safety and Monitoring Board (DSMB).
The Gene Technology Advisory Committee provides regulatory review of genetically modified products.
The process by which a Health and Disability Ethics Committee (HDEC) checks, in accordance with the Standard Operating Procedures, that a new application (or substantial amendment to a previously approved application) meets or exceeds established ethical standards.
The Health Research Council is the agency responsible for managing the Government’s investment in health research. The HRC’s committees provide advice on gene technology, accredit health and disability ethics committees and institutional ethics committees, monitor the safety of large clinical trials and review applications to use new medicines in trials.
The Health Research Council website
The Health Research Council Ethics Committee ensure that independent ethical assessment of any proposed research submitted for a Health Research Council (HRC) grant has been carried out either by the HRCEC itself, or an ethics committee approved by the HRCEC. The HRCEC approves ethics committees to carry out this function.
An organisation responsible for a hospital, health centre, surgery or other establishment or facility in New Zealand at or from which the procedures outlined in the protocol of a study are to be conducted.
‘Locality review’ is the process by which a locality assesses its suitability for the safe and effective conduct of an intervention study.
New Zealand Medicines and Medical Devices Safety Authority
SCOTT is the Standing Committee on Therapeutic Trials; a standing committee of the Health Research Council (HRC) whose function is to make recommendations to Medsafe regarding the approval of clinical trials of new medicines under section 30 of the Medicines Act 1981.
An administrative check carried out by the Health and Disability Ethics Committee (HDEC) secretariat to verify that an application or other item of business is complete and may be assigned for review through the full or expedited review pathway.
Case studies
To illustrate some clinical trial journeys with links to supporting material, we've put together two fictional case studies for example health innovations in collaboration with the New Zealand Association of Clinical Research (NZACRes), Te Tītoki Mataora and the National Institute of Health Innovation (NIHI).
Articles, commentary and blogs

Phase I Pharmaceutical: Psoriasis Treatment
Learn more about how a new treatment for psoriasis could navigate the Phase I clinical trial process, with links to further reading on the discussed topics.

Class II Medical Device: Diabetes Monitoring
Learn more about the clinical investigation process for a wearable diabetes monitoring device, with links to further reading on the discussed topics.
Connecting with the clinical trials sector in New Zealand
Search the HealthTech Activator (HTA) directory for listed clinical trials sites and supporting bodies.
.png)
Cancer Trials New Zealand
Carrying out life changing cancer research.
Cancer Trials New Zealand (CTNZ) was established in 2003 with a vision and commitment to improve cancer control through research.
The primary focus of Cancer Trials New Zealand is to support the development of new research ideas from scientists and clinicians to the point at which they are ready for submission to the relevant project-funding body (Health Research Council, Cancer Society, Cancer Research Trust, Pharmaceutical Industry etc).
Our focus is on those studies with relevance to New Zealanders, from supporting our own researchers through to improving the outcomes of New Zealanders at risk of or already facing the challenges of cancer.
The scope of trials range from, discovering new ways of preventing, diagnosing, treating and monitoring cancer, to the refinement of established treatments and understanding the delivery of care within cancer services.
Building and retaining a high-quality cancer research workforce in New Zealand is one of our great challenges if we are to keep pace with modern strategies in cancer research and care.
Establishment of Cancer Trials New Zealand was made possible by an initial three year foundation seeding grant from Auckland and Northland division of the New Zealand Cancer Society, and we are very grateful that they have been supporting our core activities annually ever since. With their valuable support we continue to work towards achieving our mission.
Our Mission
To sustain and grow a collaborative clinical research program which is responsive to the principles of equity and the priorities of those affected by cancer in Aotearoa New Zealand
Equitable and improved outcomes for those affected by cancer, emphasizing partnership with Māori and excellence in clinical research
Acknowledgements:

- Leadership Team
- Participating Process
- Participant Testimonials
- Ethics committees
- Regulatory authority
- P3 Wellington
- P3 Tauranga
- P3 Hawkes Bay
- P3 Palmerston North
- P3 Lower Hutt
Welcome to P3 Research A New Zealand leader in Clinical Trials
P3 research is a leading and respected independent clinical trials company dedicated to performing high quality clinical studies..
Our seven clinical research sites are capable of performing studies from small scale Phase 2 trials to large scale, longer term Phase 3 studies.
Our Mission
To provide independent and accurate clinical research with integrity and professionalism.
To contribute to the improvement of health outcomes for our participants and future generations.
To be progressive, leading through innovation and the quality of our work. Our core values are integrity, respect and partnership.
We have experience performing studies in the following therapeutic areas:
Participate in a clinical trial.
Help yourself while helping others in a safe environment.
Information for Sponsors
Experience super-fast timeframes in as little as 3 months to FPFV.
Our Clients


Our Research Sites
Find a clinical research site that is closest to you. Our research sites are spread across New Zealand with state-of-the-art facilities, each with patient care and experience at the heart of everything we do.

Christchurch
Forte 2, level 2, 132 peterborough st, christchurch, 8013, nz.

6 Avalon Drive, Nawton, Hamilton, 3200, NZ

469 Main Road Stoke, Nelson, 7011, NZ

New Lynn, Auckland
1 mccrae way, level 1, new lynn, auckland 0600, nz.

53 Domain Road, Papamoa Beach, Papamoa, 3118, NZ

1289 Haupapa Street, Rotorua 3010, NZ

Silverdale, Auckland
7 polarity rise, silverdale, hibiscus coast, 0932, nz.

Takapuna, Auckland
Level 2, 2 fred thomas drive, takapuna, auckland 0622, nz.

Upper Hutt Health Centre, Queen Street, Ebdentown, Upper Hutt, 5018, NZ
- Our Partners
Participants
- Current Trials
- Trial Locations
- Why Volunteer?
- Facilities & Capabilities
- Trial Highlights
- News & Insights
© PCRN, All rights reserved
Terms of Use | Privacy Policy
Site: Done By Nine

Master of Clinical Research
This course is available
Level of Study
Master's Degree
Next start date
Help improve human health. Study Clinical Research and get the skills you need to carry out evidence-based research that will advance medical knowledge.
You’ll learn to use both qualitative and quantitative research methods, and find out how to critically evaluate current literature. Gain knowledge in good study design and research practice, data analysis and research presentation. You'll find out how to carry out clinical trials and cover ethical and cultural issues in clinical research.
You‘ll be able to expand your current work to include clinical research, or move into a new career as a clinical researcher in a range of areas in the health sector. You might work for a drug company, a hospital clinical trials unit or a research institute.
If you haven’t completed the Diploma but have significant and relevant clinical research experience, you might still be admitted into the Master’s programme.
Programme requirements
For this programme you’ll need to:
Complete the 120 point thesis course Thesis in Clinical Research (CLNR 591)
Entry criteria
To be accepted into this programme you'll need:
- A Postgraduate Diploma in Clinical Research
- To be accepted by the programme director as capable of proceeding with the proposed course of study
Proof of English proficiency
To be accepted into this programme you will need to meet one of the following:
IELTS: minimum overall score of 6.5 with no sub-score below 6.0
TOEFL: minimum score of 90 for the internet-based test with a minimum of 20 in writing
Pearson Test of English: minimum score of 58 (with a ‘Communicative’ score of not less than 50)
EPP: minimum final scores of 4,4,5,5
Application Deadline - Trimester 1: 1 Dec.
Studying in NZ
Share this course
New Zealand Centre for Brain Research testing Alzheimer’s treatments on sheep
Share this article
A New Zealand geneticist says his research into the minds of sheep may hold the key to curing Alzheimer’s disease .
Auckland University’s Centre for Brain Research received more than $300,000 of funding from the US-based Cure Alzheimer’s Fund earlier this year.
Professor of genetics Russell Snell said the funding would help expand his research focusing on the development of early-onset Alzheimer’s disease in genetically modified sheep.

The goal was to improve the drug pre-screening process for Alzheimer’s disease, Snell said.
“By far and away the most value these animals will have is the pre-clinical testing of potential treatments, to throw away the ones that won’t work, [and] to refine the list to the ones that do, so that they can then go on into human clinical trials.”
Sheep served as excellent models for Alzheimer’s disease research due to their longer life span, complex brain structure and genetic similarity to humans, Snell said.
Many drugs that performed well in rodents failed in human clinical trials and those trials could cost more than a billion dollars to run, he said.
His work would be a game-changer for Alzheimer’s disease research globally, Snell said.
“Our country’s size means we are always close to the people we are working to provide real hope to. We look at things differently here; we ask, ‘What can we bring that’s unique?’
“This work is truly unique. Our group has a vision for dementia , that it is possible to fix it.”
Centre for Brain Research director and research collaborator Sir Richard Faull said the Cure Alzheimer’s Fund recognised the importance of its work.
“Our research work has not only a pioneering scientific value to it; it critically also has a human life value. This funding recognises that in the most perfect way.”

Latest from New Zealand

Hundreds lose power in Gisborne as police investigate multiple car break-ins
A 'bloody angry' victim is counting the cost.

Fiery social media - What will it be like at the table ?

Plans revealed for new stopbank at Waiohiki following Gabrielle floods

A city council legacy

Real NZ Mountains: The new ski season beckons
Home / Contact Us

Get in touch
- First Name *
- Last Name *
- Query Type * Participant/Patient Sponsor/Clinical Research Organisation Other Query
- Query related to * All Sites Auckland Hamilton Wellington Christchurch
Our Offices
Christchurch, get in touch today.

New Zealand Clinical Research (NZCR) provides state of the art research facilities and the expertise to conduct complex early phase clinical research in healthy participant and patient populations.

COMMENTS
NZCR is a research community that conducts early phase clinical trials for new medicines in New Zealand and around the world. Join their research community, get paid for your involvement, and enjoy the benefits of their state-of-the-art facilities and expert care.
The Enhancing Aotearoa New Zealand Clinical Trials research project was co-led by researchers from the University of Auckland and the University of Otago. The project was completed in July 2022 and proposes a model and roadmap for a National Clinical Trials Network for consideration by health agencies. Manatū Hauora is working closely with Te ...
The Medical Research Institute of New Zealand (MRINZ) is Aotearoa New Zealand's leading independent medical research institute. Our research is guided by a simple philosophy: it must challenge dogma, increase knowledge, and have the potential to improve clinical practice and outcomes, both in Aotearoa New Zealand, and internationally.
And in a first for the HRC, two of its three medals have gone to researchers from the same team who are working with iwi and whānau to prevent cervical cancer and improve maternity and infant outcomes. The Health Research Council of New Zealand (HRC) is a Crown agency of the New Zealand Government. It is responsible for managing the government ...
NZCR is a leading clinical research organisation in New Zealand, with a team of experts in various fields of medicine and science. Learn about their roles, experience, publications and key research interests.
NZACRes was established in 2004 as a professional association for clinical researchers in New Zealand. Membership is open to all those who have an interest in clinical research and as such, we represent a wide range of industry members from the private and public sectors. Our goal is to promote and support clinical research within New Zealand ...
Clinical Research Trials New Zealand Our experienced team is committed to conducting world-class clinical trials across a wide range of therapeutic areas to enhance the health and wellbeing of our diverse population. Learn more. Who are we.
Aoteoroa Clinical Trials is the FIRST clinical trial site network in New Zealand to achieve GCSA Certification - the Global Quality Standard for Clinical Research Sites and IAOCR Workforce Quality Accreditation (WQA). These awards acknowledge ACTT as a centre of excellence for clinical trials. Read More.
New Zealand Association of Clinical Research (NZACRes) 1,302 followers. 3mo. NZACRes, kindly supported by BeiGene, is hosting a networking event at the: Victoria University of Wellington room ...
PGDipClinRes Postgraduate Diploma in Clinical Research. Duration 2 trimesters. Fees NZ$12,439for the full programme. Schedule Seminars, videoconferences, self-directed study. Intensity Part time only. Starts 8 Jul 2024, 24 Feb 2025. (+ -1 more) Type Coursework. Location Online.
Optimal Clinical Trials is based in Auckland, New Zealand. Optimal Clinical Trials is a private clinical research centre with multiple locations, focused on providing world-class outpatient phase Ib-III clinical research studies for the pharmaceutical, biotech and CRO research industry.
At Pacific Clinical Research Network New Zealand, we are paving the way for the global availability of innovative medicines and treatments. The work we do showcases New Zealand as the country of choice for medical trials. With 9 state-of-the-art partner facilities nationwide, we attract a diverse pool of participants, producing extensive high ...
It was officially opened in 2005 by Dame Silvia Cartwright, the (then) Governor General of New Zealand. Our bright and child friendly Clinical Research Unit is fully-equipped for a wide range of clinical studies, including detailed metabolic, obesity and nutritional assessments, and neurocognitive testing in children and adults.
Good Clinical Practice (GCP) From 1 January 2011 all clinical trials conducted in New Zealand are expected to be conducted in accordance with the Note for Guidance on Good Clinical Practice (CPMP/ICH/135/95). This applies whether or not approval under the Medicines Act 1981 is required for the trial. In some cases, requirements set out in CPMP ...
New Zealand Clinical Research (NZCR) provides state of the art research facilities and the expertise to conduct complex early phase clinical research in healthy participant and patient populations. ... Grd floor, 3 Ferncroft St, Grafton Auckland 1010 New Zealand 08007883437 +64 9 3733474. Christchurch. Level 4, 264 Antigua St Christchurch 8011 ...
Cancer Trials New Zealand (CTNZ) was established in 2003 with a vision and commitment to improve cancer control through research. The primary focus of Cancer Trials New Zealand is to support the development of new research ideas from scientists and clinicians to the point at which they are ready for submission to the relevant project-funding ...
A New Zealand leader in Clinical Trials P3 Research is a leading and respected independent clinical trials company dedicated to performing high quality clinical studies. Our seven clinical research sites are capable of performing studies from small scale Phase 2 trials to large scale, longer term Phase 3 studies.
Wellington. Upper Hutt Health Centre, Queen Street, Ebdentown, Upper Hutt, 5018, NZ. Learn more. Our research sites are spread across New Zealand, each with state-of-the-art facilities. Find a clinical research site that is closest to you.
TOEFL: minimum score of 90 for the internet-based test with a minimum of 20 in writing. Pearson Test of English: minimum score of 58 (with a 'Communicative' score of not less than 50) EPP: minimum final scores of 4,4,5,5. Application Deadline - Trimester 1: 1 Dec. Study Master of Clinical Research at Victoria University of Wellington.
Donor-linking provisions in New Zealand: counselling roles, concerns and needs. Sonja Goedeke Department of Psychology and Neuroscience, School of Clinical Sciences, Auckland University of Technology, Auckland, ... In a two-day meeting stakeholders aimed to address practice and research needs around donor conception in New Zealand. There was ...
By RNZ. A New Zealand geneticist says his research into the minds of sheep may hold the key to curing Alzheimer's disease.. Auckland University's Centre for Brain Research received more than ...
New Zealand Clinical Research conducts complex early-phase clinical trials involving both healthy participant and patient populations from our state-of-the-art research units located in Auckland, Christchurch and Hamilton, New Zealand. NZCR research units provide 70 research beds. Our location close to major teaching hospitals provides access ...
17 likes, 0 comments - nzcr_akl on April 14, 2024: "Dr Chris Wynne, NZCR's Chief Scientific Officer, joined the TVNZ Breakfast team this morning to discuss clinical trials. Dr Wynne discussed how the safety for our participants, with controls from the Health and Disability Ethics Committee, Medsafe and most importantly our superb medical team, is of paramount importance.
Clinical Research Associate II / Senior CRA - New Zealand. Award winning, Global Biotech; Competitive remuneration package with phenomenal benefits; Genuine internal progression & training opportunities; Remote working role ; We are seeking a dedicated CRA II and Senior CRA to join a truly innovative and dynamic global Biotech. The role
According to Glassdoor, clinical research associates in Canada earn an average salary of $73,209 . The job outlook for clinical research associates is projected to be moderate to good in nearly all provinces and territories in Canada through 2026, according to the Job Bank . How to become a clinical research associate.
Unit 1B/300 Grey Street. Hamilton East, Hamilton 3216. New Zealand. 0800 788 3437.