- History, Facts & Figures
- YSM Dean & Deputy Deans
- YSM Administration
- Department Chairs
- YSM Executive Group
- YSM Board of Permanent Officers
- FAC Documents
- Current FAC Members
- Appointments & Promotions Committees
- Ad Hoc Committees and Working Groups
- Chair Searches
- Leadership Searches
- Organization Charts
- Faculty Demographic Data
- Professionalism Reporting Data
- 2022 Diversity Engagement Survey
- State of the School Archive
- Faculty Climate Survey: YSM Results
- Strategic Planning
- Mission Statement & Process
- Beyond Sterling Hall
- COVID-19 Series Workshops
- Previous Workshops
- Listening Meetings
- Departments & Centers
- Find People
- Biomedical Data Science
- Health Equity
- Inflammation
- Neuroscience
- Global Health
- Diabetes and Metabolism
- Policies & Procedures
- Media Relations
- A to Z YSM Lab Websites
- A-Z Faculty List
- A-Z Staff List
- A to Z Abbreviations
- Terms, Privacy & Notices
- Dept. Diversity Vice Chairs & Champions
- DEI Educational Offerings
- Dean’s Advisory Council on Lesbian, Gay, Bisexual, Transgender, Queer and Intersex Affairs Website
- Minority Organization for Retention and Expansion Website
- Office for Women in Medicine and Science
- Committee on the Status of Women in Medicine Website
- Director of Scientist Diversity and Inclusion
- Diversity Supplements
- Frequently Asked Questions
- Recruitment
- By Department & Program
- News & Events
- Executive Committee
- Aperture: Women in Medicine
- Self-Reflection
- Portraits of Strength
- Mindful: Mental Health Through Art
- Event Photo Galleries
- Individual Resources
- MD-PhD Program
- PA Online Program
- Joint MD Programs
- Advanced Health Sciences Research
- Clinical Informatics & Data Science
- Clinical Investigation
- Medical Education
- Visiting Student Programs
- Special Programs & Student Opportunities
- Residency & Fellowship Programs
- Center for Med Ed
- Organizational Chart
- House Naming Process
- Leadership & Staff
- Committee Procedural Info (Login Required)
- Faculty Affairs Department Teams
- Recent Appointments & Promotions
- Academic Clinician Track
- Clinician Educator-Scholar Track
- Clinican-Scientist Track
- Investigator Track
- Traditional Track
- Research Ranks
- Instructor/Lecturer
- Social Work Ranks
- Voluntary Ranks
- Adjunct Ranks
- Other Appt Types
- Appointments
- Reappointments
- Transfer of Track
- Term Extensions
- Timeline for A&P Processes
- Interfolio Faculty Search
- Interfolio A&P Processes
- Yale CV Part 1 (CV1)
- Yale CV Part 2 (CV2)
- Samples of Scholarship
- Teaching Evaluations
- Letters of Evaluation
- Dept A&P Narrative
- A&P Voting
- Faculty Affairs Staff Pages
- OAPD Faculty Workshops
- Leadership & Development Seminars
- List of Faculty Mentors
- Incoming Faculty Orientation
- Faculty Onboarding
- Past YSM Award Recipients
- Past PA Award Recipients
- Past YM Award Recipients
- International Award Recipients
- Nominations Calendar
- OAPD Newsletter
- Fostering a Shared Vision of Professionalism
- Academic Integrity
- Addressing Professionalism Concerns
- Consultation Support for Chairs & Section Chiefs
- Policies & Codes of Conduct
- First Fridays
- Faculty Facing Caregiving Need
- Fund for Physician-Scientist Mentorship
- Grant Library
- Grant Writing Course
- Mock Study Section
- Research Paper Writing
- Establishing a Thriving Research Program
- Funding Opportunities
- Join Our Voluntary Faculty
- Faculty Attestation
- Child Mental Health: Fostering Wellness in Children
- Faculty Resources
- Research by Keyword
- Research by Department
- Research by Global Location
- Translational Research
- Research Cores & Services
- Program for the Promotion of Interdisciplinary Team Science (POINTS)
- CEnR Steering Committee
- Experiential Learning Subcommittee
- Goals & Objectives
- Faculty & Staff
- About YSM Editorial
- Issues List
- Print Magazine PDFs
- Archive of Issues List
- Archive of Print Newsletter PDFs
- YSM Events Newsletter
- Social Media
- YSM & the Community
- Patient Care
INFORMATION FOR
- Residents & Fellows
- Researchers

Promising Trial for a Drug to Ease Uterine Fibroids
About three quarters of women will experience uterine fibroids before they reach the age of 50. With a range of often debilitating symptoms including excessive menstrual bleeding, they are the most common reason women undergo hysterectomies. And there is a lack of effective treatments—most therapies either only mask symptoms or are difficult for patients to tolerate.
Now, researchers have developed a new, more patient-friendly drug that could revolutionize the way clinicians treat some of the most common gynecologic diseases including fibroids and endometriosis. The ObsEva-funded clinical trial published September 17 in the Lancet , which included co-first author Hugh S. Taylor, MD , Anita O’Keeffe Young Professor of Obstetrics, Gynecology & Reproductive Sciences and professor of molecular, cellular, and developmental biology, found that linzagolix (brand name Yselty), an oral drug that hinders estrogen production, is an effective and customizable treatment for fibroids. The treatment not only addresses the symptoms, but also tackles the underlying problem through shrinking the fibroids themselves.
“No treatments to date for fibroid growth are something I would ever want my patients to take for a prolonged period of time, as they did not treat the underlying cause of the problem,” says Taylor. “This is an extremely well tolerated class of drugs that can control fibroid growth. We’ve never had anything like that before.”
Fibroids Can Be Debilitating and Hard to Treat
The impact of uterine fibroids can be devastating. Heavy, prolonged bleeding can significantly disrupt a person’s life—they may need to go through excessive numbers of sanitary pads or tampons, worry about bleeding through and staining clothing, or need to wake up throughout the night to change pads. And profound blood loss may lead to anemia and fatigue. “This can be an impediment to getting a good night’s sleep and being socially active, and it can even affect job performance,” says Taylor.
As fibroids grow larger, they may begin putting pressure on other organs, resulting in a range of unpleasant symptoms including diarrhea or constipation and frequent urination. Fibroids can also lead to difficulty in getting pregnant and increased risk of miscarriage.
Uterine fibroids disproportionately impact Black patients—they are more common and tend to be more aggressive in comparison to white patients.
Most drugs commonly used for uterine fibroids, including birth control pills, fail to treat the fibroids themselves and simply only lighten or stop periods. And more aggressive drugs, although they treat the root of the problem, says Taylor, are “overkill.” For example, leuprolide (brand name Lupron) is an injectable drug that puts patients into a menopausal state by initially overstimulating hormonal receptors, which eventually shuts them down and completely blocks estrogen production. Although the treatment addresses the fibroids, it also can initially exacerbate symptoms and cause harsh side effects. In more extreme cases, patients may choose to undergo a hysterectomy.
Promising Clinical Trial Results
Linzagolix is an oral medication that works similarly to leuprolide by hindering hormone production. However, unlike its predecessor, it works by directly blocking the receptors instead of overstimulating them. The drug is also titratable—clinicians can tailor how much estrogen is blocked in order to best suit an individual patient’s needs without necessarily putting them into a full-blown menopause.
This is an extremely well tolerated class of drugs that can control fibroid growth. We’ve never had anything like that before. Hugh S. Taylor, MD
The new drug may cause menopause symptoms such as hot flashes. Hormonal add-back therapy can be an effective option for mitigating these symptoms. For some patients, however, including patients with obesity, hypertension, or diabetes, this therapy has risks and may not be a suitable option. These conditions also tend to be more prevalent in Black patients. In this group, a lower dose of linzagolix without add-back therapy might be preferable.
To test the effectiveness of the drug, Taylor’s team ran two large prospective, randomized, double-blind, placebo-controlled clinical trials known as PRIMROSE 1 and PRIMROSE 2. The studies enrolled patients suffering from substantial bleeding who were randomly selected to receive a placebo or one of several different doses of the drug—100 mg alone, 100 mg with add-back therapy, 200 mg alone, or 200 mg with add-back therapy. The researchers followed the patients and tracked their symptoms for one year. The researchers considered the therapy successful if the patient’s bleeding was reduced by half and also stayed in what is considered the normal range.
Patients in all four treatment groups experienced a significant reduction in menstrual bleeding. The 200 mg with add-back therapy group worked with “amazing efficacy,” says Taylor—the clinical trials showed a 75.5 percent response rate in PRIMROSE 1 and a 93.9 response rate in PRIMROSE 2. Even the lower dose of the drug still showed promising results. There were greater than 60% response rates in both trials for the 100 mg group with add-back therapy, and the 100 mg group without add-back showed better than 50% response rates.
“What is interesting and unique about our trials, that has not been done with other drugs in this class, is that we used a low dose with or without hormones,” says Taylor. “This is a great option for patients who experience severe menopause symptoms from the high dose or have a medical problem where they can’t tolerate hormonal add-back therapy.”
Revolutionizing Treatment of Gynecologic Diseases
Linzagolix is one of several in this new class of drugs in development for the treatment for common gynecologic diseases. Taylor was also involved in the 2017 clinical trial for elagolix , (brand name Orlissa), a medication designed to suppress endometriosis that has recently become available for patients.
Linzagolix has been approved in Europe, but is not yet available in the United States. Taylor says drugs in this class will radically change how clinicians treat fibroids, and he hopes linzagolix will lead to a reduction in future hysterectomies once it becomes available.
“A good medical therapy is finally here for fibroids, and I predict that what was a very common operation will dramatically decrease within the next few years,” he says. “Reducing the need for hysterectomy is very important for patients who don’t want to undergo a major surgery, especially for younger people who may still want to preserve the potential of having children in the future.”
Featured in this article
- Hugh Taylor, MD Anita O'Keeffe Young Professor of Obstetrics, Gynecology, and Reproductive Sciences and Professor of Molecular, Cellular, and Developmental Biology; Chair, Obstetrics, Gynecology & Reproductive Sciences; Chief , Obstetrics and Gynecology, Yale New Haven Hospital
New research reveals important discovery for women who have fibroids
- 30 July 2020
New research findings will help women and doctors make an informed decision about treatment of uterine fibroids.
The NIHR-funded FEMME trial led by a collaborative group of researchers at the University of Oxford, St George’s Hospital London, and the Universities of Birmingham and Glasgow, compared two competing treatments which allow fertility for symptomatic uterine fibroids, to see which option best reduced symptoms and improved the patient’s quality of life.
The results from the trial, which published in the New England Journal of Medicine , showed that myomectomy, a surgical procedure performed to remove uterine fibroids, resulted in a small but significantly higher quality of life compared with uterine artery embolisation (UAE). UAE is a minimally invasive procedure which shrinks the fibroids by placing tiny beads into the blood vessels which supply them.
A fibroid is a non-cancerous growth of the womb, with 1 in 3 women developing them at some point in their life. They most often occur in women aged 30-50 and develop more frequently in women of African-Caribbean family origin. Approximately half of women with uterine fibroids experience significant symptoms that can include heavy menstrual bleeding, abdominal pain and bloating.
Fibroids may also be associated with infertility and problems during pregnancy, including miscarriage and preterm birth. As more women are having children at a later age, fibroids are becoming more of an issue for them and safe and effective fertility sparing treatments are needed.
Two hundred and fifty-four eligible women, wishing to reduce fibroid symptoms were recruited from over 29 UK hospitals to participate in the trial. Researchers compared the two fertility preserving treatments for uterine fibroids on two patient groups. The first patient group comprised of women with an intention to conceive whilst the second group was made up of black women (who have a particularly high incidence of uterine fibroids).
The women were randomised to receive either a myomectomy or UAE procedure. The trial revealed that contrary to popular belief, rates of conception were shown to be broadly similar between the myomectomy and UAE group. Although too few trial participants were trying to get pregnant to be able to determine with certainty whether there was an impact of either treatment on pregnancy rates, which was higher in the UAE group.
Klim McPherson, Visiting Professor of Public Health Epidemiology at Oxford University and study Chief Investigator, said: “These findings are important and reveal new evidence for our understanding of the best treatment for women with fibroids who wish to avoid a hysterectomy.
“It is worth noting that the myomectomy group reported only marginally higher quality of life score than the Uterine Artery Embolisation group, although on average women in both groups saw improvements. Interestingly, the perceived drawback associated with embolisation, that it might affect the working of the ovaries, was not supported by the evidence in this trial.”
Professor Andy Shennan, Professor of Obstetrics, and Clinical Director NIHR Clinical Research Network South London, said, “This work is a major contribution to knowledge on the management of the most common tumour in women of reproductive age. The researchers found a significant but small advantage for myomectomy in terms of quality of life, while observing slightly more pregnancies in the UAE arm, which provides wider treatment choices for women with symptomatic fibroids.”
The study was funded by the NIHR Health Technology Assessment Programme
More information about the study is available on the NIHR’s Funding & Awards website
Share this page
Latest news.

NIHR study tackles multiple long-term conditions in Sri Lanka

GPs supported to help patients with depression through new specialist clinics

NIHR hands multi-million dementia funding to top UK research institute
Breakthrough in understanding genesis of fibroids
Scientists at the University of Helsinki and Helsinki University Hospital have made a breakthrough in understanding the genesis of uterine leiomyomas, also called fibroids.
Fibroids are extremely common tumors. They are a major burden for women's health worldwide, and the most common cause of hysterectomy. The Finland Myoma Study published in Nature found that the part of the human genome that controls expression of genes, is of major importance in fibroid development.
The findings of the new study represent a significant advance in fibroids research. Without detailed knowledge on the mechanisms of tumorigenesis involved, it would be difficult to develop targeted therapies for this condition affecting hundreds of millions of women.
Genes that are poised to change expression level are important for fibroid development.
The researchers discovered that multiple tumors carried mutations in genes that were involved in trafficking certain type of histones, proteins that are important for the structure and functional properties of the genome. They next found that mutations in these same genes were important in hereditary predisposition to the disease.
The work also documented the many changes in the regulatory genome that these mutations exerted, and finally showed a strong effect on gene expression levels.
"In particular, genes that are poised to frequently turn on and off seem affected in fibroids," says Academy Professor Lauri Aaltonen.
This might explain why this new mechanism of tumorigenesis frequently affects the uterine muscle wall but rarely other tissue types, as the uterus needs to adjust to many changing external cues such as those governing the menstrual cycle and pregnancy.
"Thus, disturbances in genes that need to be poised to change expression level might harm the uterus more easily than other organs," explains Aaltonen.
"Some of the overexpressed genes might provide clues for development of new fibroid treatment options," Aaltonen points out.
- Women's Health
- Parkinson's Research
- Human Biology
- Personalized Medicine
- Medical Topics
- Uterine fibroids
- Breech birth
- Developmental psychology
- Oral contraceptive
- Somatic cell
Story Source:
Materials provided by University of Helsinki . Note: Content may be edited for style and length.
Journal Reference :
- Davide G. Berta, Heli Kuisma, Niko Välimäki, Maritta Räisänen, Maija Jäntti, Annukka Pasanen, Auli Karhu, Jaana Kaukomaa, Aurora Taira, Tatiana Cajuso, Sanna Nieminen, Rosa-Maria Penttinen, Saija Ahonen, Rainer Lehtonen, Miika Mehine, Pia Vahteristo, Jyrki Jalkanen, Biswajyoti Sahu, Janne Ravantti, Netta Mäkinen, Kristiina Rajamäki, Kimmo Palin, Jussi Taipale, Oskari Heikinheimo, Ralf Bützow, Eevi Kaasinen, Lauri A. Aaltonen. Deficient H2A.Z deposition is associated with genesis of uterine leiomyoma . Nature , 2021; DOI: 10.1038/s41586-021-03747-1
Cite This Page :
Explore More
- Earliest Evidence of Humans Using Fire
- Egyptians Drank Hallucinogenic Cocktails
- Scientific Thought On Emotions in Animals
- Genetic 'Switch' Behind Parrot Color Diversity
- Sitting Too Long Can Harm Heart Health
- Heart Shape and Risk of Cardiovascular Disease
- Living Microbes in Earth's Driest Desert
- AI Headphones Create a 'Sound Bubble'
- Backyard Birds Learn from New Neighbors
- 4 Policies to Eliminate 90% of Plastic Waste
Trending Topics
Strange & offbeat.
News Center
New genes implicated in uterine fibroid development.
Northwestern Medicine scientists have identified new genes implicated in the development of uterine fibroids, according to a study published in Nature Communications .
Uterine fibroids, a type of non-cancerous growth in the uterus, are very common: around 70 percent of women will develop a uterine fibroid in their lifetime, according to the U.S. Department of Health and Human Services Office on Women’s Health. For most women, uterine fibroids are harmless, but nearly a quarter of those afflicted will suffer from excessive uterine bleeding, anemia and recurrent pregnancy loss.
Despite their prevalence, uterine fibroids are notoriously understudied, said Mazhar Adli, PhD , the Thomas J. Watkins Memorial Professor of Tumor Genomics, associate professor of Obstetrics and Gynecology in the Division of Reproductive Science in Medicine and senior author of the study.
“Uterine fibroids are not well-studied, at least not compared to other tumors,” Adli said.

In the current study, Northwestern Medicine investigators performed a meta-analysis of existing fibroid genome-wide association studies representing more than 20,000 uterine fibroid cases. They identified 24 new risk loci, or locations on the human genome, where genetic variants increased the risk of developing uterine fibroids.
The investigators then integrated that information with single-cell gene expression data from uterine fibroid patients and identified causal cell types with increased expression of those high-risk genes. It was previously understood that smooth muscle cells contribute to uterine fibroids, but Adli and his collaborators found that certain immune cells also play a role, he said.
Previously, around 120 genes were thought to contribute to the risk of developing uterine fibroids. However, by integrating the 3D genomic organization data and epigenomic data, Adli’s group has shown that nearly 400 genes may contribute to fibroid development. To experimentally validate which of these genes are related to disease risk, the scientists used CRISPR-based epigenetic repression or activation of fibroid disease-associated genomic regions to narrow down and validate disease-associated genes.
“One of the significances of the study is to understand which population may be at a higher risk for uterine fibroids, but more critically, what are the potential genes that are being affected by this variance that are causing uterine fibroids,” Adli said.

The study also incorporated data from people with diverse ancestries, yielding a more inclusive picture of uterine fibroid risk compared to previous studies which only analyzed patients of European ancestry, Adli said.
“Historically, a lot of these studies have been done in European ancestry populations,” said Adli, who is also a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. “When we took that information and compared it to a majority Black population, or Japanese population, the regions of the genome associated with disease are unique. That really shows the importance of studying diseases in diverse ancestry populations.”
Now, Adli and his collaborators will build off this discovery and inhibit the identified genes in models of the disease to see if uterine fibroid growth can be halted, he said.
“The next step for us is to further understand the genetics of this disease to find which genes should we inhibit to block the development of uterine fibroids,” Adli said.
The study was supported in part by a pilot award from the Northwestern Uterine Leiomyoma Research Center.
Related Posts
Study finds sustained remission of obesity-related conditions a decade after adolescent weight loss surgery, improving atopic dermatitis treatment for adolescents, accelerating discoveries in immunobiology through collaboration .
Comments are closed.
Type above and press Enter to search. Press Esc to cancel.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
August 14, 2023
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Scientists develop new model for understanding uterine fibroids
by Olivia Dimmer, Northwestern University
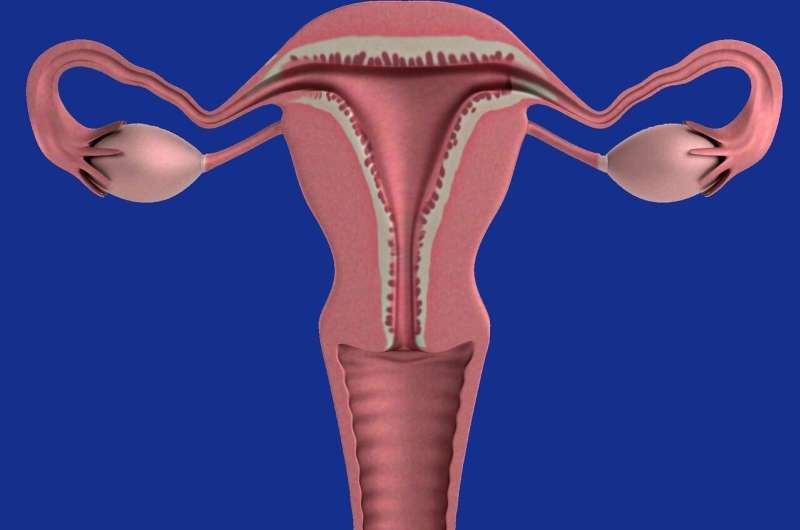
Northwestern Medicine scientists have developed a new cellular model of uterine fibroids that stem from common genetic mutations, which will accelerate further research and development of future treatments, according to findings published in Nature Communications .
It's estimated that more than half of all women will develop uterine fibroids—noncancerous muscular tumors that grow in the wall of the uterus—at some point in their lives, according to the American College of Obstetricians and Gynecologists.
It's not clear what causes uterine fibroids to develop, but many scientists believe hormonal and genetic factors play a role.
Nearly 70% of uterine fibroids are linked to a mutation in the MED12 gene, but research has been stymied by a lack of tractable cellular models to study, said Mazhar Adli, Ph.D., associate professor of Obstetrics and Gynecology in the Division of Reproductive Science in Medicine and senior author of the study.
"When cultured in the lab, the mutant cells in the fibroid tumor do not survive and hence the cellular model that mimics fibroid tumors could not be generated," said Adli, who is also a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
To address this challenge, his lab used CRISPR-based genome editing technology to precisely engineer cells that have the same mutation in the MED12 gene.
"We found that these engineered mutant cells, like fibroid tumor cells, did not proliferate well in a 2D culture, however when we culture them in 3D conditions, that better mimics the normal in vivo physiology, and they proliferated better," Adli said.
After successfully culturing the mutated fibroid cells in 3D spheres, Adli and his collaborators noticed that the cells produced heightened levels of collagen, a key feature of uterine fibroids, as well as other chromosomal abnormalities commonly seen in uterine fibroids in patients.
"Having an accurate cellular model of uterine fibroid growth will aid in future research around fibroid treatment," Adli said.
"This model opens the door for us to conduct subsequent studies to try to identify drugs that will selectively target these mutant fibroid cells," Adli said. "We could not do these experiments before because we didn't have a model system . Now that we do, we are able to conduct high-throughput CRISPR screenings to identify potential therapeutic targets."
In addition to follow-up studies into potential treatment targets for uterine fibroids , Adli's cell models will be available to other scientists and will hopefully expedite further research into the condition.
"So far, the basic biology of this disease has been lacking," Adli said. "Either there were not enough resources or there was not a good model system really to understand the molecular biology and genomic features of this disease. I think this model system and additional model systems that we are generating will really help us to understand the disease in a much better, more tractable way."
Explore further
Feedback to editors

Clinical trial finds daily tablet increases growth in children with achondroplasia
2 hours ago

Exploring how stressful life events affect internalizing and externalizing symptoms of psychopathology in childhood
Nov 17, 2024

New microfluidic device shows tumor shape predicts cancer aggressiveness
Nov 16, 2024

New study shows how salmonella tricks gut defenses to cause infection

Excessive social media use tied to substance experimentation in US pre-teens


Scientists discover a rare missense variant in STAT6 that protects against asthma
Nov 15, 2024

A new experimental infection model in flies offers a fast and cost-effective way to test drugs

Health care database analysis highlights lingering symptoms long after COVID-19 infection

Glutamine metabolic switch is key to red blood cell development and disease, researchers reveal

Zinc deficiency promotes Acinetobacter lung infection, mouse study shows
Related stories.
Mayo Clinic Minute: Know your uterine fibroid treatment options
Mar 1, 2022

New study using human fibroid cells supports use of green tea compound as treatment for uterine fibroids
Jul 13, 2023
Treatment for uterine fibroids without surgery
May 5, 2023
Women's wellness: Uterine fibroids are common noncancerous growths
Oct 21, 2020
Need relief from uterine fibroids? You may have options
Jul 20, 2021

Mayo Clinic Minute: Can uterine fibroids affect pregnancy?
May 13, 2022
Recommended for you

Study shows potential of optogenetics in treating epilepsy

New drug targets for Alzheimer's identified from cerebrospinal fluid
Nov 14, 2024

Discovery enables effective use of gene therapy for muscular dystrophies and other large-gene diseases

Chewing xylitol gum linked to decrease in preterm birth

Genetic study links heart shape to cardiovascular disease
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

IMAGES