Featured Topics
Featured series.
A series of random questions answered by Harvard experts.

Explore the Gazette
Read the latest.

Exercise cuts heart disease risk in part by lowering stress, study finds

How to realize immense promise of gene editing

Women rarely die from heart problems, right? Ask Paula.
Start of new era for alzheimer’s treatment.
Alvin Powell
Harvard Staff Writer
Expert discusses recent lecanemab trial, why it appears to offer hope for those with deadly disease
Researchers say we appear to be at the start of a new era for Alzheimer’s treatment. Trial results published in January showed that for the first time a drug has been able to slow the cognitive decline characteristic of the disease. The drug, lecanemab, is a monoclonal antibody that works by binding to a key protein linked to the malady, called amyloid-beta, and removing it from the body. Experts say the results offer hope that the slow, inexorable loss of memory and eventual death brought by Alzheimer’s may one day be a thing of the past.
The Gazette spoke with Scott McGinnis , an assistant professor of neurology at Harvard Medical School and Alzheimer’s disease expert at Brigham and Women’s Hospital , about the results and a new clinical trial testing whether the same drug given even earlier can prevent its progression.
Scott McGinnis
GAZETTE: The results of the Clarity AD trial have some saying we’ve entered a new era in Alzheimer’s treatment. Do you agree?
McGINNIS: It’s appropriate to consider it a new era in Alzheimer’s treatment. Until we obtained the results of this study, trials suggested that the only mode of treatment was what we would call a “symptomatic therapeutic.” That might give a modest boost to cognitive performance — to memory and thinking and performance in usual daily activities. But a symptomatic drug does not act on the fundamental pathophysiology, the mechanisms, of the disease. The Clarity AD study was the first that unambiguously suggested a disease-modifying effect with clear clinical benefit. A couple of weeks ago, we also learned a study with a second drug, donanemab, yielded similar results.
GAZETTE: Hasn’t amyloid beta, which forms Alzheimer’s characteristic plaques in the brain and which was the target in this study, been a target in previous trials that have not been effective?
McGINNIS: That’s true. Amyloid beta removal has been the most widely studied mechanism in the field. Over the last 15 to 20 years, we’ve been trying to lower beta amyloid, and we’ve been uncertain about the benefits until this point. Unfavorable results in study after study contributed to a debate in the field about how important beta amyloid is in the disease process. To be fair, this debate is not completely settled, and the results of Clarity AD do not suggest that lecanemab is a cure for the disease. The results do, however, provide enough evidence to support the hypothesis that there is a disease-modifying effect via amyloid removal.
GAZETTE: Do we know how much of the decline in Alzheimer’s is due to beta amyloid?
McGINNIS: There are two proteins that define Alzheimer’s disease. The gold standard for diagnosing Alzheimer’s disease is identifying amyloid beta plaques and tau neurofibrillary tangles. We know that amyloid beta plaques form in the brain early, prior to accumulation of tau and prior to changes in memory and thinking. In fact, the levels and locations of tau accumulation correlate much better with symptoms than the levels and locations of amyloid. But amyloid might directly “fuel the fire” to accelerated changes in tau and other downstream mechanisms, a hypothesis supported by basic science research and the findings in Clarity AD that treatment with lecanemab lowered levels of not just amyloid beta but also levels of tau and neurodegeneration in the blood and cerebrospinal fluid.
GAZETTE: In the Clarity AD trial, what’s the magnitude of the effect they saw?
McGINNIS: The relevant standards in the trial — set by the FDA and others — were to see two clinical benefits for the drug to be considered effective. One was a benefit on tests of memory and thinking, a cognitive benefit. The other was a benefit in terms of the performance in usual daily activities, a functional benefit. Lecanemab met both of these standards by slowing the rate of decline by approximately 25 to 35 percent compared to placebo on measures of cognitive and functional decline over the 18-month studies.
“In a perfect world, we’d have treatments that completely stop decline and even restore function. We’re not there yet, but this represents an important step toward that goal.”
Steven M. Smith
GAZETTE: What are the key questions that remain?
McGINNIS: An important question relates to the stages at which the interventions were done. The study was done in subjects with mild cognitive impairment and mild Alzheimer dementia. People who have mild cognitive impairment have retained their independence in instrumental activities of daily living — for example, driving, taking medications, managing finances, errands, chores — but have cognitive and memory changes beyond what we would attribute to normal aging. When people transition to mild dementia, they’re a bit further along. The study was for people within that spectrum but there’s some reason to believe that intervening even earlier might be more effective, as is the case with many other medical conditions.
We’re doing a study here called the AHEAD study that is investigating the effects of lecanemab when administered earlier, in cognitively normal individuals who have elevated brain amyloid, to see whether we see a preventative benefit. The hope is that we would at least see a delay to onset of cognitive impairment and a favorable effect not only on amyloid biomarkers, but other biomarkers that might capture progression of the disease.
GAZETTE: Is anybody in that study treatment yet or are you still enrolling?
McGINNIS: There’s a rolling enrollment, so there are people who are in the double-blind phase of treatment, receiving either the drug or the placebo. But the enrollment target hasn’t been reached yet so we’re still accepting new participants.
GAZETTE: Is it likely that we may see drug cocktails that go after tau and amyloid? Is that a future approach?
More like this
Newly identified genetic variant protects against Alzheimer’s
Using AI to target Alzheimer’s

Excessive napping and Alzheimer’s linked in study
McGINNIS: It has not yet been tried, but those of us in the field are very excited at the prospect of these studies. There’s been a lot of work in recent years on developing therapeutics that target tau, and I think we’re on the cusp of some important breakthroughs. This is key, considering evidence that spreading of tau from cell to cell might contribute to progression of the disease. Additionally, for some time, we’ve had a suspicion that we will likely have to target multiple different aspects of the disease process, as is the case with most types of cancer treatment. Many in our field believe that we will obtain the most success when we identify the most pertinent mechanisms for subgroups of people with Alzheimer’s disease and then specifically target those mechanisms. Examples might include metabolic dysfunction, inflammation, and problems with elements of cellular processing, including mitochondrial functioning and processing old or damaged proteins. Multi-drug trials represent a natural next step.
GAZETTE: What about side effects from this drug?
McGINNIS: We’ve known for a long time that drugs in this class, antibodies that harness the power of the immune system to remove amyloid, carry a risk of causing swelling in the brain. In most cases, it’s asymptomatic and just detected by MRI scan. In Clarity AD, while 12 to 13 percent of participants receiving lecanemab had some level of swelling detected by MRI, it was symptomatic in only about 3 percent of participants and mild in most of those cases.
Another potential side effect is bleeding in the brain or on the surface of the brain. When we see bleeding, it’s usually very small, pinpoint areas of bleeding in the brain that are also asymptomatic. A subset of people with Alzheimer’s disease who don’t receive any treatment are going to have these because they have amyloid in their blood vessels, and it’s important that we screen for this with an MRI scan before a person receives treatment. In Clarity AD, we saw a rate of 9 percent in the placebo group and about 17 percent in the treatment group, many of those cases in conjunction with swelling and mostly asymptomatic.
The scenario that everybody worries about is a hemorrhagic stroke, a larger area of bleeding. That was much less common in this study, less than 1 percent of people. Unlike similar studies, this study allowed subjects to be on anticoagulation medications, which thin the blood to prevent or treat clots. The rate of macro hemorrhage — larger bleeds — was between 2 and 3 percent in the anticoagulated participants. There were some highly publicized cases including a patient who had a stroke, presented for treatment, received a medication to dissolve clots, then had a pretty bad hemorrhage. If the drug gets full FDA approval, is covered by insurance, and becomes clinically available, most physicians are probably not going to give it to people who are on anticoagulation. These are questions that we’ll have to work out as we learn more about the drug from ongoing research.
GAZETTE: Has this study, and these recent developments in the field, had an effect on patients?
McGINNIS: It has had a considerable impact. There’s a lot of interest in the possibility of receiving this drug or a similar drug, but our patients and their family members understand that this is not a cure. They understand that we’re talking about slowing down a rate of decline. In a perfect world, we’d have treatments that completely stop decline and even restore function. We’re not there yet, but this represents an important step toward that goal. So there’s hope. There’s optimism. Our patients, particularly patients who are at earlier stages of the disease, have their lives to live and are really interested in living life fully. Anything that can help them do that for a longer period of time is welcome.
Share this article
You might like.
Benefits nearly double for people with depression

Nobel-winning CRISPR pioneer says approval of revolutionary sickle-cell therapy shows need for more efficient, less expensive process

New book traces how medical establishment’s sexism, focus on men over centuries continues to endanger women’s health, lives
So what exactly makes Taylor Swift so great?
Experts weigh in on pop superstar's cultural and financial impact as her tours and albums continue to break records.
Good genes are nice, but joy is better
Harvard study, almost 80 years old, has proved that embracing community helps us live longer, and be happier
- Skip to main content
- Keyboard shortcuts for audio player
- Your Health
- Treatments & Tests
- Health Inc.
- Public Health
Shots - Health News
Alzheimer's researchers are looking beyond plaques and tangles for new treatments.

Jon Hamilton
Scientists say research into Alzheimer's needs to take a broader view of how the disease affects the brain — whether that's changes in the cortex or the role of inflammation. Matt York/AP hide caption
Scientists say research into Alzheimer's needs to take a broader view of how the disease affects the brain — whether that's changes in the cortex or the role of inflammation.
The field of Alzheimer's research is branching out.
After decades of focusing on the sticky amyloid plaques and tangled tau fibers associated with the disease, brain researchers are searching for other potential causes of impaired memory and thinking.
That search is on full display this week at the Alzheimer's Association International Conference in San Diego, where sessions are exploring factors including genes, brain injury, clogged arteries and inflammation.
A group of researchers from Seattle even unveiled a highly detailed atlas showing how different types of brain cells change in Alzheimer's. The goal is to help scientists identify new approaches to treatment.
"Certainly, plaques and tangles are a hallmark," says Maria Carrillo , chief science officer of the Alzheimer's Association. "It doesn't mean plaques are the cause of cell death."
Plaques are clumps of a protein called beta-amyloid that appear in the spaces between neurons. Tangles are made up of a protein called tau that appears inside a neuron.
Both proteins tend to accumulate in the brains of people with Alzheimer's. But their role in killing brain cells is still unclear.
Carrillo says the Alzheimer's field needs to look to cancer research where a deeper understanding of the disease has led to better treatments.
The shift comes after a series of experimental drugs have succeeded in removing amyloid plaques and tau tangles from the brain, but failed to halt the disease.
The Food and Drug Administration has approved one amyloid drug, Aduhelm, but is still evaluating whether it actually helps patients.
An Alzheimer's Atlas
The study that produced the atlas is emblematic of how researchers are recalibrating.
"What we're trying to do with this study is to look at cell vulnerability early on in disease, before [people] have plaques and tangles, before they have cognitive impairment," says Dr. C. Dirk Keene , a neuropathologist at the University of Washington.
To create the atlas, Keene and a team of researches analyzed more than a million cells from 84 brains donated by people who'd signed up for Alzheimer's research projects run by the University of Washington and Kaiser Permanente Washington Research Institute.
The brains came from donors "at all different stages of disease" Keene says, "so we can pinpoint what's happening from the earliest levels all the way through to people with advanced disease."
The effort is funded by the National Institute on Aging and grew out of the federal BRAIN initiative launched by President Obama in 2013.
The atlas came from the realization that "If we want to treat diseases of an extremely complex cellular organ, you need to understand that organ much better than we do," says Ed Lein , a senior investigator at the Allen Institute for Brain Science, which played a key role in analyzing the brain tissue.
So the team spent years studying cells in healthy brains before looking at brains affected by Alzheimer's.
"We've defined what a normal adult brain looks like," Lein says, "and now we can use that knowledge and look for changes that are happening in specific kinds of cells."

Future Alzheimer's Treatments Aim To Do More Than Clear Plaques From The Brain
Finding vulnerable brain cells.
At the Alzheimer's meeting, the team described changes they saw in more than 100 types of cells taken from the cortex — an area of the brain which is important to memory and thinking.
One finding was that neurons that make connections within the cortex itself were much more likely to die than those that connect to distant areas of the brain.
"What we're seeing is a profound effect on cortical circuitry that very plausibly is the reason we have cognitive decline," Lein says.
If so, a treatment designed to protect those vulnerable neurons might prevent declines in memory and thinking linked to Alzheimer's.
The team also found a proliferation of brain cells that contribute to inflammation. These included certain immune cells and a type of cell that responds to injury.
"So while the neurons are lost, the non-neuronal cells are actually increasing and changing" Lein says.
The finding supports the idea that inflammation plays an important role in Alzheimer's, and that anti-inflammatory drugs might help protect the brain.
The Seattle team hopes other scientists will use the brain cell atlas to come up with new treatments for Alzheimer's.
"We've created an open-access resource where the whole community can come and look at this data," Lein says. "They can mine it to speed up progress in the field as a whole."
Speeding up progress is one reason Kyle Travaglini , a researcher at the Allen Institute, jumped at the chance to work on the Alzheimer's project.
"My grandmother started developing Alzheimer's disease when I was just going off to college," says Travaglini, who received his PhD in 2021.
Travaglini says the atlas project is appealing because it isn't based on a preconceived idea about what causes Alzheimer's.
"It's like looking at the same disease that everyone has been looking at but in an entirely different way," he says.

A substance found in young spinal fluid helps old mice remember

Scientists look to people with Down syndrome to test Alzheimer's drugs
- Open access
- Published: 02 October 2023
Clinical trials of new drugs for Alzheimer disease: a 2020–2023 update
- Li-Kai Huang 1 , 2 , 3 na1 ,
- Yi-Chun Kuan 2 , 3 , 4 , 5 na1 ,
- Ho-Wei Lin 6 &
- Chaur-Jong Hu ORCID: orcid.org/0000-0002-4900-5967 1 , 2 , 3 , 4
Journal of Biomedical Science volume 30 , Article number: 83 ( 2023 ) Cite this article
21k Accesses
20 Citations
24 Altmetric
Metrics details
Alzheimer's disease (AD) is the leading cause of dementia, presenting a significant unmet medical need worldwide. The pathogenesis of AD involves various pathophysiological events, including the accumulation of amyloid and tau, neuro-inflammation, and neuronal injury. Clinical trials focusing on new drugs for AD were documented in 2020, but subsequent developments have emerged since then. Notably, the US-FDA has approved Aducanumab and Lecanemab, both antibodies targeting amyloid, marking the end of a nearly two-decade period without new AD drugs. In this comprehensive report, we review all trials listed in clinicaltrials.gov, elucidating their underlying mechanisms and study designs. Ongoing clinical trials are investigating numerous promising new drugs for AD. The main trends in these trials involve pathophysiology-based, disease-modifying therapies and the recruitment of participants in earlier stages of the disease. These trends underscore the significance of conducting fundamental research on pathophysiology, prevention, and intervention prior to the occurrence of brain damage caused by AD.
Alzheimer disease (AD) represents a major global medical, social, and economic burden. The World Alzheimer Report 2022 revealed that more than 55 million people have AD or related conditions worldwide, and this number is projected to reach 82 million by 2030 and 138 million by 2050 [ 1 ]. Typically, AD first manifests as progressive memory decline accompanied or followed by other cognitive dysfunctions, such as visuospatial abnormalities, navigation difficulties, executive problems, and language disturbances. These cognitive impairments affect the performance of activities of daily living. During the course of AD, many behavioral and psychological symptoms of dementia (BPSD) occur [ 2 , 3 , 4 ].
Although the exact causes of AD remain unclear, the disease has two pathological hallmarks: plaques composed of amyloid-beta (Aβ) fibrils and neurofibrillary tangles (NFTs) consisting of hyperphosphorylated tau protein [ 5 , 6 , 7 ]. The key event in AD pathogenesis is believed to be Aβ accumulation. Cerebral Aβ fibril deposition may occur decades before the onset of clinical symptoms [ 8 ]. Brain atrophy, particularly in the hippocampus, is major indicator of early Aβ accumulation, particularly in the presubiculum [ 9 , 10 ]. Aβ accumulation was discovered to be crucial by three independent research groups in 1991 [ 11 , 12 , 13 ]. In familial AD, mutant autosomal-dominant genes, including the genes for amyloid precursor protein ( APP ), presenilin-1 ( PSEN1 ), and presenilin-2 ( PSEN2 ), encode the major proteins involved in amyloid metabolism [ 13 , 14 , 15 ]. Individuals with trisomy 21 (Down syndrome) have an extra copy of the APP gene, which may result in increased amyloid production and AD risk in middle age [ 16 ]. At present, the predominant theory regarding the cause of AD is the amyloid hypothesis; crucial advancements in AD therapy have been made on the basis of the proposed role of amyloid accumulation in the AD development. The United States Food and Drug Administration (US FDA) granted traditional approval for Leqembi (lecanemab-irmb) on July 6, 2023, for the treatment of AD [ 17 ]. The approval of this treatment not only affirms the pathophysiological significance of amyloid in AD but also marks a notable advance in clinical approaches to AD treatment, remedying the scarcity of new drugs in the market for nearly two decades.
Tau is a microtubule-associated protein that aids in microtubule assembly and stabilization. In AD, tau becomes hyperphosphorylated and aggregates to form paired helical filaments, a major component of NFTs within the neuronal cytoplasm. As the disease progresses, the gradual spread of tau pathology throughout brain regions has been suggested to be caused by the transfer of abnormal types of tau protein from one neuron to another [ 18 ]. The accumulation of NFTs might be initiated between the accumulation of Aβ and the development of clinical symptoms of AD [ 19 ]. NFTs and quantitative neuronal loss may be more closely correlated with disease severity and dementia progression than the amyloid plaque burden [ 20 , 21 , 22 ]. Positron emission tomography (PET) investigations have revealed a strong correlation between the binding characteristics of tau tracers and the severity of clinical manifestations in individuals with AD [ 23 ]. Molecular imaging modalities (PET) and cerebrospinal fluid (CSF) and blood–based biomarkers have extended the diagnostic scope of AD pathology to both clinical and even preclinical settings. The analysis of a combination of biomarkers such as amyloid, tau, and neurodegeneration (collectively, ATN classification) has been proposed by research on AD [ 24 , 25 ]. Furthermore, the exceptional diagnostic accuracy of plasma-based biomarkers has facilitated the clinical transition of fluid biomarkers from research settings to clinical practice. A recent presentation at the Alzheimer’s Association International Conference in 2023 highlighted the clinical and research applications of two fundamental AD biomarker categories, labeled as A and T. The A category pertains to biomarkers associated with the Aβ proteinopathy pathway, and the T category pertains to biomarkers linked to tau proteinopathy [ 26 ].
Aβ serves as a proinflammatory agent and triggers the nuclear factor κB (NF-κB) pathway in astrocytes, increasing complement C3 release. Subsequently, by binding to C3a receptors, C3 causes neuronal dysfunction and microglial activation [ 27 ]. In the early stage of AD, activated microglia may play a protective, anti-neuroinflammatory role by clearing amyloids and releasing nerve growth factors. However, activated microglia induce neurotoxic A1 astrocyte reactivity through the release of IL-1α, C1q, and TNF-α, resulting in a feedback loop of dysregulated inflammation in AD [ 28 ]. The excessive accumulation of Aβ or other toxic compounds activates proinflammatory phenotypes, resulting in neuronal damage [ 29 ]. Sustained inflammation has been observed in the brains of patients with AD [ 30 , 31 ]. The inadequate clearance of Aβ along with the aggregation of tau disrupts microglial defense mechanisms, resulting in sustained and harmful microglial activation [ 32 ]. The sequential occurrence of amyloid plaque formation, microglial activation, and the pathological phosphorylation and aggregation of tau proteins to form NFTs is the fundamental notion of the amyloid cascade–inflammation hypothesis. In the Multi-Ethnic Study of Atherosclerosis (multiple covariates were controlled for), vascular risk factor profiles and Aβ deposition significantly predicted cognitive decline [ 33 ]. Vascular risk factors can also lead to inflammation in the brain, which damages neuronal cells and further increases the likelihood of AD dementia [ 34 ].
The role of autophagy impairment is proposed in a novel hypothesis concerning plaque formation in AD. Among neurons that are compromised but still maintain some integrity, autophagic vacuoles (AVs) containing abundant Aβ are notably present. These AVs cluster within expansive membrane blebs, exhibiting a distinctive flower-like arrangement termed PANTHOS. These formations constitute the primary source of the majority of amyloid plaques found in mouse models of AD [ 35 ]. Neuroprotective therapies, including free radical scavengers, regeneration enhancers, and the suppression of excitable amino acid signaling pathways, have been proposed for preventing neuronal death or brain atrophy caused by amyloid, tau, and neuroinflammation [ 36 ]. Pathological evidence indicates that AD is also associated with degeneration in cholinergic neuron-rich regions, such as the nucleus basalis of Meynert, frontal cortex, and anterior and posterior cingulate cortex, which can lead to the symptoms of memory impairment and agitation. Acetylcholine (ACh) plays a vital role in memory function, including memory encoding, consolidation, and retrieval processes, and increasing Ach levels by using cholinesterase inhibitors (AChEIs) has become a standard therapy for the symptoms of AD [ 37 ].
Clinical trials of early or preventive interventions based on amyloid/tau theory and those targeting other pathophysiologies are ongoing or have been initiated. Many ongoing clinical trials on AD are focused on disease-modifying therapies (DMTs) that target the causes and can change the course of AD. The other trials involve symptomatic treatments—for example, enhancing cognitive function and relieving BPSD (Fig. 1 ). In this review, we summarize the new drugs being examined in ongoing trials (listed on ClinicalTrials.gov) and discuss the trends in and obstacles in AD clinical trials.

According to the amyloid hypothesis, the pathophysiology and clinical course of Alzheimer's disease progress as follows: amyloid accumulation, neuroinflammation, tau accumulation, brain metabolism dysfunction, brain atrophy, cognitive decline (from mild cognitive impairment to dementia), and the development of dementia symptoms. Novel drugs should target at least one of these events. AD Alzheimer's disease, aMCI amnestic mild cognitive impairment, BPSD behavioral psychological symptoms of dementia
Anti-amyloid therapy
Table 1 summarizes the US FDA approval status of anti-amyloid agents. Tables 2 and 3 summarize the ongoing phase 3 and phase 2 trials of anti-amyloid therapy respectively.
Aducanumab (brand name: Aduhelm) is a high-affinity, fully human immunoglobulin gamma 1 (IgG1) monoclonal antibody that binds to the N-terminus of Aβ fibrils and blocks amyloid aggregation [ 38 ]. In August 2015, two phase 3 clinical trials, namely ENGAGE and EMERGE studies, were initiated. These trials compared monthly intravenous infusions of aducanumab at one of three doses with infusions of placebo over 18 months, and the primary outcomes were cognitive and functional decline, which were assessed using the Clinical Dementia Rating (CDR) scale Sum of Boxes (CDR-SB). The secondary outcomes were other cognitive and functional measures. The trials were conducted in 150 centers across North America, Europe, Australia, and Asia. However, the findings of the EMERGE trial reached statistical significance, whereas the primary endpoint was not reached in the ENGAGE trial. An exploratory analysis revealed that a subgroup of the participants in the ENGAGE trial who received a high dose of aducanumab exhibited slow decline, which was similar to that observed among the participants in the EMERGE trial. The US FDA approved aducanumab in June 2021 on the basis of the data of the EMERGE and ENGAGE trials. Both trials presented evidence of an intermediate effect of the drug on biomarkers, indicating amyloid removal, which is likely linked to the clinical benefit of aducanumab. Further trials must be conducted to confirm the potential benefit of aducanumab [ 39 ]. The phase 3b/4 ENVISION trial (NCT05310071), which began in 2022, will enroll 1,512 patients with early AD who will receive either monthly doses of aducanumab of up to 10 mg/kg or placebo for 18 months. The aim of the trial is to determine the efficacy of aducanumab in delaying cognitive and functional decline in comparison with placebo, which would be determined on the basis of CDR-SB scores. The secondary endpoints of the trial include scores on the Alzheimer’s Disease Assessment Scale–Cognitive Subscale (ADAS-Cog) 13, Alzheimer’s Disease Cooperative Study–Activities of Daily Living Inventory (ADCS-ADL)–Mild Cognitive Impairment Version, Integrated Alzheimer’s Disease Rating Scale (iADRS), Mini-Mental State Examination, and Neuropsychiatric Inventory. The trial intends to recruit 18% of its participants from Black and Latinx populations in the United States and will have a long-term follow-up of up to 4 years, with results expected by 2026. The EMBARK trial (NCT04241068) is a phase 3b open-label study including 1,696 participants from previous aducanumab trials (from trials 221AD103, 221AD301, 221AD302, and 221AD205) that will assess aducanumab safety and tolerability over 100 weeks after a wash-out period. Participants will receive an intravenous infusion of aducanumab at 10 mg/kg monthly for 2 years, and eligible participants will continue to receive the infusion for another 52 weeks during the long-term extended treatment period. The primary outcomes are safety and tolerability, and the efficacy endpoints are the same as those in the EMERGE and ENGAGE trials, and Caregiver Global Impression of Change evaluations will be conducted every 6 months. All participants will undergo volumetric magnetic resonance imaging (MRI) scans, and a subset of the study population will undergo biomarker testing, including amyloid PET, tau PET, and CSF testing.
Lecanemab (brand name: Leqembi), a humanized IgG1 antibody derived from mAb158, selectively binds to soluble Aβ protofibrils [ 40 ]. The US FDA approved it on January 6, 2023, through an accelerated approval pathway on the basis of evidence of amyloid removal in a phase 2 trial (NCT01767311) and because it had a likelihood of having clinical benefits [ 41 ] A double-blind, placebo-controlled phase 2 trial recruited 856 patients with AD with mild cognitive impairment (MCI) or mild dementia and verified amyloid pathology through amyloid PET or CSF Aβ1-42 [ 42 ]. The results revealed a significant and dose-dependent reduction of amyloid plaques in the lecanemab group (10 mg/kg, intravenous infusion every 2 weeks) from baseline to week 79 compared with the placebo group. At the time of writing this paper, three phase 3 clinical trials on lecanemab are underway. The first trial, Clarity AD (NCT03887455), was initiated in March 2019 and was conducted at 250 sites around the world. It reported favorable outcomes for all primary and secondary measures, including ADAS-Cog14, AD Composite Score (ADCOMS), and ADCS-MCI-ADL scores [ 43 ]. The second trial is AHEAD 3–45 (NCT04468659), which was initiated in July 2020 as a 4-year trial comprising two substudies, one of which is A3, and the other one is A45. A3 is enrolling 400 people whose amyloid levels are below the brain-wide threshold for positivity; participants will receive 5 mg/kg lecanemab titrated to 10 mg/kg or placebo every month for 216 weeks. A45 is enrolling 1,000 cognitively healthy participants with positive amyloid PET scans, and they will receive lecanemab titrated to 10 mg/kg every 2 weeks for 96 weeks, followed by 10 mg/kg every month through week 216. The trial is expected to run until October 2027. The third phase 3 clinical trial is the Dominantly Inherited Alzheimer Network Trials Unit (DIAN-TU) Next Generation trial (DIAN-TU-001 (E2814), NCT05269394), in which a combination of lecanemab and the anti-tau antibody E2814 (phase 2) will be administered to 168 people with familial AD mutations. On July 6, 2023, Leqembi (lecanemab-irmb) received traditional approval from the US FDA for the treatment of AD based on Phase 3 data from the Clarity AD clinical trial [ 17 ].
The appropriate use recommendations (AURs) for lecanemab and aducanumab highlight the importance of patient selection, surveillance for adverse events, and clinician preparedness [ 44 , 45 ]. The AURs for both drugs have several similarities with respect to age criteria, biomarker requirements (positive amyloid PET or CSF findings indicative of AD), diagnosis (MCI due to AD or mild AD dementia), and MRI exclusion criteria (e.g., microhemorrhages and cortical infarction). The AURs also emphasize the importance of monitoring for amyloid-related imaging abnormalities (ARIAs), which can occur in patients receiving these drugs. APOE genotyping is recommended for informing risk discussions with candidate participants because APOE4 allele carriers, especially APOE4 homozygotes, are at a high risk of ARIAs. Patients receiving treatment must have care partners or family members who can provide necessary support and who clearly understand the nature and requirements of the therapy. Discontinuation of treatment is recommended in the following situations: when a patient is taking drugs with associated risks, such as anticoagulation agents for conditions like atrial fibrillation, deep vein thrombosis, or pulmonary embolism; or when any of the following conditions occur: a hypercoagulable state, or the development of any of the following: cerebral macrohemorrhage, multiple areas of superficial siderosis, more than 10 instances of microhemorrhages since treatment initiation, severe symptoms of ARIAs, or two or more episodes of ARIAs.
Donanemab is a humanized monoclonal antibody developed from mouse mE8-IgG2a. It recognizes Aβ (3–42), an aggregated form of Aβ found in amyloid plaques [ 46 ]. It was discovered to be bound to approximately one-third of amyloid plaques in postmortem brain samples of patients with AD or Down syndrome, and it strongly reacted with the plaque core [ 47 ]. In the phase 2 TRAILBLAZER-ALZ study, the safety, tolerability, and efficacy of donanemab alone and in combination with the Beta-Secretase 1 (BACE1) inhibitor LY3202626 (developed by Eli Lilly and Company) were evaluated over 18 months. The trial met its primary endpoint of delaying decline—which was determined on the basis of iADRS scores—by 32% compared with placebo. Amyloid burden reduction was correlated with improvement in iADRS scores only in ApoE4 carriers [ 48 ]. Donanemab reduced the tau burden in the temporal, parietal, and frontal lobes and significantly decreased plasma pTau217 by 24% in the treatment group, whereas the placebo group exhibited a 6% increase in plasma pTau217 at the end of the trial [ 49 ]. At the time of writing this paper, five phase 3 trials of donanemab are underway: TRAILBLAZER-ALZ 2, TRAILBLAZER-ALZ 3, TRAILBLAZER-ALZ 4, TRAILBLAZER-ALZ 5 and TRAILBLAZER-ALZ 6. The TRAILBLAZER-ALZ 2 (NCT04437511) trial was initially started in June 2020 as a phase 2 safety and efficacy trial, and 500 patients with early AD were recruited. Inclusion criteria of TRAILBLAZER-ALZ 2 are similar to those of TRAILBLAZER-ALZ: a ≥ 6-month history of worsening memory and positive amyloid (flortaucipir) PET. The trial was subsequently extended to a phase 3 trial with 1,800 participants. The primary outcome is iADRS, and the effectiveness of treatment is being measured using a disease-progression model rather than solely on the basis of changes at the final time point. Trial results for 1,736 participants were published to report donanemab’s impact on early symptomatic AD. Using PET imaging to categorize individuals into groups with low/medium or high tau pathology load, the study spanned 18 months and assessed cognitive and functional scales. Donanemab achieved significant cognitive improvement in the low/medium tau group (iADRS change: − 6.02 vs. − 9.27 placebo) and combined population (change: − 10.2 vs. − 13.1 placebo). The drug notably reduced decline by 60% in patients with early-stage AD, supporting the efficacy of short-term dosing. Twenty-four outcomes were evaluated, with significant findings for 23 outcomes. Adverse effects included amyloid-related imaging problems (24% donanemab vs. 2.1% placebo) and infusion-related reactions (8.7% donanemab vs. 0.5% placebo). The study findings indicated the potential of donanemab to slow AD progression, particularly in the early stage [ 50 ]. In the TRAILBLAZER-ALZ study, donanemab slowed disease progression by 32% at 18 months ( p = 0.04 vs. placebo), thus demonstrating clinical efficacy [ 51 ]. TRAILBLAZER-ALZ 3 (NCT05026866) is a placebo-controlled phase 3 prevention trial that was started in August 2021. The trial plans to enroll 3,300 cognitively healthy people aged 50–55 years who are at high risk of AD, as determined by elevated plasma pTau217 levels and Telephone Interview for Cognitive Status-modified scores. The primary outcome is the time to clinical progression, which is measured using global CDR scores. Participants are to be monitored every 6 months until cognitive impairment is noted (i.e., a score above 0 on the CDR for two consecutive evaluations) in 434 participants. The trial has a decentralized design and is being conducted at more than 200 sites in the United States, Japan, and Puerto Rico until November 2027. TRAILBLAZER-ALZ 4 (NCT05108922) is a phase 3, open-label, head-to-head comparison of amyloid clearance by either donanemab or aducanumab that began in November 2021 after the US FDA approval of aducanumab. The trial enrolled 200 people with early symptomatic AD, as indicated by a global CDR score of 0.5 or 1, at 31 sites in the United States. The primary outcome is the percentage of participants who achieve complete amyloid plaque clearance after 6 months for each treatment group, with clearance determined using amyloid (florbetapir) PET. The trial has 17 secondary outcomes, which are all related to amyloid PET measurements at up to 18 months. The preliminary results were presented at the 2022 Clinical Trial of AD (CTAD) conference: 38% of the patients on donanemab exhibited amyloid levels below the amyloid positivity threshold after 6 months, whereas only 2% of the patients on aducanumab has such findings. Plasma pTau217 levels decreased by 25% for the participants receiving donanemab, but not at all for those receiving aducanumab. The side effect of ARIA-edema occurred in 22% of the participants in both groups. TRAILBLAZER-ALZ 5 (NCT05508789) is being conducted to assess the safety and efficacy of donanemab in individuals with early symptomatic AD. The trial started in October 2022; 1,500 participants will be recruited by using the same criteria as those of TRAILBLAZER-ALZ 2 from 148 sites across China, Korea, Taiwan, and Europe; and the trial is expected to run until mid-2025. Participants will be administered monthly infusions of either donanemab or placebo, and the primary outcome will be measured on the basis of iADRS score changes after 18 months. TRAILBLAZER-ALZ 6 (NCT05738486) is a phase 3b study that will assess the impact of various dosing regimens of donanemab on the occurrence and severity of ARIA-E (ARIA with edema or effusion) in 800 adults with early symptomatic AD. The study also seeks to identify participant characteristics that predict the risk of ARIA-E. The trial is divided into four arms, each with a distinct donanemab dose.
Remternetug is a monoclonal antibody that recognizes a pyroglutamated form of Aβ that aggregates into amyloid plaques. In August 2022, Eli Lilly and Company initiated a phase 3 trial called TRAILRUNNER-ALZ1 (NCT05463731) that will randomize 600 patients with early symptomatic AD across 75 sites in the United States and 2 sites in Japan into groups receiving the antibody or placebo through intravenous infusion or subcutaneous injection for 1 year. The primary outcome is the percentage of patients whose amyloid plaques are cleared by the end of the treatment period. The secondary outcomes include the measurement of amyloid clearance, pharmacokinetics, and treatment-emergent anti-drug antibodies. The study also plans to conduct a year-long, blinded crossover extension. An additional safety cohort of 640 patients will receive open-label remternetug for 1 year.
Solanezumab is a humanized monoclonal antibody that targets the mid-domain of the Aβ peptide for increasing Aβ clearance [ 52 ]. Phase 3 trials of solanezumab, including EXPEDITION-1 and EXPEDITION-2, which enrolled 2,052 patients with mild-to-moderate AD, did not reveal improvements in ADAS-Cog11 and ADCS-ADL scores, which were the primary outcome measures. Similarly, the phase 3 trial EXPEDITION-3 demonstrated that 400 mg solanezumab administered every 4 weeks did not have significant effects on cognitive decline in patients with mild AD [ 52 ]. A4 (NCT02008357) is a phase 3 prevention trial focused on slowing memory and cognitive decline in elderly individuals without cognitive impairment or dementia. A4 is using a sensitive cognitive battery—the Alzheimer Disease Cooperative Study Preclinical Alzheimer Cognitive Composite—and was initiated in February 28, 2014. On March 8, 2023, Eli Lilly and Company reported that solanezumab did not slow cognitive decline or clear amyloid plaques in individuals with preclinical AD in the A4 study. DIAN-TU-001 (NCT01760005) is another ongoing phase 3 clinical trial that is testing the combination of solanezumab and gantenerumab in 210 asymptomatic and mildly symptomatic carriers of autosomal-dominant mutations in AD genes. However, on February 10, 2020, the study investigators announced that the primary endpoint was not achieved in the trial, namely treatment-related changes on the DIAN-Multivariate Cognitive Endpoint. The results indicated that the solanezumab-treated group had greater cognitive decline on some measures relative to placebo, and that solanezumab treatment did not exert any beneficial effects on downstream biomarkers, whereas gantenerumab significantly reduced amyloid plaques, CSF total tau, and phospho-tau181 and attenuated increases in neurofilament light chain [ 53 ]. The participants were offered an open-label extension with high-dose gantenerumab because of its positive effects on imaging and other biomarkers, such as normalized CSF Aβ42, and because it reduced CSF total tau and pTau181 levels.
ALZ-801 is a prodrug of tramiprosate, a small molecule of anti-Aβ oligomers and an aggregation inhibitor [ 54 ]. The phase 3 trial APOLLOE4 (NCT04770220) is evaluating the safety and efficacy of ALZ-801 for patients with early AD and carrying the homozygous ε4 allele on the apolipoprotein E gene ( APOE4/4 ). The recruited patients are receiving 265 mg ALZ-801 or placebo twice daily for 18 months. The trial started in May 2021. The primary endpoint is ADAS-Cog scores, and the secondary endpoints are scores of the Disability Assessment for Dementia, CDR-SB, and Amsterdam-iADL. The biomarkers of interest include the hippocampal volume, as determined through MRI and based on CSF and plasma pTau181 levels. Another phase 2 trial (NCT04693520) is investigating the effects of oral ALZ-801 administered to participants with early AD who have the APOE4/4 or APOE3/4 genotype with biomarkers of core AD pathology. The study is also assessing the efficacy, safety, and tolerability of ALZ-801.
Simufilam (PTI-125) is a drug that binds to filamin, a scaffolding protein that stabilizes the interaction between soluble Aβ42 and the α7 nicotinic acetylcholine receptor [ 55 ]. Two phase 3 trials, namely RETHINK-ALZ (NCT04994483) and REFOCUS-ALZ (NCT05026177), were commenced in November 2021. Both are safety and efficacy studies of simufilam and have enrolled participants with mild-to-moderate AD. RETHINK-ALZ will randomize 750 participants with AD and CDR scores of 0.5, 1, or 2 into groups receiving either placebo or 100 mg of simufilam twice a day for 1 year (52 weeks). The coprimary outcomes of this trial are ADAS-Cog12 and ADCS-ADL scores, and the trial is set to run through October 2023. REFOCUS-ALZ will randomize 1,083 participants into groups receiving placebo or 50 or 100 mg of simufilam (1:1:1) for 76 weeks. The primary outcome measures are similar to those of the RETHINK-ALZ trial. A phase 3 trial of simufilam (NCT05575076) was started in November 2022 to assess the long-term safety and tolerability of simufilam in participants with mild-to-moderate AD. That open-label extension study is intended to assess the long-term safety and tolerability of simufilam 100 mg twice daily in patients who have completed the RETHINK-ALZ or REFOCUS-ALZ Phase 3 clinical trials. The primary outcome measure is adverse event monitoring from baseline to week 52.
Varoglutamstat (PQ912) is a glutaminyl cyclase inhibitor that reduces pGlu-Aβ generation [ 56 ]. Glutaminyl cyclase catalyzes the cyclization of an exposed glutamate at the N-terminus of Aβ, resulting in the formation of toxic pGlu-Aβ, a major component of amyloid plaques. Two ongoing phase 2 clinical trials, namely VIVA-MIND and VIVIAD, are evaluating the safety, tolerability, and efficacy of varoglutamstat in participants with MCI and mild dementia due to AD. VIVA-MIND (NCT03919162) is a phase 2A multicenter, randomized, double-blind, placebo-controlled, parallel-group study of varoglutamstat, with a stage gate to phase 2B. Phase 2A involves an adaptive dosing evaluation of three doses of varoglutamstat or placebo for ≥ 24 weeks. VIVIAD (NCT04498650) is a phase 2B, multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-finding study being conducted to evaluate the safety, tolerability, and efficacy of varoglutamstat in 259 participants with MCI and mild dementia due to AD.
ABBV-916 is a monoclonal antibody to Aβ. It recognizes N-terminal truncated Aβ modified with pyroglutamate at position 3 (N3), a form of Aβ that is aggregated into amyloid plaques. A two-stage phase 2 trial of ABBV-916 is ongoing (NCT05291234). Stage A is a multiple ascending dose study, and participants have a 25% chance of receiving placebo. Stage B is a proof-of-concept study, and participants have a 20% chance of receiving placebo. The first 6 months of the study are a double-blinded period, which is to be followed by a 2-year extension period in which all participants receive ABBV-916. Approximately 195 participants aged 50–90 years are to be enrolled at approximately 90 sites across the world. The participants are to receive intravenous doses of ABBV-916 or placebo once every 4 weeks for 24 weeks and are to be followed up for an additional 16 weeks.
CT1812 is a ligand that targets the component 1 subunit of the sigma2/progesterone membrane receptor. It functions as a negative allosteric regulator, reducing the affinity of oligomeric Aβ and interfering with Aβ-induced synaptic toxicity [ 57 ]. START(COG0203) study (NCT05531656) is a phase 2, multicenter, randomized, double-blind, placebo-controlled trial that was initiated in September 2022 for evaluating the efficacy and safety of CT1812. START is comparing the effects of CT1812 (100 or 300 mg) with those of placebo over 18 months in 540 people with MCI or mild dementia due to AD. The SHINE (COG0201) study (NCT03507790) is a multicenter, randomized, double-blind, placebo-controlled, parallel-group, 36-week phase 2 study of two doses of CT1812 in adults with mild-to-moderate AD. The study is evaluating the safety, tolerability, pharmacokinetics, and efficacy of CT1812.
Anti-tau therapy
Table 4 summarizes the ongoing phase 2 trials of anti-tau therapy.
Bepranemab (UCB0107) is a monoclonal IgG4 antibody that targets a central tau epitope. An ongoing phase 2 trial (NCT04867616) enrolling 421 participants with prodromal or mild AD is investigating the safety, tolerability, and efficacy of bepranemab. After an 80-week double-blinded treatment period, the participants are eligible to enter a 48-week open-label extension period, in which they are to receive bepranemab treatment for 44 weeks. Subsequently, they are to participate in a safety evaluation visit 20 weeks after the last infusion. The primary outcome measure is the CDR-SB score.
JNJ-63733657 is a humanized IgG1 monoclonal antibody that targets the microtubule-binding region of tau and prevents the cell-to-cell propagation of pathogenic tau aggregates. The AUTONOMY trial (NCT04619420) is an ongoing phase 2, randomized, double-blind, placebo-controlled, parallel-group multicenter study. Participants with early AD symptoms and a positive tau PET scan are randomized to groups receiving JNJ-63733657 or placebo. This trial is enrolling 420 participants and is expected to be completed by November 2025. The primary outcome measure is clinical decline, as determined using the iADRS.
ACI-35 is a liposome-based vaccine that targets pathological conformations of phosphorylated tau. A phase 1b/2a multicenter, double-blind, randomized, placebo-controlled trial (NCT04445831) was conducted to evaluate the safety, tolerability, and immunogenicity of various doses, regimens, and combinations of tau-targeting vaccines in individuals with early AD. The vaccines tested were JACI-35.054 and ACI-35.030 at various dose levels. The findings were presented at the 2022 CTAD conference. The results indicated that participants who received ACI-35.030 exhibited a strong and sustained immune response against pathological tau proteins (pTau) and nonphosphorylated tau (ePHF), particularly in the mid- and low-dose groups. Recipients of JACI-35.054 also displayed a robust immune response against ePHF and pTau, but without a clear dose–effect relationship. The trial has been conducted across nine centers in Finland, Sweden, the Netherlands, and the United Kingdom and is expected to be completed by October 2023.
E2814 is a monoclonal IgG1 antibody that targets an HVPGG epitope in the microtubule-binding domain of tau, prevents cell-to-cell propagation, and mediates the clearance of pathogenic tau proteins. The DIAN-TU-001 (E2814) trial (NCT05269394) is a phase 2/3 multicenter, randomized, double-blind, placebo-controlled platform trial of potential disease-modifying therapies with biomarker, cognitive, and clinical endpoints. The trial is enrolling patients with dominantly inherited AD. The study design involves the use of the anti-amyloid antibody lecanemab. Some participants are receiving a matching placebo plus lecanemab, whereas others are receiving concurrent therapy with E2814 plus lecanemab.
LY3372689 is a small-molecule inhibitor of O-GlcNAcase, which promotes tau glycosylation and prevents tau aggregation [ 58 ]. A phase 2 trial (NCT05063539) was initiated in September 2021 for assessing the safety, tolerability, and efficacy of LY3372689 in 330 patients with early symptomatic AD with progressive memory changes for ≥ 6 months and who met the criterion of having a positive flortaucipir-PET scan.
BIIB080 is a tau DNA/RNA-based antisense oligonucleotide that inhibits the translation of tau mRNA into protein, thus suppressing tau expression. CELIA (NCT05399888) is an ongoing phase 2 trial that is aiming to determine whether BIIB080 can delay AD progression in comparison with placebo and to identify the most effective dose of BIIB080. In March 2019, Biogen/Ionis performed a 4-year open-label extension trial of quarterly injections for individuals who completed the randomized portion of the trial. The initial data of this trial were reported at the Alzheimer’s Association International Conference (2021), revealing no serious adverse events from the intrathecal injection of BIIB080 at either of three doses every month for 3 months or two high-dose injections 3 months apart. BIIB080 led to a dose-dependent reductions of 30%–50% in total tau and pTau181 levels in CSF.
Neuroprotectors and cognitive enhancers
Table 5 summarizes the ongoing phase 3 trials for therapies other than anti-amyloid/tau treatment.
The active metabolite of fosgonimeton (ATH-1017) is a positive modulator of hepatocyte growth factor (HGF)/MET signaling [ 59 ]. A phase 3 trial of fosgonimeton (NCT04488419) was initiated in September 2020 and is expected to be completed in February 2024. This study is evaluating the safety and efficacy of fosgonimeton in participants with mild-to-moderate AD, with double-blind, parallel-arm treatment implemented for 26 weeks. The primary outcome measure is the overall treatment effect of fosgonimeton, as measured using the Global Statistical Test, which combines cognition (ADAS-Cog) and function (ADCS-ADL) scores.
AR-1001 selectively inhibits phosphodiesterase 5 and suppresses cGMP hydrolysis, resulting in the activation of protein kinase G and the increased phosphorylation of the cAMP-responsive element-binding protein at Ser133. It can rescue long-term potentiation impairment and cognitive dysfunction in animal models of AD [ 60 ]. A phase 3 trial of AR-1001 (NCT05531526) was started in December 2022 and is estimated to be completed in December 2027. The study aims to evaluate the efficacy and safety of AR1001 in participants with early AD. The primary outcome measure is the change in the CDR-SB from baseline to week 52.
BPDO-1603 is a potential cognitive-enhancing drug for AD, but its mechanism of action remains unknown [ 61 ]. A phase 3 trial of BPDO-1603 (NCT04229927) was started in February 2020 and is estimated to be completed in March 2023. The study has been undertaken to evaluate the efficacy and safety of BPDO-1603 in patients with moderate-to-severe AD. The primary outcome measures are the change in Severe Impairment Battery total scores from baseline to week 24, and CIBIC-plus total scores at week 24.
Buntanetap is a novel translational inhibitor of multiple neurotoxic proteins, including APP, tau, and α-synuclein, by enhancing the binding of the atypical iron response element in the 5′UTR regions of the mRNA of the neurotoxic proteins to iron regulatory protein 1 [ 62 ]. In February 2023, phase 2 and 3 trials (NCT05686044) were initiated to measure the efficacy and safety of three doses of buntanetap in comparison with placebo in participants with mild-to-moderate AD. The primary outcome measures are ADAS-Cog and ADCS Clinical Global Impression of Change (ADCS-CGIC) scores.
Caffeine is an adenosine receptor antagonist that has been reported to be associated with slower cognitive decline and lower cerebral amyloid accumulation [ 63 ]. A phase 3 trial of caffeine (NCT04570085) was started in March 2021 to evaluate the efficacy of 30 weeks of caffeine intake in comparison with placebo on cognitive decline in patients with mild-to-moderate AD dementia (Mini-Mental State Examination scores: 16–24). The primary outcome measure is changes in neuropsychological test battery scores between the randomized value and the value after 30 weeks of treatment.
Hydralazine may have anti-neurodegenerative effects because it activates the Nrf2 pathway, which involves more than 200 antioxidant proteins; improves mitochondrial function; and increases respiration capacity and the production of adenosine triphosphate; hydralazine also activates autophagy, which aids in the clearance of intracellular aggregates [ 64 , 65 , 66 ]. A phase 3 trial of hydralazine (NCT04842552) was started in August 2021 and is anticipated to be completed in December 2023. The study is comparing the effects of 75 mg hydralazine versus placebo in patients with mild-to-moderate AD. Various cognitive and function tests, including olfactory tests, biochemical analyses, and adverse effect monitoring, are being conducted regularly during follow-up.
KarXT (xanomeline-trospium), comprised of muscarinic agonist xanomeline and muscarinic antagonist trospium, is designed to preferentially activate muscarinic receptor in the CNS and ameliorate the peripheral muscarinic side effects. It is reported that KarXT improves cognition in patients with AD and schizophrenia [ 67 ]. A 38-week phase 3 trial comparing the effects of KarXT (NCT05511363) and placebo in participants with psychosis associated with AD dementia was started in August 2022. The trial is analyzing the time from randomization to relapse (primary outcome) as well as the time from randomization to discontinuation for any reason and the safety and tolerability of KarXT (secondary outcomes).
Metformin, a commonly prescribed antidiabetic medication, has been reported to improve cognition or mood in many neurological disorders [ 68 , 69 ]. A phase 3 trial of metformin (NCT04098666) was started in March 2021 and is anticipated to be completed in April 2026. The primary outcome measure is the total recall of the Free and Cued Selective Reminding Test at 24 months.
Nilotinib is a tyrosine kinase inhibitor that preferentially targets discoidin domain receptors and can effectively reduce the occurrence of misfolded proteins in animal models of neurodegeneration by crossing the blood–brain barrier and promoting Aβ and tau degradation [ 70 ]. A phase 3 trial (NCT05143528) was initiated in February 2022 to investigate the safety and efficacy of nilotinib BE (bioequivalent) in individuals with early AD. The primary outcome measure is changes in CDR-SB scores between baseline and week 72.
Piromelatine is a melatonin MT1/2/3 and serotonin 5-HT-1A/1D receptor agonist and was developed as a treatment for mild AD [ 71 ]. In May 2022, a randomized trial (NCT05267535) was initiated in 225 noncarriers of a specific polymorphism, and these participants with mild dementia due to AD are allocated at a ratio of 1:1 to receive piromelatine or placebo for 26 weeks. A 12-month extension involves treating the placebo group with piromelatine to assess the drug’s disease-modifying effects. The primary analysis will be conducted after the initial 26 weeks. If efficacy is not confirmed, the study is to end without the extension phase.
Semaglutide is a peptidic GLP-1 receptor agonist that may regulate the aggregation of Aβ in AD. GLP-1 receptors are involved in cognition, synaptic transmission in hippocampal neurons, and cell apoptosis; thus, they may serve as targets for exploring candidate drugs with neuroprotective and cognition-enhancing effects [ 72 ]. A phase 3 trial of semaglutide (NCT04777396) was started in May 2021 to investigate the efficacy of semaglutide in individuals with early AD. The primary outcome measure is changes in the CDR-SB score from baseline to week 104.
Tricaprilin, a semisynthetic medium-chain triglyceride, is hydrolyzed to octanoic acid after administration and is further metabolized to ketones, which serve as an alternative energy substrate for the brain [ 73 ]. Therefore, tricaprilin can be used as a ketogenic source for the management of mild-to-moderate AD. A phase 3 trial (NCT04187547) was started in June 2022 to evaluate the efficacy and safety of tricaprilin in participants with mild-to-moderate AD. The primary outcome measure is changes in ADAS-Cog scores from baseline to week 20.
Anti-neuroinflammation therapy
Masitinib, an oral tyrosine kinase inhibitor, exerts effects by inhibiting mast cell and microglia/macrophage activity, with significant CNS penetration [ 74 ]. It is currently undergoing a phase 3 trial (NCT05564169) with 600 participants, employing a randomized, double-blind, placebo-controlled, parallel-group design over 24 weeks, followed by a 24-week extension phase. Quadruple masking ensures blinding. The study aims to evaluate Masitinib as an adjunct therapy for mild to moderate AD. Estimated to conclude on December 15, 2025, the trial assesses primary outcomes through changes from baseline in ADAS-Cog-11 and ADCS-ADL scores, measuring cognitive and functional abilities, respectively.
NE3107 is an anti-inflammatory insulin sensitizer that can cross the blood–brain barrier and bind to ERK. NE3107 can selectively inhibit inflammation-driven ERK- and NF-κB-stimulated inflammatory mediators, including TNF-α, without disturbing their homeostatic functions [ 75 ]. A multicenter phase 3 trial (NCT04669028) was started in August 2021 to investigate the safety and efficacy of NE3107 at 20 mg that was orally administered twice daily versus placebo in adult participants with mild-to-moderate AD. The primary outcome measures are changes in ADAS-Cog12 and ADCS-CGIC scores from baseline to week 30 [ 76 ].
BPSD-relieving therapy
Masupirdine, a selective 5‐HT6 receptor antagonist with favorable physicochemical properties and absorption, distribution, metabolism, and excretion properties, may have beneficial effects on agitation, aggression, and psychosis in patients with moderate AD [ 77 ]. A phase 3 trial (NCT05397639) was started in November 2022 to evaluate the efficacy, safety, tolerability, and pharmacokinetics of masupirdine in comparison with placebo for treating agitation in participants with AD dementia. The primary outcome measure is the change in the score of the Cohen–Mansfield Agitation Inventory from baseline to week 12.
Nabilone is a partial agonist of cannabinoid receptor 1 (CB1) and CB2 in the brain and in peripheral tissues, and it has been reported to provide effective treatment for agitation in patients with AD [ 78 ]. A phase 3 trial (NCT04516057) was started in February 2021 to investigate whether nabilone is an effective treatment for agitation in AD patients. The primary outcome measure is agitation (Cohen–Mansfield Agitation Inventory) between baseline and week 8.
Phase 4 and repurposing trials
Table 6 summarizes ongoing phase 4 trials.
Escitalopram, a selective-serotonin reuptake inhibitor, is a commonly used antidepressant. It ameliorates cognitive impairment and could selectively attenuate phosphorylated tau accumulation in stressed rats by regulating hypothalamic–pituitary–adrenal axis activity and the insulin receptor substrate/glycogen synthase kinase-3β signaling pathway [ 79 ]. A phase 4 trial (NCT05004987) was started in February 2022 to investigate whether a reduction in depressive symptoms owing to the administration of escitalopram oxalate is associated with the normalization of AD biomarkers in CSF and inflammatory markers in the peripheral blood. The primary outcome measures are changes in CSF Aβ40 and Aβ42 levels, vascular dysfunction biomarker levels, and scores of the Montgomery–Asberg Depression Ratio Scale at week 8.
Sodium oligomannate (GV-971), a marine-derived oligosaccharide, can reconstitute the gut microbiota, reduce bacterial metabolite–driven peripheral infiltration of immune cells into the brain, inhibit amyloid-β fibril formation, and inhibit neuroinflammation in the brain, as demonstrated in animal studies [ 80 , 81 ]. A phase 4 trial (NCT05181475) was initiated in December 2021 to examine the long-term efficacy and safety of GV-971 as well as changes in blood and gut microbiota biomarkers and thereby validate its mechanism of action and establish guidance for the more rational use of drugs in clinical practice. The primary outcome measure is changes in ADAS-Cog11 scores from baseline to week 48. Another phase 4 trial was started in July 2022 and is comparing the efficacy and safety of memantine and GV-971 monotherapy and combination therapy in patients with moderate-to-severe AD. The primary outcome measure is changes in cognitive function at weeks 12, 24, 36, and 48.
Spironolactone, an aldosterone mineralocorticoid receptor antagonist, has been commonly used to treat cardiovascular diseases, including hypertension. It has anti-inflammatory effects on the peripheral tissues and central nervous system and therefore may have beneficial effects on neurological disorders [ 82 ]. A phase 4 trial (NCT04522739) was started in September 2022 to investigate whether spironolactone can be tolerated by older Black American adults with MCI and to determine its effect on memory and thinking abilities, as measured by participant performance on cognitive tests. The primary outcome measures are the number of adverse events and the attrition rate.
Published results
Among the clinical trials newly registered in the last 4 years, four articles pertaining to two trials have been published in peer-reviewed scientific journals. The characteristics of the published randomized controlled trials are summarized in Table 7 [ 43 , 53 , 83 , 84 ]. Two articles reported the results of NCT03887455 [ 43 , 84 ], and the other two reported the results of NCT01760005 [ 53 , 83 ]. The articles were published between 2018 and 2023. The results of both NCT03887455 (Clarity AD) and NCT01760005 have been discussed in the anti-amyloid section. The methodological quality of these studies is summarized in Table 8 . Both trials (NCT03887455 and NCT01760005) had a overall low risk of bias [ 43 , 53 , 83 , 84 ].
Our understanding of AD originated from clinical research, and how pathological findings are associated with clinical presentation of AD has continued to intrigue the neuroscience research community over the past century. DMTs have become the core of new drug development, and the accumulation of knowledge is leading to the evolution of diagnostic criteria and clinical outcome measurements. The view of clinical outcomes has shifted from considering them as solely determinative to considering them to be just one of the determinants. In accordance with the 2018 NIA-AA Research Framework criteria [ 25 ] or the new 2023 NIA-AA revised criteria for AD [ 26 ], the incorporation of biomarkers is necessary in clinical practice.
This review documented that in terms of the number of AD drug trials and the number of recruited participants, the majority of trials continue to focus on mechanisms involving amyloid and tau. Our 2020 report highlighted that due to the failure of early anti-amyloid trials to achieve their intended outcomes, particularly studies involving BACE inhibitors and monoclonal antibodies, some have questioned whether amyloid remains clinically relevant in AD. This shift in perspective has led to a change in the focus of research toward populations in the prodromal or preclinical stage with positive results for diagnostic biomarkers. Additionally, the validity of the amyloid hypothesis has been contested, resulting in a significant reduction in the number of anti-amyloid phase 3 trials since 2019. However, the targets of both phase 1 and phase 2 trials are diverse, with a noticeable increase in the number of phase 1 trials focusing on neuroprotection and phase 2 trials focusing on anti-neuroinflammation [ 85 ]. Since the positive outcomes in terms of slow decline in cognitive abilities in the lecanemab Clarity AD trial [ 43 ] and the donanemab trial TRAILBLAZER-ALZ [ 86 ], the impact of amyloid and consequent pathological alterations is likely to become the main focus of clinical trials. The incorporation of amyloid-related therapy either as an add-on or as a link to specific aspects of AD pathophysiology might become an important trend in clinical trials of new drugs in the future. However, despite this expansion of research areas, the scope of indications for novel anti-amyloid monoclonal antibody therapy remains limited. The mode of treatment administration and the high monitoring costs along with the need for specialized facilities and imaging scans remain challenges. Other unmet needs, such as addressing BPSD and enhancing cognitive function, necessitate pharmaceutical research. Examining drugs with diverse mechanisms necessitates thorough evaluation that extends beyond mere clinical measurements to encompass their intermediate impact on biomarkers. It is essential to investigate the potential synergy between a new drug and existing medications approved by the US FDA. This approach could even be extended to situations where adjuvant treatment, such as tau-related treatments, is provided after amyloid clearance has been achieved. Clinical trials related to AD have also exhibited a shift in focus toward the earlier stages of AD, such as MCI, or even cognitively healthy participants for developing prevention interventions.
Successful phase 3 trials such as Clarity AD (lecanemab) and EMERGE (aducanumab) have evaluated anti-amyloid treatment in mild AD (Fig. 2 ). Trials that do not target specific pathophysiologies are becoming fewer in all phases (Figs. 2 and 3 ). However, an increasing number of early-phase trials of therapies for symptoms, including cognitive enhancers and agents for relieving BPSD, are being conducted. This reflects the unmet clinical need for such therapies (Figs. 2 and 3 ). Similarly, an increasing number of phase 1 trials involving DMTs, particularly those targeting both anti-amyloid and anti-tau mechanisms, has been noted, indicating the importance of basic research (Fig. 3 ). Outcome measurement tools have also become more diverse, which has enabled meaningful improvements in AD and the efficacy of treatments to be clearly determined in clinical trials. Overall, the field of AD clinical trials is evolving, and additional promising treatments for AD are likely to be developed in the near future.
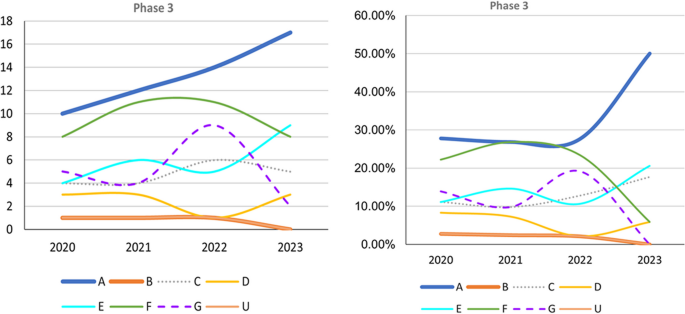
Trends in Phase 3 trials, 2020–2023, categorized according to event-related themes in ClinicalTrials.gov. Left: Number of Phase 3 trials. Right: Percentage of Phase 3 trials. A anti-amyloid therapy, B anti-tau therapy, C neuroprotection, D anti-neuroinflammation, E cognitive enhancer, F relief of behavioral psychological symptoms of dementia, G others, U undisclosed
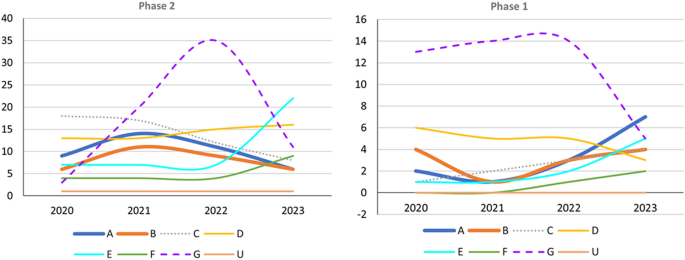
Trends in Phase 1 and 2 trials, 2020–2023, categorized according to event-related themes in ClinicalTrials.gov. Left: Number of Phase 2 trials. Right: Number of Phase 1 trials; A anti-amyloid therapy, B anti-tau therapy, C neuroprotection, D anti-neuroinflammation, E cognitive enhancer, F relief of behavioral psychological symptoms of dementia, G others, U undisclosed
Availability of data and materials
Not applicable.
Abbreviations
Amyloid-beta
Acetylcholine
Cholinesterase inhibitors
- Alzheimer disease
Alzheimer’s Disease Assessment Scale–Cognitive Subscale
Alzheimer’s Disease Cooperative Study–Activities of Daily Living Inventory–Mild Cognitive Impairment Version
Apolipoprotein gene
Amyloid precursor protein
Amyloid-related imaging abnormalities
Amyloid, tau, and neurodegeneration biomarkers
Appropriate use recommendations
Autophagic vacuoles
Beta-secretase 1
Behavioral psychological symptoms of dementia
Clinical Dementia Rating scale
Clinical Dementia Rating scale Sum of Box
Caregiver Global Impression of Change
Cerebrospinal fluid
Clinical Trial of AD
Disease-modifyung therapies
Integrated Alzheimer’s Disease Rating Scale
Immunoglobulin gamma 1
Mild cognitive impairment
Magnetic resonance imaging
Nuclear factor κB
Neurofibrillary tangles
Neuropsychiatric Inventory
Positron emission tomography
Presenilin-1
Presenilin-2
Gauthier S WC, Servaes S, Morais JA, Rosa-Neto P. World Alzheimer Report 2022: Life after diagnosis: Navigating treatment, care and support; 2022.
Apostolova LG. Alzheimer disease. Continuum (Minneap Minn). 2016;22(2 Dementia):419–34.
Mega MS, Cummings JL, Fiorello T, Gornbein J. The spectrum of behavioral changes in Alzheimer’s disease. Neurology. 1996;46(1):130–5.
Article CAS PubMed Google Scholar
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–9.
Article PubMed PubMed Central Google Scholar
Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120(3):885–90.
Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985;82(12):4245–9.
Article CAS PubMed PubMed Central Google Scholar
Goedert M, Spillantini M, Crowther R. Tau proteins and neurofibrillary degeneration. Brain Pathol. 1991;1(4):279–86.
Sperling R, Mormino E, Johnson K. The evolution of preclinical Alzheimer’s disease: implications for prevention trials. Neuron. 2014;84(3):608–22.
De Leon MJ, George AE, Golomb J, Tarshish C, Convit A, Kluger A, et al. Frequency of hippocampal formation atrophy in normal aging and Alzheimer’s disease. Neurobiol Aging. 1997;18(1):1–11.
Article PubMed Google Scholar
Parker TD, Cash DM, Lane CAS, Lu K, Malone IB, Nicholas JM, et al. Hippocampal subfield volumes and pre-clinical Alzheimer’s disease in 408 cognitively normal adults born in 1946. PLoS ONE. 2019;14(10): e0224030.
Beyreuther K, Masters CL. Amyloid precursor protein (APP) and beta A4 amyloid in the etiology of Alzheimer’s disease: precursor-product relationships in the derangement of neuronal function. Brain Pathol. 1991;1(4):241–51.
Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci. 1991;12(10):383–8.
Selkoe DJ. The molecular pathology of Alzheimer’s disease. Neuron. 1991;6(4):487–98.
Kang J, Lemaire H-G, Unterbeck A, Salbaum JM, Masters CL, Grzeschik K-H, et al. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325(6106):733–6.
Gunawardena S, Yang G, Goldstein LS. Presenilin controls kinesin-1 and dynein function during APP-vesicle transport in vivo. Hum Mol Genet. 2013;22(19):3828–43.
Kolata G. Down Syndrome—Alzheimer’s Linked: Down syndrome adults get Alzheimer-like changes in their brains and many become demented, leading researchers to ask about the connection. Science. 1985;230(4730):1152–3.
FDA Converts Novel Alzheimer’s Disease Treatment to Traditional Approval-Action Follows Confirmatory Trial to Verify Clinical Benefit. 2023.
Medina M, Avila J. The role of extracellular Tau in the spreading of neurofibrillary pathology. Front Cell Neurosci. 2014;8:113.
Jack CR, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119–28.
Gómez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC, et al. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann Neurol. 1997;41(1):17–24.
Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42(3 Pt 1):631–9.
Bierer LM, Hof PR, Purohit DP, Carlin L, Schmeidler J, Davis KL, et al. Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer’s disease. Arch Neurol. 1995;52(1):81–8.
Okamura N, Yanai K. Applications of tau PET imaging. Nat Rev Neurol. 2017;13(4):197–8.
Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87:539–47.
Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535–62.
NIA-AA Revised Clinical Criteria for Alzheimer's Disease. 2023.
Lian H, Yang L, Cole A, Sun L, Chiang AC, Fowler SW, et al. NFκB-activated astroglial release of complement C3 compromises neuronal morphology and function associated with Alzheimer’s disease. Neuron. 2015;85(1):101–15.
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–7.
Hamelin L, Lagarde J, Dorothée G, Leroy C, Labit M, Comley RA, et al. Early and protective microglial activation in Alzheimer’s disease: a prospective study using 18 F-DPA-714 PET imaging. Brain. 2016;139(4):1252–64.
Gomez-Nicola D, Boche D. Post-mortem analysis of neuroinflammatory changes in human Alzheimer’s disease. Alzheimers Res Ther. 2015;7(1):42.
Zimmer ER, Leuzy A, Benedet AL, Breitner J, Gauthier S, Rosa-Neto P. Tracking neuroinflammation in Alzheimer’s disease: the role of positron emission tomography imaging. J Neuroinflammation. 2014;11(1):1–12.
Article Google Scholar
McGeer PL, McGeer EG. The amyloid cascade-inflammatory hypothesis of Alzheimer disease: implications for therapy. Acta Neuropathol. 2013;126(4):479–97.
Lockhart SN, Schaich CL, Craft S, Sachs BC, Rapp SR, Jung Y, et al. Associations among vascular risk factors, neuroimaging biomarkers, and cognition: Preliminary analyses from the Multi-Ethnic Study of Atherosclerosis (MESA). Alzheimers Dement. 2022;18(4):551–60.
Wanleenuwat P, Iwanowski P, Kozubski W. Alzheimer’s dementia: pathogenesis and impact of cardiovascular risk factors on cognitive decline. Postgrad Med. 2019;131(7):415–22.
Lee J-H, Yang D-S, Goulbourne CN, Im E, Stavrides P, Pensalfini A, et al. Faulty autolysosome acidification in Alzheimer’s disease mouse models induces autophagic build-up of Aβ in neurons, yielding senile plaques. Nat Neurosci. 2022;25(6):688–701.
Chi H, Chang H-Y, Sang T-K. Neuronal cell death mechanisms in major neurodegenerative diseases. Int J Mol Sci. 2018;19(10):3082.
Raina P, Santaguida P, Ismaila A, Patterson C, Cowan D, Levine M, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med. 2008;148(5):379–97.
Arndt JW, Qian F, Smith BA, Quan C, Kilambi KP, Bush MW, et al. Structural and kinetic basis for the selectivity of aducanumab for aggregated forms of amyloid-β. Sci Rep. 2018;8(1):6412.
Dunn B, Stein P, Cavazzoni P. Approval of aducanumab for Alzheimer disease—the FDA’s perspective. JAMA Intern Med. 2021;181(10):1276–8.
Tucker S, Möller C, Tegerstedt K, Lord A, Laudon H, Sjödahl J, et al. The murine version of BAN2401 (mAb158) selectively reduces amyloid-β protofibrils in brain and cerebrospinal fluid of tg-ArcSwe mice. J Alzheimers Dis. 2015;43(2):575–88.
Canady VA. FDA approves new treatment for Alzheimer’s disease. Ment Heal Wkly. 2023;33(3):6–7.
Swanson CJ, Zhang Y, Dhadda S, Wang J, Kaplow J, Lai RY, et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimers Res Ther. 2021;13(1):80.
Van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9–21.
Cummings J, Rabinovici G, Atri A, Aisen P, Apostolova L, Hendrix S, et al. Aducanumab: appropriate use recommendations update. J Prev Alzheimers Di s. 2022;9(2):221–30.
CAS Google Scholar
Cummings J, Apostolova L, Rabinovici G, Atri A, Aisen P, Greenberg S, et al. Lecanemab: appropriate use recommendations. J Prev Alzheimers Dis. 2023;10(3):362–77.
CAS PubMed Google Scholar
Irizarry MC, Sims JR, Lowe SL, Nakano M, Hawdon A, Willis BA, et al. O4–08-06: Safety, pharmacokinetics (PK), and florbetapir F-18 positron emission tomography (PET) after multiple dose administration of LY3002813, a β-amyloid plaque-specific antibody, in Alzheimer’s disease. Alzheimers Dement. 2016;12:P352–3.
Bouter Y, Liekefeld H, Pichlo S, Westhoff AC, Fenn L, Bakrania P, et al. Donanemab detects a minor fraction of amyloid-β plaques in post-mortem brain tissue of patients with Alzheimer’s disease and Down syndrome. Acta Neuropathol. 2022;143(5):601–3.
Shcherbinin S, Evans CD, Lu M, Andersen SW, Pontecorvo MJ, Willis BA, et al. Association of amyloid reduction after donanemab treatment with tau pathology and clinical outcomes: the TRAILBLAZER-ALZ randomized clinical trial. JAMA Neurol. 2022;79(10):1015–24.
Pontecorvo MJ, Lu M, Burnham SC, Schade AE, Dage JL, Shcherbinin S, et al. Association of donanemab treatment with exploratory plasma biomarkers in early symptomatic Alzheimer disease: a secondary analysis of the TRAILBLAZER-ALZ randomized clinical trial. JAMA Neurol. 2022;79(12):1250–9.
Sims JR, Zimmer JA, Evans CD, Lu M, Ardayfio P, Sparks J, et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512–27.
Wessels AM, Dennehy EB, Dowsett SA, Dickson SP, Hendrix SB. Meaningful clinical changes in Alzheimer disease measured with the iADRS and illustrated using the donanemab TRAILBLAZER-ALZ study findings. Neurol Clin Pract. 2023;13(2): e200127.
Honig LS, Vellas B, Woodward M, Boada M, Bullock R, Borrie M, et al. Trial of solanezumab for mild dementia due to Alzheimer’s disease. N Engl J Med. 2018;378(4):321–30.
Salloway S, Farlow M, McDade E, Clifford DB, Wang G, Llibre-Guerra JJ, et al. A trial of gantenerumab or solanezumab in dominantly inherited Alzheimer’s disease. Nat Med. 2021;27(7):1187–96.
Hey JA, Yu JY, Versavel M, Abushakra S, Kocis P, Power A, et al. Clinical pharmacokinetics and safety of ALZ-801, a novel prodrug of tramiprosate in development for the treatment of Alzheimer’s disease. Clin Pharmacokinet. 2018;57(3):315–33.
Wang HY, Bakshi K, Frankfurt M, Stucky A, Goberdhan M, Shah SM, et al. Reducing amyloid-related Alzheimer’s disease pathogenesis by a small molecule targeting filamin A. J Neurosci. 2012;32(29):9773–84.
Lues I, Weber F, Meyer A, Bühring U, Hoffmann T, Kühn-Wache K, et al. A phase 1 study to evaluate the safety and pharmacokinetics of PQ912, a glutaminyl cyclase inhibitor, in healthy subjects. Alzheimers Dement (N Y). 2015;1(3):182–95.
Grundman M, Morgan R, Lickliter JD, Schneider LS, DeKosky S, Izzo NJ, et al. A phase 1 clinical trial of the sigma-2 receptor complex allosteric antagonist CT1812, a novel therapeutic candidate for Alzheimer’s disease. Alzheimers Dement (N Y). 2019;5:20–6.
Paul S, Haskali MB, Liow JS, Zoghbi SS, Barth VN, Kolodrubetz MC, et al. Evaluation of a PET radioligand to image O-GlcNAcase in brain and periphery of rhesus monkey and knock-out mouse. J Nucl Med. 2019;60(1):129–34.
Johnston JL, Reda SM, Setti SE, Taylor RW, Berthiaume A-A, Walker WE, et al. Fosgonimeton, a novel positive modulator of the HGF/MET system, promotes neurotrophic and procognitive effects in models of dementia. Neurotherapeutics. 2023;20(2):431–51.
Kang BW, Kim F, Cho J-Y, Kim S, Rhee J, Choung JJ. Phosphodiesterase 5 inhibitor mirodenafil ameliorates Alzheimer-like pathology and symptoms by multimodal actions. Alzheimers Res Ther. 2022;14(1):92.
Cummings J, Lee G, Nahed P, Kambar MEZN, Zhong K, Fonseca J, et al. Alzheimer’s disease drug development pipeline: 2022. Alzheimers Dement (N Y). 2022;8(1): e12295.
Fang C, Hernandez P, Liow K, Damiano E, Zetterberg H, Blennow K, et al. Buntanetap, a novel translational inhibitor of multiple neurotoxic proteins, proves to be safe and promising in both Alzheimer’s and Parkinson’s patients. J Prev Alzheimers Dis. 2023;10(1):25–33.
Gardener SL, Rainey-Smith SR, Villemagne VL, Fripp J, Doré V, Bourgeat P, et al. Higher coffee consumption is associated with slower cognitive decline and less cerebral Aβ-Amyloid accumulation over 126 months: data from the Australian imaging, biomarkers, and lifestyle study. Front Aging Neurosci. 2021;13: 744872.
Chhunchha B, Kubo E, Krueger RR, Singh D. Hydralazine revives cellular and ocular lens health-span by ameliorating the aging and oxidative-dependent loss of the Nrf2-activated cellular stress response. Antioxidants (Basel). 2023;12(1):140.
Chang T-T, Chen J-W. Potential Impacts of Hydralazine as a Novel Antioxidant on Cardiovascular and Renal Disease—Beyond Vasodilation and Blood Pressure Lowering. Antioxidants (Basel). 2022;11(11):2224.
Dehghan E, Goodarzi M, Saremi B, Lin R, Mirzaei H. Hydralazine targets cAMP-dependent protein kinase leading to sirtuin1/5 activation and lifespan extension in C. elegans . Nat Commun. 2019;10(1):4905.
Sauder C, Allen LA, Baker E, Miller AC, Paul SM, Brannan SK. Effectiveness of KarXT (xanomeline-trospium) for cognitive impairment in schizophrenia: post hoc analyses from a randomised, double-blind, placebo-controlled phase 2 study. Transl Psychiatry. 2022;12(1):491.
Li N, Zhou T, Fei E. Actions of metformin in the brain: a new perspective of metformin treatments in related neurological disorders. Int J Mol Sci. 2022;23(15):8281.
Khaleghi-Mehr M, Delshad A-A, Shafie-Damavandi S, Roghani M. Metformin mitigates amyloid β1-40-induced cognitive decline via attenuation of oxidative/nitrosative stress and neuroinflammation. Metab Brain Dis. 2023;38(4):1127–42.
Turner RS, Hebron ML, Lawler A, Mundel EE, Yusuf N, Starr JN, et al. Nilotinib effects on safety, tolerability, and biomarkers in Alzheimer’s disease. Ann Neurol. 2020;88(1):183–94.
Schneider L, Laudon M, Nir T, Caceres J, Ianniciello G, Capulli M, et al. A polymorphism cluster at the 2q12 locus may predict response to piromelatine in patients with mild Alzheimer’s disease. J Prev Alzheimers Dis. 2022;9(2):247–54.
Mahapatra MK, Karuppasamy M, Sahoo BM. Therapeutic potential of semaglutide, a newer GLP-1 receptor agonist, in abating obesity, non-alcoholic steatohepatitis and neurodegenerative diseases: a narrative review. Pharm Res. 2022;39(6):1233–48.
Li Z, et al. Modeling digestion, absorption, and ketogenesis after administration of tricaprilin formulations to humans. Eur J Pharm Biopharm. 2023;182:41–52.
Li T, Martin E, Abada YS, et al. Effects of chronic masitinib treatment in APPswe/PSEN1dE9 transgenic mice modeling Alzheimer’s disease. J Alzheimers Dis. 2020;76(4):1339–45.
Reading CL, Ahlem CN, Murphy MF. NM101 Phase III study of NE3107 in Alzheimer’s disease: rationale, design and therapeutic modulation of neuroinflammation and insulin resistance. Neurodegener Dis Manag. 2021;11(4):289–98.
Nirogi R, Jayarajan P, Benade V, Shinde A, Goyal VK, Jetta S, et al. Potential beneficial effects of masupirdine (SUVN-502) on agitation/aggression and psychosis in patients with moderate Alzheimer’s disease: exploratory post hoc analyses. Int J Geriatr Psychiatry. 2022. https://doi.org/10.1002/gps.5813 .
Herrmann N, Ruthirakuhan M, Gallagher D, Verhoeff N, Kiss A, Black SE, et al. Randomized Placebo-Controlled Trial of Nabilone for Agitation in Alzheimer’s Disease. Am J Geriatr Psychiatry. 2019;27(11):1161–73.
Wu C, Gong W-G, Wang Y-J, Sun J-J, Zhou H, Zhang Z-J, et al. Escitalopram alleviates stress-induced Alzheimer’s disease-like tau pathologies and cognitive deficits by reducing hypothalamic-pituitary-adrenal axis reactivity and insulin/GSK-3β signal pathway activity. Neurobiol Aging. 2018;67:137–47.
Xiao S, Chan P, Wang T, Hong Z, Wang S, Kuang W, et al. A 36-week multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 3 clinical trial of sodium oligomannate for mild-to-moderate Alzheimer’s dementia. Alzheimers Res Ther. 2021;13(1):62.
Lu J, Pan Q, Zhou J, Weng Y, Chen K, Shi L, et al. Pharmacokinetics, distribution, and excretion of sodium oligomannate, a recently approved anti-Alzheimer’s disease drug in China. J Pharm Anal. 2022;12(1):145–55.
Mehdipour M, Emamghoreishi M, Farrokhi MR, Amirinezhadfard E, Keshavarz M. The effect of spironolactone on β-amyloid-induced memory impairment in male rats: the role of microglial inhibition. Adv Pharm Bull. 2022;12(3):623–31.
Bateman RJ, Benzinger TL, Berry S, Clifford DB, Duggan C, Fagan AM, et al. The DIAN-TU Next Generation Alzheimer’s prevention trial: Adaptive design and disease progression model. Alzheimers Dement. 2017;13(1):8–19.
Tahami Monfared AA, Ye W, Sardesai A, Folse H, Chavan A, Aruffo E, et al. A path to improved Alzheimer’s care: simulating long-term health outcomes of lecanemab in early Alzheimer’s disease from the CLARITY AD trial. Neurol Ther. 2023;12(3):863–81.
Huang L-K, Chao S-P, Hu C-J. Clinical trials of new drugs for Alzheimer disease. J Biomed Sci. 2020;27(1):18.
Mintun MA, Lo AC, Duggan Evans C, Wessels AM, Ardayfio PA, Andersen SW, et al. Donanemab in early Alzheimer’s disease. N Engl J Med. 2021;384(18):1691–704.
Download references
Acknowledgements
This manuscript was edited by Wallace Academic Editing.
The authors have not received any funding.
Author information
Li-Kai Huang and Yi-Chun Kuan contributed equally to this work.
Authors and Affiliations
PhD Program in Medical Neuroscience, College of Medical Science and Technology, Taipei Medical University, No. 291, Zhong Zheng Road, Zhonghe District, New Taipei City, Taiwan
Li-Kai Huang & Chaur-Jong Hu
Taipei Neuroscience Institute, Taipei Medical University, New Taipei City, Taiwan
Li-Kai Huang, Yi-Chun Kuan & Chaur-Jong Hu
Dementia Center and Department of Neurology, Shuang-Ho Hospital, Taipei Medical University, New Taipei City, Taiwan
Department of Neurology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan
Yi-Chun Kuan & Chaur-Jong Hu
Department of Biomedical Engineering, National Taiwan University, Taipei, Taiwan
Yi-Chun Kuan
School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan
You can also search for this author in PubMed Google Scholar
Contributions
LKH and YCK: Conducted literature search, developed the study concept and design, extracted information from trials and studies, and contributed to manuscript drafting and revision. HWL: Extracted information from trials and studies and contributed to manuscript drafting and revision. CJH: Contributed to the study concept and design, interpreted the data and information, finalized and revised the manuscript, and provided overall supervision of the entire project.
Corresponding author
Correspondence to Chaur-Jong Hu .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Huang, LK., Kuan, YC., Lin, HW. et al. Clinical trials of new drugs for Alzheimer disease: a 2020–2023 update. J Biomed Sci 30 , 83 (2023). https://doi.org/10.1186/s12929-023-00976-6
Download citation
Received : 31 May 2023
Accepted : 26 September 2023
Published : 02 October 2023
DOI : https://doi.org/10.1186/s12929-023-00976-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Clinical trials
- Anti-amyloid
- Neuroprotection
- Cognitive enhancement
- Neuroinflammation
Journal of Biomedical Science
ISSN: 1423-0127
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Suggestions or feedback?
MIT News | Massachusetts Institute of Technology
- Machine learning
- Social justice
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
A new peptide may hold potential as an Alzheimer’s treatment
Press contact :, media download.
*Terms of Use:
Images for download on the MIT News office website are made available to non-commercial entities, press and the general public under a Creative Commons Attribution Non-Commercial No Derivatives license . You may not alter the images provided, other than to crop them to size. A credit line must be used when reproducing images; if one is not provided below, credit the images to "MIT."
Previous image Next image
MIT neuroscientists have found a way to reverse neurodegeneration and other symptoms of Alzheimer’s disease by interfering with an enzyme that is typically overactive in the brains of Alzheimer’s patients.
When the researchers treated mice with a peptide that blocks the hyperactive version of an enzyme called CDK5, they found dramatic reductions in neurodegeneration and DNA damage in the brain. These mice also showed improvements in their ability to perform tasks such as learning to navigate a water maze.
“We found that the effect of this peptide is just remarkable,” says Li-Huei Tsai, director of MIT’s Picower Institute for Learning and Memory and the senior author of the study. “We saw wonderful effects in terms of reducing neurodegeneration and neuroinflammatory responses, and even rescuing behavior deficits.”
With further testing, the researchers hope that the peptide could eventually be used as a treatment for patients with Alzheimer’s disease and other forms of dementia that have CDK5 overactivation. The peptide does not interfere with CDK1, an essential enzyme that is structurally similar to CDK5, and it is similar in size to other peptide drugs that are used in clinical applications.
Picower Institute Research Scientist Ping-Chieh Pao is the lead author of the paper , which appears this week in the Proceedings of the National Academy of Sciences .
Targeting CDK5
Tsai has been studying CDK5’s role in Alzheimer’s disease and other neurodegenerative diseases since early in her career. As a postdoc, she identified and cloned the CDK5 gene, which encodes a type of enzyme known as a cyclin-dependent kinase. Most of the other cyclin-dependent kinases are involved in controlling cell division, but CDK5 is not. Instead, it plays important roles in the development of the central nervous system, and also helps to regulate synaptic function.
CDK5 is activated by a smaller protein that it interacts with, known as P35. When P35 binds to CDK5, the enzyme’s structure changes, allowing it to phosphorylate — add a phosphate molecule to — its targets. However, in Alzheimer’s and other neurodegenerative diseases, P35 is cleaved into a smaller protein called P25, which can also bind to CDK5 but has a longer half-life than P35.
When bound to P25, CDK5 becomes more active in cells. P25 also allows CDK5 to phosphorylate molecules other than its usual targets, including the Tau protein. Hyperphosphorylated Tau proteins form the neurofibrillary tangles that are one of the characteristic features of Alzheimer’s disease.
In previous work, Tsai’s lab has shown that transgenic mice engineered to express P25 develop severe neurodegeneration. In humans, P25 has been linked to several diseases, including not only Alzheimer’s but also Parkinson’s disease and frontotemporal dementia.
Pharmaceutical companies have tried to target P25 with small-molecule drugs, but these drugs tend to cause side effects because they also interfere with other cyclin-dependent kinases, so none of them have been tested in patients.
The MIT team decided to take a different approach to targeting P25, by using a peptide instead of a small molecule. They designed their peptide with a sequence identical to that of a segment of CDK5 known as the T loop, which is a structure critical to the binding of CDK5 to P25. The entire peptide is only 12 amino acids long — slightly longer than most existing peptide drugs, which are five to 10 amino acids long.
“From a peptide drug point of view, usually smaller is better,” Tsai says. “Our peptide is almost within that ideal molecular size.”
Dramatic effects
In tests in neurons grown in a lab dish, the researchers found that treatment with the peptide led to a moderate reduction in CDK5 activity. Those tests also showed that the peptide does not inhibit the normal CDK5-P35 complex, nor does it affect other cyclin-dependent kinases.
When the researchers tested the peptide in a mouse model of Alzheimer’s disease that has hyperactive CDK5, they saw a myriad of beneficial effects, including reductions in DNA damage, neural inflammation, and neuron loss. These effects were much more pronounced in the mouse studies than in tests in cultured cells.
The peptide treatment also produced dramatic improvements in a different mouse model of Alzheimer’s, which has a mutant form of the Tau protein that leads to neurofibrillary tangles. After treatment, those mice showed reductions in both Tau pathologies and neuron loss. Along with those effects in the brain, the researchers also observed behavioral improvements. Mice treated with the peptide performed much better in a task that required learning to navigate a water maze, which relies on spatial memory, than mice that were treated with a control peptide (a scrambled version of the peptide used to inhibit CDK5-P25).
In those mouse studies, the researchers injected the peptide and found that it was able to cross the blood-brain barrier and reach neurons of the hippocampus and other parts of the brain.
The researchers also analyzed the changes in gene expression that occur in mouse neurons following treatment with the peptide. Among the changes they observed was an increase in expression of about 20 genes that are typically activated by a family of gene regulators called MEF2. Tsai’s lab has previously shown that MEF2 activation of these genes can confer resilience to cognitive impairment in the brains of people with Tau tangles, and she hypothesizes that the peptide treatment may have similar effects.
“Further development of such peptide inhibitors toward a lead therapeutic candidate, if proven to be selective for the target and relatively free of clinical side effects, may eventually lead to novel treatments for neurodegenerative disorders ranging from Alzheimer’s disease to Frontotemporal dementia to Parkinson’s disease,” says Stuart Lipton, a professor of neuroscience at Scripps Research, who was not involved in the study.
Tsai now plans to do further studies in other mouse models of diseases that involve P25-associated neurodegeneration, such as frontotemporal dementia, HIV-induced dementia, and diabetes-linked cognitive impairment.
“It’s very hard to say precisely which disease will most benefit, so I think a lot more work is needed,” she says.
The research was funded by the National Institutes of Health.
Share this news article on:
Press mentions, new york post.
In a new study, researchers at MIT showed that they “were able to interfere with an enzyme typically found to be overactive in the brains of Alzheimer’s patients,” reports Alex Mitchell for The New York Post . After using a peptide to treat the overactive enzyme, they found that “the peptide shows protective effects against loss of neurons and also appears to be able to rescue some of the behavior deficits,” says Prof. Li-Huei Tsai.
Previous item Next item
Related Links
- Li-Huei Tsai
- Department of Brain and Cognitive Sciences
Related Topics
- Neuroscience
- Alzheimer's
- Brain and cognitive sciences
- Picower Institute
- National Institutes of Health (NIH)
Related Articles

Study links gene to cognitive resilience in the elderly

Study offers an explanation for why the APOE4 gene enhances Alzheimer’s risk
Neuroscientists discover roles of gene linked to Alzheimer’s
![new research into alzheimer's disease “…[I]f humans behave similarly to mice in response to this treatment, I would say the potential is just enormous, because it’s so noninvasive, and it’s so accessible,” says Li-Huei Tsai, the Picower Professor of Neuroscience, when describing a new treatment for Alzheimer’s disease.](https://news.mit.edu/sites/default/files/styles/news_article__archive/public/images/201612/MIT-li-huei-tsai.jpg?itok=jrC2K2AI)
Unique visual stimulation may be new treatment for Alzheimer’s
More mit news.

Featured video: Moooving the needle on methane
Read full story →
The many-body dynamics of cold atoms and cross-country running

Heather Paxson named associate dean for faculty of the School of Humanities, Arts, and Social Sciences

Preparing MIT’s campus for cardiac emergencies

Researching extreme environments

To build a better AI helper, start by modeling the irrational behavior of humans
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram
Alzheimer's Research News
Top headlines, latest headlines.
- Protecting Brain Cells With Cannabinol
- HIV Treatments May Aid Alzheimer's Patients
- Tiny Brain Bubbles Carry Complete Codes
- How to Protect the Brain After Traumatic Injury
- Treatments for No. 1 Dementia in Under-60s
- Antioxidant Supplement May Improve Cognition
- What Drives Protein Build-Up in Parkinson's?
- Illuminating Oxygen's Journey in the Brain
- Familial Alzheimer's Via Bone Marrow Transplant
- Making Long-Term Memories: Nerve-Cell Damage
Earlier Headlines
Wednesday, march 27, 2024.
- Risk Factors for Faster Aging in the Brain Revealed in New Study
- Memory Self-Test Via Smartphone Can Identify Early Signs of Alzheimer's Disease
Tuesday, March 26, 2024
- New Treatment Target Identified for Alzheimer's Disease
- Common Degenerative Brain Disease May Begin to Develop in Middle Age
- Large-Scale Animal Study Links Brain pH Changes to Wide-Ranging Cognitive Issues
Monday, March 25, 2024
- Human Brains Are Getting Larger: That May Be Good News for Dementia Risk
Friday, March 22, 2024
- Physicists Develop Modeling Software to Diagnose Serious Diseases
- Movement Disorder ALS and Cognitive Disorder FTLD Show Strong Molecular Overlaps, New Study Shows
Thursday, March 21, 2024
- Novel Genetic Variants Associated With Alzheimer's Disease
- Immune Cells Identified as Key Players in Brain Health
Wednesday, March 20, 2024
- Rural and Minority Dementia Patients Face Disparities in Access to Neurologists
- Keto Diet Prevents Early Memory Decline in Mice
Tuesday, March 19, 2024
- Biomarkers of the Middle-Aged Brain Predict Cognitive Health in Old Age
Thursday, March 14, 2024
- A Healthier Diet Is Linked With a Slower Pace of Aging, Reduced Dementia Risk, Study Shows
- Alzheimer's Drug Fermented With Help from AI and Bacteria Moves Closer to Reality
Wednesday, March 13, 2024
- Blast-Related Concussions Linked to Higher Alzheimer's Risk
- Genetic Condition Haemochromatosis Linked to Higher Levels of Disease in Older People
- Integrity of Blood-Brain Barrier Depends on Protein That Is Altered in Some Neurodegenerative Diseases
- Recreational Activities Such as Golfing, Gardening May Be Associated With Increased ALS Risk Among Men
Tuesday, March 12, 2024
- 'Curved' Walking and a Depth Camera: New Tool Detects Early Cognitive Decline
Monday, March 11, 2024
- Researchers Identify Gene Involved in Neuronal Vulnerability in Alzheimer's Disease
- Wrist Device That Monitors Activity Could Help Provide Early Warning of Alzheimer's
Wednesday, March 6, 2024
- Earliest-Yet Alzheimer's Biomarker Found in Mouse Model Could Point to New Targets
- A Noninvasive Treatment for 'chemo Brain'
- Amyloid Blood Levels Associated With Brain Changes in Alzheimer's Study
Thursday, February 29, 2024
- Tiny Magnetic Particles in Air Pollution Linked to Development of Alzheimer's
- How Cognition Changes Before Dementia Hits
- Mutations in Hereditary Alzheimer's Disease Damage Neurons Without 'usual Suspect' Amyloid Plaques
- Poor Spatial Navigation Could Predict Alzheimer's Disease Years Before the Onset of Symptoms
Wednesday, February 28, 2024
- More Than Just Neurons: A New Model for Studying Human Brain Inflammation
- How 40Hz Sensory Gamma Rhythm Stimulation Clears Amyloid in Alzheimer's Mice
Monday, February 26, 2024
- Yoga Provides Unique Cognitive Benefits to Older Women at Risk of Alzheimer's Disease
Wednesday, February 21, 2024
- Air Pollution Linked to More Signs of Alzheimer's in Brain
- How AI Can Help Spot Early Risk Factors for Alzheimer's Disease
Tuesday, February 20, 2024
- Fixing Rogue Brain Cells May Hold Key to Preventing Neurodegeneration
- Blocking Key Protein May Halt Progression of Alzheimer's Disease
Friday, February 16, 2024
- New Genetic Therapy Holds Promise for ALS and Frontotemporal Dementia
Thursday, February 15, 2024
- Helping Caregivers Help People With Dementia Eat at Home
Wednesday, February 14, 2024
- Double Risk of Dementia After Mouth Ulcer Virus
- Exposure to Agent Orange Damages Brain Tissue in Ways Similar to Alzheimer's Disease
- Team Creates Novel Rabies Viral Vectors for Neural Circuit Mapping
- Gargling Away the 'bad' Bacteria in Type 2 Diabetes
Tuesday, February 13, 2024
- Are Stressed-out Brain Cells the Root Cause of Neurodegenerative Disease?
Monday, February 12, 2024
- Protein Modifications Key Influencers in Neurodegenerative Diseases
Friday, February 9, 2024
- Immune Genes Are Altered in Alzheimer's Patients' Blood
- Protein Accumulation on Fat Droplets Implicated in Late-Onset Alzheimer's Disease
- Language Barriers Could Contribute to Higher Aggression in People With Dementia
Thursday, February 8, 2024
- Analysis of Biological Networks Helps Explain the Complexity of Multiple Sclerosis
- Illuminating the Invisible: Detecting Proteins Linked to Diseases
Wednesday, February 7, 2024
- Erectile Dysfunction Drugs May Be Linked to Reduced Risk of Alzheimer's Disease
- New Direct Links Discovered Between the Brain and Its Surrounding Environment
Tuesday, February 6, 2024
- Study Finds Strongest Evidence to Date of Brain's Ability to Compensate for Age-Related Cognitive Decline
Monday, February 5, 2024
- Lighting Up Alzheimer's-Related Proteins to Allow for Earlier Disease Detection
Thursday, February 1, 2024
- Researchers 3D-Print Functional Human Brain Tissue
- Scientists Discover a Potential Way to Repair Synapses Damaged in Alzheimer's Disease
- Understanding Rapid Weight Loss in Older Women: Message from the Heart
Wednesday, January 31, 2024
- A New Way to Visualize Brain Cancer
- Did Dementia Exist in Ancient Greek and Rome?
- Researchers Find Enzyme Plays Much Larger Role in Preventing Neurodegenerative Diseases
Tuesday, January 30, 2024
- How Fasting May Protect Against Inflammation
Monday, January 29, 2024
- Re-Energizing Mitochondria to Treat Alzheimer's Disease
- Alzheimer's Disease Acquired from Historic Medical Treatments
Friday, January 26, 2024
- New Tool Helps Predict Progression of Alzheimer's
- Brain Drain: Nasopharyngeal Lymphatics Found to Be Crucial for Cerebrospinal Fluid Outflow
Tuesday, January 23, 2024
- Bioengineers on the Brink of Breaching Blood-Brain Barrier
- Could Bizarre Visual Symptoms Be a Telltale Sign of Alzheimer's?
Monday, January 22, 2024
- Liquid Laundry Detergent Packet Exposure Burden
- Thinning of Brain Region May Signal Dementia Risk 5-10 Years Before Symptoms
Friday, January 19, 2024
- Research Into the Nature of Memory Reveals How Cells That Store Information Are Stabilized Over Time
Thursday, January 18, 2024
- Wireless Drug Patch Shows Promise as Chronic Disease Treatment Delivery System
- Using Magnetized Neurons to Treat Parkinson's Disease Symptoms
- Don't Look Back: The Aftermath of a Distressing Event Is More Memorable Than the Lead-Up
- New Cause of Neuron Death in Alzheimer's Discovered
- Third Major Study Finds Evidence That Daily Multivitamin Supplements Improve Memory and Slow Cognitive Aging in Older Adults
Sunday, January 14, 2024
- Study Reveals Function of Little-Understood Synapse in the Brain
Wednesday, January 10, 2024
- ALS: Blocking Inflammation to Reduce Symptoms
Friday, January 5, 2024
- Hearing Loss Increases the Risk of Dementia
Thursday, January 4, 2024
- Even in Midlife, Disrupted Sleep Tied to Memory, Thinking Problems Later on
Wednesday, January 3, 2024
- Researchers Identify Path to Prevent Cognitive Decline After Radiation
Tuesday, January 2, 2024
- New Method Illuminates Druggable Sites on Proteins
- Healthy Omega-3 Fats May Slow Deadly Pulmonary Fibrosis
Wednesday, December 27, 2023
- Risk of Young-Onset Dementia Could Be Reduced Through Targeting Health and Lifestyle Factors
Sunday, December 24, 2023
- Predicting Alzheimer's Dementia in Oldest of the Old
Thursday, December 21, 2023
- Alzheimer's Discovery Reveals Dire Effect of Toxic Tau Protein on Brain Cells
- Integrating Research and Clinical Care to Uncover Secrets of Brain Development
- Evidence Early, but Emerging, That Gamma Rhythm Stimulation Can Treat Neurological Disorders
- Brain Lesions in Former Football Players Linked to Vascular, Brain Changes
Wednesday, December 20, 2023
- Finding That Statins Could Slow Dementia Stimulates Further Research
Tuesday, December 19, 2023
- Protein-Protein Interaction Discovery Unveils Down Syndrome's Molecular Mechanism Potential
Wednesday, December 13, 2023
- Very Irregular Sleep Linked to Higher Risk of Dementia
- Exercise Can Boost Brain Health
- Infection With Stomach Bacteria May Increase Risk of Alzheimer's Disease
Tuesday, December 12, 2023
- Smoking Causes Brain Shrinkage, Study Finds
- Saliva: A Means to Detect Pain in People With Dementia
Monday, December 11, 2023
- Clues to Preventing Alzheimer's Come from Patient Who, Despite Genetics, Evaded Disease
- Nanoparticle-Delivered RNA Reduces Neuroinflammation in Lab Tests
Friday, December 8, 2023
- New Cause of Diabetes Discovered, Offering Potential Target for New Classes of Drugs to Treat the Disease
Thursday, December 7, 2023
- Alzheimer's: Researchers Determine the Structural Basis for Interactions Between Apolipoprotein E and Amyloid Beta
- Serotonin Loss May Contribute to Cognitive Decline in the Early Stages of Alzheimer's Disease
- Protein Found in Brain Linked to Frontotemporal Dementia
- LATEST NEWS
- Health & Medicine
- Diseases & Conditions
- Alzheimer's Research
- Amyotrophic Lateral Sclerosis
- Attention Deficit Disorder
- Back and Neck Pain
- Birth Defects
- Bladder Disorders
- Blood Clots
- COVID and SARS
- Cervical Cancer
- Bladder Cancer
- Multiple Myeloma
- Pancreatic Cancer
- Brain Tumor
- Colon Cancer
- Breast Cancer
- Ovarian Cancer
- Lung Cancer
- Mesothelioma
- Skin Cancer
- Prostate Cancer
- Cerebral Palsy
- Chikungunya
- Chronic Fatigue Syndrome
- Cold and Flu
- Crohn's Disease
- Cystic Fibrosis
- Dengue Fever
- Down Syndrome
- Eating Disorder Research
- Encephalitis
- Epilepsy Research
- Erectile Dysfunction
- Fibromyalgia
- Gastrointestinal Problems
- HIV and AIDS
- Headache Research
- Hearing Loss
- Heart Health
- Cholesterol
- Stroke Prevention
- Heart Disease
- Hormone Disorders
- Hypertension
- Infectious Diseases
- Insomnia Research
- Irritable Bowel Syndrome
- Kidney Disease
- Liver Disease
- Lung Disease
- Lyme Disease
- Mental Health Research
- Multiple Sclerosis Research
- Mumps, Measles, Rubella
- Muscular Dystrophy
- Osteoporosis
- Parkinson's Research
- Prostate Health
- Restless Leg Syndrome
- Sickle Cell Anemia
- Sleep Disorder Research
- Thyroid Disease
- Triglycerides
- Tuberculosis
- Medical Topics
- Accident and Trauma
- Alternative Medicine
- Birth Control
- Bone and Spine
- Chronic Illness
- Controlled Substances
- Dietary Supplements and Minerals
- Epigenetics
- Food Additives
- Foodborne Illness
- Foot Health
- Gene Therapy
- Health Policy
- Human Biology
- Immune System
- Joint Health
- Medical Imaging
- Nervous System
- Pain Control
- Personalized Medicine
- Pharmacology
- Psychology Research
- Wounds and Healing
- PHYSICAL/TECH
- ENVIRONMENT
- SOCIETY & EDUCATION
- Two Species Interbreeding Created New Butterfly
- Warming Antarctic Deep-Sea and Sea Level Rise
- Octopus Inspires New Suction Mechanism for ...
- Cities Sinking: Urban Populations at Risk
- Puzzle Solved About Ancient Galaxy
- How 3D Printers Can Give Robots a Soft Touch
- Combo of Multiple Health Stressors Harming Bees
- Methane Emission On a Cold Brown Dwarf
- Remarkable Memories of Mountain Chickadees
- Predicting Future Marine Extinctions
Trending Topics
Strange & offbeat.
Alzheimer's disease: New research offers potential path to treatment

New research into Alzheimer's may help treatment in the future. Image: Katarzyna Grabowska/Unsplash
.chakra .wef-1c7l3mo{-webkit-transition:all 0.15s ease-out;transition:all 0.15s ease-out;cursor:pointer;-webkit-text-decoration:none;text-decoration:none;outline:none;color:inherit;}.chakra .wef-1c7l3mo:hover,.chakra .wef-1c7l3mo[data-hover]{-webkit-text-decoration:underline;text-decoration:underline;}.chakra .wef-1c7l3mo:focus,.chakra .wef-1c7l3mo[data-focus]{box-shadow:0 0 0 3px rgba(168,203,251,0.5);} Andrea Sturchio
Kariem ezzat, samir el andaloussi.
.chakra .wef-9dduvl{margin-top:16px;margin-bottom:16px;line-height:1.388;font-size:1.25rem;}@media screen and (min-width:56.5rem){.chakra .wef-9dduvl{font-size:1.125rem;}} Explore and monitor how .chakra .wef-15eoq1r{margin-top:16px;margin-bottom:16px;line-height:1.388;font-size:1.25rem;color:#F7DB5E;}@media screen and (min-width:56.5rem){.chakra .wef-15eoq1r{font-size:1.125rem;}} Health and Healthcare is affecting economies, industries and global issues

.chakra .wef-1nk5u5d{margin-top:16px;margin-bottom:16px;line-height:1.388;color:#2846F8;font-size:1.25rem;}@media screen and (min-width:56.5rem){.chakra .wef-1nk5u5d{font-size:1.125rem;}} Get involved with our crowdsourced digital platform to deliver impact at scale
Stay up to date:, health and healthcare.
- Alois Alzheimer's theory that plaques – insoluble clumps of amyloid-beta protein found in the brain – are the cause of Alzheimer’s disease still stands more than 100 years after his discovery.
- However, researchers in the field believe this fails to explain why the presence of plaques doesn't always cause neurological symptoms.
- Or why clinical trials using drugs that reduce these plaques have been largely unsuccessful.
- They recently investigated whether the amount of plaques in the brain or the amount of soluble amyloid-beta 42 remaining is more important for disease progression in people genetically predisposed to Alzheimer's.
- Those with high levels of amyloid-beta 42 in their cerebrospinal fluid (the liquid around the brain and spinal cord) were protected and their cognition was preserved over the study period, their findings show.
- This could be key in treating Alzheimer’s and other protein aggregation diseases, such as Parkinson’s and motor neuron disease, the researchers say.
In 1906, Alois Alzheimer, a psychiatrist and neuroanatomist, reported “a peculiar severe disease process of the cerebral cortex” to a gathering of psychiatrists in Tübingen, Germany. The case was a 50-year-old woman who suffered from memory loss, delusions, hallucinations, aggression and confusion – all of which worsened until her untimely death five years later.
In the autopsy, Alzheimer noticed distinctive plaques on her brain. These plaques – clumps of amyloid-beta protein – are still considered to be the cause of Alzheimer’s disease.
However, this theory has two major problems. First, it does not explain why many subjects (even old people) have plaques in their brains in the absence of any neurological symptoms, such as memory loss. Second, clinical trials for drugs that reduce these plaques have been unsuccessful – with one recent exception , but more of that later.
When amyloid-beta protein accumulates in the form of plaques (insoluble clumps), the original soluble form of the protein, which performs important functions in the brain, is consumed and lost. Some studies have shown that reduced levels of soluble amyloid-beta – called amyloid-beta 42 – have led to patients having worse clinical outcomes.
Have you read?
Driving a global effort against alzheimer’s.
In a recent study , published in the Journal of Alzheimer’s Disease , we investigated whether it’s the amount of plaques in the brain or the amount of amyloid-beta 42 remaining that is more important for Alzheimer’s disease progression.
To answer this question, we studied data on a group of people who have a rare inherited gene mutation that puts them at high risk of developing Alzheimer’s disease. The participants were from the Dominantly Inherited Alzheimer Network cohort study.
We found that the depletion of amyloid-beta 42 (the functional version of amyloid-beta) is more harmful than the amount of plaques (the insoluble clumps of amyloid beta).
Participants had an average of three years follow-up and we found that those with high levels of amyloid-beta 42 in their cerebrospinal fluid (the liquid around the brain and spinal cord) were protected and their cognition was preserved over the study period. This chimes with many studies that showed important functions of amyloid-beta 42 in memory and cognition .
It is also relevant because we studied people with the genetic mutation who develop Alzheimer’s disease, a group that is considered to provide the strongest evidence supporting the idea that amyloid-beta plaques are harmful. However, even in this group, those with higher cerebronspinal fluid (CSF) levels of amyloid-beta 42 remained cognitively normal regardless of the amount of plaques in their brains.
It is also worth mentioning that in some rare, inherited forms of Alzheimer’s disease – for example, in carriers of the so-called Osaka gene mutation or Arctic mutation – people can develop dementia having low levels of amyloid-beta 42 and no detectable plaques. This suggests that plaques aren’t the cause of their dementia, but low levels of amyloid-beta 42 might be.
Alzheimer’s Diesease, a result of rapid ageing that causes dementia, is a growing concern. Dementia, the seventh leading cause of death worldwide, cost the world $1.25 trillion in 2018, and affected about 50 million people in 2019. Without major breakthroughs, the number of people affected will triple by 2050, to 152 million.
To catalyse the fight against Alzheimer's, the World Economic Forum is partnering with the Global CEO Initiative (CEOi) to form a coalition of public and private stakeholders – including pharmaceutical manufacturers, biotech companies, governments, international organizations, foundations and research agencies.
The initiative aims to advance pre-clinical research to advance the understanding of the disease, attract more capital by lowering the risks to investment in biomarkers, develop standing clinical trial platforms, and advance healthcare system readiness in the fields of detection, diagnosis, infrastructure and access.
Lecanemab – the one recent exception
How will our findings affect drug development and clinical trials for Alzheimer’s disease? Until the recent trial with lecanemab , an antibody drug that reduces plaques, all the drug trials in Alzheimer’s disease have failed.
Some drugs were designed to reduce the levels of amyloid-beta 42, based on the rationale that if levels of the normal protein are reduced, patients will accumulate fewer plaques. Unfortunately, these drugs often made the patient’s condition worse .
Lecanemab was recently reported to have a small but significant effect in reducing cognitive decline. According to previous studies , this drug increases the levels of amyloid-beta 42 in the CSF. This is, again, in line with our hypothesis, namely that the increase of the normal amyloid protein can be beneficial.
We will know more when the results of the lecanemab trial are published. At the moment, all we have is a press release from the makers of the drug.
We think that it will be important for future trials to focus on the levels of amyloid-beta 42, and whether it is beneficial to increase and restore its levels to normal values instead of targeting it for removal. This could be achieved using proteins similar to amyloid-beta 42 – so-called “protein analogues” – but that clump together less than the natural ones.
This active protein replacement approach might become a promising new avenue of treatment for Alzheimer’s and other protein aggregation diseases, such as Parkinson’s and motor neuron disease.
Don't miss any update on this topic
Create a free account and access your personalized content collection with our latest publications and analyses.
License and Republishing
World Economic Forum articles may be republished in accordance with the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International Public License, and in accordance with our Terms of Use.
The views expressed in this article are those of the author alone and not the World Economic Forum.
Related topics:
The agenda .chakra .wef-n7bacu{margin-top:16px;margin-bottom:16px;line-height:1.388;font-weight:400;} weekly.
A weekly update of the most important issues driving the global agenda
.chakra .wef-1dtnjt5{display:-webkit-box;display:-webkit-flex;display:-ms-flexbox;display:flex;-webkit-align-items:center;-webkit-box-align:center;-ms-flex-align:center;align-items:center;-webkit-flex-wrap:wrap;-ms-flex-wrap:wrap;flex-wrap:wrap;} More on Health and Healthcare .chakra .wef-17xejub{-webkit-flex:1;-ms-flex:1;flex:1;justify-self:stretch;-webkit-align-self:stretch;-ms-flex-item-align:stretch;align-self:stretch;} .chakra .wef-nr1rr4{display:-webkit-inline-box;display:-webkit-inline-flex;display:-ms-inline-flexbox;display:inline-flex;white-space:normal;vertical-align:middle;text-transform:uppercase;font-size:0.75rem;border-radius:0.25rem;font-weight:700;-webkit-align-items:center;-webkit-box-align:center;-ms-flex-align:center;align-items:center;line-height:1.2;-webkit-letter-spacing:1.25px;-moz-letter-spacing:1.25px;-ms-letter-spacing:1.25px;letter-spacing:1.25px;background:none;padding:0px;color:#B3B3B3;-webkit-box-decoration-break:clone;box-decoration-break:clone;-webkit-box-decoration-break:clone;}@media screen and (min-width:37.5rem){.chakra .wef-nr1rr4{font-size:0.875rem;}}@media screen and (min-width:56.5rem){.chakra .wef-nr1rr4{font-size:1rem;}} See all

Scientists have invented a method to break down 'forever chemicals' in our drinking water. Here’s how
Johnny Wood
April 17, 2024

Young people are becoming unhappier, a new report finds

Promoting healthy habit formation is key to improving public health. Here's why
Adrian Gore
April 15, 2024
Why stemming the rise of antibiotic resistance will be an historic achievement
Carel du Marchie Sarvaas
April 11, 2024

Medical innovation for an aging world: the view from Hong Kong
April 10, 2024

Kidney disease ‘should be global health priority’, plus other top health stories
Shyam Bishen

Gene Discovery May Lead to Better Alzheimer's Treatments
Gene Discovery May Lead to Better Alzheimer's Treatments
By Ernie Mundell HealthDay Reporter

THURSDAY, April 11, 2024 (HealthDay News) -- The discovery of a gene variant that rids the brain of toxic plaques linked to Alzheimer's might lead to new treatments for the disease, researchers report.
The variant arises naturally in people who don't seem to get Alzheimer's disease despite having another gene, called APOEe4, that strongly promotes the illness.
“These resilient people can tell us a lot about the disease and what genetic and non-genetic factors might provide protection,” explained study co-lead author Badri Vardarajan , an assistant professor of neurological science at Columbia University in New York City.
"We hypothesized that these resilient people may have genetic variants that protect them from APOEe4," Vardarajan added in a university news release.
U.S. Cities With the Most Homelessness

The researchers believe the newly discovered gene variant may reduce the risk for Alzheimer's by more than 70% in folks lucky enough to carry it.
Breaking the blood-brain barrier
In their research, Vardarajan and colleagues conducted genomic screening on hundreds of individuals who carried the APOEe4 gene variant, which greatly raises the risk for Alzheimer's, but who had gone into old age without developing the disease.
The found a common variant shared by many of these people. It occurred in a gene charged with making fibronectin, a component used to make the "blood-brain barrier."
That's the lining that surrounds the brain and helps police which substances can get in and out.
People who develop Alzheimer's tend to produce much higher levels of fibronectin, the Columbia team noted. In contrast, folks with the newly discovered gene variant had only small amounts of fibronectin within their blood-brain barrier.
That means that certain toxins -- including amyloid plaques that are a hallmark of Alzheimer's -- can more readily escape the brain in folks who carry the newly discovered gene variant.
“Alzheimer’s disease may get started with amyloid deposits in the brain, but the disease manifestations are the result of changes that happen after the deposits appear,” noted study co-leader Caghan Kizil , an associate professor of neurological science at Columbia's Vagelos College of Physicians and Surgeons.
Excess fibronectin could encourage all that toxic amyloid to stay put.
"It’s a classic case of too much of a good thing,” Kizil said. “It made us think that excess fibronectin could be preventing the clearance of amyloid deposits from the brain.”
People with the new gene variant may have a kind of "release valve" to let amyloid drain away in blood.
“Our findings suggest that some of these changes occur in the brain’s vasculature and that we may be able to develop new types of therapies that mimic the gene’s protective effect to prevent or treat the disease," said Kizil.
Earlier clearance, better treatment?
Certain newly approved drugs, such as Leqembi , create an immune system response that can help remove some amyloid from the brain. However, the effect is weak and may come too late, said study co-leader Dr. Richard Mayeux , chair of neurology at Columbia.
“We may need to start clearing amyloid much earlier and we think that can be done through the bloodstream,” Mayeux said. “That's why we are excited about the discovery of this variant in fibronectin, which may be a good target for drug development.”
The initial findings from the Columbia cohort of Alzheimer's-resistant patients have already been replicated in a group of similar patients in Europe. That study was led by researchers at Stanford and Washington universities.
“They found the same fibronectin variant, which confirmed our finding and gave us even more confidence in our result,” Vardarajan noted.
The total number of patients studied topped 11,000, and that statistical power allowed the researchers to calculate that the gene variant cut a person's odds for Alzheimer's by 71%.
Even if Alzheimer's did develop, they estimate that the fibronectin gene variant slowed disease onset by an average of four years.
The Columbia team published its findings April 10 in the journal Acta Neuropathologica .
About 1% of all APOEe4 carriers in the United States are thought to also carry the fibronectin gene variant, the study authors noted.
“There’s a significant difference in fibronectin levels in the blood-brain barrier between cognitively healthy individuals and those with Alzheimer's disease, independent of their APOEe4 status,” Kizil said. “Anything that reduces excess fibronectin should provide some protection, and a drug that does this could be a significant step forward in the fight against this debilitating condition.”
More information
Find out more about amyloid plaques and Alzheimer's disease at the Alzheimer's Association .
SOURCE: Columbia University, news release, April 10, 2024
Copyright © 2024 HealthDay . All rights reserved.
Join the Conversation
Tags: Alzheimer's disease
America 2024

Health News Bulletin
Stay informed on the latest news on health and COVID-19 from the editors at U.S. News & World Report.
Sign in to manage your newsletters »
Sign up to receive the latest updates from U.S News & World Report and our trusted partners and sponsors. By clicking submit, you are agreeing to our Terms and Conditions & Privacy Policy .
You May Also Like
The 10 worst presidents.
U.S. News Staff Feb. 23, 2024

Cartoons on President Donald Trump
Feb. 1, 2017, at 1:24 p.m.

Photos: Obama Behind the Scenes
April 8, 2022

Photos: Who Supports Joe Biden?
March 11, 2020

House Passes $95B Foreign Aid Package
Aneeta Mathur-Ashton April 20, 2024

Title IX or Student Debt Relief?
Lauren Camera April 19, 2024

A Look at the Trump Jury
Laura Mannweiler April 19, 2024

Jury Set in Trump Hush Money Trial

Dems Save the Day for Johnson
Aneeta Mathur-Ashton April 19, 2024

What to Know: Israel’s Strike on Iran
Cecelia Smith-Schoenwalder April 19, 2024

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Comprehensive Review on Alzheimer’s Disease: Causes and Treatment
Alzheimer’s disease (AD) is a disorder that causes degeneration of the cells in the brain and it is the main cause of dementia, which is characterized by a decline in thinking and independence in personal daily activities. AD is considered a multifactorial disease: two main hypotheses were proposed as a cause for AD, cholinergic and amyloid hypotheses. Additionally, several risk factors such as increasing age, genetic factors, head injuries, vascular diseases, infections, and environmental factors play a role in the disease. Currently, there are only two classes of approved drugs to treat AD, including inhibitors to cholinesterase enzyme and antagonists to N -methyl d -aspartate (NMDA), which are effective only in treating the symptoms of AD, but do not cure or prevent the disease. Nowadays, the research is focusing on understanding AD pathology by targeting several mechanisms, such as abnormal tau protein metabolism, β-amyloid, inflammatory response, and cholinergic and free radical damage, aiming to develop successful treatments that are capable of stopping or modifying the course of AD. This review discusses currently available drugs and future theories for the development of new therapies for AD, such as disease-modifying therapeutics (DMT), chaperones, and natural compounds.
1. Introduction
Alzheimer’s disease (AD) (named after the German psychiatric Alois Alzheimer) is the most common type of dementia and can be defined as a slowly progressive neurodegenerative disease characterized by neuritic plaques and neurofibrillary tangles ( Figure 1 ) as a result of amyloid-beta peptide’s (Aβ) accumulation in the most affected area of the brain, the medial temporal lobe and neocortical structures [ 1 ]. Alois Alzheimer noticed a presence of amyloid plaques and a massive loss of neurons while examining the brain of his first patient that suffered from memory loss and change of personality before dying and described the condition as a serious disease of the cerebral cortex. Emil Kraepelin named this medical condition Alzheimer’s disease for the first time in his 8th edition psychiatry handbook [ 2 , 3 ]. Progressive loss of cognitive functions can be caused by cerebral disorder like Alzheimer’s disease (AD) or other factors such as intoxications, infections, abnormality in the pulmonary and circulatory systems, which causes a reduction in the oxygen supply to the brain, nutritional deficiency, vitamin B12 deficiency, tumors, and others [ 4 , 5 ].

The physiological structure of the brain and neurons in ( a ) healthy brain and ( b ) Alzheimer’s disease (AD) brain.
At present, there are around 50 million AD patients worldwide and this number is projected to double every 5 years and will increase to reach 152 million by 2050. AD burden affects individuals, their families, and the economy, with estimated global costs of US$1 trillion annually. At present, there is no cure for Alzheimer’s disease, although there are available treatments that just improve the symptoms [ 6 , 7 ]. The purpose of this review is to give a brief description about AD diagnosis, pathology, causes, and current treatments, and to highlight the recent development of compounds that could prevent or treat AD by targeting several pathogenic mechanisms, such as Aβ and tau aggregation, and misfolding, inflammation, oxidative damage, and others.
2. Alzheimer’s Disease Diagnostic Criteria
A patient suspected to have AD should undergo several tests, including neurological examination, magnetic resonance imaging (MRI) for neurons, laboratory examinations such as vitamin B12, and other tests besides the medical and family history of the patients [ 8 ]. Vitamin (vit.) B12 deficiency has been long known for its association with neurologic problems and increasing risks of AD, according to some studies. A special marker of vit. B12 deficiency is elevated homocysteine levels, which can cause brain damage by oxidative stress, increasing calcium influx and apoptosis. Diagnoses of vit. B12 deficiency can be done by measuring serum vit. B12 level alongside complete blood count and serum homocysteine levels tests [ 9 , 10 ].
In 1984, The National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) and the Alzheimer’s Disease and Related Disorders Association (ADRDA) formed a work group (NINCDS-ADRDA) to establish a clinical diagnostic’s criteria for Alzheimer’s disease. This criteria includes: (1) probable Alzheimer’s disease, which can be diagnosed by dementia that is confirmed by neuropsychological tests, progressive memory loss, impaired daily-life activity, and other symptoms like aphasia (impairment of a language), apraxia (a motor skills disorder), and agnosia (a loss of perception). All of these symptoms can start from age 40–90, with the absence of any systemic or brain diseases, (2) possible Alzheimer’s disease can be applied in the absence of neurologic, psychiatric disorders, and the presence of another illness like systemic or brain disorder, but they are not the primary cause of dementia, and (3) definite Alzheimer’s disease, that is confirmed by histopathologic confirmation obtained from a biopsy or autopsy [ 11 , 12 ].
In 2011, The National Institute on Aging—Alzheimer’s Association made several changes and updated the 1984 NINCDS-ADRDA criteria for higher specificity and sensitivity in the diagnosis of Alzheimer’s disease. The newly proposed criteria include probable and possible AD dementia for the use in clinical settings and probable or possible AD dementia with pathophysiological evidence for research purposes, in addition to clinical biomarkers. There are two categories of Alzheimer’s disease biomarkers: (a) markers of brain amyloid such as positron emission tomography (PET) and cerebrospinal fluid (CSF), and (b) markers of neuronal injury like cerebrospinal fluid tau, fluorodeoxyglucose (FDG) for metabolic activity, and magnetic resonance imaging (MRI) for atrophy measurement [ 13 , 14 , 15 ].
3. Alzheimer’s Disease’s Neuropathology
There are two types of neuropathological changes in AD which provide evidence about disease progress and symptoms and include: (1) positive lesions (due to accumulation), which are characterized by the accumulation of neurofibrillary tangles, amyloid plaques, dystrophic neurites, neuropil threads, and other deposits found in the brains of AD patients. In addition to (2) negative lesions (due to losses), that are characterized by large atrophy due to a neural, neuropil, and synaptic loss. Besides, other factors can cause neurodegeneration such as neuroinflammation, oxidative stress, and injury of cholinergic neurons [ 16 , 17 , 18 ].
3.1. Senile Plaques (SP)
The senile plaques are extracellular deposits of beta-amyloid protein (Aβ) with different morphological forms, including neuritic, diffuse, dense-cored, or classic and compact type plaques. Proteolytic cleavage enzymes such as β-secretase and γ-secretase are responsible for the biosynthesis of Aβ deposits from the transmembrane amyloid precursor protein (APP) [ 19 , 20 , 21 ]. These enzymes cleave APP into several amino acid fragments: 43, 45, 46, 48, 49, and 51 amino acids, which reach the final forms Aβ40 and Aβ42. There are several types of Aβ monomers, including large and insoluble amyloid fibrils which can accumulate to form amyloid plaques and soluble oligomers that can spread throughout the brain. Aβ plays a major role in neurotoxicity and neural function, therefore, accumulation of denser plaques in the hippocampus, amygdala, and cerebral cortex can cause stimulation of astrocytes and microglia, damage to axons, dendrites, and loss of synapses, in addition to cognitive impairments [ 21 , 22 , 23 ].
3.2. Neurofibrillary Tangles (NFTs)
NFT are abnormal filaments of the hyperphosphorylated tau protein that in some stages can be twisted around each other to form paired helical filament (PHF) and accumulate in neuralperikaryal cytoplasm, axons, and dendrites, which cause a loss of cytoskeletal microtubules and tubulin-associated proteins. The hyperphosphorylated tau protein is the major constituent of NFTs in the brains of AD patients, and its evolution can reflect NFTs morphological stages, which include: (1) pre-tangle phase, one type of NFT, where phosphorylated tau proteins are accumulated in the somatodendritic compartment without the formation of PHF, (2) mature NFTs, which are characterized by filament aggregation of tau protein with the displacement of the nucleus to the periphery part of the soma, and (3) the extracellular tangles, or the ghost NFTs stage, that results from a neuronal loss due to large amounts of filamentous tau protein with partial resistance to proteolysis [ 24 , 25 ].
3.3. Synaptic Loss
A synaptic damage in the neocortex and limbic system causes memory impairment and generally is observed at the early stages of AD. Synaptic loss mechanisms involve defects in axonal transport, mitochondrial damage, oxidative stress, and other processes that can contribute to small fractions, like the accumulation of Aβ and tau at the synaptic sites. These processes eventually lead to a loss of dendritic spines, pre-synaptic terminals, and axonal dystrophy [ 26 ]. Synaptic proteins serve as biomarkers for the detection of synapses loss, and severity, such as neurogranin, a postsynaptic neuronal protein, visinin-like protein-1 (VILIP-1), and synaptotagmin-1 [ 27 , 28 ].
4. The Stages of Alzheimer’s Disease
The clinical phases of Alzheimer’s disease can be classified into (1) pre-clinical or the pre-symptomatic stage, which can last for several years or more. This stage is characterized by mild memory loss and early pathological changes in cortex and hippocampus, with no functional impairment in the daily activities and absence of clinical signs and symptoms of AD [ 1 , 29 , 30 ]. (2) The mild or early stage of AD, where several symptoms start to appear in patients, such as a trouble in the daily life of the patient with a loss of concentration and memory, disorientation of place and time, a change in the mood, and a development of depression [ 30 , 31 ]. (3) Moderate AD stage, in which the disease spreads to cerebral cortex areas that results in an increased memory loss with trouble recognizing family and friends, a loss of impulse control, and difficulty in reading, writing, and speaking [ 30 ]. (4) Severe AD or late-stage, which involves the spread of the disease to the entire cortex area with a severe accumulation of neuritic plaques and neurofibrillary tangles, resulting in a progressive functional and cognitive impairment where the patients cannot recognize their family at all and may become bedridden with difficulties in swallowing and urination, and eventually leading to the patient’s death due to these complications [ 1 , 32 ].
5. Causes and Risk Factors of Alzheimer’s Disease
AD has been considered a multifactorial disease associated with several risk factors ( Figure 2 ) such as increasing age, genetic factors, head injuries, vascular diseases, infections, and environmental factors (heavy metals, trace metals, and others). The underlying cause of pathological changes in Alzheimer’s disease (Aβ, NFTs, and synaptic loss) is still unknown. Several hypotheses were proposed as a cause for AD but two of them are believed to be the main cause: some believe that an impairment in the cholinergic function is a critical risk factor for AD, while others suggest that alteration in amyloid β-protein production and processing is the main initiating factor. However, at present, there is no accepted theory for explaining the AD pathogenesis [ 33 , 34 ].

The risk factors for Alzheimer’s disease.
5.1. Alzheimer’s Disease Hypotheses
5.1.1. cholinergic hypothesis.
In the 1970s, neocortical and presynaptic cholinergic deficits were reported to be related to the enzyme choline acetyltransferase (ChAT), which is responsible for the synthesis of acetylcholine (ACh). Due to the essential role of ACh in cognitive function, a cholinergic hypothesis of AD was proposed. ACh is synthesized in the cytoplasm of cholinergic neurons from choline and acetyl-coenzyme A by the ChAT enzyme and transported to the synaptic vesicles by vesicular acetylcholine transporter (VAChT) ( Figure 3 ). In the brain, ACh is involved in several physiological processes such as memory, attention, sensory information, learning, and other critical functions. Degeneration of the cholinergic neurons was found to take place in AD and to cause alternation in cognitive function and memory loss. Β -amyloid is believed to affect cholinergic neurotransmission and to cause a reduction in the choline uptake and a release of ACh. Studies demonstrated that cholinergic synaptic loss and amyloid fibril formation are related to Aβ oligomers’ neurotoxicity and to interactions between AChE and Aβ peptide. Additional factors also contribute to the progression of AD, such as a reduction in nicotinic and muscarinic (M2) Ach receptors, located on presynaptic cholinergic terminals, and the deficit in excitatory amino acid (EAA) neurotransmission, where glutamate concentration and D-aspartate uptake are significantly reduced in many cortical areas in AD brains. This is in addition to the use of cholinergic receptor antagonists such as scopolamine, which was found to induce amnesia. This effect can be reversed by using compounds that activate acetylcholine formation [ 35 , 36 , 37 ].

The pathway for the synthesis and transportation of acetylcholine between presynaptic and postsynaptic nerve terminals.
As a result, the cholinergic hypothesis is based on three concepts: reduced presynaptic cholinergic markers in the cerebral cortex, severe neurodegeneration of nucleus basalis of Meynert (NBM) in the basal forebrain, which is the source of cortical cholinergic innervation, and the role of cholinergic antagonists in memory decline compared to the agonists, which have the opposite effect [ 38 ].
5.1.2. Amyloid Hypothesis
For decades, it was recognized that abnormal deposition of β-sheets in the central nervous system has a strong correlation with dementia, which led to the concept of the amyloid hypothesis. However, it was found that the amyloid plaques (AP) also deposit in normal healthy brains with aging, which raised the question of whether AP deposition is responsible for AD onset or not? Therefore, in the recent years, alternative hypotheses were proposed for the non-inherited form of AD (NIAD), but at present, the amyloid hypothesis remains the most accepted pathological mechanism for inherited AD (IAD). The amyloid hypothesis suggests that the degradation of Aβ, derived from APP by β- and γ-secretase, is decreased by age or pathological conditions, which leads to the accumulation of Aβ peptides (Aβ40 and Aβ42). Increasing the ratio of Aβ42/Aβ40 induces Aβ amyloid fibril formation, resulting in neurotoxicity and tau pathology induction, and consequently, leading to neuronal cell death and neurodegeneration. AD risk factors and mutations of several genes like APP, PSEN1, and PSEN2 were found to affect Aβ catabolism and anabolism, which rapidly cause an accumulation of Aβ and fast progression of neurodegeneration [ 39 , 40 , 41 ].
5.2. Alzheimer’s Disease Risk Factors
5.2.1. aging.
The most important risk factor in AD is aging. Younger individuals rarely have this disease, and most AD cases have a late onset that starts after 65 years of age [ 42 ]. Aging is a complex and irreversible process that occurs through multiple organs and cell systems with a reduction in the brain volume and weight, a loss of synapses, and ventricles’ enlargement in specific areas accompanied by SP deposition and NFT. Moreover, several conditions might emerge during aging such as glucose hypometabolism, cholesterol dyshomeostasis, mitochondria dysfunction, depression, and cognitive decline. These changes also appear in normal aging, which makes it difficult to distinguish the cases in early AD [ 43 , 44 ]. AD can be divided based on age of onset into early-onset AD (EOAD), the rare form with around 1–6% of cases, in which most of them are familial AD characterized by having more than one member in more than one generation with AD, and ranges from 30–60 or 65 years. The second type is the late-onset AD (LOAD), which is more common with age of onset above 65 years. Both types may occur in people who have a family with a positive history of AD and families with a late-onset disease [ 45 ].
5.2.2. Genetics
Genetic factors were discovered over the years and were found to play a major role in the development of AD. 70% of the AD cases were related to genetic factors: most cases of EOAD are inherited in an autosomal dominant pattern and mutations in the dominant genes such as Amyloid precursor protein (APP) , Presenilin-1 (PSEN-1), Presenilin-2 (PSEN-2) , and apolipoprotein E (ApoE) are associated with AD [ 46 , 47 ].
Herein, we discuss the strong genetic risk factors in AD.
- Amyloid Precursor Protein (APP)
APP is a type I transmembrane protein cleaved by α-, β-, and γ-secretase to release Aβ and other proteins and is encoded by the APP gene on chromosome 21. Thirty mutations have been found in the APP gene in which twenty-five of them are related to AD and cause an accumulation of Aβ with elevated amounts. Meanwhile, there is one protective mutation, A673T, which protects against AD by decreasing Aβ, Aβ40, and Aβ42 secretion [ 48 , 49 ]. All mutations surround the secretase cleavage site, for example, the KM670/671NL mutation in mouse models has shown an increasing level of amyloid plaques in the hippocampus and cortex with no NFTs. A673V, D678H, D678N, E682K, and K687N mutations have shown cortical atrophy, whereas E682K has shown hippocampal atrophy. Neuropathological reports for the A673V mutation demonstrated a presence of NFTs and Aβ, activation of microglia and astrocytes, and neuronal loss, compared to the rest of the mentioned mutations, which show no change in the intracellular Aβ according to neuropathological reports [ 48 , 50 ]. Other mutations such as T714I, V715A, V715M, V717I, V717L, L723P, K724N, and I716V affect the γ-secretase cleavage site and cause an increase in the Aβ42/Aβ40 ratio, while E693G, E693K, D694N, and A692G mutations affect the α-secretase cleavage site and cause polymorphic aggregates with the ability to disrupt bilayer integrity. Also, the E693delta is a deletion mutation that enhances the formation of synaptotoxic Aβ [ 51 , 52 ].
- Presenilin-1 (PSEN-1) and Presenilin-2 (PSEN-2)
PSEN1 and PSEN2 genes are also the autosomal dominant form of EOAD located on chromosomes 14 and 1, respectively. PSEN-2 and PSEN-1 are homologous, with 67% similarity, with a difference in the N -terminus and the hydrophilic region. Mutation in PSEN1 gene is more common, with more than 200 mutations, while a rare form with less than 40 mutations was identified in the PSEN2 gene [ 53 , 54 ].
PSEN1 is a core protein that activates the γ-secretase complex and plays an important role in the production of Aβ from APP. Knockout studies of PSEN1 showed synaptic dysfunction and memory impairment in mice, which indicate its essential role in maintaining memory and neurons [ 51 ]. PSEN1 mutations are simple ones which include single amino acid substitution, and severe mutation can result from the substitutions of two amino acids [ 55 ]. Mutations in the PSEN1 gene increase the ratio of Aβ42/Aβ40 by decreasing Aβ40 levels. The results obtained by Sun et al. study demonstrated that C410Y or L435F mutations in PSEN1 knock-in mice increased the Aβ42/Aβ40 ratio due to a greater reduction in Aβ40 [ 56 ].
In contrast, PSEN-2 mutations are rare and play a minor role in Aβ production. Any mutation in PSEN-2 might have a severe effect on the Aβ 42/40 ratio, causing familial AD in the presence of normal PSEN-1 alleles. Some of the PSEN-2 mutations cause a significant increase in γ-secretase activity with an elevation in the Aβ-42 and Aβ 42/40 ratio level, such as N141I, T122P, M239V, and M239I, while others are rare polymorphisms and have no effect on Aβ-42, -40, and Aβ 42/40 ratio levels and are not considered as pathogenic mutations [ 53 , 57 ].
- Apolipoprotein E (ApoE)
ApoE protein is a glycoprotein expressed highly in the liver and brain astrocytes and some microglia and serves as a receptor-mediated endocytosis ligand for lipoprotein particles like cholesterol, which is essential for myelin production and normal brain function. The ApoE gene located on chromosome 19 has three isoforms, ApoE2, ApoE3, and ApoE4, due to single-nucleotide polymorphisms (SNPs) which cause changes in the coding sequence. The ApoEε4 allele is a strong risk factor for both EOAD and LOAD compared to ApoEε2 and ApoEε3 alleles that are associated with a lower risk and protective effect, respectively [ 58 ]. ApoEε4 plays an important role in Aβ deposition as a senile plaque and causes cerebral amyloid angiopathy (CAA), which is known as a marker for AD [ 59 ]. ApoEε4 was also shown to be associated with vascular damage in the brain, which leads to AD pathogenesis [ 60 ].
- ATP Binding Cassette Transporter A1 (ABCA1)
Adenosine triphosphate (ATP)-binding cassette transporter A1 (ABCA1) is part of a large ABC transporters family that regulate cholesterol efflux in the circulation, like apolipoproteins-AI (ApoAI), and into the brain, like ApoE. In addition, ABCA1 maintains the stability of ApoE lipidation and serves as a mediator for high-density lipoprotein (HDL) generation, which reflects its role in atherosclerosis and cardiovascular diseases. Studies on the AD mice model showed that ABCA1 deficiency increases amyloid plaques and eliminates the lipidation of ApoE [ 61 ]. In humans, a mutation in ABCA1 results in Tangier disease, which is characterized by low levels of high-density lipoprotein (HDL) and ApoAI in plasma, accumulation of cholesterol in tissues, and AD pathogenesis [ 62 ].
- Clusterin Gene (CLU) and Bridging Integrator 1 ( BIN1 )
In contrast to PSEN1 , PSEN2 , and APP mutations, which result in familial or EOAD, clusterin ( CLU) and Bridging Integrator 1 ( BIN1 ) genes are novel risk factors for LOAD. In 2009, Genome-Wide Association Studies (GWAS) identified the CLU gene located on chromosome 8, which is upregulated in the cortex and hippocampus of AD brains, in addition to AD cerebrospinal fluid (CSF) and plasma, which make the CLU a promising biomarker for AD. The CLU may play a protective role by interacting with Aβ and promoting its clearance, or a neurotoxic role by reducing Aβ clearance. The Aβ ratio values determine whether the CLU role is neuroprotective or neurotoxic [ 63 ].
BIN1 is a Bin-Amphiphysin-Rvs (BAR) adaptor protein that is involved in the production of membrane curvature and other endocytosis cellular functions. BIN1 has several isoforms: some are found in the brain, where they interact with different proteins such as clathrin, synaptojanin, and amphiphysin 1, and others in which they regulate synaptic vesicle endocytosis. Recently, BIN1 was recognized as the second most important risk factor for LOAD after ApoE, where it plays a role in Aβ production and as a tau and NFT pathology modulator [ 64 , 65 ].
- Evolutionarily Conserved Signaling Intermediate in Toll pathway (ECSIT)
A significant accumulation of Aβ in AD brains increases protein oxidation, which reflects the critical role of mitochondria in Aβ cytotoxicity and AD pathogenesis. Evolutionarily conserved signaling intermediate in Toll pathway (ECSIT) gene is located on chromosome 19 and is associated with increasing the risk of AD. ECSIT encodes the adapting protein that functions as a cytoplasmic and signaling protein and is responsible for stabilizing the mitochondrial respiratory complex. Moreover, the adaptor protein is involved in the activation of nuclear factor (NF)-κB, interferon regulatory factors (IRFs), and activating protein-1. Also, it is involved in coupling immune toll-like receptor (TLR), homeostatic bone morphogenetic pathway (BMP), and transforming growth factor-beta (TGF-b) pathways [ 66 , 67 ].
ECSIT interacts with mitochondrial proteins such as Lon protease homolog (LONP1) and glutaryl-CoA dehydrogenase (GCDH), which are involved in intra-mitochondrial proteolysis and redox signaling respectively, followed by interactions with AD seed nitric oxide synthase (NOS3). Moreover, studies have shown certain interactions of ECSIT with the AD genes ApoE , PSEN-1 , and PSEN-2 . These interactions support the role of ECSIT as a molecular link in oxidative stress, inflammation, and mitochondrial dysfunction in AD [ 66 , 68 ].
- Estrogen Receptor Gene (ESR)
AD affects both women and men, but nearly two-thirds of AD cases are women. Several studies have shown that women with AD experience worse mental deterioration than men. Additionally, on the genetic level, some genes’ variation, like the ApoE4 allele, significantly increases AD risk in women compared to men. Other studies documented that AD risk in women is associated with the loss of ovarian hormones during menopause due to the fact that estrogen regulates several activities in the brain, such as neurotransmission, neural development, survival, protection against oxidative stress, reduction of Aβ peptide levels, and attenuation of tau hyperphosphorylation. The estrogen activity is mediated through estrogen receptors (ERs) (intracellular, transmembrane, and membrane-bound ERs). The two major subtypes of these receptors are ERα and Erβ, which are encoded by two distinct genes and are located on chromosome 6 and 14, respectively. ERα receptor is found in the hypothalamus and amygdala, whereas ERβ receptors are in the hippocampus and cortex. Single nucleotide polymorphisms (SNPs) in ERβ and ERα genes may affect exogenous estrogen in older women and influence cognitive aging. PvuII (rs9340799) and Xbal (rs223493) are examples of SNPs found in ERα and are associated with AD and cognitive impairment. Also, several SNPs in ERβ have been proven to increase the risk of AD in women [ 69 , 70 , 71 , 72 ].
- Other Genes
Other genes’ polymorphism associated with increasing the risk of AD include vitamin D receptor (VDR) gene polymorphism, which affects the affinity of vitamin D to its receptor and may cause neurodegenerative diseases and neuronal damage [ 73 ]. Moreover, epigenetic factors like DNA methylation, histone, and chromatin modifications were demonstrated to be involved in AD [ 33 , 74 ].
5.2.3. Environmental Factors
Aging and genetic risk factors cannot explain all cases of AD. Environmental risk factors including air pollution, diet, metals, infections, and many others may induce oxidative stress and inflammation and increase the risk for developing AD. Herein, we report the most important environmental factors and their relationships with AD [ 75 , 76 ].
- Air Pollution
The air pollution is characterized by modifying the nature of the atmosphere through the introduction of chemical, physical, or biological pollutants. It is associated with respiratory and cardiovascular diseases and recently, its association with AD was documented. Six air pollutants have been defined by National Ambient Air Quality Standards (NAAQSs) in the USA as a threat to human health, including ozone (O 3 ), nitrogen oxides (NO x ), carbon monoxide (CO), particulate matter (PM), sulfur dioxide (SO 2 ), and lead. Studies on animals and cellular models have shown that an exposure to high levels of air pollution can result in a damage to the olfactory mucosa and bulb, in addition to the frontal cortex region, similar to that observed in AD. In individuals exposed to air pollutants, there is a link between oxidative stress, neuroinflammation, and neurodegeneration, with the presence of hyper-phosphorylated tau and Aβ plaques in the frontal cortex. The air pollution can cause an increase in Aβ 42 formation, accumulation, and impaired cognitive function [ 77 , 78 ].
In recent years, the number of studies on the role of nutrition in AD have been increased. Several dietary supplements such as antioxidants, vitamins, polyphenols, and fish were reported to decrease the risk of AD, whereas saturated fatty acids and high-calorie intake were associated with increasing the risk of AD [ 79 ]. The food processing causes degradation of heat-sensitive micronutrients (e.g., vitamin C and folates), loss of large amounts of water, and formation of toxic secondary products (advanced glycation end products, AGEs) from non-enzymatic glycation of free amino groups in proteins, lipids, and nucleic acids. The toxic effect of AGEs is referred to as their ability to induce oxidative stress and inflammation by modifying the structure and function of the cell surface receptors and body proteins. Different studies demonstrated that elevated AGEs serum level is associated with cognitive decline and progression of AD. The AGE receptor (RAGE) is located in different places within the body, including microglia and astrocytes, and was established to be overexpressed in the brain of AD patients and serve as a transporter and a cell surface receptor for Aβ [ 80 ]. Malnutrition is another risk factor for AD. Deficiency in nutrients such as folate, vitamin B12, and vitamin D may cause a decrease in cognitive function, in addition to the fact that patients with AD suffer from problems associated with eating and swallowing, which may increase the risk of malnutrition [ 81 ].
Metals are found in nature and biological systems and can be divided into bio-metals that have a physiological function in living organisms (e.g., copper, zinc, and iron), and toxicological metals which do not possess any biological function (e.g., aluminum and lead) [ 82 ]. Aluminum is used significantly in the industries such as processed foods, cosmetics, medical preparations, medicines, and others. In the body, aluminum is bound to plasma transferrin and to citrate molecules that can mediate the transfer of aluminum to the brain. Studies demonstrated that Al accumulates in the cortex, hippocampus, and cerebellum areas, where it interacts with proteins and causes misfolding, aggregation, and phosphorylation of highly phosphorylated proteins like tau protein, characteristic of AD [ 83 ]. Lead competes with the binding site of bio-metals like calcium and can cross the blood–brain barrier (BBB) rapidly, where it can modify neural differentiation and synaptogenesis and cause severe damage. Studies revealed that an acute exposure to lead was associated with AD and caused an increase of β-secretase expression and Aβ accumulation. Cadmium is a carcinogenic water-soluble metal that can cross the BBB and cause neurological diseases like AD. Results have demonstrated that Cadmium ions are involved in the aggregation of Aβ plaques and the self-aggregation of tau in the AD brain. The data accumulated on metals support the notion that they are among the risk factors involved in the development of AD [ 84 ].
Chronic infections to the central nervous system (CNS) can cause an accumulation of Aβ plaques and NFT, therefore, they are included among the risk factors in AD. Studies by Dr. Itzhaki showed that the DNA of herpes simplex virus (HSV-1) was found in patients with ApoE-ε4 allele carriers, which explains the high risk for developing AD. HSV-1 can replicate in the brain, which can result in the activation of the inflammatory response and an increase in Aβ deposition, resulting in damage to neurons and gradual development of AD. On the other hand, the study results by Miklossy and Balin’s have revealed the role of chronic bacterial infections in AD. For example, syphilitic dementia caused by spirochete bacteria ( Treponema pallidum ), which are accumulated in the cerebral cortex, produced lesions similar to neurofibrillary tangles, which led to devastating neurodegenerative disorders. Besides, Chlamydia pneumonia bacterium can trigger late-onset AD by activation of astrocyte and cytotoxic microglia, disrupt calcium regulation and apoptosis, resulting in deterioration of cognitive function, and increase the risk of AD [ 85 , 86 , 87 ].
5.2.4. Medical Factors
Several risk factors are related to the development of Alzheimer’s disease. Adding to this list, older people with AD usually have medical conditions such as cardiovascular disease (CVD), obesity, diabetes, and others. All of these conditions are associated with increased risk of AD [ 88 , 89 ].
- Cardiovascular Disease (CVDs)
CVDs are recognized as an important risk factor for AD, such as the stroke that is associated with increased risk of dementia due to a neural tissue loss, which enhances degenerative effect and influences amyloid and tau pathology. Atrial fibrillation also causes embolisms which leads to stroke and a decrease in memory and cognitive functions. Moreover, heart failure affects the pumping function of the heart and results in insufficient blood supply to the body and hypo-perfusion of the brain that leads to hypoxia and neural damage. The coronary heart disease’s hypothesis indicates that atherosclerosis, peripheral artery disease, hypo-perfusion, and emboli are all related to increased risk of AD. Hypertension is associated with thickening of vessel walls and narrowing of the lumen which reduce the cerebral blood flow, and in chronic cases, it may cause cerebral edema, which all participate as risk factors for AD and CVD. The CVD is a modifiable risk factor and by focusing on its relationship with AD, a pathway to prevent and delay the disease can be obtained [ 89 , 90 ].
- Obesity and Diabetes
Obesity is a term used for too much body fat in individuals due to consuming more calories than they burn and can be calculated by using the body mass index (BMI). Increasing the body fat is associated with a decreased brain blood supply which promotes brain ischemia, memory loss, and vascular dementia. The obesity, unhealthy diet, and other factors can cause impaired glucose tolerance (IGT) or diabetes, which is characterized by hyperglycemia that affects peripheral tissues and blood vessels. Chronic hyperglycemia can induce cognitive impairment as a result of increasing amyloid-beta accumulation, oxidative stress, mitochondrial dysfunction, and neuroinflammation. Obesity is characterized by increasing pro-inflammatory cytokines secretions from adipose tissue, which stimulate macrophages and lymphocytes and eventually lead to local and systemic inflammation. This inflammation promotes insulin resistance, hyperinsulinemia, and as a consequence, hyperglycemia. Obesity is a well-known risk factor for type 2 diabetes, CVDs, and cancer, which are identified as risk factors for dementia and AD. The brain inflammation causes an increase in microglia and results in reduced synaptic plasticity and impaired neurogenesis. Microglia can affect insulin receptor substrate 1 (IRS-1) and block intracellular insulin signaling, which has an important role in neural health. Therefore, alteration in insulin action can result in Aβ accumulation and reduce the tau protein degradation associated with AD [ 91 , 92 , 93 , 94 ].
6. Treatment
Currently, Alzheimer’s disease cases worldwide are reported to be around 24 million, and in 2050, the total number of people with dementia is estimated to increase 4 times. Even though AD is a public health issue, as of now, there is only two classes of drugs approved to treat AD, including inhibitors to cholinesterase enzyme (naturally derived, synthetic and hybrid analogues) and antagonists to N -methyl d -aspartate (NMDA). Several physiological processes in AD destroy Ach-producing cells which reduce cholinergic transmission through the brain. Acetylcholinesterase inhibitors (AChEIs), which are classified as reversible, irreversible, and pseudo-reversible, act by blocking cholinesterase enzymes (AChE and butyrylcholinesterase (BChE)) from breaking down ACh, which results in increasing ACh levels in the synaptic cleft [ 95 , 96 , 97 ]. On the other hand, overactivation of NMDAR leads to increasing levels of influxed Ca 2+ , which promotes cell death and synaptic dysfunction. NMDAR antagonist prevents overactivation of NMDAR glutamate receptor and hence, Ca 2+ influx, and restores its normal activity. Despite the therapeutic effect of these two classes, they are effective only in treating the symptoms of AD, but do not cure or prevent the disease [ 98 , 99 ]. Unfortunately, only a few clinical trials on AD have been launched in the last decade and their outcome was a big failure. Several mechanisms have been proposed to understand AD pathology in order to modify its pathway and develop successful treatments, which include abnormal tau protein metabolism, β-amyloid, inflammatory response, and cholinergic and free radical damage [ 30 , 100 ]. On the other hand, most AD modifiable risk factors such as cardiovascular or lifestyle habits can be prevented without medical intervention. Studies showed that physical activity can improve the brain health and reduce AD by activating the brain vascularization, plasticity, neurogenesis, and reducing inflammation by decreasing Aβ production, which all result in improving cognitive function in older people. Moreover, the Mediterranean diet (MD), intellectual activity, and higher education all may reduce the progression of AD and memory loss and increase the brain capacity and cognitive functions. Several studies revealed that multi-domain intervention which includes lifestyle (diet, exercise, and cognitive training), depression of AD symptoms, and controlling cardiovascular risk factors, can increase or maintain cognitive function and prevent new cases of AD in older people [ 101 ]. Herein, we summarize the currently available drugs and theories for the development of new therapies for AD.
6.1. Symptomatic Treatment of AD
6.1.1. cholinesterase inhibitors.
According to the cholinergic hypothesis, AD is due to the reduction in acetylcholine (ACh) biosynthesis. Increasing cholinergic levels by inhibiting acetylcholinesterase (AChE) is considered one of the therapeutic strategies that increases cognitive and neural cell function. AChEIs are used to inhibit acetylcholine degradation in the synapses, which results in continuous accumulation of ACh and activation of cholinergic receptors. Tacrine (tetrahydroaminoacridine) ( 1, Figure 4 ) was the first FDA (Food and Drug Administration)-approved cholinesterase inhibitor drug for the treatment of AD, which acts by increasing ACh in muscarinic neurons, but it exited the market immediately after its introduction due to a high incidence of side effects like hepatotoxicity and a lack of benefits, which was observed in several trials. Later on, several AChEIs were introduced, such as donepezil ( 2 , Figure 4 ), rivastigmine ( 3 , Figure 4 ), and galantamine ( 4 , Figure 4 ), and are currently in use for the symptomatic treatment of AD [ 34 , 97 , 102 , 103 ]. Another strategy that may help in the treatment of AD is increasing choline reuptake and as a result, increasing acetylcholine synthesis at the presynaptic terminals. This can be achieved by targeting choline transporter (CHT1) which is responsible for supplying choline for the synthesis of ACh. Developing drugs that are capable of increasing CHT1 at the plasma membrane may become the future therapy of AD [ 36 ].

The chemical structures of approved drugs for symptomatic treatment of AD (tacrine 1 , donepezil 2 , rivastigmine 3 , galantamine 4 , and memantine 5 ) and disease-modifying compounds that entered clinical trials (semagacestat 6 , avagacestat 7 , tarenflurbil 8 , lanabecestat 9 , verubecestat 10 , atabecestat 11 , umibecestat 12 , methylene blue 13 , tideglusib 14 , and saracatinibin 15 ).
Donepezil ( 2 , Figure 4 ) is an indanonebenzylpiperidine derivative and a second generation of AChEIs and is considered the leading drug for AD treatment. Donepezil binds to acetylcholinesterase reversibly and inhibits acetylcholine hydrolysis, which leads to a higher concentration of ACh at the synapses. The drug is well-tolerated with mild and transient cholinergic side effects which are related to the gastrointestinal and nervous systems. It should be noted that donepezil is used to treat symptoms of AD such as improving cognition and behavior without altering the AD progression [ 104 , 105 , 106 ].
- Rivastigmine
Rivastigmine ( 3 , Figure 4 ) is a pseudo irreversible inhibitor of AChE and butyrylcholinesterase (BuChE) that acts by binding to the two active sites of AChE (anionic and estearic sites), which results in preventing ACh metabolism. BuChE is found mostly in glial cells with only 10% of AChE activity in the normal brain, whereas in the AD brain, its activity is increased to 40–90%, while ACh activity is reduced simultaneously, which suggests that BuChE action may indicate a moderate to severe dementia. Rivastigmine dissociates more slowly than AChE, which is why it is called a pseudo-irreversible, and it undergoes metabolism at the synapse by AChE and BuChE. The drug is used in mild to moderate AD cases. It improves cognitive functions and daily life activities. Oral administration of the drug is associated with adverse effects such as nausea, vomiting, dyspepsia, asthenia, anorexia, and weight loss. In many cases, these side effects are the main reason behind stopping taking the medicine, however, they can be settled down in time and consequently, the drug becomes more tolerated. Rivastigmine can be delivered by transdermal patches for controlled and continuous delivery of the drug through the skin, with enhanced tolerability and caregiver satisfaction. Also, the patches can deliver a lower dosage compared to pills, which results in reduced side effects. Most AD patients suffer from memory loss and swallowing problems which affect their compliance in administering oral drugs at regular intervals. Therefore, the use of transdermal patches is the most appropriate method for delivering the drug in AD patients [ 107 , 108 , 109 , 110 ].
- Galantamine (GAL)
Galantamine ( 4 , Figure 4 ) is considered a standard first-line drug for mild to moderate AD cases. GAL is a selective tertiary isoquinoline alkaloid with a dual mechanism of action in which it acts as a competitive inhibitor of AChE and can bind allosterically to the α-subunit of nicotinic acetylcholine receptors and activate them. GAL can improve behavioral symptoms, daily life activities, and cognitive performance with good efficacy and tolerability, similar to other AChE inhibitors. Several delivery systems were developed to improve the drug delivery to the brain: Wahba et al. attached GAL to ceria-containing hydroxyapatite particles for selective delivery of the drug to the affected regions in the brain. Misra et al. and Fornaguera et al. used solid-lipid nanoparticles and nano-emulsification approaches respectively, to carry GAL hydrobromide. The results of these studies demonstrated a promising strategy for safe delivery of the drug. Hanafy et al. developed nasal GAL hydrobromide/chitosan complex nanoparticles which showed good pharmacological efficacy, while Woo et al. utilized the patch system as a carrier for a controlled release dosage form of the drug [ 111 , 112 , 113 , 114 ].
6.1.2. N -methyl d -aspartate (NMDA) Antagonists
NMDAR is believed to have a dominant role in the pathophysiology of AD. NMDAR stimulation results in Ca 2+ influx which activates signal transduction and as a consequence, it triggers gene transcription essential for the formation of a long-term potentiation (LTP), which is important for synaptic neurotransmission, plasticity, and memory formation. Over-activation of NMDARs causes an abnormal level of Ca 2+ signaling and overstimulation of glutamate, which is the primary excitatory amino acid in the CNS, which results in excitotoxicity, synaptic dysfunction, neuronal cell death, and a decline in cognitive functions. Several NMDAR uncompetitive antagonists have been developed and entered clinical trials, however, most of them failed due to low efficacy and side effects. Memantine ( 5 , Figure 4 ) is the only approved drug in this category to treat moderate to severe AD; in addition, other NMDAR uncompetitive antagonist compounds are being developed, such as RL-208 (3,4,8,9-tetramethyltetracyclo [4.4.0.0 3,9 .0 4,8 ]dec-1-yl)methylamine hydrochloride), a polycyclic amine compound that may possess a promising therapeutic effect in age-related cognitive problems and AD [ 115 , 116 , 117 ].
Memantine ( 5 , Figure 4 ) is a low-affinity uncompetitive antagonist of the NMDAR, a subtype of glutamate receptor that prevents over-activation of the glutaminergic system involved in the neurotoxicity in AD cases. Memantine is used for the treatment of moderate to severe AD alone or in combination with AChEI. The drug is safe and well-tolerated, it blocks the excitatory receptor without interfering with the normal synaptic transmission due to memantine’s low affinity, where it is displaced rapidly from NMDAR by high concentrations of glutamate, thus avoiding a prolonged blockage. The latter is associated with high side effects, especially on learning and memory [ 99 , 118 ].
6.2. Promising Future Therapies
6.2.1. disease-modifying therapeutics (dmt).
Disease-modifying treatment or therapy (DMT) alter the progression of AD by working on several pathophysiological mechanisms. This is in contrast to symptomatic therapy which works on improving the cognitive functions and decreasing symptoms such as depression or delusions without affecting or modifying the disease. DMTs, either immunotherapies or small molecules, are administrated orally and are being developed to prevent AD or decrease its progression. Several DMTs have been developed and entered the clinical trials, such as AN-1792, a synthetic Aβ peptide (human Aβ 1–42 peptide of 42-amino acids with the immune adjuvant QS-21) and the first active immunotherapy for AD which entered phase II clinical trials and discontinued due to a meningoencephalitis side effect in 6% of the patients. Other drugs were also developed and failed in the clinical trials, including the anti-Aβ antibody (solanezumab and bapineuzumab), γ-Secretase inhibitors (semagacestat 6 , avagacestat 7 , and tarenflurbil 8 ) ( Figure 4 ) and β-secretase inhibitors (BACE) (Lanabecestat 9, verubecestat 10 , and atabecestat 11 ) ( Figure 4 ). DMTs failures are due to several factors, such as starting therapy too late, giving treatment for the wrong main target, use of inappropriate drug doses, and misunderstanding of the pathophysiology of AD. Several immunotherapies described in Table 1 have been developed over decades, including: CAD106, an active Aβ immunotherapy that induces Aβ antibodies in animal models and consists of multiple copies of Aβ1–6 peptide coupled to Qβ coat protein, a virus-like particle, and is still in clinical trials, and CNP520 (umibecestat, 12 ) ( Figure 4 ), a small molecule that inhibits beta-scretase-1 (BACE-1) and therefore inhibits Aβ production. CNP520 was found to reduce Aβ plaque deposition and Aβ levels in the brain and CSF in rats, dogs, and healthy adults ≥ 60 years old, and is still under clinical trials. Furthermore, aducanumab, gantenerumab, and crenezumab are all human Aβ monoclonal antibody that bind with high affinity to aggregated Aβ, and they are still under study in the clinical phases with other DMTs described in Table 1 [ 6 , 119 , 120 , 121 , 122 , 123 , 124 ].
Disease modifying agents for the treatment of Alzheimer’s disease in clinical trials.
Another class targeting the α-secretase enzyme was developed and has been considered as therapeutic agents. α-secretase modulators or activators stimulate the cleavage of APP. There is little knowledge about the activation pathway, but research assumes that it is promoted by the phosphatidylinositol 3-kinase (PI3K)/Akt pathway or by γ-aminobutyric acid (GABA) receptor signaling. Targeting these pathways may give potential therapeutic agents for AD [ 6 ].
In addition to the anti-amyloid agents, the tau aggregation inhibitors are another promising DMT. The tau is a biomarker for neurofibrillary tangles (NFT) in AD and naturally modulates microtubule stability, signaling pathways, and axonal transport. A modification in tau conformation results in toxic aggregation. Therefore, the prevention of tau aggregation becomes an interesting approach for drug discovery to reduce AD progression. Studies in mice have shown that tau oligomers cause mitochondrial damage, disruption of neuronal signaling, synaptic loss, and memory impairment. Disease-modifying therapeutics (DMT) like small molecules can be used to inhibit the initial step in the tau aggregation and thereby reduce its accumulation. Methylene blue ( 13 , Figure 4 ) is a blue dye that inhibits the tau aggregation and entered phase II clinical trials to treat mild to moderate AD. Upon administration of the drug, the color of the urine becomes blue, which indicates a lack of binding, and because of that, the study was highly criticized. Other approaches suggest that an inhibition of specific kinases such as glycogen synthase kinase 3 (GSK3β) can inhibit tau hyperphosphorylation and block tau deposition. Examples of these entities include tideglusib ( 14 , or NP-031112 (NP-12), Figure 4 ), a thiazolidinedione-derived compound, lithium, pyrazolopyridines, pyrazolopyrazines, sodium valproate, and others. Another protein kinase inhibitor is saracatinib (AZD0530) ( 15 , Figure 4 ), which acts by inhibiting tyrosine kinase and has shown good results in improving memory in transgenic mice and is currently in phase II trials [ 125 , 126 , 127 ]. Davidowitz et al. utilized the hatu mouse model of tauopathy to study the efficacy of a lead small molecule in preventing tau accumulation. The study results demonstrated a significant reduction in tau levels and its phosphorylated form levels, which indicates the ability to inhibit the entire pathway of the tau aggregation by using an optimized lead compound [ 128 ].
6.2.2. Chaperones
Protein misfolding caused by mutations or environmental factors results in aggregations that are toxic, and their accumulation causes neurodegenerative disorders like AD. Naturally, cells develop protein quality control (PQC) systems that inhibit protein misfolding before exerting their toxic effects. With age, this balance is altered and the misfolded shapes overwhelm the PQC system, which in turn activates the unfolded protein response (UPR) that stops the protein synthesis and increases chaperone production. Generally, the cells in humans have proteins that are responsible for other proteins to function and arrive to their destination in the cell. These proteins are called “chaperones”. Chaperones are involved in protein folding and improvement of the PQC system efficiency. Therefore, it is considered a promising candidate for treating neurodegenerative diseases. It can be classified into three groups: (1) molecular chaperones, which are proteins that assist other nonnative proteins in their folding or unfolding, like overexpression of heat shock proteins (Hsps) that serve as neuroprotective agents, (2) pharmacological chaperones, which are low molecular weight compounds (enzymes or receptor-ligand or selective binding molecules) that induce refolding of proteins, stabilize their structure, and restore their function, and (3) chemical chaperones, also low molecular weight compounds, which are divided into two groups, osmolytes and hydrophobic compounds. The members in these two groups have no specific mechanism of action and need high concentrations to exert their therapeutic effects [ 129 ].
- Heat Shock Proteins (Hsps)
The causes for most neurodegenerative diseases are protein misfolding and aggregation, which lead to cell death. The molecular chaperone can be intracellular, such as in the case of heat shock proteins (e.g., Hsp40, Hsp60, Hsp70, Hsp90, Hsp100, and Hsp110), and extracellular, such as clustering and alpha-macroglobulin. HSPs play an essential role in the protein folding process and protect cells from harmful stress-related events. There are two families of Hsps: (a) classic Hsps that possess an ATP-binding site with a molecular weight of 60 kD or more. This family includes Hsp100, Hsp90, Hsp70, and Hsp60, and (b) the small Hsps such as αB-crystalline, Hsp27, Hsp20, HspB8, and HspB2/B3 that lack ATP-binding site, with a molecular weight of 40 kD or less. These proteins can assist other Hsps in their refolding function. Failure of these mechanisms can lead to oxidative stress, mitochondrial dysfunction, and many other conditions that cause damage, a loss of neurons, and a progression of neurodegenerative diseases. Different HSPs can block the aggregation process of misfolded proteins, like amyloidogenic proteins (Aβ and tau), and promote their degradation [ 130 , 131 ].
Hsp60 plays an important role in mitochondrial protein folding. Its role in AD is not clear, some believe that the protein has a protective role and others think it has a harmful effect where it can be over-expressed by activated microglia, which increases pro-inflammatory factors such as toll-like receptor 4 (TLR-4) that stimulate neuronal cell death. Therefore, inhibiting activated microglia and Hsp60 expression is a promising strategy for preventing neurodegenerative diseases. Examples of compounds that inhibit Hsp60 are mizoribine (Immunosuppressant) ( 16 , Figure 5 ) and pyrazolopyrimidine EC3016 ( 17 , Figure 5 ). Both compounds act by blocking ATPase activity of Hsp60 and inhibiting protein folding. On the other hand, avrainvillamide, a fungal metabolite ( 18 , Figure 5 ), and epolactaene, a bacterial metabolite ( 19 , Figure 5 ), act by binding to the Hsp60′s cysteine residues and inhibit its folding activity. However, Hsp60’s role in AD remains controversial and there is a need for more investigations to understand its role [ 130 ].

The chemical structures of different chaperone molecules: Mizoribine 16 , EC3016 17 , Avrainvillamide 18 , Epolaztaene 19 , MKT-077 20 , YM-01 21 , JG-98 22 , Radicicol 23 , Geldanamycin 24 , 17-AAG 25 , Pochoxime C (OS47720) 26 , R55 27 , and OT1001 28 .
Studies have shown that Hsp70 binds to Aβ42 and prevents self-aggregation. Martín-Peña et al. studied two isoforms of Hsp70, cytosolic and extracellular, in Drosophila flies AD models and evaluated their protective role against memory decline that results from Aβ42 aggregation. The animal studies showed that Hsp70 has a dual function: intracellularly and extracellularly, where it protects against Aβ42 neurotoxicity and synaptic loss. In addition to its ability to bind to tau and its hyper-phosphorylated form and prevent its formation, it decreases aggregation and promotes tau binding to microtubules. Hsp70 acts by activating microglia, insulin-degrading enzyme, and tumor growth facto r- β1, which degrades β-amyloids and prevents memory impairments [ 132 , 133 ]. Some studies in AD brain tissue demonstrated an overexpression of Hsp70 levels and a correlation with the presence of activated glia and stressed neurons. Also, it was found that Hsp70 is associated with extracellular deposits in AD. Drug therapies targeting Hsp70, mainly referring to previous anticancer drugs which target and inhibit Hsp70 ATP-binding site, are considered as candidates in AD treatment due to their ability to reduce tau levels in vitro and ex vivo. MKT-077(1-ethyl-2-(( Z )-(( E )-3-ethyl-5-(3-methylbenzo [ d ]thiazol-2(3 H )-ylidene)-4-oxothiazolidin-2-ylidene)methyl)pyridin-1-ium chloride) ( 20 , Figure 5 ), is an anticancer rhodacyanine compound that binds to mortalin, a mitochondrial Hsp70 site, and acts as an anti-proliferative agent, but the use of this compound was stopped due to toxicity side effects and low BBB penetration. On the other hand, YM-01 ( 21 , Figure 5 ), a more potent MKT-077 derivative, was developed with a single replacement of the ethyl group on the pyridinium nitrogen of MKT-077 with a methyl group. JG-98 ( 22 , Figure 5 ) is also an MKT-077 derivative with a 60-fold higher binding affinity to Hsp70 than YM-01 [ 130 , 134 , 135 , 136 ].
Hsp90 is another type of HSP that regulates the tau phosphorylation and dephosphorylation. An inhibition of Hsp90 results in a decrease in phosphorylation of tau due to a reduction in tau kinases, which is thought to be responsible for tau pathogenesis when it is hyperactivated. Hsp90 inhibitors are used for cancer therapy, but recently, they are considered as promising therapy for AD. Radicicol (RDC) ( 23 , Figure 5 ) and geldanamycin (GA) ( 24 , Figure 5 ) are Hsp90 inhibitors. GA is a natural antifungal compound and the first discovered Hsp90 inhibitor. Studies on this inhibitor were stopped due to its toxicity. On the other hand, 17-AAG (17-(Allylamino)-17-demethoxygeldanamycin) ( 25 , Figure 5 ) is a GA derivative with a lower toxicity and better pharmacokinetic profile that showed a good improvement of the cognitive function by inducing other HSPs, like Hsp70, in addition to reducing NFTs in the transgenic mouse model by blocking the tau phosphorylation pathway, indirectly [ 137 , 138 ]. Pochoxime C (OS47720) ( 26 , Figure 5 ) is also a CNS-permeable Hsp90 inhibitor that showed good safety and efficacy profiles when tested in the AD mouse model. Studies revealed that OS47720 acts by strengthening synaptic function via heat shock factor (HSF-1) activation and dependent transcriptional events [ 139 ].
The combined studies demonstrate that targeting HSPs is a promising strategy to develop drugs with a new mechanism of action for reducing pathogenic tau levels and restoring normal tau homeostasis.
- Vacuolar sorting protein 35 (VPS35)
An accumulation of proteins in neurons and glial cells leads to disturbance of cellular protein homeostasis. The endosomal-lysosomal system is responsible for transporting proteins for recycling and degradation. Any malfunction in the system can lead to several diseases, such as Alzheimer’s disease. Retromer is a complex of regulator proteins composed of sorting nexin (SNX1, 2, 5, 6) and vacuolar sorting proteins (VPS 26, 29, 35), which are responsible for transporting cargo molecules from the endosome to the trans -Golgi network. A loss of retromer’s function results in the downregulation of VPS35, which can increase Aβ formation, induce cognitive impairments, and cause synaptic dysfunction, which is reported in AD patients [ 140 , 141 ]. A study on 3xTg mice brains was conducted to evaluate the effect of VPS35 overexpression on memory function. The study showed that a significant reduction of the Aβ peptide and tau neuropathology (soluble, insoluble, and phosphorylated tau) was associated with overexpression of VPS35, in addition to a reduction in neuroinflammation and ameliorating synaptic dysfunction [ 142 ]. Therefore, VPS35 is an important promising therapeutic target for AD treatment. A small pharmacological chaperones molecule called R55 (thiophene-2,5-diylbis(methylene) dicarbamimidothioatedihydrochloride) ( 27 , Figure 5 ), a thiophenethiourea derivative, can enhance retromer stability and function by increasing retromer proteins, shifting AOO from the endosome, and reducing pathogenic processing of APP, which may serve as a promising therapeutic molecule for neurodegenerative diseases [ 143 ].
Studies demonstrated that the accumulation of gangliosides has been associated with misfolding and aggregation of proteins in neurodegenerative diseases. Abnormal levels of mono-sialoganglioside (GM1, GM2, and GM3) have been reported in AD brains. Mutant forms of Aβ, like Dutch mutant APPE693Q, showed susceptibility to pro-aggregation properties of GM2 and GM3, resulting in the formation of Aβ peptides complexes with gangliosides (ganglioside-bound Aβ (GAβ) peptide) and subsequently leading to an acceleration of aggregation and accumulation of Aβ peptides.
β-hexosaminidase (β-hex) is a lysosomal enzyme that acts by catabolizing GM2 ganglioside, and increasing its activity can lead to a reduction of GM2 levels and Aβ aggregation and accumulation. Small molecules like pharmacological chaperones (PC) can selectively bind and stabilize wild-type proteins and restore their normal folding. OT1001 ( 28 , Figure 5 ) is an iminosugar PC that targets β-hex and increases its level in the brain and reduces GAβ pathology. Studies on Dutch APPE693Q transgenic mice showed that OT1001 has good pharmacokinetics, brain penetration ability, and tolerability, with lower side effects. These make the compound a good drug candidate for increasing the β-hex activity [ 144 ].
6.2.3. Natural Extract
For a long time, natural compounds have been used as therapeutic agents for several pathological diseases, and recent studies showed that they possess a neuroprotective effect. In vitro and in vivo studies have proven that natural compounds possess a therapeutic potential for AD, which allowed some of them to enter the clinical trials stages. Nicotine was the first natural compound entered in the clinical trials for AD, then other compounds like vitamins C, E, and D gained more attention and interest due to their protective role against neuroinflammation and oxidative damage. Recently, bryostatin, a macrolide lactone extract from bryozoan Bugula neritina, has been evaluated and showed the ability to induce α-secretase activity, reduce Aβ production, and enhance the learning and memory in an AD mice model [ 145 ]. Other natural compounds used in folk medicine (traditional Chinese medicine (TCM)) demonstrated a great potential in treating AD by acting on several mechanisms, as shown in Table 2 below [ 146 ].
Natural compounds used in folk medicine and their mechanism of actions.
7. Conclusions
Alzheimer’s disease is now considered a world health concern; as a consequence, the National Institute on Aging—Alzheimer’s Association reclassified and updated the 1984 NINCDS-ADRDA criteria for higher specificity, sensitivity, and early identification of patients at risk of developing AD. Several criteria have been proposed for a more accurate diagnosis of AD, including clinical biomarkers, bodily fluids, and imaging studies. Despite that, the treatment of AD remains symptomatic, without alteration in the disease’s prognosis. Inhibitors to cholinesterase enzyme such as galantamine, donepezil, and rivastigmine, and NMDA antagonists such as memantine, improve memory and alertness but do not prevent progression. Several studies have shown that modification in lifestyle habits like diet and exercise can improve brain health and reduce AD without medical intervention and is considered as a first-line intervention for all AD patients. Recently, the research is focusing on targeting the pathological features of AD such as Aβ and p-tau. Future therapies such as disease-modifying treatment can alter the progression of AD by targeting the Aβ pathway, and many drugs have entered the clinical trials, like AN-1792, solanezumab, bapineuzumab, semagacestat, avagacestat, and tarenflurbil, but failed in demonstrating efficacy in the final clinical stages. Other DMTs are still under investigation, such as those targeting Aβ and tau pathologies, such as aducanumab, gantenerumab, crenezumab, tideglusib, lithium, and others. Other promising compounds called chaperones like heat shock proteins and vacuolar sorting protein 35 (VPS35) function by assisting other proteins to function normally and to arrive at their destination in the cell safely, and therefore can be used as a treatment for neurodegenerative diseases. Moreover, the natural extracts used in folk Chinese medicine showed great potential in treating AD by acting on several mechanisms’ pathways. In conclusion, the success of AD treatment depends on its early administration and patient monitoring for disease progression using biomarkers diagnosis. Future therapies that target tau pathology and the use of combination therapy may have a potential to slow the progression of AD pathology. Designing a potent, selective, and effective drug is urgently needed to treat patients with AD and those at risk for developing the disease.
Author Contributions
Literature survey and first draft writing were done by Z.B., and final draft, including the revisions, were accomplished by R.K. All authors have read and agreed to the published version of the manuscript.
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appointments at Mayo Clinic
Alzheimer's treatments: what's on the horizon.
Despite many promising leads, new treatments for Alzheimer's are slow to emerge.
Current Alzheimer's treatments temporarily improve symptoms of memory loss and problems with thinking and reasoning.
These Alzheimer's treatments boost the performance of chemicals in the brain that carry information from one brain cell to another. They include cholinesterase inhibitors and the medicine memantine (Namenda). However, these treatments don't stop the underlying decline and death of brain cells. As more cells die, Alzheimer's disease continues to progress.
Experts are cautious but hopeful about developing treatments that can stop or delay the progression of Alzheimer's. Experts continue to better understand how the disease changes the brain. This has led to the research of potential Alzheimer's treatments that may affect the disease process.
Future Alzheimer's treatments may include a combination of medicines. This is similar to treatments for many cancers or HIV / AIDS that include more than one medicine.
These are some of the strategies currently being studied.
Taking aim at plaques
Some of the new Alzheimer's treatments target clumps of the protein beta-amyloid, known as plaques, in the brain. Plaques are a characteristic sign of Alzheimer's disease.
Strategies aimed at beta-amyloid include:
Recruiting the immune system. Medicines known as monoclonal antibodies may prevent beta-amyloid from clumping into plaques. They also may remove beta-amyloid plaques that have formed. They do this by helping the body clear them from the brain. These medicines mimic the antibodies your body naturally produces as part of your immune system's response to foreign invaders or vaccines.
In 2023, the U.S. Food and Drug Administration (FDA) approved lecanemab (Leqembi) for people with mild Alzheimer's disease and mild cognitive impairment due to Alzheimer's disease.
A phase 3 clinical trial found that the medicine slowed cognitive decline in people with early Alzheimer's disease. The medicine prevents amyloid plaques in the brain from clumping. The phase 3 trial was the largest so far to study whether clearing clumps of amyloid plaques from the brain can slow the disease.
Lecanemab is given as an IV infusion every two weeks. Your care team likely will watch for side effects and ask you or your caregiver how your body reacts to the drug. Side effects of lecanemab include infusion-related reactions such as fever, flu-like symptoms, nausea, vomiting, dizziness, changes in heart rate and shortness of breath.
Also, people taking lecanemab may have swelling in the brain or may get small bleeds in the brain. Rarely, brain swelling can be serious enough to cause seizures and other symptoms. Also in rare instances, bleeding in the brain can cause death. The FDA recommends getting a brain MRI before starting treatment. It also recommends being monitored with brain MRI s during treatment for symptoms of brain swelling or bleeding.
People who carry a certain form of a gene known as APOE e4 appear to have a higher risk of these serious complications. The FDA recommends being tested for this gene before starting treatment with lecanemab.
If you take a blood thinner or have other risk factors for brain bleeding, talk to your health care professional before taking lecanemab. Blood-thinning medicines may increase the risk of bleeds in the brain.
More research is being done on the potential risks of taking lecanemab. Other research is looking at how effective lecanemab may be for people at risk of Alzheimer's disease, including people who have a first-degree relative, such as a parent or sibling, with the disease.
Another medicine being studied is donanemab. It targets and reduces amyloid plaques and tau proteins. It was found to slow declines in thinking and functioning in people with early Alzheimer's disease.
The monoclonal antibody solanezumab did not show benefits for individuals with preclinical, mild or moderate Alzheimer's disease. Solanezumab did not lower beta-amyloid in the brain, which may be why it wasn't effective.
Preventing destruction. A medicine initially developed as a possible cancer treatment — saracatinib — is now being tested in Alzheimer's disease.
In mice, saracatinib turned off a protein that allowed synapses to start working again. Synapses are the tiny spaces between brain cells through which the cells communicate. The animals in the study experienced a reversal of some memory loss. Human trials for saracatinib as a possible Alzheimer's treatment are now underway.
Production blockers. These therapies may reduce the amount of beta-amyloid formed in the brain. Research has shown that beta-amyloid is produced from a "parent protein" in two steps performed by different enzymes.
Several experimental medicines aim to block the activity of these enzymes. They're known as beta- and gamma-secretase inhibitors. Recent studies showed that the beta-secretase inhibitors did not slow cognitive decline. They also were associated with significant side effects in those with mild or moderate Alzheimer's. This has decreased enthusiasm for the medicines.
Keeping tau from tangling
A vital brain cell transport system collapses when a protein called tau twists into tiny fibers. These fibers are called tangles. They are another common change in the brains of people with Alzheimer's. Researchers are looking at a way to prevent tau from forming tangles.
Tau aggregation inhibitors and tau vaccines are currently being studied in clinical trials.
Reducing inflammation
Alzheimer's causes chronic, low-level brain cell inflammation. Researchers are studying ways to treat the processes that lead to inflammation in Alzheimer's disease. The medicine sargramostim (Leukine) is currently in research. The medicine may stimulate the immune system to protect the brain from harmful proteins.
Researching insulin resistance
Studies are looking into how insulin may affect the brain and brain cell function. Researchers are studying how insulin changes in the brain may be related to Alzheimer's. However, a trial testing of an insulin nasal spray determined that the medicine wasn't effective in slowing the progression of Alzheimer's.
Studying the heart-head connection
Growing evidence suggests that brain health is closely linked to heart and blood vessel health. The risk of developing dementia appears to increase as a result of many conditions that damage the heart or arteries. These include high blood pressure, heart disease, stroke, diabetes and high cholesterol.
A number of studies are exploring how best to build on this connection. Strategies being researched include:
- Current medicines for heart disease risk factors. Researchers are looking into whether blood pressure medicines may benefit people with Alzheimer's. They're also studying whether the medicines may reduce the risk of dementia.
- Medicines aimed at new targets. Other studies are looking more closely at how the connection between heart disease and Alzheimer's works at the molecular level. The goal is to find new potential medicines for Alzheimer's.
- Lifestyle choices. Research suggests that lifestyle choices with known heart benefits may help prevent Alzheimer's disease or delay its onset. Those lifestyle choices include exercising on most days and eating a heart-healthy diet.
Studies during the 1990s suggested that taking hormone replacement therapy during perimenopause and menopause lowered the risk of Alzheimer's disease. But further research has been mixed. Some studies found no cognitive benefit of taking hormone replacement therapy. More research and a better understanding of the relationship between estrogen and cognitive function are needed.
Speeding treatment development
Developing new medicines is a slow process. The pace can be frustrating for people with Alzheimer's and their families who are waiting for new treatment options.
To help speed discovery, the Critical Path for Alzheimer's Disease (CPAD) consortium created a first-of-its-kind partnership to share data from Alzheimer's clinical trials. CPAD 's partners include pharmaceutical companies, nonprofit foundations and government advisers. CPAD was formerly called the Coalition Against Major Diseases.
CPAD also has collaborated with the Clinical Data Interchange Standards Consortium to create data standards. Researchers think that data standards and sharing data from thousands of study participants will speed development of more-effective therapies.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
- Treatments and research. Alzheimer's Association. https://www.alz.org/alzheimers-dementia/research_progress/treatment-horizon. Accessed March 23, 2023.
- Cummings J, et al. Alzheimer's disease drug development pipeline: 2022. Alzheimer's and Dementia. 2022; doi:10.1002/trc2.12295.
- Burns S, et al. Therapeutics of Alzheimer's disease: Recent developments. Antioxidants. 2022; doi:10.3390/antiox11122402.
- Plascencia-Villa G, et al. Lessons from antiamyloid-beta immunotherapies in Alzheimer's disease. Handbook of Clinical Neurology. 2023; doi:10.1016/B978-0-323-85555-6.00019-9.
- Brockmann R, et al. Impacts of FDA approval and Medicare restriction on antiamyloid therapies for Alzheimer's disease: Patient outcomes, healthcare costs and drug development. The Lancet Regional Health. 2023; doi:10.1016/j.lana. 2023.100467 .
- Wojtunik-Kulesza K, et al. Aducanumab — Hope or disappointment for Alzheimer's disease. International Journal of Molecular Sciences. 2023; doi:10.3390/ijms24054367.
- Can Alzheimer's disease be prevented? Alzheimer's Association. http://www.alz.org/research/science/alzheimers_prevention_and_risk.asp. Accessed March 23, 2023.
- Piscopo P, et al. A systematic review on drugs for synaptic plasticity in the treatment of dementia. Ageing Research Reviews. 2022; doi:10.1016/j.arr. 2022.101726 .
- Facile R, et al. Use of Clinical Data Interchange Standards Consortium (CDISC) standards for real-world data: Expert perspectives from a qualitative Delphi survey. JMIR Medical Informatics. 2022; doi:10.2196/30363.
- Imbimbo BP, et al. Role of monomeric amyloid-beta in cognitive performance in Alzheimer's disease: Insights from clinical trials with secretase inhibitors and monoclonal antibodies. Pharmacological Research. 2023; doi:10.1016/j.phrs. 2022.106631 .
- Conti Filho CE, et al. Advances in Alzheimer's disease's pharmacological treatment. Frontiers in Pharmacology. 2023; doi:10.3389/fphar. 2023.1101452 .
- Potter H, et al. Safety and efficacy of sargramostim (GM-CSF) in the treatment of Alzheimer's disease. Alzheimer's and Dementia. 2021; doi:10.1002/trc2.12158.
- Zhong H, et al. Effect of peroxisome proliferator-activated receptor-gamma agonists on cognitive function: A systematic review and meta-analysis. Biomedicines. 2023; doi:10.3390/biomedicines11020246.
- Grodstein F. Estrogen and cognitive function. https://www.uptodate.com/contents/search. Accessed March 23, 2023.
- Mills ZB, et al. Is hormone replacement therapy a risk factor or a therapeutic option for Alzheimer's disease? International Journal of Molecular Sciences. 2023; doi:10.3390/ijms24043205.
- Custodia A, et al. Biomarkers assessing endothelial dysfunction in Alzheimer's disease. Cells. 2023; doi:10.3390/cells12060962.
- Overview. Critical Path for Alzheimer's Disease. https://c-path.org/programs/cpad/. Accessed March 29, 2023.
- Shi M, et al. Impact of anti-amyloid-β monoclonal antibodies on the pathology and clinical profile of Alzheimer's disease: A focus on aducanumab and lecanemab. Frontiers in Aging and Neuroscience. 2022; doi:10.3389/fnagi. 2022.870517 .
- Leqembi (approval letter). Biologic License Application 761269. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=761269. Accessed July 7, 2023.
- Van Dyck CH, et al. Lecanemab in early Alzheimer's disease. New England Journal of Medicine. 2023; doi:10.1056/NEJMoa2212948.
- Leqembi (prescribing information). Eisai; 2023. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&varApplNo=761269. Accessed July 10, 2023.
Products and Services
- A Book: Mayo Clinic on Alzheimer's Disease
- Assortment of Products for Independent Living from Mayo Clinic Store
- A Book: Day to Day: Living With Dementia
- A Book: Mayo Clinic on Healthy Aging
- Give today to find Alzheimer's cures for tomorrow
- Alzheimer's sleep problems
- Alzheimer's 101
- Understanding the difference between dementia types
- Alzheimer's disease
- Alzheimer's genes
- Alzheimer's drugs
- Alzheimer's prevention: Does it exist?
- Alzheimer's stages
- Antidepressant withdrawal: Is there such a thing?
- Antidepressants and alcohol: What's the concern?
- Antidepressants and weight gain: What causes it?
- Antidepressants: Can they stop working?
- Antidepressants: Side effects
- Antidepressants: Selecting one that's right for you
- Antidepressants: Which cause the fewest sexual side effects?
- Anxiety disorders
- Atypical antidepressants
- Caregiver stress
- Clinical depression: What does that mean?
- Corticobasal degeneration (corticobasal syndrome)
- Depression and anxiety: Can I have both?
- Depression, anxiety and exercise
- What is depression? A Mayo Clinic expert explains.
- Depression in women: Understanding the gender gap
- Depression (major depressive disorder)
- Depression: Supporting a family member or friend
- Diagnosing Alzheimer's
- Did the definition of Alzheimer's disease change?
- How your brain works
- Intermittent fasting
- Lecanemab for Alzheimer's disease
- Male depression: Understanding the issues
- MAOIs and diet: Is it necessary to restrict tyramine?
- Marijuana and depression
- Mayo Clinic Minute: 3 tips to reduce your risk of Alzheimer's disease
- Mayo Clinic Minute: Alzheimer's disease risk and lifestyle
- Mayo Clinic Minute: New definition of Alzheimer's changes
- Mayo Clinic Minute: Women and Alzheimer's Disease
- Memory loss: When to seek help
- Monoamine oxidase inhibitors (MAOIs)
- Natural remedies for depression: Are they effective?
- Nervous breakdown: What does it mean?
- New Alzheimers Research
- Pain and depression: Is there a link?
- Phantosmia: What causes olfactory hallucinations?
- Positron emission tomography scan
- Posterior cortical atrophy
- Seeing inside the heart with MRI
- Selective serotonin reuptake inhibitors (SSRIs)
- Serotonin and norepinephrine reuptake inhibitors (SNRIs)
- Sundowning: Late-day confusion
- Treatment-resistant depression
- Tricyclic antidepressants and tetracyclic antidepressants
- Video: Alzheimer's drug shows early promise
- Vitamin B-12 and depression
- Young-onset Alzheimer's
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
- Alzheimer s treatments What s on the horizon
Make twice the impact
Your gift can go twice as far to advance cancer research and care!
Call our 24 hours, seven days a week helpline at 800.272.3900

- Professionals

- Younger/Early-Onset Alzheimer's
- Is Alzheimer's Genetic?
- Women and Alzheimer's
- Creutzfeldt-Jakob Disease
- Dementia with Lewy Bodies
- Down Syndrome & Alzheimer's
- Frontotemporal Dementia
- Huntington's Disease
- Mixed Dementia
- Normal Pressure Hydrocephalus
- Posterior Cortical Atrophy
- Parkinson's Disease Dementia
- Vascular Dementia
- Korsakoff Syndrome
- Traumatic Brain Injury (TBI)
- Know the 10 Signs
- Difference Between Alzheimer's & Dementia
- 10 Steps to Approach Memory Concerns in Others
- Medical Tests for Diagnosing Alzheimer's
- Why Get Checked?
- Visiting Your Doctor
- Life After Diagnosis
- Stages of Alzheimer's
- Earlier Diagnosis
- Part the Cloud
- Research Momentum
- Our Commitment to Research
- TrialMatch: Find a Clinical Trial
- What Are Clinical Trials?
- How Clinical Trials Work
- When Clinical Trials End
- Why Participate?
- Talk to Your Doctor
- Clinical Trials: Myths vs. Facts
- Can Alzheimer's Disease Be Prevented?
- Brain Donation
- Navigating Treatment Options
- Lecanemab Approved for Treatment of Early Alzheimer's Disease
- Aducanumab Discontinued as Alzheimer's Treatment
- Medicare Treatment Coverage
- Donanemab for Treatment of Early Alzheimer's Disease — News Pending FDA Review
- Questions for Your Doctor
- Medications for Memory, Cognition and Dementia-Related Behaviors
- Treatments for Behavior
- Treatments for Sleep Changes
- Alternative Treatments
- Facts and Figures
- Assessing Symptoms and Seeking Help
- Now is the Best Time to Talk about Alzheimer's Together
- Get Educated
- Just Diagnosed
- Sharing Your Diagnosis
- Changes in Relationships
- If You Live Alone
- Treatments and Research
- Legal Planning
- Financial Planning
- Building a Care Team
- End-of-Life Planning
- Programs and Support
- Overcoming Stigma
- Younger-Onset Alzheimer's
- Taking Care of Yourself
- Reducing Stress
- Tips for Daily Life
- Helping Family and Friends
- Leaving Your Legacy
- Live Well Online Resources
- Make a Difference
- Daily Care Plan
- Communication and Alzheimer's
- Food and Eating
- Art and Music
- Incontinence
- Dressing and Grooming
- Dental Care
- Working With the Doctor
- Medication Safety
- Accepting the Diagnosis
- Early-Stage Caregiving
- Middle-Stage Caregiving
- Late-Stage Caregiving
- Aggression and Anger
- Anxiety and Agitation
- Hallucinations
- Memory Loss and Confusion
- Sleep Issues and Sundowning
- Suspicions and Delusions
- In-Home Care
- Adult Day Centers
- Long-Term Care
- Respite Care
- Hospice Care
- Choosing Care Providers
- Finding a Memory Care-Certified Nursing Home or Assisted Living Community
- Changing Care Providers
- Working with Care Providers
- Creating Your Care Team
- Long-Distance Caregiving
- Community Resource Finder
- Be a Healthy Caregiver
- Caregiver Stress
- Caregiver Stress Check
- Caregiver Depression
- Changes to Your Relationship
- Grief and Loss as Alzheimer's Progresses
- Home Safety
- Dementia and Driving
- Technology 101
- Preparing for Emergencies
- Managing Money Online Program
- Planning for Care Costs
- Paying for Care
- Health Care Appeals for People with Alzheimer's and Other Dementias
- Social Security Disability
- Medicare Part D Benefits
- Tax Deductions and Credits
- Planning Ahead for Legal Matters
- Legal Documents
- ALZ Talks Virtual Events
- ALZNavigator™
- Veterans and Dementia
- The Knight Family Dementia Care Coordination Initiative
- Online Tools
- Asian Americans and Pacific Islanders and Alzheimer's
- Native Americans and Alzheimer's
- Black Americans and Alzheimer's
- Hispanic Americans and Alzheimer's
- LGBTQ+ Community Resources for Dementia
- Educational Programs and Dementia Care Resources
- Brain Facts
- 50 Activities
- For Parents and Teachers
- Resolving Family Conflicts
- Holiday Gift Guide for Caregivers and People Living with Dementia
- Trajectory Report
- Resource Lists
- Search Databases
- Publications
- Favorite Links
- 10 Healthy Habits for Your Brain
- Stay Physically Active
- Adopt a Healthy Diet
- Stay Mentally and Socially Active
- Online Community
- Support Groups
- Find Your Local Chapter
- Any Given Moment
- New IDEAS Study
- RFI Amyloid PET Depletion Following Treatment
- Bruce T. Lamb, Ph.D., Chair
- Christopher van Dyck, M.D.
- Cynthia Lemere, Ph.D.
- David Knopman, M.D.
- Lee A. Jennings, M.D. MSHS
- Karen Bell, M.D.
- Lea Grinberg, M.D., Ph.D.
- Malú Tansey, Ph.D.
- Mary Sano, Ph.D.
- Oscar Lopez, M.D.
- Suzanne Craft, Ph.D.
- About Our Grants
- Andrew Kiselica, Ph.D., ABPP-CN
- Arjun Masurkar, M.D., Ph.D.
- Benjamin Combs, Ph.D.
- Charles DeCarli, M.D.
- Damian Holsinger, Ph.D.
- David Soleimani-Meigooni, Ph.D.
- Donna M. Wilcock, Ph.D.
- Elizabeth Head, M.A, Ph.D.
- Fan Fan, M.D.
- Fayron Epps, Ph.D., R.N.
- Ganesh Babulal, Ph.D., OTD
- Hui Zheng, Ph.D.
- Jason D. Flatt, Ph.D., MPH
- Jennifer Manly, Ph.D.
- Joanna Jankowsky, Ph.D.
- Luis Medina, Ph.D.
- Marcello D’Amelio, Ph.D.
- Marcia N. Gordon, Ph.D.
- Margaret Pericak-Vance, Ph.D.
- María Llorens-Martín, Ph.D.
- Nancy Hodgson, Ph.D.
- Shana D. Stites, Psy.D., M.A., M.S.
- Walter Swardfager, Ph.D.
- ALZ WW-FNFP Grant
- Capacity Building in International Dementia Research Program
- ISTAART IGPCC
- Alzheimer’s Disease Strategic Fund: Endolysosomal Activity in Alzheimer’s (E2A) Grant Program
- Imaging Research in Alzheimer’s and Other Neurodegenerative Diseases
- Zenith Fellow Awards
- National Academy of Neuropsychology & Alzheimer’s Association Funding Opportunity
- Part the Cloud-Gates Partnership Grant Program: Bioenergetics and Inflammation
- Pilot Awards for Global Brain Health Leaders (Invitation Only)
- Robert W. Katzman, M.D., Clinical Research Training Scholarship
- Funded Studies
- How to Apply
- Portfolio Summaries
- Supporting Research in Health Disparities, Policy and Ethics in Alzheimer’s Disease and Dementia Research (HPE-ADRD)
- Diagnostic Criteria & Guidelines
- Annual Conference: AAIC
- Professional Society: ISTAART
- Alzheimer's & Dementia
- Alzheimer's & Dementia: DADM
- Alzheimer's & Dementia: TRCI
- International Network to Study SARS-CoV-2 Impact on Behavior and Cognition
- Alzheimer’s Association Business Consortium (AABC)
- Global Biomarker Standardization Consortium (GBSC)
- Global Alzheimer’s Association Interactive Network
- International Alzheimer's Disease Research Portfolio
- Alzheimer’s Disease Neuroimaging Initiative Private Partner Scientific Board (ADNI-PPSB)
- Research Roundtable
- About WW-ADNI
- North American ADNI
- European ADNI
- Australia ADNI
- Taiwan ADNI
- Argentina ADNI
- WW-ADNI Meetings
- Submit Study
- RFI Inclusive Language Guide
- Scientific Conferences
- AUC for Amyloid and Tau PET Imaging
- Make a Donation
- Walk to End Alzheimer's
- The Longest Day
- RivALZ to End ALZ
- Ride to End ALZ
- Tribute Pages
- Gift Options to Meet Your Goals
- Founders Society
- Fred Bernhardt
- Anjanette Kichline
- Lori A. Jacobson
- Pam and Bill Russell
- Gina Adelman
- Franz and Christa Boetsch
- Adrienne Edelstein
- For Professional Advisors
- Free Planning Guides
- Contact the Planned Giving Staff
- Workplace Giving
- Do Good to End ALZ
- Donate a Vehicle
- Donate Stock
- Donate Cryptocurrency
- Donate Gold & Sterling Silver
- Donor-Advised Funds
- Use of Funds
- Giving Societies
- Why We Advocate
- Ambassador Program
- About the Alzheimer’s Impact Movement
- Research Funding
- Improving Care
- Support for People Living With Dementia
- Public Policy Victories
- Planned Giving
- Community Educator
- Community Representative
- Support Group Facilitator or Mentor
- Faith Outreach Representative
- Early Stage Social Engagement Leaders
- Data Entry Volunteer
- Tech Support Volunteer
- Other Local Opportunities
- Visit the Program Volunteer Community to Learn More
- Become a Corporate Partner
- A Family Affair
- A Message from Elizabeth
- The Belin Family
- The Eliashar Family
- The Fremont Family
- The Freund Family
- Jeff and Randi Gillman
- Harold Matzner
- The Mendelson Family
- Patty and Arthur Newman
- The Ozer Family
- Salon Series
- No Shave November
- Other Philanthropic Activities
- Still Alice
- The Judy Fund E-blast Archive
- The Judy Fund in the News
- The Judy Fund Newsletter Archives
- Sigma Kappa Foundation
- Alpha Delta Kappa
- Parrot Heads in Paradise
- Tau Kappa Epsilon (TKE)
- Sigma Alpha Mu
- Alois Society Member Levels and Benefits
- Alois Society Member Resources
- Zenith Society
- Founder's Society
- Joel Berman
- JR and Emily Paterakis
- Legal Industry Leadership Council
- Accounting Industry Leadership Council

Find Local Resources
Let us connect you to professionals and support options near you. Please select an option below:
Use Current Location Use Map Selector
Search Alzheimer’s Association
As the largest nonprofit funder of Alzheimer's research, the Association is committed to accelerating the global progress of new treatments, preventions and, ultimately, a cure.
Information for Researchers
Research we fund, apply for a grant, find a clinical trial, efforts we lead, the first survivor of alzheimer's is out there, but we won't get there without you., learn how alzheimer’s disease affects the brain..
Take the Brain Tour
Don't just hope for a cure. Help us find one. Volunteer for a clinical trial.
Keep up with alzheimer’s news and events.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
March 3, 2023
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
New insights into eye damage in Alzheimer's disease patients
by Cedars-Sinai Medical Center
Cedars-Sinai investigators have produced the most extensive analysis to date of changes in the retina—a layer of tissue at the back of the eye where visual information originates—and how those retinal changes correspond to brain and cognitive changes in Alzheimer's disease patients.
Their analysis, published in Acta Neuropathologica , is an important step toward understanding the complex effects of Alzheimer's disease on the retina, especially at the earliest stages of cognitive impairment. Experts believe this understanding is key for the development of more effective treatments that could prevent progression of the disease.
More than 3 million Americans are diagnosed with Alzheimer's disease each year. The disease progressively destroys memory and cognitive ability. Currently, there is no single diagnostic test that can definitively diagnose a patient with Alzheimer's disease, and the newest treatments only slow–don't stop—progression.
"Our study is the first to provide in-depth analyses of the protein profiles and the molecular, cellular, and structural effects of Alzheimer's disease in the human retina and how they correspond with changes in the brain and cognitive function," said Maya Koronyo-Hamaoui, Ph.D., professor of Neurosurgery, Neurology, and Biomedical Sciences at Cedars-Sinai and senior author of the study.
"These findings may eventually lead to the development of imaging techniques that allow us to diagnose Alzheimer's disease earlier and more accurately and monitor its progression noninvasively by looking through the eye."
"The retina, a developmental extension of the brain, offers an unparalleled opportunity for affordable, noninvasive monitoring of the central nervous system," said Yosef Koronyo, MSc, research associate in the Cedars-Sinai Department of Neurosurgery and first author of the study. "And with the help of our collaborators, we discovered the accumulation of highly toxic proteins in the retinas of patients with Alzheimer's disease and mild cognitive impairment , causing severe degeneration of cells."
Investigators looked at retinal and brain tissue samples collected over 14 years from 86 human donors—the largest group of retinal samples from human patients with Alzheimer's disease and mild cognitive impairment thus far studied. They compared samples from donors with normal cognitive function to those with mild cognitive impairment at the earliest stages of Alzheimer's disease, and those with later-stage Alzheimer's disease dementia.
The investigators explored the physical features of the retinas of these patients, measuring and mapping markers of inflammation and functional cell loss, and analyzed the proteins present in retinal and brain tissues.
Here is what investigators found in the retinas of patients with mild cognitive impairment and Alzheimer's disease:
- An overabundance of a protein called amyloid beta 42, which in the brains of Alzheimer's disease patients clumps together to form plaques that disrupt brain function
- Accumulation of amyloid beta protein in ganglion cells, the cells that bridge visual input from the retina to the optic nerve
- Higher numbers of astrocytes and immune cells, called microglia, tightly surrounding amyloid beta plaques
- As many as 80% fewer microglial cells clearing amyloid beta proteins from the retina and brain
- Specific molecules and biological pathways responsible for inflammation, and cell and tissue death
"These changes in the retina correlated with changes in parts of the brain called the entorhinal and temporal cortices, a hub for memory, navigation and the perception of time," said Koronyo.
Retinal changes also correlated with the pathological stage of Alzheimer's disease (called Braak stage) and patients' cognitive status. And they were found even in patients who appeared cognitively normal or very mildly impaired, marking them as a possible early predictor of later cognitive decline.
"These findings give us a deeper understanding of the effects of Alzheimer's disease on the retina ," said Keith L. Black, MD, chair of the Department of Neurosurgery and the Ruth and Lawrence Harvey Chair in Neuroscience at Cedars-Sinai and a co-author of the study. "Because these changes correspond with changes in the brain and can be detected in the earliest stages of impairment, they may lead us to new diagnostics for Alzheimer's disease and a means to evaluate new forms of treatment."
Explore further
Feedback to editors

Occupations that are cognitively stimulating may be protective against later-life dementia
Apr 20, 2024

Researchers develop a new way to safely boost immune cells to fight cancer
Apr 19, 2024

New compound from blessed thistle may promote functional nerve regeneration

New research defines specific genomic changes associated with the transmissibility of the mpox virus

New study confirms community pharmacies can help people quit smoking

Researchers discover glial hyper-drive for triggering epileptic seizures

Deeper dive into the gut microbiome shows changes linked to body weight

A new therapeutic target for traumatic brain injury

Dozens of COVID virus mutations arose in man with longest known case, research finds

Researchers explore causal machine learning, a new advancement for AI in health care
Related stories.

New potential clues in diagnosing, treating Alzheimer's found in retinas
Nov 17, 2020

Predicting dementia using neural network characteristics
Jan 27, 2023

Japan approves Alzheimer's blood test kit
Dec 22, 2022
Alzheimer's disease can be diagnosed before symptoms emerge
Nov 11, 2022
Researchers investigate brain's immune cell response in Alzheimer's disease
Nov 3, 2022
Brain tissue inflammation is key to Alzheimer's disease progression
Aug 26, 2021
Recommended for you

Analyzing the progression in retinal thickness could predict cognitive progression in Parkinson's patients

Geneticists develop world's first bioinformatic tool to identify amyloids consisting of multiple copies of same protein

Potential modifier gene identified as cause of ciliary pathology in retinitis pigmentosa patient

No negative impact from prolonged eye patching on child's development or family stress levels
Apr 18, 2024

AI beats doctors in accurately assessing eye problems
Apr 17, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

- April 17, 2024 | New Research Suggests That Common HIV Drugs May Reduce Alzheimer’s Risk
- April 17, 2024 | NASA Solves Mystery: Identifies Object That Crashed Into Florida Home
- April 17, 2024 | Powerful New Tool Ushers In New Era of Quantum Materials Research
- April 17, 2024 | New Green Water Technology Could Reduce Carbon Emissions and Save $15.6 Billion
- April 17, 2024 | Why Do Humans Blink So Much? New Research Challenges Traditional Views
New Research Suggests That Common HIV Drugs May Reduce Alzheimer’s Risk
By Sanford Burnham Prebys April 17, 2024

Recent research indicates that HIV drugs may reduce the incidence of Alzheimer’s disease. The study, analyzing data from over 225,000 patients, shows promising results for using reverse transcriptase inhibitors in older, HIV-positive individuals to potentially lower Alzheimer’s occurrence.
Researchers at Sanford Burnham Prebys have discovered positive connections between certain HIV medications and Alzheimer’s disease.
Nearly seven million individuals in the U.S. are currently battling Alzheimer’s disease (AD), and projections suggest this number could rise to almost 13 million by 2050. This increasing prevalence highlights a critical gap in effective treatments for AD. Researchers at Sanford Burnham Prebys have recently made significant strides by discovering real-world evidence that common HIV medications may lower the risk of AD. The study, led by Jerold Chun, M.D., Ph.D., was published in Pharmaceuticals .
Chun’s new research builds on his lab’s landmark publication in Nature in 2018 that described how somatic gene recombination in neurons can produce thousands of new gene variants within Alzheimer’s disease brains. Importantly, it also revealed for the first time how the Alzheimer’s-linked gene, APP, is recombined by using the same type of enzyme found in HIV.
The enzyme, called reverse transcriptase (RT), copies RNA molecules and changes them into complementary DNA duplicates that can then be inserted back into DNA, producing permanent sequence changes within the cell’s DNA blueprint.

Jerold Chun, M.D. Ph.D., is a professor in the Center for Genetic Disorders and Aging Research at Sanford Burnham Prebys. Credit: Sanford Burnham Prebys
Linking HIV Treatment and Alzheimer’s Reduction
HIV and many other viruses rely on RT to hijack a host’s cells to establish a chronic infection, so drugs that block the RT enzyme’s activity have become a common part of treatment cocktails for keeping HIV at bay.
The brain appears to have its own RTs that are different from those in viruses, and the research team wondered if inhibiting brain RTs with HIV drugs actually helps AD patients.
To assess the link between real-world RT inhibitor exposure and AD in humans, the team analyzed anonymized medical records with prescription claims from more than 225,000 control and HIV-positive patients, and found that RT inhibitor exposure was associated with a statistically significant reduced incidence and prevalence of AD.
“Thus, we looked at HIV-positive individuals taking RT inhibitors and other combined antiretroviral therapies as they aged, and asked the question: How many of them got Alzheimer’s disease?” says Chun. “And the answer is that there were many fewer than might have been expected compared to the general population.”
Observational Findings and Future Directions
Of the more than 225,000 individuals with claims data in the study, just shy of 80,000 were HIV-positive individuals over the age of 60. More than 46,000 had taken RT inhibitors during a nearly three-year observation period from 2016 to 2019. The data was obtained through a collaboration with health information technology and clinical research firm IQVIA, led by Tiffany Chow, M.D.
In living persons with HIV, there were 2.46 Alzheimer’s disease diagnoses per 1,000 persons among HIV-positive individuals taking these inhibitors, versus 6.15 for the general population. This control group was represented by more than 150,000 HIV-negative patients over the age of 60 with medical insurance claims related to treatment for the common cold.
“You cannot feasibly run a prospective clinical trial with this number of patients,” Chun adds. “This approach is a way to look at how a drug can act on a large patient population.”
Chun underscores that the drugs patients took in this retrospective study were designed to counter RT activity in HIV and likely only had a limited effect on many different possible forms of the enzyme active in the brain.
“What we’re looking at now is very crude,” says Chun. “The clear next step for our lab is to identify which versions of RTs are at work in the AD brain so that more targeted treatments can be discovered, while prospective clinical trials of currently available RT inhibitors on persons with early AD should be pursued.”
Reference: “Nucleoside Reverse Transcriptase Inhibitor Exposure Is Associated with Lower Alzheimer’s Disease Risk: A Retrospective Cohort Proof-of-Concept Study” by Tiffany W. Chow, Mark Raupp, Matthew W. Reynolds, Siying Li, Gwendolyn E. Kaeser and Jerold Chun, 21 March 2024, Pharmaceuticals . DOI: 10.3390/ph17040408
The work was supported by the National Institute on Aging – NIH (R01AG071465, R01AG065541, and R56AG073965), the Shaffer Family Foundation, and the Bruce Ford & Anne Smith Bundy Foundation.
More on SciTechDaily

Ice Age Discoveries Cool Down Climate Change Alarms
Collapsing COVID: MIT Says Ultrasound Can Damage Coronaviruses

“Puzzling and Surprising” New Gas Signatures Discovered by ExoMars Orbiter in the Martian Atmosphere

Quantum Revolution: Redefining Physics With Fractional Charges

Warning: Columbia University Uncovers High Metal Levels in Blood of Marijuana Users

Hydroxyl Molecule Signature Detected in an Exoplanet Atmosphere for the First Time

Botox Breakthrough – New Discovery Could Save Lives
Esa’s φ-week: digital twin earth, quantum computing and ai take center stage, be the first to comment on "new research suggests that common hiv drugs may reduce alzheimer’s risk", leave a comment cancel reply.
Email address is optional. If provided, your email will not be published or shared.
Save my name, email, and website in this browser for the next time I comment.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 02 January 2023
Insights into Alzheimer’s disease from single-cell genomic approaches
- Mitchell H. Murdock 1 &
- Li-Huei Tsai ORCID: orcid.org/0000-0003-1262-0592 1
Nature Neuroscience volume 26 , pages 181–195 ( 2023 ) Cite this article
21k Accesses
32 Citations
150 Altmetric
Metrics details
- Cell death in the nervous system
- Cellular neuroscience
Alzheimer’s disease (AD) is an age-related disease pathologically defined by the deposition of amyloid plaques and neurofibrillary tangles in the brain parenchyma. Single-cell profiling has shown that Alzheimer’s dementia involves the complex interplay of virtually every major brain cell type. Here, we highlight cell-type-specific molecular perturbations in AD. We discuss how genomic information from single cells expands existing paradigms of AD pathogenesis and highlight new opportunities for therapeutic interventions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
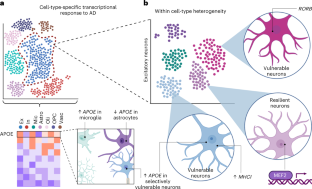
Similar content being viewed by others

A global view of aging and Alzheimer’s pathogenesis-associated cell population dynamics and molecular signatures in human and mouse brains
Andras Sziraki, Ziyu Lu, … Junyue Cao
Single-cell transcriptomic analysis of Alzheimer’s disease
Hansruedi Mathys, Jose Davila-Velderrain, … Li-Huei Tsai
Systematic phenotyping and characterization of the 5xFAD mouse model of Alzheimer’s disease
Stefania Forner, Shimako Kawauchi, … Kim N. Green
Yao, Z. et al. A transcriptomic and epigenomic cell atlas of the mouse primary motor cortex. Nature 598 , 103–110 (2021).
Article CAS Google Scholar
Zeisel, A. et al. Molecular architecture of the mouse nervous system. Cell 174 , 999–1014 (2018).
Tasic, B. et al. Shared and distinct transcriptomic cell types across neocortical areas. Nature 563 , 72–78 (2018).
Saunders, A. et al. Molecular diversity and specializations among the cells of the adult mouse brain. Cell 174 , 1015–1030 (2018).
Lake, B. B. et al. Neuronal subtypes and diversity revealed by single-nucleus RNA sequencing of the human brain. Science 352 , 1586–1590 (2016).
Zhong, S. et al. A single-cell RNA-seq survey of the developmental landscape of the human prefrontal cortex. Nature 555 , 524–528 (2018).
Grubman, A. et al. A single-cell atlas of entorhinal cortex from individuals with Alzheimer’s disease reveals cell-type-specific gene expression regulation. Nat. Neurosci. 22 , 2087–2097 (2019).
Mathys, H. et al. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 570 , 332–337 (2019).
Zhou, Y. et al. Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer’s disease. Nat. Med. 26 , 131–142 (2020).
Villa, K. L. et al. Inhibitory synapses are repeatedly assembled and removed at persistent sites in vivo. Neuron 89 , 756–769 (2016).
Kurucu, H. et al. Inhibitory synapse loss and accumulation of amyloid beta in inhibitory presynaptic terminals in Alzheimer’s disease. Eur. J. Neurol. 29 , 1311–1323.
Otero-Garcia, M. et al. Molecular signatures underlying neurofibrillary tangle susceptibility in Alzheimer’s disease. Neuron 110 , 2929–2948 (2022).
Davila-Velderrain, J. et al. Single-cell anatomical analysis of human hippocampus and entorhinal cortex uncovers early-stage molecular pathology in Alzheimer’s disease. Preprint at bioRxiv https://doi.org/10.1101/2021.07.01.450715 (2021).
Lau, S.-F., Cao, H., Fu, A. K. Y. & Ip, N. Y. Single-nucleus transcriptome analysis reveals dysregulation of angiogenic endothelial cells and neuroprotective glia in Alzheimer’s disease. Proc. Natl Acad. Sci. USA 117 , 25800–25809 (2020).
Li, S. & Sheng, Z. -H. Energy matters: presynaptic metabolism and the maintenance of synaptic transmission. Nat. Rev. Neurosci. https://doi.org/10.1038/s41583-021-00535-8 (2021).
Cheng, X.-T., Huang, N. & Sheng, Z.-H. Programming axonal mitochondrial maintenance and bioenergetics in neurodegeneration and regeneration. Neuron 110 , 1899–1923 (2022).
Fu, H. et al. A tau homeostasis signature is linked with the cellular and regional vulnerability of excitatory neurons to tau pathology. Nat. Neurosci. 22 , 47–56 (2019).
Leng, K. et al. Molecular characterization of selectively vulnerable neurons in Alzheimer’s disease. Nat. Neurosci. 24 , 276–287 (2021).
Barker, S. J. et al. MEF2 is a key regulator of cognitive potential and confers resilience to neurodegeneration. Sci. Transl. Med. 13 , eabd7695 (2021).
Zalocusky, K. A. et al. Neuronal ApoE upregulates MHC-I expression to drive selective neurodegeneration in Alzheimer’s disease. Nat. Neurosci. 24 , 786–798 (2021).
Tiscione, S. A. et al. IP3R-driven increases in mitochondrial Ca2 + promote neuronal death in NPC disease. Proc. Natl Acad. Sci. USA 118 , e2110629118 (2021).
Welch, G. & Tsai, L. -H. Mechanisms of DNA damage-mediated neurotoxicity in neurodegenerative disease. EMBO Rep. 23 , e54217 (2022).
Lodato, M. A. et al. Aging and neurodegeneration are associated with increased mutations in single human neurons. Science 359 , 555–559 (2018).
Zhu, Q., Niu, Y., Gundry, M. & Zong, C. Single-cell damagenome profiling unveils vulnerable genes and functional pathways in human genome toward DNA damage. Sci. Adv. 7 , eabf3329 (2021).
Welch, G. M. et al. Neurons burdened by DNA double-strand breaks incite microglia activation through antiviral-like signaling in neurodegeneration. Sci. Adv. 8 , eabo4662 (2022).
Wu, W. et al. Neuronal enhancers are hotspots for DNA single-strand break repair. Nature 593 , 440–444 (2021).
Madabhushi, R. et al. Activity-induced DNA breaks govern the expression of neuronal early-response genes. Cell 161 , 1592–1605 (2015).
Habib, N. et al. Div-Seq: single-nucleus RNA-seq reveals dynamics of rare adult newborn neurons. Science 353 , 925–928 (2016).
Hochgerner, H., Zeisel, A., Lönnerberg, P. & Linnarsson, S. Conserved properties of dentate gyrus neurogenesis across postnatal development revealed by single-cell RNA sequencing. Nat. Neurosci. 21 , 290–299 (2018).
Durante, M. A. et al. Single-cell analysis of olfactory neurogenesis and differentiation in adult humans. Nat. Neurosci. 23 , 323–326 (2020).
Rohrback, S. et al. Submegabase copy number variations arise during cerebral cortical neurogenesis as revealed by single-cell whole-genome sequencing. Proc. Natl Acad. Sci. USA 115 , 10804–10809 (2018).
Ayhan, F. et al. Resolving cellular and molecular diversity along the hippocampal anterior-to-posterior axis in humans. Neuron 109 , 2091–2105 (2021).
Zhou, Y. et al. Molecular landscapes of human hippocampal immature neurons across lifespan. Nature 607 , 527–533 (2022).
Franjic, D. et al. Transcriptomic taxonomy and neurogenic trajectories of adult human, macaque, and pig hippocampal and entorhinal cells. Neuron https://doi.org/10.1016/j.neuron.2021.10.036 (2021).
Article Google Scholar
Arber, C. et al. Familial Alzheimer’s disease mutations in PSEN1 lead to premature human stem cell neurogenesis. Cell Rep. 34 , 108615 (2021).
Cosacak, M. I. et al. Single-cell transcriptomics analyses of neural stem cell heterogeneity and contextual plasticity in a zebrafish brain model of amyloid toxicity. Cell Rep. 27 , 1307–1318 (2019).
Mi, D. et al. Early emergence of cortical interneuron diversity in the mouse embryo. Science 360 , 81–85 (2018).
Tasic, B. et al. Adult mouse cortical cell taxonomy revealed by single-cell transcriptomics. Nat. Neurosci. 19 , 335–346 (2016).
Krienen, F. M. et al. Innovations present in the primate interneuron repertoire. Nature 586 , 262–269 (2020).
Yu, Y. et al. Interneuron origin and molecular diversity in the human fetal brain. Nat. Neurosci. 24 , 1745–1756 (2021).
Bugeon, S. et al. A transcriptomic axis predicts state modulation of cortical interneurons. Nature 607 , 330–338 (2022).
Martinez-Losa, M. et al. Nav1.1-overexpressing interneuron transplants restore brain rhythms and cognition in a mouse model of Alzheimer’s disease. Neuron 98 , 75–89 (2018).
Nuriel, T. et al. Neuronal hyperactivity due to loss of inhibitory tone in APOE4 mice lacking Alzheimer’s disease-like pathology. Nat. Commun. 8 , 1464 (2017).
Smith, S. J. et al. Single-cell transcriptomic evidence for dense intracortical neuropeptide networks. eLife 8 , e47889 (2019).
Zhong, W. et al. The neuropeptide landscape of human prefrontal cortex. Proc. Natl Acad. Sci. USA 119 , e2123146119 (2022).
Cauli, B. et al. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J. Neurosci. 24 , 8940–8949 (2004).
Jansen, I. E. et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat. Genet. 51 , 404–413 (2019).
Chen, Y. & Colonna, M. Microglia in Alzheimer’s disease at single-cell level. Are there common patterns in humans and mice? J. Exp. Med. 218 , e20202717 (2021).
Keren-Shaul, H. et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169 , 1276–1290 (2017).
Grubman, A. et al. Transcriptional signature in microglia associated with Aβ plaque phagocytosis. Nat. Commun. 12 , 3015 (2021).
March-Diaz, R. et al. Hypoxia compromises the mitochondrial metabolism of Alzheimer’s disease microglia via HIF1. Nat. Aging 1 , 385–399 (2021).
Mathys, H. et al. Temporal tracking of microglia activation in neurodegeneration at single-cell resolution. Cell Rep. 21 , 366–380 (2017).
Friedman, B. A. et al. Diverse brain myeloid expression profiles reveal distinct microglial activation states and aspects of Alzheimer’s disease not evident in mouse models. Cell Rep. 22 , 832–847 (2018).
Sala Frigerio, C. et al. The major risk factors for Alzheimer’s disease: age, sex and genes modulate the microglia response to Aβ plaques. Cell Rep. 27 , 1293–1306 (2019).
Yang, H. S. et al. Natural genetic variation determines microglia heterogeneity in wild-derived mouse models of Alzheimer’s disease. Cell Rep. 34 , 108739 (2021).
Sayed, F. A. et al. AD-linked R47H-TREM2 mutation induces disease-enhancing microglial states via AKT hyperactivation. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.abe3947 (2021).
Jaitin, D. A. et al. Lipid-associated macrophages control metabolic homeostasis in a Trem2-dependent manner. Cell 178 , 686–698 (2019).
Krasemann, S. et al. The TREM2–APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47 , 566–581 (2017).
Shi, Y. et al. Overexpressing low-density lipoprotein receptor reduces tau-associated neurodegeneration in relation to apoE-linked mechanisms. Neuron 109 , 2413–2426 (2021).
Badimon, A. et al. Negative feedback control of neuronal activity by microglia. Nature 586 , 417–423 (2020).
Merlini, M. et al. Microglial Gi-dependent dynamics regulate brain network hyperexcitability. Nat. Neurosci. 24 , 19–23 (2021).
Hong, S. et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352 , 712–716 (2016).
Gunner, G. et al. Sensory lesioning induces microglial synapse elimination via ADAM10 and fractalkine signaling. Nat. Neurosci. 22 , 1075–1088 (2019).
Victor, M. B. et al. Lipid accumulation induced by APOE4 impairs microglial surveillance of neuronal-network activity. Cell Stem Cell 29 , 1197–1212 (2022).
Favuzzi, E. et al. GABA-receptive microglia selectively sculpt developing inhibitory circuits. Cell 184 , 4048–4063 (2021).
Stogsdill, J. A. et al. Pyramidal neuron subtype diversity governs microglia states in the neocortex. Nature https://doi.org/10.1038/s41586-022-05056-7 (2022).
Bisht, K. et al. Capillary-associated microglia regulate vascular structure and function through PANX1–P2RY12 coupling in mice. Nat. Commun. 12 , 5289 (2021).
Császár, E. et al. Microglia modulate blood flow, neurovascular coupling, and hypoperfusion via purinergic actions. J. Exp. Med. 219 , e20211071 (2022).
Masuda, T. et al. Specification of CNS macrophage subsets occurs postnatally in defined niches. Nature 604 , 740–748 (2022).
Munro, D. A. D., Movahedi, K. & Priller, J. Macrophage compartmentalization in the brain and cerebrospinal fluid system. Sci. Immunol. 7 , eabk0391 (2022).
Vanlandewijck, M. et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature 554 , 475–480 (2018).
Van Hove, H. et al. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat. Neurosci. 22 , 1021–1035 (2019).
Hernández, J. C. C. et al. Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer’s disease mouse models. Nat. Neurosci. 22 , 413 (2019).
Sankowski, R. et al. Mapping microglia states in the human brain through the integration of high-dimensional techniques. Nat. Neurosci. 22 , 2098–2110 (2019).
Safaiyan, S. et al. White matter aging drives microglial diversity. Neuron 109 , 1100–1117 (2021).
Hughes, A. N. & Appel, B. Microglia phagocytose myelin sheaths to modify developmental myelination. Nat. Neurosci. 23 , 1055–1066 (2020).
Morabito, S. et al. Single-nucleus chromatin accessibility and transcriptomic characterization of Alzheimer’s disease. Nat. Genet. 53 , 1143–1155 (2021).
Novikova, G. et al. Integration of Alzheimer’s disease genetics and myeloid genomics identifies disease risk regulatory elements and genes. Nat. Commun. 12 , 1610 (2021).
Huang, K. et al. A common haplotype lowers PU.1 expression in myeloid cells and delays onset of Alzheimer’s disease. Nat. Neurosci. 20 , 1052–1061 (2017).
Cao, H. et al. Association of SPI1 haplotypes with altered SPI1 gene expression and Alzheimer’s disease risk. J. Alzheimers Dis. 86 , 1861–1873 (2022).
Dräger, N. M. et al. A CRISPRi/a platform in human iPSC-derived microglia uncovers regulators of disease states. Nat. Neurosci. https://doi.org/10.1038/s41593-022-01131-4 (2022).
Batiuk, M. Y. et al. Identification of region-specific astrocyte subtypes at single-cell resolution. Nat. Commun. 11 , 1220 (2020).
Bayraktar, O. A. et al. Astrocyte layers in the mammalian cerebral cortex revealed by a single-cell in situ transcriptomic map. Nat. Neurosci. 23 , 500–509 (2020).
Hasel, P., Rose, I. V. L., Sadick, J. S., Kim, R. D. & Liddelow, S. A. Neuroinflammatory astrocyte subtypes in the mouse brain. Nat. Neurosci. 24 , 1475–1487 (2021).
Escartin, C. et al. Reactive astrocyte nomenclature, definitions and future directions. Nat. Neurosci. 24 , 312–325 (2021).
Habib, N. et al. Disease-associated astrocytes in Alzheimer’s disease and aging. Nat. Neurosci. 23 , 701–706 (2020).
Ioannou, M. S. et al. Neuron–astrocyte metabolic coupling protects against activity-induced fatty acid toxicity. Cell 177 , 1522–1535 (2019).
Wang, C. et al. Selective removal of astrocytic APOE4 strongly protects against tau-mediated neurodegeneration and decreases synaptic phagocytosis by microglia. Neuron 109 , 1657–1674 (2021).
Ballabio, A. & Bonifacino, J. S. Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat. Rev. Mol. Cell Biol. 21 , 101–118 (2020).
Xu, Y., Kong, J. & Hu, P. Computational drug repurposing for Alzheimer’s disease using risk genes from GWAS and single-cell RNA-sequencing studies. Front. Pharmacol . 12 , 617537 (2021).
Marques, S. et al. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 352 , 1326–1329 (2016).
Falcão, A. M. et al. Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nat. Med. 24 , 1837–1844 (2018).
Jäkel, S. et al. Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature 566 , 543–547 (2019).
Chen, J.-F. et al. Enhancing myelin renewal reverses cognitive dysfunction in a murine model of Alzheimer’s disease. Neuron 109 , 2292–2307 (2021).
Bonetto, G., Belin, D. & Káradóttir, R. T. Myelin: a gatekeeper of activity-dependent circuit plasticity? Science 374 , eaba6905 (2021).
de Faria, O. et al. Periods of synchronized myelin changes shape brain function and plasticity. Nat. Neurosci. 24 , 1508–1521 (2021).
Yang, S. M., Michel, K., Jokhi, V., Nedivi, E. & Arlotta, P. Neuron class-specific responses govern adaptive myelin remodeling in the neocortex. Science https://doi.org/10.1126/science.abd2109 (2020).
Pan, S., Mayoral, S. R., Choi, H. S., Chan, J. R. & Kheirbek, M. A. Preservation of a remote fear memory requires new myelin formation. Nat. Neurosci. 23 , 487–499 (2020).
Kirby, L. et al. Oligodendrocyte precursor cells present antigen and are cytotoxic targets in inflammatory demyelination. Nat. Commun. 10 , 3887 (2019).
Kenigsbuch, M. et al. A shared disease-associated oligodendrocyte signature among multiple CNS pathologies. Nat. Neurosci. 25 , 876–886 (2022).
Arai, K. & Lo, E. H. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J. Neurosci. 29 , 4351–4355 (2009).
Pham, L.-D. D. et al. Cross-talk between oligodendrocytes and cerebral endothelium contributes to vascular remodeling after white matter injury. Glia 60 , 875–881 (2012).
Marques, S. et al. Transcriptional convergence of oligodendrocyte lineage progenitors during development. Dev. Cell 46 , 504–517 (2018).
Beiter, R. M. et al. Evidence for oligodendrocyte progenitor cell heterogeneity in the adult mouse brain. Sci. Rep. 12 , 12921 (2022).
Huang, W. et al. Origins and proliferative states of human oligodendrocyte precursor. Cell 182 , 594–608 (2020).
Fu, Y. et al. Heterogeneity of glial progenitor cells during the neurogenesis-to-gliogenesis switch in the developing human cerebral cortex. Cell Rep. 34 , 108788 (2021).
Káradóttir, R., Hamilton, N. B., Bakiri, Y. & Attwell, D. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat. Neurosci. 11 , 450–456 (2008).
Zhang, P. et al. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat. Neurosci. 22 , 719–728 (2019).
Zhang, H. et al. Cerebral blood flow in mild cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis. Ageing Res. Rev. 71 , 101450 (2021).
Chen, M. B. et al. Brain endothelial cells are exquisite sensors of age-related circulatory cues. Cell Rep. 30 , 4418–4432 (2020).
Kalucka, J. et al. Single-cell transcriptome atlas of murine endothelial cells. Cell 180 , 764–779 (2020).
Yousef, H. et al. Aged blood impairs hippocampal neural precursor activity and activates microglia via brain endothelial cell VCAM1. Nat. Med. 25 , 988–1000 (2019).
Zhao, L. et al. Pharmacologically reversible zonation-dependent endothelial cell transcriptomic changes with neurodegenerative disease associations in the aged brain. Nat. Commun. 11 , 4413 (2020).
Wälchli, T. et al. Molecular atlas of the human brain vasculature at the single-cell level. Preprint at bioRxiv https://www.doi.org/content/10.1101/2021.10.18.464715v1 (2021).
Sun, N. et al. Single-cell multi-region dissection of brain vasculature in Alzheimer′s disease. Preprint at bioRxiv https://doi.org/10.1101/2022.02.09.479797 (2022).
Yang, A. C. et al. A human brain vascular atlas reveals diverse mediators of Alzheimer’s risk. Nature https://doi.org/10.1038/s41586-021-04369-3 (2022).
Winkler, E. A. et al. A single-cell atlas of the normal and malformed human brain vasculature. Science 375 , eabi7377 (2022).
Profaci, C. P., Munji, R. N., Pulido, R. S. & Daneman, R. The blood–brain barrier in health and disease: Important unanswered questions. J. Exp. Med. 217 , e20190062 (2020).
Zou, C. et al. Reduction of mNAT1/hNAT2 contributes to cerebral endothelial necroptosis and Aβ accumulation in Alzheimer’s disease. Cell Rep. 33 , 108447 (2020).
Gonzales, A. L. et al. Contractile pericytes determine the direction of blood flow at capillary junctions. Proc. Natl Acad. Sci. USA 117 , 27022–27033 (2020).
Hartmann, D. A. et al. Brain capillary pericytes exert a substantial but slow influence on blood flow. Nat. Neurosci. 24 , 633–645 (2021).
Nortley, R. et al. Amyloid β oligomers constrict human capillaries in Alzheimer’s disease via signaling to pericytes. Science 365 , eaav9518 (2019).
Blanchard, J. W. et al. Reconstruction of the human blood–brain barrier in vitro reveals a pathogenic mechanism of APOE4 in pericytes. Nat. Med. 26 , 952–963 (2020).
Yamazaki, Y. et al. Vascular ApoE4 impairs behavior by modulating gliovascular function. Neuron 109 , 438–447 (2021).
Barnes, L. L. Alzheimer disease in African American individuals: increased incidence or not enough data? Nat. Rev. Neurol. 18 , 56–62 (2022).
Vila-Castelar, C., Fox-Fuller, J. T., Guzmán-Vélez, E., Schoemaker, D. & Quiroz, Y. T. A cultural approach to dementia—insights from US Latino and other minoritized groups. Nat. Rev. Neurol. 18 , 307–314 (2022).
Alsema, A. M. et al. Profiling microglia from Alzheimer’s disease donors and non-demented elderly in acute human postmortem cortical tissue. Front. Mol. Neurosci. 13 , 134 (2020).
Marinaro, F. et al. Molecular and cellular pathology of monogenic Alzheimer’s disease at single-cell resolution. Preprint at bioRxiv https://doi.org/10.1101/2020.07.14.202317 (2020).
Gerrits, E. et al. Distinct amyloid-β and tau-associated microglia profiles in Alzheimer’s disease. Acta Neuropathol. 141 , 681–696 (2021).
Del-Aguila, J. L. et al. A single-nuclei RNA-sequencing study of Mendelian and sporadic AD in the human brain. Alzheimer’s Res. Ther. 11 , 71 (2019).
Olah, M. et al. Single-cell RNA sequencing of human microglia uncovers a subset associated with Alzheimer’s disease. Nat. Commun. 11 , 6129 (2020).
Xu, H. & Jia, J. Single-cell RNA sequencing of peripheral blood reveals immune cell signatures in Alzheimer’s disease. Front. Immunol. 12 , 2727 (2021).
Google Scholar
Gate, D. et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature 577 , 399–404 (2020).
Smith, A. M. et al. Diverse human astrocyte and microglial transcriptional responses to Alzheimer’s pathology. Acta Neuropathol. 143 , 75–91 (2022).
Krishnaswami, S. R. et al. Using single nuclei for RNA-seq to capture the transcriptome of postmortem neurons. Nat. Protoc. 11 , 499–524 (2016).
Thrupp, N. et al. Single-nucleus RNA-seq is not suitable for detection of microglial activation genes in humans. Cell Rep. 32 , 108189 (2020).
Marsh, S. E. et al. Dissection of artifactual and confounding glial signatures by single-cell sequencing of mouse and human brain. Nat. Neurosci. 25 , 306–316 (2022).
Zeng, H. What is a cell type and how to define it? Cell 185 , 2739–2755 (2022).
Rustenhoven, J. et al. PU.1 regulates Alzheimer’s disease-associated genes in primary human microglia. Mol. Neurodegener. 13 , 44 (2018).
Sanchez, P. E. et al. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer’s disease model. Proc. Natl Acad. Sci. USA 109 , E2895–E2903 (2012).
Vossel, K. et al. Effect of levetiracetam on cognition in patients with Alzheimer disease with and without epileptiform activity: a randomized clinical trial. JAMA Neurol. 78 , 1345–1354 (2021).
Gonzales, M. M. et al. Senolytic therapy to modulate the progression of Alzheimer’s disease (SToMP-AD): a pilot clinical trial. J. Prev. Alzheimers Dis. 9 , 22–29 (2022).
CAS Google Scholar
Wightman, D. P. et al. A genome-wide association study with 1,126,563 individuals identifies new risk loci for Alzheimer’s disease. Nat. Genet. 53 , 1276–1282 (2021).
Bellenguez, C. et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 54 , 412–436 (2022).
Hammond, T. R. et al. Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity 50 , 253–271 (2019).
Grone, B. P. et al. Early and lifelong effects of APOE4 on neuronal gene expression networks relevant to Alzheimer’s disease. Preprint at bioRxiv https://doi.org/10.1101/2022.06.16.496371 (2022).
Da Mesquita, S. et al. Aging-associated deficit in CCR7 is linked to worsened glymphatic function, cognition, neuroinflammation and β-amyloid pathology. Sci. Adv. 7 , eabe4601 (2021).
Taubes, A. et al. Experimental and real-world evidence supporting the computational repurposing of bumetanide for APOE4-related Alzheimer’s disease. Nat. Aging 1 , 932–947 (2021).
Lin, Y.-T. et al. APOE4 causes widespread molecular and cellular alterations associated with Alzheimer’s disease phenotypes in human iPSC-derived brain cell types. Neuron 98 , 1141–1154 (2018).
Download references
Acknowledgements
We are grateful to H. Mathys, M. Kellis and all members of his laboratory, and all members of the laboratory of L-H.T. for insightful discussions. We thank the following individuals for valuable discussions and helpful feedback on this paper: M. Victor, J. Penney, E. Niederst, L. Akay, D. von Maydell, P. -C. Pao, L. Bozzelli, A. Bubnys, G. Welch, D. -S. Park and J. M. Bonner. L.-H.T. acknowledges National Institutes of Health grants R01AT011460-01 and R37-NS051874-2. We thank the JPB Foundation, the Belfer Neurodegeneration Consortium, the Glenn Foundation for Medical Research, the Cure Alzheimer’s Fund and the Alana Foundation. We gratefully acknowledge generous support from the following individuals and institutions: R. A. Belfer and R. Belfer, the Ko Hahn family, the Carol and Gene Ludwig Family Foundation, the Halis Family Foundation, L. A. Gimpelson, the Dolby family, J. L. Miller and C. D. Miller, D. B. Emmes and the Marc Haas Foundation. All figures were created with BioRender.com .
Author information
Authors and affiliations.
Department of Brain and Cognitive Sciences, The Picower Institute for Learning and Memory, Massachusetts Institute of Technology, Cambridge, MA, USA
Mitchell H. Murdock & Li-Huei Tsai
You can also search for this author in PubMed Google Scholar
Contributions
M.H.M. and L.-H.T. conceived the original idea and wrote the paper.
Corresponding author
Correspondence to Li-Huei Tsai .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Neuroscience thanks Marco Colonna, Nancy Ip and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Murdock, M.H., Tsai, LH. Insights into Alzheimer’s disease from single-cell genomic approaches. Nat Neurosci 26 , 181–195 (2023). https://doi.org/10.1038/s41593-022-01222-2
Download citation
Received : 24 February 2022
Accepted : 28 October 2022
Published : 02 January 2023
Issue Date : February 2023
DOI : https://doi.org/10.1038/s41593-022-01222-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases.
- Jian Sheng Loh
- Kooi Yeong Khaw
Signal Transduction and Targeted Therapy (2024)
Spatial transcriptomics reveals molecular dysfunction associated with cortical Lewy pathology
- Thomas M. Goralski
- Lindsay Meyerdirk
- Michael X. Henderson
Nature Communications (2024)
Rare genetic variation in fibronectin 1 (FN1) protects against APOEε4 in Alzheimer’s disease
- Prabesh Bhattarai
- Tamil Iniyan Gunasekaran
- Badri N. Vardarajan
Acta Neuropathologica (2024)
Alzheimer’s genes in microglia: a risk worth investigating
- Ari Sudwarts
- Gopal Thinakaran
Molecular Neurodegeneration (2023)
Spatial transcriptomics reveals unique gene expression changes in different brain regions after sleep deprivation
- Yann Vanrobaeys
- Zeru J. Peterson
Nature Communications (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
The Alzheimer's Knowledge Base: A Knowledge Graph for Alzheimer Disease Research
Affiliations.
- 1 Institute for Biomedical Informatics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States.
- 2 Center of Excellence in Environmental Toxicology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States.
- 3 Department of Biostatistics, Epidemiology and Informatics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States.
- 4 Graduate Group in Genomics and Computational Biology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States.
- 5 Department of Genetics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States.
- 6 Medical Scientist Training Program, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States.
- 7 Department of Computational Biomedicine, Cedars-Sinai Medical Center, Los Angeles, CA, United States.
- PMID: 38635981
- DOI: 10.2196/46777
Background: As global populations age and become susceptible to neurodegenerative illnesses, new therapies for Alzheimer disease (AD) are urgently needed. Existing data resources for drug discovery and repurposing fail to capture relationships central to the disease's etiology and response to drugs.
Objective: We designed the Alzheimer's Knowledge Base (AlzKB) to alleviate this need by providing a comprehensive knowledge representation of AD etiology and candidate therapeutics.
Methods: We designed the AlzKB as a large, heterogeneous graph knowledge base assembled using 22 diverse external data sources describing biological and pharmaceutical entities at different levels of organization (eg, chemicals, genes, anatomy, and diseases). AlzKB uses a Web Ontology Language 2 ontology to enforce semantic consistency and allow for ontological inference. We provide a public version of AlzKB and allow users to run and modify local versions of the knowledge base.
Results: AlzKB is freely available on the web and currently contains 118,902 entities with 1,309,527 relationships between those entities. To demonstrate its value, we used graph data science and machine learning to (1) propose new therapeutic targets based on similarities of AD to Parkinson disease and (2) repurpose existing drugs that may treat AD. For each use case, AlzKB recovers known therapeutic associations while proposing biologically plausible new ones.
Conclusions: AlzKB is a new, publicly available knowledge resource that enables researchers to discover complex translational associations for AD drug discovery. Through 2 use cases, we show that it is a valuable tool for proposing novel therapeutic hypotheses based on public biomedical knowledge.
Keywords: Alzheimer; Alzheimer disease; artificial intelligence; drug discovery; drug repurposing; etiology; heterogeneous graph; knowledge base; knowledge graph; machine learning; open source; therapeutic discovery; therapeutic targets.
©Joseph D Romano, Van Truong, Rachit Kumar, Mythreye Venkatesan, Britney E Graham, Yun Hao, Nick Matsumoto, Xi Li, Zhiping Wang, Marylyn D Ritchie, Li Shen, Jason H Moore. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 18.04.2024.
- Today's news
- Reviews and deals
- Climate change
- 2024 election
- Fall allergies
- Health news
- Mental health
- Sexual health
- Family health
- So mini ways
- Unapologetically
- Buying guides
Entertainment
- How to Watch
- My Portfolio
- Stock Market
- Biden Economy
- Stocks: Most Actives
- Stocks: Gainers
- Stocks: Losers
- Trending Tickers
- World Indices
- US Treasury Bonds
- Top Mutual Funds
- Highest Open Interest
- Highest Implied Volatility
- Stock Comparison
- Advanced Charts
- Currency Converter
- Basic Materials
- Communication Services
- Consumer Cyclical
- Consumer Defensive
- Financial Services
- Industrials
- Real Estate
- Mutual Funds
- Credit Cards
- Balance transfer cards
- Cash-back cards
- Rewards cards
- Travel cards
- Personal Loans
- Student Loans
- Car Insurance
- Options 101
- Good Buy or Goodbye
- Options Pit
- Yahoo Finance Invest
- EV Deep Dive
- Fantasy football
- Pro Pick 'Em
- College Pick 'Em
- Fantasy baseball
- Fantasy hockey
- Fantasy basketball
- Download the app
- Daily fantasy
- Scores and schedules
- GameChannel
- World Baseball Classic
- Premier League
- CONCACAF League
- Champions League
- Motorsports
- Horse racing
- Newsletters
New on Yahoo
- Privacy Dashboard
Yahoo Finance
Bioarctic and eisai sign research evaluation agreement regarding ban2802.
STOCKHOLM , April 20, 2024 /PRNewswire/ -- BioArctic AB (publ) (Nasdaq Stockholm: BIOA B) today announced that BioArctic AB and Eisai Co., Ltd., have entered into a research evaluation agreement regarding BAN2802, a potential new treatment combining BioArctic's proprietary BrainTransporter™ technology with an undisclosed Alzheimer drug candidate. At the end of the collaboration, Eisai will evaluate the data generated and decide if they chose to exercise an option to license BAN2802 for the treatment of Alzheimer's disease.
BioArctic and Eisai have a long-standing collaboration dating back to 2005 regarding the development and commercialization of drugs for the treatment of Alzheimer's disease. This collaboration has led to Leqembi® (lecanemab) – the world's first approved drug [1] shown to slow the progression of early Alzheimer's disease. The new collaboration will build on the companies' joint knowledge in the field of Alzheimer's disease. Costs for the research evaluation program will be shared and the program will evaluate what could be the next generation disease modifying treatment for Alzheimer's disease.
"I am very happy that our Brain Transporter technology has continued to progress so well and that we have now entered into this first agreement utilizing this platform. I believe that this technology has huge potential to improve many different projects, and support companies in their pursuit of helping people with brain disorders," said Gunilla Osswald , CEO at BioArctic. "Eisai has been a valuable partner to BioArctic during the past two decades, and we are very pleased to extend and deepen our relationship with this new research evaluation agreement on BAN2802. Together, we have been able to deliver lecanemab, the first fully approved disease modifying treatment in Alzheimer's disease in the US, Japan and China , and we look forward to continuing our fruitful collaboration and lead the development of the next generation of drugs to help patients with this devastating disease."
BioArctic's proprietary BrainTransporter technology is a technology that can actively transport biologics across the blood brain barrier into the brain. The technology has the potential to create faster and stronger efficacy of treatments targeted to the brain, while reducing the burden of treatment for both patients and society. The BrainTransporter technology manifests BioArctic's commitment to sustainability by aiming for continuous improvement of clinical and commercial benefit.
This release discusses investigational uses of an agent in development and is not intended to convey conclusions about efficacy or safety. There is no guarantee that such investigational agents will successfully complete clinical development or gain health authority approval.
This information is information that BioArctic AB (publ) is obliged to disclose pursuant to the EU Market Abuse Regulation. The information was released for public disclosure, through the agency of the contact persons below, on April 20, 2024 , at 09.40 a.m. CET .
For further information, please contact: Oskar Bosson , VP Communications and IR E-mail: [email protected] Phone: +46 70 410 71 80
About the collaboration between BioArctic and Eisai Since 2005, BioArctic has a long-term collaboration with Eisai regarding the development and commercialization of drugs for the treatment of Alzheimer's disease. The most important agreements are the Development and Commercialization Agreement for the lecanemab antibody, which was signed 2007, and the Development and Commercialization agreement for the antibody LEQEMBI back-up for Alzheimer's disease, which was signed 2015. In 2014, Eisai and Biogen entered into a joint development and commercialization agreement for lecanemab. Eisai is responsible for the clinical development, application for market approval and commercialization of the products for Alzheimer's disease. BioArctic has the right to commercialize lecanemab in the Nordic region under certain conditions and is currently preparing for commercialization in the Nordics together with Eisai. BioArctic has no development costs for lecanemab in Alzheimer's disease and is entitled to payments in connection with regulatory approvals, and sales milestones as well as royalties on global sales.
About BioArctic AB BioArctic AB (publ) is a Swedish research-based biopharma company focusing on innovative treatments that can delay or stop the progression of neurodegenerative diseases. The company invented Leqembi® (lecanemab) – the world's first drug proven to slow the progression of the disease and reduce cognitive impairment in early Alzheimer's disease. Leqembi has been developed together with BioArctic's partner Eisai, who are responsible for regulatory interactions and commercialization globally. In addition to Leqembi, BioArctic has a broad research portfolio with antibodies against Parkinson's disease and ALS as well as additional projects against Alzheimer's disease. Several of the projects utilize the company's proprietary BrainTransporter™ technology, which has the potential to actively transport antibodies across the blood-brain barrier to enhance the efficacy of the treatment. BioArctic's B share (BIOA B) is listed on Nasdaq Stockholm Large Cap. For further information, please visit www.bioarctic.se .
[1] Approved in the US, Japan and China .
This information was brought to you by Cision http://news.cision.com
https://news.cision.com/bioarctic/r/bioarctic-and-eisai-sign-research-evaluation-agreement-regarding-ban2802,c3964796
The following files are available for download:
View original content: https://www.prnewswire.com/news-releases/bioarctic-and-eisai-sign-research-evaluation-agreement-regarding-ban2802-302122670.html
SOURCE BioArctic

- News Releases
Pioneering study targets Alzheimer's disease risk factors among Californians from the Middle East and North Africa
Keck School of Medicine of USC
This study is also called 1001-NAMES (North African and Middle Eastern Studies) of Aging, a play on the collection of Middle Eastern folktales One Thousand and One Nights.
Credit: USC Stevens INI
A new study led by Neda Jahanshad, PhD , a researcher at the USC Mark and Mary Stevens Neuroimaging and Informatics Institute ( Stevens INI ), is set to illuminate the underexplored domain of brain aging and risk for Alzheimer's disease and related dementias (ADRD) among Middle Eastern and North African (MENA) adults in the United States. Addressing this research gap is important because this population is projected to significantly influence global dementia prevalence.
The study, ADRD Risk Factors in Middle Eastern and North African Immigrants in the U.S., marks a groundbreaking effort to understand the mechanisms behind the high rates of ADRD among MENA immigrants—a growing and often overlooked community.
"Individuals from the MENA regions are nearing 10% of the global population and over 3% in the U.S., yet they remain significantly underrepresented in health studies. This gap in data can lead to health-related misunderstandings, misdiagnoses, and overall disparities in health care outcomes," explains Dr. Jahanshad, who is also an associate professor of neurology at the Keck School of Medicine of USC . "The lack of a MENA category in U.S. Census data to date has complicated the issue by hampering the tracking of brain health and Alzheimer's risk levels within this demographic."
The study will concentrate on the Los Angeles area, home to one of the largest MENA populations outside their home countries. Through both remote surveys and in-person assessments, the study seeks to evaluate the prevalence of ADRD risk factors in relation to daily living, different types of stress, genetics, and brain health.
The study will survey 120 MENA individuals who are 55+ years old and proficient in English and at least one other language, including Arabic, Armenian, Farsi, or Hebrew, to examine factors that may contribute to ADRD risk. Some focus areas include education, social factors, lifestyle habits such as diet and exercise, medical history, and genetic risk.
Researchers will then conduct detailed MRI-based brain imaging and cognitive assessments on a subset of participants to gain deeper insights into key brain regions, cognitive ability, and vascular burden—the cumulative effect of various factors that contribute to the dysfunction of the vascular system. Collecting and investigating brain changes in the MENA population for the first time is a crucial step in understanding neurological health within this demographic, as ethnoracial backgrounds and underlying additional pathologies can influence the pattern of changes. The research team stresses the importance of conducting cognitive assessment tests by experts fluent in the languages spoken in MENA countries and familiar with the cultural nuances, which is vital for ensuring the validity and appropriateness of these assessments.
"Cognitive tests designed primarily for Western populations may not accurately capture the cognitive abilities of individuals from MENA backgrounds due to language, education, and cultural context differences. This pilot data can then be used to modify existing cognitive tests or develop new assessments that are more culturally and linguistically appropriate for MENA populations," states Dr. Nasim Sheikh-Bahaei, MD, PhD, a double board-certified clinician scientist in internal medicine and neuroradiology at USC and the study's co-PI.
"The richness of the MENA culture and the diversity within this group pose unique opportunities for research. This research is notably timely now that MENA is added as a separate and recognized race in the U.S. for the first time on the most recent racial revision published by The White House. We believe this study will fill a significant void in our understanding of ADRD risk factors worldwide and highlight the importance of culturally sensitive health research," says Dr. Nasim Sheikh-Bahaei.
The study's outcomes could revolutionize approaches to ADRD risk, leading to more targeted interventions and support for MENA immigrants. The researchers also invite community engagement and look forward to sharing findings that could have wide-reaching implications for public health strategies and policies. "Our study may also provide crucial insights into how gender differences and social determinants of health, like discrimination and immigration-related stressors, impact MENA participants' brain and vascular health, with an overarching goal of enhancing the well-being and health care services provided to this community," notes Arpana Gupta, PhD of the UCLA David Geffen School of Medicine, also a co-principal investigator.
"Dr. Jahanshad's work is bringing crucial awareness to overlooked health differences in brain aging research, which is one of the Institute's most important commitments. We are thrilled to add this study to our suite of research initiatives on diverse brain health, says Stevens INI Director Arthur W. Toga , PhD . "I am particularly pleased that this study can directly impact MENA members of our community right here in Los Angeles."
"We hope this pilot project, supported by the Alzheimer's Association, will be a stepping stone to help us expand our future research projects in this area. More extensive research will be required to gain a comprehensive understanding of the effect of race, culture, genetics, immigration, and language barriers on cognitive assessments and various risk factors for developing Alzheimer's disease or Alzheimer's disease-related dementias in the MENA population. This all necessitates substantial funding from federal and philanthropic sources, which we will work to receive," says Dr. Jahanshad.
Arousiak V. Maraian, MD, of the Keck School of Medicine of USC, is also a co-PI on the study. This study is partially supported by an Alzheimer's Association research grant (AARG-23-1150420).
For more information on this study or to participate, please contact:
Neda Jahanshad, PhD
Disclaimer: AAAS and EurekAlert! are not responsible for the accuracy of news releases posted to EurekAlert! by contributing institutions or for the use of any information through the EurekAlert system.

IMAGES
COMMENTS
Research from UC Berkeley indicates that ongoing stress caused by protein aggregation is leading to the death of brain cells. Numerous neurodegenerative conditions, including Alzheimer's and Parkinson's, involve the buildup of protein clusters, known as aggregates, within the brain. This phenomenon has prompted researchers to hypothesize that these protein masses are responsible for the ...
1. Introduction. Alzheimer's disease (AD) is a polygenic and multifactorial disease characterized by the deposition of amyloid-β (Aβ) fibrils in the brain, leading to the formation of plaques and neurofibrillary tangles (NFTs), and ultimately resulting in dendritic dysfunction, neuronal cell death, memory loss, behavioral changes, and organ shutdown [1,2,3,4,5].
November 08, 2022. Alzheimer's Disease. NIH has released Advancing Alzheimer's Disease and Related Dementias Research for All Populations: Prevent. Diagnose. Treat. Care. (PDF, 17M), a 2022 scientific progress report. The report features science advances and related efforts made between March 2021 and early 2022 in areas including drug ...
What thrilled Sperling, who won the award for her work on clinical trials of Alzheimer's treatments, was a sense of hope, which has been conspicuously missing from research into the disease for ...
Alzheimer's disease is a progressive neurodegenerative disease that impairs memory and cognitive judgment and is often accompanied by mood swings, disorientation and eventually delirium. It is the ...
Researchers say we appear to be at the start of a new era for Alzheimer's treatment. Trial results published in January showed that for the first time a drug has been able to slow the cognitive decline characteristic of the disease. The drug, lecanemab, is a monoclonal antibody that works by binding to a key protein linked to the malady ...
Alzheimer's researchers are looking beyond plaques and tangles for new treatments. Scientists say research into Alzheimer's needs to take a broader view of how the disease affects the brain ...
Meta-analysis of genome-wide association studies on Alzheimer's disease and related dementias identifies new loci and enables generation of a new genetic risk score associated with the risk of ...
New findings from big-data and open-science research are revealing clues about the molecular mechanisms of Alzheimer's disease and new ways to discover potential therapeutic targets and biomarkers. These new discoveries were made by six research teams participating in the Accelerating Medicines Partnership Alzheimer's Disease (AMP AD) program.
Alzheimer's disease (AD) is the leading cause of dementia, presenting a significant unmet medical need worldwide. The pathogenesis of AD involves various pathophysiological events, including the accumulation of amyloid and tau, neuro-inflammation, and neuronal injury. Clinical trials focusing on new drugs for AD were documented in 2020, but subsequent developments have emerged since then.
In a new study, researchers at MIT showed that they "were able to interfere with an enzyme typically found to be overactive in the brains of Alzheimer's patients," reports Alex Mitchell for The New York Post.After using a peptide to treat the overactive enzyme, they found that "the peptide shows protective effects against loss of neurons and also appears to be able to rescue some of ...
Neuroscientists create new resource to improve Alzheimer's disease research models. ScienceDaily . Retrieved April 15, 2024 from www.sciencedaily.com / releases / 2023 / 08 / 230822151729.htm
Read the latest research on Alzheimer's disease. Learn about Alzheimer's symptoms such as memory loss and senile dementia. Find out about Alzheimer's stages, causes and new treatments.
Alzheimer's Diesease, a result of rapid ageing that causes dementia, is a growing concern. Dementia, the seventh leading cause of death worldwide, cost the world $1.25 trillion in 2018, and affected about 50 million people in 2019. Without major breakthroughs, the number of people affected will triple by 2050, to 152 million.
According to new research by scientists at the UCSF Weill Institute for Neurosciences, retinal scans can detect key changes in blood vessels that may provide an early sign of Alzheimer's disease, while offering important insights into how one of the most common Alzheimer's risk genes contributes to the disease.
Track elected officials, research health conditions, and find news you can use in politics, business, health, and education. ... risk for Alzheimer's, but who had gone into old age without ...
To speed progress even more, they've made all the data freely available online to the research community, where it promises to yield many more fundamentally important discoveries about the precise underpinnings of Alzheimer's disease in the brain and new ways to intervene in Alzheimer's dementia. References:
1. Introduction. Alzheimer's disease (AD) (named after the German psychiatric Alois Alzheimer) is the most common type of dementia and can be defined as a slowly progressive neurodegenerative disease characterized by neuritic plaques and neurofibrillary tangles (Figure 1) as a result of amyloid-beta peptide's (Aβ) accumulation in the most affected area of the brain, the medial temporal ...
Researchers are looking into whether blood pressure medicines may benefit people with Alzheimer's. ... the 1990s suggested that taking hormone replacement therapy during perimenopause and menopause lowered the risk of Alzheimer's disease. But further research has been mixed. ... et al. Lecanemab in early Alzheimer's disease. New England Journal ...
Alzheimer's and dementia research - find the latest information on research funding, grants, clinical trials and global research news. Get information and resources for Alzheimer's and other dementias from the Alzheimer's Association.
Here is what investigators found in the retinas of patients with mild cognitive impairment and Alzheimer's disease: "These changes in the retina correlated with changes in parts of the brain ...
Alzheimer's disease (AD) remains one of the most challenging and prevalent neurodegenerative disorders, affecting millions of individuals worldwide. In a new study published in Developmental Cell ...
Chun's new research builds on his lab's landmark publication in Nature in 2018 that described how somatic gene recombination in neurons can produce thousands of new gene variants within Alzheimer's disease brains. Importantly, it also revealed for the first time how the Alzheimer's-linked gene, APP, is recombined by using the same type ...
Alzheimer's disease (AD) is an age-related disease pathologically defined by the deposition of amyloid plaques and neurofibrillary tangles in the brain parenchyma. ... New insights into the ...
The federal government's Alzheimer's and related dementias research strategy focuses on engaging a cross-disciplinary team of geneticists, epidemiologists, gerontologists, behavioral scientists, disease and structural biologists, pharmacologists, clinical researchers, and others to bring the greatest and most diverse expertise to the field.
Advances in early detection and management of the disease have changed the way patients view an Alzheimer's diagnosis—and how they live with it. New developments in early detection and ...
AlzKB is a new, publicly available knowledge resource that enables researchers to discover complex translational associations for AD drug discovery. Through 2 use cases, we show that it is a valuable tool for proposing novel therapeutic hypotheses based on public biomedical knowledge. ... A Knowledge Graph for Alzheimer Disease Research J Med ...
John Roszkowski. September 28, 2022. ›. A new research model suggests that Alzheimer's disease may be a result of autoimmune disease in the brain, rather than a disease of the brain as many ...
The new collaboration will build on the companies' joint knowledge in the field of Alzheimer's disease. Costs for the research evaluation program will be shared and the program will evaluate what ...
News Release 16-Apr-2024. Pioneering study targets Alzheimer's disease risk factors among Californians from the Middle East and North Africa. Business Announcement. Keck School of Medicine of USC ...