An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List


Insights into human evolution from 60 years of research on chimpanzees at Gombe
Michael lawrence wilson.
- Author information
- Article notes
- Copyright and License information
E-mail: [email protected]
Collection date 2021.
This is an Open Access article, distributed under the terms of the Creative Commons Attribution licence ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted re-use, distribution, and reproduction in any medium, provided the original work is properly cited.

Keywords: Chimpanzee, Pan troglodytes , human evolution, Gombe National Park
Sixty years of research on chimpanzees ( Pan troglodytes ) at Gombe National Park, Tanzania have revealed many similarities with human behaviour, including hunting, tool use and coalitionary killing. The close phylogenetic relationship between chimpanzees and humans suggests that these traits were present in the last common ancestor of Pan and Homo (LCA PH ). However, findings emerging from studies of our other closest living relative, the bonobo ( Pan paniscus ), indicate that either bonobos are derived in these respects, or the many similarities between chimpanzees and humans evolved convergently. In either case, field studies provide opportunities to test hypotheses for how and why our lineage has followed its peculiar path through the adaptive landscape. Evidence from primate field studies suggests that the hominin path depends on our heritage as apes: inefficient quadrupeds with grasping hands, orthograde posture and digestive systems that require high-quality foods. Key steps along this path include: (a) changes in diet; (b) increased use of tools; (c) bipedal gait; (d) multilevel societies; (e) collective foraging, including a sexual division of labour and extensive food transfers; and (f) language. Here I consider some possible explanations for these transitions, with an emphasis on contributions from Gombe.
Media summary: Long-term study of chimpanzees at Gombe, Tanzania, begun by Jane Goodall 60 years ago, continues to shed light on key questions in human evolution.
Introduction
In 1960, Jane Goodall established the first long-term field study of chimpanzees ( Pan troglodytes ) at what is now Gombe National Park, Tanzania, just over a century after On the origin of species (Darwin, 1859 ) laid the foundation for an evolutionary understanding of human origins. Goodall's mentor, Louis Leakey, hoped that studying living apes would shed light on the behaviour of fossil apes such as Proconsul (Peterson, 2006 ). Leakey believed the ape and human lineages had diverged deep in time (Leakey, 1970 ), but nonetheless thought studies of living apes would provide essential context for understanding human evolution. Fossils provide indispensable evidence, but can offer only limited information about the living creatures that left those remains behind. What sort of societies did they have? What did they eat? How did they behave?
Leakey's hopes have been rewarded abundantly. The Gombe chimpanzee study has continued for 60 years, producing over 300 scientific publications and reaching a broad global audience through popular books, magazine articles and films. Additionally, other studies that follow Gombe's model of collaborative long-term field research have broadened our understanding of living primates, providing invaluable comparative data for testing hypotheses about how and why humans evolved our many distinctive traits.
Research at Gombe revealed that chimpanzees, like humans, make and use tools (Goodall, 1964 ), hunt in groups and share meat (Goodall, 1963 ), and have hostile intergroup relations, including fatal attacks (Goodall et al., 1979 ). Molecular evidence of the close genetic relationship between humans and chimpanzees (Sibley & Ahlquist, 1984 ) suggested that chimpanzees provide a window into the lives of our common ancestors: male-dominated, warlike, monkey hunters (Ghiglieri, 2000 ; Goodall, 1990 ; Wrangham & Peterson, 1996 ). Others have emphasized the challenges posed to this view by our other closest living relatives, bonobos ( Pan paniscus ): female-dominated pacifists that defuse tension with sex (Parish & de Waal, 2000 ). Still others have argued that neither species of Pan is particularly similar to our common ancestor (Sayers & Lovejoy, 2008 ; White et al., 2012 ). An extreme version of this argument holds that humans are too distinct for any comparisons with Pan to be useful (Fuentes, 2018 ; Marks, 2002 ). Efforts to reconstruct traits of the last common ancestor of Pan and Homo (LCA PH ) remain speculative, particularly given the sparse fossil record for African apes in the late Miocene (McNulty, 2010 ; Andrews, 2020 ). Chimpanzees and bonobos are our cousins, not our ancestors. Evolution has continued for both panins and hominins. Both living panins probably differ in important ways from our common ancestor. At the same time, the LCA PH probably resembled Pan more than Homo in many ways that are important for understanding human evolution. Moreover, the many ways in which humans differ from Pan – and indeed, from all other species on Earth – depend on features peculiar to the apes, rather than features common to primates in general. Long-term studies at Gombe and other sites have yielded rich datasets for clarifying our place in nature. These data help explain why, among the primates, only apes produced a lineage that evolved bipedal locomotion, cumulative culture, cooking and language.
Please note that a short review such as this cannot hope to do justice to Gombe's 60 years of research, much less to the numerous studies at other sites across Africa, which have revealed considerable behavioural diversity across Pan . For more breadth and depth, see Arcadi ( 2018 ), Boesch et al. ( 2019 ), Furuichi ( 2019 ), Hare and Yamamoto ( 2015 ), Hunt ( 2020 ), Nakamura et al. ( 2015 ) andStanford ( 2018 ).
Research at Gombe
Gombe National Park is located in northwestern Tanzania (latitude 4°38′–4°45′S, longitude 29°36′–29°39′E). The park covers 35.69 km 2 of land and 20.72 km 2 of Lake Tanganyika. Mountains rise from the lakeshore (766 m a.s.l.) to peaks (1,300–1,623 m a.s.l.) along the eastern boundary. Streams running west to the lake divide the park into a series of steep valleys. The valley bottoms contain evergreen forest, with semi-deciduous forest and vine tangle on the slopes, open woodland on the ridges and montane grassland at higher elevations. This mosaic habitat resembles habitats constructed for early hominins (e.g. Levin et al., 2008 ). Approximately 90 chimpanzees live in the park, divided into three communities: Mitumba, Kasekela and Kalande (Wilson et al., 2020 ). Other primates found in the park include olive baboons ( Papio anubis ), red colobus monkeys ( Piliocolobus tephrosceles ) and vervets ( Chlorocebus pygerythrus ), along with two species of guenon (red-tailed monkeys ( Cercopithecus ascanius schmidti ) and blue monkeys ( C. mitis doggetti )), and their hybrids.
Goodall's books provide vivid accounts of her early years at Gombe, the subsequent development of Gombe Stream Research Centre and the chimpanzees, with their distinct personalities, rich emotional lives, complex societies and strong social bonds (Goodall, 1986 , 1990 ; van Lawick-Goodall, 1971 ). While popular media often depict Goodall as a lone woman in the forest, she established and maintained an intensely collaborative research team. She founded the Jane Goodall Institute, which maintains a team of approximately 50 Tanzanian employees at Gombe to collect data on chimpanzees, baboons, guenon monkeys and plants. Research teams conduct focal follows of individual chimpanzees each day, endeavouring to follow the focal individual from dawn to dusk (Wilson, 2012 ). Researchers also collect observations of mothers and infants and adolescent females, monitor chimpanzee health, collect faecal and urine samples, and carry out monthly phenology transects focused on plant species important in the chimpanzee diet. Starting in the 1990s, Anne Pusey led efforts to digitize the long-term data and develop a computer database. A consortium of principal investigators at Gombe (D. Mjungu and D. A. Collins), the Jane Goodall Institute-USA (L. Pintea) and faculty at six universities (K. Detwiler, I. C. Gilby, E. V. Lonsdorf, C. M. Murray, A. E. Pusey and M. L. Wilson) now work to maintain various datasets and coordinate ongoing research.
Gombe exemplifies what has become a standard approach for primate field studies: collaborative research, collecting systematic information on identified individuals, followed throughout their entire lives. This approach was pioneered in 1948, when Kinji Imanishi and Jun'ichiro Itani began the first study of Japanese macaques ( Macaca fuscata ; Matsuzawa & McGrew, 2008 ). Like Leakey, Imanishi developed a broad vision of studying human evolution through combined studies of fossil remains, non-human primates and human hunter–gatherers, and in 1958 began expeditions to Africa, which eventually led to the establishment of multiple long-term studies of great apes, including chimpanzees at Mahale, Tanzania (Nishida, 2012 ), and bonobos at Wamba, Democratic Republic of Congo (Kano, 1992 ). However, Goodall was unaware of Japanese primatology when she began her research; although Itani and Imanishi were among her earliest scientific visitors at Gombe, lack of fluency in a common language prevented meaningful exchange of scientific ideas (Goodall, personal communication). Similarities in their research methods thus resulted from convergent cultural evolution, rather than vertical transmission.
Gombe serves multiple functions. As a site for observing animals going about their business in their natural habitat, it provides scientists with opportunities to learn about natural behaviour. Conducting field research inspires new scientific thinking, particularly when animals behave in unexpected ways. As Toshisada Nishida observed after decades of fieldwork at Mahale, ‘chimpanzees are always new to me’ (de Waal, 2011 ). As a site for systematic long-term data collection, Gombe enables scientists to test hypotheses that require more data than can easily be obtained in a single field season (or even in an entire career). As a site for training young scientists, Gombe provides opportunities for learning how to ask questions of nature in ways that (hopefully) get clear answers. At least 59 graduate students have completed doctoral dissertations based on Gombe research, and several additional dissertation projects are currently underway. Additionally, research at Gombe promotes conservation in and around the park (Wilson et al., 2020 ).
Studies at Gombe have covered many topics relevant for understanding human evolution, including diet (Goodall, 1963 ; O'Malley & Power, 2014 ; Wrangham, 1977 ), locomotion and posture (Hunt, 1994 ), tool use (Goodall, 1964 ; Musgrave et al., 2020 ; Whiten et al., 1999 ), cooperation and food sharing (Gilby, 2006 ; Silk, 1978 ; Stanford, 1996 ), sexual behaviour (Feldblum et al., 2014 ; Tutin & McGinnis, 1981 ; Wroblewski et al., 2009 ), aggression and competition (Bygott, 1979 ; Feldblum et al., 2018 ; Foerster et al. 2016 ; Goodall et al., 1979 ; Murray, 2007 ; Wilson et al., 2014 ), life histories (Alberts et al., 2013 ; Hill et al., 2001 ; Kirchhoff, 2019 ; Pusey, 1990 ; Walker et al., 2018 ; Williams et al., 2008 ) and vocal communication (Marler, 1976 ; Mitani et al., 1992 ).
The Gombe team has sought to obtain a balanced view of chimpanzee life. All-day follows of individual males and females, conducted regularly since the 1974, ensure that researchers have detailed data on both sexes and their interactions (Goodall, 1986 ). While accounts of predominantly male behaviours such as hunting and fighting might particularly draw public attention, much research at Gombe has focused on female social behaviour and ecology. Having studied chimpanzee feeding ecology at Gombe, Richard Wrangham placed females at the centre of theoretical models of primate social behaviour: fertile females are the key resource over which males compete, and competition among females for food shapes their spatial distribution and social relationships, and thus constrains male options (Wrangham 1979 , 1980 ). Dominance interactions among females are more subtle than those among males, but females can nonetheless be assigned ranks (Pusey et al., 1997 ; Foerster et al., 2016 ). High-ranking females reproduce more quickly (Pusey et al., 1997 ; Jones et al., 2010 ), and maintain a more stable body mass (Pusey et al., 2005 ), probably because they forage in core areas with more food resources (Murray et al., 2006 ; Murray, 2007 ). They do sometimes hunt, but tend to take sedentary prey (Gilby et al., 2017 ). Females occasionally engage in severe aggression, particularly towards other females and their offspring (Pusey et al., 2008 ). Chimpanzee mothers spend years caring for their offspring; yet mothers also vary in their skill and aptitude (Goodall, 1986 ). Data collection focusing on mothers and infants began in 1969 and continues to the present, yielding many insights into topics including the consequences of variation of maternal behaviour (Stanton et al., 2014 ) and relationships among siblings (Lonsdorf et al., 2018 ).
The last common ancestor of Pan and Homo
When Goodall arrived at Gombe, scientists knew little about the behaviour of apes in the wild. Gorillas and chimpanzees were assumed to be each other's closest relatives; some authorities classified them together in the genus Pan (Tuttle, 1969 ). Bonobos were known to exist but had not been studied. By the 1980s, when molecular studies began to clarify that Pan formed a clade with Homo , not Gorilla (Sibley & Ahlquist, 1984 ), field studies had documented many behavioural similarities between chimpanzees and humans. Like humans, but unlike gorillas, chimpanzees hunt, share meat, make and use tools, and live in multimale, multifemale communities with fission–fusion grouping patterns and hostile, sometimes deadly, intergroup relations (Goodall, 1986 ). Female chimpanzees typically leave their natal communities at sexual maturity (Nishida & Kawanaka, 1972 ; Pusey, 1979 ), similar to residence patterns reported for hunter–gatherers (Ember, 1978 ; although more recent analysis of hunter–gatherer residence patterns reveals a more nuanced pattern – Hill et al., 2011 ). Such female dispersal explained sex-biased patterns of cooperation: males cooperated with male kin, while dispersing females became socially more isolated (Wrangham, 1979 ). These numerous similarities seemed likely to be homologies, shared by descent from the LCA PH (Ghiglieri, 1987 ; Wrangham, 1987 ), reflected in Wrangham's ( 2001 ) proposed name for the LCA PH : Pan prior , ‘the early chimpanzee’. Muller et al. ( 2017 ) explore in detail the implications of a chimpanzee-like LCA PH for human evolution.
Field studies also revealed many differences. In contrast to chimpanzees, humans have bipedal locomotion; greater reliance on tools to acquire and prepare foods; more sharing of foods; home bases for cooking and sleeping; long-term breeding bonds and multilevel societies composed of multiple families; and language (Isaac, 1978 ).
Assuming that the LCA PH was similar to a chimpanzee provides a clear starting point for efforts to explain the origin of distinctively human traits. This view has been challenged, however, on various grounds. Some consider the fossil evidence of Miocene apes and early hominins such as Ardipithecus to be incompatible with a chimpanzee-like LCA PH (Sayers & Lovejoy, 2008 ; White et al., 2012 ). Pilbeam and Lieberman ( 2017 ) address these arguments in detail. More could be said on this topic, but here I focus on field studies of living apes.
Bonobos are just as closely related to humans as are chimpanzees, but differ in ways that complicate efforts to infer traits of the LCA PH (Parish & de Waal, 2000 ). Bonobo field studies did not begin until 1973 (Kano, 1992 ), and bonobos remain less intensively studied than chimpanzees, owing to political instability and war in Congo. Nonetheless, researchers have persevered at several long-term field sites; their findings increasingly challenge preconceptions about Pan . Bonobos rarely use tools (Furuichi et al., 2015 ). They hunt infrequently, and females catch mammalian prey as often as males (Surbeck & Hohmann, 2008 ). As in chimpanzees, male bonobos stay in their natal groups and females disperse, but in bonobos females, rather than males, form coalitions and dominate the opposite sex (Tokuyama & Furuichi, 2016 ). Bonobos sometimes engage in intergroup hostility, but members of different communities can also spend days together (Sakamaki et al., 2018 ; Pisor & Surbeck 2019 ), and have been observed to share food across borders (Fruth & Hohmann, 2018 ). Bonobos engage in less violence than chimpanzees, and have not been confirmed to kill other bonobos (Wilson et al., 2014 ). In some respects, bonobos bear a closer resemblance to humans than do chimpanzees, such as in their greater elaboration and diversity of sexual behaviour, and their lower levels of reactive aggression (Parish & de Waal, 2000 ; Wrangham, 2019 ). Surprisingly, although bonobos are commonly depicted as having relaxed sexual relations, genetic studies have revealed that high-ranking males nonetheless sire a disproportionate share of offspring, with reproductive skew higher than for chimpanzees (Surbeck et al., 2017 ). Variation among chimpanzee populations suggests that fairly subtle changes in ecology can lead to substantial differences in social behaviour, and even physiology. Western chimpanzees appear to be more bonobo-like than eastern chimpanzees, with more cohesive parties (Furuichi, 2009 ; Wrangham, 1999 ), female sexual swellings that resume sooner after giving (Deschner & Boesch, 2007 ), and a tendency towards lower rates of lethal aggression (Wilson et al., 2014 ).
Researchers have variously argued that bonobos are more derived than chimpanzees (Pilbeam & Lieberman, 2017 ), less derived than chimpanzees (Diogo et al., 2017 ) or that both species of Pan are derived compared with the LCA PH (Parish & de Waal, 2000 ). Bonobos are sometimes claimed to more closely resemble hominins, such as by having longer legs and more frequent bipedality (Zihlman et al., 1978 ). Controlling for body mass, however, bonobos and chimpanzees do not differ in limb length (Druelle et al., 2018 ), nor do bonobos engage in bipedal posture more often than chimpanzees, either in captivity (Videan & McGrew, 2001 ) or in the wild (Doran & Hunt, 1994 ). Studies of cranial development in African apes, including the chimpanzees of Gombe, challenge simple characterizations of any African ape species being either primitive or derived (Massey, 2018 ). Given that both species are our cousins, not our ancestors, both species probably differ in multiple ways from our common ancestor.
Using phylogenetic methods to infer likely traits of the LCA PH , Duda and Zrzavý ( 2013 ) conclude that both chimpanzees and bonobos are derived. For example, they conclude that prominent sexual swellings of adult females ( Figure 1 ) are a derived trait of Pan . This possibility has implications for the socioecology of the LCA PH , since such sexual swellings occur mainly in multimale, multifemale societies (Nunn, 1999 ). Duda and Zrzavý ( 2013 ) reconstruct the LCA PH as living in gorilla-like groups, with a single male and multiple females, and considerable sexual dimorphism. Chimpanzees are thought to be unable to live in such groups because their dependence on ripe fruit imposes severe feeding competition during fruit-poor seasons (Wrangham, 1979 ). Gorillas can forage together because their large size enables them to efficiently digest abundant fibrous vegetation, reducing feeding competition (Wrangham, 1979 ). Hunt ( 2016 ) argues that Miocene apes faced less feeding competition from monkeys, making stable groups viable for frugivorous apes. If this is the case, various other features of chimpanzee societies – fission–fusion dynamics, multimale groups, male coalitions, coalitionary killing – might not have been present in the LCA PH . If this is so, many similarities between humans and chimpanzees would represent homoplasies rather than homologies. While such a high rate of homoplasy may seem unlikely, Duda and Zrzavý ( 2013 ) report rates of homoplasy for their most parsimonious tree that are within the range of other biological datasets.

Kasekela male Faustino grooming Nasa, a female with a fully tumescent sexual swelling. Photo by Michael L. Wilson.
In the absence of additional evidence, such as fossils or a more detailed understanding of how genes relate to traits, reconstructions of the LCA PH are necessarily speculative. Moreover, even when a good fossil record is available, much remains unknown about soft tissue and behaviour. An improved fossil record for African apes in the late Miocene would help resolve some of these questions. Additionally, genetic studies may shed light on the evolution of traits such as exaggerated sexual swellings.
Why apes, not monkeys, became human
If many of the similarities between chimpanzees and humans result from homoplasy rather than homology, what is the use of comparing chimpanzees to humans? Fuentes ( 2018 ) argues that ‘what chimpanzees and humans do today is not directly comparable – because we have evolved independently for millions of years’. However, comparison of traits among diverse lineages is a central tool in evolutionary studies; the comparative method is one of Darwin's many valuable gifts to science.
We did not evolve from chimpanzees, but we did evolve from something that was more like a chimpanzee than it was like human (Begun, 2016 ). Our last common ancestor with chimpanzees surely didn't have language, or cooking, or habitual bipedal locomotion. If we want to understand why those traits evolved in the hominin lineage, the best way to test hypotheses is to study our modern relatives: chimpanzees, bonobos, gorillas and other primates. Not every trait, of course, is best studied in our closest relatives; a broad view of biology is needed to benefit fully from the comparative method. The dance communication of bees, for example, is more similar to human language in some respects than any non-human primate communication system (Section 4.7). However, for many traits, it matters that we evolved from a specific kind of animal: a large-bodied, orthograde, ripe fruit specialist ape.
Adaptive landscapes
To illustrate the peculiar path that hominin evolution has followed, and how it depends on our ape ancestry, Figure 2 depicts an adaptive landscape for selected African monkeys and apes, following the metaphor of Wright ( 1932 ). The x -axis represents variation in genetic space (which is vastly multidimensional, but simplified here for the sake of illustration). Moving to the right along this axis represents increasing genetic adaptation to environmental challenges, while the y -axis represents variation in cultural space, which is also vastly multidimensional, but simplified here as a single axis, representing socially learned adaptations to environmental challenges. The contour lines represent fitness. Both genetic and cultural traits increase in frequency within populations if they produce a net beneficial effect on the carrier's inclusive fitness. Over time, this causes populations to move uphill, finding nearby fitness peaks. Arrows drawn perpendicular to the contour lines would represent vectors: directions in which natural selection tends to move populations. The figure necessarily represents a static landscape, but in reality this landscape is dynamic, shifting as climate change, newly evolved species and cultural innovations create new challenges and opportunities. Research at Gombe has provided insights into some possible pathways across this adaptive landscape.
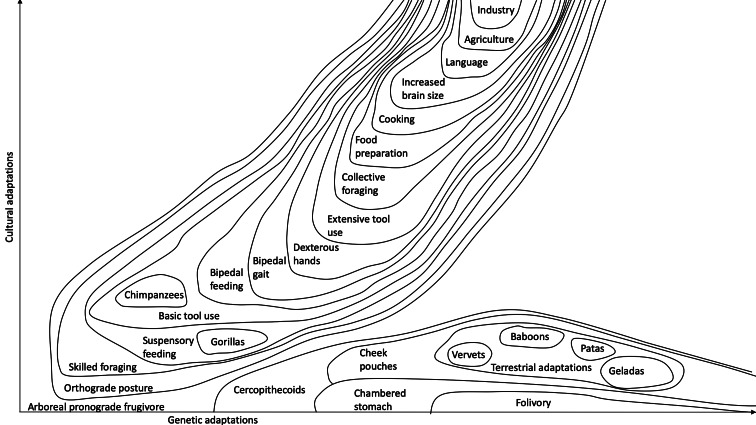
Adaptive landscape for African apes and monkeys.
Diet drives adaptations: ‘you are what you eat’. We therefore begin our tour of the primate adaptive landscape by focusing on dietary adaptations.
Goodall ( 1963 ) found that chimpanzees eat a broad range of items: leaves, fruit, flowers, seeds, pith, gums, insects, honey and a variety of smaller mammals, particularly monkeys. Further study revealed that, within this broad diet, chimpanzees, like other apes, prefer ripe fruit (Wrangham, 1977 ).
Fossil evidence indicates that the ancestor of catarrhines – apes and Old World monkeys – was probably an arboreal frugivore with the pronograde (horizontal) body posture typical of mammals (Begun, 2016 ). Studies of dentition, microwear and reconstructions of habitat indicate that apes have long specialized in eating ripe fruit (Potts, 2004 ). Tooth shape indicates that early Miocene apes probably relied on fruit to an even greater extent than present-day apes (Potts, 2004 ). Apes evolved large body size and an orthograde (upright) feeding posture, and lost their tails, which are less necessary when apes climb deliberately and hang from branches, rather than scampering along the tops of branches, and also less effective in balancing large body masses. Modern apes have suspensory adaptations (freely rotating arms, narrow shoulders and short, stiff backs), which enable them to gain access to fruits on terminal branches too small to support their weight (Hunt, 2016 ; Figure 3 ).

Kasekela juvenile male Fede using suspensory feeding posture, holding fruits of Parinari curatellifolia in hand and foot. Photo by Michael L. Wilson.
Apes once ranged widely across Eurasia as well as Africa, reaching a peak in species diversity in the Miocene, between 11 and 9.5 Ma (Potts, 2004 ). After this time, the global climate became cooler and drier. Ape diversity declined, and the range of apes contracted towards the equator, tracking the contracting range of moist forests. Begun ( 2016 ) views at least some of these European apes as having had suspensory feeding adaptations, and to have been close to the ancestry of crown hominoids such as gorillas and chimpanzees.
Modern African apes occupy distinct adaptive peaks. Gorillas evolved large body size, providing the hindgut capacity needed to ferment herbs effectively. This enables them to survive on mountains with few fruit trees, but it also traps them on a narrow fitness peak: moist forests close to the equator, with a year-round supply of readily digestible fruits and herbs. Bonobos likewise appear best adapted for moist forest; even in forest–savanna mosaics, they prefer mature forest for feeding and nesting (Serckx et al., 2015 ). Chimpanzees live across a broader swath of Africa, from Senegal to Tanzania, from the rainforests of the Congo basin to the arid savannas of Mali. Cultural adaptations help chimpanzees survive in these varied habitats, using stones to crack nuts, sticks to dig for tubers and spear bushbabies and hands to dig wells, and sheltering from the heat in pools and caves (McGrew, 2010 ). Chimpanzees have thus shifted further up the cultural dimension ( Figure 2 ).
Cercopithecoid monkeys have followed a different trajectory from apes – moving to the right along the genetic axis through a series of dietary adaptations ( Figure 2 ). Colobine monkeys evolved a cow-like chambered stomach that enables them to digest a broad range of foods that are unpalatable to apes, such as mature leaves. Cercopithecine monkeys evolved cheek pouches, where amylase-secreting glands break down the starch of unripe fruit into more readily digestible sugars (Lambert, 2005 ). Controlling for body size, cercopithecine monkeys also retain food in their digestive tracts for a longer period (Lambert, 2007 ).
These physiological adaptations have enabled monkeys to thrive in habitats unsuitable for apes. As Europe became cooler, drier and more seasonal in the late Miocene, apes declined to extinction, while monkeys diversified (Potts, 2004 ). Monkeys maintained the ancestral pronograde posture, making them efficient quadrupeds on the ground as well as in trees. As forests contracted in Africa, monkeys found multiple pathways to exploit open woodlands and savannas, represented by a range of peaks occupied by savanna monkeys (vervets and their allies: Chlorocebus spp.), patas monkeys ( Erythrocebus patas ), geladas ( Theropithecus gelada ) and baboons ( Papio spp.). Savanna monkeys show the least obvious degree of genetic adaptation, with bodies closely resembling those of their cousins, the forest guenons ( Cercopihtecus spp.; Selby, 2018 ). Patas monkeys have evolved long limbs and fleet bodies like greyhounds, enabling them to flee predators and travel efficiently among widely dispersed food resources (Selby, 2018 ). Baboons have evolved large body size and broad diets, but are also highly selective feeders (Barton & Whiten, 1994 ), and are the most widely distributed of African primates, besides humans. The one remaining species of gelada ( Theropithecus gelada ) is a specialized graminivore, eating mainly grass and herbs (Fashing et al., 2014 ). Genetic adaptations can lead populations to fitness peaks that make cultural innovation less likely; geladas do not need tools, for example, when they can graze in the meadows.
Apes lack the dental and physiological specializations that enable monkeys to subsist on the coarser foods that are readily available in more seasonal habitats. As Byrne ( 2016 ) argues, apes evolved skilled foraging methods, including tool use, to compete with monkeys. Even so, monkeys have reached higher adaptive peaks than apes, even in forests. Monkeys outmass chimpanzees at Gombe by a factor of nearly 26 ( Figure 4 ), and similarly outmass apes at other African field sites (Lambert, 2007 ).
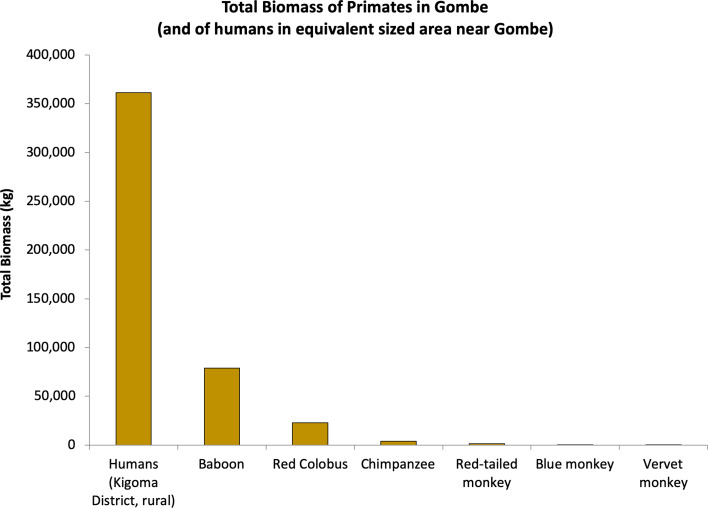
Estimated biomass of primates in Gombe (and of humans in an equivalent sized area near Gombe). Human population is calculated for areas outside the park, based on population density in rural Kigoma District (218.6 people/km 2 ); mean adult body mass of 60.7 kg, (Walpole et al., 2012 ); 44% of Tanzanian population under 15 (CIA World Factbook); and assuming that everyone above 15 years old is average adult body mass, and everyone below 15 is half that. Non-human primate data from Falk Grossman (unpublished data).
Humans followed a narrow and winding path up a mountain that towers over all the other primate peaks. In the rural landscape of Kigoma District, surrounding Gombe National Park, the human population on an average piece of Gombe-sized land outmasses the entire primate population at Gombe more than three times ( Figure 4 ). Globally, humans and our livestock constituted an estimated 97.2% of the biomass of land mammals in 2000 (Smil, 2011 ).
The pathway to this high peak depends on a series of both genetic and cultural adaptations. In many cases, cultural innovations created selection pressures for new genetic changes, and vice-versa, through gene–culture co-evolution (Richerson & Boyd, 2004 ; Henrich 2017 ). Insofar as apes learn to forage from their mothers, the suite of foods they learn to use represents cultural knowledge. Apes faced with drying habitats and shrinking forests can either retreat along with the forest or learn to acquire foods in more open landscapes. Cultural changes, such as learning to use foods that require a bipedal feeding posture, probably promoted genetic changes for improved bipedal posture, which increasingly freed the hands to make and use tools (Darwin, 1871 ). Tools and cumulative culture enabled hominins to thrive in new habitats (Richerson & Boyd, 2004 ; Henrich 2017 ). Using tools and fire to smash, grind and soften foods reduced the size of teeth, jaws and guts while providing energy to grow larger bodies and brains (Wrangham, 2011 ). Increasing brain size promoted the co-evolution of brains and symbolic communication (Deacon 1997 ). Such gene-culture co-evolution moved hominins both upwards and rightwards along the ridge, leading towards the current human fitness peak.
Human subsistence relies on cultural adaptations: weapons for hunting, knives for butchering, baskets for gathering, pots for cooking, and clothing and shelter for protection from the elements. The rapid speed of cultural evolution enabled hunter–gatherers to expand into nearly all terrestrial habitats, despite only modest changes in biological adaptations (Henrich, 2017 ). Complex tools such as hafted spears, baskets and canoes emerge through many generations of cumulative culture, in which the accumulating insights and innovations of thousands of ancestors contribute the know-how necessary to make items that would defy the powers of a lone individual (Boyd et al., 2011 ). Chimpanzees do not have cumulative culture, but research at Gombe and other sites has revealed that they make and use tools, and exhibit cultural traditions, which provides a provocative model for how material culture may have emerged in early hominins.
Tools have been a major focus of research at Gombe since the beginning. American tool manufacturer Leighton Wilkie supported Leakey's work on stone tools at Olduvai, and also provided the initial funding for Goodall's study (Peterson, 2006 ). On 4 November 1960, Jonathan and Mary Leakey discovered OH7, the type specimen for Homo habilis , whose name (‘handy man’) represented Leakey's conviction that the maker of the Oldowan tools had finally been found. That same day – in the final month of Goodall's initial study – Goodall observed the chimpanzee David Greybeard fishing for termites (Peterson, 2006 ). Goodall soon observed chimpanzees fashion wands for termite fishing, by removing leaves from a stem. This confirmation that chimpanzees make and use tools proved key to attracting funding from the National Geographic Society that extended research at Gombe into a truly long-term study.
Tool use has remained a major focus of research at Gombe (e.g. Lonsdorf et al., 2004 ; McGrew, 1979 ; Musgrave et al., 2020 ; O'Malley et al., 2012 ; Teleki, 1974 ; Whiten et al., 1999 ). Tool use gives chimpanzees access to easily digestible, energy-rich foods such as social insects, nuts, honey and brains. As Byrne ( 2016 ) notes, baboons eagerly eat termites when swarms of reproductive alates emerge from their nests, but lack tools to extract them from their nests. In contrast, chimpanzees at Gombe consume termites extensively, particularly during the wet season, when rain softens the outside of the mounds, making it easier to insert tools. Chimpanzees also use tools to eat driver ants ( Dorylus spp.), which are rich in protein (O'Malley & Power, 2014 ), but whose fierce bites dissuade most potential predators. Chimpanzees prey on these ants by ‘dipping’ for them, inserting a wand made of vegetation into a column of ants or the entrance of their nest (McGrew, 1979 ). The ants bite the wand with their mandibles – making them both easy to transport to the mouth and safe to consume.
Observations at Gombe suggested that females using perishable tools to acquire insect and plant foods played an important role in hominin evolution, in contrast to the once prevailing focus on stone tools and hunting (Zihlman, 1978 ; McGrew, 1979 ). Gombe females spend more time than males termite fishing (Pandolfi et al., 2003 ) and ant-dipping (McGrew, 1979 ). Lonsdorf and colleagues ( 2004 ) found that young females gain proficiency at tool use faster than young males. Similarly, at Taï, Côte d'Ivoire, females spend more time than males cracking nuts (Boesch-Achermann & Boesch, 1993 ). O'Malley et al. ( 2012 ) report evidence that, when females transfer among communities, they may bring with them new cultural knowledge ( Figure 5 ). However, at Taï, group differences in nut-cracking patterns persisted despite females transferring among neighbouring communities (Luncz & Boesch, 2014 ).

Flirt, a female immigrant to Mitumba from the Kasekela community, watching Mitumba-born female Flower fishing for ants (probably Camponotus spp.). This technique is habitual in Mitumba, and appears to have spread to Kasekela with immigrant female Trezia in the 1990s (O'Malley et al., 2012 ). Photo by Michael L. Wilson.
The extent to which the LCA PH made and used tools may never be known, as the tools used by chimpanzees leave only scant evidence for archaeologists. Neither gorillas nor bonobos use tools to any great extent in the wild, so perhaps extensive tool use evolved convergently in humans and chimpanzees. Nonetheless, the ape requirement for high-quality food appears to be a major factor promoting tool use in both lineages.
Tools may have been important for making possible the dietary shifts revealed by stable isotope studies. Analysis of the ratios of stable isotopes of carbon in hominin fossils has revealed a striking shift in the diets of hominins, away from C 3 plants – forest trees, shrubs, lianas and herbs – towards increasing use of C 4 plants: grasses and sedges (Cerling et al., 2011 ; Nelson & Hamilton, 2017 ; Sponheimer et al., 2013 ). Nockerts ( 2019 ) analysed stable isotope signatures of chimpanzees, baboons and their foods at Gombe. Examining these new data in context with published data on chimpanzee, baboon and hominin isotope signatures, Nockerts has found that even early hominins had distinct stable isotope signatures from chimpanzees, including ‘savanna’ chimpanzees (Nockerts, 2019 ).
As apes, how did hominins gain access to this diet, without the physiological adaptations of monkeys? Presumably hominin diets differed substantially from those of baboons, even if their stable isotope signatures indicate similar consumption of C 4 resources. The teeth and jaws of australopiths differ greatly from those of baboons – although they are similar in some respects to those of geladas (Jolly, 1970 ). Underground storage organs of plants – roots and tubers – have been proposed as a major fallback food for australopiths (Laden & Wrangham, 2005 ). Hunter–gatherers use digging sticks to gain access to these resources. Chimpanzees in western Tanzania, in the dry, open habitat of Masito–Ugalla, have been found to do this as well (Hernandez-Aguilar et al., 2007 ). Simple tools that chimpanzees are capable of using thus may have been a key factor in enabling early hominins to adapt to more open habitats.
Bipedal gait
Darwin ( 1871 ) inferred that bipedal locomotion was a key early step in human evolution. The reasons why bipedal locomotion evolved, however, remain unclear. Many hypotheses presume that bipedal gait evolved in response to increasing use of more open habitats. As noted above, monkeys have adapted to open habitats multiple times, but none have evolved bipedal gait. Why did the ancestors of humans follow a different path? One likely factor is that monkeys were already well adapted to quadrupedal locomotion. When in trees, they walk quadrupedally along the tops of limbs (Selby, 2018 ). Apes, in contrast, have evolved an orthograde posture for feeding and climbing (Selby, 2018 ). Adaptations for manoeuvring their large bodies in trees have made apes inefficient quadrupeds on the ground.
Data from Gombe and other sites indicate that chimpanzees travel shorter distances each day than human foragers. A study of the Hadza hunter–gatherers in Tanzania found that mean daily walking distances were 5.8 km/day for women and 11.4 km/day for men (Pontzer et al., 2012 ), much greater than the daily travel distances for chimpanzees at Kanyawara (females 2.0 km/day; males 2.4 km/day) or Gombe (females 3.2 km/day; males 4.6 km/day; Pontzer & Wrangham, 2004 ). Baboons, on the other hand, travel further each day than chimpanzees, and in some cases rival the distances travelled by human foragers. Yellow baboons in Amboseli, Kenya travel about 5.5 km/day, while hamadryas baboons travel an average of 13.2 km/day (Altmann, 1974 ).
Rodman and McHenry ( 1980 ) proposed that bipedal locomotion enabled early hominins to travel more efficiently between dispersed food patches, while also retaining the forelimb anatomy needed to climb into feeding trees. Consistent with this hypothesis, laboratory studies have found that humans walk more much more efficiently than chimpanzees (Sockol et al., 2007 ). The first steps onto the narrow and winding path towards hominization thus probably resulted from our ape ancestors being better adapted for climbing than walking.
Laboratory studies have found that chimpanzees are equally inefficient at bipedal and quadrupedal locomotion (Pontzer et al., 2014 ). Bipedal locomotion therefore seems unlikely to have increased efficiency of travel in the earliest hominins. Adaptation for efficient bipedal locomotion seems most likely to have occurred if it followed an earlier phase of selection for increased use of bipedal posture. The wading hypothesis, inspired by observations of baboons foraging among islands in the Okavango delta (Wrangham et al., 2009 ), proposes that water provided the support necessary to stabilize bipedal posture in early hominins. Alternatively, the postural feeding hypothesis, based on observations of geladas, suggests a source of selection pressure for feeding in drier habitats (Wrangham, 1980 ).
Because primates spend much of each day feeding, feeding posture is likely to provide an important source of selection pressure, and a mechanism by which increased bipedality would benefit early hominins before they became efficient walkers. In a study based at both Gombe and Mahale, Hunt ( 1994 ) found that chimpanzees and baboons engaged in bipedal posture most commonly when feeding ( Figure 6 ). Hunt argued that early hominins stood bipedally to forage on small objects from small trees and shrubs, which are common in open habitats. Consistent with this hypothesis, Tourkakis ( 2009 ) found that chimpanzees in the dry, open habitat of Fongoli, Senegal engaged in more bipedal standing when feeding, as well as more bipedal locomotion, compared with chimpanzees at Gombe and other less open habitats.

Kasekela female Imani standing bipedally to feed from low vegetation. Photo by Michael L. Wilson.
Multilevel societies
Field studies of apes have provided valuable insights into the evolution of human societies. Chimpanzee societies resemble human societies in having what Kummer ( 1971 ) called ‘fusion–fission’ grouping patterns: members of a group travel in subgroups of varying size, in contrast to the stable groups of gorillas and many other primates. Chimpanzees differ from humans, however, in not having stable pair-bonds between males and females, and in not having higher-level patterns of affiliation – features that lead researchers to classify humans, but not chimpanzees, as having multilevel societies (Grueter et al., 2012 ).
It took over a decade of research for researchers to gain a clear picture of chimpanzee social structure at Gombe (Goodall et al., 1979 ). As Goodall learned to recognize individuals in her first years at Gombe, she observed that chimpanzees travelled in subgroups (‘parties’) that varied in size and composition; the only stable grouping appeared to be mothers and their offspring (van Lawick-Goodall, 1968a ). Because different parties joined together without aggression, Goodall initially speculated that chimpanzees lived in unbounded societies, which she called ‘communities’ (Goodall et al., 1979 ). Nishida, who began working at Mahale in 1965, more quickly determined that chimpanzees live in closed social groups (Nishida, 1968 ). Two key factors helped Nishida gain this understanding. First, the location where Nishida planted sugarcane to attract chimpanzees happened to be located in the boundary area between two groups, enabling him to observe intergroup interactions early on (Nishida, 2012 ). In contrast, Goodall's feeding station at Gombe was located near the centre of the study group's range; research for much of the 1960s focused on behaviour in and around the feeding station, making observation of intergroup encounters unlikely. Second, Nishida came to Mahale specifically seeking to understand chimpanzee social structure (Nishida, 2012 ). His mentors, Imanishi and Itani, shared a strong interest in the evolutionary origins of human societies (Matsuzawa & McGrew, 2008 ). Imanishi initially reasoned that chimpanzees, like gorillas, might live in one-male groups, which he called ‘familoids’, thought to provide an evolutionary precursor to the human family (Imanishi, 1965 ). His student Itani later speculated that chimpanzee groups might consist of multiple familoid groups (Itani, 1966 ). Nishida found no evidence for familoid groups; instead, like at Gombe, Mahale chimpanzees travelled in extremely fluid parties. Consistent with observations suggesting closed social groups at Filabanga, Tanzania (Itani & Suzuki, 1967 ), Nishida found that Mahale chimpanzees associated with one of two distinct groups: Kajabara and Mimikire (Nishida, 1968 ). Mimikire had more members and appeared socially dominant (Nishida, 1968 ). Nishida called these bounded societies ‘unit-groups’ (Nishida, 1968 ).
At Gombe, the existence of social boundaries became apparent by the late 1960s (van Lawick-Goodall, 1971 ), and became strongly evident in the early 1970s, when the study group split into the mutually hostile Kasekela and Kahama communities (Goodall et al., 1979 ). Intergroup killings subsequently resulted in the Kahama community's extinction (Goodall, 1986 ). Goodall and other Gombe researchers continued to use the term ‘community’ to refer to chimpanzee groups, but shifted the meaning, making it synonymous with ‘unit-group’. These two terms persist in the literature, with European and American researchers tending to use ‘community’, while Japanese researchers tend to use ‘unit-group’ (Itoh & Nakamura, 2015 ). Nishida avoided using the word ‘community’ in this sense, not only because ‘unit-group’ referred specifically to closed social groups, but also because Imanishi used ‘community’ to describe a characteristic feature of human societies: ‘social units do not exist independently but are integrated into a higher unit’ (Nishida, 2012 : 3). Imanishi's understanding of ‘community’ thus appears equivalent to the term ‘multilevel society’.
The multilevel societies of humans consist of families with long-term breeding bonds that identify as members of larger groupings, such as bands, ethno-linguistic groups, tribes and nations (Grueter et al., 2012 ; Wilson & Glowacki, 2017 ). None of the other apes have a clearly multilevel society, although aggregations of groups of western gorillas, and gatherings of parties from different bonobo communities, are similar in some ways (Furuichi, 2020 ). The multilevel societies of gelada monkeys and hamadryas baboons provide better analogues of this aspect of human societies.
Why do these monkeys live in multilevel societies? Two factors appear to be key: food and sleeping sites (Grueter et al., 2012 ). Monkeys are able to find sufficient food to forage in stable groups under a broad range of conditions, but in the arid lands of the Horn of Africa and Arabia, even baboons are forced to disperse into smaller foraging parties, at least in some seasons. Geladas live at high elevation, where there is more rainfall, but seasonal variation in food availability forces large herds to scatter into smaller groups. The second factor promoting multilevel societies appears to be the scarcity of safe sleeping sites. In forested areas, primates can easily find trees to sleep in safely away from predators. In more open country, suitable refuges are sparse, consisting mainly of groves of trees along watercourses and cliffs. Geladas and hamadryas baboons both rely mainly on cliffs. Cliffs suitable for sleeping sites are commonly large enough to accommodate many individuals, and so serve as common aggregation points at the end of the day for parties that have been foraging separately. A small group cannot readily monopolize a sleeping site with room for many groups, and so scarce but capacious sleeping sites will readily be shared by multiple groups.
Given that fossils of hominins, including Australopithecus and Ardipithecus , have been found in the region now inhabited by hamadryas baboons, it seems plausible that these hominins would have sought refuge on cliffs at night, and dispersed throughout the day in smaller foraging parties. As Marlowe ( 2005 ) has noted, women in present-day hunter–gatherer societies such as the Hadza forage for many of the same resources hypothesized to be important for early hominins (berries and nuts from trees and bushes, underground storage organs), and technology such as digging sticks would also probably have been available to early hominins. Given that Hadza women generally forage in groups of about five (Marlowe, 2005 ), it seems plausible that earlier hominins would have been able to do so as well.
The long-term breeding bonds that characterize multilevel societies may result from females foraging in smaller groups, which makes fertile females easier for males to monopolize (Grueter et al., 2012 ). Simulations using agent-based computer models support this hypothesis (Crouse et al., 2019 ). Such stable breeding bonds differ strikingly from the more promiscuous mating patterns of Pan . If the LCA PH had Pan -like societies, then stable breeding bonds may have emerged from a more promiscuous mating system, as probably happened in hamadryas baboons and perhaps geladas as well. If the LCA PH instead had gorilla-like one-male societies, then stable breeding bonds would be an ancestral trait for hominins. In this case, multilevel societies would have evolved from the aggregation of smaller units, rather than the disintegration of troops.
If hominins regularly used a small number of regular sleeping sites, this could promote the evolution of another distinctive hominin trait: central place foraging. Any foods that required more extensive processing before eating might be carried back to the sleeping cliffs and worked on there. Any food preparation at a central site would be visible to other group members, and so might serve as an impetus to sharing (and/or scrounging).
Collective foraging
The human subsistence strategy – hunting and gathering – differs profoundly from that of any living primates, with its sexual division of labour (men do most of the hunting, women do most of the gathering) and transfers of food (Isaac, 1978 ). Hunting and fishing account for over half of the daily energy obtained across foraging societies (Kaplan et al., 2000 ), providing humans with food in habitats where apes would starve, such as climates where arid conditions or long winters make ripe fruit unavailable. Gathering differs from the foraging of chimpanzees and other primates in three major ways: (a) gatherers bring much of what they acquire back to a central camp, where they (b) normally prepare the food to make it more palatable and (c) share it with others. Food transfers from parents and others to offspring promote offspring survival, shorten interbirth intervals and enable human populations to reproduce more quickly than other apes (Kaplan et al., 2000 ). In contrast, other primates mainly eat as they go, and fend for themselves, rather than collecting food to share. While chimpanzees do not engage in full-scale collective foraging, studies at Gombe and other sites have revealed that chimpanzees do engage in some sharing of food, providing insights into how collective foraging may have evolved in the human lineage.
On 30 October 1960, Goodall observed David Greybeard eating an infant bushpig, and sharing the meat with a female. Hunting and meat sharing have been major research topics at Gombe ever since (Boesch, 1994 ; Stanford et al., 1994 ; Gilby et al, 2015 ; Goodall, 1963 ; Teleki, 1973 ).
Hunting presents an interesting problem for cooperation. Hunters incur energetic costs and risk of injury in pursuing prey. Individuals can cheat by opting out of the hunt, but obtaining meat afterwards, by either theft or begging. Two solutions have been proposed: reciprocity (hunters share meat more with other hunters, and/or with key allies and/or fertile females) and self-interest (males hunt if they are highly motivated to acquire meat for themselves, and they share meat to reduce harassment from beggars).
Boesch ( 1994 ) argued that hunting at Taï is maintained by reciprocity: hunters share more meat with other hunters than with bystanders. In contrast, Wrangham ( 1975 ) proposed that chimpanzee sharing is more selfish: chimpanzees share to induce beggars to leave them alone so that they can feed more efficiently. Detailed analysis of video from meat-sharing events confirmed key predictions of Wrangham's hypothesis: more persistent beggars obtained more food; beggars left after getting food; and the carcass-holder's feeding efficiency increased when fewer beggars were present (Gilby, 2006 ; Figure 8 ).

Edgar, an adult male in the Mitumba community, eating a red colobus monkey, with adolescent female Yamaha watching. Photo by Ian C. Gilby.
Boesch visited Gombe in the early 1990s and speculated that ecological differences influenced patterns of cooperation, with cooperation being more critical for success in the high canopied forest of Taï (Boesch, 1994 ). This hypothesis has not been tested systematically, but Gilby et al. ( 2006 ) confirmed Wrangham's ( 1975 ) observation that Gombe chimpanzees were more likely to hunt when in woodlands – where the broken canopy made it more difficult for monkeys to escape – than in evergreen forest ( Figure 7 ).

Adult red colobus monkey leaping across a gap in the canopy to escape from a female chimpanzee, Samwise, who is grabbing a juvenile colobus. Photo by Ian C. Gilby.
Stanford argued that male chimpanzees hunt to obtain meat to share with fertile females, based on observations that encounters with red colobus monkeys led to hunting attempts more often when sexually receptive females were present (Stanford et al., 1994 ). This suggested an intriguing analogy to food transfers in hunter–gatherers (Stanford, 1996 ). However, subsequent analysis of a larger dataset from Gombe, which included the years Stanford analysed, failed to support this hypothesis (Gilby et al., 2006 ). Instead, the presence of receptive females made males less likely to hunt – presumably because males can invest either in hunting or in mate guarding, but not both at once. Analysis of data from both Kanyawara and Gombe found that males neither shared preferentially with receptive females nor increased their mating success by sharing with receptive females (Gilby et al., 2010 ). While female chimpanzees are eager to obtain meat, they do not require meat as an inducement to mate (Gilby et al., 2010 ). Stanford's results may have differed from later analyses because during the years Stanford analysed, the sterile female Gigi was often present, and often swollen. Male chimpanzees show substantially greater interest in mating with females that have produced offspring (Muller et al., 2006 ), so the presence of Gigi may have provided little distraction from an interest in hunting.
Observations that hunting success increases with male party size appear to support the view that chimpanzee hunting is a cooperative endeavour (Boesch, 1994 ; Stanford et al., 1994 ). Gilby and colleagues have found, however, that this effect depends on the presence of a small number of key individuals: ‘impact hunters’. If an impact hunter is present, larger parties are more likely to hunt, but in the absence of these individuals, hunts are unlikely to occur (Gilby et al., 2015 ). Presumably, the risks and costs of hunting are highest for the first individual to start the hunt, but once a hunt begins, others stand to gain from participation. Hunting in chimpanzees thus appears to be a byproduct of mutualism.
Hunting was a key factor enabling hominins to navigate their adaptive landscape, climbing higher up the narrow ridge towards distinctively human adaptive peaks. Whether hominins followed that path because their ancestors hunted remains uncertain. If the LCA PH lived in single male groups, opportunities for hunting by multiple males would have been rare. Even if the LCA PH closely resembled chimpanzees, any hunting conducted by hominins probably differed from the chimpanzee pattern. As McGrew ( 2010 ) observes ‘Most chimpanzee hunting is done arboreally, by “four-handed” hunters who can leap about in the canopy, pursuing monkeys. This is not likely to be instructive about hunting by terrestrial bipeds’. Human hunting depends on bipedal locomotion – which enables hunters to travel long distances efficiently in the pursuit of prey, and frees the hands to make and use weapons and carry meat back to camp – and cumulative culture, which preserves and transmits innovations for capturing, killing and processing prey. The evolution of extensive hunting in hominins might therefore depend more on the various factors that led to bipedal locomotion and cumulative culture than on a heritage of hunting. Nonetheless, hunting and meat sharing in hominins would have posed similar problems for cooperation. Field studies of chimpanzees provide insights into how these problems can be solved.
Observations of chimpanzees suggest that, long before hominins shared meat on a large scale, mothers shared plant foods with their offspring (McGrew, 1979 ). Chimpanzee mothers commonly share foods that are difficult to open, such as Strychnos and Diplorhynchus , with their offspring (Silk, 1978 ; McGrew, 1979 ). At Taï, where chimpanzees use stones and anvils to crack nuts, mothers allow their offspring to take nuts they have opened (Boesch-Achermann and Boesch, 1993 ). When technology enables individuals to produce food surpluses, mothers have strong incentives to share food with their offspring, both to promote the growth and survival of their offspring and to offset their own lactation costs (Silk, 1978 ). Such food sharing by mothers may have played an important role in the evolution of more cooperative temperaments, by promoting an increased capacity to inhibit impulses and keep in mind the needs of others.
Language so completely permeates human life that fully understanding what it enables us to do, and what can be done without it, can be difficult. Language shapes our thinking and imagination. It helps cooperation, enabling individuals to share detailed, explicit plans and to negotiate coalitions and trade. It provides a conduit for cumulative culture, storing and transmitting the stories, knowledge and know-how of our ancestors and distant others who we will never meet. The reasons for how and why human language evolved remain unclear, but research in diverse fields suggests that an empirically grounded, comparative approach can shed light on these questions (Fitch, 2010 ).
Because chimpanzees are so similar to humans in many respects, researchers have sought evidence of language-like capacities in chimpanzee vocal communication (Fedurek & Slocombe, 2011 ). Marler recorded chimpanzee vocalizations at Gombe in 1967, and based on acoustic analysis, identified a repertoire of 13 basic types, which generally resembled those of gorillas (Marler, 1976 ). Goodall ( 1986 ) identified a richer set of 32 categories that sounded distinct to her. Classifying chimpanzee calls is challenging, because many categories grade into one another. Crockford ( 2019 ) proposed a hierarchical system that classifies most chimpanzee calls as variants of five basic categories: screams, barks, grunts, hoos and pants.
Marler's work inspired two main avenues of searches for language parallels: referential signalling and dialects. Marler ( 1977 ) considered food-associated ‘rough grunt’ calls to be the most promising candidates for referential signalling. Slocombe and Zuberbühler ( 2005 ) proposed that different categories of rough grunt signalled food preference: high-pitched, more tonal calls were given in response to more preferred foods, while low-pitched, noisier calls were given in response to less preferred foods. However, field studies in Budongo (Slocombe & Zuberbuhler, 2006 ) and Taï (Kalan et al., 2015 ) have found only limited evidence for associations between acoustic structure and food characteristics. At Gombe, O'Bryan ( 2015 ) found that acoustic features varied considerably within bouts, indicating that these calls have low potential for referential signalling. These calls may instead signal more about the caller's intentions to continue feeding (Fedurek & Slocombe. 2013; O'Bryan, 2015 ).
Chimpanzees give acoustically distinct alarm calls to snakes, which appear to be better candidates for functionally referential calls (Crockford et al., 2015 ). An intriguing possibility suggested by long-term researchers at Gombe is that chimpanzees have distinct calls for pythons and cobras.
Dialects – regional variation in acoustic structure – are thought to reflect vocal learning, which is a key component of human speech, but a rare trait in mammals. Mitani compared recordings of pant-hoots from Mahale with Marler's recordings from Gombe, and found evidence for dialects (Mitani et al., 1992 ). However, Mitani later argued that the evidence for vocal learning in chimpanzees was slim: most variation in acoustic structure depended on individual identity, rather than community membership (Mitani & Brandt, 1994 ). Subsequent studies have found evidence for dialects at Taï (Crockford et al., 2004 ). Work is currently underway to assess whether the Mitumba and Kasekela communities at Gombe have distinct pant-hoot calls; preliminary analyses indicate that they do not (Desai & Wilson, 2020 ).
Chimpanzees also produce many gestures (van Lawick-Goodall, 1968b ). Gestures have long been of interest as a possible step along the path towards language, particularly given that apes appear to have more intentional control over their gestures than their vocalizations (Pollick & de Waal, 2007 ). Perhaps challenging the view that gestures represent learned signals, however, the physical forms of gestures used by chimpanzees at Budongo, Uganda and bonobos at Wamba, overlap by approximately 90% in their physical shape, and to a considerable extent, gestures of the same form share the same apparent meaning in the two species (Graham et al., 2018 ).
The modest evidence for referential communication and vocal learning in chimpanzees suggests that the key events in language evolution occurred only in the hominin lineage, after the divergence from panins. More recent efforts to find language parallels have focused on aspects of social cognition, which surely are relevant for understanding language evolution (Crockford et al., 2017 ). Nonetheless, considering the capacity to accurately inform others about remote objects, the closest parallel to human language comes not from chimpanzees or bonobos or any other primate, but instead from social insects: the dance communication of honeybees ( Apis spp.). Worker bees ‘waggle dance’ to indicate the distance and direction to key resources such as food, water and potential nest sites (von Frisch, 1967 ; l'Anson Price & Grüter, 2015 ). That bees have evolved this capacity suggests that a key constraint on the evolution of such informative signalling is not cognitive capacity, but social structures, such as collective foraging. As Krebs and Dawkins ( 1984 ) argued, animals generally have a strong incentive to signal in ways that manipulate others to their own benefit; deception is thus rampant in animal signalling. Among collective foragers, however, group members share a common interest in one another's foraging success. Alexander ( 1987 ) noted that, because honeybees feed their sisters, honest communication about food resources promotes their inclusive fitness. Ants are collective foragers as well; they signal food location by depositing a scent trail on their way from good food sources back to the nest (Wilson, 1962 ). Bees cannot deposit a scent trail through the air when they fly, so they have evolved the waggle dance instead. As collective foraging evolved in hominins, foragers began to share a wider stake in the foraging success of other group members, and so would have had more to gain from honestly indicating the location of good food sources. Bickerton ( 2009 ) argues that hominins evolved language to communicate about the location of large animal carcasses, which they scavenged. Insofar as individuals shared food with one another, collective foraging would promote honest communication about other food sources as well. Cooking probably played an important role in language evolution as well, by providing energy needed to grow and maintain larger brains, as part of an autocatalytic cycle, whereby tool use increased the importance of social learning, resulting in greater access to energy to fuel bigger brains, which in turn provided more computational power for insights and social learning.
Living apes provide little evidence that language-like capacities existed in the LCA PH . However, insofar as tool use, food processing and collective foraging evolved in hominins because hominins shared with their ape ancestors a requirement for high-quality food, inefficient quadrupedal locomotion and a capacity for tool use, our ape ancestry may have played an important role in leading us to this otherwise improbable path.
Conclusions
Long-term field studies of chimpanzees at Gombe and other sites have amply fulfilled the hopes of visionaries such as Leakey and Imanishi. Decades of systematic study have revealed both striking similarities between chimpanzees and humans and profound differences. Gaining further insights into the details of these differences, and how and why they are likely to have emerged, has taken decades of work, and much remains to be done.
Contrary to Fuentes ( 2018 ), the relevance of chimpanzee studies for understanding human evolution does not depend upon a belief that phylogenetic inertia dooms us to behaving in the ways the LCA PH behaved millions of years ago. We know phylogenetic inertia has limited power from the many ways in which chimpanzees, bonobos and humans differ from one another: each of these living species is the product of continuing evolution. Instead, studying chimpanzees and other primates provides insights into both general factors that favour the evolution of particular traits (such as aggressive vs. friendly behaviour), and into how being a particular kind of animal – a great ape – results in particular constraints and opportunities.
As primates, we have adaptations for complex social living. Yet this alone cannot explain the peculiar trajectory of hominin evolution. Multiple lineages of primates adapted to the expanding open habitats of the Plio-Pleistocene: savanna monkeys, patas monkeys, gelada monkeys, baboons and hominins. Of these, only hominins evolved bipedal locomotion, extensive tool use and language. Monkeys are efficient quadrupeds, and so did not need to evolve bipedal locomotion. Monkeys can digest a broader range of foods, and so did not need to invent tools to obtain high-quality foods – and because they remained quadrupedal, did not evolve bipedal locomotion that freed hominin hands for more effective tool use. The reasons for how and why language evolved remain obscure, but this most distinctive of human traits is probably closely related to the consequences of increasingly elaborate tool use, including an increased importance of social learning, increased access to energy to fuel larger brains and the increased value of honest signalling among collective foragers. Thus, all of these specifically human traits depend on our having evolved from apes. The reason hominins, but not baboons or elephants or some other lineage, ended up on this peculiar path very much depends on our heritage as apes: inefficient quadrupeds with grasping hands, orthograde posture, large brains and digestive systems that require high-quality foods. Long-term studies of apes at Gombe and other sites have been, and will continue to be, essential for providing insights into our own distinctive evolutionary pathway.
Acknowledgements
Research at Gombe is conducted with the permission and support of the Tanzania Commission for Science and Technology, the Tanzania Wildlife Research Institute and the Tanzania National Parks. Thanks to Frans de Waal, Sergey Gavrilets and Peter Richerson for the invitation to contribute to this special issue. Thanks to Frans de Waal, Kevin Hunt, Anne Pusey, Richard Wrangham and an anonymous reviewer for helpful comments on the manuscript, to Larry Wilson for discussion of fitness vectors, and to Jane Goodall for answering questions about Gombe's history. Thanks to Falk Grossmann for giving permission to use unpublished data and to Ian Gilby for providing photos. Above all, the long-term study depends on the hard work of the field staff and visiting researchers at the Gombe Stream Research Centre, who have contributed countless hours to this project.
Author contributions
Michael L. Wilson wrote the paper.
Financial support
Over the past 60 years, numerous sources have provided funding for this research, including the National Institutes of Health (R00 HD057992, R01 AI050529, R01 AI120810), the National Science Foundation (DBS-9021946, SBR-9319909, BCS-0452315, IOS-1052693, IOS-1457260, BCS-0648481, BCS-1753437, BCS-1743506), the Arcus Foundation, Carnegie Corporation, the Leo S. Guthman Foundation, the Leakey Foundation, Margo Marsh, Mazuri (AAZV), the Morris Animal Foundation, the National Geographic Society, the Harris Steel Group, the Wilkie Foundation, the William T. Grant Foundation, the Windibrow Foundation, Arizona State University, Duke University, Franklin and Marshall College, George Washington University and the University of Minnesota. The Jane Goodall Institute has provided major funding and administers the long-term research.
Conflicts of interest
The author declares no conflicts of interest.
Research transparency and reproducibility
This review paper did not report new findings, apart from the data used to make Figure 4 , which are available upon reasonable request of the author.
- Alberts, S. C., Altmann, J., Brockman, D. K., Cords, M., Fedigan, L., Pusey, A., … Bronikowski, A. F. (2013). Reproductive cessation patterns in primates reveal that humans are distinct. Proceedings of the National Academy of Sciences 110(33), 13440–13445. 10.1073/pnas.1311857110 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Alexander, R. D. (1987). The biology of moral systems. AldineTransaction. [ Google Scholar ]
- Altmann, S. (1974). Baboons, space, time, and energy. American Zoologist. 14, 221–248. [ Google Scholar ]
- Andrews, P. (2020). Last common ancestor of apes and humans: Morphology and environment. Folia Primatologica, 91, 122–148. 10.1159/000501557 [ DOI ] [ PubMed ] [ Google Scholar ]
- Arcadi, A. C. (2018). Wild chimpanzees: Social behavior of an endangered species. Cambridge University Press. [ Google Scholar ]
- Barton, R. A., & Whiten, A. (1994). Reducing complex diets to simple rules: Food selection by olive baboons. Behavioral Ecology and Sociobiology, 35(4), 283–293. [ Google Scholar ]
- Begun, D. R. (2016). The real planet of the apes: A new story of human origins. Princeton University Press. https://www.jstor.org/stable/j.ctt21c4v71.13 [ Google Scholar ]
- Bickerton, D. (2009). Adam's tongue: How humans made language, how language made humans. Hill and Wang. [ Google Scholar ]
- Boesch-Achermann, H., & Boesch, C. (1993). Tool use in wild chimpanzees: New light from dark forests. Current Directions in Psychological Science, 2(1), 18–21. 10.1111/1467-8721.ep10770551 [ DOI ] [ Google Scholar ]
- Boesch, C. (1994). Cooperative hunting in wild chimpanzees. Animal Behaviour, 48, 653–667. [ Google Scholar ]
- Boesch, C., Wittig, R., Crockford, C., Vigilant, L., Dseshner, T., & Leendertz, F. (2019) The chimpanzees of the Taï Forest: 40 years of research. Cambridge University Press. [ Google Scholar ]
- Boyd, R., Richerson, P. J., & Henrich, J. (2011). The cultural niche: Why social learning is essential for human adaptation. Proceedings of the National Academy of Sciences of the United States of America, 108(Suppl. 2), 10918–10925. 10.1073/pnas.1100290108 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bygott, J. D. (1979). Agonistic behaviour, dominance, and social structure in wild chimpanzees of the Gombe National Park. In Hamburg D. A. & McCown E. R. (Eds.) The great apes (pp. 405–427). Benjamin/Cummings. [ Google Scholar ]
- Byrne, R. (2016). Evolving insight. Oxford University Press. [ Google Scholar ]
- Cerling, T. E., Manthi, F. K., Mbua, E. N., Leakey, L. N., Leakey, M. G., Leakey, R. E., Brown, F. H., … Wood, B. A. (2011). Stable isotope-based diet reconstructions of Turkana Basin hominins. Proceedings of the National Academy of Sciences, 110(26), 10501–10506. 10.1073/pnas.1222568110 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Crockford, C. (2019). Why does the chimpanzee vocal repertoire remain poorly understood and what can be done about it? In Crockford, C., Vigilant, L., Deschner, T., Leendertz, F. (Authors) & Boesch, C., Wittig, C. (Eds.) The chimpanzees of the Taï Forest: 40 years of research (pp. 394–409). Cambridge University Press. 10.1017/9781108674218.025 [ DOI ] [ Google Scholar ]
- Crockford, C., Herbinger, I., Vigilant, L., & Boesch, C. (2004). Wild chimpanzees produce group-specific calls: A case for vocal learning? Ethology, 110, 221–243. [ Google Scholar ]
- Crockford, C., Wittig, R. M., & Zuberbuehler, K. (2015). An intentional vocalization draws others’ attention: A playback experiment with wild chimpanzees. Animal Cognition, 18(3), 581–591. 10.1007/s10071-014-0827-z [ DOI ] [ PubMed ] [ Google Scholar ]
- Crockford, C., Wittig, R. M., & Zuberbühler, K. (2017). Vocalizing in chimpanzees is influenced by social-cognitive processes. Science Advances, 3(11), e1701742. 10.1126/sciadv.1701742 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Crouse, K. N., Miller, C. M., & Wilson, M. L. (2019). New approaches to modeling primate socioecology: Does small female group size beget loyal males? Journal of Human Evolution, 137, 102671. 10.1016/j.jhevol.2019.102671 [ DOI ] [ PubMed ] [ Google Scholar ]
- Darwin, C. (1859). On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. John Murray. https://www.gutenberg.org/files/1228/1228-h/1228-h.htm [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Darwin, C. (1871). The descent of man, and selection in relation to sex. John Murray. http://www.gutenberg.org/files/34967/34967-h/34967-h.htm [ Google Scholar ]
- Deacon, T. W. (1997). The symbolic species: The co-evolution of language and the brain. W.W. Norton. [ Google Scholar ]
- Desai, N., & Wilson, M. L. (2020). Do chimpanzees of Gombe National Park, Tanzania have community-specific dialects? American Journal of Physical Anthropology, 171(Suppl. 69), 70–70. 89th Annual Meeting of the American Association of Physical Anthropologists, Los Angeles, CA, 15–18 April, 2020. [ Google Scholar ]
- Deschner, T., & Boesch, C. (2007). Can the patterns of sexual swelling cycles in female Taï chimpanzees be explained by the cost-of-sexual-attraction hypothesis? International Journal of Primatology, 28(2), 389–406. [ Google Scholar ]
- de Waal, F. B. M. (2011). Toshisada Nishida (1941–2011): Chimpanzee Rapport. PLoS Biology, 9(10), e1001185. 10.1371/journal.pbio.1001185 [ DOI ] [ Google Scholar ]
- Diogo, R., Molnar, J. L., & Wood, B. (2017) Bonobo anatomy reveals stasis and mosaicism in chimpanzee evolution, and supports bonobos as the most appropriate extant model for the common ancestor of chimpanzees and humans. Scientific Reports, 7, 608. 10.1038/s41598-017-00548-3 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Doran, D. M., Hunt, K. D. (1994) The comparative locomotor behavior of chimpanzees and bonobos: species and habitat differences. In Wrangham R. W., McGrew W. C., de Waal F. B. M., & Heltne P. G. (Eds.) Chimpanzee cultures (pp. 93–108). Harvard University Press. [ Google Scholar ]
- Druelle, F., Schoonaert, K., Aerts, P., Nauwelaerts, S., Stevens, J. M. G., D'Août, K. (2018). Segmental morphometrics of bonobos ( Pan paniscus ): Are they really different from chimpanzees ( Pan troglodytes )? Journal of Anatomy, 233(6). 10.1111/joa.12894 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Duda, P., & Zrzavý, J. (2013). Evolution of life history and behavior in Hominidae: Towards phylogenetic reconstruction of the chimpanzee–human last common ancestor. Journal of Human Evolution. 65(4), 424–46. 10.1016/j.jhevol.2013.07.009 [ DOI ] [ PubMed ] [ Google Scholar ]
- Ember, C. R. (1978). Myths about hunter–gatherers. Ethnology, 17(4), 439–448. 10.2307/3773193 [ DOI ] [ Google Scholar ]
- Fashing, P. J., Nguyen, N., Venkataraman, V. V., & Kerby, J. T. (2014). Gelada feeding ecology in an intact ecosystem at Guassa, Ethiopia: Variability over time and implications for theropith and hominin dietary evolution. American Journal of Physical Anthropology, 155, 1–16. 10.1002/ajpa.22559 [ DOI ] [ PubMed ] [ Google Scholar ]
- Fedurek, P., & Slocombe, K. E. (2011). Primate vocal communication: A useful tool for understanding human speech and language evolution? Human Biology, 83(2), 153–173. 10.3378/027.083.0202 [ DOI ] [ PubMed ] [ Google Scholar ]
- Feldblum, J. T., Manfredi, S., Gilby, I. C., & Pusey, A. E. (2018). The timing and causes of a unique chimpanzee community fission preceding Gombe's ‘Four Years War’. American Journal of Physical Anthropology, 166(3), 730–744. [ DOI ] [ PubMed ] [ Google Scholar ]
- Feldblum, J. T, Wroblewski, E. E., Rudicell, R. S., Hahn, B. H., Paiva, T., Cetinkaya-Rundel, M., … Gilby, I. C. (2014). Sexually coercive male chimpanzees sire more offspring. Current Biology, 24(23), 2855–2860. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fitch, W. T. S. (2010). The evolution of language. Cambridge University Press. 10.1017/CBO9780511817779 [ DOI ] [ Google Scholar ]
- Foerster, S., Franz, M., Murray, C. M., Gilby, I. C., Feldblum, J. F., Walker, K. K., & Pusey, A. E. (2016). Chimpanzee females queue but males compete for social status. Scientific Reports, 6, 35404. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fruth, B., & Hohmann, G. (2018). Food sharing across borders. Human Nature, 29, 91–103. 10.1007/s12110-018-9311-9 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fuentes, A. (2018). Chimpanzees can't tell us much about being human. https://www.sapiens.org/evolution/chimpanzees-cant-tell-us-much-about-being-human/
- Furuichi, T. (2009). Factors underlying party size differences between chimpanzees and bonobos: a review and hypotheses for future study. Primates, 50, 197–209. 10.1007/s10329-009-0141-6 [ DOI ] [ PubMed ] [ Google Scholar ]
- Furuichi, T. (2019). Bonobo and chimpanzee: The lessons of social coexistence. Springer. [ Google Scholar ]
- Furuichi, T (2020). Variation in intergroup relationships among species and among and within local populations of African apes. International Journal of Primatology, 41, 203–223. 10.1007/s10764-020-00134-x [ DOI ] [ Google Scholar ]
- Furuichi, T., Sanz, C., Koops, K., Sakamaki, T., Ryu, H., Tokuyama, N., & Morgan, D. (2015). Why do wild bonobos not use tools like chimpanzees do? Behaviour, 152(3–4), 425–460. https://doi-org.ezp1.lib.umn.edu/10.1163/1568539X-00003226 [ Google Scholar ]
- Ghiglieri, M. P. (1987). Sociobiology of the great apes and the hominid ancestor. Journal of Human Evolution, 16(4), 319–357. 10.1016/0047-2484(87)90065-0 [ DOI ] [ Google Scholar ]
- Ghiglieri, M. P. (2000). The dark side of man: Tracing the origins of male violence. Basic Books. [ Google Scholar ]
- Gilby, I. C. (2006). Meat sharing among the Gombe chimpanzees: Harassment and reciprocal exchange. Animal Behaviour, 71, 953–963. [ Google Scholar ]
- Gilby, I. C., Eberly, L. E., Pintea, L., & Pusey, A. E. (2006). Ecological and social influences on the hunting behaviour of wild chimpanzees, Pan troglodytes schweinfurthii . Animal Behaviour, 72, 169–180. [ Google Scholar ]
- Gilby, I. C., Machanda, Z. P., Mjungu, D. C., Rosen, J., Muller, M. N., Pusey, A. E., & Wrangham, R. W. (2015). ‘Impact hunters’ catalyse cooperative hunting in two wild chimpanzee communities. Philosophical Transactions of the Royal Society B – Biological Sciences, 370(1683), 12. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gilby, I. C., Machanda, Z. P., O'Malley, R. C., Murray, C. M., Lonsdorf, E. V., Walker, K., … Wrangham, R. W. (2017). Predation by female chimpanzees: Toward an understanding of sex differences in meat acquisition in the last common ancestor of Pan and Homo . Journal of Human Evolution, 110, 82–94. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gilby, I. C., Thompson, M. E., Ruane, J. D., & Wrangham, R. W. (2010). No evidence of short-term exchange of meat for sex among chimpanzees. Journal of Human Evolution, 59(1), 44–53. [ DOI ] [ PubMed ] [ Google Scholar ]
- Goodall, J. (1963). Feeding behaviour of wild chimpanzees. A preliminary report. Symposium of the Zoological Society of London, 10, 39–47. [ Google Scholar ]
- Goodall, J. (1964). Tool-using and aimed throwing in a community of free-living chimpanzees. Nature, 201, 1264–1266. [ DOI ] [ PubMed ] [ Google Scholar ]
- Goodall, J. (1986). The chimpanzees of Gombe: Patterns of behavior. Belknap Press. [ Google Scholar ]
- Goodall, J. (1990). Through a window: My thirty years with the chimpanzees of Gombe. Houghton Mifflin. [ Google Scholar ]
- Goodall, J., Bandora, A., Bermann, E., Busse, C., Matama, H., Mpongo, E., … Riss, D. (1979). Intercommunity interactions in the chimpanzee population of the Gombe National Park. In Hamburg D. A., McCown, E. R. (Eds.) The great apes (pp. 13–53). Benjamin/Cummings. [ Google Scholar ]
- Graham, K. E., Hobaiter, C., Ounsley, J., Furuichi, T., & Byrne, R. W. (2018) Bonobo and chimpanzee gestures overlap extensively in meaning. PLoS Biology, 16(2), e2004825. 10.1371/journal.pbio.2004825 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Grueter, C. C., Chapais, B., & Zinner, D. (2012). Evolution of multilevel social systems in nonhuman primates and humans. International Journal of Primatology, 33, 1002–1037. 10.1007/s10764-012-9618-z [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hare, B., & Yamamoto, S. (2015). Bonobo cognition and behavior. Brill. [ Google Scholar ]
- Henrich, J. (2017). The secret of our success: How culture is driving human evolution, domesticating our species, and making us smarter. Princeton University Press. [ Google Scholar ]
- Hernandez-Aguilar, A., Moore, J., & Pickering, T. R. (2007). Savanna chimpanzees use tools to harvest the underground storage organs of plants. Proceedings of the National Academy of Sciences, 104, 19210–19213. 10.1073/pnas.0707929104 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hill, K., Boesch, C., Goodall, J., Pusey, A. E., Williams, J. M., & Wrangham, R. W. (2001). Mortality rates among wild-living chimpanzees. Journal of Human Evolution, 40, 437–450. [ DOI ] [ PubMed ] [ Google Scholar ]
- Hill, K. R., Walker, R. S., Bozicević, M., Eder, J., Headland, T., Hewlett, B., … Wood, B. (2011). Co-residence patterns in hunter–gatherer societies show unique human social structure. Science, 331(6022), 1286–1289. 10.1126/science.1199071 [ DOI ] [ PubMed ] [ Google Scholar ]
- Hunt, K. D. (1994). The evolution of human bipedality: ecology and functional morphology. Journal of Human Evolution, 26, 183–202. [ Google Scholar ]
- Hunt, K. D. (2016). Why are there apes? Evidence for the co-evolution of ape and monkey ecomorphology. Journal of Anatomy, 228, 630–685. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hunt, K. D. (2020). Chimpanzee: Lessons from our sister species. Cambridge University Press. [ Google Scholar ]
- Imanishi, K. (1965). The origin of the human family: A primatological approach. In Imanishi K. & Altmann S. A. (Eds.) Japanese monkeys: A collection of translations (pp. 113–140). Chicago, 1965. (Originally published in Japanese in Japanese Journal of Ethnology , 25(3), 119–138, 1961. 10.14890/minkennewseries.25.3_119) [ DOI ] [ Google Scholar ]
- Isaac, G. Ll. (1978). Food sharing and human evolution: Archaeological evidence from the Plio-Pleistocene of East Africa. Journal of Anthropological Research, 34(3), 311–325. [ Google Scholar ]
- Itani, J. (1966). The social organization of chimpanzees. Shizen, 21(8): 17–30. [ Google Scholar ]
- Itani, J., Suzuki, A. (1967). The social unit of chimpanzees. Primates, 8, 355–381. 10.1007/BF01792020 [ DOI ] [ Google Scholar ]
- Itoh, N., & Nakamura, M. (2015). Social system: Features and variations. In Nakamura M., Hosaka K, Itoh N., & Zamma K. (Eds.), Mahale chimpanzees: 50 years of research (pp. 71–81). Cambridge: Cambridge University Press. [ Google Scholar ]
- Jolly, C. J. (1970). The seed-eaters: A new model of hominid differentiation based on a baboon analogy. Man, New Series , 5(1), 5–26. [ Google Scholar ]
- Jones, J. H., Wilson, M. L., Murray, C. M., & Pusey, A. E. (2010). Phenotypic quality influences fertility in Gombe chimpanzees. Journal of Animal Ecology, 79(6), 1262–1269. 10.1111/j.1365-2656.2010.01687.x [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kalan, A. K., Mundry, R., & Boesch, C. (2015). Wild chimpanzees modify food call structure with respect to tree size for a particular fruit species. Animal Behaviour, 101, e9. 10.1016/j.anbehav.2014.12.011 [ DOI ] [ Google Scholar ]
- Kano, T. (1992). The last ape: Pygmy chimpanzee behavior and ecology. Stanford University Press. [ Google Scholar ]
- Kaplan, H., Hill, K., Lancaster, J., & Hurtado, A. M. (2000). A theory of human life history evolution: Diet, intelligence, and longevity. Evolutionary Anthropology, 9(4),156–1985. [ Google Scholar ]
- Kirchhoff, C. A. (2019). Life and death in the Gombe chimpanzees: Skeletal analysis as an insight into life history. Springer. [ Google Scholar ]
- Krebs, J. R., & Dawkins, R. (1984). Animal signals: Mind reading and manipulation. In Krebs J. R. & Davies N. B. (Eds), Behavioural ecology: An evolutionary approach (2nd ed., pp. 380–402). Oxford University Press. [ Google Scholar ]
- Kummer, H. (1971). Primate societies: Group techniques of ecological adaptation. Aldine. [ Google Scholar ]
- Laden, G., Wrangham, R. W. (2005). The rise of the hominids as an adaptive shift in fallback foods: Plant underground storage organs (USOs) and australopith origins. Journal of Human Evolution, 49, 482–498. 10.1016/j.jhevol.2005.05.007 [ DOI ] [ PubMed ] [ Google Scholar ]
- Lambert, J. E. (2005). Competition, predation, and the evolutionary significance of the cercopithecine cheek pouch: The case of Cercopithecus and Lophocebus . American Journal of Physical Anthropology, 126(2), 183–192. 10.1002/ajpa.10440 [ DOI ] [ PubMed ] [ Google Scholar ]
- Lambert, J. E. (2007). The biology and evolution of ape and monkey feeding. In: Henke W. & Tattersall I. (Eds.) Handbook of paleoanthropology (pp. 1207–1234). Springer. 10.1007/978-3-540-33761-4_39 [ DOI ] [ Google Scholar ]
- l'Anson Price, R., & Grüter, C. (2015). Why, when and where did honeybee dance communication evolve? Frontiers in Ecology and Evolution, 3, 125. 10.3389/fevo.2015.00125 [ DOI ] [ Google Scholar ]
- Leakey, L. S. B. (1970). The relationship of African apes, Man, and Old World monkeys. Proceedings of the National Academy of Sciences, 67(2), 746–748. 10.1073/pnas.67.2.746 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Levin, N. E., Simpson, S. W., Quade, J., Cerling, T. E., & Frost, S. R. (2008). Herbivore enamel carbon isotopic composition and the environmental context of Ardipithecus at Gona, Ethiopia. Geological Society of America Special Papers, 446, 215–234. 10.1130/2008.2446(10). [ DOI ] [ Google Scholar ]
- Lonsdorf, E. V., Pusey, A. E., & Eberly, L. (2004). Sex differences in learning in chimpanzees. Nature, 428, 715–716. [ DOI ] [ PubMed ] [ Google Scholar ]
- Lonsdorf, E. V., Stanton, M. A., & Murray, C. M. (2018). Sex differences in maternal sibling–infant interactions in wild chimpanzees. Behavioral Ecology and Sociobiology, 72(7), 117. [ Google Scholar ]
- Luncz, L. V., & Boesch, C. (2014). Tradition over trend: Neighboring chimpanzee communities maintain differences in cultural behavior despite frequent immigration of adult females. American Journal of Primatology, 76, 649–657. 10.1002/ajp.22259 [ DOI ] [ PubMed ] [ Google Scholar ]
- Marks, J. (2002). What it means to be 98% chimpanzee: Apes, people and their genes. University of California Press. [ Google Scholar ]
- Marler, P. (1976). Social organization, communication, and graded signals: The chimpanzee and the gorilla. In Bateson P. P. G. & Hinde R. A. (Eds), Growing points in ethology (pp. 239–280). Cambridge University Press. [ Google Scholar ]
- Marler, P. (1977). Primate vocalizations: Affective or symbolic? In Bourne G. H. (Ed.) Progress in ape research (pp. 85–96). Academic Press. [ Google Scholar ]
- Marlowe, F. W. (2005). Hunter–gatherers and human evolution. Evolutionary Anthropology, 14, 54–67. [ Google Scholar ]
- Massey, J. (2018). Pattern of cranial ontogeny in populations of Gorilla and Pan. Doctoral dissertation, University of Minnesota. https://conservancy.umn.edu/handle/11299/200300 [ Google Scholar ]
- Matsuzawa, T., & McGrew, W. C. (2008). Kinji Imanishi and 60 years of Japanese primatology. Current Biology, 18(14), R587–R591. [ DOI ] [ PubMed ] [ Google Scholar ]
- McGrew, W. C. (1979). Evolutionary implications of sex differences in chimpanzee predation and tool use. In Hamburg D. A. & McCown E. R. (Eds.) The great apes (pp. 441–463). Benjamin/Cummings. [ Google Scholar ]
- McGrew, W. C. (2010). In search of the last common ancestor: New findings on wild chimpanzees. Philosophical Transactions of the Royal Society B, 365, 3267–3276. 10.1098/rstb.2010.0067 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- McNulty, K. P. (2010). Apes and tricksters: The evolution and diversification of humans’ closest relatives. Evolution: Education and Outreach 3, 322–332. 10.1007/s12052-010-0251-z [ DOI ] [ Google Scholar ]
- Mitani, J. C., & Brandt, K. L. (1994). Social factors influence the acoustic variability in the long-distance calls of male chimpanzees. Ethology, 96, 233–252. [ Google Scholar ]
- Mitani, J. C., Hasegawa, T., Gros-Louis, J., Marler, P., & Byrne, R. (1992). Dialects in wild chimpanzees? American Journal of Primatology, 27, 233–243. [ DOI ] [ PubMed ] [ Google Scholar ]
- Muller, M. N., Emery Thompson, M., & Wrangham, R. W. (2006). Male chimpanzees prefer mating with old females. Current Biology, 16, 2234–2238. [ DOI ] [ PubMed ] [ Google Scholar ]
- Muller, M. N., Wrangham, R. W., & Pilbeam, D. R. (Eds.) (2017). Chimpanzees and human evolution. The Belknap Press. [ Google Scholar ]
- Murray, C. M. (2007). Dominance rank influences female chimpanzee space use: Towards an ideal despotic distribution. Animal Behaviour, 74, 1795–1804. [ Google Scholar ]
- Murray, C. M., Eberly, L. E., & Pusey, A. E. (2006). Foraging strategies as a function of season and rank among wild female chimpanzees ( Pan troglodytes ). Behavioral Ecology, 17, 1020–1028. [ Google Scholar ]
- Musgrave, S., Lonsdorf, E., Morgan, D., Prestipino, M., Kurtycz, L., Mundry, R., & Sanz, C. (2020). Teaching varies with task complexity in wild chimpanzees. Proceedings of the National Academy of Sciences, 117(2), 969–976. 10.1073/pnas.1907476116 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nakamura, M., Hosaka, K., Itoh, N., & Zamma, K. (2015). Mahale chimpanzees: 50 years of research. Cambridge University Press. [ Google Scholar ]
- Nelson, S. V., & Hamilton, M. I. (2017). Evolution of the human dietary niche: Initial transitions in chimpanzees and human evolution. In Muller M. N., Wrangham R. W., & Pilbeam D. R., Chimpanzees and human evolution (pp. 286–310). Harvard University Press. [ Google Scholar ]
- Nishida, T. (1968). The social group of wild chimpanzees in the Mahali Mountains. Primates, 9, 167–224. [ Google Scholar ]
- Nishida, T. (2012). Chimpanzees of the lakeshore: Natural history and culture at Mahale. Cambridge University Press. [ Google Scholar ]
- Nishida, T., & Kawanaka, K. (1972). Inter-unit-group relationships among wild chimpanzees of the Mahale Mountains. Kyoto University African Studies, 7, 131–169. [ Google Scholar ]
- Nockerts, R. S. (2019). Stable isotope ecology of Gombe National Park: A modern analogue for fossil hominins. Doctoral dissertation, University of Minnesota. [ Google Scholar ]
- Nunn, C. L. (1999). The evolution of exaggerated sexual swellings in primates and the graded-signal hypothesis. Animal Behaviour, 58, 229–246 [ DOI ] [ PubMed ] [ Google Scholar ]
- O'Bryan, L. R. (2015). The socioecology of chimpanzee foraging and food-associated calling behavior. Doctoral dissertation, University of Minnesota. http://hdl.handle.net/11299/175282 [ Google Scholar ]
- O'Malley, R. C., & Power, M. L. (2014). The energetic and nutritional yields from insectivory for Kasekela chimpanzees. Journal of Human Evolution, 71, 46–58. [ DOI ] [ PubMed ] [ Google Scholar ]
- O'Malley, R. C., Wallauer, W., Murray, C. M., & Goodall, J. (2012). The appearance and spread of ant fishing among the Kasekela chimpanzees of Gombe: A possible case of intercommunity cultural transmission. Current Anthropology, 53(5), 650–663. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pandolfi, S., van Schaik, C., Pusey, A. (2003). Sex differences in termite fishing among Gombe chimpanzees. In: De Waal F. B. M. & Tyack P. L. (Eds.) Animal social complexity: Intelligence, culture, and individualized societies (pp. 414–418). Harvard University Press. [ Google Scholar ]
- Parish, A. R., & de Waal, F. B. M. (2000). The other ‘closest living relative’: How bonobos ( Pan paniscus ) challenge traditional assumptions about females, dominance, intra- and intersexual interactions, and hominid evolution. Annals of the New York Academy of Sciences, 907, 97–113. 10.1111/j.1749-6632.2000.tb06618.x [ DOI ] [ PubMed ] [ Google Scholar ]
- Peterson, D. (2006). Jane Goodall: The woman who redefined man. Houghton Mifflin Harcourt. [ Google Scholar ]
- Pilbeam, D. R., & Lieberman, D. E. (2017). Reconstructing the last common ancestor of chimpanzees and humans. In Muller M. N., Wrangham R. W., & Pilbeam D. R. (Eds.) Chimpanzees and human evolution (pp. 22–141). Harvard University Press. [ Google Scholar ]
- Pisor, A. C., & Surbeck, M. (2019). The evolution of intergroup tolerance in nonhuman primates and humans. Evolutionary Anthropology, 28, 210–223. 10.1002/evan.21793 [ DOI ] [ PubMed ] [ Google Scholar ]
- Pollick, A. S., & de Waal, F. B. M. (2007). Ape gestures and language evolution. Proceedings of the National Academy of Sciences, 104(19), 8184–8189. 10.1073/pnas.0702624104 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pontzer, H., Raichlen, D. A., & Rodman, P. S. (2014) Bipedal and quadrupedal locomotion in chimpanzees. Journal of Human Evolution, 66, 64–82. 10.1016/j.jhevol.2013.10.002 [ DOI ] [ PubMed ] [ Google Scholar ]
- Pontzer, H., Raichlen, D. A., Wood, B. M., Mabulla, A. Z. P., Racette, S. B., & Marlowe, F. W. (2012). Hunter–gatherer energetics and human obesity. PLoS One, 7(7), e40503. 10.1371/journal.pone.0040503 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pontzer, H., & Wrangham, R. W. (2004). Climbing and the daily energy cost of locomotion in wild chimpanzees: Implications for hominoid locomotor evolution. Journal of Human Evolution, 46(3), 317–335. 10.1016/j.jhevol.2003.12.006 [ DOI ] [ PubMed ] [ Google Scholar ]
- Potts, R. (2004). Paleoenvironmental basis of cognitive evolution in great apes. American Journal of Primatology, 62, 209–228. 10.1002/ajp.20016 [ DOI ] [ PubMed ] [ Google Scholar ]
- Pusey, A. E. (1979). Intercommunity transfer of chimpanzees in Gombe National Park. In Hamburg D. A. & McCown E. R. (Eds.) The great apes (pp. 465–479), Benjamin/Cummings. [ Google Scholar ]
- Pusey, A. E. (1990). Behavioural changes at adolescence in chimpanzees. Behaviour, 115, 203–246. [ Google Scholar ]
- Pusey, A. E., Murray, C. M., Wallauer, W. R., Wilson, M. L., Wroblewski, E. E., & Goodall, J. (2008). Severe aggression among female chimpanzees at Gombe National Park, Tanzania. International Journal of Primatology, 29(4), 949–973. [ Google Scholar ]
- Pusey, A. E., Oehlert, G. W., Williams, J. M., & Goodall, J. (2005). Influence of ecological and social factors on body mass of wild chimpanzees. International Journal of Primatology, 26, 3–31. 10.1007/s10764-005-0721-2 [ DOI ] [ Google Scholar ]
- Pusey, A. E., Williams, J. M., & Goodall, J. (1997). The influence of dominance rank on the reproductive success of female chimpanzees. Science, 277, 828–831. [ DOI ] [ PubMed ] [ Google Scholar ]
- Richerson, P. J., & Boyd, R. (2004) Not by genes alone: How culture transformed human evolution. University of Chicago Press. [ Google Scholar ]
- Rodman, P., & McHenry, H. (1980). Bioenergetics and the origin of hominid bipedalism. American Journal of Physical Anthropology, 52, 103–106. [ DOI ] [ PubMed ] [ Google Scholar ]
- Sakamaki, T., Ryu, H., Toda, K., Tokuyama, N., & Furuichi, T. (2018). Increased frequency of intergroup encounters in wild bonobos ( Pan paniscus ) around the yearly peak in fruit abundance at Wamba. International Journal of Primatology, 39, 685–704. 10.1007/s10764-018-0058-2 [ DOI ] [ Google Scholar ]
- Sayers, K., & Lovejoy, C. O (2008). The chimpanzee has no clothes: A critical examination of Pan troglodytes in models of human evolution. Current Anthropology, 49(1), 87–114. [ Google Scholar ]
- Selby, M. (2018). Catarrhine locomotion. In Vonk J. & Shackelford T. K. (Eds.) Encyclopedia of animal cognition and behavior (pp. 1–7). 10.1007/978-3-319-47829-6_472-1 [ DOI ]
- Serckx, A., Kuhl, H. S., Beudels-Jamar, R. C., Poncin, P., Bastin, J. F., & Huynen, M. C. (2015). Feeding ecology of bonobos living in forest-savannah mosaics: Diet, seasonal variation, and importance of fallback foods. American Journal of Primatology, 77(9), 948–962. 10.1002/ajp.22425 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sibley, C. G., Ahlquist, J. E. (1984). The phylogeny of the hominoid primates, as indicated by DNA–DNA hybridization. Journal of Molecular Evolution, 20(1), 2–15. 10.1007/BF02101980 [ DOI ] [ PubMed ] [ Google Scholar ]
- Silk, J. B. (1978). Patterns of food sharing among mother and infant chimpanzees at Gombe National Park, Tanzania. Folia Primatologica, 29, 129–141. [ DOI ] [ PubMed ] [ Google Scholar ]
- Slocombe, K. E., & Zuberbühler, K. (2005). Functionally referential communication in a chimpanzee. Current Biology, 15, 1779–1784. 10.1016/j.cub.2005.08.068 [ DOI ] [ PubMed ] [ Google Scholar ]
- Slocombe, K. E., & Zuberbühler, K. (2006). Food-associated calls in chimpanzees: Responses to food types or food preferences? Animal Behaviour, 72(5), 989–999. [ Google Scholar ]
- Smil, V. (2011). Harvesting the biosphere: The human impact. Population and Development Review, 37(4), 613–636. [ DOI ] [ PubMed ] [ Google Scholar ]
- Sockol, M. D., Raichlen, D. A., & Pontzer, H. (2007). Chimpanzee locomotor energetics and the origin of human bipedalism. Proceedings of the National Academy of Sciences, 104(30), 12265–12269. 10.1073/pnas.0703267104 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sponheimer, M., Alemseged, Z., Cerling, T. E., Grine, F. E., Kimbel, W. H., Leakey, M. G., … Wynn, J. G. (2013). Isotopic evidence of early hominin diets. Proceedings of the National Academy of Sciences, 110, 10513–10518. 10.1073/pnas.1222579110 [ DOI ] [ Google Scholar ]
- Stanford, C. (2018). The new chimpanzee: A twenty-first-century portrait of our closest kin. Harvard University Press. [ Google Scholar ]
- Stanford, C. B. (1996). The hunting ecology of wild chimpanzees: implications for the evolutionary ecology of Pliocene hominids. American Anthropologist, 98, 96–113. [ Google Scholar ]
- Stanford, C. B., Wallis, J., Mpongo, E., & Goodall, J. (1994). Hunting decisions in wild chimpanzees. Behaviour, 131(1–2), 1–20. [ Google Scholar ]
- Stanton, M. A., Lonsdorf, E. V., Pusey, A. E., Goodall, J., & Murray, C. M. (2014). Maternal behavior by birth order in wild chimpanzees ( Pan troglodyes ). Current Anthropology, 55(4), 483–489. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Surbeck, M., & Hohmann, G. (2008). Primate hunting by bonobos at LuiKotale, Salonga National Park. Current Biology, 18(19), R906–R907. [ DOI ] [ PubMed ] [ Google Scholar ]
- Surbeck, M., Langergraber, K. E., Fruth, B. L., & Hohmann, G. (2017). Male reproductive skew is higher in bonobos than chimpanzees. Current Biology, 27(13), R640–R641. 10.1016/j.cub.2017.05.039 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Teleki, G. (1973). The predatory behavior of chimpanzees. Bucknell University Press. [ Google Scholar ]
- Teleki, G. (1974). Chimpanzee subsistence technology: Materials and skills. Journal of Human Evolution, 3, 575–594. [ Google Scholar ]
- Tokuyama, N., & Furuichi, T. (2016). Do friends help each other? Patterns of female coalition formation in wild bonobos at Wamba. Animal Behaviour, 119, 27–35. 10.1016/j.anbehav.2016.06.021 [ DOI ] [ Google Scholar ]
- Tourkakis, C. A. (2009). Savanna chimpanzees ( Pan troglodytes verus ) as a referential model for the evolution of habitual bipedalism in hominids . Master's thesis, Iowa State University. https://lib.dr.iastate.edu/etd/10584/ [ Google Scholar ]
- Tutin, C. E. G., & McGinnis, P. R. (1981). Chimpanzee reproduction in the wild. In Graham C. E. (Ed.) Reproductive biology of the great apes (pp. 239–264). Academic Press. [ Google Scholar ]
- Tuttle, R. H. (1969). Knuckle-walking and the problem of human origins. Science, 166, 953–961. [ DOI ] [ PubMed ] [ Google Scholar ]
- van Lawick-Goodall, J. (1968a). The behaviour of free-living chimpanzees in the Gombe Stream Reserve. Animal Behaviour Monographs, 1(3), 161–311. 10.1016/S0066-1856(68)80003-2 [ DOI ] [ Google Scholar ]
- van Lawick-Goodall, J. (1968b). A preliminary report on expressive movements and communication in the Gombe Stream chimpanzees. In Dolhinow P. (Ed.) Primate patterns (pp. 25–84). Holt, Rinehart and Winston. [ Google Scholar ]
- van Lawick-Goodall, J. (1971). In the shadow of man. Houghton Mifflin Company. [ Google Scholar ]
- Videan, E. N., & McGrew, W. C. (2001) Are bonobos ( Pan paniscus ) really more bipedal than chimpanzees ( Pan troglodytes )? American Journal of Primatology, 54(4), 233–239. 10.1002/ajp.1033 [ DOI ] [ PubMed ] [ Google Scholar ]
- von Frisch, K. (1967). The dance language and orientation of bees. Harvard University Press. [ Google Scholar ]
- Walker, K., Walker, C., Goodall, J., & Pusey, A. E. (2018). Maturation is prolonged and variable in female chimpanzees. Journal of Human Evolution, 114, 131–140. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Walpole, S. C, Prieto-Merino, D., Edwards, P., Cleland, J., Stevens, G., & Roberts, I. (2012). The weight of nations: An estimation of adult human biomass. BMC Public Health, 12, 439. 10.1186/1471-2458-12-439 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- White, T. D., Lovejoy, C. O., Asfaw, B., Carlson, J. P., & Suwa, G. (2012). Neither chimpanzee nor human, Ardipithecus reveals the surprising ancestry of both. Proceedings of the National Academy of Science, 112(16), 4877–4884. 10.1073/pnas.1403659111 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Whiten, A., Goodall, J., McGrew, W. C., Nishida, T., Reynolds, V., Sugiyama, Y., … Boesch, C. (1999). Chimpanzee cultures. Nature, 399, 682–685. [ DOI ] [ PubMed ] [ Google Scholar ]
- Williams, J. M., Lonsdorf, E. V., Wilson, M. L., Schumacher-Stankey, J., Goodall, J., & Pusey, A. E. (2008). Causes of death in the Kasekela chimpanzees of Gombe National Park, Tanzania. American Journal of Primatology, 70(8), 766–777. [ DOI ] [ PubMed ] [ Google Scholar ]
- Wilson, E. O. (1962). Chemical communication among workers of the fire ant Solenopsis saevissima (Fr. Smith). 3. The experimental induction of social responses. Animal Behaviour, 10, 159–164. 10.1016/0003-3472(62)90143-4 [ DOI ] [ Google Scholar ]
- Wilson, M. L. (2012). Long-term studies of the Gombe chimpanzees. In Kappeler P. & Watts D. P. (Eds.) Long-term field studies of primates (pp. 357–384). Springer. [ Google Scholar ]
- Wilson, M. L., Boesch, C., Fruth, B., Furuichi, T., Gilby, I. C., Hashimoto, C., … Wrangham, R. W. (2014). Lethal aggression in Pan is better explained by adaptive strategies than human impacts. Nature, 513, 414–417. 10.1038/nature13727 [ DOI ] [ PubMed ] [ Google Scholar ]
- Wilson, M. L., & Glowacki, L. (2017). Violent cousins: Chimpanzees, humans and the roots of war. In Muller M. N., Wrangham R. W., & Pilbeam D. R. (Eds.) Chimpanzees and human evolution (pp. 464–508). Harvard University Press. [ Google Scholar ]
- Wilson, M. L., Lonsdorf, E. V., Mjungu, D. C., Kamenya, S., Kimaro, E. W., Collins, D. A., … Goodall, J. (2020). Research and conservation in the Greater Gombe Ecosystem: Challenges and opportunities. Biological Conservation, 252, 108853. 10.1016/j.biocon.2020.108853 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wrangham, R. W. (1975). The behavioural ecology of chimpanzees in Gombe National Park, Tanzania. Doctoral dissertation, Cambridge University. [ PubMed ] [ Google Scholar ]
- Wrangham, R. W. (1977). Feeding behaviour of chimpanzees in Gombe National Park, Tanzania. In Clutton-Brock T. H., Primate ecology (pp. 503–538). Academic Press. [ Google Scholar ]
- Wrangham, R. W. (1979). On the evolution of ape social systems. Social Science Information, 18(3), 336–368. 10.1177/053901847901800301 [ DOI ] [ Google Scholar ]
- Wrangham, R. W. (1980). Bipedal locomotion as a feeding adaptation in Gelada baboons, and its implications for hominid evolution. Journal of Human Evolution, 9, 329–331. [ Google Scholar ]
- Wrangham, R. W. (1987). The significance of African apes for reconstructing human social evolution. In Kinzey W. G. (Ed.) Evolution of human behavior: Primate models (pp. 51–71). State University of New York Press. [ Google Scholar ]
- Wrangham, R. W. (2001). Out of the Pan, into the fire: From ape to human. In de Waal F. B. M. (Ed.) Tree of origin (pp. 119–143). Harvard University Press. [ Google Scholar ]
- Wrangham, R. W. (2011). Catching fire: How cooking, made us human. Basic Books [ Google Scholar ]
- Wrangham, R. W. (2019). The Goodness paradox: The strange relationship between virtue and violence in human evolution. Penguin Random House. [ Google Scholar ]
- Wrangham, R. W. (1999). Evolution of coalitionary killing. Yearbook of Physical Anthropology, 42, 1–30. [ DOI ] [ PubMed ] [ Google Scholar ]
- Wrangham, R. W., Cheney, D., Seyfarth, R., & Sarmiento, E. (2009). Shallow-water habitats as sources of fallback foods for hominins. American Journal of Physical Anthropology, 140, 630–642. 10.1002/ajpa.21122 [ DOI ] [ PubMed ] [ Google Scholar ]
- Wrangham, R. W., & Peterson, D. (1996). Demonic males: Apes and the origins of human violence. Houghton Mifflin. [ Google Scholar ]
- Wright, S. (1932). The roles of mutation, inbreeding, crossbreeding and selection in evolution. Proceedings of the Sixth International Congress of Genetics, 1, 356–366. [ Google Scholar ]
- Wroblewski, E. E., Murray, C. M., Keele, B. F., Schumacher-Stankey, J., Hahn, B. H., & Pusey, A. E. (2009). Male dominance rank and reproductive success in chimpanzees, Pan troglodytes schweinfurthii . Animal Behaviour, 77(4), 873–885. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zihlman, A. L. (1978). Women in evolution, Part II: Subsistence and social organization among early hominids. Signs: Journal of Women in Culture and Society, 4(1), 4–20. [ Google Scholar ]
- Zihlman, A. L., Sarich, V. M., Cronin, J. E., & Cramer, D. L. (1978). Pygmy chimpanzee as a possible prototype for the common ancestor of humans, chimpanzees and gorillas. Nature, 275(5682), 744–746. [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (961.5 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM

Add to Collections
- ORIGINAL SCIENTIFIC ARTICLE
- Open access
- Published: 29 July 2010
Human Origins Studies: A Historical Perspective
- Tom Gundling 1
Evolution: Education and Outreach volume 3 , pages 314–321 ( 2010 ) Cite this article
34k Accesses
1 Citations
2 Altmetric
Metrics details
Research into the deep history of the human species is a relatively young science which can be divided into two broad periods. The first spans the century between the publication of Darwin’s Origin and the end of World War II. This period is characterized by the recovery of the first non-modern human fossils and subsequent attempts at reconstructing family trees as visual representations of the transition from ape to human. The second period, from 1945 to the present, is marked by a dramatic upsurge in the quantity of research, with a concomitant increase in specialization. During this time, emphasis shifted from classification of fossil humans to paleoecology in which hominids were seen as parts of complex evolving ecosystems. This shift is in no small part due to the incorporation of neo-Darwinian synthetic theory. Finally, technological innovation and changes in social context are considered as influences on human origins studies.
Introduction
Considering the grand sweep of history, the realization that human beings gradually evolved from some non-human ancestor represents a very recent insight. Even so, the goal of this one brief essay cannot be to provide an in-depth description and analysis of every significant development within the field of paleoanthropology, but rather to identify broad patterns and highlight a collection of “events” that are most germane in shaping current understanding of our evolutionary origin. These events naturally include the accretion of fossil material, the raw data which is the direct, if mute, testimony of the past. These fossil discoveries are situated among technological breakthroughs, theoretical shifts, and changes in the sociocultural context in which human origins studies were conducted. It is only through such a contextualized historical approach that we can truly grasp our current understanding of human origins. Foibles of the past remind us to be critical in assessing newly produced knowledge, yet simultaneously we can genuinely appreciate the enormous strides that have been made.
In addition to selective coverage, a second caveat is that this review will focus on research by scientists writing in English. Non-modern hominids Footnote 1 are a cosmopolitan bunch, having been discovered throughout Africa, Asia, and Europe, and there is a significant literature in other languages. In an effort to ameliorate both of these shortcomings, numerous secondary references are included in the bibliography, providing more in-depth information on specific topics. For example, some texts approach the history of paleoanthropology by detailing a single time period (Bowler 1986 ), early human species (Walker and Shipman 1996 ), or researcher (Morell 1995 ), and there are quite a few that consider the subject more comprehensively (Leakey and Goodall 1969 ; Reader 1988 ; Lewin 1997 ; Tattersall 2008 ). In addition, there are a handful of encyclopedia format tomes (Jones et al. 1994 ; Spencer 1997 ; Delson et al. 2000 ), textbooks (Conroy 2005 ; Cela-Conde and Ayala 2007 ; Klein 2009 ), and “coffee table” popular volumes (Stringer and Andrews 2005 ; Johanson and Edgar 2006 ) that in part address the history of human origins studies. Moreover, these texts contain abundant references to the primary literature if that level of scrutiny is desired.
In seeking to provide a useful heuristic framework for the purposes of this particular essay, human origins studies can be broken down into two very broad periods. The first is roughly the century between 1850 and 1950 when research, often conducted by individuals with training outside of anthropology, focused on taxonomy and phylogeny. In other words, although scientists were cognizant that climate change (e.g., northern hemisphere glaciations) would have directly impacted the evolution of early humans, they were mainly interested in collecting “missing links,” naming them, and creating family trees. The second period, from 1950 to the present, is characterized by the relatively rapid development of paleoanthropology as it is currently practiced. Here the emphasis is only partly on the hominids themselves, with ecological context being of equal importance.
The Emergence of Human Origins Studies
This review begins with two mid-nineteenth century developments which are often conflated, but were initially distinct. The first is the acceptance of a temporal association of human material culture (stone tools), with extinct Ice Age mammals (Van Riper 1993 ; Sommer 2007 ). This was significant in that it opened up a considerable prehistory for the human species, well beyond estimates derived from literal scriptural interpretation. However, while acknowledging a lengthy antiquity for the human species, there was, at the time, no reason to suspect that the makers of the stone tools were not fully modern humans in a biological sense. The second major development was the publication of Darwin’s On the Origin of Species in 1859 (Darwin 1859 ). Darwin is rightfully credited with being the most influential, although by no means the first individual to broach the subject of descent with modification, or transmutation theory, as he put it (for an overview of pre-evolutionary ideas related to human origins, see Greene 1959 , Bowler 2003 ). Darwin’s central thesis was that all living species shared a common ancestry, with “endless forms most beautiful” having diverged via natural selection, and although he only briefly mentioned his own species the inference was clear. These two events dovetailed into the now quotidian, but then controversial, notion that humans had evolved over a vast expanse of time (Grayson 1983 ).
While Darwin was initially reticent to discuss human evolution in any detail, his colleague Thomas H. Huxley harbored no such reluctance when he published Man’s Place in Nature: Essays in 1863 (Huxley 1900 ). Darwin freely admitted that the veracity of his audacious proposal would have to withstand paleontological scrutiny and that his theory would collapse in the absence of transitional fossil forms. Huxley’s advantage, beyond his more outspoken personality, was that he actually had a fossil human to describe. The first Neandertal recognized by science was discovered in 1856; however, its description only appeared in English three years later, just as Darwin was going to press (Trinkaus and Shipman 1993 ). Huxley provided a detailed description of the eponymous cranium coupled with carefully composed line drawings (Huxley 1900 ). However, while the importance of the Neandertals in providing empirical evidence documenting an ancient and morphologically distinct human form cannot be discounted, these people hardly bridged the gap separating humans and the great apes. Although a few dissenting voices denied the close evolutionary relationship among humans and the “man-like” apes, and consequently an ape phase of human ancestry, most scientists accepted the overwhelming morphological and embryological evidence in support of just such a relationship. This acceptance was in no small part due to Huxley’s meticulous comparison of gorilla and human anatomy in which he concluded that the gorilla and its close relation, the chimpanzee, represented the nearest approach to humanity in nature.
If Neandertals were more or less human, then more distant, primitive “missing links” remained to be discovered. Just such fossils were recovered on the island of Java in the 1890s by Dutch physician Eugene Dubois, who had traveled to Indonesia as part of the army but with the express purpose of finding the remains of primitive humans (Shipman 2001 ). Java Man consisted of a skull cap, a femur, and a few isolated teeth which taken in combination suggested an early human with a much smaller cranial capacity relative to Neandertals or Homo sapiens (roughly 1,000 vs. 1,500 cubic centimeters), although the femur appeared modern. Dubois did not receive the universal accolades and acceptance he coveted, but his fossils bolstered the conventional wisdom at the time that humans first evolved somewhere in Asia.
During the early twentieth century, the early hominid fossil record grew significantly, if not exponentially, and evolution was widely accepted in scientific circles even while large segments of the lay public remained skeptical. Certainly, there were disagreements over whether natural selection was a sufficient evolutionary mechanism in itself (Bowler 1983 ), but the basic premise of biological change through time was affirmed. The recovery of additional Neandertal remains in Europe refuted lingering claims of pathology regarding the original Neander Valley specimen and solidified the interpretation that the latter was representative of a population of archaic humans occupying Ice Age Europe. Some Neandertal remains were interpreted as not only indicating intentional internment but also associated funerary ritual. The European fossil record was extended significantly with the recovery of a robust lower jaw from Mauer, Germany discovered in 1907.
In 1912 in England, heretofore devoid of non-modern hominid remains despite the prominence of several British scholars in human origins studies, the announcement of hominid fossils from Piltdown was warmly received locally, if with some incredulity abroad. Piltdown was significant since it reified the “brain first” hypothesis, in which primitive humans evolved a large brain before other key human traits evolved. Although a favorite of intelligent design creationism advocates, Piltdown is actually a beautiful example of the scientific method at work, whereby new evidence eventually calls into question prior interpretation, and in this case recognition of intentional fraud (Spencer 1990 ). It was, after all, a new relative dating method measuring the fluorine content of fossils that in 1953 exposed the non-contemporaneity of the jaw and skull. In any case, in the first decades of the twentieth century, a fairly simple human family tree was beginning to emerge (see McCown and Kennedy 1972 and especially Delisle 2007 for exceptions). Relatively small-brained Pithecanthropus led to the more capacious Neandertals and Piltdown, who in turn evolved into modern H. sapiens . Yet the truly ape-like human ancestors remained elusive.
Africa as the Cradle of Humanity
In 1921 a skull bearing superficial resemblance to European Neandertals was recovered as part of mining operations at a place called Broken Hill in Northern Rhodesia (now Zambia). Rhodesian Man marks the recovery of the first in a very long line of non-modern hominids from the African continent. A mere four years later, University of Witwatersrand anatomist Raymond Dart, Australian by birth and having been trained in England, published a brief paper describing the fossil skull of a juvenile “ape” discovered in a limestone quarry near Taung, South Africa. Dart identified certain features of the face, the teeth, the cranium, and the brain of Australopithecus africanus that foreshadowed those of H. sapiens and made the startling claim that what was essentially a bipedal ape signaled the beginning of the human lineage separate from the African great apes.
Initially, with only the one individual, and a juvenile at that, Dart found little support. His most ardent advocate, Scottish physician and paleontologist Robert Broom discovered additional fragmentary remains of the australopithecines, as they were then called, in other South African caves in the 1930s, but these were initially insufficient to sway opinion (Dart 1959 ). This was perhaps due to the near-simultaneous discovery of significant hominid remains from Zhoukoutien (Dragon Bone Hill) in China which quickly eclipsed whatever controversy the diminutive skull from Taung elicited, and despite Broom’s ongoing efforts. As was the case with Java Man, the more complete Chinese fossils fulfilled the expectations of many scientists who anticipated that earliest human ancestors evolved to the East. Comparative analysis of the Javanese and Chinese fossils revealed a great deal of similarity, and all of the fossils were ultimately subsumed in the species Homo erectus .
The Neo-Darwinian Synthesis and the New Physical Anthropology
For several disparate reasons, the decades following the end of World War II (WWII) rather quickly led to a science of paleoanthropology that is recognizably modern. One significant factor relevant in the U.S. if not everywhere, was the dramatic upsurge in enrollment at colleges and universities. The G.I. Bill and subsequent effects of Civil Rights legislation that greatly increased access to higher education meant that millions more students went to college and hence the expansion of existing campuses and programs and in some cases the appearance of entirely new colleges and universities Footnote 2 . As a result, greater numbers of faculty were required who could teach courses and supervise research in diverse academic programs, which in turn led to an attendant rise in the numbers of graduate students themselves who went on to secure positions at institutions of higher learning. Consequently, many disciplines experienced significant increases in research activity, including physical anthropology, and it is worth noting that this was the first generation of researchers whose formal training was in physical anthropology, not in some allied field such as anatomy or medicine. The dramatic rise in practitioners not only increased the knowledge base in terms of simple quantity, but specialization within the field also began to emerge.
A second crucial development that transformed human evolutionary studies was theoretical in nature. Changing ideas regarding the process of evolution had been fermenting and roiling in biology circles for several decades before they infiltrated the study of human origins. In essence, a consensus was reached among biologists ( sensu lato ) that Darwinian natural selection acting on variation arising from random mutation was a sufficient mechanism to explain evolutionary change. For anthropologists, although questions of taxonomy and phylogeny remained important, the intellectual fallout of the so-called neo-Darwinian synthesis led to the “New Physical Anthropology” in which early hominid fossils, rather than representative of some platonic archetype, were interpreted as unique members of variable populations. Focusing on evolution as a process effecting change in populations over time, in contrast to the comparatively myopic sorting of the resulting pattern , arguably represents the most significant theoretical shift in thinking about evolution since Darwin.
Given the comparative de-emphasis on iconic types, the bloated alpha taxonomy of the past was reduced to a mere handful of hominid species displaying previously under-appreciated within species variability. This great reduction in hominid names and consequent simplification of hominid family trees has led some modern scholars to lament what they see as a return to the bad old days of teleology and orthogenesis. Yet there can be little doubt that the “splitting” taxonomic philosophy of the past where almost every new specimen received a new species or quite frequently a new genus name was in dire need of revision.
Just as species types came under scrutiny, so did the concept of evolutionary grades which had up to this point made clear distinctions between the categories of ape and human. While this may have provided some welcome taxonomic clarity, it was artificial in that it ignored the evolutionary reality that at some point members of the human lineage were very ape-like. This realization, obvious in retrospect, led to the widespread acceptance of the South African australopithecines as human ancestors, and the important corollary that bipedalism preceded other distinctive human attributes (Gundling 2005 ).
In addition to increased research activity and theoretical shifts, by the early 1960s technological innovations for the first time permitted the creation of a reliable absolute timescale of human evolution. Comparative protein analysis demonstrated that the African apes were most similar genetically to H. sapiens , inferring their recent common ancestry to the exclusion of other apes and monkeys. Molecular clocks based upon mutation rates and calibrated by the fossil record suggested that this common ancestor lived as little as a few million years ago, although recent estimates put this ancestor at seven to five million years ago. Consequently, known early and middle Miocene ape species became suspect as purported human ancestors, since they preceded the split between the hominid and great ape lineages. Most notably this eventually led to the downfall of Ramapithecus , a Miocene ape genus once widely hailed as a very ancient and very primitive hominid Lewin ( 1997 ).
While molecular studies of living species effectively imposed a theoretical maximum on the age of the hominid lineage, the temporal framework of human origins was further clarified with the introduction of the new potassium argon (K-Ar) method of absolute dating. Louis and Mary Leakey had been scouring the fossil-bearing sediments in and around eastern Africa’s Great Rift Valley for decades when Mary discovered the skull of a robust australopithecine at Olduvai Gorge in 1959. Significantly, Zinjanthropus , the genus coined for the new skull, was discovered within sediments near the base of the Pleistocene Epoch. Volcanic minerals from associated strata were dated to approximately 1.75 million years ago using the K–Ar method, nearly double the age estimated from using other more crude means. This greatly expanded time range certainly bolstered claims for the australopithecines as human ancestors rather than extinct collateral cousins to the “true” human lineage, yet to be discovered.
As an aside, Louis Leakey’s interest in understanding the human past was not limited to the collection of fossils. Sherwood Washburn, a main architect of the new physical anthropology, along with Irven DeVore, conducted pioneering studies of savanna baboons, large-bodied, terrestrial, and highly social primates that served as living proxies for modeling early hominid behavioral ecology (Washburn and DeVore 1961 ). Leakey, on the other hand, took a more phylogenetically based approach and hired scholars to conduct research into the behavior of the great apes as a potential new data source informing hypotheses of early hominid behavior. Jane Goodall was the first, studying chimpanzee behavior at Gombe in Tanzania, then came Dianne Fossey who undertook a longitudinal study of mountain gorillas in Rwanda, and finally Birute Galdikas traveled to Indonesia to conduct field studies of the orangutan (see Kinzey 1987 and De Waal 2001 for more recent primate studies that explicitly address questions of human behavioral evolution).
The emergence of paleoanthropology as a truly multidisciplinary endeavor, concerned with a more holistic picture of our evolutionary past, was a logical extension of the post-WWII new physical anthropology which eschewed simple classification and promoted variable populations as the unit of study. Naturally, these hominid populations did not exist in a vacuum but were components of complex, evolving ecosystems. Hence, field work began to emphasize the collection of greater contextual data in an effort to reconstruct biological and physical environments in which these human ancestors existed and evolved. One of the first field projects to adopt this new approach was an international expedition centered around the Omo River Valley in southern Ethiopia, beginning in 1967. Remarkably, of the 50 papers collected in the resulting volume, only five primarily focus on the hominid remains themselves (Coppens et al. 1976 ).
Early Human Diet and Subsistence
One major aspect of early hominid ecology that occupied researchers engaged in such multidisciplinary efforts was subsistence, which has understandably been of great interest to paleoanthropologists, particularly after 1950 as scientists endeavored to contextualize the fossil remains of distant ancestors. What early humans ate, how food was acquired and processed, even how it was distributed among members of a social group, became viable questions. Throughout the 1950s and 1960s it was widely assumed that the social, cognitive, and technological skills associated with big-game hunting drove the evolution of the human species; in fact the allure of “Man the Hunter” is longstanding in Western thought (Cartmill 1993 ). Raymond Dart, as part of his second foray into human origins studies, proposed that Australopithecus had already developed a hunting strategy facilitated by a technology comprised of durable animal parts that he referred to as the osteodontokeratic (bone, tooth, horn) culture. This concept was enthusiastically embraced by writer Robert Ardrey, who published a series of four popular novels documenting the success of these “killer apes” in the context of a changing environment (e.g., Ardrey 1976 ). Research scientists were only slightly less enthusiastic in championing such ideas (Lee and DeVore 1968 ) which remain popular, if more nuanced today (Wrangham and Peterson 1996 ).
Mirroring changes in the broader society, by the early 1970s some anthropologists challenged the “Man the Hunter” hypothesis and developed an alternative that focused on the central role of women in child rearing and gathering of food resources (Dahlberg 1981 ). These studies used ethnographic data from extant food-foraging societies, the rarity of which injected a sense of urgency on the part of anthropologists. Not long after the “Women the Gatherer” model appeared as a second wave feminist rejoinder to the previously unquestioned authority of “Man the Hunter,” another group of researchers also began to question the big-game hunting scenario. Archeologists, geologists, and paleontologists began working on “site formation processes” to get a better understanding of how assemblages of fragmented animal bones and stone tools came to be commingled. Over the next few decades, often with recourse to modern ecosystems as analogs, one of the main conclusions drawn from the new science called taphonomy (=laws of burial) was the potential importance of scavenging. The association of “bones and stones” was no longer assumed to be the signature of hominid big-game hunting but instead interpreted as meals containing essential fat and protein scavenged by early humans. Perhaps even more disconcerting, some sites were reinterpreted as the remains of carnivore kills occasionally including early humans themselves (Brain 1981 ; Hart and Sussman 2008 ).
Here’s Lucy
If Mary and Louis Leakey’s discoveries at Olduvai put the Great Rift Valley on the map, during the 1970s eastern Africa was validated as the center of early hominid studies. The Leakey’s son Richard established himself on the east side of Lake Turkana in northern Kenya, where his expeditions uncovered a prolific cache of early hominid fossils, some of which corroborated the occasionally controversial claims made by his parents a decade earlier. Sediments around the lake yielded hominid fossils of robust australopithecines, early members of genus Homo , and an early African variant of Asian H. erectus , these days referred to as Homo ergaster (Leakey and Lewin 1978 ). The latter includes a mostly complete skeleton, KNM-WT15000, which has become iconic for the species (Walker and Shipman 1996 ).
Arguably the most significant fossil discovery of the 1970s was another partial skeleton, AL-288, from Hadar, Ethiopia, better known as Lucy (Johanson and Edey 1981 ). Here was a single individual represented by numerous skeletal elements, and although her morphology was generally similar to the “gracile” australopithecines of South Africa, she was even more primitive in some respects. Consequently her discoverers coined a new species name, Australopithecus afarensis that included not only the Hadar specimens but fossils collected by Mary Leakey’s expedition at Laetoli in Tanzania. The latter is renowned for its famous footprint trail preserved in solidified volcanic ash, imparting convincing evidence for bipedalism at 3.6 million years ago. Hadar is also replete with datable volcanic sediments, and Lucy’s status as the most primitive hominid was reinforced by firm radiometric dates which placed the fossils at greater than 3.0 million years ago, at the time astonishingly ancient.
One other significant event from the 1970s bears mentioning. Although the American Journal of Physical Anthropology was first published in 1918, it is perhaps surprising that a journal explicitly dedicated to the study of human evolution did not appear in the U.S. until 1972. Since then the Journal of Human Evolution has been the premier academic forum for publications related to human evolution, and in 1992, the Paleoanthropology Society was established, which organizes its own conference and publishes an online journal.
Modern Human Origins
The question of modern human origins has been debated for centuries, long predating paleoanthropology as a scientific discipline. One of the central issues, which became particularly evident as Renaissance and Enlightenment Europeans began to travel the globe on a regular basis, was how to explain the physical diversity of human populations. Two broad perspectives emerged, one which viewed all people as having a single origin and another which believed that supposedly distinct races had separate origins. The pre-Darwinian debates between so-called monogenists and polygenists were recast with the advent of an evolutionary paradigm in the mid-nineteenth century. Within this new theoretical context, monogenists believed that all living humans evolved from a common ancestor that was already H. sapiens , while the polygenists believed that the races had deeper roots and had descended from different non-modern ancestors (e.g., H. erectus or in a few instances different ape species). A major step towards resolving this debate came in 1987 with an analysis of living human mitochondrial DNA diversity which concluded that H. sapiens had a recent African origin. The discovery of essentially modern human fossils at the 160,000-year-old site of Herto, Ehtiopia, provides paleontological support for a recent African origin, and many subsequent genetic studies have supported this basic conclusion. However, the possibility of some gene flow between migrating early modern humans and local archaic populations remains plausible (compare Stringer and McKie 1996 and Wolpoff and Caspari 1998 , also see Relethford 2003 for a geneticist’s perspective).
Conclusion: Twenty-First Century Paleoanthropology
New fossil discoveries, technological innovations, theoretical advances, and social transformations will continue to inform knowledge of our deep past. Recovery of hominid fossils, some from previously unknown time periods and geographic locations, continues at a brisk rate. Many of the most significant recent discoveries are beginning to fill in the crucial African late Miocene time period during which our lineage ramified from that leading to the chimpanzee (Gibbons 2006 ). Of particular note, one of these fossils was discovered in Chad, quite a distance from established sites in the Great Rift Valley, challenging the long standing hypothesis that hominids evolved in the savanna grasslands of eastern Africa while the African ape ancestors remained sequestered in their tropical rainforest refugium. Moreover, botanical, faunal, and geological evidence associated with very early fossil hominids in Ethiopia and Kenya intimate a forested environment, a discovery that clearly constrains hypotheses explaining the success of the bipedal adaptation.
Other significant fossil discoveries from the early Pleistocene site of Dmanisi in the Republic of Georgia have energized discussion of the initial expansion of early humans beyond the tropics of Africa (Wong 2006 ). Not only are these fossils considerably older than prior known Eurasian specimens, but they are morphologically primitive, especially in terms of stature and cranial capacity, and are associated with very simple (“mode 1”) lithic technology. These early migrants hardly manifest the tall striding bipeds equipped with comparatively advanced Acheulian bifacial tools so often depicted in earlier “out of Africa” scenarios Footnote 3 , which are at least in part based on the iconic WT15000 skeleton mentioned earlier.
Perhaps the most surprising discovery of the last decade is the diminutive 18,000-year-old skeleton from the Indonesian island of Flores, which has sparked a spirited, occasionally acrimonious debate between those advocates of a replacement model of modern human origins and those inclined towards regional continuity (Morwood and van Oosterzee 2007 ). The former, comprised of the team who made the discovery and their allies, interpret the remains as those of a surprisingly primitive hominid akin to early Homo , and perhaps the first documented example of the effects of island dwarfing on an early human population. Other scholars believe the remains to be those of a pathological modern human, whose illness resulted in a cascade of skeletal and dental anomalies. Ongoing research on Flores and other nearby locations will undoubtedly resolve this debate.
New discoveries are not limited to the paleontological record but also include behavioral information gleaned from archaeology. Symbolic expression in the form of language, art (including music), and religion is undoubtedly one of the most distinctive human traits. Evidence for such behavior has proved elusive beyond the seeming cultural explosion perceived in the Upper Paleolithic of Europe beginning around 35,000 years before present. However, archeological evidence for at least some of these behaviors has recently been coaxed out of several sites in sub-Saharan Africa. Advanced utilitarian objects such as blades and harpoons have been recovered well back into the Middle Stone Age and use of ochre and shells for body adornment has been found at sites approaching 100 kiloannum (Balter 2009 ).
Recent advances also include a plethora of technological innovations that have allowed anthropologists to hone traditional inquiries in the areas of dating (e.g., single crystal, laser fusion, argon–argon dating), systematic analysis (e.g., geometric morphometrics), and paleoenvironmental reconstruction (e.g., stable isotope analysis). The badly distorted remains of the spectacular 4.4 megaanum skeleton of Ardipithecus ramidus from Aramis, Ethiopia was restored in part using digital imaging technology (Gibbons 2009 ). Additionally, new technology is facilitating, perhaps even driving, novel questions such as those related to the emergence of the unique human life history pattern.
While fossils provide real-time evidence for human evolution, signals from our ancient past are also encoded into our modern DNA. The groundbreaking work of the 1960s effectively demonstrated our close affinity with the African great apes, and today’s genomic analyses comparing humans and chimpanzees are beginning to reveal differences in much finer detail than heretofore possible. Already several areas within the human genome have been identified as having undergone intense selection; these regions may be related to the evolution of the especially dexterous human thumb, reduction of muscles of mastication in the wake of the ability to cook food, the greatly enlarged neo-cortex, and our ability for spoken language.
In addition to modern DNA analyses, ancient DNA analysis has informed the “Neandertal problem” providing preliminary evidence in support of the replacement hypothesis, at least in Europe, whereby modern humans arriving there equipped with Upper Paleolithic technology drove the indigenous Neandertals to extinction. Even more recent genomic analyses, however, suggest that a small but detectable degree of interbreeding occurred when expanding modern human populations emerging from the African tropics encountered Neandertal populations in the Middle East around 120,000 years before present (Gibbons 2010 ).
In conclusion, our understanding of human origins, like all scientific knowledge, is the result of an ongoing, iterative process. Over the last few decades, the accelerating pace of fossil discoveries and the incorporation of innovative technologies have corroborated and enhanced much of what we already suspected to be true, although there have been a few surprises. No doubt this pattern will continue into the foreseeable future as we slowly, yet inexorably, piece together the circumstances by which our lineage became human.
Hominidae (=hominid) is the biological group (clade) to which humans and their extinct ancestors belong. For many current scholars, this group is distinguished at some lower taxonomic level, usually the tribe Hominini (=hominin). In this study, I maintain the traditional use of Hominidae simply to be consistent with the historical literature. For the same reason, I use the subfamily designation Australopithecinae (=australopithecine) for all of the African “bipedal apes.” This group is certainly paraphyletic, to use the modern jargon, and as a result an increasing number of scholars prefer to use the less formal term australopith to lump together the various African species.
According to the U.S. Census Bureau, there were about 2.2 million students enrolled in U.S. colleges in 1950. That number doubled by 1963 to just less than 4.4 million and doubled again to over 9 million by 1972.
I usually avoid this term despite its heavy usage within the scientific community. I believe that the “Out of Africa” trope perpetuates an anti-Africa bias which seems to suggest that early humans, on several occasions, left Africa wholesale as if there was something inherently undesirable about the place. I suppose that “hominid extra-tropical range expansion” doesn’t have the same ring, but it is more accurate.
Ardrey R. The hunting hypothesis. New York: Atheneum; 1976.
Google Scholar
Balter M. On the origin of art and symbolism. Science. 2009;323:709–11.
Article CAS PubMed Google Scholar
Bowler PJ. The eclipse of darwinism. Anti-darwinian evolution theories in the decades around 1900. Baltimore: The Johns Hopkins University Press; 1983.
Bowler PJ. Theories of human evolution: a century of debate 1844–1944. Baltimore: The Johns Hopkins University Press; 1986.
Bowler PJ. Evolution: the history of an idea. 3rd ed. Berkeley: University of California Press; 2003.
Brain CK. The hunters or the hunted? An introduction to African cave taphonomy. Chicago: University of Chicago Press; 1981.
Cartmill M. A view to a death in the morning: hunting and nature through history. Cambridge: Harvard University Press; 1993.
Cela-Conde CJ, Ayala FJ. Human evolution: trails from the past. Oxford: Oxford University Press; 2007.
Conroy GC. Reconstructing human origins. 2nd ed. New York: W.W. Norton; 2005.
Coppens Y, Howell FC, Isaac GL, Leakey REF. Earliest Man and environments in the lake rudolf basin: stratigraphy, paleoecology and evolution. University of Chicago Press; 1976.
Dahlberg F, editor. Woman the gatherer. New Haven: Yale University Press; 1981.
Dart RA. Adventures with the missing link. Britain: Hamish Hamilton, Ltd.; 1959.
Darwin CR. On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. London: John Murray; 1859.
Book Google Scholar
De Waal FB, editor. Tree of origin: what primate behavior can tell us about human social evolution. Cambridge: Harvard University Press; 2001.
Delisle RG. Debating humankind’s place in nature 1860–2000: the nature of paleoanthropology. Upper Saddle River: Pearson Education, Inc.; 2007.
Delson E, Tattersall I, Van Couvering JA, Brooks AS. Encyclopedia of human evolution and prehistory. 2nd ed. New York: Garland Publishing, Inc.; 2000.
Gibbons A. The first human: the race to discover our earliest ancestors. New York: Doubleday; 2006.
Gibbons A. Breakthrough of the year: Ardipithecus ramidus . Science. 2009;326:1598–9.
Gibbons A. Close encounters of the prehistoric kind. Science. 2010;328:680–4.
Grayson DK. The establishment of human antiquity. New York: Academic; 1983.
Greene JC. The death of Adam: evolution and its impact on western thought. Ames: Iowa State University Press; 1959.
Gundling T. First in line: tracing our ape ancestry. New Haven: Yale University Press; 2005.
Hart D, Sussman RW. Man the hunted: primates, predators, and human evolution. Expandedth ed. Boulder: Westview Press; 2008.
Huxley TH. Man’s place in nature: essays. New York: D. Appleton & Co.; 1900 [1863]
Johanson D, Edey M. Lucy: the beginnings of humankind. New York: Simon and Schuster; 1981.
Johanson DJ, Edgar B. From Lucy to language. Revised, updated, and expanded. New York: Simon & Schuster; 2006.
Jones S, Martin R, Pilbeam D, editors. The cambridge encyclopedia of human evolution. Cambridge: Cambridge University Press; 1994.
Kinzey WG, editor. The evolution of human behavior: primate models. Albany: SUNY Press; 1987.
Klein RG. The human career: human biological and cultural origins. 3rd ed. Chicago: University of Chicago Press; 2009.
Leakey LSB, Goodall VM. Unveiling man’s origins. Cambridge: Schenkman Publ. Co.; 1969.
Leakey RE, Lewin R. People of the lake: mankind and its beginnings. New York: Doubleday; 1978.
Lee RB, DeVore I, editors. Man the hunter. Chicago: Aldine Publishing Co.; 1968.
Lewin RL. Bones of contention: controversies in the search for human origins. 2nd ed. Chicago: University of Chicago Press; 1997.
McCown TD, Kennedy KAR, editors. Climbing man’s family tree: a collection of major writings on human phylogeny, 1699 to 1971. Englewood Cliffs: Prentice Hall; 1972.
Morell V. Ancestral passions: the Leakey family and the quest for humankind’s beginnings. New York: Simon & Schuster; 1995.
Morwood MJ, van Oosterzee P. A new human: the startling discovery and the strange story of the “Hobbits” of Flores, Indonesia. New York: Harper Collins; 2007.
Reader J. Missing Links: the hunt for earliest man. 2nd ed. London: Pelican Books; 1988.
Relethford JH. Reflections of our past: how human history is revealed in our genes. Boulder: Westview Press; 2003.
Shipman P. The man who found the missing link: Eugene Dubois and his lifelong quest to prove Darwin right. New York: Simon and Schuster; 2001.
Sommer M. Bones and ochre: the curious afterlife of the red lady of paviland. Cambridge: Harvard University Press; 2007.
Spencer F. Piltdown: a scientific forgery. London: British Museum (Natural History); 1990.
Spencer F, editor. History of physical anthropology: an encyclopedia. New York: Garland Publishing, Inc.; 1997.
Stringer C, Andrews P. The complete world of human evolution. London: Thames and Hudson; 2005.
Stringer C, McKie R. African exodus: the origins of modern humanity. New York: Henry Holt; 1996.
Tattersall I. The fossil trail: how we know what we think we know about human evolution. 2nd ed. Oxford: Oxford University Press; 2008.
Trinkaus E, Shipman P. The Neandertals: changing the image of mankind. New York: Alfred A. Knopf; 1993.
Van Riper AB. Men among mammoths: victorian science and the discovery of human prehistory. Chicago: Chicago University Press; 1993.
Walker A, Shipman P. The wisdom of the bones: in search of human origins. New York: Alfred A. Knopf; 1996.
Washburn SL, DeVore I. The social life of baboons. Sci. Am. June; 1961
Wolpoff M, Caspari R. Race and human evolution. Boulder: Westview Press; 1998 [1997]
Wong K. Stranger in a new land. Sci Am. 2006;16(2):38–47.
Article Google Scholar
Wrangham R, Peterson D. Demonic males: apes and the origins of human violence. New York: Houghton Mifflin; 1996.
Download references
Author information
Authors and affiliations.
Department of Anthropology, William Paterson University, 300 Pompton Road, Wayne, NJ, 07470, USA
Tom Gundling
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Tom Gundling .
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Reprints and permissions
About this article
Cite this article.
Gundling, T. Human Origins Studies: A Historical Perspective. Evo Edu Outreach 3 , 314–321 (2010). https://doi.org/10.1007/s12052-010-0248-7
Download citation
Published : 29 July 2010
Issue Date : September 2010
DOI : https://doi.org/10.1007/s12052-010-0248-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Paleoanthropology
- History of science
- Human evolution
Evolution: Education and Outreach
ISSN: 1936-6434
- Submission enquiries: [email protected]

IMAGES
VIDEO