How to conduct a literature review
The purpose of literature reviews and how to execute them.

Agencja Fotograficzna Caro / Alamy Stock Photo
In recent decades, the ‘evidence-based medicine’ movement has become widely accepted and, consequently, decisions regarding what is best practice are informed by the best available evidence [1] . However, an ever-increasing quantity of evidence published in literature has meant that healthcare providers, researchers, and policy makers are inundated with unmanageable amounts of information, which can hinder, rather than inform, rational decision making [2] . It is therefore vital that healthcare professionals, including pharmacists, appreciate the importance of evaluating the available evidence when attempting to answer a clinical question [3] . A literature review can therefore be considered “the comprehensive study and interpretation of literature that relates to a particular topic” [4] .
When conducted correctly, a literature review can be viewed as more than simply a cursory overview of the literature on a given topic. Indeed, they are often viewed as a piece of research in their own right. A historical example of the value of literature reviews in informing evidence-based practice is that of streptokinase in the treatment of myocardial infarction (MI). In the 1970s, 33 separate clinical trials comparing streptokinase with a placebo for the treatment of MI had been conducted and their results published. Individually, these trials provided inconclusive evidence regarding the role of streptokinase. However, a re-analysis of their combined results clearly demonstrated the beneficial effect of streptokinase. As a result the medicine became part of the standard treatment following MI, thereby transforming care and saving lives [2] .

Narrative versus systematic literature reviews
Literature reviews can be broadly divided into narrative (descriptive) reviews and systematic reviews [5] . The most important difference between the two categories of reviews is the difference in their level of associated scientific rigour. Narrative literature reviews provide an overview of the literature, typically from the perspective of an ‘expert in the field’ [1] . Such reviews often report findings in a format that includes a brief summary of each included study. While such reviews can be informative, particularly when there has been no previous attempt to assimilate the literature in a given area, narrative reviews are often criticised as having a high risk of various types of bias associated with them [6] . Narrative reviews typically rely on subjective and non-systematic methods of selecting and reviewing data. Consequently, authors may intentionally or unintentionally cite only those literature sources that reinforce the author’s preconceived hypotheses or promote their own views on a topic [7] .
In contrast to a narrative review, a systematic literature review aims to identify all the available evidence on a topic and appraise the quality of that evidence [4] . Therefore, such reviews have the ability to either answer the research question or, failing that, identify gaps in the existing literature which highlight the need for further high quality research into a specific area [8] . Established in 1993, the Cochrane collaboration is an international organisation that produces systematic reviews, often regarded as the gold standard of evidence, concerning the effectiveness of healthcare interventions.
The purpose of a literature review
Literature reviews may be conducted for a variety of reasons, for example, to form part of a research proposal or introductory section in an academic paper, or to inform evidence-based practice [8] . The original focus of evidence-based medicine concerned mainly clinical research questions, such as the streptokinase example. However, increasingly, more research is being conducted in the area of health services research, which incorporates social science perspectives with the contribution of individuals and institutions that deliver care. Pharmacy practice research (a sub-type of health services research) is concerned with investigating how and why people access pharmacy services, new roles for pharmacists, and outcomes for patients as a result of pharmacy services [9] . Whether conducting either a clinical pharmacy or a pharmacy practice literature review, the following basic principles outlined in this article should be followed.
Searching with a strategy
While time and resource constraints may not allow for a definitive systematic review to be conducted, those attempting to undertake a literature review should nevertheless adopt a ‘systematic approach’ [10] . The first step in any literature review is to define the research question (i.e. the scope of the review). This will inform which studies or other forms of evidence are to be included and excluded from the literature review.
Questions that should be asked at this stage include:
- Which study designs are relevant to the review? For example, double-blind randomised controlled trials, observational studies or qualitative research.
- Is there a particular population of interest? For example, paediatrics, pregnant women or people living in care homes.
- Are there particular outcomes of interest? For example, mortality, costs or quality of life.
The literature search strategy is a fundamental component of any literature review, because errors in the search process could produce an incomplete and therefore biased evidence base for the review. Where possible, reviewers should consult with a pharmacy or healthcare librarian who can assist in developing and refining an appropriate search strategy [11] . The search should be conducted in more than one electronic database to help ensure all relevant papers are identified. The main medical literature databases are Medline and Embase. International Pharmaceutical Abstracts (IPA) is also an important database to consider because it indexes many pharmacy-specific journals not found in the larger databases. It should also be remembered that pharmacy literature often overlaps with other disciplines therefore consideration should be given to databases such as the Cumulative Index of Nursing and Allied Health (CINAHL), as well as condition-specific databases, for example PsycInfo.
When conducting searches on electronic databases, thought must be given to the search terms used. Several keywords can exist for each search term and all should be included in the search (e.g. older adult/elderly/aged, are all terms that could be used synonymously). Most databases also have the facility to identify all possible endings of the key terms; this is usually denoted with an asterisk. For example, by searching for ‘pharm*’, articles containing any of the following would be retrieved: pharmacist, pharmacists, pharmacy, pharmaceutical. Boolean operators (e.g. ‘and’, ‘or’, ‘not’) can also be used to combine search terms to refine the search results [12] .
Avoiding publication bias
It has been well documented that studies that report significant results are more likely than those reporting non-significant results to be published, cited by others and produce multiple publications, introducing what is known as ‘publication bias’. Consequently, such studies are also more likely to be identified and included in systematic reviews [13] .
For this reason, efforts should be made to identify all relevant literature on the review topic so the search should not be limited solely to electronic databases [14] . Additional search strategies include hand-checking relevant article reference lists and personal communication with experts in the field. Searching the ‘grey literature’ is of particular importance for pharmacy practice literature reviews because relevant articles written by non-academic pharmacists are often not published in traditional academic journals [15] .
The importance of such additional search strategies cannot be underestimated. Greenhalgh and Peacock reported that, in a literature review concerning innovations in healthcare organisations, 51% of the sources included in the eventual review were identified by hand-searching reference lists, while an additional 24% were identified through personal communications [7] . The search strategy, including databases searched, all search terms used, the limits of the search (for example, written in the English language, published in the last decade), the number of results retrieved from each resource and the date the search was conducted should be carefully documented. Although it can never be guaranteed that the entirety of relevant literature will be identified, conducting the search in a systematic manner will help avoid omissions, and where they do exist they can be said to be unintentional [9] .
Once the search has been performed, the next step is to screen the titles or abstracts (or both) of the identified articles against the predefined inclusion and exclusion criteria. If the article cannot be excluded on the basis of the information contained in the abstract, efforts must be made to access the full paper in order to reach a decision. Conducting a literature review can be a time-consuming process and the time taken to complete is directly related to number of citations identified by the initial search. Allen et al . [16] calculated that it would take a reviewer more than 1,000 hours to complete a literature review involving the screening of 2,500 articles. For this reason, it is worth considering the use of free citation management software, such as Refworks, Mendeley and Endnote, which can be used to manage references retrieved from the literature search.
Drawing conclusions
Studies identified as part of a literature review may report contradictory findings and the quality of the individual studies will have a direct impact on the overall findings of the literature review. When assimilating the evidence identified, the reviewer should attempt to assess the quality of the evidence provided by the individual studies to arrive at valid conclusions [6] . It is recommended that when conducting a literature review, the author should make a judgement of the quality of the evidence provided within the included studies or articles using appropriate assessment guidelines [17] . For example, the Cochrane Collaboration’s risk-of-bias tool attempts to aid quality assessment in terms of assessing potential sources of bias in studies.
In an effort to draw quantitative conclusions within a systematic review, data from individual studies may be pooled quantitatively and reanalysed. A meta-analysis is a statistical method used to combine the outcomes of individual studies to produce data with more power than the individual studies. By pooling the results of individual studies, the sample size is effectively increased, thereby increasing the statistical power of the analysis, which in turns narrows the confidence interval around the effect size, with the result that the overall estimate of effect is more robust [9] . Individual studies included in the analysis are assigned weights, with greater weight assigned to studies with larger sample sizes. The results of a meta-analysis are often displayed graphically in what is known as a “forest plot” [18] .
Publishing your literature review
Targeting an appropriate journal.
- Ensure the scope of your review aligns with the scope of the journal. Familiarise yourself with the journal’s mission statement and the typical content of the journal.
- Be aware of the journal’s target audience in relation to the scope of the review (e.g. does the journal have a pharmacy-specific or multidisciplinary audience? Does the journal have a national or international readership?)
- Consider whether the journal offers open-access publishing. Increasingly, funding bodies stipulate that articles are published only in open-access journals.
- Look at the journal’s impact factor. While articles published in journals with higher impact factors tend to get cited more, high-impact journals have lower article acceptance rates, making it potentially more challenging to get your review published.
Submitting the manuscript
- Familiarise yourself with the journal’s ‘guidelines for authors’. These will dictate the accepted word limit of a review in addition to other formatting and submission stipulations surrounding tables, figures and references, etc.
- Write a cover letter, addressed to the editor of the journal, to accompany your manuscript submission. The cover letter should highlight why the review is important and why you think it is a good fit for the chosen journal.
Reading this article counts towards your CPD
You can use the following forms to record your learning and action points from this article from Pharmaceutical Journal Publications.
Your CPD module results are stored against your account here at The Pharmaceutical Journal . You must be registered and logged into the site to do this. To review your module results, go to the ‘My Account’ tab and then ‘My CPD’.
Any training, learning or development activities that you undertake for CPD can also be recorded as evidence as part of your RPS Faculty practice-based portfolio when preparing for Faculty membership. To start your RPS Faculty journey today, access the portfolio and tools at www.rpharms.com/Faculty
If your learning was planned in advance, please click:
If your learning was spontaneous, please click:
[1] Akobeng AK. Understanding systematic reviews and meta-analysis. Arch Dis Child 2005;90:845–848. doi: 10.1136/adc.2004.058230
[2] Mulrow CD. Systematic Reviews: Rationale for systematic reviews. The BMJ 1994;309:597. doi: 10.1136/bmj.309.6954.597
[3] Guyatt G, Cairns J, Churchill D et al . Evidence-based medicine: a new approach to teaching the practice of medicine. JAMA 1992;268:2420–2425. doi: 10.1001/jama.268.17.2420
[4] Aveyard H. Doing a literature review in health and social care: a practical guide. 3rd Edn. Open University Press 2014. doi: 10.7748/ns.29.27.30.s33
[5] Grant MJ & Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J 2009;26:91–108. doi: 10.1111/j.1471-1842.2009.00848.x
[6] Green BN. Writing narrative literature reviews for peer-reviewed journals: secrets of the trade. J Chiropr Med 2006;5:101–117. doi: 10.1016/S0899-3467(07)60142-6
[7] Greenhalgh T & Peacock R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: audit of primary sources. The BMJ . 2005;331:1064–1065. doi: 10.1136/bmj.38636.593461.68
[8] Jesson J & Lacey F. How to do (or not to do) a critical literature review. Pharmacy Education 2006;6:139–148. doi: 10.1080/15602210600616218
[9] Babar Z. Pharmacy Practice Research Methods: Adis/Springer International Publishing, Switzerland, 2015.
[10] McKee M & Britton A. Conducting a literature review on the effectiveness of health care interventions. Health Policy Plan 1997;12:262–267. doi: 10.1093/heapol/12.3.262
[11] McGowan J & Sampson M. Systematic reviews need systematic searchers. J Med Libr Assoc 2005;93:74–80.
[12] Cronin P, Ryan F & Coughlan M. Undertaking a literature review: a step-by-step approach. BJN 2008;17:38–43. doi: 10.12968/bjon.2008.17.1.28 059
[13] Sterne J, Egger M & Smith GD. Investigating and dealing with publication and other biases in meta-analysis. The BMJ 2001;323:101. doi: 10.1136/bmj.323.7304.101
[14] Crumley ET, Wiebe N, Cramer K et al . Which resources should be used to identify RCT/CCTs for systematic reviews: a systematic review. BMC Med Res Methodol 2005;5:24. doi: 10.1186/1471-2288-5-24
[15] Charrois T, Durec T & Tsuyuki R. Systematic reviews of pharmacy practice research: methodologic issues in searching, evaluating, interpreting, and disseminating results. Ann Pharmacother 2009;43:118–122. doi: 10.1345/aph.1l302
[16] Allen E & Olkin I. Estimating time to conduct a meta-analysis from number of citations retrieved. JAMA 1999;282:634–635. PMID: 10517715
[17] Jüni P, Altman DG & Egger M. Assessing the quality of controlled clinical trials. The BMJ 2001;323:42–46. doi: 10.1136/bmj.323.7303.42
[18] Lewis S & Clarke M. Forest plots: trying to see the wood and the trees. The BMJ 2001;322:1479–1480. doi: 10.1136/bmj.322.7300.1479
You might also be interested in…

Case-based learning: social prescribing and pharmacy professionals

Government introduces ‘emergency ban’ on overseas prescriptions for puberty blockers
Our experience as pharmacists in ‘SPACE’
- University of Michigan Library
- Research Guides
Pharmacy and Pharmacology
- Literature Reviews
- Drug Information
- Point of Care Resources
- Finding & Using Data & Statistics
- Patient Safety
- Citation Management (EndNote, Mendeley, Zotero) This link opens in a new window
- Mobile Resources
- Creating a Well-Focused Question
- Searching PubMed
- PubMed - Article Abstract Page
- Searching Other Databases - Embase
- Searching Other Databases - International Pharmaceutical Abstracts
- Academic/Professional Integrity at UM
- Research Data Management This link opens in a new window
- NIH Public Access Policy
- Research Funding & Grants This link opens in a new window
Writing literature reviews - an article from Nature
Tips from 8 authors.
- How to Write a Superb Literature Review
What is a Literature Review?
A literature review surveys scholarly articles, books, dissertations, conference proceedings & other resources that are relevant to a particular issue, area of research, or theory & provides context for a dissertation by identifying past researchon a topic.
Research tells a story, & the existing literature helps us identify where we are in the story currently. It is up to those working on a research project to continue that story with new research and new perspectives, but they must first be familiar with the story before they can move forward.
Purpose of a Literature Review
A literature review:
- Helps you to discover the research that has been conducted on a topic already & identifies gaps in current knowledge
- Increases the breadth of your knowledge in your area of research
- Helps you identify seminal works in your area
- Allows you to provide the intellectual context for your work & position your research with other, related research
- Provides you with opposing viewpoints
- Helps you to discover research methods that may be applicable to your work
Greenfield, T. (2002). Research methods for postgraduates. 2nd ed. London: Arnold.
While there are many specific types of literature reviews, you will be writing a critical literature review to demonstrate your knowledge of a specific area & that your project is viable.
To read more about the different types of reviews, read this article, which provides a good overview: Maria J. Grant & Andrew Booth. "A typology of reviews: an analysis of 14 review types and associated methodologies". Health Information and Libraries Journal (26):91–108. DOI: 10.1111/j.1471-1842.2009.00848.x
The Process of Writing a Literature Review
Frequently asked questions.
What kinds of literature are appropriate for my research question? This will depend on your area of research, but in the health sciences, you will most often rely on scholarly journal articles, patents, conference proceedings, & data sets.
How much literature should I use? There is no standard answer to this question, but make sure that you have enough literature to tell your story. Discuss this question with your advisor & peers.
How will I find all appropriate information to inform my research? You should consult multiple databases & resources appropriate for your research area so that you can have a comprehensive view of the research that has already been done in your area. Browse the Pharmacy research guide for databases & other recommended resources. Also consult with your informationist at the Taubman Health Sciences Library to determine the resources you should investigate.
How will I evaluate the literature to include trustworthy information and eliminate unnecessary or untrustworthy information? Start with scholarly sources, such as peer-reviewed journal articles & books. Always pay attention to creditability of the source(s) & the author(s) you cite. Citation analysis (see research guides http://guides.lib.umich.edu/citeanalysis & http://guides.lib.umich.edu/citation ) can be useful to check the creditability of sources & authors.
How should I organize my literature? What citation management program is best for me? Citation management software, such as Mendeley, a free citation management program, helps you collect & organize references & easily insert citations & format citations & bibliographies in thousands of styles in your Word document.
To choose the program that's right for you, consider which one works best with your literature search & writing process. This guide compares different types of citation management software & provides tutorials for each type. You may also ask your library informationist for advice.
How do I ensure academic integrity (i.e., avoid plagiarism)? Familiarize yourself with different types of intentional and unintentional plagiarism and learn about the University's standards for academic integrity here. Remember, citation management tools can help you avoid unintentional plagiarism by making it easy to collect & cite sources.
What Kind of Literature Should You Choose?
Different types of information sources may be critical for particular disciplines. Please contact your library informationist for additional guidance on information sources appropriate to your research. In addition to books, reference resources, journal articles, & datasets, these sources may be helpful.
Government documents
The U.S. Government Printing Office produces a great deal of information that is useful to researchers. Congress, the Supreme Court, the Office of the President & federal agencies are rich sources of policy information, legislation, & historical records. The University of Michigan's Clark Library is a federal depository library. Librarians there can help you find documents and records created by the federal government, as well as state & local laws & legislation. International government information can be found in United Nations documents , available in print & online since 1946. Also check the Grey Literature guide for more resources.
Statistics reported by government or private sources can be useful, but can also be difficult to find. Use the Health Statistics research guide for more information on how to search & for sources.
Grey Literature
Theses & Dissertations : Dissertations on topics similar to yours may contain information & technical details not published in other forms. You may also be inspired by how others approach similar topics.
Conference proceedings : For many fields, researchers present their most up-to-date research results at professional conferences. These results will later be published in conference proceedings, abstracts, or preprints.
Other unpublished information : For all of the above and resources, including clinical & pharmaceutical research, FDA reports, & more, visit the Grey Literature research guide .
Related Research Guides
- Grey Literature Resources & strategies for searching for information not contained in databases.
- Finding Tests & Measurement Instruments Resources, tips, & tricks for finding tests & measurement instruments in the health & social sciences.
- Bioinformatics Resources on the interdisciplinary science that uses information technology to solve molecular biology problems.
- Research Impact Assessment (Health Sciences) Explore methods and tools for assessing your research impact, including citation tracking and altmetrics.
Writing Help
- Graduate Students - Sweetland Center for Writing One-on-one assistance & workshops, all free.
- Graduate Student Writing Clinic

Pharmacy research project guidance: Doing your literature review
- Project Management
- Ethical Approval
- Doing a systematic literature search
- Evaluating your sources
- Doing your literature review
- Citing references
- Using EndNote
- File and Data Management
- Your Lab/Log Book
- Reflective Writing
- Supervisory Expectations
- Frequently Asked Questions
Introduction
All projects will include a literature review:
- In a lab-based project the review may just be part of the introduction helping to outline the state of the knowledge and gap you are trying to address.
- For literature-based projects this will be the bulk of your discussion, although the way your report is structured will depend on the type of review you are doing. If you are doing a systematic review you will need to follow a specific protocol for writing it up. See ' Doing a systematic literature search ' for guidance and links.
Getting started
- Video tutorial on doing a literature review
A literature review sets up your project and positions it in relation to the background research. It also provides evidence you can refer back to later to help interpret your own results. When getting started on your literature review, it helps to know what role this plays in your overall project.
A literature review:
- Provides the background / context to your topic
- Demonstrates familiarity with previous research
- Positions your study in relation to the research
- Provides evidence that may help explain your findings later
- Highlights any gaps in the research
- Identifies your research question/s
In your literature review you should include:
- Background to the topic (e.g. general considerations, mechanisms of formation, analytical techniques, etc…)
- Why it is important (e.g. food with improve flavour, less carcinogens, more taste, less processed foods, new probiotics ......... & etc.)
- What research has been performed and what has been found out
- The specific area you are interested in (e.g. cheese, snacks, fruits, ….)
- Current ideas and hypotheses in this area
- The key research questions which remain

It can seem difficult to know where to start with your literature review, but to a certain extent it doesn’t matter where you start…as long as you do!
If you like understanding the bigger picture and seeing the whole of an idea before getting into the detail – try starting with a general text and then using the bibliography of this to find more specific journal articles.
If you like to start small with one idea or study, find a relevant journal article or single study and then build up by trying to find related studies and also contrasting studies.
Further help
For more on this view the video tutorial on the other tab in this box, or take a look at these study guides:
- Starting a literature review
- Undertaking a literature review
If you are unable to view this video on YouTube it is also available on YuJa - view the Doing your literature review video on YuJa (University username and password required)
Read the script for the video (PDF)
Note-taking
- Tips on note-taking
- Video tutorial on critical note taking
A key to a good literature review, is having a good system for recording and keeping track of what you are reading. Good notes means you will have done a lot of the thinking, synthesising, and interpreting of the literature before you come to write it up and it will hopefully make the writing process that bit smoother. Systematic note-taking will also ensure you have all the details you need to write your references and won’t accidentally plagiarise.
Have a format for recording your notes that suits you – whether this is in a table, bullet points, spider diagrams, using a programme like Evernote, or in a traditional notebook!
Tables can be a useful way of recording notes for a literature review as it enables you to compare and contrast studies side-by-side in the table. It also forces you to write a concise summary or it won’t fit into the table!
e.g. A suggested outline for a note-making table
Have a system for distinguishing quotations and your own words – you don’t want to accidentally include something only to discover it was someone else’s words and you may have plagiarised by mistake. Always make sure your quotation marks are clear in your notes (it is easy to miss them in a hurry) and it really helps to record the page number of any direct quotation so you can go back to check easily.
Avoid the temptation to copy out text – copying out large chunks of text is slow and also means you tend not to process and understand what you copy. Summarising and writing short phrases instead means you are likely to have a better understanding and will remember it and be able to use your notes more easily later.
Summarise – writing a short summary or overview of what you have just read helps you to clarify their argument and position. It also means you have a handy short reminder when you come back to it later – you don’t want to be re-reading notes that are as long as the original text in the first place!
Always record the full bibliographical details – it only takes a few moments to write down everything you need for your reference. You may think it is fine to leave it as you will be able to find these details later…but you probably won’t and you will waste time searching for them when your deadline is fast approaching.
A top tip if you find it hard to put things in your own words – try reading a longer section of the text before taking notes. It is very difficult to paraphrase something line-by-line as you go along, because everything seems important and it is too easy to just lift the phrases the author has used. Reading a longer section will give you a better overview and fuller understanding, meaning you can choose what is important and relevant to your own project.
For more on this watch the video tutorial on the other tab in this box, or take a look at these study guides:
- Managing academic reading
- Effective note-taking study guide This guide produced by the Study Advice Team gives tips on note-taking and outlines some different approaches.
If you are unable to view this video on YouTube it is also available on YuJa - view the Critical note taking video on YuJa (University username and password required)
Referencing and avoiding plagiarism
- Managing references
- Video tutorial on avoiding unintentional plagiarism
It is a good idea to keep your references up to date as you write so that you know exactly where each idea comes from (and it will save a tedious job at the end ).
Make sure you reference every idea that comes from another source, which includes things like images, diagrams, and statistics, not just word-for-word quotations.
Use the referencing style detailed in the 'Referencing' page in this guide and stick to it consistently! Don’t switch between styles or formats. It may seem petty, but meticulously formatted referencing shows you have taken care in your work and have a professional academic approach (and it will get you marks!). You could consider using a reference management tool, such as EndNote Online, for storing your references and inserting them into your report (see the 'Referencing' page ) - this will be essential if you are doing a literature-based project or a systematic review.
A top tip is to have a proof-read through for referencing only – print out your literature review as it is easier to spot mistakes on paper than on screen.
Referencing checklist
- Is every idea from another source referenced?
- Does every word-for-word quotation have quote marks and is referenced?
- Are all paraphrases in your own words (not just changing a few words) and referenced?
- Does every in-text reference match a full reference in the bibliography?
- Are all names and titles in the references spelled correctly?
- Have you followed the department’s preferred referencing style consistently?
For more on this watch the video tutorial on the other tab in this box.
For detailed help on citing references see the Referencing page in this guide:
- Referencing guidance for the Pharmacy research project
If you are unable to view this video on YouTube it is also available on YuJa - view the Avoiding Unintentional Plagiarism video on YuJa (University username and password required)
Structuring your review
A literature review compares and contrasts the research that has been done on a topic. It isn’t a chronological account of how the research has developed in the field nor is it a summary of each source in turn like a ‘book review’. Instead a literature review explores the key themes or concepts in the literature and compares what different research has found about each theme.
Use sub-headings to structure your literature review as this helps you group the different studies to compare and contrast them and avoids a straight chronological narrative.
To help find your sub-headings:
- Brainstorm all the different concepts or themes in the research that relate to your topic or title
- Identify the ones that are important to your research question – think of what the reader needs to know about to understand the different aspects of your project
- Place the themes in an order that would make sense to your reader – usually going from broad themes to themes more directly related to your project (see funnel diagram in Getting started)
- Turn these into sub-headings
- Use these sub-headings as an outline plan for your literature review – what will come under each sub-heading
Below is an example structure of a literature review that starts broad and starts to narrow by linking the concepts that are specific to this project:
For more on this see the following study guide:
Writing the literature review
When writing a literature review, you want to be comparing and contrasting the studies to build up a picture of what the research says about your topic.
This means you should be using comparative and evaluative language more than descriptive language:
For more examples of the kinds of comparative and evaluative language used in literature reviews see:
- Academic Phrasebank Use this site for examples of linking phrases and ways to refer to sources.
Be selective
Also you want to be selective in how you refer to the literature . In a literature review, you don’t have to refer to each study in the same depth. Think of the points you want to make and then include just enough detail about the study to provide evidence for this. For example, you don’t have to analyse the strengths and weaknesses of the methodology for each study in depth, you only need to do this if you are making a point which relates to the methodology or a point about the findings which depends on the methods being robust and valid (e.g. the authors claim there are wide-spread applications of their trials, but they have used a very small sample size, which suggests they can’t make such a bold assertion).
For example - the summary below maps out the state of current research and the positions taken by the key researchers. A significant amount of reading and in-depth understanding of the field has gone in to being able to summarise the research in these few sentences.
Sometimes you need to go into greater depth and refer to some sources in more detail in order to interrogate the methods and stand points expressed by these researchers. Even in this more analytical piece of writing, only the relevant points of the study and the theory are mentioned briefly - but you need a confident and thorough understanding to refer to them so concisely.
For example:
See the following study guide for more on this:
- Developing your literature review
Returning to your literature review - link to the discussion
Once you have written your literature review, its job doesn’t end there. The literature review sets up the ideas and concepts that you can draw upon later to help interpret your own findings.
Do your own findings confirm or contradict the previous research? And why might this be?
If your literature review funnels down from broad to narrow, you can think of your discussion like the other half of the hour-glass, broadening out to the wider applications of your project at the end:

So although you may draft your literature review as one of your first steps, you will probably come back to it towards the end of your project to redraft it to help fit in with your discussion. You may need to emphasise some studies that didn’t initially seem that important, but which are now more useful because of what you have found in your own experiments.
This is an example of the thinking that might go on behind interpreting a result and linking it to the previous literature:
- << Previous: Evaluating your sources
- Next: Citing references >>
- Last Updated: Jun 4, 2024 2:51 PM
- URL: https://libguides.reading.ac.uk/pharmacy-research-project-guide
- Open access
- Published: 11 May 2022
Systematic literature review of pharmacists in general practice in supporting the implementation of shared care agreements in primary care
- Naveed Iqbal ORCID: orcid.org/0000-0003-1807-1424 1 ,
- Chi Huynh ORCID: orcid.org/0000-0001-6982-6642 1 &
- Ian Maidment ORCID: orcid.org/0000-0003-4152-9704 1
Systematic Reviews volume 11 , Article number: 88 ( 2022 ) Cite this article
4871 Accesses
4 Citations
8 Altmetric
Metrics details
Rising demand for healthcare continues to impact all sectors of the health service. As a result of the growing ageing population and the burden of chronic disease, healthcare has become more complex, and the need for more efficient management of specialist medication across the healthcare interface is of paramount importance. With the rising number of pharmacists working in primary care in clinical roles, is this a role that pharmacists could support to ensure the successful execution of shared care agreement (SCA) in primary care for these patients?
Aim of the review
Systematic review to identify activities and assess the interventions provided by pharmacists in primary care on SCA provision and how it affects health-related quality of life (HRQoL) for patients.
Primary studies in English which tested the intervention or obtained views of stakeholders related to pharmacist input to shared care agreement within primary care were included. The following electronic databases were systematically searched from the date of inception to November 2021: AMED®, CINAHL®, Cochrane Database of Systematic Reviews (CDSR), EMBASE®, EMCARE®, Google Scholar, HMIC®, MEDLINE®, PsycINFO®, Scopus and Web of Science®. Grey literature sources were also searched. The search was adapted according to the respective database-specific search tools. It was searched using a combination of Medical Subject Heading terms (MeSH), free-text search terms and Boolean operators.
A total of 5244 titles/abstracts were screened after duplicates were removed, and 64 full articles were assessed for eligibility. On examination of full text, no studies met the inclusion criteria for this review.
This review highlights the need for further research to evaluate how pharmacists in general practice can support the safe and effective integration of specialist medication in primary care with the use of SCA.
Systematic review registration
NIHR PROSPERO No: 2020 CRD42020165363 .
Peer Review reports
Introduction
Healthcare systems in the world are facing significant challenges as a result of severe funding pressure, a growing ageing population, societal changes, rising demand and a limited supply of some healthcare professional groups [ 1 , 2 , 3 ]. This is compounded by the increasing prevalence of long-term conditions (LTC), in particular, people having two or more conditions which are being supported by different parts of the healthcare system [ 4 ]. The UK is home to the National Health Service (NHS), one of the largest healthcare systems in the world. In the UK, LTCs account for 70% of the NHS healthcare budget [ 4 ]. With the continued demands in the NHS, the current models of dealing with long-term conditions are not sustainable; the need to innovate in order to continue to deliver world-class healthcare outcomes within a limited financial envelope is critical [ 5 ]. Care needs to be provided in the right place and at the right time to ensure that the healthcare system meets the current and future needs of a nation’s healthcare provision [ 6 ]. In response to this new normal, care must not be fragmented between healthcare systems, e.g. pre-hospital, hospital and specialist care [ 7 ].
This is of particular importance in countries where healthcare systems are well developed where care needs to move seamlessly from the gatekeeping primary care systems to the hospital and specialist services for the benefit of patient care [ 8 ]. Effective communication and cooperation between primary and secondary care are critical to making the best use of limited resources [ 9 ] and to ensure the patient receives high-quality joined-up care [ 8 , 10 , 11 ]. A tool that has been used in the literature to support effective integrated care between primary and secondary care is the shared care agreement (SCA) [ 12 , 13 , 14 ]. The SCA was designed to provide a framework for the seamless sharing of care and to facilitate the passage of hospital-prescribed medication or a consultant-managed patient into primary care.
The term shared care agreement has various definitions, none of which is universally agreed on [ 14 , 15 , 16 , 17 , 18 ]. The primary description to describe shared care was by Hickman et al. in their seminal paper on the taxonomy of shared care in 1994 [ 15 ]. The original definition of shared care described shared care as The joint participation of general practitioners and hospital consultants in the planned delivery of care for patients with chronic inflammatory musculoskeletal disorders, informed by an enhanced information exchange over and above the routine clinic, discharge and referral letters [ 15 ]. A more recent evolved definition describes these arrangements as the joint participation of primary and speciality care practitioners in the planned delivery of care for patients with a chronic condition, informed by enhanced information exchange, over and above routine discharge and referral notices [ 16 ]. This evolved definition takes into account the changes in primary care since the primary definition was described.
Since the introduction of an executive letter EL (91) 127 by the NHS in 1991 described SCA to support prescribing between hospitals and GPs [ 19 ], several problems have marred the integration of SCA into primary care, and in 2017, the British Medical Association (BMA) stated that shared care is still not working effectively [ 20 ]. General practitioners have expressed concern about poor communication between primary and secondary care, lack of follow-up and monitoring and the medico-legal responsibilities for the prescriber when they accept shared care [ 16 , 21 , 22 , 23 ]. After 30 years, EL (91) 127 has been superseded by the new national guidance released in 2018 for England [ 24 ]. The guidance was designed to overcome the challenges of shared care that have been exhibited in the healthcare system over the last 30 years [ 21 ]. The document has described a role for the pharmacist in general practice in supporting joint working and collaboration to ensure that primary care prescribers have access to information on new or less familiar medicines and how they can support the introduction of medicines into primary care [ 21 ]. Evidence indicates that pharmacists have a significant role in medicine optimisation and improving safe and effective medication use in primary care [ 25 ]. In addition, the literature has shown that pharmacists in general practice are increasingly playing a central role in managing medicines [ 26 ], such as setting up systems for safe monitoring and prescribing high-risk medicines such as direct oral anticoagulants, lithium, non-steroidal anti-inflammatory drugs (NSAIDs) prescribing and medicine safety requirement in general practice. With the support of government initiatives to increase the number of pharmacists in general practice to support new models of care, the pharmacist could be seen as a vital component in bridging the transfer of care from secondary to primary care settings [ 4 , 27 , 28 ]. This paper aims to review the literature on the role of a pharmacist in general practice with regard to SCA support, their roles and identify the potential benefits, barriers and facilitators to their potential integral role.
This study aimed to identify activities and assess the interventions provided by pharmacists in primary care on SCA provision. The specific objectives were to determine the following:
The types of interventions/activities being provided by pharmacists in supporting SCA in a general practice setting
The effectiveness of these interventions/activities on health-related quality of life (HRQoL) for patients, to consider the impact of clinical pharmacist supporting shared care agreement in a general practice setting.
Study registration
The review protocol was registered with the International Prospective Register for Systematic Review (PROSPERO database; registration number CRD42020165363). The review was guided by the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions [ 29 ]. The reporting of the review complies with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement and checklist (refer to supplementary information S 2 and S 3 ) [ 30 ].
Eligibility criteria for study inclusion
The search was focused on locating studies eligible for inclusion or excluded based on the criteria below.
Inclusion criteria
The following are the inclusion criteria:
Only primary studies (qualitative, quantitative and mixed studies)
Studies which have tested an intervention and/or have obtained views of stakeholders (pharmacists, GPs, medical specialists, practice staff and patients) related to SCA in the primary care setting
Studies in which a pharmacist has input within a primary care setting to provide non-dispensing care
Exclusion criteria
The following are the exclusion criteria:
Studies in which the intervention has been provided only in secondary or tertiary care settings (hospitals, specialist clinics and national and regional specialist centre)
Studies written in a language other than English
Studies presented as editorials, protocols and commentaries
Information sources and search strategy
Specific search strategies were developed with expert information specialists and included broad and narrow, free-text and thesaurus-based terms. The term pharmacist was used as a general term to allow greater scope to find multiple roles provided by pharmacists, which included prescribers. Boolean operators and truncation were used to ensure we maximised our search strategy. The following eleven databases were systematically searched from date of inception to November 2021: Allied and Complementary Medicine Database (AMED®) (1985 to 07.11.2021) Platform: Ovid®; Cumulative Index to Nursing and Allied Health Literature (CINAHL®) (1950 to 07.11.2021) Platform: EBSCO®; Cochrane Database of Systematic Reviews (CDSR) (accessed on 07.11.2021) Platform: Wiley® online library; Excerpta Medica database (EMBASE®) (1974 to 07.11.2021) Platform: Ovid®; EMCARE® (1995 to 07.11.2021) Platform: Ovid®; Google Scholar (accessed on 07.11.2021) Platform: Google UK®; Healthcare Management Information Consortium (HMIC®) (1979 to November 2021) Platform: Ovid®; Medical Literature Analysis and Retrieval System Online (MEDLINE®) (1946 to 07.11.2021) Platform: Ovid®; PsycINFO®, Psychology and Behavioural Sciences Collection, Health Business Elite, Biomedica Reference Collection: Comprehensive Library, Information Science & Technology Abstracts (1967 to 07.11.2021) Platform: EBSCOhost®; and Scopus (2004 to 07.11.2021) Platform: Elsevier, Web of Science® Core Collection (1970-07.11.2021) Platform: Clarivate Analytics®. The search was adapted according to the respective database-specific search tools. It was searched using a combination of Medical Subject Heading terms (MeSH) where available, free-text search terms and Boolean operators. Refer to supplementary information S 1 ‘Search terms’ for the specific detail of the search used for each database. Search results in languages other than English were noted, but for practical reasons, only search results in English or translated into English were included in this review. In an effort to identify unpublished studies, a search of grey literature was performed ( http://www.opengrey.eu/ on 07.11.2021) to identify studies not indexed in the databases listed above. The term grey literature in this paper refers to sources used to describe a wide range of information produced outside of traditional publishing and distribution channels and which is often not well represented in indexing databases.
Data collection and analysis
All references from database search were downloaded into EndNote® X8.2 [ 31 ] reference manager, which was used to collate and remove duplicate records, to screen titles and abstracts and to store the full text of retrieved studies. Citations from OpenGrey could not be uploaded to EndNote® reference manager and therefore were uploaded to a Microsoft Excel® 2016 spreadsheet. Duplicate citations were removed by the automatic de-duplicating option in EndNote®X8.2 and were supplemented by hand-searching. Two researchers (NI and CH) examined the titles and abstracts of all eligible articles according to the inclusion and exclusion criteria listed above. References to be screened were allocated into groups and was divided into ‘include’, ‘exclude’ and ‘potential’ groupsets. The full-text articles of any abstracts classified as potentially meeting the inclusion criteria were retrieved and analysed independently by two authors (NI and CH) against the predefined inclusion and exclusion criteria with differences between reviewers resolved by consensus. The principal authors of all included papers were contacted to explore the potential for any studies considered vital to them that may have been missed in the search strategy. A data extraction form from the Cochrane collaboration was utilised to extract data from eligible papers [ 32 ]. Raw data from quantitative studies were extracted onto Microsoft Excel® 2016 spreadsheet. If data could not be pooled for meta-analysis, the plan was to undertake a narrative synthesis of results. The qualitative data synthesis methodology was decided upon after the quantity, quality, conceptual richness, and contextual thickness of the qualitative studies were determined. The intended results and data synthesis were to be conducted by authors NI and CH independently to assure the data extraction integrity.
The Mixed Method Appraisal Tool (MMAT) 2018 version was used to appraise and describe the methodological quality of included quantitative, qualitative and mixed-method studies. A pilot test of two articles was conducted to ensure consistent interpretation between the two authors (NI and CH). Discrepancies were resolved through discussion and consensus with the research team. If further information was required to appraise a particular study, an attempt was made to contact the authors by phone or email. Quality scores will be calculated using the MMAT tool. However, this did not solely determine if studies were of “low” or “high” quality, as a descriptive summary using MMAT criteria was considered [ 33 ]. If a study received a low score, it was compared with those with a higher score (higher quality studies) to consider if this modifies the outcome and interpretation of our synthesis.
The database search yielded 7727 citations. After duplicates were removed, the database search identified 3908 citations. Based on the title and abstract information, 3844 citations were ‘excluded’, leaving 64 citations identified as ‘potentially relevant’ articles requiring a full-text article text review. In addition to the 3908 citations that were yielded by the databases, 1336 additional citations were retrieved from http://www.opengrey.eu/ and were screened separately as they could not be uploaded onto EndNote® X8.2 [ 31 ] reference manager. After screening, no pertinent articles that met the inclusion and exclusion criteria were extracted from grey literature sources.
On the examination of the full text of the 64 studies in the ‘potentially relevant’ category, no publications addressing evidence to identify activities and to assess interventions provided by pharmacists in primary care on SCA provision were identified (see Table 1 , which includes a tabulated list of the 64 excluded studies along with reasons for exclusion, with some articles having more than one reason for exclusion). Out of the 64 excluded studies, one study (a conference proceeding) could not be found, and an attempt was made to contact the author to obtain further information (full set of results). The author did not respond; hence, this article was excluded from our review as the reviewers could not assess the eligibility based on the information from the abstract. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow chart (Fig. 1 ) shows the identification, screening and selection of papers for this review. There were no included studies for which to assess the risk of bias or to apply for evidence synthesis.
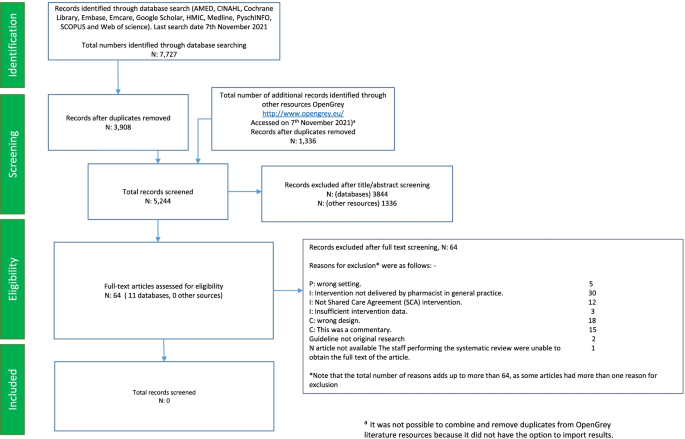
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow chart
This paper aimed to systematically assess the pharmacist’s role in general practice in supporting the implementation of SCA in primary care. No articles met our inclusion criteria. Several studies have tried to define the role of pharmacists in general practice, but the working definition has not been defined by healthcare organisations or the research community [ 98 , 99 ]. Another reason for there to be no studies available is because the GP pharmacist’s role is still a relatively new role in healthcare and little existing literature in role evolution is available [ 100 ].
Comparison with other studies
There is an agreement in the literature that the pharmacist role is developing at pace within the general practice setting and recent international systematic reviews and meta-analyses demonstrate positive effects on medication use and clinical outcomes [ 99 , 101 ]. Pharmacists integrated into general practice teams can perform a variety of roles. This includes direct patient care, medicines reconciliation and education to members of the healthcare team and in the detection and resolution of medication-related problems [ 102 , 103 , 104 ]. A recent review of the impact of integrating pharmacists into primary care teams on health systems indicators on healthcare utilisation by Hayhoe et al. did highlight the activities provided by pharmacists in general practice. Still, it failed to discover activities to support SCA in the published literature [ 105 ]. Internationally, other terminologies and contexts vary, giving one example being collaborative care agreements by Stuhec et al. in Slovenia [ 106 , 107 , 108 , 109 ]. The focus has been aimed in this international context as clinical pharmacists share a role within their own heterogeneous setting and sharing this with various physicians in managing a patient with chronic conditions, not necessarily in the same setting such as GP surgery in the UK where the GP is seen anecdotally as the main gatekeeper and first point of contact for all medication issues related to the patient. The observational studies by Stuhec et al. have focused on older patient and psychiatric patients. Further research would be required to homogenise and formalise an internationally recognised term for shared care agreements.
The most recent Cochrane systematic review, which assessed the effectiveness of shared care across the interface between primary and speciality care in the management of long-term conditions, did not consider models of integrating care between pharmacists and primary care physicians [ 18 ]. The authors of the Cochrane review stated that this limited its generalisability to all types of collaborative care due to the contextual specificity. The review did note that several other models of shared or collaborative care should be considered for review. This systematic review has highlighted that the model of care involving a pharmacist in primary care supporting SCA is not currently present in the literature and should be considered for further investigation.
Implications of the review findings on clinicians and decision-makers in healthcare and future studies
The changing needs of the population make it increasingly important that the patient’s multiple needs are met in a well-coordinated way. To respond effectively to these changing needs, healthcare teams need to utilise the skills available across our healthcare teams. To help alleviate these pressures, it has been recommended that the NHS should maximise the opportunities offered by pharmacists [ 9 , 110 , 111 ]. Pharmacists can take on significant amounts of work currently done by GPs and other staff in general practice [ 111 ]. To explore whether shared care management could be implemented successfully by the pharmacist in general practice, we need to understand the relevant and important pharmacist related barriers and facilitators concerning the implementation of this role. The influence of these factors needs to be recognised and considered and how this can inform further research into this area.
Decision-making
Collaboration between pharmacists and the healthcare team is of paramount importance, and understanding the skills of multiple healthcare professionals will help support an integrated system [ 112 ]. We need to understand the role in which pharmacists will support SCA and how general practitioners are willing to integrate pharmacists within this process. A finding from earlier research has shown that general practice has a positive experience of pharmacist recommendations in a range of conditions [ 113 , 114 , 115 ]. However, how would this apply to SCA when patients and healthcare staff need to understand the role of the pharmacist and agree to the SCA? Would patients accept pharmacists undertaking this new role within the general practice?
Funding and workload
General practice continues to be concerned with the inappropriate funding associated with supporting shared care medication [ 21 ]. Commissioners have always stated that the funding of specialist’s drugs has been agreed so cost should not be an issue. General practitioners have expressed concerns that the additional workload required to support the integration of specialised medication is a factor they believe has not been appreciated [ 116 ]. This has been exacerbated by the COVID-19 pandemic, which has seen a rise in how general practice is expected to manage care which would typically be carried out in a hospital setting which in turn has contributed to a growing workload in primary care [ 117 ]. Previous studies have suggested that the impact of practice-based pharmacists will not be on workload but quality and safety [ 118 ]. It is reasonable to ask whether GPs would support pharmacists taking on this role without appropriate funding and whether this intervention will reduce workload in general practice.
Working definition and liability
We need to consider a working definition of the practice pharmacist role in shared care. The differences in pharmacists’ primary care roles have been identified in the international literature. The lack of clarity and knowledge of this primary care role can negatively affect their potential integration into the primary care team [ 119 ]. The knowledge, skills and attitudes required to support SCA should be made readily available to practice pharmacists, primary care teams and the general public. This would enable SCA management to be developed and applied nationally across primary care. The rapid emergence of new professional roles for pharmacists also means that arrangement in respect of liability needs to keep up with the changing nature of pharmacy practice within this more complex intervention.
This empty review can act as a platform to inform policymakers in healthcare that there is a lack of robust evidence that evaluates the role and potential value of pharmacists supporting SCA in general practice. Healthcare systems must seek out the best possible evidence to support patients within this new healthcare environment. However, the absence of research in this area does not justify the rejection of this intervention [ 120 ]. As the role is relatively new, there is still work to be done to develop the evidence base of pharmacists working in general practice to support SCA and the benefits of this role within the healthcare system.
Limitations
A limitation of this review is that the search strategy included a literature search of articles only in the English language. Other articles may have been published on pharmacists supporting SCA in general practice in non-English journals. Personal commentaries, blogs and opinion pieces were excluded from this systematic review due to the research design. This may have excluded observations that are occurring in general practice but have not been critically appraised. Another limitation is that the term SCA may be used as an alternate term from an international perspective. Despite the use of 12 healthcare-related bibliographical databases and the extensive use of keywords to maximise the sensitivity of relevant studies, after removal of duplicates, this yielded a low number of citations of titles and abstracts, another limitation. This systematic review used database word stock to establish standard search terms; however, it cannot be discounted that this may not have retrieved all articles relating to this intervention. Another limitation that needs to be considered is that abstracts from conference proceedings were not included in the synthesis, which could account for the empty return due to the high evidence bar for the systematic review.
This systematic review identified no eligible studies on the interventions provided by a pharmacist in supporting SCA in general practice. It is not possible to formulate what the role pharmacists can play in supporting SCA in general practice based on scientific evidence. There is an urgent need for studies that identify, observe and evaluate GP-based pharmacists’ roles concerning SCA that currently occur in clinical practice. Comparing and contrasting each general practice’s approach will ensure the development of a consensus for the role of GP pharmacists on SCA based on the current SCAs occurring in general practice, and how to implement this intervention consistently. The role of the pharmacist is expanding in general practice, and interventions which prove beneficial for patients and the healthcare system are required to meet the ever-changing demand in healthcare and to ensure that these new interventions follow the evidence. This empty systematic review serves as a starting point for further clinical research in this area.
Availability of data and materials
We provide all supporting data in the manuscript in Fig. 1 , and Table 1 , Search Strategy S 1 , PRISMA statement (S 2 ) and abstract checklist (S 3 ).
Abbreviations
Shared care agreement
Health-related quality of life
Medical Subject Heading terms
- Long-term conditions
National Health Service
British Medical Association
Preferred Reporting Items for Systematic Review and Meta-Analysis
Mixed Method Appraisal Tool
High-level commission on health employment and economic growth. Working for health and growth: investing in the health workforce. World Health Organization. 2016. http://apps.who.int/iris/bitstream/handle/10665/250047/9789241511308-eng.pdf?sequence=1 . Accessed 03 Aug 2020.
United Nations Department of Economic and Social Affairs, Population Division. World population ageing 2019 (ST/ESA/SER.A/444): United Nations; 2020. https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/files/documents/2020/Jan/un_2019_worldpopulationageing_report.pdf . Accessed 03 Aug 2020
Book Google Scholar
Campbell J, Dussault G, Buchan J, Pozo-Martin F, Guerra Arias M, Leone C, et al. A universal truth: no health without a workforce. Forum Report 2013, Third Global Forum on Human Resources for Health, Recife, Brazil. Geneva: Global Health Workforce Alliance and World Health Organization; 2013. https://www.who.int/workforcealliance/knowledge/resources/GHWA-a_universal_truth_report.pdf?ua=1 . Accessed 10 Aug 2020
Google Scholar
NHS England, Public Health England, Care Quality Commission, Monitor, NHS Trust Development Authority, Health Education England. NHS five year forward view: NHS England; 2014. https://www.england.nhs.uk/ourwork/futurenhs/ . Accessed 10 Aug 2020
Rosen R. Delivering general practice with too few GPs: Nuffield Trust; 2019. https://www.nuffieldtrust.org.uk/files/2019-10/general-practice-without-gps-v2.pdf . Accessed 14 Aug 2020
Jones R, Jones R, Newbold M, Reilly J, Drinkwater R, Stoate H. The future of primary and secondary care. Br J Gen Pract. 2013;63:379–82. https://doi.org/10.3399/bjgp13X669400 .
Article PubMed PubMed Central Google Scholar
Scally G, Jacobson B, Abbasi K. The UK’s public health response to covid-19. BMJ. 2020. https://doi.org/10.1136/bmj.m1932 .
Sampson R, Cooper J, Barbour R, Polson R, Wilson P. Patients’ perspectives on the medical primary-secondary care interface: systematic review and synthesis of qualitative research. BMJ Open. 2015. https://doi.org/10.1136/bmjopen-2015-008708 .
Naylor C, Alderwick H, Honeyman M. Acute hospitals and integrated care: from hospitals to health systems: King’s Fund; 2015. https://www.kingsfund.org.uk/sites/default/files/field/field_publication_file/acute-hospitals-and-integrated-care-march-2015.pdf . Accessed 26 Aug 2020
Cresswell A, Hart M, Suchanek O, Young T, Leaver L, Hibbs S. Mind the gap: improving discharge communication between secondary and primary care. BMJ Qual Improv Rep. 2015. https://doi.org/10.1136/bmjquality.u207936.w3197 .
Epstein RM. Communication between primary care physicians and consultants. Arch Fam Med. 1995;4:403–9.
Article CAS Google Scholar
Drummond N, Abdalla M, Buckingham JK, et al. Integrated care for asthma: a clinical, social, and economic evaluation. Grampian asthma study of integrated care (GRASSIC). BMJ. 1994;308:559–64. https://doi.org/10.1136/bmj.308.6928.559 .
Integrated care for diabetes: a clinical, social and economic evaluation. Diabetes integrated care evaluation team. BMJ. 1994;308:1208–12. https://doi.org/10.1136/bmj.308.6938.1208 .
Midlands Therapeutics Review and Advisory Committee. Independent review of medicines for primary care. http://ccg.centreformedicinesoptimisation.co.uk/mtrac/ . Accessed 03 Aug 2020.
Hickman M, Drummond N, Grimshaw J. A taxonomy of shared care of chronic disease. J Public Health Med. 1994;16:447–54.
Horne R, Mailey E, Frost S, Lea R. Shared care: a qualitative study of GPs’ and hospital doctors’ views on prescribing specialist medicines. Br J Gen Pract. 2001;51:187–93.
CAS PubMed PubMed Central Google Scholar
Specialist Pharmacy Service. RMOC work programme: NHS England; 2020. https://www.sps.nhs.uk/topics/?with=rMOc . Accessed 03 Mar 2020
Smith SM, Cousins G, Clyne B, Allwright S, O’Dowd T. Shared care across the interface between primary and specialty care in management of long term conditions. Cochrane Database Syst Rev. 2017. https://doi.org/10.1002/14651858.CD004910.pub3 .
National Health Service Management Executive. Responsibility for prescribing between hospitals and GPs. London: NHS Management Executive; 1991.
British Medical Association. Primary and secondary care interface guidance. https://www.bma.org.uk/collective-voice/committees/general-practitioners-committee/gpc-current-issues/nhs-england-standard-hospital-contract-guidance-2017-2019/primary-and-secondary-care-interface-guidance . Accessed 03 Mar 2020.
Shared care protocols – have they had their day? Drug Ther Bull. 2017;55:133 https://dtb.bmj.com/content/55/12/133 . Accessed 03 Mar 2020.
Wilcock M, Rohilla A. Three decades of shared care guidelines: are we any further forward? Drug Ther Bull. 2020; https://dtb.bmj.com/content/58/5/67 . Accessed 03 Mar 2020.
Crowe S, Cantrill JA, Tully MP. Shared care arrangements for specialist drugs in the UK: the challenges facing GP adherence. Qual Saf Health Care. 2010. https://doi.org/10.1136/qshc.2009.035857 .
NHS England. Responsibility for prescribing between primary & secondary/tertiary care; 2018. https://www.england.nhs.uk/publication/responsibility-for-prescribing-between-primary-and-secondary-tertiary-care/ . Accessed 03 Aug 2020
Tan EC, Stewart K, Elliott RA, George J. Pharmacist services provided in general practice clinics: a systematic review and meta-analysis. Res Soc Adm Pharm. 2014:608–22. https://doi.org/10.1016/j.sapharm.2013.08.006 .
Wood S. Safer prescribing and monitoring of high-risk medicines. Prescriber. 2020;31(4):10–5.
Article Google Scholar
NHS. The NHS long term plan; 2019. https://www.longtermplan.nhs.uk/ . Accessed 03 Mar 2020
Petty D. Clinical pharmacist roles in primary care networks. Prescriber. 2019;30(11):22–6.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane; 2019. https://training.cochrane.org/cochrane-handbook-systematic-reviews-interventions . Accessed 05 May 2020.
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009. https://doi.org/10.1136/bmj.b2535 .
Clarivate Analytics. EndNote. Version X8.2. Philadelphia: Clarivate Analytics; 2018.
Cochrane Effective Practice and Organisation of Care (EPOC). Data collection form. In: EPOC Resources for review authors: Cochrane; 2017. https://epoc.cochrane.org/resources/epoc-resources-review-authors . Accessed 04 Mar 2020.
Hong QN, Pluye P, Fàbregues S, Bartlett G, Boardman F, Cargo M, et al. Mixed Methods Appraisal Tool (MMAT), version 2018. Registration of Copyright (#1148552), Canadian Intellectual Property Office, Industry Canada. Available at: - http://mixedmethodsappraisaltoolpublic.pbworks.com/w/file/fetch/127916259/MMAT_2018_criteria-manual_2018-08-01_ENG.pdf . Accessed 12 April 2022
Adams B. NHS alliance: The evolution of primary care. Prescriber. 2015;26(3):32–4. Available: - https://wileymicrositebuilder.com/prescriber/wpcontent/uploads/sites/23/2015/12/NHS-Alliance-the-evolution-of-primary-care.pdf . Accessed 12 Apr 2022.
Agyapong VI, Conway C, Guerandel A. Shared care between specialized psychiatric services and primary care: the experiences and expectations of consultant psychiatrists in Ireland. Int J Psychiatry Med. 2011;42(3):295–313. https://doi.org/10.2190/2FPM.42.3.e .
Al-Alawi K, Al Mandhari A, Johansson H. Care providers' perceptions towards challenges and opportunities for service improvement at diabetes management clinics in public primary health care in Muscat, Oman: a qualitative study. BMC Health Serv Res. 2019;19(1). https://doi.org/10.1186/s12913-019-3866-y .
Alhabib S, Aldraimly M, Alfarhan A. An evolving role of clinical pharmacists in managing diabetes: Evidence from the literature. Saudi Pharm J. 2016;24(4):441–6. https://doi.org/10.1016/j.jsps.2014.07.008 .
Article PubMed Google Scholar
Ali S, Smith TL, Mican L, Brown C. Psychiatric providers' willingness to participate in shared decision-making when prescribing psychotropic medications. J Pharm Pract. 2013;26(3):334. https://doi.org/10.1177/2F8755122515578288 .
Aljumah K, Hassali MA. Impact of pharmacist intervention on adherence and measurable patient outcomes among depressed patients: A randomised controlled study. BMC Psychiatry. 2015;15(1). https://doi.org/10.1186/s12888-015-0605-8 .
Almunef M, Mason J, Curtis C, Jalal Z. Management of chronic illness in young people aged 10-24 years: A systematic review to explore the role of primary care pharmacists. Arch Dis Child. 2019;104(7):e2.39–e2. https://doi.org/10.1136/archdischild-2019-nppc.44 .
Alshehri AA, Cheema E, Yahyouche A, et al. Evaluating the role and integration of general practice pharmacists in England: a cross-sectional study. Int J Clin Pharm. 2021;43:1609–18. https://doi.org/10.1007/s11096-021-01291-6 .
Anderson K, Freeman C, Rowett D, Burrows J, Scott I, Rigby D. Polypharmacy, deprescribing and shared decision-making in primary care: the role of the accredited pharmacist. J Pharm Pract. 2015;45(4):446–9. https://doi.org/10.1002/jppr.1164 .
General Practitioner/pharmacy interface a local initiative Pharm J. 1994;253(6812):574.
The role of pharmacists in primary care groups (PCGs): a strategic approach. Pharm J. 1999;262(7038):445–6.
Pharmacy in a new age: the role of government. Pharm J. 1995;255(6863):1995.
Shared care addiction scheme starts. Pharm J. 2003;270(7252):782.
Ashcroft DM, Clark CM, Gorman SP. Shared care: A study of patients’ experiences with erythropoietin. Int J Pharm Pract. 1998;6(3):145–9. https://doi.org/10.1111/j.2042-7174.1998.tb00930.x .
Bains S, Naqvi M, West A. S126 The pharmacist-led accelerated transfer of patients to shared care for the monitoring and prescribing of immunomodulatory therapy during covid-19. Thorax. 2021;76:A75. https://thorax.bmj.com/content/76/Suppl_1/A75.2 .
Bajramovic J, Emmerton L, Tett SE. Perceptions around concordance–focus groups and semi-structured interviews conducted with consumers, pharmacists and general practitioners. Health Expect. 2004;7(3):221–34. https://doi.org/10.1111/j.1369-7625.2004.00280.x .
Barnes E, Ashraf I, Din A. New roles for clinical pharmacists in general practice. Prescriber. 2017;28(4):26–9. https://wileymicrositebuilder.com/prescriber/wpcontent/uploads/sites/23/2017/04/Pharmacist-new-roles-EB-edit-AC-made-lsw.pdf .
Bellingham C. How to improve medicines management at the primary/secondary care interface. Pharm J. 2004;272(7287):210–1.
Berendsen AJ, de Jong GM, Meyboom-de Jong B, Dekker JH, Schuling J. Transition of care: experiences and preferences of patients across the primary/secondary interface–a qualitative study. BMC Health Serv Res. 2009;9(1):62. https://doi.org/10.1186/1472-6963-9-62 .
Bojke C, Philips Z, Sculpher M, et al. Cost-effectiveness of shared pharmaceutical care for older patients : RESPECT trial findings. Br J Gen Pract. 2010;60(570):e20–7. https://doi.org/10.3399/bjgp09X482312 .
Article PubMed Central Google Scholar
British National Association of Health and Trusts. NAHAT Update: asthma care - the challenge ahead. Conference 1994.
Cain RM, Cain RM. The physician-pharmacist interface in the clinical practice of pharmacy. Ann Pharmacother. 2006;40(12):2240–2. https://doi.org/10.1345/aph.140048 .
Carrington I, McAloon J. Why shared-care arrangements for prescribing in attention deficit hyperactivity disorder may not be accepted. Eur J Hosp Pharm. 2018;25(4):222–4.
Chana N, Porat T, Whittlesea C, Delaney B. Improving specialist drug prescribing in primary care using task and error analysis: an observational study. Br J Gen Pract. 2017;67(656):e157–67. https://doi.org/10.3399/bjgp17x689389 .
Chartrand M, Maheu A, Guenette L, Martin E, Moisan J, Gregoire JP, Lauzier S, Blais L, Perreault S, Lalonde L. Implementation and evaluation of pharmacy services through a practice-based research network (PBRN). Journal of Population Therapeutics and Clinical Pharmacology. 2013;20(3):e272–3.
Cox WM. Evaluation of a shared-care program for methadone treatment of drug abuse: An international perspective. Journal of Drug Issues. 2002;32(4):1115–23. https://doi.org/10.1177/2F002204260203200407 .
Crowe S, Tully MP, Cantrill JA. The prescribing of specialist medicines: what factors influence GPs’ decision making? Family practice. 2009;26(4):301–8.
Crowe S, Cantrill JA, Tully MP. Shared care arrangements for specialist drugs in the UK: the challenges facing GP adherence. Qual Saf Health Care. 2010;19(6):e54.
Duggan C, Beavon N, Bates I, Patel S. Shared care in the UK: Failings of the past and lessons for the future. Int J Pharm Pract. 2001;9(3):211–6. https://doi.org/10.1111/j.2042-7174.2001.tb01051.x .
Fearne J, Grech L, Inglott AS, Azzopardi LM. Development and evaluation of shared paediatric pharmaceutical care plan. Int J Clin Pharm. 2018;40(1):263–4.
Finch E, Ford C [ed]. Shared care at the primary and secondary interface: GPs and specialist drug services. Drug misuse and community pharmacy 2002 1st Edition. eBook ISBN 9780429229848.
Grixti D, Vella J, Fearne J, Grech L, Pizzuto MA, Serracino-Inglott A. Development of shared care guidelines in rheumatology. Int J Clin Pharm. 2014;36(4):850.
Gu Y, Humphrey G, Warren J, Tibby S, Bycroft J, editors. An Innovative Approach to Shared Care–New Zealand Pilot Study of a Technology-enabled National Shared Care Planning Programme. Health Informatics New Zealand Conference; 2012
James O, Cardwell K, Moriarty F, Smith SM, Clyne B, on behalf of the General Practice Pharmacist (GPP) Study Group. Pharmacists in general practice: a qualitative process evaluation of the General Practice Pharmacist (GPP) study. Family Practice. 2020;37(5):711–8. https://doi.org/10.1093/fampra/cmaa044 .
Johnson C. Adult attention deficit and hyperactivity disorder (ADHD) clinic: A collaboration between psychiatry, primary care and pharmacy to improve access, care experience and affordability. JACCP. 2018;1(2):318.
Jones BW, Clark W. Shared care agreements - How to overcome the blank page. Pharm J. 2003;270(7234):165–6.
Jones C, Robinson H, Trickey J, Rees D, Jolliffe V, Wood J, et al. UPDATE ON THE INTRODUCTION OF DOSE TAPERING TO MODERNIzE AND IMPROVE THE BIOLOGICS SERVICE IN A DISTRICT GENERAL HOSPITAL. Rheumatology. 2017;56(suppl_2):kex062.143. https://doi.org/10.1093/rheumatology/kex062.143 .
Jones E, Cuevas OA. P12 An audit on the use and monitoring of azathioprine (aza) in a paediatric gastroenterology centre. could nhs england via specialist commissioning rules (nhs-e-spr) be affecting quality of care? Arch Dis Child. 2018;103:e2. https://adc.bmj.com/content/103/2/e2.14 .
Lloyd LA, Breslin A, Jones J. An audit of methotrexate monitoring in primary care as part of a shared care agreement. Rheumatology. 2009;(1):i61.
MacLellan J, Shahmanesh M, Singh S, Morton J, Estcourt C, Asboe. Shared care: how can we do it? Findings from the BHIVA primary care project. British HIV Association BHIVA. Available at: https://www.bhiva.org/file/PsGAzexFTYXJy/Shared-Care.pdf . Accessed 12 Apr 2022.
Mercer K, Burns C, Guirguis L, Chin J, Dogba MJ, Dolovich L, et al. Physician and pharmacist medication decision-making in the time of electronic health records: Mixed-methods study. J Med Internet Res. 2018;20(9). https://doi.org/10.2196/humanfactors.9891 .
Mercer K, Neiterman E, Guirguis L, Burns C, Grindrod K. “My pharmacist”: Creating and maintaining relationship between physicians and pharmacists in primary care settings. Res Social Adm Pharm. 2020;16(1):102–7. https://doi.org/10.1016/j.sapharm.2019.03.144 .
McWilliams T, Morakinyo K. Shared care guideline for the use of Methylphenidate, Dexamfetamine, Lisdexamfetamine dimesylate & Atomoxetine for the management of Attention Deficit Hyperactivity Disorder (ADHD) in Adults (18-64years). East London NHS Foundation Trust; 2017. https://gp-website-cdnprod.s3.amazonaws.com/shared-care-downloads/1539870612-c9e7983bdf34a9c9dcc7f3063eada5e9.pdf . Accessed 12 April 2022.
Mousa Y, Kayyali R, Goldsmith C, Coughlan L, Cairns C, Evans C. Investigating the potential for improved management of patients with long term conditions through shared-care protocols. Pharmacy World and Science. 2009;31(4):502.
National Instititute of Health and Care excellence NICE) 2015. Transition between inpatient hospital settings and community or care home settings for adults with social care needs. NICE guideline [NG27]. https://www.nice.org.uk/guidance/ng27 . Accessed 12 April 2022.
Nkansah N, Mostovetsky O, Yu C, Chheng T, Beney J, Bond CM, et al. Effect of outpatient pharmacists' nondispensing roles on patient outcomes and prescribing patterns. Cochrane Database Syst Rev. 2010(7). https://doi.org/10.1002/14651858.CD000336.pub2 .
O'Halloran KA. Developing Integrated Care Teams Across the North West London System. Int J Integr Care. 2016;16(6):A150. https://doi.org/10.5334/ijic.2698 .
Petty D. Clinical pharmacist roles in primary care networks. Prescriber. 2019;30(11):22–6. https://doi.org/10.1002/psb.1802 .
Murphy K. Clozapine, concomitant medications and consumers: Assessing the accuracy of medication records and the lived experience of people prescribed clozapine under shared care arrangements. Thesis Masters. Australia: Griffith University Queensland; 2018. https://doi.org/10.25904/1912/162 .
Richmond S, Morton V, Cross B, Wong ICK, Russell I, Philips Z, et al. Effectiveness of shared pharmaceutical care for older patients: RESPECT trial findings. Br J Gen Pract. 2010;60(570):e10–9. https://doi.org/10.3399/bjgp09X473295 .
Roberts RI. The welsh sahred care prescribing project. Pharm J. 1997;258(6935).
Sibbald B, Wilkie P, Raftery J, Anderson S, Freeling P. Prescribing at the hospital-general practice interface. II: Impact of hospital outpatient dispensing policies in England on general practitioners and hospital consultants. BMJ. 1992;304(6818):31–4. https://doi.org/10.1136/bmj.304.6818.31 .
Shemilt R, Azar M, Singh G, Campbell A, Gunnell KA. White S. An evaluation into the refusal of essential shared care agreements: Quetiapine. Pharmacoepidemiol Drug Saf. 2021;30(S2):3–20. https://doi.org/10.1002/pds.5315 .
Smith SM, Cousins G, Clyne B, Allwright S, O'Dowd T. Shared care across the interface between primary and specialty care in management of long term conditions. Cochrane Database Syst Rev. 2017;2(2):CD004910. https://doi.org/10.1002/14651858.CD004910.pub3 .
Sowerby C, Taylor D. Cross-sector user and provider perceptions on experiences of shared-care clozapine: a qualitative study. BMJ Open. 2017;28;7(9):e017183. https://doi.org/10.1136/bmjopen-2017-017183 .
Steckowych K, Smith M. Lessons learned and real-world challenges of implementing clinical pharmacy services in a primary care office. J Am Pharm Assoc. 2018;58(3):e132–e3 4.
Swallow VM, Nightingale R, Williams J, et al. Multidisciplinary teams, and parents, negotiating common ground in shared-care of children with long-term conditions: A mixed methods study. BMC Health Serv Res. 2013;13:264. https://doi.org/10.1186/1472-6963-13-264 .
Taylor D, Sutton J, Dawson H. User and staff perspectives of clozapine clinic services. Int J Pharm Pract. 2010;2:72.
Terry DRP. Medicines management acrosss the primary-hospital healthcare interface: a study of paediatric patients. PhD thesis. https://publications.aston.ac.uk/id/eprint/15825/ . Accessed 12 Apr 2022.
Terry D, Sinclair A. Prescribing for children at the interfaces of care. Arch Dis Child Educ Pract Ed. 2012;97(4):152–6.
Travis SS, Bethea LS. Medication administration by family members of dependent elders in shared care arrangements. Journal of Clinical Geropsychology. 2001;7(3):231–43. https://doi.org/10.1023/A:1011395229262 .
Tolley L, Azar M, Singh G, Gunnel KA, Campbell A, White S. An evaluation into the refusal of essential shared care agreements: Aripriprazole. Pharmacoepidemiol Drug Saf. 2021;30(S2):3–20. https://doi.org/10.1002/pds.5315 .
Walker M. Shared care for opiate substance misusers in Berkshire. Pharm J. 2001;266(7144):547–52.
Yones E, Mullan J, Horwood A, Connell N, Odams S, Maloney J, et al. Prescribing dronedarone for paroxysmal atrial fibrillation: How is it done across the UK and is it safe? Eur J Hosp Pharm. 2019;26(4):220–2.
Butterworth J, Sansom A, Sims L, Healey M, Kingsland E, Campbell J. Pharmacists’ perceptions of their emerging general practice roles in UK primary care: a qualitative interview study. Br J Gen Pract. 2017;67(662):e650–8.
Tan EC, Stewart K, Elliott RA, George J. Pharmacist consultations in general practice clinics: the pharmacists in practice study (PIPS). Res Soc Adm Pharm. 2013:623–32. https://doi.org/10.1016/j.sapharm.2013.08.005 .
Bradley F, Seston E, Mannall C, Cutts C. Evolution of the general practice pharmacist’s role in England: a longitudinal study. Br J Gen Pract. 2018;68(675):e727–34.
Hazen AC, De Bont AA, Boelman L, Zwart DL, et al. The degree of integration of non-dispensing pharmacists in primary care practice and the impact on health outcomes: a systematic review. Res Soc Adm Pharm. 2018;14:228–40.
Weber ZA, Skelley J, Sachdev G, et al. Integration of pharmacists into team-based ambulatory care practice models. Am J Health Syst Pharm. 2015;72(9):745–51. https://doi.org/10.2146/ajhp140576 .
Benson H, Lucas C, Benrimoj SI, Williams KA. The development of a role description and competency map for pharmacists in an interprofessional care setting. Int J Clin Pharm. 2019;41(2):391–407. https://doi.org/10.1007/s11096-019-00808-4 .
Benson H, Lucas C, Kmet W, Benrimoj SI, Williams K. Pharmacists in general practice: a focus on drug-related problems. Int J Clin Pharm. 2018;40(3):566–72. https://doi.org/10.1007/s11096-018-0617-9 .
Hayhoe B, Cespedes JA, Foley K, Majeed A, Ruzangi J, Greenfield G. Impact of integrating pharmacists into primary care teams on health systems indicators: a systematic review. Br J Gen Pract. 2019;69:e665–74.
Stuhec M, Lah L. Clinical pharmacist interventions in elderly patients with mental disorders in primary care focused on psychotropics: a retrospective pre-post observational study. Ther Adv Psychopharmacol. 2021;11:20451253211011007.
Stuhec M, Gorenc K, Zelko E. Evaluation of a collaborative care approach between general practitioners and clinical pharmacists in primary care community settings in elderly patients on polypharmacy in Slovenia: a cohort retrospective study reveals positive evidence for implementation. BMC Health Serv Res. 2019;19(1):118.
Stuhec M, Flegar I, Zelko E, Kovačič A, Zabavnik V. Clinical pharmacist interventions in cardiovascular disease pharmacotherapy in elderly patients on excessive polypharmacy: a retrospective pre-post observational multicentric study. Wien Klin Wochenschr. 2021;133(15-16):770–9.
Stuhec M, Gorenc K. Positive impact of clinical pharmacist interventions on antipsychotic use in patients on excessive polypharmacy evidenced in a retrospective cohort study. Global Psychiatry. 2019;2:155–63. https://doi.org/10.2478/gp-2019-0013 .
NHS England, Public Health England, Health Education England, Monitor, Care Quality Commission, NHS Trust Development Authority. Five year forward view. http://www.england.nhs.uk/wp-content/uploads/2014/10/5yfv-web.pdf . Accessed 18 Aug 2020.
Mann C, Anderson C, Avery A, Waring J, Boyd M. Clinical pharmacists in general practice: pilot scheme independent evaluation report: full report: University of Nottingham; 2018. https://www.nottingham.ac.uk/pharmacy/documents/generalpracticeyearfwdrev/clinical-pharmacists-in-general-practice-pilot-scheme-full-report.pdf . Accessed 13 Jan 2020.
Reiss-Brennan B, Brunisholz KD, Dredge C, Briot P, Grazier K, Wilcox A, et al. Association of integrated team-based care with health care quality, utilization, and cost. JAMA. 2016;316:826–34.
Weeks G, George J, MacLure K, Stewart D. Non-medical pre-scribing versus medical prescribing for acute and chronic disease management in primary and secondary care. Cochrane Database Syst Rev. 2016;11:CD01122.
Anderson C, Zhan K, Boyd M, Mann C. The role of pharmacists in general practice: a realist review. Res Soc Adm Pharm. 2019;15:338–45. https://doi.org/10.1016/j.sapharm.2018.06.001 .
Raynsford J, Dada C, Stansfield D, Cullen T. Impact of a specialist mental health pharmacy team on medicines optimisation in primary care for patients on a severe mental illness register: a pilot study. Eur J Hosp Pharm. 2020;27(1):31–5.
Nkansah N, Mostovetsky O, Yu C, Chheng T, Beney J, Bond CM, et al. Effect of outpatient pharmacists’ non-dispensing roles on patient outcomes and prescribing patterns. Cochrane Database Syst Rev. 2010;2010(7):CD000336. https://doi.org/10.1002/14651858.CD000336.pub2 .
Trust GPs to lead: learning from the response to COVID-19 within general practice in England BMA. 2020. Available from: https://www.bma.org.uk/media/2652/bma-report-trust-gps-to-lead-june-2020.pdf . Accessed 01 Sep 2020.
Avery AJ. Pharmacists working in general practice: can they help tackle the current workload crisis? Br J Gen Pract. 2017;67(662):390–1.
Jorgenson D, Laubscher T, Lyons B, Palmer R. Integrating pharmacists into primary care teams: barriers and facilitators. Int J Pharm Pract. 2014;22(4):292–9. https://doi.org/10.1111/ijpp.12080 .
Schlosser RW, Sigafoos J. ‘Empty’ reviews and evidence-based practice. Evid Based Commun Assess Interv. 2009;1–3. https://doi.org/10.1080/17489530902801067 .
Download references
Acknowledgements
The authors would like to thank Andrew Doyle, library specialist at Aston University, for his guidance with the development of the search strategy.
Author information
Authors and affiliations.
Aston Pharmacy School, College of Health and Life Sciences, Aston University, Aston Triangle, Birmingham, B4 7ET, UK
Naveed Iqbal, Chi Huynh & Ian Maidment
You can also search for this author in PubMed Google Scholar
Contributions
NI was responsible for the data collection and contributed to the analysis, synthesis, interpretation and project management. He led the drafting of this article. CH provided methodological, analysis and synthesis input and contributed to the data collection and synthesis. In case any disagreement occurred, the final decision was made by a discussion with IM and the systematic reviewer (NI/CH) to reach an amicable solution. No concerns were raised. IM contributed to the analysis. NI designed and conducted the searches with support from CH and contributed to data collection. All authors contributed to revised successive drafts of this article and approved the final version submitted for publication.
Corresponding author
Correspondence to Naveed Iqbal .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
All authors agree with the publication of this article.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: s1..
List of Search Terms.
Additional file 2: S2.
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist.
Additional file 3: S3.
PRISMA 2020 for Abstracts Checklist.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Iqbal, N., Huynh, C. & Maidment, I. Systematic literature review of pharmacists in general practice in supporting the implementation of shared care agreements in primary care. Syst Rev 11 , 88 (2022). https://doi.org/10.1186/s13643-022-01933-4
Download citation
Received : 21 January 2021
Accepted : 18 March 2022
Published : 11 May 2022
DOI : https://doi.org/10.1186/s13643-022-01933-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Shared care
- Pharmacists
- Systematic review
- integrated care
- Primary Care
- Seamless care
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- USC Libraries
- Research Guides
- Health Sciences
- Pharmacy Students: Year 3
- Literature Review
Pharmacy Students: Year 3: Literature Review
- Home and Help
- 567: Pharmacy Law
- 608: Therapeutics: Oncology and Immune Disorders
- 610: Therapeutics: Special Populations
- 611: Therapeutics: Infectious Diseases
- 619: Therapeutics: Cardiovascular System
- 620/621: Practice and Professionalism
- 622/623: Case Conference
- 633: Pharmacy Management and Economics
- 650: APPE Gateway
- Develop a Question
- Data Management
- IRB and CITI
- Make a Poster
- Cannabis User Safety (pharmacy elective)
- Community Pharmacy and Business Management (pharmacy elective)
- Geriatric Pharmacy (pharmacy elective)
- Global Pharmacy (pharmacy elective)
- Interprofessional Education (pharmacy electives)
- Marketing and Development in the Pharmaceutical Industry (pharmacy elective)
- Pharmaceutical Bioinformatics (pharmacy elective)
- Pharmaceutical Development (pharmacy elective)
- Pharmacy Education (pharmacy electives)
- Spanish for Pharmacists (pharmacy elective)
- Sports and Dietary Supplements (pharmacy elective)
- Toxicology and the Media (pharmacy elective)
- Research Resources
- Spanish for Health Care Professionals
- Career Resources
- Drug Information
Why do a literature review?
A literature review is an essential part of the research process. By conducting a review-- searching for and reading articles-- it helps you to understand who else has worked on your topic, methods that have been used to investigate this topic, and results/outcomes from similar studies, and develop your own research questions. When writing a literature review, you selective choose some of the readings you identified during your exploration, and write a short paragraph to prove to readers that your research question is necessary, important, and unanswered.
You do not need to conduct a Systematic Review. A Systematic Review is a research methodology where you attempt to gather all relevant literature describing a narrowly defined question. You may use a systematic technique to ensure you have searched relevant databases thoroughly, but your literature review does not need to be as extensive as a Systematic Review.
For a literature review, expect:
- That you will read two articles or book chapters for every one included in your final written review.
- You will need to search 2-6 subject-specific databases containing articles, book chapters, dissertations/theses, and other scholarly output. This typically happens at the start of the review.
- Your goal is to be thorough: expect to use both keywords and indexing terms to search databases
- You may need to use statistical sources, newspaper articles, articles from popular press magazines, reports from government organizations, or other types of unique sources in your review. These are unlikely to be a major part of your literature review, but can help to prove that a problem is widespread and deserving of research attention.
Process of a literature review
1. Define your question-- develop a hypothesis or research question. For example, "If we teach nurses to use asthma puffers correctly, does this increase patient-reported compliance with use of puffers and increase the rate of refills?"
2. Consider which databases would best provide information on your topic. Databases tend to focus on disciplines or subjects. If your research is about clinical use of drugs, you may only need to use clinically-focused databases. If your research concerns motivation for behavior, you may want to use psychology databases. If your research question is about economics of drugs, you may want to use business/economics-focused databases. Use this list of databases on this page or talk to a librarian to find the best databases for researching your question.
2. Break apart your question into concepts. With the question above, major concepts include nurses, asthma puffers, drug compliance, technique, refills.
3. For each concept, brainstorm synonyms. For example, puffer= inhaler, Flovent (or other brand names), etc.
3a. If using an indexed database like PubMed, look for the preferred indexing term for each concept and add it to your synonym list.
4. Combine the synonyms in your database. Use OR to connect synonyms. Use AND to connect different concepts. For example: (Nurse OR nurses) AND (inhaler OR puffer) AND (education OR training OR instruction).
4a. It is sometimes easier to look for research covering parts of your question rather than the whole. It is unlikely that there has been research on exactly your topic (nurses, and asthma puffers, and instruction, and compliance, and refills). Consider looking for research on nurses and inhalers; instruction with inhalers and if that affects patient compliance; use of puffers and refill rates; or other ways to break down the question into smaller parts.
5. Examine the results in your first database by reading the abstracts. Add to your list of synonyms by seeing what terms are used in the abstracts, and consider re-running the search with additional terms. Create a list of materials from this database that seem relevant. Next, download the full text of articles, book chapters, etc. on your list. Get older articles in print from the library's print collections. Repeat this process in your other databases.
6. Read the materials you found in step 5. Consider whether the information you have found allows you to demonstrate:
- That your topic is relevant and important, and deserves you and your group spending a year in investigating it.
- That you have found articles that cover a relevant timeframe and scope for your question.
- That you have seen any landmark studies, or major studies that have changed thinking on a topic. Not every topic area has landmark studies.
- That you can summarize the research methods that have been used to study this question/problem before and their results.
7. If you cannot demonstrate each of these, repeat steps 4 and 5 until you can.
8. Write your literature review, summarizing why your question/problem is important, other methods that have been used to investigate this problem and what they have found, and explain why your research question will help fill gaps in current knowledge. You can consider using the FINER criteria (Feasible, Interesting, Novel, Ethical, Relevant) to ensure you have covered the key aspects of the topic in enough depth. You will likely cite/reference about half the items you read. Your goal here is to select enough materials to demonstrate that you know this topic, without overwhelming your audience.
Help on literature reviews
- Bailey's Research for the Health Professional, 3rd ed ISBN: 9780803645134 Includes detailed help on literature reviews
Databases to use
USC subscribes to over 1300 databases, so only the primary databases you may use are listed here. If these do not cover your topic fully, examine the A-Z list of databases and read descriptions, or ask a librarian for help.
- Databases licensed by USC Libraries This link opens in a new window A-Z list of all databases licensed by USC Libraries.
Clinical databases
- CINAHL Complete This link opens in a new window Most comprehensive database of full-text for nursing & allied health journals from 1937 to present. Includes access to scholarly journal articles, dissertations, magazines, pamphlets, evidence-based care sheets, books, and research instruments.
- Embase This link opens in a new window Embase provides access to over more than 29 million citations for biomedical articles and conference proceedings. This version has been customized for USC users to find the full-text of articles from library holdings.
Basic sciences databases
- Biosis Citation Index This link opens in a new window Life sciences and biomedical research database covering pre-clinical and experimental research, methods and instrumentation, animal studies, and more.
- International Pharmaceutical Abstracts (Ovid) This link opens in a new window Worldwide coverage of pharmaceutical science and practice literature. Includes coverage of drug development, pharmacoeconomics, toxicity, regulation, and technology.
- SciFinder This link opens in a new window Articles, patents, and white papers describing chemicals. Includes information on structures, reactions, compounds, drug development, and manufacturing. Requires separate login. Go to USC's create an account page to register.
Social Sciences databases
- ERIC (ProQuest) This link opens in a new window Education focused database.
- Global Health This link opens in a new window Public health database, focus on international coverage.
- PsycINFO This link opens in a new window Abstract and citation database of scholarly literature in psychological, social, behavioral, and health sciences. Includes journal articles, books, reports, theses, and dissertations from 1806 to present.
- EconLit This link opens in a new window Literature in economics.
General Databases These databases include multiple subjects. They are useful databases for looking for landmark studies; determining if your topic is well-studied by another subject (as this would lead you to select a database that focuses on just that subject); and ensuring you did not miss research published in prominent scientific journals.
- Scopus This link opens in a new window Large abstract and citation database of peer-reviewed literature in science, technology, medicine, social sciences, and arts and humanities. Includes bibliometrics tools to track, analyze and visualize research.
- Web of Science This link opens in a new window
- Google Scholar This link opens in a new window Filtered Google Search finding scholarly journal articles, books, citations, and case law.
Writing & Citing Help
Use these handouts to get information on citing and writing; contact the Norris Medical Library with questions.
- Using Review Articles Review this handout to make sure you're using review articles appropriately.
- Quick Citing Reference- AMA 11th ed 2 page handout covering the basics of using AMA style, 11th edition
- << Previous: Develop a Question
- Next: Data Management >>
- Last Updated: Jun 3, 2024 10:12 AM
- URL: https://libguides.usc.edu/healthsciences/pharmyr3
Systematic Reviews and Meta-Analysis in Pharmacy Practice
- First Online: 22 April 2020
Cite this chapter

- Syed Shahzad Hasan 2 ,
- Therese Kairuz 3 ,
- Kaeshaelya Thiruchelvam 3 &
- Zaheer-Ud-Din Babar 2
699 Accesses
1 Citations
A systematic review is a process of synthesizing research evidence by collecting and summarizing all empirical evidence that meets predefined inclusion and exclusion criteria. Systematic reviews are performed by using systematic methods and often include a meta-analysis component which involves statistical techniques to conduct quantitative synthesis. Pharmacists from different regions of the world and practices—such as academia, hospital, and community—are increasingly using this approach to produce evidence about their new services and interventions, comparing them with services provided by other healthcare professionals or with control groups. This chapter covers the inception of a systematic approach to reviews and their use in pharmacy practice. The quality associated with systematic reviews and meta-analyses are discussed. A quick guide outlines the important steps in conducting a systematic review, and some of the models used in the reporting of meta-analyses—such as direct and indirect evidence models and pooling effect sizes—are introduced.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions

Abbreviations
Cumulative Index to Nursing and Allied Health Literature
Evidence-based practice
Excerpta Medica Database
Grading of Recommendations, Assessment, Development and Evaluations
International Pharmaceutical Abstracts
Meta-analysis
National Institute for Health and Care Excellence
Patient, intervention, comparison, outcome
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Quality of Reporting of Meta-analyses
Randomized controlled trial
[Relative] Risk ratio
Scottish Intercollegiate Guidelines Network
Systematic review
Ahn EJ, Kang H. Introduction to systematic review and meta-analysis. Korean J Anesthesiol. 2018;71(2):103–12.
Article PubMed PubMed Central Google Scholar
Babar ZU, Kousar R, Hasan SS, Scahill S, Curley LE. Glycemic control through pharmaceutical care: a meta-analysis of randomized controlled trials. J Pharm Health Serv Res. 2019;10(1):35–44.
Article Google Scholar
Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–91.
Article CAS PubMed Google Scholar
Carter BL, Rogers M, Daly J, Zheng S, James PA. The potency of team-based care interventions for hypertension: a meta-analysis. Arch Intern Med. 2009;169(19):1748–55.
Chalmers I. The James Lind initiative. J R Soc Med. 2003;96(12):575–6.
Cochrane Consumer Network. What is a systematic review? 2019. Available from: https://consumers.cochrane.org/what-systematic-review .
Collins Dictionary. Definition of ‘review’. 2019. Available from: https://www.collinsdictionary.com/dictionary/english/review .
Debray TP, Moons KG, Abo-Zaid GM, Koffijberg H, Riley RD. Individual participant data meta-analysis for a binary outcome: one-stage or two-stage? PLoS One. 2013;8(4):e60650.
Article CAS PubMed PubMed Central Google Scholar
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Dijkers M. Introducing GRADE: a systematic approach to rating evidence in systematic reviews and to guideline development. Knowl Translat Update. 2013;1:1–9.
Google Scholar
Doi SAR, Barendregt JJ. Meta-analysis I: computational methods. In: Doi, Williams, editors. Methods of clinical epidemiology. Berlin, Heidelberg: Springer Publishing; 2013. p. 229–52.
Chapter Google Scholar
Doi SA, Barendregt JJ, Khan S, Thalib L, Williams GM. Advances in the meta-analysis of heterogeneous clinical trials I: the inverse variance heterogeneity model. Contemp Clin Trials. 2015;45(Pt A):130–8.
Article PubMed Google Scholar
Doi SA, Thalib L. A quality-effects model for meta-analysis. Epidemiology. 2008;19(1):94–100.
Effective Practice and Organisation of Care (EPOC). Norwegian Knowledge Centre for the Health Services. Suggested risk of bias criteria for EPOC reviews. 2019. Available from: https://epoc.cochrane.org/sites/epoc.cochrane.org/files/uploads/Suggested%20risk%20of%20bias%20criteria%20for%20EPOC%20reviews.pdf .
Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Inf Libr J. 2009;26(2):91–108.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6.
Hasan SS, Zaidi STR, Nirwan JS, Ghori MU, Javid F, Ahmadi K, Babar ZD. Use of central nervous system (CNS) medicines in aged care homes: a systematic review and meta-analysis. J Clin Med. 2019;8:1292.
Article PubMed Central Google Scholar
Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. Cochrane statistical methods group and the Cochrane Bias methods group. 2011. Available from: http://handbook.cochrane.org/chapter_8/8_assessing_risk_of_bias_in_included_studies.htm .
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019). Cochrane. 2019. Available from www.training.cochrane.org/handbook .
Holland R, Desbororough J, Goodyer L, Hall S, Wright D, Loke YK. Does pharmacist-led medication review help to reduce hospital admissions and deaths in older people? A systematic review and meta-analysis. Br J Clin Pharmacol. 2007;65(3):303–16.
Kang H. Statistical considerations in meta-analysis. Hanyang Med Rev. 2015;35:23–32.
Kao LS, Tyson JE, Blakely ML, Lally KP. Clinical research methodology I: introduction to randomized trials. J Am Coll Surg. 2008;206(2):361–9.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23(20):3105–24.
Melchiors AC, Correr CJ, Venson R, Pontarolo R. An analysis of quality of systematic reviews on pharmacist health interventions. Int J Clin Pharm. 2012;34:32–42.
Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet. 1999;354:1896–900.
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. https://doi.org/10.1136/bmj.b2535 .
Mulrow CD. The medical review article: state of the science. Ann Intern Med. 1987;106:485–8.
Naghavi M. Global, regional, and national burden of suicide mortality 1990 to 2016: systematic analysis for the Global Burden of Disease Study 2016. BMJ. 2019;364:194.
National Institute for Health and Clinical Excellence (NICE). Developing NICE guidelines: the manual. London: National Institute for Health and Clinical Excellence; 2014.
Petticrew M, Roberts H. Systematic reviews in the social sciences: a practical guide. Malden, MA: Blackwell Publishing; 2006.
Book Google Scholar
Scottish Intercollegiate Guidelines Network. Methodology checklist 3: cohort studies. Scottish Intercollegiate Guidelines Network: Scotland, Edinburgh; 2012.
The Institute for Health Metrics and Evaluation (IHME). Available from http://www.healthdata.org/gbd/faq#What%20is%20an%20uncertainty%20interval ? Accessed 06 Dec 2019.
Thomas PAD, Moons KGM, van Valkenhoef G, Orestis E, Hummel N, Rolf GHH, Johannes RB, on behalf of the GetReal Methods Review Group. Get real in individual participant data (IPD) meta-analysis: a review of the methodology. Res Synth Methods. 2015;6(4):293–309.
Uman LS. Systematic reviews and meta-analyses. J Can Acad Child Adolesc Psychiatry. 2011;20(1):57–9.
PubMed PubMed Central Google Scholar
Willis BH, Quigley M. The assessment of the quality of reporting of meta-analyses in diagnostic research: a systematic review. BMC Med Res Methodol. 2011;11:163.
Woolf B. On estimating the relation between blood group and disease. Ann Hum Genet. 1955;19:251–3.
Download references
Acknowledgments
We would like to thank Dr. Keivan Ahmadi from Lincoln Medical School (UK) for providing constructed feedback on meta-analysis component.
Author information
Authors and affiliations.
Department of Pharmacy, University of Huddersfield, Huddersfield, West Yorkshire, UK
Syed Shahzad Hasan & Zaheer-Ud-Din Babar
School of Biomedical Sciences & Pharmacy, University of Newcastle, Callaghan, NSW, Australia
Therese Kairuz & Kaeshaelya Thiruchelvam
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Zaheer-Ud-Din Babar .
Editor information
Editors and affiliations.
Department of Pharmacy, School of Applied Sciences, University of Huddersfield, Huddersfield, UK
Zaheer-Ud-Din Babar
Rights and permissions
Reprints and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Hasan, S.S., Kairuz, T., Thiruchelvam, K., Babar, ZUD. (2020). Systematic Reviews and Meta-Analysis in Pharmacy Practice. In: Babar, ZUD. (eds) Pharmacy Practice Research Methods. Springer, Singapore. https://doi.org/10.1007/978-981-15-2993-1_12
Download citation
DOI : https://doi.org/10.1007/978-981-15-2993-1_12
Published : 22 April 2020
Publisher Name : Springer, Singapore
Print ISBN : 978-981-15-2992-4
Online ISBN : 978-981-15-2993-1
eBook Packages : Medicine Medicine (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research

Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
4.5 Introduction to the Literature Review
Beverley Glass
What is a Literature Review?
A literature review is a type of academic writing that provides an overview of existing knowledge in a particular field of research.
A good literature review summarises, analyses, evaluates and synthesises the relevant literature within a particular field of research. It illuminates how knowledge has evolved within the field, highlighting what has already been done, what is generally accepted, what is emerging and what is the current state of thinking on the topic. Additionally, literature reviews identify the gaps in the current knowledge – that is, uninvestigated or under-researched area
What is literature in pharmacy?
- Primary sources include first-hand reports and original creations (diaries, interviews, art) as well as experimental results (from clinical trials, experiments, and scientific discoveries).
- A secondary source reports on or analyses a primary source.
While primary research articles provide a background on their subject by summarising previously conducted research, this typically occurs only in the “Introduction” section of the article. Review articles, however, will summarise previously conducted research throughout the entire paper.
1. Jesson and Lacey, How to do (or not to do) a critical literature review, Pharmacy Education, June 2006; 6(2): 139–148. Available at https://pharmacyeducation.fip.org/pharmacyeducation/article/view/103/83
Activity 1️⃣⏰30 Minutes
Research Methods in Pharmacy Practice Copyright © by Beverley Glass. All Rights Reserved.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 21 July 2023
Psychedelic therapies reconsidered: compounds, clinical indications, and cautious optimism
- Jennifer M. Mitchell ORCID: orcid.org/0000-0002-7567-8129 1 , 2 , 3 , 4 &
- Brian T. Anderson 2 , 4
Neuropsychopharmacology volume 49 , pages 96–103 ( 2024 ) Cite this article
6151 Accesses
6 Citations
24 Altmetric
Metrics details
- Neuroscience
The clinical investigation of psychedelic medicines has blossomed over the last 5 years. Data from a Phase 3 industry trial and a multicenter Phase 2 industry trial, in addition to multiple early phase investigator-initiated and industry trials, have now been published in peer-reviewed journals. This narrative review summarizes both the recent data and the current clinical trials that are being conducted with various classes of “psyche-manifesting” substances, which may prove beneficial in the treatment of a broad range of conditions. Methodological considerations, unique challenges, and next steps for research are discussed in keeping with the uniquely “experiential” nature of these therapies.
Similar content being viewed by others

Adults who microdose psychedelics report health related motivations and lower levels of anxiety and depression compared to non-microdosers

Psilocybin microdosers demonstrate greater observed improvements in mood and mental health at one month relative to non-microdosing controls

Emotions and brain function are altered up to one month after a single high dose of psilocybin
Introduction.
The past few years have ushered in a renewed wave of psychedelic interest and research that has led to the clinical testing of multiple psychedelic agents for various health conditions. Australia and Canada have even recently approved the clinical use of certain psychedelic medicines under restricted circumstances [ 1 , 2 ]. However, along with this renewed wave of interest has come a series of challenges with respect to the taxonomy of psychedelics, the implementation of best practices for the evaluation of psychedelic therapies, and the capacity to stay abreast of developments in this rapidly expanding scientific field. Here we review the contemporary clinical research to provide an overview of the different classes of psychedelics currently under investigation for clinical use, their proposed indications, and—because they are “experiential medicines”—considerations for their judicious use.
Psychedelics have long been implicated in the treatment of mood and anxiety disorders, as well as disorders related to impulsivity, repetitive behaviors, and impaired decision-making, such as alcohol and other substance use disorders [ 3 , 4 , 5 ]. Yet the clinical promise of mid-20th Century psychedelic research was ultimately overshadowed by methodological limitations [ 6 ], regulatory restrictions, and a political landscape that made psychedelic research all but untenable for several decades [ 7 ]. Current biomedical theories speculate that psychedelic therapies improve clinical functioning by regulating affective states (such as anhedonia) and self-referential cognitive processes and may therefore ultimately involve interrelated neural circuits across a broad range of conditions [ 8 , 9 ]. Along these lines, the current clinical research landscape has expanded significantly compared to even a few years ago [ 10 ], and now includes evaluations of the effects of psychedelic therapies in a wide array of diagnostic categories, including mood disorders, substance use disorders, obsessive compulsive and related disorders, trauma-related disorders, and disorders of dysfunctional coping such as pathological grief and end of life distress. Typically, when investigated as therapies for psychiatric conditions, psychedelics are administered as adjuncts to a brief course of behavioral therapy to mitigate the risk of adverse events and to augment efficacy [ 11 , 12 ].
In addition to psychiatric indications, psychedelics are also being studied more generally as neuroplastic agents, potentially capable of inducing change in intractably crystalized neurological pathways. As such, they are being pursued as therapeutics for age-related degenerative conditions—including Alzheimer’s and Parkinson’s—as well as for pain, headache and migraine, autism, and visual impairments. Only time will tell if these compounds can reverse ingrained neurological processes, perhaps by enabling neuroplasticity, to the point where previously inflexible systems can be adjusted and reset.
Defining psychedelic
The original definition of the term psychedelic , as coined by the psychiatrist Humphry Osmond, is “mind-” or “soul-manifesting”, and as such is independent of biological mechanism. With this definition in mind, drugs that activate the serotonin 5-HT2A receptor directly (typically termed classic psychedelics) [ 13 ] as well as those that activate serotonin receptors indirectly or not at all, or that work through binding to a combination of receptors (including glutamatergic, dopaminergic, and opioidergic receptors) would all be considered psychedelic if they demonstrate the capacity for allowing greater access to the psyche in a manner similar to the classic psychedelics. Therefore, this review will include a series of drugs that meet the original definition of psychedelic for which there exists preliminary evidence from modern trials of the potential to affect psychotherapeutic change in humans. Some atypical, or non-classic, psychedelics are also included here; these are drugs with psychoactive effects that are not primarily or uniquely mediated through the activation of the 5-HT2A receptor. The decades-long history of the use of substances like ketamine [ 14 , 15 ] and psychedelic amphetamines like 3,4-methelenedioxyamphetamine (MDA) [ 16 ] in psychedelic therapy suggests that a comprehensive review of psychedelic therapies should include drugs beyond the classic psychedelics.
While the term hallucinogen is often colloquially substituted for the term psychedelic, hallucinogens are a category of drugs that induce perceptions in the absence of external stimuli (as opposed to inducing perceptual distortions of extant stimuli). Therefore, although there is some overlap in the categories of hallucinogen and psychedelic, here we will only use the term psychedelic, as we are summarizing effects that are more broadly relevant to processes of psychotherapeutic change, and not limited to perceptual alterations per se. With these definitions in mind, and considering the available data and currently registered trials, the modern clinical investigation of the following classic and atypical psychedelics (henceforth, collectively referred to as “psychedelics”) will be discussed in this review: ayahuasca, N,N-dimethyltrypatmine (DMT) and 5-methoxy-DMT (5-MeO-DMT), ibogaine, ketamine, lysergic acid diethylamide (LSD), 3,4-methylenedioxymethamphetamine (MDMA), mescaline, salvinorin A, and psilocybin. In addition, as psychedelics are thought to be “experiential medicines” affected by both set and setting [ 17 , 18 , 19 ], attention will also be focused on the environmental and psychological conditions that modulate the therapeutic efficacy of psychedelics.
Many psychedelic molecules have structural similarities that may enable specific signaling mechanisms. However, molecular differences allow them to be divided into indolamines (including the ergolines and simple tryptamines), phenethylamines, diterpines, and cyclohexanones. Each of these will be discussed in turn in an attempt to provide the reader an up-to-date view of the rapidly evolving field of clinical research into psyche -manifesting drugs.
Indolamines
Ibogaine is an indolamine that is derived from the West African Tabernanthe iboga bush that has long been used as part of the Bwiti religious tradition in the jungles of Gabon [ 20 ]. Ibogaine binds dopamine and serotonin (5-HT) receptors, acts as both an NMDA and a3b4-nicotinic receptor antagonist, and acts as a kappa opioid receptor agonist [ 21 ]. Although ibogaine was originally touted as an anti-addiction medication as far back as the 1960s https://www.nytimes.com/2010/02/17/us/17lotsof.html , it took over 30 years for it to make its way into phase 1 clinical studies, which indicated that, although ibogaine has the potential to be an effective an anti-addiction therapeutic for a number of different substance use disorders [ 22 , 23 , 24 ], it also carries cardiac and neurological risks that complicate its use as a therapeutic [ 25 , 26 , 27 ]. A series of sudden and unexpected deaths halted phase 1 testing for drug abuse and dependence [ 25 , 28 ] and clinical trials have yet to resume in the United States, although there is growing political support for these studies [ 29 ].
Although ibogaine is a Schedule 1 substance and is not FDA approved, over the last few decades it has been administered at drug and alcohol treatment centers in Latin America and the Caribbean, and it is currently under investigation outside of the United States as a potential therapeutic for alcohol misuse (NCT03380728), and for drug use, dependence, and withdrawal (NCT04003948; NCT05029401). Recent data suggest that the potential negative impact of ibogaine on cardiac function can be controlled through careful screening and monitoring during drug administration [ 30 ] and that, as both ibogaine and methadone may induce QT prolongation (an alteration in cardiac ventricular repolarization that is associated with the Torsade de Pointes arrhythmia and increased risk of sudden cardiac death), care should be taken to ensure that those seeking ibogaine treatment for opioid use disorder are screened for the use of methadone and other QT prolonging drugs prior to ibogaine administration.
Elucidation of the mechanism(s) by which ibogaine exerts its clinical effects might lend insight into the contribution of various neurotransmitter systems to the clinical effectiveness of psychedelics, as ibogaine is perhaps one of the least pharmacologically specific, and yet most impactful, psychedelics currently under investigation.
Indolamines: Ergolines
Lysergic acid diethylamide (lsd).
LSD was first synthesized by the chemist Albert Hoffman during his employment with Sandoz Pharmaceuticals in the 1930s while he was searching for novel compounds to treat respiratory depression. LSD has since been shown to bind with high affinity to several 5-HT receptors [ 31 ] and also acts as a dopamine (D1, D2, D4) receptor agonist [ 32 , 33 ]. Potential clinical indications include alcohol and other substance use disorders [ 34 , 35 , 36 ], obsessive compulsive disorder [ 37 ], depression (now in phase 2), end of life anxiety [ 38 ], cluster headache [ 39 ], and attention-deficit hyperactivity disorder (ADHD) [ 40 ]. LSD has long been available through Compassionate Use laws in Switzerland as an adjunct to psychotherapy for the treatment of a number of different mental health conditions [ 41 ].
Although a substantial volume of data from the 1960s to 1970s suggest that LSD could be effective in decreasing alcohol consumption in self-ascribed, and self-selected, “alcoholics,” [ 42 ] and although a more recent meta-analysis that incorporated clinical data from 6 smaller studies demonstrated that a single dose of LSD resulted in a long-term decrease in alcohol “misuse,” [ 35 ] previous outcome measures were not collected using current methodological standards and should therefore be replicated to confirm and extend previous findings in a thoroughly characterized subject population with alcohol use disorder (AUD). Fortunately, a double-blind, placebo-controlled, randomized, multisite study is currently being planned (NCT05474989) to evaluate the effects of two doses of LSD (150 µg for first dose followed by either 150 µg or 250 µg for second dose) on prevention of relapse to alcohol in a population with AUD. These data will hopefully provide insight into whether LSD does indeed hold promise as a potential therapeutic for AUD.
LSD has also recently completed early phase clinical testing for anxiety disorders (NCT03153579; NCT00920387) and the results have shown that LSD decreases anxiety while increasing quality of life [ 43 ] and also, importantly, that these effects are long-lasting [ 38 ]. In addition, a randomized, double-blind, placebo-controlled phase 2 trial of LSD for major depression (comparing two moderate to high doses of LSD = 100 µg/100 µg or 100 µg/200 µg, and two low doses of LSD = 25 µg/25 µg) has now been completed (NCT03866252), although these data were not yet available for review at the time of this publication. Lastly, a multisite, randomized, double-blind, placebo-controlled phase 2 trial is also currently being planned to further study the use of LSD for adults with ADHD (NCT05200936).
In addition to the mental health disorders listed above, both case (40. Sewell et al., 2006) and self-reports [ 44 ] indicate that LSD could be effective in combating cluster headache, and a double-blind, randomized, placebo-controlled phase 2 trial of LSD (3 doses of 100 mcg over 3 weeks) has recently been initiated to evaluate the efficacy of LSD for treatment of cluster headache (NCT03781128). Finally, as part of a drug development program for treating Alzheimer’s Disease, a recent double-blind randomized pilot trial of repeated (every 4 days) low doses of LSD (5 mcg vs 10 mcg vs 20 mcg vs placebo) in healthy older adults (age 55–75) found the drug to be well-tolerated in this population [ 45 ].
Indolamines: Simple tryptamines
Psilocybin is an active agent in Psilocybe mushrooms, which have been used ritualistically for thousands of years by Indigenous communities in Central and North America. Psilocybin exerts its psychedelic effects primarily through activation of 5HT2A receptors [ 46 ] and through activation of a 5-HT2AR-mGluR2 receptor complex [ 47 ]. Likely due to its well-established safety profile, minimal abuse potential, and short duration of subjective perceptual effects, psilocybin is currently the most broadly studied psychedelic for mental health conditions. Clinical indications under investigation currently include major depression [ 48 ], treatment-resistant depression [ 49 ], alcohol and other substance use disorders [ 50 ], smoking cessation [ 51 ], and OCD [ 52 ]. Psilocybin has also been studied in conjunction with individual and group psychotherapy for treating distress in patients with serious illness including cancer-related mood and anxiety disorders, and demoralization in long term AIDS survivors [ 53 ].
Not only has psilocybin been successfully administered for smoking cessation [ 51 , 54 ], but, intriguingly, it has also been shown that change in tobacco consumption following psilocybin administration is correlated with the degree of “mystical-type experience” reported by study participants, such that those reporting greater intensity of mystical-type experiences also report a greater decrease in smoking [ 54 ]. While there remains great debate over the nature and assessment of a “mystical-type experience”, high-dose psilocybin has repeatedly been reported by participants to be a spiritual or transcendent event, which seems to be an important contributor to treatment effectiveness [ 55 ] and, as such, merits further attention.
A modest-sized proof of principle phase 2 trial recently demonstrated the significant and long-lasting efficacy of psilocybin when combined with psychotherapy for the treatment of AUD ( 50 . Bogenschutz et al., 2022). Mechanistic (NCT04141501), head-to-head (NCT05421065), larger, multisite (NCT05646303), and other trials (NCT04620759) of psilocybin therapy for AUD are currently recruiting.
The most detailed exploration of psilocybin for a therapeutic indication thus far has been for treatment-resistant depression (TRD), and a recent, double-blind phase 2 study found a dose-dependent reduction in depression scores in the weeks following administration of a single dose of psilocybin (1 mg, 10 mg, or 25 mg) [ 56 ]. Similarly, a trial investigating the use of two psilocybin administration sessions in conjunction with therapy for major depressive disorder (MDD) not only found a significant attenuation in depression scores at both the primary endpoint and at the 4-week follow up [ 57 ] but also noted that these effects were still durable 1 year after psilocybin administration [ 58 ]. This trial also noted a correlation between mystical-type experience at the time of psilocybin administration and increased well-being at the 12-month follow-up. As multiple studies have noted a positive correlation between the lasting impact of psilocybin on mental health measures and mystical-type experiences, it will be interesting to note whether future studies will be able to elucidate the nature of the relationship between psilocybin-induced mystical-type experiences and durable alleviation of mental health conditions.
A myriad of additional phase 2 trials with psilocybin are now underway for a variety of other indications including PTSD (NCT05554094; NCT05243329; NCT05312151), OCD (NCT05370911; NCT04882839; NCT03300947; NCT05546658; NCT03356483), depression in bipolar 2 disorder (NCT0506529; NCT04433845), anorexia nervosa (NCT04656301; NCT04052568; NCT04661514; NCT05481736; NCT04505189), binge eating (NCT05035927), fibromyalgia (NCT05548075; NCT05128162; NCT05068791), phantom limb pain (NCT05224336), migraine (NCT03341689; NCT04218539), cluster headache (NCT02981173), concussion headache (NCT03806985). Multiple trials are assessing psilocybin therapy for distress associated with serious medical illness (NCT04950608; NCT05398484; NCT05506982; NCT04522804; NCT05220046; NCT04593563; NCT05403086). In addition, a series of studies have been evaluating the potential of psilocybin to attenuate methamphetamine use disorder (NCT04982796; NCT05322954) and cocaine use disorder (NCT02037126). With any luck, the next couple of years should further quantify the myriad of potential therapeutic uses of psilocybin.
Ayahuasca is typically an admixture of the Banisteriopsis caapi vine, containing MAO-inhibiting beta-carboline alkaloids, and the DMT-containing leaves of the Psychotria viridis shrub, although other plants, such as Diplopterys cabrerana , are at times used to make decoctions that are also referred to as ayahuasca. The drink has been used ceremonially in the Amazon Basin for at least hundreds of years and is used widely today in shamanic and other religious contexts within and outside of South America [ 59 ]. Potential indications for ayahuasca include alcohol and other substance use disorders, anxiety and depression disorders [ 60 ] and possibly prolonged grief disorder [ 61 ] and eating disorders [ 62 ]. Naturalistic studies have indicated that regular users of ayahuasca consume less alcohol and other drugs compared to other populations [ 63 , 64 , 65 ], and that ritual participants self-report improved affective symptoms after drinking ayahuasca [ 66 ], however individuals with anxiety and mood disorders may also be at higher risk of experiencing adverse effects in rituals settings [ 67 ]. One recent placebo-controlled proof of principle trial has also shown that a single administration of ayahuasca can attenuate symptoms of treatment-resistant depression [ 68 ].
DMT & 5-MeO-DMT
N,N-Dimethyltryptamine (DMT) is a substituted tryptamine that constitutes one of the primary active ingredients in ayahuasca and is structurally similar to the psychedelic compounds 5-MeO-DMT and bufotenin (5-HO-DMT). In addition to high binding affinity at a number of 5-HT receptors, DMT acts as a TAAR agonist [ 69 ], and a sigma receptor agonist [ 70 ] and may mediate effects at metabotropic glutamate receptors [ 71 ]. Although the clinical data are currently limited, DMT is now being studied in a fixed order, open-label, dose-escalation study in participants with major depression (NCT04711915), and a double-blind, randomized, placebo-controlled study of intravenous DMT in subjects with major depressive disorder (MDD) has now been completed (NCT04673383). Because the subjective effects of DMT are short-lasting compared to other psychedelic compounds [ 72 ], DMT might lend itself more readily to use in clinical settings.
Phenethylamines
3,4-Methylenedioxymethamphetamine (MDMA) was originally synthesized by the pharmaceutical company Merck in 1912 as part of a research program on anticoagulating agents. Early case reports suggested that MDMA could be a remarkably effective catalyst in both individual and couples psychotherapy [ 73 , 74 ] for a variety of psychological issues. The psychedelic-like effects of MDMA were eventually immortalized by Alexander and Ann Shulgin in their book, PiHKAL [ 75 ].
MDMA acts on human monoamine transporters [ 76 ], though most of the subjective effects of MDMA are dependent on serotonin release, which MDMA potentiates through a series of different mechanisms. MDMA inhibits the 5-HT vesicular transporter (VMAT2) and activates the intracellular presynaptic terminal receptor (TAAR1), which impacts both the release and reuptake of serotonin [ 69 , 77 , 78 ]. Downstream of serotonin efflux, MDMA promotes the release of oxytocin [ 79 ], a neuromodulator shown to play a critical role in bonding and social interactions [ 80 ], which may therefore facilitate the therapeutic process by enabling participants to remain emotionally open while they explore difficult memories and subject matter.
MDMA most likely exerts its influence through effects within the amygdala, and previous human research indicates that MDMA attenuates left amygdalar responses to angry facial expressions and enhances ventral striatal responses to happy expressions [ 81 ]. More recent research has found that, when administered to subjects with severe PTSD, MDMA induces changes in functional connectivity between the left amygdala and both the left insula and bilateral posterior cingulate cortex during autobiographical memory recall [ 82 ]. Further experiments are needed to address individual differences in responsivity to MDMA and to determine if and how to maximize the effects of MDMA administration on retrieval and reconsolidation of negative memories.
MDMA is typically administered in conjunction with therapy and the combination of MDMA plus therapy is has recently been investigated for use in indications including PTSD [ 83 , 84 ], social anxiety in adults with [ 85 ] and without autism (NCT05138068), AUDs (NCT05709353), illness-related anxiety (NCT02427568), adjustment disorder (NCT05584826), fear extinction (NCT03527316), and eating disorders (NCT04454684). The most thoroughly investigated of these indications is currently PTSD. Indeed, MDMA for PTSD might well be the first psychedelic to be submitted to the FDA as part of a new drug application (NDA) for regulatory approval and is the only psychedelic to date to have completed phase 3 clinical trials. Phase 3 findings demonstrated that MDMA-therapy was both safe and effective in treating PTSD, functional disability, and symptoms of depression in a population with severe PTSD [ 83 ].
In addition to the current manualized inner-directed therapy that has been used in conjunction with MDMA administration in phase 3, several other studies are now underway to investigate the pairing of MDMA with other gold standard, manualized therapies for PTSD. Studies are being conducted to investigate the combination of MDMA plus exposure therapy for PTSD (NCT05746572), MDMA plus group therapy for Veterans with PTSD (NCT05173831), MDMA plus cognitive processing therapy for PTSD (NCT05067244), and MDMA plus cognitive behavioral conjoint therapy for couples with PTSD (NCT02876172). Also, because of its ability to potentiate self-compassion [ 86 ], MDMA could be particularly powerful in those suffering from moral injury in relation to PTSD.
Mescaline is currently found in four species of cacti: Bolivian Flame, Peruvian Flame, San Pedro, and Peyote, the last of which has been used in ritual by Native American communities for thousands of years [ 87 ]. It has long been used as a treatment for alcoholism within Native American communities [ 88 , 89 ].
Recent self-reported data (via an online questionnaire) indicate that mescaline may attenuate symptoms of anxiety, PTSD, depression, and both alcohol and substance use [ 90 , 91 ]. In keeping with studies into the mechanistic actions of psilocybin, many participants rated their experience with mescaline as one of the most spiritually significant and meaningful experiences of their lives [ 91 ]. In addition, improvements in symptoms of anxiety, PTSD, depression, and both alcohol and other substance use were associated with greater “intensity of insight”, again demonstrating that some aspect of the subjective effect of the psychedelic experience is linked to clinical outcome. While clinical research with mescaline is still in its infancy, the data thus far suggest that mescaline may hold similar promise to other phenethylamines for the treatment of multiple mental health disorders.
Salvinorin A
Salvia divinorum is a sage species that is used ritualistically among the Mazatec tribe of Mexico. The active constituent, salvinorin A, is a kappa opioid agonist that has no discernable action at the 5HT 2A receptor, giving pause to the assertion that all psychedelics act primarily through 5HT 2A receptor activation. As a kappa agonist, salvinorin A may also hold clinical potential as a treatment for pain, ischemia, cardiac damage, and addiction [ 92 ], perhaps especially in biological females who do not find kappa agonists particularly aversive [ 93 ]. While there is still a paucity of human research on salvinorin A, recent findings indicate that when smoked, salvinorin A produces intense but short acting hallucinations and out of body experiences but, notably, no significant changes in heart rate or blood pressure [ 94 ]. In keeping with the classic psychedelics, administration of salvinorin A has also been shown to reduced brain wide dynamic functional connectivity (most notably in the default mode network), while increasing between-network static functional connectivity [ 95 ]. Unlike ibogaine, which also activates kappa opioid receptors and demonstrates anti-addictive properties, salvinorin A has not, to date, been shown to induce the notable adverse effects that have curtailed ibogaine’s development as a clinical therapeutic, and emergent events are rare ( https://calpoison.org/news/salvia-divinorum ). Because of its potential safety and novel pharmacological mechanism of action, further effort should be made to evaluate the pharmacological potential of salvinorin A.
Dissociative agents (Cyclohexanones)
Ketamine is a selective NMDA antagonist that has long been used as an anesthetic and animal tranquilizer, and which has recently found new use as a fast-acting—albeit temporary—treatment for depression [ 96 ]. While ketamine is best considered an atypical psychedelic, or perhaps a drug with psychedelic-like effects, the mechanism of action of ketamine (NMDA antagonism) is a contributor to the effects of several classic psychedelics (such as ibogaine and DMT) and may prove relevant to the further development of psychedelics as therapeutics. It is therefore worth briefly mentioning the state of current research with ketamine.
In addition to its use as an antidepressant, ketamine might hold promise for the treatment of anxiety and PTSD. A recent review indicates that, under certain circumstances (e.g., specified dose and route of administration), ketamine is effective in temporarily attenuating some anxiety disorders [ 97 ] and may therefore merit further investigation. With respect to PTSD symptomology, although a randomized, double-blind active-placebo-controlled trial demonstrated that 2 weeks of 3× weekly ketamine infusions are efficacious for up to a month in those with severe PTSD [ 98 ], a larger trial found no significant effect of 4 weeks of 2× weekly ketamine infusions in a population of military Veterans and service members with comorbid depression and PTSD [ 99 ]. Furthermore, recent years have seen the publishing of promising data on the use of ketamine as a rapid-acting therapy for substance use disorders and other neuropsychiatric conditions like obsessive-compulsive disorder [ 100 ]. As with previous depression trials, even if initially efficacious under certain dosing regimens, the limited durability of positive clinical outcomes of ketamine complicates drug administration and adoption as a frontline therapeutic [ 101 ] for a number of conditions. It is possible that, as with MDMA and psilocybin, durability of ketamine’s effects on mental health indications could be potentiated with the addition of psychotherapy and, to this end, a recent study has found that an automated, computerized training protocol might extend the effects of ketamine on depression [ 102 ]. Additional data are needed to determine whether and how the coupling of ketamine administration with psychotherapy [ 103 ] renders the drug more efficacious and durable for different indications.
Set and setting are key variables
Set and setting have long been recognized as fundamental elements driving the clinical outcomes of psychedelic administration [ 104 ], but more research is needed to operationalize and investigate how best to incorporate these factors into treatment protocols. Set is typically defined as the mindset, psychosocial education, and experience that a participant brings with them as they enter treatment, and setting is defined as the environment in which the psychedelic compound is administered. The dependence of the clinical and psychological effects of psychedelics on the mindset and environment of the user suggests that they truly are “experiential medicines”. Increasingly, human studies with psychedelics are attempting to systematically modify set and setting, either to study set and setting as independent variables affecting the outcomes of the study (NCT04410913), or by making them a fixed key part of the study design, such as electing to use group psychotherapy and/or drug administration instead of individual sessions [ 105 , 106 ]. Group treatment processes likely result in qualitatively different therapeutic environments that differ from individual treatments in ways beyond economics and scalability. The long history of the group use of psychedelic substances in Indigenous and other traditional settings across North and South America suggests that, with the proper context and training, it is possible for group psychedelic experiences to be safely managed and to result in positive outcomes for the participants [ 107 , 108 ]. Nevertheless, most of the clinical data to date have been generated using a fairly homogenous clinical approach, and so specific experiments should be conducted with more variation in set and setting to determine how best to potentiate the therapeutic value of these variables, while mitigating possible harm when administering psychedelics as medical therapies.
Optimizing set while maintaining blinding
One concern that is repeatedly raised in the discussion of psychedelic trials is the conundrum around experimental blinding [ 109 ]. The prevailing belief is that psychedelic trials are difficult to blind and therefore one must always worry that expectation is coloring outcome. This is especially true when participants enroll in a trial that not only takes up weeks of their life, but also assesses whether the investigational compound occasions a particularly meaningful, and often spiritual, life experience. While maintaining a double-blind is indeed a common challenge in psychedelic trials, several methods can be utilized to minimize the impact of expectation and ensure that clinical outcome measures reflect the long-term and durable effects of psychedelic therapies. For example, the use of an active control drug and/or a psychedelic naïve subject population may make it more difficult for even a well-informed participant to be confident of their treatment assignment. In addition, the use of a centralized assessment core to evaluate outcome measures ensures that data collection is blinded and homogeneously collected across study sites while also mitigating the risk of participants inflating their improvements to please study staff with whom they have developed a therapeutic alliance. Perhaps most importantly, the collection of long-term follow-up data from study participants can partially address concerns regarding expectation effects and can speak to the potential durability of psychedelic-induced change. While it is reasonable to suggest that a participant in the throes of a clinical trial might inadvertently exaggerate their improvements when surrounded with engaged and supportive staff, it is less reasonable to assume that this effect would last months after the trial has ended and the participant is again immersed in their regular environment.
Limitations and future directions for psychedelic therapies
The recent explosion in interest in psychedelic therapies has been based on multiple preliminary reports suggesting the potential of safety and efficacy in various psychiatric and general medical conditions, especially mood disorders, alcohol use disorder, and PTSD. These data, however, are not without their limitations. As clinically effective as psychedelics can be when administered under the right conditions, it would be negligent to forego mention of the study participants who do not respond discernably to psychedelic agents. For example, while the recent phase 3 trial of MDMA therapy for PTSD showed that 67% of participants gain complete remission from PTSD, and another 21% exhibited a clinically meaningful response, this still left 12% of study participants with no clinically meaningful response. Similarly, a recent phase 2 trial of psilocybin therapy for an episode of treatment-resistant depression showed that, while 37% of participants displayed a clinically meaningful response to psilocybin at the primary endpoint (week 3), most did not [ 56 ]. While some of this can perhaps be chalked up to the impact of set and setting, some of it is undoubtedly due to differences in sensitivity to psychedelic compounds, and perhaps also to differences in the response to the uncertainty and change brought about by these therapies. In addition, genetics play a role not only in pharmacokinetics but also in suggestibility and the development and maintenance of emotional memories [ 110 ] and may therefore also impact the effects of psychedelic therapies. One would hope that, as precision medicine advances, and as adaptive trials and genetic testing enable us to better tailor treatments to individual patients, biological and behavioral factors will be used to ensure that the potential therapeutic impact of psychedelic therapies is maximized.
Psychedelics are powerful compounds that are capable of enabling great change. As such, they should be approached with care and caution. Under the best of circumstances, and when properly facilitated, psychedelic therapy can kindle the release of some of the most deeply entrenched negative affective states and thought processes, resulting in clinical recovery and positive growth. However, the experiential flipside is equally relevant: occasionally, and especially when taken under suboptimal conditions, without adequate support, or at too high a dose, psychedelics can trigger dysphoria, disorganize thought, and spark delusional perceptions [ 111 , 112 , 113 , 114 ]. In addition, given the largely explanatory trials dataset available to date, it remains to be seen how clinical outcomes will be shaped by different real-world factors such as personality disorders, significant psychiatric and medical comorbidities, and the combination of psychedelics with different behavioral therapies or even with other psychedelics (e.g., psilocybin plus MDMA). We must therefore move forward with care and forethought. These compounds may potently manifest aspects of the human psyche in a manner that can both help and harm. As such, scientific investigations into the judicious use of psychedelics test our capacity for, and professional commitment to, the proper uses of clinical power in the service of healing. It is time that psychedelic therapies be carefully reconsidered [ 115 ].
Therapeutic Goods Administration (Australian Government). 2023. Change to classification of psilocybin and MDMA to enable prescribing by authorised psychiatrists. https://www.tga.gov.au/news/media-releases/change-classification-psilocybin-and-mdma-enable-prescribing-authorised-psychiatrists Accessed 4 June 2023.
Health Canada. Notice to stakeholders: Requests to the Special Access Program (SAP) involving psychedelic-assisted psychotherapy. 2023. https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/announcements/requests-special-access-program-psychedelic-assisted-psychotherapy.html Accessed 4 June 2023.
Delay P, Lemperiere Q. Effet therapeutique de la psilocybine sur une nevrose convulsive. Annales Medico-Psychologiques: Revue Psychiatrique. Masson & CLE, editors. Paris: Libraires De L'Academie de Medicine; 1959. p. 509–15.
Mangini M. Treatment of alcoholism using psychedelic drugs: a review of the program of research. J Psychoact Drugs. 1998;30(Dec):381–418.
Article CAS Google Scholar
Rucker JJ, Iliff J, Nutt DJ. Psychiatry & the psychedelic drugs. Past, present & future. Neuropharmacology 2018;142:200–18.
Article CAS PubMed Google Scholar
O’Brien CP, Jones RT. Methodological issues in the evaluation of a medication for its potential benefits in enhancing psychotherapy. Fifty Years of LSD: Current Status and Perspectives of Hallucinogens, New York, Parthenon. 1994:213–21.
Nutt DJ, King LA, Nichols DE. Effects of Schedule I drug laws on neuroscience research and treatment innovation. Nat Rev Neurosci. 2013;14:577–85.
Barrett FS, Doss MK, Sepeda ND, Pekar JJ, Griffiths RR. Emotions and brain function are altered up to one month after a single high dose of psilocybin. Sci Rep. 2020;10:1–4.
Article Google Scholar
Ho JT, Preller KH, Lenggenhager B. Neuropharmacological modulation of the aberrant bodily self through psychedelics. Neurosci Biobehav Rev. 2020;108:526–41.
Article PubMed Google Scholar
Reiff CM, Richman EE, Nemeroff CB, Carpenter LL, Widge AS, Rodriguez CI, et al. Work Group on Biomarkers and Novel Treatments, a Division of the American Psychiatric Association Council of Research. Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry. 2020;177:391–410.
Johnson MW, Richards WA, Griffiths RR. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22:603–20.
Article CAS PubMed PubMed Central Google Scholar
Horton DM, Morrison B, Schmidt J. Systematized review of psychotherapeutic components of psilocybin-assisted psychotherapy. Am J Psychother. 2021;74:140–9.
David E. Nichols, Charles D. Nichols, and Peter S. Hendricks.Proposed Consensus Statement on Defining Psychedelic Drugs.Psychedelic Medicine.Mar 2023.12–13. https://doi.org/10.1089/psymed.2022.0008 .
Krupitsky EM, Grinenko AY. Ketamine psychedelic therapy (KPT): a review of the results of ten years of research. J Psychoact Drugs. 1997;29:165–83.
Villoldo A. An introduction to the psychodelic psychotherapy of Salvador. Roquet J Humanist Psychol 1997;17:45–58.
Google Scholar
Naranjo C, Shulgin AT, Sargent T. Evaluation of 3, 4-methylenedioxyamphetamine (MDA) as an adjunct to psychotherapy. Pharmacology 1978;17:359–64.
Haijen ECHM, Kaelen M, Roseman L, Timmermann C, Kettner H, Russ S, et al. Predicting Responses to Psychedelics: A Prospective Study. Front Pharm. 2018;9:897.
Studerus E, Gamma A, Kometer M, Vollenweider FX. Prediction of psilocybin response in healthy volunteers. PloS ONE. 2012;7:e30800.
Studerus E, Vizeli P, Harder S, Ley L, Liechti ME. Prediction of MDMA response in healthy humans: a pooled analysis of placebo-controlled studies. J Psychopharmacol. 2021;35:556–65.
Goutarel R, Gollnhofer O, Sillans R. Pharmacodynamics and therapeutic applications of iboga and ibogaine. Psychedelic Monogr Essays 1993;6:71–111.
Maillet EL, Milon N, Heghinian MD, Fishback J, Schürer SC, Garamszegi N, et al. Noribogaine is a G-protein biased κ-opioid receptor agonist. Neuropharmacology 2015;99:675–88.
Mash DC, Kovera CA, Buck BE, Norenberg MD, Shapshak P, Hearn WL, et al. Medication Development of Ibogaine as a Pharmacotherapy for Drug Dependence. Ann N. Y Acad Sci. 1998;844:274–92. https://doi.org/10.1111/j.1749-6632.1998.tb08242.x .
Mash DC, Kovera CA, Pablo J, Tyndale RF, Ervin FD, Williams IC, et al. Ibogaine: complex pharmacokinetics, concerns for safety, and preliminary efficacy measures. Ann N Y Acad Sci. 2000;914:394–401.
Schenberg EE, de Castro Comis MA, Chaves BR, da Silveira DX. Treating drug dependence with the aid of ibogaine: A retrospective study. J Psychopharmacol. 2014;28:993–1000.
Litjens RP, Brunt TM. How toxic is ibogaine? Clin Toxicol (Philos). 2016;54:297–302. https://doi.org/10.3109/15563650.2016.1138226 .
Köck P, Froelich K, Walter M, Lang U, Dürsteler KM J Subst Abuse Treat. 2022 A systematic literature review of clinical trials and therapeutic applications of ibogaine. Jul;138:108717.
Ona G, Rocha JM, Bouso JC, Hallak JEC, Borràs T, Colomina MT, et al. The adverse events of ibogaine in humans: an updated systematic review of the literature (2015–2020). Psychopharmacology (Berl). 2022;239:1977–87. https://doi.org/10.1007/s00213-021-05964-y .
KR Alper, Stajić M, JR Gill. Fatalities Temporally Associated with the Ingestion of Ibogaine. 2012;57:398–412.
Patrick M, Could an illegal psychedelic substance ease the opioid crisis? Daniel Cameron wants to find out. News from the States. 2023. https://www.newsfromthestates.com/article/could-illegal-psychedelic-substance-ease-opioid-crisis-daniel-cameron-wants-find-out Accessed, 4 June, 2023.
Knuijver T, Schellekens A, Belgers M, Donders R, van Oosteren T, Kramers K, et al. Safety of ibogaine administration in detoxification of opioid-dependent individuals: a descriptive open-label observational study. Addiction. 2022;117:118–28. https://doi.org/10.1111/add.15448 .
Rickli A, Moning OD, Hoener MC, Liechti ME. Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. Eur Neuropsychopharmacol. 2016;26:1327–37.
Watts VJ, Lawler CP, Rox DR, Neve KA, Nichols DE, Mailman RB. LSD and structural analogs: pharmacological evaluation at D 1 dopamine receptors. Psychopharmacology 1995;118:401–9.
Marona-Lewicka D, Chemel BR, Nichols DE. Dopamine D4 receptor involvement in the discriminative stimulus effects in rats of LSD, but not the phenethylamine hallucinogen DOI. Psychopharmacol (Berl). 2009;203:265–77.
Dyck E. ‘Hitting highs at rock bottom’: LSD treatment for alcoholism, 1950–1970. Soc Hist Med. 2006;19:313–29.
Krebs TS, Johansen PØ. Lysergic acid diethylamide (LSD) for alcoholism: meta-analysis of randomized controlled trials. J Psychopharmacol. 2012;26:994–1002.
B Liester M. A review of lysergic acid diethylamide (LSD) in the treatment of addictions: historical perspectives and future prospects. Curr Drug Abus Rev. 2014;7:146–56.
Leonard HL, Rapoport JL. Relief of obsessive-compulsive symptoms by LSD and psilocin. Am J Psychiatry. 1987.
Gasser P, Kirchner K, Passie T. LSD-assisted psychotherapy for anxiety associated with a life-threatening disease: a qualitative study of acute and sustained subjective effects. J Psychopharmacol. 2015;29:57–68. https://doi.org/10.1177/0269881114555249 .
Sewell RA, Halpern JH, Pope HG Jr. Response of cluster headache to psilocybin and LSD. Neurology 2006;66:1920–2. https://doi.org/10.1212/01.wnl.0000219761.05466.43 .
Haijen EC, Hurks PP, Kuypers KP. Microdosing with psychedelics to self-medicate for ADHD symptoms in adults: A prospective naturalistic study. Neurosci Appl. 2022;1:101012.
Gasser P. Psycholytic Therapy with MDMA and LSD in Switzerland. Newsletter of the Multidisciplinary Association for Psychedelic Studies MAPS - Volume 5 Number 3 Winter 1994–95 - p. 3–7.
Pahnke WN, Kurland AA, Unger S, Savage C, Grof S. The experimental use of psychedelic (LSD) psychotherapy. JAMA. 1970;212:1856–63.
Gasser P, Holstein D, Michel Y, Doblin R, Yazar-Klosinski B, Passie T, et al. Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. Nerv Ment Dis. 2014;202:513–20.
Schindler EA, Gottschalk CH, Weil MJ, Shapiro RE, Wright DA, Sewell RA. Indoleamine Hallucinogens in Cluster Headache: Results of the Clusterbusters Medication Use Survey. J Psychoact Drugs. 2015;47:372–81. https://doi.org/10.1080/02791072.2015.1107664 .
Family N, Maillet EL, Williams LT, Krediet E, Carhart-Harris RL, Williams TM, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of low dose lysergic acid diethylamide (LSD) in healthy older volunteers. Psychopharmacology 2020;237:841–53.
Passie T, Seifert J, Schneider U, Emrich HM. The pharmacology of psilocybin. Addict Biol. 2002;7:357–64. https://doi.org/10.1080/1355621021000005937 .
Moreno JL, Holloway T, Albizu L, Sealfon SC, González-Maeso J. Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci Lett. 2011;493:76–9.
Davis AK, Barrett FS, Griffiths RR. Psychological flexibility mediates the relations between acute psychedelic effects and subjective decreases in depression and anxiety. J Contextual Behav Sci. 2020;15:39–45.
Carhart-Harris RL, Bolstridge M, Rucker J, Day CM, Erritzoe D, Kaelen M, et al. Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry. 2016;3:619–27.
Bogenschutz MP, Ross S, Bhatt S, Baron T, Forcehimes AA, Laska E, et al. Percentage of Heavy Drinking Days Following Psilocybin-Assisted Psychotherapy vs Placebo in the Treatment of Adult Patients With Alcohol Use Disorder: A Randomized Clinical Trial. JAMA Psychiatry. 2022;79:953–62. https://doi.org/10.1001/jamapsychiatry.2022.2096 .
Article PubMed PubMed Central Google Scholar
Johnson MW, Garcia-Romeu A, Cosimano MP, Griffiths RR. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol. 2014;28:983–92.
Moreno FA, Wiegand CB, Taitano EK, Delgado PL. Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. J Clin Psychiatry. 2006;67:1735–40.
Anderson BT, Danforth A, Daroff PR, Stauffer C, Ekman E, Agin-Liebes G, et al. Psilocybin-assisted group therapy for demoralized older long-term AIDS survivor men: An open-label safety and feasibility pilot study. EClinicalMedicine. 2020;27:100538 https://doi.org/10.1016/j.eclinm.2020.100538 .
Johnson MW, Garcia-Romeu A, Griffiths RR. Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abus. 2017;43:55–60.
James E, Robertshaw TL, Hoskins M, Sessa B. Psilocybin occasioned mystical-type experiences. Hum Psychopharmacol Clin Exp. 2020;35:e2742.
Goodwin GM, Aaronson ST, Alvarez O, Arden PC, Baker A, Bennett JC, et al. Single-Dose Psilocybin for a Treatment-Resistant Episode of Major Depression. N Engl J Med 2022;387:1637–48. https://doi.org/10.1056/NEJMoa2206443 .
Davis AK, Barrett FS, May DG, Cosimano MP, Sepeda ND, Johnson MW, et al. Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry. 2021;78:481–9. https://doi.org/10.1001/jamapsychiatry.2020.3285 .
Gukasyan N, Davis AK, Barrett FS, Cosimano MP, Sepeda ND, Johnson MW, et al. Efficacy and safety of psilocybin-assisted treatment for major depressive disorder: Prospective 12-month follow-up. J Psychopharmacol. 2022;36:151–8.
Labate BC, Cavnar C, editors. Ayahuasca shamanism in the Amazon and beyond. Oxford Ritual Studies; 2014.
Dos Santos RG, Hallak JEC. Therapeutic use of serotoninergic hallucinogens: A review of the evidence and of the biological and psychological mechanisms. Neurosci Biobehav Rev. 2020;108:423–34. https://doi.org/10.1016/j.neubiorev.2019.12.001 .
González D, Cantillo J, Pérez I, Farré M, Feilding A, Obiols JE, et al. Therapeutic potential of ayahuasca in grief: a prospective, observational study. Psychopharmacology 2020;237:1171–82.
Lafrance A, Loizaga-Velder A, Fletcher J, Renelli M, Files N, Tupper KW. Nourishing the Spirit: Exploratory Research on Ayahuasca Experiences along the Continuum of Recovery from Eating Disorders. J Psychoact Drugs. 2017;49:427–35. https://doi.org/10.1080/02791072.2017.1361559 .
Fábregas JM, González D, Fondevila S, Cutchet M, Fernández X, Barbosa PCR, et al. "Assessment of addiction severity among ritual users of ayahuasca". Drug Alcohol Depend. 2010;111:257–61.
Harris R, Gurel L. "A Study of Ayahuasca Use in North America". J Psychoact Drugs. 2012;44:209–15.
Barbosa PC, Strassman RJ, Da Silveira DX, Areco K, Hoy R, Pommy J, et al. Psychological and neuropsychological assessment of regular hoasca users. Compr Psychiatry. 2016;71:95–105.
Sarris J, Perkins D, Cribb L, Schubert V, Opaleye E, Bouso JC, et al. Ayahuasca use and reported effects on depression and anxiety symptoms: An international cross-sectional study of 11,912 consumers. J Affect Disord Rep. 2021;4:100098.
Durante Í, Dos Santos RG, Bouso JC, Hallak JE. Risk assessment of ayahuasca use in a religious context: self-reported risk factors and adverse effects. Braz J Psychiatry. 2020;43:362–9.
Article PubMed Central Google Scholar
Palhano-Fontes F, Barreto D, Onias H, Andrade KC, Novaes MM, Pessoa JA, et al. "Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial". Psychological Med. 2018;49:655–63.
Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang G, Quigley DI, et al. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol Pharm. 2001;60:1181–8. https://doi.org/10.1124/mol.60.6.1181 .
Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science. 2009;323:934–7. https://doi.org/10.1126/science.1166127 .
Carbonaro TM, Eshleman AJ, Forster MJ, Cheng K, Rice KC, Gatch MB. The role of 5-HT2A, 5-HT 2C and mGlu2 receptors in the behavioral effects of tryptamine hallucinogens N,N-dimethyltryptamine and N,N-diisopropyltryptamine in rats and mice. Psychopharmacol (Berl). 2015;232:275–84. https://doi.org/10.1007/s00213-014-3658-3 .
Strassman RJ, Qualls CR. Dose-response study of N,N-dimethyltryptamine in humans. I. Neuroendocrine, autonomic, and cardiovascular effects. Arch Gen Psychiatry. 1994;51:85–97. https://doi.org/10.1001/archpsyc.1994.03950020009001 .
Adamson S, (Ed). Through the gateway of the heart: Accounts of experiences with MDMA and other empathogenic substances. San Francisco: Four Trees Publications; 1985.
Greer G, Tolbert R. Subjective reports of the effects of MDMA in a clinical setting. J Psychoact Drugs. 1986;18:319–27. https://doi.org/10.1080/02791072.1986.10472364 . PMID: 2880946
Shulgin AT, Shulgin A. PiHKAL: A Chemical Love Story, Transform Press; 1991.
Verrico CD, Miller GM, Madras BK. MDMA (Ecstasy) and human dopamine, norepinephrine, and serotonin transporters: implications for MDMA-induced neurotoxicity and treatment. Psychopharmacology (Berl). 2007;189:489–503. https://doi.org/10.1007/s00213-005-0174-5 .
Nichols DE, Lloyd DH, Hoffman AJ, Nichols MB, Yim G. Effects of certain hallucinogenic amphetamine analogs on the release of [3H]-serotonin from rat brain synaptosomes. J Med Chem. 1982;25:530–5.
Rudnick G, Wall SC. The molecular mechanism of “ecstasy” [3, 4-methylenedioxy-methamphetamine (MDMA)]: serotonin transporters are targets for MDMA-induced serotonin release. Proc Natl Acad Sci. 1992;89:1817–21.
Kirkpatrick MG, Francis SM, Lee R, de Wit H, Jacob S. Plasma oxytocin concentrations following MDMA or intranasal oxytocin in humans. Psychoneuroendocrinology. 2014;46:23–31. https://doi.org/10.1016/j.psyneuen.2014.04.006 .
Bos PA, Panksepp J, Bluthé RM, van Honk J. Acute effects of steroid hormones and neuropeptides on human social-emotional behavior: a review of single administration studies. Front Neuroendocrinol. 2012;33:17–35. https://doi.org/10.1016/j.yfrne.2011.01.002 .
Bedi G, Phan KL, Angstadt M, de Wit H. Effects of MDMA on sociability and neural response to social threat and social reward. Psychopharmacol (Berl). 2009;207:73–83. https://doi.org/10.1007/s00213-009-1635-z .
Singleton SP, Wang JB, Mithoefer M, Hanlon C, George MS, Mithoefer A, et al. Altered brain activity and functional connectivity after MDMA-assisted therapy for post-traumatic stress disorder. Front Psychiatry. 2023;13:947622 https://doi.org/10.3389/fpsyt.2022.947622 .
Mitchell JM, Bogenschutz M, Lilienstein A, Harrison C, Kleiman S, Parker-Guilbert K, et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. 2021;27:1025–33. https://doi.org/10.1038/s41591-021-01336-3 .
Smith KW, Sicignano DJ, Hernandez AV, White CM. MDMA-Assisted Psychotherapy for Treatment of Posttraumatic Stress Disorder: A Systematic Review with Meta-Analysis. J Clin Pharm. 2022;62:463–71. https://doi.org/10.1002/jcph.1995 .
Danforth AL, Grob CS, Struble C, Feduccia AA, Walker N, Jerome L, et al. Reduction in social anxiety after MDMA-assisted psychotherapy with autistic adults: a randomized, double-blind, placebo-controlled pilot study. Psychopharmacol (Berl). 2018;235:3137–48. https://doi.org/10.1007/s00213-018-5010-9 .
Kamboj SK, Kilford EJ, Minchin S, Moss A, Lawn W, Das RK, et al. Recreational 3,4-methylenedioxy-N-methylamphetamine (MDMA) or 'ecstasy' and self-focused compassion: Preliminary steps in the development of a therapeutic psychopharmacology of contemplative practices. J Psychopharmacol. 2015;29:961–70. https://doi.org/10.1177/0269881115587143 .
El-Seedi HR, De Smet PA, Beck O, Possnert G, Bruhn JG. "Prehistoric peyote use: alkaloid analysis and radiocarbon dating of archaeological specimens of Lophophora from Texas". J Ethnopharmacol. 2005;101:238–42.
Albaugh BJ, Anderson PO. Peyote in the treatment of alcoholism among American Indians. Am J Psychiatry. 1974;131:1247–50. https://doi.org/10.1176/ajp.131.11.1247 .
Blum K, Futterman SL, Pascarosa P. Peyote, a potential ethnopharmacologic agent for alcoholism and other drug dependencies: possible biochemical rationale. Clin Toxicol. 1977;11:459–72. https://doi.org/10.3109/15563657708988210 .
Garcia-Romeu A, Davis AK, Erowid F, Erowid E, Griffiths RR, Johnson MW. Cessation and reduction in alcohol consumption and misuse after psychedelic use. J Psychopharmacol. 2019;33:1088–101. https://doi.org/10.1177/0269881119845793 .
Agin-Liebes G, Haas TF, Lancelotta R, Uthaug MV, Ramaekers JG, Davis AK. Naturalistic Use of Mescaline Is Associated with Self-Reported Psychiatric Improvements and Enduring Positive Life Changes. ACS Pharm Transl Sci. 2021;4:543–52. https://doi.org/10.1021/acsptsci.1c00018 .
Beck TC, Hapstack MA, Beck KR, Dix TA. Therapeutic potential of kappa opioid agonists. Pharmaceuticals 2019;12:95.
Rasakham K, Liu-Chen LY. Sex differences in kappa opioid pharmacology. Life Sci 2011;88:2–16. https://doi.org/10.1016/j.lfs.2010.10.007 .
Johnson MW, Griffiths RR. P. 1. c. 057 Human psychopharmacology of the kappa opioid agonist salvinorin A. Eur Neuropsychopharmacol. 2010;20:S267–8.
Doss MK, May DG, Johnson MW, Clifton JM, Hedrick SL, Prisinzano TE, et al. The Acute Effects of the Atypical Dissociative Hallucinogen Salvinorin A on Functional Connectivity in the Human Brain. Sci Rep. 2020;10:16392 https://doi.org/10.1038/s41598-020-73216-8 .
Corriger A, Pickering G. Ketamine and depression: a narrative review. Drug Des Devel Ther. 2019;13:3051–67. https://doi.org/10.2147/DDDT.S221437 .
Tully JL, Dahlén AD, Haggarty CJ, Schiöth HB, Brooks S. Ketamine treatment for refractory anxiety: A systematic review. Br J Clin Pharm. 2022;88:4412–26. https://doi.org/10.1111/bcp.15374 .
Feder A, Costi S, Rutter SB, Collins AB, Govindarajulu U, Jha MK, et al. A Randomized Controlled Trial of Repeated Ketamine Administration for Chronic Posttraumatic Stress Disorder. Am J Psychiatry. 2021;178:193–202. https://doi.org/10.1176/appi.ajp.2020.20050596 .
Abdallah CG, Roache JD, Gueorguieva R, Averill LA, Young-McCaughan S, Shiroma PR, et al. Dose-related effects of ketamine for antidepressant-resistant symptoms of posttraumatic stress disorder in veterans and active duty military: a double-blind, randomized, placebo-controlled multi-center clinical trial. Neuropsychopharmacology. 2022;47:1574–81. https://doi.org/10.1038/s41386-022-01266-9 .
Walsh Z, Mollaahmetoglu OM, Rootman J, Golsof S, Keeler J, Marsh B, et al. Ketamine for the treatment of mental health and substance use disorders: comprehensive systematic review. BJPsych Open. 2022;8:e19.
Rodriguez CI, Kegeles LS, Levinson A, Feng T, Marcus SM, Vermes D, et al. Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology. 2013;38:2475–83. https://doi.org/10.1038/npp.2013.150 .
Price RB, Spotts C, Panny B, Griffo A, Degutis M, Cruz N, et al. A novel, brief, fully automated intervention to extend the antidepressant effect of a single ketamine infusion: a randomized clinical trial. Am J Psychiatry. 2022;179:959–68.
Drozdz SJ, Goel A, McGarr MW, Katz J, Ritvo P, Mattina GF, et al. Ketamine assisted psychotherapy: A systematic narrative review of the literature. J Pain Res. 2022:1691–706.
Leary T, Litwin GH, Metzner R. Reactions to psilocybin administered in a supportive environment. J Nerv Ment Dis. 1963;137:561–73. https://doi.org/10.1097/00005053-196312000-00007 .
Agrawal M, Emanuel E, Richards B, Richards W, Roddy K, Thambi P. Assessment of Psilocybin Therapy for Patients with Cancer and Major Depression Disorder. JAMA Oncol. 2023. https://doi.org/10.1001/jamaoncol.2023.0351 .
Gasser P. Psychedelic group therapy. In Disruptive Psychopharmacology. Cham: Springer International Publishing; 2022. p 23–34.
Bouso JC, González D, Fondevila S, Cutchet M, Fernández X, Ribeiro Barbosa PC, et al. Personality, psychopathology, life attitudes and neuropsychological performance among ritual users of ayahuasca: a longitudinal study. PLoS ONE. 2012. https://doi.org/10.1371/journal.pone.0042421 .
Csordas TJ. The Navajo healing project. Med Anthropol Q. 2000;14:463–75.
Muthukumaraswamy SD, Forsyth A, Lumley T. Blinding and expectancy confounds in psychedelic randomized controlled trials. Expert Rev Clin Pharmacol. 2021;14:1133–52.
Norrholm SD, Ressler KJ. Genetics of anxiety and trauma-related disorders. Neuroscience. 2009;164:272–87. https://doi.org/10.1016/j.neuroscience.2009.06.036 .
Strassman RJ. Adverse reactions to psychedelic drugs. A review of the literature. J Nerv Ment Dis. 1984;172:577–95.
Freedman DX. "On the Use and Abuse of LSD.". Arch Gen Psychiatry. 1968;18:330–47.
Carbonaro TM, Bradstreet MP, Barrett FS, MacLean KA, Jesse R, Johnson MW, et al. Survey study of challenging experiences after ingesting psilocybin mushrooms: Acute and enduring positive and negative consequences. J Psychopharmacol. 2016;30:1268–78.
Bender D, Hellerstein DJ. Assessing the risk–benefit profile of classical psychedelics: A clinical review of second-wave psychedelic research. Psychopharmacology. 2022:1–26.
Grinspoon L, Bakalar J. Psychedelic drugs reconsidered. New York: Basic Books; 1979.
Download references
Author information
Authors and affiliations.
Department of Neurology, University of California San Francisco, San Francisco, CA, USA
Jennifer M. Mitchell
Department of Psychiatry and Behavioral Sciences, University of California San Francisco, San Francisco, CA, USA
Jennifer M. Mitchell & Brian T. Anderson
Department of Veterans Affairs, Research Service, San Francisco VA Medical Center, San Francisco, CA, USA
Berkeley Center for the Science of Psychedelics, University of California Berkeley, Berkeley, CA, USA
You can also search for this author in PubMed Google Scholar
Contributions
The authors contributed equally to the writing of this paper.
Corresponding author
Correspondence to Jennifer M. Mitchell .
Ethics declarations
Competing interests.
In the last 3 years, BA has received consulting fees from Journey Colab and JM has received grant support from the Multidisciplinary Association for Psychedelic Studies.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Mitchell, J.M., Anderson, B.T. Psychedelic therapies reconsidered: compounds, clinical indications, and cautious optimism. Neuropsychopharmacol. 49 , 96–103 (2024). https://doi.org/10.1038/s41386-023-01656-7
Download citation
Received : 17 April 2023
Revised : 05 June 2023
Accepted : 06 July 2023
Published : 21 July 2023
Issue Date : January 2024
DOI : https://doi.org/10.1038/s41386-023-01656-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
INNOVATIONS in pharmacy
Vol. 15 No. 2 (2024)
Copyright (c) 2024 Patrick Gallegos, Salaar, Michael

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License .
Copyright of content published in INNOVATIONS in pharmacy belongs to the author(s).
Leadership and Followership in Health Professions: A Systematic Review
Patrick Gallegos
Cleveland Clinic Akron General
Muhammad Salaar Riaz
Nassau University Medical Center
Michael Peeters
University of Toledo
DOI: https://doi.org/10.24926/iip.v15i2.5987
Keywords: Leadership, Followership, Health Professions
Objective: Leadership discussion, including leadership development programs, is common. However, discussion of followership as a component of leadership seems less frequently discussed. With a focus on leadership and followership, this investigation reviewed the health-professions education literature and characterized leadership-followership within health-professions education.
Methods : Using PubMed, ERIC, and Google Scholar, two investigators independently and systematically searched health-professions education literature for articles related to leadership and followership. Reports were categorized based on the articles by type, application, profession, leadership, and followership qualities.
Results: Eighty-one articles were included. More than half (48/81, 59%) were theoretical, 27% (22/81) empirical, 7% (6/81) commentaries, and 6% (5/81) letters-to-the-editor). Empirical studies did not share outcomes that could be meaningfully combined quantitatively by meta-analysis; however, the vast majority (96%) of theoretical articles discussed a healthcare-related application of leadership and followership (e.g., improving patient care, improving communication, improving organizational efficiency). Thus, a qualitative review was completed. Of the 81 articles, 57% (n=46) involved multiple professions, while 43% (n=35) focused on a specific profession [Nursing (n=16), Medicine (n=7), Others (n=5) Surgery (n=3), Pharmacy (n=2), Veterinary Medicine (n=2)]. While most articles (75%) discussed leadership qualities (with top qualities of effective communication, visionary, and delegating tasks), fewer (57%) discussed followership qualities (with top qualities of being responsible, committed, and supportive). Of note, some qualities overlapped in both leadership and followership (with top qualities of effective communication, being supportive, and providing/receiving feedback).
Conclusions: Leadership-Followership was described in many health-professions’ education literature. However, Pharmacy and Veterinary Medicine had substantially fewer articles published on this topic. Notably, followership did not receive nearly as much attention as leadership. Leadership has a dynamic and complex interaction with followership highlighting that an effective leader must know how to be an effective follower and vice versa. To improve leadership within healthcare teamwork, education should focus on both leadership-followership.
Author Biographies
Muhammad salaar riaz, nassau university medical center.
Internal Medicine Resident
Michael Peeters, University of Toledo
Director of Interprofessional Education

Contact Publishing Services | Acceptable Use of IT Resources
The copyright of these individual works published by the University of Minnesota Libraries Publishing remains with the original creator or editorial team. For uses beyond those covered by law or the Creative Commons license, permission to reuse should be sought directly from the copyright owner listed on each article.
- Download PDF
- CME & MOC
- Share X Facebook Email LinkedIn
- Permissions
Barrett Esophagus : A Review
- 1 University of Kansas School of Medicine, VA Medical Center, Kansas City, Kansas
- Clinical Trials Update Novel Device Improves Barrett Esophagus Diagnosis in Primary Care Anita Slomski, MA JAMA
- Original Investigation Biomarkers for Human Papillomavirus and Survival With Barrett Dysplasia and Adenocarcinoma Shanmugarajah Rajendra, MBBCh, MSc, MD, FRACP; Preeti Sharma, BMedSci; Shweta Dutta Gautam, MSc, PhD; Manoj Saxena, MBBChir, PhD, FRACP; Amit Kapur, MBBS, PhD, FRACP; Prateek Sharma, MD; Neil Merrett, MBBS, FRACS; Tao Yang, PhD, FRCPA; Leonardo D. Santos, MBBS, FRCPA; Darren Pavey, MBBS, FRACP; Omar Sharaiha, MBBS, FRACP; Owen McKay, MBBS, FRACP; Hugh Dixson, MBBS, FRACP, FAANMS; Wei Xuan, MSc, PhD JAMA Network Open
Importance Barrett esophagus is characterized by the replacement of normal esophageal squamous cell epithelium with columnar metaplasia and affects approximately 5% of people in the US and approximately 1% worldwide. Approximately 3% to 5% of patients with Barrett esophagus will be diagnosed with esophageal adenocarcinoma in their lifetime.
Observations Barrett esophagus affects approximately 2.3% to 8.3% of people with gastroesophageal reflux disease (GERD) and approximately 1.2% to 5.6% of people without GERD. Characteristics associated with Barrett esophagus include older age (prevalence of approximately 1.1% in individuals older than 50 years compared with 0.3% in those 50 years or younger), male sex, and smoking (prevalence of approximately 12% in people who smoke cigarettes compared with 1.1% in those who do not smoke cigarettes). The histopathology of Barrett esophagus progresses from metaplasia to dysplasia and, without treatment, can progress to adenocarcinoma. People with Barrett esophagus have approximately a 0.2% to 0.5% annual rate of developing esophageal adenocarcinoma. Management of Barrett esophagus primarily consists of acid-suppressive medications to reduce underlying GERD symptoms and surveillance endoscopy every 3 to 5 years. In patients with Barrett esophagus and dysplasia or early cancer, endoscopic therapy consisting of resection and ablation successfully treats 80% to 90% of patients.
Conclusions and Relevance Barrett esophagus affects approximately 5% of people in the US and approximately 1% worldwide and is associated with an increased risk of esophageal adenocarcinoma. First-line therapy for Barrett esophagus consists of proton-pump inhibitors for control of reflux symptoms, but their role in chemoprevention is unclear. Surveillance with upper endoscopy is recommended by practice guidelines to monitor for progression to esophageal adenocarcinoma, but randomized clinical trials are lacking.
Read More About
Sharma P. Barrett Esophagus : A Review . JAMA. 2022;328(7):663–671. doi:10.1001/jama.2022.13298
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.193(49); 2021 Dec 13

Soviet pharmaceutical regulation (1918–1990)
What counts as robust evidence for drug regulators is influenced by social, political and cultural factors. A dramatic example of culture and politics shaping regulatory science can be found in the history of pharmaceutical testing in the Union of Soviet Socialist Republics (USSR or Soviet Union) before 1990. Regulators in the USSR did not rely on the 4-phase clinical trial model introduced in the West in the 1960s. This model was not officially supported in the USSR, because it was considered wasteful and too remote from clinical realities. In fact, several core characteristics of Western regulatory science, such as randomization, double blinding and the use of placebos, were publicly rejected in the USSR as unethical and exploitative of research participants. 1 The Soviet drug testing system prioritized testing “in the real world” and thus represented an alternative to what ultimately became the global gold standard. With the collapse of communism and the postsocialist transition in the early 1990s, Russia embraced the Western model and completely abandoned the older Soviet system for testing drugs. 2 This article interprets Soviet drug regulation as an expression of prevailing political forces that shaped what counted as authoritative knowledge.
The Soviet Union was politically centralized with a socialized economy. It had a universal health care system, supported by basic and applied research. Starting in 1918, The People’s Commissariat of Public Health of the Russian Soviet Federative Socialist Republic (also known by its abbreviated name, Narkomzdrav), oversaw all medical matters. Of importance, it took control of the pharmaceutical industry after the 1917 Revolution, which included pharmaceutical research. 3 Research on new drugs was a priority for the newly formed Soviet government, which sought to achieve independence from drugs manufactured in the “bourgeois West.”
Archival documents indicate that as early as 1921, Soviet health care authorities requested that all new drugs introduced to medical practice be tested in clinical trials (mostly observational) and evaluated by the relevant department of the People’s Commissariat of Public Health. For example, in September of 1921, the Venereological Section of the Narkomzdrav insisted that the pharmaceutical company Glavanil provide the results of clinical trials for novoarsenol (neosalvarsan) before it could be used in any health care facilities. 4 The section indicated that the Supreme Soviet of the People’s Economy, the main regulatory board in the sphere of economic relations, supported such demands.

A photo of the building in downtown Moscow (14 Solianka Street) that housed the central Soviet drug regulator, the Pharmacological Committee.
In 1923, the Soviet government decided to establish the Pharmacopoeia Commission, which was tasked with controlling the quality of pharmaceuticals. The Commission, which controlled the import and production of new pharmaceuticals, required submission of all available research data to health care authorities. 5 This was further specified in a circular decree, “On Regulation of Finished Pharmaceutical Products,” issued on May 25, 1926, by the People’s Commissariat for Public Health. The decree postulated that “new drugs can only be approved after the pharmaceutical and clinical investigation of their value.” 6 The instruction preceded the 1938 Federal Food, Drug, and Cosmetic Act in the United States. 7 It also resembled (and may have been a model for) Scandinavian drug regulation in the late 1920s and early 1930s. 8
Through the 1930s, the Pharmacopoeia Commission was reorganized several times and given new responsibilities. Under the name of the Pharmacological Committee, it ultimately took charge of reviewing clinical reports, influenced the interpretation of clinical trials and issued statements recommending or denying drug registration. Except for a brief decentralization experiment in 1958–1963 under the leadership of Nikita Khrushchev, in which decisions were delegated to the 15 constituent republics of the Soviet Union, the central Pharmacological Committee made decisions for the entire USSR. The committee initially consisted of eminent physicians based at a Moscow research university; however, after 1970, these medical experts were increasingly supplemented by representatives of the Ministry of Health, which gave the organization a more bureaucratic outlook. At their meetings (usually biweekly or monthly), the committee reviewed applications for prospective drugs (both Soviet and foreign made). Three outcomes were possible after a review: register the drug right away based on existing evidence, organize clinical trials of the substance or deny it registration altogether. Outright approvals were uncommon.
The committee selected sites for clinical trials based on the perceived expertise of the organizations in specific medical fields. Trials were conducted mostly in Moscow and Leningrad, with some participation in cities in the European part of the USSR. The direct involvement of the Pharmacological Committee in selecting sites for clinical trials was presented as a sign of coherent regulatory policy, and the potential for cronyism arising from the tight relationship between the committee and clinicians at preferred sites received little if any attention. 1 The committee granted substantial latitude to individual clinics in designing and conducting trials. Although the results of some experiments or individual trials were published in Soviet and international medical journals, reports presented to the Pharmacological Committee were kept confidential and access to this documentation remains restricted to the public.
Before 1970, most trials were observational. Placebos were generally discouraged on ethical grounds as potentially unfair to research participants, who could be denied an effective medication. 1 This approach to placebos reflects, in part, a willingness of the Soviet regulator to assume that drug development in the USSR was inherently safer than in the West, where the profit motive was seen to drive medical science, even at the expense of denying some trial participants effective treatment. Some exceptions to the no-placebo rule were made for vaccines, antiarrhythmic medications, plant-based medicines and oral contraception. 9 Vaccine research was seen as a special ethical case, because vaccines were for use in “healthy populations,” and hence placebo did not deny treatment to a person who was sick. 1 On the other hand, antiarrhythmic trials were arguably influenced by the personal interests of lead investigators, who looked favourably on Western technology and its methods of assessment. 10
Despite official resistance to placebo-controlled trials, in the early 1980s, some research institutions adopted the methods used for randomized controlled trials, describing it as “modern” or “progressive.” There is no evidence that these institutions were punished for deviating from central policy. The last decade of the Soviet Union was marked by increased interest in Western models of administration, and toward the end of the 1980s, administrators (including the Chairman of the committee, Professor Vladimir K. Lepakhin) discussed reforming the Soviet system along the lines of American, British and French regulators. This set the stage for the creation in 1990 of the All-Union Scientific Center for Pharmaceutical Expertise, and the subsequent adoption of Western-style randomized clinical trials as the gold standard in Russian drug regulation. 11
Although the history of Soviet drug regulation seems to end in 1990, recent events surrounding the limited acceptance of the Sputnik V vaccine for SARS-CoV-2 suggest it remains relevant. 12 Traces of Soviet politics are deeply embedded in Russian culture. Could it be that reports of self-experimentation by medical researchers and a lack of data transparency have rekindled memories of Soviet-era pharmaceutical regulation, and perhaps concerns that “modernization” has failed? 13 Even though Russian regulators shifted their policies in the early 1990s, what is perceived as authoritative medical knowledge cannot be reduced to experimental methods. Today, as in the past, what counts as trustworthy knowledge is inextricably bound to slowly changing social, political and cultural factors.
This article was solicited and has been peer reviewed.
Competing interests: None declared.
Funding: Pavel Vasilyev and Alexander Petrenko received a grant from the Russian Science Foundation (no. 18-78-10016).
- Case report
- Open access
- Published: 25 May 2024
Wandering spleen presenting in the form of right sided pelvic mass and pain in a patient with AD-PCKD: a case report and review of the literature
- Yitagesu aberra shibiru ORCID: orcid.org/0000-0003-3645-9115 1 ,
- Sahlu wondimu 1 &
- Wassie almaw 1
Journal of Medical Case Reports volume 18 , Article number: 259 ( 2024 ) Cite this article
310 Accesses
2 Altmetric
Metrics details
Wandering spleen is a rare clinical entity in which the spleen is hypermobile and migrate from its normal left hypochondriac position to any other abdominal or pelvic position as a result of absent or abnormal laxity of the suspensory ligaments (Puranik in Gastroenterol Rep 5:241, 2015, Evangelos in Am J Case Rep. 21, 2020) which in turn is due to either congenital laxity or precipitated by trauma, pregnancy, or connective tissue disorder (Puranik in Gastroenterol Rep 5:241, 2015, Jawad in Cureus 15, 2023). It may be asymptomatic and accidentally discovered for imaging done for other reasons or cause symptoms as a result of torsion of its pedicle and infarction or compression on adjacent viscera on its new position. It needs to be surgically treated upon discovery either by splenopexy or splectomy based on whether the spleen is mobile or not.
Case presentation
We present a case of 39 years old female Ethiopian patient who presented to us complaining constant lower abdominal pain especially on the right side associated with swelling of one year which got worse over the preceding few months of her presentation to our facility. She is primiparous with delivery by C/section and a known case of HIV infection on HAART. Physical examination revealed a right lower quadrant well defined, fairly mobile and slightly tender swelling. Hematologic investigations are unremarkable. Imaging with abdominopelvic U/S and CT-scan showed a predominantly cystic, hypo attenuating right sided pelvic mass with narrow elongated attachment to pancreatic tail and absent spleen in its normal position. CT also showed multiple different sized purely cystic lesions all over both kidneys and the pancreas compatible with AD polycystic kidney and pancreatic disease.
With a diagnosis of wandering possibly infarcted spleen, she underwent laparotomy, the finding being a fully infarcted spleen located on the right half of the upper pelvis with twisted pedicle and dense adhesions to the adjacent distal ileum and colon. Release of adhesions and splenectomy was done. Her post-operative course was uneventful.
Wandering spleen is a rare clinical condition that needs to be included in the list of differential diagnosis in patients presenting with lower abdominal and pelvic masses. As we have learnt from our case, a high index of suspicion is required to detect it early and intervene by doing splenopexy and thereby avoiding splenectomy and its related complications.
Peer Review reports
Introduction
Wandering spleen is a rare clinical entity characterized by hypermobility of the spleen as a result of absence or abnormal laxity of its suspensory ligaments which in turn can be congenital or precipitated by a number of risk factors like repeated pregnancy, trauma, surgery or connective tissue disorder. The spleen therefore migrates from its normal left hypochondriac position, to other parts of the peritoneal cavity especially the pelvis [ 3 ]. Since the first case report in 1667, there have been less than 600 cases reported in the literature so far [ 1 , 3 ].
Wandering spleen can have different clinical presentations ranging from asymptomatic incidental finding on imaging to features of acute abdomen as a result of complete torsion of the pedicle and total infarction of the spleen or complete obstruction of adjacent hollow viscus due to pressure effect. Less dramatic presentation includes chronic lower abdominal pain, swelling and symptoms of partial obstruction of bowel especially of the colon [ 3 , 4 , 5 , 6 ].
Diagnosis is confirmed by imaging usually abdominal ultrasound or CT which reveals that the spleen is absent from its normal anatomical position but seen somewhere else in the new location within the peritoneal cavity [ 3 , 9 , 10 ]. Once diagnosed, surgical intervention is required either by splenopexy or splenectomy depending on the viability of the organ [ 3 , 5 ] and can be done laparoscopically or by laparotomy.
Owing to its rarity, a high index of suspicion is required and this condition should always be considered as a possible differential diagnosis in patients presenting with lower abdominal swelling and pain. We present this case to share our experience in diagnosing and managing such a rare pathology and once again bring it to the attention of fellow clinicians handling this sort of abdominal conditions.
Case summary
Our patient is a 39 years old female Primi-para Ethiopian, who presented with lower abdominal dull aching pain of one-year duration which got worse over the last few months associated with right lower abdominal swelling, easy fatigability, LGIF, loss of appetite and weight. She is a known case of RVI on HAART for the past 18yrs and hypertensive for the last 8 years for which she was taking enalapril and atenolol. Her only child was delivered by C/section 10 years ago.
On examination , she looked chronically sick with her vitals in the normal range. The abdomen was flat with a lower midline surgical scar and a visible round mass on the right paraumblical and lower quadrant areas. The mass was well defined, smooth surfaced, slightly tender and mobile (Fig. 1 —black arrow).
Black arrow shows the splenic mass, red arrow shows the stomach, cyan arrow shows previous CS scar
Her hematologic tests revealed WBC of 8.7 × 103, Hgb of 12.3 and PLT count of 544 × 10 3 . Serum electrolyte and liver function tests were all in the normal range. Creatinine was 1.4 mg/dl.
Abdominal ultrasound
Multiple bilateral renal, liver and pancreatic cysts. An ehcocomplex mainly hypoechoic, 13 cmx8cm well defined right sided abdomino-pelvic mass, with absent color Doppler flow. Spleen was not visualized in its normal anatomic site.
Contrast enhanced abdomino-pelvic CT
Described the mass as a hypoattenuating, well circumscribed lesion with no contrast enhancement located at right abdomino pelvic cavity (Fig. 2 ). Its long torsed pedicle could be traced to the region of the tail of the pancreas and the spleen was missing from its normal location. (Fig. 3 ) Majority of the renal parenchyma is almost replaced with different sized cystic lesions with imperceptible wall causing bilateral renal enlargement. (Fig. 3 ) The liver and the pancreas too is filled with similar cysts. The portal vein were not visualized and replaced by periportal enlarged collateral vessels. (Figs. 3 , 4 ).
Infarcted spleen
Absent spleen in the splenic fossa
Spleen seen in the abdomino-pelvic cavity
With a diagnosis of wandering spleen located in the right abdomino pelvic region with torsion of the pedicle and infarction, she was admitted and underwent laparotomy. Intraoperatively, dense adhesion encountered between the anterior abdominal wall, omentum, the wandering spleen and small bowel. The spleen was whitish, distended and grossly infarcted with its long stalk torsed > 360°. (Fig. 5 ) Adhesions were gently released and splenectomy done. The splenic mass was sent for biopsy.
The intra-op picture of our patient upon exploration
She was discharged on the 3rd postoperative day and her post-operative course was uneventful. She was seen after a month on follow up clinic with no report of complication. Her biopsy result showed splenic tissue. She got her pentavelant vaccine on the third week.
Wandering spleen is a rare clinical entity characterized by splenic hypermobility from its left hypochondriac position to any other abdominal or pelvic position caused by absent or abnormal laxity of the suspensory ligaments [ 1 , 2 ].
The first case of wandering spleen was reported by Von Horne in 1667. So far less than 600 cases are reported world wide [ 1 , 3 ].
Anatomically a normal spleen is found in the left hypochondriac region suspended by ligaments to the stomach, kidney, pancreas, colon and left hemi-diaphram by the gastrosplenic, splenorenal, pancreaticosplenic, splenocolic, splenophreni ligaments and presplenic folds [ 1 ]. Our patient presented with RLQ palpable abdominal mass which is against the commonest presentation being in the LLQ of the abdomen (Fig. 1 ).
It could result from either a developmental failure of the embryonic septum transversum to fuse properly with the posterior abdominal wall which results in absent/lax ligaments [ 4 ] or from acquired conditions that result in lax suspensory ligaments as in pregnancy or connective tissue disorders [ 3 ]. The spleen is found in any quadrant of the abdomen or the pelvis though mostly in the left quadrants attached only by a long and loose vascular pedicle. Our patient presented with RLQ mass.
It is mostly seen in multiparous women [ 4 ] though the incidence is found to be nearly equal in both sexes in the prepubertal age group [ 3 ]. Our patient was a Para 1 mother and presented with 01 year history of abdominal pain which got worse in the past 06 months. Otherwise she had no any other pressure symptoms. She had visible umbilical area mass which was mobile up on examination
Wandering spleen can have different presentation ranging from asymptomatic incidental finding on imaging or upon surgical exploration for other surgical conditions to a presentation that mimics acute abdomen [ 3 , 5 ]. Mostly it presents as an on and of type acute/ subacute non-specific abdominal pain due to torsion and spontaneous de-torsion of the loose splenic pedicle [ 3 , 4 ]. This chronic torsion results in congestion and splenomegaly [ 3 , 5 ]. Hence patients could have palpable mobile mass [ 6 ] which is the typical presentation of this patient. The other presentations are usually related to the mass effect of the enlarged spleen and patients could present with GOO, bowel obstruction, pancreatitis and urinary symptoms [ 3 , 6 ].
In some cases it is reported to be associated with some other disorders like gastric volvulus [ 7 ] and distal pancreatic volvulus [ 8 ].
Ultrasound is one of the imaging modalities to investigate patients whom we suspect had wandering spleen. It usually shows absent spleen in the splenic fossa and a comma shaped spleen in the abdomen or pelvis [ 9 ]. Doppler study might help us see the vascular condition and ads up to a better preoperative plan. CT scan shows absence of the spleen in the left upper quadrant, ovoid or comma-shaped abdominal mass, enlarged spleen, a whirled appearance of non-enhancing splenic vessels and signs of splenic hypo-perfusion: homogenous or heterogeneous decreased enhancement depending on the degree of infarction [ 3 , 9 , 10 ].
Our patient was scanned with US and showed 13*8 cm large midline abdomino-pelvic well defined oval mass which was predominantly solid with areas of cystic component with absent color Doppler flow. Otherwise the spleen was not visualized in the splenic fossa. Bilateral kidney and liver has multiple different sized cystic lesions. With this image Abdomino-pelvic CT was done and shows spleen is located in the lower abdomen and appears to have torsed vascular pedicle and the whole splenic parenchyma is hypodense and no enhancement seen. Majority of the renal parenchyma is almost replaced with different sized cystic lesions with imperceptible wall causing bilateral renal enlargement. The whole liver is filled with cystic lesions with imperceptible wall. The portal veins were not visualized and replaced by periportal enlarged collateral vessels (Figs. 6 , 7 ).
Usually surgical management is the rule once a patient is diagnosed with wandering spleen [ 3 , 5 ]. Most patients; 65% as reported in some studies will have torsion of the vascular pedicle at some point of their life [ 5 , 6 ]. Hence splenopexy or splenectomy shall be considered when a wandering spleen is found incidentally up on surgical exploration for some other purposes [ 6 ]. Complicated wandering spleen like infarcted, signs of hypersplenism, huge in size and splenic vein thrombosis needs splenectomy while others can be managed with splenopexy [ 3 , 5 , 6 ]. Nowadays though laparoscopic technique is the gold standard, open technique can be used for splenopexy and splenectomy [ 3 , 5 ].
Partial infraction of a wandering spleen might necessitate partial splenectomy and splenopexy or splenectomy and splenic implantation [ 6 , 11 ].
The spleen might get fixed by different methods [ 8 , 9 ].
Simple splenic fixation involves simple tacking the splenic capsule to the peritoneum
Retroperitoneal pouch splenopexy- Tissue [ 11 , 12 ]/Mesh splenopexy (sandwich technique) [ 13 ].
Omental and peritoneal pouch splenic fixation [ 14 ].
In our case, Spleen was absent from the normal anatomic splenic fossa and the spleen in the abdomino-pelvic area looks infarcted. Hence she was managed with splenectomy and the patient was extubated on table and having a stable postoperative course .
Wandering spleen is a rare form of splenic pathology. Such a rare pathology presents commonly as an acute torsion with infarction. Spleen in the RLQ with chronic torsion and infarction is a very rare presentation for wandering spleen. In addition there is no report of such a presentation in a patient with AD-PCKD.
Recommendation
We recommend Clinicians to consider wandering spleen in their differential diagnosis in a patient presenting with RLQ abdominal mass and chronic abdominal pain.
Availability of supporting data
Data related with this case report is available at Addis ababa university, Tikur Ambesa Tertiary Hospital.
Abbreviations
Autosomal dominant polycystic kidney disease
Blood pressure
Low grade intermittent fever
High active anti-retroviral therapy
Right lower quadrant
Retro viral infection
Hypertension
White blood cell count
Puranik AK, et al . Wandering spleen: a surgical enigma. Gastroenterol Rep. 2015;5:241.
Google Scholar
Evangelos K, et al . Wandering spleen volvulus: a case report and literature review of this diagnostic challenge. Am J Case Rep. 2020;21: e925301.
Jawad M. Wandering spleen: a rare case from the emergency department. Cureus. 2023;15(1): e33246.
PubMed PubMed Central Google Scholar
Ayaz UY, et al . Wandering spleen in a child with symptoms of acute abdomen. Med Ultrasonogr. 2012;14:64.
Masroor M, Sarwari MA. Torsion of the wandering spleen as an abdominal emergency: a case report. BMC Surg. 2021;21:289.
Article PubMed PubMed Central Google Scholar
Blouhos K, et al . Ectopic spleen: an easily identifiable but commonly undiagnosed entity until manifestation of complications. Int J Surg Case Rep. 2014;8:451–4.
Article Google Scholar
Uc A. Gastric volvulus and wandering spleen. Am J Gastroenrterol. 1998;93:1146–8.
Article CAS Google Scholar
Alqadi GO, Saxena AK. Is laparoscopic approach for wandering spleen in children an option? J Min Access Surg. 2019;15:93.
Awan M, et al . Torsion of wandering spleen treated by laparoscopic splenopexy. Int J Surgery Case Rep. 2019;62:58.
Taori K, et al . Wandering spleen with torsion of vascular pedicle: early diagnosis with multiplaner reformation technique of multislice spiral CT. Abdom Imaging. 2004;29:09428925.
Fonseca AZ, et al . Torsion of a wandering spleen treated with partial splenectomy and splenopexy. J Emerg Med. 2013;44:e33.
Article PubMed Google Scholar
Seashore JH, McIntosh S. Elective splenopexy for wandering spleen. J Pediatr Surg. 1990;25:270–2.
Article CAS PubMed Google Scholar
Soleimani M. Surgical treatment of patients with wandering spleen: report of six cases with a review of the literature. Surg Today. 2007;37:261.
Peitgen K. Laparoscopic splenopexy by peritoneal and omental pouch construction for intermittent splenic torsion (“wandering spleen”). Surg Endosc. 2001;15:413.
Download references
Acknowledgements
We would like to thank the managing team of this patient including all the ward staffs who played a great role in the peri-operative management of this patient. We also appreciate the support of our consultants, residents and member of the department of surgery and HPB unit. Our kind gratitude goes to the family of this patient for their unreserved support in post-operative period that helped for the fast recovery of this patient
Funding is not applicable.
Author information
Authors and affiliations.
Addis Ababa University, Addis Ababa, Ethiopia
Yitagesu aberra shibiru, Sahlu wondimu & Wassie almaw
You can also search for this author in PubMed Google Scholar
Contributions
Dr. Yitagesu Aberra, Main author of this case report, is an HPB surgery fellow in the department of surgery, college of health science, Addis Ababa University who was the leading surgeon in the management of this patient. Dr. Sahlu Wendimu is an HPB surgery subspecialist and Assistant professor of General Surgery who was the consultant in duty during the management of this patient. Dr.Wassie Almaw is a 2nd year pediatric surgery resident attaching at HPB surgery unit who took part in the management of this patient.
Corresponding author
Correspondence to Yitagesu aberra shibiru .
Ethics declarations
Ethics approval and consent to participate.
Ethical clearance is not applicable but we took oral and written consent from the patient for case presentation and publication.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
There is no competing interest in this case presentation.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
shibiru, Y.a., wondimu, S. & almaw, W. Wandering spleen presenting in the form of right sided pelvic mass and pain in a patient with AD-PCKD: a case report and review of the literature. J Med Case Reports 18 , 259 (2024). https://doi.org/10.1186/s13256-024-04580-6
Download citation
Received : 02 December 2023
Accepted : 02 May 2024
Published : 25 May 2024
DOI : https://doi.org/10.1186/s13256-024-04580-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Wandering spleen
- Splenic torsion
- Splenic infarction
- Splenectomy
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
How to do (or not to do) a critical literature review. Pharmacy Education 2006;6:139-148. doi: 10.1080/15602210600616218. Babar Z. Pharmacy Practice Research Methods: Adis/Springer International Publishing, Switzerland, 2015. McKee M & Britton A. Conducting a literature review on the effectiveness of health care interventions.
Writing the Literature Review. In this section of your research materials the focus is on writing the literature review. The 2 types of reviews are illustrated below. In this section of resources you can explore specific information related to these types of reviews as you commence the writing process.
Explore the latest in clinical pharmacy and pharmacology, including topics in drug safety, development, pharmacogenetics, and pharmacoeconomics. This randomized clinical trial examines the efficacy of ivonescimab plus chemotherapy vs chemotherapy alone for patients with advanced or metastatic non-small cell lung cancer with the epidermal ...
The Journal of Pharmacy Practice (JPP) is a peer-reviewed journal that was established in 1988 and is published 6 times per year. The journal is read and cited both nationally and internationally. ... Review of the Literature on the Costs Associated With Acute Kidney Injury. Joseph F. Dasta; Sandra Kane-Gill; Restricted access. Research article.
There are five key steps to writing a literature review: Search for relevant literature. Evaluate sources. Identify themes, debates, and gaps. Outline the structure. Write your literature review. Activity 30 Minutes. Read The following articles to give you some assistance as you begin reading your articles for your literature review.
A literature review: Helps you to discover the research that has been conducted on a topic already & identifies gaps in current knowledge. Increases the breadth of your knowledge in your area of research. Helps you identify seminal works in your area. Allows you to provide the intellectual context for your work & position your research with ...
Quasi-experimental studies used in pharmacy literature may be classified into five major categories: (1) ... They are developed based on the literature review, aim and objectives, research questions, and propositions (Kleiber, 2004). b. ... Research strategies such as use of personal journals, audio recording and transcript auditing, and ...
Instead a literature review explores the key themes or concepts in the literature and compares what different research has found about each theme. Use sub-headings to structure your literature review as this helps you group the different studies to compare and contrast them and avoids a straight chronological narrative. To help find your sub ...
Journal of Clinical Pharmacy and Therapeutics provides a forum for clinicians, pharmacists and pharmacologists to explore and report on issues of common interest.Its scope embraces all aspects of clinical drug development and therapeutics.
Hospital Pharmacy is an independent, peer-reviewed journal. For 50 years it has been practitioner-focused and dedicated to the promotion of best practices and medication safety. Turn to HPX for essential information on medication errors, adverse reaction reporting, formulary drug reviews, original research, current FDA-related drug information, off-label drug uses, new technology, and more.
Pharmacy is an international, scientific, peer-reviewed, open access journal dealing with pharmacy education and practice and is published bimonthly online by MDPI. Open Access — free for readers, ... This scoping review aims to explore the literature on feedback for pharmacy students during experiential learning, with a focus on identifying ...
A literature review is defined as "a critical analysis of a segment of a published body of knowledge through summary, classification, and comparison of prior research studies, reviews of literature, and theoretical articles." (The Writing Center University of Winconsin-Madison 2022) A literature review is an integrated analysis, not just a summary of scholarly work on a specific topic.
The British Journal of Clinical Pharmacology is a leading international clinical pharmacology journal published by the ... A systematic literature review and meta-analysis of community pharmacist-led interventions to optimise the use of antibiotics ... (1982), 42 USA (one pharmacy serves a rural population, one pharmacy an urban population ...
Healthcare systems in the world are facing significant challenges as a result of severe funding pressure, a growing ageing population, societal changes, rising demand and a limited supply of some healthcare professional groups [1,2,3].This is compounded by the increasing prevalence of long-term conditions (LTC), in particular, people having two or more conditions which are being supported by ...
Publishing in reputable peer-reviewed journals is an integral step of the clinical pharmacy research process, allowing for knowledge transfer and advancement in clinical pharmacy practice. Writing a manuscript for publication in a journal requires several careful considerations to ensure that research findings are communicated to the satisfaction of editors and reviewers, and effectively to ...
Pharmacy research : a how-to guide for students, residents, and new practitioners by Vellurattil. ISBN: 1582122415. Publication Date: 2015. Detailed info on conducting a lit review. Bailey's Research for the Health Professional, 3rd ed. ISBN: 9780803645134. Includes detailed help on literature reviews.
The literature search was conducted in 2018 and utilized numerous sources including: OneSearch (EBSCOhost), PubMed, ScienceDirect, and the Journal of the American Pharmacists Association Opioid Topic Collection. Results. Seven articles were chosen for inclusion in this review.
Abstract. A systematic review is a process of synthesizing research evidence by collecting and summarizing all empirical evidence that meets predefined inclusion and exclusion criteria. Systematic reviews are performed by using systematic methods and often include a meta-analysis component which involves statistical techniques to conduct ...
Remember, the literature review is an iterative process. You may need to revisit parts of this search, find new or additional information, or update your research question based on what you find. 7. Provide a synthesis and overview of the literature; this can be organized by themes or chronologically.
2.1.1 Pharmacy Journal and Types of Research Papers. ... A literature review is a type of academic writing that provides an overview of existing knowledge in a particular field of research. ... How to do (or not to do) a critical literature review, Pharmacy Education, June 2006; 6(2): 139-148. Available at https://pharmacyeducation.fip.org ...
Figure 2 illustrates the physical architecture of the pharmacy area; this is often referred to as an embodiment. Multiple customers may enter and choose an available room. These rooms are individually secure as they can be locked from within [].Over and above this, the vault, which is the store for a variety of medications and dispensers, can be controlled according to specific requirements ...
The clinical investigation of psychedelic medicines has blossomed over the last 5 years. Data from a Phase 3 industry trial and a multicenter Phase 2 industry trial, in addition to multiple early ...
Abstract. This article presents the results of the analysis of peer-reviewed periodical scientific medical journals founded in Russia over the past 10 years (2010-2019). The selection process was carried out using a specially developed methodology, which made it possible to identify 84 Russian medical journals with an emphasis on practical ...
Abstract. Objective: Leadership discussion, including leadership development programs, is common. However, discussion of followership as a component of leadership seems less frequently discussed. With a focus on leadership and followership, this investigation reviewed the health-professions education literature and characterized leadership-followership within health-professions education.
Bitemark analysis involves the examination of both patterned injuries and contextual circumstances, combining morphological and positional data. Considering the uniqueness of human dentition, bitemarks caused by teeth on skin or impressions on flexible surfaces could assist in human identification. Aims: to investigate the available literature systematically and evaluate the scientific ...
This review summarizes the current evidence regarding diagnosis and management of Barrett esophagus, including how to evaluate patients for Barrett esophagus, the association with gastroesophageal reflux disease, and progression to esophageal adenocarcinoma.
Editor's Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. ... "Human Placental Schistosomiasis ...
Journal of Clinical Oncology. Volume 42, Number 16_suppl June 2024. ... Methods: A literature review from 1/1950-11/2023 was conducted for co-existing diagnoses of BC and SS in the PubMed Database. Search terms included Sweet Syndrome or Acute febrile neutrophilic dermatosis with Breast Cancer with subtypes. Key outcomes included: trigger ...
Voyez "La réglementation pharmaceutique soviétique (1918-1990)". What counts as robust evidence for drug regulators is influenced by social, political and cultural factors. A dramatic example of culture and politics shaping regulatory science can be found in the history of pharmaceutical testing in the Union of Soviet Socialist Republics ...
Wandering spleen is a rare clinical entity in which the spleen is hypermobile and migrate from its normal left hypochondriac position to any other abdominal or pelvic position as a result of absent or abnormal laxity of the suspensory ligaments (Puranik in Gastroenterol Rep 5:241, 2015, Evangelos in Am J Case Rep. 21, 2020) which in turn is due to either congenital laxity or precipitated by ...