Learn (Aprender): Anthesis

Table of Contents

Introduction
Anthesis is a term commonly used in botany to describe a specific phase in the life cycle of a flower . It marks a significant period during which a flower is fully open and functional, allowing for pollination to occur.
Definition: What does Anthesis mean?
Anthesis is defined as the period during which a flower is fully open and the sexual organs are exposed , facilitating pollination. This is the time when the flower is most receptive to pollinators such as bees, birds, bats, or the wind .
Beginner Explanation
Imagine a flower like a tiny shop that needs to open its doors to let customers in. Anthesis is like the grand opening of that shop, when the flower opens up wide so that bees and other insects can come inside to visit. When they do, they help the flower by moving pollen from one flower to another, which is how new seeds are made.
Advanced Explanation
In botanical terms, anthesis encompasses the sequence of events that occur during the maturation of a flower, leading to its full expansion and the exposure of its reproductive structures, namely the stamens and pistils . This stage can vary in duration among different plant species , ranging from a few hours to several days. Anthesis is often synchronized with the diurnal or nocturnal activity patterns of the pollinators that a particular plant species relies on for successful reproduction . Environmental factors such as temperature, light , and humidity can influence the timing of anthesis, thereby affecting the flower’s reproductive success.
Historical Background
The term anthesis is derived from the Greek word anthos, meaning flower, and has been used in botanical literature to describe the flowering stage since at least the 18th century. The study of anthesis and its patterns has been important in the field of botany for understanding plant reproduction and the evolution of flowering plants.
Practical Application
For gardeners and farmers, understanding anthesis can be very important for the cultivation of both ornamental and crop plants. Knowing when a flower is at anthesis can help in planning the planting and care schedule to optimize pollination and, consequently, fruit and seed production. For instance, if a farmer knows that a particular crop flowers and reaches anthesis at dawn, they might plan to have pollinators like bees present at that time to ensure effective pollination. Similarly, in ornamental gardening , a gardener may time the planting of different species to ensure a continuous display of flowers at anthesis, creating an aesthetically pleasing garden with a succession of blooms.
Scientific Application
In scientific research, anthesis is a critical phase for studying plant reproduction, genetics, and breeding. By understanding the timing and conditions that trigger anthesis, scientists can develop strategies to enhance crop yields, create new varieties through selective breeding , and even protect endangered plant species by ensuring successful pollination and seed set in conservation programs.
Subscribe Now
Get FREE instant access to our eBook, "13 Mistakes Beginner Gardeners Make (And How To Avoid Them)". Enter your email below.
Download our FREE eBook "13 Mistakes Beginner Gardeners Make"
"13 Mistakes Beginner Gardeners Make (And How to Avoid Them)" Get it FREE - Enter your email below.
Disclaimer - Privacy Policy - Terms and Conditions
First days of anthesis: C. texana
First days of anthesis: c. carduacea, last days of anthesis: c. texana, last days of anthesis: c. carduacea.
Words and phrases
Personal account.
- Access or purchase personal subscriptions
- Get our newsletter
- Save searches
- Set display preferences
Institutional access
Sign in with library card
Sign in with username / password
Recommend to your librarian
Institutional account management
Sign in as administrator on Oxford Academic
anthesis noun
- Hide all quotations
Earlier version
- anthesis in OED Second Edition (1989)
What does the noun anthesis mean?
There is one meaning in OED's entry for the noun anthesis . See ‘Meaning & use’ for definition, usage, and quotation evidence.
How common is the noun anthesis ?
How is the noun anthesis pronounced, british english, u.s. english, where does the noun anthesis come from.
Earliest known use
The earliest known use of the noun anthesis is in the late 1700s.
OED's earliest evidence for anthesis is from 1783, in C. Linnaeus' Syst. Veg.
anthesis is a borrowing from Latin.
Etymons: Latin anthesis .
Nearby entries
- antheridium, n. 1818–
- antheriferous, adj. 1799–
- antheriform, adj. 1802–
- antherine, n. 1689–
- antherless, adj. 1798–
- antherogenous, adj. 1847
- antheroid, adj. 1818–
- antherozoid, n. 1853–
- antherozoidal, adj. 1865–
- anther valve, n. 1839–
- anthesis, n. 1783–
- anthias, n. 1601–
- anthill, n. Old English–
- ant-hillock, n. 1656–
- ant-hilly, adj. 1796–
- anthine, n. & adj. 1601–1768
- ant-hive, n. 1817–
- antho-, comb. form
- anthobian, n. & adj. 1835–
- anthocarpous, adj. 1835–
- anthocephalous, adj. 1847
Meaning & use
The Anthesis [Latin Anthesis ] takes place, when the burnt Anthers scatter their bags of Dust upon the Stigma.
Bractea of the female flowers very much enlarged after anthesis , when the spike presents the appearance of a pine-apple; bright yellow, with red apices.
The term anthesis is sometimes used to indicate the period at which the flower-bud opens.
There were both delayed and extended antheses and most of the time the flowers were semi-open.
Histologically the ovary and style are relatively simple at anthesis .
From the time of anthesis , when the floral parts open to receive pollen, the developing grain becomes the dominant sink.
A later planting date reduced pre-anthesis moisture stress by reducing the number of days..for the crop to reach anthesis .
- efflorescence 1626– The process of producing flowers, or bursting into flower; the period of flowering.
- blow 1748– Manner, style, or time of blossoming. Also figurative .
- anthesis 1783– The stage at which a flower is open, allowing fertilization to occur. Also: an instance of this.
- florescence 1793– The process of producing flowers or bursting into flower; the period or state of flowering. Also concrete . Flowers collectively.
Pronunciation
Plural: antheses.
- ð th ee
- ɬ rhingy ll
Some consonants can take the function of the vowel in unstressed syllables. Where necessary, a syllabic marker diacritic is used, hence <petal> /ˈpɛtl/ but <petally> /ˈpɛtl̩i/.
- a trap, bath
- ɑː start, palm, bath
- ɔː thought, force
- ᵻ (/ɪ/-/ə/)
- ᵿ (/ʊ/-/ə/)
Other symbols
- The symbol ˈ at the beginning of a syllable indicates that that syllable is pronounced with primary stress.
- The symbol ˌ at the beginning of a syllable indicates that that syllable is pronounced with secondary stress.
- Round brackets ( ) in a transcription indicate that the symbol within the brackets is optional.
View the pronunciation model here .
* /d/ also represents a 'tapped' /t/ as in <bitter>
Some consonants can take the function of the vowel in unstressed syllables. Where necessary, a syllabic marker diacritic is used, hence <petal> /ˈpɛd(ə)l/ but <petally> /ˈpɛdl̩i/.
- i fleece, happ y
- æ trap, bath
- ɑ lot, palm, cloth, thought
- ɔ cloth, thought
- ɔr north, force
- ə strut, comm a
- ər nurse, lett er
- ɛ(ə)r square
- æ̃ sal on
Simple Text Respell
Simple text respell breaks words into syllables, separated by a hyphen. The syllable which carries the primary stress is written in capital letters. This key covers both British and U.S. English Simple Text Respell.
b, d, f, h, k, l, m, n, p, r, s, t, v, w and z have their standard English values
- arr carry (British only)
- a(ng) gratin
- o lot (British only)
- orr sorry (British only)
- o(ng) salon
Inflections
anthesis typically occurs about 0.2 times per million words in modern written English.
anthesis is in frequency band 4, which contains words occurring between 0.1 and 1 times per million words in modern written English. More about OED's frequency bands
Frequency of anthesis, n. , 1810–2010
* Occurrences per million words in written English
Historical frequency series are derived from Google Books Ngrams (version 2), a data set based on the Google Books corpus of several million books printed in English between 1500 and 2010.
The overall frequency for a given word is calculated by summing frequencies for the main form of the word, any plural or inflected forms, and any major spelling variations.
For sets of homographs (distinct entries that share the same word-form, e.g. mole , n.¹, mole , n.², mole , n.³, etc.), we have estimated the frequency of each homograph entry as a fraction of the total Ngrams frequency for the word-form. This may result in inaccuracies.
Smoothing has been applied to series for lower-frequency words, using a moving-average algorithm. This reduces short-term fluctuations, which may be produced by variability in the content of the Google Books corpus.
Frequency of anthesis, n. , 2017–2023
Modern frequency series are derived from a corpus of 20 billion words, covering the period from 2017 to the present. The corpus is mainly compiled from online news sources, and covers all major varieties of World English.
Smoothing has been applied to series for lower-frequency words, using a moving-average algorithm. This reduces short-term fluctuations, which may be produced by variability in the content of the corpus.
Compounds & derived words
- synanthesis , n. 1880– Simultaneous ripening of the stamens and pistils in a flower.
Entry history for anthesis, n.
anthesis, n. was revised in March 2016.
anthesis, n. was last modified in July 2023.
oed.com is a living text, updated every three months. Modifications may include:
- further revisions to definitions, pronunciation, etymology, headwords, variant spellings, quotations, and dates;
- new senses, phrases, and quotations.
Revisions and additions of this kind were last incorporated into anthesis, n. in July 2023.
Earlier versions of this entry were published in:
OED First Edition (1885)
- Find out more
OED Second Edition (1989)
- View anthesis in OED Second Edition
Please submit your feedback for anthesis, n.
Please include your email address if you are happy to be contacted about your feedback. OUP will not use this email address for any other purpose.
Citation details
Factsheet for anthesis, n., browse entry.
- Cambridge Dictionary +Plus
- Examples of anthesis
These examples are from corpora and from sources on the web. Any opinions in the examples do not represent the opinion of the Cambridge Dictionary editors or of Cambridge University Press or its licensors.
{{message}}
Please choose a part of speech and type your suggestion in the Definition field.
Help us improve the Cambridge Dictionary
anthesis doesn't have a definition yet. You can help!

Word of the Day
spin your wheels
to waste time doing things that achieve nothing

Alike and analogous (Talking about similarities, Part 1)

Learn more with +Plus
Thank you for suggesting a definition! Only you will see it until the Cambridge Dictionary team approves it, then other users will be able to see it and vote on it.
See your definition
- Recent and Recommended {{#preferredDictionaries}} {{name}} {{/preferredDictionaries}}
- Definitions Clear explanations of natural written and spoken English English Learner’s Dictionary Essential British English Essential American English
- Grammar and thesaurus Usage explanations of natural written and spoken English Grammar Thesaurus
- Pronunciation British and American pronunciations with audio English Pronunciation
- English–Chinese (Simplified) Chinese (Simplified)–English
- English–Chinese (Traditional) Chinese (Traditional)–English
- English–Dutch Dutch–English
- English–French French–English
- English–German German–English
- English–Indonesian Indonesian–English
- English–Italian Italian–English
- English–Japanese Japanese–English
- English–Norwegian Norwegian–English
- English–Polish Polish–English
- English–Portuguese Portuguese–English
- English–Spanish Spanish–English
- English–Swedish Swedish–English
- Dictionary +Plus Word Lists
There was a problem sending your report.
- Add a definition
- All translations
- TheFreeDictionary
- Word / Article
- Starts with
- Free toolbar & extensions
- Word of the Day
- Free content
an•the•sis
- development
- efflorescence
- florescence
- inflorescence
- ontogenesis
- Anthemis cotula
- Anthemis nobilis
- Anthemis tinctoria
- Anthemius of Tralles
- Antheraea mylitta
- Antheraea pernyi
- Antheraea polyphemus
- Anthericum liliago
- Anthericum torreyi
- antheridial
- antheridiophore
- antheridium
- Antheriferous
- Antheriform
- Antherogenous
- Antheropeas
- Antheropeas wallacei
- antherozoid
- Anthobranchia
- anthocarpous
- Anthoceropsida
- Anthocerotaceae
- Anthocerotales
- anthochlore
- anthocyanin
- anthoecology
- anthography
- anthological
- anthologise
- anthologist
- anthologize
- antherozoids
- antherozooid
- Anthes, Richard
- Anthesteria
- Anthetarius
- Anthoathecatae
- Anthocarpous
- anthocaulis
- Anthoceratopsida
- Anthocerophyta
- Anthocerotae
- Facebook Share
- Research article
- Open access
- Published: 16 March 2021
Spikelet movements, anther extrusion and pollen production in wheat cultivars with contrasting tendencies to cleistogamy
- Urszula Zajączkowska ORCID: orcid.org/0000-0002-7119-7547 1 ,
- Bożena Denisow ORCID: orcid.org/0000-0001-6718-7496 2 ,
- Barbara Łotocka ORCID: orcid.org/0000-0001-5913-8424 3 ,
- Alicja Dołkin-Lewko ORCID: orcid.org/0000-0003-0676-6384 1 &
- Monika Rakoczy-Trojanowska ORCID: orcid.org/0000-0001-6891-0471 4
BMC Plant Biology volume 21 , Article number: 136 ( 2021 ) Cite this article
2890 Accesses
7 Citations
1 Altmetric
Metrics details
Cleistogamic flowers are a main barrier in pollen dispersal for cross-pollination necessary in wheat hybrid breeding. The aim of our study was to gain new knowledge on the biology of wheat flowering, in particular on the differences between the cleisto- and chasmogamic forms which has certainly cognitive significance, but it can also be used in practice when seeking a female and male ideotypes for cross hybridization.
We characterized the most significant features defining the flowering specificity in two wheat cultivars with contrasting tendency to cleistogamy: Piko (chasmogamous) and Dacanto (cleistogamous). In the field observations we assessed diurnal pattern of anther extrusion and anther extrusion capacity. For the first time we adapted the time lapse method for measuring kinetics of the spikelet movement and 3-D image correlation technique for the non-invasive measurements of potential deformations of the spikelet lemmas. We found that the two cultivars differ in the potential of pollen dispersion for-cross-pollination and in the spikelet kinetics. We also described some anatomical traits that can have potential functional role in floret opening. None of the cultivars showed any symptoms of lemma surface deformation.
Conclusions
The cleistogamic and chasmogamic wheat cultivars differ significantly in the potential for pollen dispersion for cross-pollination, which is mainly related to anther extrusion capacity. Although none of these features differentiated the cultivars clearly, we assume, based on spikelet kinetics and the lack of lemmas surface deformation, that the water transport and turgor of cells is essential for the floret opening and anther extrusion in wheat. The search for parental ideotype should be supported by marker assisted selection, e.g. based of polymorphisms in genes related to aquaporin biosynthesis.
Peer Review reports
Cleistogamy is a sexual reproduction system, which is defined as the formation of closed self-pollinated flowers, has been found in many angiosperm taxa. Several plant species have mixed reproduction strategies and proportion of cleistogamous and chasmogamous flowers has been reported to be modified by environmental conditions like soil moisture, light intensity, fertilization of soil and plant density [ 1 ]. Hexaploid common wheat ( Triticum aestivum ), which is one of the most important world food plant, is a self-pollinated species with mostly cleistogamous florets adapted for self-pollination and fertilization in closed florets [ 2 , 3 , 4 ]. Cleistogamy is the main disadvantage for hybrid breeding [ 5 , 6 , 7 , 8 ]. The male components of cross hybridization, regardless of the mechanism on which it is based – natural (e.g. cms-Rf ) or artificial (chemical emasculation) - should have chasmogamous flowers that are open at anthesis which facilitate anther extrusion and, consequently, pollen shedding for cross-pollination [ 9 , 10 , 11 , 12 ]. Wheat florets are arranged in a spike inflorescence, typical for Poaceae (Gramineae). The spike main axis bear spikelets. There are two glumes at the base of each spikelet. Each spikelet consists of two-five florets. A floret is perfect and consists of a lemma and palea, three stamens, one pistil, and two lodicules located between the lemma and the ovary base [ 2 , 13 , 14 ]. The floret opening at anthesis in cereals is thought to be due to the lodicules swelling [ 2 , 15 , 16 , 17 , 18 ]. In wheat, the pair of lodicules located between the lemma and the ovary base expand rapidly at the time of anthesis, push away the rigid lemma allowing anthers and stigma to emerge [ 16 , 19 ]. The process induced by the turgid lodicules called as ‘first opening’ is rather short and usually lasts for less than 30 min [ 2 , 20 , 21 ]. The ‘second opening’ of wheat floret was recently described and the authors suggested that unfertilized ovary increase in radial dimensions and generates lateral push of the rigid lemma and palea [ 22 ]. However, it is still not clear whether during the ‘first opening’ the lemma undergoes deformations, which could increase the efficiency of floret opening and anther extrusion. Also, the kinetics of the floret opening has so far been poorly studied. It is known that plants have evolved various mechanisms of movement of certain morphological structures that are important in reproduction processes, such as floret opening, pollen dispersion or seed distribution [ 23 , 24 ]. In many cases of ferns and flowering plants, the energy that activates the onset of movement is due to the uneven cell wall thickness or the anisotropy of a specific structure that is deformed in the evaporation process [ 25 , 26 ]. To date, however, there has been no experimental data on the possibility of lemma deformation during rapid expansion of swelling lodicules and ovary base that occur at the time of the floret opening at anthesis in cereals. There is also no data on the kinetics of the flower opening process. Pollen production is also among the traits reported to be important in hybrid wheat breeding [ 4 , 10 , 27 , 28 ]. However, most cultivated forms of common wheat produce low amounts of pollen, which is indicated as a significant limitation in wheat hybrid breeding programs [ 7 , 11 , 29 ].
In this study, we surveyed the kinetics and the micromorphological and anatomical structures of spikelets in two wheat cultivars with contrasting tendency to cleistogamy. Two winter wheat cultivars - Piko (chasmogamous) and Dacanto (cleistogamous) were used. For the first time we applied the time lapse technique for measuring the kinetics of spikelets movements and the non-invasive measurements of potential deformations of the spikelet lemmas during anthesis. Moreover, we analyzed if the structural features and changes in the pre-anthesis and post-anthesis spikelets can determine the anther extrusion ability. In addition, we tried to assess to what degree the floral biology (diurnal pattern of anther extrusion, the efficiency for anther extrusion, the pollen production and pollen dispersion capability) differ between two contrasting cultivars.
Anther extrusion
The diurnal pattern of anther extrusion was similar in both genotypes grown in the field conditions (Fig. 1 , Additional file 1 : Table S1). The peak of anther extrusion was observed in early morning hours (between 5.00 h and 8.00 h). A total lack of anther extrusion was characteristic for mid-day hours. Towards the end of the day, during evening hours, a small proportion of anthers have been extruded (approximately 3–5% of the daily amount in Dacanto and 10% in Piko). The number of anthers per spike was statistically non-significant between cultivars (Mann-Whitney U test: Z = − 0.65, P = 0.516; Table 1 , Additional file 1 : Tables S3-S4). Considerable year-to-year differences were documented for the number of anthers developed in spikes (for cv. Dacanto Z = 3.74, P = 0.000; for cv. Piko Z = 3.71 P = 0.000) and lower values were established in 2019 compared to 2018. The proportion of extruded anthers differed considerably between the cultivars ( Z = − 5.39, P = 0.000; Fig. 2 , Additional file 1 : Table S2. Approximately two-fold higher proportion of extruded anthers was noted for the cv. Piko compared to the cv. Dacanto (42.2% ± 1.9 vs. 18.6% ± 3.0, respectively). The year effect was found within cultivars for the ability to anther extrusion (for cv. Dacanto Z = 1.96, P = 0.049; for cv. Piko Z = − 2.88, P = 0.004).
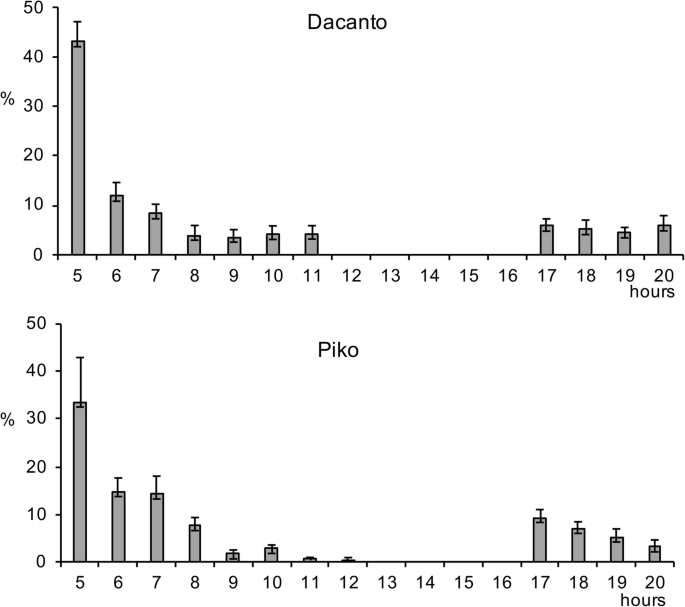
Diurnal pattern of anther extrusion in two wheat cultivars. The extrusion is expressed as the number of anthers extruded at one hour intervals (GMT + 2 h) in relation of the total number of anthers extruded per day (average from 2018 to 2019). Whiskers indicate ± SD; hours GMT + 2 h.
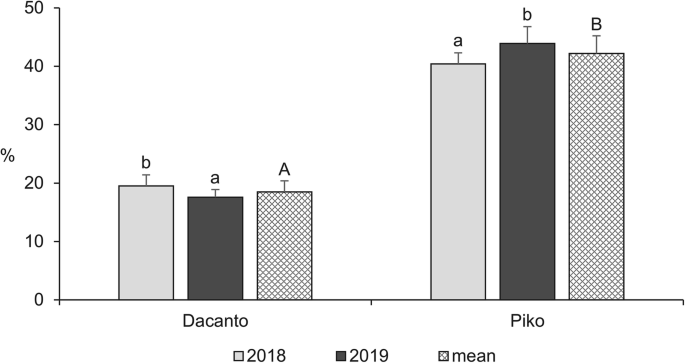
Proportion of extruded anthers in two wheat cultivars observed in 2018 and 2019. Untransformed data are presented. Whiskers indicate ± SD. Means followed by the same small letter are significantly different between the years and those followed by the same capital letter - between the cultivars at P < 0.05, according to the Mann-Whitney’s U test
Pollen production
The number of pollen grains produced per anther differed significantly between the cultivars ( t = 2.00, df = 406, P = 0.000; Table 1 ) and was higher in the cv. Dacanto. A year-to-year disparity was found in the amount of pollen in the anthers (Dacanto t = −11.79, df = 202, P = 0.000; Piko t = −13.43, df = 202, P = 0.000). In both cultivars, a statistically higher amount of pollen was produced in 2019 (ca. 2-fold higher than in 2018). The total pollen production per spike was similar in both cultivars ( t = 1.73, df = 78, P = 0.087; Fig. 3 A, Additional file 1 : Table S4). However, a year effect on the number of pollen grains produced in spikes was found (Dacanto t = − 7.58, df = 38, P = 0.000; Piko t = − 5.00, df = 38, P = 0.004). The cultivars differed significantly in the number of pollen grains released for cross-pollination ( t = − 21.15, df = 78, P = 0.000), and the cv. Piko exhibited a higher amount of dispersed pollen (Fig. 3 B, Additional file 1 : Table S4). The number of pollen grains available for cross-pollination differed between the seasons (Dacanto t = − 4.24, df = 38, P = 0.000; Piko t = − 7.58, df = 38, P = 0.000).
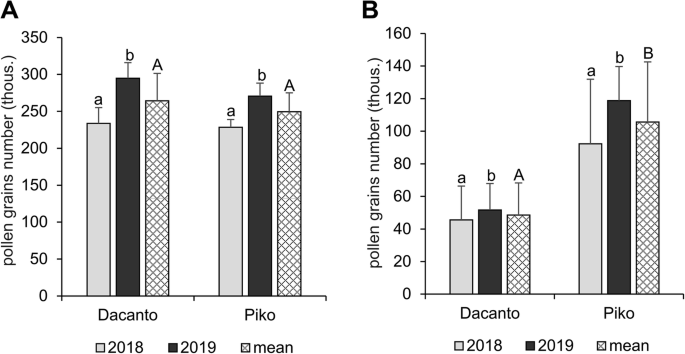
Total pollen production per spike ( A ) and pollen dispersion capacity per spike ( B ). Results of the experiments with two wheat cultivars performed in 2018 and 2019. Whiskers indicate ± SD. Means followed by the same small letter are not significantly different between the years and those followed by the same capital letter - between the cultivars at P < 0.05, according to t-test
Micromorphology and anatomy of spikelets
The spikelets of both cultivars consisted of two glumes and 4–6 florets, including 3 fertile ones in cv. Dacanto (Fig. 4 a, b), or 4–5 florets, including 2–3 fertile ones in cv. Piko (Fig. 4 c, d) set distichously and alternately on the rachilla, with antherns positioned higher within the florets in Piko. Characteristically, in every sectioned post-anthesis spikelet of the cv. Dacanto, all florets contained some non-extruded anther(s) (Fig. 4 b). In the spikelets of the cv. Piko examined post anthesis, all anthers were absent at least in the basal two florets (Fig. 4 d). In the same spikelets, the upper 1 or 2 florets retained stamens within the lemma and palea, and their filaments were slightly or non-elongated. The glumes were boat-shaped, with a massive base in both cultivars (Fig. 5 a-c). In this area, the glume abaxial epidermis in both cultivars was smooth and composed of elongated non-turgid pavement cells. In this domain, a minute and shallow transversal groove was discernible before anthesis (Fig. 5 a, c). In spikelets fixed post-anthesis, widening of the grooves within the bases of glumes was visible (Fig. 5 b). In both cultivars, the two basal florets formed the largest pistils with a well-formed gametophyte. The third flower was smaller but properly built as well, and the distal 2–3 florets were minute, apparently aborted at the early developmental stage (Fig. 4 a, b). Each floret was supported by a lemma and palea. The lemmas were boat-shaped, like the glumes, but wider and longer (Fig. 5 d-f). Like in the glumes, a groove was discernible at pre-anthesis also in the lemma bases within a domain of non-turgid pavement cells of abaxial epidermis. The two lodicules were both facing lemma. Due to such location, in the dorsoventral longitudinal sections (Fig. 4 a-d) only a single lodicule was visible in every fertile floret. At pre-anthesis, lodicules were turgid, with basal half convex and the apical one rather flat and covered with long, thick-walled and spiked trichomes (Fig. 6 a, b). The epidermis of lodicules was composed exclusively of rather short pavement cells, with straight anticlinal walls and characteristic surface microsculpture (Fig. 6 c). Post anthesis, the lodicules were turgor-less and crushed (Fig. 4 b, d).
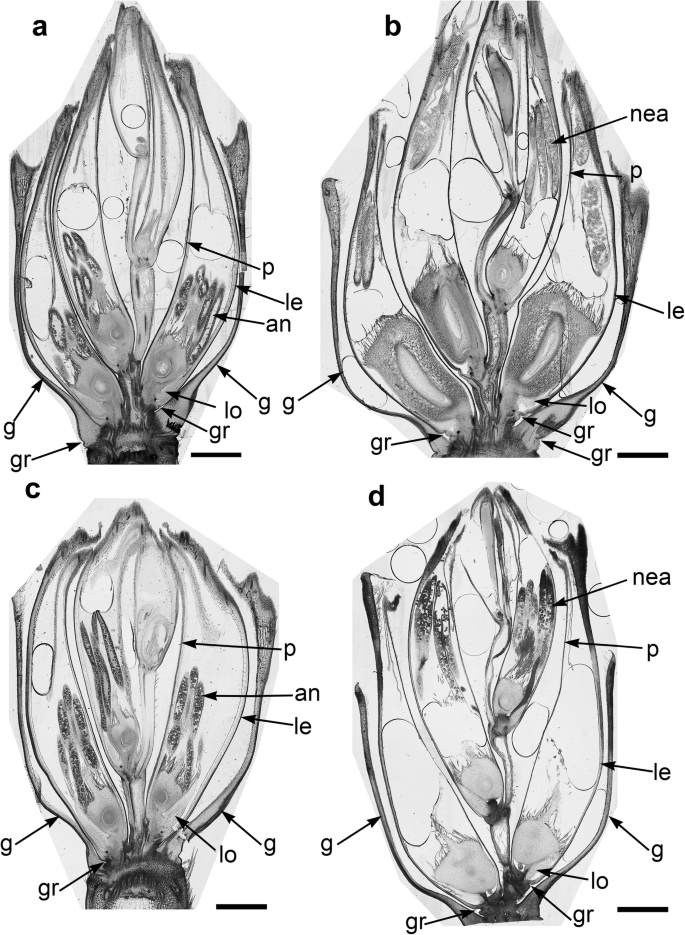
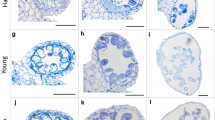
General anatomy of the spikelet in cv. Dacanto ( a , b ) and cv. Piko ( c , d ). Spikelets before anthesis ( a , c ) and after anther extrusion ( b , d ). Arrows, g- glume, le- lemma, p- palea, lo-lodicule, an-anthers, nea- non-extruded anthers, gr - grooves. Scale bars = 1500 μm
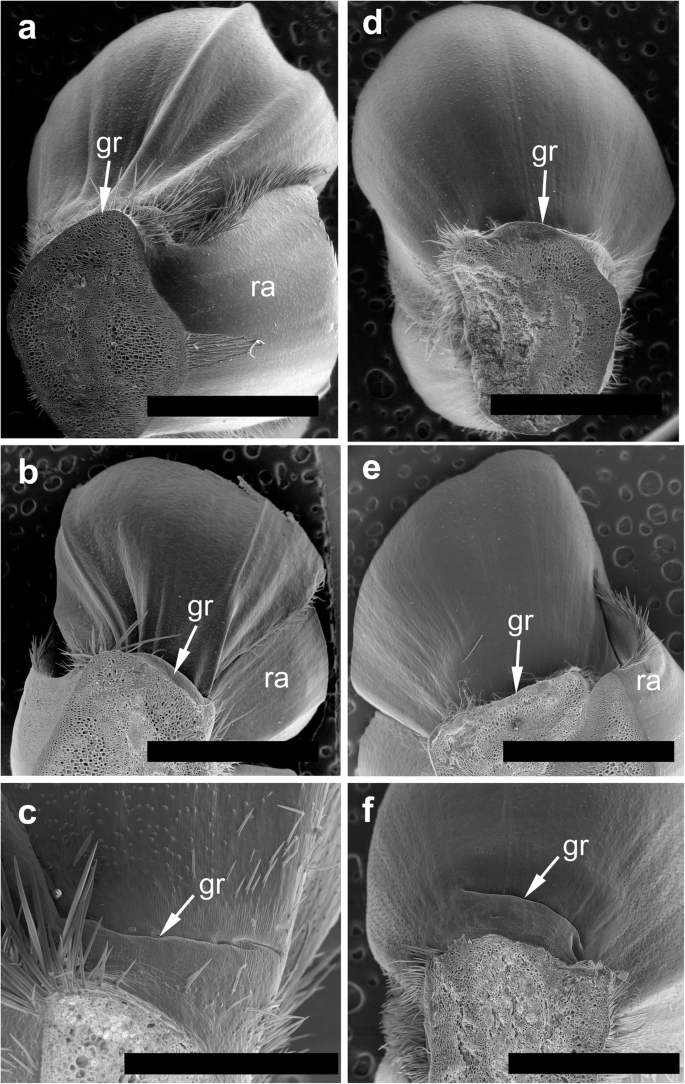
Micromorphology of glumes ( a-c ) and lemmas ( d-f ). Spikelets of cv. Dacanto ( a , b , d , e ) and cv. Piko ( c , f ) before anthesis ( a , c , d , f ) and after anther extrusion ( b , e ). Small grooves (gr) are visible in the basal parts of the glumes and lemmas already before anthesis, ra – rachilla. Scale bars in ( a ), ( b ), ( d ), ( e ) = 2000 μm, bar in ( c ) = 500 μm and bar in ( f ) = 1000 μm
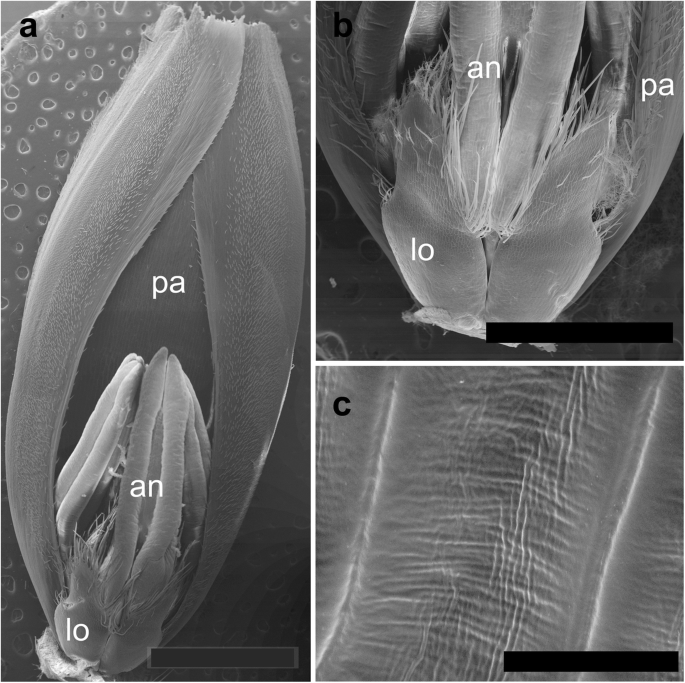
Micromorphology of cv. Dacanto spikelet before anthesis: palea (cut apically) with a floret attached ( a ). Lodicules of cv. Piko before anthesis ( b ); a criss-cross pattern of the lodicule cell surface in cv. Dacanto ( c ); pa – palea, lo – lodicules, an – anthers. Scale bar in ( a ) = 2000 μm, bar in ( b ) = 1000 μm, and bars in ( c ) =100 μm
- Lemma kinetics
The analysis of time-lapse movies shows that lemma opening period lasted on average about 5 min, and for individuals it ranged from 2 to 15 min (Fig. 7 , Additional file 1 : Table 5). The maximum lemma opening was on average about 1.5 min from the beginning of the process in the 2018 experiment and about 1.5–2 min in 2019. During this first period of flowering, the highest speed of lemmas displacement was also noted. Comparison of lemmas displacement kinetics shows that wider lemmas opening occurs in the Piko cultivar. In 2018 this was observed for almost the entire lemma opening period and in 2019 it was revealed in the period after the maximum opening time, when the degree of lemma opening was gradually decreasing. Additional movie files illustrate the original time-lapse movies recorded during the lemmas opening in two cultivars (see Additional files 2 and 3).
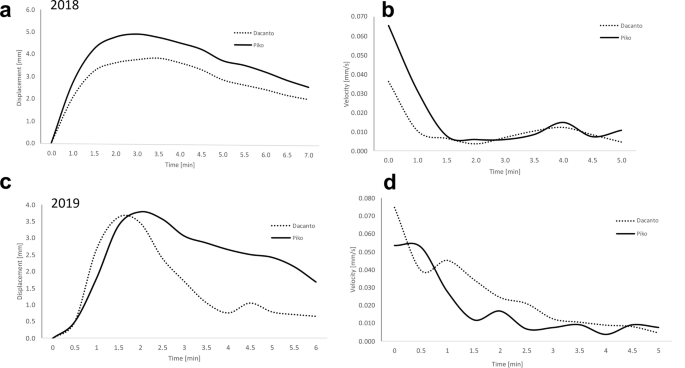
Spatial displacement ( a , b ) and speed of movement ( c , d ) of the opening lemmas. Results of two experiments during flowering of Piko and Dacanto wheat cultivars. Carried out in 2018 ( a , c ) and 2019 ( b , d ). Data obtained from the time-lapse imaging transformed in the Tracker program
Additional file 2: Movie 1. Example of the original time-lapse movie recorded during the lemmas opening in cv. Dacanto.
Additional file 3: Movie 2. Example of the original time-lapse movie recorded during the lemmas opening in cv. Piko.
Potential deformations of lemmas during the flowering process was measured using a 3-D image correlation method along two rectangular X-Y axes (Figs. 8 , 9 , 10 , 11 ). The longer Y axis was parallel to the lemma venation pattern and the X axis was oriented perpendicularly. The color maps presented in Figs. 8 c,d, 9 , 10 and 11 c,d show the Z values of the points on the whole lemma surface at the beginning of flowering process before the lemma opening and at the initial phase after opening. The end of the measurement period was limited by the depth of field of the lens of the stereoscopic microscope and occurred when during the lemma displacement the surface of the lemma became out of focus of the lens. A detailed analysis of the potential lemma surface deformation can be done on the basis of the data shown in the graphs in Figs. 8 a,b, 9 , 10 and 11 a,b where the Z values of the points along X and Y axes are presented. The comparison of the pattern of the graphs for the same lemma at the beginning and the end of measurement indicate that during initiation of the lemma opening there is a spatial position shift of whole lemma surface without any noticeable displacement between the reference points distributed within the lemma surface which suggests that there are no symptoms of the lemma surface shape deformation.
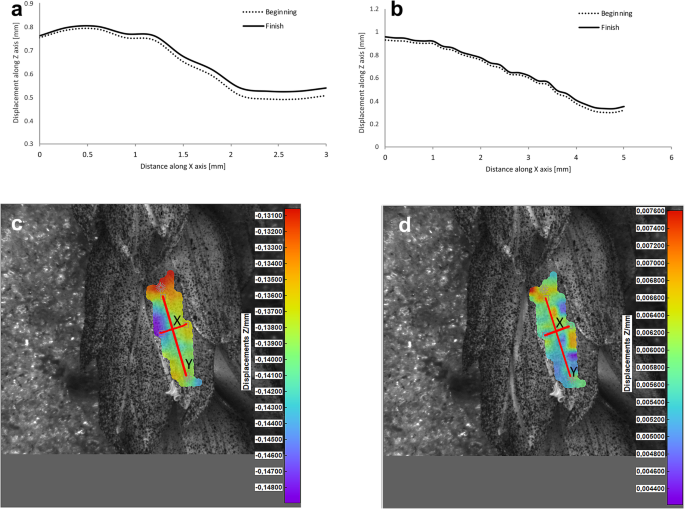
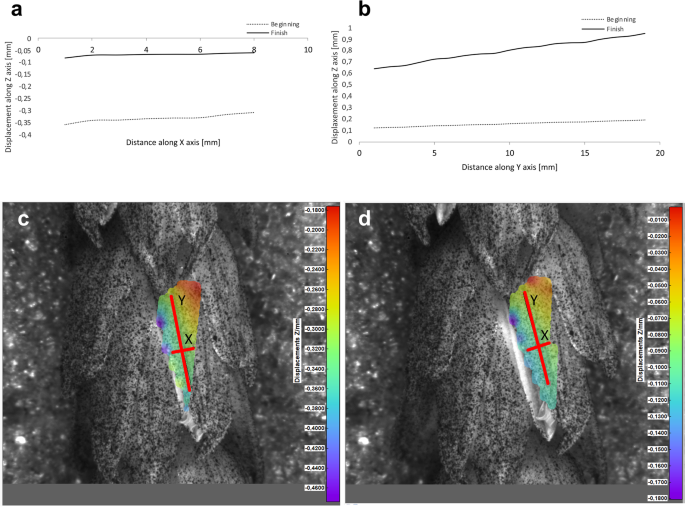
Results of the lemma surface deformation test using the 3D image correlation method. Diagrams ( a , b ) and maps ( c , d ) of the lemma surface displacement. The graphs presented in ( a ) and ( b ) show the Z values of the points on the lemma surface along X and Y axes, respectively, at the beginning of the flowering process before the lemma opening ( c ) and after the first phase of opening ( d ). Piko cultivar, lemma No. 1
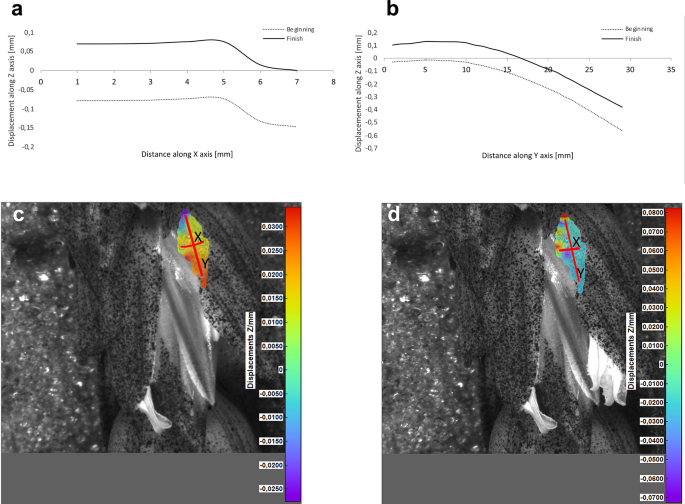
Results of the lemma surface deformation test using the 3D image correlation method. Diagrams ( a , b ) and maps ( c , d ) of the lemma surface displacement. The graphs presented in ( a ) and ( b ) show the Z values of the points on the lemma surface along X and Y axes, respectively, at the beginning of the flowering process before the lemma opening ( c ) and after the first phase of opening ( d ). Piko cultivar, lemma No. 2
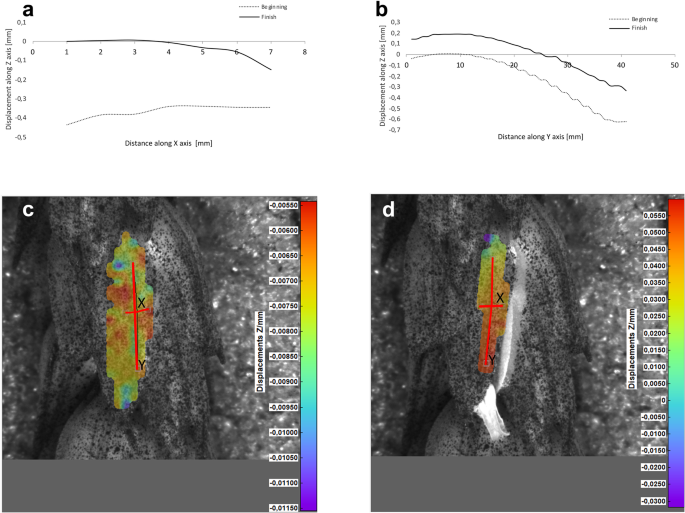
Results of the lemma surface deformation test using the 3D image correlation method. Diagrams ( a , b ) and maps ( c , d ) of the lemma surface displacement. The graphs presented in ( a ) and ( b ) show the Z values of the points on the lemma surface along X and Y axes, respectively, at the beginning of the flowering process before the lemma opening ( c ) and after the first phase of opening ( d ). Dacanto cultivar, lemma No.1
Results of the lemma surface deformation test using the 3D image correlation method. Diagrams ( a , b ) and maps ( c , d ) of the lemma surface displacement. The graphs presented in ( a ) and ( b ) show the Z values of the points on the lemma surface along X and Y axes, respectively, at the beginning of the flowering process before the lemma opening ( c ) and after the first phase of opening ( d ). Dacanto cultivar, lemma No.2
An additional movie file illustrates, as an example, the change in the position of the lemma during flowering, recorded from two cameras connected to a stereoscopic microscope, which were the basis for determining the potential lemma deformation using 3-D image correlation method (see Additional file 4).
Additional file 4: Movie 3. Movie illustrating, the change in the position of the lemma during flowering. Original records from two cameras connected to a stereoscopic microscope, which were the basis for determining the potential lemma deformation using 3-D image correlation method.
In this study we presented a detailed comparative characteristics of flowering biology of two contrasting winter wheat cultivars – cleisto- and chasmogamic, which comprised morpho-anatomical study of spikelet structure including their kinetics, anther extrusion and pollen production. Such a complex study employing different analytical approaches was performed in wheat for the first time. Our anatomical observations revealed that in every sectioned post-anthesis spikelet of cv. Dacanto, all florets contained some non-extruded anther(s), while in cv. Piko spikelets nearly all anthers were absent at least in the basal two florets. These findings correspond to field observations that exhibited two-fold higher proportion of anthers extruded from the Piko spikes.
Microscopic analysis showed that spikelets anatomy of the two cultivars, in general is similar to that reported in classical papers concerning floret anatomy of the bread wheat genotypes [ 13 , 30 ]. However, certain details of the spikelet micromorphology were not reported earlier. This novel trait that we consider as having potentially functional role in floret opening concerns formation of grooves within the glume and lemma bases and deepening of the grooves during anthesis. Although the grooves seem more pronounced (wider) in cv. Piko, which is a better extruder of anthers, from the structural point of view both cultivars can potentially extrude anthers with similar efficiency. As it is seemingly not the case, the reasons should be sought in the functioning of the spikelet elements, especially in the water transport and turgor control [ 19 ]. This suggestion is confirmed by our results of measuring the kinetics of lemmas openings in which for the first time the time lapse technique was applied. The measurements showed that the average length of this period is about 6–7 min, and for individual spikelets it is in the range of 2–15 min. The data are consistent with the reports of earlier authors indicating that the actual opening of the floret in most grasses is caused by the lodicules becoming suddenly swollen at the base, so the lemma is levered outwards [ 22 ]. The difference between the two cultivars is, however, small, but the kinetics parameters seem to be more favorable for anther extrusion and to the cross- pollination processes for the cv. Piko.
The diurnal pattern of anther extrusion can also support the importance of hydration of spikelet elements for the anther extrusion process. The peak of anther release was observed in early morning hours, when the plant cells are usually well hydrated [ 24 ]. A fundamental role of the water balance, the turgor and its regulation in flower opening have been confirmed in many studies [ 31 ]. The key role of water transport and turgor of cells in lodicules is also confirmed by the results of our studies on the possible deformations of lemmas in the process of florets opening using the non-invasive 3-D image correlation technique, which was for the first time applied to plant biological material. Due to the limited range of depth of field of camera lenses, these studies were limited to the initial lemmas opening period only, however this period seems to be crucial for the process and the results obtained can be considered to be reliable for the whole process. The clearly demonstrated for the first time the absence of any deformation of the lemma surface indicates that in the process of wheat florets opening, the potential mechanical effects associated with lemmas strains do not play a significant role, and the mechanisms related to cell turgor and water transport to lodicules should be the crucial factor. These results may suggest that in future studies on mechanisms associated with wheat flowering biology, more attention should be focused on aquaporins. Genes encoding aquaporins in wheat have been already recognized, e.g. Pandey et al. [ 32 ] indicated 13 wheat aquaporin genes, including genes coding for tonoplast aquaporins. Despite transcripts of two genes (PIP1–2 and TIP2–1, encoding the plasma membrane intrinsic proteins and tonoplast intrinsic proteins, respectively) were detected at a relatively high level in florets and inflorescences, the authors, however did not indicate the clear role of any of these genes in the processes related to wheat floret opening.
Our survey has shown that the wheat cultivars with contrasting tendency to cleistogamy differ in the potential for pollen dispersion. The cultivar Dacanto with higher pollen production per anther and similar pollen production per spike compared to Piko, has displayed lower amount of dispersed pollen (i.e. the number of pollen grains available for cross-pollination), therefore we conclude that the anther extrusion capability is particularly important for the provision of air-blown pollen for cross-pollination in wheat. Several studies have reported that the ability for anther presentation outside the floret is essential for pollen dispersal in wheat [ 4 , 7 , 10 , 29 ]. However, the number of pollen grains production per anther can not be ignored, as indicated by de Vries [ 27 ], who pointed out that pollen donor capacity is positively related to the total number of produced pollen grains. In our study, the number of pollen grains established per anther (averaged 1243–1386) is within that reported in literature for wheat cultivars, e.g. 975 to 2773 [ 33 ]. However, our values recognized for cv. Piko were lower than that reported for this cultivar by de Vries [ 27 ], who documented 2672–2933 of pollen grains per anther. These disparity can be understood as pollen production even in this same species/cultivars is sensitive to external factors and can vary greatly spatially and temporally [ 34 , 35 ]. The differences could be the results of the human activities, i.e. fertilization, irrigation or their combination. It was reported in a number of studies that decrease in nutrient supply may result in the reduction of pollen grains formed per anther [ 36 , 37 ]. Likewise the method used for the assessment of pollen grains number can impact on the records.
Similarly, the temporal (=between years) differences established in pollen production (per anthers, per spikes) in both cultivars can be associated, at least partially, with specific environmental factors occurring during pollen development. Every stages of pollen development, i.e. the differentiation of sporogenous cells and meiosis (microsporogenesis) as well as post-meiotic development of microspores are recognized as the most stress-sensitive period in plant reproduction [ 36 ]. The role of drought stress, moderate water–deficit stress, suboptimal temperature and/or oxidative stresses on pollen production has been demonstrated across wheat cultivars [ 3 , 38 ]. According to Monneveux et al. [ 39 ], pollen development in wheat is critically affected by imbalance between photosynthesis and translocation use of photoassimilates (mainly starch). Year-to-year disparity in the pollen production calculated for spikes can be associated with both the differences in the pollen production per anther and the variable number of anthers developed in inflorescences across growing seasons. Such relationships have been previously documented in many plants [ 34 , 35 ].
The aim of our study was to gain new knowledge on the biology of wheat flowering, in particular on the differences between the cleisto- and chasmogamic forms which has certainly cognitive significance, but it can also be used in practice when seeking a female and male ideotypes for cross hybridization. In conclusion, the cleistogamic and chasmogamic wheat cultivars differ significantly in the potential for pollen dispersion for cross-pollination, which is mainly related to anther extrusion capacity. We described several novel anatomical traits that can have potential functional role in floret opening. Although none of these features differentiated the cultivars clearly, we assume, based on spikelet kinetics and the lack of lemmas surface deformation, that the water transport and turgor of cells is essential for the floret opening and anther extrusion in wheat. The search for parental ideotype should be supported by marker assisted selection, e.g. based of polymorphisms in genes related to aquaporin biosynthesis.
Materials and methods
Plant material.
Two winter wheat cultivars - Piko (chasmogamous, bred by Nordsaat Saatzucht GmbH, Germany), and Dacanto (cleistogamous, bred by KWS Polska Sp. z o.o., Poland) were used.
For the field study on the floral biology the experimental plants were grown under field conditions at the University of Life Sciences (ULS) in Lublin, Poland (51° 14′ N, 22° 34′ E, altitude 238 m a.s.l) in 2018–2019. The soil is podzolic, developed from loess. The soil reaction pH in H 2 O ranged 5.8–6.8. The average concetrations of nutriens in soil at depth of 0–20 cm is presented in Table 2 . The plants used for anatomical study were grown in the collection of agricultural crop plants (Department of Agronomy) at the Warsaw University of Life Sciences (WULS) campus in Ursynów, Warsaw (52° 16′ N, 21° 05′ E) in 2018. Plants were cultivated in podzolic loam soil at pH 6–7. Caryopses of each cultivar were sown in September (Warsaw) or October (Lublin), in plots ( n = 5) 4.0 m long. From each plot 10 plants were collected for anatomical studies. No fertilization was applied. Standard procedures for winter wheat plant protection were applied. Weeds were removed by hand, if necessary.
Field study
The field observations were conducted to assess (i) diurnal pattern of anther extrusion and (ii) anther extrusion capacity. The diurnal pattern of anther extrusion was exhibited based on methods described by Langer et al. [ 4 ] and was recorded between 4.00 h (GMT + 2 h) and 20.00 h, in one-hour intervals, using n = 10 spikes per cultivar. Diurnal pattern of anther extrusion was expressed as the percentage of newly extruded anthers in relation to the total number of anthers extruded during a day. These observations were conducted in full flowering phase of each cultivar. The anther extrusion capacity was determined on the basis of the proportion of anthers released out of florets in relation to all anthers developed per spike. To establish this, we covered spikes ( n = 20 per cultivar per year) with isolators (mesh size < 1 mm) before flowering and counted the number of released anthers as well as the number of all developed anthers. Isolators were kept until the end of the spike flowering period.
Pollen dispersed for cross-pollination
Pollen production was evaluated as (i) total pollen production, i.e. the number of pollen grains produced per spike, and (ii) capability for pollen dispersion, i.e. the number of pollen grains dispersed outside the spike. The capability for pollen dispersion was calculated based on total pollen production and the anther extrusion capacity (for definition see Field study subsection).
The number of pollen grains produced per spike was calculated based on the number of anthers developed per spike ( n = 20 spikes per cultivar per year, collected from different plants) and the number of pollen grains produced per anther. The number of pollen grains per anther was established using electronic particle counter (Multisizer 4e, Beckman Coulter Counter, Inc., USA) calibrated according to the producer recommendations. The pollen grains were counted in mature anthers collected just before opening. Anthers were dissected from florets randomly selected form different spikes. In total, 408 anthers were sampled ( n = 204 for Dacanto and for Piko). Anthers were placed in Eppendorf Tubes 2.0 ml. Subsequently, pollen was removed from anthers using ether (2 times × 1 ml) and 70% ethanol (5–7 times × 1–2 ml). Finally, ethanol was evaporated, the electrolyte was added and counting of pollen grains was made automatically using an electronic sensor.
Micromorphology and anatomical study
Entire inflorescences (spikes) were taken for microscopic examination, in two terms (i) before (= pre-anthesis) and (ii) after opening (= post-anthesis) the lemmas. The inflorescences were fixed in pure methanol overnight [ 40 ], and next rinsed a few times (and stored) in absolute ethanol. During rinsing, sterile basal spikelets and the basal fertile spikelet were removed, and the next 3–4 fertile spikelets were later excised for light microscopy (LM), and the next ones for scanning electron microscopy (SEM).
Spikelets intended for LM were stained whole prior to embedding with the safranin-Astra blue (S-AB; Maácz and Vágás [ 41 ]). The most uniform staining was achieved in rehydrated spikelets, with the staining mixture slowly instilled into the spikelet using a syringe. Stained spikelets were rinsed a few times with 70% ethanol and embedded for vibratome sectioning. Since the blade pulled the spikelets from the 7% agarose blocks, the specimens were embedded in Glycid Ether 100 Epoxy Resin (SERVA) grade very soft (without the hardener, prepared according to the manufacturer’s formula), as follows (i) dehydration in graded ethanol series, with each stage prolonged to 30 min and overnight final dehydration in absolute ethanol, (ii) propylene oxide, (iii) saturation in resin-propylene oxide mixtures with each stage prolonged until sinking of most specimens, (iv) overnight saturation with pure resin and placement of samples in flat embedding molds for sectioning at the ventral-dorsal longitudinal plane, (v) preliminary polymerization at room temperature overnight to facilitate evaporation of propylene oxide residues (this stage was tested to be necessary), and final polymerization at 60 °C for 24 h. Blocks were attached to the microtome holder with commercial two-component epoxy glue and serial-sectioned at 70 μm using VT1000 S vibratome (Leica). Sections were unfolded at 70 °C, mounted in a glycerol and examined using Provis AX70 (Olympus Corporation) light microscope equipped with a digital camera UC90.
Spikelets for SEM were trimmed to expose the basal part of a glume or lemma/palea, or the adaxial side of lemma or palea. Next the specimens were critical-point dried using CPD 7501 dryer (Polaron), attached to the holders and gold sputtered in sputter coater JFC-1300 (JEOL). They were examined in the Analytical Centre of WULS using Quanta 200 (FEI Company) environmental scanning electron microscope operating at 20 kV under high vacuum. The images were saved as tiff files at 2048 × 1886 pixels resolution.
The tests were performed in two replicate experiments done on May 15–31, 2018 and May 20–June 10, 2019. Just before the start of flowering, the plants were cut off at the base of the stem, submerged with the basal ends of the stems in a container of water and placed in a plant grow box, under continuous light source (HPS Phytolite 600 W lamp, photon flux1045 lmol m-2 s-1, luminous flux 100 klm) at 21 °C. Measurements of the lemmas opening kinetics were based on films recorded using the time-lapse movie method (Ricoh GR cameras, Japan) and analyzed in the Tracker program ( https://www.cabrillo.edu/dbrown/tracker/ ) based on the Open Source Physics (OSP) Java framework. Fifteen spikelets of each of the two cultivars were used in the observations of lemmas opening kinetic in each experiment. Lemmas potential deformations during the flowering process were examined using a 3D image correlation method based on two cameras in a stereoscopic setup connected to a stereoscopic microscope. Such a technique gives the opportunity to perform measurements of 3D deformation and strain of specimens. Each camera provides a 2D view and software, through a correlation algorithm, forms a 3D image combining both camera views from different angles [ 42 ]. Present measurements of the wheat lemma deformation were made using the Dantec Q-400 system based on the Istra 4D software module with 5 MPix optics connected to the Leica M125 stereoscopic microscope. Activated carbon particles were sprayed on the lemma to create a stochastic pattern to the surface. The changes of this pattern due to deformation or movement of the object are recorded by the cameras. These images were automatically analyzed with special high accuracy correlation algorithms. As the result a set of data was generated containing the start contour of the object at beginning of the measurement and the three dimensional displacement vector of each object point due to the object deformation. In order to prevent possible other movements, not related to the lemma opening process each spikelet was inserted in a floristic sponge. The deformation measurements were made in 2019 on two replicate lemmas of each of the two cultivars.
Statistical analyses
The data are presented as means with standard deviations (SD). Statistical analyses was based on a t-test for independent groups used to compare the means of analyzed pollen data between cultivars and between years within cultivars. The pair-wise U Mann-Whitney’s non-parametric test was applied for the number of anthers per spike and the proportion of extruded anthers as the data did not fit t-test assumptions. The level of statistical significance for all the analyses was P = 0.05. The analyses were performed using Statistica ver. 13.3 software (Statsoft Polska, Kraków, Poland).
Availability of data and materials
The materials used during the current study are included in the Supplementary Information: Additional file 1 . Tables S1-S6.
Abbreviations
Light microscopy
Scanning electron microscopy
University of Life Sciences in Lublin
Warsaw University of Life Sciences - SGGW
Le Corff J. Effects of light and nutrient availability on chasmogamy and cleistogamy in an understory tropical herb, Calathea micans (Marantaceae). Am J Bot. 1993;80:1392–9.
Article Google Scholar
De Vries A. Flowering biology of wheat, particularly in view of hybrid seed production—a review. Euphytica. 1971;20:152–70.
Waddington SR, Cartwright PM, Wall PC. A qualitative scale of spike initial and pistil development in barley and wheat. Ann Bot. 1993;51:119–30.
Langer S, Longin C, Würschum T. Phenotypic evaluation of floral and flowering traits with relevance for hybrid breeding in wheat ( Triticum aestivum L.). PBI. 2014;133:433–41.
Google Scholar
Longin CF, Mühleisen J, Maurer HP, Zhang H, Gowda M, Reif JC. Hybrid breeding in autogamous cereals. Theor Appl Genet. 2012;125:1087–96.
Mette MF, Gils M, Longin CFH, Reif JC. Hybrid breeding in wheat. In: Ogihara Y, Takumi S, Handa H, editors. Advances in Wheat Genetics: From Genome to Field. Tokyo: Springer; 2015. p. 225–32.
Chapter Google Scholar
Boeven PH, Longin CF, Leiser WL, Kollers S, Ebmeyer E, Würschum T. Genetic architecture of male floral traits required for hybrid wheat breeding. Theor Appl Genet. 2016;129:2343–57.
Gupta PK, Balyan HS, Gahlaut V, Saripalli G, Pal B, Basnet BR, Joshi AK. Hybrid wheat: past, present and future. Theor Appl Genet. 2019;32:2463–83.
Gowda M, Longin CFH, Lein V, Reif JC. Relevance of specific versus general combining ability in winter wheat. Crop Sci. 2012;52:2494–500.
Whitford R, Fleury D, Reif J, Garcia M, Okada T, Korzun V, Langridge P. Hybrid breeding in wheat: technologies to improve hybrid wheat seed production. J Exp Bot. 2013;64:5411–28.
Article CAS Google Scholar
Dixon LE, Bencivenga S, Boden SA. A new opening for wheat seed production. JXB. 2018;69:341–3.
CAS Google Scholar
Liu H, Zhang G, Wang J, Li J, Song Y, Qiao L, Niu N, Wang J, Ma S, Li L. Chemical hybridizing agent SQ-1-induced male sterility in Triticum aestivum L.: a comparative analysis of the anther proteome. BMC Plant Biol. 2018;18:7. https://doi.org/10.1186/s12870-017-1225-x .
Article CAS PubMed PubMed Central Google Scholar
Percival J. The wheat plant. London: Duckworth and co.; 1921. https://doi.org/10.5962/bhl.title.25683
Yoshida H. Is the lodicule a petal: molecular evidence? Plant Sci. 2012;184:121–8.
De Vries A. Some aspects of cross-pollination in wheat ( Triticum aesticum L.). another length and number of pollen grains per anther. Euphytica. 1974;23:11–9.
Zee S, O’Brien T. The vascular tissue of the lodicules of wheat. Aust J Biol Sci. 1971;24:797–804.
Frankel R, Galun E. Pollination mechanisms, reproduction and plant breeding. Berlin: Springer-Verlag; 1977. p. 792–34.
Book Google Scholar
Virmani SS. Heterosis and hybrid rice breeding. Berlin: Springer-Verlag; 1994.
Heslop-Harrison Y, Heslop-Harrison JS. Lodicule function and filament extension in the grasses: potassium ion movement and tissue specialization. Ann Bot. 1996;77:573–82.
Kirby EJM, Fellowes G, Appleyard M. An thesis in winter barley. In: Annual report—Plant Breeding Institute. Cambridge: PBI; 1983. p. 112–3.
Pickett AA. Hybrid wheat - results and problems. Berlin and Hamburg: Paul Parey Scientific Publishers; 1993.
Okada T, Ridma JEA, Jayasinghe M, Nansamba M, Baes M, Warner P, Kouidri A, Correia D, Nguyen VY, Whitford R, Baumann U. Unfertilized ovary pushes wheat flower open for cross-pollination. J Exp Bot. 2018;69:399–412.
Barrett SC. The evolution of plant sexual diversity. Nat Rev Genet. 2002;3:274–84.
Van Doorn WG, Van Meeteren U. Flower opening and closure: a review. J Exp Bot. 2003;54:1801–12.
Ostergaard L. Fruit Development and Seed Dispersal (L. Ostergaard, Ed.). Oxford: Wiley; 2009. Annual Plant Reviews
Llorens C, Argentina M, Rojas N, Westbrook J, Dumais J, Noblin X. The fern cavitation catapult: mechanism and design principles. J R Soc. 2016;13:20150930.
De Vries A. Some aspects of cross-pollination in wheat ( Triticum aestivum L.) 1. Pollen concentration in the field as influenced by variety, diurnal pattern, weather conditions and level as compared to the height of the pollen donor. Euphytica. 1972;21:185–203.
Kempe K, Boudichevskaia A, Jerchel R, Pescianschi D, Schmidt R, Kirchhoff M, Schachschneider R, Gils M. Quantitative assessment of wheat pollen shed by digital image analysis of trapped airborne pollen grains. ACST. 2013;2(1):119.
Beri SM, Anand SC. Factors affecting pollen shedding capacity in wheat. Euphytica. 1971;20:327–32.
Arber A. The reproductive shoot in grasses – structure and anthesis. In: The Gramineae. A Study of Cereal, Bamboo and Grass, vol. 8: Cambridge University Press; 1934. https://doi.org/10.1017/CBO9780511700668.
Beuzamy L, Nakayama N, Boudaoud A. Flowers under pressure: ins and outs of turgor regulation in development. Ann Bot. 2014;114:1517–33.
Pandey B, Sharma P, Pandey DM, Sharma I, Chatrath R. Identification of new aquaporin genes and single nucleotide polymorphism in bread wheat. Evol Bioinformatics Online. 2013;9:437–52.
Singh RB, Sindhu JS. Pollen production and shedding in male fertility restorer lines of wheat. Wheat Inf Serv. 1974;39:14.
Antoń S, Denisow B, Milaniuk K. Flowering, pollen production and insect visitation in two Aconitum species (Ranunculaceae). Acta Agrobot. 2014;67:3–12.
Bhattacharya K, Datta BK. Anthesis and pollen release: anthesis and pollen release of some plants of West Bengal, India. Grana. 1992;31:67–71.
Hedhly A, Hormaza JI, Herrero M. Global warming and sexual plant reproduction. Trends Plant Sci. 2009;14:30–6.
Browne RG, Iacuone S, Li SF, Dolferus R, Parish RW. Another morphological development and stage determination in Triticum aestivum . Front Plant Sci. 2018;9:228. https://doi.org/10.3389/fpls.2018.00228 .
Article PubMed PubMed Central Google Scholar
Ji X, Shiran B, Wan J, Lewis DC, Jenkins CLD, Condon AG, Richards RA, Dolferus R. Importance of pre-anthesis anther sink strength for maintenance of grain number during reproductive stage water stress in wheat. Plant Cell Environ. 2010;33:926–42.
Monneveux P, Pastenes C, Reynolds MP. Limitations to photosynthesis under light and heat stress in three high-yielding wheat genotypes. J Plant Physiol. 2003;160:657–66.
Schwab B, Hülskamp M. Quick and easy fixation of plant tissues for scanning electron microscopy (SEM). Cold Spring Harbor Protoc. 2010;8:pdb.prot4934. https://doi.org/10.1101/pdb.prot493413 .
Maácz GJ, Vágás E. A new method for staining of cellulose and lignified cell-walls. Mikroskopie. 1961;16:40–3.
PubMed Google Scholar
Rusin T, Kopernik M. Characterization of biocompatible materials using stereo microscope 3D digital image correlation. Adv Eng Mater. 2016;18:1651–60.
Download references
Acknowledgements
We are grateful to Grażyna Szymczak PhD for providing the area for the experimental plots in the UMCS Botanical Garden in Lublin. We thank Krzysztof Pągowski (Department of Agronomy, WULS - SGGW) for expert technical assistance in growing plants, Agnieszka Ostrowska (Analytical Centre, WULS- SGGW) - SEM, Monika Strzałkowska-Abramek, Ernest Stawiarz, Jacek Jachuła, and Magdalena Kamińska (ULS Lublin) for assistance in flowering observations and pollen production assessment. Our thanks also go to Agnieszka Kubik-Komar (ULS Lublin) for statistical assistance. Special thanks are due to an unknown reviewers for substantial improvements of the manuscript.
The research was financed by BIOSTRATEG3/343665/6/NCBR/2017 grant.
Author information
Authors and affiliations.
Department of Forest Botany, Institute of Forest Sciences, Warsaw University of Life Sciences – SGGW, 159 Nowoursynowska Street, 02-776, Warszawa, Poland
Urszula Zajączkowska & Alicja Dołkin-Lewko
Department of Botany and Plant Physiology, University of Life Sciences in Lublin, 15 Akademicka Street, 20-950, Lublin, Poland
Bożena Denisow
Department of Botany, Institute of Biology, Warsaw University of Life Sciences – SGGW, 159 Nowoursynowska Street, 02-776, Warszawa, Poland
Barbara Łotocka
Department of Plant Genetics, Breeding and Biotechnology, Institute of Biology, Warsaw University of Life Sciences –SGGW, 159 Nowoursynowska Street, 02-776, Warszawa, Poland
Monika Rakoczy-Trojanowska
You can also search for this author in PubMed Google Scholar
Contributions
BD performed the anther extrusion and pollen production experiment, BŁ and AD-L made the microscopic studies, UZ and AD-L conducted the observations of lemma opening kinetics and the lemma deformation experiment, BD, BŁ, UZ, and MR-T participated in writing the manuscript, UZ prepared the final edition of the manuscript. MR-T supervised the research. All authors have read and approved the manuscript.
Corresponding author
Correspondence to Urszula Zajączkowska .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.

Supplementary Information
Additional file 1: table s1..
Diurnal pattern of anther extrusion in two wheat cultivars. Original data from two experiments in 2018 and 2019 are summarized in Fig. 1 . Table S2. Proportion of extruded anthers in two wheat cultivars. Original data from two experiments in 2018 and 2019 are summarized in Fig. 2 . Table S3. Number of pollen grains per anther in two wheat cultivars. Original data from two experiments in 2018 and 2019 are summarized in Table 1 . Table S3. Number of pollen grains per anther in two wheat cultivars. Original data from two experiments in 2018 and 2019 are summarized in Table 1 . Table S4. Components of pollen production (columns C, D,) and the number of pollen grains (E-H) in two wheat cultivars. Original data from two experiments in 2018 and 2019., Data from columns C, D and columns E-H are summarized in Table 1 and Fig. 3 , respectively. Table S5. Spatial displacement (mm) of the opening lemmas. Results of two experiments during flowering of Dacanto and Piko wheat cultivars carried in 2018 and 2019. Data of 15 replicate lemmas obtained from the time-lapse imaging. The data are summarized in Fig. 7 . Table S6. Speed of spatial displacement (mm/min) of the opening lemmas. Results of two experiments during flowering of Dacanto and Piko wheat cultivars carried in 2018 and 2019. Data of 15 replicate lemmas obtained from the time-lapse imaging. The data are summarized in Fig. 7 .
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Zajączkowska, U., Denisow, B., Łotocka, B. et al. Spikelet movements, anther extrusion and pollen production in wheat cultivars with contrasting tendencies to cleistogamy. BMC Plant Biol 21 , 136 (2021). https://doi.org/10.1186/s12870-021-02917-7
Download citation
Received : 03 December 2020
Accepted : 22 February 2021
Published : 16 March 2021
DOI : https://doi.org/10.1186/s12870-021-02917-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cleistogamy
- Flower opening
- Spikelet anatomy
- Pollen dispersion
- Triticum aestivum
- Wheat breeding
BMC Plant Biology
ISSN: 1471-2229
- General enquiries: [email protected]
Understanding the molecular mechanism of anther development under abiotic stresses
- Published: 15 September 2020
- Volume 105 , pages 1–10, ( 2021 )
Cite this article
- Zaibao Zhang ORCID: orcid.org/0000-0003-2278-0119 1 na1 ,
- Menghui Hu 1 na1 ,
- Weiwei Xu 1 ,
- Yuan Wang 1 ,
- Ke Huang 1 ,
- Chi Zhang 1 &
- Jie Wen 1
3054 Accesses
27 Citations
Explore all metrics
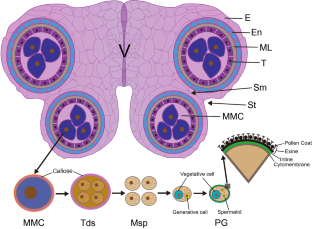
Key message
The developmental stage of anther development is generally more sensitive to abiotic stress than other stages of growth. Specific ROS levels, plant hormones and carbohydrate metabolism are disturbed in anthers subjected to abiotic stresses.
As sessile organisms, plants are often challenged to multiple extreme abiotic stresses, such as drought, heat, cold, salinity and metal stresses in the field, which reduce plant growth, productivity and yield. The development of reproductive stage is more susceptible to abiotic stresses than the vegetative stage. Anther, the male reproductive organ that generate pollen grains, is more sensitive to abiotic stresses than female organs. Abiotic stresses affect all the processes of anther development, including tapetum development and degradation, microsporogenesis and pollen development, anther dehiscence, and filament elongation. In addition, abiotic stresses significantly interrupt phytohormone, lipid and carbohydrate metabolism, alter reactive oxygen species (ROS) homeostasis in anthers, which are strongly responsible for the loss of pollen fertility. At present, the precise molecular mechanisms of anther development under adverse abiotic stresses are still not fully understood. Therefore, more emphasis should be given to understand molecular control of anther development during abiotic stresses to engineer crops with better crop yield.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
Involvement of cat in the detoxification of ht-induced ros burst in rice anther and its relation to pollen fertility.
Qian Zhao, Lujian Zhou, … Fangmin Cheng
Reactive oxygen species mediate tapetal programmed cell death in tobacco and tomato
Shi-Xia Yu, Qiang-Nan Feng, … Yan Zhang
Differential responses of anthers of stress tolerant and sensitive wheat cultivars to high temperature stress
Richard G. Browne, Song F. Li, … Roger W. Parish
Agarwal P, Khurana P (2019) Functional characterization of HSFs from wheat in response to heat and other abiotic stress conditions. Funct Integr Genom 19(3):497–513. https://doi.org/10.1007/s10142-019-00666-3
Article CAS Google Scholar
Bai B, Wu J, Sheng WT, Zhou B, Zhou LJ, Zhuang W, Yao DP, Deng QY (2015) Comparative analysis of anther transcriptome profiles of two different rice male sterile lines genotypes under cold stress. Int J Mol Sci 16(5):11398–11416. https://doi.org/10.3390/ijms160511398
Article CAS PubMed PubMed Central Google Scholar
Barton DA, Cantrill LC, Law AM, Phillips CG, Sutton BG, Overall RL (2014) Chilling to zero degrees disrupts pollen formation but not meiotic microtubule arrays in Triticum aestivum L. Plant, Cell Environ 37(12):2781–2794. https://doi.org/10.1111/pce.12358
Chang F, Wang Y, Wang S, Ma H (2011) Molecular control of microsporogenesis in Arabidopsis. Curr Opin Plant Biol 14(1):66–73
Chaturvedi P, Ischebeck T, Egelhofer V, Lichtscheidl I, Weckwerth W (2013) Cell-specific analysis of the tomato pollen proteome from pollen mother cell to mature pollen provides evidence for developmental priming. J Proteome Res 12(11):4892–4903. https://doi.org/10.1021/pr400197p
Article CAS PubMed Google Scholar
Chen J, Pan A, He S, Su P, Yuan X, Zhu S, Liu Z (2020) Different microRNA families involved in regulating high temperature stress response during cotton ( Gossypium hirsutum L.) anther development. Int J Mol Sci. https://doi.org/10.3390/ijms21041280
Article PubMed PubMed Central Google Scholar
Endo M, Tsuchiya T, Hamada K, Kawamura S, Yano K, Ohshima M, Higashitani A, Watanabe M, Kawagishi-Kobayashi M (2009) High temperatures cause male sterility in rice plants with transcriptional alterations during pollen development. Plant Cell Physiol 50(11):1911–1922. https://doi.org/10.1093/pcp/pcp135
Fang W, Cheng F-M, Liu Y, Zhong LJ, Zhang GP (2006) Dynamic changes of plant hormones in developing grains at rice filling stage under different temperatures. Acta Agron Sin 32:25–29
CAS Google Scholar
Fath A, Bethke P, Lonsdale J, Meza-Romero R, Jones R (2000) Programmed cell death in cereal aleurone. Plant Mol Biol 44(3):255–266. https://doi.org/10.1023/a:1026584207243
Fragkostefanakis S, Mesihovic A, Hu Y, Schleiff E (2016) Unfolded protein response in pollen development and heat stress tolerance. Plant Reprod 29(1–2):81–91. https://doi.org/10.1007/s00497-016-0276-8
Gao H, Brandizzi F, Benning C, Larkin RM (2008) A membrane-tethered transcription factor defines a branch of the heat stress response in Arabidopsis thaliana. Proc Natl Acad Sci USA 105(42):16398–16403. https://doi.org/10.1073/pnas.0808463105
Article PubMed Google Scholar
Giorno F, Wolters-Arts M, Mariani C, Rieu I (2013) Ensuring reproduction at high temperatures: the heat stress response during anther and pollen development. Plants 2(3):489–506. https://doi.org/10.3390/plants2030489
Gong Z, Xiong L, Shi H, Yang S, Herrera-Estrella LR, Xu G, Chao DY, Li J, Wang PY, Qin F, Li J, Ding Y, Shi Y, Wang Y, Yang Y, Guo Y, Zhu JK (2020) Plant abiotic stress response and nutrient use efficiency. Sci China 63(5):635–674. https://doi.org/10.1007/s11427-020-1683-x
Article Google Scholar
Guo C, Ge X, Ma H (2013) The rice OsDIL gene plays a role in drought tolerance at vegetative and reproductive stages. Plant Mol Biol 82(3):239–253. https://doi.org/10.1007/s11103-013-0057-9
Guo C, Yao L, You C, Wang S, Cui J, Ge X, Ma H (2016) MID1 plays an important role in response to drought stress during reproductive development. Plant J 88(2):280–293. https://doi.org/10.1111/tpj.13250
Hu L, Liang W, Yin C, Cui X, Zong J, Wang X, Hu J, Zhang D (2011) Rice MADS3 regulates ROS homeostasis during late anther development. Plant Cell 23(2):515–533. https://doi.org/10.1105/tpc.110.074369
IPCC (2014) Climate Change 2014: mitigation of climate change. In: Edenhofer O, Pichs-Madruga R, Sokona Y, Minx JC, Farahani E, Kadner S, Seyboth K, Adler A, Baum I, Brunner S, Eickemeier P, Kriemann B, Savolainen J, Schlömer S, von Stechow C, Zwickel T (eds) Contribution of working group III to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, New York, pp 1435
Ishiguro S, Ogasawara K, Fujino K, Sato Y, Kishima Y (2014) Low temperature-responsive changes in the anther transcriptome’s repeat sequences are indicative of stress sensitivity and pollen sterility in rice strains. Plant Physiol 164(2):671–682. https://doi.org/10.1104/pp.113.230656
Jagadish SV, Craufurd PQ, Wheeler TR (2007) High temperature stress and spikelet fertility in rice ( Oryza sativa L). J Exp Bot 58(7):1627–1635. https://doi.org/10.1093/jxb/erm003
Jagadish SV, Murty MV, Quick WP (2015) Rice responses to rising temperatures–challenges, perspectives and future directions. Plant, Cell Environ 38(9):1686–1698. https://doi.org/10.1111/pce.12430
Ji X, Shiran B, Wan J, Lewis DC, Jenkins CL, Condon AG, Richards RA, Dolferus R (2010) Importance of pre-anthesis anther sink strength for maintenance of grain number during reproductive stage water stress in wheat. Plant, Cell Environ 33(6):926–942. https://doi.org/10.1111/j.1365-3040.2010.02130.x
Ji X, Dong B, Shiran B, Talbot MJ, Edlington JE, Hughes T, White RG, Gubler F, Dolferus R (2011) Control of abscisic acid catabolism and abscisic acid homeostasis is important for reproductive stage stress tolerance in cereals. Plant Physiol 156(2):647–662. https://doi.org/10.1104/pp.111.176164
Jiang Y, Lahlali R, Karunakaran C, Warkentin TD, Davis AR, Bueckert RA (2019) Pollen, ovules, and pollination in pea: success, failure, and resilience in heat. Plant, Cell Environ 42(1):354–372. https://doi.org/10.1111/pce.13427
Jin Y, Yang H, Wei Z, Ma H, Ge X (2013) Rice male development under drought stress: phenotypic changes and stage-dependent transcriptomic reprogramming. Mol Plant 6(5):1630–1645. https://doi.org/10.1093/mp/sst067
Kelliher T, Walbot V (2012) Hypoxia triggers meiotic fate acquisition in maize. Science 337(6092):345–348. https://doi.org/10.1126/science.1220080
Kim SY, Hong CB, Lee I (2001) Heat shock stress causes stage-specific male sterility in Arabidopsis thaliana . J Plant Res 114(3):301–307. https://doi.org/10.1007/PL00013991
Ko H, Koichiro A, Tokunori H, Hitoshi S, Mikiko K, Angeles SR, Yasuko H, Miyako UT, Makoto M (2008) Comprehensive transcriptome analysis of phytohormone biosynthesis and signaling genes in microspore/pollen and tapetum of rice. Plant Cell Physiol 49(10):1429–1450
Kotak S, Larkindale J, Lee U, von Koskull-Doring P, Vierling E, Scharf KD (2007) Complexity of the heat stress response in plants. Curr Opin Plant Biol 10(3):310–316. https://doi.org/10.1016/j.pbi.2007.04.011
Kurusu T, Koyano T, Hanamata S, Kubo T, Noguchi Y, Yagi C, Nagata N, Yamamoto T, Ohnishi T, Okazaki Y, Kitahata N, Ando D, Ishikawa M, Wada S, Miyao A, Hirochika H, Shimada H, Makino A, Saito K, Ishida H, Kinoshita T, Kurata N, Kuchitsu K (2014) OsATG7 is required for autophagy-dependent lipid metabolism in rice postmeiotic anther development. Autophagy 10(5):878–888. https://doi.org/10.4161/auto.28279
Lalonde S, Beebe DU, Saini HS (1997) Early signs of disruption of wheat anther development associated with the induction of male sterility by meiotic-stage water deficit. Sex Plant Reprod 10(1):40–48. https://doi.org/10.1007/s004970050066
Lamers J, van der Meer T, Testerink C (2020) How plants sense and respond to stressful environments. Plant Physiol 182(4):1624–1635. https://doi.org/10.1104/pp.19.01464
Lesk C, Rowhani P, Ramankutty N (2016) Influence of extreme weather disasters on global crop production. Nature 529(7584):84–87. https://doi.org/10.1038/nature16467
Liu JX, Howell SH (2010) Endoplasmic reticulum protein quality control and its relationship to environmental stress responses in plants. Plant Cell 22(9):2930–2942. https://doi.org/10.1105/tpc.110.078154
Liu Z, Shi X, Li S, Zhang L, Song X (2018) Oxidative stress and aberrant programmed cell death are associated with pollen abortion in isonuclear alloplasmic male-sterile wheat. Front Plant Sci 9:595. https://doi.org/10.3389/fpls.2018.00595
Lu S, Su W, Li H, Guo Z (2009) Abscisic acid improves drought tolerance of triploid bermudagrass and involves H 2 O 2 - and NO-induced antioxidant enzyme activities. Plant Physiol Biochem 47(2):132–138. https://doi.org/10.1016/j.plaphy.2008.10.006
Lyu X, Chen S, Liao N, Liu J, Hu Z, Yang J, Zhang M (2019) Characterization of watermelon anther and its programmed cell death-associated events during dehiscence under cold stress. Plant Cell Rep 38(12):1551–1561. https://doi.org/10.1007/s00299-019-02466-2
Ma H (2005) Molecular genetic analyses of microsporogenesis and microgametogenesis in flowering plants. Annu Rev Plant Biol 56:393–434. https://doi.org/10.1146/annurev.arplant.55.031903.141717
Ma Y, Kang J, Wu J, Zhu Y, Wang X (2015) Identification of tapetum-specific genes by comparing global gene expression of four different male sterile lines in Brassica oleracea . Plant Mol Biol 87(6):541–554. https://doi.org/10.1007/s11103-015-0287-0
Mamun EA, Alfred S, Cantrill LC, Overall RL, Sutton BG (2006) Effects of chilling on male gametophyte development in rice. Cell Biol Int 30(7):583–591. https://doi.org/10.1016/j.cellbi.2006.03.004
Morgan JM (1980) Possible role of abscisic acid in reducing seed set in water-stressed wheat plants. Nature 289:655–657
Mousavi S, Alisoltani A, Shiran B, Fallahi H, Ebrahimie E, Imani A, Houshmand S (2014) De novo transcriptome assembly and comparative analysis of differentially expressed genes in Prunus dulcis Mill. in response to freezing stress. PLoS ONE 9(8):e104541. https://doi.org/10.1371/journal.pone.0104541
Murray F, Kalla R, Jacobsen J, Gubler F (2003) A role for HvGAMYB in anther development. Plant J 33(3):481–491. https://doi.org/10.1046/j.1365-313x.2003.01641.x
Namuco OS, O’Toole JC (1986) Reproductive stage water stress and sterility. I. Effect of stress during meiosis 1. Crop Sci 26(2):317–321
Oliver SN, Dongen JTV, Alfred SC, Mamun EA, Dolferus R (2005) Cold-induced repression of the rice anther-specific cell wall invertase gene OSINV4 is correlated with sucrose accumulation and pollen sterility. Plant, Cell Environ 28(12):1534–1551
Oliver SN, Dennis ES, Dolferus R (2007) ABA regulates apoplastic sugar transport and is a potential signal for cold-induced pollen sterility in rice. Plant Cell Physiol 48(9):1319–1330. https://doi.org/10.1093/pcp/pcm100
Peet MM, Sato S, Gardner RG (2002) Comparing heat stress effects on male-fertile and male-sterile tomatoes. Plant, Cell Environ 21:225–231. https://doi.org/10.1046/j.1365-3040.1998.00281.x
Plackett AR, Ferguson AC, Powers SJ, Wanchoo-Kohli A, Phillips AL, Wilson ZA, Hedden P, Thomas SG (2014) DELLA activity is required for successful pollen development in the Columbia ecotype of Arabidopsis. New Phytol 201(3):825–836. https://doi.org/10.1111/nph.12571
Qi J, Song CP, Wang B, Zhou J, Kangasjarvi J, Zhu JK, Gong Z (2018) Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J Integr Plant Biol 60(9):805–826. https://doi.org/10.1111/jipb.12654
Saini HS, Sedgley M, Aspinall D (1984) Development anatomy in wheat of male sterility induced by heat stress, water deficit or abscisic acid. Funct Plant Biol 11(4):243–253
Saito K, Hayanosaito Y, Kuroki M, Sato Y (2010) Map-based cloning of the rice cold tolerance gene Ctb1. Plant Sci 179(1):97–102
Sakata T, Higashitani A (2008) Male sterility accompanied with abnormal anther development in plants—genes and environmental stresses with special reference to high temperature injury. Int J Plant Dev Biol 2
Sakata T, Oshino T, Miura S, Tomabechi M, Tsunaga Y, Higashitani N, Miyazawa Y, Takahashi H, Watanabe M, Higashitani A (2010) Auxins reverse plant male sterility caused by high temperatures. Proc Natl Acad Sci USA 107(19):8569–8574. https://doi.org/10.1073/pnas.1000869107
Sakata T, Oda S, Tsunaga Y, Shomura H, Kawagishikobayashi M, Aya K, Saeki K, Endo T, Nagano K, Kojima M (2014) Reduction of gibberellin by low temperature disrupts pollen development in rice. Plant Physiol 164(4):2011–2019
Sanders PM, Bui AQ, Weterings K, Mcintire KN, Hsu YC, Pei YL, Mai TT, Beals TP, Goldberg RB (1999) Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex Plant Reprod 11(6):297–322
Sharma KD, Nayyar H (2014) Cold stress alters transcription in meiotic anthers of cold tolerant chickpea ( Cicer arietinum L.). BMC Res Notes 7:717. https://doi.org/10.1186/1756-0500-7-717
Sharma KD, Nayyar H (2016) Regulatory networks in pollen development under cold stress. Front Plant Sci 7:402. https://doi.org/10.3389/fpls.2016.00402
Sharma L, Dalal M, Verma R, Kumar SV, Yadav SK, Pushkar S, Kushwaha SR, Bhowmik A, Chinnusamy V (2018) Auxin protects spikelet fertility and grain yield under drought and heat stresses in rice. Environ Exp Bot. https://doi.org/10.1016/j.envexpbot.2018.02.013
Shimono H, Abe A, Aoki N, Koumoto T, Sato M, Yokoi S, Kuroda E, Endo T, Saeki KI, Nagano K (2016) Combining mapping of physiological quantitative trait loci and transcriptome for cold tolerance for counteracting male sterility induced by low temperatures during reproductive stage in rice. Physiol Plant 157(2):175–192. https://doi.org/10.1111/ppl.12410
Shinohara T, Leskovar DI (2014) Effects of ABA, antitranspirants, heat and drought stress on plant growth, physiology and water status of artichoke transplants. Sci Hortic 165:225–234. https://doi.org/10.1016/j.scienta.2013.10.045
Su Z, Ma X, Guo H, Sukiran NL, Guo B, Assmann SM, Ma H (2013) Flower development under drought stress: morphological and transcriptomic analyses reveal acute responses and long-term acclimation in Arabidopsis. Plant Cell 25(10):3785–3807. https://doi.org/10.1105/tpc.113.115428
Sun L, Lu SJ, Zhang SS, Zhou SF, Sun L, Liu JX (2013) The lumen-facing domain is important for the biological function and organelle-to-organelle movement of bZIP28 during ER stress in Arabidopsis. Mol Plant 6(5):1605–1615. https://doi.org/10.1093/mp/sst059
Suzuki K, Takeda H, Tsukaguchi T, Egawa Y (2001) Ultrastructural study on degeneration of tapetum in anther of snap bean ( Phaseolus vulgaris L.) under heat stress. Sex Plant Reprod 13(6):293–299
Tang RS, Zheng JC, Jin ZQ, Zhang DD, Huang YH, Chen LG (2008) Possible correlation between high temperature-induced floret sterility and endogenous levels of IAA, GAs and ABA in rice ( Oryza sativa L.). Plant Growth Regul 54(1):37–43
Vaughan MM, Christensen S, Schmelz EA, Huffaker A, McAuslane HJ, Alborn HT, Romero M, Allen LH, Teal PE (2015) Accumulation of terpenoid phytoalexins in maize roots is associated with drought tolerance. Plant, Cell Environ 38(11):2195–2207. https://doi.org/10.1111/pce.12482
Yao X, Tian L, Yang J, Zhao YN, Zhu YX, Dai X, Zhao Y, Yang ZN (2018) Auxin production in diploid microsporocytes is necessary and sufficient for early stages of pollen development. PLoS Genet 14(5):e1007397
Yu SX, Feng QN, Xie HT, Li S, Zhang Y (2017) Reactive oxygen species mediate tapetal programmed cell death in tobacco and tomato. BMC Plant Biol 17(1):76. https://doi.org/10.1186/s12870-017-1025-3
Yu J, Jiang M, Guo C (2019) Crop pollen development under drought: from the phenotype to the mechanism. Int J Mol Sci. https://doi.org/10.3390/ijms20071550
Zeng Y, Zhang Y, Xiang J, Uphoff NT, Pan X, Zhu D (2017) Effects of low temperature stress on spikelet-related parameters during anthesis in indica-Japonica hybrid rice. Front Plant Sci 8:1350. https://doi.org/10.3389/fpls.2017.01350
Zhang D, Yang L (2014) Specification of tapetum and microsporocyte cells within the anther. Curr Opin Plant Biol 17:49–55. https://doi.org/10.1016/j.pbi.2013.11.001
Zhang X, Li J, Liu A, Zou J, Zhou X, Xiang J, Rerksiri W, Peng Y, Xiong X, Chen X (2012) Expression profile in rice panicle: insights into heat response mechanism at reproductive stage. PLoS ONE 7(11):e49652. https://doi.org/10.1371/journal.pone.0049652
Zhang SS, Yang H, Ding L, Song ZT, Ma H, Chang F, Liu JX (2017) Tissue-specific transcriptomics reveals an important role of the unfolded protein response in maintaining fertility upon heat stress in Arabidopsis. Plant Cell 29(5):1007–1023. https://doi.org/10.1105/tpc.16.00916
Zhao Q, Zhou L, Liu J, Cao Z, Du X, Huang F, Pan G, Cheng F (2018a) Involvement of CAT in the detoxification of HT-induced ROS burst in rice anther and its relation to pollen fertility. Plant Cell Rep 37(5):741–757. https://doi.org/10.1007/s00299-018-2264-y
Zhao Q, Zhou L, Liu J, Du X, Asad MA, Huang F, Pan G, Cheng F (2018b) Relationship of ROS accumulation and superoxide dismutase isozymes in developing anther with floret fertility of rice under heat stress. Plant Physiol Biochem 122:90–101. https://doi.org/10.1016/j.plaphy.2017.11.009
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273. https://doi.org/10.1146/annurev.arplant.53.091401.143329
Zou C, Jiang W, Yu D (2010) Male gametophyte-specific WRKY34 transcription factor mediates cold sensitivity of mature pollen in Arabidopsis. J Exp Bot 61(14):3901–3914. https://doi.org/10.1093/jxb/erq204
Download references
Acknowledgements
This work was supported by the grants from Natural Science Foundation of Henan Provincial Science and Technology (No. 182300410063), Key Scientific Research Projects of Henan Higher Education Institutions (No. 18A180031), and Nanhu Scholars Program for Young Scholars of Xinyang Normal University.
Author information
Zaibao Zhang and Menghui Hu have contributed equally to this work.
Authors and Affiliations
College of Life Science, Xinyang Normal University, Xinyang, Henan, China
Zaibao Zhang, Menghui Hu, Weiwei Xu, Yuan Wang, Ke Huang, Chi Zhang & Jie Wen
You can also search for this author in PubMed Google Scholar
Contributions
ZZ designed the outline of the article. ZZ and MH wrote the article. MH and WX prepared the figures. CZ, JW and HY participated in manuscript preparation and revision. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Zaibao Zhang .
Ethics declarations
Conflict of interest.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Zhang, Z., Hu, M., Xu, W. et al. Understanding the molecular mechanism of anther development under abiotic stresses. Plant Mol Biol 105 , 1–10 (2021). https://doi.org/10.1007/s11103-020-01074-z
Download citation
Received : 31 March 2020
Accepted : 09 September 2020
Published : 15 September 2020
Issue Date : January 2021
DOI : https://doi.org/10.1007/s11103-020-01074-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Anther development
- Abiotic stress
- Tapetum degradation
- Phytohormones
- Reactive oxygen species
- Carbohydrate metabolism
- Find a journal
- Publish with us
- Track your research
- Dictionaries home
- American English
- Collocations
- German-English
- Grammar home
- Practical English Usage
- Learn & Practise Grammar (Beta)
- Word Lists home
- My Word Lists
- Recent additions
- Resources home
- Text Checker
Definition of antithesis noun from the Oxford Advanced Learner's Dictionary
- Love is the antithesis of selfishness.
- Students finishing their education at 16 is the very antithesis of what society needs.
- The current establishment is the antithesis of democracy.
- antithesis between
- antithesis of
Questions about grammar and vocabulary?
Find the answers with Practical English Usage online, your indispensable guide to problems in English.
- There is an antithesis between the needs of the state and the needs of the people.
- the sharp antithesis between their views
Nearby words
- TheFreeDictionary
- Word / Article
- Starts with
- Free toolbar & extensions
- Word of the Day
- Free content
- the opening of a flower bud.
- the period from flowering to fruit setting.
- anteroposterior diameter of pelvic cavity
- anteroposterior diameter of pelvic inlet
- anteroposterior diameter of pelvic outlet
- anteroposterior discrepancy
- anteroposterior position
- anteroseptal myocardial infarction
- anterosuperior
- anterosuperior sacroiliac ligament
- anterosuperior surface of body of pancreas
- anterovertical partial laryngectomy
- antesepalous
- antesystole
- anteversion
- Anthelminthic
- anthelmintic
- anthelone E
- Anthemis nobilis
- antheridium
- antherozoid
- Anthocerotae
- anthocyanin
- anthocyanins
- Anthomyia canicularis
- Anthony Nolan panel
- anthophobia
- anthoxanthins
- anthracemia
- anthracenedione
- anthracidal
- anthracomucin
- anthracosilicosis
- Antherogenous
- Antheropeas
- Antheropeas wallacei
- antherozoids
- antherozooid
- Anthes, Richard
- Anthesteria
- Anthetarius
- Anthoathecatae
- Anthobranchia
- Anthocarpous
- anthocaulis
- Anthoceratopsida
- Anthocerophyta
- Anthoceropsida
- Anthocerotaceae
- Facebook Share

Definition of 'anthesis'
Anthesis in british english.

anthesis in American English
Trends of anthesis.
View usage for: All Years Last 10 years Last 50 years Last 100 years Last 300 years
Browse alphabetically anthesis
- antherozoid
- antherozooid
- Anthesteria
- All ENGLISH words that begin with 'A'
Quick word challenge
Quiz Review
Score: 0 / 5

Wordle Helper

Scrabble Tools

Definition noun, plural: syntheses ( biochemistry ) The production of an organic compound in a living thing, especially as aided by enzymes Supplement In general, the term synthesis pertains to the creation of something. It is the process of combining two or more components to produce an entity. In biochemistry, it refers to the production of an organic compound in a living thing, especially as aided by enzymes. There are several syntheses occurring in the cell or organism. The creation of an organic compound in a living organism is referred to as biosynthesis. One of them is photosynthesis, a synthesis of complex organic material using carbon dioxide, water, inorganic salts, and light energy (from sunlight) captured by light-absorbing pigments, such as chlorophyll and other accessory pigments. In other relevant fields, such as chemistry, the term refers to the act or process of forming a complex substance by combining or integrating two or more chemical entities, especially through a chemical reaction. In psychiatry, synthesis pertains to the integration of different elements of the personality, in opposition to analysis. Word origin: Latin synthesis , from Ancient Greek synthesis (a putting together) See also:
- biosynthesis
Related term(s):
- photosynthesis
- Kiliani-fischer synthesis
- Merrifield synthesis
- Protein synthesis
- Dna synthesis
- Gene synthesis
- Synthesis of continuity
- Synthesis period
Related form(s):
- synthesize or synthesise ( verb , to produce substance by combining chemical precursors)
Last updated on July 23rd, 2021
You will also like...

Genetic Information and Protein Synthesis

Protein Synthesis
Role of Golgi Apparatus & Endoplasmic Reticulum in Protein Synthesis
Protein Activity and Cellular Metabolism
Regulation of Organic Metabolism, Growth and Energy Balance
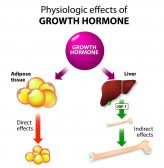
Animal Growth Hormones
Related articles....

Tools and Methods for Data Collection in Ethnobotanical Studies of Homegardens

Biodiversity

Sex Reversal – When Males Grew Ovaries Instead of Testes

A Protein Being Born – a live cell imaging of RNA translation
Cells know when to separate at mitosis
- More from M-W
- To save this word, you'll need to log in. Log In
Definition of antithesis
Did you know.
Writers and speechmakers use the traditional pattern known as antithesis for its resounding effect; John Kennedy's famous "ask not what your country can do for you—ask what you can do for your country" is an example. But antithesis normally means simply "opposite". Thus, war is the antithesis of peace, wealth is the antithesis of poverty, and love is the antithesis of hate. Holding two antithetical ideas in one's head at the same time—for example, that you're the sole master of your fate but also the helpless victim of your terrible upbringing—is so common as to be almost normal.
Examples of antithesis in a Sentence
These examples are programmatically compiled from various online sources to illustrate current usage of the word 'antithesis.' Any opinions expressed in the examples do not represent those of Merriam-Webster or its editors. Send us feedback about these examples.
Word History
Late Latin, from Greek, literally, opposition, from antitithenai to oppose, from anti- + tithenai to set — more at do
1529, in the meaning defined at sense 1b(1)
Dictionary Entries Near antithesis
anti-theoretical
Cite this Entry
“Antithesis.” Merriam-Webster.com Dictionary , Merriam-Webster, https://www.merriam-webster.com/dictionary/antithesis. Accessed 16 Apr. 2024.
Kids Definition
Kids definition of antithesis, more from merriam-webster on antithesis.
Nglish: Translation of antithesis for Spanish Speakers
Britannica English: Translation of antithesis for Arabic Speakers
Britannica.com: Encyclopedia article about antithesis
Subscribe to America's largest dictionary and get thousands more definitions and advanced search—ad free!

Can you solve 4 words at once?
Word of the day, inalienable.
See Definitions and Examples »
Get Word of the Day daily email!
Popular in Grammar & Usage
Your vs. you're: how to use them correctly, every letter is silent, sometimes: a-z list of examples, more commonly mispronounced words, how to use em dashes (—), en dashes (–) , and hyphens (-), absent letters that are heard anyway, popular in wordplay, the words of the week - apr. 12, 10 scrabble words without any vowels, 12 more bird names that sound like insults (and sometimes are), 9 superb owl words, 15 words that used to mean something different, games & quizzes.

Anthesis is a phenomenon which refers to
formation of pollen
reception of pollen by stigma
opening of flower bud
development of anther
The correct option is C opening of flower bud The correct option is C. Explanation of correct option: Anthesis refers to the period of opening of the flower bud. In this way, the flower becomes functional. In anthesis, the style is extended far beyond the upper perianth of a flower. It facilitates the pollination process. Diurnal anthesis is observed in bright-coloured flowers which attract insects. Nocturnal anthesis is found in white or pale-coloured flowers that attract several moth species. Explanation of incorrect option: Option A: The formation of pollen is known as microgametogenesis. Pollen grains are formed from microspores. Option B: Reception of pollen by stigma is termed pollination. There are three types of pollination- autogamy, geitonogamy, and xenogamy. Option D: The anther is a part of the flower which plays an important role in pollen formation. The anther contains microsporangia which are developed from a group of cells called eusporangiate. Final answer: Anthesis is a phenomenon which refers to the opening of the flower bud.

Anthesis is

- Daily Crossword
- Word Puzzle
- Word Finder
- Word of the Day
- Synonym of the Day
- Word of the Year
- Language stories
- All featured
- Gender and sexuality
- All pop culture
- Grammar Coach ™
- Writing hub
- Grammar essentials
- Commonly confused
- All writing tips
- Pop culture
- Writing tips
opposition; contrast: the antithesis of right and wrong.
the direct opposite (usually followed by of or to ): Her behavior was the very antithesis of cowardly.
the placing of a sentence or one of its parts against another to which it is opposed to form a balanced contrast of ideas, as in “Give me liberty or give me death.”
the second sentence or part thus set in opposition, as “or give me death.”
Philosophy . See under Hegelian dialectic .
Origin of antithesis
Other words for antithesis, other words from antithesis.
- self-an·tith·e·sis, noun
Words that may be confused with antithesis
- antithesis , synthesis , thesis
Words Nearby antithesis
- antisway bar
- antisymmetric
- antiterrorist
- antithetical
- antithrombin
- antithrombotic
- antitorque rotor
Dictionary.com Unabridged Based on the Random House Unabridged Dictionary, © Random House, Inc. 2024
How to use antithesis in a sentence
In the Democratic primary in his home state, he was humiliated by his old antithesis Wallace, who beat him decisively.
It cannot be stressed enough that the behavior of the sitting president is the antithesis of the ideals of American democracy, institutions or peaceful transitions.
American Christians may have chosen cynicism in 2016, but cynicism is the antithesis of the Christian faith, and cynicism won’t have the final word in America, either.
The transhuman cannot exist outside of ubuntu, of course, which is the antithesis of the colonial order for a number of reasons.
Tesla’s being touted as a go-go player in the antithesis of a go-go sector.
Belle Knox is the antithesis of Jenna Jameson—and not just in looks.
To me this is the antithesis of what travel should be about.
Married at First Sight is the antithesis of The Bachelor and The Bachelorette.
Yet its sound is the musical antithesis of a blended Frappuccino.
Now Joffrey, the Starks' black-hearted antithesis , has met a similar fate.
If you did fail, you would try Exclusion, and you would find nothing which is the antithesis of the area of New York.
Thus seen, socialism appeared as the very antithesis of law and order, of love and chastity, and of religion itself.
There is, however, but little danger of overdoing the parallel construction where there is no antithesis .
Nor is it to be wondered at, if we consider the antithesis which is presented to their usual mode of life.
He is a sentimental Classicist, and his subjects the antithesis of the Grco-Roman ideal to which he does homage in his technique.
British Dictionary definitions for antithesis
/ ( ænˈtɪθɪsɪs ) /
the exact opposite
contrast or opposition
rhetoric the juxtaposition of contrasting ideas, phrases, or words so as to produce an effect of balance, such as my words fly up, my thoughts remain below
philosophy the second stage in the Hegelian dialectic contradicting the thesis before resolution by the synthesis
Collins English Dictionary - Complete & Unabridged 2012 Digital Edition © William Collins Sons & Co. Ltd. 1979, 1986 © HarperCollins Publishers 1998, 2000, 2003, 2005, 2006, 2007, 2009, 2012
Anthesis is Opening of floral bud Maturation of anthers Development of anthers Reception of pollen by stigma
The period of anthesis refers to the opening of the floral buds. the flower becomes functional at the time of anthesis. it brings about the attraction of the pollinators, dehiscence of the anthers, receptivity of the stigma, elongation of the style etc. hence, the correct answer is 'opening of floral bud'.
Question 15 Length of pollen tube depends on the distance between (a) pollen grain and upper surface of stigma (b) pollen grain on upper surface of stigma and ovule (c) pollen grain in anther and upper surface of stigma (d) upper surface of stigma and lower part of style
The length of the pollen tube depends on the distance between : (a) pollen grain and the upper surface of the stigma (b) pollen grain on the upper surface of stigma and ovule (c) pollen grain in anther and the upper surface of the stigma (d) the upper surface of stigma and lower part of the style

IMAGES
VIDEO
COMMENTS
Anthesis is the period during which a flower is fully open and functional. It may also refer to the onset of that period. The onset of anthesis is spectacular in some species. In Banksia species, for example, anthesis involves the extension of the style far beyond the upper perianth parts.
anthesis: [noun] the action or period of opening of a flower.
The term anthesis is derived from the Greek word anthos, meaning flower, and has been used in botanical literature to describe the flowering stage since at least the 18th century. The study of anthesis and its patterns has been important in the field of botany for understanding plant reproduction and the evolution of flowering plants.
anthesis. The period from the initial display of pistillate floret style branches until all pistillate floret style branches are enveloped by pappus bristles; generally 4—7 days. This is most apparent when the pappus of the peripheral florets is exserted from the involucre. The term is here applied to the head as a whole, not individual florets.
anthesis: 1 n the time and process of budding and unfolding of blossoms Synonyms: blossoming , efflorescence , florescence , flowering , inflorescence Type of: development , growing , growth , maturation , ontogenesis , ontogeny (biology) the process of an individual organism growing organically; a purely biological unfolding of events ...
Botany. 1783-. The stage at which a flower is open, allowing fertilization to occur. Also: an instance of this. 1783. The Anthesis [Latin Anthesis] takes place, when the burnt Anthers scatter their bags of Dust upon the Stigma. translation of C. Linnaeus, Syst. Veg. (1785) vol. I. 10.
Examples of how to use "anthesis" in a sentence from Cambridge Dictionary.
anthesis 1. The time of flowering in a plant. This appears to be a response to a combination of factors including day-length, temperature, and rainfall, but may also be initiated by the addition of gibberellins, one of a group of growth-promoting substances.2. The opening of a flower bud. Source for information on anthesis: A Dictionary of Plant Sciences dictionary.
Anthesis definition: the period or act of expansion in flowers, especially the maturing of the stamens. See examples of ANTHESIS used in a sentence.
Anthesis. Flowering is the transition from vegetative to reproductive phase in the plant lifecycle. From: Molecular Plant, 2010. Related terms: Ethylene; Pollinator; ... D.-J. Yun, in International Review of Cell and Molecular Biology, 2016. 5.1 FLC Integrating Cold and ABA Into Autonomous and Vernalization Pathways. FLC, ...
Anthesis definition: The period during which a flower is fully open and functional.
Darwin named this phenomenon "antithesis," meaning that one expression is the reversal of another. He found it also in animals. For example, a dog preparing for attack bristles its hair, so as to appear larger. For the same purpose it raises its ears, and stands upright.
Define anthesis. anthesis synonyms, anthesis pronunciation, anthesis translation, English dictionary definition of anthesis. n. pl. an·the·ses The period during which a flower is fully open and functional.
Moreover, we analyzed if the structural features and changes in the pre-anthesis and post-anthesis spikelets can determine the anther extrusion ability. In addition, we tried to assess to what degree the floral biology (diurnal pattern of anther extrusion, the efficiency for anther extrusion, the pollen production and pollen dispersion ...
Key message The developmental stage of anther development is generally more sensitive to abiotic stress than other stages of growth. Specific ROS levels, plant hormones and carbohydrate metabolism are disturbed in anthers subjected to abiotic stresses. Abstract As sessile organisms, plants are often challenged to multiple extreme abiotic stresses, such as drought, heat, cold, salinity and ...
antithesis of See full entry Word Origin late Middle English (originally denoting the substitution of one grammatical case for another): from late Latin, from Greek antitithenai 'set against', from anti 'against' + tithenai 'to place'.
The determination of the time of flower opening and closing was performed in periodic visits to the experimental field of Embrapa Agrobiologia, where the exact moments in which the anthesis and the closure of flowers were observed, and the flower was only considered totally open when petals and sepals were definitively positioned, ceasing the opening movement, presenting approximate angulation ...
The time when a flower becomes sexually functional.... Click for English pronunciations, examples sentences, video.
Definition. noun, plural: syntheses. ( biochemistry) The production of an organic compound in a living thing, especially as aided by enzymes. Supplement. In general, the term synthesis pertains to the creation of something. It is the process of combining two or more components to produce an entity. In biochemistry, it refers to the production ...
antithesis: [noun] the direct opposite. the rhetorical contrast of ideas by means of parallel arrangements of words, clauses, or sentences (as in "action, not words" or "they promised freedom and provided slavery"). opposition, contrast. the second of two opposing words, clauses, or sentences that are being rhetorically contrasted.
In anthesis, the style is extended far beyond the upper perianth of a flower. It facilitates the pollination process. Diurnal anthesis is observed in bright-coloured flowers which attract insects. Nocturnal anthesis is found in white or pale-coloured flowers that attract several moth species. Explanation of incorrect option: Option A:
Antithesis definition: opposition; contrast. See examples of ANTITHESIS used in a sentence.
Biology. Structure of Male Reproductive Part. Question. Anthesis is . A. Opening of floral bud. B. Development of anthers. C. Maturation of anthers. D. ... The period of anthesis refers to the opening of the floral buds. The flower becomes functional at the time of anthesis. It brings about the attraction of the pollinators, dehiscence of the ...