Innovation in Eye Care, Research & Education
- See us on facebook
- See us on linkedin
- See us on instagram


The Program
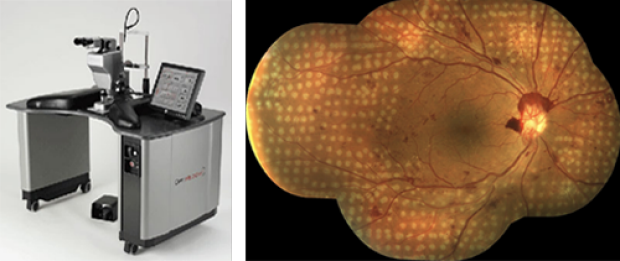
Daniel Palanker, PhD, and Mark Blumenkranz, MD, co-invented the PASCAL patterned scanning laser that is now considered the standard of care in the treatment of diabetic retinopathy and has been used to treat millions of patients worldwide. Retina photo courtesy of Daniel Lavinsky, MD.
Dr. Mark Blumenkranz established the Ophthalmic Innovation Program at the Byers Eye Institute at Stanford—the first of its kind nationally—in 2016. The Program is an immersive, year-long didactic and project-based fellowship in the conceptualization and implementation of technology to improve eye care. The Program aims to teach participants to advance innovative eye care solutions for important unmet needs and to thereby improve the lives of people living with vision loss and eye disease.
The Ophthalmic Innovation Program teaches the basic principles that facilitate the introduction of products and technical advances into clinical practice: regulatory science, clinical trial design, strategic decision making, reimbursement, protection of intellectual property, and the development of a high-performance organizational structure – topics not taught in medical school or in residency but are critical for successful medical innovation.
Recent Events:
Faculty and fellows from the Ophthalmic Innovation Program have been involved as planning committee members, panelists, and speakers on national workshops and meetings led in conjunction with the FDA’s Center for Devices and Radiological Health, including the October 2017 FDA workshop on Ophthalmic Digital Health and the April 2019 Forum on Laser-based Eye Imaging . Program participants have also been integrally involved in the Collaborative Community on Ophthalmic Imaging that was formed in response to a formal public call for proposals by the FDA .

Byers Eye Institute at Stanford hosts Collaborative Community on Ophthalmic Imaging conference
On September 3 and 4, the Byers Eye Institute at Stanford, as the convening center for the Collaborative Community on Ophthalmic Imaging (CCOI), co-hosted a two-day virtual conference on “The Future of Artificial Intelligence (AI) Enabled Ophthalmic Imaging.”

RECENT PAPERS AUTHORED BY INNOVATION FELLOWS:
Brodie F, Repka M, Burns SA, Prakalapakorn SG, Morse C, Schuman JS, Duenas MR, Afshari N, Pollack JS, Thorne JE, Vitale A, Sen HN, Myung D, Blumenkranz MS, Tu E, Hammer DX, Tarver M, Cunningham B, Kagemann L, Sadda S, Sarraf D, Jaffe GJ, Eydelman M. Development, Validation, and Innovation in Ophthalmic Laser-Based Imaging: Report From a US Food and Drug Administration-Cosponsored Forum . JAMA Ophthalmology , 2020 Nov 19. doi: 10.1001/jamaophthalmol.2020.4994. Online ahead of print. PMID: 33211074
Brodie F, Nguyen T, Eydelman M. US Food and Drug Administration Regulatory Programs for Innovative Technologies . JAMA Ophthalmology , 2019 Sep 26. doi: 10.1001/jamaophthalmol.2019.3708. Online ahead of print. PMID: 31556915
Bodnar ZM, Schuchard R, Myung D, Tarver ME, Blumenkranz M, Afshari NA, Humayun, MS, Morse C, Nischal K, Repka MX, Sprunger D, Trese M, and Eydelman M. Evaluating New Ophthalmic Digital Devices for Safety and Effectiveness in the Context of Rapid Technological Development. JAMA Ophthalmology , 2019, 137(8):939-944.PMID: 31169870
Bodnar ZM, Tarver ME, Eydelman M. Accelerating Innovation in Ophthalmic Digital Health: New Frontiers for Medical Devices. JAMA Ophthalmology , 2017 Dec 1;135(12):1291-1292. PMID: 29075778
A Tradition of Successful Innovation
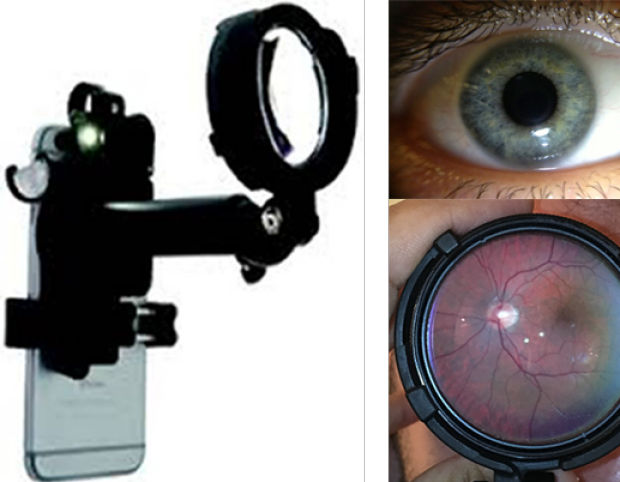
David Myung, MD, PhD, and Robert Chang, MD, co-invented the Paxos Scope™, the first combined anterior and posterior ophthalmic camera system for smartphones. Seed-funded through a grant from the Stanford Biodesign Program. Paxos Scope was registered with the FDA as a 510k Class II exempt ophthalmic camera in 2015 and received CE Mark on 2017.
Clinician-scientists and basic researchers at the Byers Eye Institute at Stanford have a rich tradition in ophthalmic innovation. Over the past several decades Stanford Ophthalmology faculty have led the way toward a number of ground breaking discoveries and technologies, many of which have been out-licensed through the Stanford Office of Technology Licensing and served as the foundation for the establishment of innovative Silicon Valley companies that translated them into practice-altering commercial products. These technologies are in various stages of evolution ranging from pre-clinical studies to full FDA approval, and have provided the roots for a number of free-standing, venture-backed companies, including Optimedica (which developed the PASCAL and Catalys laser and was acquired by AMO), PEAK Surgical (plasma-mediated surgical tools, acquired by Medtronic), Oculeve (neurostimulation devices for dry eye, acquired by Allergan), Adverum Biotechnologies (gene therapy, NASDAQ:ADVM), Pixium (artificial retinal prosthesis) and Verana Health (data science). Millions of patients worldwide have been impacted by technologies developed by inventors from Stanford Ophthalmology.

Fellowship Information
We are honored that the Byers family established the Byers Family Ophthalmic Innovation Fellowship. Brook Byers, founding member of Kleiner Perkins and legendary Silicon Valley life science investor, has been a long-time Stanford supporter and advisor, and also serves as a mentor to Innovation Fellows. Thanks to this generosity, the Byers Ophthalmic Innovation Fellow receives training in the sequential stages of development necessary for successful commercialization and adoption into patient care. The Program includes formal academic and project-based instruction including formal coursework; close mentorship and regular seminars on ophthalmic innovation by experts from academia and industry; workshops on regulatory science with leading professional societies and the FDA; networking and internship opportunities with members of the Department of Ophthalmology, other Stanford departments, Silicon Valley innovators, and colleagues at the FDA; and hands-on collaborative projects carried out longitudinally throughout the fellowship year.
Stanford University and the Department of Ophthalmology have existing collaborative educational and research programs in place with the Center for Devices and Radiological Health (CDRH) at the Food and Drug Administration (FDA) in the area of regulatory science. Fellows have the opportunity to participate in projects within these programs during the course of the year.
The Stanford Ophthalmic Innovation Program is affiliated with the Stanford Byers Center for Biodesign. Byers Ophthalmic Innovation Fellows have the opportunity to apply for the two-quarter Biodesign Innovation Course (BioE 274A/B) and participate in selected other Stanford educational programs in these basic areas offered by the university. For more information about Biodesign, please visit biodesign.stanford.edu .
HOW TO APPLY

Fellows who have completed ophthalmology residency training will have the opportunity to do up to 1 day per week of clinical work during the year. The timeframe of the fellowship is 12 months, beginning July 1, 2025 through June 30, 2026.
Applicants should prepare a CV and 1-page personal statement that includes goals for the year and career following the fellowship. We will be accepting applications through June 30th, 2025. Applications and questions should be directed to Niloufar Pirkhezri ( [email protected] )
Honoring Mark S. Blumenkranz, MD
In recognition of Dr. Blumenkranz’s pivotal role in fostering ophthalmic innovation and for teaching the principles for successfully moving those innovations into patient care, we have established The Mark Blumenkranz Ophthalmic Innovation Endowed Fund . Endowment funding will ensure long term sustainable funding for this important program. Please consider recognizing Dr. Blumenkranz and ensuring the future of this program through your generous support.
Ophthalmic Innovation Fellows
2024-2025 BYERS FAMILY OPHTHALMIC INNOVATION FELLOW

Sachin Rajpal, MD, MS
Medical School: Howard University Residency: Howard University Hospital
Sachin Rajpal grew up in the Washington D.C. area. He majored in Decision Sciences at Carnegie Mellon University and completed a master's in physiology and biophysics from Georgetown. He has experience working in health economics and outcomes research, performing economic modeling, and evaluating unmet needs in various diseases. Sachin completed his Ophthalmology residency and medical school at Howard University. He spent time in medical school and residency at a few healthcare device and technology startups where he designed and implemented clinical trials. Sachin is passionate about improving patient care and outcomes through innovative technology. In his free time, he enjoys spending time with his 2 year old daughter, Ayla, and exploring the world with her.
2023-2024 BYERS FAMILY OPHTHALMIC INNOVATION FELLOW

Kevin Jackson, MD
Medical School: Perelman School of Medicine at the University of Pennsylvania Residency: Duke Eye Center
Dr. Jackson graduated from Rice University with a degree in Bioengineering and minors in Global Health and Biochemistry. He then attended medical school at the Perelman School of Medicine at the University of Pennsylvania where he also completed a research year in ophthalmology specifically adaptive optics scanning light ophthalmoscopy. After his PGY-1 year at Pennsylvania Hospital, he completed residency at Duke Eye Center and was awarded the Edward J Isbey Jr Resident Award during his final year. He then went on to complete his Cornea Fellowship at Duke Eye Center.
2022-2023 BYERS FAMILY OPHTHALMIC INNOVATION FELLOW

Bryce Chiang, MD, PhD
Bryce Chiang grew up in Freehold, New Jersey. As an undergraduate while working under the mentorship of a physician scientist at the Johns Hopkins University, he developed an interest in developing innovative treatments for diseases with no current cure. With the goal of becoming a physician scientist himself, Bryce completed his MD at the Emory University School of Medicine and his PhD in biomedical engineering at the Georgia Institute of Technology. His PhD work was focused on ocular drug delivery, specifically the pharmacokinetics of microneedle injections into the suprachoroidal space. Dr. Chiang completed his ophthalmology residency at the Byers Eye Institute at Stanford. While in residency, he realized that targeted drug delivery to the optic nerve head could enable new treatment strategies in optic neuropathies such as glaucoma, and he developed a novel surgical technique to achieve this. Now as the 2022-2023 Byers Family Ophthalmic Innovation Fellow and the 2022-2024 glaucoma clinical and research fellow, he is furthering his work in this area. Dr. Chiang’s current interest includes targeted ocular drug delivery specifically to the optic nerve head, utilizing the suprachoroidal space to treat ocular disease, and understanding the pathogenesis of glaucoma. In his spare time, he loves exploring the Bay Area and California with his wife Pauline and son Nathan.
2021-2022 BYERS FAMILY OPHTHALMIC INNOVATION FELLOW

Eliot Dow, MD, PhD
Eliot Dow was born and raised in Ohio. He attended Ohio State University where he developed an interest in science and medicine. This led him to earn an MD at Cornell University and a PhD at The Rockefeller University studying sensory nervous system development with Jim Hudspeth. During his time in the MD/PhD program, he was also a visiting scientist and later post-doctoral researcher with Moritz Helmstaedter at the Max Planck Institute for Brain Research working on human-in-the-loop machine learning for large neuroscience data sets. He completed an ophthalmology residency at the Stein Eye Institute of UCLA. While in residency, he studied the application of machine learning to ophthalmology as an honorary research fellow at Moorfields Eye Hospital with Pearse Keane and undertook informatics training at UCLA. He joined the Byers Eye Institute as the 2021-2022 Stanford Ophthalmic Innovation Fellow. With this experience, he hopes to integrate human-centered data science and machine learning into ophthalmology to improve patient outcomes and provider satisfaction.
2020-2021 BYERS FAMILY OPHTHALMIC INNOVATION FELLOW

Michael Mbagwu, MD
Dr. Mbagwu completed his ophthalmology residency at Northwestern University and a transitional year internship at Presence Resurrection Medical Center in Chicago. He completed his MD from Northwestern University Feinberg School of Medicine. During that time, he had the opportunity to complete a grant-supported research year serving as Lead Project Coordinator for a National Eye Institute-funded study. He helped develop clinical data mining tools to characterize disparities in eye care using an assembly of electronic health records from several health care institutions in the Chicagoland area. This also gave him an interest in further exploring the intersection of technology, innovation, and healthcare delivery. Dr. Mbagwu attended The Ohio State University for his undergraduate studies, where he developed his deep interest in Big Data/informatics, working at several institutions including the Air Force Research Laboratory (AFRL), Harvard/Brigham and Women's Hospital, and Stanford University on a variety of data-driven research projects. In his free time, Dr. Mbagwu enjoys running, travel, and watching college football.
2019-2020 OPHTHALMIC INNOVATION FELLOW

Aaron Webel, MD, MBA
Aaron Webel, MD, MBA, was the 2019-2020 Ophthalmic Innovation Fellow. He recently finished his clinical glaucoma fellowship at the Bascom Palmer Eye Institute, and prior to that completed his ophthalmology residency at the University of Florida. He is a graduate of the MD/MBA dual degree program at the University of North Carolina School of Medicine and Kenan-Flagler Business School. He joined Hatteras Venture Partners in Durham, NC as the Discovery Fellow where he provided strategic ophthalmic disease target recommendations for the novel injection technology developed by one of their ophthalmology portfolio companies. He has a keen interest in ophthalmic medical device research and was recently a co-principal investigator on a University of Florida Opportunity Seed Fund grant to develop a novel implantable thin-film intraocular pressure sensor. Aaron’s current interests include development of minimally invasive glaucoma drainage device technologies with a goal of greater predictability and lower failure rates than current glaucoma surgical modalities. In his spare time, he enjoys playing ultimate frisbee and exploring the Bay Area with his wife Mackenzie and two daughters, Alta and Berit.
2018-2019 OPHTHALMIC INNOVATION FELLOW

Frank Brodie, MD, MBA
Frank Brodie, MD, MBA, joined the Department of Ophthalmology as the 2018-19 Innovation Fellow. He completed his Ophthalmology residency at the University of California San Francisco. Prior to that, Frank was at Harbor UCLA for an internal medicine internship. He completed his MD and MBA at University of Pennsylvania School of Medicine and The Wharton School respectively. Frank is very active in translational research; during his residency, in conjunction with Caltech, he developed a device to assist patient head-positioning correctly after intraocular surgery. Frank also developed a system to provide custom glasses for children with craniofacial malformations using advance imaging technology coupled with 3D printing. Additionally, he has been working with scientists at Caltech to develop a novel method to prevent amblyopia and glaucoma in children born with congenital cataracts.
2017-2018 OPHTHALMIC INNOVATION FELLOW

Alexander Kreymerman, MS, PhD
Alexander Kreymerman holds an MS in molecular biology, a PhD in neuroscience, and has received scientific training within ophthalmology departments at University of Miami, University of California San Diego, and Stanford University. His major research projects and professional interests are focused on retinal regeneration and neuroprotection, the development of novel, high resolution, and minimally invasive intraoperative imaging and microscopy devices, as well as gene therapy, stem cells, and drug development for ocular pathologies. As the Innovation Fellow in the ophthalmology department, Alex pushed forward research that focused on gene therapy and stem cell technology for modeling and designing treatments in retinal dystrophies, as well as intraoperative imaging techniques for retinal and orbital surgery.
2016-2017 OPHTHALMIC INNOVATION FELLOW

Zachary Bodnar, MD
Dr. Bodnar was born and raised in Salt Lake City, Utah. He attended the Massachusetts Institute of Technology, where he earned a BS in electrical engineering and computer science, followed by a masters of engineering, also in electrical engineering and computer science. He worked for the enterprise software startup Endeca for several years, prior to its acquisition by Oracle, until he matriculated in Dartmouth Medical School where he earned his MD. Dr. Bodnar completed the Ophthalmic Pathology and Research fellowship at the John A. Moran Eye Center, at the University of Utah, internship in general surgery at the Mayo Clinic Hospital in Phoenix and residency in ophthalmology at Saint Louis University. His academic interests include machine vision, machine learning, digital signal processing and their applications to ophthalmic imaging and medical informatics. Dr. Bodnar was the first ophthalmic innovation fellow and continued on afterward at Stanford as a vitreoretinal fellow, which he completed in 2019.

Mark Blumenkranz, MD PROGRAM FOUNDER, CO-DIRECTOR H. J. SMEAD PROFESSOR OF OPHTHALMOLOGY, EMERITUS

David Myung, MD, PhD PROGRAM DIRECTOR ASSOCIATE PROFESSOR OF OPHTHALMOLOGY, AND, BY COURTESY, CHEMICAL ENGINEERING

Darius Moshfeghi, MD FACULTY MENTOR PROFESSOR OF OPHTHALMOLOGY

Daniel Palanker, PhD FACULTY MENTOR PROFESSOR OF OPHTHALMOLOGY

Robert Chang, MD FACULTY MENTOR ASSOCIATE PROFESSOR OF OPHTHALMOLOGY

Jeffrey Goldberg, MD, PhD FACULTY MENTOR BLUMENKRANZ SMEAD PROFESSOR AND CHAIR OF OPHTHALMOLOGY
Visionary Innovation Mentors

Brook Byers, MBA PARTNER KLEINER PERKINS CAUFIELD AND BYERS

Emmett Cunningham Jr., MD, PhD, MPH SENIOR MANAGING DIRECTOR, BLACKSTONE LIFE SCIENCES ADJUNCT CLINICAL PROFESSOR, BYERS EYE INSTITUTE

Malvina Eydelman, MD DIRECTOR OF THE OFFICE OF OPHTHALMIC, ANESTHESIA, RESPIRATORY, ENT AND DENTAL DEVICES- FDA

Gil Kliman, MD, MBA MANAGING DIRECTOR INTERWEST PARTNERS

Dimitri Azar, MD, MBA CLINICAL LEAD, OPHTHALMOLOGY PROGRAMS VERILY LIFE SCIENCES

Karen Havenstrite, PhD CO-FOUNDER AND CTO TANGIBLE SCIENCE

Adrienne Graves, PhD FORMER PRESIDENT & CEO SANTEN, INC.

Leslie Bottorff, MBA PRINCIPAL BOTTORFF ADVISORS

Ranya Habash, MD CEO, LIFELONG VISION FDA DIGITAL HEALTH NETWORK OF EXPERTS

Daniel Ting, MBBS (Hons), M Med(Ophth), FAMS, PhD (UWA) SINGAPORE NATIONAL EYE CENTER (SNEC) DIRECTOR OF SINGAPORE HEALTH SERVICE (SINGHEALTH) AI OFFICE SNEC CHIEF DIGITAL AND DATA OFFICER AND HEAD OF AI AND DIGITAL INNOVATION IN SINGAPORE EYE RESEARCH INSTITUTE (SERI)

Victor Chong, MD, MBA Global Head, Retinal Disease Areas Stronghold Janssen Research & Development

IMAGES
VIDEO